User login
Higher plasma cell-free DNA tracks with worse PAH survival
NEW ORLEANS – Cell-free (cf) DNA looked like an informative biomarker for both the severity of pulmonary artery hypertension and the survival prognosis for patients with this disease, based on results from two preliminary studies involving a total of 173 people.
“Plasma levels of cell-free DNA are elevated in patients with pulmonary artery hypertension, compared with healthy controls, and may predict disease severity and mortality,” Samuel B. Brusca, MD, said at the at the annual meeting of the American College of Cardiology.
A growing biomedical literature has documented a role for cfDNA in tracking the course of cancer, septic shock, and transplanted organs (Transplantation. 2019 Feb;103[2]:273-83) (Cell-Free DNA: Applications in Different Diseases, in “Cell-free DNA as Diagnostic Markers.” [New York: Humana Press, 2018, pp. 3-12]). Based on this background Dr. Brusca and his associates decided to examine whether plasma levels of cfDNA linked with pulmonary artery hypertension (PAH) severity and survival.
Their first study included seven patients with mild PAH (defined as patients with a tricuspid annular plane systolic excursion [TAPSE] of more than 18 mm and a maximum oxygen uptake [VO2] of at least 75% of predicted), eight with severe PAH (a TAPSE of 18 mm or less and a VO2 of less than 75%), and seven healthy adult controls. Measurement of plasma cfDNA showed an average level of 19.4 ng/mL among the healthy controls (prior reports had indicated that 10-20 ng/mL were normal levels), 22.0 ng/mL among patients with mild PAH, and 36.2 ng/mL in those with severe PAH. The level among the severe PAH patients was significantly higher than the level in controls by two different statistical tests, said Dr. Brusca, a critical care medicine physician at the National Institutes of Health Clinical Center in Bethesda, Md.
The second analysis by Dr. Brusca and his associates included 151 PAH patients followed by physicians at the Clinical Center for an average of 40 months. Their analysis tracked survival of these patients relative to their baseline levels of cfDNA and divided into tertiles. Patients in the lowest tertile had a starting cfDNA level of up to 39 ng/mL, those in the middle tertile had levels of 39.1-64.0 ng/mL, and those in the top tertile had levels of at least 64.1 ng/mL. A Kaplan-Meier analysis showed statistically significant differences in survival rates between each of the tertiles. Patients in the lowest tertile had a 5-year actuarial survival rate of about 65%, those in the middle tertile had a survival rate of about 48%, and those in the tertile with the highest level of cfDNA had a survival rate of about 28%.
Additional studies of cfDNA are needed in larger numbers of PAH patients, and cfDNA levels should be compared with levels of other, more established biomarkers, such as inflammatory cytokines, Dr. Brusca said in an interview.
Dr. Brusca had no disclosures. The study received no commercial funding.
SOURCE: Brusca SB et al. J Am Coll Cardiol. 2019 March 12;73(9 Suppl 1):1897.
I was very excited to hear Dr. Brusca’s report on using cell-free (cf) DNA to track the severity of pulmonary artery hypertension and survival of these patients. I’m now using cfDNA frequently to monitor heart transplant patients, and the information it provides has been very valuable. But cfDNA may be even better suited to assessing patients with pulmonary artery hypertension (PAH) because it’s a vascular disease, and increases in cfDNA appears to reflect damage to the vascular endothelium. It’s a brilliant application of this technology. Brain natriuretic peptide and troponin are markers of right heart damage, but cfDNA appears to be able to track the progression of the vascular component of PAH. It appears to be the first disease-specific biomarker we have for PAH. It’s time to start routinely measuring levels of cfDNA in trials so we can gather more data on the clinical correlates of changing levels of this biomarker.
I was very excited to hear Dr. Brusca’s report on using cell-free (cf) DNA to track the severity of pulmonary artery hypertension and survival of these patients. I’m now using cfDNA frequently to monitor heart transplant patients, and the information it provides has been very valuable. But cfDNA may be even better suited to assessing patients with pulmonary artery hypertension (PAH) because it’s a vascular disease, and increases in cfDNA appears to reflect damage to the vascular endothelium. It’s a brilliant application of this technology. Brain natriuretic peptide and troponin are markers of right heart damage, but cfDNA appears to be able to track the progression of the vascular component of PAH. It appears to be the first disease-specific biomarker we have for PAH. It’s time to start routinely measuring levels of cfDNA in trials so we can gather more data on the clinical correlates of changing levels of this biomarker.
I was very excited to hear Dr. Brusca’s report on using cell-free (cf) DNA to track the severity of pulmonary artery hypertension and survival of these patients. I’m now using cfDNA frequently to monitor heart transplant patients, and the information it provides has been very valuable. But cfDNA may be even better suited to assessing patients with pulmonary artery hypertension (PAH) because it’s a vascular disease, and increases in cfDNA appears to reflect damage to the vascular endothelium. It’s a brilliant application of this technology. Brain natriuretic peptide and troponin are markers of right heart damage, but cfDNA appears to be able to track the progression of the vascular component of PAH. It appears to be the first disease-specific biomarker we have for PAH. It’s time to start routinely measuring levels of cfDNA in trials so we can gather more data on the clinical correlates of changing levels of this biomarker.
NEW ORLEANS – Cell-free (cf) DNA looked like an informative biomarker for both the severity of pulmonary artery hypertension and the survival prognosis for patients with this disease, based on results from two preliminary studies involving a total of 173 people.
“Plasma levels of cell-free DNA are elevated in patients with pulmonary artery hypertension, compared with healthy controls, and may predict disease severity and mortality,” Samuel B. Brusca, MD, said at the at the annual meeting of the American College of Cardiology.
A growing biomedical literature has documented a role for cfDNA in tracking the course of cancer, septic shock, and transplanted organs (Transplantation. 2019 Feb;103[2]:273-83) (Cell-Free DNA: Applications in Different Diseases, in “Cell-free DNA as Diagnostic Markers.” [New York: Humana Press, 2018, pp. 3-12]). Based on this background Dr. Brusca and his associates decided to examine whether plasma levels of cfDNA linked with pulmonary artery hypertension (PAH) severity and survival.
Their first study included seven patients with mild PAH (defined as patients with a tricuspid annular plane systolic excursion [TAPSE] of more than 18 mm and a maximum oxygen uptake [VO2] of at least 75% of predicted), eight with severe PAH (a TAPSE of 18 mm or less and a VO2 of less than 75%), and seven healthy adult controls. Measurement of plasma cfDNA showed an average level of 19.4 ng/mL among the healthy controls (prior reports had indicated that 10-20 ng/mL were normal levels), 22.0 ng/mL among patients with mild PAH, and 36.2 ng/mL in those with severe PAH. The level among the severe PAH patients was significantly higher than the level in controls by two different statistical tests, said Dr. Brusca, a critical care medicine physician at the National Institutes of Health Clinical Center in Bethesda, Md.
The second analysis by Dr. Brusca and his associates included 151 PAH patients followed by physicians at the Clinical Center for an average of 40 months. Their analysis tracked survival of these patients relative to their baseline levels of cfDNA and divided into tertiles. Patients in the lowest tertile had a starting cfDNA level of up to 39 ng/mL, those in the middle tertile had levels of 39.1-64.0 ng/mL, and those in the top tertile had levels of at least 64.1 ng/mL. A Kaplan-Meier analysis showed statistically significant differences in survival rates between each of the tertiles. Patients in the lowest tertile had a 5-year actuarial survival rate of about 65%, those in the middle tertile had a survival rate of about 48%, and those in the tertile with the highest level of cfDNA had a survival rate of about 28%.
Additional studies of cfDNA are needed in larger numbers of PAH patients, and cfDNA levels should be compared with levels of other, more established biomarkers, such as inflammatory cytokines, Dr. Brusca said in an interview.
Dr. Brusca had no disclosures. The study received no commercial funding.
SOURCE: Brusca SB et al. J Am Coll Cardiol. 2019 March 12;73(9 Suppl 1):1897.
NEW ORLEANS – Cell-free (cf) DNA looked like an informative biomarker for both the severity of pulmonary artery hypertension and the survival prognosis for patients with this disease, based on results from two preliminary studies involving a total of 173 people.
“Plasma levels of cell-free DNA are elevated in patients with pulmonary artery hypertension, compared with healthy controls, and may predict disease severity and mortality,” Samuel B. Brusca, MD, said at the at the annual meeting of the American College of Cardiology.
A growing biomedical literature has documented a role for cfDNA in tracking the course of cancer, septic shock, and transplanted organs (Transplantation. 2019 Feb;103[2]:273-83) (Cell-Free DNA: Applications in Different Diseases, in “Cell-free DNA as Diagnostic Markers.” [New York: Humana Press, 2018, pp. 3-12]). Based on this background Dr. Brusca and his associates decided to examine whether plasma levels of cfDNA linked with pulmonary artery hypertension (PAH) severity and survival.
Their first study included seven patients with mild PAH (defined as patients with a tricuspid annular plane systolic excursion [TAPSE] of more than 18 mm and a maximum oxygen uptake [VO2] of at least 75% of predicted), eight with severe PAH (a TAPSE of 18 mm or less and a VO2 of less than 75%), and seven healthy adult controls. Measurement of plasma cfDNA showed an average level of 19.4 ng/mL among the healthy controls (prior reports had indicated that 10-20 ng/mL were normal levels), 22.0 ng/mL among patients with mild PAH, and 36.2 ng/mL in those with severe PAH. The level among the severe PAH patients was significantly higher than the level in controls by two different statistical tests, said Dr. Brusca, a critical care medicine physician at the National Institutes of Health Clinical Center in Bethesda, Md.
The second analysis by Dr. Brusca and his associates included 151 PAH patients followed by physicians at the Clinical Center for an average of 40 months. Their analysis tracked survival of these patients relative to their baseline levels of cfDNA and divided into tertiles. Patients in the lowest tertile had a starting cfDNA level of up to 39 ng/mL, those in the middle tertile had levels of 39.1-64.0 ng/mL, and those in the top tertile had levels of at least 64.1 ng/mL. A Kaplan-Meier analysis showed statistically significant differences in survival rates between each of the tertiles. Patients in the lowest tertile had a 5-year actuarial survival rate of about 65%, those in the middle tertile had a survival rate of about 48%, and those in the tertile with the highest level of cfDNA had a survival rate of about 28%.
Additional studies of cfDNA are needed in larger numbers of PAH patients, and cfDNA levels should be compared with levels of other, more established biomarkers, such as inflammatory cytokines, Dr. Brusca said in an interview.
Dr. Brusca had no disclosures. The study received no commercial funding.
SOURCE: Brusca SB et al. J Am Coll Cardiol. 2019 March 12;73(9 Suppl 1):1897.
REPORTING FROM ACC 2019
CardioMEMS cuts heart failure hospitalizations in post-approval study
NEW ORLEANS – Frequent, noninvasive measurement of pulmonary artery pressure in patients with advanced heart failure and an implanted CardioMEMS device that allows this measurement led to management that produced a substantial reduction in heart failure hospitalizations, compared with each patient’s history, in a real-world study.
The Food and Drug Administration–mandated CardioMEMS Post-Approval Study included 1,200 patients who received CardioMEMS implants after it received U.S. marketing approval. The study showed that when clinicians and patients used the device in routine practice, presumably as part of a structured management system designed to take advantage of the pulmonary artery (PA) pressures the device provides, the result safely produced a 58% cut in heart failure hospitalizations during the year following device placement when compared to each patient’s own hospitalization history during the year before they got the CardioMEMS device, David M. Shavelle, MD, said at the at the annual meeting of the American College of Cardiology. This statistically significant result for the study’s primary endpoint showed an absolute reduction in the average rate of heart failure hospitalizations from 1.24 per patient during the year before the CardioMEMS placement to 0.52 hospitalizations per patient during the 12 months after placement, an average reduction of 0.72 hospitalizations/patient, said Dr. Shavelle, an interventional cardiologist at the University of Southern California in Los Angeles.
Another notable finding was that this benefit from CardioMEMS placement and use occurred at roughly similar rates in patients with New York Heart Association class III heart failure regardless of whether they had a reduced ejection fraction (40% or less), a mid-range ejection fraction (41%-50%), or preserved ejection fraction (greater than 50%), making CardioMEMS use one of the few treatments to produce any proven benefit in patients with heart failure with preserved ejection fraction. In that subgroup, 30% of the 1,200 enrolled patients had an average cut of 0.68 hospitalizations in the year after CardioMEMS implantation, a 61% drop, relative to the year before they received the device.
