User login
Should I suspect obstructive sleep apnea if a patient has hard-to-control hypertension?
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
How can I predict bleeding in my elderly patient taking anticoagulants?
We have tools to predict bleeding risk, but their predictive value is modest, and the estimated risk of bleeding is often outweighed by the benefits of anticoagulant therapy.
Anticoagulant therapy is commonly prescribed for conditions that disproportionately affect the elderly, including atrial fibrillation, venous thromboembolism, and valvular heart disease. Though anticoagulants are highly effective in preventing clots, they also significantly increase the risk of bleeding. Since older age is a risk factor for bleeding as well as thrombosis, it is essential to weigh the risks and benefits of anticoagulants for each patient.
WHAT KINDS OF BLEEDING DEVELOP IN PATIENTS ON ANTICOAGULANTS?
Patients taking anticoagulants have roughly double the risk of bleeding compared with patients not on anticoagulants.1 Bleeding rates tend to be slightly higher in patients taking anticoagulants for venous thromboembolism than in those taking them for atrial fibrillation. The average yearly risk of a “major” anticoagulant-associated bleeding event (eg, requiring transfusion or intervention or occurring in a critical anatomic site) is about 2% to 3%, with most of the bleeding being gastrointestinal.2
Intracranial hemorrhage is by far the most deadly complication of anticoagulant therapy: it causes 90% of deaths and disability from warfarin-associated hemorrhage and is associated with a death rate over 50%; however, it is much less common than gastrointestinal bleeding.3 Anticoagulant therapy increases the risk of intracranial hemorrhage by only 0.2% per year.1
RISK-PREDICTION TOOLS HAVE LIMITATIONS
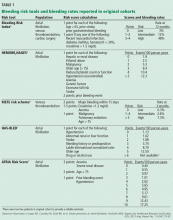
Not all patients have the same risk of bleeding when taking anticoagulants. Many factors in addition to advanced age have been associated with increased bleeding risk, including coexisting medical conditions (such as malignancy, prior stroke or bleeding event, and renal insufficiency), medications (particularly aspirin, nonsteroidal anti-inflammatory drugs, and other antiplatelet drugs), and the timing and intensity of anticoagulation therapy.4
Scoring tools have been developed to identify patients at higher risk of bleeding (Table 1).4–9 The various schemes incorporate many of the same variables, such as older age, renal impairment, and history of bleeding, but some include additional risk factors while others are more parsimonious. They also differ in how individual risk factors are weighted to generate a final risk score.
In terms of predictive ability, none of the available risk schemes appears to be vastly superior, and their ability to predict hemorrhage is modest at best. There is also no universal or well-established threshold at which the risk of bleeding is so high that one would not consider anticoagulants. In fact, a “high-risk” patient may have an aggregate bleeding rate of only 4% to 6% per year. Using risk schemes such as ATRIA,5 HEMORR2HAGES,6 and HAS-BLED7 may be more useful because they provide an estimate of bleeding risk for each point on the scale.
Moreover, the current tools to predict bleeding risk have several other limitations. They were developed in patients already taking anticoagulants and so probably underestimate the actual risk of hemorrhage, as people who could not take anticoagulants were excluded, most likely because they were at high risk of bleeding. Therefore, bleeding risk tools probably apply best to a patient for whom anticoagulation can be considered.
Some clinical variables are necessarily broad. For example, “prior bleeding” is a risk factor included in several risk scores, but does not distinguish between massive variceal bleeding and minor hemorrhoidal bleeding.
Risk scores do not effectively predict intracranial hemorrhage.
Finally, these risk tools were developed in patients taking vitamin K antagonists, and it is not yet established that they can effectively predict hemorrhage related to other, newer anticoagulants.
WHEN DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
For patients with atrial fibrillation, the net clinical benefit of anticoagulation (strokes prevented minus bleeding events induced) increases as the risk of stroke rises. Updated guidelines for managing atrial fibrillation now recommend anticoagulation for most patients.10
For most older patients with atrial fibrillation, the decision to anticoagulate may not change even if a bleeding risk tool indicates a high bleeding risk.11 For example, a patient with a history of ischemic stroke will generally derive more benefit than harm from anticoagulants. The primary exception is in patients with prior lobar intracranial hemorrhage, because of the high risk of rebleeding and the worse outcomes associated with intracranial hemorrhage.12 As a general rule, most patients with atrial fibrillation and an additional risk factor for stroke should be considered for anticoagulant therapy unless they have a history of lobar intracranial hemorrhage.
Anticoagulation may be deferred if the patient is at the lower end of the stroke risk spectrum and if the bleeding risk is calculated to be high. However, as noted before, current bleeding risk tools probably do not capture the experiences of patients at the extremes of high bleeding risk, so clinical judgment continues to be important. In addition, forgoing anticoagulation could be reasonable even in patients at high risk for recurrent stroke if their life expectancy is limited, if anticoagulation is unacceptably burdensome, or if it is not within their goals and preferences.
WHAT ABOUT FALL RISK?
Fall risk commonly deters clinicians from prescribing anticoagulants because of the fear of causing intracranial hemorrhage. In particular, falls increase the risk for subdural hematoma, which has a death rate comparable to that of ischemic stroke.13
Studies have had difficulty quantifying the exact risk associated with falls because these patients are less likely to be prescribed anticoagulants. One decision analysis estimated that a person would have to fall about 300 times per year before the risk of intracranial hemorrhage outweighed the benefits from stroke reduction.14 Studies have found that patients at high risk of falls have a higher risk of intracranial hemorrhage, but that this risk is counterbalanced by an even greater risk of ischemic stroke.15
Therefore, if the baseline risk of ischemic stroke is high, anticoagulation is still favored.
WHEN SHOULD I USE A BLEEDING RISK TOOL?
Despite their limitations, bleeding risk tools are useful in clinical practice when estimates of bleeding risk affect clinical behavior. They are most helpful for patients at the lower end of the stroke or thromboembolism risk spectrum, where the decision to anticoagulate is strongly influenced by bleeding risk. Risk tools may also be helpful when counseling patients about their bleeding risk off and on anticoagulants.
Finally, recognizing that a patient is at high bleeding risk may lead the clinician to consider closer monitoring of anticoagulants or to implement strategies to reduce the risk.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Lopes LC, Spencer FA, Neumann I, et al. Bleeding risk in atrial fibrillation patients taking vitamin K antagonists: systematic review and meta-analysis. Clin Pharmacol Ther 2013; 94:367–375.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke prevention in atrial fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC113-EF.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J 2006; 151:713–719.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Nieto JA, Solano R, Iglesias NT, et al, for the RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thrombosis Res 2013; 132:175–179.
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003; 34:1710–1716.
- Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012; 43:1795–1799.
- Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003; 163:1580–1586.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
We have tools to predict bleeding risk, but their predictive value is modest, and the estimated risk of bleeding is often outweighed by the benefits of anticoagulant therapy.
Anticoagulant therapy is commonly prescribed for conditions that disproportionately affect the elderly, including atrial fibrillation, venous thromboembolism, and valvular heart disease. Though anticoagulants are highly effective in preventing clots, they also significantly increase the risk of bleeding. Since older age is a risk factor for bleeding as well as thrombosis, it is essential to weigh the risks and benefits of anticoagulants for each patient.
WHAT KINDS OF BLEEDING DEVELOP IN PATIENTS ON ANTICOAGULANTS?
Patients taking anticoagulants have roughly double the risk of bleeding compared with patients not on anticoagulants.1 Bleeding rates tend to be slightly higher in patients taking anticoagulants for venous thromboembolism than in those taking them for atrial fibrillation. The average yearly risk of a “major” anticoagulant-associated bleeding event (eg, requiring transfusion or intervention or occurring in a critical anatomic site) is about 2% to 3%, with most of the bleeding being gastrointestinal.2
Intracranial hemorrhage is by far the most deadly complication of anticoagulant therapy: it causes 90% of deaths and disability from warfarin-associated hemorrhage and is associated with a death rate over 50%; however, it is much less common than gastrointestinal bleeding.3 Anticoagulant therapy increases the risk of intracranial hemorrhage by only 0.2% per year.1
RISK-PREDICTION TOOLS HAVE LIMITATIONS
Not all patients have the same risk of bleeding when taking anticoagulants. Many factors in addition to advanced age have been associated with increased bleeding risk, including coexisting medical conditions (such as malignancy, prior stroke or bleeding event, and renal insufficiency), medications (particularly aspirin, nonsteroidal anti-inflammatory drugs, and other antiplatelet drugs), and the timing and intensity of anticoagulation therapy.4
Scoring tools have been developed to identify patients at higher risk of bleeding (Table 1).4–9 The various schemes incorporate many of the same variables, such as older age, renal impairment, and history of bleeding, but some include additional risk factors while others are more parsimonious. They also differ in how individual risk factors are weighted to generate a final risk score.
In terms of predictive ability, none of the available risk schemes appears to be vastly superior, and their ability to predict hemorrhage is modest at best. There is also no universal or well-established threshold at which the risk of bleeding is so high that one would not consider anticoagulants. In fact, a “high-risk” patient may have an aggregate bleeding rate of only 4% to 6% per year. Using risk schemes such as ATRIA,5 HEMORR2HAGES,6 and HAS-BLED7 may be more useful because they provide an estimate of bleeding risk for each point on the scale.
Moreover, the current tools to predict bleeding risk have several other limitations. They were developed in patients already taking anticoagulants and so probably underestimate the actual risk of hemorrhage, as people who could not take anticoagulants were excluded, most likely because they were at high risk of bleeding. Therefore, bleeding risk tools probably apply best to a patient for whom anticoagulation can be considered.
Some clinical variables are necessarily broad. For example, “prior bleeding” is a risk factor included in several risk scores, but does not distinguish between massive variceal bleeding and minor hemorrhoidal bleeding.
Risk scores do not effectively predict intracranial hemorrhage.
Finally, these risk tools were developed in patients taking vitamin K antagonists, and it is not yet established that they can effectively predict hemorrhage related to other, newer anticoagulants.
WHEN DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
For patients with atrial fibrillation, the net clinical benefit of anticoagulation (strokes prevented minus bleeding events induced) increases as the risk of stroke rises. Updated guidelines for managing atrial fibrillation now recommend anticoagulation for most patients.10
For most older patients with atrial fibrillation, the decision to anticoagulate may not change even if a bleeding risk tool indicates a high bleeding risk.11 For example, a patient with a history of ischemic stroke will generally derive more benefit than harm from anticoagulants. The primary exception is in patients with prior lobar intracranial hemorrhage, because of the high risk of rebleeding and the worse outcomes associated with intracranial hemorrhage.12 As a general rule, most patients with atrial fibrillation and an additional risk factor for stroke should be considered for anticoagulant therapy unless they have a history of lobar intracranial hemorrhage.
Anticoagulation may be deferred if the patient is at the lower end of the stroke risk spectrum and if the bleeding risk is calculated to be high. However, as noted before, current bleeding risk tools probably do not capture the experiences of patients at the extremes of high bleeding risk, so clinical judgment continues to be important. In addition, forgoing anticoagulation could be reasonable even in patients at high risk for recurrent stroke if their life expectancy is limited, if anticoagulation is unacceptably burdensome, or if it is not within their goals and preferences.
WHAT ABOUT FALL RISK?
Fall risk commonly deters clinicians from prescribing anticoagulants because of the fear of causing intracranial hemorrhage. In particular, falls increase the risk for subdural hematoma, which has a death rate comparable to that of ischemic stroke.13
Studies have had difficulty quantifying the exact risk associated with falls because these patients are less likely to be prescribed anticoagulants. One decision analysis estimated that a person would have to fall about 300 times per year before the risk of intracranial hemorrhage outweighed the benefits from stroke reduction.14 Studies have found that patients at high risk of falls have a higher risk of intracranial hemorrhage, but that this risk is counterbalanced by an even greater risk of ischemic stroke.15
Therefore, if the baseline risk of ischemic stroke is high, anticoagulation is still favored.
WHEN SHOULD I USE A BLEEDING RISK TOOL?
Despite their limitations, bleeding risk tools are useful in clinical practice when estimates of bleeding risk affect clinical behavior. They are most helpful for patients at the lower end of the stroke or thromboembolism risk spectrum, where the decision to anticoagulate is strongly influenced by bleeding risk. Risk tools may also be helpful when counseling patients about their bleeding risk off and on anticoagulants.
