User login
Single-dose influenza drug baloxavir similar to oseltamivir in efficacy
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Single-dose influenza antiviral baloxavir shows efficacy similar to that of oseltamivir.
Major finding: Baloxavir shows similar time to alleviation of influenza symptoms compared with oseltamivir, but greater reductions in viral load.
Study details: Phase 2 and phase 3 randomized controlled trials in 389 and 1,366 otherwise healthy patients with influenza.
Disclosures: The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
Source: Hayden F et al. N Engl J Med 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
Service, please: Hospital setting matters for pneumonia
the National Center for Health Statistics (NCHS) reported.
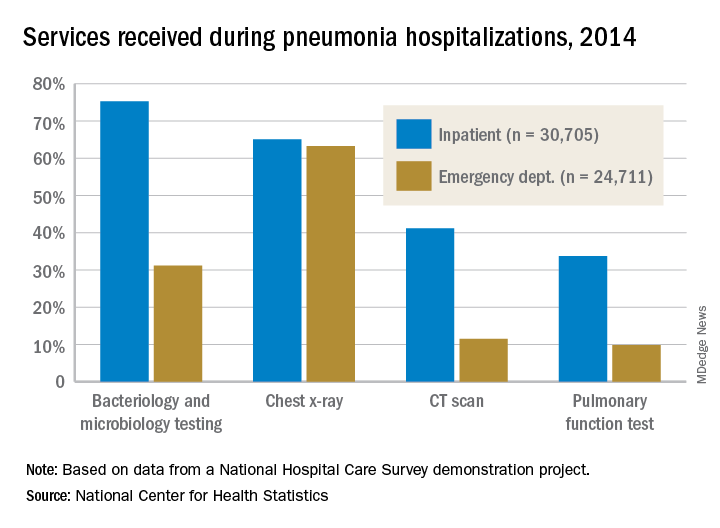
The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
the National Center for Health Statistics (NCHS) reported.

The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
the National Center for Health Statistics (NCHS) reported.

The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
Hydrocortisone plus fludrocortisone for adults with septic shock
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
United Kingdom experience provides important lessons for controlling C. auris outbreaks
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Ten additional cases occurred in the 7 months after the axillary probes were removed from the environment.
Study details: A review of the epidemiology and control of a C. auris outbreak affecting 76 patients.
Disclosures: Dr. Eyre reported having no disclosures.
Source: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
Plan now for outpatient diabetes tech in the hospital
ORLANDO –
A third or more of patients with type 1 diabetes mellitus and growing numbers of patients with insulin-dependent type 2 diabetes mellitus patients are using pumps and sensor technology. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, and there’s general consensus that continuous glucose monitors (CGM) can be used in the hospital.
All in all, it’s a good thing, according to Guillermo E. Umpierrez, MD, professor of endocrinology at Emory University and chief of endocrinology at Grady Memorial Hospital, both in Atlanta.
In a talk at the annual scientific sessions of the American Diabetes Association, Dr. Umpierrez reviewed a number of studies showing that glycemic control with the new technology is no worse in the hospital – and sometimes even better – than with traditional point-of-care glucose testing and insulin administration. There is a lack of randomized, controlled trials to prove the point definitively, but what evidence does exist is promising.
“This technology is rapidly advancing, and I am very optimistic that we are going to see more and more of these devices in the hospital. If patients can manage themselves, allow them to use CGM, allow them to use their pumps,” he said.
As for closed loop systems – automated glucose sensing and insulin administration – emerging evidence suggests they “allow you to have very good glucose control and less glycemic variability,” both inside and outside of the ICU, he said. “I am very hopeful before I retire that there will be management of a significant number of patients with closed loop systems.”
To keep up, training for hospital providers on the new technology is now “mandatory at all levels,” Dr. Umpierrez said, and if they haven’t done so already, hospitals need to put policies and procedures in place for when, and when not, to allow patients to use their diabetes equipment, and how to integrate it into care.
Among many things to consider, patients must be well enough to use their pumps and monitors, be able to demonstrate their functions, and also want to participate in their own care.
