User login
Stress in medicine: Strategies for caregivers, patients, clinicians—The burdens of caregiver stress
The number of people in the United States who spend a significant part of each week working as unpaid caregivers is considerable, and the toll exacted for such work is high. Understanding the profi le of the caregiver, the nature of the duties performed, the stress imposed by such duties, and the consequences of the stress can assist the clinician in recognizing the caregiver in need of intervention.
A PROFILE OF THE CAREGIVER
A recent survey estimated that more than 65 million Americans provide unpaid assistance annually to older adults with disabilities.1 The value of that labor has been estimated at $306 billion annually, or nearly double the combined cost of home health care and nursing home care.2,3
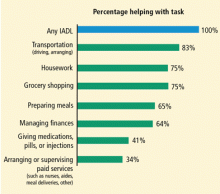
The typical caregiver is a woman, about 48 years old, with some college education, who spends 20 hours or more each week providing unpaid care to someone aged 50 years or older.1 The recipients of care often have long-term physical disabilities; mental confusion or emotional problems frequently complicate care.
PSYCHOLOGIC AND PHYSICAL COSTS
Caregiving may take a toll on the caregiver in a variety of ways: behavioral, in the form of alcohol or substance use4; psychologic, in the form of depression or other mental health problems5; and physical, in the form of chronic health conditions and impaired immune response.6 About three-fifths of caregivers report fair or poor health, compared with one-third of noncaregivers, and caregivers have approximately twice as many chronic conditions, such as heart disease, cancer, arthritis, and diabetes, compared with noncaregivers.2,7 Caregiving also exacts a financial toll, as employees who are caregivers cost their employers $13.4 billion more per year in health care expenditures.8 In addition, absenteeism, workday interruptions, and shifts from full-time to part-time work by caregivers cost businesses between $17.1 and $33.6 billion per year.9
The cost of caregiving is higher for women, who exhibit higher levels of anxiety and depression and lower levels of subjective well-being, life satisfaction, and physical health.10,11 The stress of caregiving has also been identified as a risk factor for morbidity among older (66 to 96 years old) caregivers, who have a 63% greater mortality than noncaregivers of the same age.12
PSYCHOSOCIAL STRESS, UNHEALTHY BEHAVIORS, AND ILLNESS ARE LINKED
Psychosocial stress is a predictor of disease and can lead to unhealthy behaviors such as smoking, substance abuse, overeating, poor nutrition, and a sedentary lifestyle; these, in turn, can lead to physical and psychiatric illness. Behaviors adopted initially as coping skills may persist to become chronic, thereby promoting either continued wellness (in the case of healthy coping behaviors) or worsening levels of illness (in the case of unhealthy coping behaviors).
McEwen and Gianaros13 have suggested that these stress mechanisms arise from patterns of communication between the brain and the autonomic, cardiovascular, and immune systems, which mutually influenceone another. These so-called bidirectional stress processes affect cognition, experience, and behavior.
An integrated model of stress that maps the bidirectional causal pathways among psychosocial stressors, resulting unhealthy behaviors, and illness is needed. Although the steps from unhealthy behaviors to illness are fairly well understood, the links from psychosocial stress, such as those exhibited by caregivers, to unhealthy behaviors are not as clear. Several mediators are under study:
- Personality mediators can be either ameliorative (resilience, self-confi dence, self-control, optimism, high self-esteem, a sense of mastery, and finding meaning in life) or exacerbating (neuroticism and inhibition, which together form the so-called type D personality).
- Environmental mediators include social support, financial support, a history of a significant life change, and trauma early in life, which may increase one’s subsequent vulnerability to unhealthy behaviors.
- Biologic mediators may include prolonged sympathetic activation and enhanced platelet activation, caused by increased levels of depression and anxiety in chronically stressed caregivers.14
IMPLICATIONS FOR INTERVENTION
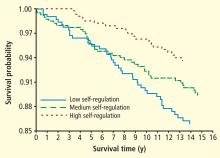
A significant percentage of caregivers do not need a clinician’s intervention to help them cope with stress or unhealthy coping skills. Among caregivers aged 50 years or older, 47% indicated in a recent study that the burden of caregiving is low (ie, 1 or 2 on a 5-point scale).1 Those who respond to stressors as challenges rather than threats tend to be resilient people who exert control over their lives, often through meditation or similar techniques, and have a strong social support network. Many report that caregiving provides them with an opportunity to act in accordance with their values and feel helpful rather than helpless.
Cognitive-behavioral interventions to alleviate stress-related symptoms appear to be more effective if offered as individual rather than group therapy. Teaching caregivers effective coping strategies, rather than merely providing social support, has been shown to improve caregiver psychologic health.15 Chief among the goals of intervention should be to alter brain function and instill optimism, a sense of control and self-esteem.13
- The National Alliance for Caregiving, in collaboration with the American Association of Retired Persons. Caregiving in the U.S. 2009. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed March 21, 2011.
- Family Caregiver Alliance. Caregiver health. A population at risk. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=1822. Published 2006. Accessed March 21, 2011.
- Family Caregiver Alliance. Prevalence, hours, and economic value of family caregiving, updated state-by-state analysis of 2004 national estimates. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content/pdfs/State_Caregiving_Data_Amo_20061107.pdf. Published 2006. Accessed March 21, 2011.
- Evercare. Study of caregivers in decline: a close-up look at the health risks of caring for a loved one. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregivers%20in%20Decline%20Study-FINAL-lowres.pdf. Published 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a metaanalysis. Psychol Aging 2003; 18:250–267.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129:946–972.
- Ho A, Collins S, Davis K, Doty M. A look at working-age caregivers’ roles, health concerns, and need for support (issue brief). New York, NY: The Commonwealth Fund; 2005.
- MetLife study of working caregivers and employer health care costs. MetLife Web site. http://www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-carecosts.pdf. Published July 2006. Accessed March 21, 2011.
- MetLife caregiving cost study: productivity losses to U.S. business. National Alliance for Caregiving Web site. http://www.caregiving. org/data/Caregiver%20Cost%20Study.pdf. Published July 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci 2006; 61:P33–P45.
- Johnson RW, Wiener JM. A profi le of frail older Americans and their caregivers. Urban Institute Web site. http://www.urban.org/UploadedPDF/311284_older_americans.pdf. Published February 2006. Accessed March 21, 2011.
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2215–2219.
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010; 1186:190–222.
- Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 2008; 22:493–502.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89.
The number of people in the United States who spend a significant part of each week working as unpaid caregivers is considerable, and the toll exacted for such work is high. Understanding the profi le of the caregiver, the nature of the duties performed, the stress imposed by such duties, and the consequences of the stress can assist the clinician in recognizing the caregiver in need of intervention.
A PROFILE OF THE CAREGIVER
A recent survey estimated that more than 65 million Americans provide unpaid assistance annually to older adults with disabilities.1 The value of that labor has been estimated at $306 billion annually, or nearly double the combined cost of home health care and nursing home care.2,3
The typical caregiver is a woman, about 48 years old, with some college education, who spends 20 hours or more each week providing unpaid care to someone aged 50 years or older.1 The recipients of care often have long-term physical disabilities; mental confusion or emotional problems frequently complicate care.
PSYCHOLOGIC AND PHYSICAL COSTS
Caregiving may take a toll on the caregiver in a variety of ways: behavioral, in the form of alcohol or substance use4; psychologic, in the form of depression or other mental health problems5; and physical, in the form of chronic health conditions and impaired immune response.6 About three-fifths of caregivers report fair or poor health, compared with one-third of noncaregivers, and caregivers have approximately twice as many chronic conditions, such as heart disease, cancer, arthritis, and diabetes, compared with noncaregivers.2,7 Caregiving also exacts a financial toll, as employees who are caregivers cost their employers $13.4 billion more per year in health care expenditures.8 In addition, absenteeism, workday interruptions, and shifts from full-time to part-time work by caregivers cost businesses between $17.1 and $33.6 billion per year.9
The cost of caregiving is higher for women, who exhibit higher levels of anxiety and depression and lower levels of subjective well-being, life satisfaction, and physical health.10,11 The stress of caregiving has also been identified as a risk factor for morbidity among older (66 to 96 years old) caregivers, who have a 63% greater mortality than noncaregivers of the same age.12
PSYCHOSOCIAL STRESS, UNHEALTHY BEHAVIORS, AND ILLNESS ARE LINKED
Psychosocial stress is a predictor of disease and can lead to unhealthy behaviors such as smoking, substance abuse, overeating, poor nutrition, and a sedentary lifestyle; these, in turn, can lead to physical and psychiatric illness. Behaviors adopted initially as coping skills may persist to become chronic, thereby promoting either continued wellness (in the case of healthy coping behaviors) or worsening levels of illness (in the case of unhealthy coping behaviors).
McEwen and Gianaros13 have suggested that these stress mechanisms arise from patterns of communication between the brain and the autonomic, cardiovascular, and immune systems, which mutually influenceone another. These so-called bidirectional stress processes affect cognition, experience, and behavior.
An integrated model of stress that maps the bidirectional causal pathways among psychosocial stressors, resulting unhealthy behaviors, and illness is needed. Although the steps from unhealthy behaviors to illness are fairly well understood, the links from psychosocial stress, such as those exhibited by caregivers, to unhealthy behaviors are not as clear. Several mediators are under study:
- Personality mediators can be either ameliorative (resilience, self-confi dence, self-control, optimism, high self-esteem, a sense of mastery, and finding meaning in life) or exacerbating (neuroticism and inhibition, which together form the so-called type D personality).
- Environmental mediators include social support, financial support, a history of a significant life change, and trauma early in life, which may increase one’s subsequent vulnerability to unhealthy behaviors.
- Biologic mediators may include prolonged sympathetic activation and enhanced platelet activation, caused by increased levels of depression and anxiety in chronically stressed caregivers.14
IMPLICATIONS FOR INTERVENTION
A significant percentage of caregivers do not need a clinician’s intervention to help them cope with stress or unhealthy coping skills. Among caregivers aged 50 years or older, 47% indicated in a recent study that the burden of caregiving is low (ie, 1 or 2 on a 5-point scale).1 Those who respond to stressors as challenges rather than threats tend to be resilient people who exert control over their lives, often through meditation or similar techniques, and have a strong social support network. Many report that caregiving provides them with an opportunity to act in accordance with their values and feel helpful rather than helpless.
Cognitive-behavioral interventions to alleviate stress-related symptoms appear to be more effective if offered as individual rather than group therapy. Teaching caregivers effective coping strategies, rather than merely providing social support, has been shown to improve caregiver psychologic health.15 Chief among the goals of intervention should be to alter brain function and instill optimism, a sense of control and self-esteem.13
The number of people in the United States who spend a significant part of each week working as unpaid caregivers is considerable, and the toll exacted for such work is high. Understanding the profi le of the caregiver, the nature of the duties performed, the stress imposed by such duties, and the consequences of the stress can assist the clinician in recognizing the caregiver in need of intervention.
A PROFILE OF THE CAREGIVER
A recent survey estimated that more than 65 million Americans provide unpaid assistance annually to older adults with disabilities.1 The value of that labor has been estimated at $306 billion annually, or nearly double the combined cost of home health care and nursing home care.2,3
The typical caregiver is a woman, about 48 years old, with some college education, who spends 20 hours or more each week providing unpaid care to someone aged 50 years or older.1 The recipients of care often have long-term physical disabilities; mental confusion or emotional problems frequently complicate care.
PSYCHOLOGIC AND PHYSICAL COSTS
Caregiving may take a toll on the caregiver in a variety of ways: behavioral, in the form of alcohol or substance use4; psychologic, in the form of depression or other mental health problems5; and physical, in the form of chronic health conditions and impaired immune response.6 About three-fifths of caregivers report fair or poor health, compared with one-third of noncaregivers, and caregivers have approximately twice as many chronic conditions, such as heart disease, cancer, arthritis, and diabetes, compared with noncaregivers.2,7 Caregiving also exacts a financial toll, as employees who are caregivers cost their employers $13.4 billion more per year in health care expenditures.8 In addition, absenteeism, workday interruptions, and shifts from full-time to part-time work by caregivers cost businesses between $17.1 and $33.6 billion per year.9
The cost of caregiving is higher for women, who exhibit higher levels of anxiety and depression and lower levels of subjective well-being, life satisfaction, and physical health.10,11 The stress of caregiving has also been identified as a risk factor for morbidity among older (66 to 96 years old) caregivers, who have a 63% greater mortality than noncaregivers of the same age.12
PSYCHOSOCIAL STRESS, UNHEALTHY BEHAVIORS, AND ILLNESS ARE LINKED
Psychosocial stress is a predictor of disease and can lead to unhealthy behaviors such as smoking, substance abuse, overeating, poor nutrition, and a sedentary lifestyle; these, in turn, can lead to physical and psychiatric illness. Behaviors adopted initially as coping skills may persist to become chronic, thereby promoting either continued wellness (in the case of healthy coping behaviors) or worsening levels of illness (in the case of unhealthy coping behaviors).
McEwen and Gianaros13 have suggested that these stress mechanisms arise from patterns of communication between the brain and the autonomic, cardiovascular, and immune systems, which mutually influenceone another. These so-called bidirectional stress processes affect cognition, experience, and behavior.
An integrated model of stress that maps the bidirectional causal pathways among psychosocial stressors, resulting unhealthy behaviors, and illness is needed. Although the steps from unhealthy behaviors to illness are fairly well understood, the links from psychosocial stress, such as those exhibited by caregivers, to unhealthy behaviors are not as clear. Several mediators are under study:
- Personality mediators can be either ameliorative (resilience, self-confi dence, self-control, optimism, high self-esteem, a sense of mastery, and finding meaning in life) or exacerbating (neuroticism and inhibition, which together form the so-called type D personality).
- Environmental mediators include social support, financial support, a history of a significant life change, and trauma early in life, which may increase one’s subsequent vulnerability to unhealthy behaviors.
- Biologic mediators may include prolonged sympathetic activation and enhanced platelet activation, caused by increased levels of depression and anxiety in chronically stressed caregivers.14
IMPLICATIONS FOR INTERVENTION
A significant percentage of caregivers do not need a clinician’s intervention to help them cope with stress or unhealthy coping skills. Among caregivers aged 50 years or older, 47% indicated in a recent study that the burden of caregiving is low (ie, 1 or 2 on a 5-point scale).1 Those who respond to stressors as challenges rather than threats tend to be resilient people who exert control over their lives, often through meditation or similar techniques, and have a strong social support network. Many report that caregiving provides them with an opportunity to act in accordance with their values and feel helpful rather than helpless.
Cognitive-behavioral interventions to alleviate stress-related symptoms appear to be more effective if offered as individual rather than group therapy. Teaching caregivers effective coping strategies, rather than merely providing social support, has been shown to improve caregiver psychologic health.15 Chief among the goals of intervention should be to alter brain function and instill optimism, a sense of control and self-esteem.13
- The National Alliance for Caregiving, in collaboration with the American Association of Retired Persons. Caregiving in the U.S. 2009. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed March 21, 2011.
- Family Caregiver Alliance. Caregiver health. A population at risk. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=1822. Published 2006. Accessed March 21, 2011.
- Family Caregiver Alliance. Prevalence, hours, and economic value of family caregiving, updated state-by-state analysis of 2004 national estimates. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content/pdfs/State_Caregiving_Data_Amo_20061107.pdf. Published 2006. Accessed March 21, 2011.
- Evercare. Study of caregivers in decline: a close-up look at the health risks of caring for a loved one. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregivers%20in%20Decline%20Study-FINAL-lowres.pdf. Published 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a metaanalysis. Psychol Aging 2003; 18:250–267.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129:946–972.
- Ho A, Collins S, Davis K, Doty M. A look at working-age caregivers’ roles, health concerns, and need for support (issue brief). New York, NY: The Commonwealth Fund; 2005.
- MetLife study of working caregivers and employer health care costs. MetLife Web site. http://www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-carecosts.pdf. Published July 2006. Accessed March 21, 2011.
- MetLife caregiving cost study: productivity losses to U.S. business. National Alliance for Caregiving Web site. http://www.caregiving. org/data/Caregiver%20Cost%20Study.pdf. Published July 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci 2006; 61:P33–P45.
- Johnson RW, Wiener JM. A profi le of frail older Americans and their caregivers. Urban Institute Web site. http://www.urban.org/UploadedPDF/311284_older_americans.pdf. Published February 2006. Accessed March 21, 2011.
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2215–2219.
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010; 1186:190–222.
- Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 2008; 22:493–502.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89.
- The National Alliance for Caregiving, in collaboration with the American Association of Retired Persons. Caregiving in the U.S. 2009. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed March 21, 2011.
- Family Caregiver Alliance. Caregiver health. A population at risk. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=1822. Published 2006. Accessed March 21, 2011.
- Family Caregiver Alliance. Prevalence, hours, and economic value of family caregiving, updated state-by-state analysis of 2004 national estimates. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content/pdfs/State_Caregiving_Data_Amo_20061107.pdf. Published 2006. Accessed March 21, 2011.
- Evercare. Study of caregivers in decline: a close-up look at the health risks of caring for a loved one. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregivers%20in%20Decline%20Study-FINAL-lowres.pdf. Published 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a metaanalysis. Psychol Aging 2003; 18:250–267.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129:946–972.
- Ho A, Collins S, Davis K, Doty M. A look at working-age caregivers’ roles, health concerns, and need for support (issue brief). New York, NY: The Commonwealth Fund; 2005.
- MetLife study of working caregivers and employer health care costs. MetLife Web site. http://www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-carecosts.pdf. Published July 2006. Accessed March 21, 2011.
- MetLife caregiving cost study: productivity losses to U.S. business. National Alliance for Caregiving Web site. http://www.caregiving. org/data/Caregiver%20Cost%20Study.pdf. Published July 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci 2006; 61:P33–P45.
- Johnson RW, Wiener JM. A profi le of frail older Americans and their caregivers. Urban Institute Web site. http://www.urban.org/UploadedPDF/311284_older_americans.pdf. Published February 2006. Accessed March 21, 2011.
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2215–2219.
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010; 1186:190–222.
- Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 2008; 22:493–502.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89.
Stress in medicine: Strategies for caregivers, patients, clinicians—Promoting better outcomes with stress and anxiety reduction
The traditional paradigm for cardiac care has emphasized the use of technology to treat disease. Our focus on technologies such as echocardiography, advanced imaging instrumentation, and cardiac catheterization mirrors the preoccupation of society as a whole with technologic advances.
Attention has only recently been given to the patient’s emotional experience and how this might relate to outcomes, recovery, and healing. An expanded paradigm of cardiac care incorporates pain relief, emotional support, spiritual healing, and a caring environment. These elements of patient-centered care aim to relieve stress and anxiety in order to achieve a better clinical outcome.
PATIENT-CENTERED CARE
The importance of patient-centered care is illustrated by the results of a 2007 survey in which 41% of patients cited elements of the patient experience as factors that most influenced their choice of hospital.1 Accepted wisdom on patient choice has historically centered on medical factors such as clinical reputation, physician recommendations, and hospital location, each of which was cited by 18% to 21% of the patients surveyed. Elements of the patient experience cited in the study include stress-reducing factors such as the appearance of the room, ease of scheduling, an environment that supports family needs, convenience and comfort of common areas, on-time performance, and simple registration procedures.
Székely et al2 found in a 4-year followup study that high levels of preoperative anxiety predicted greater mortality and cardiovascular morbidity following cardiac surgery. In a study by Tully et al,3 preoperative anxiety was also predictive of hospital readmission following cardiac surgery. Preoperative stress and anxiety are reliable predictors of postoperative distress.4
The variety and relative efficacy of interventions to reduce stress and anxiety are not well studied. Voss et al5 showed that cardiac surgery patients who were played soothing music experienced significantly reduced anxiety, pain, pain distress, and length of hospital stay. One Cleveland Clinic study of massage therapy, however, was unable to demonstrate a statistically significant therapeutic benefit, despite patient satisfaction with the therapy.6
THE ADVENT OF HEALING SERVICES
Identifying patients who exhibit significant preoperative stress and providing, as part of an expanded cardiac care paradigm, emotional care both pre- and postoperatively may ameliorate clinical outcomes. As such, the Heart and Vascular Institute at the Cleveland Clinic formed a healing services division, based on the concept that healing is more than simply physical recovery from a particular procedure. The division’s mission statement is: “To enhance the patient experience by promoting healing through a comprehensive set of coordinated services addressing the holistic needs of the patient.”
At the Cleveland Clinic, healing services are now integrated with standard services to enhance the cardiac care paradigm. Our standard medical services focus on areas of communication and pain control, both of which affect anxiety and stress. The need for enhanced communication is significant: 75% of patients admitted to a Chicago hospital were unable to name a single doctor assigned to their care, and of the remaining 25%, only 40% of responders were correct.7
It is worth noting that communicating more information to a patient is not necessarily better. Patients given detailed preoperative information about their disease and the potential complications of their cardiac surgery had levels of preoperative, perioperative, and postoperative stress, anxiety, and depression similar to those who received routine medical information.8,9 On the other hand, patients desire information about their postoperative plan of care while they are experiencing it, and value communication with physicians, nurses, healing services personnel, and other caregivers when it is presented in a calm and forthright manner. Communications should emphasize that the entire clinical team is there to help the patient get better.
THE FIFTH VITAL SIGN
Pain control is an aspect of care that was long ignored. The goal of the pain control task force at the Cleveland Clinic is the development of effective, efficient, and compassionate pain management.
The fifth vital sign, one that escapes the electronic medical record, can be addressed by this question: “How are you feeling?” Treating pain will reduce stress and anxiety. Before surgery, pain management priorities are discussed with patients, and at each daily encounter the goal is to set, refine, and exceed expectations for pain control through discussion and frequent pain assessments.
Reducing anxiety and stress is the goal of both standard care services and healing services, resulting in more satisfied patients with better clinical outcomes.
CASE: “YOU AND THE TEAM MADE ME GET OUTOF BED AND MOVE FORWARD”
Bobbi is a 78-year-old woman who was initially recovering well following cardiac surgery, including valve surgery, but had to return to the intensive care unit, which is difficult for patients. She was subsequently returned to the floor but was reluctant to walk and progressed slowly, despite normal electrocardiogram, radiographs, and blood panel results. We discovered that her husband was in hospice care in another state, causing Bobbi anxiety as she expressed concern over being her husband’s caregiver while being weakened physically herself. She was fearful of moving forward and her recovery stalled.
The primary care nurse referred her to the healing services team. The healing services team provided support for her anxiety and stress, and reviewed options for managing her husband’s care. She participated in Reiki, spiritual support, and social work services. During her admission her husband died, so the team provided appropriate support.
When asked about her experience upon leavingthe hospital, Bobbi did not mention her surgeon or the success of her heart valve procedure, but commented instead on the healing services team that enabled her to get through the experience.
- Grote KD, Newman JRS, Sutaria SS. A better hospital experience. The McKinsey Quarterly. November 2007.
- Székely A, Balog P, Benkö E, et al. Anxiety predicts mortality and morbidity after coronary artery and valve surgery—a 4-year followup study. Psychosom Med 2007; 69:625–631.
- Tully PJ, Baker RA, Turnbull D, Winefield H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. J Behav Med 2008; 31:281–290.
- Vingerhoets G. Perioperative anxiety and depression in open-heart surgery. Psychosomatics 1998; 39:30–37.
