User login
Intervention opens access to care for minority youths with type 1 diabetes
For racial or ethnic minority youths with type 1 diabetes, participating in an interventional program improves access to care, new research shows.
Youth categorized as Black, Indigenous, and other people of color (BIPOC) had significantly improved outpatient attendance during and after participating in Novel Interventions in Children’s Healthcare (NICH), a systems intervention for children with chronic health conditions and their families.
By comparison, no improvements in care access were observed among BIPOC children who were not able to access the program because of insurance or other reasons, David V. Wagner, PhD, Associate Professor and NICH research director at Oregon Health & Science University, Portland, reported at the annual scientific sessions of the American Diabetes Association.
The findings demonstrate a need for intensive, home-based services that aim to correct health inequities, said Dr. Wagner, who presented the findings along with Winniebhelle Cadiz, a scholar in the BUILD EXITO undergraduate research training program at Portland (Ore.) State University.
The NICH program hinges on trained interventionists who visit families at home, attend clinic visits, and work with schools and other contacts to help solve problems that keep children from following medical instructions, according to a program description.
“Families report having somebody by their side to help them navigate the system, address the transportation difficulties experienced, and help them and build that relationship with their health care provider seems to be hugely influential in terms of helping them navigate and access care,” Dr. Wagner said in a presentation of the study.
A NICH for youths with chronic health conditions
The NICH program differs from some other programs that have been developed in an attempt to improve health outcomes among youths in the community, according to Dr. Wagner.
“Many of the programs that exist out there are often piloted on, and seemingly built for, those who have more resources,” he said in his presentation. “Those who are in greatest need often have difficulty accessing and responding to the services.”
NICH doesn’t take the place of existing services, but is “an addition to the continuum of care” for youths and families who are struggling because of lack of resources or marginalization in the health care system, Dr. Wagner said.
While NICH is not specific to any one chronic health condition, several previous investigations have specifically looked at the impact of the NICH program on access to care in youths with type 1 diabetes.
Youths participating in the program for a year had fewer ED visits, including fewer visits with diabetic ketoacidosis (DKA), as well as fewer and shorter admissions as compared with the year prior to participating in the program, Dr. Wagner said.
In another study, youths had fewer admissions for diabetes or DKA and less frequent pediatric ICU contact during the NICH program, as compared with before the program.
Another study showed that, while NICH had no impact overall on access to care among youths with type 1 diabetes, BIPOC youths had an improvement in the mean number of outpatient visits as compared with preprogram levels. However, because none of those studies included a control group, Dr. Wagner said, it remained unclear whether this systems intervention might improve outpatient access among youths with type 1 diabetes as compared with those who did not participate.
Intervention linked to increased BIPOC care access
The latest study includes 144 youths with type 1 diabetes referred for the program. The mean age was 13.7 years, 58% were female, and 81% were non-Hispanic White. While 51 youths were able to participate in NICH, the remaining 93 were not served by the program because of insurance denial or nonresponse, according to investigators.
While participation in the program made no difference in access to care overall, results of this study suggest NICH reduced access disparities among BIPOC youths, the investigators said.
Those BIPOC youth, 28 in total, had significantly worse access to care prior to referral. However, BIPOC youth participation in NICH was associated with improved attendance at endocrinology appointments and outpatient attendance overall.
A mean change of 1.9 more appointments per year was seen among BIPOC youth who participated in NICH, compared with a mean decrease of 0.5 appointments per year among BIPOC youth not served by the program (P = .03), according to the study abstract.
Prior to NICH participation, outpatient attendance among BIPOC youths was about 2.5 visits per year, data presented by the investigators show.
Systemic changes needed
This study is representative of systemic changes that are needed to improve access to quality care for BIPOC youth, according Cynthia E. Muñoz, PhD, MPH, ADA’s president of health care and education.
“We know that there are increased risks for poor health outcomes for these children and youths, and we know that there is a risk for mental health and psychosocial challenges for youth from these communities,” said Dr. Muñoz, a bilingual licensed psychologist and assistant professor of clinical pediatrics at the University of Southern California, Los Angeles.
In his presentation, Dr. Wagner said lumping racial and ethnic minority participants under a single BIPOC header probably wasn’t ideal because of the diversity and differences among racial and ethnic minorities. However, it was necessary in this particular study because of limited sample size.
Dr. Wagner and coauthors disclosed no conflicts of interest related to the research, which was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
For racial or ethnic minority youths with type 1 diabetes, participating in an interventional program improves access to care, new research shows.
Youth categorized as Black, Indigenous, and other people of color (BIPOC) had significantly improved outpatient attendance during and after participating in Novel Interventions in Children’s Healthcare (NICH), a systems intervention for children with chronic health conditions and their families.
By comparison, no improvements in care access were observed among BIPOC children who were not able to access the program because of insurance or other reasons, David V. Wagner, PhD, Associate Professor and NICH research director at Oregon Health & Science University, Portland, reported at the annual scientific sessions of the American Diabetes Association.
The findings demonstrate a need for intensive, home-based services that aim to correct health inequities, said Dr. Wagner, who presented the findings along with Winniebhelle Cadiz, a scholar in the BUILD EXITO undergraduate research training program at Portland (Ore.) State University.
The NICH program hinges on trained interventionists who visit families at home, attend clinic visits, and work with schools and other contacts to help solve problems that keep children from following medical instructions, according to a program description.
“Families report having somebody by their side to help them navigate the system, address the transportation difficulties experienced, and help them and build that relationship with their health care provider seems to be hugely influential in terms of helping them navigate and access care,” Dr. Wagner said in a presentation of the study.
A NICH for youths with chronic health conditions
The NICH program differs from some other programs that have been developed in an attempt to improve health outcomes among youths in the community, according to Dr. Wagner.
“Many of the programs that exist out there are often piloted on, and seemingly built for, those who have more resources,” he said in his presentation. “Those who are in greatest need often have difficulty accessing and responding to the services.”
NICH doesn’t take the place of existing services, but is “an addition to the continuum of care” for youths and families who are struggling because of lack of resources or marginalization in the health care system, Dr. Wagner said.
While NICH is not specific to any one chronic health condition, several previous investigations have specifically looked at the impact of the NICH program on access to care in youths with type 1 diabetes.
Youths participating in the program for a year had fewer ED visits, including fewer visits with diabetic ketoacidosis (DKA), as well as fewer and shorter admissions as compared with the year prior to participating in the program, Dr. Wagner said.
In another study, youths had fewer admissions for diabetes or DKA and less frequent pediatric ICU contact during the NICH program, as compared with before the program.
Another study showed that, while NICH had no impact overall on access to care among youths with type 1 diabetes, BIPOC youths had an improvement in the mean number of outpatient visits as compared with preprogram levels. However, because none of those studies included a control group, Dr. Wagner said, it remained unclear whether this systems intervention might improve outpatient access among youths with type 1 diabetes as compared with those who did not participate.
Intervention linked to increased BIPOC care access
The latest study includes 144 youths with type 1 diabetes referred for the program. The mean age was 13.7 years, 58% were female, and 81% were non-Hispanic White. While 51 youths were able to participate in NICH, the remaining 93 were not served by the program because of insurance denial or nonresponse, according to investigators.
While participation in the program made no difference in access to care overall, results of this study suggest NICH reduced access disparities among BIPOC youths, the investigators said.
Those BIPOC youth, 28 in total, had significantly worse access to care prior to referral. However, BIPOC youth participation in NICH was associated with improved attendance at endocrinology appointments and outpatient attendance overall.
A mean change of 1.9 more appointments per year was seen among BIPOC youth who participated in NICH, compared with a mean decrease of 0.5 appointments per year among BIPOC youth not served by the program (P = .03), according to the study abstract.
Prior to NICH participation, outpatient attendance among BIPOC youths was about 2.5 visits per year, data presented by the investigators show.
Systemic changes needed
This study is representative of systemic changes that are needed to improve access to quality care for BIPOC youth, according Cynthia E. Muñoz, PhD, MPH, ADA’s president of health care and education.
“We know that there are increased risks for poor health outcomes for these children and youths, and we know that there is a risk for mental health and psychosocial challenges for youth from these communities,” said Dr. Muñoz, a bilingual licensed psychologist and assistant professor of clinical pediatrics at the University of Southern California, Los Angeles.