Finally, recognizing that a patient is at high bleeding risk may lead the clinician to consider closer monitoring of anticoagulants or to implement strategies to reduce the risk.
We have tools to predict bleeding risk, but their predictive value is modest, and the estimated risk of bleeding is often outweighed by the benefits of anticoagulant therapy.
Anticoagulant therapy is commonly prescribed for conditions that disproportionately affect the elderly, including atrial fibrillation, venous thromboembolism, and valvular heart disease. Though anticoagulants are highly effective in preventing clots, they also significantly increase the risk of bleeding. Since older age is a risk factor for bleeding as well as thrombosis, it is essential to weigh the risks and benefits of anticoagulants for each patient.
WHAT KINDS OF BLEEDING DEVELOP IN PATIENTS ON ANTICOAGULANTS?
Patients taking anticoagulants have roughly double the risk of bleeding compared with patients not on anticoagulants.1 Bleeding rates tend to be slightly higher in patients taking anticoagulants for venous thromboembolism than in those taking them for atrial fibrillation. The average yearly risk of a “major” anticoagulant-associated bleeding event (eg, requiring transfusion or intervention or occurring in a critical anatomic site) is about 2% to 3%, with most of the bleeding being gastrointestinal.2
Intracranial hemorrhage is by far the most deadly complication of anticoagulant therapy: it causes 90% of deaths and disability from warfarin-associated hemorrhage and is associated with a death rate over 50%; however, it is much less common than gastrointestinal bleeding.3 Anticoagulant therapy increases the risk of intracranial hemorrhage by only 0.2% per year.1
RISK-PREDICTION TOOLS HAVE LIMITATIONS
Not all patients have the same risk of bleeding when taking anticoagulants. Many factors in addition to advanced age have been associated with increased bleeding risk, including coexisting medical conditions (such as malignancy, prior stroke or bleeding event, and renal insufficiency), medications (particularly aspirin, nonsteroidal anti-inflammatory drugs, and other antiplatelet drugs), and the timing and intensity of anticoagulation therapy.4
Scoring tools have been developed to identify patients at higher risk of bleeding (Table 1).4–9 The various schemes incorporate many of the same variables, such as older age, renal impairment, and history of bleeding, but some include additional risk factors while others are more parsimonious. They also differ in how individual risk factors are weighted to generate a final risk score.
In terms of predictive ability, none of the available risk schemes appears to be vastly superior, and their ability to predict hemorrhage is modest at best. There is also no universal or well-established threshold at which the risk of bleeding is so high that one would not consider anticoagulants. In fact, a “high-risk” patient may have an aggregate bleeding rate of only 4% to 6% per year. Using risk schemes such as ATRIA,5 HEMORR2HAGES,6 and HAS-BLED7 may be more useful because they provide an estimate of bleeding risk for each point on the scale.
Moreover, the current tools to predict bleeding risk have several other limitations. They were developed in patients already taking anticoagulants and so probably underestimate the actual risk of hemorrhage, as people who could not take anticoagulants were excluded, most likely because they were at high risk of bleeding. Therefore, bleeding risk tools probably apply best to a patient for whom anticoagulation can be considered.
Some clinical variables are necessarily broad. For example, “prior bleeding” is a risk factor included in several risk scores, but does not distinguish between massive variceal bleeding and minor hemorrhoidal bleeding.
Risk scores do not effectively predict intracranial hemorrhage.
Finally, these risk tools were developed in patients taking vitamin K antagonists, and it is not yet established that they can effectively predict hemorrhage related to other, newer anticoagulants.
WHEN DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
For patients with atrial fibrillation, the net clinical benefit of anticoagulation (strokes prevented minus bleeding events induced) increases as the risk of stroke rises. Updated guidelines for managing atrial fibrillation now recommend anticoagulation for most patients.10
For most older patients with atrial fibrillation, the decision to anticoagulate may not change even if a bleeding risk tool indicates a high bleeding risk.11 For example, a patient with a history of ischemic stroke will generally derive more benefit than harm from anticoagulants. The primary exception is in patients with prior lobar intracranial hemorrhage, because of the high risk of rebleeding and the worse outcomes associated with intracranial hemorrhage.12 As a general rule, most patients with atrial fibrillation and an additional risk factor for stroke should be considered for anticoagulant therapy unless they have a history of lobar intracranial hemorrhage.
Anticoagulation may be deferred if the patient is at the lower end of the stroke risk spectrum and if the bleeding risk is calculated to be high. However, as noted before, current bleeding risk tools probably do not capture the experiences of patients at the extremes of high bleeding risk, so clinical judgment continues to be important. In addition, forgoing anticoagulation could be reasonable even in patients at high risk for recurrent stroke if their life expectancy is limited, if anticoagulation is unacceptably burdensome, or if it is not within their goals and preferences.
WHAT ABOUT FALL RISK?
Fall risk commonly deters clinicians from prescribing anticoagulants because of the fear of causing intracranial hemorrhage. In particular, falls increase the risk for subdural hematoma, which has a death rate comparable to that of ischemic stroke.13
Studies have had difficulty quantifying the exact risk associated with falls because these patients are less likely to be prescribed anticoagulants. One decision analysis estimated that a person would have to fall about 300 times per year before the risk of intracranial hemorrhage outweighed the benefits from stroke reduction.14 Studies have found that patients at high risk of falls have a higher risk of intracranial hemorrhage, but that this risk is counterbalanced by an even greater risk of ischemic stroke.15
Therefore, if the baseline risk of ischemic stroke is high, anticoagulation is still favored.
WHEN SHOULD I USE A BLEEDING RISK TOOL?
Despite their limitations, bleeding risk tools are useful in clinical practice when estimates of bleeding risk affect clinical behavior. They are most helpful for patients at the lower end of the stroke or thromboembolism risk spectrum, where the decision to anticoagulate is strongly influenced by bleeding risk. Risk tools may also be helpful when counseling patients about their bleeding risk off and on anticoagulants.
Finally, recognizing that a patient is at high bleeding risk may lead the clinician to consider closer monitoring of anticoagulants or to implement strategies to reduce the risk.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Lopes LC, Spencer FA, Neumann I, et al. Bleeding risk in atrial fibrillation patients taking vitamin K antagonists: systematic review and meta-analysis. Clin Pharmacol Ther 2013; 94:367–375.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke prevention in atrial fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC113-EF.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J 2006; 151:713–719.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Nieto JA, Solano R, Iglesias NT, et al, for the RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thrombosis Res 2013; 132:175–179.
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003; 34:1710–1716.
- Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012; 43:1795–1799.
- Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003; 163:1580–1586.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Lopes LC, Spencer FA, Neumann I, et al. Bleeding risk in atrial fibrillation patients taking vitamin K antagonists: systematic review and meta-analysis. Clin Pharmacol Ther 2013; 94:367–375.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke prevention in atrial fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC113-EF.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J 2006; 151:713–719.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Nieto JA, Solano R, Iglesias NT, et al, for the RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thrombosis Res 2013; 132:175–179.
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003; 34:1710–1716.
- Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012; 43:1795–1799.
- Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003; 163:1580–1586.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
What is the best approach to a high systolic pulmonary artery pressure on echocardiography?
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
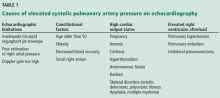
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
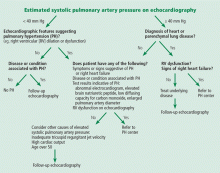
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
Can patients with infectious endocarditis be safely anticoagulated?
Managing anticoagulation in patients with infectious endocarditis requires an individualized approach, using a careful risk-benefit assessment on a case-by-case basis. There is a dearth of high-quality evidence; consequently, the recommendations also vary according to the clinical situation.
Newly diagnosed native valve infectious endocarditis in itself is not an indication for anticoagulation.1–3 The question of whether to anticoagulate arises in patients who have a preexisting or coexisting indication for anticoagulation such as atrial fibrillation, deep vein thrombosis, pulmonary embolism, or a mechanical prosthetic heart valve. The question becomes yet more complex in patients with cerebrovascular complications and a coexistent strong indication for anticoagulation, creating what is often a very thorny dilemma.
Based on a review of available evidence, recommendations for anticoagulation in patients with infectious endocarditis are summarized below.
AVAILABLE EVIDENCE IS SCARCE AND MIXED
Earlier observational studies suggested a significant risk of cerebral hemorrhage with anticoagulation in patients with native valve endocarditis, although none of these studies were recent (some of them took place in the 1940s), and none are methodologically compelling.4–8 Consequently, some experts have expressed skepticism regarding their findings, particularly in recent years.
In part, this skepticism arises from studies that showed a lower incidence of cerebrovascular complications and smaller vegetation size in patients with prosthetic valve infectious endocarditis, studies in which many of the patients received anticoagulation therapy.9,10 The mechanism responsible for this effect is theorized to be that the vegetation is an amalgam of destroyed cells, platelets, and fibrin, with anticoagulation preventing this aggregation from further growth and propagation.
How great is the benefit or the potential harm?
Some experts argue that the incidence of ischemic stroke with hemorrhagic transformation in patients with infectious endocarditis receiving anticoagulation is overestimated. According to this view, the beneficial effects of anticoagulation at least counterbalance the potential harmful effects.
In addition to the studies cited above, recent studies have shown that patients on anticoagulation tend to have smaller vegetations and fewer cerebrovascular complications.11–13 Snygg-Martin et al11 and Rasmussen et al12 found not only that cerebrovascular complications were less common in patients already on anticoagulation at the time infectious endocarditis was diagnosed, but also that no increase in the rate of hemorrhagic lesions was reported.
These were all nonrandomized studies, and most of the patients in them had native valve infectious endocarditis diagnosed at an early stage. Importantly, these studies found that the beneficial effects of anticoagulation were only present if the patient was receiving warfarin before infectious endocarditis was diagnosed and antibiotic therapy was initiated. No benefits from anticoagulation were demonstrated once antimicrobial therapy was begun.
Similarly, Anavekar et al14 showed that embolic events occurred significantly less often in those who were currently on continuous daily antiplatelet therapy, suggesting that receiving antiplatelet agents at baseline protects against cardioembolic events in patients who develop infective endocarditis. However, the only randomized trial examining the initiation of antiplatelet therapy in patients diagnosed with infectious endocarditis receiving antibiotic treatment showed that adding aspirin did not reduce the risk of embolic events and was associated with a trend toward increased risk of bleeding.15
A recent large cohort study suggested that infectious endocarditis patients who receive anticoagulation therapy may have a higher incidence of cerebrovascular complications (hazard ratio 1.31, 95% confidence interval 1.00–1.72, P = .048), with a particular association of anticoagulation therapy with intracranial bleeding (hazard ratio 2.71, 95% confidence interval 1.54–4.76, P = .001).16
Another provocative link supported by the same study was a higher incidence of hemorrhagic complications with anticoagulation in patients with infectious endocarditis caused by Staphylococcus aureus, an association also suggested by older data from Tornos et al,8 but not seen in a study by Rasmussen et al.12
Continuing anticoagulation is an individualized decision
The benefit or harm of anticoagulation in patients with infectious endocarditis may be determined at least in part by a complex mix of factors including the valve involved (embolic events are more common with mitral valve vegetations than with aortic valve vegetations), vegetation size (higher risk if > 1 cm), mobility of vegetations, and perhaps the virulence of the causative organism.16,17 The fact that antimicrobial therapy obviates any beneficial effect of anticoagulation speaks strongly against starting anticoagulation therapy in infectious endocarditis patients with the sole purpose of reducing stroke risk.
Without large randomized trials to better delineate the risks and benefits of continuing preexisting anticoagulation in all patients with infectious endocarditis, patients already receiving anticoagulants need a careful, individualized risk-benefit assessment. Current guidelines agree that newly diagnosed infectious endocarditis per se is not an indication for anticoagulation or aspirin therapy (Table 1).1–3
TAKE-HOME POINTS
- Starting antiplatelet and anticoagulation therapy for the sole purpose of stroke prevention is not recommended in patients with newly diagnosed infectious endocarditis.
- In most cases, anticoagulation and antiplatelet therapy should be temporarily discontinued in patients with infectious endocarditis and stroke or suspected stroke.
- Patients need careful assessment on a case-by-case basis, and the presence of risk factors predisposing patients to cerebrovascular complications (eg, large or very mobile vegetations, causative pathogens such as S aureus or Candida spp) may prompt temporary suspension of anticoagulation and antiplatelet therapy.
