User login
Clindamycin, TMP-SMX both of benefit for small abscesses
NEW ORLEANS – Both clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) were superior to placebo when used after incision and drainage for the treatment of small, uncomplicated abscesses in children and adults in a prospective, randomized, placebo-controlled study.
Further, the cure rates were similar with both antibiotics, except in subjects with a clindamycin-resistant Staphylococcus aureus isolate, in whom the cure rate was lower, Robert S. Daum, MD, of the University of Chicago reported at an annual scientific meeting on infectious diseases.
Small, uncomplicated skin abscesses are common in ambulatory settings, but the optimal treatment strategy in the era of community-acquired methicillin-resistant S. aureus has been unclear. A prior study showed that clindamycin and TMP-SMX are both of benefit in the setting of large skin abscesses. The current findings further demonstrate that they also are of benefit when used in conjunction with incision and drainage for the treatment of small abscesses.
In 786 outpatient subjects, including 505 adults and 281 children who were randomized to receive 10 days of treatment with either clindamycin, TMP-SMX, or placebo following incision and drainage, mean cure rates at the 10-day posttherapy test of cure visit were 83% in the clindamycin group, 82% in the TMP-SMX group, and 69% in the placebo group, he said, noting that the differences were statistically significant for both treatments vs. placebo.
Study participants had a single skin abscess of 5 cm or less in diameter. Those with significant comorbidity, such as diabetes, were excluded.
S. aureus was isolated from 527 subjects (67%), and methicillin-resistant S. aureus was isolated from 388 (49%).
In clindamycin-treated subjects with an S. aureus lesion, 54% with a clindamycin-resistant isolate were cured, compared with 85% with a clindamycin-susceptible isolate.
Of note, subjects without S. aureus did not do better with antibiotics vs. placebo, Dr. Daum said.
“Staph aureus matters,” he said. People who did not grow Staph aureus did not do better with placebo than with antibiotic ... incision and drainage was basically all that was needed [in those patients],” he said.
Adverse events were more common in the clindamycin group (22% vs. 11% with TMP-SMX and 12.5% with placebo), but all events were mild and resolved without sequelae, and among those who were cured initially, fewer new skin infections were noted at a 1-month follow-up visit among clindamycin recipients, compared with those who received TMP-SMX or placebo, he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
No cases of Clostridium difficile-associated diarrhea were reported among study subjects.
Dr. Daum reported having no disclosures.
NEW ORLEANS – Both clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) were superior to placebo when used after incision and drainage for the treatment of small, uncomplicated abscesses in children and adults in a prospective, randomized, placebo-controlled study.
Further, the cure rates were similar with both antibiotics, except in subjects with a clindamycin-resistant Staphylococcus aureus isolate, in whom the cure rate was lower, Robert S. Daum, MD, of the University of Chicago reported at an annual scientific meeting on infectious diseases.
Small, uncomplicated skin abscesses are common in ambulatory settings, but the optimal treatment strategy in the era of community-acquired methicillin-resistant S. aureus has been unclear. A prior study showed that clindamycin and TMP-SMX are both of benefit in the setting of large skin abscesses. The current findings further demonstrate that they also are of benefit when used in conjunction with incision and drainage for the treatment of small abscesses.
In 786 outpatient subjects, including 505 adults and 281 children who were randomized to receive 10 days of treatment with either clindamycin, TMP-SMX, or placebo following incision and drainage, mean cure rates at the 10-day posttherapy test of cure visit were 83% in the clindamycin group, 82% in the TMP-SMX group, and 69% in the placebo group, he said, noting that the differences were statistically significant for both treatments vs. placebo.
Study participants had a single skin abscess of 5 cm or less in diameter. Those with significant comorbidity, such as diabetes, were excluded.
S. aureus was isolated from 527 subjects (67%), and methicillin-resistant S. aureus was isolated from 388 (49%).
In clindamycin-treated subjects with an S. aureus lesion, 54% with a clindamycin-resistant isolate were cured, compared with 85% with a clindamycin-susceptible isolate.
Of note, subjects without S. aureus did not do better with antibiotics vs. placebo, Dr. Daum said.
“Staph aureus matters,” he said. People who did not grow Staph aureus did not do better with placebo than with antibiotic ... incision and drainage was basically all that was needed [in those patients],” he said.
Adverse events were more common in the clindamycin group (22% vs. 11% with TMP-SMX and 12.5% with placebo), but all events were mild and resolved without sequelae, and among those who were cured initially, fewer new skin infections were noted at a 1-month follow-up visit among clindamycin recipients, compared with those who received TMP-SMX or placebo, he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
No cases of Clostridium difficile-associated diarrhea were reported among study subjects.
Dr. Daum reported having no disclosures.
NEW ORLEANS – Both clindamycin and trimethoprim-sulfamethoxazole (TMP-SMX) were superior to placebo when used after incision and drainage for the treatment of small, uncomplicated abscesses in children and adults in a prospective, randomized, placebo-controlled study.
Further, the cure rates were similar with both antibiotics, except in subjects with a clindamycin-resistant Staphylococcus aureus isolate, in whom the cure rate was lower, Robert S. Daum, MD, of the University of Chicago reported at an annual scientific meeting on infectious diseases.
Small, uncomplicated skin abscesses are common in ambulatory settings, but the optimal treatment strategy in the era of community-acquired methicillin-resistant S. aureus has been unclear. A prior study showed that clindamycin and TMP-SMX are both of benefit in the setting of large skin abscesses. The current findings further demonstrate that they also are of benefit when used in conjunction with incision and drainage for the treatment of small abscesses.
In 786 outpatient subjects, including 505 adults and 281 children who were randomized to receive 10 days of treatment with either clindamycin, TMP-SMX, or placebo following incision and drainage, mean cure rates at the 10-day posttherapy test of cure visit were 83% in the clindamycin group, 82% in the TMP-SMX group, and 69% in the placebo group, he said, noting that the differences were statistically significant for both treatments vs. placebo.
Study participants had a single skin abscess of 5 cm or less in diameter. Those with significant comorbidity, such as diabetes, were excluded.
S. aureus was isolated from 527 subjects (67%), and methicillin-resistant S. aureus was isolated from 388 (49%).
In clindamycin-treated subjects with an S. aureus lesion, 54% with a clindamycin-resistant isolate were cured, compared with 85% with a clindamycin-susceptible isolate.
Of note, subjects without S. aureus did not do better with antibiotics vs. placebo, Dr. Daum said.
“Staph aureus matters,” he said. People who did not grow Staph aureus did not do better with placebo than with antibiotic ... incision and drainage was basically all that was needed [in those patients],” he said.
Adverse events were more common in the clindamycin group (22% vs. 11% with TMP-SMX and 12.5% with placebo), but all events were mild and resolved without sequelae, and among those who were cured initially, fewer new skin infections were noted at a 1-month follow-up visit among clindamycin recipients, compared with those who received TMP-SMX or placebo, he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
No cases of Clostridium difficile-associated diarrhea were reported among study subjects.
Dr. Daum reported having no disclosures.
AT IDWEEK 2016
Key clinical point:
Major finding: Mean cure rates were 83%, 82%, and 69% with clindamycin, TMP-SMX, and placebo, respectively.
Data source: A randomized, placebo-controlled, multicenter study of 786 subjects.
Disclosures: Dr. Daum reported having no disclosures.
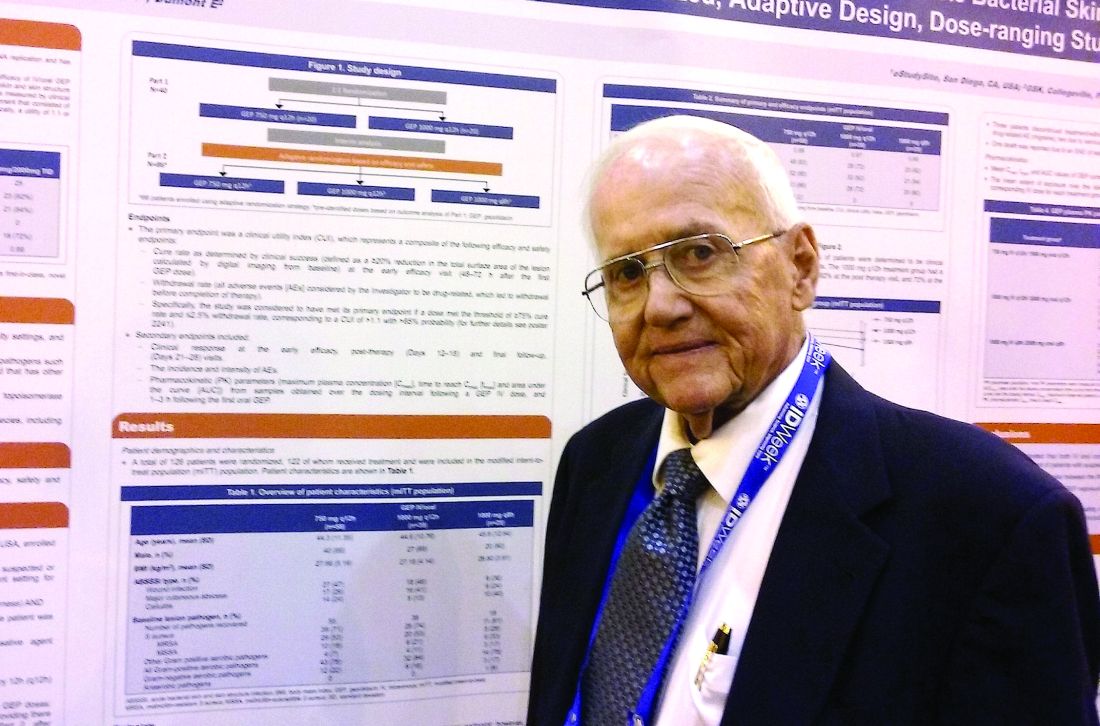
Novel antibiotic hits skin and soft tissue infections with one-two punch
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
AT IDWEEK 2016
Key clinical point: A dual-mechanism-of-action antibiotic in development shows good efficacy and a low adverse event rate in a phase II study.
Major finding: A total 71 of 122 adult patients achieved clinical success within 48 to 72 hours with gepotidacin treatment.
Data source: 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis.
Disclosures: Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
VIDEO: No effect of donor on FMT outcomes in C. difficile patients
NEW ORLEANS – Fecal microbiota transplantation, or FMT, is a highly effective treatment for Clostridium difficile infection (CDI) and other digestive and autoimmune disorders, but little is known about the role of donor characteristics with respect to outcomes in patients with recurrent CDI.
A study of nearly 1,999 patients with an 83.9% cure rate showed no significant difference between 28 donors in terms of clinical outcomes at 8 weeks, according to Majdi Osman, MD, of OpenBiome, a not-for-profit stool bank in the Boston area.
Studies in inflammatory bowel diseases have suggested that donors do matter, but that does not appear to be the case when it comes to recurrent CDI, Dr. Osman said at an annual scientific meeting on infectious diseases.
“Broadly speaking, it seems like the efficacy rate is the same amongst all of our donors,” he said in a video interview at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Potential donors are subject to a rigorous screening process, and less than 3% are accepted, but given that donors were shown in previous studies to play a role in effectiveness in some other conditions, Dr. Osman said it was worth checking to see if outcomes in CDI could be further improved through donor selection.
In fact, it appears that “the donor doesn’t matter,” he said, noting that it may be that “we are selecting for a fairly narrow spectrum of the population, and actually the stool that we’re selecting is fairly similar in composition.”
Efforts are underway to look more closely at that possibility, and Dr. Osman said he hopes to see more standardized clinical trials and clinical follow-up. He also said he is excited about an FMT registry – a joint project of the American Gastroenterology Association and the Infectious Diseases Society of America – that will follow 4,000 patients for 10 years.
“We will be working closely with them to provide material and get some really good robust clinical data going forward,” he said.
Dr. Osman reported having no disclosures.
NEW ORLEANS – Fecal microbiota transplantation, or FMT, is a highly effective treatment for Clostridium difficile infection (CDI) and other digestive and autoimmune disorders, but little is known about the role of donor characteristics with respect to outcomes in patients with recurrent CDI.
A study of nearly 1,999 patients with an 83.9% cure rate showed no significant difference between 28 donors in terms of clinical outcomes at 8 weeks, according to Majdi Osman, MD, of OpenBiome, a not-for-profit stool bank in the Boston area.
Studies in inflammatory bowel diseases have suggested that donors do matter, but that does not appear to be the case when it comes to recurrent CDI, Dr. Osman said at an annual scientific meeting on infectious diseases.
“Broadly speaking, it seems like the efficacy rate is the same amongst all of our donors,” he said in a video interview at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Potential donors are subject to a rigorous screening process, and less than 3% are accepted, but given that donors were shown in previous studies to play a role in effectiveness in some other conditions, Dr. Osman said it was worth checking to see if outcomes in CDI could be further improved through donor selection.
In fact, it appears that “the donor doesn’t matter,” he said, noting that it may be that “we are selecting for a fairly narrow spectrum of the population, and actually the stool that we’re selecting is fairly similar in composition.”
Efforts are underway to look more closely at that possibility, and Dr. Osman said he hopes to see more standardized clinical trials and clinical follow-up. He also said he is excited about an FMT registry – a joint project of the American Gastroenterology Association and the Infectious Diseases Society of America – that will follow 4,000 patients for 10 years.
“We will be working closely with them to provide material and get some really good robust clinical data going forward,” he said.
Dr. Osman reported having no disclosures.
NEW ORLEANS – Fecal microbiota transplantation, or FMT, is a highly effective treatment for Clostridium difficile infection (CDI) and other digestive and autoimmune disorders, but little is known about the role of donor characteristics with respect to outcomes in patients with recurrent CDI.
A study of nearly 1,999 patients with an 83.9% cure rate showed no significant difference between 28 donors in terms of clinical outcomes at 8 weeks, according to Majdi Osman, MD, of OpenBiome, a not-for-profit stool bank in the Boston area.
Studies in inflammatory bowel diseases have suggested that donors do matter, but that does not appear to be the case when it comes to recurrent CDI, Dr. Osman said at an annual scientific meeting on infectious diseases.
“Broadly speaking, it seems like the efficacy rate is the same amongst all of our donors,” he said in a video interview at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Potential donors are subject to a rigorous screening process, and less than 3% are accepted, but given that donors were shown in previous studies to play a role in effectiveness in some other conditions, Dr. Osman said it was worth checking to see if outcomes in CDI could be further improved through donor selection.
In fact, it appears that “the donor doesn’t matter,” he said, noting that it may be that “we are selecting for a fairly narrow spectrum of the population, and actually the stool that we’re selecting is fairly similar in composition.”
Efforts are underway to look more closely at that possibility, and Dr. Osman said he hopes to see more standardized clinical trials and clinical follow-up. He also said he is excited about an FMT registry – a joint project of the American Gastroenterology Association and the Infectious Diseases Society of America – that will follow 4,000 patients for 10 years.
“We will be working closely with them to provide material and get some really good robust clinical data going forward,” he said.
Dr. Osman reported having no disclosures.
Bezlotoxumab reduces CDI recurrence across antibiotic subgroups
NEW ORLEANS – The monoclonal antibody bezlotoxumab significantly reduces the risk of Clostridium difficile infection (CDI) recurrence in adults receiving standard of care antibiotic treatment, regardless of whether that treatment is with metronidazole, vancomycin, or fidaxomicin, according to an analysis of data from the MODIFY I and II trials.
The global, randomized, double-blind, placebo-controlled trials demonstrated that bezlotoxumab was safe and effective for preventing CDI recurrence, and the current prespecified analysis further showed that choice of standard of care antibiotic therapy did not affect the outcomes, Erik R. Dubberke, MD, of Washington University in St. Louis, reported at IDWeek, an annual scientific meeting on infectious diseases.
Consistent with the overall study results, clinical cure rates were similar with bezlotoxumab and placebo, regardless of the standard of care antibiotic received (80% vs. 80.3%, respectively, overall; 81% vs. 81.3% in the metronidazole group; 78.5% vs. 79.6% in the vancomycin group; and 86.7% vs. 76.9% in the fidaxomicin group), he said.
However, CDI recurrence rates were lower among those who received bezlotoxumab, compared with those who received placebo in all three standard of care subgroups, with 10%, 15%, and 12% fewer bezlotoxumab vs. placebo patients experiencing recurrence in the metronidazole, vancomycin, and fidaxomicin groups, respectively, and 12% fewer experiencing recurrence overall.
The primary endpoint of CDI recurrence was defined in the studies as a new episode of diarrhea (at least three unformed stools in 24 hours) and a positive stool test for toxigenic C. difficile after clinical cure of the baseline CDI episode. Clinical cure was defined as standard of care antibiotics given for 14 days or less and no diarrhea for 2 consecutive days after completing standard of care treatment.
Of note, the patients in the vancomycin group were older and sicker, and had more risk factors for CDI, he said, noting, for example, that 57% of vancomycin patients were aged at least 65 years and 33% were aged at least 75 years, vs. 46% and 26% for metronidazole, respectively, and 46% and 18% for fidaxomicin; 72% of vancomycin patients were inpatients, compared with 65% and 50% of metronidazole and fidaxomicin patients.
Further, 19% of vancomycin patients, vs. 13% and 14% of metronidazole and fidaxomicin patients met criteria for severe CDI.
The findings of the current analysis are important, because the incidence of CDI recurrence is about 25%, and the risk increases with each subsequent recurrence, Dr. Dubberke said, concluding that this novel, nonantibiotic approach to prevention of CDI recurrence using bezlotoxumab, which recently received Food and Drug Administration approval for this indication, is of benefit for reducing that risk, regardless of the antibiotic used as part of standard of care therapy for CDI.
Dr. Dubberke reported serving as an investigator, adviser and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
NEW ORLEANS – The monoclonal antibody bezlotoxumab significantly reduces the risk of Clostridium difficile infection (CDI) recurrence in adults receiving standard of care antibiotic treatment, regardless of whether that treatment is with metronidazole, vancomycin, or fidaxomicin, according to an analysis of data from the MODIFY I and II trials.
The global, randomized, double-blind, placebo-controlled trials demonstrated that bezlotoxumab was safe and effective for preventing CDI recurrence, and the current prespecified analysis further showed that choice of standard of care antibiotic therapy did not affect the outcomes, Erik R. Dubberke, MD, of Washington University in St. Louis, reported at IDWeek, an annual scientific meeting on infectious diseases.
Consistent with the overall study results, clinical cure rates were similar with bezlotoxumab and placebo, regardless of the standard of care antibiotic received (80% vs. 80.3%, respectively, overall; 81% vs. 81.3% in the metronidazole group; 78.5% vs. 79.6% in the vancomycin group; and 86.7% vs. 76.9% in the fidaxomicin group), he said.
However, CDI recurrence rates were lower among those who received bezlotoxumab, compared with those who received placebo in all three standard of care subgroups, with 10%, 15%, and 12% fewer bezlotoxumab vs. placebo patients experiencing recurrence in the metronidazole, vancomycin, and fidaxomicin groups, respectively, and 12% fewer experiencing recurrence overall.
The primary endpoint of CDI recurrence was defined in the studies as a new episode of diarrhea (at least three unformed stools in 24 hours) and a positive stool test for toxigenic C. difficile after clinical cure of the baseline CDI episode. Clinical cure was defined as standard of care antibiotics given for 14 days or less and no diarrhea for 2 consecutive days after completing standard of care treatment.
Of note, the patients in the vancomycin group were older and sicker, and had more risk factors for CDI, he said, noting, for example, that 57% of vancomycin patients were aged at least 65 years and 33% were aged at least 75 years, vs. 46% and 26% for metronidazole, respectively, and 46% and 18% for fidaxomicin; 72% of vancomycin patients were inpatients, compared with 65% and 50% of metronidazole and fidaxomicin patients.
Further, 19% of vancomycin patients, vs. 13% and 14% of metronidazole and fidaxomicin patients met criteria for severe CDI.
The findings of the current analysis are important, because the incidence of CDI recurrence is about 25%, and the risk increases with each subsequent recurrence, Dr. Dubberke said, concluding that this novel, nonantibiotic approach to prevention of CDI recurrence using bezlotoxumab, which recently received Food and Drug Administration approval for this indication, is of benefit for reducing that risk, regardless of the antibiotic used as part of standard of care therapy for CDI.
Dr. Dubberke reported serving as an investigator, adviser and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
NEW ORLEANS – The monoclonal antibody bezlotoxumab significantly reduces the risk of Clostridium difficile infection (CDI) recurrence in adults receiving standard of care antibiotic treatment, regardless of whether that treatment is with metronidazole, vancomycin, or fidaxomicin, according to an analysis of data from the MODIFY I and II trials.
The global, randomized, double-blind, placebo-controlled trials demonstrated that bezlotoxumab was safe and effective for preventing CDI recurrence, and the current prespecified analysis further showed that choice of standard of care antibiotic therapy did not affect the outcomes, Erik R. Dubberke, MD, of Washington University in St. Louis, reported at IDWeek, an annual scientific meeting on infectious diseases.
Consistent with the overall study results, clinical cure rates were similar with bezlotoxumab and placebo, regardless of the standard of care antibiotic received (80% vs. 80.3%, respectively, overall; 81% vs. 81.3% in the metronidazole group; 78.5% vs. 79.6% in the vancomycin group; and 86.7% vs. 76.9% in the fidaxomicin group), he said.
However, CDI recurrence rates were lower among those who received bezlotoxumab, compared with those who received placebo in all three standard of care subgroups, with 10%, 15%, and 12% fewer bezlotoxumab vs. placebo patients experiencing recurrence in the metronidazole, vancomycin, and fidaxomicin groups, respectively, and 12% fewer experiencing recurrence overall.
The primary endpoint of CDI recurrence was defined in the studies as a new episode of diarrhea (at least three unformed stools in 24 hours) and a positive stool test for toxigenic C. difficile after clinical cure of the baseline CDI episode. Clinical cure was defined as standard of care antibiotics given for 14 days or less and no diarrhea for 2 consecutive days after completing standard of care treatment.
Of note, the patients in the vancomycin group were older and sicker, and had more risk factors for CDI, he said, noting, for example, that 57% of vancomycin patients were aged at least 65 years and 33% were aged at least 75 years, vs. 46% and 26% for metronidazole, respectively, and 46% and 18% for fidaxomicin; 72% of vancomycin patients were inpatients, compared with 65% and 50% of metronidazole and fidaxomicin patients.
Further, 19% of vancomycin patients, vs. 13% and 14% of metronidazole and fidaxomicin patients met criteria for severe CDI.
The findings of the current analysis are important, because the incidence of CDI recurrence is about 25%, and the risk increases with each subsequent recurrence, Dr. Dubberke said, concluding that this novel, nonantibiotic approach to prevention of CDI recurrence using bezlotoxumab, which recently received Food and Drug Administration approval for this indication, is of benefit for reducing that risk, regardless of the antibiotic used as part of standard of care therapy for CDI.
Dr. Dubberke reported serving as an investigator, adviser and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
AT IDWEEK 2016
Key clinical point:
Major finding: 12% fewer bezlotoxumab vs. placebo patients experienced CDI recurrence.
Data source: A prespecified analysis of data from 1,554 subjects from the MODIFY I and II trials.
Disclosures: Dr. Dubberke reported serving as an investigator, adviser, and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
Fatal measles complication occurs more often than realized
NEW ORLEANS – A fatal complication of measles known as subacute sclerosing panencephalitis (SSPE) can develop years after measles infection and appears to occur much more often than published reports suggest, according to a review of cases in California from 1998 to 2015.
The findings underscore the vital importance of herd immunity by vaccination, Kristen Wendorf, MD, reported at an annual scientific meeting on infectious diseases.
The incidence of postmeasles SSPE was previously thought be about 1 in 100,000, according to an IDWeek press release.
“There is no cure for SSPE, and the only way to prevent it is to vaccinate everyone against measles,” the release stated.
The cases in the current study were among children with a clinically compatible illness, and either measles IgG antibody detected in cerebrospinal fluid, a characteristic pattern on electroencephalography, typical histologic findings on brain biopsy, or medical record documentation of SSPE-related complications. They were identified based on death certificates, reports from the Centers for Diseases Control and Prevention, or through investigations for undiagnosed neurologic disease. Twelve of the 17 affected children had a clinical history of a febrile rash illness compatible with measles, and all 12 of those experienced illness before age 15 months and before measles vaccination.
Most (67%) were living in the United States when they had measles, Dr. Wendorf said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The median age at diagnosis of SSPE was 12 years, although the range was 3-35 years, and the mean latency period was 9.5 years. In many cases, long-standing cognitive or motor problems were experienced prior to diagnosis, she noted.
The findings suggest that SSPE is more common than previously recognized in unvaccinated children with measles during infancy, Dr. Wendorf said.
Protection of infants younger than 12-15 months of age – before the time when measles vaccine is routinely administered – and in those who can’t be vaccinated because of immune system disorders requires avoidance of travel to endemic areas. Parents also may consider early vaccination prior to such travel.
Further, clinicians should be aware of the risk of SSPE in patients with symptoms suggestive of the disease. This is true even among older patients in whom no specific history of measles infection is known, she said.
In the press release, coauthor James D. Cherry, MD, professor of pediatrics at the University of California, Los Angeles, further stressed the importance of protecting unvaccinated infants.
“Parents of infants who have not yet been vaccinated should avoid putting their children at risk. For example, they should postpone trips overseas – including to Europe – where measles is endemic and epidemic until after their baby has been vaccinated with two doses,” he said. “It’s just not worth the risk.”
The authors reported having no disclosures.
NEW ORLEANS – A fatal complication of measles known as subacute sclerosing panencephalitis (SSPE) can develop years after measles infection and appears to occur much more often than published reports suggest, according to a review of cases in California from 1998 to 2015.
The findings underscore the vital importance of herd immunity by vaccination, Kristen Wendorf, MD, reported at an annual scientific meeting on infectious diseases.
The incidence of postmeasles SSPE was previously thought be about 1 in 100,000, according to an IDWeek press release.
“There is no cure for SSPE, and the only way to prevent it is to vaccinate everyone against measles,” the release stated.
The cases in the current study were among children with a clinically compatible illness, and either measles IgG antibody detected in cerebrospinal fluid, a characteristic pattern on electroencephalography, typical histologic findings on brain biopsy, or medical record documentation of SSPE-related complications. They were identified based on death certificates, reports from the Centers for Diseases Control and Prevention, or through investigations for undiagnosed neurologic disease. Twelve of the 17 affected children had a clinical history of a febrile rash illness compatible with measles, and all 12 of those experienced illness before age 15 months and before measles vaccination.
Most (67%) were living in the United States when they had measles, Dr. Wendorf said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The median age at diagnosis of SSPE was 12 years, although the range was 3-35 years, and the mean latency period was 9.5 years. In many cases, long-standing cognitive or motor problems were experienced prior to diagnosis, she noted.
The findings suggest that SSPE is more common than previously recognized in unvaccinated children with measles during infancy, Dr. Wendorf said.
Protection of infants younger than 12-15 months of age – before the time when measles vaccine is routinely administered – and in those who can’t be vaccinated because of immune system disorders requires avoidance of travel to endemic areas. Parents also may consider early vaccination prior to such travel.
Further, clinicians should be aware of the risk of SSPE in patients with symptoms suggestive of the disease. This is true even among older patients in whom no specific history of measles infection is known, she said.
In the press release, coauthor James D. Cherry, MD, professor of pediatrics at the University of California, Los Angeles, further stressed the importance of protecting unvaccinated infants.
“Parents of infants who have not yet been vaccinated should avoid putting their children at risk. For example, they should postpone trips overseas – including to Europe – where measles is endemic and epidemic until after their baby has been vaccinated with two doses,” he said. “It’s just not worth the risk.”
The authors reported having no disclosures.
NEW ORLEANS – A fatal complication of measles known as subacute sclerosing panencephalitis (SSPE) can develop years after measles infection and appears to occur much more often than published reports suggest, according to a review of cases in California from 1998 to 2015.
The findings underscore the vital importance of herd immunity by vaccination, Kristen Wendorf, MD, reported at an annual scientific meeting on infectious diseases.
The incidence of postmeasles SSPE was previously thought be about 1 in 100,000, according to an IDWeek press release.
“There is no cure for SSPE, and the only way to prevent it is to vaccinate everyone against measles,” the release stated.
The cases in the current study were among children with a clinically compatible illness, and either measles IgG antibody detected in cerebrospinal fluid, a characteristic pattern on electroencephalography, typical histologic findings on brain biopsy, or medical record documentation of SSPE-related complications. They were identified based on death certificates, reports from the Centers for Diseases Control and Prevention, or through investigations for undiagnosed neurologic disease. Twelve of the 17 affected children had a clinical history of a febrile rash illness compatible with measles, and all 12 of those experienced illness before age 15 months and before measles vaccination.
Most (67%) were living in the United States when they had measles, Dr. Wendorf said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The median age at diagnosis of SSPE was 12 years, although the range was 3-35 years, and the mean latency period was 9.5 years. In many cases, long-standing cognitive or motor problems were experienced prior to diagnosis, she noted.
The findings suggest that SSPE is more common than previously recognized in unvaccinated children with measles during infancy, Dr. Wendorf said.
Protection of infants younger than 12-15 months of age – before the time when measles vaccine is routinely administered – and in those who can’t be vaccinated because of immune system disorders requires avoidance of travel to endemic areas. Parents also may consider early vaccination prior to such travel.
Further, clinicians should be aware of the risk of SSPE in patients with symptoms suggestive of the disease. This is true even among older patients in whom no specific history of measles infection is known, she said.
In the press release, coauthor James D. Cherry, MD, professor of pediatrics at the University of California, Los Angeles, further stressed the importance of protecting unvaccinated infants.
“Parents of infants who have not yet been vaccinated should avoid putting their children at risk. For example, they should postpone trips overseas – including to Europe – where measles is endemic and epidemic until after their baby has been vaccinated with two doses,” he said. “It’s just not worth the risk.”
The authors reported having no disclosures.
AT IDWEEK 2016
Key clinical point:
Major finding: The incidence of SSPE among measles cases was 1 in 1,367 children under age 5 years and 1 in 609 children under age 12 months at the time of measles disease.
Data source: A review of records and 17 cases of SSPE.
Disclosures: The authors reported having no disclosures.
VIDEO: Biologic therapy for multidrug-resistant HIV offers new hope
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT IDWEEK 2016
Key clinical point: Ibalizumab, a new biologic in a phase III trial, significantly reduces viral loads in patients with multidrug-resistant HIV infection.
Major finding: At 14 days, 83% of 40 trial participants experienced at least a one-half log reduction in HIV viral load.
Data source: Single arm, 24-week study of ibalizumab plus optimized background regimen in treatment experienced patients with multidrug-resistant HIV-1.
Disclosures: TaiMed Biologics, the manufacturer of ibalizumab, sponsored the study. Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
Surgical treatment tops medical management of prosthetic valve endocarditis
NEW ORLEANS – Over the years patients with prosthetic valve endocarditis treated at Cleveland Clinic tended to fare better with surgery compared to medical management, some clinicians noted. However, there was no data to confirm their observations.
“It was not recognized widely. A lot of our colleagues continued to believe it could be adequately treated with the right antibiotic,” Nabin K. Shrestha, MD, said at the IDWeek 2016 annual meeting on infectious diseases.
So Dr Shrestha and his colleagues conducted a retrospective cohort study to compare outcomes between 253 surgically treated adults and 77 others treated medically between April 2008 and December 2012. Survival from the time of treatment decision was the primary outcome.
The groups differed on some demographic and clinical factors. For example, the medically treated group was older, had fewer men, and more patients with mitral valves. “We might think the medical patients might be too sick for surgery, and that could certainly be true, but … they could have been too well for surgery too,” Dr. Shrestha said. To control for these differences between groups, the investigators performed a number of statistical analyses, including a propensity score adjusted model and reduced Cox proportion hazards model.
“Patients with PVE have a high hazard of death if treated medically,” Dr. Shrestha said, based on a 6.68 hazard ratio. The higher risk of death associated with medical treatment remained significant when adjusted for age, sex, and other factors. “Compared to surgical treatment, medical treatment was associated with a seven-fold higher hazard of death overall,” Dr. Shrestha said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The medical treatment group also fared worse on a number of secondary outcomes. For example, this group had a five-fold higher risk of death during hospitalization compared to the surgery group (odds ratio, 4.65); a 12-fold higher risk of death within one year (OR, 11.70); a seven-fold higher risk of subsequent surgery for infective endocarditis (OR, 6.57); and an eight-fold higher odds of surgery for the same episode of infective endocarditis at a subsequent hospitalization (OR, 8.02).
A large sample size and setting the date of management decision as time zero to avoid survival selection bias “give us confidence in our findings.” Limitations include an inability to look at some important variables because of the retrospective design.
A meeting attendee commented that surgeons often request a patient be optimized medically prior to surgery, and asked if investigators looked at time from hospitalization to the operation.
“The median date from admission to surgery was six days in our database,” said Dr. Shrestha, who is a staff physician at the Cleveland Clinic in Ohio.
“Medical treatment overall is associated with significantly poorer outcomes in patients with PVE compared with surgical treatment,” Dr. Shrestha said. “Although some patients are not candidates for surgery, a definite diagnosis of PVE should prompt a surgical evaluation in the majority of patients.”
Dr. Shrestha reported having no disclosures.
NEW ORLEANS – Over the years patients with prosthetic valve endocarditis treated at Cleveland Clinic tended to fare better with surgery compared to medical management, some clinicians noted. However, there was no data to confirm their observations.
“It was not recognized widely. A lot of our colleagues continued to believe it could be adequately treated with the right antibiotic,” Nabin K. Shrestha, MD, said at the IDWeek 2016 annual meeting on infectious diseases.
So Dr Shrestha and his colleagues conducted a retrospective cohort study to compare outcomes between 253 surgically treated adults and 77 others treated medically between April 2008 and December 2012. Survival from the time of treatment decision was the primary outcome.
The groups differed on some demographic and clinical factors. For example, the medically treated group was older, had fewer men, and more patients with mitral valves. “We might think the medical patients might be too sick for surgery, and that could certainly be true, but … they could have been too well for surgery too,” Dr. Shrestha said. To control for these differences between groups, the investigators performed a number of statistical analyses, including a propensity score adjusted model and reduced Cox proportion hazards model.
“Patients with PVE have a high hazard of death if treated medically,” Dr. Shrestha said, based on a 6.68 hazard ratio. The higher risk of death associated with medical treatment remained significant when adjusted for age, sex, and other factors. “Compared to surgical treatment, medical treatment was associated with a seven-fold higher hazard of death overall,” Dr. Shrestha said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The medical treatment group also fared worse on a number of secondary outcomes. For example, this group had a five-fold higher risk of death during hospitalization compared to the surgery group (odds ratio, 4.65); a 12-fold higher risk of death within one year (OR, 11.70); a seven-fold higher risk of subsequent surgery for infective endocarditis (OR, 6.57); and an eight-fold higher odds of surgery for the same episode of infective endocarditis at a subsequent hospitalization (OR, 8.02).
A large sample size and setting the date of management decision as time zero to avoid survival selection bias “give us confidence in our findings.” Limitations include an inability to look at some important variables because of the retrospective design.
A meeting attendee commented that surgeons often request a patient be optimized medically prior to surgery, and asked if investigators looked at time from hospitalization to the operation.
“The median date from admission to surgery was six days in our database,” said Dr. Shrestha, who is a staff physician at the Cleveland Clinic in Ohio.
“Medical treatment overall is associated with significantly poorer outcomes in patients with PVE compared with surgical treatment,” Dr. Shrestha said. “Although some patients are not candidates for surgery, a definite diagnosis of PVE should prompt a surgical evaluation in the majority of patients.”
Dr. Shrestha reported having no disclosures.
NEW ORLEANS – Over the years patients with prosthetic valve endocarditis treated at Cleveland Clinic tended to fare better with surgery compared to medical management, some clinicians noted. However, there was no data to confirm their observations.
“It was not recognized widely. A lot of our colleagues continued to believe it could be adequately treated with the right antibiotic,” Nabin K. Shrestha, MD, said at the IDWeek 2016 annual meeting on infectious diseases.
So Dr Shrestha and his colleagues conducted a retrospective cohort study to compare outcomes between 253 surgically treated adults and 77 others treated medically between April 2008 and December 2012. Survival from the time of treatment decision was the primary outcome.
The groups differed on some demographic and clinical factors. For example, the medically treated group was older, had fewer men, and more patients with mitral valves. “We might think the medical patients might be too sick for surgery, and that could certainly be true, but … they could have been too well for surgery too,” Dr. Shrestha said. To control for these differences between groups, the investigators performed a number of statistical analyses, including a propensity score adjusted model and reduced Cox proportion hazards model.
“Patients with PVE have a high hazard of death if treated medically,” Dr. Shrestha said, based on a 6.68 hazard ratio. The higher risk of death associated with medical treatment remained significant when adjusted for age, sex, and other factors. “Compared to surgical treatment, medical treatment was associated with a seven-fold higher hazard of death overall,” Dr. Shrestha said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The medical treatment group also fared worse on a number of secondary outcomes. For example, this group had a five-fold higher risk of death during hospitalization compared to the surgery group (odds ratio, 4.65); a 12-fold higher risk of death within one year (OR, 11.70); a seven-fold higher risk of subsequent surgery for infective endocarditis (OR, 6.57); and an eight-fold higher odds of surgery for the same episode of infective endocarditis at a subsequent hospitalization (OR, 8.02).
A large sample size and setting the date of management decision as time zero to avoid survival selection bias “give us confidence in our findings.” Limitations include an inability to look at some important variables because of the retrospective design.
A meeting attendee commented that surgeons often request a patient be optimized medically prior to surgery, and asked if investigators looked at time from hospitalization to the operation.
“The median date from admission to surgery was six days in our database,” said Dr. Shrestha, who is a staff physician at the Cleveland Clinic in Ohio.
“Medical treatment overall is associated with significantly poorer outcomes in patients with PVE compared with surgical treatment,” Dr. Shrestha said. “Although some patients are not candidates for surgery, a definite diagnosis of PVE should prompt a surgical evaluation in the majority of patients.”
Dr. Shrestha reported having no disclosures.
Key clinical point:
Major finding: Compared to surgery, odds of death within one year higher were almost 7 times greater with medical treatment (hazard ratio, 6.68).
Data source: Presentation at IDWeek 2016
Disclosures: Dr. Nabin K. Shrestha had no relevant disclosures.