User login
Crucial Contact
If the secret to successful real estate investing is “location, location, location,” the key to maintaining good relationships with referring physicians is “communication, communication, communication.” While this may seem simplistic, the complexities of interpersonal communications can pose challenges even in the most straightforward of physician interchanges.
Recent studies and hospitalists consulted for this report maintain that hospitalists’ communications with referring physicians must be examined, practiced, and fine-tuned continually to ensure satisfaction for doctors—and their patients.
“It’s all about the communication,” says Bruce Becker, MD, chief medical officer at Medical Center Hospital in Odessa, Texas, and a family physician and professor of medicine for more than 20 years. “It’s sometimes not what you say, but how you say it.”
According to John Nelson, MD, medical director of the Hospitalist Practice at Overlake Hospital in Bellevue, Wash., a consultant for hospital medicine groups with Nelson/Flores Associates and a columnist for The Hospitalist (“Practice Management”), it is key for hospitalists to examine and revise oral and written communication processes—especially at critical points during patient handoffs—rather than to assume that good communication will just happen naturally.
Gaining Acceptance
Some primary care physicians (PCPs) are more willing than others to refer patients to a hospitalist. Initially, new programs may have to work hard to gain acceptance with referring physicians. Some referring physicians may not be ready to give up hospital visits and may want to maintain collegiality and control. On the other hand, some family physicians are “ready to step away from hospital practice,” says Dr. Becker, citing diminishing reimbursements due to diagnosis-related groups (DRGs) and managed care.
John A. Bolinger, DO, FACP, medical director of the Hospitalist Program at Terre Haute (Ind.) Regional Hospital, believes that one way to promote a hospitalist program to PCPs is to emphasize hospitalists’ levels of training and efficiency.
“I try to make them aware of how [our hospitalist program] can be advantageous to them,” he explains. “Even if they have only one patient in the hospital, by the time they drive there, get the chart, make the rounds, make their notes, and do the required paperwork, it may take them an hour to see one patient. It makes good economic sense for them to stay in their offices, where they can see a minimum of four patients in the same amount of time for equal or better reimbursements.”
When primary care physicians voice resistance to using a hospitalist program, Dr. Bolinger says he tries to impress upon them the fact that hospitalists do not have outside practices, they will “never try to steal patients,” they stay within referral patterns, and they will make sure that PCPs get pertinent records as soon as possible. Dr. Bolinger believes referring physicians’ biggest concern when dealing with hospitalists is that they “don’t want to lose control.” The way to address those fears is to make sure referring physicians are always kept in the communications loop regarding their patients’ progress.
The policy for Dr. Bolinger’s hospitalist program is to make sure all dictated reports are transcribed and faxed immediately to the referring physician’s office. All scheduled follow-up appointments and medication changes are included in discharge summaries. “If need be,” says Dr. Bolinger, “we will even hand deliver information to physicians’ offices, which we have done multiple times.”
Become User-Friendly
The methods Dr. Bolinger describes often result in referring physicians’ satisfaction with hospitalist services, followed by increased referrals. Hospitalists can ensure continued referrals from their PCPs if they remember hospital medicine’s cardinal rules of availability and prompt, thorough reporting, says Dr. Nelson. It can help to view the interface with the hospitalist service from the PCP’s point of view.
For instance, how can physicians quickly reach the hospitalist service? “If you’re a referring doctor and you’ve got a patient in your office whom you think should be admitted today, who do you call? This can actually be a little tricky for most practices,” says Dr. Nelson. “Every practice should give some thought to making that contact as easy for the referring doctors as possible.”
Some important questions to ask: When trying to reach a hospitalist, is it best to call the group’s main number? Will a voicemail message be returned within an hour? Two hours? Is it better to call the hospital operator and have the hospitalist paged? Or do PCPs have access to hospitalists’ cell phone numbers?
Dr. Nelson suggests that hospitalist groups also give thought to standardizing admission and discharge reports, as well as other forms. Often, individual members of a hospitalist group use slightly different formats for reporting to referring physicians. He points out that this can be less user friendly for the reader, who may have a harder time scanning the document quickly to find a particular piece of important information. Other useful suggestions for making reports user friendly: Use similar headings on all reports; avoid dense text; list pending tests in a prominent place in the report document; and consider highlighting or underlining key words.
Preference for In-Person Contact
For a new hospitalist practice, telephone communication between the hospitalist and the primary physician is valuable, and the hospitalist should “pick up the phone liberally,” says Dr. Nelson.
Dr. Becker believes the best way for physicians to communicate is one on one—in person. “Too often,” he says, “we get used to communicating through a third party—usually a unit clerk, a nurse, or a resident. I believe that physician-to-physician communication is the ideal. If the attending physician and the consultant [hospitalist or subspecialist] speak in person, they can explain their thinking to each other, and “within one minute of precious time, figure out which way to go.”
In this way, without wasting time, the physician gives the consultant guidance as to the appropriate track to take and can also listen to the consultant’s suggestions.
“I feel that medicine has perhaps gotten a little bit away from that communication link,” continues Dr. Becker. “When we get further away from that direct communication—whether it is between doctors and consultants, nurses and doctors, or doctors and family members—you take that little bit of risk that there will be a missed step, either on the part of the communicator or on the receiving end as the listener.”
In a study exploring barriers to effective patient handoffs, Solet and colleagues focused on the communication between physicians as a vital link in patient care continuity. The authors concluded that, regardless of the method of managing patient handoffs (e.g., computer-assisted or paper-based), the best way to ensure effective handoffs of hospitalized patients was “precise, unambiguous, face-to-face communication.”1
In a 2001 study by Hruby, Pantilat, and colleagues at UCSF, the authors found that, for the most part, hospitalized patients with PCPs wanted contact with their primary physicians even while in the hospital. Approximately half of the surveyed patients also believed that the PCP, rather than an inpatient physician, should be the first to discuss with them serious diagnoses or disease management choices.2 Preferences such as those expressed in this study may play into referring physicians’ reluctance to make use of hospitalist services, says Dr. Bolinger. They may fear that patients will feel abandoned by the primary physician. Dr. Bolinger’s response to those sentiments: “Initially, some patients [in our hospital] were a little guarded and were not sure what to expect. But after a day or two of having us there, they are generally very, very pleased to have us on board. We are part of their medical team now.”
A Need for Marketing?
Dr. Becker has been actively developing a hospital medicine program at Medical Center Hospital for the past two years and joined SHM as part of that effort. Familiarizing himself with the tenets of hospital medicine, he discovered that, as a family doctor, “unknowingly, I was actually practicing hospital medicine for 20 years!”
As part of the hospital medicine program development process, he has solicited input from local physicians as well as patients. A simple survey to assess interest in a hospitalist program asked potential referring physicians, Would you use a hospitalist? Would you use a hospitalist after waiting a while to see how the process goes? Or, would you not consider using a hospitalist?
In two years, says Dr. Becker, response from the referring physician community has changed from “bah, humbug” to one of readiness for the program.
A mass mailing can serve to introduce a hospital medicine program in a community. Dr. Bolinger’s group used this method and, in his experience, local subspecialists—orthopedists, cardiologists, endocrinologists, pulmonologists—have proved the biggest source of referrals to their program. But PCPs are starting to use the hospitalist service for vacations and call-coverage issues and are beginning to value hospitalists’ services. “Physicians like coming to the hospital, but they’re starting to realize that the hospitalist program is a better system,” says Dr. Bolinger.
Dr. Nelson has been a hospitalist for 18 years. For most of that time he has had no shortage of referred patients. In fact, the bigger problem has been finding enough doctors to join the group and handle the existing referral volume. In that situation, it has not made sense to undertake marketing with the goal of increasing referrals. However, he advises, “It is always worth spending time and energy trying to maintain good relationships with physicians with whom you regularly share patients, and perhaps this could be called ‘marketing.’”
To maintain good relationships with referring physicians, his group conducts a survey on a yearly basis. A survey, he suggests, should be very short, consisting of only a few key questions, such as:
- Do we send reports promptly to your practice?
- Are your patients satisfied with the care they receive from us?
- Do you have any comments or feedback for our group?
Although his group gains information from these surveys, Dr. Nelson notes that the greater value of conducting such surveys may be in building public relations capital. By conducting a survey, hospitalists demonstrate that they care enough to ask for their referring physicians’ input.
Another good marketing tool is a patient education brochure, given to referring physicians, that explains hospitalists and hospitalist care. These brochures can help referring physicians prepare their patients for seeing a hospitalist in the inpatient setting, thus easing the initial reluctance patients sometimes experience when encountering a new physician.
Conclusion
On the cusp of launching his medical center’s hospital medicine program, Dr. Becker sees that good communication between referring physicians and hospitalists will ensure the program’s success. He advises physicians to remember their classes in communication as third-year medical students, when most participate in videotaped patient encounters. It’s always instructive, he says, to see how we come across to others in conversation.
Both verbal and nonverbal cues play a part in good communication. A 2003 study by Griffith and colleagues concluded that better nonverbal communication skills are associated with greater patient satisfaction, and that formal instruction in nonverbal communication can be a good addition to residency training.3
“I find that doctors talk to each other, in general, very easily,” says Dr. Becker. Sometimes [good communication] is just a matter of opening that door and essentially keeping the former attending, the PCP, apprised of what is going on.”
When hospitalists attend to thorough communication and promptly deliver complete discharge summaries, family physicians can report to their patients that they know what happened in the hospital and poll their patients about their experiences in the hospital. In this way, hospitalists and referring physicians can cement their relationship as team members for the patient. The success of any hospitalist program, Dr. Becker believes, lies in “making sure you fulfill the promise of what hospital medicine generates, and that is a continuity of care … , obtaining front-end communication so that patients get the best care throughout their [hospital] stay, and then follow up with discharge summaries to the primary physician’s office.” TH
Gretchen Henkel is a regular contributor to The Hospitalist.
References
- Solet DJ, Norvell JM, Rutan GH, et al. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med. 2005 Dec;80(12): 1094-1099.
- Hruby M, Pantilat SZ, Lo B. How do patients view the role of the primary care physician in inpatient care? Am J Med. 2001;21;111(9B):21S-25S.
- Griffith CH III, Wilson JF, Langer S, et al. House staff nonverbal communication skills and standardized patient satisfaction. J Gen Intern Med. 2003 Mar;18(3):170-174.
If the secret to successful real estate investing is “location, location, location,” the key to maintaining good relationships with referring physicians is “communication, communication, communication.” While this may seem simplistic, the complexities of interpersonal communications can pose challenges even in the most straightforward of physician interchanges.
Recent studies and hospitalists consulted for this report maintain that hospitalists’ communications with referring physicians must be examined, practiced, and fine-tuned continually to ensure satisfaction for doctors—and their patients.
“It’s all about the communication,” says Bruce Becker, MD, chief medical officer at Medical Center Hospital in Odessa, Texas, and a family physician and professor of medicine for more than 20 years. “It’s sometimes not what you say, but how you say it.”
According to John Nelson, MD, medical director of the Hospitalist Practice at Overlake Hospital in Bellevue, Wash., a consultant for hospital medicine groups with Nelson/Flores Associates and a columnist for The Hospitalist (“Practice Management”), it is key for hospitalists to examine and revise oral and written communication processes—especially at critical points during patient handoffs—rather than to assume that good communication will just happen naturally.
Gaining Acceptance
Some primary care physicians (PCPs) are more willing than others to refer patients to a hospitalist. Initially, new programs may have to work hard to gain acceptance with referring physicians. Some referring physicians may not be ready to give up hospital visits and may want to maintain collegiality and control. On the other hand, some family physicians are “ready to step away from hospital practice,” says Dr. Becker, citing diminishing reimbursements due to diagnosis-related groups (DRGs) and managed care.
John A. Bolinger, DO, FACP, medical director of the Hospitalist Program at Terre Haute (Ind.) Regional Hospital, believes that one way to promote a hospitalist program to PCPs is to emphasize hospitalists’ levels of training and efficiency.
“I try to make them aware of how [our hospitalist program] can be advantageous to them,” he explains. “Even if they have only one patient in the hospital, by the time they drive there, get the chart, make the rounds, make their notes, and do the required paperwork, it may take them an hour to see one patient. It makes good economic sense for them to stay in their offices, where they can see a minimum of four patients in the same amount of time for equal or better reimbursements.”
When primary care physicians voice resistance to using a hospitalist program, Dr. Bolinger says he tries to impress upon them the fact that hospitalists do not have outside practices, they will “never try to steal patients,” they stay within referral patterns, and they will make sure that PCPs get pertinent records as soon as possible. Dr. Bolinger believes referring physicians’ biggest concern when dealing with hospitalists is that they “don’t want to lose control.” The way to address those fears is to make sure referring physicians are always kept in the communications loop regarding their patients’ progress.
The policy for Dr. Bolinger’s hospitalist program is to make sure all dictated reports are transcribed and faxed immediately to the referring physician’s office. All scheduled follow-up appointments and medication changes are included in discharge summaries. “If need be,” says Dr. Bolinger, “we will even hand deliver information to physicians’ offices, which we have done multiple times.”
Become User-Friendly
The methods Dr. Bolinger describes often result in referring physicians’ satisfaction with hospitalist services, followed by increased referrals. Hospitalists can ensure continued referrals from their PCPs if they remember hospital medicine’s cardinal rules of availability and prompt, thorough reporting, says Dr. Nelson. It can help to view the interface with the hospitalist service from the PCP’s point of view.
For instance, how can physicians quickly reach the hospitalist service? “If you’re a referring doctor and you’ve got a patient in your office whom you think should be admitted today, who do you call? This can actually be a little tricky for most practices,” says Dr. Nelson. “Every practice should give some thought to making that contact as easy for the referring doctors as possible.”
Some important questions to ask: When trying to reach a hospitalist, is it best to call the group’s main number? Will a voicemail message be returned within an hour? Two hours? Is it better to call the hospital operator and have the hospitalist paged? Or do PCPs have access to hospitalists’ cell phone numbers?
Dr. Nelson suggests that hospitalist groups also give thought to standardizing admission and discharge reports, as well as other forms. Often, individual members of a hospitalist group use slightly different formats for reporting to referring physicians. He points out that this can be less user friendly for the reader, who may have a harder time scanning the document quickly to find a particular piece of important information. Other useful suggestions for making reports user friendly: Use similar headings on all reports; avoid dense text; list pending tests in a prominent place in the report document; and consider highlighting or underlining key words.
Preference for In-Person Contact
For a new hospitalist practice, telephone communication between the hospitalist and the primary physician is valuable, and the hospitalist should “pick up the phone liberally,” says Dr. Nelson.
Dr. Becker believes the best way for physicians to communicate is one on one—in person. “Too often,” he says, “we get used to communicating through a third party—usually a unit clerk, a nurse, or a resident. I believe that physician-to-physician communication is the ideal. If the attending physician and the consultant [hospitalist or subspecialist] speak in person, they can explain their thinking to each other, and “within one minute of precious time, figure out which way to go.”
In this way, without wasting time, the physician gives the consultant guidance as to the appropriate track to take and can also listen to the consultant’s suggestions.
“I feel that medicine has perhaps gotten a little bit away from that communication link,” continues Dr. Becker. “When we get further away from that direct communication—whether it is between doctors and consultants, nurses and doctors, or doctors and family members—you take that little bit of risk that there will be a missed step, either on the part of the communicator or on the receiving end as the listener.”
In a study exploring barriers to effective patient handoffs, Solet and colleagues focused on the communication between physicians as a vital link in patient care continuity. The authors concluded that, regardless of the method of managing patient handoffs (e.g., computer-assisted or paper-based), the best way to ensure effective handoffs of hospitalized patients was “precise, unambiguous, face-to-face communication.”1
In a 2001 study by Hruby, Pantilat, and colleagues at UCSF, the authors found that, for the most part, hospitalized patients with PCPs wanted contact with their primary physicians even while in the hospital. Approximately half of the surveyed patients also believed that the PCP, rather than an inpatient physician, should be the first to discuss with them serious diagnoses or disease management choices.2 Preferences such as those expressed in this study may play into referring physicians’ reluctance to make use of hospitalist services, says Dr. Bolinger. They may fear that patients will feel abandoned by the primary physician. Dr. Bolinger’s response to those sentiments: “Initially, some patients [in our hospital] were a little guarded and were not sure what to expect. But after a day or two of having us there, they are generally very, very pleased to have us on board. We are part of their medical team now.”
A Need for Marketing?
Dr. Becker has been actively developing a hospital medicine program at Medical Center Hospital for the past two years and joined SHM as part of that effort. Familiarizing himself with the tenets of hospital medicine, he discovered that, as a family doctor, “unknowingly, I was actually practicing hospital medicine for 20 years!”
As part of the hospital medicine program development process, he has solicited input from local physicians as well as patients. A simple survey to assess interest in a hospitalist program asked potential referring physicians, Would you use a hospitalist? Would you use a hospitalist after waiting a while to see how the process goes? Or, would you not consider using a hospitalist?
In two years, says Dr. Becker, response from the referring physician community has changed from “bah, humbug” to one of readiness for the program.
A mass mailing can serve to introduce a hospital medicine program in a community. Dr. Bolinger’s group used this method and, in his experience, local subspecialists—orthopedists, cardiologists, endocrinologists, pulmonologists—have proved the biggest source of referrals to their program. But PCPs are starting to use the hospitalist service for vacations and call-coverage issues and are beginning to value hospitalists’ services. “Physicians like coming to the hospital, but they’re starting to realize that the hospitalist program is a better system,” says Dr. Bolinger.
Dr. Nelson has been a hospitalist for 18 years. For most of that time he has had no shortage of referred patients. In fact, the bigger problem has been finding enough doctors to join the group and handle the existing referral volume. In that situation, it has not made sense to undertake marketing with the goal of increasing referrals. However, he advises, “It is always worth spending time and energy trying to maintain good relationships with physicians with whom you regularly share patients, and perhaps this could be called ‘marketing.’”
To maintain good relationships with referring physicians, his group conducts a survey on a yearly basis. A survey, he suggests, should be very short, consisting of only a few key questions, such as:
- Do we send reports promptly to your practice?
- Are your patients satisfied with the care they receive from us?
- Do you have any comments or feedback for our group?
Although his group gains information from these surveys, Dr. Nelson notes that the greater value of conducting such surveys may be in building public relations capital. By conducting a survey, hospitalists demonstrate that they care enough to ask for their referring physicians’ input.
Another good marketing tool is a patient education brochure, given to referring physicians, that explains hospitalists and hospitalist care. These brochures can help referring physicians prepare their patients for seeing a hospitalist in the inpatient setting, thus easing the initial reluctance patients sometimes experience when encountering a new physician.
Conclusion
On the cusp of launching his medical center’s hospital medicine program, Dr. Becker sees that good communication between referring physicians and hospitalists will ensure the program’s success. He advises physicians to remember their classes in communication as third-year medical students, when most participate in videotaped patient encounters. It’s always instructive, he says, to see how we come across to others in conversation.
Both verbal and nonverbal cues play a part in good communication. A 2003 study by Griffith and colleagues concluded that better nonverbal communication skills are associated with greater patient satisfaction, and that formal instruction in nonverbal communication can be a good addition to residency training.3
“I find that doctors talk to each other, in general, very easily,” says Dr. Becker. Sometimes [good communication] is just a matter of opening that door and essentially keeping the former attending, the PCP, apprised of what is going on.”
When hospitalists attend to thorough communication and promptly deliver complete discharge summaries, family physicians can report to their patients that they know what happened in the hospital and poll their patients about their experiences in the hospital. In this way, hospitalists and referring physicians can cement their relationship as team members for the patient. The success of any hospitalist program, Dr. Becker believes, lies in “making sure you fulfill the promise of what hospital medicine generates, and that is a continuity of care … , obtaining front-end communication so that patients get the best care throughout their [hospital] stay, and then follow up with discharge summaries to the primary physician’s office.” TH
Gretchen Henkel is a regular contributor to The Hospitalist.
References
- Solet DJ, Norvell JM, Rutan GH, et al. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med. 2005 Dec;80(12): 1094-1099.
- Hruby M, Pantilat SZ, Lo B. How do patients view the role of the primary care physician in inpatient care? Am J Med. 2001;21;111(9B):21S-25S.
- Griffith CH III, Wilson JF, Langer S, et al. House staff nonverbal communication skills and standardized patient satisfaction. J Gen Intern Med. 2003 Mar;18(3):170-174.
If the secret to successful real estate investing is “location, location, location,” the key to maintaining good relationships with referring physicians is “communication, communication, communication.” While this may seem simplistic, the complexities of interpersonal communications can pose challenges even in the most straightforward of physician interchanges.
Recent studies and hospitalists consulted for this report maintain that hospitalists’ communications with referring physicians must be examined, practiced, and fine-tuned continually to ensure satisfaction for doctors—and their patients.
“It’s all about the communication,” says Bruce Becker, MD, chief medical officer at Medical Center Hospital in Odessa, Texas, and a family physician and professor of medicine for more than 20 years. “It’s sometimes not what you say, but how you say it.”
According to John Nelson, MD, medical director of the Hospitalist Practice at Overlake Hospital in Bellevue, Wash., a consultant for hospital medicine groups with Nelson/Flores Associates and a columnist for The Hospitalist (“Practice Management”), it is key for hospitalists to examine and revise oral and written communication processes—especially at critical points during patient handoffs—rather than to assume that good communication will just happen naturally.
Gaining Acceptance
Some primary care physicians (PCPs) are more willing than others to refer patients to a hospitalist. Initially, new programs may have to work hard to gain acceptance with referring physicians. Some referring physicians may not be ready to give up hospital visits and may want to maintain collegiality and control. On the other hand, some family physicians are “ready to step away from hospital practice,” says Dr. Becker, citing diminishing reimbursements due to diagnosis-related groups (DRGs) and managed care.
John A. Bolinger, DO, FACP, medical director of the Hospitalist Program at Terre Haute (Ind.) Regional Hospital, believes that one way to promote a hospitalist program to PCPs is to emphasize hospitalists’ levels of training and efficiency.
“I try to make them aware of how [our hospitalist program] can be advantageous to them,” he explains. “Even if they have only one patient in the hospital, by the time they drive there, get the chart, make the rounds, make their notes, and do the required paperwork, it may take them an hour to see one patient. It makes good economic sense for them to stay in their offices, where they can see a minimum of four patients in the same amount of time for equal or better reimbursements.”
When primary care physicians voice resistance to using a hospitalist program, Dr. Bolinger says he tries to impress upon them the fact that hospitalists do not have outside practices, they will “never try to steal patients,” they stay within referral patterns, and they will make sure that PCPs get pertinent records as soon as possible. Dr. Bolinger believes referring physicians’ biggest concern when dealing with hospitalists is that they “don’t want to lose control.” The way to address those fears is to make sure referring physicians are always kept in the communications loop regarding their patients’ progress.
The policy for Dr. Bolinger’s hospitalist program is to make sure all dictated reports are transcribed and faxed immediately to the referring physician’s office. All scheduled follow-up appointments and medication changes are included in discharge summaries. “If need be,” says Dr. Bolinger, “we will even hand deliver information to physicians’ offices, which we have done multiple times.”
Become User-Friendly
The methods Dr. Bolinger describes often result in referring physicians’ satisfaction with hospitalist services, followed by increased referrals. Hospitalists can ensure continued referrals from their PCPs if they remember hospital medicine’s cardinal rules of availability and prompt, thorough reporting, says Dr. Nelson. It can help to view the interface with the hospitalist service from the PCP’s point of view.
For instance, how can physicians quickly reach the hospitalist service? “If you’re a referring doctor and you’ve got a patient in your office whom you think should be admitted today, who do you call? This can actually be a little tricky for most practices,” says Dr. Nelson. “Every practice should give some thought to making that contact as easy for the referring doctors as possible.”
Some important questions to ask: When trying to reach a hospitalist, is it best to call the group’s main number? Will a voicemail message be returned within an hour? Two hours? Is it better to call the hospital operator and have the hospitalist paged? Or do PCPs have access to hospitalists’ cell phone numbers?
Dr. Nelson suggests that hospitalist groups also give thought to standardizing admission and discharge reports, as well as other forms. Often, individual members of a hospitalist group use slightly different formats for reporting to referring physicians. He points out that this can be less user friendly for the reader, who may have a harder time scanning the document quickly to find a particular piece of important information. Other useful suggestions for making reports user friendly: Use similar headings on all reports; avoid dense text; list pending tests in a prominent place in the report document; and consider highlighting or underlining key words.
Preference for In-Person Contact
For a new hospitalist practice, telephone communication between the hospitalist and the primary physician is valuable, and the hospitalist should “pick up the phone liberally,” says Dr. Nelson.
Dr. Becker believes the best way for physicians to communicate is one on one—in person. “Too often,” he says, “we get used to communicating through a third party—usually a unit clerk, a nurse, or a resident. I believe that physician-to-physician communication is the ideal. If the attending physician and the consultant [hospitalist or subspecialist] speak in person, they can explain their thinking to each other, and “within one minute of precious time, figure out which way to go.”
In this way, without wasting time, the physician gives the consultant guidance as to the appropriate track to take and can also listen to the consultant’s suggestions.
“I feel that medicine has perhaps gotten a little bit away from that communication link,” continues Dr. Becker. “When we get further away from that direct communication—whether it is between doctors and consultants, nurses and doctors, or doctors and family members—you take that little bit of risk that there will be a missed step, either on the part of the communicator or on the receiving end as the listener.”
In a study exploring barriers to effective patient handoffs, Solet and colleagues focused on the communication between physicians as a vital link in patient care continuity. The authors concluded that, regardless of the method of managing patient handoffs (e.g., computer-assisted or paper-based), the best way to ensure effective handoffs of hospitalized patients was “precise, unambiguous, face-to-face communication.”1
In a 2001 study by Hruby, Pantilat, and colleagues at UCSF, the authors found that, for the most part, hospitalized patients with PCPs wanted contact with their primary physicians even while in the hospital. Approximately half of the surveyed patients also believed that the PCP, rather than an inpatient physician, should be the first to discuss with them serious diagnoses or disease management choices.2 Preferences such as those expressed in this study may play into referring physicians’ reluctance to make use of hospitalist services, says Dr. Bolinger. They may fear that patients will feel abandoned by the primary physician. Dr. Bolinger’s response to those sentiments: “Initially, some patients [in our hospital] were a little guarded and were not sure what to expect. But after a day or two of having us there, they are generally very, very pleased to have us on board. We are part of their medical team now.”
A Need for Marketing?
Dr. Becker has been actively developing a hospital medicine program at Medical Center Hospital for the past two years and joined SHM as part of that effort. Familiarizing himself with the tenets of hospital medicine, he discovered that, as a family doctor, “unknowingly, I was actually practicing hospital medicine for 20 years!”
As part of the hospital medicine program development process, he has solicited input from local physicians as well as patients. A simple survey to assess interest in a hospitalist program asked potential referring physicians, Would you use a hospitalist? Would you use a hospitalist after waiting a while to see how the process goes? Or, would you not consider using a hospitalist?
In two years, says Dr. Becker, response from the referring physician community has changed from “bah, humbug” to one of readiness for the program.
A mass mailing can serve to introduce a hospital medicine program in a community. Dr. Bolinger’s group used this method and, in his experience, local subspecialists—orthopedists, cardiologists, endocrinologists, pulmonologists—have proved the biggest source of referrals to their program. But PCPs are starting to use the hospitalist service for vacations and call-coverage issues and are beginning to value hospitalists’ services. “Physicians like coming to the hospital, but they’re starting to realize that the hospitalist program is a better system,” says Dr. Bolinger.
Dr. Nelson has been a hospitalist for 18 years. For most of that time he has had no shortage of referred patients. In fact, the bigger problem has been finding enough doctors to join the group and handle the existing referral volume. In that situation, it has not made sense to undertake marketing with the goal of increasing referrals. However, he advises, “It is always worth spending time and energy trying to maintain good relationships with physicians with whom you regularly share patients, and perhaps this could be called ‘marketing.’”
To maintain good relationships with referring physicians, his group conducts a survey on a yearly basis. A survey, he suggests, should be very short, consisting of only a few key questions, such as:
- Do we send reports promptly to your practice?
- Are your patients satisfied with the care they receive from us?
- Do you have any comments or feedback for our group?
Although his group gains information from these surveys, Dr. Nelson notes that the greater value of conducting such surveys may be in building public relations capital. By conducting a survey, hospitalists demonstrate that they care enough to ask for their referring physicians’ input.
Another good marketing tool is a patient education brochure, given to referring physicians, that explains hospitalists and hospitalist care. These brochures can help referring physicians prepare their patients for seeing a hospitalist in the inpatient setting, thus easing the initial reluctance patients sometimes experience when encountering a new physician.
Conclusion
On the cusp of launching his medical center’s hospital medicine program, Dr. Becker sees that good communication between referring physicians and hospitalists will ensure the program’s success. He advises physicians to remember their classes in communication as third-year medical students, when most participate in videotaped patient encounters. It’s always instructive, he says, to see how we come across to others in conversation.
Both verbal and nonverbal cues play a part in good communication. A 2003 study by Griffith and colleagues concluded that better nonverbal communication skills are associated with greater patient satisfaction, and that formal instruction in nonverbal communication can be a good addition to residency training.3
“I find that doctors talk to each other, in general, very easily,” says Dr. Becker. Sometimes [good communication] is just a matter of opening that door and essentially keeping the former attending, the PCP, apprised of what is going on.”
When hospitalists attend to thorough communication and promptly deliver complete discharge summaries, family physicians can report to their patients that they know what happened in the hospital and poll their patients about their experiences in the hospital. In this way, hospitalists and referring physicians can cement their relationship as team members for the patient. The success of any hospitalist program, Dr. Becker believes, lies in “making sure you fulfill the promise of what hospital medicine generates, and that is a continuity of care … , obtaining front-end communication so that patients get the best care throughout their [hospital] stay, and then follow up with discharge summaries to the primary physician’s office.” TH
Gretchen Henkel is a regular contributor to The Hospitalist.
References
- Solet DJ, Norvell JM, Rutan GH, et al. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med. 2005 Dec;80(12): 1094-1099.
- Hruby M, Pantilat SZ, Lo B. How do patients view the role of the primary care physician in inpatient care? Am J Med. 2001;21;111(9B):21S-25S.
- Griffith CH III, Wilson JF, Langer S, et al. House staff nonverbal communication skills and standardized patient satisfaction. J Gen Intern Med. 2003 Mar;18(3):170-174.
Pregnancy Perils
Hypertension during pregnancy, of which preeclampsia and eclampsia predominate, constitutes a significant health problem both because of its high incidence (4%-11% of pregnancies in developed countries) and due to the maternal and fetal health outcomes it creates.1 Hypertensive disorders are the second leading cause of maternal mortality.1 They cause 15% of all maternal deaths and constitute considerable morbidity both during and after pregnancy.2 Fetal outcomes include premature delivery, small-for-gestational-age (SGA) infants, and fetal mortality.
The long-term cardiovascular risks for mothers who suffer from the triad of hypertension during pregnancy, SGA infants, and pre-term delivery are approximately eight times higher than for individuals without these complications during pregnancy.
Hypertension observed during pregnancy is defined according to one of the following classifications:
- Chronic hypertension, present prior to pregnancy;
- Preeclampsia/eclampsia: development of hypertension, proteinuria, and edema during pregnancy;
- Preeclampsia superimposed on preexisting renal disease; and
- Gestational hypertension: either transient mild hypertension during the third trimester or mild hypertension detected during the third trimester that does not resolve by 12 weeks postpartum.
Diagnosing Preeclampsia/Eclampsia
Of these hypertensive entities, this article focuses on the most common and potentially serious one: preeclampsia/eclampsia. Preeclampsia is characterized by blood pressure over 140/90 mm Hg—measured on two separate occasions, and proteinuria greater than 300 mg/24 hours, occurring after 20 weeks gestation. Severe preeclampsia includes the same criteria, as well as proteinuria greater than 5,000 mg/24 hours; blood pressure higher than 160/110 mm Hg; or end-organ damage such as headaches, visual changes, renal dysfunction, hepatic dysfunction, or thrombocytopenia.
Historically, the term eclampsia has been reserved to describe the symptoms of preeclampsia combined with the occurrence of seizure activity. HELLP syndrome is a severe variant of preeclampsia that affects up to 1% of pregnancies and includes the constellation of hemolysis, elevated liver enzyme levels, and a low platelet count.
Given the potential risks for these disorders during pregnancy, it seems logical to target primarily those individuals at risk and attempt to prevent the disorder. Traditionally, the higher risk populations include patients experiencing primagravida pregnancies, those with a history of hypertension or renal disease, women who have had prior episodes of preeclampsia, and multiparous individuals with different paternal partners. Other less helpful or more expensive screening procedures include evaluation for inherited thrombophilias.
Recommended Treatment
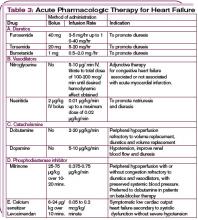
Several trials have attempted to prevent the development of preeclampsia using calcium supplementation or aspirin therapy, but current evidence demonstrates no benefit from these interventions except in selected populations.3,4 For the past several decades, the treatment for hypertension during pregnancy has consisted of either delivery of the fetus or bed rest combined with therapies involving alpha-methyl-DOPA, hydralazine, and intravenous magnesium sulfate, depending on the level of the patient’s blood pressure and proteinuria, as well as the stage of the pregnancy.
Because of the potential risks to the health of the infant (as well as that of the mother) generic interventional trials extrapolated from other hypertensive populations that may offer equivalent or more effective therapies have not been readily accomplished. In spite of this lack of clinical and outcomes studies, the following generalizations regarding care are currently advocated:
1. Continue anti-hypertensive therapy for chronic hypertension with a goal of maintaining blood pressure below 140/90 mm Hg. Though the risks of SGA infants and preeclampsia were not affected by therapy, the incidence of premature delivery was reduced. Nearly all anti-hypertensive drugs have been used in treating chronic hypertension in pregnancy, but angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are contraindicated because of teratogenecity. Diuretics and hydralazine may also have adverse outcomes on fetal and/or maternal health, respectively.
Though diuretics may reduce an already compromised placental blood flow by reducing intravascular volume, a number of randomized trials using diuretics have been reported. The population studied in these trials totaled approximately 7,000 individuals, and the results show no demonstrable adverse effects.5 Centrally acting alpha 2 adrenergic agonists (alpha-methyl-DOPA, clonidine), long-acting dihydropyridines (calcium channel blockers), and beta-adrenergic blockers with or without alpha-adrenergic blockade have been used successfully during pregnancy.6 Infants born to mothers receiving beta-blockers have a higher incidence of transient heart block and bradycardia. Newer agents, as opposed to alpha-methyl-DOPA and hydralazine, have not been shown to be more effective in controlling blood pressure but have demonstrated fewer adverse effects or events.
2. Mild preeclampsia may be treated using late-term delivery or bed rest before the pregnancy reaches 36 weeks. Close observation in a hospital is warranted until lack of progression to severe eclampsia is ensured.
3. Severe preeclampsia must be treated using either delivery or interventions to control blood pressure and prevent seizures while delivery is temporarily delayed to allow for fetal maturation. Intravenous magnesium sulfate (Mg2SO4), titrated to therapeutic concentrations of magnesium (4.5-8.5 mg/dl) along with suppression of deep tendon reflexes, has significantly lowered blood pressure and reduced the incidence of seizure activity. In direct comparison with intravenous phenytoin, there were no episodes of eclampsia in patients treated with magnesium (zero of 1,049 patients treated), while 10 of 1,089 individuals treated with phenytoin developed eclampsia.7 Similarly, Mg2SO4 decreased the incidence of eclampsia to 0.8% compared with 2.6% of individuals treated with nimodipine.8 Likewise, Mg2SO4 significantly reduced recurrent seizures compared to diazepam and phenytoin. In patients with renal insufficiency or other relative contraindications to Mg2SO4 therapy, the physician may treat the blood pressure with labetalol or a calcium channel antagonist and provide prophylaxis from seizures using phenytoin.
Because of the lack of a more complete understanding of the etiology (or etiologies) of preeclampsia/eclampsia, we are not fully able to identify individuals who are at risk. With further investigation, we will be able to provide more selective and effective interventions.
Nitrous Oxide’s Vital Role in Pregnancy
In this regard, considerable investigation has been directed toward a better understanding of eclampsia/preeclampsia in the past 20 years. The current discussion will highlight three promising lines of inquiry. Before commenting on these three areas of investigation, we feel it is important to illustrate the fundamental defect observed in all models of preeclampsia. This unifying abnormality is the presence of a placenta with inadequate uterine blood flow.
In a normal pregnancy, after implantation in the uterine wall, cytotrophoblasts invade the uterine wall, undergo a transformation of cell adhesion molecules from epithelial to endothelial expression characteristics, and induce the deep invasion of the placenta by the spiral arteries, creating large vascular sinuses in the decidua (pseudovasculogenesis). Finally, beta human chorionic gonadotropin production by the placenta stimulates the release of relaxin from the corpus luteum. Many cytokines and growth factors contribute to this normal placental development, but vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) appear to be the most important.
VEGF and PlGF play an integral role in the inducement of conversion of the cytotrophoblast cell adhesion molecules from epithelial to endothelial expression, particularly integrins and cadherins. In models of preeclampsia, there is insufficient invasion of the spiral arteries and inadequate uterine blood flow, as well as a lack of alteration in adhesion molecules of cytotrophoblasts and failed pseudovasculogenesis.9
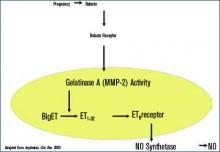
During normal pregnancy, intravascular volume increases, peripheral vascular resistance (PVR) decreases, and renal blood flow and glomerular filtration rate (GFR) increase. (See Figure 1, above left.) Concomitant with the increased plasma volume, the reduction of PVR (vasodilation) not only mitigates the effect of plasma volume on systemic blood pressure, but actually reduces the systemic blood pressure during normal pregnancy. (See Figure 2, below.) Based on seminal studies by Jeyabalan and colleagues, one explanation for the decline in PVR and blood pressure, along with the increased renal plasma flow and GFR, is the production and action of relaxin on vascular smooth muscle to stimulate gelatinase-A (matrix metalloproteinase-2), which cleaves bigET (endothelin precursor) to form ET1-32. (See Figure 3, above.) ET1-32 binds to the ETB receptor and increases nitrous oxide (NO) synthetase activity and NO production, leading to vasodilation.10
The Pathophysiology of Preeclampsia
Based on this observation of normal pregnancy, the following three lines of investigation have furthered our understanding of the potential pathophysiology of pre-eclampsia.
1. Because NO appears to play an important role in the normal vasodilation of pregnancy, abnormalities in this vascular regulatory pathway may be critical to the development of hypertension. Asymmetric dimethylarginine (ADMA) competitively inhibits NO production from arginine by nitric oxide synthetase (NOS). Savvidou and colleagues showed that women with ultrasound evidence of low uterine blood flow were more likely to develop preeclampsia and exhibited higher ADMA levels.11 There was a strong inverse relationship between ADMA levels and flow-mediated vasodilation in women who developed preeclampsia.
Similarly, dimethylarginine dimethylaminohydrolase (DDAH II), an enzyme that metabolizes ADMA, is strongly expressed in placental tissue. In states of placental insufficiency, one might speculate that levels of DDAH II would be reduced, while ADMA levels would increase. Along this same line of investigation, Noris and colleagues have shown increased arginase II activity in placental tissue from preeclamptic individuals and subsequently reduced levels of L-arginine, a substrate for NO production.12
2. A second line of promising research involves the production of a circulating inhibitor of VEGF. The growth factors VEGF and PlGF are produced by the placenta and affect vascular function by binding to two high affinity receptor tyrosine kinases: kinase insert domain-containing region (KDR) and Fms-like tyrosine kinase 1 receptor (Flt-1). Alternative splicing results in the production of an endogenously secreted protein, soluble Fms-like tyrosine kinase 1 receptor (sFlt-1), which lacks the transmembrane and cytoplasmic region of the VEGF receptor and cannot become membrane bound, but that binds VEGF and PlGF. Once VEGF has bound to sFlt-1, normal binding to the membrane-bound receptor is inhibited; thus the effects of VEGF are inhibited.
Studies have found that infusion of sFlt-1 to nonpregnant animals causes glomerular endotheliosis and hypertension similar to those found in preeclampsia; these findings support the possibility that sFlt-1 contributes to preeclampsia. Further, Levine and colleagues have shown that plasma sFlt-1 levels increase more in women with preeclampsia and precede clinical findings compared with individuals with normal pregnancies.13 In addition, PlGF levels were decreased in women with preeclampsia compared with the levels found in those experiencing normal pregnancies.
The supposition from these studies is that increased plasma levels of sFlt-1 competitively inhibit VEGF binding to VEGF receptors on vascular tissue. This inhibition causes a lack of vasodilation and increased blood pressure, with the normal fluid retention and volume expansion of pregnancy. VEGF is difficult to accurately determine in plasma, but PlGF levels, which are affected similarly, are reduced in individuals suffering from or destined to develop preeclampsia. Furthermore, since VEGF is an important determinant of normal placental development, decreased cellular binding due to competitive inhibition could contribute to abnormal placental pseudovasculogenesis.
3. Finally, Vu and colleagues have shown that pregnant animals made hypertensive by a high salt diet and deoxycorticosterone administration exhibit a higher circulating level of the Na, K-ATPase inhibitor, marinobufagenin.14 Further, blood pressure is reduced by the administration of the inhibitor of this cardenolide compound, resibufogenin. Though this is not a model of spontaneous preeclampsia, many features are similar, including reduced placental blood flow in spite of volume expansion and proteinuria.
Treatment Recommendations
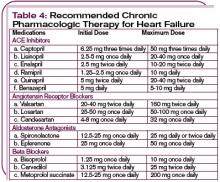
1. In individuals with hypertension prior to pregnancy, the physician may continue the same anti-hypertensive therapy used pre-pregnancy, with or without diuretics—except for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. The goal is to maintain the blood pressure at or below a level of 140/90 mm Hg. The pregnancy should be monitored as a high-risk pregnancy, and urine protein excretion, blood pressure, and fetal health should be monitored frequently, particularly after the 24th week of gestation. Urine protein excretion is most easily monitored using a spot urine protein-to-creatinine ratio. A value of less than 0.3 is equivalent to a 24-hour urine excretion of less than 300 mg.
2. Monitor individuals who develop mild preeclampsia for 24-72 hours to assess for progression. If they remain stable, the decision analysis depends on the stage of pregnancy. After 32 weeks gestation, the physician can elect to continue the pregnancy while prescribing reduced activity or bed rest with or without anti-hypertensive therapy. If anti-hypertensive therapy is elected, optimal choices include the dihydropyridine class of calcium channel antagonists, centrally acting alpha 2 agonists such as clonidine, or beta-blockers.
Though diuretics appear safe, most obstetricians do not advocate their use. Most physicians choose to initiate pharmacologic therapy if preeclampsia occurs prior to 32 weeks, in order to allow further fetal development prior to delivery. Many obstetricians will induce labor if the pregnancy is beyond 36 weeks to avoid the complications of preeclampsia/eclampsia. Delivery usually resolves the syndrome of preeclampsia within a period of time that ranges from hours to days.
3. In patients with severe preeclampsia, the risks are greater. In these circumstances, physicians are more likely to induce delivery if gestation is greater than 32 weeks. However, in pregnancies beyond 36 weeks, a physician may choose to delay delivery in order to allow fetal lung maturation, using steroids while controlling blood pressure and preventing seizures with intravenous Mg2SO4. Other options include the use of intravenous labetalol, hydralazine, or even nitroprusside, while also treating the patient with phenytoin to prevent seizures.
4. The presence of the HELLP syndrome usually necessitates urgent delivery and may have prolonged effects on blood pressure, liver function, and compromised renal function after the pregnancy has ended.
As a better understanding of the pathogenesis of preeclampsia develops in the future, more selective and definitive preventive or interventional therapy is likely. As further investigation moves toward that goal, this serious health problem in an otherwise young and healthy population should be mitigated. TH
Dr. Beach is the Paul R. Stalnaker Distinguished Professor of Internal Medicine, director, Division of Nephrology and Hypertension, and scholar, John McGovern Academy of Oslerian Medicine.
References
- Longo SA, Dola CP, Pridjian G. Preeclampsia and eclampsia revisited. South Med J. 2003 Sep;96(9): 891-899.
- Irgens HU, Reisaeter L, Irgens LM, et al. Long-term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001 Nov 24;323(7323):1213-1217.
- CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619-629.
- Hofmeyer GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Sys Rev. 2000;3:CD001059.
- Collins R, Yusuf S, Peto R. Overview of randomised trials of diuretics in pregnancy. Br Med J (Clin Res Ed). 1985 Jan 5;290 (6461):17-23.
- Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999 May 15;318 (7194):1332-1336.
- Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N Engl J Med. 1995 Jul 27;333 (4):201-205.
- Belfort MA, Anthony J, Saade GR, et al. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003 Jan 23;348 (4):304-311.
- Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005 Jun;48(2):372-386.
- Jeyabalan A, Novak J, Danielson LA, et al. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003 Dec 12;93(12):1249-1257. Epub 2003 Oct 30.
- Savvidou MD, Hingorani AD, Tsikas D, et al. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003 May 3;361(9368):1511-1517.
- Noris M, Todeschini M, Cassis P, et al. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004 Mar;43(3):614-622. Epub 2004 Jan 26.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350 (7):672-683. Epub 2004 Feb 5.
- Vu H, Ianosi-Irimie M, Danchuk S, et al. Resibufogenin corrects hypertension in a rat model of human preeclampsia. Exp Biol Med (Maywood). 2006 Feb;231(2):215-220.
Hypertension during pregnancy, of which preeclampsia and eclampsia predominate, constitutes a significant health problem both because of its high incidence (4%-11% of pregnancies in developed countries) and due to the maternal and fetal health outcomes it creates.1 Hypertensive disorders are the second leading cause of maternal mortality.1 They cause 15% of all maternal deaths and constitute considerable morbidity both during and after pregnancy.2 Fetal outcomes include premature delivery, small-for-gestational-age (SGA) infants, and fetal mortality.
The long-term cardiovascular risks for mothers who suffer from the triad of hypertension during pregnancy, SGA infants, and pre-term delivery are approximately eight times higher than for individuals without these complications during pregnancy.
Hypertension observed during pregnancy is defined according to one of the following classifications:
- Chronic hypertension, present prior to pregnancy;
- Preeclampsia/eclampsia: development of hypertension, proteinuria, and edema during pregnancy;
- Preeclampsia superimposed on preexisting renal disease; and
- Gestational hypertension: either transient mild hypertension during the third trimester or mild hypertension detected during the third trimester that does not resolve by 12 weeks postpartum.
Diagnosing Preeclampsia/Eclampsia
Of these hypertensive entities, this article focuses on the most common and potentially serious one: preeclampsia/eclampsia. Preeclampsia is characterized by blood pressure over 140/90 mm Hg—measured on two separate occasions, and proteinuria greater than 300 mg/24 hours, occurring after 20 weeks gestation. Severe preeclampsia includes the same criteria, as well as proteinuria greater than 5,000 mg/24 hours; blood pressure higher than 160/110 mm Hg; or end-organ damage such as headaches, visual changes, renal dysfunction, hepatic dysfunction, or thrombocytopenia.
Historically, the term eclampsia has been reserved to describe the symptoms of preeclampsia combined with the occurrence of seizure activity. HELLP syndrome is a severe variant of preeclampsia that affects up to 1% of pregnancies and includes the constellation of hemolysis, elevated liver enzyme levels, and a low platelet count.
Given the potential risks for these disorders during pregnancy, it seems logical to target primarily those individuals at risk and attempt to prevent the disorder. Traditionally, the higher risk populations include patients experiencing primagravida pregnancies, those with a history of hypertension or renal disease, women who have had prior episodes of preeclampsia, and multiparous individuals with different paternal partners. Other less helpful or more expensive screening procedures include evaluation for inherited thrombophilias.
Recommended Treatment
Several trials have attempted to prevent the development of preeclampsia using calcium supplementation or aspirin therapy, but current evidence demonstrates no benefit from these interventions except in selected populations.3,4 For the past several decades, the treatment for hypertension during pregnancy has consisted of either delivery of the fetus or bed rest combined with therapies involving alpha-methyl-DOPA, hydralazine, and intravenous magnesium sulfate, depending on the level of the patient’s blood pressure and proteinuria, as well as the stage of the pregnancy.
Because of the potential risks to the health of the infant (as well as that of the mother) generic interventional trials extrapolated from other hypertensive populations that may offer equivalent or more effective therapies have not been readily accomplished. In spite of this lack of clinical and outcomes studies, the following generalizations regarding care are currently advocated:
1. Continue anti-hypertensive therapy for chronic hypertension with a goal of maintaining blood pressure below 140/90 mm Hg. Though the risks of SGA infants and preeclampsia were not affected by therapy, the incidence of premature delivery was reduced. Nearly all anti-hypertensive drugs have been used in treating chronic hypertension in pregnancy, but angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are contraindicated because of teratogenecity. Diuretics and hydralazine may also have adverse outcomes on fetal and/or maternal health, respectively.
Though diuretics may reduce an already compromised placental blood flow by reducing intravascular volume, a number of randomized trials using diuretics have been reported. The population studied in these trials totaled approximately 7,000 individuals, and the results show no demonstrable adverse effects.5 Centrally acting alpha 2 adrenergic agonists (alpha-methyl-DOPA, clonidine), long-acting dihydropyridines (calcium channel blockers), and beta-adrenergic blockers with or without alpha-adrenergic blockade have been used successfully during pregnancy.6 Infants born to mothers receiving beta-blockers have a higher incidence of transient heart block and bradycardia. Newer agents, as opposed to alpha-methyl-DOPA and hydralazine, have not been shown to be more effective in controlling blood pressure but have demonstrated fewer adverse effects or events.
2. Mild preeclampsia may be treated using late-term delivery or bed rest before the pregnancy reaches 36 weeks. Close observation in a hospital is warranted until lack of progression to severe eclampsia is ensured.
3. Severe preeclampsia must be treated using either delivery or interventions to control blood pressure and prevent seizures while delivery is temporarily delayed to allow for fetal maturation. Intravenous magnesium sulfate (Mg2SO4), titrated to therapeutic concentrations of magnesium (4.5-8.5 mg/dl) along with suppression of deep tendon reflexes, has significantly lowered blood pressure and reduced the incidence of seizure activity. In direct comparison with intravenous phenytoin, there were no episodes of eclampsia in patients treated with magnesium (zero of 1,049 patients treated), while 10 of 1,089 individuals treated with phenytoin developed eclampsia.7 Similarly, Mg2SO4 decreased the incidence of eclampsia to 0.8% compared with 2.6% of individuals treated with nimodipine.8 Likewise, Mg2SO4 significantly reduced recurrent seizures compared to diazepam and phenytoin. In patients with renal insufficiency or other relative contraindications to Mg2SO4 therapy, the physician may treat the blood pressure with labetalol or a calcium channel antagonist and provide prophylaxis from seizures using phenytoin.
Because of the lack of a more complete understanding of the etiology (or etiologies) of preeclampsia/eclampsia, we are not fully able to identify individuals who are at risk. With further investigation, we will be able to provide more selective and effective interventions.
Nitrous Oxide’s Vital Role in Pregnancy
In this regard, considerable investigation has been directed toward a better understanding of eclampsia/preeclampsia in the past 20 years. The current discussion will highlight three promising lines of inquiry. Before commenting on these three areas of investigation, we feel it is important to illustrate the fundamental defect observed in all models of preeclampsia. This unifying abnormality is the presence of a placenta with inadequate uterine blood flow.
In a normal pregnancy, after implantation in the uterine wall, cytotrophoblasts invade the uterine wall, undergo a transformation of cell adhesion molecules from epithelial to endothelial expression characteristics, and induce the deep invasion of the placenta by the spiral arteries, creating large vascular sinuses in the decidua (pseudovasculogenesis). Finally, beta human chorionic gonadotropin production by the placenta stimulates the release of relaxin from the corpus luteum. Many cytokines and growth factors contribute to this normal placental development, but vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) appear to be the most important.
VEGF and PlGF play an integral role in the inducement of conversion of the cytotrophoblast cell adhesion molecules from epithelial to endothelial expression, particularly integrins and cadherins. In models of preeclampsia, there is insufficient invasion of the spiral arteries and inadequate uterine blood flow, as well as a lack of alteration in adhesion molecules of cytotrophoblasts and failed pseudovasculogenesis.9
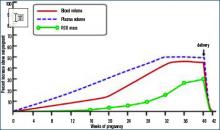
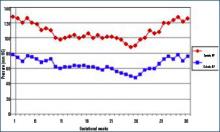
During normal pregnancy, intravascular volume increases, peripheral vascular resistance (PVR) decreases, and renal blood flow and glomerular filtration rate (GFR) increase. (See Figure 1, above left.) Concomitant with the increased plasma volume, the reduction of PVR (vasodilation) not only mitigates the effect of plasma volume on systemic blood pressure, but actually reduces the systemic blood pressure during normal pregnancy. (See Figure 2, below.) Based on seminal studies by Jeyabalan and colleagues, one explanation for the decline in PVR and blood pressure, along with the increased renal plasma flow and GFR, is the production and action of relaxin on vascular smooth muscle to stimulate gelatinase-A (matrix metalloproteinase-2), which cleaves bigET (endothelin precursor) to form ET1-32. (See Figure 3, above.) ET1-32 binds to the ETB receptor and increases nitrous oxide (NO) synthetase activity and NO production, leading to vasodilation.10
The Pathophysiology of Preeclampsia
Based on this observation of normal pregnancy, the following three lines of investigation have furthered our understanding of the potential pathophysiology of pre-eclampsia.
1. Because NO appears to play an important role in the normal vasodilation of pregnancy, abnormalities in this vascular regulatory pathway may be critical to the development of hypertension. Asymmetric dimethylarginine (ADMA) competitively inhibits NO production from arginine by nitric oxide synthetase (NOS). Savvidou and colleagues showed that women with ultrasound evidence of low uterine blood flow were more likely to develop preeclampsia and exhibited higher ADMA levels.11 There was a strong inverse relationship between ADMA levels and flow-mediated vasodilation in women who developed preeclampsia.
Similarly, dimethylarginine dimethylaminohydrolase (DDAH II), an enzyme that metabolizes ADMA, is strongly expressed in placental tissue. In states of placental insufficiency, one might speculate that levels of DDAH II would be reduced, while ADMA levels would increase. Along this same line of investigation, Noris and colleagues have shown increased arginase II activity in placental tissue from preeclamptic individuals and subsequently reduced levels of L-arginine, a substrate for NO production.12
2. A second line of promising research involves the production of a circulating inhibitor of VEGF. The growth factors VEGF and PlGF are produced by the placenta and affect vascular function by binding to two high affinity receptor tyrosine kinases: kinase insert domain-containing region (KDR) and Fms-like tyrosine kinase 1 receptor (Flt-1). Alternative splicing results in the production of an endogenously secreted protein, soluble Fms-like tyrosine kinase 1 receptor (sFlt-1), which lacks the transmembrane and cytoplasmic region of the VEGF receptor and cannot become membrane bound, but that binds VEGF and PlGF. Once VEGF has bound to sFlt-1, normal binding to the membrane-bound receptor is inhibited; thus the effects of VEGF are inhibited.
Studies have found that infusion of sFlt-1 to nonpregnant animals causes glomerular endotheliosis and hypertension similar to those found in preeclampsia; these findings support the possibility that sFlt-1 contributes to preeclampsia. Further, Levine and colleagues have shown that plasma sFlt-1 levels increase more in women with preeclampsia and precede clinical findings compared with individuals with normal pregnancies.13 In addition, PlGF levels were decreased in women with preeclampsia compared with the levels found in those experiencing normal pregnancies.
The supposition from these studies is that increased plasma levels of sFlt-1 competitively inhibit VEGF binding to VEGF receptors on vascular tissue. This inhibition causes a lack of vasodilation and increased blood pressure, with the normal fluid retention and volume expansion of pregnancy. VEGF is difficult to accurately determine in plasma, but PlGF levels, which are affected similarly, are reduced in individuals suffering from or destined to develop preeclampsia. Furthermore, since VEGF is an important determinant of normal placental development, decreased cellular binding due to competitive inhibition could contribute to abnormal placental pseudovasculogenesis.
3. Finally, Vu and colleagues have shown that pregnant animals made hypertensive by a high salt diet and deoxycorticosterone administration exhibit a higher circulating level of the Na, K-ATPase inhibitor, marinobufagenin.14 Further, blood pressure is reduced by the administration of the inhibitor of this cardenolide compound, resibufogenin. Though this is not a model of spontaneous preeclampsia, many features are similar, including reduced placental blood flow in spite of volume expansion and proteinuria.
Treatment Recommendations
1. In individuals with hypertension prior to pregnancy, the physician may continue the same anti-hypertensive therapy used pre-pregnancy, with or without diuretics—except for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. The goal is to maintain the blood pressure at or below a level of 140/90 mm Hg. The pregnancy should be monitored as a high-risk pregnancy, and urine protein excretion, blood pressure, and fetal health should be monitored frequently, particularly after the 24th week of gestation. Urine protein excretion is most easily monitored using a spot urine protein-to-creatinine ratio. A value of less than 0.3 is equivalent to a 24-hour urine excretion of less than 300 mg.
2. Monitor individuals who develop mild preeclampsia for 24-72 hours to assess for progression. If they remain stable, the decision analysis depends on the stage of pregnancy. After 32 weeks gestation, the physician can elect to continue the pregnancy while prescribing reduced activity or bed rest with or without anti-hypertensive therapy. If anti-hypertensive therapy is elected, optimal choices include the dihydropyridine class of calcium channel antagonists, centrally acting alpha 2 agonists such as clonidine, or beta-blockers.
Though diuretics appear safe, most obstetricians do not advocate their use. Most physicians choose to initiate pharmacologic therapy if preeclampsia occurs prior to 32 weeks, in order to allow further fetal development prior to delivery. Many obstetricians will induce labor if the pregnancy is beyond 36 weeks to avoid the complications of preeclampsia/eclampsia. Delivery usually resolves the syndrome of preeclampsia within a period of time that ranges from hours to days.
3. In patients with severe preeclampsia, the risks are greater. In these circumstances, physicians are more likely to induce delivery if gestation is greater than 32 weeks. However, in pregnancies beyond 36 weeks, a physician may choose to delay delivery in order to allow fetal lung maturation, using steroids while controlling blood pressure and preventing seizures with intravenous Mg2SO4. Other options include the use of intravenous labetalol, hydralazine, or even nitroprusside, while also treating the patient with phenytoin to prevent seizures.
4. The presence of the HELLP syndrome usually necessitates urgent delivery and may have prolonged effects on blood pressure, liver function, and compromised renal function after the pregnancy has ended.
As a better understanding of the pathogenesis of preeclampsia develops in the future, more selective and definitive preventive or interventional therapy is likely. As further investigation moves toward that goal, this serious health problem in an otherwise young and healthy population should be mitigated. TH
Dr. Beach is the Paul R. Stalnaker Distinguished Professor of Internal Medicine, director, Division of Nephrology and Hypertension, and scholar, John McGovern Academy of Oslerian Medicine.
References
- Longo SA, Dola CP, Pridjian G. Preeclampsia and eclampsia revisited. South Med J. 2003 Sep;96(9): 891-899.
- Irgens HU, Reisaeter L, Irgens LM, et al. Long-term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001 Nov 24;323(7323):1213-1217.
- CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619-629.
- Hofmeyer GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Sys Rev. 2000;3:CD001059.
- Collins R, Yusuf S, Peto R. Overview of randomised trials of diuretics in pregnancy. Br Med J (Clin Res Ed). 1985 Jan 5;290 (6461):17-23.
- Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999 May 15;318 (7194):1332-1336.
- Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N Engl J Med. 1995 Jul 27;333 (4):201-205.
- Belfort MA, Anthony J, Saade GR, et al. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003 Jan 23;348 (4):304-311.
- Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005 Jun;48(2):372-386.
- Jeyabalan A, Novak J, Danielson LA, et al. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003 Dec 12;93(12):1249-1257. Epub 2003 Oct 30.
- Savvidou MD, Hingorani AD, Tsikas D, et al. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003 May 3;361(9368):1511-1517.
- Noris M, Todeschini M, Cassis P, et al. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004 Mar;43(3):614-622. Epub 2004 Jan 26.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350 (7):672-683. Epub 2004 Feb 5.
- Vu H, Ianosi-Irimie M, Danchuk S, et al. Resibufogenin corrects hypertension in a rat model of human preeclampsia. Exp Biol Med (Maywood). 2006 Feb;231(2):215-220.
Hypertension during pregnancy, of which preeclampsia and eclampsia predominate, constitutes a significant health problem both because of its high incidence (4%-11% of pregnancies in developed countries) and due to the maternal and fetal health outcomes it creates.1 Hypertensive disorders are the second leading cause of maternal mortality.1 They cause 15% of all maternal deaths and constitute considerable morbidity both during and after pregnancy.2 Fetal outcomes include premature delivery, small-for-gestational-age (SGA) infants, and fetal mortality.
The long-term cardiovascular risks for mothers who suffer from the triad of hypertension during pregnancy, SGA infants, and pre-term delivery are approximately eight times higher than for individuals without these complications during pregnancy.
Hypertension observed during pregnancy is defined according to one of the following classifications:
- Chronic hypertension, present prior to pregnancy;
- Preeclampsia/eclampsia: development of hypertension, proteinuria, and edema during pregnancy;
- Preeclampsia superimposed on preexisting renal disease; and
- Gestational hypertension: either transient mild hypertension during the third trimester or mild hypertension detected during the third trimester that does not resolve by 12 weeks postpartum.
Diagnosing Preeclampsia/Eclampsia
Of these hypertensive entities, this article focuses on the most common and potentially serious one: preeclampsia/eclampsia. Preeclampsia is characterized by blood pressure over 140/90 mm Hg—measured on two separate occasions, and proteinuria greater than 300 mg/24 hours, occurring after 20 weeks gestation. Severe preeclampsia includes the same criteria, as well as proteinuria greater than 5,000 mg/24 hours; blood pressure higher than 160/110 mm Hg; or end-organ damage such as headaches, visual changes, renal dysfunction, hepatic dysfunction, or thrombocytopenia.
Historically, the term eclampsia has been reserved to describe the symptoms of preeclampsia combined with the occurrence of seizure activity. HELLP syndrome is a severe variant of preeclampsia that affects up to 1% of pregnancies and includes the constellation of hemolysis, elevated liver enzyme levels, and a low platelet count.
Given the potential risks for these disorders during pregnancy, it seems logical to target primarily those individuals at risk and attempt to prevent the disorder. Traditionally, the higher risk populations include patients experiencing primagravida pregnancies, those with a history of hypertension or renal disease, women who have had prior episodes of preeclampsia, and multiparous individuals with different paternal partners. Other less helpful or more expensive screening procedures include evaluation for inherited thrombophilias.
Recommended Treatment
Several trials have attempted to prevent the development of preeclampsia using calcium supplementation or aspirin therapy, but current evidence demonstrates no benefit from these interventions except in selected populations.3,4 For the past several decades, the treatment for hypertension during pregnancy has consisted of either delivery of the fetus or bed rest combined with therapies involving alpha-methyl-DOPA, hydralazine, and intravenous magnesium sulfate, depending on the level of the patient’s blood pressure and proteinuria, as well as the stage of the pregnancy.
Because of the potential risks to the health of the infant (as well as that of the mother) generic interventional trials extrapolated from other hypertensive populations that may offer equivalent or more effective therapies have not been readily accomplished. In spite of this lack of clinical and outcomes studies, the following generalizations regarding care are currently advocated:
1. Continue anti-hypertensive therapy for chronic hypertension with a goal of maintaining blood pressure below 140/90 mm Hg. Though the risks of SGA infants and preeclampsia were not affected by therapy, the incidence of premature delivery was reduced. Nearly all anti-hypertensive drugs have been used in treating chronic hypertension in pregnancy, but angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are contraindicated because of teratogenecity. Diuretics and hydralazine may also have adverse outcomes on fetal and/or maternal health, respectively.
Though diuretics may reduce an already compromised placental blood flow by reducing intravascular volume, a number of randomized trials using diuretics have been reported. The population studied in these trials totaled approximately 7,000 individuals, and the results show no demonstrable adverse effects.5 Centrally acting alpha 2 adrenergic agonists (alpha-methyl-DOPA, clonidine), long-acting dihydropyridines (calcium channel blockers), and beta-adrenergic blockers with or without alpha-adrenergic blockade have been used successfully during pregnancy.6 Infants born to mothers receiving beta-blockers have a higher incidence of transient heart block and bradycardia. Newer agents, as opposed to alpha-methyl-DOPA and hydralazine, have not been shown to be more effective in controlling blood pressure but have demonstrated fewer adverse effects or events.
2. Mild preeclampsia may be treated using late-term delivery or bed rest before the pregnancy reaches 36 weeks. Close observation in a hospital is warranted until lack of progression to severe eclampsia is ensured.
3. Severe preeclampsia must be treated using either delivery or interventions to control blood pressure and prevent seizures while delivery is temporarily delayed to allow for fetal maturation. Intravenous magnesium sulfate (Mg2SO4), titrated to therapeutic concentrations of magnesium (4.5-8.5 mg/dl) along with suppression of deep tendon reflexes, has significantly lowered blood pressure and reduced the incidence of seizure activity. In direct comparison with intravenous phenytoin, there were no episodes of eclampsia in patients treated with magnesium (zero of 1,049 patients treated), while 10 of 1,089 individuals treated with phenytoin developed eclampsia.7 Similarly, Mg2SO4 decreased the incidence of eclampsia to 0.8% compared with 2.6% of individuals treated with nimodipine.8 Likewise, Mg2SO4 significantly reduced recurrent seizures compared to diazepam and phenytoin. In patients with renal insufficiency or other relative contraindications to Mg2SO4 therapy, the physician may treat the blood pressure with labetalol or a calcium channel antagonist and provide prophylaxis from seizures using phenytoin.
Because of the lack of a more complete understanding of the etiology (or etiologies) of preeclampsia/eclampsia, we are not fully able to identify individuals who are at risk. With further investigation, we will be able to provide more selective and effective interventions.
Nitrous Oxide’s Vital Role in Pregnancy
In this regard, considerable investigation has been directed toward a better understanding of eclampsia/preeclampsia in the past 20 years. The current discussion will highlight three promising lines of inquiry. Before commenting on these three areas of investigation, we feel it is important to illustrate the fundamental defect observed in all models of preeclampsia. This unifying abnormality is the presence of a placenta with inadequate uterine blood flow.
In a normal pregnancy, after implantation in the uterine wall, cytotrophoblasts invade the uterine wall, undergo a transformation of cell adhesion molecules from epithelial to endothelial expression characteristics, and induce the deep invasion of the placenta by the spiral arteries, creating large vascular sinuses in the decidua (pseudovasculogenesis). Finally, beta human chorionic gonadotropin production by the placenta stimulates the release of relaxin from the corpus luteum. Many cytokines and growth factors contribute to this normal placental development, but vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) appear to be the most important.
VEGF and PlGF play an integral role in the inducement of conversion of the cytotrophoblast cell adhesion molecules from epithelial to endothelial expression, particularly integrins and cadherins. In models of preeclampsia, there is insufficient invasion of the spiral arteries and inadequate uterine blood flow, as well as a lack of alteration in adhesion molecules of cytotrophoblasts and failed pseudovasculogenesis.9
During normal pregnancy, intravascular volume increases, peripheral vascular resistance (PVR) decreases, and renal blood flow and glomerular filtration rate (GFR) increase. (See Figure 1, above left.) Concomitant with the increased plasma volume, the reduction of PVR (vasodilation) not only mitigates the effect of plasma volume on systemic blood pressure, but actually reduces the systemic blood pressure during normal pregnancy. (See Figure 2, below.) Based on seminal studies by Jeyabalan and colleagues, one explanation for the decline in PVR and blood pressure, along with the increased renal plasma flow and GFR, is the production and action of relaxin on vascular smooth muscle to stimulate gelatinase-A (matrix metalloproteinase-2), which cleaves bigET (endothelin precursor) to form ET1-32. (See Figure 3, above.) ET1-32 binds to the ETB receptor and increases nitrous oxide (NO) synthetase activity and NO production, leading to vasodilation.10
The Pathophysiology of Preeclampsia
Based on this observation of normal pregnancy, the following three lines of investigation have furthered our understanding of the potential pathophysiology of pre-eclampsia.
1. Because NO appears to play an important role in the normal vasodilation of pregnancy, abnormalities in this vascular regulatory pathway may be critical to the development of hypertension. Asymmetric dimethylarginine (ADMA) competitively inhibits NO production from arginine by nitric oxide synthetase (NOS). Savvidou and colleagues showed that women with ultrasound evidence of low uterine blood flow were more likely to develop preeclampsia and exhibited higher ADMA levels.11 There was a strong inverse relationship between ADMA levels and flow-mediated vasodilation in women who developed preeclampsia.
Similarly, dimethylarginine dimethylaminohydrolase (DDAH II), an enzyme that metabolizes ADMA, is strongly expressed in placental tissue. In states of placental insufficiency, one might speculate that levels of DDAH II would be reduced, while ADMA levels would increase. Along this same line of investigation, Noris and colleagues have shown increased arginase II activity in placental tissue from preeclamptic individuals and subsequently reduced levels of L-arginine, a substrate for NO production.12
2. A second line of promising research involves the production of a circulating inhibitor of VEGF. The growth factors VEGF and PlGF are produced by the placenta and affect vascular function by binding to two high affinity receptor tyrosine kinases: kinase insert domain-containing region (KDR) and Fms-like tyrosine kinase 1 receptor (Flt-1). Alternative splicing results in the production of an endogenously secreted protein, soluble Fms-like tyrosine kinase 1 receptor (sFlt-1), which lacks the transmembrane and cytoplasmic region of the VEGF receptor and cannot become membrane bound, but that binds VEGF and PlGF. Once VEGF has bound to sFlt-1, normal binding to the membrane-bound receptor is inhibited; thus the effects of VEGF are inhibited.
Studies have found that infusion of sFlt-1 to nonpregnant animals causes glomerular endotheliosis and hypertension similar to those found in preeclampsia; these findings support the possibility that sFlt-1 contributes to preeclampsia. Further, Levine and colleagues have shown that plasma sFlt-1 levels increase more in women with preeclampsia and precede clinical findings compared with individuals with normal pregnancies.13 In addition, PlGF levels were decreased in women with preeclampsia compared with the levels found in those experiencing normal pregnancies.
The supposition from these studies is that increased plasma levels of sFlt-1 competitively inhibit VEGF binding to VEGF receptors on vascular tissue. This inhibition causes a lack of vasodilation and increased blood pressure, with the normal fluid retention and volume expansion of pregnancy. VEGF is difficult to accurately determine in plasma, but PlGF levels, which are affected similarly, are reduced in individuals suffering from or destined to develop preeclampsia. Furthermore, since VEGF is an important determinant of normal placental development, decreased cellular binding due to competitive inhibition could contribute to abnormal placental pseudovasculogenesis.
3. Finally, Vu and colleagues have shown that pregnant animals made hypertensive by a high salt diet and deoxycorticosterone administration exhibit a higher circulating level of the Na, K-ATPase inhibitor, marinobufagenin.14 Further, blood pressure is reduced by the administration of the inhibitor of this cardenolide compound, resibufogenin. Though this is not a model of spontaneous preeclampsia, many features are similar, including reduced placental blood flow in spite of volume expansion and proteinuria.
Treatment Recommendations
1. In individuals with hypertension prior to pregnancy, the physician may continue the same anti-hypertensive therapy used pre-pregnancy, with or without diuretics—except for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. The goal is to maintain the blood pressure at or below a level of 140/90 mm Hg. The pregnancy should be monitored as a high-risk pregnancy, and urine protein excretion, blood pressure, and fetal health should be monitored frequently, particularly after the 24th week of gestation. Urine protein excretion is most easily monitored using a spot urine protein-to-creatinine ratio. A value of less than 0.3 is equivalent to a 24-hour urine excretion of less than 300 mg.
2. Monitor individuals who develop mild preeclampsia for 24-72 hours to assess for progression. If they remain stable, the decision analysis depends on the stage of pregnancy. After 32 weeks gestation, the physician can elect to continue the pregnancy while prescribing reduced activity or bed rest with or without anti-hypertensive therapy. If anti-hypertensive therapy is elected, optimal choices include the dihydropyridine class of calcium channel antagonists, centrally acting alpha 2 agonists such as clonidine, or beta-blockers.
Though diuretics appear safe, most obstetricians do not advocate their use. Most physicians choose to initiate pharmacologic therapy if preeclampsia occurs prior to 32 weeks, in order to allow further fetal development prior to delivery. Many obstetricians will induce labor if the pregnancy is beyond 36 weeks to avoid the complications of preeclampsia/eclampsia. Delivery usually resolves the syndrome of preeclampsia within a period of time that ranges from hours to days.
3. In patients with severe preeclampsia, the risks are greater. In these circumstances, physicians are more likely to induce delivery if gestation is greater than 32 weeks. However, in pregnancies beyond 36 weeks, a physician may choose to delay delivery in order to allow fetal lung maturation, using steroids while controlling blood pressure and preventing seizures with intravenous Mg2SO4. Other options include the use of intravenous labetalol, hydralazine, or even nitroprusside, while also treating the patient with phenytoin to prevent seizures.
4. The presence of the HELLP syndrome usually necessitates urgent delivery and may have prolonged effects on blood pressure, liver function, and compromised renal function after the pregnancy has ended.
As a better understanding of the pathogenesis of preeclampsia develops in the future, more selective and definitive preventive or interventional therapy is likely. As further investigation moves toward that goal, this serious health problem in an otherwise young and healthy population should be mitigated. TH
Dr. Beach is the Paul R. Stalnaker Distinguished Professor of Internal Medicine, director, Division of Nephrology and Hypertension, and scholar, John McGovern Academy of Oslerian Medicine.
References
- Longo SA, Dola CP, Pridjian G. Preeclampsia and eclampsia revisited. South Med J. 2003 Sep;96(9): 891-899.
- Irgens HU, Reisaeter L, Irgens LM, et al. Long-term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001 Nov 24;323(7323):1213-1217.
- CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619-629.
- Hofmeyer GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Sys Rev. 2000;3:CD001059.
- Collins R, Yusuf S, Peto R. Overview of randomised trials of diuretics in pregnancy. Br Med J (Clin Res Ed). 1985 Jan 5;290 (6461):17-23.
- Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999 May 15;318 (7194):1332-1336.
- Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N Engl J Med. 1995 Jul 27;333 (4):201-205.
- Belfort MA, Anthony J, Saade GR, et al. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003 Jan 23;348 (4):304-311.
- Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005 Jun;48(2):372-386.
- Jeyabalan A, Novak J, Danielson LA, et al. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003 Dec 12;93(12):1249-1257. Epub 2003 Oct 30.
- Savvidou MD, Hingorani AD, Tsikas D, et al. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003 May 3;361(9368):1511-1517.
- Noris M, Todeschini M, Cassis P, et al. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004 Mar;43(3):614-622. Epub 2004 Jan 26.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350 (7):672-683. Epub 2004 Feb 5.
- Vu H, Ianosi-Irimie M, Danchuk S, et al. Resibufogenin corrects hypertension in a rat model of human preeclampsia. Exp Biol Med (Maywood). 2006 Feb;231(2):215-220.
Diabetic Dilemma
Diabetic foot infections are common, costly, and potentially catastrophic for patients. The average patient with a diabetic foot infection undergoes three surgical procedures, including toe amputation in 19% and leg amputation in 14%.1 The annual cost of diabetic foot osteomyelitis in the United States is $2.8 billion.2
Diabetics are uniquely predisposed to foot infections. Because of neuropathy, minor repetitive injury leads to large foot ulcers, especially over the metatarsal heads. Foot deformities result in soft tissue injury from poorly fitting shoes. Because of autonomic neuropathy, the diabetic foot sweats less, leading to dry, cracked skin for bacteria to invade. Resistance to infection is lower because of neutrophil dysfunction in hyperglycemia. Vascular insufficiency impairs both wound healing and the immune response.3
The major principles of therapy are as follows:
1. Use an empiric antibiotic regimen with broad coverage against gram-positive, gram-negative, and anaerobic organisms. Diabetic foot infections are usually polymicrobial. Except for mild infections, in which therapy directed at gram-positive organisms may suffice, initial therapy should cover streptococci, methicillin-sensitive Staphylococcus aureus, E. coli, Proteus, and anaerobes.
Many studies of antibiotic therapy in diabetic foot infections have been performed, without demonstrating a clear superiority for any one regimen. The 2004 guidelines of the Infectious Diseases Society of America list 13 acceptable regimens for moderate diabetic foot infections.4 Useful single drug regimens include ampicillin-sulbactam, piperacillin-tazobactam, levofloxacin, and cefoxitin. For the penicillin-allergic patient, clindamycin and ciprofloxacin is a useful combination. Empiric vancomycin should be reserved for patients with a history of MRSA, treatment failure, or severe infection. (I would also reserve carbapenems, such as imipenem-cilastatin, for more severe infections, to prevent antibiotic resistance against a class of drugs representing our last defense against highly resistant gram-negative organisms.)
Once the patient is clinically improved and results of adequate cultures are available, consideration can be given to narrowing the course of therapy. Most diabetic foot infections can be treated with some combination of intravenous and oral therapy.
2. Have a high clinical suspicion for osteomyelitis. Osteomyelitis is extremely common in diabetic foot infections due to plantar ulceration and poor soft tissue coverage. Cure rates are reduced in osteomyelitis because dead bone acts as a nidus for persistent infection. The most useful diagnostic maneuver for osteomyelitis is deep probing of the wound with a sterile swab at the bedside. If a gritty sensation is felt, osteomyelitis is likely, and further diagnostic testing is probably unnecessary.
If the physical examination is equivocal, plain radiographs should be obtained to look for bony erosions. If these are negative, MRI is the next most useful diagnostic step. Bone scans are of limited value due to their low specificity. (Charcot arthropathy is a common cause of false-positive bone scans in this setting.)
Because of the high prevalence of osteomyelitis in diabetic foot infections, my own practice is to err on the side of longer, rather than shorter, antibiotic therapy.
3. Surgical debridement is required for many diabetic foot infections. Develop a collaborative relationship with a vascular or orthopedic surgeon with interest and expertise in the management of diabetic foot infections. Necrotic and gangrenous material should be removed. Ideally, dead bone should be debrided, both therapeutically and to help establish a bacteriologic diagnosis. (When removal of dead bone would result in loss of function or might create a non-healing wound, the option of long-term antibiotic suppression could be explored with an infectious disease specialist.)
4. When osteomyelitis is present, bone cultures help to define the optimal antibiotic therapy. Recent studies have confirmed older data regarding the poor correlation between surface cultures and bone cultures. The latter are preferred, when feasible.
5. Consider revascularization. Most diabetic foot infections arise in the setting of vascular insufficiency. At a minimum, patients with diabetic foot infections should have ankle-brachial indices performed for screening. Because diabetics may have falsely elevated ankle pressures due to calcified and non-compressible arteries, additional diagnostic studies may be useful, such as segmental pressures and Doppler pulse volume recordings.
Measurement of the transcutaneous oxygen concentration (TcP02) has been recommended, particularly in assessing which patients may benefit from hyperbaric oxygen. However, the TcP02 is not widely available, and the benefit of hyperbaric oxygen in this setting remains controversial.
6. Patients should be educated in meticulous foot care to prevent recurrences and reinfections. A diabetic foot infection may indicate that the patient lacks the knowledge, resources, or motivation for proper foot care. It also suggests that something is seriously awry with the patient’s diabetic regimen, compliance, or both. Hospital admissions for diabetic foot infections provide an opportunity to revise the patient’s diabetic medications; to educate the patient regarding wound care, skin care, and daily foot self-examination; to provide additional resources such as visiting nurses; and to refer patients for podiatric care, including tailored shoes, orthotics, and, if necessary, casting to off-load ulcers. TH
Dr. Ross is an instructor in medicine at Harvard Medical School.
References
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999 Apr;177(4):282-286.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the United States. Diabetes Care. 2003;26(6):1790-1795.
- Lazzarini L, Mader JT, Calhoun JH. Diabetic foot infection. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. New York, NY. Marcel Dekker. 2003.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004 Oct;39(7):885-910.
Diabetic foot infections are common, costly, and potentially catastrophic for patients. The average patient with a diabetic foot infection undergoes three surgical procedures, including toe amputation in 19% and leg amputation in 14%.1 The annual cost of diabetic foot osteomyelitis in the United States is $2.8 billion.2
Diabetics are uniquely predisposed to foot infections. Because of neuropathy, minor repetitive injury leads to large foot ulcers, especially over the metatarsal heads. Foot deformities result in soft tissue injury from poorly fitting shoes. Because of autonomic neuropathy, the diabetic foot sweats less, leading to dry, cracked skin for bacteria to invade. Resistance to infection is lower because of neutrophil dysfunction in hyperglycemia. Vascular insufficiency impairs both wound healing and the immune response.3
The major principles of therapy are as follows:
1. Use an empiric antibiotic regimen with broad coverage against gram-positive, gram-negative, and anaerobic organisms. Diabetic foot infections are usually polymicrobial. Except for mild infections, in which therapy directed at gram-positive organisms may suffice, initial therapy should cover streptococci, methicillin-sensitive Staphylococcus aureus, E. coli, Proteus, and anaerobes.
Many studies of antibiotic therapy in diabetic foot infections have been performed, without demonstrating a clear superiority for any one regimen. The 2004 guidelines of the Infectious Diseases Society of America list 13 acceptable regimens for moderate diabetic foot infections.4 Useful single drug regimens include ampicillin-sulbactam, piperacillin-tazobactam, levofloxacin, and cefoxitin. For the penicillin-allergic patient, clindamycin and ciprofloxacin is a useful combination. Empiric vancomycin should be reserved for patients with a history of MRSA, treatment failure, or severe infection. (I would also reserve carbapenems, such as imipenem-cilastatin, for more severe infections, to prevent antibiotic resistance against a class of drugs representing our last defense against highly resistant gram-negative organisms.)
Once the patient is clinically improved and results of adequate cultures are available, consideration can be given to narrowing the course of therapy. Most diabetic foot infections can be treated with some combination of intravenous and oral therapy.
2. Have a high clinical suspicion for osteomyelitis. Osteomyelitis is extremely common in diabetic foot infections due to plantar ulceration and poor soft tissue coverage. Cure rates are reduced in osteomyelitis because dead bone acts as a nidus for persistent infection. The most useful diagnostic maneuver for osteomyelitis is deep probing of the wound with a sterile swab at the bedside. If a gritty sensation is felt, osteomyelitis is likely, and further diagnostic testing is probably unnecessary.
If the physical examination is equivocal, plain radiographs should be obtained to look for bony erosions. If these are negative, MRI is the next most useful diagnostic step. Bone scans are of limited value due to their low specificity. (Charcot arthropathy is a common cause of false-positive bone scans in this setting.)
Because of the high prevalence of osteomyelitis in diabetic foot infections, my own practice is to err on the side of longer, rather than shorter, antibiotic therapy.
3. Surgical debridement is required for many diabetic foot infections. Develop a collaborative relationship with a vascular or orthopedic surgeon with interest and expertise in the management of diabetic foot infections. Necrotic and gangrenous material should be removed. Ideally, dead bone should be debrided, both therapeutically and to help establish a bacteriologic diagnosis. (When removal of dead bone would result in loss of function or might create a non-healing wound, the option of long-term antibiotic suppression could be explored with an infectious disease specialist.)
4. When osteomyelitis is present, bone cultures help to define the optimal antibiotic therapy. Recent studies have confirmed older data regarding the poor correlation between surface cultures and bone cultures. The latter are preferred, when feasible.
5. Consider revascularization. Most diabetic foot infections arise in the setting of vascular insufficiency. At a minimum, patients with diabetic foot infections should have ankle-brachial indices performed for screening. Because diabetics may have falsely elevated ankle pressures due to calcified and non-compressible arteries, additional diagnostic studies may be useful, such as segmental pressures and Doppler pulse volume recordings.
Measurement of the transcutaneous oxygen concentration (TcP02) has been recommended, particularly in assessing which patients may benefit from hyperbaric oxygen. However, the TcP02 is not widely available, and the benefit of hyperbaric oxygen in this setting remains controversial.
6. Patients should be educated in meticulous foot care to prevent recurrences and reinfections. A diabetic foot infection may indicate that the patient lacks the knowledge, resources, or motivation for proper foot care. It also suggests that something is seriously awry with the patient’s diabetic regimen, compliance, or both. Hospital admissions for diabetic foot infections provide an opportunity to revise the patient’s diabetic medications; to educate the patient regarding wound care, skin care, and daily foot self-examination; to provide additional resources such as visiting nurses; and to refer patients for podiatric care, including tailored shoes, orthotics, and, if necessary, casting to off-load ulcers. TH
Dr. Ross is an instructor in medicine at Harvard Medical School.
References
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999 Apr;177(4):282-286.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the United States. Diabetes Care. 2003;26(6):1790-1795.
- Lazzarini L, Mader JT, Calhoun JH. Diabetic foot infection. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. New York, NY. Marcel Dekker. 2003.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004 Oct;39(7):885-910.
Diabetic foot infections are common, costly, and potentially catastrophic for patients. The average patient with a diabetic foot infection undergoes three surgical procedures, including toe amputation in 19% and leg amputation in 14%.1 The annual cost of diabetic foot osteomyelitis in the United States is $2.8 billion.2
Diabetics are uniquely predisposed to foot infections. Because of neuropathy, minor repetitive injury leads to large foot ulcers, especially over the metatarsal heads. Foot deformities result in soft tissue injury from poorly fitting shoes. Because of autonomic neuropathy, the diabetic foot sweats less, leading to dry, cracked skin for bacteria to invade. Resistance to infection is lower because of neutrophil dysfunction in hyperglycemia. Vascular insufficiency impairs both wound healing and the immune response.3
The major principles of therapy are as follows:
1. Use an empiric antibiotic regimen with broad coverage against gram-positive, gram-negative, and anaerobic organisms. Diabetic foot infections are usually polymicrobial. Except for mild infections, in which therapy directed at gram-positive organisms may suffice, initial therapy should cover streptococci, methicillin-sensitive Staphylococcus aureus, E. coli, Proteus, and anaerobes.
Many studies of antibiotic therapy in diabetic foot infections have been performed, without demonstrating a clear superiority for any one regimen. The 2004 guidelines of the Infectious Diseases Society of America list 13 acceptable regimens for moderate diabetic foot infections.4 Useful single drug regimens include ampicillin-sulbactam, piperacillin-tazobactam, levofloxacin, and cefoxitin. For the penicillin-allergic patient, clindamycin and ciprofloxacin is a useful combination. Empiric vancomycin should be reserved for patients with a history of MRSA, treatment failure, or severe infection. (I would also reserve carbapenems, such as imipenem-cilastatin, for more severe infections, to prevent antibiotic resistance against a class of drugs representing our last defense against highly resistant gram-negative organisms.)
Once the patient is clinically improved and results of adequate cultures are available, consideration can be given to narrowing the course of therapy. Most diabetic foot infections can be treated with some combination of intravenous and oral therapy.
2. Have a high clinical suspicion for osteomyelitis. Osteomyelitis is extremely common in diabetic foot infections due to plantar ulceration and poor soft tissue coverage. Cure rates are reduced in osteomyelitis because dead bone acts as a nidus for persistent infection. The most useful diagnostic maneuver for osteomyelitis is deep probing of the wound with a sterile swab at the bedside. If a gritty sensation is felt, osteomyelitis is likely, and further diagnostic testing is probably unnecessary.
If the physical examination is equivocal, plain radiographs should be obtained to look for bony erosions. If these are negative, MRI is the next most useful diagnostic step. Bone scans are of limited value due to their low specificity. (Charcot arthropathy is a common cause of false-positive bone scans in this setting.)
Because of the high prevalence of osteomyelitis in diabetic foot infections, my own practice is to err on the side of longer, rather than shorter, antibiotic therapy.
3. Surgical debridement is required for many diabetic foot infections. Develop a collaborative relationship with a vascular or orthopedic surgeon with interest and expertise in the management of diabetic foot infections. Necrotic and gangrenous material should be removed. Ideally, dead bone should be debrided, both therapeutically and to help establish a bacteriologic diagnosis. (When removal of dead bone would result in loss of function or might create a non-healing wound, the option of long-term antibiotic suppression could be explored with an infectious disease specialist.)
4. When osteomyelitis is present, bone cultures help to define the optimal antibiotic therapy. Recent studies have confirmed older data regarding the poor correlation between surface cultures and bone cultures. The latter are preferred, when feasible.
5. Consider revascularization. Most diabetic foot infections arise in the setting of vascular insufficiency. At a minimum, patients with diabetic foot infections should have ankle-brachial indices performed for screening. Because diabetics may have falsely elevated ankle pressures due to calcified and non-compressible arteries, additional diagnostic studies may be useful, such as segmental pressures and Doppler pulse volume recordings.
Measurement of the transcutaneous oxygen concentration (TcP02) has been recommended, particularly in assessing which patients may benefit from hyperbaric oxygen. However, the TcP02 is not widely available, and the benefit of hyperbaric oxygen in this setting remains controversial.
6. Patients should be educated in meticulous foot care to prevent recurrences and reinfections. A diabetic foot infection may indicate that the patient lacks the knowledge, resources, or motivation for proper foot care. It also suggests that something is seriously awry with the patient’s diabetic regimen, compliance, or both. Hospital admissions for diabetic foot infections provide an opportunity to revise the patient’s diabetic medications; to educate the patient regarding wound care, skin care, and daily foot self-examination; to provide additional resources such as visiting nurses; and to refer patients for podiatric care, including tailored shoes, orthotics, and, if necessary, casting to off-load ulcers. TH
Dr. Ross is an instructor in medicine at Harvard Medical School.
References
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999 Apr;177(4):282-286.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the United States. Diabetes Care. 2003;26(6):1790-1795.
- Lazzarini L, Mader JT, Calhoun JH. Diabetic foot infection. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. New York, NY. Marcel Dekker. 2003.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004 Oct;39(7):885-910.
Broken Heart
Acute decompensated heart failure (ADHF) remains one of the most common reasons for hospitalization. ADHF patients who have co-morbid conditions present stubborn challenges for hospitalists. And ADHF is frequently observed in patients 65 and older. The neurohormonal activation that results as a consequence of myocardial dysfunction leads to progressive cardiac deterioration and hemodynamic disturbances that ultimately become manifest as acute decompensated heart failure.
ADHG management goals include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival.
In this article we present the case of a 26-year-old female with ADHF and highlight the management strategies that can result in stabilization and improved long-term outcome.
Introduction
Despite major advances in the treatment of heart disease, heart failure remains a growing public health problem of epidemic proportions in the United States. Approximately five million Americans have heart failure, and more than 550,000 patients are diagnosed with the disease each year.1 The annual number of hospitalizations for heart failure as a primary diagnosis has increased from approximately 810,000 in 1990 to more than 1 million in 1999, and it is the most common discharge diagnosis-related group for patients 65 and older.2 Medicare spent more dollars on the diagnosis and treatment of heart failure than on any other diagnosis—more than $27.9 billion in 2005.1
Patients presenting to the emergency department (ED) with ADHF are often hemodynamically unstable, with severe symptoms of dyspnea and fluid overload. Rapid assessment and prompt initiation of appropriate interventions are necessary to achieve clinical stability and prevent prolonged hospital stay if hospitalization is required. The in-hospital mortality rate for ADHF is 5%-8%; median duration of hospitalization is five days, and the six-month re-hospitalization rate is about 50%.1,3 Thus, it is clear that improved recognition and treatment are of paramount importance. With these goals in mind, we present a recent case that highlights many of the concerns about and treatment options for ADHF.
Case Presentation
Karen A. is a 26-year-old black female with stage III Hodgkin’s disease, diagnosed in 2000. She received chemotherapy (cisplatin, cytarabine, doxorubicin, rituxan, gemcitabine) and, as a result, in 2001 developed chemotherapy-induced cardiomyopathy with an ejection fraction of <20%. Her condition stabilized, and she remained in clinical remission until September 2002.
In 2003 she received an autologous stem cell transplant and subsequently presented to the ED with complaints of fatigue, progressive shortness of breath in the previous seven days, and lower extremity edema. She also reported right-sided pleuritic chest pain, but denied associated nausea, vomiting, or diaphoresis. In the three days preceding admission, she had gained 10 pounds. In the past six months, she had had multiple admissions for ADHF.
Her physical examination revealed an alert, obese female in moderate respiratory distress with dry mucous membranes. Her vital signs indicated a temperature of 36.5° C, a heart rate of 110 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure measuring 111/73 mm Hg, and an oxygen saturation of 92%. She had no scleral icterus, but did have jugular venous distention to the angle of the mandible at 45° upright. Her cardiac examination indicated tachycardia with distant heart sounds and an audible third heart sound (S3), as well as a grade 2/6 systolic ejection murmur at the left sternal border. Her lung examination demonstrated diffuse crackles present in both lung fields, but no wheezes. Abdominal examination was notable for tenderness to palpation at the right hypogastric region and for hepatomegaly, her skin was warm and dry with no cyanosis, and her extremities demonstrated significant bilateral pitting pre-tibial edema to her knees.
Her current medications included:
- Furosemide, 60 mg by mouth two times daily;
- Carvedilol, 12.5 mg by mouth in the morning, 6.25 mg in the evening;
- Amiodarone, 200 mg by mouth daily; and
- Digoxin, 0.125 mg by mouth daily.
Laboratory findings included normal electrolytes, blood urea nitrogen, and serum creatinine. Her hemoglobin was 11.3 gm/L, and her B-type natriuretic peptide (BNP) level was 4,837 pg/ml. Initial cardiac enzymes were negative (troponin I of <0. 03).
Her chest X-ray demonstrated moderate cardiac enlargement with bilateral thickening of subpleural septal lines and blurring of the pulmonary vasculature consistent with developing cardiogenic pulmonary edema. A 12-lead electrocardiogram indicated sinus tachycardia with a rate of 110 beats per minute and nonspecific ST-T wave changes in the inferior leads, but no Q waves were noted. Echocardiography showed severely reduced left ventricular systolic function with severe global hypokinesis of the left ventricle and a measured ejection fraction of 25%-30%. There was no pericardial effusion.
She was admitted to the telemetry floor with the diagnosis of ADHF. She was placed on supplemental oxygen and serial cardiac enzymes, and her electrocardiogram remained negative for injury or ischemia. Intravenous furosemide was initiated at 40 mg every 12 hours. After 24 hours, she was given a bolus of nesiritide (2 mcg/kg), followed by a continuous infusion at 0.01 mcg/kg/min. Cardiac medications were continued, and the dosage of carvedilol was reduced to 6.25 mg by mouth twice daily.
After a period of diuresis, the patient remained highly symptomatic; therefore, a right heart catheterization was done. The hemodynamics indicated a cardiac output of three liters per minute, a right atrial pressure of 20 mm Hg, a right ventricular pressure of 70/20 mm Hg, pulmonary artery pressure of 66/20 mm Hg with a mean pressure of 52 mm Hg, and a pulmonary capillary wedge pressure of 25 mm Hg. (See Figures 1 and 2, p. 23).
Over the next 24 hours, the patient had more than three liters of urine output and was significantly improved. The nesiritide infusion was discontinued after 48 hours of therapy, and the patient was weaned from supplemental oxygen. Lisinopril was started at 2.5 mg daily by mouth. On hospital day four, the patient walked around the nurses’ station without supplemental oxygen, and she was returned to the previous dose of furosemide, 60 mg twice daily.
The patient was enrolled in the Heart Success Program (HSP), a collaborative interdisciplinary program that is available in the institution for cancer patients with heart failure. She was provided with patient education materials that included educational videotapes on heart failure management, daily weight monitoring, diet, medications, exercise, and the emotional aspects of heart failure. Nurses with heart failure training were available to answer questions for the patient and to provide further instruction for follow-up after the patient’s discharge. The patient improved enough that she was able to enroll in a New York Heart Association (NYHA) class II and was discharged after five days, with a follow-up appointment to the outpatient clinic one week after hospital discharge.
Discussion
This case illustrates the challenges inherent in the diagnosis and management of ADHF in a cancer patient with a known history of heart failure. Rapid assessment is critical in establishing a diagnosis and initiating appropriate intervention. The goals of managing ADHF remain the same regardless of etiology. These include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival. The same principles apply—even if the patient has a major comorbidity such as cancer, and suspicion for the diagnosis must remain high.
Initial Evaluation
Early diagnosis and effective management of ADHF are critically important, as these have been shown to reduce hospitalizations and intensive care unit admissions, to decrease length of stay, and to decrease cost of hospitalization.4 A comprehensive history and physical examination must be performed to identify signs and symptoms that lead to a heart failure diagnosis.
We must evaluate such potential risk factors as history of hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, valvular disease, peripheral vascular disease, a family history of cardiomyopathy, smoking, alcohol use, thyroid problems, sleep apnea, and any recent history of infection (particularly upper respiratory tract infection, which can cause viral cardiomyopathy).
As part of further investigation with cancer patients, include the patient’s possible past exposure to cardiotoxic agents (e.g., anthracyclines, trastuzumab, high-dose cyclophosphamide) or mediastinal irradiation. Chemotherapy-induced cardiomyopathy is increasingly becoming an issue in heart failure management as a result of the growing number of long-term cancer survivors who have received treatment with anthracycline-containing chemotherapy or other aggressive therapy.
Focus on volume and perfusion status during the physical assessment of each patient. Most patients presenting to the ED with acute decompensation are volume overloaded. Volume overload is manifested by symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea, as well as jugular venous distention, hepatojugular reflux, ascites, edema, and crackles in the lungs.5 Crackles are not always present in chronic heart failure patients, however, because of the continuous movement of fluid into the interstitium associated with increased lymphatic drainage, leaving the alveoli relatively dry.6
In addition to a comprehensive history and physical examination, several tests will help establish the diagnosis. (See Table 1, top left.) A measurement of a B-type natriuretic peptide (BNP) facilitates the diagnosis of ADHF. BNP is an endogenously generated natriuretic peptide that is activated in response to atrial or ventricular expansion due to volume overload and increased wall tension.7,8 Circulating levels of endogenous BNP are significantly elevated in ADHF patients and are a valuable tool for diagnosis of heart failure in the ED.9-11 In Karen A., the BNP level was 4,837 pg/mL, which is indicative of Stage D heart failure. This diagnosis was confirmed by chest X-ray findings of cardiogenic pulmonary edema in the setting of severe left ventricular hypokinesis and an ejection fraction of 25%-30%.
Although history and physical examination may provide important clues regarding the underlying cardiac abnormality, both invasive and noninvasive testing are necessary to provide a definitive diagnosis of heart failure and to evaluate potential exacerbating conditions. A two-dimensional echocardiogram with Doppler flow study is an essential diagnostic test for evaluating myocardial contractility or ejection fraction. Echocardiogram can also evaluate other structural components such as the pericardium, valvular status, and hemodynamic parameters that may contribute to the development of ADHF. In patients with cardiac risk factors, a myocardial perfusion stress test or catheterization may identify the presence of coronary artery disease as a contributor.
A 12-lead electrocardiogram is necessary to establish the rhythm and to show evidence of acute or prior myocardial infarction, pericarditis, conduction abnormalities, or left ventricular hypertrophy as a result of prolonged uncontrolled hypertension. It is known that rhythm disturbances such as atrial fibrillation can be a precipitating factor for ADHF.
A chest X-ray is needed to uncover pulmonary edema in cases of fluid overload and to show an enlarged cardiac silhouette in cases of dilated cardiomyopathy. Identification of a definitive cause or causes that precipitate the occurrence of ADHF is crucial in devising a management plan and initiating appropriate intervention. (See Table 2, p. 22.)
Immediate Management of the ADHF
Newly developed clinical practice guidelines for the management of ADHF exist, but management is still based largely on empirical evidence.12 Begin ADHF treatment in the ED with intravenous diuretics (unless contraindicated). A majority of ADHF patients will respond to diuretics alone.13 If the patient responds poorly to diuretics, the use of nesiritide in conjunction with diuretics has proven beneficial, as shown in the analysis of data from the ADHERE registry indicating that patients treated with intravenous nesiritide had a lower hospital mortality rate than patients treated with milrinone or dobutamine.14 Other options include ultrafiltration, an intervention that has been noted to reduce lengths of stay and rehospitalization rates in patients with ADHF.
Nesiritide is a recombinant form of BNP without direct inotropic effects but with venous, arterial, and coronary vasodilatory properties that can improve symptoms in ADHF.15 The recommended dosage for nesiritide is an IV bolus of 2 mcg per kg, followed by a continuous infusion of 0.01 mcg/kg/min. In the setting of hypotension with a systolic blood pressure less than 100 mm Hg, however, an initial IV bolus dose is not recommended; instead, the patient may start with a continuous infusion of 0.01 mcg/kg/min, or consider other therapies.
In hemodynamically unstable patients with a systolic blood pressure less than 90 mm Hg, or in those with evidence of end organ hypoperfusion (cardiogenic shock), inotropic support may be considered until the patient is stabilized. (See Table 3, p. 22.) Recognize that inotropic agents have adverse effects on the neurohormonal system and are not recommended routinely but may be essential for temporary stabilization. (For more on pharmacologic management of ADHF, see Table 4, p. 24.)
Subacute Management of ADHF
Once acute decompensation has been reversed and an euvolemic state has been achieved, shift therapy to a combination of three classes of medications: diuretics, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers, unless contraindicated. The benefits of these drugs have been established by evidence from numerous large-scale clinical trials.3,12
Start ACE inhibitors in all patients with heart failure due to left ventricular systolic dysfunction (unless contraindicated or if the patient is intolerant).3 Give ACE-I to patients who have experienced a recent episode of ADHF, along with diuretics to maintain sodium balance and to prevent peripheral and pulmonary edema. ACE inhibitors are contraindicated for patients who are pregnant and for those with childbearing potential, as well as for individuals with a prior history of angioedema or renal failure after receiving the drug. Instruct patients to avoid any sudden change of position, as they may experience orthostatic hypotension while taking ACE inhibitors.
Some patients are intolerant of ACE inhibitors due to a persistent cough that occurs in approximately 5% to 10% of Caucasian patients and in up to 50% of Chinese patients.16 Angiotensin receptor blockers (ARBs) are an established alternative.17 Two ARBs (candesartan and valsartan) are recommended for treatment of heart failure based on evidence from controlled clinical trials.17,18 These drugs have demonstrated a reduction in hospitalizations, and candesartan, when used as an alternative to ACE-I, has been shown to reduce mortality. Additionally, in patients with evidence of left ventricular dysfunction after myocardial infarction, valsartan provided a benefit that was not inferior to ACE inhibitors.17
Initiate beta-blockers at very low doses and gradually increased as tolerated. Monitor patients closely for symptoms of hypotension, significant weight gain, fluid retention, bradycardia, and heart block. In addition, inform patients that they may experience generalized fatigue or weakness with the initiation of beta-blockers. In a cancer patient it is difficult to differentiate between fatigue caused by the disease and the side effects of therapy. Fatigue associated with beta-blocker therapy usually resolves spontaneously within a few days. Make every effort to achieve optimum target beta-blocker dose.
An aldosterone antagonist, such as spironolactone or eplerenone, given at a daily dose of 12.5 to 25 mg in addition to standard therapy, effectively blocks the effects of aldosterone (RALES study) to achieve comprehensive neurohormonal blockade.19 When prescribing an aldosterone antagonist, especially in combination with ACE inhibitors and loop diuretics, it is important to monitor serum potassium levels because this combination can result in hyperkalemia.
Disease Management Programs
Comprehensive management of heart failure is not only limited to hospital care during an episode of ADHF. In order to prevent repeated hospitalizations, implement additional measures through formal disease management programs. These disease management programs are often directed or coordinated by advanced practice nurses who address the comprehensive care of heart failure patients with emphasis on patient education and counseling to improve patient compliance.20
The non-pharmacologic treatment strategies emphasized in disease management programs have proven effective in achieving positive outcomes. These include counseling patients on dietary management, including encouraging a two-gram sodium diet, alcohol restriction, and adequate supplementation of electrolyte loss from diuretics. Keeping a diary of the patient’s daily weight at home and bringing it to office visits will help both the patient and the clinician monitor fluid retention efficiently.
Hypotension is a common side effect from the pharmacologic therapy for heart failure. Employ comprehensive education with both patient and family to avoid unnecessary discontinuation of the medications. A systolic blood pressure of 90 mm Hg is acceptable as long as there are no associated symptoms of dizziness or syncope.
Encourage activity guidelines, including participation in exercise programs. Attendance at support group meetings will provide a venue in which patients can share common problems and concerns with others in similar situations. One to two weeks after hospital discharge, schedule an outpatient follow-up in a heart failure clinic, where heart failure education is reinforced to prevent another episode of ADHF admission.
Summary
Despite the added challenges, managing ADHF in a patient with a serious comorbidity such as cancer involves the same goals as the treatment of ADHF in any other patient. With rapid assessment and appropriate intervention, the patient is given the best possible chance of survival. TH
The authors work at the University of Texas M.D. Anderson Cancer Center, Department of Cardiology, Houston.
References
- American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas.: American Heart Association; 2005. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Last accessed August 20, 2006.
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004 Jan;147(1):74-78.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154-235.Epub 2005 Sep 13.
- Peacock WF IV, Emerman CL, Wynne J, for the ADHERE Scientific Advisory Committee and Investigators and the ADHERE Study Group. Early use of nesiritide in the emergency department is associated with improved outcome: an ADHERE registry analysis. Ann Emerg Med. 2004;44:S78.
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail. 1999 Aug;1(3):251-257.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888.
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995 Sep;96(3):1280-1287.
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825-832.
- Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005 Jan-Feb;11(1):30-38.
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul;106(4):416-422.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161-167.
- Adams KF, Lindenfeld J, Arnold JMO, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10-38.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol. 2005 Jul;46(1):65-67.
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul;46(1):57-64.
- Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002 Dec;144(6):1102-1108.
- Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995 Aug;40(2):141-144.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003 Nov 13;349(20):1893-1906. Epub 2003 Nov 10.
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-776.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-717.
- Albert NM, Eastwood CA, Edwards ML. Evidence-based practice for acute decompensated heart failure. Crit Care Nurse. 2004 Dec;24(6):14-16, 18-24, 26-29; quiz 30-31.
Acute decompensated heart failure (ADHF) remains one of the most common reasons for hospitalization. ADHF patients who have co-morbid conditions present stubborn challenges for hospitalists. And ADHF is frequently observed in patients 65 and older. The neurohormonal activation that results as a consequence of myocardial dysfunction leads to progressive cardiac deterioration and hemodynamic disturbances that ultimately become manifest as acute decompensated heart failure.
ADHG management goals include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival.
In this article we present the case of a 26-year-old female with ADHF and highlight the management strategies that can result in stabilization and improved long-term outcome.
Introduction
Despite major advances in the treatment of heart disease, heart failure remains a growing public health problem of epidemic proportions in the United States. Approximately five million Americans have heart failure, and more than 550,000 patients are diagnosed with the disease each year.1 The annual number of hospitalizations for heart failure as a primary diagnosis has increased from approximately 810,000 in 1990 to more than 1 million in 1999, and it is the most common discharge diagnosis-related group for patients 65 and older.2 Medicare spent more dollars on the diagnosis and treatment of heart failure than on any other diagnosis—more than $27.9 billion in 2005.1
Patients presenting to the emergency department (ED) with ADHF are often hemodynamically unstable, with severe symptoms of dyspnea and fluid overload. Rapid assessment and prompt initiation of appropriate interventions are necessary to achieve clinical stability and prevent prolonged hospital stay if hospitalization is required. The in-hospital mortality rate for ADHF is 5%-8%; median duration of hospitalization is five days, and the six-month re-hospitalization rate is about 50%.1,3 Thus, it is clear that improved recognition and treatment are of paramount importance. With these goals in mind, we present a recent case that highlights many of the concerns about and treatment options for ADHF.
Case Presentation
Karen A. is a 26-year-old black female with stage III Hodgkin’s disease, diagnosed in 2000. She received chemotherapy (cisplatin, cytarabine, doxorubicin, rituxan, gemcitabine) and, as a result, in 2001 developed chemotherapy-induced cardiomyopathy with an ejection fraction of <20%. Her condition stabilized, and she remained in clinical remission until September 2002.
In 2003 she received an autologous stem cell transplant and subsequently presented to the ED with complaints of fatigue, progressive shortness of breath in the previous seven days, and lower extremity edema. She also reported right-sided pleuritic chest pain, but denied associated nausea, vomiting, or diaphoresis. In the three days preceding admission, she had gained 10 pounds. In the past six months, she had had multiple admissions for ADHF.
Her physical examination revealed an alert, obese female in moderate respiratory distress with dry mucous membranes. Her vital signs indicated a temperature of 36.5° C, a heart rate of 110 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure measuring 111/73 mm Hg, and an oxygen saturation of 92%. She had no scleral icterus, but did have jugular venous distention to the angle of the mandible at 45° upright. Her cardiac examination indicated tachycardia with distant heart sounds and an audible third heart sound (S3), as well as a grade 2/6 systolic ejection murmur at the left sternal border. Her lung examination demonstrated diffuse crackles present in both lung fields, but no wheezes. Abdominal examination was notable for tenderness to palpation at the right hypogastric region and for hepatomegaly, her skin was warm and dry with no cyanosis, and her extremities demonstrated significant bilateral pitting pre-tibial edema to her knees.
Her current medications included:
- Furosemide, 60 mg by mouth two times daily;
- Carvedilol, 12.5 mg by mouth in the morning, 6.25 mg in the evening;
- Amiodarone, 200 mg by mouth daily; and
- Digoxin, 0.125 mg by mouth daily.
Laboratory findings included normal electrolytes, blood urea nitrogen, and serum creatinine. Her hemoglobin was 11.3 gm/L, and her B-type natriuretic peptide (BNP) level was 4,837 pg/ml. Initial cardiac enzymes were negative (troponin I of <0. 03).
Her chest X-ray demonstrated moderate cardiac enlargement with bilateral thickening of subpleural septal lines and blurring of the pulmonary vasculature consistent with developing cardiogenic pulmonary edema. A 12-lead electrocardiogram indicated sinus tachycardia with a rate of 110 beats per minute and nonspecific ST-T wave changes in the inferior leads, but no Q waves were noted. Echocardiography showed severely reduced left ventricular systolic function with severe global hypokinesis of the left ventricle and a measured ejection fraction of 25%-30%. There was no pericardial effusion.
She was admitted to the telemetry floor with the diagnosis of ADHF. She was placed on supplemental oxygen and serial cardiac enzymes, and her electrocardiogram remained negative for injury or ischemia. Intravenous furosemide was initiated at 40 mg every 12 hours. After 24 hours, she was given a bolus of nesiritide (2 mcg/kg), followed by a continuous infusion at 0.01 mcg/kg/min. Cardiac medications were continued, and the dosage of carvedilol was reduced to 6.25 mg by mouth twice daily.
After a period of diuresis, the patient remained highly symptomatic; therefore, a right heart catheterization was done. The hemodynamics indicated a cardiac output of three liters per minute, a right atrial pressure of 20 mm Hg, a right ventricular pressure of 70/20 mm Hg, pulmonary artery pressure of 66/20 mm Hg with a mean pressure of 52 mm Hg, and a pulmonary capillary wedge pressure of 25 mm Hg. (See Figures 1 and 2, p. 23).
Over the next 24 hours, the patient had more than three liters of urine output and was significantly improved. The nesiritide infusion was discontinued after 48 hours of therapy, and the patient was weaned from supplemental oxygen. Lisinopril was started at 2.5 mg daily by mouth. On hospital day four, the patient walked around the nurses’ station without supplemental oxygen, and she was returned to the previous dose of furosemide, 60 mg twice daily.
The patient was enrolled in the Heart Success Program (HSP), a collaborative interdisciplinary program that is available in the institution for cancer patients with heart failure. She was provided with patient education materials that included educational videotapes on heart failure management, daily weight monitoring, diet, medications, exercise, and the emotional aspects of heart failure. Nurses with heart failure training were available to answer questions for the patient and to provide further instruction for follow-up after the patient’s discharge. The patient improved enough that she was able to enroll in a New York Heart Association (NYHA) class II and was discharged after five days, with a follow-up appointment to the outpatient clinic one week after hospital discharge.
Discussion
This case illustrates the challenges inherent in the diagnosis and management of ADHF in a cancer patient with a known history of heart failure. Rapid assessment is critical in establishing a diagnosis and initiating appropriate intervention. The goals of managing ADHF remain the same regardless of etiology. These include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival. The same principles apply—even if the patient has a major comorbidity such as cancer, and suspicion for the diagnosis must remain high.
Initial Evaluation
Early diagnosis and effective management of ADHF are critically important, as these have been shown to reduce hospitalizations and intensive care unit admissions, to decrease length of stay, and to decrease cost of hospitalization.4 A comprehensive history and physical examination must be performed to identify signs and symptoms that lead to a heart failure diagnosis.
We must evaluate such potential risk factors as history of hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, valvular disease, peripheral vascular disease, a family history of cardiomyopathy, smoking, alcohol use, thyroid problems, sleep apnea, and any recent history of infection (particularly upper respiratory tract infection, which can cause viral cardiomyopathy).
As part of further investigation with cancer patients, include the patient’s possible past exposure to cardiotoxic agents (e.g., anthracyclines, trastuzumab, high-dose cyclophosphamide) or mediastinal irradiation. Chemotherapy-induced cardiomyopathy is increasingly becoming an issue in heart failure management as a result of the growing number of long-term cancer survivors who have received treatment with anthracycline-containing chemotherapy or other aggressive therapy.
Focus on volume and perfusion status during the physical assessment of each patient. Most patients presenting to the ED with acute decompensation are volume overloaded. Volume overload is manifested by symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea, as well as jugular venous distention, hepatojugular reflux, ascites, edema, and crackles in the lungs.5 Crackles are not always present in chronic heart failure patients, however, because of the continuous movement of fluid into the interstitium associated with increased lymphatic drainage, leaving the alveoli relatively dry.6
In addition to a comprehensive history and physical examination, several tests will help establish the diagnosis. (See Table 1, top left.) A measurement of a B-type natriuretic peptide (BNP) facilitates the diagnosis of ADHF. BNP is an endogenously generated natriuretic peptide that is activated in response to atrial or ventricular expansion due to volume overload and increased wall tension.7,8 Circulating levels of endogenous BNP are significantly elevated in ADHF patients and are a valuable tool for diagnosis of heart failure in the ED.9-11 In Karen A., the BNP level was 4,837 pg/mL, which is indicative of Stage D heart failure. This diagnosis was confirmed by chest X-ray findings of cardiogenic pulmonary edema in the setting of severe left ventricular hypokinesis and an ejection fraction of 25%-30%.
Although history and physical examination may provide important clues regarding the underlying cardiac abnormality, both invasive and noninvasive testing are necessary to provide a definitive diagnosis of heart failure and to evaluate potential exacerbating conditions. A two-dimensional echocardiogram with Doppler flow study is an essential diagnostic test for evaluating myocardial contractility or ejection fraction. Echocardiogram can also evaluate other structural components such as the pericardium, valvular status, and hemodynamic parameters that may contribute to the development of ADHF. In patients with cardiac risk factors, a myocardial perfusion stress test or catheterization may identify the presence of coronary artery disease as a contributor.
A 12-lead electrocardiogram is necessary to establish the rhythm and to show evidence of acute or prior myocardial infarction, pericarditis, conduction abnormalities, or left ventricular hypertrophy as a result of prolonged uncontrolled hypertension. It is known that rhythm disturbances such as atrial fibrillation can be a precipitating factor for ADHF.
A chest X-ray is needed to uncover pulmonary edema in cases of fluid overload and to show an enlarged cardiac silhouette in cases of dilated cardiomyopathy. Identification of a definitive cause or causes that precipitate the occurrence of ADHF is crucial in devising a management plan and initiating appropriate intervention. (See Table 2, p. 22.)
Immediate Management of the ADHF
Newly developed clinical practice guidelines for the management of ADHF exist, but management is still based largely on empirical evidence.12 Begin ADHF treatment in the ED with intravenous diuretics (unless contraindicated). A majority of ADHF patients will respond to diuretics alone.13 If the patient responds poorly to diuretics, the use of nesiritide in conjunction with diuretics has proven beneficial, as shown in the analysis of data from the ADHERE registry indicating that patients treated with intravenous nesiritide had a lower hospital mortality rate than patients treated with milrinone or dobutamine.14 Other options include ultrafiltration, an intervention that has been noted to reduce lengths of stay and rehospitalization rates in patients with ADHF.
Nesiritide is a recombinant form of BNP without direct inotropic effects but with venous, arterial, and coronary vasodilatory properties that can improve symptoms in ADHF.15 The recommended dosage for nesiritide is an IV bolus of 2 mcg per kg, followed by a continuous infusion of 0.01 mcg/kg/min. In the setting of hypotension with a systolic blood pressure less than 100 mm Hg, however, an initial IV bolus dose is not recommended; instead, the patient may start with a continuous infusion of 0.01 mcg/kg/min, or consider other therapies.
In hemodynamically unstable patients with a systolic blood pressure less than 90 mm Hg, or in those with evidence of end organ hypoperfusion (cardiogenic shock), inotropic support may be considered until the patient is stabilized. (See Table 3, p. 22.) Recognize that inotropic agents have adverse effects on the neurohormonal system and are not recommended routinely but may be essential for temporary stabilization. (For more on pharmacologic management of ADHF, see Table 4, p. 24.)
Subacute Management of ADHF
Once acute decompensation has been reversed and an euvolemic state has been achieved, shift therapy to a combination of three classes of medications: diuretics, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers, unless contraindicated. The benefits of these drugs have been established by evidence from numerous large-scale clinical trials.3,12
Start ACE inhibitors in all patients with heart failure due to left ventricular systolic dysfunction (unless contraindicated or if the patient is intolerant).3 Give ACE-I to patients who have experienced a recent episode of ADHF, along with diuretics to maintain sodium balance and to prevent peripheral and pulmonary edema. ACE inhibitors are contraindicated for patients who are pregnant and for those with childbearing potential, as well as for individuals with a prior history of angioedema or renal failure after receiving the drug. Instruct patients to avoid any sudden change of position, as they may experience orthostatic hypotension while taking ACE inhibitors.
Some patients are intolerant of ACE inhibitors due to a persistent cough that occurs in approximately 5% to 10% of Caucasian patients and in up to 50% of Chinese patients.16 Angiotensin receptor blockers (ARBs) are an established alternative.17 Two ARBs (candesartan and valsartan) are recommended for treatment of heart failure based on evidence from controlled clinical trials.17,18 These drugs have demonstrated a reduction in hospitalizations, and candesartan, when used as an alternative to ACE-I, has been shown to reduce mortality. Additionally, in patients with evidence of left ventricular dysfunction after myocardial infarction, valsartan provided a benefit that was not inferior to ACE inhibitors.17
Initiate beta-blockers at very low doses and gradually increased as tolerated. Monitor patients closely for symptoms of hypotension, significant weight gain, fluid retention, bradycardia, and heart block. In addition, inform patients that they may experience generalized fatigue or weakness with the initiation of beta-blockers. In a cancer patient it is difficult to differentiate between fatigue caused by the disease and the side effects of therapy. Fatigue associated with beta-blocker therapy usually resolves spontaneously within a few days. Make every effort to achieve optimum target beta-blocker dose.
An aldosterone antagonist, such as spironolactone or eplerenone, given at a daily dose of 12.5 to 25 mg in addition to standard therapy, effectively blocks the effects of aldosterone (RALES study) to achieve comprehensive neurohormonal blockade.19 When prescribing an aldosterone antagonist, especially in combination with ACE inhibitors and loop diuretics, it is important to monitor serum potassium levels because this combination can result in hyperkalemia.
Disease Management Programs
Comprehensive management of heart failure is not only limited to hospital care during an episode of ADHF. In order to prevent repeated hospitalizations, implement additional measures through formal disease management programs. These disease management programs are often directed or coordinated by advanced practice nurses who address the comprehensive care of heart failure patients with emphasis on patient education and counseling to improve patient compliance.20
The non-pharmacologic treatment strategies emphasized in disease management programs have proven effective in achieving positive outcomes. These include counseling patients on dietary management, including encouraging a two-gram sodium diet, alcohol restriction, and adequate supplementation of electrolyte loss from diuretics. Keeping a diary of the patient’s daily weight at home and bringing it to office visits will help both the patient and the clinician monitor fluid retention efficiently.
Hypotension is a common side effect from the pharmacologic therapy for heart failure. Employ comprehensive education with both patient and family to avoid unnecessary discontinuation of the medications. A systolic blood pressure of 90 mm Hg is acceptable as long as there are no associated symptoms of dizziness or syncope.
Encourage activity guidelines, including participation in exercise programs. Attendance at support group meetings will provide a venue in which patients can share common problems and concerns with others in similar situations. One to two weeks after hospital discharge, schedule an outpatient follow-up in a heart failure clinic, where heart failure education is reinforced to prevent another episode of ADHF admission.
Summary
Despite the added challenges, managing ADHF in a patient with a serious comorbidity such as cancer involves the same goals as the treatment of ADHF in any other patient. With rapid assessment and appropriate intervention, the patient is given the best possible chance of survival. TH
The authors work at the University of Texas M.D. Anderson Cancer Center, Department of Cardiology, Houston.
References
- American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas.: American Heart Association; 2005. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Last accessed August 20, 2006.
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004 Jan;147(1):74-78.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154-235.Epub 2005 Sep 13.
- Peacock WF IV, Emerman CL, Wynne J, for the ADHERE Scientific Advisory Committee and Investigators and the ADHERE Study Group. Early use of nesiritide in the emergency department is associated with improved outcome: an ADHERE registry analysis. Ann Emerg Med. 2004;44:S78.
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail. 1999 Aug;1(3):251-257.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888.
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995 Sep;96(3):1280-1287.
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825-832.
- Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005 Jan-Feb;11(1):30-38.
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul;106(4):416-422.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161-167.
- Adams KF, Lindenfeld J, Arnold JMO, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10-38.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol. 2005 Jul;46(1):65-67.
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul;46(1):57-64.
- Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002 Dec;144(6):1102-1108.
- Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995 Aug;40(2):141-144.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003 Nov 13;349(20):1893-1906. Epub 2003 Nov 10.
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-776.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-717.
- Albert NM, Eastwood CA, Edwards ML. Evidence-based practice for acute decompensated heart failure. Crit Care Nurse. 2004 Dec;24(6):14-16, 18-24, 26-29; quiz 30-31.
Acute decompensated heart failure (ADHF) remains one of the most common reasons for hospitalization. ADHF patients who have co-morbid conditions present stubborn challenges for hospitalists. And ADHF is frequently observed in patients 65 and older. The neurohormonal activation that results as a consequence of myocardial dysfunction leads to progressive cardiac deterioration and hemodynamic disturbances that ultimately become manifest as acute decompensated heart failure.
ADHG management goals include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival.
In this article we present the case of a 26-year-old female with ADHF and highlight the management strategies that can result in stabilization and improved long-term outcome.
Introduction
Despite major advances in the treatment of heart disease, heart failure remains a growing public health problem of epidemic proportions in the United States. Approximately five million Americans have heart failure, and more than 550,000 patients are diagnosed with the disease each year.1 The annual number of hospitalizations for heart failure as a primary diagnosis has increased from approximately 810,000 in 1990 to more than 1 million in 1999, and it is the most common discharge diagnosis-related group for patients 65 and older.2 Medicare spent more dollars on the diagnosis and treatment of heart failure than on any other diagnosis—more than $27.9 billion in 2005.1
Patients presenting to the emergency department (ED) with ADHF are often hemodynamically unstable, with severe symptoms of dyspnea and fluid overload. Rapid assessment and prompt initiation of appropriate interventions are necessary to achieve clinical stability and prevent prolonged hospital stay if hospitalization is required. The in-hospital mortality rate for ADHF is 5%-8%; median duration of hospitalization is five days, and the six-month re-hospitalization rate is about 50%.1,3 Thus, it is clear that improved recognition and treatment are of paramount importance. With these goals in mind, we present a recent case that highlights many of the concerns about and treatment options for ADHF.
Case Presentation
Karen A. is a 26-year-old black female with stage III Hodgkin’s disease, diagnosed in 2000. She received chemotherapy (cisplatin, cytarabine, doxorubicin, rituxan, gemcitabine) and, as a result, in 2001 developed chemotherapy-induced cardiomyopathy with an ejection fraction of <20%. Her condition stabilized, and she remained in clinical remission until September 2002.
In 2003 she received an autologous stem cell transplant and subsequently presented to the ED with complaints of fatigue, progressive shortness of breath in the previous seven days, and lower extremity edema. She also reported right-sided pleuritic chest pain, but denied associated nausea, vomiting, or diaphoresis. In the three days preceding admission, she had gained 10 pounds. In the past six months, she had had multiple admissions for ADHF.
Her physical examination revealed an alert, obese female in moderate respiratory distress with dry mucous membranes. Her vital signs indicated a temperature of 36.5° C, a heart rate of 110 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure measuring 111/73 mm Hg, and an oxygen saturation of 92%. She had no scleral icterus, but did have jugular venous distention to the angle of the mandible at 45° upright. Her cardiac examination indicated tachycardia with distant heart sounds and an audible third heart sound (S3), as well as a grade 2/6 systolic ejection murmur at the left sternal border. Her lung examination demonstrated diffuse crackles present in both lung fields, but no wheezes. Abdominal examination was notable for tenderness to palpation at the right hypogastric region and for hepatomegaly, her skin was warm and dry with no cyanosis, and her extremities demonstrated significant bilateral pitting pre-tibial edema to her knees.
Her current medications included:
- Furosemide, 60 mg by mouth two times daily;
- Carvedilol, 12.5 mg by mouth in the morning, 6.25 mg in the evening;
- Amiodarone, 200 mg by mouth daily; and
- Digoxin, 0.125 mg by mouth daily.
Laboratory findings included normal electrolytes, blood urea nitrogen, and serum creatinine. Her hemoglobin was 11.3 gm/L, and her B-type natriuretic peptide (BNP) level was 4,837 pg/ml. Initial cardiac enzymes were negative (troponin I of <0. 03).
Her chest X-ray demonstrated moderate cardiac enlargement with bilateral thickening of subpleural septal lines and blurring of the pulmonary vasculature consistent with developing cardiogenic pulmonary edema. A 12-lead electrocardiogram indicated sinus tachycardia with a rate of 110 beats per minute and nonspecific ST-T wave changes in the inferior leads, but no Q waves were noted. Echocardiography showed severely reduced left ventricular systolic function with severe global hypokinesis of the left ventricle and a measured ejection fraction of 25%-30%. There was no pericardial effusion.
She was admitted to the telemetry floor with the diagnosis of ADHF. She was placed on supplemental oxygen and serial cardiac enzymes, and her electrocardiogram remained negative for injury or ischemia. Intravenous furosemide was initiated at 40 mg every 12 hours. After 24 hours, she was given a bolus of nesiritide (2 mcg/kg), followed by a continuous infusion at 0.01 mcg/kg/min. Cardiac medications were continued, and the dosage of carvedilol was reduced to 6.25 mg by mouth twice daily.
After a period of diuresis, the patient remained highly symptomatic; therefore, a right heart catheterization was done. The hemodynamics indicated a cardiac output of three liters per minute, a right atrial pressure of 20 mm Hg, a right ventricular pressure of 70/20 mm Hg, pulmonary artery pressure of 66/20 mm Hg with a mean pressure of 52 mm Hg, and a pulmonary capillary wedge pressure of 25 mm Hg. (See Figures 1 and 2, p. 23).
Over the next 24 hours, the patient had more than three liters of urine output and was significantly improved. The nesiritide infusion was discontinued after 48 hours of therapy, and the patient was weaned from supplemental oxygen. Lisinopril was started at 2.5 mg daily by mouth. On hospital day four, the patient walked around the nurses’ station without supplemental oxygen, and she was returned to the previous dose of furosemide, 60 mg twice daily.
The patient was enrolled in the Heart Success Program (HSP), a collaborative interdisciplinary program that is available in the institution for cancer patients with heart failure. She was provided with patient education materials that included educational videotapes on heart failure management, daily weight monitoring, diet, medications, exercise, and the emotional aspects of heart failure. Nurses with heart failure training were available to answer questions for the patient and to provide further instruction for follow-up after the patient’s discharge. The patient improved enough that she was able to enroll in a New York Heart Association (NYHA) class II and was discharged after five days, with a follow-up appointment to the outpatient clinic one week after hospital discharge.
Discussion
This case illustrates the challenges inherent in the diagnosis and management of ADHF in a cancer patient with a known history of heart failure. Rapid assessment is critical in establishing a diagnosis and initiating appropriate intervention. The goals of managing ADHF remain the same regardless of etiology. These include stabilizing the patient, managing acute hemodynamic abnormalities, reversing the symptoms of dyspnea caused by fluid overload, and initiating evidence-based therapies to decrease disease progression and improve survival. The same principles apply—even if the patient has a major comorbidity such as cancer, and suspicion for the diagnosis must remain high.
Initial Evaluation
Early diagnosis and effective management of ADHF are critically important, as these have been shown to reduce hospitalizations and intensive care unit admissions, to decrease length of stay, and to decrease cost of hospitalization.4 A comprehensive history and physical examination must be performed to identify signs and symptoms that lead to a heart failure diagnosis.
We must evaluate such potential risk factors as history of hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, valvular disease, peripheral vascular disease, a family history of cardiomyopathy, smoking, alcohol use, thyroid problems, sleep apnea, and any recent history of infection (particularly upper respiratory tract infection, which can cause viral cardiomyopathy).
As part of further investigation with cancer patients, include the patient’s possible past exposure to cardiotoxic agents (e.g., anthracyclines, trastuzumab, high-dose cyclophosphamide) or mediastinal irradiation. Chemotherapy-induced cardiomyopathy is increasingly becoming an issue in heart failure management as a result of the growing number of long-term cancer survivors who have received treatment with anthracycline-containing chemotherapy or other aggressive therapy.
Focus on volume and perfusion status during the physical assessment of each patient. Most patients presenting to the ED with acute decompensation are volume overloaded. Volume overload is manifested by symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea, as well as jugular venous distention, hepatojugular reflux, ascites, edema, and crackles in the lungs.5 Crackles are not always present in chronic heart failure patients, however, because of the continuous movement of fluid into the interstitium associated with increased lymphatic drainage, leaving the alveoli relatively dry.6
In addition to a comprehensive history and physical examination, several tests will help establish the diagnosis. (See Table 1, top left.) A measurement of a B-type natriuretic peptide (BNP) facilitates the diagnosis of ADHF. BNP is an endogenously generated natriuretic peptide that is activated in response to atrial or ventricular expansion due to volume overload and increased wall tension.7,8 Circulating levels of endogenous BNP are significantly elevated in ADHF patients and are a valuable tool for diagnosis of heart failure in the ED.9-11 In Karen A., the BNP level was 4,837 pg/mL, which is indicative of Stage D heart failure. This diagnosis was confirmed by chest X-ray findings of cardiogenic pulmonary edema in the setting of severe left ventricular hypokinesis and an ejection fraction of 25%-30%.
Although history and physical examination may provide important clues regarding the underlying cardiac abnormality, both invasive and noninvasive testing are necessary to provide a definitive diagnosis of heart failure and to evaluate potential exacerbating conditions. A two-dimensional echocardiogram with Doppler flow study is an essential diagnostic test for evaluating myocardial contractility or ejection fraction. Echocardiogram can also evaluate other structural components such as the pericardium, valvular status, and hemodynamic parameters that may contribute to the development of ADHF. In patients with cardiac risk factors, a myocardial perfusion stress test or catheterization may identify the presence of coronary artery disease as a contributor.
A 12-lead electrocardiogram is necessary to establish the rhythm and to show evidence of acute or prior myocardial infarction, pericarditis, conduction abnormalities, or left ventricular hypertrophy as a result of prolonged uncontrolled hypertension. It is known that rhythm disturbances such as atrial fibrillation can be a precipitating factor for ADHF.
A chest X-ray is needed to uncover pulmonary edema in cases of fluid overload and to show an enlarged cardiac silhouette in cases of dilated cardiomyopathy. Identification of a definitive cause or causes that precipitate the occurrence of ADHF is crucial in devising a management plan and initiating appropriate intervention. (See Table 2, p. 22.)
Immediate Management of the ADHF
Newly developed clinical practice guidelines for the management of ADHF exist, but management is still based largely on empirical evidence.12 Begin ADHF treatment in the ED with intravenous diuretics (unless contraindicated). A majority of ADHF patients will respond to diuretics alone.13 If the patient responds poorly to diuretics, the use of nesiritide in conjunction with diuretics has proven beneficial, as shown in the analysis of data from the ADHERE registry indicating that patients treated with intravenous nesiritide had a lower hospital mortality rate than patients treated with milrinone or dobutamine.14 Other options include ultrafiltration, an intervention that has been noted to reduce lengths of stay and rehospitalization rates in patients with ADHF.
Nesiritide is a recombinant form of BNP without direct inotropic effects but with venous, arterial, and coronary vasodilatory properties that can improve symptoms in ADHF.15 The recommended dosage for nesiritide is an IV bolus of 2 mcg per kg, followed by a continuous infusion of 0.01 mcg/kg/min. In the setting of hypotension with a systolic blood pressure less than 100 mm Hg, however, an initial IV bolus dose is not recommended; instead, the patient may start with a continuous infusion of 0.01 mcg/kg/min, or consider other therapies.
In hemodynamically unstable patients with a systolic blood pressure less than 90 mm Hg, or in those with evidence of end organ hypoperfusion (cardiogenic shock), inotropic support may be considered until the patient is stabilized. (See Table 3, p. 22.) Recognize that inotropic agents have adverse effects on the neurohormonal system and are not recommended routinely but may be essential for temporary stabilization. (For more on pharmacologic management of ADHF, see Table 4, p. 24.)
Subacute Management of ADHF
Once acute decompensation has been reversed and an euvolemic state has been achieved, shift therapy to a combination of three classes of medications: diuretics, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers, unless contraindicated. The benefits of these drugs have been established by evidence from numerous large-scale clinical trials.3,12
Start ACE inhibitors in all patients with heart failure due to left ventricular systolic dysfunction (unless contraindicated or if the patient is intolerant).3 Give ACE-I to patients who have experienced a recent episode of ADHF, along with diuretics to maintain sodium balance and to prevent peripheral and pulmonary edema. ACE inhibitors are contraindicated for patients who are pregnant and for those with childbearing potential, as well as for individuals with a prior history of angioedema or renal failure after receiving the drug. Instruct patients to avoid any sudden change of position, as they may experience orthostatic hypotension while taking ACE inhibitors.
Some patients are intolerant of ACE inhibitors due to a persistent cough that occurs in approximately 5% to 10% of Caucasian patients and in up to 50% of Chinese patients.16 Angiotensin receptor blockers (ARBs) are an established alternative.17 Two ARBs (candesartan and valsartan) are recommended for treatment of heart failure based on evidence from controlled clinical trials.17,18 These drugs have demonstrated a reduction in hospitalizations, and candesartan, when used as an alternative to ACE-I, has been shown to reduce mortality. Additionally, in patients with evidence of left ventricular dysfunction after myocardial infarction, valsartan provided a benefit that was not inferior to ACE inhibitors.17
Initiate beta-blockers at very low doses and gradually increased as tolerated. Monitor patients closely for symptoms of hypotension, significant weight gain, fluid retention, bradycardia, and heart block. In addition, inform patients that they may experience generalized fatigue or weakness with the initiation of beta-blockers. In a cancer patient it is difficult to differentiate between fatigue caused by the disease and the side effects of therapy. Fatigue associated with beta-blocker therapy usually resolves spontaneously within a few days. Make every effort to achieve optimum target beta-blocker dose.
An aldosterone antagonist, such as spironolactone or eplerenone, given at a daily dose of 12.5 to 25 mg in addition to standard therapy, effectively blocks the effects of aldosterone (RALES study) to achieve comprehensive neurohormonal blockade.19 When prescribing an aldosterone antagonist, especially in combination with ACE inhibitors and loop diuretics, it is important to monitor serum potassium levels because this combination can result in hyperkalemia.
Disease Management Programs
Comprehensive management of heart failure is not only limited to hospital care during an episode of ADHF. In order to prevent repeated hospitalizations, implement additional measures through formal disease management programs. These disease management programs are often directed or coordinated by advanced practice nurses who address the comprehensive care of heart failure patients with emphasis on patient education and counseling to improve patient compliance.20
The non-pharmacologic treatment strategies emphasized in disease management programs have proven effective in achieving positive outcomes. These include counseling patients on dietary management, including encouraging a two-gram sodium diet, alcohol restriction, and adequate supplementation of electrolyte loss from diuretics. Keeping a diary of the patient’s daily weight at home and bringing it to office visits will help both the patient and the clinician monitor fluid retention efficiently.
Hypotension is a common side effect from the pharmacologic therapy for heart failure. Employ comprehensive education with both patient and family to avoid unnecessary discontinuation of the medications. A systolic blood pressure of 90 mm Hg is acceptable as long as there are no associated symptoms of dizziness or syncope.
Encourage activity guidelines, including participation in exercise programs. Attendance at support group meetings will provide a venue in which patients can share common problems and concerns with others in similar situations. One to two weeks after hospital discharge, schedule an outpatient follow-up in a heart failure clinic, where heart failure education is reinforced to prevent another episode of ADHF admission.
Summary
Despite the added challenges, managing ADHF in a patient with a serious comorbidity such as cancer involves the same goals as the treatment of ADHF in any other patient. With rapid assessment and appropriate intervention, the patient is given the best possible chance of survival. TH
The authors work at the University of Texas M.D. Anderson Cancer Center, Department of Cardiology, Houston.
References
- American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Texas.: American Heart Association; 2005. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Last accessed August 20, 2006.
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004 Jan;147(1):74-78.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154-235.Epub 2005 Sep 13.
- Peacock WF IV, Emerman CL, Wynne J, for the ADHERE Scientific Advisory Committee and Investigators and the ADHERE Study Group. Early use of nesiritide in the emergency department is associated with improved outcome: an ADHERE registry analysis. Ann Emerg Med. 2004;44:S78.
- Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail. 1999 Aug;1(3):251-257.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-888.
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995 Sep;96(3):1280-1287.
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825-832.
- Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005 Jan-Feb;11(1):30-38.
- McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul;106(4):416-422.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161-167.
- Adams KF, Lindenfeld J, Arnold JMO, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10-38.
- Dec GW. Acute decompensated heart failure: the shrinking role of inotropic therapy. J Am Coll Cardiol. 2005 Jul;46(1):65-67.
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul;46(1):57-64.
- Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002 Dec;144(6):1102-1108.
- Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995 Aug;40(2):141-144.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003 Nov 13;349(20):1893-1906. Epub 2003 Nov 10.
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-776.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-717.
- Albert NM, Eastwood CA, Edwards ML. Evidence-based practice for acute decompensated heart failure. Crit Care Nurse. 2004 Dec;24(6):14-16, 18-24, 26-29; quiz 30-31.
"Testing" Your Diagnostic Skills
A72-year-old male with a history of renal transplantation underwent laparoscopic cholecystectomy for cholelithiasis. On post-operative day one, he developed worsening abdominal pain. An urgent exploratory laparotomy revealed no abnormalities. Over the next few days, he experienced worsening confusion and agitation. He developed respiratory failure, as well as a cutaneous eruption over the face, chest, abdomen, back, and upper extremities.
What is the most sensitive test for diagnosis of this eruption?
- Tzanck smear;
- Darkfield microscopy;
- Direct fluorescent antibody testing;
- Polymerase chain reaction; or
- Viral culture.
Discussion
The answer is D: polymerase chain reaction. Varicella zoster virus (VZV) was detected by rapid polymerase chain reaction from a skin biopsy specimen and from a sample of cerebrospinal fluid. It was also detected by viral culture from tracheal secretions. The patient was diagnosed with disseminated herpes zoster with VZV encephalitis and pneumonitis. He was treated with intravenous acyclovir, 1 g every eight hours for 14 days. His respiratory function and mental status gradually improved.
Herpes zoster is a painful vesicular eruption confined to the distribution of a sensory dermatome. It is caused by the reactivation of latent VZV located in the dorsal root ganglion of the affected dermatome. Triggers for reactivation include stress, trauma, fever, radiation therapy, and immunosuppression. The reactivation of VZV can also present in a disseminated fashion. Herpes zoster is classified as disseminated when more than 20 vesicular lesions occur outside of the primary and adjacent dermatomes.1 Disseminated herpes zoster can occur in immunocompetent patients, but it usually occurs in the setting of immunosuppression.2 It is seen most often in the settings of HIV, malignancy, and immunosuppressive therapy.1
The cutaneous lesions of herpes zoster begin as erythematous macules and papules that progress to vesicles. The vesicular lesions can evolve into pustules. The vesicles become crusts after seven to 10 days. Intense pain often precedes the cutaneous eruption, which can lead to misdiagnosis as myocardial infarction, pleurisy, acute surgical abdomen, or herniated intervertebral disk.2
Disseminated herpes zoster can also involve the central nervous system, lungs, liver, heart, and gastrointestinal tract. Pulmonary involvement is the most common of the possible visceral manifestations. Visceral zoster occurs in 10% of immunocompromised patients with cutaneous zoster.1
The differential diagnosis of disseminated herpes zoster includes bullous impetigo, insect bites, erythema multiforme, papular urticaria, drug eruption, contact dermatitis, and other viral exanthems such as coxsackie virus, rickettsial pox, and smallpox. The best initial test is a Tzanck smear of a scraping from the base of a lesion. The presence of multinucleated giant cells and epithelial cells with acidophilic intranuclear inclusions provides a rapid diagnosis. The most sensitive test is polymerase chain reaction of vesicular fluid, but this test is not universally available.3 The diagnosis can also be made by viral culture, but this can be difficult because VZV is a labile virus.2,3 Other diagnostic tests include direct fluorescent antibody testing, serology, and immunohistochemical stains on a skin biopsy specimen.
Treatment with intravenous acyclovir is indicated in immunocompromised patients with disseminated VZV.1 Treatment should be started within 72 hours of the onset of the vesicular eruption. Acyclovir-resistant VZV should be treated with foscarnet.2 There are also reports of physicians combining acyclovir with plasma exchange, a treatment option that may be beneficial in decreasing the viral load.4 Varicella zoster virus is transmissible to susceptible individuals by direct contact or by respiratory transmission from a patient with pulmonary involvement. Airborne and contact precautions are recommended until all of the patient’s vesicles have crusted. TH
References
- Stratman E. Visceral zoster as the presenting feature of disseminated herpes zoster. J Am Acad Dermatol. 2002;46(5):771-774.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41(1):1-14.
- Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14(1):31-36.
- Lee C, Koike M, Oshimi K, et al. Acyclovir combined with plasma exchange for disseminated varicella-zoster virus infection after bone marrow transplantation [in Japanese]. Rinsho Ketsueki. 2006;47(3):210-213.
A72-year-old male with a history of renal transplantation underwent laparoscopic cholecystectomy for cholelithiasis. On post-operative day one, he developed worsening abdominal pain. An urgent exploratory laparotomy revealed no abnormalities. Over the next few days, he experienced worsening confusion and agitation. He developed respiratory failure, as well as a cutaneous eruption over the face, chest, abdomen, back, and upper extremities.
What is the most sensitive test for diagnosis of this eruption?
- Tzanck smear;
- Darkfield microscopy;
- Direct fluorescent antibody testing;
- Polymerase chain reaction; or
- Viral culture.
Discussion
The answer is D: polymerase chain reaction. Varicella zoster virus (VZV) was detected by rapid polymerase chain reaction from a skin biopsy specimen and from a sample of cerebrospinal fluid. It was also detected by viral culture from tracheal secretions. The patient was diagnosed with disseminated herpes zoster with VZV encephalitis and pneumonitis. He was treated with intravenous acyclovir, 1 g every eight hours for 14 days. His respiratory function and mental status gradually improved.
Herpes zoster is a painful vesicular eruption confined to the distribution of a sensory dermatome. It is caused by the reactivation of latent VZV located in the dorsal root ganglion of the affected dermatome. Triggers for reactivation include stress, trauma, fever, radiation therapy, and immunosuppression. The reactivation of VZV can also present in a disseminated fashion. Herpes zoster is classified as disseminated when more than 20 vesicular lesions occur outside of the primary and adjacent dermatomes.1 Disseminated herpes zoster can occur in immunocompetent patients, but it usually occurs in the setting of immunosuppression.2 It is seen most often in the settings of HIV, malignancy, and immunosuppressive therapy.1
The cutaneous lesions of herpes zoster begin as erythematous macules and papules that progress to vesicles. The vesicular lesions can evolve into pustules. The vesicles become crusts after seven to 10 days. Intense pain often precedes the cutaneous eruption, which can lead to misdiagnosis as myocardial infarction, pleurisy, acute surgical abdomen, or herniated intervertebral disk.2
Disseminated herpes zoster can also involve the central nervous system, lungs, liver, heart, and gastrointestinal tract. Pulmonary involvement is the most common of the possible visceral manifestations. Visceral zoster occurs in 10% of immunocompromised patients with cutaneous zoster.1
The differential diagnosis of disseminated herpes zoster includes bullous impetigo, insect bites, erythema multiforme, papular urticaria, drug eruption, contact dermatitis, and other viral exanthems such as coxsackie virus, rickettsial pox, and smallpox. The best initial test is a Tzanck smear of a scraping from the base of a lesion. The presence of multinucleated giant cells and epithelial cells with acidophilic intranuclear inclusions provides a rapid diagnosis. The most sensitive test is polymerase chain reaction of vesicular fluid, but this test is not universally available.3 The diagnosis can also be made by viral culture, but this can be difficult because VZV is a labile virus.2,3 Other diagnostic tests include direct fluorescent antibody testing, serology, and immunohistochemical stains on a skin biopsy specimen.
Treatment with intravenous acyclovir is indicated in immunocompromised patients with disseminated VZV.1 Treatment should be started within 72 hours of the onset of the vesicular eruption. Acyclovir-resistant VZV should be treated with foscarnet.2 There are also reports of physicians combining acyclovir with plasma exchange, a treatment option that may be beneficial in decreasing the viral load.4 Varicella zoster virus is transmissible to susceptible individuals by direct contact or by respiratory transmission from a patient with pulmonary involvement. Airborne and contact precautions are recommended until all of the patient’s vesicles have crusted. TH
References
- Stratman E. Visceral zoster as the presenting feature of disseminated herpes zoster. J Am Acad Dermatol. 2002;46(5):771-774.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41(1):1-14.
- Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14(1):31-36.
- Lee C, Koike M, Oshimi K, et al. Acyclovir combined with plasma exchange for disseminated varicella-zoster virus infection after bone marrow transplantation [in Japanese]. Rinsho Ketsueki. 2006;47(3):210-213.
A72-year-old male with a history of renal transplantation underwent laparoscopic cholecystectomy for cholelithiasis. On post-operative day one, he developed worsening abdominal pain. An urgent exploratory laparotomy revealed no abnormalities. Over the next few days, he experienced worsening confusion and agitation. He developed respiratory failure, as well as a cutaneous eruption over the face, chest, abdomen, back, and upper extremities.
What is the most sensitive test for diagnosis of this eruption?
- Tzanck smear;
- Darkfield microscopy;
- Direct fluorescent antibody testing;
- Polymerase chain reaction; or
- Viral culture.
Discussion
The answer is D: polymerase chain reaction. Varicella zoster virus (VZV) was detected by rapid polymerase chain reaction from a skin biopsy specimen and from a sample of cerebrospinal fluid. It was also detected by viral culture from tracheal secretions. The patient was diagnosed with disseminated herpes zoster with VZV encephalitis and pneumonitis. He was treated with intravenous acyclovir, 1 g every eight hours for 14 days. His respiratory function and mental status gradually improved.
Herpes zoster is a painful vesicular eruption confined to the distribution of a sensory dermatome. It is caused by the reactivation of latent VZV located in the dorsal root ganglion of the affected dermatome. Triggers for reactivation include stress, trauma, fever, radiation therapy, and immunosuppression. The reactivation of VZV can also present in a disseminated fashion. Herpes zoster is classified as disseminated when more than 20 vesicular lesions occur outside of the primary and adjacent dermatomes.1 Disseminated herpes zoster can occur in immunocompetent patients, but it usually occurs in the setting of immunosuppression.2 It is seen most often in the settings of HIV, malignancy, and immunosuppressive therapy.1
The cutaneous lesions of herpes zoster begin as erythematous macules and papules that progress to vesicles. The vesicular lesions can evolve into pustules. The vesicles become crusts after seven to 10 days. Intense pain often precedes the cutaneous eruption, which can lead to misdiagnosis as myocardial infarction, pleurisy, acute surgical abdomen, or herniated intervertebral disk.2
Disseminated herpes zoster can also involve the central nervous system, lungs, liver, heart, and gastrointestinal tract. Pulmonary involvement is the most common of the possible visceral manifestations. Visceral zoster occurs in 10% of immunocompromised patients with cutaneous zoster.1
The differential diagnosis of disseminated herpes zoster includes bullous impetigo, insect bites, erythema multiforme, papular urticaria, drug eruption, contact dermatitis, and other viral exanthems such as coxsackie virus, rickettsial pox, and smallpox. The best initial test is a Tzanck smear of a scraping from the base of a lesion. The presence of multinucleated giant cells and epithelial cells with acidophilic intranuclear inclusions provides a rapid diagnosis. The most sensitive test is polymerase chain reaction of vesicular fluid, but this test is not universally available.3 The diagnosis can also be made by viral culture, but this can be difficult because VZV is a labile virus.2,3 Other diagnostic tests include direct fluorescent antibody testing, serology, and immunohistochemical stains on a skin biopsy specimen.
Treatment with intravenous acyclovir is indicated in immunocompromised patients with disseminated VZV.1 Treatment should be started within 72 hours of the onset of the vesicular eruption. Acyclovir-resistant VZV should be treated with foscarnet.2 There are also reports of physicians combining acyclovir with plasma exchange, a treatment option that may be beneficial in decreasing the viral load.4 Varicella zoster virus is transmissible to susceptible individuals by direct contact or by respiratory transmission from a patient with pulmonary involvement. Airborne and contact precautions are recommended until all of the patient’s vesicles have crusted. TH
References
- Stratman E. Visceral zoster as the presenting feature of disseminated herpes zoster. J Am Acad Dermatol. 2002;46(5):771-774.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41(1):1-14.
- Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14(1):31-36.
- Lee C, Koike M, Oshimi K, et al. Acyclovir combined with plasma exchange for disseminated varicella-zoster virus infection after bone marrow transplantation [in Japanese]. Rinsho Ketsueki. 2006;47(3):210-213.
Small Gains Made by Gainsharing
While pay-for-performance has become one of the hottest trends in healthcare, another program that would tie payment to performance is starting to get some notice. Gainsharing is on the horizon for hospitals and the physicians who work for them, but the advances made in this savings-sharing model are questionable.
Gainsharing Defined
What is gainsharing? It has to do with the sharing of gains—or of costs saved, to be more precise. Under a gainsharing model, a hospital will select specific best practices, including standard policies, procedures, and/or protocols that will improve quality of care and reduce financial costs. Any reduction in costs that results from physicians following these best practices is documented by the hospital over a specified period. After monitoring and noting that physicians met the predetermined benchmarks for quality of care, the hospital then pays a cash bonus to those who contributed to the cost reduction.
Gainsharing can take various forms, but generally the practice applies to services provided within a single clinical specialty such as oncology or cardiac surgery. “Gainsharing could affect anyone who cares for patients in a hospital if it became legal,” says Ron Greeno, MD, FCCP, chief medical officer, Cogent Healthcare, Irvine, Calif., “but hospitalists are the perfect group of doctors to take on projects like this.”
The most recent gainsharing programs have focused on the use of pre-approved medical devices, equipment, and supplies. For example, a hospital might recommend that physicians use less costly items with the same level of effectiveness, such as a knee-high sequential compression device rather than a thigh-high device.
How much money is involved in gainsharing? Of course, it depends on the program. “Gainsharing deals could include significant money depending on how they are structured,” explains Dr. Greeno, “if you did something that results in enough quality benefit.”
Example: A hospital asked a physician group to give an appropriate antibiotic to patients as soon as possible upon admission and was consequently able to show that they avoided a prolonged stay for five of those patients and that this saved the hospital $1 million in costs. “If the hospital gave 50% of that savings to 20 physicians who helped meet that goal, why would anyone want to regulate against that?” asks Dr. Greeno.
A Brief History of Gainsharing
There is a good reason you may not have heard much about gainsharing; the practice was banned in 1999 by the federal Office of Inspector General (OIG). The OIG was concerned that gainsharing could limit patient care and might lead to physicians “cherry picking” patients who are healthier, while sending seriously ill patients to a different hospital.
Only in September 2005 did the OIG carry out a half-hearted reversal, providing advisory opinions that allow pre-approved arrangements between individual hospitals and physician groups—as long as appropriate safeguards are adopted to protect against abuse.
“This was a very small change,” says Dr. Greeno. “Their approval is highly limited. They have basically agreed to very specific, very short trials of gainsharing, which they will approve one at a time.”
Dr. Greeno is frustrated by the baby step taken by the federal government. “The OIG equates gainsharing with denial of care,” he says. “Their limits are preventing a tremendous opportunity for hospitals and physicians to partner to provide quality care; they’re saying we can’t provide the right incentive scheme.”
The point that Dr. Greeno finds most frustrating is the vast difference of opinion on gainsharing versus another payment trend. “The irony is that all gainsharing is pay-for-performance,” says Dr. Greeno. “The difference is that instead of Medicare paying, it’s the individual hospital paying for performance. Even people who really understand healthcare haven’t connected the dots. They’re pushing for pay-for-performance, but telling hospitals they can’t do essentially the same thing. The hospital industry is developing a strategy to point that out to Capitol Hill, and in my opinion, SHM should develop a more formal approach to do the same.”
Current State of Gainsharing
There have been a few gainsharing programs that were approved by the OIG. “There are a couple of [pilot programs] that private hospitals have done,” says Dr. Greeno. “All the projects so far have targeted medical devices. One New Jersey hospital focused on the use of defibrillators. They spent a lot of money setting up the project, which only lasted one year. As I said, these [approved projects] are very limited.”
Gainsharing programs that focus on medical devices are simple because cost savings are easy to track; however, gainsharing could be built around quality indicators that are found in pay-for-performance programs. “Cutting costs is not the only way to improve quality,” says Dr. Greeno. “That’s not how you get the biggest bang for your buck. That comes when someone will invest in capabilities and processes that target the 30% of costs spent on each patient in the hospital that is waste.”
Pilot Programs Planned
Following the OIG reversal, Congress passed the Deficit Reduction Omnibus Reconciliation Act of 2005, which included funding for a gainsharing demonstration project. The Centers for Medicare and Medicaid Services (CMS) will establish six gainsharing pilot programs, including two in rural settings, by January 1, 2007. The bill states that these demonstration programs are intended to “test and evaluate methodologies and arrangements between hospitals and physicians designed to govern the utilization of inpatient hospital resources and physician work to improve quality and efficiency of care provided to Medicare beneficiaries.”
You can review details on the gainsharing demonstration on the CMS Web site at www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=3&sortOrder=ascending&itemID=CMS1186805.
A second new CMS demonstration program will go beyond the traditional concept of gainsharing. Under the program, known as the Physician-Hospital Collaboration Demonstration (PHCD), hospitals would be allowed to pay physicians a portion of the savings they reap from specific quality improvement and efficiency initiatives. This project, in particular, could eventually have direct implications for hospital medicine.
The SHM Public Policy Committee is urging members to consider soliciting involvement with PHCD, and possibly partnering with other physician groups and affiliated hospitals to compete for inclusion. You can find more on the PHCD online at www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none&filterByDID=99&sortByDID=3&sortOrder=ascending&itemID=CMS1186653. If you decide to submit a proposal to CMS for the PHCD, please let Joe Miller at SHM ([email protected]) know.
The Future of Gainsharing
Will the CMS gainsharing pilot programs lead to widespread trials? “Who knows?” asks Dr. Greeno. “It will be a slow crawl toward some type of application, but it will likely be too limited when it does happen.”
In the meantime, Dr. Greeno is urging SHM and individual physicians to keep pushing for some real advances in gainsharing. “Our best chance is to work with the hospital community to connect the dots for our federal lawmakers,” he says. “We want to work to allow hospitals to reward doctors for quality performance.” TH
Jane Jerrard regularly writes “Public Policy.”
While pay-for-performance has become one of the hottest trends in healthcare, another program that would tie payment to performance is starting to get some notice. Gainsharing is on the horizon for hospitals and the physicians who work for them, but the advances made in this savings-sharing model are questionable.
Gainsharing Defined
What is gainsharing? It has to do with the sharing of gains—or of costs saved, to be more precise. Under a gainsharing model, a hospital will select specific best practices, including standard policies, procedures, and/or protocols that will improve quality of care and reduce financial costs. Any reduction in costs that results from physicians following these best practices is documented by the hospital over a specified period. After monitoring and noting that physicians met the predetermined benchmarks for quality of care, the hospital then pays a cash bonus to those who contributed to the cost reduction.
Gainsharing can take various forms, but generally the practice applies to services provided within a single clinical specialty such as oncology or cardiac surgery. “Gainsharing could affect anyone who cares for patients in a hospital if it became legal,” says Ron Greeno, MD, FCCP, chief medical officer, Cogent Healthcare, Irvine, Calif., “but hospitalists are the perfect group of doctors to take on projects like this.”
The most recent gainsharing programs have focused on the use of pre-approved medical devices, equipment, and supplies. For example, a hospital might recommend that physicians use less costly items with the same level of effectiveness, such as a knee-high sequential compression device rather than a thigh-high device.
How much money is involved in gainsharing? Of course, it depends on the program. “Gainsharing deals could include significant money depending on how they are structured,” explains Dr. Greeno, “if you did something that results in enough quality benefit.”
Example: A hospital asked a physician group to give an appropriate antibiotic to patients as soon as possible upon admission and was consequently able to show that they avoided a prolonged stay for five of those patients and that this saved the hospital $1 million in costs. “If the hospital gave 50% of that savings to 20 physicians who helped meet that goal, why would anyone want to regulate against that?” asks Dr. Greeno.
A Brief History of Gainsharing
There is a good reason you may not have heard much about gainsharing; the practice was banned in 1999 by the federal Office of Inspector General (OIG). The OIG was concerned that gainsharing could limit patient care and might lead to physicians “cherry picking” patients who are healthier, while sending seriously ill patients to a different hospital.
Only in September 2005 did the OIG carry out a half-hearted reversal, providing advisory opinions that allow pre-approved arrangements between individual hospitals and physician groups—as long as appropriate safeguards are adopted to protect against abuse.
“This was a very small change,” says Dr. Greeno. “Their approval is highly limited. They have basically agreed to very specific, very short trials of gainsharing, which they will approve one at a time.”
Dr. Greeno is frustrated by the baby step taken by the federal government. “The OIG equates gainsharing with denial of care,” he says. “Their limits are preventing a tremendous opportunity for hospitals and physicians to partner to provide quality care; they’re saying we can’t provide the right incentive scheme.”
The point that Dr. Greeno finds most frustrating is the vast difference of opinion on gainsharing versus another payment trend. “The irony is that all gainsharing is pay-for-performance,” says Dr. Greeno. “The difference is that instead of Medicare paying, it’s the individual hospital paying for performance. Even people who really understand healthcare haven’t connected the dots. They’re pushing for pay-for-performance, but telling hospitals they can’t do essentially the same thing. The hospital industry is developing a strategy to point that out to Capitol Hill, and in my opinion, SHM should develop a more formal approach to do the same.”
Current State of Gainsharing
There have been a few gainsharing programs that were approved by the OIG. “There are a couple of [pilot programs] that private hospitals have done,” says Dr. Greeno. “All the projects so far have targeted medical devices. One New Jersey hospital focused on the use of defibrillators. They spent a lot of money setting up the project, which only lasted one year. As I said, these [approved projects] are very limited.”
Gainsharing programs that focus on medical devices are simple because cost savings are easy to track; however, gainsharing could be built around quality indicators that are found in pay-for-performance programs. “Cutting costs is not the only way to improve quality,” says Dr. Greeno. “That’s not how you get the biggest bang for your buck. That comes when someone will invest in capabilities and processes that target the 30% of costs spent on each patient in the hospital that is waste.”
Pilot Programs Planned
Following the OIG reversal, Congress passed the Deficit Reduction Omnibus Reconciliation Act of 2005, which included funding for a gainsharing demonstration project. The Centers for Medicare and Medicaid Services (CMS) will establish six gainsharing pilot programs, including two in rural settings, by January 1, 2007. The bill states that these demonstration programs are intended to “test and evaluate methodologies and arrangements between hospitals and physicians designed to govern the utilization of inpatient hospital resources and physician work to improve quality and efficiency of care provided to Medicare beneficiaries.”
You can review details on the gainsharing demonstration on the CMS Web site at www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=3&sortOrder=ascending&itemID=CMS1186805.
A second new CMS demonstration program will go beyond the traditional concept of gainsharing. Under the program, known as the Physician-Hospital Collaboration Demonstration (PHCD), hospitals would be allowed to pay physicians a portion of the savings they reap from specific quality improvement and efficiency initiatives. This project, in particular, could eventually have direct implications for hospital medicine.
The SHM Public Policy Committee is urging members to consider soliciting involvement with PHCD, and possibly partnering with other physician groups and affiliated hospitals to compete for inclusion. You can find more on the PHCD online at www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none&filterByDID=99&sortByDID=3&sortOrder=ascending&itemID=CMS1186653. If you decide to submit a proposal to CMS for the PHCD, please let Joe Miller at SHM ([email protected]) know.
The Future of Gainsharing
Will the CMS gainsharing pilot programs lead to widespread trials? “Who knows?” asks Dr. Greeno. “It will be a slow crawl toward some type of application, but it will likely be too limited when it does happen.”
In the meantime, Dr. Greeno is urging SHM and individual physicians to keep pushing for some real advances in gainsharing. “Our best chance is to work with the hospital community to connect the dots for our federal lawmakers,” he says. “We want to work to allow hospitals to reward doctors for quality performance.” TH
Jane Jerrard regularly writes “Public Policy.”
While pay-for-performance has become one of the hottest trends in healthcare, another program that would tie payment to performance is starting to get some notice. Gainsharing is on the horizon for hospitals and the physicians who work for them, but the advances made in this savings-sharing model are questionable.
Gainsharing Defined
What is gainsharing? It has to do with the sharing of gains—or of costs saved, to be more precise. Under a gainsharing model, a hospital will select specific best practices, including standard policies, procedures, and/or protocols that will improve quality of care and reduce financial costs. Any reduction in costs that results from physicians following these best practices is documented by the hospital over a specified period. After monitoring and noting that physicians met the predetermined benchmarks for quality of care, the hospital then pays a cash bonus to those who contributed to the cost reduction.
Gainsharing can take various forms, but generally the practice applies to services provided within a single clinical specialty such as oncology or cardiac surgery. “Gainsharing could affect anyone who cares for patients in a hospital if it became legal,” says Ron Greeno, MD, FCCP, chief medical officer, Cogent Healthcare, Irvine, Calif., “but hospitalists are the perfect group of doctors to take on projects like this.”
The most recent gainsharing programs have focused on the use of pre-approved medical devices, equipment, and supplies. For example, a hospital might recommend that physicians use less costly items with the same level of effectiveness, such as a knee-high sequential compression device rather than a thigh-high device.
How much money is involved in gainsharing? Of course, it depends on the program. “Gainsharing deals could include significant money depending on how they are structured,” explains Dr. Greeno, “if you did something that results in enough quality benefit.”
Example: A hospital asked a physician group to give an appropriate antibiotic to patients as soon as possible upon admission and was consequently able to show that they avoided a prolonged stay for five of those patients and that this saved the hospital $1 million in costs. “If the hospital gave 50% of that savings to 20 physicians who helped meet that goal, why would anyone want to regulate against that?” asks Dr. Greeno.
A Brief History of Gainsharing
There is a good reason you may not have heard much about gainsharing; the practice was banned in 1999 by the federal Office of Inspector General (OIG). The OIG was concerned that gainsharing could limit patient care and might lead to physicians “cherry picking” patients who are healthier, while sending seriously ill patients to a different hospital.
Only in September 2005 did the OIG carry out a half-hearted reversal, providing advisory opinions that allow pre-approved arrangements between individual hospitals and physician groups—as long as appropriate safeguards are adopted to protect against abuse.
“This was a very small change,” says Dr. Greeno. “Their approval is highly limited. They have basically agreed to very specific, very short trials of gainsharing, which they will approve one at a time.”
Dr. Greeno is frustrated by the baby step taken by the federal government. “The OIG equates gainsharing with denial of care,” he says. “Their limits are preventing a tremendous opportunity for hospitals and physicians to partner to provide quality care; they’re saying we can’t provide the right incentive scheme.”
The point that Dr. Greeno finds most frustrating is the vast difference of opinion on gainsharing versus another payment trend. “The irony is that all gainsharing is pay-for-performance,” says Dr. Greeno. “The difference is that instead of Medicare paying, it’s the individual hospital paying for performance. Even people who really understand healthcare haven’t connected the dots. They’re pushing for pay-for-performance, but telling hospitals they can’t do essentially the same thing. The hospital industry is developing a strategy to point that out to Capitol Hill, and in my opinion, SHM should develop a more formal approach to do the same.”
Current State of Gainsharing
There have been a few gainsharing programs that were approved by the OIG. “There are a couple of [pilot programs] that private hospitals have done,” says Dr. Greeno. “All the projects so far have targeted medical devices. One New Jersey hospital focused on the use of defibrillators. They spent a lot of money setting up the project, which only lasted one year. As I said, these [approved projects] are very limited.”
Gainsharing programs that focus on medical devices are simple because cost savings are easy to track; however, gainsharing could be built around quality indicators that are found in pay-for-performance programs. “Cutting costs is not the only way to improve quality,” says Dr. Greeno. “That’s not how you get the biggest bang for your buck. That comes when someone will invest in capabilities and processes that target the 30% of costs spent on each patient in the hospital that is waste.”
Pilot Programs Planned
Following the OIG reversal, Congress passed the Deficit Reduction Omnibus Reconciliation Act of 2005, which included funding for a gainsharing demonstration project. The Centers for Medicare and Medicaid Services (CMS) will establish six gainsharing pilot programs, including two in rural settings, by January 1, 2007. The bill states that these demonstration programs are intended to “test and evaluate methodologies and arrangements between hospitals and physicians designed to govern the utilization of inpatient hospital resources and physician work to improve quality and efficiency of care provided to Medicare beneficiaries.”
You can review details on the gainsharing demonstration on the CMS Web site at www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=3&sortOrder=ascending&itemID=CMS1186805.
A second new CMS demonstration program will go beyond the traditional concept of gainsharing. Under the program, known as the Physician-Hospital Collaboration Demonstration (PHCD), hospitals would be allowed to pay physicians a portion of the savings they reap from specific quality improvement and efficiency initiatives. This project, in particular, could eventually have direct implications for hospital medicine.
The SHM Public Policy Committee is urging members to consider soliciting involvement with PHCD, and possibly partnering with other physician groups and affiliated hospitals to compete for inclusion. You can find more on the PHCD online at www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none&filterByDID=99&sortByDID=3&sortOrder=ascending&itemID=CMS1186653. If you decide to submit a proposal to CMS for the PHCD, please let Joe Miller at SHM ([email protected]) know.
The Future of Gainsharing
Will the CMS gainsharing pilot programs lead to widespread trials? “Who knows?” asks Dr. Greeno. “It will be a slow crawl toward some type of application, but it will likely be too limited when it does happen.”
In the meantime, Dr. Greeno is urging SHM and individual physicians to keep pushing for some real advances in gainsharing. “Our best chance is to work with the hospital community to connect the dots for our federal lawmakers,” he says. “We want to work to allow hospitals to reward doctors for quality performance.” TH
Jane Jerrard regularly writes “Public Policy.”
Master Meetings
Meetings are a fact of life. They play an integral role in how our departments, committees, and hospitals make decisions, implement new projects and processes, convey information, and, unfortunately, take up our precious time.
Perhaps you’ve been thrust into the role of leading meetings—as the head of a quality initiative task force, or as facilitator of a brainstorming session on a quality of care issue—or maybe you’re just beginning to face that possibility. How can you hone your skills to ensure that your meetings are on track, fulfill their goals, and waste as little time as possible?
—Roger Schenke
Watch and Learn
Russell L. Holman, MD, senior vice president and national medical director, Cogent Healthcare, and president-elect, SHM, put a lot of effort into mastering his meeting leadership skills, beginning early in his career. “I was working in an organization that was meeting-rich,” recalls Dr. Holman. “It was important to me to learn that way of getting things done.”
He began with the simple act of watching how others led meetings, noting what some did right and others did wrong. “A lot of my work has been through observation, and through seeking out mentoring,” he says. It helps to have spent time sitting in many meetings before you become a leader. “You can see how things are done. When you observe someone who does well at leading a meeting, ask to sit down with them and ask how they set out to accomplish the goal of the meeting.”
Learn as You Go
As a hospitalist, you are likely to assume the role of meeting leader. You can then concentrate on refining your skills with each meeting. “Seek feedback of the senior people participating, as well as general participants,” suggests Dr. Holman. “Do a bit of analysis if there were conflicts in the meeting, or if you feel the group is not making progress.”
Tip: If you’re a clinical hospitalist without hierarchical authority and have been asked to lead a project or task force, use Dr. Holman’s “transfer of authority” method. Ask someone with recognizable importance, such as the hospital CEO or a department chair, to stop in your group’s first meeting for a few minutes. While they are there, they should offer their resources for any necessary support, and voice their confidence in you as the leader and in the group in general. This simple visit will transfer their authority to you as the group leader.
Structure Is Key
Roger Schenke, executive vice president of the American College of Physician Executives (ACPE), believes that when the right structure is imposed any meeting is easy to run—without personality conflicts or wasted time. Schenke, who teaches an introductory ACPE course on management that includes a section on meetings, explains, “A structure approach [to meetings] takes proven techniques and superimposes them on any group. Once you start using structure, you don’t have to manipulate the meeting; people will simply follow the structure. They’ll feel that they’re part of a process.”
Schenke outlines an ideal meeting scenario, which can take place as a single, long meeting within a pre-set room, or be broken into separate, sequential meetings:
- Briefing. This should last no more than 30 minutes. A single speaker or a select panel provides information to participants and then allows questions. “Everyone knows the rules for this type of meeting,” adds Schenke.
- Problem-solving or problem-identification meeting. “The room should be set up with small tables and a flip chart for each. Small groups are assigned a specific task,” says Schenke, such as solving the hospital’s nursing shortage. A committee or task force should be broken into small groups so “you can’t go above seven people when you want people to interact,” says Schenke. “Above that, and little subgroups and factions form.”
- Parliamentary meetings should take place at a conference table. “Everyone eligible to mandate policy hears recommendations from other groups, such as the problem-solving groups,” says Schenke.
Obviously, each of these meetings has a unique structure with a unique room setting. “Structure determines or controls behavior,” says Schenke. “When people are put in these situations, they understand the rules. The key is not preparation; all it takes is letting the group know the purpose of the meeting.”
Practice, Practice, Practice
There are many theories and styles regarding running successful meetings, and you’ll find a wealth of information on how to become a better meeting leader. Just as meetings are a fact of life, it’s also true that you can improve your facilitation skills with each new meeting you lead. TH
Jane Jerrard writes “Career Management” monthly for The Hospitalist.
Meetings are a fact of life. They play an integral role in how our departments, committees, and hospitals make decisions, implement new projects and processes, convey information, and, unfortunately, take up our precious time.
Perhaps you’ve been thrust into the role of leading meetings—as the head of a quality initiative task force, or as facilitator of a brainstorming session on a quality of care issue—or maybe you’re just beginning to face that possibility. How can you hone your skills to ensure that your meetings are on track, fulfill their goals, and waste as little time as possible?
—Roger Schenke
Watch and Learn
Russell L. Holman, MD, senior vice president and national medical director, Cogent Healthcare, and president-elect, SHM, put a lot of effort into mastering his meeting leadership skills, beginning early in his career. “I was working in an organization that was meeting-rich,” recalls Dr. Holman. “It was important to me to learn that way of getting things done.”
He began with the simple act of watching how others led meetings, noting what some did right and others did wrong. “A lot of my work has been through observation, and through seeking out mentoring,” he says. It helps to have spent time sitting in many meetings before you become a leader. “You can see how things are done. When you observe someone who does well at leading a meeting, ask to sit down with them and ask how they set out to accomplish the goal of the meeting.”
Learn as You Go
As a hospitalist, you are likely to assume the role of meeting leader. You can then concentrate on refining your skills with each meeting. “Seek feedback of the senior people participating, as well as general participants,” suggests Dr. Holman. “Do a bit of analysis if there were conflicts in the meeting, or if you feel the group is not making progress.”
Tip: If you’re a clinical hospitalist without hierarchical authority and have been asked to lead a project or task force, use Dr. Holman’s “transfer of authority” method. Ask someone with recognizable importance, such as the hospital CEO or a department chair, to stop in your group’s first meeting for a few minutes. While they are there, they should offer their resources for any necessary support, and voice their confidence in you as the leader and in the group in general. This simple visit will transfer their authority to you as the group leader.
Structure Is Key
Roger Schenke, executive vice president of the American College of Physician Executives (ACPE), believes that when the right structure is imposed any meeting is easy to run—without personality conflicts or wasted time. Schenke, who teaches an introductory ACPE course on management that includes a section on meetings, explains, “A structure approach [to meetings] takes proven techniques and superimposes them on any group. Once you start using structure, you don’t have to manipulate the meeting; people will simply follow the structure. They’ll feel that they’re part of a process.”
Schenke outlines an ideal meeting scenario, which can take place as a single, long meeting within a pre-set room, or be broken into separate, sequential meetings:
- Briefing. This should last no more than 30 minutes. A single speaker or a select panel provides information to participants and then allows questions. “Everyone knows the rules for this type of meeting,” adds Schenke.
- Problem-solving or problem-identification meeting. “The room should be set up with small tables and a flip chart for each. Small groups are assigned a specific task,” says Schenke, such as solving the hospital’s nursing shortage. A committee or task force should be broken into small groups so “you can’t go above seven people when you want people to interact,” says Schenke. “Above that, and little subgroups and factions form.”
- Parliamentary meetings should take place at a conference table. “Everyone eligible to mandate policy hears recommendations from other groups, such as the problem-solving groups,” says Schenke.
Obviously, each of these meetings has a unique structure with a unique room setting. “Structure determines or controls behavior,” says Schenke. “When people are put in these situations, they understand the rules. The key is not preparation; all it takes is letting the group know the purpose of the meeting.”
Practice, Practice, Practice
There are many theories and styles regarding running successful meetings, and you’ll find a wealth of information on how to become a better meeting leader. Just as meetings are a fact of life, it’s also true that you can improve your facilitation skills with each new meeting you lead. TH
Jane Jerrard writes “Career Management” monthly for The Hospitalist.
Meetings are a fact of life. They play an integral role in how our departments, committees, and hospitals make decisions, implement new projects and processes, convey information, and, unfortunately, take up our precious time.
Perhaps you’ve been thrust into the role of leading meetings—as the head of a quality initiative task force, or as facilitator of a brainstorming session on a quality of care issue—or maybe you’re just beginning to face that possibility. How can you hone your skills to ensure that your meetings are on track, fulfill their goals, and waste as little time as possible?
—Roger Schenke
Watch and Learn
Russell L. Holman, MD, senior vice president and national medical director, Cogent Healthcare, and president-elect, SHM, put a lot of effort into mastering his meeting leadership skills, beginning early in his career. “I was working in an organization that was meeting-rich,” recalls Dr. Holman. “It was important to me to learn that way of getting things done.”
He began with the simple act of watching how others led meetings, noting what some did right and others did wrong. “A lot of my work has been through observation, and through seeking out mentoring,” he says. It helps to have spent time sitting in many meetings before you become a leader. “You can see how things are done. When you observe someone who does well at leading a meeting, ask to sit down with them and ask how they set out to accomplish the goal of the meeting.”
Learn as You Go
As a hospitalist, you are likely to assume the role of meeting leader. You can then concentrate on refining your skills with each meeting. “Seek feedback of the senior people participating, as well as general participants,” suggests Dr. Holman. “Do a bit of analysis if there were conflicts in the meeting, or if you feel the group is not making progress.”
Tip: If you’re a clinical hospitalist without hierarchical authority and have been asked to lead a project or task force, use Dr. Holman’s “transfer of authority” method. Ask someone with recognizable importance, such as the hospital CEO or a department chair, to stop in your group’s first meeting for a few minutes. While they are there, they should offer their resources for any necessary support, and voice their confidence in you as the leader and in the group in general. This simple visit will transfer their authority to you as the group leader.
Structure Is Key
Roger Schenke, executive vice president of the American College of Physician Executives (ACPE), believes that when the right structure is imposed any meeting is easy to run—without personality conflicts or wasted time. Schenke, who teaches an introductory ACPE course on management that includes a section on meetings, explains, “A structure approach [to meetings] takes proven techniques and superimposes them on any group. Once you start using structure, you don’t have to manipulate the meeting; people will simply follow the structure. They’ll feel that they’re part of a process.”
Schenke outlines an ideal meeting scenario, which can take place as a single, long meeting within a pre-set room, or be broken into separate, sequential meetings:
- Briefing. This should last no more than 30 minutes. A single speaker or a select panel provides information to participants and then allows questions. “Everyone knows the rules for this type of meeting,” adds Schenke.
- Problem-solving or problem-identification meeting. “The room should be set up with small tables and a flip chart for each. Small groups are assigned a specific task,” says Schenke, such as solving the hospital’s nursing shortage. A committee or task force should be broken into small groups so “you can’t go above seven people when you want people to interact,” says Schenke. “Above that, and little subgroups and factions form.”
- Parliamentary meetings should take place at a conference table. “Everyone eligible to mandate policy hears recommendations from other groups, such as the problem-solving groups,” says Schenke.
Obviously, each of these meetings has a unique structure with a unique room setting. “Structure determines or controls behavior,” says Schenke. “When people are put in these situations, they understand the rules. The key is not preparation; all it takes is letting the group know the purpose of the meeting.”
Practice, Practice, Practice
There are many theories and styles regarding running successful meetings, and you’ll find a wealth of information on how to become a better meeting leader. Just as meetings are a fact of life, it’s also true that you can improve your facilitation skills with each new meeting you lead. TH
Jane Jerrard writes “Career Management” monthly for The Hospitalist.
World Hospice and Palliative Care Day
October 7, 2006, is World Hospice and Palliative Care Day. This day is dedicated to raising the visibility of palliative care within the global community and to providing opportunities to support hospice and palliative care in the form of a unified day of action.
According to official organizers, the event’s theme is “Access to care for all—highlighting the fact that everyone has a right to high-quality end-of-life care, but that more needs to be done to enable everyone to access it.” In creating World Hospice and Palliative Care Day 2006, the event’s organizers aim to raise awareness and understanding of the needs of those living with a terminal diagnosis, as well as the needs of their families. Other goals include calling attention to the need for increasing hospice and palliative care availability throughout the world and raising funds to be used in supporting these services.
Like the first World Hospice and Palliative Care Day, held in 2005, this event will be carried out in conjunction with Voices for Hospices, a global music effort that supports concerts held around the world to raise awareness of this important topic. The Voices for Hospices group is one of many supporters of this cause.
—World Hospice and Palliative Care Day 2006 Web site, Key Messages, page 1.
More than 1,000 events took place on World Hospice and Palliative Care Day 2005, and 74 countries supported the activities. Included in the 2005 event were a cycle rally in Nepal; art exhibitions in Australia, Hong Kong, and Austria; and palliative care conferences in Lithuania and Belarus. In addition, thousands of people from around the world signed a global petition calling for better quality care for people afflicted by terminal illness.
Hospitalists are asked frequently to lead and participate in initiatives meant to improve the identification and treatment of patients and families in need of palliative care. It is common knowledge that traditional medical training tends to focus on the efforts that must be made to cure and prevent illness. There are times when the first priority must be to look for a cure at all costs; however, it must be acknowledged that there are also times when the treatment of a patient’s symptoms should be looked upon as just as important. Conventional medical training frequently does not provide the tools needed to offer the best care for patients and their families when the latter goal becomes the higher priority.
This is why support of initiatives like World Hospice and Palliative Care Day can offer such value to the global community. Events like this one promote awareness of an important topic. For information and ideas on how to get involved in this or in future events, please consult www.worldday.org. Access the Web site’s “Get Involved” page for ideas on how to offer support. Suggested activities include campaigning, creating links and partnerships, and producing materials that will raise awareness.
On the “PR & Press” page of the Web site, in the “Key Messages 2006” section, the following question was posed: “What kinds of issues in general terms does World Hospice and Palliative Care Day hope to raise awareness of year on year?” One of the well-stated answers: “First and foremost we hope the Day helps to increase understanding of hospice and palliative care and how it supports those facing the end of life … . It’s not about ‘helping someone die’ but instead about helping someone to live as comfortably as possible with their illness. It’s about seeing them as [a] living person, not a dying patient. It’s supporting those closest to them and adding life to days, whether or not days can be added to lives.”
Smart Tools for QI Initiatives
SHM’s Quality Improvement Resource Rooms support hospitalists as QI leaders
The role and recognition of the hospitalist has evolved tremendously in the past 10 years, and hospitalists continue to be called upon to lead at their institutions, particularly in quality improvement initiatives. Based on their unique role within the hospital system (a job that requires interaction with many levels of hospital staff) hospitalists are clearly positioned to lead such efforts. As part of SHM’s dedication to promote the highest quality of care for the hospitalized patient, SHM’s Resource Rooms provide members and non-members alike access to information that will aid their knowledge in quality improvement around specific disease states. Currently, SHM provides four Resource Rooms: Venous Thromboembolism (VTE), Stroke, Antimicrobial Resistance (ABX), and Heart Failure. Two additional rooms, Geriatrics and Glycemic Control, will launch this fall.
Quality improvement for the patient will be successful if a systems-based multidisciplinary collaboration within the hospital is improved. Hospitalists are leading this challenging yet exciting opportunity to change the face of healthcare. It has been noted that medical school and residency training have failed to prepare the hospitalist for this leadership role. To this end, SHM provides users of the Resource Rooms with information describing how a specific disease state affects the population and explains why a hospitalist should act in initiating change, as well as what key knowledge, skills, and attitudes the hospitalist should possess. The user is offered information regarding didactic and bedside teaching, patient education, and opportunities for continuing medical education. These resources are useful for the novice as well as for the advanced hospitalist leader. Readers can also apply the concepts of these general mechanisms to any disease state they are seeking to improve at their institutions.
The QI workbook within each of the Resource Rooms is the most important feature and serves as a field guide to implementing a quality improvement program. The workbook includes the following aids:
- Essential first steps: garnering institutional support, assembling a team, developing team rules, and understanding the framework for improvement;
- Conducting an in-depth analysis of current processes and failures;
- Collecting data and devising metrics to assess the impact of your QI initiative;
- Moving from problems to solutions; and
- Continuing to improve: monitoring and learning from the process, as well as holding the gains and spreading your improvement
Other important resources that are common to all of the rooms and will aid in leading the QI efforts of the hospitalist are the educational features. Complex problems need multidisciplinary solutions. The “Improve” and “Educate” areas of the Resource Rooms include information that allow the hospitalist to teach and be taught. In the “Improve” section, a user can find QI Theory slide sets on the foundations of quality improvement initiatives and core measures on specific disease states.
Didactic sessions and teaching tools, as well as professional development, including core competencies and CME opportunities, are all present in the “Educate” feature of each room. For example, in the Heart Failure Resource Room, a didactic session slide set concerning the management of heart failure for hospitalized patients is provided. The slide set includes a heart failure overview that describes the epidemiology, etiologies, and objectives surrounding the management of acute congestive heart failure.
Evidence, improvement, and education tools designed to enhance inpatient outcomes can help the hospitalist develop and lead initiatives that can create a more cost-efficient approach to the treatment of hospitalized patients, while at the same time improving patient outcomes. SHM’s Quality Improvement Resource Rooms provide a compendium of resources to support the hospitalist who is embarking on this enormous task.
September Leadership Academy
New Level II a great success
The recently completed 4th SHM Leadership Academy was a true success in every sense of the word. The event was nearly a sellout, with 160 hospital medicine leaders arriving in Nashville, Tenn., in September to learn—from nationally respected leaders—tangible skills that they could take back to institute in their own practices.
The Leadership Academy Level I was designed to provide leaders in hospital medicine with the skills and resources required to lead and manage programs successfully both now and in the future. Small group sessions gave attendees a chance to interact with faculty and to share personal experiences from their own institutions. Nationally recognized speaker Jack Silversin, DMD, DrPH, presented his infamous broken squares activity, which kept the group energized and working together creatively to learn about effective communication. This course allowed attendees to evaluate personal leadership strengths and weaknesses and then apply them to everyday leadership and management challenges.
Another highlight was the self-evaluation session presented by David Javitch, PhD. His exercise gave everyone an opportunity to learn about their own personality traits and to practice working with extreme opposites, both in the workplace and in everyday life. Attendees continue to rave about the content of this meeting and are looking forward to enhancing their leadership skills by attending Level II courses, scheduled for fall 2007.
“No matter how many times I plan this course, I am amazed at the enthusiasm of the attendees and the new questions that they pose,” says Russell Holman, MD, SHM Leadership Academy course director.
Level II resulted from more than 300 Level I course evaluations that requested additional and ongoing leadership development activities. The Level II course focused on discussions about culture change, negotiation skills, and finance. Keynote speaker Leonard Marcus, PhD, defined the term “meta-leadership” in hospital medicine as a type of leadership that links individuals through their leader’s vision, creating enthusiastic followers.
The Level II course is a must have for those who want to expand upon leadership skills learned in Level I or for those who already have an MBA and want to improve upon leadership in clinical care. The skills discussed in this session are essential to effectively developing and implementing quality improvement programs, patient-safety initiatives, and other programs whose goal is to make system changes that improve patient care.
“The level of attendees participating in Level II was challenging,” says Dr. Holman. “It had us all—faculty and attendees alike—collaborating to answer questions from real-life experiences.”
The phrase “all work and no play” doesn’t describe any SHM meeting, and it certainly can’t be used in reference to the Leadership Academy. Attendees had an opportunity to network with fellow participants and exhibiting companies during the Monday night reception sponsored by Cogent Healthcare. Participants also had ample time to get out and experience some southern hospitality, while enjoying the spa, playing golf, touring on steamboats, dining, and shopping at the Gaylord Opryland Resort and Convention Center.
Leadership Academy Levels I and II were jam-packed with relevant materials and tools applicable to business and the real world. This is an outstanding opportunity for individuals just beginning their leadership journey and for those wanting to take their leadership skills to the next level.
Don’t miss out on the next opportunity to become a leader in hospital medicine. The next meeting will take place during the week of February 26–March 1, 2007, at the Gaylord Palms Resort and Convention Center in Orlando, Fla. Log on to www.hospitalmedicine.org or call (800) 843-3360 for more information.
October 7, 2006, is World Hospice and Palliative Care Day. This day is dedicated to raising the visibility of palliative care within the global community and to providing opportunities to support hospice and palliative care in the form of a unified day of action.
According to official organizers, the event’s theme is “Access to care for all—highlighting the fact that everyone has a right to high-quality end-of-life care, but that more needs to be done to enable everyone to access it.” In creating World Hospice and Palliative Care Day 2006, the event’s organizers aim to raise awareness and understanding of the needs of those living with a terminal diagnosis, as well as the needs of their families. Other goals include calling attention to the need for increasing hospice and palliative care availability throughout the world and raising funds to be used in supporting these services.
Like the first World Hospice and Palliative Care Day, held in 2005, this event will be carried out in conjunction with Voices for Hospices, a global music effort that supports concerts held around the world to raise awareness of this important topic. The Voices for Hospices group is one of many supporters of this cause.
—World Hospice and Palliative Care Day 2006 Web site, Key Messages, page 1.
More than 1,000 events took place on World Hospice and Palliative Care Day 2005, and 74 countries supported the activities. Included in the 2005 event were a cycle rally in Nepal; art exhibitions in Australia, Hong Kong, and Austria; and palliative care conferences in Lithuania and Belarus. In addition, thousands of people from around the world signed a global petition calling for better quality care for people afflicted by terminal illness.
Hospitalists are asked frequently to lead and participate in initiatives meant to improve the identification and treatment of patients and families in need of palliative care. It is common knowledge that traditional medical training tends to focus on the efforts that must be made to cure and prevent illness. There are times when the first priority must be to look for a cure at all costs; however, it must be acknowledged that there are also times when the treatment of a patient’s symptoms should be looked upon as just as important. Conventional medical training frequently does not provide the tools needed to offer the best care for patients and their families when the latter goal becomes the higher priority.
This is why support of initiatives like World Hospice and Palliative Care Day can offer such value to the global community. Events like this one promote awareness of an important topic. For information and ideas on how to get involved in this or in future events, please consult www.worldday.org. Access the Web site’s “Get Involved” page for ideas on how to offer support. Suggested activities include campaigning, creating links and partnerships, and producing materials that will raise awareness.
On the “PR & Press” page of the Web site, in the “Key Messages 2006” section, the following question was posed: “What kinds of issues in general terms does World Hospice and Palliative Care Day hope to raise awareness of year on year?” One of the well-stated answers: “First and foremost we hope the Day helps to increase understanding of hospice and palliative care and how it supports those facing the end of life … . It’s not about ‘helping someone die’ but instead about helping someone to live as comfortably as possible with their illness. It’s about seeing them as [a] living person, not a dying patient. It’s supporting those closest to them and adding life to days, whether or not days can be added to lives.”
Smart Tools for QI Initiatives
SHM’s Quality Improvement Resource Rooms support hospitalists as QI leaders
The role and recognition of the hospitalist has evolved tremendously in the past 10 years, and hospitalists continue to be called upon to lead at their institutions, particularly in quality improvement initiatives. Based on their unique role within the hospital system (a job that requires interaction with many levels of hospital staff) hospitalists are clearly positioned to lead such efforts. As part of SHM’s dedication to promote the highest quality of care for the hospitalized patient, SHM’s Resource Rooms provide members and non-members alike access to information that will aid their knowledge in quality improvement around specific disease states. Currently, SHM provides four Resource Rooms: Venous Thromboembolism (VTE), Stroke, Antimicrobial Resistance (ABX), and Heart Failure. Two additional rooms, Geriatrics and Glycemic Control, will launch this fall.
Quality improvement for the patient will be successful if a systems-based multidisciplinary collaboration within the hospital is improved. Hospitalists are leading this challenging yet exciting opportunity to change the face of healthcare. It has been noted that medical school and residency training have failed to prepare the hospitalist for this leadership role. To this end, SHM provides users of the Resource Rooms with information describing how a specific disease state affects the population and explains why a hospitalist should act in initiating change, as well as what key knowledge, skills, and attitudes the hospitalist should possess. The user is offered information regarding didactic and bedside teaching, patient education, and opportunities for continuing medical education. These resources are useful for the novice as well as for the advanced hospitalist leader. Readers can also apply the concepts of these general mechanisms to any disease state they are seeking to improve at their institutions.
The QI workbook within each of the Resource Rooms is the most important feature and serves as a field guide to implementing a quality improvement program. The workbook includes the following aids:
- Essential first steps: garnering institutional support, assembling a team, developing team rules, and understanding the framework for improvement;
- Conducting an in-depth analysis of current processes and failures;
- Collecting data and devising metrics to assess the impact of your QI initiative;
- Moving from problems to solutions; and
- Continuing to improve: monitoring and learning from the process, as well as holding the gains and spreading your improvement
Other important resources that are common to all of the rooms and will aid in leading the QI efforts of the hospitalist are the educational features. Complex problems need multidisciplinary solutions. The “Improve” and “Educate” areas of the Resource Rooms include information that allow the hospitalist to teach and be taught. In the “Improve” section, a user can find QI Theory slide sets on the foundations of quality improvement initiatives and core measures on specific disease states.
Didactic sessions and teaching tools, as well as professional development, including core competencies and CME opportunities, are all present in the “Educate” feature of each room. For example, in the Heart Failure Resource Room, a didactic session slide set concerning the management of heart failure for hospitalized patients is provided. The slide set includes a heart failure overview that describes the epidemiology, etiologies, and objectives surrounding the management of acute congestive heart failure.
Evidence, improvement, and education tools designed to enhance inpatient outcomes can help the hospitalist develop and lead initiatives that can create a more cost-efficient approach to the treatment of hospitalized patients, while at the same time improving patient outcomes. SHM’s Quality Improvement Resource Rooms provide a compendium of resources to support the hospitalist who is embarking on this enormous task.
September Leadership Academy
New Level II a great success
The recently completed 4th SHM Leadership Academy was a true success in every sense of the word. The event was nearly a sellout, with 160 hospital medicine leaders arriving in Nashville, Tenn., in September to learn—from nationally respected leaders—tangible skills that they could take back to institute in their own practices.
The Leadership Academy Level I was designed to provide leaders in hospital medicine with the skills and resources required to lead and manage programs successfully both now and in the future. Small group sessions gave attendees a chance to interact with faculty and to share personal experiences from their own institutions. Nationally recognized speaker Jack Silversin, DMD, DrPH, presented his infamous broken squares activity, which kept the group energized and working together creatively to learn about effective communication. This course allowed attendees to evaluate personal leadership strengths and weaknesses and then apply them to everyday leadership and management challenges.
Another highlight was the self-evaluation session presented by David Javitch, PhD. His exercise gave everyone an opportunity to learn about their own personality traits and to practice working with extreme opposites, both in the workplace and in everyday life. Attendees continue to rave about the content of this meeting and are looking forward to enhancing their leadership skills by attending Level II courses, scheduled for fall 2007.
“No matter how many times I plan this course, I am amazed at the enthusiasm of the attendees and the new questions that they pose,” says Russell Holman, MD, SHM Leadership Academy course director.
Level II resulted from more than 300 Level I course evaluations that requested additional and ongoing leadership development activities. The Level II course focused on discussions about culture change, negotiation skills, and finance. Keynote speaker Leonard Marcus, PhD, defined the term “meta-leadership” in hospital medicine as a type of leadership that links individuals through their leader’s vision, creating enthusiastic followers.
The Level II course is a must have for those who want to expand upon leadership skills learned in Level I or for those who already have an MBA and want to improve upon leadership in clinical care. The skills discussed in this session are essential to effectively developing and implementing quality improvement programs, patient-safety initiatives, and other programs whose goal is to make system changes that improve patient care.
“The level of attendees participating in Level II was challenging,” says Dr. Holman. “It had us all—faculty and attendees alike—collaborating to answer questions from real-life experiences.”
The phrase “all work and no play” doesn’t describe any SHM meeting, and it certainly can’t be used in reference to the Leadership Academy. Attendees had an opportunity to network with fellow participants and exhibiting companies during the Monday night reception sponsored by Cogent Healthcare. Participants also had ample time to get out and experience some southern hospitality, while enjoying the spa, playing golf, touring on steamboats, dining, and shopping at the Gaylord Opryland Resort and Convention Center.
Leadership Academy Levels I and II were jam-packed with relevant materials and tools applicable to business and the real world. This is an outstanding opportunity for individuals just beginning their leadership journey and for those wanting to take their leadership skills to the next level.
Don’t miss out on the next opportunity to become a leader in hospital medicine. The next meeting will take place during the week of February 26–March 1, 2007, at the Gaylord Palms Resort and Convention Center in Orlando, Fla. Log on to www.hospitalmedicine.org or call (800) 843-3360 for more information.
October 7, 2006, is World Hospice and Palliative Care Day. This day is dedicated to raising the visibility of palliative care within the global community and to providing opportunities to support hospice and palliative care in the form of a unified day of action.
According to official organizers, the event’s theme is “Access to care for all—highlighting the fact that everyone has a right to high-quality end-of-life care, but that more needs to be done to enable everyone to access it.” In creating World Hospice and Palliative Care Day 2006, the event’s organizers aim to raise awareness and understanding of the needs of those living with a terminal diagnosis, as well as the needs of their families. Other goals include calling attention to the need for increasing hospice and palliative care availability throughout the world and raising funds to be used in supporting these services.
Like the first World Hospice and Palliative Care Day, held in 2005, this event will be carried out in conjunction with Voices for Hospices, a global music effort that supports concerts held around the world to raise awareness of this important topic. The Voices for Hospices group is one of many supporters of this cause.
—World Hospice and Palliative Care Day 2006 Web site, Key Messages, page 1.
More than 1,000 events took place on World Hospice and Palliative Care Day 2005, and 74 countries supported the activities. Included in the 2005 event were a cycle rally in Nepal; art exhibitions in Australia, Hong Kong, and Austria; and palliative care conferences in Lithuania and Belarus. In addition, thousands of people from around the world signed a global petition calling for better quality care for people afflicted by terminal illness.
Hospitalists are asked frequently to lead and participate in initiatives meant to improve the identification and treatment of patients and families in need of palliative care. It is common knowledge that traditional medical training tends to focus on the efforts that must be made to cure and prevent illness. There are times when the first priority must be to look for a cure at all costs; however, it must be acknowledged that there are also times when the treatment of a patient’s symptoms should be looked upon as just as important. Conventional medical training frequently does not provide the tools needed to offer the best care for patients and their families when the latter goal becomes the higher priority.
This is why support of initiatives like World Hospice and Palliative Care Day can offer such value to the global community. Events like this one promote awareness of an important topic. For information and ideas on how to get involved in this or in future events, please consult www.worldday.org. Access the Web site’s “Get Involved” page for ideas on how to offer support. Suggested activities include campaigning, creating links and partnerships, and producing materials that will raise awareness.
On the “PR & Press” page of the Web site, in the “Key Messages 2006” section, the following question was posed: “What kinds of issues in general terms does World Hospice and Palliative Care Day hope to raise awareness of year on year?” One of the well-stated answers: “First and foremost we hope the Day helps to increase understanding of hospice and palliative care and how it supports those facing the end of life … . It’s not about ‘helping someone die’ but instead about helping someone to live as comfortably as possible with their illness. It’s about seeing them as [a] living person, not a dying patient. It’s supporting those closest to them and adding life to days, whether or not days can be added to lives.”
Smart Tools for QI Initiatives
SHM’s Quality Improvement Resource Rooms support hospitalists as QI leaders
The role and recognition of the hospitalist has evolved tremendously in the past 10 years, and hospitalists continue to be called upon to lead at their institutions, particularly in quality improvement initiatives. Based on their unique role within the hospital system (a job that requires interaction with many levels of hospital staff) hospitalists are clearly positioned to lead such efforts. As part of SHM’s dedication to promote the highest quality of care for the hospitalized patient, SHM’s Resource Rooms provide members and non-members alike access to information that will aid their knowledge in quality improvement around specific disease states. Currently, SHM provides four Resource Rooms: Venous Thromboembolism (VTE), Stroke, Antimicrobial Resistance (ABX), and Heart Failure. Two additional rooms, Geriatrics and Glycemic Control, will launch this fall.
Quality improvement for the patient will be successful if a systems-based multidisciplinary collaboration within the hospital is improved. Hospitalists are leading this challenging yet exciting opportunity to change the face of healthcare. It has been noted that medical school and residency training have failed to prepare the hospitalist for this leadership role. To this end, SHM provides users of the Resource Rooms with information describing how a specific disease state affects the population and explains why a hospitalist should act in initiating change, as well as what key knowledge, skills, and attitudes the hospitalist should possess. The user is offered information regarding didactic and bedside teaching, patient education, and opportunities for continuing medical education. These resources are useful for the novice as well as for the advanced hospitalist leader. Readers can also apply the concepts of these general mechanisms to any disease state they are seeking to improve at their institutions.
The QI workbook within each of the Resource Rooms is the most important feature and serves as a field guide to implementing a quality improvement program. The workbook includes the following aids:
- Essential first steps: garnering institutional support, assembling a team, developing team rules, and understanding the framework for improvement;
- Conducting an in-depth analysis of current processes and failures;
- Collecting data and devising metrics to assess the impact of your QI initiative;
- Moving from problems to solutions; and
- Continuing to improve: monitoring and learning from the process, as well as holding the gains and spreading your improvement
Other important resources that are common to all of the rooms and will aid in leading the QI efforts of the hospitalist are the educational features. Complex problems need multidisciplinary solutions. The “Improve” and “Educate” areas of the Resource Rooms include information that allow the hospitalist to teach and be taught. In the “Improve” section, a user can find QI Theory slide sets on the foundations of quality improvement initiatives and core measures on specific disease states.
Didactic sessions and teaching tools, as well as professional development, including core competencies and CME opportunities, are all present in the “Educate” feature of each room. For example, in the Heart Failure Resource Room, a didactic session slide set concerning the management of heart failure for hospitalized patients is provided. The slide set includes a heart failure overview that describes the epidemiology, etiologies, and objectives surrounding the management of acute congestive heart failure.
Evidence, improvement, and education tools designed to enhance inpatient outcomes can help the hospitalist develop and lead initiatives that can create a more cost-efficient approach to the treatment of hospitalized patients, while at the same time improving patient outcomes. SHM’s Quality Improvement Resource Rooms provide a compendium of resources to support the hospitalist who is embarking on this enormous task.
September Leadership Academy
New Level II a great success
The recently completed 4th SHM Leadership Academy was a true success in every sense of the word. The event was nearly a sellout, with 160 hospital medicine leaders arriving in Nashville, Tenn., in September to learn—from nationally respected leaders—tangible skills that they could take back to institute in their own practices.
The Leadership Academy Level I was designed to provide leaders in hospital medicine with the skills and resources required to lead and manage programs successfully both now and in the future. Small group sessions gave attendees a chance to interact with faculty and to share personal experiences from their own institutions. Nationally recognized speaker Jack Silversin, DMD, DrPH, presented his infamous broken squares activity, which kept the group energized and working together creatively to learn about effective communication. This course allowed attendees to evaluate personal leadership strengths and weaknesses and then apply them to everyday leadership and management challenges.
Another highlight was the self-evaluation session presented by David Javitch, PhD. His exercise gave everyone an opportunity to learn about their own personality traits and to practice working with extreme opposites, both in the workplace and in everyday life. Attendees continue to rave about the content of this meeting and are looking forward to enhancing their leadership skills by attending Level II courses, scheduled for fall 2007.
“No matter how many times I plan this course, I am amazed at the enthusiasm of the attendees and the new questions that they pose,” says Russell Holman, MD, SHM Leadership Academy course director.
Level II resulted from more than 300 Level I course evaluations that requested additional and ongoing leadership development activities. The Level II course focused on discussions about culture change, negotiation skills, and finance. Keynote speaker Leonard Marcus, PhD, defined the term “meta-leadership” in hospital medicine as a type of leadership that links individuals through their leader’s vision, creating enthusiastic followers.
The Level II course is a must have for those who want to expand upon leadership skills learned in Level I or for those who already have an MBA and want to improve upon leadership in clinical care. The skills discussed in this session are essential to effectively developing and implementing quality improvement programs, patient-safety initiatives, and other programs whose goal is to make system changes that improve patient care.
“The level of attendees participating in Level II was challenging,” says Dr. Holman. “It had us all—faculty and attendees alike—collaborating to answer questions from real-life experiences.”
The phrase “all work and no play” doesn’t describe any SHM meeting, and it certainly can’t be used in reference to the Leadership Academy. Attendees had an opportunity to network with fellow participants and exhibiting companies during the Monday night reception sponsored by Cogent Healthcare. Participants also had ample time to get out and experience some southern hospitality, while enjoying the spa, playing golf, touring on steamboats, dining, and shopping at the Gaylord Opryland Resort and Convention Center.
Leadership Academy Levels I and II were jam-packed with relevant materials and tools applicable to business and the real world. This is an outstanding opportunity for individuals just beginning their leadership journey and for those wanting to take their leadership skills to the next level.
Don’t miss out on the next opportunity to become a leader in hospital medicine. The next meeting will take place during the week of February 26–March 1, 2007, at the Gaylord Palms Resort and Convention Center in Orlando, Fla. Log on to www.hospitalmedicine.org or call (800) 843-3360 for more information.
The Quality Care Revolution
We are now knee deep in the quality revolution. In some ways, this should have been driven by the hospitals and doctors striving for continual quality improvement. It should have been driven by patients demanding better outcomes, more uniform processes, and the data to help them decide where to receive the best care. In reality it is being driven by those who pay for care—America’s businesses and our government, two entities that want better value for the increasingly dear dollars they spend on healthcare.
Hospitals and doctors have survived (and many have succeeded) by using the traditional compensation system, which rewards the performing of care without rewarding the best or even the better practice of medicine. Today you can do the wrong procedure and do it poorly and still get paid. The mantra of the entire performance and standards effort is to shift at least some of the rewards to those with better outcomes, to processes that are more in line with national practice standards, and to those who have the data to back that up. In marketing shorthand, this is pay for performance—or P4P—and while it seems natural in most of the rest of the American marketplace, it is somewhat revolutionary in healthcare.
While the concept of identifying best practices, measuring performance, collecting data, and then appropriately tying compensation or rewards to performance sounds clear and straightforward, many issues quickly surface to cloud any forward progress.
Decide What to Measure
Unfortunately, you can arrive quickly and efficiently at the wrong destination. Everyone knows that some of the hallmarks of physicians are that we can “perform for the test” and adapt to a new paradigm. It is important that we don’t just settle for what we can easily measure (knowing that most of our systems’ data collection efforts are geared initially to getting paid and not to measuring key performance indicators), but that we make sure that we are selecting performance measures that lead to better patient outcomes and improve care. Hospitalists must constantly examine their hospitals’ plans for data collection to ensure that achieving high marks will lead to better patient care.
Data, Data, Who Gets the Data?
There is no doubt that the by-product of the current P4P movement is that there will be more known about doctors and hospitals than ever before. Like nuclear energy, this volatile resource can be used for good or evil. It is not a trivial issue of who “owns” the data and who has access to it.
How valuable would it be to the pharmaceutical industry to know which doctors treat a lot of heart failure and which medications they use and why? How valuable would it be for insurance companies to see physician or hospital performance data not just for their insured, but for all of a physician’s or institution’s patients? Who will control access to all the data that will be collected?
This plays into another important question: Just how will individual or small independent groups of physicians pay for all this reporting? Very likely, data collection and reporting will be an additional cost of doing business for an already strapped profession. To succeed—or just to stay in the game—physicians will need to upgrade their systems with new hardware and software, while facing the prospect of having their payment diminished or of being cut off from certain patients. What if a hospital offered physicians free systems upgrades in exchange for a look at all the physicians’ data? What if pharmaceutical companies made the same offer? Would physicians potentially sell their information for a handful of beads?
Where to Be? What to Do?
For national professional societies, the greater issue may be how best to participate proactively in the P4P process and how to define their roles. Should SHM be involved in developing new standards of care for areas where we have crucial roles (e.g., transitions of care, end-of-life care) or should we simply critique the efforts of others? Is our role to be the patients’ advocate at any cost, or do we have a responsibility to stand up for the young and evolving discipline of hospital medicine? Is SHM’s main role to be a communicator to our nation’s hospitalists about what the new rules and standards will be, or should SHM develop educational resources to help hospitalists act as leaders in the implementation of the rules that flow out of this complex process?
Just as important is to try to understand where SHM can be most effective. As hospitals have seen a huge growth in the data they must collect and report on, so too has SHM observed a proliferation of organizations cropping up to take their place as key players in the P4P arena. SHM can’t be everywhere, so we have chosen to enter where we feel we can make the most impact.
Hospitalists’ Role in Improving Quality
First, SHM has created a working group on Performance and Standards to coordinate all of our relationships in this rapidly evolving and growing field. SHM has hired Jill Epstein to be the dedicated staff for this effort. SHM has decided to actively participate with the AMA Physician Consortium for Performance Improvement (PCPI) because this is where most of the specialties of medicine come together to develop and assess performance standards.
SHM is also becoming more active at the National Quality Forum (NQF), where groups such as the PCPI submit their standards for acceptance. SHM has nominated hospital medicine leaders for the NQF Steering Committee as well as for its Technical Advisory Panels on Patient Safety, Anesthesia and Surgery, and Pediatrics. The Centers for Medicare and Medicaid Services and Congress will look to NQF as a national clearinghouse for performance measurements.
SHM has had a good working relationship with the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) for years. Now, SHM looks to expand our work with JCAHO as their role in standard setting and accreditation evolves.
In this complex arena, SHM is constantly looking for other professional medical societies with similar interests and values with which to share information and strategies. SHM has found the American College of Physicians, the American Geriatrics Society, the Society of Critical Care Medicine, the American College of Chest Physicians (ACCP), and others particularly open and helpful.
But SHM won’t be content with just helping to set the standards. We believe hospitalists have a unique role in implementing change. Beginning with our Leadership Academies, which train those who can lead and manage change, SHM has also introduced a quality improvement precourse at our annual meetings, as well as the practical Resource Rooms on our Web site, which have 100-page detailed workbooks to guide hospitalist leaders in quality improvement projects in DVT and diabetes. Under the leadership of Greg Maynard, MD, of University of California at San Diego (UCSD) and Jason Stein, MD, of Emory University Hospital, Atlanta, with staff leadership by Kathleen Kerr of UCSF, SHM has just launched its DVT Mentored Implementation project, in which we will guide, support, and mentor 30 hospital medicine leaders to improve patient care at their local institutions.
SHM is actively partnering with the Institute for Healthcare Improvement (IHI) to train and support the hospitalists who will leverage IHI’s “100K Lives Saved” campaign. These hospitalist leaders will act as change agents for further quality improvements on a local level.
Not a Time to Stand Idly By
The status quo is not an option. This call for change is, in many ways, fueling the growth of hospital medicine. Change that was called for many years ago is now taking shape. SHM is playing a role in ensuring that the new standards of care that we will have to meet make sense to improve the care our patients receive. But SHM won’t just set the rules, line the field, and build the scoreboard. Spring training—a time when we will need to refine old skills and develop new ones—is upon us. Hospitalists are ready to play their part. Game on. Let’s go. TH
Dr. Wellikson has been CEO of SHM since 2000.
We are now knee deep in the quality revolution. In some ways, this should have been driven by the hospitals and doctors striving for continual quality improvement. It should have been driven by patients demanding better outcomes, more uniform processes, and the data to help them decide where to receive the best care. In reality it is being driven by those who pay for care—America’s businesses and our government, two entities that want better value for the increasingly dear dollars they spend on healthcare.
Hospitals and doctors have survived (and many have succeeded) by using the traditional compensation system, which rewards the performing of care without rewarding the best or even the better practice of medicine. Today you can do the wrong procedure and do it poorly and still get paid. The mantra of the entire performance and standards effort is to shift at least some of the rewards to those with better outcomes, to processes that are more in line with national practice standards, and to those who have the data to back that up. In marketing shorthand, this is pay for performance—or P4P—and while it seems natural in most of the rest of the American marketplace, it is somewhat revolutionary in healthcare.
While the concept of identifying best practices, measuring performance, collecting data, and then appropriately tying compensation or rewards to performance sounds clear and straightforward, many issues quickly surface to cloud any forward progress.
Decide What to Measure
Unfortunately, you can arrive quickly and efficiently at the wrong destination. Everyone knows that some of the hallmarks of physicians are that we can “perform for the test” and adapt to a new paradigm. It is important that we don’t just settle for what we can easily measure (knowing that most of our systems’ data collection efforts are geared initially to getting paid and not to measuring key performance indicators), but that we make sure that we are selecting performance measures that lead to better patient outcomes and improve care. Hospitalists must constantly examine their hospitals’ plans for data collection to ensure that achieving high marks will lead to better patient care.
Data, Data, Who Gets the Data?
There is no doubt that the by-product of the current P4P movement is that there will be more known about doctors and hospitals than ever before. Like nuclear energy, this volatile resource can be used for good or evil. It is not a trivial issue of who “owns” the data and who has access to it.
How valuable would it be to the pharmaceutical industry to know which doctors treat a lot of heart failure and which medications they use and why? How valuable would it be for insurance companies to see physician or hospital performance data not just for their insured, but for all of a physician’s or institution’s patients? Who will control access to all the data that will be collected?
This plays into another important question: Just how will individual or small independent groups of physicians pay for all this reporting? Very likely, data collection and reporting will be an additional cost of doing business for an already strapped profession. To succeed—or just to stay in the game—physicians will need to upgrade their systems with new hardware and software, while facing the prospect of having their payment diminished or of being cut off from certain patients. What if a hospital offered physicians free systems upgrades in exchange for a look at all the physicians’ data? What if pharmaceutical companies made the same offer? Would physicians potentially sell their information for a handful of beads?
Where to Be? What to Do?
For national professional societies, the greater issue may be how best to participate proactively in the P4P process and how to define their roles. Should SHM be involved in developing new standards of care for areas where we have crucial roles (e.g., transitions of care, end-of-life care) or should we simply critique the efforts of others? Is our role to be the patients’ advocate at any cost, or do we have a responsibility to stand up for the young and evolving discipline of hospital medicine? Is SHM’s main role to be a communicator to our nation’s hospitalists about what the new rules and standards will be, or should SHM develop educational resources to help hospitalists act as leaders in the implementation of the rules that flow out of this complex process?
Just as important is to try to understand where SHM can be most effective. As hospitals have seen a huge growth in the data they must collect and report on, so too has SHM observed a proliferation of organizations cropping up to take their place as key players in the P4P arena. SHM can’t be everywhere, so we have chosen to enter where we feel we can make the most impact.
Hospitalists’ Role in Improving Quality
First, SHM has created a working group on Performance and Standards to coordinate all of our relationships in this rapidly evolving and growing field. SHM has hired Jill Epstein to be the dedicated staff for this effort. SHM has decided to actively participate with the AMA Physician Consortium for Performance Improvement (PCPI) because this is where most of the specialties of medicine come together to develop and assess performance standards.
SHM is also becoming more active at the National Quality Forum (NQF), where groups such as the PCPI submit their standards for acceptance. SHM has nominated hospital medicine leaders for the NQF Steering Committee as well as for its Technical Advisory Panels on Patient Safety, Anesthesia and Surgery, and Pediatrics. The Centers for Medicare and Medicaid Services and Congress will look to NQF as a national clearinghouse for performance measurements.
SHM has had a good working relationship with the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) for years. Now, SHM looks to expand our work with JCAHO as their role in standard setting and accreditation evolves.
In this complex arena, SHM is constantly looking for other professional medical societies with similar interests and values with which to share information and strategies. SHM has found the American College of Physicians, the American Geriatrics Society, the Society of Critical Care Medicine, the American College of Chest Physicians (ACCP), and others particularly open and helpful.
But SHM won’t be content with just helping to set the standards. We believe hospitalists have a unique role in implementing change. Beginning with our Leadership Academies, which train those who can lead and manage change, SHM has also introduced a quality improvement precourse at our annual meetings, as well as the practical Resource Rooms on our Web site, which have 100-page detailed workbooks to guide hospitalist leaders in quality improvement projects in DVT and diabetes. Under the leadership of Greg Maynard, MD, of University of California at San Diego (UCSD) and Jason Stein, MD, of Emory University Hospital, Atlanta, with staff leadership by Kathleen Kerr of UCSF, SHM has just launched its DVT Mentored Implementation project, in which we will guide, support, and mentor 30 hospital medicine leaders to improve patient care at their local institutions.
SHM is actively partnering with the Institute for Healthcare Improvement (IHI) to train and support the hospitalists who will leverage IHI’s “100K Lives Saved” campaign. These hospitalist leaders will act as change agents for further quality improvements on a local level.
Not a Time to Stand Idly By
The status quo is not an option. This call for change is, in many ways, fueling the growth of hospital medicine. Change that was called for many years ago is now taking shape. SHM is playing a role in ensuring that the new standards of care that we will have to meet make sense to improve the care our patients receive. But SHM won’t just set the rules, line the field, and build the scoreboard. Spring training—a time when we will need to refine old skills and develop new ones—is upon us. Hospitalists are ready to play their part. Game on. Let’s go. TH
Dr. Wellikson has been CEO of SHM since 2000.
We are now knee deep in the quality revolution. In some ways, this should have been driven by the hospitals and doctors striving for continual quality improvement. It should have been driven by patients demanding better outcomes, more uniform processes, and the data to help them decide where to receive the best care. In reality it is being driven by those who pay for care—America’s businesses and our government, two entities that want better value for the increasingly dear dollars they spend on healthcare.
Hospitals and doctors have survived (and many have succeeded) by using the traditional compensation system, which rewards the performing of care without rewarding the best or even the better practice of medicine. Today you can do the wrong procedure and do it poorly and still get paid. The mantra of the entire performance and standards effort is to shift at least some of the rewards to those with better outcomes, to processes that are more in line with national practice standards, and to those who have the data to back that up. In marketing shorthand, this is pay for performance—or P4P—and while it seems natural in most of the rest of the American marketplace, it is somewhat revolutionary in healthcare.
While the concept of identifying best practices, measuring performance, collecting data, and then appropriately tying compensation or rewards to performance sounds clear and straightforward, many issues quickly surface to cloud any forward progress.
Decide What to Measure
Unfortunately, you can arrive quickly and efficiently at the wrong destination. Everyone knows that some of the hallmarks of physicians are that we can “perform for the test” and adapt to a new paradigm. It is important that we don’t just settle for what we can easily measure (knowing that most of our systems’ data collection efforts are geared initially to getting paid and not to measuring key performance indicators), but that we make sure that we are selecting performance measures that lead to better patient outcomes and improve care. Hospitalists must constantly examine their hospitals’ plans for data collection to ensure that achieving high marks will lead to better patient care.
Data, Data, Who Gets the Data?
There is no doubt that the by-product of the current P4P movement is that there will be more known about doctors and hospitals than ever before. Like nuclear energy, this volatile resource can be used for good or evil. It is not a trivial issue of who “owns” the data and who has access to it.
How valuable would it be to the pharmaceutical industry to know which doctors treat a lot of heart failure and which medications they use and why? How valuable would it be for insurance companies to see physician or hospital performance data not just for their insured, but for all of a physician’s or institution’s patients? Who will control access to all the data that will be collected?
This plays into another important question: Just how will individual or small independent groups of physicians pay for all this reporting? Very likely, data collection and reporting will be an additional cost of doing business for an already strapped profession. To succeed—or just to stay in the game—physicians will need to upgrade their systems with new hardware and software, while facing the prospect of having their payment diminished or of being cut off from certain patients. What if a hospital offered physicians free systems upgrades in exchange for a look at all the physicians’ data? What if pharmaceutical companies made the same offer? Would physicians potentially sell their information for a handful of beads?
Where to Be? What to Do?
For national professional societies, the greater issue may be how best to participate proactively in the P4P process and how to define their roles. Should SHM be involved in developing new standards of care for areas where we have crucial roles (e.g., transitions of care, end-of-life care) or should we simply critique the efforts of others? Is our role to be the patients’ advocate at any cost, or do we have a responsibility to stand up for the young and evolving discipline of hospital medicine? Is SHM’s main role to be a communicator to our nation’s hospitalists about what the new rules and standards will be, or should SHM develop educational resources to help hospitalists act as leaders in the implementation of the rules that flow out of this complex process?
Just as important is to try to understand where SHM can be most effective. As hospitals have seen a huge growth in the data they must collect and report on, so too has SHM observed a proliferation of organizations cropping up to take their place as key players in the P4P arena. SHM can’t be everywhere, so we have chosen to enter where we feel we can make the most impact.
Hospitalists’ Role in Improving Quality
First, SHM has created a working group on Performance and Standards to coordinate all of our relationships in this rapidly evolving and growing field. SHM has hired Jill Epstein to be the dedicated staff for this effort. SHM has decided to actively participate with the AMA Physician Consortium for Performance Improvement (PCPI) because this is where most of the specialties of medicine come together to develop and assess performance standards.
SHM is also becoming more active at the National Quality Forum (NQF), where groups such as the PCPI submit their standards for acceptance. SHM has nominated hospital medicine leaders for the NQF Steering Committee as well as for its Technical Advisory Panels on Patient Safety, Anesthesia and Surgery, and Pediatrics. The Centers for Medicare and Medicaid Services and Congress will look to NQF as a national clearinghouse for performance measurements.
SHM has had a good working relationship with the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) for years. Now, SHM looks to expand our work with JCAHO as their role in standard setting and accreditation evolves.
In this complex arena, SHM is constantly looking for other professional medical societies with similar interests and values with which to share information and strategies. SHM has found the American College of Physicians, the American Geriatrics Society, the Society of Critical Care Medicine, the American College of Chest Physicians (ACCP), and others particularly open and helpful.
But SHM won’t be content with just helping to set the standards. We believe hospitalists have a unique role in implementing change. Beginning with our Leadership Academies, which train those who can lead and manage change, SHM has also introduced a quality improvement precourse at our annual meetings, as well as the practical Resource Rooms on our Web site, which have 100-page detailed workbooks to guide hospitalist leaders in quality improvement projects in DVT and diabetes. Under the leadership of Greg Maynard, MD, of University of California at San Diego (UCSD) and Jason Stein, MD, of Emory University Hospital, Atlanta, with staff leadership by Kathleen Kerr of UCSF, SHM has just launched its DVT Mentored Implementation project, in which we will guide, support, and mentor 30 hospital medicine leaders to improve patient care at their local institutions.
SHM is actively partnering with the Institute for Healthcare Improvement (IHI) to train and support the hospitalists who will leverage IHI’s “100K Lives Saved” campaign. These hospitalist leaders will act as change agents for further quality improvements on a local level.
Not a Time to Stand Idly By
The status quo is not an option. This call for change is, in many ways, fueling the growth of hospital medicine. Change that was called for many years ago is now taking shape. SHM is playing a role in ensuring that the new standards of care that we will have to meet make sense to improve the care our patients receive. But SHM won’t just set the rules, line the field, and build the scoreboard. Spring training—a time when we will need to refine old skills and develop new ones—is upon us. Hospitalists are ready to play their part. Game on. Let’s go. TH
Dr. Wellikson has been CEO of SHM since 2000.
Learn to Lead
Everyone has it within his power to say, this I am today, that I shall be tomorrow.— Louis L’Amour
Professional advancement means different things to different people. For some, it is important to be the leader of their medical group—whether it is a hospital group or a private practice. For others, it means being associate professor or department chair. And, for a few, it will mean becoming the chief executive officer of a hospital or healthcare company. Much of this comes down to trying to make a difference to the patients and other people around us, as well as trying to bring about improvements in healthcare.
To many physicians, trying to make a difference has been limited to making sure we are doing a good job—diagnostically, pharmacologically, and emotionally—for our individual patients. However, as we become adept at serving the individual patient, we often feel a need to take on more challenges. Medical staff leadership is one way to affect the care of many by directing the actions of the group.
Healthcare is a large component of our country’s economy, and this is not likely to change. In addition, it is an area with many challenges: the aging population, the uninsured population, new pharmaceutical developments, and medical device discoveries. There will be a continued demand for individuals who can understand this very complex intersection of business and medicine.
Fill in the Gaps and Offer Your Help
How many times have we heard our colleagues complain that “the problems never change and nothing gets done around here”? No doubt, change is tough, but taking a role in your department or at your hospital is a way to start. Many hospitalists are filling in the leadership gaps as other specialists move to outpatient centers or into the office. Posts that have traditionally been held by cardiology or urology are changing.
Give some thought to where you might help out. A commitment to something as simple as the Pharmacy and Therapeutics Committee can lead to changes for all the patients as well as get you started on your new career path. Every chief of staff and vice president of medical affairs is looking for volunteers who are interested in projects and can follow through. This applies to department chairmen as well. Given the commitment we all have to our patients and our lives, the offer of help for even just one project is a breath of fresh air to those who have the responsibility for the group or department. Depending on your area of interest—patient safety, quality improvement, patient or medical student education, or process improvement—a project can be created that furthers your institution and addresses your interests.
Worried that there is no room for you at the table? Think again. If your department chair or chief of staff is not asking for help, it may be that their requests have fallen on deaf ears for so long that they have stopped asking. If you have identified a project that interests you, it may interest others also. Ask if there is a way that you can work with others on an existing project. Alternatively, ask if there is a project that needs doing that has no one to do it. Many projects need a political and medical champion; they would welcome your offer to help. Volunteer and be prepared to take on a project that is not your favorite but that may give you experience for other projects. Your initiative will certainly get the attention of the chairperson or others because you are solving a problem for them.
Ask, Listen, and Learn
In addition, this is just a beginning step that will lead to further leadership positions for many. Gaining experience with what works and with how you can accomplish initiatives will lead to bigger opportunities. Embedding yourself in the fabric of your organization provides an opportunity for others to see you work and interact. Seek the advice of those whom you trust and who appear to be successful. Listen to feedback and adjust accordingly. Take classes on leadership, financial performance, process improvement, or in areas that appeal to you and that address what you want to accomplish. Before you know it, you will be chief of staff, division chair, or chief medical officer. Knowing where you want to end up is always an advantage, but many individuals find their way through different experiences and exposures. Sometimes where you end up is not what you expected, but the journey is usually interesting.
Different career choices come at different times. Focusing on family, whether it is making time for children, caring for elderly parents, or supporting a spouse’s career choice may require less career focus for a time. However, as your responsibilities change, new opportunities arise. Finding a mentor or trusted individual who can advise you during these times is helpful.
Develop and Demonstrate Executive Expertise
As the hospital environment changes and hospitalists become the primary providers of care in the acute care setting, they will become hospital coordinators, in conjunction with the emergency department and other specialists. They will develop a knowledge that can be leveraged to improve processes, reduce errors, and improve outcomes. A different set of skills is needed to be successful as an executive. It requires a different way of problem solving; it requires studying and applying new lessons. The successful person develops this new expertise. The effect this person makes in applying these new skills will lead to increased roles and responsibilities. There will be continuing demand for individuals who can access, plan, and implement change within our complex systems. There will also be continuing challenges in healthcare, including the areas of medical education, research, the uninsured, and the aging population. Skills acquired now could be applied as the vice president of medical affairs or as chief medical officer.
Historically, the chief executive officer of a hospital or an integrated system has been a non-medical person with business expertise in healthcare. Hospitalists may fill this role more and more in the future. Many individuals are starting their careers in the hospital. This experience will allow them to develop skills their prior colleagues did not have. It will expose them to teamwork, results orientation, and mentors. We are a young group of professionals with many career years ahead of us. Hospitals are increasingly recognizing that the expertise of committed physician partners is critical to their success. This combination of interest and opportunity will groom many individuals, some of whom will affect healthcare for generations.
Bringing our knowledge of medicine to business, and creating crossroads and interactions, can advance our careers at the same time it improves the healthcare of others. This type of career path is not out of reach for you, and to think it all started with the Pharmacy and Therapeutics Committee. TH
Dr. Gorman is the president of SHM.
Everyone has it within his power to say, this I am today, that I shall be tomorrow.— Louis L’Amour
Professional advancement means different things to different people. For some, it is important to be the leader of their medical group—whether it is a hospital group or a private practice. For others, it means being associate professor or department chair. And, for a few, it will mean becoming the chief executive officer of a hospital or healthcare company. Much of this comes down to trying to make a difference to the patients and other people around us, as well as trying to bring about improvements in healthcare.
To many physicians, trying to make a difference has been limited to making sure we are doing a good job—diagnostically, pharmacologically, and emotionally—for our individual patients. However, as we become adept at serving the individual patient, we often feel a need to take on more challenges. Medical staff leadership is one way to affect the care of many by directing the actions of the group.
Healthcare is a large component of our country’s economy, and this is not likely to change. In addition, it is an area with many challenges: the aging population, the uninsured population, new pharmaceutical developments, and medical device discoveries. There will be a continued demand for individuals who can understand this very complex intersection of business and medicine.
Fill in the Gaps and Offer Your Help
How many times have we heard our colleagues complain that “the problems never change and nothing gets done around here”? No doubt, change is tough, but taking a role in your department or at your hospital is a way to start. Many hospitalists are filling in the leadership gaps as other specialists move to outpatient centers or into the office. Posts that have traditionally been held by cardiology or urology are changing.
Give some thought to where you might help out. A commitment to something as simple as the Pharmacy and Therapeutics Committee can lead to changes for all the patients as well as get you started on your new career path. Every chief of staff and vice president of medical affairs is looking for volunteers who are interested in projects and can follow through. This applies to department chairmen as well. Given the commitment we all have to our patients and our lives, the offer of help for even just one project is a breath of fresh air to those who have the responsibility for the group or department. Depending on your area of interest—patient safety, quality improvement, patient or medical student education, or process improvement—a project can be created that furthers your institution and addresses your interests.
Worried that there is no room for you at the table? Think again. If your department chair or chief of staff is not asking for help, it may be that their requests have fallen on deaf ears for so long that they have stopped asking. If you have identified a project that interests you, it may interest others also. Ask if there is a way that you can work with others on an existing project. Alternatively, ask if there is a project that needs doing that has no one to do it. Many projects need a political and medical champion; they would welcome your offer to help. Volunteer and be prepared to take on a project that is not your favorite but that may give you experience for other projects. Your initiative will certainly get the attention of the chairperson or others because you are solving a problem for them.
Ask, Listen, and Learn
In addition, this is just a beginning step that will lead to further leadership positions for many. Gaining experience with what works and with how you can accomplish initiatives will lead to bigger opportunities. Embedding yourself in the fabric of your organization provides an opportunity for others to see you work and interact. Seek the advice of those whom you trust and who appear to be successful. Listen to feedback and adjust accordingly. Take classes on leadership, financial performance, process improvement, or in areas that appeal to you and that address what you want to accomplish. Before you know it, you will be chief of staff, division chair, or chief medical officer. Knowing where you want to end up is always an advantage, but many individuals find their way through different experiences and exposures. Sometimes where you end up is not what you expected, but the journey is usually interesting.
Different career choices come at different times. Focusing on family, whether it is making time for children, caring for elderly parents, or supporting a spouse’s career choice may require less career focus for a time. However, as your responsibilities change, new opportunities arise. Finding a mentor or trusted individual who can advise you during these times is helpful.
Develop and Demonstrate Executive Expertise
As the hospital environment changes and hospitalists become the primary providers of care in the acute care setting, they will become hospital coordinators, in conjunction with the emergency department and other specialists. They will develop a knowledge that can be leveraged to improve processes, reduce errors, and improve outcomes. A different set of skills is needed to be successful as an executive. It requires a different way of problem solving; it requires studying and applying new lessons. The successful person develops this new expertise. The effect this person makes in applying these new skills will lead to increased roles and responsibilities. There will be continuing demand for individuals who can access, plan, and implement change within our complex systems. There will also be continuing challenges in healthcare, including the areas of medical education, research, the uninsured, and the aging population. Skills acquired now could be applied as the vice president of medical affairs or as chief medical officer.
Historically, the chief executive officer of a hospital or an integrated system has been a non-medical person with business expertise in healthcare. Hospitalists may fill this role more and more in the future. Many individuals are starting their careers in the hospital. This experience will allow them to develop skills their prior colleagues did not have. It will expose them to teamwork, results orientation, and mentors. We are a young group of professionals with many career years ahead of us. Hospitals are increasingly recognizing that the expertise of committed physician partners is critical to their success. This combination of interest and opportunity will groom many individuals, some of whom will affect healthcare for generations.
Bringing our knowledge of medicine to business, and creating crossroads and interactions, can advance our careers at the same time it improves the healthcare of others. This type of career path is not out of reach for you, and to think it all started with the Pharmacy and Therapeutics Committee. TH
Dr. Gorman is the president of SHM.
Everyone has it within his power to say, this I am today, that I shall be tomorrow.— Louis L’Amour
Professional advancement means different things to different people. For some, it is important to be the leader of their medical group—whether it is a hospital group or a private practice. For others, it means being associate professor or department chair. And, for a few, it will mean becoming the chief executive officer of a hospital or healthcare company. Much of this comes down to trying to make a difference to the patients and other people around us, as well as trying to bring about improvements in healthcare.
To many physicians, trying to make a difference has been limited to making sure we are doing a good job—diagnostically, pharmacologically, and emotionally—for our individual patients. However, as we become adept at serving the individual patient, we often feel a need to take on more challenges. Medical staff leadership is one way to affect the care of many by directing the actions of the group.
Healthcare is a large component of our country’s economy, and this is not likely to change. In addition, it is an area with many challenges: the aging population, the uninsured population, new pharmaceutical developments, and medical device discoveries. There will be a continued demand for individuals who can understand this very complex intersection of business and medicine.
Fill in the Gaps and Offer Your Help
How many times have we heard our colleagues complain that “the problems never change and nothing gets done around here”? No doubt, change is tough, but taking a role in your department or at your hospital is a way to start. Many hospitalists are filling in the leadership gaps as other specialists move to outpatient centers or into the office. Posts that have traditionally been held by cardiology or urology are changing.
Give some thought to where you might help out. A commitment to something as simple as the Pharmacy and Therapeutics Committee can lead to changes for all the patients as well as get you started on your new career path. Every chief of staff and vice president of medical affairs is looking for volunteers who are interested in projects and can follow through. This applies to department chairmen as well. Given the commitment we all have to our patients and our lives, the offer of help for even just one project is a breath of fresh air to those who have the responsibility for the group or department. Depending on your area of interest—patient safety, quality improvement, patient or medical student education, or process improvement—a project can be created that furthers your institution and addresses your interests.
Worried that there is no room for you at the table? Think again. If your department chair or chief of staff is not asking for help, it may be that their requests have fallen on deaf ears for so long that they have stopped asking. If you have identified a project that interests you, it may interest others also. Ask if there is a way that you can work with others on an existing project. Alternatively, ask if there is a project that needs doing that has no one to do it. Many projects need a political and medical champion; they would welcome your offer to help. Volunteer and be prepared to take on a project that is not your favorite but that may give you experience for other projects. Your initiative will certainly get the attention of the chairperson or others because you are solving a problem for them.
Ask, Listen, and Learn
In addition, this is just a beginning step that will lead to further leadership positions for many. Gaining experience with what works and with how you can accomplish initiatives will lead to bigger opportunities. Embedding yourself in the fabric of your organization provides an opportunity for others to see you work and interact. Seek the advice of those whom you trust and who appear to be successful. Listen to feedback and adjust accordingly. Take classes on leadership, financial performance, process improvement, or in areas that appeal to you and that address what you want to accomplish. Before you know it, you will be chief of staff, division chair, or chief medical officer. Knowing where you want to end up is always an advantage, but many individuals find their way through different experiences and exposures. Sometimes where you end up is not what you expected, but the journey is usually interesting.
Different career choices come at different times. Focusing on family, whether it is making time for children, caring for elderly parents, or supporting a spouse’s career choice may require less career focus for a time. However, as your responsibilities change, new opportunities arise. Finding a mentor or trusted individual who can advise you during these times is helpful.
Develop and Demonstrate Executive Expertise
As the hospital environment changes and hospitalists become the primary providers of care in the acute care setting, they will become hospital coordinators, in conjunction with the emergency department and other specialists. They will develop a knowledge that can be leveraged to improve processes, reduce errors, and improve outcomes. A different set of skills is needed to be successful as an executive. It requires a different way of problem solving; it requires studying and applying new lessons. The successful person develops this new expertise. The effect this person makes in applying these new skills will lead to increased roles and responsibilities. There will be continuing demand for individuals who can access, plan, and implement change within our complex systems. There will also be continuing challenges in healthcare, including the areas of medical education, research, the uninsured, and the aging population. Skills acquired now could be applied as the vice president of medical affairs or as chief medical officer.
Historically, the chief executive officer of a hospital or an integrated system has been a non-medical person with business expertise in healthcare. Hospitalists may fill this role more and more in the future. Many individuals are starting their careers in the hospital. This experience will allow them to develop skills their prior colleagues did not have. It will expose them to teamwork, results orientation, and mentors. We are a young group of professionals with many career years ahead of us. Hospitals are increasingly recognizing that the expertise of committed physician partners is critical to their success. This combination of interest and opportunity will groom many individuals, some of whom will affect healthcare for generations.
Bringing our knowledge of medicine to business, and creating crossroads and interactions, can advance our careers at the same time it improves the healthcare of others. This type of career path is not out of reach for you, and to think it all started with the Pharmacy and Therapeutics Committee. TH
Dr. Gorman is the president of SHM.