- If there is a clear preexisting or coexisting indication for these agents and surgery is not anticipated, consider continuing antiplatelet and anticoagulant therapy in patients with infectious endocarditis, provided they lack the risk factors described above and stroke has been excluded.
- If there is a clear preexisting or coexisting indication for these agents and surgery is being considered, consider using a short-acting anticoagulant such as intravenous or low-molecular weight heparin as a bridge to surgery.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–1486.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Rev Esp Cardiol (Engl Ed) 2016; 69:69.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e576S–e600S.
- Delahaye JP, Poncet P, Malquarti V, Beaune J, Garé JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990; 11:1074–1078.
- Loewe L. The combined use of anti-infectives and anticoagulants in the treatment of subacute bacterial endocarditis. Bull N Y Acad Med 1945; 21:59–86.
- Priest WS, Smith JM, McGee GC. The effect of anticoagulants on the penicillin therapy and the pathologic lesions of subacute bacterial endocarditis. N Engl J Med 1946; 235:699–706.
- Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine 1978; 57:329–343.
- Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999; 159:473–475.
- Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation 1978; 57:1004–1007.
- Schulz R, Werner GS, Fuchs JB, et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990’s. Eur Heart J 1996; 17:281–288.
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis 2011; 30:151–157.
- Rasmussen RV, Snygg-Martin U, Olaison L, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 2009; 114:284–291.
- Yau JW, Lee P, Wilson A, Jenkins AJ. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? Intern Med J 2011; 41:795–797.
- Anavekar NS, Tleyjeh IM, Mirzoyev Z, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis 2007; 44:1180–1186.
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42:775–780.
- Garcia-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–2284.
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112:69–75.
Managing anticoagulation in patients with infectious endocarditis requires an individualized approach, using a careful risk-benefit assessment on a case-by-case basis. There is a dearth of high-quality evidence; consequently, the recommendations also vary according to the clinical situation.
Newly diagnosed native valve infectious endocarditis in itself is not an indication for anticoagulation.1–3 The question of whether to anticoagulate arises in patients who have a preexisting or coexisting indication for anticoagulation such as atrial fibrillation, deep vein thrombosis, pulmonary embolism, or a mechanical prosthetic heart valve. The question becomes yet more complex in patients with cerebrovascular complications and a coexistent strong indication for anticoagulation, creating what is often a very thorny dilemma.
Based on a review of available evidence, recommendations for anticoagulation in patients with infectious endocarditis are summarized below.
AVAILABLE EVIDENCE IS SCARCE AND MIXED
Earlier observational studies suggested a significant risk of cerebral hemorrhage with anticoagulation in patients with native valve endocarditis, although none of these studies were recent (some of them took place in the 1940s), and none are methodologically compelling.4–8 Consequently, some experts have expressed skepticism regarding their findings, particularly in recent years.
In part, this skepticism arises from studies that showed a lower incidence of cerebrovascular complications and smaller vegetation size in patients with prosthetic valve infectious endocarditis, studies in which many of the patients received anticoagulation therapy.9,10 The mechanism responsible for this effect is theorized to be that the vegetation is an amalgam of destroyed cells, platelets, and fibrin, with anticoagulation preventing this aggregation from further growth and propagation.
How great is the benefit or the potential harm?
Some experts argue that the incidence of ischemic stroke with hemorrhagic transformation in patients with infectious endocarditis receiving anticoagulation is overestimated. According to this view, the beneficial effects of anticoagulation at least counterbalance the potential harmful effects.
In addition to the studies cited above, recent studies have shown that patients on anticoagulation tend to have smaller vegetations and fewer cerebrovascular complications.11–13 Snygg-Martin et al11 and Rasmussen et al12 found not only that cerebrovascular complications were less common in patients already on anticoagulation at the time infectious endocarditis was diagnosed, but also that no increase in the rate of hemorrhagic lesions was reported.
These were all nonrandomized studies, and most of the patients in them had native valve infectious endocarditis diagnosed at an early stage. Importantly, these studies found that the beneficial effects of anticoagulation were only present if the patient was receiving warfarin before infectious endocarditis was diagnosed and antibiotic therapy was initiated. No benefits from anticoagulation were demonstrated once antimicrobial therapy was begun.
Similarly, Anavekar et al14 showed that embolic events occurred significantly less often in those who were currently on continuous daily antiplatelet therapy, suggesting that receiving antiplatelet agents at baseline protects against cardioembolic events in patients who develop infective endocarditis. However, the only randomized trial examining the initiation of antiplatelet therapy in patients diagnosed with infectious endocarditis receiving antibiotic treatment showed that adding aspirin did not reduce the risk of embolic events and was associated with a trend toward increased risk of bleeding.15
A recent large cohort study suggested that infectious endocarditis patients who receive anticoagulation therapy may have a higher incidence of cerebrovascular complications (hazard ratio 1.31, 95% confidence interval 1.00–1.72, P = .048), with a particular association of anticoagulation therapy with intracranial bleeding (hazard ratio 2.71, 95% confidence interval 1.54–4.76, P = .001).16
Another provocative link supported by the same study was a higher incidence of hemorrhagic complications with anticoagulation in patients with infectious endocarditis caused by Staphylococcus aureus, an association also suggested by older data from Tornos et al,8 but not seen in a study by Rasmussen et al.12
Continuing anticoagulation is an individualized decision
The benefit or harm of anticoagulation in patients with infectious endocarditis may be determined at least in part by a complex mix of factors including the valve involved (embolic events are more common with mitral valve vegetations than with aortic valve vegetations), vegetation size (higher risk if > 1 cm), mobility of vegetations, and perhaps the virulence of the causative organism.16,17 The fact that antimicrobial therapy obviates any beneficial effect of anticoagulation speaks strongly against starting anticoagulation therapy in infectious endocarditis patients with the sole purpose of reducing stroke risk.
Without large randomized trials to better delineate the risks and benefits of continuing preexisting anticoagulation in all patients with infectious endocarditis, patients already receiving anticoagulants need a careful, individualized risk-benefit assessment. Current guidelines agree that newly diagnosed infectious endocarditis per se is not an indication for anticoagulation or aspirin therapy (Table 1).1–3
TAKE-HOME POINTS
- Starting antiplatelet and anticoagulation therapy for the sole purpose of stroke prevention is not recommended in patients with newly diagnosed infectious endocarditis.
- In most cases, anticoagulation and antiplatelet therapy should be temporarily discontinued in patients with infectious endocarditis and stroke or suspected stroke.
- Patients need careful assessment on a case-by-case basis, and the presence of risk factors predisposing patients to cerebrovascular complications (eg, large or very mobile vegetations, causative pathogens such as S aureus or Candida spp) may prompt temporary suspension of anticoagulation and antiplatelet therapy.
- If there is a clear preexisting or coexisting indication for these agents and surgery is not anticipated, consider continuing antiplatelet and anticoagulant therapy in patients with infectious endocarditis, provided they lack the risk factors described above and stroke has been excluded.
- If there is a clear preexisting or coexisting indication for these agents and surgery is being considered, consider using a short-acting anticoagulant such as intravenous or low-molecular weight heparin as a bridge to surgery.
Managing anticoagulation in patients with infectious endocarditis requires an individualized approach, using a careful risk-benefit assessment on a case-by-case basis. There is a dearth of high-quality evidence; consequently, the recommendations also vary according to the clinical situation.
Newly diagnosed native valve infectious endocarditis in itself is not an indication for anticoagulation.1–3 The question of whether to anticoagulate arises in patients who have a preexisting or coexisting indication for anticoagulation such as atrial fibrillation, deep vein thrombosis, pulmonary embolism, or a mechanical prosthetic heart valve. The question becomes yet more complex in patients with cerebrovascular complications and a coexistent strong indication for anticoagulation, creating what is often a very thorny dilemma.
Based on a review of available evidence, recommendations for anticoagulation in patients with infectious endocarditis are summarized below.
AVAILABLE EVIDENCE IS SCARCE AND MIXED
Earlier observational studies suggested a significant risk of cerebral hemorrhage with anticoagulation in patients with native valve endocarditis, although none of these studies were recent (some of them took place in the 1940s), and none are methodologically compelling.4–8 Consequently, some experts have expressed skepticism regarding their findings, particularly in recent years.
In part, this skepticism arises from studies that showed a lower incidence of cerebrovascular complications and smaller vegetation size in patients with prosthetic valve infectious endocarditis, studies in which many of the patients received anticoagulation therapy.9,10 The mechanism responsible for this effect is theorized to be that the vegetation is an amalgam of destroyed cells, platelets, and fibrin, with anticoagulation preventing this aggregation from further growth and propagation.
How great is the benefit or the potential harm?
Some experts argue that the incidence of ischemic stroke with hemorrhagic transformation in patients with infectious endocarditis receiving anticoagulation is overestimated. According to this view, the beneficial effects of anticoagulation at least counterbalance the potential harmful effects.
In addition to the studies cited above, recent studies have shown that patients on anticoagulation tend to have smaller vegetations and fewer cerebrovascular complications.11–13 Snygg-Martin et al11 and Rasmussen et al12 found not only that cerebrovascular complications were less common in patients already on anticoagulation at the time infectious endocarditis was diagnosed, but also that no increase in the rate of hemorrhagic lesions was reported.
These were all nonrandomized studies, and most of the patients in them had native valve infectious endocarditis diagnosed at an early stage. Importantly, these studies found that the beneficial effects of anticoagulation were only present if the patient was receiving warfarin before infectious endocarditis was diagnosed and antibiotic therapy was initiated. No benefits from anticoagulation were demonstrated once antimicrobial therapy was begun.
Similarly, Anavekar et al14 showed that embolic events occurred significantly less often in those who were currently on continuous daily antiplatelet therapy, suggesting that receiving antiplatelet agents at baseline protects against cardioembolic events in patients who develop infective endocarditis. However, the only randomized trial examining the initiation of antiplatelet therapy in patients diagnosed with infectious endocarditis receiving antibiotic treatment showed that adding aspirin did not reduce the risk of embolic events and was associated with a trend toward increased risk of bleeding.15
A recent large cohort study suggested that infectious endocarditis patients who receive anticoagulation therapy may have a higher incidence of cerebrovascular complications (hazard ratio 1.31, 95% confidence interval 1.00–1.72, P = .048), with a particular association of anticoagulation therapy with intracranial bleeding (hazard ratio 2.71, 95% confidence interval 1.54–4.76, P = .001).16
Another provocative link supported by the same study was a higher incidence of hemorrhagic complications with anticoagulation in patients with infectious endocarditis caused by Staphylococcus aureus, an association also suggested by older data from Tornos et al,8 but not seen in a study by Rasmussen et al.12
Continuing anticoagulation is an individualized decision
The benefit or harm of anticoagulation in patients with infectious endocarditis may be determined at least in part by a complex mix of factors including the valve involved (embolic events are more common with mitral valve vegetations than with aortic valve vegetations), vegetation size (higher risk if > 1 cm), mobility of vegetations, and perhaps the virulence of the causative organism.16,17 The fact that antimicrobial therapy obviates any beneficial effect of anticoagulation speaks strongly against starting anticoagulation therapy in infectious endocarditis patients with the sole purpose of reducing stroke risk.
Without large randomized trials to better delineate the risks and benefits of continuing preexisting anticoagulation in all patients with infectious endocarditis, patients already receiving anticoagulants need a careful, individualized risk-benefit assessment. Current guidelines agree that newly diagnosed infectious endocarditis per se is not an indication for anticoagulation or aspirin therapy (Table 1).1–3
TAKE-HOME POINTS
- Starting antiplatelet and anticoagulation therapy for the sole purpose of stroke prevention is not recommended in patients with newly diagnosed infectious endocarditis.
- In most cases, anticoagulation and antiplatelet therapy should be temporarily discontinued in patients with infectious endocarditis and stroke or suspected stroke.
- Patients need careful assessment on a case-by-case basis, and the presence of risk factors predisposing patients to cerebrovascular complications (eg, large or very mobile vegetations, causative pathogens such as S aureus or Candida spp) may prompt temporary suspension of anticoagulation and antiplatelet therapy.
- If there is a clear preexisting or coexisting indication for these agents and surgery is not anticipated, consider continuing antiplatelet and anticoagulant therapy in patients with infectious endocarditis, provided they lack the risk factors described above and stroke has been excluded.
