User login
Multiple Sclerosis Hub
New consensus guideline on clinical MRI use in MS
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
Multiple DMTs linked to alopecia, especially in women
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
FROM CMSC 2021
Certain DMTs in MS linked to more psoriasis
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
FROM CMSC 2021
Which agent is best for neuromyelitis optica?
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.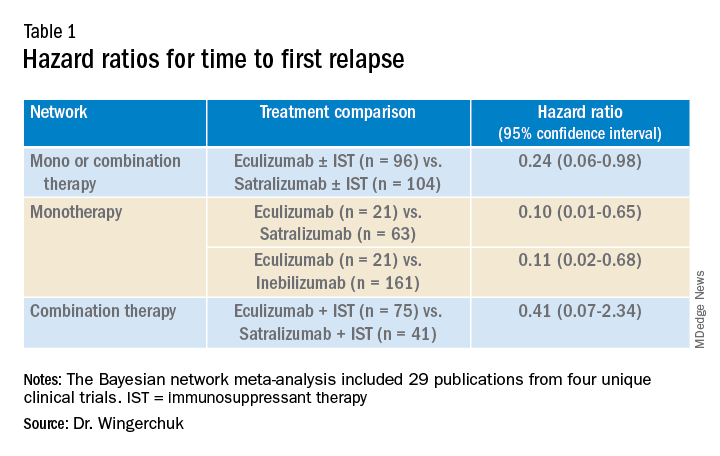
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).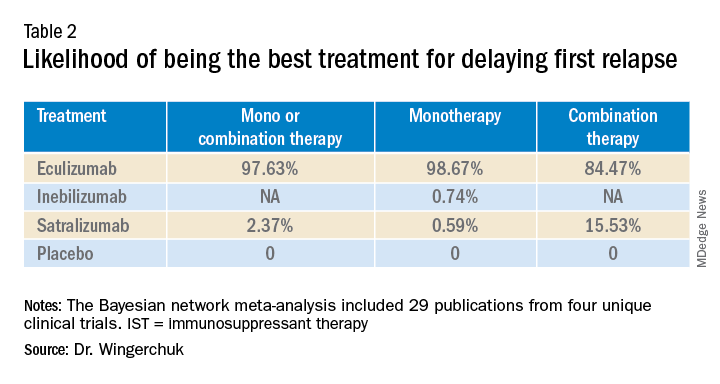
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Two diets linked to improved cognition, fatigue in MS
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
MS and COVID: Docs switched DMTs but maybe didn’t need to
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
FROM CMSC 2021
MS fundraising during a pandemic
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Good data is lacking on best first-line MS drug strategies
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
FROM CMSC 2021
Pain in MS: Focus on flexibility, multiple strategies, and nondrug treatments
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
FROM CMSC 2021
Steroid-induced psychosis in MS? Quetiapine may help
a new case review says.
“Our case-report study observed that quetiapine was effective at decreasing irritability, reducing psychological distress, and improving sleep in patients with MS who experienced psychosis symptoms compared with patients who received no treatment. This has changed our practice as we now counsel all patients about the potential side effect of steroid-induced psychosis and discuss treatment options,” said Olinka Hrebicek, MD, medical director of Vancouver Island Multiple Sclerosis Clinic in Victoria, B.C., who was scheduled to present the study findings at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
According to Dr. Hrebicek, who spoke in an interview, nursing staff and neurologists at the Canadian clinic had typically attributed symptoms such as irritability, anger, insomnia, and psychological distress to the stress of experiencing a relapse. The treatment often was a prescription for a benzodiazepine or zopiclone.
In fact, she and colleagues wrote in their report, psychosis following treatment with high-dose corticosteroids for MS may be underreported.
“The purpose of the study was to determine whether quetiapine was effective for treating symptoms of steroid-induced psychosis in patients with MS,” study coauthor and clinic research assistant Niall Murphy said in an interview. “We also wanted to highlight the importance of looking for symptoms of steroid-induced psychosis as this is likely not the primary concern when treating patients for a relapse. In addition, nurses and neurologists may have less experience with the spectrum of clinical symptoms of psychosis than psychiatrists.”
For the case review, researchers examined 10 reports (8 female) of patients who had signs of psychiatric distress after treatment with steroids. Eight of the patients were treated with quetiapine (six female, two male).
All those who took quetiapine experienced benefits, while the two others didn’t improve.
Commenting on the study, E. Sherwood Brown, MD, PhD, MBA, professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas, said in an interview that psychosis may not appear as expected in patients who develop it as a result of corticosteroid use. “Typically, psychosis refers to delusions, hallucinations, or disorganized thought processes. However, with corticosteroids severe mood and cognitive changes [for example, delirium] are also often included in the definition. Mild mood and memory changes appear to be fairly common with prescription corticosteroids. More severe symptoms are less common.”
Higher doses of corticosteroids – like those used in MS – boost the risk of psychosis, said Dr. Brown, who was not involved in the study.
As for quetiapine, Dr. Brown said it could be a good treatment option. “The use of quetiapine, a drug approved for schizophrenia and mania, is consistent with the idea suggested in the literature that the symptoms with corticosteroids tend to be similar to those of bipolar disorder and that they respond to medications for bipolar disorder,” he said. “A potential concern is that both corticosteroids and quetiapine can cause weight gain. However, this may not be a major problem with a brief course of the corticosteroids. It would be great to see a randomized, controlled trial.”
In British Columbia, the Victoria clinic has changed policy as a result of the analysis, Dr. Hrebicek said. “Nurses and physicians now ask more specific questions to decide if patients are experiencing symptoms of steroid-induced psychosis and whether they should be treated with an antipsychotic medication.”
And now, report coauthor Mr. Murphy said, “our clinic proactively offers patients a prescription for quetiapine that they can fill if they are experiencing symptoms of steroid psychosis.”
Dr. Brown supported the new policy of alerting patients to the psychosis risk. “Counseling patients about common side effects is a good idea,” he said. “I have seen data suggesting that patients may be hesitant to report psychiatric symptoms with corticosteroids to their physicians. Letting them know about the potential for these kinds of side effects might make them more forthcoming in reporting this side effect.”
No study funding is reported. The study authors reported no disclosures. Dr. Brown has a National Institutes of Health grant for studying the effect of corticosteroids on the brain.
a new case review says.
“Our case-report study observed that quetiapine was effective at decreasing irritability, reducing psychological distress, and improving sleep in patients with MS who experienced psychosis symptoms compared with patients who received no treatment. This has changed our practice as we now counsel all patients about the potential side effect of steroid-induced psychosis and discuss treatment options,” said Olinka Hrebicek, MD, medical director of Vancouver Island Multiple Sclerosis Clinic in Victoria, B.C., who was scheduled to present the study findings at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
According to Dr. Hrebicek, who spoke in an interview, nursing staff and neurologists at the Canadian clinic had typically attributed symptoms such as irritability, anger, insomnia, and psychological distress to the stress of experiencing a relapse. The treatment often was a prescription for a benzodiazepine or zopiclone.
In fact, she and colleagues wrote in their report, psychosis following treatment with high-dose corticosteroids for MS may be underreported.
“The purpose of the study was to determine whether quetiapine was effective for treating symptoms of steroid-induced psychosis in patients with MS,” study coauthor and clinic research assistant Niall Murphy said in an interview. “We also wanted to highlight the importance of looking for symptoms of steroid-induced psychosis as this is likely not the primary concern when treating patients for a relapse. In addition, nurses and neurologists may have less experience with the spectrum of clinical symptoms of psychosis than psychiatrists.”
For the case review, researchers examined 10 reports (8 female) of patients who had signs of psychiatric distress after treatment with steroids. Eight of the patients were treated with quetiapine (six female, two male).
All those who took quetiapine experienced benefits, while the two others didn’t improve.
Commenting on the study, E. Sherwood Brown, MD, PhD, MBA, professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas, said in an interview that psychosis may not appear as expected in patients who develop it as a result of corticosteroid use. “Typically, psychosis refers to delusions, hallucinations, or disorganized thought processes. However, with corticosteroids severe mood and cognitive changes [for example, delirium] are also often included in the definition. Mild mood and memory changes appear to be fairly common with prescription corticosteroids. More severe symptoms are less common.”
Higher doses of corticosteroids – like those used in MS – boost the risk of psychosis, said Dr. Brown, who was not involved in the study.
As for quetiapine, Dr. Brown said it could be a good treatment option. “The use of quetiapine, a drug approved for schizophrenia and mania, is consistent with the idea suggested in the literature that the symptoms with corticosteroids tend to be similar to those of bipolar disorder and that they respond to medications for bipolar disorder,” he said. “A potential concern is that both corticosteroids and quetiapine can cause weight gain. However, this may not be a major problem with a brief course of the corticosteroids. It would be great to see a randomized, controlled trial.”
In British Columbia, the Victoria clinic has changed policy as a result of the analysis, Dr. Hrebicek said. “Nurses and physicians now ask more specific questions to decide if patients are experiencing symptoms of steroid-induced psychosis and whether they should be treated with an antipsychotic medication.”
And now, report coauthor Mr. Murphy said, “our clinic proactively offers patients a prescription for quetiapine that they can fill if they are experiencing symptoms of steroid psychosis.”
Dr. Brown supported the new policy of alerting patients to the psychosis risk. “Counseling patients about common side effects is a good idea,” he said. “I have seen data suggesting that patients may be hesitant to report psychiatric symptoms with corticosteroids to their physicians. Letting them know about the potential for these kinds of side effects might make them more forthcoming in reporting this side effect.”
No study funding is reported. The study authors reported no disclosures. Dr. Brown has a National Institutes of Health grant for studying the effect of corticosteroids on the brain.
a new case review says.
“Our case-report study observed that quetiapine was effective at decreasing irritability, reducing psychological distress, and improving sleep in patients with MS who experienced psychosis symptoms compared with patients who received no treatment. This has changed our practice as we now counsel all patients about the potential side effect of steroid-induced psychosis and discuss treatment options,” said Olinka Hrebicek, MD, medical director of Vancouver Island Multiple Sclerosis Clinic in Victoria, B.C., who was scheduled to present the study findings at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
According to Dr. Hrebicek, who spoke in an interview, nursing staff and neurologists at the Canadian clinic had typically attributed symptoms such as irritability, anger, insomnia, and psychological distress to the stress of experiencing a relapse. The treatment often was a prescription for a benzodiazepine or zopiclone.
In fact, she and colleagues wrote in their report, psychosis following treatment with high-dose corticosteroids for MS may be underreported.
“The purpose of the study was to determine whether quetiapine was effective for treating symptoms of steroid-induced psychosis in patients with MS,” study coauthor and clinic research assistant Niall Murphy said in an interview. “We also wanted to highlight the importance of looking for symptoms of steroid-induced psychosis as this is likely not the primary concern when treating patients for a relapse. In addition, nurses and neurologists may have less experience with the spectrum of clinical symptoms of psychosis than psychiatrists.”
For the case review, researchers examined 10 reports (8 female) of patients who had signs of psychiatric distress after treatment with steroids. Eight of the patients were treated with quetiapine (six female, two male).
All those who took quetiapine experienced benefits, while the two others didn’t improve.
Commenting on the study, E. Sherwood Brown, MD, PhD, MBA, professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas, said in an interview that psychosis may not appear as expected in patients who develop it as a result of corticosteroid use. “Typically, psychosis refers to delusions, hallucinations, or disorganized thought processes. However, with corticosteroids severe mood and cognitive changes [for example, delirium] are also often included in the definition. Mild mood and memory changes appear to be fairly common with prescription corticosteroids. More severe symptoms are less common.”
Higher doses of corticosteroids – like those used in MS – boost the risk of psychosis, said Dr. Brown, who was not involved in the study.
As for quetiapine, Dr. Brown said it could be a good treatment option. “The use of quetiapine, a drug approved for schizophrenia and mania, is consistent with the idea suggested in the literature that the symptoms with corticosteroids tend to be similar to those of bipolar disorder and that they respond to medications for bipolar disorder,” he said. “A potential concern is that both corticosteroids and quetiapine can cause weight gain. However, this may not be a major problem with a brief course of the corticosteroids. It would be great to see a randomized, controlled trial.”
In British Columbia, the Victoria clinic has changed policy as a result of the analysis, Dr. Hrebicek said. “Nurses and physicians now ask more specific questions to decide if patients are experiencing symptoms of steroid-induced psychosis and whether they should be treated with an antipsychotic medication.”
And now, report coauthor Mr. Murphy said, “our clinic proactively offers patients a prescription for quetiapine that they can fill if they are experiencing symptoms of steroid psychosis.”
Dr. Brown supported the new policy of alerting patients to the psychosis risk. “Counseling patients about common side effects is a good idea,” he said. “I have seen data suggesting that patients may be hesitant to report psychiatric symptoms with corticosteroids to their physicians. Letting them know about the potential for these kinds of side effects might make them more forthcoming in reporting this side effect.”
No study funding is reported. The study authors reported no disclosures. Dr. Brown has a National Institutes of Health grant for studying the effect of corticosteroids on the brain.
FROM CMSC 2021




