User login
Prolactin, the pituitary, and pregnancy: Where’s the balance?
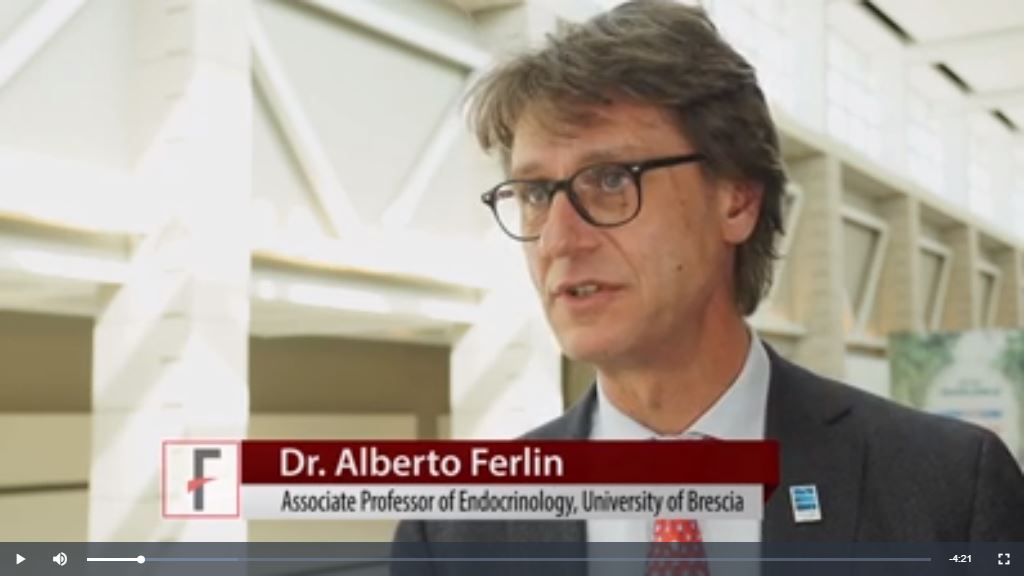
CHICAGO – Management of fertility and reproduction for women with prolactin-secreting pituitary tumors is a balancing act, often in the absence of robust data to support clinical decision making. So judgment, communication, and paying attention to the patient become paramount considerations, said endocrinologist Mark Molitch, MD, speaking at the annual meeting of the Endocrine Society.
The first step: restoring fertility
“Remember that our patients that have hyperprolactinemia are generally infertile,” said Dr. Molitch, Martha Leland Sherwin Professor of Medicine at Northwestern University, Chicago. “You really need to restore prolactin levels to close to normal, or normal, to allow ovulation to occur,” he said.
Dr. Molitch noted that up to 94% of women with hyperprolactinemia will initially have anovulation, amenorrhea, and infertility, but restoration of normal prolactin levels usually corrects these.
“If you have a patient where you are unable to restore prolactin levels to normal, there are other methods” to consider. Patients may end up using clomiphene, gonadotropin-releasing hormone (GnRH) or gonadotropins, or even moving to in vitro fertilization in these cases, said Dr. Molitch.
Preferable to any of these, though, is achieving normal prolactin levels.
In patients who are hyperprolactinemic, “the major action is occurring at the hypothalamic level,” with decreases in pulsatile secretion of GnRH, said Dr. Molitch. Next, there are resultant decreases in gonadotropin secretion, which in turn interrupt the ovary’s normal physiology. “There’s also an interruption in positive estrogen feedback in this cycle,” he said.
“So what’s new in this area is kisspeptin,” Dr. Molitch said, adding that the peptide activates the G-protein coupled receptor GPR54, found in the hypothalamus and pituitary. Infusion of kisspeptin stimulates secretion of luteinizing hormone, follicle stimulating hormone, and testosterone. Conversely, mutations that inactivate GPR43 result in hypogonadotropic hypogonadism, while activating mutations are associated with centrally caused precocious puberty (Biol Reprod. 2011 Oct;85[4]:650-60; Mol Cell Endocrinol. 2011 Oct 22;346[1-2]:29-3).
From this and other work, endocrinologists now know that kisspeptin is “very likely involved in puberty initiation and in response to fasting,” said Dr. Molitch. It’s now thought that high prolactin levels alter kisspeptin levels, “which then causes the further downstream effects,” said Dr. Molitch.
Dopamine agonists and pregnancy
Dopamine agonists are the primary therapies used for patients with prolactinomas and hyperprolactinemia. In patients seeking fertility, the dopamine agonist is usually continued just through the first few weeks of pregnancy, until the patient misses her first menstrual period, he said.
Establishing the menstrual interval is key to knowing when to stop the dopamine agonist, though. “We often use barrier contraceptives, so we can tell what the menstrual interval is – so we can tell when somebody’s missed her period,” Dr. Molitch explained.
“Most of the safety data on these drugs … are based on that relatively short period of exposure,” said Dr. Molitch. “As far as long-term use during pregnancy, only about 100 patients who took bromocriptine throughout pregnancy have been reported,” with two minor fetal anomalies reported from this series of patients, he said.
Though fewer than 20 cases have been reported of cabergoline being continued throughout a pregnancy, “there have not been any problems with that,” said Dr. Molitch.
Overall, over 6,000 pregnancies with bromocriptine exposure, as well as over 1,000 with cabergoline, have now been reported.
Dr. Molitch summarized the aggregate safety data for each dopamine agonist: “When we look at the adverse outcomes that occurred with either of these drugs, as far as spontaneous abortions or terminations, premature deliveries, multiple births, and, of course, the most important thing here being major malformations … in neither drug is there an increase in these adverse outcomes” (J Endocrinol Invest. 2018 Jan;41[1]:129-41).
“In my own mind now, I think that the number of cases with cabergoline now is quite sufficient to justify the safety of its use during pregnancy,” Dr. Molitch said. “However, this is sort of an individualized decision between you – the clinician – as well as your patient to make”: whether to trust the 1,000-case–strong data for cabergoline. “I no longer change patients from cabergoline to bromocriptine because of safety concerns. I think that cabergoline is perfectly safe,” he added.
What if the tumor grows in pregnancy?
During pregnancy, high estrogen levels from the placenta can stimulate prolactinoma growth, and the dopamine agonist’s inhibitory effect is gone once that medication’s been stopped. This means that “We have both a ‘push’ and a decrease in the ‘pull’ here, so you may have tumor enlargement,” said Dr. Molitch.
The risk for tumor enlargement in microadenomas is about 2.4%, and ranges to about 16% for enlargement of macroadenomas during pregnancy. For macroadenomas, “you might consider a prepregnancy debulking of the tumor,” Dr. Molitch said.
It’s reasonable to stop a dopamine agonist once pregnancy’s been established in a patient with a prolactinoma, and “follow the patient symptomatically every few months,” letting suspicious new symptoms like visual changes or headaches be the prompt for visual field exam and magnetic resonance imaging without contrast, said Dr. Molitch.
Though it’s not FDA approved, consideration can be given to continuing a dopamine agonist in a patient with a large prepregnancy adenoma throughout pregnancy – and if the tumor is enlarging significantly, the dopamine agonist should be restarted if it’s been withheld, said Dr. Molitch. Finally, surgery is the option if an enlarging tumor doesn’t respond to a dopamine agonist, unless the pregnancy is far enough along that delivery is a safe option.
“It’s important to actually document that there’s tumor enlargement, because if you’re going to do something like restarting a drug or even surgery, you really want to make sure that it’s the enlarging tumor that’s causing the problem,” said Dr. Molitch.
Postpartum prolactinoma considerations
Postpartum, even though prolactin secretion is upped by nursing, “there are no data to show that lactation stimulates tumor growth,” said Dr. Molitch. “I don’t see that there’s any problem with somebody nursing if they choose to do so.” However, “You certainly cannot restart the dopamine agonist, because that will lower prolactin levels and prevent that person from being able to nurse,” he said.
For reasons that are not clear, prolactin levels often drop post partum. Accordingly, it’s a reasonable approach in a nursing mother with mildly elevated prolactin levels to wait until nursing is done to see if menses resume spontaneously before restarting the dopamine agonist, said Dr. Molitch.
Dr. Molitch reported receiving fees and research funding from several pharmaceutical companies. He also disclosed that his spouse holds stock in Amgen.
SOURCE: Molitch M ENDO 2018. Abstract M02-2.
CHICAGO – Management of fertility and reproduction for women with prolactin-secreting pituitary tumors is a balancing act, often in the absence of robust data to support clinical decision making. So judgment, communication, and paying attention to the patient become paramount considerations, said endocrinologist Mark Molitch, MD, speaking at the annual meeting of the Endocrine Society.
The first step: restoring fertility
“Remember that our patients that have hyperprolactinemia are generally infertile,” said Dr. Molitch, Martha Leland Sherwin Professor of Medicine at Northwestern University, Chicago. “You really need to restore prolactin levels to close to normal, or normal, to allow ovulation to occur,” he said.
Dr. Molitch noted that up to 94% of women with hyperprolactinemia will initially have anovulation, amenorrhea, and infertility, but restoration of normal prolactin levels usually corrects these.
“If you have a patient where you are unable to restore prolactin levels to normal, there are other methods” to consider. Patients may end up using clomiphene, gonadotropin-releasing hormone (GnRH) or gonadotropins, or even moving to in vitro fertilization in these cases, said Dr. Molitch.
Preferable to any of these, though, is achieving normal prolactin levels.
In patients who are hyperprolactinemic, “the major action is occurring at the hypothalamic level,” with decreases in pulsatile secretion of GnRH, said Dr. Molitch. Next, there are resultant decreases in gonadotropin secretion, which in turn interrupt the ovary’s normal physiology. “There’s also an interruption in positive estrogen feedback in this cycle,” he said.
“So what’s new in this area is kisspeptin,” Dr. Molitch said, adding that the peptide activates the G-protein coupled receptor GPR54, found in the hypothalamus and pituitary. Infusion of kisspeptin stimulates secretion of luteinizing hormone, follicle stimulating hormone, and testosterone. Conversely, mutations that inactivate GPR43 result in hypogonadotropic hypogonadism, while activating mutations are associated with centrally caused precocious puberty (Biol Reprod. 2011 Oct;85[4]:650-60; Mol Cell Endocrinol. 2011 Oct 22;346[1-2]:29-3).
From this and other work, endocrinologists now know that kisspeptin is “very likely involved in puberty initiation and in response to fasting,” said Dr. Molitch. It’s now thought that high prolactin levels alter kisspeptin levels, “which then causes the further downstream effects,” said Dr. Molitch.
Dopamine agonists and pregnancy
Dopamine agonists are the primary therapies used for patients with prolactinomas and hyperprolactinemia. In patients seeking fertility, the dopamine agonist is usually continued just through the first few weeks of pregnancy, until the patient misses her first menstrual period, he said.
Establishing the menstrual interval is key to knowing when to stop the dopamine agonist, though. “We often use barrier contraceptives, so we can tell what the menstrual interval is – so we can tell when somebody’s missed her period,” Dr. Molitch explained.
“Most of the safety data on these drugs … are based on that relatively short period of exposure,” said Dr. Molitch. “As far as long-term use during pregnancy, only about 100 patients who took bromocriptine throughout pregnancy have been reported,” with two minor fetal anomalies reported from this series of patients, he said.
Though fewer than 20 cases have been reported of cabergoline being continued throughout a pregnancy, “there have not been any problems with that,” said Dr. Molitch.
Overall, over 6,000 pregnancies with bromocriptine exposure, as well as over 1,000 with cabergoline, have now been reported.
Dr. Molitch summarized the aggregate safety data for each dopamine agonist: “When we look at the adverse outcomes that occurred with either of these drugs, as far as spontaneous abortions or terminations, premature deliveries, multiple births, and, of course, the most important thing here being major malformations … in neither drug is there an increase in these adverse outcomes” (J Endocrinol Invest. 2018 Jan;41[1]:129-41).
“In my own mind now, I think that the number of cases with cabergoline now is quite sufficient to justify the safety of its use during pregnancy,” Dr. Molitch said. “However, this is sort of an individualized decision between you – the clinician – as well as your patient to make”: whether to trust the 1,000-case–strong data for cabergoline. “I no longer change patients from cabergoline to bromocriptine because of safety concerns. I think that cabergoline is perfectly safe,” he added.
What if the tumor grows in pregnancy?
During pregnancy, high estrogen levels from the placenta can stimulate prolactinoma growth, and the dopamine agonist’s inhibitory effect is gone once that medication’s been stopped. This means that “We have both a ‘push’ and a decrease in the ‘pull’ here, so you may have tumor enlargement,” said Dr. Molitch.
The risk for tumor enlargement in microadenomas is about 2.4%, and ranges to about 16% for enlargement of macroadenomas during pregnancy. For macroadenomas, “you might consider a prepregnancy debulking of the tumor,” Dr. Molitch said.
It’s reasonable to stop a dopamine agonist once pregnancy’s been established in a patient with a prolactinoma, and “follow the patient symptomatically every few months,” letting suspicious new symptoms like visual changes or headaches be the prompt for visual field exam and magnetic resonance imaging without contrast, said Dr. Molitch.
