User login
What is the preferred treatment for a child with mild persistent asthma?
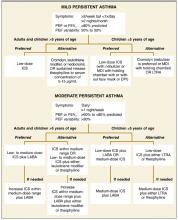
Low-dose inhaled corticosteroids are the preferred treatment for children with mild persistent asthma because they demonstrate superior reduction in severity and frequency of asthma exacerbations compared with alternatives (strength of recommendation [SOR]: A, based on multiple randomized controlled trials). As add-on therapy, nedocromil, theophylline, and cromolyn have all demonstrated a modest benefit in symptom control; leukotriene receptor antagonists are also recommended based on data from older children (SOR: B, cohort study). Unlike treatment of moderate or severe asthma, long-acting beta-agonists are not recommended (SOR: A, randomized trials).
Clear medication choices for mild asthma are supported by good evidence
John Heintzman, MD
Oregon Health and Science University, Portland
Physicians who routinely treat children with asthma are fortunate to have the body of evidence outlined in this review. Clear medication choices are supported in most instances by relatively clear comparisons with alternatives. In my practice, where many children can be classified in the “mild persistent” category, I am always surprised at how many patients’ families lack a clear understanding of the factors that trigger a child’s asthma and how to avoid them.
Another common clinical scenario among children and adolescents is exercise-induced asthma. Depending on the sport, the asthma can be classified as “mild persistent” or “mild intermittent.” for true intermittent symptoms, my clinical experience (and often parental preference) argues for pre-activity treatment with short acting beta-agonists as the most practical therapy.
Evidence summary
Mild persistent asthma is defined as forced expiratory volume over 1 second (FEV1) ≥80% predicted, with daytime symptoms more than twice per week but less than once daily, and nighttime symptoms more often than twice monthly.1
Low-dose inhaled corticosteroids
Two large randomized trials support using low-dose inhaled corticosteroids in these children. The Childhood Asthma Management Program (CAMP) study, which included 1041 children, evaluated treatment with either budesonide or nedocromil vs placebo. Patients taking budesonide had a lower rate of urgent care visits (absolute risk reduction [ARR]=10%; number needed to treat [NNT]=10; P=.02) compared with children taking nedocromil (ARR=6%; NNT=17; P=.02). The urgent care visits were reported as number of visits per 100 person-years.
In practical terms, this means that in order to decrease 1 urgent care visit, 1 patient would need to take budesonide for 10 years. However, because rates are not necessarily homogenous over time, the number of visits decreased during the first year may be different than the number of events decreased throughout the tenth year.
Children taking budesonide experienced 21.5% more episode-free days than those taking placebo (P=.01). No change was observed in the nedocromil group.2 In the inhaled Steroid Treatment As Regular Therapy (START) in early asthma study, budesonide demonstrated a 44% relative reduction in time to first severe asthma related event, compared with placebo (95% confidence interval [CI], 0.45–0.71; NNT=44; P=.0001).3
Theophylline
Theophylline is considered an alternative to inhaled corticosteroids. One study compared beclomethasone with theophylline in 195 children. This study found near-equivalent efficacy in doctor visits, hospitalizations, monthly peak expiratory flow rates, and FEV1; however, beclomethasone was superior to theophylline in maintaining symptom control and decreasing the use of inhaled bronchodilators and systemic steroids.
When compared with beclomethasone, theophylline was linked to 14% more central nervous system adverse effects (P<.001) and 17% more gastrointestinal disturbances (P<.001). Although beclomethasone induced more oral candidiasis compared with theophylline (8.9% vs 2.4%; P<.001), the incidence of this infection can be reduced by using a spacer.
Long-term systemic effects
The potential long-term adverse systemic effects of inhaled corticosteroids on growth, bone metabolism, and pituitary-adrenal function call for longer-term studies.4 A systematic review of 15 trials reported that the protective effect of leukotriene receptor antagonists is inferior to inhaled corticosteroids for adults (relative risk [RR]=1.71; 95% CI, 1.40–2.09); however, evidence is insufficient to extrapolate this to children.5
Beta-agonists
Evidence does not support use of long-acting beta-agonists as monotherapy or in combination with other medications for children with mild persistent asthma. Although 1 study showed an improvement in lung function for children taking budesonide plus formoterol compared with budesonide alone, the rate of severe exacerbations was lower for those taking budesonide alone (62% decrease vs 55.8% decrease; P=.001). Both groups had a 32% decrease in the number of rescue inhalations per day when compared with placebo (P=.0008).6
Recommendations from others
Recommendations are listed in the TABLE.1,7,8 Unlike the NAEPP and GINA asthma guidelines, the BTS/SIGN asthma guidelines define no objective measurement or staging classification to diagnose asthma among children. Diagnosis is determined by a child’s response to medication.8 Independent of any daily controller medication use, all children should have a short acting bronchodilator on hand in case of an acute attack.1,8
TABLE
Recommendations for treating mild persistent asthma
| GUIDELINE | DAILY CONTROLLER MEDICATION | ALTERNATIVE TREATMENT |
|---|---|---|
| National Asthma Education and Prevention Program (NAEPP)1 | Low-dose inhaled corticosteroids | Children <5: cromolyn, LTRAs Children >5: cromolyn, LTRAs, nedocromil, sustained release theophylline |
| Global initiative for asthma (GINA)7 | low-dose inhaled corticosteroids | All children: sustained released theophylline, Cromone, LTRAs |
| British Thoracic Society/Scottish intercollegiate Guidelines network (BTS/SIGN)8 | Inhaled steroids | All children: LTRAs, theophylline Children >5: cromones, nedocromil |
| LRTA leukotriene receptor antagonists. | ||
| Sources: NAEPP J Allergy Clin Immunol 20021; GINA Guidelines and Resources 20057 and BTS/SIGN, Thorax 2003.8 | ||
1. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics—2002. National Asthma Education and Prevention Program. J Allergy Clin Immunol 2002;110:S141-S219.
2. Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 2000;343:1054-1063.
3. Pauwels RA, Pedersen S, Busse WW, et al. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003;361:1071-1076.
4. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild-to-moderate asthma. The American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. J Allergy Clin Immunol 1998;101:14-23.
5. Ducharme FM, Salvio F, Ducharme F. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children (Cochrane review). In: The Cochrane Library. 2006 Issue 2. Chichester, UK: John Wiley and Sons, Ltd.
6. O’byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392-1397.
7. The Global Initiative for Asthma. Guidelines and Resources: 2005 Update. Available at: www.ginasthma.com/Guidelineitem.asp??I1=2&I2=1&intId=60. Accessed January 9, 2007.
8. British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. Thorax 2003;58:i1-i94.
Low-dose inhaled corticosteroids are the preferred treatment for children with mild persistent asthma because they demonstrate superior reduction in severity and frequency of asthma exacerbations compared with alternatives (strength of recommendation [SOR]: A, based on multiple randomized controlled trials). As add-on therapy, nedocromil, theophylline, and cromolyn have all demonstrated a modest benefit in symptom control; leukotriene receptor antagonists are also recommended based on data from older children (SOR: B, cohort study). Unlike treatment of moderate or severe asthma, long-acting beta-agonists are not recommended (SOR: A, randomized trials).
