User login
Serum magnesium level reflects risk of death, irrespective of CKD
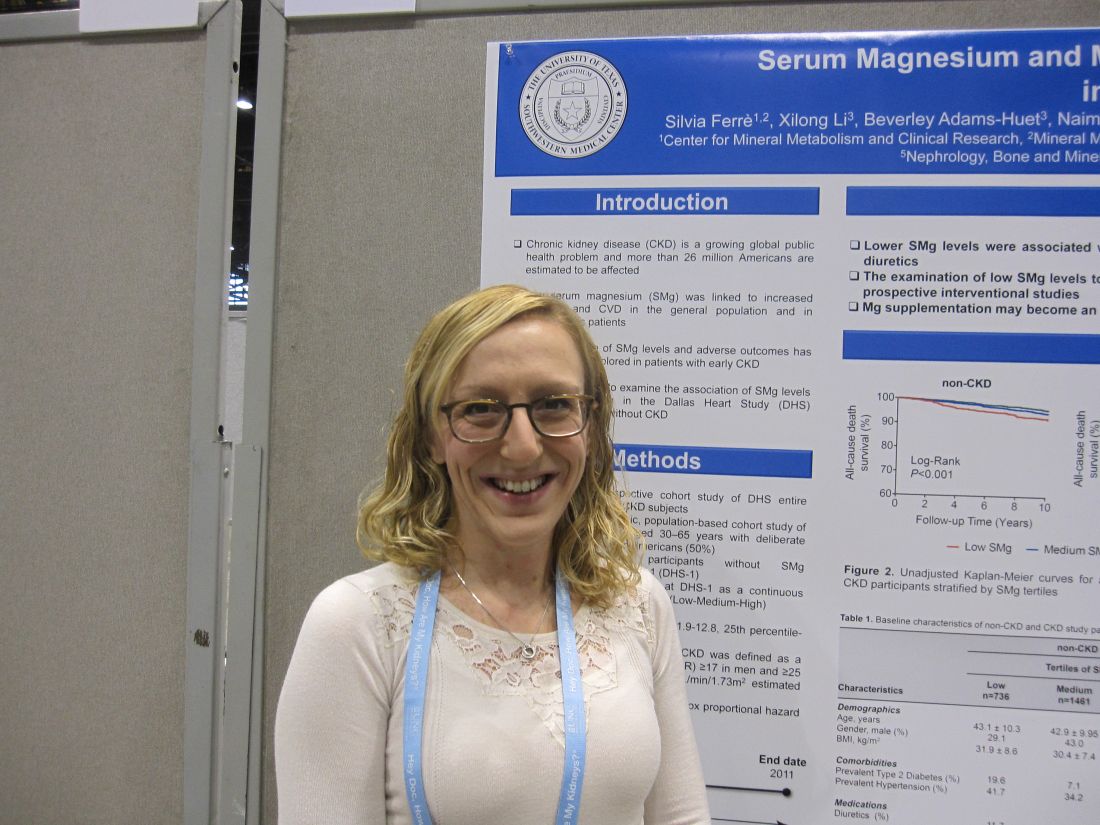
CHICAGO – Low levels of serum magnesium were associated with increased all-cause mortality, whether or not patients had chronic kidney disease, in a single center, retrospective study of 3,551 people.
The association was independent of sociodemographic factors, comorbidities, and use of diuretics.
If causality is shown, “magnesium supplementation could be a simple therapy to lessen the chance of death in CKD patients,” study investigator Silvia Ferrè, PhD, University of Texas Southwestern, Dallas, said in an interview regarding the results of the Dallas Heart Study, which was presented at the annual meeting of the American Society of Nephrology.
In both groups, the subjects with low serum magnesium were younger, more likely to be female, had a higher body mass index, and were more burdened by comorbidities including type 2 diabetes mellitus and hypertension. Subjects without CKD and low serum magnesium were significantly more likely to use diuretics. Diuretic use was comparable in subjects with CKD regardless of serum magnesium level.
Irrespective of CKD status, survival was significantly lower in subjects with low serum magnesium in the median 12.3-year follow-up compared to the other two serum magnesium tertiles (P less than 0.001 and P equal to 0.03, respectively). Following adjustment for age, gender, race/ethnicity, body mass index, phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone, total cholesterol, high-density lipoprotein, and use of diuretics and supplements, low serum magnesium was independently associated with all-cause death in subjects with CKD (Hazard Ratio, 1.92; 9%% Confidence Interval, 1.03 to 3.59; P equal to 0.04) and those without CKD (HR, 1.43; 1.43; 95% CI, 0.95 to 2.15; P equal to 0.09), when compared to high serum magnesium as the referent.
Dr. Ferrè said that screening for serum magnesium and supplementation with magnesium as part of routine blood testing might improve survival. Low magnesium level alsonhas been linked with osteoporosis, diabetes, and cardiovascular disease.
The Dallas Heart Study was a multiethnic, population-based study involving 6,101 adults residing in Dallas County. The study, which ran from 2000 to the end of 2011, was designed to explore the early detection of cardiovascular disease and the social, behavioral, and environmental factors associated with risk, with the goal of interventions that can be provided at the community level.
The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
CHICAGO – Low levels of serum magnesium were associated with increased all-cause mortality, whether or not patients had chronic kidney disease, in a single center, retrospective study of 3,551 people.
The association was independent of sociodemographic factors, comorbidities, and use of diuretics.
If causality is shown, “magnesium supplementation could be a simple therapy to lessen the chance of death in CKD patients,” study investigator Silvia Ferrè, PhD, University of Texas Southwestern, Dallas, said in an interview regarding the results of the Dallas Heart Study, which was presented at the annual meeting of the American Society of Nephrology.
In both groups, the subjects with low serum magnesium were younger, more likely to be female, had a higher body mass index, and were more burdened by comorbidities including type 2 diabetes mellitus and hypertension. Subjects without CKD and low serum magnesium were significantly more likely to use diuretics. Diuretic use was comparable in subjects with CKD regardless of serum magnesium level.
Irrespective of CKD status, survival was significantly lower in subjects with low serum magnesium in the median 12.3-year follow-up compared to the other two serum magnesium tertiles (P less than 0.001 and P equal to 0.03, respectively). Following adjustment for age, gender, race/ethnicity, body mass index, phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone, total cholesterol, high-density lipoprotein, and use of diuretics and supplements, low serum magnesium was independently associated with all-cause death in subjects with CKD (Hazard Ratio, 1.92; 9%% Confidence Interval, 1.03 to 3.59; P equal to 0.04) and those without CKD (HR, 1.43; 1.43; 95% CI, 0.95 to 2.15; P equal to 0.09), when compared to high serum magnesium as the referent.
Dr. Ferrè said that screening for serum magnesium and supplementation with magnesium as part of routine blood testing might improve survival. Low magnesium level alsonhas been linked with osteoporosis, diabetes, and cardiovascular disease.
The Dallas Heart Study was a multiethnic, population-based study involving 6,101 adults residing in Dallas County. The study, which ran from 2000 to the end of 2011, was designed to explore the early detection of cardiovascular disease and the social, behavioral, and environmental factors associated with risk, with the goal of interventions that can be provided at the community level.
The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
CHICAGO – Low levels of serum magnesium were associated with increased all-cause mortality, whether or not patients had chronic kidney disease, in a single center, retrospective study of 3,551 people.
The association was independent of sociodemographic factors, comorbidities, and use of diuretics.
If causality is shown, “magnesium supplementation could be a simple therapy to lessen the chance of death in CKD patients,” study investigator Silvia Ferrè, PhD, University of Texas Southwestern, Dallas, said in an interview regarding the results of the Dallas Heart Study, which was presented at the annual meeting of the American Society of Nephrology.
In both groups, the subjects with low serum magnesium were younger, more likely to be female, had a higher body mass index, and were more burdened by comorbidities including type 2 diabetes mellitus and hypertension. Subjects without CKD and low serum magnesium were significantly more likely to use diuretics. Diuretic use was comparable in subjects with CKD regardless of serum magnesium level.
Irrespective of CKD status, survival was significantly lower in subjects with low serum magnesium in the median 12.3-year follow-up compared to the other two serum magnesium tertiles (P less than 0.001 and P equal to 0.03, respectively). Following adjustment for age, gender, race/ethnicity, body mass index, phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone, total cholesterol, high-density lipoprotein, and use of diuretics and supplements, low serum magnesium was independently associated with all-cause death in subjects with CKD (Hazard Ratio, 1.92; 9%% Confidence Interval, 1.03 to 3.59; P equal to 0.04) and those without CKD (HR, 1.43; 1.43; 95% CI, 0.95 to 2.15; P equal to 0.09), when compared to high serum magnesium as the referent.
Dr. Ferrè said that screening for serum magnesium and supplementation with magnesium as part of routine blood testing might improve survival. Low magnesium level alsonhas been linked with osteoporosis, diabetes, and cardiovascular disease.
The Dallas Heart Study was a multiethnic, population-based study involving 6,101 adults residing in Dallas County. The study, which ran from 2000 to the end of 2011, was designed to explore the early detection of cardiovascular disease and the social, behavioral, and environmental factors associated with risk, with the goal of interventions that can be provided at the community level.
The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR NEPHROLOGY
Key clinical point: If causality is shown, magnesium supplementation could be a simple therapy to reduce mortality in patients with chronic kidney disease.
Major finding: Low serum magnesium was significantly associated with risk of death in patients without CKD (P less than 0.01) and patients with CKD (P equal to 0.03).
Data source: A single-center, retrospective cohort of 3,551 patients in the Dallas Heart Study.
