User login
Roux-en-Y bests sleeve gastrectomy for weight loss
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: At 5 years post surgery, Roux-en-Y recipients had kept off 25% of their body weight, compared with 20% for sleeve gastrectomy patients (P less than .05).
Data source: Longitudinal follow-up of 716 patients who had one of two surgical procedures for weight loss.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
For girls with Turner syndrome, experimental fertility preservation may offer the hope of a baby of their own
ORLANDO – Fertility preservation techniques pioneered in young cancer patients may someday allow some women with Turner syndrome to give birth to their own children, without relying on donated eggs.
Spontaneous conception and live birth are exceedingly rare among women with the genetic disorder. Until very recently, adoption was the only practical way for most to grow a family. In the last decade, however, fertility specialists in Europe and the United States have had good success with in vitro fertilization using donated eggs. Now, those clinicians are aiming for a higher goal: babies born from a patient’s own eggs, cryopreserved either individually or within whole ovarian tissue.
These are not pipe dreams, according to experts interviewed for this story. Autologous oocyte freezing is well established in healthy women and is now coming of age in cancer patients, with recent reports of live births. Ovarian tissue freezing and reimplantation is a much newer technique, also pioneered in cancer patients. To date, more than 70 live births have occurred from ovarian cortical tissue conservation and later transplantation in adults. Last December brought the first report of a live birth to a childhood cancer survivor who had prepubertal ovarian tissue frozen before chemotherapy. And a 2015 report detailed the case of a girl with primary ovarian failure secondary to sickle cell anemia treatment. At 25, she gave birth to a healthy child conceived from ovarian tissue removed when she was 14 years old.
Not all clinicians so enthusiastically embrace this future, however. A new set of consensus guidelines for the management of girls and women with Turner syndrome is in the works and will recommend a more conservative clinical approach, according to Nelly Mauras, MD, chief of endocrinology, diabetes, and metabolism at the Nemours Children’s Health System in Jacksonville, Fla.
A group of academic and patient advocacy stakeholders is cooperatively honing the document based on a meeting last summer in Cincinnati. These groups include the European Society of Endocrinology, the Endocrine Society, the U.S. Pediatric Endocrine Society, the European Society for Pediatric Endocrinology, Cardiology, and Reproductive Endocrinology, as well as Turner syndrome patient advocacy groups.
Dr. Mauras said the guideline will be “less discouraging” than the existing one issued in 2012 by the American Society of Reproductive Medicine. In its 2012 guidelines, the society identified Turner syndrome as a relative contraindication to pregnancy and an absolute contraindication in those with documented cardiac anomalies. However, greater experience has since been accrued in reproductive techniques of oocyte donation in Turner, with better outcomes.
The upcoming guidelines, however, will still recommend strongly against ovarian stimulation for fertility preservation for girls younger than 12, Dr. Mauras said, and will not recommend ovarian tissue conservation. “We just do not have the safety data and pregnancy outcomes that we need to give strong recommendations for these treatments,” she said. “These are still considered experimental treatments for girls with Turner syndrome.”
Turner syndrome, caused by a deletion of one X chromosome, throws a unique curve into the game – very early ovarian failure. Those with a complete deletion (45,X) begin losing their primordial ovarian follicles even before birth. Most will never experience spontaneous puberty; even if they do, their egg reserve is virtually gone soon after. Ovarian reserve may not be completely lost in girls with mosaicism, however, who have the X deletion in only a portion of their cells (46,XX/45,X). Some will enter puberty, and about 5% may even conceive spontaneously in younger years. But of the majority of Turner girls eventually experience complete ovarian failure.
This means that fertility preservation can’t be a wait-and-see issue, according to Kutluk Oktay, MD, PhD, a fertility specialist on the leading edge of this issue in the United States.
“We have to be proactive,” said Dr. Oktay, professor of obstetrics and gynecology at New York Medical College, Valhalla. “If we wait until girls are 12 or 13 to address this, a majority will have totally depleted their ovarian reserve by then. They will have no option other than an egg donor or adoption. We are suggesting that they should be screened as soon as they are diagnosed, and if they and their parents wish it, something should be done before it’s too late.”
Dr. Oktay is also the founder of fertilitypreservation.org, which specializes in advanced fertility treatments for cancer patients. He is one of a handful of physicians in the United States who advocate early oocyte harvesting in peripubertal girls and ovarian tissue harvesting in prepubertal girls with Turner syndrome. Last year, in conjunction with the Turner Syndrome Foundation, he and his colleagues published a set of guidelines for preserving fertility in these patients.
The paper recommends fertility assessment pathways for pre- and postpubertal girls. For both groups, Dr. Oktay employs serial assessments of ovarian reserve by monitoring several hormones, including follicle-stimulating hormone, luteinizing hormone, and antimüllerian hormone (AMH). Produced by primordial follicles, AMH declines as egg reserve declines over a lifetime, and is considered an accurate marker of ovarian reserve. When a girl experiences two consecutive AMH declines, egg depletion is probably accelerating. “This is the time to consider fertility preservation,” he said.
If a girl is peri- or postpubertal, the choice would probably be ovarian stimulation with the goal of retrieving mature oocytes. For prepubertal girls, the best choice is probably ovarian tissue cryopreservation. But because the egg reserve may already be spotty inside the ovary, he recommended freezing it en bloc, rather than preserving just cortical sections.
Because these techniques are only beginning to be used in young Turner patients, neither has been tested yet to see if it would result in a pregnancy. However, Dr. Oktay said, more than 80 babies have been born to women with other disorders who had ovaries frozen as adults. And European women with Turner syndrome have been successfully bearing children with donated oocytes for years.
“I don’t differentiate Europe from the U.S.,” he said. “We have no reason to believe Turner syndrome girls would be any different here than they are there.”
Pregnancy rates by egg donation in Turner syndrome patients are about half that typically seen in an otherwise healthy infertile woman, according to numerous sources; with a take-home baby rate of about 18%. There are numerous reasons for this. The miscarriage rate in Turner patients is about 44%. Women with Turner tend to have smaller uteri, with thinner endometrium, mostly because of the lack of estrogen.
But with careful management, those who do conceive can safely deliver a healthy baby, said Outi Hovatta, MD, a Finnish fertility specialist who has done extensive work in the area. “In Europe, we have been doing this quite liberally for years, and haven’t had a bad experience,” she said in an interview.
A 2013 review summarized both the success and the risks of these pregnancies. It examined obstetric and neonatal outcomes among 106 women with Turner syndrome who gave birth via egg donation from 1992 to 2011 in Sweden, Finland, and Denmark.
Most (70%) had a single embryo transferred, as virtually all guidelines recommend. Women with Turner are prone to cardiac and aortic defects that can worsen under the strain of pregnancy, or even present for the first time during gestation. Aortic dissection is a real threat; up to a third of patients with Turner experience it during adulthood, and it’s a major cause of death among them. In the Nordic series, 10 women (9%) had a known cardiac anomaly.
The multiple birth rate was 7%. More than a third (35%) developed a hypertensive disorder, including preeclampsia (20%). Four women had potentially life-threatening complications, including one with aortic dissection, one who developed mild regurgitation of the tricuspid and mitral valve, one with a mechanical heart valve who developed HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), and one who underwent a postpartum hysterectomy because of severe hemorrhaging.
The infants, nevertheless, did well. The preterm birth rate was 8%, with 9% of the singleton infants having a low birth weight. About 4% had a major birth defect. Of the 131 born, three died (2.3%), including a set of extremely preterm twins.
Close follow-up and cross-specialty cooperation are what make these positive outcomes possible, said Dr. Hovatta, who is now a professor emeritus at the Karolinska Institute, Sweden.
“We do everything we can to exclude things that could cause bad outcomes.” That includes extremely rigorous cardiac testing before pregnancy and continuous monitoring during it. “If a woman has any sign of cardiac anomaly, she is advised not to become pregnant. If she shows any signs of aortic dilation, we follow her extremely carefully with experienced cardiologists.”
Like Dr. Oktay, Dr. Hovatta and her European colleagues make fertility preservation an early topic of conversation. Unlike in the United States, where many girls aren’t diagnosed with the disorder until they fail to enter puberty, almost all Turner girls in Europe are identified very early in childhood. They receive early growth hormone treatment, and there is frequent consultation with interdisciplinary specialists. Fertility is spoken of early and often.
Early oocyte retrieval is common, Dr. Hovatta said. “Yes, it’s possible to wait until puberty, but for so many girls, most of the eggs have disappeared by then, so we typically don’t wait. We start looking at that option around 11 years, which is the same time we think about cryopreserving ovarian tissue.”
However, she added, as in the United States, the outcomes of these procedures are still unknown. But the existing data in other populations, the ability to carefully shepherd women through a successful pregnancy, and the willingness of families to provide the option all support further exploring them. Dr. Hovatta was at the Cincinnati gathering last summer and said she did not agree with the conservative tone she heard. Dr. Oktay also does not agree.
“This evidence we have so far is good evidence,” he said. “Look, where we are right now with Turner girls is where we were 15 years ago with cancer patients. People thought, ‘They have cancer. They should just be worrying about surviving cancer, not about their fertility.’ Now fertility counseling is a very important part of cancer care. We have all these tools available to us for cancer patients who don’t want to lose their fertility. This accumulated experience that has already been applied in other medical conditions … why not use that for Turner syndrome? This is my point: With all the data out there about the potential benefits and the ways to manage the risks, we shouldn’t have to tell girls, ‘Well, you have to become menopausal, and maybe you can adopt someday.’ That doesn’t sit well with parents any more. We want these girls to thrive. Not just to survive, but to have as close to a normal quality of life as possible.”
ORLANDO – Fertility preservation techniques pioneered in young cancer patients may someday allow some women with Turner syndrome to give birth to their own children, without relying on donated eggs.
Spontaneous conception and live birth are exceedingly rare among women with the genetic disorder. Until very recently, adoption was the only practical way for most to grow a family. In the last decade, however, fertility specialists in Europe and the United States have had good success with in vitro fertilization using donated eggs. Now, those clinicians are aiming for a higher goal: babies born from a patient’s own eggs, cryopreserved either individually or within whole ovarian tissue.
These are not pipe dreams, according to experts interviewed for this story. Autologous oocyte freezing is well established in healthy women and is now coming of age in cancer patients, with recent reports of live births. Ovarian tissue freezing and reimplantation is a much newer technique, also pioneered in cancer patients. To date, more than 70 live births have occurred from ovarian cortical tissue conservation and later transplantation in adults. Last December brought the first report of a live birth to a childhood cancer survivor who had prepubertal ovarian tissue frozen before chemotherapy. And a 2015 report detailed the case of a girl with primary ovarian failure secondary to sickle cell anemia treatment. At 25, she gave birth to a healthy child conceived from ovarian tissue removed when she was 14 years old.