- Voss JA, Good M, Yates B, Baun MM, Thompson A, Hertzog M. Sedative music reduces anxiety and pain during chair rest after open-heart surgery. Pain 2004; 112:197–203.
- Albert NM, Gillinov AM, Lytle BW, Feng J, Cwynar R, Blackstone EH. A randomized trial of massage therapy after heart surgery. Heart Lung 2009; 38:480–490.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med 2009; 169:199–201.
- Ivarsson B, Larsson S, Lührs C, Sjöberg T. Extended written pre-operative information about possible complications at cardiac surgery—do the patients want to know? Eur J Cardiothorac Surg 2005; 28:407–414.
- Bergmann P, Huber S, Mächler H, et al. The influence of medical information on the perioperative course of stress in cardiac surgery patients. Anesth Analg 2001; 93:1093–1099.
The traditional paradigm for cardiac care has emphasized the use of technology to treat disease. Our focus on technologies such as echocardiography, advanced imaging instrumentation, and cardiac catheterization mirrors the preoccupation of society as a whole with technologic advances.
Attention has only recently been given to the patient’s emotional experience and how this might relate to outcomes, recovery, and healing. An expanded paradigm of cardiac care incorporates pain relief, emotional support, spiritual healing, and a caring environment. These elements of patient-centered care aim to relieve stress and anxiety in order to achieve a better clinical outcome.
PATIENT-CENTERED CARE
The importance of patient-centered care is illustrated by the results of a 2007 survey in which 41% of patients cited elements of the patient experience as factors that most influenced their choice of hospital.1 Accepted wisdom on patient choice has historically centered on medical factors such as clinical reputation, physician recommendations, and hospital location, each of which was cited by 18% to 21% of the patients surveyed. Elements of the patient experience cited in the study include stress-reducing factors such as the appearance of the room, ease of scheduling, an environment that supports family needs, convenience and comfort of common areas, on-time performance, and simple registration procedures.
Székely et al2 found in a 4-year followup study that high levels of preoperative anxiety predicted greater mortality and cardiovascular morbidity following cardiac surgery. In a study by Tully et al,3 preoperative anxiety was also predictive of hospital readmission following cardiac surgery. Preoperative stress and anxiety are reliable predictors of postoperative distress.4
The variety and relative efficacy of interventions to reduce stress and anxiety are not well studied. Voss et al5 showed that cardiac surgery patients who were played soothing music experienced significantly reduced anxiety, pain, pain distress, and length of hospital stay. One Cleveland Clinic study of massage therapy, however, was unable to demonstrate a statistically significant therapeutic benefit, despite patient satisfaction with the therapy.6
THE ADVENT OF HEALING SERVICES
Identifying patients who exhibit significant preoperative stress and providing, as part of an expanded cardiac care paradigm, emotional care both pre- and postoperatively may ameliorate clinical outcomes. As such, the Heart and Vascular Institute at the Cleveland Clinic formed a healing services division, based on the concept that healing is more than simply physical recovery from a particular procedure. The division’s mission statement is: “To enhance the patient experience by promoting healing through a comprehensive set of coordinated services addressing the holistic needs of the patient.”
At the Cleveland Clinic, healing services are now integrated with standard services to enhance the cardiac care paradigm. Our standard medical services focus on areas of communication and pain control, both of which affect anxiety and stress. The need for enhanced communication is significant: 75% of patients admitted to a Chicago hospital were unable to name a single doctor assigned to their care, and of the remaining 25%, only 40% of responders were correct.7
It is worth noting that communicating more information to a patient is not necessarily better. Patients given detailed preoperative information about their disease and the potential complications of their cardiac surgery had levels of preoperative, perioperative, and postoperative stress, anxiety, and depression similar to those who received routine medical information.8,9 On the other hand, patients desire information about their postoperative plan of care while they are experiencing it, and value communication with physicians, nurses, healing services personnel, and other caregivers when it is presented in a calm and forthright manner. Communications should emphasize that the entire clinical team is there to help the patient get better.
THE FIFTH VITAL SIGN
Pain control is an aspect of care that was long ignored. The goal of the pain control task force at the Cleveland Clinic is the development of effective, efficient, and compassionate pain management.
The fifth vital sign, one that escapes the electronic medical record, can be addressed by this question: “How are you feeling?” Treating pain will reduce stress and anxiety. Before surgery, pain management priorities are discussed with patients, and at each daily encounter the goal is to set, refine, and exceed expectations for pain control through discussion and frequent pain assessments.
Reducing anxiety and stress is the goal of both standard care services and healing services, resulting in more satisfied patients with better clinical outcomes.
CASE: “YOU AND THE TEAM MADE ME GET OUTOF BED AND MOVE FORWARD”
Bobbi is a 78-year-old woman who was initially recovering well following cardiac surgery, including valve surgery, but had to return to the intensive care unit, which is difficult for patients. She was subsequently returned to the floor but was reluctant to walk and progressed slowly, despite normal electrocardiogram, radiographs, and blood panel results. We discovered that her husband was in hospice care in another state, causing Bobbi anxiety as she expressed concern over being her husband’s caregiver while being weakened physically herself. She was fearful of moving forward and her recovery stalled.
The primary care nurse referred her to the healing services team. The healing services team provided support for her anxiety and stress, and reviewed options for managing her husband’s care. She participated in Reiki, spiritual support, and social work services. During her admission her husband died, so the team provided appropriate support.
When asked about her experience upon leavingthe hospital, Bobbi did not mention her surgeon or the success of her heart valve procedure, but commented instead on the healing services team that enabled her to get through the experience.
The traditional paradigm for cardiac care has emphasized the use of technology to treat disease. Our focus on technologies such as echocardiography, advanced imaging instrumentation, and cardiac catheterization mirrors the preoccupation of society as a whole with technologic advances.
Attention has only recently been given to the patient’s emotional experience and how this might relate to outcomes, recovery, and healing. An expanded paradigm of cardiac care incorporates pain relief, emotional support, spiritual healing, and a caring environment. These elements of patient-centered care aim to relieve stress and anxiety in order to achieve a better clinical outcome.
PATIENT-CENTERED CARE
The importance of patient-centered care is illustrated by the results of a 2007 survey in which 41% of patients cited elements of the patient experience as factors that most influenced their choice of hospital.1 Accepted wisdom on patient choice has historically centered on medical factors such as clinical reputation, physician recommendations, and hospital location, each of which was cited by 18% to 21% of the patients surveyed. Elements of the patient experience cited in the study include stress-reducing factors such as the appearance of the room, ease of scheduling, an environment that supports family needs, convenience and comfort of common areas, on-time performance, and simple registration procedures.
Székely et al2 found in a 4-year followup study that high levels of preoperative anxiety predicted greater mortality and cardiovascular morbidity following cardiac surgery. In a study by Tully et al,3 preoperative anxiety was also predictive of hospital readmission following cardiac surgery. Preoperative stress and anxiety are reliable predictors of postoperative distress.4
The variety and relative efficacy of interventions to reduce stress and anxiety are not well studied. Voss et al5 showed that cardiac surgery patients who were played soothing music experienced significantly reduced anxiety, pain, pain distress, and length of hospital stay. One Cleveland Clinic study of massage therapy, however, was unable to demonstrate a statistically significant therapeutic benefit, despite patient satisfaction with the therapy.6
THE ADVENT OF HEALING SERVICES
Identifying patients who exhibit significant preoperative stress and providing, as part of an expanded cardiac care paradigm, emotional care both pre- and postoperatively may ameliorate clinical outcomes. As such, the Heart and Vascular Institute at the Cleveland Clinic formed a healing services division, based on the concept that healing is more than simply physical recovery from a particular procedure. The division’s mission statement is: “To enhance the patient experience by promoting healing through a comprehensive set of coordinated services addressing the holistic needs of the patient.”
At the Cleveland Clinic, healing services are now integrated with standard services to enhance the cardiac care paradigm. Our standard medical services focus on areas of communication and pain control, both of which affect anxiety and stress. The need for enhanced communication is significant: 75% of patients admitted to a Chicago hospital were unable to name a single doctor assigned to their care, and of the remaining 25%, only 40% of responders were correct.7
It is worth noting that communicating more information to a patient is not necessarily better. Patients given detailed preoperative information about their disease and the potential complications of their cardiac surgery had levels of preoperative, perioperative, and postoperative stress, anxiety, and depression similar to those who received routine medical information.8,9 On the other hand, patients desire information about their postoperative plan of care while they are experiencing it, and value communication with physicians, nurses, healing services personnel, and other caregivers when it is presented in a calm and forthright manner. Communications should emphasize that the entire clinical team is there to help the patient get better.
THE FIFTH VITAL SIGN
Pain control is an aspect of care that was long ignored. The goal of the pain control task force at the Cleveland Clinic is the development of effective, efficient, and compassionate pain management.
The fifth vital sign, one that escapes the electronic medical record, can be addressed by this question: “How are you feeling?” Treating pain will reduce stress and anxiety. Before surgery, pain management priorities are discussed with patients, and at each daily encounter the goal is to set, refine, and exceed expectations for pain control through discussion and frequent pain assessments.
Reducing anxiety and stress is the goal of both standard care services and healing services, resulting in more satisfied patients with better clinical outcomes.
CASE: “YOU AND THE TEAM MADE ME GET OUTOF BED AND MOVE FORWARD”
Bobbi is a 78-year-old woman who was initially recovering well following cardiac surgery, including valve surgery, but had to return to the intensive care unit, which is difficult for patients. She was subsequently returned to the floor but was reluctant to walk and progressed slowly, despite normal electrocardiogram, radiographs, and blood panel results. We discovered that her husband was in hospice care in another state, causing Bobbi anxiety as she expressed concern over being her husband’s caregiver while being weakened physically herself. She was fearful of moving forward and her recovery stalled.
The primary care nurse referred her to the healing services team. The healing services team provided support for her anxiety and stress, and reviewed options for managing her husband’s care. She participated in Reiki, spiritual support, and social work services. During her admission her husband died, so the team provided appropriate support.
When asked about her experience upon leavingthe hospital, Bobbi did not mention her surgeon or the success of her heart valve procedure, but commented instead on the healing services team that enabled her to get through the experience.
- Grote KD, Newman JRS, Sutaria SS. A better hospital experience. The McKinsey Quarterly. November 2007.
- Székely A, Balog P, Benkö E, et al. Anxiety predicts mortality and morbidity after coronary artery and valve surgery—a 4-year followup study. Psychosom Med 2007; 69:625–631.
- Tully PJ, Baker RA, Turnbull D, Winefield H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. J Behav Med 2008; 31:281–290.
- Vingerhoets G. Perioperative anxiety and depression in open-heart surgery. Psychosomatics 1998; 39:30–37.
- Voss JA, Good M, Yates B, Baun MM, Thompson A, Hertzog M. Sedative music reduces anxiety and pain during chair rest after open-heart surgery. Pain 2004; 112:197–203.
- Albert NM, Gillinov AM, Lytle BW, Feng J, Cwynar R, Blackstone EH. A randomized trial of massage therapy after heart surgery. Heart Lung 2009; 38:480–490.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med 2009; 169:199–201.
- Ivarsson B, Larsson S, Lührs C, Sjöberg T. Extended written pre-operative information about possible complications at cardiac surgery—do the patients want to know? Eur J Cardiothorac Surg 2005; 28:407–414.
- Bergmann P, Huber S, Mächler H, et al. The influence of medical information on the perioperative course of stress in cardiac surgery patients. Anesth Analg 2001; 93:1093–1099.
- Grote KD, Newman JRS, Sutaria SS. A better hospital experience. The McKinsey Quarterly. November 2007.
- Székely A, Balog P, Benkö E, et al. Anxiety predicts mortality and morbidity after coronary artery and valve surgery—a 4-year followup study. Psychosom Med 2007; 69:625–631.
- Tully PJ, Baker RA, Turnbull D, Winefield H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. J Behav Med 2008; 31:281–290.
- Vingerhoets G. Perioperative anxiety and depression in open-heart surgery. Psychosomatics 1998; 39:30–37.
- Voss JA, Good M, Yates B, Baun MM, Thompson A, Hertzog M. Sedative music reduces anxiety and pain during chair rest after open-heart surgery. Pain 2004; 112:197–203.
- Albert NM, Gillinov AM, Lytle BW, Feng J, Cwynar R, Blackstone EH. A randomized trial of massage therapy after heart surgery. Heart Lung 2009; 38:480–490.
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med 2009; 169:199–201.
- Ivarsson B, Larsson S, Lührs C, Sjöberg T. Extended written pre-operative information about possible complications at cardiac surgery—do the patients want to know? Eur J Cardiothorac Surg 2005; 28:407–414.
- Bergmann P, Huber S, Mächler H, et al. The influence of medical information on the perioperative course of stress in cardiac surgery patients. Anesth Analg 2001; 93:1093–1099.
Stress in medicine: Strategies for caregivers, patients, clinicians—Addressing the impact of clinician stress
The impact of clinician stress on the health care system is significant. It can adversely affect the patient experience, compromise patient safety, hinder the delivery of care in a manner that is inconsistent with producing quality outcomes, and increase the overall cost of care.
CLINICIAN STRESS IS PREVALENT
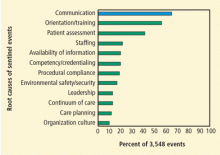
Models of health care that restore human interaction are desperately needed. Clinicians today are overwhelmed by performance assessments that are based on length of stay, use of evidence-based medication regimens, and morbidity and mortality outcomes. Yet clinicians have few opportunities to establish more than cursory relationships with their patients—relationships that would permit better understanding of patients’ emotional well-being and that would optimize the overall healing experience.
Shanafelt et al1 surveyed 7,905 surgeons and found that clinician stress is pervasive: 64% indicated that their work schedule left inadequate time for their personal or family life, 40% reported burnout, and 30% screened positive for symptoms of depression. Another survey of 763 practicing physicians in California found that 53% reported moderate to severe levels of stress.2 Nonphysician clinicians have significant levels of stress as well, with one survey of nurses finding that, of those who quit the profession, 26% cited stress as the cause.3
THE EFFECT OF CLINICIAN STRESSON QUALITY OF CARE
In the Shanafelt et al study, high levels of emotional exhaustion correlated positively with major medical errors over the previous 3 months.1 Nearly 9% of the surgeons surveyed reported making a stress-related major medical mistake in the past 3 months; among those surgeons with high levels of emotional exhaustion, that figure was nearly 15%. This study also found that every 1-point increase in the emotional exhaustion scale (range, 0 to 54) was associated with a 5% increase in the likelihood of reporting a medical error.1
In a study of internal medicine residents, fatigue and distress were associated with medical errors, which were reported by 39% of respondents.4
STRESS AND COMMUNICATION
Stress can damage the physician-nurse relationship, with a significant impact not only on clinicians, but also on delivery of care. The associated breakdowns in communication can negatively affect several areas, including critical care transitions and timely delivery of care. Stress also affects morale, job satisfaction, and job retention.5
ADDRESSING THE IMPACT OF CLINICIAN STRESS
The traditional response to complaints registered by patients has been behavioral coaching, disruptive-behavior programs, and the punitive use of satisfaction metrics, which are incorporated into the physician’s annual evaluation. These approaches do little to address the cause of the stress and can inculcate cynicism instead.
A more useful approach is to define and strive for an optimal working environment for clinicians, thereby promoting an enhanced patient experience. This approach attempts to restore balance to both the business and art of medicine and may incorporate biofeedback and other healing services to clinicians as tools to minimize and manage stress.
The business of medicine may be restored by enhancing the culture and climate of the hospital, improving communication and collaboration, reducing administrative tasks, restoring authority and autonomy, and eliminating punitive practices. The art of medicine may be restored by valuing the sacred relationship between clinician and patient, learning to listen more carefully to the patient, creating better healing environments, providing emotional support, and supporting caregivers.
- Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg 2010; 251:995–1000.
- Beck M. Checking up on the doctor. What patients can learn from the ways physicians take care of themselves. Wall Street Journal. May 25, 2010. http://online.wsj.com/article/SB10001424052748704113504575264364125574500.html?KEYWORDS=Checking+up+on+the+doctor. Accessed April 27, 2011.
- Reineck C, Furino A. Nursing career fulfillment: statistics and statements from registered nurses. Nursing Economics 2005; 23:25–30.
- West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of resident fatigue and distress with perceived medical errors. JAMA 2009; 302:1294–1300.
- Rosenstein AH. Nurse-physician relationships: Impact on nurses atisfaction and retention. Am J Nursing 2002; 102:26–34.
- Hickam DH, Severance S, Feldstein A, et al; Oregon Health & Science University Evidence-based Practice Center. The effect of health care working conditions on patient safety. Agency for Healthcare Research and Quality publication 03-E031. http://www.ahrq.gov/downloads/pub/evidence/pdf/work/work.pdf. Published May 2003. Accessed April 27, 2011.
The impact of clinician stress on the health care system is significant. It can adversely affect the patient experience, compromise patient safety, hinder the delivery of care in a manner that is inconsistent with producing quality outcomes, and increase the overall cost of care.
CLINICIAN STRESS IS PREVALENT
Models of health care that restore human interaction are desperately needed. Clinicians today are overwhelmed by performance assessments that are based on length of stay, use of evidence-based medication regimens, and morbidity and mortality outcomes. Yet clinicians have few opportunities to establish more than cursory relationships with their patients—relationships that would permit better understanding of patients’ emotional well-being and that would optimize the overall healing experience.
Shanafelt et al1 surveyed 7,905 surgeons and found that clinician stress is pervasive: 64% indicated that their work schedule left inadequate time for their personal or family life, 40% reported burnout, and 30% screened positive for symptoms of depression. Another survey of 763 practicing physicians in California found that 53% reported moderate to severe levels of stress.2 Nonphysician clinicians have significant levels of stress as well, with one survey of nurses finding that, of those who quit the profession, 26% cited stress as the cause.3
THE EFFECT OF CLINICIAN STRESSON QUALITY OF CARE
In the Shanafelt et al study, high levels of emotional exhaustion correlated positively with major medical errors over the previous 3 months.1 Nearly 9% of the surgeons surveyed reported making a stress-related major medical mistake in the past 3 months; among those surgeons with high levels of emotional exhaustion, that figure was nearly 15%. This study also found that every 1-point increase in the emotional exhaustion scale (range, 0 to 54) was associated with a 5% increase in the likelihood of reporting a medical error.1
In a study of internal medicine residents, fatigue and distress were associated with medical errors, which were reported by 39% of respondents.4
STRESS AND COMMUNICATION
Stress can damage the physician-nurse relationship, with a significant impact not only on clinicians, but also on delivery of care. The associated breakdowns in communication can negatively affect several areas, including critical care transitions and timely delivery of care. Stress also affects morale, job satisfaction, and job retention.5
ADDRESSING THE IMPACT OF CLINICIAN STRESS
The traditional response to complaints registered by patients has been behavioral coaching, disruptive-behavior programs, and the punitive use of satisfaction metrics, which are incorporated into the physician’s annual evaluation. These approaches do little to address the cause of the stress and can inculcate cynicism instead.
A more useful approach is to define and strive for an optimal working environment for clinicians, thereby promoting an enhanced patient experience. This approach attempts to restore balance to both the business and art of medicine and may incorporate biofeedback and other healing services to clinicians as tools to minimize and manage stress.
The business of medicine may be restored by enhancing the culture and climate of the hospital, improving communication and collaboration, reducing administrative tasks, restoring authority and autonomy, and eliminating punitive practices. The art of medicine may be restored by valuing the sacred relationship between clinician and patient, learning to listen more carefully to the patient, creating better healing environments, providing emotional support, and supporting caregivers.
The impact of clinician stress on the health care system is significant. It can adversely affect the patient experience, compromise patient safety, hinder the delivery of care in a manner that is inconsistent with producing quality outcomes, and increase the overall cost of care.
CLINICIAN STRESS IS PREVALENT
Models of health care that restore human interaction are desperately needed. Clinicians today are overwhelmed by performance assessments that are based on length of stay, use of evidence-based medication regimens, and morbidity and mortality outcomes. Yet clinicians have few opportunities to establish more than cursory relationships with their patients—relationships that would permit better understanding of patients’ emotional well-being and that would optimize the overall healing experience.
Shanafelt et al1 surveyed 7,905 surgeons and found that clinician stress is pervasive: 64% indicated that their work schedule left inadequate time for their personal or family life, 40% reported burnout, and 30% screened positive for symptoms of depression. Another survey of 763 practicing physicians in California found that 53% reported moderate to severe levels of stress.2 Nonphysician clinicians have significant levels of stress as well, with one survey of nurses finding that, of those who quit the profession, 26% cited stress as the cause.3
THE EFFECT OF CLINICIAN STRESSON QUALITY OF CARE
In the Shanafelt et al study, high levels of emotional exhaustion correlated positively with major medical errors over the previous 3 months.1 Nearly 9% of the surgeons surveyed reported making a stress-related major medical mistake in the past 3 months; among those surgeons with high levels of emotional exhaustion, that figure was nearly 15%. This study also found that every 1-point increase in the emotional exhaustion scale (range, 0 to 54) was associated with a 5% increase in the likelihood of reporting a medical error.1
In a study of internal medicine residents, fatigue and distress were associated with medical errors, which were reported by 39% of respondents.4
STRESS AND COMMUNICATION
Stress can damage the physician-nurse relationship, with a significant impact not only on clinicians, but also on delivery of care. The associated breakdowns in communication can negatively affect several areas, including critical care transitions and timely delivery of care. Stress also affects morale, job satisfaction, and job retention.5
ADDRESSING THE IMPACT OF CLINICIAN STRESS
The traditional response to complaints registered by patients has been behavioral coaching, disruptive-behavior programs, and the punitive use of satisfaction metrics, which are incorporated into the physician’s annual evaluation. These approaches do little to address the cause of the stress and can inculcate cynicism instead.
A more useful approach is to define and strive for an optimal working environment for clinicians, thereby promoting an enhanced patient experience. This approach attempts to restore balance to both the business and art of medicine and may incorporate biofeedback and other healing services to clinicians as tools to minimize and manage stress.
The business of medicine may be restored by enhancing the culture and climate of the hospital, improving communication and collaboration, reducing administrative tasks, restoring authority and autonomy, and eliminating punitive practices. The art of medicine may be restored by valuing the sacred relationship between clinician and patient, learning to listen more carefully to the patient, creating better healing environments, providing emotional support, and supporting caregivers.
- Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg 2010; 251:995–1000.
- Beck M. Checking up on the doctor. What patients can learn from the ways physicians take care of themselves. Wall Street Journal. May 25, 2010. http://online.wsj.com/article/SB10001424052748704113504575264364125574500.html?KEYWORDS=Checking+up+on+the+doctor. Accessed April 27, 2011.
- Reineck C, Furino A. Nursing career fulfillment: statistics and statements from registered nurses. Nursing Economics 2005; 23:25–30.
- West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of resident fatigue and distress with perceived medical errors. JAMA 2009; 302:1294–1300.
- Rosenstein AH. Nurse-physician relationships: Impact on nurses atisfaction and retention. Am J Nursing 2002; 102:26–34.
- Hickam DH, Severance S, Feldstein A, et al; Oregon Health & Science University Evidence-based Practice Center. The effect of health care working conditions on patient safety. Agency for Healthcare Research and Quality publication 03-E031. http://www.ahrq.gov/downloads/pub/evidence/pdf/work/work.pdf. Published May 2003. Accessed April 27, 2011.
- Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg 2010; 251:995–1000.
- Beck M. Checking up on the doctor. What patients can learn from the ways physicians take care of themselves. Wall Street Journal. May 25, 2010. http://online.wsj.com/article/SB10001424052748704113504575264364125574500.html?KEYWORDS=Checking+up+on+the+doctor. Accessed April 27, 2011.
- Reineck C, Furino A. Nursing career fulfillment: statistics and statements from registered nurses. Nursing Economics 2005; 23:25–30.
- West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of resident fatigue and distress with perceived medical errors. JAMA 2009; 302:1294–1300.
- Rosenstein AH. Nurse-physician relationships: Impact on nurses atisfaction and retention. Am J Nursing 2002; 102:26–34.
- Hickam DH, Severance S, Feldstein A, et al; Oregon Health & Science University Evidence-based Practice Center. The effect of health care working conditions on patient safety. Agency for Healthcare Research and Quality publication 03-E031. http://www.ahrq.gov/downloads/pub/evidence/pdf/work/work.pdf. Published May 2003. Accessed April 27, 2011.
Stress in medicine: Strategies for caregivers, patients, clinicians—Biofeedback in the treatment of stress
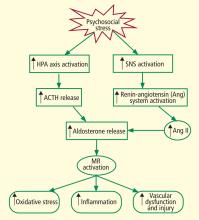
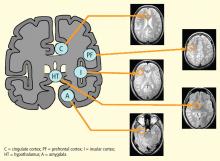
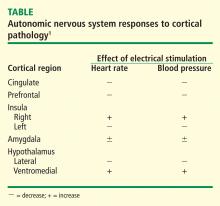
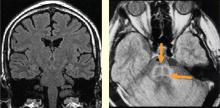
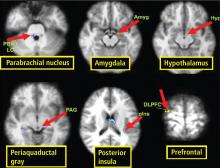
Traditionally, biofeedback was considered to be a stress management technique that targeted sympathetic nervous system (SNS) overdrive with an adrenal medullary system backup. Recent advances in autonomic physiology, however, have clarified that except in extreme situations, the SNS is not the key factor in day-to-day stress. Rather, the parasympathetic branch of the autonomic nervous system appears to be a more likely candidate for mediating routine stress because, unlike the SNS, which has slow-acting neurotransmitters (ie, catecholamines), the parasympathetic nervous system has the fast-acting transmitter acetylcholine.
VAGAL WITHDRAWAL: AN ALTERNATIVE TO SYMPATHETIC ACTIVATION
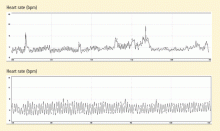
Porges1 first proposed the concept of vagal withdrawal as an indicator of stress and stress vulnerability; this contrasts with the idea that the stress response is a consequence of sympathetic activation and the hypothalamic-pituitary-adrenal axis response. In the vagal withdrawal model, the response to stress is stabilization of the sympathetic system followed by termination of parasympathetic activity, manifested as cardiac acceleration.
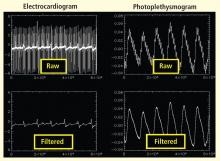
Respiratory sinus arrhythmia (RSA), or the variability in heart rate as it synchronizes with breathing, is considered an index of parasympathetic tone. In the laboratory, slow atropine infusion produces a transient paradoxical vagomimetic effect characterized by an initial increase in RSA, followed by a flattening and then a rise in the heart rate.2 This phenomenon has been measured in people during times of routine stress, such as when worrying about being late for an appointment. In such individuals, biofeedback training can result in recovery of normal RSA shortly after an episode of anxiety.
Historically, the focus of biofeedback was to cultivate low arousal, presumably reducing SNS activity, through the use of finger temperature, skin conductance training, and profound muscle relaxation. More sophisticated ways to look at both branches of the autonomic nervous system have since emerged that allow for sampling of the beat-by-beat changes in heart rate.
HEART RATE VARIABILITY BIOFEEDBACK
The concept of modifying the respiration rate (paced breathing) originated some 2,500 years ago as a component of meditation. It is being revisited today in the form of heart rate variability (HRV) biofeedback training, which is being used as a stress-management tool and a method to correct disorders in which autonomic regulation is thought to be important. HRV biofeedback involves training to increase the amplitude of HRV rhythms and thus improve autonomic homeostasis.
Normal HRV has a pattern of overlapping oscillatory frequency components, including:
- a high-frequency rhythm, 0.15 to 0.4 Hz, which is the RSA;
- a low-frequency rhythm, 0.05 to 0.15 Hz, associated with blood pressure oscillations; and
- a very-low-frequency rhythm, 0.005 to 0.05 Hz, which may regulate vascular tone and body temperature.
The goal of HRV biofeedback is to achieve respiratory rates at which resonance occurs between cardiac rhythms associated with respiration (RSA, or high-frequency oscillations) and those caused by baroreflex activity (low-frequency oscillations).
Spectral analysis has demonstrated that nearly all of the activity with HRV biofeedback occurs at a low-frequency band. The reason is that activity in the low-frequency band is related more to baroreflex activity than to HRV compared with other ranges of frequency. Breathing rates that correspond to baroreflex effects, called resonance frequency breathing, represent resonance in the cardiovascular system. Several devices are available whose mechanisms are based on the concept of achieving resonance frequency breathing. One such device is a slow-breathing monitor (Resp-e-rate) that has been approved by the US Food and Drug Administration for the adjunctive treatment of hypertension.
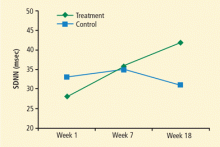
Improved HRV may suggest an improved risk status: Kleiger et al4 found that the relative risk of mortality was 5.3 times greater for people with SDNN of less than 50 msec compared with those whose SDNN was greater than 100 msec. In Del Pozo’s study, eight of 30 patients in the intervention group achieved an SDNN of greater than 50 msec (vs 0 at pretreatment) compared with three of 31 controls (vs two at pretreatment).3 As an additional benefit of HRV biofeedback, patients in the intervention group who entered the study with hypertension all became normotensive.
In a meta-analysis, van Dixhoorn and White5 found fewer cardiac events, fewer episodes of angina, and less occurrence of arrhythmia and exercise-induced ischemia from intensive supervised relaxation therapy in patients with ischemic heart disease. Improvements in scales of depression and anxiety were also observed with relaxation therapy.
Other studies have shown biofeedback to have beneficial effects based on the Posttraumatic Stress Disorder Checklist, the Hamilton Depression Rating Scale, and, in patients with mild to moderate heartfailure, the 6-minute walk test.6–8
The proposed mechanism for the beneficial effects of biofeedback found in clinical trials is improvement in baroreflex function, producing greater reflex efficiency and improved modulation of autonomic activity.
CONCLUSION
A shift in emphasis to vagal withdrawal has led to new forms of biofeedback that probably potentiate many of the same mechanisms thought to be present in Eastern practices such as yoga and tai chi. Results from small-scale trials have been promising for HRV biofeedback as a means of modifying responses to stress and promoting homeostatic processes that reduce the intensity of symptoms and improve surrogate markers associated with a number of disorders.
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233.
- Médigue C, Girard A, Laude D, Monti A, Wargon M, ElghoziJ-L. Relationship between pulse interval and respiratory sinusarrhythmia: a time- and frequency-domain analysis of the effects ofatropine. Eur J Physiol 2001; 441:650–655.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedbacktreatment increases heart rate variability in patients withknown coronary artery disease. Am Heart J 2004; 147:e11. http://download.journals.elsevierhealth.com/pdfs/journals/0002-8703/PIIS0002870303007191.pdf. Accessed May 2, 2011.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart ratevariability and its association with increased mortality after acutemyocardial infarciton. Am J Cardiol 1987; 59:256–262.
- van Dixhoorn JV, White A. Relaxation therapy for rehabilitationand prevention in ischaemic heart disease: a systematic review andmeta-analysis. Eur J Cardiovasc Prev Rehabil 2005; 12:193–202.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary resultsof an open label study of heart rate variability biofeedback for thetreatment of major depression. Appl Psychophysiol Biofeedback2007; 32:19–30.
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, GevirtzRN. The effects of respiratory sinus arrhythmia biofeedback onheart rate variability and posttraumatic stress disorder symptoms: apilot study. Appl Psychophysiol Biofeedback 2009; 34:135–143.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, StoletniyL. The effect of biofeedback on function in patients with heartfailure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
Traditionally, biofeedback was considered to be a stress management technique that targeted sympathetic nervous system (SNS) overdrive with an adrenal medullary system backup. Recent advances in autonomic physiology, however, have clarified that except in extreme situations, the SNS is not the key factor in day-to-day stress. Rather, the parasympathetic branch of the autonomic nervous system appears to be a more likely candidate for mediating routine stress because, unlike the SNS, which has slow-acting neurotransmitters (ie, catecholamines), the parasympathetic nervous system has the fast-acting transmitter acetylcholine.
VAGAL WITHDRAWAL: AN ALTERNATIVE TO SYMPATHETIC ACTIVATION
Porges1 first proposed the concept of vagal withdrawal as an indicator of stress and stress vulnerability; this contrasts with the idea that the stress response is a consequence of sympathetic activation and the hypothalamic-pituitary-adrenal axis response. In the vagal withdrawal model, the response to stress is stabilization of the sympathetic system followed by termination of parasympathetic activity, manifested as cardiac acceleration.
Respiratory sinus arrhythmia (RSA), or the variability in heart rate as it synchronizes with breathing, is considered an index of parasympathetic tone. In the laboratory, slow atropine infusion produces a transient paradoxical vagomimetic effect characterized by an initial increase in RSA, followed by a flattening and then a rise in the heart rate.2 This phenomenon has been measured in people during times of routine stress, such as when worrying about being late for an appointment. In such individuals, biofeedback training can result in recovery of normal RSA shortly after an episode of anxiety.
Historically, the focus of biofeedback was to cultivate low arousal, presumably reducing SNS activity, through the use of finger temperature, skin conductance training, and profound muscle relaxation. More sophisticated ways to look at both branches of the autonomic nervous system have since emerged that allow for sampling of the beat-by-beat changes in heart rate.
HEART RATE VARIABILITY BIOFEEDBACK
The concept of modifying the respiration rate (paced breathing) originated some 2,500 years ago as a component of meditation. It is being revisited today in the form of heart rate variability (HRV) biofeedback training, which is being used as a stress-management tool and a method to correct disorders in which autonomic regulation is thought to be important. HRV biofeedback involves training to increase the amplitude of HRV rhythms and thus improve autonomic homeostasis.
Normal HRV has a pattern of overlapping oscillatory frequency components, including:
- a high-frequency rhythm, 0.15 to 0.4 Hz, which is the RSA;
- a low-frequency rhythm, 0.05 to 0.15 Hz, associated with blood pressure oscillations; and
- a very-low-frequency rhythm, 0.005 to 0.05 Hz, which may regulate vascular tone and body temperature.
The goal of HRV biofeedback is to achieve respiratory rates at which resonance occurs between cardiac rhythms associated with respiration (RSA, or high-frequency oscillations) and those caused by baroreflex activity (low-frequency oscillations).
Spectral analysis has demonstrated that nearly all of the activity with HRV biofeedback occurs at a low-frequency band. The reason is that activity in the low-frequency band is related more to baroreflex activity than to HRV compared with other ranges of frequency. Breathing rates that correspond to baroreflex effects, called resonance frequency breathing, represent resonance in the cardiovascular system. Several devices are available whose mechanisms are based on the concept of achieving resonance frequency breathing. One such device is a slow-breathing monitor (Resp-e-rate) that has been approved by the US Food and Drug Administration for the adjunctive treatment of hypertension.
Improved HRV may suggest an improved risk status: Kleiger et al4 found that the relative risk of mortality was 5.3 times greater for people with SDNN of less than 50 msec compared with those whose SDNN was greater than 100 msec. In Del Pozo’s study, eight of 30 patients in the intervention group achieved an SDNN of greater than 50 msec (vs 0 at pretreatment) compared with three of 31 controls (vs two at pretreatment).3 As an additional benefit of HRV biofeedback, patients in the intervention group who entered the study with hypertension all became normotensive.
In a meta-analysis, van Dixhoorn and White5 found fewer cardiac events, fewer episodes of angina, and less occurrence of arrhythmia and exercise-induced ischemia from intensive supervised relaxation therapy in patients with ischemic heart disease. Improvements in scales of depression and anxiety were also observed with relaxation therapy.
Other studies have shown biofeedback to have beneficial effects based on the Posttraumatic Stress Disorder Checklist, the Hamilton Depression Rating Scale, and, in patients with mild to moderate heartfailure, the 6-minute walk test.6–8
The proposed mechanism for the beneficial effects of biofeedback found in clinical trials is improvement in baroreflex function, producing greater reflex efficiency and improved modulation of autonomic activity.
CONCLUSION
A shift in emphasis to vagal withdrawal has led to new forms of biofeedback that probably potentiate many of the same mechanisms thought to be present in Eastern practices such as yoga and tai chi. Results from small-scale trials have been promising for HRV biofeedback as a means of modifying responses to stress and promoting homeostatic processes that reduce the intensity of symptoms and improve surrogate markers associated with a number of disorders.
Traditionally, biofeedback was considered to be a stress management technique that targeted sympathetic nervous system (SNS) overdrive with an adrenal medullary system backup. Recent advances in autonomic physiology, however, have clarified that except in extreme situations, the SNS is not the key factor in day-to-day stress. Rather, the parasympathetic branch of the autonomic nervous system appears to be a more likely candidate for mediating routine stress because, unlike the SNS, which has slow-acting neurotransmitters (ie, catecholamines), the parasympathetic nervous system has the fast-acting transmitter acetylcholine.
VAGAL WITHDRAWAL: AN ALTERNATIVE TO SYMPATHETIC ACTIVATION
Porges1 first proposed the concept of vagal withdrawal as an indicator of stress and stress vulnerability; this contrasts with the idea that the stress response is a consequence of sympathetic activation and the hypothalamic-pituitary-adrenal axis response. In the vagal withdrawal model, the response to stress is stabilization of the sympathetic system followed by termination of parasympathetic activity, manifested as cardiac acceleration.
Respiratory sinus arrhythmia (RSA), or the variability in heart rate as it synchronizes with breathing, is considered an index of parasympathetic tone. In the laboratory, slow atropine infusion produces a transient paradoxical vagomimetic effect characterized by an initial increase in RSA, followed by a flattening and then a rise in the heart rate.2 This phenomenon has been measured in people during times of routine stress, such as when worrying about being late for an appointment. In such individuals, biofeedback training can result in recovery of normal RSA shortly after an episode of anxiety.
Historically, the focus of biofeedback was to cultivate low arousal, presumably reducing SNS activity, through the use of finger temperature, skin conductance training, and profound muscle relaxation. More sophisticated ways to look at both branches of the autonomic nervous system have since emerged that allow for sampling of the beat-by-beat changes in heart rate.
HEART RATE VARIABILITY BIOFEEDBACK
The concept of modifying the respiration rate (paced breathing) originated some 2,500 years ago as a component of meditation. It is being revisited today in the form of heart rate variability (HRV) biofeedback training, which is being used as a stress-management tool and a method to correct disorders in which autonomic regulation is thought to be important. HRV biofeedback involves training to increase the amplitude of HRV rhythms and thus improve autonomic homeostasis.
Normal HRV has a pattern of overlapping oscillatory frequency components, including:
- a high-frequency rhythm, 0.15 to 0.4 Hz, which is the RSA;
- a low-frequency rhythm, 0.05 to 0.15 Hz, associated with blood pressure oscillations; and
- a very-low-frequency rhythm, 0.005 to 0.05 Hz, which may regulate vascular tone and body temperature.
The goal of HRV biofeedback is to achieve respiratory rates at which resonance occurs between cardiac rhythms associated with respiration (RSA, or high-frequency oscillations) and those caused by baroreflex activity (low-frequency oscillations).
Spectral analysis has demonstrated that nearly all of the activity with HRV biofeedback occurs at a low-frequency band. The reason is that activity in the low-frequency band is related more to baroreflex activity than to HRV compared with other ranges of frequency. Breathing rates that correspond to baroreflex effects, called resonance frequency breathing, represent resonance in the cardiovascular system. Several devices are available whose mechanisms are based on the concept of achieving resonance frequency breathing. One such device is a slow-breathing monitor (Resp-e-rate) that has been approved by the US Food and Drug Administration for the adjunctive treatment of hypertension.
Improved HRV may suggest an improved risk status: Kleiger et al4 found that the relative risk of mortality was 5.3 times greater for people with SDNN of less than 50 msec compared with those whose SDNN was greater than 100 msec. In Del Pozo’s study, eight of 30 patients in the intervention group achieved an SDNN of greater than 50 msec (vs 0 at pretreatment) compared with three of 31 controls (vs two at pretreatment).3 As an additional benefit of HRV biofeedback, patients in the intervention group who entered the study with hypertension all became normotensive.
In a meta-analysis, van Dixhoorn and White5 found fewer cardiac events, fewer episodes of angina, and less occurrence of arrhythmia and exercise-induced ischemia from intensive supervised relaxation therapy in patients with ischemic heart disease. Improvements in scales of depression and anxiety were also observed with relaxation therapy.
Other studies have shown biofeedback to have beneficial effects based on the Posttraumatic Stress Disorder Checklist, the Hamilton Depression Rating Scale, and, in patients with mild to moderate heartfailure, the 6-minute walk test.6–8
The proposed mechanism for the beneficial effects of biofeedback found in clinical trials is improvement in baroreflex function, producing greater reflex efficiency and improved modulation of autonomic activity.
CONCLUSION
A shift in emphasis to vagal withdrawal has led to new forms of biofeedback that probably potentiate many of the same mechanisms thought to be present in Eastern practices such as yoga and tai chi. Results from small-scale trials have been promising for HRV biofeedback as a means of modifying responses to stress and promoting homeostatic processes that reduce the intensity of symptoms and improve surrogate markers associated with a number of disorders.
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233.
- Médigue C, Girard A, Laude D, Monti A, Wargon M, ElghoziJ-L. Relationship between pulse interval and respiratory sinusarrhythmia: a time- and frequency-domain analysis of the effects ofatropine. Eur J Physiol 2001; 441:650–655.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedbacktreatment increases heart rate variability in patients withknown coronary artery disease. Am Heart J 2004; 147:e11. http://download.journals.elsevierhealth.com/pdfs/journals/0002-8703/PIIS0002870303007191.pdf. Accessed May 2, 2011.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart ratevariability and its association with increased mortality after acutemyocardial infarciton. Am J Cardiol 1987; 59:256–262.
- van Dixhoorn JV, White A. Relaxation therapy for rehabilitationand prevention in ischaemic heart disease: a systematic review andmeta-analysis. Eur J Cardiovasc Prev Rehabil 2005; 12:193–202.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary resultsof an open label study of heart rate variability biofeedback for thetreatment of major depression. Appl Psychophysiol Biofeedback2007; 32:19–30.
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, GevirtzRN. The effects of respiratory sinus arrhythmia biofeedback onheart rate variability and posttraumatic stress disorder symptoms: apilot study. Appl Psychophysiol Biofeedback 2009; 34:135–143.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, StoletniyL. The effect of biofeedback on function in patients with heartfailure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233.
- Médigue C, Girard A, Laude D, Monti A, Wargon M, ElghoziJ-L. Relationship between pulse interval and respiratory sinusarrhythmia: a time- and frequency-domain analysis of the effects ofatropine. Eur J Physiol 2001; 441:650–655.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedbacktreatment increases heart rate variability in patients withknown coronary artery disease. Am Heart J 2004; 147:e11. http://download.journals.elsevierhealth.com/pdfs/journals/0002-8703/PIIS0002870303007191.pdf. Accessed May 2, 2011.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart ratevariability and its association with increased mortality after acutemyocardial infarciton. Am J Cardiol 1987; 59:256–262.
- van Dixhoorn JV, White A. Relaxation therapy for rehabilitationand prevention in ischaemic heart disease: a systematic review andmeta-analysis. Eur J Cardiovasc Prev Rehabil 2005; 12:193–202.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary resultsof an open label study of heart rate variability biofeedback for thetreatment of major depression. Appl Psychophysiol Biofeedback2007; 32:19–30.
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, GevirtzRN. The effects of respiratory sinus arrhythmia biofeedback onheart rate variability and posttraumatic stress disorder symptoms: apilot study. Appl Psychophysiol Biofeedback 2009; 34:135–143.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, StoletniyL. The effect of biofeedback on function in patients with heartfailure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
Stress in medicine: Strategies for caregivers, patients, clinicians—Biofeedback for extreme stress: Wounded warriors
Posttraumatic stress disorder (PTSD) is a severe anxiety disorder whose symptoms emerge following exposure to extreme stress, such as those encountered in the battlefield or as a result of sexual abuse or natural disasters. The ability to employ coping mechanisms affects the disorder’s presentation as well as the frequency, intensity, and duration of the symptoms. The “Wounded Warrior” program at East Carolina University (Greenville, NC) was developed to promote the functional independence of US Marines, including those with PTSD.
STRESS RESPONSE: INTERACTION OF THE BRAIN AND IMMUNE SYSTEM
Walter Cannon coined the “flight or fight” response to stress in the early 20th century, in which he emphasized the importance of the parasympathetic system.1 In 1988, Folkow clarified the description as an immune response to stress.2 The stress response is now understood to be a neuroendocrine function that includes a feedback loop between the hypothalamus and the pituitary and adrenal glands; stimulation of the hypothalamus promotes secretion of corticotropin-releasing hormone (CRH) into the hypophyseal portal system, which supplies the anterior pituitarywith blood. CRH stimulates the secretion of adrenocorticotropic hormone into the bloodstream by the pituitary, prompting the adrenal glands to release the stress hormone cortisol.
Cortisol mobilizes the body’s defenses to meet the challenge of an adverse situation. It modulates the stress response by inhibiting the further release of CRH by the hypothalamus. Cortisol thus protects healthy cells and tissues by inhibiting an overreaction from the immune system. Without this protective effect, the interaction between the brain and the immune system can become dysregulated, increasing the risk of immune disorders.
THE CENTRAL AUTONOMIC NETWORK
The central nervous system that regulates the overall balance of the autonomic nervous system (ANS) has been called the central autonomic network (CAN).3 The CAN helps control executive, social, affective, attentional, and motivational functions. Therefore, the old paradigm of simply decreasing hyperarousal of the ANS to treat negative affective states and dispositions is inadequate. Instead, restoring the appropriate relationship between the ANS and the central nervous system is the aim behind interventions to treat PTSD.
Autonomic, cognitive, and affective functions assist humans in maintaining balance when confronted with external challenges. The CAN controls inhibitory or negative processes that permit specific behavior and redeploy resources needed elsewhere. When negative circuits are compromised, positive circuits develop, resulting in hypervigilance, the symptoms of which can be devastating and, if not ameliorated, can develop into permanent conditions. In one study,Vietnam veterans with PTSD had an 8% reduction in the volume of their right hippocampus compared with veterans without PTSD. Another study calculated a 26% reduction in the left hippocampus and a 22% reduction in the right in veterans with the most severe PTSD compared with veterans who were in combat but had no PTSD symptoms.4
A common subcortical neural system regulates defensive behavior, including autonomic, emotional, and cognitive behavior. When the prefrontal cortex is taken “off line” for whatever reason, parasympathetic inhibitory action is withdrawn, and relative sympathetic dominance, associated with defense, occurs.
CONFRONTING HYPERAROUSAL
The question then arises of how to train the ANS to avoid hypervigilance. Growing evidence supports the use of heart rate variability as a predictor of hypervigilance and inefficient allocation of attentional and cognitive resources.
The overall objective of heart rate variability training is to decrease ANS hyperarousal and to improve its balance. “Wounded warriors” learn to control ANS responses to stress-producing stimuli (eg, thoughts, memories, and images associated with combat). The goal of training is to decrease arousal and maintain ANS balance for increasing lengths of time.
Once it was observed that alpha waves were dysfunctional in vulnerable populations, protocols were developed to train alpha and theta waves as a method of improving function. Peniston and colleagues5–9 showed that increased alpha and theta brain wave production resulted in normalized personality measures and prolonged the period of time before relapse in alcoholics. This protocol has also shown efficacy as an intervention in depression and PTSD.
BIOFEEDBACK TRAINING PROGRAM
The US Department of Defense is studying a combination of central nervous system biofeedback with ANS biofeedback, with the goal of restoring and maintaining tone between the systems.
The training program used in the study lasts 1 month, and starts with a session for preassessment, 16 biofeedback sessions (four per week), a postprogram evaluation, and a 3-month followup. Each week, participants are exposed to stress-producing stimuli that increase in intensity:
- Week 1: Stroop Color Word Test, math stressor, talk stressor/everyday events
- Week 2: Talk stressor, combat experiences
- Week 3: Images and sounds of combat
- Week 4: Virtual Baghdad or Afghanistan (virtual reality exposure)
Each biofeedback session consists of 5 minutes of baseline evaluation; 5 minutes in which the veteran is subjected to the weekly stressor; 20 minutes of heart rate variability and neurofeedback training; 5 more minutes of training with the weekly stressor; 20 more minutes of heart rate variability and neurofeedback training; and finally 5 minutes of recovery.
SUMMARY
Dysfunction in the balance of both the ANS and central nervous system is associated with symptoms of PTSD in combat veterans. Methods that are designed to restore balance in these systems are needed to ameliorate these symptoms. Biofeedback and neurofeedback are safe methods with which to achieve these goals.
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. 2nd ed. New York, NY: Appleton-Century-Crofts; 1929.
- Folkow B. Stress, hypothalamic function and neuroendocrine consequences. Acta Med Scand Suppl 1988; 723:61–69.
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 2005; 30:1050–1058.
- van der Kolk BA. The psychobiology and psychopharmacology of PTSD. Hum Psychopharmacol 2001; 16:S49–S64.
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcohol Clin Exp Res 1989;13:271–279.
- Peniston EG, Kulkosky PJ. Alcoholic personality and alpha-thetabrainwave training. Medical Psychotherapy: An International Journal1990; 3:37–55.
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave neurofeedbacktherapy for Vietnam veterans with combat-related posttraumaticstress disorder. Medical Psychotherapy: An International Journal1991; 4:47–60.
- Peniston EG, Kulkosky PJ. Alpha-theta EEG biofeedback trainingin alcoholism and posttraumatic stress disorder. The InternationalSociety for the Study of Subtle Energies and Energy Medicines1992; 2:5–7.
- Peniston EG, Marrinan DA, Deming WA, Kulkosky PJ. EEGalpha-theta brainwave synchronization in Vietnam theater veteranswith combat-related posttraumatic stress disorder and alcohol abuse.Medical Psychotherapy: An International Journal 1993; 6:37–50.
Posttraumatic stress disorder (PTSD) is a severe anxiety disorder whose symptoms emerge following exposure to extreme stress, such as those encountered in the battlefield or as a result of sexual abuse or natural disasters. The ability to employ coping mechanisms affects the disorder’s presentation as well as the frequency, intensity, and duration of the symptoms. The “Wounded Warrior” program at East Carolina University (Greenville, NC) was developed to promote the functional independence of US Marines, including those with PTSD.
STRESS RESPONSE: INTERACTION OF THE BRAIN AND IMMUNE SYSTEM
Walter Cannon coined the “flight or fight” response to stress in the early 20th century, in which he emphasized the importance of the parasympathetic system.1 In 1988, Folkow clarified the description as an immune response to stress.2 The stress response is now understood to be a neuroendocrine function that includes a feedback loop between the hypothalamus and the pituitary and adrenal glands; stimulation of the hypothalamus promotes secretion of corticotropin-releasing hormone (CRH) into the hypophyseal portal system, which supplies the anterior pituitarywith blood. CRH stimulates the secretion of adrenocorticotropic hormone into the bloodstream by the pituitary, prompting the adrenal glands to release the stress hormone cortisol.
Cortisol mobilizes the body’s defenses to meet the challenge of an adverse situation. It modulates the stress response by inhibiting the further release of CRH by the hypothalamus. Cortisol thus protects healthy cells and tissues by inhibiting an overreaction from the immune system. Without this protective effect, the interaction between the brain and the immune system can become dysregulated, increasing the risk of immune disorders.
THE CENTRAL AUTONOMIC NETWORK
The central nervous system that regulates the overall balance of the autonomic nervous system (ANS) has been called the central autonomic network (CAN).3 The CAN helps control executive, social, affective, attentional, and motivational functions. Therefore, the old paradigm of simply decreasing hyperarousal of the ANS to treat negative affective states and dispositions is inadequate. Instead, restoring the appropriate relationship between the ANS and the central nervous system is the aim behind interventions to treat PTSD.
Autonomic, cognitive, and affective functions assist humans in maintaining balance when confronted with external challenges. The CAN controls inhibitory or negative processes that permit specific behavior and redeploy resources needed elsewhere. When negative circuits are compromised, positive circuits develop, resulting in hypervigilance, the symptoms of which can be devastating and, if not ameliorated, can develop into permanent conditions. In one study,Vietnam veterans with PTSD had an 8% reduction in the volume of their right hippocampus compared with veterans without PTSD. Another study calculated a 26% reduction in the left hippocampus and a 22% reduction in the right in veterans with the most severe PTSD compared with veterans who were in combat but had no PTSD symptoms.4
A common subcortical neural system regulates defensive behavior, including autonomic, emotional, and cognitive behavior. When the prefrontal cortex is taken “off line” for whatever reason, parasympathetic inhibitory action is withdrawn, and relative sympathetic dominance, associated with defense, occurs.
CONFRONTING HYPERAROUSAL
The question then arises of how to train the ANS to avoid hypervigilance. Growing evidence supports the use of heart rate variability as a predictor of hypervigilance and inefficient allocation of attentional and cognitive resources.
The overall objective of heart rate variability training is to decrease ANS hyperarousal and to improve its balance. “Wounded warriors” learn to control ANS responses to stress-producing stimuli (eg, thoughts, memories, and images associated with combat). The goal of training is to decrease arousal and maintain ANS balance for increasing lengths of time.
Once it was observed that alpha waves were dysfunctional in vulnerable populations, protocols were developed to train alpha and theta waves as a method of improving function. Peniston and colleagues5–9 showed that increased alpha and theta brain wave production resulted in normalized personality measures and prolonged the period of time before relapse in alcoholics. This protocol has also shown efficacy as an intervention in depression and PTSD.
BIOFEEDBACK TRAINING PROGRAM
The US Department of Defense is studying a combination of central nervous system biofeedback with ANS biofeedback, with the goal of restoring and maintaining tone between the systems.
The training program used in the study lasts 1 month, and starts with a session for preassessment, 16 biofeedback sessions (four per week), a postprogram evaluation, and a 3-month followup. Each week, participants are exposed to stress-producing stimuli that increase in intensity:
- Week 1: Stroop Color Word Test, math stressor, talk stressor/everyday events
- Week 2: Talk stressor, combat experiences
- Week 3: Images and sounds of combat
- Week 4: Virtual Baghdad or Afghanistan (virtual reality exposure)
Each biofeedback session consists of 5 minutes of baseline evaluation; 5 minutes in which the veteran is subjected to the weekly stressor; 20 minutes of heart rate variability and neurofeedback training; 5 more minutes of training with the weekly stressor; 20 more minutes of heart rate variability and neurofeedback training; and finally 5 minutes of recovery.
SUMMARY
Dysfunction in the balance of both the ANS and central nervous system is associated with symptoms of PTSD in combat veterans. Methods that are designed to restore balance in these systems are needed to ameliorate these symptoms. Biofeedback and neurofeedback are safe methods with which to achieve these goals.
Posttraumatic stress disorder (PTSD) is a severe anxiety disorder whose symptoms emerge following exposure to extreme stress, such as those encountered in the battlefield or as a result of sexual abuse or natural disasters. The ability to employ coping mechanisms affects the disorder’s presentation as well as the frequency, intensity, and duration of the symptoms. The “Wounded Warrior” program at East Carolina University (Greenville, NC) was developed to promote the functional independence of US Marines, including those with PTSD.
STRESS RESPONSE: INTERACTION OF THE BRAIN AND IMMUNE SYSTEM
Walter Cannon coined the “flight or fight” response to stress in the early 20th century, in which he emphasized the importance of the parasympathetic system.1 In 1988, Folkow clarified the description as an immune response to stress.2 The stress response is now understood to be a neuroendocrine function that includes a feedback loop between the hypothalamus and the pituitary and adrenal glands; stimulation of the hypothalamus promotes secretion of corticotropin-releasing hormone (CRH) into the hypophyseal portal system, which supplies the anterior pituitarywith blood. CRH stimulates the secretion of adrenocorticotropic hormone into the bloodstream by the pituitary, prompting the adrenal glands to release the stress hormone cortisol.
Cortisol mobilizes the body’s defenses to meet the challenge of an adverse situation. It modulates the stress response by inhibiting the further release of CRH by the hypothalamus. Cortisol thus protects healthy cells and tissues by inhibiting an overreaction from the immune system. Without this protective effect, the interaction between the brain and the immune system can become dysregulated, increasing the risk of immune disorders.
THE CENTRAL AUTONOMIC NETWORK
The central nervous system that regulates the overall balance of the autonomic nervous system (ANS) has been called the central autonomic network (CAN).3 The CAN helps control executive, social, affective, attentional, and motivational functions. Therefore, the old paradigm of simply decreasing hyperarousal of the ANS to treat negative affective states and dispositions is inadequate. Instead, restoring the appropriate relationship between the ANS and the central nervous system is the aim behind interventions to treat PTSD.
Autonomic, cognitive, and affective functions assist humans in maintaining balance when confronted with external challenges. The CAN controls inhibitory or negative processes that permit specific behavior and redeploy resources needed elsewhere. When negative circuits are compromised, positive circuits develop, resulting in hypervigilance, the symptoms of which can be devastating and, if not ameliorated, can develop into permanent conditions. In one study,Vietnam veterans with PTSD had an 8% reduction in the volume of their right hippocampus compared with veterans without PTSD. Another study calculated a 26% reduction in the left hippocampus and a 22% reduction in the right in veterans with the most severe PTSD compared with veterans who were in combat but had no PTSD symptoms.4
A common subcortical neural system regulates defensive behavior, including autonomic, emotional, and cognitive behavior. When the prefrontal cortex is taken “off line” for whatever reason, parasympathetic inhibitory action is withdrawn, and relative sympathetic dominance, associated with defense, occurs.
CONFRONTING HYPERAROUSAL
The question then arises of how to train the ANS to avoid hypervigilance. Growing evidence supports the use of heart rate variability as a predictor of hypervigilance and inefficient allocation of attentional and cognitive resources.
The overall objective of heart rate variability training is to decrease ANS hyperarousal and to improve its balance. “Wounded warriors” learn to control ANS responses to stress-producing stimuli (eg, thoughts, memories, and images associated with combat). The goal of training is to decrease arousal and maintain ANS balance for increasing lengths of time.
Once it was observed that alpha waves were dysfunctional in vulnerable populations, protocols were developed to train alpha and theta waves as a method of improving function. Peniston and colleagues5–9 showed that increased alpha and theta brain wave production resulted in normalized personality measures and prolonged the period of time before relapse in alcoholics. This protocol has also shown efficacy as an intervention in depression and PTSD.
BIOFEEDBACK TRAINING PROGRAM
The US Department of Defense is studying a combination of central nervous system biofeedback with ANS biofeedback, with the goal of restoring and maintaining tone between the systems.
The training program used in the study lasts 1 month, and starts with a session for preassessment, 16 biofeedback sessions (four per week), a postprogram evaluation, and a 3-month followup. Each week, participants are exposed to stress-producing stimuli that increase in intensity:
- Week 1: Stroop Color Word Test, math stressor, talk stressor/everyday events
- Week 2: Talk stressor, combat experiences
- Week 3: Images and sounds of combat
- Week 4: Virtual Baghdad or Afghanistan (virtual reality exposure)
Each biofeedback session consists of 5 minutes of baseline evaluation; 5 minutes in which the veteran is subjected to the weekly stressor; 20 minutes of heart rate variability and neurofeedback training; 5 more minutes of training with the weekly stressor; 20 more minutes of heart rate variability and neurofeedback training; and finally 5 minutes of recovery.
SUMMARY
Dysfunction in the balance of both the ANS and central nervous system is associated with symptoms of PTSD in combat veterans. Methods that are designed to restore balance in these systems are needed to ameliorate these symptoms. Biofeedback and neurofeedback are safe methods with which to achieve these goals.
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. 2nd ed. New York, NY: Appleton-Century-Crofts; 1929.
- Folkow B. Stress, hypothalamic function and neuroendocrine consequences. Acta Med Scand Suppl 1988; 723:61–69.
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 2005; 30:1050–1058.
- van der Kolk BA. The psychobiology and psychopharmacology of PTSD. Hum Psychopharmacol 2001; 16:S49–S64.
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcohol Clin Exp Res 1989;13:271–279.
- Peniston EG, Kulkosky PJ. Alcoholic personality and alpha-thetabrainwave training. Medical Psychotherapy: An International Journal1990; 3:37–55.
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave neurofeedbacktherapy for Vietnam veterans with combat-related posttraumaticstress disorder. Medical Psychotherapy: An International Journal1991; 4:47–60.
- Peniston EG, Kulkosky PJ. Alpha-theta EEG biofeedback trainingin alcoholism and posttraumatic stress disorder. The InternationalSociety for the Study of Subtle Energies and Energy Medicines1992; 2:5–7.
- Peniston EG, Marrinan DA, Deming WA, Kulkosky PJ. EEGalpha-theta brainwave synchronization in Vietnam theater veteranswith combat-related posttraumatic stress disorder and alcohol abuse.Medical Psychotherapy: An International Journal 1993; 6:37–50.
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. 2nd ed. New York, NY: Appleton-Century-Crofts; 1929.
- Folkow B. Stress, hypothalamic function and neuroendocrine consequences. Acta Med Scand Suppl 1988; 723:61–69.
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 2005; 30:1050–1058.
- van der Kolk BA. The psychobiology and psychopharmacology of PTSD. Hum Psychopharmacol 2001; 16:S49–S64.
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcohol Clin Exp Res 1989;13:271–279.
- Peniston EG, Kulkosky PJ. Alcoholic personality and alpha-thetabrainwave training. Medical Psychotherapy: An International Journal1990; 3:37–55.
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave neurofeedbacktherapy for Vietnam veterans with combat-related posttraumaticstress disorder. Medical Psychotherapy: An International Journal1991; 4:47–60.
- Peniston EG, Kulkosky PJ. Alpha-theta EEG biofeedback trainingin alcoholism and posttraumatic stress disorder. The InternationalSociety for the Study of Subtle Energies and Energy Medicines1992; 2:5–7.
- Peniston EG, Marrinan DA, Deming WA, Kulkosky PJ. EEGalpha-theta brainwave synchronization in Vietnam theater veteranswith combat-related posttraumatic stress disorder and alcohol abuse.Medical Psychotherapy: An International Journal 1993; 6:37–50.
Stress in medicine: Strategies for caregivers, patients, clinicians—Panel discussion
Question from audience: Why does the Cleveland Clinic start its healing services program preoperatively rather than postoperatively?
Dr. Gillinov: We have a fairly well defined preoperative set of medical tests, and during this process nurses present patients with materials that explain the experience, and nurses and doctors make themselves available in special classes to answer patients’ questions. In doing so, we have increasingly identified patients preoperatively who have stress or problems.
Last week I saw a woman who had a leaking mitral valve, but her symptoms were out of proportion to her disease. She had loss of energy and appetite, and she wasn’t eating much. She was depressed and our team picked that up. She actually never had to undergo surgery. We referred her to a psychologist and, according to her son, she started to feel better. By starting preoperatively, we’re sometimes able to pick out things that we should treat instead of heart disease.
We also provide guided imagery and massage preoperatively.
Dr. Duffy: Healing services is on standing preoperative orders at the hospital. The team goes in proactively and asks, “In addition to your open heart surgery on Wednesday, is there anything we can do to support your emotional and spiritual journey here today?”
Terminology also matters. The term “healing services” is a safe umbrella under which we include biofeedback as one of the services, but it encompasses pastoral care, hospice care, and palliative care. The way it’s integrated into a care model is important. If it’s reserved for end of life, it might be viewed as defective or as a death sentence, so we want the healing services team to be proactive.
Question from audience: How does the primary care physician fit into all of this? I believe that if the physicians in the hospital want to gain patient confidence, they’ll show that they’re communicating well with the primary care physician.
Dr. Gevirtz: The primary care physicians are incredibly open to this idea. They have 12 minutes to deal with people with fibromyalgia, irritable bowel syndrome, chronic pain, noncardiac chest pain, etc. What are they going to do in 12 minutes? They’re grateful if they have a handoff, especially if it’s in the Clinic itself.
Question from audience: Are there any thoughts on making biofeedback part of general training rather than using it just for patients who’ve already experienced trauma?
Dr. Gevirtz: We did a study in which we showed that a biofeedback technician in the primary care setting saved the health maintenance system quite a lot of money, but the administration couldn’t decide whose territory to take to give us an office, so it ended the program.
Dr. Russoniello: How we enable greater access to our intervention is an important question. I see people quit the program if they can’t get access to biofeedback. In an effort to enhance compliance, we’ve incorporated biofeedback into video games, working with a couple of private companies to develop them.The idea is that persons playing the video game can accrue points to enhance their overall score if they perform paced breathing or some other form of biofeedback. Early indications from focus groups are that people will like this.
We have already shown in randomized controlled clinical studies of depression and anxiety that certain video games can improve mood and decrease stress.There is a big movement to get products in people’s hands to help them manage their health.
Question from audience: How much overlap is there between biofeedback methodologies—enhancing heart rate variability, vagal withdrawal, neurofeedback, and electroencephalographic feedback—in the systems you’re targeting and what are the unique contributions of each?
Dr. Gevirtz: We follow a stepped-care model. We start with the simplest and move on to the more complicated technologies. Two published studies with long-term followup showed the effectiveness of a learned breathing technique in alleviating noncardiac chest pain. Simple biofeedback wasn’t even needed. Three years later, the patients were better than they were at the end of the actual training. If you can do it simply, then you do it, and if it doesn’t work, then move on to more and more complicated techniques, with neurofeedback being the last resort.
Question from audience: Has anybody measured the physical impact of stimulating multiple systems on the study subject? In other words, can it be damaging to overstimulate these systems at the same time?
Dr. Gevirtz: We’ve been trying to do that. Recurrent abdominal pain or functional abdominal pain is the most common complaint to pediatric gastroenterologists. We have 1,800 patients a year who make it to the children’s hospital level with this complaint. These are kids who are suffering with very great pain and we we’re pretty sure it’s an autonomically mediated kind of phenomenon. We’re able to measure vagal activity in these kids in ambulatory settings at school and have found very little vagal activity before treatment. After training, they were able to restore vagal activity, and it correlated at the level of 0.63 with a reduction of symptoms. I think it’s important to try to tie the physiology to symptoms. It’s not always easy to do but we’re trying.
Question from audience: I’d like to pick up on two topics that Dr. Duffy raised: the business of medicine and the proposal for informed hope rather than an informed consent before surgery. Something that I see with patients and families at times is this magical expectation promoted by the business side that medicine can do these amazing and wonderful things and doesn’t have any sort of weaknesses. I wonder what role unrealistic expectations promoted by the media, advertising, and others may play in the stress of patients, caregivers, and physicians who need to try to meet the expectations of infallible medicine?
Dr. Duffy: We’ve spun so far the other way with our advanced technology that we’ve lost the human side, especially the concept of a relationship and giving people hope even though they have a terminal condition. It’s a balance between the art and the business of medicine. It’s about setting realistic expectations and realistic hope.
Question from audience: Why does the Cleveland Clinic start its healing services program preoperatively rather than postoperatively?
Dr. Gillinov: We have a fairly well defined preoperative set of medical tests, and during this process nurses present patients with materials that explain the experience, and nurses and doctors make themselves available in special classes to answer patients’ questions. In doing so, we have increasingly identified patients preoperatively who have stress or problems.
Last week I saw a woman who had a leaking mitral valve, but her symptoms were out of proportion to her disease. She had loss of energy and appetite, and she wasn’t eating much. She was depressed and our team picked that up. She actually never had to undergo surgery. We referred her to a psychologist and, according to her son, she started to feel better. By starting preoperatively, we’re sometimes able to pick out things that we should treat instead of heart disease.
We also provide guided imagery and massage preoperatively.
Dr. Duffy: Healing services is on standing preoperative orders at the hospital. The team goes in proactively and asks, “In addition to your open heart surgery on Wednesday, is there anything we can do to support your emotional and spiritual journey here today?”
Terminology also matters. The term “healing services” is a safe umbrella under which we include biofeedback as one of the services, but it encompasses pastoral care, hospice care, and palliative care. The way it’s integrated into a care model is important. If it’s reserved for end of life, it might be viewed as defective or as a death sentence, so we want the healing services team to be proactive.
Question from audience: How does the primary care physician fit into all of this? I believe that if the physicians in the hospital want to gain patient confidence, they’ll show that they’re communicating well with the primary care physician.
Dr. Gevirtz: The primary care physicians are incredibly open to this idea. They have 12 minutes to deal with people with fibromyalgia, irritable bowel syndrome, chronic pain, noncardiac chest pain, etc. What are they going to do in 12 minutes? They’re grateful if they have a handoff, especially if it’s in the Clinic itself.
Question from audience: Are there any thoughts on making biofeedback part of general training rather than using it just for patients who’ve already experienced trauma?
Dr. Gevirtz: We did a study in which we showed that a biofeedback technician in the primary care setting saved the health maintenance system quite a lot of money, but the administration couldn’t decide whose territory to take to give us an office, so it ended the program.
Dr. Russoniello: How we enable greater access to our intervention is an important question. I see people quit the program if they can’t get access to biofeedback. In an effort to enhance compliance, we’ve incorporated biofeedback into video games, working with a couple of private companies to develop them.The idea is that persons playing the video game can accrue points to enhance their overall score if they perform paced breathing or some other form of biofeedback. Early indications from focus groups are that people will like this.
We have already shown in randomized controlled clinical studies of depression and anxiety that certain video games can improve mood and decrease stress.There is a big movement to get products in people’s hands to help them manage their health.
Question from audience: How much overlap is there between biofeedback methodologies—enhancing heart rate variability, vagal withdrawal, neurofeedback, and electroencephalographic feedback—in the systems you’re targeting and what are the unique contributions of each?
Dr. Gevirtz: We follow a stepped-care model. We start with the simplest and move on to the more complicated technologies. Two published studies with long-term followup showed the effectiveness of a learned breathing technique in alleviating noncardiac chest pain. Simple biofeedback wasn’t even needed. Three years later, the patients were better than they were at the end of the actual training. If you can do it simply, then you do it, and if it doesn’t work, then move on to more and more complicated techniques, with neurofeedback being the last resort.
Question from audience: Has anybody measured the physical impact of stimulating multiple systems on the study subject? In other words, can it be damaging to overstimulate these systems at the same time?
Dr. Gevirtz: We’ve been trying to do that. Recurrent abdominal pain or functional abdominal pain is the most common complaint to pediatric gastroenterologists. We have 1,800 patients a year who make it to the children’s hospital level with this complaint. These are kids who are suffering with very great pain and we we’re pretty sure it’s an autonomically mediated kind of phenomenon. We’re able to measure vagal activity in these kids in ambulatory settings at school and have found very little vagal activity before treatment. After training, they were able to restore vagal activity, and it correlated at the level of 0.63 with a reduction of symptoms. I think it’s important to try to tie the physiology to symptoms. It’s not always easy to do but we’re trying.
Question from audience: I’d like to pick up on two topics that Dr. Duffy raised: the business of medicine and the proposal for informed hope rather than an informed consent before surgery. Something that I see with patients and families at times is this magical expectation promoted by the business side that medicine can do these amazing and wonderful things and doesn’t have any sort of weaknesses. I wonder what role unrealistic expectations promoted by the media, advertising, and others may play in the stress of patients, caregivers, and physicians who need to try to meet the expectations of infallible medicine?
Dr. Duffy: We’ve spun so far the other way with our advanced technology that we’ve lost the human side, especially the concept of a relationship and giving people hope even though they have a terminal condition. It’s a balance between the art and the business of medicine. It’s about setting realistic expectations and realistic hope.
Question from audience: Why does the Cleveland Clinic start its healing services program preoperatively rather than postoperatively?
Dr. Gillinov: We have a fairly well defined preoperative set of medical tests, and during this process nurses present patients with materials that explain the experience, and nurses and doctors make themselves available in special classes to answer patients’ questions. In doing so, we have increasingly identified patients preoperatively who have stress or problems.
Last week I saw a woman who had a leaking mitral valve, but her symptoms were out of proportion to her disease. She had loss of energy and appetite, and she wasn’t eating much. She was depressed and our team picked that up. She actually never had to undergo surgery. We referred her to a psychologist and, according to her son, she started to feel better. By starting preoperatively, we’re sometimes able to pick out things that we should treat instead of heart disease.
We also provide guided imagery and massage preoperatively.
Dr. Duffy: Healing services is on standing preoperative orders at the hospital. The team goes in proactively and asks, “In addition to your open heart surgery on Wednesday, is there anything we can do to support your emotional and spiritual journey here today?”
Terminology also matters. The term “healing services” is a safe umbrella under which we include biofeedback as one of the services, but it encompasses pastoral care, hospice care, and palliative care. The way it’s integrated into a care model is important. If it’s reserved for end of life, it might be viewed as defective or as a death sentence, so we want the healing services team to be proactive.
Question from audience: How does the primary care physician fit into all of this? I believe that if the physicians in the hospital want to gain patient confidence, they’ll show that they’re communicating well with the primary care physician.
Dr. Gevirtz: The primary care physicians are incredibly open to this idea. They have 12 minutes to deal with people with fibromyalgia, irritable bowel syndrome, chronic pain, noncardiac chest pain, etc. What are they going to do in 12 minutes? They’re grateful if they have a handoff, especially if it’s in the Clinic itself.
Question from audience: Are there any thoughts on making biofeedback part of general training rather than using it just for patients who’ve already experienced trauma?
Dr. Gevirtz: We did a study in which we showed that a biofeedback technician in the primary care setting saved the health maintenance system quite a lot of money, but the administration couldn’t decide whose territory to take to give us an office, so it ended the program.
Dr. Russoniello: How we enable greater access to our intervention is an important question. I see people quit the program if they can’t get access to biofeedback. In an effort to enhance compliance, we’ve incorporated biofeedback into video games, working with a couple of private companies to develop them.The idea is that persons playing the video game can accrue points to enhance their overall score if they perform paced breathing or some other form of biofeedback. Early indications from focus groups are that people will like this.
We have already shown in randomized controlled clinical studies of depression and anxiety that certain video games can improve mood and decrease stress.There is a big movement to get products in people’s hands to help them manage their health.
Question from audience: How much overlap is there between biofeedback methodologies—enhancing heart rate variability, vagal withdrawal, neurofeedback, and electroencephalographic feedback—in the systems you’re targeting and what are the unique contributions of each?
Dr. Gevirtz: We follow a stepped-care model. We start with the simplest and move on to the more complicated technologies. Two published studies with long-term followup showed the effectiveness of a learned breathing technique in alleviating noncardiac chest pain. Simple biofeedback wasn’t even needed. Three years later, the patients were better than they were at the end of the actual training. If you can do it simply, then you do it, and if it doesn’t work, then move on to more and more complicated techniques, with neurofeedback being the last resort.
Question from audience: Has anybody measured the physical impact of stimulating multiple systems on the study subject? In other words, can it be damaging to overstimulate these systems at the same time?
Dr. Gevirtz: We’ve been trying to do that. Recurrent abdominal pain or functional abdominal pain is the most common complaint to pediatric gastroenterologists. We have 1,800 patients a year who make it to the children’s hospital level with this complaint. These are kids who are suffering with very great pain and we we’re pretty sure it’s an autonomically mediated kind of phenomenon. We’re able to measure vagal activity in these kids in ambulatory settings at school and have found very little vagal activity before treatment. After training, they were able to restore vagal activity, and it correlated at the level of 0.63 with a reduction of symptoms. I think it’s important to try to tie the physiology to symptoms. It’s not always easy to do but we’re trying.
Question from audience: I’d like to pick up on two topics that Dr. Duffy raised: the business of medicine and the proposal for informed hope rather than an informed consent before surgery. Something that I see with patients and families at times is this magical expectation promoted by the business side that medicine can do these amazing and wonderful things and doesn’t have any sort of weaknesses. I wonder what role unrealistic expectations promoted by the media, advertising, and others may play in the stress of patients, caregivers, and physicians who need to try to meet the expectations of infallible medicine?
Dr. Duffy: We’ve spun so far the other way with our advanced technology that we’ve lost the human side, especially the concept of a relationship and giving people hope even though they have a terminal condition. It’s a balance between the art and the business of medicine. It’s about setting realistic expectations and realistic hope.
Key 2010 publications in behavioral medicine
The effect of emotion on the heart is not confined to depression, but extends to a variety of mental states; as William Harvey described in 1628, “A mental disturbance provoking pain, excessive joy, hope or anxiety extends to the heart, where it affects its temper and rate, impairing general nutrition and vigor.”
In going beyond the well-established role of depression as a risk factor for heart disease, 2010 delivered several important publications recognizing anxiety, anger, and other forms of distress as key factors in the etiology of coronary heart disease (CHD). Other papers of merit elucidated new and overlooked insights into the pathways linking psychosocial stress and cardiovascular risk, and also considered psychologic states that appear to promote healthy functioning.
IMPACT OF NEGATIVE EMOTIONS ON RISK OF INCIDENT CORONARY HEART DISEASE
In a meta-analysis of 20 prospective studies that included 249,846 persons with a mean follow-up of 11.2 years, Roest et al1 examined the impact of anxiety characterized by the presence of anxiety symptoms or a diagnosis of anxiety disorder on incident CHD. Most of the studies adjusted for a broad array of relevant potential confounders. Findings suggest the presence of anxiety increases the risk of incident CHD by 26% (P P = .003).
In a meta-analysis of 25 prospective studies of 7,160 persons with a mean follow-up exceeding 10 years, Chida and Steptoe2 found that anger increased the risk of incident CHD by 19%, after adjustment for standard coronary risk factors. The effect was less stable than that associated with anxiety and depression, and when stratified by gender, the harmful effects of anger were more evident in men than in women. The effect of anger was attenuated when controlling for behavioral covariates. The association between anger and CHD did not hold for all ways of measuring anger, which suggests that the type of anger or the ability to regulate anger may be relevant to the relationship.
A study that did account for the type of anger expression on the risk of incident CHD was conducted by Davidson and Mostofsky.3 The independent effect of three distinct types of anger expression (constructive anger, destructive anger justification, and destructive anger rumination) on 10-year incident CHD was examined, controlling for other psychosocial factors. In men, higher scores for constructive anger were associated with a lower rate of CHD; in both men and women, higher scores for destructive anger justification were associated with an increased risk of CHD.
Insights gained from these studies are as follows:
- The impact of anxiety appears to be comparable to depression, and the effects of anxiety and depression are largely independent.
- If anxiety and depression co-occur, the effect on CHD is synergistic.
- The effects of anger are less clear; its impact may be independent of or dependent on other forms of psychologic distress.
- Distress in general appears to serve as a signal that something is wrong and needs to be addressed. If ignored, it may become chronic and unremitting; because symptoms of distress may lead to systemic dysregulation and increased CHD risk, they may indicate the need for increased surveillance and intervention.
WHY FOCUS ON THE BIOLOGY OF EMOTIONS?
A clear biologic explanation for the influence of emotional factors on physical health would serve to assuage skeptics who doubt that such a link exists or who attribute a common underlying genetic trait to both negative affect and heart disease. Further, focusing on the biology may help answer key questions with respect to emotions and disease processes: What is the damage incurred by negative emotional states and is it reversible? Can compensatory pathways be activated to bypass the mechanisms causing damage or slow the progression of disease?
Cardiac response to worry and stress
In one study attempting to shed light on relevant emotion-related biologic process, the prolonged physiologic effects of worry were examined. Worry episodes and stressful events were recorded hourly along with ambulatory heart rate and heart rate variability in 73 teachers for 4 days.4 Autonomic activity, as reflected by a concurrent elevation in heart rate and a decrease in heart rate variability, was increased up to 2 hours after a worry episode. The findings also suggested that the prolonged cardiac effects of separate worry episodes were independent.
Another study sought to determine whether heightened reactivity or delayed recovery to acute stress increases risk of cardiovascular disease.5 This meta-analysis included 36 studies to assess whether acute cardiovascular response to various laboratory stressors (ie, cognitive tasks, stress interviews, public speaking). Findings indicated that heightened cardiovascular reactivity was associated with worse cardiovascular outcomes, such as incident hypertension, coronary calcification, carotid intima-media thickness, and cardiovascular events over time.
Role of aldosterone overlooked
WHY CONSIDER RESILIENCE?
Because the absence of a deficit is not the same as the presence of an asset, greater insight into dysfunction may be gained by explicitly considering what promotes healthy functioning. Ameliorating distress has proven difficult; so, in studying resilience (including the ability to regulate affect), new targets for prevention and intervention may be identified. Although no meta-analysis of resilience factors has been published to date owing to the paucity of data, the studies that have been performed are generally rigorous and have demonstrated consistent findings.
For example, one prospective, well-controlled study of 1,739 men and women demonstrated a protective effect of positive affect (as ascertained by structured interview) against 10-year incident CHD.6 The risk of fatal or nonfatal ischemic heart disease events was reduced by 22% (P = .02) for each 1-point increase in positive affect, even after controlling for depression and negative emotions.
Biology of resilience: Counteracting cellular damage
Genomic changes can be induced by the relaxation response, as evidenced by the differential gene expression profiles of long-term daily practitioners of relaxation (ie, meditation, yoga), short-term (8-week) practitioners of relaxation, and healthy controls.8 Alterations in cellular metabolism, oxidative phosphorylation, and generation of reactive oxygen species that counteract proinflammatory responses, indicative of an adaptive response, were observed in both groups that practiced relaxation.
FUTURE DIRECTIONS
Whether and how the sources and effects of psychosocial stress and response to treatment differ across men and women deserves closer examination. A review by Low et al9 summarizes the current state of knowledge with respect to psychosocial factors and heart disease in women, noting that the sources of stress associated with increased CHD risk differ across men and women; psychosocial risk factors like depression and anxiety appear to increase risk for both men and women; work-related stress has larger effects in men while stress related to relationships and family responsibilities appear to have larger effects in women.
Although responses to psychosocial stress are not clearly different between men and women, intervention targeted at reducing distress is much less effective in reducing the risk of adverse events in women versus men. The mechanism to explain this difference in effectiveness of intervention urgently requires further exploration.
In conducting this work, several factors are important. The best time to intervene to reduce psychosocial distress is unknown; a key consideration will be, what is the best etiologic window for intervention? Perhaps a life-course approach that targets individuals with chronically high levels of emotional distress who also have multiple coronary risk factors, and that enhances their capacity to regulate emotions would prove superior to waiting until late in the disease process.
Another area that may prove fruitful is to consider in more depth the biology of the placebo effect and whether and how it may inform our understanding of resilience.
More generally, considering why interventions seem to influence outcomes so differently across men and women, applying a life course approach to determine the best etiologic window for prevention and intervention strategies, and conducting a more in-depth exploration of the biology of resilience may lead to improved capacity for population-based approaches to reducing the burden of CHD.
- Roest AM, Martens E, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol 2010; 56:38–46.
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol 2009; 53:936–946.
- Davidson KW, Mostofsky E. Anger expression and risk of coronary heart disease: evidence from the Nova Scotia Health Survey. Am Heart J 2010; 159:199–206.
- Pieper S, Brosschot JF, van der Leeden R, Thayer J. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosom Med 2010; 72:570–577.
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 2010; 55:1026–1032.
- Davidson KW, Mostofsky E, Whang W. Don’t worry, be happy: positive affect and reduced 10-year incident coronary heart disease: the Canadian Nova Scotia Health Survey. Eur Heart J 2010; 31:1065–1070.
- Kubzansky LD, Park N, Peterson C, Vokonas P, Sparrow D. Healthy psychological functioning and incident coronary heart disease. Arch Gen Psychiatry 2000; 68:400–408.
- Dusek JA, Out HH, Wohlhueter AL, et al Genomic counterstress changes induced by the relaxation response. PLoS One 2008; 3:e2576.
- Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med 2010; 72:842–854.
The effect of emotion on the heart is not confined to depression, but extends to a variety of mental states; as William Harvey described in 1628, “A mental disturbance provoking pain, excessive joy, hope or anxiety extends to the heart, where it affects its temper and rate, impairing general nutrition and vigor.”
In going beyond the well-established role of depression as a risk factor for heart disease, 2010 delivered several important publications recognizing anxiety, anger, and other forms of distress as key factors in the etiology of coronary heart disease (CHD). Other papers of merit elucidated new and overlooked insights into the pathways linking psychosocial stress and cardiovascular risk, and also considered psychologic states that appear to promote healthy functioning.
IMPACT OF NEGATIVE EMOTIONS ON RISK OF INCIDENT CORONARY HEART DISEASE
In a meta-analysis of 20 prospective studies that included 249,846 persons with a mean follow-up of 11.2 years, Roest et al1 examined the impact of anxiety characterized by the presence of anxiety symptoms or a diagnosis of anxiety disorder on incident CHD. Most of the studies adjusted for a broad array of relevant potential confounders. Findings suggest the presence of anxiety increases the risk of incident CHD by 26% (P P = .003).
In a meta-analysis of 25 prospective studies of 7,160 persons with a mean follow-up exceeding 10 years, Chida and Steptoe2 found that anger increased the risk of incident CHD by 19%, after adjustment for standard coronary risk factors. The effect was less stable than that associated with anxiety and depression, and when stratified by gender, the harmful effects of anger were more evident in men than in women. The effect of anger was attenuated when controlling for behavioral covariates. The association between anger and CHD did not hold for all ways of measuring anger, which suggests that the type of anger or the ability to regulate anger may be relevant to the relationship.
A study that did account for the type of anger expression on the risk of incident CHD was conducted by Davidson and Mostofsky.3 The independent effect of three distinct types of anger expression (constructive anger, destructive anger justification, and destructive anger rumination) on 10-year incident CHD was examined, controlling for other psychosocial factors. In men, higher scores for constructive anger were associated with a lower rate of CHD; in both men and women, higher scores for destructive anger justification were associated with an increased risk of CHD.
Insights gained from these studies are as follows:
- The impact of anxiety appears to be comparable to depression, and the effects of anxiety and depression are largely independent.
- If anxiety and depression co-occur, the effect on CHD is synergistic.
- The effects of anger are less clear; its impact may be independent of or dependent on other forms of psychologic distress.
- Distress in general appears to serve as a signal that something is wrong and needs to be addressed. If ignored, it may become chronic and unremitting; because symptoms of distress may lead to systemic dysregulation and increased CHD risk, they may indicate the need for increased surveillance and intervention.
WHY FOCUS ON THE BIOLOGY OF EMOTIONS?
A clear biologic explanation for the influence of emotional factors on physical health would serve to assuage skeptics who doubt that such a link exists or who attribute a common underlying genetic trait to both negative affect and heart disease. Further, focusing on the biology may help answer key questions with respect to emotions and disease processes: What is the damage incurred by negative emotional states and is it reversible? Can compensatory pathways be activated to bypass the mechanisms causing damage or slow the progression of disease?
Cardiac response to worry and stress
In one study attempting to shed light on relevant emotion-related biologic process, the prolonged physiologic effects of worry were examined. Worry episodes and stressful events were recorded hourly along with ambulatory heart rate and heart rate variability in 73 teachers for 4 days.4 Autonomic activity, as reflected by a concurrent elevation in heart rate and a decrease in heart rate variability, was increased up to 2 hours after a worry episode. The findings also suggested that the prolonged cardiac effects of separate worry episodes were independent.
Another study sought to determine whether heightened reactivity or delayed recovery to acute stress increases risk of cardiovascular disease.5 This meta-analysis included 36 studies to assess whether acute cardiovascular response to various laboratory stressors (ie, cognitive tasks, stress interviews, public speaking). Findings indicated that heightened cardiovascular reactivity was associated with worse cardiovascular outcomes, such as incident hypertension, coronary calcification, carotid intima-media thickness, and cardiovascular events over time.
Role of aldosterone overlooked
WHY CONSIDER RESILIENCE?
Because the absence of a deficit is not the same as the presence of an asset, greater insight into dysfunction may be gained by explicitly considering what promotes healthy functioning. Ameliorating distress has proven difficult; so, in studying resilience (including the ability to regulate affect), new targets for prevention and intervention may be identified. Although no meta-analysis of resilience factors has been published to date owing to the paucity of data, the studies that have been performed are generally rigorous and have demonstrated consistent findings.
For example, one prospective, well-controlled study of 1,739 men and women demonstrated a protective effect of positive affect (as ascertained by structured interview) against 10-year incident CHD.6 The risk of fatal or nonfatal ischemic heart disease events was reduced by 22% (P = .02) for each 1-point increase in positive affect, even after controlling for depression and negative emotions.
Biology of resilience: Counteracting cellular damage
Genomic changes can be induced by the relaxation response, as evidenced by the differential gene expression profiles of long-term daily practitioners of relaxation (ie, meditation, yoga), short-term (8-week) practitioners of relaxation, and healthy controls.8 Alterations in cellular metabolism, oxidative phosphorylation, and generation of reactive oxygen species that counteract proinflammatory responses, indicative of an adaptive response, were observed in both groups that practiced relaxation.
FUTURE DIRECTIONS
Whether and how the sources and effects of psychosocial stress and response to treatment differ across men and women deserves closer examination. A review by Low et al9 summarizes the current state of knowledge with respect to psychosocial factors and heart disease in women, noting that the sources of stress associated with increased CHD risk differ across men and women; psychosocial risk factors like depression and anxiety appear to increase risk for both men and women; work-related stress has larger effects in men while stress related to relationships and family responsibilities appear to have larger effects in women.
Although responses to psychosocial stress are not clearly different between men and women, intervention targeted at reducing distress is much less effective in reducing the risk of adverse events in women versus men. The mechanism to explain this difference in effectiveness of intervention urgently requires further exploration.
In conducting this work, several factors are important. The best time to intervene to reduce psychosocial distress is unknown; a key consideration will be, what is the best etiologic window for intervention? Perhaps a life-course approach that targets individuals with chronically high levels of emotional distress who also have multiple coronary risk factors, and that enhances their capacity to regulate emotions would prove superior to waiting until late in the disease process.
Another area that may prove fruitful is to consider in more depth the biology of the placebo effect and whether and how it may inform our understanding of resilience.
More generally, considering why interventions seem to influence outcomes so differently across men and women, applying a life course approach to determine the best etiologic window for prevention and intervention strategies, and conducting a more in-depth exploration of the biology of resilience may lead to improved capacity for population-based approaches to reducing the burden of CHD.
The effect of emotion on the heart is not confined to depression, but extends to a variety of mental states; as William Harvey described in 1628, “A mental disturbance provoking pain, excessive joy, hope or anxiety extends to the heart, where it affects its temper and rate, impairing general nutrition and vigor.”
In going beyond the well-established role of depression as a risk factor for heart disease, 2010 delivered several important publications recognizing anxiety, anger, and other forms of distress as key factors in the etiology of coronary heart disease (CHD). Other papers of merit elucidated new and overlooked insights into the pathways linking psychosocial stress and cardiovascular risk, and also considered psychologic states that appear to promote healthy functioning.
IMPACT OF NEGATIVE EMOTIONS ON RISK OF INCIDENT CORONARY HEART DISEASE
In a meta-analysis of 20 prospective studies that included 249,846 persons with a mean follow-up of 11.2 years, Roest et al1 examined the impact of anxiety characterized by the presence of anxiety symptoms or a diagnosis of anxiety disorder on incident CHD. Most of the studies adjusted for a broad array of relevant potential confounders. Findings suggest the presence of anxiety increases the risk of incident CHD by 26% (P P = .003).
In a meta-analysis of 25 prospective studies of 7,160 persons with a mean follow-up exceeding 10 years, Chida and Steptoe2 found that anger increased the risk of incident CHD by 19%, after adjustment for standard coronary risk factors. The effect was less stable than that associated with anxiety and depression, and when stratified by gender, the harmful effects of anger were more evident in men than in women. The effect of anger was attenuated when controlling for behavioral covariates. The association between anger and CHD did not hold for all ways of measuring anger, which suggests that the type of anger or the ability to regulate anger may be relevant to the relationship.
A study that did account for the type of anger expression on the risk of incident CHD was conducted by Davidson and Mostofsky.3 The independent effect of three distinct types of anger expression (constructive anger, destructive anger justification, and destructive anger rumination) on 10-year incident CHD was examined, controlling for other psychosocial factors. In men, higher scores for constructive anger were associated with a lower rate of CHD; in both men and women, higher scores for destructive anger justification were associated with an increased risk of CHD.
Insights gained from these studies are as follows:
- The impact of anxiety appears to be comparable to depression, and the effects of anxiety and depression are largely independent.
- If anxiety and depression co-occur, the effect on CHD is synergistic.
- The effects of anger are less clear; its impact may be independent of or dependent on other forms of psychologic distress.
- Distress in general appears to serve as a signal that something is wrong and needs to be addressed. If ignored, it may become chronic and unremitting; because symptoms of distress may lead to systemic dysregulation and increased CHD risk, they may indicate the need for increased surveillance and intervention.
WHY FOCUS ON THE BIOLOGY OF EMOTIONS?
A clear biologic explanation for the influence of emotional factors on physical health would serve to assuage skeptics who doubt that such a link exists or who attribute a common underlying genetic trait to both negative affect and heart disease. Further, focusing on the biology may help answer key questions with respect to emotions and disease processes: What is the damage incurred by negative emotional states and is it reversible? Can compensatory pathways be activated to bypass the mechanisms causing damage or slow the progression of disease?
Cardiac response to worry and stress
In one study attempting to shed light on relevant emotion-related biologic process, the prolonged physiologic effects of worry were examined. Worry episodes and stressful events were recorded hourly along with ambulatory heart rate and heart rate variability in 73 teachers for 4 days.4 Autonomic activity, as reflected by a concurrent elevation in heart rate and a decrease in heart rate variability, was increased up to 2 hours after a worry episode. The findings also suggested that the prolonged cardiac effects of separate worry episodes were independent.
Another study sought to determine whether heightened reactivity or delayed recovery to acute stress increases risk of cardiovascular disease.5 This meta-analysis included 36 studies to assess whether acute cardiovascular response to various laboratory stressors (ie, cognitive tasks, stress interviews, public speaking). Findings indicated that heightened cardiovascular reactivity was associated with worse cardiovascular outcomes, such as incident hypertension, coronary calcification, carotid intima-media thickness, and cardiovascular events over time.
Role of aldosterone overlooked
WHY CONSIDER RESILIENCE?
Because the absence of a deficit is not the same as the presence of an asset, greater insight into dysfunction may be gained by explicitly considering what promotes healthy functioning. Ameliorating distress has proven difficult; so, in studying resilience (including the ability to regulate affect), new targets for prevention and intervention may be identified. Although no meta-analysis of resilience factors has been published to date owing to the paucity of data, the studies that have been performed are generally rigorous and have demonstrated consistent findings.
For example, one prospective, well-controlled study of 1,739 men and women demonstrated a protective effect of positive affect (as ascertained by structured interview) against 10-year incident CHD.6 The risk of fatal or nonfatal ischemic heart disease events was reduced by 22% (P = .02) for each 1-point increase in positive affect, even after controlling for depression and negative emotions.
Biology of resilience: Counteracting cellular damage
Genomic changes can be induced by the relaxation response, as evidenced by the differential gene expression profiles of long-term daily practitioners of relaxation (ie, meditation, yoga), short-term (8-week) practitioners of relaxation, and healthy controls.8 Alterations in cellular metabolism, oxidative phosphorylation, and generation of reactive oxygen species that counteract proinflammatory responses, indicative of an adaptive response, were observed in both groups that practiced relaxation.
FUTURE DIRECTIONS
Whether and how the sources and effects of psychosocial stress and response to treatment differ across men and women deserves closer examination. A review by Low et al9 summarizes the current state of knowledge with respect to psychosocial factors and heart disease in women, noting that the sources of stress associated with increased CHD risk differ across men and women; psychosocial risk factors like depression and anxiety appear to increase risk for both men and women; work-related stress has larger effects in men while stress related to relationships and family responsibilities appear to have larger effects in women.
Although responses to psychosocial stress are not clearly different between men and women, intervention targeted at reducing distress is much less effective in reducing the risk of adverse events in women versus men. The mechanism to explain this difference in effectiveness of intervention urgently requires further exploration.
In conducting this work, several factors are important. The best time to intervene to reduce psychosocial distress is unknown; a key consideration will be, what is the best etiologic window for intervention? Perhaps a life-course approach that targets individuals with chronically high levels of emotional distress who also have multiple coronary risk factors, and that enhances their capacity to regulate emotions would prove superior to waiting until late in the disease process.
Another area that may prove fruitful is to consider in more depth the biology of the placebo effect and whether and how it may inform our understanding of resilience.
More generally, considering why interventions seem to influence outcomes so differently across men and women, applying a life course approach to determine the best etiologic window for prevention and intervention strategies, and conducting a more in-depth exploration of the biology of resilience may lead to improved capacity for population-based approaches to reducing the burden of CHD.
- Roest AM, Martens E, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol 2010; 56:38–46.
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol 2009; 53:936–946.
- Davidson KW, Mostofsky E. Anger expression and risk of coronary heart disease: evidence from the Nova Scotia Health Survey. Am Heart J 2010; 159:199–206.
- Pieper S, Brosschot JF, van der Leeden R, Thayer J. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosom Med 2010; 72:570–577.
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 2010; 55:1026–1032.
- Davidson KW, Mostofsky E, Whang W. Don’t worry, be happy: positive affect and reduced 10-year incident coronary heart disease: the Canadian Nova Scotia Health Survey. Eur Heart J 2010; 31:1065–1070.
- Kubzansky LD, Park N, Peterson C, Vokonas P, Sparrow D. Healthy psychological functioning and incident coronary heart disease. Arch Gen Psychiatry 2000; 68:400–408.
- Dusek JA, Out HH, Wohlhueter AL, et al Genomic counterstress changes induced by the relaxation response. PLoS One 2008; 3:e2576.
- Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med 2010; 72:842–854.
- Roest AM, Martens E, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol 2010; 56:38–46.
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol 2009; 53:936–946.
- Davidson KW, Mostofsky E. Anger expression and risk of coronary heart disease: evidence from the Nova Scotia Health Survey. Am Heart J 2010; 159:199–206.
- Pieper S, Brosschot JF, van der Leeden R, Thayer J. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosom Med 2010; 72:570–577.
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 2010; 55:1026–1032.
- Davidson KW, Mostofsky E, Whang W. Don’t worry, be happy: positive affect and reduced 10-year incident coronary heart disease: the Canadian Nova Scotia Health Survey. Eur Heart J 2010; 31:1065–1070.
- Kubzansky LD, Park N, Peterson C, Vokonas P, Sparrow D. Healthy psychological functioning and incident coronary heart disease. Arch Gen Psychiatry 2000; 68:400–408.
- Dusek JA, Out HH, Wohlhueter AL, et al Genomic counterstress changes induced by the relaxation response. PLoS One 2008; 3:e2576.
- Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med 2010; 72:842–854.
Imaging for autonomic dysfunction
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10
FUNCTIONAL BRAIN IMAGING IN GENERAL
Direct visualization of heart-brain interactions is the goal when assessing ANS function. Positron emission tomography (PET) produces quantitative images, but spatial and temporal resolutions are vastly superior with fMRI.11 Further, radiation exposure is low with fMRI, allowing for safe repeat imaging.
Ogawa et al12 first demonstrated that in vivo images of brain microvasculature are affected by blood oxygen level, and that blood oxygenation reduced vascular signal loss. Therefore, blood oxygenation level–dependent (BOLD) contrast added to MRI could complement PET-like measurements in the study of regional brain activity.
Bilateral finger tapping with intermittent periods of rest is associated with a pattern of increasing and decreasing intensity of fMRI signals in involved brain regions that reflect the periods of activity and rest. This technique has been used to locate brain voxels with similar patterns of activity, enabling the creation of familiar color brain mapping. A challenge posed by autonomic fMRI in such brain mapping is that fMRI is susceptible to artifacts (Figure 3). For example, a movement of the head as little as 1 mm inside the MRI scanner—a distance comparable to the size of autonomic structures—can produce a motion artifact (false activation of brain regions) that can affect statistical significance. In addition, many ANS regions of the brain are near osseous structures (for example the brainstem and skull base) that cause signal distortion and loss.
REQUIREMENTS FOR AUTONOMIC fMRI
The tasks chosen to visualize brain control of autonomic function must naturally elicit an autonomic response. The difficulty is that untrained persons have little or no volitional control over autonomic functions, so the task and its analysis must be designed carefully and be MRI-compatible. Any motion will degrade the image; further, the capacity for the MRI environment to corrupt the measurements can limit the potential tasks for measurement.
Possible stimuli for eliciting a sympathetic response include pain, fear, anticipation, anxiety, concentration or memory, cold pressor, Stroop test, breathing tests, and maximal hand grip. Examples of parasympathetic stimuli are the Valsalva maneuver and paced breathing. The responses to stimuli (ie, heart rate, heart rate variability, blood pressure, galvanic skin response, papillary response) must be monitored to compare the data obtained from fMRI. MRI-compatible equipment is now available for measuring many of these responses.
Identifying areas activated during tasks
Functional neuroimaging with PET and fMRI has shown consistently that the anterior cingulate is activated during multiple tasks designed to elicit an autonomic response (gambling anticipation, emotional response to faces, Stroop test).11
In a study designed to test autonomic interoceptive awareness, subjects underwent fMRI while they were asked to judge the timing of their heartbeats to auditory tones that were either synchronized with their heartbeat or delayed by 500 msec.13 Areas of enhanced activity during the task were the right insular cortex, anterior cingulate, parietal lobes, and operculum.
Characterizing brainstem sites
It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses. These regions are small and motion artifacts are common because of brainstem movement with the cardiac pulse. With fMRI, Topolovec et al14 were able to characterize brainstem sites involved in autonomic control, demonstrating activation of the nucleus of the solitary tract and parabrachial nucleus.
A review of four fMRI studies of stressor-evoked blood pressure reactivity demonstrated activation in corticolimbic areas, including the cingulate cortex, insula, amygdala, and cortical and subcortical areas that are involved in hemodynamic and metabolic support for stress-related behavioral responses.16
FUNCTIONAL BRAIN IMAGING IN DISEASE STATES
There are few studies of functional brain imaging in patients with disease because of the challenges involved. The studies are difficult to perform on sick patients because of the unfriendly MRI environment, with struct requirements for attention and participation. Furthermore, autonomic responses may be blunted, making physiologic comparisons difficult. In addition, there is evidence that BOLD may be intrinsically impaired in disease states. Unlike fMRI studies to locate brain regions involved in simple tasks such as finger tapping, which can be performed in a single subject, detecting changes in autonomic responses in disease states requires averaging over studies of multiple patients.
Woo et al17 used fMRI to compare brain regions of activation in six patients with heart failure and 16 controls upon a forehead cold pressor challenge. Increases in heart rate were measured in the patients with heart failure with application of the cold stimulus. Larger neural fMRI signal responses in patients with heart failure were observed in 14 brain regions, whereas reduced fMRI activity was observed in 15 other brain regions in the heart failure patients. Based on the results, the investigators suggested that heart failure may be associated with altered sympathetic and parasympathetic activity, and that these dysfunctions might contribute to the progression of heart failure.
Gianaros et al18 found fMRI evidence for a correlation between carotid artery intima-media thickness, a surrogate measure for carotid artery or coronary artery disease, and altered ANS reaction to fear using a fearful faces paradigm.
CONCLUSION
Functional MRI of heart-brain interactions has strong potential for normal subjects, in whom the BOLD effect is small, within the limits of motion and susceptibility artifacts. Typically, such applications require averaging results over multiple subjects. Its potential utility in disease states is less significant because of the additional limitations of MRI with sick patients (the MRI environment, blunting of autonomic response in disease, possible impairment of BOLD), but continued investigation is warranted.
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51:725–737.
- Kodounis A, Stamboulis E, Constantinidis TS, Liolios A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112:403–408.
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999; 246:578–586.
- Kulcu DG, Akbas B, Citci B, Cihangiroglu M. Autonomic dysreflexia in a man with multiple sclerosis. J Spinal Cord Med 2009; 32:198–203.
- Abboud H, Berroir S, Labreuche J, Orjuele K, Amarenco O. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59:691–699.
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30:1301–1311.
- Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol 2003; 50:30–38.
- Lassman AB, Mayer SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol 2005; 62:1286–1288.
- Deluca C, Tinazzi M, Bovi P, Rizzuto N, Moretto G. Limb ataxia and proximal intracranial territory brain infarcts: clinical and topographical correlations. J Neurol Neurosurg Psychiatry 2007; 78:832–835.
- Gómez-Esteban JC, Berganzo K, Tijero B, Barcena J, Zarranz JJ. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J Neurol 2009; 256:1357–1359.
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872.
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 2004; 101:6333–6334.
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol 2004; 471:446–461.
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 2008; 42:169–177.
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009; 47:922–936.
- Woo MA, Macey PM, Keens PT, et al Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11:437–446.
- Gianaros PJ, Hariri AR, Sheu LK, et al Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psych 2009; 65:943–950.
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10
FUNCTIONAL BRAIN IMAGING IN GENERAL
Direct visualization of heart-brain interactions is the goal when assessing ANS function. Positron emission tomography (PET) produces quantitative images, but spatial and temporal resolutions are vastly superior with fMRI.11 Further, radiation exposure is low with fMRI, allowing for safe repeat imaging.
Ogawa et al12 first demonstrated that in vivo images of brain microvasculature are affected by blood oxygen level, and that blood oxygenation reduced vascular signal loss. Therefore, blood oxygenation level–dependent (BOLD) contrast added to MRI could complement PET-like measurements in the study of regional brain activity.
Bilateral finger tapping with intermittent periods of rest is associated with a pattern of increasing and decreasing intensity of fMRI signals in involved brain regions that reflect the periods of activity and rest. This technique has been used to locate brain voxels with similar patterns of activity, enabling the creation of familiar color brain mapping. A challenge posed by autonomic fMRI in such brain mapping is that fMRI is susceptible to artifacts (Figure 3). For example, a movement of the head as little as 1 mm inside the MRI scanner—a distance comparable to the size of autonomic structures—can produce a motion artifact (false activation of brain regions) that can affect statistical significance. In addition, many ANS regions of the brain are near osseous structures (for example the brainstem and skull base) that cause signal distortion and loss.
REQUIREMENTS FOR AUTONOMIC fMRI
The tasks chosen to visualize brain control of autonomic function must naturally elicit an autonomic response. The difficulty is that untrained persons have little or no volitional control over autonomic functions, so the task and its analysis must be designed carefully and be MRI-compatible. Any motion will degrade the image; further, the capacity for the MRI environment to corrupt the measurements can limit the potential tasks for measurement.
Possible stimuli for eliciting a sympathetic response include pain, fear, anticipation, anxiety, concentration or memory, cold pressor, Stroop test, breathing tests, and maximal hand grip. Examples of parasympathetic stimuli are the Valsalva maneuver and paced breathing. The responses to stimuli (ie, heart rate, heart rate variability, blood pressure, galvanic skin response, papillary response) must be monitored to compare the data obtained from fMRI. MRI-compatible equipment is now available for measuring many of these responses.
Identifying areas activated during tasks
Functional neuroimaging with PET and fMRI has shown consistently that the anterior cingulate is activated during multiple tasks designed to elicit an autonomic response (gambling anticipation, emotional response to faces, Stroop test).11
In a study designed to test autonomic interoceptive awareness, subjects underwent fMRI while they were asked to judge the timing of their heartbeats to auditory tones that were either synchronized with their heartbeat or delayed by 500 msec.13 Areas of enhanced activity during the task were the right insular cortex, anterior cingulate, parietal lobes, and operculum.
Characterizing brainstem sites
It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses. These regions are small and motion artifacts are common because of brainstem movement with the cardiac pulse. With fMRI, Topolovec et al14 were able to characterize brainstem sites involved in autonomic control, demonstrating activation of the nucleus of the solitary tract and parabrachial nucleus.
A review of four fMRI studies of stressor-evoked blood pressure reactivity demonstrated activation in corticolimbic areas, including the cingulate cortex, insula, amygdala, and cortical and subcortical areas that are involved in hemodynamic and metabolic support for stress-related behavioral responses.16
FUNCTIONAL BRAIN IMAGING IN DISEASE STATES
There are few studies of functional brain imaging in patients with disease because of the challenges involved. The studies are difficult to perform on sick patients because of the unfriendly MRI environment, with struct requirements for attention and participation. Furthermore, autonomic responses may be blunted, making physiologic comparisons difficult. In addition, there is evidence that BOLD may be intrinsically impaired in disease states. Unlike fMRI studies to locate brain regions involved in simple tasks such as finger tapping, which can be performed in a single subject, detecting changes in autonomic responses in disease states requires averaging over studies of multiple patients.
Woo et al17 used fMRI to compare brain regions of activation in six patients with heart failure and 16 controls upon a forehead cold pressor challenge. Increases in heart rate were measured in the patients with heart failure with application of the cold stimulus. Larger neural fMRI signal responses in patients with heart failure were observed in 14 brain regions, whereas reduced fMRI activity was observed in 15 other brain regions in the heart failure patients. Based on the results, the investigators suggested that heart failure may be associated with altered sympathetic and parasympathetic activity, and that these dysfunctions might contribute to the progression of heart failure.
Gianaros et al18 found fMRI evidence for a correlation between carotid artery intima-media thickness, a surrogate measure for carotid artery or coronary artery disease, and altered ANS reaction to fear using a fearful faces paradigm.
CONCLUSION
Functional MRI of heart-brain interactions has strong potential for normal subjects, in whom the BOLD effect is small, within the limits of motion and susceptibility artifacts. Typically, such applications require averaging results over multiple subjects. Its potential utility in disease states is less significant because of the additional limitations of MRI with sick patients (the MRI environment, blunting of autonomic response in disease, possible impairment of BOLD), but continued investigation is warranted.
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10
FUNCTIONAL BRAIN IMAGING IN GENERAL
Direct visualization of heart-brain interactions is the goal when assessing ANS function. Positron emission tomography (PET) produces quantitative images, but spatial and temporal resolutions are vastly superior with fMRI.11 Further, radiation exposure is low with fMRI, allowing for safe repeat imaging.
Ogawa et al12 first demonstrated that in vivo images of brain microvasculature are affected by blood oxygen level, and that blood oxygenation reduced vascular signal loss. Therefore, blood oxygenation level–dependent (BOLD) contrast added to MRI could complement PET-like measurements in the study of regional brain activity.
Bilateral finger tapping with intermittent periods of rest is associated with a pattern of increasing and decreasing intensity of fMRI signals in involved brain regions that reflect the periods of activity and rest. This technique has been used to locate brain voxels with similar patterns of activity, enabling the creation of familiar color brain mapping. A challenge posed by autonomic fMRI in such brain mapping is that fMRI is susceptible to artifacts (Figure 3). For example, a movement of the head as little as 1 mm inside the MRI scanner—a distance comparable to the size of autonomic structures—can produce a motion artifact (false activation of brain regions) that can affect statistical significance. In addition, many ANS regions of the brain are near osseous structures (for example the brainstem and skull base) that cause signal distortion and loss.
REQUIREMENTS FOR AUTONOMIC fMRI
The tasks chosen to visualize brain control of autonomic function must naturally elicit an autonomic response. The difficulty is that untrained persons have little or no volitional control over autonomic functions, so the task and its analysis must be designed carefully and be MRI-compatible. Any motion will degrade the image; further, the capacity for the MRI environment to corrupt the measurements can limit the potential tasks for measurement.
Possible stimuli for eliciting a sympathetic response include pain, fear, anticipation, anxiety, concentration or memory, cold pressor, Stroop test, breathing tests, and maximal hand grip. Examples of parasympathetic stimuli are the Valsalva maneuver and paced breathing. The responses to stimuli (ie, heart rate, heart rate variability, blood pressure, galvanic skin response, papillary response) must be monitored to compare the data obtained from fMRI. MRI-compatible equipment is now available for measuring many of these responses.
Identifying areas activated during tasks
Functional neuroimaging with PET and fMRI has shown consistently that the anterior cingulate is activated during multiple tasks designed to elicit an autonomic response (gambling anticipation, emotional response to faces, Stroop test).11
In a study designed to test autonomic interoceptive awareness, subjects underwent fMRI while they were asked to judge the timing of their heartbeats to auditory tones that were either synchronized with their heartbeat or delayed by 500 msec.13 Areas of enhanced activity during the task were the right insular cortex, anterior cingulate, parietal lobes, and operculum.
Characterizing brainstem sites
It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses. These regions are small and motion artifacts are common because of brainstem movement with the cardiac pulse. With fMRI, Topolovec et al14 were able to characterize brainstem sites involved in autonomic control, demonstrating activation of the nucleus of the solitary tract and parabrachial nucleus.
A review of four fMRI studies of stressor-evoked blood pressure reactivity demonstrated activation in corticolimbic areas, including the cingulate cortex, insula, amygdala, and cortical and subcortical areas that are involved in hemodynamic and metabolic support for stress-related behavioral responses.16
FUNCTIONAL BRAIN IMAGING IN DISEASE STATES
There are few studies of functional brain imaging in patients with disease because of the challenges involved. The studies are difficult to perform on sick patients because of the unfriendly MRI environment, with struct requirements for attention and participation. Furthermore, autonomic responses may be blunted, making physiologic comparisons difficult. In addition, there is evidence that BOLD may be intrinsically impaired in disease states. Unlike fMRI studies to locate brain regions involved in simple tasks such as finger tapping, which can be performed in a single subject, detecting changes in autonomic responses in disease states requires averaging over studies of multiple patients.
Woo et al17 used fMRI to compare brain regions of activation in six patients with heart failure and 16 controls upon a forehead cold pressor challenge. Increases in heart rate were measured in the patients with heart failure with application of the cold stimulus. Larger neural fMRI signal responses in patients with heart failure were observed in 14 brain regions, whereas reduced fMRI activity was observed in 15 other brain regions in the heart failure patients. Based on the results, the investigators suggested that heart failure may be associated with altered sympathetic and parasympathetic activity, and that these dysfunctions might contribute to the progression of heart failure.
Gianaros et al18 found fMRI evidence for a correlation between carotid artery intima-media thickness, a surrogate measure for carotid artery or coronary artery disease, and altered ANS reaction to fear using a fearful faces paradigm.
CONCLUSION
Functional MRI of heart-brain interactions has strong potential for normal subjects, in whom the BOLD effect is small, within the limits of motion and susceptibility artifacts. Typically, such applications require averaging results over multiple subjects. Its potential utility in disease states is less significant because of the additional limitations of MRI with sick patients (the MRI environment, blunting of autonomic response in disease, possible impairment of BOLD), but continued investigation is warranted.
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51:725–737.
- Kodounis A, Stamboulis E, Constantinidis TS, Liolios A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112:403–408.
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999; 246:578–586.
- Kulcu DG, Akbas B, Citci B, Cihangiroglu M. Autonomic dysreflexia in a man with multiple sclerosis. J Spinal Cord Med 2009; 32:198–203.
- Abboud H, Berroir S, Labreuche J, Orjuele K, Amarenco O. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59:691–699.
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30:1301–1311.
- Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol 2003; 50:30–38.
- Lassman AB, Mayer SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol 2005; 62:1286–1288.
- Deluca C, Tinazzi M, Bovi P, Rizzuto N, Moretto G. Limb ataxia and proximal intracranial territory brain infarcts: clinical and topographical correlations. J Neurol Neurosurg Psychiatry 2007; 78:832–835.
- Gómez-Esteban JC, Berganzo K, Tijero B, Barcena J, Zarranz JJ. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J Neurol 2009; 256:1357–1359.
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872.
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 2004; 101:6333–6334.
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol 2004; 471:446–461.
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 2008; 42:169–177.
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009; 47:922–936.
- Woo MA, Macey PM, Keens PT, et al Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11:437–446.
- Gianaros PJ, Hariri AR, Sheu LK, et al Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psych 2009; 65:943–950.
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51:725–737.
- Kodounis A, Stamboulis E, Constantinidis TS, Liolios A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112:403–408.
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999; 246:578–586.
- Kulcu DG, Akbas B, Citci B, Cihangiroglu M. Autonomic dysreflexia in a man with multiple sclerosis. J Spinal Cord Med 2009; 32:198–203.
- Abboud H, Berroir S, Labreuche J, Orjuele K, Amarenco O. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59:691–699.
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30:1301–1311.
- Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol 2003; 50:30–38.
- Lassman AB, Mayer SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol 2005; 62:1286–1288.
- Deluca C, Tinazzi M, Bovi P, Rizzuto N, Moretto G. Limb ataxia and proximal intracranial territory brain infarcts: clinical and topographical correlations. J Neurol Neurosurg Psychiatry 2007; 78:832–835.
- Gómez-Esteban JC, Berganzo K, Tijero B, Barcena J, Zarranz JJ. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J Neurol 2009; 256:1357–1359.
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872.
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 2004; 101:6333–6334.
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol 2004; 471:446–461.
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 2008; 42:169–177.
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009; 47:922–936.
- Woo MA, Macey PM, Keens PT, et al Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11:437–446.
- Gianaros PJ, Hariri AR, Sheu LK, et al Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psych 2009; 65:943–950.
Neurohormonal control of heart failure
We have known for more than 100 years that heart failure is characterized by excessive sympathetic nervous system (SNS) activity. Thanks to refinement of this concept in the 1980s and 1990s, we now have a good understanding of SNS activity in both experimental and clinical heart failure. During those two decades, we also realized the pathophysiologic importance of the renin-angiotensin-aldosterone system (RAAS) in patients with heart failure.1 By 2000, it was obvious that heart failure was inextricably intertwined with excessive neurohormonal activity.2,3 This understanding of the pathophysiology of heart failure took on greater importance with the ability to pharmacologically block these neurohormonal systems, thereby demonstrating the detrimental role of neurohormones in the onset and progression of heart failure.
This article is a brief historical and personal description of the study of neurohormonal control mechanisms as they relate to the clinical syndrome of heart failure. The article includes a personal account of how the story unfolded in the cardiology research laboratories at the University of Minnesota.
THE EARLY YEARS: NEUROHORMONAL HYPOTHESIS
A hypothesis emerged gradually in the 1980s suggesting that progression of heart failure was in part a product of excessive SNS and RAAS activity. Many believed that pharmacologic inhibition of these systems might mitigate against progressive cardiac remodeling and thereby reduce symptoms and extend life—the so called neurohormonal hypothesis.4 SNS blockers and RAAS blockers are now widely used in tandem as first-line therapy to treat patients with heart failure,5–11 but in 1980 we were just beginning to consider their therapeutic effects.
This major shift in thinking about neurohormonal systems and heart failure did not come about quickly. Early success was driven by the ability to quickly and precisely measure neurohormones in the laboratory coupled with the availability of drugs specifically designed to block the SNS and RAAS. It was also critically important to embrace the power of randomized controlled trials to test new therapies. Investigators, research nurses, and patients from many medical centers and laboratories should be credited with this astonishing success. I am proud to have been a part of this activity at the University of Minnesota.
THE COHN LABORATORY
Early work done in the 1960s by numerous investigators noted that the failing left ventricle (LV) was exquisitely sensitive to afterload conditions.12–15 John Ross and Eugene Braunwald explored this observation in patients in 1964.15 Jay Cohn, with his unique background in hypertension and hemodynamics, brought the concept back into the laboratory in the early 1970s, where he explored the mechanisms responsible for increased sensitivity to afterload in patients with heart failure.16
I had the good fortune to join Cohn’s laboratory in 1979, when this avenue of heart failure research was in full bloom. A team of investigators was gradually assembled that included Maria Teresa Olivari, who relocated from the Cardiovascular Research Institute in Milan, Italy, directed by Maurizio D. Guazzi. Also joining the group were T. Barry Levine from the University of Michigan, Ann Arbor; Steven Goldsmith from Ohio State University, Columbus; Susan Ziesche from the Minneapolis Veterans Affairs (VA) Medical Center; Thomas Rector, an expert statistician and pharmacologist at the University of Minnesota; and many research fellows, visitors, students, biochemists, statisticians, and research nurses. Joseph Franciosa joined the University of Minnesota group in 1974 and, after completing several important trials, left in 1979 to lead the cardiology group at the Philadelphia VA Medical Center.
The Cohn group developed a working hypothesis that activation of the SNS and RAAS in heart failure was most likely an adaptive mechanism intended for short-term circulatory support, such as in the setting of blood loss, dehydration, shock, volume depletion, or flight response. In patients with heart failure, according to the hypothesis, the SNS and RAAS activity persisted beyond that needed for adaptation, with chronic release of norepinephrine (NE), renin, angiotensin II, aldosterone, and other neurohormones. The neurohormones ultimately became “maladaptive.” Thanks to the assaying skills of Ada Simon, we had the early advantage of precise and rapid radioenzyme measurement of plasma norepinephrine and renin activity in the blood of patients and animals.
We believed that neurohormonal activation contributed in part to the excessive afterload conditions observed in heart failure. We also thought that excessive neurohormonal activation directly impaired cardiac systolic function. The obvious next step was to explore whether neurohormonal antagonists would improve myocardial performance.
Under the leadership of Steven Goldsmith, many studies were performed to investigate reflex control mechanisms and their pathogenic role in patients with heart failure. The accumulating data suggested that persistent, excessive neurohormonal activity was characteristic of heart failure and that it was associated with a poor prognosis.17 The precise mechanism that drives activation of the SNS remained elusive, however, and is poorly defined even today. In that era, when β-adrenergic blockers were believed to be contraindicated, we inhibited the central SNS with bromocriptine, clonidine, and guanfacine with modestly favorable responses. We inhibited circulating arginine vasopressin antibody (thanks to Prof. Alan Cowley for noting an acute favorable response).
THE PHARMACOLOGIC ERA
The 1980s and 1990s saw the availability of several pharmacologic tools for assessing the roles of the SNS and RAAS in heart failure. The hypotensive effects of angiotensin-converting enzyme (ACE) inhibitors and, later, angiotensin-receptor blockers (ARBs) were sources of concern, since many patients with advanced heart failure had low- to normal-range blood pressures before they received RAAS blockers. However, our group as well as others observed that abrupt blood pressure reduction occurred primarily in patients with very hyperreninemic responses to intravenous diuretics (ie, volume-depleted patients). Eventually, we learned that low baseline blood pressure did not adversely affect outcomes when vasodilators were used in patients with heart failure,18,19 leading us to titrate these drugs upward over days to weeks.
Several different combinations of vasodilators were used successfully to treat heart failure, including hydralazine, isosorbide dinitrate,20 ACE inhibitors,21,22 and ARBs.8,23–28 Direct-acting calcium channel blocking vasodilators, such as amlodipine, did not improve survival in patients with systolic heart failure, although they appeared to be safe in this setting.29 The aldosterone receptor blockers spironolactone30 and eplerenone31 were later demonstrated to improve survival of patients with advanced systolic heart failure when added to vasodilator therapy.
By the end of the 1990s, it was evident that drugs that blocked the SNS and RAAS were not just vasodilators or “afterload reducers,” similar to α-blockers, hydralazine, nitrates, and amlodipine. Neurohormonal blockers were doing something profoundly beneficial not observed with more direct-acting vasodilators.32–37 Simple afterload reduction was not enough in patients with systolic heart failure.
Neurohormonal antagonists were acting more directly on the myocardium. They were preventing the progression of LV remodeling and, in some cases, promoting reverse remodeling, thus improving myocardial function and favorably influencing the natural history of heart failure.31–39 We were astonished to discover that the failing, dilated heart could revert to normal size in response to neurohormone blockade with ACE inhibitors and β-adrenergic blockers; these findings were soon reported by other laboratories as well.
Contrary to our concept of heart failure in the 1970s, we now understood that the heart has inherent plasticity. It can dilate in response to abnormal loading conditions or myocardial injury, and it can restore itself to normal size when neurohormones are blocked and perverse loading conditions are improved. This reversal can occur spontaneously if an offending agent such as chronic alcohol use or inflammation is removed, but it is likely facilitated by SNS and RAAS blockers.
THE REMODELING ERA
Ken McDonald joined the University of Minnesota lab in 1989 as a research fellow. His skill in conducting both animal and clinical mechanistic studies was pivotal to our achieving our research goals. The inspired animal work by Boston-based Marc and Janice Pfeffer revealed the significance of the LV remodeling concept in the development of heart failure36: ventricular remodeling was a hallmark of systolic heart failure, and pharmacologic inhibition of LV remodeling by blocking neurohormones had profound clinical implications.
Under the direction of Wenda Carlyle, a molecular biology laboratory was established at the University of Minnesota whose work was dedicated solely to exploration of remodeling at a very basic level. Alan Hirsch was recruited from Victor Dzau’s laboratory at Brigham and Women’s Hospital in Boston to extend our efforts to understand the molecular basis of cardiac remodeling. Ken McDonald guided the use of magnetic resonance imaging to study remodeling in dogs.
The late 1970s saw the initiation and eventual execution of several important clinical trials, including the Vasodilator Heart Failure Trials (V-HeFT I and V-HeFT II)40,41 under our leadership, and Studies of Left Ventricular Dysfunction (SOLVD)5,6 under the leadership of Salim Yusuf and others at the National Heart Lung and Blood Institute (NHLBI). Many neuro hormonal and remodeling substudies sprang from these large clinical trials. Spencer Kubo joined our group from the Medical College of Cornell University in the mid-1980s, and he immediately demonstrated his prowess in clinical research. He also recruited Alan Bank to study the endothelium in both experimental and human heart failure.
Integrating the molecular, animal, and clinical laboratories allowed us to pursue many mechanistic studies. Laboratory meetings, often held on Saturday mornings, generated ideas for program projects that were subsequently funded by NHLBI. Birthday parties and other social events with laboratory staff and their families were part of our fabric. Late-night trips to the Post Office to send off abstracts for national meetings before the midnight deadline were a regular feature.
Our coordination of and participation in the large clinical trials allowed us to meet frequently in Bethesda with colleagues from other major centers, fostering many collaborations and friendships that continue to thrive. Susan Ziesche deserves much of the credit for coordinating many groups that were part of these large, complex trials. Cheryl Yano, our administrator, also played a key role. All National Institutes of Health (NIH) grants passed through Cheryl, and she worked tirelessly to ensure that the proposals were in the best possible shape before we submitted them. Inder Anand joined our group in the early 1990s and became a major analytical force. Jay Cohn was the intellectual leader of the group, as well as our soul and inspiration. People worked hard for him, and he taught us much in a setting that valued creativity and new ideas above all.
THE LATER YEARS
By 1997, the face of heart failure had changed. New treatments were effective, but there were new challenges to face. I moved that year to the Cleveland Clinic, where I spent 11 enjoyable and productive years. I returned to Minnesota in 2008 to help build a new cardiovascular division.
It is gratifying to look back and see what has become of the “neurohormonal hypothesis.” Today, nearly all major medical centers have heart failure programs, and certification in advanced heart failure/heart transplantation is a reality. Training programs in advanced heart failure and heart transplant are common. The Heart Failure Society of America sprang up in the early 1990s, dedicated to patients with heart failure. Jay Cohn founded the Journal of Cardiac Failure, which flourished under his leadership. Neurohormonal blockers are now considered standard, conventional therapy and are widely used throughout the world.
CONCLUSIONS
Still, there is much work to do. An increasing number of devices are being developed, largely for patients with more advanced heart failure, but attention is also being directed to prevention of heart failure. Identification and possible treatment of patients at risk for the development of heart failure, and identification of those who already have some early structural and functional perturbation without advanced symptoms, are critically important. Since event rates are so low in these patients, we need to create new strategies for studying interventions. In the long term, the best treatment for nearly any condition is early diagnosis and perhaps early treatment with a goal of prevention.
One consequence of our progress over the years may be that heart failure now primarily affects a more elderly group—patients who often have many associated comorbidities. The consequences include more frequent readmissions, large numbers of patients with intractable signs and symptoms, and the emergence of difficult end-of-life decisions. If we could truly prevent heart failure rather than forestall its emergence to a later point in life, perhaps we could do more good.
For me, the study of neurohormonal mechanisms in the setting of heart failure was the centerpiece of my early career. Jay Cohn had asked several of us early in our laboratory experience to choose a neurohormonal system and learn about it in great depth and detail. My assignment was the SNS. Since then, I have never tired of learning about its control mechanisms, how it achieves circulatory homeostasis, how its excess quantities can be directly toxic to the heart, and the variety of pharmacologic ways that we can control it. I am indeed fortunate to have been part of this amazing study group.
- Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 1981; 63:645–651.
- Francis GS, Goldsmith SR, Levine TB, Olivari MT, Cohn JN. The neurohumoral axis in congestive heart failure. Ann Intern Med 1984; 101:370–377.
- Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of the sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol 1982; 49:1659–1666.
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 1992; 20:248–254.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325:293–302.
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327:685–691.
- Pitt B, Zannand F, Remme WJ, et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- CIBIS Investigators and Committees. A randomized trial of β-blockade in heart failure: the Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994; 90:1765–1773.
- Hjalmarson A, Goldstein S, Fagerberg B, et al Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERITHF). JAMA 2000; 283:1295–1302.
- Packer M, Fowler MB, Roecker EB, et al Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002; 106:2194–2199.
- Imperial ES, Levy MN, Zieske H. Outflow resistance as an independent determinant of cardiac performance. Circ Res 1961; 9:1148–1155.
- Sonnenblick EH, Downing SE. Afterload as a primary determinant of ventricular performance. Am J Physiol 1963; 204:604–610.
- Wilcken DE, Charlier AA, Hoffman JI. Effects of alterations in aortic impedance on the performance of the ventricles. Circ Res 1964; 14:283–293.
- Ross J, Braunwald E. The study of left ventricular function in man by increasing resistance to ventricular ejection with angiotensin. Circulation 1964; 29:739–749.
- Cohn JN. Blood pressure and cardiac performance. Am J Med 1973; 55:351–361.
- Cohn JN, Levine TB, Olivari MT, et al Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311:819–823.
- Anand IS, Tam SW, Rector TS, et al Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Rouleau JL, Roecker EB, Tendra M, et al Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol 2004; 43:1423–1429.
- Taylor AL, Ziesche S, Yancy C, et al Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2:755–763.
- Pfeffer MA, Braunwald E, Moyé LA, et al Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial—the SAVE Investigators. N Eng J Med 1992; 327:669–677.
- Curtiss C, Cohn JN, Vrobel T, Franciosa J. Role of the renin-angiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation 1978; 58:763–770.
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- Young JB, Dunlap ME, Pfeffer MA, et al Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection trials. Circulation 2004; 110:2618–2626.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349:1893–1906.
- ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Konstam MA, Neaton JD, Dickstein K, et al Effects of high-dose versus lose-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomized, double-blind trial. Lancet 2009; 374:1840–1848.
- Packer M. Prospective randomized amlodipine survival evaluation 2. Presented at: 49th American College of Cardiology meeting; March 2000; Anaheim, CA.
- Pitt B, Zannand F, Remme WJ, et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannand F, et al Eplerenone, a selective aldosterone blocker in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Cohn JN. Structural basis for heart failure: ventricular remodeling and its pharmacological inhibition. Circulation 1995; 91:2504–2507.
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000; 35:569–581.
- Konstam MA, Kronenberg MW, Rousseau MF, et al Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilation in patients with asymptomatic systolic dysfunction. Circulation 1993; 88:2277–2283.
- Greenberg B, Quinones MA, Koilpillai C, et al Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation 1995; 91:2573–2581.
- Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 1985; 57:84–95.
- Cohn JN. Structural basis for heart failure: ventricular remodeling and its pharmacological inhibition. Circulation 1995; 91:2504–2507.
- McDonald KM, Garr M, Carlyle PF, et al Relative effects of α1-adrenoceptor blockade, converting enzyme inhibitor therapy, and angiotensin II sub-type 1 receptor blockade on ventricular remodeling in the dog. Circulation 1994; 90:3034–3046.
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990; 81:1161–1172.
- Cohn JN, Archibald DG, Ziesche S, et al Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 1986; 314:1547–1552.
- Cohn JN, Johnson G, Ziesche S, et al A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
We have known for more than 100 years that heart failure is characterized by excessive sympathetic nervous system (SNS) activity. Thanks to refinement of this concept in the 1980s and 1990s, we now have a good understanding of SNS activity in both experimental and clinical heart failure. During those two decades, we also realized the pathophysiologic importance of the renin-angiotensin-aldosterone system (RAAS) in patients with heart failure.1 By 2000, it was obvious that heart failure was inextricably intertwined with excessive neurohormonal activity.2,3 This understanding of the pathophysiology of heart failure took on greater importance with the ability to pharmacologically block these neurohormonal systems, thereby demonstrating the detrimental role of neurohormones in the onset and progression of heart failure.
This article is a brief historical and personal description of the study of neurohormonal control mechanisms as they relate to the clinical syndrome of heart failure. The article includes a personal account of how the story unfolded in the cardiology research laboratories at the University of Minnesota.
THE EARLY YEARS: NEUROHORMONAL HYPOTHESIS
A hypothesis emerged gradually in the 1980s suggesting that progression of heart failure was in part a product of excessive SNS and RAAS activity. Many believed that pharmacologic inhibition of these systems might mitigate against progressive cardiac remodeling and thereby reduce symptoms and extend life—the so called neurohormonal hypothesis.4 SNS blockers and RAAS blockers are now widely used in tandem as first-line therapy to treat patients with heart failure,5–11 but in 1980 we were just beginning to consider their therapeutic effects.
This major shift in thinking about neurohormonal systems and heart failure did not come about quickly. Early success was driven by the ability to quickly and precisely measure neurohormones in the laboratory coupled with the availability of drugs specifically designed to block the SNS and RAAS. It was also critically important to embrace the power of randomized controlled trials to test new therapies. Investigators, research nurses, and patients from many medical centers and laboratories should be credited with this astonishing success. I am proud to have been a part of this activity at the University of Minnesota.
THE COHN LABORATORY
Early work done in the 1960s by numerous investigators noted that the failing left ventricle (LV) was exquisitely sensitive to afterload conditions.12–15 John Ross and Eugene Braunwald explored this observation in patients in 1964.15 Jay Cohn, with his unique background in hypertension and hemodynamics, brought the concept back into the laboratory in the early 1970s, where he explored the mechanisms responsible for increased sensitivity to afterload in patients with heart failure.16
I had the good fortune to join Cohn’s laboratory in 1979, when this avenue of heart failure research was in full bloom. A team of investigators was gradually assembled that included Maria Teresa Olivari, who relocated from the Cardiovascular Research Institute in Milan, Italy, directed by Maurizio D. Guazzi. Also joining the group were T. Barry Levine from the University of Michigan, Ann Arbor; Steven Goldsmith from Ohio State University, Columbus; Susan Ziesche from the Minneapolis Veterans Affairs (VA) Medical Center; Thomas Rector, an expert statistician and pharmacologist at the University of Minnesota; and many research fellows, visitors, students, biochemists, statisticians, and research nurses. Joseph Franciosa joined the University of Minnesota group in 1974 and, after completing several important trials, left in 1979 to lead the cardiology group at the Philadelphia VA Medical Center.
The Cohn group developed a working hypothesis that activation of the SNS and RAAS in heart failure was most likely an adaptive mechanism intended for short-term circulatory support, such as in the setting of blood loss, dehydration, shock, volume depletion, or flight response. In patients with heart failure, according to the hypothesis, the SNS and RAAS activity persisted beyond that needed for adaptation, with chronic release of norepinephrine (NE), renin, angiotensin II, aldosterone, and other neurohormones. The neurohormones ultimately became “maladaptive.” Thanks to the assaying skills of Ada Simon, we had the early advantage of precise and rapid radioenzyme measurement of plasma norepinephrine and renin activity in the blood of patients and animals.
We believed that neurohormonal activation contributed in part to the excessive afterload conditions observed in heart failure. We also thought that excessive neurohormonal activation directly impaired cardiac systolic function. The obvious next step was to explore whether neurohormonal antagonists would improve myocardial performance.
Under the leadership of Steven Goldsmith, many studies were performed to investigate reflex control mechanisms and their pathogenic role in patients with heart failure. The accumulating data suggested that persistent, excessive neurohormonal activity was characteristic of heart failure and that it was associated with a poor prognosis.17 The precise mechanism that drives activation of the SNS remained elusive, however, and is poorly defined even today. In that era, when β-adrenergic blockers were believed to be contraindicated, we inhibited the central SNS with bromocriptine, clonidine, and guanfacine with modestly favorable responses. We inhibited circulating arginine vasopressin antibody (thanks to Prof. Alan Cowley for noting an acute favorable response).
THE PHARMACOLOGIC ERA
The 1980s and 1990s saw the availability of several pharmacologic tools for assessing the roles of the SNS and RAAS in heart failure. The hypotensive effects of angiotensin-converting enzyme (ACE) inhibitors and, later, angiotensin-receptor blockers (ARBs) were sources of concern, since many patients with advanced heart failure had low- to normal-range blood pressures before they received RAAS blockers. However, our group as well as others observed that abrupt blood pressure reduction occurred primarily in patients with very hyperreninemic responses to intravenous diuretics (ie, volume-depleted patients). Eventually, we learned that low baseline blood pressure did not adversely affect outcomes when vasodilators were used in patients with heart failure,18,19 leading us to titrate these drugs upward over days to weeks.
Several different combinations of vasodilators were used successfully to treat heart failure, including hydralazine, isosorbide dinitrate,20 ACE inhibitors,21,22 and ARBs.8,23–28 Direct-acting calcium channel blocking vasodilators, such as amlodipine, did not improve survival in patients with systolic heart failure, although they appeared to be safe in this setting.29 The aldosterone receptor blockers spironolactone30 and eplerenone31 were later demonstrated to improve survival of patients with advanced systolic heart failure when added to vasodilator therapy.
By the end of the 1990s, it was evident that drugs that blocked the SNS and RAAS were not just vasodilators or “afterload reducers,” similar to α-blockers, hydralazine, nitrates, and amlodipine. Neurohormonal blockers were doing something profoundly beneficial not observed with more direct-acting vasodilators.32–37 Simple afterload reduction was not enough in patients with systolic heart failure.
Neurohormonal antagonists were acting more directly on the myocardium. They were preventing the progression of LV remodeling and, in some cases, promoting reverse remodeling, thus improving myocardial function and favorably influencing the natural history of heart failure.31–39 We were astonished to discover that the failing, dilated heart could revert to normal size in response to neurohormone blockade with ACE inhibitors and β-adrenergic blockers; these findings were soon reported by other laboratories as well.
Contrary to our concept of heart failure in the 1970s, we now understood that the heart has inherent plasticity. It can dilate in response to abnormal loading conditions or myocardial injury, and it can restore itself to normal size when neurohormones are blocked and perverse loading conditions are improved. This reversal can occur spontaneously if an offending agent such as chronic alcohol use or inflammation is removed, but it is likely facilitated by SNS and RAAS blockers.
THE REMODELING ERA
Ken McDonald joined the University of Minnesota lab in 1989 as a research fellow. His skill in conducting both animal and clinical mechanistic studies was pivotal to our achieving our research goals. The inspired animal work by Boston-based Marc and Janice Pfeffer revealed the significance of the LV remodeling concept in the development of heart failure36: ventricular remodeling was a hallmark of systolic heart failure, and pharmacologic inhibition of LV remodeling by blocking neurohormones had profound clinical implications.
Under the direction of Wenda Carlyle, a molecular biology laboratory was established at the University of Minnesota whose work was dedicated solely to exploration of remodeling at a very basic level. Alan Hirsch was recruited from Victor Dzau’s laboratory at Brigham and Women’s Hospital in Boston to extend our efforts to understand the molecular basis of cardiac remodeling. Ken McDonald guided the use of magnetic resonance imaging to study remodeling in dogs.
The late 1970s saw the initiation and eventual execution of several important clinical trials, including the Vasodilator Heart Failure Trials (V-HeFT I and V-HeFT II)40,41 under our leadership, and Studies of Left Ventricular Dysfunction (SOLVD)5,6 under the leadership of Salim Yusuf and others at the National Heart Lung and Blood Institute (NHLBI). Many neuro hormonal and remodeling substudies sprang from these large clinical trials. Spencer Kubo joined our group from the Medical College of Cornell University in the mid-1980s, and he immediately demonstrated his prowess in clinical research. He also recruited Alan Bank to study the endothelium in both experimental and human heart failure.
Integrating the molecular, animal, and clinical laboratories allowed us to pursue many mechanistic studies. Laboratory meetings, often held on Saturday mornings, generated ideas for program projects that were subsequently funded by NHLBI. Birthday parties and other social events with laboratory staff and their families were part of our fabric. Late-night trips to the Post Office to send off abstracts for national meetings before the midnight deadline were a regular feature.
Our coordination of and participation in the large clinical trials allowed us to meet frequently in Bethesda with colleagues from other major centers, fostering many collaborations and friendships that continue to thrive. Susan Ziesche deserves much of the credit for coordinating many groups that were part of these large, complex trials. Cheryl Yano, our administrator, also played a key role. All National Institutes of Health (NIH) grants passed through Cheryl, and she worked tirelessly to ensure that the proposals were in the best possible shape before we submitted them. Inder Anand joined our group in the early 1990s and became a major analytical force. Jay Cohn was the intellectual leader of the group, as well as our soul and inspiration. People worked hard for him, and he taught us much in a setting that valued creativity and new ideas above all.
THE LATER YEARS
By 1997, the face of heart failure had changed. New treatments were effective, but there were new challenges to face. I moved that year to the Cleveland Clinic, where I spent 11 enjoyable and productive years. I returned to Minnesota in 2008 to help build a new cardiovascular division.
It is gratifying to look back and see what has become of the “neurohormonal hypothesis.” Today, nearly all major medical centers have heart failure programs, and certification in advanced heart failure/heart transplantation is a reality. Training programs in advanced heart failure and heart transplant are common. The Heart Failure Society of America sprang up in the early 1990s, dedicated to patients with heart failure. Jay Cohn founded the Journal of Cardiac Failure, which flourished under his leadership. Neurohormonal blockers are now considered standard, conventional therapy and are widely used throughout the world.
CONCLUSIONS
Still, there is much work to do. An increasing number of devices are being developed, largely for patients with more advanced heart failure, but attention is also being directed to prevention of heart failure. Identification and possible treatment of patients at risk for the development of heart failure, and identification of those who already have some early structural and functional perturbation without advanced symptoms, are critically important. Since event rates are so low in these patients, we need to create new strategies for studying interventions. In the long term, the best treatment for nearly any condition is early diagnosis and perhaps early treatment with a goal of prevention.
One consequence of our progress over the years may be that heart failure now primarily affects a more elderly group—patients who often have many associated comorbidities. The consequences include more frequent readmissions, large numbers of patients with intractable signs and symptoms, and the emergence of difficult end-of-life decisions. If we could truly prevent heart failure rather than forestall its emergence to a later point in life, perhaps we could do more good.
For me, the study of neurohormonal mechanisms in the setting of heart failure was the centerpiece of my early career. Jay Cohn had asked several of us early in our laboratory experience to choose a neurohormonal system and learn about it in great depth and detail. My assignment was the SNS. Since then, I have never tired of learning about its control mechanisms, how it achieves circulatory homeostasis, how its excess quantities can be directly toxic to the heart, and the variety of pharmacologic ways that we can control it. I am indeed fortunate to have been part of this amazing study group.
We have known for more than 100 years that heart failure is characterized by excessive sympathetic nervous system (SNS) activity. Thanks to refinement of this concept in the 1980s and 1990s, we now have a good understanding of SNS activity in both experimental and clinical heart failure. During those two decades, we also realized the pathophysiologic importance of the renin-angiotensin-aldosterone system (RAAS) in patients with heart failure.1 By 2000, it was obvious that heart failure was inextricably intertwined with excessive neurohormonal activity.2,3 This understanding of the pathophysiology of heart failure took on greater importance with the ability to pharmacologically block these neurohormonal systems, thereby demonstrating the detrimental role of neurohormones in the onset and progression of heart failure.
This article is a brief historical and personal description of the study of neurohormonal control mechanisms as they relate to the clinical syndrome of heart failure. The article includes a personal account of how the story unfolded in the cardiology research laboratories at the University of Minnesota.
THE EARLY YEARS: NEUROHORMONAL HYPOTHESIS
A hypothesis emerged gradually in the 1980s suggesting that progression of heart failure was in part a product of excessive SNS and RAAS activity. Many believed that pharmacologic inhibition of these systems might mitigate against progressive cardiac remodeling and thereby reduce symptoms and extend life—the so called neurohormonal hypothesis.4 SNS blockers and RAAS blockers are now widely used in tandem as first-line therapy to treat patients with heart failure,5–11 but in 1980 we were just beginning to consider their therapeutic effects.
This major shift in thinking about neurohormonal systems and heart failure did not come about quickly. Early success was driven by the ability to quickly and precisely measure neurohormones in the laboratory coupled with the availability of drugs specifically designed to block the SNS and RAAS. It was also critically important to embrace the power of randomized controlled trials to test new therapies. Investigators, research nurses, and patients from many medical centers and laboratories should be credited with this astonishing success. I am proud to have been a part of this activity at the University of Minnesota.
THE COHN LABORATORY
Early work done in the 1960s by numerous investigators noted that the failing left ventricle (LV) was exquisitely sensitive to afterload conditions.12–15 John Ross and Eugene Braunwald explored this observation in patients in 1964.15 Jay Cohn, with his unique background in hypertension and hemodynamics, brought the concept back into the laboratory in the early 1970s, where he explored the mechanisms responsible for increased sensitivity to afterload in patients with heart failure.16
I had the good fortune to join Cohn’s laboratory in 1979, when this avenue of heart failure research was in full bloom. A team of investigators was gradually assembled that included Maria Teresa Olivari, who relocated from the Cardiovascular Research Institute in Milan, Italy, directed by Maurizio D. Guazzi. Also joining the group were T. Barry Levine from the University of Michigan, Ann Arbor; Steven Goldsmith from Ohio State University, Columbus; Susan Ziesche from the Minneapolis Veterans Affairs (VA) Medical Center; Thomas Rector, an expert statistician and pharmacologist at the University of Minnesota; and many research fellows, visitors, students, biochemists, statisticians, and research nurses. Joseph Franciosa joined the University of Minnesota group in 1974 and, after completing several important trials, left in 1979 to lead the cardiology group at the Philadelphia VA Medical Center.
The Cohn group developed a working hypothesis that activation of the SNS and RAAS in heart failure was most likely an adaptive mechanism intended for short-term circulatory support, such as in the setting of blood loss, dehydration, shock, volume depletion, or flight response. In patients with heart failure, according to the hypothesis, the SNS and RAAS activity persisted beyond that needed for adaptation, with chronic release of norepinephrine (NE), renin, angiotensin II, aldosterone, and other neurohormones. The neurohormones ultimately became “maladaptive.” Thanks to the assaying skills of Ada Simon, we had the early advantage of precise and rapid radioenzyme measurement of plasma norepinephrine and renin activity in the blood of patients and animals.
We believed that neurohormonal activation contributed in part to the excessive afterload conditions observed in heart failure. We also thought that excessive neurohormonal activation directly impaired cardiac systolic function. The obvious next step was to explore whether neurohormonal antagonists would improve myocardial performance.
Under the leadership of Steven Goldsmith, many studies were performed to investigate reflex control mechanisms and their pathogenic role in patients with heart failure. The accumulating data suggested that persistent, excessive neurohormonal activity was characteristic of heart failure and that it was associated with a poor prognosis.17 The precise mechanism that drives activation of the SNS remained elusive, however, and is poorly defined even today. In that era, when β-adrenergic blockers were believed to be contraindicated, we inhibited the central SNS with bromocriptine, clonidine, and guanfacine with modestly favorable responses. We inhibited circulating arginine vasopressin antibody (thanks to Prof. Alan Cowley for noting an acute favorable response).
THE PHARMACOLOGIC ERA
The 1980s and 1990s saw the availability of several pharmacologic tools for assessing the roles of the SNS and RAAS in heart failure. The hypotensive effects of angiotensin-converting enzyme (ACE) inhibitors and, later, angiotensin-receptor blockers (ARBs) were sources of concern, since many patients with advanced heart failure had low- to normal-range blood pressures before they received RAAS blockers. However, our group as well as others observed that abrupt blood pressure reduction occurred primarily in patients with very hyperreninemic responses to intravenous diuretics (ie, volume-depleted patients). Eventually, we learned that low baseline blood pressure did not adversely affect outcomes when vasodilators were used in patients with heart failure,18,19 leading us to titrate these drugs upward over days to weeks.
Several different combinations of vasodilators were used successfully to treat heart failure, including hydralazine, isosorbide dinitrate,20 ACE inhibitors,21,22 and ARBs.8,23–28 Direct-acting calcium channel blocking vasodilators, such as amlodipine, did not improve survival in patients with systolic heart failure, although they appeared to be safe in this setting.29 The aldosterone receptor blockers spironolactone30 and eplerenone31 were later demonstrated to improve survival of patients with advanced systolic heart failure when added to vasodilator therapy.
By the end of the 1990s, it was evident that drugs that blocked the SNS and RAAS were not just vasodilators or “afterload reducers,” similar to α-blockers, hydralazine, nitrates, and amlodipine. Neurohormonal blockers were doing something profoundly beneficial not observed with more direct-acting vasodilators.32–37 Simple afterload reduction was not enough in patients with systolic heart failure.
Neurohormonal antagonists were acting more directly on the myocardium. They were preventing the progression of LV remodeling and, in some cases, promoting reverse remodeling, thus improving myocardial function and favorably influencing the natural history of heart failure.31–39 We were astonished to discover that the failing, dilated heart could revert to normal size in response to neurohormone blockade with ACE inhibitors and β-adrenergic blockers; these findings were soon reported by other laboratories as well.
Contrary to our concept of heart failure in the 1970s, we now understood that the heart has inherent plasticity. It can dilate in response to abnormal loading conditions or myocardial injury, and it can restore itself to normal size when neurohormones are blocked and perverse loading conditions are improved. This reversal can occur spontaneously if an offending agent such as chronic alcohol use or inflammation is removed, but it is likely facilitated by SNS and RAAS blockers.
THE REMODELING ERA
Ken McDonald joined the University of Minnesota lab in 1989 as a research fellow. His skill in conducting both animal and clinical mechanistic studies was pivotal to our achieving our research goals. The inspired animal work by Boston-based Marc and Janice Pfeffer revealed the significance of the LV remodeling concept in the development of heart failure36: ventricular remodeling was a hallmark of systolic heart failure, and pharmacologic inhibition of LV remodeling by blocking neurohormones had profound clinical implications.
Under the direction of Wenda Carlyle, a molecular biology laboratory was established at the University of Minnesota whose work was dedicated solely to exploration of remodeling at a very basic level. Alan Hirsch was recruited from Victor Dzau’s laboratory at Brigham and Women’s Hospital in Boston to extend our efforts to understand the molecular basis of cardiac remodeling. Ken McDonald guided the use of magnetic resonance imaging to study remodeling in dogs.
The late 1970s saw the initiation and eventual execution of several important clinical trials, including the Vasodilator Heart Failure Trials (V-HeFT I and V-HeFT II)40,41 under our leadership, and Studies of Left Ventricular Dysfunction (SOLVD)5,6 under the leadership of Salim Yusuf and others at the National Heart Lung and Blood Institute (NHLBI). Many neuro hormonal and remodeling substudies sprang from these large clinical trials. Spencer Kubo joined our group from the Medical College of Cornell University in the mid-1980s, and he immediately demonstrated his prowess in clinical research. He also recruited Alan Bank to study the endothelium in both experimental and human heart failure.
Integrating the molecular, animal, and clinical laboratories allowed us to pursue many mechanistic studies. Laboratory meetings, often held on Saturday mornings, generated ideas for program projects that were subsequently funded by NHLBI. Birthday parties and other social events with laboratory staff and their families were part of our fabric. Late-night trips to the Post Office to send off abstracts for national meetings before the midnight deadline were a regular feature.
Our coordination of and participation in the large clinical trials allowed us to meet frequently in Bethesda with colleagues from other major centers, fostering many collaborations and friendships that continue to thrive. Susan Ziesche deserves much of the credit for coordinating many groups that were part of these large, complex trials. Cheryl Yano, our administrator, also played a key role. All National Institutes of Health (NIH) grants passed through Cheryl, and she worked tirelessly to ensure that the proposals were in the best possible shape before we submitted them. Inder Anand joined our group in the early 1990s and became a major analytical force. Jay Cohn was the intellectual leader of the group, as well as our soul and inspiration. People worked hard for him, and he taught us much in a setting that valued creativity and new ideas above all.
THE LATER YEARS
By 1997, the face of heart failure had changed. New treatments were effective, but there were new challenges to face. I moved that year to the Cleveland Clinic, where I spent 11 enjoyable and productive years. I returned to Minnesota in 2008 to help build a new cardiovascular division.
It is gratifying to look back and see what has become of the “neurohormonal hypothesis.” Today, nearly all major medical centers have heart failure programs, and certification in advanced heart failure/heart transplantation is a reality. Training programs in advanced heart failure and heart transplant are common. The Heart Failure Society of America sprang up in the early 1990s, dedicated to patients with heart failure. Jay Cohn founded the Journal of Cardiac Failure, which flourished under his leadership. Neurohormonal blockers are now considered standard, conventional therapy and are widely used throughout the world.
CONCLUSIONS
Still, there is much work to do. An increasing number of devices are being developed, largely for patients with more advanced heart failure, but attention is also being directed to prevention of heart failure. Identification and possible treatment of patients at risk for the development of heart failure, and identification of those who already have some early structural and functional perturbation without advanced symptoms, are critically important. Since event rates are so low in these patients, we need to create new strategies for studying interventions. In the long term, the best treatment for nearly any condition is early diagnosis and perhaps early treatment with a goal of prevention.
One consequence of our progress over the years may be that heart failure now primarily affects a more elderly group—patients who often have many associated comorbidities. The consequences include more frequent readmissions, large numbers of patients with intractable signs and symptoms, and the emergence of difficult end-of-life decisions. If we could truly prevent heart failure rather than forestall its emergence to a later point in life, perhaps we could do more good.
For me, the study of neurohormonal mechanisms in the setting of heart failure was the centerpiece of my early career. Jay Cohn had asked several of us early in our laboratory experience to choose a neurohormonal system and learn about it in great depth and detail. My assignment was the SNS. Since then, I have never tired of learning about its control mechanisms, how it achieves circulatory homeostasis, how its excess quantities can be directly toxic to the heart, and the variety of pharmacologic ways that we can control it. I am indeed fortunate to have been part of this amazing study group.
- Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 1981; 63:645–651.
- Francis GS, Goldsmith SR, Levine TB, Olivari MT, Cohn JN. The neurohumoral axis in congestive heart failure. Ann Intern Med 1984; 101:370–377.
- Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of the sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol 1982; 49:1659–1666.
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 1992; 20:248–254.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325:293–302.
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327:685–691.
- Pitt B, Zannand F, Remme WJ, et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- CIBIS Investigators and Committees. A randomized trial of β-blockade in heart failure: the Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994; 90:1765–1773.
- Hjalmarson A, Goldstein S, Fagerberg B, et al Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERITHF). JAMA 2000; 283:1295–1302.
- Packer M, Fowler MB, Roecker EB, et al Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002; 106:2194–2199.
- Imperial ES, Levy MN, Zieske H. Outflow resistance as an independent determinant of cardiac performance. Circ Res 1961; 9:1148–1155.
- Sonnenblick EH, Downing SE. Afterload as a primary determinant of ventricular performance. Am J Physiol 1963; 204:604–610.
- Wilcken DE, Charlier AA, Hoffman JI. Effects of alterations in aortic impedance on the performance of the ventricles. Circ Res 1964; 14:283–293.
- Ross J, Braunwald E. The study of left ventricular function in man by increasing resistance to ventricular ejection with angiotensin. Circulation 1964; 29:739–749.
- Cohn JN. Blood pressure and cardiac performance. Am J Med 1973; 55:351–361.
- Cohn JN, Levine TB, Olivari MT, et al Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311:819–823.
- Anand IS, Tam SW, Rector TS, et al Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Rouleau JL, Roecker EB, Tendra M, et al Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol 2004; 43:1423–1429.
- Taylor AL, Ziesche S, Yancy C, et al Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2:755–763.
- Pfeffer MA, Braunwald E, Moyé LA, et al Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial—the SAVE Investigators. N Eng J Med 1992; 327:669–677.
- Curtiss C, Cohn JN, Vrobel T, Franciosa J. Role of the renin-angiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation 1978; 58:763–770.
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- Young JB, Dunlap ME, Pfeffer MA, et al Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection trials. Circulation 2004; 110:2618–2626.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349:1893–1906.
- ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Konstam MA, Neaton JD, Dickstein K, et al Effects of high-dose versus lose-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomized, double-blind trial. Lancet 2009; 374:1840–1848.
- Packer M. Prospective randomized amlodipine survival evaluation 2. Presented at: 49th American College of Cardiology meeting; March 2000; Anaheim, CA.
- Pitt B, Zannand F, Remme WJ, et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannand F, et al Eplerenone, a selective aldosterone blocker in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Cohn JN. Structural basis for heart failure: ventricular remodeling and its pharmacological inhibition. Circulation 1995; 91:2504–2507.
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000; 35:569–581.
- Konstam MA, Kronenberg MW, Rousseau MF, et al Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilation in patients with asymptomatic systolic dysfunction. Circulation 1993; 88:2277–2283.
- Greenberg B, Quinones MA, Koilpillai C, et al Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation 1995; 91:2573–2581.
- Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 1985; 57:84–95.
- Cohn JN. Structural basis for heart failure: ventricular remodeling and its pharmacological inhibition. Circulation 1995; 91:2504–2507.
- McDonald KM, Garr M, Carlyle PF, et al Relative effects of α1-adrenoceptor blockade, converting enzyme inhibitor therapy, and angiotensin II sub-type 1 receptor blockade on ventricular remodeling in the dog. Circulation 1994; 90:3034–3046.
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990; 81:1161–1172.
- Cohn JN, Archibald DG, Ziesche S, et al Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 1986; 314:1547–1552.
- Cohn JN, Johnson G, Ziesche S, et al A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 1981; 63:645–651.
- Francis GS, Goldsmith SR, Levine TB, Olivari MT, Cohn JN. The neurohumoral axis in congestive heart failure. Ann Intern Med 1984; 101:370–377.
- Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of the sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol 1982; 49:1659–1666.
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 1992; 20:248–254.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325:293–302.
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327:685–691.
- Pitt B, Zannand F, Remme WJ, et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- CIBIS Investigators and Committees. A randomized trial of β-blockade in heart failure: the Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 1994; 90:1765–1773.
- Hjalmarson A, Goldstein S, Fagerberg B, et al Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERITHF). JAMA 2000; 283:1295–1302.
- Packer M, Fowler MB, Roecker EB, et al Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002; 106:2194–2199.
- Imperial ES, Levy MN, Zieske H. Outflow resistance as an independent determinant of cardiac performance. Circ Res 1961; 9:1148–1155.
- Sonnenblick EH, Downing SE. Afterload as a primary determinant of ventricular performance. Am J Physiol 1963; 204:604–610.
- Wilcken DE, Charlier AA, Hoffman JI. Effects of alterations in aortic impedance on the performance of the ventricles. Circ Res 1964; 14:283–293.
- Ross J, Braunwald E. The study of left ventricular function in man by increasing resistance to ventricular ejection with angiotensin. Circulation 1964; 29:739–749.
- Cohn JN. Blood pressure and cardiac performance. Am J Med 1973; 55:351–361.
- Cohn JN, Levine TB, Olivari MT, et al Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311:819–823.
- Anand IS, Tam SW, Rector TS, et al Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Rouleau JL, Roecker EB, Tendra M, et al Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol 2004; 43:1423–1429.
- Taylor AL, Ziesche S, Yancy C, et al Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2:755–763.
- Pfeffer MA, Braunwald E, Moyé LA, et al Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial—the SAVE Investigators. N Eng J Med 1992; 327:669–677.
- Curtiss C, Cohn JN, Vrobel T, Franciosa J. Role of the renin-angiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation 1978; 58:763–770.
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- Young JB, Dunlap ME, Pfeffer MA, et al Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection trials. Circulation 2004; 110:2618–2626.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349:1893–1906.
- ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Konstam MA, Neaton JD, Dickstein K, et al Effects of high-dose versus lose-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomized, double-blind trial. Lancet 2009; 374:1840–1848.
- Packer M. Prospective randomized amlodipine survival evaluation 2. Presented at: 49th American College of Cardiology meeting; March 2000; Anaheim, CA.
- Pitt B, Zannand F, Remme WJ, et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannand F, et al Eplerenone, a selective aldosterone blocker in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Cohn JN. Structural basis for heart failure: ventricular remodeling and its pharmacological inhibition. Circulation 1995; 91:2504–2507.
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000; 35:569–581.
- Konstam MA, Kronenberg MW, Rousseau MF, et al Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilation in patients with asymptomatic systolic dysfunction. Circulation 1993; 88:2277–2283.
- Greenberg B, Quinones MA, Koilpillai C, et al Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation 1995; 91:2573–2581.
- Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 1985; 57:84–95.
- Cohn JN. Structural basis for heart failure: ventricular remodeling and its pharmacological inhibition. Circulation 1995; 91:2504–2507.
- McDonald KM, Garr M, Carlyle PF, et al Relative effects of α1-adrenoceptor blockade, converting enzyme inhibitor therapy, and angiotensin II sub-type 1 receptor blockade on ventricular remodeling in the dog. Circulation 1994; 90:3034–3046.
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990; 81:1161–1172.
- Cohn JN, Archibald DG, Ziesche S, et al Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 1986; 314:1547–1552.
- Cohn JN, Johnson G, Ziesche S, et al A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.