User login
Pediatric hospitalist certification beset by gender bias concerns
Are women unfairly penalized?
More than 1,625 pediatricians have applied to take the first pediatric hospitalist certification exam in November 2019, and approximately 93% of them have been accepted, according to a statement from the American Board of Pediatrics.
It was the rejection of the 7%, however, that set off a firestorm on the electronic discussion board for American Academy of Pediatrics (AAP) hospital medicine this summer, and led to a petition to the board to revise its eligibility requirements, ensure that the requirements are fair to women, and bring transparency to its decision process. The petition has more than 1,400 signatures.
Seattle Children’s Hospital and Yale New Haven (Conn.) Children’s Hospital have both said they will not consider board certification in hiring decisions until the situation is resolved.
The American Board of Pediatrics (ABP) declined an interview request pending its formal response to the Aug. 6 petition, but in a statement to this news organization, executive vice president Suzanne Woods, MD, said, “The percentage of women and men meeting the eligibility requirements for the exam did not differ. We stress this point because a concern about possible gender bias appears to have been the principal reason for this ... petition, and we wanted to offer immediate reassurance that no unintended bias has occurred.”
“We are carefully considering the requests and will release detailed data to hospitalists on the AAP’s [pediatric hospital medicine (PHM) electronic discussion board] ... and on the ABP’s website. We are conferring with ABP PHM subboard members as well as leaders from our volunteer community. We expect to provide a thoughtful response within the next 3 weeks,” Dr. Woods said in the Aug. 15 statement.
“Case-by-case” exceptions
The backstory is that, for better or worse depending on who you talk to, pediatric hospital medicine is becoming a board certified subspecialty. A fellowship will be required to sit for the exam after a few years, which is standard for subspecialties.
What’s generated concern is how the board is grandfathering current pediatric hospitalists into certification via a “practice pathway” until the fellowship requirement takes hold after 2023.
To qualify for the November test, hospitalists had to complete 4 years of full-time practice by June 30, 2019, which has been understood to mean 48 months of continual employment. At least 50% of that time had to be devoted to “professional activities ... related to the care of hospitalized children,” and at least 25% of that “devoted to direct patient care.” Assuming about 2,000 work hours per year, it translated to “450-500 hours” of direct patient care “per year over the most recent four years” to sit for the test, the board said.
“For individuals who have interrupted practice during the most recent four years for family leave or other such circumstances, an exception may be considered if there is substantial prior experience in pediatric hospital medicine. ... Such exceptions are made at the discretion of the ABP and will be considered on a case-by-case basis.” Specific criteria for exceptions were not spelled out.
In the end, there were more than a few surprises when denial letters went out in recent months, and scores of appeals have been filed. There’s “a lot of tension and a lot of confusion” about why some people with practice gaps during the 4 years were approved, but others were denied. There’s been “a lack of transparency on the ABP’s part,” said H. Barrett Fromme, MD, section chief of pediatric hospital medicine and a professor of pediatrics at the University of Chicago.
“The standard has to be reasonable”
There are concerns about the availability of fellowship slots and other issues, but the 4-year rule – instead of averaging clinical hours over 4 or 5 years, for instance – is the main sticking point. It’s a gender issue because “women take maternity; women move with their spouse; women take care of elders; women tend to be in these roles that require time off” more than men do, Dr. Fromme said.
Until the board releases its data, the gender breakdown of the denials and the degree to which practice gaps due to such issues led to them is unknown. There’s concern that women have been unfairly penalized.
The storm was set off on the discussion board this summer by stories from physicians such as Chandani DeZure, MD, a pediatric hospitalist currently working in the neonatal ICU at Stanford (Calif.) University. She was denied a seat at the table in November, appealed, and was denied again.
She was a full-time pediatric hospitalist at Children’s National Medical Center in Washington, from 2014, when she graduated residency, until Oct. 2018, when her husband, also a doctor, was offered a promising research position in California, and “we decided to take it,” Dr. DeZure said.
They moved to California with their young son in November. Dr. DeZure got her California medical license in 6 weeks, was hired by Stanford in January, and started her new postion in mid-April.
Because of the move, she worked only 3.5 years in the board’s 4 year practice window, but, as is common with young physicians, that time was spent in direct patient care, for a total of over 6,000 hours.
“How is that not good enough? How is a person that worked 500 hours with patients for 4 years” – for a total of 2,000 hours – “better qualified than someone who worked 100% for 3 and a half years? Nobody is saying there shouldn’t be a standard, but the standard has to be reasonable,” Dr. DeZure said.
“Illegal regardless of intent”
It’s situations like Dr. DeZure’s that led to the petition. One of demands is that ABP “revise the practice pathway criteria to be more inclusive of applicants with interrupted practice and varied clinical experience, to include clear-cut parameters rather than considering these applications on a closed-door ‘case-by-case basis...at the discretion of the ABP.’ ” Also, the petition asks the board to “clarify the appeals process and improve responsiveness to appeals and inquiries regarding denials.”
As ABP noted in its statement, however, the major demand is that the board “facilitate a timely analysis to determine if gender bias is present.” The petition noted that signers “do not suspect intentional bias on the part of the ABP; however, if gender bias is present it is unethical and potentially illegal regardless of intent.”
For now, the perception is that the board has “a hard 48-month rule” with not many exceptions; there are people who are “very concerned that, ‘Oh my gosh, I can’t have children for 4 years because I won’t be able to sit for the boards.’ No one should ever have to have that in their head,” Dr. Fromme said. At this point, it seems that 3 months off for maternity is being grandfathered in, but perhaps not 6 months for a second child; no one knows for sure.
Dr. DeZure, meanwhile, continues to study for the board exam, just in case.
Looking back over the past year, she said “I could have somehow picked up one shift a week moonlighting that would have kept me eligible, but the [board] didn’t respond to me” when contacted about her situation during the California move.
“The other option was for me was to live cross country from my husband with a small child,” she said.
Are women unfairly penalized?
Are women unfairly penalized?
More than 1,625 pediatricians have applied to take the first pediatric hospitalist certification exam in November 2019, and approximately 93% of them have been accepted, according to a statement from the American Board of Pediatrics.
It was the rejection of the 7%, however, that set off a firestorm on the electronic discussion board for American Academy of Pediatrics (AAP) hospital medicine this summer, and led to a petition to the board to revise its eligibility requirements, ensure that the requirements are fair to women, and bring transparency to its decision process. The petition has more than 1,400 signatures.
Seattle Children’s Hospital and Yale New Haven (Conn.) Children’s Hospital have both said they will not consider board certification in hiring decisions until the situation is resolved.
The American Board of Pediatrics (ABP) declined an interview request pending its formal response to the Aug. 6 petition, but in a statement to this news organization, executive vice president Suzanne Woods, MD, said, “The percentage of women and men meeting the eligibility requirements for the exam did not differ. We stress this point because a concern about possible gender bias appears to have been the principal reason for this ... petition, and we wanted to offer immediate reassurance that no unintended bias has occurred.”
“We are carefully considering the requests and will release detailed data to hospitalists on the AAP’s [pediatric hospital medicine (PHM) electronic discussion board] ... and on the ABP’s website. We are conferring with ABP PHM subboard members as well as leaders from our volunteer community. We expect to provide a thoughtful response within the next 3 weeks,” Dr. Woods said in the Aug. 15 statement.
“Case-by-case” exceptions
The backstory is that, for better or worse depending on who you talk to, pediatric hospital medicine is becoming a board certified subspecialty. A fellowship will be required to sit for the exam after a few years, which is standard for subspecialties.
What’s generated concern is how the board is grandfathering current pediatric hospitalists into certification via a “practice pathway” until the fellowship requirement takes hold after 2023.
To qualify for the November test, hospitalists had to complete 4 years of full-time practice by June 30, 2019, which has been understood to mean 48 months of continual employment. At least 50% of that time had to be devoted to “professional activities ... related to the care of hospitalized children,” and at least 25% of that “devoted to direct patient care.” Assuming about 2,000 work hours per year, it translated to “450-500 hours” of direct patient care “per year over the most recent four years” to sit for the test, the board said.
“For individuals who have interrupted practice during the most recent four years for family leave or other such circumstances, an exception may be considered if there is substantial prior experience in pediatric hospital medicine. ... Such exceptions are made at the discretion of the ABP and will be considered on a case-by-case basis.” Specific criteria for exceptions were not spelled out.
In the end, there were more than a few surprises when denial letters went out in recent months, and scores of appeals have been filed. There’s “a lot of tension and a lot of confusion” about why some people with practice gaps during the 4 years were approved, but others were denied. There’s been “a lack of transparency on the ABP’s part,” said H. Barrett Fromme, MD, section chief of pediatric hospital medicine and a professor of pediatrics at the University of Chicago.
“The standard has to be reasonable”
There are concerns about the availability of fellowship slots and other issues, but the 4-year rule – instead of averaging clinical hours over 4 or 5 years, for instance – is the main sticking point. It’s a gender issue because “women take maternity; women move with their spouse; women take care of elders; women tend to be in these roles that require time off” more than men do, Dr. Fromme said.
Until the board releases its data, the gender breakdown of the denials and the degree to which practice gaps due to such issues led to them is unknown. There’s concern that women have been unfairly penalized.
The storm was set off on the discussion board this summer by stories from physicians such as Chandani DeZure, MD, a pediatric hospitalist currently working in the neonatal ICU at Stanford (Calif.) University. She was denied a seat at the table in November, appealed, and was denied again.
She was a full-time pediatric hospitalist at Children’s National Medical Center in Washington, from 2014, when she graduated residency, until Oct. 2018, when her husband, also a doctor, was offered a promising research position in California, and “we decided to take it,” Dr. DeZure said.
They moved to California with their young son in November. Dr. DeZure got her California medical license in 6 weeks, was hired by Stanford in January, and started her new postion in mid-April.
Because of the move, she worked only 3.5 years in the board’s 4 year practice window, but, as is common with young physicians, that time was spent in direct patient care, for a total of over 6,000 hours.
“How is that not good enough? How is a person that worked 500 hours with patients for 4 years” – for a total of 2,000 hours – “better qualified than someone who worked 100% for 3 and a half years? Nobody is saying there shouldn’t be a standard, but the standard has to be reasonable,” Dr. DeZure said.