The results also fulfilled the study’s two prespecified safety measures. Among the 1,214 patients in the study assessed for safety, which included the 1,200 patients who received the device and 4 patients in whom placement failed, 4 patients had a device or system related complication during the study, a 0.3% rate, compared with a prespecified objective performance criteria of less than 20%. Among the 1,200 patients with a functioning CardioMEMS sensor, one patient (0.1%) had a device failure, compared with the study’s objective performance criteria of less than 10%.
The performance of the CardioMEMS device and the benefit it provided to patients in the post-approval study closely tracked its performance during the published pivotal trial (Lancet. 2011 Feb 19;377[9766]:658-66). On the basis of the pivotal trial results, the FDA approved CardioMEMS for U.S. marketing in 2014. Since then, the company has reported that about 10,000 U.S. heart failure patients have received these devices, Dr. Shavelle said.
“The benefit was seen across the range of ejection fractions; that’s very important,” commented Gurusher Panjrath, MD, director of advanced heart failure at George Washington University in Washington and a designated discussant for Dr. Shavelle’s report. “The safety seemed very good, and the efficacy was consistent” with prior reports. “There also was high compliance. The key to success is the structure” of patient management, Dr. Pangroth said. “The data are limited by who is monitoring patients and their data and how much of that contact influences patient outcomes.”
That final comment by Dr. Panjrath highlighted the biggest caveat that heart failure clinicians have raised about judging the efficacy of CardioMEMS. To achieve clinical efficacy, the implanted device requires diligent, virtually daily interrogation and data transmission by the patient, assessment of a large amount of data for each patient by the patient’s clinical team, and responsiveness by the patient to medication adjustments directed by the clinical team to deal with episodes of rising PA pressure.
“The device itself has no benefit. It’s the actions prompted by the device that have benefit,” noted Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University in Chicago and a second designated discussant for the report.
Dr. Shavelle agreed that for the CardioMEMS device to have an impact, one basic requirement is to identify patients who will cooperate with data collection and transmission and also with changes in their medications that are sent to them in response to PA pressure changes. This means selecting patients who appear to have problems with volume overload, including prior hospitalizations for decompensation, and patients who are comfortable interacting with their clinical-care providers. It also means excluding patients who are too sick to benefit from this intervention. He estimated that at his center more than 95% of class III heart failure patients who qualified for inclusion in the post-approval study by clinical criteria were also judged reasonable recipients of the device based on their willingness to cooperate with this system. He also estimated that at the University of Southern California the heart failure clinical team is now caring for about 150 patients with a CardioMEMS device implanted.
Another concern is teasing apart the specific benefit of collecting and using PA pressure data from the contact that the clinical team maintains with CardioMEMS patients.
“If nurses are contacting patients more often, is it the device or the communication? We need to look at that very carefully in a study that had no control group,” Dr. Yancy said in an interview. Contact with a nurse “is the best thing you can do for heart failure patients.”
Dr. Shavelle countered that several reports from past studies that assessed case management and regular monitoring of and contact with heart failure patients but without PA pressure data failed to showed any consistent benefit to patients.
“If you pick the right patients, CardioMEMS works. There is no question in my mind that the device works,” Dr. Shavelle said in an interview. “If you pick the wrong patient, who will not send the data or follow dose changes, then it won’t work.”
The study was sponsored by Abbott, the company that markets the CardioMEMS HF System. Dr. Shavelle has been a consultant to and speaker on behalf of Abbott Vascular and he has received research funding from Abbott Vascular, Abiomed, Biocardia, and V-Wave. Dr. Yancy had an unspecified financial relationship with Abbott Laboratories. Dr. Panjrath had no disclosures.
[email protected]
On Twitter @mitchelzoler
SOURCE: Shavelle DM et al. American College of Cardiology annual meeting, abstract 405-16.
NEW ORLEANS – Frequent, noninvasive measurement of pulmonary artery pressure in patients with advanced heart failure and an implanted CardioMEMS device that allows this measurement led to management that produced a substantial reduction in heart failure hospitalizations, compared with each patient’s history, in a real-world study.
The Food and Drug Administration–mandated CardioMEMS Post-Approval Study included 1,200 patients who received CardioMEMS implants after it received U.S. marketing approval. The study showed that when clinicians and patients used the device in routine practice, presumably as part of a structured management system designed to take advantage of the pulmonary artery (PA) pressures the device provides, the result safely produced a 58% cut in heart failure hospitalizations during the year following device placement when compared to each patient’s own hospitalization history during the year before they got the CardioMEMS device, David M. Shavelle, MD, said at the at the annual meeting of the American College of Cardiology. This statistically significant result for the study’s primary endpoint showed an absolute reduction in the average rate of heart failure hospitalizations from 1.24 per patient during the year before the CardioMEMS placement to 0.52 hospitalizations per patient during the 12 months after placement, an average reduction of 0.72 hospitalizations/patient, said Dr. Shavelle, an interventional cardiologist at the University of Southern California in Los Angeles.
Another notable finding was that this benefit from CardioMEMS placement and use occurred at roughly similar rates in patients with New York Heart Association class III heart failure regardless of whether they had a reduced ejection fraction (40% or less), a mid-range ejection fraction (41%-50%), or preserved ejection fraction (greater than 50%), making CardioMEMS use one of the few treatments to produce any proven benefit in patients with heart failure with preserved ejection fraction. In that subgroup, 30% of the 1,200 enrolled patients had an average cut of 0.68 hospitalizations in the year after CardioMEMS implantation, a 61% drop, relative to the year before they received the device.
The results also fulfilled the study’s two prespecified safety measures. Among the 1,214 patients in the study assessed for safety, which included the 1,200 patients who received the device and 4 patients in whom placement failed, 4 patients had a device or system related complication during the study, a 0.3% rate, compared with a prespecified objective performance criteria of less than 20%. Among the 1,200 patients with a functioning CardioMEMS sensor, one patient (0.1%) had a device failure, compared with the study’s objective performance criteria of less than 10%.
The performance of the CardioMEMS device and the benefit it provided to patients in the post-approval study closely tracked its performance during the published pivotal trial (Lancet. 2011 Feb 19;377[9766]:658-66). On the basis of the pivotal trial results, the FDA approved CardioMEMS for U.S. marketing in 2014. Since then, the company has reported that about 10,000 U.S. heart failure patients have received these devices, Dr. Shavelle said.
“The benefit was seen across the range of ejection fractions; that’s very important,” commented Gurusher Panjrath, MD, director of advanced heart failure at George Washington University in Washington and a designated discussant for Dr. Shavelle’s report. “The safety seemed very good, and the efficacy was consistent” with prior reports. “There also was high compliance. The key to success is the structure” of patient management, Dr. Pangroth said. “The data are limited by who is monitoring patients and their data and how much of that contact influences patient outcomes.”
That final comment by Dr. Panjrath highlighted the biggest caveat that heart failure clinicians have raised about judging the efficacy of CardioMEMS. To achieve clinical efficacy, the implanted device requires diligent, virtually daily interrogation and data transmission by the patient, assessment of a large amount of data for each patient by the patient’s clinical team, and responsiveness by the patient to medication adjustments directed by the clinical team to deal with episodes of rising PA pressure.
“The device itself has no benefit. It’s the actions prompted by the device that have benefit,” noted Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University in Chicago and a second designated discussant for the report.
Dr. Shavelle agreed that for the CardioMEMS device to have an impact, one basic requirement is to identify patients who will cooperate with data collection and transmission and also with changes in their medications that are sent to them in response to PA pressure changes. This means selecting patients who appear to have problems with volume overload, including prior hospitalizations for decompensation, and patients who are comfortable interacting with their clinical-care providers. It also means excluding patients who are too sick to benefit from this intervention. He estimated that at his center more than 95% of class III heart failure patients who qualified for inclusion in the post-approval study by clinical criteria were also judged reasonable recipients of the device based on their willingness to cooperate with this system. He also estimated that at the University of Southern California the heart failure clinical team is now caring for about 150 patients with a CardioMEMS device implanted.
Another concern is teasing apart the specific benefit of collecting and using PA pressure data from the contact that the clinical team maintains with CardioMEMS patients.
“If nurses are contacting patients more often, is it the device or the communication? We need to look at that very carefully in a study that had no control group,” Dr. Yancy said in an interview. Contact with a nurse “is the best thing you can do for heart failure patients.”
Dr. Shavelle countered that several reports from past studies that assessed case management and regular monitoring of and contact with heart failure patients but without PA pressure data failed to showed any consistent benefit to patients.
“If you pick the right patients, CardioMEMS works. There is no question in my mind that the device works,” Dr. Shavelle said in an interview. “If you pick the wrong patient, who will not send the data or follow dose changes, then it won’t work.”
The study was sponsored by Abbott, the company that markets the CardioMEMS HF System. Dr. Shavelle has been a consultant to and speaker on behalf of Abbott Vascular and he has received research funding from Abbott Vascular, Abiomed, Biocardia, and V-Wave. Dr. Yancy had an unspecified financial relationship with Abbott Laboratories. Dr. Panjrath had no disclosures.
[email protected]
On Twitter @mitchelzoler
SOURCE: Shavelle DM et al. American College of Cardiology annual meeting, abstract 405-16.
NEW ORLEANS – Frequent, noninvasive measurement of pulmonary artery pressure in patients with advanced heart failure and an implanted CardioMEMS device that allows this measurement led to management that produced a substantial reduction in heart failure hospitalizations, compared with each patient’s history, in a real-world study.
The Food and Drug Administration–mandated CardioMEMS Post-Approval Study included 1,200 patients who received CardioMEMS implants after it received U.S. marketing approval. The study showed that when clinicians and patients used the device in routine practice, presumably as part of a structured management system designed to take advantage of the pulmonary artery (PA) pressures the device provides, the result safely produced a 58% cut in heart failure hospitalizations during the year following device placement when compared to each patient’s own hospitalization history during the year before they got the CardioMEMS device, David M. Shavelle, MD, said at the at the annual meeting of the American College of Cardiology. This statistically significant result for the study’s primary endpoint showed an absolute reduction in the average rate of heart failure hospitalizations from 1.24 per patient during the year before the CardioMEMS placement to 0.52 hospitalizations per patient during the 12 months after placement, an average reduction of 0.72 hospitalizations/patient, said Dr. Shavelle, an interventional cardiologist at the University of Southern California in Los Angeles.
Another notable finding was that this benefit from CardioMEMS placement and use occurred at roughly similar rates in patients with New York Heart Association class III heart failure regardless of whether they had a reduced ejection fraction (40% or less), a mid-range ejection fraction (41%-50%), or preserved ejection fraction (greater than 50%), making CardioMEMS use one of the few treatments to produce any proven benefit in patients with heart failure with preserved ejection fraction. In that subgroup, 30% of the 1,200 enrolled patients had an average cut of 0.68 hospitalizations in the year after CardioMEMS implantation, a 61% drop, relative to the year before they received the device.
The results also fulfilled the study’s two prespecified safety measures. Among the 1,214 patients in the study assessed for safety, which included the 1,200 patients who received the device and 4 patients in whom placement failed, 4 patients had a device or system related complication during the study, a 0.3% rate, compared with a prespecified objective performance criteria of less than 20%. Among the 1,200 patients with a functioning CardioMEMS sensor, one patient (0.1%) had a device failure, compared with the study’s objective performance criteria of less than 10%.
The performance of the CardioMEMS device and the benefit it provided to patients in the post-approval study closely tracked its performance during the published pivotal trial (Lancet. 2011 Feb 19;377[9766]:658-66). On the basis of the pivotal trial results, the FDA approved CardioMEMS for U.S. marketing in 2014. Since then, the company has reported that about 10,000 U.S. heart failure patients have received these devices, Dr. Shavelle said.
“The benefit was seen across the range of ejection fractions; that’s very important,” commented Gurusher Panjrath, MD, director of advanced heart failure at George Washington University in Washington and a designated discussant for Dr. Shavelle’s report. “The safety seemed very good, and the efficacy was consistent” with prior reports. “There also was high compliance. The key to success is the structure” of patient management, Dr. Pangroth said. “The data are limited by who is monitoring patients and their data and how much of that contact influences patient outcomes.”
