User login
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
IV fluid weaning unnecessary after gastroenteritis rehydration
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
Burnout gets personal for 68% of physicians
by real-time market insights technology firm InCrowd.
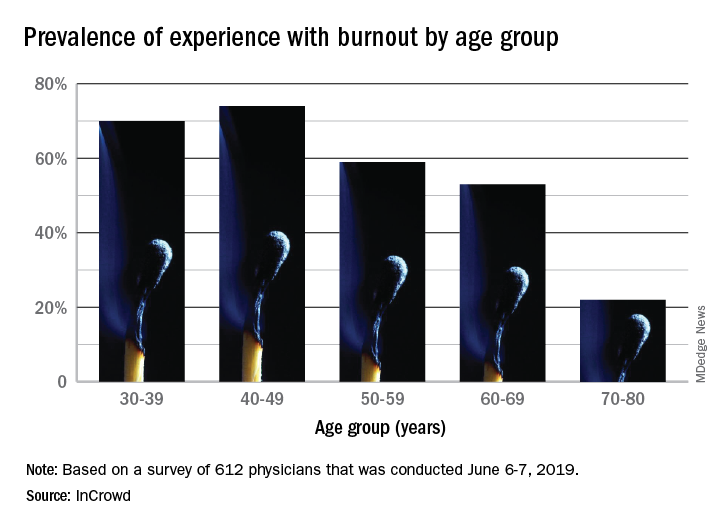
The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
by real-time market insights technology firm InCrowd.

The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
by real-time market insights technology firm InCrowd.

The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
Professional coaching keeps doctors in the game
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
FROM JAMA INTERNAL MEDICINE
Generalist knowledge is an asset
Hospitalists trained in family medicine
Lori J. Heim, MD, FAAFP, a hospitalist in practice at Scotland Memorial Hospital in Laurinburg, N.C., for the past 10 years, recalls when she first decided to pursue hospital medicine as a career. As a family physician in private practice who admitted patients to the local hospital in Pinehurst, N.C., and even followed them into the ICU, she needed a more flexible schedule when she became president-elect of the American Academy of Family Physicians (AAFP).
“My local hospital told me they had a policy against hiring family physicians as hospitalists. They didn’t consider us qualified,” Dr. Heim said. “I was incredulous when I first heard that because I already had full admitting privileges at the hospital. It made no sense, since they allowed me to manage my patients in the ICU.”
Then an opportunity opened at Scotland Memorial, located an hour away. “That has been a fabulous experience for me,” she said. The transition was relatively easy, following more than 2 decades of office practice. Dr. Heim’s hospitalist group now includes eight full-time clinicians who have a mix of family medicine and internal medicine backgrounds.
“I’ve never felt anything other than collegial support here. We go to the ER to evaluate patients and decide whether to admit them, and we do a lot of medical procedures. I’m not practicing pediatrics currently, but I have no problem conducting a gynecological exam. I think my experience in family medicine and primary care has been an asset,” Dr. Heim said. “I’m not sure I would be a hospitalist today if I had not been elected president of AAFP, but it was fortuitous.”
Respect for HTFMs is growing
Hospitalists trained in family medicine (HTFM) are a small but important segment of this field and of the membership of the Society of Hospital Medicine. The board specialties of physicians who work in the hospital are not always broken out in existing databases, but HTFMs are believed to represent about 8% of SHM members, and somewhere around 10%-15% of the total hospitalist workforce. According to SHM’s 2018 State of Hospital Medicine Report, 65% of hospital medicine groups employed at least one family medicine–trained provider in their group.1
SHM’s Special Interest Group (SIG) for HTFMs reports to the society’s Board of Directors. The American Academy of Family Medicine, with 131,400 members, also has a Member Interest Group (MIG) for HTFMs. When AAFP recently surveyed its members to identify their primary patient care practice location, only 4% named the hospital (not including the emergency department), while 3% said the hospital emergency department.2
Among 32,450 adult primary care-trained hospitalists surveyed for the June 2016 AAMC In Brief of the American Association of Medical Colleges, 81.9% of the hospitalists identified internal medicine as their specialty, while 5.2% identified themselves as family physicians.3 A 2014 Medical Group Management Association survey, which reported data for 4,200 hospitalists working in community hospitals, found that 82% were internal medicine trained, versus 10% in family medicine and 7% in pediatrics.
Family medicine hospitalists may be more common in rural areas or in small hospitals – where a clinician is often expected to wear more hats, said hospitalist David Goldstein, MD, FHM, assistant director of the family medicine residency program at Natividad Medical Center, Salinas, Calif., and cochair of SHM’s family medicine SIG. “In a smaller hospital, if there’s not sufficient volume to support full-time pediatric and adult hospital medicine services, a family medicine hospitalist might do both – and even help staff the ICU.”
A decade or so ago, much of the professional literature about the role of HTFMs suggested that some had experienced a lack of respect or of equal job opportunities, while others faced pay differentials.3-5 Since then, the field of hospital medicine has come a long way toward recognizing their contributions, although there are still hurdles to overcome, mainly involving issues of credentialing, to allow HTFMs to play equal roles in the hospital, the ICU, or in residency training. The SHM 2018 State of Hospital Medicine Report reveals that HTFMs actually made slightly higher salaries on average than their internist colleagues, $301,833 versus $300,030.
Prior to the advent of hospital medicine, both family medicine and internal medicine physicians practiced in much the same way in their medical offices, and visited their patients in the hospital, said Claudia Geyer, MD, SFHM, system chief of hospital medicine at Central Maine Healthcare in Lewiston. She is trained and boarded in both family and internal medicine. “When hospital medicine launched, its heavy academic emphasis on internists led to underrecognition of the continued contributions of family medicine. Family physicians never left the hospital setting and – in certain locales – were the predominant hospitalists. We just waited for the recognition to catch up with the reality,” Dr. Geyer said.
“I don’t feel family medicine for hospitalists is nearly the stepchild of internal medicine that it was when I first started,” Dr. Heim said. “In my multihospital hospitalist group, I haven’t seen anything to suggest that they treat family medicine hospitalists as second class.” The demand for hospitalists is greater than internists can fill, while clearly the public is not concerned about these distinctions, she said.
Whether clinicians are board certified in family medicine or internal medicine may be less important to their skills for practicing in the hospital than which residency program they completed, what emphasis it placed on working in the hospital or ICU, electives completed, and other past experience. “Some family medicine residencies offer more or less hospital experience,” Dr. Heim said.
Jasen Gundersen, MD, MBA, CPE, SFHM, president of acute and post-acute services for the national hospital services company TeamHealth, agreed that there has been dramatic improvement in the status of HTFMs. He is one, and still practices as a hospitalist at Boca Raton (Fla.) Regional Hospital when administrative responsibilities permit.
TeamHealth has long been open to family medicine doctors, Dr. Gundersen added, although some of the medical staff at hospitals that contract with TeamHealth have issues with it. “We will talk to them about it,” he said. “We hire hospitalists who can do the work, and we evaluate them based on their background and skill set, where they’ve practiced and for how long. We want people who are experienced and good at managing hospitalized patients. For new residency grads, we look at their electives and the focus of their training.”
What is home for HTFMs?
Where are HTFMs most likely to find their professional home? “That’s hard to answer,” said Patricia Seymour, MD, FHM, FAAFP, an academic hospitalist at the University of Massachusetts-Worcester. “In the last 4-5 years, SHM has worked very hard to create a space for HTFMs. AAFP has a hospital medicine track at their annual meeting, and that’s a good thing. But they also need to protect family physicians’ right to practice in any setting they choose. For those pursuing hospital medicine, there’s a different career trajectory, different CME needs, and different recertification needs.”
Dr. Seymour is the executive cochair of SHM’s family medicine SIG and serves as interim chief of a family medicine hospitalist group that provides inpatient training for a family practice residency, where up to a third of the 12 residents each year go on to pursue hospital medicine as a career. “We have the second-oldest family medicine–specific hospitalist group in the country, so our residency training has an emphasis on hospital medicine,” she explained.
“Because I’m a practicing hospitalist, the residents come to me seeking advice. I appreciate the training I received as a family physician in communication science, palliative care, geriatrics, family systems theory, and public health. I wouldn’t have done it any other way, and that’s how I counsel our students and residents,” she said. Others suggest that the generalist training and diverse experiences of family medicine can be a gift for a doctor who later chooses hospital medicine.
AAFP is a large umbrella organization and the majority of its members practice primary care, Dr. Heim said. “I don’t know the percentage of HTFMs who are members of AAFP. Some no doubt belong to both AAFP and SHM.” Even though both groups have recognized this important subset of their members who chose the field of hospital medicine and its status as a career track, it can be a stretch for family medicine to embrace hospitalists.
“It inherently goes against our training, which is to work in outpatient, inpatient, obstetric, pediatric, and adult settings,” Dr. Heim said. “It’s difficult to reconcile giving up a big part of what defined your training – that range of settings. I remember feeling like I should apologize to other family medicine doctors for choosing this path.”
Credentialing opportunities and barriers
For the diverse group of practicing HTFMs, credentialing and scope of practice represent their biggest current issues. A designation of Focused Practice in Hospital Medicine (FPHM) has been offered jointly since 2010 by the American Board of Family Medicine (ABFM) and the American Board of Internal Medicine (ABIM), although their specific requirements vary.
Eligible hospitalist candidates for the focused practice exam must have an unrestricted medical license, maintenance of current primary certification, and verification of three years of unsupervised hospital medicine practice experience. ABIM views FPHM not as a subspecialty, but as a variation of internal medicine certification, identifying diplomates who are board-certified in internal medicine with a hospital medicine specialization. They do not have to take the general internal medicine recertification exam if they qualify for FPHM.
ABFM-certified family physicians who work primarily in a hospital setting can take the same test for FPHM, with the same eligibility requirements. But ABFM does not consider focused practice a subspecialty, or the Certificate of Added Qualifications in Family Medicine as sufficient for board certification. That means family physicians also need to take its general board exam in order to maintain their ABFM board certification.
ABFM’s decision not to accept the focused practice designation as sufficient for boarding was disappointing to a lot of hospitalists, said Laura “Nell” Hodo, MD, FAAFP, chair of AAFP’s hospital medicine MIG, and a pediatric academic hospitalist at Icahn School of Medicine at Mount Sinai, New York. “Many family physicians practice hospital medicine exclusively and would prefer to take one boarding exam instead of two, and not have to do CME and board review in areas where we don’t practice anymore,” Dr. Hodo said, adding that she hopes that this decision could be revisited in the future.
A number of 1-year hospital medicine fellowships across the country provide additional training opportunities for both family practice and internal medicine residency graduates. These fellowships do not offer board certification or designated specialty credentialing for hospitalists and are not recognized by the American College of Graduate Medical Education (ACGME), which sets standards for residency and fellowship training. “But they reflect a need and an interest in optimizing the knowledge of hospital medicine and developing the specific skills needed to practice it well,” Dr. Geyer noted.
She directs a program for one to three fellows per year out of the Central Maine Family Medicine Residency program and Central Maine Medical Center in Lewiston, and is now recruiting her tenth class. At least 13 other hospital medicine fellowships, out of about 40 nationwide, are family medicine based. “We rely heavily on the Core Competencies in Hospital Medicine developed by SHM, which emphasize clinical conditions, medical procedures, and health care systems. Gaining fluency in the latter is really what makes hospital medicine unique,” Dr. Geyer said.
Often residency graduates seeking work in hospital medicine are insufficiently prepared for hospital billing and coding, enacting safe transitions of care, providing palliative care, and understanding how to impact their health care systems for quality improvement, patient safety and the like, she added.
Dr. Geyer said her fellowship does not mean just being a poorly paid hospitalist for a year. “The fellows are clearly trainees, getting the full benefit of our supervision and supplemental training focused on enhanced clinical and procedural exposure, but also on academics, quality improvement, leadership, and efficiency,” she said. “All of our fellows join SHM, go to the Annual Conference, propose case studies, do longitudinal quality or safety projects, and learn the other aspects of hospital medicine not well-taught in residency. We train them to be highly functional hospitalists right out of the gate.”
Until recently, another barrier for HTFMs was their ability to be on the faculty of internal medicine residency programs. Previous language from ACGME indicated that family medicine-trained physicians could not serve as faculty for these programs, Dr. Goldstein said. SHM has lobbied ACGME to change that rule, which could enable family medicine hospitalists who had achieved FPHM designation to be attendings and to teach internal medicine residents.
Needed in critical care – but not credentialed
One of the biggest frustrations for family medicine hospitalists is clarifying their role in the ICU. SHM’s Education Committee recently surveyed hospitalist members who practice in the ICU, finding that at least half felt obliged to practice beyond their scope, 90 percent occasionally perceived insufficient support from intensivists, and two-thirds reported moderate difficulty transferring patients to higher levels of intensive care.7 The respondents overwhelmingly indicated that they wanted more training and education in critical care medicine.
“I want to highlight the fact that in some settings family physicians are the sole providers of critical care,” Dr. Goldstein said. Meanwhile, the standards of the Leapfrog Group, a coalition of health care purchasers, call for ICUs to be staffed by physicians certified in critical care, even though there is a growing shortage of credentialed intensivists to treat an increasing number of older, sicker, critically ill patients.
Some internal medicine physicians don’t want to have anything to do with the ICU because of the medical and legal risks, said David Aymond, MD, a family physician and hospitalist at Byrd Regional Hospital in Leesville, La. “There’s a bunch of sick people in the ICU, and when some doctors like me started doing critical care, we realized we liked it. Depending on your locale, if you are doing hospital medicine, critically ill patients are going to fall in your lap,” he said. “But if you don’t have the skills, that could lead to poor outcomes and unnecessary transfers.”
Dr. Aymond started his career in family medicine. “When I got into residency, I saw how much critical care was needed in rural communities. I decided I would learn everything I could about it. I did a hospital medicine fellowship at the University of Alabama, which included considerable involvement in the ICU. When I went to Byrd Regional, a 60-bed facility with eight ICU beds, we did all of the critical care, and word started to spread in the community. My hospitalist partner and I are now on call 24/7 alternating weeks, doing the majority of the critical care and taking care of anything that goes on in an ICU at a larger center, although we often lack access to consultation services,” he explained.
“We needed to get the attention of the Society of Critical Care Medicine (SCCM) to communicate the scope of this problem. These doctors are doing critical care but there is no official medical training or recognition for them. So they’re legally out on a limb, even though often they are literally the only person available to do it,” Dr. Aymond said. “Certainly there’s a skills gap between HTFMs and board-certified intensivists, but some of that gap has to do with the volume of patients they have seen in the ICU and their comfort level,” he said.
SHM is pursuing initiatives to help address this gap, including collaborating with SCCM on developing a rigorous critical care training curriculum for internal medicine and family medicine hospitalists, with coursework drawn from existing sources, said Eric Siegal, MD, SFHM, a critical care physician in Milwaukee. “It doesn’t replace a 2-year critical care fellowship, but it will be a lot more than what’s currently out there for the nonintensivist who practices in the ICU.” SCCM has approved moving forward with the advanced training curriculum, he said.
Another priority is to try to create a pathway that could permit family medicine–trained hospitalists to apply for existing critical care fellowships, as internal medicine doctors are now able to do. SHM has lobbied ABFM to create a pathway to subspecialty certification in critical care medicine, similar to those that exist for internists and emergency physicians, Dr. Goldstein said, adding that ACGME, which controls access to fellowships, will be the next step. Dr. Aymond expects that there will be a lot of hoops to jump through.
“David Aymond is an exceptional hospitalist,” Dr. Siegal added. “He thinks and talks like an intensivist, but it took concerted and self-directed effort for him to get there. Family practitioners are a significant part of the rural critical care workforce, but their training generally does not adequately prepare them for this role – unless they have made a conscious effort to pursue additional training,” he said.
“My message to family practitioners is not that they’re not good enough to do this, but rather that they are being asked to do something they weren’t trained for. How can we help them do it well?”
References
1. Society of Hospital Medicine (SHM) Practice Analysis Committee. 2018 State of Hospital Medicine Report; Oct 2018.
2. American Academy of Family Physicians Member Census, Dec 31, 2017.
3. Jones KC et al. Hospitalists: A growing part of the primary care workforce. AAMC Analysis in Brief; June 2016; 16(5):1.
4. Berczuk C. Uniquely positioned. The Hospitalist; July 2009.
5. Iqbal Y. Family medicine hospitalists: Separate and unequal? Today’s Hospitalist; May 2007.
6. Kinnan JP. The family way. The Hospitalist; Nov 2007.
7. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Hospitalists trained in family medicine
Hospitalists trained in family medicine
Lori J. Heim, MD, FAAFP, a hospitalist in practice at Scotland Memorial Hospital in Laurinburg, N.C., for the past 10 years, recalls when she first decided to pursue hospital medicine as a career. As a family physician in private practice who admitted patients to the local hospital in Pinehurst, N.C., and even followed them into the ICU, she needed a more flexible schedule when she became president-elect of the American Academy of Family Physicians (AAFP).
“My local hospital told me they had a policy against hiring family physicians as hospitalists. They didn’t consider us qualified,” Dr. Heim said. “I was incredulous when I first heard that because I already had full admitting privileges at the hospital. It made no sense, since they allowed me to manage my patients in the ICU.”
Then an opportunity opened at Scotland Memorial, located an hour away. “That has been a fabulous experience for me,” she said. The transition was relatively easy, following more than 2 decades of office practice. Dr. Heim’s hospitalist group now includes eight full-time clinicians who have a mix of family medicine and internal medicine backgrounds.
“I’ve never felt anything other than collegial support here. We go to the ER to evaluate patients and decide whether to admit them, and we do a lot of medical procedures. I’m not practicing pediatrics currently, but I have no problem conducting a gynecological exam. I think my experience in family medicine and primary care has been an asset,” Dr. Heim said. “I’m not sure I would be a hospitalist today if I had not been elected president of AAFP, but it was fortuitous.”
Respect for HTFMs is growing
Hospitalists trained in family medicine (HTFM) are a small but important segment of this field and of the membership of the Society of Hospital Medicine. The board specialties of physicians who work in the hospital are not always broken out in existing databases, but HTFMs are believed to represent about 8% of SHM members, and somewhere around 10%-15% of the total hospitalist workforce. According to SHM’s 2018 State of Hospital Medicine Report, 65% of hospital medicine groups employed at least one family medicine–trained provider in their group.1
SHM’s Special Interest Group (SIG) for HTFMs reports to the society’s Board of Directors. The American Academy of Family Medicine, with 131,400 members, also has a Member Interest Group (MIG) for HTFMs. When AAFP recently surveyed its members to identify their primary patient care practice location, only 4% named the hospital (not including the emergency department), while 3% said the hospital emergency department.2
Among 32,450 adult primary care-trained hospitalists surveyed for the June 2016 AAMC In Brief of the American Association of Medical Colleges, 81.9% of the hospitalists identified internal medicine as their specialty, while 5.2% identified themselves as family physicians.3 A 2014 Medical Group Management Association survey, which reported data for 4,200 hospitalists working in community hospitals, found that 82% were internal medicine trained, versus 10% in family medicine and 7% in pediatrics.
Family medicine hospitalists may be more common in rural areas or in small hospitals – where a clinician is often expected to wear more hats, said hospitalist David Goldstein, MD, FHM, assistant director of the family medicine residency program at Natividad Medical Center, Salinas, Calif., and cochair of SHM’s family medicine SIG. “In a smaller hospital, if there’s not sufficient volume to support full-time pediatric and adult hospital medicine services, a family medicine hospitalist might do both – and even help staff the ICU.”
A decade or so ago, much of the professional literature about the role of HTFMs suggested that some had experienced a lack of respect or of equal job opportunities, while others faced pay differentials.3-5 Since then, the field of hospital medicine has come a long way toward recognizing their contributions, although there are still hurdles to overcome, mainly involving issues of credentialing, to allow HTFMs to play equal roles in the hospital, the ICU, or in residency training. The SHM 2018 State of Hospital Medicine Report reveals that HTFMs actually made slightly higher salaries on average than their internist colleagues, $301,833 versus $300,030.
Prior to the advent of hospital medicine, both family medicine and internal medicine physicians practiced in much the same way in their medical offices, and visited their patients in the hospital, said Claudia Geyer, MD, SFHM, system chief of hospital medicine at Central Maine Healthcare in Lewiston. She is trained and boarded in both family and internal medicine. “When hospital medicine launched, its heavy academic emphasis on internists led to underrecognition of the continued contributions of family medicine. Family physicians never left the hospital setting and – in certain locales – were the predominant hospitalists. We just waited for the recognition to catch up with the reality,” Dr. Geyer said.
“I don’t feel family medicine for hospitalists is nearly the stepchild of internal medicine that it was when I first started,” Dr. Heim said. “In my multihospital hospitalist group, I haven’t seen anything to suggest that they treat family medicine hospitalists as second class.” The demand for hospitalists is greater than internists can fill, while clearly the public is not concerned about these distinctions, she said.
Whether clinicians are board certified in family medicine or internal medicine may be less important to their skills for practicing in the hospital than which residency program they completed, what emphasis it placed on working in the hospital or ICU, electives completed, and other past experience. “Some family medicine residencies offer more or less hospital experience,” Dr. Heim said.
Jasen Gundersen, MD, MBA, CPE, SFHM, president of acute and post-acute services for the national hospital services company TeamHealth, agreed that there has been dramatic improvement in the status of HTFMs. He is one, and still practices as a hospitalist at Boca Raton (Fla.) Regional Hospital when administrative responsibilities permit.
TeamHealth has long been open to family medicine doctors, Dr. Gundersen added, although some of the medical staff at hospitals that contract with TeamHealth have issues with it. “We will talk to them about it,” he said. “We hire hospitalists who can do the work, and we evaluate them based on their background and skill set, where they’ve practiced and for how long. We want people who are experienced and good at managing hospitalized patients. For new residency grads, we look at their electives and the focus of their training.”
What is home for HTFMs?
Where are HTFMs most likely to find their professional home? “That’s hard to answer,” said Patricia Seymour, MD, FHM, FAAFP, an academic hospitalist at the University of Massachusetts-Worcester. “In the last 4-5 years, SHM has worked very hard to create a space for HTFMs. AAFP has a hospital medicine track at their annual meeting, and that’s a good thing. But they also need to protect family physicians’ right to practice in any setting they choose. For those pursuing hospital medicine, there’s a different career trajectory, different CME needs, and different recertification needs.”
Dr. Seymour is the executive cochair of SHM’s family medicine SIG and serves as interim chief of a family medicine hospitalist group that provides inpatient training for a family practice residency, where up to a third of the 12 residents each year go on to pursue hospital medicine as a career. “We have the second-oldest family medicine–specific hospitalist group in the country, so our residency training has an emphasis on hospital medicine,” she explained.
“Because I’m a practicing hospitalist, the residents come to me seeking advice. I appreciate the training I received as a family physician in communication science, palliative care, geriatrics, family systems theory, and public health. I wouldn’t have done it any other way, and that’s how I counsel our students and residents,” she said. Others suggest that the generalist training and diverse experiences of family medicine can be a gift for a doctor who later chooses hospital medicine.
AAFP is a large umbrella organization and the majority of its members practice primary care, Dr. Heim said. “I don’t know the percentage of HTFMs who are members of AAFP. Some no doubt belong to both AAFP and SHM.” Even though both groups have recognized this important subset of their members who chose the field of hospital medicine and its status as a career track, it can be a stretch for family medicine to embrace hospitalists.
“It inherently goes against our training, which is to work in outpatient, inpatient, obstetric, pediatric, and adult settings,” Dr. Heim said. “It’s difficult to reconcile giving up a big part of what defined your training – that range of settings. I remember feeling like I should apologize to other family medicine doctors for choosing this path.”
Credentialing opportunities and barriers
For the diverse group of practicing HTFMs, credentialing and scope of practice represent their biggest current issues. A designation of Focused Practice in Hospital Medicine (FPHM) has been offered jointly since 2010 by the American Board of Family Medicine (ABFM) and the American Board of Internal Medicine (ABIM), although their specific requirements vary.
Eligible hospitalist candidates for the focused practice exam must have an unrestricted medical license, maintenance of current primary certification, and verification of three years of unsupervised hospital medicine practice experience. ABIM views FPHM not as a subspecialty, but as a variation of internal medicine certification, identifying diplomates who are board-certified in internal medicine with a hospital medicine specialization. They do not have to take the general internal medicine recertification exam if they qualify for FPHM.
ABFM-certified family physicians who work primarily in a hospital setting can take the same test for FPHM, with the same eligibility requirements. But ABFM does not consider focused practice a subspecialty, or the Certificate of Added Qualifications in Family Medicine as sufficient for board certification. That means family physicians also need to take its general board exam in order to maintain their ABFM board certification.
ABFM’s decision not to accept the focused practice designation as sufficient for boarding was disappointing to a lot of hospitalists, said Laura “Nell” Hodo, MD, FAAFP, chair of AAFP’s hospital medicine MIG, and a pediatric academic hospitalist at Icahn School of Medicine at Mount Sinai, New York. “Many family physicians practice hospital medicine exclusively and would prefer to take one boarding exam instead of two, and not have to do CME and board review in areas where we don’t practice anymore,” Dr. Hodo said, adding that she hopes that this decision could be revisited in the future.
A number of 1-year hospital medicine fellowships across the country provide additional training opportunities for both family practice and internal medicine residency graduates. These fellowships do not offer board certification or designated specialty credentialing for hospitalists and are not recognized by the American College of Graduate Medical Education (ACGME), which sets standards for residency and fellowship training. “But they reflect a need and an interest in optimizing the knowledge of hospital medicine and developing the specific skills needed to practice it well,” Dr. Geyer noted.
She directs a program for one to three fellows per year out of the Central Maine Family Medicine Residency program and Central Maine Medical Center in Lewiston, and is now recruiting her tenth class. At least 13 other hospital medicine fellowships, out of about 40 nationwide, are family medicine based. “We rely heavily on the Core Competencies in Hospital Medicine developed by SHM, which emphasize clinical conditions, medical procedures, and health care systems. Gaining fluency in the latter is really what makes hospital medicine unique,” Dr. Geyer said.
Often residency graduates seeking work in hospital medicine are insufficiently prepared for hospital billing and coding, enacting safe transitions of care, providing palliative care, and understanding how to impact their health care systems for quality improvement, patient safety and the like, she added.
Dr. Geyer said her fellowship does not mean just being a poorly paid hospitalist for a year. “The fellows are clearly trainees, getting the full benefit of our supervision and supplemental training focused on enhanced clinical and procedural exposure, but also on academics, quality improvement, leadership, and efficiency,” she said. “All of our fellows join SHM, go to the Annual Conference, propose case studies, do longitudinal quality or safety projects, and learn the other aspects of hospital medicine not well-taught in residency. We train them to be highly functional hospitalists right out of the gate.”
Until recently, another barrier for HTFMs was their ability to be on the faculty of internal medicine residency programs. Previous language from ACGME indicated that family medicine-trained physicians could not serve as faculty for these programs, Dr. Goldstein said. SHM has lobbied ACGME to change that rule, which could enable family medicine hospitalists who had achieved FPHM designation to be attendings and to teach internal medicine residents.
Needed in critical care – but not credentialed
One of the biggest frustrations for family medicine hospitalists is clarifying their role in the ICU. SHM’s Education Committee recently surveyed hospitalist members who practice in the ICU, finding that at least half felt obliged to practice beyond their scope, 90 percent occasionally perceived insufficient support from intensivists, and two-thirds reported moderate difficulty transferring patients to higher levels of intensive care.7 The respondents overwhelmingly indicated that they wanted more training and education in critical care medicine.
“I want to highlight the fact that in some settings family physicians are the sole providers of critical care,” Dr. Goldstein said. Meanwhile, the standards of the Leapfrog Group, a coalition of health care purchasers, call for ICUs to be staffed by physicians certified in critical care, even though there is a growing shortage of credentialed intensivists to treat an increasing number of older, sicker, critically ill patients.
Some internal medicine physicians don’t want to have anything to do with the ICU because of the medical and legal risks, said David Aymond, MD, a family physician and hospitalist at Byrd Regional Hospital in Leesville, La. “There’s a bunch of sick people in the ICU, and when some doctors like me started doing critical care, we realized we liked it. Depending on your locale, if you are doing hospital medicine, critically ill patients are going to fall in your lap,” he said. “But if you don’t have the skills, that could lead to poor outcomes and unnecessary transfers.”
Dr. Aymond started his career in family medicine. “When I got into residency, I saw how much critical care was needed in rural communities. I decided I would learn everything I could about it. I did a hospital medicine fellowship at the University of Alabama, which included considerable involvement in the ICU. When I went to Byrd Regional, a 60-bed facility with eight ICU beds, we did all of the critical care, and word started to spread in the community. My hospitalist partner and I are now on call 24/7 alternating weeks, doing the majority of the critical care and taking care of anything that goes on in an ICU at a larger center, although we often lack access to consultation services,” he explained.
“We needed to get the attention of the Society of Critical Care Medicine (SCCM) to communicate the scope of this problem. These doctors are doing critical care but there is no official medical training or recognition for them. So they’re legally out on a limb, even though often they are literally the only person available to do it,” Dr. Aymond said. “Certainly there’s a skills gap between HTFMs and board-certified intensivists, but some of that gap has to do with the volume of patients they have seen in the ICU and their comfort level,” he said.
SHM is pursuing initiatives to help address this gap, including collaborating with SCCM on developing a rigorous critical care training curriculum for internal medicine and family medicine hospitalists, with coursework drawn from existing sources, said Eric Siegal, MD, SFHM, a critical care physician in Milwaukee. “It doesn’t replace a 2-year critical care fellowship, but it will be a lot more than what’s currently out there for the nonintensivist who practices in the ICU.” SCCM has approved moving forward with the advanced training curriculum, he said.
Another priority is to try to create a pathway that could permit family medicine–trained hospitalists to apply for existing critical care fellowships, as internal medicine doctors are now able to do. SHM has lobbied ABFM to create a pathway to subspecialty certification in critical care medicine, similar to those that exist for internists and emergency physicians, Dr. Goldstein said, adding that ACGME, which controls access to fellowships, will be the next step. Dr. Aymond expects that there will be a lot of hoops to jump through.
“David Aymond is an exceptional hospitalist,” Dr. Siegal added. “He thinks and talks like an intensivist, but it took concerted and self-directed effort for him to get there. Family practitioners are a significant part of the rural critical care workforce, but their training generally does not adequately prepare them for this role – unless they have made a conscious effort to pursue additional training,” he said.
“My message to family practitioners is not that they’re not good enough to do this, but rather that they are being asked to do something they weren’t trained for. How can we help them do it well?”
References
1. Society of Hospital Medicine (SHM) Practice Analysis Committee. 2018 State of Hospital Medicine Report; Oct 2018.
2. American Academy of Family Physicians Member Census, Dec 31, 2017.
3. Jones KC et al. Hospitalists: A growing part of the primary care workforce. AAMC Analysis in Brief; June 2016; 16(5):1.
4. Berczuk C. Uniquely positioned. The Hospitalist; July 2009.
5. Iqbal Y. Family medicine hospitalists: Separate and unequal? Today’s Hospitalist; May 2007.
6. Kinnan JP. The family way. The Hospitalist; Nov 2007.
7. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Lori J. Heim, MD, FAAFP, a hospitalist in practice at Scotland Memorial Hospital in Laurinburg, N.C., for the past 10 years, recalls when she first decided to pursue hospital medicine as a career. As a family physician in private practice who admitted patients to the local hospital in Pinehurst, N.C., and even followed them into the ICU, she needed a more flexible schedule when she became president-elect of the American Academy of Family Physicians (AAFP).
“My local hospital told me they had a policy against hiring family physicians as hospitalists. They didn’t consider us qualified,” Dr. Heim said. “I was incredulous when I first heard that because I already had full admitting privileges at the hospital. It made no sense, since they allowed me to manage my patients in the ICU.”
Then an opportunity opened at Scotland Memorial, located an hour away. “That has been a fabulous experience for me,” she said. The transition was relatively easy, following more than 2 decades of office practice. Dr. Heim’s hospitalist group now includes eight full-time clinicians who have a mix of family medicine and internal medicine backgrounds.
“I’ve never felt anything other than collegial support here. We go to the ER to evaluate patients and decide whether to admit them, and we do a lot of medical procedures. I’m not practicing pediatrics currently, but I have no problem conducting a gynecological exam. I think my experience in family medicine and primary care has been an asset,” Dr. Heim said. “I’m not sure I would be a hospitalist today if I had not been elected president of AAFP, but it was fortuitous.”
Respect for HTFMs is growing
Hospitalists trained in family medicine (HTFM) are a small but important segment of this field and of the membership of the Society of Hospital Medicine. The board specialties of physicians who work in the hospital are not always broken out in existing databases, but HTFMs are believed to represent about 8% of SHM members, and somewhere around 10%-15% of the total hospitalist workforce. According to SHM’s 2018 State of Hospital Medicine Report, 65% of hospital medicine groups employed at least one family medicine–trained provider in their group.1
SHM’s Special Interest Group (SIG) for HTFMs reports to the society’s Board of Directors. The American Academy of Family Medicine, with 131,400 members, also has a Member Interest Group (MIG) for HTFMs. When AAFP recently surveyed its members to identify their primary patient care practice location, only 4% named the hospital (not including the emergency department), while 3% said the hospital emergency department.2
Among 32,450 adult primary care-trained hospitalists surveyed for the June 2016 AAMC In Brief of the American Association of Medical Colleges, 81.9% of the hospitalists identified internal medicine as their specialty, while 5.2% identified themselves as family physicians.3 A 2014 Medical Group Management Association survey, which reported data for 4,200 hospitalists working in community hospitals, found that 82% were internal medicine trained, versus 10% in family medicine and 7% in pediatrics.
Family medicine hospitalists may be more common in rural areas or in small hospitals – where a clinician is often expected to wear more hats, said hospitalist David Goldstein, MD, FHM, assistant director of the family medicine residency program at Natividad Medical Center, Salinas, Calif., and cochair of SHM’s family medicine SIG. “In a smaller hospital, if there’s not sufficient volume to support full-time pediatric and adult hospital medicine services, a family medicine hospitalist might do both – and even help staff the ICU.”
A decade or so ago, much of the professional literature about the role of HTFMs suggested that some had experienced a lack of respect or of equal job opportunities, while others faced pay differentials.3-5 Since then, the field of hospital medicine has come a long way toward recognizing their contributions, although there are still hurdles to overcome, mainly involving issues of credentialing, to allow HTFMs to play equal roles in the hospital, the ICU, or in residency training. The SHM 2018 State of Hospital Medicine Report reveals that HTFMs actually made slightly higher salaries on average than their internist colleagues, $301,833 versus $300,030.
Prior to the advent of hospital medicine, both family medicine and internal medicine physicians practiced in much the same way in their medical offices, and visited their patients in the hospital, said Claudia Geyer, MD, SFHM, system chief of hospital medicine at Central Maine Healthcare in Lewiston. She is trained and boarded in both family and internal medicine. “When hospital medicine launched, its heavy academic emphasis on internists led to underrecognition of the continued contributions of family medicine. Family physicians never left the hospital setting and – in certain locales – were the predominant hospitalists. We just waited for the recognition to catch up with the reality,” Dr. Geyer said.
“I don’t feel family medicine for hospitalists is nearly the stepchild of internal medicine that it was when I first started,” Dr. Heim said. “In my multihospital hospitalist group, I haven’t seen anything to suggest that they treat family medicine hospitalists as second class.” The demand for hospitalists is greater than internists can fill, while clearly the public is not concerned about these distinctions, she said.
Whether clinicians are board certified in family medicine or internal medicine may be less important to their skills for practicing in the hospital than which residency program they completed, what emphasis it placed on working in the hospital or ICU, electives completed, and other past experience. “Some family medicine residencies offer more or less hospital experience,” Dr. Heim said.
Jasen Gundersen, MD, MBA, CPE, SFHM, president of acute and post-acute services for the national hospital services company TeamHealth, agreed that there has been dramatic improvement in the status of HTFMs. He is one, and still practices as a hospitalist at Boca Raton (Fla.) Regional Hospital when administrative responsibilities permit.
TeamHealth has long been open to family medicine doctors, Dr. Gundersen added, although some of the medical staff at hospitals that contract with TeamHealth have issues with it. “We will talk to them about it,” he said. “We hire hospitalists who can do the work, and we evaluate them based on their background and skill set, where they’ve practiced and for how long. We want people who are experienced and good at managing hospitalized patients. For new residency grads, we look at their electives and the focus of their training.”
What is home for HTFMs?
Where are HTFMs most likely to find their professional home? “That’s hard to answer,” said Patricia Seymour, MD, FHM, FAAFP, an academic hospitalist at the University of Massachusetts-Worcester. “In the last 4-5 years, SHM has worked very hard to create a space for HTFMs. AAFP has a hospital medicine track at their annual meeting, and that’s a good thing. But they also need to protect family physicians’ right to practice in any setting they choose. For those pursuing hospital medicine, there’s a different career trajectory, different CME needs, and different recertification needs.”
Dr. Seymour is the executive cochair of SHM’s family medicine SIG and serves as interim chief of a family medicine hospitalist group that provides inpatient training for a family practice residency, where up to a third of the 12 residents each year go on to pursue hospital medicine as a career. “We have the second-oldest family medicine–specific hospitalist group in the country, so our residency training has an emphasis on hospital medicine,” she explained.
“Because I’m a practicing hospitalist, the residents come to me seeking advice. I appreciate the training I received as a family physician in communication science, palliative care, geriatrics, family systems theory, and public health. I wouldn’t have done it any other way, and that’s how I counsel our students and residents,” she said. Others suggest that the generalist training and diverse experiences of family medicine can be a gift for a doctor who later chooses hospital medicine.
AAFP is a large umbrella organization and the majority of its members practice primary care, Dr. Heim said. “I don’t know the percentage of HTFMs who are members of AAFP. Some no doubt belong to both AAFP and SHM.” Even though both groups have recognized this important subset of their members who chose the field of hospital medicine and its status as a career track, it can be a stretch for family medicine to embrace hospitalists.
“It inherently goes against our training, which is to work in outpatient, inpatient, obstetric, pediatric, and adult settings,” Dr. Heim said. “It’s difficult to reconcile giving up a big part of what defined your training – that range of settings. I remember feeling like I should apologize to other family medicine doctors for choosing this path.”
Credentialing opportunities and barriers
For the diverse group of practicing HTFMs, credentialing and scope of practice represent their biggest current issues. A designation of Focused Practice in Hospital Medicine (FPHM) has been offered jointly since 2010 by the American Board of Family Medicine (ABFM) and the American Board of Internal Medicine (ABIM), although their specific requirements vary.
Eligible hospitalist candidates for the focused practice exam must have an unrestricted medical license, maintenance of current primary certification, and verification of three years of unsupervised hospital medicine practice experience. ABIM views FPHM not as a subspecialty, but as a variation of internal medicine certification, identifying diplomates who are board-certified in internal medicine with a hospital medicine specialization. They do not have to take the general internal medicine recertification exam if they qualify for FPHM.
ABFM-certified family physicians who work primarily in a hospital setting can take the same test for FPHM, with the same eligibility requirements. But ABFM does not consider focused practice a subspecialty, or the Certificate of Added Qualifications in Family Medicine as sufficient for board certification. That means family physicians also need to take its general board exam in order to maintain their ABFM board certification.
ABFM’s decision not to accept the focused practice designation as sufficient for boarding was disappointing to a lot of hospitalists, said Laura “Nell” Hodo, MD, FAAFP, chair of AAFP’s hospital medicine MIG, and a pediatric academic hospitalist at Icahn School of Medicine at Mount Sinai, New York. “Many family physicians practice hospital medicine exclusively and would prefer to take one boarding exam instead of two, and not have to do CME and board review in areas where we don’t practice anymore,” Dr. Hodo said, adding that she hopes that this decision could be revisited in the future.
A number of 1-year hospital medicine fellowships across the country provide additional training opportunities for both family practice and internal medicine residency graduates. These fellowships do not offer board certification or designated specialty credentialing for hospitalists and are not recognized by the American College of Graduate Medical Education (ACGME), which sets standards for residency and fellowship training. “But they reflect a need and an interest in optimizing the knowledge of hospital medicine and developing the specific skills needed to practice it well,” Dr. Geyer noted.
She directs a program for one to three fellows per year out of the Central Maine Family Medicine Residency program and Central Maine Medical Center in Lewiston, and is now recruiting her tenth class. At least 13 other hospital medicine fellowships, out of about 40 nationwide, are family medicine based. “We rely heavily on the Core Competencies in Hospital Medicine developed by SHM, which emphasize clinical conditions, medical procedures, and health care systems. Gaining fluency in the latter is really what makes hospital medicine unique,” Dr. Geyer said.
Often residency graduates seeking work in hospital medicine are insufficiently prepared for hospital billing and coding, enacting safe transitions of care, providing palliative care, and understanding how to impact their health care systems for quality improvement, patient safety and the like, she added.
Dr. Geyer said her fellowship does not mean just being a poorly paid hospitalist for a year. “The fellows are clearly trainees, getting the full benefit of our supervision and supplemental training focused on enhanced clinical and procedural exposure, but also on academics, quality improvement, leadership, and efficiency,” she said. “All of our fellows join SHM, go to the Annual Conference, propose case studies, do longitudinal quality or safety projects, and learn the other aspects of hospital medicine not well-taught in residency. We train them to be highly functional hospitalists right out of the gate.”
Until recently, another barrier for HTFMs was their ability to be on the faculty of internal medicine residency programs. Previous language from ACGME indicated that family medicine-trained physicians could not serve as faculty for these programs, Dr. Goldstein said. SHM has lobbied ACGME to change that rule, which could enable family medicine hospitalists who had achieved FPHM designation to be attendings and to teach internal medicine residents.
Needed in critical care – but not credentialed
One of the biggest frustrations for family medicine hospitalists is clarifying their role in the ICU. SHM’s Education Committee recently surveyed hospitalist members who practice in the ICU, finding that at least half felt obliged to practice beyond their scope, 90 percent occasionally perceived insufficient support from intensivists, and two-thirds reported moderate difficulty transferring patients to higher levels of intensive care.7 The respondents overwhelmingly indicated that they wanted more training and education in critical care medicine.
“I want to highlight the fact that in some settings family physicians are the sole providers of critical care,” Dr. Goldstein said. Meanwhile, the standards of the Leapfrog Group, a coalition of health care purchasers, call for ICUs to be staffed by physicians certified in critical care, even though there is a growing shortage of credentialed intensivists to treat an increasing number of older, sicker, critically ill patients.
Some internal medicine physicians don’t want to have anything to do with the ICU because of the medical and legal risks, said David Aymond, MD, a family physician and hospitalist at Byrd Regional Hospital in Leesville, La. “There’s a bunch of sick people in the ICU, and when some doctors like me started doing critical care, we realized we liked it. Depending on your locale, if you are doing hospital medicine, critically ill patients are going to fall in your lap,” he said. “But if you don’t have the skills, that could lead to poor outcomes and unnecessary transfers.”
Dr. Aymond started his career in family medicine. “When I got into residency, I saw how much critical care was needed in rural communities. I decided I would learn everything I could about it. I did a hospital medicine fellowship at the University of Alabama, which included considerable involvement in the ICU. When I went to Byrd Regional, a 60-bed facility with eight ICU beds, we did all of the critical care, and word started to spread in the community. My hospitalist partner and I are now on call 24/7 alternating weeks, doing the majority of the critical care and taking care of anything that goes on in an ICU at a larger center, although we often lack access to consultation services,” he explained.
“We needed to get the attention of the Society of Critical Care Medicine (SCCM) to communicate the scope of this problem. These doctors are doing critical care but there is no official medical training or recognition for them. So they’re legally out on a limb, even though often they are literally the only person available to do it,” Dr. Aymond said. “Certainly there’s a skills gap between HTFMs and board-certified intensivists, but some of that gap has to do with the volume of patients they have seen in the ICU and their comfort level,” he said.
SHM is pursuing initiatives to help address this gap, including collaborating with SCCM on developing a rigorous critical care training curriculum for internal medicine and family medicine hospitalists, with coursework drawn from existing sources, said Eric Siegal, MD, SFHM, a critical care physician in Milwaukee. “It doesn’t replace a 2-year critical care fellowship, but it will be a lot more than what’s currently out there for the nonintensivist who practices in the ICU.” SCCM has approved moving forward with the advanced training curriculum, he said.
Another priority is to try to create a pathway that could permit family medicine–trained hospitalists to apply for existing critical care fellowships, as internal medicine doctors are now able to do. SHM has lobbied ABFM to create a pathway to subspecialty certification in critical care medicine, similar to those that exist for internists and emergency physicians, Dr. Goldstein said, adding that ACGME, which controls access to fellowships, will be the next step. Dr. Aymond expects that there will be a lot of hoops to jump through.
“David Aymond is an exceptional hospitalist,” Dr. Siegal added. “He thinks and talks like an intensivist, but it took concerted and self-directed effort for him to get there. Family practitioners are a significant part of the rural critical care workforce, but their training generally does not adequately prepare them for this role – unless they have made a conscious effort to pursue additional training,” he said.
“My message to family practitioners is not that they’re not good enough to do this, but rather that they are being asked to do something they weren’t trained for. How can we help them do it well?”
References
1. Society of Hospital Medicine (SHM) Practice Analysis Committee. 2018 State of Hospital Medicine Report; Oct 2018.
2. American Academy of Family Physicians Member Census, Dec 31, 2017.
3. Jones KC et al. Hospitalists: A growing part of the primary care workforce. AAMC Analysis in Brief; June 2016; 16(5):1.
4. Berczuk C. Uniquely positioned. The Hospitalist; July 2009.
5. Iqbal Y. Family medicine hospitalists: Separate and unequal? Today’s Hospitalist; May 2007.
6. Kinnan JP. The family way. The Hospitalist; Nov 2007.
7. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Older patients who stop statins may be increasing their cardiovascular risk
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
FROM THE EUROPEAN HEART JOURNAL
Too many blood cultures ordered for pediatric SSTIs
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
REPORTING FROM PHM 2019
Dispatch from HM19: COPD updates
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
AI technology meets AFib detection
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
FROM THE LANCET
Enteral feeding is safe during bronchiolitis HFNC
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019



















