User login
Advocacy and Compliance Issues Impacting Dermatology in 2025
Advocacy and Compliance Issues Impacting Dermatology in 2025
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
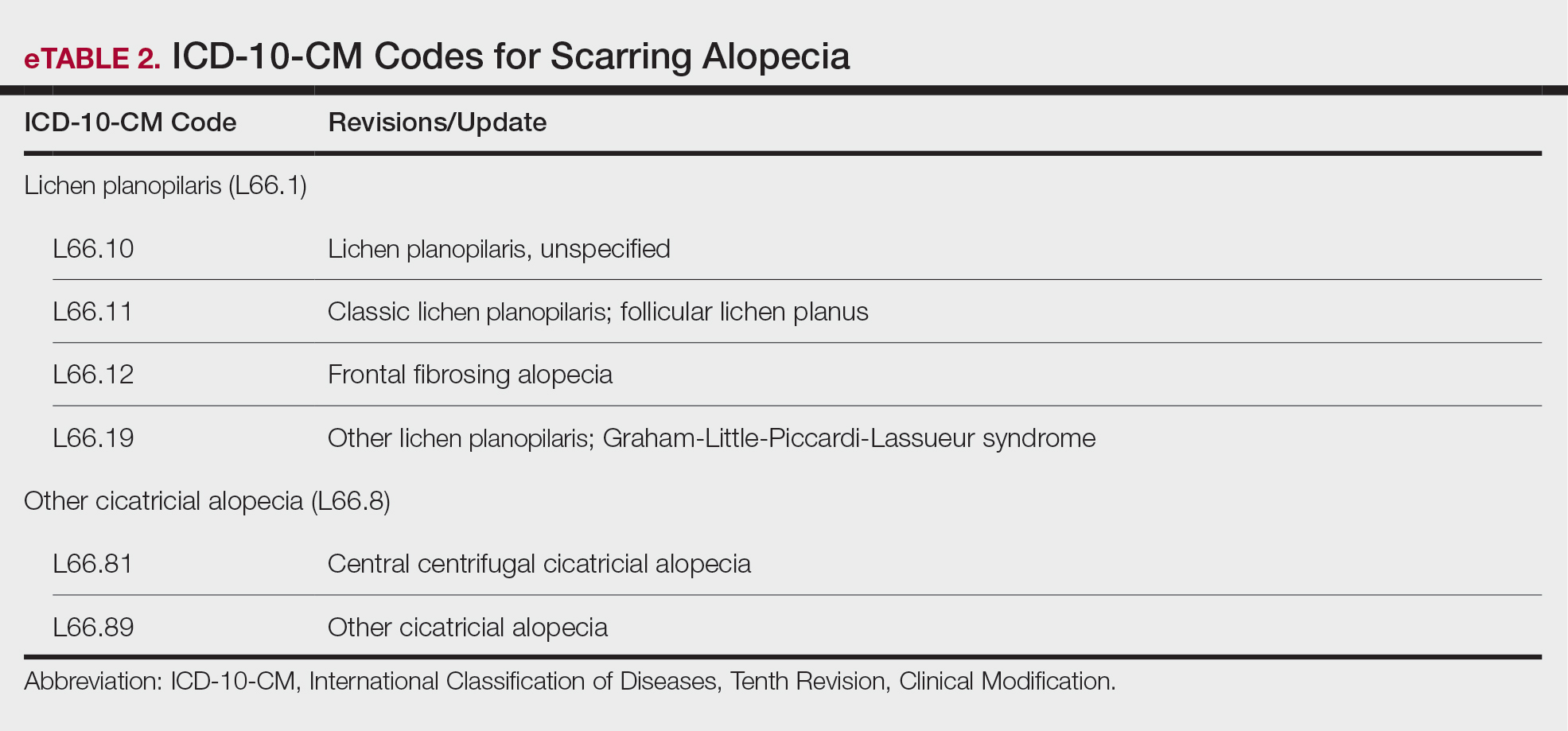
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
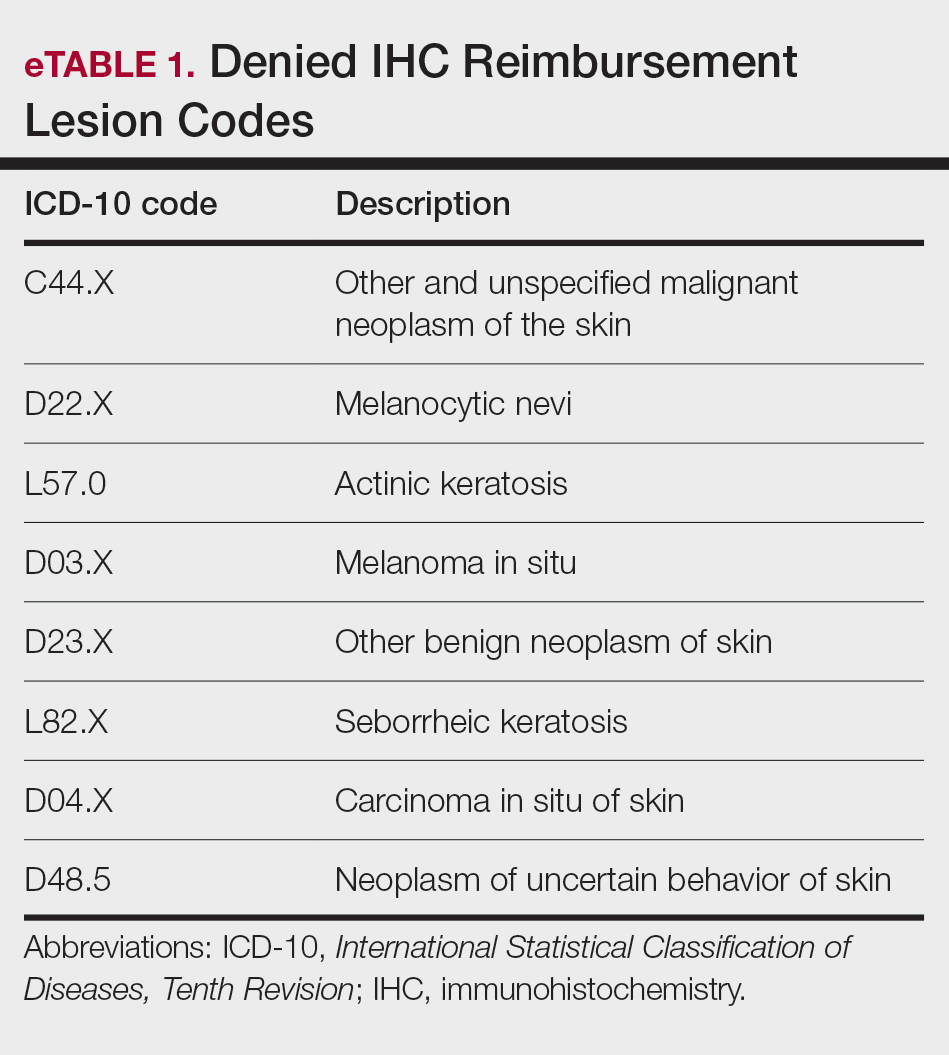
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6

Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6


Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6


Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
Advocacy and Compliance Issues Impacting Dermatology in 2025
Advocacy and Compliance Issues Impacting Dermatology in 2025
PRACTICE POINTS
- Recent advocacy efforts have led to the reversal of widespread insurer denials for immunohistochemistry stains; however, continued vigilance is necessary, as restrictive coverage policies may re-emerge.