That final comment by Dr. Panjrath highlighted the biggest caveat that heart failure clinicians have raised about judging the efficacy of CardioMEMS. To achieve clinical efficacy, the implanted device requires diligent, virtually daily interrogation and data transmission by the patient, assessment of a large amount of data for each patient by the patient’s clinical team, and responsiveness by the patient to medication adjustments directed by the clinical team to deal with episodes of rising PA pressure.
“The device itself has no benefit. It’s the actions prompted by the device that have benefit,” noted Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University in Chicago and a second designated discussant for the report.
Dr. Shavelle agreed that for the CardioMEMS device to have an impact, one basic requirement is to identify patients who will cooperate with data collection and transmission and also with changes in their medications that are sent to them in response to PA pressure changes. This means selecting patients who appear to have problems with volume overload, including prior hospitalizations for decompensation, and patients who are comfortable interacting with their clinical-care providers. It also means excluding patients who are too sick to benefit from this intervention. He estimated that at his center more than 95% of class III heart failure patients who qualified for inclusion in the post-approval study by clinical criteria were also judged reasonable recipients of the device based on their willingness to cooperate with this system. He also estimated that at the University of Southern California the heart failure clinical team is now caring for about 150 patients with a CardioMEMS device implanted.
Another concern is teasing apart the specific benefit of collecting and using PA pressure data from the contact that the clinical team maintains with CardioMEMS patients.
“If nurses are contacting patients more often, is it the device or the communication? We need to look at that very carefully in a study that had no control group,” Dr. Yancy said in an interview. Contact with a nurse “is the best thing you can do for heart failure patients.”
Dr. Shavelle countered that several reports from past studies that assessed case management and regular monitoring of and contact with heart failure patients but without PA pressure data failed to showed any consistent benefit to patients.
“If you pick the right patients, CardioMEMS works. There is no question in my mind that the device works,” Dr. Shavelle said in an interview. “If you pick the wrong patient, who will not send the data or follow dose changes, then it won’t work.”
The study was sponsored by Abbott, the company that markets the CardioMEMS HF System. Dr. Shavelle has been a consultant to and speaker on behalf of Abbott Vascular and he has received research funding from Abbott Vascular, Abiomed, Biocardia, and V-Wave. Dr. Yancy had an unspecified financial relationship with Abbott Laboratories. Dr. Panjrath had no disclosures.
[email protected]
On Twitter @mitchelzoler
SOURCE: Shavelle DM et al. American College of Cardiology annual meeting, abstract 405-16.
REPORTING FROM ACC 2019
AFib screening cuts hospitalizations and ED visits
NEW ORLEANS – People diagnosed with atrial fibrillation by screening with a wearable ECG patch had significantly fewer emergency department visits or hospital admissions, compared with similar people diagnosed with atrial fibrillation by usual-care surveillance in an observational study with 5,109 total participants.
People diagnosed with atrial fibrillation (AFib) through screening had a statistically significant 80% relative cut in hospitalizations and a 65% cut in emergency department visits during 12 months of follow-up, compared with controls in the study who had their AFib identified and diagnosed as part of routine practice, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The data also showed no difference between the screened and control patients identified with AFib in the average number of cardiologist consultations during a year of follow-up, and a trend that missed statistical significance for 16% fewer primary care physician visits in Afib patients diagnosed by screening rather than by routine surveillance.
These findings provided some insight into the potential clinical impact of AFib screening in at-risk people. Dr. Steinhubl and his associates plan to report on the incidence of strokes and MIs in the two study subgroups after 3 years of follow-up, but he noted that preliminary findings for these two outcomes after 1 year indicated that active screening for AFib also had reduced these rates, compared with waiting for the arrhythmia to become apparent by emergence of symptoms.
The data came from the mSToPS (mHealth Screening to Prevent Strokes) study, which randomized 2,659 U.S. residents enrolled in a large health plan who had risk factors for AFib to either immediate or delayed arrhythmia assessment by ECG patches. Half the participants used a patch for about 14 days immediately and then a second time 3 months later, while the other half waited 4 months and then wore an ECG patch for 2 weeks and again 3 months later. The primary endpoint, first reported at the ACC annual meeting a year before and subsequently published, was the incidence of newly diagnosed AFib during the first 4 months in the actively monitored cohort, compared with a cohort followed by usual care. The results showed that screening identified AFib in 3.9% of people, while no screening and usual-practice follow-up identified a 0.9% incidence of AFib, showing that screening worked better for AFib case identification (JAMA. 2018 Jul 10;320[2]:146-55).
To examine the clinical impact of screening and an increased incidence of diagnosed AFib cases, Dr. Steinhubl and his associates focused on 1,725 of the original 2,659 patients who underwent ECG patch assessment, either immediate or delayed, and continued through 12 months of follow-up, and compared them with 3,384 matched controls who never underwent ECG patch screening but were also followed for 12 months for incident AFib identified during routine care and surveillance. This resulted in a cumulative incidence of newly diagnosed AFib of 6.3% in those who had worn two ECG patches and 2.3% among the matched controls.
During follow-up, use of various interventions was more common among the screened people than the controls. Initiation of anticoagulation treatment started in 4.0% of the entire screened group, compared with 1.9% of the controls, The screened people also had a 0.9% rate of receiving a pacemaker or defibrillator, a 0.8% rate of starting on treatment with an antiarrhythmic drug, and a 0.3% rate of undergoing catheter ablation, compared with none, 0.3%, and one of the controls, respectively, said Dr. Steinhubl, director of digital medicine at the Scripps Research Translational Institute in La Jolla, Calif.
The mSToPS study was funded by Janssen. Dr. Steinhubl has received research funding from DynoSense, EasyG, Janssen, the Qualcomm Foundation, and Striv.
SOURCE: Steinhubl SR et al. J Am Coll Cardiol. 2019 Mar 12;73(9)suppl 1:296.
NEW ORLEANS – People diagnosed with atrial fibrillation by screening with a wearable ECG patch had significantly fewer emergency department visits or hospital admissions, compared with similar people diagnosed with atrial fibrillation by usual-care surveillance in an observational study with 5,109 total participants.
People diagnosed with atrial fibrillation (AFib) through screening had a statistically significant 80% relative cut in hospitalizations and a 65% cut in emergency department visits during 12 months of follow-up, compared with controls in the study who had their AFib identified and diagnosed as part of routine practice, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The data also showed no difference between the screened and control patients identified with AFib in the average number of cardiologist consultations during a year of follow-up, and a trend that missed statistical significance for 16% fewer primary care physician visits in Afib patients diagnosed by screening rather than by routine surveillance.
These findings provided some insight into the potential clinical impact of AFib screening in at-risk people. Dr. Steinhubl and his associates plan to report on the incidence of strokes and MIs in the two study subgroups after 3 years of follow-up, but he noted that preliminary findings for these two outcomes after 1 year indicated that active screening for AFib also had reduced these rates, compared with waiting for the arrhythmia to become apparent by emergence of symptoms.
The data came from the mSToPS (mHealth Screening to Prevent Strokes) study, which randomized 2,659 U.S. residents enrolled in a large health plan who had risk factors for AFib to either immediate or delayed arrhythmia assessment by ECG patches. Half the participants used a patch for about 14 days immediately and then a second time 3 months later, while the other half waited 4 months and then wore an ECG patch for 2 weeks and again 3 months later. The primary endpoint, first reported at the ACC annual meeting a year before and subsequently published, was the incidence of newly diagnosed AFib during the first 4 months in the actively monitored cohort, compared with a cohort followed by usual care. The results showed that screening identified AFib in 3.9% of people, while no screening and usual-practice follow-up identified a 0.9% incidence of AFib, showing that screening worked better for AFib case identification (JAMA. 2018 Jul 10;320[2]:146-55).
To examine the clinical impact of screening and an increased incidence of diagnosed AFib cases, Dr. Steinhubl and his associates focused on 1,725 of the original 2,659 patients who underwent ECG patch assessment, either immediate or delayed, and continued through 12 months of follow-up, and compared them with 3,384 matched controls who never underwent ECG patch screening but were also followed for 12 months for incident AFib identified during routine care and surveillance. This resulted in a cumulative incidence of newly diagnosed AFib of 6.3% in those who had worn two ECG patches and 2.3% among the matched controls.
During follow-up, use of various interventions was more common among the screened people than the controls. Initiation of anticoagulation treatment started in 4.0% of the entire screened group, compared with 1.9% of the controls, The screened people also had a 0.9% rate of receiving a pacemaker or defibrillator, a 0.8% rate of starting on treatment with an antiarrhythmic drug, and a 0.3% rate of undergoing catheter ablation, compared with none, 0.3%, and one of the controls, respectively, said Dr. Steinhubl, director of digital medicine at the Scripps Research Translational Institute in La Jolla, Calif.
The mSToPS study was funded by Janssen. Dr. Steinhubl has received research funding from DynoSense, EasyG, Janssen, the Qualcomm Foundation, and Striv.
SOURCE: Steinhubl SR et al. J Am Coll Cardiol. 2019 Mar 12;73(9)suppl 1:296.
NEW ORLEANS – People diagnosed with atrial fibrillation by screening with a wearable ECG patch had significantly fewer emergency department visits or hospital admissions, compared with similar people diagnosed with atrial fibrillation by usual-care surveillance in an observational study with 5,109 total participants.
People diagnosed with atrial fibrillation (AFib) through screening had a statistically significant 80% relative cut in hospitalizations and a 65% cut in emergency department visits during 12 months of follow-up, compared with controls in the study who had their AFib identified and diagnosed as part of routine practice, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The data also showed no difference between the screened and control patients identified with AFib in the average number of cardiologist consultations during a year of follow-up, and a trend that missed statistical significance for 16% fewer primary care physician visits in Afib patients diagnosed by screening rather than by routine surveillance.
These findings provided some insight into the potential clinical impact of AFib screening in at-risk people. Dr. Steinhubl and his associates plan to report on the incidence of strokes and MIs in the two study subgroups after 3 years of follow-up, but he noted that preliminary findings for these two outcomes after 1 year indicated that active screening for AFib also had reduced these rates, compared with waiting for the arrhythmia to become apparent by emergence of symptoms.
The data came from the mSToPS (mHealth Screening to Prevent Strokes) study, which randomized 2,659 U.S. residents enrolled in a large health plan who had risk factors for AFib to either immediate or delayed arrhythmia assessment by ECG patches. Half the participants used a patch for about 14 days immediately and then a second time 3 months later, while the other half waited 4 months and then wore an ECG patch for 2 weeks and again 3 months later. The primary endpoint, first reported at the ACC annual meeting a year before and subsequently published, was the incidence of newly diagnosed AFib during the first 4 months in the actively monitored cohort, compared with a cohort followed by usual care. The results showed that screening identified AFib in 3.9% of people, while no screening and usual-practice follow-up identified a 0.9% incidence of AFib, showing that screening worked better for AFib case identification (JAMA. 2018 Jul 10;320[2]:146-55).
To examine the clinical impact of screening and an increased incidence of diagnosed AFib cases, Dr. Steinhubl and his associates focused on 1,725 of the original 2,659 patients who underwent ECG patch assessment, either immediate or delayed, and continued through 12 months of follow-up, and compared them with 3,384 matched controls who never underwent ECG patch screening but were also followed for 12 months for incident AFib identified during routine care and surveillance. This resulted in a cumulative incidence of newly diagnosed AFib of 6.3% in those who had worn two ECG patches and 2.3% among the matched controls.
During follow-up, use of various interventions was more common among the screened people than the controls. Initiation of anticoagulation treatment started in 4.0% of the entire screened group, compared with 1.9% of the controls, The screened people also had a 0.9% rate of receiving a pacemaker or defibrillator, a 0.8% rate of starting on treatment with an antiarrhythmic drug, and a 0.3% rate of undergoing catheter ablation, compared with none, 0.3%, and one of the controls, respectively, said Dr. Steinhubl, director of digital medicine at the Scripps Research Translational Institute in La Jolla, Calif.
The mSToPS study was funded by Janssen. Dr. Steinhubl has received research funding from DynoSense, EasyG, Janssen, the Qualcomm Foundation, and Striv.
SOURCE: Steinhubl SR et al. J Am Coll Cardiol. 2019 Mar 12;73(9)suppl 1:296.