Though it’s not FDA approved, consideration can be given to continuing a dopamine agonist in a patient with a large prepregnancy adenoma throughout pregnancy – and if the tumor is enlarging significantly, the dopamine agonist should be restarted if it’s been withheld, said Dr. Molitch. Finally, surgery is the option if an enlarging tumor doesn’t respond to a dopamine agonist, unless the pregnancy is far enough along that delivery is a safe option.
“It’s important to actually document that there’s tumor enlargement, because if you’re going to do something like restarting a drug or even surgery, you really want to make sure that it’s the enlarging tumor that’s causing the problem,” said Dr. Molitch.
Postpartum prolactinoma considerations
Postpartum, even though prolactin secretion is upped by nursing, “there are no data to show that lactation stimulates tumor growth,” said Dr. Molitch. “I don’t see that there’s any problem with somebody nursing if they choose to do so.” However, “You certainly cannot restart the dopamine agonist, because that will lower prolactin levels and prevent that person from being able to nurse,” he said.
For reasons that are not clear, prolactin levels often drop post partum. Accordingly, it’s a reasonable approach in a nursing mother with mildly elevated prolactin levels to wait until nursing is done to see if menses resume spontaneously before restarting the dopamine agonist, said Dr. Molitch.
Dr. Molitch reported receiving fees and research funding from several pharmaceutical companies. He also disclosed that his spouse holds stock in Amgen.
SOURCE: Molitch M ENDO 2018. Abstract M02-2.
CHICAGO – Management of fertility and reproduction for women with prolactin-secreting pituitary tumors is a balancing act, often in the absence of robust data to support clinical decision making. So judgment, communication, and paying attention to the patient become paramount considerations, said endocrinologist Mark Molitch, MD, speaking at the annual meeting of the Endocrine Society.
The first step: restoring fertility
“Remember that our patients that have hyperprolactinemia are generally infertile,” said Dr. Molitch, Martha Leland Sherwin Professor of Medicine at Northwestern University, Chicago. “You really need to restore prolactin levels to close to normal, or normal, to allow ovulation to occur,” he said.
Dr. Molitch noted that up to 94% of women with hyperprolactinemia will initially have anovulation, amenorrhea, and infertility, but restoration of normal prolactin levels usually corrects these.
“If you have a patient where you are unable to restore prolactin levels to normal, there are other methods” to consider. Patients may end up using clomiphene, gonadotropin-releasing hormone (GnRH) or gonadotropins, or even moving to in vitro fertilization in these cases, said Dr. Molitch.
Preferable to any of these, though, is achieving normal prolactin levels.
In patients who are hyperprolactinemic, “the major action is occurring at the hypothalamic level,” with decreases in pulsatile secretion of GnRH, said Dr. Molitch. Next, there are resultant decreases in gonadotropin secretion, which in turn interrupt the ovary’s normal physiology. “There’s also an interruption in positive estrogen feedback in this cycle,” he said.
“So what’s new in this area is kisspeptin,” Dr. Molitch said, adding that the peptide activates the G-protein coupled receptor GPR54, found in the hypothalamus and pituitary. Infusion of kisspeptin stimulates secretion of luteinizing hormone, follicle stimulating hormone, and testosterone. Conversely, mutations that inactivate GPR43 result in hypogonadotropic hypogonadism, while activating mutations are associated with centrally caused precocious puberty (Biol Reprod. 2011 Oct;85[4]:650-60; Mol Cell Endocrinol. 2011 Oct 22;346[1-2]:29-3).
From this and other work, endocrinologists now know that kisspeptin is “very likely involved in puberty initiation and in response to fasting,” said Dr. Molitch. It’s now thought that high prolactin levels alter kisspeptin levels, “which then causes the further downstream effects,” said Dr. Molitch.
Dopamine agonists and pregnancy
Dopamine agonists are the primary therapies used for patients with prolactinomas and hyperprolactinemia. In patients seeking fertility, the dopamine agonist is usually continued just through the first few weeks of pregnancy, until the patient misses her first menstrual period, he said.
Establishing the menstrual interval is key to knowing when to stop the dopamine agonist, though. “We often use barrier contraceptives, so we can tell what the menstrual interval is – so we can tell when somebody’s missed her period,” Dr. Molitch explained.
“Most of the safety data on these drugs … are based on that relatively short period of exposure,” said Dr. Molitch. “As far as long-term use during pregnancy, only about 100 patients who took bromocriptine throughout pregnancy have been reported,” with two minor fetal anomalies reported from this series of patients, he said.
Though fewer than 20 cases have been reported of cabergoline being continued throughout a pregnancy, “there have not been any problems with that,” said Dr. Molitch.
Overall, over 6,000 pregnancies with bromocriptine exposure, as well as over 1,000 with cabergoline, have now been reported.
Dr. Molitch summarized the aggregate safety data for each dopamine agonist: “When we look at the adverse outcomes that occurred with either of these drugs, as far as spontaneous abortions or terminations, premature deliveries, multiple births, and, of course, the most important thing here being major malformations … in neither drug is there an increase in these adverse outcomes” (J Endocrinol Invest. 2018 Jan;41[1]:129-41).
“In my own mind now, I think that the number of cases with cabergoline now is quite sufficient to justify the safety of its use during pregnancy,” Dr. Molitch said. “However, this is sort of an individualized decision between you – the clinician – as well as your patient to make”: whether to trust the 1,000-case–strong data for cabergoline. “I no longer change patients from cabergoline to bromocriptine because of safety concerns. I think that cabergoline is perfectly safe,” he added.
What if the tumor grows in pregnancy?
During pregnancy, high estrogen levels from the placenta can stimulate prolactinoma growth, and the dopamine agonist’s inhibitory effect is gone once that medication’s been stopped. This means that “We have both a ‘push’ and a decrease in the ‘pull’ here, so you may have tumor enlargement,” said Dr. Molitch.
The risk for tumor enlargement in microadenomas is about 2.4%, and ranges to about 16% for enlargement of macroadenomas during pregnancy. For macroadenomas, “you might consider a prepregnancy debulking of the tumor,” Dr. Molitch said.
It’s reasonable to stop a dopamine agonist once pregnancy’s been established in a patient with a prolactinoma, and “follow the patient symptomatically every few months,” letting suspicious new symptoms like visual changes or headaches be the prompt for visual field exam and magnetic resonance imaging without contrast, said Dr. Molitch.
Though it’s not FDA approved, consideration can be given to continuing a dopamine agonist in a patient with a large prepregnancy adenoma throughout pregnancy – and if the tumor is enlarging significantly, the dopamine agonist should be restarted if it’s been withheld, said Dr. Molitch. Finally, surgery is the option if an enlarging tumor doesn’t respond to a dopamine agonist, unless the pregnancy is far enough along that delivery is a safe option.
“It’s important to actually document that there’s tumor enlargement, because if you’re going to do something like restarting a drug or even surgery, you really want to make sure that it’s the enlarging tumor that’s causing the problem,” said Dr. Molitch.
Postpartum prolactinoma considerations
Postpartum, even though prolactin secretion is upped by nursing, “there are no data to show that lactation stimulates tumor growth,” said Dr. Molitch. “I don’t see that there’s any problem with somebody nursing if they choose to do so.” However, “You certainly cannot restart the dopamine agonist, because that will lower prolactin levels and prevent that person from being able to nurse,” he said.
For reasons that are not clear, prolactin levels often drop post partum. Accordingly, it’s a reasonable approach in a nursing mother with mildly elevated prolactin levels to wait until nursing is done to see if menses resume spontaneously before restarting the dopamine agonist, said Dr. Molitch.
Dr. Molitch reported receiving fees and research funding from several pharmaceutical companies. He also disclosed that his spouse holds stock in Amgen.
SOURCE: Molitch M ENDO 2018. Abstract M02-2.
REPORTING FROM ENDO 2018
Metabolic syndrome scoring system predicts CVD in type 2 diabetes
CHICAGO – the system identified the association independent of hemoglobin A1c levels, according to work presented at the annual meeting of the Endocrine Society.
The findings may point toward an additional surveillance tool for coronary heart disease (CHD) in patients who have type 2 diabetes, according to Mark D. DeBoer, MD, and his coauthors, who had not previously applied the metabolic syndrome severity scoring system to individuals with diabetes.
When broken down by quartile, increasing severity of metabolic syndrome for individuals with type 2 diabetes was associated with an increased risk of future cardiovascular disease, even when blood glucose levels were not included in calculation of metabolic syndrome (P less than .001 with glucose levels and P = .001 without glucose levels).
Dr. DeBoer, of the department of pediatrics and the Child Health Research Center at the University of Virginia, Charlottesville, and his coinvestigators, had previously developed the continuous scoring system for metabolic syndrome. The system incorporates the components that form the diagnostic criteria for metabolic syndrome – waist circumference, systolic blood pressure, and levels of HDL cholesterol, triglycerides, and blood glucose.
However, rather than using cutoffs for a dichotomous score of 0 or 1 for each criterion, the investigators developed sex- and race/ethnicity-specific scores of severity. This approach may identify metabolic dysregulation that would not be apparent if measures of several different criteria were just short of missing the cutoff, for example.
“These scores are standardized like z scores such that 2.0 is two standard deviations above the mean,” wrote Dr. DeBoer and his colleagues. Thus, the scores are dubbed “MetS z scores;” a free online calculator is available.
In developing the model, the investigators performed single-factor confirmatory factor analyses using data from 6,870 adults from the National Health and Nutrition Examination Survey cohort, developing scores specific for non-Hispanic whites, non-Hispanic blacks, and Hispanics.
In the present work, MetS z scores were applied to data from the Atherosclerosis Risk in Communities (ARIC) study, which followed 8,660 participants aged 45-64 years for 12 years, with adjudicated follow-up for cardiovascular incidents up to 20 years. Only participants with no baseline CHD and with complete metabolic syndrome risk factor data were included.
Dr. DeBoer and his collaborators compared MetS z scores for patients who were never diagnosed with diabetes, those who had diabetes at baseline, and those who had an incident diagnosis of type 2 diabetes at the second, third, or fourth ARIC study visit. They found that individuals who entered ARIC with diabetes had the highest z scores, while those with incident type 2 diabetes had higher baseline scores, compared with those who never had a diabetes diagnosis. The difference in z scores was lowest for white men, while black men and women “exhibited increased scores after diagnosis, suggesting inadequate treatment,” wrote Dr. DeBoer and his colleagues.
The investigators also looked for an association between MetS z scores and the primary outcome measure, time to incident CHD, calculating the z score both with and without the inclusion of glucose levels.
Dr. DeBoer and his colleagues analyzed the association between MetS z score and CHD for patients with and without type 2 diabetes. They found metabolic syndrome severity as assessed by MetS z score was independently associated with increased risk for CHD in participants with diabetes (P = .001).
“We additionally assessed whether the [metabolic syndrome] z score predicted future CHD following adjustment for HbA1c and when using a similar score derived without glucose as a component,” wrote Dr. DeBoer and his collaborators.
When metabolic syndrome severity as assessed by z score was broken into quartiles, “increasing MetS severity (by quartile) increased the risk of future CVD [cardiovascular disease], both using the traditional 5-component MetS z score and the no-glucose score,” wrote Dr. DeBoer and his colleagues. “This continuous MetS severity z score confers risk for future CHD among individuals with type 2 diabetes, both with the traditional MetS score and a score without glucose. These findings were independent of HbA1c and may relate to risk associated with the pathophysiologic processes underlying MetS.”
The investigators plan to integrate an automated metabolic syndrome severity score calculator into the electronic medical record “to identify and track risk in individuals over time and identify those who may benefit from increased intervention,” wrote Dr. DeBoer and his collaborators.
The National Institutes of Health funded the study. Dr. DeBoer reported no relevant conflicts of interest.
SOURCE: DeBoer MD et al. ENDO 2018, Abstract SAT-015.
CHICAGO – the system identified the association independent of hemoglobin A1c levels, according to work presented at the annual meeting of the Endocrine Society.
The findings may point toward an additional surveillance tool for coronary heart disease (CHD) in patients who have type 2 diabetes, according to Mark D. DeBoer, MD, and his coauthors, who had not previously applied the metabolic syndrome severity scoring system to individuals with diabetes.
When broken down by quartile, increasing severity of metabolic syndrome for individuals with type 2 diabetes was associated with an increased risk of future cardiovascular disease, even when blood glucose levels were not included in calculation of metabolic syndrome (P less than .001 with glucose levels and P = .001 without glucose levels).
Dr. DeBoer, of the department of pediatrics and the Child Health Research Center at the University of Virginia, Charlottesville, and his coinvestigators, had previously developed the continuous scoring system for metabolic syndrome. The system incorporates the components that form the diagnostic criteria for metabolic syndrome – waist circumference, systolic blood pressure, and levels of HDL cholesterol, triglycerides, and blood glucose.
However, rather than using cutoffs for a dichotomous score of 0 or 1 for each criterion, the investigators developed sex- and race/ethnicity-specific scores of severity. This approach may identify metabolic dysregulation that would not be apparent if measures of several different criteria were just short of missing the cutoff, for example.
