User login
Caring for women with HIV: Unique needs and challenges
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
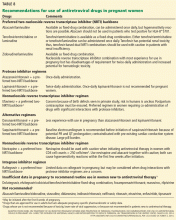
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
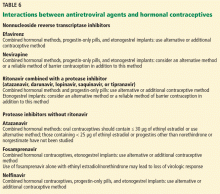
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
KEY POINTS
- The number of women living with HIV has increased over the past 30 years, and African American women bear a disproportionate burden of disease.
- Women of all ages are at risk of acquiring HIV; therefore, HIV testing should be part of routine care.
- Preconception counseling is an essential component of both primary and preventive care and should be the standard of care for all women of reproductive age with HIV.
- Women with HIV have the same gynecologic problems as all women but may be more vulnerable to certain conditions, such as human papillomavirus infection.
Women and HIV: An expanded perspective
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Short and Anderson give an overview of the epidemic of human immunodeficiency virus (HIV) infection in US women and the various aspects of health care of this group, including pregnancy.1 They introduce a much broader topic and bring to light a number of additional concerns.
HIV PREYS ON THE VULNERABLE
The authors review epidemiologic trends and the evolving demographics of HIV, which deserve specific discussion.
In the early years of the epidemic, ie, the early 1980s, HIV infection in women was overshadowed by the epidemic in men, particularly men who have sex with men. The epidemic in men who have sex with men remains the larger component of the HIV picture in the United States. But worldwide, HIV is an evenly balanced problem, with nearly half of all infections occurring in women.2 Women have received much more attention recently.
In the United States, about 300,000 women are living with HIV, and 10% of them are unaware of it. Between 1985 and 2013, the number of HIV cases in US women tripled.
The epidemic continues to disproportionately affect women of color. Two-thirds of all women with HIV are African American,2 and estimates suggest that 1 of every 32 African American women will acquire HIV during her lifetime. On a positive note, there was a 20% reduction in new infections among African American women between 2008 and 2010.3
The epidemic preys on the vulnerable and is fueled by poverty, lack of education (general and health literacy), substance abuse, and restricted access to health care. Major metropolitan areas such as New York, Washington, DC, Miami, and Los Angeles are “hot spots,” where high concentrations of infected people reside.4
Many women underestimate or do not perceive their susceptibility. They unknowingly acquire HIV infection from their male partners, many of whom are unaware of their infection. Some of their partners may lead a dual life of bisexuality. In some areas, an estimated 20% of men who have sex with men also engage in sex with women.5 If these women contract the disease, they may be diagnosed at a late stage and when they are symptomatic, or coincidentally during pregnancy and childbirth.
Negotiating safe sex practices can be difficult for a woman. She may perceive or lack empowerment to do so, fearing rejection, isolation, or violence. Sexual violence may have been initiated in childhood, through intimate partners, rape, sex trafficking, or prostitution. Patterns vary throughout the world, but sexual violence is more common than perceived.6 Because of shame, embarrassment, and isolation, many victims do not seek medical care and so may carry undiagnosed infections. Even when they access care, they are less likely to remain in the HIV care system.7 Greater efforts are needed to reach these women, make them feel supported in care, and keep them in the system.
TESTING IS CRUCIAL
Diagnosis remains a weak link in the chain of care for both men and women. Success has been noted in the form of a marked reduction in cases of mother-to-child transmission, thanks to near-universal opt-out screening during pregnancy or at delivery.
If appropriate routine testing were done for all people, as advocated by the US Centers for Disease Control and Prevention guidelines,8 more cases could be diagnosed, behaviors changed, and treatment offered. Control of HIV through treatment can lead to a 96% reduction in transmission between serodiscordant partners, as demonstrated in HPTN 052, an ongoing phase 3 trial.9 Early diagnosis and treatment offer the potential for improved immune regeneration and healthier lives.
PRE-EXPOSURE PROPHYLAXIS
Pre-exposure prophylaxis (PrEP) is one approach to empowering women and preventing HIV infection. Studies have demonstrated the efficacy of this approach, although some studies have not.10,11
An important finding in the failed studies appeared to be a lack of adherence to the regimen.11 Unless taken faithfully, PrEP will not succeed. Additionally, there may be inherent differences in outcomes for unknown reasons. Lack of access to the necessary two-drug combination regimen is another barrier.
PrEP is expensive, requires regular monitoring, and requires patients to remain engaged in medical care. Currently, not all medical programs offer PrEP, and not all insurance policies cover it. Further insight into long-term side effects and complications is needed.
Although PrEP is an attractive concept and a reality for some, it is an incomplete solution to prevention at this time.
MEN AND WOMEN ARE DIFFERENT
Men and women are different physiologically and psychologically. Women typically have a lower body mass, lower bone mass, and higher content of body fat. As a result, women may differ from men in their ability to tolerate medications, and long-term side effects may be more pronounced.
Women are also more likely to place family responsibilities above self-preservation and personal health concerns. As a result, providing for and taking care of their children takes precedence over care of their own health.
Providing care to women presents many challenges and opportunities to improve their health. Health care access, transportation, assistance with child care during medical visits, the availability of counseling to deal with shame, guilt, and depression, and maintaining women within the care system are but a few examples.
AGING WITH HIV: STUDY NEEDED
Antiretroviral therapy has enabled patients to survive and often to reach a normal life expectancy if the infection is diagnosed and treated early. As a result, HIV-associated causes of death have been replaced by non-HIV comorbidities typical of aging, such as cardiovascular disease, organ failure (heart, lung, kidney, liver), non-HIV cancers, and bone disease.
Women face unique aspects of aging with menopause, including an accelerated rate of bone loss resulting in osteoporosis. HIV itself and some antiretroviral drugs may increase the loss of bone mineral density. Alcohol abuse, sedentary lifestyle, smoking, hepatitis C co-infection, and poor nutrition also contribute to this problem. Bone disease and many other aspects of aging and HIV in women require more research and intervention.
Other areas that need to be studied are the unique mucosal immune system of the female genital tract, the interplay of sex hormones and the immune system, the role of genital tract inflammation in increasing the risk of HIV acquisition, sexual violence and HIV acquisition, and the safety and efficacy of PrEP for women. This will require prioritization and ongoing funding, which is becoming scarcer. If there is to be hope of containing this disease, our efforts to understand it must not diminish.
- Short WR, Anderson JR. Caring for women with HIV: unique needs and challenges. Cleve Clin J Med 2014; 81:691–701.
- UNAIDS. Women out loud: how women living with HIV will help the world end AIDS. www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20121211_Women_Out_Loud_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). HIV among women. www.cdc.gov/hiv/risk/gender/women/index.html. Accessed October 2, 2014.
- Hodder SL, Justman J, Hughes JP, et al; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–18.
- UNAIDS. Fact Sheet: women, girls, gender equality and HIV. www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120217_FS_WomenGirls_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). National intimate partner and sexual violence survey: 2010 summary report. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed October 2, 2014.
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr 2013; 64:32–38.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10 9:e1001511.
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Short and Anderson give an overview of the epidemic of human immunodeficiency virus (HIV) infection in US women and the various aspects of health care of this group, including pregnancy.1 They introduce a much broader topic and bring to light a number of additional concerns.
HIV PREYS ON THE VULNERABLE
The authors review epidemiologic trends and the evolving demographics of HIV, which deserve specific discussion.
In the early years of the epidemic, ie, the early 1980s, HIV infection in women was overshadowed by the epidemic in men, particularly men who have sex with men. The epidemic in men who have sex with men remains the larger component of the HIV picture in the United States. But worldwide, HIV is an evenly balanced problem, with nearly half of all infections occurring in women.2 Women have received much more attention recently.
In the United States, about 300,000 women are living with HIV, and 10% of them are unaware of it. Between 1985 and 2013, the number of HIV cases in US women tripled.
The epidemic continues to disproportionately affect women of color. Two-thirds of all women with HIV are African American,2 and estimates suggest that 1 of every 32 African American women will acquire HIV during her lifetime. On a positive note, there was a 20% reduction in new infections among African American women between 2008 and 2010.3
The epidemic preys on the vulnerable and is fueled by poverty, lack of education (general and health literacy), substance abuse, and restricted access to health care. Major metropolitan areas such as New York, Washington, DC, Miami, and Los Angeles are “hot spots,” where high concentrations of infected people reside.4
Many women underestimate or do not perceive their susceptibility. They unknowingly acquire HIV infection from their male partners, many of whom are unaware of their infection. Some of their partners may lead a dual life of bisexuality. In some areas, an estimated 20% of men who have sex with men also engage in sex with women.5 If these women contract the disease, they may be diagnosed at a late stage and when they are symptomatic, or coincidentally during pregnancy and childbirth.
Negotiating safe sex practices can be difficult for a woman. She may perceive or lack empowerment to do so, fearing rejection, isolation, or violence. Sexual violence may have been initiated in childhood, through intimate partners, rape, sex trafficking, or prostitution. Patterns vary throughout the world, but sexual violence is more common than perceived.6 Because of shame, embarrassment, and isolation, many victims do not seek medical care and so may carry undiagnosed infections. Even when they access care, they are less likely to remain in the HIV care system.7 Greater efforts are needed to reach these women, make them feel supported in care, and keep them in the system.
TESTING IS CRUCIAL
Diagnosis remains a weak link in the chain of care for both men and women. Success has been noted in the form of a marked reduction in cases of mother-to-child transmission, thanks to near-universal opt-out screening during pregnancy or at delivery.
If appropriate routine testing were done for all people, as advocated by the US Centers for Disease Control and Prevention guidelines,8 more cases could be diagnosed, behaviors changed, and treatment offered. Control of HIV through treatment can lead to a 96% reduction in transmission between serodiscordant partners, as demonstrated in HPTN 052, an ongoing phase 3 trial.9 Early diagnosis and treatment offer the potential for improved immune regeneration and healthier lives.
PRE-EXPOSURE PROPHYLAXIS
Pre-exposure prophylaxis (PrEP) is one approach to empowering women and preventing HIV infection. Studies have demonstrated the efficacy of this approach, although some studies have not.10,11
An important finding in the failed studies appeared to be a lack of adherence to the regimen.11 Unless taken faithfully, PrEP will not succeed. Additionally, there may be inherent differences in outcomes for unknown reasons. Lack of access to the necessary two-drug combination regimen is another barrier.
PrEP is expensive, requires regular monitoring, and requires patients to remain engaged in medical care. Currently, not all medical programs offer PrEP, and not all insurance policies cover it. Further insight into long-term side effects and complications is needed.
Although PrEP is an attractive concept and a reality for some, it is an incomplete solution to prevention at this time.
MEN AND WOMEN ARE DIFFERENT
Men and women are different physiologically and psychologically. Women typically have a lower body mass, lower bone mass, and higher content of body fat. As a result, women may differ from men in their ability to tolerate medications, and long-term side effects may be more pronounced.
Women are also more likely to place family responsibilities above self-preservation and personal health concerns. As a result, providing for and taking care of their children takes precedence over care of their own health.
Providing care to women presents many challenges and opportunities to improve their health. Health care access, transportation, assistance with child care during medical visits, the availability of counseling to deal with shame, guilt, and depression, and maintaining women within the care system are but a few examples.
AGING WITH HIV: STUDY NEEDED
Antiretroviral therapy has enabled patients to survive and often to reach a normal life expectancy if the infection is diagnosed and treated early. As a result, HIV-associated causes of death have been replaced by non-HIV comorbidities typical of aging, such as cardiovascular disease, organ failure (heart, lung, kidney, liver), non-HIV cancers, and bone disease.
Women face unique aspects of aging with menopause, including an accelerated rate of bone loss resulting in osteoporosis. HIV itself and some antiretroviral drugs may increase the loss of bone mineral density. Alcohol abuse, sedentary lifestyle, smoking, hepatitis C co-infection, and poor nutrition also contribute to this problem. Bone disease and many other aspects of aging and HIV in women require more research and intervention.
Other areas that need to be studied are the unique mucosal immune system of the female genital tract, the interplay of sex hormones and the immune system, the role of genital tract inflammation in increasing the risk of HIV acquisition, sexual violence and HIV acquisition, and the safety and efficacy of PrEP for women. This will require prioritization and ongoing funding, which is becoming scarcer. If there is to be hope of containing this disease, our efforts to understand it must not diminish.
