User login
Concomitant PsA tied with higher comorbidities and low treatment persistence in psoriasis
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Diagnostic role of nailfold capillaroscopy for identifying PsA in psoriasis needs further investigation
Key clinical point: Nailfold capillaroscopy (NC) outcomes could not conclusively differentiate psoriasis from psoriatic arthritis (PsA).
Major finding: In addition to altered morphology, the density of capillaries at the nailfold was significantly lower in patients with psoriasis (standardized group difference [SMD] −0.91; P = .0058; area under curve [AUC] 0.740) and PsA (SMD −1.22; P = .0432; AUC, 0.806) compared with control individuals; however, no NC outcomes conclusively differentiated between psoriasis and PsA.
Study details: Findings are from a systematic review and meta-analysis of 22 studies investigating NC as a diagnostic tool for psoriasis or PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lazar LT et al. Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 2023;147:104476 (Jan 16). Doi: 10.1016/j.mvr.2023.104476
Key clinical point: Nailfold capillaroscopy (NC) outcomes could not conclusively differentiate psoriasis from psoriatic arthritis (PsA).
Major finding: In addition to altered morphology, the density of capillaries at the nailfold was significantly lower in patients with psoriasis (standardized group difference [SMD] −0.91; P = .0058; area under curve [AUC] 0.740) and PsA (SMD −1.22; P = .0432; AUC, 0.806) compared with control individuals; however, no NC outcomes conclusively differentiated between psoriasis and PsA.
Study details: Findings are from a systematic review and meta-analysis of 22 studies investigating NC as a diagnostic tool for psoriasis or PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lazar LT et al. Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 2023;147:104476 (Jan 16). Doi: 10.1016/j.mvr.2023.104476
Key clinical point: Nailfold capillaroscopy (NC) outcomes could not conclusively differentiate psoriasis from psoriatic arthritis (PsA).
Major finding: In addition to altered morphology, the density of capillaries at the nailfold was significantly lower in patients with psoriasis (standardized group difference [SMD] −0.91; P = .0058; area under curve [AUC] 0.740) and PsA (SMD −1.22; P = .0432; AUC, 0.806) compared with control individuals; however, no NC outcomes conclusively differentiated between psoriasis and PsA.
Study details: Findings are from a systematic review and meta-analysis of 22 studies investigating NC as a diagnostic tool for psoriasis or PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lazar LT et al. Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 2023;147:104476 (Jan 16). Doi: 10.1016/j.mvr.2023.104476
Psoriatic arthritis: An independent risk factor for reduced bone density and fractures
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
PsA: Guselkumab demonstrates consistent safety profile irrespective of prior TNFi exposure
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Commentary: Concerning PsA treatments and comorbidities, March 2023
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
Treatment of Axial Psoriatic Arthritis
Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that may involve several different domains, including peripheral joints, entheses, nails, axial skeleton, and skin. A recent increased awareness of PsA has accompanied a large increase in available therapeutic options. In addition to traditional disease-modifying antirheumatic drugs (DMARDs), new biologics and targeted small molecules have now been shown to be effective in PsA. These agents include those targeting pathways involving tumor necrosis factor (TNF), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), interleukins (IL) 12, 17, 23, janus kinase (JAK), and phosphodiesterase 4 (PDE4). These agents have demonstrated efficacy in outcome measures developed for peripheral arthritis, such as the American College of Rheumatology 20 (ACR20) response. However, an ongoing question is whether these agents are equally effective in axial disease. Based on our experience and the existing literature, we believe that some of these agents, including PDE4 and IL-23 inhibitors, are not effective for axial disease.
Moll and Wright’s original description of PsA estimated that 5% of patients with PsA had axial disease1; however, they were describing patients in whom axial arthritis was the predominant, or the only, manifestation. There are many patients for whom axial symptoms are just one of several domains of disease activity. With this in mind, and depending on the cohort studied, the estimated overall prevalence of axial disease ranges from 7% to 32% in patients with PsA.2 This is in contrast to peripheral arthritis, a domain that occurs in most patients with PsA and is the most common manifestation of PsA.2 We believe there are differences in axial and peripheral response among some of the drugs used to treat PsA; therefore it is critical to consider both the presence and magnitude of axial involvement.