Contraindications to inpatient pump use include impaired consciousness, critical illness, and hyperglycemic crises because insulin requirements change too rapidly and dramatically for pumps. Lack of trained providers and supplies is another hurdle. Pumps also need to come off for MRIs.
CGM, meanwhile, has been shown to improve glycemic control, detecting both hyperglycemia and hypoglycemia more readily than point-of-care testing. It’s good at picking up trends in glucose levels, and Dr. Umpierrez anticipates a time when readings will be transmitted to nurses’ stations automatically to track blood glucose trends. “I think that’s the future,” he said.
But, as with insulin pumps, there are caveats. Among them, it’s unclear how well CGM works during hypoxia, hypothermia, and hypotension. Thrombus formation and infections have been reported with intravascular monitors, and a number of agents can throw off some CGM devices, including acetaminophen, heparin, and dopamine.
Dr. Umpierrez disclosed relationships with AstraZeneca, Merck, Novo Nordisk, Sanofi, and other companies.
ORLANDO –
A third or more of patients with type 1 diabetes mellitus and growing numbers of patients with insulin-dependent type 2 diabetes mellitus patients are using pumps and sensor technology. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, and there’s general consensus that continuous glucose monitors (CGM) can be used in the hospital.
All in all, it’s a good thing, according to Guillermo E. Umpierrez, MD, professor of endocrinology at Emory University and chief of endocrinology at Grady Memorial Hospital, both in Atlanta.
In a talk at the annual scientific sessions of the American Diabetes Association, Dr. Umpierrez reviewed a number of studies showing that glycemic control with the new technology is no worse in the hospital – and sometimes even better – than with traditional point-of-care glucose testing and insulin administration. There is a lack of randomized, controlled trials to prove the point definitively, but what evidence does exist is promising.
“This technology is rapidly advancing, and I am very optimistic that we are going to see more and more of these devices in the hospital. If patients can manage themselves, allow them to use CGM, allow them to use their pumps,” he said.
As for closed loop systems – automated glucose sensing and insulin administration – emerging evidence suggests they “allow you to have very good glucose control and less glycemic variability,” both inside and outside of the ICU, he said. “I am very hopeful before I retire that there will be management of a significant number of patients with closed loop systems.”
To keep up, training for hospital providers on the new technology is now “mandatory at all levels,” Dr. Umpierrez said, and if they haven’t done so already, hospitals need to put policies and procedures in place for when, and when not, to allow patients to use their diabetes equipment, and how to integrate it into care.
Among many things to consider, patients must be well enough to use their pumps and monitors, be able to demonstrate their functions, and also want to participate in their own care.
Contraindications to inpatient pump use include impaired consciousness, critical illness, and hyperglycemic crises because insulin requirements change too rapidly and dramatically for pumps. Lack of trained providers and supplies is another hurdle. Pumps also need to come off for MRIs.
CGM, meanwhile, has been shown to improve glycemic control, detecting both hyperglycemia and hypoglycemia more readily than point-of-care testing. It’s good at picking up trends in glucose levels, and Dr. Umpierrez anticipates a time when readings will be transmitted to nurses’ stations automatically to track blood glucose trends. “I think that’s the future,” he said.
But, as with insulin pumps, there are caveats. Among them, it’s unclear how well CGM works during hypoxia, hypothermia, and hypotension. Thrombus formation and infections have been reported with intravascular monitors, and a number of agents can throw off some CGM devices, including acetaminophen, heparin, and dopamine.
Dr. Umpierrez disclosed relationships with AstraZeneca, Merck, Novo Nordisk, Sanofi, and other companies.
ORLANDO –
A third or more of patients with type 1 diabetes mellitus and growing numbers of patients with insulin-dependent type 2 diabetes mellitus patients are using pumps and sensor technology. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, and there’s general consensus that continuous glucose monitors (CGM) can be used in the hospital.
All in all, it’s a good thing, according to Guillermo E. Umpierrez, MD, professor of endocrinology at Emory University and chief of endocrinology at Grady Memorial Hospital, both in Atlanta.
In a talk at the annual scientific sessions of the American Diabetes Association, Dr. Umpierrez reviewed a number of studies showing that glycemic control with the new technology is no worse in the hospital – and sometimes even better – than with traditional point-of-care glucose testing and insulin administration. There is a lack of randomized, controlled trials to prove the point definitively, but what evidence does exist is promising.
