User login
U.S. infant mortality continued slow decline in 2017
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.
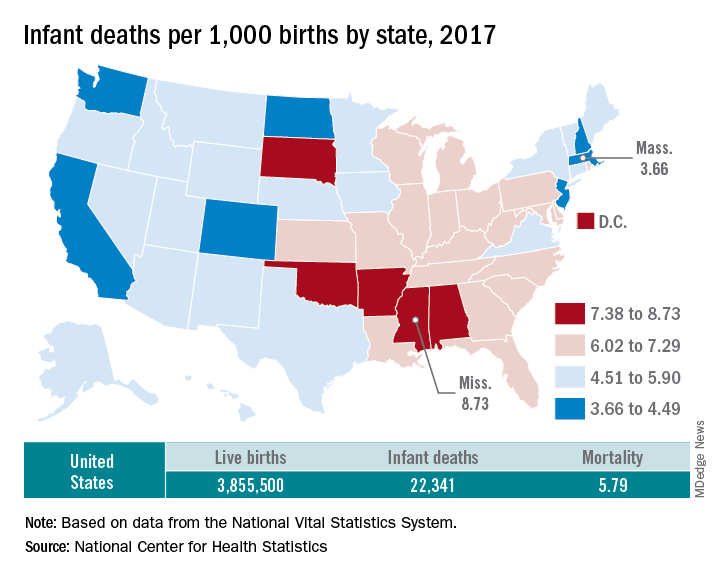
The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.

The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.

The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
Critics say hospital price transparency proposal ‘misses the mark’
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
Key clinical point: CMS proposes complete transparency in hospital prices.
Major finding: Hospitals would be required to make public the list prices, as well as all payer-negotiated prices.
Study details: CMS asserts that the disclosure of pricing data will lead to reduced prices through market competition.
Disclosures: CMS, as issuer of the proposed rule, makes no disclosures.
Source: Proposed rule updating the hospital outpatient prospective payment system for 2020.
Short-course azithromycin no benefit in pediatric asthma admissions
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
REPORTING FROM PHM 2019
CMS plans to give MIPS an overhaul
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Key clinical point: The Centers for Medicare & Medicaid Services proposes an overhaul to the Merit-based Incentive Payment System track of the Quality Payment Program.
Major finding: The move is intended to make measures more meaningful to clinicians.
Study details: Measures would be more focused to specialties through Merit-based Incentive Payment System Value Pathways, with all those reporting on a specialty or condition reporting on more streamlined measures.
Disclosures: CMS, as the issuer of the rules, makes no disclosures.
CMS proposes improved E/M payments, additional price transparency for hospitals
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
[email protected]
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
[email protected]
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
[email protected]
FDA approvals permit double-immunoassay approach to Lyme disease diagnosis
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Most patients with new hypertension under revised BP guidelines won’t need pharmacotherapy
ORLANDO – An additional 14% of Americans have been reclassified as having hypertension – an increase from 32% to 46% of adults – under the latest guidelines on management of high blood pressure released by the American College of Cardiology and American Heart Association in 2017. However, this does not mean these patients are mandated for pharmacologic therapy, since most of them have been reclassified as having stage 1 hypertension, said Leslie L. Davis, PhD, RN, ANP-BC, FPCNA, FAANP, FAHA, associate professor of nursing at the University of North Carolina, Greensboro.
According to the new guidelines, normal BP is classified as less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Elevated BP is 120-129 mm Hg systolic and under 80 mm Hg diastolic, while patients are classified as having stage 1 hypertension if their systolic BP is 130-139 mm Hg or diastolic BP is 80-90 mm Hg. Patients now have stage 2 hypertension if their systolic BP is higher than 140 mm Hg or diastolic BP is above 90 mm Hg.
This raises the importance of getting an accurate BP measurement from patients. At least two readings over two or more visits should be used before categorizing a patient. To take an ideal reading, patients should be sitting at rest with their back supported, feet positioned on a flat surface (no crossing legs), and their arm at heart level for at least 5 minutes. Patients should also refrain from tobacco or caffeine use 30 minutes before the reading. “These numbers need to be correct, because we’ve got new [BP] categories, and also for managing blood pressure, you’re making decisions based on these numbers,” said Dr. Davis at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
When taking a patient’s BP, neither the patient nor the provider should talk, and constricting clothes on the upper extremity should be removed instead of pushed up. Using a incorrectly sized cuff can artificially raise or lower a patient’s BP level, so clinicians should use one that is 80% of the length and 40% of the width of the patient’s arm circumference. If the BP reading is elevated, confirm the reading in the other arm and use the arm with the higher reading for future measurements. BP measurements taken in different settings, such as ambulatory BP monitoring or home BP monitoring, can help give context to the in-office reading and whether the patient has white-coat hypertension or masked hypertension.
After a patient has their accurate BP reading, they can calculate their atherosclerotic cardiovascular disease (ASCVD) risk using the ACC’s ASCVD Risk Estimator, said Dr. Davis. If a patient has confirmed cardiovascular disease or has a 10% or greater 10-year ASCVD risk, the target to lower BP is 130 based on high-quality evidence, but expert opinion recommends targeting 130/80 for patients with confirmed hypertension as well.
“If somebody is overweight or obese, 10 pounds is 10 points,” said Dr. Davis. “Even if you don’t get them to the appropriate body mass index over 3 months’ time, that’s as much as a low or medium dose of antihypertensive therapy. For you to be able to get double-digit reduction, that’s what a med does.”
Other nonpharmacologic interventions for these patients include a heart-healthy diet such as the DASH diet, lowering sodium and increasing potassium, structuring exercise and physical activity, lowering use of or avoiding alcohol, and smoking cessation. The goal of nonpharmacologic therapy is not only to lower BP, but make the medication work better, said Dr. Davis.
Pharmacologic therapy should be initiated when patients exceed or are above the cutoff values for the new BP categories and if a patient has already had a cardiovascular event. “Basically, if you’re above that line in the sand of your goal, that’s when to start medications,” she said.
For stage 1 hypertension, first-line therapy is lifestyle change plus thiazides, calcium-channel blockers, or ACE inhibitors, with stage 1 hypertension therapy consisting of a combination of two first-line therapy therapies to reduce systolic BP by about 20 mm Hg and diastolic BP by 10 mm Hg. Beta-blockers are not first-line antihypertension therapy, but can be considered in patients with coronary artery disease and heart failure with reduced left ventricular ejection fraction.
With regard to follow-up, patients with low ASCVD risk and stage 1 hypertension can be monitored in 3-6 months after lifestyle changes, while patients with high ASCVD risk and stage 1 hypertension should be followed up in 1 month. Patients with stage 2 hypertension should follow up with their primary care provider 1 month after beginning their therapy, and those with very high BP should promptly be started on drug treatment with lifestyle changes, with upward dose adjustments as needed.
In adults aged 65 years or older, the ACC/AHA guidelines also focused on how to prevent cognitive decline and dementia, said Dr. Davis. The goal for ambulatory, community-dwelling adults is still to have a systolic BP of less than 130 mm Hg, but clinical judgment should prevail because of comorbid conditions and limited life expectancy in these patients. Patient preference should also be considered, and clinicians should use a team-based approach with shared decision making to determine goals for each patient.
Dr. Davis reported no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – An additional 14% of Americans have been reclassified as having hypertension – an increase from 32% to 46% of adults – under the latest guidelines on management of high blood pressure released by the American College of Cardiology and American Heart Association in 2017. However, this does not mean these patients are mandated for pharmacologic therapy, since most of them have been reclassified as having stage 1 hypertension, said Leslie L. Davis, PhD, RN, ANP-BC, FPCNA, FAANP, FAHA, associate professor of nursing at the University of North Carolina, Greensboro.
According to the new guidelines, normal BP is classified as less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Elevated BP is 120-129 mm Hg systolic and under 80 mm Hg diastolic, while patients are classified as having stage 1 hypertension if their systolic BP is 130-139 mm Hg or diastolic BP is 80-90 mm Hg. Patients now have stage 2 hypertension if their systolic BP is higher than 140 mm Hg or diastolic BP is above 90 mm Hg.
This raises the importance of getting an accurate BP measurement from patients. At least two readings over two or more visits should be used before categorizing a patient. To take an ideal reading, patients should be sitting at rest with their back supported, feet positioned on a flat surface (no crossing legs), and their arm at heart level for at least 5 minutes. Patients should also refrain from tobacco or caffeine use 30 minutes before the reading. “These numbers need to be correct, because we’ve got new [BP] categories, and also for managing blood pressure, you’re making decisions based on these numbers,” said Dr. Davis at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
When taking a patient’s BP, neither the patient nor the provider should talk, and constricting clothes on the upper extremity should be removed instead of pushed up. Using a incorrectly sized cuff can artificially raise or lower a patient’s BP level, so clinicians should use one that is 80% of the length and 40% of the width of the patient’s arm circumference. If the BP reading is elevated, confirm the reading in the other arm and use the arm with the higher reading for future measurements. BP measurements taken in different settings, such as ambulatory BP monitoring or home BP monitoring, can help give context to the in-office reading and whether the patient has white-coat hypertension or masked hypertension.
After a patient has their accurate BP reading, they can calculate their atherosclerotic cardiovascular disease (ASCVD) risk using the ACC’s ASCVD Risk Estimator, said Dr. Davis. If a patient has confirmed cardiovascular disease or has a 10% or greater 10-year ASCVD risk, the target to lower BP is 130 based on high-quality evidence, but expert opinion recommends targeting 130/80 for patients with confirmed hypertension as well.
“If somebody is overweight or obese, 10 pounds is 10 points,” said Dr. Davis. “Even if you don’t get them to the appropriate body mass index over 3 months’ time, that’s as much as a low or medium dose of antihypertensive therapy. For you to be able to get double-digit reduction, that’s what a med does.”
Other nonpharmacologic interventions for these patients include a heart-healthy diet such as the DASH diet, lowering sodium and increasing potassium, structuring exercise and physical activity, lowering use of or avoiding alcohol, and smoking cessation. The goal of nonpharmacologic therapy is not only to lower BP, but make the medication work better, said Dr. Davis.
Pharmacologic therapy should be initiated when patients exceed or are above the cutoff values for the new BP categories and if a patient has already had a cardiovascular event. “Basically, if you’re above that line in the sand of your goal, that’s when to start medications,” she said.
For stage 1 hypertension, first-line therapy is lifestyle change plus thiazides, calcium-channel blockers, or ACE inhibitors, with stage 1 hypertension therapy consisting of a combination of two first-line therapy therapies to reduce systolic BP by about 20 mm Hg and diastolic BP by 10 mm Hg. Beta-blockers are not first-line antihypertension therapy, but can be considered in patients with coronary artery disease and heart failure with reduced left ventricular ejection fraction.
With regard to follow-up, patients with low ASCVD risk and stage 1 hypertension can be monitored in 3-6 months after lifestyle changes, while patients with high ASCVD risk and stage 1 hypertension should be followed up in 1 month. Patients with stage 2 hypertension should follow up with their primary care provider 1 month after beginning their therapy, and those with very high BP should promptly be started on drug treatment with lifestyle changes, with upward dose adjustments as needed.
In adults aged 65 years or older, the ACC/AHA guidelines also focused on how to prevent cognitive decline and dementia, said Dr. Davis. The goal for ambulatory, community-dwelling adults is still to have a systolic BP of less than 130 mm Hg, but clinical judgment should prevail because of comorbid conditions and limited life expectancy in these patients. Patient preference should also be considered, and clinicians should use a team-based approach with shared decision making to determine goals for each patient.
Dr. Davis reported no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – An additional 14% of Americans have been reclassified as having hypertension – an increase from 32% to 46% of adults – under the latest guidelines on management of high blood pressure released by the American College of Cardiology and American Heart Association in 2017. However, this does not mean these patients are mandated for pharmacologic therapy, since most of them have been reclassified as having stage 1 hypertension, said Leslie L. Davis, PhD, RN, ANP-BC, FPCNA, FAANP, FAHA, associate professor of nursing at the University of North Carolina, Greensboro.
According to the new guidelines, normal BP is classified as less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Elevated BP is 120-129 mm Hg systolic and under 80 mm Hg diastolic, while patients are classified as having stage 1 hypertension if their systolic BP is 130-139 mm Hg or diastolic BP is 80-90 mm Hg. Patients now have stage 2 hypertension if their systolic BP is higher than 140 mm Hg or diastolic BP is above 90 mm Hg.
This raises the importance of getting an accurate BP measurement from patients. At least two readings over two or more visits should be used before categorizing a patient. To take an ideal reading, patients should be sitting at rest with their back supported, feet positioned on a flat surface (no crossing legs), and their arm at heart level for at least 5 minutes. Patients should also refrain from tobacco or caffeine use 30 minutes before the reading. “These numbers need to be correct, because we’ve got new [BP] categories, and also for managing blood pressure, you’re making decisions based on these numbers,” said Dr. Davis at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
When taking a patient’s BP, neither the patient nor the provider should talk, and constricting clothes on the upper extremity should be removed instead of pushed up. Using a incorrectly sized cuff can artificially raise or lower a patient’s BP level, so clinicians should use one that is 80% of the length and 40% of the width of the patient’s arm circumference. If the BP reading is elevated, confirm the reading in the other arm and use the arm with the higher reading for future measurements. BP measurements taken in different settings, such as ambulatory BP monitoring or home BP monitoring, can help give context to the in-office reading and whether the patient has white-coat hypertension or masked hypertension.
After a patient has their accurate BP reading, they can calculate their atherosclerotic cardiovascular disease (ASCVD) risk using the ACC’s ASCVD Risk Estimator, said Dr. Davis. If a patient has confirmed cardiovascular disease or has a 10% or greater 10-year ASCVD risk, the target to lower BP is 130 based on high-quality evidence, but expert opinion recommends targeting 130/80 for patients with confirmed hypertension as well.
“If somebody is overweight or obese, 10 pounds is 10 points,” said Dr. Davis. “Even if you don’t get them to the appropriate body mass index over 3 months’ time, that’s as much as a low or medium dose of antihypertensive therapy. For you to be able to get double-digit reduction, that’s what a med does.”
Other nonpharmacologic interventions for these patients include a heart-healthy diet such as the DASH diet, lowering sodium and increasing potassium, structuring exercise and physical activity, lowering use of or avoiding alcohol, and smoking cessation. The goal of nonpharmacologic therapy is not only to lower BP, but make the medication work better, said Dr. Davis.
Pharmacologic therapy should be initiated when patients exceed or are above the cutoff values for the new BP categories and if a patient has already had a cardiovascular event. “Basically, if you’re above that line in the sand of your goal, that’s when to start medications,” she said.
For stage 1 hypertension, first-line therapy is lifestyle change plus thiazides, calcium-channel blockers, or ACE inhibitors, with stage 1 hypertension therapy consisting of a combination of two first-line therapy therapies to reduce systolic BP by about 20 mm Hg and diastolic BP by 10 mm Hg. Beta-blockers are not first-line antihypertension therapy, but can be considered in patients with coronary artery disease and heart failure with reduced left ventricular ejection fraction.
With regard to follow-up, patients with low ASCVD risk and stage 1 hypertension can be monitored in 3-6 months after lifestyle changes, while patients with high ASCVD risk and stage 1 hypertension should be followed up in 1 month. Patients with stage 2 hypertension should follow up with their primary care provider 1 month after beginning their therapy, and those with very high BP should promptly be started on drug treatment with lifestyle changes, with upward dose adjustments as needed.
In adults aged 65 years or older, the ACC/AHA guidelines also focused on how to prevent cognitive decline and dementia, said Dr. Davis. The goal for ambulatory, community-dwelling adults is still to have a systolic BP of less than 130 mm Hg, but clinical judgment should prevail because of comorbid conditions and limited life expectancy in these patients. Patient preference should also be considered, and clinicians should use a team-based approach with shared decision making to determine goals for each patient.
Dr. Davis reported no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Gram-negative bacteremia: Cultures, drugs, and duration
Are we doing it right?
Case
A 42-year-old woman with uncontrolled diabetes presents to the ED with fever, chills, dysuria, and flank pain for 3 days. On exam, she is febrile and tachycardic. Lab results show leukocytosis and urinalysis is consistent with infection. CT scan shows acute pyelonephritis without complication. She is admitted to the hospital and started on ceftriaxone 2 g/24 hrs. On hospital day 2, her blood cultures show gram-negative bacteria.
Brief overview
Management of gram-negative (GN) bacteremia remains a challenging clinical situation for inpatient providers. With the push for high-value care and reductions in length of stay, recent literature has focused on reviewing current practices and attempting to standardize care. Despite this, no overarching guidelines exist to direct practice and clinicians are left to make decisions based on prior experience and expert opinion. Three key clinical questions exist when caring for a hospitalized patient with GN bacteremia: Should blood cultures be repeated? When is transition to oral antibiotics appropriate? And for what duration should antibiotics be given?
Overview of the data
When considering repeating blood cultures, it is important to understand that current literature does not support the practice for all GN bacteremias.
Canzoneri et al. retrospectively studied GN bacteremia and found that it took 17 repeat blood cultures being drawn to yield 1 positive result, which suggests that they are not necessary in all cases.1 Furthermore, repeat blood cultures increase cost of hospitalization, length of stay, and inconvenience to patients.2
However, Mushtaq et al. noted that repeating blood cultures can provide valuable information to confirm the response to treatment in patients with endovascular infection. Furthermore, they found that repeated blood cultures are also reasonable when the following scenarios are suspected: endocarditis or central line–associated infection, concern for multidrug resistant GN bacilli, and ongoing evidence of sepsis or patient decompensation.3
Consideration of a transition from intravenous to oral antibiotics is a key decision point in the care of GN bacteremia. Without guidelines, clinicians are left to evaluate patients on a case-by-case basis.4 Studies have suggested that the transition should be guided by the condition of the patient, the type of infection, and the culture-derived sensitivities.5 Additionally, bioavailability of antibiotics (see Table 1) is an important consideration and a recent examination of oral antibiotic failure rates demonstrated that lower bioavailability antibiotics have an increased risk of failure (2% vs. 16%).6
In their study, Kutob et al. highlighted the importance of choosing not only an antibiotic of high bioavailability, but also an antibiotic dose which will support a high concentration of the antibiotic in the bloodstream.6 For example, they identify ciprofloxacin as a moderate bioavailability medication, but note that most cases they examined utilized 500 mg b.i.d., where the concentration-dependent killing and dose-dependent bioavailability would advocate for the use of 750 mg b.i.d. or 500 mg every 8 hours.
The heterogeneity of GN bloodstream infections also creates difficulty in standardization of care. The literature suggests that infection source plays a significant role in the type of GN bacteria isolated.6,7 The best data for the transition to oral antibiotics exists with urologic sources and it remains unclear whether bacteria from other sources have higher risks of oral antibiotic failure.8
One recent study of 66 patients examined bacteremia in the setting of cholangitis and found that, once patients had stabilized, a switch from intravenous to oral antibiotics was noninferior, but randomized, prospective trials have not been performed. Notably, patients were transitioned to orals only after they were found to have a fluoroquinolone-sensitive infection, allowing the study authors to use higher-bioavailability agents for the transition to orals.9 Multiple studies have highlighted the unique care required for certain infections, such as pseudomonal infections, which most experts agree requires a more conservative approach.5,6
Fluoroquinolones are the bedrock of therapy for GN bacteremia because of historic in vivo experience and in vitro findings about bioavailability and dose-dependent killing, but they are also the antibiotic class associated with the highest hospitalization rates for antibiotic-associated adverse events.8 A recent noninferiority trial comparing the use of beta-lactams with fluoroquinolones found that beta-lactams were noninferior, though the study was flawed by the limited number of beta-lactam–using patients identified.8 It is clear that more investigation is needed before recommendations can be made regarding ideal oral antibiotics for GN bacteremia.
The transition to oral is reasonable given the following criteria: the patient has improved on intravenous antibiotics and source control has been achieved; the culture data have demonstrated sensitivity to the oral antibiotic of choice, with special care given to higher-risk bacteria such as Pseudomonas; the patient is able to take the oral antibiotic; and the oral antibiotic of choice has the highest bioavailability possible and is given at an appropriate dose to reach its highest killing and bioavailability concentrations.7
After evaluating the appropriateness of transition to oral antibiotics, the final decision is about duration of antibiotic therapy. Current Infectious Disease Society of America guidelines are based on expert opinion and recommend 7-14 days of therapy. As with many common infections, recent studies have focused on evaluating reduction in antibiotic durations.
Chotiprasitsakul et al. demonstrated no difference in mortality or morbidity in 385 propensity-matched pairs with treatment of Enterobacteriaceae bacteremia for 8 versus 15 days.10 A mixed meta-analysis performed in 2011 evaluated 24 randomized, controlled trials and found shorter durations (5-7 days) had similar outcomes to prolonged durations (7-21 days).11 Recently, Yahav et al. performed a randomized control trial comparing 7- and 14-day regimens for uncomplicated GN bacteremia and found a 7-day course to be noninferior if patients were clinically stable by day 5 and had source control.12
It should be noted that not all studies have found that reduced durations are without harm. Nelson et al. performed a retrospective cohort analysis and found that reduced durations of antibiotics (7-10 days) increased mortality and recurrent infection when compared with a longer course (greater than 10 days).13 These contrary findings highlight the need for provider discretion in selecting a course of antibiotics as well as the need for further studies about optimal duration of antibiotics.
Application of the data
Returning to our case, on day 3, the patient’s fever had resolved and leukocytosis improved. In the absence of concern for persistent infection, repeat blood cultures were not performed. On day 4 initial blood cultures showed pan-sensitive Escherichia coli. The patient was transitioned to 750 mg oral ciprofloxacin b.i.d. to complete a 10-day course from first dose of ceftriaxone and was discharged from the hospital.
Bottom line
Management of GN bacteremia requires individualized care based on clinical presentation, but the data presented above can be used as broad guidelines to help reduce excess blood cultures, avoid prolonged use of intravenous antibiotics, and limit the duration of antibiotic exposure.
Dr. Imber is an assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, and director of the Internal Medicine Simulation Education and Hospitalist Procedural Certification. Dr. Burns is an assistant professor in the division of hospital medicine at the University of New Mexico. Dr. Chan is currently a chief resident in the department of internal medicine at the University of New Mexico.
References
1. Canzoneri CN et al. Follow-up blood cultures in gram-negative bacteremia: Are they needed? Clin Infect Dis. 2017;65(11):1776-9. doi: 10.1093/cid/cix648.
2. Kang CK et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365. doi: 10.1186/1471-2334-13-365.
3. Mushtaq A et al. Repeating blood cultures after an initial bacteremia: When and how often? Cleve Clin J Med. 2019;86(2):89-92. doi: 10.3949/ccjm.86a.18001.
4. Nimmich EB et al. Development of institutional guidelines for management of gram-negative bloodstream infections: Incorporating local evidence. Hosp Pharm. 2017;52(10):691-7. doi: 10.1177/0018578717720506.
5. Hale AJ et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018 May. doi: 10.12788/jhm.2949.
6. Kutob LF et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016. doi: 10.1016/j.ijantimicag.2016.07.013.
7. Tamma PD et al. Association of 30-day mortality with oral step-down vs. continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.6226.
8. Mercuro NJ et al. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones vs. B-lactams. Int J Antimicrob Agents. 2017. doi: 10.1016/j.ijantimicag.2017.12.007.
9. Park TY et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790-6. doi: 10.1007/s10620-014-3233-0.
10. Chotiprasitsakul D et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-7. doi:10.1093/cid/cix767.
11. Havey TC et al. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi:10.1186/cc10545.
12. Yahav D et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2018 Dec. doi:10.1093/cid/ciy1054.
13. Nelson AN et al. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection. 2017;45(5):613-20. doi:10.1007/s15010-017-1020-5.
Are we doing it right?
Are we doing it right?
Case
A 42-year-old woman with uncontrolled diabetes presents to the ED with fever, chills, dysuria, and flank pain for 3 days. On exam, she is febrile and tachycardic. Lab results show leukocytosis and urinalysis is consistent with infection. CT scan shows acute pyelonephritis without complication. She is admitted to the hospital and started on ceftriaxone 2 g/24 hrs. On hospital day 2, her blood cultures show gram-negative bacteria.
Brief overview
Management of gram-negative (GN) bacteremia remains a challenging clinical situation for inpatient providers. With the push for high-value care and reductions in length of stay, recent literature has focused on reviewing current practices and attempting to standardize care. Despite this, no overarching guidelines exist to direct practice and clinicians are left to make decisions based on prior experience and expert opinion. Three key clinical questions exist when caring for a hospitalized patient with GN bacteremia: Should blood cultures be repeated? When is transition to oral antibiotics appropriate? And for what duration should antibiotics be given?
Overview of the data
When considering repeating blood cultures, it is important to understand that current literature does not support the practice for all GN bacteremias.
Canzoneri et al. retrospectively studied GN bacteremia and found that it took 17 repeat blood cultures being drawn to yield 1 positive result, which suggests that they are not necessary in all cases.1 Furthermore, repeat blood cultures increase cost of hospitalization, length of stay, and inconvenience to patients.2
However, Mushtaq et al. noted that repeating blood cultures can provide valuable information to confirm the response to treatment in patients with endovascular infection. Furthermore, they found that repeated blood cultures are also reasonable when the following scenarios are suspected: endocarditis or central line–associated infection, concern for multidrug resistant GN bacilli, and ongoing evidence of sepsis or patient decompensation.3
Consideration of a transition from intravenous to oral antibiotics is a key decision point in the care of GN bacteremia. Without guidelines, clinicians are left to evaluate patients on a case-by-case basis.4 Studies have suggested that the transition should be guided by the condition of the patient, the type of infection, and the culture-derived sensitivities.5 Additionally, bioavailability of antibiotics (see Table 1) is an important consideration and a recent examination of oral antibiotic failure rates demonstrated that lower bioavailability antibiotics have an increased risk of failure (2% vs. 16%).6
In their study, Kutob et al. highlighted the importance of choosing not only an antibiotic of high bioavailability, but also an antibiotic dose which will support a high concentration of the antibiotic in the bloodstream.6 For example, they identify ciprofloxacin as a moderate bioavailability medication, but note that most cases they examined utilized 500 mg b.i.d., where the concentration-dependent killing and dose-dependent bioavailability would advocate for the use of 750 mg b.i.d. or 500 mg every 8 hours.
The heterogeneity of GN bloodstream infections also creates difficulty in standardization of care. The literature suggests that infection source plays a significant role in the type of GN bacteria isolated.6,7 The best data for the transition to oral antibiotics exists with urologic sources and it remains unclear whether bacteria from other sources have higher risks of oral antibiotic failure.8
One recent study of 66 patients examined bacteremia in the setting of cholangitis and found that, once patients had stabilized, a switch from intravenous to oral antibiotics was noninferior, but randomized, prospective trials have not been performed. Notably, patients were transitioned to orals only after they were found to have a fluoroquinolone-sensitive infection, allowing the study authors to use higher-bioavailability agents for the transition to orals.9 Multiple studies have highlighted the unique care required for certain infections, such as pseudomonal infections, which most experts agree requires a more conservative approach.5,6
Fluoroquinolones are the bedrock of therapy for GN bacteremia because of historic in vivo experience and in vitro findings about bioavailability and dose-dependent killing, but they are also the antibiotic class associated with the highest hospitalization rates for antibiotic-associated adverse events.8 A recent noninferiority trial comparing the use of beta-lactams with fluoroquinolones found that beta-lactams were noninferior, though the study was flawed by the limited number of beta-lactam–using patients identified.8 It is clear that more investigation is needed before recommendations can be made regarding ideal oral antibiotics for GN bacteremia.
The transition to oral is reasonable given the following criteria: the patient has improved on intravenous antibiotics and source control has been achieved; the culture data have demonstrated sensitivity to the oral antibiotic of choice, with special care given to higher-risk bacteria such as Pseudomonas; the patient is able to take the oral antibiotic; and the oral antibiotic of choice has the highest bioavailability possible and is given at an appropriate dose to reach its highest killing and bioavailability concentrations.7
After evaluating the appropriateness of transition to oral antibiotics, the final decision is about duration of antibiotic therapy. Current Infectious Disease Society of America guidelines are based on expert opinion and recommend 7-14 days of therapy. As with many common infections, recent studies have focused on evaluating reduction in antibiotic durations.
Chotiprasitsakul et al. demonstrated no difference in mortality or morbidity in 385 propensity-matched pairs with treatment of Enterobacteriaceae bacteremia for 8 versus 15 days.10 A mixed meta-analysis performed in 2011 evaluated 24 randomized, controlled trials and found shorter durations (5-7 days) had similar outcomes to prolonged durations (7-21 days).11 Recently, Yahav et al. performed a randomized control trial comparing 7- and 14-day regimens for uncomplicated GN bacteremia and found a 7-day course to be noninferior if patients were clinically stable by day 5 and had source control.12
It should be noted that not all studies have found that reduced durations are without harm. Nelson et al. performed a retrospective cohort analysis and found that reduced durations of antibiotics (7-10 days) increased mortality and recurrent infection when compared with a longer course (greater than 10 days).13 These contrary findings highlight the need for provider discretion in selecting a course of antibiotics as well as the need for further studies about optimal duration of antibiotics.
Application of the data
Returning to our case, on day 3, the patient’s fever had resolved and leukocytosis improved. In the absence of concern for persistent infection, repeat blood cultures were not performed. On day 4 initial blood cultures showed pan-sensitive Escherichia coli. The patient was transitioned to 750 mg oral ciprofloxacin b.i.d. to complete a 10-day course from first dose of ceftriaxone and was discharged from the hospital.
Bottom line
Management of GN bacteremia requires individualized care based on clinical presentation, but the data presented above can be used as broad guidelines to help reduce excess blood cultures, avoid prolonged use of intravenous antibiotics, and limit the duration of antibiotic exposure.
Dr. Imber is an assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, and director of the Internal Medicine Simulation Education and Hospitalist Procedural Certification. Dr. Burns is an assistant professor in the division of hospital medicine at the University of New Mexico. Dr. Chan is currently a chief resident in the department of internal medicine at the University of New Mexico.
References
1. Canzoneri CN et al. Follow-up blood cultures in gram-negative bacteremia: Are they needed? Clin Infect Dis. 2017;65(11):1776-9. doi: 10.1093/cid/cix648.
2. Kang CK et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365. doi: 10.1186/1471-2334-13-365.
3. Mushtaq A et al. Repeating blood cultures after an initial bacteremia: When and how often? Cleve Clin J Med. 2019;86(2):89-92. doi: 10.3949/ccjm.86a.18001.
4. Nimmich EB et al. Development of institutional guidelines for management of gram-negative bloodstream infections: Incorporating local evidence. Hosp Pharm. 2017;52(10):691-7. doi: 10.1177/0018578717720506.
5. Hale AJ et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018 May. doi: 10.12788/jhm.2949.
6. Kutob LF et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016. doi: 10.1016/j.ijantimicag.2016.07.013.
7. Tamma PD et al. Association of 30-day mortality with oral step-down vs. continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.6226.
8. Mercuro NJ et al. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones vs. B-lactams. Int J Antimicrob Agents. 2017. doi: 10.1016/j.ijantimicag.2017.12.007.
9. Park TY et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790-6. doi: 10.1007/s10620-014-3233-0.
10. Chotiprasitsakul D et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-7. doi:10.1093/cid/cix767.
11. Havey TC et al. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi:10.1186/cc10545.
12. Yahav D et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2018 Dec. doi:10.1093/cid/ciy1054.
13. Nelson AN et al. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection. 2017;45(5):613-20. doi:10.1007/s15010-017-1020-5.
Case
A 42-year-old woman with uncontrolled diabetes presents to the ED with fever, chills, dysuria, and flank pain for 3 days. On exam, she is febrile and tachycardic. Lab results show leukocytosis and urinalysis is consistent with infection. CT scan shows acute pyelonephritis without complication. She is admitted to the hospital and started on ceftriaxone 2 g/24 hrs. On hospital day 2, her blood cultures show gram-negative bacteria.
Brief overview
Management of gram-negative (GN) bacteremia remains a challenging clinical situation for inpatient providers. With the push for high-value care and reductions in length of stay, recent literature has focused on reviewing current practices and attempting to standardize care. Despite this, no overarching guidelines exist to direct practice and clinicians are left to make decisions based on prior experience and expert opinion. Three key clinical questions exist when caring for a hospitalized patient with GN bacteremia: Should blood cultures be repeated? When is transition to oral antibiotics appropriate? And for what duration should antibiotics be given?
Overview of the data
When considering repeating blood cultures, it is important to understand that current literature does not support the practice for all GN bacteremias.
Canzoneri et al. retrospectively studied GN bacteremia and found that it took 17 repeat blood cultures being drawn to yield 1 positive result, which suggests that they are not necessary in all cases.1 Furthermore, repeat blood cultures increase cost of hospitalization, length of stay, and inconvenience to patients.2
However, Mushtaq et al. noted that repeating blood cultures can provide valuable information to confirm the response to treatment in patients with endovascular infection. Furthermore, they found that repeated blood cultures are also reasonable when the following scenarios are suspected: endocarditis or central line–associated infection, concern for multidrug resistant GN bacilli, and ongoing evidence of sepsis or patient decompensation.3
Consideration of a transition from intravenous to oral antibiotics is a key decision point in the care of GN bacteremia. Without guidelines, clinicians are left to evaluate patients on a case-by-case basis.4 Studies have suggested that the transition should be guided by the condition of the patient, the type of infection, and the culture-derived sensitivities.5 Additionally, bioavailability of antibiotics (see Table 1) is an important consideration and a recent examination of oral antibiotic failure rates demonstrated that lower bioavailability antibiotics have an increased risk of failure (2% vs. 16%).6
In their study, Kutob et al. highlighted the importance of choosing not only an antibiotic of high bioavailability, but also an antibiotic dose which will support a high concentration of the antibiotic in the bloodstream.6 For example, they identify ciprofloxacin as a moderate bioavailability medication, but note that most cases they examined utilized 500 mg b.i.d., where the concentration-dependent killing and dose-dependent bioavailability would advocate for the use of 750 mg b.i.d. or 500 mg every 8 hours.
The heterogeneity of GN bloodstream infections also creates difficulty in standardization of care. The literature suggests that infection source plays a significant role in the type of GN bacteria isolated.6,7 The best data for the transition to oral antibiotics exists with urologic sources and it remains unclear whether bacteria from other sources have higher risks of oral antibiotic failure.8
One recent study of 66 patients examined bacteremia in the setting of cholangitis and found that, once patients had stabilized, a switch from intravenous to oral antibiotics was noninferior, but randomized, prospective trials have not been performed. Notably, patients were transitioned to orals only after they were found to have a fluoroquinolone-sensitive infection, allowing the study authors to use higher-bioavailability agents for the transition to orals.9 Multiple studies have highlighted the unique care required for certain infections, such as pseudomonal infections, which most experts agree requires a more conservative approach.5,6
Fluoroquinolones are the bedrock of therapy for GN bacteremia because of historic in vivo experience and in vitro findings about bioavailability and dose-dependent killing, but they are also the antibiotic class associated with the highest hospitalization rates for antibiotic-associated adverse events.8 A recent noninferiority trial comparing the use of beta-lactams with fluoroquinolones found that beta-lactams were noninferior, though the study was flawed by the limited number of beta-lactam–using patients identified.8 It is clear that more investigation is needed before recommendations can be made regarding ideal oral antibiotics for GN bacteremia.
The transition to oral is reasonable given the following criteria: the patient has improved on intravenous antibiotics and source control has been achieved; the culture data have demonstrated sensitivity to the oral antibiotic of choice, with special care given to higher-risk bacteria such as Pseudomonas; the patient is able to take the oral antibiotic; and the oral antibiotic of choice has the highest bioavailability possible and is given at an appropriate dose to reach its highest killing and bioavailability concentrations.7
After evaluating the appropriateness of transition to oral antibiotics, the final decision is about duration of antibiotic therapy. Current Infectious Disease Society of America guidelines are based on expert opinion and recommend 7-14 days of therapy. As with many common infections, recent studies have focused on evaluating reduction in antibiotic durations.
Chotiprasitsakul et al. demonstrated no difference in mortality or morbidity in 385 propensity-matched pairs with treatment of Enterobacteriaceae bacteremia for 8 versus 15 days.10 A mixed meta-analysis performed in 2011 evaluated 24 randomized, controlled trials and found shorter durations (5-7 days) had similar outcomes to prolonged durations (7-21 days).11 Recently, Yahav et al. performed a randomized control trial comparing 7- and 14-day regimens for uncomplicated GN bacteremia and found a 7-day course to be noninferior if patients were clinically stable by day 5 and had source control.12
It should be noted that not all studies have found that reduced durations are without harm. Nelson et al. performed a retrospective cohort analysis and found that reduced durations of antibiotics (7-10 days) increased mortality and recurrent infection when compared with a longer course (greater than 10 days).13 These contrary findings highlight the need for provider discretion in selecting a course of antibiotics as well as the need for further studies about optimal duration of antibiotics.
Application of the data
Returning to our case, on day 3, the patient’s fever had resolved and leukocytosis improved. In the absence of concern for persistent infection, repeat blood cultures were not performed. On day 4 initial blood cultures showed pan-sensitive Escherichia coli. The patient was transitioned to 750 mg oral ciprofloxacin b.i.d. to complete a 10-day course from first dose of ceftriaxone and was discharged from the hospital.
Bottom line
Management of GN bacteremia requires individualized care based on clinical presentation, but the data presented above can be used as broad guidelines to help reduce excess blood cultures, avoid prolonged use of intravenous antibiotics, and limit the duration of antibiotic exposure.
Dr. Imber is an assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, and director of the Internal Medicine Simulation Education and Hospitalist Procedural Certification. Dr. Burns is an assistant professor in the division of hospital medicine at the University of New Mexico. Dr. Chan is currently a chief resident in the department of internal medicine at the University of New Mexico.
References
1. Canzoneri CN et al. Follow-up blood cultures in gram-negative bacteremia: Are they needed? Clin Infect Dis. 2017;65(11):1776-9. doi: 10.1093/cid/cix648.
2. Kang CK et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365. doi: 10.1186/1471-2334-13-365.
3. Mushtaq A et al. Repeating blood cultures after an initial bacteremia: When and how often? Cleve Clin J Med. 2019;86(2):89-92. doi: 10.3949/ccjm.86a.18001.
4. Nimmich EB et al. Development of institutional guidelines for management of gram-negative bloodstream infections: Incorporating local evidence. Hosp Pharm. 2017;52(10):691-7. doi: 10.1177/0018578717720506.
5. Hale AJ et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018 May. doi: 10.12788/jhm.2949.
6. Kutob LF et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016. doi: 10.1016/j.ijantimicag.2016.07.013.
7. Tamma PD et al. Association of 30-day mortality with oral step-down vs. continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.6226.
8. Mercuro NJ et al. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones vs. B-lactams. Int J Antimicrob Agents. 2017. doi: 10.1016/j.ijantimicag.2017.12.007.
9. Park TY et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790-6. doi: 10.1007/s10620-014-3233-0.
10. Chotiprasitsakul D et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-7. doi:10.1093/cid/cix767.
11. Havey TC et al. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi:10.1186/cc10545.
12. Yahav D et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2018 Dec. doi:10.1093/cid/ciy1054.
13. Nelson AN et al. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection. 2017;45(5):613-20. doi:10.1007/s15010-017-1020-5.
Beyond C. difficile: The future of fecal microbial transplantation
SAN DIEGO – Two leading figures in microbiome research took time during the annual Digestive Disease Week to share their perspective with members of the press. Identifying key research presented at the meeting and painting a broader picture of trends and challenges in research on the interplay with the microbiota with gut health, Colleen Kelly, MD, of Brown University, Providence, R.I., cochair and principal investigator, AGA FMT Registry Steering Committee, led off the round table with a discussion of human research on fecal microbial transplantation (FMT) and obesity.
Purna Kashyap, MBBS, of the Mayo Clinic, Rochester, Minn., member, AGA Center for Gut Microbiome Research and Education Scientific Advisory Board, delved into the potential for donor microbiota transplant to address small bowel disorders, such as small intestinal bacterial overgrowth, and provided commentary regarding the potential – and limitations – of using FMT in functional bowel disorders such as irritable bowel syndrome (IBS).
Obesity
Dr. Kelly noted that two abstracts at the conference presented data about FMT in obesity. The first study was presented by Jessica Allegretti, MD, a gastroenterologist at Brigham and Women’s Hospital, Boston; the second study was presented by Elaine Yu, MD, an endocrinologist at Massachusetts General Hospital, Boston.
The two studies shared some similarities, but had some differences, said Dr. Kelly. “They both used lean donor encapsulated FMT ... and they both were placebo controlled, using placebo capsules.” The first study looked at metabolically healthy obese patients, while the second study included patients with mild to moderate insulin resistance.
Dr. Allegretti’s work looked at the effect that FMT from lean donors had on levels of a satiety peptide, glucagonlike peptide–1 (GLP-1), while also looking at changes in weight and microbiota, as well as safety. Patients received an initial 30-capsule dose as well as two later doses of 12 capsules each. The 22-patient study, in which individuals were randomized 1:1 to FMT or placebo capsules, didn’t show statistically significant changes in GLP-1 levels or body mass index with FMT over the 12-week study period. “But they were able to show engraftment, which I think is an important thing that you do wonder about – over this period of time, the bacteria that came from the lean donor actually engrafted into the recipient and affected the diversity. The recipient became more similar to the donor,” said Dr. Kelly.
There were some clues among the findings that engraftment was effecting metabolic change in the recipient, she said, including differences in bile acid conversion among gut bacteria; also, lower levels of the primary bile acid taurocholic acid in recipients after FMT. “So it was a negative study in what she was looking for, but an example of these smaller studies kind of pushing the field along.”
In discussing the study presented by Dr. Yu that examined lean donor FMT in individuals with insulin resistance, Dr. Kelly said, “It was actually pretty sophisticated.” By using hyperinsulinemic euglycemic clamping, the investigators were able to measure insulin sensitivity based on glucose load. In this study of 24 individuals randomized 1:1 to receive FMT or placebo capsules, recipients received weekly doses over a period of 6 weeks. Here again, though Dr. Yu and colleagues again found engraftment, “they did not find the big changes in metabolic parameters that they hoped that they would,” said Dr. Kelly.
These two studies furthered previous work completed in the Netherlands examining lean donor FMT for individuals with metabolic syndrome. “Those [studies] did show both engraftment and some changes in insulin resistance,” but they were also small studies, noted Dr. Kelly. Dr. Kashyap pointed out that the earlier studies had shown in a subgroup analysis that response to FMT was limited to those patients who lacked microbial diversity pretransplant.
This makes some mechanistic sense when thinking about FMT’s greatest success to date: Treating Clostridioides difficile infection, a condition whose very hallmark is dysbiosis characterized by monospecies gut domination, noted Dr. Kashyap.
Added Dr. Kelly, “I think everyone’s hoping there’s going to be this pill that’s going to make us skinny, but I don’t think we’re going to find that with FMT and obesity. I do think these studies are important, because there’s so much animal data already, and we’re kind of like, ‘How much more can you do in mice?’ ” By translating this preclinical work into humans, the mechanisms of obesity and the role of the microbiome can be better understood.
IBS
Turning to functional bowel disorders, Dr. Kelly pointed out a new study examining FMT for IBS. “So far, the research has been pretty disappointing,” she noted. The study, presented by Prashant Singh, MBBS, a gastroenterology fellow at Beth Israel Deaconess Medical Center, Boston, examined FMT for patients with moderate to severe diarrhea-predominant IBS. The study had four arms, randomizing the 44 participants to FMT alone; pretreatment with metronidazole and ciprofloxacin or rifaximin, each followed by FMT; or placebo. “It really didn’t show any differences in their severity of IBS in any group; so no effect: a negative study,” said Dr. Kelly.
“It’s challenging. FMT is an appealing strategy because we don’t have to put a lot of thought process into it,” said Dr. Kashyap, but it’s no panacea. “We aren’t going to be able to treat everybody with FMT.
“The challenge with IBS is that if we look at all the compositional studies, the majority of them show that at least a big subset of patients with IBS already have a normal-appearing microbiome,” said Dr. Kashyap. In those patients, “It’s very hard to know what FMT is doing.” He noted that IBS subclassifications are made by pathophysiology, without regard to the intestinal microbiome, so these classifications may not be useful for determining who may benefit from FMT.
“Again, there’s always an opportunity to learn from these studies,” whether they’re positive or negative, as long as they’re well done, said Dr. Kashyap. “There’s always an opportunity to go back and see, was there a specific subgroup of patients who responded, where there might be one or more causes which might be more amenable” to FMT.
Small intestine
And most intestinal microbial research to date, noted Dr. Kelly, has focused on the colon. “Most of our knowledge is of fecal microbiota.” New techniques including double-balloon enteroscopy of the small bowel have promise to “provide completely new information about patterns of bacteria throughout the small bowel,” and of the role of small bowel bacteria in overall gut health, she said.
“The role of the small bowel has been ignored because of accessibility,” agreed Dr. Kashyap. There’s a current focus on research into enteroscopy and other techniques to sample small intestine microbiota, he said.
In a podium presentation, Eugene Chang, MD, Martin Boyer Professor in the University of Chicago’s department of medicine, gave a broad overview of how the small intestine microbiome modulates lipid regulation. This choice of topic for the Charles M. Mansbach Memorial Lecture shows that gastroenterologists are recognizing the importance of microbiome along the entire span of the gut, said Dr. Kashyap. Dr. Chang’s approach, he added, represents a departure in that “it’s not simply just looking at what’s present and what’s not, but seeing what’s functionally relevant to metabolism.”
“We always have known that the small intestine is the workhorse; that’s where everything happens” in terms of motility, absorption, and digestion, said Dr. Kashyap. “But because of our inability to reach it easily we’ve always chosen to ignore it; we always go after the low-hanging fruit.” Despite challenges, more microbiome research should be small-bowel focused. “Eventually, it’s no pain, no gain.”
Dr. Kashyap is on the advisory board of uBiome, and is an ad hoc advisory board member for Salix Pharmaceuticals. Dr. Kelly reported no conflicts of interest.
This story was updated on July 30, 2019.
SAN DIEGO – Two leading figures in microbiome research took time during the annual Digestive Disease Week to share their perspective with members of the press. Identifying key research presented at the meeting and painting a broader picture of trends and challenges in research on the interplay with the microbiota with gut health, Colleen Kelly, MD, of Brown University, Providence, R.I., cochair and principal investigator, AGA FMT Registry Steering Committee, led off the round table with a discussion of human research on fecal microbial transplantation (FMT) and obesity.
Purna Kashyap, MBBS, of the Mayo Clinic, Rochester, Minn., member, AGA Center for Gut Microbiome Research and Education Scientific Advisory Board, delved into the potential for donor microbiota transplant to address small bowel disorders, such as small intestinal bacterial overgrowth, and provided commentary regarding the potential – and limitations – of using FMT in functional bowel disorders such as irritable bowel syndrome (IBS).
Obesity
Dr. Kelly noted that two abstracts at the conference presented data about FMT in obesity. The first study was presented by Jessica Allegretti, MD, a gastroenterologist at Brigham and Women’s Hospital, Boston; the second study was presented by Elaine Yu, MD, an endocrinologist at Massachusetts General Hospital, Boston.
The two studies shared some similarities, but had some differences, said Dr. Kelly. “They both used lean donor encapsulated FMT ... and they both were placebo controlled, using placebo capsules.” The first study looked at metabolically healthy obese patients, while the second study included patients with mild to moderate insulin resistance.
Dr. Allegretti’s work looked at the effect that FMT from lean donors had on levels of a satiety peptide, glucagonlike peptide–1 (GLP-1), while also looking at changes in weight and microbiota, as well as safety. Patients received an initial 30-capsule dose as well as two later doses of 12 capsules each. The 22-patient study, in which individuals were randomized 1:1 to FMT or placebo capsules, didn’t show statistically significant changes in GLP-1 levels or body mass index with FMT over the 12-week study period. “But they were able to show engraftment, which I think is an important thing that you do wonder about – over this period of time, the bacteria that came from the lean donor actually engrafted into the recipient and affected the diversity. The recipient became more similar to the donor,” said Dr. Kelly.
There were some clues among the findings that engraftment was effecting metabolic change in the recipient, she said, including differences in bile acid conversion among gut bacteria; also, lower levels of the primary bile acid taurocholic acid in recipients after FMT. “So it was a negative study in what she was looking for, but an example of these smaller studies kind of pushing the field along.”
In discussing the study presented by Dr. Yu that examined lean donor FMT in individuals with insulin resistance, Dr. Kelly said, “It was actually pretty sophisticated.” By using hyperinsulinemic euglycemic clamping, the investigators were able to measure insulin sensitivity based on glucose load. In this study of 24 individuals randomized 1:1 to receive FMT or placebo capsules, recipients received weekly doses over a period of 6 weeks. Here again, though Dr. Yu and colleagues again found engraftment, “they did not find the big changes in metabolic parameters that they hoped that they would,” said Dr. Kelly.
These two studies furthered previous work completed in the Netherlands examining lean donor FMT for individuals with metabolic syndrome. “Those [studies] did show both engraftment and some changes in insulin resistance,” but they were also small studies, noted Dr. Kelly. Dr. Kashyap pointed out that the earlier studies had shown in a subgroup analysis that response to FMT was limited to those patients who lacked microbial diversity pretransplant.
This makes some mechanistic sense when thinking about FMT’s greatest success to date: Treating Clostridioides difficile infection, a condition whose very hallmark is dysbiosis characterized by monospecies gut domination, noted Dr. Kashyap.
Added Dr. Kelly, “I think everyone’s hoping there’s going to be this pill that’s going to make us skinny, but I don’t think we’re going to find that with FMT and obesity. I do think these studies are important, because there’s so much animal data already, and we’re kind of like, ‘How much more can you do in mice?’ ” By translating this preclinical work into humans, the mechanisms of obesity and the role of the microbiome can be better understood.
IBS
Turning to functional bowel disorders, Dr. Kelly pointed out a new study examining FMT for IBS. “So far, the research has been pretty disappointing,” she noted. The study, presented by Prashant Singh, MBBS, a gastroenterology fellow at Beth Israel Deaconess Medical Center, Boston, examined FMT for patients with moderate to severe diarrhea-predominant IBS. The study had four arms, randomizing the 44 participants to FMT alone; pretreatment with metronidazole and ciprofloxacin or rifaximin, each followed by FMT; or placebo. “It really didn’t show any differences in their severity of IBS in any group; so no effect: a negative study,” said Dr. Kelly.
“It’s challenging. FMT is an appealing strategy because we don’t have to put a lot of thought process into it,” said Dr. Kashyap, but it’s no panacea. “We aren’t going to be able to treat everybody with FMT.
“The challenge with IBS is that if we look at all the compositional studies, the majority of them show that at least a big subset of patients with IBS already have a normal-appearing microbiome,” said Dr. Kashyap. In those patients, “It’s very hard to know what FMT is doing.” He noted that IBS subclassifications are made by pathophysiology, without regard to the intestinal microbiome, so these classifications may not be useful for determining who may benefit from FMT.
“Again, there’s always an opportunity to learn from these studies,” whether they’re positive or negative, as long as they’re well done, said Dr. Kashyap. “There’s always an opportunity to go back and see, was there a specific subgroup of patients who responded, where there might be one or more causes which might be more amenable” to FMT.
Small intestine
And most intestinal microbial research to date, noted Dr. Kelly, has focused on the colon. “Most of our knowledge is of fecal microbiota.” New techniques including double-balloon enteroscopy of the small bowel have promise to “provide completely new information about patterns of bacteria throughout the small bowel,” and of the role of small bowel bacteria in overall gut health, she said.
“The role of the small bowel has been ignored because of accessibility,” agreed Dr. Kashyap. There’s a current focus on research into enteroscopy and other techniques to sample small intestine microbiota, he said.
In a podium presentation, Eugene Chang, MD, Martin Boyer Professor in the University of Chicago’s department of medicine, gave a broad overview of how the small intestine microbiome modulates lipid regulation. This choice of topic for the Charles M. Mansbach Memorial Lecture shows that gastroenterologists are recognizing the importance of microbiome along the entire span of the gut, said Dr. Kashyap. Dr. Chang’s approach, he added, represents a departure in that “it’s not simply just looking at what’s present and what’s not, but seeing what’s functionally relevant to metabolism.”
“We always have known that the small intestine is the workhorse; that’s where everything happens” in terms of motility, absorption, and digestion, said Dr. Kashyap. “But because of our inability to reach it easily we’ve always chosen to ignore it; we always go after the low-hanging fruit.” Despite challenges, more microbiome research should be small-bowel focused. “Eventually, it’s no pain, no gain.”
Dr. Kashyap is on the advisory board of uBiome, and is an ad hoc advisory board member for Salix Pharmaceuticals. Dr. Kelly reported no conflicts of interest.
This story was updated on July 30, 2019.
SAN DIEGO – Two leading figures in microbiome research took time during the annual Digestive Disease Week to share their perspective with members of the press. Identifying key research presented at the meeting and painting a broader picture of trends and challenges in research on the interplay with the microbiota with gut health, Colleen Kelly, MD, of Brown University, Providence, R.I., cochair and principal investigator, AGA FMT Registry Steering Committee, led off the round table with a discussion of human research on fecal microbial transplantation (FMT) and obesity.
Purna Kashyap, MBBS, of the Mayo Clinic, Rochester, Minn., member, AGA Center for Gut Microbiome Research and Education Scientific Advisory Board, delved into the potential for donor microbiota transplant to address small bowel disorders, such as small intestinal bacterial overgrowth, and provided commentary regarding the potential – and limitations – of using FMT in functional bowel disorders such as irritable bowel syndrome (IBS).
Obesity
Dr. Kelly noted that two abstracts at the conference presented data about FMT in obesity. The first study was presented by Jessica Allegretti, MD, a gastroenterologist at Brigham and Women’s Hospital, Boston; the second study was presented by Elaine Yu, MD, an endocrinologist at Massachusetts General Hospital, Boston.
The two studies shared some similarities, but had some differences, said Dr. Kelly. “They both used lean donor encapsulated FMT ... and they both were placebo controlled, using placebo capsules.” The first study looked at metabolically healthy obese patients, while the second study included patients with mild to moderate insulin resistance.
Dr. Allegretti’s work looked at the effect that FMT from lean donors had on levels of a satiety peptide, glucagonlike peptide–1 (GLP-1), while also looking at changes in weight and microbiota, as well as safety. Patients received an initial 30-capsule dose as well as two later doses of 12 capsules each. The 22-patient study, in which individuals were randomized 1:1 to FMT or placebo capsules, didn’t show statistically significant changes in GLP-1 levels or body mass index with FMT over the 12-week study period. “But they were able to show engraftment, which I think is an important thing that you do wonder about – over this period of time, the bacteria that came from the lean donor actually engrafted into the recipient and affected the diversity. The recipient became more similar to the donor,” said Dr. Kelly.
There were some clues among the findings that engraftment was effecting metabolic change in the recipient, she said, including differences in bile acid conversion among gut bacteria; also, lower levels of the primary bile acid taurocholic acid in recipients after FMT. “So it was a negative study in what she was looking for, but an example of these smaller studies kind of pushing the field along.”
In discussing the study presented by Dr. Yu that examined lean donor FMT in individuals with insulin resistance, Dr. Kelly said, “It was actually pretty sophisticated.” By using hyperinsulinemic euglycemic clamping, the investigators were able to measure insulin sensitivity based on glucose load. In this study of 24 individuals randomized 1:1 to receive FMT or placebo capsules, recipients received weekly doses over a period of 6 weeks. Here again, though Dr. Yu and colleagues again found engraftment, “they did not find the big changes in metabolic parameters that they hoped that they would,” said Dr. Kelly.
These two studies furthered previous work completed in the Netherlands examining lean donor FMT for individuals with metabolic syndrome. “Those [studies] did show both engraftment and some changes in insulin resistance,” but they were also small studies, noted Dr. Kelly. Dr. Kashyap pointed out that the earlier studies had shown in a subgroup analysis that response to FMT was limited to those patients who lacked microbial diversity pretransplant.
This makes some mechanistic sense when thinking about FMT’s greatest success to date: Treating Clostridioides difficile infection, a condition whose very hallmark is dysbiosis characterized by monospecies gut domination, noted Dr. Kashyap.
Added Dr. Kelly, “I think everyone’s hoping there’s going to be this pill that’s going to make us skinny, but I don’t think we’re going to find that with FMT and obesity. I do think these studies are important, because there’s so much animal data already, and we’re kind of like, ‘How much more can you do in mice?’ ” By translating this preclinical work into humans, the mechanisms of obesity and the role of the microbiome can be better understood.
IBS
Turning to functional bowel disorders, Dr. Kelly pointed out a new study examining FMT for IBS. “So far, the research has been pretty disappointing,” she noted. The study, presented by Prashant Singh, MBBS, a gastroenterology fellow at Beth Israel Deaconess Medical Center, Boston, examined FMT for patients with moderate to severe diarrhea-predominant IBS. The study had four arms, randomizing the 44 participants to FMT alone; pretreatment with metronidazole and ciprofloxacin or rifaximin, each followed by FMT; or placebo. “It really didn’t show any differences in their severity of IBS in any group; so no effect: a negative study,” said Dr. Kelly.
“It’s challenging. FMT is an appealing strategy because we don’t have to put a lot of thought process into it,” said Dr. Kashyap, but it’s no panacea. “We aren’t going to be able to treat everybody with FMT.
“The challenge with IBS is that if we look at all the compositional studies, the majority of them show that at least a big subset of patients with IBS already have a normal-appearing microbiome,” said Dr. Kashyap. In those patients, “It’s very hard to know what FMT is doing.” He noted that IBS subclassifications are made by pathophysiology, without regard to the intestinal microbiome, so these classifications may not be useful for determining who may benefit from FMT.
“Again, there’s always an opportunity to learn from these studies,” whether they’re positive or negative, as long as they’re well done, said Dr. Kashyap. “There’s always an opportunity to go back and see, was there a specific subgroup of patients who responded, where there might be one or more causes which might be more amenable” to FMT.
Small intestine
And most intestinal microbial research to date, noted Dr. Kelly, has focused on the colon. “Most of our knowledge is of fecal microbiota.” New techniques including double-balloon enteroscopy of the small bowel have promise to “provide completely new information about patterns of bacteria throughout the small bowel,” and of the role of small bowel bacteria in overall gut health, she said.
“The role of the small bowel has been ignored because of accessibility,” agreed Dr. Kashyap. There’s a current focus on research into enteroscopy and other techniques to sample small intestine microbiota, he said.
In a podium presentation, Eugene Chang, MD, Martin Boyer Professor in the University of Chicago’s department of medicine, gave a broad overview of how the small intestine microbiome modulates lipid regulation. This choice of topic for the Charles M. Mansbach Memorial Lecture shows that gastroenterologists are recognizing the importance of microbiome along the entire span of the gut, said Dr. Kashyap. Dr. Chang’s approach, he added, represents a departure in that “it’s not simply just looking at what’s present and what’s not, but seeing what’s functionally relevant to metabolism.”
“We always have known that the small intestine is the workhorse; that’s where everything happens” in terms of motility, absorption, and digestion, said Dr. Kashyap. “But because of our inability to reach it easily we’ve always chosen to ignore it; we always go after the low-hanging fruit.” Despite challenges, more microbiome research should be small-bowel focused. “Eventually, it’s no pain, no gain.”
Dr. Kashyap is on the advisory board of uBiome, and is an ad hoc advisory board member for Salix Pharmaceuticals. Dr. Kelly reported no conflicts of interest.
This story was updated on July 30, 2019.
EXPERT ANALYSIS FROM DDW 2019
Older adults’ interested in conversations about deprescribing
Clinical question: Among older adults, what attitudes exist toward deprescribing?
Background: Polypharmacy in older adults is common and can be associated with increased hospitalizations and reduced quality of life.
Study design: Population-based survey study.
Setting: Medicare beneficiaries in the United States.
Synopsis: The investigators used data from the National Health and Aging Trends Study (NHATS), which collects information annually on a nationally representative sample of Medicare beneficiaries ages 65 and older. Of 1,981 responses on the NHATS Medication Attitudes module, 92% of older adults expressed willingness to stop a medication if their doctor said it was possible. While 89% agreed that all their medications were necessary, 66.6% also agreed that they would like to reduce the number of their medications. Patients taking more than six medications, compared with those taking fewer than six (adjusted odds ratio, 2.9; 95% confidence interval, 1.74-4.82) and those with three or more medical conditions, compared with patients with fewer than two (aOR 2.87; 95% CI 1.53-5.37) had greater odds of willingness to stop a medication. Importantly, the study did not collect data about specific medications.
Bottom line: A vast majority of older adults would be willing to stop one or more of their medications if considered possible by their physician, and two-thirds want to reduce the number of their medications. If appropriate, hospitalists should consider having a conversation about deprescribing with their older patients.
Citation: Reeve E et al. Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med. 2018;178(12):1673-180.
Dr. Stanley is assistant professor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Clinical question: Among older adults, what attitudes exist toward deprescribing?
Background: Polypharmacy in older adults is common and can be associated with increased hospitalizations and reduced quality of life.
Study design: Population-based survey study.
Setting: Medicare beneficiaries in the United States.
Synopsis: The investigators used data from the National Health and Aging Trends Study (NHATS), which collects information annually on a nationally representative sample of Medicare beneficiaries ages 65 and older. Of 1,981 responses on the NHATS Medication Attitudes module, 92% of older adults expressed willingness to stop a medication if their doctor said it was possible. While 89% agreed that all their medications were necessary, 66.6% also agreed that they would like to reduce the number of their medications. Patients taking more than six medications, compared with those taking fewer than six (adjusted odds ratio, 2.9; 95% confidence interval, 1.74-4.82) and those with three or more medical conditions, compared with patients with fewer than two (aOR 2.87; 95% CI 1.53-5.37) had greater odds of willingness to stop a medication. Importantly, the study did not collect data about specific medications.
Bottom line: A vast majority of older adults would be willing to stop one or more of their medications if considered possible by their physician, and two-thirds want to reduce the number of their medications. If appropriate, hospitalists should consider having a conversation about deprescribing with their older patients.
Citation: Reeve E et al. Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med. 2018;178(12):1673-180.
Dr. Stanley is assistant professor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Clinical question: Among older adults, what attitudes exist toward deprescribing?
Background: Polypharmacy in older adults is common and can be associated with increased hospitalizations and reduced quality of life.
Study design: Population-based survey study.
Setting: Medicare beneficiaries in the United States.
Synopsis: The investigators used data from the National Health and Aging Trends Study (NHATS), which collects information annually on a nationally representative sample of Medicare beneficiaries ages 65 and older. Of 1,981 responses on the NHATS Medication Attitudes module, 92% of older adults expressed willingness to stop a medication if their doctor said it was possible. While 89% agreed that all their medications were necessary, 66.6% also agreed that they would like to reduce the number of their medications. Patients taking more than six medications, compared with those taking fewer than six (adjusted odds ratio, 2.9; 95% confidence interval, 1.74-4.82) and those with three or more medical conditions, compared with patients with fewer than two (aOR 2.87; 95% CI 1.53-5.37) had greater odds of willingness to stop a medication. Importantly, the study did not collect data about specific medications.
Bottom line: A vast majority of older adults would be willing to stop one or more of their medications if considered possible by their physician, and two-thirds want to reduce the number of their medications. If appropriate, hospitalists should consider having a conversation about deprescribing with their older patients.
Citation: Reeve E et al. Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med. 2018;178(12):1673-180.
Dr. Stanley is assistant professor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.












