User login
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.
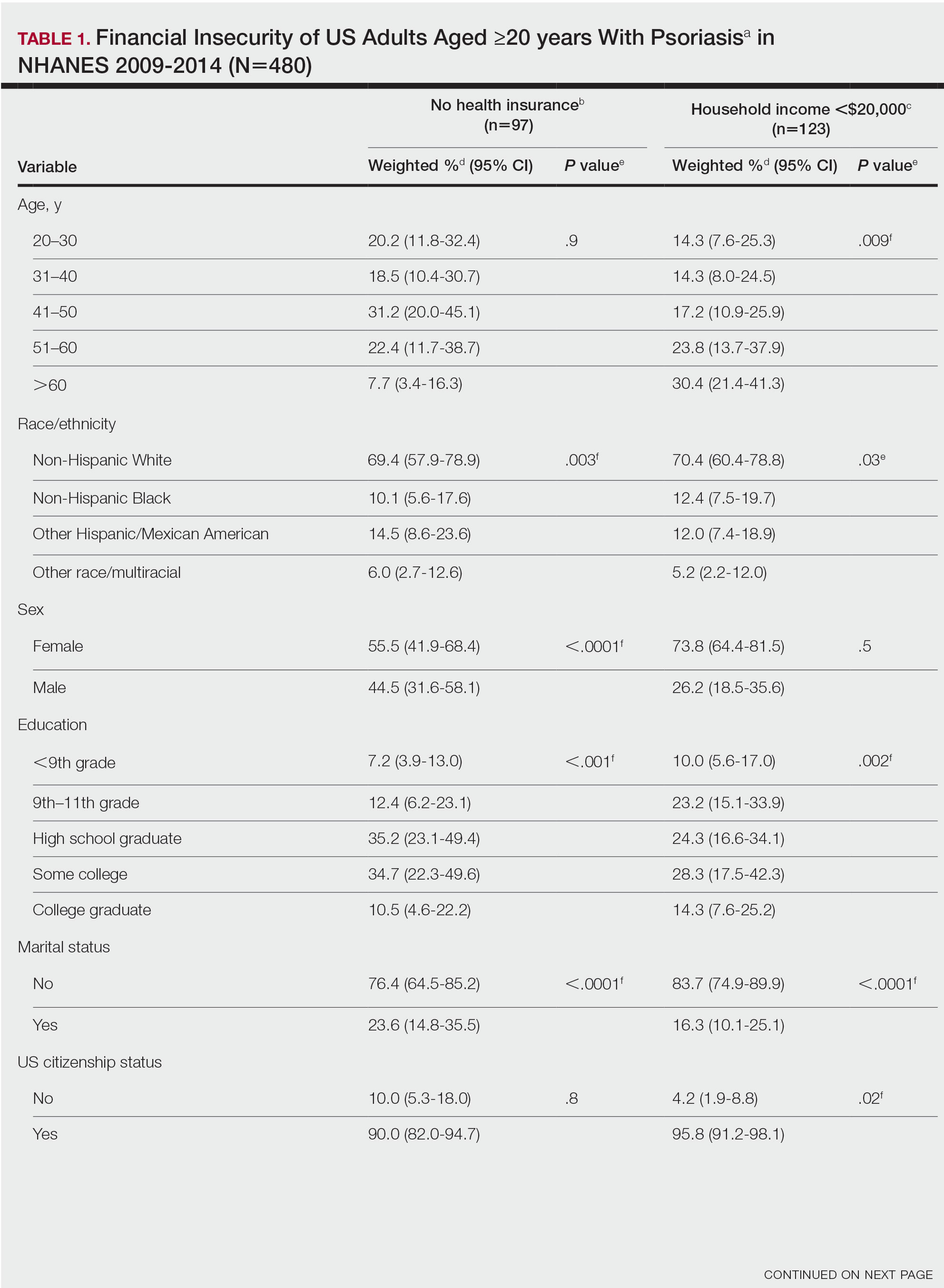

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
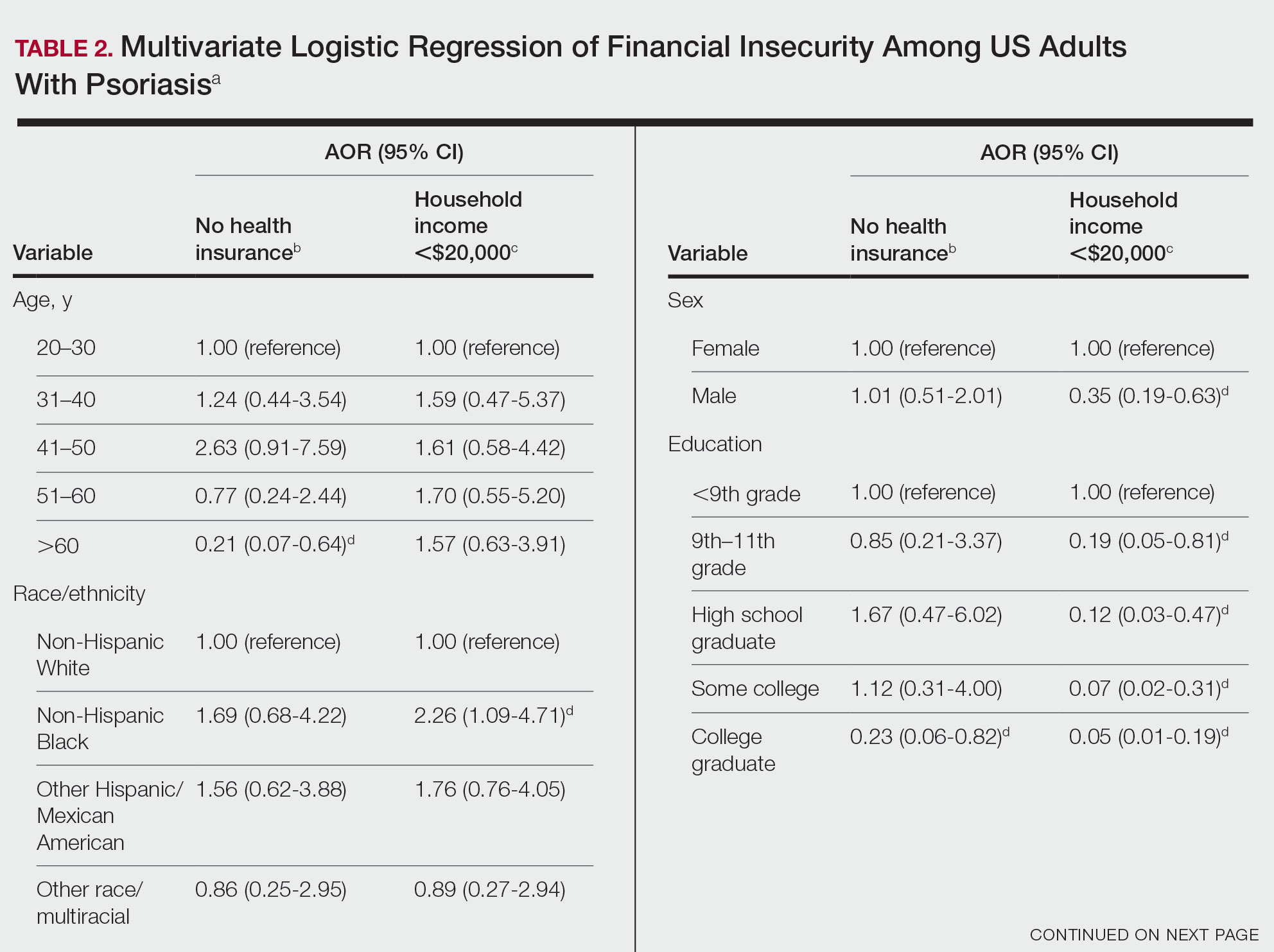
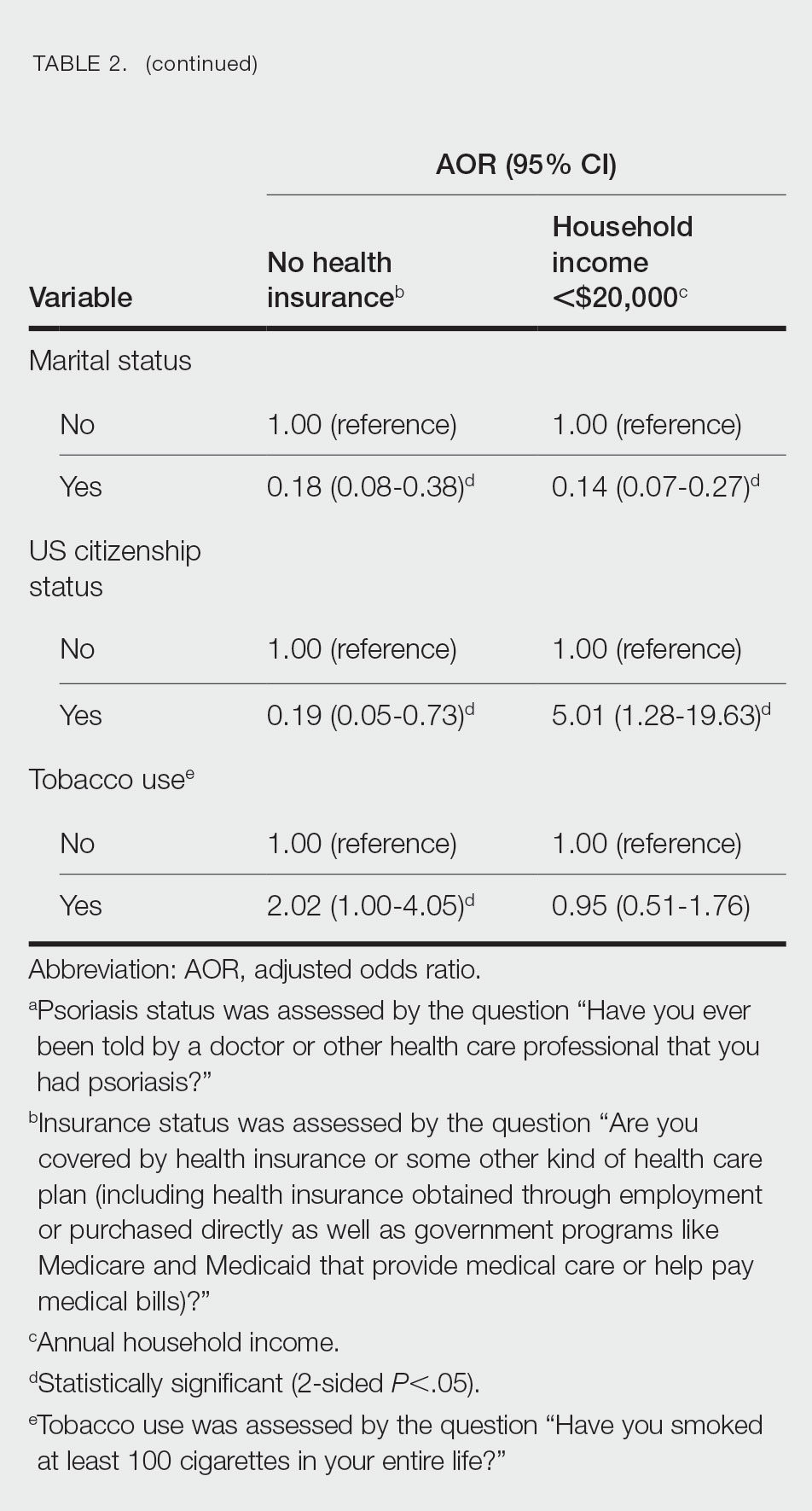
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Risky drinking common in cancer survivors
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Consider housing insecurity, other issues when managing challenging skin diseases in children, expert says
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
AT SPD 2023
Triglyceride puzzle: Do TG metabolites better predict risk?
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.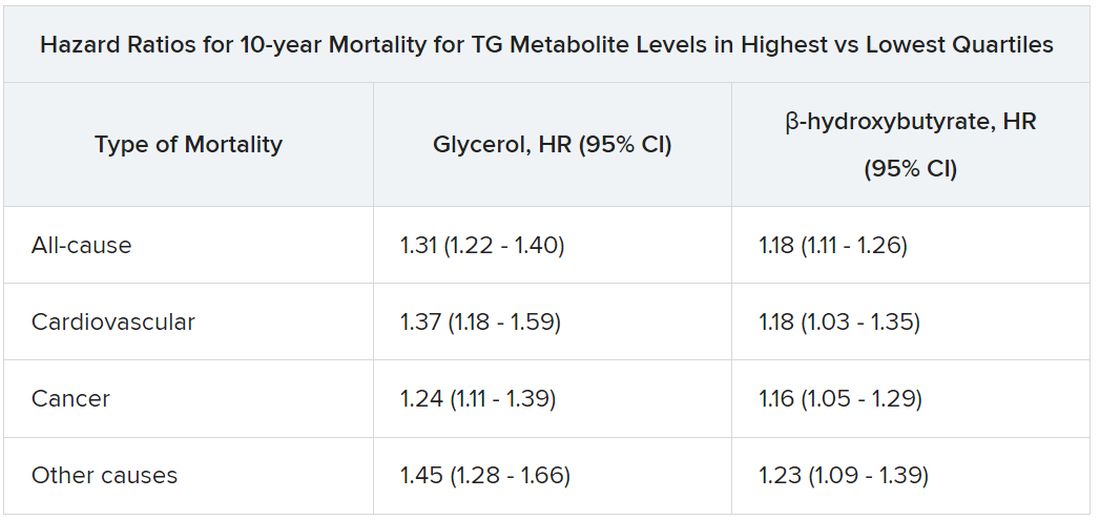
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
How should muscle mass be measured in heart failure?
, research suggests.
In a single-center, retrospective study that included more than 800 patients, high MUAC (hazard ratio for combined events, 0.590) and high AMC (HR for combined events, 0.529) were associated with significantly better prognoses than low MUAC and low AMC.
The findings were “surprising,” Kentaro Kamiya, PT, PhD, and Shota Uchida, PT, PhD, of Kitasato University School of Allied Health Sciences in Kanagawa, Japan, said in an interview.
“These findings challenge the current recommendations found in sarcopenia guidelines,” they noted. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia recommend SMI, as measured by bioelectrical impedance analysis (BIA), and CC as methods for screening skeletal muscle mass.
The study was published online in the Canadian Journal of Cardiology.
Arm measures prognostic
Sarcopenia, which is marked by a loss of skeletal muscle mass and strength, is associated with risks of adverse outcomes. Patients with heart failure have a high rate of sarcopenia, but assessing skeletal muscle mass in these patients is difficult because of the fluid retention they often have.
The investigators examined the association between skeletal muscle mass metrics, measured using bioelectrical impedance analysis, and anthropometric measures and prognosis in patients with heart failure.
SMI was calculated using the BIA by dividing appendicular skeletal muscle mass by height squared. MUAC and CC were measured to the nearest 1 mm using a plastic tape measure. AMC was calculated as follows: MUAC (cm) − (0.314 x triceps skinfold [TSF]). The TSF was measured to the nearest 2 mm with a skinfold caliper. The measuring spot for TSF was the same measuring spot for MUAC. MUAC, CC, and TSF were measured by trained physiotherapists or nurses.
The investigators identified 1,930 consecutive patients with heart failure who underwent cardiac rehabilitation during their hospitalization. They excluded from their analysis 1,013 patients who did not undergo a skeletal mass metrics evaluation and 48 who could not be followed up.
