User login
Dogs show potential as medical detectives in breast cancer
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
FROM BIOLOGY
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Wolf in sheep’s clothing: metatarsal osteosarcoma
Metatarsal bones are an unusual subsite for small bone involvement in osteosarcomas. This subgroup is often misdiagnosed and hence associated with significant treatment delays. The standard treatment of metatarsal osteosarcomas remains the same as for those treated at other sites, namely neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. Limb salvage surgery or metatarsectomy in the foot is often a challenge owing to the poor compartmentalization of the disease. We hereby describe the case of a young girl with a metatarsal osteosarcoma who was managed with neoadjuvant chemotherapy and limb salvage surgery.
Introduction
Osteosarcomas are the most common primary malignant bone tumor in children and adolescents. Although predominantly occurring in pediatric and adolescent age groups, bimodal distribution (with a second incidence peak occurring in the sixth and seventh decades) is not uncommon.1 Osteosarcomas of the foot and small bones represent a rare and distinct clinical entity. This must have been a well-known observation for years that led to Watson-Jones stating, “Sarcoma of this [metatarsal] bone has not yet been reported in thousands of years in any country.”2 The incidence of osteosarcomas of the foot is estimated to be from 0.2% to 2%.3
These tumors, owing to their rarity, often lead to diagnostic dilemmas and hence treatment delays.4 They are usually mistaken for inflammatory conditions and often treated with—but not limited to—curettages and drainage procedures.5 The following case of osteosarcoma of the metatarsal bone in a young girl highlights the importance of having a high index of clinical suspicion prior to treatment.
Case Presentation and Summary
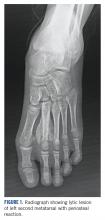
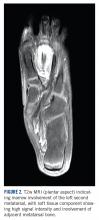
A 10-year-old girl visited our outpatient clinic with a painful progressive swelling on the dorsum of the left foot of 2 months’ duration. There was no history of antecedent trauma or fever. Physical examination revealed a bony hard swelling measuring around 5 x 6 cm on the dorsum of the left foot around the region of the second metatarsal. There was no regional lymphadenopathy or distal neurovascular deficit. She was evaluated with a plain radiograph that demonstrated a lytic lesion in the left second metatarsal associated with cortical destruction and periosteal reaction (Figure 1). A subsequent magnetic resonance image (MRI) revealed a bony lesion destroying part of the left second metatarsal with cortical destruction and marrow involvement and affecting the soft tissue around the adjacent third metatarsal (Figure 2). Needle biopsy showed chondroblastic osteosarcoma. Computed tomography (CT) of the thorax and bone scan were both negative for distant metastases.
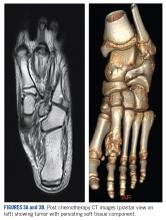
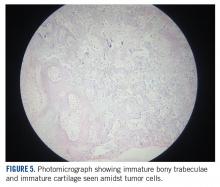
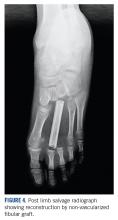
She received 3 cycles of a MAP (highdose methotrexate, doxorubicin, and cisplatin) regimen as neoadjuvant chemotherapy. Response assessment scans showed partial response (Figures 3A and B). We performed a wide excision of the second and third metatarsal with reconstruction using a segment of non-vascularized fibular graft as rigid fixation (Figure 4). The postoperative period was uneventful. She was able to begin partial weight bearing on the fourth postoperative day and her sutures were removed on the twelfth postoperative day. She received adjuvant chemotherapy following surgery. The final histopathology report showed residual disease with Huvos grade III response (>90% necrosis) with all margins negative for malignancy (Figure 5). At present, the child is disease-free at 5 months of treatment completion and is undergoing regular follow-up visits.
Discussion
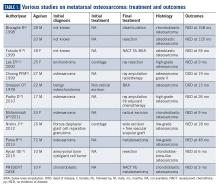
Metatarsal involvement amongst smallbone osteosarcomas is uncommon.3 There are about 32 cases of osteosarcomas reported in the literature from 1940 to 2018 involving the metatarsal bones (Table 1). According to a review article from the Mayo Clinic, the most common bone of the foot involved is the calcaneum.6 While the incidence of osteosarcomas of the foot as a whole is around 0.2% to 2%,3 metatarsal involvement is documented in 0.5% of these patients.7 However, a recent study depicted metatarsal involvement in 33% of all osteosarcomas of the foot.8
Osteosarcomas at conventional sites tend to have a bimodal age distribution with respect to disease affliction.9 Metatarsal osteosarcomas, however, are more common in an older age group.4,10 Our patient is probably the second youngest reported case of metatarsal osteosarcoma in the literature.11
Biscaglia et al propounded that osteosarcomas of the metatarsal were a distinct subgroup due to the rarity of occurrence, anatomical location, and prognosis.4 This often led to misdiagnosis and subsequent inadequate or inappropriate surgery. In six out of the ten cases (60%) described in Table 1, an incorrect pretreatment diagnosis was made that led to treatment delay. None, except one patient, received neoadjuvant chemotherapy, which is currently the standard of care. The average duration from symptom onset to diagnosis was found to be 2 years.4 However, in our case, the duration of symptoms was approximately 2 months.
Surgery for metatarsal osteosarcomas can be challenging, as the compartments of the foot are narrow spaces with poor demarcation. Limb salvage surgery in the form of metatarsectomy needs proper preoperative planning and execution. Neoadjuvant chemotherapy will serve to downstage the tumor within the fascial barriers of the metatarsal compartment.It has also been postulated that osteosarcoma of the foot may have a better prognosis and survival compared to other osteosarcoma subsites.10 This can be extrapolated from the fact that the majority are found to be low grade, and despite a long delay in treatment, there was no rapid increase in size and/or metastatic spread. However, tumor grade remains an important factor affecting survival— patients with higher grade tumors have worse survival.8
A number of differentials, including benign tumors, are to be kept in mind when diagnosing and treating such patients (Table 2). The most common benign tumors affecting the metatarsal are giant cell tumors (GCT) followed by chondromyxoid fibroma. Osteosarcomas and Ewing sarcomas constitute the malignant tumors.12 Occasionally, infections like osteomyelitis of the small bones may mimic malignancy. The absence of an extensive soft tissue component and/or calcifications with the presence of bony changes (like sequestrum) favors a diagnosis of infection/osteomyelitis. In addition, clinical findings like fever, skin redness, and presence of a painful swelling (especially after onset of fever) point to an inflammatory pathology rather than malignancy. Stress fractures rarely simulate tumors. MRI showing marrow and soft tissue edema with a visible fracture line points to the diagnosis.
A plane radiograph showing cortical bone destruction with a soft tissue component and calcification should be considered suspicious and must be thoroughly evaluated prior to surgical treatment.13 In a young patient such as ours, the important differentials that need to be considered include Ewing sarcoma, chronic osteomyelitis, and eosinophilic granuloma, which can radiologically mimic osteosarcoma at this location.
Conclusions
Osteosarcoma of the metatarsal is rare. Our case remains unique as it reports the second youngest patient in the literature. Erroneous or delayed diagnosis resulting in inadequate tumor excision and limb loss (amputation) often occurs in a majority of the cases. Proper pretreatment radiological imaging becomes imperative, and when clinical suspicion is high, a needle biopsy must follow in those cases. Early diagnosis with administration of neoadjuvant chemotherapy may allow us to perform limb salvage surgery or wide excision in these cases.
Acknowledgement
We would like to thank Dr. Sithara Aravind, Associate Professor, Department of Pathology, Malabar Cancer Center, for the photomicrographs.
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
2. Watson-Jones R. Fractures and Joint Injuries. Vol. I, 4th ed. Edinburgh and London: E & S Livingstone Ltd.1960:347.
3. Wu KK. Osteogenic sarcoma of the tarsal navicular bone. J Foot Surg. 1989;28(4):363-369.
4. Biscaglia R, Gasbarrini A, Böhling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot: an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73(9):842-847.
5. Kundu ZS, Gupta V, Sangwan SS, Rana P. Curettage of benign bone tumors and tumor like lesions: A retrospective analysis. Indian J Orthop. 2013;47(3):295-301.
6. Choong PFM, Qureshil AA, Sim FH, Unni KK. Osteosarcoma of the foot. A review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70(4):361-364.
7. Sneppen O, Dissing I, Heerfordt J, Schiödt T. Osteosarcoma of the metatarsal bones: Review of the literature and report of a case. Acta Orthop Scand. 1978;49(2):220-223.
8. Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462(1):109-120.
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology and End Results Program. Cancer.
2009;115(7):1531-1543.
10. Wang CW, Chen CY, Yang RS. Talar osteosarcoma treated with limb sparing surgery. J Bone Joint Surg Am. 2011;93:e22.
11. Aycan OE, Vanel D, Righi A, Arikan Y, Manfrini M. Chondroblastoma-like osteosarcoma:
a case report and review. Skeletal Radiol. 2015;44(6):869-873.
12. Jarkiewicz-Kochman E, Gołebiowski M, Swiatkowski J, Pacholec E, Rajewski R. Tumours of the metatarsus. Ortop Traumatol Rehabil. 2007;9(3):319-330.
13. Schatz J, Soper J, McCormack S, Healy M, Deady L, Brown W. Imaging of tumours in the ankle and foot. Top Magn Reson Imaging. 2010;21(1):37-50.
14. Fukuda K, Ushigome S, Nikaidou T, Asanuma K, Masui F. Osteosarcoma of the metatarsal. Skeletal Radiol. 1999;28(5):294-297.