- Laboratory directors must comply with updated Clinical Laboratory Improvement Amendments of 1988 and College of American Pathologists personnel requirements effective December 28, 2024, including stricter board certification and 2 years of laboratory training or experience and 20 hours of continuing education requirements.
- The American Society of Dermatopathology Appropriate Use Criteria mobile application provides physicians with evidence-based guidance for test selection in dermatopathology.
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11
Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
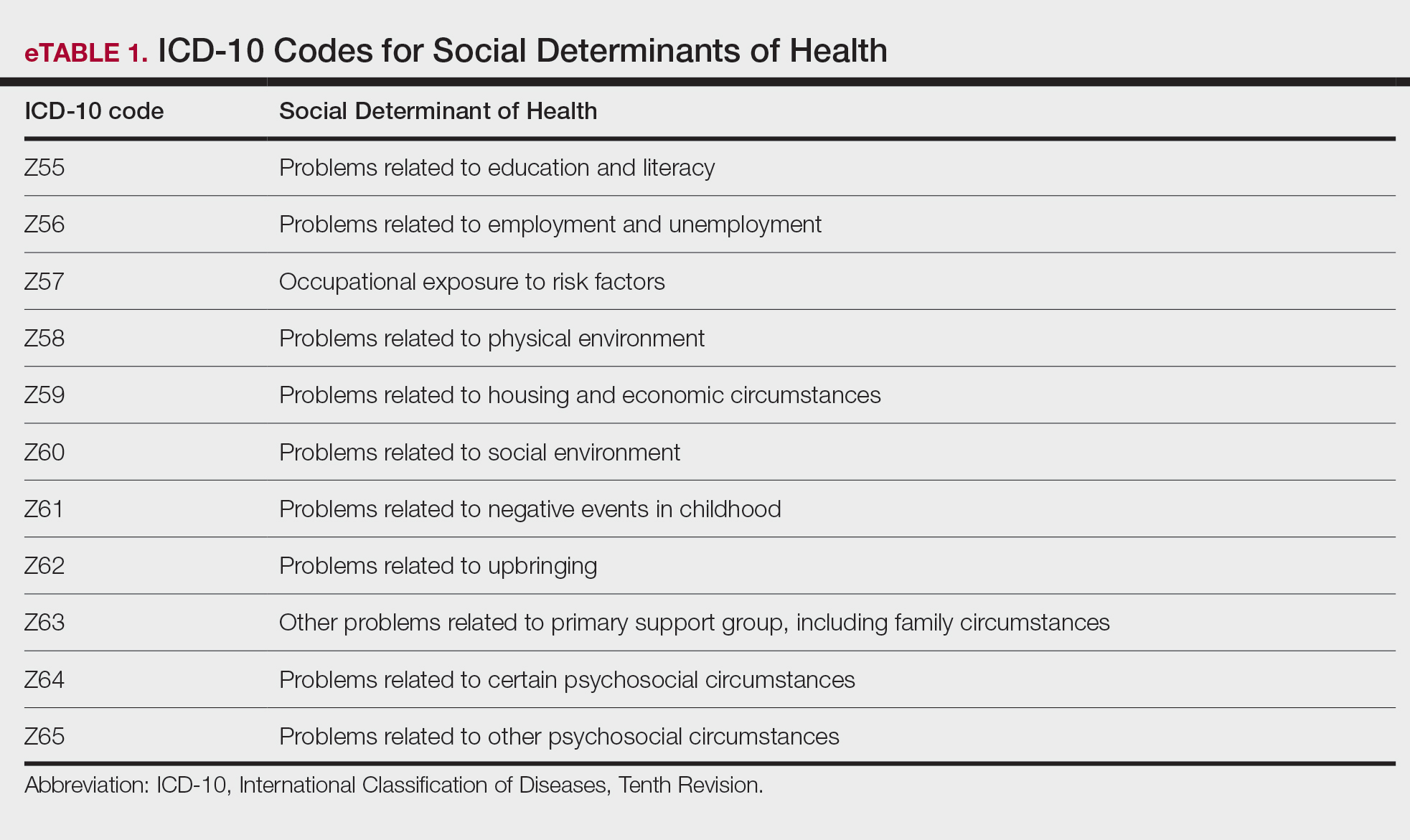
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13
New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14
Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
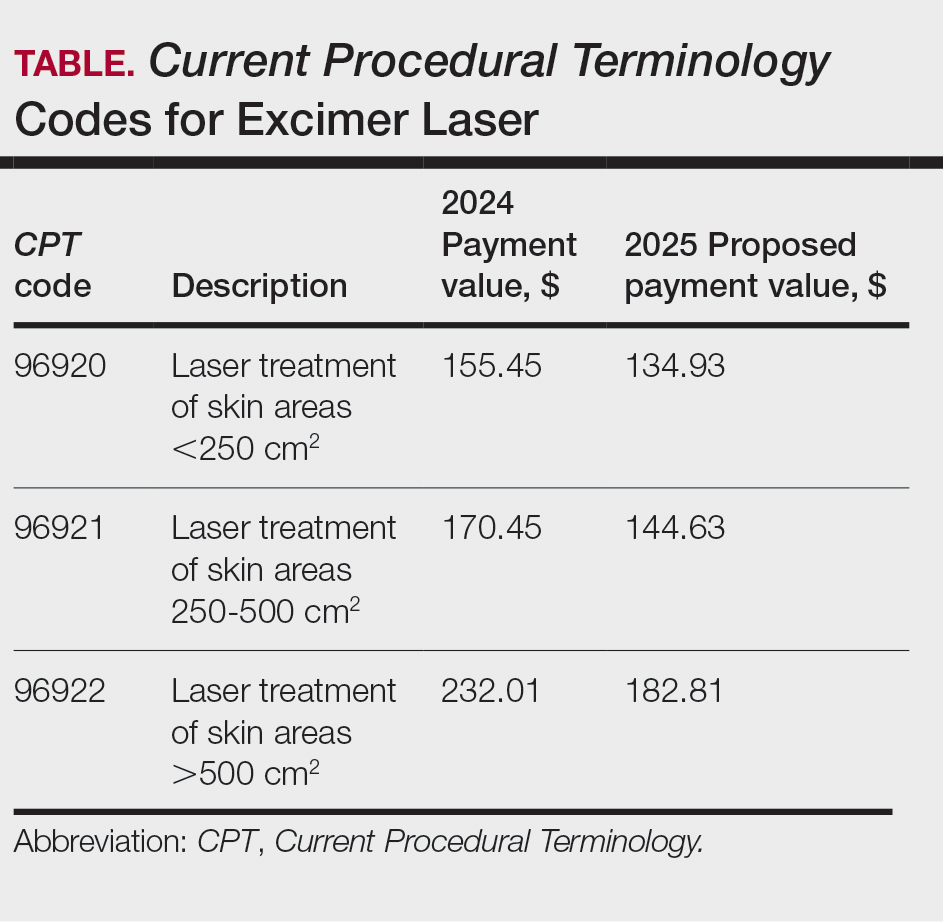
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
PRACTICE POINTS
- The Centers for Medicare & Medicaid Services released the 2025 Medicare Physician Fee Schedule final rule on November 1, 2024, setting the 2025 conversion factor at $32.35—a 2.83% reduction from 2024.
- With this change, dermatology practices may see an overall 2.83% reduction in payments in 2025 compared to 2024, although individual outcomes will vary based on practice mix.
- The American Academy of Dermatology Association continues to advocate for change, and members need to urge their federal legislators to support critical bills aimed at reforming Medicare physician payment.
Understanding the Evaluation and Management Add-on Complexity Code
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
PRACTICE POINTS
- The add-on code G2211 went into effect on January 1, 2024, and can be applied to outpatient evaluation and management visits that fulfill certain criteria.
- This code should be utilized when one is serving as the continuing focal point for all of the patient's health care needs or providing ongoing medical care related to a patient’s single, serious or complex condition.
Update on Dermatology Reimbursement in 2024
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4
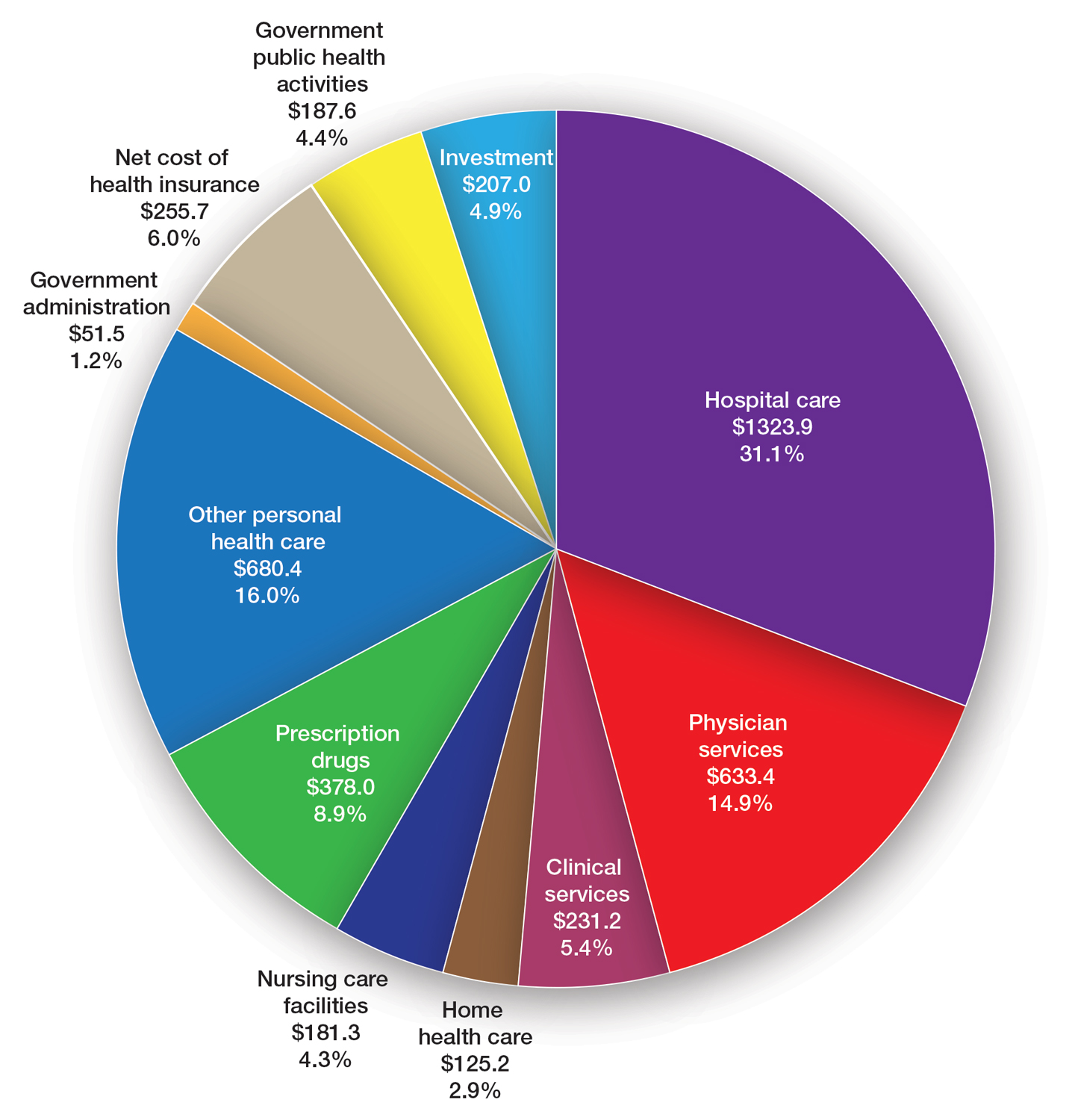
The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
PRACTICE POINTS
- The proposed 2024 Medicare physician fee schedule published by the Centers for Medicare & Medicaid Services in July 2023 will negatively impact dermatology practices.
- The final regulations are expected in November 2023.
Coding the “Spot Check”: Part 2
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Coding the “Spot Check”: Part 1
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
How to Effectively Utilize Consultation Codes: 2023 Updates
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.
Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.
To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
PRACTICE POINTS
- Updates to the inpatient and outpatient consultation codes went into effect January 1, 2023.
- For inpatient and outpatient consultation codes, level of service is now solely based on either time on the date of encounter or medical decision-making.
- Interprofessional consultation codes can be utilized when assisting in the diagnosis and/or management of a patient without face-to-face contact.
Advocacy Update: Ringing in 2023
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.
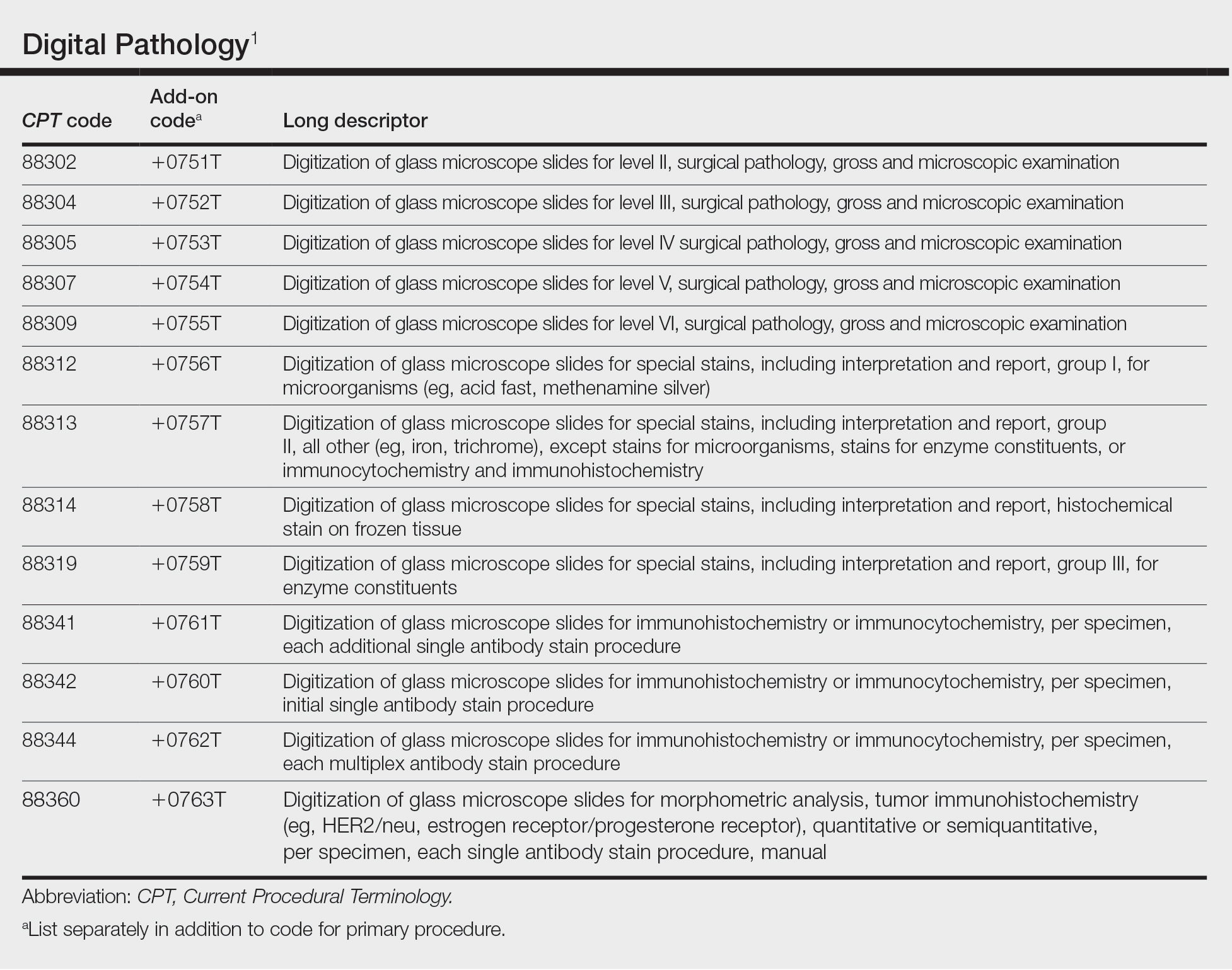
Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2
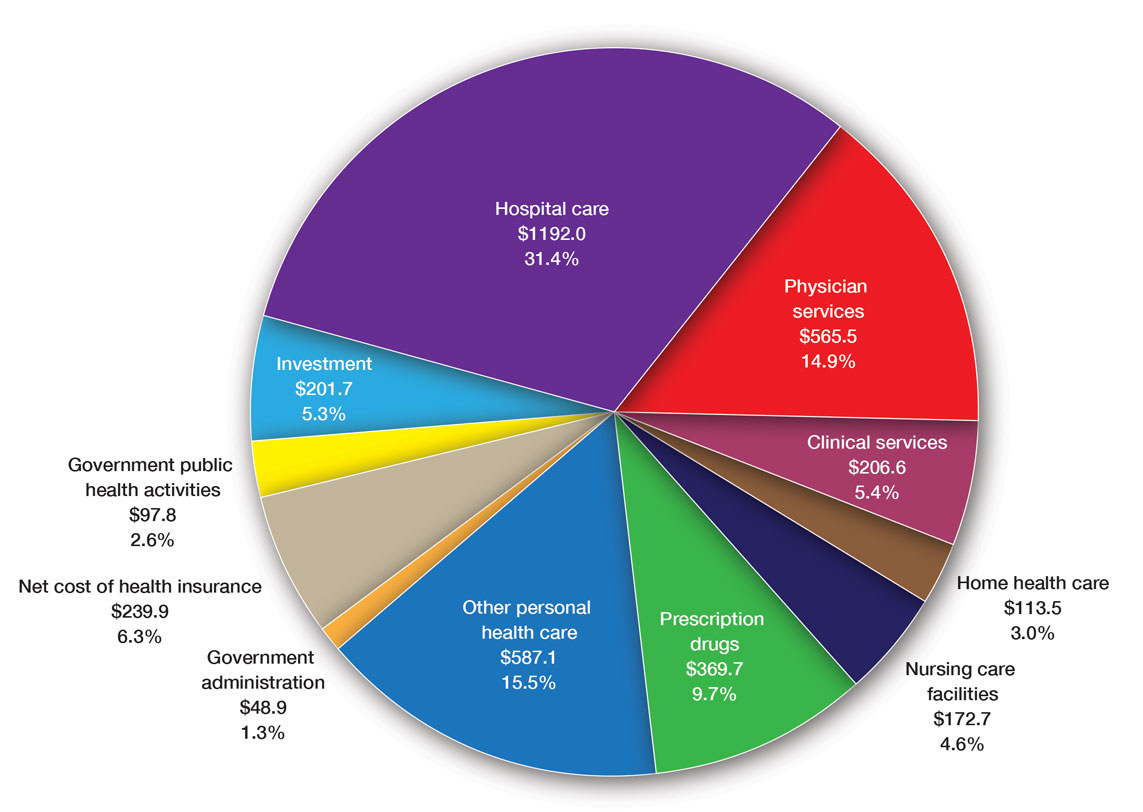
Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5
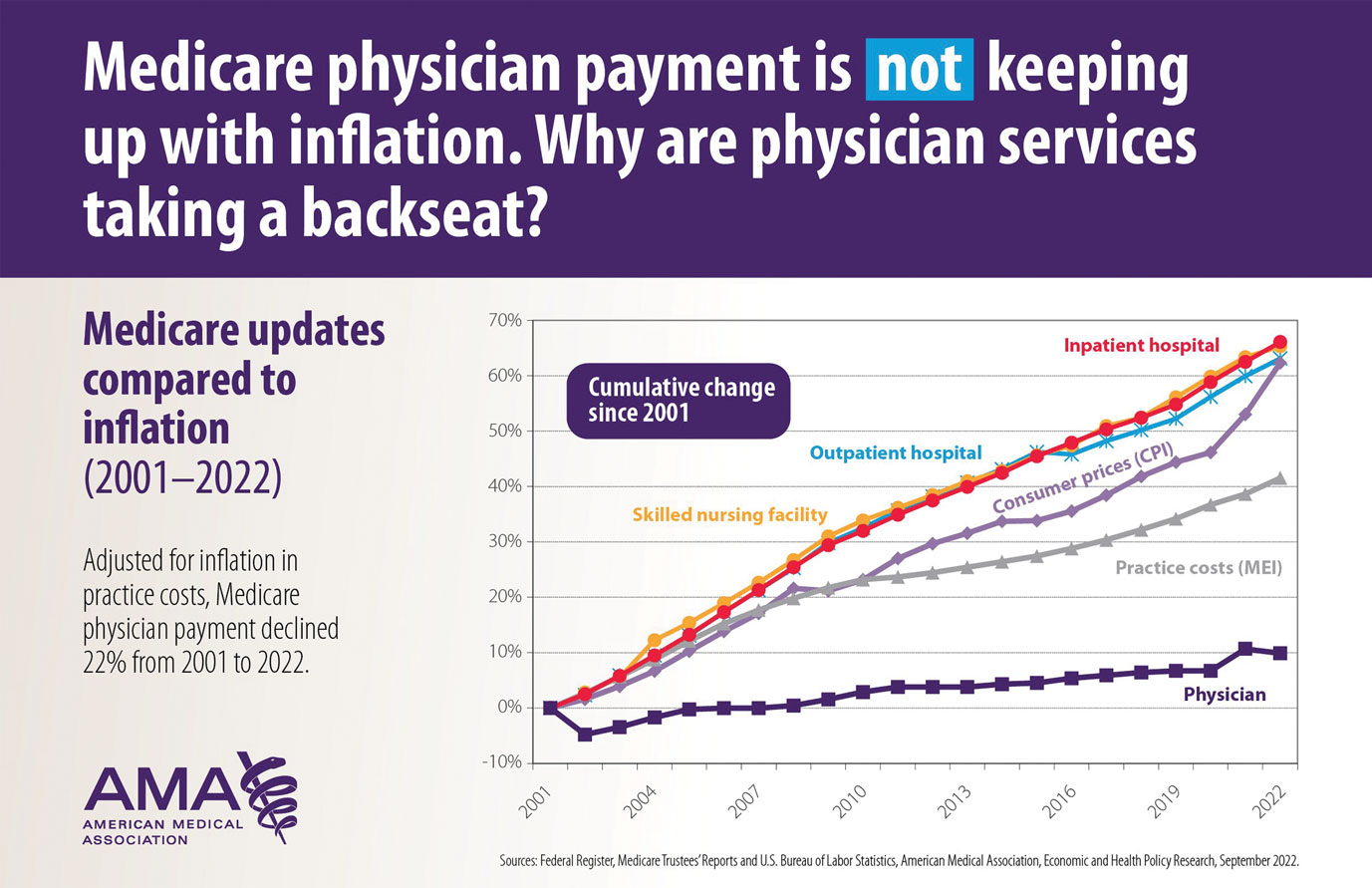
The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.
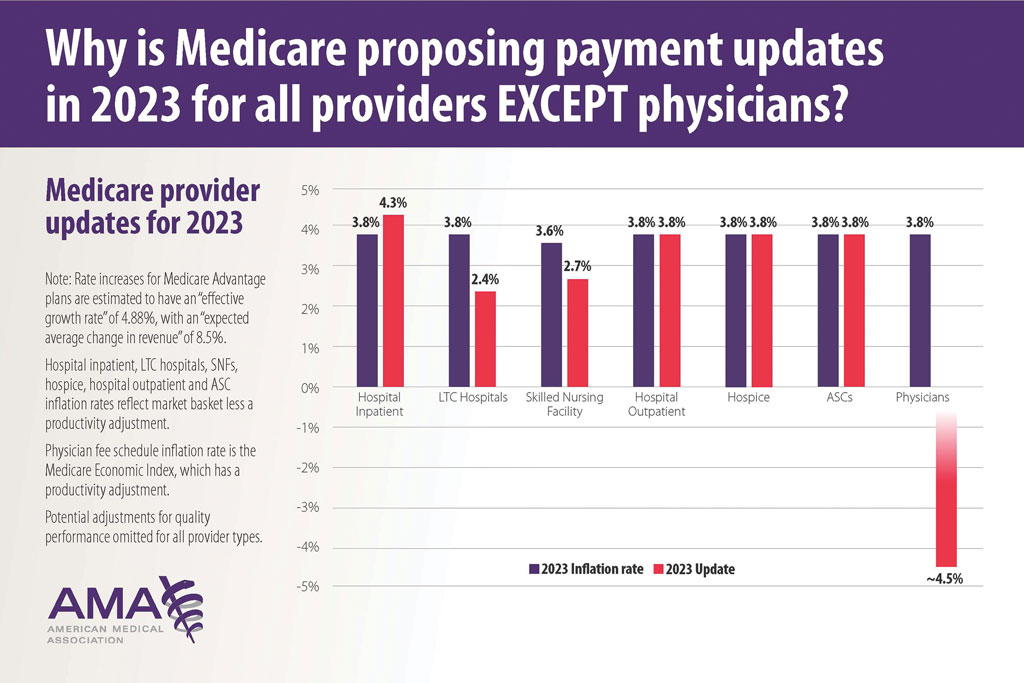
In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
Practice Points
- New digital pathology codes proposed by the American Medical Association can be used starting January 1, 2023.
- A proposed 2023 fee schedule negatively impacting dermatology practices was published by the Centers for Medicare & Medicaid Services in July 2022.
- Advocacy involvement provides a collaborative collective voice for our specialty to help our patients improve their care.
Audit Proof Your Mohs Note
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
Practice Points
- Medicare’s updated guidance for documentation of Mohs micrographic surgery (MMS) includes some new requirements that Mohs surgeons should ensure are implemented in their Mohs records.
- Per Medicare guidance, MMS records should include a justification of why MMS was the most appropriate treatment and a description of the histologic findings from the Mohs slides.
- One major improvement with the updated documentation requirements is that if no tumor is visualized in the first stage of MMS, then no histology description of the tumor is required.
Private Payer Engagement
Payer Advocacy in Dermatology
Frustrations with payers is a common source of annoyance among dermatologists. Payment rules can seem arbitrary, ever-changing, and not uniform among the various payers. Keeping track of payer requirements can be nearly impossible.
To assist members in handling these concerns, the American Academy of Dermatology Association (AADA) created the Patient Access and Payer Relations (PAPR) committee, which seeks to promote patient access to dermatologic care by addressing issues that may arise with private payers. The committee utilizes a multipronged approach to develop strategies to educate payers on the value of dermatology, addressing systematic payment issues as they arise over time, and building relationships with insurers and employers to promote coverage and payment policies allowing for the highest quality of dermatologic care. The committee is comprised of practicing dermatologists who meet regularly to help guide and implement the AADA’s payer advocacy initiatives.
Identifying payer contacts and forging working relationships is a cornerstone of payer advocacy. In addition to patient access to quality dermatologic services, fair reimbursement is always a primary concern.
Hot Topics in Payer Advocacy
How to Use Modifier −25 Appropriately—The AADA has been advocating for appropriate coverage and reimbursement for services billed by dermatologists; recent examples include assuring appropriate payment for services reported with modifier −25, which is used when a procedure such as a biopsy is performed on the same day as a separate and unrelated evaluation and management (E/M) service, such as psoriasis management. Some payers claim the concurrent nature of the services results in an overlap of office expenses such that these claims should be paid at a lesser amount; however, when procedure codes are frequently billed in association with an office visit, that overlap has already been accounted for as part of the code valuation process, negating the need for additional reduction.
The AADA PAPR committee has created numerous resources for our members to ensure they are using modifier −25 appropriately, particularly now that the US Department of Health and Human Services Office of the Inspector General (OIG) has announced a work plan to audit dermatologists claims reporting modifier −25.1 The AADA immediately formed a work group, including PAPR committee members, to develop and employ a strategy to educate key decision-makers on the correct use of modifier −25 and highlight appropriate resources to guide members. An introductory call was held with the OIG audit team to discuss the appropriate use of modifier −25 in dermatology as the OIG prepares to develop the parameters of its audit sometime in the future (AADA, unpublished data, 2021).
Working With Dermatology Societies on Payer Issues—The American Academy of Dermatology Association PAPR committee works collaboratively with members of the American Academy of Dermatology, state and local dermatology societies, and private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for furnished services, and ensure patients can access covered quality care. Collaboration with state dermatology societies is essential to address payer issues that impact their members and provide guidance on effective engagement with their state payers. Recent examples include working with dermatology societies in Massachusetts, Rhode Island, and Florida on strategies to advocate against modifier −25 payment reductions by insurance carriers (AADA, unpublished data, 2021). Additionally, the AADA PAPR committee has been able to provide guidance and technical support as needed to state dermatology societies, such as to the Rhode Island Dermatology Society and the Pennsylvania Academy of Dermatology and Dermatologic Surgery to address payer quality metrics and access to laboratory services, respectively (AADA, unpublished data, 2021).
Patient Access to Affordable Treatments—American Academy of Dermatology Association payer advocacy is anchored to published position statements and clinical guidelines. To strengthen AADA advocacy on payer-mandated drug substitutions for nonmedical reasons and to preserve patient access to medications, the PAPR committee collaborated with the American Academy of Dermatology’s Drug Pricing and Transparency Task Force to update the AADA Position Statement on Patient Access to Affordable Treatments2 to address this issue. Essentially, patients who are stable on a medication should be allowed to keep using the same medication without payers changing their coverage for nonmedical reasons or by offering financial incentives to switch.
Relationships With Major Insurance Carriers—Integral to the PAPR committee’s private payer advocacy success are our proactive relationships with major insurance carriers. In 2021, the PAPR committee established quarterly dermatology-specific meetings with the major national carriers. In nurturing these relationships, the PAPR committee has been able to expand on opportunities to provide payer policy reviews as well as identify dermatologists as subject matter experts available to payers to assist with physician panels or policy reviews. These regular contacts also have proved beneficial in addressing issues raised by members; a few such examples include when one major payer reversed its denials on dermatologists’ claims for Current Procedural Terminology code 88304 (surgical pathology, gross and microscopic tissue exam) after it was brought to their attention by the AADA (AADA, unpublished data, 2021). This payer worked with its external vendor to correct the denials. When the AADA learned that another major payer was improperly denying payment for claims for 1 stage of Mohs micrographic surgery reported using Current Procedural Terminology code 17311, we worked with contacts at this payer to resolve the issue. They were receptive to our concerns and readily researched the issue. Leadership of the PAPR committee continued working with the AADA coding team and this payer to develop training guidance to prevent future denials, and the payer has reviewed prior denials and reprocessed claims for payment (AADA, unpublished data, 2021).
E/M Coding Issues
Another issue under consideration by several national insurers is E/M-level reassignment. Payers are reviewing claims from providers who are identified as coding at a higher E/M level as compared to their specialty peers. Some insurance carriers are using proprietary algorithms that attempt to link specific diagnoses to certain levels of E/M, triggering claim edits within their claim processing systems (AADA, unpublished data, 2021). The carrier will then either deny the claim or adjust reimbursement to a lower-level E/M service. In discussions with a national carrier on its E/M Leveling Program, the AADA has offered to work with them on appropriate E/M documentation and reporting (AADA, unpublished data, 2021). The AADA also has extensive member resources for guidance on E/M reporting as well as preparing for audits and appealing payer downcoding developed by the coding staff in conjunction with the Coding and Reimbursement Committee.
Recent Efforts From the AADA
Within the AADA, the PAPR committee works closely with the coding, practice management, and regulatory teams to address payer issues and develop resources for members. Recent examples include resources for dermatology practices on the No Surprises Act and what practices need to do to comply (AADA, unpublished data, 2021). The PAPR committee also works collaboratively with other AADA committees and task forces on payer issues as needed; for example, the PAPR committee has been working with the Dermatopathology Rapid Response committee to address member concerns regarding access to the pathology laboratory of their choice. Many payers are seeking to consolidate and save money by requiring the use of preferred laboratories, which impacts patient access to physician office laboratories and physician-recommended reference laboratories. The AADA, along with other medical specialties, has advocated for payers to not create a restrictive network of pathology laboratories within their provider networks and to support dermatologists’ laboratories of choice (AADA, unpublished data, 2021).
Within the payer space, the role of employers in impacting payment and coverage policies continues to rise. In 2021, the AADA leadership approved the employer outreach strategy to engage employers. The overall objectives are to advocate to employers on the value of dermatologic care and access to care provided by board-certified dermatologists. This is a long-term project that is just getting underway (AADA, unpublished data, 2021).
Payer Resource Center for AADA Members
To ensure that AADA members have the resources they need to advocate with payers as well as to keep the PAPR committee aware of emerging payer issues, the AADA created a new private payer resource center for members (https://www.aad.org/member/advocacy/priorities/payer-advocacy), which assists AADA members with common dermatologic concerns with insurers as well as contracting issues. The website also includes an email address for members to report payer issues ([email protected]). This information helps the PAPR committee identify and prioritize issues of concern.
Final Thoughts
Given the control that private insurance companies exert over the health care that dermatology patients can access, the AADA in general and the PAPR committee specifically play a valuable role in advocating access to care for dermatology patients.
- US Department of Health and Human Services Office of the Inspector General. Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. Accessed May 16, 2022. https://www.oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- American Academy of Dermatology Association. Position Statement on Patient Access to Affordable Treatments. Updated November 4, 2017. Accessed May 24, 2022. https://server.aad.org/forms/policies/uploads/ps/ps%20-%20patient%20access%20to%20affordable%20treatments.pdf?)
Payer Advocacy in Dermatology
Frustrations with payers is a common source of annoyance among dermatologists. Payment rules can seem arbitrary, ever-changing, and not uniform among the various payers. Keeping track of payer requirements can be nearly impossible.
To assist members in handling these concerns, the American Academy of Dermatology Association (AADA) created the Patient Access and Payer Relations (PAPR) committee, which seeks to promote patient access to dermatologic care by addressing issues that may arise with private payers. The committee utilizes a multipronged approach to develop strategies to educate payers on the value of dermatology, addressing systematic payment issues as they arise over time, and building relationships with insurers and employers to promote coverage and payment policies allowing for the highest quality of dermatologic care. The committee is comprised of practicing dermatologists who meet regularly to help guide and implement the AADA’s payer advocacy initiatives.
Identifying payer contacts and forging working relationships is a cornerstone of payer advocacy. In addition to patient access to quality dermatologic services, fair reimbursement is always a primary concern.
Hot Topics in Payer Advocacy
How to Use Modifier −25 Appropriately—The AADA has been advocating for appropriate coverage and reimbursement for services billed by dermatologists; recent examples include assuring appropriate payment for services reported with modifier −25, which is used when a procedure such as a biopsy is performed on the same day as a separate and unrelated evaluation and management (E/M) service, such as psoriasis management. Some payers claim the concurrent nature of the services results in an overlap of office expenses such that these claims should be paid at a lesser amount; however, when procedure codes are frequently billed in association with an office visit, that overlap has already been accounted for as part of the code valuation process, negating the need for additional reduction.
The AADA PAPR committee has created numerous resources for our members to ensure they are using modifier −25 appropriately, particularly now that the US Department of Health and Human Services Office of the Inspector General (OIG) has announced a work plan to audit dermatologists claims reporting modifier −25.1 The AADA immediately formed a work group, including PAPR committee members, to develop and employ a strategy to educate key decision-makers on the correct use of modifier −25 and highlight appropriate resources to guide members. An introductory call was held with the OIG audit team to discuss the appropriate use of modifier −25 in dermatology as the OIG prepares to develop the parameters of its audit sometime in the future (AADA, unpublished data, 2021).
Working With Dermatology Societies on Payer Issues—The American Academy of Dermatology Association PAPR committee works collaboratively with members of the American Academy of Dermatology, state and local dermatology societies, and private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for furnished services, and ensure patients can access covered quality care. Collaboration with state dermatology societies is essential to address payer issues that impact their members and provide guidance on effective engagement with their state payers. Recent examples include working with dermatology societies in Massachusetts, Rhode Island, and Florida on strategies to advocate against modifier −25 payment reductions by insurance carriers (AADA, unpublished data, 2021). Additionally, the AADA PAPR committee has been able to provide guidance and technical support as needed to state dermatology societies, such as to the Rhode Island Dermatology Society and the Pennsylvania Academy of Dermatology and Dermatologic Surgery to address payer quality metrics and access to laboratory services, respectively (AADA, unpublished data, 2021).
Patient Access to Affordable Treatments—American Academy of Dermatology Association payer advocacy is anchored to published position statements and clinical guidelines. To strengthen AADA advocacy on payer-mandated drug substitutions for nonmedical reasons and to preserve patient access to medications, the PAPR committee collaborated with the American Academy of Dermatology’s Drug Pricing and Transparency Task Force to update the AADA Position Statement on Patient Access to Affordable Treatments2 to address this issue. Essentially, patients who are stable on a medication should be allowed to keep using the same medication without payers changing their coverage for nonmedical reasons or by offering financial incentives to switch.
Relationships With Major Insurance Carriers—Integral to the PAPR committee’s private payer advocacy success are our proactive relationships with major insurance carriers. In 2021, the PAPR committee established quarterly dermatology-specific meetings with the major national carriers. In nurturing these relationships, the PAPR committee has been able to expand on opportunities to provide payer policy reviews as well as identify dermatologists as subject matter experts available to payers to assist with physician panels or policy reviews. These regular contacts also have proved beneficial in addressing issues raised by members; a few such examples include when one major payer reversed its denials on dermatologists’ claims for Current Procedural Terminology code 88304 (surgical pathology, gross and microscopic tissue exam) after it was brought to their attention by the AADA (AADA, unpublished data, 2021). This payer worked with its external vendor to correct the denials. When the AADA learned that another major payer was improperly denying payment for claims for 1 stage of Mohs micrographic surgery reported using Current Procedural Terminology code 17311, we worked with contacts at this payer to resolve the issue. They were receptive to our concerns and readily researched the issue. Leadership of the PAPR committee continued working with the AADA coding team and this payer to develop training guidance to prevent future denials, and the payer has reviewed prior denials and reprocessed claims for payment (AADA, unpublished data, 2021).
E/M Coding Issues
Another issue under consideration by several national insurers is E/M-level reassignment. Payers are reviewing claims from providers who are identified as coding at a higher E/M level as compared to their specialty peers. Some insurance carriers are using proprietary algorithms that attempt to link specific diagnoses to certain levels of E/M, triggering claim edits within their claim processing systems (AADA, unpublished data, 2021). The carrier will then either deny the claim or adjust reimbursement to a lower-level E/M service. In discussions with a national carrier on its E/M Leveling Program, the AADA has offered to work with them on appropriate E/M documentation and reporting (AADA, unpublished data, 2021). The AADA also has extensive member resources for guidance on E/M reporting as well as preparing for audits and appealing payer downcoding developed by the coding staff in conjunction with the Coding and Reimbursement Committee.
Recent Efforts From the AADA
Within the AADA, the PAPR committee works closely with the coding, practice management, and regulatory teams to address payer issues and develop resources for members. Recent examples include resources for dermatology practices on the No Surprises Act and what practices need to do to comply (AADA, unpublished data, 2021). The PAPR committee also works collaboratively with other AADA committees and task forces on payer issues as needed; for example, the PAPR committee has been working with the Dermatopathology Rapid Response committee to address member concerns regarding access to the pathology laboratory of their choice. Many payers are seeking to consolidate and save money by requiring the use of preferred laboratories, which impacts patient access to physician office laboratories and physician-recommended reference laboratories. The AADA, along with other medical specialties, has advocated for payers to not create a restrictive network of pathology laboratories within their provider networks and to support dermatologists’ laboratories of choice (AADA, unpublished data, 2021).
Within the payer space, the role of employers in impacting payment and coverage policies continues to rise. In 2021, the AADA leadership approved the employer outreach strategy to engage employers. The overall objectives are to advocate to employers on the value of dermatologic care and access to care provided by board-certified dermatologists. This is a long-term project that is just getting underway (AADA, unpublished data, 2021).
Payer Resource Center for AADA Members
To ensure that AADA members have the resources they need to advocate with payers as well as to keep the PAPR committee aware of emerging payer issues, the AADA created a new private payer resource center for members (https://www.aad.org/member/advocacy/priorities/payer-advocacy), which assists AADA members with common dermatologic concerns with insurers as well as contracting issues. The website also includes an email address for members to report payer issues ([email protected]). This information helps the PAPR committee identify and prioritize issues of concern.
Final Thoughts
Given the control that private insurance companies exert over the health care that dermatology patients can access, the AADA in general and the PAPR committee specifically play a valuable role in advocating access to care for dermatology patients.
Payer Advocacy in Dermatology
Frustrations with payers is a common source of annoyance among dermatologists. Payment rules can seem arbitrary, ever-changing, and not uniform among the various payers. Keeping track of payer requirements can be nearly impossible.
To assist members in handling these concerns, the American Academy of Dermatology Association (AADA) created the Patient Access and Payer Relations (PAPR) committee, which seeks to promote patient access to dermatologic care by addressing issues that may arise with private payers. The committee utilizes a multipronged approach to develop strategies to educate payers on the value of dermatology, addressing systematic payment issues as they arise over time, and building relationships with insurers and employers to promote coverage and payment policies allowing for the highest quality of dermatologic care. The committee is comprised of practicing dermatologists who meet regularly to help guide and implement the AADA’s payer advocacy initiatives.
Identifying payer contacts and forging working relationships is a cornerstone of payer advocacy. In addition to patient access to quality dermatologic services, fair reimbursement is always a primary concern.
Hot Topics in Payer Advocacy
How to Use Modifier −25 Appropriately—The AADA has been advocating for appropriate coverage and reimbursement for services billed by dermatologists; recent examples include assuring appropriate payment for services reported with modifier −25, which is used when a procedure such as a biopsy is performed on the same day as a separate and unrelated evaluation and management (E/M) service, such as psoriasis management. Some payers claim the concurrent nature of the services results in an overlap of office expenses such that these claims should be paid at a lesser amount; however, when procedure codes are frequently billed in association with an office visit, that overlap has already been accounted for as part of the code valuation process, negating the need for additional reduction.
The AADA PAPR committee has created numerous resources for our members to ensure they are using modifier −25 appropriately, particularly now that the US Department of Health and Human Services Office of the Inspector General (OIG) has announced a work plan to audit dermatologists claims reporting modifier −25.1 The AADA immediately formed a work group, including PAPR committee members, to develop and employ a strategy to educate key decision-makers on the correct use of modifier −25 and highlight appropriate resources to guide members. An introductory call was held with the OIG audit team to discuss the appropriate use of modifier −25 in dermatology as the OIG prepares to develop the parameters of its audit sometime in the future (AADA, unpublished data, 2021).
Working With Dermatology Societies on Payer Issues—The American Academy of Dermatology Association PAPR committee works collaboratively with members of the American Academy of Dermatology, state and local dermatology societies, and private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for furnished services, and ensure patients can access covered quality care. Collaboration with state dermatology societies is essential to address payer issues that impact their members and provide guidance on effective engagement with their state payers. Recent examples include working with dermatology societies in Massachusetts, Rhode Island, and Florida on strategies to advocate against modifier −25 payment reductions by insurance carriers (AADA, unpublished data, 2021). Additionally, the AADA PAPR committee has been able to provide guidance and technical support as needed to state dermatology societies, such as to the Rhode Island Dermatology Society and the Pennsylvania Academy of Dermatology and Dermatologic Surgery to address payer quality metrics and access to laboratory services, respectively (AADA, unpublished data, 2021).
Patient Access to Affordable Treatments—American Academy of Dermatology Association payer advocacy is anchored to published position statements and clinical guidelines. To strengthen AADA advocacy on payer-mandated drug substitutions for nonmedical reasons and to preserve patient access to medications, the PAPR committee collaborated with the American Academy of Dermatology’s Drug Pricing and Transparency Task Force to update the AADA Position Statement on Patient Access to Affordable Treatments2 to address this issue. Essentially, patients who are stable on a medication should be allowed to keep using the same medication without payers changing their coverage for nonmedical reasons or by offering financial incentives to switch.
Relationships With Major Insurance Carriers—Integral to the PAPR committee’s private payer advocacy success are our proactive relationships with major insurance carriers. In 2021, the PAPR committee established quarterly dermatology-specific meetings with the major national carriers. In nurturing these relationships, the PAPR committee has been able to expand on opportunities to provide payer policy reviews as well as identify dermatologists as subject matter experts available to payers to assist with physician panels or policy reviews. These regular contacts also have proved beneficial in addressing issues raised by members; a few such examples include when one major payer reversed its denials on dermatologists’ claims for Current Procedural Terminology code 88304 (surgical pathology, gross and microscopic tissue exam) after it was brought to their attention by the AADA (AADA, unpublished data, 2021). This payer worked with its external vendor to correct the denials. When the AADA learned that another major payer was improperly denying payment for claims for 1 stage of Mohs micrographic surgery reported using Current Procedural Terminology code 17311, we worked with contacts at this payer to resolve the issue. They were receptive to our concerns and readily researched the issue. Leadership of the PAPR committee continued working with the AADA coding team and this payer to develop training guidance to prevent future denials, and the payer has reviewed prior denials and reprocessed claims for payment (AADA, unpublished data, 2021).
E/M Coding Issues
Another issue under consideration by several national insurers is E/M-level reassignment. Payers are reviewing claims from providers who are identified as coding at a higher E/M level as compared to their specialty peers. Some insurance carriers are using proprietary algorithms that attempt to link specific diagnoses to certain levels of E/M, triggering claim edits within their claim processing systems (AADA, unpublished data, 2021). The carrier will then either deny the claim or adjust reimbursement to a lower-level E/M service. In discussions with a national carrier on its E/M Leveling Program, the AADA has offered to work with them on appropriate E/M documentation and reporting (AADA, unpublished data, 2021). The AADA also has extensive member resources for guidance on E/M reporting as well as preparing for audits and appealing payer downcoding developed by the coding staff in conjunction with the Coding and Reimbursement Committee.
Recent Efforts From the AADA
Within the AADA, the PAPR committee works closely with the coding, practice management, and regulatory teams to address payer issues and develop resources for members. Recent examples include resources for dermatology practices on the No Surprises Act and what practices need to do to comply (AADA, unpublished data, 2021). The PAPR committee also works collaboratively with other AADA committees and task forces on payer issues as needed; for example, the PAPR committee has been working with the Dermatopathology Rapid Response committee to address member concerns regarding access to the pathology laboratory of their choice. Many payers are seeking to consolidate and save money by requiring the use of preferred laboratories, which impacts patient access to physician office laboratories and physician-recommended reference laboratories. The AADA, along with other medical specialties, has advocated for payers to not create a restrictive network of pathology laboratories within their provider networks and to support dermatologists’ laboratories of choice (AADA, unpublished data, 2021).
Within the payer space, the role of employers in impacting payment and coverage policies continues to rise. In 2021, the AADA leadership approved the employer outreach strategy to engage employers. The overall objectives are to advocate to employers on the value of dermatologic care and access to care provided by board-certified dermatologists. This is a long-term project that is just getting underway (AADA, unpublished data, 2021).
Payer Resource Center for AADA Members
To ensure that AADA members have the resources they need to advocate with payers as well as to keep the PAPR committee aware of emerging payer issues, the AADA created a new private payer resource center for members (https://www.aad.org/member/advocacy/priorities/payer-advocacy), which assists AADA members with common dermatologic concerns with insurers as well as contracting issues. The website also includes an email address for members to report payer issues ([email protected]). This information helps the PAPR committee identify and prioritize issues of concern.
Final Thoughts
Given the control that private insurance companies exert over the health care that dermatology patients can access, the AADA in general and the PAPR committee specifically play a valuable role in advocating access to care for dermatology patients.
- US Department of Health and Human Services Office of the Inspector General. Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. Accessed May 16, 2022. https://www.oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- American Academy of Dermatology Association. Position Statement on Patient Access to Affordable Treatments. Updated November 4, 2017. Accessed May 24, 2022. https://server.aad.org/forms/policies/uploads/ps/ps%20-%20patient%20access%20to%20affordable%20treatments.pdf?)
- US Department of Health and Human Services Office of the Inspector General. Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. Accessed May 16, 2022. https://www.oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- American Academy of Dermatology Association. Position Statement on Patient Access to Affordable Treatments. Updated November 4, 2017. Accessed May 24, 2022. https://server.aad.org/forms/policies/uploads/ps/ps%20-%20patient%20access%20to%20affordable%20treatments.pdf?)
Practice Points
- The American Academy of Dermatology Association routinely interacts with private medical payers on behalf of dermatologists and to insure access to dermatologic care for patients.
- Members of the American Academy of Dermatology are encouraged to work with the association when issues with payers arise.