User login
What constitutes a clinically meaningful reduction in seizure frequency?
NEW ORLEANS – according to a study described at the annual meeting of the American Epilepsy Society. A reduction in seizure frequency of between 60% and 68% is associated with Clinical Global Impression of Improvement (CGI-I) ratings of “very much improved,” as assessed by caregivers and investigators.
“Further analyses from other phase III studies in Dravet syndrome and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as nonseizure outcomes and tolerability,” said Arnold Gammaitoni, PharmD, vice president of medical and scientific affairs at Zogenix in San Diego, and his colleagues.
A 50% reduction in seizure frequency is conventionally considered to be the cutoff for a clinically meaningful change. To develop an evidence-based definition of clinically meaningful seizure reduction, Dr. Gammaitoni and colleagues examined data from a phase III, randomized, double-blind, placebo-controlled trial of fenfluramine HCl oral solution for the adjunctive treatment of seizures associated with Dravet syndrome. The investigators took an anchor-based approach and examined the percentage change in seizure frequency, along with caregiver and investigator CGI-I ratings.
A total of 119 patients with Dravet syndrome were enrolled and randomized in equal groups to placebo, 0.2 mg/kg per day of fenfluramine HCl, or 0.8 mg/kg per day of fenfluramine HCl. After a 2-week titration period, patients entered a 12-week maintenance period. Patients in the 0.8-mg/kg per day group had a 63.9% greater reduction in seizure frequency than controls did.
After the 14-week titration and maintenance period, caregivers and investigators rated the change in participants’ clinical status from baseline, using the CGI-I scale, on which responses range from 1 (very much improved) to 7 (very much worse). The investigators considered patients with CGI-I scores of 1 or 2 (much improved) to have achieved a clinically meaningful response. A score of 3 (minimally improved) was not considered meaningful. The researchers pooled the results of the three treatment groups for this analysis. They estimated the clinically meaningful percentage change in seizure frequency using receiver operating characteristic analysis of binary CGI-I score, compared with percentage change in seizure frequency, and defined it as the cut-point for which specificity and sensitivity were equal or most similar.
Caregivers and investigators provided CGI-I assessments for 112 patients and 114 patients, respectively. The receiver operating characteristic analysis identified a 44% reduction in seizure frequency as a clinically meaningful cutoff point for caregiver and investigator assessments. Using this threshold, 75%, 46%, and 12.5% of patients in the 0.8-mg/kg per day, 0.2-mg/kg per day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in the phase III study.
“The use of external anchors is one method to define a clinically meaningful change in seizure frequency,” said Dr. Gammaitoni. “Having a defined minimum clinically important difference like this allows clinicians to assess impacts of treatments on an individual patient basis.... This is a chance for others to do similar types of analyses to confirm the findings that we have had in this first study with bigger data sets, in terms of using external anchors and data to define what a clinically meaningful change is.”
Zogenix, which is developing the fenfluramine formulation examined in this study, provided funding for this research.
SOURCE: Nabbout R et al. AES 2018, Abstract 3.202.
NEW ORLEANS – according to a study described at the annual meeting of the American Epilepsy Society. A reduction in seizure frequency of between 60% and 68% is associated with Clinical Global Impression of Improvement (CGI-I) ratings of “very much improved,” as assessed by caregivers and investigators.
“Further analyses from other phase III studies in Dravet syndrome and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as nonseizure outcomes and tolerability,” said Arnold Gammaitoni, PharmD, vice president of medical and scientific affairs at Zogenix in San Diego, and his colleagues.
A 50% reduction in seizure frequency is conventionally considered to be the cutoff for a clinically meaningful change. To develop an evidence-based definition of clinically meaningful seizure reduction, Dr. Gammaitoni and colleagues examined data from a phase III, randomized, double-blind, placebo-controlled trial of fenfluramine HCl oral solution for the adjunctive treatment of seizures associated with Dravet syndrome. The investigators took an anchor-based approach and examined the percentage change in seizure frequency, along with caregiver and investigator CGI-I ratings.
A total of 119 patients with Dravet syndrome were enrolled and randomized in equal groups to placebo, 0.2 mg/kg per day of fenfluramine HCl, or 0.8 mg/kg per day of fenfluramine HCl. After a 2-week titration period, patients entered a 12-week maintenance period. Patients in the 0.8-mg/kg per day group had a 63.9% greater reduction in seizure frequency than controls did.
After the 14-week titration and maintenance period, caregivers and investigators rated the change in participants’ clinical status from baseline, using the CGI-I scale, on which responses range from 1 (very much improved) to 7 (very much worse). The investigators considered patients with CGI-I scores of 1 or 2 (much improved) to have achieved a clinically meaningful response. A score of 3 (minimally improved) was not considered meaningful. The researchers pooled the results of the three treatment groups for this analysis. They estimated the clinically meaningful percentage change in seizure frequency using receiver operating characteristic analysis of binary CGI-I score, compared with percentage change in seizure frequency, and defined it as the cut-point for which specificity and sensitivity were equal or most similar.
Caregivers and investigators provided CGI-I assessments for 112 patients and 114 patients, respectively. The receiver operating characteristic analysis identified a 44% reduction in seizure frequency as a clinically meaningful cutoff point for caregiver and investigator assessments. Using this threshold, 75%, 46%, and 12.5% of patients in the 0.8-mg/kg per day, 0.2-mg/kg per day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in the phase III study.
“The use of external anchors is one method to define a clinically meaningful change in seizure frequency,” said Dr. Gammaitoni. “Having a defined minimum clinically important difference like this allows clinicians to assess impacts of treatments on an individual patient basis.... This is a chance for others to do similar types of analyses to confirm the findings that we have had in this first study with bigger data sets, in terms of using external anchors and data to define what a clinically meaningful change is.”
Zogenix, which is developing the fenfluramine formulation examined in this study, provided funding for this research.
SOURCE: Nabbout R et al. AES 2018, Abstract 3.202.
NEW ORLEANS – according to a study described at the annual meeting of the American Epilepsy Society. A reduction in seizure frequency of between 60% and 68% is associated with Clinical Global Impression of Improvement (CGI-I) ratings of “very much improved,” as assessed by caregivers and investigators.
“Further analyses from other phase III studies in Dravet syndrome and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as nonseizure outcomes and tolerability,” said Arnold Gammaitoni, PharmD, vice president of medical and scientific affairs at Zogenix in San Diego, and his colleagues.
A 50% reduction in seizure frequency is conventionally considered to be the cutoff for a clinically meaningful change. To develop an evidence-based definition of clinically meaningful seizure reduction, Dr. Gammaitoni and colleagues examined data from a phase III, randomized, double-blind, placebo-controlled trial of fenfluramine HCl oral solution for the adjunctive treatment of seizures associated with Dravet syndrome. The investigators took an anchor-based approach and examined the percentage change in seizure frequency, along with caregiver and investigator CGI-I ratings.
A total of 119 patients with Dravet syndrome were enrolled and randomized in equal groups to placebo, 0.2 mg/kg per day of fenfluramine HCl, or 0.8 mg/kg per day of fenfluramine HCl. After a 2-week titration period, patients entered a 12-week maintenance period. Patients in the 0.8-mg/kg per day group had a 63.9% greater reduction in seizure frequency than controls did.
After the 14-week titration and maintenance period, caregivers and investigators rated the change in participants’ clinical status from baseline, using the CGI-I scale, on which responses range from 1 (very much improved) to 7 (very much worse). The investigators considered patients with CGI-I scores of 1 or 2 (much improved) to have achieved a clinically meaningful response. A score of 3 (minimally improved) was not considered meaningful. The researchers pooled the results of the three treatment groups for this analysis. They estimated the clinically meaningful percentage change in seizure frequency using receiver operating characteristic analysis of binary CGI-I score, compared with percentage change in seizure frequency, and defined it as the cut-point for which specificity and sensitivity were equal or most similar.
Caregivers and investigators provided CGI-I assessments for 112 patients and 114 patients, respectively. The receiver operating characteristic analysis identified a 44% reduction in seizure frequency as a clinically meaningful cutoff point for caregiver and investigator assessments. Using this threshold, 75%, 46%, and 12.5% of patients in the 0.8-mg/kg per day, 0.2-mg/kg per day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in the phase III study.
“The use of external anchors is one method to define a clinically meaningful change in seizure frequency,” said Dr. Gammaitoni. “Having a defined minimum clinically important difference like this allows clinicians to assess impacts of treatments on an individual patient basis.... This is a chance for others to do similar types of analyses to confirm the findings that we have had in this first study with bigger data sets, in terms of using external anchors and data to define what a clinically meaningful change is.”
Zogenix, which is developing the fenfluramine formulation examined in this study, provided funding for this research.
SOURCE: Nabbout R et al. AES 2018, Abstract 3.202.
REPORTING FROM AES 2018
Key clinical point: Data support the convention of considering a 50% reduction in seizure frequency as the cutoff for a clinically meaningful change.
Major finding: Statistical analysis indicates that a 44% reduction in seizure frequency is clinically meaningful.
Study details: A phase III, randomized, double-blind, placebo-controlled clinical trial of fenfluramine HCl that included 119 patients.
Disclosures: Zogenix provided funding for the study.
Source: Nabbout R et al. Abstract 3.202.
Resection, neurostimulation combo found successful in eloquent cortical regions
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
REPORTING FROM AES 2018
Key clinical point:
Major finding: Two patients became seizure free and one had a 62% reduction in seizures.
Study details: A three-patient case series.
Disclosures: NeuroPace makes the neurostimulator used in the study. The presenter is an employee of NeuroPace.
Source: Razavi B et al. AES 2018, Abstract 2.315.
New and established AEDs have similar tolerability
NEW ORLEANS – according to an analysis presented at the annual meeting of the American Epilepsy Society. Approximately one-third of patients with epilepsy discontinue their AEDs because of adverse drug reactions, according to the researchers. An increasing number of concomitant AEDs is associated with decreasing tolerability.
Previous research by Patrick Kwan, MBBChir, PhD, chair of neurology at the University of Melbourne and his colleagues indicated that the introduction of AEDs with new mechanisms of action in the past two decades has not changed seizure outcome overall in newly diagnosed epilepsy. Researchers had not studied the long-term tolerability of AEDs, however.
Dr. Kwan, Zhibin Chen, PhD, a biostatistician at the University of Melbourne, and their colleagues examined AED-induced adverse drug reactions over a 30-year period. They analyzed data for adults who were newly treated with AEDs at the epilepsy unit of the Western Infirmary in Glasgow during July 1, 1982–Oct. 31, 2012. All patients were followed prospectively until April 30, 2016, or death. The researchers systematically reviewed patient-reported adverse drug reactions and categorized them with the Medical Dictionary for Regulatory Activities. They defined adverse reactions that resulted in AED discontinuation as intolerable.
The investigators included 1,527 patients in their analysis. Approximately 56% of the sample was male, and the median age was 37 years. Participants tried a total of 2,766 AED regimens, including 2,028 (73%) as monotherapy and 738 (27%) as combination therapy. Among the monotherapies, 927 (46%) were established AEDs, and 1,101 (54%) were newer AEDs.
In all, 675 (44%) patients reported adverse drug reactions. These reports included 391 (26%) patients with nervous system disorders (e.g., tremor, sedation, and headaches), 272 (18%) with general disorders (e.g., fatigue, ataxia, and irritability), and 136 (9%) with psychiatric disorders (e.g., aggression, depression, and mood swings). A total of 498 (33%) patients had at least one intolerable adverse drug reaction.
The established and newer AEDs, when taken as monotherapy, had similar rates of intolerable adverse drug reactions (odds ratio, 1.09).The crude rate of intolerable adverse drug reactions appeared to increase for each additional AED regimen tried. Multivariable analysis indicated that women were more likely to report intolerable adverse drug reactions than men.
Compared with patients taking monotherapy, patients taking two AEDs had 1.67 times the risk of developing an intolerable adverse drug reaction, after data adjustments for number of previous AED regimens tried, previous intolerable adverse drug reaction, age, sex, pretreatment psychiatric comorbidity, and epilepsy type. The odds increased further in patients on three AEDs (OR, 2.38) and four AEDs (OR, 5.24). Patients who had intolerable adverse drug reactions to previous AED regimens had much greater odds of experiencing a further event (OR, 22.7).
After considering all the above factors, the researchers found that the odds of intolerable adverse drug reactions decreased for each additional AED regimen. When analyzing the 642 patients who took more than one AED regimen, they found that those who failed the first AED because of adverse drug reactions were more likely to develop intolerable adverse drug reactions to subsequent regimens (OR, 5.09). The odds of drug withdrawal because of adverse drug reaction increased 12-fold for each additional previous intolerable adverse drug reaction (OR, 13.3).
The investigators received no funding for this study.
This article was updated 12/4/18.
SOURCE: Alsfouk B et al. AES 2018, Abstract 2.275.
NEW ORLEANS – according to an analysis presented at the annual meeting of the American Epilepsy Society. Approximately one-third of patients with epilepsy discontinue their AEDs because of adverse drug reactions, according to the researchers. An increasing number of concomitant AEDs is associated with decreasing tolerability.
Previous research by Patrick Kwan, MBBChir, PhD, chair of neurology at the University of Melbourne and his colleagues indicated that the introduction of AEDs with new mechanisms of action in the past two decades has not changed seizure outcome overall in newly diagnosed epilepsy. Researchers had not studied the long-term tolerability of AEDs, however.
Dr. Kwan, Zhibin Chen, PhD, a biostatistician at the University of Melbourne, and their colleagues examined AED-induced adverse drug reactions over a 30-year period. They analyzed data for adults who were newly treated with AEDs at the epilepsy unit of the Western Infirmary in Glasgow during July 1, 1982–Oct. 31, 2012. All patients were followed prospectively until April 30, 2016, or death. The researchers systematically reviewed patient-reported adverse drug reactions and categorized them with the Medical Dictionary for Regulatory Activities. They defined adverse reactions that resulted in AED discontinuation as intolerable.
The investigators included 1,527 patients in their analysis. Approximately 56% of the sample was male, and the median age was 37 years. Participants tried a total of 2,766 AED regimens, including 2,028 (73%) as monotherapy and 738 (27%) as combination therapy. Among the monotherapies, 927 (46%) were established AEDs, and 1,101 (54%) were newer AEDs.
In all, 675 (44%) patients reported adverse drug reactions. These reports included 391 (26%) patients with nervous system disorders (e.g., tremor, sedation, and headaches), 272 (18%) with general disorders (e.g., fatigue, ataxia, and irritability), and 136 (9%) with psychiatric disorders (e.g., aggression, depression, and mood swings). A total of 498 (33%) patients had at least one intolerable adverse drug reaction.
The established and newer AEDs, when taken as monotherapy, had similar rates of intolerable adverse drug reactions (odds ratio, 1.09).The crude rate of intolerable adverse drug reactions appeared to increase for each additional AED regimen tried. Multivariable analysis indicated that women were more likely to report intolerable adverse drug reactions than men.
Compared with patients taking monotherapy, patients taking two AEDs had 1.67 times the risk of developing an intolerable adverse drug reaction, after data adjustments for number of previous AED regimens tried, previous intolerable adverse drug reaction, age, sex, pretreatment psychiatric comorbidity, and epilepsy type. The odds increased further in patients on three AEDs (OR, 2.38) and four AEDs (OR, 5.24). Patients who had intolerable adverse drug reactions to previous AED regimens had much greater odds of experiencing a further event (OR, 22.7).
After considering all the above factors, the researchers found that the odds of intolerable adverse drug reactions decreased for each additional AED regimen. When analyzing the 642 patients who took more than one AED regimen, they found that those who failed the first AED because of adverse drug reactions were more likely to develop intolerable adverse drug reactions to subsequent regimens (OR, 5.09). The odds of drug withdrawal because of adverse drug reaction increased 12-fold for each additional previous intolerable adverse drug reaction (OR, 13.3).
