User login
Racial/ethnic disparities exacerbated maternal death rise during 2020 pandemic.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
FROM JAMA NETWORK OPEN
Noninvasive brain stimulation promising for COVID-related smell loss
Noninvasive brain stimulation may help restore a sense of smell in patients with chronic anosmia or hyposmia related to COVID-19, early research suggests.
Results of a small, double-blind, sham-controlled study showed anodal transcranial direct current stimulation (A-tDCS) combined with olfactory training (OT) provided notable and durable improvement in seven patients with persistent COVID-19–related hyposmia or anosmia.
“We are proud and very excited about these results. Although seven patients is a small sample, it is still notable,” lead investigator Fabio Bandini, MD, head of the department of neurology, ASL 3 Genovese, Genoa, Italy, said in an interview.
tDCS is cheap, safe, accessible, and very easy to administer. It has been used in rehabilitative treatment for 15 years, but this is the first time it has been used for this kind of problem, Dr. Bandini added.
The study was published online in the Journal of Neurology, Neurosurgery, and Psychiatry.
First study of its kind
Approximately 1% of patients with COVID will suffer from long-term smell loss, and given the widespread global impact of COVID, this represents a substantial number who have experienced or will potentially experience chronic smell loss because of the disease.
Loss of smell associated with COVID may last anywhere from 15 to 180 days after a SAR-CoV-2 infection, the researchers noted. Research suggests there is central nervous system involvement in COVID anosmia, mostly in the orbitofrontal cortex – the neural substrate for conscious olfactory perception.
“Smell loss has important consequences in everyday life for food, for hazards, for socialization. Usually, you recover from smell loss after 2 or 3 months, but after 6 months, that is considered permanent,” said Dr. Bandini.
Some research has pointed to the activation of the orbital frontal cortex for control of olfactory perception, so Dr. Bandini and colleagues wanted to explore whether stimulating this area could improve smell disturbances in post-COVID patients.
The study included seven consecutive patients with hyposmia or anosmia from COVID-19 lasting at least 6 months and who had a score of less than 12 on the Sniffin’ Sticks identification subtest. Exclusion criteria included severe mood disorder, rhinologic diseases, epilepsy, and sensitive scalp. No medications for alleviating olfactory symptoms were permitted.
Patients’ smell performances were assessed immediately prior to stimulation (t0) and rated on a scale of 0-10, with a score of 0 indicating a complete loss of smell and a score of 10 indicating a full sense of smell as the subjective measure. Sniffin’ Sticks, a validated test that assesses smell threshold, discrimination, and validation, was used as an objective measure.
In the 20-minute OT session, patients had to sniff 10 odors (rose, eucalyptus, lemon, star anise, rosemary, strawberry, coconut, vanilla, pine tree, and bergamot) in a random order for 10 seconds each then were asked to identify the smell and rate its intensity. The training was applied once in each session.
A-tDCS or sham-transcranial direct current stimulation (S-tDCS) was administered at the same time. In the active stimulation the anode was placed over the left prefrontal cortex because the orbitofrontal cortex is not directly accessible by A-tDCS.
The patients participated in olfactory training with S-tDCS for the first 2 weeks. In the second 2 weeks of the study, they received OT with A-tDCS.
The order of sham and A-tDCS stimulation was not counterbalanced to avoid potential carryover effects if A-tDCS had been applied first. The patients and assessors collecting the data were blinded.
The smell assessment was repeated immediately after S-tDCS (t1), A-tDCS (t2) and 3 months from the end of stimulation (t3), using the same odors and the same order of the first assessment.
The Wilcoxon test was used to compare each assessment (t1, t2, and t3) with baseline, indicating a two-sided alpha less than 0.05, which was considered statistically significant.
Both the subjective and objective measures showed a statistically significant improvement at t2 and t3, with average measurements doubled or even tripled, compared with t0 and t1. In addition, all patients demonstrated notable improvement in smell performance.
This study, said Dr. Bandini, is the first to use A-tDCS to treat patients with persistent smell loss due to COVID. Not only did the results show significant improvement in all study participants, compared with baseline but the beneficial effect lasted up to 3 months after treatment, demonstrating a durable effect.
Dr. Bandini noted that the study’s small sample size is a major limitation of the research so he hopes to enlarge it in future research testing A-tDCS for COVID-related smell loss and work toward providing this therapy on an outpatient basis.
Encouraging results offer new hope
Commenting on the research, Cheng-Ying Ho, MD, associate professor of pathology at the Johns Hopkins University, Baltimore, described the study as “interesting and encouraging.
“Even though there is a small percentage of patients that suffer persistent smell loss from COVID, it’s still a large number of people who have smell dysfunction and are unable to recover.”
“So far, there is no treatment for COVID-related or viral infection–related smell loss. The only thing that can be done is olfactory training, but the effect is very limited. There is no drug or other type of therapy for smell loss so far,” said Dr. Ho, whose areas of expertise include neuromuscular pathology, pediatric neuropathology, and neuropathology of infectious diseases.
“Even though it’s a small study with only seven patients, the results are very encouraging. After 2 weeks of stimulation, almost all had smell recovery that lasted several months. The weakness of the study is that they didn’t have a control group. The next step would be to expand the study to include more participants and have an adequate control group that received the sham stimuli to see if their results still stand when they have more participants.
“This very encouraging and relatively noninvasive treatment modality can give patients with smell loss some hope that this therapy can help them recover their sense of smell to some degree. The study seems to suggest that either the tDCS can stimulate nerve regrowth or that it actually can correct the rewiring of the brain,” added Dr. Ho.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. No competing interests were declared.
A version of this article first appeared on Medscape.com.
Noninvasive brain stimulation may help restore a sense of smell in patients with chronic anosmia or hyposmia related to COVID-19, early research suggests.
Results of a small, double-blind, sham-controlled study showed anodal transcranial direct current stimulation (A-tDCS) combined with olfactory training (OT) provided notable and durable improvement in seven patients with persistent COVID-19–related hyposmia or anosmia.
“We are proud and very excited about these results. Although seven patients is a small sample, it is still notable,” lead investigator Fabio Bandini, MD, head of the department of neurology, ASL 3 Genovese, Genoa, Italy, said in an interview.
tDCS is cheap, safe, accessible, and very easy to administer. It has been used in rehabilitative treatment for 15 years, but this is the first time it has been used for this kind of problem, Dr. Bandini added.
The study was published online in the Journal of Neurology, Neurosurgery, and Psychiatry.
First study of its kind
Approximately 1% of patients with COVID will suffer from long-term smell loss, and given the widespread global impact of COVID, this represents a substantial number who have experienced or will potentially experience chronic smell loss because of the disease.
Loss of smell associated with COVID may last anywhere from 15 to 180 days after a SAR-CoV-2 infection, the researchers noted. Research suggests there is central nervous system involvement in COVID anosmia, mostly in the orbitofrontal cortex – the neural substrate for conscious olfactory perception.
“Smell loss has important consequences in everyday life for food, for hazards, for socialization. Usually, you recover from smell loss after 2 or 3 months, but after 6 months, that is considered permanent,” said Dr. Bandini.
Some research has pointed to the activation of the orbital frontal cortex for control of olfactory perception, so Dr. Bandini and colleagues wanted to explore whether stimulating this area could improve smell disturbances in post-COVID patients.
The study included seven consecutive patients with hyposmia or anosmia from COVID-19 lasting at least 6 months and who had a score of less than 12 on the Sniffin’ Sticks identification subtest. Exclusion criteria included severe mood disorder, rhinologic diseases, epilepsy, and sensitive scalp. No medications for alleviating olfactory symptoms were permitted.
Patients’ smell performances were assessed immediately prior to stimulation (t0) and rated on a scale of 0-10, with a score of 0 indicating a complete loss of smell and a score of 10 indicating a full sense of smell as the subjective measure. Sniffin’ Sticks, a validated test that assesses smell threshold, discrimination, and validation, was used as an objective measure.
In the 20-minute OT session, patients had to sniff 10 odors (rose, eucalyptus, lemon, star anise, rosemary, strawberry, coconut, vanilla, pine tree, and bergamot) in a random order for 10 seconds each then were asked to identify the smell and rate its intensity. The training was applied once in each session.
A-tDCS or sham-transcranial direct current stimulation (S-tDCS) was administered at the same time. In the active stimulation the anode was placed over the left prefrontal cortex because the orbitofrontal cortex is not directly accessible by A-tDCS.
The patients participated in olfactory training with S-tDCS for the first 2 weeks. In the second 2 weeks of the study, they received OT with A-tDCS.
The order of sham and A-tDCS stimulation was not counterbalanced to avoid potential carryover effects if A-tDCS had been applied first. The patients and assessors collecting the data were blinded.
The smell assessment was repeated immediately after S-tDCS (t1), A-tDCS (t2) and 3 months from the end of stimulation (t3), using the same odors and the same order of the first assessment.
The Wilcoxon test was used to compare each assessment (t1, t2, and t3) with baseline, indicating a two-sided alpha less than 0.05, which was considered statistically significant.
Both the subjective and objective measures showed a statistically significant improvement at t2 and t3, with average measurements doubled or even tripled, compared with t0 and t1. In addition, all patients demonstrated notable improvement in smell performance.
This study, said Dr. Bandini, is the first to use A-tDCS to treat patients with persistent smell loss due to COVID. Not only did the results show significant improvement in all study participants, compared with baseline but the beneficial effect lasted up to 3 months after treatment, demonstrating a durable effect.
Dr. Bandini noted that the study’s small sample size is a major limitation of the research so he hopes to enlarge it in future research testing A-tDCS for COVID-related smell loss and work toward providing this therapy on an outpatient basis.
Encouraging results offer new hope
Commenting on the research, Cheng-Ying Ho, MD, associate professor of pathology at the Johns Hopkins University, Baltimore, described the study as “interesting and encouraging.
“Even though there is a small percentage of patients that suffer persistent smell loss from COVID, it’s still a large number of people who have smell dysfunction and are unable to recover.”
“So far, there is no treatment for COVID-related or viral infection–related smell loss. The only thing that can be done is olfactory training, but the effect is very limited. There is no drug or other type of therapy for smell loss so far,” said Dr. Ho, whose areas of expertise include neuromuscular pathology, pediatric neuropathology, and neuropathology of infectious diseases.
“Even though it’s a small study with only seven patients, the results are very encouraging. After 2 weeks of stimulation, almost all had smell recovery that lasted several months. The weakness of the study is that they didn’t have a control group. The next step would be to expand the study to include more participants and have an adequate control group that received the sham stimuli to see if their results still stand when they have more participants.
“This very encouraging and relatively noninvasive treatment modality can give patients with smell loss some hope that this therapy can help them recover their sense of smell to some degree. The study seems to suggest that either the tDCS can stimulate nerve regrowth or that it actually can correct the rewiring of the brain,” added Dr. Ho.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. No competing interests were declared.
A version of this article first appeared on Medscape.com.
Noninvasive brain stimulation may help restore a sense of smell in patients with chronic anosmia or hyposmia related to COVID-19, early research suggests.
Results of a small, double-blind, sham-controlled study showed anodal transcranial direct current stimulation (A-tDCS) combined with olfactory training (OT) provided notable and durable improvement in seven patients with persistent COVID-19–related hyposmia or anosmia.
“We are proud and very excited about these results. Although seven patients is a small sample, it is still notable,” lead investigator Fabio Bandini, MD, head of the department of neurology, ASL 3 Genovese, Genoa, Italy, said in an interview.
tDCS is cheap, safe, accessible, and very easy to administer. It has been used in rehabilitative treatment for 15 years, but this is the first time it has been used for this kind of problem, Dr. Bandini added.
The study was published online in the Journal of Neurology, Neurosurgery, and Psychiatry.
First study of its kind
Approximately 1% of patients with COVID will suffer from long-term smell loss, and given the widespread global impact of COVID, this represents a substantial number who have experienced or will potentially experience chronic smell loss because of the disease.
Loss of smell associated with COVID may last anywhere from 15 to 180 days after a SAR-CoV-2 infection, the researchers noted. Research suggests there is central nervous system involvement in COVID anosmia, mostly in the orbitofrontal cortex – the neural substrate for conscious olfactory perception.
“Smell loss has important consequences in everyday life for food, for hazards, for socialization. Usually, you recover from smell loss after 2 or 3 months, but after 6 months, that is considered permanent,” said Dr. Bandini.
Some research has pointed to the activation of the orbital frontal cortex for control of olfactory perception, so Dr. Bandini and colleagues wanted to explore whether stimulating this area could improve smell disturbances in post-COVID patients.
The study included seven consecutive patients with hyposmia or anosmia from COVID-19 lasting at least 6 months and who had a score of less than 12 on the Sniffin’ Sticks identification subtest. Exclusion criteria included severe mood disorder, rhinologic diseases, epilepsy, and sensitive scalp. No medications for alleviating olfactory symptoms were permitted.
Patients’ smell performances were assessed immediately prior to stimulation (t0) and rated on a scale of 0-10, with a score of 0 indicating a complete loss of smell and a score of 10 indicating a full sense of smell as the subjective measure. Sniffin’ Sticks, a validated test that assesses smell threshold, discrimination, and validation, was used as an objective measure.
In the 20-minute OT session, patients had to sniff 10 odors (rose, eucalyptus, lemon, star anise, rosemary, strawberry, coconut, vanilla, pine tree, and bergamot) in a random order for 10 seconds each then were asked to identify the smell and rate its intensity. The training was applied once in each session.
A-tDCS or sham-transcranial direct current stimulation (S-tDCS) was administered at the same time. In the active stimulation the anode was placed over the left prefrontal cortex because the orbitofrontal cortex is not directly accessible by A-tDCS.
The patients participated in olfactory training with S-tDCS for the first 2 weeks. In the second 2 weeks of the study, they received OT with A-tDCS.
The order of sham and A-tDCS stimulation was not counterbalanced to avoid potential carryover effects if A-tDCS had been applied first. The patients and assessors collecting the data were blinded.
The smell assessment was repeated immediately after S-tDCS (t1), A-tDCS (t2) and 3 months from the end of stimulation (t3), using the same odors and the same order of the first assessment.
The Wilcoxon test was used to compare each assessment (t1, t2, and t3) with baseline, indicating a two-sided alpha less than 0.05, which was considered statistically significant.
Both the subjective and objective measures showed a statistically significant improvement at t2 and t3, with average measurements doubled or even tripled, compared with t0 and t1. In addition, all patients demonstrated notable improvement in smell performance.
This study, said Dr. Bandini, is the first to use A-tDCS to treat patients with persistent smell loss due to COVID. Not only did the results show significant improvement in all study participants, compared with baseline but the beneficial effect lasted up to 3 months after treatment, demonstrating a durable effect.
Dr. Bandini noted that the study’s small sample size is a major limitation of the research so he hopes to enlarge it in future research testing A-tDCS for COVID-related smell loss and work toward providing this therapy on an outpatient basis.
Encouraging results offer new hope
Commenting on the research, Cheng-Ying Ho, MD, associate professor of pathology at the Johns Hopkins University, Baltimore, described the study as “interesting and encouraging.
“Even though there is a small percentage of patients that suffer persistent smell loss from COVID, it’s still a large number of people who have smell dysfunction and are unable to recover.”
“So far, there is no treatment for COVID-related or viral infection–related smell loss. The only thing that can be done is olfactory training, but the effect is very limited. There is no drug or other type of therapy for smell loss so far,” said Dr. Ho, whose areas of expertise include neuromuscular pathology, pediatric neuropathology, and neuropathology of infectious diseases.
“Even though it’s a small study with only seven patients, the results are very encouraging. After 2 weeks of stimulation, almost all had smell recovery that lasted several months. The weakness of the study is that they didn’t have a control group. The next step would be to expand the study to include more participants and have an adequate control group that received the sham stimuli to see if their results still stand when they have more participants.