Clear medication choices for mild asthma are supported by good evidence
John Heintzman, MD
Oregon Health and Science University, Portland
Physicians who routinely treat children with asthma are fortunate to have the body of evidence outlined in this review. Clear medication choices are supported in most instances by relatively clear comparisons with alternatives. In my practice, where many children can be classified in the “mild persistent” category, I am always surprised at how many patients’ families lack a clear understanding of the factors that trigger a child’s asthma and how to avoid them.
Another common clinical scenario among children and adolescents is exercise-induced asthma. Depending on the sport, the asthma can be classified as “mild persistent” or “mild intermittent.” for true intermittent symptoms, my clinical experience (and often parental preference) argues for pre-activity treatment with short acting beta-agonists as the most practical therapy.
Evidence summary
Mild persistent asthma is defined as forced expiratory volume over 1 second (FEV1) ≥80% predicted, with daytime symptoms more than twice per week but less than once daily, and nighttime symptoms more often than twice monthly.1
Low-dose inhaled corticosteroids
Two large randomized trials support using low-dose inhaled corticosteroids in these children. The Childhood Asthma Management Program (CAMP) study, which included 1041 children, evaluated treatment with either budesonide or nedocromil vs placebo. Patients taking budesonide had a lower rate of urgent care visits (absolute risk reduction [ARR]=10%; number needed to treat [NNT]=10; P=.02) compared with children taking nedocromil (ARR=6%; NNT=17; P=.02). The urgent care visits were reported as number of visits per 100 person-years.
In practical terms, this means that in order to decrease 1 urgent care visit, 1 patient would need to take budesonide for 10 years. However, because rates are not necessarily homogenous over time, the number of visits decreased during the first year may be different than the number of events decreased throughout the tenth year.
Children taking budesonide experienced 21.5% more episode-free days than those taking placebo (P=.01). No change was observed in the nedocromil group.2 In the inhaled Steroid Treatment As Regular Therapy (START) in early asthma study, budesonide demonstrated a 44% relative reduction in time to first severe asthma related event, compared with placebo (95% confidence interval [CI], 0.45–0.71; NNT=44; P=.0001).3
Theophylline
Theophylline is considered an alternative to inhaled corticosteroids. One study compared beclomethasone with theophylline in 195 children. This study found near-equivalent efficacy in doctor visits, hospitalizations, monthly peak expiratory flow rates, and FEV1; however, beclomethasone was superior to theophylline in maintaining symptom control and decreasing the use of inhaled bronchodilators and systemic steroids.
When compared with beclomethasone, theophylline was linked to 14% more central nervous system adverse effects (P<.001) and 17% more gastrointestinal disturbances (P<.001). Although beclomethasone induced more oral candidiasis compared with theophylline (8.9% vs 2.4%; P<.001), the incidence of this infection can be reduced by using a spacer.
Long-term systemic effects
The potential long-term adverse systemic effects of inhaled corticosteroids on growth, bone metabolism, and pituitary-adrenal function call for longer-term studies.4 A systematic review of 15 trials reported that the protective effect of leukotriene receptor antagonists is inferior to inhaled corticosteroids for adults (relative risk [RR]=1.71; 95% CI, 1.40–2.09); however, evidence is insufficient to extrapolate this to children.5
Beta-agonists
Evidence does not support use of long-acting beta-agonists as monotherapy or in combination with other medications for children with mild persistent asthma. Although 1 study showed an improvement in lung function for children taking budesonide plus formoterol compared with budesonide alone, the rate of severe exacerbations was lower for those taking budesonide alone (62% decrease vs 55.8% decrease; P=.001). Both groups had a 32% decrease in the number of rescue inhalations per day when compared with placebo (P=.0008).6
Recommendations from others
Recommendations are listed in the TABLE.1,7,8 Unlike the NAEPP and GINA asthma guidelines, the BTS/SIGN asthma guidelines define no objective measurement or staging classification to diagnose asthma among children. Diagnosis is determined by a child’s response to medication.8 Independent of any daily controller medication use, all children should have a short acting bronchodilator on hand in case of an acute attack.1,8
TABLE
Recommendations for treating mild persistent asthma
| GUIDELINE | DAILY CONTROLLER MEDICATION | ALTERNATIVE TREATMENT |
|---|---|---|
| National Asthma Education and Prevention Program (NAEPP)1 | Low-dose inhaled corticosteroids | Children <5: cromolyn, LTRAs Children >5: cromolyn, LTRAs, nedocromil, sustained release theophylline |
| Global initiative for asthma (GINA)7 | low-dose inhaled corticosteroids | All children: sustained released theophylline, Cromone, LTRAs |
| British Thoracic Society/Scottish intercollegiate Guidelines network (BTS/SIGN)8 | Inhaled steroids | All children: LTRAs, theophylline Children >5: cromones, nedocromil |
| LRTA leukotriene receptor antagonists. | ||
| Sources: NAEPP J Allergy Clin Immunol 20021; GINA Guidelines and Resources 20057 and BTS/SIGN, Thorax 2003.8 | ||
Low-dose inhaled corticosteroids are the preferred treatment for children with mild persistent asthma because they demonstrate superior reduction in severity and frequency of asthma exacerbations compared with alternatives (strength of recommendation [SOR]: A, based on multiple randomized controlled trials). As add-on therapy, nedocromil, theophylline, and cromolyn have all demonstrated a modest benefit in symptom control; leukotriene receptor antagonists are also recommended based on data from older children (SOR: B, cohort study). Unlike treatment of moderate or severe asthma, long-acting beta-agonists are not recommended (SOR: A, randomized trials).
Clear medication choices for mild asthma are supported by good evidence
John Heintzman, MD
Oregon Health and Science University, Portland
Physicians who routinely treat children with asthma are fortunate to have the body of evidence outlined in this review. Clear medication choices are supported in most instances by relatively clear comparisons with alternatives. In my practice, where many children can be classified in the “mild persistent” category, I am always surprised at how many patients’ families lack a clear understanding of the factors that trigger a child’s asthma and how to avoid them.
Another common clinical scenario among children and adolescents is exercise-induced asthma. Depending on the sport, the asthma can be classified as “mild persistent” or “mild intermittent.” for true intermittent symptoms, my clinical experience (and often parental preference) argues for pre-activity treatment with short acting beta-agonists as the most practical therapy.
Evidence summary
Mild persistent asthma is defined as forced expiratory volume over 1 second (FEV1) ≥80% predicted, with daytime symptoms more than twice per week but less than once daily, and nighttime symptoms more often than twice monthly.1
Low-dose inhaled corticosteroids
Two large randomized trials support using low-dose inhaled corticosteroids in these children. The Childhood Asthma Management Program (CAMP) study, which included 1041 children, evaluated treatment with either budesonide or nedocromil vs placebo. Patients taking budesonide had a lower rate of urgent care visits (absolute risk reduction [ARR]=10%; number needed to treat [NNT]=10; P=.02) compared with children taking nedocromil (ARR=6%; NNT=17; P=.02). The urgent care visits were reported as number of visits per 100 person-years.
In practical terms, this means that in order to decrease 1 urgent care visit, 1 patient would need to take budesonide for 10 years. However, because rates are not necessarily homogenous over time, the number of visits decreased during the first year may be different than the number of events decreased throughout the tenth year.
