User login
European College of Neuropsychopharmacology (ECNP): Annual Congress
Harm-reduction approach to alcoholism gains growing credibility
BERLIN – The harm-reduction concept as an alternative to abstinence as a treatment strategy for alcohol dependence has taken on new legitimacy as a result of the recent approval of nalmefene by the European Medicines Agency.
Nalmefene, an opioid receptor antagonist, received marketing approval across Europe for use specifically in a novel as-needed dosing regimen that is under the patient’s control. One 18-mg tablet is to be taken on days when a patient anticipates a risk of drinking, or if the patient has already started drinking, then as soon as possible.
The indication was granted by European regulators on the strength of three European phase III randomized trials that showed that heavy drinkers who used nalmefene in this way reduced their alcohol consumption to a more normal level. Thus, marketing approval was an implicit endorsement of the harm-reduction strategy.
“I have been praying for this for years and years. I have been strictly against the concept of complete abstinence, because the core of the disease of addiction is relapse. It is just a natural thing that one relapses,” Rainer Spanagel, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
“It has been a major mistake to say you are only successful in the field of addiction treatment if there is abstinence. This is an almost unreachable goal. This goal, set out to achieve for decades now, has been almost impossible for most patients to achieve,” added Dr. Spanagel, director of the Institute of Psychopharmacology at the University of Heidelberg (Germany).
Dr. Robert M. Swift noted that most treatment programs in the United States still promote abstinence as the only acceptable treatment outcome. It’s a concept that dates back to the 1800s and was heavily promoted by Alcoholics Anonymous beginning in the early 1900s, a period before there was any viable treatment for alcoholism.
Today, a variety of treatments have proved effective, both psychosocial as well as pharmacologic, but people aren’t receiving them. A large epidemiologic study conducted by the National Institute on Alcohol Abuse and Alcoholism estimated that of 7.9 million Americans who are alcohol dependent, only 2.4 million have ever been diagnosed as such, 1.5 million have ever gotten any treatment for their disorder, and a mere 139,000 have ever received pharmacotherapy.
“There’s this amazing treatment gap,” commented Dr. Swift, professor of psychiatry and associate director of the Center for Alcohol and Addiction Studies at Brown University in Providence, R.I.
The main reason for this huge treatment gap was revealed in a 2012 survey conducted by the U.S. Substance Abuse and Mental Health Services Administration: 49.5% of participants who had not sought treatment for an alcohol problem within the past year even though they needed it and recognized their need for it gave as their reason that they were just not ready to stop drinking completely. This is a group where the harm-reduction strategy makes a great deal of sense, the psychiatrist said.
Harm reduction is based upon evidence that the risk of mortality because of alcohol-related causes rises exponentially as daily consumption climbs above the threshold of hazardous drinking, which has been defined by U.S. public health officials as five drinks per day or 15 per week in men and four per day or eight per week in women. The corollary is that reducing heavy drinking provides exponential health benefits. Investigators at Imperial College, London, have estimated that reducing alcohol intake from 100 g per day to 50 g per day provides an eight-fold reduction in health harms (J. Psychopharmacol. 2014;28:3-7).
“You get a lot of bang for your buck by reducing the amount of alcohol you consume,” Dr. Swift said. “The evidence really is out there that reducing drinking is an effective harm reduction strategy.”
Dr. Wim van den Brink, who led the SENSE trial, a 1-year, phase III, randomized, placebo-controlled study with positive safety and efficacy outcomes for nalmefene as-needed (J. Psychopharmacol. 2014;28:733-44), said one of the strengths of this treatment strategy is that it gives patients responsibility for their drinking and allows them to set their own treatment goals. That enhances the likelihood of successful outcomes.
The harm-reduction approach with as-needed nalmefene also is a way to bring into treatment some of the 50% of problem drinkers who don’t want to stop drinking entirely, added Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
A spokesperson for Lundbeck, which sponsored the SENSE and 6-month ESENSE 1 and ESENSE 2 trials, said the company would not seek marketing approval for nalmefene in the United States because the drug’s remaining duration of patent protection makes it commercially nonviable.
Nalmefene and naltrexone, the latter of which is available in the United States, are both mu opioid receptor antagonists that also affect the delta opioid receptor. However, nalmefene also is a partial agonist at the kappa opioid receptor.
Dr. Swift said both drugs have been shown in preclinical and clinical studies to interrupt multiple biobehavioral mechanisms responsible for initiating and maintaining alcohol dependence. They reduce alcohol craving, block the motivation to drink to relieve stress and anxiety, and might improve impulsivity.
“Naltrexone has been shown not only to affect the rewards of alcohol consumption, it also seems to have direct cognitive and attentional effects in the orbitofrontal cortex. It reduces preoccupation with alcohol and improves attention to non–alcohol related stimuli,” he said. “I think that’s a really important finding and provides a new understanding of how opioid antagonists work.”
The psychiatrist noted that a recent meta-analysis concluded that opioid antagonists are better for reducing heavy drinking than for increasing abstinence (Addiction 2013;108:275-93).
Dr. Swift reported receiving honoraria from Lundbeck and serving as an adviser or consultant to D&B Pharma and CT San Remo. Dr. van den Brink is on the advisory boards of Lundbeck and more than a half-dozen other pharmaceutical companies.
Alcohol use disorders are clinical diagnoses based on a loss of control over alcohol consumption despite adverse consequences. Remission occurs when an individual exerts control over his or her drinking behavior. Abstinence is the safest option, but, paraphrasing Voltaire, the perfect should not be the enemy of the good.
It has long been known that people with a diagnosis of alcohol dependence can moderate their drinking, and that such reductions in consumption reduce end-organ damage and other adverse health consequences. So, it makes complete sense that the opioid antagonists naltrexone or nalmefene that reduce alcohol’s euphoric reward and help alcoholic patients regain control over their drinking should be welcome additions to the pharmacopoeia.
Medicine has long accepted disease-modifying therapies as organ- and lifesaving for other disorders – gold salts and methotrexate for rheumatoid arthritis come to mind. Such remittive agents reduce disease activity without an insistence on complete, total, and lasting cure.
These medications are called effective treatment, and are not burdened by the apologist moniker of “harm reduction.” Effective remittive therapies for alcohol dependence such as naltrexone and nalmefene should not be viewed any differently.
Peter D. Friedmann, M.D., M.P.H., is professor of medicine and professor of health services, policy, and practice at Brown University, Providence, R.I. He has received research support from Alkermes and a speaker’s honorarium from Orexo.
Alcohol use disorders are clinical diagnoses based on a loss of control over alcohol consumption despite adverse consequences. Remission occurs when an individual exerts control over his or her drinking behavior. Abstinence is the safest option, but, paraphrasing Voltaire, the perfect should not be the enemy of the good.
It has long been known that people with a diagnosis of alcohol dependence can moderate their drinking, and that such reductions in consumption reduce end-organ damage and other adverse health consequences. So, it makes complete sense that the opioid antagonists naltrexone or nalmefene that reduce alcohol’s euphoric reward and help alcoholic patients regain control over their drinking should be welcome additions to the pharmacopoeia.
Medicine has long accepted disease-modifying therapies as organ- and lifesaving for other disorders – gold salts and methotrexate for rheumatoid arthritis come to mind. Such remittive agents reduce disease activity without an insistence on complete, total, and lasting cure.
These medications are called effective treatment, and are not burdened by the apologist moniker of “harm reduction.” Effective remittive therapies for alcohol dependence such as naltrexone and nalmefene should not be viewed any differently.
Peter D. Friedmann, M.D., M.P.H., is professor of medicine and professor of health services, policy, and practice at Brown University, Providence, R.I. He has received research support from Alkermes and a speaker’s honorarium from Orexo.
Alcohol use disorders are clinical diagnoses based on a loss of control over alcohol consumption despite adverse consequences. Remission occurs when an individual exerts control over his or her drinking behavior. Abstinence is the safest option, but, paraphrasing Voltaire, the perfect should not be the enemy of the good.
It has long been known that people with a diagnosis of alcohol dependence can moderate their drinking, and that such reductions in consumption reduce end-organ damage and other adverse health consequences. So, it makes complete sense that the opioid antagonists naltrexone or nalmefene that reduce alcohol’s euphoric reward and help alcoholic patients regain control over their drinking should be welcome additions to the pharmacopoeia.
Medicine has long accepted disease-modifying therapies as organ- and lifesaving for other disorders – gold salts and methotrexate for rheumatoid arthritis come to mind. Such remittive agents reduce disease activity without an insistence on complete, total, and lasting cure.
These medications are called effective treatment, and are not burdened by the apologist moniker of “harm reduction.” Effective remittive therapies for alcohol dependence such as naltrexone and nalmefene should not be viewed any differently.
Peter D. Friedmann, M.D., M.P.H., is professor of medicine and professor of health services, policy, and practice at Brown University, Providence, R.I. He has received research support from Alkermes and a speaker’s honorarium from Orexo.
BERLIN – The harm-reduction concept as an alternative to abstinence as a treatment strategy for alcohol dependence has taken on new legitimacy as a result of the recent approval of nalmefene by the European Medicines Agency.
Nalmefene, an opioid receptor antagonist, received marketing approval across Europe for use specifically in a novel as-needed dosing regimen that is under the patient’s control. One 18-mg tablet is to be taken on days when a patient anticipates a risk of drinking, or if the patient has already started drinking, then as soon as possible.
The indication was granted by European regulators on the strength of three European phase III randomized trials that showed that heavy drinkers who used nalmefene in this way reduced their alcohol consumption to a more normal level. Thus, marketing approval was an implicit endorsement of the harm-reduction strategy.
“I have been praying for this for years and years. I have been strictly against the concept of complete abstinence, because the core of the disease of addiction is relapse. It is just a natural thing that one relapses,” Rainer Spanagel, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
“It has been a major mistake to say you are only successful in the field of addiction treatment if there is abstinence. This is an almost unreachable goal. This goal, set out to achieve for decades now, has been almost impossible for most patients to achieve,” added Dr. Spanagel, director of the Institute of Psychopharmacology at the University of Heidelberg (Germany).
Dr. Robert M. Swift noted that most treatment programs in the United States still promote abstinence as the only acceptable treatment outcome. It’s a concept that dates back to the 1800s and was heavily promoted by Alcoholics Anonymous beginning in the early 1900s, a period before there was any viable treatment for alcoholism.
Today, a variety of treatments have proved effective, both psychosocial as well as pharmacologic, but people aren’t receiving them. A large epidemiologic study conducted by the National Institute on Alcohol Abuse and Alcoholism estimated that of 7.9 million Americans who are alcohol dependent, only 2.4 million have ever been diagnosed as such, 1.5 million have ever gotten any treatment for their disorder, and a mere 139,000 have ever received pharmacotherapy.
“There’s this amazing treatment gap,” commented Dr. Swift, professor of psychiatry and associate director of the Center for Alcohol and Addiction Studies at Brown University in Providence, R.I.
The main reason for this huge treatment gap was revealed in a 2012 survey conducted by the U.S. Substance Abuse and Mental Health Services Administration: 49.5% of participants who had not sought treatment for an alcohol problem within the past year even though they needed it and recognized their need for it gave as their reason that they were just not ready to stop drinking completely. This is a group where the harm-reduction strategy makes a great deal of sense, the psychiatrist said.
Harm reduction is based upon evidence that the risk of mortality because of alcohol-related causes rises exponentially as daily consumption climbs above the threshold of hazardous drinking, which has been defined by U.S. public health officials as five drinks per day or 15 per week in men and four per day or eight per week in women. The corollary is that reducing heavy drinking provides exponential health benefits. Investigators at Imperial College, London, have estimated that reducing alcohol intake from 100 g per day to 50 g per day provides an eight-fold reduction in health harms (J. Psychopharmacol. 2014;28:3-7).
“You get a lot of bang for your buck by reducing the amount of alcohol you consume,” Dr. Swift said. “The evidence really is out there that reducing drinking is an effective harm reduction strategy.”
Dr. Wim van den Brink, who led the SENSE trial, a 1-year, phase III, randomized, placebo-controlled study with positive safety and efficacy outcomes for nalmefene as-needed (J. Psychopharmacol. 2014;28:733-44), said one of the strengths of this treatment strategy is that it gives patients responsibility for their drinking and allows them to set their own treatment goals. That enhances the likelihood of successful outcomes.
The harm-reduction approach with as-needed nalmefene also is a way to bring into treatment some of the 50% of problem drinkers who don’t want to stop drinking entirely, added Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
A spokesperson for Lundbeck, which sponsored the SENSE and 6-month ESENSE 1 and ESENSE 2 trials, said the company would not seek marketing approval for nalmefene in the United States because the drug’s remaining duration of patent protection makes it commercially nonviable.
Nalmefene and naltrexone, the latter of which is available in the United States, are both mu opioid receptor antagonists that also affect the delta opioid receptor. However, nalmefene also is a partial agonist at the kappa opioid receptor.
Dr. Swift said both drugs have been shown in preclinical and clinical studies to interrupt multiple biobehavioral mechanisms responsible for initiating and maintaining alcohol dependence. They reduce alcohol craving, block the motivation to drink to relieve stress and anxiety, and might improve impulsivity.
“Naltrexone has been shown not only to affect the rewards of alcohol consumption, it also seems to have direct cognitive and attentional effects in the orbitofrontal cortex. It reduces preoccupation with alcohol and improves attention to non–alcohol related stimuli,” he said. “I think that’s a really important finding and provides a new understanding of how opioid antagonists work.”
The psychiatrist noted that a recent meta-analysis concluded that opioid antagonists are better for reducing heavy drinking than for increasing abstinence (Addiction 2013;108:275-93).
Dr. Swift reported receiving honoraria from Lundbeck and serving as an adviser or consultant to D&B Pharma and CT San Remo. Dr. van den Brink is on the advisory boards of Lundbeck and more than a half-dozen other pharmaceutical companies.
BERLIN – The harm-reduction concept as an alternative to abstinence as a treatment strategy for alcohol dependence has taken on new legitimacy as a result of the recent approval of nalmefene by the European Medicines Agency.
Nalmefene, an opioid receptor antagonist, received marketing approval across Europe for use specifically in a novel as-needed dosing regimen that is under the patient’s control. One 18-mg tablet is to be taken on days when a patient anticipates a risk of drinking, or if the patient has already started drinking, then as soon as possible.
The indication was granted by European regulators on the strength of three European phase III randomized trials that showed that heavy drinkers who used nalmefene in this way reduced their alcohol consumption to a more normal level. Thus, marketing approval was an implicit endorsement of the harm-reduction strategy.
“I have been praying for this for years and years. I have been strictly against the concept of complete abstinence, because the core of the disease of addiction is relapse. It is just a natural thing that one relapses,” Rainer Spanagel, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
“It has been a major mistake to say you are only successful in the field of addiction treatment if there is abstinence. This is an almost unreachable goal. This goal, set out to achieve for decades now, has been almost impossible for most patients to achieve,” added Dr. Spanagel, director of the Institute of Psychopharmacology at the University of Heidelberg (Germany).
Dr. Robert M. Swift noted that most treatment programs in the United States still promote abstinence as the only acceptable treatment outcome. It’s a concept that dates back to the 1800s and was heavily promoted by Alcoholics Anonymous beginning in the early 1900s, a period before there was any viable treatment for alcoholism.
Today, a variety of treatments have proved effective, both psychosocial as well as pharmacologic, but people aren’t receiving them. A large epidemiologic study conducted by the National Institute on Alcohol Abuse and Alcoholism estimated that of 7.9 million Americans who are alcohol dependent, only 2.4 million have ever been diagnosed as such, 1.5 million have ever gotten any treatment for their disorder, and a mere 139,000 have ever received pharmacotherapy.
“There’s this amazing treatment gap,” commented Dr. Swift, professor of psychiatry and associate director of the Center for Alcohol and Addiction Studies at Brown University in Providence, R.I.
The main reason for this huge treatment gap was revealed in a 2012 survey conducted by the U.S. Substance Abuse and Mental Health Services Administration: 49.5% of participants who had not sought treatment for an alcohol problem within the past year even though they needed it and recognized their need for it gave as their reason that they were just not ready to stop drinking completely. This is a group where the harm-reduction strategy makes a great deal of sense, the psychiatrist said.
Harm reduction is based upon evidence that the risk of mortality because of alcohol-related causes rises exponentially as daily consumption climbs above the threshold of hazardous drinking, which has been defined by U.S. public health officials as five drinks per day or 15 per week in men and four per day or eight per week in women. The corollary is that reducing heavy drinking provides exponential health benefits. Investigators at Imperial College, London, have estimated that reducing alcohol intake from 100 g per day to 50 g per day provides an eight-fold reduction in health harms (J. Psychopharmacol. 2014;28:3-7).
“You get a lot of bang for your buck by reducing the amount of alcohol you consume,” Dr. Swift said. “The evidence really is out there that reducing drinking is an effective harm reduction strategy.”
Dr. Wim van den Brink, who led the SENSE trial, a 1-year, phase III, randomized, placebo-controlled study with positive safety and efficacy outcomes for nalmefene as-needed (J. Psychopharmacol. 2014;28:733-44), said one of the strengths of this treatment strategy is that it gives patients responsibility for their drinking and allows them to set their own treatment goals. That enhances the likelihood of successful outcomes.
The harm-reduction approach with as-needed nalmefene also is a way to bring into treatment some of the 50% of problem drinkers who don’t want to stop drinking entirely, added Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
A spokesperson for Lundbeck, which sponsored the SENSE and 6-month ESENSE 1 and ESENSE 2 trials, said the company would not seek marketing approval for nalmefene in the United States because the drug’s remaining duration of patent protection makes it commercially nonviable.
Nalmefene and naltrexone, the latter of which is available in the United States, are both mu opioid receptor antagonists that also affect the delta opioid receptor. However, nalmefene also is a partial agonist at the kappa opioid receptor.
Dr. Swift said both drugs have been shown in preclinical and clinical studies to interrupt multiple biobehavioral mechanisms responsible for initiating and maintaining alcohol dependence. They reduce alcohol craving, block the motivation to drink to relieve stress and anxiety, and might improve impulsivity.
“Naltrexone has been shown not only to affect the rewards of alcohol consumption, it also seems to have direct cognitive and attentional effects in the orbitofrontal cortex. It reduces preoccupation with alcohol and improves attention to non–alcohol related stimuli,” he said. “I think that’s a really important finding and provides a new understanding of how opioid antagonists work.”
The psychiatrist noted that a recent meta-analysis concluded that opioid antagonists are better for reducing heavy drinking than for increasing abstinence (Addiction 2013;108:275-93).
Dr. Swift reported receiving honoraria from Lundbeck and serving as an adviser or consultant to D&B Pharma and CT San Remo. Dr. van den Brink is on the advisory boards of Lundbeck and more than a half-dozen other pharmaceutical companies.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
New insights into relationship between alcohol, depression, and suicide
BERLIN– Alcohol dependence and major depressive disorder are similarly potent yet independent risk factors for suicidal behavior, according to Dr. Philip Gorwood.
Although alcohol use disorder and major depression are extremely common and often comorbid, the mechanisms by which they boost the risk for suicidal behavior are very different, he said at the annual congress of the European College of Neuropsychopharmacology.
“The ideation about suicidal behavior comes from the mood disorder, but the organization of the behavior – the suicidal attempt, especially when it is unplanned and impulsive – is driven by the alcohol consumption,” said Dr. Gorwood, professor of psychiatry at Rene Descartes University, Paris.
“The real take-home message is the difference between suicidal ideas and impulsive acts. Probably that was not well understood before. Alcohol dependence has no relationship with suicidal ideas. But once you have those ideas, alcohol consumption becomes very important in the risk of suicidal behavior,” he said.
A recent retrospective analysis of nearly 451,000 French patients hospitalized for suicide attempts during 2004-2011 showed their most common psychiatric diagnosis was major depressive disorder, present in 52% of the men and 65% of the women. Second most common – and far ahead of the rest of the pack – was alcohol use disorder, diagnosed in 36% of the hospitalized men and 49% of the women.
Alcohol probably facilitates suicidal behaviors in a variety of ways – by generating stressful life events, weakening social supports, worsening mood, restricting cognition and thereby inhibiting coping strategies, boosting impulsivity, and promoting inaccurate assessment of harm and pain.
A recent review of epidemiologic studies on the risk factors for suicide attempts and suicide among individuals with alcohol use disorder identified a clutch of risk factors that could be useful in clinical practice as a means of targeting problem drinkers at increased risk for suicidality: major depressive disorder, greater severity of alcohol use disorder, limited social support, stressful life events, aggression, medical illness, and economic adversity (Am. J. Prev. Med. 2014;47:S204-S208).
Other studies have identified additional risk factors that distinguish individuals with alcohol dependence who do attempt suicide from those who don’t: early onset of alcohol dependence, childhood trauma, a family history of suicidal behavior, and tobacco dependence. But if a clinician is going to rely on the two strongest risk factors in order to identify increased suicidal risk among alcohol-dependent patients, those would be major depression and the greater severity of alcohol dependence, according to Dr. Gorwood.
A study from the National Comorbidity Survey Replication based on a representative sample comprised of 9,282 U.S. adults concluded that alcohol abuse or dependence was associated with a 2.1-fold increased risk of a history of a suicide attempt, while major depressive disorder was independently associated with a 2.0-fold increased risk. In a multivariate analysis, major depressive disorder was associated with a statistically significant 2.3-fold increased risk of suicidal ideation but no significant increase in planned or unplanned suicide attempts. In contrast, alcohol abuse or dependence wasn’t associated with a significant increase in suicidal ideation but did carry a 2.9-fold increased risk of unplanned, impulsive suicide attempts (Mol. Psychiatry 2010;15:868-76).
A first-of-its-kind U.S. national study of postmortem blood alcohol levels as a means of assessing alcohol use prior to suicide concluded that men and women who committed suicide were 1.8- and 2.4-fold more likely, respectively, to have consumed alcohol within 48 hours prior to their death, compared with matched living controls. More importantly, in a multivariate analysis adjusted for potential confounders, men and women who committed suicide were 6.2-fold and 10-fold more likely to have been intoxicated. The investigators concluded that it’s essential for suicide prevention programs to include components that discourage intoxication (Ann. Epidemiol. 2014;24:588-92).
Dr. Gorwood said a popular misconception among clinicians is that focusing solely on antidepressant therapy in patients with major depressive disorder and comorbid alcohol dependence will reduce their risk of suicidal behavior. A large Finnish national study scrutinizing the use of antidepressants and cause-specific mortality over the course of a decade showed this is not the case (J. Affect. Disord. 2013;148:278-85).
“The Finnish data means that probably if you are not also taking care of the alcohol dependence, then independently treating the associated mood disorder will not be an efficient way of reducing the suicide risk,” Dr. Gorwood noted.
He added, however, that getting patients with alcohol use disorder to stop drinking is hardly a panacea.
“We shouldn’t be naive. Stopping drinking alcohol will not resolve a lot of the difficulties that affect these patients. They have to build a new life and new types of functioning, and it’s difficult. There’s a lot of work to do. But it’s a very important step in reducing suicidal risk.”
Dr. Gorwood reported receiving research grants from Eli Lilly and Servier, and serving on scientific advisory boards or speakers panels for those pharmaceutical companies and eight others.
BERLIN– Alcohol dependence and major depressive disorder are similarly potent yet independent risk factors for suicidal behavior, according to Dr. Philip Gorwood.
Although alcohol use disorder and major depression are extremely common and often comorbid, the mechanisms by which they boost the risk for suicidal behavior are very different, he said at the annual congress of the European College of Neuropsychopharmacology.
“The ideation about suicidal behavior comes from the mood disorder, but the organization of the behavior – the suicidal attempt, especially when it is unplanned and impulsive – is driven by the alcohol consumption,” said Dr. Gorwood, professor of psychiatry at Rene Descartes University, Paris.
“The real take-home message is the difference between suicidal ideas and impulsive acts. Probably that was not well understood before. Alcohol dependence has no relationship with suicidal ideas. But once you have those ideas, alcohol consumption becomes very important in the risk of suicidal behavior,” he said.
A recent retrospective analysis of nearly 451,000 French patients hospitalized for suicide attempts during 2004-2011 showed their most common psychiatric diagnosis was major depressive disorder, present in 52% of the men and 65% of the women. Second most common – and far ahead of the rest of the pack – was alcohol use disorder, diagnosed in 36% of the hospitalized men and 49% of the women.
Alcohol probably facilitates suicidal behaviors in a variety of ways – by generating stressful life events, weakening social supports, worsening mood, restricting cognition and thereby inhibiting coping strategies, boosting impulsivity, and promoting inaccurate assessment of harm and pain.
A recent review of epidemiologic studies on the risk factors for suicide attempts and suicide among individuals with alcohol use disorder identified a clutch of risk factors that could be useful in clinical practice as a means of targeting problem drinkers at increased risk for suicidality: major depressive disorder, greater severity of alcohol use disorder, limited social support, stressful life events, aggression, medical illness, and economic adversity (Am. J. Prev. Med. 2014;47:S204-S208).
Other studies have identified additional risk factors that distinguish individuals with alcohol dependence who do attempt suicide from those who don’t: early onset of alcohol dependence, childhood trauma, a family history of suicidal behavior, and tobacco dependence. But if a clinician is going to rely on the two strongest risk factors in order to identify increased suicidal risk among alcohol-dependent patients, those would be major depression and the greater severity of alcohol dependence, according to Dr. Gorwood.
A study from the National Comorbidity Survey Replication based on a representative sample comprised of 9,282 U.S. adults concluded that alcohol abuse or dependence was associated with a 2.1-fold increased risk of a history of a suicide attempt, while major depressive disorder was independently associated with a 2.0-fold increased risk. In a multivariate analysis, major depressive disorder was associated with a statistically significant 2.3-fold increased risk of suicidal ideation but no significant increase in planned or unplanned suicide attempts. In contrast, alcohol abuse or dependence wasn’t associated with a significant increase in suicidal ideation but did carry a 2.9-fold increased risk of unplanned, impulsive suicide attempts (Mol. Psychiatry 2010;15:868-76).
A first-of-its-kind U.S. national study of postmortem blood alcohol levels as a means of assessing alcohol use prior to suicide concluded that men and women who committed suicide were 1.8- and 2.4-fold more likely, respectively, to have consumed alcohol within 48 hours prior to their death, compared with matched living controls. More importantly, in a multivariate analysis adjusted for potential confounders, men and women who committed suicide were 6.2-fold and 10-fold more likely to have been intoxicated. The investigators concluded that it’s essential for suicide prevention programs to include components that discourage intoxication (Ann. Epidemiol. 2014;24:588-92).
Dr. Gorwood said a popular misconception among clinicians is that focusing solely on antidepressant therapy in patients with major depressive disorder and comorbid alcohol dependence will reduce their risk of suicidal behavior. A large Finnish national study scrutinizing the use of antidepressants and cause-specific mortality over the course of a decade showed this is not the case (J. Affect. Disord. 2013;148:278-85).
“The Finnish data means that probably if you are not also taking care of the alcohol dependence, then independently treating the associated mood disorder will not be an efficient way of reducing the suicide risk,” Dr. Gorwood noted.
He added, however, that getting patients with alcohol use disorder to stop drinking is hardly a panacea.
“We shouldn’t be naive. Stopping drinking alcohol will not resolve a lot of the difficulties that affect these patients. They have to build a new life and new types of functioning, and it’s difficult. There’s a lot of work to do. But it’s a very important step in reducing suicidal risk.”
Dr. Gorwood reported receiving research grants from Eli Lilly and Servier, and serving on scientific advisory boards or speakers panels for those pharmaceutical companies and eight others.
BERLIN– Alcohol dependence and major depressive disorder are similarly potent yet independent risk factors for suicidal behavior, according to Dr. Philip Gorwood.
Although alcohol use disorder and major depression are extremely common and often comorbid, the mechanisms by which they boost the risk for suicidal behavior are very different, he said at the annual congress of the European College of Neuropsychopharmacology.
“The ideation about suicidal behavior comes from the mood disorder, but the organization of the behavior – the suicidal attempt, especially when it is unplanned and impulsive – is driven by the alcohol consumption,” said Dr. Gorwood, professor of psychiatry at Rene Descartes University, Paris.
“The real take-home message is the difference between suicidal ideas and impulsive acts. Probably that was not well understood before. Alcohol dependence has no relationship with suicidal ideas. But once you have those ideas, alcohol consumption becomes very important in the risk of suicidal behavior,” he said.
A recent retrospective analysis of nearly 451,000 French patients hospitalized for suicide attempts during 2004-2011 showed their most common psychiatric diagnosis was major depressive disorder, present in 52% of the men and 65% of the women. Second most common – and far ahead of the rest of the pack – was alcohol use disorder, diagnosed in 36% of the hospitalized men and 49% of the women.
Alcohol probably facilitates suicidal behaviors in a variety of ways – by generating stressful life events, weakening social supports, worsening mood, restricting cognition and thereby inhibiting coping strategies, boosting impulsivity, and promoting inaccurate assessment of harm and pain.
A recent review of epidemiologic studies on the risk factors for suicide attempts and suicide among individuals with alcohol use disorder identified a clutch of risk factors that could be useful in clinical practice as a means of targeting problem drinkers at increased risk for suicidality: major depressive disorder, greater severity of alcohol use disorder, limited social support, stressful life events, aggression, medical illness, and economic adversity (Am. J. Prev. Med. 2014;47:S204-S208).
Other studies have identified additional risk factors that distinguish individuals with alcohol dependence who do attempt suicide from those who don’t: early onset of alcohol dependence, childhood trauma, a family history of suicidal behavior, and tobacco dependence. But if a clinician is going to rely on the two strongest risk factors in order to identify increased suicidal risk among alcohol-dependent patients, those would be major depression and the greater severity of alcohol dependence, according to Dr. Gorwood.
A study from the National Comorbidity Survey Replication based on a representative sample comprised of 9,282 U.S. adults concluded that alcohol abuse or dependence was associated with a 2.1-fold increased risk of a history of a suicide attempt, while major depressive disorder was independently associated with a 2.0-fold increased risk. In a multivariate analysis, major depressive disorder was associated with a statistically significant 2.3-fold increased risk of suicidal ideation but no significant increase in planned or unplanned suicide attempts. In contrast, alcohol abuse or dependence wasn’t associated with a significant increase in suicidal ideation but did carry a 2.9-fold increased risk of unplanned, impulsive suicide attempts (Mol. Psychiatry 2010;15:868-76).
A first-of-its-kind U.S. national study of postmortem blood alcohol levels as a means of assessing alcohol use prior to suicide concluded that men and women who committed suicide were 1.8- and 2.4-fold more likely, respectively, to have consumed alcohol within 48 hours prior to their death, compared with matched living controls. More importantly, in a multivariate analysis adjusted for potential confounders, men and women who committed suicide were 6.2-fold and 10-fold more likely to have been intoxicated. The investigators concluded that it’s essential for suicide prevention programs to include components that discourage intoxication (Ann. Epidemiol. 2014;24:588-92).
Dr. Gorwood said a popular misconception among clinicians is that focusing solely on antidepressant therapy in patients with major depressive disorder and comorbid alcohol dependence will reduce their risk of suicidal behavior. A large Finnish national study scrutinizing the use of antidepressants and cause-specific mortality over the course of a decade showed this is not the case (J. Affect. Disord. 2013;148:278-85).
“The Finnish data means that probably if you are not also taking care of the alcohol dependence, then independently treating the associated mood disorder will not be an efficient way of reducing the suicide risk,” Dr. Gorwood noted.
He added, however, that getting patients with alcohol use disorder to stop drinking is hardly a panacea.
“We shouldn’t be naive. Stopping drinking alcohol will not resolve a lot of the difficulties that affect these patients. They have to build a new life and new types of functioning, and it’s difficult. There’s a lot of work to do. But it’s a very important step in reducing suicidal risk.”
Dr. Gorwood reported receiving research grants from Eli Lilly and Servier, and serving on scientific advisory boards or speakers panels for those pharmaceutical companies and eight others.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Redefined schizoaffective disorder in DSM-5 seen as problematic
BERLIN – The redefinition of schizoaffective disorder unveiled in the DSM-5 leaves much to be desired, experts opined at the annual congress of the European College of Neuropsychopharmacology
“We all thought we had the solution for making new criteria for schizoaffective disorder,” recalled Dr. Jim van Os, who served on the DSM-5 committee charged with reexamining the disorder. “But in the end, nothing much changed.”
Indeed, despite the lofty ambitions he and many of his fellow DSM-5 committee members held for thoroughly overhauling the criteria for what he called “this enigmatic combination of affective and psychotic dysregulation,” the difference between the DSM-IV and DSM-5 boiled down to small potatoes: Whereas the DSM-IV stated that the diagnosis of schizoaffective disorder required that the mood episode must be present for “a substantial duration of the illness,” the DSM-5 requires that the mood episode be present for “the majority” of the illness.
“The change was made to improve the reliability and stability of the diagnosis,” explained Dr. van Os, professor of psychiatric epidemiology at Maastricht (the Netherlands) University.
He was an advocate on the committee for introducing a dimensional approach to the diagnosis of schizoaffective disorder, one that would incorporate consideration of clustering of symptoms across a continuum.
“This would have been the best solution to the conundrum of the ‘fuzzy set’ problem of mental disorders. It would have been a great way of recognizing the continuity of all mental phenomena seen in mental disorders. However, it did not happen, unfortunately.”
The psychiatrist noted that committee members debated “for many hours” how best to overhaul the criteria for schizoaffective disorder in the DSM-5 before deciding to scale back their scope.
“We realized that any measure of precision in the criteria we tried to introduce for the admixture of affective dysregulation and psychosis is spurious. We really haven’t got the tools yet to make valid distinctions. That’s why we said in DSM-5 that the clinician can make the diagnosis using clinical intuition that the majority of time in the illness there is affective dysregulation. So, not spurious precision, but clinical intuition. I don’t know if that’s a good decision, but that’s what we did,” Dr. van Os said.
At the same symposium on schizoaffective disorder held during the ECNP congress, Dr. Heinz Grunze declared: “I would have welcomed as a development in DSM-5, as we’ve seen with some other disorders, basically abolishing schizoaffective disorder on its own and assigning it either to bipolar disorder with psychotic features specified or to schizophrenia with a strong affective specifier. I think that would be better suited to characterizing these patients.”
“Clearly, schizoaffective disorder has quite a polymorphic course. It’s definitely not one illness; it’s several illnesses which we put together in this random category,” added Dr. Grunze, professor of clinical psychiatry at University of Newcastle (England).
His own view is that the disorder belongs under the umbrella of bipolar disorder. A recent Spanish functional magnetic resonance brain imaging study demonstrated that schizoaffective disorder exhibits features that more closely resemble those of bipolar disorder than schizophrenia, including the finding that patients in clinical remission show failure of deactivation in the medial frontal gyrus, compared with healthy controls, Dr. Grunze noted.
“I personally can’t see a difference between a schizoaffective acute episode, manic type, and a psychotic manic episode. So my personal opinion is that schizoaffective disorder belongs under the heading of bipolar disorder. That’s obviously different from the opinion of those conducting treatment trials and the pharmaceutical industry, where schizoaffective disorder is never a subgroup in bipolar disorder trials, but a subgroup in schizophrenia trials,” Dr. Grunze said.
One audience member rose to state that while all this wrangling over how best to subclassify these patients is interesting, why not take a pragmatic approach and simply treat them symptomatically?
“Well, I think that’s what everyone in this room who’s a clinician is doing,” Dr. Grunze replied. “Because even if we assume that the diagnosis itself might be unstable, I think the greatest variance in patients switching around between bipolar, schizophrenia, and schizoaffective comes from the different doctors seeing them, each having a different understanding of schizoaffective disorder. I see a lot of patients who are classified as schizoaffective disorder because they have what I would have considered just as mood-congruent psychotic symptoms while manic. So I absolutely agree with you that this is more of an academic discussion, really, because at the moment we don’t have any consequences for treatment. We don’t have good evidence about how to treat these patients; we just extrapolate from whatever we think their symptoms come closest to.”
He reported receiving research grants from the National Institute for Health Research and the U.K. Medical Research Council, as well as more than a half-dozen pharmaceutical companies.
Dr. van Os reported having no financial conflicts.
BERLIN – The redefinition of schizoaffective disorder unveiled in the DSM-5 leaves much to be desired, experts opined at the annual congress of the European College of Neuropsychopharmacology
“We all thought we had the solution for making new criteria for schizoaffective disorder,” recalled Dr. Jim van Os, who served on the DSM-5 committee charged with reexamining the disorder. “But in the end, nothing much changed.”
Indeed, despite the lofty ambitions he and many of his fellow DSM-5 committee members held for thoroughly overhauling the criteria for what he called “this enigmatic combination of affective and psychotic dysregulation,” the difference between the DSM-IV and DSM-5 boiled down to small potatoes: Whereas the DSM-IV stated that the diagnosis of schizoaffective disorder required that the mood episode must be present for “a substantial duration of the illness,” the DSM-5 requires that the mood episode be present for “the majority” of the illness.
“The change was made to improve the reliability and stability of the diagnosis,” explained Dr. van Os, professor of psychiatric epidemiology at Maastricht (the Netherlands) University.
He was an advocate on the committee for introducing a dimensional approach to the diagnosis of schizoaffective disorder, one that would incorporate consideration of clustering of symptoms across a continuum.
“This would have been the best solution to the conundrum of the ‘fuzzy set’ problem of mental disorders. It would have been a great way of recognizing the continuity of all mental phenomena seen in mental disorders. However, it did not happen, unfortunately.”
The psychiatrist noted that committee members debated “for many hours” how best to overhaul the criteria for schizoaffective disorder in the DSM-5 before deciding to scale back their scope.
“We realized that any measure of precision in the criteria we tried to introduce for the admixture of affective dysregulation and psychosis is spurious. We really haven’t got the tools yet to make valid distinctions. That’s why we said in DSM-5 that the clinician can make the diagnosis using clinical intuition that the majority of time in the illness there is affective dysregulation. So, not spurious precision, but clinical intuition. I don’t know if that’s a good decision, but that’s what we did,” Dr. van Os said.
At the same symposium on schizoaffective disorder held during the ECNP congress, Dr. Heinz Grunze declared: “I would have welcomed as a development in DSM-5, as we’ve seen with some other disorders, basically abolishing schizoaffective disorder on its own and assigning it either to bipolar disorder with psychotic features specified or to schizophrenia with a strong affective specifier. I think that would be better suited to characterizing these patients.”
“Clearly, schizoaffective disorder has quite a polymorphic course. It’s definitely not one illness; it’s several illnesses which we put together in this random category,” added Dr. Grunze, professor of clinical psychiatry at University of Newcastle (England).
His own view is that the disorder belongs under the umbrella of bipolar disorder. A recent Spanish functional magnetic resonance brain imaging study demonstrated that schizoaffective disorder exhibits features that more closely resemble those of bipolar disorder than schizophrenia, including the finding that patients in clinical remission show failure of deactivation in the medial frontal gyrus, compared with healthy controls, Dr. Grunze noted.
“I personally can’t see a difference between a schizoaffective acute episode, manic type, and a psychotic manic episode. So my personal opinion is that schizoaffective disorder belongs under the heading of bipolar disorder. That’s obviously different from the opinion of those conducting treatment trials and the pharmaceutical industry, where schizoaffective disorder is never a subgroup in bipolar disorder trials, but a subgroup in schizophrenia trials,” Dr. Grunze said.
One audience member rose to state that while all this wrangling over how best to subclassify these patients is interesting, why not take a pragmatic approach and simply treat them symptomatically?
“Well, I think that’s what everyone in this room who’s a clinician is doing,” Dr. Grunze replied. “Because even if we assume that the diagnosis itself might be unstable, I think the greatest variance in patients switching around between bipolar, schizophrenia, and schizoaffective comes from the different doctors seeing them, each having a different understanding of schizoaffective disorder. I see a lot of patients who are classified as schizoaffective disorder because they have what I would have considered just as mood-congruent psychotic symptoms while manic. So I absolutely agree with you that this is more of an academic discussion, really, because at the moment we don’t have any consequences for treatment. We don’t have good evidence about how to treat these patients; we just extrapolate from whatever we think their symptoms come closest to.”
He reported receiving research grants from the National Institute for Health Research and the U.K. Medical Research Council, as well as more than a half-dozen pharmaceutical companies.
Dr. van Os reported having no financial conflicts.
BERLIN – The redefinition of schizoaffective disorder unveiled in the DSM-5 leaves much to be desired, experts opined at the annual congress of the European College of Neuropsychopharmacology
“We all thought we had the solution for making new criteria for schizoaffective disorder,” recalled Dr. Jim van Os, who served on the DSM-5 committee charged with reexamining the disorder. “But in the end, nothing much changed.”
Indeed, despite the lofty ambitions he and many of his fellow DSM-5 committee members held for thoroughly overhauling the criteria for what he called “this enigmatic combination of affective and psychotic dysregulation,” the difference between the DSM-IV and DSM-5 boiled down to small potatoes: Whereas the DSM-IV stated that the diagnosis of schizoaffective disorder required that the mood episode must be present for “a substantial duration of the illness,” the DSM-5 requires that the mood episode be present for “the majority” of the illness.
“The change was made to improve the reliability and stability of the diagnosis,” explained Dr. van Os, professor of psychiatric epidemiology at Maastricht (the Netherlands) University.
He was an advocate on the committee for introducing a dimensional approach to the diagnosis of schizoaffective disorder, one that would incorporate consideration of clustering of symptoms across a continuum.
“This would have been the best solution to the conundrum of the ‘fuzzy set’ problem of mental disorders. It would have been a great way of recognizing the continuity of all mental phenomena seen in mental disorders. However, it did not happen, unfortunately.”
The psychiatrist noted that committee members debated “for many hours” how best to overhaul the criteria for schizoaffective disorder in the DSM-5 before deciding to scale back their scope.
“We realized that any measure of precision in the criteria we tried to introduce for the admixture of affective dysregulation and psychosis is spurious. We really haven’t got the tools yet to make valid distinctions. That’s why we said in DSM-5 that the clinician can make the diagnosis using clinical intuition that the majority of time in the illness there is affective dysregulation. So, not spurious precision, but clinical intuition. I don’t know if that’s a good decision, but that’s what we did,” Dr. van Os said.
At the same symposium on schizoaffective disorder held during the ECNP congress, Dr. Heinz Grunze declared: “I would have welcomed as a development in DSM-5, as we’ve seen with some other disorders, basically abolishing schizoaffective disorder on its own and assigning it either to bipolar disorder with psychotic features specified or to schizophrenia with a strong affective specifier. I think that would be better suited to characterizing these patients.”
“Clearly, schizoaffective disorder has quite a polymorphic course. It’s definitely not one illness; it’s several illnesses which we put together in this random category,” added Dr. Grunze, professor of clinical psychiatry at University of Newcastle (England).
His own view is that the disorder belongs under the umbrella of bipolar disorder. A recent Spanish functional magnetic resonance brain imaging study demonstrated that schizoaffective disorder exhibits features that more closely resemble those of bipolar disorder than schizophrenia, including the finding that patients in clinical remission show failure of deactivation in the medial frontal gyrus, compared with healthy controls, Dr. Grunze noted.
“I personally can’t see a difference between a schizoaffective acute episode, manic type, and a psychotic manic episode. So my personal opinion is that schizoaffective disorder belongs under the heading of bipolar disorder. That’s obviously different from the opinion of those conducting treatment trials and the pharmaceutical industry, where schizoaffective disorder is never a subgroup in bipolar disorder trials, but a subgroup in schizophrenia trials,” Dr. Grunze said.
One audience member rose to state that while all this wrangling over how best to subclassify these patients is interesting, why not take a pragmatic approach and simply treat them symptomatically?
“Well, I think that’s what everyone in this room who’s a clinician is doing,” Dr. Grunze replied. “Because even if we assume that the diagnosis itself might be unstable, I think the greatest variance in patients switching around between bipolar, schizophrenia, and schizoaffective comes from the different doctors seeing them, each having a different understanding of schizoaffective disorder. I see a lot of patients who are classified as schizoaffective disorder because they have what I would have considered just as mood-congruent psychotic symptoms while manic. So I absolutely agree with you that this is more of an academic discussion, really, because at the moment we don’t have any consequences for treatment. We don’t have good evidence about how to treat these patients; we just extrapolate from whatever we think their symptoms come closest to.”
He reported receiving research grants from the National Institute for Health Research and the U.K. Medical Research Council, as well as more than a half-dozen pharmaceutical companies.
Dr. van Os reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Simple risk score predicts dementia risk in type 2 diabetes
BERLIN – The first-ever dementia risk score designed specifically for patients with type 2 diabetes has successfully undergone external validation and is ready for everyday use by clinicians, Rachel A. Whitmer, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
Patients with type 2 diabetes are on average twice as likely to develop dementia as are nondiabetics. But within the diabetes population, the magnitude of risk varies enormously. The Diabetes-Specific Dementia Risk Score pins down an individual’s 10-year risk more precisely. The predictive factors are easily obtained from a patient’s medical history, enabling primary care physicians, endocrinologists, psychiatrists, and neurologists to readily calculate an individualized risk score without resorting to cognitive function testing or other labor-intensive measures.
“This is a risk score that can easily be determined in a primary care setting. It can be done by self-report. It can be done using an electronic medical record. This is a way to tell someone what their risk is. Knowing your risk might encourage patients to take steps to reduce that risk. And if their risk is low you can show it to them and say it’s all the more reason to avoid getting diabetes complications – because it’s not just about your diabetes, it’s also for your brain health,” said Dr. Whitmer, a research scientist at the division of research, Kaiser Permanente Northern California, Oakland.
She and her coinvestigators harnessed the Kaiser Permanente Northern California Diabetes Registry to develop the risk score. They evaluated 45 candidate predictors in nearly 30,000 registry participants age 60 or older with type 2 diabetes, 5,173 of whom developed dementia during 10 years of follow-up. Once they’d developed the risk score, which is based upon the eight strongest predictors, they validated it in a cohort of 2,413 type 2 diabetic patients at Group Health of Puget Sound. The validation study was published last year (Lancet Diabetes Endocrinol. 2013;1:183-90).
The predictive variables are age, education, depression, microvascular disease, acute metabolic events, cerebrovascular disease, cardiovascular disease, and having a diabetic foot.
Here’s how the Diabetes-Specific Dementia Risk Score works: A patient gets 0 points for being age 60-64, 3 for being 65-69, and so on up to a maximum of 10 points for being age 85 or more. A history of an acute metabolic event is worth 2 points, as is cerebrovascular disease or depression. Microvascular disease or cardiovascular disease are 1 point each. A college education is worth minus 1 point, a high school education or less gets 0 points. The points awarded for the eight predictors are added up. In the validation study, the 10-year risk of dementia ranged from a low of 5% in patients with a net score of minus 1 to 73% in patients with a score of 12-19.
Dr. Whitmer sees the risk score as being particularly well-suited as a tool for dementia screening of type 2 diabetes patients by primary care physicians. She noted that, today, well-managed diabetic patients have a podiatrist, dietician, and dentist, as well as a physician, all working to prevent the physical complications of diabetes. But there’s not typically anyone looking out for the diabetes patient’s brain health.
“I think it’s time now when we’re taking care of diabetic individuals that we need to think about the brain,” she said.
Numerous earlier studies conducted using the Kaiser Permanente Northern California Diabetes Registry have been instrumental in helping to establish vascular complications, depression, and hypoglycemic episodes as events that markedly elevate the risk of dementia among people with type 2 diabetes.
“The question is, if we can treat depression more effectively and manage glycemic control and prevent vascular complications, can we lower the risk of dementia in these people who are at particularly high risk? That’s where the field is right now. There are trials ongoing in people with diabetes and prediabetes looking at this question,” Dr. Whitmer noted.
The question takes on some urgency, she continued, because the intersection of type 2 diabetes and dementia looms ahead as an enormous public health problem. The International Diabetes Federation estimates that there are now 392 million people worldwide with diabetes, and that by 2035 this figure will climb to 592 million. Meanwhile, other projections are that the worldwide population of individuals with dementia will jump from just under 50 million today to more than 125 million by 2050, with most of the growth coming from low- and middle-income countries where the average lifespan is increasing.
The risk score project was supported by Kaiser Permanente and the National Institutes of Health. Dr. Whitmer reported having no financial conflicts.
BERLIN – The first-ever dementia risk score designed specifically for patients with type 2 diabetes has successfully undergone external validation and is ready for everyday use by clinicians, Rachel A. Whitmer, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
Patients with type 2 diabetes are on average twice as likely to develop dementia as are nondiabetics. But within the diabetes population, the magnitude of risk varies enormously. The Diabetes-Specific Dementia Risk Score pins down an individual’s 10-year risk more precisely. The predictive factors are easily obtained from a patient’s medical history, enabling primary care physicians, endocrinologists, psychiatrists, and neurologists to readily calculate an individualized risk score without resorting to cognitive function testing or other labor-intensive measures.
“This is a risk score that can easily be determined in a primary care setting. It can be done by self-report. It can be done using an electronic medical record. This is a way to tell someone what their risk is. Knowing your risk might encourage patients to take steps to reduce that risk. And if their risk is low you can show it to them and say it’s all the more reason to avoid getting diabetes complications – because it’s not just about your diabetes, it’s also for your brain health,” said Dr. Whitmer, a research scientist at the division of research, Kaiser Permanente Northern California, Oakland.
She and her coinvestigators harnessed the Kaiser Permanente Northern California Diabetes Registry to develop the risk score. They evaluated 45 candidate predictors in nearly 30,000 registry participants age 60 or older with type 2 diabetes, 5,173 of whom developed dementia during 10 years of follow-up. Once they’d developed the risk score, which is based upon the eight strongest predictors, they validated it in a cohort of 2,413 type 2 diabetic patients at Group Health of Puget Sound. The validation study was published last year (Lancet Diabetes Endocrinol. 2013;1:183-90).
The predictive variables are age, education, depression, microvascular disease, acute metabolic events, cerebrovascular disease, cardiovascular disease, and having a diabetic foot.
Here’s how the Diabetes-Specific Dementia Risk Score works: A patient gets 0 points for being age 60-64, 3 for being 65-69, and so on up to a maximum of 10 points for being age 85 or more. A history of an acute metabolic event is worth 2 points, as is cerebrovascular disease or depression. Microvascular disease or cardiovascular disease are 1 point each. A college education is worth minus 1 point, a high school education or less gets 0 points. The points awarded for the eight predictors are added up. In the validation study, the 10-year risk of dementia ranged from a low of 5% in patients with a net score of minus 1 to 73% in patients with a score of 12-19.
Dr. Whitmer sees the risk score as being particularly well-suited as a tool for dementia screening of type 2 diabetes patients by primary care physicians. She noted that, today, well-managed diabetic patients have a podiatrist, dietician, and dentist, as well as a physician, all working to prevent the physical complications of diabetes. But there’s not typically anyone looking out for the diabetes patient’s brain health.
“I think it’s time now when we’re taking care of diabetic individuals that we need to think about the brain,” she said.
Numerous earlier studies conducted using the Kaiser Permanente Northern California Diabetes Registry have been instrumental in helping to establish vascular complications, depression, and hypoglycemic episodes as events that markedly elevate the risk of dementia among people with type 2 diabetes.
“The question is, if we can treat depression more effectively and manage glycemic control and prevent vascular complications, can we lower the risk of dementia in these people who are at particularly high risk? That’s where the field is right now. There are trials ongoing in people with diabetes and prediabetes looking at this question,” Dr. Whitmer noted.
The question takes on some urgency, she continued, because the intersection of type 2 diabetes and dementia looms ahead as an enormous public health problem. The International Diabetes Federation estimates that there are now 392 million people worldwide with diabetes, and that by 2035 this figure will climb to 592 million. Meanwhile, other projections are that the worldwide population of individuals with dementia will jump from just under 50 million today to more than 125 million by 2050, with most of the growth coming from low- and middle-income countries where the average lifespan is increasing.
The risk score project was supported by Kaiser Permanente and the National Institutes of Health. Dr. Whitmer reported having no financial conflicts.
BERLIN – The first-ever dementia risk score designed specifically for patients with type 2 diabetes has successfully undergone external validation and is ready for everyday use by clinicians, Rachel A. Whitmer, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
Patients with type 2 diabetes are on average twice as likely to develop dementia as are nondiabetics. But within the diabetes population, the magnitude of risk varies enormously. The Diabetes-Specific Dementia Risk Score pins down an individual’s 10-year risk more precisely. The predictive factors are easily obtained from a patient’s medical history, enabling primary care physicians, endocrinologists, psychiatrists, and neurologists to readily calculate an individualized risk score without resorting to cognitive function testing or other labor-intensive measures.
“This is a risk score that can easily be determined in a primary care setting. It can be done by self-report. It can be done using an electronic medical record. This is a way to tell someone what their risk is. Knowing your risk might encourage patients to take steps to reduce that risk. And if their risk is low you can show it to them and say it’s all the more reason to avoid getting diabetes complications – because it’s not just about your diabetes, it’s also for your brain health,” said Dr. Whitmer, a research scientist at the division of research, Kaiser Permanente Northern California, Oakland.
She and her coinvestigators harnessed the Kaiser Permanente Northern California Diabetes Registry to develop the risk score. They evaluated 45 candidate predictors in nearly 30,000 registry participants age 60 or older with type 2 diabetes, 5,173 of whom developed dementia during 10 years of follow-up. Once they’d developed the risk score, which is based upon the eight strongest predictors, they validated it in a cohort of 2,413 type 2 diabetic patients at Group Health of Puget Sound. The validation study was published last year (Lancet Diabetes Endocrinol. 2013;1:183-90).
The predictive variables are age, education, depression, microvascular disease, acute metabolic events, cerebrovascular disease, cardiovascular disease, and having a diabetic foot.
Here’s how the Diabetes-Specific Dementia Risk Score works: A patient gets 0 points for being age 60-64, 3 for being 65-69, and so on up to a maximum of 10 points for being age 85 or more. A history of an acute metabolic event is worth 2 points, as is cerebrovascular disease or depression. Microvascular disease or cardiovascular disease are 1 point each. A college education is worth minus 1 point, a high school education or less gets 0 points. The points awarded for the eight predictors are added up. In the validation study, the 10-year risk of dementia ranged from a low of 5% in patients with a net score of minus 1 to 73% in patients with a score of 12-19.
Dr. Whitmer sees the risk score as being particularly well-suited as a tool for dementia screening of type 2 diabetes patients by primary care physicians. She noted that, today, well-managed diabetic patients have a podiatrist, dietician, and dentist, as well as a physician, all working to prevent the physical complications of diabetes. But there’s not typically anyone looking out for the diabetes patient’s brain health.
“I think it’s time now when we’re taking care of diabetic individuals that we need to think about the brain,” she said.
Numerous earlier studies conducted using the Kaiser Permanente Northern California Diabetes Registry have been instrumental in helping to establish vascular complications, depression, and hypoglycemic episodes as events that markedly elevate the risk of dementia among people with type 2 diabetes.
“The question is, if we can treat depression more effectively and manage glycemic control and prevent vascular complications, can we lower the risk of dementia in these people who are at particularly high risk? That’s where the field is right now. There are trials ongoing in people with diabetes and prediabetes looking at this question,” Dr. Whitmer noted.
The question takes on some urgency, she continued, because the intersection of type 2 diabetes and dementia looms ahead as an enormous public health problem. The International Diabetes Federation estimates that there are now 392 million people worldwide with diabetes, and that by 2035 this figure will climb to 592 million. Meanwhile, other projections are that the worldwide population of individuals with dementia will jump from just under 50 million today to more than 125 million by 2050, with most of the growth coming from low- and middle-income countries where the average lifespan is increasing.
The risk score project was supported by Kaiser Permanente and the National Institutes of Health. Dr. Whitmer reported having no financial conflicts.
AT THE ECNP CONGRESS
Key clinical point: It’s now readily possible to predict the 10-year dementia risk for individuals with type 2 diabetes.
Major finding: The 10-year risk of dementia in patients with type 2 diabetes ranged from 5% to 73% depending upon their Diabetes-Specific Dementia Risk Score, on the basis of eight variables easily obtained from a patient’s medical history.
Data source: The risk score was developed by evaluating 45 candidate predictive variables in a longitudinal cohort of 29,961 type 2 diabetes patients, 5,173 of whom developed dementia during 10 years of follow-up.
Disclosures: The study was funded by Kaiser Permanente and the National Institutes of Health. The presenter reported having no financial conflicts.
Increased Alzheimer’s risk with long-term benzodiazepine use
BERLIN – Chronic use of benzodiazepines by elderly patients is associated with a 43%-51% increased risk of being diagnosed with Alzheimer’s disease 5-10 years later, according to a large case-control study. “Considering the extent to which benzodiazepines are prescribed in the elderly population and the growing incidence of dementia, unwarranted chronic use of benzodiazepines in the elderly should be viewed as a public health issue,” Sophie Billioti de Gage said at the annual congress of the European College of Neuropsychopharmacology.
Her case-control study used the Quebec health insurance database. The subjects were 1,796 elderly individuals diagnosed with Alzheimer’s disease, each matched with 4 controls based upon age, gender, and duration of follow-up. The Quebec database permitted identification of all subjects with prescriptions for benzodiazepines during 2000-2009, a period 5-10 years prior to diagnosis of Alzheimer’s disease. This substantial time lag was chosen because previous studies reporting a link between benzodiazepines and dementia have often been criticized for possible confounding due to reverse causality. That is, because many earlier studies featured a shorter interval between medication use and dementia diagnosis, skeptics argued that benzodiazepines might not have caused the dementia but rather were prescribed to treat early manifestations of the disease, such as anxiety, depressive symptoms, and insomnia, explained Ms. Billioti de Gage, a PhD student at the University of Bordeaux (France).
The database enabled her to determine how many cumulative days worth of benzodiazepine prescriptions participants filled during the study years, as well as whether the medications had a short or long half-life.
In a multivariate analysis adjusted for stroke, MI, hypertension, diabetes, and use of antiplatelet agents or anticoagulants during the period 5-10 years prior to diagnosis of Alzheimer’s disease, the use of benzodiazepines was independently associated with a 51% increased risk of subsequent Alzheimer’s compared with nonusers.
A dose-response relationship was apparent. Individuals with prescriptions for up to 90 days worth of benzodiazepines were not at significantly greater risk for future Alzheimer’s disease than nonusers. Those with a cumulative 91- to 180-day exposure had a 32% increased risk, compared with nonusers, however, and patients with more than 180 days of benzodiazepine use had an 84% increased risk.
The association with later Alzheimer’s disease was stronger in patients who used benzodiazepines with a half-life of 20 hours or longer. In a multivariate analysis they had a 70% greater risk of Alzheimer’s, compared with nonusers. Patients who used a benzodiazepine having a half-life of less than 20 hours had a 43% increase in risk.
When the multivariate analyses were further adjusted for anxiety, depressive symptoms, and insomnia – all of which can be prodromes of dementia – the results were not meaningfully altered, she added.
This case-control study confirms the results of an earlier prospective population-based study by Ms. Billioti de Gage and coworkers. That French study also found a roughly 50% increased risk of dementia in elderly patients who initiated chronic benzodiazepine therapy (BMJ 2012;345:e6231 [doi: 10.1136/bmj.e6231]). However, with only 1,063 participants, 253 of whom were diagnosed with dementia during 15 years of follow-up, the sample size was too small to draw firm conclusions. The current case-control study, with 8,980 subjects representative of the Quebec community-dwelling elderly population, is more persuasive, the investigator said.
Guidelines recommend preferential use of short half-life benzodiazepines and short durations of use in the elderly; however, in clinical practice the medications are often used long-term.
The biologic mechanism by which chronic use of benzodiazepines by elderly individuals might predispose to Alzheimer’s disease hasn’t been worked out, but the medications’ short-term adverse impact on memory and cognition are well recognized.
The case-control study was funded by the French Ministry of Health and the Funding Agency for Health Research of Quebec as well as by university grants.
BERLIN – Chronic use of benzodiazepines by elderly patients is associated with a 43%-51% increased risk of being diagnosed with Alzheimer’s disease 5-10 years later, according to a large case-control study. “Considering the extent to which benzodiazepines are prescribed in the elderly population and the growing incidence of dementia, unwarranted chronic use of benzodiazepines in the elderly should be viewed as a public health issue,” Sophie Billioti de Gage said at the annual congress of the European College of Neuropsychopharmacology.
Her case-control study used the Quebec health insurance database. The subjects were 1,796 elderly individuals diagnosed with Alzheimer’s disease, each matched with 4 controls based upon age, gender, and duration of follow-up. The Quebec database permitted identification of all subjects with prescriptions for benzodiazepines during 2000-2009, a period 5-10 years prior to diagnosis of Alzheimer’s disease. This substantial time lag was chosen because previous studies reporting a link between benzodiazepines and dementia have often been criticized for possible confounding due to reverse causality. That is, because many earlier studies featured a shorter interval between medication use and dementia diagnosis, skeptics argued that benzodiazepines might not have caused the dementia but rather were prescribed to treat early manifestations of the disease, such as anxiety, depressive symptoms, and insomnia, explained Ms. Billioti de Gage, a PhD student at the University of Bordeaux (France).
The database enabled her to determine how many cumulative days worth of benzodiazepine prescriptions participants filled during the study years, as well as whether the medications had a short or long half-life.
In a multivariate analysis adjusted for stroke, MI, hypertension, diabetes, and use of antiplatelet agents or anticoagulants during the period 5-10 years prior to diagnosis of Alzheimer’s disease, the use of benzodiazepines was independently associated with a 51% increased risk of subsequent Alzheimer’s compared with nonusers.
A dose-response relationship was apparent. Individuals with prescriptions for up to 90 days worth of benzodiazepines were not at significantly greater risk for future Alzheimer’s disease than nonusers. Those with a cumulative 91- to 180-day exposure had a 32% increased risk, compared with nonusers, however, and patients with more than 180 days of benzodiazepine use had an 84% increased risk.
The association with later Alzheimer’s disease was stronger in patients who used benzodiazepines with a half-life of 20 hours or longer. In a multivariate analysis they had a 70% greater risk of Alzheimer’s, compared with nonusers. Patients who used a benzodiazepine having a half-life of less than 20 hours had a 43% increase in risk.
When the multivariate analyses were further adjusted for anxiety, depressive symptoms, and insomnia – all of which can be prodromes of dementia – the results were not meaningfully altered, she added.
This case-control study confirms the results of an earlier prospective population-based study by Ms. Billioti de Gage and coworkers. That French study also found a roughly 50% increased risk of dementia in elderly patients who initiated chronic benzodiazepine therapy (BMJ 2012;345:e6231 [doi: 10.1136/bmj.e6231]). However, with only 1,063 participants, 253 of whom were diagnosed with dementia during 15 years of follow-up, the sample size was too small to draw firm conclusions. The current case-control study, with 8,980 subjects representative of the Quebec community-dwelling elderly population, is more persuasive, the investigator said.
Guidelines recommend preferential use of short half-life benzodiazepines and short durations of use in the elderly; however, in clinical practice the medications are often used long-term.
The biologic mechanism by which chronic use of benzodiazepines by elderly individuals might predispose to Alzheimer’s disease hasn’t been worked out, but the medications’ short-term adverse impact on memory and cognition are well recognized.
The case-control study was funded by the French Ministry of Health and the Funding Agency for Health Research of Quebec as well as by university grants.
BERLIN – Chronic use of benzodiazepines by elderly patients is associated with a 43%-51% increased risk of being diagnosed with Alzheimer’s disease 5-10 years later, according to a large case-control study. “Considering the extent to which benzodiazepines are prescribed in the elderly population and the growing incidence of dementia, unwarranted chronic use of benzodiazepines in the elderly should be viewed as a public health issue,” Sophie Billioti de Gage said at the annual congress of the European College of Neuropsychopharmacology.
Her case-control study used the Quebec health insurance database. The subjects were 1,796 elderly individuals diagnosed with Alzheimer’s disease, each matched with 4 controls based upon age, gender, and duration of follow-up. The Quebec database permitted identification of all subjects with prescriptions for benzodiazepines during 2000-2009, a period 5-10 years prior to diagnosis of Alzheimer’s disease. This substantial time lag was chosen because previous studies reporting a link between benzodiazepines and dementia have often been criticized for possible confounding due to reverse causality. That is, because many earlier studies featured a shorter interval between medication use and dementia diagnosis, skeptics argued that benzodiazepines might not have caused the dementia but rather were prescribed to treat early manifestations of the disease, such as anxiety, depressive symptoms, and insomnia, explained Ms. Billioti de Gage, a PhD student at the University of Bordeaux (France).
The database enabled her to determine how many cumulative days worth of benzodiazepine prescriptions participants filled during the study years, as well as whether the medications had a short or long half-life.
In a multivariate analysis adjusted for stroke, MI, hypertension, diabetes, and use of antiplatelet agents or anticoagulants during the period 5-10 years prior to diagnosis of Alzheimer’s disease, the use of benzodiazepines was independently associated with a 51% increased risk of subsequent Alzheimer’s compared with nonusers.
A dose-response relationship was apparent. Individuals with prescriptions for up to 90 days worth of benzodiazepines were not at significantly greater risk for future Alzheimer’s disease than nonusers. Those with a cumulative 91- to 180-day exposure had a 32% increased risk, compared with nonusers, however, and patients with more than 180 days of benzodiazepine use had an 84% increased risk.
The association with later Alzheimer’s disease was stronger in patients who used benzodiazepines with a half-life of 20 hours or longer. In a multivariate analysis they had a 70% greater risk of Alzheimer’s, compared with nonusers. Patients who used a benzodiazepine having a half-life of less than 20 hours had a 43% increase in risk.
When the multivariate analyses were further adjusted for anxiety, depressive symptoms, and insomnia – all of which can be prodromes of dementia – the results were not meaningfully altered, she added.
This case-control study confirms the results of an earlier prospective population-based study by Ms. Billioti de Gage and coworkers. That French study also found a roughly 50% increased risk of dementia in elderly patients who initiated chronic benzodiazepine therapy (BMJ 2012;345:e6231 [doi: 10.1136/bmj.e6231]). However, with only 1,063 participants, 253 of whom were diagnosed with dementia during 15 years of follow-up, the sample size was too small to draw firm conclusions. The current case-control study, with 8,980 subjects representative of the Quebec community-dwelling elderly population, is more persuasive, the investigator said.
Guidelines recommend preferential use of short half-life benzodiazepines and short durations of use in the elderly; however, in clinical practice the medications are often used long-term.
The biologic mechanism by which chronic use of benzodiazepines by elderly individuals might predispose to Alzheimer’s disease hasn’t been worked out, but the medications’ short-term adverse impact on memory and cognition are well recognized.
The case-control study was funded by the French Ministry of Health and the Funding Agency for Health Research of Quebec as well as by university grants.
AT THE ECNP CONGRESS
Key clinical point: Chronic use of benzodiazepines by the elderly may boost their risk of Alzheimer’s disease in a dose-dependent fashion.
Major finding: Individuals who used benzodiazepines for more than 180 days during a 6-year period had an 84% greater risk of being diagnosed with Alzheimer’s disease 5-10 years later than nonusers.
Data source: This was a case-control study involving 1,796 elderly Quebec residents diagnosed with Alzheimer’s disease and 7,184 matched controls.
Disclosures:This study was funded primarily by the French Ministry of Health and the Funding Agency for Health Research of Quebec.
Less daytime sleepiness with lurasidone for schizophrenia
BERLIN – Daytime sleepiness was reduced when patients with schizophrenia were placed on lurasidone but increased when assigned to quetiapine extended-release, in a randomized trial.
This improvement in daytime sleepiness in patients treated with lurasidone had important clinical consequences: namely, significantly better scores on measures of cognition and functional capacity, Dr. Antony Loebel reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a post hoc analysis of a 6-week, double-blind, placebo-controlled randomized trial. The study population comprised 486 patients with an acute exacerbation of schizophrenia who were randomized to fixed-dose therapy with lurasidone (Latuda) 80 mg, lurasidone 160 mg, quetiapine extended release (Seroquel XR) 600 mg, or placebo, all dosed once daily with food in the evening.
Daytime sleepiness as assessed by the Epworth Sleepiness Scale (ESS) improved significantly from baseline in the two lurasidone groups and in placebo-treated controls while worsening significantly in the quetiapine group. The mean total ESS score decreased by 0.7 points in the lower-dose lurasidone group, 1.1 points in patients on lurasidone at 160 mg/day, and 0.9 points in controls. In contrast, the mean score rose by 0.6 points in the quetiapine group.
Daytime sleepiness was associated with reduced agitation as assessed by the PANSS (Positive and Negative Syndrome Scale) excitement subscale. However, this came at the cost of reduced functional capacity.
Specifically, the quetiapine group showed significantly increased sleepiness in five of the eight daytime scenarios assessed in the ESS: dozing when talking, sitting and reading, watching television, sitting quietly after lunch without alcohol, and during afternoon resting.
Moreover, as daytime sleepiness increased, functional capacity evaluated via the UCSD Performance-Based Skills Assessment, Brief, worsened, as did cognitive performance as assessed by the CogState Computerized Schizophrenia Battery, according to Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals in Fort Lee, N.J.
Lurasidone at 160 mg/day not only yielded the greatest improvement from baseline in daytime sleepiness, it also demonstrated a significant improvement in overall cognitive performance when compared to quetiapine and placebo.
The psychiatrist concluded that the impact of daytime sleepiness on key treatment outcomes is underappreciated. Daytime sleepiness is generally not assessed with a validated scale. The ESS is a quick, practical, well-validated tool for this purpose that’s well suited for use in clinical practice, he added.
BERLIN – Daytime sleepiness was reduced when patients with schizophrenia were placed on lurasidone but increased when assigned to quetiapine extended-release, in a randomized trial.
This improvement in daytime sleepiness in patients treated with lurasidone had important clinical consequences: namely, significantly better scores on measures of cognition and functional capacity, Dr. Antony Loebel reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a post hoc analysis of a 6-week, double-blind, placebo-controlled randomized trial. The study population comprised 486 patients with an acute exacerbation of schizophrenia who were randomized to fixed-dose therapy with lurasidone (Latuda) 80 mg, lurasidone 160 mg, quetiapine extended release (Seroquel XR) 600 mg, or placebo, all dosed once daily with food in the evening.
Daytime sleepiness as assessed by the Epworth Sleepiness Scale (ESS) improved significantly from baseline in the two lurasidone groups and in placebo-treated controls while worsening significantly in the quetiapine group. The mean total ESS score decreased by 0.7 points in the lower-dose lurasidone group, 1.1 points in patients on lurasidone at 160 mg/day, and 0.9 points in controls. In contrast, the mean score rose by 0.6 points in the quetiapine group.
Daytime sleepiness was associated with reduced agitation as assessed by the PANSS (Positive and Negative Syndrome Scale) excitement subscale. However, this came at the cost of reduced functional capacity.
Specifically, the quetiapine group showed significantly increased sleepiness in five of the eight daytime scenarios assessed in the ESS: dozing when talking, sitting and reading, watching television, sitting quietly after lunch without alcohol, and during afternoon resting.
Moreover, as daytime sleepiness increased, functional capacity evaluated via the UCSD Performance-Based Skills Assessment, Brief, worsened, as did cognitive performance as assessed by the CogState Computerized Schizophrenia Battery, according to Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals in Fort Lee, N.J.
Lurasidone at 160 mg/day not only yielded the greatest improvement from baseline in daytime sleepiness, it also demonstrated a significant improvement in overall cognitive performance when compared to quetiapine and placebo.
The psychiatrist concluded that the impact of daytime sleepiness on key treatment outcomes is underappreciated. Daytime sleepiness is generally not assessed with a validated scale. The ESS is a quick, practical, well-validated tool for this purpose that’s well suited for use in clinical practice, he added.
BERLIN – Daytime sleepiness was reduced when patients with schizophrenia were placed on lurasidone but increased when assigned to quetiapine extended-release, in a randomized trial.
This improvement in daytime sleepiness in patients treated with lurasidone had important clinical consequences: namely, significantly better scores on measures of cognition and functional capacity, Dr. Antony Loebel reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a post hoc analysis of a 6-week, double-blind, placebo-controlled randomized trial. The study population comprised 486 patients with an acute exacerbation of schizophrenia who were randomized to fixed-dose therapy with lurasidone (Latuda) 80 mg, lurasidone 160 mg, quetiapine extended release (Seroquel XR) 600 mg, or placebo, all dosed once daily with food in the evening.
Daytime sleepiness as assessed by the Epworth Sleepiness Scale (ESS) improved significantly from baseline in the two lurasidone groups and in placebo-treated controls while worsening significantly in the quetiapine group. The mean total ESS score decreased by 0.7 points in the lower-dose lurasidone group, 1.1 points in patients on lurasidone at 160 mg/day, and 0.9 points in controls. In contrast, the mean score rose by 0.6 points in the quetiapine group.
Daytime sleepiness was associated with reduced agitation as assessed by the PANSS (Positive and Negative Syndrome Scale) excitement subscale. However, this came at the cost of reduced functional capacity.
Specifically, the quetiapine group showed significantly increased sleepiness in five of the eight daytime scenarios assessed in the ESS: dozing when talking, sitting and reading, watching television, sitting quietly after lunch without alcohol, and during afternoon resting.
Moreover, as daytime sleepiness increased, functional capacity evaluated via the UCSD Performance-Based Skills Assessment, Brief, worsened, as did cognitive performance as assessed by the CogState Computerized Schizophrenia Battery, according to Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals in Fort Lee, N.J.
Lurasidone at 160 mg/day not only yielded the greatest improvement from baseline in daytime sleepiness, it also demonstrated a significant improvement in overall cognitive performance when compared to quetiapine and placebo.
The psychiatrist concluded that the impact of daytime sleepiness on key treatment outcomes is underappreciated. Daytime sleepiness is generally not assessed with a validated scale. The ESS is a quick, practical, well-validated tool for this purpose that’s well suited for use in clinical practice, he added.
AT THE ECNP CONGRESS
Key clinical point: Patients with an acute exacerbation of schizophrenia treated with lurasidone experience less sleepiness during waking hours, with associated significant improvements in functional capacity and cognition, compared with patients on quetiapine extended release.
Major finding: Patients on lurasidone at 160 mg/day showed a mean 1.1-point improvement on the Epworth Sleepiness Scale total score, compared with a 0.6-point worsening on quetiapine extended release at 600 mg/day.
Data source: A post hoc analysis of a 486-patient, 6-week, randomized, double-blind, placebo-controlled clinical trial.
Disclosures: The presenter is chief medical officer of Sunovion Pharmaceuticals, which sponsored the study.
Switch to long-acting antipsychotics improved treatment attitude
BERLIN – A switch to long-acting second-generation injectable antipsychotics brought improved attitudes and beliefs regarding antipsychotic therapy among patients with schizoaffective disorder who already were stabilized on a single oral antipsychotic agent, Dr. Francesco Pietrini reported at the annual congress of the European College of Neuropsychopharmacology.
The conventional view has been that long-acting injectable antipsychotics are most appropriate as an alternative option for patients with poor adherence to oral antipsychotics. But this small observational study suggests that the switch is beneficial in a broader population, according to Dr. Pietrini of the University of Florence (Italy).
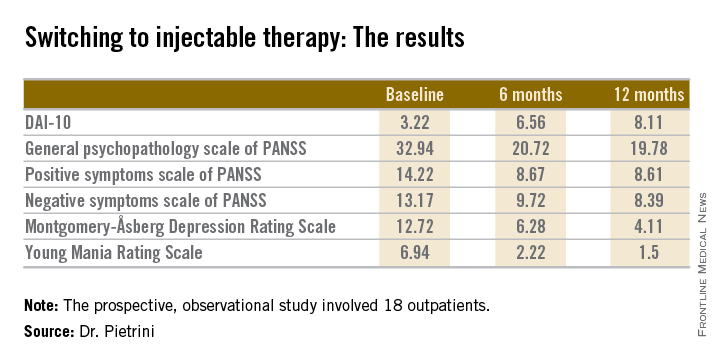
The 18 outpatients who participated in this 12-month, prospective, observational study showed significant improvements in a variety of measures of psychopathology after the switch to long-acting injectable therapy. These benefits were accompanied by a steadily improving attitude toward treatment as reflected in rising scores on the Drug Attitude Inventory-10 (DAI-10).
All patients were stable at baseline on either oral olanzapine or oral paliperidone. They were then switched to the corresponding dose of the long-acting injectable formulation of the same drug and followed prospectively for 12 months.
Mean scores on the 0-10 DAI-10 rose from 3.22 at baseline to 6.56 after 6 months on a long-acting injectable antipsychotic and 8.11 at 12 months. These improvements in scores reflect the patients’ growing endorsement of inventory items such as “taking medication will prevent me from having a breakdown,” “my thoughts are clearer on medication,” and “for me, the good things about medication outweigh the bad.”
Scores on most measures of psychopathology improved significantly from baseline during the first 6 months on long-acting therapy. The improvement was maintained but not expanded upon during the second 6 months. The exception was negative psychotic symptoms as assessed by the negative symptom scale of the Positive and Negative Syndrome Scale (PANSS), which didn’t show significant improvement during the first 6 months but did in the second half of the year-long study (see chart).
Dr. Pietrini reported having no financial conflicts regarding this study, which was conducted free of commercial support.
BERLIN – A switch to long-acting second-generation injectable antipsychotics brought improved attitudes and beliefs regarding antipsychotic therapy among patients with schizoaffective disorder who already were stabilized on a single oral antipsychotic agent, Dr. Francesco Pietrini reported at the annual congress of the European College of Neuropsychopharmacology.
The conventional view has been that long-acting injectable antipsychotics are most appropriate as an alternative option for patients with poor adherence to oral antipsychotics. But this small observational study suggests that the switch is beneficial in a broader population, according to Dr. Pietrini of the University of Florence (Italy).

The 18 outpatients who participated in this 12-month, prospective, observational study showed significant improvements in a variety of measures of psychopathology after the switch to long-acting injectable therapy. These benefits were accompanied by a steadily improving attitude toward treatment as reflected in rising scores on the Drug Attitude Inventory-10 (DAI-10).
All patients were stable at baseline on either oral olanzapine or oral paliperidone. They were then switched to the corresponding dose of the long-acting injectable formulation of the same drug and followed prospectively for 12 months.
Mean scores on the 0-10 DAI-10 rose from 3.22 at baseline to 6.56 after 6 months on a long-acting injectable antipsychotic and 8.11 at 12 months. These improvements in scores reflect the patients’ growing endorsement of inventory items such as “taking medication will prevent me from having a breakdown,” “my thoughts are clearer on medication,” and “for me, the good things about medication outweigh the bad.”
Scores on most measures of psychopathology improved significantly from baseline during the first 6 months on long-acting therapy. The improvement was maintained but not expanded upon during the second 6 months. The exception was negative psychotic symptoms as assessed by the negative symptom scale of the Positive and Negative Syndrome Scale (PANSS), which didn’t show significant improvement during the first 6 months but did in the second half of the year-long study (see chart).
Dr. Pietrini reported having no financial conflicts regarding this study, which was conducted free of commercial support.
BERLIN – A switch to long-acting second-generation injectable antipsychotics brought improved attitudes and beliefs regarding antipsychotic therapy among patients with schizoaffective disorder who already were stabilized on a single oral antipsychotic agent, Dr. Francesco Pietrini reported at the annual congress of the European College of Neuropsychopharmacology.
The conventional view has been that long-acting injectable antipsychotics are most appropriate as an alternative option for patients with poor adherence to oral antipsychotics. But this small observational study suggests that the switch is beneficial in a broader population, according to Dr. Pietrini of the University of Florence (Italy).

The 18 outpatients who participated in this 12-month, prospective, observational study showed significant improvements in a variety of measures of psychopathology after the switch to long-acting injectable therapy. These benefits were accompanied by a steadily improving attitude toward treatment as reflected in rising scores on the Drug Attitude Inventory-10 (DAI-10).
All patients were stable at baseline on either oral olanzapine or oral paliperidone. They were then switched to the corresponding dose of the long-acting injectable formulation of the same drug and followed prospectively for 12 months.
Mean scores on the 0-10 DAI-10 rose from 3.22 at baseline to 6.56 after 6 months on a long-acting injectable antipsychotic and 8.11 at 12 months. These improvements in scores reflect the patients’ growing endorsement of inventory items such as “taking medication will prevent me from having a breakdown,” “my thoughts are clearer on medication,” and “for me, the good things about medication outweigh the bad.”
Scores on most measures of psychopathology improved significantly from baseline during the first 6 months on long-acting therapy. The improvement was maintained but not expanded upon during the second 6 months. The exception was negative psychotic symptoms as assessed by the negative symptom scale of the Positive and Negative Syndrome Scale (PANSS), which didn’t show significant improvement during the first 6 months but did in the second half of the year-long study (see chart).
Dr. Pietrini reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE ECNP CONGRESS
Key clinical point: A switch to a long-acting injectable antipsychotic proved beneficial in multiple ways in patients with schizoaffective disorder who were stable on a single oral agent.
Major finding: Mean scores on the Drug Attitude Inventory-10 improved from 3.22 when patients were stabilized on an oral antipsychotic to 6.56 after 6 months on the long-term injectable formulation of the same drug and 8.11 after 12 months.
Data source: This was a 12-month observational study of 18 outpatients with schizoaffective disorder.
Disclosures: Dr. Pietrini reported having no financial conflicts regarding this study, which was conducted free of commercial support.
Injectable paliperidone palmitate reduces incarcerations in schizophrenia
BERLIN – Once-monthly intramuscular paliperidone palmitate significantly delayed the unwelcome real world consequences of schizophrenia – including contact with the criminal justice system as well as psychiatric hospitalization – compared with oral antipsychotics, in the randomized PRIDE study.
PRIDE, or Paliperidone Palmitate Research in Demonstrating Effectiveness, deliberately enrolled the sort of real world patients with schizophrenia who are familiar to every clinician and jail warden but normally excluded from participation in clinical trials, including patients with a history of arrest and incarceration, and comorbid substance abuse.
Indeed, release from incarceration within the past 90 days was a prerequisite for eligibility for the PRIDE study, Dr. H. Lynn Starr explained at the annual congress of the European College of Neuropsychopharmacology.
Meanwhile, Janssen announced Nov. 13 that the Food and Drug Administration has approved the supplemental new drug applications for the long-acting drug for treating schizoaffective disorder.
PRIDE was a prospective, open-label, multicenter single-blind study in which 450 U.S. schizophrenia patients were randomized to treatment with once-monthly injectable paliperidone palmitate (Invega Sustenna) or daily oral antipsychotics for up to 15 months. Willingness to accept the long-acting injectable medication was a precondition for eligibility. Prior to randomization, patients sat down individually with a clinician, reviewed seven widely prescribed oral antipsychotics available in this study, and eliminated up to six of them from further consideration. They were then randomly assigned to flexibly dosed therapy with either an oral antipsychotic agent or injectable paliperidone palmitate.
The primary study endpoint was time to treatment failure, broadly defined in this study as a composite of arrest or incarceration; psychiatric hospitalization; suicide; dropout because of insufficient efficacy; discontinuation because of safety or tolerability issues; and the need for treatment supplementation with an additional antipsychotic because of inadequate efficacy, suicide risk, or increased use of psychiatric services in order to prevent imminent psychiatric hospitalization.
Median time to treatment failure was 226 days in the oral antipsychotic group and nearly twice as long at 416 days in the injectable paliperidone palmitate group. The treatment failure rate was 39.8% with paliperidone palmitate, compared with 53.7% with oral antipsychotics. This translated to a 43% greater risk of treatment failure in the oral therapy group, according to Dr. Starr of Janssen Pharmaceuticals in Titusville, N.J.
A prespecified key secondary endpoint was time to first psychiatric hospitalization or arrest/incarceration, as these outcomes are associated with considerable public health impact. The risk of either event proved to be 43% higher in the oral antipsychotic group. Arrest/incarceration and psychiatric hospitalization turned out to be the two most frequently occurring elements of the primary composite endpoint. Arrest/incarceration occurred in 21.2% of the paliperidone group, compared with 29.4% on oral antipsychotics. Eight percent of patients on long-term injectable therapy underwent psychiatric hospitalization, as did 11.9% on oral antipsychotics.
Patients on oral antipsychotic therapy not only experienced arrest/incarceration or psychiatric hospitalization sooner, they also had a significantly greater cumulative number of such events during follow-up.
The study drug was discontinued because of safety or tolerability issues in 6.6% of the paliperidone palmitate group and 3.7% on an oral antipsychotic. Erectile dysfunction was reported by 8.8% of patients on long-acting therapy but none on oral agents. Among women, amenorrhea occurred in 15.2% on paliperidone palmitate, vs. 3.6% on oral antipsychotics.
Thirty-two percent of patients on paliperidone palmitate had at least a 7% weight gain, as did 14% of the oral antipsychotic group.
In the oral antipsychotic group, 22% of patients were on paliperidone; 17%, on risperidone; 16.5%, on olanzapine; 15.1%, on aripiprazole; 13.3%, on quetiapine; 9.2%, on perphenazine; and 6.9%, on haloperidol.
The PRIDE study was funded by Janssen Scientific Affairs, where Dr. Starr serves as director of clinical development.
BERLIN – Once-monthly intramuscular paliperidone palmitate significantly delayed the unwelcome real world consequences of schizophrenia – including contact with the criminal justice system as well as psychiatric hospitalization – compared with oral antipsychotics, in the randomized PRIDE study.
PRIDE, or Paliperidone Palmitate Research in Demonstrating Effectiveness, deliberately enrolled the sort of real world patients with schizophrenia who are familiar to every clinician and jail warden but normally excluded from participation in clinical trials, including patients with a history of arrest and incarceration, and comorbid substance abuse.
Indeed, release from incarceration within the past 90 days was a prerequisite for eligibility for the PRIDE study, Dr. H. Lynn Starr explained at the annual congress of the European College of Neuropsychopharmacology.
Meanwhile, Janssen announced Nov. 13 that the Food and Drug Administration has approved the supplemental new drug applications for the long-acting drug for treating schizoaffective disorder.
PRIDE was a prospective, open-label, multicenter single-blind study in which 450 U.S. schizophrenia patients were randomized to treatment with once-monthly injectable paliperidone palmitate (Invega Sustenna) or daily oral antipsychotics for up to 15 months. Willingness to accept the long-acting injectable medication was a precondition for eligibility. Prior to randomization, patients sat down individually with a clinician, reviewed seven widely prescribed oral antipsychotics available in this study, and eliminated up to six of them from further consideration. They were then randomly assigned to flexibly dosed therapy with either an oral antipsychotic agent or injectable paliperidone palmitate.
The primary study endpoint was time to treatment failure, broadly defined in this study as a composite of arrest or incarceration; psychiatric hospitalization; suicide; dropout because of insufficient efficacy; discontinuation because of safety or tolerability issues; and the need for treatment supplementation with an additional antipsychotic because of inadequate efficacy, suicide risk, or increased use of psychiatric services in order to prevent imminent psychiatric hospitalization.
Median time to treatment failure was 226 days in the oral antipsychotic group and nearly twice as long at 416 days in the injectable paliperidone palmitate group. The treatment failure rate was 39.8% with paliperidone palmitate, compared with 53.7% with oral antipsychotics. This translated to a 43% greater risk of treatment failure in the oral therapy group, according to Dr. Starr of Janssen Pharmaceuticals in Titusville, N.J.
A prespecified key secondary endpoint was time to first psychiatric hospitalization or arrest/incarceration, as these outcomes are associated with considerable public health impact. The risk of either event proved to be 43% higher in the oral antipsychotic group. Arrest/incarceration and psychiatric hospitalization turned out to be the two most frequently occurring elements of the primary composite endpoint. Arrest/incarceration occurred in 21.2% of the paliperidone group, compared with 29.4% on oral antipsychotics. Eight percent of patients on long-term injectable therapy underwent psychiatric hospitalization, as did 11.9% on oral antipsychotics.
Patients on oral antipsychotic therapy not only experienced arrest/incarceration or psychiatric hospitalization sooner, they also had a significantly greater cumulative number of such events during follow-up.
The study drug was discontinued because of safety or tolerability issues in 6.6% of the paliperidone palmitate group and 3.7% on an oral antipsychotic. Erectile dysfunction was reported by 8.8% of patients on long-acting therapy but none on oral agents. Among women, amenorrhea occurred in 15.2% on paliperidone palmitate, vs. 3.6% on oral antipsychotics.
Thirty-two percent of patients on paliperidone palmitate had at least a 7% weight gain, as did 14% of the oral antipsychotic group.
In the oral antipsychotic group, 22% of patients were on paliperidone; 17%, on risperidone; 16.5%, on olanzapine; 15.1%, on aripiprazole; 13.3%, on quetiapine; 9.2%, on perphenazine; and 6.9%, on haloperidol.
The PRIDE study was funded by Janssen Scientific Affairs, where Dr. Starr serves as director of clinical development.
BERLIN – Once-monthly intramuscular paliperidone palmitate significantly delayed the unwelcome real world consequences of schizophrenia – including contact with the criminal justice system as well as psychiatric hospitalization – compared with oral antipsychotics, in the randomized PRIDE study.
PRIDE, or Paliperidone Palmitate Research in Demonstrating Effectiveness, deliberately enrolled the sort of real world patients with schizophrenia who are familiar to every clinician and jail warden but normally excluded from participation in clinical trials, including patients with a history of arrest and incarceration, and comorbid substance abuse.
Indeed, release from incarceration within the past 90 days was a prerequisite for eligibility for the PRIDE study, Dr. H. Lynn Starr explained at the annual congress of the European College of Neuropsychopharmacology.
Meanwhile, Janssen announced Nov. 13 that the Food and Drug Administration has approved the supplemental new drug applications for the long-acting drug for treating schizoaffective disorder.
PRIDE was a prospective, open-label, multicenter single-blind study in which 450 U.S. schizophrenia patients were randomized to treatment with once-monthly injectable paliperidone palmitate (Invega Sustenna) or daily oral antipsychotics for up to 15 months. Willingness to accept the long-acting injectable medication was a precondition for eligibility. Prior to randomization, patients sat down individually with a clinician, reviewed seven widely prescribed oral antipsychotics available in this study, and eliminated up to six of them from further consideration. They were then randomly assigned to flexibly dosed therapy with either an oral antipsychotic agent or injectable paliperidone palmitate.
The primary study endpoint was time to treatment failure, broadly defined in this study as a composite of arrest or incarceration; psychiatric hospitalization; suicide; dropout because of insufficient efficacy; discontinuation because of safety or tolerability issues; and the need for treatment supplementation with an additional antipsychotic because of inadequate efficacy, suicide risk, or increased use of psychiatric services in order to prevent imminent psychiatric hospitalization.
Median time to treatment failure was 226 days in the oral antipsychotic group and nearly twice as long at 416 days in the injectable paliperidone palmitate group. The treatment failure rate was 39.8% with paliperidone palmitate, compared with 53.7% with oral antipsychotics. This translated to a 43% greater risk of treatment failure in the oral therapy group, according to Dr. Starr of Janssen Pharmaceuticals in Titusville, N.J.
A prespecified key secondary endpoint was time to first psychiatric hospitalization or arrest/incarceration, as these outcomes are associated with considerable public health impact. The risk of either event proved to be 43% higher in the oral antipsychotic group. Arrest/incarceration and psychiatric hospitalization turned out to be the two most frequently occurring elements of the primary composite endpoint. Arrest/incarceration occurred in 21.2% of the paliperidone group, compared with 29.4% on oral antipsychotics. Eight percent of patients on long-term injectable therapy underwent psychiatric hospitalization, as did 11.9% on oral antipsychotics.
Patients on oral antipsychotic therapy not only experienced arrest/incarceration or psychiatric hospitalization sooner, they also had a significantly greater cumulative number of such events during follow-up.
The study drug was discontinued because of safety or tolerability issues in 6.6% of the paliperidone palmitate group and 3.7% on an oral antipsychotic. Erectile dysfunction was reported by 8.8% of patients on long-acting therapy but none on oral agents. Among women, amenorrhea occurred in 15.2% on paliperidone palmitate, vs. 3.6% on oral antipsychotics.
Thirty-two percent of patients on paliperidone palmitate had at least a 7% weight gain, as did 14% of the oral antipsychotic group.
In the oral antipsychotic group, 22% of patients were on paliperidone; 17%, on risperidone; 16.5%, on olanzapine; 15.1%, on aripiprazole; 13.3%, on quetiapine; 9.2%, on perphenazine; and 6.9%, on haloperidol.
The PRIDE study was funded by Janssen Scientific Affairs, where Dr. Starr serves as director of clinical development.
AT THE ECNP CONGRESS
Key clinical point: Real world schizophrenia patients with a history of incarceration are less likely to return to jail if they’re on long-acting intramuscular paliperidone palmitate than an oral antipsychotic.
Major finding: The median time to treatment failure, a composite endpoint including arrest/incarceration or psychiatric hospitalization, was 416 days in patients randomized to paliperidone palmitate and 226 days in those assigned to an oral antipsychotic.
Data source: The PRIDE study was a prospective, multicenter, open-label single-blind study in which 450 schizophrenia patients with contact with the criminal justice system within the last 90 days were randomized to long-acting injectable paliperidone palmitate or daily oral antipsychotic therapy.
Disclosures: The PRIDE study was sponsored by Janssen Pharmaceuticals. The presenter is a company employee.
Once-monthly aripiprazole drastically cut psychiatric hospitalizations
BERLIN– Switching patients with schizophrenia from various oral antipsychotics to long-acting injectable aripiprazole at 400 mg once monthly resulted in a dramatic reduction in their psychiatric hospitalization rate in a phase IIIb study.
This robust 90% reduction in psychiatric hospitalizations during the first 3 months on long-acting aripiprazole, compared with participants’ last 3 months prior to making the switch, suggests that injectable aripiprazole might offer significant cost savings to the health care system, Dr. Timothy S. Peters-Strickland observed at the annual congress of the European College of Neuropsychopharmacology.
He presented the findings of a phase IIIb, multicenter, open-label, mirror-image clinical trial involving 433 schizophrenia patients. The study, conducted in a naturalistic community setting, was dubbed a mirror-image study, because it retrospectively looked back at the psychiatric hospitalization rate of participants when they were on oral antipsychotics as well as conducting a prospective assessment of participants’ psychiatric hospitalizations after they made the switch to once-monthly injectable aripiprazole. Thus, patients served as their own controls.
Of note, at enrollment all participants required a change away from their current oral antipsychotic for various reasons, including lack of efficacy, side effects, or poor compliance. This might well have been a factor in some of the psychiatric hospitalizations occurring during the period before the switch.
As part of the study protocol, all participants on an oral antipsychotic other than aripiprazole (Abilify) were converted to oral aripiprazole over the course of 1-4 weeks of cross titration before making the switch to injectable aripiprazole. The duration of the cross-titration period was left to physician discretion.
A key observation regarding this cross-titration phase is that longer proved to be better. That is, the rate of discontinuation because of adverse events during the conversion process was substantially higher – 10.4% – in patients whose cross-titration period lasted 1 week than the 2.9% rate in patients whose cross-titration continued for more than 1 week and up to 4 weeks before introducing long-acting injectable therapy, reported Dr. Peters-Strickland of Otsuka in Princeton, N.J.
The primary study endpoint was the psychiatric hospitalization rate during the last 3 months on oral antipsychotic therapy prior to cross titration, compared with the rate during the first 3 months after the switch to long-acting aripiprazole. The rate on oral therapy was 27.1%, an order of magnitude greater than the 2.7% rate once the same patients had made the switch.
A hefty 32% of patients discontinued long-acting aripiprazole within the first 6 months. A total of 9% of subjects did so because of adverse events, 8% were lost to follow-up, and 2% quit because of lack of efficacy. The most common treatment-emergent adverse events were insomnia, akathisia, and emergence of a psychotic disorder.
The psychiatric hospitalization rate during the last 6 months that patients were on oral antipsychotic therapy was 38.1%, compared with an 8.8% rate during the first 6 months after they started on injectable aripiprazole, whether they stayed on the long-acting medication for the full 6 months or not.
This mirror-image study was sponsored by Lundbeck and Otsuka. Dr. Peters-Strickland is an Otsuka employee.
BERLIN– Switching patients with schizophrenia from various oral antipsychotics to long-acting injectable aripiprazole at 400 mg once monthly resulted in a dramatic reduction in their psychiatric hospitalization rate in a phase IIIb study.
This robust 90% reduction in psychiatric hospitalizations during the first 3 months on long-acting aripiprazole, compared with participants’ last 3 months prior to making the switch, suggests that injectable aripiprazole might offer significant cost savings to the health care system, Dr. Timothy S. Peters-Strickland observed at the annual congress of the European College of Neuropsychopharmacology.
He presented the findings of a phase IIIb, multicenter, open-label, mirror-image clinical trial involving 433 schizophrenia patients. The study, conducted in a naturalistic community setting, was dubbed a mirror-image study, because it retrospectively looked back at the psychiatric hospitalization rate of participants when they were on oral antipsychotics as well as conducting a prospective assessment of participants’ psychiatric hospitalizations after they made the switch to once-monthly injectable aripiprazole. Thus, patients served as their own controls.
Of note, at enrollment all participants required a change away from their current oral antipsychotic for various reasons, including lack of efficacy, side effects, or poor compliance. This might well have been a factor in some of the psychiatric hospitalizations occurring during the period before the switch.
As part of the study protocol, all participants on an oral antipsychotic other than aripiprazole (Abilify) were converted to oral aripiprazole over the course of 1-4 weeks of cross titration before making the switch to injectable aripiprazole. The duration of the cross-titration period was left to physician discretion.
A key observation regarding this cross-titration phase is that longer proved to be better. That is, the rate of discontinuation because of adverse events during the conversion process was substantially higher – 10.4% – in patients whose cross-titration period lasted 1 week than the 2.9% rate in patients whose cross-titration continued for more than 1 week and up to 4 weeks before introducing long-acting injectable therapy, reported Dr. Peters-Strickland of Otsuka in Princeton, N.J.
The primary study endpoint was the psychiatric hospitalization rate during the last 3 months on oral antipsychotic therapy prior to cross titration, compared with the rate during the first 3 months after the switch to long-acting aripiprazole. The rate on oral therapy was 27.1%, an order of magnitude greater than the 2.7% rate once the same patients had made the switch.
A hefty 32% of patients discontinued long-acting aripiprazole within the first 6 months. A total of 9% of subjects did so because of adverse events, 8% were lost to follow-up, and 2% quit because of lack of efficacy. The most common treatment-emergent adverse events were insomnia, akathisia, and emergence of a psychotic disorder.
The psychiatric hospitalization rate during the last 6 months that patients were on oral antipsychotic therapy was 38.1%, compared with an 8.8% rate during the first 6 months after they started on injectable aripiprazole, whether they stayed on the long-acting medication for the full 6 months or not.
This mirror-image study was sponsored by Lundbeck and Otsuka. Dr. Peters-Strickland is an Otsuka employee.
BERLIN– Switching patients with schizophrenia from various oral antipsychotics to long-acting injectable aripiprazole at 400 mg once monthly resulted in a dramatic reduction in their psychiatric hospitalization rate in a phase IIIb study.
This robust 90% reduction in psychiatric hospitalizations during the first 3 months on long-acting aripiprazole, compared with participants’ last 3 months prior to making the switch, suggests that injectable aripiprazole might offer significant cost savings to the health care system, Dr. Timothy S. Peters-Strickland observed at the annual congress of the European College of Neuropsychopharmacology.
He presented the findings of a phase IIIb, multicenter, open-label, mirror-image clinical trial involving 433 schizophrenia patients. The study, conducted in a naturalistic community setting, was dubbed a mirror-image study, because it retrospectively looked back at the psychiatric hospitalization rate of participants when they were on oral antipsychotics as well as conducting a prospective assessment of participants’ psychiatric hospitalizations after they made the switch to once-monthly injectable aripiprazole. Thus, patients served as their own controls.
Of note, at enrollment all participants required a change away from their current oral antipsychotic for various reasons, including lack of efficacy, side effects, or poor compliance. This might well have been a factor in some of the psychiatric hospitalizations occurring during the period before the switch.
As part of the study protocol, all participants on an oral antipsychotic other than aripiprazole (Abilify) were converted to oral aripiprazole over the course of 1-4 weeks of cross titration before making the switch to injectable aripiprazole. The duration of the cross-titration period was left to physician discretion.
A key observation regarding this cross-titration phase is that longer proved to be better. That is, the rate of discontinuation because of adverse events during the conversion process was substantially higher – 10.4% – in patients whose cross-titration period lasted 1 week than the 2.9% rate in patients whose cross-titration continued for more than 1 week and up to 4 weeks before introducing long-acting injectable therapy, reported Dr. Peters-Strickland of Otsuka in Princeton, N.J.
The primary study endpoint was the psychiatric hospitalization rate during the last 3 months on oral antipsychotic therapy prior to cross titration, compared with the rate during the first 3 months after the switch to long-acting aripiprazole. The rate on oral therapy was 27.1%, an order of magnitude greater than the 2.7% rate once the same patients had made the switch.
A hefty 32% of patients discontinued long-acting aripiprazole within the first 6 months. A total of 9% of subjects did so because of adverse events, 8% were lost to follow-up, and 2% quit because of lack of efficacy. The most common treatment-emergent adverse events were insomnia, akathisia, and emergence of a psychotic disorder.
The psychiatric hospitalization rate during the last 6 months that patients were on oral antipsychotic therapy was 38.1%, compared with an 8.8% rate during the first 6 months after they started on injectable aripiprazole, whether they stayed on the long-acting medication for the full 6 months or not.
This mirror-image study was sponsored by Lundbeck and Otsuka. Dr. Peters-Strickland is an Otsuka employee.
AT THE ECNP CONGRESS
Key clinical point: The psychiatric hospitalization rate was reduced 10-fold after schizophrenia patients were switched from various oral antipsychotic agents to long-acting injectable aripiprazole.
Major finding: The psychiatric hospitalization rate during the last 3 months patients were on oral antipsychotic therapy was 27%, compared with a 2.7% rate in the 3 months following a switch to long-acting intramuscular aripiprazole.
Data source: This prospective, open-label, mirror-image, multicenter study included 433 patients with schizophrenia who were switched from oral antipsychotic therapy with a variety of agents to once-monthly injectable aripiprazole at 400 mg.
Disclosures: This study was supported by H. Lundbeck and Otsuka. The presenter is an Otsuka employee.
Lurasidone favorably affects metabolic syndrome risk
BERLIN – The prevalence of metabolic syndrome remained stable in outpatients with schizophrenia during 12 months of double-blind treatment with lurasidone, while the rate climbed significantly in those randomized to risperidone in a multicenter head-to head comparative study.
The study included 611 outpatients with clinically stable schizophrenia who were randomized double-blind 2:1 to 12 months of flexibly dosed oral lurasidone (Latuda) at 37-111 mg/day or to oral risperidone (Risperdal) at 2-6 mg/day.
The prevalence of metabolic syndrome at enrollment as defined by International Diabetes Federation criteria was 32.5% in the lurasidone group and virtually identical at 32.7% in the risperidone group. After 1 year of double-blind treatment, 37% of those with baseline metabolic syndrome in the lurasidone group no longer met criteria for the metabolic derangement, compared with a 28% metabolic syndrome reversal rate in the risperidone group, Dr. John W. Newcomer reported at the annual congress of the European College of Neuropsychopharmacology.
Among subjects without metabolic syndrome at baseline, the incidence of new-onset metabolic syndrome during 12 months of therapy was 16% with lurasidone, compared with 27% in those on risperidone. Thus, the 1-year on-treatment risk of developing metabolic syndrome was reduced by 48% in patients treated with lurasidone – as compared to risperidone-treated patients – observed Dr. Newcomer, a psychiatrist who serves as executive vice dean and professor of clinical biomedical science at the Florida Atlantic University College of Medicine in Boca Raton.
Patients on risperidone gained a mean of 2.6 kg over the course of the year, and those on lurasidone lost 0.9 kg. Mean waist circumference grew by 2.6 cm in the risperidone group, compared with a 0.4-cm decrease in the lurasidone group. Blood pressure remained unchanged over time in both treatment arms.
At the end of 12 months of double-blind therapy, 223 study participants continued on for a 6-month open-label extension phase on flexibly dosed lurasidone. Among patients who had been on 12 months of double-blind risperidone, the prevalence of metabolic syndrome went from 42.9% at the outset of the double-blind phase to 48.4% 12 months later, thereafter dropping to 38.5% after 6 months of open-label lurasidone. During their 6 months on lurasidone, the patients formerly on risperidone lost a mean of 2.9 kg in body weight and 1.6 cm in waist circumference.
Meanwhile, among patients on lurasidone for the entire 18 months, the prevalence of metabolic syndrome decreased from 33.3% at enrollment to 26.6% after 18 months of continuous treatment. This benefit was driven by a mean 1.7-kg weight loss and a 1.5-cm reduction in waist circumference.
This head-to-head comparative study was sponsored by Sunovion Pharmaceuticals and Takeda Pharmaceuticals. Dr. Newcomer reported having served as an independent scientific member on data safety monitoring boards for studies sponsored by a handful of other companies while having no financial relationship with the cosponsors of the current study.
BERLIN – The prevalence of metabolic syndrome remained stable in outpatients with schizophrenia during 12 months of double-blind treatment with lurasidone, while the rate climbed significantly in those randomized to risperidone in a multicenter head-to head comparative study.
The study included 611 outpatients with clinically stable schizophrenia who were randomized double-blind 2:1 to 12 months of flexibly dosed oral lurasidone (Latuda) at 37-111 mg/day or to oral risperidone (Risperdal) at 2-6 mg/day.
The prevalence of metabolic syndrome at enrollment as defined by International Diabetes Federation criteria was 32.5% in the lurasidone group and virtually identical at 32.7% in the risperidone group. After 1 year of double-blind treatment, 37% of those with baseline metabolic syndrome in the lurasidone group no longer met criteria for the metabolic derangement, compared with a 28% metabolic syndrome reversal rate in the risperidone group, Dr. John W. Newcomer reported at the annual congress of the European College of Neuropsychopharmacology.
Among subjects without metabolic syndrome at baseline, the incidence of new-onset metabolic syndrome during 12 months of therapy was 16% with lurasidone, compared with 27% in those on risperidone. Thus, the 1-year on-treatment risk of developing metabolic syndrome was reduced by 48% in patients treated with lurasidone – as compared to risperidone-treated patients – observed Dr. Newcomer, a psychiatrist who serves as executive vice dean and professor of clinical biomedical science at the Florida Atlantic University College of Medicine in Boca Raton.
Patients on risperidone gained a mean of 2.6 kg over the course of the year, and those on lurasidone lost 0.9 kg. Mean waist circumference grew by 2.6 cm in the risperidone group, compared with a 0.4-cm decrease in the lurasidone group. Blood pressure remained unchanged over time in both treatment arms.
At the end of 12 months of double-blind therapy, 223 study participants continued on for a 6-month open-label extension phase on flexibly dosed lurasidone. Among patients who had been on 12 months of double-blind risperidone, the prevalence of metabolic syndrome went from 42.9% at the outset of the double-blind phase to 48.4% 12 months later, thereafter dropping to 38.5% after 6 months of open-label lurasidone. During their 6 months on lurasidone, the patients formerly on risperidone lost a mean of 2.9 kg in body weight and 1.6 cm in waist circumference.
Meanwhile, among patients on lurasidone for the entire 18 months, the prevalence of metabolic syndrome decreased from 33.3% at enrollment to 26.6% after 18 months of continuous treatment. This benefit was driven by a mean 1.7-kg weight loss and a 1.5-cm reduction in waist circumference.
This head-to-head comparative study was sponsored by Sunovion Pharmaceuticals and Takeda Pharmaceuticals. Dr. Newcomer reported having served as an independent scientific member on data safety monitoring boards for studies sponsored by a handful of other companies while having no financial relationship with the cosponsors of the current study.
BERLIN – The prevalence of metabolic syndrome remained stable in outpatients with schizophrenia during 12 months of double-blind treatment with lurasidone, while the rate climbed significantly in those randomized to risperidone in a multicenter head-to head comparative study.
The study included 611 outpatients with clinically stable schizophrenia who were randomized double-blind 2:1 to 12 months of flexibly dosed oral lurasidone (Latuda) at 37-111 mg/day or to oral risperidone (Risperdal) at 2-6 mg/day.
The prevalence of metabolic syndrome at enrollment as defined by International Diabetes Federation criteria was 32.5% in the lurasidone group and virtually identical at 32.7% in the risperidone group. After 1 year of double-blind treatment, 37% of those with baseline metabolic syndrome in the lurasidone group no longer met criteria for the metabolic derangement, compared with a 28% metabolic syndrome reversal rate in the risperidone group, Dr. John W. Newcomer reported at the annual congress of the European College of Neuropsychopharmacology.
Among subjects without metabolic syndrome at baseline, the incidence of new-onset metabolic syndrome during 12 months of therapy was 16% with lurasidone, compared with 27% in those on risperidone. Thus, the 1-year on-treatment risk of developing metabolic syndrome was reduced by 48% in patients treated with lurasidone – as compared to risperidone-treated patients – observed Dr. Newcomer, a psychiatrist who serves as executive vice dean and professor of clinical biomedical science at the Florida Atlantic University College of Medicine in Boca Raton.
Patients on risperidone gained a mean of 2.6 kg over the course of the year, and those on lurasidone lost 0.9 kg. Mean waist circumference grew by 2.6 cm in the risperidone group, compared with a 0.4-cm decrease in the lurasidone group. Blood pressure remained unchanged over time in both treatment arms.
At the end of 12 months of double-blind therapy, 223 study participants continued on for a 6-month open-label extension phase on flexibly dosed lurasidone. Among patients who had been on 12 months of double-blind risperidone, the prevalence of metabolic syndrome went from 42.9% at the outset of the double-blind phase to 48.4% 12 months later, thereafter dropping to 38.5% after 6 months of open-label lurasidone. During their 6 months on lurasidone, the patients formerly on risperidone lost a mean of 2.9 kg in body weight and 1.6 cm in waist circumference.
Meanwhile, among patients on lurasidone for the entire 18 months, the prevalence of metabolic syndrome decreased from 33.3% at enrollment to 26.6% after 18 months of continuous treatment. This benefit was driven by a mean 1.7-kg weight loss and a 1.5-cm reduction in waist circumference.
This head-to-head comparative study was sponsored by Sunovion Pharmaceuticals and Takeda Pharmaceuticals. Dr. Newcomer reported having served as an independent scientific member on data safety monitoring boards for studies sponsored by a handful of other companies while having no financial relationship with the cosponsors of the current study.
AT THE ECNP CONGRESS
Key clinical point: Lurasidone was associated with a significantly lower metabolic syndrome rate than risperidone in schizophrenia outpatients.
Major finding: Among 157 patients without metabolic syndrome at baseline who were randomized to once-daily lurasidone or risperidone in a head-to-head comparative study, the risk of developing metabolic syndrome over 12 months of therapy was 48% less in the lurasidone group.
Data source: This was a post hoc analysis of a double-blind, multicenter study in which 611 clinically stable outpatients with schizophrenia were randomized 2:1 to flexibly dosed lurasidone or risperidone, following which 174 continued on for a 6-month open-label extension phase.
Disclosures: The study was sponsored by Sunovion Pharmaceuticals and Takeda Pharmaceuticals. Dr. Newcomer reported having no relevant financial relationships.