“These scores are standardized like z scores such that 2.0 is two standard deviations above the mean,” wrote Dr. DeBoer and his colleagues. Thus, the scores are dubbed “MetS z scores;” a free online calculator is available.
In developing the model, the investigators performed single-factor confirmatory factor analyses using data from 6,870 adults from the National Health and Nutrition Examination Survey cohort, developing scores specific for non-Hispanic whites, non-Hispanic blacks, and Hispanics.
In the present work, MetS z scores were applied to data from the Atherosclerosis Risk in Communities (ARIC) study, which followed 8,660 participants aged 45-64 years for 12 years, with adjudicated follow-up for cardiovascular incidents up to 20 years. Only participants with no baseline CHD and with complete metabolic syndrome risk factor data were included.
Dr. DeBoer and his collaborators compared MetS z scores for patients who were never diagnosed with diabetes, those who had diabetes at baseline, and those who had an incident diagnosis of type 2 diabetes at the second, third, or fourth ARIC study visit. They found that individuals who entered ARIC with diabetes had the highest z scores, while those with incident type 2 diabetes had higher baseline scores, compared with those who never had a diabetes diagnosis. The difference in z scores was lowest for white men, while black men and women “exhibited increased scores after diagnosis, suggesting inadequate treatment,” wrote Dr. DeBoer and his colleagues.
The investigators also looked for an association between MetS z scores and the primary outcome measure, time to incident CHD, calculating the z score both with and without the inclusion of glucose levels.
Dr. DeBoer and his colleagues analyzed the association between MetS z score and CHD for patients with and without type 2 diabetes. They found metabolic syndrome severity as assessed by MetS z score was independently associated with increased risk for CHD in participants with diabetes (P = .001).
“We additionally assessed whether the [metabolic syndrome] z score predicted future CHD following adjustment for HbA1c and when using a similar score derived without glucose as a component,” wrote Dr. DeBoer and his collaborators.
When metabolic syndrome severity as assessed by z score was broken into quartiles, “increasing MetS severity (by quartile) increased the risk of future CVD [cardiovascular disease], both using the traditional 5-component MetS z score and the no-glucose score,” wrote Dr. DeBoer and his colleagues. “This continuous MetS severity z score confers risk for future CHD among individuals with type 2 diabetes, both with the traditional MetS score and a score without glucose. These findings were independent of HbA1c and may relate to risk associated with the pathophysiologic processes underlying MetS.”
The investigators plan to integrate an automated metabolic syndrome severity score calculator into the electronic medical record “to identify and track risk in individuals over time and identify those who may benefit from increased intervention,” wrote Dr. DeBoer and his collaborators.
The National Institutes of Health funded the study. Dr. DeBoer reported no relevant conflicts of interest.
SOURCE: DeBoer MD et al. ENDO 2018, Abstract SAT-015.
CHICAGO – the system identified the association independent of hemoglobin A1c levels, according to work presented at the annual meeting of the Endocrine Society.
The findings may point toward an additional surveillance tool for coronary heart disease (CHD) in patients who have type 2 diabetes, according to Mark D. DeBoer, MD, and his coauthors, who had not previously applied the metabolic syndrome severity scoring system to individuals with diabetes.
When broken down by quartile, increasing severity of metabolic syndrome for individuals with type 2 diabetes was associated with an increased risk of future cardiovascular disease, even when blood glucose levels were not included in calculation of metabolic syndrome (P less than .001 with glucose levels and P = .001 without glucose levels).
Dr. DeBoer, of the department of pediatrics and the Child Health Research Center at the University of Virginia, Charlottesville, and his coinvestigators, had previously developed the continuous scoring system for metabolic syndrome. The system incorporates the components that form the diagnostic criteria for metabolic syndrome – waist circumference, systolic blood pressure, and levels of HDL cholesterol, triglycerides, and blood glucose.
However, rather than using cutoffs for a dichotomous score of 0 or 1 for each criterion, the investigators developed sex- and race/ethnicity-specific scores of severity. This approach may identify metabolic dysregulation that would not be apparent if measures of several different criteria were just short of missing the cutoff, for example.
“These scores are standardized like z scores such that 2.0 is two standard deviations above the mean,” wrote Dr. DeBoer and his colleagues. Thus, the scores are dubbed “MetS z scores;” a free online calculator is available.
In developing the model, the investigators performed single-factor confirmatory factor analyses using data from 6,870 adults from the National Health and Nutrition Examination Survey cohort, developing scores specific for non-Hispanic whites, non-Hispanic blacks, and Hispanics.
In the present work, MetS z scores were applied to data from the Atherosclerosis Risk in Communities (ARIC) study, which followed 8,660 participants aged 45-64 years for 12 years, with adjudicated follow-up for cardiovascular incidents up to 20 years. Only participants with no baseline CHD and with complete metabolic syndrome risk factor data were included.
Dr. DeBoer and his collaborators compared MetS z scores for patients who were never diagnosed with diabetes, those who had diabetes at baseline, and those who had an incident diagnosis of type 2 diabetes at the second, third, or fourth ARIC study visit. They found that individuals who entered ARIC with diabetes had the highest z scores, while those with incident type 2 diabetes had higher baseline scores, compared with those who never had a diabetes diagnosis. The difference in z scores was lowest for white men, while black men and women “exhibited increased scores after diagnosis, suggesting inadequate treatment,” wrote Dr. DeBoer and his colleagues.
The investigators also looked for an association between MetS z scores and the primary outcome measure, time to incident CHD, calculating the z score both with and without the inclusion of glucose levels.
Dr. DeBoer and his colleagues analyzed the association between MetS z score and CHD for patients with and without type 2 diabetes. They found metabolic syndrome severity as assessed by MetS z score was independently associated with increased risk for CHD in participants with diabetes (P = .001).
“We additionally assessed whether the [metabolic syndrome] z score predicted future CHD following adjustment for HbA1c and when using a similar score derived without glucose as a component,” wrote Dr. DeBoer and his collaborators.
When metabolic syndrome severity as assessed by z score was broken into quartiles, “increasing MetS severity (by quartile) increased the risk of future CVD [cardiovascular disease], both using the traditional 5-component MetS z score and the no-glucose score,” wrote Dr. DeBoer and his colleagues. “This continuous MetS severity z score confers risk for future CHD among individuals with type 2 diabetes, both with the traditional MetS score and a score without glucose. These findings were independent of HbA1c and may relate to risk associated with the pathophysiologic processes underlying MetS.”
The investigators plan to integrate an automated metabolic syndrome severity score calculator into the electronic medical record “to identify and track risk in individuals over time and identify those who may benefit from increased intervention,” wrote Dr. DeBoer and his collaborators.
The National Institutes of Health funded the study. Dr. DeBoer reported no relevant conflicts of interest.
SOURCE: DeBoer MD et al. ENDO 2018, Abstract SAT-015.
REPORTING FROM ENDO 2018
Key clinical point: Increasing metabolic syndrome severity was associated with increased risk for cardiovascular disease.
Major finding: Risk for future cardiovascular disease was upped with higher scores, even when glucose wasn’t considered (P = .001).
Study details: A retrospective analysis of Atherosclerosis Risk in Communities study data on 1,419 patients with and 7,241 patients without diabetes.
Disclosures: The National Institutes of Health sponsored the study. Dr. DeBoer reported no relevant conflicts of interest.
Source: DeBoer MD et al. ENDO 2018, Abstract SAT-015.
Sucralose sparks appetite in obese, not lean, individuals
CHICAGO – but not in lean participants of a recent study, even though hunger and satiety hormone levels didn’t change. Those with obesity also consumed more calories after ingesting the artificial sweetener, though lean participants did not.
The study compared acute effects of consuming a set amount of glucose, sucralose, or water as the control, finding that sucralose consumption resulted in a significant increase in blood flow to certain areas of the brains of study participants with obesity but not in lean individuals (2.10 mL/100g per min vs. –.079 mL/100g per min; P = .002).
Whether responses to caloric and noncaloric sweeteners are different between individuals with and without obesity has not been well established, though recent in vitro and in vivo studies have suggested an association.
“A proposed mechanism is that noncaloric sweeteners uncouple sweetness from calorie intake, which may impact neurophysiological regulators of feeding behavior,” wrote Mr. Ge and his collaborators in an abstract presented at the annual meeting of the Endocrine Society. Still, the work attempts to fill a knowledge gap: “Little evidence, however, has determined the relationship between obesity status and neurophysiological and feeding responses to caloric and non-caloric sweetener consumption,” they wrote.
Of the 30 participants aged 19-24 years, 16 were female; half were lean, with a body mass index of 19-25 kg/m2; the remainder met obesity criteria, with BMIs greater than 30 kg/m2.
For the brain-imaging portion of the study, arterial spin labeling fMRI was used to examine blood flow in a number of predetermined regions of interest. These included the hypothalamus, amygdala, dorsal striatum, insula, and anterior cingulate cortex.
Participants had three scans, spaced at least 2 days apart and occurring after a 12-hour overnight fast. A scan with arterial spin labeling acquisition was taken before and 10 minutes after participants drank a 300-mL beverage consisting of just water, or either a 75-g glucose solution or 2 mmol/L sucralose.
Twenty-five of the participants had blood drawn at 0, 40, and 60 minutes after drinking the study beverage, to track levels of serum insulin, ghrelin, GLP-1, and peptide YY – all hormones that help regulate appetite and satiety.
Hormone levels for individuals who had the non–glucose beverages were similar, regardless of BMI. However, there were significant differences in cerebral blood flow between obese and nonobese participants. Mr. Ge, an undergraduate student, and his collaborators looked at the contributions of the individual brain structures to the significantly higher activation seen after sucralose consumption by the high-BMI participants. Individuals with obesity had significantly more activity in the amygdala than did the lean participants (P = .0088) after drinking the sucralose beverage; also, in lean individuals, hypothalamic activity decreased after sucralose consumption, while activity increased slightly in the high-BMI participants (P = .017).
Eating behavior after drinking the various beverages also differed depending on beverage type and BMI status. After the overnight fast and study beverage consumption, participants were offered unlimited access to a buffet-style meal. The beverage type had no significant effect on calorie consumption at the buffet for the lean study participants. However, obese individuals consumed significantly more calories than did lean individuals after ingesting sucralose (1,191 kcal vs. 731 kcal; P = .01). Caloric intake was not significantly different between the high- and low-BMI groups after consumption of water or glucose.
None of the study authors reported conflicts of interest.
SOURCE: Ge B et al. ENDO 2018, Abstract SUN-070.
CHICAGO – but not in lean participants of a recent study, even though hunger and satiety hormone levels didn’t change. Those with obesity also consumed more calories after ingesting the artificial sweetener, though lean participants did not.
The study compared acute effects of consuming a set amount of glucose, sucralose, or water as the control, finding that sucralose consumption resulted in a significant increase in blood flow to certain areas of the brains of study participants with obesity but not in lean individuals (2.10 mL/100g per min vs. –.079 mL/100g per min; P = .002).
Whether responses to caloric and noncaloric sweeteners are different between individuals with and without obesity has not been well established, though recent in vitro and in vivo studies have suggested an association.
“A proposed mechanism is that noncaloric sweeteners uncouple sweetness from calorie intake, which may impact neurophysiological regulators of feeding behavior,” wrote Mr. Ge and his collaborators in an abstract presented at the annual meeting of the Endocrine Society. Still, the work attempts to fill a knowledge gap: “Little evidence, however, has determined the relationship between obesity status and neurophysiological and feeding responses to caloric and non-caloric sweetener consumption,” they wrote.
Of the 30 participants aged 19-24 years, 16 were female; half were lean, with a body mass index of 19-25 kg/m2; the remainder met obesity criteria, with BMIs greater than 30 kg/m2.
For the brain-imaging portion of the study, arterial spin labeling fMRI was used to examine blood flow in a number of predetermined regions of interest. These included the hypothalamus, amygdala, dorsal striatum, insula, and anterior cingulate cortex.
Participants had three scans, spaced at least 2 days apart and occurring after a 12-hour overnight fast. A scan with arterial spin labeling acquisition was taken before and 10 minutes after participants drank a 300-mL beverage consisting of just water, or either a 75-g glucose solution or 2 mmol/L sucralose.
Twenty-five of the participants had blood drawn at 0, 40, and 60 minutes after drinking the study beverage, to track levels of serum insulin, ghrelin, GLP-1, and peptide YY – all hormones that help regulate appetite and satiety.
Hormone levels for individuals who had the non–glucose beverages were similar, regardless of BMI. However, there were significant differences in cerebral blood flow between obese and nonobese participants. Mr. Ge, an undergraduate student, and his collaborators looked at the contributions of the individual brain structures to the significantly higher activation seen after sucralose consumption by the high-BMI participants. Individuals with obesity had significantly more activity in the amygdala than did the lean participants (P = .0088) after drinking the sucralose beverage; also, in lean individuals, hypothalamic activity decreased after sucralose consumption, while activity increased slightly in the high-BMI participants (P = .017).
Eating behavior after drinking the various beverages also differed depending on beverage type and BMI status. After the overnight fast and study beverage consumption, participants were offered unlimited access to a buffet-style meal. The beverage type had no significant effect on calorie consumption at the buffet for the lean study participants. However, obese individuals consumed significantly more calories than did lean individuals after ingesting sucralose (1,191 kcal vs. 731 kcal; P = .01). Caloric intake was not significantly different between the high- and low-BMI groups after consumption of water or glucose.