The investigators received no funding for this study.
This article was updated 12/4/18.
SOURCE: Alsfouk B et al. AES 2018, Abstract 2.275.
NEW ORLEANS – according to an analysis presented at the annual meeting of the American Epilepsy Society. Approximately one-third of patients with epilepsy discontinue their AEDs because of adverse drug reactions, according to the researchers. An increasing number of concomitant AEDs is associated with decreasing tolerability.
Previous research by Patrick Kwan, MBBChir, PhD, chair of neurology at the University of Melbourne and his colleagues indicated that the introduction of AEDs with new mechanisms of action in the past two decades has not changed seizure outcome overall in newly diagnosed epilepsy. Researchers had not studied the long-term tolerability of AEDs, however.
Dr. Kwan, Zhibin Chen, PhD, a biostatistician at the University of Melbourne, and their colleagues examined AED-induced adverse drug reactions over a 30-year period. They analyzed data for adults who were newly treated with AEDs at the epilepsy unit of the Western Infirmary in Glasgow during July 1, 1982–Oct. 31, 2012. All patients were followed prospectively until April 30, 2016, or death. The researchers systematically reviewed patient-reported adverse drug reactions and categorized them with the Medical Dictionary for Regulatory Activities. They defined adverse reactions that resulted in AED discontinuation as intolerable.
The investigators included 1,527 patients in their analysis. Approximately 56% of the sample was male, and the median age was 37 years. Participants tried a total of 2,766 AED regimens, including 2,028 (73%) as monotherapy and 738 (27%) as combination therapy. Among the monotherapies, 927 (46%) were established AEDs, and 1,101 (54%) were newer AEDs.
In all, 675 (44%) patients reported adverse drug reactions. These reports included 391 (26%) patients with nervous system disorders (e.g., tremor, sedation, and headaches), 272 (18%) with general disorders (e.g., fatigue, ataxia, and irritability), and 136 (9%) with psychiatric disorders (e.g., aggression, depression, and mood swings). A total of 498 (33%) patients had at least one intolerable adverse drug reaction.
The established and newer AEDs, when taken as monotherapy, had similar rates of intolerable adverse drug reactions (odds ratio, 1.09).The crude rate of intolerable adverse drug reactions appeared to increase for each additional AED regimen tried. Multivariable analysis indicated that women were more likely to report intolerable adverse drug reactions than men.
Compared with patients taking monotherapy, patients taking two AEDs had 1.67 times the risk of developing an intolerable adverse drug reaction, after data adjustments for number of previous AED regimens tried, previous intolerable adverse drug reaction, age, sex, pretreatment psychiatric comorbidity, and epilepsy type. The odds increased further in patients on three AEDs (OR, 2.38) and four AEDs (OR, 5.24). Patients who had intolerable adverse drug reactions to previous AED regimens had much greater odds of experiencing a further event (OR, 22.7).
After considering all the above factors, the researchers found that the odds of intolerable adverse drug reactions decreased for each additional AED regimen. When analyzing the 642 patients who took more than one AED regimen, they found that those who failed the first AED because of adverse drug reactions were more likely to develop intolerable adverse drug reactions to subsequent regimens (OR, 5.09). The odds of drug withdrawal because of adverse drug reaction increased 12-fold for each additional previous intolerable adverse drug reaction (OR, 13.3).
The investigators received no funding for this study.
This article was updated 12/4/18.
SOURCE: Alsfouk B et al. AES 2018, Abstract 2.275.
REPORTING FROM AES 2018
Key clinical point: Patients are no more likely to tolerate newer AEDs than established AEDs.
Major finding: One-third of patients discontinue AEDs because of adverse drug reactions.
Study details: A retrospective analysis of prospectively collected data for 1,527 patients with epilepsy.
Disclosures: The investigators received no funding.
Source: Alsfouk et al. AES 2018, Abstract 2.275.
Infertility appears to be increased among women with epilepsy
NEW ORLEANS – based on a retrospective study presented at the annual meeting of the American Epilepsy Society.
Data recorded in the 2010-2014 Epilepsy Birth Control Registry indicates a 9.2% infertility rate and a 22.5% impaired fecundity rate among American women with epilepsy. Both rates are higher than the general population infertility rate of 6.0% and the 12.1% rate of impaired fecundity cited by the Centers for Disease Control and Prevention.
However, differences between the study of women with epilepsy and the study of the general population may limit the validity of this comparison, said Devon B. MacEachern, clinical and research coordinator at Neuroendocrine Associates in Wellesley Hills, Mass.
It is likewise uncertain whether use of antiepileptic drugs (AEDs) affects women’s fertility or fecundity.
The Epilepsy Birth Control Registry collected data from an Internet-based survey of 1,144 community-dwelling women with epilepsy aged 18-47 years. Participants provided information about demographics, epilepsy, AEDs, reproduction, and contraception.
The researchers focused on rates of infertility, impaired fecundity, and live birth or unaborted pregnancy among 978 American women, and additionally examined whether these outcomes were related to AED use.
Infertility was defined as the percentage of participants who had unprotected sex but did not become pregnant by 1 year. Impaired fecundity was the percentage of participants who were infertile or did not carry a pregnancy to live birth. The study excluded from the impaired fecundity analysis the 41 respondents whose only outcomes were induced abortions. The 18% of pregnancies that terminated as induced abortions were excluded from the live birth rate analysis.
In all, 373 registry participants had 724 pregnancies and 422 births between 1981 and 2013. The women had an average of 2.15 pregnancies at a mean age of 24.9 years (range, 13-44 years). In addition, 38 women (9.2%) tried to conceive, but were infertile. Of 306 women with a first pregnancy, 222 (72.5%) had a live birth. Among 292 women with two pregnancies, 260 (89.0%) had at least one live birth, and 180 (61.6%) had two live births.
Of the 373 women, 84 (22.5%) with pregnancies had impaired fecundity. The risk of impaired fecundity tended to be higher among women on AED polytherapy than among women on no AED (risk ratio, 1.74).
The ratio of live births to pregnancy (71.0%) was similar among women on no AEDs (71.3%), those on AED monotherapy (71.8%), and those on polytherapy (69.7%). The live birth rate was 67.5% for women taking enzyme-inducing AEDs, 89.1% for women taking glucuronidated AEDs, 72.8% for women taking nonenzyme-inducing AEDs, 63.3% for women taking enzyme-inhibiting AEDs, and 69.7% for women on polytherapy. Lamotrigine use was associated with the highest ratio of live births to pregnancies at 89.1%; valproate use was associated with the lowest ratio of live births to pregnancies at 63.3%.
The investigation was funded by the Epilepsy Foundation and Lundbeck.
SOURCE: MacEachern DB et al. AES 2018, Abstract 1.426.
NEW ORLEANS – based on a retrospective study presented at the annual meeting of the American Epilepsy Society.
Data recorded in the 2010-2014 Epilepsy Birth Control Registry indicates a 9.2% infertility rate and a 22.5% impaired fecundity rate among American women with epilepsy. Both rates are higher than the general population infertility rate of 6.0% and the 12.1% rate of impaired fecundity cited by the Centers for Disease Control and Prevention.
However, differences between the study of women with epilepsy and the study of the general population may limit the validity of this comparison, said Devon B. MacEachern, clinical and research coordinator at Neuroendocrine Associates in Wellesley Hills, Mass.
It is likewise uncertain whether use of antiepileptic drugs (AEDs) affects women’s fertility or fecundity.
The Epilepsy Birth Control Registry collected data from an Internet-based survey of 1,144 community-dwelling women with epilepsy aged 18-47 years. Participants provided information about demographics, epilepsy, AEDs, reproduction, and contraception.
The researchers focused on rates of infertility, impaired fecundity, and live birth or unaborted pregnancy among 978 American women, and additionally examined whether these outcomes were related to AED use.
Infertility was defined as the percentage of participants who had unprotected sex but did not become pregnant by 1 year. Impaired fecundity was the percentage of participants who were infertile or did not carry a pregnancy to live birth. The study excluded from the impaired fecundity analysis the 41 respondents whose only outcomes were induced abortions. The 18% of pregnancies that terminated as induced abortions were excluded from the live birth rate analysis.
In all, 373 registry participants had 724 pregnancies and 422 births between 1981 and 2013. The women had an average of 2.15 pregnancies at a mean age of 24.9 years (range, 13-44 years). In addition, 38 women (9.2%) tried to conceive, but were infertile. Of 306 women with a first pregnancy, 222 (72.5%) had a live birth. Among 292 women with two pregnancies, 260 (89.0%) had at least one live birth, and 180 (61.6%) had two live births.
Of the 373 women, 84 (22.5%) with pregnancies had impaired fecundity. The risk of impaired fecundity tended to be higher among women on AED polytherapy than among women on no AED (risk ratio, 1.74).
The ratio of live births to pregnancy (71.0%) was similar among women on no AEDs (71.3%), those on AED monotherapy (71.8%), and those on polytherapy (69.7%). The live birth rate was 67.5% for women taking enzyme-inducing AEDs, 89.1% for women taking glucuronidated AEDs, 72.8% for women taking nonenzyme-inducing AEDs, 63.3% for women taking enzyme-inhibiting AEDs, and 69.7% for women on polytherapy. Lamotrigine use was associated with the highest ratio of live births to pregnancies at 89.1%; valproate use was associated with the lowest ratio of live births to pregnancies at 63.3%.
The investigation was funded by the Epilepsy Foundation and Lundbeck.
SOURCE: MacEachern DB et al. AES 2018, Abstract 1.426.
NEW ORLEANS – based on a retrospective study presented at the annual meeting of the American Epilepsy Society.
Data recorded in the 2010-2014 Epilepsy Birth Control Registry indicates a 9.2% infertility rate and a 22.5% impaired fecundity rate among American women with epilepsy. Both rates are higher than the general population infertility rate of 6.0% and the 12.1% rate of impaired fecundity cited by the Centers for Disease Control and Prevention.
However, differences between the study of women with epilepsy and the study of the general population may limit the validity of this comparison, said Devon B. MacEachern, clinical and research coordinator at Neuroendocrine Associates in Wellesley Hills, Mass.
It is likewise uncertain whether use of antiepileptic drugs (AEDs) affects women’s fertility or fecundity.
The Epilepsy Birth Control Registry collected data from an Internet-based survey of 1,144 community-dwelling women with epilepsy aged 18-47 years. Participants provided information about demographics, epilepsy, AEDs, reproduction, and contraception.
The researchers focused on rates of infertility, impaired fecundity, and live birth or unaborted pregnancy among 978 American women, and additionally examined whether these outcomes were related to AED use.
Infertility was defined as the percentage of participants who had unprotected sex but did not become pregnant by 1 year. Impaired fecundity was the percentage of participants who were infertile or did not carry a pregnancy to live birth. The study excluded from the impaired fecundity analysis the 41 respondents whose only outcomes were induced abortions. The 18% of pregnancies that terminated as induced abortions were excluded from the live birth rate analysis.
In all, 373 registry participants had 724 pregnancies and 422 births between 1981 and 2013. The women had an average of 2.15 pregnancies at a mean age of 24.9 years (range, 13-44 years). In addition, 38 women (9.2%) tried to conceive, but were infertile. Of 306 women with a first pregnancy, 222 (72.5%) had a live birth. Among 292 women with two pregnancies, 260 (89.0%) had at least one live birth, and 180 (61.6%) had two live births.
Of the 373 women, 84 (22.5%) with pregnancies had impaired fecundity. The risk of impaired fecundity tended to be higher among women on AED polytherapy than among women on no AED (risk ratio, 1.74).
The ratio of live births to pregnancy (71.0%) was similar among women on no AEDs (71.3%), those on AED monotherapy (71.8%), and those on polytherapy (69.7%). The live birth rate was 67.5% for women taking enzyme-inducing AEDs, 89.1% for women taking glucuronidated AEDs, 72.8% for women taking nonenzyme-inducing AEDs, 63.3% for women taking enzyme-inhibiting AEDs, and 69.7% for women on polytherapy. Lamotrigine use was associated with the highest ratio of live births to pregnancies at 89.1%; valproate use was associated with the lowest ratio of live births to pregnancies at 63.3%.
The investigation was funded by the Epilepsy Foundation and Lundbeck.
SOURCE: MacEachern DB et al. AES 2018, Abstract 1.426.
REPORTING FROM AES 2018
Key clinical point: Women with epilepsy may have more difficulty conceiving or carrying a pregnancy to term than women without epilepsy.
Major finding: The rate of infertility is 9.2% and the rate of impaired fecundity is 22.5% among women with epilepsy.
Study details: A retrospective analysis of 373 participants in the Epilepsy Birth Control Registry.
Disclosures: The investigation was funded by the Epilepsy Foundation and Lundbeck.
Source: MacEachern DB et al. AES 2018, Abstract 1.426.
Patients with PNES have increased mortality
NEW ORLEANS – according to data presented at the annual meeting of the American Epilepsy Society. Patients with PNES have a mortality rate comparable to that of patients with drug-resistant epilepsy.
“This [finding] emphasizes the importance of correct diagnosis and identification of relevant pathologies in order to avoid preventable deaths in an important group of patients, where medical attention is often inappropriately directed to a dramatic but ultimately irrelevant clinical feature of the condition,” said Russell Nightscales, a first-year medical student at the University of Melbourne.*
Although PNES sometimes is mistaken for epilepsy and treated accordingly, it is a form of conversion disorder. The elevated risk of death among patients with epilepsy is understood, but few researchers have studied mortality in patients with PNES.
Mr. Nightscales and his colleagues conducted a retrospective cohort study of patients who had been admitted for a comprehensive epilepsy evaluation to one of two tertiary hospital video EEG monitoring (VEM) units in Melbourne between Jan. 1, 1995, and Dec. 31, 2015. The investigators ascertained mortality and cause of death by linking patient data to the Australian National Death Index (NDI). When a coroner’s report was available, they refined the cause of death using information from the National Coronial Information System. Each patient’s diagnosis was based on the consensus opinion of experienced epileptologists at the Comprehensive Epilepsy Meeting following a review of the clinical history, VEM data, and investigations. The researchers compared mortality in patients with PNES, epilepsy, or both conditions. They extracted clinical data through medical record review. Finally, they determined lifetime history of psychiatric disorders through review of neuropsychiatric reports.
Of 3,152 patients who underwent VEM, the investigators included 2,076 patients in their analyses. Of this population, 631 patients had PNES, 1,339 had epilepsy, and 106 had both. The standardized mortality ratio (SMR) among patients with PNES was 2.6 times greater than among the general population. Patients with PNES between ages 30 and 39 had a ninefold higher risk of death, compared with the general population. The SMR of patients with epilepsy was 3.2. The investigators found no significant difference in the rate of mortality between any of the patient groups after excluding 17 patients with epilepsy and a known brain tumor at the time of VEM, who had a malignant neoplasm of the brain listed as their primary cause of death.
Death resulted from external causes in 20% of all deaths among patients with PNES and in 53% of deaths with a known cause among patients who died below the age of 50. Suicide accounted for 24% of deaths among patients with PNES in this age group. Neoplasia and cardiorespiratory causes were responsible for 51% of deaths with a known cause across all ages and 67% of those between ages 50 and 69. Among people with epilepsy, external causes accounted for 7% of all deaths. Neoplasia and cardiorespiratory causes were observed in 42% of people with epilepsy. Epilepsy was responsible for 28% of deaths with a known cause among patients with epilepsy
The research was funded by Australia’s National Health and Medical Research Council and the RMH Neuroscience Foundation.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 1.139.
*Correction 12/4/18: An earlier version of this article misstated the name of the presenter. Russell Nightscales presented this study.
NEW ORLEANS – according to data presented at the annual meeting of the American Epilepsy Society. Patients with PNES have a mortality rate comparable to that of patients with drug-resistant epilepsy.