In his presentation, Dr. Wagner said lumping racial and ethnic minority participants under a single BIPOC header probably wasn’t ideal because of the diversity and differences among racial and ethnic minorities. However, it was necessary in this particular study because of limited sample size.
Dr. Wagner and coauthors disclosed no conflicts of interest related to the research, which was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
For racial or ethnic minority youths with type 1 diabetes, participating in an interventional program improves access to care, new research shows.
Youth categorized as Black, Indigenous, and other people of color (BIPOC) had significantly improved outpatient attendance during and after participating in Novel Interventions in Children’s Healthcare (NICH), a systems intervention for children with chronic health conditions and their families.
By comparison, no improvements in care access were observed among BIPOC children who were not able to access the program because of insurance or other reasons, David V. Wagner, PhD, Associate Professor and NICH research director at Oregon Health & Science University, Portland, reported at the annual scientific sessions of the American Diabetes Association.
The findings demonstrate a need for intensive, home-based services that aim to correct health inequities, said Dr. Wagner, who presented the findings along with Winniebhelle Cadiz, a scholar in the BUILD EXITO undergraduate research training program at Portland (Ore.) State University.
The NICH program hinges on trained interventionists who visit families at home, attend clinic visits, and work with schools and other contacts to help solve problems that keep children from following medical instructions, according to a program description.
“Families report having somebody by their side to help them navigate the system, address the transportation difficulties experienced, and help them and build that relationship with their health care provider seems to be hugely influential in terms of helping them navigate and access care,” Dr. Wagner said in a presentation of the study.
A NICH for youths with chronic health conditions
The NICH program differs from some other programs that have been developed in an attempt to improve health outcomes among youths in the community, according to Dr. Wagner.
“Many of the programs that exist out there are often piloted on, and seemingly built for, those who have more resources,” he said in his presentation. “Those who are in greatest need often have difficulty accessing and responding to the services.”
NICH doesn’t take the place of existing services, but is “an addition to the continuum of care” for youths and families who are struggling because of lack of resources or marginalization in the health care system, Dr. Wagner said.
While NICH is not specific to any one chronic health condition, several previous investigations have specifically looked at the impact of the NICH program on access to care in youths with type 1 diabetes.
Youths participating in the program for a year had fewer ED visits, including fewer visits with diabetic ketoacidosis (DKA), as well as fewer and shorter admissions as compared with the year prior to participating in the program, Dr. Wagner said.
In another study, youths had fewer admissions for diabetes or DKA and less frequent pediatric ICU contact during the NICH program, as compared with before the program.
Another study showed that, while NICH had no impact overall on access to care among youths with type 1 diabetes, BIPOC youths had an improvement in the mean number of outpatient visits as compared with preprogram levels. However, because none of those studies included a control group, Dr. Wagner said, it remained unclear whether this systems intervention might improve outpatient access among youths with type 1 diabetes as compared with those who did not participate.
Intervention linked to increased BIPOC care access
The latest study includes 144 youths with type 1 diabetes referred for the program. The mean age was 13.7 years, 58% were female, and 81% were non-Hispanic White. While 51 youths were able to participate in NICH, the remaining 93 were not served by the program because of insurance denial or nonresponse, according to investigators.
While participation in the program made no difference in access to care overall, results of this study suggest NICH reduced access disparities among BIPOC youths, the investigators said.
Those BIPOC youth, 28 in total, had significantly worse access to care prior to referral. However, BIPOC youth participation in NICH was associated with improved attendance at endocrinology appointments and outpatient attendance overall.
A mean change of 1.9 more appointments per year was seen among BIPOC youth who participated in NICH, compared with a mean decrease of 0.5 appointments per year among BIPOC youth not served by the program (P = .03), according to the study abstract.
Prior to NICH participation, outpatient attendance among BIPOC youths was about 2.5 visits per year, data presented by the investigators show.
Systemic changes needed
This study is representative of systemic changes that are needed to improve access to quality care for BIPOC youth, according Cynthia E. Muñoz, PhD, MPH, ADA’s president of health care and education.
“We know that there are increased risks for poor health outcomes for these children and youths, and we know that there is a risk for mental health and psychosocial challenges for youth from these communities,” said Dr. Muñoz, a bilingual licensed psychologist and assistant professor of clinical pediatrics at the University of Southern California, Los Angeles.
In his presentation, Dr. Wagner said lumping racial and ethnic minority participants under a single BIPOC header probably wasn’t ideal because of the diversity and differences among racial and ethnic minorities. However, it was necessary in this particular study because of limited sample size.
Dr. Wagner and coauthors disclosed no conflicts of interest related to the research, which was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
FROM ADA 2021
Type 1 diabetes amputation rates fall in Sweden, rise in U.S.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
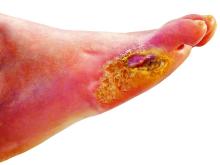
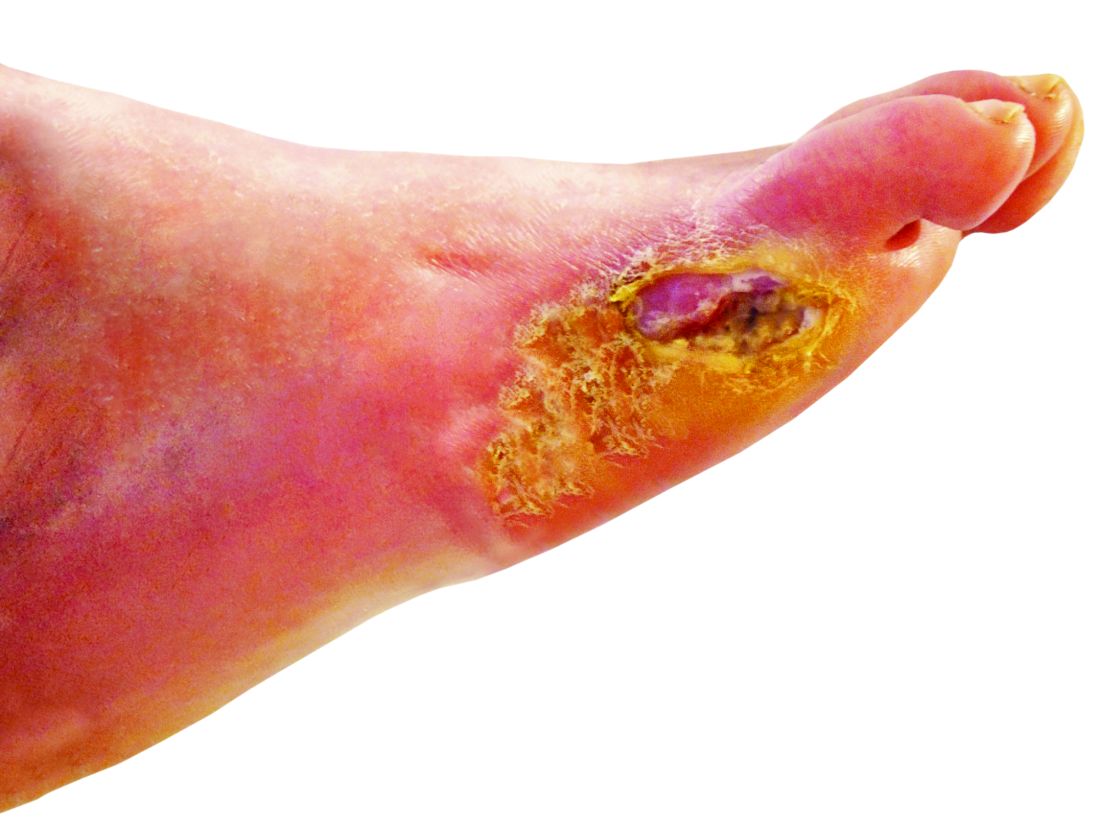
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
FROM ADA 2020
SUSTAIN FORTE: Higher-dose semaglutide safely boosts glycemic control, weight loss
Accumulating evidence shows that
Just weeks after the Food and Drug Administration approved an increased, 2.4-mg/week dose of semaglutide (Wegovy) for the indication of weight loss, results from a new randomized study with 961 patients that directly compared the standard 1.0-mg weekly dose for glycemic control with a 2.0-mg weekly dose showed that, over 40 weeks, the higher dose produced modest incremental improvements in both A1c reduction and weight loss while maintaining safety.
“Once weekly 2.0-mg subcutaneous semaglutide [Ozempic] was superior to 1.0 mg in reducing A1c, with greater weight loss and a similar safety profile,” Juan P. Frias, MD, said at the annual scientific sessions of the American Diabetes Association while presenting results of the SUSTAIN FORTE trial.
Average impact of the increased efficacy was measured. In the study’s “treatment policy estimand” analysis (considered equivalent to an intention-to-treat analysis), the primary endpoint of the cut in average A1c fell by a further 0.18% among patients on the higher dose, compared with the lower-dose arm, a significant difference in patients who entered the study with an average A1c of 8.9%. The average incremental boost for weight loss on the higher dose was about 0.8 kg, a difference that just missed significance (P = .0535).
In the study’s “trial product estimand” analysis (which censors data when patients stop the study drug or add on rescue medications), the effects were slightly more robust. The 2-mg dose produced an average 0.23% incremental decrease in A1c, compared with 1 mg, and an average incremental 0.93-kg weight reduction, both significant, reported Dr. Frias, an endocrinologist and medical director of the National Research Institute in Los Angeles.
Dr. Frias highlighted that these modest average differences had a clinical impact for some patients. In the treatment product estimand analysis, the percentage of patients achieving an A1c level of less than 7.0% increased from 58% of those who received 1 mg semaglutide to 68% of those treated with 2 mg, and achievement of an A1c of less than 6.5% occurred in 39% of patients on 1 mg and in 52% of those on 2 mg.
A similar pattern existed for weight loss in the treatment product estimand. Weight loss of at least 5% happened in 51% of patients on the 1-mg dose and in 59% of those on the higher dose.
Gradual up-titration aids tolerance
“The GLP-1 receptor agonists have so many benefits, but we were concerned in the past about pushing the dose. We’ve learned more about how to do that so that patients can better tolerate it,” commented Robert A. Gabbay, MD, PhD, chief science and medicine officer of the ADA in Arlington, Va. “The challenge with the medications from this class has been tolerability.”
A key to minimizing adverse effects, especially gastrointestinal effects, from treatment with semaglutide and other GLP-1 receptor agonists has been more gradual up-titration to the target dose, Dr. Gabbay noted in an interview, and SUSTAIN FORTE took this approach. All patients started on a 0.25-mg injection of semaglutide once weekly for the first 4 weeks, followed by a 0.5-mg dose once weekly for 4 weeks, and then a 1.0 mg weekly dose. Patients in the arm randomized to receive 2.0 mg had one further dose escalation after receiving the 1.0-mg dose for 4 weeks.
The result was that gastrointestinal adverse effects occurred in 31% of patients maintained for 32 weeks on the 1-mg dose (with 40 total weeks of semaglutide treatment), and in 34% of patients who received the 2-mg dose for 28 weeks (and 40 total weeks of semaglutide treatment). Serious adverse events of all types occurred in 5% of patients in the 1-mg arm and in 4% of those on 2 mg. Total adverse events resulting in treatment discontinuation occurred in about 4.5% of patients in both arms, and discontinuations because of gastrointestinal effects occurred in about 3% of patients in both arms.
Severe hypoglycemia episodes occurred in 1 patient maintained on 1 mg weekly and in 2 patients in the 2-mg arm, while clinically significant episodes of hypoglycemia occurred in 18 patients on the 1-mg dose (4%) and in 12 of the patients on 2 mg (3%).
“It’s reassuring that the higher dose is tolerated,” commented Dr. Gabbay.
Several doses to choose from
SUSTAIN FORTE ran during 2019-2020 at about 125 centers in 10 countries, with roughly half the sites in the United States. It randomized adults with type 2 diabetes and an A1c of 8.0%-10.0% despite ongoing metformin treatment in all patients. Just over half the patients were also maintained on a sulfonylurea agent at entry. The enrolled patients had been diagnosed with diabetes for an average of about 10 years. They averaged 58 years of age, their body mass index averaged nearly 35 kg/m2, and about 58% were men.
The new evidence in support of a 2.0-mg weekly dose of semaglutide for patients with type 2 diabetes introduces a new wrinkle in a growing menu of dose options for this drug. On June 4, 2021, the FDA approved a weekly 2.4-mg dose of semaglutide for the indication of weight loss regardless of diabetes status in patients with a body mass index of 30 or higher (or in people at 27 or more with at least one weight-related comorbidity).
Dr. Gabbay suggested that, in practice, clinicians may focus more on treatment goals for individual patients rather than drug dose, especially with an agent that’s safer with slow dose titration.
In general, “clinicians establish a goal for each patient’s A1c; you use the drug dose that gets you there,” he observed.
SUSTAIN FORTE was sponsored by Novo Nordisk, the company that markets semaglutide. Dr. Frias has been a consultant to Novo Nordisk and numerous other companies, he has been a speaker on behalf of Lilly, Merck, and Sanofi, and he has received research funding from Novo Nordisk and numerous other companies. Dr. Gabbay had no relevant disclosures.
Accumulating evidence shows that
Just weeks after the Food and Drug Administration approved an increased, 2.4-mg/week dose of semaglutide (Wegovy) for the indication of weight loss, results from a new randomized study with 961 patients that directly compared the standard 1.0-mg weekly dose for glycemic control with a 2.0-mg weekly dose showed that, over 40 weeks, the higher dose produced modest incremental improvements in both A1c reduction and weight loss while maintaining safety.
“Once weekly 2.0-mg subcutaneous semaglutide [Ozempic] was superior to 1.0 mg in reducing A1c, with greater weight loss and a similar safety profile,” Juan P. Frias, MD, said at the annual scientific sessions of the American Diabetes Association while presenting results of the SUSTAIN FORTE trial.
Average impact of the increased efficacy was measured. In the study’s “treatment policy estimand” analysis (considered equivalent to an intention-to-treat analysis), the primary endpoint of the cut in average A1c fell by a further 0.18% among patients on the higher dose, compared with the lower-dose arm, a significant difference in patients who entered the study with an average A1c of 8.9%. The average incremental boost for weight loss on the higher dose was about 0.8 kg, a difference that just missed significance (P = .0535).
In the study’s “trial product estimand” analysis (which censors data when patients stop the study drug or add on rescue medications), the effects were slightly more robust. The 2-mg dose produced an average 0.23% incremental decrease in A1c, compared with 1 mg, and an average incremental 0.93-kg weight reduction, both significant, reported Dr. Frias, an endocrinologist and medical director of the National Research Institute in Los Angeles.
Dr. Frias highlighted that these modest average differences had a clinical impact for some patients. In the treatment product estimand analysis, the percentage of patients achieving an A1c level of less than 7.0% increased from 58% of those who received 1 mg semaglutide to 68% of those treated with 2 mg, and achievement of an A1c of less than 6.5% occurred in 39% of patients on 1 mg and in 52% of those on 2 mg.
A similar pattern existed for weight loss in the treatment product estimand. Weight loss of at least 5% happened in 51% of patients on the 1-mg dose and in 59% of those on the higher dose.
Gradual up-titration aids tolerance
“The GLP-1 receptor agonists have so many benefits, but we were concerned in the past about pushing the dose. We’ve learned more about how to do that so that patients can better tolerate it,” commented Robert A. Gabbay, MD, PhD, chief science and medicine officer of the ADA in Arlington, Va. “The challenge with the medications from this class has been tolerability.”
A key to minimizing adverse effects, especially gastrointestinal effects, from treatment with semaglutide and other GLP-1 receptor agonists has been more gradual up-titration to the target dose, Dr. Gabbay noted in an interview, and SUSTAIN FORTE took this approach. All patients started on a 0.25-mg injection of semaglutide once weekly for the first 4 weeks, followed by a 0.5-mg dose once weekly for 4 weeks, and then a 1.0 mg weekly dose. Patients in the arm randomized to receive 2.0 mg had one further dose escalation after receiving the 1.0-mg dose for 4 weeks.
The result was that gastrointestinal adverse effects occurred in 31% of patients maintained for 32 weeks on the 1-mg dose (with 40 total weeks of semaglutide treatment), and in 34% of patients who received the 2-mg dose for 28 weeks (and 40 total weeks of semaglutide treatment). Serious adverse events of all types occurred in 5% of patients in the 1-mg arm and in 4% of those on 2 mg. Total adverse events resulting in treatment discontinuation occurred in about 4.5% of patients in both arms, and discontinuations because of gastrointestinal effects occurred in about 3% of patients in both arms.
Severe hypoglycemia episodes occurred in 1 patient maintained on 1 mg weekly and in 2 patients in the 2-mg arm, while clinically significant episodes of hypoglycemia occurred in 18 patients on the 1-mg dose (4%) and in 12 of the patients on 2 mg (3%).
“It’s reassuring that the higher dose is tolerated,” commented Dr. Gabbay.
Several doses to choose from
SUSTAIN FORTE ran during 2019-2020 at about 125 centers in 10 countries, with roughly half the sites in the United States. It randomized adults with type 2 diabetes and an A1c of 8.0%-10.0% despite ongoing metformin treatment in all patients. Just over half the patients were also maintained on a sulfonylurea agent at entry. The enrolled patients had been diagnosed with diabetes for an average of about 10 years. They averaged 58 years of age, their body mass index averaged nearly 35 kg/m2, and about 58% were men.
The new evidence in support of a 2.0-mg weekly dose of semaglutide for patients with type 2 diabetes introduces a new wrinkle in a growing menu of dose options for this drug. On June 4, 2021, the FDA approved a weekly 2.4-mg dose of semaglutide for the indication of weight loss regardless of diabetes status in patients with a body mass index of 30 or higher (or in people at 27 or more with at least one weight-related comorbidity).
Dr. Gabbay suggested that, in practice, clinicians may focus more on treatment goals for individual patients rather than drug dose, especially with an agent that’s safer with slow dose titration.
In general, “clinicians establish a goal for each patient’s A1c; you use the drug dose that gets you there,” he observed.
SUSTAIN FORTE was sponsored by Novo Nordisk, the company that markets semaglutide. Dr. Frias has been a consultant to Novo Nordisk and numerous other companies, he has been a speaker on behalf of Lilly, Merck, and Sanofi, and he has received research funding from Novo Nordisk and numerous other companies. Dr. Gabbay had no relevant disclosures.
Accumulating evidence shows that
Just weeks after the Food and Drug Administration approved an increased, 2.4-mg/week dose of semaglutide (Wegovy) for the indication of weight loss, results from a new randomized study with 961 patients that directly compared the standard 1.0-mg weekly dose for glycemic control with a 2.0-mg weekly dose showed that, over 40 weeks, the higher dose produced modest incremental improvements in both A1c reduction and weight loss while maintaining safety.
“Once weekly 2.0-mg subcutaneous semaglutide [Ozempic] was superior to 1.0 mg in reducing A1c, with greater weight loss and a similar safety profile,” Juan P. Frias, MD, said at the annual scientific sessions of the American Diabetes Association while presenting results of the SUSTAIN FORTE trial.
Average impact of the increased efficacy was measured. In the study’s “treatment policy estimand” analysis (considered equivalent to an intention-to-treat analysis), the primary endpoint of the cut in average A1c fell by a further 0.18% among patients on the higher dose, compared with the lower-dose arm, a significant difference in patients who entered the study with an average A1c of 8.9%. The average incremental boost for weight loss on the higher dose was about 0.8 kg, a difference that just missed significance (P = .0535).
In the study’s “trial product estimand” analysis (which censors data when patients stop the study drug or add on rescue medications), the effects were slightly more robust. The 2-mg dose produced an average 0.23% incremental decrease in A1c, compared with 1 mg, and an average incremental 0.93-kg weight reduction, both significant, reported Dr. Frias, an endocrinologist and medical director of the National Research Institute in Los Angeles.
Dr. Frias highlighted that these modest average differences had a clinical impact for some patients. In the treatment product estimand analysis, the percentage of patients achieving an A1c level of less than 7.0% increased from 58% of those who received 1 mg semaglutide to 68% of those treated with 2 mg, and achievement of an A1c of less than 6.5% occurred in 39% of patients on 1 mg and in 52% of those on 2 mg.
A similar pattern existed for weight loss in the treatment product estimand. Weight loss of at least 5% happened in 51% of patients on the 1-mg dose and in 59% of those on the higher dose.
Gradual up-titration aids tolerance
“The GLP-1 receptor agonists have so many benefits, but we were concerned in the past about pushing the dose. We’ve learned more about how to do that so that patients can better tolerate it,” commented Robert A. Gabbay, MD, PhD, chief science and medicine officer of the ADA in Arlington, Va. “The challenge with the medications from this class has been tolerability.”
A key to minimizing adverse effects, especially gastrointestinal effects, from treatment with semaglutide and other GLP-1 receptor agonists has been more gradual up-titration to the target dose, Dr. Gabbay noted in an interview, and SUSTAIN FORTE took this approach. All patients started on a 0.25-mg injection of semaglutide once weekly for the first 4 weeks, followed by a 0.5-mg dose once weekly for 4 weeks, and then a 1.0 mg weekly dose. Patients in the arm randomized to receive 2.0 mg had one further dose escalation after receiving the 1.0-mg dose for 4 weeks.
The result was that gastrointestinal adverse effects occurred in 31% of patients maintained for 32 weeks on the 1-mg dose (with 40 total weeks of semaglutide treatment), and in 34% of patients who received the 2-mg dose for 28 weeks (and 40 total weeks of semaglutide treatment). Serious adverse events of all types occurred in 5% of patients in the 1-mg arm and in 4% of those on 2 mg. Total adverse events resulting in treatment discontinuation occurred in about 4.5% of patients in both arms, and discontinuations because of gastrointestinal effects occurred in about 3% of patients in both arms.
Severe hypoglycemia episodes occurred in 1 patient maintained on 1 mg weekly and in 2 patients in the 2-mg arm, while clinically significant episodes of hypoglycemia occurred in 18 patients on the 1-mg dose (4%) and in 12 of the patients on 2 mg (3%).
“It’s reassuring that the higher dose is tolerated,” commented Dr. Gabbay.
Several doses to choose from
SUSTAIN FORTE ran during 2019-2020 at about 125 centers in 10 countries, with roughly half the sites in the United States. It randomized adults with type 2 diabetes and an A1c of 8.0%-10.0% despite ongoing metformin treatment in all patients. Just over half the patients were also maintained on a sulfonylurea agent at entry. The enrolled patients had been diagnosed with diabetes for an average of about 10 years. They averaged 58 years of age, their body mass index averaged nearly 35 kg/m2, and about 58% were men.
The new evidence in support of a 2.0-mg weekly dose of semaglutide for patients with type 2 diabetes introduces a new wrinkle in a growing menu of dose options for this drug. On June 4, 2021, the FDA approved a weekly 2.4-mg dose of semaglutide for the indication of weight loss regardless of diabetes status in patients with a body mass index of 30 or higher (or in people at 27 or more with at least one weight-related comorbidity).
Dr. Gabbay suggested that, in practice, clinicians may focus more on treatment goals for individual patients rather than drug dose, especially with an agent that’s safer with slow dose titration.
In general, “clinicians establish a goal for each patient’s A1c; you use the drug dose that gets you there,” he observed.
SUSTAIN FORTE was sponsored by Novo Nordisk, the company that markets semaglutide. Dr. Frias has been a consultant to Novo Nordisk and numerous other companies, he has been a speaker on behalf of Lilly, Merck, and Sanofi, and he has received research funding from Novo Nordisk and numerous other companies. Dr. Gabbay had no relevant disclosures.
FROM ADA 2021
‘Staggering’ doubling of type 2 diabetes in children during pandemic
The incidence of type 2 diabetes in children appears to have doubled during the COVID-19 pandemic, data from two new U.S. studies suggest, with the lead investigator of one saying she was “surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Findings from the two separate retrospective chart reviews – one conducted in Washington, D.C., and the other in Baton Rouge, La. – were presented June 25 at the annual scientific sessions of the American Diabetes Association.
Although the two studies differed somewhat in the clinical parameters examined, both revealed a similar doubling of the rates of hospitalizations for type 2 diabetes among youth during 2020, compared with the same time period in 2019, as well as greater severity of metabolic disturbance.
And, as has been previously described with type 2 diabetes in youth, African American ethnicity predominated in both cohorts.
“Although we could not assess the cause of the increases in type 2 diabetes from our data, these disparities suggest that indirect effects of social distancing measures, including school closure and unemployment, are placing undue burden on underserved communities. Decreases in well-child care and fears of seeking medical care during the pandemic may have also contributed,” lead investigator of one of the studies, pediatric endocrinologist Brynn E. Marks, MD, Children’s National Hospital, Washington, said in an interview.
More hospitalizations, racial disparities aggravated by COVID-19
Lead author of the other study, Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, said in an interview: “Since the pandemic, our data suggest that more children may be diagnosed with type 2 diabetes and may require hospitalization when they are diagnosed. Looking at both datasets, there appears to be a racial disparity in type 2 diabetes diagnoses that has only been exacerbated by the COVID-19 pandemic.”
Of concern, Dr. Hsia said, “The incidence rate of type 2 diabetes in children was already on the rise before the pandemic. While there may be a brief leveling off now that children are getting regular health care and going back to school in person, I believe these rates will continue to rise especially in light of the childhood obesity rates not improving.”
Their dataset captured all youth who were newly diagnosed with type 2 diabetes during the first full year of the COVID-19 pandemic, from March 11, 2020, to March 10, 2021, and compared those data with the time period from March 11, 2019, to March 10, 2020.
During the pandemic, the number of cases of type 2 diabetes increased by 182%, from 50 in 2019 to 141 in 2020. The average age at diagnosis was about 14 years in both time periods.
In the prepandemic period, 18 (36%) diagnosed with type 2 diabetes required inpatient admission, compared with 85 (60.3%) during the pandemic. At Children’s National, youth with suspected new-onset type 2 diabetes aren’t typically hospitalized unless they have severe hyperglycemia, ketosis, or are unable to schedule urgent outpatient follow-up, Dr. Marks noted.
The proportions of youth with new-onset type 2 diabetes who presented in diabetic ketoacidosis (DKA) rose from 2 (4%) prepandemic to 33 (23.4%) during the pandemic. Presentation with hyperosmolar DKA rose from 0 to 13 (9.2%).
However, during the pandemic only five youth were actively infected with SARS-CoV-2 at the time of type 2 diabetes diagnosis among the 90 tested.
Dr. Marks said: “We believe the increase in inpatient admissions was due to more severe presentation during the pandemic. ...We were surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Shift in diagnoses to type 2 diabetes
The pandemic also appears to have shifted the proportion of youth diagnosed with type 2 diabetes, compared with type 1 diabetes. Whereas 24% of youth with new-onset diabetes prepandemic had type 2 diabetes and the rest had type 1 diabetes, during pandemic the proportion with type 2 diabetes rose to 44%.
“Rates of type 2 diabetes rose steadily at a rate of 1.45 cases per month throughout the course of the pandemic, suggesting a cumulative effect of the indirect effects of social distancing measures,” Dr. Marks said.
Furthermore, she added, whereas 60% of youth diagnosed with type 2 diabetes before the pandemic were female, the rate fell to 40% during the pandemic. This trend might be because of activity levels in that, while male adolescents are typically more active, rates of exercise fell in both sexes during the pandemic but declined more sharply in males such that activity levels between the sexes became equal.
Although type 2 diabetes in youth has always been more common in ethnic minorities, the pandemic appears to have exacerbated these disparities.
While 58% of youth diagnosed with type 2 diabetes prepandemic identified as non-Hispanic Black, that proportion rose to 76.7% during the pandemic. Among Black youth with new-onset type 2 diabetes, 31 of 33 presented in DKA, and 12 of the 13 who presented in hyperosmolar DKA during the pandemic were Black.
“Strategies to promote health equity and address the undue burden of the COVID-19 pandemic on underserved communities must be developed to avoid worsening disparities and long-term health outcomes,” Dr. Marks said.
‘A microcosm’: Similar findings in a smaller population
Dr. Hsia and colleagues looked at a smaller number of patients in a shorter time period. In March–December 2019, the hospitalization rate for new-onset type 2 diabetes was 0.27% (8 out of 2,964 hospitalizations), compared with 0.62% (17 out of 2,729 hospitalizations) during the same period in 2020 (P < .048) – also more than a doubling. Age at admission, sex, and body mass index didn’t differ between the two groups.
Criteria for DKA were met by three children in 2019 versus eight in 2020, and hyperosmolar hyperglycemic syndrome in zero versus two, respectively. Mean hemoglobin A1c on admission was 12.4% in 2019 versus 13.1% in 2020 (P = .59), and mean serum glucose was 441 mg/dL versus 669 mg/dL (P = .14), respectively. Serum osmolality on admission was 314 mmol/kg in 2019 versus 335 mmol/kg in 2020 (P = .19).
“Clinically speaking the differences in the lab values were significant, but we did not have enough numbers ... to see a statistically significant difference. I think by looking at more centers, our site likely represents a microcosm of what is happening across the country,” Dr. Hsia said.
In 2019, 7 of the 8 children were African American, as were 16 of the 17 children in 2020. The other single child in each group was White.
Dr. Hsia said: “Larger studies that include more patients are needed to confirm our initial findings. More research is needed to understand why this increasing trend of type 2 diabetes diagnoses in children may be occurring [and] to better understand how stay-at-home orders and other restrictions due to COVID-19 have worsened risk factors for type 2 diabetes.”
“These include decreased physical activity, more screen time, disturbed sleep, and increased intake of processed foods, which can all lead to weight gain,” he concluded.
Dr. Marks reported receiving research support from Tandem, Dexcom, and the Cystic Fibrosis Foundation. Dr. Hsia reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of type 2 diabetes in children appears to have doubled during the COVID-19 pandemic, data from two new U.S. studies suggest, with the lead investigator of one saying she was “surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Findings from the two separate retrospective chart reviews – one conducted in Washington, D.C., and the other in Baton Rouge, La. – were presented June 25 at the annual scientific sessions of the American Diabetes Association.
Although the two studies differed somewhat in the clinical parameters examined, both revealed a similar doubling of the rates of hospitalizations for type 2 diabetes among youth during 2020, compared with the same time period in 2019, as well as greater severity of metabolic disturbance.
And, as has been previously described with type 2 diabetes in youth, African American ethnicity predominated in both cohorts.
“Although we could not assess the cause of the increases in type 2 diabetes from our data, these disparities suggest that indirect effects of social distancing measures, including school closure and unemployment, are placing undue burden on underserved communities. Decreases in well-child care and fears of seeking medical care during the pandemic may have also contributed,” lead investigator of one of the studies, pediatric endocrinologist Brynn E. Marks, MD, Children’s National Hospital, Washington, said in an interview.
More hospitalizations, racial disparities aggravated by COVID-19
Lead author of the other study, Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, said in an interview: “Since the pandemic, our data suggest that more children may be diagnosed with type 2 diabetes and may require hospitalization when they are diagnosed. Looking at both datasets, there appears to be a racial disparity in type 2 diabetes diagnoses that has only been exacerbated by the COVID-19 pandemic.”
Of concern, Dr. Hsia said, “The incidence rate of type 2 diabetes in children was already on the rise before the pandemic. While there may be a brief leveling off now that children are getting regular health care and going back to school in person, I believe these rates will continue to rise especially in light of the childhood obesity rates not improving.”
Their dataset captured all youth who were newly diagnosed with type 2 diabetes during the first full year of the COVID-19 pandemic, from March 11, 2020, to March 10, 2021, and compared those data with the time period from March 11, 2019, to March 10, 2020.
During the pandemic, the number of cases of type 2 diabetes increased by 182%, from 50 in 2019 to 141 in 2020. The average age at diagnosis was about 14 years in both time periods.
In the prepandemic period, 18 (36%) diagnosed with type 2 diabetes required inpatient admission, compared with 85 (60.3%) during the pandemic. At Children’s National, youth with suspected new-onset type 2 diabetes aren’t typically hospitalized unless they have severe hyperglycemia, ketosis, or are unable to schedule urgent outpatient follow-up, Dr. Marks noted.
The proportions of youth with new-onset type 2 diabetes who presented in diabetic ketoacidosis (DKA) rose from 2 (4%) prepandemic to 33 (23.4%) during the pandemic. Presentation with hyperosmolar DKA rose from 0 to 13 (9.2%).
However, during the pandemic only five youth were actively infected with SARS-CoV-2 at the time of type 2 diabetes diagnosis among the 90 tested.
Dr. Marks said: “We believe the increase in inpatient admissions was due to more severe presentation during the pandemic. ...We were surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Shift in diagnoses to type 2 diabetes
The pandemic also appears to have shifted the proportion of youth diagnosed with type 2 diabetes, compared with type 1 diabetes. Whereas 24% of youth with new-onset diabetes prepandemic had type 2 diabetes and the rest had type 1 diabetes, during pandemic the proportion with type 2 diabetes rose to 44%.
“Rates of type 2 diabetes rose steadily at a rate of 1.45 cases per month throughout the course of the pandemic, suggesting a cumulative effect of the indirect effects of social distancing measures,” Dr. Marks said.
Furthermore, she added, whereas 60% of youth diagnosed with type 2 diabetes before the pandemic were female, the rate fell to 40% during the pandemic. This trend might be because of activity levels in that, while male adolescents are typically more active, rates of exercise fell in both sexes during the pandemic but declined more sharply in males such that activity levels between the sexes became equal.
Although type 2 diabetes in youth has always been more common in ethnic minorities, the pandemic appears to have exacerbated these disparities.
While 58% of youth diagnosed with type 2 diabetes prepandemic identified as non-Hispanic Black, that proportion rose to 76.7% during the pandemic. Among Black youth with new-onset type 2 diabetes, 31 of 33 presented in DKA, and 12 of the 13 who presented in hyperosmolar DKA during the pandemic were Black.
“Strategies to promote health equity and address the undue burden of the COVID-19 pandemic on underserved communities must be developed to avoid worsening disparities and long-term health outcomes,” Dr. Marks said.
‘A microcosm’: Similar findings in a smaller population
Dr. Hsia and colleagues looked at a smaller number of patients in a shorter time period. In March–December 2019, the hospitalization rate for new-onset type 2 diabetes was 0.27% (8 out of 2,964 hospitalizations), compared with 0.62% (17 out of 2,729 hospitalizations) during the same period in 2020 (P < .048) – also more than a doubling. Age at admission, sex, and body mass index didn’t differ between the two groups.
Criteria for DKA were met by three children in 2019 versus eight in 2020, and hyperosmolar hyperglycemic syndrome in zero versus two, respectively. Mean hemoglobin A1c on admission was 12.4% in 2019 versus 13.1% in 2020 (P = .59), and mean serum glucose was 441 mg/dL versus 669 mg/dL (P = .14), respectively. Serum osmolality on admission was 314 mmol/kg in 2019 versus 335 mmol/kg in 2020 (P = .19).
“Clinically speaking the differences in the lab values were significant, but we did not have enough numbers ... to see a statistically significant difference. I think by looking at more centers, our site likely represents a microcosm of what is happening across the country,” Dr. Hsia said.
In 2019, 7 of the 8 children were African American, as were 16 of the 17 children in 2020. The other single child in each group was White.
Dr. Hsia said: “Larger studies that include more patients are needed to confirm our initial findings. More research is needed to understand why this increasing trend of type 2 diabetes diagnoses in children may be occurring [and] to better understand how stay-at-home orders and other restrictions due to COVID-19 have worsened risk factors for type 2 diabetes.”
“These include decreased physical activity, more screen time, disturbed sleep, and increased intake of processed foods, which can all lead to weight gain,” he concluded.
Dr. Marks reported receiving research support from Tandem, Dexcom, and the Cystic Fibrosis Foundation. Dr. Hsia reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of type 2 diabetes in children appears to have doubled during the COVID-19 pandemic, data from two new U.S. studies suggest, with the lead investigator of one saying she was “surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Findings from the two separate retrospective chart reviews – one conducted in Washington, D.C., and the other in Baton Rouge, La. – were presented June 25 at the annual scientific sessions of the American Diabetes Association.
Although the two studies differed somewhat in the clinical parameters examined, both revealed a similar doubling of the rates of hospitalizations for type 2 diabetes among youth during 2020, compared with the same time period in 2019, as well as greater severity of metabolic disturbance.
And, as has been previously described with type 2 diabetes in youth, African American ethnicity predominated in both cohorts.
“Although we could not assess the cause of the increases in type 2 diabetes from our data, these disparities suggest that indirect effects of social distancing measures, including school closure and unemployment, are placing undue burden on underserved communities. Decreases in well-child care and fears of seeking medical care during the pandemic may have also contributed,” lead investigator of one of the studies, pediatric endocrinologist Brynn E. Marks, MD, Children’s National Hospital, Washington, said in an interview.
More hospitalizations, racial disparities aggravated by COVID-19
Lead author of the other study, Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, said in an interview: “Since the pandemic, our data suggest that more children may be diagnosed with type 2 diabetes and may require hospitalization when they are diagnosed. Looking at both datasets, there appears to be a racial disparity in type 2 diabetes diagnoses that has only been exacerbated by the COVID-19 pandemic.”
Of concern, Dr. Hsia said, “The incidence rate of type 2 diabetes in children was already on the rise before the pandemic. While there may be a brief leveling off now that children are getting regular health care and going back to school in person, I believe these rates will continue to rise especially in light of the childhood obesity rates not improving.”
Their dataset captured all youth who were newly diagnosed with type 2 diabetes during the first full year of the COVID-19 pandemic, from March 11, 2020, to March 10, 2021, and compared those data with the time period from March 11, 2019, to March 10, 2020.
During the pandemic, the number of cases of type 2 diabetes increased by 182%, from 50 in 2019 to 141 in 2020. The average age at diagnosis was about 14 years in both time periods.
In the prepandemic period, 18 (36%) diagnosed with type 2 diabetes required inpatient admission, compared with 85 (60.3%) during the pandemic. At Children’s National, youth with suspected new-onset type 2 diabetes aren’t typically hospitalized unless they have severe hyperglycemia, ketosis, or are unable to schedule urgent outpatient follow-up, Dr. Marks noted.
The proportions of youth with new-onset type 2 diabetes who presented in diabetic ketoacidosis (DKA) rose from 2 (4%) prepandemic to 33 (23.4%) during the pandemic. Presentation with hyperosmolar DKA rose from 0 to 13 (9.2%).
However, during the pandemic only five youth were actively infected with SARS-CoV-2 at the time of type 2 diabetes diagnosis among the 90 tested.
Dr. Marks said: “We believe the increase in inpatient admissions was due to more severe presentation during the pandemic. ...We were surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Shift in diagnoses to type 2 diabetes
The pandemic also appears to have shifted the proportion of youth diagnosed with type 2 diabetes, compared with type 1 diabetes. Whereas 24% of youth with new-onset diabetes prepandemic had type 2 diabetes and the rest had type 1 diabetes, during pandemic the proportion with type 2 diabetes rose to 44%.
“Rates of type 2 diabetes rose steadily at a rate of 1.45 cases per month throughout the course of the pandemic, suggesting a cumulative effect of the indirect effects of social distancing measures,” Dr. Marks said.
Furthermore, she added, whereas 60% of youth diagnosed with type 2 diabetes before the pandemic were female, the rate fell to 40% during the pandemic. This trend might be because of activity levels in that, while male adolescents are typically more active, rates of exercise fell in both sexes during the pandemic but declined more sharply in males such that activity levels between the sexes became equal.
Although type 2 diabetes in youth has always been more common in ethnic minorities, the pandemic appears to have exacerbated these disparities.
While 58% of youth diagnosed with type 2 diabetes prepandemic identified as non-Hispanic Black, that proportion rose to 76.7% during the pandemic. Among Black youth with new-onset type 2 diabetes, 31 of 33 presented in DKA, and 12 of the 13 who presented in hyperosmolar DKA during the pandemic were Black.
“Strategies to promote health equity and address the undue burden of the COVID-19 pandemic on underserved communities must be developed to avoid worsening disparities and long-term health outcomes,” Dr. Marks said.
‘A microcosm’: Similar findings in a smaller population
Dr. Hsia and colleagues looked at a smaller number of patients in a shorter time period. In March–December 2019, the hospitalization rate for new-onset type 2 diabetes was 0.27% (8 out of 2,964 hospitalizations), compared with 0.62% (17 out of 2,729 hospitalizations) during the same period in 2020 (P < .048) – also more than a doubling. Age at admission, sex, and body mass index didn’t differ between the two groups.
Criteria for DKA were met by three children in 2019 versus eight in 2020, and hyperosmolar hyperglycemic syndrome in zero versus two, respectively. Mean hemoglobin A1c on admission was 12.4% in 2019 versus 13.1% in 2020 (P = .59), and mean serum glucose was 441 mg/dL versus 669 mg/dL (P = .14), respectively. Serum osmolality on admission was 314 mmol/kg in 2019 versus 335 mmol/kg in 2020 (P = .19).
“Clinically speaking the differences in the lab values were significant, but we did not have enough numbers ... to see a statistically significant difference. I think by looking at more centers, our site likely represents a microcosm of what is happening across the country,” Dr. Hsia said.
In 2019, 7 of the 8 children were African American, as were 16 of the 17 children in 2020. The other single child in each group was White.
Dr. Hsia said: “Larger studies that include more patients are needed to confirm our initial findings. More research is needed to understand why this increasing trend of type 2 diabetes diagnoses in children may be occurring [and] to better understand how stay-at-home orders and other restrictions due to COVID-19 have worsened risk factors for type 2 diabetes.”
“These include decreased physical activity, more screen time, disturbed sleep, and increased intake of processed foods, which can all lead to weight gain,” he concluded.
Dr. Marks reported receiving research support from Tandem, Dexcom, and the Cystic Fibrosis Foundation. Dr. Hsia reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Stunning’ twincretin beats semaglutide for A1c, weight reduction in T2D
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
FROM ADA 2021
Unmanaged diabetes, high blood glucose tied to COVID-19 severity
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
FROM ADA 2020
Time-restricted eating ‘promising, but more data are needed’
Time-restricted eating – that is, reducing the number of hours a person is allowed to eat during the day – may produce a modest 1%-4% weight loss, even without cutting calories, early studies in humans suggest. But more research is needed to provide definitive evidence.
This type of intermittent fasting also appears to improve blood glucose, blood pressure, and oxidative stress, said Courtney M. Peterson, PhD, a researcher at the University of Alabama at Birmingham, summarizing what is known about the potential weight-loss strategy at the annual scientific sessions of the American Diabetes Association.
The best results were seen with early time-restricted eating (that is, ending the nighttime fasting early in the day) and allowing a person to eat 8-10 hours each day (for example, 8 a.m. to 4 p.m. or 8 a.m. to 6 p.m.), with fasting and only water allowed the remaining hours, she reported.
However, the 3 dozen or so studies in humans to date are mainly small, pilot, or single-arm studies lasting up to 3 months, and there are only three main randomized, controlled trials with 25 or more participants in each group.
Large trials with around 260 participants are needed, Dr. Peterson said, “before drawing definitive conclusions” about the weight-loss and cardiometabolic benefits of time-restricted eating.
Invited to comment, session chair Lisa S. Chow, MD, an associate professor of medicine in the endocrine and diabetes division at the University of Minnesota, Minneapolis, similarly said: “I think time-restricted eating is promising because of its simple message and noted weight-loss benefit, yet more data are needed.”
“Many uncertainties remain,” she added, “including the potential concern that time-restricted eating may be associated with lean [muscle] mass loss and identifying the populations most likely to benefit from time-restricted eating,” she said.
36 small studies, a review, a meta-analysis, 3 RCTs
There have been about three dozen small studies of time-restricted eating in humans, which examined 4- to 11-hour eating windows, Dr. Peterson explained.
A systematic review of 23 trials of time-restricted eating reported that, on average, participants lost 3% of their initial weight. And a meta-analysis of 19 trials in 475 participants found a –0.9 kg mean difference effect for weight loss.
However, those two analyses did not compare time-restricted eating with a control treatment, she stressed.
The largest randomized, controlled trial is a 12-week study in 271 adults with nonalcoholic fatty liver disease in China, Dr. Peterson said.
The researchers compared three groups:
- Alternate-day modified fasting: healthy meal provided.
- Time-restricted eating: 8-hour window, healthy meal provided.
- Control: 20% calorie reduction, no meal provided.
At 4 and 12 weeks, adults in the two treatment groups lost more weight than those in the control group, but “this was not a fair comparison” because of the lack of a provided meal in the control group, Dr. Peterson pointed out.
The next largest randomized, controlled study is the 12-week TREAT trial, published online in JAMA Internal Medicine in October 2020.
The researchers, from the University of California, San Francisco, randomized 116 adults into two groups:
- 8-hour time-restricted eating from noon to 8 p.m..
- Control: three meals/day.
Time-restricted eating did not lead to greater weight loss, compared with three structured meals a day, which was not surprising, Dr. Chow said, as “participants just reported whether they were engaged in time-restricted eating in a yes/no answer.”
Moreover, “there was no objective measure of their eating window. From our study, we showed that the extent of eating window restriction matters, not just time-restricted eating participation.”
Also, in TREAT, the eating window was noon to 8 p.m. (considered late for time-restricted eating), and the trial also allowed noncaloric beverages outside the window, whereas most studies only allow water and medications.
Lastly, TREAT showed that time-restricted eating reduced weight, compared with baseline, but the weight loss was not significant, compared with the control group, and there was a wide spread of effects (that is, some lost a lot of weight, others didn’t lose much weight).
“That being said, the JAMA Internal Medicine paper is the largest paper to date of time-restricted eating randomized versus control, so its findings need to be acknowledged and recognized,” Dr. Chow said.
Peterson reported that her group recently completed a 14-week intervention in 90 adults with obesity divided into two groups:
- Control: Continuous energy restriction, self-selected ≥ 12-hour window.
- Early time-restricted eating: 8-hour window from 7 a.m. to 3 p.m.
The findings will provide further insight into the benefits of time-restricted eating.
How might time-restricted eating lead to weight loss?
Dr. Peterson concluded by presenting data suggesting how time-restricted eating may induce weight loss.
In a 4-day crossover study in 11 overweight adults, time-restricted eating did not affect energy expenditure, but it lessened swings in subjective hunger, improved appetite hormones including ghrelin, and increased fat oxidation.
Most trials have reported that time-restricted eating improves one or more cardiometabolic endpoints, she noted.
Early time-restricted eating was associated with improved insulin sensitivity and secretion, blood pressure, and oxidative stress, but not better lipid levels.
In contrast, compared with eating 3 meals/day (control), late time-restricted eating (eating 1 meal/day from 5 p.m. to 9 p.m.) was associated with worsened cardiometabolic health (glucose, insulin, blood pressure, and lipid levels) in an 8-week crossover study in 15 participants.
Dr. Peterson and Dr. Chow reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time-restricted eating – that is, reducing the number of hours a person is allowed to eat during the day – may produce a modest 1%-4% weight loss, even without cutting calories, early studies in humans suggest. But more research is needed to provide definitive evidence.
This type of intermittent fasting also appears to improve blood glucose, blood pressure, and oxidative stress, said Courtney M. Peterson, PhD, a researcher at the University of Alabama at Birmingham, summarizing what is known about the potential weight-loss strategy at the annual scientific sessions of the American Diabetes Association.
The best results were seen with early time-restricted eating (that is, ending the nighttime fasting early in the day) and allowing a person to eat 8-10 hours each day (for example, 8 a.m. to 4 p.m. or 8 a.m. to 6 p.m.), with fasting and only water allowed the remaining hours, she reported.
However, the 3 dozen or so studies in humans to date are mainly small, pilot, or single-arm studies lasting up to 3 months, and there are only three main randomized, controlled trials with 25 or more participants in each group.
Large trials with around 260 participants are needed, Dr. Peterson said, “before drawing definitive conclusions” about the weight-loss and cardiometabolic benefits of time-restricted eating.
Invited to comment, session chair Lisa S. Chow, MD, an associate professor of medicine in the endocrine and diabetes division at the University of Minnesota, Minneapolis, similarly said: “I think time-restricted eating is promising because of its simple message and noted weight-loss benefit, yet more data are needed.”
“Many uncertainties remain,” she added, “including the potential concern that time-restricted eating may be associated with lean [muscle] mass loss and identifying the populations most likely to benefit from time-restricted eating,” she said.
36 small studies, a review, a meta-analysis, 3 RCTs
There have been about three dozen small studies of time-restricted eating in humans, which examined 4- to 11-hour eating windows, Dr. Peterson explained.
A systematic review of 23 trials of time-restricted eating reported that, on average, participants lost 3% of their initial weight. And a meta-analysis of 19 trials in 475 participants found a –0.9 kg mean difference effect for weight loss.
However, those two analyses did not compare time-restricted eating with a control treatment, she stressed.
The largest randomized, controlled trial is a 12-week study in 271 adults with nonalcoholic fatty liver disease in China, Dr. Peterson said.
The researchers compared three groups:
- Alternate-day modified fasting: healthy meal provided.
- Time-restricted eating: 8-hour window, healthy meal provided.
- Control: 20% calorie reduction, no meal provided.
At 4 and 12 weeks, adults in the two treatment groups lost more weight than those in the control group, but “this was not a fair comparison” because of the lack of a provided meal in the control group, Dr. Peterson pointed out.
The next largest randomized, controlled study is the 12-week TREAT trial, published online in JAMA Internal Medicine in October 2020.
The researchers, from the University of California, San Francisco, randomized 116 adults into two groups:
- 8-hour time-restricted eating from noon to 8 p.m..
- Control: three meals/day.
Time-restricted eating did not lead to greater weight loss, compared with three structured meals a day, which was not surprising, Dr. Chow said, as “participants just reported whether they were engaged in time-restricted eating in a yes/no answer.”
Moreover, “there was no objective measure of their eating window. From our study, we showed that the extent of eating window restriction matters, not just time-restricted eating participation.”
Also, in TREAT, the eating window was noon to 8 p.m. (considered late for time-restricted eating), and the trial also allowed noncaloric beverages outside the window, whereas most studies only allow water and medications.
Lastly, TREAT showed that time-restricted eating reduced weight, compared with baseline, but the weight loss was not significant, compared with the control group, and there was a wide spread of effects (that is, some lost a lot of weight, others didn’t lose much weight).
“That being said, the JAMA Internal Medicine paper is the largest paper to date of time-restricted eating randomized versus control, so its findings need to be acknowledged and recognized,” Dr. Chow said.
Peterson reported that her group recently completed a 14-week intervention in 90 adults with obesity divided into two groups:
- Control: Continuous energy restriction, self-selected ≥ 12-hour window.
- Early time-restricted eating: 8-hour window from 7 a.m. to 3 p.m.
The findings will provide further insight into the benefits of time-restricted eating.
How might time-restricted eating lead to weight loss?
Dr. Peterson concluded by presenting data suggesting how time-restricted eating may induce weight loss.
In a 4-day crossover study in 11 overweight adults, time-restricted eating did not affect energy expenditure, but it lessened swings in subjective hunger, improved appetite hormones including ghrelin, and increased fat oxidation.
Most trials have reported that time-restricted eating improves one or more cardiometabolic endpoints, she noted.
Early time-restricted eating was associated with improved insulin sensitivity and secretion, blood pressure, and oxidative stress, but not better lipid levels.
In contrast, compared with eating 3 meals/day (control), late time-restricted eating (eating 1 meal/day from 5 p.m. to 9 p.m.) was associated with worsened cardiometabolic health (glucose, insulin, blood pressure, and lipid levels) in an 8-week crossover study in 15 participants.
Dr. Peterson and Dr. Chow reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time-restricted eating – that is, reducing the number of hours a person is allowed to eat during the day – may produce a modest 1%-4% weight loss, even without cutting calories, early studies in humans suggest. But more research is needed to provide definitive evidence.
This type of intermittent fasting also appears to improve blood glucose, blood pressure, and oxidative stress, said Courtney M. Peterson, PhD, a researcher at the University of Alabama at Birmingham, summarizing what is known about the potential weight-loss strategy at the annual scientific sessions of the American Diabetes Association.
The best results were seen with early time-restricted eating (that is, ending the nighttime fasting early in the day) and allowing a person to eat 8-10 hours each day (for example, 8 a.m. to 4 p.m. or 8 a.m. to 6 p.m.), with fasting and only water allowed the remaining hours, she reported.
However, the 3 dozen or so studies in humans to date are mainly small, pilot, or single-arm studies lasting up to 3 months, and there are only three main randomized, controlled trials with 25 or more participants in each group.
Large trials with around 260 participants are needed, Dr. Peterson said, “before drawing definitive conclusions” about the weight-loss and cardiometabolic benefits of time-restricted eating.
Invited to comment, session chair Lisa S. Chow, MD, an associate professor of medicine in the endocrine and diabetes division at the University of Minnesota, Minneapolis, similarly said: “I think time-restricted eating is promising because of its simple message and noted weight-loss benefit, yet more data are needed.”
“Many uncertainties remain,” she added, “including the potential concern that time-restricted eating may be associated with lean [muscle] mass loss and identifying the populations most likely to benefit from time-restricted eating,” she said.
36 small studies, a review, a meta-analysis, 3 RCTs
There have been about three dozen small studies of time-restricted eating in humans, which examined 4- to 11-hour eating windows, Dr. Peterson explained.
A systematic review of 23 trials of time-restricted eating reported that, on average, participants lost 3% of their initial weight. And a meta-analysis of 19 trials in 475 participants found a –0.9 kg mean difference effect for weight loss.
However, those two analyses did not compare time-restricted eating with a control treatment, she stressed.
The largest randomized, controlled trial is a 12-week study in 271 adults with nonalcoholic fatty liver disease in China, Dr. Peterson said.
The researchers compared three groups:
- Alternate-day modified fasting: healthy meal provided.
- Time-restricted eating: 8-hour window, healthy meal provided.
- Control: 20% calorie reduction, no meal provided.
At 4 and 12 weeks, adults in the two treatment groups lost more weight than those in the control group, but “this was not a fair comparison” because of the lack of a provided meal in the control group, Dr. Peterson pointed out.
The next largest randomized, controlled study is the 12-week TREAT trial, published online in JAMA Internal Medicine in October 2020.
The researchers, from the University of California, San Francisco, randomized 116 adults into two groups:
- 8-hour time-restricted eating from noon to 8 p.m..
- Control: three meals/day.
Time-restricted eating did not lead to greater weight loss, compared with three structured meals a day, which was not surprising, Dr. Chow said, as “participants just reported whether they were engaged in time-restricted eating in a yes/no answer.”
Moreover, “there was no objective measure of their eating window. From our study, we showed that the extent of eating window restriction matters, not just time-restricted eating participation.”
Also, in TREAT, the eating window was noon to 8 p.m. (considered late for time-restricted eating), and the trial also allowed noncaloric beverages outside the window, whereas most studies only allow water and medications.
Lastly, TREAT showed that time-restricted eating reduced weight, compared with baseline, but the weight loss was not significant, compared with the control group, and there was a wide spread of effects (that is, some lost a lot of weight, others didn’t lose much weight).
“That being said, the JAMA Internal Medicine paper is the largest paper to date of time-restricted eating randomized versus control, so its findings need to be acknowledged and recognized,” Dr. Chow said.
Peterson reported that her group recently completed a 14-week intervention in 90 adults with obesity divided into two groups:
- Control: Continuous energy restriction, self-selected ≥ 12-hour window.
- Early time-restricted eating: 8-hour window from 7 a.m. to 3 p.m.
The findings will provide further insight into the benefits of time-restricted eating.
How might time-restricted eating lead to weight loss?
Dr. Peterson concluded by presenting data suggesting how time-restricted eating may induce weight loss.
In a 4-day crossover study in 11 overweight adults, time-restricted eating did not affect energy expenditure, but it lessened swings in subjective hunger, improved appetite hormones including ghrelin, and increased fat oxidation.
Most trials have reported that time-restricted eating improves one or more cardiometabolic endpoints, she noted.
Early time-restricted eating was associated with improved insulin sensitivity and secretion, blood pressure, and oxidative stress, but not better lipid levels.
In contrast, compared with eating 3 meals/day (control), late time-restricted eating (eating 1 meal/day from 5 p.m. to 9 p.m.) was associated with worsened cardiometabolic health (glucose, insulin, blood pressure, and lipid levels) in an 8-week crossover study in 15 participants.
Dr. Peterson and Dr. Chow reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADA scientific sessions address the old and the new
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.