Disclosures: The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
Constipation severity linked with chronic kidney disease and decline in kidney function
CHICAGO – Constipation was associated with poor kidney health in a large nationwide cohort of 3.5 million United States veterans, and researchers are considering whether effectively treating constipation could help prevent or treat kidney disease.
“In this large nationwide cohort ... patients with constipation had higher risks of developing chronic kidney disease and end-stage renal disease, and were more likely to experience rapid decline in kidney function, even after adjusting for various known risk factors. We also found that more severe constipation was associated with an incrementally higher risk for both incident CKD (chronic kidney disease) and ESRD (end-stage renal disease),” said Keiichi Sumida, MD, a visiting scholar at the University of Tennessee Health Science Center in Memphis.
In a multivariable analysis, those with constipation had a 13% higher likelihood of developing CKD (Hazard Ratio, 1.13; 95% Confidence Interval, 1.11 to 1.14) and a 9% higher likelihood of developing ESRD (HR, 1.09; 95% CI, 1.01 to 1.18) compared to those without constipation. As well, those with constipation experienced a faster decline in estimated glomerular filtration ratio (eGFR).
Scrutiny of US Veterans Administration databases identified nearly 4.5 million patients with serum creatinine measurements obtained between October 2004 and September 2006. Of these, 3,504,732 patients had an eGFR greater than or equal to 60 ml/min/1.73 m2 but no other symptoms of CKD. All were followed through 2013.
Constipation was defined as at least two ICD-9-CM diagnoses for constipation made at least 60 days apart or two or more prescriptions for laxatives separated by 60 days for up to a year. The severity of constipation was based on the number of different type of laxatives prescribed, with no laxative use being considered as absence of constipation, one laxative type being indicative of mild constipation, and two or more types of laxatives being indicative of severe constipation.
Co-primary outcomes were incident CKD, incident ESRD, and change in eGFR from baseline. As expected in the propensity-matched cohort, baseline demographic and clinical characteristics were comparable for the 3,251,291 individuals who experienced constipation and the 253,441 individuals who did not.
“Our findings highlight the plausible link between the gut and the kidneys, and provide additional insights into the pathogenesis of kidney disease progression. Our results suggest the need for careful observation of kidney function in patients with constipation, particularly among those with more severe constipation,” Dr. Sumida concluded.
Dr. Sumida hypothesized that altered gut microflora in constipation may result in inflammation, changes in metabolites, or accumulation of toxins. Alternative explanations increased serotonin related to laxative use, nephrotoxicity, dehydration, or electrolyte imbalance.
These possibilities need to be examined, as does the idea that relieving constipation could prevent renal decline. “Given the high prevalence of constipation in the general population and the simplicity of its assessment in primary care settings, the management of constipation through lifestyle modifications and/or use of probiotics rather than laxatives could become a useful tool in preventing the development of CKD, or in retarding the progression of existing CKD,” Dr. Sumida said.
CHICAGO – Constipation was associated with poor kidney health in a large nationwide cohort of 3.5 million United States veterans, and researchers are considering whether effectively treating constipation could help prevent or treat kidney disease.
“In this large nationwide cohort ... patients with constipation had higher risks of developing chronic kidney disease and end-stage renal disease, and were more likely to experience rapid decline in kidney function, even after adjusting for various known risk factors. We also found that more severe constipation was associated with an incrementally higher risk for both incident CKD (chronic kidney disease) and ESRD (end-stage renal disease),” said Keiichi Sumida, MD, a visiting scholar at the University of Tennessee Health Science Center in Memphis.
In a multivariable analysis, those with constipation had a 13% higher likelihood of developing CKD (Hazard Ratio, 1.13; 95% Confidence Interval, 1.11 to 1.14) and a 9% higher likelihood of developing ESRD (HR, 1.09; 95% CI, 1.01 to 1.18) compared to those without constipation. As well, those with constipation experienced a faster decline in estimated glomerular filtration ratio (eGFR).
Scrutiny of US Veterans Administration databases identified nearly 4.5 million patients with serum creatinine measurements obtained between October 2004 and September 2006. Of these, 3,504,732 patients had an eGFR greater than or equal to 60 ml/min/1.73 m2 but no other symptoms of CKD. All were followed through 2013.
Constipation was defined as at least two ICD-9-CM diagnoses for constipation made at least 60 days apart or two or more prescriptions for laxatives separated by 60 days for up to a year. The severity of constipation was based on the number of different type of laxatives prescribed, with no laxative use being considered as absence of constipation, one laxative type being indicative of mild constipation, and two or more types of laxatives being indicative of severe constipation.
Co-primary outcomes were incident CKD, incident ESRD, and change in eGFR from baseline. As expected in the propensity-matched cohort, baseline demographic and clinical characteristics were comparable for the 3,251,291 individuals who experienced constipation and the 253,441 individuals who did not.
“Our findings highlight the plausible link between the gut and the kidneys, and provide additional insights into the pathogenesis of kidney disease progression. Our results suggest the need for careful observation of kidney function in patients with constipation, particularly among those with more severe constipation,” Dr. Sumida concluded.
Dr. Sumida hypothesized that altered gut microflora in constipation may result in inflammation, changes in metabolites, or accumulation of toxins. Alternative explanations increased serotonin related to laxative use, nephrotoxicity, dehydration, or electrolyte imbalance.
These possibilities need to be examined, as does the idea that relieving constipation could prevent renal decline. “Given the high prevalence of constipation in the general population and the simplicity of its assessment in primary care settings, the management of constipation through lifestyle modifications and/or use of probiotics rather than laxatives could become a useful tool in preventing the development of CKD, or in retarding the progression of existing CKD,” Dr. Sumida said.
CHICAGO – Constipation was associated with poor kidney health in a large nationwide cohort of 3.5 million United States veterans, and researchers are considering whether effectively treating constipation could help prevent or treat kidney disease.
“In this large nationwide cohort ... patients with constipation had higher risks of developing chronic kidney disease and end-stage renal disease, and were more likely to experience rapid decline in kidney function, even after adjusting for various known risk factors. We also found that more severe constipation was associated with an incrementally higher risk for both incident CKD (chronic kidney disease) and ESRD (end-stage renal disease),” said Keiichi Sumida, MD, a visiting scholar at the University of Tennessee Health Science Center in Memphis.
In a multivariable analysis, those with constipation had a 13% higher likelihood of developing CKD (Hazard Ratio, 1.13; 95% Confidence Interval, 1.11 to 1.14) and a 9% higher likelihood of developing ESRD (HR, 1.09; 95% CI, 1.01 to 1.18) compared to those without constipation. As well, those with constipation experienced a faster decline in estimated glomerular filtration ratio (eGFR).
Scrutiny of US Veterans Administration databases identified nearly 4.5 million patients with serum creatinine measurements obtained between October 2004 and September 2006. Of these, 3,504,732 patients had an eGFR greater than or equal to 60 ml/min/1.73 m2 but no other symptoms of CKD. All were followed through 2013.
Constipation was defined as at least two ICD-9-CM diagnoses for constipation made at least 60 days apart or two or more prescriptions for laxatives separated by 60 days for up to a year. The severity of constipation was based on the number of different type of laxatives prescribed, with no laxative use being considered as absence of constipation, one laxative type being indicative of mild constipation, and two or more types of laxatives being indicative of severe constipation.
Co-primary outcomes were incident CKD, incident ESRD, and change in eGFR from baseline. As expected in the propensity-matched cohort, baseline demographic and clinical characteristics were comparable for the 3,251,291 individuals who experienced constipation and the 253,441 individuals who did not.
“Our findings highlight the plausible link between the gut and the kidneys, and provide additional insights into the pathogenesis of kidney disease progression. Our results suggest the need for careful observation of kidney function in patients with constipation, particularly among those with more severe constipation,” Dr. Sumida concluded.
Dr. Sumida hypothesized that altered gut microflora in constipation may result in inflammation, changes in metabolites, or accumulation of toxins. Alternative explanations increased serotonin related to laxative use, nephrotoxicity, dehydration, or electrolyte imbalance.
These possibilities need to be examined, as does the idea that relieving constipation could prevent renal decline. “Given the high prevalence of constipation in the general population and the simplicity of its assessment in primary care settings, the management of constipation through lifestyle modifications and/or use of probiotics rather than laxatives could become a useful tool in preventing the development of CKD, or in retarding the progression of existing CKD,” Dr. Sumida said.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR NEPHROLOGY
Key clinical point: Presence and severity of constipation increases the risks of developing chronic kidney disease and end stage renal disease, and accelerates the decline in kidney function.
Major finding: Individuals with constipation were 13% more likely to develop chronic kidney disease and 9% more likely to develop end stage renal disease compared to those without constipation.
Data source: Retrospective analysis of Veteran’s Administration databases. The study included 3,504,732 subjects.
Disclosures: The study sponsor was the University of Tennessee Health Science Center. Funding was provided by the United States Department of Veterans Affairs. Dr. Sumida reported having no financial disclosures.
Cell ratios predict short-term possibility of death in patients beginning hemodialysis
CHICAGO – Two simple-to-calculate ratios – neutrophil lymphocyte ratio and platelet lymphocyte ratio – may be able to predict impending death in patients who have recently begun hemodialysis, based on data from 108,548 incident hemodialysis patients in the database of DaVita HealthCare Partners from 2007 to 2011.
“Neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR), and inflammatory and nutritional indices, which are calculated from complete blood count, were identified as strong predictors of impending death ... and thus are inexpensive and immediately available markers for predicting short-term mortality,” said Yoshitsugu Obi, MD, PhD, a visiting scholar at the Harold Simmons Center for Kidney Disease Research & Epidemiology, University of California Irvine School of Medicine, Irvine, California.
