User login
A female liver transplant recipient asks: Can I become pregnant?
Yes, pregnancy is possible, but not immediately after transplant, and it involves risks. Appropriate management and multidisciplinary care are necessary to optimize the outcomes.
HOW LONG SHOULD PREGNANCY BE POSTPONED?
Hypogonadism and amenorrhea are common and multifactorial in women with end-stage liver disease. Hypogonadotrophic hypogonadism, elevated estrogen level, and malnutrition all contribute to the problem.1 However, most premenopausal women experience a return of their menstrual cycle, and possibly of fertility, within the first 10 months after liver transplant,2,3 after which pregnancy is possible.
In transplant recipients of childbearing age, the need for preconception counseling and family planning should be emphasized. The timing, potential risks, and outcomes of pregnancy, and the importance of coordinated prenatal and perinatal care should be addressed.4 The National Transplant Pregnancy Registry guidelines recommend postponing conception until:
- At least 1 year has elapsed after transplant
- Graft function is stable
- Medical comorbidities such as diabetes and hypertension are well controlled
- Immunosuppression is at a low maintenance level.3
Strong evidence suggests that an appropriate liver transplant-conception interval reduces adverse maternal and fetal outcomes. In particular, the risks of a low birth weight, graft rejection, and loss during pregnancy are significantly decreased.3 Therefore, contraception must be initiated after transplant before any sexual activity, with no preference as to the form of protection used.
Limited data demonstrate the safety and efficacy of combined oral contraceptives and transdermal contraceptive patches in stable solid-organ recipients.5,6 Estrogen-containing contraceptives should, however, be avoided in recurrent liver disease after transplant because of the risk of increased hepatic toxicity.
MANAGING RISKS ASSOCIATED WITH PREGNANCY
Physicians should be alert to the possibility of a pregnancy. Early diagnosis allows the optimization of management and outcomes, as complications are increased in this population of expectant mothers.7
Well-known risks to the expectant liver transplant recipient include hypertension and preeclampsia.8 Moreover, infants born to these patients have a higher risk of prematurity and low birth weight.3,7,9 However, rates of neonatal or maternal deaths and birth defects do not differ significantly from those seen in the general population. Graft rejection is a potential complication, with rates varying between 0% and 20% in different studies.3
Multidisciplinary care is therefore crucial during these high-risk pregnancies.10 An obstetrician, a hepatologist, and a perinatalogist should collaborate to maximize outcomes.11 Frequent evaluations, preferably 2 weeks apart, are suggested for the serial assessment of fetal growth.
Furthermore, daily monitoring of the blood pressure and aggressive management of hypertension are recommended. Methyldopa appears to be the drug treatment of choice.12
Close monitoring of graft function and liver biopsy in suspected graft rejection are of essence as well.3 Routine screening for urinary tract infection, cytomegalovirus and toxoplasmosis infections, gestational diabetes, and preeclampsia should also be undertaken.
MANAGING IMMUNOSUPPRESSION IN THE PREGNANT PATIENT
The choice of immunosuppression is ideally made before pregnancy. All immunosuppressive drugs cross the placenta. Thus, in theory, all agents carry risks of teratogenicity and fetal loss. However, immunosuppression is crucial in avoiding rejection. Furthermore, the use of appropriate immunosuppressive regimens prevents negative outcomes. Drugs are classified as class A (safest to use in pregnancy), through classes B, C, D, and X.
Tacrolimus (class C) monotherapy appears to be safe, with attention to the maintenance of therapeutic levels throughout pregnancy. Allograft function and tacrolimus serum levels need to be monitored because of the change in the volume of drug distribution. Cyclosporine (a pregnancy class C drug), prednisone (class B), and azathioprine (class D) are also reasonable options and may also be used if judged necessary.13
Mycophenolic acid and mTOR (mammalian target of rapamycin) inhibitors such as sirolimus and everolimus are significantly teratogenic and should be avoided in pregnant women. They are more commonly associated with spontaneous abortion, structural abnormalities, and birth defects than other immunosuppressive drugs, especially if taken in the early stages of pregnancy. Cleft lip and palate, absent auditory canals, and microtia have been reported.2,13
- Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol 1995; 132:444–449.
- Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996; 62:476–479.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2009; 103–122.
- Parolin MB, Coelho JC, Urbanetz AA, Pampuch M. Contraception and pregnancy after liver transplantation: an update overview. Arq Gastroenterol 2009; 46:154–158. In Portuguese.
- Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010; 82:102–112.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl 2010; 16:56–63.
- Heneghan MA, Selzner M, Yoshida EM, Mullhaupt B. Pregnancy and sexual function in liver transplantation. J Hepatol 2008; 49:507–519.
- Ho JK, Ko HH, Schaeffer DF, et al. Sexual health after orthotopic liver transplantation. Liver Transpl 2006; 12:1478–1484.
- Jabiry-Zieniewicz Z, Dabrowski FA, Pietrzak B, Wielgos M. Pregnancy complications after liver transplantation. Int J Gynaecol Obstet 2015; 128:27–29.
- Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol 2012; 26:621–626.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82:1698–1702.
Yes, pregnancy is possible, but not immediately after transplant, and it involves risks. Appropriate management and multidisciplinary care are necessary to optimize the outcomes.
HOW LONG SHOULD PREGNANCY BE POSTPONED?
Hypogonadism and amenorrhea are common and multifactorial in women with end-stage liver disease. Hypogonadotrophic hypogonadism, elevated estrogen level, and malnutrition all contribute to the problem.1 However, most premenopausal women experience a return of their menstrual cycle, and possibly of fertility, within the first 10 months after liver transplant,2,3 after which pregnancy is possible.
In transplant recipients of childbearing age, the need for preconception counseling and family planning should be emphasized. The timing, potential risks, and outcomes of pregnancy, and the importance of coordinated prenatal and perinatal care should be addressed.4 The National Transplant Pregnancy Registry guidelines recommend postponing conception until:
- At least 1 year has elapsed after transplant
- Graft function is stable
- Medical comorbidities such as diabetes and hypertension are well controlled
- Immunosuppression is at a low maintenance level.3
Strong evidence suggests that an appropriate liver transplant-conception interval reduces adverse maternal and fetal outcomes. In particular, the risks of a low birth weight, graft rejection, and loss during pregnancy are significantly decreased.3 Therefore, contraception must be initiated after transplant before any sexual activity, with no preference as to the form of protection used.
Limited data demonstrate the safety and efficacy of combined oral contraceptives and transdermal contraceptive patches in stable solid-organ recipients.5,6 Estrogen-containing contraceptives should, however, be avoided in recurrent liver disease after transplant because of the risk of increased hepatic toxicity.
MANAGING RISKS ASSOCIATED WITH PREGNANCY
Physicians should be alert to the possibility of a pregnancy. Early diagnosis allows the optimization of management and outcomes, as complications are increased in this population of expectant mothers.7
Well-known risks to the expectant liver transplant recipient include hypertension and preeclampsia.8 Moreover, infants born to these patients have a higher risk of prematurity and low birth weight.3,7,9 However, rates of neonatal or maternal deaths and birth defects do not differ significantly from those seen in the general population. Graft rejection is a potential complication, with rates varying between 0% and 20% in different studies.3
Multidisciplinary care is therefore crucial during these high-risk pregnancies.10 An obstetrician, a hepatologist, and a perinatalogist should collaborate to maximize outcomes.11 Frequent evaluations, preferably 2 weeks apart, are suggested for the serial assessment of fetal growth.
Furthermore, daily monitoring of the blood pressure and aggressive management of hypertension are recommended. Methyldopa appears to be the drug treatment of choice.12
Close monitoring of graft function and liver biopsy in suspected graft rejection are of essence as well.3 Routine screening for urinary tract infection, cytomegalovirus and toxoplasmosis infections, gestational diabetes, and preeclampsia should also be undertaken.
MANAGING IMMUNOSUPPRESSION IN THE PREGNANT PATIENT
The choice of immunosuppression is ideally made before pregnancy. All immunosuppressive drugs cross the placenta. Thus, in theory, all agents carry risks of teratogenicity and fetal loss. However, immunosuppression is crucial in avoiding rejection. Furthermore, the use of appropriate immunosuppressive regimens prevents negative outcomes. Drugs are classified as class A (safest to use in pregnancy), through classes B, C, D, and X.
Tacrolimus (class C) monotherapy appears to be safe, with attention to the maintenance of therapeutic levels throughout pregnancy. Allograft function and tacrolimus serum levels need to be monitored because of the change in the volume of drug distribution. Cyclosporine (a pregnancy class C drug), prednisone (class B), and azathioprine (class D) are also reasonable options and may also be used if judged necessary.13
Mycophenolic acid and mTOR (mammalian target of rapamycin) inhibitors such as sirolimus and everolimus are significantly teratogenic and should be avoided in pregnant women. They are more commonly associated with spontaneous abortion, structural abnormalities, and birth defects than other immunosuppressive drugs, especially if taken in the early stages of pregnancy. Cleft lip and palate, absent auditory canals, and microtia have been reported.2,13
Yes, pregnancy is possible, but not immediately after transplant, and it involves risks. Appropriate management and multidisciplinary care are necessary to optimize the outcomes.
