User login
Group aims to make African blood supply safer

Credit: UAB Hospital
Three organizations have joined together to adapt a pathogen inactivation system so that it works in whole blood and can be used in sub-Saharan Africa.
The INTERCEPT Blood System is currently used to inactivate bacteria, viruses, parasites, and leukocytes in donated platelets and plasma.
However, as the common practice in many African countries is to transfuse whole blood, the organizations want to adapt the system so it can be used with whole blood.
They also want to ensure the system can function in regions that may not have the infrastructure to support complex devices or have access to controlled temperature storage. Ideally, the system will not require electricity to inactivate pathogens or leukocytes.
For this endeavor, the company that makes the INTERCEPT system, Cerus Corporation, has partnered with SRTS Geneva and Swiss Transfusion SRC.
The Humanitarian Foundation Swiss Red Cross has granted funds to Swiss Transfusion SRC for the project. The initial funding of 1.5 million Swiss Francs will support the feasibility phase of the project and the completion of in vitro studies to support clinical trials.
“We believe pathogen inactivation for whole blood has the potential to improve the safety of transfusions in sub-Saharan Africa, where diminished blood availability due to severe anemia from malaria, HIV, and obstetric bleeding is common,” said Rudolf Schwabe, chief executive officer of the Swiss Red Cross.
“Based on our experience over the past 3 years with the INTERCEPT system, we have seen first-hand the substantial impact that pathogen inactivation has had in reducing transfusion-transmitted infectious risk in platelets and plasma.”
“This technology should be made available to developing countries such as those in sub-Saharan Africa, where the risk of bacterial contamination is about 2500 times greater than in Switzerland, and 10% to 15% of HIV infections are caused by contaminated transfusions.”
Cerus currently sells the INTERCEPT Blood System for both platelets and plasma in Europe, the Commonwealth of Independent States, the Middle East, and select countries in other regions around the world.
In the US, Cerus is seeking regulatory approval of the INTERCEPT Blood System for plasma and platelets.
The INTERCEPT red blood cell system is in clinical development. For more information, see the Cerus website. ![]()

Credit: UAB Hospital
Three organizations have joined together to adapt a pathogen inactivation system so that it works in whole blood and can be used in sub-Saharan Africa.
The INTERCEPT Blood System is currently used to inactivate bacteria, viruses, parasites, and leukocytes in donated platelets and plasma.
However, as the common practice in many African countries is to transfuse whole blood, the organizations want to adapt the system so it can be used with whole blood.
They also want to ensure the system can function in regions that may not have the infrastructure to support complex devices or have access to controlled temperature storage. Ideally, the system will not require electricity to inactivate pathogens or leukocytes.
For this endeavor, the company that makes the INTERCEPT system, Cerus Corporation, has partnered with SRTS Geneva and Swiss Transfusion SRC.
The Humanitarian Foundation Swiss Red Cross has granted funds to Swiss Transfusion SRC for the project. The initial funding of 1.5 million Swiss Francs will support the feasibility phase of the project and the completion of in vitro studies to support clinical trials.
“We believe pathogen inactivation for whole blood has the potential to improve the safety of transfusions in sub-Saharan Africa, where diminished blood availability due to severe anemia from malaria, HIV, and obstetric bleeding is common,” said Rudolf Schwabe, chief executive officer of the Swiss Red Cross.
“Based on our experience over the past 3 years with the INTERCEPT system, we have seen first-hand the substantial impact that pathogen inactivation has had in reducing transfusion-transmitted infectious risk in platelets and plasma.”
“This technology should be made available to developing countries such as those in sub-Saharan Africa, where the risk of bacterial contamination is about 2500 times greater than in Switzerland, and 10% to 15% of HIV infections are caused by contaminated transfusions.”
Cerus currently sells the INTERCEPT Blood System for both platelets and plasma in Europe, the Commonwealth of Independent States, the Middle East, and select countries in other regions around the world.
In the US, Cerus is seeking regulatory approval of the INTERCEPT Blood System for plasma and platelets.
The INTERCEPT red blood cell system is in clinical development. For more information, see the Cerus website. ![]()

Credit: UAB Hospital
Three organizations have joined together to adapt a pathogen inactivation system so that it works in whole blood and can be used in sub-Saharan Africa.
The INTERCEPT Blood System is currently used to inactivate bacteria, viruses, parasites, and leukocytes in donated platelets and plasma.
However, as the common practice in many African countries is to transfuse whole blood, the organizations want to adapt the system so it can be used with whole blood.
They also want to ensure the system can function in regions that may not have the infrastructure to support complex devices or have access to controlled temperature storage. Ideally, the system will not require electricity to inactivate pathogens or leukocytes.
For this endeavor, the company that makes the INTERCEPT system, Cerus Corporation, has partnered with SRTS Geneva and Swiss Transfusion SRC.
The Humanitarian Foundation Swiss Red Cross has granted funds to Swiss Transfusion SRC for the project. The initial funding of 1.5 million Swiss Francs will support the feasibility phase of the project and the completion of in vitro studies to support clinical trials.
“We believe pathogen inactivation for whole blood has the potential to improve the safety of transfusions in sub-Saharan Africa, where diminished blood availability due to severe anemia from malaria, HIV, and obstetric bleeding is common,” said Rudolf Schwabe, chief executive officer of the Swiss Red Cross.
“Based on our experience over the past 3 years with the INTERCEPT system, we have seen first-hand the substantial impact that pathogen inactivation has had in reducing transfusion-transmitted infectious risk in platelets and plasma.”
“This technology should be made available to developing countries such as those in sub-Saharan Africa, where the risk of bacterial contamination is about 2500 times greater than in Switzerland, and 10% to 15% of HIV infections are caused by contaminated transfusions.”
Cerus currently sells the INTERCEPT Blood System for both platelets and plasma in Europe, the Commonwealth of Independent States, the Middle East, and select countries in other regions around the world.
In the US, Cerus is seeking regulatory approval of the INTERCEPT Blood System for plasma and platelets.
The INTERCEPT red blood cell system is in clinical development. For more information, see the Cerus website. ![]()
FANTOM investigators map blood cell landscape
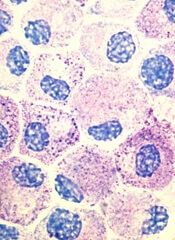
Research by an international consortium has revealed new information on hematopoiesis and shed new light on the etiology
of blood diseases.
The consortium, Functional Annotation of the Mammalian Genome (FANTOM), reported the results of this research in Blood.*
The investigators identified epigenetic regulators of hematopoiesis and uncovered the epigenetic and transcriptional changes that occur during granulopoiesis.
They “redefined” the mast cell transcriptome and mapped the enhancer and promoter landscapes of monocytes, conventional T cells, and regulatory T cells.
By pinpointing the locations of enhancers and promoters, the group was able to correlate them with activity in specific genes.
“Until this point, researchers could only recognize the unique signatures of enhancers and promoters,” said FANTOM investigator Alistair R.R. Forrest, PhD, of the RIKEN Centre for Life Science Technology in Yokohama, Japan.
“However, their exact location, as well as the association of specific enhancers to specific blood cells, remained unclear.”
Knowing the location of enhancers and promoters will improve experiments designed to determine how genes become activated, according to the investigators. And this could potentially lead to strategies for preventing or treating malignancies.
“The specific genetic alterations that are responsible for a normal cell turning into a cancer cell show up in the levels of messenger RNA in the cell, and these differences are often very subtle,” Dr Forrest said.
“Now that we have these incredibly detailed pictures of each of these cell types, we can now work backwards to compare cancer cells to the cells they came from originally to better understand what may have triggered the cells to malfunction, so we will be better equipped to develop new and more effective therapies.” ![]()
*The studies include:
High-throughput transcription profiling identifies putative epigenetic regulators of hematopoiesis
Redefinition of the human mast cell transcriptome by deep-CAGE sequencing
Transcription and enhancer profiling in human monocyte subsets
The enhancer and promoter landscape of human regulatory and conventional T-cell subpopulations.

Research by an international consortium has revealed new information on hematopoiesis and shed new light on the etiology
of blood diseases.
The consortium, Functional Annotation of the Mammalian Genome (FANTOM), reported the results of this research in Blood.*
The investigators identified epigenetic regulators of hematopoiesis and uncovered the epigenetic and transcriptional changes that occur during granulopoiesis.
They “redefined” the mast cell transcriptome and mapped the enhancer and promoter landscapes of monocytes, conventional T cells, and regulatory T cells.
By pinpointing the locations of enhancers and promoters, the group was able to correlate them with activity in specific genes.
“Until this point, researchers could only recognize the unique signatures of enhancers and promoters,” said FANTOM investigator Alistair R.R. Forrest, PhD, of the RIKEN Centre for Life Science Technology in Yokohama, Japan.
“However, their exact location, as well as the association of specific enhancers to specific blood cells, remained unclear.”
Knowing the location of enhancers and promoters will improve experiments designed to determine how genes become activated, according to the investigators. And this could potentially lead to strategies for preventing or treating malignancies.
“The specific genetic alterations that are responsible for a normal cell turning into a cancer cell show up in the levels of messenger RNA in the cell, and these differences are often very subtle,” Dr Forrest said.
“Now that we have these incredibly detailed pictures of each of these cell types, we can now work backwards to compare cancer cells to the cells they came from originally to better understand what may have triggered the cells to malfunction, so we will be better equipped to develop new and more effective therapies.” ![]()
*The studies include:
High-throughput transcription profiling identifies putative epigenetic regulators of hematopoiesis
Redefinition of the human mast cell transcriptome by deep-CAGE sequencing
Transcription and enhancer profiling in human monocyte subsets
The enhancer and promoter landscape of human regulatory and conventional T-cell subpopulations.

Research by an international consortium has revealed new information on hematopoiesis and shed new light on the etiology
of blood diseases.
The consortium, Functional Annotation of the Mammalian Genome (FANTOM), reported the results of this research in Blood.*
The investigators identified epigenetic regulators of hematopoiesis and uncovered the epigenetic and transcriptional changes that occur during granulopoiesis.
They “redefined” the mast cell transcriptome and mapped the enhancer and promoter landscapes of monocytes, conventional T cells, and regulatory T cells.
By pinpointing the locations of enhancers and promoters, the group was able to correlate them with activity in specific genes.
“Until this point, researchers could only recognize the unique signatures of enhancers and promoters,” said FANTOM investigator Alistair R.R. Forrest, PhD, of the RIKEN Centre for Life Science Technology in Yokohama, Japan.
“However, their exact location, as well as the association of specific enhancers to specific blood cells, remained unclear.”
Knowing the location of enhancers and promoters will improve experiments designed to determine how genes become activated, according to the investigators. And this could potentially lead to strategies for preventing or treating malignancies.
“The specific genetic alterations that are responsible for a normal cell turning into a cancer cell show up in the levels of messenger RNA in the cell, and these differences are often very subtle,” Dr Forrest said.
“Now that we have these incredibly detailed pictures of each of these cell types, we can now work backwards to compare cancer cells to the cells they came from originally to better understand what may have triggered the cells to malfunction, so we will be better equipped to develop new and more effective therapies.” ![]()
*The studies include:
High-throughput transcription profiling identifies putative epigenetic regulators of hematopoiesis
Redefinition of the human mast cell transcriptome by deep-CAGE sequencing
Transcription and enhancer profiling in human monocyte subsets
The enhancer and promoter landscape of human regulatory and conventional T-cell subpopulations.