Children taking budesonide experienced 21.5% more episode-free days than those taking placebo (P=.01). No change was observed in the nedocromil group.2 In the inhaled Steroid Treatment As Regular Therapy (START) in early asthma study, budesonide demonstrated a 44% relative reduction in time to first severe asthma related event, compared with placebo (95% confidence interval [CI], 0.45–0.71; NNT=44; P=.0001).3
Theophylline
Theophylline is considered an alternative to inhaled corticosteroids. One study compared beclomethasone with theophylline in 195 children. This study found near-equivalent efficacy in doctor visits, hospitalizations, monthly peak expiratory flow rates, and FEV1; however, beclomethasone was superior to theophylline in maintaining symptom control and decreasing the use of inhaled bronchodilators and systemic steroids.
When compared with beclomethasone, theophylline was linked to 14% more central nervous system adverse effects (P<.001) and 17% more gastrointestinal disturbances (P<.001). Although beclomethasone induced more oral candidiasis compared with theophylline (8.9% vs 2.4%; P<.001), the incidence of this infection can be reduced by using a spacer.
Long-term systemic effects
The potential long-term adverse systemic effects of inhaled corticosteroids on growth, bone metabolism, and pituitary-adrenal function call for longer-term studies.4 A systematic review of 15 trials reported that the protective effect of leukotriene receptor antagonists is inferior to inhaled corticosteroids for adults (relative risk [RR]=1.71; 95% CI, 1.40–2.09); however, evidence is insufficient to extrapolate this to children.5
Beta-agonists
Evidence does not support use of long-acting beta-agonists as monotherapy or in combination with other medications for children with mild persistent asthma. Although 1 study showed an improvement in lung function for children taking budesonide plus formoterol compared with budesonide alone, the rate of severe exacerbations was lower for those taking budesonide alone (62% decrease vs 55.8% decrease; P=.001). Both groups had a 32% decrease in the number of rescue inhalations per day when compared with placebo (P=.0008).6
Recommendations from others
Recommendations are listed in the TABLE.1,7,8 Unlike the NAEPP and GINA asthma guidelines, the BTS/SIGN asthma guidelines define no objective measurement or staging classification to diagnose asthma among children. Diagnosis is determined by a child’s response to medication.8 Independent of any daily controller medication use, all children should have a short acting bronchodilator on hand in case of an acute attack.1,8
TABLE
Recommendations for treating mild persistent asthma
| GUIDELINE | DAILY CONTROLLER MEDICATION | ALTERNATIVE TREATMENT |
|---|---|---|
| National Asthma Education and Prevention Program (NAEPP)1 | Low-dose inhaled corticosteroids | Children <5: cromolyn, LTRAs Children >5: cromolyn, LTRAs, nedocromil, sustained release theophylline |
| Global initiative for asthma (GINA)7 | low-dose inhaled corticosteroids | All children: sustained released theophylline, Cromone, LTRAs |
| British Thoracic Society/Scottish intercollegiate Guidelines network (BTS/SIGN)8 | Inhaled steroids | All children: LTRAs, theophylline Children >5: cromones, nedocromil |
| LRTA leukotriene receptor antagonists. | ||
| Sources: NAEPP J Allergy Clin Immunol 20021; GINA Guidelines and Resources 20057 and BTS/SIGN, Thorax 2003.8 | ||
1. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics—2002. National Asthma Education and Prevention Program. J Allergy Clin Immunol 2002;110:S141-S219.
2. Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 2000;343:1054-1063.
3. Pauwels RA, Pedersen S, Busse WW, et al. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003;361:1071-1076.
4. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild-to-moderate asthma. The American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. J Allergy Clin Immunol 1998;101:14-23.
5. Ducharme FM, Salvio F, Ducharme F. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children (Cochrane review). In: The Cochrane Library. 2006 Issue 2. Chichester, UK: John Wiley and Sons, Ltd.
6. O’byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392-1397.
7. The Global Initiative for Asthma. Guidelines and Resources: 2005 Update. Available at: www.ginasthma.com/Guidelineitem.asp??I1=2&I2=1&intId=60. Accessed January 9, 2007.
8. British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. Thorax 2003;58:i1-i94.
1. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics—2002. National Asthma Education and Prevention Program. J Allergy Clin Immunol 2002;110:S141-S219.
2. Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 2000;343:1054-1063.
3. Pauwels RA, Pedersen S, Busse WW, et al. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003;361:1071-1076.
4. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild-to-moderate asthma. The American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. J Allergy Clin Immunol 1998;101:14-23.
5. Ducharme FM, Salvio F, Ducharme F. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children (Cochrane review). In: The Cochrane Library. 2006 Issue 2. Chichester, UK: John Wiley and Sons, Ltd.
6. O’byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392-1397.
7. The Global Initiative for Asthma. Guidelines and Resources: 2005 Update. Available at: www.ginasthma.com/Guidelineitem.asp??I1=2&I2=1&intId=60. Accessed January 9, 2007.
8. British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. Thorax 2003;58:i1-i94.
Evidence-based answers from the Family Physicians Inquiries Network
When are antibiotics indicated for acute COPD exacerbations?
Antibiotics (including those given orally) reduce mortality and treatment failures for hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, based on systematic reviews). Antibiotics may be prescribed in the outpatient setting for those with severe exacerbations (SOR: C, based on expert opinion).
Antibiotics are indicated in COPD exacerbations requiring hospitalization
Julie Taraday, MD
University of Washington, Seattle
In an era when physicians aim to use antibiotics judiciously, this article clarifies that antibiotics are indicated in COPD exacerbations requiring hospitalization. In the outpatient setting, the correct action is less clear. Available guidelines, which recommend antibiotics for severe exacerbations, do not generally differentiate between the inpatient and outpatient setting. Antibiotics clearly have no role in mild exacerbations and so should be avoided in many outpatient cases.
Evidence summary
A recent Cochrane review identified 11 randomized controlled trials (RCTs) (with a total of 917 patients) addressing antibiotic therapy for COPD exacerbations characterized by 1 or more of the following: an increase in sputum purulence or volume, dyspnea, wheezing, chest tightness, or fluid retention.1 Eight trials were conducted on hospital wards, 1 was in a medical intensive care unit, and 2 trials were in the outpatient setting. Antibiotics were given orally in 9 of the 11 studies.
Overall, antibiotics reduced risk of short-term mortality by 77% (relative risk [RR]=0.23; 95% confidence interval [CI],0.10–0.52; number needed to treat [NNT]=8), treatment failure by 53% (RR=0.47; 95% CI, 0.36–0.62; NNT=3), and sputum purulence by 44% (RR=0.56; 95% CI, 0.41–0.77; NNT=8). A subgroup analysis that excluded the outpatient and intensive-care unit studies did not change the result. Another subgroup analysis of the 2 outpatient studies failed to find a significant effect, although the studies had very different designs.
These findings are more robust than those of an earlier, lower-quality meta-analysis of 9 randomized controlled trials (RCTs) with 1101 patients with presumed COPD, which also compared antibiotic therapy with placebo for acute exacerbations.2 Specific diagnostic criteria were not stated for the diagnosis of either COPD or an acute exacerbation. No single outcome measure was common to all studies. The authors found a summary beneficial effect size of antibiotic therapy of 0.22 (95% CI, 0.10–0.34), which is generally interpreted as small. One clinical parameter, peak expiratory flow rate (PEFR), was reported in 6 of the studies. Antibiotic therapy resulted in an average 10.75 L/min improvement in PEFR compared with placebo (95% CI, 4.96–16.54 L/min).