REPORTING FROM ACC 2019
TAVR for bicuspid aortic stenosis gets selective thumbs up
NEW ORLEANS – Results of the largest-ever analysis of TAVR in patients with bicuspid aortic stenosis indicate that key 30-day and 1-year outcomes are similar to those of propensity-matched TAVR patients with tricuspid disease, Raj R. Makkar, MD, said at the annual meeting of the American College of Cardiology.
“Select bicuspid anatomy is amenable to TAVR with current-generation, balloon-expandable TAVR technology with acceptable clinical outcomes. These data provide an argument for TAVR to be a reasonable alternative for bicuspid AS [aortic stenosis] patients who are at intermediate or high risk for surgical aortic valve replacement, which are the patients that are enrolled in this registry, and provide a sound basis to conduct a randomized clinical trial in young patients with bicuspid AS who are at low risk for surgery,” declared Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
The landmark randomized trials of TAVR versus SAVR (surgical aortic valve replacement) that established TAVR as the preferred treatment for patients with severe aortic stenosis who are at high, intermediate, or low surgical risk systematically excluded patients with bicuspid AS, even though bicuspid anatomy is common, particularly in younger patients with AS.
Despite the absence of supportive randomized trial data, TAVR is being done for bicuspid AS. To learn how patients with bicuspid disease have fared, Dr. Makkar and coinvestigators analyzed the real-world Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry of all patients who underwent TAVR with the balloon-expandable Sapien 3 stent in the United States during 2015-2018. They compared outcomes in 2,691 patients with high or intermediate surgical risk who underwent TAVR for bicuspid AS to an equal number of patients who had TAVR for tricuspid disease, with the two groups being propensity-matched across 25 variables.
Key outcomes were reassuringly similar in the two groups. For example, 30-day and 1-year all-cause mortality rates were 2.6% and 10.8% in patients with bicuspid valves and similar, at 2.5% and 12.1%, in those with tricuspid AS. Paravalvular leak rates at 30 days and 1 year were similar in the two groups. The Kansas City Cardiomyopathy Questionnaire scores, reflecting quality of life, improved dramatically – by nearly 30 points – from pre-TAVR baseline in both groups. The proportion of patients who were New York Heart Association functional class III or IV improved from nearly 85% at baseline to about 8% at 30 days and 1 year, again with no significant difference between the bicuspid and tricuspid AS groups. And there were other benefits, too.
“Despite the concerns regarding optimal expansion of these valves in a bicuspid anatomy, what we observed here was a significant and similar reduction in mean gradients and increase in valve area, both in the bicuspid and tricuspid AS patients. So there was no impact of bicuspid anatomy as seen here in terms of valve hemodynamics,” according to the cardiologist.
Conversion from TAVR to open surgery was required in 0.9% of bicuspid and 0.4% of tricuspid AS patients. Rates of aortic dissection and need for aortic valve reintervention were similarly low in both groups.
The 30-day stroke rate was significantly higher in the bicuspid patients – 2.4% versus 1.6% – but by 1 year there was no significant between-group difference, with stroke rates of 3.4% in the bicuspid and 3.1% in the tricuspid TAVR patients.
“I’d like to point out that more than 75% of strokes occurred in the first 3 days. These are periprocedural strokes, and there was no difference in the time distribution of strokes between the bicuspid and tricuspid groups,” Dr. Makkar said.
These stroke data make a compelling case for the routine use of cerebral protection devices in patients undergoing TAVR, something which now occurs in less than 10% of cases nationally, he continued.
“I would argue that, based on these data, it would be wise for us to use cerebral protection devices, especially when we are doing TAVR in patients with bicuspid AS, because their valves tend to be more heavily calcified than is often the case in tricuspid AS,” Dr. Makkar said.
Discussant Mayra Guerrero, MD, of the Mayo Clinic in Rochester, Minn., took issue with Dr. Makkar’s comment regarding the need for a randomized trial of TAVR in bicuspid AS patients with low surgical risk.
“Do we really need a randomized trial when we see in real-world experience with more than 2,600 patients that the outcomes are fairly similar?” she asked.
Affirmative, Dr. Makkar responded, in light of the fact that the STS/ACC TVT Registry doesn’t include low–surgical risk, typically relatively young bicuspid AS TAVR patients.
“I would say that these data are reassuring and encouraging, but we must not get carried away. I think that would be the important message that I must give,” Dr. Makkar replied. “I think for patients who are high risk and who are intermediate risk, with STS scores of what they were here – 5 and more – I think it’s reasonable to consider them for TAVR based upon CT anatomy. For young patients, as I concluded, I think we must do a randomized clinical trial to definitely establish the safety and efficacy in these patients.”
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which supported the study, as well as Abbott, Medtronic, and Boston Scientific.
[email protected]
SOURCE: Makkar RR. ACC 19, 404-15. Late-breaking clinical trials
NEW ORLEANS – Results of the largest-ever analysis of TAVR in patients with bicuspid aortic stenosis indicate that key 30-day and 1-year outcomes are similar to those of propensity-matched TAVR patients with tricuspid disease, Raj R. Makkar, MD, said at the annual meeting of the American College of Cardiology.
“Select bicuspid anatomy is amenable to TAVR with current-generation, balloon-expandable TAVR technology with acceptable clinical outcomes. These data provide an argument for TAVR to be a reasonable alternative for bicuspid AS [aortic stenosis] patients who are at intermediate or high risk for surgical aortic valve replacement, which are the patients that are enrolled in this registry, and provide a sound basis to conduct a randomized clinical trial in young patients with bicuspid AS who are at low risk for surgery,” declared Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
The landmark randomized trials of TAVR versus SAVR (surgical aortic valve replacement) that established TAVR as the preferred treatment for patients with severe aortic stenosis who are at high, intermediate, or low surgical risk systematically excluded patients with bicuspid AS, even though bicuspid anatomy is common, particularly in younger patients with AS.
Despite the absence of supportive randomized trial data, TAVR is being done for bicuspid AS. To learn how patients with bicuspid disease have fared, Dr. Makkar and coinvestigators analyzed the real-world Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry of all patients who underwent TAVR with the balloon-expandable Sapien 3 stent in the United States during 2015-2018. They compared outcomes in 2,691 patients with high or intermediate surgical risk who underwent TAVR for bicuspid AS to an equal number of patients who had TAVR for tricuspid disease, with the two groups being propensity-matched across 25 variables.
Key outcomes were reassuringly similar in the two groups. For example, 30-day and 1-year all-cause mortality rates were 2.6% and 10.8% in patients with bicuspid valves and similar, at 2.5% and 12.1%, in those with tricuspid AS. Paravalvular leak rates at 30 days and 1 year were similar in the two groups. The Kansas City Cardiomyopathy Questionnaire scores, reflecting quality of life, improved dramatically – by nearly 30 points – from pre-TAVR baseline in both groups. The proportion of patients who were New York Heart Association functional class III or IV improved from nearly 85% at baseline to about 8% at 30 days and 1 year, again with no significant difference between the bicuspid and tricuspid AS groups. And there were other benefits, too.
“Despite the concerns regarding optimal expansion of these valves in a bicuspid anatomy, what we observed here was a significant and similar reduction in mean gradients and increase in valve area, both in the bicuspid and tricuspid AS patients. So there was no impact of bicuspid anatomy as seen here in terms of valve hemodynamics,” according to the cardiologist.
Conversion from TAVR to open surgery was required in 0.9% of bicuspid and 0.4% of tricuspid AS patients. Rates of aortic dissection and need for aortic valve reintervention were similarly low in both groups.
The 30-day stroke rate was significantly higher in the bicuspid patients – 2.4% versus 1.6% – but by 1 year there was no significant between-group difference, with stroke rates of 3.4% in the bicuspid and 3.1% in the tricuspid TAVR patients.
“I’d like to point out that more than 75% of strokes occurred in the first 3 days. These are periprocedural strokes, and there was no difference in the time distribution of strokes between the bicuspid and tricuspid groups,” Dr. Makkar said.
These stroke data make a compelling case for the routine use of cerebral protection devices in patients undergoing TAVR, something which now occurs in less than 10% of cases nationally, he continued.
“I would argue that, based on these data, it would be wise for us to use cerebral protection devices, especially when we are doing TAVR in patients with bicuspid AS, because their valves tend to be more heavily calcified than is often the case in tricuspid AS,” Dr. Makkar said.
Discussant Mayra Guerrero, MD, of the Mayo Clinic in Rochester, Minn., took issue with Dr. Makkar’s comment regarding the need for a randomized trial of TAVR in bicuspid AS patients with low surgical risk.
“Do we really need a randomized trial when we see in real-world experience with more than 2,600 patients that the outcomes are fairly similar?” she asked.
Affirmative, Dr. Makkar responded, in light of the fact that the STS/ACC TVT Registry doesn’t include low–surgical risk, typically relatively young bicuspid AS TAVR patients.
“I would say that these data are reassuring and encouraging, but we must not get carried away. I think that would be the important message that I must give,” Dr. Makkar replied. “I think for patients who are high risk and who are intermediate risk, with STS scores of what they were here – 5 and more – I think it’s reasonable to consider them for TAVR based upon CT anatomy. For young patients, as I concluded, I think we must do a randomized clinical trial to definitely establish the safety and efficacy in these patients.”
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which supported the study, as well as Abbott, Medtronic, and Boston Scientific.
[email protected]
SOURCE: Makkar RR. ACC 19, 404-15. Late-breaking clinical trials
NEW ORLEANS – Results of the largest-ever analysis of TAVR in patients with bicuspid aortic stenosis indicate that key 30-day and 1-year outcomes are similar to those of propensity-matched TAVR patients with tricuspid disease, Raj R. Makkar, MD, said at the annual meeting of the American College of Cardiology.
“Select bicuspid anatomy is amenable to TAVR with current-generation, balloon-expandable TAVR technology with acceptable clinical outcomes. These data provide an argument for TAVR to be a reasonable alternative for bicuspid AS [aortic stenosis] patients who are at intermediate or high risk for surgical aortic valve replacement, which are the patients that are enrolled in this registry, and provide a sound basis to conduct a randomized clinical trial in young patients with bicuspid AS who are at low risk for surgery,” declared Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
The landmark randomized trials of TAVR versus SAVR (surgical aortic valve replacement) that established TAVR as the preferred treatment for patients with severe aortic stenosis who are at high, intermediate, or low surgical risk systematically excluded patients with bicuspid AS, even though bicuspid anatomy is common, particularly in younger patients with AS.
Despite the absence of supportive randomized trial data, TAVR is being done for bicuspid AS. To learn how patients with bicuspid disease have fared, Dr. Makkar and coinvestigators analyzed the real-world Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry of all patients who underwent TAVR with the balloon-expandable Sapien 3 stent in the United States during 2015-2018. They compared outcomes in 2,691 patients with high or intermediate surgical risk who underwent TAVR for bicuspid AS to an equal number of patients who had TAVR for tricuspid disease, with the two groups being propensity-matched across 25 variables.
Key outcomes were reassuringly similar in the two groups. For example, 30-day and 1-year all-cause mortality rates were 2.6% and 10.8% in patients with bicuspid valves and similar, at 2.5% and 12.1%, in those with tricuspid AS. Paravalvular leak rates at 30 days and 1 year were similar in the two groups. The Kansas City Cardiomyopathy Questionnaire scores, reflecting quality of life, improved dramatically – by nearly 30 points – from pre-TAVR baseline in both groups. The proportion of patients who were New York Heart Association functional class III or IV improved from nearly 85% at baseline to about 8% at 30 days and 1 year, again with no significant difference between the bicuspid and tricuspid AS groups. And there were other benefits, too.
“Despite the concerns regarding optimal expansion of these valves in a bicuspid anatomy, what we observed here was a significant and similar reduction in mean gradients and increase in valve area, both in the bicuspid and tricuspid AS patients. So there was no impact of bicuspid anatomy as seen here in terms of valve hemodynamics,” according to the cardiologist.
Conversion from TAVR to open surgery was required in 0.9% of bicuspid and 0.4% of tricuspid AS patients. Rates of aortic dissection and need for aortic valve reintervention were similarly low in both groups.
The 30-day stroke rate was significantly higher in the bicuspid patients – 2.4% versus 1.6% – but by 1 year there was no significant between-group difference, with stroke rates of 3.4% in the bicuspid and 3.1% in the tricuspid TAVR patients.