15. Parsa R, Marcus M, Orlando R, Parsa C. Low-grade central osteosarcoma of the second metatarsal in a 72 year old male. Internet J Orthop Surg. 2013;21(2): 1-8.
16. Lee EY, Seeger LL, Nelson SD, Eckardt JJ. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000;29(8):474-476.
17. Padhy D, Madhuri V, Pulimood SA, Danda S, Walter NM, Wang LL. Metatarsal osteosarcoma in Rothmund-Thomson syndrome: a case report. J Bone Joint
Surg Am. 2010;92(3):726-730.
18. Mohammadi A, Porghasem J, Noroozinia F, Ilkhanizadeh B, Ghasemi-Rad M, Khenari S. Periosteal osteosarcoma of the fifth metatarsal: A rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620-622.
19. Nishio J, Iwasaki H, Takagi S, et al. Low-grade central osteosarcoma of the metatarsal bone: A clinicopathological, immunohistochemical, cytogenetic and molecular cytogenetic analysis. Anticancer Res. 2012;32(12):5429-5435.
Metatarsal bones are an unusual subsite for small bone involvement in osteosarcomas. This subgroup is often misdiagnosed and hence associated with significant treatment delays. The standard treatment of metatarsal osteosarcomas remains the same as for those treated at other sites, namely neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. Limb salvage surgery or metatarsectomy in the foot is often a challenge owing to the poor compartmentalization of the disease. We hereby describe the case of a young girl with a metatarsal osteosarcoma who was managed with neoadjuvant chemotherapy and limb salvage surgery.
Introduction
Osteosarcomas are the most common primary malignant bone tumor in children and adolescents. Although predominantly occurring in pediatric and adolescent age groups, bimodal distribution (with a second incidence peak occurring in the sixth and seventh decades) is not uncommon.1 Osteosarcomas of the foot and small bones represent a rare and distinct clinical entity. This must have been a well-known observation for years that led to Watson-Jones stating, “Sarcoma of this [metatarsal] bone has not yet been reported in thousands of years in any country.”2 The incidence of osteosarcomas of the foot is estimated to be from 0.2% to 2%.3
These tumors, owing to their rarity, often lead to diagnostic dilemmas and hence treatment delays.4 They are usually mistaken for inflammatory conditions and often treated with—but not limited to—curettages and drainage procedures.5 The following case of osteosarcoma of the metatarsal bone in a young girl highlights the importance of having a high index of clinical suspicion prior to treatment.
Case Presentation and Summary
A 10-year-old girl visited our outpatient clinic with a painful progressive swelling on the dorsum of the left foot of 2 months’ duration. There was no history of antecedent trauma or fever. Physical examination revealed a bony hard swelling measuring around 5 x 6 cm on the dorsum of the left foot around the region of the second metatarsal. There was no regional lymphadenopathy or distal neurovascular deficit. She was evaluated with a plain radiograph that demonstrated a lytic lesion in the left second metatarsal associated with cortical destruction and periosteal reaction (Figure 1). A subsequent magnetic resonance image (MRI) revealed a bony lesion destroying part of the left second metatarsal with cortical destruction and marrow involvement and affecting the soft tissue around the adjacent third metatarsal (Figure 2). Needle biopsy showed chondroblastic osteosarcoma. Computed tomography (CT) of the thorax and bone scan were both negative for distant metastases.
She received 3 cycles of a MAP (highdose methotrexate, doxorubicin, and cisplatin) regimen as neoadjuvant chemotherapy. Response assessment scans showed partial response (Figures 3A and B). We performed a wide excision of the second and third metatarsal with reconstruction using a segment of non-vascularized fibular graft as rigid fixation (Figure 4). The postoperative period was uneventful. She was able to begin partial weight bearing on the fourth postoperative day and her sutures were removed on the twelfth postoperative day. She received adjuvant chemotherapy following surgery. The final histopathology report showed residual disease with Huvos grade III response (>90% necrosis) with all margins negative for malignancy (Figure 5). At present, the child is disease-free at 5 months of treatment completion and is undergoing regular follow-up visits.
Discussion
Metatarsal involvement amongst smallbone osteosarcomas is uncommon.3 There are about 32 cases of osteosarcomas reported in the literature from 1940 to 2018 involving the metatarsal bones (Table 1). According to a review article from the Mayo Clinic, the most common bone of the foot involved is the calcaneum.6 While the incidence of osteosarcomas of the foot as a whole is around 0.2% to 2%,3 metatarsal involvement is documented in 0.5% of these patients.7 However, a recent study depicted metatarsal involvement in 33% of all osteosarcomas of the foot.8
Osteosarcomas at conventional sites tend to have a bimodal age distribution with respect to disease affliction.9 Metatarsal osteosarcomas, however, are more common in an older age group.4,10 Our patient is probably the second youngest reported case of metatarsal osteosarcoma in the literature.11
Biscaglia et al propounded that osteosarcomas of the metatarsal were a distinct subgroup due to the rarity of occurrence, anatomical location, and prognosis.4 This often led to misdiagnosis and subsequent inadequate or inappropriate surgery. In six out of the ten cases (60%) described in Table 1, an incorrect pretreatment diagnosis was made that led to treatment delay. None, except one patient, received neoadjuvant chemotherapy, which is currently the standard of care. The average duration from symptom onset to diagnosis was found to be 2 years.4 However, in our case, the duration of symptoms was approximately 2 months.
Surgery for metatarsal osteosarcomas can be challenging, as the compartments of the foot are narrow spaces with poor demarcation. Limb salvage surgery in the form of metatarsectomy needs proper preoperative planning and execution. Neoadjuvant chemotherapy will serve to downstage the tumor within the fascial barriers of the metatarsal compartment.It has also been postulated that osteosarcoma of the foot may have a better prognosis and survival compared to other osteosarcoma subsites.10 This can be extrapolated from the fact that the majority are found to be low grade, and despite a long delay in treatment, there was no rapid increase in size and/or metastatic spread. However, tumor grade remains an important factor affecting survival— patients with higher grade tumors have worse survival.8
A number of differentials, including benign tumors, are to be kept in mind when diagnosing and treating such patients (Table 2). The most common benign tumors affecting the metatarsal are giant cell tumors (GCT) followed by chondromyxoid fibroma. Osteosarcomas and Ewing sarcomas constitute the malignant tumors.12 Occasionally, infections like osteomyelitis of the small bones may mimic malignancy. The absence of an extensive soft tissue component and/or calcifications with the presence of bony changes (like sequestrum) favors a diagnosis of infection/osteomyelitis. In addition, clinical findings like fever, skin redness, and presence of a painful swelling (especially after onset of fever) point to an inflammatory pathology rather than malignancy. Stress fractures rarely simulate tumors. MRI showing marrow and soft tissue edema with a visible fracture line points to the diagnosis.
A plane radiograph showing cortical bone destruction with a soft tissue component and calcification should be considered suspicious and must be thoroughly evaluated prior to surgical treatment.13 In a young patient such as ours, the important differentials that need to be considered include Ewing sarcoma, chronic osteomyelitis, and eosinophilic granuloma, which can radiologically mimic osteosarcoma at this location.
Conclusions
Osteosarcoma of the metatarsal is rare. Our case remains unique as it reports the second youngest patient in the literature. Erroneous or delayed diagnosis resulting in inadequate tumor excision and limb loss (amputation) often occurs in a majority of the cases. Proper pretreatment radiological imaging becomes imperative, and when clinical suspicion is high, a needle biopsy must follow in those cases. Early diagnosis with administration of neoadjuvant chemotherapy may allow us to perform limb salvage surgery or wide excision in these cases.
Acknowledgement
We would like to thank Dr. Sithara Aravind, Associate Professor, Department of Pathology, Malabar Cancer Center, for the photomicrographs.
Metatarsal bones are an unusual subsite for small bone involvement in osteosarcomas. This subgroup is often misdiagnosed and hence associated with significant treatment delays. The standard treatment of metatarsal osteosarcomas remains the same as for those treated at other sites, namely neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. Limb salvage surgery or metatarsectomy in the foot is often a challenge owing to the poor compartmentalization of the disease. We hereby describe the case of a young girl with a metatarsal osteosarcoma who was managed with neoadjuvant chemotherapy and limb salvage surgery.
Introduction
Osteosarcomas are the most common primary malignant bone tumor in children and adolescents. Although predominantly occurring in pediatric and adolescent age groups, bimodal distribution (with a second incidence peak occurring in the sixth and seventh decades) is not uncommon.1 Osteosarcomas of the foot and small bones represent a rare and distinct clinical entity. This must have been a well-known observation for years that led to Watson-Jones stating, “Sarcoma of this [metatarsal] bone has not yet been reported in thousands of years in any country.”2 The incidence of osteosarcomas of the foot is estimated to be from 0.2% to 2%.3
These tumors, owing to their rarity, often lead to diagnostic dilemmas and hence treatment delays.4 They are usually mistaken for inflammatory conditions and often treated with—but not limited to—curettages and drainage procedures.5 The following case of osteosarcoma of the metatarsal bone in a young girl highlights the importance of having a high index of clinical suspicion prior to treatment.
