User login
Meta-analysis: Lifestyle changes improve psoriasis
GENEVA – according to a systematic review and meta-analysis presented by Ching-Chi Chi, MD, at the annual congress of the European Academy of Dermatology and Venereology.
A plausible mechanism of benefit exists for these findings: “Fat tissue is known to be an endocrine organ that produces inflammatory cytokines, such as tumor necrosis factor. Reduce the amount of fat tissue and you reduce inflammation,” explained Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan, Taiwan.
Among the key findings in the meta-analysis: Participation in dietary interventions provided obese psoriasis patients with a 66% increased likelihood of achieving a PASI 75 response at week 24, compared with controls, with a number needed to treat of 3. These low-calorie diets were typically rigorous, the dermatologist noted. For example, one entailed a food intake of 1,000 kcal/day or less, while another restricted intake by 500 kcal/day less than a patient’s calculated resting energy expenditure.
Also, participants in the dietary intervention studies averaged a 14.4-point improvement from baseline in Dermatologic Life Quality Index (DLQI) scores at week 24 versus a 2.2-point improvement in controls. Researchers consider a 5-point or greater improvement in the DLQI clinically meaningful.
A combined diet and exercise program resulted in a 45% increased likelihood that obese psoriasis patients would achieve a PASI 50 response at week 16, with a number needed to treat of 7. There was a trend in the active treatment arm for higher PASI 75 and PASI 100 responses than in controls as well, but it wasn’t statistically significant.
The one randomized trial of a walking exercise program coupled with continuous health education demonstrated a significant reduction in the rate of psoriasis flares, compared with controls, over a 3-year period.
In contrast, the studies of educational programs promoting a healthy lifestyle without an associated dietary or physical activity intervention failed to show a reduction in PASI scores.
Dr. Chi reported no financial conflicts of interest regarding his study, which was funded by Chang Gung Memorial Hospital.
SOURCE: Chi C. EADV 2017.
GENEVA – according to a systematic review and meta-analysis presented by Ching-Chi Chi, MD, at the annual congress of the European Academy of Dermatology and Venereology.
A plausible mechanism of benefit exists for these findings: “Fat tissue is known to be an endocrine organ that produces inflammatory cytokines, such as tumor necrosis factor. Reduce the amount of fat tissue and you reduce inflammation,” explained Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan, Taiwan.
Among the key findings in the meta-analysis: Participation in dietary interventions provided obese psoriasis patients with a 66% increased likelihood of achieving a PASI 75 response at week 24, compared with controls, with a number needed to treat of 3. These low-calorie diets were typically rigorous, the dermatologist noted. For example, one entailed a food intake of 1,000 kcal/day or less, while another restricted intake by 500 kcal/day less than a patient’s calculated resting energy expenditure.
Also, participants in the dietary intervention studies averaged a 14.4-point improvement from baseline in Dermatologic Life Quality Index (DLQI) scores at week 24 versus a 2.2-point improvement in controls. Researchers consider a 5-point or greater improvement in the DLQI clinically meaningful.
A combined diet and exercise program resulted in a 45% increased likelihood that obese psoriasis patients would achieve a PASI 50 response at week 16, with a number needed to treat of 7. There was a trend in the active treatment arm for higher PASI 75 and PASI 100 responses than in controls as well, but it wasn’t statistically significant.
The one randomized trial of a walking exercise program coupled with continuous health education demonstrated a significant reduction in the rate of psoriasis flares, compared with controls, over a 3-year period.
In contrast, the studies of educational programs promoting a healthy lifestyle without an associated dietary or physical activity intervention failed to show a reduction in PASI scores.
Dr. Chi reported no financial conflicts of interest regarding his study, which was funded by Chang Gung Memorial Hospital.
SOURCE: Chi C. EADV 2017.
GENEVA – according to a systematic review and meta-analysis presented by Ching-Chi Chi, MD, at the annual congress of the European Academy of Dermatology and Venereology.
A plausible mechanism of benefit exists for these findings: “Fat tissue is known to be an endocrine organ that produces inflammatory cytokines, such as tumor necrosis factor. Reduce the amount of fat tissue and you reduce inflammation,” explained Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan, Taiwan.
Among the key findings in the meta-analysis: Participation in dietary interventions provided obese psoriasis patients with a 66% increased likelihood of achieving a PASI 75 response at week 24, compared with controls, with a number needed to treat of 3. These low-calorie diets were typically rigorous, the dermatologist noted. For example, one entailed a food intake of 1,000 kcal/day or less, while another restricted intake by 500 kcal/day less than a patient’s calculated resting energy expenditure.
Also, participants in the dietary intervention studies averaged a 14.4-point improvement from baseline in Dermatologic Life Quality Index (DLQI) scores at week 24 versus a 2.2-point improvement in controls. Researchers consider a 5-point or greater improvement in the DLQI clinically meaningful.
A combined diet and exercise program resulted in a 45% increased likelihood that obese psoriasis patients would achieve a PASI 50 response at week 16, with a number needed to treat of 7. There was a trend in the active treatment arm for higher PASI 75 and PASI 100 responses than in controls as well, but it wasn’t statistically significant.
The one randomized trial of a walking exercise program coupled with continuous health education demonstrated a significant reduction in the rate of psoriasis flares, compared with controls, over a 3-year period.
In contrast, the studies of educational programs promoting a healthy lifestyle without an associated dietary or physical activity intervention failed to show a reduction in PASI scores.
Dr. Chi reported no financial conflicts of interest regarding his study, which was funded by Chang Gung Memorial Hospital.
SOURCE: Chi C. EADV 2017.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Weight loss and exercise reduce psoriasis severity.
Major finding: The number needed to treat with a calorie-restricted diet in order for one additional obese patient with psoriasis on systemic therapy to achieve a PASI 75 response is 3.
Study details: This meta-analysis included 10 randomized, controlled trials totaling 1,163 patients with psoriasis.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by Chang Gung Memorial Hospital in Taoyuan, Taiwan.
Source: Chi C. EADV 2017.
Ixekizumab beats ustekinumab for fingernail psoriasis, hands down
GENEVA – Ixekizumab improved fingernail psoriasis significantly faster and with a higher complete nail clearance rate by week 24 compared with ustekinumab in a head-to-head phase 3b randomized trial, Yves Dutronc, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This is a clinically important finding because – as dermatologists and psoriasis patients well know – nail and skin psoriasis are two different animals.
He presented a prespecified secondary analysis of the randomized, phase 3b, multicenter IXORA-S trial. The study pit the interleukin-17A inhibitor ixekizumab (Taltz) head-to-head against the interleukin 12/23 inhibitor ustekinumab (Stelara). The primary endpoint, which was the PASI 90 improvement rate, has previously been reported: 73% in the ixekizumab group versus 42% in the ustekinumab group at week 12, and 83% versus 59% at week 24. And ixekizumab’s superior efficacy was achieved with a safety profile similar to that of ustekinumab (Br J Dermatol. 2017 Oct;177[4]:1014-23).
However, change in PASI score or Investigator’s Global Assessment isn’t informative regarding a patient’s change in nail psoriasis status. This was the impetus for the secondary analysis focused on the IXORA-S subgroup with baseline fingernail psoriasis. For this purpose, Dr. Dutronc and his coinvestigators used as their metric the change over time in the Nail Psoriasis Severity Index (NAPSI) total score, which entails a quadrant-by-quadrant assessment of every fingernail.
By play of chance, the 84 patients randomized to ixekizumab had slightly more severe nail psoriasis at baseline than that of the 105 ustekinumab patients. Their mean baseline NAPSI total score was 28.3, compared with 24.8 for the ustekinumab group. More than one-quarter of patients in the ixekizumab arm had a baseline NAPSI score greater than 43, whereas the top quartile of nail psoriasis severity in the ustekinumab group began with a NAPSI score above 34.
Not surprisingly, not much happened in terms of improvement in nail appearance in the first 12 weeks, since new nail grows slowly. But by week 8 the between-group difference in improvement in NAPSI score had become significant in favor of ixekizumab, with a mean 12.9-point reduction from baseline versus a 5.6-point drop in the ustekinumab group. This difference continued to grow over time, such that at week 24 the ixekizumab had a mean 19.9-point reduction, compared with a 13.2-point decrease for the ustekinumab group.
At week 12, 15.5% of the ixekizumab group and 11.3% of the ustekinumab group had reached complete clearance of their fingernail psoriasis. At week 24, complete clearance had been achieved in 48.8% of the ixekizumab group and 22.9% of patients on ustekinumab.
This is an interim analysis. Final results of the IXORA-S nail psoriasis substudy will be reported at 52 weeks of follow-up.
SOURCE: Dutronc Y. https://eadvgeneva2017.org/
GENEVA – Ixekizumab improved fingernail psoriasis significantly faster and with a higher complete nail clearance rate by week 24 compared with ustekinumab in a head-to-head phase 3b randomized trial, Yves Dutronc, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This is a clinically important finding because – as dermatologists and psoriasis patients well know – nail and skin psoriasis are two different animals.
He presented a prespecified secondary analysis of the randomized, phase 3b, multicenter IXORA-S trial. The study pit the interleukin-17A inhibitor ixekizumab (Taltz) head-to-head against the interleukin 12/23 inhibitor ustekinumab (Stelara). The primary endpoint, which was the PASI 90 improvement rate, has previously been reported: 73% in the ixekizumab group versus 42% in the ustekinumab group at week 12, and 83% versus 59% at week 24. And ixekizumab’s superior efficacy was achieved with a safety profile similar to that of ustekinumab (Br J Dermatol. 2017 Oct;177[4]:1014-23).
However, change in PASI score or Investigator’s Global Assessment isn’t informative regarding a patient’s change in nail psoriasis status. This was the impetus for the secondary analysis focused on the IXORA-S subgroup with baseline fingernail psoriasis. For this purpose, Dr. Dutronc and his coinvestigators used as their metric the change over time in the Nail Psoriasis Severity Index (NAPSI) total score, which entails a quadrant-by-quadrant assessment of every fingernail.
By play of chance, the 84 patients randomized to ixekizumab had slightly more severe nail psoriasis at baseline than that of the 105 ustekinumab patients. Their mean baseline NAPSI total score was 28.3, compared with 24.8 for the ustekinumab group. More than one-quarter of patients in the ixekizumab arm had a baseline NAPSI score greater than 43, whereas the top quartile of nail psoriasis severity in the ustekinumab group began with a NAPSI score above 34.
Not surprisingly, not much happened in terms of improvement in nail appearance in the first 12 weeks, since new nail grows slowly. But by week 8 the between-group difference in improvement in NAPSI score had become significant in favor of ixekizumab, with a mean 12.9-point reduction from baseline versus a 5.6-point drop in the ustekinumab group. This difference continued to grow over time, such that at week 24 the ixekizumab had a mean 19.9-point reduction, compared with a 13.2-point decrease for the ustekinumab group.
At week 12, 15.5% of the ixekizumab group and 11.3% of the ustekinumab group had reached complete clearance of their fingernail psoriasis. At week 24, complete clearance had been achieved in 48.8% of the ixekizumab group and 22.9% of patients on ustekinumab.
This is an interim analysis. Final results of the IXORA-S nail psoriasis substudy will be reported at 52 weeks of follow-up.
SOURCE: Dutronc Y. https://eadvgeneva2017.org/
GENEVA – Ixekizumab improved fingernail psoriasis significantly faster and with a higher complete nail clearance rate by week 24 compared with ustekinumab in a head-to-head phase 3b randomized trial, Yves Dutronc, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This is a clinically important finding because – as dermatologists and psoriasis patients well know – nail and skin psoriasis are two different animals.
