User login
Biologics during pregnancy did not affect infant vaccine response
The use of biologic therapy during pregnancy did not lower antibody titers among infants vaccinated against Haemophilus influenzae B (HiB) or tetanus toxin, according to the results of a study of 179 mothers reported in the January issue of Clinical Gastroenterology and Hepatology (2017. doi: 10.1016/j.cgh.2017.08.041).
Additionally, there was no link between median infliximab concentration in uterine cord blood and antibody titers among infants aged 7 months and older, wrote Dawn B. Beaulieu, MD, with her associates. “In a limited cohort of exposed infants given the rotavirus vaccine, there was no association with significant adverse reactions,” they also reported.
Experts now recommend against live vaccinations for infants who may have detectable concentrations of biologics, but it remained unclear whether these infants can mount adequate responses to inactive vaccines. Therefore, the researchers analyzed data from the Pregnancy in IBD and Neonatal Outcomes (PIANO) registry collected between 2007 and 2016 and surveyed women about their infants’ vaccination history. They also quantified antibodies in serum samples from infants aged 7 months and older and analyzed measured concentrations of biologics in cord blood.
Among 179 mothers with IBD, most had inactive (77%) or mild disease activity (18%) during pregnancy, the researchers said. Eleven (6%) mothers were not on immunosuppressives while pregnant, 15 (8%) were on an immunomodulator, and the rest were on biologic monotherapy (65%) or a biologic plus an immunomodulator (21%). A total of 46 infants had available HiB titer data, of whom 38 were potentially exposed to biologics; among 49 infants with available tetanus titers, 41 were potentially exposed. In all, 71% of exposed infants had protective levels of antibodies against HiB, and 80% had protective titers to tetanus toxoid. Proportions among unexposed infants were 50% and 75%, respectively. Proportions of protective antibody titers did not significantly differ between groups even after excluding infants whose mothers received certolizumab pegol, which has negligible rates of placental transfer.
A total of 39 infants received live rotavirus vaccine despite having detectable levels of biologics in cord blood at birth. Seven developed mild vaccine reactions consisting of fever (six infants) or diarrhea (one infant). This proportion (18%) resembles that from a large study (N Engl J Med. 2006;354:23-33) of healthy infants who were vaccinated against rotavirus, the researchers noted. “Despite our data suggesting a lack of severe side effects with the rotavirus vaccine in these infants, in the absence of robust evidence, one should continue to avoid live vaccines in infants born to mothers on biologic therapy (excluding certolizumab) during the first year of life or until drug clearance is confirmed,” they suggested. “With the growing availability of tests, one conceivably could test serum drug concentration in infants, and, if undetectable, consider live vaccination at that time, if appropriate for the vaccine, particularly in infants most likely to benefit from such vaccines.”
The Crohn’s and Colitis Foundation provided funding. Dr. Beaulieu disclosed a consulting relationship with AbbVie, and four coinvestigators also reported ties to pharmaceutical companies.
The use of biologic therapy during pregnancy did not lower antibody titers among infants vaccinated against Haemophilus influenzae B (HiB) or tetanus toxin, according to the results of a study of 179 mothers reported in the January issue of Clinical Gastroenterology and Hepatology (2017. doi: 10.1016/j.cgh.2017.08.041).
Additionally, there was no link between median infliximab concentration in uterine cord blood and antibody titers among infants aged 7 months and older, wrote Dawn B. Beaulieu, MD, with her associates. “In a limited cohort of exposed infants given the rotavirus vaccine, there was no association with significant adverse reactions,” they also reported.
Experts now recommend against live vaccinations for infants who may have detectable concentrations of biologics, but it remained unclear whether these infants can mount adequate responses to inactive vaccines. Therefore, the researchers analyzed data from the Pregnancy in IBD and Neonatal Outcomes (PIANO) registry collected between 2007 and 2016 and surveyed women about their infants’ vaccination history. They also quantified antibodies in serum samples from infants aged 7 months and older and analyzed measured concentrations of biologics in cord blood.
Among 179 mothers with IBD, most had inactive (77%) or mild disease activity (18%) during pregnancy, the researchers said. Eleven (6%) mothers were not on immunosuppressives while pregnant, 15 (8%) were on an immunomodulator, and the rest were on biologic monotherapy (65%) or a biologic plus an immunomodulator (21%). A total of 46 infants had available HiB titer data, of whom 38 were potentially exposed to biologics; among 49 infants with available tetanus titers, 41 were potentially exposed. In all, 71% of exposed infants had protective levels of antibodies against HiB, and 80% had protective titers to tetanus toxoid. Proportions among unexposed infants were 50% and 75%, respectively. Proportions of protective antibody titers did not significantly differ between groups even after excluding infants whose mothers received certolizumab pegol, which has negligible rates of placental transfer.
A total of 39 infants received live rotavirus vaccine despite having detectable levels of biologics in cord blood at birth. Seven developed mild vaccine reactions consisting of fever (six infants) or diarrhea (one infant). This proportion (18%) resembles that from a large study (N Engl J Med. 2006;354:23-33) of healthy infants who were vaccinated against rotavirus, the researchers noted. “Despite our data suggesting a lack of severe side effects with the rotavirus vaccine in these infants, in the absence of robust evidence, one should continue to avoid live vaccines in infants born to mothers on biologic therapy (excluding certolizumab) during the first year of life or until drug clearance is confirmed,” they suggested. “With the growing availability of tests, one conceivably could test serum drug concentration in infants, and, if undetectable, consider live vaccination at that time, if appropriate for the vaccine, particularly in infants most likely to benefit from such vaccines.”
The Crohn’s and Colitis Foundation provided funding. Dr. Beaulieu disclosed a consulting relationship with AbbVie, and four coinvestigators also reported ties to pharmaceutical companies.
The use of biologic therapy during pregnancy did not lower antibody titers among infants vaccinated against Haemophilus influenzae B (HiB) or tetanus toxin, according to the results of a study of 179 mothers reported in the January issue of Clinical Gastroenterology and Hepatology (2017. doi: 10.1016/j.cgh.2017.08.041).
Additionally, there was no link between median infliximab concentration in uterine cord blood and antibody titers among infants aged 7 months and older, wrote Dawn B. Beaulieu, MD, with her associates. “In a limited cohort of exposed infants given the rotavirus vaccine, there was no association with significant adverse reactions,” they also reported.
Experts now recommend against live vaccinations for infants who may have detectable concentrations of biologics, but it remained unclear whether these infants can mount adequate responses to inactive vaccines. Therefore, the researchers analyzed data from the Pregnancy in IBD and Neonatal Outcomes (PIANO) registry collected between 2007 and 2016 and surveyed women about their infants’ vaccination history. They also quantified antibodies in serum samples from infants aged 7 months and older and analyzed measured concentrations of biologics in cord blood.
Among 179 mothers with IBD, most had inactive (77%) or mild disease activity (18%) during pregnancy, the researchers said. Eleven (6%) mothers were not on immunosuppressives while pregnant, 15 (8%) were on an immunomodulator, and the rest were on biologic monotherapy (65%) or a biologic plus an immunomodulator (21%). A total of 46 infants had available HiB titer data, of whom 38 were potentially exposed to biologics; among 49 infants with available tetanus titers, 41 were potentially exposed. In all, 71% of exposed infants had protective levels of antibodies against HiB, and 80% had protective titers to tetanus toxoid. Proportions among unexposed infants were 50% and 75%, respectively. Proportions of protective antibody titers did not significantly differ between groups even after excluding infants whose mothers received certolizumab pegol, which has negligible rates of placental transfer.
A total of 39 infants received live rotavirus vaccine despite having detectable levels of biologics in cord blood at birth. Seven developed mild vaccine reactions consisting of fever (six infants) or diarrhea (one infant). This proportion (18%) resembles that from a large study (N Engl J Med. 2006;354:23-33) of healthy infants who were vaccinated against rotavirus, the researchers noted. “Despite our data suggesting a lack of severe side effects with the rotavirus vaccine in these infants, in the absence of robust evidence, one should continue to avoid live vaccines in infants born to mothers on biologic therapy (excluding certolizumab) during the first year of life or until drug clearance is confirmed,” they suggested. “With the growing availability of tests, one conceivably could test serum drug concentration in infants, and, if undetectable, consider live vaccination at that time, if appropriate for the vaccine, particularly in infants most likely to benefit from such vaccines.”
The Crohn’s and Colitis Foundation provided funding. Dr. Beaulieu disclosed a consulting relationship with AbbVie, and four coinvestigators also reported ties to pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: In utero biologic exposure did not prevent immune response to Haemophilus influenzae B and tetanus vaccines during infancy.
Major finding: Proportions of protective antibody titers did not significantly differ among groups.
Data source: A prospective study of 179 mothers with IBD and their infants.
Disclosures: The Crohn’s and Colitis Foundation provided funding. Dr. Beaulieu disclosed a consulting relationship with AbbVie, and four coinvestigators also reported ties to pharmaceutical companies.
VIDEO: Study supports close follow-up of patients with high-risk adenomas plus serrated polyps
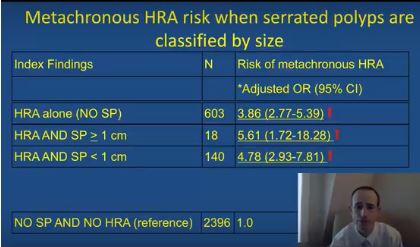
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: High-risk adenomas and the synchronous presence of serrated polyps significantly increased the risk of metachronous high-risk adenomas.
Major finding: Compared with individuals with unremarkable colonoscopies, the odds ratio was 5.6 after adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies.
Data source: Analyses of index and follow-up colonoscopies of 5,433 individuals from a population-based surveillance registry.
Disclosures: The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
Adjusting fecal immunochemical test thresholds improved their performance
Physicians can minimize the heterogeneity of fecal immunochemical colorectal cancer screening tests by adjusting thresholds for positivity, according to researchers. The report is in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2017.09.018).
“Rather than simply using thresholds recommended by the manufacturer, screening programs should choose thresholds based on intended levels of specificity and manageable positivity rates,” wrote PhD student Anton Gies of the German Cancer Research Center and the National Center for Tumor Diseases in Heidelberg, Germany, with his associates.
The investigators directly compared nine different fecal immunochemical assays using stool samples from 516 individuals, of whom 216 had advanced adenoma or colorectal cancer. Using thresholds recommended by manufacturers (2-17 mcg Hb/g feces) produced widely ranging sensitivities (22%-46%) and specificities (86%-98%). Using a uniform threshold of 15 mcg Hb/g feces narrowed the range of specificity (94%-98%), but sensitivities remained quite variable (16%-34%). Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively), the researchers reported.
Increasingly, physicians are using fecal immunochemical testing to screen for colorectal neoplasia. In a prior study (Ann Intern Med. 2009 Feb 3;150[3]:162-90) investigators evaluated the diagnostic performance of six qualitative point-of-care fecal immunochemical tests among screening colonoscopy patients in Germany, and found that the tests had highly variable sensitivities and specificities for the detection of colorectal neoplasia. Not surprisingly, the most sensitive tests were the least specific, and vice versa, which is the problem with using fixed thresholds in qualitative fecal immunochemical tests, the researchers asserted.
Quantitative fecal immunochemical tests are more flexible than qualitative assays because users can adjust thresholds based on fecal hemoglobin concentrations. However, very few studies had directly compared sensitivities and specificities among quantitative fecal immunochemical tests, and “it is unclear to what extent differences ... reflect true heterogeneity in test performance or differences in study populations or varying pre-analytical conditions,” the investigators wrote. Patients in their study underwent colonoscopies in Germany between 2005 and 2010, and fecal samples were stored at –80 °C until analysis. The researchers calculated test sensitivities and specificities by using colonoscopy and histology reports evaluated by blinded, trained research assistants.
“Apparent heterogeneity in diagnostic performance of quantitative fecal immunochemical tests can be overcome to a large extent by adjusting thresholds to yield defined levels of specificity or positivity rates,” the investigators concluded. Only 16 patients in this study had colorectal cancer, which made it difficult to pinpoint test sensitivity for this finding, they noted. However, they found similar sensitivity estimates for colorectal cancer in an ancillary clinical study.
Manufacturers provided test kits free of charge. There were no external funding sources, and the researchers reported having no conflicts of interest.
The fecal immunochemical test (FIT) is an important option for colorectal cancer screening, endorsed by guidelines and effective for mass screening using mailed outreach. Patients offered FIT or a choice between FIT and colonoscopy are more likely to be screened.
In the United States, FIT is a qualitative test (reported as positive or negative), based on Food and Drug Administration regulations, in an attempt to simplify clinical decision making. In Europe, FIT has been used quantitatively, with adjustable positivity rate and sensitivity pegged to available colonoscopy resources. Adding complexity, there are multiple FIT brands, each with varying performance, even at similar hemoglobin concentrations. Each brand has a different sensitivity, specificity, and positivity rate, because reagents, buffers, and collection devices vary. Ambient temperature during mailing and transport time to processing labs can also affect test performance.