Not all clinicians so enthusiastically embrace this future, however. A new set of consensus guidelines for the management of girls and women with Turner syndrome is in the works and will recommend a more conservative clinical approach, according to Nelly Mauras, MD, chief of endocrinology, diabetes, and metabolism at the Nemours Children’s Health System in Jacksonville, Fla.
A group of academic and patient advocacy stakeholders is cooperatively honing the document based on a meeting last summer in Cincinnati. These groups include the European Society of Endocrinology, the Endocrine Society, the U.S. Pediatric Endocrine Society, the European Society for Pediatric Endocrinology, Cardiology, and Reproductive Endocrinology, as well as Turner syndrome patient advocacy groups.
Dr. Mauras said the guideline will be “less discouraging” than the existing one issued in 2012 by the American Society of Reproductive Medicine. In its 2012 guidelines, the society identified Turner syndrome as a relative contraindication to pregnancy and an absolute contraindication in those with documented cardiac anomalies. However, greater experience has since been accrued in reproductive techniques of oocyte donation in Turner, with better outcomes.
The upcoming guidelines, however, will still recommend strongly against ovarian stimulation for fertility preservation for girls younger than 12, Dr. Mauras said, and will not recommend ovarian tissue conservation. “We just do not have the safety data and pregnancy outcomes that we need to give strong recommendations for these treatments,” she said. “These are still considered experimental treatments for girls with Turner syndrome.”
Turner syndrome, caused by a deletion of one X chromosome, throws a unique curve into the game – very early ovarian failure. Those with a complete deletion (45,X) begin losing their primordial ovarian follicles even before birth. Most will never experience spontaneous puberty; even if they do, their egg reserve is virtually gone soon after. Ovarian reserve may not be completely lost in girls with mosaicism, however, who have the X deletion in only a portion of their cells (46,XX/45,X). Some will enter puberty, and about 5% may even conceive spontaneously in younger years. But of the majority of Turner girls eventually experience complete ovarian failure.
This means that fertility preservation can’t be a wait-and-see issue, according to Kutluk Oktay, MD, PhD, a fertility specialist on the leading edge of this issue in the United States.
“We have to be proactive,” said Dr. Oktay, professor of obstetrics and gynecology at New York Medical College, Valhalla. “If we wait until girls are 12 or 13 to address this, a majority will have totally depleted their ovarian reserve by then. They will have no option other than an egg donor or adoption. We are suggesting that they should be screened as soon as they are diagnosed, and if they and their parents wish it, something should be done before it’s too late.”
Dr. Oktay is also the founder of fertilitypreservation.org, which specializes in advanced fertility treatments for cancer patients. He is one of a handful of physicians in the United States who advocate early oocyte harvesting in peripubertal girls and ovarian tissue harvesting in prepubertal girls with Turner syndrome. Last year, in conjunction with the Turner Syndrome Foundation, he and his colleagues published a set of guidelines for preserving fertility in these patients.
The paper recommends fertility assessment pathways for pre- and postpubertal girls. For both groups, Dr. Oktay employs serial assessments of ovarian reserve by monitoring several hormones, including follicle-stimulating hormone, luteinizing hormone, and antimüllerian hormone (AMH). Produced by primordial follicles, AMH declines as egg reserve declines over a lifetime, and is considered an accurate marker of ovarian reserve. When a girl experiences two consecutive AMH declines, egg depletion is probably accelerating. “This is the time to consider fertility preservation,” he said.
If a girl is peri- or postpubertal, the choice would probably be ovarian stimulation with the goal of retrieving mature oocytes. For prepubertal girls, the best choice is probably ovarian tissue cryopreservation. But because the egg reserve may already be spotty inside the ovary, he recommended freezing it en bloc, rather than preserving just cortical sections.
Because these techniques are only beginning to be used in young Turner patients, neither has been tested yet to see if it would result in a pregnancy. However, Dr. Oktay said, more than 80 babies have been born to women with other disorders who had ovaries frozen as adults. And European women with Turner syndrome have been successfully bearing children with donated oocytes for years.
“I don’t differentiate Europe from the U.S.,” he said. “We have no reason to believe Turner syndrome girls would be any different here than they are there.”
Pregnancy rates by egg donation in Turner syndrome patients are about half that typically seen in an otherwise healthy infertile woman, according to numerous sources; with a take-home baby rate of about 18%. There are numerous reasons for this. The miscarriage rate in Turner patients is about 44%. Women with Turner tend to have smaller uteri, with thinner endometrium, mostly because of the lack of estrogen.
But with careful management, those who do conceive can safely deliver a healthy baby, said Outi Hovatta, MD, a Finnish fertility specialist who has done extensive work in the area. “In Europe, we have been doing this quite liberally for years, and haven’t had a bad experience,” she said in an interview.
A 2013 review summarized both the success and the risks of these pregnancies. It examined obstetric and neonatal outcomes among 106 women with Turner syndrome who gave birth via egg donation from 1992 to 2011 in Sweden, Finland, and Denmark.
Most (70%) had a single embryo transferred, as virtually all guidelines recommend. Women with Turner are prone to cardiac and aortic defects that can worsen under the strain of pregnancy, or even present for the first time during gestation. Aortic dissection is a real threat; up to a third of patients with Turner experience it during adulthood, and it’s a major cause of death among them. In the Nordic series, 10 women (9%) had a known cardiac anomaly.
The multiple birth rate was 7%. More than a third (35%) developed a hypertensive disorder, including preeclampsia (20%). Four women had potentially life-threatening complications, including one with aortic dissection, one who developed mild regurgitation of the tricuspid and mitral valve, one with a mechanical heart valve who developed HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), and one who underwent a postpartum hysterectomy because of severe hemorrhaging.
The infants, nevertheless, did well. The preterm birth rate was 8%, with 9% of the singleton infants having a low birth weight. About 4% had a major birth defect. Of the 131 born, three died (2.3%), including a set of extremely preterm twins.
Close follow-up and cross-specialty cooperation are what make these positive outcomes possible, said Dr. Hovatta, who is now a professor emeritus at the Karolinska Institute, Sweden.
“We do everything we can to exclude things that could cause bad outcomes.” That includes extremely rigorous cardiac testing before pregnancy and continuous monitoring during it. “If a woman has any sign of cardiac anomaly, she is advised not to become pregnant. If she shows any signs of aortic dilation, we follow her extremely carefully with experienced cardiologists.”
Like Dr. Oktay, Dr. Hovatta and her European colleagues make fertility preservation an early topic of conversation. Unlike in the United States, where many girls aren’t diagnosed with the disorder until they fail to enter puberty, almost all Turner girls in Europe are identified very early in childhood. They receive early growth hormone treatment, and there is frequent consultation with interdisciplinary specialists. Fertility is spoken of early and often.
Early oocyte retrieval is common, Dr. Hovatta said. “Yes, it’s possible to wait until puberty, but for so many girls, most of the eggs have disappeared by then, so we typically don’t wait. We start looking at that option around 11 years, which is the same time we think about cryopreserving ovarian tissue.”
However, she added, as in the United States, the outcomes of these procedures are still unknown. But the existing data in other populations, the ability to carefully shepherd women through a successful pregnancy, and the willingness of families to provide the option all support further exploring them. Dr. Hovatta was at the Cincinnati gathering last summer and said she did not agree with the conservative tone she heard. Dr. Oktay also does not agree.
“This evidence we have so far is good evidence,” he said. “Look, where we are right now with Turner girls is where we were 15 years ago with cancer patients. People thought, ‘They have cancer. They should just be worrying about surviving cancer, not about their fertility.’ Now fertility counseling is a very important part of cancer care. We have all these tools available to us for cancer patients who don’t want to lose their fertility. This accumulated experience that has already been applied in other medical conditions … why not use that for Turner syndrome? This is my point: With all the data out there about the potential benefits and the ways to manage the risks, we shouldn’t have to tell girls, ‘Well, you have to become menopausal, and maybe you can adopt someday.’ That doesn’t sit well with parents any more. We want these girls to thrive. Not just to survive, but to have as close to a normal quality of life as possible.”
ORLANDO – Fertility preservation techniques pioneered in young cancer patients may someday allow some women with Turner syndrome to give birth to their own children, without relying on donated eggs.
Spontaneous conception and live birth are exceedingly rare among women with the genetic disorder. Until very recently, adoption was the only practical way for most to grow a family. In the last decade, however, fertility specialists in Europe and the United States have had good success with in vitro fertilization using donated eggs. Now, those clinicians are aiming for a higher goal: babies born from a patient’s own eggs, cryopreserved either individually or within whole ovarian tissue.
These are not pipe dreams, according to experts interviewed for this story. Autologous oocyte freezing is well established in healthy women and is now coming of age in cancer patients, with recent reports of live births. Ovarian tissue freezing and reimplantation is a much newer technique, also pioneered in cancer patients. To date, more than 70 live births have occurred from ovarian cortical tissue conservation and later transplantation in adults. Last December brought the first report of a live birth to a childhood cancer survivor who had prepubertal ovarian tissue frozen before chemotherapy. And a 2015 report detailed the case of a girl with primary ovarian failure secondary to sickle cell anemia treatment. At 25, she gave birth to a healthy child conceived from ovarian tissue removed when she was 14 years old.
Not all clinicians so enthusiastically embrace this future, however. A new set of consensus guidelines for the management of girls and women with Turner syndrome is in the works and will recommend a more conservative clinical approach, according to Nelly Mauras, MD, chief of endocrinology, diabetes, and metabolism at the Nemours Children’s Health System in Jacksonville, Fla.
A group of academic and patient advocacy stakeholders is cooperatively honing the document based on a meeting last summer in Cincinnati. These groups include the European Society of Endocrinology, the Endocrine Society, the U.S. Pediatric Endocrine Society, the European Society for Pediatric Endocrinology, Cardiology, and Reproductive Endocrinology, as well as Turner syndrome patient advocacy groups.
Dr. Mauras said the guideline will be “less discouraging” than the existing one issued in 2012 by the American Society of Reproductive Medicine. In its 2012 guidelines, the society identified Turner syndrome as a relative contraindication to pregnancy and an absolute contraindication in those with documented cardiac anomalies. However, greater experience has since been accrued in reproductive techniques of oocyte donation in Turner, with better outcomes.
The upcoming guidelines, however, will still recommend strongly against ovarian stimulation for fertility preservation for girls younger than 12, Dr. Mauras said, and will not recommend ovarian tissue conservation. “We just do not have the safety data and pregnancy outcomes that we need to give strong recommendations for these treatments,” she said. “These are still considered experimental treatments for girls with Turner syndrome.”