Two RCTs addressing antibiotic use in the outpatient setting were identified in the Cochrane review. One double-blind crossover trial performed in Canada compared antibiotic with placebo therapy for 173 outpatients with 362 exacerbations classified according to severity.3 The protocol used oral trimethoprim-sulfamethoxazole, amoxicillin, or doxycycline (according to the attending physician’s preference) or a look-alike placebo. Symptom resolution was seen by 21 days in 68% of antibiotic users vs 55% of those on placebo (P<.01, NNT=8). Ten percent of patients taking antibiotics deteriorated to the point where hospitalization or unblinding of the therapy was necessary, compared with 19% in the placebo group (P<.05, NNT=11).
For patients with all 3 cardinal COPD symptoms (increased dyspnea, sputum production, and sputum purulence) at enrollment, there was resolution at 21 days in 63% with antibiotics vs 43% for placebo (P value not given). Antibiotics did not benefit patients with 1 cardinal symptom (74% success with antibiotics vs 70% on placebo; P value not given).
The Cochrane review also identified a Danish RCT that studied 278 patients presenting to their general practitioners with subjective acute worsening of their COPD. Patients were randomized to 7 days of oral amoxicillin or placebo. There was no difference between the groups in terms of symptom resolution at 1 week (odds ratio=1.03, favoring placebo; 95% CI, 0.75–1.41) or in changes in PEFR (weighted mean difference=–0.89, favoring placebo; 95% CI, –29 to 27 L/min).
Recommendations from others
The Veterans Health Administration recommends antibiotics if a patient with COPD has changes in sputum volume or quality as well as increased dyspnea, cough, or fever; infiltrate on x-ray suggesting pneumonia should be treated as such.4
The American College of Chest Physicians recommends that with severe COPD exacerbations, narrow spectrum antibiotics are reasonable first-line agents.5 They also note that the superiority of newer, more broad-spectrum antibiotics has not been established.
1. Ram FSF, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;(2):CD004403.-
2. Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations. JAMA 1995;273:957-60.
3. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204.
4. Medical advisory panel for the pharmacy benefits management strategic healthcare group The pharmacologic management of chronic obstructive pulmonary disease. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; 2002.
5. Snow V, Lascher S. Mottur-Pilson C, and the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
Antibiotics (including those given orally) reduce mortality and treatment failures for hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, based on systematic reviews). Antibiotics may be prescribed in the outpatient setting for those with severe exacerbations (SOR: C, based on expert opinion).
Antibiotics are indicated in COPD exacerbations requiring hospitalization
Julie Taraday, MD
University of Washington, Seattle
In an era when physicians aim to use antibiotics judiciously, this article clarifies that antibiotics are indicated in COPD exacerbations requiring hospitalization. In the outpatient setting, the correct action is less clear. Available guidelines, which recommend antibiotics for severe exacerbations, do not generally differentiate between the inpatient and outpatient setting. Antibiotics clearly have no role in mild exacerbations and so should be avoided in many outpatient cases.
Evidence summary
A recent Cochrane review identified 11 randomized controlled trials (RCTs) (with a total of 917 patients) addressing antibiotic therapy for COPD exacerbations characterized by 1 or more of the following: an increase in sputum purulence or volume, dyspnea, wheezing, chest tightness, or fluid retention.1 Eight trials were conducted on hospital wards, 1 was in a medical intensive care unit, and 2 trials were in the outpatient setting. Antibiotics were given orally in 9 of the 11 studies.
Overall, antibiotics reduced risk of short-term mortality by 77% (relative risk [RR]=0.23; 95% confidence interval [CI],0.10–0.52; number needed to treat [NNT]=8), treatment failure by 53% (RR=0.47; 95% CI, 0.36–0.62; NNT=3), and sputum purulence by 44% (RR=0.56; 95% CI, 0.41–0.77; NNT=8). A subgroup analysis that excluded the outpatient and intensive-care unit studies did not change the result. Another subgroup analysis of the 2 outpatient studies failed to find a significant effect, although the studies had very different designs.
These findings are more robust than those of an earlier, lower-quality meta-analysis of 9 randomized controlled trials (RCTs) with 1101 patients with presumed COPD, which also compared antibiotic therapy with placebo for acute exacerbations.2 Specific diagnostic criteria were not stated for the diagnosis of either COPD or an acute exacerbation. No single outcome measure was common to all studies. The authors found a summary beneficial effect size of antibiotic therapy of 0.22 (95% CI, 0.10–0.34), which is generally interpreted as small. One clinical parameter, peak expiratory flow rate (PEFR), was reported in 6 of the studies. Antibiotic therapy resulted in an average 10.75 L/min improvement in PEFR compared with placebo (95% CI, 4.96–16.54 L/min).
Two RCTs addressing antibiotic use in the outpatient setting were identified in the Cochrane review. One double-blind crossover trial performed in Canada compared antibiotic with placebo therapy for 173 outpatients with 362 exacerbations classified according to severity.3 The protocol used oral trimethoprim-sulfamethoxazole, amoxicillin, or doxycycline (according to the attending physician’s preference) or a look-alike placebo. Symptom resolution was seen by 21 days in 68% of antibiotic users vs 55% of those on placebo (P<.01, NNT=8). Ten percent of patients taking antibiotics deteriorated to the point where hospitalization or unblinding of the therapy was necessary, compared with 19% in the placebo group (P<.05, NNT=11).
For patients with all 3 cardinal COPD symptoms (increased dyspnea, sputum production, and sputum purulence) at enrollment, there was resolution at 21 days in 63% with antibiotics vs 43% for placebo (P value not given). Antibiotics did not benefit patients with 1 cardinal symptom (74% success with antibiotics vs 70% on placebo; P value not given).
The Cochrane review also identified a Danish RCT that studied 278 patients presenting to their general practitioners with subjective acute worsening of their COPD. Patients were randomized to 7 days of oral amoxicillin or placebo. There was no difference between the groups in terms of symptom resolution at 1 week (odds ratio=1.03, favoring placebo; 95% CI, 0.75–1.41) or in changes in PEFR (weighted mean difference=–0.89, favoring placebo; 95% CI, –29 to 27 L/min).
Recommendations from others
The Veterans Health Administration recommends antibiotics if a patient with COPD has changes in sputum volume or quality as well as increased dyspnea, cough, or fever; infiltrate on x-ray suggesting pneumonia should be treated as such.4
The American College of Chest Physicians recommends that with severe COPD exacerbations, narrow spectrum antibiotics are reasonable first-line agents.5 They also note that the superiority of newer, more broad-spectrum antibiotics has not been established.
Antibiotics (including those given orally) reduce mortality and treatment failures for hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, based on systematic reviews). Antibiotics may be prescribed in the outpatient setting for those with severe exacerbations (SOR: C, based on expert opinion).
Antibiotics are indicated in COPD exacerbations requiring hospitalization
Julie Taraday, MD
University of Washington, Seattle
In an era when physicians aim to use antibiotics judiciously, this article clarifies that antibiotics are indicated in COPD exacerbations requiring hospitalization. In the outpatient setting, the correct action is less clear. Available guidelines, which recommend antibiotics for severe exacerbations, do not generally differentiate between the inpatient and outpatient setting. Antibiotics clearly have no role in mild exacerbations and so should be avoided in many outpatient cases.