He presented a prespecified secondary analysis of the randomized, phase 3b, multicenter IXORA-S trial. The study pit the interleukin-17A inhibitor ixekizumab (Taltz) head-to-head against the interleukin 12/23 inhibitor ustekinumab (Stelara). The primary endpoint, which was the PASI 90 improvement rate, has previously been reported: 73% in the ixekizumab group versus 42% in the ustekinumab group at week 12, and 83% versus 59% at week 24. And ixekizumab’s superior efficacy was achieved with a safety profile similar to that of ustekinumab (Br J Dermatol. 2017 Oct;177[4]:1014-23).
However, change in PASI score or Investigator’s Global Assessment isn’t informative regarding a patient’s change in nail psoriasis status. This was the impetus for the secondary analysis focused on the IXORA-S subgroup with baseline fingernail psoriasis. For this purpose, Dr. Dutronc and his coinvestigators used as their metric the change over time in the Nail Psoriasis Severity Index (NAPSI) total score, which entails a quadrant-by-quadrant assessment of every fingernail.
By play of chance, the 84 patients randomized to ixekizumab had slightly more severe nail psoriasis at baseline than that of the 105 ustekinumab patients. Their mean baseline NAPSI total score was 28.3, compared with 24.8 for the ustekinumab group. More than one-quarter of patients in the ixekizumab arm had a baseline NAPSI score greater than 43, whereas the top quartile of nail psoriasis severity in the ustekinumab group began with a NAPSI score above 34.
Not surprisingly, not much happened in terms of improvement in nail appearance in the first 12 weeks, since new nail grows slowly. But by week 8 the between-group difference in improvement in NAPSI score had become significant in favor of ixekizumab, with a mean 12.9-point reduction from baseline versus a 5.6-point drop in the ustekinumab group. This difference continued to grow over time, such that at week 24 the ixekizumab had a mean 19.9-point reduction, compared with a 13.2-point decrease for the ustekinumab group.
At week 12, 15.5% of the ixekizumab group and 11.3% of the ustekinumab group had reached complete clearance of their fingernail psoriasis. At week 24, complete clearance had been achieved in 48.8% of the ixekizumab group and 22.9% of patients on ustekinumab.
This is an interim analysis. Final results of the IXORA-S nail psoriasis substudy will be reported at 52 weeks of follow-up.
SOURCE: Dutronc Y. https://eadvgeneva2017.org/
REPORTING FROM THE EADV CONGRESS
Key clinical point:
Major finding: At week 24, complete clearance of fingernail psoriasis was documented in 49% of patients on ixekizumab and 23% on ustekinumab.
Study details: This secondary analysis of the randomized, multicenter, prospective, phase 3b IXORA-S trial included 189 patients with moderate to severe plaque psoriasis with fingernail involvement.
Disclosures: The study was sponsored by Eli Lilly and presented by a company employee.
Source: Dutronc Y. https://eadvgeneva2017.org
Huge AWARE study shows chronic spontaneous urticaria is seriously undertreated
GENEVA – Partial results of the first worldwide observational study on the burden of chronic spontaneous urticaria (CSU) and its treatment in real-world clinical practice can be summarized succinctly: “It’s worse than we thought,” Marcus Maurer, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We knew it was bad, but what comes out of looking at the daily life and treatment patterns in patients with CSU is a nightmare,” said Dr. Maurer, professor of dermatology and allergy and director of research in the department of dermatology at Charite University Hospital in Berlin.
Dr. Maurer presented the year-1 results in 1,550 AWARE participants at 256 sites across Germany. The results, he said, are sobering and reflective of what is being found in the other participating countries. The findings document a low level of adherence to the published treatment guidelines, and a lot of miserable CSU patients.
“My talk here is a clear call to action,” he declared. “The AWARE study shows we have two problems. One, patients don’t get treatment – and we can solve that. And number two, patients don’t get the right treatment. And the right treatment is treatment that controls the disease. In the guidelines we say, ‘Treat the disease until it is gone.’ That’s a great sentence. It means we should give enough treatment to control the disease: as much as needed, as little as possible, and in that order. First we establish control, then we have all the time in the world to find a treatment that gets patients to stay that way in the long run.”
He is a coauthor of the guidelines, a joint initiative of the European Academy of Allergy and Clinical Immunology, the EU-funded Global Allergy and Asthma European Network of centers of excellence, the European Dermatology Forum, and the World Allergy Organization (Allergy. 2014 Jul;69[7]:868-87).
He asked a packed hall of dermatologists how many of them like taking care of patients with CSU. One lonely hand went up. Dr. Maurer was unsurprised.
“, and it’s one where we struggle. No question, we struggle with these patients, maybe more so than with our psoriasis and atopic dermatitis patients. But the good news is there are new concepts of this disease and new treatment approaches,” he said. “We know that in 98% of patients, using the guideline algorithm, we can establish control in CSU.”
He summarized the joint guideline algorithm as follows: Routinely use a simple validated assessment tool such as the patient-reported Urticaria Control Test (UCT) (J Allergy Clin Immunol. 2014 May;133[5]:1365-72) to determine a CSU patient’s burden of disease, prescribe second-generation, nonsedating H1 antihistamines as first-line therapy, updose those agents to up to four times standard dosing as second-line therapy if the follow-up UCT results show the disease is still uncontrolled, and if it remains uncontrolled then move on to third-line treatment with add-on omalizumab (Xolair) or a maximum of 10 days of montelukast (Singulair) or cyclosporine.
When he asked how many audience members use the UCT, which assesses disease control over the previous 4 weeks, a mere handful responded affirmatively. “Please,” he urged his colleagues, “this test is so simple. It’s four questions, it’s free, it’s available online in more than 30 languages. It allows us to identify patients with uncontrolled disease. We have the ability to control the disease now, so let use this test. Patients can fill it out quickly in your waiting room so you can monitor how good your treatment is.”
AWARE study bears bad news
At study enrollment, the 1,550 German participants in the AWARE study had been diagnosed with CSU a mean of 4.9 years previously. Yet fully 78% had uncontrolled CSU as defined by a score below 12 points on the 0-16 UCT. Forty-four percent of patients had experienced angioedema within the previous 6 months, and 56% percent of patients had a baseline Dermatology Life Quality Index (DLQI) score indicating CSU had a moderate, very large, or extremely large effect on their quality of life. Yet 36% of patients weren’t receiving any treatment at baseline, and only 6.5% of patients were receiving guideline-recommended add-on third-line therapy.
In particular, most physicians fail to appreciate the negative impact of angioedema in CSU patients.
“This is not a swollen lip just once a year, this is angioedema weekly or monthly that really impairs quality of life. It’s not just a swollen lip or a swollen eye for the day or for a couple of hours, it’s the fear of going to sleep because in the morning you may wake up looking like that and not be able to go to work,” Dr. Maurer pointed out.
During the first year of the study, the overall situation improved significantly over the course of office visits every 3 months. The prevalence of DLQI scores indicative of a moderate or worse quality of life impact gradually fell from 56% to 28%. The proportion of patients on third-line treatment rose stepwise to 29.5% at 1 year. The rate of uncontrolled CSU as defined by a UCT score below 12 dropped steadily over time to 42%. At 1 year, only 16% of patients reported having an angioedema episode within the prior 12 weeks.
Still, this leaves much room for further improvement, Dr. Maurer commented.
“We’re on the right track, but patients who are in our treatment for a year should have their disease controlled. That’s our job. We have the treatments, and we should provide them. Even after 1 year there are still patients with a DLQI score above 10, and that’s not good,” he said.
Dr. Maurer was first author of the recently published interim results of the German portion of the AWARE study (Clin Exp Allergy. 2017 May;47[5]:684-52). The AWARE study is sponsored by Novartis, manufacturer of omalizumab, which is approved by the Food and Drug Administration for treating CSU. Dr. Maurer reported receiving research grants from and serving as an advisor to and paid speaker for Novartis and numerous other pharmaceutical companies.
[email protected]
GENEVA – Partial results of the first worldwide observational study on the burden of chronic spontaneous urticaria (CSU) and its treatment in real-world clinical practice can be summarized succinctly: “It’s worse than we thought,” Marcus Maurer, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We knew it was bad, but what comes out of looking at the daily life and treatment patterns in patients with CSU is a nightmare,” said Dr. Maurer, professor of dermatology and allergy and director of research in the department of dermatology at Charite University Hospital in Berlin.
Dr. Maurer presented the year-1 results in 1,550 AWARE participants at 256 sites across Germany. The results, he said, are sobering and reflective of what is being found in the other participating countries. The findings document a low level of adherence to the published treatment guidelines, and a lot of miserable CSU patients.
“My talk here is a clear call to action,” he declared. “The AWARE study shows we have two problems. One, patients don’t get treatment – and we can solve that. And number two, patients don’t get the right treatment. And the right treatment is treatment that controls the disease. In the guidelines we say, ‘Treat the disease until it is gone.’ That’s a great sentence. It means we should give enough treatment to control the disease: as much as needed, as little as possible, and in that order. First we establish control, then we have all the time in the world to find a treatment that gets patients to stay that way in the long run.”
He is a coauthor of the guidelines, a joint initiative of the European Academy of Allergy and Clinical Immunology, the EU-funded Global Allergy and Asthma European Network of centers of excellence, the European Dermatology Forum, and the World Allergy Organization (Allergy. 2014 Jul;69[7]:868-87).
He asked a packed hall of dermatologists how many of them like taking care of patients with CSU. One lonely hand went up. Dr. Maurer was unsurprised.
“, and it’s one where we struggle. No question, we struggle with these patients, maybe more so than with our psoriasis and atopic dermatitis patients. But the good news is there are new concepts of this disease and new treatment approaches,” he said. “We know that in 98% of patients, using the guideline algorithm, we can establish control in CSU.”
He summarized the joint guideline algorithm as follows: Routinely use a simple validated assessment tool such as the patient-reported Urticaria Control Test (UCT) (J Allergy Clin Immunol. 2014 May;133[5]:1365-72) to determine a CSU patient’s burden of disease, prescribe second-generation, nonsedating H1 antihistamines as first-line therapy, updose those agents to up to four times standard dosing as second-line therapy if the follow-up UCT results show the disease is still uncontrolled, and if it remains uncontrolled then move on to third-line treatment with add-on omalizumab (Xolair) or a maximum of 10 days of montelukast (Singulair) or cyclosporine.
When he asked how many audience members use the UCT, which assesses disease control over the previous 4 weeks, a mere handful responded affirmatively. “Please,” he urged his colleagues, “this test is so simple. It’s four questions, it’s free, it’s available online in more than 30 languages. It allows us to identify patients with uncontrolled disease. We have the ability to control the disease now, so let use this test. Patients can fill it out quickly in your waiting room so you can monitor how good your treatment is.”
AWARE study bears bad news
At study enrollment, the 1,550 German participants in the AWARE study had been diagnosed with CSU a mean of 4.9 years previously. Yet fully 78% had uncontrolled CSU as defined by a score below 12 points on the 0-16 UCT. Forty-four percent of patients had experienced angioedema within the previous 6 months, and 56% percent of patients had a baseline Dermatology Life Quality Index (DLQI) score indicating CSU had a moderate, very large, or extremely large effect on their quality of life. Yet 36% of patients weren’t receiving any treatment at baseline, and only 6.5% of patients were receiving guideline-recommended add-on third-line therapy.
In particular, most physicians fail to appreciate the negative impact of angioedema in CSU patients.