Turner syndrome, caused by a deletion of one X chromosome, throws a unique curve into the game – very early ovarian failure. Those with a complete deletion (45,X) begin losing their primordial ovarian follicles even before birth. Most will never experience spontaneous puberty; even if they do, their egg reserve is virtually gone soon after. Ovarian reserve may not be completely lost in girls with mosaicism, however, who have the X deletion in only a portion of their cells (46,XX/45,X). Some will enter puberty, and about 5% may even conceive spontaneously in younger years. But of the majority of Turner girls eventually experience complete ovarian failure.
This means that fertility preservation can’t be a wait-and-see issue, according to Kutluk Oktay, MD, PhD, a fertility specialist on the leading edge of this issue in the United States.
“We have to be proactive,” said Dr. Oktay, professor of obstetrics and gynecology at New York Medical College, Valhalla. “If we wait until girls are 12 or 13 to address this, a majority will have totally depleted their ovarian reserve by then. They will have no option other than an egg donor or adoption. We are suggesting that they should be screened as soon as they are diagnosed, and if they and their parents wish it, something should be done before it’s too late.”
Dr. Oktay is also the founder of fertilitypreservation.org, which specializes in advanced fertility treatments for cancer patients. He is one of a handful of physicians in the United States who advocate early oocyte harvesting in peripubertal girls and ovarian tissue harvesting in prepubertal girls with Turner syndrome. Last year, in conjunction with the Turner Syndrome Foundation, he and his colleagues published a set of guidelines for preserving fertility in these patients.
The paper recommends fertility assessment pathways for pre- and postpubertal girls. For both groups, Dr. Oktay employs serial assessments of ovarian reserve by monitoring several hormones, including follicle-stimulating hormone, luteinizing hormone, and antimüllerian hormone (AMH). Produced by primordial follicles, AMH declines as egg reserve declines over a lifetime, and is considered an accurate marker of ovarian reserve. When a girl experiences two consecutive AMH declines, egg depletion is probably accelerating. “This is the time to consider fertility preservation,” he said.
If a girl is peri- or postpubertal, the choice would probably be ovarian stimulation with the goal of retrieving mature oocytes. For prepubertal girls, the best choice is probably ovarian tissue cryopreservation. But because the egg reserve may already be spotty inside the ovary, he recommended freezing it en bloc, rather than preserving just cortical sections.
Because these techniques are only beginning to be used in young Turner patients, neither has been tested yet to see if it would result in a pregnancy. However, Dr. Oktay said, more than 80 babies have been born to women with other disorders who had ovaries frozen as adults. And European women with Turner syndrome have been successfully bearing children with donated oocytes for years.
“I don’t differentiate Europe from the U.S.,” he said. “We have no reason to believe Turner syndrome girls would be any different here than they are there.”
Pregnancy rates by egg donation in Turner syndrome patients are about half that typically seen in an otherwise healthy infertile woman, according to numerous sources; with a take-home baby rate of about 18%. There are numerous reasons for this. The miscarriage rate in Turner patients is about 44%. Women with Turner tend to have smaller uteri, with thinner endometrium, mostly because of the lack of estrogen.
But with careful management, those who do conceive can safely deliver a healthy baby, said Outi Hovatta, MD, a Finnish fertility specialist who has done extensive work in the area. “In Europe, we have been doing this quite liberally for years, and haven’t had a bad experience,” she said in an interview.
A 2013 review summarized both the success and the risks of these pregnancies. It examined obstetric and neonatal outcomes among 106 women with Turner syndrome who gave birth via egg donation from 1992 to 2011 in Sweden, Finland, and Denmark.
Most (70%) had a single embryo transferred, as virtually all guidelines recommend. Women with Turner are prone to cardiac and aortic defects that can worsen under the strain of pregnancy, or even present for the first time during gestation. Aortic dissection is a real threat; up to a third of patients with Turner experience it during adulthood, and it’s a major cause of death among them. In the Nordic series, 10 women (9%) had a known cardiac anomaly.
The multiple birth rate was 7%. More than a third (35%) developed a hypertensive disorder, including preeclampsia (20%). Four women had potentially life-threatening complications, including one with aortic dissection, one who developed mild regurgitation of the tricuspid and mitral valve, one with a mechanical heart valve who developed HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), and one who underwent a postpartum hysterectomy because of severe hemorrhaging.
The infants, nevertheless, did well. The preterm birth rate was 8%, with 9% of the singleton infants having a low birth weight. About 4% had a major birth defect. Of the 131 born, three died (2.3%), including a set of extremely preterm twins.
Close follow-up and cross-specialty cooperation are what make these positive outcomes possible, said Dr. Hovatta, who is now a professor emeritus at the Karolinska Institute, Sweden.
“We do everything we can to exclude things that could cause bad outcomes.” That includes extremely rigorous cardiac testing before pregnancy and continuous monitoring during it. “If a woman has any sign of cardiac anomaly, she is advised not to become pregnant. If she shows any signs of aortic dilation, we follow her extremely carefully with experienced cardiologists.”
Like Dr. Oktay, Dr. Hovatta and her European colleagues make fertility preservation an early topic of conversation. Unlike in the United States, where many girls aren’t diagnosed with the disorder until they fail to enter puberty, almost all Turner girls in Europe are identified very early in childhood. They receive early growth hormone treatment, and there is frequent consultation with interdisciplinary specialists. Fertility is spoken of early and often.
Early oocyte retrieval is common, Dr. Hovatta said. “Yes, it’s possible to wait until puberty, but for so many girls, most of the eggs have disappeared by then, so we typically don’t wait. We start looking at that option around 11 years, which is the same time we think about cryopreserving ovarian tissue.”
However, she added, as in the United States, the outcomes of these procedures are still unknown. But the existing data in other populations, the ability to carefully shepherd women through a successful pregnancy, and the willingness of families to provide the option all support further exploring them. Dr. Hovatta was at the Cincinnati gathering last summer and said she did not agree with the conservative tone she heard. Dr. Oktay also does not agree.
“This evidence we have so far is good evidence,” he said. “Look, where we are right now with Turner girls is where we were 15 years ago with cancer patients. People thought, ‘They have cancer. They should just be worrying about surviving cancer, not about their fertility.’ Now fertility counseling is a very important part of cancer care. We have all these tools available to us for cancer patients who don’t want to lose their fertility. This accumulated experience that has already been applied in other medical conditions … why not use that for Turner syndrome? This is my point: With all the data out there about the potential benefits and the ways to manage the risks, we shouldn’t have to tell girls, ‘Well, you have to become menopausal, and maybe you can adopt someday.’ That doesn’t sit well with parents any more. We want these girls to thrive. Not just to survive, but to have as close to a normal quality of life as possible.”
Postmenopausal hot flashes cut by 93% with novel nonhormonal treatment
AT ENDO 2017
ORLANDO –
After 12 weeks of treatment with the oral small molecule, women had a 93% reduction in moderate to severe hot flashes, compared with a 23% reduction for those taking placebo (P less than .0001). The effects of fezolinetant were seen earlier as well, with an 88% reduction in hot flashes at 4 weeks into the trial, compared with a 12% reduction for the placebo group (P less than .001).
The eight-site study enrolled 87 patients in a double-blind, randomized, placebo-controlled trial that assessed hot flash frequency and severity at study weeks 4 and 12. Postmenopausal women had to have frequent, moderate to severe hot flashes to qualify for enrollment, and they had to be otherwise healthy.
To capture data for a secondary endpoint, participants also completed a quality of life questionnaire. Dr. Fraser and his coinvestigators tracked safety and pharmacokinetic data by measuring levels of luteinizing hormone, follicle-stimulating hormone, estradiol, and sex hormone–binding globulin at baseline and 12 weeks, as well.
Evenly divided between study arms, 80 patients completed the trial. Two patients withdrew because of adverse events, one patient violated inclusion criteria, and the others withdrew for a variety of reasons. Mean age was similar between the two groups, at 53.7 years for those on placebo and 53.3 years for the fezolinetant group. Other anthropometric characteristics were similar, as well.
At baseline, patients taking placebo experienced a mean 10.3 moderate to severe hot flashes daily, compared with the fezolinetant group at a mean of 11.5. By study week 4, 14 of the 40 patients in the active arm had zero hot flashes, compared with 2 of 40 in the in the placebo arm, when the intention-to-treat population was examined. Hot flash severity dropped by 70% (P less than .0001).
Quality of life was assessed with the Hot Flash Related Daily Interference Scale (HFRDIS). When the two groups were compared, a significant (P less than .001) reduction in HFRDIS score was seen by week 4 and continued through to week 12 in the group on active treatment. Lower HFRDIS scores mean improved hot flash–related quality of life.
Using the Leeds Sleep Evaluation Questionnaire allowed Dr. Fraser and his colleagues to ask about how long it took patients to get to sleep while they were participating in the study, compared with their normal sleep latency. Patients taking fezolinetant reported getting to sleep significantly more quickly (P less than .01) than the placebo group. They also reported better quality of sleep (P less than .001) and a better awakening experience (P less than .05). However, they did not report feeling better after awakening (P = .08).
Women taking fezolinetant also showed significant improvement, compared with the placebo group, on other quality of life questionnaires, the Greene Climacteric Scale and the Sheehan Disability Scale. By week 8, a significant (P less than .001) improvement was seen on both scales and specifically at week 4 on the Sheehan Disability Scale.
Among the hormone biomarkers that were followed in the study, only plasma luteinizing-hormone levels dropped, compared with patients’ baseline levels. These were reduced 20% 12 hours after one of the two daily 60-mg oral doses, and 50% at 3 hours post dose, a point at which maximum serum fezolinetant concentration would be seen. These were all statistically significant reductions and expected effects of the drug’s mechanism of action.
Further pharmacokinetic analysis, said Dr. Fraser, “supports testing of once-daily dosing for vasomotor symptoms,” given that, when the drug was tested in premenopausal women in phase I clinical trials, there was no difference in peak and trough drug concentration.
The safety profile was good overall, said Dr. Fraser. No patients in the fezolinetant group reported serious treatment-emergent adverse events. “More patients reported treatment-emergent adverse events in the placebo group than in the fezolinetant group,” he said.
The NK3R antagonist is also under investigation for use in the treatment of polycystic ovary syndrome and uterine fibroids.