Evidence summary
A recent Cochrane review identified 11 randomized controlled trials (RCTs) (with a total of 917 patients) addressing antibiotic therapy for COPD exacerbations characterized by 1 or more of the following: an increase in sputum purulence or volume, dyspnea, wheezing, chest tightness, or fluid retention.1 Eight trials were conducted on hospital wards, 1 was in a medical intensive care unit, and 2 trials were in the outpatient setting. Antibiotics were given orally in 9 of the 11 studies.
Overall, antibiotics reduced risk of short-term mortality by 77% (relative risk [RR]=0.23; 95% confidence interval [CI],0.10–0.52; number needed to treat [NNT]=8), treatment failure by 53% (RR=0.47; 95% CI, 0.36–0.62; NNT=3), and sputum purulence by 44% (RR=0.56; 95% CI, 0.41–0.77; NNT=8). A subgroup analysis that excluded the outpatient and intensive-care unit studies did not change the result. Another subgroup analysis of the 2 outpatient studies failed to find a significant effect, although the studies had very different designs.
These findings are more robust than those of an earlier, lower-quality meta-analysis of 9 randomized controlled trials (RCTs) with 1101 patients with presumed COPD, which also compared antibiotic therapy with placebo for acute exacerbations.2 Specific diagnostic criteria were not stated for the diagnosis of either COPD or an acute exacerbation. No single outcome measure was common to all studies. The authors found a summary beneficial effect size of antibiotic therapy of 0.22 (95% CI, 0.10–0.34), which is generally interpreted as small. One clinical parameter, peak expiratory flow rate (PEFR), was reported in 6 of the studies. Antibiotic therapy resulted in an average 10.75 L/min improvement in PEFR compared with placebo (95% CI, 4.96–16.54 L/min).
Two RCTs addressing antibiotic use in the outpatient setting were identified in the Cochrane review. One double-blind crossover trial performed in Canada compared antibiotic with placebo therapy for 173 outpatients with 362 exacerbations classified according to severity.3 The protocol used oral trimethoprim-sulfamethoxazole, amoxicillin, or doxycycline (according to the attending physician’s preference) or a look-alike placebo. Symptom resolution was seen by 21 days in 68% of antibiotic users vs 55% of those on placebo (P<.01, NNT=8). Ten percent of patients taking antibiotics deteriorated to the point where hospitalization or unblinding of the therapy was necessary, compared with 19% in the placebo group (P<.05, NNT=11).
For patients with all 3 cardinal COPD symptoms (increased dyspnea, sputum production, and sputum purulence) at enrollment, there was resolution at 21 days in 63% with antibiotics vs 43% for placebo (P value not given). Antibiotics did not benefit patients with 1 cardinal symptom (74% success with antibiotics vs 70% on placebo; P value not given).
The Cochrane review also identified a Danish RCT that studied 278 patients presenting to their general practitioners with subjective acute worsening of their COPD. Patients were randomized to 7 days of oral amoxicillin or placebo. There was no difference between the groups in terms of symptom resolution at 1 week (odds ratio=1.03, favoring placebo; 95% CI, 0.75–1.41) or in changes in PEFR (weighted mean difference=–0.89, favoring placebo; 95% CI, –29 to 27 L/min).
Recommendations from others
The Veterans Health Administration recommends antibiotics if a patient with COPD has changes in sputum volume or quality as well as increased dyspnea, cough, or fever; infiltrate on x-ray suggesting pneumonia should be treated as such.4
The American College of Chest Physicians recommends that with severe COPD exacerbations, narrow spectrum antibiotics are reasonable first-line agents.5 They also note that the superiority of newer, more broad-spectrum antibiotics has not been established.
1. Ram FSF, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;(2):CD004403.-
2. Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations. JAMA 1995;273:957-60.
3. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204.
4. Medical advisory panel for the pharmacy benefits management strategic healthcare group The pharmacologic management of chronic obstructive pulmonary disease. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; 2002.
5. Snow V, Lascher S. Mottur-Pilson C, and the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
1. Ram FSF, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;(2):CD004403.-
2. Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations. JAMA 1995;273:957-60.
3. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204.
4. Medical advisory panel for the pharmacy benefits management strategic healthcare group The pharmacologic management of chronic obstructive pulmonary disease. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; 2002.
5. Snow V, Lascher S. Mottur-Pilson C, and the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
Evidence-based answers from the Family Physicians Inquiries Network
What best prevents exercise-induced bronchoconstriction for a child with asthma?
Inhaled short-acting beta-agonists (SABAs) are most effective in preventing exercise-induced bronchoconstriction, followed by inhaled mast cell stabilizers and anticholinergic agents (strength of recommendation [SOR]: A, multiple randomized control trials [RCTs]). Less evidence supports the use of leukotriene antagonists and inhaled corticosteroids, either individually or in combination (SOR: B). Underlying asthma, which commonly contributes to exercise-induced bronchoconstriction, should be diagnosed and controlled first (SOR: C).
Control the asthma and the need for pre-treatment often becomes unnecessary
Because truly isolated exercise-induced bronchoconstriction is uncommon in a nonasthmatic child, and because bronchospasm in a child during exercise more commonly indicates undiagnosed asthma, search for treatable asthma when a child wheezes with exercise. These children have sputum eosinophilia reflecting inflammation, and they are best served by addressing the underlying asthma with inhaled corticosteroids. Once the asthma is under control, their need for “the best pre-treatment” (a SABA) often becomes irrelevant. Ask the child whether he or she is having more shortness of breath and difficulty breathing after exercise than during exercise; this reveals those most likely to benefit from treatment.
Evidence summary
It is difficult to interpret studies on exercise-induced bronchoconstriction (the rather uncommon presence of exercise-induced bronchospasm in a nonasthmatic) and exercise-induced asthma (the more common situation of asthma worsened by exercise). Many studies include both types of patients.
A systematic review of 24 RCTs (of which 13 evaluated children) showed that SABAs, mast cell stabilizers, and anticholinergics provide a significant protective effect against exercise-induced bronchoconstriction with few adverse effects (the child subgroup analyses did not differ significantly from pooled results). Mast cell stabilizers were found less effective at attenuating bronchoconstriction than SABAs, with an average maximum decrease in the forced expiratory volume in 1 second (FEV1) of 11.9% compared with 4.6% for beta-agonists (child subgroup: weighted mean difference=7.3%; 95% confidence interval [CI], 3.9–10.7). Complete protection (defined in this study as maximum % decrease in FEV1 <15% post-exercise) and clinical protection (50% improvement over placebo) measures were included. Fewer children had complete protection (pooled: 66% vs 85%, odds ratio [OR]=0.3; 95% CI, 0.2–0.5) or clinical protection (pooled: 55% vs 77%, OR=0.4; 95% CI, 0.2–0.8).