Theodore R. Levin, MD, is chief of gastroenterology, Kaiser Permanente Medical Center, Walnut Creek, Calif. He has no conflicts of interest.
The fecal immunochemical test (FIT) is an important option for colorectal cancer screening, endorsed by guidelines and effective for mass screening using mailed outreach. Patients offered FIT or a choice between FIT and colonoscopy are more likely to be screened.
In the United States, FIT is a qualitative test (reported as positive or negative), based on Food and Drug Administration regulations, in an attempt to simplify clinical decision making. In Europe, FIT has been used quantitatively, with adjustable positivity rate and sensitivity pegged to available colonoscopy resources. Adding complexity, there are multiple FIT brands, each with varying performance, even at similar hemoglobin concentrations. Each brand has a different sensitivity, specificity, and positivity rate, because reagents, buffers, and collection devices vary. Ambient temperature during mailing and transport time to processing labs can also affect test performance.
Theodore R. Levin, MD, is chief of gastroenterology, Kaiser Permanente Medical Center, Walnut Creek, Calif. He has no conflicts of interest.
The fecal immunochemical test (FIT) is an important option for colorectal cancer screening, endorsed by guidelines and effective for mass screening using mailed outreach. Patients offered FIT or a choice between FIT and colonoscopy are more likely to be screened.
In the United States, FIT is a qualitative test (reported as positive or negative), based on Food and Drug Administration regulations, in an attempt to simplify clinical decision making. In Europe, FIT has been used quantitatively, with adjustable positivity rate and sensitivity pegged to available colonoscopy resources. Adding complexity, there are multiple FIT brands, each with varying performance, even at similar hemoglobin concentrations. Each brand has a different sensitivity, specificity, and positivity rate, because reagents, buffers, and collection devices vary. Ambient temperature during mailing and transport time to processing labs can also affect test performance.
Theodore R. Levin, MD, is chief of gastroenterology, Kaiser Permanente Medical Center, Walnut Creek, Calif. He has no conflicts of interest.
Physicians can minimize the heterogeneity of fecal immunochemical colorectal cancer screening tests by adjusting thresholds for positivity, according to researchers. The report is in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2017.09.018).
“Rather than simply using thresholds recommended by the manufacturer, screening programs should choose thresholds based on intended levels of specificity and manageable positivity rates,” wrote PhD student Anton Gies of the German Cancer Research Center and the National Center for Tumor Diseases in Heidelberg, Germany, with his associates.
The investigators directly compared nine different fecal immunochemical assays using stool samples from 516 individuals, of whom 216 had advanced adenoma or colorectal cancer. Using thresholds recommended by manufacturers (2-17 mcg Hb/g feces) produced widely ranging sensitivities (22%-46%) and specificities (86%-98%). Using a uniform threshold of 15 mcg Hb/g feces narrowed the range of specificity (94%-98%), but sensitivities remained quite variable (16%-34%). Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively), the researchers reported.
Increasingly, physicians are using fecal immunochemical testing to screen for colorectal neoplasia. In a prior study (Ann Intern Med. 2009 Feb 3;150[3]:162-90) investigators evaluated the diagnostic performance of six qualitative point-of-care fecal immunochemical tests among screening colonoscopy patients in Germany, and found that the tests had highly variable sensitivities and specificities for the detection of colorectal neoplasia. Not surprisingly, the most sensitive tests were the least specific, and vice versa, which is the problem with using fixed thresholds in qualitative fecal immunochemical tests, the researchers asserted.
Quantitative fecal immunochemical tests are more flexible than qualitative assays because users can adjust thresholds based on fecal hemoglobin concentrations. However, very few studies had directly compared sensitivities and specificities among quantitative fecal immunochemical tests, and “it is unclear to what extent differences ... reflect true heterogeneity in test performance or differences in study populations or varying pre-analytical conditions,” the investigators wrote. Patients in their study underwent colonoscopies in Germany between 2005 and 2010, and fecal samples were stored at –80 °C until analysis. The researchers calculated test sensitivities and specificities by using colonoscopy and histology reports evaluated by blinded, trained research assistants.
“Apparent heterogeneity in diagnostic performance of quantitative fecal immunochemical tests can be overcome to a large extent by adjusting thresholds to yield defined levels of specificity or positivity rates,” the investigators concluded. Only 16 patients in this study had colorectal cancer, which made it difficult to pinpoint test sensitivity for this finding, they noted. However, they found similar sensitivity estimates for colorectal cancer in an ancillary clinical study.
Manufacturers provided test kits free of charge. There were no external funding sources, and the researchers reported having no conflicts of interest.
Physicians can minimize the heterogeneity of fecal immunochemical colorectal cancer screening tests by adjusting thresholds for positivity, according to researchers. The report is in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2017.09.018).
“Rather than simply using thresholds recommended by the manufacturer, screening programs should choose thresholds based on intended levels of specificity and manageable positivity rates,” wrote PhD student Anton Gies of the German Cancer Research Center and the National Center for Tumor Diseases in Heidelberg, Germany, with his associates.
The investigators directly compared nine different fecal immunochemical assays using stool samples from 516 individuals, of whom 216 had advanced adenoma or colorectal cancer. Using thresholds recommended by manufacturers (2-17 mcg Hb/g feces) produced widely ranging sensitivities (22%-46%) and specificities (86%-98%). Using a uniform threshold of 15 mcg Hb/g feces narrowed the range of specificity (94%-98%), but sensitivities remained quite variable (16%-34%). Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively), the researchers reported.
Increasingly, physicians are using fecal immunochemical testing to screen for colorectal neoplasia. In a prior study (Ann Intern Med. 2009 Feb 3;150[3]:162-90) investigators evaluated the diagnostic performance of six qualitative point-of-care fecal immunochemical tests among screening colonoscopy patients in Germany, and found that the tests had highly variable sensitivities and specificities for the detection of colorectal neoplasia. Not surprisingly, the most sensitive tests were the least specific, and vice versa, which is the problem with using fixed thresholds in qualitative fecal immunochemical tests, the researchers asserted.
Quantitative fecal immunochemical tests are more flexible than qualitative assays because users can adjust thresholds based on fecal hemoglobin concentrations. However, very few studies had directly compared sensitivities and specificities among quantitative fecal immunochemical tests, and “it is unclear to what extent differences ... reflect true heterogeneity in test performance or differences in study populations or varying pre-analytical conditions,” the investigators wrote. Patients in their study underwent colonoscopies in Germany between 2005 and 2010, and fecal samples were stored at –80 °C until analysis. The researchers calculated test sensitivities and specificities by using colonoscopy and histology reports evaluated by blinded, trained research assistants.
“Apparent heterogeneity in diagnostic performance of quantitative fecal immunochemical tests can be overcome to a large extent by adjusting thresholds to yield defined levels of specificity or positivity rates,” the investigators concluded. Only 16 patients in this study had colorectal cancer, which made it difficult to pinpoint test sensitivity for this finding, they noted. However, they found similar sensitivity estimates for colorectal cancer in an ancillary clinical study.
Manufacturers provided test kits free of charge. There were no external funding sources, and the researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: To minimize the heterogeneity of fecal immunochemical screening tests, adjust thresholds to produce a predetermined specificity or a manageable rate of positivity.
Major finding: Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively).
Data source: A comparison of nine different fecal immunochemical assays in 516 patients, of whom 216 had colorectal neoplasias.
Disclosures: Manufacturers provided test kits free of charge. There were no other external sources of support, and the researchers reported having no conflicts of interest.
AGA Clinical Practice Update: Best practices for POEM in achalasia
Peroral endoscopic myotomy, or POEM, should be considered as primary therapy for type III achalasia and as a treatment option comparable with laparoscopic Heller myotomy for any of the achalasia syndromes – but only when physicians with expertise are available, according to a clinical practice update from the American Gastroenterological Association.
Further, post-POEM patients should be considered at high risk of developing reflux esophagitis and should be advised of the management considerations, including potential indefinite proton pump inhibitor therapy and/or surveillance endoscopy, prior to undergoing the procedure, Peter J. Kahrilas, MD, of Northwestern University, Chicago, and his colleagues wrote in the update, which is published in the November issue of Gastroenterology (2017. doi: 10.1053/j.gastro.2017.10.001).
In an effort to describe the place for POEM among the currently available robust treatments for achalasia, the authors conducted a literature review – their “best practice” recommendations are based on the findings from relevant publications and on expert opinion.
Additionally, they said POEM should be performed by experienced physicians in high-volume centers since the procedure is complex and an estimated 20-30 procedures are needed to achieve competence.
The update and these proposed best practices follow the evolution of POEM over the last decade: it began as an exciting concept and is now a mainstream treatment option for achalasia, the authors said.
“Uncontrolled outcome data have been very promising comparing POEM with the standard surgical treatment for achalasia, laparoscopic Heller myotomy (LHM). However, concerns remain regarding post-POEM reflux, the durability of the procedure, and the learning curve for endoscopists adopting the technique,” they wrote, which, when coupled with recent randomized controlled study data showing excellent and equivalent 5-year outcomes with pneumatic dilation and LHM, make the role of POEM somewhat controversial.
As part of the review, they considered the strengths and weaknesses of both POEM and LHM. The data comparing POEM with LHM or pneumatic dilation remain very limited, but based on those that do exist, the authors concluded that “POEM appears to be a safe, effective, and minimally invasive management option in achalasia in the short term.”
Long-term durability data are not yet available, they noted.
Dr. Kahrilas received funding from the U.S. Public Health Service.
Peroral endoscopic myotomy, or POEM, should be considered as primary therapy for type III achalasia and as a treatment option comparable with laparoscopic Heller myotomy for any of the achalasia syndromes – but only when physicians with expertise are available, according to a clinical practice update from the American Gastroenterological Association.
Further, post-POEM patients should be considered at high risk of developing reflux esophagitis and should be advised of the management considerations, including potential indefinite proton pump inhibitor therapy and/or surveillance endoscopy, prior to undergoing the procedure, Peter J. Kahrilas, MD, of Northwestern University, Chicago, and his colleagues wrote in the update, which is published in the November issue of Gastroenterology (2017. doi: 10.1053/j.gastro.2017.10.001).
In an effort to describe the place for POEM among the currently available robust treatments for achalasia, the authors conducted a literature review – their “best practice” recommendations are based on the findings from relevant publications and on expert opinion.
Additionally, they said POEM should be performed by experienced physicians in high-volume centers since the procedure is complex and an estimated 20-30 procedures are needed to achieve competence.
The update and these proposed best practices follow the evolution of POEM over the last decade: it began as an exciting concept and is now a mainstream treatment option for achalasia, the authors said.
“Uncontrolled outcome data have been very promising comparing POEM with the standard surgical treatment for achalasia, laparoscopic Heller myotomy (LHM). However, concerns remain regarding post-POEM reflux, the durability of the procedure, and the learning curve for endoscopists adopting the technique,” they wrote, which, when coupled with recent randomized controlled study data showing excellent and equivalent 5-year outcomes with pneumatic dilation and LHM, make the role of POEM somewhat controversial.
As part of the review, they considered the strengths and weaknesses of both POEM and LHM. The data comparing POEM with LHM or pneumatic dilation remain very limited, but based on those that do exist, the authors concluded that “POEM appears to be a safe, effective, and minimally invasive management option in achalasia in the short term.”
Long-term durability data are not yet available, they noted.
Dr. Kahrilas received funding from the U.S. Public Health Service.
Peroral endoscopic myotomy, or POEM, should be considered as primary therapy for type III achalasia and as a treatment option comparable with laparoscopic Heller myotomy for any of the achalasia syndromes – but only when physicians with expertise are available, according to a clinical practice update from the American Gastroenterological Association.
Further, post-POEM patients should be considered at high risk of developing reflux esophagitis and should be advised of the management considerations, including potential indefinite proton pump inhibitor therapy and/or surveillance endoscopy, prior to undergoing the procedure, Peter J. Kahrilas, MD, of Northwestern University, Chicago, and his colleagues wrote in the update, which is published in the November issue of Gastroenterology (2017. doi: 10.1053/j.gastro.2017.10.001).
In an effort to describe the place for POEM among the currently available robust treatments for achalasia, the authors conducted a literature review – their “best practice” recommendations are based on the findings from relevant publications and on expert opinion.
Additionally, they said POEM should be performed by experienced physicians in high-volume centers since the procedure is complex and an estimated 20-30 procedures are needed to achieve competence.
The update and these proposed best practices follow the evolution of POEM over the last decade: it began as an exciting concept and is now a mainstream treatment option for achalasia, the authors said.
“Uncontrolled outcome data have been very promising comparing POEM with the standard surgical treatment for achalasia, laparoscopic Heller myotomy (LHM). However, concerns remain regarding post-POEM reflux, the durability of the procedure, and the learning curve for endoscopists adopting the technique,” they wrote, which, when coupled with recent randomized controlled study data showing excellent and equivalent 5-year outcomes with pneumatic dilation and LHM, make the role of POEM somewhat controversial.
As part of the review, they considered the strengths and weaknesses of both POEM and LHM. The data comparing POEM with LHM or pneumatic dilation remain very limited, but based on those that do exist, the authors concluded that “POEM appears to be a safe, effective, and minimally invasive management option in achalasia in the short term.”
Long-term durability data are not yet available, they noted.
Dr. Kahrilas received funding from the U.S. Public Health Service.
FROM GASTROENTEROLOGY
Low tryptophan levels linked to IBD
Patients with inflammatory bowel disease (IBD) had significantly lower serum levels of the essential amino acid tryptophan than healthy controls in a large study reported in the December issue of Gastroenterology (doi: 10.1053/j.gastro.2017.08.028).
Serum tryptophan levels also correlated inversely with both disease activity and C-reactive protein levels in patients with IBD, reported Susanna Nikolaus, MD, of University Hospital Schleswig-Holstein, Kiel, Germany, with her associates. “Tryptophan deficiency could contribute to development of IBD. Studies are needed to determine whether modification of intestinal tryptophan pathways affects [its] severity,” they wrote.
Several small case series have reported low levels of tryptophan in IBD and other autoimmune disorders, the investigators noted. Removing tryptophan from the diet has been found to increase susceptibility to colitis in mice, and supplementing with tryptophan or some of its metabolites has the opposite effect. For this study, the researchers used high-performance liquid chromatography to quantify tryptophan levels in serum samples from 535 consecutive patients with IBD and 100 matched controls. They used mass spectrometry to measure metabolites of tryptophan, enzyme-linked immunosorbent assay to measure interleukin-22 (IL-22) levels, and 16S rDNA amplicon sequencing to correlate tryptophan levels with fecal microbiota species. Finally, they used real-time polymerase chain reaction to measure levels of mRNA encoding tryptophan metabolites in colonic biopsy specimens.
Serum tryptophan levels were significantly lower in patients with IBD than controls (P = 5.3 x 10–6). The difference was starker in patients with Crohn’s disease (P = 1.1 x 10–10 vs. controls) compared with those with ulcerative colitis (P = 2.8 x 10–3 vs. controls), the investigators noted. Serum tryptophan levels also correlated inversely with disease activity in patients with Crohn’s disease (P = .01), while patients with ulcerative colitis showed a similar but nonsignificant trend (P = .07). Low tryptophan levels were associated with marked, statistically significant increases in C-reactive protein levels in both Crohn’s disease and ulcerative colitis. Tryptophan level also correlated inversely with leukocyte count, although the trend was less pronounced (P = .04).IBD was associated with several aberrations in the tryptophan kynurenine pathway, which is the primary means of catabolizing the amino acid. For example, compared with controls, patients with active IBD had significantly lower levels of mRNA encoding tryptophan 2,3-dioxygenase-2 (TDO2, a key enzyme in the kynurenine pathway) and solute carrier family 6 member 19 (SLC6A19, also called B0AT1, a neutral amino acid transporter). Patients with IBD also had significantly higher levels of indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the initial, rate-limiting oxidation of tryptophan to kynurenine. Accordingly, patients with IBD had a significantly higher ratio of kynurenine to tryptophan than did controls, and this abnormality was associated with disease activity, especially in Crohn’s disease (P = .03).
Patients with IBD who had relatively higher tryptophan levels also tended to have more diverse gut microbiota than did patients with lower serum tryptophan levels, although differences among groups were not statistically significant, the investigators said. Serum concentration of IL-22 also correlated with disease activity in patients with IBD, and infliximab responders had a “significant and sustained increase” of tryptophan levels over time, compared with nonresponders.
Potsdam dietary questionnaires found no link between disease activity and dietary consumption of tryptophan, the researchers said. Additionally, they found no links between serum tryptophan levels and age, smoking status, or disease complications, such as fistulae or abscess formation.The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop drugs with inflammatory indications. The other investigators had no conflicts of interest.
In this interesting study, Nikolaus et al. found an association of decreased serum tryptophan in patients with inflammatory bowel disease (IBD), compared with control subjects. The authors also found an inverse correlation of serum tryptophan levels in patients with C-reactive protein in both ulcerative colitis and Crohn's disease and with active disease as defined by clinical disease activity scores in Crohn's disease. A validated food-frequency questionnaire found no difference in tryptophan consumption based on disease activity in a subset of patients, decreasing the likelihood that this association is secondary to altered dietary intake only and may be related to other mechanisms.
Sara Horst, MD, MPH, is an assistant professor, division of gastroenterology, hepatology & nutrition, Inflammatory Bowel Disease Center, Vanderbilt University Medical Center, Nashville, Tenn. She had no relevant conflicts of interest.
In this interesting study, Nikolaus et al. found an association of decreased serum tryptophan in patients with inflammatory bowel disease (IBD), compared with control subjects. The authors also found an inverse correlation of serum tryptophan levels in patients with C-reactive protein in both ulcerative colitis and Crohn's disease and with active disease as defined by clinical disease activity scores in Crohn's disease. A validated food-frequency questionnaire found no difference in tryptophan consumption based on disease activity in a subset of patients, decreasing the likelihood that this association is secondary to altered dietary intake only and may be related to other mechanisms.
Sara Horst, MD, MPH, is an assistant professor, division of gastroenterology, hepatology & nutrition, Inflammatory Bowel Disease Center, Vanderbilt University Medical Center, Nashville, Tenn. She had no relevant conflicts of interest.
In this interesting study, Nikolaus et al. found an association of decreased serum tryptophan in patients with inflammatory bowel disease (IBD), compared with control subjects. The authors also found an inverse correlation of serum tryptophan levels in patients with C-reactive protein in both ulcerative colitis and Crohn's disease and with active disease as defined by clinical disease activity scores in Crohn's disease. A validated food-frequency questionnaire found no difference in tryptophan consumption based on disease activity in a subset of patients, decreasing the likelihood that this association is secondary to altered dietary intake only and may be related to other mechanisms.
Sara Horst, MD, MPH, is an assistant professor, division of gastroenterology, hepatology & nutrition, Inflammatory Bowel Disease Center, Vanderbilt University Medical Center, Nashville, Tenn. She had no relevant conflicts of interest.
Patients with inflammatory bowel disease (IBD) had significantly lower serum levels of the essential amino acid tryptophan than healthy controls in a large study reported in the December issue of Gastroenterology (doi: 10.1053/j.gastro.2017.08.028).
Serum tryptophan levels also correlated inversely with both disease activity and C-reactive protein levels in patients with IBD, reported Susanna Nikolaus, MD, of University Hospital Schleswig-Holstein, Kiel, Germany, with her associates. “Tryptophan deficiency could contribute to development of IBD. Studies are needed to determine whether modification of intestinal tryptophan pathways affects [its] severity,” they wrote.
Several small case series have reported low levels of tryptophan in IBD and other autoimmune disorders, the investigators noted. Removing tryptophan from the diet has been found to increase susceptibility to colitis in mice, and supplementing with tryptophan or some of its metabolites has the opposite effect. For this study, the researchers used high-performance liquid chromatography to quantify tryptophan levels in serum samples from 535 consecutive patients with IBD and 100 matched controls. They used mass spectrometry to measure metabolites of tryptophan, enzyme-linked immunosorbent assay to measure interleukin-22 (IL-22) levels, and 16S rDNA amplicon sequencing to correlate tryptophan levels with fecal microbiota species. Finally, they used real-time polymerase chain reaction to measure levels of mRNA encoding tryptophan metabolites in colonic biopsy specimens.
Serum tryptophan levels were significantly lower in patients with IBD than controls (P = 5.3 x 10–6). The difference was starker in patients with Crohn’s disease (P = 1.1 x 10–10 vs. controls) compared with those with ulcerative colitis (P = 2.8 x 10–3 vs. controls), the investigators noted. Serum tryptophan levels also correlated inversely with disease activity in patients with Crohn’s disease (P = .01), while patients with ulcerative colitis showed a similar but nonsignificant trend (P = .07). Low tryptophan levels were associated with marked, statistically significant increases in C-reactive protein levels in both Crohn’s disease and ulcerative colitis. Tryptophan level also correlated inversely with leukocyte count, although the trend was less pronounced (P = .04).IBD was associated with several aberrations in the tryptophan kynurenine pathway, which is the primary means of catabolizing the amino acid. For example, compared with controls, patients with active IBD had significantly lower levels of mRNA encoding tryptophan 2,3-dioxygenase-2 (TDO2, a key enzyme in the kynurenine pathway) and solute carrier family 6 member 19 (SLC6A19, also called B0AT1, a neutral amino acid transporter). Patients with IBD also had significantly higher levels of indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the initial, rate-limiting oxidation of tryptophan to kynurenine. Accordingly, patients with IBD had a significantly higher ratio of kynurenine to tryptophan than did controls, and this abnormality was associated with disease activity, especially in Crohn’s disease (P = .03).
Patients with IBD who had relatively higher tryptophan levels also tended to have more diverse gut microbiota than did patients with lower serum tryptophan levels, although differences among groups were not statistically significant, the investigators said. Serum concentration of IL-22 also correlated with disease activity in patients with IBD, and infliximab responders had a “significant and sustained increase” of tryptophan levels over time, compared with nonresponders.
Potsdam dietary questionnaires found no link between disease activity and dietary consumption of tryptophan, the researchers said. Additionally, they found no links between serum tryptophan levels and age, smoking status, or disease complications, such as fistulae or abscess formation.The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop drugs with inflammatory indications. The other investigators had no conflicts of interest.
Patients with inflammatory bowel disease (IBD) had significantly lower serum levels of the essential amino acid tryptophan than healthy controls in a large study reported in the December issue of Gastroenterology (doi: 10.1053/j.gastro.2017.08.028).
Serum tryptophan levels also correlated inversely with both disease activity and C-reactive protein levels in patients with IBD, reported Susanna Nikolaus, MD, of University Hospital Schleswig-Holstein, Kiel, Germany, with her associates. “Tryptophan deficiency could contribute to development of IBD. Studies are needed to determine whether modification of intestinal tryptophan pathways affects [its] severity,” they wrote.
Several small case series have reported low levels of tryptophan in IBD and other autoimmune disorders, the investigators noted. Removing tryptophan from the diet has been found to increase susceptibility to colitis in mice, and supplementing with tryptophan or some of its metabolites has the opposite effect. For this study, the researchers used high-performance liquid chromatography to quantify tryptophan levels in serum samples from 535 consecutive patients with IBD and 100 matched controls. They used mass spectrometry to measure metabolites of tryptophan, enzyme-linked immunosorbent assay to measure interleukin-22 (IL-22) levels, and 16S rDNA amplicon sequencing to correlate tryptophan levels with fecal microbiota species. Finally, they used real-time polymerase chain reaction to measure levels of mRNA encoding tryptophan metabolites in colonic biopsy specimens.
Serum tryptophan levels were significantly lower in patients with IBD than controls (P = 5.3 x 10–6). The difference was starker in patients with Crohn’s disease (P = 1.1 x 10–10 vs. controls) compared with those with ulcerative colitis (P = 2.8 x 10–3 vs. controls), the investigators noted. Serum tryptophan levels also correlated inversely with disease activity in patients with Crohn’s disease (P = .01), while patients with ulcerative colitis showed a similar but nonsignificant trend (P = .07). Low tryptophan levels were associated with marked, statistically significant increases in C-reactive protein levels in both Crohn’s disease and ulcerative colitis. Tryptophan level also correlated inversely with leukocyte count, although the trend was less pronounced (P = .04).IBD was associated with several aberrations in the tryptophan kynurenine pathway, which is the primary means of catabolizing the amino acid. For example, compared with controls, patients with active IBD had significantly lower levels of mRNA encoding tryptophan 2,3-dioxygenase-2 (TDO2, a key enzyme in the kynurenine pathway) and solute carrier family 6 member 19 (SLC6A19, also called B0AT1, a neutral amino acid transporter). Patients with IBD also had significantly higher levels of indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the initial, rate-limiting oxidation of tryptophan to kynurenine. Accordingly, patients with IBD had a significantly higher ratio of kynurenine to tryptophan than did controls, and this abnormality was associated with disease activity, especially in Crohn’s disease (P = .03).
Patients with IBD who had relatively higher tryptophan levels also tended to have more diverse gut microbiota than did patients with lower serum tryptophan levels, although differences among groups were not statistically significant, the investigators said. Serum concentration of IL-22 also correlated with disease activity in patients with IBD, and infliximab responders had a “significant and sustained increase” of tryptophan levels over time, compared with nonresponders.
Potsdam dietary questionnaires found no link between disease activity and dietary consumption of tryptophan, the researchers said. Additionally, they found no links between serum tryptophan levels and age, smoking status, or disease complications, such as fistulae or abscess formation.The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop drugs with inflammatory indications. The other investigators had no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Patients with inflammatory bowel disease had significantly lower serum tryptophan levels than healthy controls.
Major finding: Serum tryptophan levels also correlated inversely with disease activity and C-reactive protein levels in patients with IBD.
Data source: An analysis of serum samples from 535 consecutive patients with IBD and 100 matched controls.
Disclosures: The investigators acknowledged grant support from the DFG Excellence Cluster “Inflammation at Interfaces” and BMBF e-med SYSINFLAME and H2020 SysCID. One coinvestigator reported employment by CONARIS Research Institute AG, which helps develop therapies with inflammatory indications. The other investigators had no conflicts of interest.
