User login
Biomarker testing for treatment of metastatic colorectal cancer: role of the pathologist in community practice
The past decade has been marked by significant advancements in the treatment of patients with metastatic colorectal cancer (mCRC), including the approval of novel biologic agents such as the angiogenesis inhibitors bevacizumab and afibercept and the epidermal growth factor receptor monoclonal antibodies (mAbs) cetuximab and panitumumab. Cetuximab was recently approved by the US Food and Drug Administration in combination with FOLFIRI (irinotecan, 5-fuorouracil, leucovorin) for the first-line treatment of patients with KRAS mutation-negative (wild-type) tumors as determined by an FDA-approved companion diagnostic. It was the first FDA approval in mCRC requiring use of a diagnostic test that is predictive of response prior to initiation of frontline therapy.
Click on the PDF icon at the top of this introduction to read the full article.
The past decade has been marked by significant advancements in the treatment of patients with metastatic colorectal cancer (mCRC), including the approval of novel biologic agents such as the angiogenesis inhibitors bevacizumab and afibercept and the epidermal growth factor receptor monoclonal antibodies (mAbs) cetuximab and panitumumab. Cetuximab was recently approved by the US Food and Drug Administration in combination with FOLFIRI (irinotecan, 5-fuorouracil, leucovorin) for the first-line treatment of patients with KRAS mutation-negative (wild-type) tumors as determined by an FDA-approved companion diagnostic. It was the first FDA approval in mCRC requiring use of a diagnostic test that is predictive of response prior to initiation of frontline therapy.
Click on the PDF icon at the top of this introduction to read the full article.
The past decade has been marked by significant advancements in the treatment of patients with metastatic colorectal cancer (mCRC), including the approval of novel biologic agents such as the angiogenesis inhibitors bevacizumab and afibercept and the epidermal growth factor receptor monoclonal antibodies (mAbs) cetuximab and panitumumab. Cetuximab was recently approved by the US Food and Drug Administration in combination with FOLFIRI (irinotecan, 5-fuorouracil, leucovorin) for the first-line treatment of patients with KRAS mutation-negative (wild-type) tumors as determined by an FDA-approved companion diagnostic. It was the first FDA approval in mCRC requiring use of a diagnostic test that is predictive of response prior to initiation of frontline therapy.
Click on the PDF icon at the top of this introduction to read the full article.
Current options and future directions in the systemic treatment of metastatic melanoma
Systemic treatment options for metastatic melanoma have historically been limited, with conventional cytotoxic chemotherapies demonstrating only modest benefit. Recent advances, however, have dramatically changed the treatment landscape and can be considered in 2 general categories: immunotherapeutic approaches that enhance antitumor immunity, and targeted therapeutic approaches that block oncogenic driver mutations. Immunotherapy with antibodies that block cytotoxic T-lymphocyte antigen 4 and programmed death-1 receptor can result in durable responses in a subset of patients. These treatments may be considered for patients irrespective of their mutational status, and ongoing research continues to investigate biomarkers associated with clinical outcomes. Side effects of these agents result from immune-mediated reactions involving various organ sites and can include: diarrhea, rash, hepatitis, and endocrinopathies.
Click on the PDF icon at the top of this introduction to read the full article.
Systemic treatment options for metastatic melanoma have historically been limited, with conventional cytotoxic chemotherapies demonstrating only modest benefit. Recent advances, however, have dramatically changed the treatment landscape and can be considered in 2 general categories: immunotherapeutic approaches that enhance antitumor immunity, and targeted therapeutic approaches that block oncogenic driver mutations. Immunotherapy with antibodies that block cytotoxic T-lymphocyte antigen 4 and programmed death-1 receptor can result in durable responses in a subset of patients. These treatments may be considered for patients irrespective of their mutational status, and ongoing research continues to investigate biomarkers associated with clinical outcomes. Side effects of these agents result from immune-mediated reactions involving various organ sites and can include: diarrhea, rash, hepatitis, and endocrinopathies.
Click on the PDF icon at the top of this introduction to read the full article.
Systemic treatment options for metastatic melanoma have historically been limited, with conventional cytotoxic chemotherapies demonstrating only modest benefit. Recent advances, however, have dramatically changed the treatment landscape and can be considered in 2 general categories: immunotherapeutic approaches that enhance antitumor immunity, and targeted therapeutic approaches that block oncogenic driver mutations. Immunotherapy with antibodies that block cytotoxic T-lymphocyte antigen 4 and programmed death-1 receptor can result in durable responses in a subset of patients. These treatments may be considered for patients irrespective of their mutational status, and ongoing research continues to investigate biomarkers associated with clinical outcomes. Side effects of these agents result from immune-mediated reactions involving various organ sites and can include: diarrhea, rash, hepatitis, and endocrinopathies.
Click on the PDF icon at the top of this introduction to read the full article.
Current gout guidelines stress ‘treat to target’
SNOWMASS, COLO. – The current American College of Rheumatology gout guidelines contain a number of recommendations that may come as a surprise to rheumatologists and primary care physicians alike.
The guidelines state, for example, that urate-lowering therapy should be undertaken routinely in any patient with an established diagnosis of gout who has comorbid chronic kidney disease (CKD) that is stage 2 or worse, meaning an estimated glomerular filtration rate of 89 mL/minute per 1.73 m2 or less.
The rationale is that it’s particularly important to try to prevent acute gout attacks in such patients because their renal dysfunction makes it problematic to use colchicine and NSAIDs to quell attacks. Intriguing studies suggest that lowering serum urate may actually slow progression of CKD, Dr. Michael H. Pillinger said at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The guidelines name the other indications for urate lowering in gout patients as the presence of a tophus on clinical examination or an imaging study, a history of two or more gout attacks per year, or a history of kidney stones.
Traditionally, urate-lowering therapy has been initiated during quiescent periods, but the ACR guidelines state that it also can be started during an acute attack if effective anti-inflammatory management has been instituted.
"This goes against what I was taught," observed Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
The guidelines (Arthritis Care Res. 2012;64:1431-46 and 1447-61) emphasize the importance of a treat-to-target approach.
"The primary care physicians I talk to still don’t know this. The ACR recommends a minimum serum urate target of less than 6.0 mg/dL, but the guidelines are very clear that if 6 isn’t good enough, you keep going. You go below 5. When I see patients with tophaceous gout, my target is never 6. My target is 5 or 4. That’s what I teach my fellows," explained Dr. Pillinger, who served on an expert panel that advised the guideline-writing task force.
The ACR urate-lowering algorithm begins with either allopurinol or febuxostat (Uloric) as first-line therapy. The guideline committee, which expressly excluded cost as a consideration, offered no guidance as to which xanthine oxidase inhibitor is preferred. Dr. Pillinger noted that febuxostat is a more specific xanthine oxidase inhibitor, is simpler to dose, and is far less likely to cause hypersensitivity reactions than is allopurinol. It is also more effective, although not dramatically more so. And it is considerably more expensive.
Febuxostat is approved by the Food and Drug Administration specifically for use in patients with mild to moderate CKD. Allopurinol is not. However, the gout guidelines endorse the use of allopurinol in that setting.
When allopurinol is the initial drug, the guidelines recommend dosing it in a manner that is different from how most physicians have been using it, the rheumatologist said. The recommended starting dose is lower than has been customary: 100 mg/day, and 50 mg/day in patients with stage 4 or 5 CKD. The drug is to be titrated upward every 2-5 weeks as needed to achieve the target urate level. The maximum dose is 800 mg/day, even in patients with comorbid CKD. Although the guidelines don’t provide guidance as to the size of the stepwise dosing increases, Dr. Pillinger usually boosts the allopurinol dose by 100 mg at a time, or 50 mg in patients with CKD.
"Most patients don’t get to target at 300 mg/day. You’ve got to go higher," he said.
An important innovation in the current guidelines is the recommendation for testing for the HLA-B*5801 allele in patients of Korean, Thai, or Han Chinese ancestry who are being considered for allopurinol therapy. The presence of this allele confers a several hundred–fold increased risk of allopurinol hypersensitivity.
Probenecid is endorsed as the alternative first-line urate-lowering agent, but only if at least one xanthine oxidase inhibitor is contraindicated or not tolerated. No other agents get the nod as first-line therapy.
The guidelines state that if a patient’s serum urate is not at target despite maximum-dose therapy with a first-line xanthine oxidase inhibitor, it is not appropriate to switch to the other xanthine oxidase inhibitor. Instead, it is time to add a uricosuric agent: probenecid, losartan, or fenofibrate. If the urate level still is not at target and the patient is generally well, with few gout attacks, then that’s an acceptable result. However, if the patient has moderate tophaceous gout or chronic gouty arthropathy, it’s appropriate to place the patient on pegloticase (Krystexxa) while discontinuing all other urate-lowering agents.
The ACR guidelines stress that it is vital to always try to prevent gout attacks during initiation of urate-lowering therapy. The recommended first-line agents for prophylaxis are low-dose colchicine or a low-dose NSAID, with prednisone at a dose not to exceed 10 mg/day reserved as second-line therapy in the event the first-line agents are not tolerated or are ineffective.
Prophylaxis is supposed to continue as long as a patient has any evidence of disease activity. And once all symptoms and tophi have resolved, all measures needed to keep the serum urate below 6.0 mg/dL are to be continued indefinitely.
"For most patients," Dr. Pillinger concluded, "gout treatment is almost always forever."
He reported having received research grants from Takeda, which markets febuxostat in the United States, and Savient, which markets pegloticase.
SNOWMASS, COLO. – The current American College of Rheumatology gout guidelines contain a number of recommendations that may come as a surprise to rheumatologists and primary care physicians alike.
The guidelines state, for example, that urate-lowering therapy should be undertaken routinely in any patient with an established diagnosis of gout who has comorbid chronic kidney disease (CKD) that is stage 2 or worse, meaning an estimated glomerular filtration rate of 89 mL/minute per 1.73 m2 or less.
The rationale is that it’s particularly important to try to prevent acute gout attacks in such patients because their renal dysfunction makes it problematic to use colchicine and NSAIDs to quell attacks. Intriguing studies suggest that lowering serum urate may actually slow progression of CKD, Dr. Michael H. Pillinger said at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The guidelines name the other indications for urate lowering in gout patients as the presence of a tophus on clinical examination or an imaging study, a history of two or more gout attacks per year, or a history of kidney stones.
Traditionally, urate-lowering therapy has been initiated during quiescent periods, but the ACR guidelines state that it also can be started during an acute attack if effective anti-inflammatory management has been instituted.
"This goes against what I was taught," observed Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
The guidelines (Arthritis Care Res. 2012;64:1431-46 and 1447-61) emphasize the importance of a treat-to-target approach.
"The primary care physicians I talk to still don’t know this. The ACR recommends a minimum serum urate target of less than 6.0 mg/dL, but the guidelines are very clear that if 6 isn’t good enough, you keep going. You go below 5. When I see patients with tophaceous gout, my target is never 6. My target is 5 or 4. That’s what I teach my fellows," explained Dr. Pillinger, who served on an expert panel that advised the guideline-writing task force.
The ACR urate-lowering algorithm begins with either allopurinol or febuxostat (Uloric) as first-line therapy. The guideline committee, which expressly excluded cost as a consideration, offered no guidance as to which xanthine oxidase inhibitor is preferred. Dr. Pillinger noted that febuxostat is a more specific xanthine oxidase inhibitor, is simpler to dose, and is far less likely to cause hypersensitivity reactions than is allopurinol. It is also more effective, although not dramatically more so. And it is considerably more expensive.
Febuxostat is approved by the Food and Drug Administration specifically for use in patients with mild to moderate CKD. Allopurinol is not. However, the gout guidelines endorse the use of allopurinol in that setting.
When allopurinol is the initial drug, the guidelines recommend dosing it in a manner that is different from how most physicians have been using it, the rheumatologist said. The recommended starting dose is lower than has been customary: 100 mg/day, and 50 mg/day in patients with stage 4 or 5 CKD. The drug is to be titrated upward every 2-5 weeks as needed to achieve the target urate level. The maximum dose is 800 mg/day, even in patients with comorbid CKD. Although the guidelines don’t provide guidance as to the size of the stepwise dosing increases, Dr. Pillinger usually boosts the allopurinol dose by 100 mg at a time, or 50 mg in patients with CKD.
"Most patients don’t get to target at 300 mg/day. You’ve got to go higher," he said.
An important innovation in the current guidelines is the recommendation for testing for the HLA-B*5801 allele in patients of Korean, Thai, or Han Chinese ancestry who are being considered for allopurinol therapy. The presence of this allele confers a several hundred–fold increased risk of allopurinol hypersensitivity.
Probenecid is endorsed as the alternative first-line urate-lowering agent, but only if at least one xanthine oxidase inhibitor is contraindicated or not tolerated. No other agents get the nod as first-line therapy.
The guidelines state that if a patient’s serum urate is not at target despite maximum-dose therapy with a first-line xanthine oxidase inhibitor, it is not appropriate to switch to the other xanthine oxidase inhibitor. Instead, it is time to add a uricosuric agent: probenecid, losartan, or fenofibrate. If the urate level still is not at target and the patient is generally well, with few gout attacks, then that’s an acceptable result. However, if the patient has moderate tophaceous gout or chronic gouty arthropathy, it’s appropriate to place the patient on pegloticase (Krystexxa) while discontinuing all other urate-lowering agents.
The ACR guidelines stress that it is vital to always try to prevent gout attacks during initiation of urate-lowering therapy. The recommended first-line agents for prophylaxis are low-dose colchicine or a low-dose NSAID, with prednisone at a dose not to exceed 10 mg/day reserved as second-line therapy in the event the first-line agents are not tolerated or are ineffective.
Prophylaxis is supposed to continue as long as a patient has any evidence of disease activity. And once all symptoms and tophi have resolved, all measures needed to keep the serum urate below 6.0 mg/dL are to be continued indefinitely.
"For most patients," Dr. Pillinger concluded, "gout treatment is almost always forever."
He reported having received research grants from Takeda, which markets febuxostat in the United States, and Savient, which markets pegloticase.
SNOWMASS, COLO. – The current American College of Rheumatology gout guidelines contain a number of recommendations that may come as a surprise to rheumatologists and primary care physicians alike.
The guidelines state, for example, that urate-lowering therapy should be undertaken routinely in any patient with an established diagnosis of gout who has comorbid chronic kidney disease (CKD) that is stage 2 or worse, meaning an estimated glomerular filtration rate of 89 mL/minute per 1.73 m2 or less.
The rationale is that it’s particularly important to try to prevent acute gout attacks in such patients because their renal dysfunction makes it problematic to use colchicine and NSAIDs to quell attacks. Intriguing studies suggest that lowering serum urate may actually slow progression of CKD, Dr. Michael H. Pillinger said at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The guidelines name the other indications for urate lowering in gout patients as the presence of a tophus on clinical examination or an imaging study, a history of two or more gout attacks per year, or a history of kidney stones.
Traditionally, urate-lowering therapy has been initiated during quiescent periods, but the ACR guidelines state that it also can be started during an acute attack if effective anti-inflammatory management has been instituted.
"This goes against what I was taught," observed Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
The guidelines (Arthritis Care Res. 2012;64:1431-46 and 1447-61) emphasize the importance of a treat-to-target approach.
"The primary care physicians I talk to still don’t know this. The ACR recommends a minimum serum urate target of less than 6.0 mg/dL, but the guidelines are very clear that if 6 isn’t good enough, you keep going. You go below 5. When I see patients with tophaceous gout, my target is never 6. My target is 5 or 4. That’s what I teach my fellows," explained Dr. Pillinger, who served on an expert panel that advised the guideline-writing task force.
The ACR urate-lowering algorithm begins with either allopurinol or febuxostat (Uloric) as first-line therapy. The guideline committee, which expressly excluded cost as a consideration, offered no guidance as to which xanthine oxidase inhibitor is preferred. Dr. Pillinger noted that febuxostat is a more specific xanthine oxidase inhibitor, is simpler to dose, and is far less likely to cause hypersensitivity reactions than is allopurinol. It is also more effective, although not dramatically more so. And it is considerably more expensive.
Febuxostat is approved by the Food and Drug Administration specifically for use in patients with mild to moderate CKD. Allopurinol is not. However, the gout guidelines endorse the use of allopurinol in that setting.
When allopurinol is the initial drug, the guidelines recommend dosing it in a manner that is different from how most physicians have been using it, the rheumatologist said. The recommended starting dose is lower than has been customary: 100 mg/day, and 50 mg/day in patients with stage 4 or 5 CKD. The drug is to be titrated upward every 2-5 weeks as needed to achieve the target urate level. The maximum dose is 800 mg/day, even in patients with comorbid CKD. Although the guidelines don’t provide guidance as to the size of the stepwise dosing increases, Dr. Pillinger usually boosts the allopurinol dose by 100 mg at a time, or 50 mg in patients with CKD.
"Most patients don’t get to target at 300 mg/day. You’ve got to go higher," he said.
An important innovation in the current guidelines is the recommendation for testing for the HLA-B*5801 allele in patients of Korean, Thai, or Han Chinese ancestry who are being considered for allopurinol therapy. The presence of this allele confers a several hundred–fold increased risk of allopurinol hypersensitivity.
Probenecid is endorsed as the alternative first-line urate-lowering agent, but only if at least one xanthine oxidase inhibitor is contraindicated or not tolerated. No other agents get the nod as first-line therapy.
The guidelines state that if a patient’s serum urate is not at target despite maximum-dose therapy with a first-line xanthine oxidase inhibitor, it is not appropriate to switch to the other xanthine oxidase inhibitor. Instead, it is time to add a uricosuric agent: probenecid, losartan, or fenofibrate. If the urate level still is not at target and the patient is generally well, with few gout attacks, then that’s an acceptable result. However, if the patient has moderate tophaceous gout or chronic gouty arthropathy, it’s appropriate to place the patient on pegloticase (Krystexxa) while discontinuing all other urate-lowering agents.
The ACR guidelines stress that it is vital to always try to prevent gout attacks during initiation of urate-lowering therapy. The recommended first-line agents for prophylaxis are low-dose colchicine or a low-dose NSAID, with prednisone at a dose not to exceed 10 mg/day reserved as second-line therapy in the event the first-line agents are not tolerated or are ineffective.
Prophylaxis is supposed to continue as long as a patient has any evidence of disease activity. And once all symptoms and tophi have resolved, all measures needed to keep the serum urate below 6.0 mg/dL are to be continued indefinitely.
"For most patients," Dr. Pillinger concluded, "gout treatment is almost always forever."
He reported having received research grants from Takeda, which markets febuxostat in the United States, and Savient, which markets pegloticase.
EXPERT ANALYSIS FROM THE ACR WINTER RHEUMATOLOGY SYMPOSIUM
Consortium study falls short of expectations
SAN FRANCISCO—A group’s effort to identify optimal front-line treatment for peripheral T-cell lymphomas (PTCLs) was not as successful as researchers anticipated.
The North American PTCL Consortium set out to find a treatment that could best CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone), as studies have suggested this regimen is inadequate for patients with PTCL.
So the group organized a trial testing
a potentially more promising regimen: cyclophosphamide, etoposide, vincristine, and prednisone, alternating with pralatrexate (CEOP-P).
However, CEOP-P elicited a complete response (CR) rate comparable to rates historically seen with CHOP, and progression-free survival rates with the new regimen were “not particularly encouraging.”
Ranjana Advani, MD, of Stanford University Medical Center in California, discussed this trial’s inception, execution, and results at the 6th Annual T-cell Lymphoma Forum.
Trial inception
It all began with the first meeting of the North American PTCL Consortium, which took place at the 2006 ASH Annual Meeting. Physicians from 17 centers gathered to discuss the state of PTCL research in North America.
The group realized there were too many open studies for such a rare disease, and efforts should be more focused. However, they could not agree publicly as to which studies should get priority, so they used an anonymous survey to obtain a consensus.
Survey responses were “all over the map,” Dr Advani said. But ultimately, the consensus was that ongoing trials were not sufficient, and a new trial was necessary.
The group decided to first lend their support to ongoing trials and then launch a new study. At the fourth and fifth meetings of the North American PTCL Consortium (both in 2009), they drafted the concept of a front-line trial testing CEOP-P.
“We decided to use [CEOP] as a backbone because there were reservations about anthracyclines having a role in PTCL, and there was data . . . in patients [with B-cell lymphomas] who were not anthracycline-eligible and did reasonably well when etoposide was substituted [for hydroxydaunorubicin],” Dr Advani said.
As for for the second “P” in CEOP-P, pralatrexate was the first drug approved for patients with relapsed PTCL, which provided the rationale for evaluating it in the front-line setting.
Execution and results
The primary aim of this study was to improve the CR rate from 40% to 60% with CEOP-P followed by optional transplant. A literature review had revealed that CRs with CHOP have been in the range of 40% to 50%.
The researchers enrolled a total of 34 patients, but 1 withdrew consent. Twenty-seven patients received at least 2 cycles of CEOP-P. Of the 6 patients who received a single cycle, 4 discontinued treatment due to early disease progression, and 2 discontinued because of adverse events.
Grade 3-4 adverse events associated with CEOP-P included anemia, thrombocytopenia, febrile neutropenia, mucositis, sepsis, increased creatinine, and liver transaminases.
The researchers had used a 2-stage Simon design (alpha=0.10, 90% power) to test the null hypothesis that the CR rate would be 40% or greater.
For the first stage of 20 evaluable patients, the trial would be terminated if 8 or fewer patients experienced a CR after course 2B of chemotherapy. For the second stage, 34 patients were required, and at least 17 had to achieve a CR at the end of therapy for the regimen to be considered useful.
At the end of stage 1, 50% of the patients (10/20) had achieved a CR. Ultimately, 52% of all patients (n=17) achieved a CR.
This suggests CEOP-P is a useful regimen, according to the study design. But the primary aim of improving CR from 40% to 60% was not met.
Furthermore, the estimated 1-year and 2-year progression-free survival rates were “not particularly encouraging,” according to Dr Advani. The rates were 50% and 34%, respectively. And the estimated 1-year and 2-year overall survival rate was 64%.
“So this was a lesson in working together and getting a trial from ground zero, to up and running, to a presentation, and publication underway,” Dr Advani said.
“And even though it took in all the ingredients of what everybody thought was important . . . , it’s not a regimen which has that much promise to move to a randomized setting. And so defining the optimal front-line therapy in PTCL continues to be a challenge and an unmet need.”
Now, the North American PTCL Consortium is working on a second front-line trial testing cyclophosphamide, hydroxydaunorubicin, vincristine, etoposide, and prednisone (CHOEP) plus lenalidomide in stage II, III, and IV PTCL. The final protocol has been circulated, and the group anticipates the first patient will be enrolled by June or July of this year.
Dr Advani and her colleagues also presented results of the CEOP-P trial at the 2013 ASH Annual Meeting as abstract 3044. (Information in the abstract differs from that presented at the T-cell Lymphoma Forum.) ![]()
SAN FRANCISCO—A group’s effort to identify optimal front-line treatment for peripheral T-cell lymphomas (PTCLs) was not as successful as researchers anticipated.
The North American PTCL Consortium set out to find a treatment that could best CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone), as studies have suggested this regimen is inadequate for patients with PTCL.
So the group organized a trial testing
a potentially more promising regimen: cyclophosphamide, etoposide, vincristine, and prednisone, alternating with pralatrexate (CEOP-P).
However, CEOP-P elicited a complete response (CR) rate comparable to rates historically seen with CHOP, and progression-free survival rates with the new regimen were “not particularly encouraging.”
Ranjana Advani, MD, of Stanford University Medical Center in California, discussed this trial’s inception, execution, and results at the 6th Annual T-cell Lymphoma Forum.
Trial inception
It all began with the first meeting of the North American PTCL Consortium, which took place at the 2006 ASH Annual Meeting. Physicians from 17 centers gathered to discuss the state of PTCL research in North America.
The group realized there were too many open studies for such a rare disease, and efforts should be more focused. However, they could not agree publicly as to which studies should get priority, so they used an anonymous survey to obtain a consensus.
Survey responses were “all over the map,” Dr Advani said. But ultimately, the consensus was that ongoing trials were not sufficient, and a new trial was necessary.
The group decided to first lend their support to ongoing trials and then launch a new study. At the fourth and fifth meetings of the North American PTCL Consortium (both in 2009), they drafted the concept of a front-line trial testing CEOP-P.
“We decided to use [CEOP] as a backbone because there were reservations about anthracyclines having a role in PTCL, and there was data . . . in patients [with B-cell lymphomas] who were not anthracycline-eligible and did reasonably well when etoposide was substituted [for hydroxydaunorubicin],” Dr Advani said.
As for for the second “P” in CEOP-P, pralatrexate was the first drug approved for patients with relapsed PTCL, which provided the rationale for evaluating it in the front-line setting.
Execution and results
The primary aim of this study was to improve the CR rate from 40% to 60% with CEOP-P followed by optional transplant. A literature review had revealed that CRs with CHOP have been in the range of 40% to 50%.
The researchers enrolled a total of 34 patients, but 1 withdrew consent. Twenty-seven patients received at least 2 cycles of CEOP-P. Of the 6 patients who received a single cycle, 4 discontinued treatment due to early disease progression, and 2 discontinued because of adverse events.
Grade 3-4 adverse events associated with CEOP-P included anemia, thrombocytopenia, febrile neutropenia, mucositis, sepsis, increased creatinine, and liver transaminases.
The researchers had used a 2-stage Simon design (alpha=0.10, 90% power) to test the null hypothesis that the CR rate would be 40% or greater.
For the first stage of 20 evaluable patients, the trial would be terminated if 8 or fewer patients experienced a CR after course 2B of chemotherapy. For the second stage, 34 patients were required, and at least 17 had to achieve a CR at the end of therapy for the regimen to be considered useful.
At the end of stage 1, 50% of the patients (10/20) had achieved a CR. Ultimately, 52% of all patients (n=17) achieved a CR.
This suggests CEOP-P is a useful regimen, according to the study design. But the primary aim of improving CR from 40% to 60% was not met.
Furthermore, the estimated 1-year and 2-year progression-free survival rates were “not particularly encouraging,” according to Dr Advani. The rates were 50% and 34%, respectively. And the estimated 1-year and 2-year overall survival rate was 64%.
“So this was a lesson in working together and getting a trial from ground zero, to up and running, to a presentation, and publication underway,” Dr Advani said.
“And even though it took in all the ingredients of what everybody thought was important . . . , it’s not a regimen which has that much promise to move to a randomized setting. And so defining the optimal front-line therapy in PTCL continues to be a challenge and an unmet need.”
Now, the North American PTCL Consortium is working on a second front-line trial testing cyclophosphamide, hydroxydaunorubicin, vincristine, etoposide, and prednisone (CHOEP) plus lenalidomide in stage II, III, and IV PTCL. The final protocol has been circulated, and the group anticipates the first patient will be enrolled by June or July of this year.
Dr Advani and her colleagues also presented results of the CEOP-P trial at the 2013 ASH Annual Meeting as abstract 3044. (Information in the abstract differs from that presented at the T-cell Lymphoma Forum.) ![]()
SAN FRANCISCO—A group’s effort to identify optimal front-line treatment for peripheral T-cell lymphomas (PTCLs) was not as successful as researchers anticipated.
The North American PTCL Consortium set out to find a treatment that could best CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone), as studies have suggested this regimen is inadequate for patients with PTCL.
So the group organized a trial testing
a potentially more promising regimen: cyclophosphamide, etoposide, vincristine, and prednisone, alternating with pralatrexate (CEOP-P).
However, CEOP-P elicited a complete response (CR) rate comparable to rates historically seen with CHOP, and progression-free survival rates with the new regimen were “not particularly encouraging.”
Ranjana Advani, MD, of Stanford University Medical Center in California, discussed this trial’s inception, execution, and results at the 6th Annual T-cell Lymphoma Forum.
Trial inception
It all began with the first meeting of the North American PTCL Consortium, which took place at the 2006 ASH Annual Meeting. Physicians from 17 centers gathered to discuss the state of PTCL research in North America.
The group realized there were too many open studies for such a rare disease, and efforts should be more focused. However, they could not agree publicly as to which studies should get priority, so they used an anonymous survey to obtain a consensus.
Survey responses were “all over the map,” Dr Advani said. But ultimately, the consensus was that ongoing trials were not sufficient, and a new trial was necessary.
The group decided to first lend their support to ongoing trials and then launch a new study. At the fourth and fifth meetings of the North American PTCL Consortium (both in 2009), they drafted the concept of a front-line trial testing CEOP-P.
“We decided to use [CEOP] as a backbone because there were reservations about anthracyclines having a role in PTCL, and there was data . . . in patients [with B-cell lymphomas] who were not anthracycline-eligible and did reasonably well when etoposide was substituted [for hydroxydaunorubicin],” Dr Advani said.
As for for the second “P” in CEOP-P, pralatrexate was the first drug approved for patients with relapsed PTCL, which provided the rationale for evaluating it in the front-line setting.
Execution and results
The primary aim of this study was to improve the CR rate from 40% to 60% with CEOP-P followed by optional transplant. A literature review had revealed that CRs with CHOP have been in the range of 40% to 50%.
The researchers enrolled a total of 34 patients, but 1 withdrew consent. Twenty-seven patients received at least 2 cycles of CEOP-P. Of the 6 patients who received a single cycle, 4 discontinued treatment due to early disease progression, and 2 discontinued because of adverse events.
Grade 3-4 adverse events associated with CEOP-P included anemia, thrombocytopenia, febrile neutropenia, mucositis, sepsis, increased creatinine, and liver transaminases.
The researchers had used a 2-stage Simon design (alpha=0.10, 90% power) to test the null hypothesis that the CR rate would be 40% or greater.
For the first stage of 20 evaluable patients, the trial would be terminated if 8 or fewer patients experienced a CR after course 2B of chemotherapy. For the second stage, 34 patients were required, and at least 17 had to achieve a CR at the end of therapy for the regimen to be considered useful.
At the end of stage 1, 50% of the patients (10/20) had achieved a CR. Ultimately, 52% of all patients (n=17) achieved a CR.
This suggests CEOP-P is a useful regimen, according to the study design. But the primary aim of improving CR from 40% to 60% was not met.
Furthermore, the estimated 1-year and 2-year progression-free survival rates were “not particularly encouraging,” according to Dr Advani. The rates were 50% and 34%, respectively. And the estimated 1-year and 2-year overall survival rate was 64%.
“So this was a lesson in working together and getting a trial from ground zero, to up and running, to a presentation, and publication underway,” Dr Advani said.
“And even though it took in all the ingredients of what everybody thought was important . . . , it’s not a regimen which has that much promise to move to a randomized setting. And so defining the optimal front-line therapy in PTCL continues to be a challenge and an unmet need.”
Now, the North American PTCL Consortium is working on a second front-line trial testing cyclophosphamide, hydroxydaunorubicin, vincristine, etoposide, and prednisone (CHOEP) plus lenalidomide in stage II, III, and IV PTCL. The final protocol has been circulated, and the group anticipates the first patient will be enrolled by June or July of this year.
Dr Advani and her colleagues also presented results of the CEOP-P trial at the 2013 ASH Annual Meeting as abstract 3044. (Information in the abstract differs from that presented at the T-cell Lymphoma Forum.) ![]()
Researcher status affects paper popularity, study suggests

Credit: Rhoda Baer
New research indicates that author status affects how frequently scientific papers are cited, but the size of that effect depends on a number of other factors.
Investigators found that, overall, citations increased 12% above the expected level when authors were awarded “prestigious investigator status” at the Howard Hughes Medical Institute (HHMI).
However, certain kinds of research papers benefitted more than others by this increased prestige.
“We find much more of an effect on recent papers published in a short window before the prize,” said study author Pierre Azoulay, PhD, of the MIT Sloan School of Management in Cambridge.
And the greatest gains came for papers in new areas of research and for papers published in lower-profile journals. Younger researchers who had lower profiles prior to receiving the HHMI award were more likely to see a change as well.
“The effect was much more pronounced when there was more reason to be uncertain about the quality of the science or the scientist before the prize,” Dr Azoulay noted.
This paper, titled “Matthew: Effect or Fable?,” was published in Management Science.
The “Matthew Effect” is a term coined by sociologist Robert K. Merton to describe the possibility that the work of those with high status receives greater attention than equivalent work by those who are not as well known.
Positively identifying this phenomenon in scientific paper citations is difficult, however, because it is hard to separate the status of the author from the quality of the paper. It is possible, after all, that better-known researchers are simply producing higher-quality papers, which get more attention as a result.
But Dr Azoulay and his colleagues said they’ve found a way to address this issue. They looked at papers first published before the authors became HHMI investigators, then examined the citation rates for those papers after the HHMI appointments occurred, compared to a baseline of similar papers whose authors did not receive HHMI appointments.
More specifically, each paper in the study was paired with what Dr Azoulay called a “fraternal twin,” that is, another paper published in the same journal, at the same time, with the same initial citation pattern. For good measure, the authors of the papers in this comparison group were all scientists who had received other early career awards.
In all, from 1984 through 2003, 443 scientists were named HHMI investigators. Dr Azoulay and his colleagues examined 3636 papers written by 424 of those scientists, comparing them to 3636 papers in the control group.
“You couldn’t tell [the 2 sets of papers] apart in terms of citation trajectories, up until the time of the prize,” Dr Azoulay said.
Beyond the overall 12% increase in citations, the effect was nearly twice as great for papers published in lower-profile journals.
Alternately, Dr Azoulay pointed out, “If your paper was published in Cell or Nature or Science, the HHMI [award] doesn’t add a lot.” ![]()

Credit: Rhoda Baer
New research indicates that author status affects how frequently scientific papers are cited, but the size of that effect depends on a number of other factors.
Investigators found that, overall, citations increased 12% above the expected level when authors were awarded “prestigious investigator status” at the Howard Hughes Medical Institute (HHMI).
However, certain kinds of research papers benefitted more than others by this increased prestige.
“We find much more of an effect on recent papers published in a short window before the prize,” said study author Pierre Azoulay, PhD, of the MIT Sloan School of Management in Cambridge.
And the greatest gains came for papers in new areas of research and for papers published in lower-profile journals. Younger researchers who had lower profiles prior to receiving the HHMI award were more likely to see a change as well.
“The effect was much more pronounced when there was more reason to be uncertain about the quality of the science or the scientist before the prize,” Dr Azoulay noted.
This paper, titled “Matthew: Effect or Fable?,” was published in Management Science.
The “Matthew Effect” is a term coined by sociologist Robert K. Merton to describe the possibility that the work of those with high status receives greater attention than equivalent work by those who are not as well known.
Positively identifying this phenomenon in scientific paper citations is difficult, however, because it is hard to separate the status of the author from the quality of the paper. It is possible, after all, that better-known researchers are simply producing higher-quality papers, which get more attention as a result.
But Dr Azoulay and his colleagues said they’ve found a way to address this issue. They looked at papers first published before the authors became HHMI investigators, then examined the citation rates for those papers after the HHMI appointments occurred, compared to a baseline of similar papers whose authors did not receive HHMI appointments.
More specifically, each paper in the study was paired with what Dr Azoulay called a “fraternal twin,” that is, another paper published in the same journal, at the same time, with the same initial citation pattern. For good measure, the authors of the papers in this comparison group were all scientists who had received other early career awards.
In all, from 1984 through 2003, 443 scientists were named HHMI investigators. Dr Azoulay and his colleagues examined 3636 papers written by 424 of those scientists, comparing them to 3636 papers in the control group.
“You couldn’t tell [the 2 sets of papers] apart in terms of citation trajectories, up until the time of the prize,” Dr Azoulay said.
Beyond the overall 12% increase in citations, the effect was nearly twice as great for papers published in lower-profile journals.
Alternately, Dr Azoulay pointed out, “If your paper was published in Cell or Nature or Science, the HHMI [award] doesn’t add a lot.” ![]()

Credit: Rhoda Baer
New research indicates that author status affects how frequently scientific papers are cited, but the size of that effect depends on a number of other factors.
Investigators found that, overall, citations increased 12% above the expected level when authors were awarded “prestigious investigator status” at the Howard Hughes Medical Institute (HHMI).
However, certain kinds of research papers benefitted more than others by this increased prestige.
“We find much more of an effect on recent papers published in a short window before the prize,” said study author Pierre Azoulay, PhD, of the MIT Sloan School of Management in Cambridge.
And the greatest gains came for papers in new areas of research and for papers published in lower-profile journals. Younger researchers who had lower profiles prior to receiving the HHMI award were more likely to see a change as well.
“The effect was much more pronounced when there was more reason to be uncertain about the quality of the science or the scientist before the prize,” Dr Azoulay noted.
This paper, titled “Matthew: Effect or Fable?,” was published in Management Science.
The “Matthew Effect” is a term coined by sociologist Robert K. Merton to describe the possibility that the work of those with high status receives greater attention than equivalent work by those who are not as well known.
Positively identifying this phenomenon in scientific paper citations is difficult, however, because it is hard to separate the status of the author from the quality of the paper. It is possible, after all, that better-known researchers are simply producing higher-quality papers, which get more attention as a result.
But Dr Azoulay and his colleagues said they’ve found a way to address this issue. They looked at papers first published before the authors became HHMI investigators, then examined the citation rates for those papers after the HHMI appointments occurred, compared to a baseline of similar papers whose authors did not receive HHMI appointments.
More specifically, each paper in the study was paired with what Dr Azoulay called a “fraternal twin,” that is, another paper published in the same journal, at the same time, with the same initial citation pattern. For good measure, the authors of the papers in this comparison group were all scientists who had received other early career awards.
In all, from 1984 through 2003, 443 scientists were named HHMI investigators. Dr Azoulay and his colleagues examined 3636 papers written by 424 of those scientists, comparing them to 3636 papers in the control group.
“You couldn’t tell [the 2 sets of papers] apart in terms of citation trajectories, up until the time of the prize,” Dr Azoulay said.
Beyond the overall 12% increase in citations, the effect was nearly twice as great for papers published in lower-profile journals.
Alternately, Dr Azoulay pointed out, “If your paper was published in Cell or Nature or Science, the HHMI [award] doesn’t add a lot.” ![]()
Methylation patterns can predict survival in AML, team says

Credit: Lance Liotta
Researchers have found evidence to suggest that methylation patterns in hematopoietic stem cells (HSCs) can be used to determine prognosis in patients with acute myeloid leukemia (AML).
The team discovered that patients with methylation patterns resembling those of healthy individuals lived longer than patients with substantially different patterns.
If validated in clinical trials, this finding could be used to help physicians tailor treatment according to a patient’s needs.
Ulrich Steidl, MD, PhD, of the Albert Einstein College of Medicine in New York, and his colleagues described this research in The Journal of Clinical Investigation.
The investigators knew that aberrations in HSC methylation can prevent the cells from differentiating into mature blood cells, which leads to AML.
So they speculated that comparing how closely the methylation patterns in cells from AML patients resemble the patterns found in healthy individuals’ HSCs might foretell the patients’ response to treatment.
To find out, the researchers first looked at methylation patterns in HSCs from healthy individuals. The team found that most cytosines are methylated in healthy HSCs.
And where demethylation occurs, it’s mainly limited to one particular stage of HSC differentiation—the commitment step from short-term HSC to common myeloid progenitor.
The investigators then set out to identify loci with the most significant methylation changes across differentiation stages. Their analysis revealed a set of 561 loci that distinguished between the 4 stages of HSC development they investigated.
The team next wanted to determine whether the methylation status of these loci was affected in AML. So they developed an epigenetic signature score based on loci methylation. A patient’s score increased the more his methylation pattern differed from that of a healthy individual.
The researchers tested their scoring method using data from 3 cohorts of AML patients. In each of these groups, patients with low scores had approximately twice the median survival time of patients with high scores.
Specifically, the investigators evaluated AML patients in a trial testing 2 different doses of daunorubicin (Fernandez et al, NEJM 2009).
Among patients receiving lower-dose daunorubicin, those with lower epigenetic signature scores had a median overall survival (OS) of 19 months, compared with 10.8 months for patients with higher scores (P=0.0165).
The researchers observed similar results in the patients receiving a higher dose of daunorubicin. The median OS in the group with low epigenetic signature scores was 25.4 months, compared with 13.2 months in the group with high scores (P=0.0062).
Likewise, in a third cohort of AML patients, those with a low epigenetic signature score had significantly better OS than those with a high score—a median of 28.1 months and 14.9 months, respectively (P=0.0150).
The investigators performed the same analyses using a commitment-associated gene-expression signature. And they found their epigenetic signature was more effective at predicting patient survival.
Dr Steidl and his colleagues are now studying the genes found in the aberrant epigenetic signatures to determine if they play a role in causing AML. ![]()

Credit: Lance Liotta
Researchers have found evidence to suggest that methylation patterns in hematopoietic stem cells (HSCs) can be used to determine prognosis in patients with acute myeloid leukemia (AML).
The team discovered that patients with methylation patterns resembling those of healthy individuals lived longer than patients with substantially different patterns.
If validated in clinical trials, this finding could be used to help physicians tailor treatment according to a patient’s needs.
Ulrich Steidl, MD, PhD, of the Albert Einstein College of Medicine in New York, and his colleagues described this research in The Journal of Clinical Investigation.
The investigators knew that aberrations in HSC methylation can prevent the cells from differentiating into mature blood cells, which leads to AML.
So they speculated that comparing how closely the methylation patterns in cells from AML patients resemble the patterns found in healthy individuals’ HSCs might foretell the patients’ response to treatment.
To find out, the researchers first looked at methylation patterns in HSCs from healthy individuals. The team found that most cytosines are methylated in healthy HSCs.
And where demethylation occurs, it’s mainly limited to one particular stage of HSC differentiation—the commitment step from short-term HSC to common myeloid progenitor.
The investigators then set out to identify loci with the most significant methylation changes across differentiation stages. Their analysis revealed a set of 561 loci that distinguished between the 4 stages of HSC development they investigated.
The team next wanted to determine whether the methylation status of these loci was affected in AML. So they developed an epigenetic signature score based on loci methylation. A patient’s score increased the more his methylation pattern differed from that of a healthy individual.
The researchers tested their scoring method using data from 3 cohorts of AML patients. In each of these groups, patients with low scores had approximately twice the median survival time of patients with high scores.
Specifically, the investigators evaluated AML patients in a trial testing 2 different doses of daunorubicin (Fernandez et al, NEJM 2009).
Among patients receiving lower-dose daunorubicin, those with lower epigenetic signature scores had a median overall survival (OS) of 19 months, compared with 10.8 months for patients with higher scores (P=0.0165).
The researchers observed similar results in the patients receiving a higher dose of daunorubicin. The median OS in the group with low epigenetic signature scores was 25.4 months, compared with 13.2 months in the group with high scores (P=0.0062).
Likewise, in a third cohort of AML patients, those with a low epigenetic signature score had significantly better OS than those with a high score—a median of 28.1 months and 14.9 months, respectively (P=0.0150).
The investigators performed the same analyses using a commitment-associated gene-expression signature. And they found their epigenetic signature was more effective at predicting patient survival.
Dr Steidl and his colleagues are now studying the genes found in the aberrant epigenetic signatures to determine if they play a role in causing AML. ![]()

Credit: Lance Liotta
Researchers have found evidence to suggest that methylation patterns in hematopoietic stem cells (HSCs) can be used to determine prognosis in patients with acute myeloid leukemia (AML).
The team discovered that patients with methylation patterns resembling those of healthy individuals lived longer than patients with substantially different patterns.
If validated in clinical trials, this finding could be used to help physicians tailor treatment according to a patient’s needs.
Ulrich Steidl, MD, PhD, of the Albert Einstein College of Medicine in New York, and his colleagues described this research in The Journal of Clinical Investigation.
The investigators knew that aberrations in HSC methylation can prevent the cells from differentiating into mature blood cells, which leads to AML.
So they speculated that comparing how closely the methylation patterns in cells from AML patients resemble the patterns found in healthy individuals’ HSCs might foretell the patients’ response to treatment.
To find out, the researchers first looked at methylation patterns in HSCs from healthy individuals. The team found that most cytosines are methylated in healthy HSCs.
And where demethylation occurs, it’s mainly limited to one particular stage of HSC differentiation—the commitment step from short-term HSC to common myeloid progenitor.
The investigators then set out to identify loci with the most significant methylation changes across differentiation stages. Their analysis revealed a set of 561 loci that distinguished between the 4 stages of HSC development they investigated.
The team next wanted to determine whether the methylation status of these loci was affected in AML. So they developed an epigenetic signature score based on loci methylation. A patient’s score increased the more his methylation pattern differed from that of a healthy individual.
The researchers tested their scoring method using data from 3 cohorts of AML patients. In each of these groups, patients with low scores had approximately twice the median survival time of patients with high scores.
Specifically, the investigators evaluated AML patients in a trial testing 2 different doses of daunorubicin (Fernandez et al, NEJM 2009).
Among patients receiving lower-dose daunorubicin, those with lower epigenetic signature scores had a median overall survival (OS) of 19 months, compared with 10.8 months for patients with higher scores (P=0.0165).
The researchers observed similar results in the patients receiving a higher dose of daunorubicin. The median OS in the group with low epigenetic signature scores was 25.4 months, compared with 13.2 months in the group with high scores (P=0.0062).
Likewise, in a third cohort of AML patients, those with a low epigenetic signature score had significantly better OS than those with a high score—a median of 28.1 months and 14.9 months, respectively (P=0.0150).
The investigators performed the same analyses using a commitment-associated gene-expression signature. And they found their epigenetic signature was more effective at predicting patient survival.
Dr Steidl and his colleagues are now studying the genes found in the aberrant epigenetic signatures to determine if they play a role in causing AML. ![]()
FDA approves system for GVHD prophylaxis

Credit: Miltenyi Biotec
The US Food and Drug Administration (FDA) has granted approval for a device system that can prevent graft-vs-host disease (GVHD).
The CliniMACS CD34 Reagent System is intended for use in patients with acute myeloid leukemia who are in first complete remission and undergoing stem cell transplant (SCT) from a matched, related donor.
This in vitro system enriches CD34+ hematopoietic stem cells from a donated apheresis product, while depleting other cells that can cause GVHD.
The system employs a reagent consisting of a CD34 antibody conjugated to an iron-containing nanoparticle. It enriches CD34+ cells by passing the antibody/nanoparticle-labeled cell suspension through a magnetic separation column, which is provided as part of a single-use, disposable tubing set.
Magnetically labeled CD34+ target cells are retained within the separation column, while the unlabeled cells flow through. The CD34+ cells can be recovered by removing the magnetic field and eluting the targeted CD34+ cells into a collection bag.
The FDA’s approval of this system was based on data from a phase 2 study (BMT CTN 0303) conducted by the Blood and Marrow Transplant Clinical Trials Network (Pasquini et al, JCO 2012).
The trial included 128 patients undergoing SCT from a matched, sibling donor. Forty-four patients received grafts that were T-cell depleted (TCD) using the CliniMACS system as the sole form of immune suppression. The other 84 patients received T-cell-replete grafts and pharmacologic immune suppression therapy (IST).
The 2 groups were largely similar, although more patients in the TCD arm received treatment regimens that included radiation—100% vs 50%.
Neutrophil engraftment was similar between the 2 groups. At 28 days, 96% of patients in the IST arm and 100% in the TCD arm had achieved engraftment.
Patients in the TCD arm had a significantly lower rate of chronic GVHD than those in the IST arm. The TCD patients also had a lower rate of acute GVHD, but the difference was not significant.
At 100 days, the rates of grade 2-4, acute GVHD were 39% with IST and 23% with TCD grafts (P=0.07). At 2 years, the rates of chronic GVHD were 19% with TCD grafts and 50% with IST (P<0.001).
There were no significant differences between the 2 groups with regard to graft rejection, leukemia relapse, treatment-related mortality, disease-free survival, or overall survival. However, patients in the TCD arm had a higher rate of GVHD-free survival at 2 years—41% vs 19% (P=0.006).
The CliniMACS CD34 Reagent System is manufactured by Miltenyi Biotec. For more information on the system, see the company’s website. ![]()

Credit: Miltenyi Biotec
The US Food and Drug Administration (FDA) has granted approval for a device system that can prevent graft-vs-host disease (GVHD).
The CliniMACS CD34 Reagent System is intended for use in patients with acute myeloid leukemia who are in first complete remission and undergoing stem cell transplant (SCT) from a matched, related donor.
This in vitro system enriches CD34+ hematopoietic stem cells from a donated apheresis product, while depleting other cells that can cause GVHD.
The system employs a reagent consisting of a CD34 antibody conjugated to an iron-containing nanoparticle. It enriches CD34+ cells by passing the antibody/nanoparticle-labeled cell suspension through a magnetic separation column, which is provided as part of a single-use, disposable tubing set.
Magnetically labeled CD34+ target cells are retained within the separation column, while the unlabeled cells flow through. The CD34+ cells can be recovered by removing the magnetic field and eluting the targeted CD34+ cells into a collection bag.
The FDA’s approval of this system was based on data from a phase 2 study (BMT CTN 0303) conducted by the Blood and Marrow Transplant Clinical Trials Network (Pasquini et al, JCO 2012).
The trial included 128 patients undergoing SCT from a matched, sibling donor. Forty-four patients received grafts that were T-cell depleted (TCD) using the CliniMACS system as the sole form of immune suppression. The other 84 patients received T-cell-replete grafts and pharmacologic immune suppression therapy (IST).
The 2 groups were largely similar, although more patients in the TCD arm received treatment regimens that included radiation—100% vs 50%.
Neutrophil engraftment was similar between the 2 groups. At 28 days, 96% of patients in the IST arm and 100% in the TCD arm had achieved engraftment.
Patients in the TCD arm had a significantly lower rate of chronic GVHD than those in the IST arm. The TCD patients also had a lower rate of acute GVHD, but the difference was not significant.
At 100 days, the rates of grade 2-4, acute GVHD were 39% with IST and 23% with TCD grafts (P=0.07). At 2 years, the rates of chronic GVHD were 19% with TCD grafts and 50% with IST (P<0.001).
There were no significant differences between the 2 groups with regard to graft rejection, leukemia relapse, treatment-related mortality, disease-free survival, or overall survival. However, patients in the TCD arm had a higher rate of GVHD-free survival at 2 years—41% vs 19% (P=0.006).
The CliniMACS CD34 Reagent System is manufactured by Miltenyi Biotec. For more information on the system, see the company’s website. ![]()

Credit: Miltenyi Biotec
The US Food and Drug Administration (FDA) has granted approval for a device system that can prevent graft-vs-host disease (GVHD).
The CliniMACS CD34 Reagent System is intended for use in patients with acute myeloid leukemia who are in first complete remission and undergoing stem cell transplant (SCT) from a matched, related donor.
This in vitro system enriches CD34+ hematopoietic stem cells from a donated apheresis product, while depleting other cells that can cause GVHD.
The system employs a reagent consisting of a CD34 antibody conjugated to an iron-containing nanoparticle. It enriches CD34+ cells by passing the antibody/nanoparticle-labeled cell suspension through a magnetic separation column, which is provided as part of a single-use, disposable tubing set.
Magnetically labeled CD34+ target cells are retained within the separation column, while the unlabeled cells flow through. The CD34+ cells can be recovered by removing the magnetic field and eluting the targeted CD34+ cells into a collection bag.
The FDA’s approval of this system was based on data from a phase 2 study (BMT CTN 0303) conducted by the Blood and Marrow Transplant Clinical Trials Network (Pasquini et al, JCO 2012).
The trial included 128 patients undergoing SCT from a matched, sibling donor. Forty-four patients received grafts that were T-cell depleted (TCD) using the CliniMACS system as the sole form of immune suppression. The other 84 patients received T-cell-replete grafts and pharmacologic immune suppression therapy (IST).
The 2 groups were largely similar, although more patients in the TCD arm received treatment regimens that included radiation—100% vs 50%.
Neutrophil engraftment was similar between the 2 groups. At 28 days, 96% of patients in the IST arm and 100% in the TCD arm had achieved engraftment.
Patients in the TCD arm had a significantly lower rate of chronic GVHD than those in the IST arm. The TCD patients also had a lower rate of acute GVHD, but the difference was not significant.
At 100 days, the rates of grade 2-4, acute GVHD were 39% with IST and 23% with TCD grafts (P=0.07). At 2 years, the rates of chronic GVHD were 19% with TCD grafts and 50% with IST (P<0.001).
There were no significant differences between the 2 groups with regard to graft rejection, leukemia relapse, treatment-related mortality, disease-free survival, or overall survival. However, patients in the TCD arm had a higher rate of GVHD-free survival at 2 years—41% vs 19% (P=0.006).
The CliniMACS CD34 Reagent System is manufactured by Miltenyi Biotec. For more information on the system, see the company’s website. ![]()
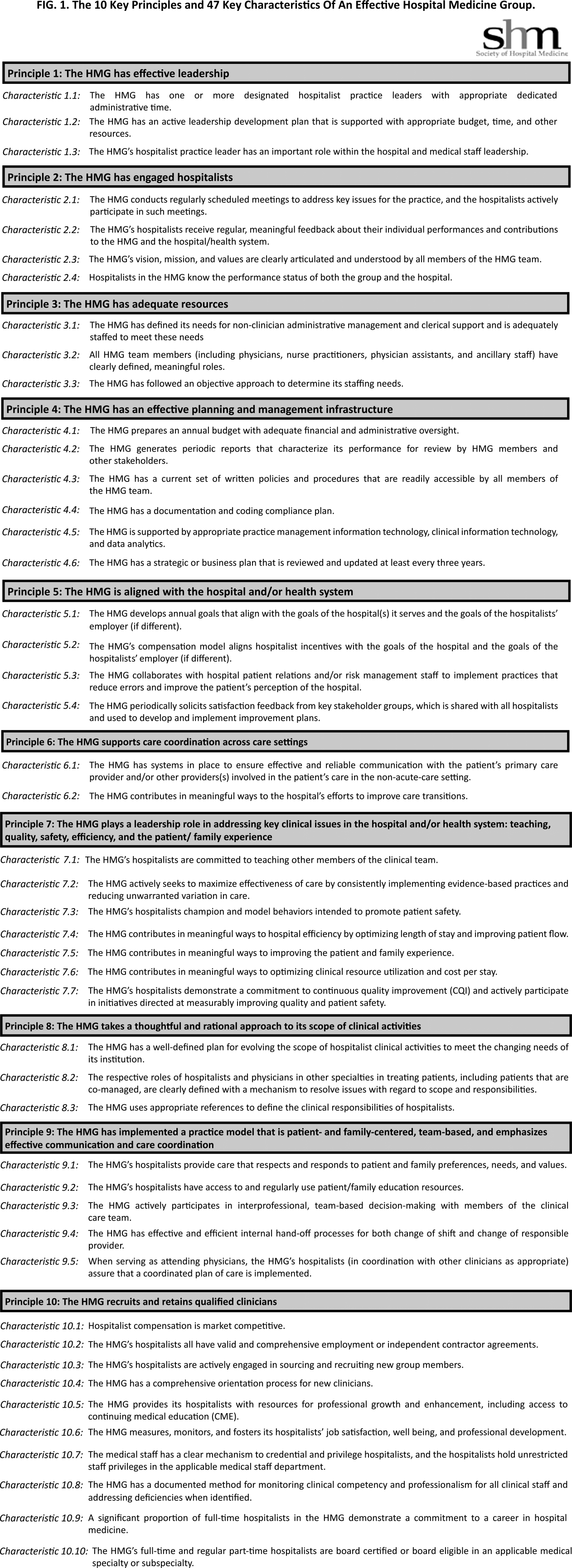
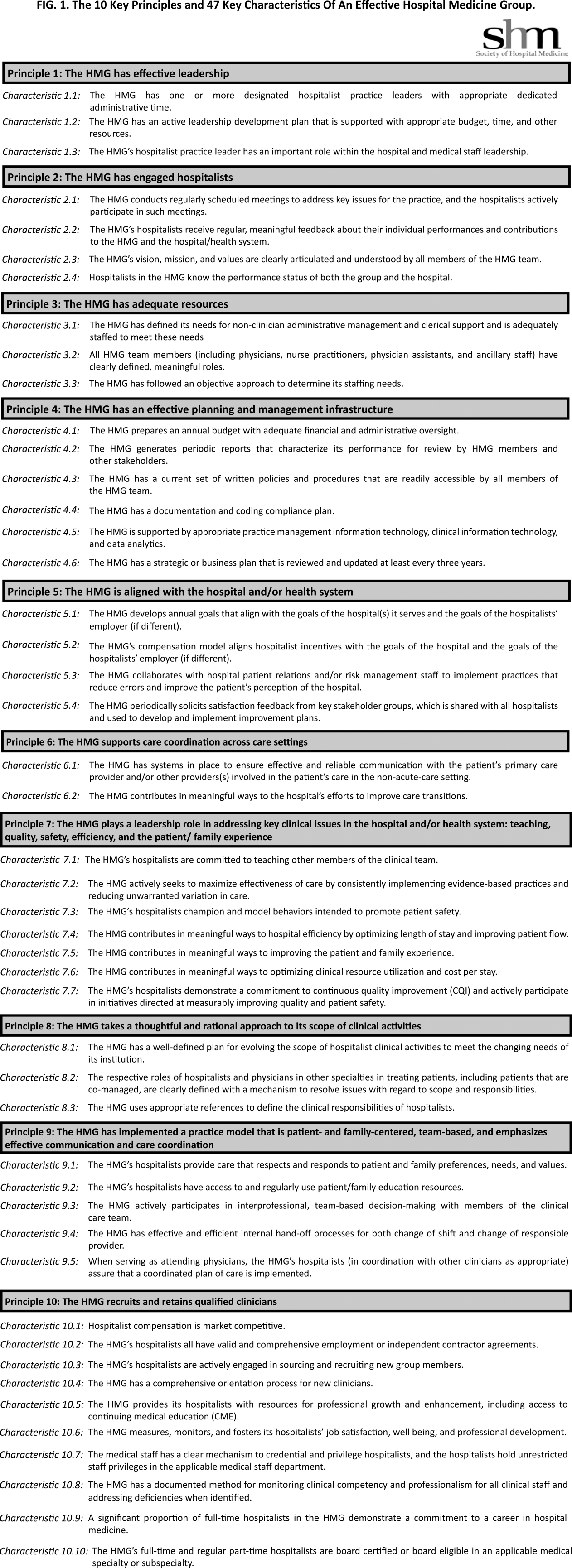
Principles and Characteristics of an HMG
With the continuing growth of the specialty of hospital medicine, the capabilities and performance of hospital medicine groups (HMGs) varies significantly. There are few guidelines that HMGs can reference as tools to guide self‐improvement. To address this deficiency, the Society of Hospital Medicine (SHM) Board of Directors authorized a process to identify the key principles and characteristics of an effective HMG.
METHODS
Topic Development and Validation Prework
In providing direction to this effort, the SHM board felt that the principles and characteristics should be directed at both hospitals and hospitalists, addressing the full range of managerial, organizational, clinical, and quality activities necessary to achieve effectiveness. Furthermore, the board defined effectiveness as consisting of 2 components. First, the HMG must assure that the patients managed by hospitalists receive high‐quality care that is sensitive to their needs and preferences. Second, the HMG must understand that the central role of the hospitalist is to coordinate patient care and foster interdisciplinary communication across the care continuum to provide optimal patient outcomes.
The SHM board appointed an HMG Characteristics Workgroup consisting of individuals who have experience with a wide array of HMG models and who could offer expert opinions on the subject. The HMG Characteristics Workgroup felt it important to review the work of other organizations that develop and administer criteria, standards, and/or requirements for healthcare organizations. Examples cited were the American College of Surgeons[1]; The Joint Commission[2]; American Nurse Credentialing Center[3]; the National Committee for Quality Assurance[4]; the American Medical Group Association[5]; and the American Association of Critical‐Care Nurses.[6]
In March 2012 and April 2012, SHM staff reviewed the websites and published materials of these organizations. For each program, information was captured on the qualifications of applicants, history of the program, timing of administering the program, the nature of recognition granted, and the program's keys to success. The summary of these findings was shared with the workgroup.
Background research and the broad scope of characteristics to be addressed led to the workgroup's decision to develop the principles and characteristics using a consensus process, emphasizing expert opinion supplemented by feedback from a broad group of stakeholders.
Initial Draft
During April 2012 and May 2012, the HMG Characteristics Workgroup identified 3 domains for the key characteristics: (1) program structure and operations, (2) clinical care delivery, and (3) organizational performance improvement. Over the course of several meetings, the HMG Characteristics Workgroup developed an initial draft of 83 characteristics, grouped into 29 subgroups within the 3 domains.
From June 2012 to November 2012, this initial draft was reviewed by a broad cross section of the hospital medicine community including members of SHM's committees, a group of academic hospitalists, focus groups in 2 communities (Philadelphia and Boston), and the leaders of several regional and national hospitalist management companies. Quantitative and qualitative feedback was obtained.
In November 2012, the SHM Board of Directors held its annual leadership meeting, attended by approximately 25 national hospitalist thought leaders and chairpersons of SHM committees. At this meeting, a series of exercises were conducted in which these leaders of the hospital medicine movement, including the SHM board members, were each assigned individual characteristics and asked to review and edit them for clarity and appropriateness.
As a result of feedback at that meeting and subsequent discussion by the SHM board, the workgroup was asked to modify the characteristics in 3 ways. First, the list should be streamlined, reducing the number of characteristics. Second, the 3 domains should be eliminated, and a better organizing framework should be created. Third, additional context should be added to the list of characteristics.
Second Draft
During the period from November 2012 to December 2012, the HMG Characteristics Workgroup went through a 2‐step Delphi process to consolidate characteristics and/or eliminate characteristics that were redundant or unnecessary. In the first step, members of the workgroup rated each characteristic from 1 to 3. A rating of 1 meant not important; good quality, but not required for an effective HMG. A rating of 2 meant important; most effective HMGs will meet requirement. A rating of 3 meant highly important; mandatory for an effective HMG. In the second step, members of the workgroup received feedback on the scores for each characteristic and came to a consensus on which characteristics should be eliminated or merged with other characteristics.
As a result, the number of characteristics was reduced and consolidated from 83 to 47, and a new framing structure was defined, replacing the 3 domains with 10 organizing principles. Finally, a rationale for each characteristic was added, defending its inclusion in the list. In addition, consideration was given to including a section describing how an HMG could demonstrate that their organization met each characteristic. However, the workgroup and the board decided that these demonstration requirements should be vetted before they were published.
From January 2013 to June 2013, the revised key principles and characteristics were reviewed by selected chairpersons of SHM committees and by 2 focus groups of HMG leaders. These reviews were conducted at the SHM Annual Meeting. Finally, in June 2013, the Committee on Clinical Leadership of the American Hospital Association reviewed and commented on the draft of the principles and characteristics.
In addition, based on feedback received from the reviewers, the wording of many of the characteristics went through revisions to assure precision and clarity. Before submission to the Journal of Hospital Medicine, a professional editor was engaged to assure that the format and language of the characteristics were clear and consistent.
Final Approval
The final draft of the 10 principles and 47 characteristics was approved for publication at a meeting of the SHM Board of Directors in September 2013 (Figure 1).
RESULTS
A recurring issue that the workgroup addressed was the applicability of the characteristics from 1 practice setting to another. Confounding factors include the HMG's employment/organizational model (eg, hospital employed, academic, multispecialty group, private practice, and management company), its population served (eg, adult vs pediatric, more than 1 hospital), and the type of hospital served (eg, academic vs community, the hospital has more than 1 HMG). The workgroup has made an effort to assure that all 47 characteristics can be applied to every type of HMG.
In developing the 10 principles, the workgroup attempted to construct a list of the basic ingredients needed to build and sustain an effective HMG. These 10 principles stand on their own, independent of the 47 key characteristics, and include issues such as effective leadership, clinician engagement, adequate resources, management infrastructure, key hospitalist roles and responsibilities, alignment with the hospital, and the recruitment and retention of qualified hospitalists.
A more detailed version of the Key Principles and Characteristics of an Effective HMG is available in the online version of this article (see Supporting Information, Appendix, in the online version of this article). The online Appendix includes the rationales for each of the characteristics, guidance on how to provide feedback to the SHM on the framework, and the SHM's plan for further development of the key principles and characteristics.
DISCUSSION
To address the variability in capabilities and performance of HMGs, these principles and characteristics are designed to provide a framework for HMGs seeking to conduct self‐assessments and develop pathways for improvement.
Although there may be HMG arrangements that do not directly involve the hospital and its executive team, and therefore alternative approaches may make sense, for most HMGs hospitals are directly involved with the HMG as either an employer or a contractor. For that reason, the Key Principles and Characteristics of an Effective HMG is written for 2 audiences: the executive leadership of the hospital (most specifically the chief medical officer or a similar role) and the hospitalists in the HMG (most specifically the practice medical director). To address the key characteristics requires the active participation of both parties. For the hospital executives, the framework establishes expectations for the HMG. For the hospitalists, the framework provides guidance in the development of an improvement plan.
Hospital executives and hospitalists can use the key characteristics in a broad spectrum of ways. The easiest and least formalized approach would be to use the framework as the basis of an ongoing dialogue between the hospital leadership and the HMG. A more formal approach would be to use the framework to guide the planning and budgeting activities of the HMG. Finally, a hospital or health system can use the key principles and characteristics as a way to evaluate their affiliated HMG(s)for example, the HMG must address 80% of the 47 characteristics.
The Key Principles and Characteristics of an Effective HMG should be considered akin to the Core Competencies in Hospital Medicine previously published in the Journal of Hospital Medicine.[7] However, instead of focusing on the competencies of individual physicians, this framework focuses on the characteristics of hospitalist groups. Just as a physician or other healthcare provider is not expected to demonstrate competency for every element in the core competencies document, an HMG does not need to have all 47 characteristics to be effective. Effective hospitalists may have skills other than those listed in the Core Competencies in Hospital Medicine. Similarly, the 47 characteristics do not represent an exhaustive list of every desirable HMG attribute. In general, effective HMGs should possess most of the characteristics.
In applying the framework, the HMG should not simply attempt to evaluate each characteristic with a yes or no assessment. For HMGs responding yes, there may be a wide range of performancefrom meeting the bare minimum requirements to employing sophisticated, expansive measures to excel in the characteristic.
SHM encourages hospital leaders and HMG leaders to use these characteristics to perform an HMG self‐assessment and to develop a plan. The plan could address implementation of selected characteristics that are not currently being addressed by the HMG or the development of additional behaviors, tools, resources, and capabilities that more fully incorporate those characteristics for which the HMG meets only minimum requirements. In addition, the plan could address the impact that a larger organization (eg, health system, hospital, or employer) may have on a given characteristic.
As outlined above, the process used to develop the Key Principles and Characteristics of an Effective HMG was grounded in expert opinion and extensive review and feedback. HMGs that use the framework should recognize that others might have a different opinion. For example, characteristic 5.2 states, The HMG's compensation model aligns hospitalist incentives with the goals of the hospital and the goals of the hospitalist's employer (if different). There are likely to be experienced hospitalist leaders who believe that an effective HMG does not need to have an incentive compensation system. However, the consensus process employed to develop the key characteristics led to the conclusion that an effective HMG should have an incentive compensation system.
The publication of the Key Principles and Characteristics of an Effective HMG may lead to negative and/or unintended consequences. A self‐assessment by an HMG using this framework could require a significant level of effort on behalf of the HMG, whereas implementing remedial efforts to address the characteristics could require an investment of time and money that could take away from other important issues facing the HMG. Many HMGs may be held accountable for addressing these characteristics without the necessary financial support from their hospital or medical group. Finally, the publication of the document could create a backlash from members of the hospitalist community who do not think that the SHM should be in the business of defining what characterizes an effective HMG, rather that this definition should be left to the marketplace.
Despite these concerns, the leadership of the SHM expects that the publication of the Key Principles and Characteristics of an Effective HMG will lead to overall improvement in the capabilities and performance of HMGs.
CONCLUSIONS
The Key Principles and Characteristics of an Effective HMG have been designed to be aspirational, helping to raise the bar for the specialty of hospital medicine. These principles and characteristics could provide a framework for HMGs seeking to conduct self‐assessments, outlining a pathway for improvement, and better defining the central role of hospitalists in coordinating team‐based, patient‐centered care in the acute care setting.
Acknowledgments
Disclosures: Patrick Cawley, MD: none; Steven Deitelzweig, MD: none; Leslie Flores, MHA: provides consulting to hospital medicine groups; Joseph A. Miller, MS: none; John Nelson, MD: provides consulting to hospital medicine groups; Scott Rissmiller, MD: none; Laurence Wellikson, MD: none; Winthrop F. Whitcomb, MD: provides consulting to hospital medicine groups.
- American College of Surgeons. New verification site visit outcomes. Available at: http://www.facs.org/trauma/verifivisitoutcomes.html. Accessed September 3, 2013.
- Hospital accreditation standards 2012. Oakbrook Terrace, IL: The Joint Commission; 2012. Available at: Amazon.com: http://www.amazon.com/Hospital‐Accreditation‐Standards‐Joint‐Commission/dp/1599404257
- The magnet model: components and sources of evidence. Silver Spring, MD: American Nurse Credentialing Center; 2011. Available at: Amazon.com: http://www.amazon.com/Magnet‐Model‐Components‐Sources‐Evidence/dp/1935213229.
- Patient Centered Medical Home Standards and Guidelines. National Committee for Quality Assurance. Available at: https://inetshop01.pub.ncqa.org/Publications/deptCate.asp?dept_id=21(suppl 1):2–95.
With the continuing growth of the specialty of hospital medicine, the capabilities and performance of hospital medicine groups (HMGs) varies significantly. There are few guidelines that HMGs can reference as tools to guide self‐improvement. To address this deficiency, the Society of Hospital Medicine (SHM) Board of Directors authorized a process to identify the key principles and characteristics of an effective HMG.
METHODS
Topic Development and Validation Prework
In providing direction to this effort, the SHM board felt that the principles and characteristics should be directed at both hospitals and hospitalists, addressing the full range of managerial, organizational, clinical, and quality activities necessary to achieve effectiveness. Furthermore, the board defined effectiveness as consisting of 2 components. First, the HMG must assure that the patients managed by hospitalists receive high‐quality care that is sensitive to their needs and preferences. Second, the HMG must understand that the central role of the hospitalist is to coordinate patient care and foster interdisciplinary communication across the care continuum to provide optimal patient outcomes.
The SHM board appointed an HMG Characteristics Workgroup consisting of individuals who have experience with a wide array of HMG models and who could offer expert opinions on the subject. The HMG Characteristics Workgroup felt it important to review the work of other organizations that develop and administer criteria, standards, and/or requirements for healthcare organizations. Examples cited were the American College of Surgeons[1]; The Joint Commission[2]; American Nurse Credentialing Center[3]; the National Committee for Quality Assurance[4]; the American Medical Group Association[5]; and the American Association of Critical‐Care Nurses.[6]
In March 2012 and April 2012, SHM staff reviewed the websites and published materials of these organizations. For each program, information was captured on the qualifications of applicants, history of the program, timing of administering the program, the nature of recognition granted, and the program's keys to success. The summary of these findings was shared with the workgroup.
Background research and the broad scope of characteristics to be addressed led to the workgroup's decision to develop the principles and characteristics using a consensus process, emphasizing expert opinion supplemented by feedback from a broad group of stakeholders.
Initial Draft
During April 2012 and May 2012, the HMG Characteristics Workgroup identified 3 domains for the key characteristics: (1) program structure and operations, (2) clinical care delivery, and (3) organizational performance improvement. Over the course of several meetings, the HMG Characteristics Workgroup developed an initial draft of 83 characteristics, grouped into 29 subgroups within the 3 domains.
From June 2012 to November 2012, this initial draft was reviewed by a broad cross section of the hospital medicine community including members of SHM's committees, a group of academic hospitalists, focus groups in 2 communities (Philadelphia and Boston), and the leaders of several regional and national hospitalist management companies. Quantitative and qualitative feedback was obtained.
In November 2012, the SHM Board of Directors held its annual leadership meeting, attended by approximately 25 national hospitalist thought leaders and chairpersons of SHM committees. At this meeting, a series of exercises were conducted in which these leaders of the hospital medicine movement, including the SHM board members, were each assigned individual characteristics and asked to review and edit them for clarity and appropriateness.
As a result of feedback at that meeting and subsequent discussion by the SHM board, the workgroup was asked to modify the characteristics in 3 ways. First, the list should be streamlined, reducing the number of characteristics. Second, the 3 domains should be eliminated, and a better organizing framework should be created. Third, additional context should be added to the list of characteristics.
Second Draft
During the period from November 2012 to December 2012, the HMG Characteristics Workgroup went through a 2‐step Delphi process to consolidate characteristics and/or eliminate characteristics that were redundant or unnecessary. In the first step, members of the workgroup rated each characteristic from 1 to 3. A rating of 1 meant not important; good quality, but not required for an effective HMG. A rating of 2 meant important; most effective HMGs will meet requirement. A rating of 3 meant highly important; mandatory for an effective HMG. In the second step, members of the workgroup received feedback on the scores for each characteristic and came to a consensus on which characteristics should be eliminated or merged with other characteristics.
As a result, the number of characteristics was reduced and consolidated from 83 to 47, and a new framing structure was defined, replacing the 3 domains with 10 organizing principles. Finally, a rationale for each characteristic was added, defending its inclusion in the list. In addition, consideration was given to including a section describing how an HMG could demonstrate that their organization met each characteristic. However, the workgroup and the board decided that these demonstration requirements should be vetted before they were published.
From January 2013 to June 2013, the revised key principles and characteristics were reviewed by selected chairpersons of SHM committees and by 2 focus groups of HMG leaders. These reviews were conducted at the SHM Annual Meeting. Finally, in June 2013, the Committee on Clinical Leadership of the American Hospital Association reviewed and commented on the draft of the principles and characteristics.
In addition, based on feedback received from the reviewers, the wording of many of the characteristics went through revisions to assure precision and clarity. Before submission to the Journal of Hospital Medicine, a professional editor was engaged to assure that the format and language of the characteristics were clear and consistent.
Final Approval
The final draft of the 10 principles and 47 characteristics was approved for publication at a meeting of the SHM Board of Directors in September 2013 (Figure 1).

RESULTS
A recurring issue that the workgroup addressed was the applicability of the characteristics from 1 practice setting to another. Confounding factors include the HMG's employment/organizational model (eg, hospital employed, academic, multispecialty group, private practice, and management company), its population served (eg, adult vs pediatric, more than 1 hospital), and the type of hospital served (eg, academic vs community, the hospital has more than 1 HMG). The workgroup has made an effort to assure that all 47 characteristics can be applied to every type of HMG.
In developing the 10 principles, the workgroup attempted to construct a list of the basic ingredients needed to build and sustain an effective HMG. These 10 principles stand on their own, independent of the 47 key characteristics, and include issues such as effective leadership, clinician engagement, adequate resources, management infrastructure, key hospitalist roles and responsibilities, alignment with the hospital, and the recruitment and retention of qualified hospitalists.
A more detailed version of the Key Principles and Characteristics of an Effective HMG is available in the online version of this article (see Supporting Information, Appendix, in the online version of this article). The online Appendix includes the rationales for each of the characteristics, guidance on how to provide feedback to the SHM on the framework, and the SHM's plan for further development of the key principles and characteristics.
DISCUSSION
To address the variability in capabilities and performance of HMGs, these principles and characteristics are designed to provide a framework for HMGs seeking to conduct self‐assessments and develop pathways for improvement.
Although there may be HMG arrangements that do not directly involve the hospital and its executive team, and therefore alternative approaches may make sense, for most HMGs hospitals are directly involved with the HMG as either an employer or a contractor. For that reason, the Key Principles and Characteristics of an Effective HMG is written for 2 audiences: the executive leadership of the hospital (most specifically the chief medical officer or a similar role) and the hospitalists in the HMG (most specifically the practice medical director). To address the key characteristics requires the active participation of both parties. For the hospital executives, the framework establishes expectations for the HMG. For the hospitalists, the framework provides guidance in the development of an improvement plan.
Hospital executives and hospitalists can use the key characteristics in a broad spectrum of ways. The easiest and least formalized approach would be to use the framework as the basis of an ongoing dialogue between the hospital leadership and the HMG. A more formal approach would be to use the framework to guide the planning and budgeting activities of the HMG. Finally, a hospital or health system can use the key principles and characteristics as a way to evaluate their affiliated HMG(s)for example, the HMG must address 80% of the 47 characteristics.
The Key Principles and Characteristics of an Effective HMG should be considered akin to the Core Competencies in Hospital Medicine previously published in the Journal of Hospital Medicine.[7] However, instead of focusing on the competencies of individual physicians, this framework focuses on the characteristics of hospitalist groups. Just as a physician or other healthcare provider is not expected to demonstrate competency for every element in the core competencies document, an HMG does not need to have all 47 characteristics to be effective. Effective hospitalists may have skills other than those listed in the Core Competencies in Hospital Medicine. Similarly, the 47 characteristics do not represent an exhaustive list of every desirable HMG attribute. In general, effective HMGs should possess most of the characteristics.
In applying the framework, the HMG should not simply attempt to evaluate each characteristic with a yes or no assessment. For HMGs responding yes, there may be a wide range of performancefrom meeting the bare minimum requirements to employing sophisticated, expansive measures to excel in the characteristic.
SHM encourages hospital leaders and HMG leaders to use these characteristics to perform an HMG self‐assessment and to develop a plan. The plan could address implementation of selected characteristics that are not currently being addressed by the HMG or the development of additional behaviors, tools, resources, and capabilities that more fully incorporate those characteristics for which the HMG meets only minimum requirements. In addition, the plan could address the impact that a larger organization (eg, health system, hospital, or employer) may have on a given characteristic.
As outlined above, the process used to develop the Key Principles and Characteristics of an Effective HMG was grounded in expert opinion and extensive review and feedback. HMGs that use the framework should recognize that others might have a different opinion. For example, characteristic 5.2 states, The HMG's compensation model aligns hospitalist incentives with the goals of the hospital and the goals of the hospitalist's employer (if different). There are likely to be experienced hospitalist leaders who believe that an effective HMG does not need to have an incentive compensation system. However, the consensus process employed to develop the key characteristics led to the conclusion that an effective HMG should have an incentive compensation system.
The publication of the Key Principles and Characteristics of an Effective HMG may lead to negative and/or unintended consequences. A self‐assessment by an HMG using this framework could require a significant level of effort on behalf of the HMG, whereas implementing remedial efforts to address the characteristics could require an investment of time and money that could take away from other important issues facing the HMG. Many HMGs may be held accountable for addressing these characteristics without the necessary financial support from their hospital or medical group. Finally, the publication of the document could create a backlash from members of the hospitalist community who do not think that the SHM should be in the business of defining what characterizes an effective HMG, rather that this definition should be left to the marketplace.
Despite these concerns, the leadership of the SHM expects that the publication of the Key Principles and Characteristics of an Effective HMG will lead to overall improvement in the capabilities and performance of HMGs.
CONCLUSIONS
The Key Principles and Characteristics of an Effective HMG have been designed to be aspirational, helping to raise the bar for the specialty of hospital medicine. These principles and characteristics could provide a framework for HMGs seeking to conduct self‐assessments, outlining a pathway for improvement, and better defining the central role of hospitalists in coordinating team‐based, patient‐centered care in the acute care setting.
Acknowledgments
Disclosures: Patrick Cawley, MD: none; Steven Deitelzweig, MD: none; Leslie Flores, MHA: provides consulting to hospital medicine groups; Joseph A. Miller, MS: none; John Nelson, MD: provides consulting to hospital medicine groups; Scott Rissmiller, MD: none; Laurence Wellikson, MD: none; Winthrop F. Whitcomb, MD: provides consulting to hospital medicine groups.
With the continuing growth of the specialty of hospital medicine, the capabilities and performance of hospital medicine groups (HMGs) varies significantly. There are few guidelines that HMGs can reference as tools to guide self‐improvement. To address this deficiency, the Society of Hospital Medicine (SHM) Board of Directors authorized a process to identify the key principles and characteristics of an effective HMG.
METHODS
Topic Development and Validation Prework
In providing direction to this effort, the SHM board felt that the principles and characteristics should be directed at both hospitals and hospitalists, addressing the full range of managerial, organizational, clinical, and quality activities necessary to achieve effectiveness. Furthermore, the board defined effectiveness as consisting of 2 components. First, the HMG must assure that the patients managed by hospitalists receive high‐quality care that is sensitive to their needs and preferences. Second, the HMG must understand that the central role of the hospitalist is to coordinate patient care and foster interdisciplinary communication across the care continuum to provide optimal patient outcomes.
The SHM board appointed an HMG Characteristics Workgroup consisting of individuals who have experience with a wide array of HMG models and who could offer expert opinions on the subject. The HMG Characteristics Workgroup felt it important to review the work of other organizations that develop and administer criteria, standards, and/or requirements for healthcare organizations. Examples cited were the American College of Surgeons[1]; The Joint Commission[2]; American Nurse Credentialing Center[3]; the National Committee for Quality Assurance[4]; the American Medical Group Association[5]; and the American Association of Critical‐Care Nurses.[6]
In March 2012 and April 2012, SHM staff reviewed the websites and published materials of these organizations. For each program, information was captured on the qualifications of applicants, history of the program, timing of administering the program, the nature of recognition granted, and the program's keys to success. The summary of these findings was shared with the workgroup.
Background research and the broad scope of characteristics to be addressed led to the workgroup's decision to develop the principles and characteristics using a consensus process, emphasizing expert opinion supplemented by feedback from a broad group of stakeholders.
Initial Draft
During April 2012 and May 2012, the HMG Characteristics Workgroup identified 3 domains for the key characteristics: (1) program structure and operations, (2) clinical care delivery, and (3) organizational performance improvement. Over the course of several meetings, the HMG Characteristics Workgroup developed an initial draft of 83 characteristics, grouped into 29 subgroups within the 3 domains.
From June 2012 to November 2012, this initial draft was reviewed by a broad cross section of the hospital medicine community including members of SHM's committees, a group of academic hospitalists, focus groups in 2 communities (Philadelphia and Boston), and the leaders of several regional and national hospitalist management companies. Quantitative and qualitative feedback was obtained.
In November 2012, the SHM Board of Directors held its annual leadership meeting, attended by approximately 25 national hospitalist thought leaders and chairpersons of SHM committees. At this meeting, a series of exercises were conducted in which these leaders of the hospital medicine movement, including the SHM board members, were each assigned individual characteristics and asked to review and edit them for clarity and appropriateness.
As a result of feedback at that meeting and subsequent discussion by the SHM board, the workgroup was asked to modify the characteristics in 3 ways. First, the list should be streamlined, reducing the number of characteristics. Second, the 3 domains should be eliminated, and a better organizing framework should be created. Third, additional context should be added to the list of characteristics.
Second Draft
During the period from November 2012 to December 2012, the HMG Characteristics Workgroup went through a 2‐step Delphi process to consolidate characteristics and/or eliminate characteristics that were redundant or unnecessary. In the first step, members of the workgroup rated each characteristic from 1 to 3. A rating of 1 meant not important; good quality, but not required for an effective HMG. A rating of 2 meant important; most effective HMGs will meet requirement. A rating of 3 meant highly important; mandatory for an effective HMG. In the second step, members of the workgroup received feedback on the scores for each characteristic and came to a consensus on which characteristics should be eliminated or merged with other characteristics.
As a result, the number of characteristics was reduced and consolidated from 83 to 47, and a new framing structure was defined, replacing the 3 domains with 10 organizing principles. Finally, a rationale for each characteristic was added, defending its inclusion in the list. In addition, consideration was given to including a section describing how an HMG could demonstrate that their organization met each characteristic. However, the workgroup and the board decided that these demonstration requirements should be vetted before they were published.
From January 2013 to June 2013, the revised key principles and characteristics were reviewed by selected chairpersons of SHM committees and by 2 focus groups of HMG leaders. These reviews were conducted at the SHM Annual Meeting. Finally, in June 2013, the Committee on Clinical Leadership of the American Hospital Association reviewed and commented on the draft of the principles and characteristics.
In addition, based on feedback received from the reviewers, the wording of many of the characteristics went through revisions to assure precision and clarity. Before submission to the Journal of Hospital Medicine, a professional editor was engaged to assure that the format and language of the characteristics were clear and consistent.
Final Approval
The final draft of the 10 principles and 47 characteristics was approved for publication at a meeting of the SHM Board of Directors in September 2013 (Figure 1).

RESULTS
A recurring issue that the workgroup addressed was the applicability of the characteristics from 1 practice setting to another. Confounding factors include the HMG's employment/organizational model (eg, hospital employed, academic, multispecialty group, private practice, and management company), its population served (eg, adult vs pediatric, more than 1 hospital), and the type of hospital served (eg, academic vs community, the hospital has more than 1 HMG). The workgroup has made an effort to assure that all 47 characteristics can be applied to every type of HMG.
In developing the 10 principles, the workgroup attempted to construct a list of the basic ingredients needed to build and sustain an effective HMG. These 10 principles stand on their own, independent of the 47 key characteristics, and include issues such as effective leadership, clinician engagement, adequate resources, management infrastructure, key hospitalist roles and responsibilities, alignment with the hospital, and the recruitment and retention of qualified hospitalists.
A more detailed version of the Key Principles and Characteristics of an Effective HMG is available in the online version of this article (see Supporting Information, Appendix, in the online version of this article). The online Appendix includes the rationales for each of the characteristics, guidance on how to provide feedback to the SHM on the framework, and the SHM's plan for further development of the key principles and characteristics.
DISCUSSION
To address the variability in capabilities and performance of HMGs, these principles and characteristics are designed to provide a framework for HMGs seeking to conduct self‐assessments and develop pathways for improvement.
Although there may be HMG arrangements that do not directly involve the hospital and its executive team, and therefore alternative approaches may make sense, for most HMGs hospitals are directly involved with the HMG as either an employer or a contractor. For that reason, the Key Principles and Characteristics of an Effective HMG is written for 2 audiences: the executive leadership of the hospital (most specifically the chief medical officer or a similar role) and the hospitalists in the HMG (most specifically the practice medical director). To address the key characteristics requires the active participation of both parties. For the hospital executives, the framework establishes expectations for the HMG. For the hospitalists, the framework provides guidance in the development of an improvement plan.
Hospital executives and hospitalists can use the key characteristics in a broad spectrum of ways. The easiest and least formalized approach would be to use the framework as the basis of an ongoing dialogue between the hospital leadership and the HMG. A more formal approach would be to use the framework to guide the planning and budgeting activities of the HMG. Finally, a hospital or health system can use the key principles and characteristics as a way to evaluate their affiliated HMG(s)for example, the HMG must address 80% of the 47 characteristics.
The Key Principles and Characteristics of an Effective HMG should be considered akin to the Core Competencies in Hospital Medicine previously published in the Journal of Hospital Medicine.[7] However, instead of focusing on the competencies of individual physicians, this framework focuses on the characteristics of hospitalist groups. Just as a physician or other healthcare provider is not expected to demonstrate competency for every element in the core competencies document, an HMG does not need to have all 47 characteristics to be effective. Effective hospitalists may have skills other than those listed in the Core Competencies in Hospital Medicine. Similarly, the 47 characteristics do not represent an exhaustive list of every desirable HMG attribute. In general, effective HMGs should possess most of the characteristics.
In applying the framework, the HMG should not simply attempt to evaluate each characteristic with a yes or no assessment. For HMGs responding yes, there may be a wide range of performancefrom meeting the bare minimum requirements to employing sophisticated, expansive measures to excel in the characteristic.
SHM encourages hospital leaders and HMG leaders to use these characteristics to perform an HMG self‐assessment and to develop a plan. The plan could address implementation of selected characteristics that are not currently being addressed by the HMG or the development of additional behaviors, tools, resources, and capabilities that more fully incorporate those characteristics for which the HMG meets only minimum requirements. In addition, the plan could address the impact that a larger organization (eg, health system, hospital, or employer) may have on a given characteristic.
As outlined above, the process used to develop the Key Principles and Characteristics of an Effective HMG was grounded in expert opinion and extensive review and feedback. HMGs that use the framework should recognize that others might have a different opinion. For example, characteristic 5.2 states, The HMG's compensation model aligns hospitalist incentives with the goals of the hospital and the goals of the hospitalist's employer (if different). There are likely to be experienced hospitalist leaders who believe that an effective HMG does not need to have an incentive compensation system. However, the consensus process employed to develop the key characteristics led to the conclusion that an effective HMG should have an incentive compensation system.
The publication of the Key Principles and Characteristics of an Effective HMG may lead to negative and/or unintended consequences. A self‐assessment by an HMG using this framework could require a significant level of effort on behalf of the HMG, whereas implementing remedial efforts to address the characteristics could require an investment of time and money that could take away from other important issues facing the HMG. Many HMGs may be held accountable for addressing these characteristics without the necessary financial support from their hospital or medical group. Finally, the publication of the document could create a backlash from members of the hospitalist community who do not think that the SHM should be in the business of defining what characterizes an effective HMG, rather that this definition should be left to the marketplace.
Despite these concerns, the leadership of the SHM expects that the publication of the Key Principles and Characteristics of an Effective HMG will lead to overall improvement in the capabilities and performance of HMGs.
CONCLUSIONS
The Key Principles and Characteristics of an Effective HMG have been designed to be aspirational, helping to raise the bar for the specialty of hospital medicine. These principles and characteristics could provide a framework for HMGs seeking to conduct self‐assessments, outlining a pathway for improvement, and better defining the central role of hospitalists in coordinating team‐based, patient‐centered care in the acute care setting.
Acknowledgments
Disclosures: Patrick Cawley, MD: none; Steven Deitelzweig, MD: none; Leslie Flores, MHA: provides consulting to hospital medicine groups; Joseph A. Miller, MS: none; John Nelson, MD: provides consulting to hospital medicine groups; Scott Rissmiller, MD: none; Laurence Wellikson, MD: none; Winthrop F. Whitcomb, MD: provides consulting to hospital medicine groups.
- American College of Surgeons. New verification site visit outcomes. Available at: http://www.facs.org/trauma/verifivisitoutcomes.html. Accessed September 3, 2013.
- Hospital accreditation standards 2012. Oakbrook Terrace, IL: The Joint Commission; 2012. Available at: Amazon.com: http://www.amazon.com/Hospital‐Accreditation‐Standards‐Joint‐Commission/dp/1599404257
- The magnet model: components and sources of evidence. Silver Spring, MD: American Nurse Credentialing Center; 2011. Available at: Amazon.com: http://www.amazon.com/Magnet‐Model‐Components‐Sources‐Evidence/dp/1935213229.
- Patient Centered Medical Home Standards and Guidelines. National Committee for Quality Assurance. Available at: https://inetshop01.pub.ncqa.org/Publications/deptCate.asp?dept_id=21(suppl 1):2–95.
- American College of Surgeons. New verification site visit outcomes. Available at: http://www.facs.org/trauma/verifivisitoutcomes.html. Accessed September 3, 2013.
- Hospital accreditation standards 2012. Oakbrook Terrace, IL: The Joint Commission; 2012. Available at: Amazon.com: http://www.amazon.com/Hospital‐Accreditation‐Standards‐Joint‐Commission/dp/1599404257
- The magnet model: components and sources of evidence. Silver Spring, MD: American Nurse Credentialing Center; 2011. Available at: Amazon.com: http://www.amazon.com/Magnet‐Model‐Components‐Sources‐Evidence/dp/1935213229.
- Patient Centered Medical Home Standards and Guidelines. National Committee for Quality Assurance. Available at: https://inetshop01.pub.ncqa.org/Publications/deptCate.asp?dept_id=21(suppl 1):2–95.
A Special Supplement on Women’s Health
While better appreciated than 2 decades ago, gender-related differences remain an important issue in clinical practice. This supplement includes 8 articles that focus on the needs and considerations in providing care to women. Four articles focus on cardiometabolic diseases: acute coronary syndrome, coronary heart disease, obesity, and diabetes mellitus. Additional topics include the early management of rheumatoid arthritis and the pharmacologic management of nausea and vomiting of pregnancy.
The last 2 articles each offer the opportunity to earn 1 free CME credit: The Pharmacologic Management of Idiopathic Overactive Bladder in Primary Care and Chronic Migraine in Women. Credit is awarded for successful completion of the quizzes at the links below; these links may also be found in the Women’s Health supplement on the first page of each article.
The Pharmacologic Management of Idiopathic Overactive Bladder in Primary Care www.pceconsortium.org/oab
Chronic Migraine in Women www.pceconsortium.org/migraine
While better appreciated than 2 decades ago, gender-related differences remain an important issue in clinical practice. This supplement includes 8 articles that focus on the needs and considerations in providing care to women. Four articles focus on cardiometabolic diseases: acute coronary syndrome, coronary heart disease, obesity, and diabetes mellitus. Additional topics include the early management of rheumatoid arthritis and the pharmacologic management of nausea and vomiting of pregnancy.
The last 2 articles each offer the opportunity to earn 1 free CME credit: The Pharmacologic Management of Idiopathic Overactive Bladder in Primary Care and Chronic Migraine in Women. Credit is awarded for successful completion of the quizzes at the links below; these links may also be found in the Women’s Health supplement on the first page of each article.
The Pharmacologic Management of Idiopathic Overactive Bladder in Primary Care www.pceconsortium.org/oab
Chronic Migraine in Women www.pceconsortium.org/migraine
While better appreciated than 2 decades ago, gender-related differences remain an important issue in clinical practice. This supplement includes 8 articles that focus on the needs and considerations in providing care to women. Four articles focus on cardiometabolic diseases: acute coronary syndrome, coronary heart disease, obesity, and diabetes mellitus. Additional topics include the early management of rheumatoid arthritis and the pharmacologic management of nausea and vomiting of pregnancy.
The last 2 articles each offer the opportunity to earn 1 free CME credit: The Pharmacologic Management of Idiopathic Overactive Bladder in Primary Care and Chronic Migraine in Women. Credit is awarded for successful completion of the quizzes at the links below; these links may also be found in the Women’s Health supplement on the first page of each article.
The Pharmacologic Management of Idiopathic Overactive Bladder in Primary Care www.pceconsortium.org/oab
Chronic Migraine in Women www.pceconsortium.org/migraine
Expert calls pegloticase a powerhouse gout drug not to be feared
SNOWMASS, COLO. – Pegloticase (Krystexxa) is a gout drug that’s expensive, inconveniently administered by intravenous infusion every 2 weeks, and saddled with a substantial rate of immunogenicity, with infusion reactions that can include anaphylaxis.
So why does gout authority Dr. Michael H. Pillinger call pegloticase "a greatly underestimated and underutilized drug"? And why do the current American College of Rheumatology gout guidelines recommend pegloticase for refractory gout?
"Nothing else we have will get rid of tophi the way this drug gets rid of tophi," Dr. Pillinger explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Why do we care about that? Because tophi are erosive, they’re damaging, and also because they’re much more extensive than we think they are," said Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
Besides, the safety concern regarding this powerhouse urate-lowering drug has been resolved. The infusion reactions are readily avoidable. Pegloticase is not a drug rheumatologists should be scared of, he emphasized.
Dr. Pillinger cited an eye-opening study that demonstrated just how much larger gout patients’ total body urate burden actually is compared with what’s apparent clinically. Investigators at the University of British Columbia, Vancouver, used dual-energy CT to assess urate deposits in 20 consecutive patients with tophaceous gout and 10 controls with other arthritic conditions. Physical evaluation of the gout patients turned up 111 areas of urate deposition; dual-energy CT revealed 440 such areas. The mean total urate volume was a hefty 40.2 cm3 (Ann. Rheum. Dis. 2009;68:1609-12).
"There’s a lot more tophi under the surface," Dr. Pillinger commented.
Pegloticase is a recombinant porcine uricase that’s modified with a baboon N-terminus. It’s pegylated to reduce immunogenicity to the uricase and increase stability and half-life. Paradoxically, the drug is still quite immunogenic because many patients develop antibodies to the polyethylene glycol used in pegylation.
The drug’s urate-lowering effect is unmatched. Within 12-24 hours of the first dose, the plasma uric acid level plummets to almost nothing.
"What happens is that very, very quickly these patients are going to split into two groups. For one group this is the greatest drug in the world; their uric acid remains almost undetectable – and that’s in our sickest refractory patients. And then there’s another group that starts to fail. It’s in the neighborhood of 30%-40% of patients, so it’s a real problem. We know they’re failing because their uric acid level starts rising. They’re making antibodies and inactivating the drug every time it’s given. And they’re the ones who get bad infusion reactions," the rheumatologist explained.
The solution to using pegloticase safely is to routinely measure serum urate 1-2 days before each infusion. If the serum urate climbs to 6 mg/dL on one or two occasions, it’s time to discontinue the drug.
"For everybody else, they’re going to do really, really well," Dr. Pillinger said.
A key point emphasized in the 2012 ACR gout guidelines (Arthritis Care Res. 2012;64:1431-46; 1447-61) is that when patients go on pegloticase, all other urate-lowering therapies must be stopped as a matter of safety. Otherwise, it’s impossible to use the pre-infusion plasma urate measurement to determine if pegloticase has stopped working.
The ACR guidelines recommend pegloticase as third-line urate-lowering therapy in patients who are not at target despite maximum-dose therapy with a xanthine oxidase inhibitor plus second-line therapy with probenecid, losartan, or fenofibrate.
Dr. Pillinger reported having received research grants from Savient, which markets pegloticase, and Takeda.
SNOWMASS, COLO. – Pegloticase (Krystexxa) is a gout drug that’s expensive, inconveniently administered by intravenous infusion every 2 weeks, and saddled with a substantial rate of immunogenicity, with infusion reactions that can include anaphylaxis.
So why does gout authority Dr. Michael H. Pillinger call pegloticase "a greatly underestimated and underutilized drug"? And why do the current American College of Rheumatology gout guidelines recommend pegloticase for refractory gout?
"Nothing else we have will get rid of tophi the way this drug gets rid of tophi," Dr. Pillinger explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Why do we care about that? Because tophi are erosive, they’re damaging, and also because they’re much more extensive than we think they are," said Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
Besides, the safety concern regarding this powerhouse urate-lowering drug has been resolved. The infusion reactions are readily avoidable. Pegloticase is not a drug rheumatologists should be scared of, he emphasized.
Dr. Pillinger cited an eye-opening study that demonstrated just how much larger gout patients’ total body urate burden actually is compared with what’s apparent clinically. Investigators at the University of British Columbia, Vancouver, used dual-energy CT to assess urate deposits in 20 consecutive patients with tophaceous gout and 10 controls with other arthritic conditions. Physical evaluation of the gout patients turned up 111 areas of urate deposition; dual-energy CT revealed 440 such areas. The mean total urate volume was a hefty 40.2 cm3 (Ann. Rheum. Dis. 2009;68:1609-12).
"There’s a lot more tophi under the surface," Dr. Pillinger commented.
Pegloticase is a recombinant porcine uricase that’s modified with a baboon N-terminus. It’s pegylated to reduce immunogenicity to the uricase and increase stability and half-life. Paradoxically, the drug is still quite immunogenic because many patients develop antibodies to the polyethylene glycol used in pegylation.
The drug’s urate-lowering effect is unmatched. Within 12-24 hours of the first dose, the plasma uric acid level plummets to almost nothing.
"What happens is that very, very quickly these patients are going to split into two groups. For one group this is the greatest drug in the world; their uric acid remains almost undetectable – and that’s in our sickest refractory patients. And then there’s another group that starts to fail. It’s in the neighborhood of 30%-40% of patients, so it’s a real problem. We know they’re failing because their uric acid level starts rising. They’re making antibodies and inactivating the drug every time it’s given. And they’re the ones who get bad infusion reactions," the rheumatologist explained.
The solution to using pegloticase safely is to routinely measure serum urate 1-2 days before each infusion. If the serum urate climbs to 6 mg/dL on one or two occasions, it’s time to discontinue the drug.
"For everybody else, they’re going to do really, really well," Dr. Pillinger said.
A key point emphasized in the 2012 ACR gout guidelines (Arthritis Care Res. 2012;64:1431-46; 1447-61) is that when patients go on pegloticase, all other urate-lowering therapies must be stopped as a matter of safety. Otherwise, it’s impossible to use the pre-infusion plasma urate measurement to determine if pegloticase has stopped working.
The ACR guidelines recommend pegloticase as third-line urate-lowering therapy in patients who are not at target despite maximum-dose therapy with a xanthine oxidase inhibitor plus second-line therapy with probenecid, losartan, or fenofibrate.
Dr. Pillinger reported having received research grants from Savient, which markets pegloticase, and Takeda.
SNOWMASS, COLO. – Pegloticase (Krystexxa) is a gout drug that’s expensive, inconveniently administered by intravenous infusion every 2 weeks, and saddled with a substantial rate of immunogenicity, with infusion reactions that can include anaphylaxis.
So why does gout authority Dr. Michael H. Pillinger call pegloticase "a greatly underestimated and underutilized drug"? And why do the current American College of Rheumatology gout guidelines recommend pegloticase for refractory gout?
"Nothing else we have will get rid of tophi the way this drug gets rid of tophi," Dr. Pillinger explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Why do we care about that? Because tophi are erosive, they’re damaging, and also because they’re much more extensive than we think they are," said Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
Besides, the safety concern regarding this powerhouse urate-lowering drug has been resolved. The infusion reactions are readily avoidable. Pegloticase is not a drug rheumatologists should be scared of, he emphasized.
Dr. Pillinger cited an eye-opening study that demonstrated just how much larger gout patients’ total body urate burden actually is compared with what’s apparent clinically. Investigators at the University of British Columbia, Vancouver, used dual-energy CT to assess urate deposits in 20 consecutive patients with tophaceous gout and 10 controls with other arthritic conditions. Physical evaluation of the gout patients turned up 111 areas of urate deposition; dual-energy CT revealed 440 such areas. The mean total urate volume was a hefty 40.2 cm3 (Ann. Rheum. Dis. 2009;68:1609-12).
"There’s a lot more tophi under the surface," Dr. Pillinger commented.
Pegloticase is a recombinant porcine uricase that’s modified with a baboon N-terminus. It’s pegylated to reduce immunogenicity to the uricase and increase stability and half-life. Paradoxically, the drug is still quite immunogenic because many patients develop antibodies to the polyethylene glycol used in pegylation.
The drug’s urate-lowering effect is unmatched. Within 12-24 hours of the first dose, the plasma uric acid level plummets to almost nothing.
"What happens is that very, very quickly these patients are going to split into two groups. For one group this is the greatest drug in the world; their uric acid remains almost undetectable – and that’s in our sickest refractory patients. And then there’s another group that starts to fail. It’s in the neighborhood of 30%-40% of patients, so it’s a real problem. We know they’re failing because their uric acid level starts rising. They’re making antibodies and inactivating the drug every time it’s given. And they’re the ones who get bad infusion reactions," the rheumatologist explained.
The solution to using pegloticase safely is to routinely measure serum urate 1-2 days before each infusion. If the serum urate climbs to 6 mg/dL on one or two occasions, it’s time to discontinue the drug.
"For everybody else, they’re going to do really, really well," Dr. Pillinger said.
A key point emphasized in the 2012 ACR gout guidelines (Arthritis Care Res. 2012;64:1431-46; 1447-61) is that when patients go on pegloticase, all other urate-lowering therapies must be stopped as a matter of safety. Otherwise, it’s impossible to use the pre-infusion plasma urate measurement to determine if pegloticase has stopped working.
The ACR guidelines recommend pegloticase as third-line urate-lowering therapy in patients who are not at target despite maximum-dose therapy with a xanthine oxidase inhibitor plus second-line therapy with probenecid, losartan, or fenofibrate.
Dr. Pillinger reported having received research grants from Savient, which markets pegloticase, and Takeda.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM