User login
Which agent is best for neuromyelitis optica?
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.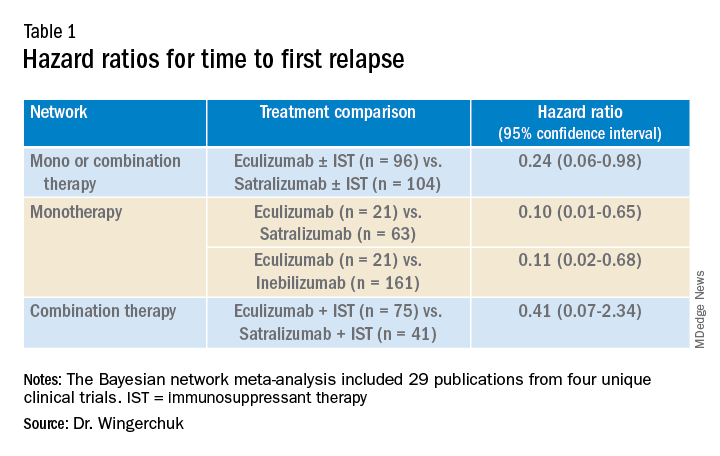
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).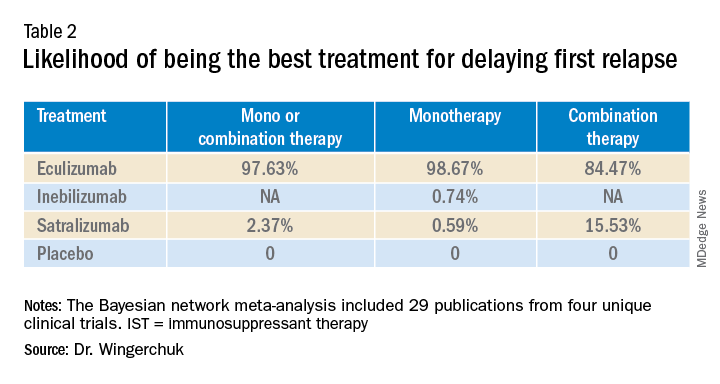
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Stem cell transplant benefits in secondary progressive MS
, a new Italian study suggests.
In the study, stem cell transplant was associated with a slowing of disability progression and a higher likelihood of disability improvement in patients with secondary progressive MS compared with other disease-modifying therapies.
“Our study shows that, although limited, sustained disability improvement is still possible during early active secondary progressive MS and seems to be more likely with stem cell transplant than other disease-modifying treatments,” said lead author Giacomo Boffa, MD, University of Genoa, Italy.
“Brain penetrating–intent immune suppression in long-term immunological reconstitution within the central nervous system could be responsible for this clinical efficacy,” he added.
Dr. Boffa presented the research at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
He explained that compartmentalized inflation in the brain parenchyma, left meninges, and the cerebral spinal fluid (CSF) is a key driver of disability worsening in secondary progressive MS.
Although initial studies did not reveal an effect of disease-modifying therapies in secondary progressive MS, recent randomized trials have established some benefit of siponimod in reducing the risk of disability worsening, and consistent with this finding, observational studies have suggested that other available immunotherapies may also be beneficial for active secondary progressive MS, Dr. Boffa noted.
“Autologous hematopoietic stem cell transplantation has been widely investigated for the treatment of refractory MS, and although the ideal candidate for this procedure is a young patient with relapsing-remitting MS, there is some evidence to suggest that the procedure could also slow down neurological disability in patients with secondary progressive MS,” Dr. Boffa said.
“Indeed, all the drugs used in the transplant technology share the ability to cross the blood-brain barrier and exert a strong immunosuppressant effect within the brain parenchyma and CSF,” he added.
Comparing treatment regimens
The aim of the current study was to compare the effect of autologous hematopoietic stem cell transplantation with other immunotherapies on disability worsening in patients with active secondary progressive MS.
Study endpoints included the Expanded Disability Status Scale (EDSS) score, 6-month worsening and improvement in disability, and sustained disability improvement over time.
The researchers studied patients with secondary progressive MS who had received autologous hematopoietic stem cell transplantation and were included in the Italian Bone Marrow Transplant Group. They were compared with a control group of patients in the Italian MS registry who had started a nontransplant disease-modifying therapy after the diagnosis of secondary progressive MS.
To control for many different variables, two separate analyses were preformed. One analyses was a propensity-score approach (patients were matched based on their propensity to receive bone marrow transplant or one of the other disease-modifying therapies). The other analysis used an overlap weighting approach (each patient was given a weight proportional to the probability of them belonging to the other treatment group).
The final cohort consisted of 79 bone marrow transplant recipients and 1,975 patients who had received other disease-modifying therapies.
Before matching, patients in the control group were older, had a longer disease duration, and had a lower annualized relapse rate than transplanted patients.
After propensity-score matching, there were 69 transplanted patients and 217 control patients who were well balanced in terms of clinical and demographic characteristics. After overlap weighting, the entire cohort was also well balanced for these variables.
In terms of the primary endpoint, stem cell transplant stabilized the EDSS score over time, while patients treated with other disease-modifying therapies had continuous progression of the EDSS score over time.
In the propensity-matched analysis, the EDSS score improved by 0.013 points per year in the stem cell transplant group compared with a worsening of 0.157 points per year in the control group. Similar results were seen in the overlap-weighting analysis.
The effect of stem cell transplant on EDSS score translated into a significantly delayed time to confirmed disability progression in the stem cell transplant group compared with the control group (HR, 0.5; P = .005), Dr. Boffa reported.
Five years after the procedure, 62% of the transplant group were free of disability progression, compared with around 20% of the control group.
Patients in the transplanted group were also more likely to show disability improvement over time. Five years after the procedure, almost 20% of the stem cell transplant group still maintained a disability improvement compared with only 4% of patients treated with other disease-modifying therapies.
“Our study population was composed of relatively young patients (average age 38 years) with clinical activity during secondary progressive MS, and the results of this study would not be applicable to patients with secondary progressive MS patients without signs of inflammatory activity,” Dr. Boffa commented.
“But on the other hand, our results reinforce the notion that ongoing inflammation during progressive MS requires adequate immunotherapy,” he added.
A version of this article first appeared on Medscape.com.
, a new Italian study suggests.
In the study, stem cell transplant was associated with a slowing of disability progression and a higher likelihood of disability improvement in patients with secondary progressive MS compared with other disease-modifying therapies.
“Our study shows that, although limited, sustained disability improvement is still possible during early active secondary progressive MS and seems to be more likely with stem cell transplant than other disease-modifying treatments,” said lead author Giacomo Boffa, MD, University of Genoa, Italy.
“Brain penetrating–intent immune suppression in long-term immunological reconstitution within the central nervous system could be responsible for this clinical efficacy,” he added.
Dr. Boffa presented the research at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
He explained that compartmentalized inflation in the brain parenchyma, left meninges, and the cerebral spinal fluid (CSF) is a key driver of disability worsening in secondary progressive MS.
Although initial studies did not reveal an effect of disease-modifying therapies in secondary progressive MS, recent randomized trials have established some benefit of siponimod in reducing the risk of disability worsening, and consistent with this finding, observational studies have suggested that other available immunotherapies may also be beneficial for active secondary progressive MS, Dr. Boffa noted.
“Autologous hematopoietic stem cell transplantation has been widely investigated for the treatment of refractory MS, and although the ideal candidate for this procedure is a young patient with relapsing-remitting MS, there is some evidence to suggest that the procedure could also slow down neurological disability in patients with secondary progressive MS,” Dr. Boffa said.
“Indeed, all the drugs used in the transplant technology share the ability to cross the blood-brain barrier and exert a strong immunosuppressant effect within the brain parenchyma and CSF,” he added.
Comparing treatment regimens
The aim of the current study was to compare the effect of autologous hematopoietic stem cell transplantation with other immunotherapies on disability worsening in patients with active secondary progressive MS.
Study endpoints included the Expanded Disability Status Scale (EDSS) score, 6-month worsening and improvement in disability, and sustained disability improvement over time.
The researchers studied patients with secondary progressive MS who had received autologous hematopoietic stem cell transplantation and were included in the Italian Bone Marrow Transplant Group. They were compared with a control group of patients in the Italian MS registry who had started a nontransplant disease-modifying therapy after the diagnosis of secondary progressive MS.
To control for many different variables, two separate analyses were preformed. One analyses was a propensity-score approach (patients were matched based on their propensity to receive bone marrow transplant or one of the other disease-modifying therapies). The other analysis used an overlap weighting approach (each patient was given a weight proportional to the probability of them belonging to the other treatment group).
The final cohort consisted of 79 bone marrow transplant recipients and 1,975 patients who had received other disease-modifying therapies.
Before matching, patients in the control group were older, had a longer disease duration, and had a lower annualized relapse rate than transplanted patients.
After propensity-score matching, there were 69 transplanted patients and 217 control patients who were well balanced in terms of clinical and demographic characteristics. After overlap weighting, the entire cohort was also well balanced for these variables.
In terms of the primary endpoint, stem cell transplant stabilized the EDSS score over time, while patients treated with other disease-modifying therapies had continuous progression of the EDSS score over time.
In the propensity-matched analysis, the EDSS score improved by 0.013 points per year in the stem cell transplant group compared with a worsening of 0.157 points per year in the control group. Similar results were seen in the overlap-weighting analysis.
The effect of stem cell transplant on EDSS score translated into a significantly delayed time to confirmed disability progression in the stem cell transplant group compared with the control group (HR, 0.5; P = .005), Dr. Boffa reported.
Five years after the procedure, 62% of the transplant group were free of disability progression, compared with around 20% of the control group.
Patients in the transplanted group were also more likely to show disability improvement over time. Five years after the procedure, almost 20% of the stem cell transplant group still maintained a disability improvement compared with only 4% of patients treated with other disease-modifying therapies.
“Our study population was composed of relatively young patients (average age 38 years) with clinical activity during secondary progressive MS, and the results of this study would not be applicable to patients with secondary progressive MS patients without signs of inflammatory activity,” Dr. Boffa commented.
“But on the other hand, our results reinforce the notion that ongoing inflammation during progressive MS requires adequate immunotherapy,” he added.
A version of this article first appeared on Medscape.com.
, a new Italian study suggests.
In the study, stem cell transplant was associated with a slowing of disability progression and a higher likelihood of disability improvement in patients with secondary progressive MS compared with other disease-modifying therapies.
“Our study shows that, although limited, sustained disability improvement is still possible during early active secondary progressive MS and seems to be more likely with stem cell transplant than other disease-modifying treatments,” said lead author Giacomo Boffa, MD, University of Genoa, Italy.
“Brain penetrating–intent immune suppression in long-term immunological reconstitution within the central nervous system could be responsible for this clinical efficacy,” he added.
Dr. Boffa presented the research at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
He explained that compartmentalized inflation in the brain parenchyma, left meninges, and the cerebral spinal fluid (CSF) is a key driver of disability worsening in secondary progressive MS.
Although initial studies did not reveal an effect of disease-modifying therapies in secondary progressive MS, recent randomized trials have established some benefit of siponimod in reducing the risk of disability worsening, and consistent with this finding, observational studies have suggested that other available immunotherapies may also be beneficial for active secondary progressive MS, Dr. Boffa noted.
“Autologous hematopoietic stem cell transplantation has been widely investigated for the treatment of refractory MS, and although the ideal candidate for this procedure is a young patient with relapsing-remitting MS, there is some evidence to suggest that the procedure could also slow down neurological disability in patients with secondary progressive MS,” Dr. Boffa said.
“Indeed, all the drugs used in the transplant technology share the ability to cross the blood-brain barrier and exert a strong immunosuppressant effect within the brain parenchyma and CSF,” he added.
Comparing treatment regimens
The aim of the current study was to compare the effect of autologous hematopoietic stem cell transplantation with other immunotherapies on disability worsening in patients with active secondary progressive MS.
Study endpoints included the Expanded Disability Status Scale (EDSS) score, 6-month worsening and improvement in disability, and sustained disability improvement over time.
The researchers studied patients with secondary progressive MS who had received autologous hematopoietic stem cell transplantation and were included in the Italian Bone Marrow Transplant Group. They were compared with a control group of patients in the Italian MS registry who had started a nontransplant disease-modifying therapy after the diagnosis of secondary progressive MS.
To control for many different variables, two separate analyses were preformed. One analyses was a propensity-score approach (patients were matched based on their propensity to receive bone marrow transplant or one of the other disease-modifying therapies). The other analysis used an overlap weighting approach (each patient was given a weight proportional to the probability of them belonging to the other treatment group).
The final cohort consisted of 79 bone marrow transplant recipients and 1,975 patients who had received other disease-modifying therapies.
Before matching, patients in the control group were older, had a longer disease duration, and had a lower annualized relapse rate than transplanted patients.
After propensity-score matching, there were 69 transplanted patients and 217 control patients who were well balanced in terms of clinical and demographic characteristics. After overlap weighting, the entire cohort was also well balanced for these variables.
In terms of the primary endpoint, stem cell transplant stabilized the EDSS score over time, while patients treated with other disease-modifying therapies had continuous progression of the EDSS score over time.
In the propensity-matched analysis, the EDSS score improved by 0.013 points per year in the stem cell transplant group compared with a worsening of 0.157 points per year in the control group. Similar results were seen in the overlap-weighting analysis.
The effect of stem cell transplant on EDSS score translated into a significantly delayed time to confirmed disability progression in the stem cell transplant group compared with the control group (HR, 0.5; P = .005), Dr. Boffa reported.
Five years after the procedure, 62% of the transplant group were free of disability progression, compared with around 20% of the control group.
Patients in the transplanted group were also more likely to show disability improvement over time. Five years after the procedure, almost 20% of the stem cell transplant group still maintained a disability improvement compared with only 4% of patients treated with other disease-modifying therapies.
“Our study population was composed of relatively young patients (average age 38 years) with clinical activity during secondary progressive MS, and the results of this study would not be applicable to patients with secondary progressive MS patients without signs of inflammatory activity,” Dr. Boffa commented.
“But on the other hand, our results reinforce the notion that ongoing inflammation during progressive MS requires adequate immunotherapy,” he added.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Updated MS guidelines advocate earlier, more aggressive treatment
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2021
DMTs linked to better pediatric MS outcomes
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
FROM ECTRIMS 2021
Motor imagery improves MS
It’s a technique that many think of as the realm of professional athletes, who use it to help mentally prepare for physical activity. But the underlying mechanism has broad applicability, even in patients with MS who have disability.
The method recruits and employs motor-related areas within the brain, which suggests that it has functional equivalence to carrying out the rehearsed movement. The mental chronometry is also similar between imagined and executed actions, said Barbara Seebacher, PhD, who discussed MI during a presentation at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
MI involves mentally rehearsing movements, and so requires working memory. The patient must also have the fundamental ability to carry out the action, so it isn’t much use having nonambulatory patients imagine themselves walking. “The mental representations need to be available,” said Dr. Seebacher, a researcher at Medical University of Innsbruck in Austria.
During MI, individuals may imagine themselves from a first- or third-person view. The experience may be visual, as in picturing oneself moving, or kinesthetic, as in imagining the feeling of movement. There can be implicit components, such as imagining a projection of the speed and distance of an approaching car, and explicit, such as imagining the personal movement of walking across the street.
The use of MI in MS rehabilitation is a relatively new development, with no reports before 2010. One early, uncontrolled study found improvements in fatigue and quality of life when MI was combined with physical practice in 20 patients. Another study published in 2012 found that MS patients had worse MI accuracy and temporal organization than that of healthy controls, and that MI accuracy was associated with cognitive impairment.
A study in 2012 used visual and rhythmic cues combined with MI, and compared results when those cues were present or absent during MI. The cues produced much better results. Dr. Seebacher’s group has used rhythmic, auditory-cued MI of walking in people with MS. Approaches included music cueing, music and verbal cueing, metronome and verbal cueing, and no cue MI. “We found significant effects after all of these approaches, but the greatest effects were shown after music and verbally-cued MI practice,” said Dr. Seebacher.
MI ability appears to be impaired by longer MS disease duration, more severe disability, depression and anxiety, and cognitive fatigue. “All of this contributes to timing deficits in performing mental movements and to deficits in the spatial organization of imagined movements,” said Dr. Seebacher.
In contrast, studies have shown that MI training improves dynamic balance, walking, perceived walking ability, balance, confidence, cognition, fatigue, anxiety and depression, quality of life, and health-related quality of life.
Rehabilitation specialists can help patients achieve success with MI by letting them select their preferred perspective, first or third person, at least during initial sessions. Patients can also be given the choice to use a more dexterous, more often-used body part, at least in initial MI sessions. External rhythmic, audio, or visual cuing can be offered.
Dr. Seebacher has developed an initial framework for helping patients to improve their MI ability. This includes assessing rhythmicity of single imagined movements to help ensure that MI and movements are functionally equivalent. Another step is to incorporate movements that are meaningful to the patients, to help ensure that they are emotionally engaged with the exercise. Research conducted primarily in stroke patients has shown that embedding physical practice into MI, or adding physical movement to MI, can enhance sensory feedback.
Motor learning principles from neurorehabilitation also apply to MI, such as beginning with simple tasks and progressing to more complex tasks, as well as use of blocked practice before turning to random practice. “All this should help our patients to perform MI more easily and to gain a greater benefit,” said Dr. Seebacher.
The potential of MI piqued the interest of comoderator Hanneke Hulst, PhD, assistant professor of neurology at Amsterdam University Medical Center, during the Q&A session. “It’s actually very intriguing and interesting,” she said, and then asked Dr. Seebacher how difficult MI is to implement in a rehabilitation program, especially for someone who isn’t a rehabilitation specialist.
Dr. Seebacher responded that it can be difficult, especially because patients and therapists usually aren’t familiar with MI. “Whenever I explain MI to patients, I compare it with athletes, because everybody knows that athletes use mental training, together with their physical training. And this is something patients can identify themselves with,” said Dr. Seebacher. It’s also vital that the patient has preserved cognitive function, and is open to a new therapeutic approach. “If somebody just wants to act the same way that they did all the time, it may not be useful for these patients,” said Dr. Seebacher.
MI is also useful for patients who have difficulty with physical training, for example following relapses, or for those who are at greater risk of falling.
Dr. Seebacher has no relevant financial disclosures. Dr. Hulst has consulted with or served on the scientific advisory boards of Biogen, Celgene, Genzyme, Merck, and Roche.
It’s a technique that many think of as the realm of professional athletes, who use it to help mentally prepare for physical activity. But the underlying mechanism has broad applicability, even in patients with MS who have disability.
The method recruits and employs motor-related areas within the brain, which suggests that it has functional equivalence to carrying out the rehearsed movement. The mental chronometry is also similar between imagined and executed actions, said Barbara Seebacher, PhD, who discussed MI during a presentation at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
MI involves mentally rehearsing movements, and so requires working memory. The patient must also have the fundamental ability to carry out the action, so it isn’t much use having nonambulatory patients imagine themselves walking. “The mental representations need to be available,” said Dr. Seebacher, a researcher at Medical University of Innsbruck in Austria.
During MI, individuals may imagine themselves from a first- or third-person view. The experience may be visual, as in picturing oneself moving, or kinesthetic, as in imagining the feeling of movement. There can be implicit components, such as imagining a projection of the speed and distance of an approaching car, and explicit, such as imagining the personal movement of walking across the street.
The use of MI in MS rehabilitation is a relatively new development, with no reports before 2010. One early, uncontrolled study found improvements in fatigue and quality of life when MI was combined with physical practice in 20 patients. Another study published in 2012 found that MS patients had worse MI accuracy and temporal organization than that of healthy controls, and that MI accuracy was associated with cognitive impairment.
A study in 2012 used visual and rhythmic cues combined with MI, and compared results when those cues were present or absent during MI. The cues produced much better results. Dr. Seebacher’s group has used rhythmic, auditory-cued MI of walking in people with MS. Approaches included music cueing, music and verbal cueing, metronome and verbal cueing, and no cue MI. “We found significant effects after all of these approaches, but the greatest effects were shown after music and verbally-cued MI practice,” said Dr. Seebacher.
MI ability appears to be impaired by longer MS disease duration, more severe disability, depression and anxiety, and cognitive fatigue. “All of this contributes to timing deficits in performing mental movements and to deficits in the spatial organization of imagined movements,” said Dr. Seebacher.
In contrast, studies have shown that MI training improves dynamic balance, walking, perceived walking ability, balance, confidence, cognition, fatigue, anxiety and depression, quality of life, and health-related quality of life.
Rehabilitation specialists can help patients achieve success with MI by letting them select their preferred perspective, first or third person, at least during initial sessions. Patients can also be given the choice to use a more dexterous, more often-used body part, at least in initial MI sessions. External rhythmic, audio, or visual cuing can be offered.
Dr. Seebacher has developed an initial framework for helping patients to improve their MI ability. This includes assessing rhythmicity of single imagined movements to help ensure that MI and movements are functionally equivalent. Another step is to incorporate movements that are meaningful to the patients, to help ensure that they are emotionally engaged with the exercise. Research conducted primarily in stroke patients has shown that embedding physical practice into MI, or adding physical movement to MI, can enhance sensory feedback.
Motor learning principles from neurorehabilitation also apply to MI, such as beginning with simple tasks and progressing to more complex tasks, as well as use of blocked practice before turning to random practice. “All this should help our patients to perform MI more easily and to gain a greater benefit,” said Dr. Seebacher.
The potential of MI piqued the interest of comoderator Hanneke Hulst, PhD, assistant professor of neurology at Amsterdam University Medical Center, during the Q&A session. “It’s actually very intriguing and interesting,” she said, and then asked Dr. Seebacher how difficult MI is to implement in a rehabilitation program, especially for someone who isn’t a rehabilitation specialist.
Dr. Seebacher responded that it can be difficult, especially because patients and therapists usually aren’t familiar with MI. “Whenever I explain MI to patients, I compare it with athletes, because everybody knows that athletes use mental training, together with their physical training. And this is something patients can identify themselves with,” said Dr. Seebacher. It’s also vital that the patient has preserved cognitive function, and is open to a new therapeutic approach. “If somebody just wants to act the same way that they did all the time, it may not be useful for these patients,” said Dr. Seebacher.
MI is also useful for patients who have difficulty with physical training, for example following relapses, or for those who are at greater risk of falling.
Dr. Seebacher has no relevant financial disclosures. Dr. Hulst has consulted with or served on the scientific advisory boards of Biogen, Celgene, Genzyme, Merck, and Roche.
It’s a technique that many think of as the realm of professional athletes, who use it to help mentally prepare for physical activity. But the underlying mechanism has broad applicability, even in patients with MS who have disability.
The method recruits and employs motor-related areas within the brain, which suggests that it has functional equivalence to carrying out the rehearsed movement. The mental chronometry is also similar between imagined and executed actions, said Barbara Seebacher, PhD, who discussed MI during a presentation at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
MI involves mentally rehearsing movements, and so requires working memory. The patient must also have the fundamental ability to carry out the action, so it isn’t much use having nonambulatory patients imagine themselves walking. “The mental representations need to be available,” said Dr. Seebacher, a researcher at Medical University of Innsbruck in Austria.
During MI, individuals may imagine themselves from a first- or third-person view. The experience may be visual, as in picturing oneself moving, or kinesthetic, as in imagining the feeling of movement. There can be implicit components, such as imagining a projection of the speed and distance of an approaching car, and explicit, such as imagining the personal movement of walking across the street.
The use of MI in MS rehabilitation is a relatively new development, with no reports before 2010. One early, uncontrolled study found improvements in fatigue and quality of life when MI was combined with physical practice in 20 patients. Another study published in 2012 found that MS patients had worse MI accuracy and temporal organization than that of healthy controls, and that MI accuracy was associated with cognitive impairment.
A study in 2012 used visual and rhythmic cues combined with MI, and compared results when those cues were present or absent during MI. The cues produced much better results. Dr. Seebacher’s group has used rhythmic, auditory-cued MI of walking in people with MS. Approaches included music cueing, music and verbal cueing, metronome and verbal cueing, and no cue MI. “We found significant effects after all of these approaches, but the greatest effects were shown after music and verbally-cued MI practice,” said Dr. Seebacher.
MI ability appears to be impaired by longer MS disease duration, more severe disability, depression and anxiety, and cognitive fatigue. “All of this contributes to timing deficits in performing mental movements and to deficits in the spatial organization of imagined movements,” said Dr. Seebacher.
In contrast, studies have shown that MI training improves dynamic balance, walking, perceived walking ability, balance, confidence, cognition, fatigue, anxiety and depression, quality of life, and health-related quality of life.
Rehabilitation specialists can help patients achieve success with MI by letting them select their preferred perspective, first or third person, at least during initial sessions. Patients can also be given the choice to use a more dexterous, more often-used body part, at least in initial MI sessions. External rhythmic, audio, or visual cuing can be offered.
Dr. Seebacher has developed an initial framework for helping patients to improve their MI ability. This includes assessing rhythmicity of single imagined movements to help ensure that MI and movements are functionally equivalent. Another step is to incorporate movements that are meaningful to the patients, to help ensure that they are emotionally engaged with the exercise. Research conducted primarily in stroke patients has shown that embedding physical practice into MI, or adding physical movement to MI, can enhance sensory feedback.
Motor learning principles from neurorehabilitation also apply to MI, such as beginning with simple tasks and progressing to more complex tasks, as well as use of blocked practice before turning to random practice. “All this should help our patients to perform MI more easily and to gain a greater benefit,” said Dr. Seebacher.
The potential of MI piqued the interest of comoderator Hanneke Hulst, PhD, assistant professor of neurology at Amsterdam University Medical Center, during the Q&A session. “It’s actually very intriguing and interesting,” she said, and then asked Dr. Seebacher how difficult MI is to implement in a rehabilitation program, especially for someone who isn’t a rehabilitation specialist.
Dr. Seebacher responded that it can be difficult, especially because patients and therapists usually aren’t familiar with MI. “Whenever I explain MI to patients, I compare it with athletes, because everybody knows that athletes use mental training, together with their physical training. And this is something patients can identify themselves with,” said Dr. Seebacher. It’s also vital that the patient has preserved cognitive function, and is open to a new therapeutic approach. “If somebody just wants to act the same way that they did all the time, it may not be useful for these patients,” said Dr. Seebacher.
MI is also useful for patients who have difficulty with physical training, for example following relapses, or for those who are at greater risk of falling.
Dr. Seebacher has no relevant financial disclosures. Dr. Hulst has consulted with or served on the scientific advisory boards of Biogen, Celgene, Genzyme, Merck, and Roche.
FROM ECTRIMS 2021
Exercise may help stall MS disability and progression
Though once regarded with suspicion,
That was the key message of a talk given by Ulrik Dalgas, PhD, professor of exercise biology at Aarhus University in Denmark, who spoke at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Rethinking the role of exercise
It used to be thought that exercise could worsen disease, but case studies in the 1960s suggested some beneficial effects. The first interventional studies were published in the 1990s. A 2008 special issue of Multiple Sclerosis Journal declared exercise safe for people with MS. Research has continued to evolve, “and now we are actually at a stage where some of us have started to believe that exercise is a medicine in multiple sclerosis,” said Dr. Dalgas, who outlined that view in a 2019 review paper.
In the early phase of MS, before the onset of a significant decline in brain volume or increase in disability, there are already measurable physical deficits. Dr. Dalgas showed data from an unpublished study from his group, which looked at 48 patients who had been diagnosed in the previous 2 years, at an average 10 months after diagnosis. “Already at this very early stage of the disease, we can actually observe impairments or deficits in walking, different walking outcome measures here between 10 and up to more than 30%, depending on the walking outcome,” said Dr. Dalgas. Similar deficits appeared in physical activity and maximum rate of oxygen consumption (VO2 max).
Animal studies suggest that exercise could improve matters at this stage. In a model of experimental autoimmune encephalomyelitis, animals allowed the opportunity to exercise had lower levels of clinical disability throughout the model disease course. That has led some to examine a “prehabilitation” approach to early MS – a term borrowed from orthopedics. “We try to prevent rather than to treat symptoms, or build reserve capacity rather than restore capacity,” said Dr. Dalgas.
Some work in this area has been done in human patients, but a review found that none of more than 70 published studies looked at patients within 5 years of onset. “That left kind of an unstudied early phase,” said Dr. Dalgas.
In the mid-phase of MS, when brain volume loss increases and mobility and other problems increase, exercise has proved to counteract some of these issues. “What we are now trying to do is figure out what are the best exercise modalities for treating different symptoms,” said Dr. Dalgas.
Resistance and aerobic training have predictable, positive effects on strength and VO2 max, but one study showed that the two modes of exercise had similar positive impacts on short and long walks, as well as fatigue, despite the fact that they have very different physiological effects.
Other studies have looked at the impact of exercise on the diseases itself. One recent study examined aerobic exercise versus a wait-list group. Gray matter volume remained stable in the exercise group, but dropped in the wait-list group, suggesting a possible protective effect .
In the later, more severe phase of MS, more specialized equipment is needed to ensure safety during exercise. A pilot study by Dr. Dalgas’ group in individuals with Expanded Disability Status Scores (EDSS) scores between 6.5 and 8 found that upper body exercise improved VO2 peak score in five out of six patients. “Even at this later stage of the disease, it seems that people can still have important improvements in health and performance markers,” said Dr. Dalgas.
A review of numerous studies found that exercise had a positive effect on quality of life, and the gains were not affected by baseline disability, disease duration, or exercise type. The study shows that “it’s never too late to improve your life through exercise,” said Dr. Dalgas.
Next steps
Challenges remain for the field. “We still need to figure out how long-term adherence is best secured in these patients, and then we really need to look further into how to provide exercise in the best possible way in severe and elderly patients,” said Dr. Dalgas.
During the Q&A session following the presentation, Dr. Dalgas was asked for advice on how to get a patient with MS started with exercise. “We normally recommend that people should find a physical therapist or sports scientist who has expertise in this field to help with getting started. If you start out wrong you can get into problems, so having the right expertise at hand is a good way to start. Then shortly afterward they will be more independent to do the exercise,” said Dr. Dalgas.
Alan Thompson, MD, who moderated the session, brought up the concept of cognitive reserve in MS, which posits that positive life experience builds up the capacity and efficiency of neural networks, which in turn act as a sort of buffer against later cognitive decline due to aging and illness. “Can you build up your exercises in a way that has a meaningful impact in delaying the onset of confirmed disability or progression?” asked Dr. Thompson, professor of clinical neurology and neurorehabilitation at University College London.
Dr. Dalgas said that there are studies that suggest this may be true, with MS diagnoses occurring later in patients who are physically active. “You can interpret that as some kind of delayed onset of the disease.”
For more information, Dr. Dalgas suggested recently published recommendations for exercise in MS patients.
Dr. Dalgas disclosed ties with Biogen Idec, Merck Serono, Sanofi Aventis, Almirall, Novartis, Bayer Schering, and Sanofi Genzyme. Dr. Thompson has no relevant financial disclosures.
Though once regarded with suspicion,
That was the key message of a talk given by Ulrik Dalgas, PhD, professor of exercise biology at Aarhus University in Denmark, who spoke at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Rethinking the role of exercise
It used to be thought that exercise could worsen disease, but case studies in the 1960s suggested some beneficial effects. The first interventional studies were published in the 1990s. A 2008 special issue of Multiple Sclerosis Journal declared exercise safe for people with MS. Research has continued to evolve, “and now we are actually at a stage where some of us have started to believe that exercise is a medicine in multiple sclerosis,” said Dr. Dalgas, who outlined that view in a 2019 review paper.
In the early phase of MS, before the onset of a significant decline in brain volume or increase in disability, there are already measurable physical deficits. Dr. Dalgas showed data from an unpublished study from his group, which looked at 48 patients who had been diagnosed in the previous 2 years, at an average 10 months after diagnosis. “Already at this very early stage of the disease, we can actually observe impairments or deficits in walking, different walking outcome measures here between 10 and up to more than 30%, depending on the walking outcome,” said Dr. Dalgas. Similar deficits appeared in physical activity and maximum rate of oxygen consumption (VO2 max).
Animal studies suggest that exercise could improve matters at this stage. In a model of experimental autoimmune encephalomyelitis, animals allowed the opportunity to exercise had lower levels of clinical disability throughout the model disease course. That has led some to examine a “prehabilitation” approach to early MS – a term borrowed from orthopedics. “We try to prevent rather than to treat symptoms, or build reserve capacity rather than restore capacity,” said Dr. Dalgas.
Some work in this area has been done in human patients, but a review found that none of more than 70 published studies looked at patients within 5 years of onset. “That left kind of an unstudied early phase,” said Dr. Dalgas.
In the mid-phase of MS, when brain volume loss increases and mobility and other problems increase, exercise has proved to counteract some of these issues. “What we are now trying to do is figure out what are the best exercise modalities for treating different symptoms,” said Dr. Dalgas.
Resistance and aerobic training have predictable, positive effects on strength and VO2 max, but one study showed that the two modes of exercise had similar positive impacts on short and long walks, as well as fatigue, despite the fact that they have very different physiological effects.
Other studies have looked at the impact of exercise on the diseases itself. One recent study examined aerobic exercise versus a wait-list group. Gray matter volume remained stable in the exercise group, but dropped in the wait-list group, suggesting a possible protective effect .
In the later, more severe phase of MS, more specialized equipment is needed to ensure safety during exercise. A pilot study by Dr. Dalgas’ group in individuals with Expanded Disability Status Scores (EDSS) scores between 6.5 and 8 found that upper body exercise improved VO2 peak score in five out of six patients. “Even at this later stage of the disease, it seems that people can still have important improvements in health and performance markers,” said Dr. Dalgas.
A review of numerous studies found that exercise had a positive effect on quality of life, and the gains were not affected by baseline disability, disease duration, or exercise type. The study shows that “it’s never too late to improve your life through exercise,” said Dr. Dalgas.
Next steps
Challenges remain for the field. “We still need to figure out how long-term adherence is best secured in these patients, and then we really need to look further into how to provide exercise in the best possible way in severe and elderly patients,” said Dr. Dalgas.
During the Q&A session following the presentation, Dr. Dalgas was asked for advice on how to get a patient with MS started with exercise. “We normally recommend that people should find a physical therapist or sports scientist who has expertise in this field to help with getting started. If you start out wrong you can get into problems, so having the right expertise at hand is a good way to start. Then shortly afterward they will be more independent to do the exercise,” said Dr. Dalgas.
Alan Thompson, MD, who moderated the session, brought up the concept of cognitive reserve in MS, which posits that positive life experience builds up the capacity and efficiency of neural networks, which in turn act as a sort of buffer against later cognitive decline due to aging and illness. “Can you build up your exercises in a way that has a meaningful impact in delaying the onset of confirmed disability or progression?” asked Dr. Thompson, professor of clinical neurology and neurorehabilitation at University College London.
Dr. Dalgas said that there are studies that suggest this may be true, with MS diagnoses occurring later in patients who are physically active. “You can interpret that as some kind of delayed onset of the disease.”
For more information, Dr. Dalgas suggested recently published recommendations for exercise in MS patients.
Dr. Dalgas disclosed ties with Biogen Idec, Merck Serono, Sanofi Aventis, Almirall, Novartis, Bayer Schering, and Sanofi Genzyme. Dr. Thompson has no relevant financial disclosures.
Though once regarded with suspicion,
That was the key message of a talk given by Ulrik Dalgas, PhD, professor of exercise biology at Aarhus University in Denmark, who spoke at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Rethinking the role of exercise
It used to be thought that exercise could worsen disease, but case studies in the 1960s suggested some beneficial effects. The first interventional studies were published in the 1990s. A 2008 special issue of Multiple Sclerosis Journal declared exercise safe for people with MS. Research has continued to evolve, “and now we are actually at a stage where some of us have started to believe that exercise is a medicine in multiple sclerosis,” said Dr. Dalgas, who outlined that view in a 2019 review paper.
In the early phase of MS, before the onset of a significant decline in brain volume or increase in disability, there are already measurable physical deficits. Dr. Dalgas showed data from an unpublished study from his group, which looked at 48 patients who had been diagnosed in the previous 2 years, at an average 10 months after diagnosis. “Already at this very early stage of the disease, we can actually observe impairments or deficits in walking, different walking outcome measures here between 10 and up to more than 30%, depending on the walking outcome,” said Dr. Dalgas. Similar deficits appeared in physical activity and maximum rate of oxygen consumption (VO2 max).
Animal studies suggest that exercise could improve matters at this stage. In a model of experimental autoimmune encephalomyelitis, animals allowed the opportunity to exercise had lower levels of clinical disability throughout the model disease course. That has led some to examine a “prehabilitation” approach to early MS – a term borrowed from orthopedics. “We try to prevent rather than to treat symptoms, or build reserve capacity rather than restore capacity,” said Dr. Dalgas.
Some work in this area has been done in human patients, but a review found that none of more than 70 published studies looked at patients within 5 years of onset. “That left kind of an unstudied early phase,” said Dr. Dalgas.
In the mid-phase of MS, when brain volume loss increases and mobility and other problems increase, exercise has proved to counteract some of these issues. “What we are now trying to do is figure out what are the best exercise modalities for treating different symptoms,” said Dr. Dalgas.
Resistance and aerobic training have predictable, positive effects on strength and VO2 max, but one study showed that the two modes of exercise had similar positive impacts on short and long walks, as well as fatigue, despite the fact that they have very different physiological effects.
Other studies have looked at the impact of exercise on the diseases itself. One recent study examined aerobic exercise versus a wait-list group. Gray matter volume remained stable in the exercise group, but dropped in the wait-list group, suggesting a possible protective effect .
In the later, more severe phase of MS, more specialized equipment is needed to ensure safety during exercise. A pilot study by Dr. Dalgas’ group in individuals with Expanded Disability Status Scores (EDSS) scores between 6.5 and 8 found that upper body exercise improved VO2 peak score in five out of six patients. “Even at this later stage of the disease, it seems that people can still have important improvements in health and performance markers,” said Dr. Dalgas.
A review of numerous studies found that exercise had a positive effect on quality of life, and the gains were not affected by baseline disability, disease duration, or exercise type. The study shows that “it’s never too late to improve your life through exercise,” said Dr. Dalgas.
Next steps
Challenges remain for the field. “We still need to figure out how long-term adherence is best secured in these patients, and then we really need to look further into how to provide exercise in the best possible way in severe and elderly patients,” said Dr. Dalgas.
During the Q&A session following the presentation, Dr. Dalgas was asked for advice on how to get a patient with MS started with exercise. “We normally recommend that people should find a physical therapist or sports scientist who has expertise in this field to help with getting started. If you start out wrong you can get into problems, so having the right expertise at hand is a good way to start. Then shortly afterward they will be more independent to do the exercise,” said Dr. Dalgas.
Alan Thompson, MD, who moderated the session, brought up the concept of cognitive reserve in MS, which posits that positive life experience builds up the capacity and efficiency of neural networks, which in turn act as a sort of buffer against later cognitive decline due to aging and illness. “Can you build up your exercises in a way that has a meaningful impact in delaying the onset of confirmed disability or progression?” asked Dr. Thompson, professor of clinical neurology and neurorehabilitation at University College London.
Dr. Dalgas said that there are studies that suggest this may be true, with MS diagnoses occurring later in patients who are physically active. “You can interpret that as some kind of delayed onset of the disease.”
For more information, Dr. Dalgas suggested recently published recommendations for exercise in MS patients.
Dr. Dalgas disclosed ties with Biogen Idec, Merck Serono, Sanofi Aventis, Almirall, Novartis, Bayer Schering, and Sanofi Genzyme. Dr. Thompson has no relevant financial disclosures.
FROM ECTRIMS 2021
MS and (non-COVID) vaccinations: consensus recommendations
by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN).
The document, announced at the annual ECTRIMS meeting, proposes a standard for vaccination in patients with MS, including a global vaccination strategy for the general MS patient population and selected subpopulations.
The document does not include any recommendations regarding vaccination against COVID-19, which is the subject of a separate report, announced at the annual meeting.
The main conclusions in the new report are as follows:
- Vaccinations in general are considered safe for patients with MS and do not modify disease activity/progression.
- Live attenuated vaccines, however, are contraindicated with immunosuppressants.
- Inactivated vaccines can be used safely, but their efficacy may be decreased with immunosuppressants.
- Vaccinations should be considered early in MS management before using immunosuppressants whenever possible.
Presenting the vaccination consensus document, Susana Otero-Romero, MD, from the Multiple Sclerosis Center of Catalonia, Spain, explained that vaccination has become an important part of the risk management strategy in patients with MS treated with highly active drugs but that questions remain as to when and whether to introduce a particular vaccine and which disease-modifying treatments affect vaccine response.
“The current reference tool has been developed to help professionals to decide on the best vaccination strategy for their patients,” she said.
The consensus document recommends that, in general, vaccination should be performed at the time of diagnosis of MS or in the early stages of the disease to prevent future delays in starting therapies.
“Ideally, vaccination should take place before the onset of disease-modifying treatment,” Dr. Otero-Romero said. The consensus document recommends inactivated vaccines to be given 2-3 weeks before immunosuppressive therapy is started, and live attenuated vaccines at least 4 weeks beforehand.
In the case of relapse, vaccination should be delayed until clinical resolution or stabilization if possible, the consensus statement recommends.
Serological testing for vaccine-induced antibody titers can be performed 1-2 months after the last dose of the vaccine (suggested for hepatitis B, measles, mumps, and varicella). For attenuated live vaccines, serological tests should be done before starting immunosuppressive therapy. In the case of insufficient response, consideration should be given to administering a booster dose of the vaccine, except for hepatitis B, in which a complete revaccination is recommended, according to the document.
As for vaccination during immunosuppressive therapy, this is considered safe for patients on interferon or glatiramer acetate when indicated, the report says.
Vaccination should ideally be avoided in patients on dimethyl fumarate, teriflunomide (Aubagio) or natalizumab (Tysabri), although it can be considered in exceptional cases when the potential risk of acquiring the infection is greater than the risk of developing vaccine-related infections (unless the absolute lymphocyte count is below 800/mm3), it adds.
Vaccination should be avoided in patients on S1P modulators (for example, fingolimod [Gilenya]), anti-CD20 therapies, and before immune restoration for cladribine (Leustatin) and alemtuzumab (Lemtrada).
In the case of patients stopping immunosuppressive therapy, inactivated vaccines can be given any time after the discontinuation of therapy but preferably after immune restoration. Live attenuated vaccines should only be administered after a safety interval ensures immune restoration has been met.
Which vaccines?
On which vaccines are needed in patients with MS, the consensus document recommends the same routine vaccination schedule as for the general population. In addition, it advises influenza and pneumococcal vaccination if patients are immunosuppressed or have significant disability.
It also recommends human papillomavirus vaccine in women and men independent of their age if they are to be treated with alemtuzumab, fingolimod, cladribine, or anti-CD20 drugs. Hepatitis B vaccination is also advised in patients treated with anti-CD20 drugs.
Special populations: pregnancy/elderly
In patients with MS who are pregnant, inactivated flu vaccine can be given in any trimester at the start of the flu season, and vaccination against diphtheria, tetanus, and pertussis can be given during the third trimester, the report says. Live attenuated vaccines should be completed at least 1 month before pregnancy or after delivery and 4-6 weeks prior to the initiation of immunosuppressive therapy.
Elderly patients with MS should receive flu and pneumococcal vaccines annually and would also benefit from the inactivated herpes zoster vaccine.
Travel vaccines
On vaccinations needed for travel, the report recommends that patients with MS consult a specialized travel clinic or vaccination expert and start immunizations 2-3 months before departure. Patients with MS with or without immunosuppressive therapy can receive hepatitis A, rabies, Japanese encephalitis, tic-borne encephalitis, polio, and inactivated typhoid vaccine. But yellow fever and oral typhoid are contraindicated in patients on immunosuppressive therapies.
A version of this article first appeared on Medscape.com.
by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN).
The document, announced at the annual ECTRIMS meeting, proposes a standard for vaccination in patients with MS, including a global vaccination strategy for the general MS patient population and selected subpopulations.
The document does not include any recommendations regarding vaccination against COVID-19, which is the subject of a separate report, announced at the annual meeting.
The main conclusions in the new report are as follows:
- Vaccinations in general are considered safe for patients with MS and do not modify disease activity/progression.
- Live attenuated vaccines, however, are contraindicated with immunosuppressants.
- Inactivated vaccines can be used safely, but their efficacy may be decreased with immunosuppressants.
- Vaccinations should be considered early in MS management before using immunosuppressants whenever possible.
Presenting the vaccination consensus document, Susana Otero-Romero, MD, from the Multiple Sclerosis Center of Catalonia, Spain, explained that vaccination has become an important part of the risk management strategy in patients with MS treated with highly active drugs but that questions remain as to when and whether to introduce a particular vaccine and which disease-modifying treatments affect vaccine response.
“The current reference tool has been developed to help professionals to decide on the best vaccination strategy for their patients,” she said.
The consensus document recommends that, in general, vaccination should be performed at the time of diagnosis of MS or in the early stages of the disease to prevent future delays in starting therapies.
“Ideally, vaccination should take place before the onset of disease-modifying treatment,” Dr. Otero-Romero said. The consensus document recommends inactivated vaccines to be given 2-3 weeks before immunosuppressive therapy is started, and live attenuated vaccines at least 4 weeks beforehand.
In the case of relapse, vaccination should be delayed until clinical resolution or stabilization if possible, the consensus statement recommends.
Serological testing for vaccine-induced antibody titers can be performed 1-2 months after the last dose of the vaccine (suggested for hepatitis B, measles, mumps, and varicella). For attenuated live vaccines, serological tests should be done before starting immunosuppressive therapy. In the case of insufficient response, consideration should be given to administering a booster dose of the vaccine, except for hepatitis B, in which a complete revaccination is recommended, according to the document.
As for vaccination during immunosuppressive therapy, this is considered safe for patients on interferon or glatiramer acetate when indicated, the report says.
Vaccination should ideally be avoided in patients on dimethyl fumarate, teriflunomide (Aubagio) or natalizumab (Tysabri), although it can be considered in exceptional cases when the potential risk of acquiring the infection is greater than the risk of developing vaccine-related infections (unless the absolute lymphocyte count is below 800/mm3), it adds.
Vaccination should be avoided in patients on S1P modulators (for example, fingolimod [Gilenya]), anti-CD20 therapies, and before immune restoration for cladribine (Leustatin) and alemtuzumab (Lemtrada).
In the case of patients stopping immunosuppressive therapy, inactivated vaccines can be given any time after the discontinuation of therapy but preferably after immune restoration. Live attenuated vaccines should only be administered after a safety interval ensures immune restoration has been met.
Which vaccines?
On which vaccines are needed in patients with MS, the consensus document recommends the same routine vaccination schedule as for the general population. In addition, it advises influenza and pneumococcal vaccination if patients are immunosuppressed or have significant disability.
It also recommends human papillomavirus vaccine in women and men independent of their age if they are to be treated with alemtuzumab, fingolimod, cladribine, or anti-CD20 drugs. Hepatitis B vaccination is also advised in patients treated with anti-CD20 drugs.
Special populations: pregnancy/elderly
In patients with MS who are pregnant, inactivated flu vaccine can be given in any trimester at the start of the flu season, and vaccination against diphtheria, tetanus, and pertussis can be given during the third trimester, the report says. Live attenuated vaccines should be completed at least 1 month before pregnancy or after delivery and 4-6 weeks prior to the initiation of immunosuppressive therapy.
Elderly patients with MS should receive flu and pneumococcal vaccines annually and would also benefit from the inactivated herpes zoster vaccine.
Travel vaccines
On vaccinations needed for travel, the report recommends that patients with MS consult a specialized travel clinic or vaccination expert and start immunizations 2-3 months before departure. Patients with MS with or without immunosuppressive therapy can receive hepatitis A, rabies, Japanese encephalitis, tic-borne encephalitis, polio, and inactivated typhoid vaccine. But yellow fever and oral typhoid are contraindicated in patients on immunosuppressive therapies.
A version of this article first appeared on Medscape.com.
by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN).
The document, announced at the annual ECTRIMS meeting, proposes a standard for vaccination in patients with MS, including a global vaccination strategy for the general MS patient population and selected subpopulations.
The document does not include any recommendations regarding vaccination against COVID-19, which is the subject of a separate report, announced at the annual meeting.
The main conclusions in the new report are as follows:
- Vaccinations in general are considered safe for patients with MS and do not modify disease activity/progression.
- Live attenuated vaccines, however, are contraindicated with immunosuppressants.
- Inactivated vaccines can be used safely, but their efficacy may be decreased with immunosuppressants.
- Vaccinations should be considered early in MS management before using immunosuppressants whenever possible.
Presenting the vaccination consensus document, Susana Otero-Romero, MD, from the Multiple Sclerosis Center of Catalonia, Spain, explained that vaccination has become an important part of the risk management strategy in patients with MS treated with highly active drugs but that questions remain as to when and whether to introduce a particular vaccine and which disease-modifying treatments affect vaccine response.
“The current reference tool has been developed to help professionals to decide on the best vaccination strategy for their patients,” she said.
The consensus document recommends that, in general, vaccination should be performed at the time of diagnosis of MS or in the early stages of the disease to prevent future delays in starting therapies.
“Ideally, vaccination should take place before the onset of disease-modifying treatment,” Dr. Otero-Romero said. The consensus document recommends inactivated vaccines to be given 2-3 weeks before immunosuppressive therapy is started, and live attenuated vaccines at least 4 weeks beforehand.
In the case of relapse, vaccination should be delayed until clinical resolution or stabilization if possible, the consensus statement recommends.
Serological testing for vaccine-induced antibody titers can be performed 1-2 months after the last dose of the vaccine (suggested for hepatitis B, measles, mumps, and varicella). For attenuated live vaccines, serological tests should be done before starting immunosuppressive therapy. In the case of insufficient response, consideration should be given to administering a booster dose of the vaccine, except for hepatitis B, in which a complete revaccination is recommended, according to the document.
As for vaccination during immunosuppressive therapy, this is considered safe for patients on interferon or glatiramer acetate when indicated, the report says.
Vaccination should ideally be avoided in patients on dimethyl fumarate, teriflunomide (Aubagio) or natalizumab (Tysabri), although it can be considered in exceptional cases when the potential risk of acquiring the infection is greater than the risk of developing vaccine-related infections (unless the absolute lymphocyte count is below 800/mm3), it adds.
Vaccination should be avoided in patients on S1P modulators (for example, fingolimod [Gilenya]), anti-CD20 therapies, and before immune restoration for cladribine (Leustatin) and alemtuzumab (Lemtrada).
In the case of patients stopping immunosuppressive therapy, inactivated vaccines can be given any time after the discontinuation of therapy but preferably after immune restoration. Live attenuated vaccines should only be administered after a safety interval ensures immune restoration has been met.
Which vaccines?
On which vaccines are needed in patients with MS, the consensus document recommends the same routine vaccination schedule as for the general population. In addition, it advises influenza and pneumococcal vaccination if patients are immunosuppressed or have significant disability.
It also recommends human papillomavirus vaccine in women and men independent of their age if they are to be treated with alemtuzumab, fingolimod, cladribine, or anti-CD20 drugs. Hepatitis B vaccination is also advised in patients treated with anti-CD20 drugs.
Special populations: pregnancy/elderly
In patients with MS who are pregnant, inactivated flu vaccine can be given in any trimester at the start of the flu season, and vaccination against diphtheria, tetanus, and pertussis can be given during the third trimester, the report says. Live attenuated vaccines should be completed at least 1 month before pregnancy or after delivery and 4-6 weeks prior to the initiation of immunosuppressive therapy.
Elderly patients with MS should receive flu and pneumococcal vaccines annually and would also benefit from the inactivated herpes zoster vaccine.
Travel vaccines
On vaccinations needed for travel, the report recommends that patients with MS consult a specialized travel clinic or vaccination expert and start immunizations 2-3 months before departure. Patients with MS with or without immunosuppressive therapy can receive hepatitis A, rabies, Japanese encephalitis, tic-borne encephalitis, polio, and inactivated typhoid vaccine. But yellow fever and oral typhoid are contraindicated in patients on immunosuppressive therapies.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2021
Rituximab more effective than other MS treatments?
, according to new research.
The risk for a first relapse was 6 times higher in patients receiving interferon beta or glatiramer acetate, compared with those receiving rituximab. But the level of disability at 3 years was only marginally different between the drugs studied.
The small differences in Expanded Disability Status Scale (EDSS) score are surprising, said investigator Peter Alping, a clinical assistant and doctoral student in the Department of Clinical Neuroscience at the Karolinska Institutet, Stockholm, as he presented the data. “It could be that we have too-short follow-up, so that EDSS doesn’t have time to diverge between therapies.”
He presented the findings at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
COMBAT-MS study
Direct comparisons of disease-modifying therapies (DMTs) for MS can help neurologists choose the most appropriate treatment for a given patient. To compare the effectiveness of the most common initial DMTs administered in Sweden, the researchers examined data from the COMBAT-MS study.
They identified all patients who initiated an injectable therapy (interferon beta or glatiramer acetate), dimethyl fumarate, natalizumab, or rituximab as a first treatment between Jan. 1, 2011, and Dec. 14, 2020. Eligible participants had prospectively recorded outcome data in the Swedish MS Register. Follow-up for a participant continued even if the participant stopped receiving therapy.
The investigators replaced missing data using multiple imputation. They adjusted for potential confounders using stabilized inverse probability of treatment weighting with baseline variables. These variables included age, sex, disease duration, geographical region, EDSS score, and relapses.
Rituximab reduced relapses
The researchers included 1,938 first-ever treatment episodes in their analysis. Of this group, 858 were associated with injectables, 339 with dimethyl fumarate, 269 with natalizumab, and 472 with rituximab.
Participants’ baseline characteristics differed by the DMT that they used. Patients who initiated natalizumab were the youngest, had the shortest disease duration, and had the most previous relapses.
For each outcome, the investigators compared all other therapies with rituximab. After they adjusted the data, they found that the hazard ratio for first relapse was 6.0 for injectables, 2.9 for dimethyl fumarate, and 1.8 for natalizumab.
In the adjusted model, the MRI lesion rate ratio for injectables, compared with rituximab, was 4.5. The rate ratio was 4.8 for dimethyl fumarate and 1.9 for natalizumab.
But differences in EDSS score at 3 years from treatment initiation were small. EDSS score in patients who received injectables was 0.24 points higher, compared with those receiving rituximab. EDSS score was 0.05 points higher in patients receiving dimethyl fumarate and 0.01 points lower in patients receiving natalizumab.
The risk for treatment discontinuation, however, differed significantly between therapies. The HR for treatment discontinuation was 32.7 for injectables, 20.3 for dimethyl fumarate, and 16.3 for natalizumab, compared with rituximab.
Among patients receiving dimethyl fumarate and injectables, the main reasons for discontinuing therapy were inadequate effect and adverse events. The main reason for discontinuation among patients receiving natalizumab was categorized as “other reason,” which mostly reflected John Cunningham virus positivity and concern for developing progressive multifocal leukoencephalopathy.
‘The uncertainty continues’
“These differences that we see in the effectiveness can be somewhat surprising, especially when it comes to natalizumab,” which is considered very effective, said Mr. Alping. The vulnerable period that occurs after switching from natalizumab may partly explain the difference. “This is something to keep in mind when starting patients on natalizumab treatment in the clinic,” Mr. Alping added.
Although rituximab is not indicated for MS, many clinics are using it in this population, said Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research at the Neurological Institute of Cleveland Clinic, both in Cleveland, Ohio. Dr. Fox was not involved in the study.
“Assessing the generalizability of the study outside Sweden will be important,” he added, “but I would be surprised if their findings did not hold up to external validation.”
The way that the researchers addressed missing data could affect the interpretation of the findings. “Depending upon how much data was missing, their imputation methods may have a high level of uncertainty,” said Dr. Fox.
The researchers’ adjustments for baseline differences also raise questions. “Even though MRI was an outcome, it doesn’t appear they adjusted for baseline differences in MRI between the groups,” Dr. Fox observed.
Moreover, the study was conducted over a long period of time. “We know there are time effects in MS, with a very different disease activity expected from patients over time,” said Dr. Fox. For example, relapse rates in placebo groups of MS trials tend to decline over time. “This time effect likely impacted their results.”
But the disability findings may be the most important part of the study, according to Dr. Fox. The lack of significant difference in disability progression between therapies “highlights that a couple relapses or lesions on MRI may be too small to translate into long-term differences in disability progression,” he said.
“The long-term implications of small differences in relapse and MRI outcomes may be very small,” Dr. Fox went on. “Thus, the uncertainty continues around escalation treatment versus initial highly effective treatment paradigms.”
The Patient-Centered Outcomes Research Institute, the Swedish Research Council, and NEURO Sweden funded this study. Mr. Alping disclosed no relevant financial relationships. Dr. Fox receives consulting fees from the companies that manufacture all the therapies analyzed in the study.
A version of this article first appeared on Medscape.com.
, according to new research.
The risk for a first relapse was 6 times higher in patients receiving interferon beta or glatiramer acetate, compared with those receiving rituximab. But the level of disability at 3 years was only marginally different between the drugs studied.
The small differences in Expanded Disability Status Scale (EDSS) score are surprising, said investigator Peter Alping, a clinical assistant and doctoral student in the Department of Clinical Neuroscience at the Karolinska Institutet, Stockholm, as he presented the data. “It could be that we have too-short follow-up, so that EDSS doesn’t have time to diverge between therapies.”
He presented the findings at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
COMBAT-MS study
Direct comparisons of disease-modifying therapies (DMTs) for MS can help neurologists choose the most appropriate treatment for a given patient. To compare the effectiveness of the most common initial DMTs administered in Sweden, the researchers examined data from the COMBAT-MS study.
They identified all patients who initiated an injectable therapy (interferon beta or glatiramer acetate), dimethyl fumarate, natalizumab, or rituximab as a first treatment between Jan. 1, 2011, and Dec. 14, 2020. Eligible participants had prospectively recorded outcome data in the Swedish MS Register. Follow-up for a participant continued even if the participant stopped receiving therapy.
The investigators replaced missing data using multiple imputation. They adjusted for potential confounders using stabilized inverse probability of treatment weighting with baseline variables. These variables included age, sex, disease duration, geographical region, EDSS score, and relapses.
Rituximab reduced relapses
The researchers included 1,938 first-ever treatment episodes in their analysis. Of this group, 858 were associated with injectables, 339 with dimethyl fumarate, 269 with natalizumab, and 472 with rituximab.
Participants’ baseline characteristics differed by the DMT that they used. Patients who initiated natalizumab were the youngest, had the shortest disease duration, and had the most previous relapses.
For each outcome, the investigators compared all other therapies with rituximab. After they adjusted the data, they found that the hazard ratio for first relapse was 6.0 for injectables, 2.9 for dimethyl fumarate, and 1.8 for natalizumab.
In the adjusted model, the MRI lesion rate ratio for injectables, compared with rituximab, was 4.5. The rate ratio was 4.8 for dimethyl fumarate and 1.9 for natalizumab.
But differences in EDSS score at 3 years from treatment initiation were small. EDSS score in patients who received injectables was 0.24 points higher, compared with those receiving rituximab. EDSS score was 0.05 points higher in patients receiving dimethyl fumarate and 0.01 points lower in patients receiving natalizumab.
The risk for treatment discontinuation, however, differed significantly between therapies. The HR for treatment discontinuation was 32.7 for injectables, 20.3 for dimethyl fumarate, and 16.3 for natalizumab, compared with rituximab.
Among patients receiving dimethyl fumarate and injectables, the main reasons for discontinuing therapy were inadequate effect and adverse events. The main reason for discontinuation among patients receiving natalizumab was categorized as “other reason,” which mostly reflected John Cunningham virus positivity and concern for developing progressive multifocal leukoencephalopathy.
‘The uncertainty continues’
“These differences that we see in the effectiveness can be somewhat surprising, especially when it comes to natalizumab,” which is considered very effective, said Mr. Alping. The vulnerable period that occurs after switching from natalizumab may partly explain the difference. “This is something to keep in mind when starting patients on natalizumab treatment in the clinic,” Mr. Alping added.
Although rituximab is not indicated for MS, many clinics are using it in this population, said Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research at the Neurological Institute of Cleveland Clinic, both in Cleveland, Ohio. Dr. Fox was not involved in the study.
“Assessing the generalizability of the study outside Sweden will be important,” he added, “but I would be surprised if their findings did not hold up to external validation.”
The way that the researchers addressed missing data could affect the interpretation of the findings. “Depending upon how much data was missing, their imputation methods may have a high level of uncertainty,” said Dr. Fox.
The researchers’ adjustments for baseline differences also raise questions. “Even though MRI was an outcome, it doesn’t appear they adjusted for baseline differences in MRI between the groups,” Dr. Fox observed.
Moreover, the study was conducted over a long period of time. “We know there are time effects in MS, with a very different disease activity expected from patients over time,” said Dr. Fox. For example, relapse rates in placebo groups of MS trials tend to decline over time. “This time effect likely impacted their results.”
But the disability findings may be the most important part of the study, according to Dr. Fox. The lack of significant difference in disability progression between therapies “highlights that a couple relapses or lesions on MRI may be too small to translate into long-term differences in disability progression,” he said.
“The long-term implications of small differences in relapse and MRI outcomes may be very small,” Dr. Fox went on. “Thus, the uncertainty continues around escalation treatment versus initial highly effective treatment paradigms.”
The Patient-Centered Outcomes Research Institute, the Swedish Research Council, and NEURO Sweden funded this study. Mr. Alping disclosed no relevant financial relationships. Dr. Fox receives consulting fees from the companies that manufacture all the therapies analyzed in the study.
A version of this article first appeared on Medscape.com.
, according to new research.
The risk for a first relapse was 6 times higher in patients receiving interferon beta or glatiramer acetate, compared with those receiving rituximab. But the level of disability at 3 years was only marginally different between the drugs studied.
The small differences in Expanded Disability Status Scale (EDSS) score are surprising, said investigator Peter Alping, a clinical assistant and doctoral student in the Department of Clinical Neuroscience at the Karolinska Institutet, Stockholm, as he presented the data. “It could be that we have too-short follow-up, so that EDSS doesn’t have time to diverge between therapies.”
He presented the findings at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
COMBAT-MS study
Direct comparisons of disease-modifying therapies (DMTs) for MS can help neurologists choose the most appropriate treatment for a given patient. To compare the effectiveness of the most common initial DMTs administered in Sweden, the researchers examined data from the COMBAT-MS study.
They identified all patients who initiated an injectable therapy (interferon beta or glatiramer acetate), dimethyl fumarate, natalizumab, or rituximab as a first treatment between Jan. 1, 2011, and Dec. 14, 2020. Eligible participants had prospectively recorded outcome data in the Swedish MS Register. Follow-up for a participant continued even if the participant stopped receiving therapy.
The investigators replaced missing data using multiple imputation. They adjusted for potential confounders using stabilized inverse probability of treatment weighting with baseline variables. These variables included age, sex, disease duration, geographical region, EDSS score, and relapses.
Rituximab reduced relapses
The researchers included 1,938 first-ever treatment episodes in their analysis. Of this group, 858 were associated with injectables, 339 with dimethyl fumarate, 269 with natalizumab, and 472 with rituximab.
Participants’ baseline characteristics differed by the DMT that they used. Patients who initiated natalizumab were the youngest, had the shortest disease duration, and had the most previous relapses.
For each outcome, the investigators compared all other therapies with rituximab. After they adjusted the data, they found that the hazard ratio for first relapse was 6.0 for injectables, 2.9 for dimethyl fumarate, and 1.8 for natalizumab.
In the adjusted model, the MRI lesion rate ratio for injectables, compared with rituximab, was 4.5. The rate ratio was 4.8 for dimethyl fumarate and 1.9 for natalizumab.
But differences in EDSS score at 3 years from treatment initiation were small. EDSS score in patients who received injectables was 0.24 points higher, compared with those receiving rituximab. EDSS score was 0.05 points higher in patients receiving dimethyl fumarate and 0.01 points lower in patients receiving natalizumab.
The risk for treatment discontinuation, however, differed significantly between therapies. The HR for treatment discontinuation was 32.7 for injectables, 20.3 for dimethyl fumarate, and 16.3 for natalizumab, compared with rituximab.
Among patients receiving dimethyl fumarate and injectables, the main reasons for discontinuing therapy were inadequate effect and adverse events. The main reason for discontinuation among patients receiving natalizumab was categorized as “other reason,” which mostly reflected John Cunningham virus positivity and concern for developing progressive multifocal leukoencephalopathy.
‘The uncertainty continues’
“These differences that we see in the effectiveness can be somewhat surprising, especially when it comes to natalizumab,” which is considered very effective, said Mr. Alping. The vulnerable period that occurs after switching from natalizumab may partly explain the difference. “This is something to keep in mind when starting patients on natalizumab treatment in the clinic,” Mr. Alping added.
Although rituximab is not indicated for MS, many clinics are using it in this population, said Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research at the Neurological Institute of Cleveland Clinic, both in Cleveland, Ohio. Dr. Fox was not involved in the study.
“Assessing the generalizability of the study outside Sweden will be important,” he added, “but I would be surprised if their findings did not hold up to external validation.”
The way that the researchers addressed missing data could affect the interpretation of the findings. “Depending upon how much data was missing, their imputation methods may have a high level of uncertainty,” said Dr. Fox.
The researchers’ adjustments for baseline differences also raise questions. “Even though MRI was an outcome, it doesn’t appear they adjusted for baseline differences in MRI between the groups,” Dr. Fox observed.
Moreover, the study was conducted over a long period of time. “We know there are time effects in MS, with a very different disease activity expected from patients over time,” said Dr. Fox. For example, relapse rates in placebo groups of MS trials tend to decline over time. “This time effect likely impacted their results.”
But the disability findings may be the most important part of the study, according to Dr. Fox. The lack of significant difference in disability progression between therapies “highlights that a couple relapses or lesions on MRI may be too small to translate into long-term differences in disability progression,” he said.
“The long-term implications of small differences in relapse and MRI outcomes may be very small,” Dr. Fox went on. “Thus, the uncertainty continues around escalation treatment versus initial highly effective treatment paradigms.”
The Patient-Centered Outcomes Research Institute, the Swedish Research Council, and NEURO Sweden funded this study. Mr. Alping disclosed no relevant financial relationships. Dr. Fox receives consulting fees from the companies that manufacture all the therapies analyzed in the study.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2021
ECTRIMS/EAN statement on COVID-19 vaccination in patients with MS
(MS).
The statement was released at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). The statement concludes that the COVID-19 vaccines that are currently available are safe for patients with MS. Further, it states that the vaccines confer the same protection for patients with MS as they do for the general population. Exceptions may be patients taking the S1P modulator fingolimod and anti-CD20 drugs. For these patients, antibody responses have been shown to be reduced.
This position statement will be published on the ECTRIMS and EAN websites. Owing to the shifting, ongoing nature of evidence, the position statement will be updated periodically.
Presenting the statement, Mauricio Farez, MD, Fundacion FLENI, Buenos Aires, concluded: “Overall, MS patients do not seem to develop more severe forms of COVID-19 as compared with healthy controls, but patients with greater disability, anti-CD20 treatment, or those with recent steroid use have a higher risk of severe disease.”
“So far there are no specific contraindications for any COVID vaccines in MS patients reported,” he added. “We should work with our patients to keep them safe with vaccines while optimizing treatment strategies and MS management, in particular for those treated with anti-CD20 and S1P modulators.”
Risk for COVID-19 among patients with MS
On the issue of whether patients with MS are at higher risk for COVID-19 or for having a more severe form of the disease, Dr. Farez noted that studies published to date are reassuring and don’t suggest major problems regarding safety.
The main factors that are associated with more serious forms of COVID in patients with MS are similar to those in the general population. These include age, obesity, diabetes, male sex, and Black race.
As for any risk associated with MS therapies, interferons and glatiramer acetate do not increase the risk of getting COVID-19 or worsen the clinical course of the disease. Fingolimod, teriflunomide, natalizumab, and dimethyl fumarate also do not seem to negatively affect risk for COVID-19, according to the statement.
However, several studies have shown that anti-CD20 therapies, such as ocrelizumab, and steroid pulses can confer an increased risk for COVID-19.
COVID-19 vaccine safety
Four COVID-19 vaccines are licensed for use in the European Union. These include two mRNA vaccines – Spikevax (Moderna) and Comimaty (Pfizer) – and two adenovirus-based vaccines, one from Janssen (J&J) and the other from AstraZeneca. Five other COVID-19 vaccines are under review and may be available in the future.
All the currently available vaccines can be administered to patients with MS, including patients receiving immunosuppressant disease-modifying therapies, the statement notes.
In real-life clinical practice, no red flags have been observed for patients with MS who have received mRNA vaccines to date. Nevertheless, because immunocompromised patients and those taking immunomodulators were excluded from trials, continued surveillance for immune-mediated adverse effects is warranted, Dr. Farez said.
Regarding possible effects of vaccines on MS relapses/disability, no significant adverse effects occurred in a study conducted in Israel (by Achiron and colleagues) that involved 435 patients with MS who were fully vaccinated with the Pfizer mRNA vaccine. The relapse rate was 1.6%, similar to the rate among patients who did not have MS. A study by Di Filippo and colleagues showed no significant changes in relapse rate in the 2 months following immunization with the Pfizer vaccine among 324 patients with MS.
“There are no specific contraindications to any of the vaccines particularly for MS patients compared with the general population,” Dr. Farez noted.
Are there different recommendations for different MS therapies?
On the issue of vaccine effects in patients taking various disease-modifying treatments, the statement says that the data on this are limited. Patients taking interferons, glatiramer acetate, teriflunomide, and fumarates whose lymphocyte counts are normal will most likely be adequately protected. Patients with moderate to severe lymphopenia may not mount an adequate immune response to COVID-19 vaccination, so absolute lymphocyte count may be checked before vaccination.
Patients taking natalizumab will also likely be protected with COVID vaccination.
It is likely that for patients taking alemtuzumab, immune cellular and humoral response to COVID-10 vaccines will be attenuated, especially in the first 6 months during maximum lymphopenia. If possible, vaccination should be delayed until at least 6 months after treatment. It is thought that patients who have completed both courses of alemtuzumab with complete immune reconstitution will mount a full immune response.
In studies, all patients with MS who were treated with cladribine demonstrated a protective humoral immune response to the COVID-19 vaccine. In those studies, the antibody response was evident about 4 months after the last treatment dose, and the titer did not differ from that of healthy persons, Dr. Farez reported.
Low antibody level with fingolimod
The majority of patients treated with fingolimod have failed to show a protective level of antibodies following COVID-19 vaccination, the statement notes.
Asked whether patients taking fingolimod should receive a COVID vaccination, Dr. Farez said that that was a good question. “We have to think about what is an immune response. Antibodies are only a small fraction of all immune responses. So, until we have data to show otherwise, I think we should vaccinate – any immunity is better than no immunity,” he said.
Dr. Farez also suggested that patients with MS who are taking fingolimod should continue to do so. “Any treatment for MS is better than none. If fingolimod is stopped, MS may rebound. So, the most likely scenario would be to keep treating with fingolimod and to give the vaccination. But these patients may need a more aggressive booster approach – we will be looking at that,” he said.
Anti-CD20 antibody drugs
Patients taking ocrelizumab also do not mount an appropriate antibody response regardless of lymphocyte count or the time interval from the last ocrelizumab dose (3-9 months), the statement says. To optimize vaccine efficacy and to balance benefits and risks, the statement advises administering COVID vaccines at least 12 weeks after administering ocrelizumab and 4-6 weeks prior to the next dose, whenever possible.
A study by Apostolidis and colleagues provides strong evidence of immune priming by COVID vaccination in patients treated with anti-CD20 medications. Although for most of these patients, antibody responses are not optimal, T-cell priming is largely intact, Dr. Farez noted.
Booster doses/antibody tests
The need for and timing of COVID vaccine booster doses have not been established. “This is being discussed now for the general population. The recommendations for MS patients will not differ significantly from those for the general population, apart from perhaps for specific populations such as those on anti-CD20 drugs or fingolimod,” Dr. Farez said.
Antibody testing is not currently recommended for assessing immunity following COVID vaccination because the clinical utility and serologic correlates of protection after vaccination have not been established. Antibody testing does not evaluate the cellular immune response, which may play a role in vaccine-mediated protection, according to the statement.
Vaccination strategy after COVID
People should be offered vaccination regardless of their history of symptomatic or asymptomatic COVID-19, including people with prolonged post-COVID symptoms. Data from clinical trials indicate that the currently authorized vaccines can be given safely to people with evidence of prior SARS-CoV-2 infection. For people who are known to be currently infected with SARS-CoV-2, vaccination should be deferred until the acute illness has passed.
Pregnancy/children
Data on the safety of COVID vaccines during pregnancy are limited. On the basis of current knowledge, experts believe that it is unlikely that COVID vaccines pose a risk to the pregnant person or fetus, and thus pregnant people with MS are eligible for and can receive a COVID-19 vaccine, the statement notes.
Adolescents aged 12-17 are eligible to receive the authorized mRNA vaccine, but children younger than 12 are not authorized to receive any COVID vaccine at this time, it adds.
A version of this article first appeared on Medscape.com.
(MS).
The statement was released at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). The statement concludes that the COVID-19 vaccines that are currently available are safe for patients with MS. Further, it states that the vaccines confer the same protection for patients with MS as they do for the general population. Exceptions may be patients taking the S1P modulator fingolimod and anti-CD20 drugs. For these patients, antibody responses have been shown to be reduced.
This position statement will be published on the ECTRIMS and EAN websites. Owing to the shifting, ongoing nature of evidence, the position statement will be updated periodically.
Presenting the statement, Mauricio Farez, MD, Fundacion FLENI, Buenos Aires, concluded: “Overall, MS patients do not seem to develop more severe forms of COVID-19 as compared with healthy controls, but patients with greater disability, anti-CD20 treatment, or those with recent steroid use have a higher risk of severe disease.”
“So far there are no specific contraindications for any COVID vaccines in MS patients reported,” he added. “We should work with our patients to keep them safe with vaccines while optimizing treatment strategies and MS management, in particular for those treated with anti-CD20 and S1P modulators.”
Risk for COVID-19 among patients with MS
On the issue of whether patients with MS are at higher risk for COVID-19 or for having a more severe form of the disease, Dr. Farez noted that studies published to date are reassuring and don’t suggest major problems regarding safety.
The main factors that are associated with more serious forms of COVID in patients with MS are similar to those in the general population. These include age, obesity, diabetes, male sex, and Black race.
As for any risk associated with MS therapies, interferons and glatiramer acetate do not increase the risk of getting COVID-19 or worsen the clinical course of the disease. Fingolimod, teriflunomide, natalizumab, and dimethyl fumarate also do not seem to negatively affect risk for COVID-19, according to the statement.
However, several studies have shown that anti-CD20 therapies, such as ocrelizumab, and steroid pulses can confer an increased risk for COVID-19.
COVID-19 vaccine safety
Four COVID-19 vaccines are licensed for use in the European Union. These include two mRNA vaccines – Spikevax (Moderna) and Comimaty (Pfizer) – and two adenovirus-based vaccines, one from Janssen (J&J) and the other from AstraZeneca. Five other COVID-19 vaccines are under review and may be available in the future.
All the currently available vaccines can be administered to patients with MS, including patients receiving immunosuppressant disease-modifying therapies, the statement notes.
In real-life clinical practice, no red flags have been observed for patients with MS who have received mRNA vaccines to date. Nevertheless, because immunocompromised patients and those taking immunomodulators were excluded from trials, continued surveillance for immune-mediated adverse effects is warranted, Dr. Farez said.
Regarding possible effects of vaccines on MS relapses/disability, no significant adverse effects occurred in a study conducted in Israel (by Achiron and colleagues) that involved 435 patients with MS who were fully vaccinated with the Pfizer mRNA vaccine. The relapse rate was 1.6%, similar to the rate among patients who did not have MS. A study by Di Filippo and colleagues showed no significant changes in relapse rate in the 2 months following immunization with the Pfizer vaccine among 324 patients with MS.
“There are no specific contraindications to any of the vaccines particularly for MS patients compared with the general population,” Dr. Farez noted.
Are there different recommendations for different MS therapies?
On the issue of vaccine effects in patients taking various disease-modifying treatments, the statement says that the data on this are limited. Patients taking interferons, glatiramer acetate, teriflunomide, and fumarates whose lymphocyte counts are normal will most likely be adequately protected. Patients with moderate to severe lymphopenia may not mount an adequate immune response to COVID-19 vaccination, so absolute lymphocyte count may be checked before vaccination.
Patients taking natalizumab will also likely be protected with COVID vaccination.
It is likely that for patients taking alemtuzumab, immune cellular and humoral response to COVID-10 vaccines will be attenuated, especially in the first 6 months during maximum lymphopenia. If possible, vaccination should be delayed until at least 6 months after treatment. It is thought that patients who have completed both courses of alemtuzumab with complete immune reconstitution will mount a full immune response.
In studies, all patients with MS who were treated with cladribine demonstrated a protective humoral immune response to the COVID-19 vaccine. In those studies, the antibody response was evident about 4 months after the last treatment dose, and the titer did not differ from that of healthy persons, Dr. Farez reported.
Low antibody level with fingolimod
The majority of patients treated with fingolimod have failed to show a protective level of antibodies following COVID-19 vaccination, the statement notes.
Asked whether patients taking fingolimod should receive a COVID vaccination, Dr. Farez said that that was a good question. “We have to think about what is an immune response. Antibodies are only a small fraction of all immune responses. So, until we have data to show otherwise, I think we should vaccinate – any immunity is better than no immunity,” he said.
Dr. Farez also suggested that patients with MS who are taking fingolimod should continue to do so. “Any treatment for MS is better than none. If fingolimod is stopped, MS may rebound. So, the most likely scenario would be to keep treating with fingolimod and to give the vaccination. But these patients may need a more aggressive booster approach – we will be looking at that,” he said.
Anti-CD20 antibody drugs
Patients taking ocrelizumab also do not mount an appropriate antibody response regardless of lymphocyte count or the time interval from the last ocrelizumab dose (3-9 months), the statement says. To optimize vaccine efficacy and to balance benefits and risks, the statement advises administering COVID vaccines at least 12 weeks after administering ocrelizumab and 4-6 weeks prior to the next dose, whenever possible.
A study by Apostolidis and colleagues provides strong evidence of immune priming by COVID vaccination in patients treated with anti-CD20 medications. Although for most of these patients, antibody responses are not optimal, T-cell priming is largely intact, Dr. Farez noted.
Booster doses/antibody tests
The need for and timing of COVID vaccine booster doses have not been established. “This is being discussed now for the general population. The recommendations for MS patients will not differ significantly from those for the general population, apart from perhaps for specific populations such as those on anti-CD20 drugs or fingolimod,” Dr. Farez said.
Antibody testing is not currently recommended for assessing immunity following COVID vaccination because the clinical utility and serologic correlates of protection after vaccination have not been established. Antibody testing does not evaluate the cellular immune response, which may play a role in vaccine-mediated protection, according to the statement.
Vaccination strategy after COVID
People should be offered vaccination regardless of their history of symptomatic or asymptomatic COVID-19, including people with prolonged post-COVID symptoms. Data from clinical trials indicate that the currently authorized vaccines can be given safely to people with evidence of prior SARS-CoV-2 infection. For people who are known to be currently infected with SARS-CoV-2, vaccination should be deferred until the acute illness has passed.
Pregnancy/children
Data on the safety of COVID vaccines during pregnancy are limited. On the basis of current knowledge, experts believe that it is unlikely that COVID vaccines pose a risk to the pregnant person or fetus, and thus pregnant people with MS are eligible for and can receive a COVID-19 vaccine, the statement notes.
Adolescents aged 12-17 are eligible to receive the authorized mRNA vaccine, but children younger than 12 are not authorized to receive any COVID vaccine at this time, it adds.
A version of this article first appeared on Medscape.com.
(MS).
The statement was released at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). The statement concludes that the COVID-19 vaccines that are currently available are safe for patients with MS. Further, it states that the vaccines confer the same protection for patients with MS as they do for the general population. Exceptions may be patients taking the S1P modulator fingolimod and anti-CD20 drugs. For these patients, antibody responses have been shown to be reduced.
This position statement will be published on the ECTRIMS and EAN websites. Owing to the shifting, ongoing nature of evidence, the position statement will be updated periodically.
Presenting the statement, Mauricio Farez, MD, Fundacion FLENI, Buenos Aires, concluded: “Overall, MS patients do not seem to develop more severe forms of COVID-19 as compared with healthy controls, but patients with greater disability, anti-CD20 treatment, or those with recent steroid use have a higher risk of severe disease.”
“So far there are no specific contraindications for any COVID vaccines in MS patients reported,” he added. “We should work with our patients to keep them safe with vaccines while optimizing treatment strategies and MS management, in particular for those treated with anti-CD20 and S1P modulators.”
Risk for COVID-19 among patients with MS
On the issue of whether patients with MS are at higher risk for COVID-19 or for having a more severe form of the disease, Dr. Farez noted that studies published to date are reassuring and don’t suggest major problems regarding safety.
The main factors that are associated with more serious forms of COVID in patients with MS are similar to those in the general population. These include age, obesity, diabetes, male sex, and Black race.
As for any risk associated with MS therapies, interferons and glatiramer acetate do not increase the risk of getting COVID-19 or worsen the clinical course of the disease. Fingolimod, teriflunomide, natalizumab, and dimethyl fumarate also do not seem to negatively affect risk for COVID-19, according to the statement.
However, several studies have shown that anti-CD20 therapies, such as ocrelizumab, and steroid pulses can confer an increased risk for COVID-19.
COVID-19 vaccine safety
Four COVID-19 vaccines are licensed for use in the European Union. These include two mRNA vaccines – Spikevax (Moderna) and Comimaty (Pfizer) – and two adenovirus-based vaccines, one from Janssen (J&J) and the other from AstraZeneca. Five other COVID-19 vaccines are under review and may be available in the future.
All the currently available vaccines can be administered to patients with MS, including patients receiving immunosuppressant disease-modifying therapies, the statement notes.
In real-life clinical practice, no red flags have been observed for patients with MS who have received mRNA vaccines to date. Nevertheless, because immunocompromised patients and those taking immunomodulators were excluded from trials, continued surveillance for immune-mediated adverse effects is warranted, Dr. Farez said.
Regarding possible effects of vaccines on MS relapses/disability, no significant adverse effects occurred in a study conducted in Israel (by Achiron and colleagues) that involved 435 patients with MS who were fully vaccinated with the Pfizer mRNA vaccine. The relapse rate was 1.6%, similar to the rate among patients who did not have MS. A study by Di Filippo and colleagues showed no significant changes in relapse rate in the 2 months following immunization with the Pfizer vaccine among 324 patients with MS.
“There are no specific contraindications to any of the vaccines particularly for MS patients compared with the general population,” Dr. Farez noted.
Are there different recommendations for different MS therapies?
On the issue of vaccine effects in patients taking various disease-modifying treatments, the statement says that the data on this are limited. Patients taking interferons, glatiramer acetate, teriflunomide, and fumarates whose lymphocyte counts are normal will most likely be adequately protected. Patients with moderate to severe lymphopenia may not mount an adequate immune response to COVID-19 vaccination, so absolute lymphocyte count may be checked before vaccination.
Patients taking natalizumab will also likely be protected with COVID vaccination.
It is likely that for patients taking alemtuzumab, immune cellular and humoral response to COVID-10 vaccines will be attenuated, especially in the first 6 months during maximum lymphopenia. If possible, vaccination should be delayed until at least 6 months after treatment. It is thought that patients who have completed both courses of alemtuzumab with complete immune reconstitution will mount a full immune response.
In studies, all patients with MS who were treated with cladribine demonstrated a protective humoral immune response to the COVID-19 vaccine. In those studies, the antibody response was evident about 4 months after the last treatment dose, and the titer did not differ from that of healthy persons, Dr. Farez reported.
Low antibody level with fingolimod
The majority of patients treated with fingolimod have failed to show a protective level of antibodies following COVID-19 vaccination, the statement notes.
Asked whether patients taking fingolimod should receive a COVID vaccination, Dr. Farez said that that was a good question. “We have to think about what is an immune response. Antibodies are only a small fraction of all immune responses. So, until we have data to show otherwise, I think we should vaccinate – any immunity is better than no immunity,” he said.
Dr. Farez also suggested that patients with MS who are taking fingolimod should continue to do so. “Any treatment for MS is better than none. If fingolimod is stopped, MS may rebound. So, the most likely scenario would be to keep treating with fingolimod and to give the vaccination. But these patients may need a more aggressive booster approach – we will be looking at that,” he said.
Anti-CD20 antibody drugs
Patients taking ocrelizumab also do not mount an appropriate antibody response regardless of lymphocyte count or the time interval from the last ocrelizumab dose (3-9 months), the statement says. To optimize vaccine efficacy and to balance benefits and risks, the statement advises administering COVID vaccines at least 12 weeks after administering ocrelizumab and 4-6 weeks prior to the next dose, whenever possible.
A study by Apostolidis and colleagues provides strong evidence of immune priming by COVID vaccination in patients treated with anti-CD20 medications. Although for most of these patients, antibody responses are not optimal, T-cell priming is largely intact, Dr. Farez noted.
Booster doses/antibody tests
The need for and timing of COVID vaccine booster doses have not been established. “This is being discussed now for the general population. The recommendations for MS patients will not differ significantly from those for the general population, apart from perhaps for specific populations such as those on anti-CD20 drugs or fingolimod,” Dr. Farez said.
Antibody testing is not currently recommended for assessing immunity following COVID vaccination because the clinical utility and serologic correlates of protection after vaccination have not been established. Antibody testing does not evaluate the cellular immune response, which may play a role in vaccine-mediated protection, according to the statement.
Vaccination strategy after COVID
People should be offered vaccination regardless of their history of symptomatic or asymptomatic COVID-19, including people with prolonged post-COVID symptoms. Data from clinical trials indicate that the currently authorized vaccines can be given safely to people with evidence of prior SARS-CoV-2 infection. For people who are known to be currently infected with SARS-CoV-2, vaccination should be deferred until the acute illness has passed.
Pregnancy/children
Data on the safety of COVID vaccines during pregnancy are limited. On the basis of current knowledge, experts believe that it is unlikely that COVID vaccines pose a risk to the pregnant person or fetus, and thus pregnant people with MS are eligible for and can receive a COVID-19 vaccine, the statement notes.
Adolescents aged 12-17 are eligible to receive the authorized mRNA vaccine, but children younger than 12 are not authorized to receive any COVID vaccine at this time, it adds.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
The Barcelona baseline risk score may predict long-term MS course
The high-risk group had the shortest time to progression to an Expanded Disability Status Score (EDSS) of 3.0, and were also more likely to progress by MRI and quality of life measures.
The ranking is based on sex, age at the first clinically isolated syndrome, CIS topography, the number of T2 lesions, and the presence of infratentorial and spinal cord lesions, contrast-enhancing lesions, and oligoclonal bands.
“What we wanted to do is merge all of the different prognostic variables for one single patient into one single score,” said Mar Tintoré, MD, PhD, in an interview. Dr. Tintoré is a professor of neurology at Vall d’Hebron University Hospital in Barcelona and a senior consultant at the Multiple Sclerosis Centre of Catalonia (Cemcat). Dr. Tintoré presented the results of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The three groups had different outcomes in MRI, clinical factors, and MRI scans, and quality of life outcomes over the course of their disease. “So this is a confirmation that this classification at baseline is really meaningful,” Dr. Tintoré said in an interview.
She attributed the success of the model to its reliance on multiple factors, but it is also designed to be simple to use. “We have been trying to use it with simple factors, [information] that you always have, like age, sex, gender, number of lesions, and topography of the region. Everybody has this information at their desk.”
Proof of concept
The study validates the approach that neurologists already utilize, according to Patricia Coyle, MD, who moderated the session. “I think the prospective study is a really unique and powerful concept,” said Dr. Coyle, who is a professor of neurology, vice chair of neurology, and director of the Stony Brook (N.Y.) Comprehensive Care Center.
The new study “kind of confirmed their concept of the initial rating, judging long-term disability progression measures in subsets. They also looked at brain atrophy, they looked at gray-matter atrophy, and that also traveled with these three different groups of severity. So it kind of gives value to looking at prognostic indicators at a first attack,” said Dr. Coyle.
The results also validate the Barcelona group’s heavy emphasis on MRI, which Dr. Coyle pointed out is common practice. “If the brain MRI looks very bad, if there are a lot of spinal cord lesions, then that’s somebody we’re much more worried about.”
Once the model is confirmed in other cohorts, the researchers plan to release the model as a generally available algorithm that clinicians could use to help manage patients. Dr. Tintoré pointed out the debate over when to begin a patient on high-efficacy disease-modifying therapies. That choice depends on a lot of factors, including patient choice, safety, and comorbidities. “But knowing that your patient is at risk of having a bad prognosis is something that is helpful” in that decision-making process, she said.
Predicting time to EDSS 3.0
The researchers used the Barcelona CIS cohort to build a Weibull survival regression in order to estimate the median time to EDSS 3.0. The model produced three categories with widely divergent predicted times from CIS to EDSS 3.0: low risk, medium risk, and high risk.
In the current report, the researchers compared the model to a “360 degree” measure of measures in 1,308 patients, including clinical milestones (McDonald 2017 MS, confirmed secondary progressive MS, progression independent of relapse activity [PIRA], EDSS disability, number of T2 MRI lesions and brain atrophy, and Patient Reported Outcomes for MS [walking speed, manual dexterity, processing speed, and contrast sensitivity]).
At 30 years after CIS, the risk of reaching EDSS 3.0 was higher in the medium risk (hazard ratio, 3.0 versus low risk) and in the high risk group (HR, 8.3 versus low risk). At 10 years, the low risk group had a 40% risk of fulfilling McDonald 2017 criteria, versus 89% in the intermediate group, and 98% in the high risk group. A similar relationship was seen for SPMS (1%, 8%, 16%) and PIRA (17%, 26%, 35%).
At 10 years, the estimated accumulated T2 lesions was 7 in the low-risk group (95% CI, 5-9), 15 in the medium-risk group (95% CI, 12-17), and 21 in the high-risk group (95% CI, 15-27).
Compared with the low- and medium-risk groups, the high-risk group had lower brain parenchymal fraction and gray-matter fraction at 5 years. They also experienced higher stigma, had worse perception of upper and lower limb function as measured by Neuro QoL, and had worse cognitive performance.
Dr. Tintoré has received compensation for consulting services and speaking honoraria from Aimirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva Pharmaceuticals, and Viela Bio. Dr. Coyle has no relevant disclosures.
The high-risk group had the shortest time to progression to an Expanded Disability Status Score (EDSS) of 3.0, and were also more likely to progress by MRI and quality of life measures.
The ranking is based on sex, age at the first clinically isolated syndrome, CIS topography, the number of T2 lesions, and the presence of infratentorial and spinal cord lesions, contrast-enhancing lesions, and oligoclonal bands.
“What we wanted to do is merge all of the different prognostic variables for one single patient into one single score,” said Mar Tintoré, MD, PhD, in an interview. Dr. Tintoré is a professor of neurology at Vall d’Hebron University Hospital in Barcelona and a senior consultant at the Multiple Sclerosis Centre of Catalonia (Cemcat). Dr. Tintoré presented the results of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The three groups had different outcomes in MRI, clinical factors, and MRI scans, and quality of life outcomes over the course of their disease. “So this is a confirmation that this classification at baseline is really meaningful,” Dr. Tintoré said in an interview.
She attributed the success of the model to its reliance on multiple factors, but it is also designed to be simple to use. “We have been trying to use it with simple factors, [information] that you always have, like age, sex, gender, number of lesions, and topography of the region. Everybody has this information at their desk.”
Proof of concept
The study validates the approach that neurologists already utilize, according to Patricia Coyle, MD, who moderated the session. “I think the prospective study is a really unique and powerful concept,” said Dr. Coyle, who is a professor of neurology, vice chair of neurology, and director of the Stony Brook (N.Y.) Comprehensive Care Center.
The new study “kind of confirmed their concept of the initial rating, judging long-term disability progression measures in subsets. They also looked at brain atrophy, they looked at gray-matter atrophy, and that also traveled with these three different groups of severity. So it kind of gives value to looking at prognostic indicators at a first attack,” said Dr. Coyle.
The results also validate the Barcelona group’s heavy emphasis on MRI, which Dr. Coyle pointed out is common practice. “If the brain MRI looks very bad, if there are a lot of spinal cord lesions, then that’s somebody we’re much more worried about.”
Once the model is confirmed in other cohorts, the researchers plan to release the model as a generally available algorithm that clinicians could use to help manage patients. Dr. Tintoré pointed out the debate over when to begin a patient on high-efficacy disease-modifying therapies. That choice depends on a lot of factors, including patient choice, safety, and comorbidities. “But knowing that your patient is at risk of having a bad prognosis is something that is helpful” in that decision-making process, she said.
Predicting time to EDSS 3.0
The researchers used the Barcelona CIS cohort to build a Weibull survival regression in order to estimate the median time to EDSS 3.0. The model produced three categories with widely divergent predicted times from CIS to EDSS 3.0: low risk, medium risk, and high risk.
In the current report, the researchers compared the model to a “360 degree” measure of measures in 1,308 patients, including clinical milestones (McDonald 2017 MS, confirmed secondary progressive MS, progression independent of relapse activity [PIRA], EDSS disability, number of T2 MRI lesions and brain atrophy, and Patient Reported Outcomes for MS [walking speed, manual dexterity, processing speed, and contrast sensitivity]).
At 30 years after CIS, the risk of reaching EDSS 3.0 was higher in the medium risk (hazard ratio, 3.0 versus low risk) and in the high risk group (HR, 8.3 versus low risk). At 10 years, the low risk group had a 40% risk of fulfilling McDonald 2017 criteria, versus 89% in the intermediate group, and 98% in the high risk group. A similar relationship was seen for SPMS (1%, 8%, 16%) and PIRA (17%, 26%, 35%).
At 10 years, the estimated accumulated T2 lesions was 7 in the low-risk group (95% CI, 5-9), 15 in the medium-risk group (95% CI, 12-17), and 21 in the high-risk group (95% CI, 15-27).
Compared with the low- and medium-risk groups, the high-risk group had lower brain parenchymal fraction and gray-matter fraction at 5 years. They also experienced higher stigma, had worse perception of upper and lower limb function as measured by Neuro QoL, and had worse cognitive performance.
Dr. Tintoré has received compensation for consulting services and speaking honoraria from Aimirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva Pharmaceuticals, and Viela Bio. Dr. Coyle has no relevant disclosures.
The high-risk group had the shortest time to progression to an Expanded Disability Status Score (EDSS) of 3.0, and were also more likely to progress by MRI and quality of life measures.
The ranking is based on sex, age at the first clinically isolated syndrome, CIS topography, the number of T2 lesions, and the presence of infratentorial and spinal cord lesions, contrast-enhancing lesions, and oligoclonal bands.
“What we wanted to do is merge all of the different prognostic variables for one single patient into one single score,” said Mar Tintoré, MD, PhD, in an interview. Dr. Tintoré is a professor of neurology at Vall d’Hebron University Hospital in Barcelona and a senior consultant at the Multiple Sclerosis Centre of Catalonia (Cemcat). Dr. Tintoré presented the results of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The three groups had different outcomes in MRI, clinical factors, and MRI scans, and quality of life outcomes over the course of their disease. “So this is a confirmation that this classification at baseline is really meaningful,” Dr. Tintoré said in an interview.
She attributed the success of the model to its reliance on multiple factors, but it is also designed to be simple to use. “We have been trying to use it with simple factors, [information] that you always have, like age, sex, gender, number of lesions, and topography of the region. Everybody has this information at their desk.”
Proof of concept
The study validates the approach that neurologists already utilize, according to Patricia Coyle, MD, who moderated the session. “I think the prospective study is a really unique and powerful concept,” said Dr. Coyle, who is a professor of neurology, vice chair of neurology, and director of the Stony Brook (N.Y.) Comprehensive Care Center.
The new study “kind of confirmed their concept of the initial rating, judging long-term disability progression measures in subsets. They also looked at brain atrophy, they looked at gray-matter atrophy, and that also traveled with these three different groups of severity. So it kind of gives value to looking at prognostic indicators at a first attack,” said Dr. Coyle.
The results also validate the Barcelona group’s heavy emphasis on MRI, which Dr. Coyle pointed out is common practice. “If the brain MRI looks very bad, if there are a lot of spinal cord lesions, then that’s somebody we’re much more worried about.”
Once the model is confirmed in other cohorts, the researchers plan to release the model as a generally available algorithm that clinicians could use to help manage patients. Dr. Tintoré pointed out the debate over when to begin a patient on high-efficacy disease-modifying therapies. That choice depends on a lot of factors, including patient choice, safety, and comorbidities. “But knowing that your patient is at risk of having a bad prognosis is something that is helpful” in that decision-making process, she said.
Predicting time to EDSS 3.0
The researchers used the Barcelona CIS cohort to build a Weibull survival regression in order to estimate the median time to EDSS 3.0. The model produced three categories with widely divergent predicted times from CIS to EDSS 3.0: low risk, medium risk, and high risk.
In the current report, the researchers compared the model to a “360 degree” measure of measures in 1,308 patients, including clinical milestones (McDonald 2017 MS, confirmed secondary progressive MS, progression independent of relapse activity [PIRA], EDSS disability, number of T2 MRI lesions and brain atrophy, and Patient Reported Outcomes for MS [walking speed, manual dexterity, processing speed, and contrast sensitivity]).
At 30 years after CIS, the risk of reaching EDSS 3.0 was higher in the medium risk (hazard ratio, 3.0 versus low risk) and in the high risk group (HR, 8.3 versus low risk). At 10 years, the low risk group had a 40% risk of fulfilling McDonald 2017 criteria, versus 89% in the intermediate group, and 98% in the high risk group. A similar relationship was seen for SPMS (1%, 8%, 16%) and PIRA (17%, 26%, 35%).
At 10 years, the estimated accumulated T2 lesions was 7 in the low-risk group (95% CI, 5-9), 15 in the medium-risk group (95% CI, 12-17), and 21 in the high-risk group (95% CI, 15-27).
Compared with the low- and medium-risk groups, the high-risk group had lower brain parenchymal fraction and gray-matter fraction at 5 years. They also experienced higher stigma, had worse perception of upper and lower limb function as measured by Neuro QoL, and had worse cognitive performance.
Dr. Tintoré has received compensation for consulting services and speaking honoraria from Aimirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva Pharmaceuticals, and Viela Bio. Dr. Coyle has no relevant disclosures.
FROM ECTRIMS 2021