HOW LONG SHOULD PREGNANCY BE POSTPONED?
Hypogonadism and amenorrhea are common and multifactorial in women with end-stage liver disease. Hypogonadotrophic hypogonadism, elevated estrogen level, and malnutrition all contribute to the problem.1 However, most premenopausal women experience a return of their menstrual cycle, and possibly of fertility, within the first 10 months after liver transplant,2,3 after which pregnancy is possible.
In transplant recipients of childbearing age, the need for preconception counseling and family planning should be emphasized. The timing, potential risks, and outcomes of pregnancy, and the importance of coordinated prenatal and perinatal care should be addressed.4 The National Transplant Pregnancy Registry guidelines recommend postponing conception until:
- At least 1 year has elapsed after transplant
- Graft function is stable
- Medical comorbidities such as diabetes and hypertension are well controlled
- Immunosuppression is at a low maintenance level.3
Strong evidence suggests that an appropriate liver transplant-conception interval reduces adverse maternal and fetal outcomes. In particular, the risks of a low birth weight, graft rejection, and loss during pregnancy are significantly decreased.3 Therefore, contraception must be initiated after transplant before any sexual activity, with no preference as to the form of protection used.
Limited data demonstrate the safety and efficacy of combined oral contraceptives and transdermal contraceptive patches in stable solid-organ recipients.5,6 Estrogen-containing contraceptives should, however, be avoided in recurrent liver disease after transplant because of the risk of increased hepatic toxicity.
MANAGING RISKS ASSOCIATED WITH PREGNANCY
Physicians should be alert to the possibility of a pregnancy. Early diagnosis allows the optimization of management and outcomes, as complications are increased in this population of expectant mothers.7
Well-known risks to the expectant liver transplant recipient include hypertension and preeclampsia.8 Moreover, infants born to these patients have a higher risk of prematurity and low birth weight.3,7,9 However, rates of neonatal or maternal deaths and birth defects do not differ significantly from those seen in the general population. Graft rejection is a potential complication, with rates varying between 0% and 20% in different studies.3
Multidisciplinary care is therefore crucial during these high-risk pregnancies.10 An obstetrician, a hepatologist, and a perinatalogist should collaborate to maximize outcomes.11 Frequent evaluations, preferably 2 weeks apart, are suggested for the serial assessment of fetal growth.
Furthermore, daily monitoring of the blood pressure and aggressive management of hypertension are recommended. Methyldopa appears to be the drug treatment of choice.12
Close monitoring of graft function and liver biopsy in suspected graft rejection are of essence as well.3 Routine screening for urinary tract infection, cytomegalovirus and toxoplasmosis infections, gestational diabetes, and preeclampsia should also be undertaken.
MANAGING IMMUNOSUPPRESSION IN THE PREGNANT PATIENT
The choice of immunosuppression is ideally made before pregnancy. All immunosuppressive drugs cross the placenta. Thus, in theory, all agents carry risks of teratogenicity and fetal loss. However, immunosuppression is crucial in avoiding rejection. Furthermore, the use of appropriate immunosuppressive regimens prevents negative outcomes. Drugs are classified as class A (safest to use in pregnancy), through classes B, C, D, and X.
Tacrolimus (class C) monotherapy appears to be safe, with attention to the maintenance of therapeutic levels throughout pregnancy. Allograft function and tacrolimus serum levels need to be monitored because of the change in the volume of drug distribution. Cyclosporine (a pregnancy class C drug), prednisone (class B), and azathioprine (class D) are also reasonable options and may also be used if judged necessary.13
Mycophenolic acid and mTOR (mammalian target of rapamycin) inhibitors such as sirolimus and everolimus are significantly teratogenic and should be avoided in pregnant women. They are more commonly associated with spontaneous abortion, structural abnormalities, and birth defects than other immunosuppressive drugs, especially if taken in the early stages of pregnancy. Cleft lip and palate, absent auditory canals, and microtia have been reported.2,13
- Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol 1995; 132:444–449.
- Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996; 62:476–479.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2009; 103–122.
- Parolin MB, Coelho JC, Urbanetz AA, Pampuch M. Contraception and pregnancy after liver transplantation: an update overview. Arq Gastroenterol 2009; 46:154–158. In Portuguese.
- Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010; 82:102–112.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl 2010; 16:56–63.
- Heneghan MA, Selzner M, Yoshida EM, Mullhaupt B. Pregnancy and sexual function in liver transplantation. J Hepatol 2008; 49:507–519.
- Ho JK, Ko HH, Schaeffer DF, et al. Sexual health after orthotopic liver transplantation. Liver Transpl 2006; 12:1478–1484.
- Jabiry-Zieniewicz Z, Dabrowski FA, Pietrzak B, Wielgos M. Pregnancy complications after liver transplantation. Int J Gynaecol Obstet 2015; 128:27–29.
- Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol 2012; 26:621–626.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82:1698–1702.
- Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol 1995; 132:444–449.
- Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996; 62:476–479.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2009; 103–122.
- Parolin MB, Coelho JC, Urbanetz AA, Pampuch M. Contraception and pregnancy after liver transplantation: an update overview. Arq Gastroenterol 2009; 46:154–158. In Portuguese.
- Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010; 82:102–112.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl 2010; 16:56–63.
- Heneghan MA, Selzner M, Yoshida EM, Mullhaupt B. Pregnancy and sexual function in liver transplantation. J Hepatol 2008; 49:507–519.
- Ho JK, Ko HH, Schaeffer DF, et al. Sexual health after orthotopic liver transplantation. Liver Transpl 2006; 12:1478–1484.
- Jabiry-Zieniewicz Z, Dabrowski FA, Pietrzak B, Wielgos M. Pregnancy complications after liver transplantation. Int J Gynaecol Obstet 2015; 128:27–29.
- Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol 2012; 26:621–626.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82:1698–1702.
When does chest CT require contrast enhancement?
Computed tomography (CT) plays an important role in the diagnosis and treatment of many clinical conditions1 involving the chest wall, mediastinum, pleura, pulmonary arteries, and lung parenchyma. The need for enhancement with intravenous (IV) contrast depends on the specific clinical indication (Table 1).
EVALUATION OF SUSPECTED CANCER
CT is commonly used to diagnose, stage, and plan treatment for lung cancer, other primary neoplastic processes involving the chest, and metastatic disease.2 The need for contrast varies on a case-by-case basis, and the benefits of contrast should be weighed against the potential risks in each patient.
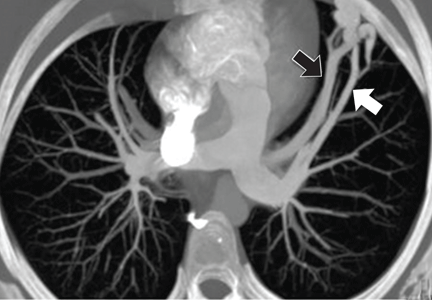
When the neoplasm has CT attenuation similar to that of adjacent structures (lymph nodes in the hilum, masses in the mediastinum or chest wall), IV contrast can improve identification of the lesion and delineation of its margins and the relationship with adjacent structures (eg, vascular structures) (Figure 1).
CT without contrast for screening
The diagnostic algorithm for lung cancer screening is evolving. The US Preventive Services Task Force currently recommends low-dose CT without contrast, along with appropriate patient counseling, for patients with a history of smoking and an age range as detailed in the Task Force statement.3
Follow-up of a solitary pulmonary nodule also typically does not require contrast enhancement, though some investigators have reported high sensitivity with dynamic contrast enhancement of pulmonary nodules.4 This represents a rare clinical application of chest CT with and without contrast.
EVALUATION OF THORACIC VASCULAR DISEASE
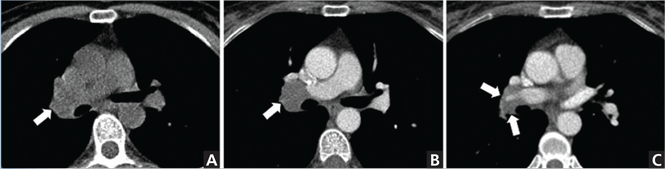
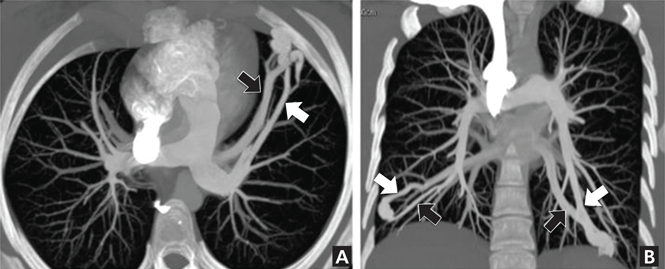
For the assessment of vascular disease, CT in most cases requires IV contrast to delineate the vessel lumen. Pulmonary embolic disease is the third most common cause of acute cardiovascular disease.5 CT pulmonary angiography is the most common way to assess for pulmonary embolic disease, as it is accurate, fast, and widely available, and can assess alternate pathologies in cases of undifferentiated chest pain. Contrast enhancement of the pulmonary arteries is key, as embolic disease is identified as abnormal filling defects within the pulmonary arteries (Figure 2).