The data were obtained from the database of a large dialysis organization; 108,548 patients who began hemodialysis from 2007 to 2011 were included. The range of NLR values were divided into 12 categories with ratios of less than 1.5 and greater than or equal to 6.5 as the bracketing ratios. The 10 other intervening ratios differed incrementally by 0.5. Eight SLR categories were created with the bracketing ratios being less than 5 and greater than or equal to 35. The intervening six ratios differed incrementally by 5.
The mean age of the cohort was 63 ± 15 years. Males predominated (56%), 59% of the subjects were diabetic, and 31% were African American. At baseline the median NLR and PLR were 3.64 and 13.12, respectively.
In an unadjusted regression analysis, the categories of NLR and PLR had a strong and linear relationship with all-cause mortality. In an analysis that adjusted for covariates, including demographics and comorbidities, as well as markers of malnutrition and inflammation, the association of the two ratios with all-cause mortality persisted.
Unlike previous small and inconclusive studies, the size of the present study makes robust the connection between these cell ratios and death in dialysis patients, he said. The plan now is to compare the mortality predictability of NLR and PLR with other established risk factors including albumin, phosphorus, and alkaline phosphatase.
CHICAGO – Two simple-to-calculate ratios – neutrophil lymphocyte ratio and platelet lymphocyte ratio – may be able to predict impending death in patients who have recently begun hemodialysis, based on data from 108,548 incident hemodialysis patients in the database of DaVita HealthCare Partners from 2007 to 2011.
“Neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR), and inflammatory and nutritional indices, which are calculated from complete blood count, were identified as strong predictors of impending death ... and thus are inexpensive and immediately available markers for predicting short-term mortality,” said Yoshitsugu Obi, MD, PhD, a visiting scholar at the Harold Simmons Center for Kidney Disease Research & Epidemiology, University of California Irvine School of Medicine, Irvine, California.
The data were obtained from the database of a large dialysis organization; 108,548 patients who began hemodialysis from 2007 to 2011 were included. The range of NLR values were divided into 12 categories with ratios of less than 1.5 and greater than or equal to 6.5 as the bracketing ratios. The 10 other intervening ratios differed incrementally by 0.5. Eight SLR categories were created with the bracketing ratios being less than 5 and greater than or equal to 35. The intervening six ratios differed incrementally by 5.
The mean age of the cohort was 63 ± 15 years. Males predominated (56%), 59% of the subjects were diabetic, and 31% were African American. At baseline the median NLR and PLR were 3.64 and 13.12, respectively.
In an unadjusted regression analysis, the categories of NLR and PLR had a strong and linear relationship with all-cause mortality. In an analysis that adjusted for covariates, including demographics and comorbidities, as well as markers of malnutrition and inflammation, the association of the two ratios with all-cause mortality persisted.
Unlike previous small and inconclusive studies, the size of the present study makes robust the connection between these cell ratios and death in dialysis patients, he said. The plan now is to compare the mortality predictability of NLR and PLR with other established risk factors including albumin, phosphorus, and alkaline phosphatase.
CHICAGO – Two simple-to-calculate ratios – neutrophil lymphocyte ratio and platelet lymphocyte ratio – may be able to predict impending death in patients who have recently begun hemodialysis, based on data from 108,548 incident hemodialysis patients in the database of DaVita HealthCare Partners from 2007 to 2011.
“Neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR), and inflammatory and nutritional indices, which are calculated from complete blood count, were identified as strong predictors of impending death ... and thus are inexpensive and immediately available markers for predicting short-term mortality,” said Yoshitsugu Obi, MD, PhD, a visiting scholar at the Harold Simmons Center for Kidney Disease Research & Epidemiology, University of California Irvine School of Medicine, Irvine, California.
The data were obtained from the database of a large dialysis organization; 108,548 patients who began hemodialysis from 2007 to 2011 were included. The range of NLR values were divided into 12 categories with ratios of less than 1.5 and greater than or equal to 6.5 as the bracketing ratios. The 10 other intervening ratios differed incrementally by 0.5. Eight SLR categories were created with the bracketing ratios being less than 5 and greater than or equal to 35. The intervening six ratios differed incrementally by 5.
The mean age of the cohort was 63 ± 15 years. Males predominated (56%), 59% of the subjects were diabetic, and 31% were African American. At baseline the median NLR and PLR were 3.64 and 13.12, respectively.
In an unadjusted regression analysis, the categories of NLR and PLR had a strong and linear relationship with all-cause mortality. In an analysis that adjusted for covariates, including demographics and comorbidities, as well as markers of malnutrition and inflammation, the association of the two ratios with all-cause mortality persisted.
Unlike previous small and inconclusive studies, the size of the present study makes robust the connection between these cell ratios and death in dialysis patients, he said. The plan now is to compare the mortality predictability of NLR and PLR with other established risk factors including albumin, phosphorus, and alkaline phosphatase.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR NEPHROLOGY
Key clinical point: The neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR) are strongly associated with imminent death in patients who have recently started hemodialysis.
Major finding: Increasing NLR and PLR were linearly associated with death in 108,548 hemodialysis patients.
Data source: Database of DaVita HealthCare Partners from 2007 to 2011.
Disclosures: The study was sponsored by University of Irvine School of Medicine. The study was funded by the National Institutes of Health. Dr. Obi had no disclosures.
Ferric citrate effective for anemia in non–dialysis-dependent CKD
CHICAGO – Ferric citrate was safe and effective for treatment of iron-deficiency anemia in patients who had non–dialysis-dependent chronic kidney disease (NDD-CKD), based on data from a phase III, randomized, double-blind study.
The responses were durable, and none of the patients received erythropoiesis-stimulating agents (ESAs), presenter Pablo Pergola, MD, PhD, of Renal Associates, San Antonio, said in an interview at a meeting sponsored by the American Society of Nephrology.
The trial involved 234 anemic adults who had NDD-CKD and had not responded to oral iron supplements. The subjects were randomized to receive oral ferric citrate (n = 117) or placebo (n = 115) with meals (one patient did not receive placebo and laboratory data were lacking for one patient). The mean dose in the treatment arm was 5 pills per day.
The primary endpoint was the proportion of patients with hemoglobin (Hgb) greater than or equal to 1.0 g/dL anytime from baseline through week 16. Secondary endpoints included mean changes from baseline in Hgb, transferrin saturation, ferritin, and serum phosphate and evidence of sustained treatment effect based on target changes in Hgb with time.
Both arms were comparable at baseline for demographic and clinical characteristics, including phosphorus and hemoglobin levels and estimated glomerular filtration rate.
The primary endpoint was met by 51.2% of patients receiving ferric citrate and 19.1% of patients receiving placebo (P less than .001). All secondary efficacy endpoints were met, with statistically significant differences between the treatment and placebo arms, Dr. Pergola reported.
Serum phosphate level was significantly reduced from baseline at week 16 (–0.21 mg/dL; 95% confidence interval, –0.39 to –0.03 mg/dL; P equal to .02) in the active treatment group, and the levels remained in the normal range, he said.
During the 16-week treatment period and subsequent 8-week, open-label safety extension period, ferric citrate was well tolerated. Treatment-emergent adverse events (AEs), most commonly diarrhea, occurred in 93 (79.5%) and 75 (64.7%) patients in the treatment and placebo arms, respectively. Serious AEs developed in 14 (12.0%) and 13 (11.2%) of patients in the same respective order. Two deaths occurred, both in the treatment group. The deaths and serious AEs were not considered drug related.
Ferric citrate binds with dietary phosphate in the gastrointestinal tract. The resulting ferric phosphate is insoluble and is excreted. The remaining unbound ferric citrate increases serum iron parameters, including ferritin and transferrin saturation.
The findings potentially extend the therapeutic reach of the drug beyond its Food and Drug Administration–approved use for control of phosphorus levels in CKD patients on dialysis, Dr. Pergola said. The trial data will be used to seek approval for the oral iron medication as a treatment for iron-deficiency anemia in adults with NDD-CKD.
The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
CHICAGO – Ferric citrate was safe and effective for treatment of iron-deficiency anemia in patients who had non–dialysis-dependent chronic kidney disease (NDD-CKD), based on data from a phase III, randomized, double-blind study.
The responses were durable, and none of the patients received erythropoiesis-stimulating agents (ESAs), presenter Pablo Pergola, MD, PhD, of Renal Associates, San Antonio, said in an interview at a meeting sponsored by the American Society of Nephrology.
The trial involved 234 anemic adults who had NDD-CKD and had not responded to oral iron supplements. The subjects were randomized to receive oral ferric citrate (n = 117) or placebo (n = 115) with meals (one patient did not receive placebo and laboratory data were lacking for one patient). The mean dose in the treatment arm was 5 pills per day.
The primary endpoint was the proportion of patients with hemoglobin (Hgb) greater than or equal to 1.0 g/dL anytime from baseline through week 16. Secondary endpoints included mean changes from baseline in Hgb, transferrin saturation, ferritin, and serum phosphate and evidence of sustained treatment effect based on target changes in Hgb with time.
Both arms were comparable at baseline for demographic and clinical characteristics, including phosphorus and hemoglobin levels and estimated glomerular filtration rate.
The primary endpoint was met by 51.2% of patients receiving ferric citrate and 19.1% of patients receiving placebo (P less than .001). All secondary efficacy endpoints were met, with statistically significant differences between the treatment and placebo arms, Dr. Pergola reported.
Serum phosphate level was significantly reduced from baseline at week 16 (–0.21 mg/dL; 95% confidence interval, –0.39 to –0.03 mg/dL; P equal to .02) in the active treatment group, and the levels remained in the normal range, he said.
