User login
Fluvastatin Improves Postoperative Cardiac Outcomes in Patients Undergoing Vascular Surgery
Clinical question: Does perioperative fluvastatin decrease adverse cardiac events after vascular surgery?
Background: Patients with atherosclerotic vascular disease who undergo vascular surgery are at high risk for postoperative cardiac events. Studies in nonsurgical populations have shown the beneficial effects of statin therapy on cardiac outcomes. However, no placebo-controlled trials have addressed the effect of statins on postoperative cardiac outcomes.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Single large academic medical center in the Netherlands.
Synopsis: The study looked at 497 statin-naïve patients 40 years or older undergoing non-cardiac vascular surgery. The patients were randomized to 80 mg of extended-release fluvastatin versus placebo; all patients received a beta-blocker. Therapy began preoperatively (median of 37 days) and continued for at least 30 days after surgery. Outcomes were assessed at 30 days post-surgery.
Postoperative myocardial infarction (MI) was significantly less common in the fluvastatin group than with placebo (10.8% vs. 19%, hazard ratio (HR) 0.55, P=0.01). In addition, the treatment group had a lower frequency of death from cardiovascular causes (4.8% vs. 10.1%, HR 0.47, P=0.03). Statin therapy was not associated with an increased rate of adverse events.
Notably, all of the patients enrolled in this study were high-risk patients undergoing high-risk (vascular) surgery. Patients already on statins were excluded.
Further studies are needed to determine whether the findings can be extrapolated to other populations, including nonvascular surgery patients.
Bottom line: Perioperative statin therapy resulted in a significant decrease in postoperative MI and death within 30 days of vascular surgery.
Citation: Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980-989.
Clinical question: Does perioperative fluvastatin decrease adverse cardiac events after vascular surgery?
Background: Patients with atherosclerotic vascular disease who undergo vascular surgery are at high risk for postoperative cardiac events. Studies in nonsurgical populations have shown the beneficial effects of statin therapy on cardiac outcomes. However, no placebo-controlled trials have addressed the effect of statins on postoperative cardiac outcomes.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Single large academic medical center in the Netherlands.
Synopsis: The study looked at 497 statin-naïve patients 40 years or older undergoing non-cardiac vascular surgery. The patients were randomized to 80 mg of extended-release fluvastatin versus placebo; all patients received a beta-blocker. Therapy began preoperatively (median of 37 days) and continued for at least 30 days after surgery. Outcomes were assessed at 30 days post-surgery.
Postoperative myocardial infarction (MI) was significantly less common in the fluvastatin group than with placebo (10.8% vs. 19%, hazard ratio (HR) 0.55, P=0.01). In addition, the treatment group had a lower frequency of death from cardiovascular causes (4.8% vs. 10.1%, HR 0.47, P=0.03). Statin therapy was not associated with an increased rate of adverse events.
Notably, all of the patients enrolled in this study were high-risk patients undergoing high-risk (vascular) surgery. Patients already on statins were excluded.
Further studies are needed to determine whether the findings can be extrapolated to other populations, including nonvascular surgery patients.
Bottom line: Perioperative statin therapy resulted in a significant decrease in postoperative MI and death within 30 days of vascular surgery.
Citation: Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980-989.
Clinical question: Does perioperative fluvastatin decrease adverse cardiac events after vascular surgery?
Background: Patients with atherosclerotic vascular disease who undergo vascular surgery are at high risk for postoperative cardiac events. Studies in nonsurgical populations have shown the beneficial effects of statin therapy on cardiac outcomes. However, no placebo-controlled trials have addressed the effect of statins on postoperative cardiac outcomes.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Single large academic medical center in the Netherlands.
Synopsis: The study looked at 497 statin-naïve patients 40 years or older undergoing non-cardiac vascular surgery. The patients were randomized to 80 mg of extended-release fluvastatin versus placebo; all patients received a beta-blocker. Therapy began preoperatively (median of 37 days) and continued for at least 30 days after surgery. Outcomes were assessed at 30 days post-surgery.
Postoperative myocardial infarction (MI) was significantly less common in the fluvastatin group than with placebo (10.8% vs. 19%, hazard ratio (HR) 0.55, P=0.01). In addition, the treatment group had a lower frequency of death from cardiovascular causes (4.8% vs. 10.1%, HR 0.47, P=0.03). Statin therapy was not associated with an increased rate of adverse events.
Notably, all of the patients enrolled in this study were high-risk patients undergoing high-risk (vascular) surgery. Patients already on statins were excluded.
Further studies are needed to determine whether the findings can be extrapolated to other populations, including nonvascular surgery patients.
Bottom line: Perioperative statin therapy resulted in a significant decrease in postoperative MI and death within 30 days of vascular surgery.
Citation: Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980-989.
New criteria for diagnosing MM could prevent organ damage

Credit: Chad McNeeley
The International Myeloma Working Group (IMWG) has published new criteria for diagnosing multiple myeloma (MM) in The Lancet Oncology.
The group has added validated biomarkers to the current clinical symptoms used for MM diagnosis—hypercalcemia, renal failure, anemia, and bone lesions.
This addition will allow physicians to diagnose MM before patients become symptomatic and, therefore, before organ damage occurs, according to the IMWG.
Lead author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota, noted that MM is always preceded sequentially by two conditions—monoclonal gammopathy of undetermined significance and smoldering MM. Since both are asymptomatic, most MM patients are not diagnosed until organ damage occurs.
“The new IMWG criteria allow for the diagnosis of myeloma to be made in patients without symptoms and before organ damage occurs, using validated biomarkers that identify patients with [smoldering] MM who have an ‘ultra-high’ risk of progression to multiple myeloma,” Dr Rajkumar said.
“These biomarkers are associated with the near-inevitable development of clinical symptoms and are important for early diagnosis and treatment, which is very important for patients.”
Other updates to the criteria used to diagnose MM include the use of CT and PET-CT scans to identify bone lesions. According to the authors, this will enable more accurate diagnosis and intervention before fractures or other serious problems arise.
“We believe that the new criteria will rectify the situation where we were unable to use the considerable advances in multiple myeloma therapy prior to organ damage,” Dr Rajkumar said. “We can now initiate therapy in some patients early on in the course of their disease.”
The IMWG’s revised diagnostic criteria for MM and smoldering MM are as follows.
Definition of MM
Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma* and one or more of the following myeloma defining events:
- Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically:
- Hypercalcemia: serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL).
- Renal insufficiency: creatinine clearance <40 mL per min (measured or estimated by validated equations) or serum creatinine >177 μmol/L (>2 mg/dL).
- Anemia: hemoglobin value of >20 g/L below the lower limit of normal or a hemoglobin value <100 g/L.
- Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT. If the bone marrow has less than 10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal marrow involvement.
- One or more of the following biomarkers:
- Clonal bone marrow plasma cell percentage ≥60%.
- Involved:uninvolved serum free light chain ratio ≥100. These values are based on the serum Freelite assay (The Binding Site Group, Birmingham, UK). The involved free light chain must be ≥100 mg/L.
- >1 focal lesions on MRI studies. Each focal lesion must be 5 mm or more in size.
*The IMWG said clonality should be established by showing κ/λ-light-chain restriction on flow cytometry, immunohistochemistry, or immunofluorescence. Bone
marrow plasma cell percentage should preferably be estimated from a core biopsy specimen. In case of a disparity between the aspirate and core biopsy, the highest value should be used.
Definition of smoldering MM
Both of the following criteria must be met:
- Serum monoclonal protein (IgG or IgA) ≥30 g/L or urinary monoclonal protein ≥500 mg per 24 hours and/or clonal bone marrow plasma cells 10%–60%.
- Absence of myeloma defining events or amyloidosis.


Credit: Chad McNeeley
The International Myeloma Working Group (IMWG) has published new criteria for diagnosing multiple myeloma (MM) in The Lancet Oncology.
The group has added validated biomarkers to the current clinical symptoms used for MM diagnosis—hypercalcemia, renal failure, anemia, and bone lesions.
This addition will allow physicians to diagnose MM before patients become symptomatic and, therefore, before organ damage occurs, according to the IMWG.
Lead author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota, noted that MM is always preceded sequentially by two conditions—monoclonal gammopathy of undetermined significance and smoldering MM. Since both are asymptomatic, most MM patients are not diagnosed until organ damage occurs.
“The new IMWG criteria allow for the diagnosis of myeloma to be made in patients without symptoms and before organ damage occurs, using validated biomarkers that identify patients with [smoldering] MM who have an ‘ultra-high’ risk of progression to multiple myeloma,” Dr Rajkumar said.
“These biomarkers are associated with the near-inevitable development of clinical symptoms and are important for early diagnosis and treatment, which is very important for patients.”
Other updates to the criteria used to diagnose MM include the use of CT and PET-CT scans to identify bone lesions. According to the authors, this will enable more accurate diagnosis and intervention before fractures or other serious problems arise.
“We believe that the new criteria will rectify the situation where we were unable to use the considerable advances in multiple myeloma therapy prior to organ damage,” Dr Rajkumar said. “We can now initiate therapy in some patients early on in the course of their disease.”
The IMWG’s revised diagnostic criteria for MM and smoldering MM are as follows.
Definition of MM
Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma* and one or more of the following myeloma defining events:
- Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically:
- Hypercalcemia: serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL).
- Renal insufficiency: creatinine clearance <40 mL per min (measured or estimated by validated equations) or serum creatinine >177 μmol/L (>2 mg/dL).
- Anemia: hemoglobin value of >20 g/L below the lower limit of normal or a hemoglobin value <100 g/L.
- Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT. If the bone marrow has less than 10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal marrow involvement.
- One or more of the following biomarkers:
- Clonal bone marrow plasma cell percentage ≥60%.
- Involved:uninvolved serum free light chain ratio ≥100. These values are based on the serum Freelite assay (The Binding Site Group, Birmingham, UK). The involved free light chain must be ≥100 mg/L.
- >1 focal lesions on MRI studies. Each focal lesion must be 5 mm or more in size.
*The IMWG said clonality should be established by showing κ/λ-light-chain restriction on flow cytometry, immunohistochemistry, or immunofluorescence. Bone
marrow plasma cell percentage should preferably be estimated from a core biopsy specimen. In case of a disparity between the aspirate and core biopsy, the highest value should be used.
Definition of smoldering MM
Both of the following criteria must be met:
- Serum monoclonal protein (IgG or IgA) ≥30 g/L or urinary monoclonal protein ≥500 mg per 24 hours and/or clonal bone marrow plasma cells 10%–60%.
- Absence of myeloma defining events or amyloidosis.


Credit: Chad McNeeley
The International Myeloma Working Group (IMWG) has published new criteria for diagnosing multiple myeloma (MM) in The Lancet Oncology.
The group has added validated biomarkers to the current clinical symptoms used for MM diagnosis—hypercalcemia, renal failure, anemia, and bone lesions.
This addition will allow physicians to diagnose MM before patients become symptomatic and, therefore, before organ damage occurs, according to the IMWG.
Lead author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota, noted that MM is always preceded sequentially by two conditions—monoclonal gammopathy of undetermined significance and smoldering MM. Since both are asymptomatic, most MM patients are not diagnosed until organ damage occurs.
“The new IMWG criteria allow for the diagnosis of myeloma to be made in patients without symptoms and before organ damage occurs, using validated biomarkers that identify patients with [smoldering] MM who have an ‘ultra-high’ risk of progression to multiple myeloma,” Dr Rajkumar said.
“These biomarkers are associated with the near-inevitable development of clinical symptoms and are important for early diagnosis and treatment, which is very important for patients.”
Other updates to the criteria used to diagnose MM include the use of CT and PET-CT scans to identify bone lesions. According to the authors, this will enable more accurate diagnosis and intervention before fractures or other serious problems arise.
“We believe that the new criteria will rectify the situation where we were unable to use the considerable advances in multiple myeloma therapy prior to organ damage,” Dr Rajkumar said. “We can now initiate therapy in some patients early on in the course of their disease.”
The IMWG’s revised diagnostic criteria for MM and smoldering MM are as follows.
Definition of MM
Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma* and one or more of the following myeloma defining events:
- Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically:
- Hypercalcemia: serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL).
- Renal insufficiency: creatinine clearance <40 mL per min (measured or estimated by validated equations) or serum creatinine >177 μmol/L (>2 mg/dL).
- Anemia: hemoglobin value of >20 g/L below the lower limit of normal or a hemoglobin value <100 g/L.
- Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT. If the bone marrow has less than 10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal marrow involvement.
- One or more of the following biomarkers:
- Clonal bone marrow plasma cell percentage ≥60%.
- Involved:uninvolved serum free light chain ratio ≥100. These values are based on the serum Freelite assay (The Binding Site Group, Birmingham, UK). The involved free light chain must be ≥100 mg/L.
- >1 focal lesions on MRI studies. Each focal lesion must be 5 mm or more in size.
*The IMWG said clonality should be established by showing κ/λ-light-chain restriction on flow cytometry, immunohistochemistry, or immunofluorescence. Bone
marrow plasma cell percentage should preferably be estimated from a core biopsy specimen. In case of a disparity between the aspirate and core biopsy, the highest value should be used.
Definition of smoldering MM
Both of the following criteria must be met:
- Serum monoclonal protein (IgG or IgA) ≥30 g/L or urinary monoclonal protein ≥500 mg per 24 hours and/or clonal bone marrow plasma cells 10%–60%.
- Absence of myeloma defining events or amyloidosis.

Device monitors methotrexate levels faster

Credit: Juan D. Alfonso
A new device can measure methotrexate levels in a patient’s blood in less than a minute, according to research published in Biosensors and Bioelectronics.
Researchers say this nanoscale device is just as accurate and 10 times less expensive than equipment currently used in hospitals.
It has an optical system that can rapidly gauge the optimal dose of methotrexate a patient needs, thereby reducing the risk of adverse effects.
“While effective, methotrexate is also highly toxic and can damage the healthy cells of patients, hence the importance of closely monitoring the drug’s concentration in the serum of treated individuals to adjust the dosage,” said study author Jean François Masson, PhD, of the University of Montreal in Quebec, Canada.
“The operation of the current [methotrexate monitoring] device is based on a cumbersome, expensive platform that requires experienced personnel because of the many samples that need to be manipulated.”
With this in mind, Dr Masson and his colleagues set out to simplify methotrexate monitoring.
In the course of their research, the team developed and manufactured a miniaturized device that works by surface plasmon resonance. It measures the concentration of serum methotrexate through gold nanoparticles on the surface of a receptacle.
In “competing” with methotrexate to block the enzyme dihydrofolate reductase, the gold nanoparticles change the color of the light detected by the instrument. And the color of the light detected reflects the exact concentration of the drug in the blood sample.
The researchers compared the accuracy of measurements taken with the new device to those taken with equipment used at the Maisonneuve-Rosemont Hospital in Montreal.
“Testing was conclusive,” Dr Masson said. “Not only were the measurements as accurate, but our device took less than 60 seconds to produce results, compared to 30 minutes for current devices.”
Moreover, the comparative tests were performed by lab technicians who were not experienced with surface plasmon resonance and did not encounter major difficulties in operating the new equipment or obtaining the same conclusive results as Dr Masson and his research team.
“In the near future, we can foresee the device in doctors’ offices or even at the bedside, where patients would receive individualized and optimal doses while minimizing the risk of complications,” Dr Masson said.
“While traditional equipment requires an investment of around $100,000, the new mobile device would likely cost 10 times less, around $10,000.” ![]()

Credit: Juan D. Alfonso
A new device can measure methotrexate levels in a patient’s blood in less than a minute, according to research published in Biosensors and Bioelectronics.
Researchers say this nanoscale device is just as accurate and 10 times less expensive than equipment currently used in hospitals.
It has an optical system that can rapidly gauge the optimal dose of methotrexate a patient needs, thereby reducing the risk of adverse effects.
“While effective, methotrexate is also highly toxic and can damage the healthy cells of patients, hence the importance of closely monitoring the drug’s concentration in the serum of treated individuals to adjust the dosage,” said study author Jean François Masson, PhD, of the University of Montreal in Quebec, Canada.
“The operation of the current [methotrexate monitoring] device is based on a cumbersome, expensive platform that requires experienced personnel because of the many samples that need to be manipulated.”
With this in mind, Dr Masson and his colleagues set out to simplify methotrexate monitoring.
In the course of their research, the team developed and manufactured a miniaturized device that works by surface plasmon resonance. It measures the concentration of serum methotrexate through gold nanoparticles on the surface of a receptacle.
In “competing” with methotrexate to block the enzyme dihydrofolate reductase, the gold nanoparticles change the color of the light detected by the instrument. And the color of the light detected reflects the exact concentration of the drug in the blood sample.
The researchers compared the accuracy of measurements taken with the new device to those taken with equipment used at the Maisonneuve-Rosemont Hospital in Montreal.
“Testing was conclusive,” Dr Masson said. “Not only were the measurements as accurate, but our device took less than 60 seconds to produce results, compared to 30 minutes for current devices.”
Moreover, the comparative tests were performed by lab technicians who were not experienced with surface plasmon resonance and did not encounter major difficulties in operating the new equipment or obtaining the same conclusive results as Dr Masson and his research team.
“In the near future, we can foresee the device in doctors’ offices or even at the bedside, where patients would receive individualized and optimal doses while minimizing the risk of complications,” Dr Masson said.
“While traditional equipment requires an investment of around $100,000, the new mobile device would likely cost 10 times less, around $10,000.” ![]()

Credit: Juan D. Alfonso
A new device can measure methotrexate levels in a patient’s blood in less than a minute, according to research published in Biosensors and Bioelectronics.
Researchers say this nanoscale device is just as accurate and 10 times less expensive than equipment currently used in hospitals.
It has an optical system that can rapidly gauge the optimal dose of methotrexate a patient needs, thereby reducing the risk of adverse effects.
“While effective, methotrexate is also highly toxic and can damage the healthy cells of patients, hence the importance of closely monitoring the drug’s concentration in the serum of treated individuals to adjust the dosage,” said study author Jean François Masson, PhD, of the University of Montreal in Quebec, Canada.
“The operation of the current [methotrexate monitoring] device is based on a cumbersome, expensive platform that requires experienced personnel because of the many samples that need to be manipulated.”
With this in mind, Dr Masson and his colleagues set out to simplify methotrexate monitoring.
In the course of their research, the team developed and manufactured a miniaturized device that works by surface plasmon resonance. It measures the concentration of serum methotrexate through gold nanoparticles on the surface of a receptacle.
In “competing” with methotrexate to block the enzyme dihydrofolate reductase, the gold nanoparticles change the color of the light detected by the instrument. And the color of the light detected reflects the exact concentration of the drug in the blood sample.
The researchers compared the accuracy of measurements taken with the new device to those taken with equipment used at the Maisonneuve-Rosemont Hospital in Montreal.
“Testing was conclusive,” Dr Masson said. “Not only were the measurements as accurate, but our device took less than 60 seconds to produce results, compared to 30 minutes for current devices.”
Moreover, the comparative tests were performed by lab technicians who were not experienced with surface plasmon resonance and did not encounter major difficulties in operating the new equipment or obtaining the same conclusive results as Dr Masson and his research team.
“In the near future, we can foresee the device in doctors’ offices or even at the bedside, where patients would receive individualized and optimal doses while minimizing the risk of complications,” Dr Masson said.
“While traditional equipment requires an investment of around $100,000, the new mobile device would likely cost 10 times less, around $10,000.” ![]()
Drug gets orphan status for PNH in US

The US Food and Drug Administration (FDA) has granted the complement inhibitor AMY-101 orphan status as a treatment for paroxysmal nocturnal
hemoglobinuria (PNH).
Roughly 2 months ago, the European Medicines Agency (EMA) did the same.
Orphan designation will allow Amyndas Pharmaceuticals, the company developing AMY-101, to proceed with expedited clinical development. The company is
planning to move the drug into clinical trials in 2015.
If AMY-101 is approved by the FDA, orphan status will allow for a 7-year period of market exclusivity from product launch in the US. It will also allow Amyndas to apply for research funding, tax credits for certain research expenses, and assistance for clinical research study design. It provides a waiver from the FDA’s Prescription Drug User Fee as well.
“Receiving the orphan drug designation from both the FDA and the EMA is an important achievement and a key milestone in the development pathway of AMY-101, and we are optimistic regarding the long-term potential of this potent complement inhibitor,” said John Lambris, PhD, of the University of Pennsylvania.
Dr Lambris developed AMY-101 at the University of Pennsylvania, and the university licensed the drug to Amyndas Pharmaceuticals. Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals.
About AMY-101 and PNH
PNH is caused by the defective expression of regulatory proteins on the surface of blood cells, which leaves them vulnerable to complement attack. This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
The monoclonal antibody eculizumab is often successful in treating PNH, but roughly a third of patients do not respond well to the drug and still require blood transfusions to manage their anemia.
Research has suggested this lack of response is due to fragments of complement C3 proteins on the surface of the patients’ red blood cells, which are eventually attacked by immune cells.
In an attempt to overcome this problem, Dr Lambris and his colleagues developed AMY-101. The drug is designed to inhibit C3, thereby preventing hemolysis and immune cell recognition.
The researchers have investigated the effects of AMY-101 on self-attack and the resulting hemolysis in human PNH cells and found the drug to be active.
These results have not been published, but the group has published results with a C3 inhibitor known as Cp40, and AMY-101 is based on Cp40.
The researchers reported in Blood that Cp40 and its long-acting form, PEG-Cp40, effectively inhibited hemolysis and efficiently prevented the deposition of C3 fragments on red blood cells from patients with PNH. ![]()

The US Food and Drug Administration (FDA) has granted the complement inhibitor AMY-101 orphan status as a treatment for paroxysmal nocturnal
hemoglobinuria (PNH).
Roughly 2 months ago, the European Medicines Agency (EMA) did the same.
Orphan designation will allow Amyndas Pharmaceuticals, the company developing AMY-101, to proceed with expedited clinical development. The company is
planning to move the drug into clinical trials in 2015.
If AMY-101 is approved by the FDA, orphan status will allow for a 7-year period of market exclusivity from product launch in the US. It will also allow Amyndas to apply for research funding, tax credits for certain research expenses, and assistance for clinical research study design. It provides a waiver from the FDA’s Prescription Drug User Fee as well.
“Receiving the orphan drug designation from both the FDA and the EMA is an important achievement and a key milestone in the development pathway of AMY-101, and we are optimistic regarding the long-term potential of this potent complement inhibitor,” said John Lambris, PhD, of the University of Pennsylvania.
Dr Lambris developed AMY-101 at the University of Pennsylvania, and the university licensed the drug to Amyndas Pharmaceuticals. Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals.
About AMY-101 and PNH
PNH is caused by the defective expression of regulatory proteins on the surface of blood cells, which leaves them vulnerable to complement attack. This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
The monoclonal antibody eculizumab is often successful in treating PNH, but roughly a third of patients do not respond well to the drug and still require blood transfusions to manage their anemia.
Research has suggested this lack of response is due to fragments of complement C3 proteins on the surface of the patients’ red blood cells, which are eventually attacked by immune cells.
In an attempt to overcome this problem, Dr Lambris and his colleagues developed AMY-101. The drug is designed to inhibit C3, thereby preventing hemolysis and immune cell recognition.
The researchers have investigated the effects of AMY-101 on self-attack and the resulting hemolysis in human PNH cells and found the drug to be active.
These results have not been published, but the group has published results with a C3 inhibitor known as Cp40, and AMY-101 is based on Cp40.
The researchers reported in Blood that Cp40 and its long-acting form, PEG-Cp40, effectively inhibited hemolysis and efficiently prevented the deposition of C3 fragments on red blood cells from patients with PNH. ![]()

The US Food and Drug Administration (FDA) has granted the complement inhibitor AMY-101 orphan status as a treatment for paroxysmal nocturnal
hemoglobinuria (PNH).
Roughly 2 months ago, the European Medicines Agency (EMA) did the same.
Orphan designation will allow Amyndas Pharmaceuticals, the company developing AMY-101, to proceed with expedited clinical development. The company is
planning to move the drug into clinical trials in 2015.
If AMY-101 is approved by the FDA, orphan status will allow for a 7-year period of market exclusivity from product launch in the US. It will also allow Amyndas to apply for research funding, tax credits for certain research expenses, and assistance for clinical research study design. It provides a waiver from the FDA’s Prescription Drug User Fee as well.
“Receiving the orphan drug designation from both the FDA and the EMA is an important achievement and a key milestone in the development pathway of AMY-101, and we are optimistic regarding the long-term potential of this potent complement inhibitor,” said John Lambris, PhD, of the University of Pennsylvania.
Dr Lambris developed AMY-101 at the University of Pennsylvania, and the university licensed the drug to Amyndas Pharmaceuticals. Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals.
About AMY-101 and PNH
PNH is caused by the defective expression of regulatory proteins on the surface of blood cells, which leaves them vulnerable to complement attack. This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
The monoclonal antibody eculizumab is often successful in treating PNH, but roughly a third of patients do not respond well to the drug and still require blood transfusions to manage their anemia.
Research has suggested this lack of response is due to fragments of complement C3 proteins on the surface of the patients’ red blood cells, which are eventually attacked by immune cells.
In an attempt to overcome this problem, Dr Lambris and his colleagues developed AMY-101. The drug is designed to inhibit C3, thereby preventing hemolysis and immune cell recognition.
The researchers have investigated the effects of AMY-101 on self-attack and the resulting hemolysis in human PNH cells and found the drug to be active.
These results have not been published, but the group has published results with a C3 inhibitor known as Cp40, and AMY-101 is based on Cp40.
The researchers reported in Blood that Cp40 and its long-acting form, PEG-Cp40, effectively inhibited hemolysis and efficiently prevented the deposition of C3 fragments on red blood cells from patients with PNH. ![]()
Texts improve malaria treatment adherence

Credit: Ed Yourdon
Text messages reminding patients to take malaria medication can improve treatment adherence, according to a study published in PLOS ONE.
“When patients don’t complete their full medication regimen, diseases can develop resistance to treatment,” said study author Julia Raifman, a PhD candidate at the Harvard School of Public Health in Boston.
“And with infectious diseases like malaria, drug-resistant diseases can spread to others. We’ve already begun to see resistance to artemisinin in Southeast Asia. It would be catastrophic if that became widespread and there was no effective treatment for the most deadly form of malaria.”
Working with researchers at the non-profit Innovations for Poverty Action in Ghana, Raifman and her colleagues drew on previous research using SMS reminders in situations where people fail to follow through on intentions, such as saving money, paying back loans, or completing college financial aid forms.
The researchers recruited 1140 people in Ghana who were taking artemisinin-based combination therapy to treat malaria.
Participants used their mobile phones to enroll in an automated system, and the system randomly assigned half of them to receive the text message reminders to take their medication.
Local researchers followed up with the participants several days later at their homes to see how many pills they had taken. Subjects who received the texts were significantly more likely to finish the full regimen.
The researchers also tested whether a short or longer, more informative message would be more effective. They were surprised to find the shorter messages had a significant impact, but the longer ones did not.
“SMS reminders are a ‘nudge,’ not a ‘shove,’” said Aaron Dibner-Dunlap, of Innovations for Poverty Action. “They can help people follow through on something they originally intended to do, but human nature is tricky, and the science is still young.”
“We’re optimistic because the technology has become so widespread and inexpensive to administer, that for programs like this one that work, there’s huge potential for helping people at very low cost.” ![]()

Credit: Ed Yourdon
Text messages reminding patients to take malaria medication can improve treatment adherence, according to a study published in PLOS ONE.
“When patients don’t complete their full medication regimen, diseases can develop resistance to treatment,” said study author Julia Raifman, a PhD candidate at the Harvard School of Public Health in Boston.
“And with infectious diseases like malaria, drug-resistant diseases can spread to others. We’ve already begun to see resistance to artemisinin in Southeast Asia. It would be catastrophic if that became widespread and there was no effective treatment for the most deadly form of malaria.”
Working with researchers at the non-profit Innovations for Poverty Action in Ghana, Raifman and her colleagues drew on previous research using SMS reminders in situations where people fail to follow through on intentions, such as saving money, paying back loans, or completing college financial aid forms.
The researchers recruited 1140 people in Ghana who were taking artemisinin-based combination therapy to treat malaria.
Participants used their mobile phones to enroll in an automated system, and the system randomly assigned half of them to receive the text message reminders to take their medication.
Local researchers followed up with the participants several days later at their homes to see how many pills they had taken. Subjects who received the texts were significantly more likely to finish the full regimen.
The researchers also tested whether a short or longer, more informative message would be more effective. They were surprised to find the shorter messages had a significant impact, but the longer ones did not.
“SMS reminders are a ‘nudge,’ not a ‘shove,’” said Aaron Dibner-Dunlap, of Innovations for Poverty Action. “They can help people follow through on something they originally intended to do, but human nature is tricky, and the science is still young.”
“We’re optimistic because the technology has become so widespread and inexpensive to administer, that for programs like this one that work, there’s huge potential for helping people at very low cost.” ![]()

Credit: Ed Yourdon
Text messages reminding patients to take malaria medication can improve treatment adherence, according to a study published in PLOS ONE.
“When patients don’t complete their full medication regimen, diseases can develop resistance to treatment,” said study author Julia Raifman, a PhD candidate at the Harvard School of Public Health in Boston.
“And with infectious diseases like malaria, drug-resistant diseases can spread to others. We’ve already begun to see resistance to artemisinin in Southeast Asia. It would be catastrophic if that became widespread and there was no effective treatment for the most deadly form of malaria.”
Working with researchers at the non-profit Innovations for Poverty Action in Ghana, Raifman and her colleagues drew on previous research using SMS reminders in situations where people fail to follow through on intentions, such as saving money, paying back loans, or completing college financial aid forms.
The researchers recruited 1140 people in Ghana who were taking artemisinin-based combination therapy to treat malaria.
Participants used their mobile phones to enroll in an automated system, and the system randomly assigned half of them to receive the text message reminders to take their medication.
Local researchers followed up with the participants several days later at their homes to see how many pills they had taken. Subjects who received the texts were significantly more likely to finish the full regimen.
The researchers also tested whether a short or longer, more informative message would be more effective. They were surprised to find the shorter messages had a significant impact, but the longer ones did not.
“SMS reminders are a ‘nudge,’ not a ‘shove,’” said Aaron Dibner-Dunlap, of Innovations for Poverty Action. “They can help people follow through on something they originally intended to do, but human nature is tricky, and the science is still young.”
“We’re optimistic because the technology has become so widespread and inexpensive to administer, that for programs like this one that work, there’s huge potential for helping people at very low cost.” ![]()
Smartphone‐Enabled Communication System
Previous studies have advocated the importance of effective communication between clinicians as a critical component in the provision of high‐quality patient care.[1, 2, 3, 4] There is increasing interest in the use of information and communication technologies to improve how clinicians communicate in hospital settings. A number of hospitals have implemented different solutions to improve communication. These solutions include alphanumeric pagers,[5] smartphones,[6] e‐mail,[7] secure text messaging,[8] and a Web‐based interdisciplinary communication tool.[9]
These systems have different limitations that render them inefficient and likely inhibit collaborative care. Current systems, such as pagers, rely on the sender to ensure the message was received and are successful in delivering messages approximately 67% of the time.[5, 9, 10] Although alphanumeric pagers and secure text messaging can increase the likelihood of delivery, these messages are often isolated and not easily viewable by the whole care team.[11] Improved systems should also reduce unnecessary interruptions by providing support for both urgent and delayed messages. Finally, messages should be stored and retrievable to enable increased accountability and allow for review for quality improvement initiatives.
It is also important to consider the unintended consequences of technology implementations.[12] Moving communication to text messages and smartphones has the potential to reduce interprofessional relations and can increase confusion if used for complex issues.[10, 13] In this article, we present a system designed to improve interprofessional communication on general internal medicine wards by incorporating these desired features and describe the usage and attitudes toward the system, specifically assessing for effects on multiple domains including efficiency, interprofessional collaboration, and relationships.
METHODS
Research Question
Will nurses and physicians use a system designed to improve interprofessional communication and will they perceive it to be effective and improve workflow?
Setting
The study took place on the general internal medicine wards at Toronto General Hospital and Toronto Western Hospital, 2 large academic teaching hospitals. There are several general internal medicine wards at each site with approximately 80 beds at each site. At each site there are 4 clinical teaching units and 1 hospitalist team. The study was approved by the research ethics board at the University Health Network.
Intervention
To address issues with communication, we developed a systemClinical Message (CM)that included 2 main components: a physician handover tool and secure messaging module. The focus of CM was to improve communication and information flow among different healthcare providers (physicians, nurses, pharmacists, social workers and therapists) through a secure, shared platform.
Physician Handover
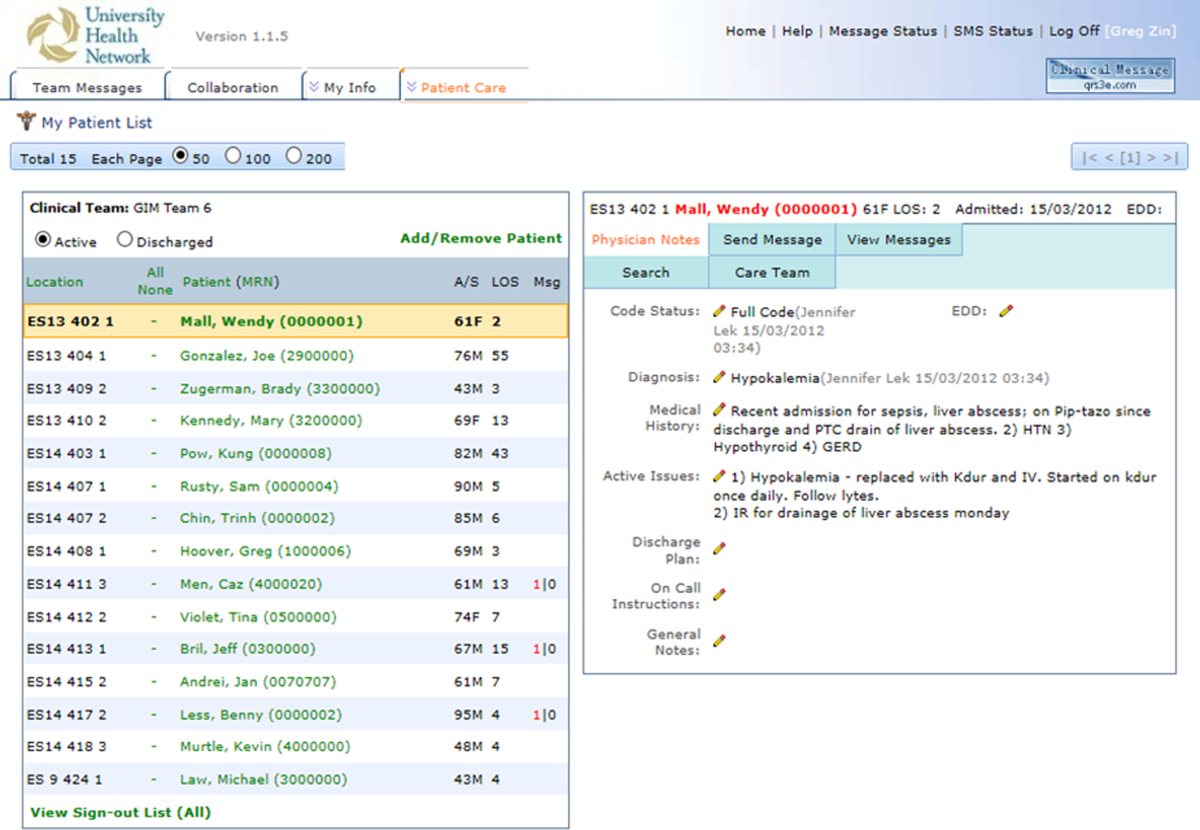
The physician handover tool was designed to facilitate the physician handover process at shift change and is used as a patient rounding tool for day‐to‐day management of patients. It is also accessed by nurses and other clinicians to view the physicians' notes and to stay informed on the overall care plan. The tool contains standard elements including a list of patients with the following information on each patient: demographics, diagnosis, code status, medical history, active issues, and discharge plans (Figure 1).

Secure Messaging
Secure messaging was designed around our dominant communication: nurses sending messages to physicians who would then respond. Nurses and other health professionals sent messages to the medical teams by accessing CM, selecting the appropriate patient, and filling out a message template. The system automatically populated the To field with the team assigned to the selected patient. Messaging for each team was centralized around a single team smartphone that was carried 24 hours a day, 7 days a week by a physician on that team. This removed the guesswork of trying to identify the individual physician responsible for that patient. For each message, a subject or issue and content were entered (Figure 2). Logic was also incorporated to reduce the amount of unnecessary interruptions. Senders would choose to send the message immediately as an interrupt message (urgent) for urgent/time sensitive issues or as an allow time to respond message (delayed). For the latter, the message was posted to the system where physicians could check and answer them. Interrupt messages were sent to the team smartphone using the Short Message Service (SMS) protocol. To try and ensure the communication loop on any issues was closed, when a message requested a response and did not receive it, the system sent another message. For urgent messages, a repeat message was initiated after 15 minutes. For delayed messages, the sender defined when they needed a response, typically within 2 to 8 hours. Senders were also able to select the mode of response that would best meet their needs from a workflow perspective: call back, text reply, or to specify that a reply was not required. Senders were also able to verify if the messages were received by the physician's smartphone. Physicians could view the messages within CM and reply. For messages that went to their team smartphone, physicians could respond from the smartphone through a secure Web link.
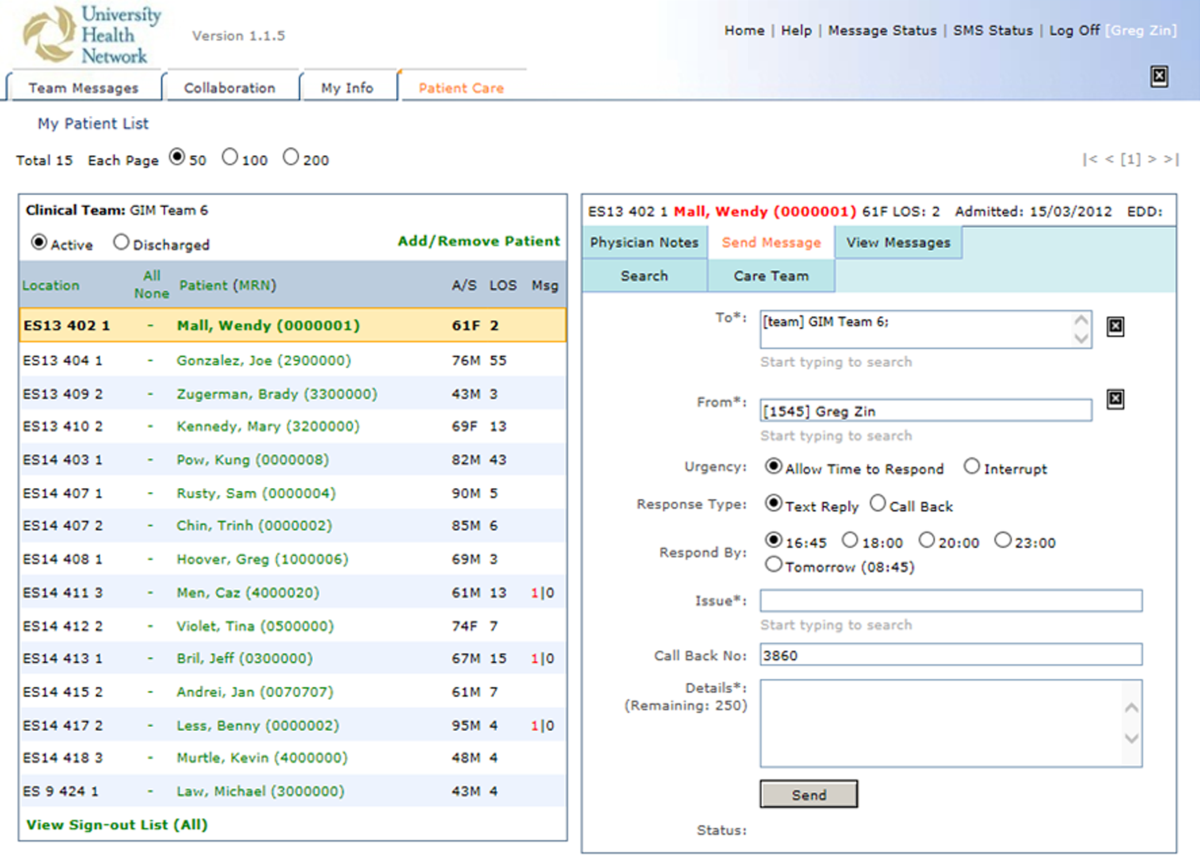
Because the messages were linked to the patients, they were visible to the entire care team, not just the message sender and recipient. If the care of the patient was transferred from 1 clinician to the next, the new clinician could easily review prior messages to understand recent patient events. The system was accessible through a browser on the intranet. The system regularly pulled patient demographic details such as name, age, medical record number, and location from our electronic medical record through a 1‐way interface. Information from this communication system was not considered part of the medical record but was retrievable.
The system was introduced as the new standard method of communication for nurses to reach physicians for all of the general internal medicine wards and for all medical teams at site 1 on May 2, 2011 and site 2 on June 6, 2011. The system replaced a text‐based Web‐paging system and supplemented the numeric pager carried by residents. Initial training of a half hour was provided to all nurses and residents.
Message Analysis for Usage Statistics
We analyzed messages created and sent via the CM system from May 2011 until August 2012. The extracted message information included date and time sent, issue, level of urgency, response type requested, roles of clinicians involved from the associated team, hospital site (senders and receivers), and message details. The following inclusion criteria were used for the analyses: (1) the senders and receivers of the messages could not be CM support staff, and (2) the messages sent were intended for the team smartphones used by the respective medical teams, not individual clinicians. Descriptive statistics and frequency analysis were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and IBM SPSS (IBM, Armonk, NY).
Survey
Development of the Survey
We used standard methods to develop a survey to assess staff perceptions on the impact of the new communication system. Relevant questionnaire items were compiled from a systematic review of the literature for communication surveys and communication issues that included the following domains: efficiency, accountability, accuracy, collaboration, timeliness, richness of the communication medium, and impact on interprofessional relationships and verbal communication.[10, 14, 15] We carried out pilot testing with 5 nurses and physicians, and modified the questionnaires based on their feedback.
Sampling and Data Collection of the Survey
Survey participants consisted of 2 groups of clinicians: (1) medical trainees that included medical residents, medical interns, and clinical fellows, and (2) nursing staff that included part‐time and full‐time nurses. To qualify for inclusion, participants had to have used the CM system for at least a month prior to administration of the questionnaire.
Data Analysis
Responses were recorded into an Excel spreadsheet that was imported into SPSS for analysis. Categorical variables were described using proportions. Survey comments were grouped into common themes, and themes mentioned by more than 1 respondent were reported.
RESULTS
Usage Analysis
A total of 60,969 messages were sent using CM between May 2, 2011 and August 19, 2012. On average, a team would receive 14.8 messages per day. Of all messages, 76.5% requested a text reply, 7.7% requested a call‐back, and 15.7% did not request a response. More than two‐thirds of messages at both hospitals were sent as immediate. Of the nonurgent messages, 86% were not replied to within the desired time, requiring a repeat message to be sent. Examples of different types of messages are shown in Table 1.
| Sender | Issue | Details | Priority | Desired Response Type | Time Created | Time Sent | Reply | Time Replied |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Nurse | Vital sign | Pt's BP is 182/95, HR is 108 now. Previous at 0800 was 165/78; HR was 99. PT is not on antihypertensive meds. | Allow time to respond (23:00) | Text reply | 21:43 | 23:02 | OK. Will assess. | 23:03 |
| Nurse | NG tube | NG tube is in place. Can you please enter portable chest x‐ray to check placement ASAP? | Immediate | Text reply | 16:58 | 16:58 | Will do. | 17:00 |
| Nurse | Bloodwork | Pt creat=216. Pt has NS @ 75 cc/hr. Pt has noted crackles throughout lung fields and has productive cough; eating and drinking well. Would you like it continued as well? Pt O2Sat 93% RA; would you like 4 L of O2 continued? Pls call for telephone order. | Immediate | Call back | 12:53 | 13:04 | Dealt with it on phone. | 13:05 |
| Nurse | Pain control | Hello! Pt has been getting 1 mg hydromorphone IV q 1 hr and pain is still not controlled. Pt remains awake and alert. Thanks! | Immediate | Info only | 15:41 | 15:41 | Thank you. | 15:42 |
For messages requesting a text reply, 8.6% did not receive a reply. The median response time was 2.3 minutes (interquartile range of 5.8 minutes), but some messages did not receive a response even after a week, which skewed the distribution of response times. For those messages that did receive a reply, 68.9% of them were responded to within 5 minutes, and 84.5% were responded to within 15 minutes. Messages were predominantly received between 9 am and midnight (see Supporting Figure 1 in the online version of this article). Because the sending of some messages was delayed, there appeared to be fewer messages received during protected educational times (89 am and 121 pm) as well as between midnight and 7 am compared to other times.
Survey Results
Between April 2013 and June 2013, 82 of 86 medical trainees (95.3%) and 83 of 116 nurses (71.6%) completed the survey, for an overall response rate of 81.7%. Clinicians perceived that CM appeared to have a positive impact on efficiency. In particular, 82.8% of physicians and 78.3% of nurses agreed or strongly agreed that CM helped speed up daily work tasks (Table 2). The majority of physicians and nurses agreed that the system increased accountability, increased timeliness of communication, and improved interprofessional relationships. It was not seen to be effective for communicating complex patient issues.
| No. of Subitems in Survey | Physician (% Agree, Strongly Agree), n=82 | Nurse (% Agree, Strongly Agree), n=83 | |
|---|---|---|---|
| |||
| Positive impact on efficiency. | 7 | 58.9% | 66.6% |
| The CM system helps speed up my daily work tasks. | 82.8% | 78.3% | |
| Positive impact on physician‐nurse collaboration. | 6 | 55.3% | 58.5% |
| The CM system increases the amount of communication between nurses and physicians. | 50.6% | 67.1% | |
| Improved timeliness of communication. | 5 | 54.2% | 50.5% |
| Communication through the CM system helps me resolve patient issues within the appropriate time frames. | 66.7% | 55.6% | |
| Increased accountability. | 2 | 67.1% | 73.2% |
| Improved accuracy of communications. | 3 | 41.6% | 50.7% |
| Improved interprofessional relationships. | 2 | 62.2% | 53.6% |
| Increased verbal communications. | 2 | 35.1% | 25.3% |
| Richness of the communication medium. | 6 | 40.7% | 48.3% |
| I find the CM system useful for communicating complex patient issues. | 35.8% | 26.3% | |
| I would prefer CM over standard hospital communication methods such as numeric paging. | 1 | 68.3% | 76.5% |
| I enjoy using the CM system for clinical communication on the wards. | 1 | 63.0% | 79.0% |
| Communication through the CM system helps to reduce interruptions for physicians. | 1 | 45.7% | |
Survey comments revealed that nurses perceived a lack of desired response, whereas physicians noted being interrupted with low‐value information through the system (Table 3). Both commented that further functionality, such as an active message stream, would be of benefit. Difficulty in communicating complex issues was also noted.
| Issue | Occurrences | Example | ||
|---|---|---|---|---|
| MD | RN | Total | ||
| ||||
| Lack of response | 1 | 10 | 11 | It depends if they respond quickly or not. A few times I send the 2nd message to remind them of the issue. I also spend more time to check if they answer it or not. I even call their Blackberries at last to get a response. |
| Message stream | 3 | 4 | 7 | I wish that I could see follow‐up messages after my initial reply (ie, it would be nice to have an open message stream). |
| Difficult to communicate complex issues | 1 | 5 | 6 | Difficult to communicate complex issues. Takes a lot of time to respond, and it becomes inefficient when responding to nonurgent CM because it interrupts workflow. |
| Many messages are low‐value interrupts | 3 | 0 | 3 | CM is useful for handover between clinicians, but often it slows down the clinician when they are used for information‐related low‐value/noncritical messages between nurses and clinicians |
| Lack of detailed response | 0 | 3 | 3 | Specific messages regarding response to care is required most times. For example, acknowledged is not a favorable response. |
| Technical issues | 2 | 0 | 2 | I find CM very useful. We have had multiple issues with our Blackberry this month, and CM was not working. When it is up and running, however, it is a wonderful tool. |
| Discrepancy in perceived urgency | 2 | 0 | 2 | Discrepancy between what nurses find urgent and what we find urgent. |
DISCUSSION
We describe an implementation of a system to improve clinical communication in hospitals. The system was highly used and was perceived to improve communication by both nurses and physicians. Specifically, users found that the system increased efficiency, accountability, timeliness, and collaboration, but that there were issues with message clarity for complex medical issues.
Other systems and approaches have been implemented to improve communication. These included the use of alphanumeric pagers, e‐mail, secure texting, and smartphones. There is evidence that more advanced systems can improve efficiency for senders.[16] A recent randomized trial of secure text messaging found that it was perceived to be more efficient than paging, but overall usage was low and inconsistent.[8] There is also evidence that smartphones may increase interruptions, worsen interprofessional relationships, and cause issues with professional behavior.[10] Unfortunately, there are a limited number of interventions that improve communication, with some improving efficiency but none demonstrating improved patient‐oriented outcomes.[16, 17] This study evaluated a novel system, with functionality to link communication to patients, and created a system that aligned with the workflow of the clinicians. Messages were linked to the patient, not the sender or receiver, so other clinicians in the patient's circle of care could easily view the communication. Moreover, the system was designed to improve message response rates and allow for nonurgent messages.
Our communication system uses standard, commercially available components (smartphones, SMS), and relatively basic functionality (handover, secure messaging). Important findings are that the current system of paging can be transformed to a more efficient system that users will readily adopt. We found positive effects with components of the system. It appeared to improve efficiency and increase accountability. Accountability is crucial and moves from undocumented conversation to fully documented details of interactions. This can be used for both incident review and to review for quality improvement.
Using the system, physicians perceived that they were bothered by low‐value information, whereas nurses perceived a lack of response, and both found that the system was not ideal for complex messages. The mismatch between what physicians and nurses perceive as important has been attributed to their different timeframes and context.[18] For nurses with an upcoming change of shift, they wanted resolution of issues before handover. A physician on a different ward may not appreciate the context of a nurse having to directly interact with an irate family member. These different perceptions likely contributed to the lack of response to 8.6% of text messages. This is still better than other systems, such as paging, which can be as high as 33%.[10] For nonurgent items, clinicians would ideally check and clear items regularly from the system using a desktop computer, responding within the allotted timeframe. Unfortunately, this never became part of routine physician workflow, likely due to their busy workload, so many physicians would only respond when items became overdue. However, having a method to deal with nonurgent messages may have prevented some interruptions during protected educational times of trainees. The system was also not ideal for urgent or complex items. Complex items can be difficult to convey using the rarified communication medium of text messages.[19, 20] Urgent or complex issues are likely best resolved with a face‐to‐face or telephone conversation.
There are several limitations in our study that should be considered when interpreting the results. It is a study of usage and perceptions after implementation. Although more rigorous study is required to evaluate the effects, we see this as a first step in process improvement. Future research should measure the impact on improving patient care of this system and on patient outcomes such as adverse events. The study and intervention was limited to general internal medicine wards in 2 academic hospital settings where there are frequent rotations of medical personnel. The findings may not be generalizable to other hospital settings.
Future directions should be to further improve on the communication system and to educate and train staff on how to effectively communicate. Survey results showed that although users perceived increased efficiency, there was still significant opportunity to improve. One way to improve would be to have a mobile application in which physicians can easily review nonurgent items. Improvements could also be realized by educating clinicians on the use of the system and providing immediate feedback. Providing feedback to physicians on how well they respond could address nurses' issues around lack of timely response. By creating consensus between nurses and physicians on what is of high and low value to communicate could increase satisfaction for all users.
In summary, we present the usage and perceptions of a system designed to improve hospital communication. We found that there was high uptake, and that users perceived it to improve efficiency, collaboration, and accountability, but it may not be useful for communicating complex issues.
ACKNOWLEDGEMENTS
The authors acknowledge the nurses, physicians, residents, and other health professions on the general internal medicine ward for their patience and support as we continue to try to innovate. The authors also acknowledge the members of the information systems department (Shared Information Management Systems, University Health Network) who helped to support the Communication System, and the software developer, QRS, that helped to codevelop the software system.
Disclosures: The hospital was in a codevelopment agreement that has since terminated. No researcher or hospital received any funds from private industry for any purpose including personal or research. The authors report no conflicts of interest.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277–286.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370–376.
- , , , et al. Gaps in pediatric clinician communication and opportunities for improvement. J Healthc Qual. 2008;30(5):43–54.
- , , , , . Quality in Australian health care study. Med J Aust. 1996;164(12):754.
- , , , . Implementation and evaluation of an alphanumeric paging system on a resident inpatient teaching service. J Hosp Med. 2009;4(8):E34–E40.
- , , , et al. Demonstrating the BlackBerry as a clinical communication tool: a pilot study conducted through the Centre for Innovation in Complex Care. Healthc Q. 2008;11(4):94–98.
- , , , . The use of wireless email to improve healthcare team communication. J Am Med Inform Assoc. 2009;16(5):705–713.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9(9):573–578.
- , , , , . Beyond paging: building a web‐based communication tool for nurses and physicians. J Gen Intern Med. 2009;24(1):105–110.
- , , , et al. The intended and unintended consequences of communication systems on general internal medicine inpatient care delivery: a prospective observational case study of five teaching hospitals. J Am Med Inform Assoc. 2013;20(4):766–777.
- , , , et al. Improving hospital care and collaborative communications for the 21st century: key recommendations for general internal medicine. Interact J Med Res. 2012;1(2):e9.
- , , , , , . Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18(1):82–90.
- , , , et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed‐methods study. J Med Internet Res. 2011;13(3):e59.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Impact of communication medium on task performance and satisfaction: an examination of media‐richness theory. Inform Manag. 1999;35:295–312.
- , , , et al. Effects of clinical communication interventions in hospitals: a systematic review of information and communication technology adoptions for improved communication between clinicians. Int J Med Inform. 2012;81(11):723–732.
- , , , et al. Provider‐to‐provider electronic communication in the era of meaningful use: a review of the evidence. J Hosp Med. 2013;8(10):589–597.
- , , , et al. Perceptions of urgency: defining the gap between what physicians and nurses perceive to be an urgent issue. Int J Med Inform. 2013;82(5):378–386.
- , , , , , . Short message service or disService: issues with text messaging in a complex medical environment. Int J Med Inform. 2014;83(4):278–284.
- , , . Instant messaging at the hospital: supporting articulation work? Int J Med Inform. 2013;82(9):753–761.
Previous studies have advocated the importance of effective communication between clinicians as a critical component in the provision of high‐quality patient care.[1, 2, 3, 4] There is increasing interest in the use of information and communication technologies to improve how clinicians communicate in hospital settings. A number of hospitals have implemented different solutions to improve communication. These solutions include alphanumeric pagers,[5] smartphones,[6] e‐mail,[7] secure text messaging,[8] and a Web‐based interdisciplinary communication tool.[9]
These systems have different limitations that render them inefficient and likely inhibit collaborative care. Current systems, such as pagers, rely on the sender to ensure the message was received and are successful in delivering messages approximately 67% of the time.[5, 9, 10] Although alphanumeric pagers and secure text messaging can increase the likelihood of delivery, these messages are often isolated and not easily viewable by the whole care team.[11] Improved systems should also reduce unnecessary interruptions by providing support for both urgent and delayed messages. Finally, messages should be stored and retrievable to enable increased accountability and allow for review for quality improvement initiatives.
It is also important to consider the unintended consequences of technology implementations.[12] Moving communication to text messages and smartphones has the potential to reduce interprofessional relations and can increase confusion if used for complex issues.[10, 13] In this article, we present a system designed to improve interprofessional communication on general internal medicine wards by incorporating these desired features and describe the usage and attitudes toward the system, specifically assessing for effects on multiple domains including efficiency, interprofessional collaboration, and relationships.
METHODS
Research Question
Will nurses and physicians use a system designed to improve interprofessional communication and will they perceive it to be effective and improve workflow?
Setting
The study took place on the general internal medicine wards at Toronto General Hospital and Toronto Western Hospital, 2 large academic teaching hospitals. There are several general internal medicine wards at each site with approximately 80 beds at each site. At each site there are 4 clinical teaching units and 1 hospitalist team. The study was approved by the research ethics board at the University Health Network.
Intervention
To address issues with communication, we developed a systemClinical Message (CM)that included 2 main components: a physician handover tool and secure messaging module. The focus of CM was to improve communication and information flow among different healthcare providers (physicians, nurses, pharmacists, social workers and therapists) through a secure, shared platform.
Physician Handover
The physician handover tool was designed to facilitate the physician handover process at shift change and is used as a patient rounding tool for day‐to‐day management of patients. It is also accessed by nurses and other clinicians to view the physicians' notes and to stay informed on the overall care plan. The tool contains standard elements including a list of patients with the following information on each patient: demographics, diagnosis, code status, medical history, active issues, and discharge plans (Figure 1).

Secure Messaging
Secure messaging was designed around our dominant communication: nurses sending messages to physicians who would then respond. Nurses and other health professionals sent messages to the medical teams by accessing CM, selecting the appropriate patient, and filling out a message template. The system automatically populated the To field with the team assigned to the selected patient. Messaging for each team was centralized around a single team smartphone that was carried 24 hours a day, 7 days a week by a physician on that team. This removed the guesswork of trying to identify the individual physician responsible for that patient. For each message, a subject or issue and content were entered (Figure 2). Logic was also incorporated to reduce the amount of unnecessary interruptions. Senders would choose to send the message immediately as an interrupt message (urgent) for urgent/time sensitive issues or as an allow time to respond message (delayed). For the latter, the message was posted to the system where physicians could check and answer them. Interrupt messages were sent to the team smartphone using the Short Message Service (SMS) protocol. To try and ensure the communication loop on any issues was closed, when a message requested a response and did not receive it, the system sent another message. For urgent messages, a repeat message was initiated after 15 minutes. For delayed messages, the sender defined when they needed a response, typically within 2 to 8 hours. Senders were also able to select the mode of response that would best meet their needs from a workflow perspective: call back, text reply, or to specify that a reply was not required. Senders were also able to verify if the messages were received by the physician's smartphone. Physicians could view the messages within CM and reply. For messages that went to their team smartphone, physicians could respond from the smartphone through a secure Web link.

Because the messages were linked to the patients, they were visible to the entire care team, not just the message sender and recipient. If the care of the patient was transferred from 1 clinician to the next, the new clinician could easily review prior messages to understand recent patient events. The system was accessible through a browser on the intranet. The system regularly pulled patient demographic details such as name, age, medical record number, and location from our electronic medical record through a 1‐way interface. Information from this communication system was not considered part of the medical record but was retrievable.
The system was introduced as the new standard method of communication for nurses to reach physicians for all of the general internal medicine wards and for all medical teams at site 1 on May 2, 2011 and site 2 on June 6, 2011. The system replaced a text‐based Web‐paging system and supplemented the numeric pager carried by residents. Initial training of a half hour was provided to all nurses and residents.
Message Analysis for Usage Statistics
We analyzed messages created and sent via the CM system from May 2011 until August 2012. The extracted message information included date and time sent, issue, level of urgency, response type requested, roles of clinicians involved from the associated team, hospital site (senders and receivers), and message details. The following inclusion criteria were used for the analyses: (1) the senders and receivers of the messages could not be CM support staff, and (2) the messages sent were intended for the team smartphones used by the respective medical teams, not individual clinicians. Descriptive statistics and frequency analysis were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and IBM SPSS (IBM, Armonk, NY).
Survey
Development of the Survey
We used standard methods to develop a survey to assess staff perceptions on the impact of the new communication system. Relevant questionnaire items were compiled from a systematic review of the literature for communication surveys and communication issues that included the following domains: efficiency, accountability, accuracy, collaboration, timeliness, richness of the communication medium, and impact on interprofessional relationships and verbal communication.[10, 14, 15] We carried out pilot testing with 5 nurses and physicians, and modified the questionnaires based on their feedback.
Sampling and Data Collection of the Survey
Survey participants consisted of 2 groups of clinicians: (1) medical trainees that included medical residents, medical interns, and clinical fellows, and (2) nursing staff that included part‐time and full‐time nurses. To qualify for inclusion, participants had to have used the CM system for at least a month prior to administration of the questionnaire.
Data Analysis
Responses were recorded into an Excel spreadsheet that was imported into SPSS for analysis. Categorical variables were described using proportions. Survey comments were grouped into common themes, and themes mentioned by more than 1 respondent were reported.
RESULTS
Usage Analysis
A total of 60,969 messages were sent using CM between May 2, 2011 and August 19, 2012. On average, a team would receive 14.8 messages per day. Of all messages, 76.5% requested a text reply, 7.7% requested a call‐back, and 15.7% did not request a response. More than two‐thirds of messages at both hospitals were sent as immediate. Of the nonurgent messages, 86% were not replied to within the desired time, requiring a repeat message to be sent. Examples of different types of messages are shown in Table 1.
| Sender | Issue | Details | Priority | Desired Response Type | Time Created | Time Sent | Reply | Time Replied |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Nurse | Vital sign | Pt's BP is 182/95, HR is 108 now. Previous at 0800 was 165/78; HR was 99. PT is not on antihypertensive meds. | Allow time to respond (23:00) | Text reply | 21:43 | 23:02 | OK. Will assess. | 23:03 |
| Nurse | NG tube | NG tube is in place. Can you please enter portable chest x‐ray to check placement ASAP? | Immediate | Text reply | 16:58 | 16:58 | Will do. | 17:00 |
| Nurse | Bloodwork | Pt creat=216. Pt has NS @ 75 cc/hr. Pt has noted crackles throughout lung fields and has productive cough; eating and drinking well. Would you like it continued as well? Pt O2Sat 93% RA; would you like 4 L of O2 continued? Pls call for telephone order. | Immediate | Call back | 12:53 | 13:04 | Dealt with it on phone. | 13:05 |
| Nurse | Pain control | Hello! Pt has been getting 1 mg hydromorphone IV q 1 hr and pain is still not controlled. Pt remains awake and alert. Thanks! | Immediate | Info only | 15:41 | 15:41 | Thank you. | 15:42 |
For messages requesting a text reply, 8.6% did not receive a reply. The median response time was 2.3 minutes (interquartile range of 5.8 minutes), but some messages did not receive a response even after a week, which skewed the distribution of response times. For those messages that did receive a reply, 68.9% of them were responded to within 5 minutes, and 84.5% were responded to within 15 minutes. Messages were predominantly received between 9 am and midnight (see Supporting Figure 1 in the online version of this article). Because the sending of some messages was delayed, there appeared to be fewer messages received during protected educational times (89 am and 121 pm) as well as between midnight and 7 am compared to other times.
Survey Results
Between April 2013 and June 2013, 82 of 86 medical trainees (95.3%) and 83 of 116 nurses (71.6%) completed the survey, for an overall response rate of 81.7%. Clinicians perceived that CM appeared to have a positive impact on efficiency. In particular, 82.8% of physicians and 78.3% of nurses agreed or strongly agreed that CM helped speed up daily work tasks (Table 2). The majority of physicians and nurses agreed that the system increased accountability, increased timeliness of communication, and improved interprofessional relationships. It was not seen to be effective for communicating complex patient issues.
| No. of Subitems in Survey | Physician (% Agree, Strongly Agree), n=82 | Nurse (% Agree, Strongly Agree), n=83 | |
|---|---|---|---|
| |||
| Positive impact on efficiency. | 7 | 58.9% | 66.6% |
| The CM system helps speed up my daily work tasks. | 82.8% | 78.3% | |
| Positive impact on physician‐nurse collaboration. | 6 | 55.3% | 58.5% |
| The CM system increases the amount of communication between nurses and physicians. | 50.6% | 67.1% | |
| Improved timeliness of communication. | 5 | 54.2% | 50.5% |
| Communication through the CM system helps me resolve patient issues within the appropriate time frames. | 66.7% | 55.6% | |
| Increased accountability. | 2 | 67.1% | 73.2% |
| Improved accuracy of communications. | 3 | 41.6% | 50.7% |
| Improved interprofessional relationships. | 2 | 62.2% | 53.6% |
| Increased verbal communications. | 2 | 35.1% | 25.3% |
| Richness of the communication medium. | 6 | 40.7% | 48.3% |
| I find the CM system useful for communicating complex patient issues. | 35.8% | 26.3% | |
| I would prefer CM over standard hospital communication methods such as numeric paging. | 1 | 68.3% | 76.5% |
| I enjoy using the CM system for clinical communication on the wards. | 1 | 63.0% | 79.0% |
| Communication through the CM system helps to reduce interruptions for physicians. | 1 | 45.7% | |
Survey comments revealed that nurses perceived a lack of desired response, whereas physicians noted being interrupted with low‐value information through the system (Table 3). Both commented that further functionality, such as an active message stream, would be of benefit. Difficulty in communicating complex issues was also noted.
| Issue | Occurrences | Example | ||
|---|---|---|---|---|
| MD | RN | Total | ||
| ||||
| Lack of response | 1 | 10 | 11 | It depends if they respond quickly or not. A few times I send the 2nd message to remind them of the issue. I also spend more time to check if they answer it or not. I even call their Blackberries at last to get a response. |
| Message stream | 3 | 4 | 7 | I wish that I could see follow‐up messages after my initial reply (ie, it would be nice to have an open message stream). |
| Difficult to communicate complex issues | 1 | 5 | 6 | Difficult to communicate complex issues. Takes a lot of time to respond, and it becomes inefficient when responding to nonurgent CM because it interrupts workflow. |
| Many messages are low‐value interrupts | 3 | 0 | 3 | CM is useful for handover between clinicians, but often it slows down the clinician when they are used for information‐related low‐value/noncritical messages between nurses and clinicians |
| Lack of detailed response | 0 | 3 | 3 | Specific messages regarding response to care is required most times. For example, acknowledged is not a favorable response. |
| Technical issues | 2 | 0 | 2 | I find CM very useful. We have had multiple issues with our Blackberry this month, and CM was not working. When it is up and running, however, it is a wonderful tool. |
| Discrepancy in perceived urgency | 2 | 0 | 2 | Discrepancy between what nurses find urgent and what we find urgent. |
DISCUSSION
We describe an implementation of a system to improve clinical communication in hospitals. The system was highly used and was perceived to improve communication by both nurses and physicians. Specifically, users found that the system increased efficiency, accountability, timeliness, and collaboration, but that there were issues with message clarity for complex medical issues.
Other systems and approaches have been implemented to improve communication. These included the use of alphanumeric pagers, e‐mail, secure texting, and smartphones. There is evidence that more advanced systems can improve efficiency for senders.[16] A recent randomized trial of secure text messaging found that it was perceived to be more efficient than paging, but overall usage was low and inconsistent.[8] There is also evidence that smartphones may increase interruptions, worsen interprofessional relationships, and cause issues with professional behavior.[10] Unfortunately, there are a limited number of interventions that improve communication, with some improving efficiency but none demonstrating improved patient‐oriented outcomes.[16, 17] This study evaluated a novel system, with functionality to link communication to patients, and created a system that aligned with the workflow of the clinicians. Messages were linked to the patient, not the sender or receiver, so other clinicians in the patient's circle of care could easily view the communication. Moreover, the system was designed to improve message response rates and allow for nonurgent messages.
Our communication system uses standard, commercially available components (smartphones, SMS), and relatively basic functionality (handover, secure messaging). Important findings are that the current system of paging can be transformed to a more efficient system that users will readily adopt. We found positive effects with components of the system. It appeared to improve efficiency and increase accountability. Accountability is crucial and moves from undocumented conversation to fully documented details of interactions. This can be used for both incident review and to review for quality improvement.
Using the system, physicians perceived that they were bothered by low‐value information, whereas nurses perceived a lack of response, and both found that the system was not ideal for complex messages. The mismatch between what physicians and nurses perceive as important has been attributed to their different timeframes and context.[18] For nurses with an upcoming change of shift, they wanted resolution of issues before handover. A physician on a different ward may not appreciate the context of a nurse having to directly interact with an irate family member. These different perceptions likely contributed to the lack of response to 8.6% of text messages. This is still better than other systems, such as paging, which can be as high as 33%.[10] For nonurgent items, clinicians would ideally check and clear items regularly from the system using a desktop computer, responding within the allotted timeframe. Unfortunately, this never became part of routine physician workflow, likely due to their busy workload, so many physicians would only respond when items became overdue. However, having a method to deal with nonurgent messages may have prevented some interruptions during protected educational times of trainees. The system was also not ideal for urgent or complex items. Complex items can be difficult to convey using the rarified communication medium of text messages.[19, 20] Urgent or complex issues are likely best resolved with a face‐to‐face or telephone conversation.
There are several limitations in our study that should be considered when interpreting the results. It is a study of usage and perceptions after implementation. Although more rigorous study is required to evaluate the effects, we see this as a first step in process improvement. Future research should measure the impact on improving patient care of this system and on patient outcomes such as adverse events. The study and intervention was limited to general internal medicine wards in 2 academic hospital settings where there are frequent rotations of medical personnel. The findings may not be generalizable to other hospital settings.
Future directions should be to further improve on the communication system and to educate and train staff on how to effectively communicate. Survey results showed that although users perceived increased efficiency, there was still significant opportunity to improve. One way to improve would be to have a mobile application in which physicians can easily review nonurgent items. Improvements could also be realized by educating clinicians on the use of the system and providing immediate feedback. Providing feedback to physicians on how well they respond could address nurses' issues around lack of timely response. By creating consensus between nurses and physicians on what is of high and low value to communicate could increase satisfaction for all users.
In summary, we present the usage and perceptions of a system designed to improve hospital communication. We found that there was high uptake, and that users perceived it to improve efficiency, collaboration, and accountability, but it may not be useful for communicating complex issues.
ACKNOWLEDGEMENTS
The authors acknowledge the nurses, physicians, residents, and other health professions on the general internal medicine ward for their patience and support as we continue to try to innovate. The authors also acknowledge the members of the information systems department (Shared Information Management Systems, University Health Network) who helped to support the Communication System, and the software developer, QRS, that helped to codevelop the software system.
Disclosures: The hospital was in a codevelopment agreement that has since terminated. No researcher or hospital received any funds from private industry for any purpose including personal or research. The authors report no conflicts of interest.
Previous studies have advocated the importance of effective communication between clinicians as a critical component in the provision of high‐quality patient care.[1, 2, 3, 4] There is increasing interest in the use of information and communication technologies to improve how clinicians communicate in hospital settings. A number of hospitals have implemented different solutions to improve communication. These solutions include alphanumeric pagers,[5] smartphones,[6] e‐mail,[7] secure text messaging,[8] and a Web‐based interdisciplinary communication tool.[9]
These systems have different limitations that render them inefficient and likely inhibit collaborative care. Current systems, such as pagers, rely on the sender to ensure the message was received and are successful in delivering messages approximately 67% of the time.[5, 9, 10] Although alphanumeric pagers and secure text messaging can increase the likelihood of delivery, these messages are often isolated and not easily viewable by the whole care team.[11] Improved systems should also reduce unnecessary interruptions by providing support for both urgent and delayed messages. Finally, messages should be stored and retrievable to enable increased accountability and allow for review for quality improvement initiatives.
It is also important to consider the unintended consequences of technology implementations.[12] Moving communication to text messages and smartphones has the potential to reduce interprofessional relations and can increase confusion if used for complex issues.[10, 13] In this article, we present a system designed to improve interprofessional communication on general internal medicine wards by incorporating these desired features and describe the usage and attitudes toward the system, specifically assessing for effects on multiple domains including efficiency, interprofessional collaboration, and relationships.
METHODS
Research Question
Will nurses and physicians use a system designed to improve interprofessional communication and will they perceive it to be effective and improve workflow?
Setting
The study took place on the general internal medicine wards at Toronto General Hospital and Toronto Western Hospital, 2 large academic teaching hospitals. There are several general internal medicine wards at each site with approximately 80 beds at each site. At each site there are 4 clinical teaching units and 1 hospitalist team. The study was approved by the research ethics board at the University Health Network.
Intervention
To address issues with communication, we developed a systemClinical Message (CM)that included 2 main components: a physician handover tool and secure messaging module. The focus of CM was to improve communication and information flow among different healthcare providers (physicians, nurses, pharmacists, social workers and therapists) through a secure, shared platform.
Physician Handover
The physician handover tool was designed to facilitate the physician handover process at shift change and is used as a patient rounding tool for day‐to‐day management of patients. It is also accessed by nurses and other clinicians to view the physicians' notes and to stay informed on the overall care plan. The tool contains standard elements including a list of patients with the following information on each patient: demographics, diagnosis, code status, medical history, active issues, and discharge plans (Figure 1).

Secure Messaging
Secure messaging was designed around our dominant communication: nurses sending messages to physicians who would then respond. Nurses and other health professionals sent messages to the medical teams by accessing CM, selecting the appropriate patient, and filling out a message template. The system automatically populated the To field with the team assigned to the selected patient. Messaging for each team was centralized around a single team smartphone that was carried 24 hours a day, 7 days a week by a physician on that team. This removed the guesswork of trying to identify the individual physician responsible for that patient. For each message, a subject or issue and content were entered (Figure 2). Logic was also incorporated to reduce the amount of unnecessary interruptions. Senders would choose to send the message immediately as an interrupt message (urgent) for urgent/time sensitive issues or as an allow time to respond message (delayed). For the latter, the message was posted to the system where physicians could check and answer them. Interrupt messages were sent to the team smartphone using the Short Message Service (SMS) protocol. To try and ensure the communication loop on any issues was closed, when a message requested a response and did not receive it, the system sent another message. For urgent messages, a repeat message was initiated after 15 minutes. For delayed messages, the sender defined when they needed a response, typically within 2 to 8 hours. Senders were also able to select the mode of response that would best meet their needs from a workflow perspective: call back, text reply, or to specify that a reply was not required. Senders were also able to verify if the messages were received by the physician's smartphone. Physicians could view the messages within CM and reply. For messages that went to their team smartphone, physicians could respond from the smartphone through a secure Web link.

Because the messages were linked to the patients, they were visible to the entire care team, not just the message sender and recipient. If the care of the patient was transferred from 1 clinician to the next, the new clinician could easily review prior messages to understand recent patient events. The system was accessible through a browser on the intranet. The system regularly pulled patient demographic details such as name, age, medical record number, and location from our electronic medical record through a 1‐way interface. Information from this communication system was not considered part of the medical record but was retrievable.
The system was introduced as the new standard method of communication for nurses to reach physicians for all of the general internal medicine wards and for all medical teams at site 1 on May 2, 2011 and site 2 on June 6, 2011. The system replaced a text‐based Web‐paging system and supplemented the numeric pager carried by residents. Initial training of a half hour was provided to all nurses and residents.
Message Analysis for Usage Statistics
We analyzed messages created and sent via the CM system from May 2011 until August 2012. The extracted message information included date and time sent, issue, level of urgency, response type requested, roles of clinicians involved from the associated team, hospital site (senders and receivers), and message details. The following inclusion criteria were used for the analyses: (1) the senders and receivers of the messages could not be CM support staff, and (2) the messages sent were intended for the team smartphones used by the respective medical teams, not individual clinicians. Descriptive statistics and frequency analysis were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and IBM SPSS (IBM, Armonk, NY).
Survey
Development of the Survey
We used standard methods to develop a survey to assess staff perceptions on the impact of the new communication system. Relevant questionnaire items were compiled from a systematic review of the literature for communication surveys and communication issues that included the following domains: efficiency, accountability, accuracy, collaboration, timeliness, richness of the communication medium, and impact on interprofessional relationships and verbal communication.[10, 14, 15] We carried out pilot testing with 5 nurses and physicians, and modified the questionnaires based on their feedback.
Sampling and Data Collection of the Survey
Survey participants consisted of 2 groups of clinicians: (1) medical trainees that included medical residents, medical interns, and clinical fellows, and (2) nursing staff that included part‐time and full‐time nurses. To qualify for inclusion, participants had to have used the CM system for at least a month prior to administration of the questionnaire.
Data Analysis
Responses were recorded into an Excel spreadsheet that was imported into SPSS for analysis. Categorical variables were described using proportions. Survey comments were grouped into common themes, and themes mentioned by more than 1 respondent were reported.
RESULTS
Usage Analysis
A total of 60,969 messages were sent using CM between May 2, 2011 and August 19, 2012. On average, a team would receive 14.8 messages per day. Of all messages, 76.5% requested a text reply, 7.7% requested a call‐back, and 15.7% did not request a response. More than two‐thirds of messages at both hospitals were sent as immediate. Of the nonurgent messages, 86% were not replied to within the desired time, requiring a repeat message to be sent. Examples of different types of messages are shown in Table 1.
| Sender | Issue | Details | Priority | Desired Response Type | Time Created | Time Sent | Reply | Time Replied |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Nurse | Vital sign | Pt's BP is 182/95, HR is 108 now. Previous at 0800 was 165/78; HR was 99. PT is not on antihypertensive meds. | Allow time to respond (23:00) | Text reply | 21:43 | 23:02 | OK. Will assess. | 23:03 |
| Nurse | NG tube | NG tube is in place. Can you please enter portable chest x‐ray to check placement ASAP? | Immediate | Text reply | 16:58 | 16:58 | Will do. | 17:00 |
| Nurse | Bloodwork | Pt creat=216. Pt has NS @ 75 cc/hr. Pt has noted crackles throughout lung fields and has productive cough; eating and drinking well. Would you like it continued as well? Pt O2Sat 93% RA; would you like 4 L of O2 continued? Pls call for telephone order. | Immediate | Call back | 12:53 | 13:04 | Dealt with it on phone. | 13:05 |
| Nurse | Pain control | Hello! Pt has been getting 1 mg hydromorphone IV q 1 hr and pain is still not controlled. Pt remains awake and alert. Thanks! | Immediate | Info only | 15:41 | 15:41 | Thank you. | 15:42 |
For messages requesting a text reply, 8.6% did not receive a reply. The median response time was 2.3 minutes (interquartile range of 5.8 minutes), but some messages did not receive a response even after a week, which skewed the distribution of response times. For those messages that did receive a reply, 68.9% of them were responded to within 5 minutes, and 84.5% were responded to within 15 minutes. Messages were predominantly received between 9 am and midnight (see Supporting Figure 1 in the online version of this article). Because the sending of some messages was delayed, there appeared to be fewer messages received during protected educational times (89 am and 121 pm) as well as between midnight and 7 am compared to other times.
Survey Results
Between April 2013 and June 2013, 82 of 86 medical trainees (95.3%) and 83 of 116 nurses (71.6%) completed the survey, for an overall response rate of 81.7%. Clinicians perceived that CM appeared to have a positive impact on efficiency. In particular, 82.8% of physicians and 78.3% of nurses agreed or strongly agreed that CM helped speed up daily work tasks (Table 2). The majority of physicians and nurses agreed that the system increased accountability, increased timeliness of communication, and improved interprofessional relationships. It was not seen to be effective for communicating complex patient issues.
| No. of Subitems in Survey | Physician (% Agree, Strongly Agree), n=82 | Nurse (% Agree, Strongly Agree), n=83 | |
|---|---|---|---|
| |||
| Positive impact on efficiency. | 7 | 58.9% | 66.6% |
| The CM system helps speed up my daily work tasks. | 82.8% | 78.3% | |
| Positive impact on physician‐nurse collaboration. | 6 | 55.3% | 58.5% |
| The CM system increases the amount of communication between nurses and physicians. | 50.6% | 67.1% | |
| Improved timeliness of communication. | 5 | 54.2% | 50.5% |
| Communication through the CM system helps me resolve patient issues within the appropriate time frames. | 66.7% | 55.6% | |
| Increased accountability. | 2 | 67.1% | 73.2% |
| Improved accuracy of communications. | 3 | 41.6% | 50.7% |
| Improved interprofessional relationships. | 2 | 62.2% | 53.6% |
| Increased verbal communications. | 2 | 35.1% | 25.3% |
| Richness of the communication medium. | 6 | 40.7% | 48.3% |
| I find the CM system useful for communicating complex patient issues. | 35.8% | 26.3% | |
| I would prefer CM over standard hospital communication methods such as numeric paging. | 1 | 68.3% | 76.5% |
| I enjoy using the CM system for clinical communication on the wards. | 1 | 63.0% | 79.0% |
| Communication through the CM system helps to reduce interruptions for physicians. | 1 | 45.7% | |
Survey comments revealed that nurses perceived a lack of desired response, whereas physicians noted being interrupted with low‐value information through the system (Table 3). Both commented that further functionality, such as an active message stream, would be of benefit. Difficulty in communicating complex issues was also noted.
| Issue | Occurrences | Example | ||
|---|---|---|---|---|
| MD | RN | Total | ||
| ||||
| Lack of response | 1 | 10 | 11 | It depends if they respond quickly or not. A few times I send the 2nd message to remind them of the issue. I also spend more time to check if they answer it or not. I even call their Blackberries at last to get a response. |
| Message stream | 3 | 4 | 7 | I wish that I could see follow‐up messages after my initial reply (ie, it would be nice to have an open message stream). |
| Difficult to communicate complex issues | 1 | 5 | 6 | Difficult to communicate complex issues. Takes a lot of time to respond, and it becomes inefficient when responding to nonurgent CM because it interrupts workflow. |
| Many messages are low‐value interrupts | 3 | 0 | 3 | CM is useful for handover between clinicians, but often it slows down the clinician when they are used for information‐related low‐value/noncritical messages between nurses and clinicians |
| Lack of detailed response | 0 | 3 | 3 | Specific messages regarding response to care is required most times. For example, acknowledged is not a favorable response. |
| Technical issues | 2 | 0 | 2 | I find CM very useful. We have had multiple issues with our Blackberry this month, and CM was not working. When it is up and running, however, it is a wonderful tool. |
| Discrepancy in perceived urgency | 2 | 0 | 2 | Discrepancy between what nurses find urgent and what we find urgent. |
DISCUSSION
We describe an implementation of a system to improve clinical communication in hospitals. The system was highly used and was perceived to improve communication by both nurses and physicians. Specifically, users found that the system increased efficiency, accountability, timeliness, and collaboration, but that there were issues with message clarity for complex medical issues.
Other systems and approaches have been implemented to improve communication. These included the use of alphanumeric pagers, e‐mail, secure texting, and smartphones. There is evidence that more advanced systems can improve efficiency for senders.[16] A recent randomized trial of secure text messaging found that it was perceived to be more efficient than paging, but overall usage was low and inconsistent.[8] There is also evidence that smartphones may increase interruptions, worsen interprofessional relationships, and cause issues with professional behavior.[10] Unfortunately, there are a limited number of interventions that improve communication, with some improving efficiency but none demonstrating improved patient‐oriented outcomes.[16, 17] This study evaluated a novel system, with functionality to link communication to patients, and created a system that aligned with the workflow of the clinicians. Messages were linked to the patient, not the sender or receiver, so other clinicians in the patient's circle of care could easily view the communication. Moreover, the system was designed to improve message response rates and allow for nonurgent messages.
Our communication system uses standard, commercially available components (smartphones, SMS), and relatively basic functionality (handover, secure messaging). Important findings are that the current system of paging can be transformed to a more efficient system that users will readily adopt. We found positive effects with components of the system. It appeared to improve efficiency and increase accountability. Accountability is crucial and moves from undocumented conversation to fully documented details of interactions. This can be used for both incident review and to review for quality improvement.
Using the system, physicians perceived that they were bothered by low‐value information, whereas nurses perceived a lack of response, and both found that the system was not ideal for complex messages. The mismatch between what physicians and nurses perceive as important has been attributed to their different timeframes and context.[18] For nurses with an upcoming change of shift, they wanted resolution of issues before handover. A physician on a different ward may not appreciate the context of a nurse having to directly interact with an irate family member. These different perceptions likely contributed to the lack of response to 8.6% of text messages. This is still better than other systems, such as paging, which can be as high as 33%.[10] For nonurgent items, clinicians would ideally check and clear items regularly from the system using a desktop computer, responding within the allotted timeframe. Unfortunately, this never became part of routine physician workflow, likely due to their busy workload, so many physicians would only respond when items became overdue. However, having a method to deal with nonurgent messages may have prevented some interruptions during protected educational times of trainees. The system was also not ideal for urgent or complex items. Complex items can be difficult to convey using the rarified communication medium of text messages.[19, 20] Urgent or complex issues are likely best resolved with a face‐to‐face or telephone conversation.
There are several limitations in our study that should be considered when interpreting the results. It is a study of usage and perceptions after implementation. Although more rigorous study is required to evaluate the effects, we see this as a first step in process improvement. Future research should measure the impact on improving patient care of this system and on patient outcomes such as adverse events. The study and intervention was limited to general internal medicine wards in 2 academic hospital settings where there are frequent rotations of medical personnel. The findings may not be generalizable to other hospital settings.
Future directions should be to further improve on the communication system and to educate and train staff on how to effectively communicate. Survey results showed that although users perceived increased efficiency, there was still significant opportunity to improve. One way to improve would be to have a mobile application in which physicians can easily review nonurgent items. Improvements could also be realized by educating clinicians on the use of the system and providing immediate feedback. Providing feedback to physicians on how well they respond could address nurses' issues around lack of timely response. By creating consensus between nurses and physicians on what is of high and low value to communicate could increase satisfaction for all users.
In summary, we present the usage and perceptions of a system designed to improve hospital communication. We found that there was high uptake, and that users perceived it to improve efficiency, collaboration, and accountability, but it may not be useful for communicating complex issues.
ACKNOWLEDGEMENTS
The authors acknowledge the nurses, physicians, residents, and other health professions on the general internal medicine ward for their patience and support as we continue to try to innovate. The authors also acknowledge the members of the information systems department (Shared Information Management Systems, University Health Network) who helped to support the Communication System, and the software developer, QRS, that helped to codevelop the software system.
Disclosures: The hospital was in a codevelopment agreement that has since terminated. No researcher or hospital received any funds from private industry for any purpose including personal or research. The authors report no conflicts of interest.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277–286.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370–376.
- , , , et al. Gaps in pediatric clinician communication and opportunities for improvement. J Healthc Qual. 2008;30(5):43–54.
- , , , , . Quality in Australian health care study. Med J Aust. 1996;164(12):754.
- , , , . Implementation and evaluation of an alphanumeric paging system on a resident inpatient teaching service. J Hosp Med. 2009;4(8):E34–E40.
- , , , et al. Demonstrating the BlackBerry as a clinical communication tool: a pilot study conducted through the Centre for Innovation in Complex Care. Healthc Q. 2008;11(4):94–98.
- , , , . The use of wireless email to improve healthcare team communication. J Am Med Inform Assoc. 2009;16(5):705–713.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9(9):573–578.
- , , , , . Beyond paging: building a web‐based communication tool for nurses and physicians. J Gen Intern Med. 2009;24(1):105–110.
- , , , et al. The intended and unintended consequences of communication systems on general internal medicine inpatient care delivery: a prospective observational case study of five teaching hospitals. J Am Med Inform Assoc. 2013;20(4):766–777.
- , , , et al. Improving hospital care and collaborative communications for the 21st century: key recommendations for general internal medicine. Interact J Med Res. 2012;1(2):e9.
- , , , , , . Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18(1):82–90.
- , , , et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed‐methods study. J Med Internet Res. 2011;13(3):e59.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Impact of communication medium on task performance and satisfaction: an examination of media‐richness theory. Inform Manag. 1999;35:295–312.
- , , , et al. Effects of clinical communication interventions in hospitals: a systematic review of information and communication technology adoptions for improved communication between clinicians. Int J Med Inform. 2012;81(11):723–732.
- , , , et al. Provider‐to‐provider electronic communication in the era of meaningful use: a review of the evidence. J Hosp Med. 2013;8(10):589–597.
- , , , et al. Perceptions of urgency: defining the gap between what physicians and nurses perceive to be an urgent issue. Int J Med Inform. 2013;82(5):378–386.
- , , , , , . Short message service or disService: issues with text messaging in a complex medical environment. Int J Med Inform. 2014;83(4):278–284.
- , , . Instant messaging at the hospital: supporting articulation work? Int J Med Inform. 2013;82(9):753–761.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277–286.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370–376.
- , , , et al. Gaps in pediatric clinician communication and opportunities for improvement. J Healthc Qual. 2008;30(5):43–54.
- , , , , . Quality in Australian health care study. Med J Aust. 1996;164(12):754.
- , , , . Implementation and evaluation of an alphanumeric paging system on a resident inpatient teaching service. J Hosp Med. 2009;4(8):E34–E40.
- , , , et al. Demonstrating the BlackBerry as a clinical communication tool: a pilot study conducted through the Centre for Innovation in Complex Care. Healthc Q. 2008;11(4):94–98.
- , , , . The use of wireless email to improve healthcare team communication. J Am Med Inform Assoc. 2009;16(5):705–713.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9(9):573–578.
- , , , , . Beyond paging: building a web‐based communication tool for nurses and physicians. J Gen Intern Med. 2009;24(1):105–110.
- , , , et al. The intended and unintended consequences of communication systems on general internal medicine inpatient care delivery: a prospective observational case study of five teaching hospitals. J Am Med Inform Assoc. 2013;20(4):766–777.
- , , , et al. Improving hospital care and collaborative communications for the 21st century: key recommendations for general internal medicine. Interact J Med Res. 2012;1(2):e9.
- , , , , , . Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18(1):82–90.
- , , , et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed‐methods study. J Med Internet Res. 2011;13(3):e59.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Impact of communication medium on task performance and satisfaction: an examination of media‐richness theory. Inform Manag. 1999;35:295–312.
- , , , et al. Effects of clinical communication interventions in hospitals: a systematic review of information and communication technology adoptions for improved communication between clinicians. Int J Med Inform. 2012;81(11):723–732.
- , , , et al. Provider‐to‐provider electronic communication in the era of meaningful use: a review of the evidence. J Hosp Med. 2013;8(10):589–597.
- , , , et al. Perceptions of urgency: defining the gap between what physicians and nurses perceive to be an urgent issue. Int J Med Inform. 2013;82(5):378–386.
- , , , , , . Short message service or disService: issues with text messaging in a complex medical environment. Int J Med Inform. 2014;83(4):278–284.
- , , . Instant messaging at the hospital: supporting articulation work? Int J Med Inform. 2013;82(9):753–761.
© 2014 Society of Hospital Medicine

Impaired Arousal and Mortality
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).
| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.
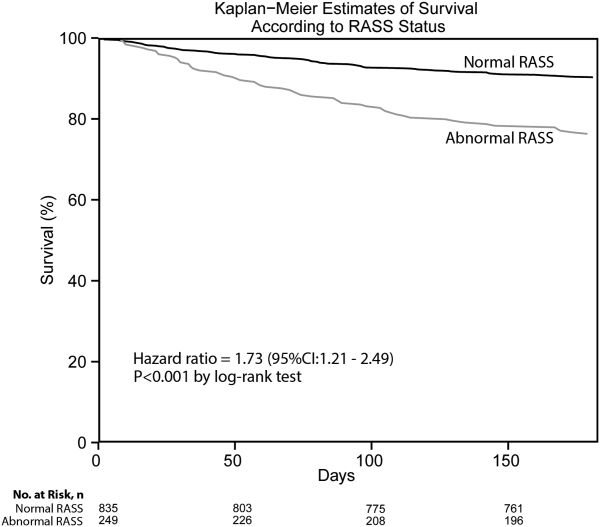
Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).

| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.


Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).

| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.


Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
© 2014 Society of Hospital Medicine
Electronic Cigarettes
Electronic cigarettes are increasingly prevalent battery‐operated devices that heat a solution to generate an inhalable nicotine‐containing aerosol.[1, 2] Despite a diverse array of devices on the market, the US Food and Drug Administration (FDA) has only recently proposed expanding its regulatory ability to include electronic cigarettes.[3] States, municipalities, and institutions have enacted variable regulations on electronic nicotine delivery systems.[4, 5] Advocates of electronic cigarettes propose that they are a less‐toxic alternative to tobacco cigarettes, with potential for use as a nicotine replacement therapy (NRT).[6, 7, 8] Opponents argue that electronic cigarettes may undermine tobacco cessation goals and potentially expose nonusers to secondhand nicotine vapor.[9, 10]
Hospital providers frequently care for nicotine‐dependent patients.[11] We investigated inpatient healthcare providers' knowledge, perceptions, and experience with electronic cigarettes, with the goals of informing educational efforts and guiding policy decisions around hospital‐based use of electronic nicotine delivery systems.
METHODS
The study was conducted at a 183‐bed urban safety‐net medical center affiliated with a residency training program using a cross‐sectional survey to query a diverse array of inpatient providers (Table 1). Respondents who had not cared for an inpatient in the past 5 years were excluded. Surveys were designed based on prior literature, personal experience, and expert suggestions.[12] Surveys were disseminated in March 2014 via e‐mail, with embedded informed consent and a link that connected anonymously to the online survey (Qualtrics, Provo, UT). We did not collect unique identifiers and offered no incentive for participation. Data were downloaded to a secure database and analyzed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and GraphPad Prism version 6.04 (GraphPad Software, Inc., La Jolla, CA). The study was approved by the institutional review board.
| Group (No.) | Do you know what an electronic cigarette is?* | Has a hospitalized patient ever asked you if he or she could use an electronic cigarette on hospital grounds?* | Do you see electronic cigarettes as a nicotine replacement option for hospitalized patients? | If you were caring for a patient, would you be okay with the patient using an electronic cigarette while hospitalized? | If you were hospitalized in a shared hospital room, would you be okay with your roommate using an electronic cigarette? | Should electronic cigarettes be banned from healthcare settings? | Should electronic cigarettes be banned in the same locations as traditional cigarettes? | Should electronic cigarettes be regulated by the US Food and Drug Administration? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Faculty MD (32) | 96.9% | 12.5% | 28.1% | 34.4% | 12.5% | 37.5% | 53.1% | 100% |
| Resident MD (33) | 97.0% | 9.1% | 27.3% | 45.5% | 24.2% | 45.5% | 36.4% | 93.9% |
| Registered nurse (35) | 94.3% | 42.9% | 25.7% | 28.6% | 25.7% | 40.0% | 54.3% | 68.6% |
| Rehabilitation staff (18) | 88.9% | 11.1% | 11.1% | 5.6% | 5.6% | 66.7% | 55.6% | 88.9% |
| Social worker (6) | 100% | 33.3% | 16.7% | 16.7% | 0.0% | 50.0% | 50.0% | 83.3% |
| Pharmacist (18) | 100% | 5.6% | 11.1% | 27.8% | 22.2% | 61.1% | 50.0% | 83.3% |
| All respondents (142) | 95.8% | 19.0% | 22.5% | 30.3% | 18.3% | 47.2% | 49.3% | 86.6% |
RESULTS
Study Participants
There were 242 survey respondents (response rate of 41%), of whom 100 were excluded based on study criteria. The median age of the 142 included participants was 34.0 years. There were significantly more female respondents (69%, P=0.001, 2 test), equally over‐represented across all inpatient provider groups. Only 1.4% of all respondents reported personal active tobacco use, whereas 24.6% of study participants reported prior tobacco use. Tobacco use history was similar across inpatient provider groups and gender. Respondents over 50 years of age demonstrated a higher rate of current or prior tobacco use compared with participants from other age groups combined (53% vs 23%, P=0.01, 2 test).
Electronic Cigarette Familiarity and Patient Requests
Of the participants, 95.8% reported familiarity with electronic cigarettes, without differences across age or gender. Of all of the providers, 19.0% reported being asked by a hospitalized patient for permission to use an electronic cigarette in the hospital. Registered nurses were significantly more likely to have been asked by patients compared to all other study participants (43% vs 11%, P0.001, 2 test).
Electronic Cigarettes as NRT
Whereas 22.5% of study participants felt that electronic cigarettes could serve as a viable in‐hospital NRT, 48.6% felt that electronic cigarettes should not be used, and 28.9% were unsure (Table 1), irrespective of demographics or personal tobacco use history. One‐third of respondents would allow an inpatient under their care to use an electronic cigarette. Groups most likely to permit use were faculty (34.4%) and resident physicians (45.5%), though this difference was not statistically significant.
Perspectives on Exposure
Only 18.3% of study participants would agree to share a hospital room with a patient using an electronic cigarette. Of all participants, 47.2% and 49.3% felt that electronic cigarettes should be banned from healthcare settings and from the same locations as traditional cigarettes, respectively. There were no significant differences in perspectives when stratified by age or gender. Current or prior tobacco users were more likely to be accepting of the use of electronic cigarettes in healthcare settings compared to nonusers (50% vs 29%, P=0.02, Fisher exact test).
FDA Regulation
Of all study participants, 86.6% responded that electronic cigarettes should be regulated by the FDA. Physicians most strongly agreed with this statement compared with all other provider groups (97% vs 78%, P=0.004, 2 test). Conversely, registered nurses were least likely to feel that electronic cigarettes should be FDA‐regulated compared to all other provider groups (69% vs 93%, P0.005, 2 test).
DISCUSSION
Our study is the first to provide hospital‐based providers' experience and perspectives surrounding electronic cigarette use. The vast majority of participants reported familiarity with electronic cigarettes, consistent with prior findings.[13] Though electronic cigarettes have yet to achieve a use in the hospital setting, 19% of our respondents reported receiving requests from hospitalized patients to use these devices. With increasing patient demand for electronic cigarettes, hospitals will need to update their tobacco policies to include these novel devices as well as target educational efforts toward front‐line providers, such as nurses, who receive the majority of requests.
Participants perceived traditional cigarettes to be significantly more harmful than electronic cigarettes, while established forms of NRT were felt to be less harmful than electronic cigarettes (data not shown). Concern about the health effects of electronic cigarettes is further reflected in providers' hesitancy to view these devices as an NRT option in the hospital, reluctance to consider sharing a room with an electronic cigarette user, and near majority opinion that electronic cigarettes should be banned from healthcare settings altogether. Current regulation by the US Department of Transportation bans electronic cigarette use on airplanes, whereas a host of states currently ban electronic cigarette use in similarly enclosed spaces such as correctional facilities and commuter trains.[14] More knowledge is needed on the health effects of electronic cigarettes on the primary user, secondhand exposure range, and their potential to aid in short‐ and long‐term nicotine cessation before providers and hospitals can make an informed risk‐benefit analysis for appropriate inpatient use. As current or past tobacco users were more accepting of the use of electronic cigarettes in hospital settings, these providers' opinions should be sought for a unique understanding of the interplay between electronic cigarettes and the healthcare environment.
Concern over the unknown safety effects can also be seen in the overwhelming provider support for FDA regulation. Healthcare advocacy groups, such as the American Heart Association, the American Lung Association, and the Legacy Foundation already support federal regulation.[15, 16, 17] FDA regulation may lead to the ability to standardize device content, regulate purchasing and marketing requirements, and ensure that claims to health effects are supported by scientific evidence, though agency involvement may also slow the process of integration into hospital use. Perhaps reflective of the immediacy of the problem, nurses who receive the majority of requests for electronic cigarettes from patients are least likely to want FDA regulation. Until more is known, patients and staff may benefit from pairing vaporizing patients in shared rooms or providing users with designated inhaling spaces.
Nicotine addiction is a strong driving force and, due to a strict no‐smoking policy at our institution, we have witnessed patients making unsafe decisions to leave the hospital (in some cases against medical advice) in an effort to continue smoking. Patients may be starting to look toward electronic cigarettes as an NRT option that more closely satisfies nicotine cravings as well as the ritualistic and tactile components of cigarette use. Electronic cigarettes could have the potential to act as a harm reduction method for nicotine‐dependent inpatients by decreasing the nicotine‐withdrawal related impetus for unsafe hospital discharges. Institutions should take this into account when formulating new policy.
Our study has several limitations. First, it was a single‐center study that may not be representative of provider perspectives at other institutions. Second, the survey was a cross‐sectional sample, missing providers who did not receive the e‐mail during the enrollment period. Third, responses may not accurately reflect perspectives of smaller responding groups such as social workers. Fourth, the survey did not include all types of physicians who deal with smoking cessation, though internal and family medicine physicians provide the majority of care for hospitalized patients at our institution. Fifth, we recorded self‐reported familiarity with electronic cigarettes and did not formally test providers' knowledge of the subject.
Our study provides new perspectives and data on electronic cigarettes to inform future research as well as hospital and healthcare policy. Hospitals should educate patients and front‐line providers around the paucity of health information on these novel devices, while formulating policy that acknowledges patient demand for electronic cigarettes and their potential for cessation therapy and harm reduction. Further research should focus on the effects of nicotine vapor inhalation on patients, the consequences of secondhand nicotine vapor, and the potential for electronic nicotine delivery systems to act as a novel NRT for hospital use.
Disclosure
Nothing to report.
- , , . E‐cigarettes: a scientific review. Circulation. 2014;129:1972–1986.
- , , , , . E‐Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766.
- U.S. Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e‐cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394667.htm. Accessed May 5, 2014.
- . Electronic cigarettes: smoke‐free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18.
- American Nonsmokers' Rights Foundation. US state and local laws regulating use of electronic cigarettes. Available at: http://www.no‐smoke.org/pdf/ecigslaws.pdf. Accessed September 2, 2014.
- , . Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028.
- , , , , , . Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health. 2011;11:786.
- , , , , . Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109(9):1531–1540.
- , . The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686.
- . Promise and peril of e‐Cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136.
- , , , et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine Tob Res. 2014;16(11):1512–1517.
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions From the Global Adult Tobacco Survey (GATS). 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. Accessed April 23, 2014.
- , , . Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683.
- U.S. Department of Transportation. DOT policy on e‐cigarettes. Available at: http://www.dot.gov/sites/dot.gov/files/docs/PolicyOnECigarettes.pdf. Accessed September 2, 2014.
- American Heart Association. AHA: E‐cigarettes threaten to addict next generation of smokers; regulation, further study needed. Available at: http://blog.heart.org/aha‐e‐cigarettes‐threaten‐to‐addict‐next‐generation‐of‐smokers‐regulation‐further‐study‐needed/. Accessed August 25, 2014.
- American Lung Association. American Lung Association statement on e‐cigarettes. Available at: http://www.lung.org/stop‐smoking/tobacco‐control‐advocacy/federal/e‐cigarettes.html. Accesses August 25, 2014.
- Legacy for Health. E‐cigarette policy: the FDA should promptly exercise regulatory authority and over e‐cigarettes. Available at: http://www.legacyforhealth.org/content/download/3962/56088/version/1/file/LEG‐Policy_Statement‐ECigarette‐JAN2014.pdf. Accessed August 25, 2014.
Electronic cigarettes are increasingly prevalent battery‐operated devices that heat a solution to generate an inhalable nicotine‐containing aerosol.[1, 2] Despite a diverse array of devices on the market, the US Food and Drug Administration (FDA) has only recently proposed expanding its regulatory ability to include electronic cigarettes.[3] States, municipalities, and institutions have enacted variable regulations on electronic nicotine delivery systems.[4, 5] Advocates of electronic cigarettes propose that they are a less‐toxic alternative to tobacco cigarettes, with potential for use as a nicotine replacement therapy (NRT).[6, 7, 8] Opponents argue that electronic cigarettes may undermine tobacco cessation goals and potentially expose nonusers to secondhand nicotine vapor.[9, 10]
Hospital providers frequently care for nicotine‐dependent patients.[11] We investigated inpatient healthcare providers' knowledge, perceptions, and experience with electronic cigarettes, with the goals of informing educational efforts and guiding policy decisions around hospital‐based use of electronic nicotine delivery systems.
METHODS
The study was conducted at a 183‐bed urban safety‐net medical center affiliated with a residency training program using a cross‐sectional survey to query a diverse array of inpatient providers (Table 1). Respondents who had not cared for an inpatient in the past 5 years were excluded. Surveys were designed based on prior literature, personal experience, and expert suggestions.[12] Surveys were disseminated in March 2014 via e‐mail, with embedded informed consent and a link that connected anonymously to the online survey (Qualtrics, Provo, UT). We did not collect unique identifiers and offered no incentive for participation. Data were downloaded to a secure database and analyzed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and GraphPad Prism version 6.04 (GraphPad Software, Inc., La Jolla, CA). The study was approved by the institutional review board.
| Group (No.) | Do you know what an electronic cigarette is?* | Has a hospitalized patient ever asked you if he or she could use an electronic cigarette on hospital grounds?* | Do you see electronic cigarettes as a nicotine replacement option for hospitalized patients? | If you were caring for a patient, would you be okay with the patient using an electronic cigarette while hospitalized? | If you were hospitalized in a shared hospital room, would you be okay with your roommate using an electronic cigarette? | Should electronic cigarettes be banned from healthcare settings? | Should electronic cigarettes be banned in the same locations as traditional cigarettes? | Should electronic cigarettes be regulated by the US Food and Drug Administration? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Faculty MD (32) | 96.9% | 12.5% | 28.1% | 34.4% | 12.5% | 37.5% | 53.1% | 100% |
| Resident MD (33) | 97.0% | 9.1% | 27.3% | 45.5% | 24.2% | 45.5% | 36.4% | 93.9% |
| Registered nurse (35) | 94.3% | 42.9% | 25.7% | 28.6% | 25.7% | 40.0% | 54.3% | 68.6% |
| Rehabilitation staff (18) | 88.9% | 11.1% | 11.1% | 5.6% | 5.6% | 66.7% | 55.6% | 88.9% |
| Social worker (6) | 100% | 33.3% | 16.7% | 16.7% | 0.0% | 50.0% | 50.0% | 83.3% |
| Pharmacist (18) | 100% | 5.6% | 11.1% | 27.8% | 22.2% | 61.1% | 50.0% | 83.3% |
| All respondents (142) | 95.8% | 19.0% | 22.5% | 30.3% | 18.3% | 47.2% | 49.3% | 86.6% |
RESULTS
Study Participants
There were 242 survey respondents (response rate of 41%), of whom 100 were excluded based on study criteria. The median age of the 142 included participants was 34.0 years. There were significantly more female respondents (69%, P=0.001, 2 test), equally over‐represented across all inpatient provider groups. Only 1.4% of all respondents reported personal active tobacco use, whereas 24.6% of study participants reported prior tobacco use. Tobacco use history was similar across inpatient provider groups and gender. Respondents over 50 years of age demonstrated a higher rate of current or prior tobacco use compared with participants from other age groups combined (53% vs 23%, P=0.01, 2 test).
Electronic Cigarette Familiarity and Patient Requests
Of the participants, 95.8% reported familiarity with electronic cigarettes, without differences across age or gender. Of all of the providers, 19.0% reported being asked by a hospitalized patient for permission to use an electronic cigarette in the hospital. Registered nurses were significantly more likely to have been asked by patients compared to all other study participants (43% vs 11%, P0.001, 2 test).
Electronic Cigarettes as NRT
Whereas 22.5% of study participants felt that electronic cigarettes could serve as a viable in‐hospital NRT, 48.6% felt that electronic cigarettes should not be used, and 28.9% were unsure (Table 1), irrespective of demographics or personal tobacco use history. One‐third of respondents would allow an inpatient under their care to use an electronic cigarette. Groups most likely to permit use were faculty (34.4%) and resident physicians (45.5%), though this difference was not statistically significant.
Perspectives on Exposure
Only 18.3% of study participants would agree to share a hospital room with a patient using an electronic cigarette. Of all participants, 47.2% and 49.3% felt that electronic cigarettes should be banned from healthcare settings and from the same locations as traditional cigarettes, respectively. There were no significant differences in perspectives when stratified by age or gender. Current or prior tobacco users were more likely to be accepting of the use of electronic cigarettes in healthcare settings compared to nonusers (50% vs 29%, P=0.02, Fisher exact test).
FDA Regulation
Of all study participants, 86.6% responded that electronic cigarettes should be regulated by the FDA. Physicians most strongly agreed with this statement compared with all other provider groups (97% vs 78%, P=0.004, 2 test). Conversely, registered nurses were least likely to feel that electronic cigarettes should be FDA‐regulated compared to all other provider groups (69% vs 93%, P0.005, 2 test).
DISCUSSION
Our study is the first to provide hospital‐based providers' experience and perspectives surrounding electronic cigarette use. The vast majority of participants reported familiarity with electronic cigarettes, consistent with prior findings.[13] Though electronic cigarettes have yet to achieve a use in the hospital setting, 19% of our respondents reported receiving requests from hospitalized patients to use these devices. With increasing patient demand for electronic cigarettes, hospitals will need to update their tobacco policies to include these novel devices as well as target educational efforts toward front‐line providers, such as nurses, who receive the majority of requests.
Participants perceived traditional cigarettes to be significantly more harmful than electronic cigarettes, while established forms of NRT were felt to be less harmful than electronic cigarettes (data not shown). Concern about the health effects of electronic cigarettes is further reflected in providers' hesitancy to view these devices as an NRT option in the hospital, reluctance to consider sharing a room with an electronic cigarette user, and near majority opinion that electronic cigarettes should be banned from healthcare settings altogether. Current regulation by the US Department of Transportation bans electronic cigarette use on airplanes, whereas a host of states currently ban electronic cigarette use in similarly enclosed spaces such as correctional facilities and commuter trains.[14] More knowledge is needed on the health effects of electronic cigarettes on the primary user, secondhand exposure range, and their potential to aid in short‐ and long‐term nicotine cessation before providers and hospitals can make an informed risk‐benefit analysis for appropriate inpatient use. As current or past tobacco users were more accepting of the use of electronic cigarettes in hospital settings, these providers' opinions should be sought for a unique understanding of the interplay between electronic cigarettes and the healthcare environment.
Concern over the unknown safety effects can also be seen in the overwhelming provider support for FDA regulation. Healthcare advocacy groups, such as the American Heart Association, the American Lung Association, and the Legacy Foundation already support federal regulation.[15, 16, 17] FDA regulation may lead to the ability to standardize device content, regulate purchasing and marketing requirements, and ensure that claims to health effects are supported by scientific evidence, though agency involvement may also slow the process of integration into hospital use. Perhaps reflective of the immediacy of the problem, nurses who receive the majority of requests for electronic cigarettes from patients are least likely to want FDA regulation. Until more is known, patients and staff may benefit from pairing vaporizing patients in shared rooms or providing users with designated inhaling spaces.
Nicotine addiction is a strong driving force and, due to a strict no‐smoking policy at our institution, we have witnessed patients making unsafe decisions to leave the hospital (in some cases against medical advice) in an effort to continue smoking. Patients may be starting to look toward electronic cigarettes as an NRT option that more closely satisfies nicotine cravings as well as the ritualistic and tactile components of cigarette use. Electronic cigarettes could have the potential to act as a harm reduction method for nicotine‐dependent inpatients by decreasing the nicotine‐withdrawal related impetus for unsafe hospital discharges. Institutions should take this into account when formulating new policy.
Our study has several limitations. First, it was a single‐center study that may not be representative of provider perspectives at other institutions. Second, the survey was a cross‐sectional sample, missing providers who did not receive the e‐mail during the enrollment period. Third, responses may not accurately reflect perspectives of smaller responding groups such as social workers. Fourth, the survey did not include all types of physicians who deal with smoking cessation, though internal and family medicine physicians provide the majority of care for hospitalized patients at our institution. Fifth, we recorded self‐reported familiarity with electronic cigarettes and did not formally test providers' knowledge of the subject.
Our study provides new perspectives and data on electronic cigarettes to inform future research as well as hospital and healthcare policy. Hospitals should educate patients and front‐line providers around the paucity of health information on these novel devices, while formulating policy that acknowledges patient demand for electronic cigarettes and their potential for cessation therapy and harm reduction. Further research should focus on the effects of nicotine vapor inhalation on patients, the consequences of secondhand nicotine vapor, and the potential for electronic nicotine delivery systems to act as a novel NRT for hospital use.
Disclosure
Nothing to report.
Electronic cigarettes are increasingly prevalent battery‐operated devices that heat a solution to generate an inhalable nicotine‐containing aerosol.[1, 2] Despite a diverse array of devices on the market, the US Food and Drug Administration (FDA) has only recently proposed expanding its regulatory ability to include electronic cigarettes.[3] States, municipalities, and institutions have enacted variable regulations on electronic nicotine delivery systems.[4, 5] Advocates of electronic cigarettes propose that they are a less‐toxic alternative to tobacco cigarettes, with potential for use as a nicotine replacement therapy (NRT).[6, 7, 8] Opponents argue that electronic cigarettes may undermine tobacco cessation goals and potentially expose nonusers to secondhand nicotine vapor.[9, 10]
Hospital providers frequently care for nicotine‐dependent patients.[11] We investigated inpatient healthcare providers' knowledge, perceptions, and experience with electronic cigarettes, with the goals of informing educational efforts and guiding policy decisions around hospital‐based use of electronic nicotine delivery systems.
METHODS
The study was conducted at a 183‐bed urban safety‐net medical center affiliated with a residency training program using a cross‐sectional survey to query a diverse array of inpatient providers (Table 1). Respondents who had not cared for an inpatient in the past 5 years were excluded. Surveys were designed based on prior literature, personal experience, and expert suggestions.[12] Surveys were disseminated in March 2014 via e‐mail, with embedded informed consent and a link that connected anonymously to the online survey (Qualtrics, Provo, UT). We did not collect unique identifiers and offered no incentive for participation. Data were downloaded to a secure database and analyzed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and GraphPad Prism version 6.04 (GraphPad Software, Inc., La Jolla, CA). The study was approved by the institutional review board.
| Group (No.) | Do you know what an electronic cigarette is?* | Has a hospitalized patient ever asked you if he or she could use an electronic cigarette on hospital grounds?* | Do you see electronic cigarettes as a nicotine replacement option for hospitalized patients? | If you were caring for a patient, would you be okay with the patient using an electronic cigarette while hospitalized? | If you were hospitalized in a shared hospital room, would you be okay with your roommate using an electronic cigarette? | Should electronic cigarettes be banned from healthcare settings? | Should electronic cigarettes be banned in the same locations as traditional cigarettes? | Should electronic cigarettes be regulated by the US Food and Drug Administration? |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Faculty MD (32) | 96.9% | 12.5% | 28.1% | 34.4% | 12.5% | 37.5% | 53.1% | 100% |
| Resident MD (33) | 97.0% | 9.1% | 27.3% | 45.5% | 24.2% | 45.5% | 36.4% | 93.9% |
| Registered nurse (35) | 94.3% | 42.9% | 25.7% | 28.6% | 25.7% | 40.0% | 54.3% | 68.6% |
| Rehabilitation staff (18) | 88.9% | 11.1% | 11.1% | 5.6% | 5.6% | 66.7% | 55.6% | 88.9% |
| Social worker (6) | 100% | 33.3% | 16.7% | 16.7% | 0.0% | 50.0% | 50.0% | 83.3% |
| Pharmacist (18) | 100% | 5.6% | 11.1% | 27.8% | 22.2% | 61.1% | 50.0% | 83.3% |
| All respondents (142) | 95.8% | 19.0% | 22.5% | 30.3% | 18.3% | 47.2% | 49.3% | 86.6% |
RESULTS
Study Participants
There were 242 survey respondents (response rate of 41%), of whom 100 were excluded based on study criteria. The median age of the 142 included participants was 34.0 years. There were significantly more female respondents (69%, P=0.001, 2 test), equally over‐represented across all inpatient provider groups. Only 1.4% of all respondents reported personal active tobacco use, whereas 24.6% of study participants reported prior tobacco use. Tobacco use history was similar across inpatient provider groups and gender. Respondents over 50 years of age demonstrated a higher rate of current or prior tobacco use compared with participants from other age groups combined (53% vs 23%, P=0.01, 2 test).
Electronic Cigarette Familiarity and Patient Requests
Of the participants, 95.8% reported familiarity with electronic cigarettes, without differences across age or gender. Of all of the providers, 19.0% reported being asked by a hospitalized patient for permission to use an electronic cigarette in the hospital. Registered nurses were significantly more likely to have been asked by patients compared to all other study participants (43% vs 11%, P0.001, 2 test).
Electronic Cigarettes as NRT
Whereas 22.5% of study participants felt that electronic cigarettes could serve as a viable in‐hospital NRT, 48.6% felt that electronic cigarettes should not be used, and 28.9% were unsure (Table 1), irrespective of demographics or personal tobacco use history. One‐third of respondents would allow an inpatient under their care to use an electronic cigarette. Groups most likely to permit use were faculty (34.4%) and resident physicians (45.5%), though this difference was not statistically significant.
Perspectives on Exposure
Only 18.3% of study participants would agree to share a hospital room with a patient using an electronic cigarette. Of all participants, 47.2% and 49.3% felt that electronic cigarettes should be banned from healthcare settings and from the same locations as traditional cigarettes, respectively. There were no significant differences in perspectives when stratified by age or gender. Current or prior tobacco users were more likely to be accepting of the use of electronic cigarettes in healthcare settings compared to nonusers (50% vs 29%, P=0.02, Fisher exact test).
FDA Regulation
Of all study participants, 86.6% responded that electronic cigarettes should be regulated by the FDA. Physicians most strongly agreed with this statement compared with all other provider groups (97% vs 78%, P=0.004, 2 test). Conversely, registered nurses were least likely to feel that electronic cigarettes should be FDA‐regulated compared to all other provider groups (69% vs 93%, P0.005, 2 test).
DISCUSSION
Our study is the first to provide hospital‐based providers' experience and perspectives surrounding electronic cigarette use. The vast majority of participants reported familiarity with electronic cigarettes, consistent with prior findings.[13] Though electronic cigarettes have yet to achieve a use in the hospital setting, 19% of our respondents reported receiving requests from hospitalized patients to use these devices. With increasing patient demand for electronic cigarettes, hospitals will need to update their tobacco policies to include these novel devices as well as target educational efforts toward front‐line providers, such as nurses, who receive the majority of requests.
Participants perceived traditional cigarettes to be significantly more harmful than electronic cigarettes, while established forms of NRT were felt to be less harmful than electronic cigarettes (data not shown). Concern about the health effects of electronic cigarettes is further reflected in providers' hesitancy to view these devices as an NRT option in the hospital, reluctance to consider sharing a room with an electronic cigarette user, and near majority opinion that electronic cigarettes should be banned from healthcare settings altogether. Current regulation by the US Department of Transportation bans electronic cigarette use on airplanes, whereas a host of states currently ban electronic cigarette use in similarly enclosed spaces such as correctional facilities and commuter trains.[14] More knowledge is needed on the health effects of electronic cigarettes on the primary user, secondhand exposure range, and their potential to aid in short‐ and long‐term nicotine cessation before providers and hospitals can make an informed risk‐benefit analysis for appropriate inpatient use. As current or past tobacco users were more accepting of the use of electronic cigarettes in hospital settings, these providers' opinions should be sought for a unique understanding of the interplay between electronic cigarettes and the healthcare environment.
Concern over the unknown safety effects can also be seen in the overwhelming provider support for FDA regulation. Healthcare advocacy groups, such as the American Heart Association, the American Lung Association, and the Legacy Foundation already support federal regulation.[15, 16, 17] FDA regulation may lead to the ability to standardize device content, regulate purchasing and marketing requirements, and ensure that claims to health effects are supported by scientific evidence, though agency involvement may also slow the process of integration into hospital use. Perhaps reflective of the immediacy of the problem, nurses who receive the majority of requests for electronic cigarettes from patients are least likely to want FDA regulation. Until more is known, patients and staff may benefit from pairing vaporizing patients in shared rooms or providing users with designated inhaling spaces.
Nicotine addiction is a strong driving force and, due to a strict no‐smoking policy at our institution, we have witnessed patients making unsafe decisions to leave the hospital (in some cases against medical advice) in an effort to continue smoking. Patients may be starting to look toward electronic cigarettes as an NRT option that more closely satisfies nicotine cravings as well as the ritualistic and tactile components of cigarette use. Electronic cigarettes could have the potential to act as a harm reduction method for nicotine‐dependent inpatients by decreasing the nicotine‐withdrawal related impetus for unsafe hospital discharges. Institutions should take this into account when formulating new policy.
Our study has several limitations. First, it was a single‐center study that may not be representative of provider perspectives at other institutions. Second, the survey was a cross‐sectional sample, missing providers who did not receive the e‐mail during the enrollment period. Third, responses may not accurately reflect perspectives of smaller responding groups such as social workers. Fourth, the survey did not include all types of physicians who deal with smoking cessation, though internal and family medicine physicians provide the majority of care for hospitalized patients at our institution. Fifth, we recorded self‐reported familiarity with electronic cigarettes and did not formally test providers' knowledge of the subject.
Our study provides new perspectives and data on electronic cigarettes to inform future research as well as hospital and healthcare policy. Hospitals should educate patients and front‐line providers around the paucity of health information on these novel devices, while formulating policy that acknowledges patient demand for electronic cigarettes and their potential for cessation therapy and harm reduction. Further research should focus on the effects of nicotine vapor inhalation on patients, the consequences of secondhand nicotine vapor, and the potential for electronic nicotine delivery systems to act as a novel NRT for hospital use.
Disclosure
Nothing to report.
- , , . E‐cigarettes: a scientific review. Circulation. 2014;129:1972–1986.
- , , , , . E‐Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766.
- U.S. Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e‐cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394667.htm. Accessed May 5, 2014.
- . Electronic cigarettes: smoke‐free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18.
- American Nonsmokers' Rights Foundation. US state and local laws regulating use of electronic cigarettes. Available at: http://www.no‐smoke.org/pdf/ecigslaws.pdf. Accessed September 2, 2014.
- , . Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028.
- , , , , , . Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health. 2011;11:786.
- , , , , . Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109(9):1531–1540.
- , . The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686.
- . Promise and peril of e‐Cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136.
- , , , et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine Tob Res. 2014;16(11):1512–1517.
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions From the Global Adult Tobacco Survey (GATS). 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. Accessed April 23, 2014.
- , , . Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683.
- U.S. Department of Transportation. DOT policy on e‐cigarettes. Available at: http://www.dot.gov/sites/dot.gov/files/docs/PolicyOnECigarettes.pdf. Accessed September 2, 2014.
- American Heart Association. AHA: E‐cigarettes threaten to addict next generation of smokers; regulation, further study needed. Available at: http://blog.heart.org/aha‐e‐cigarettes‐threaten‐to‐addict‐next‐generation‐of‐smokers‐regulation‐further‐study‐needed/. Accessed August 25, 2014.
- American Lung Association. American Lung Association statement on e‐cigarettes. Available at: http://www.lung.org/stop‐smoking/tobacco‐control‐advocacy/federal/e‐cigarettes.html. Accesses August 25, 2014.
- Legacy for Health. E‐cigarette policy: the FDA should promptly exercise regulatory authority and over e‐cigarettes. Available at: http://www.legacyforhealth.org/content/download/3962/56088/version/1/file/LEG‐Policy_Statement‐ECigarette‐JAN2014.pdf. Accessed August 25, 2014.
- , , . E‐cigarettes: a scientific review. Circulation. 2014;129:1972–1986.
- , , , , . E‐Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766.
- U.S. Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e‐cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394667.htm. Accessed May 5, 2014.
- . Electronic cigarettes: smoke‐free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18.
- American Nonsmokers' Rights Foundation. US state and local laws regulating use of electronic cigarettes. Available at: http://www.no‐smoke.org/pdf/ecigslaws.pdf. Accessed September 2, 2014.
- , . Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028.
- , , , , , . Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health. 2011;11:786.
- , , , , . Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109(9):1531–1540.
- , . The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686.
- . Promise and peril of e‐Cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136.
- , , , et al. Electronic cigarette awareness, use history, and expected future use among hospitalized cigarette smokers. Nicotine Tob Res. 2014;16(11):1512–1517.
- Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions From the Global Adult Tobacco Survey (GATS). 2nd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf. Accessed April 23, 2014.
- , , . Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683.
- U.S. Department of Transportation. DOT policy on e‐cigarettes. Available at: http://www.dot.gov/sites/dot.gov/files/docs/PolicyOnECigarettes.pdf. Accessed September 2, 2014.
- American Heart Association. AHA: E‐cigarettes threaten to addict next generation of smokers; regulation, further study needed. Available at: http://blog.heart.org/aha‐e‐cigarettes‐threaten‐to‐addict‐next‐generation‐of‐smokers‐regulation‐further‐study‐needed/. Accessed August 25, 2014.
- American Lung Association. American Lung Association statement on e‐cigarettes. Available at: http://www.lung.org/stop‐smoking/tobacco‐control‐advocacy/federal/e‐cigarettes.html. Accesses August 25, 2014.
- Legacy for Health. E‐cigarette policy: the FDA should promptly exercise regulatory authority and over e‐cigarettes. Available at: http://www.legacyforhealth.org/content/download/3962/56088/version/1/file/LEG‐Policy_Statement‐ECigarette‐JAN2014.pdf. Accessed August 25, 2014.
Master Class: Obesity
Obesity not only increases a patient’s lifetime risk of numerous chronic conditions, such as diabetes, heart disease and kidney disease, but it also is a major health issue during pregnancy. Women who are obese in pregnancy have a significantly higher chance of developing adverse perinatal outcomes and experiencing various complications that affect both their health and that of their babies.
With an ever-increasing population of overweight and obese women of reproductive age, as key caregivers for women, we must reexamine our approaches and do much more than microfocusing on a woman’s pre- and postdelivery health. We must play a more active role in helping our patients establish and maintain a healthy lifestyle – one that will help ward off and reduce the incidence of this concerning condition.
Over the previous two Master Class installments on obstetrics, we discussed the extent of the obesity epidemic and its link to diabetes, the alarming number of infants, children, and adolescents who are obese, and the implications of these societal and medical trends for ob.gyns.
In the July Master Class, we discussed the importance of appropriately counseling patients on healthy weight gain and physical activity in pregnancy. Because ob.gyns. may be the only health care professionals that many women may see, it is becoming more important that we help our patients and their children attain and maintain positive health and well-being.
In our September installment, Dr. Thomas R. Moore looked at obesity trends through the lens of the Barker Hypothesis, which got us thinking more than 3 decades ago about the role of intrauterine environment in short- and long-term health of offspring. Dr. Moore discussed how obesity in pregnancy appears to program offspring for downstream cardiovascular risk in adulthood.
He told us that we must not only liberally treat gestational diabetes and optimize glucose control during pregnancy, but, most importantly, we also must emphasize to women the importance of having healthy weights at the time of conception.
This month’s Master Class examines this latter concept in more depth. Dr. Patrick Catalano, professor in the department of obstetrics and gynecology and director of the Center for Reproductive Health at MetroHealth Medical Center, Case Western Reserve University, Cleveland, has been at the forefront of research on the physiologic impact of obesity on the placenta and the fetus, and on approaches for addressing maternal obesity and improving perinatal outcomes.
Dr. Catalano explains here why weight loss before pregnancy appears to be important for preventing adverse perinatal outcomes and breaking the intergenerational transfer of obesity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Obesity not only increases a patient’s lifetime risk of numerous chronic conditions, such as diabetes, heart disease and kidney disease, but it also is a major health issue during pregnancy. Women who are obese in pregnancy have a significantly higher chance of developing adverse perinatal outcomes and experiencing various complications that affect both their health and that of their babies.
With an ever-increasing population of overweight and obese women of reproductive age, as key caregivers for women, we must reexamine our approaches and do much more than microfocusing on a woman’s pre- and postdelivery health. We must play a more active role in helping our patients establish and maintain a healthy lifestyle – one that will help ward off and reduce the incidence of this concerning condition.
Over the previous two Master Class installments on obstetrics, we discussed the extent of the obesity epidemic and its link to diabetes, the alarming number of infants, children, and adolescents who are obese, and the implications of these societal and medical trends for ob.gyns.
In the July Master Class, we discussed the importance of appropriately counseling patients on healthy weight gain and physical activity in pregnancy. Because ob.gyns. may be the only health care professionals that many women may see, it is becoming more important that we help our patients and their children attain and maintain positive health and well-being.
In our September installment, Dr. Thomas R. Moore looked at obesity trends through the lens of the Barker Hypothesis, which got us thinking more than 3 decades ago about the role of intrauterine environment in short- and long-term health of offspring. Dr. Moore discussed how obesity in pregnancy appears to program offspring for downstream cardiovascular risk in adulthood.
He told us that we must not only liberally treat gestational diabetes and optimize glucose control during pregnancy, but, most importantly, we also must emphasize to women the importance of having healthy weights at the time of conception.
This month’s Master Class examines this latter concept in more depth. Dr. Patrick Catalano, professor in the department of obstetrics and gynecology and director of the Center for Reproductive Health at MetroHealth Medical Center, Case Western Reserve University, Cleveland, has been at the forefront of research on the physiologic impact of obesity on the placenta and the fetus, and on approaches for addressing maternal obesity and improving perinatal outcomes.
Dr. Catalano explains here why weight loss before pregnancy appears to be important for preventing adverse perinatal outcomes and breaking the intergenerational transfer of obesity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Obesity not only increases a patient’s lifetime risk of numerous chronic conditions, such as diabetes, heart disease and kidney disease, but it also is a major health issue during pregnancy. Women who are obese in pregnancy have a significantly higher chance of developing adverse perinatal outcomes and experiencing various complications that affect both their health and that of their babies.
With an ever-increasing population of overweight and obese women of reproductive age, as key caregivers for women, we must reexamine our approaches and do much more than microfocusing on a woman’s pre- and postdelivery health. We must play a more active role in helping our patients establish and maintain a healthy lifestyle – one that will help ward off and reduce the incidence of this concerning condition.
Over the previous two Master Class installments on obstetrics, we discussed the extent of the obesity epidemic and its link to diabetes, the alarming number of infants, children, and adolescents who are obese, and the implications of these societal and medical trends for ob.gyns.
In the July Master Class, we discussed the importance of appropriately counseling patients on healthy weight gain and physical activity in pregnancy. Because ob.gyns. may be the only health care professionals that many women may see, it is becoming more important that we help our patients and their children attain and maintain positive health and well-being.
In our September installment, Dr. Thomas R. Moore looked at obesity trends through the lens of the Barker Hypothesis, which got us thinking more than 3 decades ago about the role of intrauterine environment in short- and long-term health of offspring. Dr. Moore discussed how obesity in pregnancy appears to program offspring for downstream cardiovascular risk in adulthood.
He told us that we must not only liberally treat gestational diabetes and optimize glucose control during pregnancy, but, most importantly, we also must emphasize to women the importance of having healthy weights at the time of conception.
This month’s Master Class examines this latter concept in more depth. Dr. Patrick Catalano, professor in the department of obstetrics and gynecology and director of the Center for Reproductive Health at MetroHealth Medical Center, Case Western Reserve University, Cleveland, has been at the forefront of research on the physiologic impact of obesity on the placenta and the fetus, and on approaches for addressing maternal obesity and improving perinatal outcomes.
Dr. Catalano explains here why weight loss before pregnancy appears to be important for preventing adverse perinatal outcomes and breaking the intergenerational transfer of obesity.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Neonatal Physeal Separation of Distal Humerus During Cesarean Section
Physeal separation of the distal humerus in a newborn is a rare and severe injury that requires immediate treatment. This fracture was reported as an extremely rare complication of cesarean section.1 The correct diagnosis can be established by clinical and radiologic findings. However, this injury can be easily overlooked and misdiagnosed. Presentation often involves swelling, tenderness, and agitation with movement of the elbow.
We report a case in which neonatal physeal separation of the distal humerus occurred during cesarean section. The diagnosis was based on clinical and radiologic/arthrographic findings and treated with closed reduction and percutaneous fixation. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A full-term (40-week gestation) male neonate weighing 3690 g was born through cesarean section at the mother’s request. Apgar score was 9 at 1 minute and 10 at 5 minutes. The vertex position of the fetus was confirmed with preoperative ultrasonography. This was the mother’s first pregnancy and an in vitro fertilization. On his second day of life, the patient was referred to the orthopedic department for evaluation of local swelling and diminished spontaneous motion of the right elbow.
Examination revealed local tenderness and swelling in the anterior and lateral aspects of the elbow. Passive elbow range of motion (ROM) caused agitation, and elbow instability was present. A complete neurovascular examination was performed, and neurovascular injury and compartment syndrome were ruled out. Hematologic workup showed no signs of septic arthritis. Radiographs showed posteromedial displacement of the humeroulnar joint. The patient was placed in a long-arm splint, and no reduction was attempted initially.
The patient was taken to the operating room the same day. With the patient under general anesthesia, an arthrogram of the right elbow was obtained. It showed posteromedial displacement of the distal humeral epiphysis (Figure 1A). Closed reduction was performed, and the quality of the reduction was confirmed by intraoperative imaging. Percutaneously, a single 2-mm Kirschner wire (K-wire) was placed in an oblique fashion from the inferolateral aspect of the distal fragment to the contralateral metaphysis of the humerus (Figure 1B). The patient was put in a long-arm splint with the elbow flexed at 90° and the forearm in midpronation.
Follow-up visits were scheduled for 1 week, 3 weeks, and 5 weeks after surgery. Three weeks after surgery, callus formation was confirmed, and the K-wire was removed. Five weeks after surgery, the long-arm splint was removed.
At 6-month follow-up, the patient was pain-free and had full elbow ROM, and radiographs (Figures 2A, 2B) confirmed anatomical restoration of the fracture.
Discussion
Madsen2 reported the incidence of birth-related long-bone fractures, including fractures of the humerus, the femur, and the tibia (< 0.1%). According to that review, only 1 of 105,119 patients sustained traumatic physeal separation of the distal humerus.
Different mechanisms have been described for this rare fracture. As the physeal region is the weakest part of the distal humerus, it is prone to injury by rotational shear forces,3,4 hyperextension of the elbow, or a backward thrust on the forearm with the elbow flexed.5 Excessive traction applied during cesarean delivery might cause physeal separation, which was the possible cause in the present case. Most patients have a complicated birth history.
This injury should be suspected in an irritable newborn with swelling, tenderness, and reduced mobility of the upper extremity. Osteomyelitis and septic arthritis should be considered in the differential diagnosis. Brachial plexus injury and dislocation of the elbow joint should also be kept in mind. Child abuse and metabolic bone diseases (eg, osteogenesis imperfecta) should also be considered.
Anteroposterior and lateral plain radiographs of the elbow usually establish the diagnosis. Alteration of humeroulnar alignment and displacement of the proximal forearm are the key points leading to the diagnosis.
The cartilaginous part of the distal humerus and humeroulnar alignment can be demonstrated by ultrasonography.6 Magnetic resonance imaging (MRI) can be helpful in diagnosis but is seldom required,7 and the sedation or general anesthesia used is a disadvantage. Arthrography is useful not only in diagnosis but in determining the quality of the reduction.8 An arthrogram may show that open reduction is unnecessary.
Treatment differs widely. In neonates, who have a tremendous healing capability, this fracture almost always heals uneventfully. An effective treatment method is closed reduction and cast immobilization. However, valgus malalignment and limited elbow ROM were noted in 5% of the patients treated with this method.4
Jacobsen and colleagues4 reported on 6 neonates who sustained traumatic separation of the distal epiphysis of the humerus at birth and who were treated with casting with or without closed reduction. The authors described good results. One patient had varus malalignment, which was attributed to fragment internal rotation caused by rotational instability.
As our patient’s instability was noted during surgery, we performed percutaneous pinning after arthrography-assisted closed reduction. We considered using 2 lateral pins for fixation, but, after the first pin was placed, fluoroscopic stress testing with the patient under anesthesia demonstrated adequate stability. A second, smaller pin could have been used to control rotation, if needed. Medial pin placement that avoids the ulnar nerve is difficult in the newborn elbow; medial pins should probably be avoided in the newborn, if possible.
Early diagnosis and treatment are essential. Late diagnosis was reported to lead to complications such as varus deformity and restriction of joint ROM.4
Our patient healed without any complications and achieved full ROM. Long-term follow-up is needed to diagnose any physeal bar that might lead to secondary deformities.
Conclusion
Cesarean section is reported to reduce birth complications, but it might cause fractures of the femur and humerus.1 Avoiding application of excessive traction to the forearm can prevent physeal separation of the distal humerus. This entity should be kept in mind as a potential complication of cesarean section. Arthrography is helpful in treatment and may help avoid unnecessary open reduction.
1. Sabat D, Maini L, Gautam VK. Neonatal separation of distal humeral epiphysis during caesarean section: a case report. J Orthop Surg (Hong Kong). 2011;19(3):376-378.
2. Madsen ET. Fractures of the extremities in the newborn. Acta Obstet Gynecol Scand. 1955;34(1):41-74.
3. Peterson HA. Physeal fractures. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia, PA: Saunders; 1985:222-236.
4. Jacobsen S, Hansson G, Nathorst-Westfelt J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg Br. 2009;91(6):797-802.
5. Siffert RS. Displacement of distal humeral epiphysis in the newborn. J Bone Joint Surg Am. 1963;45(1):165-169.
6. Davidson RS, Markowitz RI, Dormans J, Drummond DS. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76(12):1804-1813.
7. Sawant MR, Narayanan S, O‘Neill K, Hudson I. Distal humeral epiphysis fracture separation in neonates—diagnosis using MRI scan. Injury. 2002;33(2):179-181.
8. Akbarnia BA, Silberstein MJ, Rende RJ, Graviss ER, Luisiri A. Arthrography in the diagnosis of fractures of the distal end of the humerus in infants. J Bone Joint Surg Am. 1986;68(4):599-602.
Physeal separation of the distal humerus in a newborn is a rare and severe injury that requires immediate treatment. This fracture was reported as an extremely rare complication of cesarean section.1 The correct diagnosis can be established by clinical and radiologic findings. However, this injury can be easily overlooked and misdiagnosed. Presentation often involves swelling, tenderness, and agitation with movement of the elbow.
We report a case in which neonatal physeal separation of the distal humerus occurred during cesarean section. The diagnosis was based on clinical and radiologic/arthrographic findings and treated with closed reduction and percutaneous fixation. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A full-term (40-week gestation) male neonate weighing 3690 g was born through cesarean section at the mother’s request. Apgar score was 9 at 1 minute and 10 at 5 minutes. The vertex position of the fetus was confirmed with preoperative ultrasonography. This was the mother’s first pregnancy and an in vitro fertilization. On his second day of life, the patient was referred to the orthopedic department for evaluation of local swelling and diminished spontaneous motion of the right elbow.
Examination revealed local tenderness and swelling in the anterior and lateral aspects of the elbow. Passive elbow range of motion (ROM) caused agitation, and elbow instability was present. A complete neurovascular examination was performed, and neurovascular injury and compartment syndrome were ruled out. Hematologic workup showed no signs of septic arthritis. Radiographs showed posteromedial displacement of the humeroulnar joint. The patient was placed in a long-arm splint, and no reduction was attempted initially.
The patient was taken to the operating room the same day. With the patient under general anesthesia, an arthrogram of the right elbow was obtained. It showed posteromedial displacement of the distal humeral epiphysis (Figure 1A). Closed reduction was performed, and the quality of the reduction was confirmed by intraoperative imaging. Percutaneously, a single 2-mm Kirschner wire (K-wire) was placed in an oblique fashion from the inferolateral aspect of the distal fragment to the contralateral metaphysis of the humerus (Figure 1B). The patient was put in a long-arm splint with the elbow flexed at 90° and the forearm in midpronation.
Follow-up visits were scheduled for 1 week, 3 weeks, and 5 weeks after surgery. Three weeks after surgery, callus formation was confirmed, and the K-wire was removed. Five weeks after surgery, the long-arm splint was removed.
At 6-month follow-up, the patient was pain-free and had full elbow ROM, and radiographs (Figures 2A, 2B) confirmed anatomical restoration of the fracture.
Discussion
Madsen2 reported the incidence of birth-related long-bone fractures, including fractures of the humerus, the femur, and the tibia (< 0.1%). According to that review, only 1 of 105,119 patients sustained traumatic physeal separation of the distal humerus.
Different mechanisms have been described for this rare fracture. As the physeal region is the weakest part of the distal humerus, it is prone to injury by rotational shear forces,3,4 hyperextension of the elbow, or a backward thrust on the forearm with the elbow flexed.5 Excessive traction applied during cesarean delivery might cause physeal separation, which was the possible cause in the present case. Most patients have a complicated birth history.
This injury should be suspected in an irritable newborn with swelling, tenderness, and reduced mobility of the upper extremity. Osteomyelitis and septic arthritis should be considered in the differential diagnosis. Brachial plexus injury and dislocation of the elbow joint should also be kept in mind. Child abuse and metabolic bone diseases (eg, osteogenesis imperfecta) should also be considered.
Anteroposterior and lateral plain radiographs of the elbow usually establish the diagnosis. Alteration of humeroulnar alignment and displacement of the proximal forearm are the key points leading to the diagnosis.
The cartilaginous part of the distal humerus and humeroulnar alignment can be demonstrated by ultrasonography.6 Magnetic resonance imaging (MRI) can be helpful in diagnosis but is seldom required,7 and the sedation or general anesthesia used is a disadvantage. Arthrography is useful not only in diagnosis but in determining the quality of the reduction.8 An arthrogram may show that open reduction is unnecessary.
Treatment differs widely. In neonates, who have a tremendous healing capability, this fracture almost always heals uneventfully. An effective treatment method is closed reduction and cast immobilization. However, valgus malalignment and limited elbow ROM were noted in 5% of the patients treated with this method.4
Jacobsen and colleagues4 reported on 6 neonates who sustained traumatic separation of the distal epiphysis of the humerus at birth and who were treated with casting with or without closed reduction. The authors described good results. One patient had varus malalignment, which was attributed to fragment internal rotation caused by rotational instability.
As our patient’s instability was noted during surgery, we performed percutaneous pinning after arthrography-assisted closed reduction. We considered using 2 lateral pins for fixation, but, after the first pin was placed, fluoroscopic stress testing with the patient under anesthesia demonstrated adequate stability. A second, smaller pin could have been used to control rotation, if needed. Medial pin placement that avoids the ulnar nerve is difficult in the newborn elbow; medial pins should probably be avoided in the newborn, if possible.
Early diagnosis and treatment are essential. Late diagnosis was reported to lead to complications such as varus deformity and restriction of joint ROM.4
Our patient healed without any complications and achieved full ROM. Long-term follow-up is needed to diagnose any physeal bar that might lead to secondary deformities.
Conclusion
Cesarean section is reported to reduce birth complications, but it might cause fractures of the femur and humerus.1 Avoiding application of excessive traction to the forearm can prevent physeal separation of the distal humerus. This entity should be kept in mind as a potential complication of cesarean section. Arthrography is helpful in treatment and may help avoid unnecessary open reduction.
Physeal separation of the distal humerus in a newborn is a rare and severe injury that requires immediate treatment. This fracture was reported as an extremely rare complication of cesarean section.1 The correct diagnosis can be established by clinical and radiologic findings. However, this injury can be easily overlooked and misdiagnosed. Presentation often involves swelling, tenderness, and agitation with movement of the elbow.
We report a case in which neonatal physeal separation of the distal humerus occurred during cesarean section. The diagnosis was based on clinical and radiologic/arthrographic findings and treated with closed reduction and percutaneous fixation. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A full-term (40-week gestation) male neonate weighing 3690 g was born through cesarean section at the mother’s request. Apgar score was 9 at 1 minute and 10 at 5 minutes. The vertex position of the fetus was confirmed with preoperative ultrasonography. This was the mother’s first pregnancy and an in vitro fertilization. On his second day of life, the patient was referred to the orthopedic department for evaluation of local swelling and diminished spontaneous motion of the right elbow.
Examination revealed local tenderness and swelling in the anterior and lateral aspects of the elbow. Passive elbow range of motion (ROM) caused agitation, and elbow instability was present. A complete neurovascular examination was performed, and neurovascular injury and compartment syndrome were ruled out. Hematologic workup showed no signs of septic arthritis. Radiographs showed posteromedial displacement of the humeroulnar joint. The patient was placed in a long-arm splint, and no reduction was attempted initially.
The patient was taken to the operating room the same day. With the patient under general anesthesia, an arthrogram of the right elbow was obtained. It showed posteromedial displacement of the distal humeral epiphysis (Figure 1A). Closed reduction was performed, and the quality of the reduction was confirmed by intraoperative imaging. Percutaneously, a single 2-mm Kirschner wire (K-wire) was placed in an oblique fashion from the inferolateral aspect of the distal fragment to the contralateral metaphysis of the humerus (Figure 1B). The patient was put in a long-arm splint with the elbow flexed at 90° and the forearm in midpronation.
Follow-up visits were scheduled for 1 week, 3 weeks, and 5 weeks after surgery. Three weeks after surgery, callus formation was confirmed, and the K-wire was removed. Five weeks after surgery, the long-arm splint was removed.
At 6-month follow-up, the patient was pain-free and had full elbow ROM, and radiographs (Figures 2A, 2B) confirmed anatomical restoration of the fracture.
Discussion
Madsen2 reported the incidence of birth-related long-bone fractures, including fractures of the humerus, the femur, and the tibia (< 0.1%). According to that review, only 1 of 105,119 patients sustained traumatic physeal separation of the distal humerus.
Different mechanisms have been described for this rare fracture. As the physeal region is the weakest part of the distal humerus, it is prone to injury by rotational shear forces,3,4 hyperextension of the elbow, or a backward thrust on the forearm with the elbow flexed.5 Excessive traction applied during cesarean delivery might cause physeal separation, which was the possible cause in the present case. Most patients have a complicated birth history.
This injury should be suspected in an irritable newborn with swelling, tenderness, and reduced mobility of the upper extremity. Osteomyelitis and septic arthritis should be considered in the differential diagnosis. Brachial plexus injury and dislocation of the elbow joint should also be kept in mind. Child abuse and metabolic bone diseases (eg, osteogenesis imperfecta) should also be considered.
Anteroposterior and lateral plain radiographs of the elbow usually establish the diagnosis. Alteration of humeroulnar alignment and displacement of the proximal forearm are the key points leading to the diagnosis.
The cartilaginous part of the distal humerus and humeroulnar alignment can be demonstrated by ultrasonography.6 Magnetic resonance imaging (MRI) can be helpful in diagnosis but is seldom required,7 and the sedation or general anesthesia used is a disadvantage. Arthrography is useful not only in diagnosis but in determining the quality of the reduction.8 An arthrogram may show that open reduction is unnecessary.
Treatment differs widely. In neonates, who have a tremendous healing capability, this fracture almost always heals uneventfully. An effective treatment method is closed reduction and cast immobilization. However, valgus malalignment and limited elbow ROM were noted in 5% of the patients treated with this method.4
Jacobsen and colleagues4 reported on 6 neonates who sustained traumatic separation of the distal epiphysis of the humerus at birth and who were treated with casting with or without closed reduction. The authors described good results. One patient had varus malalignment, which was attributed to fragment internal rotation caused by rotational instability.
As our patient’s instability was noted during surgery, we performed percutaneous pinning after arthrography-assisted closed reduction. We considered using 2 lateral pins for fixation, but, after the first pin was placed, fluoroscopic stress testing with the patient under anesthesia demonstrated adequate stability. A second, smaller pin could have been used to control rotation, if needed. Medial pin placement that avoids the ulnar nerve is difficult in the newborn elbow; medial pins should probably be avoided in the newborn, if possible.
Early diagnosis and treatment are essential. Late diagnosis was reported to lead to complications such as varus deformity and restriction of joint ROM.4
Our patient healed without any complications and achieved full ROM. Long-term follow-up is needed to diagnose any physeal bar that might lead to secondary deformities.
Conclusion
Cesarean section is reported to reduce birth complications, but it might cause fractures of the femur and humerus.1 Avoiding application of excessive traction to the forearm can prevent physeal separation of the distal humerus. This entity should be kept in mind as a potential complication of cesarean section. Arthrography is helpful in treatment and may help avoid unnecessary open reduction.
1. Sabat D, Maini L, Gautam VK. Neonatal separation of distal humeral epiphysis during caesarean section: a case report. J Orthop Surg (Hong Kong). 2011;19(3):376-378.
2. Madsen ET. Fractures of the extremities in the newborn. Acta Obstet Gynecol Scand. 1955;34(1):41-74.
3. Peterson HA. Physeal fractures. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia, PA: Saunders; 1985:222-236.
4. Jacobsen S, Hansson G, Nathorst-Westfelt J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg Br. 2009;91(6):797-802.
5. Siffert RS. Displacement of distal humeral epiphysis in the newborn. J Bone Joint Surg Am. 1963;45(1):165-169.
6. Davidson RS, Markowitz RI, Dormans J, Drummond DS. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76(12):1804-1813.
7. Sawant MR, Narayanan S, O‘Neill K, Hudson I. Distal humeral epiphysis fracture separation in neonates—diagnosis using MRI scan. Injury. 2002;33(2):179-181.
8. Akbarnia BA, Silberstein MJ, Rende RJ, Graviss ER, Luisiri A. Arthrography in the diagnosis of fractures of the distal end of the humerus in infants. J Bone Joint Surg Am. 1986;68(4):599-602.
1. Sabat D, Maini L, Gautam VK. Neonatal separation of distal humeral epiphysis during caesarean section: a case report. J Orthop Surg (Hong Kong). 2011;19(3):376-378.
2. Madsen ET. Fractures of the extremities in the newborn. Acta Obstet Gynecol Scand. 1955;34(1):41-74.
3. Peterson HA. Physeal fractures. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia, PA: Saunders; 1985:222-236.
4. Jacobsen S, Hansson G, Nathorst-Westfelt J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg Br. 2009;91(6):797-802.
5. Siffert RS. Displacement of distal humeral epiphysis in the newborn. J Bone Joint Surg Am. 1963;45(1):165-169.
6. Davidson RS, Markowitz RI, Dormans J, Drummond DS. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76(12):1804-1813.
7. Sawant MR, Narayanan S, O‘Neill K, Hudson I. Distal humeral epiphysis fracture separation in neonates—diagnosis using MRI scan. Injury. 2002;33(2):179-181.
8. Akbarnia BA, Silberstein MJ, Rende RJ, Graviss ER, Luisiri A. Arthrography in the diagnosis of fractures of the distal end of the humerus in infants. J Bone Joint Surg Am. 1986;68(4):599-602.