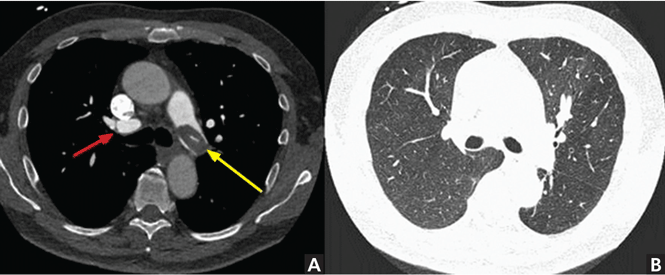
Contrast enhancement is also used to evaluate superior vena cava syndrome. At our institution, the CT protocol includes concomitant injections in the upper-extremity veins, with imaging timed for venous phase enhancement (pulmonary venogram). In cases of suspected arteriovenous malformation, a protocol similar to that used for suspected pulmonary embolus is used (Figure 3), although in some instances, the imaging features of arteriovenous malformation may be detectable without IV contrast.
EVALUATION OF PULMONARY PARENCHYMAL DISEASE
Infection, inflammation, and edema of the lung parenchyma are usually well depicted on CT without contrast enhancement. However, contrast may be helpful if there are concerns about complications such as chest wall involvement, where contrast enhancement may help further delineate the extent of complications.
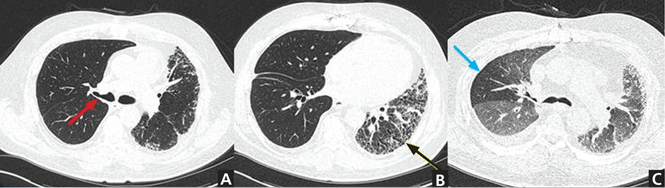
Assessment of interstitial lung disease does not require use of IV contrast; rather, a tailored protocol with thinner slices and noncontiguous expiratory images can be used to evaluate for air-trapping and dynamic airway compromise (Figure 4). Evaluation of chronic obstructive pulmonary disease also does not require IV contrast.
EVALUATION OF THE PLEURA
In pleural effusion, CT assessment for the presence, location, and extent of the effusion does not require contrast. However, contrast enhancement is used to evaluate suspected or known exudative effusions and empyema.6 It also aids the evaluation of metastatic or primary malignancy of the pleura, particularly in cases of occult disease, as enhancement and thickening of the pleura are of diagnostic interest.
EVALUATION OF AIRWAY DISEASE
Diseases of the large airway, such as stenosis and thickening, and diseases of the small airways, such as bronchiolitis, typically do not require contrast enhancement. At our institution, to assess dynamic airway narrowing, we use a dedicated airway protocol, including inspiratory and expiratory phases and multiplanar reformatted images.
EVALUATION OF STERNAL AND MEDIASTINAL INFECTIONS
Postoperative sternal wound infections are not uncommon and range from cellulitis to frank osteomyelitis. Mediastinitis may likewise be iatrogenic or may spread from the oropharynx. CT with contrast can help to depict infection of the chest wall or mediastinum and in some instances can also delineate the route of spread.7
TYPES OF IV CONTRAST MEDIA
Contrast media used in CT contain iodine, which causes increased absorption and scattering of radiation in body tissues and blood. Other contrast media, such as those used for magnetic resonance imaging or barium enemas, do not contain iodine. This absorption and scattering in turn results in higher CT attenuation values, or “enhancement” on CT images. The extent of enhancement depends on the amount and rate of contrast material administered, as well as on patient factors (eg, tissue vascularity, permeability, interstitial space) and the energy (tube voltage) of the incident x-rays.8
Adverse reactions
Contrast materials are generally safe; however, as with any pharmaceutical, there is the potential for adverse reactions. These reactions are relatively rare and are usually mild but occasionally can be severe.9 Anaphylactoid reactions have an unclear etiology but mimic allergic reactions, and they are more likely to occur in patients with a previous reaction to contrast and in patients with asthma or cardiovascular or renal disease.
