User login
Decision rule identifies unprovoked VTE patients who can halt anticoagulation
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
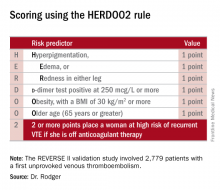
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
AT THE ESC CONGRESS 2016
Key clinical point: Half of women who have a first unprovoked venous thromboembolism can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule.
Major finding: Women with a first unprovoked venous thromboembolism identified as being at low risk of recurrence on the basis of the HERDOO2 decision rule had a 3% recurrence rate in the year after stopping anticoagulation therapy, while those identified as high risk had an 8.1% recurrence rate if they discontinued anticoagulants.
Data source: This was a prospective, multinational, observational study involving 2,779 patients with a first unprovoked venous thromboembolism.
Disclosures: The presenter reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study.
First-generation DES looking good at 10 years
ROME – There’s good news for the millions of patients living with a first-generation metallic drug-eluting stent for coronary revascularization implanted in years past: The devices perform reassuringly well a full decade after implantation, Lorenz Räber, MD, reported at the annual congress of the European Society of Cardiology.
That’s the key message of the SIRTAX VERY LATE study, the only randomized trial of first-generation drug-eluting stents (DES) that didn’t turn out the lights at a maximum of 5 years of follow-up. In fact, at the 10-year mark, SIRTAX VERY LATE shows that regardless of whether the first-generation DES was paclitaxel- or sirolimus-eluting, the risk of major adverse cardiac events due to device failure was substantially lower in the second half-decade than in the first 5 years after deployment, according to Dr. Räber of Bern (Switzerland) University Hospital.
More specifically, the cumulative risk of ischemia-driven target lesion revascularization was 14.6% at 5 years and 17.7% at 10 years, while the 5- and 10-year cumulative risks of definite stent thrombosis were 4.5% and 5.6%, respectively.
The annual risk of ischemia-driven target lesion revascularization dropped by 64%, from 1.8%/year during years 1-5 to 0.7% during years 6-10. Similarly, the annual risk of definite stent thrombosis fell from 0.67%/year to 0.23%/year after year 5, a 69% relative risk reduction. And importantly, these attenuations in risk occurred independent of age.
“The lower risk of late clinical events suggests stabilization of delayed arterial healing over time after first-generation DES implantation, with reduced chronic inflammation and neoatherosclerosis,” he said.
This is reassuring in light of the stormy history of the first-generation DES. Three years after the devices came on the U.S. market, the so-called ESC firestorm erupted. At the 2006 ESC congress, investigators presented meta-analyses suggesting the devices carried a possible late increased thrombotic risk beyond the then-recommended 3-6 months of prescribed dual-antiplatelet therapy. The use of these devices declined sharply in response, even though a Food and Drug Administration advisory panel charged with looking at the totality of evidence concluded that concerns about thrombosis didn’t outweigh the benefits of the first-generation DES over bare-metal stents.
The reductions in very late stent thrombosis and ischemia-driven target lesion revascularization beyond 5 years seen in the SIRTAX VERY LATE trial occurred despite the fact that only 15% of patients were on dual-antiplatelet therapy throughout the first 5 years and 11% were on dual-antiplatelet therapy afterwards, Dr. Räber noted.
“Our findings may have implications for secondary prevention after PCI with a first-generation DES, including the need for long-term antiplatelet therapy,” the cardiologist added.
The previously reported 5-year results of SIRTAX (the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization trial) showed a steady increase over time in late lumen loss and an ongoing risk of very late stent thrombosis (Circulation. 2011 Jun 21;123(24):2819-28). Much the same was seen at the 5-year mark in the other major trials of first-generation DES, including RAVEL, SIRIUS, and TAXUS.
However, all those studies ended at 5 years, leaving unanswered the key question of what happens later. Cardiologists have wondered if the first-generation DES they put in their patients years ago were associated with a continued steady climb in the risk of device-related adverse events, or if the risk plateaued or even dropped off. The SIRTAX VERY LATE study was conducted in order to provide answers.
SIRTAX included 1,012 Swiss patients randomized to coronary revascularization using a first-generation sirolimus- or paclitaxel-eluting stent in 2003-2004. Roughly half had stable coronary artery disease and half presented with acute coronary syndromes. The 10-year follow-up conducted in the SIRTAX VERY LATE study captured 895 (88%) of the original 1,012 subjects.
The cumulative incidence of major cardiac adverse events – a composite of cardiac death, MI, and ischemia-driven target lesion revascularization – was 20.8% at 5 years and 33.8% at 10 years. The rate was similar between years 1-5 and 6-10.
The cumulative all-cause mortality rate was 10.4% at 5 years and 24.2% at 10 years. The rate was 2.0%/year during years 1-5 and accelerated significantly to 3.1%/year in years 6-10. However, this increase appears to be largely due to the background impact of advancing age rather than to any effect of having a first-generation DES. The 5- and 10-year all-cause mortality rates in the age- and sex-matched general Swiss population are similar to those seen in SIRTAX VERY LATE, at 9.6% and 22.1%, respectively, the cardiologist observed.
The cumulative incidence of MI in the study population was 7% at 5 years and 9.7% at 10 years. Between years 1-5 the rate was 0.9%/year, dropping to 0.6%/year during years 6-10.
One of the useful potential purposes for the new SIRTAX VERY LATE follow-up data beyond 5 years is that the results could serve as a benchmark in evaluating the long-term safety and efficacy of the much newer drug-eluting fully bioresorbable vascular scaffolds, since the potential benefits of these new devices may not appear until relatively late, after the devices themselves have disappeared. SIRTAX VERY LATE sets the bar for stent-related adverse events 5 years or more after device implantation at an annual risk of less than 0.3%/year for stent thrombosis and less than 1%/year for ischemia-driven target lesion revascularization.
Session co-chair Hector Bueno, MD, drew attention to the fact that no significant differences in clinical outcomes were seen at either 5 or 10 years between the sirolimus- and paclitaxel-eluting stent recipients. That’s noteworthy because more than a decade ago when the primary endpoint of SIRTAX was reported, much was made of the finding that the 9-month rate of major adverse cardiac events was significantly lower in the sirolimus-eluting stent group (N Engl J Med. 2005 Aug 18;353[7]:653-62). Over time, any outcome differences between the two devices were erased, observed Dr. Bueno of Complutense University of Madrid.
The SIRTAX VERY LATE study was funded by grants from Bern University Hospital. Dr. Räber reported having no relevant financial interests.
Simultaneously with his presentation in Rome at ESC 2016, the study results were published online (Eur Heart J. 2016 Aug 30. doi: 10.1093/eurheartj/ehw343).
ROME – There’s good news for the millions of patients living with a first-generation metallic drug-eluting stent for coronary revascularization implanted in years past: The devices perform reassuringly well a full decade after implantation, Lorenz Räber, MD, reported at the annual congress of the European Society of Cardiology.
That’s the key message of the SIRTAX VERY LATE study, the only randomized trial of first-generation drug-eluting stents (DES) that didn’t turn out the lights at a maximum of 5 years of follow-up. In fact, at the 10-year mark, SIRTAX VERY LATE shows that regardless of whether the first-generation DES was paclitaxel- or sirolimus-eluting, the risk of major adverse cardiac events due to device failure was substantially lower in the second half-decade than in the first 5 years after deployment, according to Dr. Räber of Bern (Switzerland) University Hospital.
More specifically, the cumulative risk of ischemia-driven target lesion revascularization was 14.6% at 5 years and 17.7% at 10 years, while the 5- and 10-year cumulative risks of definite stent thrombosis were 4.5% and 5.6%, respectively.
The annual risk of ischemia-driven target lesion revascularization dropped by 64%, from 1.8%/year during years 1-5 to 0.7% during years 6-10. Similarly, the annual risk of definite stent thrombosis fell from 0.67%/year to 0.23%/year after year 5, a 69% relative risk reduction. And importantly, these attenuations in risk occurred independent of age.
“The lower risk of late clinical events suggests stabilization of delayed arterial healing over time after first-generation DES implantation, with reduced chronic inflammation and neoatherosclerosis,” he said.
This is reassuring in light of the stormy history of the first-generation DES. Three years after the devices came on the U.S. market, the so-called ESC firestorm erupted. At the 2006 ESC congress, investigators presented meta-analyses suggesting the devices carried a possible late increased thrombotic risk beyond the then-recommended 3-6 months of prescribed dual-antiplatelet therapy. The use of these devices declined sharply in response, even though a Food and Drug Administration advisory panel charged with looking at the totality of evidence concluded that concerns about thrombosis didn’t outweigh the benefits of the first-generation DES over bare-metal stents.
The reductions in very late stent thrombosis and ischemia-driven target lesion revascularization beyond 5 years seen in the SIRTAX VERY LATE trial occurred despite the fact that only 15% of patients were on dual-antiplatelet therapy throughout the first 5 years and 11% were on dual-antiplatelet therapy afterwards, Dr. Räber noted.
“Our findings may have implications for secondary prevention after PCI with a first-generation DES, including the need for long-term antiplatelet therapy,” the cardiologist added.
The previously reported 5-year results of SIRTAX (the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization trial) showed a steady increase over time in late lumen loss and an ongoing risk of very late stent thrombosis (Circulation. 2011 Jun 21;123(24):2819-28). Much the same was seen at the 5-year mark in the other major trials of first-generation DES, including RAVEL, SIRIUS, and TAXUS.
However, all those studies ended at 5 years, leaving unanswered the key question of what happens later. Cardiologists have wondered if the first-generation DES they put in their patients years ago were associated with a continued steady climb in the risk of device-related adverse events, or if the risk plateaued or even dropped off. The SIRTAX VERY LATE study was conducted in order to provide answers.
SIRTAX included 1,012 Swiss patients randomized to coronary revascularization using a first-generation sirolimus- or paclitaxel-eluting stent in 2003-2004. Roughly half had stable coronary artery disease and half presented with acute coronary syndromes. The 10-year follow-up conducted in the SIRTAX VERY LATE study captured 895 (88%) of the original 1,012 subjects.
The cumulative incidence of major cardiac adverse events – a composite of cardiac death, MI, and ischemia-driven target lesion revascularization – was 20.8% at 5 years and 33.8% at 10 years. The rate was similar between years 1-5 and 6-10.
The cumulative all-cause mortality rate was 10.4% at 5 years and 24.2% at 10 years. The rate was 2.0%/year during years 1-5 and accelerated significantly to 3.1%/year in years 6-10. However, this increase appears to be largely due to the background impact of advancing age rather than to any effect of having a first-generation DES. The 5- and 10-year all-cause mortality rates in the age- and sex-matched general Swiss population are similar to those seen in SIRTAX VERY LATE, at 9.6% and 22.1%, respectively, the cardiologist observed.
The cumulative incidence of MI in the study population was 7% at 5 years and 9.7% at 10 years. Between years 1-5 the rate was 0.9%/year, dropping to 0.6%/year during years 6-10.
One of the useful potential purposes for the new SIRTAX VERY LATE follow-up data beyond 5 years is that the results could serve as a benchmark in evaluating the long-term safety and efficacy of the much newer drug-eluting fully bioresorbable vascular scaffolds, since the potential benefits of these new devices may not appear until relatively late, after the devices themselves have disappeared. SIRTAX VERY LATE sets the bar for stent-related adverse events 5 years or more after device implantation at an annual risk of less than 0.3%/year for stent thrombosis and less than 1%/year for ischemia-driven target lesion revascularization.
Session co-chair Hector Bueno, MD, drew attention to the fact that no significant differences in clinical outcomes were seen at either 5 or 10 years between the sirolimus- and paclitaxel-eluting stent recipients. That’s noteworthy because more than a decade ago when the primary endpoint of SIRTAX was reported, much was made of the finding that the 9-month rate of major adverse cardiac events was significantly lower in the sirolimus-eluting stent group (N Engl J Med. 2005 Aug 18;353[7]:653-62). Over time, any outcome differences between the two devices were erased, observed Dr. Bueno of Complutense University of Madrid.
The SIRTAX VERY LATE study was funded by grants from Bern University Hospital. Dr. Räber reported having no relevant financial interests.
Simultaneously with his presentation in Rome at ESC 2016, the study results were published online (Eur Heart J. 2016 Aug 30. doi: 10.1093/eurheartj/ehw343).
ROME – There’s good news for the millions of patients living with a first-generation metallic drug-eluting stent for coronary revascularization implanted in years past: The devices perform reassuringly well a full decade after implantation, Lorenz Räber, MD, reported at the annual congress of the European Society of Cardiology.
That’s the key message of the SIRTAX VERY LATE study, the only randomized trial of first-generation drug-eluting stents (DES) that didn’t turn out the lights at a maximum of 5 years of follow-up. In fact, at the 10-year mark, SIRTAX VERY LATE shows that regardless of whether the first-generation DES was paclitaxel- or sirolimus-eluting, the risk of major adverse cardiac events due to device failure was substantially lower in the second half-decade than in the first 5 years after deployment, according to Dr. Räber of Bern (Switzerland) University Hospital.
More specifically, the cumulative risk of ischemia-driven target lesion revascularization was 14.6% at 5 years and 17.7% at 10 years, while the 5- and 10-year cumulative risks of definite stent thrombosis were 4.5% and 5.6%, respectively.
The annual risk of ischemia-driven target lesion revascularization dropped by 64%, from 1.8%/year during years 1-5 to 0.7% during years 6-10. Similarly, the annual risk of definite stent thrombosis fell from 0.67%/year to 0.23%/year after year 5, a 69% relative risk reduction. And importantly, these attenuations in risk occurred independent of age.
“The lower risk of late clinical events suggests stabilization of delayed arterial healing over time after first-generation DES implantation, with reduced chronic inflammation and neoatherosclerosis,” he said.
This is reassuring in light of the stormy history of the first-generation DES. Three years after the devices came on the U.S. market, the so-called ESC firestorm erupted. At the 2006 ESC congress, investigators presented meta-analyses suggesting the devices carried a possible late increased thrombotic risk beyond the then-recommended 3-6 months of prescribed dual-antiplatelet therapy. The use of these devices declined sharply in response, even though a Food and Drug Administration advisory panel charged with looking at the totality of evidence concluded that concerns about thrombosis didn’t outweigh the benefits of the first-generation DES over bare-metal stents.
The reductions in very late stent thrombosis and ischemia-driven target lesion revascularization beyond 5 years seen in the SIRTAX VERY LATE trial occurred despite the fact that only 15% of patients were on dual-antiplatelet therapy throughout the first 5 years and 11% were on dual-antiplatelet therapy afterwards, Dr. Räber noted.
“Our findings may have implications for secondary prevention after PCI with a first-generation DES, including the need for long-term antiplatelet therapy,” the cardiologist added.
The previously reported 5-year results of SIRTAX (the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization trial) showed a steady increase over time in late lumen loss and an ongoing risk of very late stent thrombosis (Circulation. 2011 Jun 21;123(24):2819-28). Much the same was seen at the 5-year mark in the other major trials of first-generation DES, including RAVEL, SIRIUS, and TAXUS.
However, all those studies ended at 5 years, leaving unanswered the key question of what happens later. Cardiologists have wondered if the first-generation DES they put in their patients years ago were associated with a continued steady climb in the risk of device-related adverse events, or if the risk plateaued or even dropped off. The SIRTAX VERY LATE study was conducted in order to provide answers.
SIRTAX included 1,012 Swiss patients randomized to coronary revascularization using a first-generation sirolimus- or paclitaxel-eluting stent in 2003-2004. Roughly half had stable coronary artery disease and half presented with acute coronary syndromes. The 10-year follow-up conducted in the SIRTAX VERY LATE study captured 895 (88%) of the original 1,012 subjects.