- If there is a clear preexisting or coexisting indication for these agents and surgery is being considered, consider using a short-acting anticoagulant such as intravenous or low-molecular weight heparin as a bridge to surgery.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–1486.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Rev Esp Cardiol (Engl Ed) 2016; 69:69.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e576S–e600S.
- Delahaye JP, Poncet P, Malquarti V, Beaune J, Garé JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990; 11:1074–1078.
- Loewe L. The combined use of anti-infectives and anticoagulants in the treatment of subacute bacterial endocarditis. Bull N Y Acad Med 1945; 21:59–86.
- Priest WS, Smith JM, McGee GC. The effect of anticoagulants on the penicillin therapy and the pathologic lesions of subacute bacterial endocarditis. N Engl J Med 1946; 235:699–706.
- Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine 1978; 57:329–343.
- Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999; 159:473–475.
- Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation 1978; 57:1004–1007.
- Schulz R, Werner GS, Fuchs JB, et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990’s. Eur Heart J 1996; 17:281–288.
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis 2011; 30:151–157.
- Rasmussen RV, Snygg-Martin U, Olaison L, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 2009; 114:284–291.
- Yau JW, Lee P, Wilson A, Jenkins AJ. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? Intern Med J 2011; 41:795–797.
- Anavekar NS, Tleyjeh IM, Mirzoyev Z, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis 2007; 44:1180–1186.
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42:775–780.
- Garcia-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–2284.
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112:69–75.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–1486.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Rev Esp Cardiol (Engl Ed) 2016; 69:69.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e576S–e600S.
- Delahaye JP, Poncet P, Malquarti V, Beaune J, Garé JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990; 11:1074–1078.
- Loewe L. The combined use of anti-infectives and anticoagulants in the treatment of subacute bacterial endocarditis. Bull N Y Acad Med 1945; 21:59–86.
- Priest WS, Smith JM, McGee GC. The effect of anticoagulants on the penicillin therapy and the pathologic lesions of subacute bacterial endocarditis. N Engl J Med 1946; 235:699–706.
- Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine 1978; 57:329–343.
- Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999; 159:473–475.
- Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation 1978; 57:1004–1007.
- Schulz R, Werner GS, Fuchs JB, et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990’s. Eur Heart J 1996; 17:281–288.
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis 2011; 30:151–157.
- Rasmussen RV, Snygg-Martin U, Olaison L, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 2009; 114:284–291.
- Yau JW, Lee P, Wilson A, Jenkins AJ. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? Intern Med J 2011; 41:795–797.
- Anavekar NS, Tleyjeh IM, Mirzoyev Z, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis 2007; 44:1180–1186.
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42:775–780.
- Garcia-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–2284.
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112:69–75.
Can patients opt to turn off implantable cardioverter-defibrillators near the end of life?
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Obstructive sleep apnea: Who should be tested, and how?
Patients who have risk factors for obstructive sleep apnea (OSA) or who report symptoms of OSA should be screened for it, first with a complete sleep history and standardized questionnaire, and then by objective testing if indicated. The gold standard test for OSA is polysomnography performed overnight in a sleep laboratory. Home testing is an option in certain instances.
Common risk factors include obesity, resistant hypertension, retrognathia, large neck circumference (> 17 inches in men, > 16 inches in women), and history of stroke, atrial fibrillation, nocturnal arrhythmias, heart failure, and pulmonary hypertension. Screening is also recommended for any patient who is found on physical examination to have upper-airway narrowing or who reports symptoms such as loud snoring, observed episodes of apnea, gasping or choking at night, unrefreshing sleep, morning headaches, unexplained fatigue, and excessive tiredness during the day.
The American Academy of Sleep Medicine suggests three opportunities to screen for OSA1:
- At routine health maintenance visits
- If the patient reports clinical symptoms of OSA
- If the patient has risk factors.
A DISMAL STATISTIC
The prevalence of OSA in the United States is high, estimated to be 2% in women and 4% in men in the middle-aged work force,2 and even more in blacks, Asians, and older adults.3 Yet only 10% of people with OSA are diagnosed4—a dismal statistic considering the association of OSA with resistant hypertension5 and with a greater risk of stroke,6 cardiovascular disease, and death.7
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is associated with a number of conditions7:
- Hypertension. OSA is one of the most common conditions associated with resistant hypertension. Patients with severe OSA and resistant hypertension who comply with continuous positive airway pressure (CPAP) treatment have significant reductions in blood pressure.
- Coronary artery disease. OSA is twice as common in people with coronary artery disease as in those with no coronary artery disease. In patients with coronary artery disease and OSA, CPAP may reduce the rate of nonfatal and fatal cardiovascular events.
- Heart failure. OSA is common in patients with systolic dysfunction (11% to 37%). OSA also has been detected in more than 50% of patients with heart failure with preserved systolic function. CPAP treatment can improve ejection fraction in patients with systolic dysfunction.
- Arrythmias. Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy have been reported to be significantly more common in people with OSA.8 If the underlying cardiac conduction system is normal and there is no significant thyroid dysfunction, bradyarrhythmias and heart block may be treated effectively with CPAP.7 Treatment of OSA may decrease the incidence and severity of ventricular arrhythmias.7
- Sudden cardiac death. OSA was independently associated with sudden cardiac death in a longitudinal study.9
- Stroke. The Sleep Heart Health Study6 showed that OSA is 30% more common in patients who developed ischemic stroke. Long-term CPAP treatment in moderate to severe OSA and ischemic stroke is associated with a reduction in the mortality rate.10
- Diabetes. The Sleep Heart Health Study showed that OSA is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus.11
A QUESTIONNAIRE HELPS IDENTIFY WHO NEEDS TESTING
If you suspect OSA, consider administering a sleep disorder questionnaire such as the Berlin,12 the Epworth Sleepiness Scale, or the STOP-Bang questionnaire (Table 1). The STOP-Bang questionnaire is an easy-to-use tool that expands on the STOP questionnaire (snoring, tiredness, observed apnea, high blood pressure) with the addition of body mass index, age, neck size, and gender. The Berlin questionnaire has been validated in the primary care setting.12 The STOP-Bang questionnaire has been validated in preoperative settings13 but not in the primary care setting (although it has been commonly used in primary care).
WHICH TEST TO ORDER?
If the score on the questionnaire indicates a moderate or high risk of OSA, the patient should undergo objective testing with polysomnography or, in certain instances, home testing.1 Polysomnography is the gold standard. Home testing costs less and is easier to arrange, but the American Academy of Sleep Medicine recommends it as an alternative to polysomnography, in conjunction with a comprehensive sleep evaluation, only in the following situations14:
- If the patient has a high pretest probability of moderate to severe OSA
- If immobility or critical illness makes polysomnography unfeasible
- If direct monitoring of the response to non-CPAP treatments for sleep apnea is needed.
Home testing for OSA should not be used in the following situations:
- If the patient has significant morbidity such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure
- In evaluating a patient suspected of having comorbid sleep disorders such as central sleep apnea, periodic limb movement disorder, insomnia, parasomnias, circadian rhythm disorder, or narcolepsy
- In screening of asymptomatic patients.
Home testing has important drawbacks. It may underestimate the severity of sleep apnea. The rate of false-negative results may be as high as 17%. If the home test was thought to be technically inadequate or the results were inconsistent with those that were expected—ie, if the patient has a high pretest probability of OSA based on risk factors or symptoms but negative results on home testing—then the patient should undergo polysomnography.14
DIAGNOSIS
The diagnosis of OSA is confirmed if the number of apnea events per hour (ie, the apnea-hypopnea index) on polysomnography or home testing is more than 15, regardless of symptoms, or more than 5 in a patient who reports OSA symptoms. An apnea-hypopnea index of 5 to 14 indicates mild OSA, 15 to 30 indicates moderate OSA, and greater than 30 indicates severe OSA.
BENEFITS OF TREATMENT
Treatment of OSA with CPAP reduces the 10-year risk of fatal and nonfatal motor vehicle accidents by 52%, the 10-year expected number of myocardial infarctions by 49%, and the 10-year risk of stroke by 31%.7 It has also been found to be cost-effective, for men and women of all ages with moderate to severe OSA.15
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Somers VK, White DP, Amin R, et al; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616.
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009; 180:36–41.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Netzer NC, Hoegel JJ, Loube D, et al; Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003; 124:1406–1414.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011; 34:695–709.
Patients who have risk factors for obstructive sleep apnea (OSA) or who report symptoms of OSA should be screened for it, first with a complete sleep history and standardized questionnaire, and then by objective testing if indicated. The gold standard test for OSA is polysomnography performed overnight in a sleep laboratory. Home testing is an option in certain instances.
Common risk factors include obesity, resistant hypertension, retrognathia, large neck circumference (> 17 inches in men, > 16 inches in women), and history of stroke, atrial fibrillation, nocturnal arrhythmias, heart failure, and pulmonary hypertension. Screening is also recommended for any patient who is found on physical examination to have upper-airway narrowing or who reports symptoms such as loud snoring, observed episodes of apnea, gasping or choking at night, unrefreshing sleep, morning headaches, unexplained fatigue, and excessive tiredness during the day.
The American Academy of Sleep Medicine suggests three opportunities to screen for OSA1:
- At routine health maintenance visits
- If the patient reports clinical symptoms of OSA
- If the patient has risk factors.
A DISMAL STATISTIC
The prevalence of OSA in the United States is high, estimated to be 2% in women and 4% in men in the middle-aged work force,2 and even more in blacks, Asians, and older adults.3 Yet only 10% of people with OSA are diagnosed4—a dismal statistic considering the association of OSA with resistant hypertension5 and with a greater risk of stroke,6 cardiovascular disease, and death.7
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is associated with a number of conditions7:
- Hypertension. OSA is one of the most common conditions associated with resistant hypertension. Patients with severe OSA and resistant hypertension who comply with continuous positive airway pressure (CPAP) treatment have significant reductions in blood pressure.
- Coronary artery disease. OSA is twice as common in people with coronary artery disease as in those with no coronary artery disease. In patients with coronary artery disease and OSA, CPAP may reduce the rate of nonfatal and fatal cardiovascular events.
- Heart failure. OSA is common in patients with systolic dysfunction (11% to 37%). OSA also has been detected in more than 50% of patients with heart failure with preserved systolic function. CPAP treatment can improve ejection fraction in patients with systolic dysfunction.
- Arrythmias. Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy have been reported to be significantly more common in people with OSA.8 If the underlying cardiac conduction system is normal and there is no significant thyroid dysfunction, bradyarrhythmias and heart block may be treated effectively with CPAP.7 Treatment of OSA may decrease the incidence and severity of ventricular arrhythmias.7
- Sudden cardiac death. OSA was independently associated with sudden cardiac death in a longitudinal study.9
- Stroke. The Sleep Heart Health Study6 showed that OSA is 30% more common in patients who developed ischemic stroke. Long-term CPAP treatment in moderate to severe OSA and ischemic stroke is associated with a reduction in the mortality rate.10
- Diabetes. The Sleep Heart Health Study showed that OSA is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus.11
A QUESTIONNAIRE HELPS IDENTIFY WHO NEEDS TESTING
If you suspect OSA, consider administering a sleep disorder questionnaire such as the Berlin,12 the Epworth Sleepiness Scale, or the STOP-Bang questionnaire (Table 1). The STOP-Bang questionnaire is an easy-to-use tool that expands on the STOP questionnaire (snoring, tiredness, observed apnea, high blood pressure) with the addition of body mass index, age, neck size, and gender. The Berlin questionnaire has been validated in the primary care setting.12 The STOP-Bang questionnaire has been validated in preoperative settings13 but not in the primary care setting (although it has been commonly used in primary care).
WHICH TEST TO ORDER?
If the score on the questionnaire indicates a moderate or high risk of OSA, the patient should undergo objective testing with polysomnography or, in certain instances, home testing.1 Polysomnography is the gold standard. Home testing costs less and is easier to arrange, but the American Academy of Sleep Medicine recommends it as an alternative to polysomnography, in conjunction with a comprehensive sleep evaluation, only in the following situations14:
- If the patient has a high pretest probability of moderate to severe OSA
- If immobility or critical illness makes polysomnography unfeasible
- If direct monitoring of the response to non-CPAP treatments for sleep apnea is needed.