During the 16-week treatment period and subsequent 8-week, open-label safety extension period, ferric citrate was well tolerated. Treatment-emergent adverse events (AEs), most commonly diarrhea, occurred in 93 (79.5%) and 75 (64.7%) patients in the treatment and placebo arms, respectively. Serious AEs developed in 14 (12.0%) and 13 (11.2%) of patients in the same respective order. Two deaths occurred, both in the treatment group. The deaths and serious AEs were not considered drug related.
Ferric citrate binds with dietary phosphate in the gastrointestinal tract. The resulting ferric phosphate is insoluble and is excreted. The remaining unbound ferric citrate increases serum iron parameters, including ferritin and transferrin saturation.
The findings potentially extend the therapeutic reach of the drug beyond its Food and Drug Administration–approved use for control of phosphorus levels in CKD patients on dialysis, Dr. Pergola said. The trial data will be used to seek approval for the oral iron medication as a treatment for iron-deficiency anemia in adults with NDD-CKD.
The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
CHICAGO – Ferric citrate was safe and effective for treatment of iron-deficiency anemia in patients who had non–dialysis-dependent chronic kidney disease (NDD-CKD), based on data from a phase III, randomized, double-blind study.
The responses were durable, and none of the patients received erythropoiesis-stimulating agents (ESAs), presenter Pablo Pergola, MD, PhD, of Renal Associates, San Antonio, said in an interview at a meeting sponsored by the American Society of Nephrology.
The trial involved 234 anemic adults who had NDD-CKD and had not responded to oral iron supplements. The subjects were randomized to receive oral ferric citrate (n = 117) or placebo (n = 115) with meals (one patient did not receive placebo and laboratory data were lacking for one patient). The mean dose in the treatment arm was 5 pills per day.
The primary endpoint was the proportion of patients with hemoglobin (Hgb) greater than or equal to 1.0 g/dL anytime from baseline through week 16. Secondary endpoints included mean changes from baseline in Hgb, transferrin saturation, ferritin, and serum phosphate and evidence of sustained treatment effect based on target changes in Hgb with time.
Both arms were comparable at baseline for demographic and clinical characteristics, including phosphorus and hemoglobin levels and estimated glomerular filtration rate.
The primary endpoint was met by 51.2% of patients receiving ferric citrate and 19.1% of patients receiving placebo (P less than .001). All secondary efficacy endpoints were met, with statistically significant differences between the treatment and placebo arms, Dr. Pergola reported.
Serum phosphate level was significantly reduced from baseline at week 16 (–0.21 mg/dL; 95% confidence interval, –0.39 to –0.03 mg/dL; P equal to .02) in the active treatment group, and the levels remained in the normal range, he said.
During the 16-week treatment period and subsequent 8-week, open-label safety extension period, ferric citrate was well tolerated. Treatment-emergent adverse events (AEs), most commonly diarrhea, occurred in 93 (79.5%) and 75 (64.7%) patients in the treatment and placebo arms, respectively. Serious AEs developed in 14 (12.0%) and 13 (11.2%) of patients in the same respective order. Two deaths occurred, both in the treatment group. The deaths and serious AEs were not considered drug related.
Ferric citrate binds with dietary phosphate in the gastrointestinal tract. The resulting ferric phosphate is insoluble and is excreted. The remaining unbound ferric citrate increases serum iron parameters, including ferritin and transferrin saturation.
The findings potentially extend the therapeutic reach of the drug beyond its Food and Drug Administration–approved use for control of phosphorus levels in CKD patients on dialysis, Dr. Pergola said. The trial data will be used to seek approval for the oral iron medication as a treatment for iron-deficiency anemia in adults with NDD-CKD.
The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
AT KIDNEY WEEK 2016
Key clinical point: Ferric citrate appears to be safe and effective for treating anemia in non–dialysis-dependent CKD patients.
Major finding: Prevalence of increased hemoglobin was 52.1% in patients receiving the active drug and 19.1% in those given placebo.
Data source: Randomized, double-blind, placebo-controlled, phase III trial with 234 patients.
Disclosures: The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
Smoking might affect response to ACE inhibitor in chronic kidney disease
CHICAGO – Smoking appears to be a modifiable risk factor for progression of chronic kidney disease associated with primary hypertension in patients treated with ACE inhibitors, Bethany Roehm, MD, reported at a meeting sponsored by the American Society of Nephrology.
Significantly increased albuminuria was noted in patients with chronic kidney disease who continued to smoke after initiating ACE inhibitor therapy, based on results from a 5-year follow-up study of 108 patients who smoked cigarettes at study entry – 25 of whom quit smoking within the first year of the study – and 108 patients who never smoked.
Further, smokers who were able to quit had improvements in measures of kidney function, said Dr. Roehm, of Tufts Medical Center, Boston, who presented the study findings.
It’s important that “we motivate our patients to stop smoking even though this can be challenging in the outpatient setting, she said. “More studies are needed to further investigate the relationship between the kidney protective effects of ACE inhibitors and the impact cigarette smoking may have on these effects.”
In addition to primary hypertension, study subjects had an estimated glomerular filtration ratio (eGFR) of 60 to 89 mL/min per 1.73 m2, and a urine albumin–to-creatinine ratio (UACR) greater than 200 mg/g. At baseline, the 108 smokers had at least a 1-year history of smoking more than a pack of cigarettes daily. They were matched with 108 people who had never smoked.
The smokers received smoking cessation information and guidance; 25 quit smoking. The nonsmokers, continued smokers, and quitters were comparable at baseline. The three groups were followed for 5 years after starting treatment with an ACE inhibitor, usually enalapril.
At 5 years, average eGFR was lower (P less than .01) in continued smokers (54.9 mL/min) than in nonsmokers (66.8 mL/min) and quitters (64.1 mL/min).
Baseline levels of urine (mcg)-to-creatinine (g) isoprostane 8-isoprostaglandin F2-alpha (8-iso/cr), an indicator of lipid peroxidation, were higher in smokers than in nonsmokers. In those who quit smoking, the level had declined at 1 year and remained at a level almost identical to that seen in the nonsmokers. One-year mean urine 8-iso/cr was higher in continued smokers (3.6) than in nonsmokers (1.6, P less than .01) and quitters (1.6, P less than .01).
Systolic blood pressure declined similarly in all three groups over the follow-up.
“The smokers had a faster decline in kidney function over time than the nonsmokers and the subjects who quit smoking ... [and] our continued smokers actually had an increase in albuminuria despite being placed on an ACE inhibitor,” Dr. Roehm said in an interview. Continued smoking appeared to interfere with the decrease in urinary protein excretion that typically accompanies ACE inhibitor therapy. “Higher urine 8-iso excretion, consistent with higher oxidative stress, was present in continued smokers, suggesting oxidative stress as a factor.”
The findings need to be confirmed in larger studies and in patients with CKD due to a wider variety of causes, Dr. Roehm said. Funding for the study was provided by pharmaceutical company support to Texas Tech University, Lubbock, and the Larry and Jane Woirhaye Memorial Endowment in Renal Research. Dr. Roehm reported having no financial disclosures.
CHICAGO – Smoking appears to be a modifiable risk factor for progression of chronic kidney disease associated with primary hypertension in patients treated with ACE inhibitors, Bethany Roehm, MD, reported at a meeting sponsored by the American Society of Nephrology.
Significantly increased albuminuria was noted in patients with chronic kidney disease who continued to smoke after initiating ACE inhibitor therapy, based on results from a 5-year follow-up study of 108 patients who smoked cigarettes at study entry – 25 of whom quit smoking within the first year of the study – and 108 patients who never smoked.
Further, smokers who were able to quit had improvements in measures of kidney function, said Dr. Roehm, of Tufts Medical Center, Boston, who presented the study findings.
It’s important that “we motivate our patients to stop smoking even though this can be challenging in the outpatient setting, she said. “More studies are needed to further investigate the relationship between the kidney protective effects of ACE inhibitors and the impact cigarette smoking may have on these effects.”
In addition to primary hypertension, study subjects had an estimated glomerular filtration ratio (eGFR) of 60 to 89 mL/min per 1.73 m2, and a urine albumin–to-creatinine ratio (UACR) greater than 200 mg/g. At baseline, the 108 smokers had at least a 1-year history of smoking more than a pack of cigarettes daily. They were matched with 108 people who had never smoked.
The smokers received smoking cessation information and guidance; 25 quit smoking. The nonsmokers, continued smokers, and quitters were comparable at baseline. The three groups were followed for 5 years after starting treatment with an ACE inhibitor, usually enalapril.
At 5 years, average eGFR was lower (P less than .01) in continued smokers (54.9 mL/min) than in nonsmokers (66.8 mL/min) and quitters (64.1 mL/min).
Baseline levels of urine (mcg)-to-creatinine (g) isoprostane 8-isoprostaglandin F2-alpha (8-iso/cr), an indicator of lipid peroxidation, were higher in smokers than in nonsmokers. In those who quit smoking, the level had declined at 1 year and remained at a level almost identical to that seen in the nonsmokers. One-year mean urine 8-iso/cr was higher in continued smokers (3.6) than in nonsmokers (1.6, P less than .01) and quitters (1.6, P less than .01).
Systolic blood pressure declined similarly in all three groups over the follow-up.
“The smokers had a faster decline in kidney function over time than the nonsmokers and the subjects who quit smoking ... [and] our continued smokers actually had an increase in albuminuria despite being placed on an ACE inhibitor,” Dr. Roehm said in an interview. Continued smoking appeared to interfere with the decrease in urinary protein excretion that typically accompanies ACE inhibitor therapy. “Higher urine 8-iso excretion, consistent with higher oxidative stress, was present in continued smokers, suggesting oxidative stress as a factor.”