None of the study authors reported conflicts of interest.
SOURCE: Ge B et al. ENDO 2018, Abstract SUN-070.
CHICAGO – but not in lean participants of a recent study, even though hunger and satiety hormone levels didn’t change. Those with obesity also consumed more calories after ingesting the artificial sweetener, though lean participants did not.
The study compared acute effects of consuming a set amount of glucose, sucralose, or water as the control, finding that sucralose consumption resulted in a significant increase in blood flow to certain areas of the brains of study participants with obesity but not in lean individuals (2.10 mL/100g per min vs. –.079 mL/100g per min; P = .002).
Whether responses to caloric and noncaloric sweeteners are different between individuals with and without obesity has not been well established, though recent in vitro and in vivo studies have suggested an association.
“A proposed mechanism is that noncaloric sweeteners uncouple sweetness from calorie intake, which may impact neurophysiological regulators of feeding behavior,” wrote Mr. Ge and his collaborators in an abstract presented at the annual meeting of the Endocrine Society. Still, the work attempts to fill a knowledge gap: “Little evidence, however, has determined the relationship between obesity status and neurophysiological and feeding responses to caloric and non-caloric sweetener consumption,” they wrote.
Of the 30 participants aged 19-24 years, 16 were female; half were lean, with a body mass index of 19-25 kg/m2; the remainder met obesity criteria, with BMIs greater than 30 kg/m2.
For the brain-imaging portion of the study, arterial spin labeling fMRI was used to examine blood flow in a number of predetermined regions of interest. These included the hypothalamus, amygdala, dorsal striatum, insula, and anterior cingulate cortex.
Participants had three scans, spaced at least 2 days apart and occurring after a 12-hour overnight fast. A scan with arterial spin labeling acquisition was taken before and 10 minutes after participants drank a 300-mL beverage consisting of just water, or either a 75-g glucose solution or 2 mmol/L sucralose.
Twenty-five of the participants had blood drawn at 0, 40, and 60 minutes after drinking the study beverage, to track levels of serum insulin, ghrelin, GLP-1, and peptide YY – all hormones that help regulate appetite and satiety.
Hormone levels for individuals who had the non–glucose beverages were similar, regardless of BMI. However, there were significant differences in cerebral blood flow between obese and nonobese participants. Mr. Ge, an undergraduate student, and his collaborators looked at the contributions of the individual brain structures to the significantly higher activation seen after sucralose consumption by the high-BMI participants. Individuals with obesity had significantly more activity in the amygdala than did the lean participants (P = .0088) after drinking the sucralose beverage; also, in lean individuals, hypothalamic activity decreased after sucralose consumption, while activity increased slightly in the high-BMI participants (P = .017).
Eating behavior after drinking the various beverages also differed depending on beverage type and BMI status. After the overnight fast and study beverage consumption, participants were offered unlimited access to a buffet-style meal. The beverage type had no significant effect on calorie consumption at the buffet for the lean study participants. However, obese individuals consumed significantly more calories than did lean individuals after ingesting sucralose (1,191 kcal vs. 731 kcal; P = .01). Caloric intake was not significantly different between the high- and low-BMI groups after consumption of water or glucose.
None of the study authors reported conflicts of interest.
SOURCE: Ge B et al. ENDO 2018, Abstract SUN-070.
REPORTING FROM ENDO 2018
Key clinical point: Sucralose ingestion upped activity in brain appetite centers only for those with obesity.
Major finding: Cerebral blood flow for regions of interest was 2.10 mL/100g per min versus –0.79 mL/100g per min after sucralose consumption by obese individuals (P = .002).
Study details: Randomized placebo-controlled trial in 15 lean participants and 15 with obesity.
Disclosures: The authors reported no external sources of funding and no conflicts of interest.
Source: Ge B et al. ENDO 2018, Abstract SUN-070.
Estrogen patch counters eating disorders in women athletes
CHICAGO – It’s a good idea to look for eating disorders in young, athletic women who present with oligomenorrhea; they are at high risk for them and can be helped with estrogen replacement, especially with a patch, according to investigators from Massachusetts General Hospital, Boston.
Estrogen replacement will also help with stress fractures and other bone problems, but young, athletic women might resist the idea. They worry that it might hurt their performance and are often proud that they work out hard enough to stop their periods, according to investigator Vibha Singhal, MD, a pediatric endocrinologist and eating disorder specialist at the hospital.
Earlier research done at Massachusetts General had shown that replacing estrogen to physiological levels helped reduce anxiety and body dissatisfaction in anorexia nervosa. Both are key drivers of the condition, along with the drive for thinness, lead investigator Franziska Plessow, PhD, a neuroendocrine researcher at the hospital, said at the Endocrine Society’s annual meeting.
But the researchers wondered whether estrogen also helps healthy weight women with nascent eating disorders, so the investigators turned to female athletes aged 14-25 years who had stopped menstruating or were about to.
The 117 oligomenorrheic athletes (OA) they investigated – none of whom had formally diagnosed eating disorders – scored significantly higher on measures of body dissatisfaction, drive for thinness, perfectionism, and “cognitive restraint of eating,” compared with 50 female athletes and 41 nonathletic women, both with normal periods. For instance, OA women scored a mean of 4.21 on the drive for thinness scale of the Eating Disorder Inventory–2, a low score, but still significantly higher than the 1.66 points in eumenorrheic athletes and 1.61 points in nonathletes (P = .0005).
Oligomenorrheic female athletes “show more disordered eating behavior and psychopathology at the subclinical level,” Dr. Plessow said.
Seventy OA women were then randomized to three groups: 25 to physiological estrogen replacement with an estradiol patch and cyclic progesterone for 12 days/month; 19 to replacement with contraceptive pills on the standard monthly schedule; and 26 to no replacement.
Over 12 months, women on the patch dropped their drive for thinness, body dissatisfaction, and uncontrolled eating scores. Body dissatisfaction scores on the Eating Disorder Inventory–2, for example, fell about two points, compared with staying about the same in the pill group and increasing by about two points in the no-estrogen group.
The pill seemed to help a bit on some measures, too, but the benefit of the pill versus that of no estrogen wasn’t generally statistically significant. Meanwhile, symptoms worsened in women who didn’t get estrogen. “The practice right now is the pill. We are shifting our practice with these data to the patch,” Dr. Singhal said.
Overall, “these findings emphasize the importance of normalizing estrogen levels in this population,” she and her colleagues concluded.
It’s a mystery why estrogen helps with eating disorders. Maybe it has something to do with the estrogen receptors in the appetite centers of the brain. Maybe the patch works better than the pill because there’s no first-pass through the liver, Dr. Singhal said.
Subjects were aged about 20 years, on average. OA women had a mean body mass index of 20.6 kg/m2 and a mean estradiol level of 45 pg/mL. Subjects with regular periods had mean BMIs of about 22 kg/m2; mean estradiol levels were 70.2 pg/mL in eumenorrheic athletes and 83.6 pg/mL in nonathletic women.
The work was funded by the National Institutes of Health. The investigators didn’t have any relevant disclosures.
SOURCE: Plessow F et al. ENDO 2018, Abstract SAT-290.
CHICAGO – It’s a good idea to look for eating disorders in young, athletic women who present with oligomenorrhea; they are at high risk for them and can be helped with estrogen replacement, especially with a patch, according to investigators from Massachusetts General Hospital, Boston.
Estrogen replacement will also help with stress fractures and other bone problems, but young, athletic women might resist the idea. They worry that it might hurt their performance and are often proud that they work out hard enough to stop their periods, according to investigator Vibha Singhal, MD, a pediatric endocrinologist and eating disorder specialist at the hospital.
Earlier research done at Massachusetts General had shown that replacing estrogen to physiological levels helped reduce anxiety and body dissatisfaction in anorexia nervosa. Both are key drivers of the condition, along with the drive for thinness, lead investigator Franziska Plessow, PhD, a neuroendocrine researcher at the hospital, said at the Endocrine Society’s annual meeting.
But the researchers wondered whether estrogen also helps healthy weight women with nascent eating disorders, so the investigators turned to female athletes aged 14-25 years who had stopped menstruating or were about to.
The 117 oligomenorrheic athletes (OA) they investigated – none of whom had formally diagnosed eating disorders – scored significantly higher on measures of body dissatisfaction, drive for thinness, perfectionism, and “cognitive restraint of eating,” compared with 50 female athletes and 41 nonathletic women, both with normal periods. For instance, OA women scored a mean of 4.21 on the drive for thinness scale of the Eating Disorder Inventory–2, a low score, but still significantly higher than the 1.66 points in eumenorrheic athletes and 1.61 points in nonathletes (P = .0005).
Oligomenorrheic female athletes “show more disordered eating behavior and psychopathology at the subclinical level,” Dr. Plessow said.
Seventy OA women were then randomized to three groups: 25 to physiological estrogen replacement with an estradiol patch and cyclic progesterone for 12 days/month; 19 to replacement with contraceptive pills on the standard monthly schedule; and 26 to no replacement.
Over 12 months, women on the patch dropped their drive for thinness, body dissatisfaction, and uncontrolled eating scores. Body dissatisfaction scores on the Eating Disorder Inventory–2, for example, fell about two points, compared with staying about the same in the pill group and increasing by about two points in the no-estrogen group.
The pill seemed to help a bit on some measures, too, but the benefit of the pill versus that of no estrogen wasn’t generally statistically significant. Meanwhile, symptoms worsened in women who didn’t get estrogen. “The practice right now is the pill. We are shifting our practice with these data to the patch,” Dr. Singhal said.
Overall, “these findings emphasize the importance of normalizing estrogen levels in this population,” she and her colleagues concluded.
It’s a mystery why estrogen helps with eating disorders. Maybe it has something to do with the estrogen receptors in the appetite centers of the brain. Maybe the patch works better than the pill because there’s no first-pass through the liver, Dr. Singhal said.
Subjects were aged about 20 years, on average. OA women had a mean body mass index of 20.6 kg/m2 and a mean estradiol level of 45 pg/mL. Subjects with regular periods had mean BMIs of about 22 kg/m2; mean estradiol levels were 70.2 pg/mL in eumenorrheic athletes and 83.6 pg/mL in nonathletic women.
The work was funded by the National Institutes of Health. The investigators didn’t have any relevant disclosures.
SOURCE: Plessow F et al. ENDO 2018, Abstract SAT-290.
CHICAGO – It’s a good idea to look for eating disorders in young, athletic women who present with oligomenorrhea; they are at high risk for them and can be helped with estrogen replacement, especially with a patch, according to investigators from Massachusetts General Hospital, Boston.
Estrogen replacement will also help with stress fractures and other bone problems, but young, athletic women might resist the idea. They worry that it might hurt their performance and are often proud that they work out hard enough to stop their periods, according to investigator Vibha Singhal, MD, a pediatric endocrinologist and eating disorder specialist at the hospital.
Earlier research done at Massachusetts General had shown that replacing estrogen to physiological levels helped reduce anxiety and body dissatisfaction in anorexia nervosa. Both are key drivers of the condition, along with the drive for thinness, lead investigator Franziska Plessow, PhD, a neuroendocrine researcher at the hospital, said at the Endocrine Society’s annual meeting.
But the researchers wondered whether estrogen also helps healthy weight women with nascent eating disorders, so the investigators turned to female athletes aged 14-25 years who had stopped menstruating or were about to.
The 117 oligomenorrheic athletes (OA) they investigated – none of whom had formally diagnosed eating disorders – scored significantly higher on measures of body dissatisfaction, drive for thinness, perfectionism, and “cognitive restraint of eating,” compared with 50 female athletes and 41 nonathletic women, both with normal periods. For instance, OA women scored a mean of 4.21 on the drive for thinness scale of the Eating Disorder Inventory–2, a low score, but still significantly higher than the 1.66 points in eumenorrheic athletes and 1.61 points in nonathletes (P = .0005).
Oligomenorrheic female athletes “show more disordered eating behavior and psychopathology at the subclinical level,” Dr. Plessow said.
Seventy OA women were then randomized to three groups: 25 to physiological estrogen replacement with an estradiol patch and cyclic progesterone for 12 days/month; 19 to replacement with contraceptive pills on the standard monthly schedule; and 26 to no replacement.
Over 12 months, women on the patch dropped their drive for thinness, body dissatisfaction, and uncontrolled eating scores. Body dissatisfaction scores on the Eating Disorder Inventory–2, for example, fell about two points, compared with staying about the same in the pill group and increasing by about two points in the no-estrogen group.
The pill seemed to help a bit on some measures, too, but the benefit of the pill versus that of no estrogen wasn’t generally statistically significant. Meanwhile, symptoms worsened in women who didn’t get estrogen. “The practice right now is the pill. We are shifting our practice with these data to the patch,” Dr. Singhal said.
Overall, “these findings emphasize the importance of normalizing estrogen levels in this population,” she and her colleagues concluded.
It’s a mystery why estrogen helps with eating disorders. Maybe it has something to do with the estrogen receptors in the appetite centers of the brain. Maybe the patch works better than the pill because there’s no first-pass through the liver, Dr. Singhal said.