Mast cell stabilizers were more effective than anticholinergic agents, with average maximum FEV1 decrease of 9.4% compared with 16.0% on anticholinergics (child subgroup: weighted mean difference=6.6%; 95% CI, 1.0–12.2). They also provided more individuals with complete protection (pooled: 73% vs 56%, OR=2.2; 95% CI, 1.3–3.7) and clinical protection (pooled: 73% vs 52%, OR=2.7; 95% CI, 1.1–6.4). Combining mast cell stabilizers with SABAs did not produce significant advantages in pulmonary function over SABAs alone. No significant subgroup differences were seen based on age, severity, or study quality.1
Another systematic review of 20 RCTs (15 studying children and 5 studying adults) with patients aged >6 years showed that 4 mg of nedocromil (Tilade) inhaled 15 to 60 minutes before exercise significantly reduced the severity and duration of exercise-induced bronchoconstriction compared with placebo. It had a greater effect on patients with severe exercise-induced bronchoconstriction (defined as an exercise-induced fall in lung function >30% from baseline).2
Eight RCTs (5 studying children) were included in a systematic review of patients aged >6 years that found no significant difference between nedocromil and cromoglycate with regards to decrease in FEV1, complete protection, clinical protection, or side effects.3
Leukotriene antagonists have been recommended on a trial basis with follow-up to evaluate the treatment response.4 Although there are several long-term studies of leukotriene antagonists for adults, few have studied children. A recent study assessed the effects of montelukast (Singulair) on 64 children with exercise-induced bronchoconstriction. After 8 weeks of treatment, the montelukast group showed significant improvements (compared with placebo) in asthma symptom scores (24.3±8.2 before vs 17.8±6.8 after 8 weeks of montelukast treatment, P<.05; vs 17.7±6.7 8 weeks after stopping treatment, P<.05), maximum percent fall in FEV1 after exercise (36.5±10.2% before vs 27.6±14.4% after 8 wks of treatment, P<.01; vs 26.7±19.4% 8 weeks after stopping treatment, P<.01), and time to recovery (41.8±8.1 min before vs 25.3±23.3 min after 8 weeks of treatment, P<.01; vs 27.7±26.5 min 8 weeks after stopping, P<.05).5
Therapies awaiting further study include a combination of budesonide (Pulmicort) and formoterol (Foradil), which is similar to the currently available preparation of fluticasone and salmeterol (Advair Diskus) but contains a long-acting beta-agonist with quicker onset. The phosphodiesterase-4 inhibitors roflumilast (Daxas) and cilomilast (Ariflo)—neither of which have been FDA-approved—and inhaled low-molecular-weight heparin have potential efficacy.6 Other options suggested for this problem—including inhaled furosemide, vitamin C, antihistamines, calcium channel blockers, and reduced dietary salt intake—need further study.7
Recommendations from others
Review articles on this topic suggest the following to prevent exercise-induced bronchoconstriction: controlling baseline asthma, avoiding known allergens, choosing appropriate sports with short bursts of activity, and selecting warm, humid environments for the activities.6-8 Some authorities recommend warm-up before athletic events to take advantage of a 30- to 90-minute refractory period. This can help prevent exercise-induced bronchoconstriction; however, effects vary considerably from person to person.7,8
The National Asthma Education and Prevention Program recommends prevention of exercise-induced bronchoconstriction by optimally controlling underlying asthma. If a patient remains symptomatic during exercise, you should review medication usage, understanding of dosage instructions, and administration technique before any changes in the treatment regimen.9
1. Spooner CH, Spooner GR, Rowe BH. Mast-cell stabilising agents to prevent exercise-induced bronchoconstriction. Cochrane Database Syst Rev 2003;(4):CD002307.
2. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction. Cochrane Database Syst Rev 2002;(1):CD001183.
3. Kelly K, Spooner CH, Rowe BH. Nedocromil sodium versus sodium cromoglycate for preventing exercise-induced bronchoconstriction in asthmatics. Cochrane Database Syst Rev 2000;(4):CD002731.
4. Moraes TJ, Selvadurai H. Management of exercise-induced bronchospasm in children: the role of leukotriene antagonists. Treat Respir Med 2004;3:9-15.
5. Kim JH, Lee SY, Kim HB, et al. Prolonged effect of montelukast in asthmatic children with exercise-induced bronchoconstriction. Pediatr Pulmonol 2005;39(2):162-166.
6. Storms WW. Asthma associated with exercise. Immunol Allergy Clin North Am 2005;25:31-43.
7. Sinha T, David AK. Recognition and management of exercise-induced bronchospasm. Am Fam Physician 2003;67(4):769-774, 675.
8. DYNAMED [database online]. Columbia, Mo: Dynamic Medical Information Systems, LLC;1995, continuous daily updating. Updated December 2, 2004.
9. Williams SG, Schmidt DK, Redd SC, Storms W. Key clinical activities for quality asthma care: recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003;52(RR-6):1-8.
Inhaled short-acting beta-agonists (SABAs) are most effective in preventing exercise-induced bronchoconstriction, followed by inhaled mast cell stabilizers and anticholinergic agents (strength of recommendation [SOR]: A, multiple randomized control trials [RCTs]). Less evidence supports the use of leukotriene antagonists and inhaled corticosteroids, either individually or in combination (SOR: B). Underlying asthma, which commonly contributes to exercise-induced bronchoconstriction, should be diagnosed and controlled first (SOR: C).
Control the asthma and the need for pre-treatment often becomes unnecessary
Because truly isolated exercise-induced bronchoconstriction is uncommon in a nonasthmatic child, and because bronchospasm in a child during exercise more commonly indicates undiagnosed asthma, search for treatable asthma when a child wheezes with exercise. These children have sputum eosinophilia reflecting inflammation, and they are best served by addressing the underlying asthma with inhaled corticosteroids. Once the asthma is under control, their need for “the best pre-treatment” (a SABA) often becomes irrelevant. Ask the child whether he or she is having more shortness of breath and difficulty breathing after exercise than during exercise; this reveals those most likely to benefit from treatment.
Evidence summary
It is difficult to interpret studies on exercise-induced bronchoconstriction (the rather uncommon presence of exercise-induced bronchospasm in a nonasthmatic) and exercise-induced asthma (the more common situation of asthma worsened by exercise). Many studies include both types of patients.
A systematic review of 24 RCTs (of which 13 evaluated children) showed that SABAs, mast cell stabilizers, and anticholinergics provide a significant protective effect against exercise-induced bronchoconstriction with few adverse effects (the child subgroup analyses did not differ significantly from pooled results). Mast cell stabilizers were found less effective at attenuating bronchoconstriction than SABAs, with an average maximum decrease in the forced expiratory volume in 1 second (FEV1) of 11.9% compared with 4.6% for beta-agonists (child subgroup: weighted mean difference=7.3%; 95% confidence interval [CI], 3.9–10.7). Complete protection (defined in this study as maximum % decrease in FEV1 <15% post-exercise) and clinical protection (50% improvement over placebo) measures were included. Fewer children had complete protection (pooled: 66% vs 85%, odds ratio [OR]=0.3; 95% CI, 0.2–0.5) or clinical protection (pooled: 55% vs 77%, OR=0.4; 95% CI, 0.2–0.8).