VIDEO: High-volume endoscopists, centers produced better ERCP outcomes
Endoscopists who performed endoscopic retrograde cholangiopancreatography (ERCP) at high-volume centers had a 60% greater odds of procedure success compared with those at low-volume centers, according to the results of a systematic review and meta-analysis.
High-volume endoscopists also had a 30% lower odds of performing ERCP that led to adverse events such as pancreatitis, perforation, and bleeding, reported Rajesh N. Keswani, MD, MS, of Northwestern University, Chicago, and his associates. High-volume centers themselves also were associated with a significantly higher odds of successful ERCP (odds ratio, 2.0; 95% CI, 1.6 to 2.5), although they were not associated with a significantly lower risk of adverse events, the reviewers wrote. The study was published in the December issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.002).
Diagnostic ERCP has fallen sevenfold in the past 30 years while therapeutic use has increased 30-fold, the researchers noted. Therapeutic use spans several complex pancreaticobiliary conditions, including chronic pancreatitis, malignant jaundice, and complications of liver transplantation. This shift from diagnostic to therapeutic has naturally increased the complexity of ERCP, the need for expert endoscopy, and the potential risk of adverse events. “As health care continues to shift toward rewarding value rather than volume, it will be increasingly important to deliver care that is effective and efficient,” the reviewers wrote. “Thus, understanding factors associated with unsuccessful interventions, such as a failed ERCP, will be of critical importance to payers and patients (Clin Gastroenterol Hepatol. 2017 Jun 7;218:237-45).
Therefore, they searched MEDLINE, EMBASE, and the Cochrane register of controlled trials for prospective and retrospective studies published through January 2017. In all, the researchers identified 13 studies that stratified outcomes by volume per endoscopist or center. These studies comprised 59,437 procedures and patients. Definitions of low volume varied by study, ranging from less than 25 to less than 156 annual ERCPs per endoscopist and from less than 87 to less than 200 annual ERCPs per center. Endoscopists who achieved this threshold were significantly more likely to perform successful ERCPs than were low-volume endoscopists (OR, 1.6; 95% CI, 1.2 to 2.1), and were significantly less likely to have patients develop pancreatitis, perforation, or bleeding after ERCP (OR, 0.7; 95% CI, 0.5 to 0.8).
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
“Given these compelling findings, we propose that providers and payers consider consolidating ERCP to high-volume endoscopists and centers to improve ERCP outcomes and value,” the reviewers wrote. Minimum thresholds for endoscopists and centers to maintain ERCP skills and optimize outcomes have not been defined, they noted. Intuitively, there is no “critical volume threshold” at which “outcomes suddenly improve,” but the studies in this analysis used widely varying definitions of low volume, they added. It also remains unclear whether a low-volume endoscopist can achieve optimal outcomes at a high-volume center, or vice versa, they said. They recommended studies to better define procedure success and the appropriate use of ERCP in therapeutic settings.
One reviewer acknowledged support from the University of Colorado Department of Medicine Outstanding Early Career Faculty Program. The reviewers reported having no conflicts of interest.
With the increasing proportion of complex therapeutic ERCPs, the field is shifting toward performance of these procedures by those who have had advanced training and who make them the focus of their clinical practice. Consistent with this, the meta-analysis by Keswani et al. highlights benefits of higher-volume centers and endoscopists - improved ERCP success rate (at the provider and practice level) and reduced adverse events (provider level only). It is unclear, however, if higher-volume endoscopists received additional training that translated into better outcomes. Other variables, including case complexity and provider experience, could not be fully assessed in this study.
Overall, however, this large, well-performed meta-analysis adds to the growing chorus that endoscopists and endoscopic centers will have better results if the endoscopists are specially trained and routinely perform these procedures. Future studies are needed to more accurately define procedure success (significant variation in the meta-analysis) and assess other variables that affect outcomes for which volume may only be a proxy. In an era of reporting and demonstrating value in endoscopic care, quality metrics for ERCP performance may not be fully appreciated but eventually may become the driving force in consolidation of these procedures to particular centers or providers, regardless of volume.
Avinash Ketwaroo, MD, MSc, is assistant professor in the division of gastroenterology and hepatology at Baylor College of Medicine, Houston, and an associate editor of GI & Hepatology News. He has no relevant conflicts of interest.
With the increasing proportion of complex therapeutic ERCPs, the field is shifting toward performance of these procedures by those who have had advanced training and who make them the focus of their clinical practice. Consistent with this, the meta-analysis by Keswani et al. highlights benefits of higher-volume centers and endoscopists - improved ERCP success rate (at the provider and practice level) and reduced adverse events (provider level only). It is unclear, however, if higher-volume endoscopists received additional training that translated into better outcomes. Other variables, including case complexity and provider experience, could not be fully assessed in this study.
Overall, however, this large, well-performed meta-analysis adds to the growing chorus that endoscopists and endoscopic centers will have better results if the endoscopists are specially trained and routinely perform these procedures. Future studies are needed to more accurately define procedure success (significant variation in the meta-analysis) and assess other variables that affect outcomes for which volume may only be a proxy. In an era of reporting and demonstrating value in endoscopic care, quality metrics for ERCP performance may not be fully appreciated but eventually may become the driving force in consolidation of these procedures to particular centers or providers, regardless of volume.
Avinash Ketwaroo, MD, MSc, is assistant professor in the division of gastroenterology and hepatology at Baylor College of Medicine, Houston, and an associate editor of GI & Hepatology News. He has no relevant conflicts of interest.
With the increasing proportion of complex therapeutic ERCPs, the field is shifting toward performance of these procedures by those who have had advanced training and who make them the focus of their clinical practice. Consistent with this, the meta-analysis by Keswani et al. highlights benefits of higher-volume centers and endoscopists - improved ERCP success rate (at the provider and practice level) and reduced adverse events (provider level only). It is unclear, however, if higher-volume endoscopists received additional training that translated into better outcomes. Other variables, including case complexity and provider experience, could not be fully assessed in this study.
Overall, however, this large, well-performed meta-analysis adds to the growing chorus that endoscopists and endoscopic centers will have better results if the endoscopists are specially trained and routinely perform these procedures. Future studies are needed to more accurately define procedure success (significant variation in the meta-analysis) and assess other variables that affect outcomes for which volume may only be a proxy. In an era of reporting and demonstrating value in endoscopic care, quality metrics for ERCP performance may not be fully appreciated but eventually may become the driving force in consolidation of these procedures to particular centers or providers, regardless of volume.
Avinash Ketwaroo, MD, MSc, is assistant professor in the division of gastroenterology and hepatology at Baylor College of Medicine, Houston, and an associate editor of GI & Hepatology News. He has no relevant conflicts of interest.
Endoscopists who performed endoscopic retrograde cholangiopancreatography (ERCP) at high-volume centers had a 60% greater odds of procedure success compared with those at low-volume centers, according to the results of a systematic review and meta-analysis.
High-volume endoscopists also had a 30% lower odds of performing ERCP that led to adverse events such as pancreatitis, perforation, and bleeding, reported Rajesh N. Keswani, MD, MS, of Northwestern University, Chicago, and his associates. High-volume centers themselves also were associated with a significantly higher odds of successful ERCP (odds ratio, 2.0; 95% CI, 1.6 to 2.5), although they were not associated with a significantly lower risk of adverse events, the reviewers wrote. The study was published in the December issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.002).
Diagnostic ERCP has fallen sevenfold in the past 30 years while therapeutic use has increased 30-fold, the researchers noted. Therapeutic use spans several complex pancreaticobiliary conditions, including chronic pancreatitis, malignant jaundice, and complications of liver transplantation. This shift from diagnostic to therapeutic has naturally increased the complexity of ERCP, the need for expert endoscopy, and the potential risk of adverse events. “As health care continues to shift toward rewarding value rather than volume, it will be increasingly important to deliver care that is effective and efficient,” the reviewers wrote. “Thus, understanding factors associated with unsuccessful interventions, such as a failed ERCP, will be of critical importance to payers and patients (Clin Gastroenterol Hepatol. 2017 Jun 7;218:237-45).
Therefore, they searched MEDLINE, EMBASE, and the Cochrane register of controlled trials for prospective and retrospective studies published through January 2017. In all, the researchers identified 13 studies that stratified outcomes by volume per endoscopist or center. These studies comprised 59,437 procedures and patients. Definitions of low volume varied by study, ranging from less than 25 to less than 156 annual ERCPs per endoscopist and from less than 87 to less than 200 annual ERCPs per center. Endoscopists who achieved this threshold were significantly more likely to perform successful ERCPs than were low-volume endoscopists (OR, 1.6; 95% CI, 1.2 to 2.1), and were significantly less likely to have patients develop pancreatitis, perforation, or bleeding after ERCP (OR, 0.7; 95% CI, 0.5 to 0.8).
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
“Given these compelling findings, we propose that providers and payers consider consolidating ERCP to high-volume endoscopists and centers to improve ERCP outcomes and value,” the reviewers wrote. Minimum thresholds for endoscopists and centers to maintain ERCP skills and optimize outcomes have not been defined, they noted. Intuitively, there is no “critical volume threshold” at which “outcomes suddenly improve,” but the studies in this analysis used widely varying definitions of low volume, they added. It also remains unclear whether a low-volume endoscopist can achieve optimal outcomes at a high-volume center, or vice versa, they said. They recommended studies to better define procedure success and the appropriate use of ERCP in therapeutic settings.
One reviewer acknowledged support from the University of Colorado Department of Medicine Outstanding Early Career Faculty Program. The reviewers reported having no conflicts of interest.
Endoscopists who performed endoscopic retrograde cholangiopancreatography (ERCP) at high-volume centers had a 60% greater odds of procedure success compared with those at low-volume centers, according to the results of a systematic review and meta-analysis.
High-volume endoscopists also had a 30% lower odds of performing ERCP that led to adverse events such as pancreatitis, perforation, and bleeding, reported Rajesh N. Keswani, MD, MS, of Northwestern University, Chicago, and his associates. High-volume centers themselves also were associated with a significantly higher odds of successful ERCP (odds ratio, 2.0; 95% CI, 1.6 to 2.5), although they were not associated with a significantly lower risk of adverse events, the reviewers wrote. The study was published in the December issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.002).
Diagnostic ERCP has fallen sevenfold in the past 30 years while therapeutic use has increased 30-fold, the researchers noted. Therapeutic use spans several complex pancreaticobiliary conditions, including chronic pancreatitis, malignant jaundice, and complications of liver transplantation. This shift from diagnostic to therapeutic has naturally increased the complexity of ERCP, the need for expert endoscopy, and the potential risk of adverse events. “As health care continues to shift toward rewarding value rather than volume, it will be increasingly important to deliver care that is effective and efficient,” the reviewers wrote. “Thus, understanding factors associated with unsuccessful interventions, such as a failed ERCP, will be of critical importance to payers and patients (Clin Gastroenterol Hepatol. 2017 Jun 7;218:237-45).
Therefore, they searched MEDLINE, EMBASE, and the Cochrane register of controlled trials for prospective and retrospective studies published through January 2017. In all, the researchers identified 13 studies that stratified outcomes by volume per endoscopist or center. These studies comprised 59,437 procedures and patients. Definitions of low volume varied by study, ranging from less than 25 to less than 156 annual ERCPs per endoscopist and from less than 87 to less than 200 annual ERCPs per center. Endoscopists who achieved this threshold were significantly more likely to perform successful ERCPs than were low-volume endoscopists (OR, 1.6; 95% CI, 1.2 to 2.1), and were significantly less likely to have patients develop pancreatitis, perforation, or bleeding after ERCP (OR, 0.7; 95% CI, 0.5 to 0.8).
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
“Given these compelling findings, we propose that providers and payers consider consolidating ERCP to high-volume endoscopists and centers to improve ERCP outcomes and value,” the reviewers wrote. Minimum thresholds for endoscopists and centers to maintain ERCP skills and optimize outcomes have not been defined, they noted. Intuitively, there is no “critical volume threshold” at which “outcomes suddenly improve,” but the studies in this analysis used widely varying definitions of low volume, they added. It also remains unclear whether a low-volume endoscopist can achieve optimal outcomes at a high-volume center, or vice versa, they said. They recommended studies to better define procedure success and the appropriate use of ERCP in therapeutic settings.
One reviewer acknowledged support from the University of Colorado Department of Medicine Outstanding Early Career Faculty Program. The reviewers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: High endoscopic retrograde cholangiopancreatography (ERCP) volume predicted procedure success.
Major finding: High-volume endoscopists were significantly more likely to achieve success with ERCP than were low-volume endoscopists (odds ratio, 1.6; 95% confidence interval, 1.2 to 2.1). High-volume centers also had greater odds of successful ERCP than did low-volume centers (OR, 2; 95% CI, 1.6 to 2.5).
Data source: A systematic review and meta-analysis of 13 studies comprising 59,437 procedures and patients.
Disclosures: One coinvestigator acknowledged support from the University of Colorado Department of Medicine Outstanding Early Career Faculty Program. The researchers reported having no conflicts of interest.
Biofeedback significantly improves abdominal distension in small study
An electromyographic biofeedback program significantly improved abdominothoracic muscle control and abdominal distension compared with placebo in a randomized trial of patients fulfilling Rome III criteria for functional intestinal disorders.
Sensations of abdominal distension improved by 56% with biofeedback (standard deviation, 1%) versus 13% (SD, 8%) with placebo, wrote Elizabeth Barba, MD, of University Hospital Vall d’Hebron in Barcelona, and her associates. The study was published in the December issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.052). Biofeedback also led to a doubling of anterior wall muscle activity (101%; SD, 10%) compared with a 4% (SD, 2%) improvement with placebo. Finally, biofeedback lowered intercostal muscle activity by a mean of 45% (SD, 3%) compared with 5% (SD, 2%) with placebo (all P values less than .001).
“Biofeedback in this trial was applied using a complex technique that provided effective guidance to patients and allowed close control of the mechanistic effects of the intervention on postural tone,” the researchers noted. “Having proved the [efficacy] of this treatment, the next steps are to develop and then to properly validate a simpler technique for widespread application.”
Episodic abdominal distension is a primary reason for visiting gastroenterology clinics. Patients typically experience an objective, visible increase in girth with no detectable cause, although they often have irritable bowel syndrome, functional dyspepsia, or both. Past work has linked abdominal distension with increased diaphragmatic tone and ventral protrusion and decreased muscle tone of the abdominal wall, the researchers noted.
Therefore, they developed an electromyography (EMG) biofeedback program to help patients learn to correct abdominothoracic muscular dystony. The trial comprised 48 patients (47 women and 1 man) ranging in age from 21 to 74 years. During each 30-minute session, patients sat upright in a quiet room while EMG recorded the activity of the intercostal muscles, anterior abdominal wall (external oblique, upper rectus, lower rectus, and internal oblique muscles), and diaphragm. Patients reported their sensation of abdominal distension on a visual rating scale ranging from 0 (no distension) to 6 (extreme distension). Those in the intervention group watched the EMG readout and were taught to reduce their intercostal and diaphragm activity while increasing the activity of the anterior abdominal muscles. Three training sessions occurred over 10 days and patients performed similar exercises at home for 5 minutes before each meal. Patients in the placebo group underwent the same instrumental interventions but did not watch the EMG recording, received no instructions about muscle control, and were given oral simethicone.
Symptoms associated with abdominal distension lessened by 57% (SD, 9%) in the biofeedback group and by 23% (4%) in the placebo group (P = .02). Treatment outcomes did not vary based on symptoms and there were no adverse effects of treatment, the researchers said. Furthermore, 19 patients in the placebo group who did not improve underwent biofeedback training and experienced benefits similar to those of the original intervention group. Sensations of abdominal distension and associated symptoms improved significantly immediately after biofeedback compared with baseline and continued to improve significantly over 6 months of follow-up.
The researchers described the complexity of the intervention, which, they acknowledged, would need to be simplified before it could be deployed widely. They measured the activity of the external oblique, upper rectus, lower rectus, and internal oblique muscles with bipolar leads, and recorded intercostal muscle activity by placing a monopolar electrode at the second intercostal space on the right midclavicular line, with a ground electrode over the central sternum. To verify correct placement, they recorded EMG responses to a Valsalva maneuver and a deep inhalation. They measured diaphragmatic activity by mounting electrodes on a polyvinyl tube, performing nasal intubation, and positioning the electrodes across the diaphragmatic hiatus under fluoroscopic control.
Funders included the Spanish Ministry of Economy and Competitiveness and the Instituto de Salud Carlos III. The researchers reported having no conflicts of interest.
An electromyographic biofeedback program significantly improved abdominothoracic muscle control and abdominal distension compared with placebo in a randomized trial of patients fulfilling Rome III criteria for functional intestinal disorders.
Sensations of abdominal distension improved by 56% with biofeedback (standard deviation, 1%) versus 13% (SD, 8%) with placebo, wrote Elizabeth Barba, MD, of University Hospital Vall d’Hebron in Barcelona, and her associates. The study was published in the December issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.052). Biofeedback also led to a doubling of anterior wall muscle activity (101%; SD, 10%) compared with a 4% (SD, 2%) improvement with placebo. Finally, biofeedback lowered intercostal muscle activity by a mean of 45% (SD, 3%) compared with 5% (SD, 2%) with placebo (all P values less than .001).
“Biofeedback in this trial was applied using a complex technique that provided effective guidance to patients and allowed close control of the mechanistic effects of the intervention on postural tone,” the researchers noted. “Having proved the [efficacy] of this treatment, the next steps are to develop and then to properly validate a simpler technique for widespread application.”
Episodic abdominal distension is a primary reason for visiting gastroenterology clinics. Patients typically experience an objective, visible increase in girth with no detectable cause, although they often have irritable bowel syndrome, functional dyspepsia, or both. Past work has linked abdominal distension with increased diaphragmatic tone and ventral protrusion and decreased muscle tone of the abdominal wall, the researchers noted.
Therefore, they developed an electromyography (EMG) biofeedback program to help patients learn to correct abdominothoracic muscular dystony. The trial comprised 48 patients (47 women and 1 man) ranging in age from 21 to 74 years. During each 30-minute session, patients sat upright in a quiet room while EMG recorded the activity of the intercostal muscles, anterior abdominal wall (external oblique, upper rectus, lower rectus, and internal oblique muscles), and diaphragm. Patients reported their sensation of abdominal distension on a visual rating scale ranging from 0 (no distension) to 6 (extreme distension). Those in the intervention group watched the EMG readout and were taught to reduce their intercostal and diaphragm activity while increasing the activity of the anterior abdominal muscles. Three training sessions occurred over 10 days and patients performed similar exercises at home for 5 minutes before each meal. Patients in the placebo group underwent the same instrumental interventions but did not watch the EMG recording, received no instructions about muscle control, and were given oral simethicone.
Symptoms associated with abdominal distension lessened by 57% (SD, 9%) in the biofeedback group and by 23% (4%) in the placebo group (P = .02). Treatment outcomes did not vary based on symptoms and there were no adverse effects of treatment, the researchers said. Furthermore, 19 patients in the placebo group who did not improve underwent biofeedback training and experienced benefits similar to those of the original intervention group. Sensations of abdominal distension and associated symptoms improved significantly immediately after biofeedback compared with baseline and continued to improve significantly over 6 months of follow-up.
The researchers described the complexity of the intervention, which, they acknowledged, would need to be simplified before it could be deployed widely. They measured the activity of the external oblique, upper rectus, lower rectus, and internal oblique muscles with bipolar leads, and recorded intercostal muscle activity by placing a monopolar electrode at the second intercostal space on the right midclavicular line, with a ground electrode over the central sternum. To verify correct placement, they recorded EMG responses to a Valsalva maneuver and a deep inhalation. They measured diaphragmatic activity by mounting electrodes on a polyvinyl tube, performing nasal intubation, and positioning the electrodes across the diaphragmatic hiatus under fluoroscopic control.
Funders included the Spanish Ministry of Economy and Competitiveness and the Instituto de Salud Carlos III. The researchers reported having no conflicts of interest.
An electromyographic biofeedback program significantly improved abdominothoracic muscle control and abdominal distension compared with placebo in a randomized trial of patients fulfilling Rome III criteria for functional intestinal disorders.
Sensations of abdominal distension improved by 56% with biofeedback (standard deviation, 1%) versus 13% (SD, 8%) with placebo, wrote Elizabeth Barba, MD, of University Hospital Vall d’Hebron in Barcelona, and her associates. The study was published in the December issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.052). Biofeedback also led to a doubling of anterior wall muscle activity (101%; SD, 10%) compared with a 4% (SD, 2%) improvement with placebo. Finally, biofeedback lowered intercostal muscle activity by a mean of 45% (SD, 3%) compared with 5% (SD, 2%) with placebo (all P values less than .001).
“Biofeedback in this trial was applied using a complex technique that provided effective guidance to patients and allowed close control of the mechanistic effects of the intervention on postural tone,” the researchers noted. “Having proved the [efficacy] of this treatment, the next steps are to develop and then to properly validate a simpler technique for widespread application.”
Episodic abdominal distension is a primary reason for visiting gastroenterology clinics. Patients typically experience an objective, visible increase in girth with no detectable cause, although they often have irritable bowel syndrome, functional dyspepsia, or both. Past work has linked abdominal distension with increased diaphragmatic tone and ventral protrusion and decreased muscle tone of the abdominal wall, the researchers noted.
Therefore, they developed an electromyography (EMG) biofeedback program to help patients learn to correct abdominothoracic muscular dystony. The trial comprised 48 patients (47 women and 1 man) ranging in age from 21 to 74 years. During each 30-minute session, patients sat upright in a quiet room while EMG recorded the activity of the intercostal muscles, anterior abdominal wall (external oblique, upper rectus, lower rectus, and internal oblique muscles), and diaphragm. Patients reported their sensation of abdominal distension on a visual rating scale ranging from 0 (no distension) to 6 (extreme distension). Those in the intervention group watched the EMG readout and were taught to reduce their intercostal and diaphragm activity while increasing the activity of the anterior abdominal muscles. Three training sessions occurred over 10 days and patients performed similar exercises at home for 5 minutes before each meal. Patients in the placebo group underwent the same instrumental interventions but did not watch the EMG recording, received no instructions about muscle control, and were given oral simethicone.
Symptoms associated with abdominal distension lessened by 57% (SD, 9%) in the biofeedback group and by 23% (4%) in the placebo group (P = .02). Treatment outcomes did not vary based on symptoms and there were no adverse effects of treatment, the researchers said. Furthermore, 19 patients in the placebo group who did not improve underwent biofeedback training and experienced benefits similar to those of the original intervention group. Sensations of abdominal distension and associated symptoms improved significantly immediately after biofeedback compared with baseline and continued to improve significantly over 6 months of follow-up.
The researchers described the complexity of the intervention, which, they acknowledged, would need to be simplified before it could be deployed widely. They measured the activity of the external oblique, upper rectus, lower rectus, and internal oblique muscles with bipolar leads, and recorded intercostal muscle activity by placing a monopolar electrode at the second intercostal space on the right midclavicular line, with a ground electrode over the central sternum. To verify correct placement, they recorded EMG responses to a Valsalva maneuver and a deep inhalation. They measured diaphragmatic activity by mounting electrodes on a polyvinyl tube, performing nasal intubation, and positioning the electrodes across the diaphragmatic hiatus under fluoroscopic control.
Funders included the Spanish Ministry of Economy and Competitiveness and the Instituto de Salud Carlos III. The researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Biofeedback training significantly improved abdominal distension in patients with functional intestinal disorders.
Major finding: Subjective abdominal distension improved by 56% in the intervention group and by 13% in the placebo group (P less than .001).
Data source: A randomized, placebo-controlled trial of 48 patients meeting Rome III criteria for functional intestinal disorders.
Disclosures: Funders included the Spanish Ministry of Economy and Competitiveness and Instituto de Salud Carlos III. The researchers reported having no conflicts of interest.
Dabigatran, rivaroxaban linked to slight increase in GI bleeding risk
Compared with conventional anticoagulants, both dabigatran and rivaroxaban conferred small but statistically significant increases in the risk of major gastrointestinal bleeding in a systematic review and meta-analysis of randomized trials reported in the November issue of Clinical Gastroenterology and Hepatology. (doi: 10.1016/j.cgh.2017.04.031)
But other novel oral anticoagulants (NOACs) showed no such effect compared with warfarin, aspirin, or placebo, reported Corey S. Miller, MD, of McGill University, Montreal, and his associates. “The potentially increased risk of GI bleeding associated with dabigatran and rivaroxaban observed in some of our subgroup analyses merits further consideration,” they wrote.
The NOACs (also known as non–vitamin K antagonist oral anticoagulants) help prevent stroke in patients with atrial fibrillation and prevent and treat venous thromboembolism. However, large AF trials have linked all except apixaban to an increased risk of major GI bleeding compared with warfarin. Dabigatran currently is the only NOAC with an approved reversal agent, “making the question of GI bleeding risk even more consequential,” the authors wrote.
They searched the MEDLINE, EMBASE, Cochrane, and ISI Web of Knowledge databases for reports of randomized trials of NOACs for approved indications published between 1980 and January 2016, which identified 43 trials of 166,289 patients. Most used warfarin as the comparator, but one study compared apixaban with aspirin and six studies compared apixaban, rivaroxaban, or dabigatran with placebo. Fifteen trials failed to specify bleeding sources and therefore could not be evaluated for the primary endpoint, the reviewers noted.
In the remaining 28 trials, 1.5% of NOAC recipients developed major GI bleeding, compared with 1.3% of recipients of conventional anticoagulants (odds ratio, 0.98; 95% confidence interval, 0.80-1.21). Five trials of dabigatran showed a 2% risk of major GI bleeding, compared with 1.4% with conventional anticoagulation, a slight but significant increase (OR, 1.27; 95% CI, 1.04-1.55). Eight trials of rivaroxaban showed a similar trend (bleeding risk, 1.7% vs. 1.3%; OR, 1.40; 95% CI, 1.15-1.70). In contrast, subgroup analyses of apixaban and edoxaban found no difference in risk of major GI bleeding versus conventional treatment.
Subgroup analyses by region found no differences except in Asia, where NOACs were associated with a significantly lower odds of major GI bleeding (0.5% and 1.2%, respectively; OR, 0.45; 95% CI, 0.22-0.91).
Most studies did not report minor or nonsevere bleeds or specify bleeding location within the GI tract, the reviewers noted. Given those caveats, NOACs and conventional anticoagulants conferred similar risks of clinically relevant nonmajor bleeding (0.6% and 0.6%, respectively), upper GI bleeding (1.5% and 1.6%), and lower GI bleeding (1.0% and 1.0%).
A post hoc analysis using a random-effects model found no significant difference in risk of major GI bleeding between either rivaroxaban or dabigatran and conventional therapy, the reviewers said. In addition, the increased risk of bleeding with dabigatran was confined to the RELY and ROCKET trials of AF, both of which exposed patients to longer treatment periods. Dabigatran is coated with tartaric acid, which might have a “direct caustic effect on the intestinal lumen,” they wrote. Also, NOACs are incompletely absorbed across the GI mucosa and therefore have some anticoagulant activity in the GI lumen, unlike warfarin or parenteral anticoagulants.
The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
Novel oral anticoagulants (NOACs) receive a lot of press now. In randomized controlled trials (RCTs) comparing NOACs to warfarin for prevention of strokes and thromboembolism in atrial fibrillation (AF) and venous thromboembolism (VTE), fewer thromboembolisms are reported, but risks of gastrointestinal bleeding vary. To expand analyses for gastrointestinal bleeding, several systematic reviews and meta-analyses are reported, including this one by CS Miller et al. Their goals were to delineate risks of gastrointestinal bleeding for different NOACs compared with warfarin. What can GI clinicians now recommend about gastrointestinal bleeding for patients requiring anticoagulants? While we lack RCTs to give the highest quality of evidence about GIB as a primary outcome, conclusions now depend on the weight of evidence from recent secondary data analyses and I have some recommendations. First, although there may be differences among NOACs in risks of bleeding, all are likely to increase the risk of GI bleeding, comparable with warfarin. Some report that dabigatran and rivaroxaban have a higher risk of GI bleeding than other NOACs or warfarin, but differences are small. Second, some patients who need NOACs/warfarin have increased risks of ulcer bleeds including elderly patients and those with a history of upper GI bleeding, renal or hepatic impairment, low body weight, and concomitant antiplatelet agents. Such high-risk patients warrant treatment with a proton pump inhibitor or histamine2-receptor agonists for primary prevention while on anticoagulants. Finally, for patients with severe ulcer bleeding who require anticoagulation, warfarin or NOACs should be restarted after successful endoscopic hemostasis and proton pump inhibitors, usually within 3-5 days.
Dr. Jensen is professor of medicine at the University of California, Los Angeles; associate director of the CURE: DDRC, where he directs the Human Studies Core; a full-time staff physician in the UCLA division of digestive diseases; and a part-time staff physician in the GI section of the VA Greater Los Angeles Healthcare Center.
Novel oral anticoagulants (NOACs) receive a lot of press now. In randomized controlled trials (RCTs) comparing NOACs to warfarin for prevention of strokes and thromboembolism in atrial fibrillation (AF) and venous thromboembolism (VTE), fewer thromboembolisms are reported, but risks of gastrointestinal bleeding vary. To expand analyses for gastrointestinal bleeding, several systematic reviews and meta-analyses are reported, including this one by CS Miller et al. Their goals were to delineate risks of gastrointestinal bleeding for different NOACs compared with warfarin. What can GI clinicians now recommend about gastrointestinal bleeding for patients requiring anticoagulants? While we lack RCTs to give the highest quality of evidence about GIB as a primary outcome, conclusions now depend on the weight of evidence from recent secondary data analyses and I have some recommendations. First, although there may be differences among NOACs in risks of bleeding, all are likely to increase the risk of GI bleeding, comparable with warfarin. Some report that dabigatran and rivaroxaban have a higher risk of GI bleeding than other NOACs or warfarin, but differences are small. Second, some patients who need NOACs/warfarin have increased risks of ulcer bleeds including elderly patients and those with a history of upper GI bleeding, renal or hepatic impairment, low body weight, and concomitant antiplatelet agents. Such high-risk patients warrant treatment with a proton pump inhibitor or histamine2-receptor agonists for primary prevention while on anticoagulants. Finally, for patients with severe ulcer bleeding who require anticoagulation, warfarin or NOACs should be restarted after successful endoscopic hemostasis and proton pump inhibitors, usually within 3-5 days.
Dr. Jensen is professor of medicine at the University of California, Los Angeles; associate director of the CURE: DDRC, where he directs the Human Studies Core; a full-time staff physician in the UCLA division of digestive diseases; and a part-time staff physician in the GI section of the VA Greater Los Angeles Healthcare Center.
Novel oral anticoagulants (NOACs) receive a lot of press now. In randomized controlled trials (RCTs) comparing NOACs to warfarin for prevention of strokes and thromboembolism in atrial fibrillation (AF) and venous thromboembolism (VTE), fewer thromboembolisms are reported, but risks of gastrointestinal bleeding vary. To expand analyses for gastrointestinal bleeding, several systematic reviews and meta-analyses are reported, including this one by CS Miller et al. Their goals were to delineate risks of gastrointestinal bleeding for different NOACs compared with warfarin. What can GI clinicians now recommend about gastrointestinal bleeding for patients requiring anticoagulants? While we lack RCTs to give the highest quality of evidence about GIB as a primary outcome, conclusions now depend on the weight of evidence from recent secondary data analyses and I have some recommendations. First, although there may be differences among NOACs in risks of bleeding, all are likely to increase the risk of GI bleeding, comparable with warfarin. Some report that dabigatran and rivaroxaban have a higher risk of GI bleeding than other NOACs or warfarin, but differences are small. Second, some patients who need NOACs/warfarin have increased risks of ulcer bleeds including elderly patients and those with a history of upper GI bleeding, renal or hepatic impairment, low body weight, and concomitant antiplatelet agents. Such high-risk patients warrant treatment with a proton pump inhibitor or histamine2-receptor agonists for primary prevention while on anticoagulants. Finally, for patients with severe ulcer bleeding who require anticoagulation, warfarin or NOACs should be restarted after successful endoscopic hemostasis and proton pump inhibitors, usually within 3-5 days.
Dr. Jensen is professor of medicine at the University of California, Los Angeles; associate director of the CURE: DDRC, where he directs the Human Studies Core; a full-time staff physician in the UCLA division of digestive diseases; and a part-time staff physician in the GI section of the VA Greater Los Angeles Healthcare Center.
Compared with conventional anticoagulants, both dabigatran and rivaroxaban conferred small but statistically significant increases in the risk of major gastrointestinal bleeding in a systematic review and meta-analysis of randomized trials reported in the November issue of Clinical Gastroenterology and Hepatology. (doi: 10.1016/j.cgh.2017.04.031)
But other novel oral anticoagulants (NOACs) showed no such effect compared with warfarin, aspirin, or placebo, reported Corey S. Miller, MD, of McGill University, Montreal, and his associates. “The potentially increased risk of GI bleeding associated with dabigatran and rivaroxaban observed in some of our subgroup analyses merits further consideration,” they wrote.
The NOACs (also known as non–vitamin K antagonist oral anticoagulants) help prevent stroke in patients with atrial fibrillation and prevent and treat venous thromboembolism. However, large AF trials have linked all except apixaban to an increased risk of major GI bleeding compared with warfarin. Dabigatran currently is the only NOAC with an approved reversal agent, “making the question of GI bleeding risk even more consequential,” the authors wrote.
They searched the MEDLINE, EMBASE, Cochrane, and ISI Web of Knowledge databases for reports of randomized trials of NOACs for approved indications published between 1980 and January 2016, which identified 43 trials of 166,289 patients. Most used warfarin as the comparator, but one study compared apixaban with aspirin and six studies compared apixaban, rivaroxaban, or dabigatran with placebo. Fifteen trials failed to specify bleeding sources and therefore could not be evaluated for the primary endpoint, the reviewers noted.
In the remaining 28 trials, 1.5% of NOAC recipients developed major GI bleeding, compared with 1.3% of recipients of conventional anticoagulants (odds ratio, 0.98; 95% confidence interval, 0.80-1.21). Five trials of dabigatran showed a 2% risk of major GI bleeding, compared with 1.4% with conventional anticoagulation, a slight but significant increase (OR, 1.27; 95% CI, 1.04-1.55). Eight trials of rivaroxaban showed a similar trend (bleeding risk, 1.7% vs. 1.3%; OR, 1.40; 95% CI, 1.15-1.70). In contrast, subgroup analyses of apixaban and edoxaban found no difference in risk of major GI bleeding versus conventional treatment.
Subgroup analyses by region found no differences except in Asia, where NOACs were associated with a significantly lower odds of major GI bleeding (0.5% and 1.2%, respectively; OR, 0.45; 95% CI, 0.22-0.91).
Most studies did not report minor or nonsevere bleeds or specify bleeding location within the GI tract, the reviewers noted. Given those caveats, NOACs and conventional anticoagulants conferred similar risks of clinically relevant nonmajor bleeding (0.6% and 0.6%, respectively), upper GI bleeding (1.5% and 1.6%), and lower GI bleeding (1.0% and 1.0%).
A post hoc analysis using a random-effects model found no significant difference in risk of major GI bleeding between either rivaroxaban or dabigatran and conventional therapy, the reviewers said. In addition, the increased risk of bleeding with dabigatran was confined to the RELY and ROCKET trials of AF, both of which exposed patients to longer treatment periods. Dabigatran is coated with tartaric acid, which might have a “direct caustic effect on the intestinal lumen,” they wrote. Also, NOACs are incompletely absorbed across the GI mucosa and therefore have some anticoagulant activity in the GI lumen, unlike warfarin or parenteral anticoagulants.
The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
Compared with conventional anticoagulants, both dabigatran and rivaroxaban conferred small but statistically significant increases in the risk of major gastrointestinal bleeding in a systematic review and meta-analysis of randomized trials reported in the November issue of Clinical Gastroenterology and Hepatology. (doi: 10.1016/j.cgh.2017.04.031)
But other novel oral anticoagulants (NOACs) showed no such effect compared with warfarin, aspirin, or placebo, reported Corey S. Miller, MD, of McGill University, Montreal, and his associates. “The potentially increased risk of GI bleeding associated with dabigatran and rivaroxaban observed in some of our subgroup analyses merits further consideration,” they wrote.
The NOACs (also known as non–vitamin K antagonist oral anticoagulants) help prevent stroke in patients with atrial fibrillation and prevent and treat venous thromboembolism. However, large AF trials have linked all except apixaban to an increased risk of major GI bleeding compared with warfarin. Dabigatran currently is the only NOAC with an approved reversal agent, “making the question of GI bleeding risk even more consequential,” the authors wrote.
They searched the MEDLINE, EMBASE, Cochrane, and ISI Web of Knowledge databases for reports of randomized trials of NOACs for approved indications published between 1980 and January 2016, which identified 43 trials of 166,289 patients. Most used warfarin as the comparator, but one study compared apixaban with aspirin and six studies compared apixaban, rivaroxaban, or dabigatran with placebo. Fifteen trials failed to specify bleeding sources and therefore could not be evaluated for the primary endpoint, the reviewers noted.
In the remaining 28 trials, 1.5% of NOAC recipients developed major GI bleeding, compared with 1.3% of recipients of conventional anticoagulants (odds ratio, 0.98; 95% confidence interval, 0.80-1.21). Five trials of dabigatran showed a 2% risk of major GI bleeding, compared with 1.4% with conventional anticoagulation, a slight but significant increase (OR, 1.27; 95% CI, 1.04-1.55). Eight trials of rivaroxaban showed a similar trend (bleeding risk, 1.7% vs. 1.3%; OR, 1.40; 95% CI, 1.15-1.70). In contrast, subgroup analyses of apixaban and edoxaban found no difference in risk of major GI bleeding versus conventional treatment.
Subgroup analyses by region found no differences except in Asia, where NOACs were associated with a significantly lower odds of major GI bleeding (0.5% and 1.2%, respectively; OR, 0.45; 95% CI, 0.22-0.91).
Most studies did not report minor or nonsevere bleeds or specify bleeding location within the GI tract, the reviewers noted. Given those caveats, NOACs and conventional anticoagulants conferred similar risks of clinically relevant nonmajor bleeding (0.6% and 0.6%, respectively), upper GI bleeding (1.5% and 1.6%), and lower GI bleeding (1.0% and 1.0%).
A post hoc analysis using a random-effects model found no significant difference in risk of major GI bleeding between either rivaroxaban or dabigatran and conventional therapy, the reviewers said. In addition, the increased risk of bleeding with dabigatran was confined to the RELY and ROCKET trials of AF, both of which exposed patients to longer treatment periods. Dabigatran is coated with tartaric acid, which might have a “direct caustic effect on the intestinal lumen,” they wrote. Also, NOACs are incompletely absorbed across the GI mucosa and therefore have some anticoagulant activity in the GI lumen, unlike warfarin or parenteral anticoagulants.
The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Compared with conventional anticoagulants, novel oral anticoagulants (NOACs) were not associated with increased risk of major gastrointestinal bleeding, with the possible exception of dabigatran and rivaroxaban.
Major finding: In the overall analysis, risk of major GI bleeding was 1.5% with NOACs and 1.3% with conventional anticoagulants (OR, 0.98; 95% CI, 0.80-1.21). In subgroup analyses, dabigatran conferred a 2% risk of major GI bleeding (OR, 1.3; 95% CI, 1.04-1.55), rivaroxaban conferred a 1.7% risk (OR, 1.40; 95% CI, 1.15-1.70).
Data source: A systematic review and meta-analysis of 43 randomized trials, comprising 166,289 patients.
Disclosures: The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
Branch duct intraductal papillar mucinous neoplasms confer increased malignancy risk
Patients with branch duct intraductal papillary mucinous neoplasms were about 19 times more likely to develop malignancies over 5 years compared with the general population, although they lacked worrisome features of malignancy at baseline.
The overall risk of malignancy in this cohort approached 8% and persisted for 10 years or more, said Ilaria Pergolini, MD, of Massachusetts General Hospital, Boston, and her associates. The findings support surveillance of this population past 5 years, although cysts that remain 1.5 cm or smaller for more than 5 years might be regarded as unlikely to become malignant, they wrote. The report appears in the November issue of Gastroenterology (doi: 10.1053/j.gastro.2017.07.019).
Few studies have explored branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), which the researchers defined as unilocular or multilocular pancreatic cysts with a nondilated main pancreatic duct (smaller than 5 mm). To begin filling this gap, they retrospectively studied 577 patients with suspected or presumed BD-IPMNs followed at Massachusetts General Hospital. Patients underwent cross-sectional imaging 3 months or more after initial diagnosis at least once thereafter. Standardized incidence ratios were calculated based on population-level data for the United States from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
Patients tended to be in their mid-60s at diagnosis (range, 21-90 years) and 59% were female, said the researchers. Median follow-up time was 82 months and ranged between 6 and 329 months, but 63% of patients were followed for at least 5 years (median, 107 months), and about one in five were followed for more than a decade. Fully 83% of patients were asymptomatic at initial diagnosis, of which 10% subsequently became symptomatic. Most patients underwent diagnostic CT, but nearly half underwent MRI/MRCP and about a third underwent endoscopic ultrasound. At diagnosis, median cyst size was 14 mm (range, 2-54 mm) and 9% of patients had cysts measuring at least 3 cm. By the end of follow-up, 55% had larger cysts than at baseline, and cysts grew by a median of 0.9 mm per year.
At diagnosis, only 1% of patients had high-risk stigmata while 12% had worrisome features such as acute pancreatitis, cysts measuring at least 3 cm, thickened or enhancing cyst walls, nonenhancing mural nodules, main pancreatic duct size of 5-9 mm, an abrupt change in caliber of the main pancreatic duct, and lymphadenopathy. During follow-up, another 13% of patients developed new worrisome features and 9% developed high-risk stigmata, while 2% experienced regression of a nodule. In all, 36% of patients had cysts with either worrisome features, high-risk stigmata, or both at some point during the study.
Among 363 patients followed for at least 5 years, 20 (5.5%) were diagnosed with high-risk dysplasia or invasive neoplasms and 4.4% developed invasive cancer, for a standardized incidence ratio of 18.8 (95% confidence interval, 9.7-32.8; P less than .001). Among 108 patients who had cysts measuring 1.5 cm or less, only one individual developed a distinct ductal adenocarcinoma during 5 or more years of follow-up. But of 255 patients with larger cysts, the 5-year rate of malignancy was 7.5% (P = .01).
“The absence of worrisome features or high-risk stigmata at a 5-year time point does not exclude the development of pancreatic malignancy, and the risk in these patients is 18.8 times higher than that of the general population,” the researchers concluded. “Because of this, we strongly support continued surveillance after 5 years from the initial diagnosis.” Cysts that remain 1.5 cm or smaller for at least 5 years are probably low risk, they said. “This is an important issue for further investigation, since it may help reduce costs related to surveillance and improve patients’ quality of life.”
The investigators did not disclose external funding sources. They reported having no conflicts of interest.
The appropriate surveillance strategy for branch duct IPMNs is a point of debate, and numerous guidelines have offered recommendations for managing these potentially malignant neoplasms. Among the contested topics is the appropriateness of ceasing imaging surveillance of lesions that are stable over years. In 2015, an American Gastroenterological Association guideline made a conditional recommendation for cessation of imaging surveillance of pancreatic cysts that have remained stable after 5 years, noting that only very low-quality evidence was available. Given the paucity of data on this topic, this recommendation has been debated.
Dr. Pergolini and colleagues shed new light on this question with this retrospective review. Their study demonstrates that a dramatically increased risk of developing pancreatic malignancy persists even when a branch duct IPMN demonstrates no worrisome features or growth after 5 years of imaging surveillance. In fact, in their cohort, the risk of malignancy not only persisted among patients with branch duct IPMNs compared to population-based controls, but in fact, the risk was even greater after 5 years of follow-up. The risk persisted even after 10 years of follow-up. This study lends credibility to the opinion that branch duct type IPMNs should undergo ongoing surveillance even after 5 years of stability on imaging. Furthermore, it invites further study on smaller (less than 1.5 cm) branch duct IPMNs that remain stable over 5 years, as they appear to be very low risk and may represent a category of IPMNs that do not require indefinite surveillance.
Anthony Gamboa, MD, is assistant professor of medicine, program director of advanced endoscopy fellowship, division of gastroenterology, hepatology and nutrition, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
The appropriate surveillance strategy for branch duct IPMNs is a point of debate, and numerous guidelines have offered recommendations for managing these potentially malignant neoplasms. Among the contested topics is the appropriateness of ceasing imaging surveillance of lesions that are stable over years. In 2015, an American Gastroenterological Association guideline made a conditional recommendation for cessation of imaging surveillance of pancreatic cysts that have remained stable after 5 years, noting that only very low-quality evidence was available. Given the paucity of data on this topic, this recommendation has been debated.
Dr. Pergolini and colleagues shed new light on this question with this retrospective review. Their study demonstrates that a dramatically increased risk of developing pancreatic malignancy persists even when a branch duct IPMN demonstrates no worrisome features or growth after 5 years of imaging surveillance. In fact, in their cohort, the risk of malignancy not only persisted among patients with branch duct IPMNs compared to population-based controls, but in fact, the risk was even greater after 5 years of follow-up. The risk persisted even after 10 years of follow-up. This study lends credibility to the opinion that branch duct type IPMNs should undergo ongoing surveillance even after 5 years of stability on imaging. Furthermore, it invites further study on smaller (less than 1.5 cm) branch duct IPMNs that remain stable over 5 years, as they appear to be very low risk and may represent a category of IPMNs that do not require indefinite surveillance.
Anthony Gamboa, MD, is assistant professor of medicine, program director of advanced endoscopy fellowship, division of gastroenterology, hepatology and nutrition, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
The appropriate surveillance strategy for branch duct IPMNs is a point of debate, and numerous guidelines have offered recommendations for managing these potentially malignant neoplasms. Among the contested topics is the appropriateness of ceasing imaging surveillance of lesions that are stable over years. In 2015, an American Gastroenterological Association guideline made a conditional recommendation for cessation of imaging surveillance of pancreatic cysts that have remained stable after 5 years, noting that only very low-quality evidence was available. Given the paucity of data on this topic, this recommendation has been debated.
Dr. Pergolini and colleagues shed new light on this question with this retrospective review. Their study demonstrates that a dramatically increased risk of developing pancreatic malignancy persists even when a branch duct IPMN demonstrates no worrisome features or growth after 5 years of imaging surveillance. In fact, in their cohort, the risk of malignancy not only persisted among patients with branch duct IPMNs compared to population-based controls, but in fact, the risk was even greater after 5 years of follow-up. The risk persisted even after 10 years of follow-up. This study lends credibility to the opinion that branch duct type IPMNs should undergo ongoing surveillance even after 5 years of stability on imaging. Furthermore, it invites further study on smaller (less than 1.5 cm) branch duct IPMNs that remain stable over 5 years, as they appear to be very low risk and may represent a category of IPMNs that do not require indefinite surveillance.
Anthony Gamboa, MD, is assistant professor of medicine, program director of advanced endoscopy fellowship, division of gastroenterology, hepatology and nutrition, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
Patients with branch duct intraductal papillary mucinous neoplasms were about 19 times more likely to develop malignancies over 5 years compared with the general population, although they lacked worrisome features of malignancy at baseline.
The overall risk of malignancy in this cohort approached 8% and persisted for 10 years or more, said Ilaria Pergolini, MD, of Massachusetts General Hospital, Boston, and her associates. The findings support surveillance of this population past 5 years, although cysts that remain 1.5 cm or smaller for more than 5 years might be regarded as unlikely to become malignant, they wrote. The report appears in the November issue of Gastroenterology (doi: 10.1053/j.gastro.2017.07.019).
Few studies have explored branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), which the researchers defined as unilocular or multilocular pancreatic cysts with a nondilated main pancreatic duct (smaller than 5 mm). To begin filling this gap, they retrospectively studied 577 patients with suspected or presumed BD-IPMNs followed at Massachusetts General Hospital. Patients underwent cross-sectional imaging 3 months or more after initial diagnosis at least once thereafter. Standardized incidence ratios were calculated based on population-level data for the United States from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
Patients tended to be in their mid-60s at diagnosis (range, 21-90 years) and 59% were female, said the researchers. Median follow-up time was 82 months and ranged between 6 and 329 months, but 63% of patients were followed for at least 5 years (median, 107 months), and about one in five were followed for more than a decade. Fully 83% of patients were asymptomatic at initial diagnosis, of which 10% subsequently became symptomatic. Most patients underwent diagnostic CT, but nearly half underwent MRI/MRCP and about a third underwent endoscopic ultrasound. At diagnosis, median cyst size was 14 mm (range, 2-54 mm) and 9% of patients had cysts measuring at least 3 cm. By the end of follow-up, 55% had larger cysts than at baseline, and cysts grew by a median of 0.9 mm per year.
At diagnosis, only 1% of patients had high-risk stigmata while 12% had worrisome features such as acute pancreatitis, cysts measuring at least 3 cm, thickened or enhancing cyst walls, nonenhancing mural nodules, main pancreatic duct size of 5-9 mm, an abrupt change in caliber of the main pancreatic duct, and lymphadenopathy. During follow-up, another 13% of patients developed new worrisome features and 9% developed high-risk stigmata, while 2% experienced regression of a nodule. In all, 36% of patients had cysts with either worrisome features, high-risk stigmata, or both at some point during the study.
Among 363 patients followed for at least 5 years, 20 (5.5%) were diagnosed with high-risk dysplasia or invasive neoplasms and 4.4% developed invasive cancer, for a standardized incidence ratio of 18.8 (95% confidence interval, 9.7-32.8; P less than .001). Among 108 patients who had cysts measuring 1.5 cm or less, only one individual developed a distinct ductal adenocarcinoma during 5 or more years of follow-up. But of 255 patients with larger cysts, the 5-year rate of malignancy was 7.5% (P = .01).
“The absence of worrisome features or high-risk stigmata at a 5-year time point does not exclude the development of pancreatic malignancy, and the risk in these patients is 18.8 times higher than that of the general population,” the researchers concluded. “Because of this, we strongly support continued surveillance after 5 years from the initial diagnosis.” Cysts that remain 1.5 cm or smaller for at least 5 years are probably low risk, they said. “This is an important issue for further investigation, since it may help reduce costs related to surveillance and improve patients’ quality of life.”
The investigators did not disclose external funding sources. They reported having no conflicts of interest.
Patients with branch duct intraductal papillary mucinous neoplasms were about 19 times more likely to develop malignancies over 5 years compared with the general population, although they lacked worrisome features of malignancy at baseline.
The overall risk of malignancy in this cohort approached 8% and persisted for 10 years or more, said Ilaria Pergolini, MD, of Massachusetts General Hospital, Boston, and her associates. The findings support surveillance of this population past 5 years, although cysts that remain 1.5 cm or smaller for more than 5 years might be regarded as unlikely to become malignant, they wrote. The report appears in the November issue of Gastroenterology (doi: 10.1053/j.gastro.2017.07.019).
Few studies have explored branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), which the researchers defined as unilocular or multilocular pancreatic cysts with a nondilated main pancreatic duct (smaller than 5 mm). To begin filling this gap, they retrospectively studied 577 patients with suspected or presumed BD-IPMNs followed at Massachusetts General Hospital. Patients underwent cross-sectional imaging 3 months or more after initial diagnosis at least once thereafter. Standardized incidence ratios were calculated based on population-level data for the United States from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
Patients tended to be in their mid-60s at diagnosis (range, 21-90 years) and 59% were female, said the researchers. Median follow-up time was 82 months and ranged between 6 and 329 months, but 63% of patients were followed for at least 5 years (median, 107 months), and about one in five were followed for more than a decade. Fully 83% of patients were asymptomatic at initial diagnosis, of which 10% subsequently became symptomatic. Most patients underwent diagnostic CT, but nearly half underwent MRI/MRCP and about a third underwent endoscopic ultrasound. At diagnosis, median cyst size was 14 mm (range, 2-54 mm) and 9% of patients had cysts measuring at least 3 cm. By the end of follow-up, 55% had larger cysts than at baseline, and cysts grew by a median of 0.9 mm per year.
At diagnosis, only 1% of patients had high-risk stigmata while 12% had worrisome features such as acute pancreatitis, cysts measuring at least 3 cm, thickened or enhancing cyst walls, nonenhancing mural nodules, main pancreatic duct size of 5-9 mm, an abrupt change in caliber of the main pancreatic duct, and lymphadenopathy. During follow-up, another 13% of patients developed new worrisome features and 9% developed high-risk stigmata, while 2% experienced regression of a nodule. In all, 36% of patients had cysts with either worrisome features, high-risk stigmata, or both at some point during the study.
Among 363 patients followed for at least 5 years, 20 (5.5%) were diagnosed with high-risk dysplasia or invasive neoplasms and 4.4% developed invasive cancer, for a standardized incidence ratio of 18.8 (95% confidence interval, 9.7-32.8; P less than .001). Among 108 patients who had cysts measuring 1.5 cm or less, only one individual developed a distinct ductal adenocarcinoma during 5 or more years of follow-up. But of 255 patients with larger cysts, the 5-year rate of malignancy was 7.5% (P = .01).
“The absence of worrisome features or high-risk stigmata at a 5-year time point does not exclude the development of pancreatic malignancy, and the risk in these patients is 18.8 times higher than that of the general population,” the researchers concluded. “Because of this, we strongly support continued surveillance after 5 years from the initial diagnosis.” Cysts that remain 1.5 cm or smaller for at least 5 years are probably low risk, they said. “This is an important issue for further investigation, since it may help reduce costs related to surveillance and improve patients’ quality of life.”
The investigators did not disclose external funding sources. They reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Branch duct intraductal papillary mucinous neoplasms conferred a markedly increased risk of malignancy even when they lacked worrisome features at baseline.
Major finding: At 5 years, the standardized incidence ratio for malignancy was 18.8 compared with the general population.
Data source: A retrospective study of 577 patients with suspected branch duct intraductal papillary mucinous neoplasms.
Disclosures: The investigators did not disclose external funding sources. They reported having no relevant conflicts of interest.
VIDEO: Trial posts null results for Helicobacter screening, eradication
Compared with usual care, screening for and the eradication of Helicobacter pylori infection did not significantly improve the risk of dyspepsia or peptic ulcer disease, use of health care services, or quality of life in a large randomized, controlled trial reported in the November issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.006).
After 13 years of follow-up, the prevalence of dyspepsia was 19% in both arms (adjusted odds ratio, 0.93; 95% confidence interval, 0.82-1.04), reported Maria Bomme, MD, of University of Southern Denmark (Odense), and her associates. The cumulative risk of the coprimary endpoint, peptic ulcer disease, was 3% in both groups (risk ratio, 0.93; 95% confidence interval, 0.79-1.09). Screening and eradication also did not affect secondary endpoints such as rates of gastroesophageal reflux, endoscopy, antacid use, or health care utilization, or mental and physical quality of life.
The study “was designed to provide evidence on the effect of H. pylori screening at a population scale,” the researchers wrote. “It showed no significant long-term effect of population screening when compared with current clinical practice in a low-prevalence area.”
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Prior studies have suggested that eradicating H. pylori infection might help prevent peptic ulcers and dyspepsia and could reduce the risk of gastric cancer, the researchers noted. For this trial, they randomly assigned 20,011 adults aged 40-65 years from a single county in Denmark to receive H. pylori screening or usual care. Screening consisted of an outpatient blood test for H. pylori, which was confirmed by 13C-urea breath test (UBT) if positive. Individuals with confirmed infections were offered triple eradication therapy (20 mg omeprazole, 500 mg clarithromycin, and either 1 g amoxicillin or 500 mg metronidazole) twice daily for 1 week. This regimen eradicated 95% of infections, based on UBT results from a subset of 200 individuals.
Compared with nonparticipants, the 12,530 (63%) study enrollees were significantly more likely to be female, 50 years or older, married, and to have a history of peptic ulcer disease. Rates of follow-up were 92% at 1 year, 83% at 5 years, and 69% (8,658 individuals) at 13 years. Among 5,749 screened participants, 17.5% tested positive for H. pylori. Nearly all underwent eradication therapy. At 5 years, screening and eradication were associated with a significant reduction in the incidence of peptic ulcers and associated complications and with modest improvements in dyspepsia, health care visits for dyspepsia, and sick leave days, compared with usual care. But the prevalence of dyspepsia waned in both groups over time and did not significantly differ between groups at 13 years in either the intention-to-treat or per-protocol analysis. Likewise, annual rates of peptic ulcer disease were very similar (1.9 cases/1,000 screened individuals and 2.2 cases/1,000 controls; incidence rate ratio, 0.87; 95% CI, 0.69-1.10). Rates of gastroesophageal cancer also were similar among groups throughout the study.
Screening for and eradicating H. pylori also did not affect the likelihood of dyspepsia or peptic ulcer disease at 13 years among individuals who were dyspeptic at baseline, the researchers said. The relatively low prevalence of H. pylori infection in Denmark might have diluted the effects of screening and eradication, they added.
Funders included the Region of Southern Denmark, the department of clinical research at the University of Southern Denmark, the Odense University Hospital research board, and the Aase and Ejnar Danielsens Foundation, Beckett- Fonden, and Helsefonden. The researchers reported having no conflicts of interest.
Compared with usual care, screening for and the eradication of Helicobacter pylori infection did not significantly improve the risk of dyspepsia or peptic ulcer disease, use of health care services, or quality of life in a large randomized, controlled trial reported in the November issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.006).
After 13 years of follow-up, the prevalence of dyspepsia was 19% in both arms (adjusted odds ratio, 0.93; 95% confidence interval, 0.82-1.04), reported Maria Bomme, MD, of University of Southern Denmark (Odense), and her associates. The cumulative risk of the coprimary endpoint, peptic ulcer disease, was 3% in both groups (risk ratio, 0.93; 95% confidence interval, 0.79-1.09). Screening and eradication also did not affect secondary endpoints such as rates of gastroesophageal reflux, endoscopy, antacid use, or health care utilization, or mental and physical quality of life.
The study “was designed to provide evidence on the effect of H. pylori screening at a population scale,” the researchers wrote. “It showed no significant long-term effect of population screening when compared with current clinical practice in a low-prevalence area.”
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Prior studies have suggested that eradicating H. pylori infection might help prevent peptic ulcers and dyspepsia and could reduce the risk of gastric cancer, the researchers noted. For this trial, they randomly assigned 20,011 adults aged 40-65 years from a single county in Denmark to receive H. pylori screening or usual care. Screening consisted of an outpatient blood test for H. pylori, which was confirmed by 13C-urea breath test (UBT) if positive. Individuals with confirmed infections were offered triple eradication therapy (20 mg omeprazole, 500 mg clarithromycin, and either 1 g amoxicillin or 500 mg metronidazole) twice daily for 1 week. This regimen eradicated 95% of infections, based on UBT results from a subset of 200 individuals.
Compared with nonparticipants, the 12,530 (63%) study enrollees were significantly more likely to be female, 50 years or older, married, and to have a history of peptic ulcer disease. Rates of follow-up were 92% at 1 year, 83% at 5 years, and 69% (8,658 individuals) at 13 years. Among 5,749 screened participants, 17.5% tested positive for H. pylori. Nearly all underwent eradication therapy. At 5 years, screening and eradication were associated with a significant reduction in the incidence of peptic ulcers and associated complications and with modest improvements in dyspepsia, health care visits for dyspepsia, and sick leave days, compared with usual care. But the prevalence of dyspepsia waned in both groups over time and did not significantly differ between groups at 13 years in either the intention-to-treat or per-protocol analysis. Likewise, annual rates of peptic ulcer disease were very similar (1.9 cases/1,000 screened individuals and 2.2 cases/1,000 controls; incidence rate ratio, 0.87; 95% CI, 0.69-1.10). Rates of gastroesophageal cancer also were similar among groups throughout the study.
Screening for and eradicating H. pylori also did not affect the likelihood of dyspepsia or peptic ulcer disease at 13 years among individuals who were dyspeptic at baseline, the researchers said. The relatively low prevalence of H. pylori infection in Denmark might have diluted the effects of screening and eradication, they added.
Funders included the Region of Southern Denmark, the department of clinical research at the University of Southern Denmark, the Odense University Hospital research board, and the Aase and Ejnar Danielsens Foundation, Beckett- Fonden, and Helsefonden. The researchers reported having no conflicts of interest.
Compared with usual care, screening for and the eradication of Helicobacter pylori infection did not significantly improve the risk of dyspepsia or peptic ulcer disease, use of health care services, or quality of life in a large randomized, controlled trial reported in the November issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.06.006).
After 13 years of follow-up, the prevalence of dyspepsia was 19% in both arms (adjusted odds ratio, 0.93; 95% confidence interval, 0.82-1.04), reported Maria Bomme, MD, of University of Southern Denmark (Odense), and her associates. The cumulative risk of the coprimary endpoint, peptic ulcer disease, was 3% in both groups (risk ratio, 0.93; 95% confidence interval, 0.79-1.09). Screening and eradication also did not affect secondary endpoints such as rates of gastroesophageal reflux, endoscopy, antacid use, or health care utilization, or mental and physical quality of life.
The study “was designed to provide evidence on the effect of H. pylori screening at a population scale,” the researchers wrote. “It showed no significant long-term effect of population screening when compared with current clinical practice in a low-prevalence area.”
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Prior studies have suggested that eradicating H. pylori infection might help prevent peptic ulcers and dyspepsia and could reduce the risk of gastric cancer, the researchers noted. For this trial, they randomly assigned 20,011 adults aged 40-65 years from a single county in Denmark to receive H. pylori screening or usual care. Screening consisted of an outpatient blood test for H. pylori, which was confirmed by 13C-urea breath test (UBT) if positive. Individuals with confirmed infections were offered triple eradication therapy (20 mg omeprazole, 500 mg clarithromycin, and either 1 g amoxicillin or 500 mg metronidazole) twice daily for 1 week. This regimen eradicated 95% of infections, based on UBT results from a subset of 200 individuals.
Compared with nonparticipants, the 12,530 (63%) study enrollees were significantly more likely to be female, 50 years or older, married, and to have a history of peptic ulcer disease. Rates of follow-up were 92% at 1 year, 83% at 5 years, and 69% (8,658 individuals) at 13 years. Among 5,749 screened participants, 17.5% tested positive for H. pylori. Nearly all underwent eradication therapy. At 5 years, screening and eradication were associated with a significant reduction in the incidence of peptic ulcers and associated complications and with modest improvements in dyspepsia, health care visits for dyspepsia, and sick leave days, compared with usual care. But the prevalence of dyspepsia waned in both groups over time and did not significantly differ between groups at 13 years in either the intention-to-treat or per-protocol analysis. Likewise, annual rates of peptic ulcer disease were very similar (1.9 cases/1,000 screened individuals and 2.2 cases/1,000 controls; incidence rate ratio, 0.87; 95% CI, 0.69-1.10). Rates of gastroesophageal cancer also were similar among groups throughout the study.
Screening for and eradicating H. pylori also did not affect the likelihood of dyspepsia or peptic ulcer disease at 13 years among individuals who were dyspeptic at baseline, the researchers said. The relatively low prevalence of H. pylori infection in Denmark might have diluted the effects of screening and eradication, they added.
Funders included the Region of Southern Denmark, the department of clinical research at the University of Southern Denmark, the Odense University Hospital research board, and the Aase and Ejnar Danielsens Foundation, Beckett- Fonden, and Helsefonden. The researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Screening for and eradicating H. pylori infections did not significantly improve long-term prevalence of dyspepsia, incidence of peptic ulcer disease, use of health care services, or quality of life.
Major finding: At 13-year follow-up, both arms had a 19% prevalence of dyspepsia (aOR, 0.93; 95% CI, 0.82-1.04) and a 3% cumulative incidence of peptic ulcer disease (RR, 0.93; 95% CI, 0.79-1.09).
Data source: A randomized controlled trial of 8,658 adults aged 40-65 years
Disclosures: Funders included the Region of Southern Denmark, the department of clinical research at the University of Southern Denmark, the Odense University Hospital research board, and the Aase and Ejnar Danielsens Foundation, Beckett- Fonden, and Helsefonden. The researchers reported having no conflicts of interest.