“This [finding] emphasizes the importance of correct diagnosis and identification of relevant pathologies in order to avoid preventable deaths in an important group of patients, where medical attention is often inappropriately directed to a dramatic but ultimately irrelevant clinical feature of the condition,” said Russell Nightscales, a first-year medical student at the University of Melbourne.*
Although PNES sometimes is mistaken for epilepsy and treated accordingly, it is a form of conversion disorder. The elevated risk of death among patients with epilepsy is understood, but few researchers have studied mortality in patients with PNES.
Mr. Nightscales and his colleagues conducted a retrospective cohort study of patients who had been admitted for a comprehensive epilepsy evaluation to one of two tertiary hospital video EEG monitoring (VEM) units in Melbourne between Jan. 1, 1995, and Dec. 31, 2015. The investigators ascertained mortality and cause of death by linking patient data to the Australian National Death Index (NDI). When a coroner’s report was available, they refined the cause of death using information from the National Coronial Information System. Each patient’s diagnosis was based on the consensus opinion of experienced epileptologists at the Comprehensive Epilepsy Meeting following a review of the clinical history, VEM data, and investigations. The researchers compared mortality in patients with PNES, epilepsy, or both conditions. They extracted clinical data through medical record review. Finally, they determined lifetime history of psychiatric disorders through review of neuropsychiatric reports.
Of 3,152 patients who underwent VEM, the investigators included 2,076 patients in their analyses. Of this population, 631 patients had PNES, 1,339 had epilepsy, and 106 had both. The standardized mortality ratio (SMR) among patients with PNES was 2.6 times greater than among the general population. Patients with PNES between ages 30 and 39 had a ninefold higher risk of death, compared with the general population. The SMR of patients with epilepsy was 3.2. The investigators found no significant difference in the rate of mortality between any of the patient groups after excluding 17 patients with epilepsy and a known brain tumor at the time of VEM, who had a malignant neoplasm of the brain listed as their primary cause of death.
Death resulted from external causes in 20% of all deaths among patients with PNES and in 53% of deaths with a known cause among patients who died below the age of 50. Suicide accounted for 24% of deaths among patients with PNES in this age group. Neoplasia and cardiorespiratory causes were responsible for 51% of deaths with a known cause across all ages and 67% of those between ages 50 and 69. Among people with epilepsy, external causes accounted for 7% of all deaths. Neoplasia and cardiorespiratory causes were observed in 42% of people with epilepsy. Epilepsy was responsible for 28% of deaths with a known cause among patients with epilepsy
The research was funded by Australia’s National Health and Medical Research Council and the RMH Neuroscience Foundation.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 1.139.
*Correction 12/4/18: An earlier version of this article misstated the name of the presenter. Russell Nightscales presented this study.
NEW ORLEANS – according to data presented at the annual meeting of the American Epilepsy Society. Patients with PNES have a mortality rate comparable to that of patients with drug-resistant epilepsy.
“This [finding] emphasizes the importance of correct diagnosis and identification of relevant pathologies in order to avoid preventable deaths in an important group of patients, where medical attention is often inappropriately directed to a dramatic but ultimately irrelevant clinical feature of the condition,” said Russell Nightscales, a first-year medical student at the University of Melbourne.*
Although PNES sometimes is mistaken for epilepsy and treated accordingly, it is a form of conversion disorder. The elevated risk of death among patients with epilepsy is understood, but few researchers have studied mortality in patients with PNES.
Mr. Nightscales and his colleagues conducted a retrospective cohort study of patients who had been admitted for a comprehensive epilepsy evaluation to one of two tertiary hospital video EEG monitoring (VEM) units in Melbourne between Jan. 1, 1995, and Dec. 31, 2015. The investigators ascertained mortality and cause of death by linking patient data to the Australian National Death Index (NDI). When a coroner’s report was available, they refined the cause of death using information from the National Coronial Information System. Each patient’s diagnosis was based on the consensus opinion of experienced epileptologists at the Comprehensive Epilepsy Meeting following a review of the clinical history, VEM data, and investigations. The researchers compared mortality in patients with PNES, epilepsy, or both conditions. They extracted clinical data through medical record review. Finally, they determined lifetime history of psychiatric disorders through review of neuropsychiatric reports.
Of 3,152 patients who underwent VEM, the investigators included 2,076 patients in their analyses. Of this population, 631 patients had PNES, 1,339 had epilepsy, and 106 had both. The standardized mortality ratio (SMR) among patients with PNES was 2.6 times greater than among the general population. Patients with PNES between ages 30 and 39 had a ninefold higher risk of death, compared with the general population. The SMR of patients with epilepsy was 3.2. The investigators found no significant difference in the rate of mortality between any of the patient groups after excluding 17 patients with epilepsy and a known brain tumor at the time of VEM, who had a malignant neoplasm of the brain listed as their primary cause of death.
Death resulted from external causes in 20% of all deaths among patients with PNES and in 53% of deaths with a known cause among patients who died below the age of 50. Suicide accounted for 24% of deaths among patients with PNES in this age group. Neoplasia and cardiorespiratory causes were responsible for 51% of deaths with a known cause across all ages and 67% of those between ages 50 and 69. Among people with epilepsy, external causes accounted for 7% of all deaths. Neoplasia and cardiorespiratory causes were observed in 42% of people with epilepsy. Epilepsy was responsible for 28% of deaths with a known cause among patients with epilepsy
The research was funded by Australia’s National Health and Medical Research Council and the RMH Neuroscience Foundation.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 1.139.
*Correction 12/4/18: An earlier version of this article misstated the name of the presenter. Russell Nightscales presented this study.
REPORTING FROM AES 2018
Key clinical point: Mortality among patients with PNES is similar to that among patients with drug-resistant epilepsy.
Major finding: The standardized mortality ratio of patients with PNES is 2.6, compared with that of the general population.
Study details: A retrospective cohort study of 2,076 patients.
Disclosures: The research was funded by Australia’s National Health and Medical Research Council and the RMH Neuroscience Foundation.
Source: O’Brien TJ et al. AES 2018, Abstract 1.139.
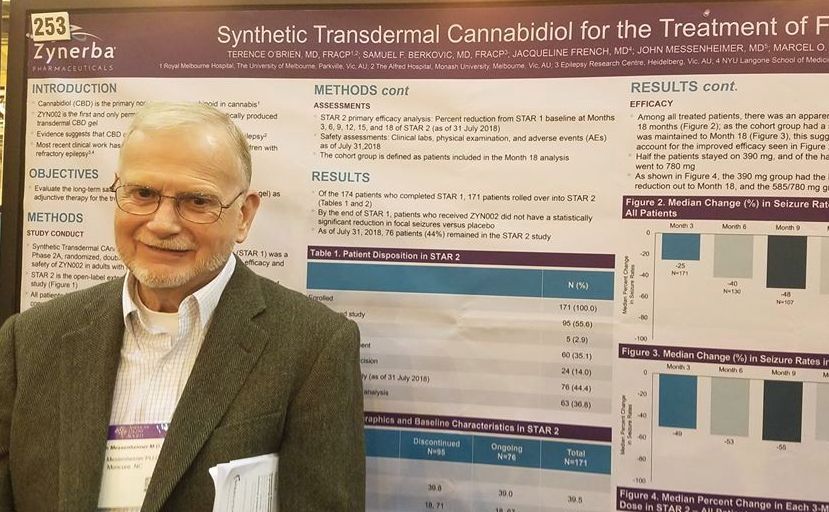
Transdermal CBD gel decreases recalcitrant focal seizures
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
REPORTING FROM AES 2018
Key clinical point:
Major finding: The gel reduced uncontrolled focal seizures by a median of about 50%.
Study details: The open-label extension study comprised 171 subjects.
Disclosures: Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
Source: O’Brien TJ et al. AES 2018, Abstract 2.253
Frontal lobe epilepsy elevates seizure risk during pregnancy
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
REPORTING FROM AES 2018
Key clinical point: Women with focal epilepsy, especially frontal lobe epilepsy, may need closer monitoring during pregnancy.
Major finding: Compared with baseline, seizures were more frequent during pregnancy in 53% of women with frontal lobe epilepsy.