Home testing for OSA should not be used in the following situations:
- If the patient has significant morbidity such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure
- In evaluating a patient suspected of having comorbid sleep disorders such as central sleep apnea, periodic limb movement disorder, insomnia, parasomnias, circadian rhythm disorder, or narcolepsy
- In screening of asymptomatic patients.
Home testing has important drawbacks. It may underestimate the severity of sleep apnea. The rate of false-negative results may be as high as 17%. If the home test was thought to be technically inadequate or the results were inconsistent with those that were expected—ie, if the patient has a high pretest probability of OSA based on risk factors or symptoms but negative results on home testing—then the patient should undergo polysomnography.14
DIAGNOSIS
The diagnosis of OSA is confirmed if the number of apnea events per hour (ie, the apnea-hypopnea index) on polysomnography or home testing is more than 15, regardless of symptoms, or more than 5 in a patient who reports OSA symptoms. An apnea-hypopnea index of 5 to 14 indicates mild OSA, 15 to 30 indicates moderate OSA, and greater than 30 indicates severe OSA.
BENEFITS OF TREATMENT
Treatment of OSA with CPAP reduces the 10-year risk of fatal and nonfatal motor vehicle accidents by 52%, the 10-year expected number of myocardial infarctions by 49%, and the 10-year risk of stroke by 31%.7 It has also been found to be cost-effective, for men and women of all ages with moderate to severe OSA.15
Patients who have risk factors for obstructive sleep apnea (OSA) or who report symptoms of OSA should be screened for it, first with a complete sleep history and standardized questionnaire, and then by objective testing if indicated. The gold standard test for OSA is polysomnography performed overnight in a sleep laboratory. Home testing is an option in certain instances.
Common risk factors include obesity, resistant hypertension, retrognathia, large neck circumference (> 17 inches in men, > 16 inches in women), and history of stroke, atrial fibrillation, nocturnal arrhythmias, heart failure, and pulmonary hypertension. Screening is also recommended for any patient who is found on physical examination to have upper-airway narrowing or who reports symptoms such as loud snoring, observed episodes of apnea, gasping or choking at night, unrefreshing sleep, morning headaches, unexplained fatigue, and excessive tiredness during the day.
The American Academy of Sleep Medicine suggests three opportunities to screen for OSA1:
- At routine health maintenance visits
- If the patient reports clinical symptoms of OSA
- If the patient has risk factors.
A DISMAL STATISTIC
The prevalence of OSA in the United States is high, estimated to be 2% in women and 4% in men in the middle-aged work force,2 and even more in blacks, Asians, and older adults.3 Yet only 10% of people with OSA are diagnosed4—a dismal statistic considering the association of OSA with resistant hypertension5 and with a greater risk of stroke,6 cardiovascular disease, and death.7
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is associated with a number of conditions7:
- Hypertension. OSA is one of the most common conditions associated with resistant hypertension. Patients with severe OSA and resistant hypertension who comply with continuous positive airway pressure (CPAP) treatment have significant reductions in blood pressure.
- Coronary artery disease. OSA is twice as common in people with coronary artery disease as in those with no coronary artery disease. In patients with coronary artery disease and OSA, CPAP may reduce the rate of nonfatal and fatal cardiovascular events.
- Heart failure. OSA is common in patients with systolic dysfunction (11% to 37%). OSA also has been detected in more than 50% of patients with heart failure with preserved systolic function. CPAP treatment can improve ejection fraction in patients with systolic dysfunction.
- Arrythmias. Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy have been reported to be significantly more common in people with OSA.8 If the underlying cardiac conduction system is normal and there is no significant thyroid dysfunction, bradyarrhythmias and heart block may be treated effectively with CPAP.7 Treatment of OSA may decrease the incidence and severity of ventricular arrhythmias.7
- Sudden cardiac death. OSA was independently associated with sudden cardiac death in a longitudinal study.9
- Stroke. The Sleep Heart Health Study6 showed that OSA is 30% more common in patients who developed ischemic stroke. Long-term CPAP treatment in moderate to severe OSA and ischemic stroke is associated with a reduction in the mortality rate.10
- Diabetes. The Sleep Heart Health Study showed that OSA is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus.11
A QUESTIONNAIRE HELPS IDENTIFY WHO NEEDS TESTING
If you suspect OSA, consider administering a sleep disorder questionnaire such as the Berlin,12 the Epworth Sleepiness Scale, or the STOP-Bang questionnaire (Table 1). The STOP-Bang questionnaire is an easy-to-use tool that expands on the STOP questionnaire (snoring, tiredness, observed apnea, high blood pressure) with the addition of body mass index, age, neck size, and gender. The Berlin questionnaire has been validated in the primary care setting.12 The STOP-Bang questionnaire has been validated in preoperative settings13 but not in the primary care setting (although it has been commonly used in primary care).
WHICH TEST TO ORDER?
If the score on the questionnaire indicates a moderate or high risk of OSA, the patient should undergo objective testing with polysomnography or, in certain instances, home testing.1 Polysomnography is the gold standard. Home testing costs less and is easier to arrange, but the American Academy of Sleep Medicine recommends it as an alternative to polysomnography, in conjunction with a comprehensive sleep evaluation, only in the following situations14:
- If the patient has a high pretest probability of moderate to severe OSA
- If immobility or critical illness makes polysomnography unfeasible
- If direct monitoring of the response to non-CPAP treatments for sleep apnea is needed.
Home testing for OSA should not be used in the following situations:
- If the patient has significant morbidity such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure
- In evaluating a patient suspected of having comorbid sleep disorders such as central sleep apnea, periodic limb movement disorder, insomnia, parasomnias, circadian rhythm disorder, or narcolepsy
- In screening of asymptomatic patients.
Home testing has important drawbacks. It may underestimate the severity of sleep apnea. The rate of false-negative results may be as high as 17%. If the home test was thought to be technically inadequate or the results were inconsistent with those that were expected—ie, if the patient has a high pretest probability of OSA based on risk factors or symptoms but negative results on home testing—then the patient should undergo polysomnography.14
DIAGNOSIS
The diagnosis of OSA is confirmed if the number of apnea events per hour (ie, the apnea-hypopnea index) on polysomnography or home testing is more than 15, regardless of symptoms, or more than 5 in a patient who reports OSA symptoms. An apnea-hypopnea index of 5 to 14 indicates mild OSA, 15 to 30 indicates moderate OSA, and greater than 30 indicates severe OSA.
BENEFITS OF TREATMENT
Treatment of OSA with CPAP reduces the 10-year risk of fatal and nonfatal motor vehicle accidents by 52%, the 10-year expected number of myocardial infarctions by 49%, and the 10-year risk of stroke by 31%.7 It has also been found to be cost-effective, for men and women of all ages with moderate to severe OSA.15
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Somers VK, White DP, Amin R, et al; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616.
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009; 180:36–41.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Netzer NC, Hoegel JJ, Loube D, et al; Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003; 124:1406–1414.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011; 34:695–709.
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Somers VK, White DP, Amin R, et al; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616.
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009; 180:36–41.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Netzer NC, Hoegel JJ, Loube D, et al; Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003; 124:1406–1414.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011; 34:695–709.
Does allergic conjunctivitis always require prescription eyedrops?
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
Should all patients with significant proteinuria take a renin-angiotensin inhibitor?
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
Are breast and pelvic exams necessary when prescribing hormonal contraception?
No. According to 2013 guidelines of the US Centers for Disease Control and Prevention (CDC),1 there is little evidence of benefit for many of the tests commonly mandated by healthcare providers before prescribing hormonal contraception (pill, ring, patch). These tests include breast and pelvic examinations, screening for cervical and sexually transmitted infections, laboratory testing, and mammography.
Only a medical history and blood pressure measurement are needed before prescribing estrogen-containing contraceptives. Patients who have elevated blood pressure but have not been previously diagnosed with hypertension should be preferentially offered other forms of contraception to avoid an additional risk of stroke or myocardial infarction, such as progestin-only products and intrauterine devices (IUDs). Women with blood pressures between 140/90 and 160/100 mm Hg may use estrogen-containing contraceptives only if other options are not appropriate. The CDC guidelines further state that if a patient is unable to come to the office for blood pressure assessment, then a community reading reported by the patient may be used to guide decision-making.
IS A PELVIC EXAMINATION NEEDED?
A pelvic examination (cervical inspection and bimanual examination) will not affect decisions related to prescribing contraceptives, except when prescribing female barrier methods (diaphragm, cervical cap) or IUDs.
Based on a systematic review of the literature between 1946 and 2014, the American College of Physicians now recommends against a screening pelvic examination in asymptomatic, nonpregnant, adult women when a Papanicolaou test is not otherwise indicated.2
The American College of Obstetricians and Gynecologists (ACOG) acknowledges that no current scientific evidence supports or refutes the need for an annual pelvic examination for an asymptomatic, low-risk patient. But ACOG supports pelvic examinations as a way to establish open communication with patients about sexual health and reproduction.3 ACOG also recommends an annual health visit for all women. Whether or not a pelvic examination is performed, women should be counseled annually about birth control and offered contraception.
Patients should also be encouraged to keep their preventive care up-to-date, including cervical cancer screening with a Papanicolaou test or a human papillomavirus test (or both) at appropriate intervals, especially if the patient has cervical abnormalities requiring follow-up. However, falling behind on preventive care should not be a barrier to obtaining contraception.
IMPROVING ADHERENCE, DECREASING UNINTENDED PREGNANCY
One goal of the CDC’s 2013 guidelines was to remove unnecessary barriers to women’s access to contraceptives. In the United States, half of all pregnancies are unintended, and almost half of unintended pregnancies lead to abortion.4 Only half of women who have had an abortion used any contraceptive method within the last month.5 This suggests high levels of unprotected and underprotected sex.
For most patients, several national societies now recommend long-acting reversible contraceptive (LARC) methods, which include IUDs and progestin-only arm implants, because they have lower failure rates in a real-world setting.1,6,7 LARC methods offer the advantage of the patient’s not having to remember to take, apply, or insert the contraceptive (ie, they are worry-free), and of not having to rely on a yearly appointment for refills.
The Contraceptive CHOICE Project8 was a large prospective cohort study that assessed the impact of offering contraception free of charge in St. Louis, Missouri. Most of the 9,256 women who participated selected a LARC method.8 Those taking combined hormonal contraceptives (ie, birth control pill, patch, or ring) had a higher contraceptive failure rate than those using LARC methods (4.55 vs 0.27 per 100 participant-years; hazard ratio after adjustment for age, education, and unintended pregnancy history, 21.8; 95% confidence interval 13.7–34.9). The rate of unintended pregnancy in those under age 21 using combined hormonal contraceptives was almost twice as high as in older participants. Subsequent analyses showed that the abortion rates in the St. Louis region decreased to less than a quarter of the national average after the start of this project.9
Given that the failure rate with combined hormonal contraceptives averages 9% per year,1 it is of the utmost importance that providers not limit access to patients’ prescriptions by requesting unnecessary visits and tests. If oral contraception is selected, women who are dispensed a full year’s supply of pill packs are more likely to continue with their contraceptive in the long term.10
THE PATIENT WITH A COMPLEX MEDICAL HISTORY
Limiting a woman’s contraceptive choices can increase her odds of experiencing an unintended pregnancy, which is associated with a far greater risk of adverse events than any contraceptive.11 Thus, the CDC developed separate guidelines in 2010 to help determine all available options for the patient with medical comorbidities and with a concerning family history (ie, breast cancer, venous thromboembolism).12 It can be helpful to consult the 2010 CDC medical eligibility criteria before offering contraception to these patients. Compared with the 2013 guidelines, which provide practical advice on how to use each contraceptive, the 2010 guidelines give guidance on when it is appropriate to prescribe each contraceptive—eg, which contraceptives are preferred based on a patient’s risk factors, medical history, and medication use. In addition to a two-page color summary chart of the 2010 medical eligibility criteria on the CDC website (https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf), a free mobile app is also available to guide decision-making.13
Pregnancy should be ruled out before initiating any contraceptive. This can be done through a detailed history. The six-item checklist in Table 1 has a 99.8% negative predictive value, so healthcare providers may be confident that a woman is not pregnant if pregnancy is excluded based on this history.14 A pregnancy test is needed in those who test positive on the checklist if they wish to start a LARC method such as an IUD or a progestin-only arm implant. However, because the test has a high false-positive rate, initiation of shorter-acting methods such as combined hormonal contraceptives should not be delayed on the basis of a positive checklist screen alone.1
Emergency contraception taken orally should be offered without an office visit, as its short duration of use allows women with traditional contraindications to hormonal contraceptives to safely use this birth control method.1,12 Because all emergency contraceptives must be used within 5 days of intercourse (the earlier the better), unnecessary office visits delay access and effectiveness.