“I’d like to point out that more than 75% of strokes occurred in the first 3 days. These are periprocedural strokes, and there was no difference in the time distribution of strokes between the bicuspid and tricuspid groups,” Dr. Makkar said.
These stroke data make a compelling case for the routine use of cerebral protection devices in patients undergoing TAVR, something which now occurs in less than 10% of cases nationally, he continued.
“I would argue that, based on these data, it would be wise for us to use cerebral protection devices, especially when we are doing TAVR in patients with bicuspid AS, because their valves tend to be more heavily calcified than is often the case in tricuspid AS,” Dr. Makkar said.
Discussant Mayra Guerrero, MD, of the Mayo Clinic in Rochester, Minn., took issue with Dr. Makkar’s comment regarding the need for a randomized trial of TAVR in bicuspid AS patients with low surgical risk.
“Do we really need a randomized trial when we see in real-world experience with more than 2,600 patients that the outcomes are fairly similar?” she asked.
Affirmative, Dr. Makkar responded, in light of the fact that the STS/ACC TVT Registry doesn’t include low–surgical risk, typically relatively young bicuspid AS TAVR patients.
“I would say that these data are reassuring and encouraging, but we must not get carried away. I think that would be the important message that I must give,” Dr. Makkar replied. “I think for patients who are high risk and who are intermediate risk, with STS scores of what they were here – 5 and more – I think it’s reasonable to consider them for TAVR based upon CT anatomy. For young patients, as I concluded, I think we must do a randomized clinical trial to definitely establish the safety and efficacy in these patients.”
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which supported the study, as well as Abbott, Medtronic, and Boston Scientific.
[email protected]
SOURCE: Makkar RR. ACC 19, 404-15. Late-breaking clinical trials
REPORTING FROM ACC 19
WISE sheds light on angina in INOCA
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM ACC 19
SGLT2 inhibitors prevent HF hospitalization regardless of baseline LVEF
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.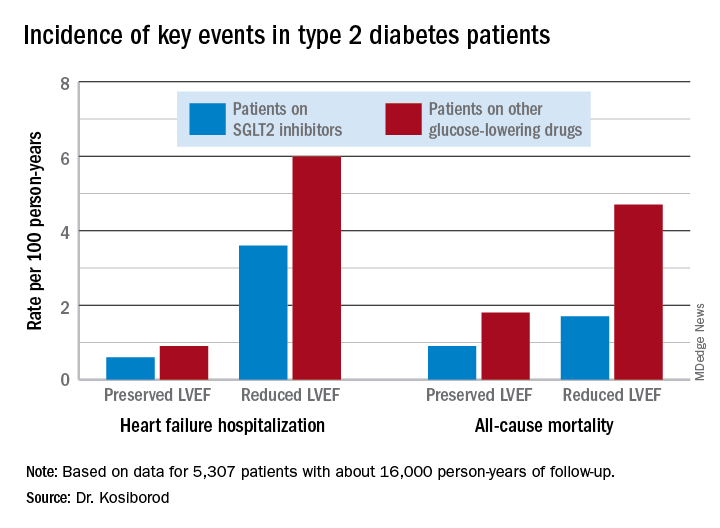
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
REPORTING FROM ACC 19
Unrecognized focal stenosis after angiographically successful PCI
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
Moreover, in 82% of DEFINE PCI participants with post-PCI residual ischemia as defined by instantaneous wave-free ratio (iFR) with pullback evaluation of the whole coronary vessel, the impaired physiology was due to an angiographically unrecognized focal stenosis that’s usually potentially treatable, Allen Jeremias, MD, observed in presenting the DEFINE PCI results at the annual meeting of the American College of Cardiology.
“We estimated that if all residual focal lesions could be treated with additional PCI, the rate of significant ischemia could theoretically be reduced from 24% to 5%, said Dr. Jeremias, director of the physiology core laboratory at the Cardiovascular Research Foundation in New York.
Post-PCI ischemia has been associated with recurrent angina and repeat PCI. The 24% prevalence of residual impaired physiology and ischemia despite a successful angiographic result may seem startlingly high to some, but it really shouldn’t be, according to Dr. Jeremias, who is also director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
“There are a lot of physiologic studies looking at FFR [fractional flow reserve] before PCI to determine if we should do it. And we learned how unreliable the angiogram is to make those decisions. So I think obviously we shouldn’t be surprised if the angiogram afterwards is just as unreliable,” he said.
DEFINE PCI was a prospective observational study of 500 patients who underwent PCI for stable or unstable angina at 27 U.S. and European sites. An iFR was done prior to PCI in all vessels with an angiographic lesion severity of 40% or more. Participating interventionalists performed angiographically guided PCI and confirmed their procedural success with post-PCI angiography before the patient left the cath lab. They also performed an iFR manual pullback interrogation of the entire treated vessel. Although the iFR data are linked to the angiographic images via a technology known as co-registration, the operators were blinded to the iFR results, which along with the angiograms were interpreted in a core laboratory. All patients received guideline-directed medical therapy.
The iFR improved on average from 0.69 pre-PCI to 0.93 post treatment. To put that in perspective, an iFR value of 0.89 or less defines hemodynamically significant ischemia.
Residual physiologic impairment post PCI was deemed due to a missed focal stenosis in 82% of cases and to diffuse atherosclerotic disease in the other 18%. Untreated focal lesions were located within the stent in 38% of cases, proximally in 31%, and distal to the stent in the remainder.
Dr. Jeremias said the investigators looked in vain for possible predictors of post-PCI residual impaired physiology. Post-PCI angiographic results were poorly correlated with iFR. For example, 30% of patients with a residual diameter stenosis of 50% or more had a post-PCI iFR of 0.89 or less, as did 21% of those with a residual diameter stenosis of less than 50%, a nonsignificant difference. Moreover, there were no procedural predictors of poor physiologic outcome.
“I don’t think that the answer is more angiograms or procedural changes guided by the angiogram, but rather guiding of the procedure with physiology and also intravascular imaging,” the cardiologist said.
Session cochair J. Dawn Abbott, MD, an interventional cardiologist at Brown University in Providence, R.I., said DEFINE PCI “really brings up the importance of co-registration of iFR, be it with optical coherence tomography or angiography, because we need the information together. If we can’t see these lesions on the angiogram, we need to be doing more complicated combined physiology and anatomy.”
“This is a very interesting study that I think generates a lot of provocative information,” said discussant John J. Warner, MD.
“It certainly challenges, once again, our definition of angiographic success.” The key remaining question is whether additional PCI addressing the residual focal stenoses causing ischemia will result in improved clinical outcomes, added Dr. Warner, an interventional cardiologist and CEO of the University of Texas Southwestern Medical Center Hospitals, Dallas.
Dr. Jeremias noted that DEFINE PCI participants are in the process of being followed through 12 months to see the impact of residual ischemia on recurrent angina, major adverse cardiovascular events, and quality of life. Moreover, a large randomized trial known as DEFINE GPS (Guided Physiologic Stenting) will soon get underway. Participants will be randomized to unblinded iFR-guided therapy with pullback in order to optimize the physiologic result or to conventional angiographically guided PCI. This trial will define the clinical value of PCI with iFR pullback and should answer the question of whether the more important iFR number is the magnitude of the iFR gain achieved via revascularization or the absolute iFR number achieved at the end.
DEFINE PCI and DEFINE GPS are funded by Volcano/Philips. Dr. Jeremias reported serving as a consultant to that company and a handful of others.
SOURCE: Jeremias A. ACC 19 Abstract 408-10.
REPORTING FROM ACC 19
Noninvasive FFRCT called ADVANCE in chest pain assessment
NEW ORLEANS – Fractional flow reserve derived noninvasively from coronary CT angiography showed clinical merit as a practical tool for evaluation of chest pain at 1 year of follow-up in the ADVANCE registry, Manesh R. Patel, MD, reported at the annual meeting of the American College of Cardiology.
In ADVANCE, a fractional flow reserve value greater than 0.80 derived from CT angiography, or FFRCT, was associated with a significantly lower rate of cardiovascular death or MI at 1 year than in patients with an FFRCT of 0.80 or lower, according to Dr. Patel, professor of medicine and chief of the division of cardiology at Duke University, Durham, N.C.
“The lower rates of revascularization and clinical events in patients with FFRCT who were managed conservatively provide reassurance regarding this clinical strategy if you were to put it into your practice,” he observed.
ADVANCE is in an international, real-world, prospective registry of more than 5,000 patients in Europe, Japan, and North America. All had clinically suspected ischemic coronary artery disease (CAD). They also had at least 30% atherosclerosis documented on coronary CT angiography as a trigger for noninvasive assessment of FFR calculated from computational fluid dynamics. The idea behind FFRCT is that by combining the anatomic information provided by CT angiography with the physiological, functional data from FFR, the result is a better guide to need for revascularization of true obstructive CAD than with conventional invasive coronary angiography alone. Indeed, FFRCT could eventually prove to be a cost-effective gatekeeper to the cardiac catheterization laboratory by cutting down on high rates of invasive coronary angiography for nonactionable CAD.
That point was suggested by the previously reported 90-day outcomes of the ADVANCE registry, the cardiologist explained. Participating physicians first classified patients and made a revascularization/no-revascularization management plan on the basis of the core laboratory CT angiography results alone. But when they received the FFRCT results, they reclassified patients and changed the management plan in 67% of cases. That’s because the prevalence of nonobstructive CAD was 44% in patients with an FFRCT greater than 0.80 in all coronary arteries, compared with just 14% in those with an FFRCT of 0.80 or less. As a result, 72% of patients with an FFRCT of 0.80 or less underwent revascularization, while the vast majority of patients with an FFRCT greater than 0.80 were initially managed conservatively (Eur Heart J. 2018 Nov 1;39[41]:3701-11).
The 1-year outcomes from ADVANCE as presented by Dr. Patel showed low rates of major adverse cardiovascular events overall. Of note, the composite endpoint of cardiovascular death or MI occurred significantly more often in patients with an FFRCT of 0.80 or less, by a margin of 0.8% versus 0.2%, for a 320% increased relative risk. The patients with a FFRCT greater than 0.80 continued to have a much lower revascularization rate from 90 days through 1 year: 5.8% versus 38.4% in the lower-FFRCT group. And 93% of patients placed on medical therapy alone after receiving their FFRCT results remained on medical therapy without revascularization or a major adverse cardiovascular event at 1 year.
Discussant Matthew J. Budoff, MD, commented that it’s time to move beyond observational studies and conduct randomized trials of an FFRCT-based screening strategy in patients with clinical suspicion of obstructive CAD.
“We want to understand the enormous advantages of having FFR-like data before we take patients to the cath lab. And I do think that adding physiology to the anatomy is going to be the approach that we’re going to be predominantly using in the future,” said Dr. Budoff, professor of medicine at the University of California, Los Angeles.
Dr. Patel noted that the ongoing, randomized, 2,100-patient PRECISE study is directed at determining in a more definitive way the clinical and cost-effectiveness of an FFRCT strategy.
The ADVANCE registry is funded by HeartFlow. Dr. Patel reported receiving research grants from that company and several others, as well as the National Institutes of Health. He serves on advisory boards for Bayer, Janssen, and Amgen.
Simultaneous with Dr. Patel’s presentation at ACC 2019, the 1-year ADVANCE registry results were published online (JACC Cardiovasc Imag. 2019 Mar 17. doi: 10.1016/j.jcmg.2019.03.003).
NEW ORLEANS – Fractional flow reserve derived noninvasively from coronary CT angiography showed clinical merit as a practical tool for evaluation of chest pain at 1 year of follow-up in the ADVANCE registry, Manesh R. Patel, MD, reported at the annual meeting of the American College of Cardiology.
In ADVANCE, a fractional flow reserve value greater than 0.80 derived from CT angiography, or FFRCT, was associated with a significantly lower rate of cardiovascular death or MI at 1 year than in patients with an FFRCT of 0.80 or lower, according to Dr. Patel, professor of medicine and chief of the division of cardiology at Duke University, Durham, N.C.
“The lower rates of revascularization and clinical events in patients with FFRCT who were managed conservatively provide reassurance regarding this clinical strategy if you were to put it into your practice,” he observed.