“This technology is rapidly advancing, and I am very optimistic that we are going to see more and more of these devices in the hospital. If patients can manage themselves, allow them to use CGM, allow them to use their pumps,” he said.
As for closed loop systems – automated glucose sensing and insulin administration – emerging evidence suggests they “allow you to have very good glucose control and less glycemic variability,” both inside and outside of the ICU, he said. “I am very hopeful before I retire that there will be management of a significant number of patients with closed loop systems.”
To keep up, training for hospital providers on the new technology is now “mandatory at all levels,” Dr. Umpierrez said, and if they haven’t done so already, hospitals need to put policies and procedures in place for when, and when not, to allow patients to use their diabetes equipment, and how to integrate it into care.
Among many things to consider, patients must be well enough to use their pumps and monitors, be able to demonstrate their functions, and also want to participate in their own care.
Contraindications to inpatient pump use include impaired consciousness, critical illness, and hyperglycemic crises because insulin requirements change too rapidly and dramatically for pumps. Lack of trained providers and supplies is another hurdle. Pumps also need to come off for MRIs.
CGM, meanwhile, has been shown to improve glycemic control, detecting both hyperglycemia and hypoglycemia more readily than point-of-care testing. It’s good at picking up trends in glucose levels, and Dr. Umpierrez anticipates a time when readings will be transmitted to nurses’ stations automatically to track blood glucose trends. “I think that’s the future,” he said.
But, as with insulin pumps, there are caveats. Among them, it’s unclear how well CGM works during hypoxia, hypothermia, and hypotension. Thrombus formation and infections have been reported with intravascular monitors, and a number of agents can throw off some CGM devices, including acetaminophen, heparin, and dopamine.
Dr. Umpierrez disclosed relationships with AstraZeneca, Merck, Novo Nordisk, Sanofi, and other companies.
EXPERT ANALYSIS FROM ADA 2018
Policy responses to opioid epidemic may have benefits, harms
Some policy responses to the opioid epidemic have immediate, beneficial effects, while others lead to short-term harms that might be offset by long-term health benefits, according to researchers who have mathematically modeled the impact of 11 interventions.
Policies that expand addiction treatment or curb harmful effects of addiction such as overdose and infection were immediately beneficial in the model, with no negative effects on life years (LYs), quality-adjusted life years (QALYs), or addiction deaths, the researchers reported.
In contrast, policies that constrain prescription opioid supply resulted in some benefits, but also short-term harms because of inadequate pain control and users switching to heroin.
However, the modeling study also suggests those harms might be mitigated over the long term as new addictions are averted, according to Allison L. Pitt, a PhD candidate in the department of management science and engineering at Stanford (Calif.) University, and her coauthors.
Combining different interventions had additive benefits in the model, prompting Ms. Pitt and her coauthors to recommend a multifaceted policy approach to curb opioid abuse and reduce addiction deaths.
“,” they wrote in the American Journal of Public Health. “Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects.”
In their study, Ms. Pitt and her colleagues projected the impact of 11 policies aimed at curbing opioid addiction and reducing addiction deaths. They used dynamic compartmental modeling, a technique commonly used for evaluating the spread of contagious disease.
This technique is appropriate for studying the opioid epidemic, because it allows for dynamic modeling of addiction incidence that reflects a changing number of prescription holders, the authors said in their report, which focused on projected outcomes of various interventions at 5 and 10 years.
None of the policies substantially reduced opioid-related deaths in 5-year outcomes projections, they found. Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over that time period.
However, interventions focused on providing services for people with addictions did generally provide uniform benefits over the 5-year horizon: “Naloxone availability, needle exchange, medication-assisted treatment, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group,” Ms. Pitt and her coauthors said.
Interventions that reduced opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, increased LYs and QALYs while decreasing total addiction deaths over 5 years. However, the investigators said, those benefits were partly offset by increases in heroin-related deaths.
Drug rescheduling was associated with a 45.6% increase in heroin-related deaths over 5 years in the model, the highest percentage increase of any intervention in the published data.
Over the 10-year horizon, addiction deaths continued to decrease proportionally for naloxone availability and needle-exchange policies, authors said. By comparison, policies focused on opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, averted substantially more deaths over 10 years than would be expected, compared with the 5-year results, investigators said.
Acute pain prescribing, which increased opioid-related deaths over 5 years in the model, was associated with a decrease in opioid-related deaths over 10 years, they added.
The report coauthors were Keith Humphreys, PhD, of Stanford’s department of psychiatry and behavioral sciences, and Margaret L. Brandeau, PhD, of the university’s department of management science and engineering.
The coauthors cited several limitations. One is that the opioid epidemic is changing in unpredictable ways. Therefore, numerous assumptions about the epidemic were made based on the opinions of clinicians and scientists.
The study was supported by a grant from the National Institute on Drug Abuse. Dr. Humphreys reported support through a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
SOURCE: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
Some policy responses to the opioid epidemic have immediate, beneficial effects, while others lead to short-term harms that might be offset by long-term health benefits, according to researchers who have mathematically modeled the impact of 11 interventions.
Policies that expand addiction treatment or curb harmful effects of addiction such as overdose and infection were immediately beneficial in the model, with no negative effects on life years (LYs), quality-adjusted life years (QALYs), or addiction deaths, the researchers reported.
In contrast, policies that constrain prescription opioid supply resulted in some benefits, but also short-term harms because of inadequate pain control and users switching to heroin.
However, the modeling study also suggests those harms might be mitigated over the long term as new addictions are averted, according to Allison L. Pitt, a PhD candidate in the department of management science and engineering at Stanford (Calif.) University, and her coauthors.
Combining different interventions had additive benefits in the model, prompting Ms. Pitt and her coauthors to recommend a multifaceted policy approach to curb opioid abuse and reduce addiction deaths.
“,” they wrote in the American Journal of Public Health. “Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects.”
In their study, Ms. Pitt and her colleagues projected the impact of 11 policies aimed at curbing opioid addiction and reducing addiction deaths. They used dynamic compartmental modeling, a technique commonly used for evaluating the spread of contagious disease.
This technique is appropriate for studying the opioid epidemic, because it allows for dynamic modeling of addiction incidence that reflects a changing number of prescription holders, the authors said in their report, which focused on projected outcomes of various interventions at 5 and 10 years.
None of the policies substantially reduced opioid-related deaths in 5-year outcomes projections, they found. Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over that time period.
However, interventions focused on providing services for people with addictions did generally provide uniform benefits over the 5-year horizon: “Naloxone availability, needle exchange, medication-assisted treatment, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group,” Ms. Pitt and her coauthors said.
Interventions that reduced opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, increased LYs and QALYs while decreasing total addiction deaths over 5 years. However, the investigators said, those benefits were partly offset by increases in heroin-related deaths.
Drug rescheduling was associated with a 45.6% increase in heroin-related deaths over 5 years in the model, the highest percentage increase of any intervention in the published data.
Over the 10-year horizon, addiction deaths continued to decrease proportionally for naloxone availability and needle-exchange policies, authors said. By comparison, policies focused on opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, averted substantially more deaths over 10 years than would be expected, compared with the 5-year results, investigators said.
Acute pain prescribing, which increased opioid-related deaths over 5 years in the model, was associated with a decrease in opioid-related deaths over 10 years, they added.
The report coauthors were Keith Humphreys, PhD, of Stanford’s department of psychiatry and behavioral sciences, and Margaret L. Brandeau, PhD, of the university’s department of management science and engineering.
The coauthors cited several limitations. One is that the opioid epidemic is changing in unpredictable ways. Therefore, numerous assumptions about the epidemic were made based on the opinions of clinicians and scientists.
The study was supported by a grant from the National Institute on Drug Abuse. Dr. Humphreys reported support through a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
SOURCE: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
Some policy responses to the opioid epidemic have immediate, beneficial effects, while others lead to short-term harms that might be offset by long-term health benefits, according to researchers who have mathematically modeled the impact of 11 interventions.
Policies that expand addiction treatment or curb harmful effects of addiction such as overdose and infection were immediately beneficial in the model, with no negative effects on life years (LYs), quality-adjusted life years (QALYs), or addiction deaths, the researchers reported.
In contrast, policies that constrain prescription opioid supply resulted in some benefits, but also short-term harms because of inadequate pain control and users switching to heroin.
However, the modeling study also suggests those harms might be mitigated over the long term as new addictions are averted, according to Allison L. Pitt, a PhD candidate in the department of management science and engineering at Stanford (Calif.) University, and her coauthors.
Combining different interventions had additive benefits in the model, prompting Ms. Pitt and her coauthors to recommend a multifaceted policy approach to curb opioid abuse and reduce addiction deaths.
“,” they wrote in the American Journal of Public Health. “Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects.”
In their study, Ms. Pitt and her colleagues projected the impact of 11 policies aimed at curbing opioid addiction and reducing addiction deaths. They used dynamic compartmental modeling, a technique commonly used for evaluating the spread of contagious disease.
This technique is appropriate for studying the opioid epidemic, because it allows for dynamic modeling of addiction incidence that reflects a changing number of prescription holders, the authors said in their report, which focused on projected outcomes of various interventions at 5 and 10 years.
None of the policies substantially reduced opioid-related deaths in 5-year outcomes projections, they found. Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over that time period.
However, interventions focused on providing services for people with addictions did generally provide uniform benefits over the 5-year horizon: “Naloxone availability, needle exchange, medication-assisted treatment, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group,” Ms. Pitt and her coauthors said.
Interventions that reduced opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, increased LYs and QALYs while decreasing total addiction deaths over 5 years. However, the investigators said, those benefits were partly offset by increases in heroin-related deaths.
Drug rescheduling was associated with a 45.6% increase in heroin-related deaths over 5 years in the model, the highest percentage increase of any intervention in the published data.
Over the 10-year horizon, addiction deaths continued to decrease proportionally for naloxone availability and needle-exchange policies, authors said. By comparison, policies focused on opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, averted substantially more deaths over 10 years than would be expected, compared with the 5-year results, investigators said.
Acute pain prescribing, which increased opioid-related deaths over 5 years in the model, was associated with a decrease in opioid-related deaths over 10 years, they added.
The report coauthors were Keith Humphreys, PhD, of Stanford’s department of psychiatry and behavioral sciences, and Margaret L. Brandeau, PhD, of the university’s department of management science and engineering.
The coauthors cited several limitations. One is that the opioid epidemic is changing in unpredictable ways. Therefore, numerous assumptions about the epidemic were made based on the opinions of clinicians and scientists.
The study was supported by a grant from the National Institute on Drug Abuse. Dr. Humphreys reported support through a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
SOURCE: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
Key clinical point: Interventions focused on providing services for people with addictions generally provided uniform benefits over the 5-year horizon.
Major finding: Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over a 5-year time period modeled in the study.
Study details: Mathematical modeling of 11 policy interventions and their effects on life years, quality-adjusted life years, and deaths over 5- and 10-year time horizons.
Disclosures: The study was supported by grant from the National Institute on Drug Abuse. One study author reported support from the VA Health Services Research and Development Service.
Source: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
Eat/sleep/console approach almost eliminates morphine for NAS
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
REPORTING FROM PHM 2018
Key clinical point: When it comes to neonatal abstinence syndrome, treat the infant, not the Finnegan score.
Major finding: The University of North Carolina Children’s Hospital dropped the length of stay for neonatal abstinence syndrome from about 11 to 5 days by moving from scheduled to PRN morphine and abandoning Finnegan scoring. Morphine use fell more than 80%.
Study details: Review of a 7-month quality improvement project
Disclosures: There was no industry funding for the work. The lead investigator didn’t have any disclosures.
Balanced crystalloid solution improves efficacy outcomes in critically sick adults
Clinical question: Does a balanced crystalloid solution lead to better outcomes than does normal saline when used in critically sick adults?
Background: Balanced crystalloids are considered more physiological, with a composition closer to plasma. Observational studies have shown lower rates of hyperchloremic acidosis, renal failure, and death with use of balanced crystalloids. In spite of this, normal saline has been the most commonly used fluid. Differences in effects on important patient-related outcomes of safety and efficacy between these two interventions remain unknown.
Study design: Pragmatic, unblinded, cluster-randomized, multiple-crossover trial.
Setting: Vanderbilt University Health Center, Nashville, Tenn.
Synopsis: This study comprised 15,802 adults with mean age of 58 admitted to ICU who were cluster randomized to receive either balanced crystalloid or normal saline. Primary outcome was a composite of death from any cause, renal replacement therapy, or persistent renal dysfunction at 30 days and was observed less frequently in the balanced crystalloid group (adjusted odds ratio, 0.90; 95% confidence interval, 0.82-0.99; P = .04).
Since the trial was cluster randomized, prognostic imbalance between the groups caused by confounding factors was a big risk. Results could not be generalized because the study was done in a university health center. Mean fluid amount received was modest in both groups. Questions still remain about the efficacy and safety of balanced fluids, and hospitalists should weigh their decisions in light of this new information.
Bottom line: Balanced crystalloid solution decreased 30-day composite outcome of death, renal replacement therapy, or persistent renal dysfunction.
Citation: Semler MW et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
Dr. Parasramka is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Does a balanced crystalloid solution lead to better outcomes than does normal saline when used in critically sick adults?
Background: Balanced crystalloids are considered more physiological, with a composition closer to plasma. Observational studies have shown lower rates of hyperchloremic acidosis, renal failure, and death with use of balanced crystalloids. In spite of this, normal saline has been the most commonly used fluid. Differences in effects on important patient-related outcomes of safety and efficacy between these two interventions remain unknown.
Study design: Pragmatic, unblinded, cluster-randomized, multiple-crossover trial.
Setting: Vanderbilt University Health Center, Nashville, Tenn.
Synopsis: This study comprised 15,802 adults with mean age of 58 admitted to ICU who were cluster randomized to receive either balanced crystalloid or normal saline. Primary outcome was a composite of death from any cause, renal replacement therapy, or persistent renal dysfunction at 30 days and was observed less frequently in the balanced crystalloid group (adjusted odds ratio, 0.90; 95% confidence interval, 0.82-0.99; P = .04).
Since the trial was cluster randomized, prognostic imbalance between the groups caused by confounding factors was a big risk. Results could not be generalized because the study was done in a university health center. Mean fluid amount received was modest in both groups. Questions still remain about the efficacy and safety of balanced fluids, and hospitalists should weigh their decisions in light of this new information.
Bottom line: Balanced crystalloid solution decreased 30-day composite outcome of death, renal replacement therapy, or persistent renal dysfunction.
Citation: Semler MW et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
Dr. Parasramka is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Does a balanced crystalloid solution lead to better outcomes than does normal saline when used in critically sick adults?
Background: Balanced crystalloids are considered more physiological, with a composition closer to plasma. Observational studies have shown lower rates of hyperchloremic acidosis, renal failure, and death with use of balanced crystalloids. In spite of this, normal saline has been the most commonly used fluid. Differences in effects on important patient-related outcomes of safety and efficacy between these two interventions remain unknown.
Study design: Pragmatic, unblinded, cluster-randomized, multiple-crossover trial.
Setting: Vanderbilt University Health Center, Nashville, Tenn.
Synopsis: This study comprised 15,802 adults with mean age of 58 admitted to ICU who were cluster randomized to receive either balanced crystalloid or normal saline. Primary outcome was a composite of death from any cause, renal replacement therapy, or persistent renal dysfunction at 30 days and was observed less frequently in the balanced crystalloid group (adjusted odds ratio, 0.90; 95% confidence interval, 0.82-0.99; P = .04).
Since the trial was cluster randomized, prognostic imbalance between the groups caused by confounding factors was a big risk. Results could not be generalized because the study was done in a university health center. Mean fluid amount received was modest in both groups. Questions still remain about the efficacy and safety of balanced fluids, and hospitalists should weigh their decisions in light of this new information.
Bottom line: Balanced crystalloid solution decreased 30-day composite outcome of death, renal replacement therapy, or persistent renal dysfunction.
Citation: Semler MW et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39.
Dr. Parasramka is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Nonpharmacologic strategies for acute pain
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Haloperidol and delirium in critically ill patients
Clinical question: Does prophylactic use of haloperidol increase survival in critically ill adults with high risk of delirium?
Background: Delirium is common and associated with high mortality. Studies evaluating prophylactic use of haloperidol have been inconclusive.
Study design: Randomized, double-blind, placebo-controlled, multicenter, investigator-driven study.
Setting: ICUs at university hospitals and teaching and nonteaching hospitals in the Netherlands.
Synopsis: 1,789 adults with mean age 66.6 years were randomized to receive either 1 or 2 mg of haloperidol or placebo. The 1-mg haloperidol group was prematurely stopped because of futility.
There was no difference in the primary outcome of survival days within a 28-day period between the 2-mg haloperidol group and the placebo group (hazard ratio, 1.003; 95% confidence interval, 0.78-1.30; P = .93).
Secondary outcome of survival was also the same at 90 days. No significant difference was seen in the delirium incidence, delirium- and coma-free days, and the delirium-related outcome measures.
Patients were already undergoing nonpharmacologic interventions for delirium prevention, and this likely attenuated the effects of haloperidol. The dose and duration of haloperidol used might have also been too low and short to have an effect on the outcomes considering the severity of patient illness.
Bottom line: Haloperidol has no role in prophylactic use to increase survival among critically ill patients with a high risk of delirium.
Citation: van den Boogaard M et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018 Feb 20;319(7):680-90.
Dr. Chikkanna is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Does prophylactic use of haloperidol increase survival in critically ill adults with high risk of delirium?
Background: Delirium is common and associated with high mortality. Studies evaluating prophylactic use of haloperidol have been inconclusive.
Study design: Randomized, double-blind, placebo-controlled, multicenter, investigator-driven study.
Setting: ICUs at university hospitals and teaching and nonteaching hospitals in the Netherlands.
Synopsis: 1,789 adults with mean age 66.6 years were randomized to receive either 1 or 2 mg of haloperidol or placebo. The 1-mg haloperidol group was prematurely stopped because of futility.
There was no difference in the primary outcome of survival days within a 28-day period between the 2-mg haloperidol group and the placebo group (hazard ratio, 1.003; 95% confidence interval, 0.78-1.30; P = .93).
Secondary outcome of survival was also the same at 90 days. No significant difference was seen in the delirium incidence, delirium- and coma-free days, and the delirium-related outcome measures.
Patients were already undergoing nonpharmacologic interventions for delirium prevention, and this likely attenuated the effects of haloperidol. The dose and duration of haloperidol used might have also been too low and short to have an effect on the outcomes considering the severity of patient illness.
Bottom line: Haloperidol has no role in prophylactic use to increase survival among critically ill patients with a high risk of delirium.
Citation: van den Boogaard M et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018 Feb 20;319(7):680-90.
Dr. Chikkanna is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Does prophylactic use of haloperidol increase survival in critically ill adults with high risk of delirium?
Background: Delirium is common and associated with high mortality. Studies evaluating prophylactic use of haloperidol have been inconclusive.
Study design: Randomized, double-blind, placebo-controlled, multicenter, investigator-driven study.
Setting: ICUs at university hospitals and teaching and nonteaching hospitals in the Netherlands.
Synopsis: 1,789 adults with mean age 66.6 years were randomized to receive either 1 or 2 mg of haloperidol or placebo. The 1-mg haloperidol group was prematurely stopped because of futility.
There was no difference in the primary outcome of survival days within a 28-day period between the 2-mg haloperidol group and the placebo group (hazard ratio, 1.003; 95% confidence interval, 0.78-1.30; P = .93).
Secondary outcome of survival was also the same at 90 days. No significant difference was seen in the delirium incidence, delirium- and coma-free days, and the delirium-related outcome measures.
Patients were already undergoing nonpharmacologic interventions for delirium prevention, and this likely attenuated the effects of haloperidol. The dose and duration of haloperidol used might have also been too low and short to have an effect on the outcomes considering the severity of patient illness.
Bottom line: Haloperidol has no role in prophylactic use to increase survival among critically ill patients with a high risk of delirium.
Citation: van den Boogaard M et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018 Feb 20;319(7):680-90.
Dr. Chikkanna is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.