The study was funded by Ogeda, which employs Dr. Fraser. Another author reported serving as a clinical adviser for Ogeda.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO –
After 12 weeks of treatment with the oral small molecule, women had a 93% reduction in moderate to severe hot flashes, compared with a 23% reduction for those taking placebo (P less than .0001). The effects of fezolinetant were seen earlier as well, with an 88% reduction in hot flashes at 4 weeks into the trial, compared with a 12% reduction for the placebo group (P less than .001).
The eight-site study enrolled 87 patients in a double-blind, randomized, placebo-controlled trial that assessed hot flash frequency and severity at study weeks 4 and 12. Postmenopausal women had to have frequent, moderate to severe hot flashes to qualify for enrollment, and they had to be otherwise healthy.
To capture data for a secondary endpoint, participants also completed a quality of life questionnaire. Dr. Fraser and his coinvestigators tracked safety and pharmacokinetic data by measuring levels of luteinizing hormone, follicle-stimulating hormone, estradiol, and sex hormone–binding globulin at baseline and 12 weeks, as well.
Evenly divided between study arms, 80 patients completed the trial. Two patients withdrew because of adverse events, one patient violated inclusion criteria, and the others withdrew for a variety of reasons. Mean age was similar between the two groups, at 53.7 years for those on placebo and 53.3 years for the fezolinetant group. Other anthropometric characteristics were similar, as well.
At baseline, patients taking placebo experienced a mean 10.3 moderate to severe hot flashes daily, compared with the fezolinetant group at a mean of 11.5. By study week 4, 14 of the 40 patients in the active arm had zero hot flashes, compared with 2 of 40 in the in the placebo arm, when the intention-to-treat population was examined. Hot flash severity dropped by 70% (P less than .0001).
Quality of life was assessed with the Hot Flash Related Daily Interference Scale (HFRDIS). When the two groups were compared, a significant (P less than .001) reduction in HFRDIS score was seen by week 4 and continued through to week 12 in the group on active treatment. Lower HFRDIS scores mean improved hot flash–related quality of life.
Using the Leeds Sleep Evaluation Questionnaire allowed Dr. Fraser and his colleagues to ask about how long it took patients to get to sleep while they were participating in the study, compared with their normal sleep latency. Patients taking fezolinetant reported getting to sleep significantly more quickly (P less than .01) than the placebo group. They also reported better quality of sleep (P less than .001) and a better awakening experience (P less than .05). However, they did not report feeling better after awakening (P = .08).
Women taking fezolinetant also showed significant improvement, compared with the placebo group, on other quality of life questionnaires, the Greene Climacteric Scale and the Sheehan Disability Scale. By week 8, a significant (P less than .001) improvement was seen on both scales and specifically at week 4 on the Sheehan Disability Scale.
Among the hormone biomarkers that were followed in the study, only plasma luteinizing-hormone levels dropped, compared with patients’ baseline levels. These were reduced 20% 12 hours after one of the two daily 60-mg oral doses, and 50% at 3 hours post dose, a point at which maximum serum fezolinetant concentration would be seen. These were all statistically significant reductions and expected effects of the drug’s mechanism of action.
Further pharmacokinetic analysis, said Dr. Fraser, “supports testing of once-daily dosing for vasomotor symptoms,” given that, when the drug was tested in premenopausal women in phase I clinical trials, there was no difference in peak and trough drug concentration.
The safety profile was good overall, said Dr. Fraser. No patients in the fezolinetant group reported serious treatment-emergent adverse events. “More patients reported treatment-emergent adverse events in the placebo group than in the fezolinetant group,” he said.
The NK3R antagonist is also under investigation for use in the treatment of polycystic ovary syndrome and uterine fibroids.
The study was funded by Ogeda, which employs Dr. Fraser. Another author reported serving as a clinical adviser for Ogeda.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO –
After 12 weeks of treatment with the oral small molecule, women had a 93% reduction in moderate to severe hot flashes, compared with a 23% reduction for those taking placebo (P less than .0001). The effects of fezolinetant were seen earlier as well, with an 88% reduction in hot flashes at 4 weeks into the trial, compared with a 12% reduction for the placebo group (P less than .001).
The eight-site study enrolled 87 patients in a double-blind, randomized, placebo-controlled trial that assessed hot flash frequency and severity at study weeks 4 and 12. Postmenopausal women had to have frequent, moderate to severe hot flashes to qualify for enrollment, and they had to be otherwise healthy.
To capture data for a secondary endpoint, participants also completed a quality of life questionnaire. Dr. Fraser and his coinvestigators tracked safety and pharmacokinetic data by measuring levels of luteinizing hormone, follicle-stimulating hormone, estradiol, and sex hormone–binding globulin at baseline and 12 weeks, as well.
Evenly divided between study arms, 80 patients completed the trial. Two patients withdrew because of adverse events, one patient violated inclusion criteria, and the others withdrew for a variety of reasons. Mean age was similar between the two groups, at 53.7 years for those on placebo and 53.3 years for the fezolinetant group. Other anthropometric characteristics were similar, as well.
At baseline, patients taking placebo experienced a mean 10.3 moderate to severe hot flashes daily, compared with the fezolinetant group at a mean of 11.5. By study week 4, 14 of the 40 patients in the active arm had zero hot flashes, compared with 2 of 40 in the in the placebo arm, when the intention-to-treat population was examined. Hot flash severity dropped by 70% (P less than .0001).
Quality of life was assessed with the Hot Flash Related Daily Interference Scale (HFRDIS). When the two groups were compared, a significant (P less than .001) reduction in HFRDIS score was seen by week 4 and continued through to week 12 in the group on active treatment. Lower HFRDIS scores mean improved hot flash–related quality of life.
Using the Leeds Sleep Evaluation Questionnaire allowed Dr. Fraser and his colleagues to ask about how long it took patients to get to sleep while they were participating in the study, compared with their normal sleep latency. Patients taking fezolinetant reported getting to sleep significantly more quickly (P less than .01) than the placebo group. They also reported better quality of sleep (P less than .001) and a better awakening experience (P less than .05). However, they did not report feeling better after awakening (P = .08).
Women taking fezolinetant also showed significant improvement, compared with the placebo group, on other quality of life questionnaires, the Greene Climacteric Scale and the Sheehan Disability Scale. By week 8, a significant (P less than .001) improvement was seen on both scales and specifically at week 4 on the Sheehan Disability Scale.
Among the hormone biomarkers that were followed in the study, only plasma luteinizing-hormone levels dropped, compared with patients’ baseline levels. These were reduced 20% 12 hours after one of the two daily 60-mg oral doses, and 50% at 3 hours post dose, a point at which maximum serum fezolinetant concentration would be seen. These were all statistically significant reductions and expected effects of the drug’s mechanism of action.
Further pharmacokinetic analysis, said Dr. Fraser, “supports testing of once-daily dosing for vasomotor symptoms,” given that, when the drug was tested in premenopausal women in phase I clinical trials, there was no difference in peak and trough drug concentration.
The safety profile was good overall, said Dr. Fraser. No patients in the fezolinetant group reported serious treatment-emergent adverse events. “More patients reported treatment-emergent adverse events in the placebo group than in the fezolinetant group,” he said.
The NK3R antagonist is also under investigation for use in the treatment of polycystic ovary syndrome and uterine fibroids.
The study was funded by Ogeda, which employs Dr. Fraser. Another author reported serving as a clinical adviser for Ogeda.
[email protected]
On Twitter @karioakes
Key clinical point: .
Major finding: Women on fezolinetant had a 93% drop in hot flash frequency, compared with a 23% reduction for those on placebo (P less than .0001).
Data source: A phase II randomized, double-blind, placebo-controlled clinical trial of 87 postmenopausal women with moderate to severe hot flashes.
Disclosures: The study was funded by Ogeda, which employs Dr. Fraser. Another author reported serving as a clinical adviser for Ogeda.
In PCOS, too much sitting means higher glucose levels
ORLANDO – Prolonged time spent sitting was associated with higher post–glucose tolerance test blood glucose levels among less-active women with polycystic ovary syndrome (PCOS).
The trend toward this effect persisted even after researchers controlled for age and body mass index, and was not seen in women who were more active.
The results showed a “compounded adverse metabolic effect of prolonged sitting time in women with PCOS who do not achieve exercise goals,” according to Eleni Greenwood, MD, and her colleagues at the University of California, San Francisco, department of obstetrics, gynecology, and reproductive sciences.
“In PCOS, insulin resistance is tissue specific and occurs in the skeletal muscle,” said Dr. Greenwood, a fellow in the reproductive endocrinology and infertility program at the University of California, San Francisco. Diet and exercise are primary interventions to help with these sequelae. In the general population, prolonged time spent being sedentary is associated with type 2 diabetes, cardiovascular disease, and even some cancers.
Because “the adverse effects of sitting are not reversed through exercise,” as Dr. Greenwood and her colleagues pointed out, the study sought to ascertain whether worse metabolic parameters would be seen in women with PCOS who had had more sedentary time, and whether the association would be independent of exercise status.
Accordingly, the investigators conducted a cross-sectional study of 324 women who met Rotterdam criteria for PCOS. The results were presented during a poster session at the annual meeting of the Endocrine Society.
Patients took the International Physical Activity Questionnaire, and responses were used to determine activity levels and amounts of sedentary times. Other measurements taken at the interdisciplinary clinic where the patients were seen included anthropometric measurements, as well as the results of serum lipid levels, fasting glucose and insulin levels, and the results of a 75-g, 2-hour oral glucose tolerance test (OGTT).
In their analysis, the investigators calculated homeostasis model assessment of insulin resistance (HOMA-IR), and used multivariable analysis to find correlations and eliminate potentially confounding variables. In a further attempt to eliminate confounders, Dr. Greenwood and her colleagues asked patients to stop hormonal contraceptive methods and insulin sensitizing medications 30 days before beginning the study.
The investigators looked at the women’s exercise status, meaning whether they had achieved the level of activity recommended by the U.S. Department of Health & Human Services. However, they also asked the women to report how sedentary they were, measured by the number of hours spent sitting in a day.
It would theoretically be possible for an individual to be very “active,” exercising vigorously for 2 hours each day, but also very “sedentary,” sitting for much of the rest of her waking hours.
Overall, two-thirds of the women (217, 67%) met the activity goals outlined by the HHS. That consisted of exercising enough to achieve 600 metabolic equivalents per week. If the women sat for more than 6 hours a day, they were judged to be sedentary. Of the inactive women, 35% (37) sat for 6 or fewer hours per day, compared with 44% (94) of the active women.
Though the results did not reach statistical significance, HOMA-IR and post-OGTT glucose levels tended to be lower among those who sat less (1.93 vs. 2.73, P = .10; 99 mg/dL vs. 107 mg/dL, P = .09).