The findings need to be confirmed in larger studies and in patients with CKD due to a wider variety of causes, Dr. Roehm said. Funding for the study was provided by pharmaceutical company support to Texas Tech University, Lubbock, and the Larry and Jane Woirhaye Memorial Endowment in Renal Research. Dr. Roehm reported having no financial disclosures.
CHICAGO – Smoking appears to be a modifiable risk factor for progression of chronic kidney disease associated with primary hypertension in patients treated with ACE inhibitors, Bethany Roehm, MD, reported at a meeting sponsored by the American Society of Nephrology.
Significantly increased albuminuria was noted in patients with chronic kidney disease who continued to smoke after initiating ACE inhibitor therapy, based on results from a 5-year follow-up study of 108 patients who smoked cigarettes at study entry – 25 of whom quit smoking within the first year of the study – and 108 patients who never smoked.
Further, smokers who were able to quit had improvements in measures of kidney function, said Dr. Roehm, of Tufts Medical Center, Boston, who presented the study findings.
It’s important that “we motivate our patients to stop smoking even though this can be challenging in the outpatient setting, she said. “More studies are needed to further investigate the relationship between the kidney protective effects of ACE inhibitors and the impact cigarette smoking may have on these effects.”
In addition to primary hypertension, study subjects had an estimated glomerular filtration ratio (eGFR) of 60 to 89 mL/min per 1.73 m2, and a urine albumin–to-creatinine ratio (UACR) greater than 200 mg/g. At baseline, the 108 smokers had at least a 1-year history of smoking more than a pack of cigarettes daily. They were matched with 108 people who had never smoked.
The smokers received smoking cessation information and guidance; 25 quit smoking. The nonsmokers, continued smokers, and quitters were comparable at baseline. The three groups were followed for 5 years after starting treatment with an ACE inhibitor, usually enalapril.
At 5 years, average eGFR was lower (P less than .01) in continued smokers (54.9 mL/min) than in nonsmokers (66.8 mL/min) and quitters (64.1 mL/min).
Baseline levels of urine (mcg)-to-creatinine (g) isoprostane 8-isoprostaglandin F2-alpha (8-iso/cr), an indicator of lipid peroxidation, were higher in smokers than in nonsmokers. In those who quit smoking, the level had declined at 1 year and remained at a level almost identical to that seen in the nonsmokers. One-year mean urine 8-iso/cr was higher in continued smokers (3.6) than in nonsmokers (1.6, P less than .01) and quitters (1.6, P less than .01).
Systolic blood pressure declined similarly in all three groups over the follow-up.
“The smokers had a faster decline in kidney function over time than the nonsmokers and the subjects who quit smoking ... [and] our continued smokers actually had an increase in albuminuria despite being placed on an ACE inhibitor,” Dr. Roehm said in an interview. Continued smoking appeared to interfere with the decrease in urinary protein excretion that typically accompanies ACE inhibitor therapy. “Higher urine 8-iso excretion, consistent with higher oxidative stress, was present in continued smokers, suggesting oxidative stress as a factor.”
The findings need to be confirmed in larger studies and in patients with CKD due to a wider variety of causes, Dr. Roehm said. Funding for the study was provided by pharmaceutical company support to Texas Tech University, Lubbock, and the Larry and Jane Woirhaye Memorial Endowment in Renal Research. Dr. Roehm reported having no financial disclosures.
AT KIDNEY WEEK 2016
Key clinical point: Smoking cessation could improve therapeutic response in patients who have chronic kidney disease and are treated with ACE inhibitors.
Major finding: At 5 years, eGFR was lower (P less than .01) in continued smokers (54.9 mL/min) than in nonsmokers (66.8 mL/min) and quitters (64.1 mL/min).
Data source: Prospective case-control study involving 216 subjects.
Disclosures: Funding for the study was provided by pharmaceutical company support to Texas Tech University, Lubbock, and the Larry and Jane Woirhaye Memorial Endowment in Renal Research. Dr. Roehm reported having no financial disclosures.
Tinzaparin is a safe, effective anticoagulant in patients on dialysis
CHICAGO – Tinzaparin was safe and effective as an anticoagulant for hemodialysis patients based on results from the Intermittent Hemodialysis Anticoagulation with Tinzaparin (HEMO-TIN) trial presented at the annual meeting sponsored by the American Society for Nephrology.
In the multicenter randomized controlled trial of 192 adults on hemodialysis, tinzaparin, a low molecular weight heparin with antithrombotic properties, was compared with unfractionated heparin. Tinzaparin has been considered for hemodialysis patients because it is thought to be less dependent on renal clearance than are other low molecular weight heparins, Christine Ribic, MD, MSc, of McMaster University, Hamilton, Ont., said in reporting the results.
After 3 months, the 78 patients remaining in the tinzaparin group crossed over to receive unfractionated heparin for 3 months. The 79 patients remaining in the unfractionated heparin group crossed over to receive tinzaparin for 3 months. Of these 156 patients, 125 completed the 3-month crossover phase.
There were 421 bleeding events in the 12,125 hemodialysis sessions studied. They were evenly distributed in the groups, with 212 (50.4%) in those receiving unfractionated heparin and 209 (49.6%) in those receiving tinzaparin. The prevalence of major bleeds (2.1 vs 1.6%), clinically important nonmajor bleeds (1.2% vs 0.2%), and minor bleeds (47.0% vs 47.7%) was also similar between the unfractionated heparin and tinzaparin groups.
Anti-Xa heparin levels were used as a surrogate measure of low molecular weight heparin activity levels and bleeding risk due to bioaccumulation. In tinzaparin-treated patients, anti-Xa heparin levels never exceeded a value of 0.2 either before or after dialysis. This value was considered the threshold between safety and increased risk for bleeding. This threshold level was routinely exceeded pre- and post-dialysis in patients receiving unfractionated heparin at baseline and both before and after crossover.
Grade 4 clotting was similar for tinzaparin and unfractionated heparin, occurring in 23 of 6,095 (0.4%) unfractionated heparin hemodialysis sessions and 41 of 6030 (0.7%) tinzaparin hemodialysis sessions. Mean dialyzer clotting scores and mean air trap clotting scores were also comparable.
The trial was supported by Leo Pharma, the maker of tinzaparin (innohep), in collaboration with McMaster University. Dr. Ribic is the sponsor of the trial.
CHICAGO – Tinzaparin was safe and effective as an anticoagulant for hemodialysis patients based on results from the Intermittent Hemodialysis Anticoagulation with Tinzaparin (HEMO-TIN) trial presented at the annual meeting sponsored by the American Society for Nephrology.
In the multicenter randomized controlled trial of 192 adults on hemodialysis, tinzaparin, a low molecular weight heparin with antithrombotic properties, was compared with unfractionated heparin. Tinzaparin has been considered for hemodialysis patients because it is thought to be less dependent on renal clearance than are other low molecular weight heparins, Christine Ribic, MD, MSc, of McMaster University, Hamilton, Ont., said in reporting the results.
After 3 months, the 78 patients remaining in the tinzaparin group crossed over to receive unfractionated heparin for 3 months. The 79 patients remaining in the unfractionated heparin group crossed over to receive tinzaparin for 3 months. Of these 156 patients, 125 completed the 3-month crossover phase.
There were 421 bleeding events in the 12,125 hemodialysis sessions studied. They were evenly distributed in the groups, with 212 (50.4%) in those receiving unfractionated heparin and 209 (49.6%) in those receiving tinzaparin. The prevalence of major bleeds (2.1 vs 1.6%), clinically important nonmajor bleeds (1.2% vs 0.2%), and minor bleeds (47.0% vs 47.7%) was also similar between the unfractionated heparin and tinzaparin groups.
Anti-Xa heparin levels were used as a surrogate measure of low molecular weight heparin activity levels and bleeding risk due to bioaccumulation. In tinzaparin-treated patients, anti-Xa heparin levels never exceeded a value of 0.2 either before or after dialysis. This value was considered the threshold between safety and increased risk for bleeding. This threshold level was routinely exceeded pre- and post-dialysis in patients receiving unfractionated heparin at baseline and both before and after crossover.
Grade 4 clotting was similar for tinzaparin and unfractionated heparin, occurring in 23 of 6,095 (0.4%) unfractionated heparin hemodialysis sessions and 41 of 6030 (0.7%) tinzaparin hemodialysis sessions. Mean dialyzer clotting scores and mean air trap clotting scores were also comparable.
The trial was supported by Leo Pharma, the maker of tinzaparin (innohep), in collaboration with McMaster University. Dr. Ribic is the sponsor of the trial.
CHICAGO – Tinzaparin was safe and effective as an anticoagulant for hemodialysis patients based on results from the Intermittent Hemodialysis Anticoagulation with Tinzaparin (HEMO-TIN) trial presented at the annual meeting sponsored by the American Society for Nephrology.
In the multicenter randomized controlled trial of 192 adults on hemodialysis, tinzaparin, a low molecular weight heparin with antithrombotic properties, was compared with unfractionated heparin. Tinzaparin has been considered for hemodialysis patients because it is thought to be less dependent on renal clearance than are other low molecular weight heparins, Christine Ribic, MD, MSc, of McMaster University, Hamilton, Ont., said in reporting the results.
After 3 months, the 78 patients remaining in the tinzaparin group crossed over to receive unfractionated heparin for 3 months. The 79 patients remaining in the unfractionated heparin group crossed over to receive tinzaparin for 3 months. Of these 156 patients, 125 completed the 3-month crossover phase.
There were 421 bleeding events in the 12,125 hemodialysis sessions studied. They were evenly distributed in the groups, with 212 (50.4%) in those receiving unfractionated heparin and 209 (49.6%) in those receiving tinzaparin. The prevalence of major bleeds (2.1 vs 1.6%), clinically important nonmajor bleeds (1.2% vs 0.2%), and minor bleeds (47.0% vs 47.7%) was also similar between the unfractionated heparin and tinzaparin groups.