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Short and Anderson give an overview of the epidemic of human immunodeficiency virus (HIV) infection in US women and the various aspects of health care of this group, including pregnancy.1 They introduce a much broader topic and bring to light a number of additional concerns.
HIV PREYS ON THE VULNERABLE
The authors review epidemiologic trends and the evolving demographics of HIV, which deserve specific discussion.
In the early years of the epidemic, ie, the early 1980s, HIV infection in women was overshadowed by the epidemic in men, particularly men who have sex with men. The epidemic in men who have sex with men remains the larger component of the HIV picture in the United States. But worldwide, HIV is an evenly balanced problem, with nearly half of all infections occurring in women.2 Women have received much more attention recently.
In the United States, about 300,000 women are living with HIV, and 10% of them are unaware of it. Between 1985 and 2013, the number of HIV cases in US women tripled.
The epidemic continues to disproportionately affect women of color. Two-thirds of all women with HIV are African American,2 and estimates suggest that 1 of every 32 African American women will acquire HIV during her lifetime. On a positive note, there was a 20% reduction in new infections among African American women between 2008 and 2010.3
The epidemic preys on the vulnerable and is fueled by poverty, lack of education (general and health literacy), substance abuse, and restricted access to health care. Major metropolitan areas such as New York, Washington, DC, Miami, and Los Angeles are “hot spots,” where high concentrations of infected people reside.4
Many women underestimate or do not perceive their susceptibility. They unknowingly acquire HIV infection from their male partners, many of whom are unaware of their infection. Some of their partners may lead a dual life of bisexuality. In some areas, an estimated 20% of men who have sex with men also engage in sex with women.5 If these women contract the disease, they may be diagnosed at a late stage and when they are symptomatic, or coincidentally during pregnancy and childbirth.
Negotiating safe sex practices can be difficult for a woman. She may perceive or lack empowerment to do so, fearing rejection, isolation, or violence. Sexual violence may have been initiated in childhood, through intimate partners, rape, sex trafficking, or prostitution. Patterns vary throughout the world, but sexual violence is more common than perceived.6 Because of shame, embarrassment, and isolation, many victims do not seek medical care and so may carry undiagnosed infections. Even when they access care, they are less likely to remain in the HIV care system.7 Greater efforts are needed to reach these women, make them feel supported in care, and keep them in the system.
TESTING IS CRUCIAL
Diagnosis remains a weak link in the chain of care for both men and women. Success has been noted in the form of a marked reduction in cases of mother-to-child transmission, thanks to near-universal opt-out screening during pregnancy or at delivery.
If appropriate routine testing were done for all people, as advocated by the US Centers for Disease Control and Prevention guidelines,8 more cases could be diagnosed, behaviors changed, and treatment offered. Control of HIV through treatment can lead to a 96% reduction in transmission between serodiscordant partners, as demonstrated in HPTN 052, an ongoing phase 3 trial.9 Early diagnosis and treatment offer the potential for improved immune regeneration and healthier lives.
PRE-EXPOSURE PROPHYLAXIS
Pre-exposure prophylaxis (PrEP) is one approach to empowering women and preventing HIV infection. Studies have demonstrated the efficacy of this approach, although some studies have not.10,11
An important finding in the failed studies appeared to be a lack of adherence to the regimen.11 Unless taken faithfully, PrEP will not succeed. Additionally, there may be inherent differences in outcomes for unknown reasons. Lack of access to the necessary two-drug combination regimen is another barrier.
PrEP is expensive, requires regular monitoring, and requires patients to remain engaged in medical care. Currently, not all medical programs offer PrEP, and not all insurance policies cover it. Further insight into long-term side effects and complications is needed.
Although PrEP is an attractive concept and a reality for some, it is an incomplete solution to prevention at this time.
MEN AND WOMEN ARE DIFFERENT
Men and women are different physiologically and psychologically. Women typically have a lower body mass, lower bone mass, and higher content of body fat. As a result, women may differ from men in their ability to tolerate medications, and long-term side effects may be more pronounced.
Women are also more likely to place family responsibilities above self-preservation and personal health concerns. As a result, providing for and taking care of their children takes precedence over care of their own health.
Providing care to women presents many challenges and opportunities to improve their health. Health care access, transportation, assistance with child care during medical visits, the availability of counseling to deal with shame, guilt, and depression, and maintaining women within the care system are but a few examples.
AGING WITH HIV: STUDY NEEDED
Antiretroviral therapy has enabled patients to survive and often to reach a normal life expectancy if the infection is diagnosed and treated early. As a result, HIV-associated causes of death have been replaced by non-HIV comorbidities typical of aging, such as cardiovascular disease, organ failure (heart, lung, kidney, liver), non-HIV cancers, and bone disease.
Women face unique aspects of aging with menopause, including an accelerated rate of bone loss resulting in osteoporosis. HIV itself and some antiretroviral drugs may increase the loss of bone mineral density. Alcohol abuse, sedentary lifestyle, smoking, hepatitis C co-infection, and poor nutrition also contribute to this problem. Bone disease and many other aspects of aging and HIV in women require more research and intervention.
Other areas that need to be studied are the unique mucosal immune system of the female genital tract, the interplay of sex hormones and the immune system, the role of genital tract inflammation in increasing the risk of HIV acquisition, sexual violence and HIV acquisition, and the safety and efficacy of PrEP for women. This will require prioritization and ongoing funding, which is becoming scarcer. If there is to be hope of containing this disease, our efforts to understand it must not diminish.
- Short WR, Anderson JR. Caring for women with HIV: unique needs and challenges. Cleve Clin J Med 2014; 81:691–701.
- UNAIDS. Women out loud: how women living with HIV will help the world end AIDS. www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20121211_Women_Out_Loud_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). HIV among women. www.cdc.gov/hiv/risk/gender/women/index.html. Accessed October 2, 2014.
- Hodder SL, Justman J, Hughes JP, et al; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–18.
- UNAIDS. Fact Sheet: women, girls, gender equality and HIV. www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120217_FS_WomenGirls_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). National intimate partner and sexual violence survey: 2010 summary report. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed October 2, 2014.
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr 2013; 64:32–38.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10 9:e1001511.
- Short WR, Anderson JR. Caring for women with HIV: unique needs and challenges. Cleve Clin J Med 2014; 81:691–701.
- UNAIDS. Women out loud: how women living with HIV will help the world end AIDS. www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20121211_Women_Out_Loud_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). HIV among women. www.cdc.gov/hiv/risk/gender/women/index.html. Accessed October 2, 2014.
- Hodder SL, Justman J, Hughes JP, et al; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–18.
- UNAIDS. Fact Sheet: women, girls, gender equality and HIV. www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120217_FS_WomenGirls_en.pdf. Accessed October 2, 2014.
- Centers for Disease Control and Prevention (CDC). National intimate partner and sexual violence survey: 2010 summary report. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed October 2, 2014.
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr 2013; 64:32–38.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10 9:e1001511.
Acute respiratory distress syndrome: Implications of recent studies
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
KEY POINTS
- The new definition of ARDS categorizes it as mild, moderate, or severe on the basis of oxygenation, specifically, the PaO2/FiO2 ratio.
- Neuromuscular blockade and prone positioning, used early in moderate or severe cases of ARDS, have shown some promise in trials, but questions remain about their application in critically ill patients.
- Based on two large trials, HFOV is no longer recommended as a primary therapy for ARDS, but it may still be considered as a rescue therapy in patients with refractory hypoxemia.
- In light of observational studies and randomized trials, ECMO should be considered an option in cases of refractory hypoxemia.
A 61-year-old man with fluctuating hypertension
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
Why are we doing cardiovascular outcome trials in type 2 diabetes?
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
KEY POINTS
- The worldwide burden of type 2 diabetes is increasing dramatically as obesity rates increase, populations age, and people around the world adopt a Western diet.
- Diabetes increases the risk of atherosclerotic cardiovascular disease, which remains the leading cause of death in diabetic patients.
- Lowering blood glucose alone may not necessarily amount to reduction in adverse cardiovascular events.
- Clinical trials of new drugs for type 2 diabetes must prove cardiovascular safety in addition to glucose-lowering potential before the drugs gain final regulatory approval.
- Aggressive risk factor modification (smoking cessation, control of hypertension, and treatment of hyperlipidemia with statins) remains paramount in reducing cardiovascular risk in people with diabetes.
Diabetes management: More than just cardiovascular risk?
Diabetes mellitus and its management have become the center of controversy in recent years. More emphasis is being placed on the potential for adverse cardiovascular outcomes with more aggressive glycemic control as well as on the potential for adverse cardiovascular events with newer antidiabetic therapies, and less on the importance of glycemic control, particularly early in the disease course.
Although it is important to take new data into consideration when managing diabetes, it appears that the results of recent clinical trials are being misinterpreted and incorrectly applied to the wrong patient populations, and in the process, the results of older landmark clinical trials are being neglected. Inadequate glycemic control not only plays a role in cardiovascular risk, it also remains the leading cause of blindness, kidney failure, and nontraumatic lower-limb amputations in the United States.1
Although we need to recognize the potential for adverse cardiovascular outcomes with diabetes and its management, we cannot lose sight of the big picture—ie, that inadequate glycemic control confers both microvascular and macrovascular risk, and that the available data show that restoring near-euglycemia in patients with diabetes considerably reduces the risk of microvascular and macrovascular complications.
Several recently published clinical trials—the Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 the Veterans Affairs Diabetes Trial (VADT),3 and the Action in Diabetes and Vascular Disease (ADVANCE)4—failed to demonstrate improved cardiovascular outcomes with improved glycemic control. However, we should not take this to mean that glycemic control is unimportant.
In this article, we will discuss why the results of these recent clinical trials are not valid for the general population of patients with diabetes. We will review evidence from landmark clinical trials that clearly demonstrates that better glycemic control reduces both microvascular and macrovascular complications of diabetes (the “glucose hypothesis”). We contend that excellent glycemic control clearly decreases the microvascular complications of diabetes, and that results from long-term follow-up studies in both type 1 and type 2 diabetes show reduced rates of heart attack and stroke in patients treated intensively earlier in the course of their disease.5,6
EVIDENCE FOR THE GLUCOSE HYPOTHESIS
Diabetes Control and Complications Trial
The first major trial demonstrating that improved glycemic control provides benefit was the Diabetes Control and Complications Trial (DCCT).7 This study enrolled 1,441 patients with insulin-dependent diabetes mellitus, 726 of whom had no retinopathy at baseline (the primary-prevention cohort) and 715 of whom had mild retinopathy (the secondary-intervention cohort).
Patients were randomly assigned to intensive therapy (three or more insulin injections per day or an insulin pump) or to conventional therapy with one or two daily insulin injections. They were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly.
During the study, the hemoglobin A1c level averaged 9% in the control group and 7% in the intensively treated group. The cumulative incidence of retinopathy was defined as a change of three steps or more on fundus photography that was sustained over a 6-month period.
Effect on retinopathy. At study completion, the cumulative incidence of retinopathy in the intensive-therapy group was approximately 50% less than in the conventional-therapy group. Intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% confidence interval [CI] 62%–85%) in the primary-prevention cohort. In the secondary-prevention cohort, intensive therapy reduced the average risk of progression by 54% (95% CI 39%–66%). Intensive therapy reduced the adjusted risk of proliferative or severe nonproliferative retinopathy by 47% (P = .011) and that of treatment with photocoagulation by 56% (P = .002).
Effect on nephropathy. Intensive therapy reduced the mean adjusted risk of microalbuminuria by 34% (P = .04) in the primary-prevention cohort and by 43% (P = .001) in the secondary-intervention cohort. The risk of macroalbuminuria was reduced by 56% (P = .01) in the secondary-intervention cohort.
Effect on neuropathy. In the patients in the primary-prevention cohort who did not have neuropathy at baseline, intensive therapy reduced the incidence of neuropathy at 5 years by 69% (to 3%, vs 10% in the conventional-therapy group; P = .006). Similarly, in the secondary-intervention cohort, intensive therapy reduced the incidence of clinical neuropathy at 5 years by 57% (to 7%, vs 16%; P < .001).
Effect on macrovascular events. In the initial trial, a nonsignificant 41% reduction in combined cardiovascular and peripheral vascular disease events was observed.
DCCT long-term follow-up
After DCCT concluded, the control and treatment groups’ hemoglobin A1c levels converged to approximately 8%. The two groups were then followed to determine the long-term effects of their prior separation of glycemic levels on micro- and macrovascular out comes.5 More than 90% of the original DCCT patients were followed for a mean of 17 years.
Intensive treatment reduced the risk of any cardiovascular disease event by 42% (95% CI 9%–63%; P = .02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (95% CI 12%– 79%; P = .02). This result was observed despite separation of glucose control in the two groups only for the first 6.5 years. This beneficial effect of intensive early glycemic control has been termed metabolic memory.
United Kingdom Prospective Diabetes Study
A second major trial, the United Kingdom Prospective Diabetes Study (UKPDS),8 assessed the effect of excellent diabetes control on diabetes complications in patients with type 2 diabetes. A total of 3,867 patients newly diagnosed with type 2 diabetes, median age 54, who after 3 months of diet treatment had mean fasting plasma glucose concentrations of 110 to 270 mg/dL, were randomly assigned to an intensive policy (with a sulfonylurea or insulin or, if overweight, metformin) or a conventional policy with diet. The aim in the intensive group was a fasting plasma glucose less than 108 mg/dL. In the conventional group, the aim was the best achievable fasting plasma glucose with diet alone; drugs were added only if there were hyperglycemic symptoms or a fasting plasma glucose greater than 270 mg/dL.
Over 10 years, the median hemoglobin A1c level was 7.0% (interquartile range 6.2%–8.2%) in the intensive group compared with 7.9% (6.9%–8.8%) in the conventional group. Compared with the conventional group, the risk of any diabetes-related end point was 12% lower in the intensive group (95% CI 1%–21%, P = .029), the risk of any diabetes-related death was 10% lower (−11% to 27%, P = .34), and the rate of all-cause mortality was 6% lower (−10% to 20%, P = .44). Most of the reduction in risk of any diabetes-related end point was from a 25% risk reduction (95% CI 7%–40%, P = .0099) in microvascular end points, including the need for retinal photocoagulation.
UKPDS long-term follow-up
In 2008, Holman et al published the results of long-term follow-up of patients included in the UKPDS.6 In posttrial monitoring, 3,277 patients were asked to attend annual UKPDS clinics for 5 years, but no attempts were made to maintain their previously assigned therapies. Annual questionnaires were used to follow patients who were unable to attend the clinics, and all patients in years 6 to 10 were assessed through questionnaires.
Between-group differences in hemoglobin A1c levels were lost after the first year. However, in the sulfonylurea-insulin group, relative reductions in risk persisted at 10 years for any diabetes-related end point (9%, P = .04) and microvascular disease (24%, P = .001), while risk reductions for myocardial infarction (15%, P = .01) and death from any cause (13%, P = .007) emerged over time as more events occurred. In the metformin group, significant risk reductions persisted for any diabetes-related end point (21%, P = .01), myocardial infarction (33%, P = .005), and death from any cause (27%, P = .002).
The long-term follow-up to the UKPDS, like the long-term follow-up to the DCCT, demonstrated metabolic memory: that is, despite an early loss of glycemic differences after completion of the trial, a continued reduction in microvascular risk and an emergent risk reduction for myocardial infarction and death from any cause were observed.
These long-term randomized prospective trials in patients with type 1 and type 2 diabetes clearly show that the glucose hypothesis is in fact correct: intensive glucose control lowers the risk of both microvascular and macrovascular complications of diabetes.
IS THERE DISCORDANCE BETWEEN OLDER AND MORE RECENT TRIALS?
If the results of these older landmark clinical trials are true, why did the more recent clinical trials fail to show cardiovascular benefit with stricter glycemic control, and in one trial2 demonstrate the potential for harm? (ACCORD2 found an increased death rate in patients who received intensive therapy, targeting a hemoglobin A1c below 6.0%.)
The answer lies in the populations studied. ACCORD,2 VADT,3 and ADVANCE4 were performed in older patients with prior cardiac events or with several risk factors for cardiovascular events. The study populations were picked to increase the number of cardiac events in a short time frame. Therefore, extrapolating the results of these studies to the younger population of patients with diabetes, most of whom have yet to develop macrovascular disease, is inappropriate.
The available evidence suggests that early aggressive management of diabetes reduces the risk of macrovascular disease, but that this benefit is delayed. In the UKPDS and DCCT trials, it took 10 to 17 years to show cardiac benefit in younger patients.
The results of ACCORD,2 VADT,3 and ADVANCE4 are important when considered in the correct clinical context. Two of these trials did demonstrate some microvascular benefit as a result of better glycemic control in older patients, many of whom had longstanding diabetes. These studies suggest that, in patients who already have established cardiovascular disease or have several risk factors for cardiovascular events, a less-strict glycemic target may be warranted.
These trials should not be interpreted as saying that glycemic control is unimportant in older patients at higher risk. Rather, they suggest that an individualized approach to diabetes management, supported by the most recent American Diabetes Association guidelines,9 is more appropriate.
Physicians may reasonably suggest a stricter A1c goal (ie, < 6.5%) in certain patients if it can be achieved without significant hypoglycemia. Stricter glycemic targets would seem appropriate in patients recently diagnosed with diabetes, those who have a long life expectancy, and those who have not yet developed significant cardiovascular disease.9
However, in patients who already have developed advanced microvascular and macrovascular complications, who have long-standing diabetes, who have a history of severe hypoglycemia (or hypoglycemia unawareness), or who have a limited life expectancy or numerous adverse comorbidities, a less strict glycemic target (hemoglobin A1c < 8%) may be more appropriate.9
CARDIOVASCULAR RISK, HYPOGLYCEMIA, AND ATTAINING GLYCEMIC TARGETS
Metformin, in the absence of contraindications or intolerability, is generally the recommended first-line therapy to manage glycemia in patients with type 2 diabetes mellitus.10,11 However, there are only limited data to direct clinicians as to which antidiabetic medication to use if further therapy is required to obtain glycemic control.
Much of the cardiovascular and mortality risk associated with aggressive diabetes management (ie, lower A1c targets) is related to hypoglycemia. Thus, antidiabetic therapies that pose no risk or only a low risk of hypoglycemia should be chosen, particularly in older patients and in those with known cardiovascular disease. This may allow for better glycemic control without the risk of hypoglycemia and adverse cardiovascular outcomes.
However, in practice, clinicians continue to use a sulfonylurea as the second-line agent. Although sulfonylureas may appear to be a great option because of their low cost, they are associated with a higher risk of hypoglycemic episodes than other classes of diabetes drugs. We need to consider the frequency and cost of hypoglycemic episodes and the potential morbidity associated with them, because these episodes are a barrier to our efforts to achieve better glycemic control.
Budnitz et al12 reported that from 2007 through 2009, in US adults age 65 and older, insulins were implicated in 13.9% of hospitalizations related to adverse drug events, and oral hypoglycemic agents (ie, insulin secretagogues) in 10.7%.
Quilliam et al13 reported that hypoglycemia resulted in a mean cost of $17,564 for an inpatient admission, $1,387 for an emergency department visit, and $394 for an outpatient visit. Thus, the cost savings associated with prescribing a sulfonylurea vs one of the newer oral antidiabetic agents that do not increase the risk of hypoglycemia (unless used concurrently with insulin or an insulin secretagogue) can quickly be eroded by severe hypoglycemic episodes requiring medical care.
Moreover, once patients start to experience hypoglycemic episodes, they are very reluctant, as are their physicians, to intensify therapy, even if it is indicated by their elevated A1c.
There are now seven classes of oral antidiabetic therapies other than insulin secretagogues (ie, other than sulfonylureas and the meglitinides nateglinide and repaglinide), as well as a few noninsulin injectable therapies (glucagon-like peptide-1 agonists), that are not associated with hypoglycemia. We believe these agents should be tried before prescribing an agent that carries the risk of hypoglycemia (ie, sulfonylureas).
If agents that do not cause hypoglycemia are used, more-aggressive glycemic targets may be achieved safely. The ACCORD study,2 which included patients at high cardiovascular risk and aimed at an aggressive glycemic target of 6%, may have yielded much different results had agents that carry a high risk of hypoglycemia been excluded.
Of importance, cardiovascular risk is also influenced by the common comorbidities seen in patients with diabetes, such as hypertension and hypercholesterolemia. Intensive, multifactorial interventions that address not only glycemic control but also blood pressure and lipids and that include low-dose aspirin therapy have been shown to lower the risk of death from cardiovascular causes and the risk of cardiovascular events.14 Likewise, smoking cessation is very important in reducing cardiovascular risk, especially in patients with diabetes.15
CLINICAL TRIALS IN CONTEXT
In conclusion, there is more to diabetes management than cardiovascular complications. Clearly, improved glycemic control decreases the risk of retinopathy, nephropathy, and neuropathy in patients with type 1 and type 2 diabetes. The DCCT and UKPDS extension studies further found that excellent glycemic control decreases rates of cardiac events.
The best way to treat diabetes may be different in otherwise healthy younger patients who have yet to develop significant complications than it is in older patients known to have cardiovascular disease or several risk factors for cardiovascular events. The available evidence suggests it would be reasonable to aim for stricter glycemic targets in the younger patients and less stringent targets in the older patients, particularly in those with long-standing diabetes who have already developed significant micro- and macrovascular complications.
We should interpret clinical trials within their narrow clinical context, emphasizing the actual population of patients included in the study, so as to avoid the inappropriate extrapolation of the results to all.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 7, 2014.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(Suppl 1):S11–S66.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011; 17:673–680.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
Diabetes mellitus and its management have become the center of controversy in recent years. More emphasis is being placed on the potential for adverse cardiovascular outcomes with more aggressive glycemic control as well as on the potential for adverse cardiovascular events with newer antidiabetic therapies, and less on the importance of glycemic control, particularly early in the disease course.
Although it is important to take new data into consideration when managing diabetes, it appears that the results of recent clinical trials are being misinterpreted and incorrectly applied to the wrong patient populations, and in the process, the results of older landmark clinical trials are being neglected. Inadequate glycemic control not only plays a role in cardiovascular risk, it also remains the leading cause of blindness, kidney failure, and nontraumatic lower-limb amputations in the United States.1
Although we need to recognize the potential for adverse cardiovascular outcomes with diabetes and its management, we cannot lose sight of the big picture—ie, that inadequate glycemic control confers both microvascular and macrovascular risk, and that the available data show that restoring near-euglycemia in patients with diabetes considerably reduces the risk of microvascular and macrovascular complications.
Several recently published clinical trials—the Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 the Veterans Affairs Diabetes Trial (VADT),3 and the Action in Diabetes and Vascular Disease (ADVANCE)4—failed to demonstrate improved cardiovascular outcomes with improved glycemic control. However, we should not take this to mean that glycemic control is unimportant.
In this article, we will discuss why the results of these recent clinical trials are not valid for the general population of patients with diabetes. We will review evidence from landmark clinical trials that clearly demonstrates that better glycemic control reduces both microvascular and macrovascular complications of diabetes (the “glucose hypothesis”). We contend that excellent glycemic control clearly decreases the microvascular complications of diabetes, and that results from long-term follow-up studies in both type 1 and type 2 diabetes show reduced rates of heart attack and stroke in patients treated intensively earlier in the course of their disease.5,6
EVIDENCE FOR THE GLUCOSE HYPOTHESIS
Diabetes Control and Complications Trial
The first major trial demonstrating that improved glycemic control provides benefit was the Diabetes Control and Complications Trial (DCCT).7 This study enrolled 1,441 patients with insulin-dependent diabetes mellitus, 726 of whom had no retinopathy at baseline (the primary-prevention cohort) and 715 of whom had mild retinopathy (the secondary-intervention cohort).
Patients were randomly assigned to intensive therapy (three or more insulin injections per day or an insulin pump) or to conventional therapy with one or two daily insulin injections. They were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly.
During the study, the hemoglobin A1c level averaged 9% in the control group and 7% in the intensively treated group. The cumulative incidence of retinopathy was defined as a change of three steps or more on fundus photography that was sustained over a 6-month period.
Effect on retinopathy. At study completion, the cumulative incidence of retinopathy in the intensive-therapy group was approximately 50% less than in the conventional-therapy group. Intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% confidence interval [CI] 62%–85%) in the primary-prevention cohort. In the secondary-prevention cohort, intensive therapy reduced the average risk of progression by 54% (95% CI 39%–66%). Intensive therapy reduced the adjusted risk of proliferative or severe nonproliferative retinopathy by 47% (P = .011) and that of treatment with photocoagulation by 56% (P = .002).
Effect on nephropathy. Intensive therapy reduced the mean adjusted risk of microalbuminuria by 34% (P = .04) in the primary-prevention cohort and by 43% (P = .001) in the secondary-intervention cohort. The risk of macroalbuminuria was reduced by 56% (P = .01) in the secondary-intervention cohort.
Effect on neuropathy. In the patients in the primary-prevention cohort who did not have neuropathy at baseline, intensive therapy reduced the incidence of neuropathy at 5 years by 69% (to 3%, vs 10% in the conventional-therapy group; P = .006). Similarly, in the secondary-intervention cohort, intensive therapy reduced the incidence of clinical neuropathy at 5 years by 57% (to 7%, vs 16%; P < .001).
Effect on macrovascular events. In the initial trial, a nonsignificant 41% reduction in combined cardiovascular and peripheral vascular disease events was observed.
DCCT long-term follow-up
After DCCT concluded, the control and treatment groups’ hemoglobin A1c levels converged to approximately 8%. The two groups were then followed to determine the long-term effects of their prior separation of glycemic levels on micro- and macrovascular out comes.5 More than 90% of the original DCCT patients were followed for a mean of 17 years.
Intensive treatment reduced the risk of any cardiovascular disease event by 42% (95% CI 9%–63%; P = .02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (95% CI 12%– 79%; P = .02). This result was observed despite separation of glucose control in the two groups only for the first 6.5 years. This beneficial effect of intensive early glycemic control has been termed metabolic memory.
United Kingdom Prospective Diabetes Study
A second major trial, the United Kingdom Prospective Diabetes Study (UKPDS),8 assessed the effect of excellent diabetes control on diabetes complications in patients with type 2 diabetes. A total of 3,867 patients newly diagnosed with type 2 diabetes, median age 54, who after 3 months of diet treatment had mean fasting plasma glucose concentrations of 110 to 270 mg/dL, were randomly assigned to an intensive policy (with a sulfonylurea or insulin or, if overweight, metformin) or a conventional policy with diet. The aim in the intensive group was a fasting plasma glucose less than 108 mg/dL. In the conventional group, the aim was the best achievable fasting plasma glucose with diet alone; drugs were added only if there were hyperglycemic symptoms or a fasting plasma glucose greater than 270 mg/dL.
Over 10 years, the median hemoglobin A1c level was 7.0% (interquartile range 6.2%–8.2%) in the intensive group compared with 7.9% (6.9%–8.8%) in the conventional group. Compared with the conventional group, the risk of any diabetes-related end point was 12% lower in the intensive group (95% CI 1%–21%, P = .029), the risk of any diabetes-related death was 10% lower (−11% to 27%, P = .34), and the rate of all-cause mortality was 6% lower (−10% to 20%, P = .44). Most of the reduction in risk of any diabetes-related end point was from a 25% risk reduction (95% CI 7%–40%, P = .0099) in microvascular end points, including the need for retinal photocoagulation.
UKPDS long-term follow-up
In 2008, Holman et al published the results of long-term follow-up of patients included in the UKPDS.6 In posttrial monitoring, 3,277 patients were asked to attend annual UKPDS clinics for 5 years, but no attempts were made to maintain their previously assigned therapies. Annual questionnaires were used to follow patients who were unable to attend the clinics, and all patients in years 6 to 10 were assessed through questionnaires.
Between-group differences in hemoglobin A1c levels were lost after the first year. However, in the sulfonylurea-insulin group, relative reductions in risk persisted at 10 years for any diabetes-related end point (9%, P = .04) and microvascular disease (24%, P = .001), while risk reductions for myocardial infarction (15%, P = .01) and death from any cause (13%, P = .007) emerged over time as more events occurred. In the metformin group, significant risk reductions persisted for any diabetes-related end point (21%, P = .01), myocardial infarction (33%, P = .005), and death from any cause (27%, P = .002).
The long-term follow-up to the UKPDS, like the long-term follow-up to the DCCT, demonstrated metabolic memory: that is, despite an early loss of glycemic differences after completion of the trial, a continued reduction in microvascular risk and an emergent risk reduction for myocardial infarction and death from any cause were observed.
These long-term randomized prospective trials in patients with type 1 and type 2 diabetes clearly show that the glucose hypothesis is in fact correct: intensive glucose control lowers the risk of both microvascular and macrovascular complications of diabetes.
IS THERE DISCORDANCE BETWEEN OLDER AND MORE RECENT TRIALS?
If the results of these older landmark clinical trials are true, why did the more recent clinical trials fail to show cardiovascular benefit with stricter glycemic control, and in one trial2 demonstrate the potential for harm? (ACCORD2 found an increased death rate in patients who received intensive therapy, targeting a hemoglobin A1c below 6.0%.)
The answer lies in the populations studied. ACCORD,2 VADT,3 and ADVANCE4 were performed in older patients with prior cardiac events or with several risk factors for cardiovascular events. The study populations were picked to increase the number of cardiac events in a short time frame. Therefore, extrapolating the results of these studies to the younger population of patients with diabetes, most of whom have yet to develop macrovascular disease, is inappropriate.
The available evidence suggests that early aggressive management of diabetes reduces the risk of macrovascular disease, but that this benefit is delayed. In the UKPDS and DCCT trials, it took 10 to 17 years to show cardiac benefit in younger patients.
The results of ACCORD,2 VADT,3 and ADVANCE4 are important when considered in the correct clinical context. Two of these trials did demonstrate some microvascular benefit as a result of better glycemic control in older patients, many of whom had longstanding diabetes. These studies suggest that, in patients who already have established cardiovascular disease or have several risk factors for cardiovascular events, a less-strict glycemic target may be warranted.
These trials should not be interpreted as saying that glycemic control is unimportant in older patients at higher risk. Rather, they suggest that an individualized approach to diabetes management, supported by the most recent American Diabetes Association guidelines,9 is more appropriate.
Physicians may reasonably suggest a stricter A1c goal (ie, < 6.5%) in certain patients if it can be achieved without significant hypoglycemia. Stricter glycemic targets would seem appropriate in patients recently diagnosed with diabetes, those who have a long life expectancy, and those who have not yet developed significant cardiovascular disease.9
However, in patients who already have developed advanced microvascular and macrovascular complications, who have long-standing diabetes, who have a history of severe hypoglycemia (or hypoglycemia unawareness), or who have a limited life expectancy or numerous adverse comorbidities, a less strict glycemic target (hemoglobin A1c < 8%) may be more appropriate.9
CARDIOVASCULAR RISK, HYPOGLYCEMIA, AND ATTAINING GLYCEMIC TARGETS
Metformin, in the absence of contraindications or intolerability, is generally the recommended first-line therapy to manage glycemia in patients with type 2 diabetes mellitus.10,11 However, there are only limited data to direct clinicians as to which antidiabetic medication to use if further therapy is required to obtain glycemic control.
Much of the cardiovascular and mortality risk associated with aggressive diabetes management (ie, lower A1c targets) is related to hypoglycemia. Thus, antidiabetic therapies that pose no risk or only a low risk of hypoglycemia should be chosen, particularly in older patients and in those with known cardiovascular disease. This may allow for better glycemic control without the risk of hypoglycemia and adverse cardiovascular outcomes.
However, in practice, clinicians continue to use a sulfonylurea as the second-line agent. Although sulfonylureas may appear to be a great option because of their low cost, they are associated with a higher risk of hypoglycemic episodes than other classes of diabetes drugs. We need to consider the frequency and cost of hypoglycemic episodes and the potential morbidity associated with them, because these episodes are a barrier to our efforts to achieve better glycemic control.
Budnitz et al12 reported that from 2007 through 2009, in US adults age 65 and older, insulins were implicated in 13.9% of hospitalizations related to adverse drug events, and oral hypoglycemic agents (ie, insulin secretagogues) in 10.7%.
Quilliam et al13 reported that hypoglycemia resulted in a mean cost of $17,564 for an inpatient admission, $1,387 for an emergency department visit, and $394 for an outpatient visit. Thus, the cost savings associated with prescribing a sulfonylurea vs one of the newer oral antidiabetic agents that do not increase the risk of hypoglycemia (unless used concurrently with insulin or an insulin secretagogue) can quickly be eroded by severe hypoglycemic episodes requiring medical care.
Moreover, once patients start to experience hypoglycemic episodes, they are very reluctant, as are their physicians, to intensify therapy, even if it is indicated by their elevated A1c.
There are now seven classes of oral antidiabetic therapies other than insulin secretagogues (ie, other than sulfonylureas and the meglitinides nateglinide and repaglinide), as well as a few noninsulin injectable therapies (glucagon-like peptide-1 agonists), that are not associated with hypoglycemia. We believe these agents should be tried before prescribing an agent that carries the risk of hypoglycemia (ie, sulfonylureas).
If agents that do not cause hypoglycemia are used, more-aggressive glycemic targets may be achieved safely. The ACCORD study,2 which included patients at high cardiovascular risk and aimed at an aggressive glycemic target of 6%, may have yielded much different results had agents that carry a high risk of hypoglycemia been excluded.
Of importance, cardiovascular risk is also influenced by the common comorbidities seen in patients with diabetes, such as hypertension and hypercholesterolemia. Intensive, multifactorial interventions that address not only glycemic control but also blood pressure and lipids and that include low-dose aspirin therapy have been shown to lower the risk of death from cardiovascular causes and the risk of cardiovascular events.14 Likewise, smoking cessation is very important in reducing cardiovascular risk, especially in patients with diabetes.15
CLINICAL TRIALS IN CONTEXT
In conclusion, there is more to diabetes management than cardiovascular complications. Clearly, improved glycemic control decreases the risk of retinopathy, nephropathy, and neuropathy in patients with type 1 and type 2 diabetes. The DCCT and UKPDS extension studies further found that excellent glycemic control decreases rates of cardiac events.
The best way to treat diabetes may be different in otherwise healthy younger patients who have yet to develop significant complications than it is in older patients known to have cardiovascular disease or several risk factors for cardiovascular events. The available evidence suggests it would be reasonable to aim for stricter glycemic targets in the younger patients and less stringent targets in the older patients, particularly in those with long-standing diabetes who have already developed significant micro- and macrovascular complications.
We should interpret clinical trials within their narrow clinical context, emphasizing the actual population of patients included in the study, so as to avoid the inappropriate extrapolation of the results to all.
Diabetes mellitus and its management have become the center of controversy in recent years. More emphasis is being placed on the potential for adverse cardiovascular outcomes with more aggressive glycemic control as well as on the potential for adverse cardiovascular events with newer antidiabetic therapies, and less on the importance of glycemic control, particularly early in the disease course.
Although it is important to take new data into consideration when managing diabetes, it appears that the results of recent clinical trials are being misinterpreted and incorrectly applied to the wrong patient populations, and in the process, the results of older landmark clinical trials are being neglected. Inadequate glycemic control not only plays a role in cardiovascular risk, it also remains the leading cause of blindness, kidney failure, and nontraumatic lower-limb amputations in the United States.1
Although we need to recognize the potential for adverse cardiovascular outcomes with diabetes and its management, we cannot lose sight of the big picture—ie, that inadequate glycemic control confers both microvascular and macrovascular risk, and that the available data show that restoring near-euglycemia in patients with diabetes considerably reduces the risk of microvascular and macrovascular complications.
Several recently published clinical trials—the Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 the Veterans Affairs Diabetes Trial (VADT),3 and the Action in Diabetes and Vascular Disease (ADVANCE)4—failed to demonstrate improved cardiovascular outcomes with improved glycemic control. However, we should not take this to mean that glycemic control is unimportant.
In this article, we will discuss why the results of these recent clinical trials are not valid for the general population of patients with diabetes. We will review evidence from landmark clinical trials that clearly demonstrates that better glycemic control reduces both microvascular and macrovascular complications of diabetes (the “glucose hypothesis”). We contend that excellent glycemic control clearly decreases the microvascular complications of diabetes, and that results from long-term follow-up studies in both type 1 and type 2 diabetes show reduced rates of heart attack and stroke in patients treated intensively earlier in the course of their disease.5,6
EVIDENCE FOR THE GLUCOSE HYPOTHESIS
Diabetes Control and Complications Trial
The first major trial demonstrating that improved glycemic control provides benefit was the Diabetes Control and Complications Trial (DCCT).7 This study enrolled 1,441 patients with insulin-dependent diabetes mellitus, 726 of whom had no retinopathy at baseline (the primary-prevention cohort) and 715 of whom had mild retinopathy (the secondary-intervention cohort).
Patients were randomly assigned to intensive therapy (three or more insulin injections per day or an insulin pump) or to conventional therapy with one or two daily insulin injections. They were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly.
During the study, the hemoglobin A1c level averaged 9% in the control group and 7% in the intensively treated group. The cumulative incidence of retinopathy was defined as a change of three steps or more on fundus photography that was sustained over a 6-month period.
Effect on retinopathy. At study completion, the cumulative incidence of retinopathy in the intensive-therapy group was approximately 50% less than in the conventional-therapy group. Intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% confidence interval [CI] 62%–85%) in the primary-prevention cohort. In the secondary-prevention cohort, intensive therapy reduced the average risk of progression by 54% (95% CI 39%–66%). Intensive therapy reduced the adjusted risk of proliferative or severe nonproliferative retinopathy by 47% (P = .011) and that of treatment with photocoagulation by 56% (P = .002).
Effect on nephropathy. Intensive therapy reduced the mean adjusted risk of microalbuminuria by 34% (P = .04) in the primary-prevention cohort and by 43% (P = .001) in the secondary-intervention cohort. The risk of macroalbuminuria was reduced by 56% (P = .01) in the secondary-intervention cohort.
Effect on neuropathy. In the patients in the primary-prevention cohort who did not have neuropathy at baseline, intensive therapy reduced the incidence of neuropathy at 5 years by 69% (to 3%, vs 10% in the conventional-therapy group; P = .006). Similarly, in the secondary-intervention cohort, intensive therapy reduced the incidence of clinical neuropathy at 5 years by 57% (to 7%, vs 16%; P < .001).
Effect on macrovascular events. In the initial trial, a nonsignificant 41% reduction in combined cardiovascular and peripheral vascular disease events was observed.
DCCT long-term follow-up
After DCCT concluded, the control and treatment groups’ hemoglobin A1c levels converged to approximately 8%. The two groups were then followed to determine the long-term effects of their prior separation of glycemic levels on micro- and macrovascular out comes.5 More than 90% of the original DCCT patients were followed for a mean of 17 years.
Intensive treatment reduced the risk of any cardiovascular disease event by 42% (95% CI 9%–63%; P = .02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (95% CI 12%– 79%; P = .02). This result was observed despite separation of glucose control in the two groups only for the first 6.5 years. This beneficial effect of intensive early glycemic control has been termed metabolic memory.
United Kingdom Prospective Diabetes Study
A second major trial, the United Kingdom Prospective Diabetes Study (UKPDS),8 assessed the effect of excellent diabetes control on diabetes complications in patients with type 2 diabetes. A total of 3,867 patients newly diagnosed with type 2 diabetes, median age 54, who after 3 months of diet treatment had mean fasting plasma glucose concentrations of 110 to 270 mg/dL, were randomly assigned to an intensive policy (with a sulfonylurea or insulin or, if overweight, metformin) or a conventional policy with diet. The aim in the intensive group was a fasting plasma glucose less than 108 mg/dL. In the conventional group, the aim was the best achievable fasting plasma glucose with diet alone; drugs were added only if there were hyperglycemic symptoms or a fasting plasma glucose greater than 270 mg/dL.
Over 10 years, the median hemoglobin A1c level was 7.0% (interquartile range 6.2%–8.2%) in the intensive group compared with 7.9% (6.9%–8.8%) in the conventional group. Compared with the conventional group, the risk of any diabetes-related end point was 12% lower in the intensive group (95% CI 1%–21%, P = .029), the risk of any diabetes-related death was 10% lower (−11% to 27%, P = .34), and the rate of all-cause mortality was 6% lower (−10% to 20%, P = .44). Most of the reduction in risk of any diabetes-related end point was from a 25% risk reduction (95% CI 7%–40%, P = .0099) in microvascular end points, including the need for retinal photocoagulation.
UKPDS long-term follow-up
In 2008, Holman et al published the results of long-term follow-up of patients included in the UKPDS.6 In posttrial monitoring, 3,277 patients were asked to attend annual UKPDS clinics for 5 years, but no attempts were made to maintain their previously assigned therapies. Annual questionnaires were used to follow patients who were unable to attend the clinics, and all patients in years 6 to 10 were assessed through questionnaires.
Between-group differences in hemoglobin A1c levels were lost after the first year. However, in the sulfonylurea-insulin group, relative reductions in risk persisted at 10 years for any diabetes-related end point (9%, P = .04) and microvascular disease (24%, P = .001), while risk reductions for myocardial infarction (15%, P = .01) and death from any cause (13%, P = .007) emerged over time as more events occurred. In the metformin group, significant risk reductions persisted for any diabetes-related end point (21%, P = .01), myocardial infarction (33%, P = .005), and death from any cause (27%, P = .002).
The long-term follow-up to the UKPDS, like the long-term follow-up to the DCCT, demonstrated metabolic memory: that is, despite an early loss of glycemic differences after completion of the trial, a continued reduction in microvascular risk and an emergent risk reduction for myocardial infarction and death from any cause were observed.
These long-term randomized prospective trials in patients with type 1 and type 2 diabetes clearly show that the glucose hypothesis is in fact correct: intensive glucose control lowers the risk of both microvascular and macrovascular complications of diabetes.
IS THERE DISCORDANCE BETWEEN OLDER AND MORE RECENT TRIALS?
If the results of these older landmark clinical trials are true, why did the more recent clinical trials fail to show cardiovascular benefit with stricter glycemic control, and in one trial2 demonstrate the potential for harm? (ACCORD2 found an increased death rate in patients who received intensive therapy, targeting a hemoglobin A1c below 6.0%.)
The answer lies in the populations studied. ACCORD,2 VADT,3 and ADVANCE4 were performed in older patients with prior cardiac events or with several risk factors for cardiovascular events. The study populations were picked to increase the number of cardiac events in a short time frame. Therefore, extrapolating the results of these studies to the younger population of patients with diabetes, most of whom have yet to develop macrovascular disease, is inappropriate.
The available evidence suggests that early aggressive management of diabetes reduces the risk of macrovascular disease, but that this benefit is delayed. In the UKPDS and DCCT trials, it took 10 to 17 years to show cardiac benefit in younger patients.
The results of ACCORD,2 VADT,3 and ADVANCE4 are important when considered in the correct clinical context. Two of these trials did demonstrate some microvascular benefit as a result of better glycemic control in older patients, many of whom had longstanding diabetes. These studies suggest that, in patients who already have established cardiovascular disease or have several risk factors for cardiovascular events, a less-strict glycemic target may be warranted.
These trials should not be interpreted as saying that glycemic control is unimportant in older patients at higher risk. Rather, they suggest that an individualized approach to diabetes management, supported by the most recent American Diabetes Association guidelines,9 is more appropriate.
Physicians may reasonably suggest a stricter A1c goal (ie, < 6.5%) in certain patients if it can be achieved without significant hypoglycemia. Stricter glycemic targets would seem appropriate in patients recently diagnosed with diabetes, those who have a long life expectancy, and those who have not yet developed significant cardiovascular disease.9
However, in patients who already have developed advanced microvascular and macrovascular complications, who have long-standing diabetes, who have a history of severe hypoglycemia (or hypoglycemia unawareness), or who have a limited life expectancy or numerous adverse comorbidities, a less strict glycemic target (hemoglobin A1c < 8%) may be more appropriate.9
CARDIOVASCULAR RISK, HYPOGLYCEMIA, AND ATTAINING GLYCEMIC TARGETS
Metformin, in the absence of contraindications or intolerability, is generally the recommended first-line therapy to manage glycemia in patients with type 2 diabetes mellitus.10,11 However, there are only limited data to direct clinicians as to which antidiabetic medication to use if further therapy is required to obtain glycemic control.
Much of the cardiovascular and mortality risk associated with aggressive diabetes management (ie, lower A1c targets) is related to hypoglycemia. Thus, antidiabetic therapies that pose no risk or only a low risk of hypoglycemia should be chosen, particularly in older patients and in those with known cardiovascular disease. This may allow for better glycemic control without the risk of hypoglycemia and adverse cardiovascular outcomes.
However, in practice, clinicians continue to use a sulfonylurea as the second-line agent. Although sulfonylureas may appear to be a great option because of their low cost, they are associated with a higher risk of hypoglycemic episodes than other classes of diabetes drugs. We need to consider the frequency and cost of hypoglycemic episodes and the potential morbidity associated with them, because these episodes are a barrier to our efforts to achieve better glycemic control.
Budnitz et al12 reported that from 2007 through 2009, in US adults age 65 and older, insulins were implicated in 13.9% of hospitalizations related to adverse drug events, and oral hypoglycemic agents (ie, insulin secretagogues) in 10.7%.
Quilliam et al13 reported that hypoglycemia resulted in a mean cost of $17,564 for an inpatient admission, $1,387 for an emergency department visit, and $394 for an outpatient visit. Thus, the cost savings associated with prescribing a sulfonylurea vs one of the newer oral antidiabetic agents that do not increase the risk of hypoglycemia (unless used concurrently with insulin or an insulin secretagogue) can quickly be eroded by severe hypoglycemic episodes requiring medical care.
Moreover, once patients start to experience hypoglycemic episodes, they are very reluctant, as are their physicians, to intensify therapy, even if it is indicated by their elevated A1c.
There are now seven classes of oral antidiabetic therapies other than insulin secretagogues (ie, other than sulfonylureas and the meglitinides nateglinide and repaglinide), as well as a few noninsulin injectable therapies (glucagon-like peptide-1 agonists), that are not associated with hypoglycemia. We believe these agents should be tried before prescribing an agent that carries the risk of hypoglycemia (ie, sulfonylureas).
If agents that do not cause hypoglycemia are used, more-aggressive glycemic targets may be achieved safely. The ACCORD study,2 which included patients at high cardiovascular risk and aimed at an aggressive glycemic target of 6%, may have yielded much different results had agents that carry a high risk of hypoglycemia been excluded.
Of importance, cardiovascular risk is also influenced by the common comorbidities seen in patients with diabetes, such as hypertension and hypercholesterolemia. Intensive, multifactorial interventions that address not only glycemic control but also blood pressure and lipids and that include low-dose aspirin therapy have been shown to lower the risk of death from cardiovascular causes and the risk of cardiovascular events.14 Likewise, smoking cessation is very important in reducing cardiovascular risk, especially in patients with diabetes.15
CLINICAL TRIALS IN CONTEXT
In conclusion, there is more to diabetes management than cardiovascular complications. Clearly, improved glycemic control decreases the risk of retinopathy, nephropathy, and neuropathy in patients with type 1 and type 2 diabetes. The DCCT and UKPDS extension studies further found that excellent glycemic control decreases rates of cardiac events.
The best way to treat diabetes may be different in otherwise healthy younger patients who have yet to develop significant complications than it is in older patients known to have cardiovascular disease or several risk factors for cardiovascular events. The available evidence suggests it would be reasonable to aim for stricter glycemic targets in the younger patients and less stringent targets in the older patients, particularly in those with long-standing diabetes who have already developed significant micro- and macrovascular complications.
We should interpret clinical trials within their narrow clinical context, emphasizing the actual population of patients included in the study, so as to avoid the inappropriate extrapolation of the results to all.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 7, 2014.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(Suppl 1):S11–S66.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011; 17:673–680.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 7, 2014.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(Suppl 1):S11–S66.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011; 17:673–680.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
Does massive hemoptysis always merit diagnostic bronchoscopy?
Yes, all patients with massive hemoptysis should undergo diagnostic bronchoscopy. The procedure plays an important role in protecting the airway, maintaining ventilation, finding the site and underlying cause of the bleeding, and in some cases stopping the bleeding, either temporarily or definitively.
Frightening to patients, massive hemoptysis is a medical emergency and demands immediate attention by an experienced pulmonary team.1 Hemoptysis can be the initial presentation of an underlying infectious, autoimmune, or malignant disorder (Table 1).2 Fortunately, most cases of hemoptysis are not massive or life-threatening.1
WHAT IS ‘MASSIVE’ HEMOPTYSIS?
Numerous studies have defined massive hemoptysis on the basis of the volume of blood lost over time, eg, more than 1 L in 24 hours or more than 400 mL in 6 hours.
Ibrahim3 has proposed that we move away from using the word “massive,” which is not useful, and instead think in terms of “life-threatening” hemoptysis, defined as any of the following:
- More than 100 mL of blood lost in 24 hours (a low number, but blood loss is hard to estimate accurately)
- Causing abnormal gas exchange due to airway obstruction
- Causing hemodynamic instability.
In this article, we use the traditional “massive” terminology.
BRONCHOSCOPY IS SUPERIOR TO IMAGING FOR DIAGNOSIS
Radiography can help identify the side or the site of bleeding in 33% to 82% of patients, and computed tomography can in 70% to 88.5%.4 Magnetic resonance imaging may also have a role; one study found it useful in cases of thoracic endometriosis during the quiescent stage.5 However, transferring a patient who is actively bleeding out of the intensive care unit for imaging can be challenging.
Flexible bronchoscopy is superior to radiographic imaging in evaluating massive hemoptysis: it can be performed at the bed-side and can include therapeutic procedures to control the bleeding until the patient can undergo a definitive therapeutic procedure.6 It has been found helpful in identifying the side of bleeding in 73% to 93% of cases of massive hemoptysis.6
However, one should consider starting the procedure with a rigid bronchoscope, which protects the airway better and allows for better ventilation during the procedure than a flexible one. One can use it to isolate the nonbleeding lung and to apply pressure to the bleeding site if it is in the main bronchus.7 Measuring 12 mm in diameter, a rigid scope cannot go as far into the lung as a flexible bronchoscope (measuring 6.4 mm), but a flexible bronchoscope can be introduced through the rigid bronchoscope to go further in.
MANAGEMENT OPTIONS
The management team should include an anesthesiologist, an intensivist, a thoracic surgeon, an interventional radiologist, and an interventional pulmonologist.
In the intensive care unit, the patient should be placed in the lateral decubitus position on the bleeding side. To maintain ventilation, the nonbleeding lung should be intubated with a large-bore endotracheal tube (internal diameter 8.5–9.0 mm) or, ideally, with a rigid bronchoscope.6 Meanwhile, the patient’s circulatory status should be stabilized with adequate fluid resuscitation and transfusion of blood products, with close monitoring.
Once the bleeding site is found, a bronchoscopic treatment is selected based on the cause of the bleeding. Massive hemoptysis usually arises from high-pressure bronchial vessels (90%) or, less commonly, from non-bronchial vessels or capillaries (10%).8 A variety of agents (eg, cold saline lavage, epinephrine 1:20,000) can be instilled through the bronchoscope to slow the bleeding and offer better visualization of the airway.6
If a bleeding intrabronchial lesion is identified, such as a malignant tracheobronchial tumor, local coagulation therapy can be applied through the bronchoscope. Options include laser treatment, argon plasma coagulation, cryotherapy, and electrocautery (Figure 1).9,10
If the bleeding persists or cannot be localized to a particular subsegment, an endobronchial balloon plug can be placed proximally (Figure 2). This can be left in place to isolate the bleeding and apply tamponade until a definitive procedure can be performed, such as bronchial artery embolization, radiation therapy, or surgery.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28:1642–1647.
- Abi Khalil S, Gourdier AL, Aoun N, et al. Cystic and cavitary lesions of the lung: imaging characteristics and differential diagnosis [in French]. J Radiol 2010; 91:465–473.
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008; 32:1131–1132.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest 1997; 111:1447–1450.
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80:38–58.
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35:901–904.
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135:463–481.
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001; 119:781–787.
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999; 20:123–138.
Yes, all patients with massive hemoptysis should undergo diagnostic bronchoscopy. The procedure plays an important role in protecting the airway, maintaining ventilation, finding the site and underlying cause of the bleeding, and in some cases stopping the bleeding, either temporarily or definitively.
Frightening to patients, massive hemoptysis is a medical emergency and demands immediate attention by an experienced pulmonary team.1 Hemoptysis can be the initial presentation of an underlying infectious, autoimmune, or malignant disorder (Table 1).2 Fortunately, most cases of hemoptysis are not massive or life-threatening.1
WHAT IS ‘MASSIVE’ HEMOPTYSIS?
Numerous studies have defined massive hemoptysis on the basis of the volume of blood lost over time, eg, more than 1 L in 24 hours or more than 400 mL in 6 hours.
Ibrahim3 has proposed that we move away from using the word “massive,” which is not useful, and instead think in terms of “life-threatening” hemoptysis, defined as any of the following:
- More than 100 mL of blood lost in 24 hours (a low number, but blood loss is hard to estimate accurately)
- Causing abnormal gas exchange due to airway obstruction
- Causing hemodynamic instability.
In this article, we use the traditional “massive” terminology.
BRONCHOSCOPY IS SUPERIOR TO IMAGING FOR DIAGNOSIS
Radiography can help identify the side or the site of bleeding in 33% to 82% of patients, and computed tomography can in 70% to 88.5%.4 Magnetic resonance imaging may also have a role; one study found it useful in cases of thoracic endometriosis during the quiescent stage.5 However, transferring a patient who is actively bleeding out of the intensive care unit for imaging can be challenging.
Flexible bronchoscopy is superior to radiographic imaging in evaluating massive hemoptysis: it can be performed at the bed-side and can include therapeutic procedures to control the bleeding until the patient can undergo a definitive therapeutic procedure.6 It has been found helpful in identifying the side of bleeding in 73% to 93% of cases of massive hemoptysis.6
However, one should consider starting the procedure with a rigid bronchoscope, which protects the airway better and allows for better ventilation during the procedure than a flexible one. One can use it to isolate the nonbleeding lung and to apply pressure to the bleeding site if it is in the main bronchus.7 Measuring 12 mm in diameter, a rigid scope cannot go as far into the lung as a flexible bronchoscope (measuring 6.4 mm), but a flexible bronchoscope can be introduced through the rigid bronchoscope to go further in.
MANAGEMENT OPTIONS
The management team should include an anesthesiologist, an intensivist, a thoracic surgeon, an interventional radiologist, and an interventional pulmonologist.
In the intensive care unit, the patient should be placed in the lateral decubitus position on the bleeding side. To maintain ventilation, the nonbleeding lung should be intubated with a large-bore endotracheal tube (internal diameter 8.5–9.0 mm) or, ideally, with a rigid bronchoscope.6 Meanwhile, the patient’s circulatory status should be stabilized with adequate fluid resuscitation and transfusion of blood products, with close monitoring.
Once the bleeding site is found, a bronchoscopic treatment is selected based on the cause of the bleeding. Massive hemoptysis usually arises from high-pressure bronchial vessels (90%) or, less commonly, from non-bronchial vessels or capillaries (10%).8 A variety of agents (eg, cold saline lavage, epinephrine 1:20,000) can be instilled through the bronchoscope to slow the bleeding and offer better visualization of the airway.6
If a bleeding intrabronchial lesion is identified, such as a malignant tracheobronchial tumor, local coagulation therapy can be applied through the bronchoscope. Options include laser treatment, argon plasma coagulation, cryotherapy, and electrocautery (Figure 1).9,10
If the bleeding persists or cannot be localized to a particular subsegment, an endobronchial balloon plug can be placed proximally (Figure 2). This can be left in place to isolate the bleeding and apply tamponade until a definitive procedure can be performed, such as bronchial artery embolization, radiation therapy, or surgery.
Yes, all patients with massive hemoptysis should undergo diagnostic bronchoscopy. The procedure plays an important role in protecting the airway, maintaining ventilation, finding the site and underlying cause of the bleeding, and in some cases stopping the bleeding, either temporarily or definitively.
Frightening to patients, massive hemoptysis is a medical emergency and demands immediate attention by an experienced pulmonary team.1 Hemoptysis can be the initial presentation of an underlying infectious, autoimmune, or malignant disorder (Table 1).2 Fortunately, most cases of hemoptysis are not massive or life-threatening.1
WHAT IS ‘MASSIVE’ HEMOPTYSIS?
Numerous studies have defined massive hemoptysis on the basis of the volume of blood lost over time, eg, more than 1 L in 24 hours or more than 400 mL in 6 hours.
Ibrahim3 has proposed that we move away from using the word “massive,” which is not useful, and instead think in terms of “life-threatening” hemoptysis, defined as any of the following:
- More than 100 mL of blood lost in 24 hours (a low number, but blood loss is hard to estimate accurately)
- Causing abnormal gas exchange due to airway obstruction
- Causing hemodynamic instability.
In this article, we use the traditional “massive” terminology.
BRONCHOSCOPY IS SUPERIOR TO IMAGING FOR DIAGNOSIS
Radiography can help identify the side or the site of bleeding in 33% to 82% of patients, and computed tomography can in 70% to 88.5%.4 Magnetic resonance imaging may also have a role; one study found it useful in cases of thoracic endometriosis during the quiescent stage.5 However, transferring a patient who is actively bleeding out of the intensive care unit for imaging can be challenging.
Flexible bronchoscopy is superior to radiographic imaging in evaluating massive hemoptysis: it can be performed at the bed-side and can include therapeutic procedures to control the bleeding until the patient can undergo a definitive therapeutic procedure.6 It has been found helpful in identifying the side of bleeding in 73% to 93% of cases of massive hemoptysis.6
However, one should consider starting the procedure with a rigid bronchoscope, which protects the airway better and allows for better ventilation during the procedure than a flexible one. One can use it to isolate the nonbleeding lung and to apply pressure to the bleeding site if it is in the main bronchus.7 Measuring 12 mm in diameter, a rigid scope cannot go as far into the lung as a flexible bronchoscope (measuring 6.4 mm), but a flexible bronchoscope can be introduced through the rigid bronchoscope to go further in.
MANAGEMENT OPTIONS
The management team should include an anesthesiologist, an intensivist, a thoracic surgeon, an interventional radiologist, and an interventional pulmonologist.
In the intensive care unit, the patient should be placed in the lateral decubitus position on the bleeding side. To maintain ventilation, the nonbleeding lung should be intubated with a large-bore endotracheal tube (internal diameter 8.5–9.0 mm) or, ideally, with a rigid bronchoscope.6 Meanwhile, the patient’s circulatory status should be stabilized with adequate fluid resuscitation and transfusion of blood products, with close monitoring.
Once the bleeding site is found, a bronchoscopic treatment is selected based on the cause of the bleeding. Massive hemoptysis usually arises from high-pressure bronchial vessels (90%) or, less commonly, from non-bronchial vessels or capillaries (10%).8 A variety of agents (eg, cold saline lavage, epinephrine 1:20,000) can be instilled through the bronchoscope to slow the bleeding and offer better visualization of the airway.6
If a bleeding intrabronchial lesion is identified, such as a malignant tracheobronchial tumor, local coagulation therapy can be applied through the bronchoscope. Options include laser treatment, argon plasma coagulation, cryotherapy, and electrocautery (Figure 1).9,10
If the bleeding persists or cannot be localized to a particular subsegment, an endobronchial balloon plug can be placed proximally (Figure 2). This can be left in place to isolate the bleeding and apply tamponade until a definitive procedure can be performed, such as bronchial artery embolization, radiation therapy, or surgery.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28:1642–1647.
- Abi Khalil S, Gourdier AL, Aoun N, et al. Cystic and cavitary lesions of the lung: imaging characteristics and differential diagnosis [in French]. J Radiol 2010; 91:465–473.
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008; 32:1131–1132.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest 1997; 111:1447–1450.
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80:38–58.
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35:901–904.
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135:463–481.
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001; 119:781–787.
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999; 20:123–138.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28:1642–1647.
- Abi Khalil S, Gourdier AL, Aoun N, et al. Cystic and cavitary lesions of the lung: imaging characteristics and differential diagnosis [in French]. J Radiol 2010; 91:465–473.
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008; 32:1131–1132.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest 1997; 111:1447–1450.
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80:38–58.
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35:901–904.
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135:463–481.
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001; 119:781–787.
- Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med 1999; 20:123–138.
Miss the ear, and you may miss the diagnosis
A 52-year-old woman presented with pain in both ears associated with redness and swelling. The symptoms appeared 3 weeks earlier. The pain had started on one side, then spread to the other over a period of 2 weeks. She denied fever, chills, rigor, rash, or upper respiratory symptoms. She had experienced similar but unilateral ear pain months before. Her medical history included bilateral knee pain and swelling (treated as osteoarthritis), hypertension, hyperlipidemia, and hypothyroidism. She also reported progressive bilateral hearing loss, for which she now uses hearing aids. She had no history of conjunctivitis or uveitis.
Physical examination showed swelling and erythema of both ears, sparing the earlobes (Figure 1), as well as bilateral knee-joint tenderness and restricted joint movement. The erythrocyte sedimentation rate was elevated at 52 mm/h (reference range 0–20); the complete blood cell count, creatinine, and liver enzyme levels were normal. An autoimmune panel was negative for antinuclear antibody, antineutrophil cytoplasmic antibody, and rheumatoid factor.
A clinical diagnosis of relapsing polychondritis was made based on the McAdam criteria.1 The patient was initially started on steroids and then was maintained on methotrexate. Her symptoms improved dramatically by 3 weeks.
RELAPSING POLYCHONDRITIS
Relapsing polychondritis is a rare, chronic, and potentially multisystem disorder characterized by recurrent episodes of cartilaginous inflammation that often lead to progressive destruction of the cartilage.2,3
Auricular chondritis is the initial presentation in 43% of cases and eventually develops in 89% of patients.2,4 The earlobes are spared, as they are devoid of cartilage, and this feature helps to differentiate the condition from an infection.
If the condition is not treated, recurrent attacks can result in irreversible cartilage damage and drooping of the pinna (ie, “cauliflower ear”). Biopsy is usually avoided, as it may further damage the ear. The diagnostic criteria for relapsing polychondritis formulated by McAdam et al1 accommodate the different presentations in order to limit the need for biopsy. Systemic involvement may include external eye structures, vasculitis affecting the eighth cranial (vestibulocochlear) nerve, noninflammatory large-joint arthritis, and the trachea. There is also an association with myelodysplasia.
- McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976; 55:193–215.
- Mathew SD, Battafarano DF, Morris MJ. Relapsing polychondritis in the Department of Defense population and review of the literature. Semin Arthritis Rheum 2012; 42:70–83.
- Letko E, Zafirakis P, Baltatzis S, Voudouri A, Livir-Rallatos C, Foster CS. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum 2002; 31:384–395.
- Kent PD, Michet CJ, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol 2004; 16:56–61.
A 52-year-old woman presented with pain in both ears associated with redness and swelling. The symptoms appeared 3 weeks earlier. The pain had started on one side, then spread to the other over a period of 2 weeks. She denied fever, chills, rigor, rash, or upper respiratory symptoms. She had experienced similar but unilateral ear pain months before. Her medical history included bilateral knee pain and swelling (treated as osteoarthritis), hypertension, hyperlipidemia, and hypothyroidism. She also reported progressive bilateral hearing loss, for which she now uses hearing aids. She had no history of conjunctivitis or uveitis.
Physical examination showed swelling and erythema of both ears, sparing the earlobes (Figure 1), as well as bilateral knee-joint tenderness and restricted joint movement. The erythrocyte sedimentation rate was elevated at 52 mm/h (reference range 0–20); the complete blood cell count, creatinine, and liver enzyme levels were normal. An autoimmune panel was negative for antinuclear antibody, antineutrophil cytoplasmic antibody, and rheumatoid factor.
A clinical diagnosis of relapsing polychondritis was made based on the McAdam criteria.1 The patient was initially started on steroids and then was maintained on methotrexate. Her symptoms improved dramatically by 3 weeks.
RELAPSING POLYCHONDRITIS
Relapsing polychondritis is a rare, chronic, and potentially multisystem disorder characterized by recurrent episodes of cartilaginous inflammation that often lead to progressive destruction of the cartilage.2,3
Auricular chondritis is the initial presentation in 43% of cases and eventually develops in 89% of patients.2,4 The earlobes are spared, as they are devoid of cartilage, and this feature helps to differentiate the condition from an infection.
If the condition is not treated, recurrent attacks can result in irreversible cartilage damage and drooping of the pinna (ie, “cauliflower ear”). Biopsy is usually avoided, as it may further damage the ear. The diagnostic criteria for relapsing polychondritis formulated by McAdam et al1 accommodate the different presentations in order to limit the need for biopsy. Systemic involvement may include external eye structures, vasculitis affecting the eighth cranial (vestibulocochlear) nerve, noninflammatory large-joint arthritis, and the trachea. There is also an association with myelodysplasia.
A 52-year-old woman presented with pain in both ears associated with redness and swelling. The symptoms appeared 3 weeks earlier. The pain had started on one side, then spread to the other over a period of 2 weeks. She denied fever, chills, rigor, rash, or upper respiratory symptoms. She had experienced similar but unilateral ear pain months before. Her medical history included bilateral knee pain and swelling (treated as osteoarthritis), hypertension, hyperlipidemia, and hypothyroidism. She also reported progressive bilateral hearing loss, for which she now uses hearing aids. She had no history of conjunctivitis or uveitis.
Physical examination showed swelling and erythema of both ears, sparing the earlobes (Figure 1), as well as bilateral knee-joint tenderness and restricted joint movement. The erythrocyte sedimentation rate was elevated at 52 mm/h (reference range 0–20); the complete blood cell count, creatinine, and liver enzyme levels were normal. An autoimmune panel was negative for antinuclear antibody, antineutrophil cytoplasmic antibody, and rheumatoid factor.
A clinical diagnosis of relapsing polychondritis was made based on the McAdam criteria.1 The patient was initially started on steroids and then was maintained on methotrexate. Her symptoms improved dramatically by 3 weeks.
RELAPSING POLYCHONDRITIS
Relapsing polychondritis is a rare, chronic, and potentially multisystem disorder characterized by recurrent episodes of cartilaginous inflammation that often lead to progressive destruction of the cartilage.2,3
Auricular chondritis is the initial presentation in 43% of cases and eventually develops in 89% of patients.2,4 The earlobes are spared, as they are devoid of cartilage, and this feature helps to differentiate the condition from an infection.
If the condition is not treated, recurrent attacks can result in irreversible cartilage damage and drooping of the pinna (ie, “cauliflower ear”). Biopsy is usually avoided, as it may further damage the ear. The diagnostic criteria for relapsing polychondritis formulated by McAdam et al1 accommodate the different presentations in order to limit the need for biopsy. Systemic involvement may include external eye structures, vasculitis affecting the eighth cranial (vestibulocochlear) nerve, noninflammatory large-joint arthritis, and the trachea. There is also an association with myelodysplasia.
- McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976; 55:193–215.
- Mathew SD, Battafarano DF, Morris MJ. Relapsing polychondritis in the Department of Defense population and review of the literature. Semin Arthritis Rheum 2012; 42:70–83.
- Letko E, Zafirakis P, Baltatzis S, Voudouri A, Livir-Rallatos C, Foster CS. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum 2002; 31:384–395.
- Kent PD, Michet CJ, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol 2004; 16:56–61.
- McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976; 55:193–215.
- Mathew SD, Battafarano DF, Morris MJ. Relapsing polychondritis in the Department of Defense population and review of the literature. Semin Arthritis Rheum 2012; 42:70–83.
- Letko E, Zafirakis P, Baltatzis S, Voudouri A, Livir-Rallatos C, Foster CS. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum 2002; 31:384–395.
- Kent PD, Michet CJ, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol 2004; 16:56–61.
Screening guidelines: A matter of perspective
Medical screening consists of trying to detect an occult disease at a point in its course—earlier than if diagnosed by clinical manifestations—when treatment offers a meaningful benefit to the patient. If the cost is acceptable, one would think that most care providers and patients would embrace the concept. So why are there such heated controversies surrounding screening for breast, prostate, and lung cancer?
The answer to that question is interpretive and philosophical and depends in part on the frame of reference. Are we looking at screening from the perspective of the health care system or from the perspective of the individual patient who is contemplating being screened?
The US Preventive Services Task Force (USPSTF), whose guidelines on screening are reviewed by Dr. Craig Nielsen in this issue of the Journal, went to great lengths to generate evidence-based guidelines based on rigorously conducted trials. They did not consider observational information or the emotional contextual biases of individual patients. Since their guidelines carry great weight, they have a big impact, sometimes including effects on insurance reimbursement for certain screening tests.
As with all “evidence-based” decisions, when applying guidelines or trial data in the clinic, we weigh the effect of our recommendations on individual patients, not on populations. Is a test worthwhile if it offers a 1 in 250 (or fill in your own number) chance of prolonging a specific patient’s life but is expensive and uncomfortable and poses the possible stress of a false-positive result that will warrant more testing? Which is actually more stressful: undergoing additional testing (with expense and discomfort) or not knowing whether you have a potentially lethal tumor? What is a reasonable cost to the patient and to a financially failing health system in attempting to delay the end of life to some time in the future when the patient may well be frail and perhaps even incapacitated?
People may differ in how they answer these questions, some of which may not even be answerable. The USPSTF guidelines, I believe, offer solid scaffolding for informed discussion. But we and our patients should use the offered evidence-based guidelines, and perhaps assume some costs, within a personalized context. Guidelines are only guidelines.
Medical screening consists of trying to detect an occult disease at a point in its course—earlier than if diagnosed by clinical manifestations—when treatment offers a meaningful benefit to the patient. If the cost is acceptable, one would think that most care providers and patients would embrace the concept. So why are there such heated controversies surrounding screening for breast, prostate, and lung cancer?
The answer to that question is interpretive and philosophical and depends in part on the frame of reference. Are we looking at screening from the perspective of the health care system or from the perspective of the individual patient who is contemplating being screened?
The US Preventive Services Task Force (USPSTF), whose guidelines on screening are reviewed by Dr. Craig Nielsen in this issue of the Journal, went to great lengths to generate evidence-based guidelines based on rigorously conducted trials. They did not consider observational information or the emotional contextual biases of individual patients. Since their guidelines carry great weight, they have a big impact, sometimes including effects on insurance reimbursement for certain screening tests.
As with all “evidence-based” decisions, when applying guidelines or trial data in the clinic, we weigh the effect of our recommendations on individual patients, not on populations. Is a test worthwhile if it offers a 1 in 250 (or fill in your own number) chance of prolonging a specific patient’s life but is expensive and uncomfortable and poses the possible stress of a false-positive result that will warrant more testing? Which is actually more stressful: undergoing additional testing (with expense and discomfort) or not knowing whether you have a potentially lethal tumor? What is a reasonable cost to the patient and to a financially failing health system in attempting to delay the end of life to some time in the future when the patient may well be frail and perhaps even incapacitated?
People may differ in how they answer these questions, some of which may not even be answerable. The USPSTF guidelines, I believe, offer solid scaffolding for informed discussion. But we and our patients should use the offered evidence-based guidelines, and perhaps assume some costs, within a personalized context. Guidelines are only guidelines.
Medical screening consists of trying to detect an occult disease at a point in its course—earlier than if diagnosed by clinical manifestations—when treatment offers a meaningful benefit to the patient. If the cost is acceptable, one would think that most care providers and patients would embrace the concept. So why are there such heated controversies surrounding screening for breast, prostate, and lung cancer?
The answer to that question is interpretive and philosophical and depends in part on the frame of reference. Are we looking at screening from the perspective of the health care system or from the perspective of the individual patient who is contemplating being screened?
The US Preventive Services Task Force (USPSTF), whose guidelines on screening are reviewed by Dr. Craig Nielsen in this issue of the Journal, went to great lengths to generate evidence-based guidelines based on rigorously conducted trials. They did not consider observational information or the emotional contextual biases of individual patients. Since their guidelines carry great weight, they have a big impact, sometimes including effects on insurance reimbursement for certain screening tests.
As with all “evidence-based” decisions, when applying guidelines or trial data in the clinic, we weigh the effect of our recommendations on individual patients, not on populations. Is a test worthwhile if it offers a 1 in 250 (or fill in your own number) chance of prolonging a specific patient’s life but is expensive and uncomfortable and poses the possible stress of a false-positive result that will warrant more testing? Which is actually more stressful: undergoing additional testing (with expense and discomfort) or not knowing whether you have a potentially lethal tumor? What is a reasonable cost to the patient and to a financially failing health system in attempting to delay the end of life to some time in the future when the patient may well be frail and perhaps even incapacitated?
People may differ in how they answer these questions, some of which may not even be answerable. The USPSTF guidelines, I believe, offer solid scaffolding for informed discussion. But we and our patients should use the offered evidence-based guidelines, and perhaps assume some costs, within a personalized context. Guidelines are only guidelines.
Six screening tests for adults: What’s recommended? What’s controversial?
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
A 68-year-old man with a history of hyperlipidemia is evaluated during a routine examination. He has a 25-pack-year cigarette smoking history but quit 12 years ago. He has no history of hypertension, diabetes mellitus, or stroke. A review of systems is unremarkable, and he has no family history of heart disease or cancer. He has noted no change in his bowel movements, and his most recent screening colonoscopy, done at age 60, was normal. His only current medication is lovastatin.
Physical examination reveals no abnormalities. His blood pressure is 130/82 mm Hg, and his body mass index is 24 kg/m2. His total cholesterol level is 213 mg/dL, and his high-density lipoprotein level is 48 mg/dL.
Which screening tests, if any, would be appropriate for this patient?
The advent in recent years of several new screening tests, along with changing and conflicting screening recommendations, has made it a challenge to manage this aspect of patient care. This article reviews six common screening tests and presents the current recommendations for their use (Table 1).
SCREENING CAN HARM
Screening is used to detect a disease in people who have no signs or symptoms of that disease; if signs or symptoms are present, diagnostic testing is indicated instead. Ideally, screening allows for early treatment to reduce the risk of illness and death associated with a disease.
Problems with screening relate to lead-time bias (detection of disease earlier in its course without actually affecting survival time), length-time bias (detection of indolent and benign cancers rather than aggressive ones), and overdiagnosis (detection of abnormalities that would not cause a problem in the patient’s lifetime, causing unnecessary concern, cost, or treatment).
The leading advisory groups on screening are the US Preventive Services Task Force (USPSTF),1 which is stringently evidence-based in its recommendations, and subspecialty societies, which often rely on expert opinion.2,3
ULTRASONOGRAPHY FOR ABDOMINAL AORTIC ANEURYSM
In 2005, the USPSTF gave a grade-B recommendation (recommended; benefit outweighs harm) for one-time ultrasonographic screening for abdominal aortic aneurysm in men ages 65 to 75 who have ever smoked at least 100 cigarettes over a lifetime. For men in the same age range who have never smoked, they gave a grade-C recommendation (no recommendation; small net benefit). The USPSTF updated its recommendation in 2014. For women ages 65 to 75 who smoke, the USPSTF thinks the evidence is insufficient to recommend for or against screening (grade-I recommendation).
Our patient described above—male, age 68, and with a 25 pack-year smoking history—is a candidate for screening for abdominal aortic aneurysm.
CT SCREENING FOR LUNG CANCER
In December 2013, the USPSTF gave a B-grade recommendation for annual screening for lung cancer with low-dose computed tomography (CT) for adults ages 55 to 80 who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that limits life expectancy or the ability to undergo curative lung surgery.
These recommendations were based on the outcomes of the National Lung Screening Trial.4 However, whereas this trial was in people ages 55 to 74, the USPSTF boosted the upper age limit to 80 based on computer modeling, a decision that was somewhat controversial.
Patz et al5 analyzed data from the National Lung Screening Trial and found that about 18% of lung cancers detected by low-dose CT appeared to be indolent and were unlikely to become clinically apparent during the patient’s lifetime. The authors concluded that overdiagnosis should be considered when guidelines for mass screening programs are developed.
Our 68-year-old patient would not qualify for CT screening for lung cancer, since his smoking history is less than 30 pack-years.
COLORECTAL CANCER SCREENING AND PREVENTION
Unlike other cancer screening tests, colorectal cancer screening can also be a preventive measure; removing polyps found during screening with colonoscopy or sigmoidoscopy is an effective strategy in preventing colon cancer.
The USPSTF last updated its colorectal screening recommendations in 2008, giving a grade-A recommendation (strongly recommended; benefit far outweighs harm) to screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy for adults ages 50 to 75. The risks and benefits of these screening methods vary. For adults ages 76 to 85, the task force recommends against routine screening but gives a grade-C recommendation for screening in that age group in some circumstances. They give a grade-D recommendation for screening after age 85.
The USPSTF concluded that the evidence is insufficient to assess the benefits and harms of CT colonography and fecal DNA testing for colorectal cancer screening.
The American Cancer Society issued similar guidelines in 2013, recommending that starting at age 50, men and women at low risk of colorectal cancer should be screened using one of the following schedules (the first four methods help detect both polyps and cancers, and the others detect only cancer)6:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- A double-contrast barium enema every 5 years
- CT colonography (“virtual colonoscopy”) every 5 years
- A guaiac-based fecal occult blood test annually
- A fecal immunochemical test annually.
Those at moderate or high risk of colorectal cancer are advised to talk with a doctor about a different testing schedule. (eg, colonoscopy every 5 years in patients with a significant family history of colon cancer).
Our patient last underwent colonoscopy 8 years ago and so does not need to be screened again for another 2 years.
CERVICAL CANCER SCREENING: MOVING TOWARD HPV TESTING FIRST?
Cervical cancer screening recommendations are fairly uniform across the major guideline-setting organizations.7 In general, they are:
- Ages 21–29: Check cytology every 3 years
- Ages 30–65: Cytology plus human papillomavirus (HPV) testing every 5 years (or cytology alone every 3 years)
- After age 65: Stop screening if prior screenings have been adequate and negative over the past 20 years.
Women who have been vaccinated against HPV have the same screening recommendations as above. Women who have had a hysterectomy for benign reasons do not need further screening.
The future of cervical cancer screening may be “reflex testing.” Rather than checking cervical samples for cytologic study (Papanicolaou smear) and HPV status together, we may one day screen samples first for HPV and, if that is positive, follow up with cytologic study. Easy-to-use home tests for HPV will likely be developed and should increase screening rates.
PROSTATE CANCER SCREENING: A SHARED DECISION
Prostate cancer screening remains controversial. Different guideline-setting bodies have different recommendations, creating confusion for patients. Physicians must follow what fits their own practice and beliefs.
The USPSTF in 2012 gave a grade-D recommendation to prostate-specific antigen (PSA) testing to screen for prostate cancer, stating that it did more harm than good. However, some men continue to be screened for PSA.
The American Cancer Society in 2013 recommended against routine testing for prostate cancer without a full discussion between physician and patient of the pros and cons of testing.8 If screening is decided upon, it should be done with annual PSA measurement or digital rectal examination, or both, starting at age 50. Men at high risk (ie, African American men, and men with a first-degree relative diagnosed with prostate cancer before age 65) should begin screening at age 45.
The American College of Physicians in 2013 issued a statement that clinicians should inform men between the ages of 50 and 69 about the limited potential benefits and substantial harms of prostate cancer screening.9 They recommended against PSA screening in men of average risk who are younger than age 50 or older than age 69, or those whose life expectancy is less than 10 to 15 years.
The American Urological Association in 2013 advised that10:
- PSA screening is not recommended in men younger than 40.
- Routine screening is not recommended in men between ages 40 and 54 at average risk.
- In men ages 55 to 69, decisions about PSA screening should be shared and based on each patient’s values and preferences. The decision to undergo PSA screening involves weighing the benefits of preventing death from prostate cancer in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment.
- To reduce the harm of screening, a routine interval of 2 years may be chosen over annual screening; such a schedule may preserve most benefits and reduce overdiagnosis and false-positive results.
- Routine PSA screening is not recommended in men ages 70 and older or with less than a 10- to 15-year life expectancy.
Shared decision-making. Many of the guidelines for prostate cancer screening are based on the concept of shared decision-making. However, studies indicate that many patients do not receive a full discussion of the issue,11 and in any event, patient education may make little difference in PSA testing rates.12,13
On the horizon for prostate cancer screening is the hope of finding a more predictable test. There is also discussion of using the PSA test earlier: some evidence shows that a very low result at age 45 predicts a less than 1% chance of developing metastatic prostate cancer by age 75, so it is possible that screening could stop in that population.
BREAST CANCER SCREENING: DIVERGENT RECOMMENDATIONS
The USPSTF created considerable controversy a few years ago when it recommended screening mammography from ages 50 to 74, and then only every 2 years—a departure from the traditional practice of starting screening at age 40. Few doctors heed the USPSTF guideline: most of the other guideline-setting organizations (eg, the American Cancer Society, the American Congress of Obstetricians and Gynecologists) recommend annual mammography for women starting at age 40.
Overdiagnosis is an especially pertinent issue with screening mammography for breast cancer because some cancers are indolent and will not cause a problem during a lifetime. Falk et al14 analyzed a Norwegian breast cancer screening program and found that overdiagnosis occurred in 10% to 20% of cases. Welch and Passow15 quantified the benefits and harms of screening mammography in 50-year-old women in the United States and found that of 1,000 women screened annually for a decade, 0.3 to 3.2 will avoid a breast cancer death, 490 to 670 will have at least one false alarm, and 3 to 14 will be overdiagnosed and treated needlessly.
Mammography screening for breast cancer will likely stay controversial for some time as we await additional data.
OTHER CANCERS: SCREENING NOT RECOMMENDED
The USPSTF currently does not recommend screening for ovarian cancer (guideline issued in 2012), pancreatic cancer (2004), or testicular cancer (2011), giving each a grade-D recommendation, indicating that screening does more harm than good. It also stated that there is insufficient evidence to recommend screening for oral cancer (2013), skin cancer (2009), and bladder cancer (2011).
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
- US Preventive Services Task Force. www.uspreventiveservicestask-force.org. Accessed August 11, 2014.
- Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831–841. Erratum in: JAMA 2009; 301:1544.
- Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med 2011; 171:18–22.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Patz EF, Pinsky P, Gatsonis C, et al; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274.
- American Cancer Society. Colorectal cancer screening and surveillance guidelines. www.cancer.org/healthy/informationforhealth-careprofessionals/colonmdclinicansinformationsource/colorec-talcancerscreeningandsurveillanceguidelines/index. Accessed August 11, 2014.
- Jin XW, Lipold L, McKenzie M, Sikon A. Cervical cancer screening: what’s new and what’s coming? Cleve Clin J Med 2013; 80:153–160.
- American Cancer Society. Prostate cancer screening guidelines. www.cancer.org/healthy/informationforhealthcareprofessionals/pros-tatemdcliniciansinformationsource/prostatecancerscreeningguide-lines/index. Accessed August 11, 2014.
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013; 158:761–769.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed September 5, 2014.
- Han PK, Kobrin S, Breen N, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med 2013; 11:306–314.
- Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 2013; 173:1704–1712.
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health 2013; 4:67–74.
- Falk RS, Hofvind S, Skaane P, Haldorsen T. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013; 133:705–712.
- Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174:448–454.
KEY POINTS
- The USPSTF has stringent standards of evidence and therefore its recommendations tend to be more conservative than those of other organizations that issue guidelines. Recommendations are available at www.uspreventiveservicestaskforce.org.
- Because screening can result in harm as well as benefit, screening should be done after shared decision-making with the patient, especially if the screening is controversial, as is the case with mammography for breast cancer and prostate-specific antigen testing for prostate cancer.
- Screening for lung cancer using low-dose computed tomography is recommended yearly beginning at age 55 for people who have at least a 30-pack-year smoking history.
- In women over age 30, cervical cancer screening with Papanicolaou (Pap) and human papillomavirus (HPV) testing is now recommended every 5 years rather than every 3 years. Testing for HPV infection may soon become the first-line screening test, with Pap testing reserved for patients who have a positive HPV result.
- Although the USPSTF no longer recommends mammography for women ages 40 to 49, other organizations continue to do so.