User login
MET/MEK inhibitor duo shows activity in resistant NSCLC
ATLANTA – A combination of the epidermal growth factor receptor–targeted agent osimertinib (Tagrisso) with selumetinib, an investigational inhibitor of MEK1/2, was safe and associated with partial responses in about one-third of patients with non–small cell lung cancer in the phase 1b TATTON trial.
In the dose-finding phase of the trial, the objective response rate was 42% for 36 patients treated with the combination either as a second- or third-line therapy, or following prior therapy for the epidermal growth factor receptor (EGFR) T70M mutation. In the dose-expansion phase of the trial, the ORR was 34% for 47 patients treated regardless of mutational status, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute at Emory University, Atlanta.
“We conclude that combining osimertinib with intermittent selumetinib is feasible with manageable toxicity and has already demonstrated promising preliminary anticancer activity,” he said at the annual meeting of the American Association for Cancer Research.
The EGFR T790M mutation is the most common cause of resistance in patients with non–small cell lung cancer (NSCLC) bearing EGFR mutations who are treated with first- or second-generation, EGFR-targeted tyrosine kinase inhibitors (TKI). Up-regulation of the RAS/RAF/MEK/ERK signaling pathway has also been implicated in NSCLC resistance to EGFR-targeted TKIs.
Selumetinib is an oral, potent, and selective inhibitor of MEK1/2 with a short half-life.
In the phase 3 SELECT-1 trial, selumetinib in combination with docetaxel did not significantly improve progression-free survival, compared with docetaxel alone as second-line therapy for patients with KRAS-mutated NSCLC.
Therapeutic rationale
Invited discussant Roy S. Herbst, MD, PhD, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn., said that there is sound rationale for combining osimertinib and selumetinib in EGFR-mutant NSCLC.
In addition to the up-regulation of the RAS/RAF/MEK/ERK pathway noted before, resistance to osimertinib has been shown in models of EGFR-mutant NSCLC to develop from aberrant ERK signaling mediation in part by MEK1 amplification, and MEK kinase inhibitors can restore sensitivity to osimertinib in resistant cells, he said.
As previously reported, the TATTON investigators are evaluating in separate cohorts combinations of osimertinib with savolitinib, an investigational MET inhibitor for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents, or with selumetinib for patients with advanced EGFR-mutated NSCLC that had progressed on prior therapy, including EGFR-targeted TKIs, irrespective of T790M or KRAS status.
In part A, the dose-finding phase, patients received osimertinib 80 mg daily plus intermittent or continuous selumetinib. Asian patients received continuous selumetinib 25/50 mg twice daily, while other patients received continuous selumetinib 50/75 mg twice daily, or intermittent selumetinib 75 mg twice daily 4 days on, 3 days off or on days 1 and 4 of each week of treatment.
In part B, the dose-expansion phase, patients received osimertinib plus intermittent selumetinib 75 mg twice daily on the 4 days on/3 days off schedule.
An analysis of preliminary antitumor activity in part A showed an ORR in 15 of 36 patients (42%); all were partial responses (PR). In addition, 14 patients (39%) had stable disease at 6 weeks, 3 had progressive disease, 2 died, and 2 were not evaluable. The median duration of response was 16.6 months; 77% of the patients had responses lasting at least 12 months.
In part B, 16 of 47 patients enrolled (34%) had confirmed PR, and 16 had stable disease. Of the remaining patients, 11 had disease progression, 2 died, and 2 were not evaluable. The median duration of response in this group was 9.1 months, and 31% of patients remained in response at 12 months.
The most common treatment-related adverse events in the dose-finding phase were diarrhea in 75% of patients, nausea in 39% and fatigue in 33%. Dose-limiting toxicities occurred in six patients, all of whom had been treated with continuous selumetinib. These toxicities, all grade 3, include liver enzyme increases, diarrhea, asthenia, dizziness, nausea, and pneumonitis.
The most common treatment-related adverse events in the dose-expansion phase were diarrhea in 81%, stomatitis in 32%, and paronychia in 30% of patients.
Results ‘okay’
In his discussion, Dr. Herbst commented that the part A results “look okay, until you realize that the most of the activity is in those patients who are T790M-positive, who have not been exposed in this cohort to a third-generation T790M inhibitor.” Patients with the mutation who are treated with third-generation inhibitors would be expected to have a 78% response rate.
Part B included a few more patients with responses who were negative for T790M. “My thought here is that perhaps there is a biomarker” for selecting patients most likely to benefit from the combination, he said.
For MET-negative patients, the combination appears to have manageable toxicities with noncontinuous dosing of selumetinib, and there may be benefit to using it in the first-line setting in select patients, but that will require further trials and identification of suitable biomarkers, Dr. Herbst summarized.
TATTON was sponsored by AstraZeneca. Dr. Ramalingam reported receiving research support from the company and consulting/contracting with others. Dr. Herbst reported receiving research support from AstraZeneca, Eli Lilly, and Merck, and serving as a consultant for AstraZeneca, Eli Lilly, Genentech/Roche, Merck, NextCure, and Pfizer.
SOURCE: Ramalingam SS et al. AACR 2019, Abstract CT034.
ATLANTA – A combination of the epidermal growth factor receptor–targeted agent osimertinib (Tagrisso) with selumetinib, an investigational inhibitor of MEK1/2, was safe and associated with partial responses in about one-third of patients with non–small cell lung cancer in the phase 1b TATTON trial.
In the dose-finding phase of the trial, the objective response rate was 42% for 36 patients treated with the combination either as a second- or third-line therapy, or following prior therapy for the epidermal growth factor receptor (EGFR) T70M mutation. In the dose-expansion phase of the trial, the ORR was 34% for 47 patients treated regardless of mutational status, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute at Emory University, Atlanta.
“We conclude that combining osimertinib with intermittent selumetinib is feasible with manageable toxicity and has already demonstrated promising preliminary anticancer activity,” he said at the annual meeting of the American Association for Cancer Research.
The EGFR T790M mutation is the most common cause of resistance in patients with non–small cell lung cancer (NSCLC) bearing EGFR mutations who are treated with first- or second-generation, EGFR-targeted tyrosine kinase inhibitors (TKI). Up-regulation of the RAS/RAF/MEK/ERK signaling pathway has also been implicated in NSCLC resistance to EGFR-targeted TKIs.
Selumetinib is an oral, potent, and selective inhibitor of MEK1/2 with a short half-life.
In the phase 3 SELECT-1 trial, selumetinib in combination with docetaxel did not significantly improve progression-free survival, compared with docetaxel alone as second-line therapy for patients with KRAS-mutated NSCLC.
Therapeutic rationale
Invited discussant Roy S. Herbst, MD, PhD, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn., said that there is sound rationale for combining osimertinib and selumetinib in EGFR-mutant NSCLC.
In addition to the up-regulation of the RAS/RAF/MEK/ERK pathway noted before, resistance to osimertinib has been shown in models of EGFR-mutant NSCLC to develop from aberrant ERK signaling mediation in part by MEK1 amplification, and MEK kinase inhibitors can restore sensitivity to osimertinib in resistant cells, he said.
As previously reported, the TATTON investigators are evaluating in separate cohorts combinations of osimertinib with savolitinib, an investigational MET inhibitor for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents, or with selumetinib for patients with advanced EGFR-mutated NSCLC that had progressed on prior therapy, including EGFR-targeted TKIs, irrespective of T790M or KRAS status.
In part A, the dose-finding phase, patients received osimertinib 80 mg daily plus intermittent or continuous selumetinib. Asian patients received continuous selumetinib 25/50 mg twice daily, while other patients received continuous selumetinib 50/75 mg twice daily, or intermittent selumetinib 75 mg twice daily 4 days on, 3 days off or on days 1 and 4 of each week of treatment.
In part B, the dose-expansion phase, patients received osimertinib plus intermittent selumetinib 75 mg twice daily on the 4 days on/3 days off schedule.
An analysis of preliminary antitumor activity in part A showed an ORR in 15 of 36 patients (42%); all were partial responses (PR). In addition, 14 patients (39%) had stable disease at 6 weeks, 3 had progressive disease, 2 died, and 2 were not evaluable. The median duration of response was 16.6 months; 77% of the patients had responses lasting at least 12 months.
In part B, 16 of 47 patients enrolled (34%) had confirmed PR, and 16 had stable disease. Of the remaining patients, 11 had disease progression, 2 died, and 2 were not evaluable. The median duration of response in this group was 9.1 months, and 31% of patients remained in response at 12 months.
The most common treatment-related adverse events in the dose-finding phase were diarrhea in 75% of patients, nausea in 39% and fatigue in 33%. Dose-limiting toxicities occurred in six patients, all of whom had been treated with continuous selumetinib. These toxicities, all grade 3, include liver enzyme increases, diarrhea, asthenia, dizziness, nausea, and pneumonitis.
The most common treatment-related adverse events in the dose-expansion phase were diarrhea in 81%, stomatitis in 32%, and paronychia in 30% of patients.
Results ‘okay’
In his discussion, Dr. Herbst commented that the part A results “look okay, until you realize that the most of the activity is in those patients who are T790M-positive, who have not been exposed in this cohort to a third-generation T790M inhibitor.” Patients with the mutation who are treated with third-generation inhibitors would be expected to have a 78% response rate.
Part B included a few more patients with responses who were negative for T790M. “My thought here is that perhaps there is a biomarker” for selecting patients most likely to benefit from the combination, he said.
For MET-negative patients, the combination appears to have manageable toxicities with noncontinuous dosing of selumetinib, and there may be benefit to using it in the first-line setting in select patients, but that will require further trials and identification of suitable biomarkers, Dr. Herbst summarized.
TATTON was sponsored by AstraZeneca. Dr. Ramalingam reported receiving research support from the company and consulting/contracting with others. Dr. Herbst reported receiving research support from AstraZeneca, Eli Lilly, and Merck, and serving as a consultant for AstraZeneca, Eli Lilly, Genentech/Roche, Merck, NextCure, and Pfizer.
SOURCE: Ramalingam SS et al. AACR 2019, Abstract CT034.
ATLANTA – A combination of the epidermal growth factor receptor–targeted agent osimertinib (Tagrisso) with selumetinib, an investigational inhibitor of MEK1/2, was safe and associated with partial responses in about one-third of patients with non–small cell lung cancer in the phase 1b TATTON trial.
In the dose-finding phase of the trial, the objective response rate was 42% for 36 patients treated with the combination either as a second- or third-line therapy, or following prior therapy for the epidermal growth factor receptor (EGFR) T70M mutation. In the dose-expansion phase of the trial, the ORR was 34% for 47 patients treated regardless of mutational status, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute at Emory University, Atlanta.
“We conclude that combining osimertinib with intermittent selumetinib is feasible with manageable toxicity and has already demonstrated promising preliminary anticancer activity,” he said at the annual meeting of the American Association for Cancer Research.
The EGFR T790M mutation is the most common cause of resistance in patients with non–small cell lung cancer (NSCLC) bearing EGFR mutations who are treated with first- or second-generation, EGFR-targeted tyrosine kinase inhibitors (TKI). Up-regulation of the RAS/RAF/MEK/ERK signaling pathway has also been implicated in NSCLC resistance to EGFR-targeted TKIs.
Selumetinib is an oral, potent, and selective inhibitor of MEK1/2 with a short half-life.
In the phase 3 SELECT-1 trial, selumetinib in combination with docetaxel did not significantly improve progression-free survival, compared with docetaxel alone as second-line therapy for patients with KRAS-mutated NSCLC.
Therapeutic rationale
Invited discussant Roy S. Herbst, MD, PhD, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn., said that there is sound rationale for combining osimertinib and selumetinib in EGFR-mutant NSCLC.
In addition to the up-regulation of the RAS/RAF/MEK/ERK pathway noted before, resistance to osimertinib has been shown in models of EGFR-mutant NSCLC to develop from aberrant ERK signaling mediation in part by MEK1 amplification, and MEK kinase inhibitors can restore sensitivity to osimertinib in resistant cells, he said.
As previously reported, the TATTON investigators are evaluating in separate cohorts combinations of osimertinib with savolitinib, an investigational MET inhibitor for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents, or with selumetinib for patients with advanced EGFR-mutated NSCLC that had progressed on prior therapy, including EGFR-targeted TKIs, irrespective of T790M or KRAS status.
In part A, the dose-finding phase, patients received osimertinib 80 mg daily plus intermittent or continuous selumetinib. Asian patients received continuous selumetinib 25/50 mg twice daily, while other patients received continuous selumetinib 50/75 mg twice daily, or intermittent selumetinib 75 mg twice daily 4 days on, 3 days off or on days 1 and 4 of each week of treatment.
In part B, the dose-expansion phase, patients received osimertinib plus intermittent selumetinib 75 mg twice daily on the 4 days on/3 days off schedule.
An analysis of preliminary antitumor activity in part A showed an ORR in 15 of 36 patients (42%); all were partial responses (PR). In addition, 14 patients (39%) had stable disease at 6 weeks, 3 had progressive disease, 2 died, and 2 were not evaluable. The median duration of response was 16.6 months; 77% of the patients had responses lasting at least 12 months.
In part B, 16 of 47 patients enrolled (34%) had confirmed PR, and 16 had stable disease. Of the remaining patients, 11 had disease progression, 2 died, and 2 were not evaluable. The median duration of response in this group was 9.1 months, and 31% of patients remained in response at 12 months.
The most common treatment-related adverse events in the dose-finding phase were diarrhea in 75% of patients, nausea in 39% and fatigue in 33%. Dose-limiting toxicities occurred in six patients, all of whom had been treated with continuous selumetinib. These toxicities, all grade 3, include liver enzyme increases, diarrhea, asthenia, dizziness, nausea, and pneumonitis.
The most common treatment-related adverse events in the dose-expansion phase were diarrhea in 81%, stomatitis in 32%, and paronychia in 30% of patients.
Results ‘okay’
In his discussion, Dr. Herbst commented that the part A results “look okay, until you realize that the most of the activity is in those patients who are T790M-positive, who have not been exposed in this cohort to a third-generation T790M inhibitor.” Patients with the mutation who are treated with third-generation inhibitors would be expected to have a 78% response rate.
Part B included a few more patients with responses who were negative for T790M. “My thought here is that perhaps there is a biomarker” for selecting patients most likely to benefit from the combination, he said.
For MET-negative patients, the combination appears to have manageable toxicities with noncontinuous dosing of selumetinib, and there may be benefit to using it in the first-line setting in select patients, but that will require further trials and identification of suitable biomarkers, Dr. Herbst summarized.
TATTON was sponsored by AstraZeneca. Dr. Ramalingam reported receiving research support from the company and consulting/contracting with others. Dr. Herbst reported receiving research support from AstraZeneca, Eli Lilly, and Merck, and serving as a consultant for AstraZeneca, Eli Lilly, Genentech/Roche, Merck, NextCure, and Pfizer.
SOURCE: Ramalingam SS et al. AACR 2019, Abstract CT034.
REPORTING FROM AACR 2019
Preclinical findings highlight value of Lynch syndrome for cancer vaccine development
ATLANTA – Lynch syndrome serves as an excellent platform for the development of immunoprevention cancer vaccines, and findings from a preclinical Lynch syndrome mouse model support ongoing research, according to Steven M. Lipkin, MD, PhD.
A novel vaccine, which included peptides encoding four intestinal cancer frameshift peptide (FSP) neoantigens derived from coding microsatellite (cMS) mutations in the genes Nacad, Maz, Xirp1, and Senp6 elicited strong antigen-specific cellular immune responses in the model, Dr. Lipkin, the Gladys and Roland Harriman Professor of Medicine and vice chair for research in the Sanford and Joan Weill Department of Medicine, Weill Cornell Medical College, New York, reported at the annual meeting of the American Association for Cancer Research.
CD4-specific T cell responses were detected for Maz, Nacad, and Senp6, and CD8-positive T cells were detected for Xirp1 and Nacad, he noted, explaining that the findings come in the wake of a recently completed clinical phase 1/2a trial that successfully demonstrated safety and immunogenicity of an FSP neoantigen-based vaccine in microsatellite unstable (MSI) colorectal cancer patients.
The current effort to further develop a cancer preventive vaccine against MSI cancers in Lynch syndrome using a preclinical mouse model involved a systematic database search to identify cMS sequences in the murine genome. Intestinal tumors obtained from Lynch syndrome mice were evaluated for mutations affecting these candidate cMS, and of 13 with a mutation frequency of 15% or higher, the 4 FSP neoantigens ultimately included in the vaccine elicited strong antigen-specific cellular immune responses.
Vaccination with peptides encoding these four intestinal cancer FSP neoantigens promoted antineoantigen immunity, reduced intestinal tumorigenicity, and prolonged overall survival, Dr. Lipkin said.
Further, based on preclinical data suggesting that naproxen in this setting might provide better risk-reducing effects, compared with aspirin (which has previously been shown to reduce colorectal cancer risk in Lynch syndrome patients), its addition to the vaccine did, indeed, improve response, he noted, explaining that naproxen worked as “sort of a super-aspirin,” that improved overall survival, compared with vaccine alone or nonsteroidal anti-inflammatory agents alone.
In a video interview, Dr. Lipkin describes his research and its potential implications for the immunoprevention of Lynch syndrome and other cancers.
Vaccination with as few as four mutations that occur across Lynch syndrome tumors induced complete cures in some mice and delays in disease onset in others, he said.
“[This is] a very simple approach, very effective,” he added, noting that the T cells are now being studied to better understand the biology of the effects. “The idea of immunoprevention ... is actually very exciting and ... can be expanded beyond this.”
Lynch syndrome is a “great place to start,” because of the high rate of mutations, which are the most immunogenic types of mutations, he said.
“If we can get this basic paradigm to work, I think we can expand it to other types of mutations – for example, KRAS or BRAF, which are seen frequently in lung cancers, colon cancers, stomach cancers, pancreatic cancers, and others,” he said, noting that a proposal for a phase 1 clinical trial has been submitted.
ATLANTA – Lynch syndrome serves as an excellent platform for the development of immunoprevention cancer vaccines, and findings from a preclinical Lynch syndrome mouse model support ongoing research, according to Steven M. Lipkin, MD, PhD.
A novel vaccine, which included peptides encoding four intestinal cancer frameshift peptide (FSP) neoantigens derived from coding microsatellite (cMS) mutations in the genes Nacad, Maz, Xirp1, and Senp6 elicited strong antigen-specific cellular immune responses in the model, Dr. Lipkin, the Gladys and Roland Harriman Professor of Medicine and vice chair for research in the Sanford and Joan Weill Department of Medicine, Weill Cornell Medical College, New York, reported at the annual meeting of the American Association for Cancer Research.
CD4-specific T cell responses were detected for Maz, Nacad, and Senp6, and CD8-positive T cells were detected for Xirp1 and Nacad, he noted, explaining that the findings come in the wake of a recently completed clinical phase 1/2a trial that successfully demonstrated safety and immunogenicity of an FSP neoantigen-based vaccine in microsatellite unstable (MSI) colorectal cancer patients.
The current effort to further develop a cancer preventive vaccine against MSI cancers in Lynch syndrome using a preclinical mouse model involved a systematic database search to identify cMS sequences in the murine genome. Intestinal tumors obtained from Lynch syndrome mice were evaluated for mutations affecting these candidate cMS, and of 13 with a mutation frequency of 15% or higher, the 4 FSP neoantigens ultimately included in the vaccine elicited strong antigen-specific cellular immune responses.
Vaccination with peptides encoding these four intestinal cancer FSP neoantigens promoted antineoantigen immunity, reduced intestinal tumorigenicity, and prolonged overall survival, Dr. Lipkin said.
Further, based on preclinical data suggesting that naproxen in this setting might provide better risk-reducing effects, compared with aspirin (which has previously been shown to reduce colorectal cancer risk in Lynch syndrome patients), its addition to the vaccine did, indeed, improve response, he noted, explaining that naproxen worked as “sort of a super-aspirin,” that improved overall survival, compared with vaccine alone or nonsteroidal anti-inflammatory agents alone.
In a video interview, Dr. Lipkin describes his research and its potential implications for the immunoprevention of Lynch syndrome and other cancers.
Vaccination with as few as four mutations that occur across Lynch syndrome tumors induced complete cures in some mice and delays in disease onset in others, he said.
“[This is] a very simple approach, very effective,” he added, noting that the T cells are now being studied to better understand the biology of the effects. “The idea of immunoprevention ... is actually very exciting and ... can be expanded beyond this.”
Lynch syndrome is a “great place to start,” because of the high rate of mutations, which are the most immunogenic types of mutations, he said.
“If we can get this basic paradigm to work, I think we can expand it to other types of mutations – for example, KRAS or BRAF, which are seen frequently in lung cancers, colon cancers, stomach cancers, pancreatic cancers, and others,” he said, noting that a proposal for a phase 1 clinical trial has been submitted.
ATLANTA – Lynch syndrome serves as an excellent platform for the development of immunoprevention cancer vaccines, and findings from a preclinical Lynch syndrome mouse model support ongoing research, according to Steven M. Lipkin, MD, PhD.
A novel vaccine, which included peptides encoding four intestinal cancer frameshift peptide (FSP) neoantigens derived from coding microsatellite (cMS) mutations in the genes Nacad, Maz, Xirp1, and Senp6 elicited strong antigen-specific cellular immune responses in the model, Dr. Lipkin, the Gladys and Roland Harriman Professor of Medicine and vice chair for research in the Sanford and Joan Weill Department of Medicine, Weill Cornell Medical College, New York, reported at the annual meeting of the American Association for Cancer Research.
CD4-specific T cell responses were detected for Maz, Nacad, and Senp6, and CD8-positive T cells were detected for Xirp1 and Nacad, he noted, explaining that the findings come in the wake of a recently completed clinical phase 1/2a trial that successfully demonstrated safety and immunogenicity of an FSP neoantigen-based vaccine in microsatellite unstable (MSI) colorectal cancer patients.
The current effort to further develop a cancer preventive vaccine against MSI cancers in Lynch syndrome using a preclinical mouse model involved a systematic database search to identify cMS sequences in the murine genome. Intestinal tumors obtained from Lynch syndrome mice were evaluated for mutations affecting these candidate cMS, and of 13 with a mutation frequency of 15% or higher, the 4 FSP neoantigens ultimately included in the vaccine elicited strong antigen-specific cellular immune responses.
Vaccination with peptides encoding these four intestinal cancer FSP neoantigens promoted antineoantigen immunity, reduced intestinal tumorigenicity, and prolonged overall survival, Dr. Lipkin said.
Further, based on preclinical data suggesting that naproxen in this setting might provide better risk-reducing effects, compared with aspirin (which has previously been shown to reduce colorectal cancer risk in Lynch syndrome patients), its addition to the vaccine did, indeed, improve response, he noted, explaining that naproxen worked as “sort of a super-aspirin,” that improved overall survival, compared with vaccine alone or nonsteroidal anti-inflammatory agents alone.
In a video interview, Dr. Lipkin describes his research and its potential implications for the immunoprevention of Lynch syndrome and other cancers.
Vaccination with as few as four mutations that occur across Lynch syndrome tumors induced complete cures in some mice and delays in disease onset in others, he said.
“[This is] a very simple approach, very effective,” he added, noting that the T cells are now being studied to better understand the biology of the effects. “The idea of immunoprevention ... is actually very exciting and ... can be expanded beyond this.”
Lynch syndrome is a “great place to start,” because of the high rate of mutations, which are the most immunogenic types of mutations, he said.
“If we can get this basic paradigm to work, I think we can expand it to other types of mutations – for example, KRAS or BRAF, which are seen frequently in lung cancers, colon cancers, stomach cancers, pancreatic cancers, and others,” he said, noting that a proposal for a phase 1 clinical trial has been submitted.
REPORTING FROM AACR 2019
CAR T cells home in on HER2 in advanced sarcomas
ATLANTA – A novel chimeric antigen receptor (CAR) T-cell construct centered on HER2 as the target antigen was safe and showed early promise in the treatment of advanced sarcomas of bone and soft tissues in a phase I trial.
One patient, a 16-year-old girl with advanced osteosarcoma metastatic to her lungs, had a complete response to the therapy that is ongoing out to nearly 3 years, reported Shoba A. Navai, MD, from Baylor College of Medicine in Houston.
A second patient, an 8-year-old boy with rhabdomyosarcoma metastatic to bone marrow, had a complete response lasting 12 months. Upon relapse he was re-enrolled, received additional CAR T-cell infusions, and had a second complete response that has been ongoing for 17 months.
“HER2 CAR T cells can induce objective clinical responses in some patients with sarcoma, and engagement of endogenous immunity may aid in generation of tumor responses. We are currently working to validate these findings in other patients who were treated,” she said at a briefing at the annual meeting of the American Association for Cancer Research.
HER2 is a member of the human epidermal growth factor receptor family that is primarily expressed on the surface of tumor cells but is largely absent from nonmalignant tissues. HER2 can be expressed in a variety of sarcomas, including osteosarcoma, and HER2 expression in osteosarcoma correlates with worse overall survival.
Unlike HER2-positive breast cancers, however, HER2 expression levels in osteosarcoma are too low to be effectively targeted by anti-HER2 agents such as trastuzumab (Hereceptin).
But as Dr. Navai and colleagues have found, HER2 appears to be a valid target for CAR T-cell therapy in otherwise antigenically “cold” tumors – that is, tumors with few targetable antigens.
Old target, new weapon
They have developed a CAR T-cell construct using a HER2-directed antibody coupled with CD28 as the costimulatory molecule. As with other CAR T therapies, the patient’s T cells or selected T cell subsets are collected, transfected to express the antigen, and are then expanded and returned to the patient following lymphodepletion with either fludarabine alone or with cyclophosphamide.
Each patient received up to three infusions of autologous CAR T cells at a dose of 1 x 108 cells/m2, and eligible patients received up to five additional infusions without additional lymphodepletion.
Dr. Navai presented data on 10 patients treated to date, including the two mentioned before; the boy with rhabdomyosarcoma was counted as two separate patients for the purpose of the efficacy analysis.
All patients had metastatic disease, including five with osteosarcoma, three with rhabdomyosarcoma, one with Ewing sarcoma, and one with synovial sarcoma.
The lymphodepletion regimens did their job, inducing neutropenia (defined as an absolute neutrophil count less than 500 per milliliter ) for up to 14 days.
Eight patients developed grade 1 or 2 cytokine release syndrome within 24 hours of CAR T-cell infusion, and all cases completely resolved with supportive care within 5 days of onset.
In nine patients, T cells were successfully expanded, with a median peak expansion on day 7.
In all 10 patients, CAR T cells were detected by quantitative polymerase chain reaction 6 weeks after infusion.
In addition to the two patients with complete remissions already described, three patients had stable disease. The remaining patients had disease progression. At the most recent analysis, five patients were still alive, and five had died.
The infusions were safe, with no dose-limiting toxicities reported. No patient required a transfusion, and there were no opportunistic, infections, no neurotoxicities, and no lasting pulmonary or cardiac toxicities, Dr. Navai reported.
Some fare better than others
Nilofer S. Azad, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, who moderated the briefing, commented that the study had “very small numbers, but is still very exciting.”
She noted that the patients who benefited most from the therapy either had minimal residual disease or bone marrow disease without visceral disease; she asked Dr. Navai how this could be addressed going forward.
“The patients who seemed to have had responses both in this trial, as well as in our previous trial without lymphodepletion, tended to have less disease or more accessible disease. So we hypothesized that disease that’s in the bone marrow because it’s more accessible, or in the lungs, where also CAR T cells go after they are first infused, may be more amenable to treatment,” Dr. Navai said.
In contrast, larger tumors and more invasive disease may emit immune inhibitory signals that dampen the efficacy of CAR T cells, she added.
Development of the CAR T-cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai and Dr. Azad reported having no disclosures relevant to the work.
SOURCE: Navai SA et al. AACR 2019, Abstract LB-147.
ATLANTA – A novel chimeric antigen receptor (CAR) T-cell construct centered on HER2 as the target antigen was safe and showed early promise in the treatment of advanced sarcomas of bone and soft tissues in a phase I trial.
One patient, a 16-year-old girl with advanced osteosarcoma metastatic to her lungs, had a complete response to the therapy that is ongoing out to nearly 3 years, reported Shoba A. Navai, MD, from Baylor College of Medicine in Houston.
A second patient, an 8-year-old boy with rhabdomyosarcoma metastatic to bone marrow, had a complete response lasting 12 months. Upon relapse he was re-enrolled, received additional CAR T-cell infusions, and had a second complete response that has been ongoing for 17 months.
“HER2 CAR T cells can induce objective clinical responses in some patients with sarcoma, and engagement of endogenous immunity may aid in generation of tumor responses. We are currently working to validate these findings in other patients who were treated,” she said at a briefing at the annual meeting of the American Association for Cancer Research.
HER2 is a member of the human epidermal growth factor receptor family that is primarily expressed on the surface of tumor cells but is largely absent from nonmalignant tissues. HER2 can be expressed in a variety of sarcomas, including osteosarcoma, and HER2 expression in osteosarcoma correlates with worse overall survival.
Unlike HER2-positive breast cancers, however, HER2 expression levels in osteosarcoma are too low to be effectively targeted by anti-HER2 agents such as trastuzumab (Hereceptin).
But as Dr. Navai and colleagues have found, HER2 appears to be a valid target for CAR T-cell therapy in otherwise antigenically “cold” tumors – that is, tumors with few targetable antigens.
Old target, new weapon
They have developed a CAR T-cell construct using a HER2-directed antibody coupled with CD28 as the costimulatory molecule. As with other CAR T therapies, the patient’s T cells or selected T cell subsets are collected, transfected to express the antigen, and are then expanded and returned to the patient following lymphodepletion with either fludarabine alone or with cyclophosphamide.
Each patient received up to three infusions of autologous CAR T cells at a dose of 1 x 108 cells/m2, and eligible patients received up to five additional infusions without additional lymphodepletion.
Dr. Navai presented data on 10 patients treated to date, including the two mentioned before; the boy with rhabdomyosarcoma was counted as two separate patients for the purpose of the efficacy analysis.
All patients had metastatic disease, including five with osteosarcoma, three with rhabdomyosarcoma, one with Ewing sarcoma, and one with synovial sarcoma.
The lymphodepletion regimens did their job, inducing neutropenia (defined as an absolute neutrophil count less than 500 per milliliter ) for up to 14 days.
Eight patients developed grade 1 or 2 cytokine release syndrome within 24 hours of CAR T-cell infusion, and all cases completely resolved with supportive care within 5 days of onset.
In nine patients, T cells were successfully expanded, with a median peak expansion on day 7.
In all 10 patients, CAR T cells were detected by quantitative polymerase chain reaction 6 weeks after infusion.
In addition to the two patients with complete remissions already described, three patients had stable disease. The remaining patients had disease progression. At the most recent analysis, five patients were still alive, and five had died.
The infusions were safe, with no dose-limiting toxicities reported. No patient required a transfusion, and there were no opportunistic, infections, no neurotoxicities, and no lasting pulmonary or cardiac toxicities, Dr. Navai reported.
Some fare better than others
Nilofer S. Azad, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, who moderated the briefing, commented that the study had “very small numbers, but is still very exciting.”
She noted that the patients who benefited most from the therapy either had minimal residual disease or bone marrow disease without visceral disease; she asked Dr. Navai how this could be addressed going forward.
“The patients who seemed to have had responses both in this trial, as well as in our previous trial without lymphodepletion, tended to have less disease or more accessible disease. So we hypothesized that disease that’s in the bone marrow because it’s more accessible, or in the lungs, where also CAR T cells go after they are first infused, may be more amenable to treatment,” Dr. Navai said.
In contrast, larger tumors and more invasive disease may emit immune inhibitory signals that dampen the efficacy of CAR T cells, she added.
Development of the CAR T-cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai and Dr. Azad reported having no disclosures relevant to the work.
SOURCE: Navai SA et al. AACR 2019, Abstract LB-147.
ATLANTA – A novel chimeric antigen receptor (CAR) T-cell construct centered on HER2 as the target antigen was safe and showed early promise in the treatment of advanced sarcomas of bone and soft tissues in a phase I trial.
One patient, a 16-year-old girl with advanced osteosarcoma metastatic to her lungs, had a complete response to the therapy that is ongoing out to nearly 3 years, reported Shoba A. Navai, MD, from Baylor College of Medicine in Houston.
A second patient, an 8-year-old boy with rhabdomyosarcoma metastatic to bone marrow, had a complete response lasting 12 months. Upon relapse he was re-enrolled, received additional CAR T-cell infusions, and had a second complete response that has been ongoing for 17 months.
“HER2 CAR T cells can induce objective clinical responses in some patients with sarcoma, and engagement of endogenous immunity may aid in generation of tumor responses. We are currently working to validate these findings in other patients who were treated,” she said at a briefing at the annual meeting of the American Association for Cancer Research.
HER2 is a member of the human epidermal growth factor receptor family that is primarily expressed on the surface of tumor cells but is largely absent from nonmalignant tissues. HER2 can be expressed in a variety of sarcomas, including osteosarcoma, and HER2 expression in osteosarcoma correlates with worse overall survival.
Unlike HER2-positive breast cancers, however, HER2 expression levels in osteosarcoma are too low to be effectively targeted by anti-HER2 agents such as trastuzumab (Hereceptin).
But as Dr. Navai and colleagues have found, HER2 appears to be a valid target for CAR T-cell therapy in otherwise antigenically “cold” tumors – that is, tumors with few targetable antigens.
Old target, new weapon
They have developed a CAR T-cell construct using a HER2-directed antibody coupled with CD28 as the costimulatory molecule. As with other CAR T therapies, the patient’s T cells or selected T cell subsets are collected, transfected to express the antigen, and are then expanded and returned to the patient following lymphodepletion with either fludarabine alone or with cyclophosphamide.
Each patient received up to three infusions of autologous CAR T cells at a dose of 1 x 108 cells/m2, and eligible patients received up to five additional infusions without additional lymphodepletion.
Dr. Navai presented data on 10 patients treated to date, including the two mentioned before; the boy with rhabdomyosarcoma was counted as two separate patients for the purpose of the efficacy analysis.
All patients had metastatic disease, including five with osteosarcoma, three with rhabdomyosarcoma, one with Ewing sarcoma, and one with synovial sarcoma.
The lymphodepletion regimens did their job, inducing neutropenia (defined as an absolute neutrophil count less than 500 per milliliter ) for up to 14 days.
Eight patients developed grade 1 or 2 cytokine release syndrome within 24 hours of CAR T-cell infusion, and all cases completely resolved with supportive care within 5 days of onset.
In nine patients, T cells were successfully expanded, with a median peak expansion on day 7.
In all 10 patients, CAR T cells were detected by quantitative polymerase chain reaction 6 weeks after infusion.
In addition to the two patients with complete remissions already described, three patients had stable disease. The remaining patients had disease progression. At the most recent analysis, five patients were still alive, and five had died.
The infusions were safe, with no dose-limiting toxicities reported. No patient required a transfusion, and there were no opportunistic, infections, no neurotoxicities, and no lasting pulmonary or cardiac toxicities, Dr. Navai reported.
Some fare better than others
Nilofer S. Azad, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, who moderated the briefing, commented that the study had “very small numbers, but is still very exciting.”
She noted that the patients who benefited most from the therapy either had minimal residual disease or bone marrow disease without visceral disease; she asked Dr. Navai how this could be addressed going forward.
“The patients who seemed to have had responses both in this trial, as well as in our previous trial without lymphodepletion, tended to have less disease or more accessible disease. So we hypothesized that disease that’s in the bone marrow because it’s more accessible, or in the lungs, where also CAR T cells go after they are first infused, may be more amenable to treatment,” Dr. Navai said.
In contrast, larger tumors and more invasive disease may emit immune inhibitory signals that dampen the efficacy of CAR T cells, she added.
Development of the CAR T-cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai and Dr. Azad reported having no disclosures relevant to the work.
SOURCE: Navai SA et al. AACR 2019, Abstract LB-147.
REPORTING FROM AACR 2019
Novel chemo/PARP inhibitor strategy promising for advanced pancreatic cancer
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
REPORTING FROM AACR 2019
UNITY-NHL: Interim findings show activity, tolerability of umbralisib for R/R MZL
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
REPORTING FROM AACR 2019
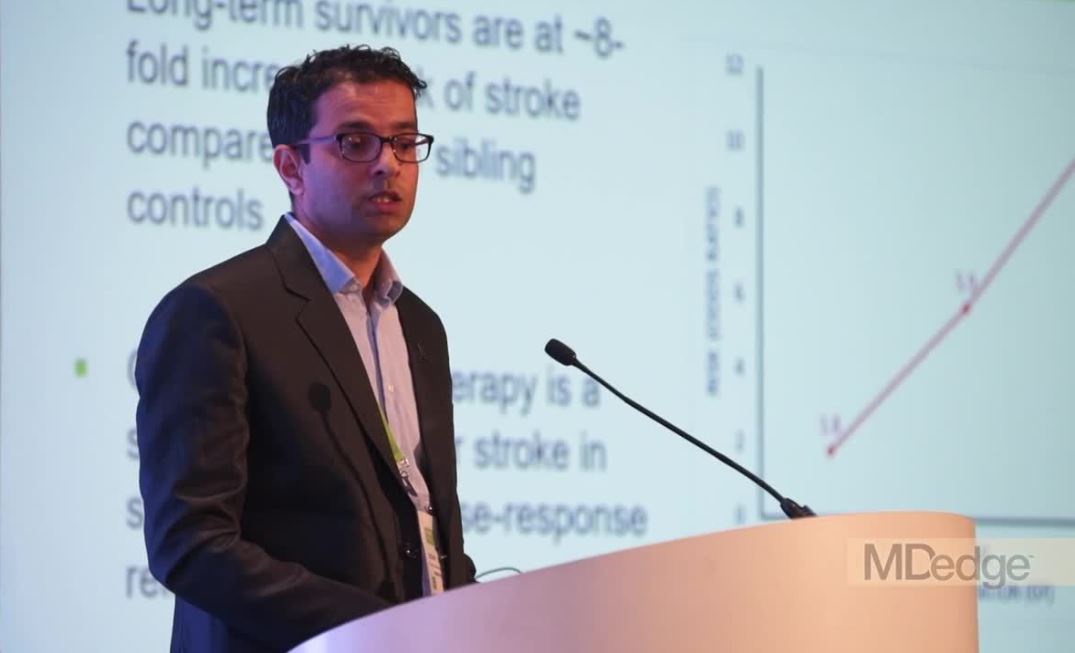
Genetic variant increases stroke risk in childhood cancer survivors
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
REPORTING FROM AACR 2019
Anti-EGFR TKI, MET inhibitor team up against drug-resistant NSCLC
ATLANTA – About 10%-25% of patients with epithelial growth factor receptor–(EGFR) mutant non–small cell lung cancer (NSCLC) have tumors with either MET amplification or another MET-based mechanism that leads to drug resistance.
In the TATTON trial, investigators are evaluating a combination of the EGFR-targeted tyrosine kinase inhibitor (TKI) osimertinib (Tagrisso) with savolitinib, an investigational MET inhibitor, for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents.
In a video interview at the annual meeting of the American Association for Cancer Research, Lecia Sequist, MD, from the Massachusetts General Hospital Cancer Center in Boston, discusses early results with the osimertinib/savolitinib combination in patients with disease progression after a first and/or second-generation EGFR TKI, or after a third-generation agent.
Dr. Sequist said results of TATTON suggest that it may be possible to overcome MET-driven drug-resistance mechanisms.
The TATTON trial is sponsored by AstraZeneca. Dr. Sequist reported serving as an advisory board member and receiving research support and honoraria from the company.
ATLANTA – About 10%-25% of patients with epithelial growth factor receptor–(EGFR) mutant non–small cell lung cancer (NSCLC) have tumors with either MET amplification or another MET-based mechanism that leads to drug resistance.
In the TATTON trial, investigators are evaluating a combination of the EGFR-targeted tyrosine kinase inhibitor (TKI) osimertinib (Tagrisso) with savolitinib, an investigational MET inhibitor, for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents.
In a video interview at the annual meeting of the American Association for Cancer Research, Lecia Sequist, MD, from the Massachusetts General Hospital Cancer Center in Boston, discusses early results with the osimertinib/savolitinib combination in patients with disease progression after a first and/or second-generation EGFR TKI, or after a third-generation agent.
Dr. Sequist said results of TATTON suggest that it may be possible to overcome MET-driven drug-resistance mechanisms.
The TATTON trial is sponsored by AstraZeneca. Dr. Sequist reported serving as an advisory board member and receiving research support and honoraria from the company.
ATLANTA – About 10%-25% of patients with epithelial growth factor receptor–(EGFR) mutant non–small cell lung cancer (NSCLC) have tumors with either MET amplification or another MET-based mechanism that leads to drug resistance.
In the TATTON trial, investigators are evaluating a combination of the EGFR-targeted tyrosine kinase inhibitor (TKI) osimertinib (Tagrisso) with savolitinib, an investigational MET inhibitor, for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents.
In a video interview at the annual meeting of the American Association for Cancer Research, Lecia Sequist, MD, from the Massachusetts General Hospital Cancer Center in Boston, discusses early results with the osimertinib/savolitinib combination in patients with disease progression after a first and/or second-generation EGFR TKI, or after a third-generation agent.
Dr. Sequist said results of TATTON suggest that it may be possible to overcome MET-driven drug-resistance mechanisms.
The TATTON trial is sponsored by AstraZeneca. Dr. Sequist reported serving as an advisory board member and receiving research support and honoraria from the company.
REPORTING FROM AACR 2019
CAR T cells target HER2 expression in advanced sarcomas
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
REPORTING FROM AACR 2019