User login
Pediatricians Called Upon for More STD Screening
VAIL, COLO. – National data indicate general pediatricians now provide care for more adolescents than ever before – and that means they need to keep current with new trends in STD management, according to Dr. Ann-Christine Nyquist.
The authoritative source on STD diagnosis, treatment, and prevention is the Centers for Disease Control and Prevention’s "Sexually Transmitted Diseases Treatment Guidelines, 2010." But the "2010" is misleading; the guidelines are actually a living document that is constantly being updated. Thus, it’s best to consult the online version (www.cdc.gov/std/treatment) rather than a hard copy, noted Dr. Nyquist, professor of pediatrics at the University of Colorado at Denver.
As evidence that pediatricians need to be adept at STD management, she cited a major study funded by the American Board of Pediatrics showing that during the years 2000-2006, the proportion of office visits to general pediatricians made by the nation’s 11- to 17-year-olds climbed from 38% to 53%.
Meanwhile, the proportion of office visits to family physicians and other nonpediatric generalists declined from 38% to 30%, while the proportion of visits to nonpediatric specialists dropped from 28% to 22% and visits to pediatric specialists inched up from 2% to 5% (J. Pediatr. 2010;157:148-52e1), DrNyquist noted at a conference on pediatric infectious diseases, sponsored by Children’s Hospital Colorado.
The data came from the CDC’s National Ambulatory Medical Care Survey, with the analysis conducted by pediatricians at the University of Michigan, Ann Arbor. The study was a follow-up to an earlier report on national trends showing who is providing medical care for America’s children, conducted by the same research group (Arch. Pediatr. Adolesc. Med. 2004;158:22-6). The latest study confirms an accelerating trend seen during 1980-2000 for a greater proportion of health care visits for children and adolescents in the United States being provided by general pediatricians.
Nearly 50% of all STDs occur in 15- to 24-year-olds. The CDC guidelines recommend annual screening for chlamydia and gonorrhea in all sexually active females under age 25; the available data aren’t strong enough at present to support routine annual screening of all similarly aged males.
The preferred screening assay is a DNA-based nucleic acid amplification test because of its superior sensitivity and specificity. The assay can be done using a urine sample or a vaginal, rectal, or pharyngeal swab specimen. That’s a boon for pediatricians who are uncomfortable doing a pelvic exam to collect a cervical specimen.
It’s vital to talk with the sexually active adolescent about his or her specific sexual behaviors in order to know what sites to test. The considerable diversity in patterns of adolescent sexual initiation was underscored in a recent study of nearly 14,000 18-year-old participants in the National Longitudinal Study of Adolescent Health. Four in five had engaged in vaginal intercourse, oral-genital sexual activity, and/or anal intercourse by age 18. One in 10 reported anal intercourse. One-third of the youths initiated two or more of the sexual behaviors within a 1-year period (Am. J. Public Health 2012;102:1221-8).
Dr. Nyquist reported having no relevant financial conflicts.
VAIL, COLO. – National data indicate general pediatricians now provide care for more adolescents than ever before – and that means they need to keep current with new trends in STD management, according to Dr. Ann-Christine Nyquist.
The authoritative source on STD diagnosis, treatment, and prevention is the Centers for Disease Control and Prevention’s "Sexually Transmitted Diseases Treatment Guidelines, 2010." But the "2010" is misleading; the guidelines are actually a living document that is constantly being updated. Thus, it’s best to consult the online version (www.cdc.gov/std/treatment) rather than a hard copy, noted Dr. Nyquist, professor of pediatrics at the University of Colorado at Denver.
As evidence that pediatricians need to be adept at STD management, she cited a major study funded by the American Board of Pediatrics showing that during the years 2000-2006, the proportion of office visits to general pediatricians made by the nation’s 11- to 17-year-olds climbed from 38% to 53%.
Meanwhile, the proportion of office visits to family physicians and other nonpediatric generalists declined from 38% to 30%, while the proportion of visits to nonpediatric specialists dropped from 28% to 22% and visits to pediatric specialists inched up from 2% to 5% (J. Pediatr. 2010;157:148-52e1), DrNyquist noted at a conference on pediatric infectious diseases, sponsored by Children’s Hospital Colorado.
The data came from the CDC’s National Ambulatory Medical Care Survey, with the analysis conducted by pediatricians at the University of Michigan, Ann Arbor. The study was a follow-up to an earlier report on national trends showing who is providing medical care for America’s children, conducted by the same research group (Arch. Pediatr. Adolesc. Med. 2004;158:22-6). The latest study confirms an accelerating trend seen during 1980-2000 for a greater proportion of health care visits for children and adolescents in the United States being provided by general pediatricians.
Nearly 50% of all STDs occur in 15- to 24-year-olds. The CDC guidelines recommend annual screening for chlamydia and gonorrhea in all sexually active females under age 25; the available data aren’t strong enough at present to support routine annual screening of all similarly aged males.
The preferred screening assay is a DNA-based nucleic acid amplification test because of its superior sensitivity and specificity. The assay can be done using a urine sample or a vaginal, rectal, or pharyngeal swab specimen. That’s a boon for pediatricians who are uncomfortable doing a pelvic exam to collect a cervical specimen.
It’s vital to talk with the sexually active adolescent about his or her specific sexual behaviors in order to know what sites to test. The considerable diversity in patterns of adolescent sexual initiation was underscored in a recent study of nearly 14,000 18-year-old participants in the National Longitudinal Study of Adolescent Health. Four in five had engaged in vaginal intercourse, oral-genital sexual activity, and/or anal intercourse by age 18. One in 10 reported anal intercourse. One-third of the youths initiated two or more of the sexual behaviors within a 1-year period (Am. J. Public Health 2012;102:1221-8).
Dr. Nyquist reported having no relevant financial conflicts.
VAIL, COLO. – National data indicate general pediatricians now provide care for more adolescents than ever before – and that means they need to keep current with new trends in STD management, according to Dr. Ann-Christine Nyquist.
The authoritative source on STD diagnosis, treatment, and prevention is the Centers for Disease Control and Prevention’s "Sexually Transmitted Diseases Treatment Guidelines, 2010." But the "2010" is misleading; the guidelines are actually a living document that is constantly being updated. Thus, it’s best to consult the online version (www.cdc.gov/std/treatment) rather than a hard copy, noted Dr. Nyquist, professor of pediatrics at the University of Colorado at Denver.
As evidence that pediatricians need to be adept at STD management, she cited a major study funded by the American Board of Pediatrics showing that during the years 2000-2006, the proportion of office visits to general pediatricians made by the nation’s 11- to 17-year-olds climbed from 38% to 53%.
Meanwhile, the proportion of office visits to family physicians and other nonpediatric generalists declined from 38% to 30%, while the proportion of visits to nonpediatric specialists dropped from 28% to 22% and visits to pediatric specialists inched up from 2% to 5% (J. Pediatr. 2010;157:148-52e1), DrNyquist noted at a conference on pediatric infectious diseases, sponsored by Children’s Hospital Colorado.
The data came from the CDC’s National Ambulatory Medical Care Survey, with the analysis conducted by pediatricians at the University of Michigan, Ann Arbor. The study was a follow-up to an earlier report on national trends showing who is providing medical care for America’s children, conducted by the same research group (Arch. Pediatr. Adolesc. Med. 2004;158:22-6). The latest study confirms an accelerating trend seen during 1980-2000 for a greater proportion of health care visits for children and adolescents in the United States being provided by general pediatricians.
Nearly 50% of all STDs occur in 15- to 24-year-olds. The CDC guidelines recommend annual screening for chlamydia and gonorrhea in all sexually active females under age 25; the available data aren’t strong enough at present to support routine annual screening of all similarly aged males.
The preferred screening assay is a DNA-based nucleic acid amplification test because of its superior sensitivity and specificity. The assay can be done using a urine sample or a vaginal, rectal, or pharyngeal swab specimen. That’s a boon for pediatricians who are uncomfortable doing a pelvic exam to collect a cervical specimen.
It’s vital to talk with the sexually active adolescent about his or her specific sexual behaviors in order to know what sites to test. The considerable diversity in patterns of adolescent sexual initiation was underscored in a recent study of nearly 14,000 18-year-old participants in the National Longitudinal Study of Adolescent Health. Four in five had engaged in vaginal intercourse, oral-genital sexual activity, and/or anal intercourse by age 18. One in 10 reported anal intercourse. One-third of the youths initiated two or more of the sexual behaviors within a 1-year period (Am. J. Public Health 2012;102:1221-8).
Dr. Nyquist reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES
Epic Progress Seen in Reducing Pneumococcal Infections
VAIL, COLO. - American medicine emphatically surpassed the Healthy People 2010 goal for reduction of invasive Streptococcus pneumoniae infections well ahead of schedule in both of the highest-risk target groups: children under age 5 years and seniors. And the tougher Healthy People 2020 objectives are already well within striking distance.
"We get an A+ on this," Dr. Mary P. Glodé commented at the annual pediatric infectious diseases conference sponsored by Children’s Hospital Colorado.
The Healthy People 2010 objective was to reduce the incidence of invasive S. pneumoniae infections to 46 cases per 100,000 among children under age 5 years and to 42 per 100,000 persons age 65 or older. The actual 2010 rates were 19 and 36 per 100,000, respectively.
Between 1999 and 2010, the annual rate of invasive pneumococcal disease in children younger age 5 plummeted by 86% as a consequence of the licensure in 2000 of the pneumococcal conjugate vaccine 7 (PCV 7) vaccine.
Most impressively, the rate also fell by 50% during that period among seniors, even though they didn’t receive the vaccine. This is ascribed to herd immunity. The presumed mechanism is that once immunized, young children were far less likely to become colonized by virulent pneumococcal serotypes, with resultant diminished opportunity for transmission of the pathogens to older children and adults, explained Dr. Glodé, professor of pediatrics and head of the section of pediatric infectious disease at the University of Colorado, Denver, and Children’s Hospital Colorado.
The Healthy People 2020 goal is to further reduce the incidence of invasive pneumococcal infections in children under age 5 from the 2010 rate of 19 down to 12 per 100,000, and in seniors from the 2010 figure of 36 per 100,000 to 31 per 100,000.
The expectation is that the target in children will be handily reached, and early on, as a consequence of the spring 2010 recommendation by the American Academy of Pediatrics and the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) that all children aged 2-59 months be routinely vaccinated with the PCV 13 vaccine, which contains the PCV 7 serotypes plus six others causing invasive disease. Early unpublished CDC data suggest there has already been some decline in disease in children as a result of the added serotypes included in PCV 13, especially in cases involving serotypes 19A and 7F, according to Dr. Glodé.
The difficult unanswered question concerns the best way to get the nation’s seniors to the Healthy People 2020 target. The rate of invasive pneumococcal disease is higher in persons aged 65 and older than in any other age group, as is the associated mortality. Indeed, of an estimated 44,000 cases of invasive pneumococcal disease in the United States during 2009, 37,000 were in adults, including 15,000 cases in individuals age 65 or more.
Late last year the Food and Drug Administration licensed the PCV 13 vaccine for use in people aged 50 and up. But that doesn’t necessarily mean it will see widespread use. There has been no official recommendation from the ACIP that the vaccine be routinely used in this population. The expert panel has noted that to date there are no data showing that the PCV 13 vaccine is effective in preventing pneumococcal pneumonia in adults, although a Dutch trial in 85,000 people over age 65 is underway.
The committee also noted that one-quarter of all cases of invasive pneumococcal disease in seniors are caused by 11 serotypes in the PPSV 23 vaccine that are not included in the PCV 13 vaccine, which further complicates the situation. The PPSV 23 vaccine has been approved since 1983 and is recommended for use in all adults over age 65 and in younger adults with certain underlying medical conditions, including diabetes and chronic lung disease.
Also, if the serotypes contained in the PCV 13 vaccine are going to largely go away in the senior population as a consequence of universal pediatric immunization, which could happen based upon the earlier herd immunity experience noted with the PCV 7 vaccine, then it may not be reasonable to give PCV 13 to all older adults, Dr. Glodé noted.
She reported serving on the data safety monitoring board for trials of an unrelated Pfizer vaccine.
VAIL, COLO. - American medicine emphatically surpassed the Healthy People 2010 goal for reduction of invasive Streptococcus pneumoniae infections well ahead of schedule in both of the highest-risk target groups: children under age 5 years and seniors. And the tougher Healthy People 2020 objectives are already well within striking distance.
"We get an A+ on this," Dr. Mary P. Glodé commented at the annual pediatric infectious diseases conference sponsored by Children’s Hospital Colorado.
The Healthy People 2010 objective was to reduce the incidence of invasive S. pneumoniae infections to 46 cases per 100,000 among children under age 5 years and to 42 per 100,000 persons age 65 or older. The actual 2010 rates were 19 and 36 per 100,000, respectively.
Between 1999 and 2010, the annual rate of invasive pneumococcal disease in children younger age 5 plummeted by 86% as a consequence of the licensure in 2000 of the pneumococcal conjugate vaccine 7 (PCV 7) vaccine.
Most impressively, the rate also fell by 50% during that period among seniors, even though they didn’t receive the vaccine. This is ascribed to herd immunity. The presumed mechanism is that once immunized, young children were far less likely to become colonized by virulent pneumococcal serotypes, with resultant diminished opportunity for transmission of the pathogens to older children and adults, explained Dr. Glodé, professor of pediatrics and head of the section of pediatric infectious disease at the University of Colorado, Denver, and Children’s Hospital Colorado.
The Healthy People 2020 goal is to further reduce the incidence of invasive pneumococcal infections in children under age 5 from the 2010 rate of 19 down to 12 per 100,000, and in seniors from the 2010 figure of 36 per 100,000 to 31 per 100,000.
The expectation is that the target in children will be handily reached, and early on, as a consequence of the spring 2010 recommendation by the American Academy of Pediatrics and the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) that all children aged 2-59 months be routinely vaccinated with the PCV 13 vaccine, which contains the PCV 7 serotypes plus six others causing invasive disease. Early unpublished CDC data suggest there has already been some decline in disease in children as a result of the added serotypes included in PCV 13, especially in cases involving serotypes 19A and 7F, according to Dr. Glodé.
The difficult unanswered question concerns the best way to get the nation’s seniors to the Healthy People 2020 target. The rate of invasive pneumococcal disease is higher in persons aged 65 and older than in any other age group, as is the associated mortality. Indeed, of an estimated 44,000 cases of invasive pneumococcal disease in the United States during 2009, 37,000 were in adults, including 15,000 cases in individuals age 65 or more.
Late last year the Food and Drug Administration licensed the PCV 13 vaccine for use in people aged 50 and up. But that doesn’t necessarily mean it will see widespread use. There has been no official recommendation from the ACIP that the vaccine be routinely used in this population. The expert panel has noted that to date there are no data showing that the PCV 13 vaccine is effective in preventing pneumococcal pneumonia in adults, although a Dutch trial in 85,000 people over age 65 is underway.
The committee also noted that one-quarter of all cases of invasive pneumococcal disease in seniors are caused by 11 serotypes in the PPSV 23 vaccine that are not included in the PCV 13 vaccine, which further complicates the situation. The PPSV 23 vaccine has been approved since 1983 and is recommended for use in all adults over age 65 and in younger adults with certain underlying medical conditions, including diabetes and chronic lung disease.
Also, if the serotypes contained in the PCV 13 vaccine are going to largely go away in the senior population as a consequence of universal pediatric immunization, which could happen based upon the earlier herd immunity experience noted with the PCV 7 vaccine, then it may not be reasonable to give PCV 13 to all older adults, Dr. Glodé noted.