Study details: An analysis of prospectively collected data from 114 pregnancies.
Disclosures: Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
Source: Voinescu PE et al. AES 2018, Abstract 3.236.
Program decreased seizure frequency for people with epilepsy
NEW ORLEANS – A self-management program that focused on medication adherence, sleep, nutrition, and stress reduction was associated with decreased seizures and improved quality of life for adults with epilepsy.
SMART (Self‐management for people with epilepsy and a history of negative health events) also was associated with improved depression scores and overall quality of life measures in participants, compared with a wait-listed control group, Martha Sajatovic, MD, said at the annual meeting of the American Epilepsy Society.
“I believe what we’re seeing is a result of improved self-management,” said Dr. Sajatovic, the Willard Brown Chair in Neurological Outcomes Research at Case Western Reserve University, Cleveland. “This is multimodal, including better medication adherence, which in turn is related to better communication with the clinician. For example, if patients are not sleeping well or their medicine makes them nauseated or they experience sexual dysfunction, we encourage them to talk to their docs about what they can live with, and what they can’t.”
Presented as a poster during the meeting, the SMART study was also published in Epilepsia.
SMART is an 8-week online educational program delivered by a nurse educator and a “peer educator,” a person with epilepsy who has had at least three negative health events. The first session is an in-person visit during which the team gets acquainted and discusses goals. The remaining sessions are self-paced and delivered on computer tablets provided by the investigators.
SMART didn’t just focus on the physical issues of living with epilepsy, Dr. Sajatovic said in an interview. Sessions also discussed the stigma still associated with the disorder, and myths that unnecessarily inflate perceptions. Discussions include goal setting, epilepsy complications and how to manage them, the importance of good sleep hygiene, problem-solving skills, nutrition and substance abuse, exercise, and how to deal with medication side effects.
“One thing we really stressed was sharing information in a way that was accessible to all patients and fostered self-motivation,” she said. “Most of our participants had never been in a program like this before. It was very empowering for many.”
The researchers chose participants who were socioeconomically challenged for this project; 88% made less than $25,000 per year and 74% were unemployed. The mean age of participants was 41 years, 70% were black, and most had been living with epilepsy at least half of their life. About 70% lived alone, and 70% had experienced at least one seizure within the month before enrolling. Mental health comorbidities were common; 69% had depression, 32% had anxiety, and 13% had PTSD.
The study enrolled 120 people, who were evenly divided between the intervention group and the wait-list group. The primary outcome was the change in total negative health events from baseline to the study’s end. Negative health events were seizures and ED or hospital admissions for any other causes including attempts at self-harm, falls, and accidents.
Secondary outcomes included changes in depression scores as measured by the Montgomery-Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire. Quality of life was measured using the 10-item Quality of Life in Epilepsy; functional status was measured using the 36-Item Short-Form Health Survey.
At baseline, the total mean 6-month negative health events count was 15, with 13 events being seizures. The other events were hospital or ED visits for other reasons.
At the end of the study, the intervention group experienced a significant mean decrease of 10 fewer negative health events, compared with a decrease of 2 in the wait-listed group. This was largely driven by a mean of 7.8 fewer seizures in the active group, compared with a decrease of about 1.0 in the wait-listed group. The 6-month ER and hospitalization counts did not significantly change.
Among the secondary outcomes, depression, overall health, and quality of life all improved significantly in the intervention group, compared with the wait-listed group. The intervention group also had significant decreases in depression measures and improvements in daily function measures, Dr. Sajatovic said.
“It was so gratifying to see this. Most of our participants had never been in a program like this before. It was a chance for them to take control of their epilepsy, instead of simply having it control them,” she said.
This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic had no financial disclosures related to this presentation.
SOURCE: Sajatovic M et al.
NEW ORLEANS – A self-management program that focused on medication adherence, sleep, nutrition, and stress reduction was associated with decreased seizures and improved quality of life for adults with epilepsy.
SMART (Self‐management for people with epilepsy and a history of negative health events) also was associated with improved depression scores and overall quality of life measures in participants, compared with a wait-listed control group, Martha Sajatovic, MD, said at the annual meeting of the American Epilepsy Society.
“I believe what we’re seeing is a result of improved self-management,” said Dr. Sajatovic, the Willard Brown Chair in Neurological Outcomes Research at Case Western Reserve University, Cleveland. “This is multimodal, including better medication adherence, which in turn is related to better communication with the clinician. For example, if patients are not sleeping well or their medicine makes them nauseated or they experience sexual dysfunction, we encourage them to talk to their docs about what they can live with, and what they can’t.”
Presented as a poster during the meeting, the SMART study was also published in Epilepsia.
SMART is an 8-week online educational program delivered by a nurse educator and a “peer educator,” a person with epilepsy who has had at least three negative health events. The first session is an in-person visit during which the team gets acquainted and discusses goals. The remaining sessions are self-paced and delivered on computer tablets provided by the investigators.
SMART didn’t just focus on the physical issues of living with epilepsy, Dr. Sajatovic said in an interview. Sessions also discussed the stigma still associated with the disorder, and myths that unnecessarily inflate perceptions. Discussions include goal setting, epilepsy complications and how to manage them, the importance of good sleep hygiene, problem-solving skills, nutrition and substance abuse, exercise, and how to deal with medication side effects.
“One thing we really stressed was sharing information in a way that was accessible to all patients and fostered self-motivation,” she said. “Most of our participants had never been in a program like this before. It was very empowering for many.”
The researchers chose participants who were socioeconomically challenged for this project; 88% made less than $25,000 per year and 74% were unemployed. The mean age of participants was 41 years, 70% were black, and most had been living with epilepsy at least half of their life. About 70% lived alone, and 70% had experienced at least one seizure within the month before enrolling. Mental health comorbidities were common; 69% had depression, 32% had anxiety, and 13% had PTSD.
The study enrolled 120 people, who were evenly divided between the intervention group and the wait-list group. The primary outcome was the change in total negative health events from baseline to the study’s end. Negative health events were seizures and ED or hospital admissions for any other causes including attempts at self-harm, falls, and accidents.
Secondary outcomes included changes in depression scores as measured by the Montgomery-Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire. Quality of life was measured using the 10-item Quality of Life in Epilepsy; functional status was measured using the 36-Item Short-Form Health Survey.
At baseline, the total mean 6-month negative health events count was 15, with 13 events being seizures. The other events were hospital or ED visits for other reasons.
At the end of the study, the intervention group experienced a significant mean decrease of 10 fewer negative health events, compared with a decrease of 2 in the wait-listed group. This was largely driven by a mean of 7.8 fewer seizures in the active group, compared with a decrease of about 1.0 in the wait-listed group. The 6-month ER and hospitalization counts did not significantly change.
Among the secondary outcomes, depression, overall health, and quality of life all improved significantly in the intervention group, compared with the wait-listed group. The intervention group also had significant decreases in depression measures and improvements in daily function measures, Dr. Sajatovic said.
“It was so gratifying to see this. Most of our participants had never been in a program like this before. It was a chance for them to take control of their epilepsy, instead of simply having it control them,” she said.
This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic had no financial disclosures related to this presentation.
SOURCE: Sajatovic M et al.
NEW ORLEANS – A self-management program that focused on medication adherence, sleep, nutrition, and stress reduction was associated with decreased seizures and improved quality of life for adults with epilepsy.
SMART (Self‐management for people with epilepsy and a history of negative health events) also was associated with improved depression scores and overall quality of life measures in participants, compared with a wait-listed control group, Martha Sajatovic, MD, said at the annual meeting of the American Epilepsy Society.
“I believe what we’re seeing is a result of improved self-management,” said Dr. Sajatovic, the Willard Brown Chair in Neurological Outcomes Research at Case Western Reserve University, Cleveland. “This is multimodal, including better medication adherence, which in turn is related to better communication with the clinician. For example, if patients are not sleeping well or their medicine makes them nauseated or they experience sexual dysfunction, we encourage them to talk to their docs about what they can live with, and what they can’t.”
Presented as a poster during the meeting, the SMART study was also published in Epilepsia.
SMART is an 8-week online educational program delivered by a nurse educator and a “peer educator,” a person with epilepsy who has had at least three negative health events. The first session is an in-person visit during which the team gets acquainted and discusses goals. The remaining sessions are self-paced and delivered on computer tablets provided by the investigators.
SMART didn’t just focus on the physical issues of living with epilepsy, Dr. Sajatovic said in an interview. Sessions also discussed the stigma still associated with the disorder, and myths that unnecessarily inflate perceptions. Discussions include goal setting, epilepsy complications and how to manage them, the importance of good sleep hygiene, problem-solving skills, nutrition and substance abuse, exercise, and how to deal with medication side effects.
“One thing we really stressed was sharing information in a way that was accessible to all patients and fostered self-motivation,” she said. “Most of our participants had never been in a program like this before. It was very empowering for many.”
The researchers chose participants who were socioeconomically challenged for this project; 88% made less than $25,000 per year and 74% were unemployed. The mean age of participants was 41 years, 70% were black, and most had been living with epilepsy at least half of their life. About 70% lived alone, and 70% had experienced at least one seizure within the month before enrolling. Mental health comorbidities were common; 69% had depression, 32% had anxiety, and 13% had PTSD.
The study enrolled 120 people, who were evenly divided between the intervention group and the wait-list group. The primary outcome was the change in total negative health events from baseline to the study’s end. Negative health events were seizures and ED or hospital admissions for any other causes including attempts at self-harm, falls, and accidents.
Secondary outcomes included changes in depression scores as measured by the Montgomery-Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire. Quality of life was measured using the 10-item Quality of Life in Epilepsy; functional status was measured using the 36-Item Short-Form Health Survey.
At baseline, the total mean 6-month negative health events count was 15, with 13 events being seizures. The other events were hospital or ED visits for other reasons.
At the end of the study, the intervention group experienced a significant mean decrease of 10 fewer negative health events, compared with a decrease of 2 in the wait-listed group. This was largely driven by a mean of 7.8 fewer seizures in the active group, compared with a decrease of about 1.0 in the wait-listed group. The 6-month ER and hospitalization counts did not significantly change.
Among the secondary outcomes, depression, overall health, and quality of life all improved significantly in the intervention group, compared with the wait-listed group. The intervention group also had significant decreases in depression measures and improvements in daily function measures, Dr. Sajatovic said.
“It was so gratifying to see this. Most of our participants had never been in a program like this before. It was a chance for them to take control of their epilepsy, instead of simply having it control them,” she said.
This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic had no financial disclosures related to this presentation.
SOURCE: Sajatovic M et al.
REPORTING FROM AES 2018
Key clinical point: Patients with epilepsy had fewer seizures and improved quality of life after 6 months of participation in a program that teaches self-management techniques.
Major finding: The intervention group had a mean of 7.8 fewer seizures, compared with their baseline count during the 6-month study.
Study details: The prospective study randomized 120 people to either the intervention group or a wait-list group.
Disclosures: This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic reported no financial disclosures related to this presentation.
Source: Sajatovic M et al. AES
Patients with epilepsy may develop tolerance to CBD-enriched oil
NEW ORLEANS – according to a study presented at the annual meeting of the American Epilepsy Society.
“CBD is a good option for children and adults with certain kinds of epilepsy, but as with antiepileptic drugs, it can become less effective over time, and the dose may need to be increased to manage the seizures,” said Shimrit Uliel-Sibony, MD, lead author of the study and head of the pediatric epilepsy service at Tel Aviv Sourasky Medical Center’s Dana-Dwek Children’s Hospital.
Prior studies have found that the efficacy of cannabinoids may wane when used for pain management. Efficacy also declines in animals with seizures.
To assess the tolerance rate of cannabinoids in the treatment of children and adults with epilepsy, researchers in Israel conducted a prospective review of 92 consecutive patients with treatment-resistant epilepsy. Patients were aged 1-37 years (mean age, 11.8 years) and were treated with cannabis oil extract during March 1, 2014–Dec. 31, 2017. The researchers defined tolerance as the need to increase the dose by at least 30% following a reduction in efficacy, or a more than 30% reduction in treatment response.
The patients had various forms of epilepsy (e.g., Dravet syndrome, Lennox-Gastaut syndrome, and epilepsy caused by stroke) and used cannabis oil extract for an average of 19.8 months. Of the 84 patients included in the tolerance analysis, 21 patients (25%) developed tolerance after an average of 7.3 months (range, 1-24 months) at an average dose of 12.6 mg/kg per day. After patients with tolerance received an increased dose, 4 patients returned to their previous response levels, and 10 patients had a response that was “satisfying but less than [the] prior response level,” Dr. Uliel-Sibony and colleagues said.
About a third of patients discontinued treatment because of side effects or lack of efficacy. Side effects included sleepiness, nausea, decreased appetite, and vomiting. In addition, seizures worsened in two patients, and one patient had signs of psychosis; treatment was stopped immediately in those three patients.
The investigators had no disclosures and received no funding for this study.
SOURCE: Uliel-Sibony S et al., AES 2018, Abstract 2.233.
NEW ORLEANS – according to a study presented at the annual meeting of the American Epilepsy Society.
“CBD is a good option for children and adults with certain kinds of epilepsy, but as with antiepileptic drugs, it can become less effective over time, and the dose may need to be increased to manage the seizures,” said Shimrit Uliel-Sibony, MD, lead author of the study and head of the pediatric epilepsy service at Tel Aviv Sourasky Medical Center’s Dana-Dwek Children’s Hospital.
Prior studies have found that the efficacy of cannabinoids may wane when used for pain management. Efficacy also declines in animals with seizures.
To assess the tolerance rate of cannabinoids in the treatment of children and adults with epilepsy, researchers in Israel conducted a prospective review of 92 consecutive patients with treatment-resistant epilepsy. Patients were aged 1-37 years (mean age, 11.8 years) and were treated with cannabis oil extract during March 1, 2014–Dec. 31, 2017. The researchers defined tolerance as the need to increase the dose by at least 30% following a reduction in efficacy, or a more than 30% reduction in treatment response.
The patients had various forms of epilepsy (e.g., Dravet syndrome, Lennox-Gastaut syndrome, and epilepsy caused by stroke) and used cannabis oil extract for an average of 19.8 months. Of the 84 patients included in the tolerance analysis, 21 patients (25%) developed tolerance after an average of 7.3 months (range, 1-24 months) at an average dose of 12.6 mg/kg per day. After patients with tolerance received an increased dose, 4 patients returned to their previous response levels, and 10 patients had a response that was “satisfying but less than [the] prior response level,” Dr. Uliel-Sibony and colleagues said.
About a third of patients discontinued treatment because of side effects or lack of efficacy. Side effects included sleepiness, nausea, decreased appetite, and vomiting. In addition, seizures worsened in two patients, and one patient had signs of psychosis; treatment was stopped immediately in those three patients.
The investigators had no disclosures and received no funding for this study.
SOURCE: Uliel-Sibony S et al., AES 2018, Abstract 2.233.
NEW ORLEANS – according to a study presented at the annual meeting of the American Epilepsy Society.
“CBD is a good option for children and adults with certain kinds of epilepsy, but as with antiepileptic drugs, it can become less effective over time, and the dose may need to be increased to manage the seizures,” said Shimrit Uliel-Sibony, MD, lead author of the study and head of the pediatric epilepsy service at Tel Aviv Sourasky Medical Center’s Dana-Dwek Children’s Hospital.
Prior studies have found that the efficacy of cannabinoids may wane when used for pain management. Efficacy also declines in animals with seizures.
To assess the tolerance rate of cannabinoids in the treatment of children and adults with epilepsy, researchers in Israel conducted a prospective review of 92 consecutive patients with treatment-resistant epilepsy. Patients were aged 1-37 years (mean age, 11.8 years) and were treated with cannabis oil extract during March 1, 2014–Dec. 31, 2017. The researchers defined tolerance as the need to increase the dose by at least 30% following a reduction in efficacy, or a more than 30% reduction in treatment response.
The patients had various forms of epilepsy (e.g., Dravet syndrome, Lennox-Gastaut syndrome, and epilepsy caused by stroke) and used cannabis oil extract for an average of 19.8 months. Of the 84 patients included in the tolerance analysis, 21 patients (25%) developed tolerance after an average of 7.3 months (range, 1-24 months) at an average dose of 12.6 mg/kg per day. After patients with tolerance received an increased dose, 4 patients returned to their previous response levels, and 10 patients had a response that was “satisfying but less than [the] prior response level,” Dr. Uliel-Sibony and colleagues said.
About a third of patients discontinued treatment because of side effects or lack of efficacy. Side effects included sleepiness, nausea, decreased appetite, and vomiting. In addition, seizures worsened in two patients, and one patient had signs of psychosis; treatment was stopped immediately in those three patients.
The investigators had no disclosures and received no funding for this study.
SOURCE: Uliel-Sibony S et al., AES 2018, Abstract 2.233.
REPORTING FROM AES 2018
Key clinical point: Cannabis oil extract may become less effective, and the dose may need to be increased to manage seizures.
Major finding: About a quarter of patients who received cannabis oil extract developed tolerance.
Study details: Prospective review of 92 consecutive patients with treatment-resistant epilepsy.
Disclosures: The investigators had no disclosures and received no funding for this study.
Source: Uliel-Sibony S et al. AES 2018, Abstract 2.233.
SUDEP risk may change over time
, based on study results presented at the annual meeting of the American Epilepsy Society.
Based on 3 years of data collected from over 12,000 people with epilepsy, 27.0% who had been at high risk (three or more generalized tonic-clonic seizures [GTCs] per year) at baseline moved out of the high-risk category. In addition, 32.5% at medium risk (one to two GTCs per year) at baseline changed categories. Finally, 7.0% in the low-risk category (no GTC seizures in the last year) at baseline moved to a higher-risk category.
“An individual’s risk [of SUDEP] is not set in stone,” said Neishay Ayub, MD, of Beth Israel Deaconess Medical Center, Boston, who presented the data at the meeting. “Our findings support the recommendation that for people with epilepsy who have ongoing generalized tonic-clonic seizures, the goal of treatment is to reduce GTCs and thereby lower SUDEP risk.”
A 2017 practice guideline summary from the American Academy of Neurology and the American Epilepsy Society identified the presence and frequency of GTCs and absence of seizure freedom as risk factors for SUDEP. Using these measures, Dr. Ayub and colleagues sought to stratify patients according to their risk of SUDEP and monitor how risk changed over time. They collected information about more than 1.4 million seizures that occurred from December 2007 to February 2018 in 12,402 users of the electronic diary Seizure Tracker.
For each user, the researchers calculated the number of generalized seizures for each year since the initial seizure diary entry. They categorized each user as being at low, medium, or high risk of SUDEP during each year. Low risk was defined as no generalized seizures in a year. Medium risk was defined as one or two generalized seizures in a year. High risk was defined as three or more generalized seizures in a year.
“The next step would be to see if we can confirm this patient-reported data with an objective study to determine when seizures did or did not occur,” said Daniel Goldenholz, MD, PhD, also of Beth Israel Deaconess Medical Center and senior author of the study. “For example, assessing information using new FDA [Food and Drug Administration]-approved wearable seizure tracker devices could give us a more comprehensive picture.”
The study was funded by the Harvard School of Public Health, Boston.
SOURCE: Ayub N et al. AES 2018, Abstract 2.158.
, based on study results presented at the annual meeting of the American Epilepsy Society.
Based on 3 years of data collected from over 12,000 people with epilepsy, 27.0% who had been at high risk (three or more generalized tonic-clonic seizures [GTCs] per year) at baseline moved out of the high-risk category. In addition, 32.5% at medium risk (one to two GTCs per year) at baseline changed categories. Finally, 7.0% in the low-risk category (no GTC seizures in the last year) at baseline moved to a higher-risk category.
“An individual’s risk [of SUDEP] is not set in stone,” said Neishay Ayub, MD, of Beth Israel Deaconess Medical Center, Boston, who presented the data at the meeting. “Our findings support the recommendation that for people with epilepsy who have ongoing generalized tonic-clonic seizures, the goal of treatment is to reduce GTCs and thereby lower SUDEP risk.”
A 2017 practice guideline summary from the American Academy of Neurology and the American Epilepsy Society identified the presence and frequency of GTCs and absence of seizure freedom as risk factors for SUDEP. Using these measures, Dr. Ayub and colleagues sought to stratify patients according to their risk of SUDEP and monitor how risk changed over time. They collected information about more than 1.4 million seizures that occurred from December 2007 to February 2018 in 12,402 users of the electronic diary Seizure Tracker.
For each user, the researchers calculated the number of generalized seizures for each year since the initial seizure diary entry. They categorized each user as being at low, medium, or high risk of SUDEP during each year. Low risk was defined as no generalized seizures in a year. Medium risk was defined as one or two generalized seizures in a year. High risk was defined as three or more generalized seizures in a year.
“The next step would be to see if we can confirm this patient-reported data with an objective study to determine when seizures did or did not occur,” said Daniel Goldenholz, MD, PhD, also of Beth Israel Deaconess Medical Center and senior author of the study. “For example, assessing information using new FDA [Food and Drug Administration]-approved wearable seizure tracker devices could give us a more comprehensive picture.”
The study was funded by the Harvard School of Public Health, Boston.
SOURCE: Ayub N et al. AES 2018, Abstract 2.158.
, based on study results presented at the annual meeting of the American Epilepsy Society.
Based on 3 years of data collected from over 12,000 people with epilepsy, 27.0% who had been at high risk (three or more generalized tonic-clonic seizures [GTCs] per year) at baseline moved out of the high-risk category. In addition, 32.5% at medium risk (one to two GTCs per year) at baseline changed categories. Finally, 7.0% in the low-risk category (no GTC seizures in the last year) at baseline moved to a higher-risk category.
“An individual’s risk [of SUDEP] is not set in stone,” said Neishay Ayub, MD, of Beth Israel Deaconess Medical Center, Boston, who presented the data at the meeting. “Our findings support the recommendation that for people with epilepsy who have ongoing generalized tonic-clonic seizures, the goal of treatment is to reduce GTCs and thereby lower SUDEP risk.”
A 2017 practice guideline summary from the American Academy of Neurology and the American Epilepsy Society identified the presence and frequency of GTCs and absence of seizure freedom as risk factors for SUDEP. Using these measures, Dr. Ayub and colleagues sought to stratify patients according to their risk of SUDEP and monitor how risk changed over time. They collected information about more than 1.4 million seizures that occurred from December 2007 to February 2018 in 12,402 users of the electronic diary Seizure Tracker.
For each user, the researchers calculated the number of generalized seizures for each year since the initial seizure diary entry. They categorized each user as being at low, medium, or high risk of SUDEP during each year. Low risk was defined as no generalized seizures in a year. Medium risk was defined as one or two generalized seizures in a year. High risk was defined as three or more generalized seizures in a year.
“The next step would be to see if we can confirm this patient-reported data with an objective study to determine when seizures did or did not occur,” said Daniel Goldenholz, MD, PhD, also of Beth Israel Deaconess Medical Center and senior author of the study. “For example, assessing information using new FDA [Food and Drug Administration]-approved wearable seizure tracker devices could give us a more comprehensive picture.”
The study was funded by the Harvard School of Public Health, Boston.
SOURCE: Ayub N et al. AES 2018, Abstract 2.158.
REPORTING FROM AES 2018
Key clinical point: Yearly patient risk assessments for sudden unexpected death in epilepsy are advisable.
Major finding: About 7% of people with no generalized tonic-clonic seizures in the last year at baseline moved to a higher-risk category.
Study details: An analysis of self-reported seizures by 12,402 users of Seizure Tracker.
Disclosures: The Harvard School of Public Health, Boston, funded the study.
Source: Ayub N et al. AES 2018, Abstract 2.158.