Looking at just the inactive group, less sitting time was associated with significantly lower post-OGTT glucose levels (99.1 mg/dL vs. 117.6 mg/dL, P = .03). This difference was not seen among the group of women judged to be active.
“Our results indicate a compounded adverse metabolic effect of prolonged sitting time in women with PCOS who do not achieve exercise goals,” wrote Dr. Greenwood and her collaborators.
Because PCOS pathophysiology can be “disrupted” by an improvement in insulin sensitivity and overall metabolic health, “women with PCOS should be counseled regarding strategies for reducing sedentary time, in addition to improving exercise and diet, as a means of improving metabolic health,” they said.
Dr. Greenwood and her colleagues reported no relevant disclosures.
[email protected]
On Twitter @karioakes
ORLANDO – Prolonged time spent sitting was associated with higher post–glucose tolerance test blood glucose levels among less-active women with polycystic ovary syndrome (PCOS).
The trend toward this effect persisted even after researchers controlled for age and body mass index, and was not seen in women who were more active.
The results showed a “compounded adverse metabolic effect of prolonged sitting time in women with PCOS who do not achieve exercise goals,” according to Eleni Greenwood, MD, and her colleagues at the University of California, San Francisco, department of obstetrics, gynecology, and reproductive sciences.
“In PCOS, insulin resistance is tissue specific and occurs in the skeletal muscle,” said Dr. Greenwood, a fellow in the reproductive endocrinology and infertility program at the University of California, San Francisco. Diet and exercise are primary interventions to help with these sequelae. In the general population, prolonged time spent being sedentary is associated with type 2 diabetes, cardiovascular disease, and even some cancers.
Because “the adverse effects of sitting are not reversed through exercise,” as Dr. Greenwood and her colleagues pointed out, the study sought to ascertain whether worse metabolic parameters would be seen in women with PCOS who had had more sedentary time, and whether the association would be independent of exercise status.
Accordingly, the investigators conducted a cross-sectional study of 324 women who met Rotterdam criteria for PCOS. The results were presented during a poster session at the annual meeting of the Endocrine Society.
Patients took the International Physical Activity Questionnaire, and responses were used to determine activity levels and amounts of sedentary times. Other measurements taken at the interdisciplinary clinic where the patients were seen included anthropometric measurements, as well as the results of serum lipid levels, fasting glucose and insulin levels, and the results of a 75-g, 2-hour oral glucose tolerance test (OGTT).
In their analysis, the investigators calculated homeostasis model assessment of insulin resistance (HOMA-IR), and used multivariable analysis to find correlations and eliminate potentially confounding variables. In a further attempt to eliminate confounders, Dr. Greenwood and her colleagues asked patients to stop hormonal contraceptive methods and insulin sensitizing medications 30 days before beginning the study.
The investigators looked at the women’s exercise status, meaning whether they had achieved the level of activity recommended by the U.S. Department of Health & Human Services. However, they also asked the women to report how sedentary they were, measured by the number of hours spent sitting in a day.
It would theoretically be possible for an individual to be very “active,” exercising vigorously for 2 hours each day, but also very “sedentary,” sitting for much of the rest of her waking hours.
Overall, two-thirds of the women (217, 67%) met the activity goals outlined by the HHS. That consisted of exercising enough to achieve 600 metabolic equivalents per week. If the women sat for more than 6 hours a day, they were judged to be sedentary. Of the inactive women, 35% (37) sat for 6 or fewer hours per day, compared with 44% (94) of the active women.
Though the results did not reach statistical significance, HOMA-IR and post-OGTT glucose levels tended to be lower among those who sat less (1.93 vs. 2.73, P = .10; 99 mg/dL vs. 107 mg/dL, P = .09).
Looking at just the inactive group, less sitting time was associated with significantly lower post-OGTT glucose levels (99.1 mg/dL vs. 117.6 mg/dL, P = .03). This difference was not seen among the group of women judged to be active.
“Our results indicate a compounded adverse metabolic effect of prolonged sitting time in women with PCOS who do not achieve exercise goals,” wrote Dr. Greenwood and her collaborators.
Because PCOS pathophysiology can be “disrupted” by an improvement in insulin sensitivity and overall metabolic health, “women with PCOS should be counseled regarding strategies for reducing sedentary time, in addition to improving exercise and diet, as a means of improving metabolic health,” they said.
Dr. Greenwood and her colleagues reported no relevant disclosures.
[email protected]
On Twitter @karioakes
ORLANDO – Prolonged time spent sitting was associated with higher post–glucose tolerance test blood glucose levels among less-active women with polycystic ovary syndrome (PCOS).
The trend toward this effect persisted even after researchers controlled for age and body mass index, and was not seen in women who were more active.
The results showed a “compounded adverse metabolic effect of prolonged sitting time in women with PCOS who do not achieve exercise goals,” according to Eleni Greenwood, MD, and her colleagues at the University of California, San Francisco, department of obstetrics, gynecology, and reproductive sciences.
“In PCOS, insulin resistance is tissue specific and occurs in the skeletal muscle,” said Dr. Greenwood, a fellow in the reproductive endocrinology and infertility program at the University of California, San Francisco. Diet and exercise are primary interventions to help with these sequelae. In the general population, prolonged time spent being sedentary is associated with type 2 diabetes, cardiovascular disease, and even some cancers.
Because “the adverse effects of sitting are not reversed through exercise,” as Dr. Greenwood and her colleagues pointed out, the study sought to ascertain whether worse metabolic parameters would be seen in women with PCOS who had had more sedentary time, and whether the association would be independent of exercise status.
Accordingly, the investigators conducted a cross-sectional study of 324 women who met Rotterdam criteria for PCOS. The results were presented during a poster session at the annual meeting of the Endocrine Society.
Patients took the International Physical Activity Questionnaire, and responses were used to determine activity levels and amounts of sedentary times. Other measurements taken at the interdisciplinary clinic where the patients were seen included anthropometric measurements, as well as the results of serum lipid levels, fasting glucose and insulin levels, and the results of a 75-g, 2-hour oral glucose tolerance test (OGTT).
In their analysis, the investigators calculated homeostasis model assessment of insulin resistance (HOMA-IR), and used multivariable analysis to find correlations and eliminate potentially confounding variables. In a further attempt to eliminate confounders, Dr. Greenwood and her colleagues asked patients to stop hormonal contraceptive methods and insulin sensitizing medications 30 days before beginning the study.
The investigators looked at the women’s exercise status, meaning whether they had achieved the level of activity recommended by the U.S. Department of Health & Human Services. However, they also asked the women to report how sedentary they were, measured by the number of hours spent sitting in a day.
It would theoretically be possible for an individual to be very “active,” exercising vigorously for 2 hours each day, but also very “sedentary,” sitting for much of the rest of her waking hours.
Overall, two-thirds of the women (217, 67%) met the activity goals outlined by the HHS. That consisted of exercising enough to achieve 600 metabolic equivalents per week. If the women sat for more than 6 hours a day, they were judged to be sedentary. Of the inactive women, 35% (37) sat for 6 or fewer hours per day, compared with 44% (94) of the active women.
Though the results did not reach statistical significance, HOMA-IR and post-OGTT glucose levels tended to be lower among those who sat less (1.93 vs. 2.73, P = .10; 99 mg/dL vs. 107 mg/dL, P = .09).
Looking at just the inactive group, less sitting time was associated with significantly lower post-OGTT glucose levels (99.1 mg/dL vs. 117.6 mg/dL, P = .03). This difference was not seen among the group of women judged to be active.
“Our results indicate a compounded adverse metabolic effect of prolonged sitting time in women with PCOS who do not achieve exercise goals,” wrote Dr. Greenwood and her collaborators.
Because PCOS pathophysiology can be “disrupted” by an improvement in insulin sensitivity and overall metabolic health, “women with PCOS should be counseled regarding strategies for reducing sedentary time, in addition to improving exercise and diet, as a means of improving metabolic health,” they said.
Dr. Greenwood and her colleagues reported no relevant disclosures.
[email protected]
On Twitter @karioakes
AT ENDO 2017
Key clinical point:
Major finding: Less-active women who also had prolonged sitting time had significantly higher post–oral glucose tolerance test levels (99.1 mg/dL vs. 117.6 mg/dL, P = .03).
Data source: Cross-sectional study of 324 women who met Rotterdam criteria for PCOS.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
One in four practitioners doing FNAs are endocrinologists
AT ENDO 2017
ORLANDO – Endocrinologists made up about one in four of the practitioners performing fine needle aspiration (FNA) biopsies between 2012 and 2014, according to a review of data from the Centers for Medicare & Medicaid Services.
Similarly, endocrinologists performed 25% of image-guided thyroid biopsies.
Endocrine surgeons represent only a small percentage of all practitioners performing head and neck ultrasound exams and image-guided FNA, lead author Mamoona Khokhar, MD, said during a poster presentation at the annual meeting of the Endocrine Society. This is true even though the more portable nature of ultrasound has made it easier for motivated surgeons to incorporate its use into their practice, she said.
Examining 3 years of data from a provider utilization and payment database, Dr. Khokhar and her colleagues identified the types of practitioners who performed head and neck ultrasound, as well as image-guided FNA.
In their analysis, the researchers broadly divided practitioners into surgeons and nonsurgeons. Overall, of the 14,750 median annual practitioners performing head and neck ultrasound between 2012 and 2014, 97.2% were nonsurgeon practitioners, reported Dr. Khokhar, an endocrine surgery fellow at Columbia University Medical Center in New York.
Of all practitioners performing head and neck ultrasound, most (81%) were radiologists. Endocrinologists made up 8% of the overall pool performing ultrasounds.
Breaking the surgeon group down further showed that endocrine surgeons represented 14.7% of surgeons performing head and neck ultrasound, meaning that they made up just 0.4% of the practitioner pool for this procedure. Just over half (52%) of the surgeons performing ultrasounds were otolaryngologists.
The number of practitioners performing image-guided FNA was smaller, at a median 3,695 per year during the study period. Surgeons made up 10.7% of this number. Of the surgeons who performed image-guided FNA, 10.5% were endocrine surgeons. Endocrine surgeons made up 1.1% of all practitioners who billed for FNA.
Again, radiologists made up the majority (58%) of the practitioners performing FNA, and one in four (25%) of practitioners performing FNAs were endocrinologists. Just 5% of the practitioners performing FNAs were otolaryngologists.
More endocrine surgeons performed ultrasound than advanced practice providers (nurse practitioners or physician assistants, 0.2%), pathologists (0.1%), and surgical oncologists (0.04%; P for all, less than .0001). However, advanced practice providers and pathologists both performed significantly more FNAs than did endocrine surgeons (2.1% and 1.8%, P less than .0001).