“This very encouraging and relatively noninvasive treatment modality can give patients with smell loss some hope that this therapy can help them recover their sense of smell to some degree. The study seems to suggest that either the tDCS can stimulate nerve regrowth or that it actually can correct the rewiring of the brain,” added Dr. Ho.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. No competing interests were declared.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY, AND PSYCHIATRY
Children and COVID: Vaccines now available to all ages
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.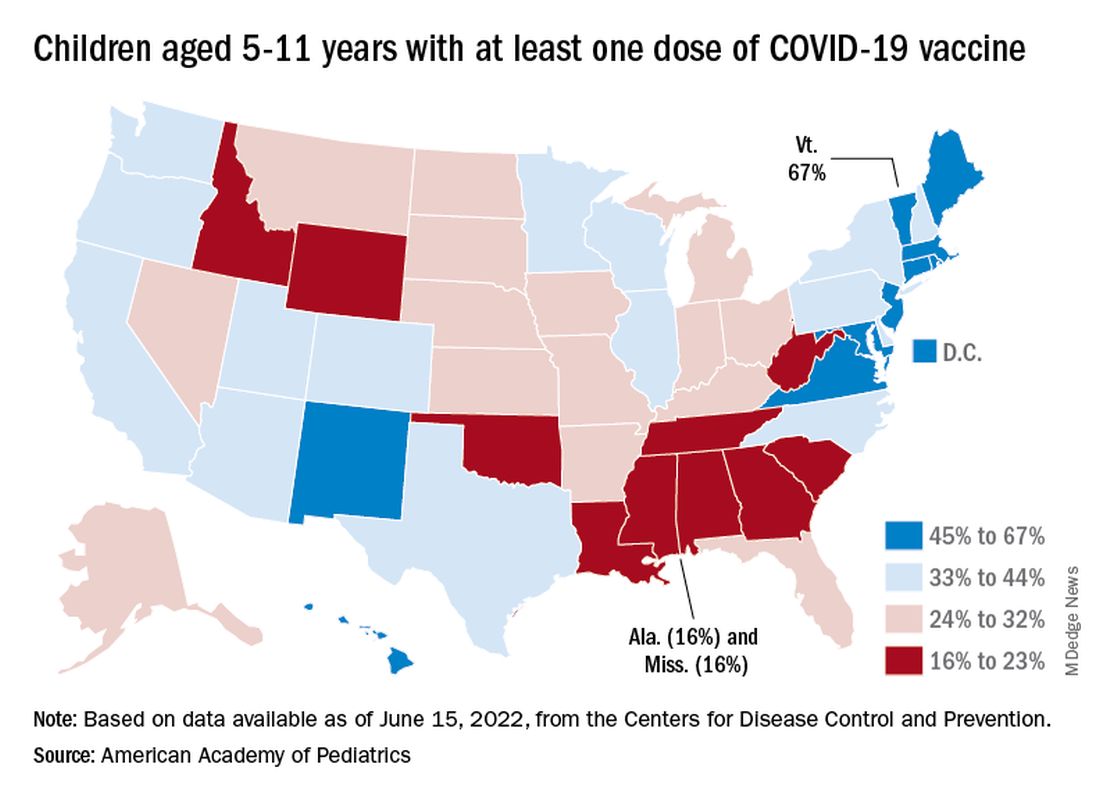
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.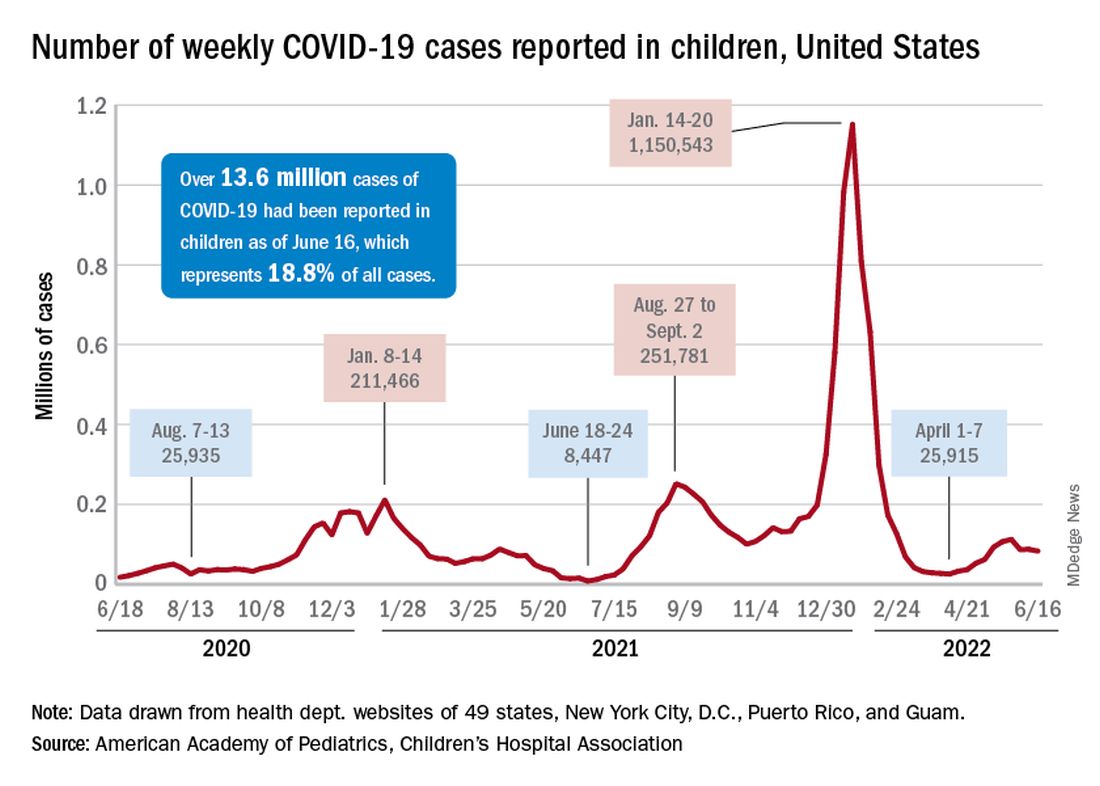
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
New saliva-based COVID-19 test provides rapid results
A rapid, saliva-based test for COVID-19 could enable testing, diagnosis, and prescribing to take place in a single office visit by immediately confirming whether a patient has the infection and needs to be treated, researchers say. The test has sparked commercial interest and earned additional funding from the Canadian government.
The test uses a DNA aptamer – a short, synthetic oligonucleotide that binds to a specific molecular target – that shows high affinity for the SARS-CoV-2 spike protein and its variants. The approach “can be rapidly adapted to different threats,” as well, Leyla Soleymani, PhD, an associate professor of engineering physics at McMaster University, Hamilton, Ontario, Canada, told this news organization. Her team invented the approach.
Adaptable to other pathogens
Current gold-standard COVID-19 tests are based on reverse transcription-polymerase chain reaction (RT-PCR), which are sensitive but costly, complicated, and require waiting at least a couple of days for results, according to Dr. Soleymani and colleagues. Rapid nucleic acid and antigen tests have only “moderate” sensitivity and specificity, particularly when viral loads are low. None have been shown to work well with saliva samples.
By contrast, the new test “uses a reader and test cartridges, similar to the glucose reader,” said Dr. Soleymani, who is also Canada Research chair in Miniaturized Biomedical Devices. A small sample of saliva is added to a chemical reagent and inserted into the reader, which is attached to a smartphone. Once commercialized, the point-of-care test is expected to be performed quickly in a physician’s office or in a clinic.