Nonanaphylactoid reactions are dependent on contrast osmolality and on the volume and route of injection (unlike anaphylactoid reactions).10 Typical symptoms include warmth, metallic taste, and nausea or vomiting.
Contrast-related nephrotoxicity has been reported,11 although this has been challenged more recently.12 Suspected risk factors for this complication include advanced age, cardiovascular disease, treatment with chemotherapy, elevated serum creatinine level, dehydration, diabetes, use of nonsteroidal anti-inflammatory medications, myeloma,13 renal disease, and kidney transplant.
Detailed protocols for premedication and management of contrast adverse reactions are beyond the scope of this review and the reader is advised to refer to dedicated manuals.10
Acknowledgment: We are grateful for the editorial assistance of Megan M. Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic.
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014; 273(suppl 2):S45–S74.
- American College of Radiology. ACR-SCBT-MR-SPR practice parameter for the performance of thoracic computed tomography (CT). www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Thoracic.pdf. Accessed March 30, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199.
- Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013; 201:971–976.
- Kraus GJ. The split pleura sign. Radiology 2007; 243:297–298.
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30:1335–1352.
- Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008; 36:69–74.
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.1. 2015. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed March 29, 2016.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol 1994; 5:125–137.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014; 273:714–725.
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology 1992; 183:519–521.
Computed tomography (CT) plays an important role in the diagnosis and treatment of many clinical conditions1 involving the chest wall, mediastinum, pleura, pulmonary arteries, and lung parenchyma. The need for enhancement with intravenous (IV) contrast depends on the specific clinical indication (Table 1).
EVALUATION OF SUSPECTED CANCER
CT is commonly used to diagnose, stage, and plan treatment for lung cancer, other primary neoplastic processes involving the chest, and metastatic disease.2 The need for contrast varies on a case-by-case basis, and the benefits of contrast should be weighed against the potential risks in each patient.
When the neoplasm has CT attenuation similar to that of adjacent structures (lymph nodes in the hilum, masses in the mediastinum or chest wall), IV contrast can improve identification of the lesion and delineation of its margins and the relationship with adjacent structures (eg, vascular structures) (Figure 1).

CT without contrast for screening
The diagnostic algorithm for lung cancer screening is evolving. The US Preventive Services Task Force currently recommends low-dose CT without contrast, along with appropriate patient counseling, for patients with a history of smoking and an age range as detailed in the Task Force statement.3
Follow-up of a solitary pulmonary nodule also typically does not require contrast enhancement, though some investigators have reported high sensitivity with dynamic contrast enhancement of pulmonary nodules.4 This represents a rare clinical application of chest CT with and without contrast.
EVALUATION OF THORACIC VASCULAR DISEASE
For the assessment of vascular disease, CT in most cases requires IV contrast to delineate the vessel lumen. Pulmonary embolic disease is the third most common cause of acute cardiovascular disease.5 CT pulmonary angiography is the most common way to assess for pulmonary embolic disease, as it is accurate, fast, and widely available, and can assess alternate pathologies in cases of undifferentiated chest pain. Contrast enhancement of the pulmonary arteries is key, as embolic disease is identified as abnormal filling defects within the pulmonary arteries (Figure 2).

Contrast enhancement is also used to evaluate superior vena cava syndrome. At our institution, the CT protocol includes concomitant injections in the upper-extremity veins, with imaging timed for venous phase enhancement (pulmonary venogram). In cases of suspected arteriovenous malformation, a protocol similar to that used for suspected pulmonary embolus is used (Figure 3), although in some instances, the imaging features of arteriovenous malformation may be detectable without IV contrast.

EVALUATION OF PULMONARY PARENCHYMAL DISEASE
Infection, inflammation, and edema of the lung parenchyma are usually well depicted on CT without contrast enhancement. However, contrast may be helpful if there are concerns about complications such as chest wall involvement, where contrast enhancement may help further delineate the extent of complications.
Assessment of interstitial lung disease does not require use of IV contrast; rather, a tailored protocol with thinner slices and noncontiguous expiratory images can be used to evaluate for air-trapping and dynamic airway compromise (Figure 4). Evaluation of chronic obstructive pulmonary disease also does not require IV contrast.

EVALUATION OF THE PLEURA
In pleural effusion, CT assessment for the presence, location, and extent of the effusion does not require contrast. However, contrast enhancement is used to evaluate suspected or known exudative effusions and empyema.6 It also aids the evaluation of metastatic or primary malignancy of the pleura, particularly in cases of occult disease, as enhancement and thickening of the pleura are of diagnostic interest.
EVALUATION OF AIRWAY DISEASE
Diseases of the large airway, such as stenosis and thickening, and diseases of the small airways, such as bronchiolitis, typically do not require contrast enhancement. At our institution, to assess dynamic airway narrowing, we use a dedicated airway protocol, including inspiratory and expiratory phases and multiplanar reformatted images.
EVALUATION OF STERNAL AND MEDIASTINAL INFECTIONS
Postoperative sternal wound infections are not uncommon and range from cellulitis to frank osteomyelitis. Mediastinitis may likewise be iatrogenic or may spread from the oropharynx. CT with contrast can help to depict infection of the chest wall or mediastinum and in some instances can also delineate the route of spread.7
TYPES OF IV CONTRAST MEDIA
Contrast media used in CT contain iodine, which causes increased absorption and scattering of radiation in body tissues and blood. Other contrast media, such as those used for magnetic resonance imaging or barium enemas, do not contain iodine. This absorption and scattering in turn results in higher CT attenuation values, or “enhancement” on CT images. The extent of enhancement depends on the amount and rate of contrast material administered, as well as on patient factors (eg, tissue vascularity, permeability, interstitial space) and the energy (tube voltage) of the incident x-rays.8
Adverse reactions
Contrast materials are generally safe; however, as with any pharmaceutical, there is the potential for adverse reactions. These reactions are relatively rare and are usually mild but occasionally can be severe.9 Anaphylactoid reactions have an unclear etiology but mimic allergic reactions, and they are more likely to occur in patients with a previous reaction to contrast and in patients with asthma or cardiovascular or renal disease.
Nonanaphylactoid reactions are dependent on contrast osmolality and on the volume and route of injection (unlike anaphylactoid reactions).10 Typical symptoms include warmth, metallic taste, and nausea or vomiting.
Contrast-related nephrotoxicity has been reported,11 although this has been challenged more recently.12 Suspected risk factors for this complication include advanced age, cardiovascular disease, treatment with chemotherapy, elevated serum creatinine level, dehydration, diabetes, use of nonsteroidal anti-inflammatory medications, myeloma,13 renal disease, and kidney transplant.
Detailed protocols for premedication and management of contrast adverse reactions are beyond the scope of this review and the reader is advised to refer to dedicated manuals.10
Acknowledgment: We are grateful for the editorial assistance of Megan M. Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic.
Computed tomography (CT) plays an important role in the diagnosis and treatment of many clinical conditions1 involving the chest wall, mediastinum, pleura, pulmonary arteries, and lung parenchyma. The need for enhancement with intravenous (IV) contrast depends on the specific clinical indication (Table 1).
EVALUATION OF SUSPECTED CANCER
CT is commonly used to diagnose, stage, and plan treatment for lung cancer, other primary neoplastic processes involving the chest, and metastatic disease.2 The need for contrast varies on a case-by-case basis, and the benefits of contrast should be weighed against the potential risks in each patient.
When the neoplasm has CT attenuation similar to that of adjacent structures (lymph nodes in the hilum, masses in the mediastinum or chest wall), IV contrast can improve identification of the lesion and delineation of its margins and the relationship with adjacent structures (eg, vascular structures) (Figure 1).

CT without contrast for screening
The diagnostic algorithm for lung cancer screening is evolving. The US Preventive Services Task Force currently recommends low-dose CT without contrast, along with appropriate patient counseling, for patients with a history of smoking and an age range as detailed in the Task Force statement.3
Follow-up of a solitary pulmonary nodule also typically does not require contrast enhancement, though some investigators have reported high sensitivity with dynamic contrast enhancement of pulmonary nodules.4 This represents a rare clinical application of chest CT with and without contrast.
EVALUATION OF THORACIC VASCULAR DISEASE
For the assessment of vascular disease, CT in most cases requires IV contrast to delineate the vessel lumen. Pulmonary embolic disease is the third most common cause of acute cardiovascular disease.5 CT pulmonary angiography is the most common way to assess for pulmonary embolic disease, as it is accurate, fast, and widely available, and can assess alternate pathologies in cases of undifferentiated chest pain. Contrast enhancement of the pulmonary arteries is key, as embolic disease is identified as abnormal filling defects within the pulmonary arteries (Figure 2).

Contrast enhancement is also used to evaluate superior vena cava syndrome. At our institution, the CT protocol includes concomitant injections in the upper-extremity veins, with imaging timed for venous phase enhancement (pulmonary venogram). In cases of suspected arteriovenous malformation, a protocol similar to that used for suspected pulmonary embolus is used (Figure 3), although in some instances, the imaging features of arteriovenous malformation may be detectable without IV contrast.

EVALUATION OF PULMONARY PARENCHYMAL DISEASE
Infection, inflammation, and edema of the lung parenchyma are usually well depicted on CT without contrast enhancement. However, contrast may be helpful if there are concerns about complications such as chest wall involvement, where contrast enhancement may help further delineate the extent of complications.
Assessment of interstitial lung disease does not require use of IV contrast; rather, a tailored protocol with thinner slices and noncontiguous expiratory images can be used to evaluate for air-trapping and dynamic airway compromise (Figure 4). Evaluation of chronic obstructive pulmonary disease also does not require IV contrast.

EVALUATION OF THE PLEURA
In pleural effusion, CT assessment for the presence, location, and extent of the effusion does not require contrast. However, contrast enhancement is used to evaluate suspected or known exudative effusions and empyema.6 It also aids the evaluation of metastatic or primary malignancy of the pleura, particularly in cases of occult disease, as enhancement and thickening of the pleura are of diagnostic interest.
EVALUATION OF AIRWAY DISEASE
Diseases of the large airway, such as stenosis and thickening, and diseases of the small airways, such as bronchiolitis, typically do not require contrast enhancement. At our institution, to assess dynamic airway narrowing, we use a dedicated airway protocol, including inspiratory and expiratory phases and multiplanar reformatted images.
EVALUATION OF STERNAL AND MEDIASTINAL INFECTIONS
Postoperative sternal wound infections are not uncommon and range from cellulitis to frank osteomyelitis. Mediastinitis may likewise be iatrogenic or may spread from the oropharynx. CT with contrast can help to depict infection of the chest wall or mediastinum and in some instances can also delineate the route of spread.7
TYPES OF IV CONTRAST MEDIA
Contrast media used in CT contain iodine, which causes increased absorption and scattering of radiation in body tissues and blood. Other contrast media, such as those used for magnetic resonance imaging or barium enemas, do not contain iodine. This absorption and scattering in turn results in higher CT attenuation values, or “enhancement” on CT images. The extent of enhancement depends on the amount and rate of contrast material administered, as well as on patient factors (eg, tissue vascularity, permeability, interstitial space) and the energy (tube voltage) of the incident x-rays.8
Adverse reactions
Contrast materials are generally safe; however, as with any pharmaceutical, there is the potential for adverse reactions. These reactions are relatively rare and are usually mild but occasionally can be severe.9 Anaphylactoid reactions have an unclear etiology but mimic allergic reactions, and they are more likely to occur in patients with a previous reaction to contrast and in patients with asthma or cardiovascular or renal disease.
Nonanaphylactoid reactions are dependent on contrast osmolality and on the volume and route of injection (unlike anaphylactoid reactions).10 Typical symptoms include warmth, metallic taste, and nausea or vomiting.
Contrast-related nephrotoxicity has been reported,11 although this has been challenged more recently.12 Suspected risk factors for this complication include advanced age, cardiovascular disease, treatment with chemotherapy, elevated serum creatinine level, dehydration, diabetes, use of nonsteroidal anti-inflammatory medications, myeloma,13 renal disease, and kidney transplant.
Detailed protocols for premedication and management of contrast adverse reactions are beyond the scope of this review and the reader is advised to refer to dedicated manuals.10
Acknowledgment: We are grateful for the editorial assistance of Megan M. Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic.
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014; 273(suppl 2):S45–S74.
- American College of Radiology. ACR-SCBT-MR-SPR practice parameter for the performance of thoracic computed tomography (CT). www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Thoracic.pdf. Accessed March 30, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199.
- Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013; 201:971–976.
- Kraus GJ. The split pleura sign. Radiology 2007; 243:297–298.
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30:1335–1352.
- Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008; 36:69–74.
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.1. 2015. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed March 29, 2016.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol 1994; 5:125–137.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014; 273:714–725.
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology 1992; 183:519–521.
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014; 273(suppl 2):S45–S74.
- American College of Radiology. ACR-SCBT-MR-SPR practice parameter for the performance of thoracic computed tomography (CT). www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Thoracic.pdf. Accessed March 30, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199.
- Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013; 201:971–976.
- Kraus GJ. The split pleura sign. Radiology 2007; 243:297–298.
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30:1335–1352.
- Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008; 36:69–74.
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.1. 2015. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed March 29, 2016.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol 1994; 5:125–137.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014; 273:714–725.
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology 1992; 183:519–521.