User login
Does vitamin D deficiency play a role in the pathogenesis of chronic heart failure? Do supplements improve survival?
Vitamin D deficiency may play a role in the pathogenesis of chronic heart failure, but whether giving patients supplements to raise their vitamin D levels into the normal range improves their survival is not clear.
ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND OTHER DISORDERS
In the mid-17th century, Whistler and Glisson independently described rickets as a severe bone-deforming disease characterized by growth retardation, bending of the spine, deformities of the legs, and weak and toneless muscles. Histologically, rickets is characterized by impaired mineralization of the cartilage in the epiphyseal growth plates in children. In 1919, Sir Edward Mellanby identified vitamin D deficiency as the cause.
Osteomalacia, another disease caused by vitamin D deficiency, is a disorder of mineralization of newly formed bone matrix in adults. Vitamin D, therefore, has well-known roles in maintaining bone health and calcium and phosphorus homeostasis.
In addition, vitamin D deficiency has been shown in recent years to be associated with myocardial dysfunction.1,2
VITAMIN D METABOLISM IS COMPLEX
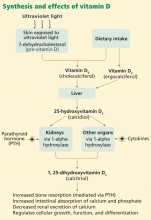
In skin exposed to ultraviolet B light, the provitamin 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Vitamin D3 is also obtained from dietary sources. However, many scientists consider vitamin D more a hormone than a classic vitamin, as adequate exposure to sunlight may negate the need for dietary supplements.
The active form of vitamin D is synthesized by hydroxylation in the liver and kidney. In the liver, hepatic enzymes add a hydroxyl (OH) group to vitamin D3, changing it to 25-hydroxyvitamin D3. In the kidney, 25-hydroxyvitamin D3 receives another hydroxyl group, converting it to the biologically active metabolite 1,25-dihydroxyvitamin D3 (calcitriol). This renal hydroxylation is via 1-alpha-hydroxylase activity and is directly under control of parathyroid hormone (PTH), and indirectly under control of the serum concentrations of calcium.
Interestingly, a number of different organ cells, including cardiomyocytes, also express 1-alpha-hydroxylase and therefore also convert 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3. Unlike the renal hydroxylation, this extrarenal process depends on cytokine activation and on serum levels of 25-hydroxyvitamin D3.3 Low levels of 25-hydroxyvitamin D3 lead to alterations in cellular control over growth, differentiation, and function.
The active form of vitamin D is transported protein-bound in the blood to various target organs, where it is delivered in free form to cells. Specific nuclear receptor proteins are found in many organs not classically considered target organs for vitamin D, including the skin, brain, skeletal muscles, cardiomyocytes, vascular endothelial cells, circulating monocytes, and activated B and T lymphocytes. Vitamin D plays a significant role in the autocrine and paracrine regulation of cellular function, growth, and differentiation in various organs.3
MOST HEART FAILURE PATIENTS HAVE LOW VITAMIN D LEVELS
More than 40% of men and 50% of women in the United States have low vitamin D levels (< 30 ng/mL [75 nmol/L])—and low levels in adults are associated with both coronary artery disease and heart failure.4 Most patients with heart failure have low levels.5,6 Therefore, screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
Low vitamin D levels carry a poor prognosis. Pilz et al5 measured baseline 25-hydroxyvitamin D3 levels in 3,299 patients referred for elective coronary angiography and followed them prospectively for a median of 7.7 years. Even after adjustment for cardiac risk factors, patients who had low 25-hydroxyvitamin D3 levels were more likely to die of heart failure or sudden cardiac death than patients with normal levels.
Boxer et al7 found an association between low 25-hydroxyvitamin D3 levels and low exercise capacity and frailty in patients with systolic heart failure.
LOW VITAMIN D CONTRIBUTES TO THE PATHOGENESIS OF HEART FAILURE
In recent years, ideas about the pathophysiology of heart failure have expanded from a purely hemodynamic view to a more complex concept involving inflammatory cytokines and neurohormonal overactivation.8
Animal studies first showed vitamin D to inhibit the renin-angiotensin-aldosterone system, activation of which contributes to the salt and water retention seen in heart failure.4,9
In addition, vitamin D has a number of effects that should help prevent hypertension, an important risk factor for heart failure. It protects the kidney by suppressing the reninangiotensin-aldosterone system, prevents secondary hyperparathyroidism and its effects on vascular stiffness, prevents insulin resistance, and suppresses inflammation, which protects vascular endothelial cells.10
The first studies to show a connection between cardiovascular homeostasis and vitamin D status were in animal models more than 20 years ago. These studies showed that 1,25-dihydroxyvitamin D3 acts directly on cardiomyocyte vitamin D receptors, which are widely distributed throughout the body in several tissue types.11
Excess PTH levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart.12 Animal studies have found that vitamin D suppresses cardiac hypertrophy.13 Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with diastolic heart failure.2,14
Currently, it is unclear whether vitamin D deficiency is a causative risk factor for heart failure or simply a reflection of the poor functional status of patients with heart failure that leads to decreased exposure to sunlight. This debate will continue until further randomized clinical trials address this association.
VITAMIN D AND HEART TRANSPLANTATION
One would expect that patients with endstage organ failure would be at high risk of vitamin D deficiency because of limited sunlight exposure. However, few studies have evaluated the role of this vitamin in heart transplant recipients.
Stein and colleagues15 measured serum 25-hydroxyvitamin D3 immediately after transplantation in 46 heart and 23 liver transplant recipients. Levels were low in both types of transplant recipients, but liver transplant recipients had significantly lower levels than heart transplant patients. This could be explained by malabsorption and impaired synthesis of 25-hydroxyvitamin D3 in end-stage liver disease.
Also, an important point is that osteoporosis is prevalent in postcardiac transplant patients and likely related to the immunosuppressive agents these patients must take.16 In theory, increasing the body’s stores of vitamin D during the pretransplant period could lower the rate of bone loss and osteoporosis after cardiac transplantation.
Further investigation is needed to determine whether restoring adequate levels of vitamin D at the time of or after transplantation prevents graft rejection or improves survival.
VITAMIN D SUPPLEMENTATION AND SURVIVAL IN HEART FAILURE
The best laboratory test to assess vitamin D levels is the serum 25-hydroxyvitamin D3 concentration. A level between 20 and 30 ng/mL (50–75 nmol/L) is considered insufficient, and a level below 20 ng/mL (50 nmol/L) represents vitamin D deficiency.4,5,11
Vitamin D insufficiency is typically treated with 800 to 1,000 IU of vitamin D3 daily, whereas deficiency requires 50,000 IU of vitamin D3 weekly for 6 to 8 weeks, followed by 800 to 1,000 IU daily.19 The goal of therapy is to increase the serum 25-hydroxyvitamin D3 level above 30 ng/mL.19
Currently, it is unknown if vitamin D supplementation improves survival in heart failure. We recommend testing for vitamin D deficiency in all patients with heart failure and treating them as described above. For heart failure patients that are not deficient, daily intake of 800 to 1,000 IU of vitamin D is reasonable. Our review underscores the need for more studies to evaluate the efficacy of vitamin D replacement in improving survival in patients with heart failure.
KEY POINTS
- Screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
- Vitamin D deficiency is common in patients with heart failure and in heart transplant recipients.
- In theory, achieving adequate levels of vitamin D would have a beneficial effect on patients with heart failure.
- Randomized controlled trials are needed to determine if vitamin D supplementation confers a survival benefit in patients with heart failure who have deficient vitamin D levels.
- Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007; 103:533–537.
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008; 149:558–564.
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35:290–304.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 2008; 102:1540–1544.
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33.
- Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008; 56:454–461.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR; Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–630.
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009; 29:691–708.
- Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392.
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996; 97:1577–1588.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006; 41:350–359.
- Stein EM, Cohen A, Freeby M, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 2009; (Epub ahead of print)
- Shane E, Rivas M, McMahon DJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82:1497–1506.
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2007; 103:204–205.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16:713–716.
Vitamin D deficiency may play a role in the pathogenesis of chronic heart failure, but whether giving patients supplements to raise their vitamin D levels into the normal range improves their survival is not clear.
ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND OTHER DISORDERS
In the mid-17th century, Whistler and Glisson independently described rickets as a severe bone-deforming disease characterized by growth retardation, bending of the spine, deformities of the legs, and weak and toneless muscles. Histologically, rickets is characterized by impaired mineralization of the cartilage in the epiphyseal growth plates in children. In 1919, Sir Edward Mellanby identified vitamin D deficiency as the cause.
Osteomalacia, another disease caused by vitamin D deficiency, is a disorder of mineralization of newly formed bone matrix in adults. Vitamin D, therefore, has well-known roles in maintaining bone health and calcium and phosphorus homeostasis.
In addition, vitamin D deficiency has been shown in recent years to be associated with myocardial dysfunction.1,2
VITAMIN D METABOLISM IS COMPLEX
In skin exposed to ultraviolet B light, the provitamin 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Vitamin D3 is also obtained from dietary sources. However, many scientists consider vitamin D more a hormone than a classic vitamin, as adequate exposure to sunlight may negate the need for dietary supplements.
The active form of vitamin D is synthesized by hydroxylation in the liver and kidney. In the liver, hepatic enzymes add a hydroxyl (OH) group to vitamin D3, changing it to 25-hydroxyvitamin D3. In the kidney, 25-hydroxyvitamin D3 receives another hydroxyl group, converting it to the biologically active metabolite 1,25-dihydroxyvitamin D3 (calcitriol). This renal hydroxylation is via 1-alpha-hydroxylase activity and is directly under control of parathyroid hormone (PTH), and indirectly under control of the serum concentrations of calcium.
Interestingly, a number of different organ cells, including cardiomyocytes, also express 1-alpha-hydroxylase and therefore also convert 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3. Unlike the renal hydroxylation, this extrarenal process depends on cytokine activation and on serum levels of 25-hydroxyvitamin D3.3 Low levels of 25-hydroxyvitamin D3 lead to alterations in cellular control over growth, differentiation, and function.
The active form of vitamin D is transported protein-bound in the blood to various target organs, where it is delivered in free form to cells. Specific nuclear receptor proteins are found in many organs not classically considered target organs for vitamin D, including the skin, brain, skeletal muscles, cardiomyocytes, vascular endothelial cells, circulating monocytes, and activated B and T lymphocytes. Vitamin D plays a significant role in the autocrine and paracrine regulation of cellular function, growth, and differentiation in various organs.3
MOST HEART FAILURE PATIENTS HAVE LOW VITAMIN D LEVELS
More than 40% of men and 50% of women in the United States have low vitamin D levels (< 30 ng/mL [75 nmol/L])—and low levels in adults are associated with both coronary artery disease and heart failure.4 Most patients with heart failure have low levels.5,6 Therefore, screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
Low vitamin D levels carry a poor prognosis. Pilz et al5 measured baseline 25-hydroxyvitamin D3 levels in 3,299 patients referred for elective coronary angiography and followed them prospectively for a median of 7.7 years. Even after adjustment for cardiac risk factors, patients who had low 25-hydroxyvitamin D3 levels were more likely to die of heart failure or sudden cardiac death than patients with normal levels.
Boxer et al7 found an association between low 25-hydroxyvitamin D3 levels and low exercise capacity and frailty in patients with systolic heart failure.
LOW VITAMIN D CONTRIBUTES TO THE PATHOGENESIS OF HEART FAILURE
In recent years, ideas about the pathophysiology of heart failure have expanded from a purely hemodynamic view to a more complex concept involving inflammatory cytokines and neurohormonal overactivation.8
Animal studies first showed vitamin D to inhibit the renin-angiotensin-aldosterone system, activation of which contributes to the salt and water retention seen in heart failure.4,9
In addition, vitamin D has a number of effects that should help prevent hypertension, an important risk factor for heart failure. It protects the kidney by suppressing the reninangiotensin-aldosterone system, prevents secondary hyperparathyroidism and its effects on vascular stiffness, prevents insulin resistance, and suppresses inflammation, which protects vascular endothelial cells.10
The first studies to show a connection between cardiovascular homeostasis and vitamin D status were in animal models more than 20 years ago. These studies showed that 1,25-dihydroxyvitamin D3 acts directly on cardiomyocyte vitamin D receptors, which are widely distributed throughout the body in several tissue types.11
Excess PTH levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart.12 Animal studies have found that vitamin D suppresses cardiac hypertrophy.13 Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with diastolic heart failure.2,14
Currently, it is unclear whether vitamin D deficiency is a causative risk factor for heart failure or simply a reflection of the poor functional status of patients with heart failure that leads to decreased exposure to sunlight. This debate will continue until further randomized clinical trials address this association.
VITAMIN D AND HEART TRANSPLANTATION
One would expect that patients with endstage organ failure would be at high risk of vitamin D deficiency because of limited sunlight exposure. However, few studies have evaluated the role of this vitamin in heart transplant recipients.
Stein and colleagues15 measured serum 25-hydroxyvitamin D3 immediately after transplantation in 46 heart and 23 liver transplant recipients. Levels were low in both types of transplant recipients, but liver transplant recipients had significantly lower levels than heart transplant patients. This could be explained by malabsorption and impaired synthesis of 25-hydroxyvitamin D3 in end-stage liver disease.
Also, an important point is that osteoporosis is prevalent in postcardiac transplant patients and likely related to the immunosuppressive agents these patients must take.16 In theory, increasing the body’s stores of vitamin D during the pretransplant period could lower the rate of bone loss and osteoporosis after cardiac transplantation.
Further investigation is needed to determine whether restoring adequate levels of vitamin D at the time of or after transplantation prevents graft rejection or improves survival.
VITAMIN D SUPPLEMENTATION AND SURVIVAL IN HEART FAILURE
The best laboratory test to assess vitamin D levels is the serum 25-hydroxyvitamin D3 concentration. A level between 20 and 30 ng/mL (50–75 nmol/L) is considered insufficient, and a level below 20 ng/mL (50 nmol/L) represents vitamin D deficiency.4,5,11
Vitamin D insufficiency is typically treated with 800 to 1,000 IU of vitamin D3 daily, whereas deficiency requires 50,000 IU of vitamin D3 weekly for 6 to 8 weeks, followed by 800 to 1,000 IU daily.19 The goal of therapy is to increase the serum 25-hydroxyvitamin D3 level above 30 ng/mL.19
Currently, it is unknown if vitamin D supplementation improves survival in heart failure. We recommend testing for vitamin D deficiency in all patients with heart failure and treating them as described above. For heart failure patients that are not deficient, daily intake of 800 to 1,000 IU of vitamin D is reasonable. Our review underscores the need for more studies to evaluate the efficacy of vitamin D replacement in improving survival in patients with heart failure.
KEY POINTS
- Screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
- Vitamin D deficiency is common in patients with heart failure and in heart transplant recipients.
- In theory, achieving adequate levels of vitamin D would have a beneficial effect on patients with heart failure.
- Randomized controlled trials are needed to determine if vitamin D supplementation confers a survival benefit in patients with heart failure who have deficient vitamin D levels.
Vitamin D deficiency may play a role in the pathogenesis of chronic heart failure, but whether giving patients supplements to raise their vitamin D levels into the normal range improves their survival is not clear.
ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND OTHER DISORDERS
In the mid-17th century, Whistler and Glisson independently described rickets as a severe bone-deforming disease characterized by growth retardation, bending of the spine, deformities of the legs, and weak and toneless muscles. Histologically, rickets is characterized by impaired mineralization of the cartilage in the epiphyseal growth plates in children. In 1919, Sir Edward Mellanby identified vitamin D deficiency as the cause.
Osteomalacia, another disease caused by vitamin D deficiency, is a disorder of mineralization of newly formed bone matrix in adults. Vitamin D, therefore, has well-known roles in maintaining bone health and calcium and phosphorus homeostasis.
In addition, vitamin D deficiency has been shown in recent years to be associated with myocardial dysfunction.1,2
VITAMIN D METABOLISM IS COMPLEX
In skin exposed to ultraviolet B light, the provitamin 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Vitamin D3 is also obtained from dietary sources. However, many scientists consider vitamin D more a hormone than a classic vitamin, as adequate exposure to sunlight may negate the need for dietary supplements.
The active form of vitamin D is synthesized by hydroxylation in the liver and kidney. In the liver, hepatic enzymes add a hydroxyl (OH) group to vitamin D3, changing it to 25-hydroxyvitamin D3. In the kidney, 25-hydroxyvitamin D3 receives another hydroxyl group, converting it to the biologically active metabolite 1,25-dihydroxyvitamin D3 (calcitriol). This renal hydroxylation is via 1-alpha-hydroxylase activity and is directly under control of parathyroid hormone (PTH), and indirectly under control of the serum concentrations of calcium.
Interestingly, a number of different organ cells, including cardiomyocytes, also express 1-alpha-hydroxylase and therefore also convert 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3. Unlike the renal hydroxylation, this extrarenal process depends on cytokine activation and on serum levels of 25-hydroxyvitamin D3.3 Low levels of 25-hydroxyvitamin D3 lead to alterations in cellular control over growth, differentiation, and function.
The active form of vitamin D is transported protein-bound in the blood to various target organs, where it is delivered in free form to cells. Specific nuclear receptor proteins are found in many organs not classically considered target organs for vitamin D, including the skin, brain, skeletal muscles, cardiomyocytes, vascular endothelial cells, circulating monocytes, and activated B and T lymphocytes. Vitamin D plays a significant role in the autocrine and paracrine regulation of cellular function, growth, and differentiation in various organs.3
MOST HEART FAILURE PATIENTS HAVE LOW VITAMIN D LEVELS
More than 40% of men and 50% of women in the United States have low vitamin D levels (< 30 ng/mL [75 nmol/L])—and low levels in adults are associated with both coronary artery disease and heart failure.4 Most patients with heart failure have low levels.5,6 Therefore, screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
Low vitamin D levels carry a poor prognosis. Pilz et al5 measured baseline 25-hydroxyvitamin D3 levels in 3,299 patients referred for elective coronary angiography and followed them prospectively for a median of 7.7 years. Even after adjustment for cardiac risk factors, patients who had low 25-hydroxyvitamin D3 levels were more likely to die of heart failure or sudden cardiac death than patients with normal levels.
Boxer et al7 found an association between low 25-hydroxyvitamin D3 levels and low exercise capacity and frailty in patients with systolic heart failure.
LOW VITAMIN D CONTRIBUTES TO THE PATHOGENESIS OF HEART FAILURE
In recent years, ideas about the pathophysiology of heart failure have expanded from a purely hemodynamic view to a more complex concept involving inflammatory cytokines and neurohormonal overactivation.8
Animal studies first showed vitamin D to inhibit the renin-angiotensin-aldosterone system, activation of which contributes to the salt and water retention seen in heart failure.4,9
In addition, vitamin D has a number of effects that should help prevent hypertension, an important risk factor for heart failure. It protects the kidney by suppressing the reninangiotensin-aldosterone system, prevents secondary hyperparathyroidism and its effects on vascular stiffness, prevents insulin resistance, and suppresses inflammation, which protects vascular endothelial cells.10
The first studies to show a connection between cardiovascular homeostasis and vitamin D status were in animal models more than 20 years ago. These studies showed that 1,25-dihydroxyvitamin D3 acts directly on cardiomyocyte vitamin D receptors, which are widely distributed throughout the body in several tissue types.11
Excess PTH levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart.12 Animal studies have found that vitamin D suppresses cardiac hypertrophy.13 Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with diastolic heart failure.2,14
Currently, it is unclear whether vitamin D deficiency is a causative risk factor for heart failure or simply a reflection of the poor functional status of patients with heart failure that leads to decreased exposure to sunlight. This debate will continue until further randomized clinical trials address this association.
VITAMIN D AND HEART TRANSPLANTATION
One would expect that patients with endstage organ failure would be at high risk of vitamin D deficiency because of limited sunlight exposure. However, few studies have evaluated the role of this vitamin in heart transplant recipients.
Stein and colleagues15 measured serum 25-hydroxyvitamin D3 immediately after transplantation in 46 heart and 23 liver transplant recipients. Levels were low in both types of transplant recipients, but liver transplant recipients had significantly lower levels than heart transplant patients. This could be explained by malabsorption and impaired synthesis of 25-hydroxyvitamin D3 in end-stage liver disease.
Also, an important point is that osteoporosis is prevalent in postcardiac transplant patients and likely related to the immunosuppressive agents these patients must take.16 In theory, increasing the body’s stores of vitamin D during the pretransplant period could lower the rate of bone loss and osteoporosis after cardiac transplantation.
Further investigation is needed to determine whether restoring adequate levels of vitamin D at the time of or after transplantation prevents graft rejection or improves survival.
VITAMIN D SUPPLEMENTATION AND SURVIVAL IN HEART FAILURE
The best laboratory test to assess vitamin D levels is the serum 25-hydroxyvitamin D3 concentration. A level between 20 and 30 ng/mL (50–75 nmol/L) is considered insufficient, and a level below 20 ng/mL (50 nmol/L) represents vitamin D deficiency.4,5,11
Vitamin D insufficiency is typically treated with 800 to 1,000 IU of vitamin D3 daily, whereas deficiency requires 50,000 IU of vitamin D3 weekly for 6 to 8 weeks, followed by 800 to 1,000 IU daily.19 The goal of therapy is to increase the serum 25-hydroxyvitamin D3 level above 30 ng/mL.19
Currently, it is unknown if vitamin D supplementation improves survival in heart failure. We recommend testing for vitamin D deficiency in all patients with heart failure and treating them as described above. For heart failure patients that are not deficient, daily intake of 800 to 1,000 IU of vitamin D is reasonable. Our review underscores the need for more studies to evaluate the efficacy of vitamin D replacement in improving survival in patients with heart failure.
KEY POINTS
- Screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
- Vitamin D deficiency is common in patients with heart failure and in heart transplant recipients.
- In theory, achieving adequate levels of vitamin D would have a beneficial effect on patients with heart failure.
- Randomized controlled trials are needed to determine if vitamin D supplementation confers a survival benefit in patients with heart failure who have deficient vitamin D levels.
- Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007; 103:533–537.
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008; 149:558–564.
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35:290–304.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 2008; 102:1540–1544.
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33.
- Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008; 56:454–461.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR; Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–630.
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009; 29:691–708.
- Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392.
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996; 97:1577–1588.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006; 41:350–359.
- Stein EM, Cohen A, Freeby M, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 2009; (Epub ahead of print)
- Shane E, Rivas M, McMahon DJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82:1497–1506.
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2007; 103:204–205.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16:713–716.
- Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007; 103:533–537.
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008; 149:558–564.
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35:290–304.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 2008; 102:1540–1544.
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33.
- Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008; 56:454–461.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR; Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–630.
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009; 29:691–708.
- Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392.
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996; 97:1577–1588.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006; 41:350–359.
- Stein EM, Cohen A, Freeby M, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 2009; (Epub ahead of print)
- Shane E, Rivas M, McMahon DJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82:1497–1506.
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2007; 103:204–205.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16:713–716.
Safe, Effective Breast Irradiation In Less Time
Grand Rounds: Man, 65, With Delayed Pain After Hand Injury
A 65-year-old man presented to the emergency department (ED) with a two-week history of progressively severe pain in his right hand and difficulty moving his fingers. He reported that approximately two weeks earlier, while shoveling snow, he slipped and fell, landing on his right hand. Initially, he had no problems with his hand. He finished his shoveling and continued his normal daily activities.
Within two to three days he started to experience pain in his right hand, which grew progressively worse.
Because he did not have a primary care provider, the patient had a limited medical history. He reported having a mildly elevated prostate-specific antigen test years earlier. He underwent an appendectomy at age 15. He denied any other medical problems.
The patient was taking no medications and reported no known allergies to medications. He denied the use of tobacco, said he had one or two beers on an average day, and denied IV drug use. He was an artist and was married with one adult child. His family history was unremarkable with the exception of an alcoholic sister who died of cirrhosis at age 70.
During triage, vital signs were essentially normal: blood pressure, 142/74 mm Hg; heart rate, 78 beats/min; and respiratory rate, 20 breaths/min. The patient was afebrile at 37.2°C (98.9°F). Physical examination was remarkable for some edema and warmth of the right hand without any notable erythema. There was no evidence of any wound. Fingers all had good sensation; however, flexion of the index and long fingers elicited a significant increase in pain.
The remainder of the exam was unremarkable. The patient’s head was normocephalic and atraumatic. Pupils were equal, round, and reactive to light. Eyes were anicteric, and no rhinorrhea was evident. The neck was supple without palpable lymphadenopathy. Lungs were clear to auscultation bilaterally. No wheezes, rales, or rhonchi were appreciated. The heart had a regular rate and rhythm; no murmurs, rubs, or gallups were noted. The abdomen was soft and non-tender. The extremities, except as previously stated, were normal, with good pulses, sensation, and strength.
Initially, only radiographs of the right hand were ordered (see Figures 1 and 2). These demonstrated soft tissue swelling on the dorsum of the hand, and an area of hypodensity between the first and second metacarpals. There were no fractures, dislocations, or other bone or joint abnormalities.
After a review of the radiographs, it was clear that the patient’s diagnosis was not a simple answer of hand contusion or fracture; thus, the evaluation was expanded. Vital signs were repeated three hours after triage: blood pressure, 128/74 mm Hg; heart rate, 76 beats/min; and respiration, 20 breaths/min. The patient was now febrile at 37.6°C (99.7°F). Because of his fever and the anomaly on the patient’s hand radiograph, expansion of the evaluation continued.
Laboratory studies included a complete blood count: white blood cells (WBCs), 30,700/mcL (reference range,1 4,500 to 11,000/mcL); hemoglobin, 13.3 g/dL (13.8 to 17.2 g/dL for men); hematocrit, 40.0% (41% to 50% for men); platelets, 217,000/mcL (130 to 400 x 103/mcL). Initial chemistry panel results were normal except for serum glucose, 143 mg/dL (70 to 125 mg/dL).
Liver function test results were normal except for aspartate aminotransferase, 33 U/L (reference range,1 10 to 30 U/L) and albumin, 2.5 g/dL (3.5 to 5.0 g/dL). Once WBCs were found to exceed 30,000/mcL, the search for a cause was widened once more.
The continued studies included a chest radiograph with normal results, unremarkable CT of the abdomen and pelvis with IV contrast, blood cultures, and urinalysis. The urinalysis showed: blood, moderate; protein, trace; nitrites, positive; leukocytes, large; WBCs > 50/high-power field (reference range,1 5/high-power field or less); and numerous bacteria.
The final study performed in the ED evaluation of the patient was a CT of the right hand with IV contrast (see Figure 3). It demonstrated diffuse edema and a 9.0-mm area of low attenuation with some rim enhancement. The differential for these findings includes an abscess or a foreign body; the latter was deemed unlikely in light of the patient’s physical exam. In consideration of his elevated WBC count, the high number of WBCs in his urine, the fever, and the CT results, the patient was diagnosed with an abscess in his right hand that had been seeded, it was surmised, by an occult urosepsis after his fall.
Before the patient’s admission, a hand surgeon was consulted. The surgeon agreed with the diagnosis, and the patient was taken to the operating room (OR). He had been given piperacillin/tazobactam in the ED.
In the OR, the surgeon made a 3.0-cm incision, conducted an exploration, and identified a cavity that contained a small amount of purulence. He determined the lesion to be a resolving abscess. The wound was washed out, and the area was closed with a Penrose drain.
The patient was continued on the piperacillin/tazobactam. His blood culture was positive for gram-positive rods, and a low-grade fever persisted. An infectious disease specialist was consulted, and levofloxacin was added to the patient’s regimen.
After 24 hours of treatment, findings on urinalysis improved: blood, small; protein, trace; nitrites, negative; leukocytes, small; WBCs, 15 to 20/high-power field; and no bacteria. Over the next three days, the patient’s condition continued to improve. His hand drain was removed, and the pain and swelling subsided. He became afebrile, and his WBC count fell to 24,700/mcL. He was discharged to home with prescriptions for cephalexin and levofloxacin. Follow-up for postoperative care was arranged with the hand surgeon.
Discussion
Pyomyositis is defined as abscess formation deep within large striated muscles.1 Although this condition is uncommon, it is believed that an occult bacteremia can seed an area of damaged muscle (compared with healthy muscle, which ordinarily resists infection), allowing an abscess to form.1,2
Epidemiology
In a 2002 review involving 676 patients with primary pyomyositis, Bickels et al3 reported the condition in ages ranging from two months to 82 years (mean, 28.1 years). In a majority of cases, only a single muscle was involved; 112 patients (16.6%) were identified with multiple-site involvement. Only seven cases (0.1%) involved the hand.
In 452 cases (66.9%), a bacterial agent was identified. Among these, 350 (77%) had a positive culture for Staphylococcus aureus. Other isolates included Streptococcus pyogenes, Escherichia coli, Salmonella enteritidus, and Mycobacterium tuberculosis.1,3 It should be noted that community-acquired methicillin-resistant S aureus (CA-MRSA) is being implicated with increasing frequency in cases of pyomyositis.4-6
Because pyomyositis is not a reportable disease and has not been studied in large clinical trials, its incidence is uncertain, and proposed risk factors have not all been confirmed2 (see Table2,7).
Pathophysiology
While the etiology of primary pyomyositis is unclear, it is believed to be caused by a combination of bacteremia (chronic or transient) and damaged muscle. In a 1960 study published in the Lancet, Smith and Vickers8 performed autopsies on 327 patients who had died of culture-positive septicemia. Only two patients were found to have a muscle abscess. At that time, the investigators concluded that both muscle injury and bacteremia would need to be present in order for an abscess to form. In animal studies, bacteremia (eg, S aureus) does not appear to lead to pyomyositis except in cases of muscle abnormality or trauma (eg, electric shock, pinching injury).9,10
When a history of trauma can be identified in patients with pyomyositis, the condition typically develops near the affected muscle, and the infection appears within days to weeks.3 In cases in which an antecedent infection is identified and hematogenous spread of the bacteria to the skeletal muscle occurs, this is termed secondary pyomyositis.11
Disease Progression
Pyomyositis generally progresses in three stages, beginning with inflammation and advancing to a focal abscess, then to a septic state.3 The first stage develops between seven and 21 days after the initial incident, is typically subacute, involves mild pain and swelling with a “woody” texture, and is occasionally associated with fevers.2
Diagnosis of pyomyositis is usually made during the second stage, 10 to 21 days after the initial incident; by that time, the pain has increased, and the fever is more pronounced. Third-stage infection usually involves fluctuance and sepsis.2
Although MRI is considered most useful in the diagnosis of pyomyositis, CT and ultrasound allow for percutaneous needle aspiration and drainage.3
Treatment
The correct treatment for pyomyositis depends upon the stage at which the disease is identified. During the first stage (before formation of an abscess), antibiotic treatment alone may be sufficient.1 Once an abscess has formed, an incision and drainage will be required, in conjunction with or followed by appropriate antibiotic therapy.