She reported serving on the data safety monitoring board for trials of an unrelated Pfizer vaccine.
VAIL, COLO. - American medicine emphatically surpassed the Healthy People 2010 goal for reduction of invasive Streptococcus pneumoniae infections well ahead of schedule in both of the highest-risk target groups: children under age 5 years and seniors. And the tougher Healthy People 2020 objectives are already well within striking distance.
"We get an A+ on this," Dr. Mary P. Glodé commented at the annual pediatric infectious diseases conference sponsored by Children’s Hospital Colorado.
The Healthy People 2010 objective was to reduce the incidence of invasive S. pneumoniae infections to 46 cases per 100,000 among children under age 5 years and to 42 per 100,000 persons age 65 or older. The actual 2010 rates were 19 and 36 per 100,000, respectively.
Between 1999 and 2010, the annual rate of invasive pneumococcal disease in children younger age 5 plummeted by 86% as a consequence of the licensure in 2000 of the pneumococcal conjugate vaccine 7 (PCV 7) vaccine.
Most impressively, the rate also fell by 50% during that period among seniors, even though they didn’t receive the vaccine. This is ascribed to herd immunity. The presumed mechanism is that once immunized, young children were far less likely to become colonized by virulent pneumococcal serotypes, with resultant diminished opportunity for transmission of the pathogens to older children and adults, explained Dr. Glodé, professor of pediatrics and head of the section of pediatric infectious disease at the University of Colorado, Denver, and Children’s Hospital Colorado.
The Healthy People 2020 goal is to further reduce the incidence of invasive pneumococcal infections in children under age 5 from the 2010 rate of 19 down to 12 per 100,000, and in seniors from the 2010 figure of 36 per 100,000 to 31 per 100,000.
The expectation is that the target in children will be handily reached, and early on, as a consequence of the spring 2010 recommendation by the American Academy of Pediatrics and the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) that all children aged 2-59 months be routinely vaccinated with the PCV 13 vaccine, which contains the PCV 7 serotypes plus six others causing invasive disease. Early unpublished CDC data suggest there has already been some decline in disease in children as a result of the added serotypes included in PCV 13, especially in cases involving serotypes 19A and 7F, according to Dr. Glodé.
The difficult unanswered question concerns the best way to get the nation’s seniors to the Healthy People 2020 target. The rate of invasive pneumococcal disease is higher in persons aged 65 and older than in any other age group, as is the associated mortality. Indeed, of an estimated 44,000 cases of invasive pneumococcal disease in the United States during 2009, 37,000 were in adults, including 15,000 cases in individuals age 65 or more.
Late last year the Food and Drug Administration licensed the PCV 13 vaccine for use in people aged 50 and up. But that doesn’t necessarily mean it will see widespread use. There has been no official recommendation from the ACIP that the vaccine be routinely used in this population. The expert panel has noted that to date there are no data showing that the PCV 13 vaccine is effective in preventing pneumococcal pneumonia in adults, although a Dutch trial in 85,000 people over age 65 is underway.
The committee also noted that one-quarter of all cases of invasive pneumococcal disease in seniors are caused by 11 serotypes in the PPSV 23 vaccine that are not included in the PCV 13 vaccine, which further complicates the situation. The PPSV 23 vaccine has been approved since 1983 and is recommended for use in all adults over age 65 and in younger adults with certain underlying medical conditions, including diabetes and chronic lung disease.
Also, if the serotypes contained in the PCV 13 vaccine are going to largely go away in the senior population as a consequence of universal pediatric immunization, which could happen based upon the earlier herd immunity experience noted with the PCV 7 vaccine, then it may not be reasonable to give PCV 13 to all older adults, Dr. Glodé noted.
She reported serving on the data safety monitoring board for trials of an unrelated Pfizer vaccine.
EXPERT ANALYSIS FROM THE ANNUAL PEDIATRIC INFECTIOUS DISEASES CONFERENCE SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Guideline Reflects New Thinking on Renal Scarring
VAIL, COLO. – The most recent American Academy of Pediatrics guidelines on management of urinary tract infections do an abrupt about-face by recommending that a voiding cystourethrogram no longer be routinely performed after a first febrile UTI in children aged 2 months to 2 years.
"This is confusing. On a dime, we have changed our recommendations regarding radiographic imaging," noted Dr. John W. Ogle, vice chair and director of pediatrics at Denver Health.
The previous 1999 AAP guidelines on UTIs stated unequivocally that "infants and children 2 months through 2 years of age who have the expected response to antimicrobials should have a sonogram and either voiding cystourethrogram or radionuclide scan at the earliest convenient time" (Pediatrics 1999;10[4 Pt 1]:843-52).
This sharp shift away from this stance in the current guidelines reflects new thinking regarding the pathogenesis of renal scarring, Dr. Ogle said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"The assumption that has been made in the past is that UTI in the presence of reflux is the primary thing that leads to renal scarring. That’s not so clear anymore. Some of what we call scarring may be congenital cortical defects in the kidney that we discover because we’ve studied the child. There are good studies demonstrating that you can have scarring from pyelonephritis with complete absence of reflux," said Dr. Ogle, who is also a professor of pediatrics at the University of Colorado, Denver.
The operative hypothesis up until the current guidelines were released in 2011 (Pediatrics 2011;128:595-610) was that identifying reflux via a voiding cystourethrogram (VCUG) allowed physicians to intervene surgically or with prophylactic antimicrobials to prevent further reflux nephropathy with further scarring.
"The evidence seems to be quite clear now that the interventions don’t change those important outcomes of renal scarring. So why look for the reflux? Why do the study, with its cost and discomfort, if you don’t have data that an intervention is going to positively affect the child?" Dr. Ogle said.
A key piece of evidence that triggered the change in AAP recommendations was a Cochrane review of 11 studies totaling 1,148 children. The analysis concluded that correction of vesicoureteral reflux by surgery or medical interventions did not reduce the risk of renal scarring (Cochrane Database Syst. Rev. 2007 July 18;(3):CD001532).
The shift in the AAP guidelines has generated controversy among urologists, some of whom are in agreement with the change while others are opposed.
"I think for pediatricians, adopting these guidelines is an easy step," according to Dr. Ogle. "Many pediatricians in office practice had already adopted this years ago. They have not routinely done a VCUG on every child that presented with a febrile UTI. So for them, the impact of the 2011 Academy guidelines has been to say, ‘What you’ve been doing for the last 10 years is probably appropriate.’ "
He predicted that the current guidelines won’t be the final word on the topic of imaging in patients with febrile UTIs.
"What the guidelines don’t tell us is who exactly you should worry about. They don’t tell you which first-time UTIs you should consider VCUG in, and what’s the strategy for second-time UTIs. So stay tuned, there’s going to be further debate with regard to this, and hopefully further evidence," Dr. Ogle said.
"Remember," he continued, "the great history of American medicine is that first we adopt strategies for interventions in medical conditions, then we study them to see if our interventions are right."
Dr. Ogle reported having no relevant financial conflicts.
VAIL, COLO. – The most recent American Academy of Pediatrics guidelines on management of urinary tract infections do an abrupt about-face by recommending that a voiding cystourethrogram no longer be routinely performed after a first febrile UTI in children aged 2 months to 2 years.
"This is confusing. On a dime, we have changed our recommendations regarding radiographic imaging," noted Dr. John W. Ogle, vice chair and director of pediatrics at Denver Health.
The previous 1999 AAP guidelines on UTIs stated unequivocally that "infants and children 2 months through 2 years of age who have the expected response to antimicrobials should have a sonogram and either voiding cystourethrogram or radionuclide scan at the earliest convenient time" (Pediatrics 1999;10[4 Pt 1]:843-52).
This sharp shift away from this stance in the current guidelines reflects new thinking regarding the pathogenesis of renal scarring, Dr. Ogle said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"The assumption that has been made in the past is that UTI in the presence of reflux is the primary thing that leads to renal scarring. That’s not so clear anymore. Some of what we call scarring may be congenital cortical defects in the kidney that we discover because we’ve studied the child. There are good studies demonstrating that you can have scarring from pyelonephritis with complete absence of reflux," said Dr. Ogle, who is also a professor of pediatrics at the University of Colorado, Denver.
The operative hypothesis up until the current guidelines were released in 2011 (Pediatrics 2011;128:595-610) was that identifying reflux via a voiding cystourethrogram (VCUG) allowed physicians to intervene surgically or with prophylactic antimicrobials to prevent further reflux nephropathy with further scarring.
"The evidence seems to be quite clear now that the interventions don’t change those important outcomes of renal scarring. So why look for the reflux? Why do the study, with its cost and discomfort, if you don’t have data that an intervention is going to positively affect the child?" Dr. Ogle said.
A key piece of evidence that triggered the change in AAP recommendations was a Cochrane review of 11 studies totaling 1,148 children. The analysis concluded that correction of vesicoureteral reflux by surgery or medical interventions did not reduce the risk of renal scarring (Cochrane Database Syst. Rev. 2007 July 18;(3):CD001532).
The shift in the AAP guidelines has generated controversy among urologists, some of whom are in agreement with the change while others are opposed.
"I think for pediatricians, adopting these guidelines is an easy step," according to Dr. Ogle. "Many pediatricians in office practice had already adopted this years ago. They have not routinely done a VCUG on every child that presented with a febrile UTI. So for them, the impact of the 2011 Academy guidelines has been to say, ‘What you’ve been doing for the last 10 years is probably appropriate.’ "
He predicted that the current guidelines won’t be the final word on the topic of imaging in patients with febrile UTIs.
"What the guidelines don’t tell us is who exactly you should worry about. They don’t tell you which first-time UTIs you should consider VCUG in, and what’s the strategy for second-time UTIs. So stay tuned, there’s going to be further debate with regard to this, and hopefully further evidence," Dr. Ogle said.
"Remember," he continued, "the great history of American medicine is that first we adopt strategies for interventions in medical conditions, then we study them to see if our interventions are right."
Dr. Ogle reported having no relevant financial conflicts.
VAIL, COLO. – The most recent American Academy of Pediatrics guidelines on management of urinary tract infections do an abrupt about-face by recommending that a voiding cystourethrogram no longer be routinely performed after a first febrile UTI in children aged 2 months to 2 years.
"This is confusing. On a dime, we have changed our recommendations regarding radiographic imaging," noted Dr. John W. Ogle, vice chair and director of pediatrics at Denver Health.
The previous 1999 AAP guidelines on UTIs stated unequivocally that "infants and children 2 months through 2 years of age who have the expected response to antimicrobials should have a sonogram and either voiding cystourethrogram or radionuclide scan at the earliest convenient time" (Pediatrics 1999;10[4 Pt 1]:843-52).
This sharp shift away from this stance in the current guidelines reflects new thinking regarding the pathogenesis of renal scarring, Dr. Ogle said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"The assumption that has been made in the past is that UTI in the presence of reflux is the primary thing that leads to renal scarring. That’s not so clear anymore. Some of what we call scarring may be congenital cortical defects in the kidney that we discover because we’ve studied the child. There are good studies demonstrating that you can have scarring from pyelonephritis with complete absence of reflux," said Dr. Ogle, who is also a professor of pediatrics at the University of Colorado, Denver.
The operative hypothesis up until the current guidelines were released in 2011 (Pediatrics 2011;128:595-610) was that identifying reflux via a voiding cystourethrogram (VCUG) allowed physicians to intervene surgically or with prophylactic antimicrobials to prevent further reflux nephropathy with further scarring.
"The evidence seems to be quite clear now that the interventions don’t change those important outcomes of renal scarring. So why look for the reflux? Why do the study, with its cost and discomfort, if you don’t have data that an intervention is going to positively affect the child?" Dr. Ogle said.
A key piece of evidence that triggered the change in AAP recommendations was a Cochrane review of 11 studies totaling 1,148 children. The analysis concluded that correction of vesicoureteral reflux by surgery or medical interventions did not reduce the risk of renal scarring (Cochrane Database Syst. Rev. 2007 July 18;(3):CD001532).
The shift in the AAP guidelines has generated controversy among urologists, some of whom are in agreement with the change while others are opposed.
"I think for pediatricians, adopting these guidelines is an easy step," according to Dr. Ogle. "Many pediatricians in office practice had already adopted this years ago. They have not routinely done a VCUG on every child that presented with a febrile UTI. So for them, the impact of the 2011 Academy guidelines has been to say, ‘What you’ve been doing for the last 10 years is probably appropriate.’ "
He predicted that the current guidelines won’t be the final word on the topic of imaging in patients with febrile UTIs.
"What the guidelines don’t tell us is who exactly you should worry about. They don’t tell you which first-time UTIs you should consider VCUG in, and what’s the strategy for second-time UTIs. So stay tuned, there’s going to be further debate with regard to this, and hopefully further evidence," Dr. Ogle said.
"Remember," he continued, "the great history of American medicine is that first we adopt strategies for interventions in medical conditions, then we study them to see if our interventions are right."
Dr. Ogle reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
HPV Vaccine's Benefits Are Mainly Extracervical
VAIL, COLO. – The human papillomavirus vaccine is still widely perceived as a tool aimed at preventing cervical cancer, yet in fact roughly 55% of all the cancers it should protect against occur at other sites, according to Dr. Myron J. Levin.
The extracervical malignancies associated with HPV types covered by the two commercially available vaccines include anorectal and oropharyngeal cancers in both women and men, as well as penile cancer.
Indeed, one-third of all HPV-related cancers occur in men, not in women, which is one reason that last year the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended routine HPV vaccination for 11- to 12-year-old boys, as is already the case for girls of the same age. Also, protecting boys will secondarily increase protection against cervical cancer in girls.
In light of the vaccine’s impressive clinical benefits, favorable cost-benefit estimates, and excellent safety record to date, the lagging U.S. HPV vaccination rates are disturbing, Dr. Levin said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado. Data from the CDC’s 2010 National Immunization Survey – Teen indicate only 23% of 13-year-old girls had received the three-dose series. The Healthy People 2020 goal is for 80% of 13- to 15-year-olds to have received three doses.
Even though the recommendation is for routine immunization at age 11-12, it’s Dr. Levin’s impression that many physicians are putting it off until their patients are 15-17 years old.
"I think we’re making a mistake. I think we’re missing a big opportunity. A significant number of girls become sexually active before age 15, and waiting until they’re that age to immunize them may compromise their chance of protection. All those favorable cost-benefit analyses don’t count if you don’t get the vaccine," said Dr. Levin, professor of pediatrics and medicine at the University of Colorado at Denver.
HPV is the most common sexually transmitted infection worldwide. Three-quarters of the general population become infected, and three-quarters of those infections occur at 15-24 years of age. Moreover, more than 50% of those who become infected do so within 2 years after becoming sexually active – and studies show that more than 20% of males and females have already had vaginal sex by age 15.
Beyond the whole issue of vaccine-preventable HPV-associated cancers, there is the matter of genital warts, or condylomata acuminata. The incidence of genital warts is about 1% per year among sexually active people. In 2010 there were 376,000 initial physician office visits for genital warts, according to data from the National Disease and Therapeutic Index. The cost of treatment is $300-$1,000 per case, and recurrences are common. Up to 90% of cases of genital warts are caused by HPV types 6 and 11, two of the four types targeted by one of the two commercially available vaccines. The incubation period for genital warts is just a few months, compared with years or decades for HPV-related malignancies.
