User login
Choroid plexuses may play a role in migraine
according to a new study. The molecule could be a novel biomarker for the study of the mechanisms that underlie migraine. The work also suggests that the barrier between blood and CSF, sometimes described as leaky, is in fact selectively permeable.
The findings complement recent PET and dynamic contrast-enhanced MRI studies that have shown no sign of damage to the blood brain barrier (BBB) in migraine. Instead, there may be heightened transport of some molecules from blood to the CSF, evidenced by greater increases in fibrinogen levels in CSF than albumin. sVCAM1 might influence BBB or blood-CSF barrier permeability, possibly as a protective measure against fibrinogen, according to Michael Harrington, MD, scientific director of neuroscience at the Huntington Medical Research Institutes, Pasadena, Calif., who presented the findings in a poster at the virtual annual meeting of the American Headache Society.
BBB disruption?
The BBB is a well-known structure that regulates what molecules enter the brain, but the blood-CSF barrier, while lesser known, is also important. It comprises choroid plexus epithelial cells that oversee selective exchange of waste products, ions, and nutrients. Acute inflammation or chronic effects from conditions like stroke, multiple sclerosis, and Alzheimer’s disease can alter the function of this barrier.
No other capillary biomarkers were different between controls and patients with migraine – only sVCAM1. “My data supports a highly selective transport change from blood to CSF, which I propose is less likely to come from brain capillaries than choroid plexuses, especially since choroid plexuses produce the bulk of the CSF. It’s a work in progress, but based on this likelihood of choroid plexus involvement, I am accumulating more data that support the choroid plexuses as the primary source of change in migraine,” said Dr. Harrington in an interview.
“The most important finding of the study is that the blood brain barrier is not compromised in people with migraine,” said Rami Burstein, PhD, professor of neuroscience at Harvard Medical School, Boston, who was asked to comment on the findings. “Most unwanted adverse events are caused by drug action inside the brain, and thus, peripherally acting drugs become more favorable as they usually have fewer side effects. Given that the headache aspect of migraine could be intercepted outside the brain, the fact that the BBB is not compromised is a very good news,” Dr. Burstein added.
Dr. Harrington’s team recruited 74 subjects: 14 nonmigraine controls, 16 who were experiencing migraine illness (ictal), 27 not experiencing migraine illness (interictal), and 17 with chronic migraine. The CSF/serum quotient for albumen was higher in the 60 migraineurs than in the 14 controls (5.6 g/L vs. 4.1 g/L; P = .04), as was the CSF/serum quotient for fibrinogen (161.5 g/L vs. 86.1 g/L; P = .007). CSF levels of plasminogen were also higher in patients with migraine (240.7 ng/mL vs. 186.2 ng/mL; P = .03).
When the researchers compared ictal to interictal subjects, they found no difference in fibrinogen or albumen. That suggested that these values are generally increased in migraine patients compared with controls, rather than spiking during attacks. They also divided subjects by annual frequency, including groups experiencing fewer than 24 migraines per year, 24-180 attacks per year, and more than 180 attacks per year. The quotient for fibrinogen increased in migraineurs in general, compared with controls, but then decreased as the frequency of migraine went up (198.6 g/L, 167.0 g/L, and 121.6 g/L, respectively; P = .004).
CSF levels of sVCAM1 were 4.7 ng/mL in controls, 4.5 in the group with fewer than 24 migraines per year, 5.5 in the 24-180 group, and 7.1 in the group with more than 180 (P = .004).
Implications for therapy
The research, though at a very early stage, could have implications for therapies. Most drugs that treat migraine remain something of a mystery because researchers don’t know for sure where they act. In the brain? Systemically? The question of permeability of various molecules through both barriers could lend insight into what’s happening. “That’s why there is interest in barrier transport, and we’re showing there is a selective change of transport in migraineurs,” said Dr. Harrington.
As for more general therapeutic implications, “I can only speculate, but clearly there is baseline altered transport, probably in the choroid plexuses of these people,” said Dr. Harrington. He added that in time researchers might test drugs to see if they alter sVCAM1 levels or even develop novel drug candidates to act directly on it.
But he also sounded a note of caution because of the exploratory nature of the study. “These are all really early speculations.”
The study was funded by NIH, the Sunstar Foundation, Wyngs Foundation, and the Higgins Family. Dr. Harrington has no relevant disclosures.
SOURCE: Harrington M et al. AHS 2020, Abstract 842752.
according to a new study. The molecule could be a novel biomarker for the study of the mechanisms that underlie migraine. The work also suggests that the barrier between blood and CSF, sometimes described as leaky, is in fact selectively permeable.
The findings complement recent PET and dynamic contrast-enhanced MRI studies that have shown no sign of damage to the blood brain barrier (BBB) in migraine. Instead, there may be heightened transport of some molecules from blood to the CSF, evidenced by greater increases in fibrinogen levels in CSF than albumin. sVCAM1 might influence BBB or blood-CSF barrier permeability, possibly as a protective measure against fibrinogen, according to Michael Harrington, MD, scientific director of neuroscience at the Huntington Medical Research Institutes, Pasadena, Calif., who presented the findings in a poster at the virtual annual meeting of the American Headache Society.
BBB disruption?
The BBB is a well-known structure that regulates what molecules enter the brain, but the blood-CSF barrier, while lesser known, is also important. It comprises choroid plexus epithelial cells that oversee selective exchange of waste products, ions, and nutrients. Acute inflammation or chronic effects from conditions like stroke, multiple sclerosis, and Alzheimer’s disease can alter the function of this barrier.
No other capillary biomarkers were different between controls and patients with migraine – only sVCAM1. “My data supports a highly selective transport change from blood to CSF, which I propose is less likely to come from brain capillaries than choroid plexuses, especially since choroid plexuses produce the bulk of the CSF. It’s a work in progress, but based on this likelihood of choroid plexus involvement, I am accumulating more data that support the choroid plexuses as the primary source of change in migraine,” said Dr. Harrington in an interview.
“The most important finding of the study is that the blood brain barrier is not compromised in people with migraine,” said Rami Burstein, PhD, professor of neuroscience at Harvard Medical School, Boston, who was asked to comment on the findings. “Most unwanted adverse events are caused by drug action inside the brain, and thus, peripherally acting drugs become more favorable as they usually have fewer side effects. Given that the headache aspect of migraine could be intercepted outside the brain, the fact that the BBB is not compromised is a very good news,” Dr. Burstein added.
Dr. Harrington’s team recruited 74 subjects: 14 nonmigraine controls, 16 who were experiencing migraine illness (ictal), 27 not experiencing migraine illness (interictal), and 17 with chronic migraine. The CSF/serum quotient for albumen was higher in the 60 migraineurs than in the 14 controls (5.6 g/L vs. 4.1 g/L; P = .04), as was the CSF/serum quotient for fibrinogen (161.5 g/L vs. 86.1 g/L; P = .007). CSF levels of plasminogen were also higher in patients with migraine (240.7 ng/mL vs. 186.2 ng/mL; P = .03).
When the researchers compared ictal to interictal subjects, they found no difference in fibrinogen or albumen. That suggested that these values are generally increased in migraine patients compared with controls, rather than spiking during attacks. They also divided subjects by annual frequency, including groups experiencing fewer than 24 migraines per year, 24-180 attacks per year, and more than 180 attacks per year. The quotient for fibrinogen increased in migraineurs in general, compared with controls, but then decreased as the frequency of migraine went up (198.6 g/L, 167.0 g/L, and 121.6 g/L, respectively; P = .004).
CSF levels of sVCAM1 were 4.7 ng/mL in controls, 4.5 in the group with fewer than 24 migraines per year, 5.5 in the 24-180 group, and 7.1 in the group with more than 180 (P = .004).
Implications for therapy
The research, though at a very early stage, could have implications for therapies. Most drugs that treat migraine remain something of a mystery because researchers don’t know for sure where they act. In the brain? Systemically? The question of permeability of various molecules through both barriers could lend insight into what’s happening. “That’s why there is interest in barrier transport, and we’re showing there is a selective change of transport in migraineurs,” said Dr. Harrington.
As for more general therapeutic implications, “I can only speculate, but clearly there is baseline altered transport, probably in the choroid plexuses of these people,” said Dr. Harrington. He added that in time researchers might test drugs to see if they alter sVCAM1 levels or even develop novel drug candidates to act directly on it.
But he also sounded a note of caution because of the exploratory nature of the study. “These are all really early speculations.”
The study was funded by NIH, the Sunstar Foundation, Wyngs Foundation, and the Higgins Family. Dr. Harrington has no relevant disclosures.
SOURCE: Harrington M et al. AHS 2020, Abstract 842752.
according to a new study. The molecule could be a novel biomarker for the study of the mechanisms that underlie migraine. The work also suggests that the barrier between blood and CSF, sometimes described as leaky, is in fact selectively permeable.
The findings complement recent PET and dynamic contrast-enhanced MRI studies that have shown no sign of damage to the blood brain barrier (BBB) in migraine. Instead, there may be heightened transport of some molecules from blood to the CSF, evidenced by greater increases in fibrinogen levels in CSF than albumin. sVCAM1 might influence BBB or blood-CSF barrier permeability, possibly as a protective measure against fibrinogen, according to Michael Harrington, MD, scientific director of neuroscience at the Huntington Medical Research Institutes, Pasadena, Calif., who presented the findings in a poster at the virtual annual meeting of the American Headache Society.
BBB disruption?
The BBB is a well-known structure that regulates what molecules enter the brain, but the blood-CSF barrier, while lesser known, is also important. It comprises choroid plexus epithelial cells that oversee selective exchange of waste products, ions, and nutrients. Acute inflammation or chronic effects from conditions like stroke, multiple sclerosis, and Alzheimer’s disease can alter the function of this barrier.
No other capillary biomarkers were different between controls and patients with migraine – only sVCAM1. “My data supports a highly selective transport change from blood to CSF, which I propose is less likely to come from brain capillaries than choroid plexuses, especially since choroid plexuses produce the bulk of the CSF. It’s a work in progress, but based on this likelihood of choroid plexus involvement, I am accumulating more data that support the choroid plexuses as the primary source of change in migraine,” said Dr. Harrington in an interview.
“The most important finding of the study is that the blood brain barrier is not compromised in people with migraine,” said Rami Burstein, PhD, professor of neuroscience at Harvard Medical School, Boston, who was asked to comment on the findings. “Most unwanted adverse events are caused by drug action inside the brain, and thus, peripherally acting drugs become more favorable as they usually have fewer side effects. Given that the headache aspect of migraine could be intercepted outside the brain, the fact that the BBB is not compromised is a very good news,” Dr. Burstein added.
Dr. Harrington’s team recruited 74 subjects: 14 nonmigraine controls, 16 who were experiencing migraine illness (ictal), 27 not experiencing migraine illness (interictal), and 17 with chronic migraine. The CSF/serum quotient for albumen was higher in the 60 migraineurs than in the 14 controls (5.6 g/L vs. 4.1 g/L; P = .04), as was the CSF/serum quotient for fibrinogen (161.5 g/L vs. 86.1 g/L; P = .007). CSF levels of plasminogen were also higher in patients with migraine (240.7 ng/mL vs. 186.2 ng/mL; P = .03).