Anti-Xa heparin levels were used as a surrogate measure of low molecular weight heparin activity levels and bleeding risk due to bioaccumulation. In tinzaparin-treated patients, anti-Xa heparin levels never exceeded a value of 0.2 either before or after dialysis. This value was considered the threshold between safety and increased risk for bleeding. This threshold level was routinely exceeded pre- and post-dialysis in patients receiving unfractionated heparin at baseline and both before and after crossover.
Grade 4 clotting was similar for tinzaparin and unfractionated heparin, occurring in 23 of 6,095 (0.4%) unfractionated heparin hemodialysis sessions and 41 of 6030 (0.7%) tinzaparin hemodialysis sessions. Mean dialyzer clotting scores and mean air trap clotting scores were also comparable.
The trial was supported by Leo Pharma, the maker of tinzaparin (innohep), in collaboration with McMaster University. Dr. Ribic is the sponsor of the trial.
AT KIDNEY WEEK 2016
Key clinical point: Tinzaparin outcomes compare to those with low molecular weight heparin, and tinzaparin may be safer because it is less dependent on renal clearance than are low molecular weight heparins.
Major finding: Mean anti-Xa levels post-hemodialysis did not exceed 0.2 for tinzaparin, indicating no residual anticoagulant effect.
Data source: Randomized, double-dummy, blinded crossover controlled trial involving 192 patients.
Disclosures: Study sponsor was McMaster University, Hamilton, Ont. The study was funded by Leo Pharma. Dr. Ribic reported having no financial disclosures.
Liraglutide lessens risk of kidney disease progression in type 2 diabetes
CHICAGO – Liraglutide reduced the risk of kidney disease progression in patients with type 2 diabetes, a study showed.
The latest results from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial build on the previously reported success of liraglutide in reducing the risk of adverse cardiovascular events in people with type 2 diabetes.
“We now have drugs that not only lower blood sugar but also have an impact on new development of diabetic kidney disease and cardiovascular disease,” said Johannes Mann, MD, of the University of Erlangen-Nürnberg, Erlangen, Germany, in a plenary presentation at the meeting sponsored by the American Society of Nephrology.
The LEADER trial involved people with type 2 diabetes mellitus (mean duration, about 13 years) with a baseline hemoglobin A1c level greater than or equal to 7%. Some had never taken antidiabetic drugs, and some were taking oral antidiabetic drugs and/or basal/premixed insulin. They were either 50 years of age or older with established cardiovascular disease and chronic kidney disease, or 60 years and older with risk factors for cardiovascular disease.
Exclusion criteria included type 1 diabetes; a history of medication with glucagonlike peptide–1 receptor agonists, dipeptidyl peptidase–4 inhibitors, pramlintide, or rapid-acting insulin; and a family/personal history of multiple endocrine neoplasia type 2 or medullary thyroid cancer.
The 9,340 subjects were randomized in a double-blind fashion to daily subcutaneous injection with 0.6-1.8 mg of liraglutide (4,668) or placebo (4,672) for at least 3.5 years to a maximum treatment time of 5 years. The mean follow-up was 3.8 years.
At baseline, microalbuminuria had been diagnosed in 26.4% and 26.6% of those randomized to liraglutide or placebo, respectively. The respective baseline rates of macroalbuminuria were 10% and 11%. An estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2 was present in 23.9% of the liraglutide group and 22.3% of the control group.
In this analysis of the LEADER results, the primary renal outcome was a composite of the development of macroalbuminuria, doubling of serum creatinine, end-stage renal disease, or renal death. Liraglutide was superior to placebo in delaying the time to the primary outcome (hazard ratio, 0.78; 95% confidence interval, 0.67-0.92; P equal to .003). The outcome was driven by the reduction in development of macroalbuminuria (HR, 0.74; 95% CI, 0.60-0.91; P = .004), with treatment not being significantly effective for doubling of serum creatinine (HR, 0.89; 95% CI, 0.67-1.19) or the need for dialysis (HR, 0.87; 95% CI, 0.61-1.24).
The eGFR declined less in the liraglutide arm. The renal protection of the drug was restricted to subjects with a baseline eGFR of 30-59 mL/min per 1.73 m2. Liragutide was not associated with an increased risk of adverse renal events.
The latest results extend the potential indications of the therapeutic prowess of liraglutide in type 2 diabetes patients with chronic kidney disease, with the caveat that the significance of the primary outcome was due to macroalbuminuria rather than the arguably more important outcomes of doubling of serum creatinine and development of end-stage renal disease.
The trial was sponsored and funded by Novo Nordisk, the maker of liraglutide (Victoza) and the National Institutes of Health. Dr. Mann disclosed financial relationships with various drug companies, including Novo Nordisk.
CHICAGO – Liraglutide reduced the risk of kidney disease progression in patients with type 2 diabetes, a study showed.
The latest results from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial build on the previously reported success of liraglutide in reducing the risk of adverse cardiovascular events in people with type 2 diabetes.
“We now have drugs that not only lower blood sugar but also have an impact on new development of diabetic kidney disease and cardiovascular disease,” said Johannes Mann, MD, of the University of Erlangen-Nürnberg, Erlangen, Germany, in a plenary presentation at the meeting sponsored by the American Society of Nephrology.
The LEADER trial involved people with type 2 diabetes mellitus (mean duration, about 13 years) with a baseline hemoglobin A1c level greater than or equal to 7%. Some had never taken antidiabetic drugs, and some were taking oral antidiabetic drugs and/or basal/premixed insulin. They were either 50 years of age or older with established cardiovascular disease and chronic kidney disease, or 60 years and older with risk factors for cardiovascular disease.
Exclusion criteria included type 1 diabetes; a history of medication with glucagonlike peptide–1 receptor agonists, dipeptidyl peptidase–4 inhibitors, pramlintide, or rapid-acting insulin; and a family/personal history of multiple endocrine neoplasia type 2 or medullary thyroid cancer.
The 9,340 subjects were randomized in a double-blind fashion to daily subcutaneous injection with 0.6-1.8 mg of liraglutide (4,668) or placebo (4,672) for at least 3.5 years to a maximum treatment time of 5 years. The mean follow-up was 3.8 years.
At baseline, microalbuminuria had been diagnosed in 26.4% and 26.6% of those randomized to liraglutide or placebo, respectively. The respective baseline rates of macroalbuminuria were 10% and 11%. An estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2 was present in 23.9% of the liraglutide group and 22.3% of the control group.
In this analysis of the LEADER results, the primary renal outcome was a composite of the development of macroalbuminuria, doubling of serum creatinine, end-stage renal disease, or renal death. Liraglutide was superior to placebo in delaying the time to the primary outcome (hazard ratio, 0.78; 95% confidence interval, 0.67-0.92; P equal to .003). The outcome was driven by the reduction in development of macroalbuminuria (HR, 0.74; 95% CI, 0.60-0.91; P = .004), with treatment not being significantly effective for doubling of serum creatinine (HR, 0.89; 95% CI, 0.67-1.19) or the need for dialysis (HR, 0.87; 95% CI, 0.61-1.24).
The eGFR declined less in the liraglutide arm. The renal protection of the drug was restricted to subjects with a baseline eGFR of 30-59 mL/min per 1.73 m2. Liragutide was not associated with an increased risk of adverse renal events.
The latest results extend the potential indications of the therapeutic prowess of liraglutide in type 2 diabetes patients with chronic kidney disease, with the caveat that the significance of the primary outcome was due to macroalbuminuria rather than the arguably more important outcomes of doubling of serum creatinine and development of end-stage renal disease.
The trial was sponsored and funded by Novo Nordisk, the maker of liraglutide (Victoza) and the National Institutes of Health. Dr. Mann disclosed financial relationships with various drug companies, including Novo Nordisk.
CHICAGO – Liraglutide reduced the risk of kidney disease progression in patients with type 2 diabetes, a study showed.
The latest results from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial build on the previously reported success of liraglutide in reducing the risk of adverse cardiovascular events in people with type 2 diabetes.
“We now have drugs that not only lower blood sugar but also have an impact on new development of diabetic kidney disease and cardiovascular disease,” said Johannes Mann, MD, of the University of Erlangen-Nürnberg, Erlangen, Germany, in a plenary presentation at the meeting sponsored by the American Society of Nephrology.
The LEADER trial involved people with type 2 diabetes mellitus (mean duration, about 13 years) with a baseline hemoglobin A1c level greater than or equal to 7%. Some had never taken antidiabetic drugs, and some were taking oral antidiabetic drugs and/or basal/premixed insulin. They were either 50 years of age or older with established cardiovascular disease and chronic kidney disease, or 60 years and older with risk factors for cardiovascular disease.
Exclusion criteria included type 1 diabetes; a history of medication with glucagonlike peptide–1 receptor agonists, dipeptidyl peptidase–4 inhibitors, pramlintide, or rapid-acting insulin; and a family/personal history of multiple endocrine neoplasia type 2 or medullary thyroid cancer.
The 9,340 subjects were randomized in a double-blind fashion to daily subcutaneous injection with 0.6-1.8 mg of liraglutide (4,668) or placebo (4,672) for at least 3.5 years to a maximum treatment time of 5 years. The mean follow-up was 3.8 years.
At baseline, microalbuminuria had been diagnosed in 26.4% and 26.6% of those randomized to liraglutide or placebo, respectively. The respective baseline rates of macroalbuminuria were 10% and 11%. An estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2 was present in 23.9% of the liraglutide group and 22.3% of the control group.
In this analysis of the LEADER results, the primary renal outcome was a composite of the development of macroalbuminuria, doubling of serum creatinine, end-stage renal disease, or renal death. Liraglutide was superior to placebo in delaying the time to the primary outcome (hazard ratio, 0.78; 95% confidence interval, 0.67-0.92; P equal to .003). The outcome was driven by the reduction in development of macroalbuminuria (HR, 0.74; 95% CI, 0.60-0.91; P = .004), with treatment not being significantly effective for doubling of serum creatinine (HR, 0.89; 95% CI, 0.67-1.19) or the need for dialysis (HR, 0.87; 95% CI, 0.61-1.24).
The eGFR declined less in the liraglutide arm. The renal protection of the drug was restricted to subjects with a baseline eGFR of 30-59 mL/min per 1.73 m2. Liragutide was not associated with an increased risk of adverse renal events.
The latest results extend the potential indications of the therapeutic prowess of liraglutide in type 2 diabetes patients with chronic kidney disease, with the caveat that the significance of the primary outcome was due to macroalbuminuria rather than the arguably more important outcomes of doubling of serum creatinine and development of end-stage renal disease.
The trial was sponsored and funded by Novo Nordisk, the maker of liraglutide (Victoza) and the National Institutes of Health. Dr. Mann disclosed financial relationships with various drug companies, including Novo Nordisk.
AT KIDNEY WEEK 2016
Key clinical point:
Major finding: Liraglutide significantly lessened all-cause death, compared with placebo.
Data source: LEADER, a multicenter, randomized, double-blind placebo-controlled trial involving 9,340 patients.
Disclosures: The trial was sponsored and funded by Novo Nordisk, the maker of liraglutide (Victoza) and the National Institutes of Health. Dr. Mann disclosed financial relationships with various drug companies, including Novo Nordisk.
Adjustment for fluid balance improved detection of AKI in critically ill children
CHICAGO – Adjustment for fluid balance increased the detection rate of acute kidney injury, more accurately staged the kidney damage, and distinguished false-positive cases in critically ill children, based on a secondary analysis of the Study of the Prediction of Acute Kidney Injury in Children Using Risk Stratification and Biomarkers (AKI-CHERUB).
Fluid overload can mask acute kidney injury (AKI) in critically ill children. “The failure to correct serum creatinine measure for fluid overload dilutes the impact of AKI on outcomes,” David T. Selewski, MD, of the University of Michigan, Ann Arbor, said at the annual meeting sponsored by the American Society for Nephrology.
The primary outcome was ICU mortality. Secondary outcomes were length of mechanical ventilation, and length of stay in the ICU and the hospital.
The original study documented an ICU mortality rate of 7.1%. AKI was identified in 77 (41.8%) of the 184 patients. The median peak fluid overload during ICU admission was 12.9 (interquartile range, 7.4-20.8).
The serum creatinine data were corrected for fluid balance and these rates were reassessed. Following the adjustment, the rate of AKI increased from 41.8% to 53.4%, with 30 new cases identified according to standard defined criteria. The mean fluid overload was now 11.2 (interquartile range, 5.7-17.7).
In the original cohort, there were 40 cases of severe AKI (stage 2 and 3). Following the creatinine correction, 13 more cases were judged to be severe. Of these, five cases were associated with a worse outcome in terms of ICU mortality. Additionally, 10 cases that had been diagnosed as AKI were found to be false positives.
The results need to be studied in larger studies and in other populations, such as neonates, Dr. Selewski said.
CHICAGO – Adjustment for fluid balance increased the detection rate of acute kidney injury, more accurately staged the kidney damage, and distinguished false-positive cases in critically ill children, based on a secondary analysis of the Study of the Prediction of Acute Kidney Injury in Children Using Risk Stratification and Biomarkers (AKI-CHERUB).
Fluid overload can mask acute kidney injury (AKI) in critically ill children. “The failure to correct serum creatinine measure for fluid overload dilutes the impact of AKI on outcomes,” David T. Selewski, MD, of the University of Michigan, Ann Arbor, said at the annual meeting sponsored by the American Society for Nephrology.
The primary outcome was ICU mortality. Secondary outcomes were length of mechanical ventilation, and length of stay in the ICU and the hospital.
The original study documented an ICU mortality rate of 7.1%. AKI was identified in 77 (41.8%) of the 184 patients. The median peak fluid overload during ICU admission was 12.9 (interquartile range, 7.4-20.8).
The serum creatinine data were corrected for fluid balance and these rates were reassessed. Following the adjustment, the rate of AKI increased from 41.8% to 53.4%, with 30 new cases identified according to standard defined criteria. The mean fluid overload was now 11.2 (interquartile range, 5.7-17.7).
In the original cohort, there were 40 cases of severe AKI (stage 2 and 3). Following the creatinine correction, 13 more cases were judged to be severe. Of these, five cases were associated with a worse outcome in terms of ICU mortality. Additionally, 10 cases that had been diagnosed as AKI were found to be false positives.
The results need to be studied in larger studies and in other populations, such as neonates, Dr. Selewski said.
CHICAGO – Adjustment for fluid balance increased the detection rate of acute kidney injury, more accurately staged the kidney damage, and distinguished false-positive cases in critically ill children, based on a secondary analysis of the Study of the Prediction of Acute Kidney Injury in Children Using Risk Stratification and Biomarkers (AKI-CHERUB).
Fluid overload can mask acute kidney injury (AKI) in critically ill children. “The failure to correct serum creatinine measure for fluid overload dilutes the impact of AKI on outcomes,” David T. Selewski, MD, of the University of Michigan, Ann Arbor, said at the annual meeting sponsored by the American Society for Nephrology.
The primary outcome was ICU mortality. Secondary outcomes were length of mechanical ventilation, and length of stay in the ICU and the hospital.
The original study documented an ICU mortality rate of 7.1%. AKI was identified in 77 (41.8%) of the 184 patients. The median peak fluid overload during ICU admission was 12.9 (interquartile range, 7.4-20.8).
The serum creatinine data were corrected for fluid balance and these rates were reassessed. Following the adjustment, the rate of AKI increased from 41.8% to 53.4%, with 30 new cases identified according to standard defined criteria. The mean fluid overload was now 11.2 (interquartile range, 5.7-17.7).
In the original cohort, there were 40 cases of severe AKI (stage 2 and 3). Following the creatinine correction, 13 more cases were judged to be severe. Of these, five cases were associated with a worse outcome in terms of ICU mortality. Additionally, 10 cases that had been diagnosed as AKI were found to be false positives.
The results need to be studied in larger studies and in other populations, such as neonates, Dr. Selewski said.
AT KIDNEY WEEK 2016
Key clinical point: Acute kidney injury can be detected more accurately in critically ill children by correcting for fluid overload.
Major finding: Fluid overload masked diagnosis of over 40% of patients with acute kidney injury in an observational study.
Data source: Secondary analysis of AKI-CHERUB single-center observational study involving 181 critically ill children.
Disclosures: The AKI-CHERUB study was funded by NIH. Dr. Selewski reported having no financial disclosures.
Acute kidney injury common in children, young adults in ICU
Acute kidney injury is common in children and young adults admitted to ICUs, and cannot always be identified by plasma creatinine level alone, according to the authors of a study presented at the meeting sponsored by the American Society of Nephrology.
The Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) study was a prospective, international, observational study in 4,683 patients aged 3 months to 25 years, recruited from 32 pediatric ICUs over the course of 3 months.
Ahmad Kaddourah, MD, from the Center for Acute Care Nephrology at the Cincinnati Children’s Hospital Medical Center, and his coauthors found that 27% of the participants developed acute kidney injury and 12% developed severe acute kidney injury – defined as stage 2 or 3 acute kidney injury – within the first 7 days after admission.
The risk of death within 28 days was 77% higher among individuals with severe acute kidney injury, even after accounting for their original diagnosis when they were admitted to the ICU. Mortality among these individuals was 11%, compared with 2.5% among patients without severe acute kidney injury. These patients also had an increased use of renal replacement therapy and mechanical ventilation, and were more likely to have longer stays in hospital.
Researchers also saw a stepwise increase in 28-day mortality associated with maximum stage of acute kidney injury.
“The common and early occurrence of acute kidney injury reinforces the need for systematic surveillance for acute kidney injury at the time of admission to the ICU,” Dr. Kaddourah and his associates wrote. “Early identification of modifiable risk factors for acute kidney injury (e.g., nephrotoxic medications) or adverse sequelae (e.g., fluid overload) has the potential to decrease morbidity and mortality.”
Of particular note was the observation that 67% of the patients who met the urine-output criteria for acute kidney injury would not have been diagnosed using the plasma creatinine criteria alone. Furthermore, “mortality was higher among patients diagnosed with stage 3 acute kidney injury according to urine output than among those diagnosed according to plasma creatinine levels,” the authors reported.
There was a steady increase in the daily prevalence of acute kidney disease, from 15% on day 1 after admission to 20% by day 7. Patients with stage 1 acute kidney injury on day 1 also were more likely to progress to stage 2 or 3 by day 7, compared with patients who did not have acute kidney injury on admission.
However, around three-quarters of this increase in stage occurred within the first 4 days after admission, which the authors suggested would support a 4-day time frame for future studies on acute kidney injury in children. They also stressed that as their assessments for acute kidney injury stopped at day 7 after admission, there may have been incidents that were missed.
Dr. Kaddourah and his associates noted that although the rates of severe and acute kidney injury seen in the study were slightly lower than those observed in studies in adults, the associations with morbidity and mortality were similar.
“The presence of chronic systemic diseases contributes to residual confounding in studies of acute kidney injury in adults,” they wrote. “Children have a low prevalence of such chronic diseases; thus, although the incremental association between acute kidney injury and risk of death mirrors that seen in adults, our study suggests that acute kidney injury itself may be key to the associated morbidity and mortality.”
The study was supported by the Pediatric Nephrology Center for Excellence at Cincinnati Children’s Hospital Medical Center. The authors declared grants, consultancies, speaking engagements, and other support from private industry, some related to and some outside of the submitted work.
A strength of this study is the definition of acute kidney injury, with the use of precise and validated criteria. Limitations of the study, beyond its observational nature, include the lack of data about diuretic and other treatment that may have influenced urine output, and the requirement for just a single baseline plasma creatinine level for study entry.
However, the study results indicate that acute injury is not only common among critically ill children and young adults, but is associated with adverse outcomes, implying that we should look more carefully for markers of acute kidney injury. Given the link between acute kidney injury and subsequent chronic kidney disease, it possible that identifying and treating acute kidney injury promptly might reduce the prevalence of chronic kidney disease, now estimated as roughly one in eight adults in the United States.
Julie R. Ingelfinger, MD, is a pediatric nephrologist at Massachusetts General Hospital and deputy editor of the New England Journal of Medicine. These comments are excerpted from an accompanying editorial (N Eng J Med. 2016 Nov 18. doi: 10.1056/NEJMe613456). No conflicts of interest were declared.
A strength of this study is the definition of acute kidney injury, with the use of precise and validated criteria. Limitations of the study, beyond its observational nature, include the lack of data about diuretic and other treatment that may have influenced urine output, and the requirement for just a single baseline plasma creatinine level for study entry.
However, the study results indicate that acute injury is not only common among critically ill children and young adults, but is associated with adverse outcomes, implying that we should look more carefully for markers of acute kidney injury. Given the link between acute kidney injury and subsequent chronic kidney disease, it possible that identifying and treating acute kidney injury promptly might reduce the prevalence of chronic kidney disease, now estimated as roughly one in eight adults in the United States.
Julie R. Ingelfinger, MD, is a pediatric nephrologist at Massachusetts General Hospital and deputy editor of the New England Journal of Medicine. These comments are excerpted from an accompanying editorial (N Eng J Med. 2016 Nov 18. doi: 10.1056/NEJMe613456). No conflicts of interest were declared.
A strength of this study is the definition of acute kidney injury, with the use of precise and validated criteria. Limitations of the study, beyond its observational nature, include the lack of data about diuretic and other treatment that may have influenced urine output, and the requirement for just a single baseline plasma creatinine level for study entry.
However, the study results indicate that acute injury is not only common among critically ill children and young adults, but is associated with adverse outcomes, implying that we should look more carefully for markers of acute kidney injury. Given the link between acute kidney injury and subsequent chronic kidney disease, it possible that identifying and treating acute kidney injury promptly might reduce the prevalence of chronic kidney disease, now estimated as roughly one in eight adults in the United States.
Julie R. Ingelfinger, MD, is a pediatric nephrologist at Massachusetts General Hospital and deputy editor of the New England Journal of Medicine. These comments are excerpted from an accompanying editorial (N Eng J Med. 2016 Nov 18. doi: 10.1056/NEJMe613456). No conflicts of interest were declared.
Acute kidney injury is common in children and young adults admitted to ICUs, and cannot always be identified by plasma creatinine level alone, according to the authors of a study presented at the meeting sponsored by the American Society of Nephrology.
The Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) study was a prospective, international, observational study in 4,683 patients aged 3 months to 25 years, recruited from 32 pediatric ICUs over the course of 3 months.
Ahmad Kaddourah, MD, from the Center for Acute Care Nephrology at the Cincinnati Children’s Hospital Medical Center, and his coauthors found that 27% of the participants developed acute kidney injury and 12% developed severe acute kidney injury – defined as stage 2 or 3 acute kidney injury – within the first 7 days after admission.
The risk of death within 28 days was 77% higher among individuals with severe acute kidney injury, even after accounting for their original diagnosis when they were admitted to the ICU. Mortality among these individuals was 11%, compared with 2.5% among patients without severe acute kidney injury. These patients also had an increased use of renal replacement therapy and mechanical ventilation, and were more likely to have longer stays in hospital.
Researchers also saw a stepwise increase in 28-day mortality associated with maximum stage of acute kidney injury.
“The common and early occurrence of acute kidney injury reinforces the need for systematic surveillance for acute kidney injury at the time of admission to the ICU,” Dr. Kaddourah and his associates wrote. “Early identification of modifiable risk factors for acute kidney injury (e.g., nephrotoxic medications) or adverse sequelae (e.g., fluid overload) has the potential to decrease morbidity and mortality.”
Of particular note was the observation that 67% of the patients who met the urine-output criteria for acute kidney injury would not have been diagnosed using the plasma creatinine criteria alone. Furthermore, “mortality was higher among patients diagnosed with stage 3 acute kidney injury according to urine output than among those diagnosed according to plasma creatinine levels,” the authors reported.
There was a steady increase in the daily prevalence of acute kidney disease, from 15% on day 1 after admission to 20% by day 7. Patients with stage 1 acute kidney injury on day 1 also were more likely to progress to stage 2 or 3 by day 7, compared with patients who did not have acute kidney injury on admission.
However, around three-quarters of this increase in stage occurred within the first 4 days after admission, which the authors suggested would support a 4-day time frame for future studies on acute kidney injury in children. They also stressed that as their assessments for acute kidney injury stopped at day 7 after admission, there may have been incidents that were missed.
Dr. Kaddourah and his associates noted that although the rates of severe and acute kidney injury seen in the study were slightly lower than those observed in studies in adults, the associations with morbidity and mortality were similar.
“The presence of chronic systemic diseases contributes to residual confounding in studies of acute kidney injury in adults,” they wrote. “Children have a low prevalence of such chronic diseases; thus, although the incremental association between acute kidney injury and risk of death mirrors that seen in adults, our study suggests that acute kidney injury itself may be key to the associated morbidity and mortality.”
The study was supported by the Pediatric Nephrology Center for Excellence at Cincinnati Children’s Hospital Medical Center. The authors declared grants, consultancies, speaking engagements, and other support from private industry, some related to and some outside of the submitted work.
Acute kidney injury is common in children and young adults admitted to ICUs, and cannot always be identified by plasma creatinine level alone, according to the authors of a study presented at the meeting sponsored by the American Society of Nephrology.
The Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) study was a prospective, international, observational study in 4,683 patients aged 3 months to 25 years, recruited from 32 pediatric ICUs over the course of 3 months.
Ahmad Kaddourah, MD, from the Center for Acute Care Nephrology at the Cincinnati Children’s Hospital Medical Center, and his coauthors found that 27% of the participants developed acute kidney injury and 12% developed severe acute kidney injury – defined as stage 2 or 3 acute kidney injury – within the first 7 days after admission.
The risk of death within 28 days was 77% higher among individuals with severe acute kidney injury, even after accounting for their original diagnosis when they were admitted to the ICU. Mortality among these individuals was 11%, compared with 2.5% among patients without severe acute kidney injury. These patients also had an increased use of renal replacement therapy and mechanical ventilation, and were more likely to have longer stays in hospital.
Researchers also saw a stepwise increase in 28-day mortality associated with maximum stage of acute kidney injury.
“The common and early occurrence of acute kidney injury reinforces the need for systematic surveillance for acute kidney injury at the time of admission to the ICU,” Dr. Kaddourah and his associates wrote. “Early identification of modifiable risk factors for acute kidney injury (e.g., nephrotoxic medications) or adverse sequelae (e.g., fluid overload) has the potential to decrease morbidity and mortality.”
Of particular note was the observation that 67% of the patients who met the urine-output criteria for acute kidney injury would not have been diagnosed using the plasma creatinine criteria alone. Furthermore, “mortality was higher among patients diagnosed with stage 3 acute kidney injury according to urine output than among those diagnosed according to plasma creatinine levels,” the authors reported.
There was a steady increase in the daily prevalence of acute kidney disease, from 15% on day 1 after admission to 20% by day 7. Patients with stage 1 acute kidney injury on day 1 also were more likely to progress to stage 2 or 3 by day 7, compared with patients who did not have acute kidney injury on admission.
However, around three-quarters of this increase in stage occurred within the first 4 days after admission, which the authors suggested would support a 4-day time frame for future studies on acute kidney injury in children. They also stressed that as their assessments for acute kidney injury stopped at day 7 after admission, there may have been incidents that were missed.
Dr. Kaddourah and his associates noted that although the rates of severe and acute kidney injury seen in the study were slightly lower than those observed in studies in adults, the associations with morbidity and mortality were similar.
“The presence of chronic systemic diseases contributes to residual confounding in studies of acute kidney injury in adults,” they wrote. “Children have a low prevalence of such chronic diseases; thus, although the incremental association between acute kidney injury and risk of death mirrors that seen in adults, our study suggests that acute kidney injury itself may be key to the associated morbidity and mortality.”
The study was supported by the Pediatric Nephrology Center for Excellence at Cincinnati Children’s Hospital Medical Center. The authors declared grants, consultancies, speaking engagements, and other support from private industry, some related to and some outside of the submitted work.
FROM KIDNEY WEEK 2016
Key clinical point: Acute kidney injury is common in children and young adults admitted to ICU, but many cases may be missed using plasma creatinine criteria alone.
Major finding: Among children and young adults admitted to intensive care, as many as 1 in 4 may have acute kidney injury and 1 in 10 may have severe acute kidney injury.
Data source: Prospective observational study in 4,683 patients aged 3 months to 25 years admitted to pediatric intensive care.
Disclosures: The study was supported by the Pediatric Nephrology Center for Excellence at Cincinnati Children’s Hospital Medical Center. The authors declared grants, consultancies, speaking engagements and other support from private industry, some related to and some outside of the submitted work.