NK cell findings may have treatment implications

Credit: St Jude Children’s
Research Hospital
Researchers say they’ve gained new insight into the production of natural killer (NK) cells.
And their findings may help them generate greater numbers of the cells in culture, which could have implications for the treatment of leukemia and other malignancies.
A previous study conducted by the same team revealed that the gene E4bp4 must be switched on to allow the immune system to produce NK cells.
Their new work suggests that E4bp4 expression is required for progenitor cells to commit to the NK lineage. And the gene promotes NK-cell development by regulating expression of the transcription factors Eomes and Id2.
The researchers described these discoveries in the Journal of Experimental Medicine.
“We are excited to find that E4bp4 has such a crucial role in determining the decisive point where blood progenitor cells become NK cells,” said study author Hugh Brady, of Imperial College London in the UK.
“We are now starting to apply this to human blood stem cells to work out how switching on E4bp4 can allow us to make lots of robust human NK cells in culture. We are hoping to make human NK cells that will have improved survival and be very toxic to cancer cells when transfused into patients. Hopefully, this will allow a big reduction in the number of NK cells needed to treat an individual patient.”
To gain insight into NK-cell production, Dr Brady and his colleagues evaluated 2 types of mice with NK-cell deficiencies. The Il15ra knockout mouse model cannot mediate IL-15 signaling, which is critical for NK-cell production. And the T-bet (Tbx21) knockout model lacks a transcription factor that’s crucial for NK-cell development.
Analysis of the Il15ra model revealed that the absence of E4bp4 perturbs NK-cell development earlier than the absence of IL-15 signaling. This suggests E4bp4 acts before IL-15, which was previously considered the definitive factor required for NK-cell production.
The researchers also found that E4bp4 is required for the production of NK progenitors, but T-bet is not. And this suggests E4bp4 acts before T-bet in NK-cell development.
To investigate these findings further, the team took cells at various stages of NK-cell differentiation from wild-type bone marrow and measured their expression of transcription factor mRNAs.
They detected E4bp4 transcript in both lymphoid-primed multipotent progenitors (LMPPs) and common lymphoid progenitors (CLPs), and E4bp4 expression increased at later stages of NK-cell development.
Based on these results, the researchers speculated that E4bp4 might be a lineage commitment factor controlling the development of NK progenitors from CLPs. To test that theory, they restored E4bp4 expression in purified E4bp4-/- CLPs to see if this could re-establish NK-cell development.
The team sorted CLPs from E4bp4-/- bone marrow, cultured them in lymphocyte-inducing conditions, transduced them with E4bp4 or empty vector, and moved on to NK-cell-inducing conditions. But neither cell type produced NK cells.
So the researchers decided to initiate the culture at an earlier developmental stage, using LMPPs. They cultured LMPPs, which exhibited a CLP phenotype at the time of transduction. And CLPs transduced with E4bp4 gave rise to NK cells, but CLPs transduced with empty vector did not.
As these results suggest that E4bp4 acts at the earliest possible point in NK-cell development, the team wanted to characterize E4bp4’s relationship with transcription factors that are likely to act downstream.
They tested several transcription factors known to play a part in NK-cell production and function. But only Eomes and Id2 proved essential for E4bp4 to direct the production of fully functional, mature NK cells. ![]()

Credit: St Jude Children’s
Research Hospital
Researchers say they’ve gained new insight into the production of natural killer (NK) cells.
And their findings may help them generate greater numbers of the cells in culture, which could have implications for the treatment of leukemia and other malignancies.
A previous study conducted by the same team revealed that the gene E4bp4 must be switched on to allow the immune system to produce NK cells.
Their new work suggests that E4bp4 expression is required for progenitor cells to commit to the NK lineage. And the gene promotes NK-cell development by regulating expression of the transcription factors Eomes and Id2.
The researchers described these discoveries in the Journal of Experimental Medicine.
“We are excited to find that E4bp4 has such a crucial role in determining the decisive point where blood progenitor cells become NK cells,” said study author Hugh Brady, of Imperial College London in the UK.
“We are now starting to apply this to human blood stem cells to work out how switching on E4bp4 can allow us to make lots of robust human NK cells in culture. We are hoping to make human NK cells that will have improved survival and be very toxic to cancer cells when transfused into patients. Hopefully, this will allow a big reduction in the number of NK cells needed to treat an individual patient.”
To gain insight into NK-cell production, Dr Brady and his colleagues evaluated 2 types of mice with NK-cell deficiencies. The Il15ra knockout mouse model cannot mediate IL-15 signaling, which is critical for NK-cell production. And the T-bet (Tbx21) knockout model lacks a transcription factor that’s crucial for NK-cell development.
Analysis of the Il15ra model revealed that the absence of E4bp4 perturbs NK-cell development earlier than the absence of IL-15 signaling. This suggests E4bp4 acts before IL-15, which was previously considered the definitive factor required for NK-cell production.
The researchers also found that E4bp4 is required for the production of NK progenitors, but T-bet is not. And this suggests E4bp4 acts before T-bet in NK-cell development.
To investigate these findings further, the team took cells at various stages of NK-cell differentiation from wild-type bone marrow and measured their expression of transcription factor mRNAs.
They detected E4bp4 transcript in both lymphoid-primed multipotent progenitors (LMPPs) and common lymphoid progenitors (CLPs), and E4bp4 expression increased at later stages of NK-cell development.
Based on these results, the researchers speculated that E4bp4 might be a lineage commitment factor controlling the development of NK progenitors from CLPs. To test that theory, they restored E4bp4 expression in purified E4bp4-/- CLPs to see if this could re-establish NK-cell development.
The team sorted CLPs from E4bp4-/- bone marrow, cultured them in lymphocyte-inducing conditions, transduced them with E4bp4 or empty vector, and moved on to NK-cell-inducing conditions. But neither cell type produced NK cells.
So the researchers decided to initiate the culture at an earlier developmental stage, using LMPPs. They cultured LMPPs, which exhibited a CLP phenotype at the time of transduction. And CLPs transduced with E4bp4 gave rise to NK cells, but CLPs transduced with empty vector did not.
As these results suggest that E4bp4 acts at the earliest possible point in NK-cell development, the team wanted to characterize E4bp4’s relationship with transcription factors that are likely to act downstream.
They tested several transcription factors known to play a part in NK-cell production and function. But only Eomes and Id2 proved essential for E4bp4 to direct the production of fully functional, mature NK cells. ![]()

Credit: St Jude Children’s
Research Hospital
Researchers say they’ve gained new insight into the production of natural killer (NK) cells.
And their findings may help them generate greater numbers of the cells in culture, which could have implications for the treatment of leukemia and other malignancies.
A previous study conducted by the same team revealed that the gene E4bp4 must be switched on to allow the immune system to produce NK cells.
Their new work suggests that E4bp4 expression is required for progenitor cells to commit to the NK lineage. And the gene promotes NK-cell development by regulating expression of the transcription factors Eomes and Id2.
The researchers described these discoveries in the Journal of Experimental Medicine.
“We are excited to find that E4bp4 has such a crucial role in determining the decisive point where blood progenitor cells become NK cells,” said study author Hugh Brady, of Imperial College London in the UK.
“We are now starting to apply this to human blood stem cells to work out how switching on E4bp4 can allow us to make lots of robust human NK cells in culture. We are hoping to make human NK cells that will have improved survival and be very toxic to cancer cells when transfused into patients. Hopefully, this will allow a big reduction in the number of NK cells needed to treat an individual patient.”
To gain insight into NK-cell production, Dr Brady and his colleagues evaluated 2 types of mice with NK-cell deficiencies. The Il15ra knockout mouse model cannot mediate IL-15 signaling, which is critical for NK-cell production. And the T-bet (Tbx21) knockout model lacks a transcription factor that’s crucial for NK-cell development.
Analysis of the Il15ra model revealed that the absence of E4bp4 perturbs NK-cell development earlier than the absence of IL-15 signaling. This suggests E4bp4 acts before IL-15, which was previously considered the definitive factor required for NK-cell production.
The researchers also found that E4bp4 is required for the production of NK progenitors, but T-bet is not. And this suggests E4bp4 acts before T-bet in NK-cell development.
To investigate these findings further, the team took cells at various stages of NK-cell differentiation from wild-type bone marrow and measured their expression of transcription factor mRNAs.
They detected E4bp4 transcript in both lymphoid-primed multipotent progenitors (LMPPs) and common lymphoid progenitors (CLPs), and E4bp4 expression increased at later stages of NK-cell development.
Based on these results, the researchers speculated that E4bp4 might be a lineage commitment factor controlling the development of NK progenitors from CLPs. To test that theory, they restored E4bp4 expression in purified E4bp4-/- CLPs to see if this could re-establish NK-cell development.
The team sorted CLPs from E4bp4-/- bone marrow, cultured them in lymphocyte-inducing conditions, transduced them with E4bp4 or empty vector, and moved on to NK-cell-inducing conditions. But neither cell type produced NK cells.
So the researchers decided to initiate the culture at an earlier developmental stage, using LMPPs. They cultured LMPPs, which exhibited a CLP phenotype at the time of transduction. And CLPs transduced with E4bp4 gave rise to NK cells, but CLPs transduced with empty vector did not.
As these results suggest that E4bp4 acts at the earliest possible point in NK-cell development, the team wanted to characterize E4bp4’s relationship with transcription factors that are likely to act downstream.
They tested several transcription factors known to play a part in NK-cell production and function. But only Eomes and Id2 proved essential for E4bp4 to direct the production of fully functional, mature NK cells. ![]()
Chest CT in Patients with Pneumonia
Pneumonia remains one of the most common indications for hospital admissions. In the United States in 2010, more than 1 million patients were discharged with a diagnosis of pneumonia.[1] A diagnosis of pneumonia is based on typical clinical findings with recommendations to identify a demonstrable infiltrate on appropriate imaging modalities.[2] Although computed tomography (CT) imaging of the chest is much more sensitive than plain radiography at detecting infiltrates, the greater cost and higher radiation exposure limits its use as a screening modality.[3, 4] Additional imaging studies are recommended for patients who fail to respond to therapy.[2] There are, however, no published studies to determine the exact impact of chest CT scans on the management of pneumonia.
We conducted a retrospective assessment of CT scan use in patients admitted with a diagnosis of pneumonia. The study was designed to assess (1) the overall utilization rate of chest CT scans at our institution and (2) the impact of CT findings on patient management.
METHODS
This retrospective study was conducted at St. John Hospital and Medical Center, an 808‐bed tertiary care community teaching hospital in Detroit. The study was approved by the St. John Hospital and Medical Center's institutional review board.
Patients admitted to our institution between January 1, 2008 and November 1, 2011 were evaluated for study inclusion by searching the hospital's computer database using the discharge International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes for pneumonia, pleural effusion, and empyema. Patients were included for initial review if the appropriate ICD‐9‐CM codes were included within the list of discharge diagnoses and were not restricted based on hierarchy within that list. Patients were included in further analysis if they were 18 years of age, a diagnosis of pneumonia was made within 48 hours of admission, and records were available for review. Patients were excluded if they did not meet the above criteria or a diagnosis of pneumonia could not be confirmed by chart review. The electronic medical record was reviewed and patient demographics, hospital admission source, microbiology results, radiographic findings, and outcomes were recorded. Additional procedures such as thoracentesis, open lung biopsy and/or chest tube placement were recorded for patients if performed. The Charlson Weighted Index of Comorbidity and Confusion, Urea, Respiratory rate, Blood pressure, Age > 65 (CURB 65) scores were calculated as described elsewhere.[5, 6] CT scans were assessed for time and date of study after admission along with all relevant findings.
Data Analysis
Descriptive statistics were generated for the overall population. The associations between categorical variables and whether or not a CT scan was performed were assessed using the 2 test. Student t test or analysis of variance, followed by the Bonferroni correction of the P value, were used to compare mean values. Logistic regression was used to predict the probability of having a chest CT done, given the variables found to be related on univariate analysis. All data were analyzed using SPSS version 22.0 (IBM, Armonk, NY), and a P value of 0.05 or less was considered to indicate statistical significance.
RESULTS
A total of 264 patients were identified by discharge diagnosis, and 195 (73.9%) patients met the inclusion criteria. Among the 69 patients who were excluded, 37 patients were diagnosed more than 48 hours after admission, 19 patients did not have a radiographically demonstrable abnormality, 5 patients had an incomplete medical record, and 8 patients received no antibiotics. The overall mean age of the cases was 63.4 19.1 years, with an average length of stay of 7.4 5.7 days. Sixty‐nine (35.3%) of the case patients had a chest CT scan performed. A CT scan was performed more often in younger patients (58.1 19.0 vs 66.8 18.6, P = 0.002) and in patients with lower CURB 65 scores (1.7 1.4 vs 2.2 1.4, P = 0.037). A CT scan was also performed more often in patients with no infiltrates or consolidation on plain radiographs (26.9% vs 7.1%, P 0.0001). Patients were also more likely to have a procedure performed if they had a CT performed (21.7% vs 3.1%, P 0.0001) and were admitted from home versus a long‐term care facility or other healthcare institution (92.8% vs 78.6%, P = 0.011). Comparisons are shown in Table 1. After controlling for age, CURB 65 score on admission, admission source, and the presence of consolidation or infiltrates on initial chest radiograph (CXR), individuals were 4.76 times less likely to have a CT scan performed if the CXR showed consolidation and/or infiltrates (odds ratio: 0.21, P = 0.001; 95% confidence interval: 0.08‐0.53) (Table 2).
| Characteristics | Chest CT Scan Performed, n = 69 (35.4%) | Chest CT Scan Not Performed, n = 126 (64.6%) | P Value |
|---|---|---|---|
| |||
| Mean age, y SD | 58.1 19.0 | 66.8 18.6 | 0.002 |
| Gender, male | 52.2% (36) | 45.2% (57) | 0.35 |
| Average length of stay, d SD | 8.6 7.4 | 6.9 4.5 | 0.08 |
| Charlson Comorbidity Index SD | 1.77 2.0 | 2.02 1.89 | 0.38 |
| CURB 65 score on admission SD | 1.7 1.4 | 2.2 1.4 | 0.037 |
| Fever on admission | 34.8% (24) | 36.5% (46) | 0.81 |
| Sepsis within 48 hours of CT | 81.2% (56) | 78.6% (99) | 0.67 |
| ICU admission within 48 hours of admission | 21.7% (15) | 15.1% (19) | 0.24 |
| No consolidation or infiltrates on CXR, n = 67a | 26.9% (18) | 7.1% (9) | 0.0001 |
| Procedure performed | 21.7% (15) | 3.1% (4) | 0.0001 |
| Source of admission | |||
| Home | 92.8% (64) | 78.6% (99) | 0.011 |
| Extended care facility | 7.2% (5) | 21.4% (27) | |
| Positive blood cultureb | 4.1% (2) | 8.9% (7) | 0.30 |
| Positive sputum culturec | 11.1% (3) | 11.4% (4) | 0.97 |
| Discharged alived | 91.3% (63) | 88.9% (112) | 0.60 |
| Characteristic | Odds Ratio | P Value | 95% CI |
|---|---|---|---|
| |||
| Age | 0.99 | 0.29 | 0.971.01 |
| CURB 65 at admission | 0.89 | 0.41 | 0.671.18 |
| Admission source (healthcare facility) | 0.36 | 0.07 | 0.121.09 |
| Consolidation or infiltrates | 0.21 | 0.001 | 0.080.53 |
Procedure Performed
Among the 195 patients, pneumonia‐related procedures were performed on only 19 (9.7%) patients. The procedures performed included bronchoscopy (n = 4), percutaneous biopsy (n = 3), thoracentesis (n = 7), and open lung biopsy (n = 5). Fifteen (78.9%) of the patients who had a pneumonia‐related procedure had a CT scan. Table 3 shows the characteristics of patients who had a procedure performed compared to those patients who did not have a procedure performed among all individuals who had a CT scan. Only average length of stay differed significantly between these 2 groups of patients (15.3 11.9 vs 6.8 4.1, P = 0.016).
| Characteristic | Procedure Performed, n = 15 (21.7%) | Procedure Not Performed, n = 54 (78.3%) | P Value |
|---|---|---|---|
| |||
| Mean age, y SD | 56.9 19.5 | 58.5 19.1 | 0.77 |
| Male gender | 53.3% (8) | 51.1% (28) | 0.92 |
| Average length of stay, d SD | 15.3 11.9 | 6.8 4.1 | 0.016 |
| Admission CURB 65 score, mean SD | 1.7 1.4 | 1.7 1.5 | 0.98 |
| Fever on admission | 40% (6) | 33.3% (18) | 0.63 |
| Sepsis within 48 hours of procedure | 93.3% (14) | 77.8% (42) | 0.17 |
| ICU admit within 48 hours of admission | 26.7% (4) | 20.4% (11) | 0.60 |
| No consolidation or infiltrates on CXR | 21.4% (3) | 7.8% (4) | 0.65 |
| Source of admission | |||
| Home | 15% (100) | 90.7% (49) | NSa |
| Extended care facility | 0% (0) | 9.3% (5) | |
| Discharge aliveb | 80% (12) | 94.4% (51) | 0.08 |
DISCUSSION
Chest radiography plays an essential role in diagnosing pneumonia. Chest CT scans are more sensitive in diagnosing pneumonia and may be more specific for certain pathogens, but objective indicators or guidelines regarding test performance are lacking.[7] There are few available studies that evaluate the benefit of chest CT scans in adults with pneumonia. Beall et al. noted 57% of immunocompetent hosts, 22% of human immunodeficiency virus (HIV) patients, and 45% of immunocompromised hosts had a new finding on CT.[8] In 40% of the cases, there was an overall change in management based on the findings. Nyamande et al. showed that high‐resolution CT scans identified abnormalities missed on plain radiographs in 82% (n = 40) of HIV patients in sub‐Saharan Africa.[9] A study by Syrjl et al. highlights the fact that high‐resolution CT scanning improves the diagnosis of community‐acquired pneumonia in patients with negative chest radiographs.[10] In the right clinical setting, additional imaging, such as high‐resolution CT scanning, is more sensitive at detecting abnormalities consistent with pneumonia.[10] We found that a CT scan was more likely to be performed in patients with no infiltrates or consolidation consistent with that finding. However, the authors did not attempt to evaluate improved clinical outcomes or management changes. Other investigators have tried to demonstrate unique or specific findings on CT scans compared to plain radiography for particular pathogens.[11, 12, 13]
We attempted to identify specific features of patients presenting with pneumonia that could assist clinicians in the decision‐making process as it relates to ordering a CT scan. CT scans were performed more frequently on subjects who were younger, had lower severity of illness, and were admitted from the community. We were unable to assess the radiographic and/or clinical findings that led the providers to order the CT scans. It is interesting to note, however, that Metlay et al. demonstrated a decreasing prevalence of pneumonia‐associated symptoms with increasing age.[14] One could speculate that patients who are younger and tend to have more symptoms may be more likely to get ancillary testing.
In our study, 35% of patients admitted with pneumonia had a CT scan performed that led to an additional procedure 22% of the time. We were unable to accurately evaluate the impact of CT on antibiotic modification, duration, or some outcomes. Although a number of studies demonstrated new or missed findings by CT compared to plain radiography, only Beall et al. reported outcome changes.[8, 9, 10, 12] They found that 39% (21/54) of patients had a change in their treatment plan including antibiotic alterations.
A number of factors impact outcomes such as length of stay and mortality in patients admitted with community‐acquired pneumonia. Empyema contributes to additional length of stay and pleural effusions are new findings identified by CT scans.[8, 9, 15, 16] Unfortunately, the number of patients with pleural effusions and even empyema (data not shown) was too small for us to analyze. Better prospective observational studies will be necessary to define specific CT findings leading to actual changes in management. The optimal timing of CT scanning could also be determined from these studies. The retrospective nature of our study is a key limitation to our results. It is difficult to determine retrospectively the clinical decision‐making process used when ordering additional diagnostic tests or procedures. Whether the CT scans ordered on our patients truly resulted in additional procedures or whether the procedures were preplanned cannot be elucidated. Our current electronic medical record and ordering process has significant drop‐down list selection bias for test indications. A postorder research‐based survey tool would be required to further evaluate the clinician's decision‐making process. In addition, as a single center study, the decision to perform CT scans and pneumonia‐related procedures reflects only the practice patterns among a relatively small number of physicians with a wide variety of practice levels and specialties. Although length of stay was not affected by performing a CT scan, patients who had a procedure did have a prolonged hospital stay consistent with a complicated course as confirmed by others.[16]
Our study results could be the first step in developing prospective studies to evaluate the indications and utility of ancillary imaging in patients with pneumonia. Prospective, multicenter observational studies, which include a clinical decision‐making survey tool as noted above, would be tremendously beneficial. Pathogen‐specific indications and outcomes will be facilitated by the deployment of more rapid and effective molecular diagnostic capabilities. Furthermore, the cost of the test, radiation exposure, impact on clinical outcomes, and overall risk/benefit would need to be calculated from these future studies.
- National Hospital Discharge Survey. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data/nhds/2average/ 2010ave2_firstlist.pdf. Accessed December 10, 2013.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , . Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266–270.
- American College of Radiology. RadiologyInfo.org website. Radiation dose in x‐ray and CT exams. Available at: http://www.radiologyinfo.org/en/safety/?pg=sfty_xray. Accessed February 24, 2014.
- , , , et al. Prospective comparison of three validated prediction rules for prognosis in community‐acquired pneumonia. Am J Med. 2005;118(4):384–392.
- , , , et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- , . Imaging pneumonia in immunocompetent and immunocompromised individuals. Curr Opin Pulm Med. 2012;18(3):194–201.
- , , , , , . Utilization of computed tomography in patients hospitalized with community‐acquired pneumonia. Md Med J. 1998;47(4):182–187.
- , , . Comparison of plain chest radiography and high‐resolution CT in human immunodeficiency virus infected patients with community‐acquired pneumonia: a sub‐Saharan Africa study. Br J Radiol. 2007;80(953):302–306.
- , , , , . High‐resolution computed tomography for the diagnosis of community‐acquired pneumonia. Clin Infect Dis. 1998;27(2):358–363.
- , , , et al. Pulmonary computed tomography findings in 39 cases of Streptococcus pneumoniae pneumonia. Intern Med. 2012;51(24):3343–3349.
- , , , et al. High‐resolution CT findings in Streptococcus milleri pulmonary infection. Clin Radiol. 2013;68(6):e331–e337.
- , , , et al. Thin‐section CT findings in Pseudomonas aeruginosa pulmonary infection. Br J Radiol. 2012;85(1020):1533–1538.
- , , , et al. Influence of age on symptoms at presentation in patients with community‐acquired pneumonia. Arch Intern Med. 1997;157(13):1453–1459.
- , , . Factors associated with length of stay in hospital for suspected community‐acquired pneumonia. Can Respir J. 2006;13(6):317–324.
- , , , , , . Predictors for length of hospital stay in patients with community‐acquired pneumonia: results from a Swiss multicenter study. BMC Pulm Med. 2012;12:21.
Pneumonia remains one of the most common indications for hospital admissions. In the United States in 2010, more than 1 million patients were discharged with a diagnosis of pneumonia.[1] A diagnosis of pneumonia is based on typical clinical findings with recommendations to identify a demonstrable infiltrate on appropriate imaging modalities.[2] Although computed tomography (CT) imaging of the chest is much more sensitive than plain radiography at detecting infiltrates, the greater cost and higher radiation exposure limits its use as a screening modality.[3, 4] Additional imaging studies are recommended for patients who fail to respond to therapy.[2] There are, however, no published studies to determine the exact impact of chest CT scans on the management of pneumonia.
We conducted a retrospective assessment of CT scan use in patients admitted with a diagnosis of pneumonia. The study was designed to assess (1) the overall utilization rate of chest CT scans at our institution and (2) the impact of CT findings on patient management.
METHODS
This retrospective study was conducted at St. John Hospital and Medical Center, an 808‐bed tertiary care community teaching hospital in Detroit. The study was approved by the St. John Hospital and Medical Center's institutional review board.
Patients admitted to our institution between January 1, 2008 and November 1, 2011 were evaluated for study inclusion by searching the hospital's computer database using the discharge International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes for pneumonia, pleural effusion, and empyema. Patients were included for initial review if the appropriate ICD‐9‐CM codes were included within the list of discharge diagnoses and were not restricted based on hierarchy within that list. Patients were included in further analysis if they were 18 years of age, a diagnosis of pneumonia was made within 48 hours of admission, and records were available for review. Patients were excluded if they did not meet the above criteria or a diagnosis of pneumonia could not be confirmed by chart review. The electronic medical record was reviewed and patient demographics, hospital admission source, microbiology results, radiographic findings, and outcomes were recorded. Additional procedures such as thoracentesis, open lung biopsy and/or chest tube placement were recorded for patients if performed. The Charlson Weighted Index of Comorbidity and Confusion, Urea, Respiratory rate, Blood pressure, Age > 65 (CURB 65) scores were calculated as described elsewhere.[5, 6] CT scans were assessed for time and date of study after admission along with all relevant findings.
Data Analysis
Descriptive statistics were generated for the overall population. The associations between categorical variables and whether or not a CT scan was performed were assessed using the 2 test. Student t test or analysis of variance, followed by the Bonferroni correction of the P value, were used to compare mean values. Logistic regression was used to predict the probability of having a chest CT done, given the variables found to be related on univariate analysis. All data were analyzed using SPSS version 22.0 (IBM, Armonk, NY), and a P value of 0.05 or less was considered to indicate statistical significance.
RESULTS
A total of 264 patients were identified by discharge diagnosis, and 195 (73.9%) patients met the inclusion criteria. Among the 69 patients who were excluded, 37 patients were diagnosed more than 48 hours after admission, 19 patients did not have a radiographically demonstrable abnormality, 5 patients had an incomplete medical record, and 8 patients received no antibiotics. The overall mean age of the cases was 63.4 19.1 years, with an average length of stay of 7.4 5.7 days. Sixty‐nine (35.3%) of the case patients had a chest CT scan performed. A CT scan was performed more often in younger patients (58.1 19.0 vs 66.8 18.6, P = 0.002) and in patients with lower CURB 65 scores (1.7 1.4 vs 2.2 1.4, P = 0.037). A CT scan was also performed more often in patients with no infiltrates or consolidation on plain radiographs (26.9% vs 7.1%, P 0.0001). Patients were also more likely to have a procedure performed if they had a CT performed (21.7% vs 3.1%, P 0.0001) and were admitted from home versus a long‐term care facility or other healthcare institution (92.8% vs 78.6%, P = 0.011). Comparisons are shown in Table 1. After controlling for age, CURB 65 score on admission, admission source, and the presence of consolidation or infiltrates on initial chest radiograph (CXR), individuals were 4.76 times less likely to have a CT scan performed if the CXR showed consolidation and/or infiltrates (odds ratio: 0.21, P = 0.001; 95% confidence interval: 0.08‐0.53) (Table 2).
| Characteristics | Chest CT Scan Performed, n = 69 (35.4%) | Chest CT Scan Not Performed, n = 126 (64.6%) | P Value |
|---|---|---|---|
| |||
| Mean age, y SD | 58.1 19.0 | 66.8 18.6 | 0.002 |
| Gender, male | 52.2% (36) | 45.2% (57) | 0.35 |
| Average length of stay, d SD | 8.6 7.4 | 6.9 4.5 | 0.08 |
| Charlson Comorbidity Index SD | 1.77 2.0 | 2.02 1.89 | 0.38 |
| CURB 65 score on admission SD | 1.7 1.4 | 2.2 1.4 | 0.037 |
| Fever on admission | 34.8% (24) | 36.5% (46) | 0.81 |
| Sepsis within 48 hours of CT | 81.2% (56) | 78.6% (99) | 0.67 |
| ICU admission within 48 hours of admission | 21.7% (15) | 15.1% (19) | 0.24 |
| No consolidation or infiltrates on CXR, n = 67a | 26.9% (18) | 7.1% (9) | 0.0001 |
| Procedure performed | 21.7% (15) | 3.1% (4) | 0.0001 |
| Source of admission | |||
| Home | 92.8% (64) | 78.6% (99) | 0.011 |
| Extended care facility | 7.2% (5) | 21.4% (27) | |
| Positive blood cultureb | 4.1% (2) | 8.9% (7) | 0.30 |
| Positive sputum culturec | 11.1% (3) | 11.4% (4) | 0.97 |
| Discharged alived | 91.3% (63) | 88.9% (112) | 0.60 |
| Characteristic | Odds Ratio | P Value | 95% CI |
|---|---|---|---|
| |||
| Age | 0.99 | 0.29 | 0.971.01 |
| CURB 65 at admission | 0.89 | 0.41 | 0.671.18 |
| Admission source (healthcare facility) | 0.36 | 0.07 | 0.121.09 |
| Consolidation or infiltrates | 0.21 | 0.001 | 0.080.53 |
Procedure Performed
Among the 195 patients, pneumonia‐related procedures were performed on only 19 (9.7%) patients. The procedures performed included bronchoscopy (n = 4), percutaneous biopsy (n = 3), thoracentesis (n = 7), and open lung biopsy (n = 5). Fifteen (78.9%) of the patients who had a pneumonia‐related procedure had a CT scan. Table 3 shows the characteristics of patients who had a procedure performed compared to those patients who did not have a procedure performed among all individuals who had a CT scan. Only average length of stay differed significantly between these 2 groups of patients (15.3 11.9 vs 6.8 4.1, P = 0.016).
| Characteristic | Procedure Performed, n = 15 (21.7%) | Procedure Not Performed, n = 54 (78.3%) | P Value |
|---|---|---|---|
| |||
| Mean age, y SD | 56.9 19.5 | 58.5 19.1 | 0.77 |
| Male gender | 53.3% (8) | 51.1% (28) | 0.92 |
| Average length of stay, d SD | 15.3 11.9 | 6.8 4.1 | 0.016 |
| Admission CURB 65 score, mean SD | 1.7 1.4 | 1.7 1.5 | 0.98 |
| Fever on admission | 40% (6) | 33.3% (18) | 0.63 |
| Sepsis within 48 hours of procedure | 93.3% (14) | 77.8% (42) | 0.17 |
| ICU admit within 48 hours of admission | 26.7% (4) | 20.4% (11) | 0.60 |
| No consolidation or infiltrates on CXR | 21.4% (3) | 7.8% (4) | 0.65 |
| Source of admission | |||
| Home | 15% (100) | 90.7% (49) | NSa |
| Extended care facility | 0% (0) | 9.3% (5) | |
| Discharge aliveb | 80% (12) | 94.4% (51) | 0.08 |
DISCUSSION
Chest radiography plays an essential role in diagnosing pneumonia. Chest CT scans are more sensitive in diagnosing pneumonia and may be more specific for certain pathogens, but objective indicators or guidelines regarding test performance are lacking.[7] There are few available studies that evaluate the benefit of chest CT scans in adults with pneumonia. Beall et al. noted 57% of immunocompetent hosts, 22% of human immunodeficiency virus (HIV) patients, and 45% of immunocompromised hosts had a new finding on CT.[8] In 40% of the cases, there was an overall change in management based on the findings. Nyamande et al. showed that high‐resolution CT scans identified abnormalities missed on plain radiographs in 82% (n = 40) of HIV patients in sub‐Saharan Africa.[9] A study by Syrjl et al. highlights the fact that high‐resolution CT scanning improves the diagnosis of community‐acquired pneumonia in patients with negative chest radiographs.[10] In the right clinical setting, additional imaging, such as high‐resolution CT scanning, is more sensitive at detecting abnormalities consistent with pneumonia.[10] We found that a CT scan was more likely to be performed in patients with no infiltrates or consolidation consistent with that finding. However, the authors did not attempt to evaluate improved clinical outcomes or management changes. Other investigators have tried to demonstrate unique or specific findings on CT scans compared to plain radiography for particular pathogens.[11, 12, 13]
We attempted to identify specific features of patients presenting with pneumonia that could assist clinicians in the decision‐making process as it relates to ordering a CT scan. CT scans were performed more frequently on subjects who were younger, had lower severity of illness, and were admitted from the community. We were unable to assess the radiographic and/or clinical findings that led the providers to order the CT scans. It is interesting to note, however, that Metlay et al. demonstrated a decreasing prevalence of pneumonia‐associated symptoms with increasing age.[14] One could speculate that patients who are younger and tend to have more symptoms may be more likely to get ancillary testing.
In our study, 35% of patients admitted with pneumonia had a CT scan performed that led to an additional procedure 22% of the time. We were unable to accurately evaluate the impact of CT on antibiotic modification, duration, or some outcomes. Although a number of studies demonstrated new or missed findings by CT compared to plain radiography, only Beall et al. reported outcome changes.[8, 9, 10, 12] They found that 39% (21/54) of patients had a change in their treatment plan including antibiotic alterations.
A number of factors impact outcomes such as length of stay and mortality in patients admitted with community‐acquired pneumonia. Empyema contributes to additional length of stay and pleural effusions are new findings identified by CT scans.[8, 9, 15, 16] Unfortunately, the number of patients with pleural effusions and even empyema (data not shown) was too small for us to analyze. Better prospective observational studies will be necessary to define specific CT findings leading to actual changes in management. The optimal timing of CT scanning could also be determined from these studies. The retrospective nature of our study is a key limitation to our results. It is difficult to determine retrospectively the clinical decision‐making process used when ordering additional diagnostic tests or procedures. Whether the CT scans ordered on our patients truly resulted in additional procedures or whether the procedures were preplanned cannot be elucidated. Our current electronic medical record and ordering process has significant drop‐down list selection bias for test indications. A postorder research‐based survey tool would be required to further evaluate the clinician's decision‐making process. In addition, as a single center study, the decision to perform CT scans and pneumonia‐related procedures reflects only the practice patterns among a relatively small number of physicians with a wide variety of practice levels and specialties. Although length of stay was not affected by performing a CT scan, patients who had a procedure did have a prolonged hospital stay consistent with a complicated course as confirmed by others.[16]
Our study results could be the first step in developing prospective studies to evaluate the indications and utility of ancillary imaging in patients with pneumonia. Prospective, multicenter observational studies, which include a clinical decision‐making survey tool as noted above, would be tremendously beneficial. Pathogen‐specific indications and outcomes will be facilitated by the deployment of more rapid and effective molecular diagnostic capabilities. Furthermore, the cost of the test, radiation exposure, impact on clinical outcomes, and overall risk/benefit would need to be calculated from these future studies.
Pneumonia remains one of the most common indications for hospital admissions. In the United States in 2010, more than 1 million patients were discharged with a diagnosis of pneumonia.[1] A diagnosis of pneumonia is based on typical clinical findings with recommendations to identify a demonstrable infiltrate on appropriate imaging modalities.[2] Although computed tomography (CT) imaging of the chest is much more sensitive than plain radiography at detecting infiltrates, the greater cost and higher radiation exposure limits its use as a screening modality.[3, 4] Additional imaging studies are recommended for patients who fail to respond to therapy.[2] There are, however, no published studies to determine the exact impact of chest CT scans on the management of pneumonia.
We conducted a retrospective assessment of CT scan use in patients admitted with a diagnosis of pneumonia. The study was designed to assess (1) the overall utilization rate of chest CT scans at our institution and (2) the impact of CT findings on patient management.
METHODS
This retrospective study was conducted at St. John Hospital and Medical Center, an 808‐bed tertiary care community teaching hospital in Detroit. The study was approved by the St. John Hospital and Medical Center's institutional review board.
Patients admitted to our institution between January 1, 2008 and November 1, 2011 were evaluated for study inclusion by searching the hospital's computer database using the discharge International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes for pneumonia, pleural effusion, and empyema. Patients were included for initial review if the appropriate ICD‐9‐CM codes were included within the list of discharge diagnoses and were not restricted based on hierarchy within that list. Patients were included in further analysis if they were 18 years of age, a diagnosis of pneumonia was made within 48 hours of admission, and records were available for review. Patients were excluded if they did not meet the above criteria or a diagnosis of pneumonia could not be confirmed by chart review. The electronic medical record was reviewed and patient demographics, hospital admission source, microbiology results, radiographic findings, and outcomes were recorded. Additional procedures such as thoracentesis, open lung biopsy and/or chest tube placement were recorded for patients if performed. The Charlson Weighted Index of Comorbidity and Confusion, Urea, Respiratory rate, Blood pressure, Age > 65 (CURB 65) scores were calculated as described elsewhere.[5, 6] CT scans were assessed for time and date of study after admission along with all relevant findings.
Data Analysis
Descriptive statistics were generated for the overall population. The associations between categorical variables and whether or not a CT scan was performed were assessed using the 2 test. Student t test or analysis of variance, followed by the Bonferroni correction of the P value, were used to compare mean values. Logistic regression was used to predict the probability of having a chest CT done, given the variables found to be related on univariate analysis. All data were analyzed using SPSS version 22.0 (IBM, Armonk, NY), and a P value of 0.05 or less was considered to indicate statistical significance.
RESULTS
A total of 264 patients were identified by discharge diagnosis, and 195 (73.9%) patients met the inclusion criteria. Among the 69 patients who were excluded, 37 patients were diagnosed more than 48 hours after admission, 19 patients did not have a radiographically demonstrable abnormality, 5 patients had an incomplete medical record, and 8 patients received no antibiotics. The overall mean age of the cases was 63.4 19.1 years, with an average length of stay of 7.4 5.7 days. Sixty‐nine (35.3%) of the case patients had a chest CT scan performed. A CT scan was performed more often in younger patients (58.1 19.0 vs 66.8 18.6, P = 0.002) and in patients with lower CURB 65 scores (1.7 1.4 vs 2.2 1.4, P = 0.037). A CT scan was also performed more often in patients with no infiltrates or consolidation on plain radiographs (26.9% vs 7.1%, P 0.0001). Patients were also more likely to have a procedure performed if they had a CT performed (21.7% vs 3.1%, P 0.0001) and were admitted from home versus a long‐term care facility or other healthcare institution (92.8% vs 78.6%, P = 0.011). Comparisons are shown in Table 1. After controlling for age, CURB 65 score on admission, admission source, and the presence of consolidation or infiltrates on initial chest radiograph (CXR), individuals were 4.76 times less likely to have a CT scan performed if the CXR showed consolidation and/or infiltrates (odds ratio: 0.21, P = 0.001; 95% confidence interval: 0.08‐0.53) (Table 2).
| Characteristics | Chest CT Scan Performed, n = 69 (35.4%) | Chest CT Scan Not Performed, n = 126 (64.6%) | P Value |
|---|---|---|---|
| |||
| Mean age, y SD | 58.1 19.0 | 66.8 18.6 | 0.002 |
| Gender, male | 52.2% (36) | 45.2% (57) | 0.35 |
| Average length of stay, d SD | 8.6 7.4 | 6.9 4.5 | 0.08 |
| Charlson Comorbidity Index SD | 1.77 2.0 | 2.02 1.89 | 0.38 |
| CURB 65 score on admission SD | 1.7 1.4 | 2.2 1.4 | 0.037 |
| Fever on admission | 34.8% (24) | 36.5% (46) | 0.81 |
| Sepsis within 48 hours of CT | 81.2% (56) | 78.6% (99) | 0.67 |
| ICU admission within 48 hours of admission | 21.7% (15) | 15.1% (19) | 0.24 |
| No consolidation or infiltrates on CXR, n = 67a | 26.9% (18) | 7.1% (9) | 0.0001 |
| Procedure performed | 21.7% (15) | 3.1% (4) | 0.0001 |
| Source of admission | |||
| Home | 92.8% (64) | 78.6% (99) | 0.011 |
| Extended care facility | 7.2% (5) | 21.4% (27) | |
| Positive blood cultureb | 4.1% (2) | 8.9% (7) | 0.30 |
| Positive sputum culturec | 11.1% (3) | 11.4% (4) | 0.97 |
| Discharged alived | 91.3% (63) | 88.9% (112) | 0.60 |
| Characteristic | Odds Ratio | P Value | 95% CI |
|---|---|---|---|
| |||
| Age | 0.99 | 0.29 | 0.971.01 |
| CURB 65 at admission | 0.89 | 0.41 | 0.671.18 |
| Admission source (healthcare facility) | 0.36 | 0.07 | 0.121.09 |
| Consolidation or infiltrates | 0.21 | 0.001 | 0.080.53 |
Procedure Performed
Among the 195 patients, pneumonia‐related procedures were performed on only 19 (9.7%) patients. The procedures performed included bronchoscopy (n = 4), percutaneous biopsy (n = 3), thoracentesis (n = 7), and open lung biopsy (n = 5). Fifteen (78.9%) of the patients who had a pneumonia‐related procedure had a CT scan. Table 3 shows the characteristics of patients who had a procedure performed compared to those patients who did not have a procedure performed among all individuals who had a CT scan. Only average length of stay differed significantly between these 2 groups of patients (15.3 11.9 vs 6.8 4.1, P = 0.016).
| Characteristic | Procedure Performed, n = 15 (21.7%) | Procedure Not Performed, n = 54 (78.3%) | P Value |
|---|---|---|---|
| |||
| Mean age, y SD | 56.9 19.5 | 58.5 19.1 | 0.77 |
| Male gender | 53.3% (8) | 51.1% (28) | 0.92 |
| Average length of stay, d SD | 15.3 11.9 | 6.8 4.1 | 0.016 |
| Admission CURB 65 score, mean SD | 1.7 1.4 | 1.7 1.5 | 0.98 |
| Fever on admission | 40% (6) | 33.3% (18) | 0.63 |
| Sepsis within 48 hours of procedure | 93.3% (14) | 77.8% (42) | 0.17 |
| ICU admit within 48 hours of admission | 26.7% (4) | 20.4% (11) | 0.60 |
| No consolidation or infiltrates on CXR | 21.4% (3) | 7.8% (4) | 0.65 |
| Source of admission | |||
| Home | 15% (100) | 90.7% (49) | NSa |
| Extended care facility | 0% (0) | 9.3% (5) | |
| Discharge aliveb | 80% (12) | 94.4% (51) | 0.08 |
DISCUSSION
Chest radiography plays an essential role in diagnosing pneumonia. Chest CT scans are more sensitive in diagnosing pneumonia and may be more specific for certain pathogens, but objective indicators or guidelines regarding test performance are lacking.[7] There are few available studies that evaluate the benefit of chest CT scans in adults with pneumonia. Beall et al. noted 57% of immunocompetent hosts, 22% of human immunodeficiency virus (HIV) patients, and 45% of immunocompromised hosts had a new finding on CT.[8] In 40% of the cases, there was an overall change in management based on the findings. Nyamande et al. showed that high‐resolution CT scans identified abnormalities missed on plain radiographs in 82% (n = 40) of HIV patients in sub‐Saharan Africa.[9] A study by Syrjl et al. highlights the fact that high‐resolution CT scanning improves the diagnosis of community‐acquired pneumonia in patients with negative chest radiographs.[10] In the right clinical setting, additional imaging, such as high‐resolution CT scanning, is more sensitive at detecting abnormalities consistent with pneumonia.[10] We found that a CT scan was more likely to be performed in patients with no infiltrates or consolidation consistent with that finding. However, the authors did not attempt to evaluate improved clinical outcomes or management changes. Other investigators have tried to demonstrate unique or specific findings on CT scans compared to plain radiography for particular pathogens.[11, 12, 13]
We attempted to identify specific features of patients presenting with pneumonia that could assist clinicians in the decision‐making process as it relates to ordering a CT scan. CT scans were performed more frequently on subjects who were younger, had lower severity of illness, and were admitted from the community. We were unable to assess the radiographic and/or clinical findings that led the providers to order the CT scans. It is interesting to note, however, that Metlay et al. demonstrated a decreasing prevalence of pneumonia‐associated symptoms with increasing age.[14] One could speculate that patients who are younger and tend to have more symptoms may be more likely to get ancillary testing.
In our study, 35% of patients admitted with pneumonia had a CT scan performed that led to an additional procedure 22% of the time. We were unable to accurately evaluate the impact of CT on antibiotic modification, duration, or some outcomes. Although a number of studies demonstrated new or missed findings by CT compared to plain radiography, only Beall et al. reported outcome changes.[8, 9, 10, 12] They found that 39% (21/54) of patients had a change in their treatment plan including antibiotic alterations.
A number of factors impact outcomes such as length of stay and mortality in patients admitted with community‐acquired pneumonia. Empyema contributes to additional length of stay and pleural effusions are new findings identified by CT scans.[8, 9, 15, 16] Unfortunately, the number of patients with pleural effusions and even empyema (data not shown) was too small for us to analyze. Better prospective observational studies will be necessary to define specific CT findings leading to actual changes in management. The optimal timing of CT scanning could also be determined from these studies. The retrospective nature of our study is a key limitation to our results. It is difficult to determine retrospectively the clinical decision‐making process used when ordering additional diagnostic tests or procedures. Whether the CT scans ordered on our patients truly resulted in additional procedures or whether the procedures were preplanned cannot be elucidated. Our current electronic medical record and ordering process has significant drop‐down list selection bias for test indications. A postorder research‐based survey tool would be required to further evaluate the clinician's decision‐making process. In addition, as a single center study, the decision to perform CT scans and pneumonia‐related procedures reflects only the practice patterns among a relatively small number of physicians with a wide variety of practice levels and specialties. Although length of stay was not affected by performing a CT scan, patients who had a procedure did have a prolonged hospital stay consistent with a complicated course as confirmed by others.[16]
Our study results could be the first step in developing prospective studies to evaluate the indications and utility of ancillary imaging in patients with pneumonia. Prospective, multicenter observational studies, which include a clinical decision‐making survey tool as noted above, would be tremendously beneficial. Pathogen‐specific indications and outcomes will be facilitated by the deployment of more rapid and effective molecular diagnostic capabilities. Furthermore, the cost of the test, radiation exposure, impact on clinical outcomes, and overall risk/benefit would need to be calculated from these future studies.
- National Hospital Discharge Survey. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data/nhds/2average/ 2010ave2_firstlist.pdf. Accessed December 10, 2013.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , . Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266–270.
- American College of Radiology. RadiologyInfo.org website. Radiation dose in x‐ray and CT exams. Available at: http://www.radiologyinfo.org/en/safety/?pg=sfty_xray. Accessed February 24, 2014.
- , , , et al. Prospective comparison of three validated prediction rules for prognosis in community‐acquired pneumonia. Am J Med. 2005;118(4):384–392.
- , , , et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- , . Imaging pneumonia in immunocompetent and immunocompromised individuals. Curr Opin Pulm Med. 2012;18(3):194–201.
- , , , , , . Utilization of computed tomography in patients hospitalized with community‐acquired pneumonia. Md Med J. 1998;47(4):182–187.
- , , . Comparison of plain chest radiography and high‐resolution CT in human immunodeficiency virus infected patients with community‐acquired pneumonia: a sub‐Saharan Africa study. Br J Radiol. 2007;80(953):302–306.
- , , , , . High‐resolution computed tomography for the diagnosis of community‐acquired pneumonia. Clin Infect Dis. 1998;27(2):358–363.
- , , , et al. Pulmonary computed tomography findings in 39 cases of Streptococcus pneumoniae pneumonia. Intern Med. 2012;51(24):3343–3349.
- , , , et al. High‐resolution CT findings in Streptococcus milleri pulmonary infection. Clin Radiol. 2013;68(6):e331–e337.
- , , , et al. Thin‐section CT findings in Pseudomonas aeruginosa pulmonary infection. Br J Radiol. 2012;85(1020):1533–1538.
- , , , et al. Influence of age on symptoms at presentation in patients with community‐acquired pneumonia. Arch Intern Med. 1997;157(13):1453–1459.
- , , . Factors associated with length of stay in hospital for suspected community‐acquired pneumonia. Can Respir J. 2006;13(6):317–324.
- , , , , , . Predictors for length of hospital stay in patients with community‐acquired pneumonia: results from a Swiss multicenter study. BMC Pulm Med. 2012;12:21.
- National Hospital Discharge Survey. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data/nhds/2average/ 2010ave2_firstlist.pdf. Accessed December 10, 2013.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , . Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266–270.
- American College of Radiology. RadiologyInfo.org website. Radiation dose in x‐ray and CT exams. Available at: http://www.radiologyinfo.org/en/safety/?pg=sfty_xray. Accessed February 24, 2014.
- , , , et al. Prospective comparison of three validated prediction rules for prognosis in community‐acquired pneumonia. Am J Med. 2005;118(4):384–392.
- , , , et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- , . Imaging pneumonia in immunocompetent and immunocompromised individuals. Curr Opin Pulm Med. 2012;18(3):194–201.
- , , , , , . Utilization of computed tomography in patients hospitalized with community‐acquired pneumonia. Md Med J. 1998;47(4):182–187.
- , , . Comparison of plain chest radiography and high‐resolution CT in human immunodeficiency virus infected patients with community‐acquired pneumonia: a sub‐Saharan Africa study. Br J Radiol. 2007;80(953):302–306.
- , , , , . High‐resolution computed tomography for the diagnosis of community‐acquired pneumonia. Clin Infect Dis. 1998;27(2):358–363.
- , , , et al. Pulmonary computed tomography findings in 39 cases of Streptococcus pneumoniae pneumonia. Intern Med. 2012;51(24):3343–3349.
- , , , et al. High‐resolution CT findings in Streptococcus milleri pulmonary infection. Clin Radiol. 2013;68(6):e331–e337.
- , , , et al. Thin‐section CT findings in Pseudomonas aeruginosa pulmonary infection. Br J Radiol. 2012;85(1020):1533–1538.
- , , , et al. Influence of age on symptoms at presentation in patients with community‐acquired pneumonia. Arch Intern Med. 1997;157(13):1453–1459.
- , , . Factors associated with length of stay in hospital for suspected community‐acquired pneumonia. Can Respir J. 2006;13(6):317–324.
- , , , , , . Predictors for length of hospital stay in patients with community‐acquired pneumonia: results from a Swiss multicenter study. BMC Pulm Med. 2012;12:21.
HM14 Special Report: Creation of a Pediatric Hospital Medicine Dashboard Across a Four Hospital Network
Presenter: Lindsay Fox, MD
Summation: A dashboard is a visual representation of the key performance indicators. A dashboard can give a hospitalist team real-time feedback on desired measures. The Floating Hospital for Children Center in Boston created a network dashboard across four hospital sites. The areas measured in the pilot dashboard included descriptive quality metrics, value added activities, productivity, and group sustainability.
An example of improvement in sustainability measures was documentation of the need for more staffing by evaluating staff to RVU ratios. More staff was provided to one site that had a disproportionate ratio. An example of improvement in value added activities was hospital throughput. Discharge by 10 a.m. improved to more than 90% at several sites after implementation of this dashboard and distribution of data.
Takeaways:
• A dashboard can give a hospitalist team real time feedback on desired measures.
• A dashboard can effect change by engaging individual hospitalists.
• Dashboards are tools that are useful for several parts of the care delivery system including individual hospitalists, hospitalist programs, and for hospital administration.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston, and a member of Team Hospitalist.
Presenter: Lindsay Fox, MD
Summation: A dashboard is a visual representation of the key performance indicators. A dashboard can give a hospitalist team real-time feedback on desired measures. The Floating Hospital for Children Center in Boston created a network dashboard across four hospital sites. The areas measured in the pilot dashboard included descriptive quality metrics, value added activities, productivity, and group sustainability.
An example of improvement in sustainability measures was documentation of the need for more staffing by evaluating staff to RVU ratios. More staff was provided to one site that had a disproportionate ratio. An example of improvement in value added activities was hospital throughput. Discharge by 10 a.m. improved to more than 90% at several sites after implementation of this dashboard and distribution of data.
Takeaways:
• A dashboard can give a hospitalist team real time feedback on desired measures.
• A dashboard can effect change by engaging individual hospitalists.
• Dashboards are tools that are useful for several parts of the care delivery system including individual hospitalists, hospitalist programs, and for hospital administration.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston, and a member of Team Hospitalist.
Presenter: Lindsay Fox, MD
Summation: A dashboard is a visual representation of the key performance indicators. A dashboard can give a hospitalist team real-time feedback on desired measures. The Floating Hospital for Children Center in Boston created a network dashboard across four hospital sites. The areas measured in the pilot dashboard included descriptive quality metrics, value added activities, productivity, and group sustainability.
An example of improvement in sustainability measures was documentation of the need for more staffing by evaluating staff to RVU ratios. More staff was provided to one site that had a disproportionate ratio. An example of improvement in value added activities was hospital throughput. Discharge by 10 a.m. improved to more than 90% at several sites after implementation of this dashboard and distribution of data.
Takeaways:
• A dashboard can give a hospitalist team real time feedback on desired measures.
• A dashboard can effect change by engaging individual hospitalists.
• Dashboards are tools that are useful for several parts of the care delivery system including individual hospitalists, hospitalist programs, and for hospital administration.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston, and a member of Team Hospitalist.
HM14 Report: Perioperative Care of the Pediatric Patient
Presenter: Moises Auron, MD, and David Rappaport, MD
Summation: Pediatric hospitalist involvement in perioperative pediatric care covered six areas of consideration.
1) Preoperative risk. Patient-related factors, including prematurity, reflux, congenital diseases, and intercurrent illnesses increase operative risks. For many of these factors no specific remedies are available other than heightened attention to care, need, and timing of surgery.
2) Perioperative lab testing. Published data show that absent specific clinical indications there is no need for routine preop studies—including coagulation testing for T&A's. Certain circumstances: complex/prolonged surgeries or fertile females may merit limited testing.
3) Intravenous Fluids. Isotonic fluids carry lower risks of hyponatremia than hypotonic fluids.
4) VTE. VTE is the second most common hospital acquired complication. Risk factors included intubation, CVL, infection, cancer, immobility and dehydration. A graded approach to prophylaxis with more aggressive interventions for higher risk patients should be used.
5) GI stress ulcer prophylaxis. No published data are available to clearly demonstrate benefit outweighs potential risk for routine use of prophylactic antacid therapy. There is a weak recommendation for antacid prophylaxis in critically ill children. PPIs are probably equivalent to H2 blockers.
6) Pulmonary Complications. Atelectasis does not cause fever. Lots of strategies to try to prevent atelectasis—no clear data on what works. Most likely to be effective are positive pressure, either IPPV or CPAP and preoperative incentive spirometry.
Many areas of pediatric perioperative medicine lack high-quality, published data to guide care.
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenter: Moises Auron, MD, and David Rappaport, MD
Summation: Pediatric hospitalist involvement in perioperative pediatric care covered six areas of consideration.
1) Preoperative risk. Patient-related factors, including prematurity, reflux, congenital diseases, and intercurrent illnesses increase operative risks. For many of these factors no specific remedies are available other than heightened attention to care, need, and timing of surgery.
2) Perioperative lab testing. Published data show that absent specific clinical indications there is no need for routine preop studies—including coagulation testing for T&A's. Certain circumstances: complex/prolonged surgeries or fertile females may merit limited testing.
3) Intravenous Fluids. Isotonic fluids carry lower risks of hyponatremia than hypotonic fluids.
4) VTE. VTE is the second most common hospital acquired complication. Risk factors included intubation, CVL, infection, cancer, immobility and dehydration. A graded approach to prophylaxis with more aggressive interventions for higher risk patients should be used.
5) GI stress ulcer prophylaxis. No published data are available to clearly demonstrate benefit outweighs potential risk for routine use of prophylactic antacid therapy. There is a weak recommendation for antacid prophylaxis in critically ill children. PPIs are probably equivalent to H2 blockers.
6) Pulmonary Complications. Atelectasis does not cause fever. Lots of strategies to try to prevent atelectasis—no clear data on what works. Most likely to be effective are positive pressure, either IPPV or CPAP and preoperative incentive spirometry.
Many areas of pediatric perioperative medicine lack high-quality, published data to guide care.
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenter: Moises Auron, MD, and David Rappaport, MD
Summation: Pediatric hospitalist involvement in perioperative pediatric care covered six areas of consideration.
1) Preoperative risk. Patient-related factors, including prematurity, reflux, congenital diseases, and intercurrent illnesses increase operative risks. For many of these factors no specific remedies are available other than heightened attention to care, need, and timing of surgery.
2) Perioperative lab testing. Published data show that absent specific clinical indications there is no need for routine preop studies—including coagulation testing for T&A's. Certain circumstances: complex/prolonged surgeries or fertile females may merit limited testing.
3) Intravenous Fluids. Isotonic fluids carry lower risks of hyponatremia than hypotonic fluids.
4) VTE. VTE is the second most common hospital acquired complication. Risk factors included intubation, CVL, infection, cancer, immobility and dehydration. A graded approach to prophylaxis with more aggressive interventions for higher risk patients should be used.
5) GI stress ulcer prophylaxis. No published data are available to clearly demonstrate benefit outweighs potential risk for routine use of prophylactic antacid therapy. There is a weak recommendation for antacid prophylaxis in critically ill children. PPIs are probably equivalent to H2 blockers.
6) Pulmonary Complications. Atelectasis does not cause fever. Lots of strategies to try to prevent atelectasis—no clear data on what works. Most likely to be effective are positive pressure, either IPPV or CPAP and preoperative incentive spirometry.
Many areas of pediatric perioperative medicine lack high-quality, published data to guide care.
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
HM14 Special Report: Measurement and Clinical Decision Support Strategies that Work—Going Beyond Core Measures
“You have got to get it right up front,” Greg Maynard, MD, UCSD, told hospitalists at SHM's HM14 annual meeting when discussing how to leverage the electronic health record (EHR) to perform active surveillance for quality and safety deficits. “This method can be labor intensive up front, but [it] leverages the EHR and has the potential to disseminate improvement efficiently,” Dr. Maynard said. He went on to provide many specific tips and techniques for providers to use in order to design successful clinical decision support strategies.
Key Points
• You need to be willing to redesign the system to go beyond current process measures to achieve optimal care. Currently, there can be a poor association between process measures and outcomes measures;
• You need real-time data to be able to perform a “measure-vention,” or measurement with concurrent intervention. You need to be able to collect data and then act on it for a particular patient that day;
• You need to determine who is going to act on the quality or safety deficits that are discovered once you develop measure-ventions, or real-time measures. There needs to be someone tasked with reviewing and acting on these daily reports; and
• Some institutions have developed “dynamic dashboards” that highlight active, ongoing surveillance of multiple quality improvement metrics. These help to create shared situational awareness for all providers involved in a patient’s care.
James O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
“You have got to get it right up front,” Greg Maynard, MD, UCSD, told hospitalists at SHM's HM14 annual meeting when discussing how to leverage the electronic health record (EHR) to perform active surveillance for quality and safety deficits. “This method can be labor intensive up front, but [it] leverages the EHR and has the potential to disseminate improvement efficiently,” Dr. Maynard said. He went on to provide many specific tips and techniques for providers to use in order to design successful clinical decision support strategies.
Key Points
• You need to be willing to redesign the system to go beyond current process measures to achieve optimal care. Currently, there can be a poor association between process measures and outcomes measures;
• You need real-time data to be able to perform a “measure-vention,” or measurement with concurrent intervention. You need to be able to collect data and then act on it for a particular patient that day;
• You need to determine who is going to act on the quality or safety deficits that are discovered once you develop measure-ventions, or real-time measures. There needs to be someone tasked with reviewing and acting on these daily reports; and
• Some institutions have developed “dynamic dashboards” that highlight active, ongoing surveillance of multiple quality improvement metrics. These help to create shared situational awareness for all providers involved in a patient’s care.
James O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
“You have got to get it right up front,” Greg Maynard, MD, UCSD, told hospitalists at SHM's HM14 annual meeting when discussing how to leverage the electronic health record (EHR) to perform active surveillance for quality and safety deficits. “This method can be labor intensive up front, but [it] leverages the EHR and has the potential to disseminate improvement efficiently,” Dr. Maynard said. He went on to provide many specific tips and techniques for providers to use in order to design successful clinical decision support strategies.
Key Points
• You need to be willing to redesign the system to go beyond current process measures to achieve optimal care. Currently, there can be a poor association between process measures and outcomes measures;
• You need real-time data to be able to perform a “measure-vention,” or measurement with concurrent intervention. You need to be able to collect data and then act on it for a particular patient that day;
• You need to determine who is going to act on the quality or safety deficits that are discovered once you develop measure-ventions, or real-time measures. There needs to be someone tasked with reviewing and acting on these daily reports; and
• Some institutions have developed “dynamic dashboards” that highlight active, ongoing surveillance of multiple quality improvement metrics. These help to create shared situational awareness for all providers involved in a patient’s care.
James O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
HM14 Special Report: How to Determine the Best Hospitalist Scheduling Model
Presenters: Todd Kislak, MBA, Brian Hazen, MD, Troy Ahlstrom, MD
Summation: Two hospitalist group directors shared their scheduling tools and philosophies regarding optimal scheduling options. Dr. Hazen does the scheduling himself and Dr. Ahlstrom uses a web-based scheduling program. They both acknowledged that provider retention and satisfaction are tightly wrapped up in how you schedule providers. They recommend that groups accommodate individual preference but also be fair and equitable to the entire group.
Key Takeaways
Dr. Hazen recommended:
- Find good providers first and then try to determine their desires and fit that into the schedule as best as possible.
- Protect your nocturnists- they burn out easily and provide a key service to the hospital and your group.
- Find each person's strengths and try to cater to those to make them most successful.
- Design a weighting (or point system) for various shifts and ensure equality by using that system.
- The last day on service is a day focused on "discharges" and other patients begin with a new provider coming on service (ensures continuity and lowers LOS).
Dr. Ahlstrom recommended:
- Block schedules are hard to make flexible and do not always fit with the flux of patient loads and urgent needs of the group.
- Designing and managing the schedule is a costly endeavor and they shifted this from a physician duty to an administrator's duty. They used the Lightning Bolt solution and have enjoyed significant savings and profit.
- The ROI is made up of increased encounters, increased provider retention, and decreased locums usage.
Greg Harlan is a pediatric hospitalist, medical director for IPC The Hospitalist Company, and a member of Team Hospitalist.
Presenters: Todd Kislak, MBA, Brian Hazen, MD, Troy Ahlstrom, MD
Summation: Two hospitalist group directors shared their scheduling tools and philosophies regarding optimal scheduling options. Dr. Hazen does the scheduling himself and Dr. Ahlstrom uses a web-based scheduling program. They both acknowledged that provider retention and satisfaction are tightly wrapped up in how you schedule providers. They recommend that groups accommodate individual preference but also be fair and equitable to the entire group.
Key Takeaways
Dr. Hazen recommended:
- Find good providers first and then try to determine their desires and fit that into the schedule as best as possible.
- Protect your nocturnists- they burn out easily and provide a key service to the hospital and your group.
- Find each person's strengths and try to cater to those to make them most successful.
- Design a weighting (or point system) for various shifts and ensure equality by using that system.
- The last day on service is a day focused on "discharges" and other patients begin with a new provider coming on service (ensures continuity and lowers LOS).
Dr. Ahlstrom recommended:
- Block schedules are hard to make flexible and do not always fit with the flux of patient loads and urgent needs of the group.
- Designing and managing the schedule is a costly endeavor and they shifted this from a physician duty to an administrator's duty. They used the Lightning Bolt solution and have enjoyed significant savings and profit.
- The ROI is made up of increased encounters, increased provider retention, and decreased locums usage.
Greg Harlan is a pediatric hospitalist, medical director for IPC The Hospitalist Company, and a member of Team Hospitalist.
Presenters: Todd Kislak, MBA, Brian Hazen, MD, Troy Ahlstrom, MD
Summation: Two hospitalist group directors shared their scheduling tools and philosophies regarding optimal scheduling options. Dr. Hazen does the scheduling himself and Dr. Ahlstrom uses a web-based scheduling program. They both acknowledged that provider retention and satisfaction are tightly wrapped up in how you schedule providers. They recommend that groups accommodate individual preference but also be fair and equitable to the entire group.
Key Takeaways
Dr. Hazen recommended:
- Find good providers first and then try to determine their desires and fit that into the schedule as best as possible.
- Protect your nocturnists- they burn out easily and provide a key service to the hospital and your group.
- Find each person's strengths and try to cater to those to make them most successful.
- Design a weighting (or point system) for various shifts and ensure equality by using that system.
- The last day on service is a day focused on "discharges" and other patients begin with a new provider coming on service (ensures continuity and lowers LOS).
Dr. Ahlstrom recommended:
- Block schedules are hard to make flexible and do not always fit with the flux of patient loads and urgent needs of the group.
- Designing and managing the schedule is a costly endeavor and they shifted this from a physician duty to an administrator's duty. They used the Lightning Bolt solution and have enjoyed significant savings and profit.
- The ROI is made up of increased encounters, increased provider retention, and decreased locums usage.
Greg Harlan is a pediatric hospitalist, medical director for IPC The Hospitalist Company, and a member of Team Hospitalist.
Three Join Ranks of Masters in Hospital Medicine
LAS VEGAS—For three hospitalists at SHM's annual meeting at the Mandalay Bay Resort and Casino, today will be a masterful day. Patrick Conway, MD, MSc, FAAP, MHM, Steven Pantilat, MD, MHM, and Jack Percelay, MD, MPH, MHM, will be designated Masters in Hospital Medicine (MHM), the growing cadre of hospitalists who have attained SHM’s highest rank. Sixteen hospitalists have attained the MHM designation. The 2014 designees will be honored on stage today during ceremonies that include the announcement of all of SHM’s new fellows and SHM’s Annual Awards of Excellence.
Dr. Pantilat, who was also a member of the inaugural class of Senior Fellows in Hospital Medicine (SFHM), is a professor of medicine in the department of medicine at the University of California at San Francisco (UCSF). He’s the founding director of the UCSF Palliative Care Program and serves as director of its Leadership Center, which trains hospitalists nationwide about how to establish palliative-care services. He is also a former SHM board member and the first recipient of the SHM Excellence in Teaching Award.
"I've never been a master of anything and despite my increasing age, somehow still feel too young to be a master," Dr. Pantilat adds. "Being bestowed with this highest honor in hospital medicine definitely ranks at the top."
Dr. Conway is used to being honored, but he says he still is humbled by the designation. As a former chairman of SHM's Public Policy Committee and the current chief medical officer for the Centers for Medicare & Medicaid Services (CMS) and its deputy administrator for innovation and quality, Dr. Conway is well known—and lauded—for giving HM a voice in Washington, D.C. He views his MHM as the latest sign that his specialty continues to grow and spur positive change in health-care delivery.
"I think the designation demonstrates that hospital medicine is a maturing specialty with strong leaders," he wrote in an email. "In addition, when you look at the current and former masters…it is a stellar group of leaders who are not only advancing hospital medicine, but also changing the face of health care across our nation and improving our health system."
Dr. Percelay is so humbled that he brought his wife, daughter, and mother with him to celebrate the moment. He’s also particularly honored that he and Dr. Conway represent the field of pediatric HM, which he believes has grown tremendously in reputation over the course of his career.
"We've been able to build upon the successes of adult hospital medicine and nurture the inherent 'playing nice in the sandbox' attitude of pediatrics to grow the discipline and work force to the point it's at now where pediatric hospitalists are seen as the experts and innovators for high value pediatric inpatient care," he wrote in an email.
Dr. Percelay is a hospitalist in the pediatric ICU at Saint Barnabas Medical Center in Livingston, N.J., and teaches in the department of physician studies at Pace University of College of Health Professions in New York. He was the founding chairman for the American Academy of Pediatrics Section on Hospital Medicine, an SHM board member from 2005 to 2012, and an associate editor of the Journal of Hospital Medicine. He says attaining the rank of master ranks just behind “being my daughter's father" on his list of personal accomplishments.
"I've received scholarships in high school, college, and medical school, but those acknowledged at most four years of work," he writes. "This award recognizes 20-plus years of work practicing as a pediatric hospitalist in community settings, caring for children and their families, and contributing to the field as a whole."
LAS VEGAS—For three hospitalists at SHM's annual meeting at the Mandalay Bay Resort and Casino, today will be a masterful day. Patrick Conway, MD, MSc, FAAP, MHM, Steven Pantilat, MD, MHM, and Jack Percelay, MD, MPH, MHM, will be designated Masters in Hospital Medicine (MHM), the growing cadre of hospitalists who have attained SHM’s highest rank. Sixteen hospitalists have attained the MHM designation. The 2014 designees will be honored on stage today during ceremonies that include the announcement of all of SHM’s new fellows and SHM’s Annual Awards of Excellence.
Dr. Pantilat, who was also a member of the inaugural class of Senior Fellows in Hospital Medicine (SFHM), is a professor of medicine in the department of medicine at the University of California at San Francisco (UCSF). He’s the founding director of the UCSF Palliative Care Program and serves as director of its Leadership Center, which trains hospitalists nationwide about how to establish palliative-care services. He is also a former SHM board member and the first recipient of the SHM Excellence in Teaching Award.
"I've never been a master of anything and despite my increasing age, somehow still feel too young to be a master," Dr. Pantilat adds. "Being bestowed with this highest honor in hospital medicine definitely ranks at the top."
Dr. Conway is used to being honored, but he says he still is humbled by the designation. As a former chairman of SHM's Public Policy Committee and the current chief medical officer for the Centers for Medicare & Medicaid Services (CMS) and its deputy administrator for innovation and quality, Dr. Conway is well known—and lauded—for giving HM a voice in Washington, D.C. He views his MHM as the latest sign that his specialty continues to grow and spur positive change in health-care delivery.
"I think the designation demonstrates that hospital medicine is a maturing specialty with strong leaders," he wrote in an email. "In addition, when you look at the current and former masters…it is a stellar group of leaders who are not only advancing hospital medicine, but also changing the face of health care across our nation and improving our health system."
Dr. Percelay is so humbled that he brought his wife, daughter, and mother with him to celebrate the moment. He’s also particularly honored that he and Dr. Conway represent the field of pediatric HM, which he believes has grown tremendously in reputation over the course of his career.
"We've been able to build upon the successes of adult hospital medicine and nurture the inherent 'playing nice in the sandbox' attitude of pediatrics to grow the discipline and work force to the point it's at now where pediatric hospitalists are seen as the experts and innovators for high value pediatric inpatient care," he wrote in an email.
Dr. Percelay is a hospitalist in the pediatric ICU at Saint Barnabas Medical Center in Livingston, N.J., and teaches in the department of physician studies at Pace University of College of Health Professions in New York. He was the founding chairman for the American Academy of Pediatrics Section on Hospital Medicine, an SHM board member from 2005 to 2012, and an associate editor of the Journal of Hospital Medicine. He says attaining the rank of master ranks just behind “being my daughter's father" on his list of personal accomplishments.
"I've received scholarships in high school, college, and medical school, but those acknowledged at most four years of work," he writes. "This award recognizes 20-plus years of work practicing as a pediatric hospitalist in community settings, caring for children and their families, and contributing to the field as a whole."
LAS VEGAS—For three hospitalists at SHM's annual meeting at the Mandalay Bay Resort and Casino, today will be a masterful day. Patrick Conway, MD, MSc, FAAP, MHM, Steven Pantilat, MD, MHM, and Jack Percelay, MD, MPH, MHM, will be designated Masters in Hospital Medicine (MHM), the growing cadre of hospitalists who have attained SHM’s highest rank. Sixteen hospitalists have attained the MHM designation. The 2014 designees will be honored on stage today during ceremonies that include the announcement of all of SHM’s new fellows and SHM’s Annual Awards of Excellence.
Dr. Pantilat, who was also a member of the inaugural class of Senior Fellows in Hospital Medicine (SFHM), is a professor of medicine in the department of medicine at the University of California at San Francisco (UCSF). He’s the founding director of the UCSF Palliative Care Program and serves as director of its Leadership Center, which trains hospitalists nationwide about how to establish palliative-care services. He is also a former SHM board member and the first recipient of the SHM Excellence in Teaching Award.
"I've never been a master of anything and despite my increasing age, somehow still feel too young to be a master," Dr. Pantilat adds. "Being bestowed with this highest honor in hospital medicine definitely ranks at the top."
Dr. Conway is used to being honored, but he says he still is humbled by the designation. As a former chairman of SHM's Public Policy Committee and the current chief medical officer for the Centers for Medicare & Medicaid Services (CMS) and its deputy administrator for innovation and quality, Dr. Conway is well known—and lauded—for giving HM a voice in Washington, D.C. He views his MHM as the latest sign that his specialty continues to grow and spur positive change in health-care delivery.
"I think the designation demonstrates that hospital medicine is a maturing specialty with strong leaders," he wrote in an email. "In addition, when you look at the current and former masters…it is a stellar group of leaders who are not only advancing hospital medicine, but also changing the face of health care across our nation and improving our health system."
Dr. Percelay is so humbled that he brought his wife, daughter, and mother with him to celebrate the moment. He’s also particularly honored that he and Dr. Conway represent the field of pediatric HM, which he believes has grown tremendously in reputation over the course of his career.
"We've been able to build upon the successes of adult hospital medicine and nurture the inherent 'playing nice in the sandbox' attitude of pediatrics to grow the discipline and work force to the point it's at now where pediatric hospitalists are seen as the experts and innovators for high value pediatric inpatient care," he wrote in an email.
Dr. Percelay is a hospitalist in the pediatric ICU at Saint Barnabas Medical Center in Livingston, N.J., and teaches in the department of physician studies at Pace University of College of Health Professions in New York. He was the founding chairman for the American Academy of Pediatrics Section on Hospital Medicine, an SHM board member from 2005 to 2012, and an associate editor of the Journal of Hospital Medicine. He says attaining the rank of master ranks just behind “being my daughter's father" on his list of personal accomplishments.
"I've received scholarships in high school, college, and medical school, but those acknowledged at most four years of work," he writes. "This award recognizes 20-plus years of work practicing as a pediatric hospitalist in community settings, caring for children and their families, and contributing to the field as a whole."
Hospitalists Central To U.S. Health System Transformation
LAS VEGAS—Hospitalists are poised to be industry leaders and change agents as the rigmarole of healthcare reform shakes out over the next few years, a keynote speaker told a standing-room-only crowd Tuesday at HM14.
Ian Morrison, PhD, a founding partner of Strategic Health Perspectives, a forecasting service for the health-care industry that includes joint-venture partners Harris Interactive and the Harvard School of Public Health’s department of health policy and management, says that while the Affordable Care Act struggled with the rollout of its health exchanges, the broader movement from fee-for-service payment structures to population-based has "turned the corner…and we ain’t going back."
"You, as a society, you, as a group, need to take the long view," Dr. Morrison told 3,500 hospitalists at the Mandalay Bay Resort and Casino. "You are going to be central to this transformation."
Morrison, a native of Scotland whose delivery is half stand-up comic, half policy wonk, says hospitalists will be on the front lines as health care shifts from local health systems to just 100 to 200 regional or super-regional systems.
And while politicians and pundits dicker over how a generational shift in policies will be implemented, hospitalists will be the ones balancing that change with patients' needs.
"This is the work of the future," Morrison says. "And it is not policy wonk work; it is clinical work. It is about the transformation of the delivery system. That is the central challenge of the future. We've got to integrate across the continuum of care, using all the innovation that both public and private sectors can deliver.
"This is not going to be determined by CMS, in my view, but by the kind of innovation that America is always good at."
LAS VEGAS—Hospitalists are poised to be industry leaders and change agents as the rigmarole of healthcare reform shakes out over the next few years, a keynote speaker told a standing-room-only crowd Tuesday at HM14.
Ian Morrison, PhD, a founding partner of Strategic Health Perspectives, a forecasting service for the health-care industry that includes joint-venture partners Harris Interactive and the Harvard School of Public Health’s department of health policy and management, says that while the Affordable Care Act struggled with the rollout of its health exchanges, the broader movement from fee-for-service payment structures to population-based has "turned the corner…and we ain’t going back."
"You, as a society, you, as a group, need to take the long view," Dr. Morrison told 3,500 hospitalists at the Mandalay Bay Resort and Casino. "You are going to be central to this transformation."
Morrison, a native of Scotland whose delivery is half stand-up comic, half policy wonk, says hospitalists will be on the front lines as health care shifts from local health systems to just 100 to 200 regional or super-regional systems.
And while politicians and pundits dicker over how a generational shift in policies will be implemented, hospitalists will be the ones balancing that change with patients' needs.
"This is the work of the future," Morrison says. "And it is not policy wonk work; it is clinical work. It is about the transformation of the delivery system. That is the central challenge of the future. We've got to integrate across the continuum of care, using all the innovation that both public and private sectors can deliver.
"This is not going to be determined by CMS, in my view, but by the kind of innovation that America is always good at."
LAS VEGAS—Hospitalists are poised to be industry leaders and change agents as the rigmarole of healthcare reform shakes out over the next few years, a keynote speaker told a standing-room-only crowd Tuesday at HM14.
Ian Morrison, PhD, a founding partner of Strategic Health Perspectives, a forecasting service for the health-care industry that includes joint-venture partners Harris Interactive and the Harvard School of Public Health’s department of health policy and management, says that while the Affordable Care Act struggled with the rollout of its health exchanges, the broader movement from fee-for-service payment structures to population-based has "turned the corner…and we ain’t going back."
"You, as a society, you, as a group, need to take the long view," Dr. Morrison told 3,500 hospitalists at the Mandalay Bay Resort and Casino. "You are going to be central to this transformation."
Morrison, a native of Scotland whose delivery is half stand-up comic, half policy wonk, says hospitalists will be on the front lines as health care shifts from local health systems to just 100 to 200 regional or super-regional systems.
And while politicians and pundits dicker over how a generational shift in policies will be implemented, hospitalists will be the ones balancing that change with patients' needs.
"This is the work of the future," Morrison says. "And it is not policy wonk work; it is clinical work. It is about the transformation of the delivery system. That is the central challenge of the future. We've got to integrate across the continuum of care, using all the innovation that both public and private sectors can deliver.
"This is not going to be determined by CMS, in my view, but by the kind of innovation that America is always good at."