When the researchers compared ictal to interictal subjects, they found no difference in fibrinogen or albumen. That suggested that these values are generally increased in migraine patients compared with controls, rather than spiking during attacks. They also divided subjects by annual frequency, including groups experiencing fewer than 24 migraines per year, 24-180 attacks per year, and more than 180 attacks per year. The quotient for fibrinogen increased in migraineurs in general, compared with controls, but then decreased as the frequency of migraine went up (198.6 g/L, 167.0 g/L, and 121.6 g/L, respectively; P = .004).
CSF levels of sVCAM1 were 4.7 ng/mL in controls, 4.5 in the group with fewer than 24 migraines per year, 5.5 in the 24-180 group, and 7.1 in the group with more than 180 (P = .004).
Implications for therapy
The research, though at a very early stage, could have implications for therapies. Most drugs that treat migraine remain something of a mystery because researchers don’t know for sure where they act. In the brain? Systemically? The question of permeability of various molecules through both barriers could lend insight into what’s happening. “That’s why there is interest in barrier transport, and we’re showing there is a selective change of transport in migraineurs,” said Dr. Harrington.
As for more general therapeutic implications, “I can only speculate, but clearly there is baseline altered transport, probably in the choroid plexuses of these people,” said Dr. Harrington. He added that in time researchers might test drugs to see if they alter sVCAM1 levels or even develop novel drug candidates to act directly on it.
But he also sounded a note of caution because of the exploratory nature of the study. “These are all really early speculations.”
The study was funded by NIH, the Sunstar Foundation, Wyngs Foundation, and the Higgins Family. Dr. Harrington has no relevant disclosures.
SOURCE: Harrington M et al. AHS 2020, Abstract 842752.
FROM AHS 2020
Migraine is often a deciding factor in pregnancy planning
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
Although women with migraine who avoided pregnancy believed their migraines would worsen during pregnancy or make their pregnancy difficult, previous observational research indicates that migraine often improves during pregnancy.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix, Arizona.
The findings were presented at the virtual annual meeting of the American Headache Society.
Plans for the future
There is a paucity of research on the effects of migraine on pregnancy planning, the researchers noted. The few studies that have investigated this issue have focused on women’s previous family planning decisions and experience rather than on plans for the future, the researchers noted.
To evaluate how migraine in women influences pregnancy planning, the investigators analyzed data from the American Registry for Migraine Research (ARMR). The registry, which was established by the American Migraine Foundation, collects clinical data about individuals with migraine and other headache disorders from multiple centers.
Participants eligible for the current analysis were women who had been diagnosed with migraine on the basis of the International Classification of Headache Disorders–3 criteria. All completed the ARMR questionnaire between February 2016 and September 2019. The investigators excluded patients with trigeminal autonomic cephalalgia, secondary headache, painful cranial neuropathies, other facial pain, and other headaches.
They identified 895 eligible women with migraine. Of these, 607 completed the pregnancy question. Among those participants, 121 women (19.9%) reported that migraine was a factor in their decision to not become pregnant. Of this group, 70 (11.5%) reported that migraine was a “significant” factor in deciding to not have children, and 8.4% said it was “somewhat” of a factor. The remainder of the cohort (479) reported that migraine had no influence on their pregnancy plans.
There were no between-group differences by race, marital status, employment, or income. This finding suggests that sociodemographic differences “have less impact on pregnancy planning than migraine-specific characteristics like headache frequency and experience with having migraine attacks triggered by menstruation,” Dr. Ishii said.
“Substantial burden”
Not surprisingly, women who avoided pregnancy had fewer children than the rest of the sample. About 60% of those who made the decision to not become pregnant had no children, and 72% had not been pregnant since they began experiencing migraine.
Compared with women who reported that migraine had no influence on their pregnancy plans, those who avoided pregnancy were more likely to have chronic migraine at 81.8% versus 70.2%. They were also more likely to have menstrual migraine at 4.1% versus 1%. In addition, women who decided to not have children because of migraine were significantly younger at an average age of 37.5 versus 47.2 years.
The number of days with headache per 3-month interval was 53.9 among women who avoided pregnancy versus 42.5 among the other women. The Migraine Disability Assessment score was also higher for women who avoided pregnancy (132.5) than for it was the other women (91.7), indicating more severe disability.
In addition, more of the women who avoided pregnancy had a history of depression (48.8%) compared with the other women (37.7%). The average score on the Patient Health Questionnaire–4 was higher among women who avoided pregnancy (4.0) than among other women (3.1), which indicates greater anxiety or depression. Among women who avoided pregnancy, 72.5% believed their migraine would worsen during pregnancy, and 68.3% believed that migraine would make pregnancy very difficult.
“Clinicians need to recognize that migraine often has a substantial burden on multiple aspects of life, including one’s plans for having children,” Dr. Ishii said.
“Clinicians should educate their patients who are considering pregnancy about the most likely course of migraine during pregnancy, migraine treatment during pregnancy, and the potential impacts of migraine and its treatment on pregnancy outcomes,” he added.
More education needed
Commenting on the study, Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center, Irvine, California, said that not knowing how pregnancy is going to affect patients’ migraines can be “very scary” for women. In addition, patients often wonder what migraine treatments they can safely take once they do become pregnant, said Dr. Hutchinson, who was not involved in the research.
She noted that advantages of the ARMR data are that they are derived from a multicenter study and that migraine diagnoses were made by a headache specialist. A potential limitation of the study is that the population may not reflect outcomes of the millions of women who have migraine and become pregnant but never see a specialist.
“These findings show that more education is needed,” Dr. Hutchinson said.
Most women, especially those who have migraine without aura, note improvement with migraine during pregnancy, primarily because of the high, steady levels of estradiol, especially in the second and third trimesters, she said. In light of this, neurologists should reassure women that migraine is not a contraindication to pregnancy, she added.
There is also a need for additional research to assess how past experience with migraine and pregnancy influences a woman’s comfort level with additional pregnancies. Studies as to which treatments are safest for acute and preventive treatment of migraine during prepregnancy, pregnancy, and lactation are also needed, Dr. Hutchinson noted.
“If women knew they had treatment options that were evidence-based, they might be much more comfortable contemplating a pregnancy,” she said.
Dr. Ishii and Dr. Hutchinson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Visualization tool aids migraine management
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.
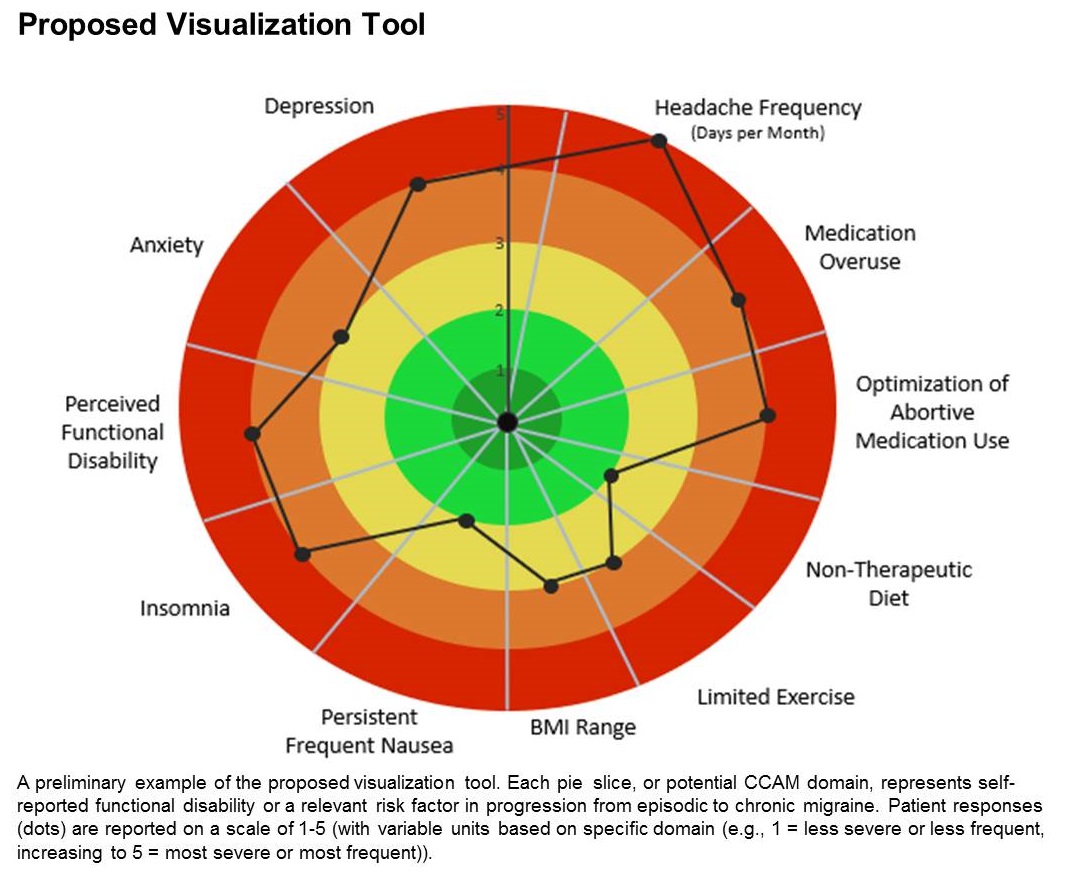
A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.

A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.

A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
FROM AHS 2020
Consistent effects for galcanezumab in cluster headache
new research suggests. A post hoc analysis of patients from the phase 3 CGAL study who also entered the open-label CGAR extension study was conducted. Results showed that the majority of participants whose scores on the Patient Global Impression of Improvement (PGI-I) showed improvement 1 month after the initial dose of galcanezumab in the CGAL study also showed improvement after treatment for subsequent cluster bouts during the CGAR study.
“There was good agreement between PGI-I between the two [cluster headache] periods,” noted the investigators, led by Brian Plato, DO, a neurologist at Norton Neuroscience Institute in Louisville, Ky.
The findings were presented at the virtual annual meeting of the American Headache Society.
Two cluster periods
Galcanezumab was approved by the Food and Drug Administration in 2019 for the treatment of episodic cluster headache in adults.
In cluster headache, attacks of recurrent, unilateral headaches with cranial autonomic symptoms last for weeks or months and are followed by periods of remission. Most studies of therapies for cluster headache examine only one cluster period. Few data about the consistency of treatment response throughout consecutive cluster periods are available, the investigators noted.
The current analysis was undertaken to examine the consistency of galcanezumab’s effect in episodic cluster headache during two cluster periods. Patients eligible for inclusion in the analysis had completed the double-blind phase of the CGAL study and had entered the open-label CGAR study.
CGAL was a phase 3, multicenter, randomized, double-blind study in which patients with episodic cluster headache were assigned to receive galcanezumab 300 mg per month or placebo. Patients who completed the double-blind and washout phases of this study were eligible for enrollment into CGAR, a phase 3b, single-arm safety study. The investigators determined the dose of galcanezumab in accordance with each patient’s symptoms and clinical response.
Response agreement
In both studies, the PGI-I was administered 1 month after the initial dose of galcanezumab. Only patients who were in an active cluster bout on entry into CGAR and who had valid PGI-I results 1 month after the first dose in CGAL and CGAR were included in the analysis.
PGI-I responses ranged from 1, signifying very much better, to 7, signifying very much worse. The investigators summarized the proportions of patients who reported each level of PGI-I score in CGAR and analyzed the results by dichotomizing PGI-I scores at both time points in two ways.
Fifty patients entered CGAR (78% men; mean age, 46.8 years). Of this group, Dr. Plato and colleagues included 39 in their analysis. Of the 17 patients who had a PGI-I score of 1 or 2 in CGAL, 12 (70.6%) had a score in the same range in CGAR. All four participants who had a score of 3 or higher in CGAL had a score in the same range in CGAR. Eighteen participants had a PGI-I score of 1, 2, or 3 in CGAL. Of this group, 15 patients (83.3%) had a score in the same range in CGAR. Of the three patients who had a score above 3 in CGAL, two (66.7%) had a score in the same range in CGAR.
The results indicate that most patients whose PGI-I score improved in one cluster bout, such as in CGAL, also improved in a subsequent bout, such as in CGAR, the investigators noted.
‘Encouraging’ results
Commenting on the study, Brian E. McGeeney, MD, a neurologist at the John R. Graham Headache Center, Brigham and Women’s Faulkner Hospital, Boston, noted that the PGI-I is an “easy-to-understand” outcome that has been widely used in headache medicine.
“Patient-assessed outcomes have become increasingly important and are an important complement to other outcomes,” said Dr. McGeeney, who was not involved in the research. However, a disadvantage is that “it is entirely subjective and may or may not reflect a change on other outcome measures that reflect the disorder itself,” he said.
“It can be difficult to demonstrate how much usefulness a treatment has with the helpful but simple outcome measures that are seen in CGAL and CGAR,” Dr. McGeeney added. “This is due to the nature of cluster headache and not to any methodological shortcomings of those studies.”
He said this is a core problem in general with cluster headache studies, “of which there are very few.”
In addition, CGAR only included episodic cluster headache, and the study period was relatively short; and CGAL only explored one cluster period per patient, Dr. McGeeney noted.
The current research attempts to provide insight that was previously unavailable, he said. “Many headache medicine clinical trial results reflect only one episode, and in general, we infer repeated usefulness – although it is not demonstrated in clinical trials,” said Dr. McGeeney.
“In this recent presentation, the authors attempt to go further and demonstrate some consistency across multiple cluster periods. The results are encouraging and what one might expect,” he said. However, “the small numbers and ad hoc nature preclude much inference from this study alone.”
Dr. Plato has received honoraria for speaking from Allergan, Amgen/Novartis, and Eli Lilly. He has also received research grants and support from Electrocore and Teva. Dr. McGeeney has consulted for Upsher-Smith and Theranica.
A version of this article originally appeared on Medscape.com.
new research suggests. A post hoc analysis of patients from the phase 3 CGAL study who also entered the open-label CGAR extension study was conducted. Results showed that the majority of participants whose scores on the Patient Global Impression of Improvement (PGI-I) showed improvement 1 month after the initial dose of galcanezumab in the CGAL study also showed improvement after treatment for subsequent cluster bouts during the CGAR study.
“There was good agreement between PGI-I between the two [cluster headache] periods,” noted the investigators, led by Brian Plato, DO, a neurologist at Norton Neuroscience Institute in Louisville, Ky.
The findings were presented at the virtual annual meeting of the American Headache Society.
Two cluster periods
Galcanezumab was approved by the Food and Drug Administration in 2019 for the treatment of episodic cluster headache in adults.
In cluster headache, attacks of recurrent, unilateral headaches with cranial autonomic symptoms last for weeks or months and are followed by periods of remission. Most studies of therapies for cluster headache examine only one cluster period. Few data about the consistency of treatment response throughout consecutive cluster periods are available, the investigators noted.
The current analysis was undertaken to examine the consistency of galcanezumab’s effect in episodic cluster headache during two cluster periods. Patients eligible for inclusion in the analysis had completed the double-blind phase of the CGAL study and had entered the open-label CGAR study.
CGAL was a phase 3, multicenter, randomized, double-blind study in which patients with episodic cluster headache were assigned to receive galcanezumab 300 mg per month or placebo. Patients who completed the double-blind and washout phases of this study were eligible for enrollment into CGAR, a phase 3b, single-arm safety study. The investigators determined the dose of galcanezumab in accordance with each patient’s symptoms and clinical response.
Response agreement
In both studies, the PGI-I was administered 1 month after the initial dose of galcanezumab. Only patients who were in an active cluster bout on entry into CGAR and who had valid PGI-I results 1 month after the first dose in CGAL and CGAR were included in the analysis.
PGI-I responses ranged from 1, signifying very much better, to 7, signifying very much worse. The investigators summarized the proportions of patients who reported each level of PGI-I score in CGAR and analyzed the results by dichotomizing PGI-I scores at both time points in two ways.
Fifty patients entered CGAR (78% men; mean age, 46.8 years). Of this group, Dr. Plato and colleagues included 39 in their analysis. Of the 17 patients who had a PGI-I score of 1 or 2 in CGAL, 12 (70.6%) had a score in the same range in CGAR. All four participants who had a score of 3 or higher in CGAL had a score in the same range in CGAR. Eighteen participants had a PGI-I score of 1, 2, or 3 in CGAL. Of this group, 15 patients (83.3%) had a score in the same range in CGAR. Of the three patients who had a score above 3 in CGAL, two (66.7%) had a score in the same range in CGAR.
The results indicate that most patients whose PGI-I score improved in one cluster bout, such as in CGAL, also improved in a subsequent bout, such as in CGAR, the investigators noted.
‘Encouraging’ results
Commenting on the study, Brian E. McGeeney, MD, a neurologist at the John R. Graham Headache Center, Brigham and Women’s Faulkner Hospital, Boston, noted that the PGI-I is an “easy-to-understand” outcome that has been widely used in headache medicine.
“Patient-assessed outcomes have become increasingly important and are an important complement to other outcomes,” said Dr. McGeeney, who was not involved in the research. However, a disadvantage is that “it is entirely subjective and may or may not reflect a change on other outcome measures that reflect the disorder itself,” he said.
“It can be difficult to demonstrate how much usefulness a treatment has with the helpful but simple outcome measures that are seen in CGAL and CGAR,” Dr. McGeeney added. “This is due to the nature of cluster headache and not to any methodological shortcomings of those studies.”
He said this is a core problem in general with cluster headache studies, “of which there are very few.”
In addition, CGAR only included episodic cluster headache, and the study period was relatively short; and CGAL only explored one cluster period per patient, Dr. McGeeney noted.
The current research attempts to provide insight that was previously unavailable, he said. “Many headache medicine clinical trial results reflect only one episode, and in general, we infer repeated usefulness – although it is not demonstrated in clinical trials,” said Dr. McGeeney.
“In this recent presentation, the authors attempt to go further and demonstrate some consistency across multiple cluster periods. The results are encouraging and what one might expect,” he said. However, “the small numbers and ad hoc nature preclude much inference from this study alone.”
Dr. Plato has received honoraria for speaking from Allergan, Amgen/Novartis, and Eli Lilly. He has also received research grants and support from Electrocore and Teva. Dr. McGeeney has consulted for Upsher-Smith and Theranica.
A version of this article originally appeared on Medscape.com.
new research suggests. A post hoc analysis of patients from the phase 3 CGAL study who also entered the open-label CGAR extension study was conducted. Results showed that the majority of participants whose scores on the Patient Global Impression of Improvement (PGI-I) showed improvement 1 month after the initial dose of galcanezumab in the CGAL study also showed improvement after treatment for subsequent cluster bouts during the CGAR study.
“There was good agreement between PGI-I between the two [cluster headache] periods,” noted the investigators, led by Brian Plato, DO, a neurologist at Norton Neuroscience Institute in Louisville, Ky.
The findings were presented at the virtual annual meeting of the American Headache Society.
Two cluster periods
Galcanezumab was approved by the Food and Drug Administration in 2019 for the treatment of episodic cluster headache in adults.
In cluster headache, attacks of recurrent, unilateral headaches with cranial autonomic symptoms last for weeks or months and are followed by periods of remission. Most studies of therapies for cluster headache examine only one cluster period. Few data about the consistency of treatment response throughout consecutive cluster periods are available, the investigators noted.
The current analysis was undertaken to examine the consistency of galcanezumab’s effect in episodic cluster headache during two cluster periods. Patients eligible for inclusion in the analysis had completed the double-blind phase of the CGAL study and had entered the open-label CGAR study.
CGAL was a phase 3, multicenter, randomized, double-blind study in which patients with episodic cluster headache were assigned to receive galcanezumab 300 mg per month or placebo. Patients who completed the double-blind and washout phases of this study were eligible for enrollment into CGAR, a phase 3b, single-arm safety study. The investigators determined the dose of galcanezumab in accordance with each patient’s symptoms and clinical response.
Response agreement
In both studies, the PGI-I was administered 1 month after the initial dose of galcanezumab. Only patients who were in an active cluster bout on entry into CGAR and who had valid PGI-I results 1 month after the first dose in CGAL and CGAR were included in the analysis.
PGI-I responses ranged from 1, signifying very much better, to 7, signifying very much worse. The investigators summarized the proportions of patients who reported each level of PGI-I score in CGAR and analyzed the results by dichotomizing PGI-I scores at both time points in two ways.
Fifty patients entered CGAR (78% men; mean age, 46.8 years). Of this group, Dr. Plato and colleagues included 39 in their analysis. Of the 17 patients who had a PGI-I score of 1 or 2 in CGAL, 12 (70.6%) had a score in the same range in CGAR. All four participants who had a score of 3 or higher in CGAL had a score in the same range in CGAR. Eighteen participants had a PGI-I score of 1, 2, or 3 in CGAL. Of this group, 15 patients (83.3%) had a score in the same range in CGAR. Of the three patients who had a score above 3 in CGAL, two (66.7%) had a score in the same range in CGAR.
The results indicate that most patients whose PGI-I score improved in one cluster bout, such as in CGAL, also improved in a subsequent bout, such as in CGAR, the investigators noted.
‘Encouraging’ results
Commenting on the study, Brian E. McGeeney, MD, a neurologist at the John R. Graham Headache Center, Brigham and Women’s Faulkner Hospital, Boston, noted that the PGI-I is an “easy-to-understand” outcome that has been widely used in headache medicine.
“Patient-assessed outcomes have become increasingly important and are an important complement to other outcomes,” said Dr. McGeeney, who was not involved in the research. However, a disadvantage is that “it is entirely subjective and may or may not reflect a change on other outcome measures that reflect the disorder itself,” he said.
“It can be difficult to demonstrate how much usefulness a treatment has with the helpful but simple outcome measures that are seen in CGAL and CGAR,” Dr. McGeeney added. “This is due to the nature of cluster headache and not to any methodological shortcomings of those studies.”
He said this is a core problem in general with cluster headache studies, “of which there are very few.”
In addition, CGAR only included episodic cluster headache, and the study period was relatively short; and CGAL only explored one cluster period per patient, Dr. McGeeney noted.
The current research attempts to provide insight that was previously unavailable, he said. “Many headache medicine clinical trial results reflect only one episode, and in general, we infer repeated usefulness – although it is not demonstrated in clinical trials,” said Dr. McGeeney.
“In this recent presentation, the authors attempt to go further and demonstrate some consistency across multiple cluster periods. The results are encouraging and what one might expect,” he said. However, “the small numbers and ad hoc nature preclude much inference from this study alone.”
Dr. Plato has received honoraria for speaking from Allergan, Amgen/Novartis, and Eli Lilly. He has also received research grants and support from Electrocore and Teva. Dr. McGeeney has consulted for Upsher-Smith and Theranica.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Marijuana for migraine? Some tentative evidence
according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
Many patients ask about medical marijuana, but there is relatively little data on its effects on headache. Studies are generally retrospective, and often focus on marijuana use for general pain, with subset analyses looking at headache, according to coauthor Claire Ceriani, MD, who is a headache fellow at Jefferson. “A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Dr. Ceriani in an interview.
Although the research is far from a final word on the subject, it did have some take-home messages, said Dr. Ceriani. “Most people seem to find it effective as an abortive medication that might be able to take the place of some of the prescription medications that they were previously using,” she said.
The study was part of the virtual annual meeting of the American Headache Society.
An effective abortive therapy?
The study began shortly after the Jefferson Headache Center became certified to offer medical marijuana around the beginning of 2019. “We wanted to start keeping track of these patients from the get-go so we’d be able to learn as much as possible from them and help guide the recommendations we give to patients in the future,” said Dr. Ceriani.
The study included 48 patients with migraine or other types of chronic headache who received medical marijuana treatment between January and September 2019. After collecting baseline information from medical records and questionnaires filled out at marijuana treatment initiation, the researchers followed up periodically with telephone questionnaires to assess treatment response and side effects. About half of the participants (56.3%) reported daily headache. 14.6% had posttraumatic headache, 10.4% new daily persistent headache, and 4.2% tension-type headache. Additional symptoms were common, including anxiety (72.9%) and insomnia (62.5%).
A total of 28 subjects completed a follow-up questionnaire over the phone. Out of the 28 participants , 3 had stopped using marijuana. Of 25 subjects who continued use, 71.4% used it two or more times per week, and 25.0% used it every day. Among participants, 50% used a THC-dominant strain of marijuana. Overall, 71.4% used an inhaled form.
Side effects included dry mouth/throat (46.4%), dry/red eyes (35.7%), fatigue/lethargy (35.7%), and increased appetite (35.7%).
Before starting on marijuana, 46.4% of the subjects used abortive medications at least 10 days per month. After starting marijuana treatment, the rate dropped to 25.0%. Marijuana use was associated with improvements in anxiety: 57.1% who had anxiety reported improvement with marijuana use, as did 78.6% with insomnia. On a scale of 10, the average rating of marijuana’s usefulness was 5.9, and 17.9% rated it as 10.
Several concerns
The study has numerous limitations. It has a small sample size, it is from a single center, and the patient population had relatively severe symptoms. Such studies are “fraught with possible bias,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, when asked to comment.
He pointed out that one key concern for marijuana is concerns over worsening of the condition or refractoriness caused by medication overuse. The cannabinoid receptors it acts on bear some similarity to opioid receptors, and opioid overuse headache is well known. The recent changes in marijuana laws makes it an important issue, one that patients often asked about. But prospective clinical trials face a range of roadblocks: Marijuana remains a controlled substance, it would be difficult to create a placebo control, and no large companies are likely to sponsor such a trial.
“But I think it’s important to keep talking about and developing evidence as much as we can and addressing not just the benefits but also being keenly aware of the possible adverse effects, especially medication overuse,” said Dr. Charles.
The authors also acknowledged the study’s limitations, “but I think there is value, because there are definitely specific patterns we were able to find in terms of what’s helpful for patients, and we also found that a lot of patients also have other disorders in addition to headache, like anxiety and insomnia. And we found that those patients in particular seemed to have more benefit than most with medical marijuana,” said coauthor Angela Hou, MD, who is also a headache fellow at Jefferson.
Dr. Hou and Dr. Ceriani cautioned against use of marijuana in any patient with a substance use disorder, as well as the inhaled form in patients with chronic lung conditions.
The study received no funding. Dr. Ceriani and Dr. Hou had no relevant financial disclosures. Dr. Charles has consulted for Amgen, Biohaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Marmura MJ et al. AHS 2020, Abstract 842679.
according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
Many patients ask about medical marijuana, but there is relatively little data on its effects on headache. Studies are generally retrospective, and often focus on marijuana use for general pain, with subset analyses looking at headache, according to coauthor Claire Ceriani, MD, who is a headache fellow at Jefferson. “A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Dr. Ceriani in an interview.
Although the research is far from a final word on the subject, it did have some take-home messages, said Dr. Ceriani. “Most people seem to find it effective as an abortive medication that might be able to take the place of some of the prescription medications that they were previously using,” she said.
The study was part of the virtual annual meeting of the American Headache Society.
An effective abortive therapy?
The study began shortly after the Jefferson Headache Center became certified to offer medical marijuana around the beginning of 2019. “We wanted to start keeping track of these patients from the get-go so we’d be able to learn as much as possible from them and help guide the recommendations we give to patients in the future,” said Dr. Ceriani.
The study included 48 patients with migraine or other types of chronic headache who received medical marijuana treatment between January and September 2019. After collecting baseline information from medical records and questionnaires filled out at marijuana treatment initiation, the researchers followed up periodically with telephone questionnaires to assess treatment response and side effects. About half of the participants (56.3%) reported daily headache. 14.6% had posttraumatic headache, 10.4% new daily persistent headache, and 4.2% tension-type headache. Additional symptoms were common, including anxiety (72.9%) and insomnia (62.5%).
A total of 28 subjects completed a follow-up questionnaire over the phone. Out of the 28 participants , 3 had stopped using marijuana. Of 25 subjects who continued use, 71.4% used it two or more times per week, and 25.0% used it every day. Among participants, 50% used a THC-dominant strain of marijuana. Overall, 71.4% used an inhaled form.
Side effects included dry mouth/throat (46.4%), dry/red eyes (35.7%), fatigue/lethargy (35.7%), and increased appetite (35.7%).
Before starting on marijuana, 46.4% of the subjects used abortive medications at least 10 days per month. After starting marijuana treatment, the rate dropped to 25.0%. Marijuana use was associated with improvements in anxiety: 57.1% who had anxiety reported improvement with marijuana use, as did 78.6% with insomnia. On a scale of 10, the average rating of marijuana’s usefulness was 5.9, and 17.9% rated it as 10.
Several concerns
The study has numerous limitations. It has a small sample size, it is from a single center, and the patient population had relatively severe symptoms. Such studies are “fraught with possible bias,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, when asked to comment.
He pointed out that one key concern for marijuana is concerns over worsening of the condition or refractoriness caused by medication overuse. The cannabinoid receptors it acts on bear some similarity to opioid receptors, and opioid overuse headache is well known. The recent changes in marijuana laws makes it an important issue, one that patients often asked about. But prospective clinical trials face a range of roadblocks: Marijuana remains a controlled substance, it would be difficult to create a placebo control, and no large companies are likely to sponsor such a trial.
“But I think it’s important to keep talking about and developing evidence as much as we can and addressing not just the benefits but also being keenly aware of the possible adverse effects, especially medication overuse,” said Dr. Charles.
The authors also acknowledged the study’s limitations, “but I think there is value, because there are definitely specific patterns we were able to find in terms of what’s helpful for patients, and we also found that a lot of patients also have other disorders in addition to headache, like anxiety and insomnia. And we found that those patients in particular seemed to have more benefit than most with medical marijuana,” said coauthor Angela Hou, MD, who is also a headache fellow at Jefferson.
Dr. Hou and Dr. Ceriani cautioned against use of marijuana in any patient with a substance use disorder, as well as the inhaled form in patients with chronic lung conditions.
The study received no funding. Dr. Ceriani and Dr. Hou had no relevant financial disclosures. Dr. Charles has consulted for Amgen, Biohaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Marmura MJ et al. AHS 2020, Abstract 842679.
according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
Many patients ask about medical marijuana, but there is relatively little data on its effects on headache. Studies are generally retrospective, and often focus on marijuana use for general pain, with subset analyses looking at headache, according to coauthor Claire Ceriani, MD, who is a headache fellow at Jefferson. “A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Dr. Ceriani in an interview.
Although the research is far from a final word on the subject, it did have some take-home messages, said Dr. Ceriani. “Most people seem to find it effective as an abortive medication that might be able to take the place of some of the prescription medications that they were previously using,” she said.
The study was part of the virtual annual meeting of the American Headache Society.
An effective abortive therapy?
The study began shortly after the Jefferson Headache Center became certified to offer medical marijuana around the beginning of 2019. “We wanted to start keeping track of these patients from the get-go so we’d be able to learn as much as possible from them and help guide the recommendations we give to patients in the future,” said Dr. Ceriani.
The study included 48 patients with migraine or other types of chronic headache who received medical marijuana treatment between January and September 2019. After collecting baseline information from medical records and questionnaires filled out at marijuana treatment initiation, the researchers followed up periodically with telephone questionnaires to assess treatment response and side effects. About half of the participants (56.3%) reported daily headache. 14.6% had posttraumatic headache, 10.4% new daily persistent headache, and 4.2% tension-type headache. Additional symptoms were common, including anxiety (72.9%) and insomnia (62.5%).
A total of 28 subjects completed a follow-up questionnaire over the phone. Out of the 28 participants , 3 had stopped using marijuana. Of 25 subjects who continued use, 71.4% used it two or more times per week, and 25.0% used it every day. Among participants, 50% used a THC-dominant strain of marijuana. Overall, 71.4% used an inhaled form.
Side effects included dry mouth/throat (46.4%), dry/red eyes (35.7%), fatigue/lethargy (35.7%), and increased appetite (35.7%).
Before starting on marijuana, 46.4% of the subjects used abortive medications at least 10 days per month. After starting marijuana treatment, the rate dropped to 25.0%. Marijuana use was associated with improvements in anxiety: 57.1% who had anxiety reported improvement with marijuana use, as did 78.6% with insomnia. On a scale of 10, the average rating of marijuana’s usefulness was 5.9, and 17.9% rated it as 10.
Several concerns
The study has numerous limitations. It has a small sample size, it is from a single center, and the patient population had relatively severe symptoms. Such studies are “fraught with possible bias,” said Andrew Charles, MD, professor of neurology and director of the UCLA Goldberg Migraine Program, when asked to comment.
He pointed out that one key concern for marijuana is concerns over worsening of the condition or refractoriness caused by medication overuse. The cannabinoid receptors it acts on bear some similarity to opioid receptors, and opioid overuse headache is well known. The recent changes in marijuana laws makes it an important issue, one that patients often asked about. But prospective clinical trials face a range of roadblocks: Marijuana remains a controlled substance, it would be difficult to create a placebo control, and no large companies are likely to sponsor such a trial.
“But I think it’s important to keep talking about and developing evidence as much as we can and addressing not just the benefits but also being keenly aware of the possible adverse effects, especially medication overuse,” said Dr. Charles.
The authors also acknowledged the study’s limitations, “but I think there is value, because there are definitely specific patterns we were able to find in terms of what’s helpful for patients, and we also found that a lot of patients also have other disorders in addition to headache, like anxiety and insomnia. And we found that those patients in particular seemed to have more benefit than most with medical marijuana,” said coauthor Angela Hou, MD, who is also a headache fellow at Jefferson.
Dr. Hou and Dr. Ceriani cautioned against use of marijuana in any patient with a substance use disorder, as well as the inhaled form in patients with chronic lung conditions.
The study received no funding. Dr. Ceriani and Dr. Hou had no relevant financial disclosures. Dr. Charles has consulted for Amgen, Biohaven, Eli Lilly, Novartis, and Lundbeck.
SOURCE: Marmura MJ et al. AHS 2020, Abstract 842679.
FROM AHS 2020
Headache may predict clinical evolution of COVID-19
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Adding CGRP to Botox is safe and effective for migraine prevention
Investigators found the CGRP-mAbs significantly reduced the number of headache days and pain severity with adverse event rates similar to those reported in previous trials of these medications.
“The addition of a CGRP monoclonal antibody provided statistically significantly fewer monthly headache days,” said study investigator Fred Cohen, MD, an internal medicine resident physician at Montefiore Health System, New York. “However, this was a retrospective chart review, which is hindered by elements such as recall bias. Therefore, future prospective studies are warranted for higher quality data.”
The findings were presented at the virtual annual meeting of the American Headache Society.
Fewer headache days
Although Botox is associated with significant clinical improvement in chronic migraine, it often fails to adequately control headache frequency and additional medications are needed.
The CGRP-mAbs fremanezumab, galcanezumab, and erenumab, have recently been approved for migraine prevention, with results from clinical trials demonstrating they are effective for both chronic and episodic migraine. However, patients treated with Botox were excluded from these trials and to date there are no data on combination treatment with Botox and CGRP-mAbs.
To determine whether adjunctive treatment with CGRP-mAbs augments Botox therapy in chronic migraine the investigators conducted a retrospective chart review of patients receiving Botox and prescribed a CGRP-mAb.
Eligible patients met the International Classification of Headache Disorders, 3rd edition, criteria for chronic migraine; were age 18 years or older; and presented at a single headache center between May 2018 and May 2019. Patients who received another new therapy during the study or those taking CGRP-mAb treatment for less than 2 months were excluded.
The study’s primary outcome was change in the number of reported monthly headache days, and change in pain severity was the secondary outcome.
The final analysis included data on 153 patients. The population’s mean age was 47.1 years, and 139 patients (90.8%) were women. In all, 89 patients (58.0%) received erenumab (35 received 70 mg and 54 received 140 mg), 51 (33.0%) received galcanezumab, and 13 (9.0%) received fremanezumab.
Overall, 114 (74.5%) patients reported a decrease in monthly headache days or pain severity. In the group of 66 patients for whom quantitative data were available, the average number of monthly headache days before Botox treatment was 25.7. After Botox treatment, patients had an average decrease of 10.9 monthly headache days, a 42.4% reduction, so on average study participants continued to have an average of 14.8 monthly headache days.
After treatment with a CGRP-mAb the number decreased by 5.6 additional days (37.8%). Patients receiving combined therapy had an average of 9.1 monthly headache days. The total decrease from baseline was 16.6 fewer monthly headache days, a 64.6% reduction.
The number of headache days per month was reduced to 9.3 for erenumab and galcanezumab and 5.8 for fremanezumab. However, few patients in the study took fremanezumab so this result had less statistical power than the results for the other CGRP-mAbs.
A total of 13 patients (8.5%) reported side effects associated with the CGRP-mAbs, which included constipation, injection-site reaction, and fatigue.
More evidence is needed
Commenting on the findings, Peter McAllister, MD, medical director of the New England Institute for Neurology and Headache in Stamford, Conn., said the study’s main limitation is that it is a retrospective chart review, which yields lower level evidence than a prospective, double-blind, placebo-controlled study. Dr. McAllister, who was not involved in the research, also noted that the sample size was small, particularly with respect to fremanezumab.
“This study, despite its limitations, shows that addition of a monoclonal antibody to onabotulinumtoxinA is safe and well tolerated, and may confer additional reduction in migraine or headache days. The authors correctly state that more evidence via prospective study is warranted,” said Dr. McAllister, who is also chief medical officer of the New England Institute for Clinical Research and was not involved in the investigation.
Dr. Cohen has reported no relevant financial relationships. Dr. McAllister was an investigator in the PREEMPT trial of onabotulinumtoxinA, as well as in all of the phase 3 monoclonal antibody studies.
A version of this article originally appeared on Medscape.com.
Investigators found the CGRP-mAbs significantly reduced the number of headache days and pain severity with adverse event rates similar to those reported in previous trials of these medications.
“The addition of a CGRP monoclonal antibody provided statistically significantly fewer monthly headache days,” said study investigator Fred Cohen, MD, an internal medicine resident physician at Montefiore Health System, New York. “However, this was a retrospective chart review, which is hindered by elements such as recall bias. Therefore, future prospective studies are warranted for higher quality data.”
The findings were presented at the virtual annual meeting of the American Headache Society.
Fewer headache days
Although Botox is associated with significant clinical improvement in chronic migraine, it often fails to adequately control headache frequency and additional medications are needed.
The CGRP-mAbs fremanezumab, galcanezumab, and erenumab, have recently been approved for migraine prevention, with results from clinical trials demonstrating they are effective for both chronic and episodic migraine. However, patients treated with Botox were excluded from these trials and to date there are no data on combination treatment with Botox and CGRP-mAbs.
To determine whether adjunctive treatment with CGRP-mAbs augments Botox therapy in chronic migraine the investigators conducted a retrospective chart review of patients receiving Botox and prescribed a CGRP-mAb.
Eligible patients met the International Classification of Headache Disorders, 3rd edition, criteria for chronic migraine; were age 18 years or older; and presented at a single headache center between May 2018 and May 2019. Patients who received another new therapy during the study or those taking CGRP-mAb treatment for less than 2 months were excluded.
The study’s primary outcome was change in the number of reported monthly headache days, and change in pain severity was the secondary outcome.
The final analysis included data on 153 patients. The population’s mean age was 47.1 years, and 139 patients (90.8%) were women. In all, 89 patients (58.0%) received erenumab (35 received 70 mg and 54 received 140 mg), 51 (33.0%) received galcanezumab, and 13 (9.0%) received fremanezumab.
Overall, 114 (74.5%) patients reported a decrease in monthly headache days or pain severity. In the group of 66 patients for whom quantitative data were available, the average number of monthly headache days before Botox treatment was 25.7. After Botox treatment, patients had an average decrease of 10.9 monthly headache days, a 42.4% reduction, so on average study participants continued to have an average of 14.8 monthly headache days.
After treatment with a CGRP-mAb the number decreased by 5.6 additional days (37.8%). Patients receiving combined therapy had an average of 9.1 monthly headache days. The total decrease from baseline was 16.6 fewer monthly headache days, a 64.6% reduction.
The number of headache days per month was reduced to 9.3 for erenumab and galcanezumab and 5.8 for fremanezumab. However, few patients in the study took fremanezumab so this result had less statistical power than the results for the other CGRP-mAbs.
A total of 13 patients (8.5%) reported side effects associated with the CGRP-mAbs, which included constipation, injection-site reaction, and fatigue.
More evidence is needed
Commenting on the findings, Peter McAllister, MD, medical director of the New England Institute for Neurology and Headache in Stamford, Conn., said the study’s main limitation is that it is a retrospective chart review, which yields lower level evidence than a prospective, double-blind, placebo-controlled study. Dr. McAllister, who was not involved in the research, also noted that the sample size was small, particularly with respect to fremanezumab.
“This study, despite its limitations, shows that addition of a monoclonal antibody to onabotulinumtoxinA is safe and well tolerated, and may confer additional reduction in migraine or headache days. The authors correctly state that more evidence via prospective study is warranted,” said Dr. McAllister, who is also chief medical officer of the New England Institute for Clinical Research and was not involved in the investigation.
Dr. Cohen has reported no relevant financial relationships. Dr. McAllister was an investigator in the PREEMPT trial of onabotulinumtoxinA, as well as in all of the phase 3 monoclonal antibody studies.
A version of this article originally appeared on Medscape.com.
Investigators found the CGRP-mAbs significantly reduced the number of headache days and pain severity with adverse event rates similar to those reported in previous trials of these medications.
“The addition of a CGRP monoclonal antibody provided statistically significantly fewer monthly headache days,” said study investigator Fred Cohen, MD, an internal medicine resident physician at Montefiore Health System, New York. “However, this was a retrospective chart review, which is hindered by elements such as recall bias. Therefore, future prospective studies are warranted for higher quality data.”
The findings were presented at the virtual annual meeting of the American Headache Society.
Fewer headache days
Although Botox is associated with significant clinical improvement in chronic migraine, it often fails to adequately control headache frequency and additional medications are needed.
The CGRP-mAbs fremanezumab, galcanezumab, and erenumab, have recently been approved for migraine prevention, with results from clinical trials demonstrating they are effective for both chronic and episodic migraine. However, patients treated with Botox were excluded from these trials and to date there are no data on combination treatment with Botox and CGRP-mAbs.
To determine whether adjunctive treatment with CGRP-mAbs augments Botox therapy in chronic migraine the investigators conducted a retrospective chart review of patients receiving Botox and prescribed a CGRP-mAb.
Eligible patients met the International Classification of Headache Disorders, 3rd edition, criteria for chronic migraine; were age 18 years or older; and presented at a single headache center between May 2018 and May 2019. Patients who received another new therapy during the study or those taking CGRP-mAb treatment for less than 2 months were excluded.
The study’s primary outcome was change in the number of reported monthly headache days, and change in pain severity was the secondary outcome.
The final analysis included data on 153 patients. The population’s mean age was 47.1 years, and 139 patients (90.8%) were women. In all, 89 patients (58.0%) received erenumab (35 received 70 mg and 54 received 140 mg), 51 (33.0%) received galcanezumab, and 13 (9.0%) received fremanezumab.
Overall, 114 (74.5%) patients reported a decrease in monthly headache days or pain severity. In the group of 66 patients for whom quantitative data were available, the average number of monthly headache days before Botox treatment was 25.7. After Botox treatment, patients had an average decrease of 10.9 monthly headache days, a 42.4% reduction, so on average study participants continued to have an average of 14.8 monthly headache days.
After treatment with a CGRP-mAb the number decreased by 5.6 additional days (37.8%). Patients receiving combined therapy had an average of 9.1 monthly headache days. The total decrease from baseline was 16.6 fewer monthly headache days, a 64.6% reduction.
The number of headache days per month was reduced to 9.3 for erenumab and galcanezumab and 5.8 for fremanezumab. However, few patients in the study took fremanezumab so this result had less statistical power than the results for the other CGRP-mAbs.
A total of 13 patients (8.5%) reported side effects associated with the CGRP-mAbs, which included constipation, injection-site reaction, and fatigue.
More evidence is needed
Commenting on the findings, Peter McAllister, MD, medical director of the New England Institute for Neurology and Headache in Stamford, Conn., said the study’s main limitation is that it is a retrospective chart review, which yields lower level evidence than a prospective, double-blind, placebo-controlled study. Dr. McAllister, who was not involved in the research, also noted that the sample size was small, particularly with respect to fremanezumab.
“This study, despite its limitations, shows that addition of a monoclonal antibody to onabotulinumtoxinA is safe and well tolerated, and may confer additional reduction in migraine or headache days. The authors correctly state that more evidence via prospective study is warranted,” said Dr. McAllister, who is also chief medical officer of the New England Institute for Clinical Research and was not involved in the investigation.
Dr. Cohen has reported no relevant financial relationships. Dr. McAllister was an investigator in the PREEMPT trial of onabotulinumtoxinA, as well as in all of the phase 3 monoclonal antibody studies.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Commonalities challenge the threshold of high-frequency episodic and low-frequency chronic migraine
according to an analysis of almost 17,000 patients from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study presented at the virtual annual meeting of the American Headache Society.
“The results showed substantial overlap in levels of burden, anxiety, depression and health utilization, including outpatient, inpatient and emergency department visits, among CaMEO respondents with high-frequency episodic migraine and those with low-frequency chronic migraine,” said Richard B. Lipton, MD, of the Albert Einstein College of Medicine, New York.
The study analyzed data on 16,789 respondents to CaMEO, the longitudinal, web-based study designed to characterize the course of episodic and chronic migraine. The study population consisted of four subgroups based on the number of self-reporting monthly headache days (MHDs):
- Low- and moderate-frequency episodic migraine (LFEM; zero to seven MHDs; n = 13,473).
- High-frequency episodic migraine (HFEM; 8-14 MHDs; n = 1,840).
- Low-frequency chronic migraine (LFCM; 15-23 MHDs; n = 1,035).
- High-frequency chronic migraine (HFCM; 24 or more MHDs; n = 441).
Dr. Lipton pointed out that the International Classification of Headache Disorders, 3rd edition, defines chronic migraine as 15 or more MHDs for 3 months or more with criteria for migraine with or without aura met on 8 days a month or more. It defines episodic migraine as less than 15 MHDs.
The study characterized migraine subgroups by various demographics. “The more frequent headache categories were associated with slightly older age of onset with a higher proportion of BMI [body mass index] in the obese range and overall with lower levels of household income and education,” Dr. Lipton said.
Similar headache characteristics
A comparison of headache characteristics and headache-related disabilities across subgroups revealed a number of commonalities between the HFEM and LFCM subgroups, Dr. Lipton said. Among them were presence of mild to severe allodynia, disability grade, interictal burden, and anxiety and depression scores. For example, 47.3% of the HFEM subgroup and 54.9% of the LFCM subgroup had Patient Health Questionnaire–9 depression test scores greater than 10.
The study also evaluated patterns of consultation, diagnosis, and health resource utilization and found similar rates between the HFEM and LCFM subgroups, Dr. Lipton said. Rates of overnight hospital stay in the past 6 months were almost identical between the two subgroups: 4.1% for the former and 4.2% for the latter. One striking difference between the two subgroups: the rate of medication overuse per ICHD-3 recommendations was 40.5% in HFEM and 63% in LFCM.
“These finding suggest that the treatment needs of people with HFEM may be similar to those of people with LFCM, suggesting that the 15-MHD threshold currently recommended by the ICHD-3 may merit reconsideration,” Dr. Lipton said.
An arbitrary cutoff?
The findings raise a valid point about reevaluating the thresholds for low- and high-frequency migraine, said Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles. “My own personal view is that they’re the same thing,” he said of HFEM and LFCM; The 15-day cutoff, he said, is “somewhat arbitrary.”
Dr. Charles suggested migraine categories address frequency and not characteristics – episodic versus chronic – and use a range rather than a threshold. “Define a range that’s more like 10-20 days per month rather than having that point at 15,” Dr. Charles said. “People sometimes make the mistake of thinking that that classification reflects some underlying pathophysiology, and that may not be necessarily true.”
Dr. Lipton disclosed financial relationships with Alder Biopharmaceuticals, Allergan (now AbbVie), Amgen, Biohaven Pharmaceuticals, Dr. Reddy’s/Promius, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Lundbeck (Alder), Merck, Pernix Therapeutics, Pfizer, Supernus, Teva, Trigemina, Axsome Therapeutics, Vector, and Vedanta. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
according to an analysis of almost 17,000 patients from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study presented at the virtual annual meeting of the American Headache Society.
“The results showed substantial overlap in levels of burden, anxiety, depression and health utilization, including outpatient, inpatient and emergency department visits, among CaMEO respondents with high-frequency episodic migraine and those with low-frequency chronic migraine,” said Richard B. Lipton, MD, of the Albert Einstein College of Medicine, New York.
The study analyzed data on 16,789 respondents to CaMEO, the longitudinal, web-based study designed to characterize the course of episodic and chronic migraine. The study population consisted of four subgroups based on the number of self-reporting monthly headache days (MHDs):
- Low- and moderate-frequency episodic migraine (LFEM; zero to seven MHDs; n = 13,473).
- High-frequency episodic migraine (HFEM; 8-14 MHDs; n = 1,840).
- Low-frequency chronic migraine (LFCM; 15-23 MHDs; n = 1,035).
- High-frequency chronic migraine (HFCM; 24 or more MHDs; n = 441).
Dr. Lipton pointed out that the International Classification of Headache Disorders, 3rd edition, defines chronic migraine as 15 or more MHDs for 3 months or more with criteria for migraine with or without aura met on 8 days a month or more. It defines episodic migraine as less than 15 MHDs.
The study characterized migraine subgroups by various demographics. “The more frequent headache categories were associated with slightly older age of onset with a higher proportion of BMI [body mass index] in the obese range and overall with lower levels of household income and education,” Dr. Lipton said.
Similar headache characteristics
A comparison of headache characteristics and headache-related disabilities across subgroups revealed a number of commonalities between the HFEM and LFCM subgroups, Dr. Lipton said. Among them were presence of mild to severe allodynia, disability grade, interictal burden, and anxiety and depression scores. For example, 47.3% of the HFEM subgroup and 54.9% of the LFCM subgroup had Patient Health Questionnaire–9 depression test scores greater than 10.
The study also evaluated patterns of consultation, diagnosis, and health resource utilization and found similar rates between the HFEM and LCFM subgroups, Dr. Lipton said. Rates of overnight hospital stay in the past 6 months were almost identical between the two subgroups: 4.1% for the former and 4.2% for the latter. One striking difference between the two subgroups: the rate of medication overuse per ICHD-3 recommendations was 40.5% in HFEM and 63% in LFCM.
“These finding suggest that the treatment needs of people with HFEM may be similar to those of people with LFCM, suggesting that the 15-MHD threshold currently recommended by the ICHD-3 may merit reconsideration,” Dr. Lipton said.
An arbitrary cutoff?
The findings raise a valid point about reevaluating the thresholds for low- and high-frequency migraine, said Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles. “My own personal view is that they’re the same thing,” he said of HFEM and LFCM; The 15-day cutoff, he said, is “somewhat arbitrary.”
Dr. Charles suggested migraine categories address frequency and not characteristics – episodic versus chronic – and use a range rather than a threshold. “Define a range that’s more like 10-20 days per month rather than having that point at 15,” Dr. Charles said. “People sometimes make the mistake of thinking that that classification reflects some underlying pathophysiology, and that may not be necessarily true.”
Dr. Lipton disclosed financial relationships with Alder Biopharmaceuticals, Allergan (now AbbVie), Amgen, Biohaven Pharmaceuticals, Dr. Reddy’s/Promius, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Lundbeck (Alder), Merck, Pernix Therapeutics, Pfizer, Supernus, Teva, Trigemina, Axsome Therapeutics, Vector, and Vedanta. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
according to an analysis of almost 17,000 patients from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study presented at the virtual annual meeting of the American Headache Society.
“The results showed substantial overlap in levels of burden, anxiety, depression and health utilization, including outpatient, inpatient and emergency department visits, among CaMEO respondents with high-frequency episodic migraine and those with low-frequency chronic migraine,” said Richard B. Lipton, MD, of the Albert Einstein College of Medicine, New York.
The study analyzed data on 16,789 respondents to CaMEO, the longitudinal, web-based study designed to characterize the course of episodic and chronic migraine. The study population consisted of four subgroups based on the number of self-reporting monthly headache days (MHDs):
- Low- and moderate-frequency episodic migraine (LFEM; zero to seven MHDs; n = 13,473).
- High-frequency episodic migraine (HFEM; 8-14 MHDs; n = 1,840).
- Low-frequency chronic migraine (LFCM; 15-23 MHDs; n = 1,035).
- High-frequency chronic migraine (HFCM; 24 or more MHDs; n = 441).
Dr. Lipton pointed out that the International Classification of Headache Disorders, 3rd edition, defines chronic migraine as 15 or more MHDs for 3 months or more with criteria for migraine with or without aura met on 8 days a month or more. It defines episodic migraine as less than 15 MHDs.
The study characterized migraine subgroups by various demographics. “The more frequent headache categories were associated with slightly older age of onset with a higher proportion of BMI [body mass index] in the obese range and overall with lower levels of household income and education,” Dr. Lipton said.
Similar headache characteristics
A comparison of headache characteristics and headache-related disabilities across subgroups revealed a number of commonalities between the HFEM and LFCM subgroups, Dr. Lipton said. Among them were presence of mild to severe allodynia, disability grade, interictal burden, and anxiety and depression scores. For example, 47.3% of the HFEM subgroup and 54.9% of the LFCM subgroup had Patient Health Questionnaire–9 depression test scores greater than 10.
The study also evaluated patterns of consultation, diagnosis, and health resource utilization and found similar rates between the HFEM and LCFM subgroups, Dr. Lipton said. Rates of overnight hospital stay in the past 6 months were almost identical between the two subgroups: 4.1% for the former and 4.2% for the latter. One striking difference between the two subgroups: the rate of medication overuse per ICHD-3 recommendations was 40.5% in HFEM and 63% in LFCM.
“These finding suggest that the treatment needs of people with HFEM may be similar to those of people with LFCM, suggesting that the 15-MHD threshold currently recommended by the ICHD-3 may merit reconsideration,” Dr. Lipton said.
An arbitrary cutoff?
The findings raise a valid point about reevaluating the thresholds for low- and high-frequency migraine, said Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles. “My own personal view is that they’re the same thing,” he said of HFEM and LFCM; The 15-day cutoff, he said, is “somewhat arbitrary.”
Dr. Charles suggested migraine categories address frequency and not characteristics – episodic versus chronic – and use a range rather than a threshold. “Define a range that’s more like 10-20 days per month rather than having that point at 15,” Dr. Charles said. “People sometimes make the mistake of thinking that that classification reflects some underlying pathophysiology, and that may not be necessarily true.”
Dr. Lipton disclosed financial relationships with Alder Biopharmaceuticals, Allergan (now AbbVie), Amgen, Biohaven Pharmaceuticals, Dr. Reddy’s/Promius, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Lundbeck (Alder), Merck, Pernix Therapeutics, Pfizer, Supernus, Teva, Trigemina, Axsome Therapeutics, Vector, and Vedanta. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
FROM AHS 2020
NSAID/triptan combination improves treatment-resistant migraine
The combination (AXS-07), in development by Axsome Therapeutics, was also safe and well tolerated, according to Cedric O’Gorman, MD, Axsome senior vice president for clinical development and medical affairs. It was tested in subjects who had inadequately responded to previous treatment and who had an average of 2-8 migraines per month.
The therapy combines 10-mg rizatriptan with 20-mg meloxicam delivered by the company’s MoSEIC technology. “Treatment with AXS-07 resulted in rapid, sustained, substantial, and statistically significant effect as compared with rizatriptan and placebo. The enhanced effect of AXS-07 may be especially relevant for patients with more difficult-to-treat migraine,” said Dr. O’Gorman during a presentation of the study at the virtual annual meeting of the American Headache Society.
Matthew Robbins, MD, said in an interview, “This combination may be particularly useful for patients who want to take an oral medication but still need rapid and sustained pain freedom.” Dr. Robbins is the neurology residency program director at New York Presbyterian Hospital and an associate professor of neurology at Weill Cornell Medicine, New York. He was not involved in the research.
The study randomized 1,594 patients 2:2:2:1 to AXS-07, rizatriptan alone, MoSEIC meloxicam alone, or placebo, which could be administered immediately after a migraine event. Between 35% and 40% of participants across the groups had previously used triptans. The mean migraine treatment optimization questionnaire (mTOQ4) score was 3.6, indicating that the population was made up of people with poor responses to medication. Among patients in the study group, 37%-43% had severe pain intensity, 41%-47% were obese, and 35%-37% had morning migraine.
At 2 hours, more patients in the AXS-07 group than in the placebo group were pain free (19.9% vs. 6.7%; P < 0.001). They were also more likely to experience freedom from the most bothersome symptom at 2 hours (36.9% vs. 24.4%; P = 0.002). Secondary outcome measures favored the AXS-07 group when compared with the rizatriptan-only group, including 1-hour pain relief (44% vs. 37%; P = 0.04), 2- to 24-hour sustained pain relief (53% vs. 44%; P = 0.006), 2- to 48-hour sustained pain relief (47% vs. 37%; P = 0.003), 2- to 24-hour sustained pain freedom (16% vs. 11%; P = 0.038), 2- to 48-hour sustained pain freedom (15% vs. 8.8%; P = 0.003), rescue medication use (23% vs. 35%; P < 0.001), a rating of much or very much improved on the Patient Global Impression of Change (PGI-C) scale (47% vs. 39%; P = 0.022), and functional improvement at 24 hours (64% vs. 56%; P = 0.027).
“The percentage of patients achieving pain relief with AXS-07 was numerically greater than with rizatriptan at every time point measure, starting at 15 minutes, and was statistically significant by 60 minutes. This is significant because rizatriptan is widely recognized as the fastest-acting and one of the most effective oral triptans,” said Dr. O’Gorman.
The frequency of adverse events was 11.0% in the AXS-07, 15.4% in the rizatriptan group, 11.5% in the meloxicam group, and 6.0% in the placebo group.
“The added benefit of this study was the demonstration of efficacy in patients who have previously failed other acute treatments. We know that ineffective acute treatments are a likely risk factor for the progression of episodic migraine to chronic migraine, and the more options that we have for our patients, the better,” Dr. Robbins commented.
He remains concerned about cost and access, however. A limited number of tablets per month for acute treatments prompt clinicians to prescribe the medications individually and advise patients to take them in combination. “Rizatriptan is generally available in 12 monthly tablets by many coverage plans, and I would hope that, if ultimately FDA approved, a similar allotment is made affordable and accessible,” he said.
The study was funded by Axsome Therapeutics. Dr. O’Gorman is an employee of Axsome. Dr. Robbins has no relevant financial disclosures.
SOURCE: O’Gorman C et al. AHS 2020, Abstract 840673.
The combination (AXS-07), in development by Axsome Therapeutics, was also safe and well tolerated, according to Cedric O’Gorman, MD, Axsome senior vice president for clinical development and medical affairs. It was tested in subjects who had inadequately responded to previous treatment and who had an average of 2-8 migraines per month.
The therapy combines 10-mg rizatriptan with 20-mg meloxicam delivered by the company’s MoSEIC technology. “Treatment with AXS-07 resulted in rapid, sustained, substantial, and statistically significant effect as compared with rizatriptan and placebo. The enhanced effect of AXS-07 may be especially relevant for patients with more difficult-to-treat migraine,” said Dr. O’Gorman during a presentation of the study at the virtual annual meeting of the American Headache Society.
Matthew Robbins, MD, said in an interview, “This combination may be particularly useful for patients who want to take an oral medication but still need rapid and sustained pain freedom.” Dr. Robbins is the neurology residency program director at New York Presbyterian Hospital and an associate professor of neurology at Weill Cornell Medicine, New York. He was not involved in the research.
The study randomized 1,594 patients 2:2:2:1 to AXS-07, rizatriptan alone, MoSEIC meloxicam alone, or placebo, which could be administered immediately after a migraine event. Between 35% and 40% of participants across the groups had previously used triptans. The mean migraine treatment optimization questionnaire (mTOQ4) score was 3.6, indicating that the population was made up of people with poor responses to medication. Among patients in the study group, 37%-43% had severe pain intensity, 41%-47% were obese, and 35%-37% had morning migraine.
At 2 hours, more patients in the AXS-07 group than in the placebo group were pain free (19.9% vs. 6.7%; P < 0.001). They were also more likely to experience freedom from the most bothersome symptom at 2 hours (36.9% vs. 24.4%; P = 0.002). Secondary outcome measures favored the AXS-07 group when compared with the rizatriptan-only group, including 1-hour pain relief (44% vs. 37%; P = 0.04), 2- to 24-hour sustained pain relief (53% vs. 44%; P = 0.006), 2- to 48-hour sustained pain relief (47% vs. 37%; P = 0.003), 2- to 24-hour sustained pain freedom (16% vs. 11%; P = 0.038), 2- to 48-hour sustained pain freedom (15% vs. 8.8%; P = 0.003), rescue medication use (23% vs. 35%; P < 0.001), a rating of much or very much improved on the Patient Global Impression of Change (PGI-C) scale (47% vs. 39%; P = 0.022), and functional improvement at 24 hours (64% vs. 56%; P = 0.027).
“The percentage of patients achieving pain relief with AXS-07 was numerically greater than with rizatriptan at every time point measure, starting at 15 minutes, and was statistically significant by 60 minutes. This is significant because rizatriptan is widely recognized as the fastest-acting and one of the most effective oral triptans,” said Dr. O’Gorman.
The frequency of adverse events was 11.0% in the AXS-07, 15.4% in the rizatriptan group, 11.5% in the meloxicam group, and 6.0% in the placebo group.
“The added benefit of this study was the demonstration of efficacy in patients who have previously failed other acute treatments. We know that ineffective acute treatments are a likely risk factor for the progression of episodic migraine to chronic migraine, and the more options that we have for our patients, the better,” Dr. Robbins commented.
He remains concerned about cost and access, however. A limited number of tablets per month for acute treatments prompt clinicians to prescribe the medications individually and advise patients to take them in combination. “Rizatriptan is generally available in 12 monthly tablets by many coverage plans, and I would hope that, if ultimately FDA approved, a similar allotment is made affordable and accessible,” he said.
The study was funded by Axsome Therapeutics. Dr. O’Gorman is an employee of Axsome. Dr. Robbins has no relevant financial disclosures.
SOURCE: O’Gorman C et al. AHS 2020, Abstract 840673.
The combination (AXS-07), in development by Axsome Therapeutics, was also safe and well tolerated, according to Cedric O’Gorman, MD, Axsome senior vice president for clinical development and medical affairs. It was tested in subjects who had inadequately responded to previous treatment and who had an average of 2-8 migraines per month.
The therapy combines 10-mg rizatriptan with 20-mg meloxicam delivered by the company’s MoSEIC technology. “Treatment with AXS-07 resulted in rapid, sustained, substantial, and statistically significant effect as compared with rizatriptan and placebo. The enhanced effect of AXS-07 may be especially relevant for patients with more difficult-to-treat migraine,” said Dr. O’Gorman during a presentation of the study at the virtual annual meeting of the American Headache Society.
Matthew Robbins, MD, said in an interview, “This combination may be particularly useful for patients who want to take an oral medication but still need rapid and sustained pain freedom.” Dr. Robbins is the neurology residency program director at New York Presbyterian Hospital and an associate professor of neurology at Weill Cornell Medicine, New York. He was not involved in the research.
The study randomized 1,594 patients 2:2:2:1 to AXS-07, rizatriptan alone, MoSEIC meloxicam alone, or placebo, which could be administered immediately after a migraine event. Between 35% and 40% of participants across the groups had previously used triptans. The mean migraine treatment optimization questionnaire (mTOQ4) score was 3.6, indicating that the population was made up of people with poor responses to medication. Among patients in the study group, 37%-43% had severe pain intensity, 41%-47% were obese, and 35%-37% had morning migraine.
At 2 hours, more patients in the AXS-07 group than in the placebo group were pain free (19.9% vs. 6.7%; P < 0.001). They were also more likely to experience freedom from the most bothersome symptom at 2 hours (36.9% vs. 24.4%; P = 0.002). Secondary outcome measures favored the AXS-07 group when compared with the rizatriptan-only group, including 1-hour pain relief (44% vs. 37%; P = 0.04), 2- to 24-hour sustained pain relief (53% vs. 44%; P = 0.006), 2- to 48-hour sustained pain relief (47% vs. 37%; P = 0.003), 2- to 24-hour sustained pain freedom (16% vs. 11%; P = 0.038), 2- to 48-hour sustained pain freedom (15% vs. 8.8%; P = 0.003), rescue medication use (23% vs. 35%; P < 0.001), a rating of much or very much improved on the Patient Global Impression of Change (PGI-C) scale (47% vs. 39%; P = 0.022), and functional improvement at 24 hours (64% vs. 56%; P = 0.027).
“The percentage of patients achieving pain relief with AXS-07 was numerically greater than with rizatriptan at every time point measure, starting at 15 minutes, and was statistically significant by 60 minutes. This is significant because rizatriptan is widely recognized as the fastest-acting and one of the most effective oral triptans,” said Dr. O’Gorman.
The frequency of adverse events was 11.0% in the AXS-07, 15.4% in the rizatriptan group, 11.5% in the meloxicam group, and 6.0% in the placebo group.
“The added benefit of this study was the demonstration of efficacy in patients who have previously failed other acute treatments. We know that ineffective acute treatments are a likely risk factor for the progression of episodic migraine to chronic migraine, and the more options that we have for our patients, the better,” Dr. Robbins commented.
He remains concerned about cost and access, however. A limited number of tablets per month for acute treatments prompt clinicians to prescribe the medications individually and advise patients to take them in combination. “Rizatriptan is generally available in 12 monthly tablets by many coverage plans, and I would hope that, if ultimately FDA approved, a similar allotment is made affordable and accessible,” he said.
The study was funded by Axsome Therapeutics. Dr. O’Gorman is an employee of Axsome. Dr. Robbins has no relevant financial disclosures.
SOURCE: O’Gorman C et al. AHS 2020, Abstract 840673.
FROM AHS 2020
CGRPs in real world: Similar efficacy, more AEs
and has found that patients who fail on one of the treatments are likely to fail again if they’re switched to another.
At the virtual annual meeting of the American Headache Society, Larry Robbins, MD, assistant professor of neurology at Chicago Medical School, North Chicago, reported on the results of his postapproval study of 369 migraine patients taking one of the three approved CGRP mAbs. “If patients do not do well on one mAb, it is sometimes worthwhile to switch, but most patients do not do well from the second or third mAb as well,” Dr. Robbins said in an interview. “In addition, there are numerous adverse effects that were not captured in the official phase 3 studies. Efficacy has held up well, but for a number of reasons, the true adverse event profile is often missed.”
Assessing efficacy and adverse events
In evaluating the efficacy of the three approved CGRP mAbs, Dr. Robbins used measures of degree of relief based on percentage decrease of symptoms versus baseline and the number of migraine days, combined with the number of moderate or severe headache days. Most of the patients kept calendars and were interviewed by two headache specialists. The study also utilized a 10-point visual analog scale and averaged relief over 3 months.
Of the patients on erenumab (n = 220), 10% described 95%-100% relief of symptoms, 24% reported 71%-100% relief, 34% described 31%-70% relief, and 43% experienced 0%-30% relief. Adverse events among this group included constipation (20%), nausea (7%), increased headache and fatigue (5% for each), and joint pain and depression (3% for each). Three patients on erenumab experienced unspecified serious adverse reactions.
In the fremanezumab group (n = 79), 8% described 95%-100% relief, 18% had 71%-100% relief, 33% experienced 31%-70% improvement, and 50% had 30% improvement or less. Adverse events in these patients included nausea, constipation, and depression (6% each); increased headache and muscle pain or cramps (5% each); rash, joint pain, anxiety, fatigue, or weight gain (4% for each ); and injection-site reactions, irritability, or alopecia (3% combined).
Patients taking galcanezumab (n = 70) reported the following outcomes: 3% had 95%-100% relief of symptoms, 14% had 71%-100% relief, 46% with 31%-70% relief, and 40% had 0%-30% relief. This group’s adverse events included constipation (10%); depression and increased headache (6% for each); nausea, fatigue, or injection-site reactions (4% each ); and muscle pain or cramps, rash, anxiety, weight gain, or alopecia (3% each).
Dr. Robbins also assessed switching from one CGRP mAb to another for various reasons. “When the reason for switching was poor efficacy, only 27% of patients did well,” he stated in the presentation. “If the reason was adverse events, 33% did well. When insurance/financial reasons alone were the reason, but efficacy was adequate, 58% did well after switching.”
Overall, postapproval efficacy of the medications “held up well,” Dr. Robbins noted. “Efficacy after 2 months somewhat predicted how patients would do after 6 months.” Among the predictors of poor response his study identified were opioid use and moderate or severe refractory chronic migraine at baseline.
However, the rates of adverse events he reported were significantly greater than those reported in the clinical trials, Dr. Robbins said. He noted four reasons to explain this discrepancy: the trials did not use an 18-item supplemental checklist that he has advocated to identify patients at risk of side effects, the trials weren’t powered for adverse events, patients in the trials tended to be less refractory than those in the clinic, and that adverse events tend to be underreported in trials.
“Adverse events become disaggregated, with the same descriptors used for an adverse event,” Dr. Robbins said. “Examples include fatigue, somnolence, and tiredness; all may be 1%, while different patients are describing the same adverse event. It is possible to reaggregate the adverse events after the study, but this is fraught with error.”
Uncovering shortcomings in clinical trials
Emily Rubenstein Engel, MD, director of the Dalessio Headache Center at the Scripps Clinic in La Jolla, Calif., noted that Dr. Robbins’ findings are significant for two reasons. “Dr. Robbins has uncovered a general flaw in clinical trials, whereby the lack of consistency of adverse event terminology as well as the lack of a standardized questionnaire format for adverse events can result in significant under-reporting of adverse events,” she said.
“Specifically for the CGRPs,” Dr. Engel continued, “he has raised awareness that this new class of medication, however promising from an efficacy standpoint, has side effects that are much more frequent and severe than seen in the initial clinical trials.”
Dr. Robbins reported financial relationships with Allergan, Amgen and Teva. Dr. Engel has no financial relationships to disclose.
and has found that patients who fail on one of the treatments are likely to fail again if they’re switched to another.
At the virtual annual meeting of the American Headache Society, Larry Robbins, MD, assistant professor of neurology at Chicago Medical School, North Chicago, reported on the results of his postapproval study of 369 migraine patients taking one of the three approved CGRP mAbs. “If patients do not do well on one mAb, it is sometimes worthwhile to switch, but most patients do not do well from the second or third mAb as well,” Dr. Robbins said in an interview. “In addition, there are numerous adverse effects that were not captured in the official phase 3 studies. Efficacy has held up well, but for a number of reasons, the true adverse event profile is often missed.”
Assessing efficacy and adverse events
In evaluating the efficacy of the three approved CGRP mAbs, Dr. Robbins used measures of degree of relief based on percentage decrease of symptoms versus baseline and the number of migraine days, combined with the number of moderate or severe headache days. Most of the patients kept calendars and were interviewed by two headache specialists. The study also utilized a 10-point visual analog scale and averaged relief over 3 months.
Of the patients on erenumab (n = 220), 10% described 95%-100% relief of symptoms, 24% reported 71%-100% relief, 34% described 31%-70% relief, and 43% experienced 0%-30% relief. Adverse events among this group included constipation (20%), nausea (7%), increased headache and fatigue (5% for each), and joint pain and depression (3% for each). Three patients on erenumab experienced unspecified serious adverse reactions.
In the fremanezumab group (n = 79), 8% described 95%-100% relief, 18% had 71%-100% relief, 33% experienced 31%-70% improvement, and 50% had 30% improvement or less. Adverse events in these patients included nausea, constipation, and depression (6% each); increased headache and muscle pain or cramps (5% each); rash, joint pain, anxiety, fatigue, or weight gain (4% for each ); and injection-site reactions, irritability, or alopecia (3% combined).
Patients taking galcanezumab (n = 70) reported the following outcomes: 3% had 95%-100% relief of symptoms, 14% had 71%-100% relief, 46% with 31%-70% relief, and 40% had 0%-30% relief. This group’s adverse events included constipation (10%); depression and increased headache (6% for each); nausea, fatigue, or injection-site reactions (4% each ); and muscle pain or cramps, rash, anxiety, weight gain, or alopecia (3% each).
Dr. Robbins also assessed switching from one CGRP mAb to another for various reasons. “When the reason for switching was poor efficacy, only 27% of patients did well,” he stated in the presentation. “If the reason was adverse events, 33% did well. When insurance/financial reasons alone were the reason, but efficacy was adequate, 58% did well after switching.”
Overall, postapproval efficacy of the medications “held up well,” Dr. Robbins noted. “Efficacy after 2 months somewhat predicted how patients would do after 6 months.” Among the predictors of poor response his study identified were opioid use and moderate or severe refractory chronic migraine at baseline.
However, the rates of adverse events he reported were significantly greater than those reported in the clinical trials, Dr. Robbins said. He noted four reasons to explain this discrepancy: the trials did not use an 18-item supplemental checklist that he has advocated to identify patients at risk of side effects, the trials weren’t powered for adverse events, patients in the trials tended to be less refractory than those in the clinic, and that adverse events tend to be underreported in trials.
“Adverse events become disaggregated, with the same descriptors used for an adverse event,” Dr. Robbins said. “Examples include fatigue, somnolence, and tiredness; all may be 1%, while different patients are describing the same adverse event. It is possible to reaggregate the adverse events after the study, but this is fraught with error.”
Uncovering shortcomings in clinical trials
Emily Rubenstein Engel, MD, director of the Dalessio Headache Center at the Scripps Clinic in La Jolla, Calif., noted that Dr. Robbins’ findings are significant for two reasons. “Dr. Robbins has uncovered a general flaw in clinical trials, whereby the lack of consistency of adverse event terminology as well as the lack of a standardized questionnaire format for adverse events can result in significant under-reporting of adverse events,” she said.
“Specifically for the CGRPs,” Dr. Engel continued, “he has raised awareness that this new class of medication, however promising from an efficacy standpoint, has side effects that are much more frequent and severe than seen in the initial clinical trials.”
Dr. Robbins reported financial relationships with Allergan, Amgen and Teva. Dr. Engel has no financial relationships to disclose.
and has found that patients who fail on one of the treatments are likely to fail again if they’re switched to another.
At the virtual annual meeting of the American Headache Society, Larry Robbins, MD, assistant professor of neurology at Chicago Medical School, North Chicago, reported on the results of his postapproval study of 369 migraine patients taking one of the three approved CGRP mAbs. “If patients do not do well on one mAb, it is sometimes worthwhile to switch, but most patients do not do well from the second or third mAb as well,” Dr. Robbins said in an interview. “In addition, there are numerous adverse effects that were not captured in the official phase 3 studies. Efficacy has held up well, but for a number of reasons, the true adverse event profile is often missed.”
Assessing efficacy and adverse events
In evaluating the efficacy of the three approved CGRP mAbs, Dr. Robbins used measures of degree of relief based on percentage decrease of symptoms versus baseline and the number of migraine days, combined with the number of moderate or severe headache days. Most of the patients kept calendars and were interviewed by two headache specialists. The study also utilized a 10-point visual analog scale and averaged relief over 3 months.
Of the patients on erenumab (n = 220), 10% described 95%-100% relief of symptoms, 24% reported 71%-100% relief, 34% described 31%-70% relief, and 43% experienced 0%-30% relief. Adverse events among this group included constipation (20%), nausea (7%), increased headache and fatigue (5% for each), and joint pain and depression (3% for each). Three patients on erenumab experienced unspecified serious adverse reactions.
In the fremanezumab group (n = 79), 8% described 95%-100% relief, 18% had 71%-100% relief, 33% experienced 31%-70% improvement, and 50% had 30% improvement or less. Adverse events in these patients included nausea, constipation, and depression (6% each); increased headache and muscle pain or cramps (5% each); rash, joint pain, anxiety, fatigue, or weight gain (4% for each ); and injection-site reactions, irritability, or alopecia (3% combined).
Patients taking galcanezumab (n = 70) reported the following outcomes: 3% had 95%-100% relief of symptoms, 14% had 71%-100% relief, 46% with 31%-70% relief, and 40% had 0%-30% relief. This group’s adverse events included constipation (10%); depression and increased headache (6% for each); nausea, fatigue, or injection-site reactions (4% each ); and muscle pain or cramps, rash, anxiety, weight gain, or alopecia (3% each).
Dr. Robbins also assessed switching from one CGRP mAb to another for various reasons. “When the reason for switching was poor efficacy, only 27% of patients did well,” he stated in the presentation. “If the reason was adverse events, 33% did well. When insurance/financial reasons alone were the reason, but efficacy was adequate, 58% did well after switching.”
Overall, postapproval efficacy of the medications “held up well,” Dr. Robbins noted. “Efficacy after 2 months somewhat predicted how patients would do after 6 months.” Among the predictors of poor response his study identified were opioid use and moderate or severe refractory chronic migraine at baseline.
However, the rates of adverse events he reported were significantly greater than those reported in the clinical trials, Dr. Robbins said. He noted four reasons to explain this discrepancy: the trials did not use an 18-item supplemental checklist that he has advocated to identify patients at risk of side effects, the trials weren’t powered for adverse events, patients in the trials tended to be less refractory than those in the clinic, and that adverse events tend to be underreported in trials.
“Adverse events become disaggregated, with the same descriptors used for an adverse event,” Dr. Robbins said. “Examples include fatigue, somnolence, and tiredness; all may be 1%, while different patients are describing the same adverse event. It is possible to reaggregate the adverse events after the study, but this is fraught with error.”
Uncovering shortcomings in clinical trials
Emily Rubenstein Engel, MD, director of the Dalessio Headache Center at the Scripps Clinic in La Jolla, Calif., noted that Dr. Robbins’ findings are significant for two reasons. “Dr. Robbins has uncovered a general flaw in clinical trials, whereby the lack of consistency of adverse event terminology as well as the lack of a standardized questionnaire format for adverse events can result in significant under-reporting of adverse events,” she said.
“Specifically for the CGRPs,” Dr. Engel continued, “he has raised awareness that this new class of medication, however promising from an efficacy standpoint, has side effects that are much more frequent and severe than seen in the initial clinical trials.”
Dr. Robbins reported financial relationships with Allergan, Amgen and Teva. Dr. Engel has no financial relationships to disclose.
FROM AHS 2020