ADVANCE is in an international, real-world, prospective registry of more than 5,000 patients in Europe, Japan, and North America. All had clinically suspected ischemic coronary artery disease (CAD). They also had at least 30% atherosclerosis documented on coronary CT angiography as a trigger for noninvasive assessment of FFR calculated from computational fluid dynamics. The idea behind FFRCT is that by combining the anatomic information provided by CT angiography with the physiological, functional data from FFR, the result is a better guide to need for revascularization of true obstructive CAD than with conventional invasive coronary angiography alone. Indeed, FFRCT could eventually prove to be a cost-effective gatekeeper to the cardiac catheterization laboratory by cutting down on high rates of invasive coronary angiography for nonactionable CAD.
That point was suggested by the previously reported 90-day outcomes of the ADVANCE registry, the cardiologist explained. Participating physicians first classified patients and made a revascularization/no-revascularization management plan on the basis of the core laboratory CT angiography results alone. But when they received the FFRCT results, they reclassified patients and changed the management plan in 67% of cases. That’s because the prevalence of nonobstructive CAD was 44% in patients with an FFRCT greater than 0.80 in all coronary arteries, compared with just 14% in those with an FFRCT of 0.80 or less. As a result, 72% of patients with an FFRCT of 0.80 or less underwent revascularization, while the vast majority of patients with an FFRCT greater than 0.80 were initially managed conservatively (Eur Heart J. 2018 Nov 1;39[41]:3701-11).
The 1-year outcomes from ADVANCE as presented by Dr. Patel showed low rates of major adverse cardiovascular events overall. Of note, the composite endpoint of cardiovascular death or MI occurred significantly more often in patients with an FFRCT of 0.80 or less, by a margin of 0.8% versus 0.2%, for a 320% increased relative risk. The patients with a FFRCT greater than 0.80 continued to have a much lower revascularization rate from 90 days through 1 year: 5.8% versus 38.4% in the lower-FFRCT group. And 93% of patients placed on medical therapy alone after receiving their FFRCT results remained on medical therapy without revascularization or a major adverse cardiovascular event at 1 year.
Discussant Matthew J. Budoff, MD, commented that it’s time to move beyond observational studies and conduct randomized trials of an FFRCT-based screening strategy in patients with clinical suspicion of obstructive CAD.
“We want to understand the enormous advantages of having FFR-like data before we take patients to the cath lab. And I do think that adding physiology to the anatomy is going to be the approach that we’re going to be predominantly using in the future,” said Dr. Budoff, professor of medicine at the University of California, Los Angeles.
Dr. Patel noted that the ongoing, randomized, 2,100-patient PRECISE study is directed at determining in a more definitive way the clinical and cost-effectiveness of an FFRCT strategy.
The ADVANCE registry is funded by HeartFlow. Dr. Patel reported receiving research grants from that company and several others, as well as the National Institutes of Health. He serves on advisory boards for Bayer, Janssen, and Amgen.
Simultaneous with Dr. Patel’s presentation at ACC 2019, the 1-year ADVANCE registry results were published online (JACC Cardiovasc Imag. 2019 Mar 17. doi: 10.1016/j.jcmg.2019.03.003).
NEW ORLEANS – Fractional flow reserve derived noninvasively from coronary CT angiography showed clinical merit as a practical tool for evaluation of chest pain at 1 year of follow-up in the ADVANCE registry, Manesh R. Patel, MD, reported at the annual meeting of the American College of Cardiology.
In ADVANCE, a fractional flow reserve value greater than 0.80 derived from CT angiography, or FFRCT, was associated with a significantly lower rate of cardiovascular death or MI at 1 year than in patients with an FFRCT of 0.80 or lower, according to Dr. Patel, professor of medicine and chief of the division of cardiology at Duke University, Durham, N.C.
“The lower rates of revascularization and clinical events in patients with FFRCT who were managed conservatively provide reassurance regarding this clinical strategy if you were to put it into your practice,” he observed.
ADVANCE is in an international, real-world, prospective registry of more than 5,000 patients in Europe, Japan, and North America. All had clinically suspected ischemic coronary artery disease (CAD). They also had at least 30% atherosclerosis documented on coronary CT angiography as a trigger for noninvasive assessment of FFR calculated from computational fluid dynamics. The idea behind FFRCT is that by combining the anatomic information provided by CT angiography with the physiological, functional data from FFR, the result is a better guide to need for revascularization of true obstructive CAD than with conventional invasive coronary angiography alone. Indeed, FFRCT could eventually prove to be a cost-effective gatekeeper to the cardiac catheterization laboratory by cutting down on high rates of invasive coronary angiography for nonactionable CAD.
That point was suggested by the previously reported 90-day outcomes of the ADVANCE registry, the cardiologist explained. Participating physicians first classified patients and made a revascularization/no-revascularization management plan on the basis of the core laboratory CT angiography results alone. But when they received the FFRCT results, they reclassified patients and changed the management plan in 67% of cases. That’s because the prevalence of nonobstructive CAD was 44% in patients with an FFRCT greater than 0.80 in all coronary arteries, compared with just 14% in those with an FFRCT of 0.80 or less. As a result, 72% of patients with an FFRCT of 0.80 or less underwent revascularization, while the vast majority of patients with an FFRCT greater than 0.80 were initially managed conservatively (Eur Heart J. 2018 Nov 1;39[41]:3701-11).
The 1-year outcomes from ADVANCE as presented by Dr. Patel showed low rates of major adverse cardiovascular events overall. Of note, the composite endpoint of cardiovascular death or MI occurred significantly more often in patients with an FFRCT of 0.80 or less, by a margin of 0.8% versus 0.2%, for a 320% increased relative risk. The patients with a FFRCT greater than 0.80 continued to have a much lower revascularization rate from 90 days through 1 year: 5.8% versus 38.4% in the lower-FFRCT group. And 93% of patients placed on medical therapy alone after receiving their FFRCT results remained on medical therapy without revascularization or a major adverse cardiovascular event at 1 year.
Discussant Matthew J. Budoff, MD, commented that it’s time to move beyond observational studies and conduct randomized trials of an FFRCT-based screening strategy in patients with clinical suspicion of obstructive CAD.
“We want to understand the enormous advantages of having FFR-like data before we take patients to the cath lab. And I do think that adding physiology to the anatomy is going to be the approach that we’re going to be predominantly using in the future,” said Dr. Budoff, professor of medicine at the University of California, Los Angeles.
Dr. Patel noted that the ongoing, randomized, 2,100-patient PRECISE study is directed at determining in a more definitive way the clinical and cost-effectiveness of an FFRCT strategy.
The ADVANCE registry is funded by HeartFlow. Dr. Patel reported receiving research grants from that company and several others, as well as the National Institutes of Health. He serves on advisory boards for Bayer, Janssen, and Amgen.
Simultaneous with Dr. Patel’s presentation at ACC 2019, the 1-year ADVANCE registry results were published online (JACC Cardiovasc Imag. 2019 Mar 17. doi: 10.1016/j.jcmg.2019.03.003).
REPORTING FROM ACC 19
Cardiac PET/CT can guide CAD therapy
NEW ORLEANS – A PET/CT-derived myocardial ischemic burden in excess of 10% defines a subset of patients with symptomatic CAD who derive significantly greater benefit from an invasive management strategy than a noninvasive one, Kent G. Meredith, MD, reported at the annual meeting of the American College of Cardiology.
Conversely, patients with an ischemia burden of 10% or less have a lower major adverse event rate if they undergo noninvasive treatment.
“We see that cardiac PET/CT-derived ischemic burden provides a convenient and useful tool for predicting clinical outcomes of invasive and noninvasive treatment strategies,”said Dr. Meredith, a cardiologist at Intermountain Medical Center in Murray, Utah.
He presented a retrospective single-center study of 5,528 consecutive patients with symptomatic CAD referred for PET/CT at Intermountain. As a condition for study inclusion, they needed to survive for at least 90 days after imaging, have no elevation of troponin, and have no prior history of CAD.
This was a study of real-world clinical practice featuring standardized institutional protocols. Dr. Meredith explained that the 10% ischemic burden threshold used by cardiologists at Intermountain to help determine an individual’s optimal treatment strategy is based upon “a very important study” in which investigators at Cedars-Sinai Medical Center in Los Angeles showed 16 years ago, in nearly 11,000 consecutive patients, that revascularization had a survival benefit over medical therapy alone at an ischemic burden in excess of 10% as measured by stress myocardial perfusion single photon emission CT (Circulation. 2003 Jun 17;107[23]:2900-7).
Dr. Meredith and his coinvestigators carried out their study to make sure this ischemic burden cutoff is still valid today in view of the considerable changes in imaging technology and optimal medical therapy in the intervening years.
Among the study population, 203 patients had a PET/CT-derived ischemic burden greater than 10% using a well-established scoring system (J Nucl Cardiol. 2006 Nov;13[6]:e157-71), while 5,325 had a lesser ischemic burden. Fifty-six percent of patients with an ischemic burden above the 10% threshold underwent coronary revascularization, 26% had coronary angiography without revascularization, and 18% were managed by optimal medical therapy alone.
The group with an ischemic burden of 10% or less was managed very differently: One percent had revascularization, 3% had angiography without revascularization, and 96% were managed medically.
The higher a patient’s baseline ischemic burden, the higher the major adverse cardiovascular event (MACE) rate during 1-4 years of follow-up. The composite MACE rate, comprising death, hospitalization for acute MI, or late revascularization after 90 days, was 3.9% in patients with an ischemic burden of 10% of less, compared with 8.9% in those above the 10% threshold.
In a multivariate analysis adjusted for demographics, hyperlipidemia, heart failure, and diabetes, patients with an ischemic burden greater than 10% had a 4.6-fold greater risk of MACE if managed medically than if they underwent revascularization. And among those with an ischemic burden of 10% or less, the adjusted risk of MACE was increased 2.8-fold if they received revascularization instead of medical management.
Dr. Meredith reported having no financial conflicts regarding his study, conducted free of commercial sponsorship.
NEW ORLEANS – A PET/CT-derived myocardial ischemic burden in excess of 10% defines a subset of patients with symptomatic CAD who derive significantly greater benefit from an invasive management strategy than a noninvasive one, Kent G. Meredith, MD, reported at the annual meeting of the American College of Cardiology.
Conversely, patients with an ischemia burden of 10% or less have a lower major adverse event rate if they undergo noninvasive treatment.
“We see that cardiac PET/CT-derived ischemic burden provides a convenient and useful tool for predicting clinical outcomes of invasive and noninvasive treatment strategies,”said Dr. Meredith, a cardiologist at Intermountain Medical Center in Murray, Utah.
He presented a retrospective single-center study of 5,528 consecutive patients with symptomatic CAD referred for PET/CT at Intermountain. As a condition for study inclusion, they needed to survive for at least 90 days after imaging, have no elevation of troponin, and have no prior history of CAD.
This was a study of real-world clinical practice featuring standardized institutional protocols. Dr. Meredith explained that the 10% ischemic burden threshold used by cardiologists at Intermountain to help determine an individual’s optimal treatment strategy is based upon “a very important study” in which investigators at Cedars-Sinai Medical Center in Los Angeles showed 16 years ago, in nearly 11,000 consecutive patients, that revascularization had a survival benefit over medical therapy alone at an ischemic burden in excess of 10% as measured by stress myocardial perfusion single photon emission CT (Circulation. 2003 Jun 17;107[23]:2900-7).
Dr. Meredith and his coinvestigators carried out their study to make sure this ischemic burden cutoff is still valid today in view of the considerable changes in imaging technology and optimal medical therapy in the intervening years.
Among the study population, 203 patients had a PET/CT-derived ischemic burden greater than 10% using a well-established scoring system (J Nucl Cardiol. 2006 Nov;13[6]:e157-71), while 5,325 had a lesser ischemic burden. Fifty-six percent of patients with an ischemic burden above the 10% threshold underwent coronary revascularization, 26% had coronary angiography without revascularization, and 18% were managed by optimal medical therapy alone.
The group with an ischemic burden of 10% or less was managed very differently: One percent had revascularization, 3% had angiography without revascularization, and 96% were managed medically.
The higher a patient’s baseline ischemic burden, the higher the major adverse cardiovascular event (MACE) rate during 1-4 years of follow-up. The composite MACE rate, comprising death, hospitalization for acute MI, or late revascularization after 90 days, was 3.9% in patients with an ischemic burden of 10% of less, compared with 8.9% in those above the 10% threshold.
In a multivariate analysis adjusted for demographics, hyperlipidemia, heart failure, and diabetes, patients with an ischemic burden greater than 10% had a 4.6-fold greater risk of MACE if managed medically than if they underwent revascularization. And among those with an ischemic burden of 10% or less, the adjusted risk of MACE was increased 2.8-fold if they received revascularization instead of medical management.
Dr. Meredith reported having no financial conflicts regarding his study, conducted free of commercial sponsorship.
NEW ORLEANS – A PET/CT-derived myocardial ischemic burden in excess of 10% defines a subset of patients with symptomatic CAD who derive significantly greater benefit from an invasive management strategy than a noninvasive one, Kent G. Meredith, MD, reported at the annual meeting of the American College of Cardiology.
Conversely, patients with an ischemia burden of 10% or less have a lower major adverse event rate if they undergo noninvasive treatment.
“We see that cardiac PET/CT-derived ischemic burden provides a convenient and useful tool for predicting clinical outcomes of invasive and noninvasive treatment strategies,”said Dr. Meredith, a cardiologist at Intermountain Medical Center in Murray, Utah.
He presented a retrospective single-center study of 5,528 consecutive patients with symptomatic CAD referred for PET/CT at Intermountain. As a condition for study inclusion, they needed to survive for at least 90 days after imaging, have no elevation of troponin, and have no prior history of CAD.
This was a study of real-world clinical practice featuring standardized institutional protocols. Dr. Meredith explained that the 10% ischemic burden threshold used by cardiologists at Intermountain to help determine an individual’s optimal treatment strategy is based upon “a very important study” in which investigators at Cedars-Sinai Medical Center in Los Angeles showed 16 years ago, in nearly 11,000 consecutive patients, that revascularization had a survival benefit over medical therapy alone at an ischemic burden in excess of 10% as measured by stress myocardial perfusion single photon emission CT (Circulation. 2003 Jun 17;107[23]:2900-7).
Dr. Meredith and his coinvestigators carried out their study to make sure this ischemic burden cutoff is still valid today in view of the considerable changes in imaging technology and optimal medical therapy in the intervening years.
Among the study population, 203 patients had a PET/CT-derived ischemic burden greater than 10% using a well-established scoring system (J Nucl Cardiol. 2006 Nov;13[6]:e157-71), while 5,325 had a lesser ischemic burden. Fifty-six percent of patients with an ischemic burden above the 10% threshold underwent coronary revascularization, 26% had coronary angiography without revascularization, and 18% were managed by optimal medical therapy alone.
The group with an ischemic burden of 10% or less was managed very differently: One percent had revascularization, 3% had angiography without revascularization, and 96% were managed medically.
The higher a patient’s baseline ischemic burden, the higher the major adverse cardiovascular event (MACE) rate during 1-4 years of follow-up. The composite MACE rate, comprising death, hospitalization for acute MI, or late revascularization after 90 days, was 3.9% in patients with an ischemic burden of 10% of less, compared with 8.9% in those above the 10% threshold.
In a multivariate analysis adjusted for demographics, hyperlipidemia, heart failure, and diabetes, patients with an ischemic burden greater than 10% had a 4.6-fold greater risk of MACE if managed medically than if they underwent revascularization. And among those with an ischemic burden of 10% or less, the adjusted risk of MACE was increased 2.8-fold if they received revascularization instead of medical management.
Dr. Meredith reported having no financial conflicts regarding his study, conducted free of commercial sponsorship.
REPORTING FROM ACC 19
REDUCE-IT results suggest rethinking what’s elevated triglyceride
NEW ORLEANS – The success of icosapent ethyl in cutting triglyceride levels and reducing cardiovascular disease events in at-risk patients in the REDUCE-IT trial may make clinicians rethink the threshold for an unhealthy triglyceride level that merits intervention.
Study results are also showing that the patients enrolled in REDUCE-IT are common, with apparently millions of Americans who could potentially receive the icosapent ethyl–processed fish oil used in the study if the Food and Drug Administration were to approve new labeling for the drug that the manufacturer filed for in late March 2019. Icosapent ethyl (Vascepa) already has U.S. marketing approval for reducing triglyceride (TG) levels in patients with baseline values of 500 mg/dL or greater, while the REDUCE-IT trial enrolled patients with established cardiovascular disease or diabetes plus at least one more risk factor with a TG level of 150-499 mg/dL. REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) enrolled only patients already on statin treatment and with a LDL cholesterol level of 41-100 mg/dL.
In reality, the clinicians who enrolled the 8,139 participants at 473 worldwide sites included patients with a TG level as low as 81 mg/dL, and 10% of entered patients had levels below the minimum threshold in the trial’s written design of at least 150 mg/dL. Initial results reported with the primary endpoint finding suggested that the icosapent ethyl treatment benefit extended to these patients who entered with what are currently considered normal TG values, and additional analyses reported by the study’s lead investigator, Deepak L. Bhatt, MD, which used a larger endpoint dataset that included total cardiovascular events rather than just first events, further confirmed that patients with lower baseline TG levels had reductions in their cardiovascular disease events that matched what was seen in patients who entered with substantially higher TG levels.
In the analysis that included total events, the tertile of patients with a baseline TG of 81-190 mg/dL had a statistically significant 26% relative reduction in events during an average 3.5-year follow-up, compared with the tertile of patients with a baseline level of 251 mg/dL or higher, who had a 40% reduction in their events during follow-up, reported Dr. Bhatt, professor of medicine at Harvard Medical School, Boston.
“We had patients [in REDUCE-IT] with lower triglycerides than the inclusion criteria. This shows that the study results apply to a broader range of patients,” he said in a talk at the annual meeting of the American College of Cardiology. “The total-event analysis gives us an appreciation of the large burden of ischemic events that statin-treated patients still have with baseline triglyceride levels of about 100 mg/dL.” Further analysis of the REDUCE-IT data, as well as future studies of TG-lowering drugs like icosapent ethyl, “may help redefine normal TG levels” in a manner similar to what happened over a 2-decade span as serial studies of statins and other drugs that reduced levels of LDL cholesterol led to incremental reductions in goal lipid levels.
In addition to providing greater precision in defining the impact of icosapent ethyl on events in patients with lower baseline TG levels, the total-event analysis “provided a better sense of what is actually going on” with patients clinically as they experience multiple cardiovascular events during follow-up, as well as the impact of treatment on reducing health-related costs. Concurrently with Dr. Bhatt’s report of the total-event analysis at the meeting, some of the new findings he presented also appeared online (J Am Coll Cardiol. 2019 March 18. doi: 10.1016/j.jacc.2019.02.032).
Recent analyses have also begun to assess the scope of patients who could potentially receive icosapent ethyl based on the enrollment criteria of REDUCE-IT. One analysis of more than 1 million people in the U.S. Veterans Affairs Health System during 2010 identified 439,019 people on statin treatment and with an LDL cholesterol of 41-100 mg/dL, the cardiovascular disease history or risk pattern that matched the trial, and not on treatment that could reduce TG levels such as fish oil. Among these people, 30% had a TG level at or above 150 mg/dL that would have qualified them to enter REDUCE-IT, said William E. Boden, MD, professor of medicine at Boston University. Among the 132,203 patients in this group who were on statin treatment and at their target LDL cholesterol level, the 5-year rate of cardiovascular disease events was 8.5% in those with higher TG levels and 6.3% in those with levels below 150 mg/dL, a statistically significant 19% increased risk after adjustment for some potential confounders, Dr. Boden reported in a poster he presented at the meeting. This analysis hinted at the magnitude of patients who are candidates for icosapent ethyl treatment based on REDUCE-IT, and the 19% residual increased risk they displayed showed what this treatment could address.
Analysis of another database identified 16% of more than 24,000 patients with stable coronary artery disease in the CLARIFY registry who would qualify for icosapent ethyl treatment by matching the REDUCE-IT enrollment criteria (J Am Coll Cardiol. 2019 March;73[11];doi: 10.1016/j.jacc.2019.01.016).
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Bhatt is an adviser to Cardax, PhaseBio, and Regado Biosciences, he is on the board of TobeSoft, and he has received research funding from several companies. Dr. Boden reported no disclosures.
Potential "game-changing" trials appear infrequently, but needless to say, they make a huge impact when they are validated. The atherosclerotic cardiovascular disease (ASCVD)/diabetes community fortunately has benefited from several lipid-lowering outcomes in recent years. Clinical trials with the PCSK9 and SGLT2 inhibitors already have had substantial impact on how we approach the patient with ASCVD, and now REDUCE-IT promises to move the needle substantially in both primary and secondary ASCVD prevention and in how we view triglyceride elevations.
After achieving LDL levels of 40-100 mg/dL with statin therapy, the 8,000-patient, 4.8-year trial demonstrated a robust 25% reduction in both primary and secondary outcomes in patients treated with 4 g/day of icosapent ethyl. Patients without previous cardiovascular disease (CVD) events with diabetes and one additional CVD risk factor (primary prevention cohort) achieved the same benefit. The "total event" as opposed to the first event analysis demonstrated a 30% overall risk reduction in patients having subsequent events.
The most stunning finding in REDUCE-IT was clearly that the benefit was not directly related to the baseline triglyceride level even though icosapent ethyl is a triglyceride-lowering agent. The subgroups with baseline triglycerides 150 mg/dL or less and those with 151-200 mg/dL had a comparable CVD benefit. The "total event" analysis, however, did demonstrate a more robust relative risk reduction in patients with baseline triglycerides of 251 mg/dL or higher.
Based on the REDUCE-IT findings, pure icosapent ethyl can be added to ezetimibe and PCSK9 inhibitors as agents that amplify the statin benefit and robustly reduce the elusive statin residual risk. The role of triglycerides in CVD remains unclear, although subgroup analysis from several fibrate studies suggest a CVD benefit from treating hypertriglyceridemia in patients with triglycerides of greater than 200 mg/dL. REDUCE-IT points to a largely independent action of icosapent ethyl. Laboratory studies suggest icosapent ethyl has potent plaque-stabilizing properties. The lowering of triglycerides may well play an additive but not primary role.
Just as was the case with LDL, as studies like this evolve our understanding of "normal" triglyceride levels will also evolve. Triglycerides may eventually join LDL with strong evidence that "lower is better." Stay tuned.
Paul Jellinger, MD, MACE, is a member of the editorial advisory board for Clinical Endocrinology News. He is professor of clinical medicine on the voluntary faculty at the University of Miami Miller School of Medicine and a practicing endocrinologist at The Center for Diabetes & Endocrine Care in Hollywood, Fla. He is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology.
This comment was added 6/10/2019.
Potential "game-changing" trials appear infrequently, but needless to say, they make a huge impact when they are validated. The atherosclerotic cardiovascular disease (ASCVD)/diabetes community fortunately has benefited from several lipid-lowering outcomes in recent years. Clinical trials with the PCSK9 and SGLT2 inhibitors already have had substantial impact on how we approach the patient with ASCVD, and now REDUCE-IT promises to move the needle substantially in both primary and secondary ASCVD prevention and in how we view triglyceride elevations.
After achieving LDL levels of 40-100 mg/dL with statin therapy, the 8,000-patient, 4.8-year trial demonstrated a robust 25% reduction in both primary and secondary outcomes in patients treated with 4 g/day of icosapent ethyl. Patients without previous cardiovascular disease (CVD) events with diabetes and one additional CVD risk factor (primary prevention cohort) achieved the same benefit. The "total event" as opposed to the first event analysis demonstrated a 30% overall risk reduction in patients having subsequent events.
The most stunning finding in REDUCE-IT was clearly that the benefit was not directly related to the baseline triglyceride level even though icosapent ethyl is a triglyceride-lowering agent. The subgroups with baseline triglycerides 150 mg/dL or less and those with 151-200 mg/dL had a comparable CVD benefit. The "total event" analysis, however, did demonstrate a more robust relative risk reduction in patients with baseline triglycerides of 251 mg/dL or higher.
Based on the REDUCE-IT findings, pure icosapent ethyl can be added to ezetimibe and PCSK9 inhibitors as agents that amplify the statin benefit and robustly reduce the elusive statin residual risk. The role of triglycerides in CVD remains unclear, although subgroup analysis from several fibrate studies suggest a CVD benefit from treating hypertriglyceridemia in patients with triglycerides of greater than 200 mg/dL. REDUCE-IT points to a largely independent action of icosapent ethyl. Laboratory studies suggest icosapent ethyl has potent plaque-stabilizing properties. The lowering of triglycerides may well play an additive but not primary role.