“The same reader can be applied to a variety of infectious diseases or infection panels by developing new cartridges,” Dr. Soleymani explained. “Noroviruses and bacteria such as C. difficile are on our list” to examine next.What’s more, she added, “this test is ideally positioned for settings where access to centralized labs is not possible, such as less developed countries.”
The team’s recent studies seem to support the promise. A study published last year in the international edition of Angewandte Chemie documents the development of the test, which at that point could detect wild-type SARS-CoV-2 and its Alpha and Delta variants in unprocessed saliva samples in 10 minutes with 80.5% sensitivity and 100% specificity.
This study was followed in January 2022 by a paper in Chemistry showing that the device also detected Alpha, Gamma, Epsilon, Kappa, and Omicron variants, demonstrating its potential for recognizing rapidly evolving targets such as those found in SARS-CoV-2.
In another demonstration of its versatility, the technology was recently adapted and successfully detected animal viruses from saliva samples.
Commercial and government funding
The findings prompted Zentek, an intellectual property development and commercialization company in Guelph, Ont., to license the technology, with plans to invest more than $1 million in the next 5 years to scale up production of the test components and adapt the technology for other forms of infection.
Furthermore, the collaborative efforts required to develop the test and move it forward gained funding from Canada’s Natural Sciences and Engineering Research Council, which is investing nearly $1.5 million in the form of two grants: $1 million to further streamline the technology development in preparation for the next pandemic and $488,440 (including $140,000 from Zentek) to get the current test to market as quickly as possible.
Meanwhile, Dr. Soleymani is urging clinicians “to be open to nontraditional diagnostic approaches even if the traditional tests do the job. Such tests are more rapid and can be used to enable personalized medicine. Our success relies on collaboration and support from clinicians.”
Further validation needed
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and the Harriet Ryan Albee Professor of Medicine at Harvard Medical School, Boston, commented on the study in response to a request from this news organization.
While “it’s always good to have more testing options available,” he said, “we don’t yet have very much information about performance characteristics of the test – that is, its sensitivity and specificity. I’d like to see the performance characteristics of this test compared to PCR tests and to the current rapid antigen tests using a large number of patient samples with currently circulating variants, and tests over time to see how soon tests become positive after symptom onset and for how long they remain positive.”
“Further validation studies and emergency use authorization or approval by regulatory authorities are needed before we will see this test implemented in the field,” Dr. Kuritzkes concluded.
A version of this article first appeared on Medscape.com.
A rapid, saliva-based test for COVID-19 could enable testing, diagnosis, and prescribing to take place in a single office visit by immediately confirming whether a patient has the infection and needs to be treated, researchers say. The test has sparked commercial interest and earned additional funding from the Canadian government.
The test uses a DNA aptamer – a short, synthetic oligonucleotide that binds to a specific molecular target – that shows high affinity for the SARS-CoV-2 spike protein and its variants. The approach “can be rapidly adapted to different threats,” as well, Leyla Soleymani, PhD, an associate professor of engineering physics at McMaster University, Hamilton, Ontario, Canada, told this news organization. Her team invented the approach.
Adaptable to other pathogens
Current gold-standard COVID-19 tests are based on reverse transcription-polymerase chain reaction (RT-PCR), which are sensitive but costly, complicated, and require waiting at least a couple of days for results, according to Dr. Soleymani and colleagues. Rapid nucleic acid and antigen tests have only “moderate” sensitivity and specificity, particularly when viral loads are low. None have been shown to work well with saliva samples.
By contrast, the new test “uses a reader and test cartridges, similar to the glucose reader,” said Dr. Soleymani, who is also Canada Research chair in Miniaturized Biomedical Devices. A small sample of saliva is added to a chemical reagent and inserted into the reader, which is attached to a smartphone. Once commercialized, the point-of-care test is expected to be performed quickly in a physician’s office or in a clinic.
“The same reader can be applied to a variety of infectious diseases or infection panels by developing new cartridges,” Dr. Soleymani explained. “Noroviruses and bacteria such as C. difficile are on our list” to examine next.What’s more, she added, “this test is ideally positioned for settings where access to centralized labs is not possible, such as less developed countries.”
The team’s recent studies seem to support the promise. A study published last year in the international edition of Angewandte Chemie documents the development of the test, which at that point could detect wild-type SARS-CoV-2 and its Alpha and Delta variants in unprocessed saliva samples in 10 minutes with 80.5% sensitivity and 100% specificity.
This study was followed in January 2022 by a paper in Chemistry showing that the device also detected Alpha, Gamma, Epsilon, Kappa, and Omicron variants, demonstrating its potential for recognizing rapidly evolving targets such as those found in SARS-CoV-2.
In another demonstration of its versatility, the technology was recently adapted and successfully detected animal viruses from saliva samples.
Commercial and government funding
The findings prompted Zentek, an intellectual property development and commercialization company in Guelph, Ont., to license the technology, with plans to invest more than $1 million in the next 5 years to scale up production of the test components and adapt the technology for other forms of infection.
Furthermore, the collaborative efforts required to develop the test and move it forward gained funding from Canada’s Natural Sciences and Engineering Research Council, which is investing nearly $1.5 million in the form of two grants: $1 million to further streamline the technology development in preparation for the next pandemic and $488,440 (including $140,000 from Zentek) to get the current test to market as quickly as possible.
Meanwhile, Dr. Soleymani is urging clinicians “to be open to nontraditional diagnostic approaches even if the traditional tests do the job. Such tests are more rapid and can be used to enable personalized medicine. Our success relies on collaboration and support from clinicians.”
Further validation needed
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and the Harriet Ryan Albee Professor of Medicine at Harvard Medical School, Boston, commented on the study in response to a request from this news organization.
While “it’s always good to have more testing options available,” he said, “we don’t yet have very much information about performance characteristics of the test – that is, its sensitivity and specificity. I’d like to see the performance characteristics of this test compared to PCR tests and to the current rapid antigen tests using a large number of patient samples with currently circulating variants, and tests over time to see how soon tests become positive after symptom onset and for how long they remain positive.”
“Further validation studies and emergency use authorization or approval by regulatory authorities are needed before we will see this test implemented in the field,” Dr. Kuritzkes concluded.
A version of this article first appeared on Medscape.com.
A rapid, saliva-based test for COVID-19 could enable testing, diagnosis, and prescribing to take place in a single office visit by immediately confirming whether a patient has the infection and needs to be treated, researchers say. The test has sparked commercial interest and earned additional funding from the Canadian government.
The test uses a DNA aptamer – a short, synthetic oligonucleotide that binds to a specific molecular target – that shows high affinity for the SARS-CoV-2 spike protein and its variants. The approach “can be rapidly adapted to different threats,” as well, Leyla Soleymani, PhD, an associate professor of engineering physics at McMaster University, Hamilton, Ontario, Canada, told this news organization. Her team invented the approach.
Adaptable to other pathogens
Current gold-standard COVID-19 tests are based on reverse transcription-polymerase chain reaction (RT-PCR), which are sensitive but costly, complicated, and require waiting at least a couple of days for results, according to Dr. Soleymani and colleagues. Rapid nucleic acid and antigen tests have only “moderate” sensitivity and specificity, particularly when viral loads are low. None have been shown to work well with saliva samples.
By contrast, the new test “uses a reader and test cartridges, similar to the glucose reader,” said Dr. Soleymani, who is also Canada Research chair in Miniaturized Biomedical Devices. A small sample of saliva is added to a chemical reagent and inserted into the reader, which is attached to a smartphone. Once commercialized, the point-of-care test is expected to be performed quickly in a physician’s office or in a clinic.
“The same reader can be applied to a variety of infectious diseases or infection panels by developing new cartridges,” Dr. Soleymani explained. “Noroviruses and bacteria such as C. difficile are on our list” to examine next.What’s more, she added, “this test is ideally positioned for settings where access to centralized labs is not possible, such as less developed countries.”
The team’s recent studies seem to support the promise. A study published last year in the international edition of Angewandte Chemie documents the development of the test, which at that point could detect wild-type SARS-CoV-2 and its Alpha and Delta variants in unprocessed saliva samples in 10 minutes with 80.5% sensitivity and 100% specificity.
This study was followed in January 2022 by a paper in Chemistry showing that the device also detected Alpha, Gamma, Epsilon, Kappa, and Omicron variants, demonstrating its potential for recognizing rapidly evolving targets such as those found in SARS-CoV-2.
In another demonstration of its versatility, the technology was recently adapted and successfully detected animal viruses from saliva samples.
Commercial and government funding
The findings prompted Zentek, an intellectual property development and commercialization company in Guelph, Ont., to license the technology, with plans to invest more than $1 million in the next 5 years to scale up production of the test components and adapt the technology for other forms of infection.
Furthermore, the collaborative efforts required to develop the test and move it forward gained funding from Canada’s Natural Sciences and Engineering Research Council, which is investing nearly $1.5 million in the form of two grants: $1 million to further streamline the technology development in preparation for the next pandemic and $488,440 (including $140,000 from Zentek) to get the current test to market as quickly as possible.
Meanwhile, Dr. Soleymani is urging clinicians “to be open to nontraditional diagnostic approaches even if the traditional tests do the job. Such tests are more rapid and can be used to enable personalized medicine. Our success relies on collaboration and support from clinicians.”
Further validation needed
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and the Harriet Ryan Albee Professor of Medicine at Harvard Medical School, Boston, commented on the study in response to a request from this news organization.
While “it’s always good to have more testing options available,” he said, “we don’t yet have very much information about performance characteristics of the test – that is, its sensitivity and specificity. I’d like to see the performance characteristics of this test compared to PCR tests and to the current rapid antigen tests using a large number of patient samples with currently circulating variants, and tests over time to see how soon tests become positive after symptom onset and for how long they remain positive.”
“Further validation studies and emergency use authorization or approval by regulatory authorities are needed before we will see this test implemented in the field,” Dr. Kuritzkes concluded.
A version of this article first appeared on Medscape.com.
COVID-19 Pandemic stress affected ovulation, not menstruation
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ENDO 2022
What are the signs of post–acute infection syndromes?
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
FDA authorizes COVID vaccines in kids as young as 6 months
, one of the final steps in a long-awaited authorization process to extend protection to the youngest of Americans.
The agency’s move comes after a closely watched FDA advisory group vote earlier this week, which resulted in a unanimous vote in favor of the FDA authorizing both vaccines in this age group.
“The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs,” the agency said in a news release.
The data show that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older.
Now all eyes are on the Centers for Disease Control and Prevention, which is expected to decide on the final regulatory hurdle at a meeting June 18. The CDC’s Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter.
Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as June 20.
A version of this article first appeared on WebMD.com.
, one of the final steps in a long-awaited authorization process to extend protection to the youngest of Americans.
The agency’s move comes after a closely watched FDA advisory group vote earlier this week, which resulted in a unanimous vote in favor of the FDA authorizing both vaccines in this age group.
“The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs,” the agency said in a news release.
The data show that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older.
Now all eyes are on the Centers for Disease Control and Prevention, which is expected to decide on the final regulatory hurdle at a meeting June 18. The CDC’s Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter.
Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as June 20.
A version of this article first appeared on WebMD.com.
, one of the final steps in a long-awaited authorization process to extend protection to the youngest of Americans.
The agency’s move comes after a closely watched FDA advisory group vote earlier this week, which resulted in a unanimous vote in favor of the FDA authorizing both vaccines in this age group.
“The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs,” the agency said in a news release.
The data show that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older.
Now all eyes are on the Centers for Disease Control and Prevention, which is expected to decide on the final regulatory hurdle at a meeting June 18. The CDC’s Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter.
Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as June 20.
A version of this article first appeared on WebMD.com.
Diabetes tied to risk of long COVID, too
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
FROM ADA 2022
Blood test aims to measure COVID immunity
Scientists created a test that indirectly measures T-cell response – an important, long-term component of immunity that can last long after antibody levels fall off – to a challenge by the virus in whole blood.
The test mimics what can be done in a formal laboratory now but avoids some complicated steps and specialized training for lab personnel. This test, researchers said, is faster, can scale up to test many more people, and can be adapted to detect viral mutations as they emerge in the future.
The study explaining how all this works was published online in Nature Biotechnology.
The test, called dqTACT, could help predict the likelihood of “breakthrough” infections in people who are fully vaccinated and could help determine how frequently people who are immunocompromised might need to be revaccinated, the authors noted.
Infection with the coronavirus and other viruses can trigger a one-two punch from the immunity system – a fast antibody response followed by longer-lasting cellular immunity, including T cells, which “remember” the virus. Cellular immunity can trigger a quick response if the same virus ever shows up again.
The new test adds synthetic viral peptides – strings of amino acids that make up proteins – from the coronavirus to a blood sample. If there is no T-cell reaction within 24 hours, the test is negative. If the peptides trigger T cells, the test can measure the strength of the immune response.
The researchers validated the new test against traditional laboratory testing in 91 people, about half of whom never had COVID-19 and another half who were infected and recovered. The results matched well.
They also found the test predicted immune strength up to 8 months following a second dose of COVID-19 vaccine. Furthermore, T-cell response was greater among people who received two doses of a vaccine versus others who received only one immunization.
Studies are ongoing and designed to meet authorization requirements as part of future licensing from the Food and Drug Administration.
A version of this article first appeared on WebMD.com.
Scientists created a test that indirectly measures T-cell response – an important, long-term component of immunity that can last long after antibody levels fall off – to a challenge by the virus in whole blood.
The test mimics what can be done in a formal laboratory now but avoids some complicated steps and specialized training for lab personnel. This test, researchers said, is faster, can scale up to test many more people, and can be adapted to detect viral mutations as they emerge in the future.
The study explaining how all this works was published online in Nature Biotechnology.
The test, called dqTACT, could help predict the likelihood of “breakthrough” infections in people who are fully vaccinated and could help determine how frequently people who are immunocompromised might need to be revaccinated, the authors noted.
Infection with the coronavirus and other viruses can trigger a one-two punch from the immunity system – a fast antibody response followed by longer-lasting cellular immunity, including T cells, which “remember” the virus. Cellular immunity can trigger a quick response if the same virus ever shows up again.
The new test adds synthetic viral peptides – strings of amino acids that make up proteins – from the coronavirus to a blood sample. If there is no T-cell reaction within 24 hours, the test is negative. If the peptides trigger T cells, the test can measure the strength of the immune response.
The researchers validated the new test against traditional laboratory testing in 91 people, about half of whom never had COVID-19 and another half who were infected and recovered. The results matched well.
They also found the test predicted immune strength up to 8 months following a second dose of COVID-19 vaccine. Furthermore, T-cell response was greater among people who received two doses of a vaccine versus others who received only one immunization.
Studies are ongoing and designed to meet authorization requirements as part of future licensing from the Food and Drug Administration.
A version of this article first appeared on WebMD.com.
Scientists created a test that indirectly measures T-cell response – an important, long-term component of immunity that can last long after antibody levels fall off – to a challenge by the virus in whole blood.
The test mimics what can be done in a formal laboratory now but avoids some complicated steps and specialized training for lab personnel. This test, researchers said, is faster, can scale up to test many more people, and can be adapted to detect viral mutations as they emerge in the future.
The study explaining how all this works was published online in Nature Biotechnology.
The test, called dqTACT, could help predict the likelihood of “breakthrough” infections in people who are fully vaccinated and could help determine how frequently people who are immunocompromised might need to be revaccinated, the authors noted.
Infection with the coronavirus and other viruses can trigger a one-two punch from the immunity system – a fast antibody response followed by longer-lasting cellular immunity, including T cells, which “remember” the virus. Cellular immunity can trigger a quick response if the same virus ever shows up again.
The new test adds synthetic viral peptides – strings of amino acids that make up proteins – from the coronavirus to a blood sample. If there is no T-cell reaction within 24 hours, the test is negative. If the peptides trigger T cells, the test can measure the strength of the immune response.
The researchers validated the new test against traditional laboratory testing in 91 people, about half of whom never had COVID-19 and another half who were infected and recovered. The results matched well.
They also found the test predicted immune strength up to 8 months following a second dose of COVID-19 vaccine. Furthermore, T-cell response was greater among people who received two doses of a vaccine versus others who received only one immunization.
Studies are ongoing and designed to meet authorization requirements as part of future licensing from the Food and Drug Administration.
A version of this article first appeared on WebMD.com.
FROM NATURE BIOTECHNOLOGY
Children and COVID: New cases hold steady in nonholiday week
The new-case count for the most recent reporting week – 87,644 for June 3-9 – did go up from the previous week, but by only 270 cases, the American Academy of Pediatrics and Children’s Hospital Association said in their weekly COVID report. That’s just 0.31% higher than a week ago and probably is affected by reduced testing and reporting because of Memorial Day, as the AAP and CHA noted earlier.
That hint of a continued decline accompanies the latest trend for new cases for all age groups: They have leveled out over the last month, with the moving 7-day daily average hovering around 100,000-110,000 since mid-May, data from the Centers for Disease Control and Prevention show.
The Food and Drug Administration, meanwhile, is in the news this week as two of its advisory panels take the next steps toward pediatric approvals of vaccines from Pfizer/BioNTtech and Moderna. The panels could advance the approvals of the Pfizer vaccine for children under the age of 5 years and the Moderna vaccine for children aged 6 months to 17 years.
Matthew Harris, MD, medical director of the COVID-19 vaccination program for Northwell Health in New Hyde Park, N.Y., emphasized the importance of vaccinations, as well as the continued challenge of convincing parents to get the shots for eligible children. “We still have a long way to go for primary vaccines and boosters for children 5 years and above,” he said in an interview.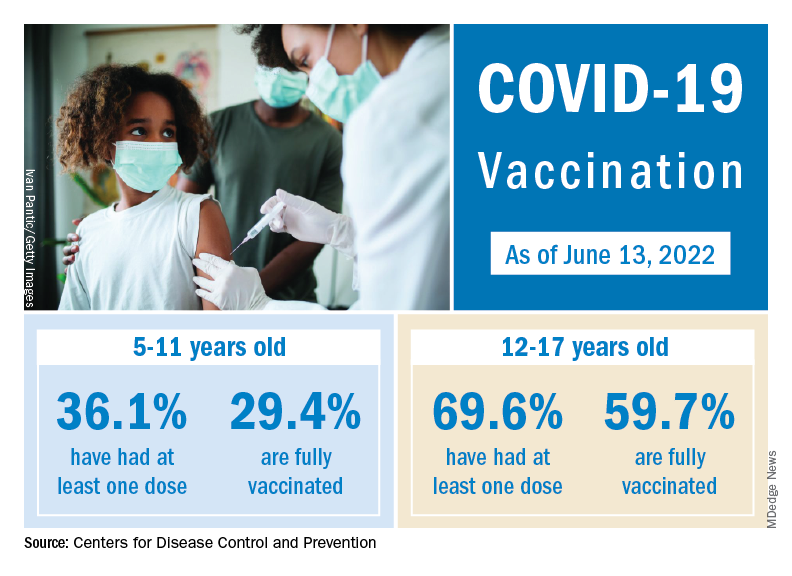
The vaccination effort against COVID-19 has stalled somewhat as interest has waned since the Omicron surge. Weekly initial vaccinations for children aged 5-11 years, which topped 100,000 as recently as mid-March, have been about 43,000 a week for the last 3 weeks, while 12- to 17-year-olds had around 27,000 or 28,000 initial vaccinations per week over that span, the AAP said in a separate report.
The latest data available from the CDC show that overall vaccine coverage levels for the younger group are only about half those of the 12- to 17-year-olds, both in terms of initial doses and completions. The 5- to 11-year-olds are not eligible for boosters yet, but 26.5% of the older children had received one as of June 13, according to the CDC’s COVID Data Tracker.
The new-case count for the most recent reporting week – 87,644 for June 3-9 – did go up from the previous week, but by only 270 cases, the American Academy of Pediatrics and Children’s Hospital Association said in their weekly COVID report. That’s just 0.31% higher than a week ago and probably is affected by reduced testing and reporting because of Memorial Day, as the AAP and CHA noted earlier.
That hint of a continued decline accompanies the latest trend for new cases for all age groups: They have leveled out over the last month, with the moving 7-day daily average hovering around 100,000-110,000 since mid-May, data from the Centers for Disease Control and Prevention show.
The Food and Drug Administration, meanwhile, is in the news this week as two of its advisory panels take the next steps toward pediatric approvals of vaccines from Pfizer/BioNTtech and Moderna. The panels could advance the approvals of the Pfizer vaccine for children under the age of 5 years and the Moderna vaccine for children aged 6 months to 17 years.
Matthew Harris, MD, medical director of the COVID-19 vaccination program for Northwell Health in New Hyde Park, N.Y., emphasized the importance of vaccinations, as well as the continued challenge of convincing parents to get the shots for eligible children. “We still have a long way to go for primary vaccines and boosters for children 5 years and above,” he said in an interview.
The vaccination effort against COVID-19 has stalled somewhat as interest has waned since the Omicron surge. Weekly initial vaccinations for children aged 5-11 years, which topped 100,000 as recently as mid-March, have been about 43,000 a week for the last 3 weeks, while 12- to 17-year-olds had around 27,000 or 28,000 initial vaccinations per week over that span, the AAP said in a separate report.
The latest data available from the CDC show that overall vaccine coverage levels for the younger group are only about half those of the 12- to 17-year-olds, both in terms of initial doses and completions. The 5- to 11-year-olds are not eligible for boosters yet, but 26.5% of the older children had received one as of June 13, according to the CDC’s COVID Data Tracker.
The new-case count for the most recent reporting week – 87,644 for June 3-9 – did go up from the previous week, but by only 270 cases, the American Academy of Pediatrics and Children’s Hospital Association said in their weekly COVID report. That’s just 0.31% higher than a week ago and probably is affected by reduced testing and reporting because of Memorial Day, as the AAP and CHA noted earlier.
That hint of a continued decline accompanies the latest trend for new cases for all age groups: They have leveled out over the last month, with the moving 7-day daily average hovering around 100,000-110,000 since mid-May, data from the Centers for Disease Control and Prevention show.
The Food and Drug Administration, meanwhile, is in the news this week as two of its advisory panels take the next steps toward pediatric approvals of vaccines from Pfizer/BioNTtech and Moderna. The panels could advance the approvals of the Pfizer vaccine for children under the age of 5 years and the Moderna vaccine for children aged 6 months to 17 years.
Matthew Harris, MD, medical director of the COVID-19 vaccination program for Northwell Health in New Hyde Park, N.Y., emphasized the importance of vaccinations, as well as the continued challenge of convincing parents to get the shots for eligible children. “We still have a long way to go for primary vaccines and boosters for children 5 years and above,” he said in an interview.
The vaccination effort against COVID-19 has stalled somewhat as interest has waned since the Omicron surge. Weekly initial vaccinations for children aged 5-11 years, which topped 100,000 as recently as mid-March, have been about 43,000 a week for the last 3 weeks, while 12- to 17-year-olds had around 27,000 or 28,000 initial vaccinations per week over that span, the AAP said in a separate report.
The latest data available from the CDC show that overall vaccine coverage levels for the younger group are only about half those of the 12- to 17-year-olds, both in terms of initial doses and completions. The 5- to 11-year-olds are not eligible for boosters yet, but 26.5% of the older children had received one as of June 13, according to the CDC’s COVID Data Tracker.