The cumulative incidence of major cardiac adverse events – a composite of cardiac death, MI, and ischemia-driven target lesion revascularization – was 20.8% at 5 years and 33.8% at 10 years. The rate was similar between years 1-5 and 6-10.
The cumulative all-cause mortality rate was 10.4% at 5 years and 24.2% at 10 years. The rate was 2.0%/year during years 1-5 and accelerated significantly to 3.1%/year in years 6-10. However, this increase appears to be largely due to the background impact of advancing age rather than to any effect of having a first-generation DES. The 5- and 10-year all-cause mortality rates in the age- and sex-matched general Swiss population are similar to those seen in SIRTAX VERY LATE, at 9.6% and 22.1%, respectively, the cardiologist observed.
The cumulative incidence of MI in the study population was 7% at 5 years and 9.7% at 10 years. Between years 1-5 the rate was 0.9%/year, dropping to 0.6%/year during years 6-10.
One of the useful potential purposes for the new SIRTAX VERY LATE follow-up data beyond 5 years is that the results could serve as a benchmark in evaluating the long-term safety and efficacy of the much newer drug-eluting fully bioresorbable vascular scaffolds, since the potential benefits of these new devices may not appear until relatively late, after the devices themselves have disappeared. SIRTAX VERY LATE sets the bar for stent-related adverse events 5 years or more after device implantation at an annual risk of less than 0.3%/year for stent thrombosis and less than 1%/year for ischemia-driven target lesion revascularization.
Session co-chair Hector Bueno, MD, drew attention to the fact that no significant differences in clinical outcomes were seen at either 5 or 10 years between the sirolimus- and paclitaxel-eluting stent recipients. That’s noteworthy because more than a decade ago when the primary endpoint of SIRTAX was reported, much was made of the finding that the 9-month rate of major adverse cardiac events was significantly lower in the sirolimus-eluting stent group (N Engl J Med. 2005 Aug 18;353[7]:653-62). Over time, any outcome differences between the two devices were erased, observed Dr. Bueno of Complutense University of Madrid.
The SIRTAX VERY LATE study was funded by grants from Bern University Hospital. Dr. Räber reported having no relevant financial interests.
Simultaneously with his presentation in Rome at ESC 2016, the study results were published online (Eur Heart J. 2016 Aug 30. doi: 10.1093/eurheartj/ehw343).
AT THE ESC CONGRESS 2016
Key clinical point: The annual risks of ischemia-driven target lesion revascularization and stent thrombosis significantly decreased starting 5 years after implantation of a first-generation sirolimus- or paclitaxel-eluting stent.
Major finding: The annual risk of ischemia-driven target lesion revascularization was 1.8%/year between 1 and 5 years after implantation of a first-generation drug-eluting stent, but only 0.7%/year during years 6-10.
Data source: This was a unique extended 10-year follow-up of 88% of the original 1,012 participants in a randomized, assessor-blinded Swiss trial of coronary revascularization using a first-generation sirolimus- or paclitaxel-eluting stent.
Disclosures: The SIRTAX VERY LATE study was funded by grants from Bern University Hospital. The presenter reported having no relevant financial interests.
ANTARCTIC results chill enthusiasm for platelet monitoring
ROME – Measuring platelet function in order to tailor antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention for acute coronary syndromes did not improve their clinical outcomes in the randomized ANTARCTIC trial, Gilles Montalescot, MD, reported at the annual congress of the European Society of Cardiology.
“We found absolutely no benefit for this strategy of adjustment of antiplatelet therapy based upon platelet function testing. The study was completely neutral on all types of endpoints, ischemic as well as bleeding,” said Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Pitié-Salpêtrière Hospital.
This was a disappointing result in what was the largest-ever randomized clinical trial involving PCI in elderly patients, he said. This was a high-risk population, not only by virtue of everyone being over age 75 years, but because they all presented with ACS. Indeed, one-third of ANTARCTIC participants underwent primary PCI for ST-segment elevation myocardial infarction.
ANTARCTIC (Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged Over 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis, and Ischemic Complications) was carried out as a follow-up to the earlier ARCTIC randomized trial, also conducted by Dr. Montalescot and his coinvestigators. Like ANTARCTIC, ARCTIC, too, showed no clinical benefit for platelet function testing in order to adjust antiplatelet therapy (N Engl J Med. 2012;367:2100-9). At the time, ARCTIC’s critics argued that this individualized strategy didn’t achieve the expected improved outcomes because the trial was conducted in low-risk, stable patients undergoing elective scheduled PCI. In contrast, if there was ever a high-risk population in which platelet function testing and tailored antiplatelet therapy should work, it was in the very high-risk ANTARCTIC population, he said.
ANTARCTIC included 877 elderly patients undergoing urgent PCI for ACS who were placed on low-dose aspirin and randomized to standard antiplatelet therapy with prasugrel (Effient) at 5 mg/day, the European approved dose for long-term maintenance therapy in elderly patients, or to tailored antiplatelet therapy.
Patients in the tailored therapy arm received prasugrel at 5 mg/day for the first 14 days, then underwent platelet function testing with the VerifyNow P2Y12 system. If they demonstrated high on-drug platelet activity, defined as at least 208 P2Y12 reaction units (PRU), their prasugrel was bumped up to 10 mg/day. If their PRU measurement was in what is considered the optimal range for quelling ischemia without promoting bleeding – that is, less than 208 but more than 85 PRU – they remained on prasugrel at 5 mg/day. And if they scored less than 85 PRU, exposing them to excess bleeding risk due to high suppression of platelets, they were switched to clopidogrel (Plavix) at 75 mg/day, a less potent antiplatelet regimen.
Two weeks after their first platelet function measurement, participants in the tailored therapy arm returned for a second round of platelet activity testing, with their antiplatelet regimen once again being adjusted on the basis of the results.
The primary study endpoint was net clinical benefit over a 12-month follow-up period. This was defined as the composite of cardiovascular death, MI, stroke, urgent revascularization, stent thrombosis, and Bleeding Academic Research Consortium (BARC) types 2, 3, or 5. This composite endpoint occurred in 27.6% of the platelet monitoring group and a near-identical 27.8% of conventionally managed patients.
Of note, 42% of patients in the actively monitored group were within the target platelet inhibition range when tested 14 days into the study. At study’s end, 55% of patients remained on prasugrel at 5 mg/day, 39% were on clopidogrel at 75 mg/day, and less than 4% were on prasugrel at 10 mg/day. Thus, most patients who underwent a dose adjustment on the basis of their VerifyNow results were downgraded to a less-potent antiplatelet regimen. Very few required enhanced platelet suppression in the form of 10 mg/day of prasugrel.
“Platelet function monitoring is difficult to use. Patients have to come back twice to be monitored. It’s costly. It’s time consuming. And platelet function monitoring clearly does not help,” the cardiologist said.
The ANTARCTIC results will likely lead to a revision of the American and European guidelines, which currently give a class IIb/level of evidence C recommendation for platelet function testing in high-risk situations.
“There is a huge literature showing that platelet reactivity affects clinical outcomes,” Dr. Montalescot continued. “One hypothesis now is that platelet reactivity may be only a marker of risk; you can modify it, but that has no impact on patient outcomes. We may be in the same situation here as with HDL cholesterol, for example.”
Discussant Steen Dalby Kristensen, MD, noted that ANTARCTIC is just the latest in a slew of negative randomized clinical trials of individualized antiplatelet therapy for coronary artery disease. In addition to ARCTIC, others include GRAVITAS, TRIGGER PCI, and ASCET. One study, the German/Austrian TROPICAL ACS trial, remains ongoing.
“It really is an intriguing concept that many of us have been fascinated by for years: to identify the sweet spot where, by measuring platelet aggregation and maybe changing the therapy, we can find just the right balance between bleeding and ischemia. The ANTARCTIC results are quite disappointing for platelet-monitoring enthusiasts. Is the whole concept wrong?” said Dr. Kristensen, professor of cardiology and head of the cardiovascular research center at Aarhus (Denmark) University Hospital.
“I think even more disappointing for me than the lack of impact on ischemic events was the bleeding. I would have anticipated that maybe bleeding could be avoided by adjusting the dose, but this was not the case,” he added.
But Stephan Gielen, MD, saw a silver lining in the negative results for ANTARCTIC.
“From my perspective as a clinical interventionalist, I’m happy that you ended up in the way you did. Putting things positively, this study confirms the safety of dual-platelet inhibition with prasugrel at the reduced dose of 5 mg in an elderly population. There is no need to go to the trouble of monitoring platelet function even in this elderly population, which I think for clinical practice is a good message,” said Dr. Gielen of Detmold (Germany) Hospital, who cochaired a press conference where Dr. Montalescot presented the ANTARCTIC results.
Simultaneously with Dr. Montalescot’s presentation at ESC 2016 in Rome, the ANTARCTIC results were published online (Lancet. 2016 Aug 26. doi: 10.1016/S0140-6736(16)31323-X).
ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
ROME – Measuring platelet function in order to tailor antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention for acute coronary syndromes did not improve their clinical outcomes in the randomized ANTARCTIC trial, Gilles Montalescot, MD, reported at the annual congress of the European Society of Cardiology.
“We found absolutely no benefit for this strategy of adjustment of antiplatelet therapy based upon platelet function testing. The study was completely neutral on all types of endpoints, ischemic as well as bleeding,” said Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Pitié-Salpêtrière Hospital.
This was a disappointing result in what was the largest-ever randomized clinical trial involving PCI in elderly patients, he said. This was a high-risk population, not only by virtue of everyone being over age 75 years, but because they all presented with ACS. Indeed, one-third of ANTARCTIC participants underwent primary PCI for ST-segment elevation myocardial infarction.
ANTARCTIC (Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged Over 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis, and Ischemic Complications) was carried out as a follow-up to the earlier ARCTIC randomized trial, also conducted by Dr. Montalescot and his coinvestigators. Like ANTARCTIC, ARCTIC, too, showed no clinical benefit for platelet function testing in order to adjust antiplatelet therapy (N Engl J Med. 2012;367:2100-9). At the time, ARCTIC’s critics argued that this individualized strategy didn’t achieve the expected improved outcomes because the trial was conducted in low-risk, stable patients undergoing elective scheduled PCI. In contrast, if there was ever a high-risk population in which platelet function testing and tailored antiplatelet therapy should work, it was in the very high-risk ANTARCTIC population, he said.
ANTARCTIC included 877 elderly patients undergoing urgent PCI for ACS who were placed on low-dose aspirin and randomized to standard antiplatelet therapy with prasugrel (Effient) at 5 mg/day, the European approved dose for long-term maintenance therapy in elderly patients, or to tailored antiplatelet therapy.
Patients in the tailored therapy arm received prasugrel at 5 mg/day for the first 14 days, then underwent platelet function testing with the VerifyNow P2Y12 system. If they demonstrated high on-drug platelet activity, defined as at least 208 P2Y12 reaction units (PRU), their prasugrel was bumped up to 10 mg/day. If their PRU measurement was in what is considered the optimal range for quelling ischemia without promoting bleeding – that is, less than 208 but more than 85 PRU – they remained on prasugrel at 5 mg/day. And if they scored less than 85 PRU, exposing them to excess bleeding risk due to high suppression of platelets, they were switched to clopidogrel (Plavix) at 75 mg/day, a less potent antiplatelet regimen.
Two weeks after their first platelet function measurement, participants in the tailored therapy arm returned for a second round of platelet activity testing, with their antiplatelet regimen once again being adjusted on the basis of the results.
The primary study endpoint was net clinical benefit over a 12-month follow-up period. This was defined as the composite of cardiovascular death, MI, stroke, urgent revascularization, stent thrombosis, and Bleeding Academic Research Consortium (BARC) types 2, 3, or 5. This composite endpoint occurred in 27.6% of the platelet monitoring group and a near-identical 27.8% of conventionally managed patients.
Of note, 42% of patients in the actively monitored group were within the target platelet inhibition range when tested 14 days into the study. At study’s end, 55% of patients remained on prasugrel at 5 mg/day, 39% were on clopidogrel at 75 mg/day, and less than 4% were on prasugrel at 10 mg/day. Thus, most patients who underwent a dose adjustment on the basis of their VerifyNow results were downgraded to a less-potent antiplatelet regimen. Very few required enhanced platelet suppression in the form of 10 mg/day of prasugrel.
“Platelet function monitoring is difficult to use. Patients have to come back twice to be monitored. It’s costly. It’s time consuming. And platelet function monitoring clearly does not help,” the cardiologist said.
The ANTARCTIC results will likely lead to a revision of the American and European guidelines, which currently give a class IIb/level of evidence C recommendation for platelet function testing in high-risk situations.
“There is a huge literature showing that platelet reactivity affects clinical outcomes,” Dr. Montalescot continued. “One hypothesis now is that platelet reactivity may be only a marker of risk; you can modify it, but that has no impact on patient outcomes. We may be in the same situation here as with HDL cholesterol, for example.”
Discussant Steen Dalby Kristensen, MD, noted that ANTARCTIC is just the latest in a slew of negative randomized clinical trials of individualized antiplatelet therapy for coronary artery disease. In addition to ARCTIC, others include GRAVITAS, TRIGGER PCI, and ASCET. One study, the German/Austrian TROPICAL ACS trial, remains ongoing.
“It really is an intriguing concept that many of us have been fascinated by for years: to identify the sweet spot where, by measuring platelet aggregation and maybe changing the therapy, we can find just the right balance between bleeding and ischemia. The ANTARCTIC results are quite disappointing for platelet-monitoring enthusiasts. Is the whole concept wrong?” said Dr. Kristensen, professor of cardiology and head of the cardiovascular research center at Aarhus (Denmark) University Hospital.
“I think even more disappointing for me than the lack of impact on ischemic events was the bleeding. I would have anticipated that maybe bleeding could be avoided by adjusting the dose, but this was not the case,” he added.
But Stephan Gielen, MD, saw a silver lining in the negative results for ANTARCTIC.
“From my perspective as a clinical interventionalist, I’m happy that you ended up in the way you did. Putting things positively, this study confirms the safety of dual-platelet inhibition with prasugrel at the reduced dose of 5 mg in an elderly population. There is no need to go to the trouble of monitoring platelet function even in this elderly population, which I think for clinical practice is a good message,” said Dr. Gielen of Detmold (Germany) Hospital, who cochaired a press conference where Dr. Montalescot presented the ANTARCTIC results.
Simultaneously with Dr. Montalescot’s presentation at ESC 2016 in Rome, the ANTARCTIC results were published online (Lancet. 2016 Aug 26. doi: 10.1016/S0140-6736(16)31323-X).
ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
ROME – Measuring platelet function in order to tailor antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention for acute coronary syndromes did not improve their clinical outcomes in the randomized ANTARCTIC trial, Gilles Montalescot, MD, reported at the annual congress of the European Society of Cardiology.
“We found absolutely no benefit for this strategy of adjustment of antiplatelet therapy based upon platelet function testing. The study was completely neutral on all types of endpoints, ischemic as well as bleeding,” said Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Pitié-Salpêtrière Hospital.
This was a disappointing result in what was the largest-ever randomized clinical trial involving PCI in elderly patients, he said. This was a high-risk population, not only by virtue of everyone being over age 75 years, but because they all presented with ACS. Indeed, one-third of ANTARCTIC participants underwent primary PCI for ST-segment elevation myocardial infarction.
ANTARCTIC (Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged Over 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis, and Ischemic Complications) was carried out as a follow-up to the earlier ARCTIC randomized trial, also conducted by Dr. Montalescot and his coinvestigators. Like ANTARCTIC, ARCTIC, too, showed no clinical benefit for platelet function testing in order to adjust antiplatelet therapy (N Engl J Med. 2012;367:2100-9). At the time, ARCTIC’s critics argued that this individualized strategy didn’t achieve the expected improved outcomes because the trial was conducted in low-risk, stable patients undergoing elective scheduled PCI. In contrast, if there was ever a high-risk population in which platelet function testing and tailored antiplatelet therapy should work, it was in the very high-risk ANTARCTIC population, he said.
ANTARCTIC included 877 elderly patients undergoing urgent PCI for ACS who were placed on low-dose aspirin and randomized to standard antiplatelet therapy with prasugrel (Effient) at 5 mg/day, the European approved dose for long-term maintenance therapy in elderly patients, or to tailored antiplatelet therapy.
Patients in the tailored therapy arm received prasugrel at 5 mg/day for the first 14 days, then underwent platelet function testing with the VerifyNow P2Y12 system. If they demonstrated high on-drug platelet activity, defined as at least 208 P2Y12 reaction units (PRU), their prasugrel was bumped up to 10 mg/day. If their PRU measurement was in what is considered the optimal range for quelling ischemia without promoting bleeding – that is, less than 208 but more than 85 PRU – they remained on prasugrel at 5 mg/day. And if they scored less than 85 PRU, exposing them to excess bleeding risk due to high suppression of platelets, they were switched to clopidogrel (Plavix) at 75 mg/day, a less potent antiplatelet regimen.
Two weeks after their first platelet function measurement, participants in the tailored therapy arm returned for a second round of platelet activity testing, with their antiplatelet regimen once again being adjusted on the basis of the results.
The primary study endpoint was net clinical benefit over a 12-month follow-up period. This was defined as the composite of cardiovascular death, MI, stroke, urgent revascularization, stent thrombosis, and Bleeding Academic Research Consortium (BARC) types 2, 3, or 5. This composite endpoint occurred in 27.6% of the platelet monitoring group and a near-identical 27.8% of conventionally managed patients.
Of note, 42% of patients in the actively monitored group were within the target platelet inhibition range when tested 14 days into the study. At study’s end, 55% of patients remained on prasugrel at 5 mg/day, 39% were on clopidogrel at 75 mg/day, and less than 4% were on prasugrel at 10 mg/day. Thus, most patients who underwent a dose adjustment on the basis of their VerifyNow results were downgraded to a less-potent antiplatelet regimen. Very few required enhanced platelet suppression in the form of 10 mg/day of prasugrel.
“Platelet function monitoring is difficult to use. Patients have to come back twice to be monitored. It’s costly. It’s time consuming. And platelet function monitoring clearly does not help,” the cardiologist said.
The ANTARCTIC results will likely lead to a revision of the American and European guidelines, which currently give a class IIb/level of evidence C recommendation for platelet function testing in high-risk situations.
“There is a huge literature showing that platelet reactivity affects clinical outcomes,” Dr. Montalescot continued. “One hypothesis now is that platelet reactivity may be only a marker of risk; you can modify it, but that has no impact on patient outcomes. We may be in the same situation here as with HDL cholesterol, for example.”
Discussant Steen Dalby Kristensen, MD, noted that ANTARCTIC is just the latest in a slew of negative randomized clinical trials of individualized antiplatelet therapy for coronary artery disease. In addition to ARCTIC, others include GRAVITAS, TRIGGER PCI, and ASCET. One study, the German/Austrian TROPICAL ACS trial, remains ongoing.
“It really is an intriguing concept that many of us have been fascinated by for years: to identify the sweet spot where, by measuring platelet aggregation and maybe changing the therapy, we can find just the right balance between bleeding and ischemia. The ANTARCTIC results are quite disappointing for platelet-monitoring enthusiasts. Is the whole concept wrong?” said Dr. Kristensen, professor of cardiology and head of the cardiovascular research center at Aarhus (Denmark) University Hospital.
“I think even more disappointing for me than the lack of impact on ischemic events was the bleeding. I would have anticipated that maybe bleeding could be avoided by adjusting the dose, but this was not the case,” he added.
But Stephan Gielen, MD, saw a silver lining in the negative results for ANTARCTIC.
“From my perspective as a clinical interventionalist, I’m happy that you ended up in the way you did. Putting things positively, this study confirms the safety of dual-platelet inhibition with prasugrel at the reduced dose of 5 mg in an elderly population. There is no need to go to the trouble of monitoring platelet function even in this elderly population, which I think for clinical practice is a good message,” said Dr. Gielen of Detmold (Germany) Hospital, who cochaired a press conference where Dr. Montalescot presented the ANTARCTIC results.
Simultaneously with Dr. Montalescot’s presentation at ESC 2016 in Rome, the ANTARCTIC results were published online (Lancet. 2016 Aug 26. doi: 10.1016/S0140-6736(16)31323-X).
ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
AT THE ESC CONGRESS 2016
Key clinical point: Researchers have just about given up on the notion that monitoring platelet function in order to individualize antiplatelet therapy in patients undergoing PCI provides any clinical benefit.
Major finding: Individualized antiplatelet therapy based upon serial measurements of platelet function did not result in improved outcomes in elderly patients undergoing PCI for acute coronary syndrome (27.6% in the platelet monitoring group and 27.8% in conventionally managed patients).
Data source: ANTARCTIC was an open-label, blinded-endpoint randomized trial of tailored versus standard antiplatelet therapy in 877 elderly patients undergoing PCI for ACS.
Disclosures: ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
Ticagrelor slashes first stroke risk after MI
ROME – Adding ticagrelor at 60 mg twice daily in patients on low-dose aspirin due to a prior MI reduced their risk of a first stroke by 25% in a secondary analysis of the landmark PEGASUS-TIMI 54 trial, Marc P. Bonaca, MD, reported at the annual congress of the European Society of Cardiology.
PEGASUS-TIMI 54 was a randomized, double-blind, placebo-controlled clinical trial conducted in more than 21,000 stable patients on low-dose aspirin with a history of an acute MI 1-3 years earlier. The significant reduction in secondary cardiovascular events seen in this study during a median 33 months of follow-up (N Engl J Med. 2015 May 7;372[19]:1791-800) led to approval of ticagrelor (Brilinta) at 60 mg twice daily for long-term secondary prevention.
But while PEGASUS-TIMI 54 was a secondary prevention study in terms of cardiovascular events, it was actually a primary prevention study in terms of stroke, since patients with a history of stroke weren’t eligible for enrollment. And in this trial, recipients of ticagrelor at 50 mg twice daily experienced a 25% reduction in the risk of stroke relative to placebo, from 1.94% at 3 years to 1.47%. This benefit was driven by fewer ischemic strokes, with no increase in hemorrhagic strokes seen with ticagrelor. And therein lies a clinical take home point: “When evaluating the overall benefits and risks of long-term ticagrelor in patients with prior MI, stroke reduction should also be considered,” according to Dr. Bonaca of Brigham and Women’s Hospital, Boston.
All strokes were adjudicated and subclassified by a blinded central committee. A total of 213 stroke events occurred during follow-up: 81% ischemic, 7% hemorrhagic, 4% ischemic with hemorrhagic conversion, and 8% unknown; 18% of the strokes were fatal. Another 15% resulted in moderate or severe disability at 30 days. All PEGASUS-TIMI 54 participants were on aspirin and more than 90% were on statin therapy.
The strokes that occurred in patients on ticagrelor were generally less severe than in controls. The risk of having a modified Rankin score of 3-6, which encompasses outcomes ranging from moderate disability to death, was reduced by 43% in stroke patients on ticagrelor relative to those on placebo, the cardiologist continued.
To ensure that the stroke benefit with ticagrelor seen in PEGASUS-TIMI 54 wasn’t a fluke, Dr. Bonaca and his coinvestigators performed a meta-analysis of four placebo-controlled randomized trials of more intensive versus less intensive antiplatelet therapy in nearly 45,000 participants with coronary disease in the CHARISMA, DAPT, PEGASUS-TIMI 54, and TRA 2*P-TIMI 50 trials. A total of 532 strokes occurred in this enlarged analysis. More intensive antiplatelet therapy – typically adding a second drug to low-dose aspirin – resulted in a 34% reduction in ischemic stroke, compared with low-dose aspirin and placebo.
Excluding from the meta-analysis the large subgroup of patients in TRA 2*P-TIMI 50 who were on triple-drug antiplatelet therapy, investigators were left with 32,348 participants in the four trials who were randomized to dual-antiplatelet therapy or monotherapy with aspirin. In this population, there was no increase in the risk of hemorrhagic stroke associated with more intensive antiplatelet therapy, according to Dr. Bonaca.
Session co-chair Keith A.A. Fox, MD, of the University of Edinburgh, noted that various studies have shown monotherapy with aspirin or another antiplatelet agent reduces stroke risk by about 15%, and now PEGASUS-TIMI 54 shows that ticagrelor plus aspirin decreases stroke risk by 25%. He posed a direct question: “How much is too much?”
“More and more antiplatelet therapy begets more bleeding, so I think that more than two agents may be approaching too much, although it really depends on what agents you’re using and in what dosages,” Dr. Bonaca replied.
He reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Simultaneous with Dr. Bonaca’s presentation at ESC 2016 in Rome, the new report from PEGASUS-TIMI 54 including the four-trial meta-analysis was published online (Circulation. 2016 Aug 30. doi: circulationaha.116.024637).
ROME – Adding ticagrelor at 60 mg twice daily in patients on low-dose aspirin due to a prior MI reduced their risk of a first stroke by 25% in a secondary analysis of the landmark PEGASUS-TIMI 54 trial, Marc P. Bonaca, MD, reported at the annual congress of the European Society of Cardiology.
PEGASUS-TIMI 54 was a randomized, double-blind, placebo-controlled clinical trial conducted in more than 21,000 stable patients on low-dose aspirin with a history of an acute MI 1-3 years earlier. The significant reduction in secondary cardiovascular events seen in this study during a median 33 months of follow-up (N Engl J Med. 2015 May 7;372[19]:1791-800) led to approval of ticagrelor (Brilinta) at 60 mg twice daily for long-term secondary prevention.
But while PEGASUS-TIMI 54 was a secondary prevention study in terms of cardiovascular events, it was actually a primary prevention study in terms of stroke, since patients with a history of stroke weren’t eligible for enrollment. And in this trial, recipients of ticagrelor at 50 mg twice daily experienced a 25% reduction in the risk of stroke relative to placebo, from 1.94% at 3 years to 1.47%. This benefit was driven by fewer ischemic strokes, with no increase in hemorrhagic strokes seen with ticagrelor. And therein lies a clinical take home point: “When evaluating the overall benefits and risks of long-term ticagrelor in patients with prior MI, stroke reduction should also be considered,” according to Dr. Bonaca of Brigham and Women’s Hospital, Boston.
All strokes were adjudicated and subclassified by a blinded central committee. A total of 213 stroke events occurred during follow-up: 81% ischemic, 7% hemorrhagic, 4% ischemic with hemorrhagic conversion, and 8% unknown; 18% of the strokes were fatal. Another 15% resulted in moderate or severe disability at 30 days. All PEGASUS-TIMI 54 participants were on aspirin and more than 90% were on statin therapy.
The strokes that occurred in patients on ticagrelor were generally less severe than in controls. The risk of having a modified Rankin score of 3-6, which encompasses outcomes ranging from moderate disability to death, was reduced by 43% in stroke patients on ticagrelor relative to those on placebo, the cardiologist continued.
To ensure that the stroke benefit with ticagrelor seen in PEGASUS-TIMI 54 wasn’t a fluke, Dr. Bonaca and his coinvestigators performed a meta-analysis of four placebo-controlled randomized trials of more intensive versus less intensive antiplatelet therapy in nearly 45,000 participants with coronary disease in the CHARISMA, DAPT, PEGASUS-TIMI 54, and TRA 2*P-TIMI 50 trials. A total of 532 strokes occurred in this enlarged analysis. More intensive antiplatelet therapy – typically adding a second drug to low-dose aspirin – resulted in a 34% reduction in ischemic stroke, compared with low-dose aspirin and placebo.
Excluding from the meta-analysis the large subgroup of patients in TRA 2*P-TIMI 50 who were on triple-drug antiplatelet therapy, investigators were left with 32,348 participants in the four trials who were randomized to dual-antiplatelet therapy or monotherapy with aspirin. In this population, there was no increase in the risk of hemorrhagic stroke associated with more intensive antiplatelet therapy, according to Dr. Bonaca.
Session co-chair Keith A.A. Fox, MD, of the University of Edinburgh, noted that various studies have shown monotherapy with aspirin or another antiplatelet agent reduces stroke risk by about 15%, and now PEGASUS-TIMI 54 shows that ticagrelor plus aspirin decreases stroke risk by 25%. He posed a direct question: “How much is too much?”
“More and more antiplatelet therapy begets more bleeding, so I think that more than two agents may be approaching too much, although it really depends on what agents you’re using and in what dosages,” Dr. Bonaca replied.
He reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Simultaneous with Dr. Bonaca’s presentation at ESC 2016 in Rome, the new report from PEGASUS-TIMI 54 including the four-trial meta-analysis was published online (Circulation. 2016 Aug 30. doi: circulationaha.116.024637).
ROME – Adding ticagrelor at 60 mg twice daily in patients on low-dose aspirin due to a prior MI reduced their risk of a first stroke by 25% in a secondary analysis of the landmark PEGASUS-TIMI 54 trial, Marc P. Bonaca, MD, reported at the annual congress of the European Society of Cardiology.
PEGASUS-TIMI 54 was a randomized, double-blind, placebo-controlled clinical trial conducted in more than 21,000 stable patients on low-dose aspirin with a history of an acute MI 1-3 years earlier. The significant reduction in secondary cardiovascular events seen in this study during a median 33 months of follow-up (N Engl J Med. 2015 May 7;372[19]:1791-800) led to approval of ticagrelor (Brilinta) at 60 mg twice daily for long-term secondary prevention.
But while PEGASUS-TIMI 54 was a secondary prevention study in terms of cardiovascular events, it was actually a primary prevention study in terms of stroke, since patients with a history of stroke weren’t eligible for enrollment. And in this trial, recipients of ticagrelor at 50 mg twice daily experienced a 25% reduction in the risk of stroke relative to placebo, from 1.94% at 3 years to 1.47%. This benefit was driven by fewer ischemic strokes, with no increase in hemorrhagic strokes seen with ticagrelor. And therein lies a clinical take home point: “When evaluating the overall benefits and risks of long-term ticagrelor in patients with prior MI, stroke reduction should also be considered,” according to Dr. Bonaca of Brigham and Women’s Hospital, Boston.
All strokes were adjudicated and subclassified by a blinded central committee. A total of 213 stroke events occurred during follow-up: 81% ischemic, 7% hemorrhagic, 4% ischemic with hemorrhagic conversion, and 8% unknown; 18% of the strokes were fatal. Another 15% resulted in moderate or severe disability at 30 days. All PEGASUS-TIMI 54 participants were on aspirin and more than 90% were on statin therapy.
The strokes that occurred in patients on ticagrelor were generally less severe than in controls. The risk of having a modified Rankin score of 3-6, which encompasses outcomes ranging from moderate disability to death, was reduced by 43% in stroke patients on ticagrelor relative to those on placebo, the cardiologist continued.
To ensure that the stroke benefit with ticagrelor seen in PEGASUS-TIMI 54 wasn’t a fluke, Dr. Bonaca and his coinvestigators performed a meta-analysis of four placebo-controlled randomized trials of more intensive versus less intensive antiplatelet therapy in nearly 45,000 participants with coronary disease in the CHARISMA, DAPT, PEGASUS-TIMI 54, and TRA 2*P-TIMI 50 trials. A total of 532 strokes occurred in this enlarged analysis. More intensive antiplatelet therapy – typically adding a second drug to low-dose aspirin – resulted in a 34% reduction in ischemic stroke, compared with low-dose aspirin and placebo.
Excluding from the meta-analysis the large subgroup of patients in TRA 2*P-TIMI 50 who were on triple-drug antiplatelet therapy, investigators were left with 32,348 participants in the four trials who were randomized to dual-antiplatelet therapy or monotherapy with aspirin. In this population, there was no increase in the risk of hemorrhagic stroke associated with more intensive antiplatelet therapy, according to Dr. Bonaca.
Session co-chair Keith A.A. Fox, MD, of the University of Edinburgh, noted that various studies have shown monotherapy with aspirin or another antiplatelet agent reduces stroke risk by about 15%, and now PEGASUS-TIMI 54 shows that ticagrelor plus aspirin decreases stroke risk by 25%. He posed a direct question: “How much is too much?”
“More and more antiplatelet therapy begets more bleeding, so I think that more than two agents may be approaching too much, although it really depends on what agents you’re using and in what dosages,” Dr. Bonaca replied.
He reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Simultaneous with Dr. Bonaca’s presentation at ESC 2016 in Rome, the new report from PEGASUS-TIMI 54 including the four-trial meta-analysis was published online (Circulation. 2016 Aug 30. doi: circulationaha.116.024637).
AT THE ESC CONGRESS 2016
Key clinical point: Ticagrelor reduced the risk of a first stroke by 25% in patients with a prior MI.
Major finding: Ticagrelor, at the approved dose of 60 mg twice daily for long-term secondary cardiovascular prevention, reduced the risk of a first stroke by 25% in patients with a prior MI.
Data source: This secondary analysis of a randomized, double-blind, placebo-controlled trial included 14,112 stable patients with a prior MI 1-3 years earlier who were randomized to ticagrelor at 60 mg twice daily or placebo and followed prospectively for a median of 33 months.
Disclosures: PEGASUS-TIMI 54 was supported by AstraZeneca. The presenter of the updated analysis reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Trials offer lessons despite negative primary endpoints
Conventional wisdom holds that for randomized, controlled trials, it’s all about the primary endpoint. If it’s negative or neutral, then none of the other results means much beyond “hypothesis generating.”
This strict-constructionist thinking has now been called into question. A recent article in the New England Journal of Medicine declared “an unreasonable yet widespread practice is the labeling of all randomized trials as either positive or negative on the basis of whether the P value for the primary outcome is less than .05. This view is overly simplistic.” (2016 Sept 1;375[9]:861-70).
The article, by the highly experienced and respected trialists Stuart J. Pocock, PhD, and Gregg W. Stone, MD, adds this: “If the primary outcome is negative, positive findings for secondary outcomes are usually considered to be hypothesis generating. Certainly, regulatory approval of a new drug is unlikely to follow. However, in some instances, secondary findings are compelling enough to affect guidelines and practice.”
This unconventional take from a pair of high-level trialists was especially timely given the buzz around the results from two studies reported at the European Society of Cardiology annual congress in late August, DANISH and NORSTENT.
The DANISH trial compared the impact of implantable cardioverter-defibrillators (ICDs) plus optimal care against optimal care without ICDs in 1,116 patients with nonischemic systolic heart failure. The primary outcome, all-cause death during more than 5 years of follow-up, was a relative 13% less with ICD use, a difference that was not statistically significant, and one secondary outcome, cardiovascular death, was cut by a relative 25% with ICD use, also not statistically significant.
But for the study’s second prespecified secondary endpoint of sudden cardiac death, treatment with ICDs cut the rate in half, compared with nonischemic heart failure patients who did not receive an ICD, a 4-percentage-point difference that was statistically significant.
And in a prespecified secondary analysis of the primary endpoint that broke down the study group by age, the two-thirds of patients younger than 68 years had a significant reduction in all-cause mortality with ICD use, a benefit not seen in patients aged 68 or older.
Discussion of the results at the meeting mainly focused on what meaning, if any, could be drawn from these strongly positive secondary outcomes in a trial neutral for its primary outcome.
“The ICDs did what they were supposed to, prevent sudden cardiac death,” said the lead investigator of the study, Lars Køber, MD. “As a principle I say don’t believe in a subgroup, but guidelines are often based on subgroup analyses.”
“The primary outcome was neutral, but the reduction in sudden cardiac death, the primary objective of an ICD, was significant, so an ICD should be taken into consideration,” commented Michel Komajda, MD, a discussant for the report.
After I wrote a news article about the DANISH report at ESC, I received an email from a reader who objected to spinning the results this way and insisted that no valid lessons can be drawn from the DANISH results because the study’s primary endpoint failed to show a statistical significance. This purist view misses the important, relevant lessons from the DANISH results. The DANISH trial was not designed to provide pivotal data for regulatory approval of ICDs in these patients. Rather, Dr. Køber and his associates designed DANISH to see whether ICD use in these patients could cut all-cause death over a fairly long follow-up. It was a very high bar and ICDs failed, but the deck was stacked against an ICD win. Enrolled patients averaged 64 years old at entry into the study, and they all had New York Heart Association class II or III heart failure. “The overall survival curves start to diverge, but then converge after 5 years because of the comorbidities and patients dying for other reasons,” Dr. Køber noted.
“The message is, in younger patients with less morbidity and more life expectancy, sudden cardiac death is a bigger problem, and they had a substantial drop in mortality” with ICD use, commented heart failure specialist Javed Butler, MD. “It’s very consistent with the way we think about providing ICD treatment to patients.”
In other words, the DANISH results showed that all patients with nonischemic systolic heart failure can’t expect to live substantially longer during extended follow-up if they get an ICD, because the cut in sudden cardiac death the devices provide eventually gets washed out by the many other risks for death these patients face. But younger, relatively healthier patients might very well see their reduced rate of sudden cardiac death translate into an overall mortality benefit even when they are followed for at least 5 years. That’s important information to help an individual patient decide whether to have an ICD placed, and an important message from the DANISH trial despite the neutral primary endpoint.
NORSTENT involved a similar scenario in a trial that addressed a totally different issue: Should patients with either stable or unstable coronary artery disease who are undergoing coronary stenting receive a drug-eluting stent (DES) or a bare metal stent (BMS)? The trial randomized 9,013 patients to receive either of the two stent types plus optimal medical therapy. The primary endpoint was the rate of all-cause death or nonfatal MI during 5 years of follow-up, and the results showed no statistically significant difference between the patients who received a DES and those who got a BMS (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
But for the secondary endpoint of repeat revascularizations performed during follow-up, the use of a DES cut the procedure rate by 3.3 percentage points, a 17% relative risk reduction that was statistically significant. The use of a DES also cut the stent thrombosis rate by 0.4 percentage points, a one-third relative drop in these events that was also statistically significant.
In short, despite the neutral primary endpoint for the trial, the results showed that drug-eluting stents did what they were designed to do relative to bare metal stents: cut the rate of target lesion restenosis and the need for repeat revascularization. Several interventional cardiologists who heard the results at the meeting said that the findings would not change their practice and that they would continue to use the DES as their default device for percutaneous coronary interventions. Although “the long-term benefit of contemporary DES over BMS was less than expected,” said Kaare H. Bønaa, MD, lead investigator for the NORSTENT trial, the secondary benefit of significantly reduced repeat revascularization and the very modest price difference that now exists between drug-eluting stents and bare metal stents means that many interventionalists will continue to use a DES for most patients.
The message from Dr. Pocock and Dr. Stone, underscored by the DANISH and NORSTENT results, is that large and well-run randomized trials can yield important evidence to inform practice that transcends a simple black or white statistical assessment of the primary endpoint.
On Twitter @mitchelzoler
Conventional wisdom holds that for randomized, controlled trials, it’s all about the primary endpoint. If it’s negative or neutral, then none of the other results means much beyond “hypothesis generating.”
This strict-constructionist thinking has now been called into question. A recent article in the New England Journal of Medicine declared “an unreasonable yet widespread practice is the labeling of all randomized trials as either positive or negative on the basis of whether the P value for the primary outcome is less than .05. This view is overly simplistic.” (2016 Sept 1;375[9]:861-70).
The article, by the highly experienced and respected trialists Stuart J. Pocock, PhD, and Gregg W. Stone, MD, adds this: “If the primary outcome is negative, positive findings for secondary outcomes are usually considered to be hypothesis generating. Certainly, regulatory approval of a new drug is unlikely to follow. However, in some instances, secondary findings are compelling enough to affect guidelines and practice.”
This unconventional take from a pair of high-level trialists was especially timely given the buzz around the results from two studies reported at the European Society of Cardiology annual congress in late August, DANISH and NORSTENT.
The DANISH trial compared the impact of implantable cardioverter-defibrillators (ICDs) plus optimal care against optimal care without ICDs in 1,116 patients with nonischemic systolic heart failure. The primary outcome, all-cause death during more than 5 years of follow-up, was a relative 13% less with ICD use, a difference that was not statistically significant, and one secondary outcome, cardiovascular death, was cut by a relative 25% with ICD use, also not statistically significant.
But for the study’s second prespecified secondary endpoint of sudden cardiac death, treatment with ICDs cut the rate in half, compared with nonischemic heart failure patients who did not receive an ICD, a 4-percentage-point difference that was statistically significant.
And in a prespecified secondary analysis of the primary endpoint that broke down the study group by age, the two-thirds of patients younger than 68 years had a significant reduction in all-cause mortality with ICD use, a benefit not seen in patients aged 68 or older.
Discussion of the results at the meeting mainly focused on what meaning, if any, could be drawn from these strongly positive secondary outcomes in a trial neutral for its primary outcome.
“The ICDs did what they were supposed to, prevent sudden cardiac death,” said the lead investigator of the study, Lars Køber, MD. “As a principle I say don’t believe in a subgroup, but guidelines are often based on subgroup analyses.”
“The primary outcome was neutral, but the reduction in sudden cardiac death, the primary objective of an ICD, was significant, so an ICD should be taken into consideration,” commented Michel Komajda, MD, a discussant for the report.
After I wrote a news article about the DANISH report at ESC, I received an email from a reader who objected to spinning the results this way and insisted that no valid lessons can be drawn from the DANISH results because the study’s primary endpoint failed to show a statistical significance. This purist view misses the important, relevant lessons from the DANISH results. The DANISH trial was not designed to provide pivotal data for regulatory approval of ICDs in these patients. Rather, Dr. Køber and his associates designed DANISH to see whether ICD use in these patients could cut all-cause death over a fairly long follow-up. It was a very high bar and ICDs failed, but the deck was stacked against an ICD win. Enrolled patients averaged 64 years old at entry into the study, and they all had New York Heart Association class II or III heart failure. “The overall survival curves start to diverge, but then converge after 5 years because of the comorbidities and patients dying for other reasons,” Dr. Køber noted.
“The message is, in younger patients with less morbidity and more life expectancy, sudden cardiac death is a bigger problem, and they had a substantial drop in mortality” with ICD use, commented heart failure specialist Javed Butler, MD. “It’s very consistent with the way we think about providing ICD treatment to patients.”
In other words, the DANISH results showed that all patients with nonischemic systolic heart failure can’t expect to live substantially longer during extended follow-up if they get an ICD, because the cut in sudden cardiac death the devices provide eventually gets washed out by the many other risks for death these patients face. But younger, relatively healthier patients might very well see their reduced rate of sudden cardiac death translate into an overall mortality benefit even when they are followed for at least 5 years. That’s important information to help an individual patient decide whether to have an ICD placed, and an important message from the DANISH trial despite the neutral primary endpoint.
NORSTENT involved a similar scenario in a trial that addressed a totally different issue: Should patients with either stable or unstable coronary artery disease who are undergoing coronary stenting receive a drug-eluting stent (DES) or a bare metal stent (BMS)? The trial randomized 9,013 patients to receive either of the two stent types plus optimal medical therapy. The primary endpoint was the rate of all-cause death or nonfatal MI during 5 years of follow-up, and the results showed no statistically significant difference between the patients who received a DES and those who got a BMS (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
But for the secondary endpoint of repeat revascularizations performed during follow-up, the use of a DES cut the procedure rate by 3.3 percentage points, a 17% relative risk reduction that was statistically significant. The use of a DES also cut the stent thrombosis rate by 0.4 percentage points, a one-third relative drop in these events that was also statistically significant.
In short, despite the neutral primary endpoint for the trial, the results showed that drug-eluting stents did what they were designed to do relative to bare metal stents: cut the rate of target lesion restenosis and the need for repeat revascularization. Several interventional cardiologists who heard the results at the meeting said that the findings would not change their practice and that they would continue to use the DES as their default device for percutaneous coronary interventions. Although “the long-term benefit of contemporary DES over BMS was less than expected,” said Kaare H. Bønaa, MD, lead investigator for the NORSTENT trial, the secondary benefit of significantly reduced repeat revascularization and the very modest price difference that now exists between drug-eluting stents and bare metal stents means that many interventionalists will continue to use a DES for most patients.
The message from Dr. Pocock and Dr. Stone, underscored by the DANISH and NORSTENT results, is that large and well-run randomized trials can yield important evidence to inform practice that transcends a simple black or white statistical assessment of the primary endpoint.
On Twitter @mitchelzoler
Conventional wisdom holds that for randomized, controlled trials, it’s all about the primary endpoint. If it’s negative or neutral, then none of the other results means much beyond “hypothesis generating.”
This strict-constructionist thinking has now been called into question. A recent article in the New England Journal of Medicine declared “an unreasonable yet widespread practice is the labeling of all randomized trials as either positive or negative on the basis of whether the P value for the primary outcome is less than .05. This view is overly simplistic.” (2016 Sept 1;375[9]:861-70).
The article, by the highly experienced and respected trialists Stuart J. Pocock, PhD, and Gregg W. Stone, MD, adds this: “If the primary outcome is negative, positive findings for secondary outcomes are usually considered to be hypothesis generating. Certainly, regulatory approval of a new drug is unlikely to follow. However, in some instances, secondary findings are compelling enough to affect guidelines and practice.”
This unconventional take from a pair of high-level trialists was especially timely given the buzz around the results from two studies reported at the European Society of Cardiology annual congress in late August, DANISH and NORSTENT.
The DANISH trial compared the impact of implantable cardioverter-defibrillators (ICDs) plus optimal care against optimal care without ICDs in 1,116 patients with nonischemic systolic heart failure. The primary outcome, all-cause death during more than 5 years of follow-up, was a relative 13% less with ICD use, a difference that was not statistically significant, and one secondary outcome, cardiovascular death, was cut by a relative 25% with ICD use, also not statistically significant.
But for the study’s second prespecified secondary endpoint of sudden cardiac death, treatment with ICDs cut the rate in half, compared with nonischemic heart failure patients who did not receive an ICD, a 4-percentage-point difference that was statistically significant.
And in a prespecified secondary analysis of the primary endpoint that broke down the study group by age, the two-thirds of patients younger than 68 years had a significant reduction in all-cause mortality with ICD use, a benefit not seen in patients aged 68 or older.
Discussion of the results at the meeting mainly focused on what meaning, if any, could be drawn from these strongly positive secondary outcomes in a trial neutral for its primary outcome.
“The ICDs did what they were supposed to, prevent sudden cardiac death,” said the lead investigator of the study, Lars Køber, MD. “As a principle I say don’t believe in a subgroup, but guidelines are often based on subgroup analyses.”
“The primary outcome was neutral, but the reduction in sudden cardiac death, the primary objective of an ICD, was significant, so an ICD should be taken into consideration,” commented Michel Komajda, MD, a discussant for the report.
After I wrote a news article about the DANISH report at ESC, I received an email from a reader who objected to spinning the results this way and insisted that no valid lessons can be drawn from the DANISH results because the study’s primary endpoint failed to show a statistical significance. This purist view misses the important, relevant lessons from the DANISH results. The DANISH trial was not designed to provide pivotal data for regulatory approval of ICDs in these patients. Rather, Dr. Køber and his associates designed DANISH to see whether ICD use in these patients could cut all-cause death over a fairly long follow-up. It was a very high bar and ICDs failed, but the deck was stacked against an ICD win. Enrolled patients averaged 64 years old at entry into the study, and they all had New York Heart Association class II or III heart failure. “The overall survival curves start to diverge, but then converge after 5 years because of the comorbidities and patients dying for other reasons,” Dr. Køber noted.
“The message is, in younger patients with less morbidity and more life expectancy, sudden cardiac death is a bigger problem, and they had a substantial drop in mortality” with ICD use, commented heart failure specialist Javed Butler, MD. “It’s very consistent with the way we think about providing ICD treatment to patients.”
In other words, the DANISH results showed that all patients with nonischemic systolic heart failure can’t expect to live substantially longer during extended follow-up if they get an ICD, because the cut in sudden cardiac death the devices provide eventually gets washed out by the many other risks for death these patients face. But younger, relatively healthier patients might very well see their reduced rate of sudden cardiac death translate into an overall mortality benefit even when they are followed for at least 5 years. That’s important information to help an individual patient decide whether to have an ICD placed, and an important message from the DANISH trial despite the neutral primary endpoint.
NORSTENT involved a similar scenario in a trial that addressed a totally different issue: Should patients with either stable or unstable coronary artery disease who are undergoing coronary stenting receive a drug-eluting stent (DES) or a bare metal stent (BMS)? The trial randomized 9,013 patients to receive either of the two stent types plus optimal medical therapy. The primary endpoint was the rate of all-cause death or nonfatal MI during 5 years of follow-up, and the results showed no statistically significant difference between the patients who received a DES and those who got a BMS (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
But for the secondary endpoint of repeat revascularizations performed during follow-up, the use of a DES cut the procedure rate by 3.3 percentage points, a 17% relative risk reduction that was statistically significant. The use of a DES also cut the stent thrombosis rate by 0.4 percentage points, a one-third relative drop in these events that was also statistically significant.
In short, despite the neutral primary endpoint for the trial, the results showed that drug-eluting stents did what they were designed to do relative to bare metal stents: cut the rate of target lesion restenosis and the need for repeat revascularization. Several interventional cardiologists who heard the results at the meeting said that the findings would not change their practice and that they would continue to use the DES as their default device for percutaneous coronary interventions. Although “the long-term benefit of contemporary DES over BMS was less than expected,” said Kaare H. Bønaa, MD, lead investigator for the NORSTENT trial, the secondary benefit of significantly reduced repeat revascularization and the very modest price difference that now exists between drug-eluting stents and bare metal stents means that many interventionalists will continue to use a DES for most patients.
The message from Dr. Pocock and Dr. Stone, underscored by the DANISH and NORSTENT results, is that large and well-run randomized trials can yield important evidence to inform practice that transcends a simple black or white statistical assessment of the primary endpoint.
On Twitter @mitchelzoler
Longer DAPT better for PAD, study suggests

Photo by Sage Ross
ROME—A subanalysis of the PRODIGY study suggests a longer duration of dual antiplatelet therapy (DAPT) improves outcomes after percutaneous coronary intervention (PCI) for patients with peripheral arterial disease (PAD).
Receiving long-term DAPT after PCI reduced the risk of atherothrombotic events and death in patients with PAD, without increasing the risk of actionable bleeding episodes.
However, patients without PAD fared better with short-term DAPT.
These results were presented at ESC Congress 2016 (abstract 5154) and published in JAMA Cardiology.
Marco Valgimigli, MD, PhD, of Bern University Hospital in Bern, Switzerland, and his colleagues performed this analysis of PRODIGY data.
The study included patients from tertiary care hospitals who had stable coronary artery disease or acute coronary syndromes, with or without concomitant PAD, and were undergoing PCI.
There were 246 patients with PAD—118 who were randomized to receive DAPT for 24 months after PCI and 128 who were randomized to DAPT for 6 months or less.
There were 1724 patients without PAD—869 who were randomized to receive DAPT for 24 months after PCI and 855 who were randomized to DAPT for 6 months or less.
The patients with PAD were older and more frequently underwent multivessel intervention. They were also more likely to have hypertension, type 1 or 2 diabetes, previous myocardial infarction, previous coronary artery bypass grafting, non-ST-segment elevation myocardial infarction, and more complex coronary artery disease.
At 30 days, patients with PAD were more often taking diuretics, and patients without PAD were more often taking beta-blockers and statins.
Patients with PAD who were randomized to long-term DAPT were younger, had a higher body mass index, and less frequently underwent PCI of the left main coronary artery than PAD patients randomized to short-term DAPT.
Having PAD was associated with a higher risk of death and ischemic events, with a hazard ratio (HR) of 2.80 (95% CI, 2.05-3.83; P<0.001).
Results
The primary efficacy endpoint of this study was a composite of death, myocardial infarction, and cerebrovascular accidents.
Among patients with PAD, those who received long-term DAPT had a lower risk of this endpoint than those who received short-term DAPT—16.1% and 27.3%, respectively. The HR was 0.54 (95% CI, 0.31-0.95; P=0.03).
Among patients without PAD, there was no significant difference in the incidence of the primary endpoint according to DAPT duration. It occurred in 9.3% of patients who received long-term DAPT and 7.4% of patients who received short-term DAPT. The HR was 1.28 (95% CI, 0.92-1.77; P=0.15).
The key safety endpoint was a composite of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding.
There was no significant difference in this endpoint according to DAPT duration for patients with PAD, but long-term DAPT was associated with a significant increase in this endpoint for patients without PAD.
Among patients with PAD, BARC type 2, 3, or 5 bleeding occurred in 5.2% of those receiving long-term DAPT and 6.9% of those receiving short-term DAPT. The HR was 0.77 (95% CI, 0.27-2.21; P=0.62).
Among patients without PAD, BARC type 2, 3, or 5 bleeding occurred in 8% of those receiving long-term DAPT and 3.1% of those receiving short-term DAPT. The HR was 2.61 (95% CI, 0.27-2.21; P<0.001).
The researchers said the apparent neutral effect of long-term DAPT on bleeding risk in PAD patients requires further evaluation in adequately powered studies, but this research suggests patients with PAD will benefit from prolonged DAPT after PCI. ![]()

Photo by Sage Ross
ROME—A subanalysis of the PRODIGY study suggests a longer duration of dual antiplatelet therapy (DAPT) improves outcomes after percutaneous coronary intervention (PCI) for patients with peripheral arterial disease (PAD).
Receiving long-term DAPT after PCI reduced the risk of atherothrombotic events and death in patients with PAD, without increasing the risk of actionable bleeding episodes.
However, patients without PAD fared better with short-term DAPT.
These results were presented at ESC Congress 2016 (abstract 5154) and published in JAMA Cardiology.
Marco Valgimigli, MD, PhD, of Bern University Hospital in Bern, Switzerland, and his colleagues performed this analysis of PRODIGY data.
The study included patients from tertiary care hospitals who had stable coronary artery disease or acute coronary syndromes, with or without concomitant PAD, and were undergoing PCI.
There were 246 patients with PAD—118 who were randomized to receive DAPT for 24 months after PCI and 128 who were randomized to DAPT for 6 months or less.
There were 1724 patients without PAD—869 who were randomized to receive DAPT for 24 months after PCI and 855 who were randomized to DAPT for 6 months or less.
The patients with PAD were older and more frequently underwent multivessel intervention. They were also more likely to have hypertension, type 1 or 2 diabetes, previous myocardial infarction, previous coronary artery bypass grafting, non-ST-segment elevation myocardial infarction, and more complex coronary artery disease.
At 30 days, patients with PAD were more often taking diuretics, and patients without PAD were more often taking beta-blockers and statins.
Patients with PAD who were randomized to long-term DAPT were younger, had a higher body mass index, and less frequently underwent PCI of the left main coronary artery than PAD patients randomized to short-term DAPT.
Having PAD was associated with a higher risk of death and ischemic events, with a hazard ratio (HR) of 2.80 (95% CI, 2.05-3.83; P<0.001).
Results
The primary efficacy endpoint of this study was a composite of death, myocardial infarction, and cerebrovascular accidents.
Among patients with PAD, those who received long-term DAPT had a lower risk of this endpoint than those who received short-term DAPT—16.1% and 27.3%, respectively. The HR was 0.54 (95% CI, 0.31-0.95; P=0.03).
Among patients without PAD, there was no significant difference in the incidence of the primary endpoint according to DAPT duration. It occurred in 9.3% of patients who received long-term DAPT and 7.4% of patients who received short-term DAPT. The HR was 1.28 (95% CI, 0.92-1.77; P=0.15).
The key safety endpoint was a composite of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding.
There was no significant difference in this endpoint according to DAPT duration for patients with PAD, but long-term DAPT was associated with a significant increase in this endpoint for patients without PAD.
Among patients with PAD, BARC type 2, 3, or 5 bleeding occurred in 5.2% of those receiving long-term DAPT and 6.9% of those receiving short-term DAPT. The HR was 0.77 (95% CI, 0.27-2.21; P=0.62).
Among patients without PAD, BARC type 2, 3, or 5 bleeding occurred in 8% of those receiving long-term DAPT and 3.1% of those receiving short-term DAPT. The HR was 2.61 (95% CI, 0.27-2.21; P<0.001).
The researchers said the apparent neutral effect of long-term DAPT on bleeding risk in PAD patients requires further evaluation in adequately powered studies, but this research suggests patients with PAD will benefit from prolonged DAPT after PCI. ![]()

Photo by Sage Ross
ROME—A subanalysis of the PRODIGY study suggests a longer duration of dual antiplatelet therapy (DAPT) improves outcomes after percutaneous coronary intervention (PCI) for patients with peripheral arterial disease (PAD).
Receiving long-term DAPT after PCI reduced the risk of atherothrombotic events and death in patients with PAD, without increasing the risk of actionable bleeding episodes.
However, patients without PAD fared better with short-term DAPT.
These results were presented at ESC Congress 2016 (abstract 5154) and published in JAMA Cardiology.
Marco Valgimigli, MD, PhD, of Bern University Hospital in Bern, Switzerland, and his colleagues performed this analysis of PRODIGY data.
The study included patients from tertiary care hospitals who had stable coronary artery disease or acute coronary syndromes, with or without concomitant PAD, and were undergoing PCI.
There were 246 patients with PAD—118 who were randomized to receive DAPT for 24 months after PCI and 128 who were randomized to DAPT for 6 months or less.
There were 1724 patients without PAD—869 who were randomized to receive DAPT for 24 months after PCI and 855 who were randomized to DAPT for 6 months or less.
The patients with PAD were older and more frequently underwent multivessel intervention. They were also more likely to have hypertension, type 1 or 2 diabetes, previous myocardial infarction, previous coronary artery bypass grafting, non-ST-segment elevation myocardial infarction, and more complex coronary artery disease.
At 30 days, patients with PAD were more often taking diuretics, and patients without PAD were more often taking beta-blockers and statins.
Patients with PAD who were randomized to long-term DAPT were younger, had a higher body mass index, and less frequently underwent PCI of the left main coronary artery than PAD patients randomized to short-term DAPT.
Having PAD was associated with a higher risk of death and ischemic events, with a hazard ratio (HR) of 2.80 (95% CI, 2.05-3.83; P<0.001).
Results
The primary efficacy endpoint of this study was a composite of death, myocardial infarction, and cerebrovascular accidents.
Among patients with PAD, those who received long-term DAPT had a lower risk of this endpoint than those who received short-term DAPT—16.1% and 27.3%, respectively. The HR was 0.54 (95% CI, 0.31-0.95; P=0.03).
Among patients without PAD, there was no significant difference in the incidence of the primary endpoint according to DAPT duration. It occurred in 9.3% of patients who received long-term DAPT and 7.4% of patients who received short-term DAPT. The HR was 1.28 (95% CI, 0.92-1.77; P=0.15).
The key safety endpoint was a composite of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding.
There was no significant difference in this endpoint according to DAPT duration for patients with PAD, but long-term DAPT was associated with a significant increase in this endpoint for patients without PAD.
Among patients with PAD, BARC type 2, 3, or 5 bleeding occurred in 5.2% of those receiving long-term DAPT and 6.9% of those receiving short-term DAPT. The HR was 0.77 (95% CI, 0.27-2.21; P=0.62).
Among patients without PAD, BARC type 2, 3, or 5 bleeding occurred in 8% of those receiving long-term DAPT and 3.1% of those receiving short-term DAPT. The HR was 2.61 (95% CI, 0.27-2.21; P<0.001).
The researchers said the apparent neutral effect of long-term DAPT on bleeding risk in PAD patients requires further evaluation in adequately powered studies, but this research suggests patients with PAD will benefit from prolonged DAPT after PCI. ![]()
Simple algorithm can rule out PE, team says

Image courtesy of
Medical College of Georgia
ROME—A new study suggests the YEARS algorithm may provide a simple method for ruling out pulmonary embolism (PE) and therefore reduce the need for computed tomography pulmonary angiography (CTPA).
“[The YEARS algorithm] can replace current diagnostic algorithms, which, although safe and accurate, are often not used in busy emergency departments because they are too complex,” said study investigator Tom Van der Hulle, MD, of Leiden University Medical Center in the Netherlands.
“The advantage of the YEARS algorithm over existing algorithms is a 14% reduction in the need for CTPA imaging and, with that, reduced potential for radiation-induced harm and overdiagnosis.”
Dr Van der Hulle described his team’s results with the YEARS algorithm at ESC Congress 2016 (abstract 5727).
About the algorithm
The YEARS algorithm consists of a blood test and 3 items of the original Wells rule.
Patients presenting to the emergency department can be evaluated based on:
- Clinical signs of deep vein thrombosis (eg, swelling, edema)
- Hemoptysis
- Whether the clinician considers PE to be “the most likely diagnosis.”
Using this information combined with results of a blood test measuring D-dimer, clinicians can either exclude PE or recommend CTPA for definitive diagnosis.
Based on the algorithm, PE can be excluded in patients who have either:
- None of the 3 YEARS items and a D-dimer level <1000 ng/mL
- One or more YEARS items and a D-dimer level <500 ng/mL.
Testing the algorithm
Dr Van der Hulle and his colleagues prospectively evaluated the YEARS algorithm in 3465 patients. They had a mean age of 53, and 88% were outpatients.
If patients did not have PE excluded via the algorithm, they went on to CTPA. If PE was confirmed, patients received anticoagulant therapy.
Most of the patients in whom PE was excluded (either by algorithm or CTPA) were left untreated and were followed for 3 months, although 60 patients who had PE excluded received anticoagulants anyway.
In all, 1651 patients had PE excluded with the YEARS algorithm, and 1633 of them did not receive anticoagulation.
A total of 1814 patients did not have PE excluded via the YEARS algorithm and went on to CTPA. Of these patients, 456 had PE, 42 had PE excluded via CTPA but received anticoagulation, and 1316 had PE excluded but did not receive anticoagulation.
Five patients were lost to follow-up—4 with PE excluded via the YEARS algorithm and 1 who went on to CTPA.
Patient outcomes
The primary outcome of this study was the 3-month incidence of symptomatic venous thromboembolism (VTE).
A total of 2944 patients were evaluable (because they had PE excluded, did not receive anticoagulation, and were not lost to follow-up)—1629 who had PE excluded via the YEARS algorithm and 1315 who had PE excluded via CTPA.
Symptomatic VTE occurred in 0.61% of all evaluable patients, 0.43% of patients who had PE excluded via YEARS, and 0.84% of patients who had PE excluded via CTPA.
Fatal PE occurred in 0.20% of all evaluable patients, 0.12% of patients who had PE excluded via YEARS, and 0.30% of patients who had PE excluded via CTPA.
“This is fully in line with that observed in studies using traditional, sequential algorithms such as the 2-level Wells score and a fixed cut-off level of D-dimer of 500 ng/mL,” Dr Van der Hulle said.
“Using the YEARS algorithm, CTPA was not indicated in 48% of our patients at baseline, but this would have been only 34% of patients using the traditional algorithm. This shows that the YEARS algorithm can safely exclude PE and resulted in an absolute reduction of required CTPA of 14%.”
“We expect that the YEARS algorithm can be easily implemented outside the participating study sites and that these safety and efficacy outcomes are representative of what could be expected in regular clinical settings.” ![]()

Image courtesy of
Medical College of Georgia
ROME—A new study suggests the YEARS algorithm may provide a simple method for ruling out pulmonary embolism (PE) and therefore reduce the need for computed tomography pulmonary angiography (CTPA).
“[The YEARS algorithm] can replace current diagnostic algorithms, which, although safe and accurate, are often not used in busy emergency departments because they are too complex,” said study investigator Tom Van der Hulle, MD, of Leiden University Medical Center in the Netherlands.
“The advantage of the YEARS algorithm over existing algorithms is a 14% reduction in the need for CTPA imaging and, with that, reduced potential for radiation-induced harm and overdiagnosis.”
Dr Van der Hulle described his team’s results with the YEARS algorithm at ESC Congress 2016 (abstract 5727).
About the algorithm
The YEARS algorithm consists of a blood test and 3 items of the original Wells rule.
Patients presenting to the emergency department can be evaluated based on:
- Clinical signs of deep vein thrombosis (eg, swelling, edema)
- Hemoptysis
- Whether the clinician considers PE to be “the most likely diagnosis.”
Using this information combined with results of a blood test measuring D-dimer, clinicians can either exclude PE or recommend CTPA for definitive diagnosis.
Based on the algorithm, PE can be excluded in patients who have either:
- None of the 3 YEARS items and a D-dimer level <1000 ng/mL
- One or more YEARS items and a D-dimer level <500 ng/mL.
Testing the algorithm
Dr Van der Hulle and his colleagues prospectively evaluated the YEARS algorithm in 3465 patients. They had a mean age of 53, and 88% were outpatients.
If patients did not have PE excluded via the algorithm, they went on to CTPA. If PE was confirmed, patients received anticoagulant therapy.
Most of the patients in whom PE was excluded (either by algorithm or CTPA) were left untreated and were followed for 3 months, although 60 patients who had PE excluded received anticoagulants anyway.
In all, 1651 patients had PE excluded with the YEARS algorithm, and 1633 of them did not receive anticoagulation.
A total of 1814 patients did not have PE excluded via the YEARS algorithm and went on to CTPA. Of these patients, 456 had PE, 42 had PE excluded via CTPA but received anticoagulation, and 1316 had PE excluded but did not receive anticoagulation.
Five patients were lost to follow-up—4 with PE excluded via the YEARS algorithm and 1 who went on to CTPA.
Patient outcomes
The primary outcome of this study was the 3-month incidence of symptomatic venous thromboembolism (VTE).
A total of 2944 patients were evaluable (because they had PE excluded, did not receive anticoagulation, and were not lost to follow-up)—1629 who had PE excluded via the YEARS algorithm and 1315 who had PE excluded via CTPA.
Symptomatic VTE occurred in 0.61% of all evaluable patients, 0.43% of patients who had PE excluded via YEARS, and 0.84% of patients who had PE excluded via CTPA.
Fatal PE occurred in 0.20% of all evaluable patients, 0.12% of patients who had PE excluded via YEARS, and 0.30% of patients who had PE excluded via CTPA.
“This is fully in line with that observed in studies using traditional, sequential algorithms such as the 2-level Wells score and a fixed cut-off level of D-dimer of 500 ng/mL,” Dr Van der Hulle said.
“Using the YEARS algorithm, CTPA was not indicated in 48% of our patients at baseline, but this would have been only 34% of patients using the traditional algorithm. This shows that the YEARS algorithm can safely exclude PE and resulted in an absolute reduction of required CTPA of 14%.”
“We expect that the YEARS algorithm can be easily implemented outside the participating study sites and that these safety and efficacy outcomes are representative of what could be expected in regular clinical settings.” ![]()

Image courtesy of
Medical College of Georgia
ROME—A new study suggests the YEARS algorithm may provide a simple method for ruling out pulmonary embolism (PE) and therefore reduce the need for computed tomography pulmonary angiography (CTPA).
“[The YEARS algorithm] can replace current diagnostic algorithms, which, although safe and accurate, are often not used in busy emergency departments because they are too complex,” said study investigator Tom Van der Hulle, MD, of Leiden University Medical Center in the Netherlands.
“The advantage of the YEARS algorithm over existing algorithms is a 14% reduction in the need for CTPA imaging and, with that, reduced potential for radiation-induced harm and overdiagnosis.”
Dr Van der Hulle described his team’s results with the YEARS algorithm at ESC Congress 2016 (abstract 5727).
About the algorithm
The YEARS algorithm consists of a blood test and 3 items of the original Wells rule.
Patients presenting to the emergency department can be evaluated based on:
- Clinical signs of deep vein thrombosis (eg, swelling, edema)
- Hemoptysis
- Whether the clinician considers PE to be “the most likely diagnosis.”
Using this information combined with results of a blood test measuring D-dimer, clinicians can either exclude PE or recommend CTPA for definitive diagnosis.
Based on the algorithm, PE can be excluded in patients who have either:
- None of the 3 YEARS items and a D-dimer level <1000 ng/mL
- One or more YEARS items and a D-dimer level <500 ng/mL.
Testing the algorithm
Dr Van der Hulle and his colleagues prospectively evaluated the YEARS algorithm in 3465 patients. They had a mean age of 53, and 88% were outpatients.
If patients did not have PE excluded via the algorithm, they went on to CTPA. If PE was confirmed, patients received anticoagulant therapy.
Most of the patients in whom PE was excluded (either by algorithm or CTPA) were left untreated and were followed for 3 months, although 60 patients who had PE excluded received anticoagulants anyway.
In all, 1651 patients had PE excluded with the YEARS algorithm, and 1633 of them did not receive anticoagulation.
A total of 1814 patients did not have PE excluded via the YEARS algorithm and went on to CTPA. Of these patients, 456 had PE, 42 had PE excluded via CTPA but received anticoagulation, and 1316 had PE excluded but did not receive anticoagulation.
Five patients were lost to follow-up—4 with PE excluded via the YEARS algorithm and 1 who went on to CTPA.
Patient outcomes
The primary outcome of this study was the 3-month incidence of symptomatic venous thromboembolism (VTE).
A total of 2944 patients were evaluable (because they had PE excluded, did not receive anticoagulation, and were not lost to follow-up)—1629 who had PE excluded via the YEARS algorithm and 1315 who had PE excluded via CTPA.
Symptomatic VTE occurred in 0.61% of all evaluable patients, 0.43% of patients who had PE excluded via YEARS, and 0.84% of patients who had PE excluded via CTPA.
Fatal PE occurred in 0.20% of all evaluable patients, 0.12% of patients who had PE excluded via YEARS, and 0.30% of patients who had PE excluded via CTPA.
“This is fully in line with that observed in studies using traditional, sequential algorithms such as the 2-level Wells score and a fixed cut-off level of D-dimer of 500 ng/mL,” Dr Van der Hulle said.
“Using the YEARS algorithm, CTPA was not indicated in 48% of our patients at baseline, but this would have been only 34% of patients using the traditional algorithm. This shows that the YEARS algorithm can safely exclude PE and resulted in an absolute reduction of required CTPA of 14%.”
“We expect that the YEARS algorithm can be easily implemented outside the participating study sites and that these safety and efficacy outcomes are representative of what could be expected in regular clinical settings.” ![]()
Edoxaban appears comparable to standard therapy

ROME—The novel oral anticoagulant edoxaban is a feasible treatment option for patients with atrial fibrillation (AF) who need anticoagulation before cardioversion, according to researchers.
Results of the ENSURE-AF trial suggest edoxaban is “an effective and safe alternative” to standard therapy with warfarin and enoxaparin and may allow for prompt cardioversion with transesophageal echocardiography (TEE), said Andreas Goette, MD, of St. Vincenz-Hospital in Paderborn, Germany.
“These results may have important clinical implications for newly diagnosed, non-anticoagulated AF patients undergoing cardioversion,” Dr Goette noted.
“According to the study protocol, a newly diagnosed, non-anticoagulated AF patient was started on edoxaban, and the cardioversion procedure was scheduled as early as 2 hours following the start of treatment, when applying a TEE-guided approach.”
Dr Goette presented results from ENSURE-AF at ESC Congress 2016 (abstract 5715). The data were simultaneously published in The Lancet. The study was supported by Daiichi Sankyo.
This phase 3b study included 2199 patients with documented non-valvular AF who were scheduled for electrical cardioversion after anticoagulation therapy.
Patients were randomized to receive edoxaban (n=1095) or warfarin with enoxaparin bridging (n=1104). Edoxaban was dosed at 60 mg once daily, but the dose was reduced to 30 mg if one or more of the following factors were present: renal impairment, low body weight, or concomitant use of certain P-glycoprotein inhibitors.
Patients were stratified according to the cardioversion approach (TEE or non-TEE), a patient’s prior experience taking anticoagulants at the time of randomization (ie, anticoagulant-experienced or naïve), and edoxaban dose (60 mg or 30 mg). Patients were randomized in a 1:1 ratio to 2 treatment groups within each stratum.
The primary efficacy endpoint was the composite of stroke, systemic embolic event, myocardial infarction, and cardiovascular death at day 28.
This endpoint occurred at a comparable rate in both treatment arms—0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin arm (odds ratio [OR]=0.46; 95% CI, 0.12–1.43).
The primary safety endpoint was the composite of major and clinically relevant non-major bleeding events at 30 days.
This endpoint also occurred at a comparable rate in both arms—1.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin arm (OR=1.48; 95% CI, 0.64–3.55).
The result for the net clinical outcome—a composite of stroke, systemic embolic event, myocardial infarction, cardiovascular mortality, and major bleeding—was 0.7% in the edoxaban arm and 1.4% in the enoxaparin/warfarin arm (OR=0.50; 95% CI, 0.19–1.25) during the overall study period.
The researchers said the ORs recorded in this trial should be viewed with caution because the trial was not adequately powered to show statistically significant differences for efficacy or safety endpoints.
Still, the team said the results suggest edoxaban can provide an alternative to standard therapy.
“At a practical level, our study results show that newly diagnosed, non-anticoagulated AF patients can start edoxaban as early as 2 hours prior to their cardioversion procedure if they have access to [TEE] or 3 weeks prior without,” Dr Goette said. ![]()

ROME—The novel oral anticoagulant edoxaban is a feasible treatment option for patients with atrial fibrillation (AF) who need anticoagulation before cardioversion, according to researchers.
Results of the ENSURE-AF trial suggest edoxaban is “an effective and safe alternative” to standard therapy with warfarin and enoxaparin and may allow for prompt cardioversion with transesophageal echocardiography (TEE), said Andreas Goette, MD, of St. Vincenz-Hospital in Paderborn, Germany.
“These results may have important clinical implications for newly diagnosed, non-anticoagulated AF patients undergoing cardioversion,” Dr Goette noted.
“According to the study protocol, a newly diagnosed, non-anticoagulated AF patient was started on edoxaban, and the cardioversion procedure was scheduled as early as 2 hours following the start of treatment, when applying a TEE-guided approach.”
Dr Goette presented results from ENSURE-AF at ESC Congress 2016 (abstract 5715). The data were simultaneously published in The Lancet. The study was supported by Daiichi Sankyo.
This phase 3b study included 2199 patients with documented non-valvular AF who were scheduled for electrical cardioversion after anticoagulation therapy.
Patients were randomized to receive edoxaban (n=1095) or warfarin with enoxaparin bridging (n=1104). Edoxaban was dosed at 60 mg once daily, but the dose was reduced to 30 mg if one or more of the following factors were present: renal impairment, low body weight, or concomitant use of certain P-glycoprotein inhibitors.
Patients were stratified according to the cardioversion approach (TEE or non-TEE), a patient’s prior experience taking anticoagulants at the time of randomization (ie, anticoagulant-experienced or naïve), and edoxaban dose (60 mg or 30 mg). Patients were randomized in a 1:1 ratio to 2 treatment groups within each stratum.
The primary efficacy endpoint was the composite of stroke, systemic embolic event, myocardial infarction, and cardiovascular death at day 28.
This endpoint occurred at a comparable rate in both treatment arms—0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin arm (odds ratio [OR]=0.46; 95% CI, 0.12–1.43).
The primary safety endpoint was the composite of major and clinically relevant non-major bleeding events at 30 days.
This endpoint also occurred at a comparable rate in both arms—1.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin arm (OR=1.48; 95% CI, 0.64–3.55).
The result for the net clinical outcome—a composite of stroke, systemic embolic event, myocardial infarction, cardiovascular mortality, and major bleeding—was 0.7% in the edoxaban arm and 1.4% in the enoxaparin/warfarin arm (OR=0.50; 95% CI, 0.19–1.25) during the overall study period.
The researchers said the ORs recorded in this trial should be viewed with caution because the trial was not adequately powered to show statistically significant differences for efficacy or safety endpoints.
Still, the team said the results suggest edoxaban can provide an alternative to standard therapy.
“At a practical level, our study results show that newly diagnosed, non-anticoagulated AF patients can start edoxaban as early as 2 hours prior to their cardioversion procedure if they have access to [TEE] or 3 weeks prior without,” Dr Goette said. ![]()

ROME—The novel oral anticoagulant edoxaban is a feasible treatment option for patients with atrial fibrillation (AF) who need anticoagulation before cardioversion, according to researchers.
Results of the ENSURE-AF trial suggest edoxaban is “an effective and safe alternative” to standard therapy with warfarin and enoxaparin and may allow for prompt cardioversion with transesophageal echocardiography (TEE), said Andreas Goette, MD, of St. Vincenz-Hospital in Paderborn, Germany.
“These results may have important clinical implications for newly diagnosed, non-anticoagulated AF patients undergoing cardioversion,” Dr Goette noted.
“According to the study protocol, a newly diagnosed, non-anticoagulated AF patient was started on edoxaban, and the cardioversion procedure was scheduled as early as 2 hours following the start of treatment, when applying a TEE-guided approach.”
Dr Goette presented results from ENSURE-AF at ESC Congress 2016 (abstract 5715). The data were simultaneously published in The Lancet. The study was supported by Daiichi Sankyo.
This phase 3b study included 2199 patients with documented non-valvular AF who were scheduled for electrical cardioversion after anticoagulation therapy.
Patients were randomized to receive edoxaban (n=1095) or warfarin with enoxaparin bridging (n=1104). Edoxaban was dosed at 60 mg once daily, but the dose was reduced to 30 mg if one or more of the following factors were present: renal impairment, low body weight, or concomitant use of certain P-glycoprotein inhibitors.
Patients were stratified according to the cardioversion approach (TEE or non-TEE), a patient’s prior experience taking anticoagulants at the time of randomization (ie, anticoagulant-experienced or naïve), and edoxaban dose (60 mg or 30 mg). Patients were randomized in a 1:1 ratio to 2 treatment groups within each stratum.
The primary efficacy endpoint was the composite of stroke, systemic embolic event, myocardial infarction, and cardiovascular death at day 28.
This endpoint occurred at a comparable rate in both treatment arms—0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin arm (odds ratio [OR]=0.46; 95% CI, 0.12–1.43).
The primary safety endpoint was the composite of major and clinically relevant non-major bleeding events at 30 days.
This endpoint also occurred at a comparable rate in both arms—1.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin arm (OR=1.48; 95% CI, 0.64–3.55).
The result for the net clinical outcome—a composite of stroke, systemic embolic event, myocardial infarction, cardiovascular mortality, and major bleeding—was 0.7% in the edoxaban arm and 1.4% in the enoxaparin/warfarin arm (OR=0.50; 95% CI, 0.19–1.25) during the overall study period.
The researchers said the ORs recorded in this trial should be viewed with caution because the trial was not adequately powered to show statistically significant differences for efficacy or safety endpoints.
Still, the team said the results suggest edoxaban can provide an alternative to standard therapy.
“At a practical level, our study results show that newly diagnosed, non-anticoagulated AF patients can start edoxaban as early as 2 hours prior to their cardioversion procedure if they have access to [TEE] or 3 weeks prior without,” Dr Goette said. ![]()
VIDEO: Coronary DES outperform BMS mostly on restenosis
ROME – The difference between contemporary drug-eluting coronary stents and bare-metal stents is not very great, a large Norwegian coronary stent trial showed.
Today’s drug-eluting stents (DES), often called second-generation DES, largely do only what they were designed to do, compared with bare-metal stents (BMS): reduce the rate of stent restenosis and the need for target-lesion revascularization.
“The long-term benefit of contemporary DES over BMS was less that expected,” Kaare H. Bønaa, MD, reported at the annual congress of the European Society of Cardiology.
Results from the Norwegian Coronary Stent Trial (NORSTENT), run with 9,013 patients, showed that patients who received one or more drug-eluting stents had, during nearly 5 years of follow-up, a 5% absolute drop in target-lesion revascularizations (a 53% relative risk reduction), and a 3.3% reduction in all revascularizations (a 24% relative risk reduction), compared with patients who received bare-metal stents, said Dr. Bønaa.
The results also showed that patients who received DES had a 0.4% reduced rate of stent thrombosis (a 36% relative risk reduction), compared with patients treated with BMS during nearly 5 years of follow-up. All three differences were statistically significant.
But the NORSTENT findings also documented that the patients who received either DES or BMS had virtually identical rates of all-cause deaths and nonfatal myocardial infarctions. And, on average, the two different types of coronary stents produced identical improvements in patients’ quality of life, reported Dr. Bønaa, a professor and researcher in the Clinic for Heart Disease at St. Olav’s University Hospital in Trondheim, Norway.
The study’s primary endpoint was the combined rate of death or nonfatal MI, and so the nonsignificant difference in that outcome between the two study arms meant that, formally, the NORSTENT trial produced a neutral result. Concurrently with his report, the results appeared in an article online (New Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
“The difference between the two stent types is not as great as we thought. Patients who get DES do not live longer or better” than those who receive BMS, Dr. Bønaa said. “We suggest that both contemporary DES and BMS can be recommended for contemporary revascularization. The results open up use of BMS for certain patients,” such as those scheduled for surgery or patients who cannot tolerate or afford the drugs used for dual antiplatelet therapy following coronary stent placement.
But the designated discussant for the study, Stefan James, MD, insisted that recent-generation DES “should remain recommended over BMS,” particularly the specific DES that underwent testing in randomized trials that used hard clinical endpoints. The 2014 revascularization guidelines of the European Society of Cardiology recommend new-generation DES over BMS, he noted.
In addition, “BMS should not be specifically recommended for patients at high risk of stent thrombosis or for patients who do not tolerate dual-antiplatelet therapy,” said Dr. James, professor of cardiology at Uppsala University in Sweden.
NORSTENT ran at eight centers in Norway during 2008-2011, and enrolled patients either had acute coronary syndrome (71% of those in the study) or stable coronary disease. Patients averaged 63 years old. The trial excluded patients with prior stents or bifurcated coronary lesions. Enrolled patients received, on average, 1.7 stents. The specific stent in each class that patients received was left to the discretion of each operator, and 95% of patients in the DES arm received a second-generation device. All patients in both arms of the study received dual-antiplatelet therapy for 9 months.
The finding that DES cut the rate of revascularization procedures by 3.3%, compared with patients treated with BMS, means that, on average, clinicians would need to treat 30 patients with DES to avoid the need for one additional repeat revascularization procedure that would occur if BMS were used instead.
That number needed to treat of 30 to avoid one repeat revascularization may seem high, but the money saved that way would still counterbalance the incremental cost of a DES over a BMS, which today in Europe would be about 50-100 euros, noted one cardiologist.
If you multiply 30 procedures by 100 extra euros per stent and by an average of 1.7 stents per patient, you may spend 5,100 euros, less than the cost of a repeat revascularization procedure, commented Carlo Di Mario, MD, a professor of cardiology and an interventional cardiologist at Royal Brompton & Harefield Hospitals in London.
In a video interview, Steen D. Kristensen, MD, of Aarhus University, Denmark, discussed the NORSTENT findings and their implications.
NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
NORSTENT was a very well-performed trial. It produced a neutral result for its primary endpoint, but for the secondary endpoint of repeat revascularization, there were significantly more events using bare-metal stents. This is a major finding, and NORSTENT’s design make the results very generalizable.
It may be slightly surprising that the newer drug-eluting stents did not perform better for the primary endpoint of reducing deaths and MIs during 5 years of follow-up, but seeing a difference in the revascularization rate is not surprising; that is what we would expect. We use DES to reduce the problem of restenosis. Results from several earlier studies that had compared DES with BMS had suggested other benefits from DES, and that is also what the European Society of Cardiology guidelines say.
I will not go home now and start using BMS in my own practice. I will continue to use DES, because they have an advantage. I use BMS in patients who cannot tolerate long-term treatment with dual antiplatelet therapy. The results are encouraging for centers where there is a large price difference between DES and BMS, but that is not the case where I practice in Denmark.
Steen D. Kristensen, MD, is a professor of interventional cardiologist at Aarhus University, Denmark. He made these comments in an interview. He had no relevant disclosures.
NORSTENT was a very well-performed trial. It produced a neutral result for its primary endpoint, but for the secondary endpoint of repeat revascularization, there were significantly more events using bare-metal stents. This is a major finding, and NORSTENT’s design make the results very generalizable.
It may be slightly surprising that the newer drug-eluting stents did not perform better for the primary endpoint of reducing deaths and MIs during 5 years of follow-up, but seeing a difference in the revascularization rate is not surprising; that is what we would expect. We use DES to reduce the problem of restenosis. Results from several earlier studies that had compared DES with BMS had suggested other benefits from DES, and that is also what the European Society of Cardiology guidelines say.
I will not go home now and start using BMS in my own practice. I will continue to use DES, because they have an advantage. I use BMS in patients who cannot tolerate long-term treatment with dual antiplatelet therapy. The results are encouraging for centers where there is a large price difference between DES and BMS, but that is not the case where I practice in Denmark.
Steen D. Kristensen, MD, is a professor of interventional cardiologist at Aarhus University, Denmark. He made these comments in an interview. He had no relevant disclosures.
NORSTENT was a very well-performed trial. It produced a neutral result for its primary endpoint, but for the secondary endpoint of repeat revascularization, there were significantly more events using bare-metal stents. This is a major finding, and NORSTENT’s design make the results very generalizable.
It may be slightly surprising that the newer drug-eluting stents did not perform better for the primary endpoint of reducing deaths and MIs during 5 years of follow-up, but seeing a difference in the revascularization rate is not surprising; that is what we would expect. We use DES to reduce the problem of restenosis. Results from several earlier studies that had compared DES with BMS had suggested other benefits from DES, and that is also what the European Society of Cardiology guidelines say.
I will not go home now and start using BMS in my own practice. I will continue to use DES, because they have an advantage. I use BMS in patients who cannot tolerate long-term treatment with dual antiplatelet therapy. The results are encouraging for centers where there is a large price difference between DES and BMS, but that is not the case where I practice in Denmark.
Steen D. Kristensen, MD, is a professor of interventional cardiologist at Aarhus University, Denmark. He made these comments in an interview. He had no relevant disclosures.
ROME – The difference between contemporary drug-eluting coronary stents and bare-metal stents is not very great, a large Norwegian coronary stent trial showed.
Today’s drug-eluting stents (DES), often called second-generation DES, largely do only what they were designed to do, compared with bare-metal stents (BMS): reduce the rate of stent restenosis and the need for target-lesion revascularization.
“The long-term benefit of contemporary DES over BMS was less that expected,” Kaare H. Bønaa, MD, reported at the annual congress of the European Society of Cardiology.
Results from the Norwegian Coronary Stent Trial (NORSTENT), run with 9,013 patients, showed that patients who received one or more drug-eluting stents had, during nearly 5 years of follow-up, a 5% absolute drop in target-lesion revascularizations (a 53% relative risk reduction), and a 3.3% reduction in all revascularizations (a 24% relative risk reduction), compared with patients who received bare-metal stents, said Dr. Bønaa.
The results also showed that patients who received DES had a 0.4% reduced rate of stent thrombosis (a 36% relative risk reduction), compared with patients treated with BMS during nearly 5 years of follow-up. All three differences were statistically significant.
But the NORSTENT findings also documented that the patients who received either DES or BMS had virtually identical rates of all-cause deaths and nonfatal myocardial infarctions. And, on average, the two different types of coronary stents produced identical improvements in patients’ quality of life, reported Dr. Bønaa, a professor and researcher in the Clinic for Heart Disease at St. Olav’s University Hospital in Trondheim, Norway.
The study’s primary endpoint was the combined rate of death or nonfatal MI, and so the nonsignificant difference in that outcome between the two study arms meant that, formally, the NORSTENT trial produced a neutral result. Concurrently with his report, the results appeared in an article online (New Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
“The difference between the two stent types is not as great as we thought. Patients who get DES do not live longer or better” than those who receive BMS, Dr. Bønaa said. “We suggest that both contemporary DES and BMS can be recommended for contemporary revascularization. The results open up use of BMS for certain patients,” such as those scheduled for surgery or patients who cannot tolerate or afford the drugs used for dual antiplatelet therapy following coronary stent placement.
But the designated discussant for the study, Stefan James, MD, insisted that recent-generation DES “should remain recommended over BMS,” particularly the specific DES that underwent testing in randomized trials that used hard clinical endpoints. The 2014 revascularization guidelines of the European Society of Cardiology recommend new-generation DES over BMS, he noted.
In addition, “BMS should not be specifically recommended for patients at high risk of stent thrombosis or for patients who do not tolerate dual-antiplatelet therapy,” said Dr. James, professor of cardiology at Uppsala University in Sweden.
NORSTENT ran at eight centers in Norway during 2008-2011, and enrolled patients either had acute coronary syndrome (71% of those in the study) or stable coronary disease. Patients averaged 63 years old. The trial excluded patients with prior stents or bifurcated coronary lesions. Enrolled patients received, on average, 1.7 stents. The specific stent in each class that patients received was left to the discretion of each operator, and 95% of patients in the DES arm received a second-generation device. All patients in both arms of the study received dual-antiplatelet therapy for 9 months.
The finding that DES cut the rate of revascularization procedures by 3.3%, compared with patients treated with BMS, means that, on average, clinicians would need to treat 30 patients with DES to avoid the need for one additional repeat revascularization procedure that would occur if BMS were used instead.
That number needed to treat of 30 to avoid one repeat revascularization may seem high, but the money saved that way would still counterbalance the incremental cost of a DES over a BMS, which today in Europe would be about 50-100 euros, noted one cardiologist.
If you multiply 30 procedures by 100 extra euros per stent and by an average of 1.7 stents per patient, you may spend 5,100 euros, less than the cost of a repeat revascularization procedure, commented Carlo Di Mario, MD, a professor of cardiology and an interventional cardiologist at Royal Brompton & Harefield Hospitals in London.
In a video interview, Steen D. Kristensen, MD, of Aarhus University, Denmark, discussed the NORSTENT findings and their implications.
NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ROME – The difference between contemporary drug-eluting coronary stents and bare-metal stents is not very great, a large Norwegian coronary stent trial showed.
Today’s drug-eluting stents (DES), often called second-generation DES, largely do only what they were designed to do, compared with bare-metal stents (BMS): reduce the rate of stent restenosis and the need for target-lesion revascularization.
“The long-term benefit of contemporary DES over BMS was less that expected,” Kaare H. Bønaa, MD, reported at the annual congress of the European Society of Cardiology.
Results from the Norwegian Coronary Stent Trial (NORSTENT), run with 9,013 patients, showed that patients who received one or more drug-eluting stents had, during nearly 5 years of follow-up, a 5% absolute drop in target-lesion revascularizations (a 53% relative risk reduction), and a 3.3% reduction in all revascularizations (a 24% relative risk reduction), compared with patients who received bare-metal stents, said Dr. Bønaa.
The results also showed that patients who received DES had a 0.4% reduced rate of stent thrombosis (a 36% relative risk reduction), compared with patients treated with BMS during nearly 5 years of follow-up. All three differences were statistically significant.
But the NORSTENT findings also documented that the patients who received either DES or BMS had virtually identical rates of all-cause deaths and nonfatal myocardial infarctions. And, on average, the two different types of coronary stents produced identical improvements in patients’ quality of life, reported Dr. Bønaa, a professor and researcher in the Clinic for Heart Disease at St. Olav’s University Hospital in Trondheim, Norway.
The study’s primary endpoint was the combined rate of death or nonfatal MI, and so the nonsignificant difference in that outcome between the two study arms meant that, formally, the NORSTENT trial produced a neutral result. Concurrently with his report, the results appeared in an article online (New Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
“The difference between the two stent types is not as great as we thought. Patients who get DES do not live longer or better” than those who receive BMS, Dr. Bønaa said. “We suggest that both contemporary DES and BMS can be recommended for contemporary revascularization. The results open up use of BMS for certain patients,” such as those scheduled for surgery or patients who cannot tolerate or afford the drugs used for dual antiplatelet therapy following coronary stent placement.
But the designated discussant for the study, Stefan James, MD, insisted that recent-generation DES “should remain recommended over BMS,” particularly the specific DES that underwent testing in randomized trials that used hard clinical endpoints. The 2014 revascularization guidelines of the European Society of Cardiology recommend new-generation DES over BMS, he noted.
In addition, “BMS should not be specifically recommended for patients at high risk of stent thrombosis or for patients who do not tolerate dual-antiplatelet therapy,” said Dr. James, professor of cardiology at Uppsala University in Sweden.
NORSTENT ran at eight centers in Norway during 2008-2011, and enrolled patients either had acute coronary syndrome (71% of those in the study) or stable coronary disease. Patients averaged 63 years old. The trial excluded patients with prior stents or bifurcated coronary lesions. Enrolled patients received, on average, 1.7 stents. The specific stent in each class that patients received was left to the discretion of each operator, and 95% of patients in the DES arm received a second-generation device. All patients in both arms of the study received dual-antiplatelet therapy for 9 months.
The finding that DES cut the rate of revascularization procedures by 3.3%, compared with patients treated with BMS, means that, on average, clinicians would need to treat 30 patients with DES to avoid the need for one additional repeat revascularization procedure that would occur if BMS were used instead.
That number needed to treat of 30 to avoid one repeat revascularization may seem high, but the money saved that way would still counterbalance the incremental cost of a DES over a BMS, which today in Europe would be about 50-100 euros, noted one cardiologist.
If you multiply 30 procedures by 100 extra euros per stent and by an average of 1.7 stents per patient, you may spend 5,100 euros, less than the cost of a repeat revascularization procedure, commented Carlo Di Mario, MD, a professor of cardiology and an interventional cardiologist at Royal Brompton & Harefield Hospitals in London.
In a video interview, Steen D. Kristensen, MD, of Aarhus University, Denmark, discussed the NORSTENT findings and their implications.
NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2016
Key clinical point: The benefit from coronary revascularization with drug-eluting stents, compared with bare-metal stents, was mostly in a reduced need for repeat revascularization, with no difference in mortality or MIs during 5 years of follow-up.
Major finding: Thirty patients need to be treated with drug-eluting stents to prevent one repeat revascularization, compared with bare-metal stents.
Data source: NORSTENT, a randomized, multicenter trial with 9,013 patients.
Disclosures: NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
Antidote to factor Xa inhibitors exhibits efficacy in patients with major bleeding

ROME—Preliminary results from the ANNEXA-4 study suggest that andexanet alfa, an investigational antidote to factor Xa inhibitors, can be effective in patients with acute major bleeding.
The drug reversed the anticoagulant effects of rivaroxaban, apixaban, and enoxaparin in this study, providing “excellent” or “good” hemostatic efficacy in 79% of patients over 12 hours.
Thrombotic events occurred in 18% of patients, and 15% died during the 30-day follow-up period.
According to investigators, these events occurred within the range expected in this patient population, given the severity of their bleeding, their underlying thrombotic risk, and the low percentage of patients who restarted anticoagulant therapy following their bleeding episode.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented results from ANNEXA-4 at ESC Congress 2016 (abstract 5718).
Results were also published in NEJM. The study was funded by Portola Pharmaceuticals Inc.
Patients and treatment
The preliminary analysis of the phase 3/4 ANNEXA-4 trial included 67 patients. All of these patients were evaluated for safety, and 47 were evaluated for efficacy. The mean age of both populations was 77.1, and slightly more than half of the patients were male.
All patients received andexanet alfa given as a bolus dose over 30 minutes, followed by a 2-hour infusion. Patients received a low or high dose depending on which factor Xa inhibitor they received and the time they received it. The patients were evaluated for 30 days following andexanet alfa administration.
The co-primary efficacy endpoints are the percent change in anti-factor Xa activity at 2 hours and the assessment of hemostasis over 12 hours following the infusion. Hemostatic efficacy is assessed by an independent endpoint adjudication committee as excellent, good, or poor/none.
Efficacy
“In this preliminary analysis, [andexanet alfa] was effective in rapidly reversing anti-factor Xa inhibitor activity and restoring normal blood clotting in real-world patients with factor Xa inhibitor-related bleeding,” Dr Connolly said.
Of the 47 patients evaluable for efficacy, 32 were receiving an anticoagulant due to atrial fibrillation, 12 had venous thromboembolism (VTE), and 3 had both atrial fibrillation and VTE.
Twenty-six patients were receiving rivaroxaban, 20 were receiving apixaban, and 1 was receiving enoxaparin. Twenty-five patients had gastrointestinal bleeding, 20 had intracranial bleeding, and 2 had bleeding at other sites.
Forty-two patients received a low dose of andexanet alfa, and 5 received a high dose. The mean time from presentation to the emergency department and the administration of the andexanet alfa bolus was 4.8 ± 1.8 hours.
After the bolus, the median anti-factor Xa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among those receiving apixaban. At the end of the 2-hour infusion, the decrease from baseline was 86% and 92%, respectively.
Twelve hours after the infusion ended, the median anti-factor Xa activity had decreased 64% from baseline among patients receiving rivaroxaban and 31% among those receiving apixaban.
Overall, at 12 hours, clinical hemostasis was rated excellent or good in 79% of patients. Hemostatic efficacy was rated as excellent or good in 81% of the patients on rivaroxaban, 75% of the patients on apixaban, and in the 1 patient on edoxaban.
Safety
Of the 67 patients in the safety population, 47 were receiving an anticoagulant due to atrial fibrillation, 15 had VTE, and 5 had both atrial fibrillation and VTE.
Thirty-two patients were receiving rivaroxaban, 31 were receiving apixaban, and 4 were receiving edoxaban. Thirty-eight patients had gastrointestinal bleeding, 28 had intracranial bleeding, and 6 had bleeding at other sites.
There were no infusion reactions, no antibodies to factors Xa or X, and no neutralizing antibodies to andexanet alfa.
Twelve patients (18%) experienced thrombotic events—1 with myocardial infarction, 5 with stroke, 7 with deep-vein thrombosis, and 1 with pulmonary embolism. (Some patients had more than 1 event.)
“This rate of events is not unexpected, considering the thrombotic potential of the patients and the fact that, in most of them, anticoagulation was discontinued at the time of bleeding and not restarted,” Dr Connolly said.
Four patients had a thrombotic event within 3 days of andexanet alfa treatment, and the rest occurred between 4 days and 30 days.
Eighteen patients (27%) resumed anticoagulant therapy within 30 days. One of the 12 patients with a thrombotic event restarted anticoagulation at a therapeutic dose before the event. One other patient received prophylactic doses of enoxaparin before developing a deep-vein thrombosis.
There were 10 deaths (15%), 6 due to cardiovascular events.
Andexanet alfa development
Andexanet alfa is being developed as a reversal agent for apixaban, rivaroxaban, edoxaban, and enoxaparin. Andexanet alfa is intended to be used when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
The drug is under review by the US Food and Drug Administration (FDA) and the European Medicines Agency. The FDA recently issued a complete response letter regarding the biologics license application for andexanet alfa.
Portola said it plans to meet with the FDA as soon as possible to resolve the outstanding questions in the letter and determine the appropriate next steps. ![]()

ROME—Preliminary results from the ANNEXA-4 study suggest that andexanet alfa, an investigational antidote to factor Xa inhibitors, can be effective in patients with acute major bleeding.
The drug reversed the anticoagulant effects of rivaroxaban, apixaban, and enoxaparin in this study, providing “excellent” or “good” hemostatic efficacy in 79% of patients over 12 hours.
Thrombotic events occurred in 18% of patients, and 15% died during the 30-day follow-up period.
According to investigators, these events occurred within the range expected in this patient population, given the severity of their bleeding, their underlying thrombotic risk, and the low percentage of patients who restarted anticoagulant therapy following their bleeding episode.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented results from ANNEXA-4 at ESC Congress 2016 (abstract 5718).
Results were also published in NEJM. The study was funded by Portola Pharmaceuticals Inc.
Patients and treatment
The preliminary analysis of the phase 3/4 ANNEXA-4 trial included 67 patients. All of these patients were evaluated for safety, and 47 were evaluated for efficacy. The mean age of both populations was 77.1, and slightly more than half of the patients were male.
All patients received andexanet alfa given as a bolus dose over 30 minutes, followed by a 2-hour infusion. Patients received a low or high dose depending on which factor Xa inhibitor they received and the time they received it. The patients were evaluated for 30 days following andexanet alfa administration.
The co-primary efficacy endpoints are the percent change in anti-factor Xa activity at 2 hours and the assessment of hemostasis over 12 hours following the infusion. Hemostatic efficacy is assessed by an independent endpoint adjudication committee as excellent, good, or poor/none.
Efficacy
“In this preliminary analysis, [andexanet alfa] was effective in rapidly reversing anti-factor Xa inhibitor activity and restoring normal blood clotting in real-world patients with factor Xa inhibitor-related bleeding,” Dr Connolly said.
Of the 47 patients evaluable for efficacy, 32 were receiving an anticoagulant due to atrial fibrillation, 12 had venous thromboembolism (VTE), and 3 had both atrial fibrillation and VTE.
Twenty-six patients were receiving rivaroxaban, 20 were receiving apixaban, and 1 was receiving enoxaparin. Twenty-five patients had gastrointestinal bleeding, 20 had intracranial bleeding, and 2 had bleeding at other sites.
Forty-two patients received a low dose of andexanet alfa, and 5 received a high dose. The mean time from presentation to the emergency department and the administration of the andexanet alfa bolus was 4.8 ± 1.8 hours.
After the bolus, the median anti-factor Xa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among those receiving apixaban. At the end of the 2-hour infusion, the decrease from baseline was 86% and 92%, respectively.
Twelve hours after the infusion ended, the median anti-factor Xa activity had decreased 64% from baseline among patients receiving rivaroxaban and 31% among those receiving apixaban.
Overall, at 12 hours, clinical hemostasis was rated excellent or good in 79% of patients. Hemostatic efficacy was rated as excellent or good in 81% of the patients on rivaroxaban, 75% of the patients on apixaban, and in the 1 patient on edoxaban.
Safety
Of the 67 patients in the safety population, 47 were receiving an anticoagulant due to atrial fibrillation, 15 had VTE, and 5 had both atrial fibrillation and VTE.
Thirty-two patients were receiving rivaroxaban, 31 were receiving apixaban, and 4 were receiving edoxaban. Thirty-eight patients had gastrointestinal bleeding, 28 had intracranial bleeding, and 6 had bleeding at other sites.
There were no infusion reactions, no antibodies to factors Xa or X, and no neutralizing antibodies to andexanet alfa.
Twelve patients (18%) experienced thrombotic events—1 with myocardial infarction, 5 with stroke, 7 with deep-vein thrombosis, and 1 with pulmonary embolism. (Some patients had more than 1 event.)
“This rate of events is not unexpected, considering the thrombotic potential of the patients and the fact that, in most of them, anticoagulation was discontinued at the time of bleeding and not restarted,” Dr Connolly said.
Four patients had a thrombotic event within 3 days of andexanet alfa treatment, and the rest occurred between 4 days and 30 days.
Eighteen patients (27%) resumed anticoagulant therapy within 30 days. One of the 12 patients with a thrombotic event restarted anticoagulation at a therapeutic dose before the event. One other patient received prophylactic doses of enoxaparin before developing a deep-vein thrombosis.
There were 10 deaths (15%), 6 due to cardiovascular events.
Andexanet alfa development
Andexanet alfa is being developed as a reversal agent for apixaban, rivaroxaban, edoxaban, and enoxaparin. Andexanet alfa is intended to be used when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
The drug is under review by the US Food and Drug Administration (FDA) and the European Medicines Agency. The FDA recently issued a complete response letter regarding the biologics license application for andexanet alfa.
Portola said it plans to meet with the FDA as soon as possible to resolve the outstanding questions in the letter and determine the appropriate next steps. ![]()

ROME—Preliminary results from the ANNEXA-4 study suggest that andexanet alfa, an investigational antidote to factor Xa inhibitors, can be effective in patients with acute major bleeding.
The drug reversed the anticoagulant effects of rivaroxaban, apixaban, and enoxaparin in this study, providing “excellent” or “good” hemostatic efficacy in 79% of patients over 12 hours.
Thrombotic events occurred in 18% of patients, and 15% died during the 30-day follow-up period.
According to investigators, these events occurred within the range expected in this patient population, given the severity of their bleeding, their underlying thrombotic risk, and the low percentage of patients who restarted anticoagulant therapy following their bleeding episode.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented results from ANNEXA-4 at ESC Congress 2016 (abstract 5718).
Results were also published in NEJM. The study was funded by Portola Pharmaceuticals Inc.
Patients and treatment
The preliminary analysis of the phase 3/4 ANNEXA-4 trial included 67 patients. All of these patients were evaluated for safety, and 47 were evaluated for efficacy. The mean age of both populations was 77.1, and slightly more than half of the patients were male.
All patients received andexanet alfa given as a bolus dose over 30 minutes, followed by a 2-hour infusion. Patients received a low or high dose depending on which factor Xa inhibitor they received and the time they received it. The patients were evaluated for 30 days following andexanet alfa administration.
The co-primary efficacy endpoints are the percent change in anti-factor Xa activity at 2 hours and the assessment of hemostasis over 12 hours following the infusion. Hemostatic efficacy is assessed by an independent endpoint adjudication committee as excellent, good, or poor/none.
Efficacy
“In this preliminary analysis, [andexanet alfa] was effective in rapidly reversing anti-factor Xa inhibitor activity and restoring normal blood clotting in real-world patients with factor Xa inhibitor-related bleeding,” Dr Connolly said.
Of the 47 patients evaluable for efficacy, 32 were receiving an anticoagulant due to atrial fibrillation, 12 had venous thromboembolism (VTE), and 3 had both atrial fibrillation and VTE.
Twenty-six patients were receiving rivaroxaban, 20 were receiving apixaban, and 1 was receiving enoxaparin. Twenty-five patients had gastrointestinal bleeding, 20 had intracranial bleeding, and 2 had bleeding at other sites.
Forty-two patients received a low dose of andexanet alfa, and 5 received a high dose. The mean time from presentation to the emergency department and the administration of the andexanet alfa bolus was 4.8 ± 1.8 hours.
After the bolus, the median anti-factor Xa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among those receiving apixaban. At the end of the 2-hour infusion, the decrease from baseline was 86% and 92%, respectively.
Twelve hours after the infusion ended, the median anti-factor Xa activity had decreased 64% from baseline among patients receiving rivaroxaban and 31% among those receiving apixaban.
Overall, at 12 hours, clinical hemostasis was rated excellent or good in 79% of patients. Hemostatic efficacy was rated as excellent or good in 81% of the patients on rivaroxaban, 75% of the patients on apixaban, and in the 1 patient on edoxaban.
Safety
Of the 67 patients in the safety population, 47 were receiving an anticoagulant due to atrial fibrillation, 15 had VTE, and 5 had both atrial fibrillation and VTE.
Thirty-two patients were receiving rivaroxaban, 31 were receiving apixaban, and 4 were receiving edoxaban. Thirty-eight patients had gastrointestinal bleeding, 28 had intracranial bleeding, and 6 had bleeding at other sites.
There were no infusion reactions, no antibodies to factors Xa or X, and no neutralizing antibodies to andexanet alfa.
Twelve patients (18%) experienced thrombotic events—1 with myocardial infarction, 5 with stroke, 7 with deep-vein thrombosis, and 1 with pulmonary embolism. (Some patients had more than 1 event.)
“This rate of events is not unexpected, considering the thrombotic potential of the patients and the fact that, in most of them, anticoagulation was discontinued at the time of bleeding and not restarted,” Dr Connolly said.
Four patients had a thrombotic event within 3 days of andexanet alfa treatment, and the rest occurred between 4 days and 30 days.
Eighteen patients (27%) resumed anticoagulant therapy within 30 days. One of the 12 patients with a thrombotic event restarted anticoagulation at a therapeutic dose before the event. One other patient received prophylactic doses of enoxaparin before developing a deep-vein thrombosis.
There were 10 deaths (15%), 6 due to cardiovascular events.
Andexanet alfa development
Andexanet alfa is being developed as a reversal agent for apixaban, rivaroxaban, edoxaban, and enoxaparin. Andexanet alfa is intended to be used when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
The drug is under review by the US Food and Drug Administration (FDA) and the European Medicines Agency. The FDA recently issued a complete response letter regarding the biologics license application for andexanet alfa.
Portola said it plans to meet with the FDA as soon as possible to resolve the outstanding questions in the letter and determine the appropriate next steps. ![]()