Subjects were aged about 20 years, on average. OA women had a mean body mass index of 20.6 kg/m2 and a mean estradiol level of 45 pg/mL. Subjects with regular periods had mean BMIs of about 22 kg/m2; mean estradiol levels were 70.2 pg/mL in eumenorrheic athletes and 83.6 pg/mL in nonathletic women.
The work was funded by the National Institutes of Health. The investigators didn’t have any relevant disclosures.
SOURCE: Plessow F et al. ENDO 2018, Abstract SAT-290.
REPORTING FROM ENDO 2018
Key clinical point: It’s a good idea to look for eating disorders in young, athletic women who present with oligo-amenorrhea; they’re at high risk for them and can be helped with estrogen replacement, especially with a patch.
Major finding: Over 12 months, body dissatisfaction scores fell about 2 points among women on the patch, versus staying about the same in the pill group, and increasing about 2 points in the no-estrogen group.
Study details: Combined cross-sectional and randomized investigation
Disclosures: The work was supported by the National Institutes of Health. The investigators didn’t have any disclosures.
Source: Plessow F et al. ENDO 2018 abstract SAT-290
Transgender women on HT have lower bone density, more fat mass than men
CHICAGO – according to findings from a recent Brazilian study.
“Lumbar spine density was lower than in reference men but similar to that of reference women,” said Tayane Muniz Fighera, MD, speaking at the annual meeting of the Endocrine Society.
Lower lumbar spine density in transgender women was associated with lower appendicular lean mass and higher total fat mass, with correlation coefficients of 0.327 and 0.334, respectively (P = .0001 for both).
Dr. Fighera and her colleagues looked at the independent contribution of age, estradiol level, appendicular lean mass, and fat mass to bone mineral density (BMD) in the transgender patients, using linear regression analysis. Total fat mass and appendicular lean mass were both independent predictors of bone mineral density (P = .001 and P = .022, respectively). For femur BMD, age, and total fat mass were predictors (P = .001 and P = .000, respectively).
The study aimed to assess bone mineral density as well as other aspects of body composition within a cohort of transgender women initiating hormone therapy in order to determine how estrogen therapy affected BMD and assess the prevalence of low bone mass among this population.
The hypothesis, said Dr. Fighera, was that hormone therapy for transgender women might decrease muscle mass and increase fat mass, “leading to less bone surface strain and smaller bone size over time,” said Dr. Fighera, of the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Previous work has shown conflicting results, she said. “While some studies report that estrogen therapy is able to increase bone mass, others have observed no difference in BMD” despite the use of hormone therapy. The studies showing an association between estrogen therapy and decreased bone mass were those that followed patients for longer periods of time – 2 years or longer, she said.
Dr. Fighera explained that in Brazil, individuals with gender dysphoria have free access to hormone therapy and gender-affirming surgery through the public health service.
A total of 142 transgender women enrolled in the study, conducted at outpatient endocrine clinics for transgender people in Porto Alegre, Brazil. The clinics’ standardized hormone therapy protocol used daily estradiol valerate 1-4 mg, daily conjugated equine estrogen 0.625-2.5 mg, or daily transdermal 17 beta estradiol 0.5-2 mg. The estrogen therapy was accompanied by either spironolactone 50-150 mg per day, or cyproterone acetate 50-100 mg per day.
For comparison, the investigators enrolled 22 men and 17 women aged 18-40 years. All participants received a dual-energy x-ray absorptiometry (DXA) scan 3 months after those in the transgender arm began hormone therapy, and a second scan at 12 months. For the first year, participants were seen for clinical evaluation and lab studies every 3 months; they were seen every 6 months thereafter.
Although ranges were wide, estradiol levels in transgender women were, on average, approximately intermediate between the female and male control values. Total testosterone for transgender women was an average 1.17 nmol/L, closer to female (0.79 nmol/L) than male (16.39 nmol/L) values.
In a subgroup of 46 participants, Dr. Fighera and her colleagues also examined change over time for transgender women who remained on hormone therapy. Though they did find that appendicular lean mass declined and total fat mass increased from baseline, these changes in body composition were not associated with significant decreases in any BMD measurement when the DXA scan was repeated at 31 months.
Participants’ mean age was 33.7 years, and the mean BMI was 25.4 kg/m2. One-third of participants had already undergone gender-affirming surgery , and most (86.6%) had some previous exposure to hormone therapy. Almost all (96%) of study participants were white.
At 18%, “the prevalence of low bone mass for age was fairly high in this sample of [transgender women] from southern Brazil,” said Dr. Fighera. She called for more work to track change over time in hormone therapy–related bone loss for transgender women. “Until then, monitoring of bone mass should be considered in this population; nonpharmacological lifestyle-related strategies for preventing bone loss may benefit transgender women” who receive long-term hormone therapy, she said.
None of the study authors had disclosures to report.
SOURCE: Fighera T et al. ENDO 2018, Abstract OR 25-5.
CHICAGO – according to findings from a recent Brazilian study.
“Lumbar spine density was lower than in reference men but similar to that of reference women,” said Tayane Muniz Fighera, MD, speaking at the annual meeting of the Endocrine Society.
Lower lumbar spine density in transgender women was associated with lower appendicular lean mass and higher total fat mass, with correlation coefficients of 0.327 and 0.334, respectively (P = .0001 for both).
Dr. Fighera and her colleagues looked at the independent contribution of age, estradiol level, appendicular lean mass, and fat mass to bone mineral density (BMD) in the transgender patients, using linear regression analysis. Total fat mass and appendicular lean mass were both independent predictors of bone mineral density (P = .001 and P = .022, respectively). For femur BMD, age, and total fat mass were predictors (P = .001 and P = .000, respectively).
The study aimed to assess bone mineral density as well as other aspects of body composition within a cohort of transgender women initiating hormone therapy in order to determine how estrogen therapy affected BMD and assess the prevalence of low bone mass among this population.
The hypothesis, said Dr. Fighera, was that hormone therapy for transgender women might decrease muscle mass and increase fat mass, “leading to less bone surface strain and smaller bone size over time,” said Dr. Fighera, of the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Previous work has shown conflicting results, she said. “While some studies report that estrogen therapy is able to increase bone mass, others have observed no difference in BMD” despite the use of hormone therapy. The studies showing an association between estrogen therapy and decreased bone mass were those that followed patients for longer periods of time – 2 years or longer, she said.
Dr. Fighera explained that in Brazil, individuals with gender dysphoria have free access to hormone therapy and gender-affirming surgery through the public health service.
A total of 142 transgender women enrolled in the study, conducted at outpatient endocrine clinics for transgender people in Porto Alegre, Brazil. The clinics’ standardized hormone therapy protocol used daily estradiol valerate 1-4 mg, daily conjugated equine estrogen 0.625-2.5 mg, or daily transdermal 17 beta estradiol 0.5-2 mg. The estrogen therapy was accompanied by either spironolactone 50-150 mg per day, or cyproterone acetate 50-100 mg per day.
For comparison, the investigators enrolled 22 men and 17 women aged 18-40 years. All participants received a dual-energy x-ray absorptiometry (DXA) scan 3 months after those in the transgender arm began hormone therapy, and a second scan at 12 months. For the first year, participants were seen for clinical evaluation and lab studies every 3 months; they were seen every 6 months thereafter.
Although ranges were wide, estradiol levels in transgender women were, on average, approximately intermediate between the female and male control values. Total testosterone for transgender women was an average 1.17 nmol/L, closer to female (0.79 nmol/L) than male (16.39 nmol/L) values.
In a subgroup of 46 participants, Dr. Fighera and her colleagues also examined change over time for transgender women who remained on hormone therapy. Though they did find that appendicular lean mass declined and total fat mass increased from baseline, these changes in body composition were not associated with significant decreases in any BMD measurement when the DXA scan was repeated at 31 months.
Participants’ mean age was 33.7 years, and the mean BMI was 25.4 kg/m2. One-third of participants had already undergone gender-affirming surgery , and most (86.6%) had some previous exposure to hormone therapy. Almost all (96%) of study participants were white.
At 18%, “the prevalence of low bone mass for age was fairly high in this sample of [transgender women] from southern Brazil,” said Dr. Fighera. She called for more work to track change over time in hormone therapy–related bone loss for transgender women. “Until then, monitoring of bone mass should be considered in this population; nonpharmacological lifestyle-related strategies for preventing bone loss may benefit transgender women” who receive long-term hormone therapy, she said.
None of the study authors had disclosures to report.
SOURCE: Fighera T et al. ENDO 2018, Abstract OR 25-5.
CHICAGO – according to findings from a recent Brazilian study.
“Lumbar spine density was lower than in reference men but similar to that of reference women,” said Tayane Muniz Fighera, MD, speaking at the annual meeting of the Endocrine Society.
Lower lumbar spine density in transgender women was associated with lower appendicular lean mass and higher total fat mass, with correlation coefficients of 0.327 and 0.334, respectively (P = .0001 for both).
Dr. Fighera and her colleagues looked at the independent contribution of age, estradiol level, appendicular lean mass, and fat mass to bone mineral density (BMD) in the transgender patients, using linear regression analysis. Total fat mass and appendicular lean mass were both independent predictors of bone mineral density (P = .001 and P = .022, respectively). For femur BMD, age, and total fat mass were predictors (P = .001 and P = .000, respectively).
The study aimed to assess bone mineral density as well as other aspects of body composition within a cohort of transgender women initiating hormone therapy in order to determine how estrogen therapy affected BMD and assess the prevalence of low bone mass among this population.
The hypothesis, said Dr. Fighera, was that hormone therapy for transgender women might decrease muscle mass and increase fat mass, “leading to less bone surface strain and smaller bone size over time,” said Dr. Fighera, of the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Previous work has shown conflicting results, she said. “While some studies report that estrogen therapy is able to increase bone mass, others have observed no difference in BMD” despite the use of hormone therapy. The studies showing an association between estrogen therapy and decreased bone mass were those that followed patients for longer periods of time – 2 years or longer, she said.
Dr. Fighera explained that in Brazil, individuals with gender dysphoria have free access to hormone therapy and gender-affirming surgery through the public health service.
A total of 142 transgender women enrolled in the study, conducted at outpatient endocrine clinics for transgender people in Porto Alegre, Brazil. The clinics’ standardized hormone therapy protocol used daily estradiol valerate 1-4 mg, daily conjugated equine estrogen 0.625-2.5 mg, or daily transdermal 17 beta estradiol 0.5-2 mg. The estrogen therapy was accompanied by either spironolactone 50-150 mg per day, or cyproterone acetate 50-100 mg per day.
For comparison, the investigators enrolled 22 men and 17 women aged 18-40 years. All participants received a dual-energy x-ray absorptiometry (DXA) scan 3 months after those in the transgender arm began hormone therapy, and a second scan at 12 months. For the first year, participants were seen for clinical evaluation and lab studies every 3 months; they were seen every 6 months thereafter.
Although ranges were wide, estradiol levels in transgender women were, on average, approximately intermediate between the female and male control values. Total testosterone for transgender women was an average 1.17 nmol/L, closer to female (0.79 nmol/L) than male (16.39 nmol/L) values.
In a subgroup of 46 participants, Dr. Fighera and her colleagues also examined change over time for transgender women who remained on hormone therapy. Though they did find that appendicular lean mass declined and total fat mass increased from baseline, these changes in body composition were not associated with significant decreases in any BMD measurement when the DXA scan was repeated at 31 months.
Participants’ mean age was 33.7 years, and the mean BMI was 25.4 kg/m2. One-third of participants had already undergone gender-affirming surgery , and most (86.6%) had some previous exposure to hormone therapy. Almost all (96%) of study participants were white.
At 18%, “the prevalence of low bone mass for age was fairly high in this sample of [transgender women] from southern Brazil,” said Dr. Fighera. She called for more work to track change over time in hormone therapy–related bone loss for transgender women. “Until then, monitoring of bone mass should be considered in this population; nonpharmacological lifestyle-related strategies for preventing bone loss may benefit transgender women” who receive long-term hormone therapy, she said.
None of the study authors had disclosures to report.
SOURCE: Fighera T et al. ENDO 2018, Abstract OR 25-5.
REPORTING FROM ENDO 2018
Key clinical point: Transgender women on hormone therapy have bone mass more similar to women than men.
Major finding: Lower lumbar spine density was associated with higher total fat mass (P = .001).
Study details: Study of 142 transgender women receiving hormone therapy, tracked over time and compared with 22 men and 17 women for reference.
Disclosures: The study was sponsored by the Brazilian government. The authors reported that they have no conflicts of interest.
Source: Fighera T et al. ENDO 2018, Abstract OR 25-5.
Empiric fluid restriction cuts transsphenoidal surgery readmissions
CHICAGO – A 70% drop in 30-day was achieved at the University of Colorado, Aurora, after endocrinologists there restricted fluid for the first 2 weeks postop, according to a report at the Endocrine Society annual meeting.
The antidiuretic hormone (ADH) rebound following pituitary adenoma resection often leads to fluid retention, and potentially dangerous hyponatremia, in about 25% of patients. It’s the leading cause of readmission for this procedure, occurring in up to 15% of patients.
To counter the problem, endocrinology fellow Kelsi Deaver, MD, and her colleagues limited patients to 1.5 L of fluid for 2 weeks after discharge, with a serum sodium check at day 7. If the sodium level was normal, patients remained on 1.5 L until the 2-week postop visit. If levels trended upward – a sign of dehydration – restrictions were eased to 2 or even 3 L, which is about the normal daily intake. If sodium levels trended downward, fluids were tightened to 1-1.2 L, or patients were brought in for further workup. The discharge packet included a 1.5-L cup so patients could track their intake.
Among 118 patients studied before the protocol was implemented in September 2015, 9 (7.6%) were readmitted for symptomatic hyponatremia within 30 days. Among 169 studied after the implementation of the fluid restriction protocol, just 4 (2.4%) were readmitted for hyponatremia (P = .044).
At present, there are no widely accepted postop fluid management guidelines for transsphenoidal surgery, but some hospitals have taken similar steps, she said.
It was the fluid restriction, not the 7-day sodium check, that drove the results. Among the four readmissions after the protocol took effect, two patients had their sodium checked, and two did not because their sodium drop was so precipitous that they were back in the hospital before the week was out. Overall, only about 70% of patients got their sodium checked as instructed.
Fluid restriction isn’t easy for patients. “The last day before discharge, we try to coach them through it,” with tips about sucking on ice chips and other strategies; “anything really to help them through it,” Dr. Deaver said.
Readmitted patients were no different from others in terms of pituitary tumor subtype, tumor size, gender, and other factors. “We couldn’t find any predictors,” she said. There were a higher percentage of macroadenomas in the preimplementation patients (91.5% versus 81.7%), but they were otherwise similar to postimplementation patients.
Those with evidence of diabetes insipidus at discharge were excluded from the study.
The National Institutes of Health funded the study. The investigators did not have any disclosures.
SOURCE: Deaver KE et al. Endocrine Society 2018 annual meeting abstract SUN-572.
CHICAGO – A 70% drop in 30-day was achieved at the University of Colorado, Aurora, after endocrinologists there restricted fluid for the first 2 weeks postop, according to a report at the Endocrine Society annual meeting.
The antidiuretic hormone (ADH) rebound following pituitary adenoma resection often leads to fluid retention, and potentially dangerous hyponatremia, in about 25% of patients. It’s the leading cause of readmission for this procedure, occurring in up to 15% of patients.
To counter the problem, endocrinology fellow Kelsi Deaver, MD, and her colleagues limited patients to 1.5 L of fluid for 2 weeks after discharge, with a serum sodium check at day 7. If the sodium level was normal, patients remained on 1.5 L until the 2-week postop visit. If levels trended upward – a sign of dehydration – restrictions were eased to 2 or even 3 L, which is about the normal daily intake. If sodium levels trended downward, fluids were tightened to 1-1.2 L, or patients were brought in for further workup. The discharge packet included a 1.5-L cup so patients could track their intake.
Among 118 patients studied before the protocol was implemented in September 2015, 9 (7.6%) were readmitted for symptomatic hyponatremia within 30 days. Among 169 studied after the implementation of the fluid restriction protocol, just 4 (2.4%) were readmitted for hyponatremia (P = .044).
At present, there are no widely accepted postop fluid management guidelines for transsphenoidal surgery, but some hospitals have taken similar steps, she said.
It was the fluid restriction, not the 7-day sodium check, that drove the results. Among the four readmissions after the protocol took effect, two patients had their sodium checked, and two did not because their sodium drop was so precipitous that they were back in the hospital before the week was out. Overall, only about 70% of patients got their sodium checked as instructed.
Fluid restriction isn’t easy for patients. “The last day before discharge, we try to coach them through it,” with tips about sucking on ice chips and other strategies; “anything really to help them through it,” Dr. Deaver said.
Readmitted patients were no different from others in terms of pituitary tumor subtype, tumor size, gender, and other factors. “We couldn’t find any predictors,” she said. There were a higher percentage of macroadenomas in the preimplementation patients (91.5% versus 81.7%), but they were otherwise similar to postimplementation patients.
Those with evidence of diabetes insipidus at discharge were excluded from the study.
The National Institutes of Health funded the study. The investigators did not have any disclosures.
SOURCE: Deaver KE et al. Endocrine Society 2018 annual meeting abstract SUN-572.
CHICAGO – A 70% drop in 30-day was achieved at the University of Colorado, Aurora, after endocrinologists there restricted fluid for the first 2 weeks postop, according to a report at the Endocrine Society annual meeting.
The antidiuretic hormone (ADH) rebound following pituitary adenoma resection often leads to fluid retention, and potentially dangerous hyponatremia, in about 25% of patients. It’s the leading cause of readmission for this procedure, occurring in up to 15% of patients.
To counter the problem, endocrinology fellow Kelsi Deaver, MD, and her colleagues limited patients to 1.5 L of fluid for 2 weeks after discharge, with a serum sodium check at day 7. If the sodium level was normal, patients remained on 1.5 L until the 2-week postop visit. If levels trended upward – a sign of dehydration – restrictions were eased to 2 or even 3 L, which is about the normal daily intake. If sodium levels trended downward, fluids were tightened to 1-1.2 L, or patients were brought in for further workup. The discharge packet included a 1.5-L cup so patients could track their intake.
Among 118 patients studied before the protocol was implemented in September 2015, 9 (7.6%) were readmitted for symptomatic hyponatremia within 30 days. Among 169 studied after the implementation of the fluid restriction protocol, just 4 (2.4%) were readmitted for hyponatremia (P = .044).
At present, there are no widely accepted postop fluid management guidelines for transsphenoidal surgery, but some hospitals have taken similar steps, she said.
It was the fluid restriction, not the 7-day sodium check, that drove the results. Among the four readmissions after the protocol took effect, two patients had their sodium checked, and two did not because their sodium drop was so precipitous that they were back in the hospital before the week was out. Overall, only about 70% of patients got their sodium checked as instructed.
Fluid restriction isn’t easy for patients. “The last day before discharge, we try to coach them through it,” with tips about sucking on ice chips and other strategies; “anything really to help them through it,” Dr. Deaver said.
Readmitted patients were no different from others in terms of pituitary tumor subtype, tumor size, gender, and other factors. “We couldn’t find any predictors,” she said. There were a higher percentage of macroadenomas in the preimplementation patients (91.5% versus 81.7%), but they were otherwise similar to postimplementation patients.
Those with evidence of diabetes insipidus at discharge were excluded from the study.
The National Institutes of Health funded the study. The investigators did not have any disclosures.
SOURCE: Deaver KE et al. Endocrine Society 2018 annual meeting abstract SUN-572.
REPORTING FROM ENDO 2018
Key clinical point: A simple fluid restriction protocol cuts readmissions 70% following transsphenoidal surgery.
Major finding: The readmission rate among the transsphenoidal surgery patients was 7.6% before the fluid restriction protocol was implemented, compared with 2.4% (P = .044) afterward.
Study details: Review of 287 transsphenoidal surgery patients.
Disclosures: The National Institutes of Health supported the work. The investigators did not have any disclosures.
Source: Deaver KE et al. Abstract SUN-572
Diabetes from checkpoint inhibitors probably means lifelong insulin
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
REPORTING FROM ENDO 2018
Key clinical point: Be on the lookout for new-onset diabetes when patients start immune checkpoint inhibitors.
Major finding: In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs.
Study details: Review of 24 cases.
Disclosures: The investigators had no disclosures. A funding source was not reported.
Source: Iyer PC et al. Abstract OR05-5.
VIDEO: Poorer cardiometabolic health seen in men with low sperm count
CHICAGO – Low testosterone levels alone didn’t account for the finding, said Alberto Ferlin, MD, PhD, professor of reproductive endocrinology at the University of Brescia, Italy.
“So at the end, we showed that, independent of testosterone, low sperm count could be a marker of general male health, in particular for cardiovascular risk factors or metabolic derangement,” said Dr. Ferlin in an interview following a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The Italian study, which Dr. Ferlin said was the largest of its kind to date, studied 5,177 males who were part of an infertile couple, comparing men with low sperm count (less than 39 million sperm per ejaculate) with those with normal sperm count (at least 39 million sperm per ejaculate). In all, 2,583 of the participants had low sperm counts.
“Our main aim was to understand if semen analysis and, in general, the reproductive function of a man, could be a marker of his general cardiovascular and metabolic health,” said Dr. Ferlin.
Only men with a comprehensive work-up were included, so all participants had a medical history and physical exam, and semen analysis and culture. Additional components of the evaluation included blood lipid and glucose metabolism testing, reproductive hormone levels, ultrasound of the testes and, for men diagnosed with hypogonadism, bone densitometry.
The study, said Dr. Ferlin, found that among men with a low total sperm count, there was a high prevalence of hypogonadism, defined as both low testosterone and elevated levels of luteinizing hormone. Additionally, these men had a high prevalence of elevated luteinizing hormones with normal testosterone – “so-called subclinical hypogonadism,” said Dr. Ferlin.
In men with a low sperm count – defined as fewer than 39 million sperm per ejaculate – the prevalence of biochemical hypogonadism was about 45%, compared with just 6% in men with normal sperm counts, said Dr. Ferlin. Men with infertility had an odds ratio for hypogonadism of 12.2, said Dr. Ferlin (95% confidence interval, 10.2-14.6).
Additionally, Dr. Ferlin reported that 35% of men with hypogonadism had osteopenia, and 17% met criteria for osteoporosis. The numbers surprised the investigators. “These are very young men – about 30 years old,” said Dr. Ferlin.
Dr. Ferlin and his collaborators also looked at the subset of eugonadal men in the study, comparing those with normal sperm counts (n = 2,431) to those who had low sperm counts, (n = 1,423). They found that men with low sperm counts had significantly higher body mass index, waist circumference, systolic blood pressure, hemoglobin A1c, and homeostatic model assessment of insulin resistance (HOMA-IR) levels (P less than .001 for all).
High density lipoprotein (HDL) cholesterol, testosterone, and follicle stimulating hormone levels were also significantly lower for men with low sperm count. “Men with oligozoospermia … have an increased risk of metabolic derangement – so, altered lipid profile with higher LDL cholesterol and lower HDL [cholesterol], higher triglycerides, higher insulin resistance,” said Dr. Ferlin.
The findings have implications for reproductive endocrinologists caring for couples with infertility, said Dr. Ferlin. “Infertile men should be studied comprehensively, and the diagnosis cannot be limited to just one semen analysis,” given the study’s findings, he said. “All these men should be counseled, should be treated … for worsening of these cardiovascular and metabolic risk factors that are present in such frequency in oligozoospermic men.”
Dr. Ferlin reported no conflicts of interest.
CHICAGO – Low testosterone levels alone didn’t account for the finding, said Alberto Ferlin, MD, PhD, professor of reproductive endocrinology at the University of Brescia, Italy.
“So at the end, we showed that, independent of testosterone, low sperm count could be a marker of general male health, in particular for cardiovascular risk factors or metabolic derangement,” said Dr. Ferlin in an interview following a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The Italian study, which Dr. Ferlin said was the largest of its kind to date, studied 5,177 males who were part of an infertile couple, comparing men with low sperm count (less than 39 million sperm per ejaculate) with those with normal sperm count (at least 39 million sperm per ejaculate). In all, 2,583 of the participants had low sperm counts.
“Our main aim was to understand if semen analysis and, in general, the reproductive function of a man, could be a marker of his general cardiovascular and metabolic health,” said Dr. Ferlin.
Only men with a comprehensive work-up were included, so all participants had a medical history and physical exam, and semen analysis and culture. Additional components of the evaluation included blood lipid and glucose metabolism testing, reproductive hormone levels, ultrasound of the testes and, for men diagnosed with hypogonadism, bone densitometry.
The study, said Dr. Ferlin, found that among men with a low total sperm count, there was a high prevalence of hypogonadism, defined as both low testosterone and elevated levels of luteinizing hormone. Additionally, these men had a high prevalence of elevated luteinizing hormones with normal testosterone – “so-called subclinical hypogonadism,” said Dr. Ferlin.
In men with a low sperm count – defined as fewer than 39 million sperm per ejaculate – the prevalence of biochemical hypogonadism was about 45%, compared with just 6% in men with normal sperm counts, said Dr. Ferlin. Men with infertility had an odds ratio for hypogonadism of 12.2, said Dr. Ferlin (95% confidence interval, 10.2-14.6).
Additionally, Dr. Ferlin reported that 35% of men with hypogonadism had osteopenia, and 17% met criteria for osteoporosis. The numbers surprised the investigators. “These are very young men – about 30 years old,” said Dr. Ferlin.
Dr. Ferlin and his collaborators also looked at the subset of eugonadal men in the study, comparing those with normal sperm counts (n = 2,431) to those who had low sperm counts, (n = 1,423). They found that men with low sperm counts had significantly higher body mass index, waist circumference, systolic blood pressure, hemoglobin A1c, and homeostatic model assessment of insulin resistance (HOMA-IR) levels (P less than .001 for all).
High density lipoprotein (HDL) cholesterol, testosterone, and follicle stimulating hormone levels were also significantly lower for men with low sperm count. “Men with oligozoospermia … have an increased risk of metabolic derangement – so, altered lipid profile with higher LDL cholesterol and lower HDL [cholesterol], higher triglycerides, higher insulin resistance,” said Dr. Ferlin.
The findings have implications for reproductive endocrinologists caring for couples with infertility, said Dr. Ferlin. “Infertile men should be studied comprehensively, and the diagnosis cannot be limited to just one semen analysis,” given the study’s findings, he said. “All these men should be counseled, should be treated … for worsening of these cardiovascular and metabolic risk factors that are present in such frequency in oligozoospermic men.”
Dr. Ferlin reported no conflicts of interest.
CHICAGO – Low testosterone levels alone didn’t account for the finding, said Alberto Ferlin, MD, PhD, professor of reproductive endocrinology at the University of Brescia, Italy.
“So at the end, we showed that, independent of testosterone, low sperm count could be a marker of general male health, in particular for cardiovascular risk factors or metabolic derangement,” said Dr. Ferlin in an interview following a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The Italian study, which Dr. Ferlin said was the largest of its kind to date, studied 5,177 males who were part of an infertile couple, comparing men with low sperm count (less than 39 million sperm per ejaculate) with those with normal sperm count (at least 39 million sperm per ejaculate). In all, 2,583 of the participants had low sperm counts.
“Our main aim was to understand if semen analysis and, in general, the reproductive function of a man, could be a marker of his general cardiovascular and metabolic health,” said Dr. Ferlin.
Only men with a comprehensive work-up were included, so all participants had a medical history and physical exam, and semen analysis and culture. Additional components of the evaluation included blood lipid and glucose metabolism testing, reproductive hormone levels, ultrasound of the testes and, for men diagnosed with hypogonadism, bone densitometry.
The study, said Dr. Ferlin, found that among men with a low total sperm count, there was a high prevalence of hypogonadism, defined as both low testosterone and elevated levels of luteinizing hormone. Additionally, these men had a high prevalence of elevated luteinizing hormones with normal testosterone – “so-called subclinical hypogonadism,” said Dr. Ferlin.
In men with a low sperm count – defined as fewer than 39 million sperm per ejaculate – the prevalence of biochemical hypogonadism was about 45%, compared with just 6% in men with normal sperm counts, said Dr. Ferlin. Men with infertility had an odds ratio for hypogonadism of 12.2, said Dr. Ferlin (95% confidence interval, 10.2-14.6).
Additionally, Dr. Ferlin reported that 35% of men with hypogonadism had osteopenia, and 17% met criteria for osteoporosis. The numbers surprised the investigators. “These are very young men – about 30 years old,” said Dr. Ferlin.
Dr. Ferlin and his collaborators also looked at the subset of eugonadal men in the study, comparing those with normal sperm counts (n = 2,431) to those who had low sperm counts, (n = 1,423). They found that men with low sperm counts had significantly higher body mass index, waist circumference, systolic blood pressure, hemoglobin A1c, and homeostatic model assessment of insulin resistance (HOMA-IR) levels (P less than .001 for all).
High density lipoprotein (HDL) cholesterol, testosterone, and follicle stimulating hormone levels were also significantly lower for men with low sperm count. “Men with oligozoospermia … have an increased risk of metabolic derangement – so, altered lipid profile with higher LDL cholesterol and lower HDL [cholesterol], higher triglycerides, higher insulin resistance,” said Dr. Ferlin.
The findings have implications for reproductive endocrinologists caring for couples with infertility, said Dr. Ferlin. “Infertile men should be studied comprehensively, and the diagnosis cannot be limited to just one semen analysis,” given the study’s findings, he said. “All these men should be counseled, should be treated … for worsening of these cardiovascular and metabolic risk factors that are present in such frequency in oligozoospermic men.”
Dr. Ferlin reported no conflicts of interest.
REPORTING FROM ENDO 2018
Levothyroxine may increase mortality in older patients
CHICAGO – according to a study presented at the annual meeting of the Endocrine Society.
With increased mortality and similar prevalence of atrial fibrillation and femoral fractures between study and control groups, physicians may want to reevaluate giving their elderly patients levothyroxine until more information is available, according to presenter Joseph Meyerovitch, MD, of Schneider Children’s Medical Center, Ramat Hasharon, Israel.
The case-control study included 416 patients 65 years or older with TSH levels of 4.2-10 mIU/L who died between 2012 and 2016, and 1,461 patients with comparable TSH levels who did not die during that time.
Most of the patients in both the control and study group were women. The average age of the patients was 84 years, and most had some form of dementia or senility (86.4%).
Mortality was 19% more likely in the group taking levothyroxine, according to an analysis by Dr. Meyerovitch and his fellow investigators.
When broken down further, presence of certain comorbidities increased mortality dramatically, including dementia (odds ratio, 1.61), heart failure (OR, 2.67), chronic renal failure (OR, 1.89), and cerebrovascular disease (OR, 1.94).
There was no significant difference in prevalence of atrial fibrillation between the test and control groups with thyroid stimulating hormone (TSH) testing, nor any difference in femur fracture prevalence.
Patients were given a TSH test three times during follow-up and showed significantly lower TSH levels compared with controls, according to Dr. Meyerovitch.
Dr. Meyerovitch acknowledged that the data he and his team used did not include the reason for a TSH evaluation, nor why patients began levothyroxine treatment. This leaves unanswered questions about the initial baseline mortality risk in patients included in the study.
“This may have resulted in treatment of patients with a higher risk, since we don’t know the reason for the treatment,” he said. “There may have been other reasons that were not included in the database that caused the physician to treat with levothyroxine.”
Details on the cause of death were not included.
Despite these limitations, Dr. Meyerovitch and his team stressed the need for more research before continuing to recommend this treatment to their elderly patients.
Dr. Meyerovitch reported no relevant financial disclosures.
Source: Meyerovitch J et al. ENDO 2018 Abstract OR34-2.
CHICAGO – according to a study presented at the annual meeting of the Endocrine Society.
With increased mortality and similar prevalence of atrial fibrillation and femoral fractures between study and control groups, physicians may want to reevaluate giving their elderly patients levothyroxine until more information is available, according to presenter Joseph Meyerovitch, MD, of Schneider Children’s Medical Center, Ramat Hasharon, Israel.
The case-control study included 416 patients 65 years or older with TSH levels of 4.2-10 mIU/L who died between 2012 and 2016, and 1,461 patients with comparable TSH levels who did not die during that time.
Most of the patients in both the control and study group were women. The average age of the patients was 84 years, and most had some form of dementia or senility (86.4%).
Mortality was 19% more likely in the group taking levothyroxine, according to an analysis by Dr. Meyerovitch and his fellow investigators.
When broken down further, presence of certain comorbidities increased mortality dramatically, including dementia (odds ratio, 1.61), heart failure (OR, 2.67), chronic renal failure (OR, 1.89), and cerebrovascular disease (OR, 1.94).
There was no significant difference in prevalence of atrial fibrillation between the test and control groups with thyroid stimulating hormone (TSH) testing, nor any difference in femur fracture prevalence.
Patients were given a TSH test three times during follow-up and showed significantly lower TSH levels compared with controls, according to Dr. Meyerovitch.
Dr. Meyerovitch acknowledged that the data he and his team used did not include the reason for a TSH evaluation, nor why patients began levothyroxine treatment. This leaves unanswered questions about the initial baseline mortality risk in patients included in the study.
“This may have resulted in treatment of patients with a higher risk, since we don’t know the reason for the treatment,” he said. “There may have been other reasons that were not included in the database that caused the physician to treat with levothyroxine.”
Details on the cause of death were not included.
Despite these limitations, Dr. Meyerovitch and his team stressed the need for more research before continuing to recommend this treatment to their elderly patients.
Dr. Meyerovitch reported no relevant financial disclosures.
Source: Meyerovitch J et al. ENDO 2018 Abstract OR34-2.
CHICAGO – according to a study presented at the annual meeting of the Endocrine Society.
With increased mortality and similar prevalence of atrial fibrillation and femoral fractures between study and control groups, physicians may want to reevaluate giving their elderly patients levothyroxine until more information is available, according to presenter Joseph Meyerovitch, MD, of Schneider Children’s Medical Center, Ramat Hasharon, Israel.
The case-control study included 416 patients 65 years or older with TSH levels of 4.2-10 mIU/L who died between 2012 and 2016, and 1,461 patients with comparable TSH levels who did not die during that time.
Most of the patients in both the control and study group were women. The average age of the patients was 84 years, and most had some form of dementia or senility (86.4%).
Mortality was 19% more likely in the group taking levothyroxine, according to an analysis by Dr. Meyerovitch and his fellow investigators.
When broken down further, presence of certain comorbidities increased mortality dramatically, including dementia (odds ratio, 1.61), heart failure (OR, 2.67), chronic renal failure (OR, 1.89), and cerebrovascular disease (OR, 1.94).
There was no significant difference in prevalence of atrial fibrillation between the test and control groups with thyroid stimulating hormone (TSH) testing, nor any difference in femur fracture prevalence.
Patients were given a TSH test three times during follow-up and showed significantly lower TSH levels compared with controls, according to Dr. Meyerovitch.
Dr. Meyerovitch acknowledged that the data he and his team used did not include the reason for a TSH evaluation, nor why patients began levothyroxine treatment. This leaves unanswered questions about the initial baseline mortality risk in patients included in the study.
“This may have resulted in treatment of patients with a higher risk, since we don’t know the reason for the treatment,” he said. “There may have been other reasons that were not included in the database that caused the physician to treat with levothyroxine.”
Details on the cause of death were not included.
Despite these limitations, Dr. Meyerovitch and his team stressed the need for more research before continuing to recommend this treatment to their elderly patients.
Dr. Meyerovitch reported no relevant financial disclosures.
Source: Meyerovitch J et al. ENDO 2018 Abstract OR34-2.
REPORTING FROM ENDO 2018
Key clinical point: Levothyroxine is associated with increased mortality in hypothyroidism patients 65 years and older.
Major finding: Patients who were treated with levothyroxine had an increased rate of mortality of 19% (HR = 1.19) compared to those treated with other methods.
Data source: Case-control study of 416 hypothyroidism patients 65 years or older who died between 2012 and 2016, compared with 1,461 hypothyroidism patients treated in the same period who did not die.
Disclosures: Dr. Meyerovitch reported no relevant financial disclosures.
Source: Meyerovitch J et al. ENDO 2018 Abstract OR34-2.
Metformin reduces preterm births, late miscarriages in PCOS
CHICAGO – , preterm births and late miscarriages were reduced, but researchers saw no effect on blood glucose levels or other diabetes-related maternal outcomes.
A Nordic study of pregnant women, dubbed PregMet 2, followed the course of 487 women with PCOS who were randomized to take 2,000 mg of metformin or placebo daily during pregnancy.
Among the entire study population, those with PCOS who took placebo had a 9.6% incidence of late miscarriage or preterm birth. For those taking metformin, the figure was 5%, similar to the 5.2% risk seen in the population-wide Norwegian Birth Registry, said Dr. Løvvik, of the Norwegian University of Science and Technology, Trondheim.
This analysis yielded a number needed to treat (NNT) for benefit of metformin of 22, she said.
However, Dr. Løvvik said that “the elephant in the room” is the lack of benefit of metformin for rates of gestational diabetes, hypertension in pregnancy, or preeclampsia in PregMet 2.
Of the various endpoint analyses, “the most surprising and striking one is the absolute lack of effect on gestational diabetes in this high-risk population,” said Dr. Løvvik. “There’s not even a tendency towards effect. … Metformin had no effect on prevention, treatment, or the need for additional insulin, indicating it was actually as effective as placebo.”
Considering that “metformin is now a part of the standard treatment for gestational diabetes, according to many national guidelines, we think that it’s questionable that it’s never, ever been tested against placebo for this diagnosis,” said Dr. Løvvik.
Pregnant women with PCOS have an increased risk of complications, and metformin is often prescribed off label to attempt to address some of these complications. Some previous work had shown metformin to be helpful, but previous studies have been underpowered, said Dr. Løvvik.
PregMet 2’s protocol attempted to address the literature gap; over 3 years, it called for enrollment of 1,000 women in the first trimester of pregnancy, randomized 1:1 to receive metformin or placebo in pregnancy.
At that enrollment level, the study would aim to show a 50% reduction in late miscarriages and preterm birth, with a power of 85%. Two predetermined analysis were planned at the end of the 3-year study, one for the intent-to-treat population and one for the per-protocol group.
In the end, however, just 487 patients were enrolled and randomized over a period of 5 years, despite recruitment at 14 centers in Iceland, Norway, and Sweden.
Richard Legro, MD, an ob.gyn. from Pennsylvania State University, Hershey, was in attendance. While praising the overall high quality of the study, he commented, “The primary concern is that you stopped the study when you hadn’t even achieved half your sample size.”
Dr. Løvvik said that a variety of factors contributed to the low study enrollment. One primary issue was that participation required additional visits on the part of participants; the study was not part of routine prenatal care, she said. Also, the placebo used in the study expired before enrollment was completed, complicating execution of the study.
She also noted that nearly a quarter of patients had a history of depression, and one in five had a history of migraine. More than half of patients overall had at least one previous or ongoing chronic medical condition.
A small number of patients in each group were excluded for protocol violations, pregnancy termination, intrauterine fetal demise, or loss to follow-up. The final intent-to-treat population included 238 in the metformin group and 240 who received placebo.
The per-protocol analysis was conducted two ways: In the first analysis, only those who dropped out were excluded, leaving 209 in the metformin group and 223 in the placebo group. The second analysis included those whose adherence to taking the study medication was assessed at 90% or better. For this second per-protocol analysis, just 142 metformin takers and 156 in the placebo group remained.
In the intent-to-treat population, the primary endpoint – a composite outcome of late miscarriage and preterm delivery – yielded an odds ratio of 1.99 favoring metformin over placebo, a figure that fell short of statistical significance (95% confidence interval, 0.93-4.51; P = .08).
However, there was significantly less weight gain in those taking metformin in this population (8.7 vs. 11.48 kg; P less than .0001). And newborns had significantly larger head circumferences if their mothers with PCOS took metformin (35.4 vs. 35.0 cm; P = .006).
Dr. Løvvik and her colleagues subsequently performed the same analysis on the first per-protocol group, one that excluded the 46 patients who were early dropouts after randomization. Here, the odds ratio favoring metformin for the composite primary outcome measure was a significant 2.55 (n = 432; 95% CI, 1.10-6.42; P = .03). Results for the final per-protocol analysis that excluded dropouts and those with less than 90% adherence to study medication were similar, with an odds ratio of 2.76 in favor of metformin use during pregnancy (n = 298; 95% CI, 1.0-8.82; P = .05).
In response to an audience question, Dr. Løvvik said that, though a per-protocol analysis of some sort had been predetermined, the decision to perform the narrower analysis on the highly adherent group was made later. However, compared to thresholds for adherence in other studies, “ours was way higher – so it’s too strict,” she acknowledged.
At baseline, the median age overall was about 30 years, and the median body mass index was about 28 kg/m2. Patients were at a median 74 days gestational age of pregnancy at randomization, and 53%-61% of patients were on metformin at the time of conception, with no significant difference between the metformin and placebo group.
A little less than half (40%-46%) of participants had a spontaneous conception, and most participants (56%-59%) had no prior term deliveries. Participants were overwhelmingly (91%-97%) white and Nordic.
There were no serious maternal, fetal, or neonatal safety signals in either study group.
The study followed the earlier PregMet study, which also tracked use of metformin in pregnancy for women with PCOS.
CHICAGO – , preterm births and late miscarriages were reduced, but researchers saw no effect on blood glucose levels or other diabetes-related maternal outcomes.
A Nordic study of pregnant women, dubbed PregMet 2, followed the course of 487 women with PCOS who were randomized to take 2,000 mg of metformin or placebo daily during pregnancy.
Among the entire study population, those with PCOS who took placebo had a 9.6% incidence of late miscarriage or preterm birth. For those taking metformin, the figure was 5%, similar to the 5.2% risk seen in the population-wide Norwegian Birth Registry, said Dr. Løvvik, of the Norwegian University of Science and Technology, Trondheim.
This analysis yielded a number needed to treat (NNT) for benefit of metformin of 22, she said.
However, Dr. Løvvik said that “the elephant in the room” is the lack of benefit of metformin for rates of gestational diabetes, hypertension in pregnancy, or preeclampsia in PregMet 2.
Of the various endpoint analyses, “the most surprising and striking one is the absolute lack of effect on gestational diabetes in this high-risk population,” said Dr. Løvvik. “There’s not even a tendency towards effect. … Metformin had no effect on prevention, treatment, or the need for additional insulin, indicating it was actually as effective as placebo.”
Considering that “metformin is now a part of the standard treatment for gestational diabetes, according to many national guidelines, we think that it’s questionable that it’s never, ever been tested against placebo for this diagnosis,” said Dr. Løvvik.
Pregnant women with PCOS have an increased risk of complications, and metformin is often prescribed off label to attempt to address some of these complications. Some previous work had shown metformin to be helpful, but previous studies have been underpowered, said Dr. Løvvik.
PregMet 2’s protocol attempted to address the literature gap; over 3 years, it called for enrollment of 1,000 women in the first trimester of pregnancy, randomized 1:1 to receive metformin or placebo in pregnancy.
At that enrollment level, the study would aim to show a 50% reduction in late miscarriages and preterm birth, with a power of 85%. Two predetermined analysis were planned at the end of the 3-year study, one for the intent-to-treat population and one for the per-protocol group.
In the end, however, just 487 patients were enrolled and randomized over a period of 5 years, despite recruitment at 14 centers in Iceland, Norway, and Sweden.
Richard Legro, MD, an ob.gyn. from Pennsylvania State University, Hershey, was in attendance. While praising the overall high quality of the study, he commented, “The primary concern is that you stopped the study when you hadn’t even achieved half your sample size.”
Dr. Løvvik said that a variety of factors contributed to the low study enrollment. One primary issue was that participation required additional visits on the part of participants; the study was not part of routine prenatal care, she said. Also, the placebo used in the study expired before enrollment was completed, complicating execution of the study.
She also noted that nearly a quarter of patients had a history of depression, and one in five had a history of migraine. More than half of patients overall had at least one previous or ongoing chronic medical condition.
A small number of patients in each group were excluded for protocol violations, pregnancy termination, intrauterine fetal demise, or loss to follow-up. The final intent-to-treat population included 238 in the metformin group and 240 who received placebo.
The per-protocol analysis was conducted two ways: In the first analysis, only those who dropped out were excluded, leaving 209 in the metformin group and 223 in the placebo group. The second analysis included those whose adherence to taking the study medication was assessed at 90% or better. For this second per-protocol analysis, just 142 metformin takers and 156 in the placebo group remained.
In the intent-to-treat population, the primary endpoint – a composite outcome of late miscarriage and preterm delivery – yielded an odds ratio of 1.99 favoring metformin over placebo, a figure that fell short of statistical significance (95% confidence interval, 0.93-4.51; P = .08).
However, there was significantly less weight gain in those taking metformin in this population (8.7 vs. 11.48 kg; P less than .0001). And newborns had significantly larger head circumferences if their mothers with PCOS took metformin (35.4 vs. 35.0 cm; P = .006).
Dr. Løvvik and her colleagues subsequently performed the same analysis on the first per-protocol group, one that excluded the 46 patients who were early dropouts after randomization. Here, the odds ratio favoring metformin for the composite primary outcome measure was a significant 2.55 (n = 432; 95% CI, 1.10-6.42; P = .03). Results for the final per-protocol analysis that excluded dropouts and those with less than 90% adherence to study medication were similar, with an odds ratio of 2.76 in favor of metformin use during pregnancy (n = 298; 95% CI, 1.0-8.82; P = .05).
In response to an audience question, Dr. Løvvik said that, though a per-protocol analysis of some sort had been predetermined, the decision to perform the narrower analysis on the highly adherent group was made later. However, compared to thresholds for adherence in other studies, “ours was way higher – so it’s too strict,” she acknowledged.
At baseline, the median age overall was about 30 years, and the median body mass index was about 28 kg/m2. Patients were at a median 74 days gestational age of pregnancy at randomization, and 53%-61% of patients were on metformin at the time of conception, with no significant difference between the metformin and placebo group.
A little less than half (40%-46%) of participants had a spontaneous conception, and most participants (56%-59%) had no prior term deliveries. Participants were overwhelmingly (91%-97%) white and Nordic.
There were no serious maternal, fetal, or neonatal safety signals in either study group.
The study followed the earlier PregMet study, which also tracked use of metformin in pregnancy for women with PCOS.
CHICAGO – , preterm births and late miscarriages were reduced, but researchers saw no effect on blood glucose levels or other diabetes-related maternal outcomes.
A Nordic study of pregnant women, dubbed PregMet 2, followed the course of 487 women with PCOS who were randomized to take 2,000 mg of metformin or placebo daily during pregnancy.
Among the entire study population, those with PCOS who took placebo had a 9.6% incidence of late miscarriage or preterm birth. For those taking metformin, the figure was 5%, similar to the 5.2% risk seen in the population-wide Norwegian Birth Registry, said Dr. Løvvik, of the Norwegian University of Science and Technology, Trondheim.
This analysis yielded a number needed to treat (NNT) for benefit of metformin of 22, she said.
However, Dr. Løvvik said that “the elephant in the room” is the lack of benefit of metformin for rates of gestational diabetes, hypertension in pregnancy, or preeclampsia in PregMet 2.
Of the various endpoint analyses, “the most surprising and striking one is the absolute lack of effect on gestational diabetes in this high-risk population,” said Dr. Løvvik. “There’s not even a tendency towards effect. … Metformin had no effect on prevention, treatment, or the need for additional insulin, indicating it was actually as effective as placebo.”
Considering that “metformin is now a part of the standard treatment for gestational diabetes, according to many national guidelines, we think that it’s questionable that it’s never, ever been tested against placebo for this diagnosis,” said Dr. Løvvik.
Pregnant women with PCOS have an increased risk of complications, and metformin is often prescribed off label to attempt to address some of these complications. Some previous work had shown metformin to be helpful, but previous studies have been underpowered, said Dr. Løvvik.
PregMet 2’s protocol attempted to address the literature gap; over 3 years, it called for enrollment of 1,000 women in the first trimester of pregnancy, randomized 1:1 to receive metformin or placebo in pregnancy.
At that enrollment level, the study would aim to show a 50% reduction in late miscarriages and preterm birth, with a power of 85%. Two predetermined analysis were planned at the end of the 3-year study, one for the intent-to-treat population and one for the per-protocol group.
In the end, however, just 487 patients were enrolled and randomized over a period of 5 years, despite recruitment at 14 centers in Iceland, Norway, and Sweden.
Richard Legro, MD, an ob.gyn. from Pennsylvania State University, Hershey, was in attendance. While praising the overall high quality of the study, he commented, “The primary concern is that you stopped the study when you hadn’t even achieved half your sample size.”
Dr. Løvvik said that a variety of factors contributed to the low study enrollment. One primary issue was that participation required additional visits on the part of participants; the study was not part of routine prenatal care, she said. Also, the placebo used in the study expired before enrollment was completed, complicating execution of the study.
She also noted that nearly a quarter of patients had a history of depression, and one in five had a history of migraine. More than half of patients overall had at least one previous or ongoing chronic medical condition.
A small number of patients in each group were excluded for protocol violations, pregnancy termination, intrauterine fetal demise, or loss to follow-up. The final intent-to-treat population included 238 in the metformin group and 240 who received placebo.
The per-protocol analysis was conducted two ways: In the first analysis, only those who dropped out were excluded, leaving 209 in the metformin group and 223 in the placebo group. The second analysis included those whose adherence to taking the study medication was assessed at 90% or better. For this second per-protocol analysis, just 142 metformin takers and 156 in the placebo group remained.
In the intent-to-treat population, the primary endpoint – a composite outcome of late miscarriage and preterm delivery – yielded an odds ratio of 1.99 favoring metformin over placebo, a figure that fell short of statistical significance (95% confidence interval, 0.93-4.51; P = .08).
However, there was significantly less weight gain in those taking metformin in this population (8.7 vs. 11.48 kg; P less than .0001). And newborns had significantly larger head circumferences if their mothers with PCOS took metformin (35.4 vs. 35.0 cm; P = .006).
Dr. Løvvik and her colleagues subsequently performed the same analysis on the first per-protocol group, one that excluded the 46 patients who were early dropouts after randomization. Here, the odds ratio favoring metformin for the composite primary outcome measure was a significant 2.55 (n = 432; 95% CI, 1.10-6.42; P = .03). Results for the final per-protocol analysis that excluded dropouts and those with less than 90% adherence to study medication were similar, with an odds ratio of 2.76 in favor of metformin use during pregnancy (n = 298; 95% CI, 1.0-8.82; P = .05).
In response to an audience question, Dr. Løvvik said that, though a per-protocol analysis of some sort had been predetermined, the decision to perform the narrower analysis on the highly adherent group was made later. However, compared to thresholds for adherence in other studies, “ours was way higher – so it’s too strict,” she acknowledged.
At baseline, the median age overall was about 30 years, and the median body mass index was about 28 kg/m2. Patients were at a median 74 days gestational age of pregnancy at randomization, and 53%-61% of patients were on metformin at the time of conception, with no significant difference between the metformin and placebo group.
A little less than half (40%-46%) of participants had a spontaneous conception, and most participants (56%-59%) had no prior term deliveries. Participants were overwhelmingly (91%-97%) white and Nordic.
There were no serious maternal, fetal, or neonatal safety signals in either study group.
The study followed the earlier PregMet study, which also tracked use of metformin in pregnancy for women with PCOS.
REPORTING FROM ENDO 2018
Key clinical point: Metformin reduced the rate of late preterm births and miscarriages in pregnant women with PCOS.
Major finding: The rate of the composite outcome was 5% for those on metformin, with a NNT of 22.
Study details: Randomized placebo-controlled trial of 487 pregnant women.
Disclosures: None of the study authors reported conflicts of interest.
Source: Løvvik T et al. ENDO 2018, Abstract 33-4.