“Illegal regardless of intent”
It’s situations like Dr. DeZure’s that led to the petition. One of demands is that ABP “revise the practice pathway criteria to be more inclusive of applicants with interrupted practice and varied clinical experience, to include clear-cut parameters rather than considering these applications on a closed-door ‘case-by-case basis...at the discretion of the ABP.’ ” Also, the petition asks the board to “clarify the appeals process and improve responsiveness to appeals and inquiries regarding denials.”
As ABP noted in its statement, however, the major demand is that the board “facilitate a timely analysis to determine if gender bias is present.” The petition noted that signers “do not suspect intentional bias on the part of the ABP; however, if gender bias is present it is unethical and potentially illegal regardless of intent.”
For now, the perception is that the board has “a hard 48-month rule” with not many exceptions; there are people who are “very concerned that, ‘Oh my gosh, I can’t have children for 4 years because I won’t be able to sit for the boards.’ No one should ever have to have that in their head,” Dr. Fromme said. At this point, it seems that 3 months off for maternity is being grandfathered in, but perhaps not 6 months for a second child; no one knows for sure.
Dr. DeZure, meanwhile, continues to study for the board exam, just in case.
Looking back over the past year, she said “I could have somehow picked up one shift a week moonlighting that would have kept me eligible, but the [board] didn’t respond to me” when contacted about her situation during the California move.
“The other option was for me was to live cross country from my husband with a small child,” she said.
More than 1,625 pediatricians have applied to take the first pediatric hospitalist certification exam in November 2019, and approximately 93% of them have been accepted, according to a statement from the American Board of Pediatrics.
It was the rejection of the 7%, however, that set off a firestorm on the electronic discussion board for American Academy of Pediatrics (AAP) hospital medicine this summer, and led to a petition to the board to revise its eligibility requirements, ensure that the requirements are fair to women, and bring transparency to its decision process. The petition has more than 1,400 signatures.
Seattle Children’s Hospital and Yale New Haven (Conn.) Children’s Hospital have both said they will not consider board certification in hiring decisions until the situation is resolved.
The American Board of Pediatrics (ABP) declined an interview request pending its formal response to the Aug. 6 petition, but in a statement to this news organization, executive vice president Suzanne Woods, MD, said, “The percentage of women and men meeting the eligibility requirements for the exam did not differ. We stress this point because a concern about possible gender bias appears to have been the principal reason for this ... petition, and we wanted to offer immediate reassurance that no unintended bias has occurred.”
“We are carefully considering the requests and will release detailed data to hospitalists on the AAP’s [pediatric hospital medicine (PHM) electronic discussion board] ... and on the ABP’s website. We are conferring with ABP PHM subboard members as well as leaders from our volunteer community. We expect to provide a thoughtful response within the next 3 weeks,” Dr. Woods said in the Aug. 15 statement.
“Case-by-case” exceptions
The backstory is that, for better or worse depending on who you talk to, pediatric hospital medicine is becoming a board certified subspecialty. A fellowship will be required to sit for the exam after a few years, which is standard for subspecialties.
What’s generated concern is how the board is grandfathering current pediatric hospitalists into certification via a “practice pathway” until the fellowship requirement takes hold after 2023.
To qualify for the November test, hospitalists had to complete 4 years of full-time practice by June 30, 2019, which has been understood to mean 48 months of continual employment. At least 50% of that time had to be devoted to “professional activities ... related to the care of hospitalized children,” and at least 25% of that “devoted to direct patient care.” Assuming about 2,000 work hours per year, it translated to “450-500 hours” of direct patient care “per year over the most recent four years” to sit for the test, the board said.
“For individuals who have interrupted practice during the most recent four years for family leave or other such circumstances, an exception may be considered if there is substantial prior experience in pediatric hospital medicine. ... Such exceptions are made at the discretion of the ABP and will be considered on a case-by-case basis.” Specific criteria for exceptions were not spelled out.
In the end, there were more than a few surprises when denial letters went out in recent months, and scores of appeals have been filed. There’s “a lot of tension and a lot of confusion” about why some people with practice gaps during the 4 years were approved, but others were denied. There’s been “a lack of transparency on the ABP’s part,” said H. Barrett Fromme, MD, section chief of pediatric hospital medicine and a professor of pediatrics at the University of Chicago.
“The standard has to be reasonable”
There are concerns about the availability of fellowship slots and other issues, but the 4-year rule – instead of averaging clinical hours over 4 or 5 years, for instance – is the main sticking point. It’s a gender issue because “women take maternity; women move with their spouse; women take care of elders; women tend to be in these roles that require time off” more than men do, Dr. Fromme said.
Until the board releases its data, the gender breakdown of the denials and the degree to which practice gaps due to such issues led to them is unknown. There’s concern that women have been unfairly penalized.
The storm was set off on the discussion board this summer by stories from physicians such as Chandani DeZure, MD, a pediatric hospitalist currently working in the neonatal ICU at Stanford (Calif.) University. She was denied a seat at the table in November, appealed, and was denied again.
She was a full-time pediatric hospitalist at Children’s National Medical Center in Washington, from 2014, when she graduated residency, until Oct. 2018, when her husband, also a doctor, was offered a promising research position in California, and “we decided to take it,” Dr. DeZure said.
They moved to California with their young son in November. Dr. DeZure got her California medical license in 6 weeks, was hired by Stanford in January, and started her new postion in mid-April.
Because of the move, she worked only 3.5 years in the board’s 4 year practice window, but, as is common with young physicians, that time was spent in direct patient care, for a total of over 6,000 hours.
“How is that not good enough? How is a person that worked 500 hours with patients for 4 years” – for a total of 2,000 hours – “better qualified than someone who worked 100% for 3 and a half years? Nobody is saying there shouldn’t be a standard, but the standard has to be reasonable,” Dr. DeZure said.
“Illegal regardless of intent”
It’s situations like Dr. DeZure’s that led to the petition. One of demands is that ABP “revise the practice pathway criteria to be more inclusive of applicants with interrupted practice and varied clinical experience, to include clear-cut parameters rather than considering these applications on a closed-door ‘case-by-case basis...at the discretion of the ABP.’ ” Also, the petition asks the board to “clarify the appeals process and improve responsiveness to appeals and inquiries regarding denials.”
As ABP noted in its statement, however, the major demand is that the board “facilitate a timely analysis to determine if gender bias is present.” The petition noted that signers “do not suspect intentional bias on the part of the ABP; however, if gender bias is present it is unethical and potentially illegal regardless of intent.”
For now, the perception is that the board has “a hard 48-month rule” with not many exceptions; there are people who are “very concerned that, ‘Oh my gosh, I can’t have children for 4 years because I won’t be able to sit for the boards.’ No one should ever have to have that in their head,” Dr. Fromme said. At this point, it seems that 3 months off for maternity is being grandfathered in, but perhaps not 6 months for a second child; no one knows for sure.
Dr. DeZure, meanwhile, continues to study for the board exam, just in case.
Looking back over the past year, she said “I could have somehow picked up one shift a week moonlighting that would have kept me eligible, but the [board] didn’t respond to me” when contacted about her situation during the California move.
“The other option was for me was to live cross country from my husband with a small child,” she said.
Anticoagulant therapy for AFib in patients with end-stage renal disease
Warfarin or apixaban are sensible options
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Warfarin or apixaban are sensible options
Warfarin or apixaban are sensible options
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
Case
A 78-year-old woman with end-stage renal disease (ESRD) is hospitalized with cellulitis and is incidentally found to be in atrial fibrillation. She does not have a history of mitral stenosis, nor does she have a prosthetic valve. She does have a history of hypertension, diabetes, and prior stroke without residual deficits.
After counseling her about the risk of stroke associated with atrial fibrillation (AFib) she makes it clear she is interested in pharmacologic therapy to minimize her risk of stroke and asks what medication you would recommend for anticoagulation.
Brief overview of the issue
Anticoagulation for AFib is indicated for stroke prophylaxis in patients with an elevated risk of stroke. The CHA2DS2-VASc score is useful in calculating an individual patient’s risk of stroke and as a decision tool to determine who would benefit from anticoagulation, and it is recommended in the American Heart Association guidelines.1
Low-risk patients (CHA2DS2-VASc score of 0 in men or 1 in women) should not be started on anticoagulation for stroke prophylaxis. For anyone with a risk factor, other than being female, anticoagulation is indicated and should be considered.
The guideline recommends anticoagulant therapy, not antiplatelet agents. For most of the recent past, this has meant a vitamin K antagonist (warfarin) or sometimes a low-molecular-weight heparin injected subcutaneously. Over the past decade, however, with the approval of multiple direct oral anticoagulants (DOACs), nonwarfarin oral anticoagulation has grown in popularity as the prophylactic medication of choice.2
While the data for patients with preserved renal function is robust, there is far less data to guide decision making for patients with end-stage renal disease.
Overview of the data
Until the introduction of DOACs, warfarin was the main agent used for stroke prophylaxis in patients with end-stage kidney disease and AFib. Professional guidelines favored warfarin for these patients who were mostly excluded from DOAC trials. Specialized conferences also looked at this issue.
The Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference, which reviewed chronic kidney disease and arrhythmias, noted that there were no randomized controlled trials that examined the efficacy and safety of anticoagulation in chronic kidney disease patients with estimated creatinine clearance less than 30 mL/min. They remarked that there was insufficient high-quality evidence to recommend warfarin for the prevention of stroke in patients with AFib and dialysis-dependent chronic kidney disease.
Since, according to other trials, DOACs had better safety profiles in other populations, the conference noted that lower-dose apixaban (2.5 mg orally twice daily) or rivaroxaban (15 mg daily) may be considered in this population until clinical safety data were available. Furthermore, the conference recommended that these patients be treated with a multidisciplinary approach in regards to anticoagulation and have an annual reevaluation of treatment goals, along with a risk-benefit assessment.3
Since the publication of the 2018 AHA guidelines and the guidance document that resulted from the KDIGO conference, additional research has been published comparing anticoagulation with a DOAC versus warfarin for AFib in patients with ESRD.
“Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States” was an observational, retrospective, cohort study that compared outcomes in dialysis patients who took warfarin for AFib with those who took apixaban.4 Patients’ data was taken from the U.S. Renal Data System database and were included in the final analysis if they had ESRD, a recent diagnosis of AFib or atrial flutter, and a new prescription for either warfarin or apixaban. Outcome measures were stroke or systemic embolism, major bleeding (critical site, transfusion, or death), gastrointestinal bleeding, intracranial bleeding, or death. Drug usage and compliance were assessed using Medicare Part D prescription information.
A total of 25,523 patients met the inclusion/exclusion criteria and had taken either warfarin (n = 23,172) or apixaban (n = 2,351). To account for selection bias in these cohorts, a subset of the warfarin patients was selected based on prognostic score matching. The prognostic score was calculated from the baseline characteristics (which included age, stroke history, diabetes, smoking, antiplatelet medication, liver disease, prior bleeding, and CHA2DS2-VASc score). Kaplan-Meier and Cox regression analysis were used to give hazard ratios and 95% confidence intervals for each outcome measure. Prespecified subgroup analyses were conducted to compare apixaban doses, where 44% were prescribed 5 mg b.i.d. and 56% were prescribed 2.5 mg b.i.d..
In the study, patients in the apixaban group had a significantly lower risk of major bleeding as compared with the warfarin group (HR, 0.72; 95% CI, 0.59-0.87; P less than .001) with overall high rates of major bleeding in both groups at 19.7 and 22.9 per 100 patient-years in the apixaban group and warfarin group, respectively. There was no difference in the rate of stroke/systemic embolism between patients receiving apixaban and warfarin (HR, 0.88; 95% CI, 0.69-1.12; P = .29). There was a nonsignificant trend toward decreased risk of GI bleeding in the apixaban group and no significant differences between the groups in the rates of intracranial bleeding. Apixaban was also associated with a nonsignificant trend toward lower risk of mortality (HR, 0.85; 95% CI, 0.71-1.01; P = .06).
Notably, censoring rates because of expired prescriptions or a 1-month gap between prescriptions were high in both groups and the majority of censoring occurred within the first 12 months. Additionally, in dose specific analyses, patients receiving the 5-mg, twice-daily dose were found to have statistically significant decreases in risk of stroke/systemic embolism (P = .035) and mortality (P = .005) as compared with the 2.5-mg, twice-daily dose without significant differences in GI or intracranial bleeding.
There are three ongoing, open-label, randomized, controlled trials examining anticoagulation for nonvalvular AFib in patients with ESRD on hemodialysis with two comparing apixaban to warfarin (or derivative) and the other warfarin versus no anticoagulation.5 All trials are in adult patients with documented AFib and CHA2DS2-VASc score of at least 2. AKADIA (Germany based) plans to enroll 222 patients and compares a vitamin K antagonist (INR goal, 2-3) with 2.5-mg b.i.d. apixaban patients with ESRD on hemodialysis for at least 3 months with primary outcome of major and clinically relevant nonmajor bleeding and secondary outcome of thromboembolic events, as well as apixaban levels pre- and post hemodialysis.
RENAL-AF (U.S. based) plans to enrolled 762 patients and compares 5-mg b.i.d. apixaban (with 2.5 mg for selected patients) with warfarin in people of chronic hemodialysis with primary outcome of days to first major or clinically relevant nonmajor bleeding event and secondary outcome of stroke, systemic embolism, mortality, adherence and plasma apixaban levels. AVKDIAL (France based) plans to enroll 855 patients and compares no anticoagulation with vitamin K antagonists in patients on hemodialysis for at least 1 month, with primary outcome of cumulative incidence of severe bleeding and thrombosis.
Application of the data to our original case
Our patient is Medicare age with ESRD and newly diagnosed nonvalvular AFib. Recent data suggests apixaban could be used for stroke prevention instead of the prior standard of care, warfarin. This approach is supported in the 2019 guidelines.1
Patients with ESRD have an increased risk of bleeding and apixaban was shown to have less bleeding complications than warfarin in this analysis. However, only standard-dose apixaban was associated with a statistically significant lower risk of stroke/systemic embolism, major bleeding, and death. Reduced-dose apixaban had a lower risk of major bleeding but no difference for stroke/systemic embolism or death. Reduced-dose apixaban is used for patients who have two out of the following three criteria: aged at least 80 years, weight of at least 60 kg, and creatinine of at least 1.5 mg/dL. Therefore, many Medicare-age patients with ESRD would not be indicated for the dose of apixaban that was shown to improve the most important outcomes of stroke/SE and death.
It may still be beneficial to use apixaban in this patient since it appears to work as well as warfarin for stroke/systemic embolism prevention with less bleeding complications.
Bottom line
For patients who have decided to pursue an anticoagulation strategy for stroke prevention in AFib and have end-stage renal disease, either warfarin or apixaban are sensible options.
Dr. Farber is a medical instructor at Duke University Health System in Durham, N.C. Dr. Stafford is a medical instructor at Duke University. Dr. Sata is assistant professor of medicine at Duke University. Dr. Abdo and Dr. Menon are hospitalists at Duke University. Dr. Brooks is assistant professor of medicine at Duke University. Dr. Wachter is associate medical director at Duke Regional Hospital and assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and assistant professor of medicine at Duke University.
References
1. January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
2. Lippi G et al. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017 Aug; 5(16):322. doi: 10.21037/atm.2017.06.65.
3. Turakhia MP et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018 Jun 21;39(24):2314-25. doi: 10.1093/eurheartj/ehy060.
4. Siontis KC et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018 Oct 9;138(15):1519-29. doi: 10.1161/CIRCULATIONAHA.118.035418.
5. Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit-to-risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Key points
- According to 2019 American Heart Association guidelines, warfarin or apixaban are reasonable options for stroke prevention for patients who have end-stage renal disease and who plan for anticoagulation because of atrial fibrillation.
- Recent observational data suggests that apixaban may be safer than warfarin in this population.
- Several randomized, controlled trials are ongoing that may help determine the optimal agent to use in this setting.
- Until more definitive data is available, a reasonable approach is to discuss the risks and benefits of various treatment strategies with patients, and engage a multidisciplinary team (cardiologist, nephrologist, primary care provider, pharmacist) in the decision making process.
Additional reading
January CT et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;139. doi: 1161/CIR.0000000000000665.
Nigwekar SU et al. Long-term anticoagulation for patient receiving dialysis: Tilting the benefit to risk ratio? Circulation. 2018 Oct 9;138(15):1530-3. doi: 10.1161/CIRCULATIONAHA.118.037091.
Garlo KG et al. Demystifying the benefits and harms of anticoagulation for atrial fibrillation in chronic kidney disease. Clin J Am Soc Nephrol 2019;14:125-36. doi: 10.2215/CJN.06430518.
Quiz
Two days ago you admitted a 72-year-old woman with end-stage renal disease on dialysis who had developed new-onset atrial fibrillation causing a mild acute diastolic congestive heart failure exacerbation. Transthoracic ECG showed a preserved left ventricular ejection fraction and no significant valvular disease. After two sessions of dialysis in the hospital and initiation of a beta-blocker for control of her heart rate, she is stable and ready for discharge. Her discharge weight is 75 kg.
Which of the following recommendations should you make to this patient regarding anticoagulation for prevention of stroke and systemic embolism from atrial fibrillation?
A. Take warfarin with a international normalized ratio goal of 2.5.
B. Take apixaban 2.5 mg twice a day.
C. Take apixaban 5 mg twice a day.
D. Discuss the risks/benefits of various treatment approaches with the patient, and involve the hospital pharmacist as well as the patient’s nephrologist, cardiologist, and/or primary care provider in the decision making process to reach a consensus and to ensure a safe follow-up plan.
The best answer is D. While A, B, and C are all reasonable approaches based on the available data and current guidelines, the best approach is to involve the patient and the multidisciplinary team in the decision making process. When more clinical trial data becomes available in the future, the optimal approach to managing patients such as this one may become clearer, but until then it makes sense to take into account individual patient characteristics and patient preferences.
FUO, pneumonia often distinguishes influenza from RSV in hospitalized young children
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.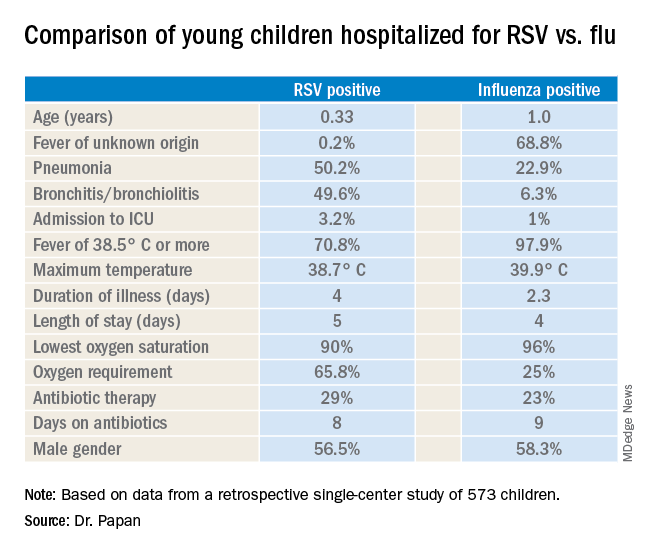
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
REPORTING FROM ESPID 2019
Treating children with Kawasaki disease and coronary enlargement
IVIG plus steroids or infliximab, or IVIG alone?
Clinical question
Does use of corticosteroids or infliximab in addition to intravenous immunoglobulin improve cardiac outcomes in children with Kawasaki disease and enlarged coronary arteries?
Background
Kawasaki disease is a medium-vessel vasculitis primarily of young children. While the underlying cause remains unknown, treatment with intravenous immunoglobulin (IVIG) substantially lowers the risk of coronary artery aneurysms (CAA), the most serious sequelae of Kawasaki disease. Recent studies have suggested that – in cases of high-risk or treatment-resistant Kawasaki disease – using an immunomodulator, such as a corticosteroid or a TNF-alpha blocker, may improve outcomes, though these studies involved relatively small and homogeneous patient populations. It is unknown if these medications could prevent progression of CAA.
Study design
Retrospective multicenter study.
Setting
Two freestanding children’s hospitals and one mother-child hospital.
Synopsis
The study identified 121 children diagnosed with Kawasaki disease with CAA (z score 2.5-10) from 2008 through 2017 treated at the three study hospitals. Children with giant CAA at the time of diagnosis (z score greater than 10) or significant preexisting congenital heart disease were excluded.
All study hospitals had protocols for treatment of Kawasaki disease: Center 1 used IVIG and corticosteroids, Center 2 used IVIG and infliximab, and Center 3 used IVIG alone. Patients at all centers also received aspirin. Center 1 used methylprednisolone IV initially, changing to oral prednisolone after clinical improvement. The researchers reviewed the charts of each patient and classified them as having complete or incomplete Kawasaki disease. They assigned z scores for CAA size based on both initial and follow-up echocardiograms. The primary outcome was change in z score of CAA over the first year.
The population of patients treated at each center was significantly different. Center 1 reported older patients (median age 2.6 vs. 2.0 and 1.1), as well as a higher rate of male patients (83% vs. 77% and 58%). However, there was no difference in baseline z scores between centers. Patients who initially received IVIG and corticosteroids were less likely to require additional therapy because of persistent fever versus those receiving IVIG only, or IVIG and infliximab (0% vs. 21% vs. 14%, P = .03).
Patients receiving IVIG and corticosteroids, or IVIG and infliximab, were less likely to have progression of CAA size, with 23% and 24% having an increase in z score of more than 1 versus 58% of those who received IVIG alone. No group had significant differences in maximum z score, the rate of giant aneurysms, or the rate of regression of CAA.
Bottom line
Using IVIG + corticosteroids or IVIG + infliximab versus IVIG alone for children with Kawasaki disease with coronary artery aneurysms decreases the rate of aneurysm enlargement.
Citation
Dionne A et al. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. May 2019:e20183341. doi: 10.1542/peds.2018-3341.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and clinical assistant professor of pediatrics at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia.
IVIG plus steroids or infliximab, or IVIG alone?
IVIG plus steroids or infliximab, or IVIG alone?
Clinical question
Does use of corticosteroids or infliximab in addition to intravenous immunoglobulin improve cardiac outcomes in children with Kawasaki disease and enlarged coronary arteries?
Background
Kawasaki disease is a medium-vessel vasculitis primarily of young children. While the underlying cause remains unknown, treatment with intravenous immunoglobulin (IVIG) substantially lowers the risk of coronary artery aneurysms (CAA), the most serious sequelae of Kawasaki disease. Recent studies have suggested that – in cases of high-risk or treatment-resistant Kawasaki disease – using an immunomodulator, such as a corticosteroid or a TNF-alpha blocker, may improve outcomes, though these studies involved relatively small and homogeneous patient populations. It is unknown if these medications could prevent progression of CAA.
Study design
Retrospective multicenter study.
Setting
Two freestanding children’s hospitals and one mother-child hospital.
Synopsis
The study identified 121 children diagnosed with Kawasaki disease with CAA (z score 2.5-10) from 2008 through 2017 treated at the three study hospitals. Children with giant CAA at the time of diagnosis (z score greater than 10) or significant preexisting congenital heart disease were excluded.
All study hospitals had protocols for treatment of Kawasaki disease: Center 1 used IVIG and corticosteroids, Center 2 used IVIG and infliximab, and Center 3 used IVIG alone. Patients at all centers also received aspirin. Center 1 used methylprednisolone IV initially, changing to oral prednisolone after clinical improvement. The researchers reviewed the charts of each patient and classified them as having complete or incomplete Kawasaki disease. They assigned z scores for CAA size based on both initial and follow-up echocardiograms. The primary outcome was change in z score of CAA over the first year.
The population of patients treated at each center was significantly different. Center 1 reported older patients (median age 2.6 vs. 2.0 and 1.1), as well as a higher rate of male patients (83% vs. 77% and 58%). However, there was no difference in baseline z scores between centers. Patients who initially received IVIG and corticosteroids were less likely to require additional therapy because of persistent fever versus those receiving IVIG only, or IVIG and infliximab (0% vs. 21% vs. 14%, P = .03).
Patients receiving IVIG and corticosteroids, or IVIG and infliximab, were less likely to have progression of CAA size, with 23% and 24% having an increase in z score of more than 1 versus 58% of those who received IVIG alone. No group had significant differences in maximum z score, the rate of giant aneurysms, or the rate of regression of CAA.
Bottom line
Using IVIG + corticosteroids or IVIG + infliximab versus IVIG alone for children with Kawasaki disease with coronary artery aneurysms decreases the rate of aneurysm enlargement.
Citation
Dionne A et al. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. May 2019:e20183341. doi: 10.1542/peds.2018-3341.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and clinical assistant professor of pediatrics at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia.
Clinical question
Does use of corticosteroids or infliximab in addition to intravenous immunoglobulin improve cardiac outcomes in children with Kawasaki disease and enlarged coronary arteries?
Background
Kawasaki disease is a medium-vessel vasculitis primarily of young children. While the underlying cause remains unknown, treatment with intravenous immunoglobulin (IVIG) substantially lowers the risk of coronary artery aneurysms (CAA), the most serious sequelae of Kawasaki disease. Recent studies have suggested that – in cases of high-risk or treatment-resistant Kawasaki disease – using an immunomodulator, such as a corticosteroid or a TNF-alpha blocker, may improve outcomes, though these studies involved relatively small and homogeneous patient populations. It is unknown if these medications could prevent progression of CAA.
Study design
Retrospective multicenter study.
Setting
Two freestanding children’s hospitals and one mother-child hospital.
Synopsis
The study identified 121 children diagnosed with Kawasaki disease with CAA (z score 2.5-10) from 2008 through 2017 treated at the three study hospitals. Children with giant CAA at the time of diagnosis (z score greater than 10) or significant preexisting congenital heart disease were excluded.
All study hospitals had protocols for treatment of Kawasaki disease: Center 1 used IVIG and corticosteroids, Center 2 used IVIG and infliximab, and Center 3 used IVIG alone. Patients at all centers also received aspirin. Center 1 used methylprednisolone IV initially, changing to oral prednisolone after clinical improvement. The researchers reviewed the charts of each patient and classified them as having complete or incomplete Kawasaki disease. They assigned z scores for CAA size based on both initial and follow-up echocardiograms. The primary outcome was change in z score of CAA over the first year.
The population of patients treated at each center was significantly different. Center 1 reported older patients (median age 2.6 vs. 2.0 and 1.1), as well as a higher rate of male patients (83% vs. 77% and 58%). However, there was no difference in baseline z scores between centers. Patients who initially received IVIG and corticosteroids were less likely to require additional therapy because of persistent fever versus those receiving IVIG only, or IVIG and infliximab (0% vs. 21% vs. 14%, P = .03).
Patients receiving IVIG and corticosteroids, or IVIG and infliximab, were less likely to have progression of CAA size, with 23% and 24% having an increase in z score of more than 1 versus 58% of those who received IVIG alone. No group had significant differences in maximum z score, the rate of giant aneurysms, or the rate of regression of CAA.
Bottom line
Using IVIG + corticosteroids or IVIG + infliximab versus IVIG alone for children with Kawasaki disease with coronary artery aneurysms decreases the rate of aneurysm enlargement.
Citation
Dionne A et al. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. May 2019:e20183341. doi: 10.1542/peds.2018-3341.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and clinical assistant professor of pediatrics at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia.
Obtain proper reimbursements with more effective documentation and coding
SHM webinar series provides hospitalists with best practices to improve accuracy and compliance
Hospitalists cannot bill for everything they do, but they can document and code to obtain appropriate reimbursements. It is important for hospitalists to know the factors that influence coding to ensure accuracy and compliance.
The Society of Hospital Medicine developed the Clinical Documentation & Coding for Hospitalists webinar series (formerly known as CODE-H) to provide hospitalists with the latest information on best practices in coding, documentation, and compliance from nationally recognized experts, along with the opportunity to claim CME.
The Hospitalist recently spoke with Carol Pohlig, BSN, RN, CPC, ACS, course director of the webinar series and a coding and documentation expert at the University of Pennsylvania Medical Center in Philadelphia. She was instrumental in developing the content in the series to ensure it was specifically designed to address challenges regularly faced by hospitalists.
What inspired the creation of Clinical Documentation & Coding for Hospitalists?
Providers are so busy trying to keep up with regulations for their institution, such as malpractice and quality issues, that the focus isn’t always on the documentation required for reimbursement. The creation of the series rose out of a need for providers to understand key issues related to documentation and billing and some of the hurdles that they need to overcome – or need to be aware of in the first place.
This series brings awareness and solutions to some of these problems. It is available on an ongoing basis, so viewers can move at their own pace. Given the wealth of information in the series, it made sense to create it in this format.
What are some common challenges that hospitalists encounter when coding, and how does this webinar series help to address these challenges?
Some common challenges relate to concurrent care or comanagement. Hospitalists are hired to be the gatekeepers – the ones overseeing patient care. When other consultants are on board, they wind up sharing responsibilities, which can muddy the waters at times, especially with billing and coding. It is important for hospitalists to understand their role in comanagement and, in turn, how the payers view their role.
We highlight everything – including requirements for history, exam, and medical decision making – and review each component in depth. We also discuss billing based on these key components or, when it is appropriate, billing based on time. However, when billing time-based services, you have to meet certain qualifications because it is different from the standard way of reporting, which is something we break down in the series.
Related to mitigating risk, EMRs and their copy and paste function is another topic we delve into. It’s easy to copy and paste and pull forward information from a previous note to help save time. However, it is important to understand what the ramifications are. Each of these copied and pasted encounters must be modified to make it applicable to the current day’s patient and ensure care is not being misrepresented.
Those are just a few of the items covered, but we believe that each of the eight modules in the series offers something unique that will help improve documentation and coding practices.
How can this webinar series go beyond the hospital medicine care team and more broadly affect the institution as a whole?
Hospitalists are often involved in a number of different categories of services, including observation and same-day admission/discharge. The series reviews rules and challenges specific to those sites of service, which on a broader scale, impact not only providers in other service lines but also those who work in the revenue cycle at the parent institution. How each of these parties understands the nuances explained in the series can directly affect the successful processing of the submitted claims.
In addition, interpretation of rules when it comes to coding and documentation can vary at a local level. We raise awareness of local interpretations to ensure everyone involved in the documentation and coding process knows things to look out for when reading rules. You might think it means one thing when, in reality, it could mean another. With this series, everyone involved with billing and coding can reflect on the implications that incorrect or inaccurate coding may have on their hospital.
Who would benefit from viewing this webinar series?
Although we primarily had hospitalists of all types – including physicians, nurse practitioners, and physician assistants – in mind during the development of course content, anyone who works as a practice manager, biller, coder, or internal auditor has the potential to benefit from the series. If they understand broader challenges in coding, it could help them proactively prevent issues throughout the process with more accurate documentation that could reduce claims denials.
Let SHM’s Clinical Documentation & Coding for Hospitalists webinar series bolster your and your team’s accuracy and compliance. Individual and group subscriptions are available. For more information, visit hospitalmedicine.org/coding.
SHM webinar series provides hospitalists with best practices to improve accuracy and compliance
SHM webinar series provides hospitalists with best practices to improve accuracy and compliance
Hospitalists cannot bill for everything they do, but they can document and code to obtain appropriate reimbursements. It is important for hospitalists to know the factors that influence coding to ensure accuracy and compliance.
The Society of Hospital Medicine developed the Clinical Documentation & Coding for Hospitalists webinar series (formerly known as CODE-H) to provide hospitalists with the latest information on best practices in coding, documentation, and compliance from nationally recognized experts, along with the opportunity to claim CME.
The Hospitalist recently spoke with Carol Pohlig, BSN, RN, CPC, ACS, course director of the webinar series and a coding and documentation expert at the University of Pennsylvania Medical Center in Philadelphia. She was instrumental in developing the content in the series to ensure it was specifically designed to address challenges regularly faced by hospitalists.
What inspired the creation of Clinical Documentation & Coding for Hospitalists?
Providers are so busy trying to keep up with regulations for their institution, such as malpractice and quality issues, that the focus isn’t always on the documentation required for reimbursement. The creation of the series rose out of a need for providers to understand key issues related to documentation and billing and some of the hurdles that they need to overcome – or need to be aware of in the first place.
This series brings awareness and solutions to some of these problems. It is available on an ongoing basis, so viewers can move at their own pace. Given the wealth of information in the series, it made sense to create it in this format.
What are some common challenges that hospitalists encounter when coding, and how does this webinar series help to address these challenges?
Some common challenges relate to concurrent care or comanagement. Hospitalists are hired to be the gatekeepers – the ones overseeing patient care. When other consultants are on board, they wind up sharing responsibilities, which can muddy the waters at times, especially with billing and coding. It is important for hospitalists to understand their role in comanagement and, in turn, how the payers view their role.
We highlight everything – including requirements for history, exam, and medical decision making – and review each component in depth. We also discuss billing based on these key components or, when it is appropriate, billing based on time. However, when billing time-based services, you have to meet certain qualifications because it is different from the standard way of reporting, which is something we break down in the series.
Related to mitigating risk, EMRs and their copy and paste function is another topic we delve into. It’s easy to copy and paste and pull forward information from a previous note to help save time. However, it is important to understand what the ramifications are. Each of these copied and pasted encounters must be modified to make it applicable to the current day’s patient and ensure care is not being misrepresented.
Those are just a few of the items covered, but we believe that each of the eight modules in the series offers something unique that will help improve documentation and coding practices.
How can this webinar series go beyond the hospital medicine care team and more broadly affect the institution as a whole?
Hospitalists are often involved in a number of different categories of services, including observation and same-day admission/discharge. The series reviews rules and challenges specific to those sites of service, which on a broader scale, impact not only providers in other service lines but also those who work in the revenue cycle at the parent institution. How each of these parties understands the nuances explained in the series can directly affect the successful processing of the submitted claims.
In addition, interpretation of rules when it comes to coding and documentation can vary at a local level. We raise awareness of local interpretations to ensure everyone involved in the documentation and coding process knows things to look out for when reading rules. You might think it means one thing when, in reality, it could mean another. With this series, everyone involved with billing and coding can reflect on the implications that incorrect or inaccurate coding may have on their hospital.
Who would benefit from viewing this webinar series?
Although we primarily had hospitalists of all types – including physicians, nurse practitioners, and physician assistants – in mind during the development of course content, anyone who works as a practice manager, biller, coder, or internal auditor has the potential to benefit from the series. If they understand broader challenges in coding, it could help them proactively prevent issues throughout the process with more accurate documentation that could reduce claims denials.
Let SHM’s Clinical Documentation & Coding for Hospitalists webinar series bolster your and your team’s accuracy and compliance. Individual and group subscriptions are available. For more information, visit hospitalmedicine.org/coding.
Hospitalists cannot bill for everything they do, but they can document and code to obtain appropriate reimbursements. It is important for hospitalists to know the factors that influence coding to ensure accuracy and compliance.
The Society of Hospital Medicine developed the Clinical Documentation & Coding for Hospitalists webinar series (formerly known as CODE-H) to provide hospitalists with the latest information on best practices in coding, documentation, and compliance from nationally recognized experts, along with the opportunity to claim CME.
The Hospitalist recently spoke with Carol Pohlig, BSN, RN, CPC, ACS, course director of the webinar series and a coding and documentation expert at the University of Pennsylvania Medical Center in Philadelphia. She was instrumental in developing the content in the series to ensure it was specifically designed to address challenges regularly faced by hospitalists.
What inspired the creation of Clinical Documentation & Coding for Hospitalists?
Providers are so busy trying to keep up with regulations for their institution, such as malpractice and quality issues, that the focus isn’t always on the documentation required for reimbursement. The creation of the series rose out of a need for providers to understand key issues related to documentation and billing and some of the hurdles that they need to overcome – or need to be aware of in the first place.
This series brings awareness and solutions to some of these problems. It is available on an ongoing basis, so viewers can move at their own pace. Given the wealth of information in the series, it made sense to create it in this format.
What are some common challenges that hospitalists encounter when coding, and how does this webinar series help to address these challenges?
Some common challenges relate to concurrent care or comanagement. Hospitalists are hired to be the gatekeepers – the ones overseeing patient care. When other consultants are on board, they wind up sharing responsibilities, which can muddy the waters at times, especially with billing and coding. It is important for hospitalists to understand their role in comanagement and, in turn, how the payers view their role.
We highlight everything – including requirements for history, exam, and medical decision making – and review each component in depth. We also discuss billing based on these key components or, when it is appropriate, billing based on time. However, when billing time-based services, you have to meet certain qualifications because it is different from the standard way of reporting, which is something we break down in the series.
Related to mitigating risk, EMRs and their copy and paste function is another topic we delve into. It’s easy to copy and paste and pull forward information from a previous note to help save time. However, it is important to understand what the ramifications are. Each of these copied and pasted encounters must be modified to make it applicable to the current day’s patient and ensure care is not being misrepresented.
Those are just a few of the items covered, but we believe that each of the eight modules in the series offers something unique that will help improve documentation and coding practices.
How can this webinar series go beyond the hospital medicine care team and more broadly affect the institution as a whole?
Hospitalists are often involved in a number of different categories of services, including observation and same-day admission/discharge. The series reviews rules and challenges specific to those sites of service, which on a broader scale, impact not only providers in other service lines but also those who work in the revenue cycle at the parent institution. How each of these parties understands the nuances explained in the series can directly affect the successful processing of the submitted claims.
In addition, interpretation of rules when it comes to coding and documentation can vary at a local level. We raise awareness of local interpretations to ensure everyone involved in the documentation and coding process knows things to look out for when reading rules. You might think it means one thing when, in reality, it could mean another. With this series, everyone involved with billing and coding can reflect on the implications that incorrect or inaccurate coding may have on their hospital.
Who would benefit from viewing this webinar series?
Although we primarily had hospitalists of all types – including physicians, nurse practitioners, and physician assistants – in mind during the development of course content, anyone who works as a practice manager, biller, coder, or internal auditor has the potential to benefit from the series. If they understand broader challenges in coding, it could help them proactively prevent issues throughout the process with more accurate documentation that could reduce claims denials.
Let SHM’s Clinical Documentation & Coding for Hospitalists webinar series bolster your and your team’s accuracy and compliance. Individual and group subscriptions are available. For more information, visit hospitalmedicine.org/coding.
Dapagliflozin meets primary endpoint in the DAPA-HF trial
The SGLT2 inhibitor dapagliflozin (Farxiga) successfully met the primary endpoint of the phase 3 DAPA-HF trial in patients with heart failure, according to a press release from AstraZeneca.
DAPA-HF is an international, multicenter, parallel group, randomized, double-blind trial in patients with heart failure and reduced ejection fraction with or without type 2 diabetes. Patients in the study received either 10 mg dapagliflozin or placebo, and the primary outcome was time to a worsening heart failure event or to cardiovascular death. Patients who received dapagliflozin had a statistically significant reduction in incidence of cardiovascular death and an increase in time to a heart failure event.
The adverse events reported for dapagliflozin in DAPA-HF matched the established safety profile for the drug, AstraZeneca noted in the press release.
“The benefits of dapagliflozin in DAPA-HF are very impressive, with a substantial reduction in the primary composite outcome of cardiovascular death or hospital admission. We hope these exciting new findings will ultimately help reduce the terrible burden of disease caused by heart failure and help improve outcomes for our patients,” said John McMurray, MD, of the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Another dapagliflozin trial, called DELIVER, is focused on 4,700 patients with heart failure with preserved ejection fraction randomized to dapagliflozin (Farxiga) or placebo. That is due to be completed next year.
The full results from DAPA-HF will be presented at a later date.
The SGLT2 inhibitor dapagliflozin (Farxiga) successfully met the primary endpoint of the phase 3 DAPA-HF trial in patients with heart failure, according to a press release from AstraZeneca.
DAPA-HF is an international, multicenter, parallel group, randomized, double-blind trial in patients with heart failure and reduced ejection fraction with or without type 2 diabetes. Patients in the study received either 10 mg dapagliflozin or placebo, and the primary outcome was time to a worsening heart failure event or to cardiovascular death. Patients who received dapagliflozin had a statistically significant reduction in incidence of cardiovascular death and an increase in time to a heart failure event.
The adverse events reported for dapagliflozin in DAPA-HF matched the established safety profile for the drug, AstraZeneca noted in the press release.
“The benefits of dapagliflozin in DAPA-HF are very impressive, with a substantial reduction in the primary composite outcome of cardiovascular death or hospital admission. We hope these exciting new findings will ultimately help reduce the terrible burden of disease caused by heart failure and help improve outcomes for our patients,” said John McMurray, MD, of the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Another dapagliflozin trial, called DELIVER, is focused on 4,700 patients with heart failure with preserved ejection fraction randomized to dapagliflozin (Farxiga) or placebo. That is due to be completed next year.
The full results from DAPA-HF will be presented at a later date.
The SGLT2 inhibitor dapagliflozin (Farxiga) successfully met the primary endpoint of the phase 3 DAPA-HF trial in patients with heart failure, according to a press release from AstraZeneca.
DAPA-HF is an international, multicenter, parallel group, randomized, double-blind trial in patients with heart failure and reduced ejection fraction with or without type 2 diabetes. Patients in the study received either 10 mg dapagliflozin or placebo, and the primary outcome was time to a worsening heart failure event or to cardiovascular death. Patients who received dapagliflozin had a statistically significant reduction in incidence of cardiovascular death and an increase in time to a heart failure event.
The adverse events reported for dapagliflozin in DAPA-HF matched the established safety profile for the drug, AstraZeneca noted in the press release.
“The benefits of dapagliflozin in DAPA-HF are very impressive, with a substantial reduction in the primary composite outcome of cardiovascular death or hospital admission. We hope these exciting new findings will ultimately help reduce the terrible burden of disease caused by heart failure and help improve outcomes for our patients,” said John McMurray, MD, of the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Another dapagliflozin trial, called DELIVER, is focused on 4,700 patients with heart failure with preserved ejection fraction randomized to dapagliflozin (Farxiga) or placebo. That is due to be completed next year.
The full results from DAPA-HF will be presented at a later date.
Am I still a hospitalist?
HM as a force for change
I wear a suit every day to work. I count the time between shifts in months, not days. Rather than looking for subtle diagnostic clues hidden in clinical information, I find myself up to my elbows in performance and financial data. Instead of meetings complicated by challenging family dynamics, I spend my time calming the waters between clinical departments that each feel slighted.
And yet, when people ask me what I do, I do not say I am a health system CEO. Rather, I am a hospitalist. I say it, not out of habit, but with pride and clear intention. Almost 20 years ago, I had to explain to my parents what a hospitalist was as I made the transition from primary care doctor to hospitalist. I told them that hospitalists take care of sick people who are in the hospital, but also are charged with making the hospital a better place to take care of people. I hope that in some small way, in every role I have had over the past 20 years as a hospitalist, I have been able to do that.
While the small changes we can all make every day are important, massive changes to health care, hospitals, and providers are coming. The forces driving these changes are manifold, complex, and powerful. Individual hospitalists, hospital groups, and hospitals will be challenged to keep up with responding to these changes. I hope, though, that our field, hospital medicine, will not be sitting there, waiting for the changes to come, but will instead be one of the forces for change.
I also believe that hospital medicine and health care delivery systems should drive the change in a coordinated and collaborative partnership. A partnership not built on self-advocacy but one in which we remember why we exist – to take care of people. A force for change that preserves the essential, evolves what needs improvement, and revolutionizes the archaic.
Partnerships between hospitalist groups and health care administration will always face the day-to-day challenges of balancing the need for resources with the ability to provide them, agreeing on how to measure and assess quality, and aligning rewards with priorities. However, by working together in venues that allow us to think beyond the day-to-day issues, we in hospital medicine will be leaders in the change that is coming. I believe that today, the Society of Hospital Medicine must be one of those venues. Through its committees, meetings, advocacy, publications, and most importantly, members, SHM will continue to shape the future of care delivery in this country and beyond.
SHM has been my professional home for almost 20 years, helping me think about how to make the hospital a better place to take care of people. Recent examples of SHM and its members partnering in this area include advocacy work to improve alternative payment models, such as Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), as well as educational efforts for its members on how to navigate the current rules around MACRA.
For many years, SHM has been the leader in professional organizations for leading the way on quality improvement. Through the Center for Quality Improvement, SHM not only offers robust educational tools to better enable members to lead efforts at their home institutions but also has led multi-institutional efforts to reduce harm that have been recognized nationally for their impact.
As we move further down the path from volume to value toward population health, the SHM Board will be sure that the society continues to be a leader for both its members and the health system at large as we face these changes. We have the opportunity in front of us to collectively embrace and create the changes coming toward us with that shared purpose of making wherever it is that we care for people better places to provide that care. How could one not be proud to say, with intent, “I am a hospitalist,” regardless of what it is that brings each of us to SHM.
Dr. Whelan is CEO of Banner–University Medical Center Tucson (Ariz.) and a member of the SHM Board of Directors.
HM as a force for change
HM as a force for change
I wear a suit every day to work. I count the time between shifts in months, not days. Rather than looking for subtle diagnostic clues hidden in clinical information, I find myself up to my elbows in performance and financial data. Instead of meetings complicated by challenging family dynamics, I spend my time calming the waters between clinical departments that each feel slighted.
And yet, when people ask me what I do, I do not say I am a health system CEO. Rather, I am a hospitalist. I say it, not out of habit, but with pride and clear intention. Almost 20 years ago, I had to explain to my parents what a hospitalist was as I made the transition from primary care doctor to hospitalist. I told them that hospitalists take care of sick people who are in the hospital, but also are charged with making the hospital a better place to take care of people. I hope that in some small way, in every role I have had over the past 20 years as a hospitalist, I have been able to do that.
While the small changes we can all make every day are important, massive changes to health care, hospitals, and providers are coming. The forces driving these changes are manifold, complex, and powerful. Individual hospitalists, hospital groups, and hospitals will be challenged to keep up with responding to these changes. I hope, though, that our field, hospital medicine, will not be sitting there, waiting for the changes to come, but will instead be one of the forces for change.
I also believe that hospital medicine and health care delivery systems should drive the change in a coordinated and collaborative partnership. A partnership not built on self-advocacy but one in which we remember why we exist – to take care of people. A force for change that preserves the essential, evolves what needs improvement, and revolutionizes the archaic.
Partnerships between hospitalist groups and health care administration will always face the day-to-day challenges of balancing the need for resources with the ability to provide them, agreeing on how to measure and assess quality, and aligning rewards with priorities. However, by working together in venues that allow us to think beyond the day-to-day issues, we in hospital medicine will be leaders in the change that is coming. I believe that today, the Society of Hospital Medicine must be one of those venues. Through its committees, meetings, advocacy, publications, and most importantly, members, SHM will continue to shape the future of care delivery in this country and beyond.
SHM has been my professional home for almost 20 years, helping me think about how to make the hospital a better place to take care of people. Recent examples of SHM and its members partnering in this area include advocacy work to improve alternative payment models, such as Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), as well as educational efforts for its members on how to navigate the current rules around MACRA.
For many years, SHM has been the leader in professional organizations for leading the way on quality improvement. Through the Center for Quality Improvement, SHM not only offers robust educational tools to better enable members to lead efforts at their home institutions but also has led multi-institutional efforts to reduce harm that have been recognized nationally for their impact.
As we move further down the path from volume to value toward population health, the SHM Board will be sure that the society continues to be a leader for both its members and the health system at large as we face these changes. We have the opportunity in front of us to collectively embrace and create the changes coming toward us with that shared purpose of making wherever it is that we care for people better places to provide that care. How could one not be proud to say, with intent, “I am a hospitalist,” regardless of what it is that brings each of us to SHM.
Dr. Whelan is CEO of Banner–University Medical Center Tucson (Ariz.) and a member of the SHM Board of Directors.
I wear a suit every day to work. I count the time between shifts in months, not days. Rather than looking for subtle diagnostic clues hidden in clinical information, I find myself up to my elbows in performance and financial data. Instead of meetings complicated by challenging family dynamics, I spend my time calming the waters between clinical departments that each feel slighted.
And yet, when people ask me what I do, I do not say I am a health system CEO. Rather, I am a hospitalist. I say it, not out of habit, but with pride and clear intention. Almost 20 years ago, I had to explain to my parents what a hospitalist was as I made the transition from primary care doctor to hospitalist. I told them that hospitalists take care of sick people who are in the hospital, but also are charged with making the hospital a better place to take care of people. I hope that in some small way, in every role I have had over the past 20 years as a hospitalist, I have been able to do that.
While the small changes we can all make every day are important, massive changes to health care, hospitals, and providers are coming. The forces driving these changes are manifold, complex, and powerful. Individual hospitalists, hospital groups, and hospitals will be challenged to keep up with responding to these changes. I hope, though, that our field, hospital medicine, will not be sitting there, waiting for the changes to come, but will instead be one of the forces for change.
I also believe that hospital medicine and health care delivery systems should drive the change in a coordinated and collaborative partnership. A partnership not built on self-advocacy but one in which we remember why we exist – to take care of people. A force for change that preserves the essential, evolves what needs improvement, and revolutionizes the archaic.
Partnerships between hospitalist groups and health care administration will always face the day-to-day challenges of balancing the need for resources with the ability to provide them, agreeing on how to measure and assess quality, and aligning rewards with priorities. However, by working together in venues that allow us to think beyond the day-to-day issues, we in hospital medicine will be leaders in the change that is coming. I believe that today, the Society of Hospital Medicine must be one of those venues. Through its committees, meetings, advocacy, publications, and most importantly, members, SHM will continue to shape the future of care delivery in this country and beyond.
SHM has been my professional home for almost 20 years, helping me think about how to make the hospital a better place to take care of people. Recent examples of SHM and its members partnering in this area include advocacy work to improve alternative payment models, such as Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), as well as educational efforts for its members on how to navigate the current rules around MACRA.
For many years, SHM has been the leader in professional organizations for leading the way on quality improvement. Through the Center for Quality Improvement, SHM not only offers robust educational tools to better enable members to lead efforts at their home institutions but also has led multi-institutional efforts to reduce harm that have been recognized nationally for their impact.
As we move further down the path from volume to value toward population health, the SHM Board will be sure that the society continues to be a leader for both its members and the health system at large as we face these changes. We have the opportunity in front of us to collectively embrace and create the changes coming toward us with that shared purpose of making wherever it is that we care for people better places to provide that care. How could one not be proud to say, with intent, “I am a hospitalist,” regardless of what it is that brings each of us to SHM.
Dr. Whelan is CEO of Banner–University Medical Center Tucson (Ariz.) and a member of the SHM Board of Directors.
New biomarker model outmatches conventional risk factors for predicting mortality
A new model using 14 biomarkers may be more accurate at predicting longer-term mortality than a model comprising conventional risk factors, based on the largest metabolomics study to date.
The prognostic model was more accurate at predicting 5- and 10-year mortality across all ages, reported Joris Deelen, PhD, of Leiden (the Netherlands) University Medical Center and colleagues.
“These results suggest that metabolic biomarker profiling could potentially be used to guide patient care, if further validated in relevant clinical settings,” the investigators wrote in Nature Communications.
“There is no consensus on the ultimate set of predictors of longer-term [5-10 years] mortality risk, since the predictive power of the currently used risk factors is limited, especially at higher ages,” the investigators wrote. “However, it is especially this age group and follow-up time window for which a robust tool would aid clinicians in assessing whether treatment is still sensible.”
The current study was a survival meta-analysis of 44,168 individuals from 12 cohorts aged between 18 and 109 years at baseline. First, the investigators looked for associations between 226 metabolic biomarkers and all-cause mortality in the 5,512 people who died during follow-up. This revealed associations between mortality and 136 biomarkers, which increased to 159 biomarkers after adjusting for recently reported all-cause mortality associations with albumin, very low-density lipoprotein (VLDL) particle size, citrate, and glycoprotein acetyls. Because of strong correlations between many of the biomarkers evaluated, the investigators pared the field down to 63 biomarkers, then used a forward-backward procedure to ultimately identify 14 biomarkers independently associated with mortality. Of the four recently described biomarkers, citrate was excluded from the final model because of its minimal contribution to mortality estimates.
The 14 biomarkers were total lipids in chylomicrons and extremely large VLDL cholesterol, total lipids in small HDL cholesterol, mean diameter for VLDL cholesterol particles, ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, isoleucine, leucine, valine, phenylalanine, acetoacetate, albumin, and glycoprotein acetyls.
“The 14 identified biomarkers are involved in various processes, such as lipoprotein and fatty acid metabolism, glycolysis, fluid balance, and inflammation. Although the majority of these biomarkers have been associated with mortality before, this is the first study that shows their independent effect when combined into one model,” the researchers wrote.
Implementation of the new biomarker model led to a score that typically ranged from –2 to 3. A 1-point increase was associated with a 173% increased risk of death (hazard ratio, 2.73; P less than 1 x 10–132). Analysis of cause-specific mortality revealed that most biomarkers were predictive of multiple causes of death. Some biomarkers were more focused; glucose, for example, was more predictive of cardiovascular-related death than of death because of cancer or nonlocalized infections. Compared with a model incorporating conventional risk factors, the biomarker model more accurately predicted 5- and 10-year mortality, with respective C-statistics of 0.837 versus 0.772 and 0.830 versus 0.790. This superiority was even more pronounced when only individuals aged older than 60 years were included.
The study was funded by Biobanking and BioMolecular resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
SOURCE: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
A new model using 14 biomarkers may be more accurate at predicting longer-term mortality than a model comprising conventional risk factors, based on the largest metabolomics study to date.
The prognostic model was more accurate at predicting 5- and 10-year mortality across all ages, reported Joris Deelen, PhD, of Leiden (the Netherlands) University Medical Center and colleagues.
“These results suggest that metabolic biomarker profiling could potentially be used to guide patient care, if further validated in relevant clinical settings,” the investigators wrote in Nature Communications.
“There is no consensus on the ultimate set of predictors of longer-term [5-10 years] mortality risk, since the predictive power of the currently used risk factors is limited, especially at higher ages,” the investigators wrote. “However, it is especially this age group and follow-up time window for which a robust tool would aid clinicians in assessing whether treatment is still sensible.”
The current study was a survival meta-analysis of 44,168 individuals from 12 cohorts aged between 18 and 109 years at baseline. First, the investigators looked for associations between 226 metabolic biomarkers and all-cause mortality in the 5,512 people who died during follow-up. This revealed associations between mortality and 136 biomarkers, which increased to 159 biomarkers after adjusting for recently reported all-cause mortality associations with albumin, very low-density lipoprotein (VLDL) particle size, citrate, and glycoprotein acetyls. Because of strong correlations between many of the biomarkers evaluated, the investigators pared the field down to 63 biomarkers, then used a forward-backward procedure to ultimately identify 14 biomarkers independently associated with mortality. Of the four recently described biomarkers, citrate was excluded from the final model because of its minimal contribution to mortality estimates.
The 14 biomarkers were total lipids in chylomicrons and extremely large VLDL cholesterol, total lipids in small HDL cholesterol, mean diameter for VLDL cholesterol particles, ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, isoleucine, leucine, valine, phenylalanine, acetoacetate, albumin, and glycoprotein acetyls.
“The 14 identified biomarkers are involved in various processes, such as lipoprotein and fatty acid metabolism, glycolysis, fluid balance, and inflammation. Although the majority of these biomarkers have been associated with mortality before, this is the first study that shows their independent effect when combined into one model,” the researchers wrote.
Implementation of the new biomarker model led to a score that typically ranged from –2 to 3. A 1-point increase was associated with a 173% increased risk of death (hazard ratio, 2.73; P less than 1 x 10–132). Analysis of cause-specific mortality revealed that most biomarkers were predictive of multiple causes of death. Some biomarkers were more focused; glucose, for example, was more predictive of cardiovascular-related death than of death because of cancer or nonlocalized infections. Compared with a model incorporating conventional risk factors, the biomarker model more accurately predicted 5- and 10-year mortality, with respective C-statistics of 0.837 versus 0.772 and 0.830 versus 0.790. This superiority was even more pronounced when only individuals aged older than 60 years were included.
The study was funded by Biobanking and BioMolecular resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
SOURCE: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
A new model using 14 biomarkers may be more accurate at predicting longer-term mortality than a model comprising conventional risk factors, based on the largest metabolomics study to date.
The prognostic model was more accurate at predicting 5- and 10-year mortality across all ages, reported Joris Deelen, PhD, of Leiden (the Netherlands) University Medical Center and colleagues.
“These results suggest that metabolic biomarker profiling could potentially be used to guide patient care, if further validated in relevant clinical settings,” the investigators wrote in Nature Communications.
“There is no consensus on the ultimate set of predictors of longer-term [5-10 years] mortality risk, since the predictive power of the currently used risk factors is limited, especially at higher ages,” the investigators wrote. “However, it is especially this age group and follow-up time window for which a robust tool would aid clinicians in assessing whether treatment is still sensible.”
The current study was a survival meta-analysis of 44,168 individuals from 12 cohorts aged between 18 and 109 years at baseline. First, the investigators looked for associations between 226 metabolic biomarkers and all-cause mortality in the 5,512 people who died during follow-up. This revealed associations between mortality and 136 biomarkers, which increased to 159 biomarkers after adjusting for recently reported all-cause mortality associations with albumin, very low-density lipoprotein (VLDL) particle size, citrate, and glycoprotein acetyls. Because of strong correlations between many of the biomarkers evaluated, the investigators pared the field down to 63 biomarkers, then used a forward-backward procedure to ultimately identify 14 biomarkers independently associated with mortality. Of the four recently described biomarkers, citrate was excluded from the final model because of its minimal contribution to mortality estimates.
The 14 biomarkers were total lipids in chylomicrons and extremely large VLDL cholesterol, total lipids in small HDL cholesterol, mean diameter for VLDL cholesterol particles, ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, isoleucine, leucine, valine, phenylalanine, acetoacetate, albumin, and glycoprotein acetyls.
“The 14 identified biomarkers are involved in various processes, such as lipoprotein and fatty acid metabolism, glycolysis, fluid balance, and inflammation. Although the majority of these biomarkers have been associated with mortality before, this is the first study that shows their independent effect when combined into one model,” the researchers wrote.
Implementation of the new biomarker model led to a score that typically ranged from –2 to 3. A 1-point increase was associated with a 173% increased risk of death (hazard ratio, 2.73; P less than 1 x 10–132). Analysis of cause-specific mortality revealed that most biomarkers were predictive of multiple causes of death. Some biomarkers were more focused; glucose, for example, was more predictive of cardiovascular-related death than of death because of cancer or nonlocalized infections. Compared with a model incorporating conventional risk factors, the biomarker model more accurately predicted 5- and 10-year mortality, with respective C-statistics of 0.837 versus 0.772 and 0.830 versus 0.790. This superiority was even more pronounced when only individuals aged older than 60 years were included.
The study was funded by Biobanking and BioMolecular resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
SOURCE: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
FROM NATURE COMMUNICATIONS
Key clinical point: A new model using 14 biomarkers may be more accurate at predicting 5- and 10-year mortality than a model comprising conventional risk factors.
Major finding: The biomarker model better predicted 5-year mortality than the conventional model (C-statistic, 0.837 vs. 0.772).
Study details: A retrospective metabolomics study involving 44,168 individuals.
Disclosures: The study was funded by Biobanking and BioMolecular Resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
Source: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
FDA approves Xenleta for community-acquired bacterial pneumonia treatment
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
Short-term parenteral antibiotics effective for bacteremic UTI in young infants
according to a study.
While previous studies have shown short-term parenteral antibiotic therapy to be safe and equally effective in uncomplicated urinary tract infections (UTIs), short-term therapy safety in bacteremic UTI had not been established, Sanyukta Desai, MD, of the University of Cincinnati and Cincinnati Children’s Hospital and associates wrote in Pediatrics.
“As a result, infants with bacteremic UTI often receive prolonged courses of parenteral antibiotics, which can lead to long hospitalizations and increased costs,” they said.
In a multicenter, retrospective cohort study, Dr. Desai and associates analyzed a group of 115 infants aged 60 days or younger who were admitted to a group of 11 participating EDs between July 1, 2011, and June 30, 2016, if they had a UTI caused by a bacterial pathogen. Half of the infants were administered parenteral antibiotics for 7 days or less before being switched to oral antibiotics, and the rest were given parenteral antibiotics for more than 7 days before switching to oral. Infants were more likely to receive long-term parenteral treatment if they were ill appearing and had growth of a non–Escherichia coli organism.
Six infants (two in the short-term group, four in the long-term group) had a recurrent UTI, each one diagnosed between 15 and 30 days after discharge; the adjusted risk difference between the two groups was 3% (95% confidence interval, –5.8 to 12.7). Two of the infants in the long-term group with a recurrent UTI had a different organism than during the index infection. When comparing only the infants with growth of the same pathogen that caused the index UTI, the adjusted risk difference between the two groups was 0.2% (95% CI, –7.8 to 8.3).
A total of 15 infants (6 in the short-term group, 9 in the long-term group) had 30-day all-cause reutilization, with no significant difference between groups (adjusted risk difference, 3%; 95% CI, –14.6 to 20.4).
Mean length of stay was significantly longer in the long-term treatment group, compared with the short-term group (11 days vs. 5 days; adjusted mean difference, 6 days; 95% CI, 4.0-8.8).
No infants experienced a serious adverse event such as ICU readmission, need for mechanical ventilation or vasopressor use, or signs of neurologic sequelae within 30 days of discharge from the index hospitalization, the investigators noted. Peripherally inserted central catheters were required in 13 infants; of these, 1 infant had to revisit an ED because of a related mechanical complication.
“Researchers in future prospective studies should seek to establish the bioavailability and optimal dosing of oral antibiotics in young infants and assess if there are particular subpopulations of infants with bacteremic UTI who may benefit from longer courses of parenteral antibiotic therapy,” Dr. Desai and associates concluded.
In a related editorial, Natalia V. Leva, MD, and Hillary L. Copp, MD, of the University of California, San Francisco, noted that the study represents a “critical piece of a complicated puzzle that not only includes minimum duration of parenteral antibiotic treatment but also involves bioavailability of antimicrobial agents in infants and total treatment duration, which includes parenteral and oral antibiotic therapy.”
The question that remains is how long a duration of parenteral antibiotic is necessary, Dr. Leva and Dr. Copp wrote. “Desai et al. used a relatively arbitrary cutoff of 7 days on the basis of the distribution of antibiotic course among their patient population; however, this is likely more a reflection of clinical practice than it is evidence based.” They concluded that this study provided evidence that a “short course of parenteral antibiotics in infants [aged 60 days or younger] with bacteremic UTI is safe and effective. Although the current study does not address total duration of antibiotics [parenteral and oral], it does shine a light on where we should focus future research endeavors.”
The study authors reported that they had no conflicts of interest. The study was supported in part by a National Center for Advancing Translational Sciences grant and an Agency for Healthcare Research and Quality grant. The editorialists had no relevant conflicts of interest and received no external funding.
SOURCEs: Desai S et al. Pediatrics. 2019 Aug 20. doi: 10.1542/peds.2018-3844; Leva et al. 2019 Aug 20. doi: 10.1542/peds.2019-1611.
according to a study.
While previous studies have shown short-term parenteral antibiotic therapy to be safe and equally effective in uncomplicated urinary tract infections (UTIs), short-term therapy safety in bacteremic UTI had not been established, Sanyukta Desai, MD, of the University of Cincinnati and Cincinnati Children’s Hospital and associates wrote in Pediatrics.
“As a result, infants with bacteremic UTI often receive prolonged courses of parenteral antibiotics, which can lead to long hospitalizations and increased costs,” they said.
In a multicenter, retrospective cohort study, Dr. Desai and associates analyzed a group of 115 infants aged 60 days or younger who were admitted to a group of 11 participating EDs between July 1, 2011, and June 30, 2016, if they had a UTI caused by a bacterial pathogen. Half of the infants were administered parenteral antibiotics for 7 days or less before being switched to oral antibiotics, and the rest were given parenteral antibiotics for more than 7 days before switching to oral. Infants were more likely to receive long-term parenteral treatment if they were ill appearing and had growth of a non–Escherichia coli organism.
Six infants (two in the short-term group, four in the long-term group) had a recurrent UTI, each one diagnosed between 15 and 30 days after discharge; the adjusted risk difference between the two groups was 3% (95% confidence interval, –5.8 to 12.7). Two of the infants in the long-term group with a recurrent UTI had a different organism than during the index infection. When comparing only the infants with growth of the same pathogen that caused the index UTI, the adjusted risk difference between the two groups was 0.2% (95% CI, –7.8 to 8.3).
A total of 15 infants (6 in the short-term group, 9 in the long-term group) had 30-day all-cause reutilization, with no significant difference between groups (adjusted risk difference, 3%; 95% CI, –14.6 to 20.4).
Mean length of stay was significantly longer in the long-term treatment group, compared with the short-term group (11 days vs. 5 days; adjusted mean difference, 6 days; 95% CI, 4.0-8.8).
No infants experienced a serious adverse event such as ICU readmission, need for mechanical ventilation or vasopressor use, or signs of neurologic sequelae within 30 days of discharge from the index hospitalization, the investigators noted. Peripherally inserted central catheters were required in 13 infants; of these, 1 infant had to revisit an ED because of a related mechanical complication.
“Researchers in future prospective studies should seek to establish the bioavailability and optimal dosing of oral antibiotics in young infants and assess if there are particular subpopulations of infants with bacteremic UTI who may benefit from longer courses of parenteral antibiotic therapy,” Dr. Desai and associates concluded.
In a related editorial, Natalia V. Leva, MD, and Hillary L. Copp, MD, of the University of California, San Francisco, noted that the study represents a “critical piece of a complicated puzzle that not only includes minimum duration of parenteral antibiotic treatment but also involves bioavailability of antimicrobial agents in infants and total treatment duration, which includes parenteral and oral antibiotic therapy.”
The question that remains is how long a duration of parenteral antibiotic is necessary, Dr. Leva and Dr. Copp wrote. “Desai et al. used a relatively arbitrary cutoff of 7 days on the basis of the distribution of antibiotic course among their patient population; however, this is likely more a reflection of clinical practice than it is evidence based.” They concluded that this study provided evidence that a “short course of parenteral antibiotics in infants [aged 60 days or younger] with bacteremic UTI is safe and effective. Although the current study does not address total duration of antibiotics [parenteral and oral], it does shine a light on where we should focus future research endeavors.”
The study authors reported that they had no conflicts of interest. The study was supported in part by a National Center for Advancing Translational Sciences grant and an Agency for Healthcare Research and Quality grant. The editorialists had no relevant conflicts of interest and received no external funding.
SOURCEs: Desai S et al. Pediatrics. 2019 Aug 20. doi: 10.1542/peds.2018-3844; Leva et al. 2019 Aug 20. doi: 10.1542/peds.2019-1611.
according to a study.
While previous studies have shown short-term parenteral antibiotic therapy to be safe and equally effective in uncomplicated urinary tract infections (UTIs), short-term therapy safety in bacteremic UTI had not been established, Sanyukta Desai, MD, of the University of Cincinnati and Cincinnati Children’s Hospital and associates wrote in Pediatrics.
“As a result, infants with bacteremic UTI often receive prolonged courses of parenteral antibiotics, which can lead to long hospitalizations and increased costs,” they said.
In a multicenter, retrospective cohort study, Dr. Desai and associates analyzed a group of 115 infants aged 60 days or younger who were admitted to a group of 11 participating EDs between July 1, 2011, and June 30, 2016, if they had a UTI caused by a bacterial pathogen. Half of the infants were administered parenteral antibiotics for 7 days or less before being switched to oral antibiotics, and the rest were given parenteral antibiotics for more than 7 days before switching to oral. Infants were more likely to receive long-term parenteral treatment if they were ill appearing and had growth of a non–Escherichia coli organism.
Six infants (two in the short-term group, four in the long-term group) had a recurrent UTI, each one diagnosed between 15 and 30 days after discharge; the adjusted risk difference between the two groups was 3% (95% confidence interval, –5.8 to 12.7). Two of the infants in the long-term group with a recurrent UTI had a different organism than during the index infection. When comparing only the infants with growth of the same pathogen that caused the index UTI, the adjusted risk difference between the two groups was 0.2% (95% CI, –7.8 to 8.3).
A total of 15 infants (6 in the short-term group, 9 in the long-term group) had 30-day all-cause reutilization, with no significant difference between groups (adjusted risk difference, 3%; 95% CI, –14.6 to 20.4).
Mean length of stay was significantly longer in the long-term treatment group, compared with the short-term group (11 days vs. 5 days; adjusted mean difference, 6 days; 95% CI, 4.0-8.8).
No infants experienced a serious adverse event such as ICU readmission, need for mechanical ventilation or vasopressor use, or signs of neurologic sequelae within 30 days of discharge from the index hospitalization, the investigators noted. Peripherally inserted central catheters were required in 13 infants; of these, 1 infant had to revisit an ED because of a related mechanical complication.
“Researchers in future prospective studies should seek to establish the bioavailability and optimal dosing of oral antibiotics in young infants and assess if there are particular subpopulations of infants with bacteremic UTI who may benefit from longer courses of parenteral antibiotic therapy,” Dr. Desai and associates concluded.
In a related editorial, Natalia V. Leva, MD, and Hillary L. Copp, MD, of the University of California, San Francisco, noted that the study represents a “critical piece of a complicated puzzle that not only includes minimum duration of parenteral antibiotic treatment but also involves bioavailability of antimicrobial agents in infants and total treatment duration, which includes parenteral and oral antibiotic therapy.”
The question that remains is how long a duration of parenteral antibiotic is necessary, Dr. Leva and Dr. Copp wrote. “Desai et al. used a relatively arbitrary cutoff of 7 days on the basis of the distribution of antibiotic course among their patient population; however, this is likely more a reflection of clinical practice than it is evidence based.” They concluded that this study provided evidence that a “short course of parenteral antibiotics in infants [aged 60 days or younger] with bacteremic UTI is safe and effective. Although the current study does not address total duration of antibiotics [parenteral and oral], it does shine a light on where we should focus future research endeavors.”
The study authors reported that they had no conflicts of interest. The study was supported in part by a National Center for Advancing Translational Sciences grant and an Agency for Healthcare Research and Quality grant. The editorialists had no relevant conflicts of interest and received no external funding.
SOURCEs: Desai S et al. Pediatrics. 2019 Aug 20. doi: 10.1542/peds.2018-3844; Leva et al. 2019 Aug 20. doi: 10.1542/peds.2019-1611.
FROM PEDIATRICS
Key clinical point: Urinary tract infection (UTI) recurrence and hospital reutilization was similar in infants with bacteremic UTIs, regardless of parenteral antibiotic treatment duration of 7 days or less or greater than 7 days prior to oral antibiotics.
Major finding: The adjusted risk difference for both infection recurrence and hospital reutilization was 3% and was nonsignificant in both cases.
Study details: A group of 115 infants aged 60 days or younger who were admitted to an ED with a bacteremic UTI.
Disclosures: The study authors reported that they had no conflicts of interest. The funding of the study was supported in part by a National Center for Advancing Translational Sciences grant and an Agency for Healthcare Research and Quality grant.
Source: Desai S et al. Pediatrics. 2019 Aug 20. doi: 10.1542/peds.2018-3844.