Dr. Levin highlighted landmark research from Australia demonstrating the profound impact widespread adoption of the quadrivalent HPV vaccine can have at the population level. Australia was the first country to fund a vaccination program for all females aged 12-26 years, starting in July 2007. A national surveillance program demonstrated a 59% reduction in new diagnoses of genital warts among women eligible for the free vaccine during the first 2 years after the program started (Lancet Infect. Dis. 2011;11:39-44).
Interestingly, there was also a 39% drop in new cases among heterosexual Australian males aged 12-26, even though they weren’t included in the vaccine program. This is evidence of herd immunity, Dr. Levin said. In contrast, rates remained unchanged among men who have sex with men.
In a subsequent report with updated data through mid-2011, Australian investigators described "the dramatic decline and near disappearance" of genital warts in women and heterosexual men under age 21 years 4 years after the start of the national HPV vaccination program targeting females (Sex. Transm. Infect. 2011;87:544-7).
One development worth keeping an eye on is the possibility that patients may not really need three doses of HPV vaccine to be protected, Dr. Levin said. This prospect was raised by investigators at the National Cancer Institute, who observed in a large Costa Rican randomized clinical trial that the efficacy of GlaxoSmithKline’s bivalent HPV 16/18 vaccine was comparable after a median 4.2 years of follow-up in women who didn’t come back for their third dose and in those who received all three. Since the three-dose regimen is expensive and difficult to complete, a two-dose strategy could be particularly important in resource-poor countries (J. Natl. Cancer Inst. 2011;103:1444-51).
"We don’t know that two doses would be as good as three for the duration of a woman’s life or a man’s life, but it may be good enough. You really want to protect people when they’re most sexually active and most likely to have multiple partners. Then when they settle down, they’re less likely to be at risk, and it doesn’t matter as much if they’re not as protected," Dr. Levin explained.
He reported that he serves as a consultant to Merck and GlaxoSmithKline and holds intellectual property rights involving Merck’s Zostavax shingles vaccine.
VAIL, COLO. – The human papillomavirus vaccine is still widely perceived as a tool aimed at preventing cervical cancer, yet in fact roughly 55% of all the cancers it should protect against occur at other sites, according to Dr. Myron J. Levin.
The extracervical malignancies associated with HPV types covered by the two commercially available vaccines include anorectal and oropharyngeal cancers in both women and men, as well as penile cancer.
Indeed, one-third of all HPV-related cancers occur in men, not in women, which is one reason that last year the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended routine HPV vaccination for 11- to 12-year-old boys, as is already the case for girls of the same age. Also, protecting boys will secondarily increase protection against cervical cancer in girls.
In light of the vaccine’s impressive clinical benefits, favorable cost-benefit estimates, and excellent safety record to date, the lagging U.S. HPV vaccination rates are disturbing, Dr. Levin said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado. Data from the CDC’s 2010 National Immunization Survey – Teen indicate only 23% of 13-year-old girls had received the three-dose series. The Healthy People 2020 goal is for 80% of 13- to 15-year-olds to have received three doses.
Even though the recommendation is for routine immunization at age 11-12, it’s Dr. Levin’s impression that many physicians are putting it off until their patients are 15-17 years old.
"I think we’re making a mistake. I think we’re missing a big opportunity. A significant number of girls become sexually active before age 15, and waiting until they’re that age to immunize them may compromise their chance of protection. All those favorable cost-benefit analyses don’t count if you don’t get the vaccine," said Dr. Levin, professor of pediatrics and medicine at the University of Colorado at Denver.
HPV is the most common sexually transmitted infection worldwide. Three-quarters of the general population become infected, and three-quarters of those infections occur at 15-24 years of age. Moreover, more than 50% of those who become infected do so within 2 years after becoming sexually active – and studies show that more than 20% of males and females have already had vaginal sex by age 15.
Beyond the whole issue of vaccine-preventable HPV-associated cancers, there is the matter of genital warts, or condylomata acuminata. The incidence of genital warts is about 1% per year among sexually active people. In 2010 there were 376,000 initial physician office visits for genital warts, according to data from the National Disease and Therapeutic Index. The cost of treatment is $300-$1,000 per case, and recurrences are common. Up to 90% of cases of genital warts are caused by HPV types 6 and 11, two of the four types targeted by one of the two commercially available vaccines. The incubation period for genital warts is just a few months, compared with years or decades for HPV-related malignancies.
Dr. Levin highlighted landmark research from Australia demonstrating the profound impact widespread adoption of the quadrivalent HPV vaccine can have at the population level. Australia was the first country to fund a vaccination program for all females aged 12-26 years, starting in July 2007. A national surveillance program demonstrated a 59% reduction in new diagnoses of genital warts among women eligible for the free vaccine during the first 2 years after the program started (Lancet Infect. Dis. 2011;11:39-44).
Interestingly, there was also a 39% drop in new cases among heterosexual Australian males aged 12-26, even though they weren’t included in the vaccine program. This is evidence of herd immunity, Dr. Levin said. In contrast, rates remained unchanged among men who have sex with men.
In a subsequent report with updated data through mid-2011, Australian investigators described "the dramatic decline and near disappearance" of genital warts in women and heterosexual men under age 21 years 4 years after the start of the national HPV vaccination program targeting females (Sex. Transm. Infect. 2011;87:544-7).
One development worth keeping an eye on is the possibility that patients may not really need three doses of HPV vaccine to be protected, Dr. Levin said. This prospect was raised by investigators at the National Cancer Institute, who observed in a large Costa Rican randomized clinical trial that the efficacy of GlaxoSmithKline’s bivalent HPV 16/18 vaccine was comparable after a median 4.2 years of follow-up in women who didn’t come back for their third dose and in those who received all three. Since the three-dose regimen is expensive and difficult to complete, a two-dose strategy could be particularly important in resource-poor countries (J. Natl. Cancer Inst. 2011;103:1444-51).
"We don’t know that two doses would be as good as three for the duration of a woman’s life or a man’s life, but it may be good enough. You really want to protect people when they’re most sexually active and most likely to have multiple partners. Then when they settle down, they’re less likely to be at risk, and it doesn’t matter as much if they’re not as protected," Dr. Levin explained.
He reported that he serves as a consultant to Merck and GlaxoSmithKline and holds intellectual property rights involving Merck’s Zostavax shingles vaccine.
VAIL, COLO. – The human papillomavirus vaccine is still widely perceived as a tool aimed at preventing cervical cancer, yet in fact roughly 55% of all the cancers it should protect against occur at other sites, according to Dr. Myron J. Levin.
The extracervical malignancies associated with HPV types covered by the two commercially available vaccines include anorectal and oropharyngeal cancers in both women and men, as well as penile cancer.
Indeed, one-third of all HPV-related cancers occur in men, not in women, which is one reason that last year the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended routine HPV vaccination for 11- to 12-year-old boys, as is already the case for girls of the same age. Also, protecting boys will secondarily increase protection against cervical cancer in girls.
In light of the vaccine’s impressive clinical benefits, favorable cost-benefit estimates, and excellent safety record to date, the lagging U.S. HPV vaccination rates are disturbing, Dr. Levin said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado. Data from the CDC’s 2010 National Immunization Survey – Teen indicate only 23% of 13-year-old girls had received the three-dose series. The Healthy People 2020 goal is for 80% of 13- to 15-year-olds to have received three doses.
Even though the recommendation is for routine immunization at age 11-12, it’s Dr. Levin’s impression that many physicians are putting it off until their patients are 15-17 years old.
"I think we’re making a mistake. I think we’re missing a big opportunity. A significant number of girls become sexually active before age 15, and waiting until they’re that age to immunize them may compromise their chance of protection. All those favorable cost-benefit analyses don’t count if you don’t get the vaccine," said Dr. Levin, professor of pediatrics and medicine at the University of Colorado at Denver.
HPV is the most common sexually transmitted infection worldwide. Three-quarters of the general population become infected, and three-quarters of those infections occur at 15-24 years of age. Moreover, more than 50% of those who become infected do so within 2 years after becoming sexually active – and studies show that more than 20% of males and females have already had vaginal sex by age 15.
Beyond the whole issue of vaccine-preventable HPV-associated cancers, there is the matter of genital warts, or condylomata acuminata. The incidence of genital warts is about 1% per year among sexually active people. In 2010 there were 376,000 initial physician office visits for genital warts, according to data from the National Disease and Therapeutic Index. The cost of treatment is $300-$1,000 per case, and recurrences are common. Up to 90% of cases of genital warts are caused by HPV types 6 and 11, two of the four types targeted by one of the two commercially available vaccines. The incubation period for genital warts is just a few months, compared with years or decades for HPV-related malignancies.
Dr. Levin highlighted landmark research from Australia demonstrating the profound impact widespread adoption of the quadrivalent HPV vaccine can have at the population level. Australia was the first country to fund a vaccination program for all females aged 12-26 years, starting in July 2007. A national surveillance program demonstrated a 59% reduction in new diagnoses of genital warts among women eligible for the free vaccine during the first 2 years after the program started (Lancet Infect. Dis. 2011;11:39-44).
Interestingly, there was also a 39% drop in new cases among heterosexual Australian males aged 12-26, even though they weren’t included in the vaccine program. This is evidence of herd immunity, Dr. Levin said. In contrast, rates remained unchanged among men who have sex with men.
In a subsequent report with updated data through mid-2011, Australian investigators described "the dramatic decline and near disappearance" of genital warts in women and heterosexual men under age 21 years 4 years after the start of the national HPV vaccination program targeting females (Sex. Transm. Infect. 2011;87:544-7).
One development worth keeping an eye on is the possibility that patients may not really need three doses of HPV vaccine to be protected, Dr. Levin said. This prospect was raised by investigators at the National Cancer Institute, who observed in a large Costa Rican randomized clinical trial that the efficacy of GlaxoSmithKline’s bivalent HPV 16/18 vaccine was comparable after a median 4.2 years of follow-up in women who didn’t come back for their third dose and in those who received all three. Since the three-dose regimen is expensive and difficult to complete, a two-dose strategy could be particularly important in resource-poor countries (J. Natl. Cancer Inst. 2011;103:1444-51).
"We don’t know that two doses would be as good as three for the duration of a woman’s life or a man’s life, but it may be good enough. You really want to protect people when they’re most sexually active and most likely to have multiple partners. Then when they settle down, they’re less likely to be at risk, and it doesn’t matter as much if they’re not as protected," Dr. Levin explained.
He reported that he serves as a consultant to Merck and GlaxoSmithKline and holds intellectual property rights involving Merck’s Zostavax shingles vaccine.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
STDs: Expedited Partner Therapy Garners Attention
VAIL, COLO. – Expedited partner therapy is a novel approach to the treatment of sexually transmitted infections that’s catching on across much of the United States.
In expedited partner therapy, the physician treats the sexual partners of a patient diagnosed with chlamydia or gonorrhea by providing prescriptions or actual medications for the patient to pass along to his or her partners without prior examination of the partners by the health care provider.
"It’s a new concept that sometimes makes people uncomfortable. You have no relationship with the patient’s partners. But when you think about it from the public health perspective, you’re treating the sexual partners – and you may be preventing your patient from being reinfected," Dr. Ann-Christine Nyquist said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
Ideally, the patient’s partner or partners should come in for a complete sexually transmitted disease (STD) evaluation and counseling plus treatment, but many times it’s just not possible to get them into care, said Dr. Nyquist, professor of pediatrics and associate dean at the University of Colorado, Denver.
Experts in the Division of STD Prevention at the Centers for Disease Control and Prevention are enthusiastic about expedited partner therapy. They see it as a way to put a dent in the estimated 3 million new cases of chlamydia and 700,000 of gonorrhea per year in the United States. Together with legal scholars at the Arizona State University College of Law in Tempe, the CDC has created a "Legal/Policy Toolkit for Adoption and Implementation of Expedited Partner Therapy."
The toolkit provides model legislation for consideration by state lawmakers. It also includes answers to frequently asked questions from physicians and pharmacists regarding issues such as prescription labeling requirements, breach of confidentiality considerations, and how the HIPAA Privacy Rule bears on expedited partner therapy.
At present, expedited partner therapy is permissible in 32 states, potentially allowable in 11, and prohibited in 7: Arkansas, Florida, Kentucky, Michigan, Ohio, Oklahoma, and West Virginia.
"You need to find out what the legal landscape is in your state," Dr. Nyquist advised.
She reported having no financial conflicts of interest.
VAIL, COLO. – Expedited partner therapy is a novel approach to the treatment of sexually transmitted infections that’s catching on across much of the United States.
In expedited partner therapy, the physician treats the sexual partners of a patient diagnosed with chlamydia or gonorrhea by providing prescriptions or actual medications for the patient to pass along to his or her partners without prior examination of the partners by the health care provider.
"It’s a new concept that sometimes makes people uncomfortable. You have no relationship with the patient’s partners. But when you think about it from the public health perspective, you’re treating the sexual partners – and you may be preventing your patient from being reinfected," Dr. Ann-Christine Nyquist said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
Ideally, the patient’s partner or partners should come in for a complete sexually transmitted disease (STD) evaluation and counseling plus treatment, but many times it’s just not possible to get them into care, said Dr. Nyquist, professor of pediatrics and associate dean at the University of Colorado, Denver.
Experts in the Division of STD Prevention at the Centers for Disease Control and Prevention are enthusiastic about expedited partner therapy. They see it as a way to put a dent in the estimated 3 million new cases of chlamydia and 700,000 of gonorrhea per year in the United States. Together with legal scholars at the Arizona State University College of Law in Tempe, the CDC has created a "Legal/Policy Toolkit for Adoption and Implementation of Expedited Partner Therapy."
The toolkit provides model legislation for consideration by state lawmakers. It also includes answers to frequently asked questions from physicians and pharmacists regarding issues such as prescription labeling requirements, breach of confidentiality considerations, and how the HIPAA Privacy Rule bears on expedited partner therapy.
At present, expedited partner therapy is permissible in 32 states, potentially allowable in 11, and prohibited in 7: Arkansas, Florida, Kentucky, Michigan, Ohio, Oklahoma, and West Virginia.
"You need to find out what the legal landscape is in your state," Dr. Nyquist advised.
She reported having no financial conflicts of interest.
VAIL, COLO. – Expedited partner therapy is a novel approach to the treatment of sexually transmitted infections that’s catching on across much of the United States.
In expedited partner therapy, the physician treats the sexual partners of a patient diagnosed with chlamydia or gonorrhea by providing prescriptions or actual medications for the patient to pass along to his or her partners without prior examination of the partners by the health care provider.
"It’s a new concept that sometimes makes people uncomfortable. You have no relationship with the patient’s partners. But when you think about it from the public health perspective, you’re treating the sexual partners – and you may be preventing your patient from being reinfected," Dr. Ann-Christine Nyquist said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
Ideally, the patient’s partner or partners should come in for a complete sexually transmitted disease (STD) evaluation and counseling plus treatment, but many times it’s just not possible to get them into care, said Dr. Nyquist, professor of pediatrics and associate dean at the University of Colorado, Denver.
Experts in the Division of STD Prevention at the Centers for Disease Control and Prevention are enthusiastic about expedited partner therapy. They see it as a way to put a dent in the estimated 3 million new cases of chlamydia and 700,000 of gonorrhea per year in the United States. Together with legal scholars at the Arizona State University College of Law in Tempe, the CDC has created a "Legal/Policy Toolkit for Adoption and Implementation of Expedited Partner Therapy."