An absence of axial PsA–specific clinical trials complicates navigating this treatment domain. Most considerations regarding treatment options for axial disease in PsA are extrapolated from ankylosing spondylitis (AS) trials and experience, as is the case for the TNF and JAK inhibitors. To our knowledge, only one high-quality randomized trial, MAXIMISE, looked specifically at the treatment of axial PsA, in this case with the IL-17 inhibitor secukinumab.3 This trial demonstrated efficacy of secukinumab in reducing symptoms and acute phase reactants in patients with PsA who were categorized as having active axial disease using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Other than conclusions drawn from AS trials and from this single axial PsA randomized controlled trial, data on the treatment of axial PsA are drawn entirely from observational and post-hoc analyses. As there are no consensus criteria for axial PsA, the cohorts included in these data may vary. This heterogeneity showcases the diversity in patients with PsA with axial disease but complicates the generalizability of the findings to individual patients.
Another challenge in understanding axial response to medication is the lack of specific, validated outcome measures for axial PsA. The BASDAI and, more recently, the Assessment in Ankylosing Spondylitis (ASAS) and the Ankylosing Spondylitis Disease Activity Score (ASDAS), all developed specifically for AS, are often used to measure treatment response. The BASDAI incorporates patient-reported symptoms which include fatigue, peripheral joint pain/swelling, tenderness, and morning stiffness not specifically localized to the back. The ASDAS also includes a C-reactive protein measurement.
When used to assess response in PsA, however, these patient-reported outcomes may not be precise enough to separate the impact of axial disease or symptoms from that of peripheral disease. Only question 2 on the BASDAI specifically addresses axial complaints: “How would you describe the overall level of AS-related pain you have had in your neck, back, or hips?” Even this question is vulnerable to confounding from noninflammatory causes of back pain. Although these issues exist with patient-reported outcomes, objective spinal mobility measures used in evaluation of AS, including the modified Schober test, lumbar side flexion, and cervical rotation, have been demonstrated also to perform well in axial PsA.4
This was corroborated in the INSPIRE study, which showed adequate interobserver reliability in primary AS that was equally reproducible in axial PsA, with most measures, including occiput to wall, modified Schober test, cervical rotation, lateral bending, and hip mobility, performing in a “good to excellent” manner.5 Therefore, the inclusion of these objective measures in future therapeutic studies may enhance the external validation of available data.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has established therapeutic guidelines for psoriatic disease based on currently available literature and data. Similar to previous iterations of guidelines, GRAPPA continues to recommend agents with TNF inhibition or IL-17 inhibition for patients with PsA with axial disease who have failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and/or glucocorticoid injections. Newly recommended in the latest iteration of the GRAPPA guidelines, based on the efficacy of these agents in AS, is the use of JAK inhibitors for axial PsA.6
Although TNF, IL-17, and JAK targeted therapies have demonstrated more likely benefit, albeit subject to the trial limitations previously discussed, the question remains whether agents targeting PDE4 and IL-23 are an effective option for axial PsA. Studies of both PDE4 and IL-23 inhibitors in AS have not demonstrated adequate benefit, which, importantly, contrasts with the previously mentioned and recommended therapies. Additionally, there are no primary randomized control trials that have directly evaluated the efficacy of IL-23 therapy in axial PsA.
Existing data about potential benefit come from post-hoc analyses of the PSUMMIT 1 and 2 trials7-10 with ustekinumab (which inhibits IL-12 and IL-23) and the DISCOVER trials11-13 with guselkumab (a pure IL-23 inhibitor). However, these analyses relied on a physician-reported diagnosis of axial disease and not on prespecified entry criteria. This lack of uniform diagnostic criteria may introduce bias into the interpretation of the results and limit external validation. All patients in these trials had a significant burden of peripheral arthritis; therefore it is hard to know whether, even in patients with physician-reported axial disease, improvement in general outcome measures were due to true amelioration of axial disease or were confounded by improvement in peripheral and skin domains. The analysis of these trials did look specifically at patient answers to BASDAI question 2 regarding level of neck, back, or hip pain. However, it remains difficult to be certain that the results are truly a reflection of axial symptoms and are not driven by patient-perceived improvement in other disease domains and an overall positive trajectory in well-being.