The analysis included 869 patients (median age, 73 years; 62% men). Patients were separated into three groups on the basis of the sex-specific tertiles of skeletal muscle mass. The study endpoint was all-cause death or readmission due to heart failure, and the median follow-up period was 1.24 years.
After the investigators adjusted the data for age, sex, New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF), ischemic etiology, prior heart failure, diabetes, chronic obstructive pulmonary disease, log-transformed B-type natriuretic peptide (BNP), and estimated glomerular filtration rate (eGFR), the high MUAC and high AMC groups were associated with significantly better prognoses than their respective low groups. By contrast, high SMI and high CC were not associated with better prognoses.
Subgroup analyses showed no interactions between MUAC and age, sex, LVEF, BNP, eGFR, prior heart failure, beta-blocker use, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. However, diuretic agents significantly interacted with AMC (P = .03).
“These results support the use of MUAC and AMC to determine the risk stratification of sarcopenia and a poor prognosis in patients with heart failure and suggest that they may be useful in developing treatment strategies in patients with heart failure,” wrote the authors.
“When caring for patients with heart failure, it seems that the often overlooked and simple measure of arm circumference might carry significant prognostic value,” said Dr. Kamiya and Dr. Uchida. “So, as you cuff the arm for routine blood pressure measurement, it might be worthwhile to also pay attention to arm girth.”
Although the findings provide valuable insights, they should be approached with caution, Dr. Kamiya and Dr. Uchida added. “Before considering them practice-changing, further research is needed to validate these results in diverse patient cohorts.”
Prospective study needed
Commenting on the study for this news organization, Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation in New York, expressed concerns about the study methodology.
Methodologic weaknesses include the retrospective, observational nature of the study and the fact that incomplete data collection led to the exclusion of more than half of the potential participants, he said. In addition, “anthropometric measurements are prone to inter-rater error. It is not clear if the same or different researchers conducted these measurements.
“Furthermore, hospitalized patients may not be clinically stable when discharged,” said Dr. Whiteson. “It is recommended that biometrics for muscle mass and fluid retention be done when patients are at an optimized clinical state: that is, stabilized outpatients.”
For now, Dr. Whiteson concluded, the findings should be considered “interesting and suggestive of further study.” What’s needed is “a prospective study including all patients admitted with heart failure, but measurements done when the patient is stabilized as an outpatient.”
The study was partially supported by the Japan Society for the Promotion of Science KAKENHI. Dr. Kamiya, Dr. Uchida, and Dr. Whiteson reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, research suggests.
In a single-center, retrospective study that included more than 800 patients, high MUAC (hazard ratio for combined events, 0.590) and high AMC (HR for combined events, 0.529) were associated with significantly better prognoses than low MUAC and low AMC.
The findings were “surprising,” Kentaro Kamiya, PT, PhD, and Shota Uchida, PT, PhD, of Kitasato University School of Allied Health Sciences in Kanagawa, Japan, said in an interview.
“These findings challenge the current recommendations found in sarcopenia guidelines,” they noted. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia recommend SMI, as measured by bioelectrical impedance analysis (BIA), and CC as methods for screening skeletal muscle mass.
The study was published online in the Canadian Journal of Cardiology.
Arm measures prognostic
Sarcopenia, which is marked by a loss of skeletal muscle mass and strength, is associated with risks of adverse outcomes. Patients with heart failure have a high rate of sarcopenia, but assessing skeletal muscle mass in these patients is difficult because of the fluid retention they often have.
The investigators examined the association between skeletal muscle mass metrics, measured using bioelectrical impedance analysis, and anthropometric measures and prognosis in patients with heart failure.
SMI was calculated using the BIA by dividing appendicular skeletal muscle mass by height squared. MUAC and CC were measured to the nearest 1 mm using a plastic tape measure. AMC was calculated as follows: MUAC (cm) − (0.314 x triceps skinfold [TSF]). The TSF was measured to the nearest 2 mm with a skinfold caliper. The measuring spot for TSF was the same measuring spot for MUAC. MUAC, CC, and TSF were measured by trained physiotherapists or nurses.
The investigators identified 1,930 consecutive patients with heart failure who underwent cardiac rehabilitation during their hospitalization. They excluded from their analysis 1,013 patients who did not undergo a skeletal mass metrics evaluation and 48 who could not be followed up.
The analysis included 869 patients (median age, 73 years; 62% men). Patients were separated into three groups on the basis of the sex-specific tertiles of skeletal muscle mass. The study endpoint was all-cause death or readmission due to heart failure, and the median follow-up period was 1.24 years.
After the investigators adjusted the data for age, sex, New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF), ischemic etiology, prior heart failure, diabetes, chronic obstructive pulmonary disease, log-transformed B-type natriuretic peptide (BNP), and estimated glomerular filtration rate (eGFR), the high MUAC and high AMC groups were associated with significantly better prognoses than their respective low groups. By contrast, high SMI and high CC were not associated with better prognoses.
Subgroup analyses showed no interactions between MUAC and age, sex, LVEF, BNP, eGFR, prior heart failure, beta-blocker use, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. However, diuretic agents significantly interacted with AMC (P = .03).
“These results support the use of MUAC and AMC to determine the risk stratification of sarcopenia and a poor prognosis in patients with heart failure and suggest that they may be useful in developing treatment strategies in patients with heart failure,” wrote the authors.
“When caring for patients with heart failure, it seems that the often overlooked and simple measure of arm circumference might carry significant prognostic value,” said Dr. Kamiya and Dr. Uchida. “So, as you cuff the arm for routine blood pressure measurement, it might be worthwhile to also pay attention to arm girth.”
Although the findings provide valuable insights, they should be approached with caution, Dr. Kamiya and Dr. Uchida added. “Before considering them practice-changing, further research is needed to validate these results in diverse patient cohorts.”
Prospective study needed
Commenting on the study for this news organization, Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation in New York, expressed concerns about the study methodology.
Methodologic weaknesses include the retrospective, observational nature of the study and the fact that incomplete data collection led to the exclusion of more than half of the potential participants, he said. In addition, “anthropometric measurements are prone to inter-rater error. It is not clear if the same or different researchers conducted these measurements.
“Furthermore, hospitalized patients may not be clinically stable when discharged,” said Dr. Whiteson. “It is recommended that biometrics for muscle mass and fluid retention be done when patients are at an optimized clinical state: that is, stabilized outpatients.”
For now, Dr. Whiteson concluded, the findings should be considered “interesting and suggestive of further study.” What’s needed is “a prospective study including all patients admitted with heart failure, but measurements done when the patient is stabilized as an outpatient.”
The study was partially supported by the Japan Society for the Promotion of Science KAKENHI. Dr. Kamiya, Dr. Uchida, and Dr. Whiteson reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, research suggests.
In a single-center, retrospective study that included more than 800 patients, high MUAC (hazard ratio for combined events, 0.590) and high AMC (HR for combined events, 0.529) were associated with significantly better prognoses than low MUAC and low AMC.
The findings were “surprising,” Kentaro Kamiya, PT, PhD, and Shota Uchida, PT, PhD, of Kitasato University School of Allied Health Sciences in Kanagawa, Japan, said in an interview.
“These findings challenge the current recommendations found in sarcopenia guidelines,” they noted. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia recommend SMI, as measured by bioelectrical impedance analysis (BIA), and CC as methods for screening skeletal muscle mass.
The study was published online in the Canadian Journal of Cardiology.
Arm measures prognostic
Sarcopenia, which is marked by a loss of skeletal muscle mass and strength, is associated with risks of adverse outcomes. Patients with heart failure have a high rate of sarcopenia, but assessing skeletal muscle mass in these patients is difficult because of the fluid retention they often have.
The investigators examined the association between skeletal muscle mass metrics, measured using bioelectrical impedance analysis, and anthropometric measures and prognosis in patients with heart failure.
SMI was calculated using the BIA by dividing appendicular skeletal muscle mass by height squared. MUAC and CC were measured to the nearest 1 mm using a plastic tape measure. AMC was calculated as follows: MUAC (cm) − (0.314 x triceps skinfold [TSF]). The TSF was measured to the nearest 2 mm with a skinfold caliper. The measuring spot for TSF was the same measuring spot for MUAC. MUAC, CC, and TSF were measured by trained physiotherapists or nurses.
The investigators identified 1,930 consecutive patients with heart failure who underwent cardiac rehabilitation during their hospitalization. They excluded from their analysis 1,013 patients who did not undergo a skeletal mass metrics evaluation and 48 who could not be followed up.
The analysis included 869 patients (median age, 73 years; 62% men). Patients were separated into three groups on the basis of the sex-specific tertiles of skeletal muscle mass. The study endpoint was all-cause death or readmission due to heart failure, and the median follow-up period was 1.24 years.
After the investigators adjusted the data for age, sex, New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF), ischemic etiology, prior heart failure, diabetes, chronic obstructive pulmonary disease, log-transformed B-type natriuretic peptide (BNP), and estimated glomerular filtration rate (eGFR), the high MUAC and high AMC groups were associated with significantly better prognoses than their respective low groups. By contrast, high SMI and high CC were not associated with better prognoses.
Subgroup analyses showed no interactions between MUAC and age, sex, LVEF, BNP, eGFR, prior heart failure, beta-blocker use, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. However, diuretic agents significantly interacted with AMC (P = .03).
“These results support the use of MUAC and AMC to determine the risk stratification of sarcopenia and a poor prognosis in patients with heart failure and suggest that they may be useful in developing treatment strategies in patients with heart failure,” wrote the authors.
“When caring for patients with heart failure, it seems that the often overlooked and simple measure of arm circumference might carry significant prognostic value,” said Dr. Kamiya and Dr. Uchida. “So, as you cuff the arm for routine blood pressure measurement, it might be worthwhile to also pay attention to arm girth.”
Although the findings provide valuable insights, they should be approached with caution, Dr. Kamiya and Dr. Uchida added. “Before considering them practice-changing, further research is needed to validate these results in diverse patient cohorts.”
Prospective study needed
Commenting on the study for this news organization, Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation in New York, expressed concerns about the study methodology.
Methodologic weaknesses include the retrospective, observational nature of the study and the fact that incomplete data collection led to the exclusion of more than half of the potential participants, he said. In addition, “anthropometric measurements are prone to inter-rater error. It is not clear if the same or different researchers conducted these measurements.
“Furthermore, hospitalized patients may not be clinically stable when discharged,” said Dr. Whiteson. “It is recommended that biometrics for muscle mass and fluid retention be done when patients are at an optimized clinical state: that is, stabilized outpatients.”
For now, Dr. Whiteson concluded, the findings should be considered “interesting and suggestive of further study.” What’s needed is “a prospective study including all patients admitted with heart failure, but measurements done when the patient is stabilized as an outpatient.”
The study was partially supported by the Japan Society for the Promotion of Science KAKENHI. Dr. Kamiya, Dr. Uchida, and Dr. Whiteson reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
New RSV shot is a monoclonal antibody, not a vaccine
For the first time in the fall of 2023, families will be offered season-long protection for infants and some children against respiratory syncytial virus (RSV).
The Food and Drug Administration in July approved a prevention called nirsevimab (Beyfortus, AstraZeneca/Sanofi) and it is expected to be widely rolled out in the coming weeks as the RSV season begins.
And monoclonal antibodies are often used for treatment rather than prevention.
Adding to potential confusion is the fact the Centers for Disease Control and Prevention has included nirsevimab in the Vaccines for Children program, which covers the costs for uninsured kids and makes it more accessible.
Nirsevimab is approved for infants (up to 8 months old) born during or entering their first RSV season, and in children up to 2 years of age who are still vulnerable to severe RSV through their second season.
It’s recommended that all infants get one injection in their first 8 months for prevention instead of the previous monthly shots used to help prevent kids at high risk from getting severe RSV.
If monoclonal antibodies can be used for preventing disease in infants, could they become a viable vaccine alternative for adults?
Specialists say no.
That’s partly because of the difference in body size. Although an injection is an option for a newborn, pediatricians suggest, it would take far too much of the treatment to work as a shot for adults.
Ruth Karron, MD, an expert in pediatric infectious diseases at Johns Hopkins Medicine, Baltimore, said that, while vaccines come in small amounts and activate immune cells, monoclonal antibodies are more like a drug, with the dose based on weight.
“You’d have to give it intravenously,” for larger doses, she explained, which has never been studied before and would also be very expensive. “It really couldn’t be an option for adults.”
What’s the difference between vaccines and antibodies?
Monoclonal antibodies are proteins made in a lab to mimic the immune system’s ability to fight pathogens such as viruses.
Dr. Karron explained that a wide variety of monoclonal antibodies have long been used to treat diseases such as cancers and autoimmune disease. In recent years, the antibodies have been used to treat COVID.
Monoclonal antibodies have also been used to treat RSV in children, but the effects don’t last long – they confer passive immunity and “when it’s gone, it’s gone,” Dr. Karron said.
That means kids at high risk for severe RSV have had to get monthly injections.
But with nirsevimab, the mutated antibodies stay in circulation longer so they can last 5 or 6 months, enough to cover the RSV season, Dr. Karron explained. “It’s highly, highly effective.”
Vaccines train the body
“The idea with vaccines is that you engage the individual’s immune system. You teach it to make antibodies,” Dr. Karron said. Conversely, “you give an antibody and it’s good for as long as the antibody lasts. It’s not teaching your body anything.”
Frank Esper, MD, a pediatric infectious disease specialist at Cleveland Clinic Children’s Hospital, said monoclonal antibody protection for RSV is particularly welcome. “We’ve been trying to make an RSV vaccine since the 1960s and have done nothing but fail miserably.”
“The best thing is always a vaccine,” Dr. Esper said, explaining that vaccines teach the body to make its own antibodies and confer long-term protection and are “probably more efficacious than anything that’s ever manmade.
“But since we’ve really not done very well for pediatric RSV vaccines, nirsevimab is certainly something I’m looking forward to,” he said.
Fast-acting monoclonal antibodies
An advantage for monoclonal antibodies is that they start working almost immediately.
Children can get sick with RSV in the first few months of life so the speed of the monoclonal antibodies to begin protection is important, Dr. Esper said, adding that RSV “is the worst during the first year of life.”
The peak age for babies getting infected enough to require hospitalization is about 2 months, he said.
By 14 months, he said, kids’ immune systems and airways have matured enough “that it’s not nearly as bad.”
To get protection from a vaccine, he added, “usually takes 2-4 weeks from the time you get your shot to the time you see some benefit. With an antibody, you’re bypassing the processing that the body has to do, and it goes straight to ‘protection’ mode,” Dr. Esper said. “You get protected pretty much as soon as you get the antibody.”
A version of this article first appeared on Medscape.com.
For the first time in the fall of 2023, families will be offered season-long protection for infants and some children against respiratory syncytial virus (RSV).
The Food and Drug Administration in July approved a prevention called nirsevimab (Beyfortus, AstraZeneca/Sanofi) and it is expected to be widely rolled out in the coming weeks as the RSV season begins.
And monoclonal antibodies are often used for treatment rather than prevention.
Adding to potential confusion is the fact the Centers for Disease Control and Prevention has included nirsevimab in the Vaccines for Children program, which covers the costs for uninsured kids and makes it more accessible.
Nirsevimab is approved for infants (up to 8 months old) born during or entering their first RSV season, and in children up to 2 years of age who are still vulnerable to severe RSV through their second season.
It’s recommended that all infants get one injection in their first 8 months for prevention instead of the previous monthly shots used to help prevent kids at high risk from getting severe RSV.
If monoclonal antibodies can be used for preventing disease in infants, could they become a viable vaccine alternative for adults?
Specialists say no.
That’s partly because of the difference in body size. Although an injection is an option for a newborn, pediatricians suggest, it would take far too much of the treatment to work as a shot for adults.
Ruth Karron, MD, an expert in pediatric infectious diseases at Johns Hopkins Medicine, Baltimore, said that, while vaccines come in small amounts and activate immune cells, monoclonal antibodies are more like a drug, with the dose based on weight.
“You’d have to give it intravenously,” for larger doses, she explained, which has never been studied before and would also be very expensive. “It really couldn’t be an option for adults.”
What’s the difference between vaccines and antibodies?
Monoclonal antibodies are proteins made in a lab to mimic the immune system’s ability to fight pathogens such as viruses.
Dr. Karron explained that a wide variety of monoclonal antibodies have long been used to treat diseases such as cancers and autoimmune disease. In recent years, the antibodies have been used to treat COVID.
Monoclonal antibodies have also been used to treat RSV in children, but the effects don’t last long – they confer passive immunity and “when it’s gone, it’s gone,” Dr. Karron said.
That means kids at high risk for severe RSV have had to get monthly injections.
But with nirsevimab, the mutated antibodies stay in circulation longer so they can last 5 or 6 months, enough to cover the RSV season, Dr. Karron explained. “It’s highly, highly effective.”
Vaccines train the body
“The idea with vaccines is that you engage the individual’s immune system. You teach it to make antibodies,” Dr. Karron said. Conversely, “you give an antibody and it’s good for as long as the antibody lasts. It’s not teaching your body anything.”
Frank Esper, MD, a pediatric infectious disease specialist at Cleveland Clinic Children’s Hospital, said monoclonal antibody protection for RSV is particularly welcome. “We’ve been trying to make an RSV vaccine since the 1960s and have done nothing but fail miserably.”
“The best thing is always a vaccine,” Dr. Esper said, explaining that vaccines teach the body to make its own antibodies and confer long-term protection and are “probably more efficacious than anything that’s ever manmade.
“But since we’ve really not done very well for pediatric RSV vaccines, nirsevimab is certainly something I’m looking forward to,” he said.
Fast-acting monoclonal antibodies
An advantage for monoclonal antibodies is that they start working almost immediately.
Children can get sick with RSV in the first few months of life so the speed of the monoclonal antibodies to begin protection is important, Dr. Esper said, adding that RSV “is the worst during the first year of life.”
The peak age for babies getting infected enough to require hospitalization is about 2 months, he said.
By 14 months, he said, kids’ immune systems and airways have matured enough “that it’s not nearly as bad.”
To get protection from a vaccine, he added, “usually takes 2-4 weeks from the time you get your shot to the time you see some benefit. With an antibody, you’re bypassing the processing that the body has to do, and it goes straight to ‘protection’ mode,” Dr. Esper said. “You get protected pretty much as soon as you get the antibody.”
A version of this article first appeared on Medscape.com.
For the first time in the fall of 2023, families will be offered season-long protection for infants and some children against respiratory syncytial virus (RSV).
The Food and Drug Administration in July approved a prevention called nirsevimab (Beyfortus, AstraZeneca/Sanofi) and it is expected to be widely rolled out in the coming weeks as the RSV season begins.
And monoclonal antibodies are often used for treatment rather than prevention.
Adding to potential confusion is the fact the Centers for Disease Control and Prevention has included nirsevimab in the Vaccines for Children program, which covers the costs for uninsured kids and makes it more accessible.
Nirsevimab is approved for infants (up to 8 months old) born during or entering their first RSV season, and in children up to 2 years of age who are still vulnerable to severe RSV through their second season.
It’s recommended that all infants get one injection in their first 8 months for prevention instead of the previous monthly shots used to help prevent kids at high risk from getting severe RSV.
If monoclonal antibodies can be used for preventing disease in infants, could they become a viable vaccine alternative for adults?
Specialists say no.
That’s partly because of the difference in body size. Although an injection is an option for a newborn, pediatricians suggest, it would take far too much of the treatment to work as a shot for adults.
Ruth Karron, MD, an expert in pediatric infectious diseases at Johns Hopkins Medicine, Baltimore, said that, while vaccines come in small amounts and activate immune cells, monoclonal antibodies are more like a drug, with the dose based on weight.
“You’d have to give it intravenously,” for larger doses, she explained, which has never been studied before and would also be very expensive. “It really couldn’t be an option for adults.”
What’s the difference between vaccines and antibodies?
Monoclonal antibodies are proteins made in a lab to mimic the immune system’s ability to fight pathogens such as viruses.
Dr. Karron explained that a wide variety of monoclonal antibodies have long been used to treat diseases such as cancers and autoimmune disease. In recent years, the antibodies have been used to treat COVID.
Monoclonal antibodies have also been used to treat RSV in children, but the effects don’t last long – they confer passive immunity and “when it’s gone, it’s gone,” Dr. Karron said.
That means kids at high risk for severe RSV have had to get monthly injections.
But with nirsevimab, the mutated antibodies stay in circulation longer so they can last 5 or 6 months, enough to cover the RSV season, Dr. Karron explained. “It’s highly, highly effective.”
Vaccines train the body
“The idea with vaccines is that you engage the individual’s immune system. You teach it to make antibodies,” Dr. Karron said. Conversely, “you give an antibody and it’s good for as long as the antibody lasts. It’s not teaching your body anything.”
Frank Esper, MD, a pediatric infectious disease specialist at Cleveland Clinic Children’s Hospital, said monoclonal antibody protection for RSV is particularly welcome. “We’ve been trying to make an RSV vaccine since the 1960s and have done nothing but fail miserably.”
“The best thing is always a vaccine,” Dr. Esper said, explaining that vaccines teach the body to make its own antibodies and confer long-term protection and are “probably more efficacious than anything that’s ever manmade.
“But since we’ve really not done very well for pediatric RSV vaccines, nirsevimab is certainly something I’m looking forward to,” he said.
Fast-acting monoclonal antibodies
An advantage for monoclonal antibodies is that they start working almost immediately.
Children can get sick with RSV in the first few months of life so the speed of the monoclonal antibodies to begin protection is important, Dr. Esper said, adding that RSV “is the worst during the first year of life.”
The peak age for babies getting infected enough to require hospitalization is about 2 months, he said.
By 14 months, he said, kids’ immune systems and airways have matured enough “that it’s not nearly as bad.”
To get protection from a vaccine, he added, “usually takes 2-4 weeks from the time you get your shot to the time you see some benefit. With an antibody, you’re bypassing the processing that the body has to do, and it goes straight to ‘protection’ mode,” Dr. Esper said. “You get protected pretty much as soon as you get the antibody.”
A version of this article first appeared on Medscape.com.
Expert calls for sparing oxygen use for dyspnea in the emergency department
PARIS – , as per the current guidelines. Florian Negrello, MD, an emergency medicine specialist at University Hospital of Martinique in Fort-de-France, reiterated this message at the 2023 conference held by France’s emergency medicine society (Urgences 2023). The recommendation is intended to prevent hyperoxia; increasing evidence indicates the harmful effects of such a state on the body.
“This is a real problem. Oxygen therapy is given all too readily despite studies now showing that excess oxygen is harmful, especially in patients with head trauma, ischemic stroke, or cardiac arrest,” stated the session’s moderator, Patrick Plaisance, MD, PhD, a doctor at Lariboisière Hospital in Paris.
No proven hypoxia
Described as difficulty breathing or shortness of breath, dyspnea is common in the emergency department, occurring in 5%-9% of patients. Close to 20% of intensive care unit admissions involve patients with dyspnea. “Since this is a very subjective symptom, it’s possible it’s being underdiagnosed,” said Dr. Negrello.
Lower respiratory tract infection, acute heart failure, chronic obstructive pulmonary disease, and exacerbation of asthma are the four main diagnoses linked to dyspnea, but this symptom is also seen in several medical conditions (gastrointestinal, metabolic, neurologic, etc.), he noted.
Often seen as a harmless treatment option, oxygen therapy is commonly administered to patients with breathing difficulties even when no hypoxemia is documented. This is particularly the case for patients brought into hospital via ambulance who are treated with oxygen without even having had their blood oxygen levels, SpO2, and partial pressure of oxygen checked.
In the United States, one of the few studies published on the topic showed that one-third of patients transported via ambulance are put on oxygen, with SpO2 being measured in just 5% of these cases. Finally, just 17% of patients receiving oxygen were experiencing hypoxia, defined as SpO2 < 94%.
Oxidative stress
Recently, several research studies have revealed the potential dangers of unjustified use of oxygen, which can lead to hyperoxia and increased mortality in hospitalized patients.
A meta-analysis reported a linear relationship between severe hyperoxia, in-hospital mortality, and length of stay in intensive care. Another study revealed a greater mortality rate in patients with acute respiratory distress syndrome (ARDS) experiencing an episode of hyperoxia, regardless of the severity of ARDS.
Oxygen toxicity in intensive care is said to be linked to oxidative stress caused by increased growth of reactive oxygen species but also to the systemic inflammation caused by hyperoxia, explained Dr. Negrello. Excess oxygen may also cause lung lesions with necrosis, the severity of which is proportional to the fraction of inspired oxygen and the length of exposure.
According to the most up-to-date international recommendations published in 2018 on the use of oxygen therapy in treating acute conditions, oxygen should not be used when SpO2 ≥ 93%. When treatment has been started, it must be stopped when SpO2 reaches 96%. SpO2 cannot be maintained above 96%, according to experts.
These threshold values can be found in the COVID-19 treatment guidelines produced by the French-Language Society of Respiratory Medicine, with oxygen therapy being recommended when SpO2 < 92%, added Dr. Negrello. The aim is to maintain normal oxygen levels, with SpO2 between 92% and 96%.
Use sparingly
For patients with COPD, the target levels are lower, due to the risk of hypercapnia (higher than normal carbon dioxide levels in the blood). Oxygen saturation levels should then be kept between 88% and 92%, “by using the minimum amount of oxygen necessary,” per the guidelines.
“Oxygen should be used sparingly,” concluded Dr. Negrello. “To treat our patients without harming them, we must be able to use it at the right time, meaning when a patient really has low blood oxygen, by focusing on normal saturation levels as the end goal.”
SpO2 measurement is the first step to be taken to determine oxygen requirements, followed by, if necessary, blood gas analysis once the patient has been admitted, he explained.
Questioned at the end of his session on how long oxygen therapy can be given for, Dr. Negrello reiterated that the risk for death is correlated with the length of time spent in a state of hyperoxia but that it is difficult to establish a maximum timeframe to be adhered to strictly.
Given that excess oxygen is harmful to patients in intensive care, “it would be better, when in doubt, to focus on physiological levels” and simply stop treatment when target saturation levels are reached.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – , as per the current guidelines. Florian Negrello, MD, an emergency medicine specialist at University Hospital of Martinique in Fort-de-France, reiterated this message at the 2023 conference held by France’s emergency medicine society (Urgences 2023). The recommendation is intended to prevent hyperoxia; increasing evidence indicates the harmful effects of such a state on the body.
“This is a real problem. Oxygen therapy is given all too readily despite studies now showing that excess oxygen is harmful, especially in patients with head trauma, ischemic stroke, or cardiac arrest,” stated the session’s moderator, Patrick Plaisance, MD, PhD, a doctor at Lariboisière Hospital in Paris.
No proven hypoxia
Described as difficulty breathing or shortness of breath, dyspnea is common in the emergency department, occurring in 5%-9% of patients. Close to 20% of intensive care unit admissions involve patients with dyspnea. “Since this is a very subjective symptom, it’s possible it’s being underdiagnosed,” said Dr. Negrello.
Lower respiratory tract infection, acute heart failure, chronic obstructive pulmonary disease, and exacerbation of asthma are the four main diagnoses linked to dyspnea, but this symptom is also seen in several medical conditions (gastrointestinal, metabolic, neurologic, etc.), he noted.
Often seen as a harmless treatment option, oxygen therapy is commonly administered to patients with breathing difficulties even when no hypoxemia is documented. This is particularly the case for patients brought into hospital via ambulance who are treated with oxygen without even having had their blood oxygen levels, SpO2, and partial pressure of oxygen checked.
In the United States, one of the few studies published on the topic showed that one-third of patients transported via ambulance are put on oxygen, with SpO2 being measured in just 5% of these cases. Finally, just 17% of patients receiving oxygen were experiencing hypoxia, defined as SpO2 < 94%.
Oxidative stress
Recently, several research studies have revealed the potential dangers of unjustified use of oxygen, which can lead to hyperoxia and increased mortality in hospitalized patients.
A meta-analysis reported a linear relationship between severe hyperoxia, in-hospital mortality, and length of stay in intensive care. Another study revealed a greater mortality rate in patients with acute respiratory distress syndrome (ARDS) experiencing an episode of hyperoxia, regardless of the severity of ARDS.
Oxygen toxicity in intensive care is said to be linked to oxidative stress caused by increased growth of reactive oxygen species but also to the systemic inflammation caused by hyperoxia, explained Dr. Negrello. Excess oxygen may also cause lung lesions with necrosis, the severity of which is proportional to the fraction of inspired oxygen and the length of exposure.
According to the most up-to-date international recommendations published in 2018 on the use of oxygen therapy in treating acute conditions, oxygen should not be used when SpO2 ≥ 93%. When treatment has been started, it must be stopped when SpO2 reaches 96%. SpO2 cannot be maintained above 96%, according to experts.
These threshold values can be found in the COVID-19 treatment guidelines produced by the French-Language Society of Respiratory Medicine, with oxygen therapy being recommended when SpO2 < 92%, added Dr. Negrello. The aim is to maintain normal oxygen levels, with SpO2 between 92% and 96%.
Use sparingly
For patients with COPD, the target levels are lower, due to the risk of hypercapnia (higher than normal carbon dioxide levels in the blood). Oxygen saturation levels should then be kept between 88% and 92%, “by using the minimum amount of oxygen necessary,” per the guidelines.
“Oxygen should be used sparingly,” concluded Dr. Negrello. “To treat our patients without harming them, we must be able to use it at the right time, meaning when a patient really has low blood oxygen, by focusing on normal saturation levels as the end goal.”
SpO2 measurement is the first step to be taken to determine oxygen requirements, followed by, if necessary, blood gas analysis once the patient has been admitted, he explained.
Questioned at the end of his session on how long oxygen therapy can be given for, Dr. Negrello reiterated that the risk for death is correlated with the length of time spent in a state of hyperoxia but that it is difficult to establish a maximum timeframe to be adhered to strictly.
Given that excess oxygen is harmful to patients in intensive care, “it would be better, when in doubt, to focus on physiological levels” and simply stop treatment when target saturation levels are reached.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – , as per the current guidelines. Florian Negrello, MD, an emergency medicine specialist at University Hospital of Martinique in Fort-de-France, reiterated this message at the 2023 conference held by France’s emergency medicine society (Urgences 2023). The recommendation is intended to prevent hyperoxia; increasing evidence indicates the harmful effects of such a state on the body.
“This is a real problem. Oxygen therapy is given all too readily despite studies now showing that excess oxygen is harmful, especially in patients with head trauma, ischemic stroke, or cardiac arrest,” stated the session’s moderator, Patrick Plaisance, MD, PhD, a doctor at Lariboisière Hospital in Paris.
No proven hypoxia
Described as difficulty breathing or shortness of breath, dyspnea is common in the emergency department, occurring in 5%-9% of patients. Close to 20% of intensive care unit admissions involve patients with dyspnea. “Since this is a very subjective symptom, it’s possible it’s being underdiagnosed,” said Dr. Negrello.
Lower respiratory tract infection, acute heart failure, chronic obstructive pulmonary disease, and exacerbation of asthma are the four main diagnoses linked to dyspnea, but this symptom is also seen in several medical conditions (gastrointestinal, metabolic, neurologic, etc.), he noted.
Often seen as a harmless treatment option, oxygen therapy is commonly administered to patients with breathing difficulties even when no hypoxemia is documented. This is particularly the case for patients brought into hospital via ambulance who are treated with oxygen without even having had their blood oxygen levels, SpO2, and partial pressure of oxygen checked.
In the United States, one of the few studies published on the topic showed that one-third of patients transported via ambulance are put on oxygen, with SpO2 being measured in just 5% of these cases. Finally, just 17% of patients receiving oxygen were experiencing hypoxia, defined as SpO2 < 94%.
Oxidative stress
Recently, several research studies have revealed the potential dangers of unjustified use of oxygen, which can lead to hyperoxia and increased mortality in hospitalized patients.
A meta-analysis reported a linear relationship between severe hyperoxia, in-hospital mortality, and length of stay in intensive care. Another study revealed a greater mortality rate in patients with acute respiratory distress syndrome (ARDS) experiencing an episode of hyperoxia, regardless of the severity of ARDS.
Oxygen toxicity in intensive care is said to be linked to oxidative stress caused by increased growth of reactive oxygen species but also to the systemic inflammation caused by hyperoxia, explained Dr. Negrello. Excess oxygen may also cause lung lesions with necrosis, the severity of which is proportional to the fraction of inspired oxygen and the length of exposure.
According to the most up-to-date international recommendations published in 2018 on the use of oxygen therapy in treating acute conditions, oxygen should not be used when SpO2 ≥ 93%. When treatment has been started, it must be stopped when SpO2 reaches 96%. SpO2 cannot be maintained above 96%, according to experts.
These threshold values can be found in the COVID-19 treatment guidelines produced by the French-Language Society of Respiratory Medicine, with oxygen therapy being recommended when SpO2 < 92%, added Dr. Negrello. The aim is to maintain normal oxygen levels, with SpO2 between 92% and 96%.
Use sparingly
For patients with COPD, the target levels are lower, due to the risk of hypercapnia (higher than normal carbon dioxide levels in the blood). Oxygen saturation levels should then be kept between 88% and 92%, “by using the minimum amount of oxygen necessary,” per the guidelines.
“Oxygen should be used sparingly,” concluded Dr. Negrello. “To treat our patients without harming them, we must be able to use it at the right time, meaning when a patient really has low blood oxygen, by focusing on normal saturation levels as the end goal.”
SpO2 measurement is the first step to be taken to determine oxygen requirements, followed by, if necessary, blood gas analysis once the patient has been admitted, he explained.
Questioned at the end of his session on how long oxygen therapy can be given for, Dr. Negrello reiterated that the risk for death is correlated with the length of time spent in a state of hyperoxia but that it is difficult to establish a maximum timeframe to be adhered to strictly.
Given that excess oxygen is harmful to patients in intensive care, “it would be better, when in doubt, to focus on physiological levels” and simply stop treatment when target saturation levels are reached.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
FDA approves new tubeless insulin pump
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
One in five women report mistreatment during maternity care
“We have to do better at providing respectful and unbiased care to all mothers,” Debra E. Houry, MD, chief medical officer of the Centers for Disease Control and Prevention, said in a press briefing announcing the findings, which were published as a Vital Signs report in the CDC’s Morbidity and Mortality Weekly Report.
Previous research showed an increase in maternal deaths in the United States from 17.4 to 32.9 per 100,000 live births between 2018 and 2021, but approximately 80% of these deaths are preventable, wrote Yousra A. Mohamoud, PhD, of the CDC’s division of reproductive health, and colleagues.
“Maternal mortality review committees have identified discrimination as one factor contributing to pregnancy-related deaths,” the researchers wrote. Respectful care must be part of a larger strategy to prevent these deaths, they emphasized.
In the report, researchers reviewed data from 2,402 women who responded to an opt-in survey. The survey was conducted for the CDC through Porter Novelli, and no personally identifying information was included. Nearly 70% of the participants were White, 10.7% were Black, 10.2% were Hispanic, 4.8% were Asian, 1.5% were American Indian, Alaska Native, Pacific Islander, or Native Hawaiian, 2.8% were multiracial, and 0.5% were another race.
The survey included questions about maternity care experiences during pregnancy and delivery of the youngest child. For 65.5% of respondents, their youngest child was 5 years or older at the time of the survey.
Mistreatment during maternity care was defined using seven validated questions, including questions about violations of physical privacy, verbal abuse, and inattention to requests for help. Satisfaction with maternity care was defined as “very satisfied” or “somewhat satisfied.”
Participants also responded to questions about discrimination during maternity care based on factors such as race, ethnicity, skin color, age, and weight. Finally, participants were asked whether they refrained from asking questions about their health or raising concerns with health care providers.
Overall, 20.4% of respondents reported experiencing one of the defined forms of mistreatment during maternity care. The most common mistreatment reported by the women was being ignored by providers when they requested help (9.7%), followed by being shouted at or scolded (6.7%), having physical privacy violated (5.1%), and being forced to accept unwanted treatment or threatened with withholding of treatment (4.6%).
However, approximately 90% of women overall and 75% of those who reported any mistreatment were very or somewhat satisfied with their maternity care.
When stratified by race, mistreatment was reported most frequently by Black, Hispanic, and multiracial women (30%, 29%, and 27%, respectively).
Overall, 29% of women reported experiencing some type of discrimination; the most frequently reported reasons were age, weight, and income. Black women reported the highest rates of discrimination (40%) followed by multiracial women (39%) and Hispanic women (37%).
With regard to self-advocacy, 45% of women reported holding back from asking questions of health care providers; the most common reasons were thinking their health concerns were normal for pregnancy, being embarrassed, and being concerned that health care providers would consider them difficult.
In addition, more women with no insurance or public insurance at the time of delivery reported mistreatment during their maternity care than did women with private insurance (28%, 26%, and 16%, respectively).
The findings were limited by several factors, including the opt-in nature of the survey, which means that the data are likely not representative of the birthing population in the United States, the researchers noted. Other limitations included the reliance on self-reports, potential recall bias, use of English language only, and use of a combined category for respondents of American Indian, Alaska Native, Native Hawaiian, and Pacific Islander ethnicity.
However, the results highlight the need for improving respectful care as part of a larger strategy to reduce pregnancy-related deaths, the researchers said. At the system level, quality improvement programs are needed to standardize care and support providers in recognizing and reducing biases and increasing cultural awareness and communication. At the provider level, clinicians at all points in the maternity care process can improve patient experiences by providing equitable and respectful care, and by listening to and addressing patients’ concerns.
In addition, communication campaigns and community engagement can include perspectives of patients, families, and communities to support women and encourage them to ask questions and express concerns, the researchers said.
Improving respectful care can be part of actions to reduce mortality at all levels, the researchers noted. The Hear Her campaign, developed by the CDC Foundation with funding from Merck, provides resources for pregnant and postpartum women and their support networks to help reduce pregnancy-related deaths and complications by encouraging women to share concerns with providers and to recognize urgent maternal warning signs.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“We have to do better at providing respectful and unbiased care to all mothers,” Debra E. Houry, MD, chief medical officer of the Centers for Disease Control and Prevention, said in a press briefing announcing the findings, which were published as a Vital Signs report in the CDC’s Morbidity and Mortality Weekly Report.
Previous research showed an increase in maternal deaths in the United States from 17.4 to 32.9 per 100,000 live births between 2018 and 2021, but approximately 80% of these deaths are preventable, wrote Yousra A. Mohamoud, PhD, of the CDC’s division of reproductive health, and colleagues.
“Maternal mortality review committees have identified discrimination as one factor contributing to pregnancy-related deaths,” the researchers wrote. Respectful care must be part of a larger strategy to prevent these deaths, they emphasized.
In the report, researchers reviewed data from 2,402 women who responded to an opt-in survey. The survey was conducted for the CDC through Porter Novelli, and no personally identifying information was included. Nearly 70% of the participants were White, 10.7% were Black, 10.2% were Hispanic, 4.8% were Asian, 1.5% were American Indian, Alaska Native, Pacific Islander, or Native Hawaiian, 2.8% were multiracial, and 0.5% were another race.
The survey included questions about maternity care experiences during pregnancy and delivery of the youngest child. For 65.5% of respondents, their youngest child was 5 years or older at the time of the survey.
Mistreatment during maternity care was defined using seven validated questions, including questions about violations of physical privacy, verbal abuse, and inattention to requests for help. Satisfaction with maternity care was defined as “very satisfied” or “somewhat satisfied.”
Participants also responded to questions about discrimination during maternity care based on factors such as race, ethnicity, skin color, age, and weight. Finally, participants were asked whether they refrained from asking questions about their health or raising concerns with health care providers.
Overall, 20.4% of respondents reported experiencing one of the defined forms of mistreatment during maternity care. The most common mistreatment reported by the women was being ignored by providers when they requested help (9.7%), followed by being shouted at or scolded (6.7%), having physical privacy violated (5.1%), and being forced to accept unwanted treatment or threatened with withholding of treatment (4.6%).
However, approximately 90% of women overall and 75% of those who reported any mistreatment were very or somewhat satisfied with their maternity care.
When stratified by race, mistreatment was reported most frequently by Black, Hispanic, and multiracial women (30%, 29%, and 27%, respectively).
Overall, 29% of women reported experiencing some type of discrimination; the most frequently reported reasons were age, weight, and income. Black women reported the highest rates of discrimination (40%) followed by multiracial women (39%) and Hispanic women (37%).
With regard to self-advocacy, 45% of women reported holding back from asking questions of health care providers; the most common reasons were thinking their health concerns were normal for pregnancy, being embarrassed, and being concerned that health care providers would consider them difficult.
In addition, more women with no insurance or public insurance at the time of delivery reported mistreatment during their maternity care than did women with private insurance (28%, 26%, and 16%, respectively).
The findings were limited by several factors, including the opt-in nature of the survey, which means that the data are likely not representative of the birthing population in the United States, the researchers noted. Other limitations included the reliance on self-reports, potential recall bias, use of English language only, and use of a combined category for respondents of American Indian, Alaska Native, Native Hawaiian, and Pacific Islander ethnicity.
However, the results highlight the need for improving respectful care as part of a larger strategy to reduce pregnancy-related deaths, the researchers said. At the system level, quality improvement programs are needed to standardize care and support providers in recognizing and reducing biases and increasing cultural awareness and communication. At the provider level, clinicians at all points in the maternity care process can improve patient experiences by providing equitable and respectful care, and by listening to and addressing patients’ concerns.
In addition, communication campaigns and community engagement can include perspectives of patients, families, and communities to support women and encourage them to ask questions and express concerns, the researchers said.
Improving respectful care can be part of actions to reduce mortality at all levels, the researchers noted. The Hear Her campaign, developed by the CDC Foundation with funding from Merck, provides resources for pregnant and postpartum women and their support networks to help reduce pregnancy-related deaths and complications by encouraging women to share concerns with providers and to recognize urgent maternal warning signs.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“We have to do better at providing respectful and unbiased care to all mothers,” Debra E. Houry, MD, chief medical officer of the Centers for Disease Control and Prevention, said in a press briefing announcing the findings, which were published as a Vital Signs report in the CDC’s Morbidity and Mortality Weekly Report.
Previous research showed an increase in maternal deaths in the United States from 17.4 to 32.9 per 100,000 live births between 2018 and 2021, but approximately 80% of these deaths are preventable, wrote Yousra A. Mohamoud, PhD, of the CDC’s division of reproductive health, and colleagues.
“Maternal mortality review committees have identified discrimination as one factor contributing to pregnancy-related deaths,” the researchers wrote. Respectful care must be part of a larger strategy to prevent these deaths, they emphasized.
In the report, researchers reviewed data from 2,402 women who responded to an opt-in survey. The survey was conducted for the CDC through Porter Novelli, and no personally identifying information was included. Nearly 70% of the participants were White, 10.7% were Black, 10.2% were Hispanic, 4.8% were Asian, 1.5% were American Indian, Alaska Native, Pacific Islander, or Native Hawaiian, 2.8% were multiracial, and 0.5% were another race.
The survey included questions about maternity care experiences during pregnancy and delivery of the youngest child. For 65.5% of respondents, their youngest child was 5 years or older at the time of the survey.
Mistreatment during maternity care was defined using seven validated questions, including questions about violations of physical privacy, verbal abuse, and inattention to requests for help. Satisfaction with maternity care was defined as “very satisfied” or “somewhat satisfied.”
Participants also responded to questions about discrimination during maternity care based on factors such as race, ethnicity, skin color, age, and weight. Finally, participants were asked whether they refrained from asking questions about their health or raising concerns with health care providers.
Overall, 20.4% of respondents reported experiencing one of the defined forms of mistreatment during maternity care. The most common mistreatment reported by the women was being ignored by providers when they requested help (9.7%), followed by being shouted at or scolded (6.7%), having physical privacy violated (5.1%), and being forced to accept unwanted treatment or threatened with withholding of treatment (4.6%).
However, approximately 90% of women overall and 75% of those who reported any mistreatment were very or somewhat satisfied with their maternity care.
When stratified by race, mistreatment was reported most frequently by Black, Hispanic, and multiracial women (30%, 29%, and 27%, respectively).
Overall, 29% of women reported experiencing some type of discrimination; the most frequently reported reasons were age, weight, and income. Black women reported the highest rates of discrimination (40%) followed by multiracial women (39%) and Hispanic women (37%).
With regard to self-advocacy, 45% of women reported holding back from asking questions of health care providers; the most common reasons were thinking their health concerns were normal for pregnancy, being embarrassed, and being concerned that health care providers would consider them difficult.
In addition, more women with no insurance or public insurance at the time of delivery reported mistreatment during their maternity care than did women with private insurance (28%, 26%, and 16%, respectively).
The findings were limited by several factors, including the opt-in nature of the survey, which means that the data are likely not representative of the birthing population in the United States, the researchers noted. Other limitations included the reliance on self-reports, potential recall bias, use of English language only, and use of a combined category for respondents of American Indian, Alaska Native, Native Hawaiian, and Pacific Islander ethnicity.
However, the results highlight the need for improving respectful care as part of a larger strategy to reduce pregnancy-related deaths, the researchers said. At the system level, quality improvement programs are needed to standardize care and support providers in recognizing and reducing biases and increasing cultural awareness and communication. At the provider level, clinicians at all points in the maternity care process can improve patient experiences by providing equitable and respectful care, and by listening to and addressing patients’ concerns.
In addition, communication campaigns and community engagement can include perspectives of patients, families, and communities to support women and encourage them to ask questions and express concerns, the researchers said.
Improving respectful care can be part of actions to reduce mortality at all levels, the researchers noted. The Hear Her campaign, developed by the CDC Foundation with funding from Merck, provides resources for pregnant and postpartum women and their support networks to help reduce pregnancy-related deaths and complications by encouraging women to share concerns with providers and to recognize urgent maternal warning signs.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM MMWR
Commentary: Age and breast cancer, and cardiometabolic comorbidities, September 2023
Studies have shown that breast cancer survivors have increased rates of age-related conditions, including cardiovascular disease and osteoporosis among others, therefore postulating that the biological aging process may be accelerated in this population.2 Among 417 women enrolled in the prospective Sister Study cohort, paired blood samples collected an average of 7.7 years apart compared three epigenetic metrics of biological aging (calculated on the basis of DNA methylation data) between women who were diagnosed and treated for breast cancer (n = 190) vs those who remained breast cancer–free (n = 227) (Kresovich et al). Women diagnosed and treated for breast cancer had higher biological aging metrics than women who were cancer-free at the time of follow-up: PhenoAgeAccel3 (standardized mean difference [β] = 0.13; P = .04), GrimAgeAccel4 (β = 0.14; P = .01), and DunedinPACE5 (β = 0.37; P < .001). Regarding breast cancer therapies received, the increases in biological aging were most striking for those women who underwent radiation. The effect of cancer treatments, specifically chemotherapy and radiation, on DNA methylation profiles and accelerating the aging process has been demonstrated in prior studies as well.6 Future research should strive to improve our understanding of the specific mechanisms underlying these age-related changes, identify ways to affect those which are modifiable, and positively influence long-term cognitive and functional consequences.
The association between cardiometabolic abnormalities, including obesity, hyperinsulinemia, diabetes, hypertension, and dyslipidemia, and an elevated breast cancer risk has been demonstrated in various studies.7 Furthermore, dysregulation of obesity-related proteins plays a role in breast cancer development and progression. A study by Xu and colleagues evaluated the temporal relationships and longitudinal associations of body mass index (BMI), cardiometabolic risk score (CRS), and obesity-related protein score (OPS) among 444 healthy women in a breast cancer screening cohort. After adjustment for demographics, lifestyle, and reproductive factors, a 1-kg/m2 increase in BMI per year increased CRS in both premenopausal (0.057 unit; P = .025) and postmenopausal women (0.054 unit; P = .033) and increased OPS by 0.588 unit (P = .001) in postmenopausal women. A significant association was also observed between CRS and OPS in postmenopausal women (β = 0.281; P = .034). These results support the importance of weight management and its effect on cardiometabolic and obesity-related parameters in breast cancer prevention. Research focused on lifestyle interventions to modify risk factors and effective implementation of these techniques will contribute to further reducing breast cancer risk.
Additional References
- García-Albéniz X, Hernán MA, Logan RW, et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172(6):381-389. doi: 10.7326/M18-1199
- Greenlee H, Iribarren C, Rana JS, et al. Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. J Clin Oncol. 2022;40(15):1647-1658. doi: 10.1200/JCO.21.01736
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022:11:e73420. doi: 10.7554/eLife.73420
- Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. doi: 10.1038/s41523-020-0161-3
- Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: Systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503
Studies have shown that breast cancer survivors have increased rates of age-related conditions, including cardiovascular disease and osteoporosis among others, therefore postulating that the biological aging process may be accelerated in this population.2 Among 417 women enrolled in the prospective Sister Study cohort, paired blood samples collected an average of 7.7 years apart compared three epigenetic metrics of biological aging (calculated on the basis of DNA methylation data) between women who were diagnosed and treated for breast cancer (n = 190) vs those who remained breast cancer–free (n = 227) (Kresovich et al). Women diagnosed and treated for breast cancer had higher biological aging metrics than women who were cancer-free at the time of follow-up: PhenoAgeAccel3 (standardized mean difference [β] = 0.13; P = .04), GrimAgeAccel4 (β = 0.14; P = .01), and DunedinPACE5 (β = 0.37; P < .001). Regarding breast cancer therapies received, the increases in biological aging were most striking for those women who underwent radiation. The effect of cancer treatments, specifically chemotherapy and radiation, on DNA methylation profiles and accelerating the aging process has been demonstrated in prior studies as well.6 Future research should strive to improve our understanding of the specific mechanisms underlying these age-related changes, identify ways to affect those which are modifiable, and positively influence long-term cognitive and functional consequences.
The association between cardiometabolic abnormalities, including obesity, hyperinsulinemia, diabetes, hypertension, and dyslipidemia, and an elevated breast cancer risk has been demonstrated in various studies.7 Furthermore, dysregulation of obesity-related proteins plays a role in breast cancer development and progression. A study by Xu and colleagues evaluated the temporal relationships and longitudinal associations of body mass index (BMI), cardiometabolic risk score (CRS), and obesity-related protein score (OPS) among 444 healthy women in a breast cancer screening cohort. After adjustment for demographics, lifestyle, and reproductive factors, a 1-kg/m2 increase in BMI per year increased CRS in both premenopausal (0.057 unit; P = .025) and postmenopausal women (0.054 unit; P = .033) and increased OPS by 0.588 unit (P = .001) in postmenopausal women. A significant association was also observed between CRS and OPS in postmenopausal women (β = 0.281; P = .034). These results support the importance of weight management and its effect on cardiometabolic and obesity-related parameters in breast cancer prevention. Research focused on lifestyle interventions to modify risk factors and effective implementation of these techniques will contribute to further reducing breast cancer risk.
Additional References
- García-Albéniz X, Hernán MA, Logan RW, et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172(6):381-389. doi: 10.7326/M18-1199
- Greenlee H, Iribarren C, Rana JS, et al. Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. J Clin Oncol. 2022;40(15):1647-1658. doi: 10.1200/JCO.21.01736
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022:11:e73420. doi: 10.7554/eLife.73420
- Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. doi: 10.1038/s41523-020-0161-3
- Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: Systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503
Studies have shown that breast cancer survivors have increased rates of age-related conditions, including cardiovascular disease and osteoporosis among others, therefore postulating that the biological aging process may be accelerated in this population.2 Among 417 women enrolled in the prospective Sister Study cohort, paired blood samples collected an average of 7.7 years apart compared three epigenetic metrics of biological aging (calculated on the basis of DNA methylation data) between women who were diagnosed and treated for breast cancer (n = 190) vs those who remained breast cancer–free (n = 227) (Kresovich et al). Women diagnosed and treated for breast cancer had higher biological aging metrics than women who were cancer-free at the time of follow-up: PhenoAgeAccel3 (standardized mean difference [β] = 0.13; P = .04), GrimAgeAccel4 (β = 0.14; P = .01), and DunedinPACE5 (β = 0.37; P < .001). Regarding breast cancer therapies received, the increases in biological aging were most striking for those women who underwent radiation. The effect of cancer treatments, specifically chemotherapy and radiation, on DNA methylation profiles and accelerating the aging process has been demonstrated in prior studies as well.6 Future research should strive to improve our understanding of the specific mechanisms underlying these age-related changes, identify ways to affect those which are modifiable, and positively influence long-term cognitive and functional consequences.
The association between cardiometabolic abnormalities, including obesity, hyperinsulinemia, diabetes, hypertension, and dyslipidemia, and an elevated breast cancer risk has been demonstrated in various studies.7 Furthermore, dysregulation of obesity-related proteins plays a role in breast cancer development and progression. A study by Xu and colleagues evaluated the temporal relationships and longitudinal associations of body mass index (BMI), cardiometabolic risk score (CRS), and obesity-related protein score (OPS) among 444 healthy women in a breast cancer screening cohort. After adjustment for demographics, lifestyle, and reproductive factors, a 1-kg/m2 increase in BMI per year increased CRS in both premenopausal (0.057 unit; P = .025) and postmenopausal women (0.054 unit; P = .033) and increased OPS by 0.588 unit (P = .001) in postmenopausal women. A significant association was also observed between CRS and OPS in postmenopausal women (β = 0.281; P = .034). These results support the importance of weight management and its effect on cardiometabolic and obesity-related parameters in breast cancer prevention. Research focused on lifestyle interventions to modify risk factors and effective implementation of these techniques will contribute to further reducing breast cancer risk.
Additional References
- García-Albéniz X, Hernán MA, Logan RW, et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172(6):381-389. doi: 10.7326/M18-1199
- Greenlee H, Iribarren C, Rana JS, et al. Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. J Clin Oncol. 2022;40(15):1647-1658. doi: 10.1200/JCO.21.01736
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022:11:e73420. doi: 10.7554/eLife.73420
- Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. doi: 10.1038/s41523-020-0161-3
- Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: Systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503