Although a levonorgestrel-based emergency contraceptive is available over the counter, ulipristal acetate is more effective, especially in women who are overweight.15 A copper IUD placed within 5 days of intercourse is the most effective form of emergency contraception15 but requires an office visit. This method is an option for most women but should be strongly considered for women at highest risk of pregnancy (previous unintended pregnancy, intercourse at midcycle, obesity).
In summary, most women may safely begin their hormonal contraceptive with a detailed medical history alone, without additional office visits, examinations, or screening tests.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013; 62:1–60.
- Qaseem A, Humphrey LL, Harris R, et al; Clinical Guidelines Committee of the American College of Physicians. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:67–72.
- American Congress of Obstetricians and Gynecologists. ACOG practice advisory on annual pelvic examination recommendations; 2014. www.acog.org/About-ACOG/News-Room/Practice-Advisories/ACOG-Practice-Advisory-on-Annual-Pelvic-Examination-Recommendations. Accessed September 8, 2015.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000-2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Committee on Health Care for Underserved Women. Committee opinion no. 615: access to contraception. Obstet Gynecol 2015; 125:250–255.
- Committee on Adolescent Health Care. Committee opinion no. 598: the initial reproductive health visit. Obstet Gynecol 2014; 123:1143–1147.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014; 371:1316–1323.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Over-the-counter access to oral contraceptives. Committee opinion no 544. Obstet Gynecol 2012; 120:1527–1531.
- Committee on Gynecologic Practice. ACOG committee opinion number 540: risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol 2012; 120:1239–1242.
- Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep 2010; 59:1–86.
- Centers for Disease Control and Prevention (CDC). United States medical eligibility criteria (US MEC) for contraceptive use, 2010. www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm. Accessed September 8, 2015.
- Min J, Buckel C, Secura GM, Peipert JF, Madden T. Performance of a checklist to exclude pregnancy at the time of contraceptive initiation among women with a negative urine pregnancy test. Contraception 2015; 91:80–84.
- Batur P. Emergency contraception: separating fact from fiction. Cleve Clin J Med 2012; 79:771–776.
No. According to 2013 guidelines of the US Centers for Disease Control and Prevention (CDC),1 there is little evidence of benefit for many of the tests commonly mandated by healthcare providers before prescribing hormonal contraception (pill, ring, patch). These tests include breast and pelvic examinations, screening for cervical and sexually transmitted infections, laboratory testing, and mammography.
Only a medical history and blood pressure measurement are needed before prescribing estrogen-containing contraceptives. Patients who have elevated blood pressure but have not been previously diagnosed with hypertension should be preferentially offered other forms of contraception to avoid an additional risk of stroke or myocardial infarction, such as progestin-only products and intrauterine devices (IUDs). Women with blood pressures between 140/90 and 160/100 mm Hg may use estrogen-containing contraceptives only if other options are not appropriate. The CDC guidelines further state that if a patient is unable to come to the office for blood pressure assessment, then a community reading reported by the patient may be used to guide decision-making.
IS A PELVIC EXAMINATION NEEDED?
A pelvic examination (cervical inspection and bimanual examination) will not affect decisions related to prescribing contraceptives, except when prescribing female barrier methods (diaphragm, cervical cap) or IUDs.
Based on a systematic review of the literature between 1946 and 2014, the American College of Physicians now recommends against a screening pelvic examination in asymptomatic, nonpregnant, adult women when a Papanicolaou test is not otherwise indicated.2
The American College of Obstetricians and Gynecologists (ACOG) acknowledges that no current scientific evidence supports or refutes the need for an annual pelvic examination for an asymptomatic, low-risk patient. But ACOG supports pelvic examinations as a way to establish open communication with patients about sexual health and reproduction.3 ACOG also recommends an annual health visit for all women. Whether or not a pelvic examination is performed, women should be counseled annually about birth control and offered contraception.
Patients should also be encouraged to keep their preventive care up-to-date, including cervical cancer screening with a Papanicolaou test or a human papillomavirus test (or both) at appropriate intervals, especially if the patient has cervical abnormalities requiring follow-up. However, falling behind on preventive care should not be a barrier to obtaining contraception.
IMPROVING ADHERENCE, DECREASING UNINTENDED PREGNANCY
One goal of the CDC’s 2013 guidelines was to remove unnecessary barriers to women’s access to contraceptives. In the United States, half of all pregnancies are unintended, and almost half of unintended pregnancies lead to abortion.4 Only half of women who have had an abortion used any contraceptive method within the last month.5 This suggests high levels of unprotected and underprotected sex.
For most patients, several national societies now recommend long-acting reversible contraceptive (LARC) methods, which include IUDs and progestin-only arm implants, because they have lower failure rates in a real-world setting.1,6,7 LARC methods offer the advantage of the patient’s not having to remember to take, apply, or insert the contraceptive (ie, they are worry-free), and of not having to rely on a yearly appointment for refills.
The Contraceptive CHOICE Project8 was a large prospective cohort study that assessed the impact of offering contraception free of charge in St. Louis, Missouri. Most of the 9,256 women who participated selected a LARC method.8 Those taking combined hormonal contraceptives (ie, birth control pill, patch, or ring) had a higher contraceptive failure rate than those using LARC methods (4.55 vs 0.27 per 100 participant-years; hazard ratio after adjustment for age, education, and unintended pregnancy history, 21.8; 95% confidence interval 13.7–34.9). The rate of unintended pregnancy in those under age 21 using combined hormonal contraceptives was almost twice as high as in older participants. Subsequent analyses showed that the abortion rates in the St. Louis region decreased to less than a quarter of the national average after the start of this project.9
Given that the failure rate with combined hormonal contraceptives averages 9% per year,1 it is of the utmost importance that providers not limit access to patients’ prescriptions by requesting unnecessary visits and tests. If oral contraception is selected, women who are dispensed a full year’s supply of pill packs are more likely to continue with their contraceptive in the long term.10
THE PATIENT WITH A COMPLEX MEDICAL HISTORY
Limiting a woman’s contraceptive choices can increase her odds of experiencing an unintended pregnancy, which is associated with a far greater risk of adverse events than any contraceptive.11 Thus, the CDC developed separate guidelines in 2010 to help determine all available options for the patient with medical comorbidities and with a concerning family history (ie, breast cancer, venous thromboembolism).12 It can be helpful to consult the 2010 CDC medical eligibility criteria before offering contraception to these patients. Compared with the 2013 guidelines, which provide practical advice on how to use each contraceptive, the 2010 guidelines give guidance on when it is appropriate to prescribe each contraceptive—eg, which contraceptives are preferred based on a patient’s risk factors, medical history, and medication use. In addition to a two-page color summary chart of the 2010 medical eligibility criteria on the CDC website (https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf), a free mobile app is also available to guide decision-making.13
Pregnancy should be ruled out before initiating any contraceptive. This can be done through a detailed history. The six-item checklist in Table 1 has a 99.8% negative predictive value, so healthcare providers may be confident that a woman is not pregnant if pregnancy is excluded based on this history.14 A pregnancy test is needed in those who test positive on the checklist if they wish to start a LARC method such as an IUD or a progestin-only arm implant. However, because the test has a high false-positive rate, initiation of shorter-acting methods such as combined hormonal contraceptives should not be delayed on the basis of a positive checklist screen alone.1
Emergency contraception taken orally should be offered without an office visit, as its short duration of use allows women with traditional contraindications to hormonal contraceptives to safely use this birth control method.1,12 Because all emergency contraceptives must be used within 5 days of intercourse (the earlier the better), unnecessary office visits delay access and effectiveness.
Although a levonorgestrel-based emergency contraceptive is available over the counter, ulipristal acetate is more effective, especially in women who are overweight.15 A copper IUD placed within 5 days of intercourse is the most effective form of emergency contraception15 but requires an office visit. This method is an option for most women but should be strongly considered for women at highest risk of pregnancy (previous unintended pregnancy, intercourse at midcycle, obesity).
In summary, most women may safely begin their hormonal contraceptive with a detailed medical history alone, without additional office visits, examinations, or screening tests.
No. According to 2013 guidelines of the US Centers for Disease Control and Prevention (CDC),1 there is little evidence of benefit for many of the tests commonly mandated by healthcare providers before prescribing hormonal contraception (pill, ring, patch). These tests include breast and pelvic examinations, screening for cervical and sexually transmitted infections, laboratory testing, and mammography.
Only a medical history and blood pressure measurement are needed before prescribing estrogen-containing contraceptives. Patients who have elevated blood pressure but have not been previously diagnosed with hypertension should be preferentially offered other forms of contraception to avoid an additional risk of stroke or myocardial infarction, such as progestin-only products and intrauterine devices (IUDs). Women with blood pressures between 140/90 and 160/100 mm Hg may use estrogen-containing contraceptives only if other options are not appropriate. The CDC guidelines further state that if a patient is unable to come to the office for blood pressure assessment, then a community reading reported by the patient may be used to guide decision-making.
IS A PELVIC EXAMINATION NEEDED?
A pelvic examination (cervical inspection and bimanual examination) will not affect decisions related to prescribing contraceptives, except when prescribing female barrier methods (diaphragm, cervical cap) or IUDs.
Based on a systematic review of the literature between 1946 and 2014, the American College of Physicians now recommends against a screening pelvic examination in asymptomatic, nonpregnant, adult women when a Papanicolaou test is not otherwise indicated.2
The American College of Obstetricians and Gynecologists (ACOG) acknowledges that no current scientific evidence supports or refutes the need for an annual pelvic examination for an asymptomatic, low-risk patient. But ACOG supports pelvic examinations as a way to establish open communication with patients about sexual health and reproduction.3 ACOG also recommends an annual health visit for all women. Whether or not a pelvic examination is performed, women should be counseled annually about birth control and offered contraception.
Patients should also be encouraged to keep their preventive care up-to-date, including cervical cancer screening with a Papanicolaou test or a human papillomavirus test (or both) at appropriate intervals, especially if the patient has cervical abnormalities requiring follow-up. However, falling behind on preventive care should not be a barrier to obtaining contraception.
IMPROVING ADHERENCE, DECREASING UNINTENDED PREGNANCY
One goal of the CDC’s 2013 guidelines was to remove unnecessary barriers to women’s access to contraceptives. In the United States, half of all pregnancies are unintended, and almost half of unintended pregnancies lead to abortion.4 Only half of women who have had an abortion used any contraceptive method within the last month.5 This suggests high levels of unprotected and underprotected sex.
For most patients, several national societies now recommend long-acting reversible contraceptive (LARC) methods, which include IUDs and progestin-only arm implants, because they have lower failure rates in a real-world setting.1,6,7 LARC methods offer the advantage of the patient’s not having to remember to take, apply, or insert the contraceptive (ie, they are worry-free), and of not having to rely on a yearly appointment for refills.
The Contraceptive CHOICE Project8 was a large prospective cohort study that assessed the impact of offering contraception free of charge in St. Louis, Missouri. Most of the 9,256 women who participated selected a LARC method.8 Those taking combined hormonal contraceptives (ie, birth control pill, patch, or ring) had a higher contraceptive failure rate than those using LARC methods (4.55 vs 0.27 per 100 participant-years; hazard ratio after adjustment for age, education, and unintended pregnancy history, 21.8; 95% confidence interval 13.7–34.9). The rate of unintended pregnancy in those under age 21 using combined hormonal contraceptives was almost twice as high as in older participants. Subsequent analyses showed that the abortion rates in the St. Louis region decreased to less than a quarter of the national average after the start of this project.9
Given that the failure rate with combined hormonal contraceptives averages 9% per year,1 it is of the utmost importance that providers not limit access to patients’ prescriptions by requesting unnecessary visits and tests. If oral contraception is selected, women who are dispensed a full year’s supply of pill packs are more likely to continue with their contraceptive in the long term.10
THE PATIENT WITH A COMPLEX MEDICAL HISTORY
Limiting a woman’s contraceptive choices can increase her odds of experiencing an unintended pregnancy, which is associated with a far greater risk of adverse events than any contraceptive.11 Thus, the CDC developed separate guidelines in 2010 to help determine all available options for the patient with medical comorbidities and with a concerning family history (ie, breast cancer, venous thromboembolism).12 It can be helpful to consult the 2010 CDC medical eligibility criteria before offering contraception to these patients. Compared with the 2013 guidelines, which provide practical advice on how to use each contraceptive, the 2010 guidelines give guidance on when it is appropriate to prescribe each contraceptive—eg, which contraceptives are preferred based on a patient’s risk factors, medical history, and medication use. In addition to a two-page color summary chart of the 2010 medical eligibility criteria on the CDC website (https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf), a free mobile app is also available to guide decision-making.13
Pregnancy should be ruled out before initiating any contraceptive. This can be done through a detailed history. The six-item checklist in Table 1 has a 99.8% negative predictive value, so healthcare providers may be confident that a woman is not pregnant if pregnancy is excluded based on this history.14 A pregnancy test is needed in those who test positive on the checklist if they wish to start a LARC method such as an IUD or a progestin-only arm implant. However, because the test has a high false-positive rate, initiation of shorter-acting methods such as combined hormonal contraceptives should not be delayed on the basis of a positive checklist screen alone.1
Emergency contraception taken orally should be offered without an office visit, as its short duration of use allows women with traditional contraindications to hormonal contraceptives to safely use this birth control method.1,12 Because all emergency contraceptives must be used within 5 days of intercourse (the earlier the better), unnecessary office visits delay access and effectiveness.
Although a levonorgestrel-based emergency contraceptive is available over the counter, ulipristal acetate is more effective, especially in women who are overweight.15 A copper IUD placed within 5 days of intercourse is the most effective form of emergency contraception15 but requires an office visit. This method is an option for most women but should be strongly considered for women at highest risk of pregnancy (previous unintended pregnancy, intercourse at midcycle, obesity).
In summary, most women may safely begin their hormonal contraceptive with a detailed medical history alone, without additional office visits, examinations, or screening tests.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013; 62:1–60.
- Qaseem A, Humphrey LL, Harris R, et al; Clinical Guidelines Committee of the American College of Physicians. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:67–72.
- American Congress of Obstetricians and Gynecologists. ACOG practice advisory on annual pelvic examination recommendations; 2014. www.acog.org/About-ACOG/News-Room/Practice-Advisories/ACOG-Practice-Advisory-on-Annual-Pelvic-Examination-Recommendations. Accessed September 8, 2015.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000-2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Committee on Health Care for Underserved Women. Committee opinion no. 615: access to contraception. Obstet Gynecol 2015; 125:250–255.
- Committee on Adolescent Health Care. Committee opinion no. 598: the initial reproductive health visit. Obstet Gynecol 2014; 123:1143–1147.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014; 371:1316–1323.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Over-the-counter access to oral contraceptives. Committee opinion no 544. Obstet Gynecol 2012; 120:1527–1531.
- Committee on Gynecologic Practice. ACOG committee opinion number 540: risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol 2012; 120:1239–1242.
- Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep 2010; 59:1–86.
- Centers for Disease Control and Prevention (CDC). United States medical eligibility criteria (US MEC) for contraceptive use, 2010. www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm. Accessed September 8, 2015.
- Min J, Buckel C, Secura GM, Peipert JF, Madden T. Performance of a checklist to exclude pregnancy at the time of contraceptive initiation among women with a negative urine pregnancy test. Contraception 2015; 91:80–84.
- Batur P. Emergency contraception: separating fact from fiction. Cleve Clin J Med 2012; 79:771–776.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013; 62:1–60.
- Qaseem A, Humphrey LL, Harris R, et al; Clinical Guidelines Committee of the American College of Physicians. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:67–72.
- American Congress of Obstetricians and Gynecologists. ACOG practice advisory on annual pelvic examination recommendations; 2014. www.acog.org/About-ACOG/News-Room/Practice-Advisories/ACOG-Practice-Advisory-on-Annual-Pelvic-Examination-Recommendations. Accessed September 8, 2015.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000-2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Committee on Health Care for Underserved Women. Committee opinion no. 615: access to contraception. Obstet Gynecol 2015; 125:250–255.
- Committee on Adolescent Health Care. Committee opinion no. 598: the initial reproductive health visit. Obstet Gynecol 2014; 123:1143–1147.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014; 371:1316–1323.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Over-the-counter access to oral contraceptives. Committee opinion no 544. Obstet Gynecol 2012; 120:1527–1531.
- Committee on Gynecologic Practice. ACOG committee opinion number 540: risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol 2012; 120:1239–1242.
- Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep 2010; 59:1–86.
- Centers for Disease Control and Prevention (CDC). United States medical eligibility criteria (US MEC) for contraceptive use, 2010. www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm. Accessed September 8, 2015.
- Min J, Buckel C, Secura GM, Peipert JF, Madden T. Performance of a checklist to exclude pregnancy at the time of contraceptive initiation among women with a negative urine pregnancy test. Contraception 2015; 91:80–84.
- Batur P. Emergency contraception: separating fact from fiction. Cleve Clin J Med 2012; 79:771–776.
What can we offer patients with mild cognitive impairment?
We can promote healthy nutrition, physical activity, socialization, and mental activity. These interventions help stabilize and even improve cognition, as well as enhance quality of life and mood, delay institutionalization, and reduce disruptive behaviors. However, no medication is approved by the US Food and Drug Administration for treating mild cognitive impairment (MCI).
WHAT IS MILD COGNITIVE IMPAIRMENT?
MCI is a dynamic stage between normal aging and dementia. It is diagnosed in patients with an objective cognitive deficit but preserved function.
Population-based studies have found a wide range of rates of MCI incidence (21.5–71.3 per 1,000 person-years) and prevalence (3%–42%).1 The risk of progression from MCI to dementia ranges from 5% to 25% per year and is highest with MCI that involves memory loss (amnestic MCI).2,3
MCI can be regarded as a syndrome that is often associated with Alzheimer pathology and that has variable outcomes. In MCI due to Alzheimer disease, the primary complaint is short-term memory loss.4 Patients who have multiple impaired cognitive domains with prominent deficits in attention and executive function and relatively unimpaired short-term memory (nonamnestic MCI) are more likely to have vascular or Lewy body pathologies.5 Although distinctions between amnestic and nonamnestic MCI can be useful for counseling patients, both subtypes have similar proportions of “pure” Alzheimer disease pathology, vascular infarcts, and other pathologies at autopsy.5,6
GENERAL MANAGEMENT—IMPROVE OVERALL HEALTH
Primary management of MCI should focus on improving lifestyle factors and treating comorbid conditions that can affect cognition (eg, depression, nutritional deficiencies).
An important goal of management is to preserve working memory, ie, the ability to maintain and manipulate information while ignoring distractions. Preservation of working memory but not short-term memory is associated with slower functional decline in MCI and early Alzheimer disease.7 Lifestyle factors including sleep, stress, and exercise affect working memory performance and, thus, functional ability.
Minimizing the risk of traumatic brain injury by reducing the risk of falling is also important. Although the role of alcohol consumption as it relates to cognition is controversial, physicians may counsel older adults with MCI to reduce their alcohol consumption even if they are consuming no more than one standard drink in a 24-hour period, in order to reduce the risk of falls and their sequelae.
Optimally controlling blood pressure, lipids, and blood sugar can reduce cardiovascular risk and may slow progression of MCI to dementia.2
Smoking should be stopped and polypharmacy avoided, with particular emphasis on eliminating medications included in the Beers criteria.8
A HEALTHY DIET MAY HELP
Although evidence supporting the benefits of various diets for MCI remains scarce with mixed results, a healthy diet may favorably affect cognition. A 2009 systematic review found that observational studies showed that long-chain omega-3 fatty acids had a positive influence on cognition, but results from clinical trials were equivocal.9 Studies investigating the impact on cognition of the Mediterranean diet—rich in vegetables, fruits, whole grains, lean protein, and olive oil—remain mixed (possibly because of dietary and cognitive measurement variations between studies) but suggest that it promotes slower cognitive decline.10
PHYSICAL ACTIVITY HAS MULTIPLE BENEFITS
Physical activity has many health benefits in the elderly: it reduces muscle loss, increases functional capacity, and decreases the risk of falls.11 Several randomized controlled trials have explored the relationship between physical activity and cognition in patients with varying degrees of cognitive impairment. Although the optimal type and duration of exercise needed to achieve a specific benefit remains unclear, physical activity has been found to be helpful in more studies than not.12 Baker et al13 found that 45 to 60 minutes of high-intensity aerobic activity 4 days a week for 6 months improved executive function.
MAINTAIN SOCIAL ACTIVITIES
Social engagement—which can include a range of activities from conversation to structured group activities—is important for maintaining cognitive function.
A prospective cohort study14 that followed participants for 1 to 3 years after MCI diagnosis found that those who progressed from mild to severe cognitive impairment were less likely to attend a place of worship, work, or volunteer.
A longitudinal study of 89 elderly people without known dementia evaluated measures of socialization, global cognitive function, and Alzheimer disease pathology seen on brain autopsy. Lower cognitive function was associated with more disease pathology, but social network size modified this relationship: cognitive function was less impaired than expected for those with a large social network, even for those with a high burden of brain pathology.15
ENCOURAGE BRAIN EXERCISE
Activities can include “cognitive hobbies” such as playing board games, reading, playing a musical instrument, and doing crossword puzzles. Specific cognitive training strategies (eg, mnemonics, calligraphy therapy, computer-based interventions) have shown benefits, although it is unclear if some interventions are more effective than others.12
MULTIMODAL STRATEGIES
There are no data supporting strategies that combine multiple interventions compared with a single intervention on cognitive outcome. However, most single interventions likely contain socialization as an unstated intervention. For example, group settings for a cognitive or physical activity may include interactions with an instructor and interactions with other participants. It is thus difficult to identify truly unimodal interventions.
An example of a multimodal approach for cognitive impairment is tai chi. Physical activity in tai chi is used for coordinated movements and balance; attention, visual imagery, and memory provide cognitive stimulation; and it is frequently performed in a group setting or with an instructor. A 1-year trial in 389 MCI patients found that those who practiced tai chi had lower clinical dementia rating scale scores than the control group who participated in stretching and toning exercises.16
Table 1 summarizes recommendations for patients with MCI. In addition, referral to a geriatrician should be considered for assistance with evaluation and management, particularly if the patient lacks a capable caregiver or if the caregiver is under stress.
- Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 2012; 8:14–21.
- Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis 2007; 12:23–35.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009; 66:200–208.
- Ferman TJ, Smith GE, Kantarci K, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 2013; 81:2032–2038.
- Pillai JA, Bonner-Jackson A, Walker E, Mourany L, Cummings JL. Higher working memory predicts slower functional decline in autopsy-confirmed Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 38:224–233.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 2009; 5:140–152.
- Kuczmarski MF, Allegro D, Stave E. The association of healthful diets and cognitive function: a review. J Nutr Gerontol Geriatr 2014; 33:69–90.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004; 85:1694–1704.
- Horr T, Messinger-Rapport B, Pillai JA. Systematic review of strengths and limitations of randomized controlled trials for non-pharmacological interventions in mild cognitive impairment: focus on Alzheimer’s disease. J Nutr Health Aging 2015; 19:141–153.
- Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010; 67:71-79.
- Hughes TF, Flatt JD, Fu B, Chang CC, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. Int Psychogeriatr 2013; 25:587–595.
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006; 5:406–412.
- Lam LC, Chau RC, Wong BM, et al. A 1-year randomized controlled trial comparing mind body exercise (tai chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc 2012; 13:568.e15–568.e20.
We can promote healthy nutrition, physical activity, socialization, and mental activity. These interventions help stabilize and even improve cognition, as well as enhance quality of life and mood, delay institutionalization, and reduce disruptive behaviors. However, no medication is approved by the US Food and Drug Administration for treating mild cognitive impairment (MCI).
WHAT IS MILD COGNITIVE IMPAIRMENT?
MCI is a dynamic stage between normal aging and dementia. It is diagnosed in patients with an objective cognitive deficit but preserved function.
Population-based studies have found a wide range of rates of MCI incidence (21.5–71.3 per 1,000 person-years) and prevalence (3%–42%).1 The risk of progression from MCI to dementia ranges from 5% to 25% per year and is highest with MCI that involves memory loss (amnestic MCI).2,3
MCI can be regarded as a syndrome that is often associated with Alzheimer pathology and that has variable outcomes. In MCI due to Alzheimer disease, the primary complaint is short-term memory loss.4 Patients who have multiple impaired cognitive domains with prominent deficits in attention and executive function and relatively unimpaired short-term memory (nonamnestic MCI) are more likely to have vascular or Lewy body pathologies.5 Although distinctions between amnestic and nonamnestic MCI can be useful for counseling patients, both subtypes have similar proportions of “pure” Alzheimer disease pathology, vascular infarcts, and other pathologies at autopsy.5,6
GENERAL MANAGEMENT—IMPROVE OVERALL HEALTH
Primary management of MCI should focus on improving lifestyle factors and treating comorbid conditions that can affect cognition (eg, depression, nutritional deficiencies).
An important goal of management is to preserve working memory, ie, the ability to maintain and manipulate information while ignoring distractions. Preservation of working memory but not short-term memory is associated with slower functional decline in MCI and early Alzheimer disease.7 Lifestyle factors including sleep, stress, and exercise affect working memory performance and, thus, functional ability.
Minimizing the risk of traumatic brain injury by reducing the risk of falling is also important. Although the role of alcohol consumption as it relates to cognition is controversial, physicians may counsel older adults with MCI to reduce their alcohol consumption even if they are consuming no more than one standard drink in a 24-hour period, in order to reduce the risk of falls and their sequelae.
Optimally controlling blood pressure, lipids, and blood sugar can reduce cardiovascular risk and may slow progression of MCI to dementia.2
Smoking should be stopped and polypharmacy avoided, with particular emphasis on eliminating medications included in the Beers criteria.8
A HEALTHY DIET MAY HELP
Although evidence supporting the benefits of various diets for MCI remains scarce with mixed results, a healthy diet may favorably affect cognition. A 2009 systematic review found that observational studies showed that long-chain omega-3 fatty acids had a positive influence on cognition, but results from clinical trials were equivocal.9 Studies investigating the impact on cognition of the Mediterranean diet—rich in vegetables, fruits, whole grains, lean protein, and olive oil—remain mixed (possibly because of dietary and cognitive measurement variations between studies) but suggest that it promotes slower cognitive decline.10
PHYSICAL ACTIVITY HAS MULTIPLE BENEFITS
Physical activity has many health benefits in the elderly: it reduces muscle loss, increases functional capacity, and decreases the risk of falls.11 Several randomized controlled trials have explored the relationship between physical activity and cognition in patients with varying degrees of cognitive impairment. Although the optimal type and duration of exercise needed to achieve a specific benefit remains unclear, physical activity has been found to be helpful in more studies than not.12 Baker et al13 found that 45 to 60 minutes of high-intensity aerobic activity 4 days a week for 6 months improved executive function.
MAINTAIN SOCIAL ACTIVITIES
Social engagement—which can include a range of activities from conversation to structured group activities—is important for maintaining cognitive function.
A prospective cohort study14 that followed participants for 1 to 3 years after MCI diagnosis found that those who progressed from mild to severe cognitive impairment were less likely to attend a place of worship, work, or volunteer.
A longitudinal study of 89 elderly people without known dementia evaluated measures of socialization, global cognitive function, and Alzheimer disease pathology seen on brain autopsy. Lower cognitive function was associated with more disease pathology, but social network size modified this relationship: cognitive function was less impaired than expected for those with a large social network, even for those with a high burden of brain pathology.15
ENCOURAGE BRAIN EXERCISE
Activities can include “cognitive hobbies” such as playing board games, reading, playing a musical instrument, and doing crossword puzzles. Specific cognitive training strategies (eg, mnemonics, calligraphy therapy, computer-based interventions) have shown benefits, although it is unclear if some interventions are more effective than others.12
MULTIMODAL STRATEGIES
There are no data supporting strategies that combine multiple interventions compared with a single intervention on cognitive outcome. However, most single interventions likely contain socialization as an unstated intervention. For example, group settings for a cognitive or physical activity may include interactions with an instructor and interactions with other participants. It is thus difficult to identify truly unimodal interventions.
An example of a multimodal approach for cognitive impairment is tai chi. Physical activity in tai chi is used for coordinated movements and balance; attention, visual imagery, and memory provide cognitive stimulation; and it is frequently performed in a group setting or with an instructor. A 1-year trial in 389 MCI patients found that those who practiced tai chi had lower clinical dementia rating scale scores than the control group who participated in stretching and toning exercises.16
Table 1 summarizes recommendations for patients with MCI. In addition, referral to a geriatrician should be considered for assistance with evaluation and management, particularly if the patient lacks a capable caregiver or if the caregiver is under stress.
We can promote healthy nutrition, physical activity, socialization, and mental activity. These interventions help stabilize and even improve cognition, as well as enhance quality of life and mood, delay institutionalization, and reduce disruptive behaviors. However, no medication is approved by the US Food and Drug Administration for treating mild cognitive impairment (MCI).
WHAT IS MILD COGNITIVE IMPAIRMENT?
MCI is a dynamic stage between normal aging and dementia. It is diagnosed in patients with an objective cognitive deficit but preserved function.
Population-based studies have found a wide range of rates of MCI incidence (21.5–71.3 per 1,000 person-years) and prevalence (3%–42%).1 The risk of progression from MCI to dementia ranges from 5% to 25% per year and is highest with MCI that involves memory loss (amnestic MCI).2,3
MCI can be regarded as a syndrome that is often associated with Alzheimer pathology and that has variable outcomes. In MCI due to Alzheimer disease, the primary complaint is short-term memory loss.4 Patients who have multiple impaired cognitive domains with prominent deficits in attention and executive function and relatively unimpaired short-term memory (nonamnestic MCI) are more likely to have vascular or Lewy body pathologies.5 Although distinctions between amnestic and nonamnestic MCI can be useful for counseling patients, both subtypes have similar proportions of “pure” Alzheimer disease pathology, vascular infarcts, and other pathologies at autopsy.5,6
GENERAL MANAGEMENT—IMPROVE OVERALL HEALTH
Primary management of MCI should focus on improving lifestyle factors and treating comorbid conditions that can affect cognition (eg, depression, nutritional deficiencies).
An important goal of management is to preserve working memory, ie, the ability to maintain and manipulate information while ignoring distractions. Preservation of working memory but not short-term memory is associated with slower functional decline in MCI and early Alzheimer disease.7 Lifestyle factors including sleep, stress, and exercise affect working memory performance and, thus, functional ability.
Minimizing the risk of traumatic brain injury by reducing the risk of falling is also important. Although the role of alcohol consumption as it relates to cognition is controversial, physicians may counsel older adults with MCI to reduce their alcohol consumption even if they are consuming no more than one standard drink in a 24-hour period, in order to reduce the risk of falls and their sequelae.
Optimally controlling blood pressure, lipids, and blood sugar can reduce cardiovascular risk and may slow progression of MCI to dementia.2
Smoking should be stopped and polypharmacy avoided, with particular emphasis on eliminating medications included in the Beers criteria.8
A HEALTHY DIET MAY HELP
Although evidence supporting the benefits of various diets for MCI remains scarce with mixed results, a healthy diet may favorably affect cognition. A 2009 systematic review found that observational studies showed that long-chain omega-3 fatty acids had a positive influence on cognition, but results from clinical trials were equivocal.9 Studies investigating the impact on cognition of the Mediterranean diet—rich in vegetables, fruits, whole grains, lean protein, and olive oil—remain mixed (possibly because of dietary and cognitive measurement variations between studies) but suggest that it promotes slower cognitive decline.10
PHYSICAL ACTIVITY HAS MULTIPLE BENEFITS
Physical activity has many health benefits in the elderly: it reduces muscle loss, increases functional capacity, and decreases the risk of falls.11 Several randomized controlled trials have explored the relationship between physical activity and cognition in patients with varying degrees of cognitive impairment. Although the optimal type and duration of exercise needed to achieve a specific benefit remains unclear, physical activity has been found to be helpful in more studies than not.12 Baker et al13 found that 45 to 60 minutes of high-intensity aerobic activity 4 days a week for 6 months improved executive function.
MAINTAIN SOCIAL ACTIVITIES
Social engagement—which can include a range of activities from conversation to structured group activities—is important for maintaining cognitive function.
A prospective cohort study14 that followed participants for 1 to 3 years after MCI diagnosis found that those who progressed from mild to severe cognitive impairment were less likely to attend a place of worship, work, or volunteer.
A longitudinal study of 89 elderly people without known dementia evaluated measures of socialization, global cognitive function, and Alzheimer disease pathology seen on brain autopsy. Lower cognitive function was associated with more disease pathology, but social network size modified this relationship: cognitive function was less impaired than expected for those with a large social network, even for those with a high burden of brain pathology.15
ENCOURAGE BRAIN EXERCISE
Activities can include “cognitive hobbies” such as playing board games, reading, playing a musical instrument, and doing crossword puzzles. Specific cognitive training strategies (eg, mnemonics, calligraphy therapy, computer-based interventions) have shown benefits, although it is unclear if some interventions are more effective than others.12
MULTIMODAL STRATEGIES
There are no data supporting strategies that combine multiple interventions compared with a single intervention on cognitive outcome. However, most single interventions likely contain socialization as an unstated intervention. For example, group settings for a cognitive or physical activity may include interactions with an instructor and interactions with other participants. It is thus difficult to identify truly unimodal interventions.
An example of a multimodal approach for cognitive impairment is tai chi. Physical activity in tai chi is used for coordinated movements and balance; attention, visual imagery, and memory provide cognitive stimulation; and it is frequently performed in a group setting or with an instructor. A 1-year trial in 389 MCI patients found that those who practiced tai chi had lower clinical dementia rating scale scores than the control group who participated in stretching and toning exercises.16
Table 1 summarizes recommendations for patients with MCI. In addition, referral to a geriatrician should be considered for assistance with evaluation and management, particularly if the patient lacks a capable caregiver or if the caregiver is under stress.
- Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 2012; 8:14–21.
- Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis 2007; 12:23–35.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009; 66:200–208.
- Ferman TJ, Smith GE, Kantarci K, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 2013; 81:2032–2038.
- Pillai JA, Bonner-Jackson A, Walker E, Mourany L, Cummings JL. Higher working memory predicts slower functional decline in autopsy-confirmed Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 38:224–233.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 2009; 5:140–152.
- Kuczmarski MF, Allegro D, Stave E. The association of healthful diets and cognitive function: a review. J Nutr Gerontol Geriatr 2014; 33:69–90.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004; 85:1694–1704.
- Horr T, Messinger-Rapport B, Pillai JA. Systematic review of strengths and limitations of randomized controlled trials for non-pharmacological interventions in mild cognitive impairment: focus on Alzheimer’s disease. J Nutr Health Aging 2015; 19:141–153.
- Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010; 67:71-79.
- Hughes TF, Flatt JD, Fu B, Chang CC, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. Int Psychogeriatr 2013; 25:587–595.
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006; 5:406–412.
- Lam LC, Chau RC, Wong BM, et al. A 1-year randomized controlled trial comparing mind body exercise (tai chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc 2012; 13:568.e15–568.e20.
- Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 2012; 8:14–21.
- Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis 2007; 12:23–35.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009; 66:200–208.
- Ferman TJ, Smith GE, Kantarci K, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 2013; 81:2032–2038.
- Pillai JA, Bonner-Jackson A, Walker E, Mourany L, Cummings JL. Higher working memory predicts slower functional decline in autopsy-confirmed Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 38:224–233.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 2009; 5:140–152.
- Kuczmarski MF, Allegro D, Stave E. The association of healthful diets and cognitive function: a review. J Nutr Gerontol Geriatr 2014; 33:69–90.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004; 85:1694–1704.
- Horr T, Messinger-Rapport B, Pillai JA. Systematic review of strengths and limitations of randomized controlled trials for non-pharmacological interventions in mild cognitive impairment: focus on Alzheimer’s disease. J Nutr Health Aging 2015; 19:141–153.
- Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010; 67:71-79.
- Hughes TF, Flatt JD, Fu B, Chang CC, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. Int Psychogeriatr 2013; 25:587–595.
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006; 5:406–412.
- Lam LC, Chau RC, Wong BM, et al. A 1-year randomized controlled trial comparing mind body exercise (tai chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc 2012; 13:568.e15–568.e20.