Case Presentation and Summary
A 10-year-old girl visited our outpatient clinic with a painful progressive swelling on the dorsum of the left foot of 2 months’ duration. There was no history of antecedent trauma or fever. Physical examination revealed a bony hard swelling measuring around 5 x 6 cm on the dorsum of the left foot around the region of the second metatarsal. There was no regional lymphadenopathy or distal neurovascular deficit. She was evaluated with a plain radiograph that demonstrated a lytic lesion in the left second metatarsal associated with cortical destruction and periosteal reaction (Figure 1). A subsequent magnetic resonance image (MRI) revealed a bony lesion destroying part of the left second metatarsal with cortical destruction and marrow involvement and affecting the soft tissue around the adjacent third metatarsal (Figure 2). Needle biopsy showed chondroblastic osteosarcoma. Computed tomography (CT) of the thorax and bone scan were both negative for distant metastases.
She received 3 cycles of a MAP (highdose methotrexate, doxorubicin, and cisplatin) regimen as neoadjuvant chemotherapy. Response assessment scans showed partial response (Figures 3A and B). We performed a wide excision of the second and third metatarsal with reconstruction using a segment of non-vascularized fibular graft as rigid fixation (Figure 4). The postoperative period was uneventful. She was able to begin partial weight bearing on the fourth postoperative day and her sutures were removed on the twelfth postoperative day. She received adjuvant chemotherapy following surgery. The final histopathology report showed residual disease with Huvos grade III response (>90% necrosis) with all margins negative for malignancy (Figure 5). At present, the child is disease-free at 5 months of treatment completion and is undergoing regular follow-up visits.
Discussion
Metatarsal involvement amongst smallbone osteosarcomas is uncommon.3 There are about 32 cases of osteosarcomas reported in the literature from 1940 to 2018 involving the metatarsal bones (Table 1). According to a review article from the Mayo Clinic, the most common bone of the foot involved is the calcaneum.6 While the incidence of osteosarcomas of the foot as a whole is around 0.2% to 2%,3 metatarsal involvement is documented in 0.5% of these patients.7 However, a recent study depicted metatarsal involvement in 33% of all osteosarcomas of the foot.8
Osteosarcomas at conventional sites tend to have a bimodal age distribution with respect to disease affliction.9 Metatarsal osteosarcomas, however, are more common in an older age group.4,10 Our patient is probably the second youngest reported case of metatarsal osteosarcoma in the literature.11
Biscaglia et al propounded that osteosarcomas of the metatarsal were a distinct subgroup due to the rarity of occurrence, anatomical location, and prognosis.4 This often led to misdiagnosis and subsequent inadequate or inappropriate surgery. In six out of the ten cases (60%) described in Table 1, an incorrect pretreatment diagnosis was made that led to treatment delay. None, except one patient, received neoadjuvant chemotherapy, which is currently the standard of care. The average duration from symptom onset to diagnosis was found to be 2 years.4 However, in our case, the duration of symptoms was approximately 2 months.
Surgery for metatarsal osteosarcomas can be challenging, as the compartments of the foot are narrow spaces with poor demarcation. Limb salvage surgery in the form of metatarsectomy needs proper preoperative planning and execution. Neoadjuvant chemotherapy will serve to downstage the tumor within the fascial barriers of the metatarsal compartment.It has also been postulated that osteosarcoma of the foot may have a better prognosis and survival compared to other osteosarcoma subsites.10 This can be extrapolated from the fact that the majority are found to be low grade, and despite a long delay in treatment, there was no rapid increase in size and/or metastatic spread. However, tumor grade remains an important factor affecting survival— patients with higher grade tumors have worse survival.8
A number of differentials, including benign tumors, are to be kept in mind when diagnosing and treating such patients (Table 2). The most common benign tumors affecting the metatarsal are giant cell tumors (GCT) followed by chondromyxoid fibroma. Osteosarcomas and Ewing sarcomas constitute the malignant tumors.12 Occasionally, infections like osteomyelitis of the small bones may mimic malignancy. The absence of an extensive soft tissue component and/or calcifications with the presence of bony changes (like sequestrum) favors a diagnosis of infection/osteomyelitis. In addition, clinical findings like fever, skin redness, and presence of a painful swelling (especially after onset of fever) point to an inflammatory pathology rather than malignancy. Stress fractures rarely simulate tumors. MRI showing marrow and soft tissue edema with a visible fracture line points to the diagnosis.
A plane radiograph showing cortical bone destruction with a soft tissue component and calcification should be considered suspicious and must be thoroughly evaluated prior to surgical treatment.13 In a young patient such as ours, the important differentials that need to be considered include Ewing sarcoma, chronic osteomyelitis, and eosinophilic granuloma, which can radiologically mimic osteosarcoma at this location.
Conclusions
Osteosarcoma of the metatarsal is rare. Our case remains unique as it reports the second youngest patient in the literature. Erroneous or delayed diagnosis resulting in inadequate tumor excision and limb loss (amputation) often occurs in a majority of the cases. Proper pretreatment radiological imaging becomes imperative, and when clinical suspicion is high, a needle biopsy must follow in those cases. Early diagnosis with administration of neoadjuvant chemotherapy may allow us to perform limb salvage surgery or wide excision in these cases.
Acknowledgement
We would like to thank Dr. Sithara Aravind, Associate Professor, Department of Pathology, Malabar Cancer Center, for the photomicrographs.
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
2. Watson-Jones R. Fractures and Joint Injuries. Vol. I, 4th ed. Edinburgh and London: E & S Livingstone Ltd.1960:347.
3. Wu KK. Osteogenic sarcoma of the tarsal navicular bone. J Foot Surg. 1989;28(4):363-369.
4. Biscaglia R, Gasbarrini A, Böhling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot: an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73(9):842-847.
5. Kundu ZS, Gupta V, Sangwan SS, Rana P. Curettage of benign bone tumors and tumor like lesions: A retrospective analysis. Indian J Orthop. 2013;47(3):295-301.
6. Choong PFM, Qureshil AA, Sim FH, Unni KK. Osteosarcoma of the foot. A review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70(4):361-364.
7. Sneppen O, Dissing I, Heerfordt J, Schiödt T. Osteosarcoma of the metatarsal bones: Review of the literature and report of a case. Acta Orthop Scand. 1978;49(2):220-223.
8. Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462(1):109-120.
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology and End Results Program. Cancer.
2009;115(7):1531-1543.
10. Wang CW, Chen CY, Yang RS. Talar osteosarcoma treated with limb sparing surgery. J Bone Joint Surg Am. 2011;93:e22.
11. Aycan OE, Vanel D, Righi A, Arikan Y, Manfrini M. Chondroblastoma-like osteosarcoma:
a case report and review. Skeletal Radiol. 2015;44(6):869-873.
12. Jarkiewicz-Kochman E, Gołebiowski M, Swiatkowski J, Pacholec E, Rajewski R. Tumours of the metatarsus. Ortop Traumatol Rehabil. 2007;9(3):319-330.
13. Schatz J, Soper J, McCormack S, Healy M, Deady L, Brown W. Imaging of tumours in the ankle and foot. Top Magn Reson Imaging. 2010;21(1):37-50.
14. Fukuda K, Ushigome S, Nikaidou T, Asanuma K, Masui F. Osteosarcoma of the metatarsal. Skeletal Radiol. 1999;28(5):294-297.
15. Parsa R, Marcus M, Orlando R, Parsa C. Low-grade central osteosarcoma of the second metatarsal in a 72 year old male. Internet J Orthop Surg. 2013;21(2): 1-8.
16. Lee EY, Seeger LL, Nelson SD, Eckardt JJ. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000;29(8):474-476.
17. Padhy D, Madhuri V, Pulimood SA, Danda S, Walter NM, Wang LL. Metatarsal osteosarcoma in Rothmund-Thomson syndrome: a case report. J Bone Joint
Surg Am. 2010;92(3):726-730.
18. Mohammadi A, Porghasem J, Noroozinia F, Ilkhanizadeh B, Ghasemi-Rad M, Khenari S. Periosteal osteosarcoma of the fifth metatarsal: A rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620-622.
19. Nishio J, Iwasaki H, Takagi S, et al. Low-grade central osteosarcoma of the metatarsal bone: A clinicopathological, immunohistochemical, cytogenetic and molecular cytogenetic analysis. Anticancer Res. 2012;32(12):5429-5435.
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
2. Watson-Jones R. Fractures and Joint Injuries. Vol. I, 4th ed. Edinburgh and London: E & S Livingstone Ltd.1960:347.
3. Wu KK. Osteogenic sarcoma of the tarsal navicular bone. J Foot Surg. 1989;28(4):363-369.
4. Biscaglia R, Gasbarrini A, Böhling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot: an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73(9):842-847.
5. Kundu ZS, Gupta V, Sangwan SS, Rana P. Curettage of benign bone tumors and tumor like lesions: A retrospective analysis. Indian J Orthop. 2013;47(3):295-301.
6. Choong PFM, Qureshil AA, Sim FH, Unni KK. Osteosarcoma of the foot. A review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70(4):361-364.
7. Sneppen O, Dissing I, Heerfordt J, Schiödt T. Osteosarcoma of the metatarsal bones: Review of the literature and report of a case. Acta Orthop Scand. 1978;49(2):220-223.
8. Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462(1):109-120.
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology and End Results Program. Cancer.
2009;115(7):1531-1543.
10. Wang CW, Chen CY, Yang RS. Talar osteosarcoma treated with limb sparing surgery. J Bone Joint Surg Am. 2011;93:e22.
11. Aycan OE, Vanel D, Righi A, Arikan Y, Manfrini M. Chondroblastoma-like osteosarcoma:
a case report and review. Skeletal Radiol. 2015;44(6):869-873.
12. Jarkiewicz-Kochman E, Gołebiowski M, Swiatkowski J, Pacholec E, Rajewski R. Tumours of the metatarsus. Ortop Traumatol Rehabil. 2007;9(3):319-330.
13. Schatz J, Soper J, McCormack S, Healy M, Deady L, Brown W. Imaging of tumours in the ankle and foot. Top Magn Reson Imaging. 2010;21(1):37-50.
14. Fukuda K, Ushigome S, Nikaidou T, Asanuma K, Masui F. Osteosarcoma of the metatarsal. Skeletal Radiol. 1999;28(5):294-297.
15. Parsa R, Marcus M, Orlando R, Parsa C. Low-grade central osteosarcoma of the second metatarsal in a 72 year old male. Internet J Orthop Surg. 2013;21(2): 1-8.
16. Lee EY, Seeger LL, Nelson SD, Eckardt JJ. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000;29(8):474-476.
17. Padhy D, Madhuri V, Pulimood SA, Danda S, Walter NM, Wang LL. Metatarsal osteosarcoma in Rothmund-Thomson syndrome: a case report. J Bone Joint
Surg Am. 2010;92(3):726-730.
18. Mohammadi A, Porghasem J, Noroozinia F, Ilkhanizadeh B, Ghasemi-Rad M, Khenari S. Periosteal osteosarcoma of the fifth metatarsal: A rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620-622.
19. Nishio J, Iwasaki H, Takagi S, et al. Low-grade central osteosarcoma of the metatarsal bone: A clinicopathological, immunohistochemical, cytogenetic and molecular cytogenetic analysis. Anticancer Res. 2012;32(12):5429-5435.
An unusual presentation of low-grade clavicle osteosarcoma: a case report and literature review
Osteosarcoma (OS) is a rare disease with approximately 800- 900 newly diagnosed cases each year in the United States. Of those, the majority occur about the knee. The distal femur is the most common site, followed by the proximal tibia, with the proximal humerus being a distant third. OS of the clavicle has been reported, with the earliest case report dating from 1975.1 Since then, additional case reports of high-grade OS of the clavicle have been published.2,3 We describe the case of a 16-year-old female who presented with a mass on her right medial clavicle, which was confirmed to be a low-grade central OS.
Case Presentation
The patient is a 16-year-old female who presented to the Emergency Department (ED) for evaluation of a mass on her right clavicle, after being evaluated by her primary care physician (PCP). She noted an enlarging mass over the previous 2 months but stated that it had been asymptomatic until 4 days prior to presentation to her PCP, at which time she had developed tenderness to palpation and pain with range of motion of the right arm. X-rays were obtained at the PCP’s office and she was referred to the ED for further evaluation. She denied constitutional symptoms.
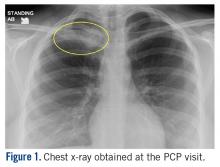
At the ED visit, she was noted to have an area of erythema and tenderness over the medial aspect of the right clavicle with increased bony prominence. A chest x-ray demonstrated medial clavicle enlargement with periosteal reaction and sclerosis (Figure 1).
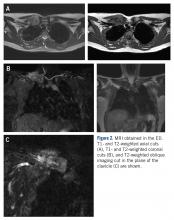
MRI demonstrated a 6-cm x 3.8-cm x 4.1-cm mass arising from the right medial clavicle with cortical destruction and concomitant displacement of the right subclavian and brachiocephalic veins (Figure 2). A CT-guided biopsy was performed 1 week later and demonstrated low-grade OS. The pathologist was concerned about the possibility of sampling error and the presence of a higher-grade component, as low-grade OS of the clavicle had not been reported.
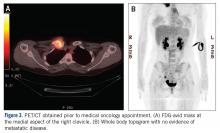
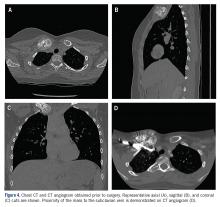
The patient was evaluated by a pediatric hematologist/oncologist 2 weeks later after having obtained the biopsy and a PET/CT scan. At that time, the PET/CT showed an FDG-avid mass at the clavicle without evidence of pulmonary metastatic disease (Figure 3). She was subsequently evaluated by orthopedic oncology, at which time a discussion was had regarding further treatment. There was essentially no literature to guide the surgical and medical teams, as low-grade clavicular OS is unknown. Based on the evidence of localized, low-grade disease, the determination was made to proceedwith surgical resection. In the event that high-grade disease was identified at the time of final pathological evaluation, the pediatric hematology/oncology team felt that administering all of the patient’s chemotherapy postoperatively would be acceptable and not affect her long-term prognosis. CT and CT angiogram were obtained for further operative planning (Figure 4).
Given the intimacy of the mass to the subclavian vessels, she was also seen preoperatively by pediatric general and cardiothoracic surgeons. The plan was formulated to have them in the operating room for mobilization of the subclavian vessels and in the event that a sternotomy was required for proximal control of the vessels. Following this visit, the case was discussed at the multidisciplinary pediatric tumor board and the consensus was to proceed with surgical resection.
Surgical Technique
General endotracheal anesthesia was administered without complication. The patient was positioned supine with a soft bump under her shoulders to place her neck in slight extension and thus facilitate access to the clavicle and great vessels. A 14-cm oblique incision was made over the subcutaneous clavicle extending to the contralateral sternoclavicular joint. Dissection was carried down to the fascia and the biopsy site was excised with the skin paddle. Dissection was carried through the sternocleidomastoid superiorly and the pectoralis major inferiorly, to 8 cm lateral from the right sternoclavicular joint. The clavicle was osteotomized well lateral of the palpable tumor and a marrow margin was sent for frozen section, which was found to be negative.
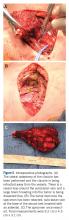
Dissection was continued circumferentially. Assistance from pediatric general and cardiothoracic surgery was required at the inferior aspect of the mass to assist with exposure and control of the subclavian vein (Figure 5A). A large branch of the subclavian vein near its junction with the internal jugular vein was found to be involved with the tumor and thus required suture ligation. The subclavian vein was noted to be intimate with the mass and somewhat friable. With the vein mobilized, a cuff of normal tissue was obtained inferiorly and superiorly to the mass. Medially, the sternoclavicular joint was disarticulated (Figure 5B). At this point, the specimen was delivered from the operative field and tagged in the usual fashion (Figure 5C). A medial soft tissue margin from the sternal side of the sternoclavicular joint was also sent and found to be negative for tumor. The wound was closed in layered fashion over a ¼” Penrose drain. A soft dressing was placed, and the patient was successfully extubated and transferred to the post-anesthesia care unit in stable condition.
Postoperative Course
The patient was found to be neurologically and vascularly intact on postoperative exam and was discharged on postoperative day 1.
She was seen 14 days postoperatively and was doing well at that time, with full range of motion of the shoulder, elbow, wrist, and hand. Final pathology confirmed a low-grade OS with extraosseous extension. All margins were negative except the medial (sternoclavicular joint) margin and the inferior margin adjacent to the subclavian vein. The intraoperative frozen section from the medial margin was negative for tumor.
The pediatric hematology/oncology team determined that, as no high-grade areas were identified, chemotherapy should be deferred. The positive margins were also discussed with the patient and her family specifically regarding further possible treatments. The findings from the pathology were discussed in a multidisciplinary tumor board and it was felt that, given the low-grade nature of the lesion as well as the high morbidity and risk of mortality with further surgery, additional surgery would be potentially more harmful than helpful. Additionally, low-grade OS is extremely resistant to radiotherapy. The plan remains to monitor her for local recurrence as well as metastases with serial imaging.
Discussion
The clavicle is one of the first bones in the body to ossify but one of the last to have final physeal closure. Its unique characteristics have led to various descriptions, such as a “short tubular bone” versus a “flat bone.”4,5 Of note are its paucity of a true intramedullary space and scanty red marrow, which make it an unlikely site for a primarily intramedullary- based neoplasm to arise.4 However, it has also been noted that malignant lesions are more common in the clavicle than benign lesions, and special attention should be paid to aggressiveappearing lesions in the clavicle.
Radiographs can be misleading as well. Prior studies have demonstrated that low-grade central OS can be readily misdiagnosed as fibrous dysplasia, desmoplastic fibroma, nonossifying fibroma, osteoblastoma, and aneurysmal bone cyst.6 Findings found in low-grade OS can include evidence of cortical interruption, local soft tissue mass development, intramedullary involvement, cortical destruction, and poor margination; however, low-grade OS is typically sclerotic and highly trabeculated. Cross-sectional imaging can help differentiate between OS and other more benign pathologies and should be considered in the clavicle where biopsy may be perilous.5
The difficulty of clavicular biopsy has been reported. Not only does clavicular anatomy make biopsy hazardous, but also the potential for sampling error does exist. In a case report of one patient with a highgrade lesion, fine needle aspiration biopsy was initially diagnosed as an aneurysmal bone cyst but was ultimately found to be osteosarcoma.2 Histology of low-grade lesions usually demonstrates minimal cytological atypia, rare mitotic activity, and variable osteoid production.5 Lower mitotic indices typically make wide resection curative for these patients, without the need for chemotherapy.
In this case, wide resection was carried out with the subclavian vein as the posterior-inferior margin and the sternoclavicular joint as the medial margin. Though the intra-operative medial margin was clear of disease, final pathology demonstrated focal (microscopic) involvement of the posterior and medial margins. A study of soft tissue sarcoma evaluated positive margins and concluded that the imperative of preservation of vital structures supersedes the need for negative margins.7,8 The rate of metastasis and overall survival was similar to surgical resections with positive margins. In the case of our patient, further resection would have carried significant morbidity and possibly mortality, including sacrifice of the major vessels to the arm below and entering into the sternum and thoracic cavity. The likely disability as well as the hazards of surgery were deemed to be too great to justify further excision. Frequent cross-sectional imaging will be necessary to evaluate the presence of recurrent or metastatic disease. To our knowledge, this is the first documented case of low-grade clavicle OS. This report demonstrates the need for multidisciplinary sarcoma care at a center of excellence, particularly in instances of unusual diagnoses.
1. Zinghi G. Osteosarcoma of the clavicle (description of a case) [in Italian]. Chir Organi Mov. 1975;62(6):671-674.
2. Cundy WJ, Carter C, Dhatrak D, Clayer M. Primary osteosarcoma of the clavicle and the perils of bone biopsy. BMJ Case Rep. 2015;2015:bcr2014208859.
3. Greenspan A, Unni KK, Mann J. Case report 804: Chondroblastic osteosarcoma grade 3 of the left clavicle. Skeletal Radiol. 1993;22(6):469-471.
4. Rossi B, Fabbriciani C, Chalidis BE, Visci F, Maccauro G. Primary malignant clavicular tumours: a clinicopathological analysis of six cases and evaluation of surgical management. Arch Orthop Trauma Surg. 2011;131(7):935-939.
5. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004;33(7):373-379.
6. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: A difficult condition to diagnose. Sarcoma. 2012; 2012:764796.
7. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866-2875.
8. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165-172.
Osteosarcoma (OS) is a rare disease with approximately 800- 900 newly diagnosed cases each year in the United States. Of those, the majority occur about the knee. The distal femur is the most common site, followed by the proximal tibia, with the proximal humerus being a distant third. OS of the clavicle has been reported, with the earliest case report dating from 1975.1 Since then, additional case reports of high-grade OS of the clavicle have been published.2,3 We describe the case of a 16-year-old female who presented with a mass on her right medial clavicle, which was confirmed to be a low-grade central OS.
Case Presentation
The patient is a 16-year-old female who presented to the Emergency Department (ED) for evaluation of a mass on her right clavicle, after being evaluated by her primary care physician (PCP). She noted an enlarging mass over the previous 2 months but stated that it had been asymptomatic until 4 days prior to presentation to her PCP, at which time she had developed tenderness to palpation and pain with range of motion of the right arm. X-rays were obtained at the PCP’s office and she was referred to the ED for further evaluation. She denied constitutional symptoms.
At the ED visit, she was noted to have an area of erythema and tenderness over the medial aspect of the right clavicle with increased bony prominence. A chest x-ray demonstrated medial clavicle enlargement with periosteal reaction and sclerosis (Figure 1).
MRI demonstrated a 6-cm x 3.8-cm x 4.1-cm mass arising from the right medial clavicle with cortical destruction and concomitant displacement of the right subclavian and brachiocephalic veins (Figure 2). A CT-guided biopsy was performed 1 week later and demonstrated low-grade OS. The pathologist was concerned about the possibility of sampling error and the presence of a higher-grade component, as low-grade OS of the clavicle had not been reported.
The patient was evaluated by a pediatric hematologist/oncologist 2 weeks later after having obtained the biopsy and a PET/CT scan. At that time, the PET/CT showed an FDG-avid mass at the clavicle without evidence of pulmonary metastatic disease (Figure 3). She was subsequently evaluated by orthopedic oncology, at which time a discussion was had regarding further treatment. There was essentially no literature to guide the surgical and medical teams, as low-grade clavicular OS is unknown. Based on the evidence of localized, low-grade disease, the determination was made to proceedwith surgical resection. In the event that high-grade disease was identified at the time of final pathological evaluation, the pediatric hematology/oncology team felt that administering all of the patient’s chemotherapy postoperatively would be acceptable and not affect her long-term prognosis. CT and CT angiogram were obtained for further operative planning (Figure 4).
Given the intimacy of the mass to the subclavian vessels, she was also seen preoperatively by pediatric general and cardiothoracic surgeons. The plan was formulated to have them in the operating room for mobilization of the subclavian vessels and in the event that a sternotomy was required for proximal control of the vessels. Following this visit, the case was discussed at the multidisciplinary pediatric tumor board and the consensus was to proceed with surgical resection.
Surgical Technique
General endotracheal anesthesia was administered without complication. The patient was positioned supine with a soft bump under her shoulders to place her neck in slight extension and thus facilitate access to the clavicle and great vessels. A 14-cm oblique incision was made over the subcutaneous clavicle extending to the contralateral sternoclavicular joint. Dissection was carried down to the fascia and the biopsy site was excised with the skin paddle. Dissection was carried through the sternocleidomastoid superiorly and the pectoralis major inferiorly, to 8 cm lateral from the right sternoclavicular joint. The clavicle was osteotomized well lateral of the palpable tumor and a marrow margin was sent for frozen section, which was found to be negative.
Dissection was continued circumferentially. Assistance from pediatric general and cardiothoracic surgery was required at the inferior aspect of the mass to assist with exposure and control of the subclavian vein (Figure 5A). A large branch of the subclavian vein near its junction with the internal jugular vein was found to be involved with the tumor and thus required suture ligation. The subclavian vein was noted to be intimate with the mass and somewhat friable. With the vein mobilized, a cuff of normal tissue was obtained inferiorly and superiorly to the mass. Medially, the sternoclavicular joint was disarticulated (Figure 5B). At this point, the specimen was delivered from the operative field and tagged in the usual fashion (Figure 5C). A medial soft tissue margin from the sternal side of the sternoclavicular joint was also sent and found to be negative for tumor. The wound was closed in layered fashion over a ¼” Penrose drain. A soft dressing was placed, and the patient was successfully extubated and transferred to the post-anesthesia care unit in stable condition.
Postoperative Course
The patient was found to be neurologically and vascularly intact on postoperative exam and was discharged on postoperative day 1.
She was seen 14 days postoperatively and was doing well at that time, with full range of motion of the shoulder, elbow, wrist, and hand. Final pathology confirmed a low-grade OS with extraosseous extension. All margins were negative except the medial (sternoclavicular joint) margin and the inferior margin adjacent to the subclavian vein. The intraoperative frozen section from the medial margin was negative for tumor.
The pediatric hematology/oncology team determined that, as no high-grade areas were identified, chemotherapy should be deferred. The positive margins were also discussed with the patient and her family specifically regarding further possible treatments. The findings from the pathology were discussed in a multidisciplinary tumor board and it was felt that, given the low-grade nature of the lesion as well as the high morbidity and risk of mortality with further surgery, additional surgery would be potentially more harmful than helpful. Additionally, low-grade OS is extremely resistant to radiotherapy. The plan remains to monitor her for local recurrence as well as metastases with serial imaging.
Discussion
The clavicle is one of the first bones in the body to ossify but one of the last to have final physeal closure. Its unique characteristics have led to various descriptions, such as a “short tubular bone” versus a “flat bone.”4,5 Of note are its paucity of a true intramedullary space and scanty red marrow, which make it an unlikely site for a primarily intramedullary- based neoplasm to arise.4 However, it has also been noted that malignant lesions are more common in the clavicle than benign lesions, and special attention should be paid to aggressiveappearing lesions in the clavicle.
Radiographs can be misleading as well. Prior studies have demonstrated that low-grade central OS can be readily misdiagnosed as fibrous dysplasia, desmoplastic fibroma, nonossifying fibroma, osteoblastoma, and aneurysmal bone cyst.6 Findings found in low-grade OS can include evidence of cortical interruption, local soft tissue mass development, intramedullary involvement, cortical destruction, and poor margination; however, low-grade OS is typically sclerotic and highly trabeculated. Cross-sectional imaging can help differentiate between OS and other more benign pathologies and should be considered in the clavicle where biopsy may be perilous.5
The difficulty of clavicular biopsy has been reported. Not only does clavicular anatomy make biopsy hazardous, but also the potential for sampling error does exist. In a case report of one patient with a highgrade lesion, fine needle aspiration biopsy was initially diagnosed as an aneurysmal bone cyst but was ultimately found to be osteosarcoma.2 Histology of low-grade lesions usually demonstrates minimal cytological atypia, rare mitotic activity, and variable osteoid production.5 Lower mitotic indices typically make wide resection curative for these patients, without the need for chemotherapy.
In this case, wide resection was carried out with the subclavian vein as the posterior-inferior margin and the sternoclavicular joint as the medial margin. Though the intra-operative medial margin was clear of disease, final pathology demonstrated focal (microscopic) involvement of the posterior and medial margins. A study of soft tissue sarcoma evaluated positive margins and concluded that the imperative of preservation of vital structures supersedes the need for negative margins.7,8 The rate of metastasis and overall survival was similar to surgical resections with positive margins. In the case of our patient, further resection would have carried significant morbidity and possibly mortality, including sacrifice of the major vessels to the arm below and entering into the sternum and thoracic cavity. The likely disability as well as the hazards of surgery were deemed to be too great to justify further excision. Frequent cross-sectional imaging will be necessary to evaluate the presence of recurrent or metastatic disease. To our knowledge, this is the first documented case of low-grade clavicle OS. This report demonstrates the need for multidisciplinary sarcoma care at a center of excellence, particularly in instances of unusual diagnoses.
Osteosarcoma (OS) is a rare disease with approximately 800- 900 newly diagnosed cases each year in the United States. Of those, the majority occur about the knee. The distal femur is the most common site, followed by the proximal tibia, with the proximal humerus being a distant third. OS of the clavicle has been reported, with the earliest case report dating from 1975.1 Since then, additional case reports of high-grade OS of the clavicle have been published.2,3 We describe the case of a 16-year-old female who presented with a mass on her right medial clavicle, which was confirmed to be a low-grade central OS.
Case Presentation
The patient is a 16-year-old female who presented to the Emergency Department (ED) for evaluation of a mass on her right clavicle, after being evaluated by her primary care physician (PCP). She noted an enlarging mass over the previous 2 months but stated that it had been asymptomatic until 4 days prior to presentation to her PCP, at which time she had developed tenderness to palpation and pain with range of motion of the right arm. X-rays were obtained at the PCP’s office and she was referred to the ED for further evaluation. She denied constitutional symptoms.
At the ED visit, she was noted to have an area of erythema and tenderness over the medial aspect of the right clavicle with increased bony prominence. A chest x-ray demonstrated medial clavicle enlargement with periosteal reaction and sclerosis (Figure 1).
MRI demonstrated a 6-cm x 3.8-cm x 4.1-cm mass arising from the right medial clavicle with cortical destruction and concomitant displacement of the right subclavian and brachiocephalic veins (Figure 2). A CT-guided biopsy was performed 1 week later and demonstrated low-grade OS. The pathologist was concerned about the possibility of sampling error and the presence of a higher-grade component, as low-grade OS of the clavicle had not been reported.
The patient was evaluated by a pediatric hematologist/oncologist 2 weeks later after having obtained the biopsy and a PET/CT scan. At that time, the PET/CT showed an FDG-avid mass at the clavicle without evidence of pulmonary metastatic disease (Figure 3). She was subsequently evaluated by orthopedic oncology, at which time a discussion was had regarding further treatment. There was essentially no literature to guide the surgical and medical teams, as low-grade clavicular OS is unknown. Based on the evidence of localized, low-grade disease, the determination was made to proceedwith surgical resection. In the event that high-grade disease was identified at the time of final pathological evaluation, the pediatric hematology/oncology team felt that administering all of the patient’s chemotherapy postoperatively would be acceptable and not affect her long-term prognosis. CT and CT angiogram were obtained for further operative planning (Figure 4).
Given the intimacy of the mass to the subclavian vessels, she was also seen preoperatively by pediatric general and cardiothoracic surgeons. The plan was formulated to have them in the operating room for mobilization of the subclavian vessels and in the event that a sternotomy was required for proximal control of the vessels. Following this visit, the case was discussed at the multidisciplinary pediatric tumor board and the consensus was to proceed with surgical resection.
Surgical Technique
General endotracheal anesthesia was administered without complication. The patient was positioned supine with a soft bump under her shoulders to place her neck in slight extension and thus facilitate access to the clavicle and great vessels. A 14-cm oblique incision was made over the subcutaneous clavicle extending to the contralateral sternoclavicular joint. Dissection was carried down to the fascia and the biopsy site was excised with the skin paddle. Dissection was carried through the sternocleidomastoid superiorly and the pectoralis major inferiorly, to 8 cm lateral from the right sternoclavicular joint. The clavicle was osteotomized well lateral of the palpable tumor and a marrow margin was sent for frozen section, which was found to be negative.
Dissection was continued circumferentially. Assistance from pediatric general and cardiothoracic surgery was required at the inferior aspect of the mass to assist with exposure and control of the subclavian vein (Figure 5A). A large branch of the subclavian vein near its junction with the internal jugular vein was found to be involved with the tumor and thus required suture ligation. The subclavian vein was noted to be intimate with the mass and somewhat friable. With the vein mobilized, a cuff of normal tissue was obtained inferiorly and superiorly to the mass. Medially, the sternoclavicular joint was disarticulated (Figure 5B). At this point, the specimen was delivered from the operative field and tagged in the usual fashion (Figure 5C). A medial soft tissue margin from the sternal side of the sternoclavicular joint was also sent and found to be negative for tumor. The wound was closed in layered fashion over a ¼” Penrose drain. A soft dressing was placed, and the patient was successfully extubated and transferred to the post-anesthesia care unit in stable condition.
Postoperative Course
The patient was found to be neurologically and vascularly intact on postoperative exam and was discharged on postoperative day 1.
She was seen 14 days postoperatively and was doing well at that time, with full range of motion of the shoulder, elbow, wrist, and hand. Final pathology confirmed a low-grade OS with extraosseous extension. All margins were negative except the medial (sternoclavicular joint) margin and the inferior margin adjacent to the subclavian vein. The intraoperative frozen section from the medial margin was negative for tumor.
The pediatric hematology/oncology team determined that, as no high-grade areas were identified, chemotherapy should be deferred. The positive margins were also discussed with the patient and her family specifically regarding further possible treatments. The findings from the pathology were discussed in a multidisciplinary tumor board and it was felt that, given the low-grade nature of the lesion as well as the high morbidity and risk of mortality with further surgery, additional surgery would be potentially more harmful than helpful. Additionally, low-grade OS is extremely resistant to radiotherapy. The plan remains to monitor her for local recurrence as well as metastases with serial imaging.
Discussion
The clavicle is one of the first bones in the body to ossify but one of the last to have final physeal closure. Its unique characteristics have led to various descriptions, such as a “short tubular bone” versus a “flat bone.”4,5 Of note are its paucity of a true intramedullary space and scanty red marrow, which make it an unlikely site for a primarily intramedullary- based neoplasm to arise.4 However, it has also been noted that malignant lesions are more common in the clavicle than benign lesions, and special attention should be paid to aggressiveappearing lesions in the clavicle.
Radiographs can be misleading as well. Prior studies have demonstrated that low-grade central OS can be readily misdiagnosed as fibrous dysplasia, desmoplastic fibroma, nonossifying fibroma, osteoblastoma, and aneurysmal bone cyst.6 Findings found in low-grade OS can include evidence of cortical interruption, local soft tissue mass development, intramedullary involvement, cortical destruction, and poor margination; however, low-grade OS is typically sclerotic and highly trabeculated. Cross-sectional imaging can help differentiate between OS and other more benign pathologies and should be considered in the clavicle where biopsy may be perilous.5
The difficulty of clavicular biopsy has been reported. Not only does clavicular anatomy make biopsy hazardous, but also the potential for sampling error does exist. In a case report of one patient with a highgrade lesion, fine needle aspiration biopsy was initially diagnosed as an aneurysmal bone cyst but was ultimately found to be osteosarcoma.2 Histology of low-grade lesions usually demonstrates minimal cytological atypia, rare mitotic activity, and variable osteoid production.5 Lower mitotic indices typically make wide resection curative for these patients, without the need for chemotherapy.
In this case, wide resection was carried out with the subclavian vein as the posterior-inferior margin and the sternoclavicular joint as the medial margin. Though the intra-operative medial margin was clear of disease, final pathology demonstrated focal (microscopic) involvement of the posterior and medial margins. A study of soft tissue sarcoma evaluated positive margins and concluded that the imperative of preservation of vital structures supersedes the need for negative margins.7,8 The rate of metastasis and overall survival was similar to surgical resections with positive margins. In the case of our patient, further resection would have carried significant morbidity and possibly mortality, including sacrifice of the major vessels to the arm below and entering into the sternum and thoracic cavity. The likely disability as well as the hazards of surgery were deemed to be too great to justify further excision. Frequent cross-sectional imaging will be necessary to evaluate the presence of recurrent or metastatic disease. To our knowledge, this is the first documented case of low-grade clavicle OS. This report demonstrates the need for multidisciplinary sarcoma care at a center of excellence, particularly in instances of unusual diagnoses.
1. Zinghi G. Osteosarcoma of the clavicle (description of a case) [in Italian]. Chir Organi Mov. 1975;62(6):671-674.
2. Cundy WJ, Carter C, Dhatrak D, Clayer M. Primary osteosarcoma of the clavicle and the perils of bone biopsy. BMJ Case Rep. 2015;2015:bcr2014208859.
3. Greenspan A, Unni KK, Mann J. Case report 804: Chondroblastic osteosarcoma grade 3 of the left clavicle. Skeletal Radiol. 1993;22(6):469-471.
4. Rossi B, Fabbriciani C, Chalidis BE, Visci F, Maccauro G. Primary malignant clavicular tumours: a clinicopathological analysis of six cases and evaluation of surgical management. Arch Orthop Trauma Surg. 2011;131(7):935-939.
5. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004;33(7):373-379.
6. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: A difficult condition to diagnose. Sarcoma. 2012; 2012:764796.
7. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866-2875.
8. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165-172.
1. Zinghi G. Osteosarcoma of the clavicle (description of a case) [in Italian]. Chir Organi Mov. 1975;62(6):671-674.
2. Cundy WJ, Carter C, Dhatrak D, Clayer M. Primary osteosarcoma of the clavicle and the perils of bone biopsy. BMJ Case Rep. 2015;2015:bcr2014208859.
3. Greenspan A, Unni KK, Mann J. Case report 804: Chondroblastic osteosarcoma grade 3 of the left clavicle. Skeletal Radiol. 1993;22(6):469-471.
4. Rossi B, Fabbriciani C, Chalidis BE, Visci F, Maccauro G. Primary malignant clavicular tumours: a clinicopathological analysis of six cases and evaluation of surgical management. Arch Orthop Trauma Surg. 2011;131(7):935-939.
5. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004;33(7):373-379.
6. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: A difficult condition to diagnose. Sarcoma. 2012; 2012:764796.
7. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866-2875.
8. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165-172.
CAR T cells target HER2 expression in advanced sarcomas
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
REPORTING FROM AACR 2019
REGOBONE: Regorafenib shows efficacy in metastatic osteosarcoma
Regorafenib appears to be active in patients with metastatic osteosarcomas, based on results from REGOBONE a non-comparative phase 2, double-blind, placebo-controlled trial.
Among 38 efficacy-evaluable patients (12 given placebo and 26 given regorafenib), 17 patients (65.4%) were non-progressive at 8 weeks in the regorafenib arm and 0 in the placebo arm, Florence Duffaud, MD, of La Timone University Hospital, Marseille, France, reported at the annual meeting of the American Society of Clinical Oncology.
Median progression-free survival (PFS) was 13.7 weeks for patients given regorafenib and 4 weeks for those given placebo. The PFS rate at 24 weeks was 35% with regorafenib and 0 with placebo. The 1-year overall survival was 53% and 33% for regorafenib and placebo, respectively.
Ten patients in the placebo arm crossed-over to the regorafenib arm of the study after centrally-confirmed disease progression. The most common adverse events of Grade 3 or greater with regorafenib were hypertension (24%), hand-foot skin reaction (17%), asthenia (10%), and diarrhea (7%).
REGOBONE consists of 4 independent cohorts: patients with either metastatic osteosarcoma, Ewing sarcoma, chondrosarcoma, or chordoma. The results were reported for 43 patients with metastatic osteosarcoma who were randomized 2:1 to receive either regorafinib (160 mg/day for 21 days of a 28 day cycle) or to placebo with the option to cross over at the time of confirmed central review of progressive disease.
Dr. Duffaud and several of her co-authors received funding from various drug companies including Bayer, the maker of regorafenib (Stivarga). Clinical trial information: NCT02389244
SOURCE: Duffaud F et al. ASCO 2018 (annual meeting of the American Society of Clinical Oncology), Abstract 11504.
Regorafenib appears to be active in patients with metastatic osteosarcomas, based on results from REGOBONE a non-comparative phase 2, double-blind, placebo-controlled trial.
Among 38 efficacy-evaluable patients (12 given placebo and 26 given regorafenib), 17 patients (65.4%) were non-progressive at 8 weeks in the regorafenib arm and 0 in the placebo arm, Florence Duffaud, MD, of La Timone University Hospital, Marseille, France, reported at the annual meeting of the American Society of Clinical Oncology.
Median progression-free survival (PFS) was 13.7 weeks for patients given regorafenib and 4 weeks for those given placebo. The PFS rate at 24 weeks was 35% with regorafenib and 0 with placebo. The 1-year overall survival was 53% and 33% for regorafenib and placebo, respectively.
Ten patients in the placebo arm crossed-over to the regorafenib arm of the study after centrally-confirmed disease progression. The most common adverse events of Grade 3 or greater with regorafenib were hypertension (24%), hand-foot skin reaction (17%), asthenia (10%), and diarrhea (7%).
REGOBONE consists of 4 independent cohorts: patients with either metastatic osteosarcoma, Ewing sarcoma, chondrosarcoma, or chordoma. The results were reported for 43 patients with metastatic osteosarcoma who were randomized 2:1 to receive either regorafinib (160 mg/day for 21 days of a 28 day cycle) or to placebo with the option to cross over at the time of confirmed central review of progressive disease.
Dr. Duffaud and several of her co-authors received funding from various drug companies including Bayer, the maker of regorafenib (Stivarga). Clinical trial information: NCT02389244
SOURCE: Duffaud F et al. ASCO 2018 (annual meeting of the American Society of Clinical Oncology), Abstract 11504.
Regorafenib appears to be active in patients with metastatic osteosarcomas, based on results from REGOBONE a non-comparative phase 2, double-blind, placebo-controlled trial.
Among 38 efficacy-evaluable patients (12 given placebo and 26 given regorafenib), 17 patients (65.4%) were non-progressive at 8 weeks in the regorafenib arm and 0 in the placebo arm, Florence Duffaud, MD, of La Timone University Hospital, Marseille, France, reported at the annual meeting of the American Society of Clinical Oncology.
Median progression-free survival (PFS) was 13.7 weeks for patients given regorafenib and 4 weeks for those given placebo. The PFS rate at 24 weeks was 35% with regorafenib and 0 with placebo. The 1-year overall survival was 53% and 33% for regorafenib and placebo, respectively.
Ten patients in the placebo arm crossed-over to the regorafenib arm of the study after centrally-confirmed disease progression. The most common adverse events of Grade 3 or greater with regorafenib were hypertension (24%), hand-foot skin reaction (17%), asthenia (10%), and diarrhea (7%).
REGOBONE consists of 4 independent cohorts: patients with either metastatic osteosarcoma, Ewing sarcoma, chondrosarcoma, or chordoma. The results were reported for 43 patients with metastatic osteosarcoma who were randomized 2:1 to receive either regorafinib (160 mg/day for 21 days of a 28 day cycle) or to placebo with the option to cross over at the time of confirmed central review of progressive disease.
Dr. Duffaud and several of her co-authors received funding from various drug companies including Bayer, the maker of regorafenib (Stivarga). Clinical trial information: NCT02389244
SOURCE: Duffaud F et al. ASCO 2018 (annual meeting of the American Society of Clinical Oncology), Abstract 11504.
FROM ASCO 2018
cfDNA reveals targetable mutations in pediatric neuroblastoma, sarcoma
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
REPORTING FROM ASPHO 2018
Key clinical point: Circulating free DNA analysis is a noninvasive method for detecting potential therapeutic targets.
Major finding: cfDNA revealed potentially targetable new mutations in 6 of 15 patients with neuroblastoma.
Study details: Retrospective analysis of tumor and plasma samples in 30 patients with neuroblastoma, osteosarcoma, or Wilms tumor.
Disclosures: The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
Source: Kothari P et al. ASPHO 2018. Abstract #809.
Fractures in adult osteosarcoma patients presage worse survival
AMSTERDAM – Pathological fractures are prognostic of poor outcomes in adults with osteosarcoma, but not in children with osteosarcoma, investigators have found.
A retrospective review of data on consecutive patients treated over the course of 30 years showed that among patients of all ages, both 5-year and 10-year overall survival (OS) rates were worse among patients who had pathological fractures.
But in an analysis stratified by age, the survival difference attributable to fractures was limited entirely to patients who were 18 or older at the time of an osteosarcoma diagnosis, reported Lisa Kelley, a medical student at the Ludwig-Maximilians Universität in Munich.
“It’s important to understand what are the prognostic factors [in osteosarcoma], and while many of the factors have been thoroughly researched, on pathological fractures the data are still fairly inconclusive due to the results of studies contradicting each other, and also having a relatively small patient number due to the fact that osteosarcoma itself is rare, and the pathological fractures occur only in approximately 10% of cases,” she said at an annual congress sponsored by the European Cancer Organisation.
To get a better handle on possible correlations between pathological fractures and prognosis in patients with central high-grade osteosarcoma, Ms. Kelley and her coauthors collected data on consecutive patients treated for localized or metastatic osteosarcoma of the extremities from 1980 through 2010 at one of the member institutions of the Cooperative Osteosarcoma Study Group (COSS).
They identified 2,847 patients, of whom 2,193 (77%) were 18 or younger at the time of diagnosis. Of the entire cohort, 321 patients (11.3%) had a pathological fracture either at presentation or soon after diagnosis.
Comparing patients with and without pathological fractures, the investigators found that factors significantly associated with fracture risk included tumor location, especially the humerus (P less than .001), tumors occurring proximally and in a diaphysis (P less than .001), telangiectatic subtype (P less than .001), the presence of primary metastases (P = .025). and tumors comprising more than one-third of the affected bone (P less than .001).
There were no significant differences in the cohort as a whole between patients with or without fractures in either age in years, sex, body-mass index, history of pain or swelling symptoms, local surgical remission at the main tumor site, total surgical remission including metastases, response to chemotherapy, use of adjuvant chemotherapy rather than neoadjuvant, or type of surgery.
Among adult patients only, however, factors associated with pathological fractures included age (P less than .001). BMI (P = .021), tumor site (P less than .001), histologic subtype (P less than .001), primary metastases (P = .011), relative tumor size (P = .047), and total surgical remission (P = .015).
Among pediatric patients, factors associated with fracture risk were (P less than .001 for all unless otherwise specified) age, BMI (P = .018), history or symptoms (P = .001). tumor site, localization within bone, histologic subtype, and relative tumor size.
In univariate analysis, 5-year OS rates were 70.6% for patients without fractures compared with 63.0% for those with fractures, and respective 10-year OS rates were 64.9% vs. 58.1% (P = .007 for both comparisons).
Among pediatric patients, the Kaplan-Meier survival curves of patients with and without pathological fractures overlapped. But for adult patients, survival of those with fractures was significantly worse, with 5-year OS of 69.3% with no fractures vs. 45.9% with fractures, and respective 10-year OS rates of 62.1% vs. 36.8% (P less than .001 for both comparisons).
Also in univariate analysis, 5-year and 10-year event-free survival (EFS) rates were significantly lower for patients who experienced pathological fractures.
Finally, in multivariable analysis of overall survival by age group, the investigators found that among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio [HR] 1.893, P = .013). Other factors were primary metastases (HR 2.486, P = .001), response to chemotherapy (P less than .001) and total surgical remission (P less than .001).
As noted before, pathological fracture among pediatric patients was not associated with worse OS. Factors significantly associated with OS in younger patients were primary metastases (HR 2.187, P less than .001), relative tumor size (HR 1.239, P = .024), response to chemotherapy (HR 2.295, P less than .001), total surgical remission (HR 4.253, P less than .001), and type of surgery (HR 1.282, P = .008).
In contrast, pathological fracture was not associated with EFS among either adult or pediatric patients. Factors significantly associated with EFS in both children and adults were primary metastases and response to chemotherapy. Among pediatric patients only, tumor site and relative tumor size were also associated with EFS.
Ms. Kelley acknowledged that the study was limited by the retrospective design.
She said that exploration of the discrepancies between adult and pediatric patients in regard to the influence of pathological fracture on survival and of the role of pathologic fracture as a negative prognostic factor for OS but not EFS in adults is warranted.
The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
AMSTERDAM – Pathological fractures are prognostic of poor outcomes in adults with osteosarcoma, but not in children with osteosarcoma, investigators have found.
A retrospective review of data on consecutive patients treated over the course of 30 years showed that among patients of all ages, both 5-year and 10-year overall survival (OS) rates were worse among patients who had pathological fractures.
But in an analysis stratified by age, the survival difference attributable to fractures was limited entirely to patients who were 18 or older at the time of an osteosarcoma diagnosis, reported Lisa Kelley, a medical student at the Ludwig-Maximilians Universität in Munich.
“It’s important to understand what are the prognostic factors [in osteosarcoma], and while many of the factors have been thoroughly researched, on pathological fractures the data are still fairly inconclusive due to the results of studies contradicting each other, and also having a relatively small patient number due to the fact that osteosarcoma itself is rare, and the pathological fractures occur only in approximately 10% of cases,” she said at an annual congress sponsored by the European Cancer Organisation.
To get a better handle on possible correlations between pathological fractures and prognosis in patients with central high-grade osteosarcoma, Ms. Kelley and her coauthors collected data on consecutive patients treated for localized or metastatic osteosarcoma of the extremities from 1980 through 2010 at one of the member institutions of the Cooperative Osteosarcoma Study Group (COSS).
They identified 2,847 patients, of whom 2,193 (77%) were 18 or younger at the time of diagnosis. Of the entire cohort, 321 patients (11.3%) had a pathological fracture either at presentation or soon after diagnosis.
Comparing patients with and without pathological fractures, the investigators found that factors significantly associated with fracture risk included tumor location, especially the humerus (P less than .001), tumors occurring proximally and in a diaphysis (P less than .001), telangiectatic subtype (P less than .001), the presence of primary metastases (P = .025). and tumors comprising more than one-third of the affected bone (P less than .001).
There were no significant differences in the cohort as a whole between patients with or without fractures in either age in years, sex, body-mass index, history of pain or swelling symptoms, local surgical remission at the main tumor site, total surgical remission including metastases, response to chemotherapy, use of adjuvant chemotherapy rather than neoadjuvant, or type of surgery.
Among adult patients only, however, factors associated with pathological fractures included age (P less than .001). BMI (P = .021), tumor site (P less than .001), histologic subtype (P less than .001), primary metastases (P = .011), relative tumor size (P = .047), and total surgical remission (P = .015).
Among pediatric patients, factors associated with fracture risk were (P less than .001 for all unless otherwise specified) age, BMI (P = .018), history or symptoms (P = .001). tumor site, localization within bone, histologic subtype, and relative tumor size.
In univariate analysis, 5-year OS rates were 70.6% for patients without fractures compared with 63.0% for those with fractures, and respective 10-year OS rates were 64.9% vs. 58.1% (P = .007 for both comparisons).
Among pediatric patients, the Kaplan-Meier survival curves of patients with and without pathological fractures overlapped. But for adult patients, survival of those with fractures was significantly worse, with 5-year OS of 69.3% with no fractures vs. 45.9% with fractures, and respective 10-year OS rates of 62.1% vs. 36.8% (P less than .001 for both comparisons).
Also in univariate analysis, 5-year and 10-year event-free survival (EFS) rates were significantly lower for patients who experienced pathological fractures.
Finally, in multivariable analysis of overall survival by age group, the investigators found that among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio [HR] 1.893, P = .013). Other factors were primary metastases (HR 2.486, P = .001), response to chemotherapy (P less than .001) and total surgical remission (P less than .001).
As noted before, pathological fracture among pediatric patients was not associated with worse OS. Factors significantly associated with OS in younger patients were primary metastases (HR 2.187, P less than .001), relative tumor size (HR 1.239, P = .024), response to chemotherapy (HR 2.295, P less than .001), total surgical remission (HR 4.253, P less than .001), and type of surgery (HR 1.282, P = .008).
In contrast, pathological fracture was not associated with EFS among either adult or pediatric patients. Factors significantly associated with EFS in both children and adults were primary metastases and response to chemotherapy. Among pediatric patients only, tumor site and relative tumor size were also associated with EFS.
Ms. Kelley acknowledged that the study was limited by the retrospective design.
She said that exploration of the discrepancies between adult and pediatric patients in regard to the influence of pathological fracture on survival and of the role of pathologic fracture as a negative prognostic factor for OS but not EFS in adults is warranted.
The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
AMSTERDAM – Pathological fractures are prognostic of poor outcomes in adults with osteosarcoma, but not in children with osteosarcoma, investigators have found.
A retrospective review of data on consecutive patients treated over the course of 30 years showed that among patients of all ages, both 5-year and 10-year overall survival (OS) rates were worse among patients who had pathological fractures.
But in an analysis stratified by age, the survival difference attributable to fractures was limited entirely to patients who were 18 or older at the time of an osteosarcoma diagnosis, reported Lisa Kelley, a medical student at the Ludwig-Maximilians Universität in Munich.
“It’s important to understand what are the prognostic factors [in osteosarcoma], and while many of the factors have been thoroughly researched, on pathological fractures the data are still fairly inconclusive due to the results of studies contradicting each other, and also having a relatively small patient number due to the fact that osteosarcoma itself is rare, and the pathological fractures occur only in approximately 10% of cases,” she said at an annual congress sponsored by the European Cancer Organisation.
To get a better handle on possible correlations between pathological fractures and prognosis in patients with central high-grade osteosarcoma, Ms. Kelley and her coauthors collected data on consecutive patients treated for localized or metastatic osteosarcoma of the extremities from 1980 through 2010 at one of the member institutions of the Cooperative Osteosarcoma Study Group (COSS).
They identified 2,847 patients, of whom 2,193 (77%) were 18 or younger at the time of diagnosis. Of the entire cohort, 321 patients (11.3%) had a pathological fracture either at presentation or soon after diagnosis.
Comparing patients with and without pathological fractures, the investigators found that factors significantly associated with fracture risk included tumor location, especially the humerus (P less than .001), tumors occurring proximally and in a diaphysis (P less than .001), telangiectatic subtype (P less than .001), the presence of primary metastases (P = .025). and tumors comprising more than one-third of the affected bone (P less than .001).
There were no significant differences in the cohort as a whole between patients with or without fractures in either age in years, sex, body-mass index, history of pain or swelling symptoms, local surgical remission at the main tumor site, total surgical remission including metastases, response to chemotherapy, use of adjuvant chemotherapy rather than neoadjuvant, or type of surgery.
Among adult patients only, however, factors associated with pathological fractures included age (P less than .001). BMI (P = .021), tumor site (P less than .001), histologic subtype (P less than .001), primary metastases (P = .011), relative tumor size (P = .047), and total surgical remission (P = .015).
Among pediatric patients, factors associated with fracture risk were (P less than .001 for all unless otherwise specified) age, BMI (P = .018), history or symptoms (P = .001). tumor site, localization within bone, histologic subtype, and relative tumor size.
In univariate analysis, 5-year OS rates were 70.6% for patients without fractures compared with 63.0% for those with fractures, and respective 10-year OS rates were 64.9% vs. 58.1% (P = .007 for both comparisons).
Among pediatric patients, the Kaplan-Meier survival curves of patients with and without pathological fractures overlapped. But for adult patients, survival of those with fractures was significantly worse, with 5-year OS of 69.3% with no fractures vs. 45.9% with fractures, and respective 10-year OS rates of 62.1% vs. 36.8% (P less than .001 for both comparisons).
Also in univariate analysis, 5-year and 10-year event-free survival (EFS) rates were significantly lower for patients who experienced pathological fractures.
Finally, in multivariable analysis of overall survival by age group, the investigators found that among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio [HR] 1.893, P = .013). Other factors were primary metastases (HR 2.486, P = .001), response to chemotherapy (P less than .001) and total surgical remission (P less than .001).
As noted before, pathological fracture among pediatric patients was not associated with worse OS. Factors significantly associated with OS in younger patients were primary metastases (HR 2.187, P less than .001), relative tumor size (HR 1.239, P = .024), response to chemotherapy (HR 2.295, P less than .001), total surgical remission (HR 4.253, P less than .001), and type of surgery (HR 1.282, P = .008).
In contrast, pathological fracture was not associated with EFS among either adult or pediatric patients. Factors significantly associated with EFS in both children and adults were primary metastases and response to chemotherapy. Among pediatric patients only, tumor site and relative tumor size were also associated with EFS.
Ms. Kelley acknowledged that the study was limited by the retrospective design.
She said that exploration of the discrepancies between adult and pediatric patients in regard to the influence of pathological fracture on survival and of the role of pathologic fracture as a negative prognostic factor for OS but not EFS in adults is warranted.
The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
AT ECCO2017
Key clinical point: Pathological fractures are associated with worse survival of adults but not children with osteosarcoma of the extremities.
Major finding: Among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio, 1.893, P = .013).
Data source: Retrospective review of data on 2,847 consecutive patients with osteosarcoma.
Disclosures: The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.