User login
Study questions if blue light–blocking glasses really work
Despite claims by their makers, The glasses probably don’t improve wearers’ sleep habits either, according to the study.
Blue light glasses are usually marketed as being able to filter out the potentially harmful effects of blue light from screens, such as eyestrain, dry eye, and sleep problems. Interest in blue light glasses increased during the COVID-19 pandemic as more people stayed home and looked at their computers and phones. They’re often prescribed by optometrists.
The study, published in the Cochrane Database of Systematic Reviews, looked at data collected from 17 clinical trials in six countries that recruited 619 people.
“We found there may be no short-term advantages with using blue light–filtering spectacle lenses to reduce visual fatigue associated with computer use, compared to non–blue-light–filtering lenses,” senior author Laura Downie, PhD, an associate professor of optometry and vision sciences at the University of Melbourne, said in a statement.
“It is also currently unclear whether these lenses affect vision quality or sleep-related outcomes, and no conclusions could be drawn about any potential effects on retinal health in the longer term. People should be aware of these findings when deciding whether to purchase these spectacles.”
Researchers noted that one reason the glasses don’t help is that the amount of blue light received from computer screens and other artificial sources is only about a thousandth of what people get from natural daylight. On top of that, blue light lenses usually filter out only about 10%-25% of blue light.
“Our findings do not support the prescription of blue light–filtering lenses to the general population,” Dr. Downie said.
Eye experts say people can cut down on eyestrain by simply cutting down on the amount of time they look at screens, or by taking regular breaks. To improve sleep, stop looking at screens for a few hours before bedtime.
The researchers noted limitations in their analysis. None of the studies investigated contrast sensitivity, color discrimination, discomfort glare, macular health, serum melatonin levels, or overall patient visual satisfaction.
Also, the length of the different studies varied. More studies of the use of blue light–filtering glasses is needed, the researchers said.
The study received funding from Australia’s National Health and Medical Research Council, the Public Health Agency in the United Kingdom, and Queen’s University Belfast. Two coauthors reported receiving payment from the College of Optometrists.
A version of this article first appeared on WebMD.com.
Despite claims by their makers, The glasses probably don’t improve wearers’ sleep habits either, according to the study.
Blue light glasses are usually marketed as being able to filter out the potentially harmful effects of blue light from screens, such as eyestrain, dry eye, and sleep problems. Interest in blue light glasses increased during the COVID-19 pandemic as more people stayed home and looked at their computers and phones. They’re often prescribed by optometrists.
The study, published in the Cochrane Database of Systematic Reviews, looked at data collected from 17 clinical trials in six countries that recruited 619 people.
“We found there may be no short-term advantages with using blue light–filtering spectacle lenses to reduce visual fatigue associated with computer use, compared to non–blue-light–filtering lenses,” senior author Laura Downie, PhD, an associate professor of optometry and vision sciences at the University of Melbourne, said in a statement.
“It is also currently unclear whether these lenses affect vision quality or sleep-related outcomes, and no conclusions could be drawn about any potential effects on retinal health in the longer term. People should be aware of these findings when deciding whether to purchase these spectacles.”
Researchers noted that one reason the glasses don’t help is that the amount of blue light received from computer screens and other artificial sources is only about a thousandth of what people get from natural daylight. On top of that, blue light lenses usually filter out only about 10%-25% of blue light.
“Our findings do not support the prescription of blue light–filtering lenses to the general population,” Dr. Downie said.
Eye experts say people can cut down on eyestrain by simply cutting down on the amount of time they look at screens, or by taking regular breaks. To improve sleep, stop looking at screens for a few hours before bedtime.
The researchers noted limitations in their analysis. None of the studies investigated contrast sensitivity, color discrimination, discomfort glare, macular health, serum melatonin levels, or overall patient visual satisfaction.
Also, the length of the different studies varied. More studies of the use of blue light–filtering glasses is needed, the researchers said.
The study received funding from Australia’s National Health and Medical Research Council, the Public Health Agency in the United Kingdom, and Queen’s University Belfast. Two coauthors reported receiving payment from the College of Optometrists.
A version of this article first appeared on WebMD.com.
Despite claims by their makers, The glasses probably don’t improve wearers’ sleep habits either, according to the study.
Blue light glasses are usually marketed as being able to filter out the potentially harmful effects of blue light from screens, such as eyestrain, dry eye, and sleep problems. Interest in blue light glasses increased during the COVID-19 pandemic as more people stayed home and looked at their computers and phones. They’re often prescribed by optometrists.
The study, published in the Cochrane Database of Systematic Reviews, looked at data collected from 17 clinical trials in six countries that recruited 619 people.
“We found there may be no short-term advantages with using blue light–filtering spectacle lenses to reduce visual fatigue associated with computer use, compared to non–blue-light–filtering lenses,” senior author Laura Downie, PhD, an associate professor of optometry and vision sciences at the University of Melbourne, said in a statement.
“It is also currently unclear whether these lenses affect vision quality or sleep-related outcomes, and no conclusions could be drawn about any potential effects on retinal health in the longer term. People should be aware of these findings when deciding whether to purchase these spectacles.”
Researchers noted that one reason the glasses don’t help is that the amount of blue light received from computer screens and other artificial sources is only about a thousandth of what people get from natural daylight. On top of that, blue light lenses usually filter out only about 10%-25% of blue light.
“Our findings do not support the prescription of blue light–filtering lenses to the general population,” Dr. Downie said.
Eye experts say people can cut down on eyestrain by simply cutting down on the amount of time they look at screens, or by taking regular breaks. To improve sleep, stop looking at screens for a few hours before bedtime.
The researchers noted limitations in their analysis. None of the studies investigated contrast sensitivity, color discrimination, discomfort glare, macular health, serum melatonin levels, or overall patient visual satisfaction.
Also, the length of the different studies varied. More studies of the use of blue light–filtering glasses is needed, the researchers said.
The study received funding from Australia’s National Health and Medical Research Council, the Public Health Agency in the United Kingdom, and Queen’s University Belfast. Two coauthors reported receiving payment from the College of Optometrists.
A version of this article first appeared on WebMD.com.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Simple blood test may predict heart and kidney risk in T2D
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Low-dose oral minoxidil for female pattern hair loss: Benefits, impact on BP, heart rate evaluated
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
FROM JAAD INTERNATIONAL
SCD: Survival disparities seen across insurance types
“To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with sickle cell disease covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states,” the authors report in the study, published in Blood Advances.
“Our study underscores the persistent life expectancy shortfall for patients with sickle cell disease, the burden of premature mortality during adulthood, and survival disparities by insurance status,” they write.
SCD, which disproportionately affects people of African descent, significantly increases the risk of various acute and chronic life-threatening complications, including acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, infections, and organ damage.
In recent years, advances ranging from newborn screening to prophylactic antibiotics have significantly improved the survival rates of children with SCD in the United States. However, premature mortality in adulthood has remained well above that of the general public.
A recent study of a U.S. birth cohort predicted life expectancy with SCD to be approximately 2 decades shorter than in those without the disease. However, those projections were based on a simulation model, and other life expectancy estimates have had sample sizes with state or regional limitations.
With no population-level individual data–based periodic life tables existing for individuals with SCD, the authors turned to nationwide data on Medicare and Medicaid beneficiaries to get a better idea of survival predictions.
For the study, they identified 94,616 individuals diagnosed with SCD who had not undergone transplant and were receiving common care, based on nationwide Medicare and Medicaid claim data from 2008 to 2016 on beneficiaries in all 50 states.
Of the patients, 74% were Black and 53% resided in the South at the index date. The patients had a mean entry age overall of 26.6 years across insurance types.
The results showed projected life expectancy at birth with SCD to be 52.6 years, with the life expectancy at birth for females significantly longer, compared with that of males (55.0 versus 49.3 years). For the general population, U.S. life expectancy estimates have been reported to be about 76 years.
Specifically, life expectancy in the cohort into adulthood was 35.4 years at 18 years of age; 24.1 years at 35 years of age; 19.6 years at 45 years old; 13.2 years at 65 years of age; and 5.4 years at 85 years old.
Likewise, survival probability rates were high during childhood, with survival probability at 18 years being 0.98, declining to 0.804 at age 30; 0.628 at age 45; 0.267 at age 65 and 0.70 at age 85.
Black individuals had a significantly shorter life expectancy at birth than non-Black individuals (52.2 vs. 55.1 years), with the survival expectancy for Black individuals significantly shorter than the 75-year life expectancy estimated by the 2016 U.S. Centers for Disease Control and Prevention life table for the Black population in general.
Of the patients, only 5% had Medicare old age and survivor’s insurance, and 4% had Medicare disability insurance benefits or end-stage renal disease (ESRD), while 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid. The racial and regional distributions were similar across insurance types.
Adults covered by Medicare had a life expectancy of 39.8 years at 18 years of age, an improvement over life expectancy for those with Medicare for disabilities or ESRD and those dually insured by Medicare and Medicaid.
And the shorter life expectancy with Medicare and Medicaid was also observed among beneficiaries aged 65 or older, compared with those enrolled in Medicare old age and survivor’s insurance.
“Evidently, the life expectancy gap persists among patients with sickle cell disease, even though they are protected by public insurance,” the authors report.
The shorter life expectancy among those with dual eligibility for Medicare and Medicaid is consistent with that of the general population, the authors add.
“It is well-recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs,” they write. “They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligible.”
First author Boshen Jiao, PhD, MPH, of the Harvard T.H. Chan School of Public Health, in Boston, noted that socioeconomic factors, on a broader level, have an important impact on SCD.
“Even the first and most established FDA-approved medication for sickle cell disease, hydroxyurea, suffers from underutilization,” he told MDedge. “This issue can be associated with disparities along racial lines, particularly concerning access to treatments.”
Such factors can also play a role in the lack of a well-coordinated transition program needed to prevent gaps in care between youth and adulthood, as well as a lack of access to specialized care needed to potentially initiate new therapies.
Ultimately, the decline in survival seen from childhood to adulthood in SCD “emphasizes the pivotal role of health care during the critical transition phase from childhood to adulthood for individuals with sickle cell disease,” Dr. Jiao said. “Moreover, the prospect of a curative therapy that can intervene in the early stages of life, prior to adulthood, stands as a desirable goal.”
The authors urge that “future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.” They had no disclosures to report.
A version of this article first appeared on Medscape.com.
“To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with sickle cell disease covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states,” the authors report in the study, published in Blood Advances.
“Our study underscores the persistent life expectancy shortfall for patients with sickle cell disease, the burden of premature mortality during adulthood, and survival disparities by insurance status,” they write.
SCD, which disproportionately affects people of African descent, significantly increases the risk of various acute and chronic life-threatening complications, including acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, infections, and organ damage.
In recent years, advances ranging from newborn screening to prophylactic antibiotics have significantly improved the survival rates of children with SCD in the United States. However, premature mortality in adulthood has remained well above that of the general public.
A recent study of a U.S. birth cohort predicted life expectancy with SCD to be approximately 2 decades shorter than in those without the disease. However, those projections were based on a simulation model, and other life expectancy estimates have had sample sizes with state or regional limitations.
With no population-level individual data–based periodic life tables existing for individuals with SCD, the authors turned to nationwide data on Medicare and Medicaid beneficiaries to get a better idea of survival predictions.
For the study, they identified 94,616 individuals diagnosed with SCD who had not undergone transplant and were receiving common care, based on nationwide Medicare and Medicaid claim data from 2008 to 2016 on beneficiaries in all 50 states.
Of the patients, 74% were Black and 53% resided in the South at the index date. The patients had a mean entry age overall of 26.6 years across insurance types.
The results showed projected life expectancy at birth with SCD to be 52.6 years, with the life expectancy at birth for females significantly longer, compared with that of males (55.0 versus 49.3 years). For the general population, U.S. life expectancy estimates have been reported to be about 76 years.
Specifically, life expectancy in the cohort into adulthood was 35.4 years at 18 years of age; 24.1 years at 35 years of age; 19.6 years at 45 years old; 13.2 years at 65 years of age; and 5.4 years at 85 years old.
Likewise, survival probability rates were high during childhood, with survival probability at 18 years being 0.98, declining to 0.804 at age 30; 0.628 at age 45; 0.267 at age 65 and 0.70 at age 85.
Black individuals had a significantly shorter life expectancy at birth than non-Black individuals (52.2 vs. 55.1 years), with the survival expectancy for Black individuals significantly shorter than the 75-year life expectancy estimated by the 2016 U.S. Centers for Disease Control and Prevention life table for the Black population in general.
Of the patients, only 5% had Medicare old age and survivor’s insurance, and 4% had Medicare disability insurance benefits or end-stage renal disease (ESRD), while 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid. The racial and regional distributions were similar across insurance types.
Adults covered by Medicare had a life expectancy of 39.8 years at 18 years of age, an improvement over life expectancy for those with Medicare for disabilities or ESRD and those dually insured by Medicare and Medicaid.
And the shorter life expectancy with Medicare and Medicaid was also observed among beneficiaries aged 65 or older, compared with those enrolled in Medicare old age and survivor’s insurance.
“Evidently, the life expectancy gap persists among patients with sickle cell disease, even though they are protected by public insurance,” the authors report.
The shorter life expectancy among those with dual eligibility for Medicare and Medicaid is consistent with that of the general population, the authors add.
“It is well-recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs,” they write. “They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligible.”
First author Boshen Jiao, PhD, MPH, of the Harvard T.H. Chan School of Public Health, in Boston, noted that socioeconomic factors, on a broader level, have an important impact on SCD.
“Even the first and most established FDA-approved medication for sickle cell disease, hydroxyurea, suffers from underutilization,” he told MDedge. “This issue can be associated with disparities along racial lines, particularly concerning access to treatments.”
Such factors can also play a role in the lack of a well-coordinated transition program needed to prevent gaps in care between youth and adulthood, as well as a lack of access to specialized care needed to potentially initiate new therapies.
Ultimately, the decline in survival seen from childhood to adulthood in SCD “emphasizes the pivotal role of health care during the critical transition phase from childhood to adulthood for individuals with sickle cell disease,” Dr. Jiao said. “Moreover, the prospect of a curative therapy that can intervene in the early stages of life, prior to adulthood, stands as a desirable goal.”
The authors urge that “future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.” They had no disclosures to report.
A version of this article first appeared on Medscape.com.
“To the best of our knowledge, this is the first United States nationwide investigation into lifetime survival for individuals with sickle cell disease covered by Medicare and Medicaid and disparities in survival by public insurance type, using comprehensive claims data collected from all 50 states,” the authors report in the study, published in Blood Advances.
“Our study underscores the persistent life expectancy shortfall for patients with sickle cell disease, the burden of premature mortality during adulthood, and survival disparities by insurance status,” they write.
SCD, which disproportionately affects people of African descent, significantly increases the risk of various acute and chronic life-threatening complications, including acute pain crisis, stroke, acute chest syndrome, chronic pain, symptomatic anemia, infections, and organ damage.
In recent years, advances ranging from newborn screening to prophylactic antibiotics have significantly improved the survival rates of children with SCD in the United States. However, premature mortality in adulthood has remained well above that of the general public.
A recent study of a U.S. birth cohort predicted life expectancy with SCD to be approximately 2 decades shorter than in those without the disease. However, those projections were based on a simulation model, and other life expectancy estimates have had sample sizes with state or regional limitations.
With no population-level individual data–based periodic life tables existing for individuals with SCD, the authors turned to nationwide data on Medicare and Medicaid beneficiaries to get a better idea of survival predictions.
For the study, they identified 94,616 individuals diagnosed with SCD who had not undergone transplant and were receiving common care, based on nationwide Medicare and Medicaid claim data from 2008 to 2016 on beneficiaries in all 50 states.
Of the patients, 74% were Black and 53% resided in the South at the index date. The patients had a mean entry age overall of 26.6 years across insurance types.
The results showed projected life expectancy at birth with SCD to be 52.6 years, with the life expectancy at birth for females significantly longer, compared with that of males (55.0 versus 49.3 years). For the general population, U.S. life expectancy estimates have been reported to be about 76 years.
Specifically, life expectancy in the cohort into adulthood was 35.4 years at 18 years of age; 24.1 years at 35 years of age; 19.6 years at 45 years old; 13.2 years at 65 years of age; and 5.4 years at 85 years old.
Likewise, survival probability rates were high during childhood, with survival probability at 18 years being 0.98, declining to 0.804 at age 30; 0.628 at age 45; 0.267 at age 65 and 0.70 at age 85.
Black individuals had a significantly shorter life expectancy at birth than non-Black individuals (52.2 vs. 55.1 years), with the survival expectancy for Black individuals significantly shorter than the 75-year life expectancy estimated by the 2016 U.S. Centers for Disease Control and Prevention life table for the Black population in general.
Of the patients, only 5% had Medicare old age and survivor’s insurance, and 4% had Medicare disability insurance benefits or end-stage renal disease (ESRD), while 48% had Medicaid, and 43% were dually eligible for Medicare and Medicaid. The racial and regional distributions were similar across insurance types.
Adults covered by Medicare had a life expectancy of 39.8 years at 18 years of age, an improvement over life expectancy for those with Medicare for disabilities or ESRD and those dually insured by Medicare and Medicaid.
And the shorter life expectancy with Medicare and Medicaid was also observed among beneficiaries aged 65 or older, compared with those enrolled in Medicare old age and survivor’s insurance.
“Evidently, the life expectancy gap persists among patients with sickle cell disease, even though they are protected by public insurance,” the authors report.
The shorter life expectancy among those with dual eligibility for Medicare and Medicaid is consistent with that of the general population, the authors add.
“It is well-recognized that the dual-eligible individuals are a vulnerable population with some of the most complex and expensive health care needs,” they write. “They are more likely to have fewer socioeconomic resources, more chronic conditions, and poorer survival outcomes than single eligible.”
First author Boshen Jiao, PhD, MPH, of the Harvard T.H. Chan School of Public Health, in Boston, noted that socioeconomic factors, on a broader level, have an important impact on SCD.
“Even the first and most established FDA-approved medication for sickle cell disease, hydroxyurea, suffers from underutilization,” he told MDedge. “This issue can be associated with disparities along racial lines, particularly concerning access to treatments.”
Such factors can also play a role in the lack of a well-coordinated transition program needed to prevent gaps in care between youth and adulthood, as well as a lack of access to specialized care needed to potentially initiate new therapies.
Ultimately, the decline in survival seen from childhood to adulthood in SCD “emphasizes the pivotal role of health care during the critical transition phase from childhood to adulthood for individuals with sickle cell disease,” Dr. Jiao said. “Moreover, the prospect of a curative therapy that can intervene in the early stages of life, prior to adulthood, stands as a desirable goal.”
The authors urge that “future studies should uncover factors influencing survival outcomes and explore policy options to address the unmet needs of the disabled or dually eligible population with SCD.” They had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM BLOOD ADVANCES
COVID may increase risk of high blood pressure
High blood pressure already impacts about half of U.S. adults, and the study researchers expressed concern about the sheer number of people who have newly developed the condition.
Among people in the study who had COVID but didn’t have a history of high blood pressure:
- One in five who had been hospitalized with COVID developed high blood pressure within 6 months.
- One in 10 who had COVID but were not hospitalized developed high blood pressure within 6 months.
The study appeared in Hypertension, a journal published by the American Heart Association. The researchers analyzed data for more than 45,000 people who had COVID from March 2020 to August 2022. The people did not have a history of high blood pressure. All of them were treated at the Montefiore Health System in New York, and had returned to the hospital system for any medical reason within an average of 6 months.
In an analysis to evaluate the impact of COVID, the researchers compared the likelihood of new high blood pressure in people who had the flu to the people who had COVID. The hospitalized COVID patients were more than twice as likely to get high blood pressure, compared with hospitalized flu patients. People who had COVID but weren’t hospitalized were 1.5 times more likely to get high blood pressure, compared with nonhospitalized flu patients.
People at greatest risk were age 40 or older or men, or had conditions such as chronic obstructive pulmonary disease (COPD), coronary artery disease, and chronic kidney disease.
The authors noted that the people in the study mostly lived in a low socioeconomic area, which can be a risk factor for high blood pressure. Aspects of the pandemic other than the virus itself could have impacted high blood pressure risk, too, like isolation, low activity levels, poor diet, and psychological stress. The researchers said further study is needed to overcome limitations of their research, in particular that it only included people who interacted with the health care system, and that they didn’t know if some people already had high blood pressure that was just undiagnosed.
“Given the sheer number of people affected by COVID-19, compared to influenza, these statistics are alarming and suggest that many more patients will likely develop high blood pressure in the future, which may present a major public health burden,” researcher Tim Q. Duong, PhD, professor of radiology at Albert Einstein College of Medicine and Montefiore Health System, New York, said in a statement. “These findings should heighten awareness to screen at-risk patients for hypertension after COVID-19 illness to enable earlier identification and treatment for hypertension-related complications, such as cardiovascular and kidney disease.”
A version of this article appeared on WebMD.com.
High blood pressure already impacts about half of U.S. adults, and the study researchers expressed concern about the sheer number of people who have newly developed the condition.
Among people in the study who had COVID but didn’t have a history of high blood pressure:
- One in five who had been hospitalized with COVID developed high blood pressure within 6 months.
- One in 10 who had COVID but were not hospitalized developed high blood pressure within 6 months.
The study appeared in Hypertension, a journal published by the American Heart Association. The researchers analyzed data for more than 45,000 people who had COVID from March 2020 to August 2022. The people did not have a history of high blood pressure. All of them were treated at the Montefiore Health System in New York, and had returned to the hospital system for any medical reason within an average of 6 months.
In an analysis to evaluate the impact of COVID, the researchers compared the likelihood of new high blood pressure in people who had the flu to the people who had COVID. The hospitalized COVID patients were more than twice as likely to get high blood pressure, compared with hospitalized flu patients. People who had COVID but weren’t hospitalized were 1.5 times more likely to get high blood pressure, compared with nonhospitalized flu patients.
People at greatest risk were age 40 or older or men, or had conditions such as chronic obstructive pulmonary disease (COPD), coronary artery disease, and chronic kidney disease.
The authors noted that the people in the study mostly lived in a low socioeconomic area, which can be a risk factor for high blood pressure. Aspects of the pandemic other than the virus itself could have impacted high blood pressure risk, too, like isolation, low activity levels, poor diet, and psychological stress. The researchers said further study is needed to overcome limitations of their research, in particular that it only included people who interacted with the health care system, and that they didn’t know if some people already had high blood pressure that was just undiagnosed.
“Given the sheer number of people affected by COVID-19, compared to influenza, these statistics are alarming and suggest that many more patients will likely develop high blood pressure in the future, which may present a major public health burden,” researcher Tim Q. Duong, PhD, professor of radiology at Albert Einstein College of Medicine and Montefiore Health System, New York, said in a statement. “These findings should heighten awareness to screen at-risk patients for hypertension after COVID-19 illness to enable earlier identification and treatment for hypertension-related complications, such as cardiovascular and kidney disease.”
A version of this article appeared on WebMD.com.
High blood pressure already impacts about half of U.S. adults, and the study researchers expressed concern about the sheer number of people who have newly developed the condition.
Among people in the study who had COVID but didn’t have a history of high blood pressure:
- One in five who had been hospitalized with COVID developed high blood pressure within 6 months.
- One in 10 who had COVID but were not hospitalized developed high blood pressure within 6 months.
The study appeared in Hypertension, a journal published by the American Heart Association. The researchers analyzed data for more than 45,000 people who had COVID from March 2020 to August 2022. The people did not have a history of high blood pressure. All of them were treated at the Montefiore Health System in New York, and had returned to the hospital system for any medical reason within an average of 6 months.
In an analysis to evaluate the impact of COVID, the researchers compared the likelihood of new high blood pressure in people who had the flu to the people who had COVID. The hospitalized COVID patients were more than twice as likely to get high blood pressure, compared with hospitalized flu patients. People who had COVID but weren’t hospitalized were 1.5 times more likely to get high blood pressure, compared with nonhospitalized flu patients.
People at greatest risk were age 40 or older or men, or had conditions such as chronic obstructive pulmonary disease (COPD), coronary artery disease, and chronic kidney disease.
The authors noted that the people in the study mostly lived in a low socioeconomic area, which can be a risk factor for high blood pressure. Aspects of the pandemic other than the virus itself could have impacted high blood pressure risk, too, like isolation, low activity levels, poor diet, and psychological stress. The researchers said further study is needed to overcome limitations of their research, in particular that it only included people who interacted with the health care system, and that they didn’t know if some people already had high blood pressure that was just undiagnosed.
“Given the sheer number of people affected by COVID-19, compared to influenza, these statistics are alarming and suggest that many more patients will likely develop high blood pressure in the future, which may present a major public health burden,” researcher Tim Q. Duong, PhD, professor of radiology at Albert Einstein College of Medicine and Montefiore Health System, New York, said in a statement. “These findings should heighten awareness to screen at-risk patients for hypertension after COVID-19 illness to enable earlier identification and treatment for hypertension-related complications, such as cardiovascular and kidney disease.”
A version of this article appeared on WebMD.com.
FROM HYPERTENSION
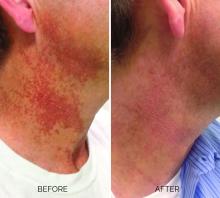
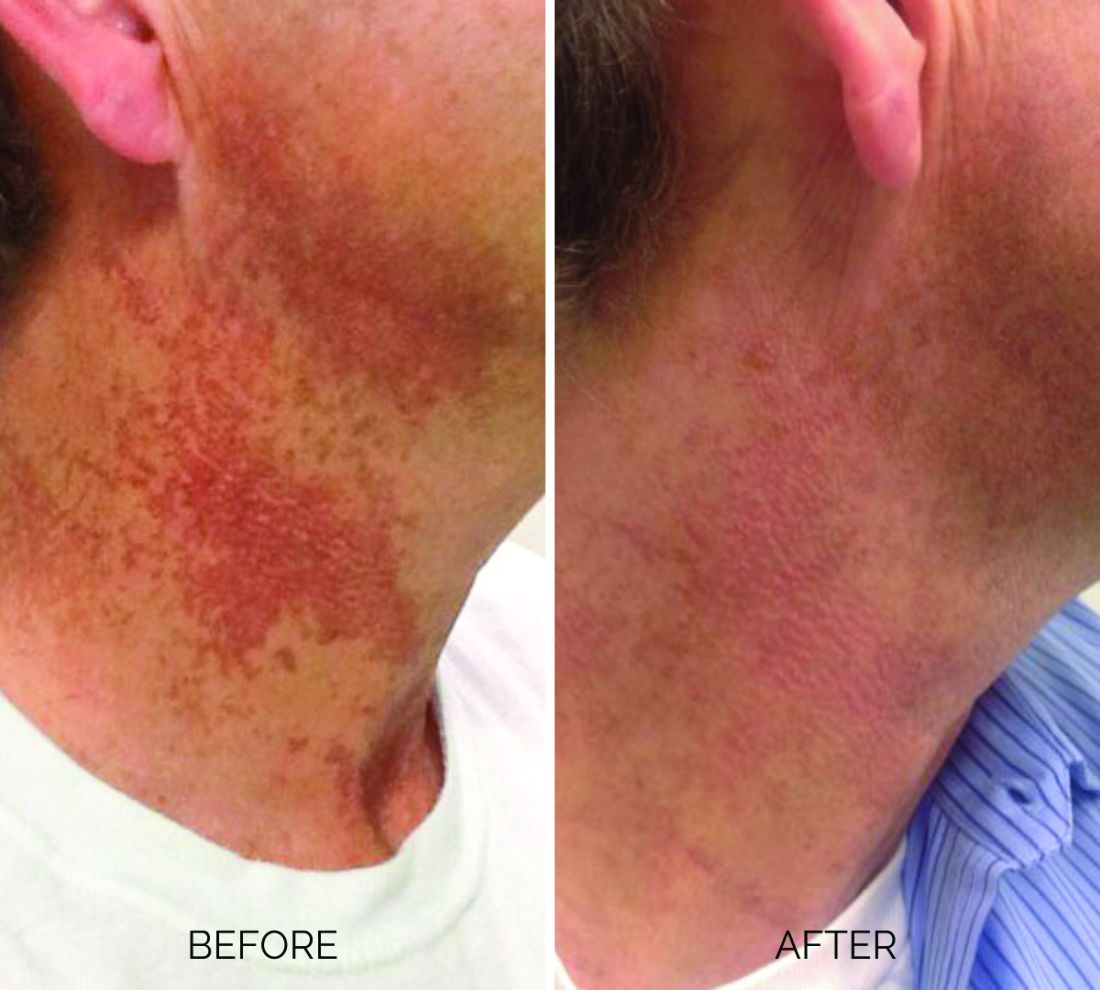
Treating poikiloderma
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
Elevated serum levels of thymic stromal lymphopoietin in patients with atopic dermatitis
Key clinical point: Serum levels of thymic stromal lymphopoietin (TSLP) are significantly elevated in patients with atopic dermatitis (AD), with the levels being significantly higher in adults than in children and increasing significantly corresponding to the severity of AD.
Major finding: Patients with AD vs control individuals had significantly higher serum levels of TSLP (standardized mean difference [SMD] 2.21; P < .001). Serum TSLP levels were significantly higher in adults vs children with AD (P = .02) and had a significant positive association with AD severity (mild: SMD 1.15; P = .025; moderate: SMD 2.48; P = .024; severe: SMD 8.28; P = .000002).
Study details: The data come from a meta-analysis of 14 studies involving 1032 patients with AD and 416 control individuals without AD.
Disclosures: This study was funded by the Universidad Nacional Autónoma de México. The authors declared no conflicts of interest.
Source: García-Reyes MM et al. Serum thymic stromal lymphopoietin (TSLP) levels in atopic dermatitis patients: A systematic review and meta-analysis. Clin Exp Med. 2023 (Jul 29). doi: 10.1007/s10238-023-01147-5
Key clinical point: Serum levels of thymic stromal lymphopoietin (TSLP) are significantly elevated in patients with atopic dermatitis (AD), with the levels being significantly higher in adults than in children and increasing significantly corresponding to the severity of AD.
Major finding: Patients with AD vs control individuals had significantly higher serum levels of TSLP (standardized mean difference [SMD] 2.21; P < .001). Serum TSLP levels were significantly higher in adults vs children with AD (P = .02) and had a significant positive association with AD severity (mild: SMD 1.15; P = .025; moderate: SMD 2.48; P = .024; severe: SMD 8.28; P = .000002).
Study details: The data come from a meta-analysis of 14 studies involving 1032 patients with AD and 416 control individuals without AD.
Disclosures: This study was funded by the Universidad Nacional Autónoma de México. The authors declared no conflicts of interest.
Source: García-Reyes MM et al. Serum thymic stromal lymphopoietin (TSLP) levels in atopic dermatitis patients: A systematic review and meta-analysis. Clin Exp Med. 2023 (Jul 29). doi: 10.1007/s10238-023-01147-5
Key clinical point: Serum levels of thymic stromal lymphopoietin (TSLP) are significantly elevated in patients with atopic dermatitis (AD), with the levels being significantly higher in adults than in children and increasing significantly corresponding to the severity of AD.
Major finding: Patients with AD vs control individuals had significantly higher serum levels of TSLP (standardized mean difference [SMD] 2.21; P < .001). Serum TSLP levels were significantly higher in adults vs children with AD (P = .02) and had a significant positive association with AD severity (mild: SMD 1.15; P = .025; moderate: SMD 2.48; P = .024; severe: SMD 8.28; P = .000002).
Study details: The data come from a meta-analysis of 14 studies involving 1032 patients with AD and 416 control individuals without AD.
Disclosures: This study was funded by the Universidad Nacional Autónoma de México. The authors declared no conflicts of interest.
Source: García-Reyes MM et al. Serum thymic stromal lymphopoietin (TSLP) levels in atopic dermatitis patients: A systematic review and meta-analysis. Clin Exp Med. 2023 (Jul 29). doi: 10.1007/s10238-023-01147-5
Long-term dupilumab therapy safe and effective in pediatric atopic dermatitis, shows meta-analysis
Key clinical point: Dupilumab demonstrates favorable efficacy and manageable safety in pediatric patients with atopic dermatitis (AD), with greater improvements in clinical signs observed with an increase in the treatment duration.
Major finding: The overall pooled Eczema Area and Severity Index (EASI) 75 response rate was 57.4% (95% CI 48.1%-66.2%), whereas an Investigator's Global Assessment score of 0 or 1 was achieved by 35.2% (29.3%-41.5%) of patients. EASI 75 response rates were 26.8% (95% CI 20.7%-33.9%) and 84.9% (95% CI 79.6%-89.0%) after 2-8 weeks and 32-52 weeks of treatment, respectively. Treatment-emergent adverse events were mostly mild-to-moderate in severity.
Study details: This meta-analysis included 18 studies (7 clinical and 11 observational) involving 1275 children and adolescents with AD.
Disclosures: This study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Xu Y et al. Efficacy and safety profile of dupilumab for the treatment of atopic dermatitis in children and adolescents: A systematic review and meta-analysis. Pediatr Dermatol. 2023 (Aug 2). doi: 10.1111/pde.15398
Key clinical point: Dupilumab demonstrates favorable efficacy and manageable safety in pediatric patients with atopic dermatitis (AD), with greater improvements in clinical signs observed with an increase in the treatment duration.
Major finding: The overall pooled Eczema Area and Severity Index (EASI) 75 response rate was 57.4% (95% CI 48.1%-66.2%), whereas an Investigator's Global Assessment score of 0 or 1 was achieved by 35.2% (29.3%-41.5%) of patients. EASI 75 response rates were 26.8% (95% CI 20.7%-33.9%) and 84.9% (95% CI 79.6%-89.0%) after 2-8 weeks and 32-52 weeks of treatment, respectively. Treatment-emergent adverse events were mostly mild-to-moderate in severity.
Study details: This meta-analysis included 18 studies (7 clinical and 11 observational) involving 1275 children and adolescents with AD.
Disclosures: This study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Xu Y et al. Efficacy and safety profile of dupilumab for the treatment of atopic dermatitis in children and adolescents: A systematic review and meta-analysis. Pediatr Dermatol. 2023 (Aug 2). doi: 10.1111/pde.15398
Key clinical point: Dupilumab demonstrates favorable efficacy and manageable safety in pediatric patients with atopic dermatitis (AD), with greater improvements in clinical signs observed with an increase in the treatment duration.
Major finding: The overall pooled Eczema Area and Severity Index (EASI) 75 response rate was 57.4% (95% CI 48.1%-66.2%), whereas an Investigator's Global Assessment score of 0 or 1 was achieved by 35.2% (29.3%-41.5%) of patients. EASI 75 response rates were 26.8% (95% CI 20.7%-33.9%) and 84.9% (95% CI 79.6%-89.0%) after 2-8 weeks and 32-52 weeks of treatment, respectively. Treatment-emergent adverse events were mostly mild-to-moderate in severity.
Study details: This meta-analysis included 18 studies (7 clinical and 11 observational) involving 1275 children and adolescents with AD.
Disclosures: This study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Xu Y et al. Efficacy and safety profile of dupilumab for the treatment of atopic dermatitis in children and adolescents: A systematic review and meta-analysis. Pediatr Dermatol. 2023 (Aug 2). doi: 10.1111/pde.15398
Dupilumab maintains efficacy despite dose reduction in persistently-controlled atopic dermatitis
Key clinical point: Reduction in dupilumab dose to every 3 or 4 weeks does not decrease its efficacy in patients with persistently-controlled atopic dermatitis (AD), with clinical outcome measures remaining stable over time.
Major finding: After 144 weeks, similar to patients receiving 300 mg dupilumab every 2 weeks, those receiving 300 mg dupilumab every 3 and 4 weeks had significantly decreased mean Eczema Area and Severity Index, Severity Scoring of Atopic Dermatitis, Pruritus Numerical Rating Scale, and Sleep Numerical Rating Scale scores (all P < .05).
Study details: This multicenter retrospective observational study included 54 patients age ≥ 16 years with controlled moderate-to-severe AD treated with dupilumab for ≥ 1 year who were assigned to receive 300 mg dupilumab every 2 weeks (n = 27), 3 weeks (n = 17), or 4 weeks (n = 10).
Disclosures: This study received no specific funding from any sources. The authors declared no conflicts of interest.
Source: Sánchez-García V et al. Evaluation of dupilumab dosing regimen in patients with persistently controlled atopic dermatitis. J Eur Acad Dermatol Venereol. 2023 (Jul 17). doi: 10.1111/jdv.19348
Key clinical point: Reduction in dupilumab dose to every 3 or 4 weeks does not decrease its efficacy in patients with persistently-controlled atopic dermatitis (AD), with clinical outcome measures remaining stable over time.
Major finding: After 144 weeks, similar to patients receiving 300 mg dupilumab every 2 weeks, those receiving 300 mg dupilumab every 3 and 4 weeks had significantly decreased mean Eczema Area and Severity Index, Severity Scoring of Atopic Dermatitis, Pruritus Numerical Rating Scale, and Sleep Numerical Rating Scale scores (all P < .05).
Study details: This multicenter retrospective observational study included 54 patients age ≥ 16 years with controlled moderate-to-severe AD treated with dupilumab for ≥ 1 year who were assigned to receive 300 mg dupilumab every 2 weeks (n = 27), 3 weeks (n = 17), or 4 weeks (n = 10).
Disclosures: This study received no specific funding from any sources. The authors declared no conflicts of interest.
Source: Sánchez-García V et al. Evaluation of dupilumab dosing regimen in patients with persistently controlled atopic dermatitis. J Eur Acad Dermatol Venereol. 2023 (Jul 17). doi: 10.1111/jdv.19348
Key clinical point: Reduction in dupilumab dose to every 3 or 4 weeks does not decrease its efficacy in patients with persistently-controlled atopic dermatitis (AD), with clinical outcome measures remaining stable over time.
Major finding: After 144 weeks, similar to patients receiving 300 mg dupilumab every 2 weeks, those receiving 300 mg dupilumab every 3 and 4 weeks had significantly decreased mean Eczema Area and Severity Index, Severity Scoring of Atopic Dermatitis, Pruritus Numerical Rating Scale, and Sleep Numerical Rating Scale scores (all P < .05).
Study details: This multicenter retrospective observational study included 54 patients age ≥ 16 years with controlled moderate-to-severe AD treated with dupilumab for ≥ 1 year who were assigned to receive 300 mg dupilumab every 2 weeks (n = 27), 3 weeks (n = 17), or 4 weeks (n = 10).
Disclosures: This study received no specific funding from any sources. The authors declared no conflicts of interest.
Source: Sánchez-García V et al. Evaluation of dupilumab dosing regimen in patients with persistently controlled atopic dermatitis. J Eur Acad Dermatol Venereol. 2023 (Jul 17). doi: 10.1111/jdv.19348
Dupilumab + TCS confers rapid and sustained improvement in atopic dermatitis severity in children
Key clinical point: Dupilumab + low-potency topical corticosteroids (TCS) led to rapid and sustained improvement in disease severity in all anatomical regions (head and neck, trunk, upper extremities, and lower extremities) in children with moderate-to-severe atopic dermatitis (AD).
Major finding: The dupilumab + TCS vs placebo + TCS group had a significant improvement in least-squares mean Eczema Area and Severity Index scores in all 4 anatomical regions by week 2 (all P < .0001) that sustained throughout the 16-week treatment.
Study details: This post hoc analysis of the LIBERTY AD PRESCHOOL trial included 162 children (age, 6 months-5 years) with moderate-to-severe AD who were randomly assigned to receive dupilumab + low-potency TCS or placebo + low-potency TCS every 4 weeks.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Five authors declared being employees or stockholders of Sanofi/Regeneron. The rest declared serving as consultants, investigators, speakers, or advisory board members for and receiving grants or personal fees from various sources, including Sanofi and Regeneron.
Source: Siegfried EC et al. Dupilumab treatment leads to rapid and consistent improvement of atopic dermatitis in all anatomical regions in patients aged 6 months to 5 years. Dermatol Ther (Heidelb). 2023 (Jul 22). doi: 10.1007/s13555-023-00960-w
Key clinical point: Dupilumab + low-potency topical corticosteroids (TCS) led to rapid and sustained improvement in disease severity in all anatomical regions (head and neck, trunk, upper extremities, and lower extremities) in children with moderate-to-severe atopic dermatitis (AD).
Major finding: The dupilumab + TCS vs placebo + TCS group had a significant improvement in least-squares mean Eczema Area and Severity Index scores in all 4 anatomical regions by week 2 (all P < .0001) that sustained throughout the 16-week treatment.
Study details: This post hoc analysis of the LIBERTY AD PRESCHOOL trial included 162 children (age, 6 months-5 years) with moderate-to-severe AD who were randomly assigned to receive dupilumab + low-potency TCS or placebo + low-potency TCS every 4 weeks.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Five authors declared being employees or stockholders of Sanofi/Regeneron. The rest declared serving as consultants, investigators, speakers, or advisory board members for and receiving grants or personal fees from various sources, including Sanofi and Regeneron.
Source: Siegfried EC et al. Dupilumab treatment leads to rapid and consistent improvement of atopic dermatitis in all anatomical regions in patients aged 6 months to 5 years. Dermatol Ther (Heidelb). 2023 (Jul 22). doi: 10.1007/s13555-023-00960-w
Key clinical point: Dupilumab + low-potency topical corticosteroids (TCS) led to rapid and sustained improvement in disease severity in all anatomical regions (head and neck, trunk, upper extremities, and lower extremities) in children with moderate-to-severe atopic dermatitis (AD).
Major finding: The dupilumab + TCS vs placebo + TCS group had a significant improvement in least-squares mean Eczema Area and Severity Index scores in all 4 anatomical regions by week 2 (all P < .0001) that sustained throughout the 16-week treatment.
Study details: This post hoc analysis of the LIBERTY AD PRESCHOOL trial included 162 children (age, 6 months-5 years) with moderate-to-severe AD who were randomly assigned to receive dupilumab + low-potency TCS or placebo + low-potency TCS every 4 weeks.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Five authors declared being employees or stockholders of Sanofi/Regeneron. The rest declared serving as consultants, investigators, speakers, or advisory board members for and receiving grants or personal fees from various sources, including Sanofi and Regeneron.
Source: Siegfried EC et al. Dupilumab treatment leads to rapid and consistent improvement of atopic dermatitis in all anatomical regions in patients aged 6 months to 5 years. Dermatol Ther (Heidelb). 2023 (Jul 22). doi: 10.1007/s13555-023-00960-w