User login
Inattention to heightened CV risk common theme in clozapine deaths teaser
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.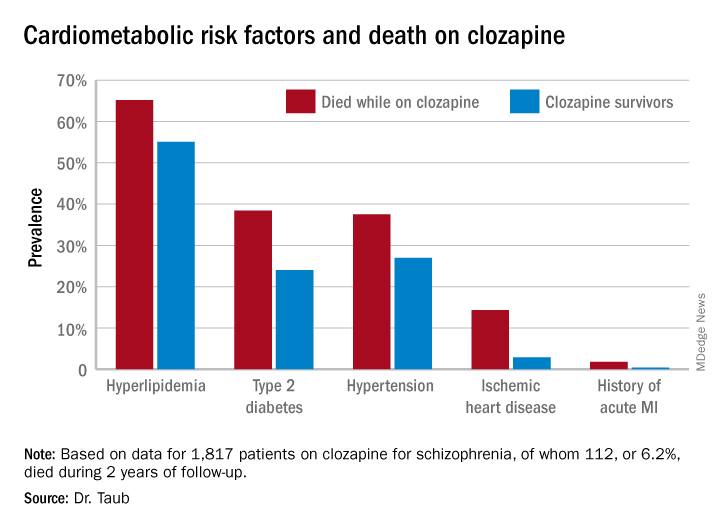
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
FROM ECNP 2020
Siblings of patients with bipolar disorder at increased risk
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
FROM ECNP 2020
Smartphones can differentiate bipolar from borderline personality disorder
There’s a reason they’re called smartphones.
Indeed, how patients use their smartphones and where they take them provides insight into what has been termed their “digital phenotype.” It’s information that, analyzed correctly, becomes useful in differentiating bipolar disorder from borderline personality disorder, a distinction that’s often challenging in clinical practice, Kate E.A. Saunders, MD, DPhil, said at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Saunders, a psychiatrist at the University of Oxford (England), and colleagues have developed a smartphone app enabling patients to briefly characterize their current mood on a daily basis, as well as a machine learning model to analyze this data stream as patients’ moods evolve over time. In their prospective longitudinal Automated Monitoring of Symptom Severity (AMoSS) study of 48 patients with a confirmed diagnosis of bipolar disorder, 31 with borderline personality disorder, and 51 healthy volunteers, the tool correctly classified 75% of participants into the correct diagnostic category on the basis of 20 daily mood ratings (Transl Psychiatry. 2018 Dec 13;81:274. doi: 10.1038/s41398-018-0334-0).
The app also monitors activity via accelerometry and geolocation to assess an individual’s circadian rest-activity patterns, as well as telephone use and texting behavior. In another report from AMoSS, Dr. Saunders and coinvestigators showed that these patterns also distinguish persons with bipolar disorder from those with borderline personality disorder, who in turn differ from healthy controls (Transl Psychiatry. 2019 Aug 20;91:195. doi: 10.1038/s41398-019-0526-2).
It doesn’t replace doctors, but clearly it can add to diagnostic accuracy,” she said.
Borderline personality disorder and bipolar disorder are common diagnoses with quite different treatment approaches and prognoses. Studies have shown that rates of misdiagnosis of the two disorders are significant. The challenge is that they share overlapping diagnostic criteria, including prominent mood instability, which is difficult to assess reliably in clinical practice. That’s because the assessment relies on retrospective self-report of how patients felt in the past, which is often colored by their present mood state. The smartphone app sidesteps that limitation by having patients rate their mood daily digitally across six categories – anxiety, elation, sadness, anger, irritability, and energy – on a 1-7 scale.
The machine learning model that analyzes this information organizes the voluminous data into what Dr. Saunders called “signatures of mood” and breaks them down using rough path theory, a mathematical concept based upon differential equations. Dr. Saunders and colleagues have demonstrated that the shifting daily mood self-rating patterns can be used not only to sharpen the differential diagnosis between bipolar disorder and borderline personality disorder, but also to predict future mood. Automated analysis of the past 20 previous mood self-ratings predicted the next day’s mood in healthy controls with 89%-98% accuracy, depending upon which of the six mood categories was under scrutiny.
The predictive power in patients with bipolar disorder was also good, ranging from 82% accuracy for the energetic and anxious domains to 90% for the angry mood category. This ability to predict future mood states could have clinical value by assisting bipolar patients in enhancing proactive self-management and managing their mood stability to avoid depressive or manic relapse, although this has yet to be studied.
“For borderline personality disorder the predictive accuracy was not so good – 70%-78% – but perhaps that doesn’t matter,” Dr. Saunders said. “Perhaps that difficulty in predicting mood may actually be quite a useful diagnostic marker.”
‘Mr. Jones, the doctor is ready to see your phone now.’
The app’s accelerometry and geolocation capabilities can also enhance diagnostic accuracy, as has been shown in the AMoSS study.
The geolocation analysis generates data on the places a patient has gone and how much time was spent there. Feeding that information into the machine learning model predicted the presence or absence of depression with 85% accuracy for bipolar disorder, but couldn’t predict depression at all in borderline personality disorder.
“So we get a sense that people with bipolar disorder have behavioral manifestations of their mood symptoms which are much more consistent with one another and appear to change very consistently with their mood state, whereas borderline personality disorder seems to be characterized by something that’s much more unstable and unpredictable – and we can pick up these predictive variables using our smartphones,” Dr. Saunders said.
As depressive symptoms arise in patients with bipolar disorder, affected individuals display much less day-to-day variability in movement as measured by accelerometry. These changes predicted bipolar disorder with 76% specificity and 48% sensitivity.
“That’s OK. But we can’t do that at all in people with borderline personality disorder, again highlighting the fact that behavioral manifestations and symptoms in these groups are very, very different,” Dr. Saunders observed.
In AMoSS, analysis of activity, geolocation, and distal temperature rhythms showed that the individuals with borderline personality disorder displayed evidence of delayed circadian function, with a distinctive rest-activity pattern that differed from persons with bipolar disorder. This delayed circadian function might provide a novel therapeutic target in borderline personality disorder, a condition for which there is a notable lack of effective pharmacologic and psychotherapeutic interventions.
Phone use patterns were revealing. Patients with bipolar disorder had an increased total telephone call frequency relative to the healthy controls, whereas those with borderline personality disorder used text messaging much more frequently, consistent with the notion that borderline patients have difficulty in interpersonal communication.
Smartphone-based diagnostic differentiation between bipolar disorder and borderline personality disorder isn’t ready for prime time use in clinical practice, Dr. Saunders said. This is groundbreaking work that needs to be refined and replicated in larger studies. There are important ethical and data protection issues that require attention. But patients are gung-ho. Dr. Saunders noted that participant compliance in AMoSS was “extraordinarily good,” at 82%. Moreover, even though the study lasted for 3 months, more than 60% of subjects continued filing mood reports for 12 months.
“Smartphones may also give us an improved understanding of the lived experience of people with mental health problems. That’s certainly the feedback we got a lot from patients. They enjoy using this technology. They feel it’s helpful to be able to show their clinician this is what it’s like for them,” Dr. Saunders said.
Clinical usefulness is limited
The study was interesting as a pilot, and it is technologically very innovative. However, at this stage, it is unclear how the results can be used clinically, said Igor Galynker, MD, PhD, when asked about the findings.
There is a place for using this type of technology for patients living in remote areas, for example. However, Dr. Galynker, director of the Richard and Cynthia Zirinsky Center for Bipolar Disorder in New York, said such technology should be viewed as augmentation rather than as a substitute for face-to-face treatment.
“Typically, if clinicians have enough time to speak to the patient and to take history, they can differentiate between bipolar disorder and borderline personality disorder: The former is cyclical, the latter is less so. However, this is hard to do without face-to-face contact, or when you only have 10 minutes,” said Dr. Galynker, professor of psychiatry at the Icahn School of Medicine and director of the Galynker Research and Prevention Laboratory, both at Mount Sinai in New York.
Dr. Saunders’ work is funded by the Wellcome Trust and the National Institute for Health Research. Dr. Galynker reported receiving funding from the National Institute of Mental Health and the American Foundation for Suicide Prevention. He has no other disclosures.
SOURCE: ECNP 2020. Session S21.
There’s a reason they’re called smartphones.
Indeed, how patients use their smartphones and where they take them provides insight into what has been termed their “digital phenotype.” It’s information that, analyzed correctly, becomes useful in differentiating bipolar disorder from borderline personality disorder, a distinction that’s often challenging in clinical practice, Kate E.A. Saunders, MD, DPhil, said at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Saunders, a psychiatrist at the University of Oxford (England), and colleagues have developed a smartphone app enabling patients to briefly characterize their current mood on a daily basis, as well as a machine learning model to analyze this data stream as patients’ moods evolve over time. In their prospective longitudinal Automated Monitoring of Symptom Severity (AMoSS) study of 48 patients with a confirmed diagnosis of bipolar disorder, 31 with borderline personality disorder, and 51 healthy volunteers, the tool correctly classified 75% of participants into the correct diagnostic category on the basis of 20 daily mood ratings (Transl Psychiatry. 2018 Dec 13;81:274. doi: 10.1038/s41398-018-0334-0).
The app also monitors activity via accelerometry and geolocation to assess an individual’s circadian rest-activity patterns, as well as telephone use and texting behavior. In another report from AMoSS, Dr. Saunders and coinvestigators showed that these patterns also distinguish persons with bipolar disorder from those with borderline personality disorder, who in turn differ from healthy controls (Transl Psychiatry. 2019 Aug 20;91:195. doi: 10.1038/s41398-019-0526-2).
It doesn’t replace doctors, but clearly it can add to diagnostic accuracy,” she said.
Borderline personality disorder and bipolar disorder are common diagnoses with quite different treatment approaches and prognoses. Studies have shown that rates of misdiagnosis of the two disorders are significant. The challenge is that they share overlapping diagnostic criteria, including prominent mood instability, which is difficult to assess reliably in clinical practice. That’s because the assessment relies on retrospective self-report of how patients felt in the past, which is often colored by their present mood state. The smartphone app sidesteps that limitation by having patients rate their mood daily digitally across six categories – anxiety, elation, sadness, anger, irritability, and energy – on a 1-7 scale.
The machine learning model that analyzes this information organizes the voluminous data into what Dr. Saunders called “signatures of mood” and breaks them down using rough path theory, a mathematical concept based upon differential equations. Dr. Saunders and colleagues have demonstrated that the shifting daily mood self-rating patterns can be used not only to sharpen the differential diagnosis between bipolar disorder and borderline personality disorder, but also to predict future mood. Automated analysis of the past 20 previous mood self-ratings predicted the next day’s mood in healthy controls with 89%-98% accuracy, depending upon which of the six mood categories was under scrutiny.
The predictive power in patients with bipolar disorder was also good, ranging from 82% accuracy for the energetic and anxious domains to 90% for the angry mood category. This ability to predict future mood states could have clinical value by assisting bipolar patients in enhancing proactive self-management and managing their mood stability to avoid depressive or manic relapse, although this has yet to be studied.
“For borderline personality disorder the predictive accuracy was not so good – 70%-78% – but perhaps that doesn’t matter,” Dr. Saunders said. “Perhaps that difficulty in predicting mood may actually be quite a useful diagnostic marker.”
‘Mr. Jones, the doctor is ready to see your phone now.’
The app’s accelerometry and geolocation capabilities can also enhance diagnostic accuracy, as has been shown in the AMoSS study.
The geolocation analysis generates data on the places a patient has gone and how much time was spent there. Feeding that information into the machine learning model predicted the presence or absence of depression with 85% accuracy for bipolar disorder, but couldn’t predict depression at all in borderline personality disorder.
“So we get a sense that people with bipolar disorder have behavioral manifestations of their mood symptoms which are much more consistent with one another and appear to change very consistently with their mood state, whereas borderline personality disorder seems to be characterized by something that’s much more unstable and unpredictable – and we can pick up these predictive variables using our smartphones,” Dr. Saunders said.
As depressive symptoms arise in patients with bipolar disorder, affected individuals display much less day-to-day variability in movement as measured by accelerometry. These changes predicted bipolar disorder with 76% specificity and 48% sensitivity.
“That’s OK. But we can’t do that at all in people with borderline personality disorder, again highlighting the fact that behavioral manifestations and symptoms in these groups are very, very different,” Dr. Saunders observed.
In AMoSS, analysis of activity, geolocation, and distal temperature rhythms showed that the individuals with borderline personality disorder displayed evidence of delayed circadian function, with a distinctive rest-activity pattern that differed from persons with bipolar disorder. This delayed circadian function might provide a novel therapeutic target in borderline personality disorder, a condition for which there is a notable lack of effective pharmacologic and psychotherapeutic interventions.
Phone use patterns were revealing. Patients with bipolar disorder had an increased total telephone call frequency relative to the healthy controls, whereas those with borderline personality disorder used text messaging much more frequently, consistent with the notion that borderline patients have difficulty in interpersonal communication.
Smartphone-based diagnostic differentiation between bipolar disorder and borderline personality disorder isn’t ready for prime time use in clinical practice, Dr. Saunders said. This is groundbreaking work that needs to be refined and replicated in larger studies. There are important ethical and data protection issues that require attention. But patients are gung-ho. Dr. Saunders noted that participant compliance in AMoSS was “extraordinarily good,” at 82%. Moreover, even though the study lasted for 3 months, more than 60% of subjects continued filing mood reports for 12 months.
“Smartphones may also give us an improved understanding of the lived experience of people with mental health problems. That’s certainly the feedback we got a lot from patients. They enjoy using this technology. They feel it’s helpful to be able to show their clinician this is what it’s like for them,” Dr. Saunders said.
Clinical usefulness is limited
The study was interesting as a pilot, and it is technologically very innovative. However, at this stage, it is unclear how the results can be used clinically, said Igor Galynker, MD, PhD, when asked about the findings.
There is a place for using this type of technology for patients living in remote areas, for example. However, Dr. Galynker, director of the Richard and Cynthia Zirinsky Center for Bipolar Disorder in New York, said such technology should be viewed as augmentation rather than as a substitute for face-to-face treatment.
“Typically, if clinicians have enough time to speak to the patient and to take history, they can differentiate between bipolar disorder and borderline personality disorder: The former is cyclical, the latter is less so. However, this is hard to do without face-to-face contact, or when you only have 10 minutes,” said Dr. Galynker, professor of psychiatry at the Icahn School of Medicine and director of the Galynker Research and Prevention Laboratory, both at Mount Sinai in New York.
Dr. Saunders’ work is funded by the Wellcome Trust and the National Institute for Health Research. Dr. Galynker reported receiving funding from the National Institute of Mental Health and the American Foundation for Suicide Prevention. He has no other disclosures.
SOURCE: ECNP 2020. Session S21.
There’s a reason they’re called smartphones.
Indeed, how patients use their smartphones and where they take them provides insight into what has been termed their “digital phenotype.” It’s information that, analyzed correctly, becomes useful in differentiating bipolar disorder from borderline personality disorder, a distinction that’s often challenging in clinical practice, Kate E.A. Saunders, MD, DPhil, said at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Saunders, a psychiatrist at the University of Oxford (England), and colleagues have developed a smartphone app enabling patients to briefly characterize their current mood on a daily basis, as well as a machine learning model to analyze this data stream as patients’ moods evolve over time. In their prospective longitudinal Automated Monitoring of Symptom Severity (AMoSS) study of 48 patients with a confirmed diagnosis of bipolar disorder, 31 with borderline personality disorder, and 51 healthy volunteers, the tool correctly classified 75% of participants into the correct diagnostic category on the basis of 20 daily mood ratings (Transl Psychiatry. 2018 Dec 13;81:274. doi: 10.1038/s41398-018-0334-0).
The app also monitors activity via accelerometry and geolocation to assess an individual’s circadian rest-activity patterns, as well as telephone use and texting behavior. In another report from AMoSS, Dr. Saunders and coinvestigators showed that these patterns also distinguish persons with bipolar disorder from those with borderline personality disorder, who in turn differ from healthy controls (Transl Psychiatry. 2019 Aug 20;91:195. doi: 10.1038/s41398-019-0526-2).
It doesn’t replace doctors, but clearly it can add to diagnostic accuracy,” she said.
Borderline personality disorder and bipolar disorder are common diagnoses with quite different treatment approaches and prognoses. Studies have shown that rates of misdiagnosis of the two disorders are significant. The challenge is that they share overlapping diagnostic criteria, including prominent mood instability, which is difficult to assess reliably in clinical practice. That’s because the assessment relies on retrospective self-report of how patients felt in the past, which is often colored by their present mood state. The smartphone app sidesteps that limitation by having patients rate their mood daily digitally across six categories – anxiety, elation, sadness, anger, irritability, and energy – on a 1-7 scale.
The machine learning model that analyzes this information organizes the voluminous data into what Dr. Saunders called “signatures of mood” and breaks them down using rough path theory, a mathematical concept based upon differential equations. Dr. Saunders and colleagues have demonstrated that the shifting daily mood self-rating patterns can be used not only to sharpen the differential diagnosis between bipolar disorder and borderline personality disorder, but also to predict future mood. Automated analysis of the past 20 previous mood self-ratings predicted the next day’s mood in healthy controls with 89%-98% accuracy, depending upon which of the six mood categories was under scrutiny.
The predictive power in patients with bipolar disorder was also good, ranging from 82% accuracy for the energetic and anxious domains to 90% for the angry mood category. This ability to predict future mood states could have clinical value by assisting bipolar patients in enhancing proactive self-management and managing their mood stability to avoid depressive or manic relapse, although this has yet to be studied.
“For borderline personality disorder the predictive accuracy was not so good – 70%-78% – but perhaps that doesn’t matter,” Dr. Saunders said. “Perhaps that difficulty in predicting mood may actually be quite a useful diagnostic marker.”
‘Mr. Jones, the doctor is ready to see your phone now.’
The app’s accelerometry and geolocation capabilities can also enhance diagnostic accuracy, as has been shown in the AMoSS study.
The geolocation analysis generates data on the places a patient has gone and how much time was spent there. Feeding that information into the machine learning model predicted the presence or absence of depression with 85% accuracy for bipolar disorder, but couldn’t predict depression at all in borderline personality disorder.
“So we get a sense that people with bipolar disorder have behavioral manifestations of their mood symptoms which are much more consistent with one another and appear to change very consistently with their mood state, whereas borderline personality disorder seems to be characterized by something that’s much more unstable and unpredictable – and we can pick up these predictive variables using our smartphones,” Dr. Saunders said.
As depressive symptoms arise in patients with bipolar disorder, affected individuals display much less day-to-day variability in movement as measured by accelerometry. These changes predicted bipolar disorder with 76% specificity and 48% sensitivity.
“That’s OK. But we can’t do that at all in people with borderline personality disorder, again highlighting the fact that behavioral manifestations and symptoms in these groups are very, very different,” Dr. Saunders observed.
In AMoSS, analysis of activity, geolocation, and distal temperature rhythms showed that the individuals with borderline personality disorder displayed evidence of delayed circadian function, with a distinctive rest-activity pattern that differed from persons with bipolar disorder. This delayed circadian function might provide a novel therapeutic target in borderline personality disorder, a condition for which there is a notable lack of effective pharmacologic and psychotherapeutic interventions.
Phone use patterns were revealing. Patients with bipolar disorder had an increased total telephone call frequency relative to the healthy controls, whereas those with borderline personality disorder used text messaging much more frequently, consistent with the notion that borderline patients have difficulty in interpersonal communication.
Smartphone-based diagnostic differentiation between bipolar disorder and borderline personality disorder isn’t ready for prime time use in clinical practice, Dr. Saunders said. This is groundbreaking work that needs to be refined and replicated in larger studies. There are important ethical and data protection issues that require attention. But patients are gung-ho. Dr. Saunders noted that participant compliance in AMoSS was “extraordinarily good,” at 82%. Moreover, even though the study lasted for 3 months, more than 60% of subjects continued filing mood reports for 12 months.
“Smartphones may also give us an improved understanding of the lived experience of people with mental health problems. That’s certainly the feedback we got a lot from patients. They enjoy using this technology. They feel it’s helpful to be able to show their clinician this is what it’s like for them,” Dr. Saunders said.
Clinical usefulness is limited
The study was interesting as a pilot, and it is technologically very innovative. However, at this stage, it is unclear how the results can be used clinically, said Igor Galynker, MD, PhD, when asked about the findings.
There is a place for using this type of technology for patients living in remote areas, for example. However, Dr. Galynker, director of the Richard and Cynthia Zirinsky Center for Bipolar Disorder in New York, said such technology should be viewed as augmentation rather than as a substitute for face-to-face treatment.
“Typically, if clinicians have enough time to speak to the patient and to take history, they can differentiate between bipolar disorder and borderline personality disorder: The former is cyclical, the latter is less so. However, this is hard to do without face-to-face contact, or when you only have 10 minutes,” said Dr. Galynker, professor of psychiatry at the Icahn School of Medicine and director of the Galynker Research and Prevention Laboratory, both at Mount Sinai in New York.
Dr. Saunders’ work is funded by the Wellcome Trust and the National Institute for Health Research. Dr. Galynker reported receiving funding from the National Institute of Mental Health and the American Foundation for Suicide Prevention. He has no other disclosures.
SOURCE: ECNP 2020. Session S21.
FROM ECNP 2020
Melancholic, psychotic depression may protect against ECT cognitive effects
Patients with severe melancholic or psychotic depression are more likely to respond to ECT, and preliminary evidence indicates they’re also protected against ECT-induced cognitive impairment, Linda van Diermen, MD, PhD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Over the decades many small, underpowered studies have looked at possible predictors of ECT response and remission, with no consensus being reached. In an effort to bring a measure of clarity, Dr. van Diermen and her coinvestigators performed a meta-analysis of 34 published studies in accord with the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-analysis Protocols) guidelines and published their findings in the British Journal of Psychiatry. They scrutinized three potential predictors of response: the presence of psychotic features, melancholic depression with psychomotor symptoms, and older age.
Psychotic depression was associated with a 1.7-fold increased likelihood of response to ECT and a 1.5-fold increased odds of remission, compared with that of ECT-treated patients without psychotic depression. Older age was also a statistically significant predictor of response. However, the findings on melancholic depression were inconclusive, with only five studies with inconsistent results being available, said Dr. van Diermen, a psychiatrist at the University of Antwerp (Belgium).
She was quick to point out that, although psychotic depression and older age were statistically significant predictors of heightened likelihood of ECT response, they are of only limited clinical significance in treatment decision-making. The ECT response rate was 79% in patients with psychotic depression but still quite good at 71% in those without psychotic depression. Moreover, the average age of remitters was 59.7 years, compared with 55.4 years in nonresponders, a difference too small to be useful in guiding clinical treatment decisions.
“Although we did a meta-analysis in more than 3,200 patients that confirmed the superior effects of ECT in older patients and we recommended it at that time as one of the elements to guide decision-making when you consider ECT, our present, more detailed look at the interdependence of the predictors leads us to reconsider this statement. We now venture that age has been given too much weight in the past decades.”
A closer look at ECT response predictors
The studies included in the meta-analysis assessed psychotic depression and melancholic features as ECT response predictors in the typical binary way employed in clinical practice: yes/no, either present or absent. Dr. van Diermer hypothesized that a more in-depth assessment of the severity of those factors would boost their predictive power.
She found that this was indeed the case for melancholic depression as evaluated by three tools for measuring psychomotor symptoms, a core feature of this form of depression. She and her coinvestigators assessed psychomotor functioning in 65 adults with major depressive disorder before, during, and after ECT using the clinician-rated CORE scale, which measures psychomotor retardation, agitation, and noninteractiveness. In addition, the investigators had the subjects wear an accelerometer and complete a timed fine-motor drawing test.
The 41 patients with melancholic depression with psychomotor symptoms as defined by a CORE score of 8 or more were 4.9-fold more likely to reach an ECT response than were those with nonmelancholic depression. A lower baseline daytime activity level as assessed by accelerometer was also a significant predictor of increased likelihood of response, as were slower times on the drawing test.
In contrast, the investigators found that more detailed assessment of psychotic depression using the validated Psychotic Depression Assessment Scale (PDAS) was predictive of the likelihood of ECT response, but not any more so than the simple presence or absence of psychotic symptoms (J ECT. 2019 Dec;35[4]:238-44).
“In our sample, better measurement of psychotic symptoms did not improve prediction, but better measurement of psychomotor symptoms did seem to be valuable,” according to the psychiatrist.
Protection against ECT’s cognitive side effects?
Dr. van Diermen and colleagues assessed short- and long-term changes in global cognitive functioning in 65 consecutive patients treated with ECT for a major depressive episode by administering the Montreal Cognitive Assessment (MoCA) at baseline, before the third ECT session, and 1 week, 3 months, and 6 months after completing their treatment course.
During ECT, the investigators documented a limited decrease in cognitive functioning at the group level, which rebounded during the 6 months after ECT. But although there was no significant difference between MoCA scores at baseline and 6 months follow-up after ECT in the overall group of study participants, that doesn’t tell the full story. Six months after completing their course of ECT, 18% of patients demonstrated improved cognitive functioning, compared with baseline, but 8% had significantly worse cognitive functioning than pretreatment.
“Saying that ECT has no cognitive effects seems to be somewhat wrong to me. It has cognitive effects for certain people, and it will be interesting to know which people,” Dr. van Diermen said.
In what she termed “a very, very preliminary analysis,” she found that the patients with psychotic or melancholic depression were markedly less likely to have long-term cognitive impairment as defined by a worse MoCA score, compared with baseline, both at 6 months and one or more intermediate time points. Only 1 of 31 patients with psychotic depression fell into that poor cognitive outcome category, as did 4 patients with melancholic depression, compared with 12 patients without psychotic depression and 9 without melancholic depression. This, Dr. van Diermen believes, is the first report of an apparent protective effect of melancholic or psychotic depression against ECT-induced long-term cognitive worsening.
“Replication of our results is definitely necessary in larger patient samples,” she cautioned.
Dr. van Diermen reported having no financial conflicts regarding her presentation.
SOURCE: van Diermen L. ECNP 2020, Session EDU03.
Patients with severe melancholic or psychotic depression are more likely to respond to ECT, and preliminary evidence indicates they’re also protected against ECT-induced cognitive impairment, Linda van Diermen, MD, PhD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Over the decades many small, underpowered studies have looked at possible predictors of ECT response and remission, with no consensus being reached. In an effort to bring a measure of clarity, Dr. van Diermen and her coinvestigators performed a meta-analysis of 34 published studies in accord with the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-analysis Protocols) guidelines and published their findings in the British Journal of Psychiatry. They scrutinized three potential predictors of response: the presence of psychotic features, melancholic depression with psychomotor symptoms, and older age.
Psychotic depression was associated with a 1.7-fold increased likelihood of response to ECT and a 1.5-fold increased odds of remission, compared with that of ECT-treated patients without psychotic depression. Older age was also a statistically significant predictor of response. However, the findings on melancholic depression were inconclusive, with only five studies with inconsistent results being available, said Dr. van Diermen, a psychiatrist at the University of Antwerp (Belgium).
She was quick to point out that, although psychotic depression and older age were statistically significant predictors of heightened likelihood of ECT response, they are of only limited clinical significance in treatment decision-making. The ECT response rate was 79% in patients with psychotic depression but still quite good at 71% in those without psychotic depression. Moreover, the average age of remitters was 59.7 years, compared with 55.4 years in nonresponders, a difference too small to be useful in guiding clinical treatment decisions.
“Although we did a meta-analysis in more than 3,200 patients that confirmed the superior effects of ECT in older patients and we recommended it at that time as one of the elements to guide decision-making when you consider ECT, our present, more detailed look at the interdependence of the predictors leads us to reconsider this statement. We now venture that age has been given too much weight in the past decades.”
A closer look at ECT response predictors
The studies included in the meta-analysis assessed psychotic depression and melancholic features as ECT response predictors in the typical binary way employed in clinical practice: yes/no, either present or absent. Dr. van Diermer hypothesized that a more in-depth assessment of the severity of those factors would boost their predictive power.
She found that this was indeed the case for melancholic depression as evaluated by three tools for measuring psychomotor symptoms, a core feature of this form of depression. She and her coinvestigators assessed psychomotor functioning in 65 adults with major depressive disorder before, during, and after ECT using the clinician-rated CORE scale, which measures psychomotor retardation, agitation, and noninteractiveness. In addition, the investigators had the subjects wear an accelerometer and complete a timed fine-motor drawing test.
The 41 patients with melancholic depression with psychomotor symptoms as defined by a CORE score of 8 or more were 4.9-fold more likely to reach an ECT response than were those with nonmelancholic depression. A lower baseline daytime activity level as assessed by accelerometer was also a significant predictor of increased likelihood of response, as were slower times on the drawing test.
In contrast, the investigators found that more detailed assessment of psychotic depression using the validated Psychotic Depression Assessment Scale (PDAS) was predictive of the likelihood of ECT response, but not any more so than the simple presence or absence of psychotic symptoms (J ECT. 2019 Dec;35[4]:238-44).
“In our sample, better measurement of psychotic symptoms did not improve prediction, but better measurement of psychomotor symptoms did seem to be valuable,” according to the psychiatrist.
Protection against ECT’s cognitive side effects?
Dr. van Diermen and colleagues assessed short- and long-term changes in global cognitive functioning in 65 consecutive patients treated with ECT for a major depressive episode by administering the Montreal Cognitive Assessment (MoCA) at baseline, before the third ECT session, and 1 week, 3 months, and 6 months after completing their treatment course.
During ECT, the investigators documented a limited decrease in cognitive functioning at the group level, which rebounded during the 6 months after ECT. But although there was no significant difference between MoCA scores at baseline and 6 months follow-up after ECT in the overall group of study participants, that doesn’t tell the full story. Six months after completing their course of ECT, 18% of patients demonstrated improved cognitive functioning, compared with baseline, but 8% had significantly worse cognitive functioning than pretreatment.
“Saying that ECT has no cognitive effects seems to be somewhat wrong to me. It has cognitive effects for certain people, and it will be interesting to know which people,” Dr. van Diermen said.
In what she termed “a very, very preliminary analysis,” she found that the patients with psychotic or melancholic depression were markedly less likely to have long-term cognitive impairment as defined by a worse MoCA score, compared with baseline, both at 6 months and one or more intermediate time points. Only 1 of 31 patients with psychotic depression fell into that poor cognitive outcome category, as did 4 patients with melancholic depression, compared with 12 patients without psychotic depression and 9 without melancholic depression. This, Dr. van Diermen believes, is the first report of an apparent protective effect of melancholic or psychotic depression against ECT-induced long-term cognitive worsening.
“Replication of our results is definitely necessary in larger patient samples,” she cautioned.
Dr. van Diermen reported having no financial conflicts regarding her presentation.
SOURCE: van Diermen L. ECNP 2020, Session EDU03.
Patients with severe melancholic or psychotic depression are more likely to respond to ECT, and preliminary evidence indicates they’re also protected against ECT-induced cognitive impairment, Linda van Diermen, MD, PhD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Over the decades many small, underpowered studies have looked at possible predictors of ECT response and remission, with no consensus being reached. In an effort to bring a measure of clarity, Dr. van Diermen and her coinvestigators performed a meta-analysis of 34 published studies in accord with the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-analysis Protocols) guidelines and published their findings in the British Journal of Psychiatry. They scrutinized three potential predictors of response: the presence of psychotic features, melancholic depression with psychomotor symptoms, and older age.
Psychotic depression was associated with a 1.7-fold increased likelihood of response to ECT and a 1.5-fold increased odds of remission, compared with that of ECT-treated patients without psychotic depression. Older age was also a statistically significant predictor of response. However, the findings on melancholic depression were inconclusive, with only five studies with inconsistent results being available, said Dr. van Diermen, a psychiatrist at the University of Antwerp (Belgium).
She was quick to point out that, although psychotic depression and older age were statistically significant predictors of heightened likelihood of ECT response, they are of only limited clinical significance in treatment decision-making. The ECT response rate was 79% in patients with psychotic depression but still quite good at 71% in those without psychotic depression. Moreover, the average age of remitters was 59.7 years, compared with 55.4 years in nonresponders, a difference too small to be useful in guiding clinical treatment decisions.
“Although we did a meta-analysis in more than 3,200 patients that confirmed the superior effects of ECT in older patients and we recommended it at that time as one of the elements to guide decision-making when you consider ECT, our present, more detailed look at the interdependence of the predictors leads us to reconsider this statement. We now venture that age has been given too much weight in the past decades.”
A closer look at ECT response predictors
The studies included in the meta-analysis assessed psychotic depression and melancholic features as ECT response predictors in the typical binary way employed in clinical practice: yes/no, either present or absent. Dr. van Diermer hypothesized that a more in-depth assessment of the severity of those factors would boost their predictive power.
She found that this was indeed the case for melancholic depression as evaluated by three tools for measuring psychomotor symptoms, a core feature of this form of depression. She and her coinvestigators assessed psychomotor functioning in 65 adults with major depressive disorder before, during, and after ECT using the clinician-rated CORE scale, which measures psychomotor retardation, agitation, and noninteractiveness. In addition, the investigators had the subjects wear an accelerometer and complete a timed fine-motor drawing test.
The 41 patients with melancholic depression with psychomotor symptoms as defined by a CORE score of 8 or more were 4.9-fold more likely to reach an ECT response than were those with nonmelancholic depression. A lower baseline daytime activity level as assessed by accelerometer was also a significant predictor of increased likelihood of response, as were slower times on the drawing test.
In contrast, the investigators found that more detailed assessment of psychotic depression using the validated Psychotic Depression Assessment Scale (PDAS) was predictive of the likelihood of ECT response, but not any more so than the simple presence or absence of psychotic symptoms (J ECT. 2019 Dec;35[4]:238-44).
“In our sample, better measurement of psychotic symptoms did not improve prediction, but better measurement of psychomotor symptoms did seem to be valuable,” according to the psychiatrist.
Protection against ECT’s cognitive side effects?
Dr. van Diermen and colleagues assessed short- and long-term changes in global cognitive functioning in 65 consecutive patients treated with ECT for a major depressive episode by administering the Montreal Cognitive Assessment (MoCA) at baseline, before the third ECT session, and 1 week, 3 months, and 6 months after completing their treatment course.
During ECT, the investigators documented a limited decrease in cognitive functioning at the group level, which rebounded during the 6 months after ECT. But although there was no significant difference between MoCA scores at baseline and 6 months follow-up after ECT in the overall group of study participants, that doesn’t tell the full story. Six months after completing their course of ECT, 18% of patients demonstrated improved cognitive functioning, compared with baseline, but 8% had significantly worse cognitive functioning than pretreatment.
“Saying that ECT has no cognitive effects seems to be somewhat wrong to me. It has cognitive effects for certain people, and it will be interesting to know which people,” Dr. van Diermen said.
In what she termed “a very, very preliminary analysis,” she found that the patients with psychotic or melancholic depression were markedly less likely to have long-term cognitive impairment as defined by a worse MoCA score, compared with baseline, both at 6 months and one or more intermediate time points. Only 1 of 31 patients with psychotic depression fell into that poor cognitive outcome category, as did 4 patients with melancholic depression, compared with 12 patients without psychotic depression and 9 without melancholic depression. This, Dr. van Diermen believes, is the first report of an apparent protective effect of melancholic or psychotic depression against ECT-induced long-term cognitive worsening.
“Replication of our results is definitely necessary in larger patient samples,” she cautioned.
Dr. van Diermen reported having no financial conflicts regarding her presentation.
SOURCE: van Diermen L. ECNP 2020, Session EDU03.
FROM ECNP 2020
Include irritability in ADHD suicidality risk assessments
Irritability appears to be a potent independent predictor of increased risk for suicidality in children and adolescents with ADHD, Tomer Levy, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
While there is ample evidence that ADHD is associated with increased suicidality, Dr. Levy’s recent study involving 1,516 youths aged 6-17 years attending an outpatient ADHD clinic demonstrated that this increased risk is mediated by depression and irritability in roughly equal measures. Moreover, upon controlling for those two factors in a multivariate analysis, ADHD symptoms, per se, had no direct effect on risk of suicidality as defined by suidical ideation, attempts, or self-harm.
The clinical take-home message is that assessing irritability, as well as depression, may bolster an estimate of suicidality and help in managing suicidal risk in ADHD, according to Dr. Levy, a child and adolescent psychiatrist at the Hospital for Sick Children, Toronto, and head of behavioral regulation services at the Geha Mental Health Center in Petah Tikva, Israel.
The study included separate parent- and teacher-structured reports of the youths’ ADHD symptoms, suicidality, depression, irritability, and anxiety.
In multivariate analyses, parent-reported depression accounted for 39.1% of the association between ADHD symptoms and suicidality, while irritability symptoms mediated 36.8% of the total effect. In the teachers’ reports, depression and irritability symptoms accounted for 45.3% and 38.4% of the association. Anxiety symptoms mediated 19% of the relationship between ADHD and suicidality by parental report but had no significant impact on the association according to teacher report in the recently published study.
Dr. Levy noted that, in the DSM-5, irritability cuts across diagnostic categories. It is not only a core dimension of ADHD, but of the other externalizing disorders – conduct disorder and oppositional defiant disorder – as well, and also of neurodevelopmental, internalizing, and stress-related disorders.
Interventional studies aimed at dampening irritability as a potential strategy to reduce suicidality haven’t yet been done, but they deserve research priority status, in Dr. Levy’s view. Numerous functional dimensions that influence irritability are potential targets, including aggression, negative affect, low tolerance of frustration, skewed threat perception, and impaired self-regulation, according to the psychiatrist.
Most suicidal youths are attempting to cope with mental disorders. The most prevalent of these are major depressive disorder and dysthymia, followed by externalizing disorders. And among the externalizing disorders, conduct disorder stands out in terms of the magnitude of associated suicidality risk. In a large Taiwanese national study including 3,711 adolescents with conduct disorder and 14,844 age- and sex-matched controls, conduct disorder was associated with an adjusted 5.17-fold increased risk of subsequent suicide attempts over the next 10 years in a multivariate regression analysis adjusted for other psychiatric comorbidities and demographics.
In addition to depression, irritability symptoms, and conduct problems, other risk factors that should be part of a suicidality assessment in children and adolescents with ADHD include substance use, anxiety, poor family support, and bullying and/or being bullied. But, perhaps surprisingly, not impulsivity, Dr. Levy said.
“There is a widely held perception that impulsivity imparts a risk for suicidality, and especially in the transition from ideation to attempt. However, more recent evidence fails to show a convincing association,” according to Dr. Levy.
He reported having no financial conflicts regarding his presentation.
SOURCE: Levy T. ECNP 2020, Session EDU.02.
Irritability appears to be a potent independent predictor of increased risk for suicidality in children and adolescents with ADHD, Tomer Levy, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
While there is ample evidence that ADHD is associated with increased suicidality, Dr. Levy’s recent study involving 1,516 youths aged 6-17 years attending an outpatient ADHD clinic demonstrated that this increased risk is mediated by depression and irritability in roughly equal measures. Moreover, upon controlling for those two factors in a multivariate analysis, ADHD symptoms, per se, had no direct effect on risk of suicidality as defined by suidical ideation, attempts, or self-harm.
The clinical take-home message is that assessing irritability, as well as depression, may bolster an estimate of suicidality and help in managing suicidal risk in ADHD, according to Dr. Levy, a child and adolescent psychiatrist at the Hospital for Sick Children, Toronto, and head of behavioral regulation services at the Geha Mental Health Center in Petah Tikva, Israel.
The study included separate parent- and teacher-structured reports of the youths’ ADHD symptoms, suicidality, depression, irritability, and anxiety.
In multivariate analyses, parent-reported depression accounted for 39.1% of the association between ADHD symptoms and suicidality, while irritability symptoms mediated 36.8% of the total effect. In the teachers’ reports, depression and irritability symptoms accounted for 45.3% and 38.4% of the association. Anxiety symptoms mediated 19% of the relationship between ADHD and suicidality by parental report but had no significant impact on the association according to teacher report in the recently published study.
Dr. Levy noted that, in the DSM-5, irritability cuts across diagnostic categories. It is not only a core dimension of ADHD, but of the other externalizing disorders – conduct disorder and oppositional defiant disorder – as well, and also of neurodevelopmental, internalizing, and stress-related disorders.
Interventional studies aimed at dampening irritability as a potential strategy to reduce suicidality haven’t yet been done, but they deserve research priority status, in Dr. Levy’s view. Numerous functional dimensions that influence irritability are potential targets, including aggression, negative affect, low tolerance of frustration, skewed threat perception, and impaired self-regulation, according to the psychiatrist.
Most suicidal youths are attempting to cope with mental disorders. The most prevalent of these are major depressive disorder and dysthymia, followed by externalizing disorders. And among the externalizing disorders, conduct disorder stands out in terms of the magnitude of associated suicidality risk. In a large Taiwanese national study including 3,711 adolescents with conduct disorder and 14,844 age- and sex-matched controls, conduct disorder was associated with an adjusted 5.17-fold increased risk of subsequent suicide attempts over the next 10 years in a multivariate regression analysis adjusted for other psychiatric comorbidities and demographics.
In addition to depression, irritability symptoms, and conduct problems, other risk factors that should be part of a suicidality assessment in children and adolescents with ADHD include substance use, anxiety, poor family support, and bullying and/or being bullied. But, perhaps surprisingly, not impulsivity, Dr. Levy said.
“There is a widely held perception that impulsivity imparts a risk for suicidality, and especially in the transition from ideation to attempt. However, more recent evidence fails to show a convincing association,” according to Dr. Levy.
He reported having no financial conflicts regarding his presentation.
SOURCE: Levy T. ECNP 2020, Session EDU.02.
Irritability appears to be a potent independent predictor of increased risk for suicidality in children and adolescents with ADHD, Tomer Levy, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
While there is ample evidence that ADHD is associated with increased suicidality, Dr. Levy’s recent study involving 1,516 youths aged 6-17 years attending an outpatient ADHD clinic demonstrated that this increased risk is mediated by depression and irritability in roughly equal measures. Moreover, upon controlling for those two factors in a multivariate analysis, ADHD symptoms, per se, had no direct effect on risk of suicidality as defined by suidical ideation, attempts, or self-harm.
The clinical take-home message is that assessing irritability, as well as depression, may bolster an estimate of suicidality and help in managing suicidal risk in ADHD, according to Dr. Levy, a child and adolescent psychiatrist at the Hospital for Sick Children, Toronto, and head of behavioral regulation services at the Geha Mental Health Center in Petah Tikva, Israel.
The study included separate parent- and teacher-structured reports of the youths’ ADHD symptoms, suicidality, depression, irritability, and anxiety.
In multivariate analyses, parent-reported depression accounted for 39.1% of the association between ADHD symptoms and suicidality, while irritability symptoms mediated 36.8% of the total effect. In the teachers’ reports, depression and irritability symptoms accounted for 45.3% and 38.4% of the association. Anxiety symptoms mediated 19% of the relationship between ADHD and suicidality by parental report but had no significant impact on the association according to teacher report in the recently published study.
Dr. Levy noted that, in the DSM-5, irritability cuts across diagnostic categories. It is not only a core dimension of ADHD, but of the other externalizing disorders – conduct disorder and oppositional defiant disorder – as well, and also of neurodevelopmental, internalizing, and stress-related disorders.
Interventional studies aimed at dampening irritability as a potential strategy to reduce suicidality haven’t yet been done, but they deserve research priority status, in Dr. Levy’s view. Numerous functional dimensions that influence irritability are potential targets, including aggression, negative affect, low tolerance of frustration, skewed threat perception, and impaired self-regulation, according to the psychiatrist.
Most suicidal youths are attempting to cope with mental disorders. The most prevalent of these are major depressive disorder and dysthymia, followed by externalizing disorders. And among the externalizing disorders, conduct disorder stands out in terms of the magnitude of associated suicidality risk. In a large Taiwanese national study including 3,711 adolescents with conduct disorder and 14,844 age- and sex-matched controls, conduct disorder was associated with an adjusted 5.17-fold increased risk of subsequent suicide attempts over the next 10 years in a multivariate regression analysis adjusted for other psychiatric comorbidities and demographics.
In addition to depression, irritability symptoms, and conduct problems, other risk factors that should be part of a suicidality assessment in children and adolescents with ADHD include substance use, anxiety, poor family support, and bullying and/or being bullied. But, perhaps surprisingly, not impulsivity, Dr. Levy said.
“There is a widely held perception that impulsivity imparts a risk for suicidality, and especially in the transition from ideation to attempt. However, more recent evidence fails to show a convincing association,” according to Dr. Levy.
He reported having no financial conflicts regarding his presentation.
SOURCE: Levy T. ECNP 2020, Session EDU.02.
FROM ECNP 2020
Key clinical point: Assessment of irritability symptoms and depression may be helpful in managing suicidality risk in ADHD.
Major finding: Parent- and teacher-reported depression and irritability symptoms mediated up to 84% of the association between pediatric ADHD and suicidality.
Study details: This cross-sectional study examined the role of irritability, depression, and anxiety in suicidality among 1,516 children and adolescents at an outpatient ADHD clinic.
Disclosures: The presenter reported having no financial conflicts regarding his study.
Source: Levy T. ECNP 2020, Session EDU.02.
Strategies offered for optimizing ECT anesthesia
General anesthesia for ECT gets short shrift in the psychiatric literature, yet it’s an indispensable part of the procedure, with a major impact on its safety and outcomes, Alexander Sartorius, MD, asserted at the virtual congress of the European College of Neuropsychopharmacology.
Just how neglected is the topic?
“The two bibles of ECT – the American Psychiatric Association’s ‘The Practice of Electroconvulsive Therapy’ and Richard Abrams’s ‘Electroconvulsive Therapy,’ contain only three pages on anesthesia out of several hundred pages,” noted Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
Dr. Sartorius, who has published extensively on the management of general anesthesia in ECT, offered fresh insights into its optimization. He also shared how to swiftly identify and deal with its main side effects.
General anesthesia is an essential part of ECT for only one reason: Not to spare the patient from pain or trauma, as is widely supposed, but simply to avoid awareness of the muscle relaxant that’s given to prevent bone fractures and other injuries caused by motor seizure, the psychiatrist explained.
Four anesthetic agents traditionally used for ECT have fallen by the wayside. The two barbiturates, thiopental and methohexital, have problematic anticonvulsant properties that complicate their use in a procedure whose whole purpose is to induce a seizure. Plus, they have black-box warnings in some countries. Etomidate, in contrast, has no anticonvulsant effect; however, anesthesiologists are increasingly leery of the drug. A single dose completely suppresses the hypothalamic-pituitary-adrenal axis for more than 24 hours, and mounting evidence suggests that etomidate may be associated with increased mortality.
Dr. Sartorius is a fan of ketofol, a combination of two anesthetic agents – ketamine and propofol – that provide rapid onset and cessation of action, pharmacokinetic predictability, synergistic efficacy, and minimal adverse effects when the two drugs are given in doses lower than standard as monotherapy.
Propofol has attractive qualities as an anesthetic, but it is a very potent anticonvulsant with an adverse effect on seizure quality and duration. When used alone for general anesthesia in ECT, a higher stimulation dose is often necessary to achieve adequate seizure quality, which in turn may produce worse cognitive side effects. In contrast, ketamine, which is listed as an essential drug by the World Health Organization, has no anticonvulsive effects.
“My conclusion about ketamine alone is it has less side effects than feared, and it’s probably not more but definitely not less effective than the grand old four anesthetic agents,” Dr. Sartorius said.
Plus, ketamine shows promise as an antidepressant agent in and of itself. Moreover, the fact that patients require a lower ECT stimulation dose while under the influence of ketamine could result in fewer cognitive side effects, although that’s conjecture at this point, he added.
Ketofol is often administered in a 1:1 ratio of propofol to ketamine. That’s not optimum for each individual patient undergoing ECT, as in many cases it results in so much propofol that seizure quality is diminished, in Dr. Sartorius’s experience. He, therefore, recently published a retrospective study of 52 patients who received 919 ECT sessions with empirically determined doses of S-ketamine plus propofol for anesthesia. The endpoints were time in the recovery room and seizure duration and quality. Seizure quality was assessed as a composite of the ratio of duration of motor response to EEG seizure duration, peak heart rate, midictal amplitude, maximal interhemispheric coherence, and postictal suppression index.
The optimal S-ketamine/propofol ratio in terms of seizure quality was 1.52:1, with a mean relative dose of 0.72 mg/kg of S-ketamine and 0.54 mg/kg of propofol.
His team uses only the S-enantiomer of ketamine, not the racemic mixture known as ketamine, but his study results would translate to a 3:1 ratio of racemic ketamine to propofol, Dr. Sartorius said.
Time in the recovery room was dependent upon return of cardiorespiratory function and orientation status to baseline pre-ECT levels. Longer recovery room time proved to be significantly related to older age. The S-ketamine dose wasn’t a significant factor.
Propofol was injected prior to S-ketamine in all patients. This was followed 1-2 minutes later by administration of succinylcholine as a muscle relaxant. It’s important to then wait for at least another 2-3 minutes before delivering the ECT stimulation. Dr. Sartorius and others have demonstrated that waiting at least 4 minutes between anesthesia induction and delivery of the ECT charge results in a better-quality seizure.
“We have a timer running so we can be sure to wait longer than 4 minutes. That’s a large advantage if you want to reduce the anticonvulsant property of propofol,” he explained.
Anesthesia-related side effects
Dr. Sartorius addressed postictal agitation syndrome, postanesthetic shivering, cardiac arrhythmias, and hypersalivation.
Postictal agitation syndrome: The deeper the level of sedation, the less likely this complication. Historically, in ECT without anesthesia, the incidence of postictal agitation was as high as 50%. At the center where Dr. Sartorius works, it’s 2%-3%. The use of intraprocedural bispectral index monitoring of the achieved deepest level of sedation allows highly accurate prediction of postictal agitation.
“Do not restrain,” he advised. “Patients are aware of this problematic situation. You have to keep everything calm and use the least possible amount of physical limitation. The good thing is that it’s self-limited within 20 minutes in most cases. But in severe cases you have to escalate staff immediately, and you may want to use 10 mg of IV diazepam. ; a lower dose of anesthetic is not the solution.”
It is also important to watch for these possible complications:
- Postanesthetic shivering: This is a rare but potentially fatal complication. It’s important to be familiar with the grading system, and to recognize that grade 3 or 4 post-anesthetic shivering requires treatment. “The treatment of choice is clonidine. That should always be with you when you do ECT,” Dr. Sartorius observed.
- Cardiac arrhythmias: “ECT is a proarrhythmic intervention; don’t forget that,” he said.
- Poststimulation asystole: This occurs in more than half of treated patients. It’s caused by the current, not the seizure, and it stops within a few seconds after the current halts. If the asystoles bother the patient, try switching to bifrontal electrode placement. Right unilateral stimulation has been shown to increase the likelihood of asystole by 207-fold, compared with bifrontal stimulation.
- Tachycardia: This is another common complication of ECT. It responds well to a short-acting beta-blocker.
- Hypersalivation: The treatment of choice is glycopyrrolate, a muscarinic receptor antagonist that doesn’t cross the blood-brain barrier.
Dr. Sartorius reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A et al. ECNP 2020, Session EDU03.02.
General anesthesia for ECT gets short shrift in the psychiatric literature, yet it’s an indispensable part of the procedure, with a major impact on its safety and outcomes, Alexander Sartorius, MD, asserted at the virtual congress of the European College of Neuropsychopharmacology.
Just how neglected is the topic?
“The two bibles of ECT – the American Psychiatric Association’s ‘The Practice of Electroconvulsive Therapy’ and Richard Abrams’s ‘Electroconvulsive Therapy,’ contain only three pages on anesthesia out of several hundred pages,” noted Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
Dr. Sartorius, who has published extensively on the management of general anesthesia in ECT, offered fresh insights into its optimization. He also shared how to swiftly identify and deal with its main side effects.
General anesthesia is an essential part of ECT for only one reason: Not to spare the patient from pain or trauma, as is widely supposed, but simply to avoid awareness of the muscle relaxant that’s given to prevent bone fractures and other injuries caused by motor seizure, the psychiatrist explained.
Four anesthetic agents traditionally used for ECT have fallen by the wayside. The two barbiturates, thiopental and methohexital, have problematic anticonvulsant properties that complicate their use in a procedure whose whole purpose is to induce a seizure. Plus, they have black-box warnings in some countries. Etomidate, in contrast, has no anticonvulsant effect; however, anesthesiologists are increasingly leery of the drug. A single dose completely suppresses the hypothalamic-pituitary-adrenal axis for more than 24 hours, and mounting evidence suggests that etomidate may be associated with increased mortality.
Dr. Sartorius is a fan of ketofol, a combination of two anesthetic agents – ketamine and propofol – that provide rapid onset and cessation of action, pharmacokinetic predictability, synergistic efficacy, and minimal adverse effects when the two drugs are given in doses lower than standard as monotherapy.
Propofol has attractive qualities as an anesthetic, but it is a very potent anticonvulsant with an adverse effect on seizure quality and duration. When used alone for general anesthesia in ECT, a higher stimulation dose is often necessary to achieve adequate seizure quality, which in turn may produce worse cognitive side effects. In contrast, ketamine, which is listed as an essential drug by the World Health Organization, has no anticonvulsive effects.
“My conclusion about ketamine alone is it has less side effects than feared, and it’s probably not more but definitely not less effective than the grand old four anesthetic agents,” Dr. Sartorius said.
Plus, ketamine shows promise as an antidepressant agent in and of itself. Moreover, the fact that patients require a lower ECT stimulation dose while under the influence of ketamine could result in fewer cognitive side effects, although that’s conjecture at this point, he added.
Ketofol is often administered in a 1:1 ratio of propofol to ketamine. That’s not optimum for each individual patient undergoing ECT, as in many cases it results in so much propofol that seizure quality is diminished, in Dr. Sartorius’s experience. He, therefore, recently published a retrospective study of 52 patients who received 919 ECT sessions with empirically determined doses of S-ketamine plus propofol for anesthesia. The endpoints were time in the recovery room and seizure duration and quality. Seizure quality was assessed as a composite of the ratio of duration of motor response to EEG seizure duration, peak heart rate, midictal amplitude, maximal interhemispheric coherence, and postictal suppression index.
The optimal S-ketamine/propofol ratio in terms of seizure quality was 1.52:1, with a mean relative dose of 0.72 mg/kg of S-ketamine and 0.54 mg/kg of propofol.
His team uses only the S-enantiomer of ketamine, not the racemic mixture known as ketamine, but his study results would translate to a 3:1 ratio of racemic ketamine to propofol, Dr. Sartorius said.
Time in the recovery room was dependent upon return of cardiorespiratory function and orientation status to baseline pre-ECT levels. Longer recovery room time proved to be significantly related to older age. The S-ketamine dose wasn’t a significant factor.
Propofol was injected prior to S-ketamine in all patients. This was followed 1-2 minutes later by administration of succinylcholine as a muscle relaxant. It’s important to then wait for at least another 2-3 minutes before delivering the ECT stimulation. Dr. Sartorius and others have demonstrated that waiting at least 4 minutes between anesthesia induction and delivery of the ECT charge results in a better-quality seizure.
“We have a timer running so we can be sure to wait longer than 4 minutes. That’s a large advantage if you want to reduce the anticonvulsant property of propofol,” he explained.
Anesthesia-related side effects
Dr. Sartorius addressed postictal agitation syndrome, postanesthetic shivering, cardiac arrhythmias, and hypersalivation.
Postictal agitation syndrome: The deeper the level of sedation, the less likely this complication. Historically, in ECT without anesthesia, the incidence of postictal agitation was as high as 50%. At the center where Dr. Sartorius works, it’s 2%-3%. The use of intraprocedural bispectral index monitoring of the achieved deepest level of sedation allows highly accurate prediction of postictal agitation.
“Do not restrain,” he advised. “Patients are aware of this problematic situation. You have to keep everything calm and use the least possible amount of physical limitation. The good thing is that it’s self-limited within 20 minutes in most cases. But in severe cases you have to escalate staff immediately, and you may want to use 10 mg of IV diazepam. ; a lower dose of anesthetic is not the solution.”
It is also important to watch for these possible complications:
- Postanesthetic shivering: This is a rare but potentially fatal complication. It’s important to be familiar with the grading system, and to recognize that grade 3 or 4 post-anesthetic shivering requires treatment. “The treatment of choice is clonidine. That should always be with you when you do ECT,” Dr. Sartorius observed.
- Cardiac arrhythmias: “ECT is a proarrhythmic intervention; don’t forget that,” he said.
- Poststimulation asystole: This occurs in more than half of treated patients. It’s caused by the current, not the seizure, and it stops within a few seconds after the current halts. If the asystoles bother the patient, try switching to bifrontal electrode placement. Right unilateral stimulation has been shown to increase the likelihood of asystole by 207-fold, compared with bifrontal stimulation.
- Tachycardia: This is another common complication of ECT. It responds well to a short-acting beta-blocker.
- Hypersalivation: The treatment of choice is glycopyrrolate, a muscarinic receptor antagonist that doesn’t cross the blood-brain barrier.
Dr. Sartorius reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A et al. ECNP 2020, Session EDU03.02.
General anesthesia for ECT gets short shrift in the psychiatric literature, yet it’s an indispensable part of the procedure, with a major impact on its safety and outcomes, Alexander Sartorius, MD, asserted at the virtual congress of the European College of Neuropsychopharmacology.
Just how neglected is the topic?
“The two bibles of ECT – the American Psychiatric Association’s ‘The Practice of Electroconvulsive Therapy’ and Richard Abrams’s ‘Electroconvulsive Therapy,’ contain only three pages on anesthesia out of several hundred pages,” noted Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
Dr. Sartorius, who has published extensively on the management of general anesthesia in ECT, offered fresh insights into its optimization. He also shared how to swiftly identify and deal with its main side effects.
General anesthesia is an essential part of ECT for only one reason: Not to spare the patient from pain or trauma, as is widely supposed, but simply to avoid awareness of the muscle relaxant that’s given to prevent bone fractures and other injuries caused by motor seizure, the psychiatrist explained.
Four anesthetic agents traditionally used for ECT have fallen by the wayside. The two barbiturates, thiopental and methohexital, have problematic anticonvulsant properties that complicate their use in a procedure whose whole purpose is to induce a seizure. Plus, they have black-box warnings in some countries. Etomidate, in contrast, has no anticonvulsant effect; however, anesthesiologists are increasingly leery of the drug. A single dose completely suppresses the hypothalamic-pituitary-adrenal axis for more than 24 hours, and mounting evidence suggests that etomidate may be associated with increased mortality.
Dr. Sartorius is a fan of ketofol, a combination of two anesthetic agents – ketamine and propofol – that provide rapid onset and cessation of action, pharmacokinetic predictability, synergistic efficacy, and minimal adverse effects when the two drugs are given in doses lower than standard as monotherapy.
Propofol has attractive qualities as an anesthetic, but it is a very potent anticonvulsant with an adverse effect on seizure quality and duration. When used alone for general anesthesia in ECT, a higher stimulation dose is often necessary to achieve adequate seizure quality, which in turn may produce worse cognitive side effects. In contrast, ketamine, which is listed as an essential drug by the World Health Organization, has no anticonvulsive effects.
“My conclusion about ketamine alone is it has less side effects than feared, and it’s probably not more but definitely not less effective than the grand old four anesthetic agents,” Dr. Sartorius said.
Plus, ketamine shows promise as an antidepressant agent in and of itself. Moreover, the fact that patients require a lower ECT stimulation dose while under the influence of ketamine could result in fewer cognitive side effects, although that’s conjecture at this point, he added.
Ketofol is often administered in a 1:1 ratio of propofol to ketamine. That’s not optimum for each individual patient undergoing ECT, as in many cases it results in so much propofol that seizure quality is diminished, in Dr. Sartorius’s experience. He, therefore, recently published a retrospective study of 52 patients who received 919 ECT sessions with empirically determined doses of S-ketamine plus propofol for anesthesia. The endpoints were time in the recovery room and seizure duration and quality. Seizure quality was assessed as a composite of the ratio of duration of motor response to EEG seizure duration, peak heart rate, midictal amplitude, maximal interhemispheric coherence, and postictal suppression index.
The optimal S-ketamine/propofol ratio in terms of seizure quality was 1.52:1, with a mean relative dose of 0.72 mg/kg of S-ketamine and 0.54 mg/kg of propofol.
His team uses only the S-enantiomer of ketamine, not the racemic mixture known as ketamine, but his study results would translate to a 3:1 ratio of racemic ketamine to propofol, Dr. Sartorius said.
Time in the recovery room was dependent upon return of cardiorespiratory function and orientation status to baseline pre-ECT levels. Longer recovery room time proved to be significantly related to older age. The S-ketamine dose wasn’t a significant factor.
Propofol was injected prior to S-ketamine in all patients. This was followed 1-2 minutes later by administration of succinylcholine as a muscle relaxant. It’s important to then wait for at least another 2-3 minutes before delivering the ECT stimulation. Dr. Sartorius and others have demonstrated that waiting at least 4 minutes between anesthesia induction and delivery of the ECT charge results in a better-quality seizure.
“We have a timer running so we can be sure to wait longer than 4 minutes. That’s a large advantage if you want to reduce the anticonvulsant property of propofol,” he explained.
Anesthesia-related side effects
Dr. Sartorius addressed postictal agitation syndrome, postanesthetic shivering, cardiac arrhythmias, and hypersalivation.
Postictal agitation syndrome: The deeper the level of sedation, the less likely this complication. Historically, in ECT without anesthesia, the incidence of postictal agitation was as high as 50%. At the center where Dr. Sartorius works, it’s 2%-3%. The use of intraprocedural bispectral index monitoring of the achieved deepest level of sedation allows highly accurate prediction of postictal agitation.
“Do not restrain,” he advised. “Patients are aware of this problematic situation. You have to keep everything calm and use the least possible amount of physical limitation. The good thing is that it’s self-limited within 20 minutes in most cases. But in severe cases you have to escalate staff immediately, and you may want to use 10 mg of IV diazepam. ; a lower dose of anesthetic is not the solution.”
It is also important to watch for these possible complications:
- Postanesthetic shivering: This is a rare but potentially fatal complication. It’s important to be familiar with the grading system, and to recognize that grade 3 or 4 post-anesthetic shivering requires treatment. “The treatment of choice is clonidine. That should always be with you when you do ECT,” Dr. Sartorius observed.
- Cardiac arrhythmias: “ECT is a proarrhythmic intervention; don’t forget that,” he said.
- Poststimulation asystole: This occurs in more than half of treated patients. It’s caused by the current, not the seizure, and it stops within a few seconds after the current halts. If the asystoles bother the patient, try switching to bifrontal electrode placement. Right unilateral stimulation has been shown to increase the likelihood of asystole by 207-fold, compared with bifrontal stimulation.
- Tachycardia: This is another common complication of ECT. It responds well to a short-acting beta-blocker.
- Hypersalivation: The treatment of choice is glycopyrrolate, a muscarinic receptor antagonist that doesn’t cross the blood-brain barrier.
Dr. Sartorius reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A et al. ECNP 2020, Session EDU03.02.
FROM ECNP 2020
Adjunctive pimavanserin looks promising for anxious depression
Adjunctive pimavanserin brought clinically meaningful improvement in patients with anxious major depressive disorder inadequately responsive to standard antidepressants alone in a post hoc analysis of the CLARITY trial, Bryan Dirks, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
This is an intriguing observation, because it’s estimated that roughly 50% of individuals with major depressive disorder (MDD) have comorbid anxiety disorders or a high level of anxiety symptoms. Moreover, anxious depression has been associated with increased risk of suicidality, high unemployment, and impaired functioning.
CLARITY was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial whose positive results for the primary outcome have been published (J Clin Psychiatry. 2019 Sep 24;80[6]:19m12928. doi: 10.4088/JCP.19m12928). Because the encouraging findings regarding pimavanserin’s impact on anxious depression came from a post hoc analysis, the results need replication. That’s ongoing in a phase 3 trial of adjunctive pimavanserin versus placebo in patients with MDD, according to Dr. Dirks, director of clinical research at Acadia Pharmaceuticals, San Diego.
The CLARITY post hoc analysis included 104 patients with baseline MDD inadequately responsive to an SSRI or a serotonin norepinephrine reuptake inhibitor and anxious depression as defined by a Hamilton Depression Rating Scale (HAMD-17) anxiety/somatization factor subscale score of 7 or more. Twenty-nine of the patients were randomized to 34 mg of adjunctive oral pimavanserin once daily, and 75 to placebo. At 5 weeks, the HAMD-17 anxiety/somatization factor score in the pimavanserin group had dropped by a mean of 5 points from a baseline of 8.8, a significantly greater effect than the 2.8-point drop in placebo-treated controls.
By week 5, the treatment response rate as defined by at least a 50% reduction in HAMD-17 total score from baseline was 55% with pimavanserin and 22% with placebo. The remission rate as indicated by a HAMD-17 total score below 7 was 24% in the pimavanserin group, compared with 5% with placebo. These results translated into an effect size of 0.78, considered by statisticians to be on the border between medium and large. Those response and remission rates in patients with anxious depression were higher with pimavanserin and lower with placebo than in the overall CLARITY trial.
as defined by a HAMD-17 total score of 24 or more plus an anxiety/somatization factor score of 7 or greater. Seventeen such patients were randomized to adjunctive pimavanserin, 36 to placebo. At 5 weeks, the mean HAMD total score had dropped by 17.4 points from a baseline of 27.6 in the pimavanserin group, compared with a 9.3-point reduction in controls.
“Of note, significant differences from placebo were observed as early as week 2 with pimavanserin,” Dr. Dirks said.
Pimavanserin is a novel selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors. At present pimavanserin is Food Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, but because of the drug’s unique mechanism of action it is under study for a variety of other mental disorders. Indeed, pimavanserin is now under FDA review for a possible expanded indication for treatment of dementia-related psychosis. The drug is also under study for schizophrenia as well as for MDD.
The CLARITY trial and this post hoc analysis were sponsored by Acadia Pharmaceuticals.
SOURCE: Dirks B. ECNP 2020. Abstract P 094.
Adjunctive pimavanserin brought clinically meaningful improvement in patients with anxious major depressive disorder inadequately responsive to standard antidepressants alone in a post hoc analysis of the CLARITY trial, Bryan Dirks, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
This is an intriguing observation, because it’s estimated that roughly 50% of individuals with major depressive disorder (MDD) have comorbid anxiety disorders or a high level of anxiety symptoms. Moreover, anxious depression has been associated with increased risk of suicidality, high unemployment, and impaired functioning.
CLARITY was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial whose positive results for the primary outcome have been published (J Clin Psychiatry. 2019 Sep 24;80[6]:19m12928. doi: 10.4088/JCP.19m12928). Because the encouraging findings regarding pimavanserin’s impact on anxious depression came from a post hoc analysis, the results need replication. That’s ongoing in a phase 3 trial of adjunctive pimavanserin versus placebo in patients with MDD, according to Dr. Dirks, director of clinical research at Acadia Pharmaceuticals, San Diego.
The CLARITY post hoc analysis included 104 patients with baseline MDD inadequately responsive to an SSRI or a serotonin norepinephrine reuptake inhibitor and anxious depression as defined by a Hamilton Depression Rating Scale (HAMD-17) anxiety/somatization factor subscale score of 7 or more. Twenty-nine of the patients were randomized to 34 mg of adjunctive oral pimavanserin once daily, and 75 to placebo. At 5 weeks, the HAMD-17 anxiety/somatization factor score in the pimavanserin group had dropped by a mean of 5 points from a baseline of 8.8, a significantly greater effect than the 2.8-point drop in placebo-treated controls.
By week 5, the treatment response rate as defined by at least a 50% reduction in HAMD-17 total score from baseline was 55% with pimavanserin and 22% with placebo. The remission rate as indicated by a HAMD-17 total score below 7 was 24% in the pimavanserin group, compared with 5% with placebo. These results translated into an effect size of 0.78, considered by statisticians to be on the border between medium and large. Those response and remission rates in patients with anxious depression were higher with pimavanserin and lower with placebo than in the overall CLARITY trial.
as defined by a HAMD-17 total score of 24 or more plus an anxiety/somatization factor score of 7 or greater. Seventeen such patients were randomized to adjunctive pimavanserin, 36 to placebo. At 5 weeks, the mean HAMD total score had dropped by 17.4 points from a baseline of 27.6 in the pimavanserin group, compared with a 9.3-point reduction in controls.
“Of note, significant differences from placebo were observed as early as week 2 with pimavanserin,” Dr. Dirks said.
Pimavanserin is a novel selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors. At present pimavanserin is Food Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, but because of the drug’s unique mechanism of action it is under study for a variety of other mental disorders. Indeed, pimavanserin is now under FDA review for a possible expanded indication for treatment of dementia-related psychosis. The drug is also under study for schizophrenia as well as for MDD.
The CLARITY trial and this post hoc analysis were sponsored by Acadia Pharmaceuticals.
SOURCE: Dirks B. ECNP 2020. Abstract P 094.
Adjunctive pimavanserin brought clinically meaningful improvement in patients with anxious major depressive disorder inadequately responsive to standard antidepressants alone in a post hoc analysis of the CLARITY trial, Bryan Dirks, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
This is an intriguing observation, because it’s estimated that roughly 50% of individuals with major depressive disorder (MDD) have comorbid anxiety disorders or a high level of anxiety symptoms. Moreover, anxious depression has been associated with increased risk of suicidality, high unemployment, and impaired functioning.
CLARITY was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial whose positive results for the primary outcome have been published (J Clin Psychiatry. 2019 Sep 24;80[6]:19m12928. doi: 10.4088/JCP.19m12928). Because the encouraging findings regarding pimavanserin’s impact on anxious depression came from a post hoc analysis, the results need replication. That’s ongoing in a phase 3 trial of adjunctive pimavanserin versus placebo in patients with MDD, according to Dr. Dirks, director of clinical research at Acadia Pharmaceuticals, San Diego.
The CLARITY post hoc analysis included 104 patients with baseline MDD inadequately responsive to an SSRI or a serotonin norepinephrine reuptake inhibitor and anxious depression as defined by a Hamilton Depression Rating Scale (HAMD-17) anxiety/somatization factor subscale score of 7 or more. Twenty-nine of the patients were randomized to 34 mg of adjunctive oral pimavanserin once daily, and 75 to placebo. At 5 weeks, the HAMD-17 anxiety/somatization factor score in the pimavanserin group had dropped by a mean of 5 points from a baseline of 8.8, a significantly greater effect than the 2.8-point drop in placebo-treated controls.
By week 5, the treatment response rate as defined by at least a 50% reduction in HAMD-17 total score from baseline was 55% with pimavanserin and 22% with placebo. The remission rate as indicated by a HAMD-17 total score below 7 was 24% in the pimavanserin group, compared with 5% with placebo. These results translated into an effect size of 0.78, considered by statisticians to be on the border between medium and large. Those response and remission rates in patients with anxious depression were higher with pimavanserin and lower with placebo than in the overall CLARITY trial.
as defined by a HAMD-17 total score of 24 or more plus an anxiety/somatization factor score of 7 or greater. Seventeen such patients were randomized to adjunctive pimavanserin, 36 to placebo. At 5 weeks, the mean HAMD total score had dropped by 17.4 points from a baseline of 27.6 in the pimavanserin group, compared with a 9.3-point reduction in controls.
“Of note, significant differences from placebo were observed as early as week 2 with pimavanserin,” Dr. Dirks said.
Pimavanserin is a novel selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors. At present pimavanserin is Food Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, but because of the drug’s unique mechanism of action it is under study for a variety of other mental disorders. Indeed, pimavanserin is now under FDA review for a possible expanded indication for treatment of dementia-related psychosis. The drug is also under study for schizophrenia as well as for MDD.
The CLARITY trial and this post hoc analysis were sponsored by Acadia Pharmaceuticals.
SOURCE: Dirks B. ECNP 2020. Abstract P 094.
FROM ECNP 2020
Key clinical point: Pimavanserin may have a future as a novel treatment for anxious depression.
Major finding: Twenty-four percent of patients with anxious major depressive disorder inadequately responsive to standard antidepressant therapy achieved remission with 5 weeks of adjunctive pimavanserin, compared with 5% with placebo.
Study details: This was a post hoc analysis of the phase 2, multicenter, randomized, double-blind CLARITY trial.
Disclosures: The study was sponsored by Acadia Pharmaceuticals and presented by a company employee.
Source: Dirks B. ECNP 2020. Abstract P 094.
Repurposing cardiovascular drugs for serious mental illness
One of the hottest topics now in psychiatry is the possibility of repurposing long-established cardiovascular medications for treatment of patients with serious mental illness, Livia De Picker, MD, PhD, said at the virtual congress of the European College of Neuropsychopharmacology.

The appeal is multifold. A huge unmet need exists in psychiatry for new and better treatments with novel mechanisms of action. Many guideline-recommended cardiovascular medications have a long track record, including a well-established safety profile with no surprises, and are available in generic versions. They can be developed for a new indication at minimal cost, noted Dr. De Picker, a psychiatrist at the University of Antwerp (Belgium).
The idea of psychiatric repurposing of drugs originally developed for nonpsychiatric indications is nothing new, she added. Examples include lithium for gout, valproate for epilepsy, and ketamine for anesthesiology.
One hitch in efforts to repurpose cardiovascular medications is that, when psychiatric patients have been included in randomized trials of the drugs’ cardiovascular effects, the psychiatric outcomes often went untallied.
Indeed, the only high-quality randomized trial evidence of psychiatric benefits for any class of cardiovascular medications is for statins, where a modest-sized meta-analysis of six placebo-controlled trials in 339 patients with schizophrenia showed the lipid-lowering agents had benefit for both positive and negative symptoms (Psychiatry Res. 2018 Apr;262:84-93). But that’s not a body of data of sufficient size to be definitive, in Dr. De Picker’s view.
Much of the recent enthusiasm for exploring the potential of cardiovascular drugs for psychiatric conditions comes from hypothesis-generating big data analyses drawn from Scandinavian national patient registries. Danish investigators scrutinized all 1.6 million Danes exposed to six classes of drugs of interest during 2005-2015 and determined that those on long-term statins, low-dose aspirin, ACE inhibitors, angiotensin receptor blockers, or allopurinol were associated with a decreased rate of new-onset depression, while high-dose aspirin and non-aspirin NSAIDs were associated with an increased rate, compared with a 30% random sample of the country’s population (Acta Psychiatr Scand. 2019 Jan;1391:68-77).
Similarly, the Danish group found that continued use of statins, angiotensin agents, or low-dose aspirin was associated with a decreased rate of new-onset bipolar disorder, while high-dose aspirin and other NSAIDs were linked to increased risk (Bipolar Disord. 2019 Aug;[15]:410-8). What these agents have in common, the investigators observed, is that they act on inflammation and potentially on the stress response system.
Meanwhile, Swedish investigators examined the course of 142,691 Swedes with a diagnosis of bipolar disorder, schizophrenia, or nonaffective psychosis during 2005-2016. They determined that, during periods when those individuals were on a statin, calcium channel blocker, or metformin, they had reduced rates of psychiatric hospitalization and self-harm (JAMA Psychiatry. 2019 Apr 1;76[4]:382-90).
Scottish researchers analyzed the health records of 144,066 patients placed on monotherapy for hypertension and determined that the lowest risk for hospitalization for a mood disorder during follow-up was in those prescribed an ACE inhibitor or angiotensin receptor blocker. The risk was significantly higher in patients on a beta-blocker or calcium channel blocker, and intermediate in those on a thiazide diuretic (Hypertension. 2016 Nov;68[5:1132-8).
“Obviously, this is all at a very macro scale and we have no idea whatsoever what this means for individual patients, number needed to treat, or which type of patients would benefit, but it does provide us with some guidance for future research,” according to Dr. De Picker.
In the meantime, while physicians await definitive evidence of any impact of cardiovascular drugs might have on psychiatric outcomes, abundant data exist underscoring what she called “shockingly high levels” of inadequate management of cardiovascular risk factors in patients with serious mental illness. That problem needs to be addressed, and Dr. De Picker offered her personal recommendations for doing so in a manner consistent with the evidence to date suggestive of potential mental health benefits of some cardiovascular medications.
She advised that, for treatment of hypertension in patients with bipolar disorder or major depression, an ACE inhibitor or angiotensin-converting enzyme inhibitor is preferred as first-line. There is some evidence to suggest lipophilic beta-blockers, which cross the blood-brain barrier, improve anxiety symptoms and panic attacks, and prevent memory consolidation in patients with posttraumatic stress disorder. But the Scottish data suggest that they may worsen mood disorders.
“I would be careful in using beta-blockers as first-line treatment for hypertension. They’re not in the guidelines for anxiety disorders. British guidelines recommend them to prevent memory consolidation in PTSD, but do not use them as first-line in patients with major depressive disorder or bipolar disorder,” she said. As for calcium channel blockers, the jury is still out, with mixed and inconsistent evidence to date as to the impact of this drug class on mental illness outcomes.
She recommended a very low threshold for prescribing statin therapy in patients with serious mental illness in light of the superb risk/benefit ratio for this drug class. In her younger patients, she turns for guidance to an online calculator of an individual’s 10-year risk of a first acute MI or stroke.
Metformin has been shown to be beneficial for addressing the weight gain and other adverse metabolic effects caused by antipsychotic agents, and there is some preliminary evidence of improved psychiatric outcomes in patients with serious mental illness.
Christian Otte, MD, who also spoke at the session, noted that not only do emerging data point to the possibility that cardiovascular drugs might have benefit in terms of psychiatric outcomes, there is also some evidence, albeit mixed, that the converse is true: that is, psychiatric drugs may have cardiovascular benefits. He pointed to a South Korean trial in which 300 patients with a recent acute coronary syndrome and major depression were randomized to 24 weeks of escitalopram or placebo. At median 8.1 years of follow-up, the group that received the SSRI had a 31% relative risk reduction in the primary composite endpoint of all-cause mortality, acute MI, or percutaneous coronary intervention (JAMA. 2018 Jul 24; 320[4]:350–7).
“Potentially independent of their antidepressant effects, some SSRIs’ antiplatelet effects could be beneficial for patients with coronary heart disease, although the jury is still open regarding this question, with evidence in both directions,” said Dr. Otte, professor of psychiatry at Charite University Medical Center in Berlin.
Dr. De Picker offered an example as well: Finnish psychiatrists recently reported that cardiovascular mortality was reduced by an adjusted 38% during periods when 62,250 Finnish schizophrenia patients were on antipsychotic agents, compared with periods of nonuse of the drugs in a national study with a median 14.1 years of follow-up (World Psychiatry. 2020 Feb;19[1]:61-8).
“What they discovered – and this is quite contrary to what we are used to hearing about antipsychotic medication and cardiovascular risk – is that while the number of cardiovascular hospitalizations was not different in periods with or without antipsychotic use, the cardiovascular mortality was quite strikingly reduced when patients were on antipsychotic medication,” she said.
Asked by an audience member whether she personally prescribes metformin, Dr. De Picker replied: “Well, yes, why not? One of the very nice things about metformin is that it is actually a very safe drug, even in the hands of nonspecialists.
“I understand that maybe psychiatrists may not feel very comfortable in starting patients on metformin due to a lack of experience. But there are really only two things you need to take into account. About one-quarter of patients will experience GI side effects – nausea, vomiting, abdominal discomfort – and this can be reduced by gradually uptitrating the dose, dosing at mealtime, and using an extended-release formulation. And the second thing is that metformin can impair vitamin B12 absorption, so I think, especially in psychiatric patients, it would be good to do an annual measurement of vitamin B12 level and, if necessary, administer intramuscular supplements,” Dr. De Picker said.
She reported having no financial conflicts regarding her presentation.
SOURCE: De Picker L. ECNP 2020. Session EDU.05.
One of the hottest topics now in psychiatry is the possibility of repurposing long-established cardiovascular medications for treatment of patients with serious mental illness, Livia De Picker, MD, PhD, said at the virtual congress of the European College of Neuropsychopharmacology.

The appeal is multifold. A huge unmet need exists in psychiatry for new and better treatments with novel mechanisms of action. Many guideline-recommended cardiovascular medications have a long track record, including a well-established safety profile with no surprises, and are available in generic versions. They can be developed for a new indication at minimal cost, noted Dr. De Picker, a psychiatrist at the University of Antwerp (Belgium).
The idea of psychiatric repurposing of drugs originally developed for nonpsychiatric indications is nothing new, she added. Examples include lithium for gout, valproate for epilepsy, and ketamine for anesthesiology.
One hitch in efforts to repurpose cardiovascular medications is that, when psychiatric patients have been included in randomized trials of the drugs’ cardiovascular effects, the psychiatric outcomes often went untallied.
Indeed, the only high-quality randomized trial evidence of psychiatric benefits for any class of cardiovascular medications is for statins, where a modest-sized meta-analysis of six placebo-controlled trials in 339 patients with schizophrenia showed the lipid-lowering agents had benefit for both positive and negative symptoms (Psychiatry Res. 2018 Apr;262:84-93). But that’s not a body of data of sufficient size to be definitive, in Dr. De Picker’s view.
Much of the recent enthusiasm for exploring the potential of cardiovascular drugs for psychiatric conditions comes from hypothesis-generating big data analyses drawn from Scandinavian national patient registries. Danish investigators scrutinized all 1.6 million Danes exposed to six classes of drugs of interest during 2005-2015 and determined that those on long-term statins, low-dose aspirin, ACE inhibitors, angiotensin receptor blockers, or allopurinol were associated with a decreased rate of new-onset depression, while high-dose aspirin and non-aspirin NSAIDs were associated with an increased rate, compared with a 30% random sample of the country’s population (Acta Psychiatr Scand. 2019 Jan;1391:68-77).
Similarly, the Danish group found that continued use of statins, angiotensin agents, or low-dose aspirin was associated with a decreased rate of new-onset bipolar disorder, while high-dose aspirin and other NSAIDs were linked to increased risk (Bipolar Disord. 2019 Aug;[15]:410-8). What these agents have in common, the investigators observed, is that they act on inflammation and potentially on the stress response system.
Meanwhile, Swedish investigators examined the course of 142,691 Swedes with a diagnosis of bipolar disorder, schizophrenia, or nonaffective psychosis during 2005-2016. They determined that, during periods when those individuals were on a statin, calcium channel blocker, or metformin, they had reduced rates of psychiatric hospitalization and self-harm (JAMA Psychiatry. 2019 Apr 1;76[4]:382-90).
Scottish researchers analyzed the health records of 144,066 patients placed on monotherapy for hypertension and determined that the lowest risk for hospitalization for a mood disorder during follow-up was in those prescribed an ACE inhibitor or angiotensin receptor blocker. The risk was significantly higher in patients on a beta-blocker or calcium channel blocker, and intermediate in those on a thiazide diuretic (Hypertension. 2016 Nov;68[5:1132-8).
“Obviously, this is all at a very macro scale and we have no idea whatsoever what this means for individual patients, number needed to treat, or which type of patients would benefit, but it does provide us with some guidance for future research,” according to Dr. De Picker.
In the meantime, while physicians await definitive evidence of any impact of cardiovascular drugs might have on psychiatric outcomes, abundant data exist underscoring what she called “shockingly high levels” of inadequate management of cardiovascular risk factors in patients with serious mental illness. That problem needs to be addressed, and Dr. De Picker offered her personal recommendations for doing so in a manner consistent with the evidence to date suggestive of potential mental health benefits of some cardiovascular medications.
She advised that, for treatment of hypertension in patients with bipolar disorder or major depression, an ACE inhibitor or angiotensin-converting enzyme inhibitor is preferred as first-line. There is some evidence to suggest lipophilic beta-blockers, which cross the blood-brain barrier, improve anxiety symptoms and panic attacks, and prevent memory consolidation in patients with posttraumatic stress disorder. But the Scottish data suggest that they may worsen mood disorders.
“I would be careful in using beta-blockers as first-line treatment for hypertension. They’re not in the guidelines for anxiety disorders. British guidelines recommend them to prevent memory consolidation in PTSD, but do not use them as first-line in patients with major depressive disorder or bipolar disorder,” she said. As for calcium channel blockers, the jury is still out, with mixed and inconsistent evidence to date as to the impact of this drug class on mental illness outcomes.
She recommended a very low threshold for prescribing statin therapy in patients with serious mental illness in light of the superb risk/benefit ratio for this drug class. In her younger patients, she turns for guidance to an online calculator of an individual’s 10-year risk of a first acute MI or stroke.
Metformin has been shown to be beneficial for addressing the weight gain and other adverse metabolic effects caused by antipsychotic agents, and there is some preliminary evidence of improved psychiatric outcomes in patients with serious mental illness.
Christian Otte, MD, who also spoke at the session, noted that not only do emerging data point to the possibility that cardiovascular drugs might have benefit in terms of psychiatric outcomes, there is also some evidence, albeit mixed, that the converse is true: that is, psychiatric drugs may have cardiovascular benefits. He pointed to a South Korean trial in which 300 patients with a recent acute coronary syndrome and major depression were randomized to 24 weeks of escitalopram or placebo. At median 8.1 years of follow-up, the group that received the SSRI had a 31% relative risk reduction in the primary composite endpoint of all-cause mortality, acute MI, or percutaneous coronary intervention (JAMA. 2018 Jul 24; 320[4]:350–7).
“Potentially independent of their antidepressant effects, some SSRIs’ antiplatelet effects could be beneficial for patients with coronary heart disease, although the jury is still open regarding this question, with evidence in both directions,” said Dr. Otte, professor of psychiatry at Charite University Medical Center in Berlin.
Dr. De Picker offered an example as well: Finnish psychiatrists recently reported that cardiovascular mortality was reduced by an adjusted 38% during periods when 62,250 Finnish schizophrenia patients were on antipsychotic agents, compared with periods of nonuse of the drugs in a national study with a median 14.1 years of follow-up (World Psychiatry. 2020 Feb;19[1]:61-8).
“What they discovered – and this is quite contrary to what we are used to hearing about antipsychotic medication and cardiovascular risk – is that while the number of cardiovascular hospitalizations was not different in periods with or without antipsychotic use, the cardiovascular mortality was quite strikingly reduced when patients were on antipsychotic medication,” she said.
Asked by an audience member whether she personally prescribes metformin, Dr. De Picker replied: “Well, yes, why not? One of the very nice things about metformin is that it is actually a very safe drug, even in the hands of nonspecialists.
“I understand that maybe psychiatrists may not feel very comfortable in starting patients on metformin due to a lack of experience. But there are really only two things you need to take into account. About one-quarter of patients will experience GI side effects – nausea, vomiting, abdominal discomfort – and this can be reduced by gradually uptitrating the dose, dosing at mealtime, and using an extended-release formulation. And the second thing is that metformin can impair vitamin B12 absorption, so I think, especially in psychiatric patients, it would be good to do an annual measurement of vitamin B12 level and, if necessary, administer intramuscular supplements,” Dr. De Picker said.
She reported having no financial conflicts regarding her presentation.
SOURCE: De Picker L. ECNP 2020. Session EDU.05.
One of the hottest topics now in psychiatry is the possibility of repurposing long-established cardiovascular medications for treatment of patients with serious mental illness, Livia De Picker, MD, PhD, said at the virtual congress of the European College of Neuropsychopharmacology.

The appeal is multifold. A huge unmet need exists in psychiatry for new and better treatments with novel mechanisms of action. Many guideline-recommended cardiovascular medications have a long track record, including a well-established safety profile with no surprises, and are available in generic versions. They can be developed for a new indication at minimal cost, noted Dr. De Picker, a psychiatrist at the University of Antwerp (Belgium).
The idea of psychiatric repurposing of drugs originally developed for nonpsychiatric indications is nothing new, she added. Examples include lithium for gout, valproate for epilepsy, and ketamine for anesthesiology.
One hitch in efforts to repurpose cardiovascular medications is that, when psychiatric patients have been included in randomized trials of the drugs’ cardiovascular effects, the psychiatric outcomes often went untallied.
Indeed, the only high-quality randomized trial evidence of psychiatric benefits for any class of cardiovascular medications is for statins, where a modest-sized meta-analysis of six placebo-controlled trials in 339 patients with schizophrenia showed the lipid-lowering agents had benefit for both positive and negative symptoms (Psychiatry Res. 2018 Apr;262:84-93). But that’s not a body of data of sufficient size to be definitive, in Dr. De Picker’s view.
Much of the recent enthusiasm for exploring the potential of cardiovascular drugs for psychiatric conditions comes from hypothesis-generating big data analyses drawn from Scandinavian national patient registries. Danish investigators scrutinized all 1.6 million Danes exposed to six classes of drugs of interest during 2005-2015 and determined that those on long-term statins, low-dose aspirin, ACE inhibitors, angiotensin receptor blockers, or allopurinol were associated with a decreased rate of new-onset depression, while high-dose aspirin and non-aspirin NSAIDs were associated with an increased rate, compared with a 30% random sample of the country’s population (Acta Psychiatr Scand. 2019 Jan;1391:68-77).
Similarly, the Danish group found that continued use of statins, angiotensin agents, or low-dose aspirin was associated with a decreased rate of new-onset bipolar disorder, while high-dose aspirin and other NSAIDs were linked to increased risk (Bipolar Disord. 2019 Aug;[15]:410-8). What these agents have in common, the investigators observed, is that they act on inflammation and potentially on the stress response system.
Meanwhile, Swedish investigators examined the course of 142,691 Swedes with a diagnosis of bipolar disorder, schizophrenia, or nonaffective psychosis during 2005-2016. They determined that, during periods when those individuals were on a statin, calcium channel blocker, or metformin, they had reduced rates of psychiatric hospitalization and self-harm (JAMA Psychiatry. 2019 Apr 1;76[4]:382-90).
Scottish researchers analyzed the health records of 144,066 patients placed on monotherapy for hypertension and determined that the lowest risk for hospitalization for a mood disorder during follow-up was in those prescribed an ACE inhibitor or angiotensin receptor blocker. The risk was significantly higher in patients on a beta-blocker or calcium channel blocker, and intermediate in those on a thiazide diuretic (Hypertension. 2016 Nov;68[5:1132-8).
“Obviously, this is all at a very macro scale and we have no idea whatsoever what this means for individual patients, number needed to treat, or which type of patients would benefit, but it does provide us with some guidance for future research,” according to Dr. De Picker.
In the meantime, while physicians await definitive evidence of any impact of cardiovascular drugs might have on psychiatric outcomes, abundant data exist underscoring what she called “shockingly high levels” of inadequate management of cardiovascular risk factors in patients with serious mental illness. That problem needs to be addressed, and Dr. De Picker offered her personal recommendations for doing so in a manner consistent with the evidence to date suggestive of potential mental health benefits of some cardiovascular medications.
She advised that, for treatment of hypertension in patients with bipolar disorder or major depression, an ACE inhibitor or angiotensin-converting enzyme inhibitor is preferred as first-line. There is some evidence to suggest lipophilic beta-blockers, which cross the blood-brain barrier, improve anxiety symptoms and panic attacks, and prevent memory consolidation in patients with posttraumatic stress disorder. But the Scottish data suggest that they may worsen mood disorders.
“I would be careful in using beta-blockers as first-line treatment for hypertension. They’re not in the guidelines for anxiety disorders. British guidelines recommend them to prevent memory consolidation in PTSD, but do not use them as first-line in patients with major depressive disorder or bipolar disorder,” she said. As for calcium channel blockers, the jury is still out, with mixed and inconsistent evidence to date as to the impact of this drug class on mental illness outcomes.
She recommended a very low threshold for prescribing statin therapy in patients with serious mental illness in light of the superb risk/benefit ratio for this drug class. In her younger patients, she turns for guidance to an online calculator of an individual’s 10-year risk of a first acute MI or stroke.
Metformin has been shown to be beneficial for addressing the weight gain and other adverse metabolic effects caused by antipsychotic agents, and there is some preliminary evidence of improved psychiatric outcomes in patients with serious mental illness.
Christian Otte, MD, who also spoke at the session, noted that not only do emerging data point to the possibility that cardiovascular drugs might have benefit in terms of psychiatric outcomes, there is also some evidence, albeit mixed, that the converse is true: that is, psychiatric drugs may have cardiovascular benefits. He pointed to a South Korean trial in which 300 patients with a recent acute coronary syndrome and major depression were randomized to 24 weeks of escitalopram or placebo. At median 8.1 years of follow-up, the group that received the SSRI had a 31% relative risk reduction in the primary composite endpoint of all-cause mortality, acute MI, or percutaneous coronary intervention (JAMA. 2018 Jul 24; 320[4]:350–7).
“Potentially independent of their antidepressant effects, some SSRIs’ antiplatelet effects could be beneficial for patients with coronary heart disease, although the jury is still open regarding this question, with evidence in both directions,” said Dr. Otte, professor of psychiatry at Charite University Medical Center in Berlin.
Dr. De Picker offered an example as well: Finnish psychiatrists recently reported that cardiovascular mortality was reduced by an adjusted 38% during periods when 62,250 Finnish schizophrenia patients were on antipsychotic agents, compared with periods of nonuse of the drugs in a national study with a median 14.1 years of follow-up (World Psychiatry. 2020 Feb;19[1]:61-8).
“What they discovered – and this is quite contrary to what we are used to hearing about antipsychotic medication and cardiovascular risk – is that while the number of cardiovascular hospitalizations was not different in periods with or without antipsychotic use, the cardiovascular mortality was quite strikingly reduced when patients were on antipsychotic medication,” she said.
Asked by an audience member whether she personally prescribes metformin, Dr. De Picker replied: “Well, yes, why not? One of the very nice things about metformin is that it is actually a very safe drug, even in the hands of nonspecialists.
“I understand that maybe psychiatrists may not feel very comfortable in starting patients on metformin due to a lack of experience. But there are really only two things you need to take into account. About one-quarter of patients will experience GI side effects – nausea, vomiting, abdominal discomfort – and this can be reduced by gradually uptitrating the dose, dosing at mealtime, and using an extended-release formulation. And the second thing is that metformin can impair vitamin B12 absorption, so I think, especially in psychiatric patients, it would be good to do an annual measurement of vitamin B12 level and, if necessary, administer intramuscular supplements,” Dr. De Picker said.
She reported having no financial conflicts regarding her presentation.
SOURCE: De Picker L. ECNP 2020. Session EDU.05.
FROM ECNP 2020
LSD microdosing to boost attention: Too soon to tell?
Microdosing with lysergic acid diethylamide (LSD) is associated with improved mood and increased attention, early research suggests. However, at least one expert believes it’s far too soon to tell and warns against endorsing patient microdosing.
In a dose-finding exploratory study, three low doses of LSD were compared with placebo in healthy volunteers who were all recreational drug users. Adjusted results showed that the highest dose boosted attention and mood, although participants were aware of psychedelic effects, prompting researchers to conclude the results demonstrated “selective, beneficial effects.”
“The majority of participants have improved attention,” study investigator Nadia Hutten, PhD, Department of Neuropsychology and Psychopharmacology, Maastricht University, the Netherlands, told Medscape Medical News.
“So we think that patients with attention deficits might have more beneficial effects,” she added, noting her team plans to study LSD microdosing in patients with attention deficit hyperactivity disorder.
The study was presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Growing interest
Over the past 10 years there has been growing interest in psychedelic microdosing, which is defined as a dose that aims to enhance mood and/or performance but does not affect perception.
However, there has been considerable debate over what constitutes a “microdose.” One tenth of a “full” psychedelic dose is typically suggested, but users report a much wider dose range in practice, suggesting potential “individual variation in response to low doses,” the researchers note.
In the current dose-finding study, the researchers explored whether the effects of LSD on cognition and subjective measures differed between individuals.
The study included 24 healthy recreational drug users and compared the acute effects of 5 mcg, 20 mcg, and 20 mcg LSD with placebo on a computer-based psychomotor vigilance task (PVT) that measured attention and on a Digit Symbol Substitution Test (DSST).
Participants also completed the 72-item Profile of Mood States (POMS) questionnaire, a visual analog scale (VAS) on mood, and the 94-item 5-Dimensional Altered States of Consciousness Rating scales (5D-ASC).
Unadjusted results showed that the 20-mcg LSD dose significantly reduced correct substitutions on the DSST vs placebo (P < .05), but had no effect on attentional lapses on the PCT or on positive mood on the POMS.
Correcting the DSST score for the number of total responses revealed no dose effect of LSD. This suggested that participants were no less accurate when under the influence of LSD, even though they encoded fewer digits, the researchers note.
Participants also reported that both the 10-mcg and 20-mcg dose of LSD increased subjective experiences on the VAS and alternated states of consciousness on the 5D-ASC compared with placebo.
After stratifying the results by dose and participant, the effect of LSD differed between individuals. For example, both the 5-mcg and 20-mcg doses were associated with improvement in attention on the PVT (P < .05), but not the 10-mcg dose.
These results also indicated that the 20-mcg dose was associated with a significant increase in the correct number of substitutions on the DSST and with a significant increase in positive mood on the POMS (P < .05 for both outcomes).
The findings suggest that future studies in patient populations with impaired attention are needed, “including biological parameters involved in LSD receptor-binding and metabolism, in order to understand the inter-individual variation in response to LSD,” the investigators note.
In an educational session at the meeting, the study’s lead researcher, Kim Kuypers, PhD, associate professor at Maastricht University, said research shows individuals are already self-medicating with psychedelic microdosing to treat a wide range of mental health problems, and rated it as significantly more effective than conventional therapy at alleviating symptoms and improving quality of life.
Nevertheless, Kuypers noted there have been fewer than 20 published placebo-controlled studies examining psychedelic microdosing in humans – and much of the current evidence is anecdotal.
However, there is some clinical research suggesting that low-dose LSD is associated with improved mood and cognitive performance and that it also has an effect on resting-state amygdala functional connectivity and acutely increases brain-derived neurotrophic factor plasma levels.
Furthermore, said Kuypers, the evidence in healthy volunteers thus far suggests microdosing is “safe.”
Jumping ahead of the science?
Commenting on the study for Medscape Medical News, Jeffrey A. Lieberman, MD, professor and chair of psychiatry at Columbia University, New York City, said he “gives the investigators credit for doing such a study” but does not believe anything can be gleaned from the findings.
He said he is also concerned that the resurgence of psychedelic research is not congruent with “the methodologic rigor and scientific thinking that accompanies treatment development in other disease areas.”
Lieberman, who is also psychiatrist-in-chief at the NewYork–Presbyterian Hospital Columbia Medical Center and was not involved with the study, added that some of the research is also being conducted in individuals who are “true believers and not sufficiently dispassionate and objective.”
“ But because these are such notorious and interesting compounds, they have attracted a lot of peripheral interest to promote and to disseminate; and the risk is that it will be done in the wrong way and there may be consequences,” he said.
Moreover, Lieberman noted that the psychedelic drugs may be used in practice ahead of strong evidence of safety and efficacy. As an example, he pointed to ketamine, a drug that was identified as a treatment for people with depression who had not responded to standard treatments, he noted.
“But before you knew it, there were clinics being opened up all over the place by anesthesiologists or other people that were trying to make a quick buck,” he said.
“That was alarming because they were stretching the criteria for whom the treatment was appropriate; there were no protocols for dosing, for frequency of administration, and there was inadequate psychiatric follow-up,” Lieberman added.
Preliminary but promising
He agreed with Kuypers that cases of microdosing with psychedelics are largely anecdotal.
“So in that context, when these investigators tried to put it to a test, which is commendable, the results in no way tell you whether it’s good, bad, or indifferent,” Lieberman said. In fact, the results are “disappointing in terms of suggesting any beneficial effect.”
Lieberman said more and larger studies are needed in order to determine whether LSD microdosing is beneficial.
In response to Lieberman’s comments, Kuypers told Medscape Medical News that the investigators tried to base their placebo-controlled research on previous anecdotal research.
She emphasized that the “whole field is still in its infancy,” including research on the use of “full” doses of psychedelics.
“I sometimes think that the message is too positive. We should never forget to communicate that not a lot of research has been done.” In addition, she agreed that researchers should “keep a balanced message.”
“All the data to date is preliminary, in my view, but promising,” she stressed, “and the evidence is growing.”
The study received financial support from the Beckley Foundation. The study authors and Lieberman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Microdosing with lysergic acid diethylamide (LSD) is associated with improved mood and increased attention, early research suggests. However, at least one expert believes it’s far too soon to tell and warns against endorsing patient microdosing.
In a dose-finding exploratory study, three low doses of LSD were compared with placebo in healthy volunteers who were all recreational drug users. Adjusted results showed that the highest dose boosted attention and mood, although participants were aware of psychedelic effects, prompting researchers to conclude the results demonstrated “selective, beneficial effects.”
“The majority of participants have improved attention,” study investigator Nadia Hutten, PhD, Department of Neuropsychology and Psychopharmacology, Maastricht University, the Netherlands, told Medscape Medical News.
“So we think that patients with attention deficits might have more beneficial effects,” she added, noting her team plans to study LSD microdosing in patients with attention deficit hyperactivity disorder.
The study was presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Growing interest
Over the past 10 years there has been growing interest in psychedelic microdosing, which is defined as a dose that aims to enhance mood and/or performance but does not affect perception.
However, there has been considerable debate over what constitutes a “microdose.” One tenth of a “full” psychedelic dose is typically suggested, but users report a much wider dose range in practice, suggesting potential “individual variation in response to low doses,” the researchers note.
In the current dose-finding study, the researchers explored whether the effects of LSD on cognition and subjective measures differed between individuals.
The study included 24 healthy recreational drug users and compared the acute effects of 5 mcg, 20 mcg, and 20 mcg LSD with placebo on a computer-based psychomotor vigilance task (PVT) that measured attention and on a Digit Symbol Substitution Test (DSST).
Participants also completed the 72-item Profile of Mood States (POMS) questionnaire, a visual analog scale (VAS) on mood, and the 94-item 5-Dimensional Altered States of Consciousness Rating scales (5D-ASC).
Unadjusted results showed that the 20-mcg LSD dose significantly reduced correct substitutions on the DSST vs placebo (P < .05), but had no effect on attentional lapses on the PCT or on positive mood on the POMS.
Correcting the DSST score for the number of total responses revealed no dose effect of LSD. This suggested that participants were no less accurate when under the influence of LSD, even though they encoded fewer digits, the researchers note.
Participants also reported that both the 10-mcg and 20-mcg dose of LSD increased subjective experiences on the VAS and alternated states of consciousness on the 5D-ASC compared with placebo.
After stratifying the results by dose and participant, the effect of LSD differed between individuals. For example, both the 5-mcg and 20-mcg doses were associated with improvement in attention on the PVT (P < .05), but not the 10-mcg dose.
These results also indicated that the 20-mcg dose was associated with a significant increase in the correct number of substitutions on the DSST and with a significant increase in positive mood on the POMS (P < .05 for both outcomes).
The findings suggest that future studies in patient populations with impaired attention are needed, “including biological parameters involved in LSD receptor-binding and metabolism, in order to understand the inter-individual variation in response to LSD,” the investigators note.
In an educational session at the meeting, the study’s lead researcher, Kim Kuypers, PhD, associate professor at Maastricht University, said research shows individuals are already self-medicating with psychedelic microdosing to treat a wide range of mental health problems, and rated it as significantly more effective than conventional therapy at alleviating symptoms and improving quality of life.
Nevertheless, Kuypers noted there have been fewer than 20 published placebo-controlled studies examining psychedelic microdosing in humans – and much of the current evidence is anecdotal.
However, there is some clinical research suggesting that low-dose LSD is associated with improved mood and cognitive performance and that it also has an effect on resting-state amygdala functional connectivity and acutely increases brain-derived neurotrophic factor plasma levels.
Furthermore, said Kuypers, the evidence in healthy volunteers thus far suggests microdosing is “safe.”
Jumping ahead of the science?
Commenting on the study for Medscape Medical News, Jeffrey A. Lieberman, MD, professor and chair of psychiatry at Columbia University, New York City, said he “gives the investigators credit for doing such a study” but does not believe anything can be gleaned from the findings.
He said he is also concerned that the resurgence of psychedelic research is not congruent with “the methodologic rigor and scientific thinking that accompanies treatment development in other disease areas.”
Lieberman, who is also psychiatrist-in-chief at the NewYork–Presbyterian Hospital Columbia Medical Center and was not involved with the study, added that some of the research is also being conducted in individuals who are “true believers and not sufficiently dispassionate and objective.”
“ But because these are such notorious and interesting compounds, they have attracted a lot of peripheral interest to promote and to disseminate; and the risk is that it will be done in the wrong way and there may be consequences,” he said.
Moreover, Lieberman noted that the psychedelic drugs may be used in practice ahead of strong evidence of safety and efficacy. As an example, he pointed to ketamine, a drug that was identified as a treatment for people with depression who had not responded to standard treatments, he noted.
“But before you knew it, there were clinics being opened up all over the place by anesthesiologists or other people that were trying to make a quick buck,” he said.
“That was alarming because they were stretching the criteria for whom the treatment was appropriate; there were no protocols for dosing, for frequency of administration, and there was inadequate psychiatric follow-up,” Lieberman added.
Preliminary but promising
He agreed with Kuypers that cases of microdosing with psychedelics are largely anecdotal.
“So in that context, when these investigators tried to put it to a test, which is commendable, the results in no way tell you whether it’s good, bad, or indifferent,” Lieberman said. In fact, the results are “disappointing in terms of suggesting any beneficial effect.”
Lieberman said more and larger studies are needed in order to determine whether LSD microdosing is beneficial.
In response to Lieberman’s comments, Kuypers told Medscape Medical News that the investigators tried to base their placebo-controlled research on previous anecdotal research.
She emphasized that the “whole field is still in its infancy,” including research on the use of “full” doses of psychedelics.
“I sometimes think that the message is too positive. We should never forget to communicate that not a lot of research has been done.” In addition, she agreed that researchers should “keep a balanced message.”
“All the data to date is preliminary, in my view, but promising,” she stressed, “and the evidence is growing.”
The study received financial support from the Beckley Foundation. The study authors and Lieberman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Microdosing with lysergic acid diethylamide (LSD) is associated with improved mood and increased attention, early research suggests. However, at least one expert believes it’s far too soon to tell and warns against endorsing patient microdosing.
In a dose-finding exploratory study, three low doses of LSD were compared with placebo in healthy volunteers who were all recreational drug users. Adjusted results showed that the highest dose boosted attention and mood, although participants were aware of psychedelic effects, prompting researchers to conclude the results demonstrated “selective, beneficial effects.”
“The majority of participants have improved attention,” study investigator Nadia Hutten, PhD, Department of Neuropsychology and Psychopharmacology, Maastricht University, the Netherlands, told Medscape Medical News.
“So we think that patients with attention deficits might have more beneficial effects,” she added, noting her team plans to study LSD microdosing in patients with attention deficit hyperactivity disorder.
The study was presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Growing interest
Over the past 10 years there has been growing interest in psychedelic microdosing, which is defined as a dose that aims to enhance mood and/or performance but does not affect perception.
However, there has been considerable debate over what constitutes a “microdose.” One tenth of a “full” psychedelic dose is typically suggested, but users report a much wider dose range in practice, suggesting potential “individual variation in response to low doses,” the researchers note.
In the current dose-finding study, the researchers explored whether the effects of LSD on cognition and subjective measures differed between individuals.
The study included 24 healthy recreational drug users and compared the acute effects of 5 mcg, 20 mcg, and 20 mcg LSD with placebo on a computer-based psychomotor vigilance task (PVT) that measured attention and on a Digit Symbol Substitution Test (DSST).
Participants also completed the 72-item Profile of Mood States (POMS) questionnaire, a visual analog scale (VAS) on mood, and the 94-item 5-Dimensional Altered States of Consciousness Rating scales (5D-ASC).
Unadjusted results showed that the 20-mcg LSD dose significantly reduced correct substitutions on the DSST vs placebo (P < .05), but had no effect on attentional lapses on the PCT or on positive mood on the POMS.
Correcting the DSST score for the number of total responses revealed no dose effect of LSD. This suggested that participants were no less accurate when under the influence of LSD, even though they encoded fewer digits, the researchers note.
Participants also reported that both the 10-mcg and 20-mcg dose of LSD increased subjective experiences on the VAS and alternated states of consciousness on the 5D-ASC compared with placebo.
After stratifying the results by dose and participant, the effect of LSD differed between individuals. For example, both the 5-mcg and 20-mcg doses were associated with improvement in attention on the PVT (P < .05), but not the 10-mcg dose.
These results also indicated that the 20-mcg dose was associated with a significant increase in the correct number of substitutions on the DSST and with a significant increase in positive mood on the POMS (P < .05 for both outcomes).
The findings suggest that future studies in patient populations with impaired attention are needed, “including biological parameters involved in LSD receptor-binding and metabolism, in order to understand the inter-individual variation in response to LSD,” the investigators note.
In an educational session at the meeting, the study’s lead researcher, Kim Kuypers, PhD, associate professor at Maastricht University, said research shows individuals are already self-medicating with psychedelic microdosing to treat a wide range of mental health problems, and rated it as significantly more effective than conventional therapy at alleviating symptoms and improving quality of life.
Nevertheless, Kuypers noted there have been fewer than 20 published placebo-controlled studies examining psychedelic microdosing in humans – and much of the current evidence is anecdotal.
However, there is some clinical research suggesting that low-dose LSD is associated with improved mood and cognitive performance and that it also has an effect on resting-state amygdala functional connectivity and acutely increases brain-derived neurotrophic factor plasma levels.
Furthermore, said Kuypers, the evidence in healthy volunteers thus far suggests microdosing is “safe.”
Jumping ahead of the science?
Commenting on the study for Medscape Medical News, Jeffrey A. Lieberman, MD, professor and chair of psychiatry at Columbia University, New York City, said he “gives the investigators credit for doing such a study” but does not believe anything can be gleaned from the findings.
He said he is also concerned that the resurgence of psychedelic research is not congruent with “the methodologic rigor and scientific thinking that accompanies treatment development in other disease areas.”
Lieberman, who is also psychiatrist-in-chief at the NewYork–Presbyterian Hospital Columbia Medical Center and was not involved with the study, added that some of the research is also being conducted in individuals who are “true believers and not sufficiently dispassionate and objective.”
“ But because these are such notorious and interesting compounds, they have attracted a lot of peripheral interest to promote and to disseminate; and the risk is that it will be done in the wrong way and there may be consequences,” he said.
Moreover, Lieberman noted that the psychedelic drugs may be used in practice ahead of strong evidence of safety and efficacy. As an example, he pointed to ketamine, a drug that was identified as a treatment for people with depression who had not responded to standard treatments, he noted.
“But before you knew it, there were clinics being opened up all over the place by anesthesiologists or other people that were trying to make a quick buck,” he said.
“That was alarming because they were stretching the criteria for whom the treatment was appropriate; there were no protocols for dosing, for frequency of administration, and there was inadequate psychiatric follow-up,” Lieberman added.
Preliminary but promising
He agreed with Kuypers that cases of microdosing with psychedelics are largely anecdotal.
“So in that context, when these investigators tried to put it to a test, which is commendable, the results in no way tell you whether it’s good, bad, or indifferent,” Lieberman said. In fact, the results are “disappointing in terms of suggesting any beneficial effect.”
Lieberman said more and larger studies are needed in order to determine whether LSD microdosing is beneficial.
In response to Lieberman’s comments, Kuypers told Medscape Medical News that the investigators tried to base their placebo-controlled research on previous anecdotal research.
She emphasized that the “whole field is still in its infancy,” including research on the use of “full” doses of psychedelics.
“I sometimes think that the message is too positive. We should never forget to communicate that not a lot of research has been done.” In addition, she agreed that researchers should “keep a balanced message.”
“All the data to date is preliminary, in my view, but promising,” she stressed, “and the evidence is growing.”
The study received financial support from the Beckley Foundation. The study authors and Lieberman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Deep brain stimulation ‘promising’ in severe schizophrenia
Deep brain stimulation (DBS) may be an effective option for patients with treatment-resistant schizophrenia (TRS), new research suggests. However, until further studies are conducted, the treatment should only be considered for the most severe cases.
The first clinical trial to assess DBS in this challenging patient population included eight patients initially randomly assigned to receive electrode placement in one of two locations in the brain. Once a clinical response was achieved and participants were stabilized, they were randomly assigned to a second crossover phase.
Preliminary findings from the first phase of the DBS-SCHIZO pilot study, which were reported in 2017, showed promising efficacy.
The newly released final results revealed an association between DBS and significant improvements in Positive and Negative Symptoms Scale (PANSS) scores, as well as reductions in doses of antipsychotic medication. Moreover, the effect reversed when the electrode was switched off.
“DBS may be a potential option for severe treatment-resistant schizophrenia patients,” lead investigator Iluminada Corripio, MD, PhD, department of psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, said during her presentation at the virtual congress of the European College of Neuropsychopharmacology. The new data were updated results of a study published in EBioMedicine earlier this year.
Dr. Corripio underlined that it is important to balance the risks and benefits of the intervention. DBS is “not useful for all phenotypes,” and benefits have been seen in patients with hallucinations but not in those with a disorganized phenotype, she added.
High economic burden
Managing TRS is challenging and is associated with a high clinical and economic burden, Corripio noted. Relapse rates can reach 80%, increasing resource use by between 200% and 900%.
There is a strong rationale for studying the use of DBS in schizophrenia, because schizophrenia shares a neurologic basis with other neurologic and psychiatric disorders centered around the cortical-striatal-thalamic-cortical circuit, said Dr. Corripio.
The study included eight patients with a DSM-IV-TR diagnosis of schizophrenia whose conditions were resistant to at least two different atypical antipsychotics and who had not responded to clozapine monotherapy, combination therapy, or electroconvulsive therapy.
All were randomly assigned in a 1:1 ratio to DBS electrode implantation in one of two locations. Investigators chose the nucleus accumbens (NAcc), because recent studies have shown that DBS can increase dopamine levels there, and the subgenual anterior cingulate cortex (ACC). Deactivation failure in the ACC region has been observed in patients with schizophrenia and other mental illnesses.
Stimulation began 48-72 hours postoperatively with unilateral left stimulation at 2.5 volts. It was increased in 0.5 volt increments to a maximum of 7.5 volts. Patients who did not respond were switched to bilateral stimulation.
Follow-up was conducted every 2 weeks for up to 20 months. The study’s primary outcome was a symptomatic response, defined as an improvement of at least 25% on the PANSS.
Once that was achieved, patients could enter a second randomization phase in which they were assigned, in a 24-week, double-blind crossover design, to on- or off-treatment DBS arms such that patients received stimulation for 12 weeks before the device was turned off for 12 weeks, or vice versa.
Those who experienced relapse while off treatment were crossed over to the on-treatment arm; those who experienced relapsed while on treatment were withdrawn from the study. The patients’ average age was 42.5 years, and 50% were women. All were taking clozapine in combination with another antipsychotic.
Adverse events
Five patients experienced adverse events during the first phase, four of which were associated with rechargeable battery replacement. One experienced akathisia, another experienced behavioral changes, and a third experienced electrical disturbances.
A fourth patient experienced postsurgical hemorrhage of the right internal capsule on day 4, followed by encephalitis at week 8. He had a clinical improvement but experienced relapsed during follow-up.
The fifth patient accidentally switched off the device and withdrew from the study.
During the first randomization phase, DBS was associated with significant improvements on total, positive, and negative PANSS scores in comparison with the postoperative baseline measure in the seven remaining patients (P < .001).
When the team compared the baseline measure with the last observation, the improvement in PANSS scores remained significant for total scores (P = .007) and positive scores (P = .002), but not for negative scores (P = .18).
Three patients entered the second crossover phase of the study. Two began in the off-treatment arm and experienced relapsed within 1 and 2 weeks, respectively. Total PANSS scores increased from 79 to 98 for the first patient and from 47 to 93 for the second patient.
Neuroimaging showed that, among patients who responded to DBS, brain metabolism increased in some brain areas and decreased in others. Dr. Corripio said this suggests a “rebalancing” of neural circuits.
As of July 2020, one of three patients with an electrode placed in the NAcc had experienced remission of positive symptoms and now has predominant negative symptoms. Another experienced significant improvements in negative symptoms. Two patients currently require psychosocial rehabilitation.
Patients for whom an electrode was placed in the ACC required higher voltages and more time to achieve an effect in comparison with those for whom an electrode was placed in the NAcc. Two patients required bilateral stimulation.
However, for all three patients who remained in the study, their clozapine dose was reduced.
Dr. Corripio reported that the team has observed negative thoughts and obsessive symptoms in patients with electrodes in the ACC, and all have needed either psychosocial rehabilitation or cognitive-behavioral therapy.
The investigators are now planning another DBS study involving patients with TRS, although this one will include a clinical recovery program focusing on family interventions and cognitive-behavioral therapy.
“Last-resort” treatment
In the postpresentation debate, Damiaan Denys, PhD, professor and chair of the department of psychiatry at the Academic Medical Canter, University of Amsterdam, said that DBS remains a treatment of “last resort” in TRS.
This is because it is both costly and invasive, and although the associated risk of bleeding and infection is low, he noted that the consequences are significant.
Dr. Denys added that patients need to have the potential for improvement; electrodes can be easily implanted, and the approach may tempt clinicians who sometimes “struggle with a huge amount of treatment-refractory cases.”
He also pointed to results achieved in studies of obsessive-compulsive disorder and depression, in which around 50% of patients responded to DBS.
“I think that’s the reason why we should be reluctant and not treat anyone at any stage, but first look for the more severe cases,” Dr. Denys said.
Unmet need
Judith M. Gault, PhD, associate research professor of neurosurgery at the University of Colorado at Denver, Aurora, also took part in the debate.
She said in an interview that patients with TRS have a lot of unmet needs and that DBS is worth trying in this patient population, with the goal being to “conduct a really good clinical trial” similar to the current study.
Antipsychotic drugs work well in responsive patients, but “in some cases the person is treatment refractory ... and in other cases the patient relapses,” Dr. Gault said.
She believes that DBS has the “potential to be more potent than antipsychotics in modulating the circuit of interest” and so fulfills the unmet needs of these patients while alleviating their symptoms.
Dr. Gault added that some patients experience “breakthrough symptoms” even while they are medication adherent. “That is a call for an intervention that is more potent” and suggests another potential role for DBS.
Overall, there are “a lot of really compelling reasons to pursue” DBS. However, there are also questions about how motivated patients with TRS are to participate in a clinical trial, Dr. Gault noted.
Patients with schizophrenia “tend not to be very motivated, especially if they have negative symptoms.” However, “if you were able to consider more of the population and not just the most severely affected, eventually you would find more people who are interested,” she said.
Still, it will take a better understanding of the efficacy and safety of the intervention for more people to be interested in trying it, said Dr. Gault.
“I think it’s hard early on, when you don’t actually know what the outcomes would be, if it’s even effective at all. But as you get more and more data in the population and at the different targets, people would be more open to it,” she said.
Another issue in generating interest among patients with schizophrenia is that many have not considered DBS as an option.
“It takes a while to think about it,” she noted. “You don’t want to rush into something that you just heard about, and so part of it is just education.”
The study was funded by Instituto Carlos III. Dr. Corripio reported having received research grants and conducting consultancy for Otsuka, Ferrer, Janssen, and Lilly. No other relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Deep brain stimulation (DBS) may be an effective option for patients with treatment-resistant schizophrenia (TRS), new research suggests. However, until further studies are conducted, the treatment should only be considered for the most severe cases.
The first clinical trial to assess DBS in this challenging patient population included eight patients initially randomly assigned to receive electrode placement in one of two locations in the brain. Once a clinical response was achieved and participants were stabilized, they were randomly assigned to a second crossover phase.
Preliminary findings from the first phase of the DBS-SCHIZO pilot study, which were reported in 2017, showed promising efficacy.
The newly released final results revealed an association between DBS and significant improvements in Positive and Negative Symptoms Scale (PANSS) scores, as well as reductions in doses of antipsychotic medication. Moreover, the effect reversed when the electrode was switched off.
“DBS may be a potential option for severe treatment-resistant schizophrenia patients,” lead investigator Iluminada Corripio, MD, PhD, department of psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, said during her presentation at the virtual congress of the European College of Neuropsychopharmacology. The new data were updated results of a study published in EBioMedicine earlier this year.
Dr. Corripio underlined that it is important to balance the risks and benefits of the intervention. DBS is “not useful for all phenotypes,” and benefits have been seen in patients with hallucinations but not in those with a disorganized phenotype, she added.
High economic burden
Managing TRS is challenging and is associated with a high clinical and economic burden, Corripio noted. Relapse rates can reach 80%, increasing resource use by between 200% and 900%.
There is a strong rationale for studying the use of DBS in schizophrenia, because schizophrenia shares a neurologic basis with other neurologic and psychiatric disorders centered around the cortical-striatal-thalamic-cortical circuit, said Dr. Corripio.
The study included eight patients with a DSM-IV-TR diagnosis of schizophrenia whose conditions were resistant to at least two different atypical antipsychotics and who had not responded to clozapine monotherapy, combination therapy, or electroconvulsive therapy.
All were randomly assigned in a 1:1 ratio to DBS electrode implantation in one of two locations. Investigators chose the nucleus accumbens (NAcc), because recent studies have shown that DBS can increase dopamine levels there, and the subgenual anterior cingulate cortex (ACC). Deactivation failure in the ACC region has been observed in patients with schizophrenia and other mental illnesses.
Stimulation began 48-72 hours postoperatively with unilateral left stimulation at 2.5 volts. It was increased in 0.5 volt increments to a maximum of 7.5 volts. Patients who did not respond were switched to bilateral stimulation.
Follow-up was conducted every 2 weeks for up to 20 months. The study’s primary outcome was a symptomatic response, defined as an improvement of at least 25% on the PANSS.
Once that was achieved, patients could enter a second randomization phase in which they were assigned, in a 24-week, double-blind crossover design, to on- or off-treatment DBS arms such that patients received stimulation for 12 weeks before the device was turned off for 12 weeks, or vice versa.
Those who experienced relapse while off treatment were crossed over to the on-treatment arm; those who experienced relapsed while on treatment were withdrawn from the study. The patients’ average age was 42.5 years, and 50% were women. All were taking clozapine in combination with another antipsychotic.
Adverse events
Five patients experienced adverse events during the first phase, four of which were associated with rechargeable battery replacement. One experienced akathisia, another experienced behavioral changes, and a third experienced electrical disturbances.
A fourth patient experienced postsurgical hemorrhage of the right internal capsule on day 4, followed by encephalitis at week 8. He had a clinical improvement but experienced relapsed during follow-up.
The fifth patient accidentally switched off the device and withdrew from the study.
During the first randomization phase, DBS was associated with significant improvements on total, positive, and negative PANSS scores in comparison with the postoperative baseline measure in the seven remaining patients (P < .001).
When the team compared the baseline measure with the last observation, the improvement in PANSS scores remained significant for total scores (P = .007) and positive scores (P = .002), but not for negative scores (P = .18).
Three patients entered the second crossover phase of the study. Two began in the off-treatment arm and experienced relapsed within 1 and 2 weeks, respectively. Total PANSS scores increased from 79 to 98 for the first patient and from 47 to 93 for the second patient.
Neuroimaging showed that, among patients who responded to DBS, brain metabolism increased in some brain areas and decreased in others. Dr. Corripio said this suggests a “rebalancing” of neural circuits.
As of July 2020, one of three patients with an electrode placed in the NAcc had experienced remission of positive symptoms and now has predominant negative symptoms. Another experienced significant improvements in negative symptoms. Two patients currently require psychosocial rehabilitation.
Patients for whom an electrode was placed in the ACC required higher voltages and more time to achieve an effect in comparison with those for whom an electrode was placed in the NAcc. Two patients required bilateral stimulation.
However, for all three patients who remained in the study, their clozapine dose was reduced.
Dr. Corripio reported that the team has observed negative thoughts and obsessive symptoms in patients with electrodes in the ACC, and all have needed either psychosocial rehabilitation or cognitive-behavioral therapy.
The investigators are now planning another DBS study involving patients with TRS, although this one will include a clinical recovery program focusing on family interventions and cognitive-behavioral therapy.
“Last-resort” treatment
In the postpresentation debate, Damiaan Denys, PhD, professor and chair of the department of psychiatry at the Academic Medical Canter, University of Amsterdam, said that DBS remains a treatment of “last resort” in TRS.
This is because it is both costly and invasive, and although the associated risk of bleeding and infection is low, he noted that the consequences are significant.
Dr. Denys added that patients need to have the potential for improvement; electrodes can be easily implanted, and the approach may tempt clinicians who sometimes “struggle with a huge amount of treatment-refractory cases.”
He also pointed to results achieved in studies of obsessive-compulsive disorder and depression, in which around 50% of patients responded to DBS.
“I think that’s the reason why we should be reluctant and not treat anyone at any stage, but first look for the more severe cases,” Dr. Denys said.
Unmet need
Judith M. Gault, PhD, associate research professor of neurosurgery at the University of Colorado at Denver, Aurora, also took part in the debate.
She said in an interview that patients with TRS have a lot of unmet needs and that DBS is worth trying in this patient population, with the goal being to “conduct a really good clinical trial” similar to the current study.
Antipsychotic drugs work well in responsive patients, but “in some cases the person is treatment refractory ... and in other cases the patient relapses,” Dr. Gault said.
She believes that DBS has the “potential to be more potent than antipsychotics in modulating the circuit of interest” and so fulfills the unmet needs of these patients while alleviating their symptoms.
Dr. Gault added that some patients experience “breakthrough symptoms” even while they are medication adherent. “That is a call for an intervention that is more potent” and suggests another potential role for DBS.
Overall, there are “a lot of really compelling reasons to pursue” DBS. However, there are also questions about how motivated patients with TRS are to participate in a clinical trial, Dr. Gault noted.
Patients with schizophrenia “tend not to be very motivated, especially if they have negative symptoms.” However, “if you were able to consider more of the population and not just the most severely affected, eventually you would find more people who are interested,” she said.
Still, it will take a better understanding of the efficacy and safety of the intervention for more people to be interested in trying it, said Dr. Gault.
“I think it’s hard early on, when you don’t actually know what the outcomes would be, if it’s even effective at all. But as you get more and more data in the population and at the different targets, people would be more open to it,” she said.
Another issue in generating interest among patients with schizophrenia is that many have not considered DBS as an option.
“It takes a while to think about it,” she noted. “You don’t want to rush into something that you just heard about, and so part of it is just education.”
The study was funded by Instituto Carlos III. Dr. Corripio reported having received research grants and conducting consultancy for Otsuka, Ferrer, Janssen, and Lilly. No other relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Deep brain stimulation (DBS) may be an effective option for patients with treatment-resistant schizophrenia (TRS), new research suggests. However, until further studies are conducted, the treatment should only be considered for the most severe cases.
The first clinical trial to assess DBS in this challenging patient population included eight patients initially randomly assigned to receive electrode placement in one of two locations in the brain. Once a clinical response was achieved and participants were stabilized, they were randomly assigned to a second crossover phase.
Preliminary findings from the first phase of the DBS-SCHIZO pilot study, which were reported in 2017, showed promising efficacy.
The newly released final results revealed an association between DBS and significant improvements in Positive and Negative Symptoms Scale (PANSS) scores, as well as reductions in doses of antipsychotic medication. Moreover, the effect reversed when the electrode was switched off.
“DBS may be a potential option for severe treatment-resistant schizophrenia patients,” lead investigator Iluminada Corripio, MD, PhD, department of psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, said during her presentation at the virtual congress of the European College of Neuropsychopharmacology. The new data were updated results of a study published in EBioMedicine earlier this year.
Dr. Corripio underlined that it is important to balance the risks and benefits of the intervention. DBS is “not useful for all phenotypes,” and benefits have been seen in patients with hallucinations but not in those with a disorganized phenotype, she added.
High economic burden
Managing TRS is challenging and is associated with a high clinical and economic burden, Corripio noted. Relapse rates can reach 80%, increasing resource use by between 200% and 900%.
There is a strong rationale for studying the use of DBS in schizophrenia, because schizophrenia shares a neurologic basis with other neurologic and psychiatric disorders centered around the cortical-striatal-thalamic-cortical circuit, said Dr. Corripio.
The study included eight patients with a DSM-IV-TR diagnosis of schizophrenia whose conditions were resistant to at least two different atypical antipsychotics and who had not responded to clozapine monotherapy, combination therapy, or electroconvulsive therapy.
All were randomly assigned in a 1:1 ratio to DBS electrode implantation in one of two locations. Investigators chose the nucleus accumbens (NAcc), because recent studies have shown that DBS can increase dopamine levels there, and the subgenual anterior cingulate cortex (ACC). Deactivation failure in the ACC region has been observed in patients with schizophrenia and other mental illnesses.
Stimulation began 48-72 hours postoperatively with unilateral left stimulation at 2.5 volts. It was increased in 0.5 volt increments to a maximum of 7.5 volts. Patients who did not respond were switched to bilateral stimulation.
Follow-up was conducted every 2 weeks for up to 20 months. The study’s primary outcome was a symptomatic response, defined as an improvement of at least 25% on the PANSS.
Once that was achieved, patients could enter a second randomization phase in which they were assigned, in a 24-week, double-blind crossover design, to on- or off-treatment DBS arms such that patients received stimulation for 12 weeks before the device was turned off for 12 weeks, or vice versa.
Those who experienced relapse while off treatment were crossed over to the on-treatment arm; those who experienced relapsed while on treatment were withdrawn from the study. The patients’ average age was 42.5 years, and 50% were women. All were taking clozapine in combination with another antipsychotic.
Adverse events
Five patients experienced adverse events during the first phase, four of which were associated with rechargeable battery replacement. One experienced akathisia, another experienced behavioral changes, and a third experienced electrical disturbances.
A fourth patient experienced postsurgical hemorrhage of the right internal capsule on day 4, followed by encephalitis at week 8. He had a clinical improvement but experienced relapsed during follow-up.
The fifth patient accidentally switched off the device and withdrew from the study.
During the first randomization phase, DBS was associated with significant improvements on total, positive, and negative PANSS scores in comparison with the postoperative baseline measure in the seven remaining patients (P < .001).
When the team compared the baseline measure with the last observation, the improvement in PANSS scores remained significant for total scores (P = .007) and positive scores (P = .002), but not for negative scores (P = .18).
Three patients entered the second crossover phase of the study. Two began in the off-treatment arm and experienced relapsed within 1 and 2 weeks, respectively. Total PANSS scores increased from 79 to 98 for the first patient and from 47 to 93 for the second patient.
Neuroimaging showed that, among patients who responded to DBS, brain metabolism increased in some brain areas and decreased in others. Dr. Corripio said this suggests a “rebalancing” of neural circuits.
As of July 2020, one of three patients with an electrode placed in the NAcc had experienced remission of positive symptoms and now has predominant negative symptoms. Another experienced significant improvements in negative symptoms. Two patients currently require psychosocial rehabilitation.
Patients for whom an electrode was placed in the ACC required higher voltages and more time to achieve an effect in comparison with those for whom an electrode was placed in the NAcc. Two patients required bilateral stimulation.
However, for all three patients who remained in the study, their clozapine dose was reduced.
Dr. Corripio reported that the team has observed negative thoughts and obsessive symptoms in patients with electrodes in the ACC, and all have needed either psychosocial rehabilitation or cognitive-behavioral therapy.
The investigators are now planning another DBS study involving patients with TRS, although this one will include a clinical recovery program focusing on family interventions and cognitive-behavioral therapy.
“Last-resort” treatment
In the postpresentation debate, Damiaan Denys, PhD, professor and chair of the department of psychiatry at the Academic Medical Canter, University of Amsterdam, said that DBS remains a treatment of “last resort” in TRS.
This is because it is both costly and invasive, and although the associated risk of bleeding and infection is low, he noted that the consequences are significant.
Dr. Denys added that patients need to have the potential for improvement; electrodes can be easily implanted, and the approach may tempt clinicians who sometimes “struggle with a huge amount of treatment-refractory cases.”
He also pointed to results achieved in studies of obsessive-compulsive disorder and depression, in which around 50% of patients responded to DBS.
“I think that’s the reason why we should be reluctant and not treat anyone at any stage, but first look for the more severe cases,” Dr. Denys said.
Unmet need
Judith M. Gault, PhD, associate research professor of neurosurgery at the University of Colorado at Denver, Aurora, also took part in the debate.
She said in an interview that patients with TRS have a lot of unmet needs and that DBS is worth trying in this patient population, with the goal being to “conduct a really good clinical trial” similar to the current study.
Antipsychotic drugs work well in responsive patients, but “in some cases the person is treatment refractory ... and in other cases the patient relapses,” Dr. Gault said.
She believes that DBS has the “potential to be more potent than antipsychotics in modulating the circuit of interest” and so fulfills the unmet needs of these patients while alleviating their symptoms.
Dr. Gault added that some patients experience “breakthrough symptoms” even while they are medication adherent. “That is a call for an intervention that is more potent” and suggests another potential role for DBS.
Overall, there are “a lot of really compelling reasons to pursue” DBS. However, there are also questions about how motivated patients with TRS are to participate in a clinical trial, Dr. Gault noted.
Patients with schizophrenia “tend not to be very motivated, especially if they have negative symptoms.” However, “if you were able to consider more of the population and not just the most severely affected, eventually you would find more people who are interested,” she said.
Still, it will take a better understanding of the efficacy and safety of the intervention for more people to be interested in trying it, said Dr. Gault.
“I think it’s hard early on, when you don’t actually know what the outcomes would be, if it’s even effective at all. But as you get more and more data in the population and at the different targets, people would be more open to it,” she said.
Another issue in generating interest among patients with schizophrenia is that many have not considered DBS as an option.
“It takes a while to think about it,” she noted. “You don’t want to rush into something that you just heard about, and so part of it is just education.”
The study was funded by Instituto Carlos III. Dr. Corripio reported having received research grants and conducting consultancy for Otsuka, Ferrer, Janssen, and Lilly. No other relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.