“This is not a swollen lip just once a year, this is angioedema weekly or monthly that really impairs quality of life. It’s not just a swollen lip or a swollen eye for the day or for a couple of hours, it’s the fear of going to sleep because in the morning you may wake up looking like that and not be able to go to work,” Dr. Maurer pointed out.
During the first year of the study, the overall situation improved significantly over the course of office visits every 3 months. The prevalence of DLQI scores indicative of a moderate or worse quality of life impact gradually fell from 56% to 28%. The proportion of patients on third-line treatment rose stepwise to 29.5% at 1 year. The rate of uncontrolled CSU as defined by a UCT score below 12 dropped steadily over time to 42%. At 1 year, only 16% of patients reported having an angioedema episode within the prior 12 weeks.
Still, this leaves much room for further improvement, Dr. Maurer commented.
“We’re on the right track, but patients who are in our treatment for a year should have their disease controlled. That’s our job. We have the treatments, and we should provide them. Even after 1 year there are still patients with a DLQI score above 10, and that’s not good,” he said.
Dr. Maurer was first author of the recently published interim results of the German portion of the AWARE study (Clin Exp Allergy. 2017 May;47[5]:684-52). The AWARE study is sponsored by Novartis, manufacturer of omalizumab, which is approved by the Food and Drug Administration for treating CSU. Dr. Maurer reported receiving research grants from and serving as an advisor to and paid speaker for Novartis and numerous other pharmaceutical companies.
[email protected]
GENEVA – Partial results of the first worldwide observational study on the burden of chronic spontaneous urticaria (CSU) and its treatment in real-world clinical practice can be summarized succinctly: “It’s worse than we thought,” Marcus Maurer, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We knew it was bad, but what comes out of looking at the daily life and treatment patterns in patients with CSU is a nightmare,” said Dr. Maurer, professor of dermatology and allergy and director of research in the department of dermatology at Charite University Hospital in Berlin.
Dr. Maurer presented the year-1 results in 1,550 AWARE participants at 256 sites across Germany. The results, he said, are sobering and reflective of what is being found in the other participating countries. The findings document a low level of adherence to the published treatment guidelines, and a lot of miserable CSU patients.
“My talk here is a clear call to action,” he declared. “The AWARE study shows we have two problems. One, patients don’t get treatment – and we can solve that. And number two, patients don’t get the right treatment. And the right treatment is treatment that controls the disease. In the guidelines we say, ‘Treat the disease until it is gone.’ That’s a great sentence. It means we should give enough treatment to control the disease: as much as needed, as little as possible, and in that order. First we establish control, then we have all the time in the world to find a treatment that gets patients to stay that way in the long run.”
He is a coauthor of the guidelines, a joint initiative of the European Academy of Allergy and Clinical Immunology, the EU-funded Global Allergy and Asthma European Network of centers of excellence, the European Dermatology Forum, and the World Allergy Organization (Allergy. 2014 Jul;69[7]:868-87).
He asked a packed hall of dermatologists how many of them like taking care of patients with CSU. One lonely hand went up. Dr. Maurer was unsurprised.
“, and it’s one where we struggle. No question, we struggle with these patients, maybe more so than with our psoriasis and atopic dermatitis patients. But the good news is there are new concepts of this disease and new treatment approaches,” he said. “We know that in 98% of patients, using the guideline algorithm, we can establish control in CSU.”
He summarized the joint guideline algorithm as follows: Routinely use a simple validated assessment tool such as the patient-reported Urticaria Control Test (UCT) (J Allergy Clin Immunol. 2014 May;133[5]:1365-72) to determine a CSU patient’s burden of disease, prescribe second-generation, nonsedating H1 antihistamines as first-line therapy, updose those agents to up to four times standard dosing as second-line therapy if the follow-up UCT results show the disease is still uncontrolled, and if it remains uncontrolled then move on to third-line treatment with add-on omalizumab (Xolair) or a maximum of 10 days of montelukast (Singulair) or cyclosporine.
When he asked how many audience members use the UCT, which assesses disease control over the previous 4 weeks, a mere handful responded affirmatively. “Please,” he urged his colleagues, “this test is so simple. It’s four questions, it’s free, it’s available online in more than 30 languages. It allows us to identify patients with uncontrolled disease. We have the ability to control the disease now, so let use this test. Patients can fill it out quickly in your waiting room so you can monitor how good your treatment is.”
AWARE study bears bad news
At study enrollment, the 1,550 German participants in the AWARE study had been diagnosed with CSU a mean of 4.9 years previously. Yet fully 78% had uncontrolled CSU as defined by a score below 12 points on the 0-16 UCT. Forty-four percent of patients had experienced angioedema within the previous 6 months, and 56% percent of patients had a baseline Dermatology Life Quality Index (DLQI) score indicating CSU had a moderate, very large, or extremely large effect on their quality of life. Yet 36% of patients weren’t receiving any treatment at baseline, and only 6.5% of patients were receiving guideline-recommended add-on third-line therapy.
In particular, most physicians fail to appreciate the negative impact of angioedema in CSU patients.
“This is not a swollen lip just once a year, this is angioedema weekly or monthly that really impairs quality of life. It’s not just a swollen lip or a swollen eye for the day or for a couple of hours, it’s the fear of going to sleep because in the morning you may wake up looking like that and not be able to go to work,” Dr. Maurer pointed out.
During the first year of the study, the overall situation improved significantly over the course of office visits every 3 months. The prevalence of DLQI scores indicative of a moderate or worse quality of life impact gradually fell from 56% to 28%. The proportion of patients on third-line treatment rose stepwise to 29.5% at 1 year. The rate of uncontrolled CSU as defined by a UCT score below 12 dropped steadily over time to 42%. At 1 year, only 16% of patients reported having an angioedema episode within the prior 12 weeks.
Still, this leaves much room for further improvement, Dr. Maurer commented.
“We’re on the right track, but patients who are in our treatment for a year should have their disease controlled. That’s our job. We have the treatments, and we should provide them. Even after 1 year there are still patients with a DLQI score above 10, and that’s not good,” he said.
Dr. Maurer was first author of the recently published interim results of the German portion of the AWARE study (Clin Exp Allergy. 2017 May;47[5]:684-52). The AWARE study is sponsored by Novartis, manufacturer of omalizumab, which is approved by the Food and Drug Administration for treating CSU. Dr. Maurer reported receiving research grants from and serving as an advisor to and paid speaker for Novartis and numerous other pharmaceutical companies.
[email protected]
EXPERT ANALYSIS FROM THE EADV CONGRESS
Neutrophilic urticarial dermatosis is usually misdiagnosed
GENEVA – Neutrophilic urticarial dermatosis (NUD) in patients with systemic lupus erythematosus (SLE) is “almost always” initially misdiagnosed as a lupus flare and treated inappropriately, Dan Lipsker, MD, PhD, said in a plenary address at the annual congress of the European Academy of Dermatology and Venereology.
“This is a condition that is underdiagnosed and overtreated,” declared Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
NUD is not rare. Dr. Lipsker estimates it occurs in 1%-2% of patients with SLE. In a retrospective study of seven patients with NUD and SLE, he and his colleagues reported that NUD was initially misdiagnosed as a lupus flare in 4 patients, who were then treated with immunosuppressive drugs (Medicine. 2014 Dec;93[29]:e351]). “That’s quite logical because the patients had a rash, fever, and joint pain,” the dermatologist noted.
However, : It won’t alleviate the symptoms and needlessly exposes the patient to drug toxicities.
The treatment for NUD is not prednisone, mycophenolate mofetil, an antimalarial, or other drugs conventionally prescribed for SLE; it’s a drug that inhibits neutrophil migration, such as dapsone at 50-200 mg per day or colchicine at 0.5-1.0 mg per day. Typically, within just a few days after starting the appropriate therapy, the joint pain and rash of NUD are gone, according to Dr. Lipsker.
Making the diagnosis
The rash of NUD is distinctly different from a classic lupus rash. It consists of pale red macules or slightly raised nonpruritic papules. Individual lesions will disappear spontaneously within 24-48 hours.
The histopathology of NUD is characteristic of a neutrophilic dermatosis. On biopsy, an intense neutrophilic perivascular and interstitial infiltrate with leukocytoclasia is seen. There is no damage to the blood vessel walls, which readily distinguishes NUD from urticarial vasculitis.
Other neutrophilic dermatoses have also been reported with increased frequency in patients with SLE. These include Sweet syndrome, pyoderma gangrenosum, bullous SLE, amicrobial pustulosis of the folds, and palisaded neutrophilic granulomatous dermatitis. Dr. Lipsker lumps them, together with NUD, as neutrophilic cutaneous lupus erythematosus. Affected SLE patients have an exaggerated innate immune response. It is as yet unclear if these neutrophilic dermatoses have prognostic significance in the setting of SLE, he said.
Dr. Lipsker reported having no financial conflicts of interest regarding his presentation.
GENEVA – Neutrophilic urticarial dermatosis (NUD) in patients with systemic lupus erythematosus (SLE) is “almost always” initially misdiagnosed as a lupus flare and treated inappropriately, Dan Lipsker, MD, PhD, said in a plenary address at the annual congress of the European Academy of Dermatology and Venereology.
“This is a condition that is underdiagnosed and overtreated,” declared Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
NUD is not rare. Dr. Lipsker estimates it occurs in 1%-2% of patients with SLE. In a retrospective study of seven patients with NUD and SLE, he and his colleagues reported that NUD was initially misdiagnosed as a lupus flare in 4 patients, who were then treated with immunosuppressive drugs (Medicine. 2014 Dec;93[29]:e351]). “That’s quite logical because the patients had a rash, fever, and joint pain,” the dermatologist noted.
However, : It won’t alleviate the symptoms and needlessly exposes the patient to drug toxicities.
The treatment for NUD is not prednisone, mycophenolate mofetil, an antimalarial, or other drugs conventionally prescribed for SLE; it’s a drug that inhibits neutrophil migration, such as dapsone at 50-200 mg per day or colchicine at 0.5-1.0 mg per day. Typically, within just a few days after starting the appropriate therapy, the joint pain and rash of NUD are gone, according to Dr. Lipsker.
Making the diagnosis
The rash of NUD is distinctly different from a classic lupus rash. It consists of pale red macules or slightly raised nonpruritic papules. Individual lesions will disappear spontaneously within 24-48 hours.
The histopathology of NUD is characteristic of a neutrophilic dermatosis. On biopsy, an intense neutrophilic perivascular and interstitial infiltrate with leukocytoclasia is seen. There is no damage to the blood vessel walls, which readily distinguishes NUD from urticarial vasculitis.
Other neutrophilic dermatoses have also been reported with increased frequency in patients with SLE. These include Sweet syndrome, pyoderma gangrenosum, bullous SLE, amicrobial pustulosis of the folds, and palisaded neutrophilic granulomatous dermatitis. Dr. Lipsker lumps them, together with NUD, as neutrophilic cutaneous lupus erythematosus. Affected SLE patients have an exaggerated innate immune response. It is as yet unclear if these neutrophilic dermatoses have prognostic significance in the setting of SLE, he said.
Dr. Lipsker reported having no financial conflicts of interest regarding his presentation.
GENEVA – Neutrophilic urticarial dermatosis (NUD) in patients with systemic lupus erythematosus (SLE) is “almost always” initially misdiagnosed as a lupus flare and treated inappropriately, Dan Lipsker, MD, PhD, said in a plenary address at the annual congress of the European Academy of Dermatology and Venereology.
“This is a condition that is underdiagnosed and overtreated,” declared Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
NUD is not rare. Dr. Lipsker estimates it occurs in 1%-2% of patients with SLE. In a retrospective study of seven patients with NUD and SLE, he and his colleagues reported that NUD was initially misdiagnosed as a lupus flare in 4 patients, who were then treated with immunosuppressive drugs (Medicine. 2014 Dec;93[29]:e351]). “That’s quite logical because the patients had a rash, fever, and joint pain,” the dermatologist noted.