Just as was the case with LDL, as studies like this evolve our understanding of "normal" triglyceride levels will also evolve. Triglycerides may eventually join LDL with strong evidence that "lower is better." Stay tuned.
Paul Jellinger, MD, MACE, is a member of the editorial advisory board for Clinical Endocrinology News. He is professor of clinical medicine on the voluntary faculty at the University of Miami Miller School of Medicine and a practicing endocrinologist at The Center for Diabetes & Endocrine Care in Hollywood, Fla. He is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology.
This comment was added 6/10/2019.
Potential "game-changing" trials appear infrequently, but needless to say, they make a huge impact when they are validated. The atherosclerotic cardiovascular disease (ASCVD)/diabetes community fortunately has benefited from several lipid-lowering outcomes in recent years. Clinical trials with the PCSK9 and SGLT2 inhibitors already have had substantial impact on how we approach the patient with ASCVD, and now REDUCE-IT promises to move the needle substantially in both primary and secondary ASCVD prevention and in how we view triglyceride elevations.
After achieving LDL levels of 40-100 mg/dL with statin therapy, the 8,000-patient, 4.8-year trial demonstrated a robust 25% reduction in both primary and secondary outcomes in patients treated with 4 g/day of icosapent ethyl. Patients without previous cardiovascular disease (CVD) events with diabetes and one additional CVD risk factor (primary prevention cohort) achieved the same benefit. The "total event" as opposed to the first event analysis demonstrated a 30% overall risk reduction in patients having subsequent events.
The most stunning finding in REDUCE-IT was clearly that the benefit was not directly related to the baseline triglyceride level even though icosapent ethyl is a triglyceride-lowering agent. The subgroups with baseline triglycerides 150 mg/dL or less and those with 151-200 mg/dL had a comparable CVD benefit. The "total event" analysis, however, did demonstrate a more robust relative risk reduction in patients with baseline triglycerides of 251 mg/dL or higher.
Based on the REDUCE-IT findings, pure icosapent ethyl can be added to ezetimibe and PCSK9 inhibitors as agents that amplify the statin benefit and robustly reduce the elusive statin residual risk. The role of triglycerides in CVD remains unclear, although subgroup analysis from several fibrate studies suggest a CVD benefit from treating hypertriglyceridemia in patients with triglycerides of greater than 200 mg/dL. REDUCE-IT points to a largely independent action of icosapent ethyl. Laboratory studies suggest icosapent ethyl has potent plaque-stabilizing properties. The lowering of triglycerides may well play an additive but not primary role.
Just as was the case with LDL, as studies like this evolve our understanding of "normal" triglyceride levels will also evolve. Triglycerides may eventually join LDL with strong evidence that "lower is better." Stay tuned.
Paul Jellinger, MD, MACE, is a member of the editorial advisory board for Clinical Endocrinology News. He is professor of clinical medicine on the voluntary faculty at the University of Miami Miller School of Medicine and a practicing endocrinologist at The Center for Diabetes & Endocrine Care in Hollywood, Fla. He is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology.
This comment was added 6/10/2019.
NEW ORLEANS – The success of icosapent ethyl in cutting triglyceride levels and reducing cardiovascular disease events in at-risk patients in the REDUCE-IT trial may make clinicians rethink the threshold for an unhealthy triglyceride level that merits intervention.
Study results are also showing that the patients enrolled in REDUCE-IT are common, with apparently millions of Americans who could potentially receive the icosapent ethyl–processed fish oil used in the study if the Food and Drug Administration were to approve new labeling for the drug that the manufacturer filed for in late March 2019. Icosapent ethyl (Vascepa) already has U.S. marketing approval for reducing triglyceride (TG) levels in patients with baseline values of 500 mg/dL or greater, while the REDUCE-IT trial enrolled patients with established cardiovascular disease or diabetes plus at least one more risk factor with a TG level of 150-499 mg/dL. REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) enrolled only patients already on statin treatment and with a LDL cholesterol level of 41-100 mg/dL.
In reality, the clinicians who enrolled the 8,139 participants at 473 worldwide sites included patients with a TG level as low as 81 mg/dL, and 10% of entered patients had levels below the minimum threshold in the trial’s written design of at least 150 mg/dL. Initial results reported with the primary endpoint finding suggested that the icosapent ethyl treatment benefit extended to these patients who entered with what are currently considered normal TG values, and additional analyses reported by the study’s lead investigator, Deepak L. Bhatt, MD, which used a larger endpoint dataset that included total cardiovascular events rather than just first events, further confirmed that patients with lower baseline TG levels had reductions in their cardiovascular disease events that matched what was seen in patients who entered with substantially higher TG levels.
In the analysis that included total events, the tertile of patients with a baseline TG of 81-190 mg/dL had a statistically significant 26% relative reduction in events during an average 3.5-year follow-up, compared with the tertile of patients with a baseline level of 251 mg/dL or higher, who had a 40% reduction in their events during follow-up, reported Dr. Bhatt, professor of medicine at Harvard Medical School, Boston.
“We had patients [in REDUCE-IT] with lower triglycerides than the inclusion criteria. This shows that the study results apply to a broader range of patients,” he said in a talk at the annual meeting of the American College of Cardiology. “The total-event analysis gives us an appreciation of the large burden of ischemic events that statin-treated patients still have with baseline triglyceride levels of about 100 mg/dL.” Further analysis of the REDUCE-IT data, as well as future studies of TG-lowering drugs like icosapent ethyl, “may help redefine normal TG levels” in a manner similar to what happened over a 2-decade span as serial studies of statins and other drugs that reduced levels of LDL cholesterol led to incremental reductions in goal lipid levels.
In addition to providing greater precision in defining the impact of icosapent ethyl on events in patients with lower baseline TG levels, the total-event analysis “provided a better sense of what is actually going on” with patients clinically as they experience multiple cardiovascular events during follow-up, as well as the impact of treatment on reducing health-related costs. Concurrently with Dr. Bhatt’s report of the total-event analysis at the meeting, some of the new findings he presented also appeared online (J Am Coll Cardiol. 2019 March 18. doi: 10.1016/j.jacc.2019.02.032).
Recent analyses have also begun to assess the scope of patients who could potentially receive icosapent ethyl based on the enrollment criteria of REDUCE-IT. One analysis of more than 1 million people in the U.S. Veterans Affairs Health System during 2010 identified 439,019 people on statin treatment and with an LDL cholesterol of 41-100 mg/dL, the cardiovascular disease history or risk pattern that matched the trial, and not on treatment that could reduce TG levels such as fish oil. Among these people, 30% had a TG level at or above 150 mg/dL that would have qualified them to enter REDUCE-IT, said William E. Boden, MD, professor of medicine at Boston University. Among the 132,203 patients in this group who were on statin treatment and at their target LDL cholesterol level, the 5-year rate of cardiovascular disease events was 8.5% in those with higher TG levels and 6.3% in those with levels below 150 mg/dL, a statistically significant 19% increased risk after adjustment for some potential confounders, Dr. Boden reported in a poster he presented at the meeting. This analysis hinted at the magnitude of patients who are candidates for icosapent ethyl treatment based on REDUCE-IT, and the 19% residual increased risk they displayed showed what this treatment could address.
Analysis of another database identified 16% of more than 24,000 patients with stable coronary artery disease in the CLARIFY registry who would qualify for icosapent ethyl treatment by matching the REDUCE-IT enrollment criteria (J Am Coll Cardiol. 2019 March;73[11];doi: 10.1016/j.jacc.2019.01.016).
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Bhatt is an adviser to Cardax, PhaseBio, and Regado Biosciences, he is on the board of TobeSoft, and he has received research funding from several companies. Dr. Boden reported no disclosures.
NEW ORLEANS – The success of icosapent ethyl in cutting triglyceride levels and reducing cardiovascular disease events in at-risk patients in the REDUCE-IT trial may make clinicians rethink the threshold for an unhealthy triglyceride level that merits intervention.
Study results are also showing that the patients enrolled in REDUCE-IT are common, with apparently millions of Americans who could potentially receive the icosapent ethyl–processed fish oil used in the study if the Food and Drug Administration were to approve new labeling for the drug that the manufacturer filed for in late March 2019. Icosapent ethyl (Vascepa) already has U.S. marketing approval for reducing triglyceride (TG) levels in patients with baseline values of 500 mg/dL or greater, while the REDUCE-IT trial enrolled patients with established cardiovascular disease or diabetes plus at least one more risk factor with a TG level of 150-499 mg/dL. REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) enrolled only patients already on statin treatment and with a LDL cholesterol level of 41-100 mg/dL.
In reality, the clinicians who enrolled the 8,139 participants at 473 worldwide sites included patients with a TG level as low as 81 mg/dL, and 10% of entered patients had levels below the minimum threshold in the trial’s written design of at least 150 mg/dL. Initial results reported with the primary endpoint finding suggested that the icosapent ethyl treatment benefit extended to these patients who entered with what are currently considered normal TG values, and additional analyses reported by the study’s lead investigator, Deepak L. Bhatt, MD, which used a larger endpoint dataset that included total cardiovascular events rather than just first events, further confirmed that patients with lower baseline TG levels had reductions in their cardiovascular disease events that matched what was seen in patients who entered with substantially higher TG levels.
In the analysis that included total events, the tertile of patients with a baseline TG of 81-190 mg/dL had a statistically significant 26% relative reduction in events during an average 3.5-year follow-up, compared with the tertile of patients with a baseline level of 251 mg/dL or higher, who had a 40% reduction in their events during follow-up, reported Dr. Bhatt, professor of medicine at Harvard Medical School, Boston.
“We had patients [in REDUCE-IT] with lower triglycerides than the inclusion criteria. This shows that the study results apply to a broader range of patients,” he said in a talk at the annual meeting of the American College of Cardiology. “The total-event analysis gives us an appreciation of the large burden of ischemic events that statin-treated patients still have with baseline triglyceride levels of about 100 mg/dL.” Further analysis of the REDUCE-IT data, as well as future studies of TG-lowering drugs like icosapent ethyl, “may help redefine normal TG levels” in a manner similar to what happened over a 2-decade span as serial studies of statins and other drugs that reduced levels of LDL cholesterol led to incremental reductions in goal lipid levels.
In addition to providing greater precision in defining the impact of icosapent ethyl on events in patients with lower baseline TG levels, the total-event analysis “provided a better sense of what is actually going on” with patients clinically as they experience multiple cardiovascular events during follow-up, as well as the impact of treatment on reducing health-related costs. Concurrently with Dr. Bhatt’s report of the total-event analysis at the meeting, some of the new findings he presented also appeared online (J Am Coll Cardiol. 2019 March 18. doi: 10.1016/j.jacc.2019.02.032).
Recent analyses have also begun to assess the scope of patients who could potentially receive icosapent ethyl based on the enrollment criteria of REDUCE-IT. One analysis of more than 1 million people in the U.S. Veterans Affairs Health System during 2010 identified 439,019 people on statin treatment and with an LDL cholesterol of 41-100 mg/dL, the cardiovascular disease history or risk pattern that matched the trial, and not on treatment that could reduce TG levels such as fish oil. Among these people, 30% had a TG level at or above 150 mg/dL that would have qualified them to enter REDUCE-IT, said William E. Boden, MD, professor of medicine at Boston University. Among the 132,203 patients in this group who were on statin treatment and at their target LDL cholesterol level, the 5-year rate of cardiovascular disease events was 8.5% in those with higher TG levels and 6.3% in those with levels below 150 mg/dL, a statistically significant 19% increased risk after adjustment for some potential confounders, Dr. Boden reported in a poster he presented at the meeting. This analysis hinted at the magnitude of patients who are candidates for icosapent ethyl treatment based on REDUCE-IT, and the 19% residual increased risk they displayed showed what this treatment could address.
Analysis of another database identified 16% of more than 24,000 patients with stable coronary artery disease in the CLARIFY registry who would qualify for icosapent ethyl treatment by matching the REDUCE-IT enrollment criteria (J Am Coll Cardiol. 2019 March;73[11];doi: 10.1016/j.jacc.2019.01.016).
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Bhatt is an adviser to Cardax, PhaseBio, and Regado Biosciences, he is on the board of TobeSoft, and he has received research funding from several companies. Dr. Boden reported no disclosures.
REPORTING FROM ACC 2019
