In our years of practice, when we turned to biologic agents, the IL-23 inhibitors and the IL-12/23 inhibitor have not been as effective in patients with PsA who have axial-predominant symptoms. The lack of efficacy of these agents in AS, in contrast to their benefit in psoriatic skin and peripheral joint disease, raises questions about the pathophysiologic role of IL-23 in axial disease, which is yet to be fully understood. For patients with a significant burden of axial pain, in concordance with the consensus from GRAPPA,6 our strategy is to start with TNF, IL-17, or JAK targeted therapies, with the choice based on patient-specific factors, including patient comorbidities, patient administration preference, and insurance coverage. We do believe it is reasonable to try IL-23–targeted therapies in patients who have mild axial symptoms when their predominant symptoms are in other domains, such as the peripheral joints or skin. In our opinion, more convincing data supporting IL-23 inhibition are required to move this into the forefront of axial-predominant PsA therapy. Clearly the investigation of axial disease in PsA lags behind that of peripheral and skin domains. Specific classification criteria for axial PsA, as are being currently developed by GRAPPA, should facilitate more focused therapeutic trials that can better inform optimal treatment of patients with this subset of disease.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55-78. doi:10.1016/0049-0172(73)90035-8
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi:10.1016/j.rdc.2015.07.001
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590. doi:10.1136/annrheumdis-2020-218808
- Fernández-Sueiro JL, Willisch A, Pértega-Díaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum. 2009;61(3):386-392. doi:10.1002/art.24280
- Gladman DD, Inman RD, Cook RJ, et al. International spondyloarthritis interobserver reliability exercise—the INSPIRE study: I. Assessment of spinal measures. J Rheumatol. 2007;34(8):1733-1739.
- Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022;49(6 suppl 1):52-54. doi:10.3899/jrheum.211331
- McInnes IB, Kavanaugh A, Gottlieb AB, et al; PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. doi:10.1016/S0140-6736(13)60594-2
- Ritchlin C, Rahman P, Kavanaugh A, et al; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990-999. doi:10.1136/annrheumdis-2013-204655
- Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984-1988. doi:10.1136/annrheumdis-2015-209068
- McInnes IB, Chakravarty SD, Apaolaza I, et al. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: data from PSUMMIT 1 and PSUMMIT 2. RMD Open. 2019;5(2):e000990. doi:10.1136/rmdopen-2019-000990
- Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER-1 Study Group. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. doi:10.1016/S0140-6736(20)30265-8
- Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. doi:10.1016/S0140-6736(20)30263-4
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 discover-1 and discover-2 studies. Lancet Rheumatol. 2021;3(10). doi:https://doi.org/10.1016/S2665-9913(21)00105-3
Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that may involve several different domains, including peripheral joints, entheses, nails, axial skeleton, and skin. A recent increased awareness of PsA has accompanied a large increase in available therapeutic options. In addition to traditional disease-modifying antirheumatic drugs (DMARDs), new biologics and targeted small molecules have now been shown to be effective in PsA. These agents include those targeting pathways involving tumor necrosis factor (TNF), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), interleukins (IL) 12, 17, 23, janus kinase (JAK), and phosphodiesterase 4 (PDE4). These agents have demonstrated efficacy in outcome measures developed for peripheral arthritis, such as the American College of Rheumatology 20 (ACR20) response. However, an ongoing question is whether these agents are equally effective in axial disease. Based on our experience and the existing literature, we believe that some of these agents, including PDE4 and IL-23 inhibitors, are not effective for axial disease.
Moll and Wright’s original description of PsA estimated that 5% of patients with PsA had axial disease1; however, they were describing patients in whom axial arthritis was the predominant, or the only, manifestation. There are many patients for whom axial symptoms are just one of several domains of disease activity. With this in mind, and depending on the cohort studied, the estimated overall prevalence of axial disease ranges from 7% to 32% in patients with PsA.2 This is in contrast to peripheral arthritis, a domain that occurs in most patients with PsA and is the most common manifestation of PsA.2 We believe there are differences in axial and peripheral response among some of the drugs used to treat PsA; therefore it is critical to consider both the presence and magnitude of axial involvement.
An absence of axial PsA–specific clinical trials complicates navigating this treatment domain. Most considerations regarding treatment options for axial disease in PsA are extrapolated from ankylosing spondylitis (AS) trials and experience, as is the case for the TNF and JAK inhibitors. To our knowledge, only one high-quality randomized trial, MAXIMISE, looked specifically at the treatment of axial PsA, in this case with the IL-17 inhibitor secukinumab.3 This trial demonstrated efficacy of secukinumab in reducing symptoms and acute phase reactants in patients with PsA who were categorized as having active axial disease using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Other than conclusions drawn from AS trials and from this single axial PsA randomized controlled trial, data on the treatment of axial PsA are drawn entirely from observational and post-hoc analyses. As there are no consensus criteria for axial PsA, the cohorts included in these data may vary. This heterogeneity showcases the diversity in patients with PsA with axial disease but complicates the generalizability of the findings to individual patients.
Another challenge in understanding axial response to medication is the lack of specific, validated outcome measures for axial PsA. The BASDAI and, more recently, the Assessment in Ankylosing Spondylitis (ASAS) and the Ankylosing Spondylitis Disease Activity Score (ASDAS), all developed specifically for AS, are often used to measure treatment response. The BASDAI incorporates patient-reported symptoms which include fatigue, peripheral joint pain/swelling, tenderness, and morning stiffness not specifically localized to the back. The ASDAS also includes a C-reactive protein measurement.
When used to assess response in PsA, however, these patient-reported outcomes may not be precise enough to separate the impact of axial disease or symptoms from that of peripheral disease. Only question 2 on the BASDAI specifically addresses axial complaints: “How would you describe the overall level of AS-related pain you have had in your neck, back, or hips?” Even this question is vulnerable to confounding from noninflammatory causes of back pain. Although these issues exist with patient-reported outcomes, objective spinal mobility measures used in evaluation of AS, including the modified Schober test, lumbar side flexion, and cervical rotation, have been demonstrated also to perform well in axial PsA.4
This was corroborated in the INSPIRE study, which showed adequate interobserver reliability in primary AS that was equally reproducible in axial PsA, with most measures, including occiput to wall, modified Schober test, cervical rotation, lateral bending, and hip mobility, performing in a “good to excellent” manner.5 Therefore, the inclusion of these objective measures in future therapeutic studies may enhance the external validation of available data.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has established therapeutic guidelines for psoriatic disease based on currently available literature and data. Similar to previous iterations of guidelines, GRAPPA continues to recommend agents with TNF inhibition or IL-17 inhibition for patients with PsA with axial disease who have failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and/or glucocorticoid injections. Newly recommended in the latest iteration of the GRAPPA guidelines, based on the efficacy of these agents in AS, is the use of JAK inhibitors for axial PsA.6
Although TNF, IL-17, and JAK targeted therapies have demonstrated more likely benefit, albeit subject to the trial limitations previously discussed, the question remains whether agents targeting PDE4 and IL-23 are an effective option for axial PsA. Studies of both PDE4 and IL-23 inhibitors in AS have not demonstrated adequate benefit, which, importantly, contrasts with the previously mentioned and recommended therapies. Additionally, there are no primary randomized control trials that have directly evaluated the efficacy of IL-23 therapy in axial PsA.
Existing data about potential benefit come from post-hoc analyses of the PSUMMIT 1 and 2 trials7-10 with ustekinumab (which inhibits IL-12 and IL-23) and the DISCOVER trials11-13 with guselkumab (a pure IL-23 inhibitor). However, these analyses relied on a physician-reported diagnosis of axial disease and not on prespecified entry criteria. This lack of uniform diagnostic criteria may introduce bias into the interpretation of the results and limit external validation. All patients in these trials had a significant burden of peripheral arthritis; therefore it is hard to know whether, even in patients with physician-reported axial disease, improvement in general outcome measures were due to true amelioration of axial disease or were confounded by improvement in peripheral and skin domains. The analysis of these trials did look specifically at patient answers to BASDAI question 2 regarding level of neck, back, or hip pain. However, it remains difficult to be certain that the results are truly a reflection of axial symptoms and are not driven by patient-perceived improvement in other disease domains and an overall positive trajectory in well-being.
In our years of practice, when we turned to biologic agents, the IL-23 inhibitors and the IL-12/23 inhibitor have not been as effective in patients with PsA who have axial-predominant symptoms. The lack of efficacy of these agents in AS, in contrast to their benefit in psoriatic skin and peripheral joint disease, raises questions about the pathophysiologic role of IL-23 in axial disease, which is yet to be fully understood. For patients with a significant burden of axial pain, in concordance with the consensus from GRAPPA,6 our strategy is to start with TNF, IL-17, or JAK targeted therapies, with the choice based on patient-specific factors, including patient comorbidities, patient administration preference, and insurance coverage. We do believe it is reasonable to try IL-23–targeted therapies in patients who have mild axial symptoms when their predominant symptoms are in other domains, such as the peripheral joints or skin. In our opinion, more convincing data supporting IL-23 inhibition are required to move this into the forefront of axial-predominant PsA therapy. Clearly the investigation of axial disease in PsA lags behind that of peripheral and skin domains. Specific classification criteria for axial PsA, as are being currently developed by GRAPPA, should facilitate more focused therapeutic trials that can better inform optimal treatment of patients with this subset of disease.
Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that may involve several different domains, including peripheral joints, entheses, nails, axial skeleton, and skin. A recent increased awareness of PsA has accompanied a large increase in available therapeutic options. In addition to traditional disease-modifying antirheumatic drugs (DMARDs), new biologics and targeted small molecules have now been shown to be effective in PsA. These agents include those targeting pathways involving tumor necrosis factor (TNF), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), interleukins (IL) 12, 17, 23, janus kinase (JAK), and phosphodiesterase 4 (PDE4). These agents have demonstrated efficacy in outcome measures developed for peripheral arthritis, such as the American College of Rheumatology 20 (ACR20) response. However, an ongoing question is whether these agents are equally effective in axial disease. Based on our experience and the existing literature, we believe that some of these agents, including PDE4 and IL-23 inhibitors, are not effective for axial disease.
Moll and Wright’s original description of PsA estimated that 5% of patients with PsA had axial disease1; however, they were describing patients in whom axial arthritis was the predominant, or the only, manifestation. There are many patients for whom axial symptoms are just one of several domains of disease activity. With this in mind, and depending on the cohort studied, the estimated overall prevalence of axial disease ranges from 7% to 32% in patients with PsA.2 This is in contrast to peripheral arthritis, a domain that occurs in most patients with PsA and is the most common manifestation of PsA.2 We believe there are differences in axial and peripheral response among some of the drugs used to treat PsA; therefore it is critical to consider both the presence and magnitude of axial involvement.
An absence of axial PsA–specific clinical trials complicates navigating this treatment domain. Most considerations regarding treatment options for axial disease in PsA are extrapolated from ankylosing spondylitis (AS) trials and experience, as is the case for the TNF and JAK inhibitors. To our knowledge, only one high-quality randomized trial, MAXIMISE, looked specifically at the treatment of axial PsA, in this case with the IL-17 inhibitor secukinumab.3 This trial demonstrated efficacy of secukinumab in reducing symptoms and acute phase reactants in patients with PsA who were categorized as having active axial disease using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Other than conclusions drawn from AS trials and from this single axial PsA randomized controlled trial, data on the treatment of axial PsA are drawn entirely from observational and post-hoc analyses. As there are no consensus criteria for axial PsA, the cohorts included in these data may vary. This heterogeneity showcases the diversity in patients with PsA with axial disease but complicates the generalizability of the findings to individual patients.
Another challenge in understanding axial response to medication is the lack of specific, validated outcome measures for axial PsA. The BASDAI and, more recently, the Assessment in Ankylosing Spondylitis (ASAS) and the Ankylosing Spondylitis Disease Activity Score (ASDAS), all developed specifically for AS, are often used to measure treatment response. The BASDAI incorporates patient-reported symptoms which include fatigue, peripheral joint pain/swelling, tenderness, and morning stiffness not specifically localized to the back. The ASDAS also includes a C-reactive protein measurement.
When used to assess response in PsA, however, these patient-reported outcomes may not be precise enough to separate the impact of axial disease or symptoms from that of peripheral disease. Only question 2 on the BASDAI specifically addresses axial complaints: “How would you describe the overall level of AS-related pain you have had in your neck, back, or hips?” Even this question is vulnerable to confounding from noninflammatory causes of back pain. Although these issues exist with patient-reported outcomes, objective spinal mobility measures used in evaluation of AS, including the modified Schober test, lumbar side flexion, and cervical rotation, have been demonstrated also to perform well in axial PsA.4
This was corroborated in the INSPIRE study, which showed adequate interobserver reliability in primary AS that was equally reproducible in axial PsA, with most measures, including occiput to wall, modified Schober test, cervical rotation, lateral bending, and hip mobility, performing in a “good to excellent” manner.5 Therefore, the inclusion of these objective measures in future therapeutic studies may enhance the external validation of available data.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has established therapeutic guidelines for psoriatic disease based on currently available literature and data. Similar to previous iterations of guidelines, GRAPPA continues to recommend agents with TNF inhibition or IL-17 inhibition for patients with PsA with axial disease who have failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and/or glucocorticoid injections. Newly recommended in the latest iteration of the GRAPPA guidelines, based on the efficacy of these agents in AS, is the use of JAK inhibitors for axial PsA.6
Although TNF, IL-17, and JAK targeted therapies have demonstrated more likely benefit, albeit subject to the trial limitations previously discussed, the question remains whether agents targeting PDE4 and IL-23 are an effective option for axial PsA. Studies of both PDE4 and IL-23 inhibitors in AS have not demonstrated adequate benefit, which, importantly, contrasts with the previously mentioned and recommended therapies. Additionally, there are no primary randomized control trials that have directly evaluated the efficacy of IL-23 therapy in axial PsA.
Existing data about potential benefit come from post-hoc analyses of the PSUMMIT 1 and 2 trials7-10 with ustekinumab (which inhibits IL-12 and IL-23) and the DISCOVER trials11-13 with guselkumab (a pure IL-23 inhibitor). However, these analyses relied on a physician-reported diagnosis of axial disease and not on prespecified entry criteria. This lack of uniform diagnostic criteria may introduce bias into the interpretation of the results and limit external validation. All patients in these trials had a significant burden of peripheral arthritis; therefore it is hard to know whether, even in patients with physician-reported axial disease, improvement in general outcome measures were due to true amelioration of axial disease or were confounded by improvement in peripheral and skin domains. The analysis of these trials did look specifically at patient answers to BASDAI question 2 regarding level of neck, back, or hip pain. However, it remains difficult to be certain that the results are truly a reflection of axial symptoms and are not driven by patient-perceived improvement in other disease domains and an overall positive trajectory in well-being.
In our years of practice, when we turned to biologic agents, the IL-23 inhibitors and the IL-12/23 inhibitor have not been as effective in patients with PsA who have axial-predominant symptoms. The lack of efficacy of these agents in AS, in contrast to their benefit in psoriatic skin and peripheral joint disease, raises questions about the pathophysiologic role of IL-23 in axial disease, which is yet to be fully understood. For patients with a significant burden of axial pain, in concordance with the consensus from GRAPPA,6 our strategy is to start with TNF, IL-17, or JAK targeted therapies, with the choice based on patient-specific factors, including patient comorbidities, patient administration preference, and insurance coverage. We do believe it is reasonable to try IL-23–targeted therapies in patients who have mild axial symptoms when their predominant symptoms are in other domains, such as the peripheral joints or skin. In our opinion, more convincing data supporting IL-23 inhibition are required to move this into the forefront of axial-predominant PsA therapy. Clearly the investigation of axial disease in PsA lags behind that of peripheral and skin domains. Specific classification criteria for axial PsA, as are being currently developed by GRAPPA, should facilitate more focused therapeutic trials that can better inform optimal treatment of patients with this subset of disease.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55-78. doi:10.1016/0049-0172(73)90035-8
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi:10.1016/j.rdc.2015.07.001
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590. doi:10.1136/annrheumdis-2020-218808
- Fernández-Sueiro JL, Willisch A, Pértega-Díaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum. 2009;61(3):386-392. doi:10.1002/art.24280
- Gladman DD, Inman RD, Cook RJ, et al. International spondyloarthritis interobserver reliability exercise—the INSPIRE study: I. Assessment of spinal measures. J Rheumatol. 2007;34(8):1733-1739.
- Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022;49(6 suppl 1):52-54. doi:10.3899/jrheum.211331
- McInnes IB, Kavanaugh A, Gottlieb AB, et al; PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. doi:10.1016/S0140-6736(13)60594-2
- Ritchlin C, Rahman P, Kavanaugh A, et al; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990-999. doi:10.1136/annrheumdis-2013-204655
- Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984-1988. doi:10.1136/annrheumdis-2015-209068
- McInnes IB, Chakravarty SD, Apaolaza I, et al. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: data from PSUMMIT 1 and PSUMMIT 2. RMD Open. 2019;5(2):e000990. doi:10.1136/rmdopen-2019-000990
- Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER-1 Study Group. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. doi:10.1016/S0140-6736(20)30265-8
- Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. doi:10.1016/S0140-6736(20)30263-4
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 discover-1 and discover-2 studies. Lancet Rheumatol. 2021;3(10). doi:https://doi.org/10.1016/S2665-9913(21)00105-3
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55-78. doi:10.1016/0049-0172(73)90035-8
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi:10.1016/j.rdc.2015.07.001
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590. doi:10.1136/annrheumdis-2020-218808
- Fernández-Sueiro JL, Willisch A, Pértega-Díaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum. 2009;61(3):386-392. doi:10.1002/art.24280
- Gladman DD, Inman RD, Cook RJ, et al. International spondyloarthritis interobserver reliability exercise—the INSPIRE study: I. Assessment of spinal measures. J Rheumatol. 2007;34(8):1733-1739.
- Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022;49(6 suppl 1):52-54. doi:10.3899/jrheum.211331
- McInnes IB, Kavanaugh A, Gottlieb AB, et al; PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. doi:10.1016/S0140-6736(13)60594-2
- Ritchlin C, Rahman P, Kavanaugh A, et al; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990-999. doi:10.1136/annrheumdis-2013-204655
- Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984-1988. doi:10.1136/annrheumdis-2015-209068
- McInnes IB, Chakravarty SD, Apaolaza I, et al. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: data from PSUMMIT 1 and PSUMMIT 2. RMD Open. 2019;5(2):e000990. doi:10.1136/rmdopen-2019-000990
- Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER-1 Study Group. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. doi:10.1016/S0140-6736(20)30265-8
- Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. doi:10.1016/S0140-6736(20)30263-4
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 discover-1 and discover-2 studies. Lancet Rheumatol. 2021;3(10). doi:https://doi.org/10.1016/S2665-9913(21)00105-3
Isolated nail psoriasis may bring arthritis into play
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.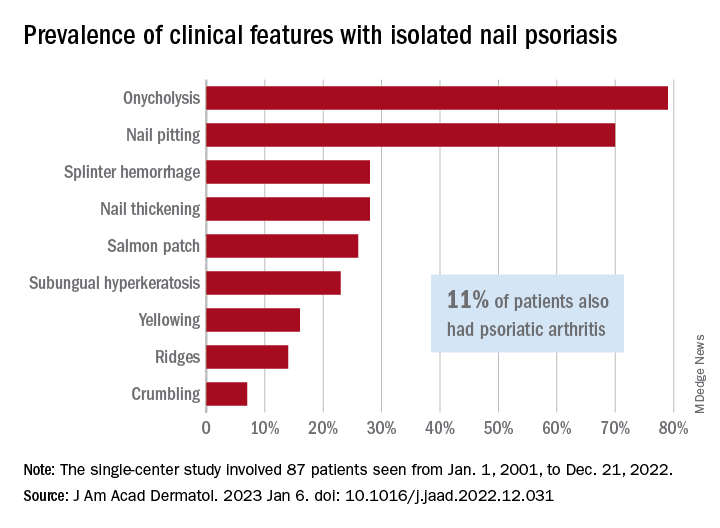
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
What’s holding back physicians from prescribing biosimilars? Four specialties weigh in
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
Are repeat radiographs necessary in rheumatoid and psoriatic arthritis?
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
FROM RWCS 2023
PsA prediction tool approaches clinical utility
Easily collected variables establish risk
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
Easily collected variables establish risk
Easily collected variables establish risk
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
FROM CRA 2023