Although the raw proportion of endocrine surgeons billing for these procedures increased during the study period, the increases were not statistically significant. Dr. Khokhar and her colleagues found that the proportion of American Association of Endocrine Surgeons members who performed head and neck ultrasound grew from 59% in 2012 to 72% in 2014 (P = 0.37), while the proportion performing FNA also increased, from 36% in 2012 to 46% in 2014 (P = .40).
Surgeons, however, may face a number of obstacles in setting up office-based ultrasound, which can lead to underutilization by surgeons, Dr. Khokhar noted, adding that “the results of this study suggest that endocrine surgeons may not be fully utilizing this critical tool in their clinical practice.”
The authors reported no outside sources of funding, and had no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Endocrinologists made up about one in four of the practitioners performing fine needle aspiration (FNA) biopsies between 2012 and 2014, according to a review of data from the Centers for Medicare & Medicaid Services.
Similarly, endocrinologists performed 25% of image-guided thyroid biopsies.
Endocrine surgeons represent only a small percentage of all practitioners performing head and neck ultrasound exams and image-guided FNA, lead author Mamoona Khokhar, MD, said during a poster presentation at the annual meeting of the Endocrine Society. This is true even though the more portable nature of ultrasound has made it easier for motivated surgeons to incorporate its use into their practice, she said.
Examining 3 years of data from a provider utilization and payment database, Dr. Khokhar and her colleagues identified the types of practitioners who performed head and neck ultrasound, as well as image-guided FNA.
In their analysis, the researchers broadly divided practitioners into surgeons and nonsurgeons. Overall, of the 14,750 median annual practitioners performing head and neck ultrasound between 2012 and 2014, 97.2% were nonsurgeon practitioners, reported Dr. Khokhar, an endocrine surgery fellow at Columbia University Medical Center in New York.
Of all practitioners performing head and neck ultrasound, most (81%) were radiologists. Endocrinologists made up 8% of the overall pool performing ultrasounds.
Breaking the surgeon group down further showed that endocrine surgeons represented 14.7% of surgeons performing head and neck ultrasound, meaning that they made up just 0.4% of the practitioner pool for this procedure. Just over half (52%) of the surgeons performing ultrasounds were otolaryngologists.
The number of practitioners performing image-guided FNA was smaller, at a median 3,695 per year during the study period. Surgeons made up 10.7% of this number. Of the surgeons who performed image-guided FNA, 10.5% were endocrine surgeons. Endocrine surgeons made up 1.1% of all practitioners who billed for FNA.
Again, radiologists made up the majority (58%) of the practitioners performing FNA, and one in four (25%) of practitioners performing FNAs were endocrinologists. Just 5% of the practitioners performing FNAs were otolaryngologists.
More endocrine surgeons performed ultrasound than advanced practice providers (nurse practitioners or physician assistants, 0.2%), pathologists (0.1%), and surgical oncologists (0.04%; P for all, less than .0001). However, advanced practice providers and pathologists both performed significantly more FNAs than did endocrine surgeons (2.1% and 1.8%, P less than .0001).
Although the raw proportion of endocrine surgeons billing for these procedures increased during the study period, the increases were not statistically significant. Dr. Khokhar and her colleagues found that the proportion of American Association of Endocrine Surgeons members who performed head and neck ultrasound grew from 59% in 2012 to 72% in 2014 (P = 0.37), while the proportion performing FNA also increased, from 36% in 2012 to 46% in 2014 (P = .40).
Surgeons, however, may face a number of obstacles in setting up office-based ultrasound, which can lead to underutilization by surgeons, Dr. Khokhar noted, adding that “the results of this study suggest that endocrine surgeons may not be fully utilizing this critical tool in their clinical practice.”
The authors reported no outside sources of funding, and had no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Endocrinologists made up about one in four of the practitioners performing fine needle aspiration (FNA) biopsies between 2012 and 2014, according to a review of data from the Centers for Medicare & Medicaid Services.
Similarly, endocrinologists performed 25% of image-guided thyroid biopsies.
Endocrine surgeons represent only a small percentage of all practitioners performing head and neck ultrasound exams and image-guided FNA, lead author Mamoona Khokhar, MD, said during a poster presentation at the annual meeting of the Endocrine Society. This is true even though the more portable nature of ultrasound has made it easier for motivated surgeons to incorporate its use into their practice, she said.
Examining 3 years of data from a provider utilization and payment database, Dr. Khokhar and her colleagues identified the types of practitioners who performed head and neck ultrasound, as well as image-guided FNA.
In their analysis, the researchers broadly divided practitioners into surgeons and nonsurgeons. Overall, of the 14,750 median annual practitioners performing head and neck ultrasound between 2012 and 2014, 97.2% were nonsurgeon practitioners, reported Dr. Khokhar, an endocrine surgery fellow at Columbia University Medical Center in New York.
Of all practitioners performing head and neck ultrasound, most (81%) were radiologists. Endocrinologists made up 8% of the overall pool performing ultrasounds.
Breaking the surgeon group down further showed that endocrine surgeons represented 14.7% of surgeons performing head and neck ultrasound, meaning that they made up just 0.4% of the practitioner pool for this procedure. Just over half (52%) of the surgeons performing ultrasounds were otolaryngologists.
The number of practitioners performing image-guided FNA was smaller, at a median 3,695 per year during the study period. Surgeons made up 10.7% of this number. Of the surgeons who performed image-guided FNA, 10.5% were endocrine surgeons. Endocrine surgeons made up 1.1% of all practitioners who billed for FNA.
Again, radiologists made up the majority (58%) of the practitioners performing FNA, and one in four (25%) of practitioners performing FNAs were endocrinologists. Just 5% of the practitioners performing FNAs were otolaryngologists.
More endocrine surgeons performed ultrasound than advanced practice providers (nurse practitioners or physician assistants, 0.2%), pathologists (0.1%), and surgical oncologists (0.04%; P for all, less than .0001). However, advanced practice providers and pathologists both performed significantly more FNAs than did endocrine surgeons (2.1% and 1.8%, P less than .0001).
Although the raw proportion of endocrine surgeons billing for these procedures increased during the study period, the increases were not statistically significant. Dr. Khokhar and her colleagues found that the proportion of American Association of Endocrine Surgeons members who performed head and neck ultrasound grew from 59% in 2012 to 72% in 2014 (P = 0.37), while the proportion performing FNA also increased, from 36% in 2012 to 46% in 2014 (P = .40).
Surgeons, however, may face a number of obstacles in setting up office-based ultrasound, which can lead to underutilization by surgeons, Dr. Khokhar noted, adding that “the results of this study suggest that endocrine surgeons may not be fully utilizing this critical tool in their clinical practice.”
The authors reported no outside sources of funding, and had no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: Endocrinologists made up 25% of the practitioners performing FNAs.
Data source: A retrospective analysis of 3 years’ worth of data on head and neck ultrasound and fine needle aspiration from the Centers for Medicare & Medicaid Services.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
Reduced-intensity conditioning may not preserve fertility in young girls after bone marrow transplant
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Anti-Müllerian hormone was abnormally low or absent in all treated girls, whether they had reduced-intensity or high-intensity conditioning.
Data source: The prospective study is following 49 females aged 1-40 years.
Disclosures: Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Low-calorie sweeteners may allow more glucose to enter fat cells
ORLANDO – Consumption of low-calorie sweeteners was associated with upregulation of gene expression for glucose transporters in experiments using human mesenchymal stromal cells and adipose tissue, according to new research.
The effect was strongest in individuals with obesity.
Using human subcutaneous fat tissue taken from individuals who consumed low-calorie sweeteners, Sabyasachi Sen, MD, of George Washington University, Washington, and his colleagues showed that these cells had at least a twofold overexpression of glucose transporters. Also overexpressed were sweet taste receptors and adipogenic genes.
Dr. Sen and his colleagues first tested the effects of both physiologic and supraphysiologic doses of sucralose on human adipose-derived mesenchymal stromal cells (MSCs). In the presence of adipogenic media, sucralose was added to the MSCs for a period of 12 days.
When sucralose was added at a concentration of 0.2 millimoles (mM), the investigators detected “small but reproducible” increases in expression of adiponectin, as well as genes that encode for the production of fatty acid binding proteins (FABP4) and proteins implicated in body weight homeostasis (CEBPA). Increases ranged from 1.04- to 1.32-fold.
Dr. Sen explained that the 0.2 mM concentration approximates the amount of sucralose in the tissues of individuals with high low-calorie sweetener consumption.
These effects were more pronounced when the sucralose concentration was raised to a supraphysiologic 1 mM. Then, CEBPA expression increased by a factor of 3.45, adiponectin by 4.39, and FABP4 by 4.06. More intracellular fat droplets were seen with higher sucralose concentrations in a “dose dependent fashion,” said Dr. Sen and his colleagues.
The researchers also took subcutaneous fat biopsies from four individuals of healthy weight (body mass index [BMI] less than 24.9 kg/m2) and from four individuals with obesity (BMI greater than 24.9). Participants ranged in age from 36 years to 60 years.
Individuals in both groups had kept a 7-day food diary before the biopsies were taken, so Dr. Sen and his coinvestigators knew whether or not they had consumed low-calorie sweeteners and in what quantity. Reported sweeteners were sucralose, acesulfame-potassium, and aspartame.
The adipose tissue biopsies were examined for mRNA expression of adipogenic genes, as well as for glucose transporters and inflammatory markers.
In individuals with low-calorie sweetener exposure, the glucose transporter GLUT1 was overexpressed by 2- to 2.2-fold, while the glucose transporter GLUT4 was overexpressed by a factor of 2.7-4.3.
In addition, low-calorie sweetener consumption was associated with up to 2.5-fold overexpression of the sweet taste receptor TAS1R3. Sweet taste receptors are G protein–coupled receptors that are present in taste buds and in other tissues, serving as carbohydrate sensors.
Adipogenic genes were also overexpressed; a 1.5- to 2.4-fold increase was seen in PPARG, for example.
“This expression pattern was most apparent in obese individuals,” the investigators wrote in the abstract accompanying the study, which was presented at the annual meeting of the Endocrine Society.
Further and larger studies are called for, especially in individuals with diabetes and obesity, Dr. Sen said. “However, from our study, we believe that low-calorie sweeteners promote additional fat formation by allowing more glucose to enter the cells, and promote inflammation, which may be more detrimental to obese individuals.”
Dr. Sen and his collaborators reported no relevant disclosures.
[email protected]
On Twitter @karioakes
ORLANDO – Consumption of low-calorie sweeteners was associated with upregulation of gene expression for glucose transporters in experiments using human mesenchymal stromal cells and adipose tissue, according to new research.
The effect was strongest in individuals with obesity.
Using human subcutaneous fat tissue taken from individuals who consumed low-calorie sweeteners, Sabyasachi Sen, MD, of George Washington University, Washington, and his colleagues showed that these cells had at least a twofold overexpression of glucose transporters. Also overexpressed were sweet taste receptors and adipogenic genes.
Dr. Sen and his colleagues first tested the effects of both physiologic and supraphysiologic doses of sucralose on human adipose-derived mesenchymal stromal cells (MSCs). In the presence of adipogenic media, sucralose was added to the MSCs for a period of 12 days.
When sucralose was added at a concentration of 0.2 millimoles (mM), the investigators detected “small but reproducible” increases in expression of adiponectin, as well as genes that encode for the production of fatty acid binding proteins (FABP4) and proteins implicated in body weight homeostasis (CEBPA). Increases ranged from 1.04- to 1.32-fold.
Dr. Sen explained that the 0.2 mM concentration approximates the amount of sucralose in the tissues of individuals with high low-calorie sweetener consumption.
These effects were more pronounced when the sucralose concentration was raised to a supraphysiologic 1 mM. Then, CEBPA expression increased by a factor of 3.45, adiponectin by 4.39, and FABP4 by 4.06. More intracellular fat droplets were seen with higher sucralose concentrations in a “dose dependent fashion,” said Dr. Sen and his colleagues.
The researchers also took subcutaneous fat biopsies from four individuals of healthy weight (body mass index [BMI] less than 24.9 kg/m2) and from four individuals with obesity (BMI greater than 24.9). Participants ranged in age from 36 years to 60 years.
Individuals in both groups had kept a 7-day food diary before the biopsies were taken, so Dr. Sen and his coinvestigators knew whether or not they had consumed low-calorie sweeteners and in what quantity. Reported sweeteners were sucralose, acesulfame-potassium, and aspartame.
The adipose tissue biopsies were examined for mRNA expression of adipogenic genes, as well as for glucose transporters and inflammatory markers.
In individuals with low-calorie sweetener exposure, the glucose transporter GLUT1 was overexpressed by 2- to 2.2-fold, while the glucose transporter GLUT4 was overexpressed by a factor of 2.7-4.3.
In addition, low-calorie sweetener consumption was associated with up to 2.5-fold overexpression of the sweet taste receptor TAS1R3. Sweet taste receptors are G protein–coupled receptors that are present in taste buds and in other tissues, serving as carbohydrate sensors.
Adipogenic genes were also overexpressed; a 1.5- to 2.4-fold increase was seen in PPARG, for example.
“This expression pattern was most apparent in obese individuals,” the investigators wrote in the abstract accompanying the study, which was presented at the annual meeting of the Endocrine Society.
Further and larger studies are called for, especially in individuals with diabetes and obesity, Dr. Sen said. “However, from our study, we believe that low-calorie sweeteners promote additional fat formation by allowing more glucose to enter the cells, and promote inflammation, which may be more detrimental to obese individuals.”
Dr. Sen and his collaborators reported no relevant disclosures.
[email protected]
On Twitter @karioakes
ORLANDO – Consumption of low-calorie sweeteners was associated with upregulation of gene expression for glucose transporters in experiments using human mesenchymal stromal cells and adipose tissue, according to new research.
The effect was strongest in individuals with obesity.
Using human subcutaneous fat tissue taken from individuals who consumed low-calorie sweeteners, Sabyasachi Sen, MD, of George Washington University, Washington, and his colleagues showed that these cells had at least a twofold overexpression of glucose transporters. Also overexpressed were sweet taste receptors and adipogenic genes.
Dr. Sen and his colleagues first tested the effects of both physiologic and supraphysiologic doses of sucralose on human adipose-derived mesenchymal stromal cells (MSCs). In the presence of adipogenic media, sucralose was added to the MSCs for a period of 12 days.
When sucralose was added at a concentration of 0.2 millimoles (mM), the investigators detected “small but reproducible” increases in expression of adiponectin, as well as genes that encode for the production of fatty acid binding proteins (FABP4) and proteins implicated in body weight homeostasis (CEBPA). Increases ranged from 1.04- to 1.32-fold.
Dr. Sen explained that the 0.2 mM concentration approximates the amount of sucralose in the tissues of individuals with high low-calorie sweetener consumption.
These effects were more pronounced when the sucralose concentration was raised to a supraphysiologic 1 mM. Then, CEBPA expression increased by a factor of 3.45, adiponectin by 4.39, and FABP4 by 4.06. More intracellular fat droplets were seen with higher sucralose concentrations in a “dose dependent fashion,” said Dr. Sen and his colleagues.
The researchers also took subcutaneous fat biopsies from four individuals of healthy weight (body mass index [BMI] less than 24.9 kg/m2) and from four individuals with obesity (BMI greater than 24.9). Participants ranged in age from 36 years to 60 years.
Individuals in both groups had kept a 7-day food diary before the biopsies were taken, so Dr. Sen and his coinvestigators knew whether or not they had consumed low-calorie sweeteners and in what quantity. Reported sweeteners were sucralose, acesulfame-potassium, and aspartame.
The adipose tissue biopsies were examined for mRNA expression of adipogenic genes, as well as for glucose transporters and inflammatory markers.
In individuals with low-calorie sweetener exposure, the glucose transporter GLUT1 was overexpressed by 2- to 2.2-fold, while the glucose transporter GLUT4 was overexpressed by a factor of 2.7-4.3.
In addition, low-calorie sweetener consumption was associated with up to 2.5-fold overexpression of the sweet taste receptor TAS1R3. Sweet taste receptors are G protein–coupled receptors that are present in taste buds and in other tissues, serving as carbohydrate sensors.
Adipogenic genes were also overexpressed; a 1.5- to 2.4-fold increase was seen in PPARG, for example.
“This expression pattern was most apparent in obese individuals,” the investigators wrote in the abstract accompanying the study, which was presented at the annual meeting of the Endocrine Society.
Further and larger studies are called for, especially in individuals with diabetes and obesity, Dr. Sen said. “However, from our study, we believe that low-calorie sweeteners promote additional fat formation by allowing more glucose to enter the cells, and promote inflammation, which may be more detrimental to obese individuals.”
Dr. Sen and his collaborators reported no relevant disclosures.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: Glucose transporters and sweet taste receptors were upregulated by 2.0- to 4.3-fold in participants who used low-calorie sweeteners.
Data source: In vitro examination of human adipose tissue-derived mesenchymal stromal cells, and examination of biopsied adipose tissue from eight individuals with varying low-calorie sweetener intake.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
Simple changes shorten door-to-insulin time for diabetic ketoacidosis
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Overall door-to-insulin time decreased from 168 minutes to 146 minutes.
Data source: A 2-year quality improvement project involving 291 patients.
Disclosures: Dr. Vajravelu had no relevant financial disclosures.
Neurokinin receptor antagonist nearly halves hot flashes
ORLANDO – A novel antagonist of a neurokinin pathway effectively reduced hot flashes in postmenopausal women, according to results of a phase II trial.
The neurokinin 3 receptor (NK3R) antagonist, termed MLE4901, cut the number of hot flashes nearly in half compared to the effect of a placebo, and also improved overall menopause-related quality of life symptoms.
The medication has the potential to fulfill an unmet clinical need, since 70% of women experience hot flashes and the median duration of symptoms is more than 7 years, Julia K. Prague, MD, said at the annual meeting of the Endocrine Society.
The study results were published concurrently with Dr. Prague’s presentation (Lancet. 2017 Apr 3. doi: 10.1016/S0140-6736[17]30823-1).
Evidence from postmortem studies of postmenopausal women, said Dr. Prague, pointed to NKB (neurokinin B)/NK3R signaling as playing a role in hot flashes. The brains of these women showed hypertrophy of NKB neurons and increased neuronal activity, as well as increased NKB gene expression. The same effects were seen in monkeys whose ovaries had been removed, and the effect was reversed when the monkeys were given estrogen.
Also, previous work has shown that administering NKB to premenopausal women gave them hot flashes (Sci Rep. 2015 Feb 6;5:8466). The preclinical work laid the groundwork for the hypothesis that taking an oral NK3R antagonist could mitigate hot flashes in menopausal women.
The randomized, placebo-controlled, double-blind 2-way crossover study enrolled 37 healthy women aged 40-62 who were at least 12 months out from their last menses. To qualify for enrollment, they needed to be experiencing at least seven hot flashes per 24 hours; the actual mean total number in the study group was 13 per 24 hours and 85 per week. A total of 28 women completed the full protocol.
The mean age of the women was 55 years, and the mean body mass index was 25.8 kg/m2. Three quarters of the women were white.
The women had an initial 2-week baseline period, during which they kept a symptom diary, and then were randomized to receive either an oral NK3R antagonist or a placebo twice daily. Then, each patient went through a 2-week washout period, after which they were crossed over to the other arm of the study. Finally, patients were monitored for a further 2 weeks after taking the study drug.
The NK3R antagonist, said Dr. Prague, “significantly reduced the total weekly number of hot [flashes] compared to placebo in the fourth week of treatment.” Patients taking placebo experienced 49.01 hot flashes per week (range, 40.81-58.86), while those taking the NK3R antagonist had 19.35 (range, 15.99-23.42, P less than .0001). This amounted to a 45% reduction in number of hot flashes compared to placebo.
Dr. Prague, a clinical research fellow at Imperial College, London, said that the treatment effect size seen was similar regardless of whether patients received the active study drug or placebo first.
Using the Menopause-Related Quality of Life questionnaire, Dr. Prague and her colleagues determined that when women were taking the NK3R antagonist, in addition to a significant reduction in vasomotor symptoms, menopause-related psychosocial symptoms were reduced by 15% (P = .0083) and physical symptoms by 19% (P = .0002). As expected for the nonhormonal medication, she said, sexual symptoms were reduced by a nonsignificant 8% (P = .24).
A subset of patients received more intensive study, with external validation of hot flashes and a series of 3-day-long stays during which an intravenous catheter was placed for blood sampling every 10 minutes.
Data from this group of patients showed that, while the NK3R antagonist did not significantly affect the number of luteinizing hormone pulses detected compared with placebo (P = .41), it did increase the mean amplitude of each pulse (P = .0243). Also, the active drug made the luteinizing hormone pulses more orderly (P = .0006 compared to placebo).
The NK3R antagonist was well tolerated. Though no serious adverse events were seen, three patients taking the NK3R antagonist did have a transient and asymptomatic elevation of transaminases without hyperbilirubinemia at 28 days after starting treatment. The elevation resolved for all patients within 90 days of stopping the medication.
The novel drug could represent “a potentially practice-changing therapeutic,” said Dr. Prague, since it “significantly relieves hot [flash] symptoms without the need for estrogen exposure.” She added that planning is underway for longer studies involving more patients.
The study was supported by AstraZeneca and Millendo Therapeutics, which are involved with manufacturing MLE4901.
[email protected]
On Twitter @karioakes
ORLANDO – A novel antagonist of a neurokinin pathway effectively reduced hot flashes in postmenopausal women, according to results of a phase II trial.
The neurokinin 3 receptor (NK3R) antagonist, termed MLE4901, cut the number of hot flashes nearly in half compared to the effect of a placebo, and also improved overall menopause-related quality of life symptoms.
The medication has the potential to fulfill an unmet clinical need, since 70% of women experience hot flashes and the median duration of symptoms is more than 7 years, Julia K. Prague, MD, said at the annual meeting of the Endocrine Society.
The study results were published concurrently with Dr. Prague’s presentation (Lancet. 2017 Apr 3. doi: 10.1016/S0140-6736[17]30823-1).
Evidence from postmortem studies of postmenopausal women, said Dr. Prague, pointed to NKB (neurokinin B)/NK3R signaling as playing a role in hot flashes. The brains of these women showed hypertrophy of NKB neurons and increased neuronal activity, as well as increased NKB gene expression. The same effects were seen in monkeys whose ovaries had been removed, and the effect was reversed when the monkeys were given estrogen.
Also, previous work has shown that administering NKB to premenopausal women gave them hot flashes (Sci Rep. 2015 Feb 6;5:8466). The preclinical work laid the groundwork for the hypothesis that taking an oral NK3R antagonist could mitigate hot flashes in menopausal women.
The randomized, placebo-controlled, double-blind 2-way crossover study enrolled 37 healthy women aged 40-62 who were at least 12 months out from their last menses. To qualify for enrollment, they needed to be experiencing at least seven hot flashes per 24 hours; the actual mean total number in the study group was 13 per 24 hours and 85 per week. A total of 28 women completed the full protocol.
The mean age of the women was 55 years, and the mean body mass index was 25.8 kg/m2. Three quarters of the women were white.
The women had an initial 2-week baseline period, during which they kept a symptom diary, and then were randomized to receive either an oral NK3R antagonist or a placebo twice daily. Then, each patient went through a 2-week washout period, after which they were crossed over to the other arm of the study. Finally, patients were monitored for a further 2 weeks after taking the study drug.
The NK3R antagonist, said Dr. Prague, “significantly reduced the total weekly number of hot [flashes] compared to placebo in the fourth week of treatment.” Patients taking placebo experienced 49.01 hot flashes per week (range, 40.81-58.86), while those taking the NK3R antagonist had 19.35 (range, 15.99-23.42, P less than .0001). This amounted to a 45% reduction in number of hot flashes compared to placebo.
Dr. Prague, a clinical research fellow at Imperial College, London, said that the treatment effect size seen was similar regardless of whether patients received the active study drug or placebo first.
Using the Menopause-Related Quality of Life questionnaire, Dr. Prague and her colleagues determined that when women were taking the NK3R antagonist, in addition to a significant reduction in vasomotor symptoms, menopause-related psychosocial symptoms were reduced by 15% (P = .0083) and physical symptoms by 19% (P = .0002). As expected for the nonhormonal medication, she said, sexual symptoms were reduced by a nonsignificant 8% (P = .24).
A subset of patients received more intensive study, with external validation of hot flashes and a series of 3-day-long stays during which an intravenous catheter was placed for blood sampling every 10 minutes.
Data from this group of patients showed that, while the NK3R antagonist did not significantly affect the number of luteinizing hormone pulses detected compared with placebo (P = .41), it did increase the mean amplitude of each pulse (P = .0243). Also, the active drug made the luteinizing hormone pulses more orderly (P = .0006 compared to placebo).
The NK3R antagonist was well tolerated. Though no serious adverse events were seen, three patients taking the NK3R antagonist did have a transient and asymptomatic elevation of transaminases without hyperbilirubinemia at 28 days after starting treatment. The elevation resolved for all patients within 90 days of stopping the medication.
The novel drug could represent “a potentially practice-changing therapeutic,” said Dr. Prague, since it “significantly relieves hot [flash] symptoms without the need for estrogen exposure.” She added that planning is underway for longer studies involving more patients.
The study was supported by AstraZeneca and Millendo Therapeutics, which are involved with manufacturing MLE4901.
[email protected]
On Twitter @karioakes
ORLANDO – A novel antagonist of a neurokinin pathway effectively reduced hot flashes in postmenopausal women, according to results of a phase II trial.
The neurokinin 3 receptor (NK3R) antagonist, termed MLE4901, cut the number of hot flashes nearly in half compared to the effect of a placebo, and also improved overall menopause-related quality of life symptoms.
The medication has the potential to fulfill an unmet clinical need, since 70% of women experience hot flashes and the median duration of symptoms is more than 7 years, Julia K. Prague, MD, said at the annual meeting of the Endocrine Society.
The study results were published concurrently with Dr. Prague’s presentation (Lancet. 2017 Apr 3. doi: 10.1016/S0140-6736[17]30823-1).
Evidence from postmortem studies of postmenopausal women, said Dr. Prague, pointed to NKB (neurokinin B)/NK3R signaling as playing a role in hot flashes. The brains of these women showed hypertrophy of NKB neurons and increased neuronal activity, as well as increased NKB gene expression. The same effects were seen in monkeys whose ovaries had been removed, and the effect was reversed when the monkeys were given estrogen.
Also, previous work has shown that administering NKB to premenopausal women gave them hot flashes (Sci Rep. 2015 Feb 6;5:8466). The preclinical work laid the groundwork for the hypothesis that taking an oral NK3R antagonist could mitigate hot flashes in menopausal women.
The randomized, placebo-controlled, double-blind 2-way crossover study enrolled 37 healthy women aged 40-62 who were at least 12 months out from their last menses. To qualify for enrollment, they needed to be experiencing at least seven hot flashes per 24 hours; the actual mean total number in the study group was 13 per 24 hours and 85 per week. A total of 28 women completed the full protocol.
The mean age of the women was 55 years, and the mean body mass index was 25.8 kg/m2. Three quarters of the women were white.
The women had an initial 2-week baseline period, during which they kept a symptom diary, and then were randomized to receive either an oral NK3R antagonist or a placebo twice daily. Then, each patient went through a 2-week washout period, after which they were crossed over to the other arm of the study. Finally, patients were monitored for a further 2 weeks after taking the study drug.
The NK3R antagonist, said Dr. Prague, “significantly reduced the total weekly number of hot [flashes] compared to placebo in the fourth week of treatment.” Patients taking placebo experienced 49.01 hot flashes per week (range, 40.81-58.86), while those taking the NK3R antagonist had 19.35 (range, 15.99-23.42, P less than .0001). This amounted to a 45% reduction in number of hot flashes compared to placebo.
Dr. Prague, a clinical research fellow at Imperial College, London, said that the treatment effect size seen was similar regardless of whether patients received the active study drug or placebo first.
Using the Menopause-Related Quality of Life questionnaire, Dr. Prague and her colleagues determined that when women were taking the NK3R antagonist, in addition to a significant reduction in vasomotor symptoms, menopause-related psychosocial symptoms were reduced by 15% (P = .0083) and physical symptoms by 19% (P = .0002). As expected for the nonhormonal medication, she said, sexual symptoms were reduced by a nonsignificant 8% (P = .24).
A subset of patients received more intensive study, with external validation of hot flashes and a series of 3-day-long stays during which an intravenous catheter was placed for blood sampling every 10 minutes.
Data from this group of patients showed that, while the NK3R antagonist did not significantly affect the number of luteinizing hormone pulses detected compared with placebo (P = .41), it did increase the mean amplitude of each pulse (P = .0243). Also, the active drug made the luteinizing hormone pulses more orderly (P = .0006 compared to placebo).
The NK3R antagonist was well tolerated. Though no serious adverse events were seen, three patients taking the NK3R antagonist did have a transient and asymptomatic elevation of transaminases without hyperbilirubinemia at 28 days after starting treatment. The elevation resolved for all patients within 90 days of stopping the medication.
The novel drug could represent “a potentially practice-changing therapeutic,” said Dr. Prague, since it “significantly relieves hot [flash] symptoms without the need for estrogen exposure.” She added that planning is underway for longer studies involving more patients.
The study was supported by AstraZeneca and Millendo Therapeutics, which are involved with manufacturing MLE4901.
[email protected]
On Twitter @karioakes
AT ENDO 2017
Key clinical point:
Major finding: The neurokinin 3 receptor antagonist reduced hot flashes from a mean of 85 per week at baseline to a mean of 49 per week.
Data source: Phase II randomized, double-blind, placebo-controlled two-way crossover study of 37 postmenopausal women with moderate to severe hot flashes.
Disclosures: The study was funded by AstraZeneca and Millendo Therapeutics, which are involved with the manufacture of the medicine, termed MLE4901.
Dulera inhaler linked to adrenocorticotropic suppression in small case series
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.
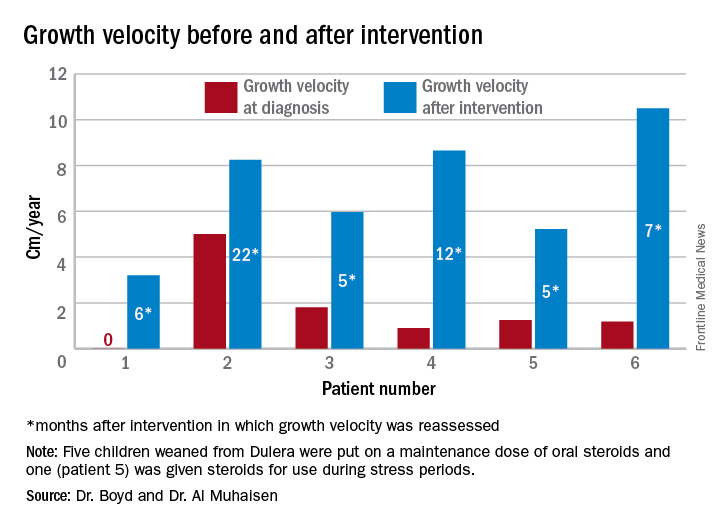
The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.

The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.

The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Of eight children who had an adrenal workup at an endocrinology clinic, six had adrenal suppression.
Data source: The case series comprised 18 children taking Dulera who presented with growth failure.
Disclosures: Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.