When pyomyositis is properly treated during the first or second stage, a full recovery is likely.2,3 By the third stage, surgical debridement is required. Additionally, osteomyelitis may develop in the adjacent bones, followed by muscle scarring, residual weakness, and functional impairment.2,3 Reported pyomyositis-associated mortality ranges between less than 1% and 4%.2,12
The Case Patient
The case presented here was of particular interest for two reasons. First, the patient had a traumatic injury that initially caused him no concern but worsened progressively over 14 days. Although this is not the typical presentation of a traumatic injury, the ED staff could very easily have performed a radiograph, made a diagnosis of traumatic hand injury, and discharged the patient.
Second, men in their 60s do not commonly have urinary tract infections.13 The patient was questioned frequently by several providers about sexual behaviors, medical problems, and urinary symptoms. Repeatedly, he denied all of these issues. While a urinalysis may be omitted in the evaluation of an otherwise healthy, asymptomatic patient, its results in this case were a key piece of data.
It should be noted that the patient thought it inappropriate to be asked for urine samples. He repeatedly said, “It’s my hand!”
Conclusion
Even in patients presenting with the most routine complaint, a careful evaluation can reveal unexpected, serious problems. This patient complained of pain in his hand some time after a fall and ultimately was treated for an occult urosepsis and hand abscess—pyomyositis, which rarely occurs in small muscles, such as those of the hand. Either condition, left untreated, could have led to serious morbidity or even mortality.
1. Beers MH, Berkow R, eds. Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories, 2006:1142-1143.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494.
3. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Current concepts review: primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277-2286.
4. Lo BM, Fickenscher BA. Primary pyomyositis caused by ca-MRSA. Int J Emerg Med. 2008;1(4):331-332.
5. Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352(14):1488–1489.
6. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43(8):953–960.
7. Ükinç K, Bayraktar M, Uzun O. A case of type 2 diabetes complicated with primary pyomyositis. Endocrinologist. 2009;19(3):129-130.
8. Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1(7138):1318-1322.
9. Phoon E-S, Sebastin SJ, Tay S-C. Primary pyomyositis (bacterial myositis) of the pronator quadratus. J Hand Surg Eur Vol. 2009;34(4):549-551.
10. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992; 15(4):668-677.
11. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006;29(11):1030-1032.
12. Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117(6):420–428.
13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 suppl 1A:5S-13S.
A 65-year-old man presented to the emergency department (ED) with a two-week history of progressively severe pain in his right hand and difficulty moving his fingers. He reported that approximately two weeks earlier, while shoveling snow, he slipped and fell, landing on his right hand. Initially, he had no problems with his hand. He finished his shoveling and continued his normal daily activities.
Within two to three days he started to experience pain in his right hand, which grew progressively worse.
Because he did not have a primary care provider, the patient had a limited medical history. He reported having a mildly elevated prostate-specific antigen test years earlier. He underwent an appendectomy at age 15. He denied any other medical problems.
The patient was taking no medications and reported no known allergies to medications. He denied the use of tobacco, said he had one or two beers on an average day, and denied IV drug use. He was an artist and was married with one adult child. His family history was unremarkable with the exception of an alcoholic sister who died of cirrhosis at age 70.
During triage, vital signs were essentially normal: blood pressure, 142/74 mm Hg; heart rate, 78 beats/min; and respiratory rate, 20 breaths/min. The patient was afebrile at 37.2°C (98.9°F). Physical examination was remarkable for some edema and warmth of the right hand without any notable erythema. There was no evidence of any wound. Fingers all had good sensation; however, flexion of the index and long fingers elicited a significant increase in pain.
The remainder of the exam was unremarkable. The patient’s head was normocephalic and atraumatic. Pupils were equal, round, and reactive to light. Eyes were anicteric, and no rhinorrhea was evident. The neck was supple without palpable lymphadenopathy. Lungs were clear to auscultation bilaterally. No wheezes, rales, or rhonchi were appreciated. The heart had a regular rate and rhythm; no murmurs, rubs, or gallups were noted. The abdomen was soft and non-tender. The extremities, except as previously stated, were normal, with good pulses, sensation, and strength.
Initially, only radiographs of the right hand were ordered (see Figures 1 and 2). These demonstrated soft tissue swelling on the dorsum of the hand, and an area of hypodensity between the first and second metacarpals. There were no fractures, dislocations, or other bone or joint abnormalities.
After a review of the radiographs, it was clear that the patient’s diagnosis was not a simple answer of hand contusion or fracture; thus, the evaluation was expanded. Vital signs were repeated three hours after triage: blood pressure, 128/74 mm Hg; heart rate, 76 beats/min; and respiration, 20 breaths/min. The patient was now febrile at 37.6°C (99.7°F). Because of his fever and the anomaly on the patient’s hand radiograph, expansion of the evaluation continued.
Laboratory studies included a complete blood count: white blood cells (WBCs), 30,700/mcL (reference range,1 4,500 to 11,000/mcL); hemoglobin, 13.3 g/dL (13.8 to 17.2 g/dL for men); hematocrit, 40.0% (41% to 50% for men); platelets, 217,000/mcL (130 to 400 x 103/mcL). Initial chemistry panel results were normal except for serum glucose, 143 mg/dL (70 to 125 mg/dL).
Liver function test results were normal except for aspartate aminotransferase, 33 U/L (reference range,1 10 to 30 U/L) and albumin, 2.5 g/dL (3.5 to 5.0 g/dL). Once WBCs were found to exceed 30,000/mcL, the search for a cause was widened once more.
The continued studies included a chest radiograph with normal results, unremarkable CT of the abdomen and pelvis with IV contrast, blood cultures, and urinalysis. The urinalysis showed: blood, moderate; protein, trace; nitrites, positive; leukocytes, large; WBCs > 50/high-power field (reference range,1 5/high-power field or less); and numerous bacteria.
The final study performed in the ED evaluation of the patient was a CT of the right hand with IV contrast (see Figure 3). It demonstrated diffuse edema and a 9.0-mm area of low attenuation with some rim enhancement. The differential for these findings includes an abscess or a foreign body; the latter was deemed unlikely in light of the patient’s physical exam. In consideration of his elevated WBC count, the high number of WBCs in his urine, the fever, and the CT results, the patient was diagnosed with an abscess in his right hand that had been seeded, it was surmised, by an occult urosepsis after his fall.
Before the patient’s admission, a hand surgeon was consulted. The surgeon agreed with the diagnosis, and the patient was taken to the operating room (OR). He had been given piperacillin/tazobactam in the ED.
In the OR, the surgeon made a 3.0-cm incision, conducted an exploration, and identified a cavity that contained a small amount of purulence. He determined the lesion to be a resolving abscess. The wound was washed out, and the area was closed with a Penrose drain.
The patient was continued on the piperacillin/tazobactam. His blood culture was positive for gram-positive rods, and a low-grade fever persisted. An infectious disease specialist was consulted, and levofloxacin was added to the patient’s regimen.
After 24 hours of treatment, findings on urinalysis improved: blood, small; protein, trace; nitrites, negative; leukocytes, small; WBCs, 15 to 20/high-power field; and no bacteria. Over the next three days, the patient’s condition continued to improve. His hand drain was removed, and the pain and swelling subsided. He became afebrile, and his WBC count fell to 24,700/mcL. He was discharged to home with prescriptions for cephalexin and levofloxacin. Follow-up for postoperative care was arranged with the hand surgeon.
Discussion
Pyomyositis is defined as abscess formation deep within large striated muscles.1 Although this condition is uncommon, it is believed that an occult bacteremia can seed an area of damaged muscle (compared with healthy muscle, which ordinarily resists infection), allowing an abscess to form.1,2
Epidemiology
In a 2002 review involving 676 patients with primary pyomyositis, Bickels et al3 reported the condition in ages ranging from two months to 82 years (mean, 28.1 years). In a majority of cases, only a single muscle was involved; 112 patients (16.6%) were identified with multiple-site involvement. Only seven cases (0.1%) involved the hand.
In 452 cases (66.9%), a bacterial agent was identified. Among these, 350 (77%) had a positive culture for Staphylococcus aureus. Other isolates included Streptococcus pyogenes, Escherichia coli, Salmonella enteritidus, and Mycobacterium tuberculosis.1,3 It should be noted that community-acquired methicillin-resistant S aureus (CA-MRSA) is being implicated with increasing frequency in cases of pyomyositis.4-6
Because pyomyositis is not a reportable disease and has not been studied in large clinical trials, its incidence is uncertain, and proposed risk factors have not all been confirmed2 (see Table2,7).
Pathophysiology
While the etiology of primary pyomyositis is unclear, it is believed to be caused by a combination of bacteremia (chronic or transient) and damaged muscle. In a 1960 study published in the Lancet, Smith and Vickers8 performed autopsies on 327 patients who had died of culture-positive septicemia. Only two patients were found to have a muscle abscess. At that time, the investigators concluded that both muscle injury and bacteremia would need to be present in order for an abscess to form. In animal studies, bacteremia (eg, S aureus) does not appear to lead to pyomyositis except in cases of muscle abnormality or trauma (eg, electric shock, pinching injury).9,10
When a history of trauma can be identified in patients with pyomyositis, the condition typically develops near the affected muscle, and the infection appears within days to weeks.3 In cases in which an antecedent infection is identified and hematogenous spread of the bacteria to the skeletal muscle occurs, this is termed secondary pyomyositis.11
Disease Progression
Pyomyositis generally progresses in three stages, beginning with inflammation and advancing to a focal abscess, then to a septic state.3 The first stage develops between seven and 21 days after the initial incident, is typically subacute, involves mild pain and swelling with a “woody” texture, and is occasionally associated with fevers.2
Diagnosis of pyomyositis is usually made during the second stage, 10 to 21 days after the initial incident; by that time, the pain has increased, and the fever is more pronounced. Third-stage infection usually involves fluctuance and sepsis.2
Although MRI is considered most useful in the diagnosis of pyomyositis, CT and ultrasound allow for percutaneous needle aspiration and drainage.3
Treatment
The correct treatment for pyomyositis depends upon the stage at which the disease is identified. During the first stage (before formation of an abscess), antibiotic treatment alone may be sufficient.1 Once an abscess has formed, an incision and drainage will be required, in conjunction with or followed by appropriate antibiotic therapy.
When pyomyositis is properly treated during the first or second stage, a full recovery is likely.2,3 By the third stage, surgical debridement is required. Additionally, osteomyelitis may develop in the adjacent bones, followed by muscle scarring, residual weakness, and functional impairment.2,3 Reported pyomyositis-associated mortality ranges between less than 1% and 4%.2,12
The Case Patient
The case presented here was of particular interest for two reasons. First, the patient had a traumatic injury that initially caused him no concern but worsened progressively over 14 days. Although this is not the typical presentation of a traumatic injury, the ED staff could very easily have performed a radiograph, made a diagnosis of traumatic hand injury, and discharged the patient.
Second, men in their 60s do not commonly have urinary tract infections.13 The patient was questioned frequently by several providers about sexual behaviors, medical problems, and urinary symptoms. Repeatedly, he denied all of these issues. While a urinalysis may be omitted in the evaluation of an otherwise healthy, asymptomatic patient, its results in this case were a key piece of data.
It should be noted that the patient thought it inappropriate to be asked for urine samples. He repeatedly said, “It’s my hand!”
Conclusion
Even in patients presenting with the most routine complaint, a careful evaluation can reveal unexpected, serious problems. This patient complained of pain in his hand some time after a fall and ultimately was treated for an occult urosepsis and hand abscess—pyomyositis, which rarely occurs in small muscles, such as those of the hand. Either condition, left untreated, could have led to serious morbidity or even mortality.
A 65-year-old man presented to the emergency department (ED) with a two-week history of progressively severe pain in his right hand and difficulty moving his fingers. He reported that approximately two weeks earlier, while shoveling snow, he slipped and fell, landing on his right hand. Initially, he had no problems with his hand. He finished his shoveling and continued his normal daily activities.
Within two to three days he started to experience pain in his right hand, which grew progressively worse.
Because he did not have a primary care provider, the patient had a limited medical history. He reported having a mildly elevated prostate-specific antigen test years earlier. He underwent an appendectomy at age 15. He denied any other medical problems.
The patient was taking no medications and reported no known allergies to medications. He denied the use of tobacco, said he had one or two beers on an average day, and denied IV drug use. He was an artist and was married with one adult child. His family history was unremarkable with the exception of an alcoholic sister who died of cirrhosis at age 70.
During triage, vital signs were essentially normal: blood pressure, 142/74 mm Hg; heart rate, 78 beats/min; and respiratory rate, 20 breaths/min. The patient was afebrile at 37.2°C (98.9°F). Physical examination was remarkable for some edema and warmth of the right hand without any notable erythema. There was no evidence of any wound. Fingers all had good sensation; however, flexion of the index and long fingers elicited a significant increase in pain.
The remainder of the exam was unremarkable. The patient’s head was normocephalic and atraumatic. Pupils were equal, round, and reactive to light. Eyes were anicteric, and no rhinorrhea was evident. The neck was supple without palpable lymphadenopathy. Lungs were clear to auscultation bilaterally. No wheezes, rales, or rhonchi were appreciated. The heart had a regular rate and rhythm; no murmurs, rubs, or gallups were noted. The abdomen was soft and non-tender. The extremities, except as previously stated, were normal, with good pulses, sensation, and strength.
Initially, only radiographs of the right hand were ordered (see Figures 1 and 2). These demonstrated soft tissue swelling on the dorsum of the hand, and an area of hypodensity between the first and second metacarpals. There were no fractures, dislocations, or other bone or joint abnormalities.
After a review of the radiographs, it was clear that the patient’s diagnosis was not a simple answer of hand contusion or fracture; thus, the evaluation was expanded. Vital signs were repeated three hours after triage: blood pressure, 128/74 mm Hg; heart rate, 76 beats/min; and respiration, 20 breaths/min. The patient was now febrile at 37.6°C (99.7°F). Because of his fever and the anomaly on the patient’s hand radiograph, expansion of the evaluation continued.
Laboratory studies included a complete blood count: white blood cells (WBCs), 30,700/mcL (reference range,1 4,500 to 11,000/mcL); hemoglobin, 13.3 g/dL (13.8 to 17.2 g/dL for men); hematocrit, 40.0% (41% to 50% for men); platelets, 217,000/mcL (130 to 400 x 103/mcL). Initial chemistry panel results were normal except for serum glucose, 143 mg/dL (70 to 125 mg/dL).
Liver function test results were normal except for aspartate aminotransferase, 33 U/L (reference range,1 10 to 30 U/L) and albumin, 2.5 g/dL (3.5 to 5.0 g/dL). Once WBCs were found to exceed 30,000/mcL, the search for a cause was widened once more.
The continued studies included a chest radiograph with normal results, unremarkable CT of the abdomen and pelvis with IV contrast, blood cultures, and urinalysis. The urinalysis showed: blood, moderate; protein, trace; nitrites, positive; leukocytes, large; WBCs > 50/high-power field (reference range,1 5/high-power field or less); and numerous bacteria.
The final study performed in the ED evaluation of the patient was a CT of the right hand with IV contrast (see Figure 3). It demonstrated diffuse edema and a 9.0-mm area of low attenuation with some rim enhancement. The differential for these findings includes an abscess or a foreign body; the latter was deemed unlikely in light of the patient’s physical exam. In consideration of his elevated WBC count, the high number of WBCs in his urine, the fever, and the CT results, the patient was diagnosed with an abscess in his right hand that had been seeded, it was surmised, by an occult urosepsis after his fall.
Before the patient’s admission, a hand surgeon was consulted. The surgeon agreed with the diagnosis, and the patient was taken to the operating room (OR). He had been given piperacillin/tazobactam in the ED.
In the OR, the surgeon made a 3.0-cm incision, conducted an exploration, and identified a cavity that contained a small amount of purulence. He determined the lesion to be a resolving abscess. The wound was washed out, and the area was closed with a Penrose drain.
The patient was continued on the piperacillin/tazobactam. His blood culture was positive for gram-positive rods, and a low-grade fever persisted. An infectious disease specialist was consulted, and levofloxacin was added to the patient’s regimen.
After 24 hours of treatment, findings on urinalysis improved: blood, small; protein, trace; nitrites, negative; leukocytes, small; WBCs, 15 to 20/high-power field; and no bacteria. Over the next three days, the patient’s condition continued to improve. His hand drain was removed, and the pain and swelling subsided. He became afebrile, and his WBC count fell to 24,700/mcL. He was discharged to home with prescriptions for cephalexin and levofloxacin. Follow-up for postoperative care was arranged with the hand surgeon.
Discussion
Pyomyositis is defined as abscess formation deep within large striated muscles.1 Although this condition is uncommon, it is believed that an occult bacteremia can seed an area of damaged muscle (compared with healthy muscle, which ordinarily resists infection), allowing an abscess to form.1,2
Epidemiology
In a 2002 review involving 676 patients with primary pyomyositis, Bickels et al3 reported the condition in ages ranging from two months to 82 years (mean, 28.1 years). In a majority of cases, only a single muscle was involved; 112 patients (16.6%) were identified with multiple-site involvement. Only seven cases (0.1%) involved the hand.
In 452 cases (66.9%), a bacterial agent was identified. Among these, 350 (77%) had a positive culture for Staphylococcus aureus. Other isolates included Streptococcus pyogenes, Escherichia coli, Salmonella enteritidus, and Mycobacterium tuberculosis.1,3 It should be noted that community-acquired methicillin-resistant S aureus (CA-MRSA) is being implicated with increasing frequency in cases of pyomyositis.4-6
Because pyomyositis is not a reportable disease and has not been studied in large clinical trials, its incidence is uncertain, and proposed risk factors have not all been confirmed2 (see Table2,7).
Pathophysiology
While the etiology of primary pyomyositis is unclear, it is believed to be caused by a combination of bacteremia (chronic or transient) and damaged muscle. In a 1960 study published in the Lancet, Smith and Vickers8 performed autopsies on 327 patients who had died of culture-positive septicemia. Only two patients were found to have a muscle abscess. At that time, the investigators concluded that both muscle injury and bacteremia would need to be present in order for an abscess to form. In animal studies, bacteremia (eg, S aureus) does not appear to lead to pyomyositis except in cases of muscle abnormality or trauma (eg, electric shock, pinching injury).9,10
When a history of trauma can be identified in patients with pyomyositis, the condition typically develops near the affected muscle, and the infection appears within days to weeks.3 In cases in which an antecedent infection is identified and hematogenous spread of the bacteria to the skeletal muscle occurs, this is termed secondary pyomyositis.11
Disease Progression
Pyomyositis generally progresses in three stages, beginning with inflammation and advancing to a focal abscess, then to a septic state.3 The first stage develops between seven and 21 days after the initial incident, is typically subacute, involves mild pain and swelling with a “woody” texture, and is occasionally associated with fevers.2
Diagnosis of pyomyositis is usually made during the second stage, 10 to 21 days after the initial incident; by that time, the pain has increased, and the fever is more pronounced. Third-stage infection usually involves fluctuance and sepsis.2
Although MRI is considered most useful in the diagnosis of pyomyositis, CT and ultrasound allow for percutaneous needle aspiration and drainage.3
Treatment
The correct treatment for pyomyositis depends upon the stage at which the disease is identified. During the first stage (before formation of an abscess), antibiotic treatment alone may be sufficient.1 Once an abscess has formed, an incision and drainage will be required, in conjunction with or followed by appropriate antibiotic therapy.
When pyomyositis is properly treated during the first or second stage, a full recovery is likely.2,3 By the third stage, surgical debridement is required. Additionally, osteomyelitis may develop in the adjacent bones, followed by muscle scarring, residual weakness, and functional impairment.2,3 Reported pyomyositis-associated mortality ranges between less than 1% and 4%.2,12
The Case Patient
The case presented here was of particular interest for two reasons. First, the patient had a traumatic injury that initially caused him no concern but worsened progressively over 14 days. Although this is not the typical presentation of a traumatic injury, the ED staff could very easily have performed a radiograph, made a diagnosis of traumatic hand injury, and discharged the patient.
Second, men in their 60s do not commonly have urinary tract infections.13 The patient was questioned frequently by several providers about sexual behaviors, medical problems, and urinary symptoms. Repeatedly, he denied all of these issues. While a urinalysis may be omitted in the evaluation of an otherwise healthy, asymptomatic patient, its results in this case were a key piece of data.
It should be noted that the patient thought it inappropriate to be asked for urine samples. He repeatedly said, “It’s my hand!”
Conclusion
Even in patients presenting with the most routine complaint, a careful evaluation can reveal unexpected, serious problems. This patient complained of pain in his hand some time after a fall and ultimately was treated for an occult urosepsis and hand abscess—pyomyositis, which rarely occurs in small muscles, such as those of the hand. Either condition, left untreated, could have led to serious morbidity or even mortality.
1. Beers MH, Berkow R, eds. Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories, 2006:1142-1143.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494.
3. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Current concepts review: primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277-2286.
4. Lo BM, Fickenscher BA. Primary pyomyositis caused by ca-MRSA. Int J Emerg Med. 2008;1(4):331-332.
5. Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352(14):1488–1489.
6. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43(8):953–960.
7. Ükinç K, Bayraktar M, Uzun O. A case of type 2 diabetes complicated with primary pyomyositis. Endocrinologist. 2009;19(3):129-130.
8. Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1(7138):1318-1322.
9. Phoon E-S, Sebastin SJ, Tay S-C. Primary pyomyositis (bacterial myositis) of the pronator quadratus. J Hand Surg Eur Vol. 2009;34(4):549-551.
10. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992; 15(4):668-677.
11. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006;29(11):1030-1032.
12. Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117(6):420–428.
13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 suppl 1A:5S-13S.
1. Beers MH, Berkow R, eds. Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories, 2006:1142-1143.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494.
3. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Current concepts review: primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277-2286.
4. Lo BM, Fickenscher BA. Primary pyomyositis caused by ca-MRSA. Int J Emerg Med. 2008;1(4):331-332.
5. Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352(14):1488–1489.
6. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43(8):953–960.
7. Ükinç K, Bayraktar M, Uzun O. A case of type 2 diabetes complicated with primary pyomyositis. Endocrinologist. 2009;19(3):129-130.
8. Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1(7138):1318-1322.
9. Phoon E-S, Sebastin SJ, Tay S-C. Primary pyomyositis (bacterial myositis) of the pronator quadratus. J Hand Surg Eur Vol. 2009;34(4):549-551.
10. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992; 15(4):668-677.
11. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006;29(11):1030-1032.
12. Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117(6):420–428.
13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 suppl 1A:5S-13S.
Structural Bone Allograft in Pediatric Foot Surgery
Bone Graft Placement by Modified Plastic Syringe
What's Eating You? Cat Flea (Ctenocephalides felis), Part 1: Clinical Features and Role as a Disease Vector
USPSTF recommendations you may have missed amid the breast cancer controversy
Late in 2009, a change in the recommendations of the US Preventive Services Task Force (USPSTF) brought more public attention to this panel than it had ever experienced before. This publicity centered on revised recommendations on breast cancer screening that pointed out that mammograms benefit a few women under 50, but are also associated with some harms. The Task Force recommended that patients and physicians discuss these potential benefits and harms and make an individual decision about whether to have a mammogram.1
Even though the criticism was loud—and harsh—from some sectors, many professional organizations, including the American Academy of Family Physicians, the American College of Physicians, and the American College of Preventive Medicine, came to the defense of the Task Force and its rigorous, evidence-based methodology.2-4 Both the Journal of the American Medical Association and the Annals of Internal Medicine have since published a series of articles and opinions on the controversy, most of them favorable to the Task Force and its methods.2-9
Lost in all the brouhaha were a number of other, less controversial recommendations that the Task Force made in 2009 (and early 2010). You can find them at www.ahrq.gov/clinic/uspstfix.htm. They are categorized by strength of recommendation (TABLE 1) and listed in TABLES 2 and 3. Family physicians should review the A and B recommendations and try to incorporate those into practice. At the same time, we should avoid services in the D category, as the evidence is strong that they are not effective or cause more harm than benefit. The C and I recommendations leave more discretion for physicians and patients to decide on these interventions based on personal values and risks. A C recommendation means the service can benefit some individuals, but the totality of benefit is small. An I recommendation means that evidence is insufficient to evaluate benefits vs harms.
TABLE 1
US Preventive Services Task Force recommendation categories
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| Source: Agency for Healthcare Research and Quality. US Preventive Services Task Force (USPSTF) ratings. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/ratingsv2.htm. Accessed September 5, 2013. | |
TABLE 2
USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| PREGNANCY |
|
| CANCER SCREENING |
|
| DEPRESSION |
|
| OBESITY |
|
TABLE 3
USPSTF recommends AGAINST routinely
|
| USPSTF recommends AGAINST |
|
| USPSTF indicates the evidence is INSUFFICIENT to assess the balance of benefits and harms of |
|
| Source: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed April 2, 2010. |
The A and B recommendations you may have missed
The major additions to the A and B recommendations pertained to the use of aspirin to prevent cardiovascular disease, routine screening for depression in adults and adolescents, and screening for obesity in children ages 6 and older. The other recommendations in these categories were reaffirmations of previous recommendations (asking about smoking and providing smoking cessation guidance to adults and pregnant women, advising folic acid supplementation for women planning or capable of pregnancy, and screening pregnant women for syphilis and hepatitis B virus) and the more controversial recommendation for biennial rather than annual mammography for women ages 50 to 74.
The use of aspirin to prevent myocardial infarction in men ages 45 to 79 and ischemic strokes in women ages 55 to 79 was endorsed if a patient’s risk of these cardiovascular events exceeds the risk of bleeding from regular aspirin use. The Task Force recommendation statement is available athttp://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htmand provides links to tools for calculating the risk of a myocardial infarction (MI) and ischemic stroke, as well as 2 tables to compare the risks and benefits of aspirin therapy for prevention.
Screening adults for depression is endorsed if “staff-assisted depression care supports” are in place to assure accurate diagnosis, effective treatment, and follow-up. Such support includes the presence of clinical staff members who can assist the primary care provider with care support or coordination, case management, or mental health treatment. The definition can be accessed athttp://www.ahrq.gov/clinic/uspstf09/adultdepression/addeprrs.htm.
One example in the statement describes “a successful study designed for practices without ready access to mental health specialty care, (in which) office staff recruited, screened, and enrolled participants who screened positive for depression before a clinic visit. If the physician confirmed the depression diagnosis, the participant was scheduled for a return visit with the physician and to meet with the nurse specialist in 1 week. The nurse specialist reassessed the patient’s level of depression, discussed treatment options and preferences, and asked the participant to complete a homework assignment. Participants completed up to 8 additional sessions that followed the same pattern, either by phone or in person.”
Screening for major depressive disorder (MDD) in adolescents 12 to 18 years of age is recommended when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive-behavioral or interpersonal), and follow-up. The Task Force addressed screening for MDD only—not for less severe depression. The instruments the group recommended using included the Patient Health Questionnaire for Adolescents (PHQ-A) and the Beck Depression Inventory-Primary Care Version (BDI-PC).
The recommendation for screening for obesity in children ages 6 and older reflects the difficulty in achieving long-term, sustainable weight loss in this group. Effective comprehensive weight-management programs include counseling and other interventions that target both diet and physical activity. Behavioral interventions and parental involvement are also encouraged. Moderate- to high-intensity programs include more than 25 hours of contact with the child and/or the family over a 6-month period; less than this does not result in sustained improvement.
What about the D and I categories?
Two interventions received a D recommendation: Use of aspirin for stroke prevention in women <55 years and for MI prevention in men <45 years, and teaching breast self-examination (BSE) to women. The BSE recommendation has been misinterpreted as recommending against women performing self-breast exams. The recommendation is against formalized teaching of the procedure by physicians, as this leads to increased false positives and no improvement in outcomes when compared to women performing exams on their own.
The list of interventions receiving an I recommendation include some services that are commonly offered in the belief that they are effective. The Task Force is attempting to develop methodologies to decrease the number of interventions that receive an I recommendation. Currently, about 40% of all recommendations end up in this category, and physicians and patients alike could use more guidance on them. This plethora of recommendations made with insufficient evidence reflects the “ready, shoot, aim” philosophy of American medicine. We tend to accept and adopt new interventions before they are proven effective. The I recommendations are valuable reminders that, while many interventions are in common use, we often do not know as much as we should about their benefits and harms.
1. Agency for Healthcare Research and Quality. Screening for breast cancer. Updated December 2009. Available at: www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 17, 2010.
2. Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303:162-163.
3. Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164-165.
4. Murphy AM. Mammography screening for breast cancer: a view from 2 worlds. JAMA. 2010;303:166-167.
5. Berg WA. Benefits of screening mammography. JAMA. 2010;303:168-169.
6. DeAngelis CF, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303:172-173.
7. Editors’ note on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00209.full. Accessed April 7, 2010.
8. Begg CB. Comments and response on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00203.full. Accessed April 7, 2010.
9. Jorgensen KJ, Gotzsche PC. The background review for the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00198.full. Accessed April 7, 2010.
Late in 2009, a change in the recommendations of the US Preventive Services Task Force (USPSTF) brought more public attention to this panel than it had ever experienced before. This publicity centered on revised recommendations on breast cancer screening that pointed out that mammograms benefit a few women under 50, but are also associated with some harms. The Task Force recommended that patients and physicians discuss these potential benefits and harms and make an individual decision about whether to have a mammogram.1
Even though the criticism was loud—and harsh—from some sectors, many professional organizations, including the American Academy of Family Physicians, the American College of Physicians, and the American College of Preventive Medicine, came to the defense of the Task Force and its rigorous, evidence-based methodology.2-4 Both the Journal of the American Medical Association and the Annals of Internal Medicine have since published a series of articles and opinions on the controversy, most of them favorable to the Task Force and its methods.2-9
Lost in all the brouhaha were a number of other, less controversial recommendations that the Task Force made in 2009 (and early 2010). You can find them at www.ahrq.gov/clinic/uspstfix.htm. They are categorized by strength of recommendation (TABLE 1) and listed in TABLES 2 and 3. Family physicians should review the A and B recommendations and try to incorporate those into practice. At the same time, we should avoid services in the D category, as the evidence is strong that they are not effective or cause more harm than benefit. The C and I recommendations leave more discretion for physicians and patients to decide on these interventions based on personal values and risks. A C recommendation means the service can benefit some individuals, but the totality of benefit is small. An I recommendation means that evidence is insufficient to evaluate benefits vs harms.
TABLE 1
US Preventive Services Task Force recommendation categories
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| Source: Agency for Healthcare Research and Quality. US Preventive Services Task Force (USPSTF) ratings. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/ratingsv2.htm. Accessed September 5, 2013. | |
TABLE 2
USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| PREGNANCY |
|
| CANCER SCREENING |
|
| DEPRESSION |
|
| OBESITY |
|
TABLE 3
USPSTF recommends AGAINST routinely
|
| USPSTF recommends AGAINST |
|
| USPSTF indicates the evidence is INSUFFICIENT to assess the balance of benefits and harms of |
|
| Source: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed April 2, 2010. |
The A and B recommendations you may have missed
The major additions to the A and B recommendations pertained to the use of aspirin to prevent cardiovascular disease, routine screening for depression in adults and adolescents, and screening for obesity in children ages 6 and older. The other recommendations in these categories were reaffirmations of previous recommendations (asking about smoking and providing smoking cessation guidance to adults and pregnant women, advising folic acid supplementation for women planning or capable of pregnancy, and screening pregnant women for syphilis and hepatitis B virus) and the more controversial recommendation for biennial rather than annual mammography for women ages 50 to 74.
The use of aspirin to prevent myocardial infarction in men ages 45 to 79 and ischemic strokes in women ages 55 to 79 was endorsed if a patient’s risk of these cardiovascular events exceeds the risk of bleeding from regular aspirin use. The Task Force recommendation statement is available athttp://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htmand provides links to tools for calculating the risk of a myocardial infarction (MI) and ischemic stroke, as well as 2 tables to compare the risks and benefits of aspirin therapy for prevention.
Screening adults for depression is endorsed if “staff-assisted depression care supports” are in place to assure accurate diagnosis, effective treatment, and follow-up. Such support includes the presence of clinical staff members who can assist the primary care provider with care support or coordination, case management, or mental health treatment. The definition can be accessed athttp://www.ahrq.gov/clinic/uspstf09/adultdepression/addeprrs.htm.
One example in the statement describes “a successful study designed for practices without ready access to mental health specialty care, (in which) office staff recruited, screened, and enrolled participants who screened positive for depression before a clinic visit. If the physician confirmed the depression diagnosis, the participant was scheduled for a return visit with the physician and to meet with the nurse specialist in 1 week. The nurse specialist reassessed the patient’s level of depression, discussed treatment options and preferences, and asked the participant to complete a homework assignment. Participants completed up to 8 additional sessions that followed the same pattern, either by phone or in person.”
Screening for major depressive disorder (MDD) in adolescents 12 to 18 years of age is recommended when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive-behavioral or interpersonal), and follow-up. The Task Force addressed screening for MDD only—not for less severe depression. The instruments the group recommended using included the Patient Health Questionnaire for Adolescents (PHQ-A) and the Beck Depression Inventory-Primary Care Version (BDI-PC).
The recommendation for screening for obesity in children ages 6 and older reflects the difficulty in achieving long-term, sustainable weight loss in this group. Effective comprehensive weight-management programs include counseling and other interventions that target both diet and physical activity. Behavioral interventions and parental involvement are also encouraged. Moderate- to high-intensity programs include more than 25 hours of contact with the child and/or the family over a 6-month period; less than this does not result in sustained improvement.
What about the D and I categories?
Two interventions received a D recommendation: Use of aspirin for stroke prevention in women <55 years and for MI prevention in men <45 years, and teaching breast self-examination (BSE) to women. The BSE recommendation has been misinterpreted as recommending against women performing self-breast exams. The recommendation is against formalized teaching of the procedure by physicians, as this leads to increased false positives and no improvement in outcomes when compared to women performing exams on their own.
The list of interventions receiving an I recommendation include some services that are commonly offered in the belief that they are effective. The Task Force is attempting to develop methodologies to decrease the number of interventions that receive an I recommendation. Currently, about 40% of all recommendations end up in this category, and physicians and patients alike could use more guidance on them. This plethora of recommendations made with insufficient evidence reflects the “ready, shoot, aim” philosophy of American medicine. We tend to accept and adopt new interventions before they are proven effective. The I recommendations are valuable reminders that, while many interventions are in common use, we often do not know as much as we should about their benefits and harms.
Late in 2009, a change in the recommendations of the US Preventive Services Task Force (USPSTF) brought more public attention to this panel than it had ever experienced before. This publicity centered on revised recommendations on breast cancer screening that pointed out that mammograms benefit a few women under 50, but are also associated with some harms. The Task Force recommended that patients and physicians discuss these potential benefits and harms and make an individual decision about whether to have a mammogram.1
Even though the criticism was loud—and harsh—from some sectors, many professional organizations, including the American Academy of Family Physicians, the American College of Physicians, and the American College of Preventive Medicine, came to the defense of the Task Force and its rigorous, evidence-based methodology.2-4 Both the Journal of the American Medical Association and the Annals of Internal Medicine have since published a series of articles and opinions on the controversy, most of them favorable to the Task Force and its methods.2-9
Lost in all the brouhaha were a number of other, less controversial recommendations that the Task Force made in 2009 (and early 2010). You can find them at www.ahrq.gov/clinic/uspstfix.htm. They are categorized by strength of recommendation (TABLE 1) and listed in TABLES 2 and 3. Family physicians should review the A and B recommendations and try to incorporate those into practice. At the same time, we should avoid services in the D category, as the evidence is strong that they are not effective or cause more harm than benefit. The C and I recommendations leave more discretion for physicians and patients to decide on these interventions based on personal values and risks. A C recommendation means the service can benefit some individuals, but the totality of benefit is small. An I recommendation means that evidence is insufficient to evaluate benefits vs harms.
TABLE 1
US Preventive Services Task Force recommendation categories
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| Source: Agency for Healthcare Research and Quality. US Preventive Services Task Force (USPSTF) ratings. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/ratingsv2.htm. Accessed September 5, 2013. | |
TABLE 2
USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| PREGNANCY |
|
| CANCER SCREENING |
|
| DEPRESSION |
|
| OBESITY |
|
TABLE 3
USPSTF recommends AGAINST routinely
|
| USPSTF recommends AGAINST |
|
| USPSTF indicates the evidence is INSUFFICIENT to assess the balance of benefits and harms of |
|
| Source: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed April 2, 2010. |
The A and B recommendations you may have missed
The major additions to the A and B recommendations pertained to the use of aspirin to prevent cardiovascular disease, routine screening for depression in adults and adolescents, and screening for obesity in children ages 6 and older. The other recommendations in these categories were reaffirmations of previous recommendations (asking about smoking and providing smoking cessation guidance to adults and pregnant women, advising folic acid supplementation for women planning or capable of pregnancy, and screening pregnant women for syphilis and hepatitis B virus) and the more controversial recommendation for biennial rather than annual mammography for women ages 50 to 74.
The use of aspirin to prevent myocardial infarction in men ages 45 to 79 and ischemic strokes in women ages 55 to 79 was endorsed if a patient’s risk of these cardiovascular events exceeds the risk of bleeding from regular aspirin use. The Task Force recommendation statement is available athttp://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htmand provides links to tools for calculating the risk of a myocardial infarction (MI) and ischemic stroke, as well as 2 tables to compare the risks and benefits of aspirin therapy for prevention.
Screening adults for depression is endorsed if “staff-assisted depression care supports” are in place to assure accurate diagnosis, effective treatment, and follow-up. Such support includes the presence of clinical staff members who can assist the primary care provider with care support or coordination, case management, or mental health treatment. The definition can be accessed athttp://www.ahrq.gov/clinic/uspstf09/adultdepression/addeprrs.htm.
One example in the statement describes “a successful study designed for practices without ready access to mental health specialty care, (in which) office staff recruited, screened, and enrolled participants who screened positive for depression before a clinic visit. If the physician confirmed the depression diagnosis, the participant was scheduled for a return visit with the physician and to meet with the nurse specialist in 1 week. The nurse specialist reassessed the patient’s level of depression, discussed treatment options and preferences, and asked the participant to complete a homework assignment. Participants completed up to 8 additional sessions that followed the same pattern, either by phone or in person.”
Screening for major depressive disorder (MDD) in adolescents 12 to 18 years of age is recommended when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive-behavioral or interpersonal), and follow-up. The Task Force addressed screening for MDD only—not for less severe depression. The instruments the group recommended using included the Patient Health Questionnaire for Adolescents (PHQ-A) and the Beck Depression Inventory-Primary Care Version (BDI-PC).
The recommendation for screening for obesity in children ages 6 and older reflects the difficulty in achieving long-term, sustainable weight loss in this group. Effective comprehensive weight-management programs include counseling and other interventions that target both diet and physical activity. Behavioral interventions and parental involvement are also encouraged. Moderate- to high-intensity programs include more than 25 hours of contact with the child and/or the family over a 6-month period; less than this does not result in sustained improvement.
What about the D and I categories?
Two interventions received a D recommendation: Use of aspirin for stroke prevention in women <55 years and for MI prevention in men <45 years, and teaching breast self-examination (BSE) to women. The BSE recommendation has been misinterpreted as recommending against women performing self-breast exams. The recommendation is against formalized teaching of the procedure by physicians, as this leads to increased false positives and no improvement in outcomes when compared to women performing exams on their own.
The list of interventions receiving an I recommendation include some services that are commonly offered in the belief that they are effective. The Task Force is attempting to develop methodologies to decrease the number of interventions that receive an I recommendation. Currently, about 40% of all recommendations end up in this category, and physicians and patients alike could use more guidance on them. This plethora of recommendations made with insufficient evidence reflects the “ready, shoot, aim” philosophy of American medicine. We tend to accept and adopt new interventions before they are proven effective. The I recommendations are valuable reminders that, while many interventions are in common use, we often do not know as much as we should about their benefits and harms.
1. Agency for Healthcare Research and Quality. Screening for breast cancer. Updated December 2009. Available at: www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 17, 2010.
2. Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303:162-163.
3. Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164-165.
4. Murphy AM. Mammography screening for breast cancer: a view from 2 worlds. JAMA. 2010;303:166-167.
5. Berg WA. Benefits of screening mammography. JAMA. 2010;303:168-169.
6. DeAngelis CF, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303:172-173.
7. Editors’ note on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00209.full. Accessed April 7, 2010.
8. Begg CB. Comments and response on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00203.full. Accessed April 7, 2010.
9. Jorgensen KJ, Gotzsche PC. The background review for the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00198.full. Accessed April 7, 2010.
1. Agency for Healthcare Research and Quality. Screening for breast cancer. Updated December 2009. Available at: www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 17, 2010.
2. Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303:162-163.
3. Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164-165.
4. Murphy AM. Mammography screening for breast cancer: a view from 2 worlds. JAMA. 2010;303:166-167.
5. Berg WA. Benefits of screening mammography. JAMA. 2010;303:168-169.
6. DeAngelis CF, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303:172-173.
7. Editors’ note on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00209.full. Accessed April 7, 2010.
8. Begg CB. Comments and response on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00203.full. Accessed April 7, 2010.
9. Jorgensen KJ, Gotzsche PC. The background review for the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00198.full. Accessed April 7, 2010.
Playing God (part 2)
I am sitting in a Budget car rental lounge waiting for my daughter to arrive from Denver. Tomorrow we will memorialize my mother.
It is interesting to reflect on reader reaction to the editorials I’ve written about my mom’s final year. Some of you lament the resources spent in prolonged ventilator care, repeated hospitalizations, and seemingly futile interventions. And while I can relate to this concern, both my brother and I agreed that following her wishes was what mattered most—all expenses aside.
I recently came across an article on the front page of the Sunday New York Times, about a charismatic leader in palliative care who, when faced with her own metastatic cancer, did everything possible to keep death at bay. Perhaps that should not be surprising. Who is to know what any of us would do when faced with such personal choices? And who better to make such decisions than the patient herself? I remain convinced that predicting the course of severe, even life-threatening illness, is highly chancy. Neither my brother nor I—nor Mom’s physicians—would ever have predicted her lengthy survival.
Other readers share my concern that palliative care remains the exception rather than the rule, and that services such as hospice continue to carry a stigma. A close family friend recently died at a local hospice. Ironically, her final days of inpatient hospice care were deemed unnecessary, and her husband was left with a significant bill. I am sure the peace of mind afforded this friend by the caring, experienced hospice staff was well worth the expense. But it is very disappointing to realize that our health care system is still more prepared to pay for a stint in the ICU than to provide a distraught spouse with the help he so desperately craves. I can’t help but think that this reflects our misplaced values—it’s as if one’s final week is somehow less worthy of support than the previous 75 years.
Over the course of the year, many of you—readers whom I have never met—have provided me with words of encouragement and support. For this, I am indebted. I trust that you—indeed, that all of us who care for patients (and families) at the end of life—will remain dedicated to making their last days as comfortable as possible, in accordance with their wishes.
I am sitting in a Budget car rental lounge waiting for my daughter to arrive from Denver. Tomorrow we will memorialize my mother.
It is interesting to reflect on reader reaction to the editorials I’ve written about my mom’s final year. Some of you lament the resources spent in prolonged ventilator care, repeated hospitalizations, and seemingly futile interventions. And while I can relate to this concern, both my brother and I agreed that following her wishes was what mattered most—all expenses aside.
I recently came across an article on the front page of the Sunday New York Times, about a charismatic leader in palliative care who, when faced with her own metastatic cancer, did everything possible to keep death at bay. Perhaps that should not be surprising. Who is to know what any of us would do when faced with such personal choices? And who better to make such decisions than the patient herself? I remain convinced that predicting the course of severe, even life-threatening illness, is highly chancy. Neither my brother nor I—nor Mom’s physicians—would ever have predicted her lengthy survival.
Other readers share my concern that palliative care remains the exception rather than the rule, and that services such as hospice continue to carry a stigma. A close family friend recently died at a local hospice. Ironically, her final days of inpatient hospice care were deemed unnecessary, and her husband was left with a significant bill. I am sure the peace of mind afforded this friend by the caring, experienced hospice staff was well worth the expense. But it is very disappointing to realize that our health care system is still more prepared to pay for a stint in the ICU than to provide a distraught spouse with the help he so desperately craves. I can’t help but think that this reflects our misplaced values—it’s as if one’s final week is somehow less worthy of support than the previous 75 years.
Over the course of the year, many of you—readers whom I have never met—have provided me with words of encouragement and support. For this, I am indebted. I trust that you—indeed, that all of us who care for patients (and families) at the end of life—will remain dedicated to making their last days as comfortable as possible, in accordance with their wishes.
I am sitting in a Budget car rental lounge waiting for my daughter to arrive from Denver. Tomorrow we will memorialize my mother.
It is interesting to reflect on reader reaction to the editorials I’ve written about my mom’s final year. Some of you lament the resources spent in prolonged ventilator care, repeated hospitalizations, and seemingly futile interventions. And while I can relate to this concern, both my brother and I agreed that following her wishes was what mattered most—all expenses aside.
I recently came across an article on the front page of the Sunday New York Times, about a charismatic leader in palliative care who, when faced with her own metastatic cancer, did everything possible to keep death at bay. Perhaps that should not be surprising. Who is to know what any of us would do when faced with such personal choices? And who better to make such decisions than the patient herself? I remain convinced that predicting the course of severe, even life-threatening illness, is highly chancy. Neither my brother nor I—nor Mom’s physicians—would ever have predicted her lengthy survival.
Other readers share my concern that palliative care remains the exception rather than the rule, and that services such as hospice continue to carry a stigma. A close family friend recently died at a local hospice. Ironically, her final days of inpatient hospice care were deemed unnecessary, and her husband was left with a significant bill. I am sure the peace of mind afforded this friend by the caring, experienced hospice staff was well worth the expense. But it is very disappointing to realize that our health care system is still more prepared to pay for a stint in the ICU than to provide a distraught spouse with the help he so desperately craves. I can’t help but think that this reflects our misplaced values—it’s as if one’s final week is somehow less worthy of support than the previous 75 years.
Over the course of the year, many of you—readers whom I have never met—have provided me with words of encouragement and support. For this, I am indebted. I trust that you—indeed, that all of us who care for patients (and families) at the end of life—will remain dedicated to making their last days as comfortable as possible, in accordance with their wishes.
A look at the long-term safety of an extended-regimen OC
Abstract
Background: Oral contraceptives (OCs) are the most widely used method of reversible contraception. Recent alterations of the standard 28-day regimen have included shortening the traditional hormone-free interval (HFI), supplementing the HFI with low-dose estrogen, or increasing the number of active pills administered, thus extending the time between withdrawal bleeding episodes by a variable number of months. In light of these changes in regimens, clinicians may be seeking evidence that the new regimens are safe and will not result in unexpected adverse events.
Methods: We initiated a long-term extension trial to evaluate the safety of a 91-day extended-regimen OC containing 150 mcg levonorgestrel/30 mcg ethinyl estradiol (EE) for 84 days, followed by 7 days of 10 mcg EE. After participation in a 1-year, open-label, phase 3 contraceptive program, 320 women qualified for enrollment in a multicenter, nonrandomized study of 91-day extended-regimen OCs for up to 3 additional consecutive years; 116 completed the study. We evaluated incidence of reported adverse events (AEs), rates of study discontinuation, and reported bleeding patterns.
Results: Total exposure was equivalent to 8292 28-day cycles. Participants reported no thromboembolic events. Thirty-one (9.7%) women discontinued treatment due to AEs. Unscheduled bleeding and spotting diminished during the course of the trial. Overall rates of study discontinuation and incidence of AEs were consistent with those observed in the phase 3 clinical program.
Conclusion: This study demonstrated that the AE profile of the 91-day extended-regimen OC over 4 years was similar to that seen in the 1-year clinical trials, with no unexpected adverse events.
Two Phase 3 studies assessed a 91-day oral contraceptive (OC) regimen for 1 year—a multicenter, open-label trial that studied safety and efficacy,1 and a multicenter trial that evaluated endometrial safety.2 Results of both studies showed the regimen to be safe, effective, and well tolerated. The regimen: 84 days of combination tablets containing 150 mcg levonorgestrel (LNG) and 30 mcg ethinyl estradiol (EE), followed by 7 days of 10 mcg EE alone instead of placebo to maintain ovarian suppression,3,4 potentially reducing the incidence of intermenstrual bleeding or spotting. To gain longer experience with this regimen, we enrolled selected subjects from both studies in a 3-year extension trial.
Methods
Study design and population
In this nonrandomized, multicenter, open-label extension study, we invited women who had successfully completed 1 year of treatment in either of the Phase 3 trials to participate as part of a convenience sample for an additional 3 years of follow-up. We conducted this study in accordance with ethical guidelines for human subjects and applicable guidelines for good clinical practice.5
Inclusion and exclusion criteria were similar to those used in the Phase 3 studies.1,2 Participants agreed to use the study medication as their primary method of birth control throughout the study. We excluded women who were using a medication that might interfere with the efficacy of OCs, or who had any medical or lifestyle contraindications to OC use (eg, clinically significant abnormal Pap smear; cigarette use if older than 35 years).
We enrolled 320 subjects whose demographic characteristics were similar to those in the earlier Phase 3 trials (TABLE 1).2
TABLE 1
Demographic characteristics of all treated participants (N=320)
| Age at screening, y | |
| Mean (SD) | 28.1 (6.0) |
| Median | 27.5 |
| Min, Max | 18.2, 40.2 |
| Weight, lb | |
| Mean (SD) | 152.3 (37.6) |
| Median | 143.5 |
| Min, Max | 94.0, 360.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 25.5 (5.8) |
| Median | 24.1 |
| Min, Max | 16.8, 56.5 |
| OC use history, n (%) | |
| Recent user | 225 (70.3%) |
| Prior user | 67 (20.9%) |
| New start | 28 (8.8%) |
| Race, n (%) | |
| African American | 40 (12.5%) |
| Asian | 7 (2.2%) |
| Caucasian | 262 (81.9%) |
| Hispanic | 4 (1.3%) |
| Other | 7 (2.2%) |
| Cigarette use status, n (%) | |
| Nonsmoker | 269 (84.1%) |
| Smoker | 51 (15.9%) |
| OC, oral contraceptive; SD, standard deviation. | |
Regular evaluation of adherence and AEs
Every 3 months at the study site, we assessed adherence with the drug regimen by reviewing participants’ daily diaries and by counting pills in returned used pill packs. We also evaluated subject-reported adverse events (AEs)—side effects, as well as serious adverse events (SAEs) requiring treatment or drug discontinuation—and use of concomitant medications or cigarettes.
Factors in our safety assessment
Our safety analysis included any subject who took at least 1 dose of the study drug. We calculated the incidence rates of subject-reported AEs, overall rates of discontinuation, and cycles of exposure. These included incidence rates of AEs the investigators deemed to be at least “remotely” related to treatment. Safety analyses also included annual changes in laboratory values (complete blood count, serum chemistry, lipid profile, and urinalysis), vital signs, occurrence of pregnancy, and rates of reported bleeding or spotting.
The evaluation included bleeding/spotting that was scheduled—occurring on cycle days 85 through 91 (EE-only tablets)—and unscheduled—intermenstrual or “breakthrough” blood loss occurring on cycle days 1 through 84. We defined bleeding as any vaginal blood loss requiring the use of sanitary protection (pads or tampons); spotting was defined as vaginal blood loss not necessitating sanitary protection.
Statistical analysis
Descriptive statistics included the number of subjects, and the mean, median (where appropriate), standard deviation or standard error of the mean (SE), or minimum and maximum values of patient characteristics. We summarized discrete events using frequencies or percentages. As this study was designed primarily to be observational and to gain further long-term experience with the regimen, we did not conduct formal power analyses and sample size calculations. For contraceptive trials, the US Food and Drug Administration typically requires a minimum exposure of 200 women using the method for 1 year. We also omitted a formal efficacy analysis, as efficacy was established in the Phase 3 clinical program.1
Results
Of the 320 subjects enrolled and treated, 244 (76.3%) completed at least 1 year of treatment; 173 (54.1%) completed at least 2 years of treatment; and 85 (26.6%) completed 3 years of treatment in this extension study, beyond the 1 year completed in the Phase 3 clinical trials (FIGURE). A total of 204 women (63.8%) discontinued treatment; primarily due to personal decisions (26.6%), becoming lost to follow-up (11.3%), and adverse events (9.7%). These discontinuation rates are consistent with those in other long-term studies.6-8
FIGURE
Of the 320 participants enrolled, 116 completed the study
* In the pregnancy prevention study, 979 patients completed 1 year; in the endometrial safety study, 177 completed 1 year. Only 11 of the original 36 sites participated in the extension study, so not all 1156 subjects had the option of enrolling in the extension.
† Not all subjects enrolled at the same time. Thirty-one patients were participating in the study with various durations of exposure when the study was ended. Although they did not complete 3 full years of use, they did participate in the full course of the study that was available to them and were therefore classified as “completers.”
Serious adverse events were few
SAEs were reported by 12 subjects; 3 were possibly related to treatment—spontaneous abortion in a 33-year-old subject, nonthrombotic coronary artery spasm in a 40-year-old subject, and acute cholecystitis in a 37-year old subject. No venous thromboembolic events (VTEs) occurred; however, such events are rare (approximately 7-18 VTEs/100,000 OC users annually9) and would be unlikely in a study of 320 subjects.
Nonurgent adverse events comparable to earlier studies
The most commonly reported treatment-related AEs were headache (9.4%), metrorrhagia (9.1%), increased weight (6.9%), and dysmenorrhea (4.4%), as noted in TABLE 2. The most frequently reported treatment-emergent AEs (ie, regardless of relationship to study medication) were headache (21.9%), upper respiratory tract infection (18.4%), nasopharyngitis (15.0%), sinusitis (12.2%), and back pain (11.6%). A total of 31 subjects (9.7%) discontinued the study due to AEs. The incidence rates of treatment-emergent and treatment-related AEs in this study were not substantially higher than those in the Phase 3 trials.1
TABLE 2
Adverse events attributable to treatment occurred in ≥2% of participants (N=320)
| MedDRA System organ class and preferred term | n (%) |
|---|---|
| Reproductive system and breast disorders Metrorrhagia Dysmenorrhea | 29 (9.1) 14 (4.4) |
| Nervous system disorders Headache | 30 (9.4) |
| Investigations Weight increased | 22 (6.9) |
| Infections and infestations Vulvovaginal mycotic infection Vaginitis, bacterial Fungal infection | 13 (4.1) 9 (2.8) 7 (2.2) |
| Skin and subcutaneous tissue disorders Acne | 7 (2.2) |
| MedDRA, Medical Dictionary for Regulatory Activities. | |
Pregnancies due mostly to nonadherence
We conducted no formal efficacy analyses. Pregnancy was determined by a positive result on a pregnancy test conducted at the study site. Six subjects (1.9%) became pregnant during the study; 4 were noncompliant with the study medication, and 2 became pregnant at least 14 days after completing the study medication. One spontaneous abortion was reported. Among those participants who continued their pregnancies, none reported abnormal outcomes.
Laboratory values changed minimally, if at all
No notable changes occurred in serum chemistry, hematology, or urinalysis values. Specific mean changes from baseline included increases of 5.0 mg/dL for total cholesterol, 2.4 mg/dL for high-density lipoproteins, and 4.0 mg/dL for low-density lipoproteins; and decreases of 5.9 mg/dL for triglycerides and 0.1 g/dL for hemoglobin.
Vital signs remained stable
No notable changes occurred in systolic or diastolic blood pressure, heart rate, or temperature. The increase in mean weight that we observed (10.4 lb) is not unexpected, as the time period of evaluation was as long as 4 years after documentation of the baseline value.
Reported bleeding or spotting diminished over time
Median rates of unscheduled bleeding or spotting declined over the course of the study, from 4 days in 91 during cycle 1 to 1 day in 91 during cycle 11. In most of the 91-day cycles, participants consistently reported a median of 3 days of scheduled (withdrawal) bleeding or spotting.
Discussion
This 3-year study increased our experience with a novel extended-regimen OC to 4 years of continuous use. The results should reassure clinicians who are prescribing extended-regimen OCs that their patients are unlikely to experience side effects that differ significantly from traditional 28-day OC regimens. In other long-term studies of 28-day regimens, the most common AEs were headache, back pain, nausea, pharyngitis, and upper respiratory infection.7,8
Overall rates of study discontinuation and the incidence of AEs (including SAEs and AEs leading to discontinuation) were consistent with those observed in 1-year1,2,10,11 and 2-year6 studies of extended-regimen OCs.
There was no suggestion of increased risk of serious estrogen-related AEs. There were no reports of endometrial abnormalities or hyperplasia, which is consistent with the results of endometrial biopsies in a previous study that compared before- and after-treatment biopsy samples from 63 subjects in the 1-year Phase 3 trial.2
A pharmacokinetic analysis of a similar extended-regimen OC demonstrated that estrogen levels, measured on days 1, 21, 84, and 91 of a 91-day extended-regimen cycle, did not build up over the course of the regimen.12
The risk of thromboembolic disease associated with OCs is not related to the length of use, and a 5-year case-control study found significantly decreasing odds ratios for reports of VTE in OC users over time.13 In this extension study, there were no reported thromboembolic AEs and there was no suggestion of an increased risk of thrombosis with the long-term use of this regimen, although such findings are not unexpected for a small-scale study.
Acknowledgements
The principal investigators and their locations are as follows: Angeli Adamczyk, Paige Brainard (Tucson, Ariz), Ted Anderson, Robert Rosenfeld, Shali Scott (Nashville, Tenn), Matthew Davis (Rochester, NY), William Gibbons, Laurel Stadtmauer (Norfolk, Va), James Lackey (Oklahoma City, Okla), Sooji Lee-Rugh (Arlington, Va), Thomas Littlejohn (Winston-Salem, NC), James Maly (Lincoln, Neb), David Portman (Columbus, Ohio), George Raad (Charlotte, NC), and Mark Shepard (Washington, DC).
CORRESPONDENCE Kathleen Reape, MD, Teva Branded Pharmaceutical Products R&D, Inc., 425 Privet Road, Horsham, PA 19044; Kathleen. [email protected]
1. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
2. Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception. 2008;77:91-96.
3. Vandever MA, Kuehl TJ, Sulak P, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77:162-170.
4. Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception. 2008;77:34-39.
5. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed April 6, 2010.
6. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. Am J Obstet Gynecol. 2006;195:92-96.
7. Zahradnik HP, Hanjalic-Beck A. Efficacy, safety, and sustainability of treatment continuation and results of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg chlormadinone acetate, in long-term usage (up to 45 cycles)—an open-label, prospective, noncontrolled, office-based Phase III study. Contraception. 2008;77:337-343.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Burkman RT. Venous thromboembolism and oral contraceptives: Current status and clinical implications. Treat Endocrinol. 2002;1:143-147.
10. Anderson FD, Hait H. The Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception. 2005;71:55-59.
12. Reape KZ, DiLiberti C. Steady-state pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen [abstract]. Obstet Gynecol. 2007;109(suppl 4):13S.-
13. Lidegaard O, Edstrom E, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65:187-196.
Abstract
Background: Oral contraceptives (OCs) are the most widely used method of reversible contraception. Recent alterations of the standard 28-day regimen have included shortening the traditional hormone-free interval (HFI), supplementing the HFI with low-dose estrogen, or increasing the number of active pills administered, thus extending the time between withdrawal bleeding episodes by a variable number of months. In light of these changes in regimens, clinicians may be seeking evidence that the new regimens are safe and will not result in unexpected adverse events.
Methods: We initiated a long-term extension trial to evaluate the safety of a 91-day extended-regimen OC containing 150 mcg levonorgestrel/30 mcg ethinyl estradiol (EE) for 84 days, followed by 7 days of 10 mcg EE. After participation in a 1-year, open-label, phase 3 contraceptive program, 320 women qualified for enrollment in a multicenter, nonrandomized study of 91-day extended-regimen OCs for up to 3 additional consecutive years; 116 completed the study. We evaluated incidence of reported adverse events (AEs), rates of study discontinuation, and reported bleeding patterns.
Results: Total exposure was equivalent to 8292 28-day cycles. Participants reported no thromboembolic events. Thirty-one (9.7%) women discontinued treatment due to AEs. Unscheduled bleeding and spotting diminished during the course of the trial. Overall rates of study discontinuation and incidence of AEs were consistent with those observed in the phase 3 clinical program.
Conclusion: This study demonstrated that the AE profile of the 91-day extended-regimen OC over 4 years was similar to that seen in the 1-year clinical trials, with no unexpected adverse events.
Two Phase 3 studies assessed a 91-day oral contraceptive (OC) regimen for 1 year—a multicenter, open-label trial that studied safety and efficacy,1 and a multicenter trial that evaluated endometrial safety.2 Results of both studies showed the regimen to be safe, effective, and well tolerated. The regimen: 84 days of combination tablets containing 150 mcg levonorgestrel (LNG) and 30 mcg ethinyl estradiol (EE), followed by 7 days of 10 mcg EE alone instead of placebo to maintain ovarian suppression,3,4 potentially reducing the incidence of intermenstrual bleeding or spotting. To gain longer experience with this regimen, we enrolled selected subjects from both studies in a 3-year extension trial.
Methods
Study design and population
In this nonrandomized, multicenter, open-label extension study, we invited women who had successfully completed 1 year of treatment in either of the Phase 3 trials to participate as part of a convenience sample for an additional 3 years of follow-up. We conducted this study in accordance with ethical guidelines for human subjects and applicable guidelines for good clinical practice.5
Inclusion and exclusion criteria were similar to those used in the Phase 3 studies.1,2 Participants agreed to use the study medication as their primary method of birth control throughout the study. We excluded women who were using a medication that might interfere with the efficacy of OCs, or who had any medical or lifestyle contraindications to OC use (eg, clinically significant abnormal Pap smear; cigarette use if older than 35 years).
We enrolled 320 subjects whose demographic characteristics were similar to those in the earlier Phase 3 trials (TABLE 1).2
TABLE 1
Demographic characteristics of all treated participants (N=320)
| Age at screening, y | |
| Mean (SD) | 28.1 (6.0) |
| Median | 27.5 |
| Min, Max | 18.2, 40.2 |
| Weight, lb | |
| Mean (SD) | 152.3 (37.6) |
| Median | 143.5 |
| Min, Max | 94.0, 360.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 25.5 (5.8) |
| Median | 24.1 |
| Min, Max | 16.8, 56.5 |
| OC use history, n (%) | |
| Recent user | 225 (70.3%) |
| Prior user | 67 (20.9%) |
| New start | 28 (8.8%) |
| Race, n (%) | |
| African American | 40 (12.5%) |
| Asian | 7 (2.2%) |
| Caucasian | 262 (81.9%) |
| Hispanic | 4 (1.3%) |
| Other | 7 (2.2%) |
| Cigarette use status, n (%) | |
| Nonsmoker | 269 (84.1%) |
| Smoker | 51 (15.9%) |
| OC, oral contraceptive; SD, standard deviation. | |
Regular evaluation of adherence and AEs
Every 3 months at the study site, we assessed adherence with the drug regimen by reviewing participants’ daily diaries and by counting pills in returned used pill packs. We also evaluated subject-reported adverse events (AEs)—side effects, as well as serious adverse events (SAEs) requiring treatment or drug discontinuation—and use of concomitant medications or cigarettes.
Factors in our safety assessment
Our safety analysis included any subject who took at least 1 dose of the study drug. We calculated the incidence rates of subject-reported AEs, overall rates of discontinuation, and cycles of exposure. These included incidence rates of AEs the investigators deemed to be at least “remotely” related to treatment. Safety analyses also included annual changes in laboratory values (complete blood count, serum chemistry, lipid profile, and urinalysis), vital signs, occurrence of pregnancy, and rates of reported bleeding or spotting.
The evaluation included bleeding/spotting that was scheduled—occurring on cycle days 85 through 91 (EE-only tablets)—and unscheduled—intermenstrual or “breakthrough” blood loss occurring on cycle days 1 through 84. We defined bleeding as any vaginal blood loss requiring the use of sanitary protection (pads or tampons); spotting was defined as vaginal blood loss not necessitating sanitary protection.
Statistical analysis
Descriptive statistics included the number of subjects, and the mean, median (where appropriate), standard deviation or standard error of the mean (SE), or minimum and maximum values of patient characteristics. We summarized discrete events using frequencies or percentages. As this study was designed primarily to be observational and to gain further long-term experience with the regimen, we did not conduct formal power analyses and sample size calculations. For contraceptive trials, the US Food and Drug Administration typically requires a minimum exposure of 200 women using the method for 1 year. We also omitted a formal efficacy analysis, as efficacy was established in the Phase 3 clinical program.1
Results
Of the 320 subjects enrolled and treated, 244 (76.3%) completed at least 1 year of treatment; 173 (54.1%) completed at least 2 years of treatment; and 85 (26.6%) completed 3 years of treatment in this extension study, beyond the 1 year completed in the Phase 3 clinical trials (FIGURE). A total of 204 women (63.8%) discontinued treatment; primarily due to personal decisions (26.6%), becoming lost to follow-up (11.3%), and adverse events (9.7%). These discontinuation rates are consistent with those in other long-term studies.6-8
FIGURE
Of the 320 participants enrolled, 116 completed the study
* In the pregnancy prevention study, 979 patients completed 1 year; in the endometrial safety study, 177 completed 1 year. Only 11 of the original 36 sites participated in the extension study, so not all 1156 subjects had the option of enrolling in the extension.
† Not all subjects enrolled at the same time. Thirty-one patients were participating in the study with various durations of exposure when the study was ended. Although they did not complete 3 full years of use, they did participate in the full course of the study that was available to them and were therefore classified as “completers.”
Serious adverse events were few
SAEs were reported by 12 subjects; 3 were possibly related to treatment—spontaneous abortion in a 33-year-old subject, nonthrombotic coronary artery spasm in a 40-year-old subject, and acute cholecystitis in a 37-year old subject. No venous thromboembolic events (VTEs) occurred; however, such events are rare (approximately 7-18 VTEs/100,000 OC users annually9) and would be unlikely in a study of 320 subjects.
Nonurgent adverse events comparable to earlier studies
The most commonly reported treatment-related AEs were headache (9.4%), metrorrhagia (9.1%), increased weight (6.9%), and dysmenorrhea (4.4%), as noted in TABLE 2. The most frequently reported treatment-emergent AEs (ie, regardless of relationship to study medication) were headache (21.9%), upper respiratory tract infection (18.4%), nasopharyngitis (15.0%), sinusitis (12.2%), and back pain (11.6%). A total of 31 subjects (9.7%) discontinued the study due to AEs. The incidence rates of treatment-emergent and treatment-related AEs in this study were not substantially higher than those in the Phase 3 trials.1
TABLE 2
Adverse events attributable to treatment occurred in ≥2% of participants (N=320)
| MedDRA System organ class and preferred term | n (%) |
|---|---|
| Reproductive system and breast disorders Metrorrhagia Dysmenorrhea | 29 (9.1) 14 (4.4) |
| Nervous system disorders Headache | 30 (9.4) |
| Investigations Weight increased | 22 (6.9) |
| Infections and infestations Vulvovaginal mycotic infection Vaginitis, bacterial Fungal infection | 13 (4.1) 9 (2.8) 7 (2.2) |
| Skin and subcutaneous tissue disorders Acne | 7 (2.2) |
| MedDRA, Medical Dictionary for Regulatory Activities. | |
Pregnancies due mostly to nonadherence
We conducted no formal efficacy analyses. Pregnancy was determined by a positive result on a pregnancy test conducted at the study site. Six subjects (1.9%) became pregnant during the study; 4 were noncompliant with the study medication, and 2 became pregnant at least 14 days after completing the study medication. One spontaneous abortion was reported. Among those participants who continued their pregnancies, none reported abnormal outcomes.
Laboratory values changed minimally, if at all
No notable changes occurred in serum chemistry, hematology, or urinalysis values. Specific mean changes from baseline included increases of 5.0 mg/dL for total cholesterol, 2.4 mg/dL for high-density lipoproteins, and 4.0 mg/dL for low-density lipoproteins; and decreases of 5.9 mg/dL for triglycerides and 0.1 g/dL for hemoglobin.
Vital signs remained stable
No notable changes occurred in systolic or diastolic blood pressure, heart rate, or temperature. The increase in mean weight that we observed (10.4 lb) is not unexpected, as the time period of evaluation was as long as 4 years after documentation of the baseline value.
Reported bleeding or spotting diminished over time
Median rates of unscheduled bleeding or spotting declined over the course of the study, from 4 days in 91 during cycle 1 to 1 day in 91 during cycle 11. In most of the 91-day cycles, participants consistently reported a median of 3 days of scheduled (withdrawal) bleeding or spotting.
Discussion
This 3-year study increased our experience with a novel extended-regimen OC to 4 years of continuous use. The results should reassure clinicians who are prescribing extended-regimen OCs that their patients are unlikely to experience side effects that differ significantly from traditional 28-day OC regimens. In other long-term studies of 28-day regimens, the most common AEs were headache, back pain, nausea, pharyngitis, and upper respiratory infection.7,8
Overall rates of study discontinuation and the incidence of AEs (including SAEs and AEs leading to discontinuation) were consistent with those observed in 1-year1,2,10,11 and 2-year6 studies of extended-regimen OCs.
There was no suggestion of increased risk of serious estrogen-related AEs. There were no reports of endometrial abnormalities or hyperplasia, which is consistent with the results of endometrial biopsies in a previous study that compared before- and after-treatment biopsy samples from 63 subjects in the 1-year Phase 3 trial.2
A pharmacokinetic analysis of a similar extended-regimen OC demonstrated that estrogen levels, measured on days 1, 21, 84, and 91 of a 91-day extended-regimen cycle, did not build up over the course of the regimen.12
The risk of thromboembolic disease associated with OCs is not related to the length of use, and a 5-year case-control study found significantly decreasing odds ratios for reports of VTE in OC users over time.13 In this extension study, there were no reported thromboembolic AEs and there was no suggestion of an increased risk of thrombosis with the long-term use of this regimen, although such findings are not unexpected for a small-scale study.
Acknowledgements
The principal investigators and their locations are as follows: Angeli Adamczyk, Paige Brainard (Tucson, Ariz), Ted Anderson, Robert Rosenfeld, Shali Scott (Nashville, Tenn), Matthew Davis (Rochester, NY), William Gibbons, Laurel Stadtmauer (Norfolk, Va), James Lackey (Oklahoma City, Okla), Sooji Lee-Rugh (Arlington, Va), Thomas Littlejohn (Winston-Salem, NC), James Maly (Lincoln, Neb), David Portman (Columbus, Ohio), George Raad (Charlotte, NC), and Mark Shepard (Washington, DC).
CORRESPONDENCE Kathleen Reape, MD, Teva Branded Pharmaceutical Products R&D, Inc., 425 Privet Road, Horsham, PA 19044; Kathleen. [email protected]
Abstract
Background: Oral contraceptives (OCs) are the most widely used method of reversible contraception. Recent alterations of the standard 28-day regimen have included shortening the traditional hormone-free interval (HFI), supplementing the HFI with low-dose estrogen, or increasing the number of active pills administered, thus extending the time between withdrawal bleeding episodes by a variable number of months. In light of these changes in regimens, clinicians may be seeking evidence that the new regimens are safe and will not result in unexpected adverse events.
Methods: We initiated a long-term extension trial to evaluate the safety of a 91-day extended-regimen OC containing 150 mcg levonorgestrel/30 mcg ethinyl estradiol (EE) for 84 days, followed by 7 days of 10 mcg EE. After participation in a 1-year, open-label, phase 3 contraceptive program, 320 women qualified for enrollment in a multicenter, nonrandomized study of 91-day extended-regimen OCs for up to 3 additional consecutive years; 116 completed the study. We evaluated incidence of reported adverse events (AEs), rates of study discontinuation, and reported bleeding patterns.
Results: Total exposure was equivalent to 8292 28-day cycles. Participants reported no thromboembolic events. Thirty-one (9.7%) women discontinued treatment due to AEs. Unscheduled bleeding and spotting diminished during the course of the trial. Overall rates of study discontinuation and incidence of AEs were consistent with those observed in the phase 3 clinical program.
Conclusion: This study demonstrated that the AE profile of the 91-day extended-regimen OC over 4 years was similar to that seen in the 1-year clinical trials, with no unexpected adverse events.
Two Phase 3 studies assessed a 91-day oral contraceptive (OC) regimen for 1 year—a multicenter, open-label trial that studied safety and efficacy,1 and a multicenter trial that evaluated endometrial safety.2 Results of both studies showed the regimen to be safe, effective, and well tolerated. The regimen: 84 days of combination tablets containing 150 mcg levonorgestrel (LNG) and 30 mcg ethinyl estradiol (EE), followed by 7 days of 10 mcg EE alone instead of placebo to maintain ovarian suppression,3,4 potentially reducing the incidence of intermenstrual bleeding or spotting. To gain longer experience with this regimen, we enrolled selected subjects from both studies in a 3-year extension trial.
Methods
Study design and population
In this nonrandomized, multicenter, open-label extension study, we invited women who had successfully completed 1 year of treatment in either of the Phase 3 trials to participate as part of a convenience sample for an additional 3 years of follow-up. We conducted this study in accordance with ethical guidelines for human subjects and applicable guidelines for good clinical practice.5
Inclusion and exclusion criteria were similar to those used in the Phase 3 studies.1,2 Participants agreed to use the study medication as their primary method of birth control throughout the study. We excluded women who were using a medication that might interfere with the efficacy of OCs, or who had any medical or lifestyle contraindications to OC use (eg, clinically significant abnormal Pap smear; cigarette use if older than 35 years).
We enrolled 320 subjects whose demographic characteristics were similar to those in the earlier Phase 3 trials (TABLE 1).2
TABLE 1
Demographic characteristics of all treated participants (N=320)
| Age at screening, y | |
| Mean (SD) | 28.1 (6.0) |
| Median | 27.5 |
| Min, Max | 18.2, 40.2 |
| Weight, lb | |
| Mean (SD) | 152.3 (37.6) |
| Median | 143.5 |
| Min, Max | 94.0, 360.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 25.5 (5.8) |
| Median | 24.1 |
| Min, Max | 16.8, 56.5 |
| OC use history, n (%) | |
| Recent user | 225 (70.3%) |
| Prior user | 67 (20.9%) |
| New start | 28 (8.8%) |
| Race, n (%) | |
| African American | 40 (12.5%) |
| Asian | 7 (2.2%) |
| Caucasian | 262 (81.9%) |
| Hispanic | 4 (1.3%) |
| Other | 7 (2.2%) |
| Cigarette use status, n (%) | |
| Nonsmoker | 269 (84.1%) |
| Smoker | 51 (15.9%) |
| OC, oral contraceptive; SD, standard deviation. | |
Regular evaluation of adherence and AEs
Every 3 months at the study site, we assessed adherence with the drug regimen by reviewing participants’ daily diaries and by counting pills in returned used pill packs. We also evaluated subject-reported adverse events (AEs)—side effects, as well as serious adverse events (SAEs) requiring treatment or drug discontinuation—and use of concomitant medications or cigarettes.
Factors in our safety assessment
Our safety analysis included any subject who took at least 1 dose of the study drug. We calculated the incidence rates of subject-reported AEs, overall rates of discontinuation, and cycles of exposure. These included incidence rates of AEs the investigators deemed to be at least “remotely” related to treatment. Safety analyses also included annual changes in laboratory values (complete blood count, serum chemistry, lipid profile, and urinalysis), vital signs, occurrence of pregnancy, and rates of reported bleeding or spotting.
The evaluation included bleeding/spotting that was scheduled—occurring on cycle days 85 through 91 (EE-only tablets)—and unscheduled—intermenstrual or “breakthrough” blood loss occurring on cycle days 1 through 84. We defined bleeding as any vaginal blood loss requiring the use of sanitary protection (pads or tampons); spotting was defined as vaginal blood loss not necessitating sanitary protection.
Statistical analysis
Descriptive statistics included the number of subjects, and the mean, median (where appropriate), standard deviation or standard error of the mean (SE), or minimum and maximum values of patient characteristics. We summarized discrete events using frequencies or percentages. As this study was designed primarily to be observational and to gain further long-term experience with the regimen, we did not conduct formal power analyses and sample size calculations. For contraceptive trials, the US Food and Drug Administration typically requires a minimum exposure of 200 women using the method for 1 year. We also omitted a formal efficacy analysis, as efficacy was established in the Phase 3 clinical program.1
Results
Of the 320 subjects enrolled and treated, 244 (76.3%) completed at least 1 year of treatment; 173 (54.1%) completed at least 2 years of treatment; and 85 (26.6%) completed 3 years of treatment in this extension study, beyond the 1 year completed in the Phase 3 clinical trials (FIGURE). A total of 204 women (63.8%) discontinued treatment; primarily due to personal decisions (26.6%), becoming lost to follow-up (11.3%), and adverse events (9.7%). These discontinuation rates are consistent with those in other long-term studies.6-8
FIGURE
Of the 320 participants enrolled, 116 completed the study
* In the pregnancy prevention study, 979 patients completed 1 year; in the endometrial safety study, 177 completed 1 year. Only 11 of the original 36 sites participated in the extension study, so not all 1156 subjects had the option of enrolling in the extension.
† Not all subjects enrolled at the same time. Thirty-one patients were participating in the study with various durations of exposure when the study was ended. Although they did not complete 3 full years of use, they did participate in the full course of the study that was available to them and were therefore classified as “completers.”
Serious adverse events were few
SAEs were reported by 12 subjects; 3 were possibly related to treatment—spontaneous abortion in a 33-year-old subject, nonthrombotic coronary artery spasm in a 40-year-old subject, and acute cholecystitis in a 37-year old subject. No venous thromboembolic events (VTEs) occurred; however, such events are rare (approximately 7-18 VTEs/100,000 OC users annually9) and would be unlikely in a study of 320 subjects.
Nonurgent adverse events comparable to earlier studies
The most commonly reported treatment-related AEs were headache (9.4%), metrorrhagia (9.1%), increased weight (6.9%), and dysmenorrhea (4.4%), as noted in TABLE 2. The most frequently reported treatment-emergent AEs (ie, regardless of relationship to study medication) were headache (21.9%), upper respiratory tract infection (18.4%), nasopharyngitis (15.0%), sinusitis (12.2%), and back pain (11.6%). A total of 31 subjects (9.7%) discontinued the study due to AEs. The incidence rates of treatment-emergent and treatment-related AEs in this study were not substantially higher than those in the Phase 3 trials.1
TABLE 2
Adverse events attributable to treatment occurred in ≥2% of participants (N=320)
| MedDRA System organ class and preferred term | n (%) |
|---|---|
| Reproductive system and breast disorders Metrorrhagia Dysmenorrhea | 29 (9.1) 14 (4.4) |
| Nervous system disorders Headache | 30 (9.4) |
| Investigations Weight increased | 22 (6.9) |
| Infections and infestations Vulvovaginal mycotic infection Vaginitis, bacterial Fungal infection | 13 (4.1) 9 (2.8) 7 (2.2) |
| Skin and subcutaneous tissue disorders Acne | 7 (2.2) |
| MedDRA, Medical Dictionary for Regulatory Activities. | |
Pregnancies due mostly to nonadherence
We conducted no formal efficacy analyses. Pregnancy was determined by a positive result on a pregnancy test conducted at the study site. Six subjects (1.9%) became pregnant during the study; 4 were noncompliant with the study medication, and 2 became pregnant at least 14 days after completing the study medication. One spontaneous abortion was reported. Among those participants who continued their pregnancies, none reported abnormal outcomes.
Laboratory values changed minimally, if at all
No notable changes occurred in serum chemistry, hematology, or urinalysis values. Specific mean changes from baseline included increases of 5.0 mg/dL for total cholesterol, 2.4 mg/dL for high-density lipoproteins, and 4.0 mg/dL for low-density lipoproteins; and decreases of 5.9 mg/dL for triglycerides and 0.1 g/dL for hemoglobin.
Vital signs remained stable
No notable changes occurred in systolic or diastolic blood pressure, heart rate, or temperature. The increase in mean weight that we observed (10.4 lb) is not unexpected, as the time period of evaluation was as long as 4 years after documentation of the baseline value.
Reported bleeding or spotting diminished over time
Median rates of unscheduled bleeding or spotting declined over the course of the study, from 4 days in 91 during cycle 1 to 1 day in 91 during cycle 11. In most of the 91-day cycles, participants consistently reported a median of 3 days of scheduled (withdrawal) bleeding or spotting.
Discussion
This 3-year study increased our experience with a novel extended-regimen OC to 4 years of continuous use. The results should reassure clinicians who are prescribing extended-regimen OCs that their patients are unlikely to experience side effects that differ significantly from traditional 28-day OC regimens. In other long-term studies of 28-day regimens, the most common AEs were headache, back pain, nausea, pharyngitis, and upper respiratory infection.7,8
Overall rates of study discontinuation and the incidence of AEs (including SAEs and AEs leading to discontinuation) were consistent with those observed in 1-year1,2,10,11 and 2-year6 studies of extended-regimen OCs.
There was no suggestion of increased risk of serious estrogen-related AEs. There were no reports of endometrial abnormalities or hyperplasia, which is consistent with the results of endometrial biopsies in a previous study that compared before- and after-treatment biopsy samples from 63 subjects in the 1-year Phase 3 trial.2
A pharmacokinetic analysis of a similar extended-regimen OC demonstrated that estrogen levels, measured on days 1, 21, 84, and 91 of a 91-day extended-regimen cycle, did not build up over the course of the regimen.12
The risk of thromboembolic disease associated with OCs is not related to the length of use, and a 5-year case-control study found significantly decreasing odds ratios for reports of VTE in OC users over time.13 In this extension study, there were no reported thromboembolic AEs and there was no suggestion of an increased risk of thrombosis with the long-term use of this regimen, although such findings are not unexpected for a small-scale study.
Acknowledgements
The principal investigators and their locations are as follows: Angeli Adamczyk, Paige Brainard (Tucson, Ariz), Ted Anderson, Robert Rosenfeld, Shali Scott (Nashville, Tenn), Matthew Davis (Rochester, NY), William Gibbons, Laurel Stadtmauer (Norfolk, Va), James Lackey (Oklahoma City, Okla), Sooji Lee-Rugh (Arlington, Va), Thomas Littlejohn (Winston-Salem, NC), James Maly (Lincoln, Neb), David Portman (Columbus, Ohio), George Raad (Charlotte, NC), and Mark Shepard (Washington, DC).
CORRESPONDENCE Kathleen Reape, MD, Teva Branded Pharmaceutical Products R&D, Inc., 425 Privet Road, Horsham, PA 19044; Kathleen. [email protected]
1. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
2. Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception. 2008;77:91-96.
3. Vandever MA, Kuehl TJ, Sulak P, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77:162-170.
4. Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception. 2008;77:34-39.
5. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed April 6, 2010.
6. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. Am J Obstet Gynecol. 2006;195:92-96.
7. Zahradnik HP, Hanjalic-Beck A. Efficacy, safety, and sustainability of treatment continuation and results of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg chlormadinone acetate, in long-term usage (up to 45 cycles)—an open-label, prospective, noncontrolled, office-based Phase III study. Contraception. 2008;77:337-343.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Burkman RT. Venous thromboembolism and oral contraceptives: Current status and clinical implications. Treat Endocrinol. 2002;1:143-147.
10. Anderson FD, Hait H. The Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception. 2005;71:55-59.
12. Reape KZ, DiLiberti C. Steady-state pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen [abstract]. Obstet Gynecol. 2007;109(suppl 4):13S.-
13. Lidegaard O, Edstrom E, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65:187-196.
1. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
2. Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception. 2008;77:91-96.
3. Vandever MA, Kuehl TJ, Sulak P, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77:162-170.
4. Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception. 2008;77:34-39.
5. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed April 6, 2010.
6. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. Am J Obstet Gynecol. 2006;195:92-96.
7. Zahradnik HP, Hanjalic-Beck A. Efficacy, safety, and sustainability of treatment continuation and results of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg chlormadinone acetate, in long-term usage (up to 45 cycles)—an open-label, prospective, noncontrolled, office-based Phase III study. Contraception. 2008;77:337-343.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Burkman RT. Venous thromboembolism and oral contraceptives: Current status and clinical implications. Treat Endocrinol. 2002;1:143-147.
10. Anderson FD, Hait H. The Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception. 2005;71:55-59.
12. Reape KZ, DiLiberti C. Steady-state pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen [abstract]. Obstet Gynecol. 2007;109(suppl 4):13S.-
13. Lidegaard O, Edstrom E, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65:187-196.
Bedtime battles: When patients act out their dreams
REM sleep behavior disorder (RBD) patients act out their dreams during sleep and could injure themselves or bed partner. In RBD, loss of skeletal muscle atonia during rapid eye movement (REM) sleep allows the patient’s motor activity to reflect dream content. During sleep, patients appear to punch, kick, or choke a bed partner or jump out of bed.
RBD is more common in elderly males and individuals with neurodegenerative disorders of alpha-synuclein accumulation, such as Parkinson’s disease, Lewy body dementia, and multiple system atrophy.1 RBD may be a precursor to these diseases.
Most antidepressants can cause or increase RBD movements.2 RBD also is associated with sedative-hypnotic withdrawal.
Differential diagnosis
When patients report striking out while asleep, differential diagnoses include RBD, periodic limb movement disorder (PLMD), sleepwalking disorder, and restless legs syndrome (RLS). Polysomnography with electromyography may distinguish among these disorders.
PLMD movements are repetitive, stereotyped motions of the foot and leg, and manifest as a repetitive partial flexion of the joints of the great toe, ankle, knee, and occasionally hip. Upper extremity movements are less common. Movements appear similar to myoclonus. Periodic limb movements occur in rhythmic patterns, every 20 to 60 seconds, continuing for 10 minutes to several hours.
Sleepwalking disorder movements occur without an associated dream during non-REM sleep. Individuals with RBD may jump out of bed, but usually don’t walk in their sleep.
RLS movement occurs prior to and in early stages of sleep, whereas in RBD, PLMD, and sleepwalking disorder, motor activity is limited to sleep. Patients perceive unpleasant sensations and an urge to move the feet and legs. Movement temporarily soothes these uncomfortable sensations. Patients are aware of these sensations before sleep; however, RBD patients are not conscious of movements until they wake and find themselves acting out a dream. RLS and PLMD often are comorbid.
Treatment
Clonazepam is most effective for RBD; however, also consider lorazepam, pramipexole, or melatonin. If clinically feasible, consider discontinuing antidepressants because this may decrease movements.3
To reduce risk of injury, recommend sleeping in separate beds, moving objects away from the bed, or padding the headboard and floor. Encourage patients with severe RBD to sleep in a zipped sleeping bag.
1. Salah Uddin ABM, Jarmi T. REM sleep behavior disorder. Available at: http://emedicine.medscape.com/article/1188651-overview. Accessed March 22, 2010.
2. Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders; 2006.
3. Buysse DJ. Sleep disorders and psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2005.
REM sleep behavior disorder (RBD) patients act out their dreams during sleep and could injure themselves or bed partner. In RBD, loss of skeletal muscle atonia during rapid eye movement (REM) sleep allows the patient’s motor activity to reflect dream content. During sleep, patients appear to punch, kick, or choke a bed partner or jump out of bed.
RBD is more common in elderly males and individuals with neurodegenerative disorders of alpha-synuclein accumulation, such as Parkinson’s disease, Lewy body dementia, and multiple system atrophy.1 RBD may be a precursor to these diseases.
Most antidepressants can cause or increase RBD movements.2 RBD also is associated with sedative-hypnotic withdrawal.
Differential diagnosis
When patients report striking out while asleep, differential diagnoses include RBD, periodic limb movement disorder (PLMD), sleepwalking disorder, and restless legs syndrome (RLS). Polysomnography with electromyography may distinguish among these disorders.
PLMD movements are repetitive, stereotyped motions of the foot and leg, and manifest as a repetitive partial flexion of the joints of the great toe, ankle, knee, and occasionally hip. Upper extremity movements are less common. Movements appear similar to myoclonus. Periodic limb movements occur in rhythmic patterns, every 20 to 60 seconds, continuing for 10 minutes to several hours.
Sleepwalking disorder movements occur without an associated dream during non-REM sleep. Individuals with RBD may jump out of bed, but usually don’t walk in their sleep.
RLS movement occurs prior to and in early stages of sleep, whereas in RBD, PLMD, and sleepwalking disorder, motor activity is limited to sleep. Patients perceive unpleasant sensations and an urge to move the feet and legs. Movement temporarily soothes these uncomfortable sensations. Patients are aware of these sensations before sleep; however, RBD patients are not conscious of movements until they wake and find themselves acting out a dream. RLS and PLMD often are comorbid.
Treatment
Clonazepam is most effective for RBD; however, also consider lorazepam, pramipexole, or melatonin. If clinically feasible, consider discontinuing antidepressants because this may decrease movements.3
To reduce risk of injury, recommend sleeping in separate beds, moving objects away from the bed, or padding the headboard and floor. Encourage patients with severe RBD to sleep in a zipped sleeping bag.
REM sleep behavior disorder (RBD) patients act out their dreams during sleep and could injure themselves or bed partner. In RBD, loss of skeletal muscle atonia during rapid eye movement (REM) sleep allows the patient’s motor activity to reflect dream content. During sleep, patients appear to punch, kick, or choke a bed partner or jump out of bed.
RBD is more common in elderly males and individuals with neurodegenerative disorders of alpha-synuclein accumulation, such as Parkinson’s disease, Lewy body dementia, and multiple system atrophy.1 RBD may be a precursor to these diseases.
Most antidepressants can cause or increase RBD movements.2 RBD also is associated with sedative-hypnotic withdrawal.
Differential diagnosis
When patients report striking out while asleep, differential diagnoses include RBD, periodic limb movement disorder (PLMD), sleepwalking disorder, and restless legs syndrome (RLS). Polysomnography with electromyography may distinguish among these disorders.
PLMD movements are repetitive, stereotyped motions of the foot and leg, and manifest as a repetitive partial flexion of the joints of the great toe, ankle, knee, and occasionally hip. Upper extremity movements are less common. Movements appear similar to myoclonus. Periodic limb movements occur in rhythmic patterns, every 20 to 60 seconds, continuing for 10 minutes to several hours.
Sleepwalking disorder movements occur without an associated dream during non-REM sleep. Individuals with RBD may jump out of bed, but usually don’t walk in their sleep.
RLS movement occurs prior to and in early stages of sleep, whereas in RBD, PLMD, and sleepwalking disorder, motor activity is limited to sleep. Patients perceive unpleasant sensations and an urge to move the feet and legs. Movement temporarily soothes these uncomfortable sensations. Patients are aware of these sensations before sleep; however, RBD patients are not conscious of movements until they wake and find themselves acting out a dream. RLS and PLMD often are comorbid.
Treatment
Clonazepam is most effective for RBD; however, also consider lorazepam, pramipexole, or melatonin. If clinically feasible, consider discontinuing antidepressants because this may decrease movements.3
To reduce risk of injury, recommend sleeping in separate beds, moving objects away from the bed, or padding the headboard and floor. Encourage patients with severe RBD to sleep in a zipped sleeping bag.
1. Salah Uddin ABM, Jarmi T. REM sleep behavior disorder. Available at: http://emedicine.medscape.com/article/1188651-overview. Accessed March 22, 2010.
2. Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders; 2006.
3. Buysse DJ. Sleep disorders and psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2005.
1. Salah Uddin ABM, Jarmi T. REM sleep behavior disorder. Available at: http://emedicine.medscape.com/article/1188651-overview. Accessed March 22, 2010.
2. Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders; 2006.
3. Buysse DJ. Sleep disorders and psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2005.