The toolkit provides model legislation for consideration by state lawmakers. It also includes answers to frequently asked questions from physicians and pharmacists regarding issues such as prescription labeling requirements, breach of confidentiality considerations, and how the HIPAA Privacy Rule bears on expedited partner therapy.
At present, expedited partner therapy is permissible in 32 states, potentially allowable in 11, and prohibited in 7: Arkansas, Florida, Kentucky, Michigan, Ohio, Oklahoma, and West Virginia.
"You need to find out what the legal landscape is in your state," Dr. Nyquist advised.
She reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Pediatric CAP Guidelines: 'It's All About Pneumococcus'
VAIL, COLO. – A major theme running through the latest guidelines for management of community-acquired pneumonia in children is that Streptococcus pneumoniae is the most common bacterial pathogen – and the best target for empiric therapy.
"It’s really all about pneumococcus," declared Dr. Mark J. Abzug, professor of pediatrics at the University of Colorado, Denver.
The guidelines put forth jointly by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America endorse high-dose amoxicillin as first-line therapy for previously healthy, appropriately immunized infants, preschoolers, school-aged children, and adolescents with mild to moderate community-acquired pneumonia (CAP) of suspected bacterial origin (Clin. Infect. Dis. 2011; 53: e25-76).
High-dose oral amoxicillin at 90 mg/kg per day covers 87%-95% of S. pneumoniae isolates nationally, whereas most second- and third-generation oral cephalosporins cover only 60%-70%. Azithromycin isn’t recommended for suspected pneumococcal CAP because of an associated resistance rate of up to 40%. Amoxicillin/clavulanic acid offers no incremental benefit over amoxicillin alone for pneumococcus, Dr. Abzug observed at the conference, which was sponsored by Children’s Hospital Colorado.
The guidelines recommend b.i.d. dosing of amoxicillin based largely on extrapolation from experience in acute otitis media. But Dr. Abzug takes issue with that guidance.
"I’m going to beg to differ with that recommendation and suggest that for pneumonia, which is a bit different from otitis, dividing t.i.d. is going to be better," the pediatrician asserted.
Modeling studies indicate b.i.d. dosing of amoxicillin at 90 mg/kg per day is effective for 99% of highly susceptible (minimal inhibitory concentration of 0.5 mcg/mL) S. pneumococcus isolates, but only for 65% of strains with a minimum inhibitory concentration (MIC) of 2 mcg/mL, whereas t.i.d. dosing is sufficient for 90% of such strains. And at Children’s Hospital Colorado, nearly 20% of S. pneumoniae isolates in 2011 were intermediately susceptible (MIC = 4 mcg/mL) or resistant (MIC = 8 mcg/mL) to penicillin.
"For the 20% or so that are intermediate or resistant, b.i.d. dosing is not going to be the answer," Dr. Abzug stressed.
For patients with nonserious amoxicillin allergies, the guidelines recommend cefuroxime, cefprozil, or cefpodoxime as oral alternatives; nationally, 67%-80% of S. pneumoniae strains are susceptible to these agents. Clindamycin is an alternative option. Levofloxacin and linezolid are effective for close to 100% of isolates, but are best reserved for second- or third-line therapy.
It may come as a surprise to many physicians that the guidelines deem routine chest x-rays "not necessary" for suspected CAP in the outpatient setting.
"Will this encourage antibiotic overuse? The guidelines don’t address this," the pediatrician noted.
The guidelines also recommend against routine complete blood counts, blood cultures, and urinary antigen detection tests in outpatients.
In fully immunized children with suspected bacterial CAP sufficiently serious for hospitalization, the recommendation is for parenteral ampicillin or penicillin G so long as local epidemiologic data indicate a lack of substantial resistance for invasive pneumococci as defined by an MIC greater than 8 mcg/mL and the patient doesn’t have empyema or other potentially life-threatening complications. When those conditions aren’t met, however, the guidelines endorse the third-generation cephalosporins ceftriaxone or cefotaxime.
Dr. Abzug applauded a recent study by pediatricians at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., that provided support in everyday clinical practice for the ampicillin-first management strategy recommended in the national guidelines. The retrospective study included 1,033 patients admitted with CAP to the tertiary referral hospital during the 12 months before and after the 2008 introduction of a clinical practice guideline encouraging the use of ampicillin as the first-line empiric antibiotic in previously healthy children with uncomplicated CAP.
The Kansas City pediatricians noted that the use of ampicillin as first-line therapy has historically been uncommon at tertiary children’s hospitals, as evidenced by a mere 5.5% rate in a study using the Pediatric Hospital Information Systems database (Pediatrics 2011;127:e255-63). The goal was to turn that situation around at Children’s Mercy Hospitals and Clinics, since ampicillin is a narrower-spectrum antibiotic than the third-generation cephalosporins, and hence less likely to promote antibiotic resistance.
Prior to introduction of the hospital guideline, ceftriaxone was prescribed as empiric therapy for CAP in 72% of cases, with ampicillin being the second most commonly prescribed antibiotic at 13%. In the year after the guideline was introduced, ampicillin was the most common antibiotic, prescribed in 63% of cases, with ceftriaxone prescribed in 21%. And even though the prevalence of S. pneumoniae isolates with intermediate susceptibility or resistance to penicillin was 24% at the hospital during that time period, the change in therapy didn’t result in an increase in adverse outcomes: The preguideline treatment failure rate was 1.5% and the postguideline rate was similar at 1% (Pediatrics 2012;129:e597-604).
"Here’s some real-world data that suggest ampicillin for the most part is probably sufficient as the parenteral empiric drug of choice for uncomplicated CAP," Dr. Abzug commented.
Dr. Abzug reported having no financial conflicts.
VAIL, COLO. – A major theme running through the latest guidelines for management of community-acquired pneumonia in children is that Streptococcus pneumoniae is the most common bacterial pathogen – and the best target for empiric therapy.
"It’s really all about pneumococcus," declared Dr. Mark J. Abzug, professor of pediatrics at the University of Colorado, Denver.
The guidelines put forth jointly by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America endorse high-dose amoxicillin as first-line therapy for previously healthy, appropriately immunized infants, preschoolers, school-aged children, and adolescents with mild to moderate community-acquired pneumonia (CAP) of suspected bacterial origin (Clin. Infect. Dis. 2011; 53: e25-76).
High-dose oral amoxicillin at 90 mg/kg per day covers 87%-95% of S. pneumoniae isolates nationally, whereas most second- and third-generation oral cephalosporins cover only 60%-70%. Azithromycin isn’t recommended for suspected pneumococcal CAP because of an associated resistance rate of up to 40%. Amoxicillin/clavulanic acid offers no incremental benefit over amoxicillin alone for pneumococcus, Dr. Abzug observed at the conference, which was sponsored by Children’s Hospital Colorado.
The guidelines recommend b.i.d. dosing of amoxicillin based largely on extrapolation from experience in acute otitis media. But Dr. Abzug takes issue with that guidance.
"I’m going to beg to differ with that recommendation and suggest that for pneumonia, which is a bit different from otitis, dividing t.i.d. is going to be better," the pediatrician asserted.
Modeling studies indicate b.i.d. dosing of amoxicillin at 90 mg/kg per day is effective for 99% of highly susceptible (minimal inhibitory concentration of 0.5 mcg/mL) S. pneumococcus isolates, but only for 65% of strains with a minimum inhibitory concentration (MIC) of 2 mcg/mL, whereas t.i.d. dosing is sufficient for 90% of such strains. And at Children’s Hospital Colorado, nearly 20% of S. pneumoniae isolates in 2011 were intermediately susceptible (MIC = 4 mcg/mL) or resistant (MIC = 8 mcg/mL) to penicillin.
"For the 20% or so that are intermediate or resistant, b.i.d. dosing is not going to be the answer," Dr. Abzug stressed.
For patients with nonserious amoxicillin allergies, the guidelines recommend cefuroxime, cefprozil, or cefpodoxime as oral alternatives; nationally, 67%-80% of S. pneumoniae strains are susceptible to these agents. Clindamycin is an alternative option. Levofloxacin and linezolid are effective for close to 100% of isolates, but are best reserved for second- or third-line therapy.
It may come as a surprise to many physicians that the guidelines deem routine chest x-rays "not necessary" for suspected CAP in the outpatient setting.
"Will this encourage antibiotic overuse? The guidelines don’t address this," the pediatrician noted.
The guidelines also recommend against routine complete blood counts, blood cultures, and urinary antigen detection tests in outpatients.
In fully immunized children with suspected bacterial CAP sufficiently serious for hospitalization, the recommendation is for parenteral ampicillin or penicillin G so long as local epidemiologic data indicate a lack of substantial resistance for invasive pneumococci as defined by an MIC greater than 8 mcg/mL and the patient doesn’t have empyema or other potentially life-threatening complications. When those conditions aren’t met, however, the guidelines endorse the third-generation cephalosporins ceftriaxone or cefotaxime.
Dr. Abzug applauded a recent study by pediatricians at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., that provided support in everyday clinical practice for the ampicillin-first management strategy recommended in the national guidelines. The retrospective study included 1,033 patients admitted with CAP to the tertiary referral hospital during the 12 months before and after the 2008 introduction of a clinical practice guideline encouraging the use of ampicillin as the first-line empiric antibiotic in previously healthy children with uncomplicated CAP.
The Kansas City pediatricians noted that the use of ampicillin as first-line therapy has historically been uncommon at tertiary children’s hospitals, as evidenced by a mere 5.5% rate in a study using the Pediatric Hospital Information Systems database (Pediatrics 2011;127:e255-63). The goal was to turn that situation around at Children’s Mercy Hospitals and Clinics, since ampicillin is a narrower-spectrum antibiotic than the third-generation cephalosporins, and hence less likely to promote antibiotic resistance.
Prior to introduction of the hospital guideline, ceftriaxone was prescribed as empiric therapy for CAP in 72% of cases, with ampicillin being the second most commonly prescribed antibiotic at 13%. In the year after the guideline was introduced, ampicillin was the most common antibiotic, prescribed in 63% of cases, with ceftriaxone prescribed in 21%. And even though the prevalence of S. pneumoniae isolates with intermediate susceptibility or resistance to penicillin was 24% at the hospital during that time period, the change in therapy didn’t result in an increase in adverse outcomes: The preguideline treatment failure rate was 1.5% and the postguideline rate was similar at 1% (Pediatrics 2012;129:e597-604).
"Here’s some real-world data that suggest ampicillin for the most part is probably sufficient as the parenteral empiric drug of choice for uncomplicated CAP," Dr. Abzug commented.
Dr. Abzug reported having no financial conflicts.
VAIL, COLO. – A major theme running through the latest guidelines for management of community-acquired pneumonia in children is that Streptococcus pneumoniae is the most common bacterial pathogen – and the best target for empiric therapy.
"It’s really all about pneumococcus," declared Dr. Mark J. Abzug, professor of pediatrics at the University of Colorado, Denver.
The guidelines put forth jointly by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America endorse high-dose amoxicillin as first-line therapy for previously healthy, appropriately immunized infants, preschoolers, school-aged children, and adolescents with mild to moderate community-acquired pneumonia (CAP) of suspected bacterial origin (Clin. Infect. Dis. 2011; 53: e25-76).
High-dose oral amoxicillin at 90 mg/kg per day covers 87%-95% of S. pneumoniae isolates nationally, whereas most second- and third-generation oral cephalosporins cover only 60%-70%. Azithromycin isn’t recommended for suspected pneumococcal CAP because of an associated resistance rate of up to 40%. Amoxicillin/clavulanic acid offers no incremental benefit over amoxicillin alone for pneumococcus, Dr. Abzug observed at the conference, which was sponsored by Children’s Hospital Colorado.
The guidelines recommend b.i.d. dosing of amoxicillin based largely on extrapolation from experience in acute otitis media. But Dr. Abzug takes issue with that guidance.
"I’m going to beg to differ with that recommendation and suggest that for pneumonia, which is a bit different from otitis, dividing t.i.d. is going to be better," the pediatrician asserted.
Modeling studies indicate b.i.d. dosing of amoxicillin at 90 mg/kg per day is effective for 99% of highly susceptible (minimal inhibitory concentration of 0.5 mcg/mL) S. pneumococcus isolates, but only for 65% of strains with a minimum inhibitory concentration (MIC) of 2 mcg/mL, whereas t.i.d. dosing is sufficient for 90% of such strains. And at Children’s Hospital Colorado, nearly 20% of S. pneumoniae isolates in 2011 were intermediately susceptible (MIC = 4 mcg/mL) or resistant (MIC = 8 mcg/mL) to penicillin.
"For the 20% or so that are intermediate or resistant, b.i.d. dosing is not going to be the answer," Dr. Abzug stressed.
For patients with nonserious amoxicillin allergies, the guidelines recommend cefuroxime, cefprozil, or cefpodoxime as oral alternatives; nationally, 67%-80% of S. pneumoniae strains are susceptible to these agents. Clindamycin is an alternative option. Levofloxacin and linezolid are effective for close to 100% of isolates, but are best reserved for second- or third-line therapy.
It may come as a surprise to many physicians that the guidelines deem routine chest x-rays "not necessary" for suspected CAP in the outpatient setting.
"Will this encourage antibiotic overuse? The guidelines don’t address this," the pediatrician noted.
The guidelines also recommend against routine complete blood counts, blood cultures, and urinary antigen detection tests in outpatients.
In fully immunized children with suspected bacterial CAP sufficiently serious for hospitalization, the recommendation is for parenteral ampicillin or penicillin G so long as local epidemiologic data indicate a lack of substantial resistance for invasive pneumococci as defined by an MIC greater than 8 mcg/mL and the patient doesn’t have empyema or other potentially life-threatening complications. When those conditions aren’t met, however, the guidelines endorse the third-generation cephalosporins ceftriaxone or cefotaxime.
Dr. Abzug applauded a recent study by pediatricians at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., that provided support in everyday clinical practice for the ampicillin-first management strategy recommended in the national guidelines. The retrospective study included 1,033 patients admitted with CAP to the tertiary referral hospital during the 12 months before and after the 2008 introduction of a clinical practice guideline encouraging the use of ampicillin as the first-line empiric antibiotic in previously healthy children with uncomplicated CAP.
The Kansas City pediatricians noted that the use of ampicillin as first-line therapy has historically been uncommon at tertiary children’s hospitals, as evidenced by a mere 5.5% rate in a study using the Pediatric Hospital Information Systems database (Pediatrics 2011;127:e255-63). The goal was to turn that situation around at Children’s Mercy Hospitals and Clinics, since ampicillin is a narrower-spectrum antibiotic than the third-generation cephalosporins, and hence less likely to promote antibiotic resistance.
Prior to introduction of the hospital guideline, ceftriaxone was prescribed as empiric therapy for CAP in 72% of cases, with ampicillin being the second most commonly prescribed antibiotic at 13%. In the year after the guideline was introduced, ampicillin was the most common antibiotic, prescribed in 63% of cases, with ceftriaxone prescribed in 21%. And even though the prevalence of S. pneumoniae isolates with intermediate susceptibility or resistance to penicillin was 24% at the hospital during that time period, the change in therapy didn’t result in an increase in adverse outcomes: The preguideline treatment failure rate was 1.5% and the postguideline rate was similar at 1% (Pediatrics 2012;129:e597-604).
"Here’s some real-world data that suggest ampicillin for the most part is probably sufficient as the parenteral empiric drug of choice for uncomplicated CAP," Dr. Abzug commented.
Dr. Abzug reported having no financial conflicts.
EXPERT ANALYSIS FROM AN ANNUAL PEDIATRIC INFECTIOUS DISEASES CONFERENCE
Arboviral Disease Season: It's On!
VAIL, COLO. – The number one cause of pediatric neuroinvasive arboviral disease in the United States turns out to be – to the surprise of most physicians – La Crosse virus.
That’s right. Not West Nile, but La Crosse.
West Nile infections draw most of the national attention and fear, and rightfully so. A total of 10,237 cases of West Nile meningitis, encephalitis, or myelitis in children and adults were reported to ArboNET, the Centers for Disease Control and Prevention’s (CDC’s) national surveillance system, between 2003 and 2011. That’s over 10 times more than the combined number of reported cases of neuroinvasive disease caused by the other arthropod-borne viruses or arboviruses: La Crosse virus, eastern equine encephalitis virus, St. Louis encephalitis virus, and Powassan virus, Dr. Marc Fischer said at a conference on pediatric infectious diseases sponsored by the Children’s Hospital Colorado.
Cases of neuroinvasive La Crosse virus disease outnumber West Nile virus disease in the pediatric age group because the age distribution of the infections is dramatically different. While fully 88% of all cases of neuroinvasive disease caused by La Crosse virus occurred in patients under age 18, that was true for only 4% of cases of West Nile neuroinvasive disease, noted Dr. Fischer, a medical epidemiologist at the CDC’s Arboviral Disease Branch in Fort Collins, Colo.
This translates to an average annual incidence of 0.9 cases of La Crosse virus neuroinvasive disease per million U.S. children and adolescents for 2003-2011, compared with 0.63 cases per million for West Nile virus. Trailing far behind are eastern equine encephalitis virus at 0.04 per million, followed by Powassan virus and St. Louis encephalitis virus, tied at 0.01 cases of neuroinvasive disease per million individuals under age 18, he reported.
These incidence figures greatly underestimate the actual risk of these diseases in regions where the arboviruses are endemic. La Crosse virus, for example, is extremely focal in its distribution. During 2003-2011, more than 80% of all cases of La Crosse virus pediatric neuroinvasive disease were reported from four states: Ohio, West Virginia, North Carolina, and Tennessee.
"These are the states where you really need to be thinking about La Crosse virus when you have a child with meningitis or encephalitis," Dr. Fischer said.
In contrast to the other disease-causing arboviruses, West Nile virus is endemic all across the country. Yet it, too, is focal in its distribution. While cases of West Nile virus pediatric neuroinvasive disease were reported from nearly every state during 2003-2011, those cases occurred in only about 8% of the more than 3,000 U.S. counties in the continental United States.
Nationally, the annual incidence of both West Nile and La Crosse virus pediatric neuroinvasive disease dropped steadily through 2007 from a peak in 2003. Since 2007, however, reported La Crosse virus disease cases have increased, while West Nile cases have continued to drop. However, in 2012 there have been more reported cases of West Nile virus neuroinvasive disease to date than on the same date in any year since 2004, with most of the activity being concentrated in Texas, Oklahoma, and Mississippi, according to Dr. Fischer.
The clinical syndrome of pediatric arboviral disease tends to be expressed differently depending upon whether the infectious agent is La Crosse or West Nile virus. In all, 78% of La Crosse cases reported to ArboNET in 2003-2011 were classified as encephalitis and 20% were meningitis, while West Nile virus cases were split 47% encephalitis and 50% meningitis.
The most lethal pediatric neuroinvasive arboviral disease is eastern equine encephalitis virus infection, with a 38% mortality rate. It’s such a rare disease, however, that this very high mortality rate translated to only 26 deaths nationwide in 2003-2011. The mortality rate was just 1% each for La Crosse and West Nile virus pediatric neuroinvasive disease.
The clinical spectrum of most domestic arboviral infections follows the same general pattern: 70%-80% of infections are asymptomatic, 20%-30% result in a generally self-limited, systemic, flulike febrile illness, and less than 1% of infections result in neuroinvasive disease.
Diagnostic laboratory testing for suspected neuroinvasive arboviral disease entails testing for IgM antibodies in the serum or cerebrospinal fluid (CSF), then confirmatory testing for neutralizing antibodies. Commercial IgM antibody assays for West Nile virus are readily available. Physicians have three options for testing for the other arboviruses: a commercially available immunofluorescent assay kit marketed by Focus Diagnostics; most state health departments will perform testing; and the CDC accepts specimens for testing at its Fort Collins laboratory.
Diagnostic testing of CSF offers two advantages over serologic testing. One is that if testing is done early – say, 4-5 days after illness onset – IgM antibodies may not be present yet in serum, but they will likely be in the CSF.
"When you get out to 9 or 10 days, though, there’s not really a difference. Everybody should have IgM antibodies in their CSF and serum," Dr. Fischer explained.
The other advantage of testing the CSF is that IgM antibodies to West Nile virus can be maintained in the serum for a long time. Their presence could be due to an asymptomatic infection that occurred the previous year. In contrast, finding IgM antibodies in the CSF of a patient with neurologic symptoms boosts the likelihood that the patient has a focal viral infection.
In response to an audience question as to why West Nile virus neuroinvasive disease has been on the wane in the United States for most of the past decade, Dr. Fischer was quick to reply that no one knows. The most popular hypothesis is that it’s due to human herd immunity, but he considers that unlikely. More plausible in his view is that the vertebrate hosts of the virus – birds – have become immune in a given area.
Or there may be an entirely different and as yet unrecognized explanation, he continued. After all, western equine encephalitis virus was a not uncommon cause of neuroinvasive disease in the western and central United States between the 1960s and the early 1990s, yet for unknown reasons no cases have been reported since 1999.
"If you look back historically, St. Louis encephalitis virus infection, when it did occur more in the United States, occurred in very large outbreaks with 20- to 30-year intervals in between. The first U.S. case of West Nile disease in humans was identified in New York in 1999, so we’re really only 12 years into our West Nile outbreak. Maybe it’ll be like St. Louis encephalitis virus: quiet for 20-30 years before coming back with a big year," he said.
Dr. Fischer stressed that the views he expressed are his own and not necessarily those of the CDC. He reported having no financial conflicts.
VAIL, COLO. – The number one cause of pediatric neuroinvasive arboviral disease in the United States turns out to be – to the surprise of most physicians – La Crosse virus.
That’s right. Not West Nile, but La Crosse.
West Nile infections draw most of the national attention and fear, and rightfully so. A total of 10,237 cases of West Nile meningitis, encephalitis, or myelitis in children and adults were reported to ArboNET, the Centers for Disease Control and Prevention’s (CDC’s) national surveillance system, between 2003 and 2011. That’s over 10 times more than the combined number of reported cases of neuroinvasive disease caused by the other arthropod-borne viruses or arboviruses: La Crosse virus, eastern equine encephalitis virus, St. Louis encephalitis virus, and Powassan virus, Dr. Marc Fischer said at a conference on pediatric infectious diseases sponsored by the Children’s Hospital Colorado.
Cases of neuroinvasive La Crosse virus disease outnumber West Nile virus disease in the pediatric age group because the age distribution of the infections is dramatically different. While fully 88% of all cases of neuroinvasive disease caused by La Crosse virus occurred in patients under age 18, that was true for only 4% of cases of West Nile neuroinvasive disease, noted Dr. Fischer, a medical epidemiologist at the CDC’s Arboviral Disease Branch in Fort Collins, Colo.
This translates to an average annual incidence of 0.9 cases of La Crosse virus neuroinvasive disease per million U.S. children and adolescents for 2003-2011, compared with 0.63 cases per million for West Nile virus. Trailing far behind are eastern equine encephalitis virus at 0.04 per million, followed by Powassan virus and St. Louis encephalitis virus, tied at 0.01 cases of neuroinvasive disease per million individuals under age 18, he reported.
These incidence figures greatly underestimate the actual risk of these diseases in regions where the arboviruses are endemic. La Crosse virus, for example, is extremely focal in its distribution. During 2003-2011, more than 80% of all cases of La Crosse virus pediatric neuroinvasive disease were reported from four states: Ohio, West Virginia, North Carolina, and Tennessee.
"These are the states where you really need to be thinking about La Crosse virus when you have a child with meningitis or encephalitis," Dr. Fischer said.
In contrast to the other disease-causing arboviruses, West Nile virus is endemic all across the country. Yet it, too, is focal in its distribution. While cases of West Nile virus pediatric neuroinvasive disease were reported from nearly every state during 2003-2011, those cases occurred in only about 8% of the more than 3,000 U.S. counties in the continental United States.
Nationally, the annual incidence of both West Nile and La Crosse virus pediatric neuroinvasive disease dropped steadily through 2007 from a peak in 2003. Since 2007, however, reported La Crosse virus disease cases have increased, while West Nile cases have continued to drop. However, in 2012 there have been more reported cases of West Nile virus neuroinvasive disease to date than on the same date in any year since 2004, with most of the activity being concentrated in Texas, Oklahoma, and Mississippi, according to Dr. Fischer.
The clinical syndrome of pediatric arboviral disease tends to be expressed differently depending upon whether the infectious agent is La Crosse or West Nile virus. In all, 78% of La Crosse cases reported to ArboNET in 2003-2011 were classified as encephalitis and 20% were meningitis, while West Nile virus cases were split 47% encephalitis and 50% meningitis.
The most lethal pediatric neuroinvasive arboviral disease is eastern equine encephalitis virus infection, with a 38% mortality rate. It’s such a rare disease, however, that this very high mortality rate translated to only 26 deaths nationwide in 2003-2011. The mortality rate was just 1% each for La Crosse and West Nile virus pediatric neuroinvasive disease.
The clinical spectrum of most domestic arboviral infections follows the same general pattern: 70%-80% of infections are asymptomatic, 20%-30% result in a generally self-limited, systemic, flulike febrile illness, and less than 1% of infections result in neuroinvasive disease.
Diagnostic laboratory testing for suspected neuroinvasive arboviral disease entails testing for IgM antibodies in the serum or cerebrospinal fluid (CSF), then confirmatory testing for neutralizing antibodies. Commercial IgM antibody assays for West Nile virus are readily available. Physicians have three options for testing for the other arboviruses: a commercially available immunofluorescent assay kit marketed by Focus Diagnostics; most state health departments will perform testing; and the CDC accepts specimens for testing at its Fort Collins laboratory.
Diagnostic testing of CSF offers two advantages over serologic testing. One is that if testing is done early – say, 4-5 days after illness onset – IgM antibodies may not be present yet in serum, but they will likely be in the CSF.
"When you get out to 9 or 10 days, though, there’s not really a difference. Everybody should have IgM antibodies in their CSF and serum," Dr. Fischer explained.
The other advantage of testing the CSF is that IgM antibodies to West Nile virus can be maintained in the serum for a long time. Their presence could be due to an asymptomatic infection that occurred the previous year. In contrast, finding IgM antibodies in the CSF of a patient with neurologic symptoms boosts the likelihood that the patient has a focal viral infection.
In response to an audience question as to why West Nile virus neuroinvasive disease has been on the wane in the United States for most of the past decade, Dr. Fischer was quick to reply that no one knows. The most popular hypothesis is that it’s due to human herd immunity, but he considers that unlikely. More plausible in his view is that the vertebrate hosts of the virus – birds – have become immune in a given area.
Or there may be an entirely different and as yet unrecognized explanation, he continued. After all, western equine encephalitis virus was a not uncommon cause of neuroinvasive disease in the western and central United States between the 1960s and the early 1990s, yet for unknown reasons no cases have been reported since 1999.
"If you look back historically, St. Louis encephalitis virus infection, when it did occur more in the United States, occurred in very large outbreaks with 20- to 30-year intervals in between. The first U.S. case of West Nile disease in humans was identified in New York in 1999, so we’re really only 12 years into our West Nile outbreak. Maybe it’ll be like St. Louis encephalitis virus: quiet for 20-30 years before coming back with a big year," he said.
Dr. Fischer stressed that the views he expressed are his own and not necessarily those of the CDC. He reported having no financial conflicts.
VAIL, COLO. – The number one cause of pediatric neuroinvasive arboviral disease in the United States turns out to be – to the surprise of most physicians – La Crosse virus.
That’s right. Not West Nile, but La Crosse.
West Nile infections draw most of the national attention and fear, and rightfully so. A total of 10,237 cases of West Nile meningitis, encephalitis, or myelitis in children and adults were reported to ArboNET, the Centers for Disease Control and Prevention’s (CDC’s) national surveillance system, between 2003 and 2011. That’s over 10 times more than the combined number of reported cases of neuroinvasive disease caused by the other arthropod-borne viruses or arboviruses: La Crosse virus, eastern equine encephalitis virus, St. Louis encephalitis virus, and Powassan virus, Dr. Marc Fischer said at a conference on pediatric infectious diseases sponsored by the Children’s Hospital Colorado.
Cases of neuroinvasive La Crosse virus disease outnumber West Nile virus disease in the pediatric age group because the age distribution of the infections is dramatically different. While fully 88% of all cases of neuroinvasive disease caused by La Crosse virus occurred in patients under age 18, that was true for only 4% of cases of West Nile neuroinvasive disease, noted Dr. Fischer, a medical epidemiologist at the CDC’s Arboviral Disease Branch in Fort Collins, Colo.
This translates to an average annual incidence of 0.9 cases of La Crosse virus neuroinvasive disease per million U.S. children and adolescents for 2003-2011, compared with 0.63 cases per million for West Nile virus. Trailing far behind are eastern equine encephalitis virus at 0.04 per million, followed by Powassan virus and St. Louis encephalitis virus, tied at 0.01 cases of neuroinvasive disease per million individuals under age 18, he reported.
These incidence figures greatly underestimate the actual risk of these diseases in regions where the arboviruses are endemic. La Crosse virus, for example, is extremely focal in its distribution. During 2003-2011, more than 80% of all cases of La Crosse virus pediatric neuroinvasive disease were reported from four states: Ohio, West Virginia, North Carolina, and Tennessee.
"These are the states where you really need to be thinking about La Crosse virus when you have a child with meningitis or encephalitis," Dr. Fischer said.
In contrast to the other disease-causing arboviruses, West Nile virus is endemic all across the country. Yet it, too, is focal in its distribution. While cases of West Nile virus pediatric neuroinvasive disease were reported from nearly every state during 2003-2011, those cases occurred in only about 8% of the more than 3,000 U.S. counties in the continental United States.
Nationally, the annual incidence of both West Nile and La Crosse virus pediatric neuroinvasive disease dropped steadily through 2007 from a peak in 2003. Since 2007, however, reported La Crosse virus disease cases have increased, while West Nile cases have continued to drop. However, in 2012 there have been more reported cases of West Nile virus neuroinvasive disease to date than on the same date in any year since 2004, with most of the activity being concentrated in Texas, Oklahoma, and Mississippi, according to Dr. Fischer.
The clinical syndrome of pediatric arboviral disease tends to be expressed differently depending upon whether the infectious agent is La Crosse or West Nile virus. In all, 78% of La Crosse cases reported to ArboNET in 2003-2011 were classified as encephalitis and 20% were meningitis, while West Nile virus cases were split 47% encephalitis and 50% meningitis.
The most lethal pediatric neuroinvasive arboviral disease is eastern equine encephalitis virus infection, with a 38% mortality rate. It’s such a rare disease, however, that this very high mortality rate translated to only 26 deaths nationwide in 2003-2011. The mortality rate was just 1% each for La Crosse and West Nile virus pediatric neuroinvasive disease.
The clinical spectrum of most domestic arboviral infections follows the same general pattern: 70%-80% of infections are asymptomatic, 20%-30% result in a generally self-limited, systemic, flulike febrile illness, and less than 1% of infections result in neuroinvasive disease.
Diagnostic laboratory testing for suspected neuroinvasive arboviral disease entails testing for IgM antibodies in the serum or cerebrospinal fluid (CSF), then confirmatory testing for neutralizing antibodies. Commercial IgM antibody assays for West Nile virus are readily available. Physicians have three options for testing for the other arboviruses: a commercially available immunofluorescent assay kit marketed by Focus Diagnostics; most state health departments will perform testing; and the CDC accepts specimens for testing at its Fort Collins laboratory.
Diagnostic testing of CSF offers two advantages over serologic testing. One is that if testing is done early – say, 4-5 days after illness onset – IgM antibodies may not be present yet in serum, but they will likely be in the CSF.
"When you get out to 9 or 10 days, though, there’s not really a difference. Everybody should have IgM antibodies in their CSF and serum," Dr. Fischer explained.
The other advantage of testing the CSF is that IgM antibodies to West Nile virus can be maintained in the serum for a long time. Their presence could be due to an asymptomatic infection that occurred the previous year. In contrast, finding IgM antibodies in the CSF of a patient with neurologic symptoms boosts the likelihood that the patient has a focal viral infection.
In response to an audience question as to why West Nile virus neuroinvasive disease has been on the wane in the United States for most of the past decade, Dr. Fischer was quick to reply that no one knows. The most popular hypothesis is that it’s due to human herd immunity, but he considers that unlikely. More plausible in his view is that the vertebrate hosts of the virus – birds – have become immune in a given area.
Or there may be an entirely different and as yet unrecognized explanation, he continued. After all, western equine encephalitis virus was a not uncommon cause of neuroinvasive disease in the western and central United States between the 1960s and the early 1990s, yet for unknown reasons no cases have been reported since 1999.
"If you look back historically, St. Louis encephalitis virus infection, when it did occur more in the United States, occurred in very large outbreaks with 20- to 30-year intervals in between. The first U.S. case of West Nile disease in humans was identified in New York in 1999, so we’re really only 12 years into our West Nile outbreak. Maybe it’ll be like St. Louis encephalitis virus: quiet for 20-30 years before coming back with a big year," he said.
Dr. Fischer stressed that the views he expressed are his own and not necessarily those of the CDC. He reported having no financial conflicts.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY THE CHILDREN'S HOSPITAL COLORADO
Lyme Arthritis: Highly Treatable, Slow to Resolve
VAIL, COLO. – Arthritis is far and away the most common manifestation of late Lyme disease, affecting up to 30% of children whose early-stage disease went untreated.
Lyme arthritis presents with a distinctive clinical picture. Nevertheless, this common condition is often initially mismanaged as a joint sprain or other orthopedic injury, with the correct diagnosis coming only following referral after a month or more with no improvement, according to Dr. Roberta L. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center, Washington.
"We see tons of kids with Lyme arthritis. We get two or three cases every week in our clinic," she said at the conference.
Lyme arthritis occurs months to years after an untreated exposure to Borrelia burgdorferi. Affected patients typically present with a history of recurrent, weeks- or months-long attacks of joint swelling in one or a few joints. The knee is by far the most common site, but other large joints can be involved, as can the temporomandibular joint. An involved knee may swell up to literally the size of a basketball, yet the child has no fever, erythema, or systemic complaints, and surprisingly little pain given the effusion size.
Chronic progressive arthritis that’s not preceded by brief waxing and waning attacks doesn’t fulfil the criteria for Lyme arthritis. Neither does chronic symmetric polyarthritis, Dr. DeBiasi stressed.
Patients with suspected Lyme arthritis typically undergo arthrocentesis because there are other possible explanations for reactive arthritis besides Lyme disease. The synovial fluid in a patient with Lyme arthritis shows prominent neutrophils, mild to moderate inflammation, and a median WBC of 25,000/mm3. But diagnostic confirmation of Lyme arthritis requires serologic testing, which will invariably be enzyme immunoassay-positive, IgG-positive on the Western blot test, and IgM-negative on the Western blot because arthritis is a late manifestation of infection. Dr. DeBiasi doesn’t consider synovial fluid PCR a useful adjunctive diagnostic test.
The first-line treatment recommended in the Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) is 28 days of oral therapy with doxycycline at 4 mg/kg per day divided b.i.d., to a maximum of 100 mg b.i.d.; amoxicillin at 50 mg/kg per day divided t.i.d., to a maximum of 500 mg t.i.d.; or cefuroxime axetil at 30 mg/kg per day divided b.i.d., to a maximum of 500 mg b.i.d. Unlike in late central nervous system Lyme disease, which responds swiftly to treatment, expect slow resolution of the joint inflammation.
"This is a situation in which you can avoid giving someone a PICC line. Patients do great with oral therapy. You just need to do it for quite a while. The joint may show no change after the first week of therapy and may be only slightly smaller after 2 weeks. I give anti-inflammatory drugs concurrently to speed improvement in swelling and range of motion," Dr. DeBiasi explained.
If, however, joint swelling persists at the end of 28 days of oral therapy, two options are available. Both involve a 1-month wait off treatment before considering retreatment.
The first option, which is preferred if the patient’s joint swelling is substantially improved but not completely resolved, is another 28-day course of oral antibiotic therapy.
The alternative is to initiate 2-4 weeks of intravenous ceftriaxone at 50-75 mg/kg per day in a single dose, to a maximum of 2 g. This is the favored option when a patient has shown no improvement at all or is worse after the first course of oral therapy.
"That’s happened once in the 6 years I’ve been taking care of kids with Lyme arthritis. The vast majority do well with oral therapy," she said.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Arthritis is far and away the most common manifestation of late Lyme disease, affecting up to 30% of children whose early-stage disease went untreated.
Lyme arthritis presents with a distinctive clinical picture. Nevertheless, this common condition is often initially mismanaged as a joint sprain or other orthopedic injury, with the correct diagnosis coming only following referral after a month or more with no improvement, according to Dr. Roberta L. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center, Washington.
"We see tons of kids with Lyme arthritis. We get two or three cases every week in our clinic," she said at the conference.
Lyme arthritis occurs months to years after an untreated exposure to Borrelia burgdorferi. Affected patients typically present with a history of recurrent, weeks- or months-long attacks of joint swelling in one or a few joints. The knee is by far the most common site, but other large joints can be involved, as can the temporomandibular joint. An involved knee may swell up to literally the size of a basketball, yet the child has no fever, erythema, or systemic complaints, and surprisingly little pain given the effusion size.
Chronic progressive arthritis that’s not preceded by brief waxing and waning attacks doesn’t fulfil the criteria for Lyme arthritis. Neither does chronic symmetric polyarthritis, Dr. DeBiasi stressed.
Patients with suspected Lyme arthritis typically undergo arthrocentesis because there are other possible explanations for reactive arthritis besides Lyme disease. The synovial fluid in a patient with Lyme arthritis shows prominent neutrophils, mild to moderate inflammation, and a median WBC of 25,000/mm3. But diagnostic confirmation of Lyme arthritis requires serologic testing, which will invariably be enzyme immunoassay-positive, IgG-positive on the Western blot test, and IgM-negative on the Western blot because arthritis is a late manifestation of infection. Dr. DeBiasi doesn’t consider synovial fluid PCR a useful adjunctive diagnostic test.
The first-line treatment recommended in the Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) is 28 days of oral therapy with doxycycline at 4 mg/kg per day divided b.i.d., to a maximum of 100 mg b.i.d.; amoxicillin at 50 mg/kg per day divided t.i.d., to a maximum of 500 mg t.i.d.; or cefuroxime axetil at 30 mg/kg per day divided b.i.d., to a maximum of 500 mg b.i.d. Unlike in late central nervous system Lyme disease, which responds swiftly to treatment, expect slow resolution of the joint inflammation.
"This is a situation in which you can avoid giving someone a PICC line. Patients do great with oral therapy. You just need to do it for quite a while. The joint may show no change after the first week of therapy and may be only slightly smaller after 2 weeks. I give anti-inflammatory drugs concurrently to speed improvement in swelling and range of motion," Dr. DeBiasi explained.
If, however, joint swelling persists at the end of 28 days of oral therapy, two options are available. Both involve a 1-month wait off treatment before considering retreatment.
The first option, which is preferred if the patient’s joint swelling is substantially improved but not completely resolved, is another 28-day course of oral antibiotic therapy.
The alternative is to initiate 2-4 weeks of intravenous ceftriaxone at 50-75 mg/kg per day in a single dose, to a maximum of 2 g. This is the favored option when a patient has shown no improvement at all or is worse after the first course of oral therapy.
"That’s happened once in the 6 years I’ve been taking care of kids with Lyme arthritis. The vast majority do well with oral therapy," she said.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Arthritis is far and away the most common manifestation of late Lyme disease, affecting up to 30% of children whose early-stage disease went untreated.
Lyme arthritis presents with a distinctive clinical picture. Nevertheless, this common condition is often initially mismanaged as a joint sprain or other orthopedic injury, with the correct diagnosis coming only following referral after a month or more with no improvement, according to Dr. Roberta L. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center, Washington.
"We see tons of kids with Lyme arthritis. We get two or three cases every week in our clinic," she said at the conference.
Lyme arthritis occurs months to years after an untreated exposure to Borrelia burgdorferi. Affected patients typically present with a history of recurrent, weeks- or months-long attacks of joint swelling in one or a few joints. The knee is by far the most common site, but other large joints can be involved, as can the temporomandibular joint. An involved knee may swell up to literally the size of a basketball, yet the child has no fever, erythema, or systemic complaints, and surprisingly little pain given the effusion size.
Chronic progressive arthritis that’s not preceded by brief waxing and waning attacks doesn’t fulfil the criteria for Lyme arthritis. Neither does chronic symmetric polyarthritis, Dr. DeBiasi stressed.
Patients with suspected Lyme arthritis typically undergo arthrocentesis because there are other possible explanations for reactive arthritis besides Lyme disease. The synovial fluid in a patient with Lyme arthritis shows prominent neutrophils, mild to moderate inflammation, and a median WBC of 25,000/mm3. But diagnostic confirmation of Lyme arthritis requires serologic testing, which will invariably be enzyme immunoassay-positive, IgG-positive on the Western blot test, and IgM-negative on the Western blot because arthritis is a late manifestation of infection. Dr. DeBiasi doesn’t consider synovial fluid PCR a useful adjunctive diagnostic test.
The first-line treatment recommended in the Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) is 28 days of oral therapy with doxycycline at 4 mg/kg per day divided b.i.d., to a maximum of 100 mg b.i.d.; amoxicillin at 50 mg/kg per day divided t.i.d., to a maximum of 500 mg t.i.d.; or cefuroxime axetil at 30 mg/kg per day divided b.i.d., to a maximum of 500 mg b.i.d. Unlike in late central nervous system Lyme disease, which responds swiftly to treatment, expect slow resolution of the joint inflammation.
"This is a situation in which you can avoid giving someone a PICC line. Patients do great with oral therapy. You just need to do it for quite a while. The joint may show no change after the first week of therapy and may be only slightly smaller after 2 weeks. I give anti-inflammatory drugs concurrently to speed improvement in swelling and range of motion," Dr. DeBiasi explained.
If, however, joint swelling persists at the end of 28 days of oral therapy, two options are available. Both involve a 1-month wait off treatment before considering retreatment.
The first option, which is preferred if the patient’s joint swelling is substantially improved but not completely resolved, is another 28-day course of oral antibiotic therapy.
The alternative is to initiate 2-4 weeks of intravenous ceftriaxone at 50-75 mg/kg per day in a single dose, to a maximum of 2 g. This is the favored option when a patient has shown no improvement at all or is worse after the first course of oral therapy.
"That’s happened once in the 6 years I’ve been taking care of kids with Lyme arthritis. The vast majority do well with oral therapy," she said.
Dr. DeBiasi reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL PEDIATRIC INFECTIOUS DISEASES CONFERENCE SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Lyme Disease: Avoiding Inappropriate Serologic Testing
VAIL, COLO. – Misconceptions abound regarding the appropriate use of diagnostic serologic testing for Lyme disease. The end result is needless difficulties for patients, families, and their physicians, Dr. Roberta L. DeBiasi asserted at a conference on pediatric infectious diseases.
The first point to understand about serologic testing is that it isn’t even guideline recommended in patients who have early localized Lyme disease in the form of erythema migrans. Erythema migrans is a clinical diagnosis that in and of itself is sufficient to trigger the decision to treat.
"This is a major pitfall we see: A child or adult comes in with a classic rash of erythema migrans and the physician decides to do the serology. It comes back negative because the sensitivity and specificity of serologic testing at that stage is abysmal. So the patient’s Lyme disease goes untreated," she explained.
Children with untreated early-stage Lyme disease have up to a 50% risk of later developing Lyme arthritis, a 10% risk of Lyme meningitis, and a 5% risk of Lyme chronic carditis, according to Dr. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center in Washington.
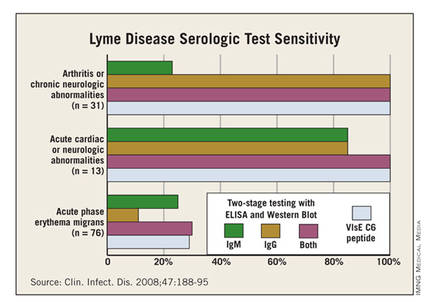
While there’s absolutely no point in doing serology in patients with early cutaneous disease manifestations only, the test sensitivity is far better in patients with extracutaneous manifestations of disease, such as meningitis, carditis, or arthritis. This was demonstrated in a classic study led by Dr. Allen C. Steere, professor of rheumatology at Harvard Medical School, Boston, who is credited with recognizing and naming the full syndrome he called Lyme disease (Clin. Infect. Dis. 2008;47:188-95).
Dr. Steere and his colleagues showed that, while the sensitivity of standard two-tiered testing was a mere 30% in the setting of acute-phase erythema migrans, it jumped to 100% in patients with arthritis, chronic neurologic abnormalities, or acute cardiac or neurologic abnormalities.
Erythema migrans appears 3-30 days and a median of 11 days after an Ixodes tick bite. It starts as a red macule or papule at the bite site and expands over days to weeks – not hours – eventually reaching a diameter of 5 cm or more, and in some cases as large as 30 cm. Circular or oval-shaped rashes are the most common. Partial clearing in the center, known as a bull’s eye, is common but not required. Up to 10% of patients with erythema migrans will have a small necrotic or vesicular/pustular are in the center of the lesion.
The most helpful features in distinguishing erythema migrans from similar-looking skin lesions are that it’s neither itchy nor painful.
"If it’s really itchy it’s usually an insect bite. If it really hurts it’s usually cellulitis," according to Dr. DeBiasi.
Differentiating erythema migrans from a tick bite hypersensitivity reaction, which is not an indicator of Borrelia burgdorferi infection, is a matter of the timing of the lesion’s onset and disappearance. Tick bite hypersensitivity reactions are erythematous lesions which appear while the tick is still attached or within 48 hours after detachment. That’s earlier than erythema migrans. And the hypersensitivity reaction typically begins to disappear 24-48 hours after its arrival; in contrast, erythema migrans grows larger during those first days after its initial appearance.
The Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) recommend treating erythema migrans without neurologic or cardiac involvement with 14 days of oral therapy. The American Academy of Pediatrics Red Book recommends 14-21 days. The first-line options are doxycycline in patients above age 8 years, amoxicillin in those under that age, and cefuroxime. Macrolide antibiotics aren’t recommended as first-line therapy because they proved less effective in clinical trials. First-generation cephalosporins are ineffective. Intravenous ceftriaxone is no more effective than oral agents.
"You might be thinking, ‘Who in the world would use IV [intravenous] ceftriaxone for erythema migrans?’ but it happens all the time. In high-incidence areas for Lyme disease, we have lots of people who call themselves Lyme specialists who set up shop and put ads in the phone book. Patients who don’t know any better go to them and can get very dangerous treatments," Dr. DeBiasi explained.
Erythema migrans resolves within the first several days of appropriate antibiotic therapy, although the oft-accompanying flu-like systemic symptoms may linger for several weeks.
Dr. DeBiasi conceded Lyme serology is confusing, even for infectious disease physicians. The key message is that it must always be a two-step process: Use an enzyme immunoassay or immunofluorescence assay as a screening test and, if it’s positive or equivocal, send the specimen for a standard IgM/IgG Western immunoblot. Antibodies aren’t detectable in the first few weeks of infection but by week 4 the screening test will pick up nearly all B. burgdorferi–infected individuals, albeit with a 5%-7% false-positive rate.
The Western immunoblot is interpreted as either positive or negative; there is no "indeterminate" with this test. IgG testing is considered positive on the Western blot if at least 5 out of 10 bands are confirmatory, while IgM requires 2 out 3 confirmatory bands.
Most patients with early disseminated or late Lyme disease have persistent antibodies for months to years.
"In no way does that correlate with the efficacy of treatment," Dr. DeBiasi stressed. "That’s a common reason people come in to the clinic. They say, ‘I want you to prove that I’m cured, and this test is negative.’ You have to explain to patients that it’s a test of antibody response, not a test for bacteria in their blood, and that just like with a vaccine, the fact that they have an antibody response is a good thing."
Many patients come in requesting the relatively new Vls C6 antibody test. It’s licensed for the diagnosis of Lyme disease, but studies indicate its sensitivity and specificity aren’t superior to the standard two-step protocol. Dr. DeBiasi reserves the Vls C6 test for travelers who may have been infected with European strains of B. burgdorferi, a situation in which it is advantageous.
She urged her colleagues not to order Lyme serologic testing for patients with fatigue, achiness, and other nonspecific symptoms in the absence of accompanying specific, objective signs of infection, such as erythema migrans or arthritis. It’s a widespread practice, but positive test results in this setting are nearly always false-positives.
Misinformation abounds regarding Lyme disease, especially on the internet. Two resources she recommends as reliable to patients are www.cdc.gov/lyme and www.idsociety.org/lyme.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Misconceptions abound regarding the appropriate use of diagnostic serologic testing for Lyme disease. The end result is needless difficulties for patients, families, and their physicians, Dr. Roberta L. DeBiasi asserted at a conference on pediatric infectious diseases.
The first point to understand about serologic testing is that it isn’t even guideline recommended in patients who have early localized Lyme disease in the form of erythema migrans. Erythema migrans is a clinical diagnosis that in and of itself is sufficient to trigger the decision to treat.
"This is a major pitfall we see: A child or adult comes in with a classic rash of erythema migrans and the physician decides to do the serology. It comes back negative because the sensitivity and specificity of serologic testing at that stage is abysmal. So the patient’s Lyme disease goes untreated," she explained.
Children with untreated early-stage Lyme disease have up to a 50% risk of later developing Lyme arthritis, a 10% risk of Lyme meningitis, and a 5% risk of Lyme chronic carditis, according to Dr. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center in Washington.

While there’s absolutely no point in doing serology in patients with early cutaneous disease manifestations only, the test sensitivity is far better in patients with extracutaneous manifestations of disease, such as meningitis, carditis, or arthritis. This was demonstrated in a classic study led by Dr. Allen C. Steere, professor of rheumatology at Harvard Medical School, Boston, who is credited with recognizing and naming the full syndrome he called Lyme disease (Clin. Infect. Dis. 2008;47:188-95).
Dr. Steere and his colleagues showed that, while the sensitivity of standard two-tiered testing was a mere 30% in the setting of acute-phase erythema migrans, it jumped to 100% in patients with arthritis, chronic neurologic abnormalities, or acute cardiac or neurologic abnormalities.
Erythema migrans appears 3-30 days and a median of 11 days after an Ixodes tick bite. It starts as a red macule or papule at the bite site and expands over days to weeks – not hours – eventually reaching a diameter of 5 cm or more, and in some cases as large as 30 cm. Circular or oval-shaped rashes are the most common. Partial clearing in the center, known as a bull’s eye, is common but not required. Up to 10% of patients with erythema migrans will have a small necrotic or vesicular/pustular are in the center of the lesion.
The most helpful features in distinguishing erythema migrans from similar-looking skin lesions are that it’s neither itchy nor painful.
"If it’s really itchy it’s usually an insect bite. If it really hurts it’s usually cellulitis," according to Dr. DeBiasi.
Differentiating erythema migrans from a tick bite hypersensitivity reaction, which is not an indicator of Borrelia burgdorferi infection, is a matter of the timing of the lesion’s onset and disappearance. Tick bite hypersensitivity reactions are erythematous lesions which appear while the tick is still attached or within 48 hours after detachment. That’s earlier than erythema migrans. And the hypersensitivity reaction typically begins to disappear 24-48 hours after its arrival; in contrast, erythema migrans grows larger during those first days after its initial appearance.
The Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) recommend treating erythema migrans without neurologic or cardiac involvement with 14 days of oral therapy. The American Academy of Pediatrics Red Book recommends 14-21 days. The first-line options are doxycycline in patients above age 8 years, amoxicillin in those under that age, and cefuroxime. Macrolide antibiotics aren’t recommended as first-line therapy because they proved less effective in clinical trials. First-generation cephalosporins are ineffective. Intravenous ceftriaxone is no more effective than oral agents.
"You might be thinking, ‘Who in the world would use IV [intravenous] ceftriaxone for erythema migrans?’ but it happens all the time. In high-incidence areas for Lyme disease, we have lots of people who call themselves Lyme specialists who set up shop and put ads in the phone book. Patients who don’t know any better go to them and can get very dangerous treatments," Dr. DeBiasi explained.
Erythema migrans resolves within the first several days of appropriate antibiotic therapy, although the oft-accompanying flu-like systemic symptoms may linger for several weeks.
Dr. DeBiasi conceded Lyme serology is confusing, even for infectious disease physicians. The key message is that it must always be a two-step process: Use an enzyme immunoassay or immunofluorescence assay as a screening test and, if it’s positive or equivocal, send the specimen for a standard IgM/IgG Western immunoblot. Antibodies aren’t detectable in the first few weeks of infection but by week 4 the screening test will pick up nearly all B. burgdorferi–infected individuals, albeit with a 5%-7% false-positive rate.
The Western immunoblot is interpreted as either positive or negative; there is no "indeterminate" with this test. IgG testing is considered positive on the Western blot if at least 5 out of 10 bands are confirmatory, while IgM requires 2 out 3 confirmatory bands.
Most patients with early disseminated or late Lyme disease have persistent antibodies for months to years.
"In no way does that correlate with the efficacy of treatment," Dr. DeBiasi stressed. "That’s a common reason people come in to the clinic. They say, ‘I want you to prove that I’m cured, and this test is negative.’ You have to explain to patients that it’s a test of antibody response, not a test for bacteria in their blood, and that just like with a vaccine, the fact that they have an antibody response is a good thing."
Many patients come in requesting the relatively new Vls C6 antibody test. It’s licensed for the diagnosis of Lyme disease, but studies indicate its sensitivity and specificity aren’t superior to the standard two-step protocol. Dr. DeBiasi reserves the Vls C6 test for travelers who may have been infected with European strains of B. burgdorferi, a situation in which it is advantageous.
She urged her colleagues not to order Lyme serologic testing for patients with fatigue, achiness, and other nonspecific symptoms in the absence of accompanying specific, objective signs of infection, such as erythema migrans or arthritis. It’s a widespread practice, but positive test results in this setting are nearly always false-positives.
Misinformation abounds regarding Lyme disease, especially on the internet. Two resources she recommends as reliable to patients are www.cdc.gov/lyme and www.idsociety.org/lyme.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Misconceptions abound regarding the appropriate use of diagnostic serologic testing for Lyme disease. The end result is needless difficulties for patients, families, and their physicians, Dr. Roberta L. DeBiasi asserted at a conference on pediatric infectious diseases.
The first point to understand about serologic testing is that it isn’t even guideline recommended in patients who have early localized Lyme disease in the form of erythema migrans. Erythema migrans is a clinical diagnosis that in and of itself is sufficient to trigger the decision to treat.
"This is a major pitfall we see: A child or adult comes in with a classic rash of erythema migrans and the physician decides to do the serology. It comes back negative because the sensitivity and specificity of serologic testing at that stage is abysmal. So the patient’s Lyme disease goes untreated," she explained.
Children with untreated early-stage Lyme disease have up to a 50% risk of later developing Lyme arthritis, a 10% risk of Lyme meningitis, and a 5% risk of Lyme chronic carditis, according to Dr. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center in Washington.

While there’s absolutely no point in doing serology in patients with early cutaneous disease manifestations only, the test sensitivity is far better in patients with extracutaneous manifestations of disease, such as meningitis, carditis, or arthritis. This was demonstrated in a classic study led by Dr. Allen C. Steere, professor of rheumatology at Harvard Medical School, Boston, who is credited with recognizing and naming the full syndrome he called Lyme disease (Clin. Infect. Dis. 2008;47:188-95).
Dr. Steere and his colleagues showed that, while the sensitivity of standard two-tiered testing was a mere 30% in the setting of acute-phase erythema migrans, it jumped to 100% in patients with arthritis, chronic neurologic abnormalities, or acute cardiac or neurologic abnormalities.
Erythema migrans appears 3-30 days and a median of 11 days after an Ixodes tick bite. It starts as a red macule or papule at the bite site and expands over days to weeks – not hours – eventually reaching a diameter of 5 cm or more, and in some cases as large as 30 cm. Circular or oval-shaped rashes are the most common. Partial clearing in the center, known as a bull’s eye, is common but not required. Up to 10% of patients with erythema migrans will have a small necrotic or vesicular/pustular are in the center of the lesion.
The most helpful features in distinguishing erythema migrans from similar-looking skin lesions are that it’s neither itchy nor painful.
"If it’s really itchy it’s usually an insect bite. If it really hurts it’s usually cellulitis," according to Dr. DeBiasi.
Differentiating erythema migrans from a tick bite hypersensitivity reaction, which is not an indicator of Borrelia burgdorferi infection, is a matter of the timing of the lesion’s onset and disappearance. Tick bite hypersensitivity reactions are erythematous lesions which appear while the tick is still attached or within 48 hours after detachment. That’s earlier than erythema migrans. And the hypersensitivity reaction typically begins to disappear 24-48 hours after its arrival; in contrast, erythema migrans grows larger during those first days after its initial appearance.
The Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) recommend treating erythema migrans without neurologic or cardiac involvement with 14 days of oral therapy. The American Academy of Pediatrics Red Book recommends 14-21 days. The first-line options are doxycycline in patients above age 8 years, amoxicillin in those under that age, and cefuroxime. Macrolide antibiotics aren’t recommended as first-line therapy because they proved less effective in clinical trials. First-generation cephalosporins are ineffective. Intravenous ceftriaxone is no more effective than oral agents.
"You might be thinking, ‘Who in the world would use IV [intravenous] ceftriaxone for erythema migrans?’ but it happens all the time. In high-incidence areas for Lyme disease, we have lots of people who call themselves Lyme specialists who set up shop and put ads in the phone book. Patients who don’t know any better go to them and can get very dangerous treatments," Dr. DeBiasi explained.
Erythema migrans resolves within the first several days of appropriate antibiotic therapy, although the oft-accompanying flu-like systemic symptoms may linger for several weeks.
Dr. DeBiasi conceded Lyme serology is confusing, even for infectious disease physicians. The key message is that it must always be a two-step process: Use an enzyme immunoassay or immunofluorescence assay as a screening test and, if it’s positive or equivocal, send the specimen for a standard IgM/IgG Western immunoblot. Antibodies aren’t detectable in the first few weeks of infection but by week 4 the screening test will pick up nearly all B. burgdorferi–infected individuals, albeit with a 5%-7% false-positive rate.
The Western immunoblot is interpreted as either positive or negative; there is no "indeterminate" with this test. IgG testing is considered positive on the Western blot if at least 5 out of 10 bands are confirmatory, while IgM requires 2 out 3 confirmatory bands.
Most patients with early disseminated or late Lyme disease have persistent antibodies for months to years.
"In no way does that correlate with the efficacy of treatment," Dr. DeBiasi stressed. "That’s a common reason people come in to the clinic. They say, ‘I want you to prove that I’m cured, and this test is negative.’ You have to explain to patients that it’s a test of antibody response, not a test for bacteria in their blood, and that just like with a vaccine, the fact that they have an antibody response is a good thing."
Many patients come in requesting the relatively new Vls C6 antibody test. It’s licensed for the diagnosis of Lyme disease, but studies indicate its sensitivity and specificity aren’t superior to the standard two-step protocol. Dr. DeBiasi reserves the Vls C6 test for travelers who may have been infected with European strains of B. burgdorferi, a situation in which it is advantageous.
She urged her colleagues not to order Lyme serologic testing for patients with fatigue, achiness, and other nonspecific symptoms in the absence of accompanying specific, objective signs of infection, such as erythema migrans or arthritis. It’s a widespread practice, but positive test results in this setting are nearly always false-positives.
Misinformation abounds regarding Lyme disease, especially on the internet. Two resources she recommends as reliable to patients are www.cdc.gov/lyme and www.idsociety.org/lyme.
Dr. DeBiasi reported having no financial conflicts.
EXPERT OPINION FROM THE ANNUAL PEDIATRIC INFECTIOUS DISEASES CONFERENCE SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Vaccines in the Pipeline For Diseases Affecting Children Worldwide
VAIL, COLO. – Effective vaccines for two of the top causes of mortality and morbidity in children worldwide – malaria and dengue fever – are now in pivotal phase III clinical trials and could reach the market within 3 years.
In addition, promising vaccines for tuberculosis and group B streptococcus are in earlier-stage clinical trials, Dr. Edwin J. Asturias noted at a conference on pediatric infectious diseases, which was sponsored by the Children’s Hospital Colorado in Aurora.
Malaria
"The malaria vaccine is coming. It is close. It will be the most important vaccine for killer diseases in children. There is a lot of hope for that vaccine," according to Dr. Asturias of the center for global health at the University of Colorado, Denver.
Malaria is endemic in 99 countries. An estimated 216 million new cases and 655,000 deaths resulting from the infection occurred in 2010. More than 90% of the world’s malaria deaths are in Africa, with most of them in children younger than age 5 years.
The malaria vaccine – now in the pivotal phase III MAL059 trial that’s expected to be completed by 2014 – has been in development by GlaxoSmithKline for 2 decades. The vaccine is known as RTS,S. It is designed for children living in endemic areas. The vaccine focuses on the pre-erythrocytic stage of infection, aiming to prevent the malaria parasite from multiplying in the liver and reentering the bloodstream.
In an interim report from the phase III trial, the three-dose series of RTS,S achieved a 56% reduction in clinical malaria among 5- to 17-month-olds (N. Engl. J. Med. 2008;359:2521-32).
"The question everybody has is, is 56% enough, or should we aim for higher efficacy in malaria?" the pediatric infectious disease specialist said.
For him, the answer is clear: "If we can reduce mortality from malaria by 50%, that would be a great success in terms of preventing this important killer around the world," Dr. Asturias said.
But the benefits will extend well beyond mortality reduction to include nationwide favorable impacts upon lost days of productivity and strain on health care systems. "In countries where malaria is endemic, every case of fever gets treated as malaria, and that’s a huge burden in terms of health resource utilization," he added.
It’s also hypothetically possible that herd immunity could amplify the clinical efficacy of RTS,S beyond that 56% figure. It all depends upon how good the vaccine is at quelling parasitemia, an issue that hasn’t been well studied as yet.
Frequent boosting may be necessary for several years after the three-dose series is given. In studies in African infants and children, the protective effect has been sustained for only about 18 months.
Much of the credit for the recent major advances in developing new vaccines for important childhood diseases worldwide belongs to an ongoing UNICEF/WHO initiative and to the Bill and Melinda Gates Foundation, which Dr. Asturias said "has been a game changer." During this decade, the foundation’s declared aim is to utilize existing vaccines and to support development of new ones in order to prevent the deaths of 7.6 million children younger than age 5 years.
Dengue Virus
Sanofi Pasteur’s chimeric flavivirus vaccine is in phase III studies totaling more than 30,000 participants in Latin America and Asia. These pivotal trials will be completed in 2014. The company is sufficiently confident that it has a winner that it has built a $440 million factory to produce the vaccine.
In a phase IIB clinical trial conducted in Thai children, the vaccine – which has received fast-track development status from the Food and Drug Administration – protected against three of the four dengue virus serotypes, which means it should decrease the incidence of dengue hemorrhagic fever, the most feared manifestation of the viral infection.
Other dengue vaccines using alternative immunization strategies are in earlier stages of development.
In countries where the mosquito-borne dengue virus is endemic and reporting is adequate, the incidence of infection is about 5% per year (Vaccine 2002;20:3043-6). Three-quarters of infections are asymptomatic or entail only a few days of mild flulike illness. Among the 25% of infections that are symptomatic, 98%-99% result in dengue fever, also known as "breakbone fever," whereas 1%-2% manifest as dengue hemorrhagic fever, which has a mortality rate of up to 5% depending upon the availability of supportive care. The infection is more severe in school-age youths and in individuals infected for the second time.
Tuberculosis
Twelve new candidate TB vaccines have entered clinical trials. Seven are subunit vaccines, two utilize immunotherapy as an adjunct to chemotherapy, and three are recombinant bacille Calmette-Guérin vaccines designed to replace the current BCG vaccine, which doesn’t protect adults from reactivation or reinfection decades after their first exposure to the pathogen. The two most advanced candidate vaccines are now in phase IIB trials; they are designed to enhance immunity in individuals who received BCG vaccine early in life. Phase II studies are likely to continue to 2020.
"We’re still far away from a good tuberculosis vaccine at this point," according to Dr. Asturias.
The most pressing need now is to define markers for TB infection and reactivation so that researchers can demonstrate vaccine efficacy without having to wait 30 years for pulmonary TB or other clinical disease to develop, he added.
Group B Streptococcus
The push to develop a group B strep vaccine stems from the fact that in developing countries, this infection is a leading cause of sepsis and meningitis in the first 3 months of life. And worldwide, the proportion of deaths in children younger than age 5 years that occur during the neonatal period hasn’t changed in 2 decades. A UNICEF/WHO estimate concluded that of 8.8 million deaths in children younger than age 5 years in 2008, 42% occurred in neonates. A maternal group B strep vaccine is key to reducing that disturbing figure, in Dr. Asturias’s view.
There are at least 10 group B strep serotypes, but most disease is caused by three of them. A Novartis trivalent vaccine showed "very promising" results in a phase I study; a phase III clinical trial for prevention of disease in pregnant women is scheduled to get underway late next year, he said.
Dr. Asturias reported having conducted vaccine research studies sponsored by Sanofi Pasteur and Crucell, serving on data safety monitoring boards for Inviragen, Sanofi Pasteur, and PATH, and acting as a consultant to the Bill and Melinda Gates Foundation.
VAIL, COLO. – Effective vaccines for two of the top causes of mortality and morbidity in children worldwide – malaria and dengue fever – are now in pivotal phase III clinical trials and could reach the market within 3 years.
In addition, promising vaccines for tuberculosis and group B streptococcus are in earlier-stage clinical trials, Dr. Edwin J. Asturias noted at a conference on pediatric infectious diseases, which was sponsored by the Children’s Hospital Colorado in Aurora.
Malaria
"The malaria vaccine is coming. It is close. It will be the most important vaccine for killer diseases in children. There is a lot of hope for that vaccine," according to Dr. Asturias of the center for global health at the University of Colorado, Denver.
Malaria is endemic in 99 countries. An estimated 216 million new cases and 655,000 deaths resulting from the infection occurred in 2010. More than 90% of the world’s malaria deaths are in Africa, with most of them in children younger than age 5 years.
The malaria vaccine – now in the pivotal phase III MAL059 trial that’s expected to be completed by 2014 – has been in development by GlaxoSmithKline for 2 decades. The vaccine is known as RTS,S. It is designed for children living in endemic areas. The vaccine focuses on the pre-erythrocytic stage of infection, aiming to prevent the malaria parasite from multiplying in the liver and reentering the bloodstream.
In an interim report from the phase III trial, the three-dose series of RTS,S achieved a 56% reduction in clinical malaria among 5- to 17-month-olds (N. Engl. J. Med. 2008;359:2521-32).
"The question everybody has is, is 56% enough, or should we aim for higher efficacy in malaria?" the pediatric infectious disease specialist said.
For him, the answer is clear: "If we can reduce mortality from malaria by 50%, that would be a great success in terms of preventing this important killer around the world," Dr. Asturias said.
But the benefits will extend well beyond mortality reduction to include nationwide favorable impacts upon lost days of productivity and strain on health care systems. "In countries where malaria is endemic, every case of fever gets treated as malaria, and that’s a huge burden in terms of health resource utilization," he added.
It’s also hypothetically possible that herd immunity could amplify the clinical efficacy of RTS,S beyond that 56% figure. It all depends upon how good the vaccine is at quelling parasitemia, an issue that hasn’t been well studied as yet.
Frequent boosting may be necessary for several years after the three-dose series is given. In studies in African infants and children, the protective effect has been sustained for only about 18 months.
Much of the credit for the recent major advances in developing new vaccines for important childhood diseases worldwide belongs to an ongoing UNICEF/WHO initiative and to the Bill and Melinda Gates Foundation, which Dr. Asturias said "has been a game changer." During this decade, the foundation’s declared aim is to utilize existing vaccines and to support development of new ones in order to prevent the deaths of 7.6 million children younger than age 5 years.
Dengue Virus
Sanofi Pasteur’s chimeric flavivirus vaccine is in phase III studies totaling more than 30,000 participants in Latin America and Asia. These pivotal trials will be completed in 2014. The company is sufficiently confident that it has a winner that it has built a $440 million factory to produce the vaccine.
In a phase IIB clinical trial conducted in Thai children, the vaccine – which has received fast-track development status from the Food and Drug Administration – protected against three of the four dengue virus serotypes, which means it should decrease the incidence of dengue hemorrhagic fever, the most feared manifestation of the viral infection.
Other dengue vaccines using alternative immunization strategies are in earlier stages of development.
In countries where the mosquito-borne dengue virus is endemic and reporting is adequate, the incidence of infection is about 5% per year (Vaccine 2002;20:3043-6). Three-quarters of infections are asymptomatic or entail only a few days of mild flulike illness. Among the 25% of infections that are symptomatic, 98%-99% result in dengue fever, also known as "breakbone fever," whereas 1%-2% manifest as dengue hemorrhagic fever, which has a mortality rate of up to 5% depending upon the availability of supportive care. The infection is more severe in school-age youths and in individuals infected for the second time.
Tuberculosis
Twelve new candidate TB vaccines have entered clinical trials. Seven are subunit vaccines, two utilize immunotherapy as an adjunct to chemotherapy, and three are recombinant bacille Calmette-Guérin vaccines designed to replace the current BCG vaccine, which doesn’t protect adults from reactivation or reinfection decades after their first exposure to the pathogen. The two most advanced candidate vaccines are now in phase IIB trials; they are designed to enhance immunity in individuals who received BCG vaccine early in life. Phase II studies are likely to continue to 2020.
"We’re still far away from a good tuberculosis vaccine at this point," according to Dr. Asturias.
The most pressing need now is to define markers for TB infection and reactivation so that researchers can demonstrate vaccine efficacy without having to wait 30 years for pulmonary TB or other clinical disease to develop, he added.
Group B Streptococcus
The push to develop a group B strep vaccine stems from the fact that in developing countries, this infection is a leading cause of sepsis and meningitis in the first 3 months of life. And worldwide, the proportion of deaths in children younger than age 5 years that occur during the neonatal period hasn’t changed in 2 decades. A UNICEF/WHO estimate concluded that of 8.8 million deaths in children younger than age 5 years in 2008, 42% occurred in neonates. A maternal group B strep vaccine is key to reducing that disturbing figure, in Dr. Asturias’s view.
There are at least 10 group B strep serotypes, but most disease is caused by three of them. A Novartis trivalent vaccine showed "very promising" results in a phase I study; a phase III clinical trial for prevention of disease in pregnant women is scheduled to get underway late next year, he said.
Dr. Asturias reported having conducted vaccine research studies sponsored by Sanofi Pasteur and Crucell, serving on data safety monitoring boards for Inviragen, Sanofi Pasteur, and PATH, and acting as a consultant to the Bill and Melinda Gates Foundation.
VAIL, COLO. – Effective vaccines for two of the top causes of mortality and morbidity in children worldwide – malaria and dengue fever – are now in pivotal phase III clinical trials and could reach the market within 3 years.
In addition, promising vaccines for tuberculosis and group B streptococcus are in earlier-stage clinical trials, Dr. Edwin J. Asturias noted at a conference on pediatric infectious diseases, which was sponsored by the Children’s Hospital Colorado in Aurora.
Malaria
"The malaria vaccine is coming. It is close. It will be the most important vaccine for killer diseases in children. There is a lot of hope for that vaccine," according to Dr. Asturias of the center for global health at the University of Colorado, Denver.
Malaria is endemic in 99 countries. An estimated 216 million new cases and 655,000 deaths resulting from the infection occurred in 2010. More than 90% of the world’s malaria deaths are in Africa, with most of them in children younger than age 5 years.
The malaria vaccine – now in the pivotal phase III MAL059 trial that’s expected to be completed by 2014 – has been in development by GlaxoSmithKline for 2 decades. The vaccine is known as RTS,S. It is designed for children living in endemic areas. The vaccine focuses on the pre-erythrocytic stage of infection, aiming to prevent the malaria parasite from multiplying in the liver and reentering the bloodstream.
In an interim report from the phase III trial, the three-dose series of RTS,S achieved a 56% reduction in clinical malaria among 5- to 17-month-olds (N. Engl. J. Med. 2008;359:2521-32).
"The question everybody has is, is 56% enough, or should we aim for higher efficacy in malaria?" the pediatric infectious disease specialist said.
For him, the answer is clear: "If we can reduce mortality from malaria by 50%, that would be a great success in terms of preventing this important killer around the world," Dr. Asturias said.
But the benefits will extend well beyond mortality reduction to include nationwide favorable impacts upon lost days of productivity and strain on health care systems. "In countries where malaria is endemic, every case of fever gets treated as malaria, and that’s a huge burden in terms of health resource utilization," he added.
It’s also hypothetically possible that herd immunity could amplify the clinical efficacy of RTS,S beyond that 56% figure. It all depends upon how good the vaccine is at quelling parasitemia, an issue that hasn’t been well studied as yet.
Frequent boosting may be necessary for several years after the three-dose series is given. In studies in African infants and children, the protective effect has been sustained for only about 18 months.
Much of the credit for the recent major advances in developing new vaccines for important childhood diseases worldwide belongs to an ongoing UNICEF/WHO initiative and to the Bill and Melinda Gates Foundation, which Dr. Asturias said "has been a game changer." During this decade, the foundation’s declared aim is to utilize existing vaccines and to support development of new ones in order to prevent the deaths of 7.6 million children younger than age 5 years.
Dengue Virus
Sanofi Pasteur’s chimeric flavivirus vaccine is in phase III studies totaling more than 30,000 participants in Latin America and Asia. These pivotal trials will be completed in 2014. The company is sufficiently confident that it has a winner that it has built a $440 million factory to produce the vaccine.
In a phase IIB clinical trial conducted in Thai children, the vaccine – which has received fast-track development status from the Food and Drug Administration – protected against three of the four dengue virus serotypes, which means it should decrease the incidence of dengue hemorrhagic fever, the most feared manifestation of the viral infection.
Other dengue vaccines using alternative immunization strategies are in earlier stages of development.
In countries where the mosquito-borne dengue virus is endemic and reporting is adequate, the incidence of infection is about 5% per year (Vaccine 2002;20:3043-6). Three-quarters of infections are asymptomatic or entail only a few days of mild flulike illness. Among the 25% of infections that are symptomatic, 98%-99% result in dengue fever, also known as "breakbone fever," whereas 1%-2% manifest as dengue hemorrhagic fever, which has a mortality rate of up to 5% depending upon the availability of supportive care. The infection is more severe in school-age youths and in individuals infected for the second time.
Tuberculosis
Twelve new candidate TB vaccines have entered clinical trials. Seven are subunit vaccines, two utilize immunotherapy as an adjunct to chemotherapy, and three are recombinant bacille Calmette-Guérin vaccines designed to replace the current BCG vaccine, which doesn’t protect adults from reactivation or reinfection decades after their first exposure to the pathogen. The two most advanced candidate vaccines are now in phase IIB trials; they are designed to enhance immunity in individuals who received BCG vaccine early in life. Phase II studies are likely to continue to 2020.
"We’re still far away from a good tuberculosis vaccine at this point," according to Dr. Asturias.
The most pressing need now is to define markers for TB infection and reactivation so that researchers can demonstrate vaccine efficacy without having to wait 30 years for pulmonary TB or other clinical disease to develop, he added.
Group B Streptococcus
The push to develop a group B strep vaccine stems from the fact that in developing countries, this infection is a leading cause of sepsis and meningitis in the first 3 months of life. And worldwide, the proportion of deaths in children younger than age 5 years that occur during the neonatal period hasn’t changed in 2 decades. A UNICEF/WHO estimate concluded that of 8.8 million deaths in children younger than age 5 years in 2008, 42% occurred in neonates. A maternal group B strep vaccine is key to reducing that disturbing figure, in Dr. Asturias’s view.
There are at least 10 group B strep serotypes, but most disease is caused by three of them. A Novartis trivalent vaccine showed "very promising" results in a phase I study; a phase III clinical trial for prevention of disease in pregnant women is scheduled to get underway late next year, he said.
Dr. Asturias reported having conducted vaccine research studies sponsored by Sanofi Pasteur and Crucell, serving on data safety monitoring boards for Inviragen, Sanofi Pasteur, and PATH, and acting as a consultant to the Bill and Melinda Gates Foundation.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES