User login
Hair loss on scalp
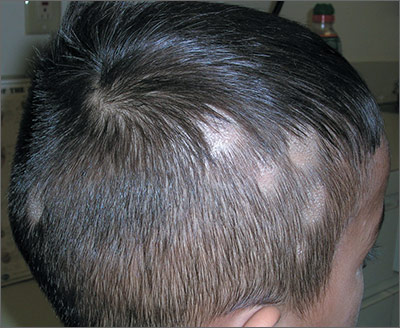
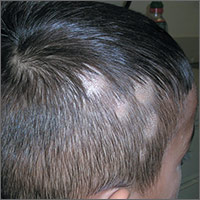
The FP noted patchy alopecia with scaling of the scalp and made the presumptive diagnosis of tinea capitis. A woods lamp examination did not demonstrate fluorescence. The child was very cooperative and the doctor was able to perform a potassium hydroxide (KOH) preparation by scraping the areas of alopecia with the edge of one glass slide while catching the scale on another slide. Microscopic examination revealed branching hyphae and some broken hairs with fungal elements within the hair shaft. (See video on how to perform a KOH preparation.) This microscopic picture was consistent with Trichophyton tonsurans, the most common cause of tinea capitis in the United States. The reason that the infected area did not fluoresce was that the dermatophyte was within the hair shaft (endothrix) rather than external to the hair (exothrix).
Topical antifungal therapy is not adequate for tinea capitis; oral treatment is needed. Oral antifungal choices include griseofulvin, terbinafine, and fluconazole. Griseofulvin comes in an oral suspension making it a desirable option for children who can’t swallow pills. However, at least 6 to 8 weeks of treatment (20 mg/kg/d) is required. Oral terbinafine tablets are less expensive and shorter courses of therapy may be used. Tablets of 250 mg terbinafine (most affordable of all the choices) can be broken in half for younger children and the dose should always be calculated based on weight. Fluconazole comes in various tablets, strengths, and liquid formulations and can be prescribed for 3 to 6 weeks, as needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Yao C. Tinea capitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:782-787.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP noted patchy alopecia with scaling of the scalp and made the presumptive diagnosis of tinea capitis. A woods lamp examination did not demonstrate fluorescence. The child was very cooperative and the doctor was able to perform a potassium hydroxide (KOH) preparation by scraping the areas of alopecia with the edge of one glass slide while catching the scale on another slide. Microscopic examination revealed branching hyphae and some broken hairs with fungal elements within the hair shaft. (See video on how to perform a KOH preparation.) This microscopic picture was consistent with Trichophyton tonsurans, the most common cause of tinea capitis in the United States. The reason that the infected area did not fluoresce was that the dermatophyte was within the hair shaft (endothrix) rather than external to the hair (exothrix).
Topical antifungal therapy is not adequate for tinea capitis; oral treatment is needed. Oral antifungal choices include griseofulvin, terbinafine, and fluconazole. Griseofulvin comes in an oral suspension making it a desirable option for children who can’t swallow pills. However, at least 6 to 8 weeks of treatment (20 mg/kg/d) is required. Oral terbinafine tablets are less expensive and shorter courses of therapy may be used. Tablets of 250 mg terbinafine (most affordable of all the choices) can be broken in half for younger children and the dose should always be calculated based on weight. Fluconazole comes in various tablets, strengths, and liquid formulations and can be prescribed for 3 to 6 weeks, as needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Yao C. Tinea capitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:782-787.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP noted patchy alopecia with scaling of the scalp and made the presumptive diagnosis of tinea capitis. A woods lamp examination did not demonstrate fluorescence. The child was very cooperative and the doctor was able to perform a potassium hydroxide (KOH) preparation by scraping the areas of alopecia with the edge of one glass slide while catching the scale on another slide. Microscopic examination revealed branching hyphae and some broken hairs with fungal elements within the hair shaft. (See video on how to perform a KOH preparation.) This microscopic picture was consistent with Trichophyton tonsurans, the most common cause of tinea capitis in the United States. The reason that the infected area did not fluoresce was that the dermatophyte was within the hair shaft (endothrix) rather than external to the hair (exothrix).
Topical antifungal therapy is not adequate for tinea capitis; oral treatment is needed. Oral antifungal choices include griseofulvin, terbinafine, and fluconazole. Griseofulvin comes in an oral suspension making it a desirable option for children who can’t swallow pills. However, at least 6 to 8 weeks of treatment (20 mg/kg/d) is required. Oral terbinafine tablets are less expensive and shorter courses of therapy may be used. Tablets of 250 mg terbinafine (most affordable of all the choices) can be broken in half for younger children and the dose should always be calculated based on weight. Fluconazole comes in various tablets, strengths, and liquid formulations and can be prescribed for 3 to 6 weeks, as needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Yao C. Tinea capitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:782-787.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Heart failure risk with individual NSAIDs examined in study
Nine popular painkillers – including traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors – are associated with an increased risk of hospitalization for heart failure in adults, based on data from a case-control study of approximately 92,000 hospital admissions. The findings were published online Sept. 28 in BMJ.
Although data from previous large studies suggest that high doses of NSAIDs as well as COX-2 inhibitors increase the risk of hospital admission for heart failure, “there is still limited information on the risk of heart failure associated with the use of individual NSAIDs in clinical practice,” wrote Andrea Arfè of the University of Milano-Bicocca, Milan, and his colleagues (BMJ. 2016 Sep;354:i4857 doi: 10.1136/bmj.i4857).
The researchers reviewed data from five electronic health databases in the Netherlands, Italy, Germany, and the United Kingdom as part of the SOS (Safety of Non-Steroidal Anti-Inflammatory Drugs) project and conducted a nested, case-control study including 92,163 hospital admissions for heart failure and 8,246,403 controls matched for age, sex, and year of study entry. The study included 23 traditional NSAIDs and four selective COX-2 inhibitors.
Overall, individual use of any of nine different NSAIDs within 14 days was associated with a nearly 20% higher likelihood of hospital admission for heart failure, compared with NSAID use more than 183 days in the past (odds ratio, 1.19). For seven traditional NSAIDS (diclofenac, ibuprofen, indomethacin, ketorolac, naproxen, nimesulide, and piroxicam) and the COX-2 inhibitors etoricoxib and rofecoxib, the odds ratios for heart failure with current use ranged from 1.16 for naproxen to 1.83 for ketorolac, compared with past use.
In addition, the odds of hospitalization for heart failure doubled for diclofenac, etoricoxib, indomethacin, piroxicam, and rofecoxib when dosed at two or more daily dose equivalents, the researchers noted. There was no increase in the odds of hospitalization for heart failure with celecoxib when dosed at standard levels, but “indomethacin and etoricoxib seemed to increase the risk of hospital admission for heart failure, even if used at medium doses,” they said. Other lesser-used NSAIDs were associated with an increased risk, but it was not statistically significant.
“The effect of individual NSAIDs could depend on a complex interaction of pharmacological properties, including duration and extent of platelet inhibition, extent of blood pressure increase, and properties possibly unique to the molecule,” the researchers said.
The findings were limited by several factors including misclassification of outcomes and the observational nature of the study, which prevents conclusions about cause and effect, the researchers noted. However, “Because any potential increased risk could have a considerable impact on public health, the risk effect estimates provided by this study may help inform both clinical practice and regulatory activities,” they said.
The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
This report provides additional weight backing the association between increased risk of heart failure with NSAID use and gives better insight on the dose-response relationship between individual NSAIDs and heart failure. However, beyond that, its clinical impact is hurt by the lack of data on the magnitude of excess absolute risk of heart failure with NSAID use, which varies according to baseline cardiovascular risk.
Even though the risk of heart failure associated with NSAID use in the study occurred independent of a history of heart failure, it still is prudent to restrict NSAID use in patients with heart failure because of the high risk noted in this group in other studies.
The widespread use and ease of access to NSAIDs fuels the common misconception that NSAIDs are harmless drugs that are safe for everyone, and this warrants a more restrictive policy by regulatory authorities on the availability of NSAIDs and requirements for health care professionals providing advice on their use.
Gunnar H. Gislason, MD, PhD, is a professor of cardiology at Copenhagen University Hospital Herlev and Gentofte, Denmark, and Christian Torp-Pedersen, MD, is a professor of cardiology at Aalborg (Denmark) University. Dr. Gislason had no disclosures and Dr. Torp-Pedersen advises Bayer on anticoagulation for atrial fibrillation. Their remarks are taken from an editorial accompanying the study by Mr. Arfè and his colleagues (BMJ. 2016 Sep;354:i5163 doi: 10.1136/bmj.i5163).
This report provides additional weight backing the association between increased risk of heart failure with NSAID use and gives better insight on the dose-response relationship between individual NSAIDs and heart failure. However, beyond that, its clinical impact is hurt by the lack of data on the magnitude of excess absolute risk of heart failure with NSAID use, which varies according to baseline cardiovascular risk.
Even though the risk of heart failure associated with NSAID use in the study occurred independent of a history of heart failure, it still is prudent to restrict NSAID use in patients with heart failure because of the high risk noted in this group in other studies.
The widespread use and ease of access to NSAIDs fuels the common misconception that NSAIDs are harmless drugs that are safe for everyone, and this warrants a more restrictive policy by regulatory authorities on the availability of NSAIDs and requirements for health care professionals providing advice on their use.
Gunnar H. Gislason, MD, PhD, is a professor of cardiology at Copenhagen University Hospital Herlev and Gentofte, Denmark, and Christian Torp-Pedersen, MD, is a professor of cardiology at Aalborg (Denmark) University. Dr. Gislason had no disclosures and Dr. Torp-Pedersen advises Bayer on anticoagulation for atrial fibrillation. Their remarks are taken from an editorial accompanying the study by Mr. Arfè and his colleagues (BMJ. 2016 Sep;354:i5163 doi: 10.1136/bmj.i5163).
This report provides additional weight backing the association between increased risk of heart failure with NSAID use and gives better insight on the dose-response relationship between individual NSAIDs and heart failure. However, beyond that, its clinical impact is hurt by the lack of data on the magnitude of excess absolute risk of heart failure with NSAID use, which varies according to baseline cardiovascular risk.
Even though the risk of heart failure associated with NSAID use in the study occurred independent of a history of heart failure, it still is prudent to restrict NSAID use in patients with heart failure because of the high risk noted in this group in other studies.
The widespread use and ease of access to NSAIDs fuels the common misconception that NSAIDs are harmless drugs that are safe for everyone, and this warrants a more restrictive policy by regulatory authorities on the availability of NSAIDs and requirements for health care professionals providing advice on their use.
Gunnar H. Gislason, MD, PhD, is a professor of cardiology at Copenhagen University Hospital Herlev and Gentofte, Denmark, and Christian Torp-Pedersen, MD, is a professor of cardiology at Aalborg (Denmark) University. Dr. Gislason had no disclosures and Dr. Torp-Pedersen advises Bayer on anticoagulation for atrial fibrillation. Their remarks are taken from an editorial accompanying the study by Mr. Arfè and his colleagues (BMJ. 2016 Sep;354:i5163 doi: 10.1136/bmj.i5163).
Nine popular painkillers – including traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors – are associated with an increased risk of hospitalization for heart failure in adults, based on data from a case-control study of approximately 92,000 hospital admissions. The findings were published online Sept. 28 in BMJ.
Although data from previous large studies suggest that high doses of NSAIDs as well as COX-2 inhibitors increase the risk of hospital admission for heart failure, “there is still limited information on the risk of heart failure associated with the use of individual NSAIDs in clinical practice,” wrote Andrea Arfè of the University of Milano-Bicocca, Milan, and his colleagues (BMJ. 2016 Sep;354:i4857 doi: 10.1136/bmj.i4857).
The researchers reviewed data from five electronic health databases in the Netherlands, Italy, Germany, and the United Kingdom as part of the SOS (Safety of Non-Steroidal Anti-Inflammatory Drugs) project and conducted a nested, case-control study including 92,163 hospital admissions for heart failure and 8,246,403 controls matched for age, sex, and year of study entry. The study included 23 traditional NSAIDs and four selective COX-2 inhibitors.
Overall, individual use of any of nine different NSAIDs within 14 days was associated with a nearly 20% higher likelihood of hospital admission for heart failure, compared with NSAID use more than 183 days in the past (odds ratio, 1.19). For seven traditional NSAIDS (diclofenac, ibuprofen, indomethacin, ketorolac, naproxen, nimesulide, and piroxicam) and the COX-2 inhibitors etoricoxib and rofecoxib, the odds ratios for heart failure with current use ranged from 1.16 for naproxen to 1.83 for ketorolac, compared with past use.
In addition, the odds of hospitalization for heart failure doubled for diclofenac, etoricoxib, indomethacin, piroxicam, and rofecoxib when dosed at two or more daily dose equivalents, the researchers noted. There was no increase in the odds of hospitalization for heart failure with celecoxib when dosed at standard levels, but “indomethacin and etoricoxib seemed to increase the risk of hospital admission for heart failure, even if used at medium doses,” they said. Other lesser-used NSAIDs were associated with an increased risk, but it was not statistically significant.
“The effect of individual NSAIDs could depend on a complex interaction of pharmacological properties, including duration and extent of platelet inhibition, extent of blood pressure increase, and properties possibly unique to the molecule,” the researchers said.
The findings were limited by several factors including misclassification of outcomes and the observational nature of the study, which prevents conclusions about cause and effect, the researchers noted. However, “Because any potential increased risk could have a considerable impact on public health, the risk effect estimates provided by this study may help inform both clinical practice and regulatory activities,” they said.
The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
Nine popular painkillers – including traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors – are associated with an increased risk of hospitalization for heart failure in adults, based on data from a case-control study of approximately 92,000 hospital admissions. The findings were published online Sept. 28 in BMJ.
Although data from previous large studies suggest that high doses of NSAIDs as well as COX-2 inhibitors increase the risk of hospital admission for heart failure, “there is still limited information on the risk of heart failure associated with the use of individual NSAIDs in clinical practice,” wrote Andrea Arfè of the University of Milano-Bicocca, Milan, and his colleagues (BMJ. 2016 Sep;354:i4857 doi: 10.1136/bmj.i4857).
The researchers reviewed data from five electronic health databases in the Netherlands, Italy, Germany, and the United Kingdom as part of the SOS (Safety of Non-Steroidal Anti-Inflammatory Drugs) project and conducted a nested, case-control study including 92,163 hospital admissions for heart failure and 8,246,403 controls matched for age, sex, and year of study entry. The study included 23 traditional NSAIDs and four selective COX-2 inhibitors.
Overall, individual use of any of nine different NSAIDs within 14 days was associated with a nearly 20% higher likelihood of hospital admission for heart failure, compared with NSAID use more than 183 days in the past (odds ratio, 1.19). For seven traditional NSAIDS (diclofenac, ibuprofen, indomethacin, ketorolac, naproxen, nimesulide, and piroxicam) and the COX-2 inhibitors etoricoxib and rofecoxib, the odds ratios for heart failure with current use ranged from 1.16 for naproxen to 1.83 for ketorolac, compared with past use.
In addition, the odds of hospitalization for heart failure doubled for diclofenac, etoricoxib, indomethacin, piroxicam, and rofecoxib when dosed at two or more daily dose equivalents, the researchers noted. There was no increase in the odds of hospitalization for heart failure with celecoxib when dosed at standard levels, but “indomethacin and etoricoxib seemed to increase the risk of hospital admission for heart failure, even if used at medium doses,” they said. Other lesser-used NSAIDs were associated with an increased risk, but it was not statistically significant.
“The effect of individual NSAIDs could depend on a complex interaction of pharmacological properties, including duration and extent of platelet inhibition, extent of blood pressure increase, and properties possibly unique to the molecule,” the researchers said.
The findings were limited by several factors including misclassification of outcomes and the observational nature of the study, which prevents conclusions about cause and effect, the researchers noted. However, “Because any potential increased risk could have a considerable impact on public health, the risk effect estimates provided by this study may help inform both clinical practice and regulatory activities,” they said.
The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
FROM BMJ
Key clinical point: Patients taking high doses of certain NSAIDS had significantly higher odds of hospital admission for heart failure, compared with controls not currently taking the medications.
Major finding: The odds of hospitalization for heart failure increased by 19% overall for adults currently using certain NSAIDS and doubled for users of certain NSAIDs at high doses.
Data source: The data come from approximately 10 million hospital admissions taken from databases in the Netherlands, Italy, Germany, and the United Kingdom.
Disclosures: The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
Cognitive Behavioral Therapy Eases Postconcussive Symptoms in Teens
Adolescents who underwent cognitive behavioral therapy (CBT) as part of postconcussion care reported significantly lower levels of postconcussive and depressive symptoms, according to the results of a randomized trial published online ahead of print September 12 in Pediatrics.
“Affective symptoms, including depression and anxiety, commonly co-occur with cognitive and somatic symptoms and may prolong recovery from postconcussive symptoms, wrote Carolyn A. McCarty, PhD, Research Associate Professor of Pediatrics and Adjunct Research Associate Professor of Psychology at Seattle Children’s Hospital Center for Child Health Behavior and Development in Seattle, and her colleagues.
“The complexities of managing persistent postconcussive symptoms in conjunction with comorbid psychological symptoms create a significant burden for injured children and adolescents, their families, and schools.”
To determine the impact of CBT on persistent symptoms in adolescents with concussions, the researchers randomized 49 patients, ages 11 to 17, to usual care or a collaborative care plan that included usual care plus CBT.
Concussions were diagnosed by sports medicine or rehabilitative medicine specialists. The patients assigned to CBT received usual care management, CBT, and possible psychopharmacologic consultation. Control patients received usual concussion care, generally defined as an initial visit with a sports medicine physician and assessments at one, three, and six months. Usual care also could include MRI, sleep medication, and subthreshold exercise, depending on the patient. No serious adverse events were reported. The average age of the patients was 15, approximately 65% were girls, and 76% were white.
After six months, approximately 13% of the teens in the CBT group reported high levels of postconcussive symptoms, compared with 42% of controls. In addition, 78% of patients receiving CBT reported a depressive symptom reduction of more than 50%, compared with 46% of controls.
Overall, 83% of the patients receiving CBT and 87% of their parents were “very satisfied” with their care, compared with 46% of patients and 29% of parents in the control group.
“Although patients in both groups showed symptom reduction in the first three months, only those who received collaborative care demonstrated sustained improvements through six months of follow-up,” Dr. McCarty and her colleagues wrote.
The results were limited by several factors, including the small size of the study, the researchers said. However, the findings “prompt more investigation into the role of affective symptoms in perpetuating physical symptoms secondary to prolonged recovery from sports-related concussion” and also suggest that collaborative care can help improve persistent postconcussive symptoms in teens.The Seattle Sports Concussion Research Collaborative supported the study.
—Heidi Splete
Suggested Reading
McCarty CA, Zatzick D, Stein E, et al. Collaborative care for adolescents with persistent postconcussive symptoms: a randomized trial. Pediatrics. 2016 Sept 13 [Epub ahead of print].
Cordingley D, Girardin R, Reimer K, et al. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. J Neurosurg Pediatr. 2016 September 13 [Epub ahead of print].
Adolescents who underwent cognitive behavioral therapy (CBT) as part of postconcussion care reported significantly lower levels of postconcussive and depressive symptoms, according to the results of a randomized trial published online ahead of print September 12 in Pediatrics.
“Affective symptoms, including depression and anxiety, commonly co-occur with cognitive and somatic symptoms and may prolong recovery from postconcussive symptoms, wrote Carolyn A. McCarty, PhD, Research Associate Professor of Pediatrics and Adjunct Research Associate Professor of Psychology at Seattle Children’s Hospital Center for Child Health Behavior and Development in Seattle, and her colleagues.
“The complexities of managing persistent postconcussive symptoms in conjunction with comorbid psychological symptoms create a significant burden for injured children and adolescents, their families, and schools.”
To determine the impact of CBT on persistent symptoms in adolescents with concussions, the researchers randomized 49 patients, ages 11 to 17, to usual care or a collaborative care plan that included usual care plus CBT.
Concussions were diagnosed by sports medicine or rehabilitative medicine specialists. The patients assigned to CBT received usual care management, CBT, and possible psychopharmacologic consultation. Control patients received usual concussion care, generally defined as an initial visit with a sports medicine physician and assessments at one, three, and six months. Usual care also could include MRI, sleep medication, and subthreshold exercise, depending on the patient. No serious adverse events were reported. The average age of the patients was 15, approximately 65% were girls, and 76% were white.
After six months, approximately 13% of the teens in the CBT group reported high levels of postconcussive symptoms, compared with 42% of controls. In addition, 78% of patients receiving CBT reported a depressive symptom reduction of more than 50%, compared with 46% of controls.
Overall, 83% of the patients receiving CBT and 87% of their parents were “very satisfied” with their care, compared with 46% of patients and 29% of parents in the control group.
“Although patients in both groups showed symptom reduction in the first three months, only those who received collaborative care demonstrated sustained improvements through six months of follow-up,” Dr. McCarty and her colleagues wrote.
The results were limited by several factors, including the small size of the study, the researchers said. However, the findings “prompt more investigation into the role of affective symptoms in perpetuating physical symptoms secondary to prolonged recovery from sports-related concussion” and also suggest that collaborative care can help improve persistent postconcussive symptoms in teens.The Seattle Sports Concussion Research Collaborative supported the study.
—Heidi Splete
Suggested Reading
McCarty CA, Zatzick D, Stein E, et al. Collaborative care for adolescents with persistent postconcussive symptoms: a randomized trial. Pediatrics. 2016 Sept 13 [Epub ahead of print].
Cordingley D, Girardin R, Reimer K, et al. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. J Neurosurg Pediatr. 2016 September 13 [Epub ahead of print].
Adolescents who underwent cognitive behavioral therapy (CBT) as part of postconcussion care reported significantly lower levels of postconcussive and depressive symptoms, according to the results of a randomized trial published online ahead of print September 12 in Pediatrics.
“Affective symptoms, including depression and anxiety, commonly co-occur with cognitive and somatic symptoms and may prolong recovery from postconcussive symptoms, wrote Carolyn A. McCarty, PhD, Research Associate Professor of Pediatrics and Adjunct Research Associate Professor of Psychology at Seattle Children’s Hospital Center for Child Health Behavior and Development in Seattle, and her colleagues.
“The complexities of managing persistent postconcussive symptoms in conjunction with comorbid psychological symptoms create a significant burden for injured children and adolescents, their families, and schools.”
To determine the impact of CBT on persistent symptoms in adolescents with concussions, the researchers randomized 49 patients, ages 11 to 17, to usual care or a collaborative care plan that included usual care plus CBT.
Concussions were diagnosed by sports medicine or rehabilitative medicine specialists. The patients assigned to CBT received usual care management, CBT, and possible psychopharmacologic consultation. Control patients received usual concussion care, generally defined as an initial visit with a sports medicine physician and assessments at one, three, and six months. Usual care also could include MRI, sleep medication, and subthreshold exercise, depending on the patient. No serious adverse events were reported. The average age of the patients was 15, approximately 65% were girls, and 76% were white.
After six months, approximately 13% of the teens in the CBT group reported high levels of postconcussive symptoms, compared with 42% of controls. In addition, 78% of patients receiving CBT reported a depressive symptom reduction of more than 50%, compared with 46% of controls.
Overall, 83% of the patients receiving CBT and 87% of their parents were “very satisfied” with their care, compared with 46% of patients and 29% of parents in the control group.
“Although patients in both groups showed symptom reduction in the first three months, only those who received collaborative care demonstrated sustained improvements through six months of follow-up,” Dr. McCarty and her colleagues wrote.
The results were limited by several factors, including the small size of the study, the researchers said. However, the findings “prompt more investigation into the role of affective symptoms in perpetuating physical symptoms secondary to prolonged recovery from sports-related concussion” and also suggest that collaborative care can help improve persistent postconcussive symptoms in teens.The Seattle Sports Concussion Research Collaborative supported the study.
—Heidi Splete
Suggested Reading
McCarty CA, Zatzick D, Stein E, et al. Collaborative care for adolescents with persistent postconcussive symptoms: a randomized trial. Pediatrics. 2016 Sept 13 [Epub ahead of print].
Cordingley D, Girardin R, Reimer K, et al. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. J Neurosurg Pediatr. 2016 September 13 [Epub ahead of print].
Adjunctive azithromycin cuts postcesarean infection
Adding a single intravenous dose of azithromycin to standard antibiotic prophylaxis further reduces maternal infections without increasing neonatal adverse outcomes after nonelective cesarean delivery, according to a report published in the New England Journal of Medicine.
The adjunctive azithromycin also significantly decreased rates of postpartum fever and of readmission or unscheduled office visits, wrote Alan T.N. Tita, MD, PhD, of the University of Alabama at Birmingham, and his colleagues.
Recent studies have suggested that extended-spectrum prophylaxis using azithromycin, when added to standard cephalosporin prophylaxis, would further reduce the incidence of post-cesarean infection, chiefly because of azithromycin’s coverage of ureaplasma species that are frequently associated with these infections. The C/SOAP (Cesarean Section Optimal Antibiotic Prophylaxis) trial tested this hypothesis in 2,013 women who underwent nonelective cesarean delivery of singleton neonates at 14 U.S. hospitals during a 3.5-year period.
All the women received standard antibiotic prophylaxis (usually with cefazolin) and were randomly assigned to receive either a 500-mg dose of azithromycin (1,019 participants) or a matching placebo (994 participants) before surgical incision.
The primary outcome measure – a composite of endometritis; wound infection; or other infections such as abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis occurring up to 6 weeks after surgery – developed in half as many women in the azithromycin group (6.1%) as in the placebo group (12.0%). The relative risk (RR) was 0.51 (P less than .001).
Azithromycin, in particular, was associated with significantly lower rates of endometritis (3.8% vs. 6.1%; RR, 0.62; P = .02) and wound infection (2.4% vs. 6.6%; RR, 0.35; P less than .001). This benefit extended across all subgroups of patients regardless of study site, maternal obesity status, the presence or absence of membrane rupture at randomization, preterm or term delivery, or maternal diabetes status.
The number of patients who would need to be treated to prevent one study outcome was 17 for the primary outcome, 43 for endometritis, and 24 for wound infections, the researchers reported (N Engl J Med. 2016 Sep 29;375:1231-41).
Serious maternal adverse events also were less common with azithromycin (1.5%) than with placebo (2.9%). Neonatal outcomes did not differ between the study groups. The rate of combined neonatal death or complications was 14.3% with azithromycin and 13.6% with placebo, a nonsignificant difference.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Pfizer donated the azithromycin used in the trial. Dr. Tita reported having no relevant financial disclosures; his colleagues reported ties to numerous industry sources.
This well-designed, pragmatic, multicenter trial shows that a single adjunctive dose of azithromycin likely would reduce the number of infectious complications for women undergoing nonelective cesarean section.
The addition of azithromycin may have been particularly effective for the 73% of this study population who had a body mass index of 30 kg/m2 or more. Obesity is known to double the risk of infectious complications, and previous studies have suggested that cefazolin may be underdosed in women with increased BMI values. It appears that azithromycin improved outcomes on the basis of the additive effects of the two drugs against common surgical pathogens, such as staphylococcus species. Information provided in the Supplementary Appendix accompanying the article indicates that routine bacterial cultures, when done, were much-less-frequently positive in the azithromycin group.
Robert A. Weinstein, MD, and Kenneth M. Boyer, MD, are at Rush University Medical Center and Cook County Health and Hospital System, both in Chicago. Dr. Weinstein reported receiving support from Merck outside of this work, and Dr. Boyer reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Sept 29. doi: 10.1056/NEJMe1610010).
This well-designed, pragmatic, multicenter trial shows that a single adjunctive dose of azithromycin likely would reduce the number of infectious complications for women undergoing nonelective cesarean section.
The addition of azithromycin may have been particularly effective for the 73% of this study population who had a body mass index of 30 kg/m2 or more. Obesity is known to double the risk of infectious complications, and previous studies have suggested that cefazolin may be underdosed in women with increased BMI values. It appears that azithromycin improved outcomes on the basis of the additive effects of the two drugs against common surgical pathogens, such as staphylococcus species. Information provided in the Supplementary Appendix accompanying the article indicates that routine bacterial cultures, when done, were much-less-frequently positive in the azithromycin group.
Robert A. Weinstein, MD, and Kenneth M. Boyer, MD, are at Rush University Medical Center and Cook County Health and Hospital System, both in Chicago. Dr. Weinstein reported receiving support from Merck outside of this work, and Dr. Boyer reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Sept 29. doi: 10.1056/NEJMe1610010).
This well-designed, pragmatic, multicenter trial shows that a single adjunctive dose of azithromycin likely would reduce the number of infectious complications for women undergoing nonelective cesarean section.
The addition of azithromycin may have been particularly effective for the 73% of this study population who had a body mass index of 30 kg/m2 or more. Obesity is known to double the risk of infectious complications, and previous studies have suggested that cefazolin may be underdosed in women with increased BMI values. It appears that azithromycin improved outcomes on the basis of the additive effects of the two drugs against common surgical pathogens, such as staphylococcus species. Information provided in the Supplementary Appendix accompanying the article indicates that routine bacterial cultures, when done, were much-less-frequently positive in the azithromycin group.
Robert A. Weinstein, MD, and Kenneth M. Boyer, MD, are at Rush University Medical Center and Cook County Health and Hospital System, both in Chicago. Dr. Weinstein reported receiving support from Merck outside of this work, and Dr. Boyer reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Sept 29. doi: 10.1056/NEJMe1610010).
Adding a single intravenous dose of azithromycin to standard antibiotic prophylaxis further reduces maternal infections without increasing neonatal adverse outcomes after nonelective cesarean delivery, according to a report published in the New England Journal of Medicine.
The adjunctive azithromycin also significantly decreased rates of postpartum fever and of readmission or unscheduled office visits, wrote Alan T.N. Tita, MD, PhD, of the University of Alabama at Birmingham, and his colleagues.
Recent studies have suggested that extended-spectrum prophylaxis using azithromycin, when added to standard cephalosporin prophylaxis, would further reduce the incidence of post-cesarean infection, chiefly because of azithromycin’s coverage of ureaplasma species that are frequently associated with these infections. The C/SOAP (Cesarean Section Optimal Antibiotic Prophylaxis) trial tested this hypothesis in 2,013 women who underwent nonelective cesarean delivery of singleton neonates at 14 U.S. hospitals during a 3.5-year period.
All the women received standard antibiotic prophylaxis (usually with cefazolin) and were randomly assigned to receive either a 500-mg dose of azithromycin (1,019 participants) or a matching placebo (994 participants) before surgical incision.
The primary outcome measure – a composite of endometritis; wound infection; or other infections such as abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis occurring up to 6 weeks after surgery – developed in half as many women in the azithromycin group (6.1%) as in the placebo group (12.0%). The relative risk (RR) was 0.51 (P less than .001).
Azithromycin, in particular, was associated with significantly lower rates of endometritis (3.8% vs. 6.1%; RR, 0.62; P = .02) and wound infection (2.4% vs. 6.6%; RR, 0.35; P less than .001). This benefit extended across all subgroups of patients regardless of study site, maternal obesity status, the presence or absence of membrane rupture at randomization, preterm or term delivery, or maternal diabetes status.
The number of patients who would need to be treated to prevent one study outcome was 17 for the primary outcome, 43 for endometritis, and 24 for wound infections, the researchers reported (N Engl J Med. 2016 Sep 29;375:1231-41).
Serious maternal adverse events also were less common with azithromycin (1.5%) than with placebo (2.9%). Neonatal outcomes did not differ between the study groups. The rate of combined neonatal death or complications was 14.3% with azithromycin and 13.6% with placebo, a nonsignificant difference.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Pfizer donated the azithromycin used in the trial. Dr. Tita reported having no relevant financial disclosures; his colleagues reported ties to numerous industry sources.
Adding a single intravenous dose of azithromycin to standard antibiotic prophylaxis further reduces maternal infections without increasing neonatal adverse outcomes after nonelective cesarean delivery, according to a report published in the New England Journal of Medicine.
The adjunctive azithromycin also significantly decreased rates of postpartum fever and of readmission or unscheduled office visits, wrote Alan T.N. Tita, MD, PhD, of the University of Alabama at Birmingham, and his colleagues.
Recent studies have suggested that extended-spectrum prophylaxis using azithromycin, when added to standard cephalosporin prophylaxis, would further reduce the incidence of post-cesarean infection, chiefly because of azithromycin’s coverage of ureaplasma species that are frequently associated with these infections. The C/SOAP (Cesarean Section Optimal Antibiotic Prophylaxis) trial tested this hypothesis in 2,013 women who underwent nonelective cesarean delivery of singleton neonates at 14 U.S. hospitals during a 3.5-year period.
All the women received standard antibiotic prophylaxis (usually with cefazolin) and were randomly assigned to receive either a 500-mg dose of azithromycin (1,019 participants) or a matching placebo (994 participants) before surgical incision.
The primary outcome measure – a composite of endometritis; wound infection; or other infections such as abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis occurring up to 6 weeks after surgery – developed in half as many women in the azithromycin group (6.1%) as in the placebo group (12.0%). The relative risk (RR) was 0.51 (P less than .001).
Azithromycin, in particular, was associated with significantly lower rates of endometritis (3.8% vs. 6.1%; RR, 0.62; P = .02) and wound infection (2.4% vs. 6.6%; RR, 0.35; P less than .001). This benefit extended across all subgroups of patients regardless of study site, maternal obesity status, the presence or absence of membrane rupture at randomization, preterm or term delivery, or maternal diabetes status.
The number of patients who would need to be treated to prevent one study outcome was 17 for the primary outcome, 43 for endometritis, and 24 for wound infections, the researchers reported (N Engl J Med. 2016 Sep 29;375:1231-41).
Serious maternal adverse events also were less common with azithromycin (1.5%) than with placebo (2.9%). Neonatal outcomes did not differ between the study groups. The rate of combined neonatal death or complications was 14.3% with azithromycin and 13.6% with placebo, a nonsignificant difference.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Pfizer donated the azithromycin used in the trial. Dr. Tita reported having no relevant financial disclosures; his colleagues reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Strategies for maintaining resilience to the burnout threat
It sometimes seems that the pace of life, and its stresses, have spiraled out of control: There just never seems to be enough time to deal with all the directions in which we are pulled. This easily can lead to the exhaustion of physical or emotional strength or motivation, otherwise known as “burnout.” Burnout is physical or mental collapse caused by overwork or stress and we are all at risk of suffering it. Conflicting demands on our time, loss of control (real or imagined), and a diminishing sense of worth grind at us from every direction.
In general, having some control over schedule and hours worked is associated with reductions in burnout and improved job satisfaction.1 But this is not always the case. Well-intentioned efforts to reduce workload, such as the electronic medical records or physician order entry systems, have actually made the problem worse.2 The seeming level of control that comes with being the chair of an obstetrics and gynecology department does not necessarily reduce burnout rates,3 and neither does the perceived resilience of mental health professionals, who still report burnout rates that approach 25%.4
This article continues the focus on recalibrating work/life balance that began last month with “ObGyn burnout: ACOG takes aim,” by Lucia DiVenere, MA, and the peer-to-peer audiocast with Ms. DiVenere and myself titled “Is burnout on the rise and what are the signs ObGyns should be on the lookout for?” Here, I identify the causes and symptoms of burnout and provide specific tools to help you develop resilience.
Who is most at risk for burnout?
Estimates range from 40% to 75% of ObGyns currently suffer from professional burnout, making the lifetime risk a virtual certainty.1−3 The idea of professional burnout is not new, but wider recognition of the alarming rates of burnout is very current.4,5 A recent survey of gynecologic oncologists6 found that of those studied 30% scored high for emotional exhaustion, 10% high for depersonalization, and 11% low for personal accomplishment. Overall, 32% of physicians had scores indicating burnout. More worrisome was that 33% screened positive for depression, 13% had a history of suicidal ideation, 15% screened positive for alcohol abuse, and 34% reported impaired quality of life. Almost 40% would not encourage their children to enter medicine and more than 10% said that they would not enter medicine again if they had to do it over.
Residents and those at mid-career are particularly vulnerable,7 with resident burnout rates reported to be as high as 75%.8 Of surveyed residents in a 2012 study, 13% satisfied all 3 subscale scores for high burnout and greater than 50% had high levels of depersonalization and emotional exhaustion. Those with high levels of emotional exhaustion were less satisfied with their careers, regretted choosing obstetrics and gynecology, and had higher rates of depression—all findings consistent with older studies.
9,10
References
- Peckham C. Medscape Lifestyle Report 2016: Bias and Burnout. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=1. Published January 13, 2016. Accessed July 7, 2016.
- Shanafelt TD, Boone, S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385.
- Martini S, Arfken CL, Churchill A, Balon R. Burnout comparison among residents in different medical specialties. Acad Psychiatry. 2004;28(3):240–242.
- Lee YY, Medford AR, Halim AS. Burnout in physicians. J R Coll Physicians Edinb. 2015;45(2):104–107.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600–1613.
- Rath KS, Huffman LB, Phillips GS, Carpenter KM, Fowler JM. Burnout and associated factors among members of the Society of Gynecologic Oncology. Am J Obstet Gynecol. 2015;213(6):824.e1–e9.
- Dyrbye LN, Varkey P, Boone SL, Satele DV, Sloan JA, Shanafelt TD. Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358–1367.
- Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389–395.
- Becker JL, Milad MP, Klock SC. Burnout, depression, and career satisfaction: cross-sectional study of obstetrics and gynecology residents. Am J Obstet Gynecol. 2006;195(5):1444–1449.
- Castelo-Branco C, Figueras F, Eixarch E, et al. Stress symptoms and burnout in obstetric and gynaecology residents. BJOG. 2007;114(1):94–98
Why burnout occurs
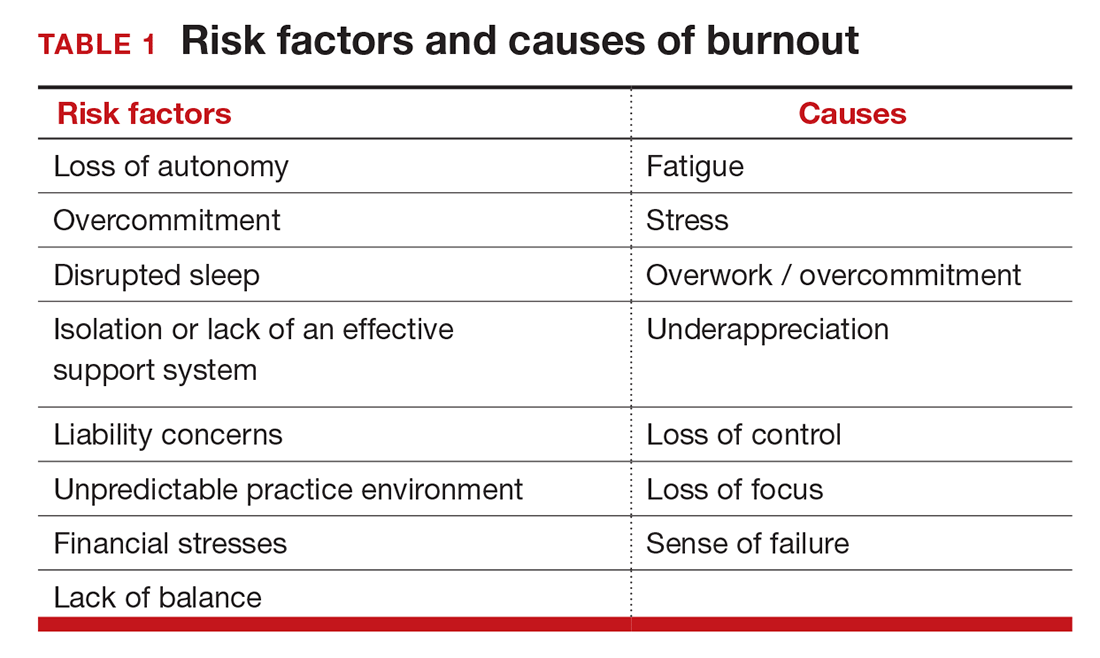
Simply identifying ourselves as professionals and the same attributes that make us successful as physicians (type-A behavior, obsessive-compulsive commitment to our profession) put us at risk for professional burnout (see “Who is most at risk for burnout?”). Those predilections combine with the forces from the world in which we live and practice to increase this threat (TABLE 1). Conditions in which there are weak retention rates, high turnover, heavy workloads, and low staffing levels or staffing shortages increase the risk of burnout and, when burnout is present, are associated with a degraded quality of care.5
Does stress cause burnout?
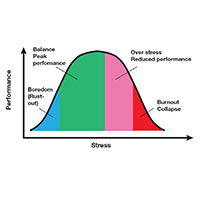
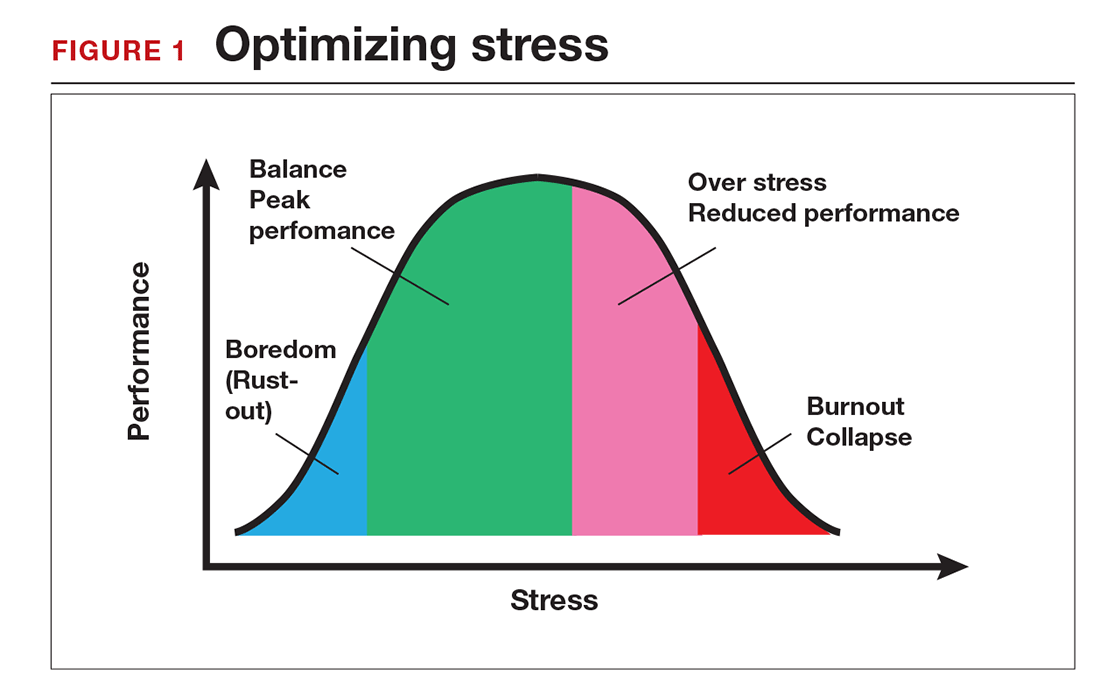
Stress is often seen as the reason for burnout. Research shows that there is no single source of burnout,6 however, and a number of factors combine to cause this physical or mental collapse. Stress can be a positive or negative factor in our performance. Too little stress and we feel underutilized; too much stress and we collapse from the strain.
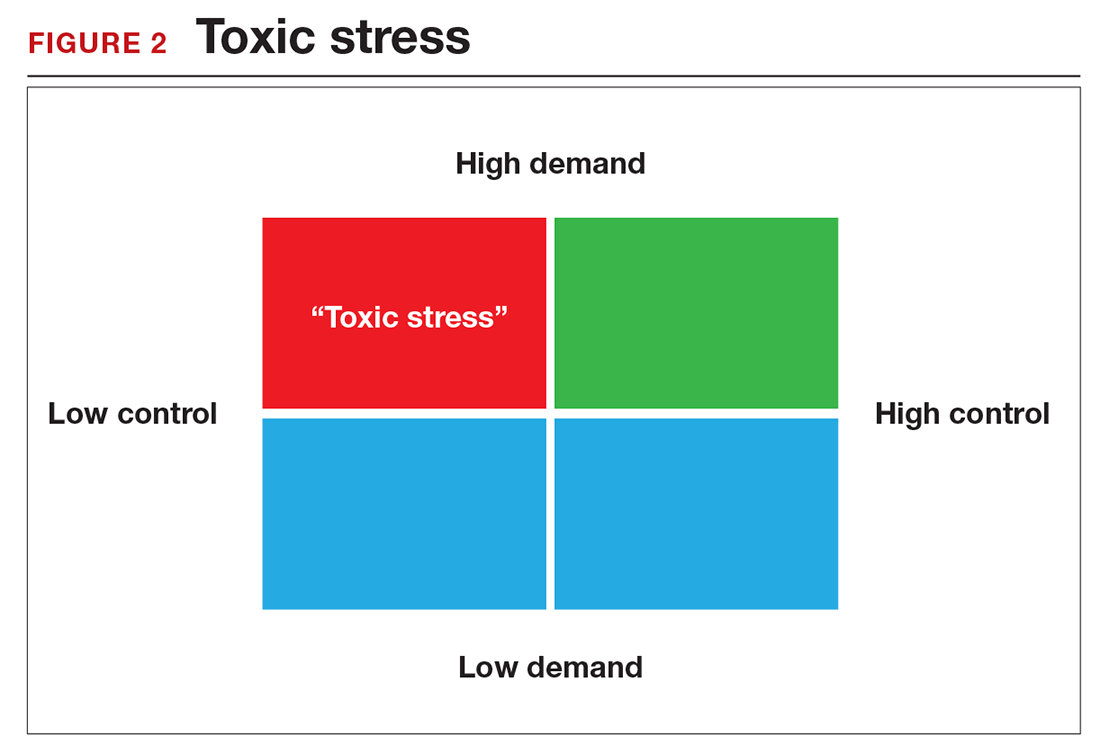
There is a middle ground where stress and expectations keep us focused and at peak productivity (FIGURE 1). The key is the balance between control and demand: When we have a greater level of control, we can handle high demands (FIGURE 2). It is when we lack that control that high demands result in what has been called “toxic stress,” and we collapse under the strain.
The impact of burnout
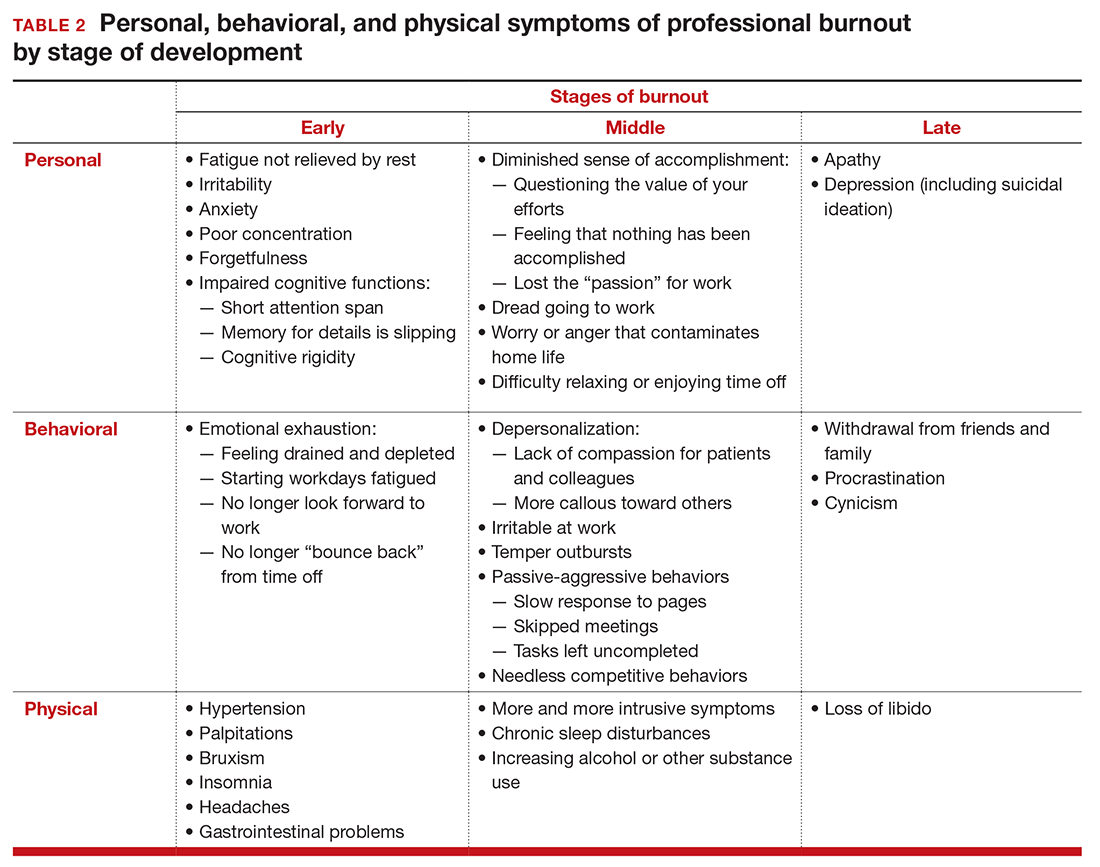
Burnout is associated with reduced performance and job satisfaction, increased rates of illness and absenteeism, accidents, premature retirement, and even premature death. Physically, stress induces the dry mouth, dilated pupils, and release of adrenalin and noradrenalin associated with the “fight-or-flight” reaction. The degree to which the physical, emotional, and professional symptoms are manifest depends on the depth or stage of burnout present (TABLE 2). Overall, burnout is associated with an increased risk for physical illness.7 Economically, the impact of physician burnout (for physicians practicing in Canada) has been estimated to be $213.1 million,8 which includes $185.2 million due to early retirement and $27.9 million due to reduced clinical hours.
“Do I have burnout?”
We all suffer from fatigue and have stress, but do we have burnout? With so many myths surrounding stress and burnout, it is sometimes hard to know where the truth lies. Some of those myths say that:
- you can leave your troubles at home
- mental stress does not affect physical performance
- stress is only for wimps
- stress and burnout are chemical imbalances that can be treated with medications
- stress is always bad
- burnout will get better if you just give it more time.
Maslach Burnout Inventory. The effective “gold standard” for diagnosing burnout is the Maslach Burnout Inventory,9 which operationalizes burnout as a 3-dimensional syndrome made up of exhaustion, cynicism, and inefficacy. Other diagnostic tools have been introduced10 but have not gained the wide acceptance of the Maslach Inventory. Some authors have argued that burnout and depression represent different, closely spaced points along a spectrum and that any effort to separate them may be artificial.11,12
The Maslach Burnout Inventory consists of a survey of 22 items; it requires a fee to take and is interpreted by a qualified individual. A simpler screening test consists of 10 questions (TABLE 3). If you answer “yes” to 5 or more of the questions, you probably have burnout. An even quicker test is to see, when you go on vacation, if your symptoms disappear. If so, you are not depressed; you have burnout. (If you cannot even go on vacation, then it is almost certain.)

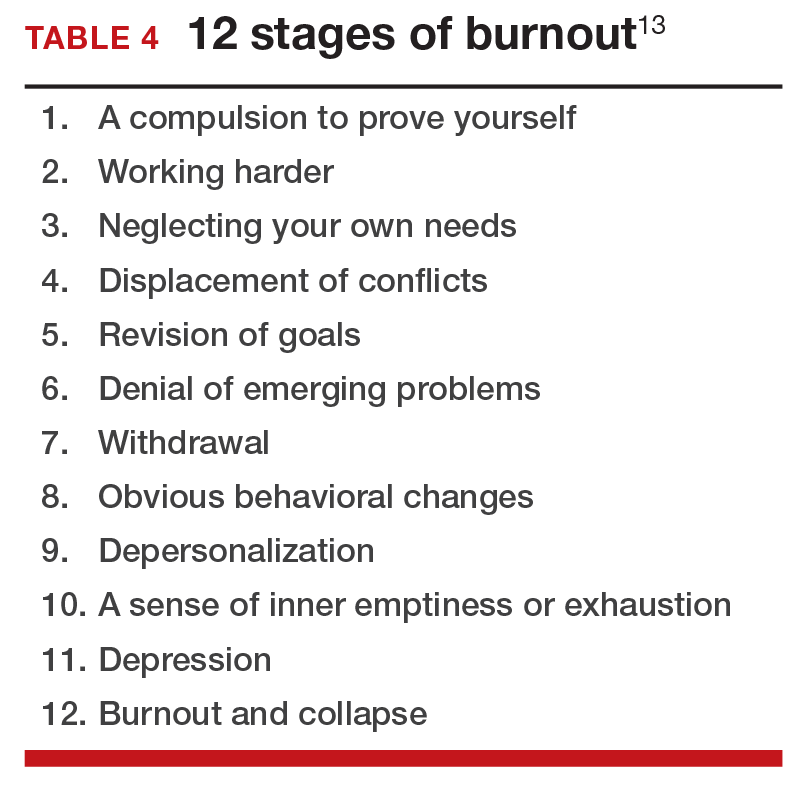
12 stages of burnout. Psychologists Herbert Freudenberger and Gail North have theorized that the burnout process can be divided into 12 phases (TABLE 4).13 These stages are not necessarily sequential—some may be absent and others may present simultaneously. It is easy to see how these can represent stages in a potentially spiraling series of behaviors and changes that result in complete dysfunction. It is also easy to understand that the characteristics that are associated with success in medical school, clinical training, and practice, such as high expectations, placing the needs of others above our own, and a desire to prove oneself, virtually define the first 3 stages.
Approaches for burnout control and prevention
There are some simple steps we can take to reduce the risk of burnout or to reverse its effects. Because fatigue and stress are 2 of the greatest risk factors, reducing these is a good place to start.
Prioritize sleep. When it comes to fatigue, that one is easy: get some sleep. Physicians tend to sleep fewer hours than the general population and what we get is often not the type that is restful and restorative.14 Just reducing the number of hours worked is not enough, as a number of studies have found.15 The rest must result in relaxation.
e Stress reduction may seem a more difficult goal than getting more sleep. In reality, there are several simple approaches to use to reduce stress:
- Even though we all have busy clinical schedules, take short breaks to rest, sing, laugh, and exercise. Even breaks as short as 10 minutes can be effective.16
- Separate work from private life by taking a short break to resolve issues before heading home. Avoiding “baggage” or homework will go a long way to giving you the perspective you need from your time off. This may also mean that you have to delegate tasks, share chores, or get carry-out for dinner.
- Set meaningful and realistic goals for yourself professionally and personally. Do not expect or demand more than is possible. This will mean setting priorities and recognizing that some tasks may have to wait.
- Finally, do not forget to pay yourself with hobbies and activities that you enjoy.
Take action
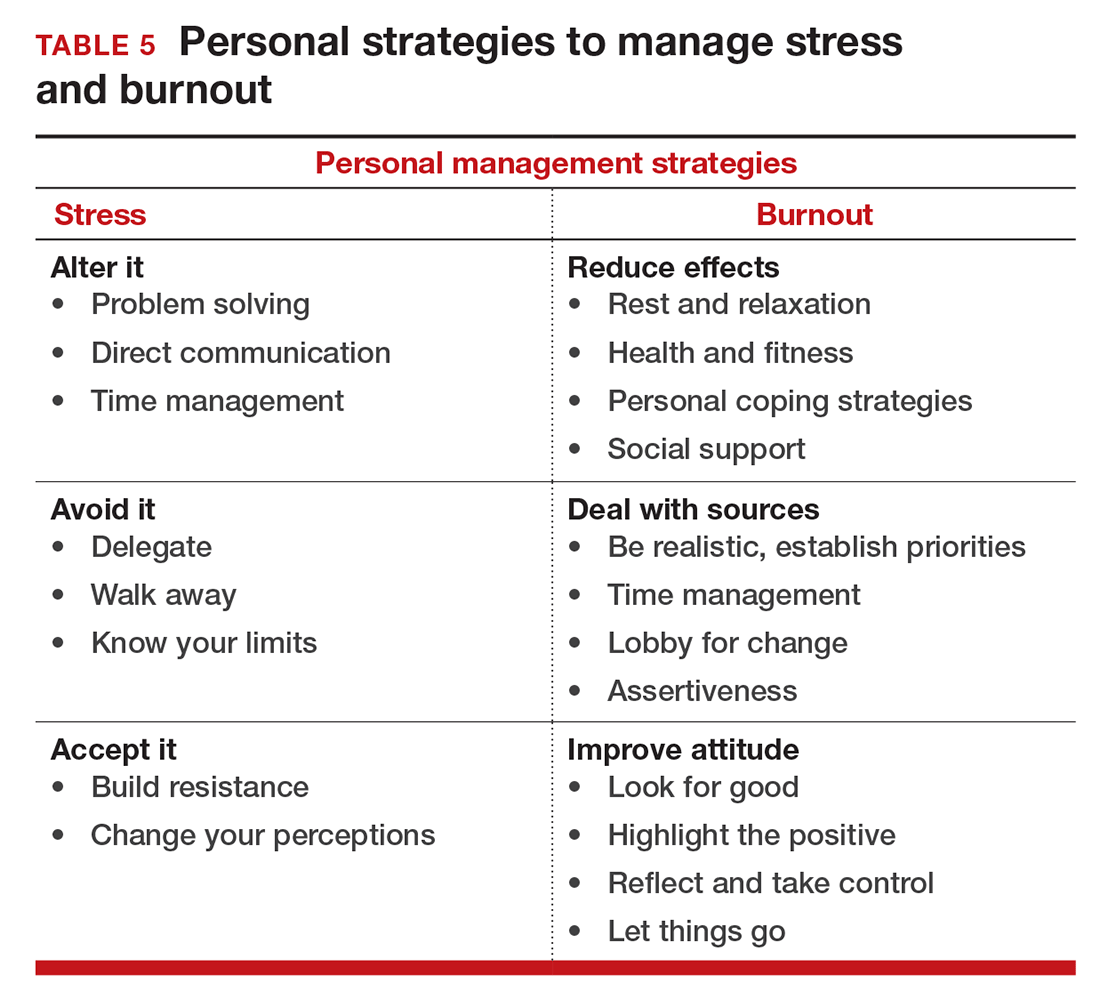
If you feel the effects of burnout tugging at your coattails, you can reduce the effects, deal with the sources, and improve your attitude (TABLE 5). Rest and relaxation will go a long way to helping, but do not forget to take care of your physical well-being with a healthy diet, exercise, and health checkups. Deal with the sources of burnout by identifying the stressors, setting realistic priorities, and practicing good time management.
You also should lobby for changes that will increase your control and reduce unnecessary obstacles to completing your goals. Be your own best advocate. Look for the good and try to identify at least one instance during the day where your presence or acts made a difference. In the end, it is like Smokey the Bear says, “Only you can prevent burnout.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Keeton K, Fenner DE, Johnson TR, Hayward RA. Predictors of physician career satisfaction, work-life balance, and burnout. Obstet Gynecol. 2007;109(4):949-955.
- Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836-848.
- Gabbe SG, Melville J, Mandel L, Walker E. Burnout in chairs of obstetrics and gynecology: diagnosis, treatment, and prevention. Am J Obstet Gynecol. 2002;186(4):601-612.
- Kok BC, Herrell RK, Grossman SH, West JC, Wilk JE. Prevalence of professional burnout among military mental health service providers. Psychiatr Serv. 2016;67(1):137-140.
- Humphries N, Morgan K, Conry MC, McGowan Y, Montgomery A, McGee H. Quality of care and health professional burnout: narrative literature review. Int J Health Care Qual Assur. 2014;27(4):293-307.
- Streu R, Hansen J, Abrahamse P, Alderman AK. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
- Honkonen T, Ahola K, Pertovaara M, et al. The association between burnout and physical illness in the general population--results from the Finnish Health 2000 Study. J Psychosom Res. 2006;61(1):59-66.
- Dewa CS, Jacobs P, Thanh NX, Loong D. An estimate of the cost of burnout on early retirement and reduction in clinical hours of practicing physicians in Canada. BMC Health Serv Res. 2014;14:254.
- Maslach C, Jackson SE, Leiter MP. The Maslach Burnout Inventory Manual. Palo Alto, California: Consulting Psychologists Press, 1996.
- Kristensen TS, Borritz M, Villadsen E, Christensen KB. The Copenhagen Burnout Inventory: A new tool for the assessment of burnout. Work & Stress. 2005;19(3):192-207.
- Bianchi R, Boffy C, Hingray C, Truchot D, Laurent E. Comparative symptomatology of burnout and depression. J Health Psychol. 2013;18(6):782-787.
- Bianchi R, Schonfeld I S, Laurent E. Is burnout a depressive disorder? A re-examination with special focus on atypical depression. Intl J Stress Manag. 2014;21(4):307-324.
- Freudenberger HJ, North G. Women's burnout: How to spot it, how to reverse it, and how to prevent it. New York, New York: Doubleday, 1985.
- Abrams RM. Sleep deprivation. Obstet Gynecol Clin North Am. 2015;42(3):493-506.
- Williams D, Tricomi G, Gupta J, Janise A. Efficacy of burnout interventions in the medical education pipeline. Acad Psychiatry. 2015;39(1):47-54.
- Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: The personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
It sometimes seems that the pace of life, and its stresses, have spiraled out of control: There just never seems to be enough time to deal with all the directions in which we are pulled. This easily can lead to the exhaustion of physical or emotional strength or motivation, otherwise known as “burnout.” Burnout is physical or mental collapse caused by overwork or stress and we are all at risk of suffering it. Conflicting demands on our time, loss of control (real or imagined), and a diminishing sense of worth grind at us from every direction.
In general, having some control over schedule and hours worked is associated with reductions in burnout and improved job satisfaction.1 But this is not always the case. Well-intentioned efforts to reduce workload, such as the electronic medical records or physician order entry systems, have actually made the problem worse.2 The seeming level of control that comes with being the chair of an obstetrics and gynecology department does not necessarily reduce burnout rates,3 and neither does the perceived resilience of mental health professionals, who still report burnout rates that approach 25%.4
This article continues the focus on recalibrating work/life balance that began last month with “ObGyn burnout: ACOG takes aim,” by Lucia DiVenere, MA, and the peer-to-peer audiocast with Ms. DiVenere and myself titled “Is burnout on the rise and what are the signs ObGyns should be on the lookout for?” Here, I identify the causes and symptoms of burnout and provide specific tools to help you develop resilience.
Who is most at risk for burnout?
Estimates range from 40% to 75% of ObGyns currently suffer from professional burnout, making the lifetime risk a virtual certainty.1−3 The idea of professional burnout is not new, but wider recognition of the alarming rates of burnout is very current.4,5 A recent survey of gynecologic oncologists6 found that of those studied 30% scored high for emotional exhaustion, 10% high for depersonalization, and 11% low for personal accomplishment. Overall, 32% of physicians had scores indicating burnout. More worrisome was that 33% screened positive for depression, 13% had a history of suicidal ideation, 15% screened positive for alcohol abuse, and 34% reported impaired quality of life. Almost 40% would not encourage their children to enter medicine and more than 10% said that they would not enter medicine again if they had to do it over.
Residents and those at mid-career are particularly vulnerable,7 with resident burnout rates reported to be as high as 75%.8 Of surveyed residents in a 2012 study, 13% satisfied all 3 subscale scores for high burnout and greater than 50% had high levels of depersonalization and emotional exhaustion. Those with high levels of emotional exhaustion were less satisfied with their careers, regretted choosing obstetrics and gynecology, and had higher rates of depression—all findings consistent with older studies.
9,10
References
- Peckham C. Medscape Lifestyle Report 2016: Bias and Burnout. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=1. Published January 13, 2016. Accessed July 7, 2016.
- Shanafelt TD, Boone, S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385.
- Martini S, Arfken CL, Churchill A, Balon R. Burnout comparison among residents in different medical specialties. Acad Psychiatry. 2004;28(3):240–242.
- Lee YY, Medford AR, Halim AS. Burnout in physicians. J R Coll Physicians Edinb. 2015;45(2):104–107.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600–1613.
- Rath KS, Huffman LB, Phillips GS, Carpenter KM, Fowler JM. Burnout and associated factors among members of the Society of Gynecologic Oncology. Am J Obstet Gynecol. 2015;213(6):824.e1–e9.
- Dyrbye LN, Varkey P, Boone SL, Satele DV, Sloan JA, Shanafelt TD. Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358–1367.
- Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389–395.
- Becker JL, Milad MP, Klock SC. Burnout, depression, and career satisfaction: cross-sectional study of obstetrics and gynecology residents. Am J Obstet Gynecol. 2006;195(5):1444–1449.
- Castelo-Branco C, Figueras F, Eixarch E, et al. Stress symptoms and burnout in obstetric and gynaecology residents. BJOG. 2007;114(1):94–98
Why burnout occurs
Simply identifying ourselves as professionals and the same attributes that make us successful as physicians (type-A behavior, obsessive-compulsive commitment to our profession) put us at risk for professional burnout (see “Who is most at risk for burnout?”). Those predilections combine with the forces from the world in which we live and practice to increase this threat (TABLE 1). Conditions in which there are weak retention rates, high turnover, heavy workloads, and low staffing levels or staffing shortages increase the risk of burnout and, when burnout is present, are associated with a degraded quality of care.5

Does stress cause burnout?
Stress is often seen as the reason for burnout. Research shows that there is no single source of burnout,6 however, and a number of factors combine to cause this physical or mental collapse. Stress can be a positive or negative factor in our performance. Too little stress and we feel underutilized; too much stress and we collapse from the strain.
There is a middle ground where stress and expectations keep us focused and at peak productivity (FIGURE 1). The key is the balance between control and demand: When we have a greater level of control, we can handle high demands (FIGURE 2). It is when we lack that control that high demands result in what has been called “toxic stress,” and we collapse under the strain.


The impact of burnout
Burnout is associated with reduced performance and job satisfaction, increased rates of illness and absenteeism, accidents, premature retirement, and even premature death. Physically, stress induces the dry mouth, dilated pupils, and release of adrenalin and noradrenalin associated with the “fight-or-flight” reaction. The degree to which the physical, emotional, and professional symptoms are manifest depends on the depth or stage of burnout present (TABLE 2). Overall, burnout is associated with an increased risk for physical illness.7 Economically, the impact of physician burnout (for physicians practicing in Canada) has been estimated to be $213.1 million,8 which includes $185.2 million due to early retirement and $27.9 million due to reduced clinical hours.

“Do I have burnout?”
We all suffer from fatigue and have stress, but do we have burnout? With so many myths surrounding stress and burnout, it is sometimes hard to know where the truth lies. Some of those myths say that:
- you can leave your troubles at home
- mental stress does not affect physical performance
- stress is only for wimps
- stress and burnout are chemical imbalances that can be treated with medications
- stress is always bad
- burnout will get better if you just give it more time.
Maslach Burnout Inventory. The effective “gold standard” for diagnosing burnout is the Maslach Burnout Inventory,9 which operationalizes burnout as a 3-dimensional syndrome made up of exhaustion, cynicism, and inefficacy. Other diagnostic tools have been introduced10 but have not gained the wide acceptance of the Maslach Inventory. Some authors have argued that burnout and depression represent different, closely spaced points along a spectrum and that any effort to separate them may be artificial.11,12
The Maslach Burnout Inventory consists of a survey of 22 items; it requires a fee to take and is interpreted by a qualified individual. A simpler screening test consists of 10 questions (TABLE 3). If you answer “yes” to 5 or more of the questions, you probably have burnout. An even quicker test is to see, when you go on vacation, if your symptoms disappear. If so, you are not depressed; you have burnout. (If you cannot even go on vacation, then it is almost certain.)

12 stages of burnout. Psychologists Herbert Freudenberger and Gail North have theorized that the burnout process can be divided into 12 phases (TABLE 4).13 These stages are not necessarily sequential—some may be absent and others may present simultaneously. It is easy to see how these can represent stages in a potentially spiraling series of behaviors and changes that result in complete dysfunction. It is also easy to understand that the characteristics that are associated with success in medical school, clinical training, and practice, such as high expectations, placing the needs of others above our own, and a desire to prove oneself, virtually define the first 3 stages.

Approaches for burnout control and prevention
There are some simple steps we can take to reduce the risk of burnout or to reverse its effects. Because fatigue and stress are 2 of the greatest risk factors, reducing these is a good place to start.
Prioritize sleep. When it comes to fatigue, that one is easy: get some sleep. Physicians tend to sleep fewer hours than the general population and what we get is often not the type that is restful and restorative.14 Just reducing the number of hours worked is not enough, as a number of studies have found.15 The rest must result in relaxation.
e Stress reduction may seem a more difficult goal than getting more sleep. In reality, there are several simple approaches to use to reduce stress:
- Even though we all have busy clinical schedules, take short breaks to rest, sing, laugh, and exercise. Even breaks as short as 10 minutes can be effective.16
- Separate work from private life by taking a short break to resolve issues before heading home. Avoiding “baggage” or homework will go a long way to giving you the perspective you need from your time off. This may also mean that you have to delegate tasks, share chores, or get carry-out for dinner.
- Set meaningful and realistic goals for yourself professionally and personally. Do not expect or demand more than is possible. This will mean setting priorities and recognizing that some tasks may have to wait.
- Finally, do not forget to pay yourself with hobbies and activities that you enjoy.
Take action
If you feel the effects of burnout tugging at your coattails, you can reduce the effects, deal with the sources, and improve your attitude (TABLE 5). Rest and relaxation will go a long way to helping, but do not forget to take care of your physical well-being with a healthy diet, exercise, and health checkups. Deal with the sources of burnout by identifying the stressors, setting realistic priorities, and practicing good time management.

You also should lobby for changes that will increase your control and reduce unnecessary obstacles to completing your goals. Be your own best advocate. Look for the good and try to identify at least one instance during the day where your presence or acts made a difference. In the end, it is like Smokey the Bear says, “Only you can prevent burnout.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It sometimes seems that the pace of life, and its stresses, have spiraled out of control: There just never seems to be enough time to deal with all the directions in which we are pulled. This easily can lead to the exhaustion of physical or emotional strength or motivation, otherwise known as “burnout.” Burnout is physical or mental collapse caused by overwork or stress and we are all at risk of suffering it. Conflicting demands on our time, loss of control (real or imagined), and a diminishing sense of worth grind at us from every direction.
In general, having some control over schedule and hours worked is associated with reductions in burnout and improved job satisfaction.1 But this is not always the case. Well-intentioned efforts to reduce workload, such as the electronic medical records or physician order entry systems, have actually made the problem worse.2 The seeming level of control that comes with being the chair of an obstetrics and gynecology department does not necessarily reduce burnout rates,3 and neither does the perceived resilience of mental health professionals, who still report burnout rates that approach 25%.4
This article continues the focus on recalibrating work/life balance that began last month with “ObGyn burnout: ACOG takes aim,” by Lucia DiVenere, MA, and the peer-to-peer audiocast with Ms. DiVenere and myself titled “Is burnout on the rise and what are the signs ObGyns should be on the lookout for?” Here, I identify the causes and symptoms of burnout and provide specific tools to help you develop resilience.
Who is most at risk for burnout?
Estimates range from 40% to 75% of ObGyns currently suffer from professional burnout, making the lifetime risk a virtual certainty.1−3 The idea of professional burnout is not new, but wider recognition of the alarming rates of burnout is very current.4,5 A recent survey of gynecologic oncologists6 found that of those studied 30% scored high for emotional exhaustion, 10% high for depersonalization, and 11% low for personal accomplishment. Overall, 32% of physicians had scores indicating burnout. More worrisome was that 33% screened positive for depression, 13% had a history of suicidal ideation, 15% screened positive for alcohol abuse, and 34% reported impaired quality of life. Almost 40% would not encourage their children to enter medicine and more than 10% said that they would not enter medicine again if they had to do it over.
Residents and those at mid-career are particularly vulnerable,7 with resident burnout rates reported to be as high as 75%.8 Of surveyed residents in a 2012 study, 13% satisfied all 3 subscale scores for high burnout and greater than 50% had high levels of depersonalization and emotional exhaustion. Those with high levels of emotional exhaustion were less satisfied with their careers, regretted choosing obstetrics and gynecology, and had higher rates of depression—all findings consistent with older studies.
9,10
References
- Peckham C. Medscape Lifestyle Report 2016: Bias and Burnout. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=1. Published January 13, 2016. Accessed July 7, 2016.
- Shanafelt TD, Boone, S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385.
- Martini S, Arfken CL, Churchill A, Balon R. Burnout comparison among residents in different medical specialties. Acad Psychiatry. 2004;28(3):240–242.
- Lee YY, Medford AR, Halim AS. Burnout in physicians. J R Coll Physicians Edinb. 2015;45(2):104–107.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600–1613.
- Rath KS, Huffman LB, Phillips GS, Carpenter KM, Fowler JM. Burnout and associated factors among members of the Society of Gynecologic Oncology. Am J Obstet Gynecol. 2015;213(6):824.e1–e9.
- Dyrbye LN, Varkey P, Boone SL, Satele DV, Sloan JA, Shanafelt TD. Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358–1367.
- Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389–395.
- Becker JL, Milad MP, Klock SC. Burnout, depression, and career satisfaction: cross-sectional study of obstetrics and gynecology residents. Am J Obstet Gynecol. 2006;195(5):1444–1449.
- Castelo-Branco C, Figueras F, Eixarch E, et al. Stress symptoms and burnout in obstetric and gynaecology residents. BJOG. 2007;114(1):94–98
Why burnout occurs
Simply identifying ourselves as professionals and the same attributes that make us successful as physicians (type-A behavior, obsessive-compulsive commitment to our profession) put us at risk for professional burnout (see “Who is most at risk for burnout?”). Those predilections combine with the forces from the world in which we live and practice to increase this threat (TABLE 1). Conditions in which there are weak retention rates, high turnover, heavy workloads, and low staffing levels or staffing shortages increase the risk of burnout and, when burnout is present, are associated with a degraded quality of care.5

Does stress cause burnout?
Stress is often seen as the reason for burnout. Research shows that there is no single source of burnout,6 however, and a number of factors combine to cause this physical or mental collapse. Stress can be a positive or negative factor in our performance. Too little stress and we feel underutilized; too much stress and we collapse from the strain.
There is a middle ground where stress and expectations keep us focused and at peak productivity (FIGURE 1). The key is the balance between control and demand: When we have a greater level of control, we can handle high demands (FIGURE 2). It is when we lack that control that high demands result in what has been called “toxic stress,” and we collapse under the strain.


The impact of burnout
Burnout is associated with reduced performance and job satisfaction, increased rates of illness and absenteeism, accidents, premature retirement, and even premature death. Physically, stress induces the dry mouth, dilated pupils, and release of adrenalin and noradrenalin associated with the “fight-or-flight” reaction. The degree to which the physical, emotional, and professional symptoms are manifest depends on the depth or stage of burnout present (TABLE 2). Overall, burnout is associated with an increased risk for physical illness.7 Economically, the impact of physician burnout (for physicians practicing in Canada) has been estimated to be $213.1 million,8 which includes $185.2 million due to early retirement and $27.9 million due to reduced clinical hours.

“Do I have burnout?”
We all suffer from fatigue and have stress, but do we have burnout? With so many myths surrounding stress and burnout, it is sometimes hard to know where the truth lies. Some of those myths say that:
- you can leave your troubles at home
- mental stress does not affect physical performance
- stress is only for wimps
- stress and burnout are chemical imbalances that can be treated with medications
- stress is always bad
- burnout will get better if you just give it more time.
Maslach Burnout Inventory. The effective “gold standard” for diagnosing burnout is the Maslach Burnout Inventory,9 which operationalizes burnout as a 3-dimensional syndrome made up of exhaustion, cynicism, and inefficacy. Other diagnostic tools have been introduced10 but have not gained the wide acceptance of the Maslach Inventory. Some authors have argued that burnout and depression represent different, closely spaced points along a spectrum and that any effort to separate them may be artificial.11,12
The Maslach Burnout Inventory consists of a survey of 22 items; it requires a fee to take and is interpreted by a qualified individual. A simpler screening test consists of 10 questions (TABLE 3). If you answer “yes” to 5 or more of the questions, you probably have burnout. An even quicker test is to see, when you go on vacation, if your symptoms disappear. If so, you are not depressed; you have burnout. (If you cannot even go on vacation, then it is almost certain.)

12 stages of burnout. Psychologists Herbert Freudenberger and Gail North have theorized that the burnout process can be divided into 12 phases (TABLE 4).13 These stages are not necessarily sequential—some may be absent and others may present simultaneously. It is easy to see how these can represent stages in a potentially spiraling series of behaviors and changes that result in complete dysfunction. It is also easy to understand that the characteristics that are associated with success in medical school, clinical training, and practice, such as high expectations, placing the needs of others above our own, and a desire to prove oneself, virtually define the first 3 stages.

Approaches for burnout control and prevention
There are some simple steps we can take to reduce the risk of burnout or to reverse its effects. Because fatigue and stress are 2 of the greatest risk factors, reducing these is a good place to start.
Prioritize sleep. When it comes to fatigue, that one is easy: get some sleep. Physicians tend to sleep fewer hours than the general population and what we get is often not the type that is restful and restorative.14 Just reducing the number of hours worked is not enough, as a number of studies have found.15 The rest must result in relaxation.
e Stress reduction may seem a more difficult goal than getting more sleep. In reality, there are several simple approaches to use to reduce stress:
- Even though we all have busy clinical schedules, take short breaks to rest, sing, laugh, and exercise. Even breaks as short as 10 minutes can be effective.16
- Separate work from private life by taking a short break to resolve issues before heading home. Avoiding “baggage” or homework will go a long way to giving you the perspective you need from your time off. This may also mean that you have to delegate tasks, share chores, or get carry-out for dinner.
- Set meaningful and realistic goals for yourself professionally and personally. Do not expect or demand more than is possible. This will mean setting priorities and recognizing that some tasks may have to wait.
- Finally, do not forget to pay yourself with hobbies and activities that you enjoy.
Take action
If you feel the effects of burnout tugging at your coattails, you can reduce the effects, deal with the sources, and improve your attitude (TABLE 5). Rest and relaxation will go a long way to helping, but do not forget to take care of your physical well-being with a healthy diet, exercise, and health checkups. Deal with the sources of burnout by identifying the stressors, setting realistic priorities, and practicing good time management.

You also should lobby for changes that will increase your control and reduce unnecessary obstacles to completing your goals. Be your own best advocate. Look for the good and try to identify at least one instance during the day where your presence or acts made a difference. In the end, it is like Smokey the Bear says, “Only you can prevent burnout.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Keeton K, Fenner DE, Johnson TR, Hayward RA. Predictors of physician career satisfaction, work-life balance, and burnout. Obstet Gynecol. 2007;109(4):949-955.
- Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836-848.
- Gabbe SG, Melville J, Mandel L, Walker E. Burnout in chairs of obstetrics and gynecology: diagnosis, treatment, and prevention. Am J Obstet Gynecol. 2002;186(4):601-612.
- Kok BC, Herrell RK, Grossman SH, West JC, Wilk JE. Prevalence of professional burnout among military mental health service providers. Psychiatr Serv. 2016;67(1):137-140.
- Humphries N, Morgan K, Conry MC, McGowan Y, Montgomery A, McGee H. Quality of care and health professional burnout: narrative literature review. Int J Health Care Qual Assur. 2014;27(4):293-307.
- Streu R, Hansen J, Abrahamse P, Alderman AK. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
- Honkonen T, Ahola K, Pertovaara M, et al. The association between burnout and physical illness in the general population--results from the Finnish Health 2000 Study. J Psychosom Res. 2006;61(1):59-66.
- Dewa CS, Jacobs P, Thanh NX, Loong D. An estimate of the cost of burnout on early retirement and reduction in clinical hours of practicing physicians in Canada. BMC Health Serv Res. 2014;14:254.
- Maslach C, Jackson SE, Leiter MP. The Maslach Burnout Inventory Manual. Palo Alto, California: Consulting Psychologists Press, 1996.
- Kristensen TS, Borritz M, Villadsen E, Christensen KB. The Copenhagen Burnout Inventory: A new tool for the assessment of burnout. Work & Stress. 2005;19(3):192-207.
- Bianchi R, Boffy C, Hingray C, Truchot D, Laurent E. Comparative symptomatology of burnout and depression. J Health Psychol. 2013;18(6):782-787.
- Bianchi R, Schonfeld I S, Laurent E. Is burnout a depressive disorder? A re-examination with special focus on atypical depression. Intl J Stress Manag. 2014;21(4):307-324.
- Freudenberger HJ, North G. Women's burnout: How to spot it, how to reverse it, and how to prevent it. New York, New York: Doubleday, 1985.
- Abrams RM. Sleep deprivation. Obstet Gynecol Clin North Am. 2015;42(3):493-506.
- Williams D, Tricomi G, Gupta J, Janise A. Efficacy of burnout interventions in the medical education pipeline. Acad Psychiatry. 2015;39(1):47-54.
- Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: The personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
- Keeton K, Fenner DE, Johnson TR, Hayward RA. Predictors of physician career satisfaction, work-life balance, and burnout. Obstet Gynecol. 2007;109(4):949-955.
- Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836-848.
- Gabbe SG, Melville J, Mandel L, Walker E. Burnout in chairs of obstetrics and gynecology: diagnosis, treatment, and prevention. Am J Obstet Gynecol. 2002;186(4):601-612.
- Kok BC, Herrell RK, Grossman SH, West JC, Wilk JE. Prevalence of professional burnout among military mental health service providers. Psychiatr Serv. 2016;67(1):137-140.
- Humphries N, Morgan K, Conry MC, McGowan Y, Montgomery A, McGee H. Quality of care and health professional burnout: narrative literature review. Int J Health Care Qual Assur. 2014;27(4):293-307.
- Streu R, Hansen J, Abrahamse P, Alderman AK. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
- Honkonen T, Ahola K, Pertovaara M, et al. The association between burnout and physical illness in the general population--results from the Finnish Health 2000 Study. J Psychosom Res. 2006;61(1):59-66.
- Dewa CS, Jacobs P, Thanh NX, Loong D. An estimate of the cost of burnout on early retirement and reduction in clinical hours of practicing physicians in Canada. BMC Health Serv Res. 2014;14:254.
- Maslach C, Jackson SE, Leiter MP. The Maslach Burnout Inventory Manual. Palo Alto, California: Consulting Psychologists Press, 1996.
- Kristensen TS, Borritz M, Villadsen E, Christensen KB. The Copenhagen Burnout Inventory: A new tool for the assessment of burnout. Work & Stress. 2005;19(3):192-207.
- Bianchi R, Boffy C, Hingray C, Truchot D, Laurent E. Comparative symptomatology of burnout and depression. J Health Psychol. 2013;18(6):782-787.
- Bianchi R, Schonfeld I S, Laurent E. Is burnout a depressive disorder? A re-examination with special focus on atypical depression. Intl J Stress Manag. 2014;21(4):307-324.
- Freudenberger HJ, North G. Women's burnout: How to spot it, how to reverse it, and how to prevent it. New York, New York: Doubleday, 1985.
- Abrams RM. Sleep deprivation. Obstet Gynecol Clin North Am. 2015;42(3):493-506.
- Williams D, Tricomi G, Gupta J, Janise A. Efficacy of burnout interventions in the medical education pipeline. Acad Psychiatry. 2015;39(1):47-54.
- Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: The personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
In this Article
- Symptoms by stage of burnout
- Tips to reduce stress and burnout
- Who is most at risk for burnout?
2016 Update on cancer
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
In this Article
- The prognostic significance of tumors with POLE mutations
- Can the immune system kill MMR-deficient endometrial cancer?
4 Supreme Court decisions important to ObGyns from the 2015−2016 term
Each year, the decisions of the Supreme Court have a significant impact on ObGyn practice. During the 2015–2016 term, which ended in June, the Court issued important rulings on abortion facilities, Affordable Care Act (ACA) contraception coverage, health care False Claims Act (FCA) liability, and state health care data collection. The American Medical Association (AMA), the Association of American Medical Colleges (AAMC), the American College of Obstetricians and Gynecologists (ACOG), and other organizations that represent health care professionals play an important role in health-related Supreme Court cases. For example, amicus curiae (“friend of the Court”) briefs are filed not by parties to a case but by organizations that have a special insight into or interest in a case. Although the extent to which amicus briefs influence cases is often unclear, organization representatives think their briefs make a difference, and briefs undoubtedly do in some cases.
The 2016 presidential election will determine the Supreme Court make-up for the next term, but in this article we consider recent cases that affect ObGyns’ practice in particular. We start with the cases in which professional organizations filed amicus briefs and then turn to other notable cases.
1. Abortion access in Texas and other states
The most important ObGyn case of the 2015–2016 term was Whole Woman’s Health v Hellerstedt.1
At stake. Texas adopted a statute requiring 1) that physicians who perform abortions have admitting privileges at a hospital within 30 miles of the clinic and 2) that abortion clinics meet the state’s standards for ambulatory surgical centers. The current law, upheld by the Court some years ago, is that state laws affecting abortion are unconstitutional if they “unduly burden” the right to abortion. By undue burden, the Court meant, “Regulations that have the purpose or effect of presenting a substantial obstacle to a woman seeking an abortion impose an undue burden on the right.” The question in the Texas case was whether the statute’s 2 requirements were undue.
ACOG, AMA, and other groups filed a brief stating that the Texas law did not promote the welfare of women but instead was unnecessary and not “supported by accepted medical practice or scientific evidence.”2 In another brief the Society of Hospital Medicine and the Society of ObGyn Hospitalists also indicated that having admitting privileges is appropriate only for physicians who regularly admit patients to a hospital.3
A brief filed by the American Association of Pro-Life Obstetricians and Gynecologists and several other organizations argued the other side: “The surgical center and admitting privileges requirements imposed by the Act reflect the professional standard of practice for outpatient gynecological and similar surgery.”4
Final ruling. In a 5−3 decision, the Court struck down the Texas law for providing little or no health benefits while significantly burdening abortion facility access. Many clinics had closed or were in plans to because of the difficulty and expense of complying with the law. This case has national implications. Similar laws, either in place or being considered in other states, will almost certainly be ruled unconstitutional.
2. Contraceptive coverage
The case of Zubik v Burwell was closely watched this past year.
At stake. Under the ACA, a nonprofit religious organization may certify its objection to its insurance plan’s contraception coverage, at which point other arrangements are made to provide contraceptive coverage through the same plan. Religious organizations objected to the certification requirement.
A brief filed by ACOG, Physicians for Reproductive Health, and other groups emphasized the importance of providing contraceptives and contraceptive counseling as part of regular health care and suggested that the current accommodation for religious organizations is appropriate.5
After hearing the formal oral arguments, the Court asked for additional briefs on “whether contraceptive coverage could be provided to petitioners’ employees, through petitioners’ insurance companies, without any such notice from petitioners.”6
Final ruling. The parties agreed such a system would resolve the issue, so the Court sent the case back to the lower court to work out the details. In effect, the case was mediated—an unusual if not unique action for the Court. The resolution probably will achieve what the briefs sought—access to contraceptives and continuity of care.
3. Fraud and abuse litigation
The FCA, which provides for triple damages (3 times actual damages) and stiff civil penalties for anyone who presents the federal government (Medicare, Medicaid) with false claims for goods or services, is a major means of uncovering and punishing health care fraud and abuse. In health care, this law has been used to prosecute cases involving services paid for but not provided, unnecessary services, and off-label pharmaceutical promotion.
An important part of the FCA is that it allows a private intervenor (whistleblower) to initiate an action against a health care provider. The government may then take up the case. If not, the intervenor may pursue it; the incentive is 15% to 30% of the damages the government is awarded.
At stake. The Court was asked if “implied certification” applies to FCA cases.7 Implied certification means that requesting a payment from Medicare or Medicaid implies that the provider is not knowingly withholding information material to the government’s decision to pay the claim. In separately filed briefs, AMA et al8 and American Hospital Association (AHA) et al9 argued that applying implied certification to FCA cases would expand FCA litigation (particularly by intervenors), which is already expensive for health care institutions.
Final ruling. The Court unanimously adopted implied certification but noted that nondisclosure of information must be shown to be a material misrepresentation rather than a trivial regulatory or contractual violation. Furthermore, the Court emphasized that the basis for a claim must be an allegation of fraud, not of malpractice. These findings, which certainly are not what the health care organizations had hoped for, likely will lead to an increase in FCA cases.
4. Collection of state health care data
In Vermont, and about 20 other states that collect data on health care utilization and costs, health insurers and other entities are required to submit detailed reports about health care claims.10 Some insurers objected to this requirement.
At stake. An AHA–AAMC brief noted the importance of health care data and of Vermont’s collecting these data as contributing to better, more efficient health care delivery.11 Another brief, filed by AMA and the Vermont Medical Society, presented more legal or statutory arguments.12
Final ruling. The Court held that the Vermont plan and similar plans violate the federal Employee Retirement Income Security Act of 1974. As health insurance companies and other entities already provide detailed utilization and cost data to the federal government, producing up to 50 additional reports for state governments would be burdensome. Any state that wants the information, the Court said, should obtain it from the federal government.
More notable 2015-2016 Supreme Court decisions.
The Court:
- permitted limited consideration of race in university admissions. ACOG, AAMC, and AMA with many other groups filed an amicus brief supporting medical school and university affirmative action programs.1
- held that a state must give full faith and credit to the adoption orders of the courts of other states (this case involved an LGBT couple).2
- held that states may require (without a search warrant) a breathalyzer test, but not a blood test, for a driver suspected of drinking.3
- narrowed the ability of the federal government to seize or restrain (before trial) the assets of a person charged with criminal health care offenses.4
- temporarily stayed the August 2016 US Department of Education order to schools to allow transgender students to use the facilities in which they feel "most comfortable." The Court likely will take up this case very soon.5
- Fisher v University of Texas at Austin et al, No. 14-981 (2016). https://www.supremecourt.gov/opinions/15pdf/14-981_4g15.pdf. Accessed August 30, 2016.
- V.L. v E.L. et al, No. 15-648 (2016). https://www.supremecourt.gov/opinions/15pdf/15-648_d18e.pdf. Accessed August 30, 2016.
- Birchfield v North Dakota, No. 14-1468 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1468_8n59.pdf. Accessed August 30, 2016.
- Luis v United States, No. 14-419 (2016). https://www.supremecourt.gov/opinions/15pdf/14-419_nmip.pdf. Accessed August 30, 2016.
- Gloucester County School Board v G.G., by his next friend and mother, Deidre Grimm, No. 16A52 (2016). https://www.supremecourt.gov/opinions/15pdf/16a52_8759.pdf. Accessed August 30, 2016.
What’s to come
The Court’s recent decisions on access to abortion services and contraceptives were good for patients of ObGyns, but its decisions on health care FCA liability and state health care data collection were, arguably, not as good for ObGyn business practices.
The Court itself had an unusual year. Justice Scalia died in February, and Congress’s inaction on seating a replacement meant that most of the term’s cases were decided by an 8-member Court. Nevertheless, the Court was deadlocked 4−4 on only 4 of the 80 cases it heard. In addition, it was relatively agreed on outcomes; in only about one-third of cases were there more than 2 justices disagreeing with the outcome.
It is unlikely that a replacement for Justice Scalia will be confirmed before the Court begins its new term in October. The need to replace Justice Scalia and the potential turnover of other Court members—Justice Ginsburg is 83, Justice Kennedy is 80, and Justice Breyer is 78—are reminders of the importance of this year’s presidential election. In the meantime, the Court is accepting the cases that will make up the coming term’s docket, and ObGyns undoubtedly will play a role in cases that involve health care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). https://www.supremecourt.gov/opinions/15pdf/15-274_new_e18f.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Osteopathic Association, and American Academy of Pediatrics in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/ACOG-WilmerHale.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by Society of Hospital Medicine and Society of Ob/Gyn Hospitalists in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/Society-of-Hospital-Medicine-Crowell.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Association of Pro-Life Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Association, Catholic Medical Association, and Physicians for Life in support of respondents. Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al. http://www.scotusblog.com/wp-content/uploads/2016/02/15-274-bsac-American-Association-of-Pro-Life-Obstetricians-and-Gynecolog....pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, American Academy of Family Physicians, American Nurses Association, et al in support of the government and affirmance. Zubik et al v Burwell, Secretary of Health and Human Services, et al, Nos. 14-1418, 14-1458, 14-1505, 15-35, 15-105, 15-119, and 15-191. http://www.scotusblog.com/wp-content/uploads/2016/02/Docfoc.com-Amicus-Brief-Zubik-v.-Burwell.pdf. Accessed August 30, 2016.
- Zubik et al v Burwell, Secretary of Health and Human Services, et al, No. 14-1418 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1418_8758.pdf. Accessed August 30, 2016.
- Universal Health Services, Inc v United States et al ex rel. Escobar et al, No. 15-7 (2016). https://www.supremecourt.gov/opinions/15pdf/15-7_a074.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association, National Association of Chain Drug Stores, National Association of Manufacturers, American Tort Reform Association and NFIB Small Business Legal Center in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7-tsac-American-Medical-Association.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7tsacAHAFAHAAMC.pdf. Accessed August 30, 2016.
- Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). https://www.supremecourt.gov/opinions/15pdf/14-181_5426.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association and Association of American Medical Colleges in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/150904-amicus-gobeille-liberty.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association and Vermont Medical Society in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/VHCURES-Amicus-Brief-of-American-Medical-Association.pdf. Accessed August 30, 2016.
Each year, the decisions of the Supreme Court have a significant impact on ObGyn practice. During the 2015–2016 term, which ended in June, the Court issued important rulings on abortion facilities, Affordable Care Act (ACA) contraception coverage, health care False Claims Act (FCA) liability, and state health care data collection. The American Medical Association (AMA), the Association of American Medical Colleges (AAMC), the American College of Obstetricians and Gynecologists (ACOG), and other organizations that represent health care professionals play an important role in health-related Supreme Court cases. For example, amicus curiae (“friend of the Court”) briefs are filed not by parties to a case but by organizations that have a special insight into or interest in a case. Although the extent to which amicus briefs influence cases is often unclear, organization representatives think their briefs make a difference, and briefs undoubtedly do in some cases.
The 2016 presidential election will determine the Supreme Court make-up for the next term, but in this article we consider recent cases that affect ObGyns’ practice in particular. We start with the cases in which professional organizations filed amicus briefs and then turn to other notable cases.
1. Abortion access in Texas and other states
The most important ObGyn case of the 2015–2016 term was Whole Woman’s Health v Hellerstedt.1
At stake. Texas adopted a statute requiring 1) that physicians who perform abortions have admitting privileges at a hospital within 30 miles of the clinic and 2) that abortion clinics meet the state’s standards for ambulatory surgical centers. The current law, upheld by the Court some years ago, is that state laws affecting abortion are unconstitutional if they “unduly burden” the right to abortion. By undue burden, the Court meant, “Regulations that have the purpose or effect of presenting a substantial obstacle to a woman seeking an abortion impose an undue burden on the right.” The question in the Texas case was whether the statute’s 2 requirements were undue.
ACOG, AMA, and other groups filed a brief stating that the Texas law did not promote the welfare of women but instead was unnecessary and not “supported by accepted medical practice or scientific evidence.”2 In another brief the Society of Hospital Medicine and the Society of ObGyn Hospitalists also indicated that having admitting privileges is appropriate only for physicians who regularly admit patients to a hospital.3
A brief filed by the American Association of Pro-Life Obstetricians and Gynecologists and several other organizations argued the other side: “The surgical center and admitting privileges requirements imposed by the Act reflect the professional standard of practice for outpatient gynecological and similar surgery.”4
Final ruling. In a 5−3 decision, the Court struck down the Texas law for providing little or no health benefits while significantly burdening abortion facility access. Many clinics had closed or were in plans to because of the difficulty and expense of complying with the law. This case has national implications. Similar laws, either in place or being considered in other states, will almost certainly be ruled unconstitutional.
2. Contraceptive coverage
The case of Zubik v Burwell was closely watched this past year.
At stake. Under the ACA, a nonprofit religious organization may certify its objection to its insurance plan’s contraception coverage, at which point other arrangements are made to provide contraceptive coverage through the same plan. Religious organizations objected to the certification requirement.
A brief filed by ACOG, Physicians for Reproductive Health, and other groups emphasized the importance of providing contraceptives and contraceptive counseling as part of regular health care and suggested that the current accommodation for religious organizations is appropriate.5
After hearing the formal oral arguments, the Court asked for additional briefs on “whether contraceptive coverage could be provided to petitioners’ employees, through petitioners’ insurance companies, without any such notice from petitioners.”6
Final ruling. The parties agreed such a system would resolve the issue, so the Court sent the case back to the lower court to work out the details. In effect, the case was mediated—an unusual if not unique action for the Court. The resolution probably will achieve what the briefs sought—access to contraceptives and continuity of care.
3. Fraud and abuse litigation
The FCA, which provides for triple damages (3 times actual damages) and stiff civil penalties for anyone who presents the federal government (Medicare, Medicaid) with false claims for goods or services, is a major means of uncovering and punishing health care fraud and abuse. In health care, this law has been used to prosecute cases involving services paid for but not provided, unnecessary services, and off-label pharmaceutical promotion.
An important part of the FCA is that it allows a private intervenor (whistleblower) to initiate an action against a health care provider. The government may then take up the case. If not, the intervenor may pursue it; the incentive is 15% to 30% of the damages the government is awarded.
At stake. The Court was asked if “implied certification” applies to FCA cases.7 Implied certification means that requesting a payment from Medicare or Medicaid implies that the provider is not knowingly withholding information material to the government’s decision to pay the claim. In separately filed briefs, AMA et al8 and American Hospital Association (AHA) et al9 argued that applying implied certification to FCA cases would expand FCA litigation (particularly by intervenors), which is already expensive for health care institutions.
Final ruling. The Court unanimously adopted implied certification but noted that nondisclosure of information must be shown to be a material misrepresentation rather than a trivial regulatory or contractual violation. Furthermore, the Court emphasized that the basis for a claim must be an allegation of fraud, not of malpractice. These findings, which certainly are not what the health care organizations had hoped for, likely will lead to an increase in FCA cases.
4. Collection of state health care data
In Vermont, and about 20 other states that collect data on health care utilization and costs, health insurers and other entities are required to submit detailed reports about health care claims.10 Some insurers objected to this requirement.
At stake. An AHA–AAMC brief noted the importance of health care data and of Vermont’s collecting these data as contributing to better, more efficient health care delivery.11 Another brief, filed by AMA and the Vermont Medical Society, presented more legal or statutory arguments.12
Final ruling. The Court held that the Vermont plan and similar plans violate the federal Employee Retirement Income Security Act of 1974. As health insurance companies and other entities already provide detailed utilization and cost data to the federal government, producing up to 50 additional reports for state governments would be burdensome. Any state that wants the information, the Court said, should obtain it from the federal government.
More notable 2015-2016 Supreme Court decisions.
The Court:
- permitted limited consideration of race in university admissions. ACOG, AAMC, and AMA with many other groups filed an amicus brief supporting medical school and university affirmative action programs.1
- held that a state must give full faith and credit to the adoption orders of the courts of other states (this case involved an LGBT couple).2
- held that states may require (without a search warrant) a breathalyzer test, but not a blood test, for a driver suspected of drinking.3
- narrowed the ability of the federal government to seize or restrain (before trial) the assets of a person charged with criminal health care offenses.4
- temporarily stayed the August 2016 US Department of Education order to schools to allow transgender students to use the facilities in which they feel "most comfortable." The Court likely will take up this case very soon.5
- Fisher v University of Texas at Austin et al, No. 14-981 (2016). https://www.supremecourt.gov/opinions/15pdf/14-981_4g15.pdf. Accessed August 30, 2016.
- V.L. v E.L. et al, No. 15-648 (2016). https://www.supremecourt.gov/opinions/15pdf/15-648_d18e.pdf. Accessed August 30, 2016.
- Birchfield v North Dakota, No. 14-1468 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1468_8n59.pdf. Accessed August 30, 2016.
- Luis v United States, No. 14-419 (2016). https://www.supremecourt.gov/opinions/15pdf/14-419_nmip.pdf. Accessed August 30, 2016.
- Gloucester County School Board v G.G., by his next friend and mother, Deidre Grimm, No. 16A52 (2016). https://www.supremecourt.gov/opinions/15pdf/16a52_8759.pdf. Accessed August 30, 2016.
What’s to come
The Court’s recent decisions on access to abortion services and contraceptives were good for patients of ObGyns, but its decisions on health care FCA liability and state health care data collection were, arguably, not as good for ObGyn business practices.
The Court itself had an unusual year. Justice Scalia died in February, and Congress’s inaction on seating a replacement meant that most of the term’s cases were decided by an 8-member Court. Nevertheless, the Court was deadlocked 4−4 on only 4 of the 80 cases it heard. In addition, it was relatively agreed on outcomes; in only about one-third of cases were there more than 2 justices disagreeing with the outcome.
It is unlikely that a replacement for Justice Scalia will be confirmed before the Court begins its new term in October. The need to replace Justice Scalia and the potential turnover of other Court members—Justice Ginsburg is 83, Justice Kennedy is 80, and Justice Breyer is 78—are reminders of the importance of this year’s presidential election. In the meantime, the Court is accepting the cases that will make up the coming term’s docket, and ObGyns undoubtedly will play a role in cases that involve health care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Each year, the decisions of the Supreme Court have a significant impact on ObGyn practice. During the 2015–2016 term, which ended in June, the Court issued important rulings on abortion facilities, Affordable Care Act (ACA) contraception coverage, health care False Claims Act (FCA) liability, and state health care data collection. The American Medical Association (AMA), the Association of American Medical Colleges (AAMC), the American College of Obstetricians and Gynecologists (ACOG), and other organizations that represent health care professionals play an important role in health-related Supreme Court cases. For example, amicus curiae (“friend of the Court”) briefs are filed not by parties to a case but by organizations that have a special insight into or interest in a case. Although the extent to which amicus briefs influence cases is often unclear, organization representatives think their briefs make a difference, and briefs undoubtedly do in some cases.
The 2016 presidential election will determine the Supreme Court make-up for the next term, but in this article we consider recent cases that affect ObGyns’ practice in particular. We start with the cases in which professional organizations filed amicus briefs and then turn to other notable cases.
1. Abortion access in Texas and other states
The most important ObGyn case of the 2015–2016 term was Whole Woman’s Health v Hellerstedt.1
At stake. Texas adopted a statute requiring 1) that physicians who perform abortions have admitting privileges at a hospital within 30 miles of the clinic and 2) that abortion clinics meet the state’s standards for ambulatory surgical centers. The current law, upheld by the Court some years ago, is that state laws affecting abortion are unconstitutional if they “unduly burden” the right to abortion. By undue burden, the Court meant, “Regulations that have the purpose or effect of presenting a substantial obstacle to a woman seeking an abortion impose an undue burden on the right.” The question in the Texas case was whether the statute’s 2 requirements were undue.
ACOG, AMA, and other groups filed a brief stating that the Texas law did not promote the welfare of women but instead was unnecessary and not “supported by accepted medical practice or scientific evidence.”2 In another brief the Society of Hospital Medicine and the Society of ObGyn Hospitalists also indicated that having admitting privileges is appropriate only for physicians who regularly admit patients to a hospital.3
A brief filed by the American Association of Pro-Life Obstetricians and Gynecologists and several other organizations argued the other side: “The surgical center and admitting privileges requirements imposed by the Act reflect the professional standard of practice for outpatient gynecological and similar surgery.”4
Final ruling. In a 5−3 decision, the Court struck down the Texas law for providing little or no health benefits while significantly burdening abortion facility access. Many clinics had closed or were in plans to because of the difficulty and expense of complying with the law. This case has national implications. Similar laws, either in place or being considered in other states, will almost certainly be ruled unconstitutional.
2. Contraceptive coverage
The case of Zubik v Burwell was closely watched this past year.
At stake. Under the ACA, a nonprofit religious organization may certify its objection to its insurance plan’s contraception coverage, at which point other arrangements are made to provide contraceptive coverage through the same plan. Religious organizations objected to the certification requirement.
A brief filed by ACOG, Physicians for Reproductive Health, and other groups emphasized the importance of providing contraceptives and contraceptive counseling as part of regular health care and suggested that the current accommodation for religious organizations is appropriate.5
After hearing the formal oral arguments, the Court asked for additional briefs on “whether contraceptive coverage could be provided to petitioners’ employees, through petitioners’ insurance companies, without any such notice from petitioners.”6
Final ruling. The parties agreed such a system would resolve the issue, so the Court sent the case back to the lower court to work out the details. In effect, the case was mediated—an unusual if not unique action for the Court. The resolution probably will achieve what the briefs sought—access to contraceptives and continuity of care.
3. Fraud and abuse litigation
The FCA, which provides for triple damages (3 times actual damages) and stiff civil penalties for anyone who presents the federal government (Medicare, Medicaid) with false claims for goods or services, is a major means of uncovering and punishing health care fraud and abuse. In health care, this law has been used to prosecute cases involving services paid for but not provided, unnecessary services, and off-label pharmaceutical promotion.
An important part of the FCA is that it allows a private intervenor (whistleblower) to initiate an action against a health care provider. The government may then take up the case. If not, the intervenor may pursue it; the incentive is 15% to 30% of the damages the government is awarded.
At stake. The Court was asked if “implied certification” applies to FCA cases.7 Implied certification means that requesting a payment from Medicare or Medicaid implies that the provider is not knowingly withholding information material to the government’s decision to pay the claim. In separately filed briefs, AMA et al8 and American Hospital Association (AHA) et al9 argued that applying implied certification to FCA cases would expand FCA litigation (particularly by intervenors), which is already expensive for health care institutions.
Final ruling. The Court unanimously adopted implied certification but noted that nondisclosure of information must be shown to be a material misrepresentation rather than a trivial regulatory or contractual violation. Furthermore, the Court emphasized that the basis for a claim must be an allegation of fraud, not of malpractice. These findings, which certainly are not what the health care organizations had hoped for, likely will lead to an increase in FCA cases.
4. Collection of state health care data
In Vermont, and about 20 other states that collect data on health care utilization and costs, health insurers and other entities are required to submit detailed reports about health care claims.10 Some insurers objected to this requirement.
At stake. An AHA–AAMC brief noted the importance of health care data and of Vermont’s collecting these data as contributing to better, more efficient health care delivery.11 Another brief, filed by AMA and the Vermont Medical Society, presented more legal or statutory arguments.12
Final ruling. The Court held that the Vermont plan and similar plans violate the federal Employee Retirement Income Security Act of 1974. As health insurance companies and other entities already provide detailed utilization and cost data to the federal government, producing up to 50 additional reports for state governments would be burdensome. Any state that wants the information, the Court said, should obtain it from the federal government.
More notable 2015-2016 Supreme Court decisions.
The Court:
- permitted limited consideration of race in university admissions. ACOG, AAMC, and AMA with many other groups filed an amicus brief supporting medical school and university affirmative action programs.1
- held that a state must give full faith and credit to the adoption orders of the courts of other states (this case involved an LGBT couple).2
- held that states may require (without a search warrant) a breathalyzer test, but not a blood test, for a driver suspected of drinking.3
- narrowed the ability of the federal government to seize or restrain (before trial) the assets of a person charged with criminal health care offenses.4
- temporarily stayed the August 2016 US Department of Education order to schools to allow transgender students to use the facilities in which they feel "most comfortable." The Court likely will take up this case very soon.5
- Fisher v University of Texas at Austin et al, No. 14-981 (2016). https://www.supremecourt.gov/opinions/15pdf/14-981_4g15.pdf. Accessed August 30, 2016.
- V.L. v E.L. et al, No. 15-648 (2016). https://www.supremecourt.gov/opinions/15pdf/15-648_d18e.pdf. Accessed August 30, 2016.
- Birchfield v North Dakota, No. 14-1468 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1468_8n59.pdf. Accessed August 30, 2016.
- Luis v United States, No. 14-419 (2016). https://www.supremecourt.gov/opinions/15pdf/14-419_nmip.pdf. Accessed August 30, 2016.
- Gloucester County School Board v G.G., by his next friend and mother, Deidre Grimm, No. 16A52 (2016). https://www.supremecourt.gov/opinions/15pdf/16a52_8759.pdf. Accessed August 30, 2016.
What’s to come
The Court’s recent decisions on access to abortion services and contraceptives were good for patients of ObGyns, but its decisions on health care FCA liability and state health care data collection were, arguably, not as good for ObGyn business practices.
The Court itself had an unusual year. Justice Scalia died in February, and Congress’s inaction on seating a replacement meant that most of the term’s cases were decided by an 8-member Court. Nevertheless, the Court was deadlocked 4−4 on only 4 of the 80 cases it heard. In addition, it was relatively agreed on outcomes; in only about one-third of cases were there more than 2 justices disagreeing with the outcome.
It is unlikely that a replacement for Justice Scalia will be confirmed before the Court begins its new term in October. The need to replace Justice Scalia and the potential turnover of other Court members—Justice Ginsburg is 83, Justice Kennedy is 80, and Justice Breyer is 78—are reminders of the importance of this year’s presidential election. In the meantime, the Court is accepting the cases that will make up the coming term’s docket, and ObGyns undoubtedly will play a role in cases that involve health care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). https://www.supremecourt.gov/opinions/15pdf/15-274_new_e18f.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Osteopathic Association, and American Academy of Pediatrics in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/ACOG-WilmerHale.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by Society of Hospital Medicine and Society of Ob/Gyn Hospitalists in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/Society-of-Hospital-Medicine-Crowell.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Association of Pro-Life Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Association, Catholic Medical Association, and Physicians for Life in support of respondents. Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al. http://www.scotusblog.com/wp-content/uploads/2016/02/15-274-bsac-American-Association-of-Pro-Life-Obstetricians-and-Gynecolog....pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, American Academy of Family Physicians, American Nurses Association, et al in support of the government and affirmance. Zubik et al v Burwell, Secretary of Health and Human Services, et al, Nos. 14-1418, 14-1458, 14-1505, 15-35, 15-105, 15-119, and 15-191. http://www.scotusblog.com/wp-content/uploads/2016/02/Docfoc.com-Amicus-Brief-Zubik-v.-Burwell.pdf. Accessed August 30, 2016.
- Zubik et al v Burwell, Secretary of Health and Human Services, et al, No. 14-1418 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1418_8758.pdf. Accessed August 30, 2016.
- Universal Health Services, Inc v United States et al ex rel. Escobar et al, No. 15-7 (2016). https://www.supremecourt.gov/opinions/15pdf/15-7_a074.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association, National Association of Chain Drug Stores, National Association of Manufacturers, American Tort Reform Association and NFIB Small Business Legal Center in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7-tsac-American-Medical-Association.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7tsacAHAFAHAAMC.pdf. Accessed August 30, 2016.
- Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). https://www.supremecourt.gov/opinions/15pdf/14-181_5426.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association and Association of American Medical Colleges in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/150904-amicus-gobeille-liberty.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association and Vermont Medical Society in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/VHCURES-Amicus-Brief-of-American-Medical-Association.pdf. Accessed August 30, 2016.
- Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). https://www.supremecourt.gov/opinions/15pdf/15-274_new_e18f.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Osteopathic Association, and American Academy of Pediatrics in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/ACOG-WilmerHale.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by Society of Hospital Medicine and Society of Ob/Gyn Hospitalists in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/Society-of-Hospital-Medicine-Crowell.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Association of Pro-Life Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Association, Catholic Medical Association, and Physicians for Life in support of respondents. Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al. http://www.scotusblog.com/wp-content/uploads/2016/02/15-274-bsac-American-Association-of-Pro-Life-Obstetricians-and-Gynecolog....pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, American Academy of Family Physicians, American Nurses Association, et al in support of the government and affirmance. Zubik et al v Burwell, Secretary of Health and Human Services, et al, Nos. 14-1418, 14-1458, 14-1505, 15-35, 15-105, 15-119, and 15-191. http://www.scotusblog.com/wp-content/uploads/2016/02/Docfoc.com-Amicus-Brief-Zubik-v.-Burwell.pdf. Accessed August 30, 2016.
- Zubik et al v Burwell, Secretary of Health and Human Services, et al, No. 14-1418 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1418_8758.pdf. Accessed August 30, 2016.
- Universal Health Services, Inc v United States et al ex rel. Escobar et al, No. 15-7 (2016). https://www.supremecourt.gov/opinions/15pdf/15-7_a074.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association, National Association of Chain Drug Stores, National Association of Manufacturers, American Tort Reform Association and NFIB Small Business Legal Center in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7-tsac-American-Medical-Association.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7tsacAHAFAHAAMC.pdf. Accessed August 30, 2016.
- Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). https://www.supremecourt.gov/opinions/15pdf/14-181_5426.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association and Association of American Medical Colleges in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/150904-amicus-gobeille-liberty.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association and Vermont Medical Society in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/VHCURES-Amicus-Brief-of-American-Medical-Association.pdf. Accessed August 30, 2016.
In this Article
- Whole Woman’s Health v Hellerstedt
- Fraud and abuse litigation
- What’s to come
What is the ideal gestational age for twin delivery to minimize perinatal deaths?
EXPERT COMMENTARY
Cheong-See and colleagues conducted a comprehensive review and analysis of 32 studies of uncomplicated dichorionic and monochorionic twin pregnancies to determine the risks of stillbirth and neonatal complications by gestational age.
Details of the study
The authors searched major databases for studies on twin pregnancies that reported rates of stillbirth as well as neonatal outcomes (neonatal mortality was defined as death up to 28 days after delivery). A total of 32 studies were included in the analysis, with 29,685 dichorionic and 5,486 monochorionic pregnancies in 35,171 women. The authors estimated the gestational-age specific differences in risk for stillbirths and neonatal deaths after 34 weeks’ gestation.
In dichorionic pregnancies, the prospective weekly pooled risk of stillbirths from expectant management and the risk of neonatal mortality from delivery were balanced at 37 weeks of gestation (risk difference, 1.2/1,000, 95% CI, −1.3 to 3.6; I2 = 0%). In monochorionic pregnancies, after 36 weeks there was a trend toward an increase in stillbirths compared with neonatal deaths, with a pooled risk difference of 2.5/1,000 (95% CI, −12.4 to 17.4; I2 = 0%). Neonatal morbidity rates were consistently reduced with increasing gestational age in both monochorionic and dichorionic pregnancies.
The researchers’ recommendations
The authors recommended that dichorionic pregnancies be delivered at 37 weeks and that the evidence for delivery of monochorionic twins prior to 36 weeks is lacking. While the analysis is comprehensive and well done, it cannot escape the limitations that afflict all systematic reviews and meta-analyses, and these limitations are well addressed by the authors. Several factors, however, warrant caution regarding the adoption of the authors’ recommendations.
Cautions. First, determination of chorionicity may not have been accurate in all of the studies reviewed. Additionally, we have no data on how these pregnancies were managed with respect to antepartum fetal surveillance, ultrasound surveillance for growth and discordancy, and management of labor and delivery. There are no data on the quality of the ultrasound examinations being performed at each of the centers.
Also, the factors that may increase the risk of stillbirth are not necessarily the same factors that may influence the neonatal death rate, and this review moves between the use of these terms (stillbirth rate, neonatal mortality rate, and perinatal mortality rate) fairly frequently. For example, an improperly managed labor, an unanticipated difficult breech extraction, or the need for emergent cesarean delivery of the second twin might contribute to the neonatal death rate irrespective of gestational age at delivery. The authors acknowledge that outcomes may have been influenced by differences in obstetric and neonatal management of twin pregnancies that were observed between centers. Another concern is the authors’ use of unpublished aggregate and individual patient data.
While this comprehensive and very well conducted review and meta-analysis highlights the complexity of balancing stillbirth risk against neonatal mortality risk, the limitations of the study are too substantial to allow for any change in current practice. My recommendation for the timing of twin delivery is to adhere to the guidelines that are currently supported by both the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists.1 These guidelines recommend that, for dichorionic-diamniotic twin pregnancy, the general timing of delivery be at early term, with suggested specific timing at 38 0/7 to 38 6/7 weeks of gestation. For monochorionic-diamniotic twin pregnancy, the general timing of delivery may be at late preterm/early term, with suggested specific timing at 34 0/7 to 37 6/7 weeks of gestation. Delivery decisions made within these date ranges depend on numerous factors discussed in the guidelines, and timing of delivery should be individualized.1
-- JOHN T. REPKE, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG Committee Opinion No. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910. Reaffirmed 2015.
EXPERT COMMENTARY
Cheong-See and colleagues conducted a comprehensive review and analysis of 32 studies of uncomplicated dichorionic and monochorionic twin pregnancies to determine the risks of stillbirth and neonatal complications by gestational age.
Details of the study
The authors searched major databases for studies on twin pregnancies that reported rates of stillbirth as well as neonatal outcomes (neonatal mortality was defined as death up to 28 days after delivery). A total of 32 studies were included in the analysis, with 29,685 dichorionic and 5,486 monochorionic pregnancies in 35,171 women. The authors estimated the gestational-age specific differences in risk for stillbirths and neonatal deaths after 34 weeks’ gestation.
In dichorionic pregnancies, the prospective weekly pooled risk of stillbirths from expectant management and the risk of neonatal mortality from delivery were balanced at 37 weeks of gestation (risk difference, 1.2/1,000, 95% CI, −1.3 to 3.6; I2 = 0%). In monochorionic pregnancies, after 36 weeks there was a trend toward an increase in stillbirths compared with neonatal deaths, with a pooled risk difference of 2.5/1,000 (95% CI, −12.4 to 17.4; I2 = 0%). Neonatal morbidity rates were consistently reduced with increasing gestational age in both monochorionic and dichorionic pregnancies.
The researchers’ recommendations
The authors recommended that dichorionic pregnancies be delivered at 37 weeks and that the evidence for delivery of monochorionic twins prior to 36 weeks is lacking. While the analysis is comprehensive and well done, it cannot escape the limitations that afflict all systematic reviews and meta-analyses, and these limitations are well addressed by the authors. Several factors, however, warrant caution regarding the adoption of the authors’ recommendations.
Cautions. First, determination of chorionicity may not have been accurate in all of the studies reviewed. Additionally, we have no data on how these pregnancies were managed with respect to antepartum fetal surveillance, ultrasound surveillance for growth and discordancy, and management of labor and delivery. There are no data on the quality of the ultrasound examinations being performed at each of the centers.
Also, the factors that may increase the risk of stillbirth are not necessarily the same factors that may influence the neonatal death rate, and this review moves between the use of these terms (stillbirth rate, neonatal mortality rate, and perinatal mortality rate) fairly frequently. For example, an improperly managed labor, an unanticipated difficult breech extraction, or the need for emergent cesarean delivery of the second twin might contribute to the neonatal death rate irrespective of gestational age at delivery. The authors acknowledge that outcomes may have been influenced by differences in obstetric and neonatal management of twin pregnancies that were observed between centers. Another concern is the authors’ use of unpublished aggregate and individual patient data.
While this comprehensive and very well conducted review and meta-analysis highlights the complexity of balancing stillbirth risk against neonatal mortality risk, the limitations of the study are too substantial to allow for any change in current practice. My recommendation for the timing of twin delivery is to adhere to the guidelines that are currently supported by both the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists.1 These guidelines recommend that, for dichorionic-diamniotic twin pregnancy, the general timing of delivery be at early term, with suggested specific timing at 38 0/7 to 38 6/7 weeks of gestation. For monochorionic-diamniotic twin pregnancy, the general timing of delivery may be at late preterm/early term, with suggested specific timing at 34 0/7 to 37 6/7 weeks of gestation. Delivery decisions made within these date ranges depend on numerous factors discussed in the guidelines, and timing of delivery should be individualized.1
-- JOHN T. REPKE, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Cheong-See and colleagues conducted a comprehensive review and analysis of 32 studies of uncomplicated dichorionic and monochorionic twin pregnancies to determine the risks of stillbirth and neonatal complications by gestational age.
Details of the study
The authors searched major databases for studies on twin pregnancies that reported rates of stillbirth as well as neonatal outcomes (neonatal mortality was defined as death up to 28 days after delivery). A total of 32 studies were included in the analysis, with 29,685 dichorionic and 5,486 monochorionic pregnancies in 35,171 women. The authors estimated the gestational-age specific differences in risk for stillbirths and neonatal deaths after 34 weeks’ gestation.
In dichorionic pregnancies, the prospective weekly pooled risk of stillbirths from expectant management and the risk of neonatal mortality from delivery were balanced at 37 weeks of gestation (risk difference, 1.2/1,000, 95% CI, −1.3 to 3.6; I2 = 0%). In monochorionic pregnancies, after 36 weeks there was a trend toward an increase in stillbirths compared with neonatal deaths, with a pooled risk difference of 2.5/1,000 (95% CI, −12.4 to 17.4; I2 = 0%). Neonatal morbidity rates were consistently reduced with increasing gestational age in both monochorionic and dichorionic pregnancies.
The researchers’ recommendations
The authors recommended that dichorionic pregnancies be delivered at 37 weeks and that the evidence for delivery of monochorionic twins prior to 36 weeks is lacking. While the analysis is comprehensive and well done, it cannot escape the limitations that afflict all systematic reviews and meta-analyses, and these limitations are well addressed by the authors. Several factors, however, warrant caution regarding the adoption of the authors’ recommendations.
Cautions. First, determination of chorionicity may not have been accurate in all of the studies reviewed. Additionally, we have no data on how these pregnancies were managed with respect to antepartum fetal surveillance, ultrasound surveillance for growth and discordancy, and management of labor and delivery. There are no data on the quality of the ultrasound examinations being performed at each of the centers.
Also, the factors that may increase the risk of stillbirth are not necessarily the same factors that may influence the neonatal death rate, and this review moves between the use of these terms (stillbirth rate, neonatal mortality rate, and perinatal mortality rate) fairly frequently. For example, an improperly managed labor, an unanticipated difficult breech extraction, or the need for emergent cesarean delivery of the second twin might contribute to the neonatal death rate irrespective of gestational age at delivery. The authors acknowledge that outcomes may have been influenced by differences in obstetric and neonatal management of twin pregnancies that were observed between centers. Another concern is the authors’ use of unpublished aggregate and individual patient data.
While this comprehensive and very well conducted review and meta-analysis highlights the complexity of balancing stillbirth risk against neonatal mortality risk, the limitations of the study are too substantial to allow for any change in current practice. My recommendation for the timing of twin delivery is to adhere to the guidelines that are currently supported by both the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists.1 These guidelines recommend that, for dichorionic-diamniotic twin pregnancy, the general timing of delivery be at early term, with suggested specific timing at 38 0/7 to 38 6/7 weeks of gestation. For monochorionic-diamniotic twin pregnancy, the general timing of delivery may be at late preterm/early term, with suggested specific timing at 34 0/7 to 37 6/7 weeks of gestation. Delivery decisions made within these date ranges depend on numerous factors discussed in the guidelines, and timing of delivery should be individualized.1
-- JOHN T. REPKE, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG Committee Opinion No. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910. Reaffirmed 2015.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG Committee Opinion No. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910. Reaffirmed 2015.
Have you measured lactate in your sick obstetrics and gynecology patients during the past year?
Lactate measurement is widely used in emergency departments (EDs) and intensive care units (ICUs) to facilitate the early diagnosis and management of sepsis, severe trauma, ischemic bowel, and necrotizing fasciitis. Measuring lactate levels is much less commonly utilized in the practice of obstetrics and gynecology; increasing measurement in our practices may improve our early recognition and treatment of women with severe sepsis and other serious diseases.
Lactate physiology
The metabolism of glucose in the Embden-Meyerhof pathway results in the production of pyruvate and the high-energy compounds ATP and NADH. Pyruvate can enter 3 alternative metabolic pathways: 1) the mitochondrial Krebs cycle, 2) conversion to lactate in the cell cytosol, or 3) conversion back to glucose in the process of gluconeogenesis.
Under aerobic conditions, most pyruvate enters the Krebs cycle and little is converted to lactate. Molecular oxygen is an absolute requirement for Krebs cycle activity. Under anaerobic conditions, pyruvate cannot enter the Krebs cycle and is preferentially converted to lactate.1
An elevated lactate level is a sensitive marker for tissue hypoxia caused by a variety of diseases, including sepsis, trauma, ischemic bowel, and necrotizing fasciitis. With sepsis, additional mechanisms also contribute to the increase in lactate, including increased glycolysis, impaired lactate clearance, and activation of inflammatory cells that shift cellular metabolism toward lactate production.2,3
The normal range for venous plasma lactate in adults is 0.5 to 2.2 mM, although the normal range may vary because of differences in local laboratory methods. Arterial, capillary, and venous lactate are all highly positively correlated.4 Venous lactate concentrations between 2.3 and 3.9 mM are suggestive of mild physiologic dysfunction, and values ≥4.0 mM are consistent with severe physiologic dysfunction. In hospitalized patients, sepsis is one of the most common causes of a lactate level ≥4 mM.5
In many patients with an elevated lactate concentration the anion gap is also increased—but this is not always the case. In fact, in one large observational study, among patients with sepsis and a lactate concentration ≥4 mM, approximately 25% had a normal bicarbonate level and normal anion gap.6
Elevated lactate levels applied in obstetric and gynecologic practice
CASE 1. Obstetric practice: Hernia identified during labor
A 30-year-old woman (G1P0) presents in early labor at 37 weeks’ gestation. Two years prior to the pregnancy she had a Roux-en-Y gastric bypass and lost more than 100 lb. In addition to reporting lower abdominal pain occurring during contractions, she reports the new onset of mid-epigastric pain. A surgical consult is requested. The initial white blood cell count is 6,290 per uL, and the lactate level is 1.0 mM.
The surgeon consulted orders a computed tomography (CT) scan with oral contrast, but the patient has difficulty retaining the oral contrast due to her nausea, delaying the performance of the CT scan. Three hours following admission a follow-up lactate measurement is 3.3 mM, and an emergency CT scan is performed.
The CT scan shows an internal hernia with swirling of the mesenteric vessels and twisting of the small bowel mesentery. An urgent cesarean delivery and repair of the internal hernia is performed.
The patient and her newborn do well postoperatively. The postoperative lactate level is 0.8 mM.
In pregnant women with a past history of a Roux-en-Y gastric bypass and abdominal discomfort who are in labor it is challenging to rapidly diagnose internal hernias and other bowel problems.7−9 In this case, the increased lactate level from 1.0 mM to 3.3 mM raised concern for ischemic bowel and triggered the emergency CT and urgent exploratory laparotomy and cesarean delivery.
Up to 14% of maternal deaths in the United States are due to infection.10 In many of these cases, there is a delay in sepsis recognition because previously healthy pregnant women with sepsis may not manifest classic signs such as fever, hypotension, or mental status changes until late in the disease course. Measurement of lactate can facilitate the early recognition of severe sepsis in pregnant women, thereby accelerating and focusing their treatment.11
To reduce mortality due to sepsis, aggressive intervention needs to occur within the first 6 hours following the onset of the infection.
CASE 2. Gynecologic practice: Bacterial infection identified in the presence of abdominal pain and vomiting
A 40-year-old woman presents to the ED 5 days following a myomectomy, with nausea, vomiting, and abdominal pain. Her vital signs reveal: temperature, 98.4°F (36.9°C); heart rate, 122 bpm; blood pressure, 115/70 mm Hg; and white blood cell count, 6,270 per uL. Her lactate level is 4.0 mM. She is admitted to the ICU with a presumptive diagnosis of severe sepsis and treatment with broad-spectrum antibiotics is initiated. Twenty-four hours following admission, gram-negative rods are identified in blood cultures that are later identified to be Bacteroides fragilis.
For the past 2 decades there has been a concerted national effort to reduce mortality caused by sepsis through early diagnosis and aggressive treatment of sepsis in an ICU setting. Observational studies have reported that an elevated lactate level is an excellent early biomarker for sepsis and may be observed prior to the onset of fever, elevated white blood cell count, or hypotension.6 For example, in one large study of patients with sepsis and a lactate measurement ≥4 mM, only 50% of patients had a systolic blood pressure <90 mm Hg.
Elevated lactate levels also are associated with an increased risk of death. Among 13,932 consecutive patients admitted to an ICU in Alberta, Canada, the mortality rate among patients with a venous or arterial lactate >2 mM was 20%, compared with a mortality rate of 5% for patients with a lactate level ≤2 mM.12 In a study of 1,278 patients with infection admitted to the hospital from the ED, mortality increased as baseline lactate concentration rose. For lactate concentrations of 0 to 2.4, 2.5 to 3.9, and ≥4.0 mM, mortality rates were 5%, 9%, and 28%, respectively.13
In patients with sepsis, serial measurement of lactate can help to guide treatment. In a randomized trial, 348 patients admitted to an ICU with a lactate ≥3 mM were randomly assigned to standard treatment, in which the clinicians had no knowledge of patients’ lactate levels, or to an experimental group, in which the clinicians were provided lactate measurement results every 2 hours. Compared with clinicians in the control group, the clinicians with access to frequent lactate measurements administered more fluids and vasodilators to their patients. Compared with patients in the control group, the hospital mortality rate was lower when the clinicians had access to frequent lactate measurements (34% vs 44%, respectively; adjusted hazard ratio, 0.61; 95% confidence interval, 0.43−0.87; P = .0006).14
Elevated lactate levels in the fetus and newborn
The physiologic status of the newborn is routinely assessed with the Apgar score. Umbilical artery and venous blood gases, including measurement of pH, are often used as a corroborating biomarker. Most studies report that umbilical artery or vein lactate measurement is as useful as a pH measurement in assessing newborn physiologic status. The normal range of lactate in fetuses and newborns is not precisely defined, with values between 3.5 and 7 mM being cited as the upper limit of normal.15−18
In many countries (but not the United States), in utero fetal status during labor is assessed by fetal scalp sampling of blood and measurement of either pH or lactate. Fetal scalp sampling is difficult and often very little blood is obtained, making it difficult to measure pH. A Cochrane review reported that in 2 randomized trials, fetal scalp sampling produced a successful measurement of lactate in 99% of attempts, while a pH result could only be obtained in 79% of cases due to an inadequate volume of blood or clotted blood.19
Increased lactate measurement can help our patients
Measuring lactate in order to rapidly identify patients with major physiologic derangements is practiced widely in EDs and ICUs. There is significant opportunity to increase the use of lactate measurement in obstetrics and gynecology. Increasing this use will help to rapidly identify women with severe sepsis and other diseases, leading to more rapid intervention and improved outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clinic Proc. 2013;88(10):1127−1140.
- Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39.
- Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252−261.
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567−573.
- Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368−1377.
- Caranta DG, Lee AM, Pennington D, Zelig CM. Complications from Roux-en-Y gastric bypass mistaken for medical complications in gravid patients. Obstet Gynecol. 2014;124(2 part 2 suppl 1):464−466.
- Moore KA, Ouyang DW, Whang EE. Maternal and fetal deaths after gastric bypass surgery for morbid obesity. N Engl J Med. 2004;351(7):721−722.
- Loar PV 3rd, Sanchez-Ramos L, Kaunitz AM, Kerwin AJ, Diaz J. Maternal death caused by midgut volvulus after bariatric surgery. Am J Obstet Gynecol. 2005;193(5):1748−1749.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
- Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(5):481−486.
- Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperprolactinemia in the critically ill. Crit Care. 2009;13(3):R90.
- Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524−528.
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752−761.
- Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33−37.
- Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical cord venous lactate for predicting arterial lactic acidemia and neonatal morbidity at term. Obstet Gynecol. 2016;127(4):674−680.
- Shirey T, St. Pierre J, Winkelman J. Cord lactate, pH, and blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15−19.
- Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107−1114.
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB, Lau R. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2015;(5):CD006174.
Lactate measurement is widely used in emergency departments (EDs) and intensive care units (ICUs) to facilitate the early diagnosis and management of sepsis, severe trauma, ischemic bowel, and necrotizing fasciitis. Measuring lactate levels is much less commonly utilized in the practice of obstetrics and gynecology; increasing measurement in our practices may improve our early recognition and treatment of women with severe sepsis and other serious diseases.
Lactate physiology
The metabolism of glucose in the Embden-Meyerhof pathway results in the production of pyruvate and the high-energy compounds ATP and NADH. Pyruvate can enter 3 alternative metabolic pathways: 1) the mitochondrial Krebs cycle, 2) conversion to lactate in the cell cytosol, or 3) conversion back to glucose in the process of gluconeogenesis.
Under aerobic conditions, most pyruvate enters the Krebs cycle and little is converted to lactate. Molecular oxygen is an absolute requirement for Krebs cycle activity. Under anaerobic conditions, pyruvate cannot enter the Krebs cycle and is preferentially converted to lactate.1
An elevated lactate level is a sensitive marker for tissue hypoxia caused by a variety of diseases, including sepsis, trauma, ischemic bowel, and necrotizing fasciitis. With sepsis, additional mechanisms also contribute to the increase in lactate, including increased glycolysis, impaired lactate clearance, and activation of inflammatory cells that shift cellular metabolism toward lactate production.2,3
The normal range for venous plasma lactate in adults is 0.5 to 2.2 mM, although the normal range may vary because of differences in local laboratory methods. Arterial, capillary, and venous lactate are all highly positively correlated.4 Venous lactate concentrations between 2.3 and 3.9 mM are suggestive of mild physiologic dysfunction, and values ≥4.0 mM are consistent with severe physiologic dysfunction. In hospitalized patients, sepsis is one of the most common causes of a lactate level ≥4 mM.5
In many patients with an elevated lactate concentration the anion gap is also increased—but this is not always the case. In fact, in one large observational study, among patients with sepsis and a lactate concentration ≥4 mM, approximately 25% had a normal bicarbonate level and normal anion gap.6
Elevated lactate levels applied in obstetric and gynecologic practice
CASE 1. Obstetric practice: Hernia identified during labor
A 30-year-old woman (G1P0) presents in early labor at 37 weeks’ gestation. Two years prior to the pregnancy she had a Roux-en-Y gastric bypass and lost more than 100 lb. In addition to reporting lower abdominal pain occurring during contractions, she reports the new onset of mid-epigastric pain. A surgical consult is requested. The initial white blood cell count is 6,290 per uL, and the lactate level is 1.0 mM.
The surgeon consulted orders a computed tomography (CT) scan with oral contrast, but the patient has difficulty retaining the oral contrast due to her nausea, delaying the performance of the CT scan. Three hours following admission a follow-up lactate measurement is 3.3 mM, and an emergency CT scan is performed.
The CT scan shows an internal hernia with swirling of the mesenteric vessels and twisting of the small bowel mesentery. An urgent cesarean delivery and repair of the internal hernia is performed.
The patient and her newborn do well postoperatively. The postoperative lactate level is 0.8 mM.
In pregnant women with a past history of a Roux-en-Y gastric bypass and abdominal discomfort who are in labor it is challenging to rapidly diagnose internal hernias and other bowel problems.7−9 In this case, the increased lactate level from 1.0 mM to 3.3 mM raised concern for ischemic bowel and triggered the emergency CT and urgent exploratory laparotomy and cesarean delivery.
Up to 14% of maternal deaths in the United States are due to infection.10 In many of these cases, there is a delay in sepsis recognition because previously healthy pregnant women with sepsis may not manifest classic signs such as fever, hypotension, or mental status changes until late in the disease course. Measurement of lactate can facilitate the early recognition of severe sepsis in pregnant women, thereby accelerating and focusing their treatment.11
To reduce mortality due to sepsis, aggressive intervention needs to occur within the first 6 hours following the onset of the infection.
CASE 2. Gynecologic practice: Bacterial infection identified in the presence of abdominal pain and vomiting
A 40-year-old woman presents to the ED 5 days following a myomectomy, with nausea, vomiting, and abdominal pain. Her vital signs reveal: temperature, 98.4°F (36.9°C); heart rate, 122 bpm; blood pressure, 115/70 mm Hg; and white blood cell count, 6,270 per uL. Her lactate level is 4.0 mM. She is admitted to the ICU with a presumptive diagnosis of severe sepsis and treatment with broad-spectrum antibiotics is initiated. Twenty-four hours following admission, gram-negative rods are identified in blood cultures that are later identified to be Bacteroides fragilis.
For the past 2 decades there has been a concerted national effort to reduce mortality caused by sepsis through early diagnosis and aggressive treatment of sepsis in an ICU setting. Observational studies have reported that an elevated lactate level is an excellent early biomarker for sepsis and may be observed prior to the onset of fever, elevated white blood cell count, or hypotension.6 For example, in one large study of patients with sepsis and a lactate measurement ≥4 mM, only 50% of patients had a systolic blood pressure <90 mm Hg.
Elevated lactate levels also are associated with an increased risk of death. Among 13,932 consecutive patients admitted to an ICU in Alberta, Canada, the mortality rate among patients with a venous or arterial lactate >2 mM was 20%, compared with a mortality rate of 5% for patients with a lactate level ≤2 mM.12 In a study of 1,278 patients with infection admitted to the hospital from the ED, mortality increased as baseline lactate concentration rose. For lactate concentrations of 0 to 2.4, 2.5 to 3.9, and ≥4.0 mM, mortality rates were 5%, 9%, and 28%, respectively.13
In patients with sepsis, serial measurement of lactate can help to guide treatment. In a randomized trial, 348 patients admitted to an ICU with a lactate ≥3 mM were randomly assigned to standard treatment, in which the clinicians had no knowledge of patients’ lactate levels, or to an experimental group, in which the clinicians were provided lactate measurement results every 2 hours. Compared with clinicians in the control group, the clinicians with access to frequent lactate measurements administered more fluids and vasodilators to their patients. Compared with patients in the control group, the hospital mortality rate was lower when the clinicians had access to frequent lactate measurements (34% vs 44%, respectively; adjusted hazard ratio, 0.61; 95% confidence interval, 0.43−0.87; P = .0006).14
Elevated lactate levels in the fetus and newborn
The physiologic status of the newborn is routinely assessed with the Apgar score. Umbilical artery and venous blood gases, including measurement of pH, are often used as a corroborating biomarker. Most studies report that umbilical artery or vein lactate measurement is as useful as a pH measurement in assessing newborn physiologic status. The normal range of lactate in fetuses and newborns is not precisely defined, with values between 3.5 and 7 mM being cited as the upper limit of normal.15−18
In many countries (but not the United States), in utero fetal status during labor is assessed by fetal scalp sampling of blood and measurement of either pH or lactate. Fetal scalp sampling is difficult and often very little blood is obtained, making it difficult to measure pH. A Cochrane review reported that in 2 randomized trials, fetal scalp sampling produced a successful measurement of lactate in 99% of attempts, while a pH result could only be obtained in 79% of cases due to an inadequate volume of blood or clotted blood.19
Increased lactate measurement can help our patients
Measuring lactate in order to rapidly identify patients with major physiologic derangements is practiced widely in EDs and ICUs. There is significant opportunity to increase the use of lactate measurement in obstetrics and gynecology. Increasing this use will help to rapidly identify women with severe sepsis and other diseases, leading to more rapid intervention and improved outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Lactate measurement is widely used in emergency departments (EDs) and intensive care units (ICUs) to facilitate the early diagnosis and management of sepsis, severe trauma, ischemic bowel, and necrotizing fasciitis. Measuring lactate levels is much less commonly utilized in the practice of obstetrics and gynecology; increasing measurement in our practices may improve our early recognition and treatment of women with severe sepsis and other serious diseases.
Lactate physiology
The metabolism of glucose in the Embden-Meyerhof pathway results in the production of pyruvate and the high-energy compounds ATP and NADH. Pyruvate can enter 3 alternative metabolic pathways: 1) the mitochondrial Krebs cycle, 2) conversion to lactate in the cell cytosol, or 3) conversion back to glucose in the process of gluconeogenesis.
Under aerobic conditions, most pyruvate enters the Krebs cycle and little is converted to lactate. Molecular oxygen is an absolute requirement for Krebs cycle activity. Under anaerobic conditions, pyruvate cannot enter the Krebs cycle and is preferentially converted to lactate.1
An elevated lactate level is a sensitive marker for tissue hypoxia caused by a variety of diseases, including sepsis, trauma, ischemic bowel, and necrotizing fasciitis. With sepsis, additional mechanisms also contribute to the increase in lactate, including increased glycolysis, impaired lactate clearance, and activation of inflammatory cells that shift cellular metabolism toward lactate production.2,3
The normal range for venous plasma lactate in adults is 0.5 to 2.2 mM, although the normal range may vary because of differences in local laboratory methods. Arterial, capillary, and venous lactate are all highly positively correlated.4 Venous lactate concentrations between 2.3 and 3.9 mM are suggestive of mild physiologic dysfunction, and values ≥4.0 mM are consistent with severe physiologic dysfunction. In hospitalized patients, sepsis is one of the most common causes of a lactate level ≥4 mM.5
In many patients with an elevated lactate concentration the anion gap is also increased—but this is not always the case. In fact, in one large observational study, among patients with sepsis and a lactate concentration ≥4 mM, approximately 25% had a normal bicarbonate level and normal anion gap.6
Elevated lactate levels applied in obstetric and gynecologic practice
CASE 1. Obstetric practice: Hernia identified during labor
A 30-year-old woman (G1P0) presents in early labor at 37 weeks’ gestation. Two years prior to the pregnancy she had a Roux-en-Y gastric bypass and lost more than 100 lb. In addition to reporting lower abdominal pain occurring during contractions, she reports the new onset of mid-epigastric pain. A surgical consult is requested. The initial white blood cell count is 6,290 per uL, and the lactate level is 1.0 mM.
The surgeon consulted orders a computed tomography (CT) scan with oral contrast, but the patient has difficulty retaining the oral contrast due to her nausea, delaying the performance of the CT scan. Three hours following admission a follow-up lactate measurement is 3.3 mM, and an emergency CT scan is performed.
The CT scan shows an internal hernia with swirling of the mesenteric vessels and twisting of the small bowel mesentery. An urgent cesarean delivery and repair of the internal hernia is performed.
The patient and her newborn do well postoperatively. The postoperative lactate level is 0.8 mM.
In pregnant women with a past history of a Roux-en-Y gastric bypass and abdominal discomfort who are in labor it is challenging to rapidly diagnose internal hernias and other bowel problems.7−9 In this case, the increased lactate level from 1.0 mM to 3.3 mM raised concern for ischemic bowel and triggered the emergency CT and urgent exploratory laparotomy and cesarean delivery.
Up to 14% of maternal deaths in the United States are due to infection.10 In many of these cases, there is a delay in sepsis recognition because previously healthy pregnant women with sepsis may not manifest classic signs such as fever, hypotension, or mental status changes until late in the disease course. Measurement of lactate can facilitate the early recognition of severe sepsis in pregnant women, thereby accelerating and focusing their treatment.11
To reduce mortality due to sepsis, aggressive intervention needs to occur within the first 6 hours following the onset of the infection.
CASE 2. Gynecologic practice: Bacterial infection identified in the presence of abdominal pain and vomiting
A 40-year-old woman presents to the ED 5 days following a myomectomy, with nausea, vomiting, and abdominal pain. Her vital signs reveal: temperature, 98.4°F (36.9°C); heart rate, 122 bpm; blood pressure, 115/70 mm Hg; and white blood cell count, 6,270 per uL. Her lactate level is 4.0 mM. She is admitted to the ICU with a presumptive diagnosis of severe sepsis and treatment with broad-spectrum antibiotics is initiated. Twenty-four hours following admission, gram-negative rods are identified in blood cultures that are later identified to be Bacteroides fragilis.
For the past 2 decades there has been a concerted national effort to reduce mortality caused by sepsis through early diagnosis and aggressive treatment of sepsis in an ICU setting. Observational studies have reported that an elevated lactate level is an excellent early biomarker for sepsis and may be observed prior to the onset of fever, elevated white blood cell count, or hypotension.6 For example, in one large study of patients with sepsis and a lactate measurement ≥4 mM, only 50% of patients had a systolic blood pressure <90 mm Hg.
Elevated lactate levels also are associated with an increased risk of death. Among 13,932 consecutive patients admitted to an ICU in Alberta, Canada, the mortality rate among patients with a venous or arterial lactate >2 mM was 20%, compared with a mortality rate of 5% for patients with a lactate level ≤2 mM.12 In a study of 1,278 patients with infection admitted to the hospital from the ED, mortality increased as baseline lactate concentration rose. For lactate concentrations of 0 to 2.4, 2.5 to 3.9, and ≥4.0 mM, mortality rates were 5%, 9%, and 28%, respectively.13
In patients with sepsis, serial measurement of lactate can help to guide treatment. In a randomized trial, 348 patients admitted to an ICU with a lactate ≥3 mM were randomly assigned to standard treatment, in which the clinicians had no knowledge of patients’ lactate levels, or to an experimental group, in which the clinicians were provided lactate measurement results every 2 hours. Compared with clinicians in the control group, the clinicians with access to frequent lactate measurements administered more fluids and vasodilators to their patients. Compared with patients in the control group, the hospital mortality rate was lower when the clinicians had access to frequent lactate measurements (34% vs 44%, respectively; adjusted hazard ratio, 0.61; 95% confidence interval, 0.43−0.87; P = .0006).14
Elevated lactate levels in the fetus and newborn
The physiologic status of the newborn is routinely assessed with the Apgar score. Umbilical artery and venous blood gases, including measurement of pH, are often used as a corroborating biomarker. Most studies report that umbilical artery or vein lactate measurement is as useful as a pH measurement in assessing newborn physiologic status. The normal range of lactate in fetuses and newborns is not precisely defined, with values between 3.5 and 7 mM being cited as the upper limit of normal.15−18
In many countries (but not the United States), in utero fetal status during labor is assessed by fetal scalp sampling of blood and measurement of either pH or lactate. Fetal scalp sampling is difficult and often very little blood is obtained, making it difficult to measure pH. A Cochrane review reported that in 2 randomized trials, fetal scalp sampling produced a successful measurement of lactate in 99% of attempts, while a pH result could only be obtained in 79% of cases due to an inadequate volume of blood or clotted blood.19
Increased lactate measurement can help our patients
Measuring lactate in order to rapidly identify patients with major physiologic derangements is practiced widely in EDs and ICUs. There is significant opportunity to increase the use of lactate measurement in obstetrics and gynecology. Increasing this use will help to rapidly identify women with severe sepsis and other diseases, leading to more rapid intervention and improved outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clinic Proc. 2013;88(10):1127−1140.
- Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39.
- Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252−261.
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567−573.
- Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368−1377.
- Caranta DG, Lee AM, Pennington D, Zelig CM. Complications from Roux-en-Y gastric bypass mistaken for medical complications in gravid patients. Obstet Gynecol. 2014;124(2 part 2 suppl 1):464−466.
- Moore KA, Ouyang DW, Whang EE. Maternal and fetal deaths after gastric bypass surgery for morbid obesity. N Engl J Med. 2004;351(7):721−722.
- Loar PV 3rd, Sanchez-Ramos L, Kaunitz AM, Kerwin AJ, Diaz J. Maternal death caused by midgut volvulus after bariatric surgery. Am J Obstet Gynecol. 2005;193(5):1748−1749.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
- Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(5):481−486.
- Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperprolactinemia in the critically ill. Crit Care. 2009;13(3):R90.
- Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524−528.
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752−761.
- Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33−37.
- Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical cord venous lactate for predicting arterial lactic acidemia and neonatal morbidity at term. Obstet Gynecol. 2016;127(4):674−680.
- Shirey T, St. Pierre J, Winkelman J. Cord lactate, pH, and blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15−19.
- Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107−1114.
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB, Lau R. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2015;(5):CD006174.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clinic Proc. 2013;88(10):1127−1140.
- Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39.
- Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252−261.
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567−573.
- Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368−1377.
- Caranta DG, Lee AM, Pennington D, Zelig CM. Complications from Roux-en-Y gastric bypass mistaken for medical complications in gravid patients. Obstet Gynecol. 2014;124(2 part 2 suppl 1):464−466.
- Moore KA, Ouyang DW, Whang EE. Maternal and fetal deaths after gastric bypass surgery for morbid obesity. N Engl J Med. 2004;351(7):721−722.
- Loar PV 3rd, Sanchez-Ramos L, Kaunitz AM, Kerwin AJ, Diaz J. Maternal death caused by midgut volvulus after bariatric surgery. Am J Obstet Gynecol. 2005;193(5):1748−1749.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
- Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(5):481−486.
- Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperprolactinemia in the critically ill. Crit Care. 2009;13(3):R90.
- Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524−528.
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752−761.
- Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33−37.
- Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical cord venous lactate for predicting arterial lactic acidemia and neonatal morbidity at term. Obstet Gynecol. 2016;127(4):674−680.
- Shirey T, St. Pierre J, Winkelman J. Cord lactate, pH, and blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15−19.
- Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107−1114.
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB, Lau R. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2015;(5):CD006174.
Menstrual migraines: Which options and when?
› Consider recommending that patients with menstrual migraines try using prophylactic triptans 2 days before the onset of menses. B
› Advise against estrogen-containing contraception for women who have menstrual migraines with aura, who smoke, or are over 35, due to the increased risk of stroke (absolute contraindication). A
› Consider estrogen-containing contraception if the benefits outweigh the risks for women with migraines who are under 35 and do not have aura (relative contraindication). A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mary, a 34-year-old woman, is a new patient to your practice after moving to the area for a job. She has a history of migraine headaches triggered by her menstrual periods. She has been taking combined oral contraceptives (COCs) since she was 17, with a few years off when she had 2 children. Her migraines improved when she was pregnant, but worsened postpartum with each of her daughters to a point where she had to stop breastfeeding at 4 months to go back on the pills.
On the COCs, she gets one or 2 mild-to-moderate headaches a month. She uses sumatriptan for abortive treatment with good relief. She has not missed work in the past 4 years because of her migraines. During the 6 months she was off COCs when trying to get pregnant, she routinely missed 2 to 3 workdays per month due to migraines. She knows when she is going to get a headache because she sees flashing lights in her left visual field. She has no other neurologic symptoms with the headaches, and the character of the headaches has not changed. She is a non-smoker, has normal blood pressure and lipid levels, and no other vascular risk factors.
You review her history and talk to her about the risk of stroke with migraines and with COCs. She is almost 35 years of age and you recommend stopping the COCs due to the risk. She feels strongly that she wants to continue taking the COCs, saying her quality of life is poor when she is off the pills. What should you do?
Migraine headaches are 2 to 3 times more prevalent in women than in men,1 with a lifetime risk of 43% vs 18%, respectively.2 Women account for about 80% of the $1 billion spent each year in the United States in medical expenses and lost work productivity related to migraines.1,2
Clinical patterns suggestive of menstrual migraine. About half of women affected by migraine have menstrually-related migraines (MRM); 3% to 12% have pure menstrual migraines (PMM).3 MRM and PMM are both characterized by the presence of symptoms in at least 2 to 3 consecutive cycles, with symptoms occurring from between 2 days before to 3 days after the onset of menstruation. However, in PMM, symptoms do not occur at any other time of the menstrual cycle; in MRM, symptoms can occur at other times of the cycle. PMM is more likely to respond to hormone therapy than is MRM.
Multiple studies in the United States, Europe, and Asia have noted that migraines related to menses typically last longer, are more severe, less likely to be associated with aura, and more likely to be recurrent and recalcitrant to treatment than non-menstrual migraines.1 TABLE 13 describes diagnostic criteria for migraine without aura.
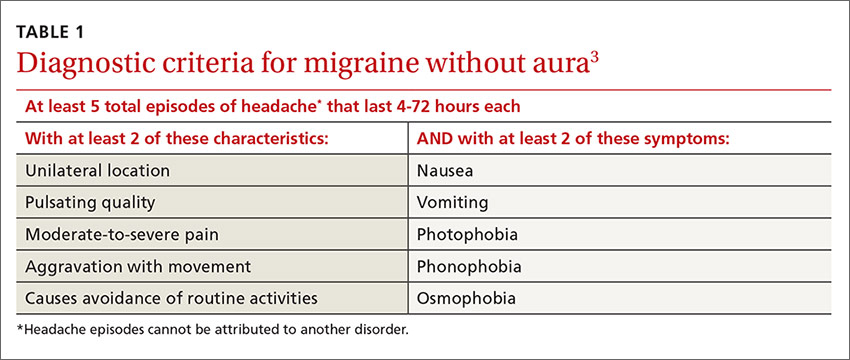
Possible mechanisms of MRM and PMM. The etiology of migraine is not well understood and is likely multifactorial.4 Incidence of menstrual migraines is related to cyclic changes in female hormones—specifically, the decreasing levels of estrogen that typically happen the week before onset of menses.1 The mechanism is not yet clear, though it is thought that a decline in estrogen levels triggers a decline in serotonin levels, which may lead to cranial vasodilation and sensitization of the trigeminal nerve.5,6 Estrogen decline has also been linked to increased cranial nociception as well as decreased endogenous opioid activity. A study using positron emission tomography found increased activity of serotonergic neurons in migraineurs.7 The evidence that triptans and serotonin receptor agonists are effective in the treatment of migraine also supports the theory that serotonin neurohormonal signaling pathways play a critical role in the pathogenesis of migraines.7
Prevalence patterns point to the role of estrogen. The prevalence of migraines in women increases around puberty, peaks between ages 30 and 40, and decreases after natural menopause.6 Migraine prevalence increases during the first week postpartum, when levels of estrogen and progesterone decrease suddenly and significantly.1 Migraine frequency and intensity decrease in the second and third trimesters of pregnancy and after menopause, when estrogen levels fluctuate significantly less.1 In the Women’s Health Initiative study, women who used hormone replacement therapy (HRT) had a 42% increased risk of migraines compared with women in the study who had never used HRT.8
The association of migraine with female hormones was further supported by a Dutch study of male-to-female transgender patients on estrogen therapy, who had a 26% incidence of migraine, equivalent to the 25% prevalence in natal female controls in this study, compared with just 7.5% in male controls.9 The association between migraine and estrogen withdrawal was investigated in studies performed more than 40 years ago, when women experiencing migraines around the time of menses were given intramuscular estradiol and experienced a delay in symptom onset.10
Abortive and prophylactic treatments: Factors that guide selection
In considering probable menstrual migraine, take a detailed history, review headache diaries if available to determine association of headaches with menses, and perform a thorough neurologic examination. If a diagnosis of menstrual migraine is established, discuss the benefits of different treatment options, both abortive and prophylactic.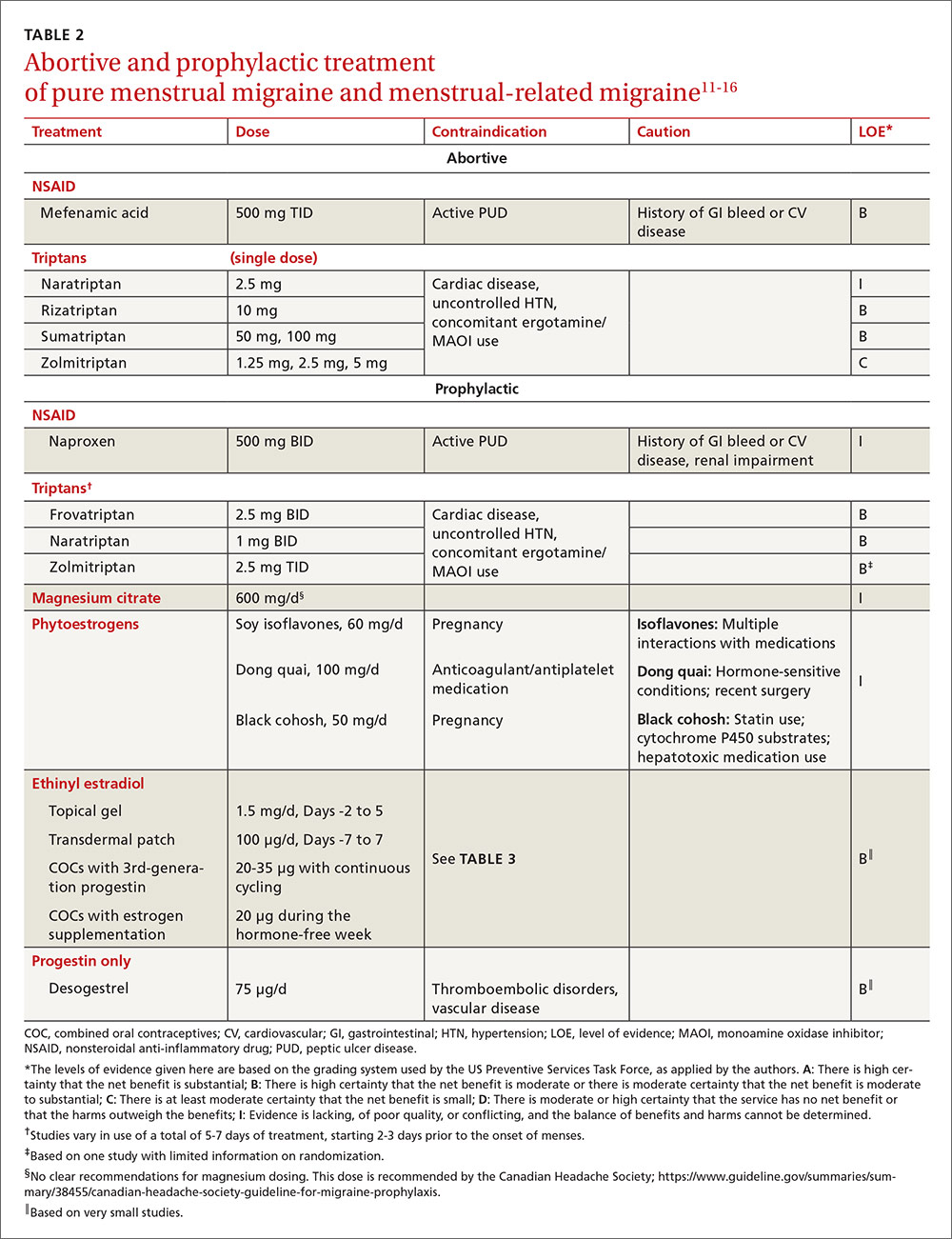
For the patient with MRM, take into account frequency of symptoms, predictability of menstruation, medication costs, and comorbidities. Both triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) can be effective treatments for MRM.11 Abortive therapy may be appropriate if a patient prefers to take medication intermittently, if her menses are unpredictable, or if she does not get migraine headaches with every menses. Mefenamic acid, sumatriptan, and rizatriptan have category B recommendations for abortive treatment for menstrual migraines (TABLE 211-16). (For the patient who has regular MRM but unpredictable menses, ovulation predictor kits can be used to help predict the onset of menses, although this would involve additional cost.)
For the patient who has predictable menses and regularly occurring menstrual migraine, some data show that a short-term prophylactic regimen with triptans started 2 to 3 days before the onset of menses and continued for 5 to 7 days total can reduce the incidence of menstrual migraine (TABLE 211-16). At least one high-quality randomized controlled trial (RCT) showed a significant reduction in the incidence of MRM when women were treated prophylactically with frovatriptan, a long-acting triptan with a half-life of approximately 26 hours. Participants received frovatriptan 2.5 mg once a day or twice a day or placebo in the perimenstrual period (day -2 to +3). The incidence of MRM was 52%, 41%, and 67%, respectively (P<.0001).11,17
Another RCT of fair quality examined the effect of naratriptan (half-life 6-8 hours) on the median number of menstrual migraines over 4 menstrual cycles. Women who received 1 mg of naratriptan BID for 2 to 3 days before menses had 2 MRM episodes over the 4 cycles compared with 4 MRM episodes in women who received placebo over the same time period (P<.05).11,18 A third RCT, also of fair quality, compared 2 different regimens of zolmitriptan (half-life 3 hours) with placebo and found that women who received 2.5 mg of zolmitriptan either BID or TID 2 to 3 days prior to menses had a reduction both in frequency of menstrual migraines and in the mean number of breakthrough headaches per menstrual cycle, as well as a reduction in the need for rescue medications.12,19 Triptans are contraindicated in women with a history of cardiac disease or uncontrolled hypertension. Also, triptans can be expensive, precluding their use for some patients.
Evidence is insufficient to recommend for or against the use of NSAIDs as prophylaxis for MRM.11 NSAIDs may be contraindicated in women with a history of peptic ulcer disease or gastrointestinal bleeding. That said, if NSAIDs are not contraindicated, a trial may be reasonable given their low cost.
Data are sparse on the use of vitamins and supplements in treating and preventing PMM or MRM. In one very small double-blind, placebo-controlled study in 1991 (N=24, with efficacy data for 20), participants received a 2-week course of oral magnesium premenstrually. There was a statistically significant reduction in the number of days with headache per month (from 4.7±3.1 days to 2.4±2.2 days; P<.01) and in the total pain index (P<.03).20 A number of studies have demonstrated a correlation between hypomagnesemia and migraine headaches.5,21 The exact mechanism for this relationship is unclear.
Some recent evidence-based reviews have examined the efficacy of nutraceuticals such as magnesium, feverfew, butterbur, coenzyme Q10, and riboflavin on typical migraine, but it is not clear if these results are translatable to the treatment and prophylaxis of menstrual migraine.11,22 A multicenter, single-blind, RCT is underway to examine the efficacy of acupuncture as prophylaxis for MRM.23
Estrogen: Prescribing criteria are strict
Most studies examining the role of exogenous estrogen in reducing menstrual migraines have used topical estrogen (either in patch or gel formulations) in the perimenstrual window (TABLE 211-16). The topical estrogen route has been examined, in particular, as it is presumed to confer less risk of hypercoagulability by avoiding first-pass metabolism. However, there is conflicting evidence on this issue, in particular regarding premenopausal women.13,25 Additionally, many of the studies of estrogen supplementation show a trend toward increased headache once estrogen is discontinued, presumably due to estrogen withdrawal.10,24
That said, one study by MacGregor, et al demonstrates that the use of estradiol gel in the perimenstrual window leads to a 22% reduction in migraine days as well as less severe migrainous symptoms.26 This trend has been demonstrated in other studies examining estrogen supplementation. Of note, the estrogen studies generally are small, older, and of fair to poor quality.11 These studies have used higher doses of estrogen than are commonly used for contraception today because lower doses of estrogen seem not to have the same impact on migraine.5,24
As for COCs, with either normal or extended cycling, data are more mixed than for estrogen supplementation alone; equivalent numbers of women experience improvement, no change, or worsening of their headache pattern. Many women have continuing or worsening migraines in the hormone-free week, and thus most studies have examined the use of extended cycling COCs.5 Sulak, et al demonstrated a statistically significant reduction in headache frequency using extended-cycling COCs, though they did not examine MRM in particular.27 The efficacy of extended-cycling COCs for reduction of MRM was confirmed by Coffee and colleagues with a small but statistically significant decrease in daily headache scores.28
Adverse effects. All estrogen therapies pose the risk of adverse effects (deep vein thrombosis, hypertension, breast tenderness, nausea, etc). Additionally, estrogen supplementation may actually trigger migraines in some women if, when it is discontinued, the blood estrogen level does not remain above a threshold concentration.5,10,24 Estrogen may also trigger migraine in previously headache-free women and may convert migraine without aura into migraine with aura. In either case, therapy should be stopped.5,24
There is promising evidence from 2 small RCTs and one observational trial that progestin-only contraceptive pills (POP) may reduce the frequency and severity of menstrual migraines (TABLE 211-16). More prospective data are needed to confirm this reduction, as there have not been specific studies examining other progesterone-only preparations to prevent menstrual migraines.
Risk of ischemic stroke. Unfortunately, there are population data showing that second-generation and, to a smaller degree, third-generation progestins, which include the desogestrel used in the above studies, may increase the risk of ischemic stroke. This is a particular concern in women who experience migraine.29 Second-generation progestins include levonorgestrel, which is in the levonorgestrel IUD; however, there is no direct evidence for increased ischemic stroke in this particular preparation, and the circulating plasma levels are low. Etonorgestrel, the active ingredient in the contraceptive implant, is a third-generation progestin, though there is no direct evidence of increased ischemic stroke with use of the etonorgestrel implant.
There is a 2- to 4-fold increased risk of ischemic stroke in women who experience migraine.1,5,30 As stated above, this risk may be further increased by some progesterone formulations. But there is also a demonstrable increase in ischemic stroke risk with the use of estrogen, particularly at the higher concentrations that have been shown to prevent MRM.31,32 The overall incidence of ischemic stroke in menstrual-age women is low, which has limited the number of studies with enough power to quantify the absolute increased risk of stroke in conjunction with estrogen use. Nevertheless, exogenous estrogen is thought to increase the risk of ischemic stroke an additional 2- to 4-fold.1,5,29,30,32-34
Women who experience aura. MRM, as it is defined, typically excludes women who experience aura; however, the number of women who experience aura with migraine either in proximity to their menses or throughout the month has not been well documented. The risk of ischemic stroke is higher for women who experience migraine with aura than those with migraine alone, possibly because aura is associated with reduced regional vascular flow leading to hypoperfusion, which sets the stage for a possible ischemic event.4,5,35 The risk of ischemic stroke is amplified further for women who are over 35, who smoke, or who have additional vascular risk factors (eg, uncontrolled hypertension, diabetes, or known vascular or cardiac disease).1,5,34 This array of evidence serves as the basis for the US Medical Eligibility Criteria (USMEC) recommendations36 for hormonal contraceptive use, in particular the absolute contraindication for estrogen use in women who experience migraine with aura (TABLE 336-38).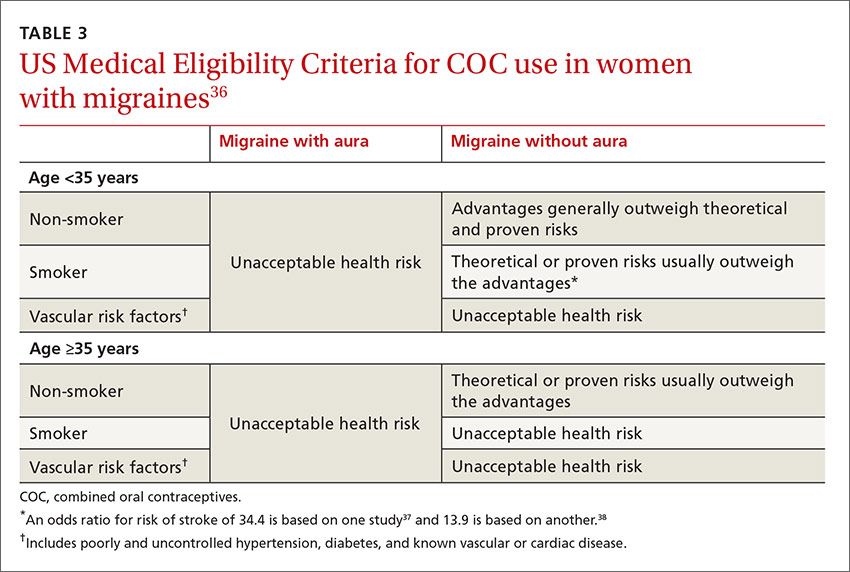
CASE › Given Mary’s experience of aura with migraine, you talk with her at length about the risk of ischemic stroke and the USMEC recommendation that she absolutely should not be taking COCs. You suggest a progestin-only method of contraception such as depot medroxyprogesterone acetate, a progestin intrauterine device, or a hormonal implant, which may suppress ovulation and decrease her headaches. You discuss that while some women may have headaches with these progestin-only methods, stroke risk is significantly reduced. You also suggest a trial of prophylactic triptans as another possible option.
She says she understands the increased risk of stroke but is still unwilling to try anything else right now due to worries about her quality of life. You decide jointly to refill COCs for 3 months, and you document the shared decision process in the chart. After advising the patient that you will not continue to prescribe COCs for an extended period of time, you also schedule a follow-up appointment to further discuss risks and benefits of migraine treatment and means of reducing other risk factors for stroke.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin, Department of Family Medicine, 1100 Delaplaine Ct, Madison, WI 53715; [email protected].
1. MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
2. Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170-1178.
3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
4. Garza I, Swanson JW, Cheshire WP Jr, et al. Headache and other craniofacial pain. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1703-1744.
5. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365-386.
6. Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006; 295:1824-1830.
7. Loder EW. Menstrual migraine: pathophysiology, diagnosis and impact. Headache. 2006;46 (Suppl 2):S55-S60.
8. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Women’s Health (Larchmt). 2003;12:1027-1036.
9. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593-594.
10. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355-365.
11. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70:1555-1563.
12. Hu Y, Guan X, Fan L, et al. Triptans in prevention of menstrual migraine: a systematic review with meta-analysis. J Headache Pain. 2013;14:7.
13. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227-1231.
14. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33:340-346.
15.
16. Morotti M, Remorgida V, Venturini PL, et al. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178-182.
17. Silberstein SD, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized double-blind, placebo-controlled study. Headache. 2001;41:248-256.
19. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
20. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
21. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2014;35:912-922.
22. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51:484-501.
23. Zhang XZ, Zhang L, Guo J, et al. Acupuncture as prophylaxis for menstrual-related migraine: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:374.
24. MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
25. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
26. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double blind placebo-controlled crossover study. Neurology. 2006;67:2159-2163.
27. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47:27-37.
28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health. 2014;23:310-317.
29. Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65:197-205.
30. Bousser MG. Estrogen, migraine, and stroke. Stroke. 2004;35(Suppl 1):2652-2656.
31. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72-78.
32. Donaghy M, Chang CL, Poulter N. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747-750.
33. Sacco S, Ricci S, Degan D. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;12:177-189.
34. Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362-368.
35. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
36. Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep
37. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
38. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
› Consider recommending that patients with menstrual migraines try using prophylactic triptans 2 days before the onset of menses. B
› Advise against estrogen-containing contraception for women who have menstrual migraines with aura, who smoke, or are over 35, due to the increased risk of stroke (absolute contraindication). A
› Consider estrogen-containing contraception if the benefits outweigh the risks for women with migraines who are under 35 and do not have aura (relative contraindication). A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mary, a 34-year-old woman, is a new patient to your practice after moving to the area for a job. She has a history of migraine headaches triggered by her menstrual periods. She has been taking combined oral contraceptives (COCs) since she was 17, with a few years off when she had 2 children. Her migraines improved when she was pregnant, but worsened postpartum with each of her daughters to a point where she had to stop breastfeeding at 4 months to go back on the pills.
On the COCs, she gets one or 2 mild-to-moderate headaches a month. She uses sumatriptan for abortive treatment with good relief. She has not missed work in the past 4 years because of her migraines. During the 6 months she was off COCs when trying to get pregnant, she routinely missed 2 to 3 workdays per month due to migraines. She knows when she is going to get a headache because she sees flashing lights in her left visual field. She has no other neurologic symptoms with the headaches, and the character of the headaches has not changed. She is a non-smoker, has normal blood pressure and lipid levels, and no other vascular risk factors.
You review her history and talk to her about the risk of stroke with migraines and with COCs. She is almost 35 years of age and you recommend stopping the COCs due to the risk. She feels strongly that she wants to continue taking the COCs, saying her quality of life is poor when she is off the pills. What should you do?
Migraine headaches are 2 to 3 times more prevalent in women than in men,1 with a lifetime risk of 43% vs 18%, respectively.2 Women account for about 80% of the $1 billion spent each year in the United States in medical expenses and lost work productivity related to migraines.1,2
Clinical patterns suggestive of menstrual migraine. About half of women affected by migraine have menstrually-related migraines (MRM); 3% to 12% have pure menstrual migraines (PMM).3 MRM and PMM are both characterized by the presence of symptoms in at least 2 to 3 consecutive cycles, with symptoms occurring from between 2 days before to 3 days after the onset of menstruation. However, in PMM, symptoms do not occur at any other time of the menstrual cycle; in MRM, symptoms can occur at other times of the cycle. PMM is more likely to respond to hormone therapy than is MRM.
Multiple studies in the United States, Europe, and Asia have noted that migraines related to menses typically last longer, are more severe, less likely to be associated with aura, and more likely to be recurrent and recalcitrant to treatment than non-menstrual migraines.1 TABLE 13 describes diagnostic criteria for migraine without aura.

Possible mechanisms of MRM and PMM. The etiology of migraine is not well understood and is likely multifactorial.4 Incidence of menstrual migraines is related to cyclic changes in female hormones—specifically, the decreasing levels of estrogen that typically happen the week before onset of menses.1 The mechanism is not yet clear, though it is thought that a decline in estrogen levels triggers a decline in serotonin levels, which may lead to cranial vasodilation and sensitization of the trigeminal nerve.5,6 Estrogen decline has also been linked to increased cranial nociception as well as decreased endogenous opioid activity. A study using positron emission tomography found increased activity of serotonergic neurons in migraineurs.7 The evidence that triptans and serotonin receptor agonists are effective in the treatment of migraine also supports the theory that serotonin neurohormonal signaling pathways play a critical role in the pathogenesis of migraines.7
Prevalence patterns point to the role of estrogen. The prevalence of migraines in women increases around puberty, peaks between ages 30 and 40, and decreases after natural menopause.6 Migraine prevalence increases during the first week postpartum, when levels of estrogen and progesterone decrease suddenly and significantly.1 Migraine frequency and intensity decrease in the second and third trimesters of pregnancy and after menopause, when estrogen levels fluctuate significantly less.1 In the Women’s Health Initiative study, women who used hormone replacement therapy (HRT) had a 42% increased risk of migraines compared with women in the study who had never used HRT.8
The association of migraine with female hormones was further supported by a Dutch study of male-to-female transgender patients on estrogen therapy, who had a 26% incidence of migraine, equivalent to the 25% prevalence in natal female controls in this study, compared with just 7.5% in male controls.9 The association between migraine and estrogen withdrawal was investigated in studies performed more than 40 years ago, when women experiencing migraines around the time of menses were given intramuscular estradiol and experienced a delay in symptom onset.10
Abortive and prophylactic treatments: Factors that guide selection
In considering probable menstrual migraine, take a detailed history, review headache diaries if available to determine association of headaches with menses, and perform a thorough neurologic examination. If a diagnosis of menstrual migraine is established, discuss the benefits of different treatment options, both abortive and prophylactic.
For the patient with MRM, take into account frequency of symptoms, predictability of menstruation, medication costs, and comorbidities. Both triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) can be effective treatments for MRM.11 Abortive therapy may be appropriate if a patient prefers to take medication intermittently, if her menses are unpredictable, or if she does not get migraine headaches with every menses. Mefenamic acid, sumatriptan, and rizatriptan have category B recommendations for abortive treatment for menstrual migraines (TABLE 211-16). (For the patient who has regular MRM but unpredictable menses, ovulation predictor kits can be used to help predict the onset of menses, although this would involve additional cost.)
For the patient who has predictable menses and regularly occurring menstrual migraine, some data show that a short-term prophylactic regimen with triptans started 2 to 3 days before the onset of menses and continued for 5 to 7 days total can reduce the incidence of menstrual migraine (TABLE 211-16). At least one high-quality randomized controlled trial (RCT) showed a significant reduction in the incidence of MRM when women were treated prophylactically with frovatriptan, a long-acting triptan with a half-life of approximately 26 hours. Participants received frovatriptan 2.5 mg once a day or twice a day or placebo in the perimenstrual period (day -2 to +3). The incidence of MRM was 52%, 41%, and 67%, respectively (P<.0001).11,17
Another RCT of fair quality examined the effect of naratriptan (half-life 6-8 hours) on the median number of menstrual migraines over 4 menstrual cycles. Women who received 1 mg of naratriptan BID for 2 to 3 days before menses had 2 MRM episodes over the 4 cycles compared with 4 MRM episodes in women who received placebo over the same time period (P<.05).11,18 A third RCT, also of fair quality, compared 2 different regimens of zolmitriptan (half-life 3 hours) with placebo and found that women who received 2.5 mg of zolmitriptan either BID or TID 2 to 3 days prior to menses had a reduction both in frequency of menstrual migraines and in the mean number of breakthrough headaches per menstrual cycle, as well as a reduction in the need for rescue medications.12,19 Triptans are contraindicated in women with a history of cardiac disease or uncontrolled hypertension. Also, triptans can be expensive, precluding their use for some patients.
Evidence is insufficient to recommend for or against the use of NSAIDs as prophylaxis for MRM.11 NSAIDs may be contraindicated in women with a history of peptic ulcer disease or gastrointestinal bleeding. That said, if NSAIDs are not contraindicated, a trial may be reasonable given their low cost.
Data are sparse on the use of vitamins and supplements in treating and preventing PMM or MRM. In one very small double-blind, placebo-controlled study in 1991 (N=24, with efficacy data for 20), participants received a 2-week course of oral magnesium premenstrually. There was a statistically significant reduction in the number of days with headache per month (from 4.7±3.1 days to 2.4±2.2 days; P<.01) and in the total pain index (P<.03).20 A number of studies have demonstrated a correlation between hypomagnesemia and migraine headaches.5,21 The exact mechanism for this relationship is unclear.
Some recent evidence-based reviews have examined the efficacy of nutraceuticals such as magnesium, feverfew, butterbur, coenzyme Q10, and riboflavin on typical migraine, but it is not clear if these results are translatable to the treatment and prophylaxis of menstrual migraine.11,22 A multicenter, single-blind, RCT is underway to examine the efficacy of acupuncture as prophylaxis for MRM.23
Estrogen: Prescribing criteria are strict
Most studies examining the role of exogenous estrogen in reducing menstrual migraines have used topical estrogen (either in patch or gel formulations) in the perimenstrual window (TABLE 211-16). The topical estrogen route has been examined, in particular, as it is presumed to confer less risk of hypercoagulability by avoiding first-pass metabolism. However, there is conflicting evidence on this issue, in particular regarding premenopausal women.13,25 Additionally, many of the studies of estrogen supplementation show a trend toward increased headache once estrogen is discontinued, presumably due to estrogen withdrawal.10,24
That said, one study by MacGregor, et al demonstrates that the use of estradiol gel in the perimenstrual window leads to a 22% reduction in migraine days as well as less severe migrainous symptoms.26 This trend has been demonstrated in other studies examining estrogen supplementation. Of note, the estrogen studies generally are small, older, and of fair to poor quality.11 These studies have used higher doses of estrogen than are commonly used for contraception today because lower doses of estrogen seem not to have the same impact on migraine.5,24
As for COCs, with either normal or extended cycling, data are more mixed than for estrogen supplementation alone; equivalent numbers of women experience improvement, no change, or worsening of their headache pattern. Many women have continuing or worsening migraines in the hormone-free week, and thus most studies have examined the use of extended cycling COCs.5 Sulak, et al demonstrated a statistically significant reduction in headache frequency using extended-cycling COCs, though they did not examine MRM in particular.27 The efficacy of extended-cycling COCs for reduction of MRM was confirmed by Coffee and colleagues with a small but statistically significant decrease in daily headache scores.28
Adverse effects. All estrogen therapies pose the risk of adverse effects (deep vein thrombosis, hypertension, breast tenderness, nausea, etc). Additionally, estrogen supplementation may actually trigger migraines in some women if, when it is discontinued, the blood estrogen level does not remain above a threshold concentration.5,10,24 Estrogen may also trigger migraine in previously headache-free women and may convert migraine without aura into migraine with aura. In either case, therapy should be stopped.5,24
There is promising evidence from 2 small RCTs and one observational trial that progestin-only contraceptive pills (POP) may reduce the frequency and severity of menstrual migraines (TABLE 211-16). More prospective data are needed to confirm this reduction, as there have not been specific studies examining other progesterone-only preparations to prevent menstrual migraines.
Risk of ischemic stroke. Unfortunately, there are population data showing that second-generation and, to a smaller degree, third-generation progestins, which include the desogestrel used in the above studies, may increase the risk of ischemic stroke. This is a particular concern in women who experience migraine.29 Second-generation progestins include levonorgestrel, which is in the levonorgestrel IUD; however, there is no direct evidence for increased ischemic stroke in this particular preparation, and the circulating plasma levels are low. Etonorgestrel, the active ingredient in the contraceptive implant, is a third-generation progestin, though there is no direct evidence of increased ischemic stroke with use of the etonorgestrel implant.
There is a 2- to 4-fold increased risk of ischemic stroke in women who experience migraine.1,5,30 As stated above, this risk may be further increased by some progesterone formulations. But there is also a demonstrable increase in ischemic stroke risk with the use of estrogen, particularly at the higher concentrations that have been shown to prevent MRM.31,32 The overall incidence of ischemic stroke in menstrual-age women is low, which has limited the number of studies with enough power to quantify the absolute increased risk of stroke in conjunction with estrogen use. Nevertheless, exogenous estrogen is thought to increase the risk of ischemic stroke an additional 2- to 4-fold.1,5,29,30,32-34
Women who experience aura. MRM, as it is defined, typically excludes women who experience aura; however, the number of women who experience aura with migraine either in proximity to their menses or throughout the month has not been well documented. The risk of ischemic stroke is higher for women who experience migraine with aura than those with migraine alone, possibly because aura is associated with reduced regional vascular flow leading to hypoperfusion, which sets the stage for a possible ischemic event.4,5,35 The risk of ischemic stroke is amplified further for women who are over 35, who smoke, or who have additional vascular risk factors (eg, uncontrolled hypertension, diabetes, or known vascular or cardiac disease).1,5,34 This array of evidence serves as the basis for the US Medical Eligibility Criteria (USMEC) recommendations36 for hormonal contraceptive use, in particular the absolute contraindication for estrogen use in women who experience migraine with aura (TABLE 336-38).
CASE › Given Mary’s experience of aura with migraine, you talk with her at length about the risk of ischemic stroke and the USMEC recommendation that she absolutely should not be taking COCs. You suggest a progestin-only method of contraception such as depot medroxyprogesterone acetate, a progestin intrauterine device, or a hormonal implant, which may suppress ovulation and decrease her headaches. You discuss that while some women may have headaches with these progestin-only methods, stroke risk is significantly reduced. You also suggest a trial of prophylactic triptans as another possible option.
She says she understands the increased risk of stroke but is still unwilling to try anything else right now due to worries about her quality of life. You decide jointly to refill COCs for 3 months, and you document the shared decision process in the chart. After advising the patient that you will not continue to prescribe COCs for an extended period of time, you also schedule a follow-up appointment to further discuss risks and benefits of migraine treatment and means of reducing other risk factors for stroke.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin, Department of Family Medicine, 1100 Delaplaine Ct, Madison, WI 53715; [email protected].
› Consider recommending that patients with menstrual migraines try using prophylactic triptans 2 days before the onset of menses. B
› Advise against estrogen-containing contraception for women who have menstrual migraines with aura, who smoke, or are over 35, due to the increased risk of stroke (absolute contraindication). A
› Consider estrogen-containing contraception if the benefits outweigh the risks for women with migraines who are under 35 and do not have aura (relative contraindication). A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mary, a 34-year-old woman, is a new patient to your practice after moving to the area for a job. She has a history of migraine headaches triggered by her menstrual periods. She has been taking combined oral contraceptives (COCs) since she was 17, with a few years off when she had 2 children. Her migraines improved when she was pregnant, but worsened postpartum with each of her daughters to a point where she had to stop breastfeeding at 4 months to go back on the pills.
On the COCs, she gets one or 2 mild-to-moderate headaches a month. She uses sumatriptan for abortive treatment with good relief. She has not missed work in the past 4 years because of her migraines. During the 6 months she was off COCs when trying to get pregnant, she routinely missed 2 to 3 workdays per month due to migraines. She knows when she is going to get a headache because she sees flashing lights in her left visual field. She has no other neurologic symptoms with the headaches, and the character of the headaches has not changed. She is a non-smoker, has normal blood pressure and lipid levels, and no other vascular risk factors.
You review her history and talk to her about the risk of stroke with migraines and with COCs. She is almost 35 years of age and you recommend stopping the COCs due to the risk. She feels strongly that she wants to continue taking the COCs, saying her quality of life is poor when she is off the pills. What should you do?
Migraine headaches are 2 to 3 times more prevalent in women than in men,1 with a lifetime risk of 43% vs 18%, respectively.2 Women account for about 80% of the $1 billion spent each year in the United States in medical expenses and lost work productivity related to migraines.1,2
Clinical patterns suggestive of menstrual migraine. About half of women affected by migraine have menstrually-related migraines (MRM); 3% to 12% have pure menstrual migraines (PMM).3 MRM and PMM are both characterized by the presence of symptoms in at least 2 to 3 consecutive cycles, with symptoms occurring from between 2 days before to 3 days after the onset of menstruation. However, in PMM, symptoms do not occur at any other time of the menstrual cycle; in MRM, symptoms can occur at other times of the cycle. PMM is more likely to respond to hormone therapy than is MRM.
Multiple studies in the United States, Europe, and Asia have noted that migraines related to menses typically last longer, are more severe, less likely to be associated with aura, and more likely to be recurrent and recalcitrant to treatment than non-menstrual migraines.1 TABLE 13 describes diagnostic criteria for migraine without aura.

Possible mechanisms of MRM and PMM. The etiology of migraine is not well understood and is likely multifactorial.4 Incidence of menstrual migraines is related to cyclic changes in female hormones—specifically, the decreasing levels of estrogen that typically happen the week before onset of menses.1 The mechanism is not yet clear, though it is thought that a decline in estrogen levels triggers a decline in serotonin levels, which may lead to cranial vasodilation and sensitization of the trigeminal nerve.5,6 Estrogen decline has also been linked to increased cranial nociception as well as decreased endogenous opioid activity. A study using positron emission tomography found increased activity of serotonergic neurons in migraineurs.7 The evidence that triptans and serotonin receptor agonists are effective in the treatment of migraine also supports the theory that serotonin neurohormonal signaling pathways play a critical role in the pathogenesis of migraines.7
Prevalence patterns point to the role of estrogen. The prevalence of migraines in women increases around puberty, peaks between ages 30 and 40, and decreases after natural menopause.6 Migraine prevalence increases during the first week postpartum, when levels of estrogen and progesterone decrease suddenly and significantly.1 Migraine frequency and intensity decrease in the second and third trimesters of pregnancy and after menopause, when estrogen levels fluctuate significantly less.1 In the Women’s Health Initiative study, women who used hormone replacement therapy (HRT) had a 42% increased risk of migraines compared with women in the study who had never used HRT.8
The association of migraine with female hormones was further supported by a Dutch study of male-to-female transgender patients on estrogen therapy, who had a 26% incidence of migraine, equivalent to the 25% prevalence in natal female controls in this study, compared with just 7.5% in male controls.9 The association between migraine and estrogen withdrawal was investigated in studies performed more than 40 years ago, when women experiencing migraines around the time of menses were given intramuscular estradiol and experienced a delay in symptom onset.10
Abortive and prophylactic treatments: Factors that guide selection
In considering probable menstrual migraine, take a detailed history, review headache diaries if available to determine association of headaches with menses, and perform a thorough neurologic examination. If a diagnosis of menstrual migraine is established, discuss the benefits of different treatment options, both abortive and prophylactic.
For the patient with MRM, take into account frequency of symptoms, predictability of menstruation, medication costs, and comorbidities. Both triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) can be effective treatments for MRM.11 Abortive therapy may be appropriate if a patient prefers to take medication intermittently, if her menses are unpredictable, or if she does not get migraine headaches with every menses. Mefenamic acid, sumatriptan, and rizatriptan have category B recommendations for abortive treatment for menstrual migraines (TABLE 211-16). (For the patient who has regular MRM but unpredictable menses, ovulation predictor kits can be used to help predict the onset of menses, although this would involve additional cost.)
For the patient who has predictable menses and regularly occurring menstrual migraine, some data show that a short-term prophylactic regimen with triptans started 2 to 3 days before the onset of menses and continued for 5 to 7 days total can reduce the incidence of menstrual migraine (TABLE 211-16). At least one high-quality randomized controlled trial (RCT) showed a significant reduction in the incidence of MRM when women were treated prophylactically with frovatriptan, a long-acting triptan with a half-life of approximately 26 hours. Participants received frovatriptan 2.5 mg once a day or twice a day or placebo in the perimenstrual period (day -2 to +3). The incidence of MRM was 52%, 41%, and 67%, respectively (P<.0001).11,17
Another RCT of fair quality examined the effect of naratriptan (half-life 6-8 hours) on the median number of menstrual migraines over 4 menstrual cycles. Women who received 1 mg of naratriptan BID for 2 to 3 days before menses had 2 MRM episodes over the 4 cycles compared with 4 MRM episodes in women who received placebo over the same time period (P<.05).11,18 A third RCT, also of fair quality, compared 2 different regimens of zolmitriptan (half-life 3 hours) with placebo and found that women who received 2.5 mg of zolmitriptan either BID or TID 2 to 3 days prior to menses had a reduction both in frequency of menstrual migraines and in the mean number of breakthrough headaches per menstrual cycle, as well as a reduction in the need for rescue medications.12,19 Triptans are contraindicated in women with a history of cardiac disease or uncontrolled hypertension. Also, triptans can be expensive, precluding their use for some patients.
Evidence is insufficient to recommend for or against the use of NSAIDs as prophylaxis for MRM.11 NSAIDs may be contraindicated in women with a history of peptic ulcer disease or gastrointestinal bleeding. That said, if NSAIDs are not contraindicated, a trial may be reasonable given their low cost.
Data are sparse on the use of vitamins and supplements in treating and preventing PMM or MRM. In one very small double-blind, placebo-controlled study in 1991 (N=24, with efficacy data for 20), participants received a 2-week course of oral magnesium premenstrually. There was a statistically significant reduction in the number of days with headache per month (from 4.7±3.1 days to 2.4±2.2 days; P<.01) and in the total pain index (P<.03).20 A number of studies have demonstrated a correlation between hypomagnesemia and migraine headaches.5,21 The exact mechanism for this relationship is unclear.
Some recent evidence-based reviews have examined the efficacy of nutraceuticals such as magnesium, feverfew, butterbur, coenzyme Q10, and riboflavin on typical migraine, but it is not clear if these results are translatable to the treatment and prophylaxis of menstrual migraine.11,22 A multicenter, single-blind, RCT is underway to examine the efficacy of acupuncture as prophylaxis for MRM.23
Estrogen: Prescribing criteria are strict
Most studies examining the role of exogenous estrogen in reducing menstrual migraines have used topical estrogen (either in patch or gel formulations) in the perimenstrual window (TABLE 211-16). The topical estrogen route has been examined, in particular, as it is presumed to confer less risk of hypercoagulability by avoiding first-pass metabolism. However, there is conflicting evidence on this issue, in particular regarding premenopausal women.13,25 Additionally, many of the studies of estrogen supplementation show a trend toward increased headache once estrogen is discontinued, presumably due to estrogen withdrawal.10,24
That said, one study by MacGregor, et al demonstrates that the use of estradiol gel in the perimenstrual window leads to a 22% reduction in migraine days as well as less severe migrainous symptoms.26 This trend has been demonstrated in other studies examining estrogen supplementation. Of note, the estrogen studies generally are small, older, and of fair to poor quality.11 These studies have used higher doses of estrogen than are commonly used for contraception today because lower doses of estrogen seem not to have the same impact on migraine.5,24
As for COCs, with either normal or extended cycling, data are more mixed than for estrogen supplementation alone; equivalent numbers of women experience improvement, no change, or worsening of their headache pattern. Many women have continuing or worsening migraines in the hormone-free week, and thus most studies have examined the use of extended cycling COCs.5 Sulak, et al demonstrated a statistically significant reduction in headache frequency using extended-cycling COCs, though they did not examine MRM in particular.27 The efficacy of extended-cycling COCs for reduction of MRM was confirmed by Coffee and colleagues with a small but statistically significant decrease in daily headache scores.28
Adverse effects. All estrogen therapies pose the risk of adverse effects (deep vein thrombosis, hypertension, breast tenderness, nausea, etc). Additionally, estrogen supplementation may actually trigger migraines in some women if, when it is discontinued, the blood estrogen level does not remain above a threshold concentration.5,10,24 Estrogen may also trigger migraine in previously headache-free women and may convert migraine without aura into migraine with aura. In either case, therapy should be stopped.5,24
There is promising evidence from 2 small RCTs and one observational trial that progestin-only contraceptive pills (POP) may reduce the frequency and severity of menstrual migraines (TABLE 211-16). More prospective data are needed to confirm this reduction, as there have not been specific studies examining other progesterone-only preparations to prevent menstrual migraines.
Risk of ischemic stroke. Unfortunately, there are population data showing that second-generation and, to a smaller degree, third-generation progestins, which include the desogestrel used in the above studies, may increase the risk of ischemic stroke. This is a particular concern in women who experience migraine.29 Second-generation progestins include levonorgestrel, which is in the levonorgestrel IUD; however, there is no direct evidence for increased ischemic stroke in this particular preparation, and the circulating plasma levels are low. Etonorgestrel, the active ingredient in the contraceptive implant, is a third-generation progestin, though there is no direct evidence of increased ischemic stroke with use of the etonorgestrel implant.
There is a 2- to 4-fold increased risk of ischemic stroke in women who experience migraine.1,5,30 As stated above, this risk may be further increased by some progesterone formulations. But there is also a demonstrable increase in ischemic stroke risk with the use of estrogen, particularly at the higher concentrations that have been shown to prevent MRM.31,32 The overall incidence of ischemic stroke in menstrual-age women is low, which has limited the number of studies with enough power to quantify the absolute increased risk of stroke in conjunction with estrogen use. Nevertheless, exogenous estrogen is thought to increase the risk of ischemic stroke an additional 2- to 4-fold.1,5,29,30,32-34
Women who experience aura. MRM, as it is defined, typically excludes women who experience aura; however, the number of women who experience aura with migraine either in proximity to their menses or throughout the month has not been well documented. The risk of ischemic stroke is higher for women who experience migraine with aura than those with migraine alone, possibly because aura is associated with reduced regional vascular flow leading to hypoperfusion, which sets the stage for a possible ischemic event.4,5,35 The risk of ischemic stroke is amplified further for women who are over 35, who smoke, or who have additional vascular risk factors (eg, uncontrolled hypertension, diabetes, or known vascular or cardiac disease).1,5,34 This array of evidence serves as the basis for the US Medical Eligibility Criteria (USMEC) recommendations36 for hormonal contraceptive use, in particular the absolute contraindication for estrogen use in women who experience migraine with aura (TABLE 336-38).
CASE › Given Mary’s experience of aura with migraine, you talk with her at length about the risk of ischemic stroke and the USMEC recommendation that she absolutely should not be taking COCs. You suggest a progestin-only method of contraception such as depot medroxyprogesterone acetate, a progestin intrauterine device, or a hormonal implant, which may suppress ovulation and decrease her headaches. You discuss that while some women may have headaches with these progestin-only methods, stroke risk is significantly reduced. You also suggest a trial of prophylactic triptans as another possible option.
She says she understands the increased risk of stroke but is still unwilling to try anything else right now due to worries about her quality of life. You decide jointly to refill COCs for 3 months, and you document the shared decision process in the chart. After advising the patient that you will not continue to prescribe COCs for an extended period of time, you also schedule a follow-up appointment to further discuss risks and benefits of migraine treatment and means of reducing other risk factors for stroke.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin, Department of Family Medicine, 1100 Delaplaine Ct, Madison, WI 53715; [email protected].
1. MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
2. Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170-1178.
3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
4. Garza I, Swanson JW, Cheshire WP Jr, et al. Headache and other craniofacial pain. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1703-1744.
5. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365-386.
6. Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006; 295:1824-1830.
7. Loder EW. Menstrual migraine: pathophysiology, diagnosis and impact. Headache. 2006;46 (Suppl 2):S55-S60.
8. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Women’s Health (Larchmt). 2003;12:1027-1036.
9. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593-594.
10. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355-365.
11. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70:1555-1563.
12. Hu Y, Guan X, Fan L, et al. Triptans in prevention of menstrual migraine: a systematic review with meta-analysis. J Headache Pain. 2013;14:7.
13. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227-1231.
14. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33:340-346.
15.
16. Morotti M, Remorgida V, Venturini PL, et al. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178-182.
17. Silberstein SD, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized double-blind, placebo-controlled study. Headache. 2001;41:248-256.
19. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
20. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
21. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2014;35:912-922.
22. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51:484-501.
23. Zhang XZ, Zhang L, Guo J, et al. Acupuncture as prophylaxis for menstrual-related migraine: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:374.
24. MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
25. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
26. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double blind placebo-controlled crossover study. Neurology. 2006;67:2159-2163.
27. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47:27-37.
28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health. 2014;23:310-317.
29. Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65:197-205.
30. Bousser MG. Estrogen, migraine, and stroke. Stroke. 2004;35(Suppl 1):2652-2656.
31. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72-78.
32. Donaghy M, Chang CL, Poulter N. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747-750.
33. Sacco S, Ricci S, Degan D. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;12:177-189.
34. Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362-368.
35. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
36. Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep
37. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
38. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.
1. MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
2. Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170-1178.
3. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
4. Garza I, Swanson JW, Cheshire WP Jr, et al. Headache and other craniofacial pain. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2012:1703-1744.
5. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365-386.
6. Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006; 295:1824-1830.
7. Loder EW. Menstrual migraine: pathophysiology, diagnosis and impact. Headache. 2006;46 (Suppl 2):S55-S60.
8. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Women’s Health (Larchmt). 2003;12:1027-1036.
9. Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593-594.
10. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355-365.
11. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70:1555-1563.
12. Hu Y, Guan X, Fan L, et al. Triptans in prevention of menstrual migraine: a systematic review with meta-analysis. J Headache Pain. 2013;14:7.
13. Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227-1231.
14. Merki-Feld GS, Imthurn B, Langner R, et al. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33:340-346.
15.
16. Morotti M, Remorgida V, Venturini PL, et al. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178-182.
17. Silberstein SD, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized double-blind, placebo-controlled study. Headache. 2001;41:248-256.
19. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
20. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
21. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2014;35:912-922.
22. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51:484-501.
23. Zhang XZ, Zhang L, Guo J, et al. Acupuncture as prophylaxis for menstrual-related migraine: study protocol for a multicenter randomized controlled trial. Trials. 2013;14:374.
24. MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354-361.
25. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
26. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double blind placebo-controlled crossover study. Neurology. 2006;67:2159-2163.
27. Sulak P, Willis S, Kuehl T, et al. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47:27-37.
28. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health. 2014;23:310-317.
29. Lidegaard Ø, Kreiner S. Contraceptives and cerebral thrombosis: a five-year national case-control study. Contraception. 2002;65:197-205.
30. Bousser MG. Estrogen, migraine, and stroke. Stroke. 2004;35(Suppl 1):2652-2656.
31. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284:72-78.
32. Donaghy M, Chang CL, Poulter N. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747-750.
33. Sacco S, Ricci S, Degan D. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;12:177-189.
34. Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362-368.
35. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2445.
36. Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep
37. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
38. Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833.