However, : It won’t alleviate the symptoms and needlessly exposes the patient to drug toxicities.
The treatment for NUD is not prednisone, mycophenolate mofetil, an antimalarial, or other drugs conventionally prescribed for SLE; it’s a drug that inhibits neutrophil migration, such as dapsone at 50-200 mg per day or colchicine at 0.5-1.0 mg per day. Typically, within just a few days after starting the appropriate therapy, the joint pain and rash of NUD are gone, according to Dr. Lipsker.
Making the diagnosis
The rash of NUD is distinctly different from a classic lupus rash. It consists of pale red macules or slightly raised nonpruritic papules. Individual lesions will disappear spontaneously within 24-48 hours.
The histopathology of NUD is characteristic of a neutrophilic dermatosis. On biopsy, an intense neutrophilic perivascular and interstitial infiltrate with leukocytoclasia is seen. There is no damage to the blood vessel walls, which readily distinguishes NUD from urticarial vasculitis.
Other neutrophilic dermatoses have also been reported with increased frequency in patients with SLE. These include Sweet syndrome, pyoderma gangrenosum, bullous SLE, amicrobial pustulosis of the folds, and palisaded neutrophilic granulomatous dermatitis. Dr. Lipsker lumps them, together with NUD, as neutrophilic cutaneous lupus erythematosus. Affected SLE patients have an exaggerated innate immune response. It is as yet unclear if these neutrophilic dermatoses have prognostic significance in the setting of SLE, he said.
Dr. Lipsker reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Omalizumab for chronic urticaria quells suffocation fears
GENEVA – a widely underappreciated dimension of the impairment caused by this disease, Karsten Weller, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Virtually all clinical studies of CSU in recent decades focus on the wheal and pruritus components and not on the angioedema component, even though angioedema is a frequent symptom in the disease. Roughly half of patients with CSU experience wheals and angioedema, and up to 13% experience angioedema only,” said Dr. Weller, a dermatologist at Charité University Hospital in Berlin.
“Angioedema is a major driver of quality-of-life impairment in CSU,” he continued. “We know that these are the patients who particularly suffer from the unpredictability of the disease, from disfigurement, from embarrassment. These are the patients who come to the emergency rooms, who lose working days, and these are the patients who often have the feeling of losing control over their lives.”
X-ACT was a multicenter German study which included 91 patients with moderate to severe CSU marked by at least four angioedema episodes during the 6 months prior to enrollment. Participants also had to be refractory to second-generation H1 antihistamines at two to four times the approved dose. The subjects were randomized to subcutaneous omalizumab at 300 mg every 4 weeks or placebo for 28 weeks; they were then further assessed for changes in quality of life during 8 weeks off omalizumab.
Because assessment of quality of life was such a major part of X-ACT, the investigators pulled out all the stops. Their multimodal evaluation included the Angioedema Quality of Life questionnaire – a patient-reported, 17-item instrument that is the first validated tool for evaluation of angioedema-specific quality of life – as well as the Dermatology Life Quality Index and the weekly Angioedema Activity Score.
Patients were also asked to rate on a 0-4 scale their degree of fearfulness of life-threatening swelling episodes and also their degree of fearfulness of angioedema-related suffocation. “To my knowledge, this is the first time this has been done in a randomized clinical trial,” Dr. Weller noted.
The patient reports were striking: At baseline, 49% indicated that they occasionally, often, or very often were afraid of suffocating caused by swelling episodes; only 4% of patients expressed that fear after 28 weeks on omalizumab, compared with 25% of placebo-treated controls. Similarly, at baseline two-thirds of patients reported occasionally, often, or very often being fearful of life-threatening swelling episodes, a rate that fell to 14% after 28 weeks on omalizumab, compared with 42% for controls.
Scores on the Angioedema Quality of Life Questionnaire improved continuously from a baseline of roughly 60 on a 0-100 scale – indicative of severe impairment – to less than 20 after 28 weeks on omalizumab; these scores steadily worsened again during the 8 weeks following treatment discontinuation. The Dermatology Life Quality Index scores dropped from a mean baseline of 15.6 down to 5 by week 4, remained in the 3-5 range for the remainder of the treatment period, then increased again when treatment was discontinued. The Angioedema Activity Score followed a similar pattern.
One audience member observed that the placebo response was quite strong in the study, with the percentage of patients reporting fear of suffocating caused by angioedema episodes falling from 49% at baseline to 25% after 28 weeks on placebo.
Dr. Weller replied that a potent placebo response is a consistent feature of all clinical trials of CSU therapies. The explanation, he added, is unknown.
He reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
SOURCE: Weller K et al. EADV Congress 2017.
GENEVA – a widely underappreciated dimension of the impairment caused by this disease, Karsten Weller, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Virtually all clinical studies of CSU in recent decades focus on the wheal and pruritus components and not on the angioedema component, even though angioedema is a frequent symptom in the disease. Roughly half of patients with CSU experience wheals and angioedema, and up to 13% experience angioedema only,” said Dr. Weller, a dermatologist at Charité University Hospital in Berlin.
“Angioedema is a major driver of quality-of-life impairment in CSU,” he continued. “We know that these are the patients who particularly suffer from the unpredictability of the disease, from disfigurement, from embarrassment. These are the patients who come to the emergency rooms, who lose working days, and these are the patients who often have the feeling of losing control over their lives.”
X-ACT was a multicenter German study which included 91 patients with moderate to severe CSU marked by at least four angioedema episodes during the 6 months prior to enrollment. Participants also had to be refractory to second-generation H1 antihistamines at two to four times the approved dose. The subjects were randomized to subcutaneous omalizumab at 300 mg every 4 weeks or placebo for 28 weeks; they were then further assessed for changes in quality of life during 8 weeks off omalizumab.
Because assessment of quality of life was such a major part of X-ACT, the investigators pulled out all the stops. Their multimodal evaluation included the Angioedema Quality of Life questionnaire – a patient-reported, 17-item instrument that is the first validated tool for evaluation of angioedema-specific quality of life – as well as the Dermatology Life Quality Index and the weekly Angioedema Activity Score.
Patients were also asked to rate on a 0-4 scale their degree of fearfulness of life-threatening swelling episodes and also their degree of fearfulness of angioedema-related suffocation. “To my knowledge, this is the first time this has been done in a randomized clinical trial,” Dr. Weller noted.
The patient reports were striking: At baseline, 49% indicated that they occasionally, often, or very often were afraid of suffocating caused by swelling episodes; only 4% of patients expressed that fear after 28 weeks on omalizumab, compared with 25% of placebo-treated controls. Similarly, at baseline two-thirds of patients reported occasionally, often, or very often being fearful of life-threatening swelling episodes, a rate that fell to 14% after 28 weeks on omalizumab, compared with 42% for controls.
Scores on the Angioedema Quality of Life Questionnaire improved continuously from a baseline of roughly 60 on a 0-100 scale – indicative of severe impairment – to less than 20 after 28 weeks on omalizumab; these scores steadily worsened again during the 8 weeks following treatment discontinuation. The Dermatology Life Quality Index scores dropped from a mean baseline of 15.6 down to 5 by week 4, remained in the 3-5 range for the remainder of the treatment period, then increased again when treatment was discontinued. The Angioedema Activity Score followed a similar pattern.
One audience member observed that the placebo response was quite strong in the study, with the percentage of patients reporting fear of suffocating caused by angioedema episodes falling from 49% at baseline to 25% after 28 weeks on placebo.
Dr. Weller replied that a potent placebo response is a consistent feature of all clinical trials of CSU therapies. The explanation, he added, is unknown.
He reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
SOURCE: Weller K et al. EADV Congress 2017.
GENEVA – a widely underappreciated dimension of the impairment caused by this disease, Karsten Weller, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Virtually all clinical studies of CSU in recent decades focus on the wheal and pruritus components and not on the angioedema component, even though angioedema is a frequent symptom in the disease. Roughly half of patients with CSU experience wheals and angioedema, and up to 13% experience angioedema only,” said Dr. Weller, a dermatologist at Charité University Hospital in Berlin.
“Angioedema is a major driver of quality-of-life impairment in CSU,” he continued. “We know that these are the patients who particularly suffer from the unpredictability of the disease, from disfigurement, from embarrassment. These are the patients who come to the emergency rooms, who lose working days, and these are the patients who often have the feeling of losing control over their lives.”
X-ACT was a multicenter German study which included 91 patients with moderate to severe CSU marked by at least four angioedema episodes during the 6 months prior to enrollment. Participants also had to be refractory to second-generation H1 antihistamines at two to four times the approved dose. The subjects were randomized to subcutaneous omalizumab at 300 mg every 4 weeks or placebo for 28 weeks; they were then further assessed for changes in quality of life during 8 weeks off omalizumab.
Because assessment of quality of life was such a major part of X-ACT, the investigators pulled out all the stops. Their multimodal evaluation included the Angioedema Quality of Life questionnaire – a patient-reported, 17-item instrument that is the first validated tool for evaluation of angioedema-specific quality of life – as well as the Dermatology Life Quality Index and the weekly Angioedema Activity Score.
Patients were also asked to rate on a 0-4 scale their degree of fearfulness of life-threatening swelling episodes and also their degree of fearfulness of angioedema-related suffocation. “To my knowledge, this is the first time this has been done in a randomized clinical trial,” Dr. Weller noted.
The patient reports were striking: At baseline, 49% indicated that they occasionally, often, or very often were afraid of suffocating caused by swelling episodes; only 4% of patients expressed that fear after 28 weeks on omalizumab, compared with 25% of placebo-treated controls. Similarly, at baseline two-thirds of patients reported occasionally, often, or very often being fearful of life-threatening swelling episodes, a rate that fell to 14% after 28 weeks on omalizumab, compared with 42% for controls.
Scores on the Angioedema Quality of Life Questionnaire improved continuously from a baseline of roughly 60 on a 0-100 scale – indicative of severe impairment – to less than 20 after 28 weeks on omalizumab; these scores steadily worsened again during the 8 weeks following treatment discontinuation. The Dermatology Life Quality Index scores dropped from a mean baseline of 15.6 down to 5 by week 4, remained in the 3-5 range for the remainder of the treatment period, then increased again when treatment was discontinued. The Angioedema Activity Score followed a similar pattern.
One audience member observed that the placebo response was quite strong in the study, with the percentage of patients reporting fear of suffocating caused by angioedema episodes falling from 49% at baseline to 25% after 28 weeks on placebo.
Dr. Weller replied that a potent placebo response is a consistent feature of all clinical trials of CSU therapies. The explanation, he added, is unknown.
He reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
SOURCE: Weller K et al. EADV Congress 2017.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Omalizumab relieves the heavy quality-of-life burden associated with CSU.
Major finding: At baseline, 49% of CSU patients indicated they occasionally, often, or very often were afraid of suffocating due to swelling episodes; after 28 weeks on omalizumab, only 4% expressed that fear.
Study details: The X-ACT trial was a phase 3 double-blind, multicenter, placebo-controlled randomized trial including 91 patients with CSU.
Disclosures: The presenter reported receiving research grants from and serving as a consultant to Novartis, which sponsored the X-ACT trial.
Source: Weller K et al. EADV Congress 2017.
Prior biologic exposure doesn’t diminish ixekizumab’s efficacy
REPORTING FROM THE EADV CONGRESS
GENEVA – Psoriasis patients switched to ixekizumab after previous exposure to another interleukin-17 inhibitor respond as well as those who are IL-17 antagonist naive, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This finding is of importance in real-world clinical practice because it’s not at all uncommon for psoriasis patients on one biologic to have to switch to another because of insufficient efficacy, side effects, or a change in insurance coverage. Physicians would like to know what sort of responses can be expected to whatever agent they prescribe next.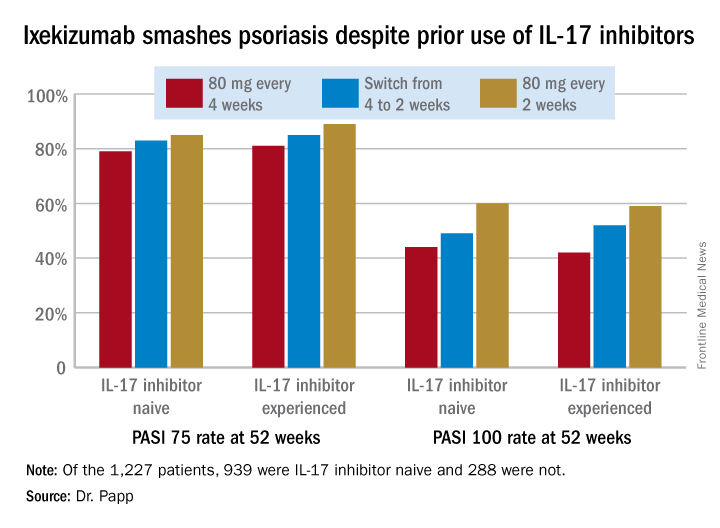
However, that was not a problem in this secondary analysis of a large clinical trial whose primary purpose was to evaluate the relative safety and efficacy of ixekizumab (Taltz) when dosed every 2 weeks versus every 4 weeks.
“I think what we have seen here is a very compelling story: , nor for that matter does it appear to impact safety,” the dermatologist said.
He reported on 1,227 patients with moderate to severe plaque psoriasis who were randomized to ixekizumab at 80 mg every 2 or 4 weeks following an initial 160 mg loading dose. Among those who started out on ixekizumab every 4 weeks, 306 patients got a per-protocol dose adjustment to biweekly therapy because of an insufficient response to monthly dosing as defined by a Physician’s Global Assessment score of 2 or more on two consecutive office visits during study weeks 12-40.
A total of 939 patients were IL-17 inhibitor naive. The other 288 had previously been on the IL-17 antagonists brodalumab (Siliq) or secukinumab (Cosentyx). The two groups had similar baseline demographics with the exception that the experienced cohort had on average a 22.2-year duration of psoriasis, 3.7 years more than IL-17 antagonist-naive patients.
In an intent-to-treat analysis, Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses at week 52 didn’t differ significantly between the IL-17 inhibitor-naive and -experienced groups. In fact, patients with prior exposure to other IL-17 antagonists showed a consistent trend for slightly higher response rates (see graphic).
It was clear from this analysis that dosing ixekizumab every 2 weeks provides significantly better efficacy than was dosing every 4 weeks, Dr. Papp noted. Yet the approved dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
No new safety issues arose in this study. The only difference between the naive and experienced groups was a lower rate of allergic reactions/hypersensitivity in the experienced group. For example, in patients on ixekizumab every 2 weeks for the entire 52-week study period the incidence of such reactions was 11.5% in the IL-17 antagonist-naive group, compared with 4.1% in the experienced cohort. This isn’t really surprising, according to Dr. Papp.
“Most injection site reactions occur in the newbies,” he said.
The study was sponsored by Eli Lilly. Dr. Papp serves as a consultant and/or adviser to Lilly and numerous other pharmaceutical companies involved in the development of dermatologic therapies.
REPORTING FROM THE EADV CONGRESS
GENEVA – Psoriasis patients switched to ixekizumab after previous exposure to another interleukin-17 inhibitor respond as well as those who are IL-17 antagonist naive, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This finding is of importance in real-world clinical practice because it’s not at all uncommon for psoriasis patients on one biologic to have to switch to another because of insufficient efficacy, side effects, or a change in insurance coverage. Physicians would like to know what sort of responses can be expected to whatever agent they prescribe next.
However, that was not a problem in this secondary analysis of a large clinical trial whose primary purpose was to evaluate the relative safety and efficacy of ixekizumab (Taltz) when dosed every 2 weeks versus every 4 weeks.
“I think what we have seen here is a very compelling story: , nor for that matter does it appear to impact safety,” the dermatologist said.
He reported on 1,227 patients with moderate to severe plaque psoriasis who were randomized to ixekizumab at 80 mg every 2 or 4 weeks following an initial 160 mg loading dose. Among those who started out on ixekizumab every 4 weeks, 306 patients got a per-protocol dose adjustment to biweekly therapy because of an insufficient response to monthly dosing as defined by a Physician’s Global Assessment score of 2 or more on two consecutive office visits during study weeks 12-40.
A total of 939 patients were IL-17 inhibitor naive. The other 288 had previously been on the IL-17 antagonists brodalumab (Siliq) or secukinumab (Cosentyx). The two groups had similar baseline demographics with the exception that the experienced cohort had on average a 22.2-year duration of psoriasis, 3.7 years more than IL-17 antagonist-naive patients.
In an intent-to-treat analysis, Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses at week 52 didn’t differ significantly between the IL-17 inhibitor-naive and -experienced groups. In fact, patients with prior exposure to other IL-17 antagonists showed a consistent trend for slightly higher response rates (see graphic).
It was clear from this analysis that dosing ixekizumab every 2 weeks provides significantly better efficacy than was dosing every 4 weeks, Dr. Papp noted. Yet the approved dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
No new safety issues arose in this study. The only difference between the naive and experienced groups was a lower rate of allergic reactions/hypersensitivity in the experienced group. For example, in patients on ixekizumab every 2 weeks for the entire 52-week study period the incidence of such reactions was 11.5% in the IL-17 antagonist-naive group, compared with 4.1% in the experienced cohort. This isn’t really surprising, according to Dr. Papp.
“Most injection site reactions occur in the newbies,” he said.
The study was sponsored by Eli Lilly. Dr. Papp serves as a consultant and/or adviser to Lilly and numerous other pharmaceutical companies involved in the development of dermatologic therapies.
REPORTING FROM THE EADV CONGRESS
GENEVA – Psoriasis patients switched to ixekizumab after previous exposure to another interleukin-17 inhibitor respond as well as those who are IL-17 antagonist naive, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This finding is of importance in real-world clinical practice because it’s not at all uncommon for psoriasis patients on one biologic to have to switch to another because of insufficient efficacy, side effects, or a change in insurance coverage. Physicians would like to know what sort of responses can be expected to whatever agent they prescribe next.
However, that was not a problem in this secondary analysis of a large clinical trial whose primary purpose was to evaluate the relative safety and efficacy of ixekizumab (Taltz) when dosed every 2 weeks versus every 4 weeks.
“I think what we have seen here is a very compelling story: , nor for that matter does it appear to impact safety,” the dermatologist said.
He reported on 1,227 patients with moderate to severe plaque psoriasis who were randomized to ixekizumab at 80 mg every 2 or 4 weeks following an initial 160 mg loading dose. Among those who started out on ixekizumab every 4 weeks, 306 patients got a per-protocol dose adjustment to biweekly therapy because of an insufficient response to monthly dosing as defined by a Physician’s Global Assessment score of 2 or more on two consecutive office visits during study weeks 12-40.
A total of 939 patients were IL-17 inhibitor naive. The other 288 had previously been on the IL-17 antagonists brodalumab (Siliq) or secukinumab (Cosentyx). The two groups had similar baseline demographics with the exception that the experienced cohort had on average a 22.2-year duration of psoriasis, 3.7 years more than IL-17 antagonist-naive patients.
In an intent-to-treat analysis, Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses at week 52 didn’t differ significantly between the IL-17 inhibitor-naive and -experienced groups. In fact, patients with prior exposure to other IL-17 antagonists showed a consistent trend for slightly higher response rates (see graphic).
It was clear from this analysis that dosing ixekizumab every 2 weeks provides significantly better efficacy than was dosing every 4 weeks, Dr. Papp noted. Yet the approved dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
No new safety issues arose in this study. The only difference between the naive and experienced groups was a lower rate of allergic reactions/hypersensitivity in the experienced group. For example, in patients on ixekizumab every 2 weeks for the entire 52-week study period the incidence of such reactions was 11.5% in the IL-17 antagonist-naive group, compared with 4.1% in the experienced cohort. This isn’t really surprising, according to Dr. Papp.
“Most injection site reactions occur in the newbies,” he said.
The study was sponsored by Eli Lilly. Dr. Papp serves as a consultant and/or adviser to Lilly and numerous other pharmaceutical companies involved in the development of dermatologic therapies.
Updosing omalizumab for chronic urticaria pays off
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
REPORTING FROM THE EADV CONGRESS
Key clinical point: Updosing of omalizumab to a maximum of twice the approved dose is safe and effective in chronic spontaneous urticaria patients unresponsive to the licensed dose.
Major finding: Upon updosing, 75% of nonresponders to the approved dose achieved good disease control with no increase in adverse events.
Study details: This multicenter study of an omalizumab updosing algorithm included 286 patients with chronic spontaneous urticaria.
Disclosures: The study presenter reported having no financial conflicts.
Skin signs spotlight highest-risk SLE patients
GENEVA – Careful warranting prompt initiation of long-term antiplatelet therapy, Dan Lipsker, MD, PhD, said in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
“Those patients are at very high risk. Those are the lupus patients with the poorest prognosis. Those are the lupus patients who still die today,” said Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
Cutaneous clues suggestive of thrombosis in SLE patients include atrophie blanche, pseudo-Degos lesions, livedo racemosa, acral nonpalpable purpura or reticulate erythema, cutaneous necrosis, splinter hemorrhage, thrombophlebitis, and nailfold telangiectasias. These skin findings can occur simultaneously with or after potentially life-threatening thrombotic events, or in the best of all scenarios, beforehand.
Dr. Lipsker told his audience of dermatologists that, by demonstrating facility in identifying these cutaneous disorders, they can make themselves “indispensable” to the rheumatologists, nephrologists, internists, and/or pediatricians who often provide the bulk of specialized care for SLE patients.
“We know today that, 5 years after initial diagnosis of SLE, the chief causes of morbidity and mortality are thrombotic events. And it can be extremely difficult to distinguish between an acute autoimmune lupus flare and a thrombotic event when, for example, the CNS or eyes are involved. But you will find direct evidence of thrombosis by carefully examining the skin,” the dermatologist maintained.
Some of these cutaneous signs constitute unequivocal evidence of thrombosis in SLE patients. Others are more ambiguous but should raise suspicion of an ongoing systemic thrombotic process unless another explanation is found.
One example of a skin finding that always indicates thrombosis is pseudo-Degos lesions: ivory-colored or white depressed atrophic papules with a raised border composed of telangiectasias. Biopsy shows evidence of a cone-shaped dermal arteriolar infarct.
“We have biopsied dozens of patients with this presentation, and you always find occluded vessels without a single inflammatory cell. These lesions are usually painful, and when you put those patients on low-dose aspirin they do better,” Dr. Lipsker said.
Atrophie blanche in a patient with SLE is also strong evidence of thrombotic vasculopathy. Atrophie blanche is a porcelain-white atrophic white scar with surrounding hyperpigmentation on the lower leg that occurs after skin injury in an area with venous insufficiency.
Livedo racemosa in a patient with SLE is also highly suggestive of a systemic thrombotic process. Characteristic of this dermatologic disorder is an irregular, netlike mottling surrounding pale skin.
“All of these skin signs allow identification of patients who have very high risk of thrombotic events,” Dr. Lipsker stressed.
He reported having no financial conflicts of interest regarding his presentation.
GENEVA – Careful warranting prompt initiation of long-term antiplatelet therapy, Dan Lipsker, MD, PhD, said in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
“Those patients are at very high risk. Those are the lupus patients with the poorest prognosis. Those are the lupus patients who still die today,” said Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
Cutaneous clues suggestive of thrombosis in SLE patients include atrophie blanche, pseudo-Degos lesions, livedo racemosa, acral nonpalpable purpura or reticulate erythema, cutaneous necrosis, splinter hemorrhage, thrombophlebitis, and nailfold telangiectasias. These skin findings can occur simultaneously with or after potentially life-threatening thrombotic events, or in the best of all scenarios, beforehand.
Dr. Lipsker told his audience of dermatologists that, by demonstrating facility in identifying these cutaneous disorders, they can make themselves “indispensable” to the rheumatologists, nephrologists, internists, and/or pediatricians who often provide the bulk of specialized care for SLE patients.
“We know today that, 5 years after initial diagnosis of SLE, the chief causes of morbidity and mortality are thrombotic events. And it can be extremely difficult to distinguish between an acute autoimmune lupus flare and a thrombotic event when, for example, the CNS or eyes are involved. But you will find direct evidence of thrombosis by carefully examining the skin,” the dermatologist maintained.
Some of these cutaneous signs constitute unequivocal evidence of thrombosis in SLE patients. Others are more ambiguous but should raise suspicion of an ongoing systemic thrombotic process unless another explanation is found.
One example of a skin finding that always indicates thrombosis is pseudo-Degos lesions: ivory-colored or white depressed atrophic papules with a raised border composed of telangiectasias. Biopsy shows evidence of a cone-shaped dermal arteriolar infarct.
“We have biopsied dozens of patients with this presentation, and you always find occluded vessels without a single inflammatory cell. These lesions are usually painful, and when you put those patients on low-dose aspirin they do better,” Dr. Lipsker said.
Atrophie blanche in a patient with SLE is also strong evidence of thrombotic vasculopathy. Atrophie blanche is a porcelain-white atrophic white scar with surrounding hyperpigmentation on the lower leg that occurs after skin injury in an area with venous insufficiency.
Livedo racemosa in a patient with SLE is also highly suggestive of a systemic thrombotic process. Characteristic of this dermatologic disorder is an irregular, netlike mottling surrounding pale skin.
“All of these skin signs allow identification of patients who have very high risk of thrombotic events,” Dr. Lipsker stressed.
He reported having no financial conflicts of interest regarding his presentation.
GENEVA – Careful warranting prompt initiation of long-term antiplatelet therapy, Dan Lipsker, MD, PhD, said in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
“Those patients are at very high risk. Those are the lupus patients with the poorest prognosis. Those are the lupus patients who still die today,” said Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
Cutaneous clues suggestive of thrombosis in SLE patients include atrophie blanche, pseudo-Degos lesions, livedo racemosa, acral nonpalpable purpura or reticulate erythema, cutaneous necrosis, splinter hemorrhage, thrombophlebitis, and nailfold telangiectasias. These skin findings can occur simultaneously with or after potentially life-threatening thrombotic events, or in the best of all scenarios, beforehand.
Dr. Lipsker told his audience of dermatologists that, by demonstrating facility in identifying these cutaneous disorders, they can make themselves “indispensable” to the rheumatologists, nephrologists, internists, and/or pediatricians who often provide the bulk of specialized care for SLE patients.
“We know today that, 5 years after initial diagnosis of SLE, the chief causes of morbidity and mortality are thrombotic events. And it can be extremely difficult to distinguish between an acute autoimmune lupus flare and a thrombotic event when, for example, the CNS or eyes are involved. But you will find direct evidence of thrombosis by carefully examining the skin,” the dermatologist maintained.
Some of these cutaneous signs constitute unequivocal evidence of thrombosis in SLE patients. Others are more ambiguous but should raise suspicion of an ongoing systemic thrombotic process unless another explanation is found.
One example of a skin finding that always indicates thrombosis is pseudo-Degos lesions: ivory-colored or white depressed atrophic papules with a raised border composed of telangiectasias. Biopsy shows evidence of a cone-shaped dermal arteriolar infarct.
“We have biopsied dozens of patients with this presentation, and you always find occluded vessels without a single inflammatory cell. These lesions are usually painful, and when you put those patients on low-dose aspirin they do better,” Dr. Lipsker said.
Atrophie blanche in a patient with SLE is also strong evidence of thrombotic vasculopathy. Atrophie blanche is a porcelain-white atrophic white scar with surrounding hyperpigmentation on the lower leg that occurs after skin injury in an area with venous insufficiency.
Livedo racemosa in a patient with SLE is also highly suggestive of a systemic thrombotic process. Characteristic of this dermatologic disorder is an irregular, netlike mottling surrounding pale skin.
“All of these skin signs allow identification of patients who have very high risk of thrombotic events,” Dr. Lipsker stressed.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Guselkumab crushes skin disease in psoriatic arthritis patients
GENEVA – The interleukin-23 inhibitor guselkumab generates the same impressive improvement in skin disease in psoriatic arthritis patients as has been seen in psoriasis without joint disease, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
However, psoriatic arthritis patients’ improvement in Dermatology Life Quality Index (DLQI) scores is less robust than in patients with psoriasis only, added Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, and CEO of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center.
The psoriatic arthritis group as a whole had more severe psoriasis, with a baseline mean PASI score of 24.3 and involvement of 32.7% of their body surface area as compared with a PASI score of 21.2 and 27.2% BSA in psoriasis patients without arthritis. A total of 28% of the psoriatic arthritis patients had previously been on other biologics and 77% had been on nonbiologic systemic agents, compared with 19% and 60% of the psoriasis patients, respectively. The psoriatic arthritis group had a mean 19.2-year history of psoriasis, 1.9 years longer than the psoriasis-only group.
Participants were randomized to 100 mg of guselkumab administered subcutaneously at weeks 0, 4, 12, and 20; placebo through week 12, followed by a switch to adalimumab (Humira); or adalimumab at 80 mg at week 0, then 40 mg at week 2 and 40 mg again every 2 weeks until week 23.
The key findings:
The PASI 90 response rate – that is, at least a 90% improvement in Psoriasis Area and Severity Index – in guselkumab-treated patients at week 16 was 72% in patients with psoriatic arthritis and 71% in those without. At week 24, the PASI 90 rate was 74% in guselkumab-treated patients with psoriatic arthritis and similar at 78% in those without. In contrast, the PASI 90 rate at week 24 in patients on adalimumab was significantly lower: 48% in the psoriatic arthritis group and 55% in those with psoriasis only. The PASI 90 rate in placebo-treated controls was single digit.
At week 24, 82% of psoriatic arthritis patients on guselkumab had clear or almost clear skin as reflected in an Investigator’s Global Assessment score of 0 or 1, as did 84% of psoriasis-only patients.
A DLQI score of 0 or 1, meaning the dermatologic disease had no impact on patient quality of life, was documented at week 16 in 46% of psoriatic arthritis patients and 55% of psoriasis-only patients, a trend that didn’t achieve statistical significance. However, by week 24 the difference became significant, with a DLQI of 0 or 1 in 48% of the psoriatic arthritis patients, compared with 62% of psoriasis-only patients.
VOYAGE 1 and 2 were dermatologic studies that didn’t measure changes in joint symptom scores or other psoriatic arthritis outcomes. Guselkumab as a potential treatment for psoriatic arthritis is under investigation in other studies.
The VOYAGE trials and this analysis were sponsored by Janssen. Dr. Kimball reported receiving research funding from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
SOURCE: Kimball A et al. https://eadvgeneva2017.org/
GENEVA – The interleukin-23 inhibitor guselkumab generates the same impressive improvement in skin disease in psoriatic arthritis patients as has been seen in psoriasis without joint disease, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
However, psoriatic arthritis patients’ improvement in Dermatology Life Quality Index (DLQI) scores is less robust than in patients with psoriasis only, added Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, and CEO of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center.
The psoriatic arthritis group as a whole had more severe psoriasis, with a baseline mean PASI score of 24.3 and involvement of 32.7% of their body surface area as compared with a PASI score of 21.2 and 27.2% BSA in psoriasis patients without arthritis. A total of 28% of the psoriatic arthritis patients had previously been on other biologics and 77% had been on nonbiologic systemic agents, compared with 19% and 60% of the psoriasis patients, respectively. The psoriatic arthritis group had a mean 19.2-year history of psoriasis, 1.9 years longer than the psoriasis-only group.
Participants were randomized to 100 mg of guselkumab administered subcutaneously at weeks 0, 4, 12, and 20; placebo through week 12, followed by a switch to adalimumab (Humira); or adalimumab at 80 mg at week 0, then 40 mg at week 2 and 40 mg again every 2 weeks until week 23.
The key findings:
The PASI 90 response rate – that is, at least a 90% improvement in Psoriasis Area and Severity Index – in guselkumab-treated patients at week 16 was 72% in patients with psoriatic arthritis and 71% in those without. At week 24, the PASI 90 rate was 74% in guselkumab-treated patients with psoriatic arthritis and similar at 78% in those without. In contrast, the PASI 90 rate at week 24 in patients on adalimumab was significantly lower: 48% in the psoriatic arthritis group and 55% in those with psoriasis only. The PASI 90 rate in placebo-treated controls was single digit.
At week 24, 82% of psoriatic arthritis patients on guselkumab had clear or almost clear skin as reflected in an Investigator’s Global Assessment score of 0 or 1, as did 84% of psoriasis-only patients.
A DLQI score of 0 or 1, meaning the dermatologic disease had no impact on patient quality of life, was documented at week 16 in 46% of psoriatic arthritis patients and 55% of psoriasis-only patients, a trend that didn’t achieve statistical significance. However, by week 24 the difference became significant, with a DLQI of 0 or 1 in 48% of the psoriatic arthritis patients, compared with 62% of psoriasis-only patients.
VOYAGE 1 and 2 were dermatologic studies that didn’t measure changes in joint symptom scores or other psoriatic arthritis outcomes. Guselkumab as a potential treatment for psoriatic arthritis is under investigation in other studies.
The VOYAGE trials and this analysis were sponsored by Janssen. Dr. Kimball reported receiving research funding from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
SOURCE: Kimball A et al. https://eadvgeneva2017.org/
GENEVA – The interleukin-23 inhibitor guselkumab generates the same impressive improvement in skin disease in psoriatic arthritis patients as has been seen in psoriasis without joint disease, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
However, psoriatic arthritis patients’ improvement in Dermatology Life Quality Index (DLQI) scores is less robust than in patients with psoriasis only, added Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, and CEO of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center.
The psoriatic arthritis group as a whole had more severe psoriasis, with a baseline mean PASI score of 24.3 and involvement of 32.7% of their body surface area as compared with a PASI score of 21.2 and 27.2% BSA in psoriasis patients without arthritis. A total of 28% of the psoriatic arthritis patients had previously been on other biologics and 77% had been on nonbiologic systemic agents, compared with 19% and 60% of the psoriasis patients, respectively. The psoriatic arthritis group had a mean 19.2-year history of psoriasis, 1.9 years longer than the psoriasis-only group.
Participants were randomized to 100 mg of guselkumab administered subcutaneously at weeks 0, 4, 12, and 20; placebo through week 12, followed by a switch to adalimumab (Humira); or adalimumab at 80 mg at week 0, then 40 mg at week 2 and 40 mg again every 2 weeks until week 23.
The key findings:
The PASI 90 response rate – that is, at least a 90% improvement in Psoriasis Area and Severity Index – in guselkumab-treated patients at week 16 was 72% in patients with psoriatic arthritis and 71% in those without. At week 24, the PASI 90 rate was 74% in guselkumab-treated patients with psoriatic arthritis and similar at 78% in those without. In contrast, the PASI 90 rate at week 24 in patients on adalimumab was significantly lower: 48% in the psoriatic arthritis group and 55% in those with psoriasis only. The PASI 90 rate in placebo-treated controls was single digit.
At week 24, 82% of psoriatic arthritis patients on guselkumab had clear or almost clear skin as reflected in an Investigator’s Global Assessment score of 0 or 1, as did 84% of psoriasis-only patients.
A DLQI score of 0 or 1, meaning the dermatologic disease had no impact on patient quality of life, was documented at week 16 in 46% of psoriatic arthritis patients and 55% of psoriasis-only patients, a trend that didn’t achieve statistical significance. However, by week 24 the difference became significant, with a DLQI of 0 or 1 in 48% of the psoriatic arthritis patients, compared with 62% of psoriasis-only patients.
VOYAGE 1 and 2 were dermatologic studies that didn’t measure changes in joint symptom scores or other psoriatic arthritis outcomes. Guselkumab as a potential treatment for psoriatic arthritis is under investigation in other studies.
The VOYAGE trials and this analysis were sponsored by Janssen. Dr. Kimball reported receiving research funding from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
SOURCE: Kimball A et al. https://eadvgeneva2017.org/
REPORTING FROM THE EADV CONGRESS
Key clinical point:
Major finding: After 16 weeks on guselkumab, 72% of psoriatic arthritis patients and 71% with psoriasis-only had a PASI 90 response.
Study details: This was a comparison of skin and DLQI outcomes in 335 patients with psoriatic arthritis and 1,494 with psoriasis only who participated in two randomized, double-blind, phase 3 clinical trials.
Disclosures: Janssen sponsored the study. The presenter reported receiving research grants from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
Source: Kimball A et al. https://eadvgeneva2017.org/.
JAK inhibitors for atopic dermatitis might hit JAK-pot
GENEVA – at the annual congress of the European Academy of Dermatology and Venereology, with three positive phase 2 randomized trials featuring one topical and two oral agents presented to enthusiastic audiences.
The way has already been paved for dermatologic researchers by veterinarians, who developed oclacitinib (Apoquel), a relatively selective Janus kinase 1 (JAK1) inhibitor, for canine AD. The medication was approved by the Food and Drug Administration in 2013 for treating AD and for controlling pruritus associated with allergic dermatitis in dogs.
PF-04965842
“Get out your pencils, everyone. This is why you’re all here at 8 o’clock on a Sunday morning,” Melinda Gooderham, MD, said, standing before a packed house at the main arena of the Geneva Convention Center, as she launched into the results of a phase II randomized, double-blind, placebo-controlled, 12-week trial of a JAK inhibitor known for now as PF-04965842. This is a JAK1-selective agent with a good effect on interleukin-4 and -13, key mediators of the Th2 cytokines implicated in the pathogenesis of AD.
The dose-ranging study included 250 adults with AD and an inadequate response to or intolerance of topical therapy. Their mean baseline Eczema Area and Severity Index (EASI) score was 25 with a 60/40 ratio of moderate to severe AD. The five-arm trial randomized patients to PF-04965842 at 10 mg, 30 mg, 100 mg, or 200 mg once daily or placebo.
The primary endpoint was the proportion of patients achieving an Investigator Global Assessment (IGA) score of 0 or 1 – clear or almost clear – along with at least a 2-grade improvement from baseline at week 12. A clear dose-response effect was evident, with the 100- and 200-mg doses achieving response rates of 28% and 45%, respectively, compared with 6% in placebo-treated controls, reported Dr. Gooderham, medical director of the Skin Center for Dermatology in Peterborough, Ont., and a dermatologist at Queen’s University in Kingston, Ont.
Onset of action was speedy: patients in the 200-mg group reached their full improvement in IGA score by week 4 and maintained that response through week 12. Maximum improvement in EASI score – a mean 80% reduction – was achieved by week 6 and sustained thereafter. The proportion of patients in the 200-mg group achieving at least a 4-point improvement on the Pruritus Numeric Rating Scale significantly exceeded that in the placebo group as early as day 2 of the trial. At week 12, 64% of patients in the 200-mg group had achieved this level of improvement in itch, compared with 26% of controls.
A dose-dependent drop in platelet count occurred in the study, reaching a 30% decline at the 4-week nadir in the 200-mg group, followed by gradual on-treatment recovery. Both LDL and HDL cholesterol rose on active therapy – a class effect of JAK inhibitors – but the ratio between the two lipid levels remained unchanged. The two serious adverse events deemed treatment related were a case of eczema herpeticum in a patient on the 100-mg dose and pneumonia in a patient on the 200-mg dose.
Baricitinib
This once-daily oral JAK1/2 inhibitor is approved for treatment of rheumatoid arthritis in Europe and Japan. Emma Guttman-Yassky, MD, PhD, presented a phase 2 study of baricitinib in 124 adults with moderate to severe AD. Notably, prior to enrollment, all participants had to have failed to respond to a 4-week run-in period of supervised treatment with 0.1% triamcinolone cream, a midpotency topical steroid. They were then randomized to 2 mg or 4 mg of once-daily baricitinib or placebo, in all cases supplemented as needed with the topical steroid. Their median baseline EASI score was 21.
The primary endpoint was the proportion of patients achieving at least a 50% improvement in EASI score, or EASI 50 response, by week 16 from baseline in a nonresponder imputation analysis. This was achieved in 65% of patients on the 4-mg dose of baricitinib, 64% on the 2-mg dose, and 46% of controls on placebo plus the topical steroid. A statistically significant difference in EASI 50 response between the baricitinib groups and controls was seen at 1 week, with nearly the maximum effect achieved at week 4. Patients with a baseline EASI score above the median had a much more impressive treatment response because the placebo effect was smaller in participants with more severe AD.
“I think this drug can be an exciting new addition to the field,” declared Dr. Guttman-Yassky, professor and vice chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
From a baseline total SCORAD (Scoring Atopic Dermatitis) score of 55, major improvements were seen in the JAK inhibitor–treated patients by week 4. At week 16, the average reduction from baseline was 47% in the 4-mg group, 41% in the 2-mg group, and 21% in the placebo group. Both the SCORAD pruritus and sleep loss subscores showed significantly more robust improvement in the baricitinib groups than in controls. Indeed, a significant drop in pruritus scores was noted within the first week.
The 4-mg dose was associated with greater improvement and faster onset of action than the 2-mg dose on some but not all disease measures.
Dr. Guttman-Yassky described baricitinib as having “an overall acceptable safety profile,” with no serious treatment-related adverse events noted. Headache, nasopharyngitis, and asymptomatic increases in serum creatinine phosphokinase were more common in baricitinib-treated patients than with placebo.
JTE-052
Hidemi Nakagawa, MD, presented a phase 2 study of topical JTE-052 ointment in 327 Japanese adults with moderate to severe AD. The drug inhibits JAK1/2/3 as well as the tyrosine kinase pathway. It also promotes keratinocyte production of filaggrin in the skin barrier. Participants were randomized to twice-daily application of JTE-052 ointment (at 0.25%, 0.5%, 1%, or 3%), vehicle ointment, or 0.1% tacrolimus ointment twice a day for 4 weeks. The primary outcome was the change from baseline in modified EASI score in the active treatment groups compared with placebo. All doses of JTE-052 proved significantly more effective than vehicle. A dose-response effect was noted, with a 42% reduction from baseline in modified EASI score in the 0.25% JTE-052 group, a 57% reduction with 0.5%, a 55% reduction with 1% ointment, and a 73% reduction with 3%, compared with a 12% reduction decrease in patients who received vehicle. The topical tacrolimus group showed a 62% reduction from baseline, reported Dr. Nakagawa, professor and head of the division of dermatology at Jikei University, Tokyo.
All doses of JTE-052 were also significantly more effective than placebo on all secondary endpoints, which included IGA, percent body surface area affected, and Pruritus Numeric Rating Scale score.
At all but the weakest concentration, JTE-052 resulted in significant reduction in pruritus starting with the second dose on day 1 of the trial, he added.
Mild nasopharyngitis occurred in 3.4% of JTE-052–treated patients. There were no serious adverse events and no changes in laboratory parameters in the study. One patient discontinued JTE-052 because of application-site contact dermatitis, another because of application-site irritation. The results of this study were recently published in the British Journal of Dermatology (Br J Dermatol. 2017 Sep 28. doi: 10.1111/bjd.16014).
Dr. Nakagawa reported receiving research grants from and serving as a consultant to Japan Tobacco, which is developing JTE-052. Dr. Guttman-Yassky reported having financial relationships with Eli Lilly and Incyte, which sponsored the baricitinib study, as well as most other pharmaceutical companies developing therapies for AD. Dr. Gooderham reported receiving research funding from and serving as a consultant to Pfizer, which sponsored the PF-04965842 study, as well as numerous other pharmaceutical companies.
GENEVA – at the annual congress of the European Academy of Dermatology and Venereology, with three positive phase 2 randomized trials featuring one topical and two oral agents presented to enthusiastic audiences.
The way has already been paved for dermatologic researchers by veterinarians, who developed oclacitinib (Apoquel), a relatively selective Janus kinase 1 (JAK1) inhibitor, for canine AD. The medication was approved by the Food and Drug Administration in 2013 for treating AD and for controlling pruritus associated with allergic dermatitis in dogs.
PF-04965842
“Get out your pencils, everyone. This is why you’re all here at 8 o’clock on a Sunday morning,” Melinda Gooderham, MD, said, standing before a packed house at the main arena of the Geneva Convention Center, as she launched into the results of a phase II randomized, double-blind, placebo-controlled, 12-week trial of a JAK inhibitor known for now as PF-04965842. This is a JAK1-selective agent with a good effect on interleukin-4 and -13, key mediators of the Th2 cytokines implicated in the pathogenesis of AD.
The dose-ranging study included 250 adults with AD and an inadequate response to or intolerance of topical therapy. Their mean baseline Eczema Area and Severity Index (EASI) score was 25 with a 60/40 ratio of moderate to severe AD. The five-arm trial randomized patients to PF-04965842 at 10 mg, 30 mg, 100 mg, or 200 mg once daily or placebo.
The primary endpoint was the proportion of patients achieving an Investigator Global Assessment (IGA) score of 0 or 1 – clear or almost clear – along with at least a 2-grade improvement from baseline at week 12. A clear dose-response effect was evident, with the 100- and 200-mg doses achieving response rates of 28% and 45%, respectively, compared with 6% in placebo-treated controls, reported Dr. Gooderham, medical director of the Skin Center for Dermatology in Peterborough, Ont., and a dermatologist at Queen’s University in Kingston, Ont.
Onset of action was speedy: patients in the 200-mg group reached their full improvement in IGA score by week 4 and maintained that response through week 12. Maximum improvement in EASI score – a mean 80% reduction – was achieved by week 6 and sustained thereafter. The proportion of patients in the 200-mg group achieving at least a 4-point improvement on the Pruritus Numeric Rating Scale significantly exceeded that in the placebo group as early as day 2 of the trial. At week 12, 64% of patients in the 200-mg group had achieved this level of improvement in itch, compared with 26% of controls.
A dose-dependent drop in platelet count occurred in the study, reaching a 30% decline at the 4-week nadir in the 200-mg group, followed by gradual on-treatment recovery. Both LDL and HDL cholesterol rose on active therapy – a class effect of JAK inhibitors – but the ratio between the two lipid levels remained unchanged. The two serious adverse events deemed treatment related were a case of eczema herpeticum in a patient on the 100-mg dose and pneumonia in a patient on the 200-mg dose.
Baricitinib
This once-daily oral JAK1/2 inhibitor is approved for treatment of rheumatoid arthritis in Europe and Japan. Emma Guttman-Yassky, MD, PhD, presented a phase 2 study of baricitinib in 124 adults with moderate to severe AD. Notably, prior to enrollment, all participants had to have failed to respond to a 4-week run-in period of supervised treatment with 0.1% triamcinolone cream, a midpotency topical steroid. They were then randomized to 2 mg or 4 mg of once-daily baricitinib or placebo, in all cases supplemented as needed with the topical steroid. Their median baseline EASI score was 21.
The primary endpoint was the proportion of patients achieving at least a 50% improvement in EASI score, or EASI 50 response, by week 16 from baseline in a nonresponder imputation analysis. This was achieved in 65% of patients on the 4-mg dose of baricitinib, 64% on the 2-mg dose, and 46% of controls on placebo plus the topical steroid. A statistically significant difference in EASI 50 response between the baricitinib groups and controls was seen at 1 week, with nearly the maximum effect achieved at week 4. Patients with a baseline EASI score above the median had a much more impressive treatment response because the placebo effect was smaller in participants with more severe AD.
“I think this drug can be an exciting new addition to the field,” declared Dr. Guttman-Yassky, professor and vice chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
From a baseline total SCORAD (Scoring Atopic Dermatitis) score of 55, major improvements were seen in the JAK inhibitor–treated patients by week 4. At week 16, the average reduction from baseline was 47% in the 4-mg group, 41% in the 2-mg group, and 21% in the placebo group. Both the SCORAD pruritus and sleep loss subscores showed significantly more robust improvement in the baricitinib groups than in controls. Indeed, a significant drop in pruritus scores was noted within the first week.
The 4-mg dose was associated with greater improvement and faster onset of action than the 2-mg dose on some but not all disease measures.
Dr. Guttman-Yassky described baricitinib as having “an overall acceptable safety profile,” with no serious treatment-related adverse events noted. Headache, nasopharyngitis, and asymptomatic increases in serum creatinine phosphokinase were more common in baricitinib-treated patients than with placebo.
JTE-052
Hidemi Nakagawa, MD, presented a phase 2 study of topical JTE-052 ointment in 327 Japanese adults with moderate to severe AD. The drug inhibits JAK1/2/3 as well as the tyrosine kinase pathway. It also promotes keratinocyte production of filaggrin in the skin barrier. Participants were randomized to twice-daily application of JTE-052 ointment (at 0.25%, 0.5%, 1%, or 3%), vehicle ointment, or 0.1% tacrolimus ointment twice a day for 4 weeks. The primary outcome was the change from baseline in modified EASI score in the active treatment groups compared with placebo. All doses of JTE-052 proved significantly more effective than vehicle. A dose-response effect was noted, with a 42% reduction from baseline in modified EASI score in the 0.25% JTE-052 group, a 57% reduction with 0.5%, a 55% reduction with 1% ointment, and a 73% reduction with 3%, compared with a 12% reduction decrease in patients who received vehicle. The topical tacrolimus group showed a 62% reduction from baseline, reported Dr. Nakagawa, professor and head of the division of dermatology at Jikei University, Tokyo.
All doses of JTE-052 were also significantly more effective than placebo on all secondary endpoints, which included IGA, percent body surface area affected, and Pruritus Numeric Rating Scale score.
At all but the weakest concentration, JTE-052 resulted in significant reduction in pruritus starting with the second dose on day 1 of the trial, he added.
Mild nasopharyngitis occurred in 3.4% of JTE-052–treated patients. There were no serious adverse events and no changes in laboratory parameters in the study. One patient discontinued JTE-052 because of application-site contact dermatitis, another because of application-site irritation. The results of this study were recently published in the British Journal of Dermatology (Br J Dermatol. 2017 Sep 28. doi: 10.1111/bjd.16014).
Dr. Nakagawa reported receiving research grants from and serving as a consultant to Japan Tobacco, which is developing JTE-052. Dr. Guttman-Yassky reported having financial relationships with Eli Lilly and Incyte, which sponsored the baricitinib study, as well as most other pharmaceutical companies developing therapies for AD. Dr. Gooderham reported receiving research funding from and serving as a consultant to Pfizer, which sponsored the PF-04965842 study, as well as numerous other pharmaceutical companies.
GENEVA – at the annual congress of the European Academy of Dermatology and Venereology, with three positive phase 2 randomized trials featuring one topical and two oral agents presented to enthusiastic audiences.
The way has already been paved for dermatologic researchers by veterinarians, who developed oclacitinib (Apoquel), a relatively selective Janus kinase 1 (JAK1) inhibitor, for canine AD. The medication was approved by the Food and Drug Administration in 2013 for treating AD and for controlling pruritus associated with allergic dermatitis in dogs.
PF-04965842
“Get out your pencils, everyone. This is why you’re all here at 8 o’clock on a Sunday morning,” Melinda Gooderham, MD, said, standing before a packed house at the main arena of the Geneva Convention Center, as she launched into the results of a phase II randomized, double-blind, placebo-controlled, 12-week trial of a JAK inhibitor known for now as PF-04965842. This is a JAK1-selective agent with a good effect on interleukin-4 and -13, key mediators of the Th2 cytokines implicated in the pathogenesis of AD.
The dose-ranging study included 250 adults with AD and an inadequate response to or intolerance of topical therapy. Their mean baseline Eczema Area and Severity Index (EASI) score was 25 with a 60/40 ratio of moderate to severe AD. The five-arm trial randomized patients to PF-04965842 at 10 mg, 30 mg, 100 mg, or 200 mg once daily or placebo.
The primary endpoint was the proportion of patients achieving an Investigator Global Assessment (IGA) score of 0 or 1 – clear or almost clear – along with at least a 2-grade improvement from baseline at week 12. A clear dose-response effect was evident, with the 100- and 200-mg doses achieving response rates of 28% and 45%, respectively, compared with 6% in placebo-treated controls, reported Dr. Gooderham, medical director of the Skin Center for Dermatology in Peterborough, Ont., and a dermatologist at Queen’s University in Kingston, Ont.
Onset of action was speedy: patients in the 200-mg group reached their full improvement in IGA score by week 4 and maintained that response through week 12. Maximum improvement in EASI score – a mean 80% reduction – was achieved by week 6 and sustained thereafter. The proportion of patients in the 200-mg group achieving at least a 4-point improvement on the Pruritus Numeric Rating Scale significantly exceeded that in the placebo group as early as day 2 of the trial. At week 12, 64% of patients in the 200-mg group had achieved this level of improvement in itch, compared with 26% of controls.
A dose-dependent drop in platelet count occurred in the study, reaching a 30% decline at the 4-week nadir in the 200-mg group, followed by gradual on-treatment recovery. Both LDL and HDL cholesterol rose on active therapy – a class effect of JAK inhibitors – but the ratio between the two lipid levels remained unchanged. The two serious adverse events deemed treatment related were a case of eczema herpeticum in a patient on the 100-mg dose and pneumonia in a patient on the 200-mg dose.
Baricitinib
This once-daily oral JAK1/2 inhibitor is approved for treatment of rheumatoid arthritis in Europe and Japan. Emma Guttman-Yassky, MD, PhD, presented a phase 2 study of baricitinib in 124 adults with moderate to severe AD. Notably, prior to enrollment, all participants had to have failed to respond to a 4-week run-in period of supervised treatment with 0.1% triamcinolone cream, a midpotency topical steroid. They were then randomized to 2 mg or 4 mg of once-daily baricitinib or placebo, in all cases supplemented as needed with the topical steroid. Their median baseline EASI score was 21.
The primary endpoint was the proportion of patients achieving at least a 50% improvement in EASI score, or EASI 50 response, by week 16 from baseline in a nonresponder imputation analysis. This was achieved in 65% of patients on the 4-mg dose of baricitinib, 64% on the 2-mg dose, and 46% of controls on placebo plus the topical steroid. A statistically significant difference in EASI 50 response between the baricitinib groups and controls was seen at 1 week, with nearly the maximum effect achieved at week 4. Patients with a baseline EASI score above the median had a much more impressive treatment response because the placebo effect was smaller in participants with more severe AD.
“I think this drug can be an exciting new addition to the field,” declared Dr. Guttman-Yassky, professor and vice chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
From a baseline total SCORAD (Scoring Atopic Dermatitis) score of 55, major improvements were seen in the JAK inhibitor–treated patients by week 4. At week 16, the average reduction from baseline was 47% in the 4-mg group, 41% in the 2-mg group, and 21% in the placebo group. Both the SCORAD pruritus and sleep loss subscores showed significantly more robust improvement in the baricitinib groups than in controls. Indeed, a significant drop in pruritus scores was noted within the first week.
The 4-mg dose was associated with greater improvement and faster onset of action than the 2-mg dose on some but not all disease measures.
Dr. Guttman-Yassky described baricitinib as having “an overall acceptable safety profile,” with no serious treatment-related adverse events noted. Headache, nasopharyngitis, and asymptomatic increases in serum creatinine phosphokinase were more common in baricitinib-treated patients than with placebo.
JTE-052
Hidemi Nakagawa, MD, presented a phase 2 study of topical JTE-052 ointment in 327 Japanese adults with moderate to severe AD. The drug inhibits JAK1/2/3 as well as the tyrosine kinase pathway. It also promotes keratinocyte production of filaggrin in the skin barrier. Participants were randomized to twice-daily application of JTE-052 ointment (at 0.25%, 0.5%, 1%, or 3%), vehicle ointment, or 0.1% tacrolimus ointment twice a day for 4 weeks. The primary outcome was the change from baseline in modified EASI score in the active treatment groups compared with placebo. All doses of JTE-052 proved significantly more effective than vehicle. A dose-response effect was noted, with a 42% reduction from baseline in modified EASI score in the 0.25% JTE-052 group, a 57% reduction with 0.5%, a 55% reduction with 1% ointment, and a 73% reduction with 3%, compared with a 12% reduction decrease in patients who received vehicle. The topical tacrolimus group showed a 62% reduction from baseline, reported Dr. Nakagawa, professor and head of the division of dermatology at Jikei University, Tokyo.
All doses of JTE-052 were also significantly more effective than placebo on all secondary endpoints, which included IGA, percent body surface area affected, and Pruritus Numeric Rating Scale score.
At all but the weakest concentration, JTE-052 resulted in significant reduction in pruritus starting with the second dose on day 1 of the trial, he added.
Mild nasopharyngitis occurred in 3.4% of JTE-052–treated patients. There were no serious adverse events and no changes in laboratory parameters in the study. One patient discontinued JTE-052 because of application-site contact dermatitis, another because of application-site irritation. The results of this study were recently published in the British Journal of Dermatology (Br J Dermatol. 2017 Sep 28. doi: 10.1111/bjd.16014).
Dr. Nakagawa reported receiving research grants from and serving as a consultant to Japan Tobacco, which is developing JTE-052. Dr. Guttman-Yassky reported having financial relationships with Eli Lilly and Incyte, which sponsored the baricitinib study, as well as most other pharmaceutical companies developing therapies for AD. Dr. Gooderham reported receiving research funding from and serving as a consultant to Pfizer, which sponsored the PF-04965842 study, as well as numerous other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS