User login
PPI cuts GI events from low- and high-dose aspirin
CHICAGO – Six months of treatment with a proton pump inhibitor (PPI) is a safe way to cut the incidence of major gastrointestinal events in cardiovascular disease patients on dual-antiplatelet therapy regardless of whether they receive low-dose or high-dose aspirin, according to a post-hoc analysis of data from more than 3,700 patients enrolled in the multicenter, randomized COGENT trial.
“Short-term, prophylactic PPI therapy consistently reduced rates of adjudicated upper-gastrointestinal events without increasing cardiovascular events, regardless of the aspirin dose,” Dr. Muthiah Vaduganathan said while presenting his study at the annual meeting of the American College of Cardiology. “Gastroprotection with PPI therapy should be used in appropriately selected patients with coronary artery disease who require dual-antiplatelet therapy even if they are on low-dose aspirin.”
In addition to documenting the safety and efficacy of 6 months of PPI treatment for patients at high risk for cardiovascular events and low or moderate risk for a GI event, the results from the analysis also documented how common GI events are in this population, even when patients receive low-dose aspirin. Nearly two-thirds of the 3,752 patients included in the analysis took low-dose aspirin, either 75 mg or 81 mg per day. Their incidence of an adjudicated upper GI bleed, the study’s primary GI endpoint, occurred in 3.1% of patients on placebo, and in 1.2% of patients taking a prophylactic PPI. Among the other 34% of patients on high-dose aspirin – a daily dosage of at least 150 mg – the rate of adjudicated upper-GI bleeds was 2.6% without a PPI and 0.9% in those on a PPI.
In other words, even among patients deemed to have a relatively low risk for upper GI complications from aspirin (because their entry into this study required no history of major GI bleeds or recent treatment with a gastroprotection agent), treatment with low-dose aspirin resulted in upper-GI bleeds at the same rate, about 3%, as high-dose aspirin. And in both of these aspirin subgroups 6 months of concurrent treatment with a PPI cut the incidence of major GI bleeds by more than half.
The findings are especially notable because the enrollment criteria stacked the deck toward patients with high cardiovascular disease risk and relatively low GI risk. The study enrolled “a unique population at high risk for cardiovascular disease – 71% had previously undergone a percutaneous coronary intervention, and 42% had a history of an acute coronary syndrome – and low GI risk, but even in this population enriched for cardiovascular disease risk, there was no increased rate of cardiovascular disease events” during a median follow-up while on PPI treatment of 110 days, Dr. Vaduganathan said.
Among patients on low-dose aspirin, the rate of cardiovascular death, MI, stroke, or coronary revascularization was 5.6% with PPI treatment and 5.5% without, and in the high-dose aspirin patients the rates were 4.2% with PPI treatment and 5.5% without. Neither of these differences between the subgroups taking or not taking a PPI were statistically significant.
Concurrent with Dr. Vaduganathan’s report at the meeting the results also appeared online (J Am Coll Cardiol. 2016 April 12;67[14]:661-71).
“There appeared to be no adverse clinical effect from PPI treatment. When used short-term, for up to 6 months, PPI treatment appears to be safe in patients with cardiovascular disease,” Dr. Vaduganathan concluded.
The analysis used data collected in COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial), a phase 3 study designed to compare a single-pill formulation of 20 mg omeprazole and 75 mg clopidogrel taken orally once daily with 75 mg clopidogrel against a background of all patients taking aspirin. COGENT stopped prematurely in late 2008 as the company developing this formulation and sponsoring the trial, Cogentus Pharmaceuticals, filed for bankruptcy. Despite its abrupt conclusion, the trial had enrolled and followed enough patients to show that treatment with omeprazole plus clopidogrel and aspirin led to a significant reduction in upper GI bleeding without increasing the rate of cardiovascular disease events, compared with clopidogrel plus aspirin (N Engl J Med. 2010 Nov 11;363[20]:1909-17).
The new analysis focused on the greater than 99% of patients in the total COGENT cohort for whom information was available on whether they received high- or low-dose aspirin.
Although the primary findings from COGENT, reported in 2010, documented the safety and efficacy of concomitant PPI treatment during dual-antiplatelet therapy, and despite guidelines revised in 2010 that called for PPI treatment when appropriate, this strategy for preventing GI complications remains underused, Dr. Vaduganathan said. The most recent U.S. recommendations that address this issue called for assessing the potential risk and benefit from PPI treatment in patients receiving dual-antiplatelet therapy: “The risk reduction with PPIs is substantial in patients with risk factors for GI bleeding and may outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug-drug interaction (J Am Coll Cardiol. 2010 Dec;56[24]:2051-66).”
The only caveat Dr. Vaduganathan placed on PPI use was that the COGENT data addressed only 6 months of PPI use; the safety of longer-term use has not been studied. But “the trend is to use PPIs for as short a period as possible,” and the risk for adverse effects from PPI treatment on cardiovascular disease events is likely greatest during the first 6 months of PPI treatment, he noted. If PPI treatment needs to continue beyond 6 months, he suggested systematically reassessing the risk-benefit balance for individual patients from continued PPI treatment every 3 months.*
*Changes were made to this story on 4/20/2016.
On Twitter @mitchelzoler
The new analysis of COGENT provides important insights into patients treated with clopidogrel and aspirin. The data show that patients on low-dose aspirin do not have an increased risk of cardiovascular events, and that patients who take low-dose aspirin still face a significant risk for upper-gastrointestinal events. Patients taking low-dose aspirin have about the same rate of upper-GI events as patients on high-dose aspirin.
The issue of GI safety for patients on low-dose aspirin as part of dual-antiplatelet therapy has been long overshadowed by concern over a hypothetical interaction between clopidogrel and proton pump inhibitors. The issue has also been distorted by a false sense of security that when patients receive low-dose aspirin they do not require protection against GI events.
Treatment of patients taking low-dose aspirin with a PPI is underutilized. The confirmation this analysis provides, that PPI treatment gives GI protection without causing an excess of cardiovascular events, calls for a change in current practice when clinicians prescribe low-dose aspirin. I’m concerned by the apparent lack of enthusiasm by clinicians to prescribe PPIs to their patients on low-dose aspirin despite their significant risk for GI events. The real question is whether all patients on low-dose aspirin should receive a PPI long term or only the subgroup of patients with high risk for an upper-GI bleed.
Dr. Michael E. Farkouh is a cardiologist at Mount Sinai Hospital in Toronto. He has no disclosures. He made these comments in an editorial that accompanied the published report (J Am Coll Cardiol. 2016 April 12;67[14]:1672-3).
The new analysis of COGENT provides important insights into patients treated with clopidogrel and aspirin. The data show that patients on low-dose aspirin do not have an increased risk of cardiovascular events, and that patients who take low-dose aspirin still face a significant risk for upper-gastrointestinal events. Patients taking low-dose aspirin have about the same rate of upper-GI events as patients on high-dose aspirin.
The issue of GI safety for patients on low-dose aspirin as part of dual-antiplatelet therapy has been long overshadowed by concern over a hypothetical interaction between clopidogrel and proton pump inhibitors. The issue has also been distorted by a false sense of security that when patients receive low-dose aspirin they do not require protection against GI events.
Treatment of patients taking low-dose aspirin with a PPI is underutilized. The confirmation this analysis provides, that PPI treatment gives GI protection without causing an excess of cardiovascular events, calls for a change in current practice when clinicians prescribe low-dose aspirin. I’m concerned by the apparent lack of enthusiasm by clinicians to prescribe PPIs to their patients on low-dose aspirin despite their significant risk for GI events. The real question is whether all patients on low-dose aspirin should receive a PPI long term or only the subgroup of patients with high risk for an upper-GI bleed.
Dr. Michael E. Farkouh is a cardiologist at Mount Sinai Hospital in Toronto. He has no disclosures. He made these comments in an editorial that accompanied the published report (J Am Coll Cardiol. 2016 April 12;67[14]:1672-3).
The new analysis of COGENT provides important insights into patients treated with clopidogrel and aspirin. The data show that patients on low-dose aspirin do not have an increased risk of cardiovascular events, and that patients who take low-dose aspirin still face a significant risk for upper-gastrointestinal events. Patients taking low-dose aspirin have about the same rate of upper-GI events as patients on high-dose aspirin.
The issue of GI safety for patients on low-dose aspirin as part of dual-antiplatelet therapy has been long overshadowed by concern over a hypothetical interaction between clopidogrel and proton pump inhibitors. The issue has also been distorted by a false sense of security that when patients receive low-dose aspirin they do not require protection against GI events.
Treatment of patients taking low-dose aspirin with a PPI is underutilized. The confirmation this analysis provides, that PPI treatment gives GI protection without causing an excess of cardiovascular events, calls for a change in current practice when clinicians prescribe low-dose aspirin. I’m concerned by the apparent lack of enthusiasm by clinicians to prescribe PPIs to their patients on low-dose aspirin despite their significant risk for GI events. The real question is whether all patients on low-dose aspirin should receive a PPI long term or only the subgroup of patients with high risk for an upper-GI bleed.
Dr. Michael E. Farkouh is a cardiologist at Mount Sinai Hospital in Toronto. He has no disclosures. He made these comments in an editorial that accompanied the published report (J Am Coll Cardiol. 2016 April 12;67[14]:1672-3).
CHICAGO – Six months of treatment with a proton pump inhibitor (PPI) is a safe way to cut the incidence of major gastrointestinal events in cardiovascular disease patients on dual-antiplatelet therapy regardless of whether they receive low-dose or high-dose aspirin, according to a post-hoc analysis of data from more than 3,700 patients enrolled in the multicenter, randomized COGENT trial.
“Short-term, prophylactic PPI therapy consistently reduced rates of adjudicated upper-gastrointestinal events without increasing cardiovascular events, regardless of the aspirin dose,” Dr. Muthiah Vaduganathan said while presenting his study at the annual meeting of the American College of Cardiology. “Gastroprotection with PPI therapy should be used in appropriately selected patients with coronary artery disease who require dual-antiplatelet therapy even if they are on low-dose aspirin.”
In addition to documenting the safety and efficacy of 6 months of PPI treatment for patients at high risk for cardiovascular events and low or moderate risk for a GI event, the results from the analysis also documented how common GI events are in this population, even when patients receive low-dose aspirin. Nearly two-thirds of the 3,752 patients included in the analysis took low-dose aspirin, either 75 mg or 81 mg per day. Their incidence of an adjudicated upper GI bleed, the study’s primary GI endpoint, occurred in 3.1% of patients on placebo, and in 1.2% of patients taking a prophylactic PPI. Among the other 34% of patients on high-dose aspirin – a daily dosage of at least 150 mg – the rate of adjudicated upper-GI bleeds was 2.6% without a PPI and 0.9% in those on a PPI.
In other words, even among patients deemed to have a relatively low risk for upper GI complications from aspirin (because their entry into this study required no history of major GI bleeds or recent treatment with a gastroprotection agent), treatment with low-dose aspirin resulted in upper-GI bleeds at the same rate, about 3%, as high-dose aspirin. And in both of these aspirin subgroups 6 months of concurrent treatment with a PPI cut the incidence of major GI bleeds by more than half.
The findings are especially notable because the enrollment criteria stacked the deck toward patients with high cardiovascular disease risk and relatively low GI risk. The study enrolled “a unique population at high risk for cardiovascular disease – 71% had previously undergone a percutaneous coronary intervention, and 42% had a history of an acute coronary syndrome – and low GI risk, but even in this population enriched for cardiovascular disease risk, there was no increased rate of cardiovascular disease events” during a median follow-up while on PPI treatment of 110 days, Dr. Vaduganathan said.
Among patients on low-dose aspirin, the rate of cardiovascular death, MI, stroke, or coronary revascularization was 5.6% with PPI treatment and 5.5% without, and in the high-dose aspirin patients the rates were 4.2% with PPI treatment and 5.5% without. Neither of these differences between the subgroups taking or not taking a PPI were statistically significant.
Concurrent with Dr. Vaduganathan’s report at the meeting the results also appeared online (J Am Coll Cardiol. 2016 April 12;67[14]:661-71).
“There appeared to be no adverse clinical effect from PPI treatment. When used short-term, for up to 6 months, PPI treatment appears to be safe in patients with cardiovascular disease,” Dr. Vaduganathan concluded.
The analysis used data collected in COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial), a phase 3 study designed to compare a single-pill formulation of 20 mg omeprazole and 75 mg clopidogrel taken orally once daily with 75 mg clopidogrel against a background of all patients taking aspirin. COGENT stopped prematurely in late 2008 as the company developing this formulation and sponsoring the trial, Cogentus Pharmaceuticals, filed for bankruptcy. Despite its abrupt conclusion, the trial had enrolled and followed enough patients to show that treatment with omeprazole plus clopidogrel and aspirin led to a significant reduction in upper GI bleeding without increasing the rate of cardiovascular disease events, compared with clopidogrel plus aspirin (N Engl J Med. 2010 Nov 11;363[20]:1909-17).
The new analysis focused on the greater than 99% of patients in the total COGENT cohort for whom information was available on whether they received high- or low-dose aspirin.
Although the primary findings from COGENT, reported in 2010, documented the safety and efficacy of concomitant PPI treatment during dual-antiplatelet therapy, and despite guidelines revised in 2010 that called for PPI treatment when appropriate, this strategy for preventing GI complications remains underused, Dr. Vaduganathan said. The most recent U.S. recommendations that address this issue called for assessing the potential risk and benefit from PPI treatment in patients receiving dual-antiplatelet therapy: “The risk reduction with PPIs is substantial in patients with risk factors for GI bleeding and may outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug-drug interaction (J Am Coll Cardiol. 2010 Dec;56[24]:2051-66).”
The only caveat Dr. Vaduganathan placed on PPI use was that the COGENT data addressed only 6 months of PPI use; the safety of longer-term use has not been studied. But “the trend is to use PPIs for as short a period as possible,” and the risk for adverse effects from PPI treatment on cardiovascular disease events is likely greatest during the first 6 months of PPI treatment, he noted. If PPI treatment needs to continue beyond 6 months, he suggested systematically reassessing the risk-benefit balance for individual patients from continued PPI treatment every 3 months.*
*Changes were made to this story on 4/20/2016.
On Twitter @mitchelzoler
CHICAGO – Six months of treatment with a proton pump inhibitor (PPI) is a safe way to cut the incidence of major gastrointestinal events in cardiovascular disease patients on dual-antiplatelet therapy regardless of whether they receive low-dose or high-dose aspirin, according to a post-hoc analysis of data from more than 3,700 patients enrolled in the multicenter, randomized COGENT trial.
“Short-term, prophylactic PPI therapy consistently reduced rates of adjudicated upper-gastrointestinal events without increasing cardiovascular events, regardless of the aspirin dose,” Dr. Muthiah Vaduganathan said while presenting his study at the annual meeting of the American College of Cardiology. “Gastroprotection with PPI therapy should be used in appropriately selected patients with coronary artery disease who require dual-antiplatelet therapy even if they are on low-dose aspirin.”
In addition to documenting the safety and efficacy of 6 months of PPI treatment for patients at high risk for cardiovascular events and low or moderate risk for a GI event, the results from the analysis also documented how common GI events are in this population, even when patients receive low-dose aspirin. Nearly two-thirds of the 3,752 patients included in the analysis took low-dose aspirin, either 75 mg or 81 mg per day. Their incidence of an adjudicated upper GI bleed, the study’s primary GI endpoint, occurred in 3.1% of patients on placebo, and in 1.2% of patients taking a prophylactic PPI. Among the other 34% of patients on high-dose aspirin – a daily dosage of at least 150 mg – the rate of adjudicated upper-GI bleeds was 2.6% without a PPI and 0.9% in those on a PPI.
In other words, even among patients deemed to have a relatively low risk for upper GI complications from aspirin (because their entry into this study required no history of major GI bleeds or recent treatment with a gastroprotection agent), treatment with low-dose aspirin resulted in upper-GI bleeds at the same rate, about 3%, as high-dose aspirin. And in both of these aspirin subgroups 6 months of concurrent treatment with a PPI cut the incidence of major GI bleeds by more than half.
The findings are especially notable because the enrollment criteria stacked the deck toward patients with high cardiovascular disease risk and relatively low GI risk. The study enrolled “a unique population at high risk for cardiovascular disease – 71% had previously undergone a percutaneous coronary intervention, and 42% had a history of an acute coronary syndrome – and low GI risk, but even in this population enriched for cardiovascular disease risk, there was no increased rate of cardiovascular disease events” during a median follow-up while on PPI treatment of 110 days, Dr. Vaduganathan said.
Among patients on low-dose aspirin, the rate of cardiovascular death, MI, stroke, or coronary revascularization was 5.6% with PPI treatment and 5.5% without, and in the high-dose aspirin patients the rates were 4.2% with PPI treatment and 5.5% without. Neither of these differences between the subgroups taking or not taking a PPI were statistically significant.
Concurrent with Dr. Vaduganathan’s report at the meeting the results also appeared online (J Am Coll Cardiol. 2016 April 12;67[14]:661-71).
“There appeared to be no adverse clinical effect from PPI treatment. When used short-term, for up to 6 months, PPI treatment appears to be safe in patients with cardiovascular disease,” Dr. Vaduganathan concluded.
The analysis used data collected in COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial), a phase 3 study designed to compare a single-pill formulation of 20 mg omeprazole and 75 mg clopidogrel taken orally once daily with 75 mg clopidogrel against a background of all patients taking aspirin. COGENT stopped prematurely in late 2008 as the company developing this formulation and sponsoring the trial, Cogentus Pharmaceuticals, filed for bankruptcy. Despite its abrupt conclusion, the trial had enrolled and followed enough patients to show that treatment with omeprazole plus clopidogrel and aspirin led to a significant reduction in upper GI bleeding without increasing the rate of cardiovascular disease events, compared with clopidogrel plus aspirin (N Engl J Med. 2010 Nov 11;363[20]:1909-17).
The new analysis focused on the greater than 99% of patients in the total COGENT cohort for whom information was available on whether they received high- or low-dose aspirin.
Although the primary findings from COGENT, reported in 2010, documented the safety and efficacy of concomitant PPI treatment during dual-antiplatelet therapy, and despite guidelines revised in 2010 that called for PPI treatment when appropriate, this strategy for preventing GI complications remains underused, Dr. Vaduganathan said. The most recent U.S. recommendations that address this issue called for assessing the potential risk and benefit from PPI treatment in patients receiving dual-antiplatelet therapy: “The risk reduction with PPIs is substantial in patients with risk factors for GI bleeding and may outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug-drug interaction (J Am Coll Cardiol. 2010 Dec;56[24]:2051-66).”
The only caveat Dr. Vaduganathan placed on PPI use was that the COGENT data addressed only 6 months of PPI use; the safety of longer-term use has not been studied. But “the trend is to use PPIs for as short a period as possible,” and the risk for adverse effects from PPI treatment on cardiovascular disease events is likely greatest during the first 6 months of PPI treatment, he noted. If PPI treatment needs to continue beyond 6 months, he suggested systematically reassessing the risk-benefit balance for individual patients from continued PPI treatment every 3 months.*
*Changes were made to this story on 4/20/2016.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: In patients at high risk of cardiovascular disease on dual-antiplatelet therapy, concurrent proton pump inhibitor treatment cut gastrointestinal events, regardless of whether patients received a low or high aspirin dosage.
Major finding: Omeprazole cut the rate of upper-GI bleeds by more than half in patients taking low- or high-dose aspirin.
Data source: Post-hoc analysis of data in COGENT, a multicenter, randomized trial with 3,762 patients.
Disclosures: Cogent was sponsored by Cogentus Pharmaceuticals; however, the company went bankrupt and provided no support for the current analysis. Dr. Vaduganathan had no disclosures.
Pre-PCI beta-blockers offer no clinical benefit
CHICAGO – Early intravenous administration of the beta-blocker metoprolol before primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
No difference was seen in infarct size, as measured by magnetic resonance imaging at 30 days, between 336 patients with STEMI who presented within 12 hours of symptom onset and were randomized to receive intravenous metoprolol (2 vials with 5 mg) before undergoing angioplasty, and 347 such patients who received placebo (left ventricular volume, 15.3% and 14.9%, respectively), Dr. Vincent Roolvink of Isala Hospital, Zwolle, the Netherlands, reported at the annual meeting of the American College of Cardiology.
No differences were seen between the groups for the secondary endpoints of blood flow from the left ventricle or levels of cardiac enzymes, Dr. Roolvink noted.
Further, while significantly fewer cases of ventricular arrhythmia occurred in the metoprolol patients (3.6% vs. 6.9%), this difference was not clinically significant, he said.
No significant differences were seen with respect to safety endpoints, including abnormally slow heart rate, low blood pressure, or cardiogenic shock.
The Early-BAMI subjects had a mean age of 62 years, and most (75%) were men. They were enrolled at centers throughout the Netherlands and Spain.
“In this nonrestricted STEMI population, early intravenous metoprolol before primary percutaneous intervention did not reduce infarct size,” Dr Roolvink said, noting that the findings follow conflicting results from prior studies, with some suggesting that beta-blockers could reduce heart attack severity or improve blood flow from the left ventricle when given to STEMI patients prior to angioplasty.
However only one randomized trial took place in the primary percutaneous coronary intervention era, and that trial – METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) – involved only patients with STEMIs involving the anterior wall of the left ventricle (J Am Coll Cardiol. 2014;63[22]:2356-62).
Early-BAMI (The Effect of Early Administration of Intravenous Beta Blockers in Patients with ST-elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) was the first double blind, placebo-controlled international multicenter study to test this approach.
“Our results do not confirm the effect observed in the METOCARD-CNIC trial,” Dr. Roolvink said.
He noted, however, that the current findings are limited by the fact that study subjects had lower than expected overall heart attack severity.
Additional large randomized trials are needed to clarify whether early beta-blocker treatment is of benefit before angioplasty in STEMI patients. The safety profile, low cost of beta-blocker administration, and the reduction of acute malignant arrhythmias among those receiving beta-blocker treatment in the current trial should encourage the performance of additional larger trials, he said.
The findings were simultaneously published online (J Am Coll Cardiol. 2016 Apr 3. doi:10.1016/j.jacc.2016.03.522)
Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
CHICAGO – Early intravenous administration of the beta-blocker metoprolol before primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
No difference was seen in infarct size, as measured by magnetic resonance imaging at 30 days, between 336 patients with STEMI who presented within 12 hours of symptom onset and were randomized to receive intravenous metoprolol (2 vials with 5 mg) before undergoing angioplasty, and 347 such patients who received placebo (left ventricular volume, 15.3% and 14.9%, respectively), Dr. Vincent Roolvink of Isala Hospital, Zwolle, the Netherlands, reported at the annual meeting of the American College of Cardiology.
No differences were seen between the groups for the secondary endpoints of blood flow from the left ventricle or levels of cardiac enzymes, Dr. Roolvink noted.
Further, while significantly fewer cases of ventricular arrhythmia occurred in the metoprolol patients (3.6% vs. 6.9%), this difference was not clinically significant, he said.
No significant differences were seen with respect to safety endpoints, including abnormally slow heart rate, low blood pressure, or cardiogenic shock.
The Early-BAMI subjects had a mean age of 62 years, and most (75%) were men. They were enrolled at centers throughout the Netherlands and Spain.
“In this nonrestricted STEMI population, early intravenous metoprolol before primary percutaneous intervention did not reduce infarct size,” Dr Roolvink said, noting that the findings follow conflicting results from prior studies, with some suggesting that beta-blockers could reduce heart attack severity or improve blood flow from the left ventricle when given to STEMI patients prior to angioplasty.
However only one randomized trial took place in the primary percutaneous coronary intervention era, and that trial – METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) – involved only patients with STEMIs involving the anterior wall of the left ventricle (J Am Coll Cardiol. 2014;63[22]:2356-62).
Early-BAMI (The Effect of Early Administration of Intravenous Beta Blockers in Patients with ST-elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) was the first double blind, placebo-controlled international multicenter study to test this approach.
“Our results do not confirm the effect observed in the METOCARD-CNIC trial,” Dr. Roolvink said.
He noted, however, that the current findings are limited by the fact that study subjects had lower than expected overall heart attack severity.
Additional large randomized trials are needed to clarify whether early beta-blocker treatment is of benefit before angioplasty in STEMI patients. The safety profile, low cost of beta-blocker administration, and the reduction of acute malignant arrhythmias among those receiving beta-blocker treatment in the current trial should encourage the performance of additional larger trials, he said.
The findings were simultaneously published online (J Am Coll Cardiol. 2016 Apr 3. doi:10.1016/j.jacc.2016.03.522)
Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
CHICAGO – Early intravenous administration of the beta-blocker metoprolol before primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
No difference was seen in infarct size, as measured by magnetic resonance imaging at 30 days, between 336 patients with STEMI who presented within 12 hours of symptom onset and were randomized to receive intravenous metoprolol (2 vials with 5 mg) before undergoing angioplasty, and 347 such patients who received placebo (left ventricular volume, 15.3% and 14.9%, respectively), Dr. Vincent Roolvink of Isala Hospital, Zwolle, the Netherlands, reported at the annual meeting of the American College of Cardiology.
No differences were seen between the groups for the secondary endpoints of blood flow from the left ventricle or levels of cardiac enzymes, Dr. Roolvink noted.
Further, while significantly fewer cases of ventricular arrhythmia occurred in the metoprolol patients (3.6% vs. 6.9%), this difference was not clinically significant, he said.
No significant differences were seen with respect to safety endpoints, including abnormally slow heart rate, low blood pressure, or cardiogenic shock.
The Early-BAMI subjects had a mean age of 62 years, and most (75%) were men. They were enrolled at centers throughout the Netherlands and Spain.
“In this nonrestricted STEMI population, early intravenous metoprolol before primary percutaneous intervention did not reduce infarct size,” Dr Roolvink said, noting that the findings follow conflicting results from prior studies, with some suggesting that beta-blockers could reduce heart attack severity or improve blood flow from the left ventricle when given to STEMI patients prior to angioplasty.
However only one randomized trial took place in the primary percutaneous coronary intervention era, and that trial – METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) – involved only patients with STEMIs involving the anterior wall of the left ventricle (J Am Coll Cardiol. 2014;63[22]:2356-62).
Early-BAMI (The Effect of Early Administration of Intravenous Beta Blockers in Patients with ST-elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) was the first double blind, placebo-controlled international multicenter study to test this approach.
“Our results do not confirm the effect observed in the METOCARD-CNIC trial,” Dr. Roolvink said.
He noted, however, that the current findings are limited by the fact that study subjects had lower than expected overall heart attack severity.
Additional large randomized trials are needed to clarify whether early beta-blocker treatment is of benefit before angioplasty in STEMI patients. The safety profile, low cost of beta-blocker administration, and the reduction of acute malignant arrhythmias among those receiving beta-blocker treatment in the current trial should encourage the performance of additional larger trials, he said.
The findings were simultaneously published online (J Am Coll Cardiol. 2016 Apr 3. doi:10.1016/j.jacc.2016.03.522)
Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
AT ACC 16
Key clinical point: Early intravenous administration of metoprolol before primary percutaneous coronary intervention in patients with STEMI was safe but did not reduce infarct size in the randomized, placebo-controlled Early-BAMI trial.
Major finding: Infarct size on 30-day MRI was 15.3% and 14.9% of left ventricular volume in patients who received metoprolol and placebo, respectively.
Data source: The randomized, placebo-controlled Early-BAMI trial of 683 patients.
Disclosures: Early-BAMI was funded by the Dutch Heart Foundation and Medtronic. Dr. Roolvink reported having no disclosures.
Ticagrelor Cuts Post-MI Events in Diabetes Patients
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
AT ACC 2016
Ticagrelor cuts post-MI events in diabetes patients
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
On Twitter @mitchelzoler
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
On Twitter @mitchelzoler
CHICAGO – The benefit from dual-antiplatelet therapy in high-risk patients following a myocardial infarction was especially apparent in post-MI patients with diabetes in a prespecified secondary analysis from a multicenter trial of ticagrelor with more than 21,000 patients.
Among post-MI patients with diabetes, treatment with ticagrelor plus aspirin led to an absolute 1.5% reduction in the rate of cardiovascular death, MI, or stroke during a median 33-month follow-up, compared with an absolute 1.1% cut in patients without diabetes, Dr. Deepak L. Bhatt said at the annual meeting of the American College of Cardiology. The relative risk reduction, compared with placebo was 16% in both the diabetes and no diabetes subgroups, statistically significant differences in both subgroups.
“Long-term treatment with ticagrelor reduced the composite of cardiovascular death, MI, or stroke in diabetic patients with a greater absolute risk reduction than in nondiabetic patients,” said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. Treatment with ticagrelor plus aspirin in post-MI patients with diabetes also led to an increased number of major bleeding episodes, compared with patients on aspirin alone, but no excess of intracerebral hemorrhages or fatal bleeds, he noted.
This finding of a significant benefit from ticagrelor in post-MI patients with diabetes confirms similar, prior findings with other antiplatelet drugs (including clopidogrel, prasugrel, and vorapaxar) and prior findings with ticagrelor, Dr. Bhatt noted.
The new analysis used data collected in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. The primary results from PEGASUS-TIMI 54 had shown that adding ticagrelor to aspirin treatment of high-risk post-MI patients, including those who both had or did not have diabetes, significantly cut the composite rate of cardiovascular death, MI, and stroke, compared with aspirin alone (N Engl J Med. 2015 May 7;372[19]:1791-800). The study group included 6,806 patients with diabetes (type 2 diabetes in 99% of these patients), and 14,355 without diabetes. All patients had their MI 1-3 years before entering the study.
Dr. Bhatt and his associates examined the incidence of the various clinical endpoints measured in the study among only the patients with diabetes divided into those who received any dosage of ticagrelor (60 mg b.i.d. or 90 mg b.i.d.) or placebo, and also among the patients without diabetes. In addition to the primary endpoint, the new analysis showed that the rate of cardiovascular death during follow-up was 3.9% in the diabetes patients on dual therapy and 5.0% among the diabetes patients on aspirin only, a 22% relative risk reduction with ticagrelor added that was statistically significant. In contrast, among patients without diabetes the rates of cardiovascular death between those on and not on ticagrelor only differed by 0.2%, a 9% relative risk reduction that was not statistically significant. The same pattern occurred for the endpoint of death from coronary artery disease.
Concurrent with Dr. Bhatt’s report, the results appeared in an article published online (J Am Coll Cardiol. 2016 Apr; doi: 10.1016/S0735-1097[16]30023-7).
A new study, THEMIS, is examining the safety and efficacy of combined ticagrelor and aspirin treatment in a lower-risk group of patients with diabetes, those with coronary artery disease who have not had a prior MI. Those results may be available in 2018.
PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: Among post-MI patients with diabetes, dual-antiplatelet therapy with aspirin and ticagrelor produced a significant drop in the rate of cardiovascular death and other ischemic events.
Major finding: Post-MI patients with diabetes had a 10.1% combined endpoint rate on ticagrelor and a 11.6% rate on placebo.
Data source: Prespecified secondary analysis of data from PEGASUS-TIMI 54, a multicenter randomized trial with 21,162 patients.
Disclosures: PEGASUS-TIMI 54 was sponsored by AstraZeneca, the company that markets ticagrelor (Brilinta). Dr. Bhatt has been an advisor to Cardax and Regado Biosciences and has received research support from AstraZeneca and several other companies.
VIDEO: Serial lung fluid measurement improved heart failure outcomes
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
The very exciting results reported by Dr. Shochat came from a small, positive trial that showed impedance monitoring was an effective way to detect an increased amount of fluid in a heart failure patient’s lungs. This resulted in improved outcomes, compared with patients managed using usual care, including fewer hospitalizations and reduced mortality.
These results suggest that when physicians had lung impedance information, they identified episodes of acute heart failure decompensation sooner and that they used this alert to change treatment and prevent patient worsening. Heart failure exacerbations and decompensation events are a recurring problem for heart failure patients, and the earlier they are identified and addressed with altered treatment, the better it is for the patient’s well being. The next step is to see if these positive results can be confirmed by other research groups and in larger numbers of patients.
These results contrast with the findings from a German study reported in 2015 that used lung impedance information collected by implantable cardioverter defibrillators in heart failure patients to identify episodes of fluid buildup and decompensation. That study failed to show a statistically significant impact on patient outcomes. The researchers speculated that this may have been because patients often did not go online to allow their information to get transmitted to their physician, and physicians often did not act on the information because the patients reported no coincident change in symptoms.
This problem with the German study highlights that collecting lung impedance information will only improve outcomes if physicians then act on the information and modify a patient’s treatment. In the new study reported by Dr. Shochat, patients consistently underwent evaluation for their lung impedance status every month, and when the results suggested a growing problem of fluid overload the physicians consistently acted on the information by adjusting medication dosages.
Use of lung impedance measurement is similar to another approach for monitoring patients with heart failure that recently entered routine U.S. practice, an implanted device to monitor pulmonary artery pressure and identify episodes of fluid overload and acute decompensation. In the future, it will be interesting to compare the efficacy and ease of use of managing heart failure patients with pulmonary artery pressure monitoring with an implanted device and monitoring fluid build up in the lungs with lung impedance.
Dr. John A. Jarcho is a cardiologist at Brigham and Women’s Hospital, Boston. He had no disclosures. He made these comments as a discussant of Dr. Shochat’s report and in an interview.
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
CHICAGO – Regular assessment of a heart failure patient’s lung fluid volume using a device that measures electrical conduction through the chest – lung impedance – helped guide clinicians to make timely adjustments in a patient’s medications and thereby significantly reduce mortality and hospitalizations during an average 4 years of follow-up in a randomized, controlled study with 256 patients.
Monthly measurement of lung impedance and medication adjustments based on the information led to a 58% reduction in hospitalizations for acute heart failure during the first year of the study, compared with control patients, and a 56% reduction in heart failure hospitalizations, compared with controls, during the entire course of the study, the study’s two primary endpoints, Dr. Michael K. Shochat reported at the annual meeting of the American College of Cardiology.
The results also showed that performing regular lung impedance measurements and using the results to guide treatment led to a 43% reduction in all-cause mortality and a 62% drop in heart failure mortality during the average 4-year course of the study, said Dr. Shochat, a cardiologist at the Heart Institute of Hillel Yaffe Medical Center in Hadera, Israel. Concurrent with Dr. Shochat’s report at the meeting the results also appeared in an article published online (J Card Failure. 2016;doi:10.1016/j.cardfail.2016.03.015).
A key aspect of the study was that the clinicians who treated the enrolled patients who underwent lung impedance monitoring used this information to adjust medications the patients received. Overall, patients who underwent monitoring had more than twice the number of medication dose adjustments, compared with the control patients. These adjustments particularly focused on diuretic dosages, which changed three times as often in the monitored patients, compared with controls, Dr. Shochat reported. Changes in the dosages of beta-blockers and ACE inhibitors also showed marked increases in the monitored patients, compared with the controls.
The Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) trial enrolled 256 patients at two centers in Israel during 2005-2014. Patients had New York Heart Association class II-IV heart failure and a left ventricular ejection fraction of 35% or less. The enrolled patients averaged 67 years of age, and 80% were men.
Clinicians measured lung impedance using a proprietary device that places external electrodes on opposite sides of the patient’s chest. Calculation of impedance used a formula that eliminated the noise from chest wall impedance and focused exclusively on lung impedance. Once the electrodes are placed collection of the impedance data takes about 1 minute, Dr. Shochat said. The study protocol called for impedance data to be collected monthly, and in practice it occurred about 11 times a year during the study.
The investigators calculated for each patient in the active arm of the study a “basal” lung impedance level that reflected their level of lung conductivity when their lungs were clear of excess fluid. Participating clinicians were instructed to intervene by altering medications when the impedance level dropped more than 18% below the basal level. Their goal was to prevent impedance from dropping to more than 24% below the basal level, which correlated with when heart failure patients usually required hospitalization for acute decompensation. The specifics of how to adjust medications to manage patients who showed these signs of fluid overload were left to the discretion of each attending physician.
MPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: Monthly, noninvasive measurement of lung fluid levels using lung impedance produced better fluid control in heart failure patients and significantly fewer deaths and heart failure hospitalizations.
Major finding: Lung impedance–based management produced a 56% cut in heart failure hospitalizations, compared with standard care.
Data source: IMPEDANCE-HF, a randomized study with 256 heart failure patients at two Israeli centers.
Disclosures: IMPEDANCE-HF was sponsored by the RSMM Company, which is developing the lung impedance measurement device used in the study. Dr. Shochat is a cofounder of RSMM and is a member of the company’s board of directors.
DANAMI 3-iPOST: No significant benefit with ischemic postconditioning after STEMI
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
AT ACC 16
Key clinical point: Ischemic postconditioning in patients with STEMI failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
Major finding: No significant difference was seen in the primary composite endpoint of death from any cause and hospitalization for heart failure in standard angioplasty and ischemic postconditioning patients (HR, 0.93).
Data source: A randomized, controlled, open-label study of 1,234 patients from the DANAMI 3-iPOST trial.
Disclosures: The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
VIDEO: HOPE-3 bolsters primary prevention in intermediate-risk patients
CHICAGO – Results from the HOPE-3 trial confirm what guidelines have already recommended: patients with intermediate risk for cardiovascular disease should be treated for primary prevention of coronary event, Dr. Prakash Deedwania said in an interview at the annual meeting of the American College of Cardiology.
In the Heart Outcomes Prevention Evaluation (HOPE)-3 trial, nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease were randomized to either lipid lowering with rosuvastatin at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke, compared with placebo-treated controls, regardless of baseline LDL-cholesterol level. However, only subjects with a baseline pressure of greater than 143.5 mm Hg benefited from the dual-antihypertensive therapy.
In a video interview, Dr. Deedwania, professor of medicine at the University of California, San Francisco, Fresno, gave three takeaways from the HOPE-3 trial regarding primary prevention of cardiovascular events in patients at intermediate risk, how the results of the dual-antihypertensive treatment arm match up to guidelines, and whether there’s a future for the polypill.
Dr. Deedwania has received consultant fees and/or honoraria from Amgen, Pfizer, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results from the HOPE-3 trial confirm what guidelines have already recommended: patients with intermediate risk for cardiovascular disease should be treated for primary prevention of coronary event, Dr. Prakash Deedwania said in an interview at the annual meeting of the American College of Cardiology.
In the Heart Outcomes Prevention Evaluation (HOPE)-3 trial, nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease were randomized to either lipid lowering with rosuvastatin at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke, compared with placebo-treated controls, regardless of baseline LDL-cholesterol level. However, only subjects with a baseline pressure of greater than 143.5 mm Hg benefited from the dual-antihypertensive therapy.
In a video interview, Dr. Deedwania, professor of medicine at the University of California, San Francisco, Fresno, gave three takeaways from the HOPE-3 trial regarding primary prevention of cardiovascular events in patients at intermediate risk, how the results of the dual-antihypertensive treatment arm match up to guidelines, and whether there’s a future for the polypill.
Dr. Deedwania has received consultant fees and/or honoraria from Amgen, Pfizer, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results from the HOPE-3 trial confirm what guidelines have already recommended: patients with intermediate risk for cardiovascular disease should be treated for primary prevention of coronary event, Dr. Prakash Deedwania said in an interview at the annual meeting of the American College of Cardiology.
In the Heart Outcomes Prevention Evaluation (HOPE)-3 trial, nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease were randomized to either lipid lowering with rosuvastatin at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke, compared with placebo-treated controls, regardless of baseline LDL-cholesterol level. However, only subjects with a baseline pressure of greater than 143.5 mm Hg benefited from the dual-antihypertensive therapy.
In a video interview, Dr. Deedwania, professor of medicine at the University of California, San Francisco, Fresno, gave three takeaways from the HOPE-3 trial regarding primary prevention of cardiovascular events in patients at intermediate risk, how the results of the dual-antihypertensive treatment arm match up to guidelines, and whether there’s a future for the polypill.
Dr. Deedwania has received consultant fees and/or honoraria from Amgen, Pfizer, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Drug-eluting Stent Recipients Can Safely Have Surgery Sooner
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.
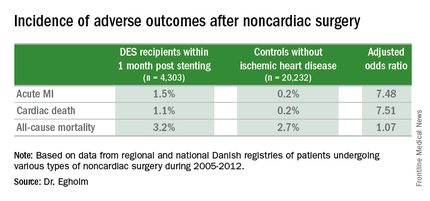
Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
AT ACC 16
Drug-eluting stent recipients can safely have surgery sooner
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.
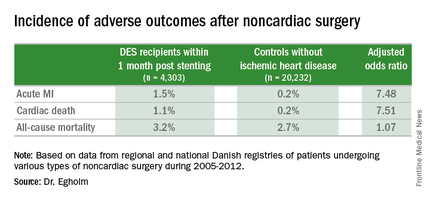
Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
AT ACC 16
Key clinical point: The risk of noncardiac surgery is elevated only when the operation occurs during the first month after stenting.
Major finding: Danish drug-eluting stent recipients who underwent noncardiac surgery within 1 month after stent placement were at 7.5-fold increased risks of acute MI and cardiac death, but surgery performed 2-12 months post stenting carried no increased risks.
Data source: This retrospective observational study based upon large Danish patient registries compared outcomes of noncardiac surgery performed within 12 months after drug-eluting stent placement in 4,303 patients with 20,232 matched controls without ischemic heart disease who underwent the same operations.
Disclosures: The study was supported by Danish research funds. The presenter reported having no financial conflicts of interest.
DANAMI 3-DEFER: No benefit with delayed stenting for STEMI
CHICAGO – Delaying stent implantation in patients with ST-segment elevation myocardial infarction failed to reduce the rate of mortality, heart failure, myocardial infarction, or repeat revascularization, compared with conventional percutaneous intervention in the randomized, controlled DANAMI 3-DEFER trial.
Among 1,215 patients with ST-segment elevation MI (STEMI) who were randomized to receive either standard primary percutaneous coronary intervention (PCI) with immediate stent implantation or deferred stent implantation 48 hours after the index procedure, the rate of the primary composite endpoint of all-cause mortality, hospital admission for heart failure, recurrent infarction, or any unplanned revascularization of the target vessel within 2 years was 18% in the immediate treatment group and 17% in the deferred stent implantation group, a nonsignificant difference, Dr. Henning Kelbæk reported at the annual meeting of the American College of Cardiology.
Procedure-related myocardial infarction, bleeding requiring transfusion or surgery, contrast-induced nephropathy, or stroke occurred in 5% and 4% of patients in the groups, respectively, he said.
Although some might be relieved to know there won’t be a need for doing a second procedure, the findings are a disappointment in that preliminary findings suggested a benefit when stenting is delayed for several hours to several days after angioplasty, said Dr. Kelbæk of Roskilde Hospital (Denmark).
The thinking was that medication given during the delay might help diminish residual blood clots, thereby reducing the risk of distal embolization, which occurs in 7% of cases, and which can occur despite successful treatment of the culprit artery lesion by primary PCI with stent implantation, he explained, noting that slow- or no-flow occurs in 10% of cases.
It is possible that the study may not have been large enough to detect overall differences in the two treatment groups. It is also possible that patients at the highest risk for developing another arterial blockage could potentially benefit from a delay, especially given that a small but significant improvement in left ventricular function was detected 18 months after treatment among patients who underwent deferred stenting (left ventricular ejection fraction, 60% vs. 57% in the immediate treatment group), but such patients were excluded from DANAMI 3-DEFER (the Third Danish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: Deferred stent implantation in connection with primary PCI), he said.
He added that he and his coinvestigators will “look carefully for possible ‘hypothesis-generating’ findings in subsets of patients – both those who might have benefited from the deferred-treatment strategy and, equally important, those in whom this strategy might have worsened their condition.”
Patients were enrolled into DANAMI 3-DEFER during March 2011–February 2014 at four primary PCI centers in Denmark. All were adults with acute onset symptoms lasting 12 hours or less, and ST-segment elevation of 0.1 mV or more in at least 2 contiguous electrocardiographic leads, or newly developed left bundle branch block. Those in the deferred treatment group were only randomized to that group if stabilized flow could be obtained in the infarct-related artery. Median follow-up was 42 months.
The findings indicate that at this point, deferred stent implantation cannot be recommended as a routine procedure for STEMI patients treated with primary PCI, Dr. Kelbæk concluded. The findings were published online simultaneously with the presentation (Lancet. 2016 Apr 3. doi: 10.1016/S0140-6736[16]30072-1).
The DANAMI-3-DEFER trial was funded by the Danish Agency for Science, Technology and Innovation and Danish Council for Strategic Research. Dr. Kelbæk reported having no disclosures.
CHICAGO – Delaying stent implantation in patients with ST-segment elevation myocardial infarction failed to reduce the rate of mortality, heart failure, myocardial infarction, or repeat revascularization, compared with conventional percutaneous intervention in the randomized, controlled DANAMI 3-DEFER trial.
Among 1,215 patients with ST-segment elevation MI (STEMI) who were randomized to receive either standard primary percutaneous coronary intervention (PCI) with immediate stent implantation or deferred stent implantation 48 hours after the index procedure, the rate of the primary composite endpoint of all-cause mortality, hospital admission for heart failure, recurrent infarction, or any unplanned revascularization of the target vessel within 2 years was 18% in the immediate treatment group and 17% in the deferred stent implantation group, a nonsignificant difference, Dr. Henning Kelbæk reported at the annual meeting of the American College of Cardiology.
Procedure-related myocardial infarction, bleeding requiring transfusion or surgery, contrast-induced nephropathy, or stroke occurred in 5% and 4% of patients in the groups, respectively, he said.
Although some might be relieved to know there won’t be a need for doing a second procedure, the findings are a disappointment in that preliminary findings suggested a benefit when stenting is delayed for several hours to several days after angioplasty, said Dr. Kelbæk of Roskilde Hospital (Denmark).
The thinking was that medication given during the delay might help diminish residual blood clots, thereby reducing the risk of distal embolization, which occurs in 7% of cases, and which can occur despite successful treatment of the culprit artery lesion by primary PCI with stent implantation, he explained, noting that slow- or no-flow occurs in 10% of cases.
It is possible that the study may not have been large enough to detect overall differences in the two treatment groups. It is also possible that patients at the highest risk for developing another arterial blockage could potentially benefit from a delay, especially given that a small but significant improvement in left ventricular function was detected 18 months after treatment among patients who underwent deferred stenting (left ventricular ejection fraction, 60% vs. 57% in the immediate treatment group), but such patients were excluded from DANAMI 3-DEFER (the Third Danish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: Deferred stent implantation in connection with primary PCI), he said.
He added that he and his coinvestigators will “look carefully for possible ‘hypothesis-generating’ findings in subsets of patients – both those who might have benefited from the deferred-treatment strategy and, equally important, those in whom this strategy might have worsened their condition.”
Patients were enrolled into DANAMI 3-DEFER during March 2011–February 2014 at four primary PCI centers in Denmark. All were adults with acute onset symptoms lasting 12 hours or less, and ST-segment elevation of 0.1 mV or more in at least 2 contiguous electrocardiographic leads, or newly developed left bundle branch block. Those in the deferred treatment group were only randomized to that group if stabilized flow could be obtained in the infarct-related artery. Median follow-up was 42 months.
The findings indicate that at this point, deferred stent implantation cannot be recommended as a routine procedure for STEMI patients treated with primary PCI, Dr. Kelbæk concluded. The findings were published online simultaneously with the presentation (Lancet. 2016 Apr 3. doi: 10.1016/S0140-6736[16]30072-1).
The DANAMI-3-DEFER trial was funded by the Danish Agency for Science, Technology and Innovation and Danish Council for Strategic Research. Dr. Kelbæk reported having no disclosures.
CHICAGO – Delaying stent implantation in patients with ST-segment elevation myocardial infarction failed to reduce the rate of mortality, heart failure, myocardial infarction, or repeat revascularization, compared with conventional percutaneous intervention in the randomized, controlled DANAMI 3-DEFER trial.
Among 1,215 patients with ST-segment elevation MI (STEMI) who were randomized to receive either standard primary percutaneous coronary intervention (PCI) with immediate stent implantation or deferred stent implantation 48 hours after the index procedure, the rate of the primary composite endpoint of all-cause mortality, hospital admission for heart failure, recurrent infarction, or any unplanned revascularization of the target vessel within 2 years was 18% in the immediate treatment group and 17% in the deferred stent implantation group, a nonsignificant difference, Dr. Henning Kelbæk reported at the annual meeting of the American College of Cardiology.
Procedure-related myocardial infarction, bleeding requiring transfusion or surgery, contrast-induced nephropathy, or stroke occurred in 5% and 4% of patients in the groups, respectively, he said.
Although some might be relieved to know there won’t be a need for doing a second procedure, the findings are a disappointment in that preliminary findings suggested a benefit when stenting is delayed for several hours to several days after angioplasty, said Dr. Kelbæk of Roskilde Hospital (Denmark).
The thinking was that medication given during the delay might help diminish residual blood clots, thereby reducing the risk of distal embolization, which occurs in 7% of cases, and which can occur despite successful treatment of the culprit artery lesion by primary PCI with stent implantation, he explained, noting that slow- or no-flow occurs in 10% of cases.
It is possible that the study may not have been large enough to detect overall differences in the two treatment groups. It is also possible that patients at the highest risk for developing another arterial blockage could potentially benefit from a delay, especially given that a small but significant improvement in left ventricular function was detected 18 months after treatment among patients who underwent deferred stenting (left ventricular ejection fraction, 60% vs. 57% in the immediate treatment group), but such patients were excluded from DANAMI 3-DEFER (the Third Danish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: Deferred stent implantation in connection with primary PCI), he said.
He added that he and his coinvestigators will “look carefully for possible ‘hypothesis-generating’ findings in subsets of patients – both those who might have benefited from the deferred-treatment strategy and, equally important, those in whom this strategy might have worsened their condition.”
Patients were enrolled into DANAMI 3-DEFER during March 2011–February 2014 at four primary PCI centers in Denmark. All were adults with acute onset symptoms lasting 12 hours or less, and ST-segment elevation of 0.1 mV or more in at least 2 contiguous electrocardiographic leads, or newly developed left bundle branch block. Those in the deferred treatment group were only randomized to that group if stabilized flow could be obtained in the infarct-related artery. Median follow-up was 42 months.
The findings indicate that at this point, deferred stent implantation cannot be recommended as a routine procedure for STEMI patients treated with primary PCI, Dr. Kelbæk concluded. The findings were published online simultaneously with the presentation (Lancet. 2016 Apr 3. doi: 10.1016/S0140-6736[16]30072-1).
The DANAMI-3-DEFER trial was funded by the Danish Agency for Science, Technology and Innovation and Danish Council for Strategic Research. Dr. Kelbæk reported having no disclosures.
AT ACC 16
Key clinical point: Delaying stent implantation in patients with STEMI failed to improve outcomes, compared with conventional percutaneous intervention in the randomized, controlled DANAMI 3-DEFER trial.
Major finding: The rate of the primary composite endpoint was 18% in the immediate treatment group and 17% in the deferred stent implantation group.
Data source: The open-label, randomized, controlled DANAMI 3-DEFER trial of 1,215 patients.
Disclosures: The DANAMI 3-DEFER trial was funded by the Danish Agency for Science, Technology, and Innovation, and the Danish Council for Strategic Research. Dr. Kelbæk reported having no disclosures.


