Mast cell stabilizers were more effective than anticholinergic agents, with average maximum FEV1 decrease of 9.4% compared with 16.0% on anticholinergics (child subgroup: weighted mean difference=6.6%; 95% CI, 1.0–12.2). They also provided more individuals with complete protection (pooled: 73% vs 56%, OR=2.2; 95% CI, 1.3–3.7) and clinical protection (pooled: 73% vs 52%, OR=2.7; 95% CI, 1.1–6.4). Combining mast cell stabilizers with SABAs did not produce significant advantages in pulmonary function over SABAs alone. No significant subgroup differences were seen based on age, severity, or study quality.1
Another systematic review of 20 RCTs (15 studying children and 5 studying adults) with patients aged >6 years showed that 4 mg of nedocromil (Tilade) inhaled 15 to 60 minutes before exercise significantly reduced the severity and duration of exercise-induced bronchoconstriction compared with placebo. It had a greater effect on patients with severe exercise-induced bronchoconstriction (defined as an exercise-induced fall in lung function >30% from baseline).2
Eight RCTs (5 studying children) were included in a systematic review of patients aged >6 years that found no significant difference between nedocromil and cromoglycate with regards to decrease in FEV1, complete protection, clinical protection, or side effects.3
Leukotriene antagonists have been recommended on a trial basis with follow-up to evaluate the treatment response.4 Although there are several long-term studies of leukotriene antagonists for adults, few have studied children. A recent study assessed the effects of montelukast (Singulair) on 64 children with exercise-induced bronchoconstriction. After 8 weeks of treatment, the montelukast group showed significant improvements (compared with placebo) in asthma symptom scores (24.3±8.2 before vs 17.8±6.8 after 8 weeks of montelukast treatment, P<.05; vs 17.7±6.7 8 weeks after stopping treatment, P<.05), maximum percent fall in FEV1 after exercise (36.5±10.2% before vs 27.6±14.4% after 8 wks of treatment, P<.01; vs 26.7±19.4% 8 weeks after stopping treatment, P<.01), and time to recovery (41.8±8.1 min before vs 25.3±23.3 min after 8 weeks of treatment, P<.01; vs 27.7±26.5 min 8 weeks after stopping, P<.05).5
Therapies awaiting further study include a combination of budesonide (Pulmicort) and formoterol (Foradil), which is similar to the currently available preparation of fluticasone and salmeterol (Advair Diskus) but contains a long-acting beta-agonist with quicker onset. The phosphodiesterase-4 inhibitors roflumilast (Daxas) and cilomilast (Ariflo)—neither of which have been FDA-approved—and inhaled low-molecular-weight heparin have potential efficacy.6 Other options suggested for this problem—including inhaled furosemide, vitamin C, antihistamines, calcium channel blockers, and reduced dietary salt intake—need further study.7
Recommendations from others
Review articles on this topic suggest the following to prevent exercise-induced bronchoconstriction: controlling baseline asthma, avoiding known allergens, choosing appropriate sports with short bursts of activity, and selecting warm, humid environments for the activities.6-8 Some authorities recommend warm-up before athletic events to take advantage of a 30- to 90-minute refractory period. This can help prevent exercise-induced bronchoconstriction; however, effects vary considerably from person to person.7,8
The National Asthma Education and Prevention Program recommends prevention of exercise-induced bronchoconstriction by optimally controlling underlying asthma. If a patient remains symptomatic during exercise, you should review medication usage, understanding of dosage instructions, and administration technique before any changes in the treatment regimen.9
Inhaled short-acting beta-agonists (SABAs) are most effective in preventing exercise-induced bronchoconstriction, followed by inhaled mast cell stabilizers and anticholinergic agents (strength of recommendation [SOR]: A, multiple randomized control trials [RCTs]). Less evidence supports the use of leukotriene antagonists and inhaled corticosteroids, either individually or in combination (SOR: B). Underlying asthma, which commonly contributes to exercise-induced bronchoconstriction, should be diagnosed and controlled first (SOR: C).
Control the asthma and the need for pre-treatment often becomes unnecessary
Because truly isolated exercise-induced bronchoconstriction is uncommon in a nonasthmatic child, and because bronchospasm in a child during exercise more commonly indicates undiagnosed asthma, search for treatable asthma when a child wheezes with exercise. These children have sputum eosinophilia reflecting inflammation, and they are best served by addressing the underlying asthma with inhaled corticosteroids. Once the asthma is under control, their need for “the best pre-treatment” (a SABA) often becomes irrelevant. Ask the child whether he or she is having more shortness of breath and difficulty breathing after exercise than during exercise; this reveals those most likely to benefit from treatment.
Evidence summary
It is difficult to interpret studies on exercise-induced bronchoconstriction (the rather uncommon presence of exercise-induced bronchospasm in a nonasthmatic) and exercise-induced asthma (the more common situation of asthma worsened by exercise). Many studies include both types of patients.
A systematic review of 24 RCTs (of which 13 evaluated children) showed that SABAs, mast cell stabilizers, and anticholinergics provide a significant protective effect against exercise-induced bronchoconstriction with few adverse effects (the child subgroup analyses did not differ significantly from pooled results). Mast cell stabilizers were found less effective at attenuating bronchoconstriction than SABAs, with an average maximum decrease in the forced expiratory volume in 1 second (FEV1) of 11.9% compared with 4.6% for beta-agonists (child subgroup: weighted mean difference=7.3%; 95% confidence interval [CI], 3.9–10.7). Complete protection (defined in this study as maximum % decrease in FEV1 <15% post-exercise) and clinical protection (50% improvement over placebo) measures were included. Fewer children had complete protection (pooled: 66% vs 85%, odds ratio [OR]=0.3; 95% CI, 0.2–0.5) or clinical protection (pooled: 55% vs 77%, OR=0.4; 95% CI, 0.2–0.8).
Mast cell stabilizers were more effective than anticholinergic agents, with average maximum FEV1 decrease of 9.4% compared with 16.0% on anticholinergics (child subgroup: weighted mean difference=6.6%; 95% CI, 1.0–12.2). They also provided more individuals with complete protection (pooled: 73% vs 56%, OR=2.2; 95% CI, 1.3–3.7) and clinical protection (pooled: 73% vs 52%, OR=2.7; 95% CI, 1.1–6.4). Combining mast cell stabilizers with SABAs did not produce significant advantages in pulmonary function over SABAs alone. No significant subgroup differences were seen based on age, severity, or study quality.1
Another systematic review of 20 RCTs (15 studying children and 5 studying adults) with patients aged >6 years showed that 4 mg of nedocromil (Tilade) inhaled 15 to 60 minutes before exercise significantly reduced the severity and duration of exercise-induced bronchoconstriction compared with placebo. It had a greater effect on patients with severe exercise-induced bronchoconstriction (defined as an exercise-induced fall in lung function >30% from baseline).2
Eight RCTs (5 studying children) were included in a systematic review of patients aged >6 years that found no significant difference between nedocromil and cromoglycate with regards to decrease in FEV1, complete protection, clinical protection, or side effects.3
Leukotriene antagonists have been recommended on a trial basis with follow-up to evaluate the treatment response.4 Although there are several long-term studies of leukotriene antagonists for adults, few have studied children. A recent study assessed the effects of montelukast (Singulair) on 64 children with exercise-induced bronchoconstriction. After 8 weeks of treatment, the montelukast group showed significant improvements (compared with placebo) in asthma symptom scores (24.3±8.2 before vs 17.8±6.8 after 8 weeks of montelukast treatment, P<.05; vs 17.7±6.7 8 weeks after stopping treatment, P<.05), maximum percent fall in FEV1 after exercise (36.5±10.2% before vs 27.6±14.4% after 8 wks of treatment, P<.01; vs 26.7±19.4% 8 weeks after stopping treatment, P<.01), and time to recovery (41.8±8.1 min before vs 25.3±23.3 min after 8 weeks of treatment, P<.01; vs 27.7±26.5 min 8 weeks after stopping, P<.05).5
Therapies awaiting further study include a combination of budesonide (Pulmicort) and formoterol (Foradil), which is similar to the currently available preparation of fluticasone and salmeterol (Advair Diskus) but contains a long-acting beta-agonist with quicker onset. The phosphodiesterase-4 inhibitors roflumilast (Daxas) and cilomilast (Ariflo)—neither of which have been FDA-approved—and inhaled low-molecular-weight heparin have potential efficacy.6 Other options suggested for this problem—including inhaled furosemide, vitamin C, antihistamines, calcium channel blockers, and reduced dietary salt intake—need further study.7
Recommendations from others
Review articles on this topic suggest the following to prevent exercise-induced bronchoconstriction: controlling baseline asthma, avoiding known allergens, choosing appropriate sports with short bursts of activity, and selecting warm, humid environments for the activities.6-8 Some authorities recommend warm-up before athletic events to take advantage of a 30- to 90-minute refractory period. This can help prevent exercise-induced bronchoconstriction; however, effects vary considerably from person to person.7,8
The National Asthma Education and Prevention Program recommends prevention of exercise-induced bronchoconstriction by optimally controlling underlying asthma. If a patient remains symptomatic during exercise, you should review medication usage, understanding of dosage instructions, and administration technique before any changes in the treatment regimen.9
1. Spooner CH, Spooner GR, Rowe BH. Mast-cell stabilising agents to prevent exercise-induced bronchoconstriction. Cochrane Database Syst Rev 2003;(4):CD002307.
2. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction. Cochrane Database Syst Rev 2002;(1):CD001183.
3. Kelly K, Spooner CH, Rowe BH. Nedocromil sodium versus sodium cromoglycate for preventing exercise-induced bronchoconstriction in asthmatics. Cochrane Database Syst Rev 2000;(4):CD002731.
4. Moraes TJ, Selvadurai H. Management of exercise-induced bronchospasm in children: the role of leukotriene antagonists. Treat Respir Med 2004;3:9-15.
5. Kim JH, Lee SY, Kim HB, et al. Prolonged effect of montelukast in asthmatic children with exercise-induced bronchoconstriction. Pediatr Pulmonol 2005;39(2):162-166.
6. Storms WW. Asthma associated with exercise. Immunol Allergy Clin North Am 2005;25:31-43.
7. Sinha T, David AK. Recognition and management of exercise-induced bronchospasm. Am Fam Physician 2003;67(4):769-774, 675.
8. DYNAMED [database online]. Columbia, Mo: Dynamic Medical Information Systems, LLC;1995, continuous daily updating. Updated December 2, 2004.
9. Williams SG, Schmidt DK, Redd SC, Storms W. Key clinical activities for quality asthma care: recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003;52(RR-6):1-8.
1. Spooner CH, Spooner GR, Rowe BH. Mast-cell stabilising agents to prevent exercise-induced bronchoconstriction. Cochrane Database Syst Rev 2003;(4):CD002307.
2. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction. Cochrane Database Syst Rev 2002;(1):CD001183.
3. Kelly K, Spooner CH, Rowe BH. Nedocromil sodium versus sodium cromoglycate for preventing exercise-induced bronchoconstriction in asthmatics. Cochrane Database Syst Rev 2000;(4):CD002731.
4. Moraes TJ, Selvadurai H. Management of exercise-induced bronchospasm in children: the role of leukotriene antagonists. Treat Respir Med 2004;3:9-15.
5. Kim JH, Lee SY, Kim HB, et al. Prolonged effect of montelukast in asthmatic children with exercise-induced bronchoconstriction. Pediatr Pulmonol 2005;39(2):162-166.
6. Storms WW. Asthma associated with exercise. Immunol Allergy Clin North Am 2005;25:31-43.
7. Sinha T, David AK. Recognition and management of exercise-induced bronchospasm. Am Fam Physician 2003;67(4):769-774, 675.
8. DYNAMED [database online]. Columbia, Mo: Dynamic Medical Information Systems, LLC;1995, continuous daily updating. Updated December 2, 2004.
9. Williams SG, Schmidt DK, Redd SC, Storms W. Key clinical activities for quality asthma care: recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003;52(RR-6):1-8.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for pertussis?
A short-term course of erythromycin, azithromycin, or clarithromycin is as effective as a long-term (2-week) erythromycin therapy in eradicating Bordetella pertussis from the nasopharynx (strength of recommendation [SOR]: A; based on one meta-analysis of randomized controlled trials [RCTs]). Evidence is insufficient to determine the benefit of antibiotic prophylaxis for pertussis contacts. However, due to high mortality and morbidity, prophylaxis is recommended for families who have an infant less than 6 months old (SOR: C; based on expert opinion).
Fewer doses and lower cost make compliance more likely
Marcia Warren, MD
Departments of Family and Community Medicine and Pediatrics, Baylor College of Medicine, Houston, Tex
I found this Clinical Inquiry on the treatment and prophylaxis of Bordetella pertussis invaluable as it addresses ease of dosing and cost, 2 things important in my pediatric community health practice with its inherent financial and social constraints. The alternatives suggested are easy to use and are as equally effective as the first-line therapy of erythromycin estolate, the long-term treatment recommended by the CDC and the AAP. These alternatives, clarithromycin and azithromycin, require either twice a day or a once a day dosing for 7 days or 3 days respectively, can be accommodated in busy households, thus promoting better compliance.
The cost of medication also relates to compliance. The cost difference between the first-line therapy and the alternative therapy is significant, and may be as much as $89. In an underinsured population, this out-of-pocket cost for the alternatives would prove prohibitive, resulting in decreased compliance. Where cost is not a great issue and concerns of compliance important, choosing the short-term treatment may be a preferable option. For the financially strapped, the 1-week regimen of erythromycin estolate would be preferable. The importance of counseling cannot be overstated in all dosing regimens, especially in those with a more difficult dosing schedule and in cases of prophylaxis in a household with an infant less than 6 months old.
Evidence summary
A 2005 Cochrane review of 11 RCTs and 1 quasi-randomized trial, with a total of 1720 adults and children, investigated several antibiotics for treatment and prophylaxis of pertussis. The outcome measures used to assess the efficacy of antibiotic treatment or prophylaxis vary between the trials and most of them did not report the immunization status of the participants. The Cochrane review included 1 meta-analysis of 3 studies with 252 participants, comparing azithromycin for 3 days, erythromycin estolate for 7 days, and clarithromycin for 7 days (short-term treatment) with erythromycin estolate for fourteen days (long-term treatment). The study showed equal efficacy in eradication of B pertussis from the nasopharynx of 99.2% to 97.7% (absolute risk reduction [ARR]=1.44%; 95% confidence interval [CI], –1.58 to 4.46). There were fewer side effects with the short-term treatment (32.1% vs 48.9%; ARR=16%; 95% CI, 7.84 to 25.84).1
A large, multicenter RCT of 477 children of 6 months to 16 years of age demonstrated that a 5-day treatment with azithromycin eradicated B pertussis from the nasopharynx as effectively as a 10-day course of erythromycin estolate.2 Similarly, trimethoprim/sulfamethoxazole proved as effective as erythromycin in eliminating B pertussis from the nasopharynx.
Although tetracycline and chloramphenicol are effective treatments for pertussis, they are not recommended because of their side effects.1 Six randomized trials failed to show any statistically significant difference between antibiotics and placebo on frequency and severity of cough or duration of pertussis disease.1 A randomized, placebo-controlled trial studied 300 household contacts of children with culture-positive pertussis. There was no statistically significant difference in either the frequency of pertussis disease or rate of positive cultures in household contacts between the erythromycin group (2.1%) and the placebo group (5.1%) (ARR=2.95%; 95% CI, –1.21 to 7.11).1
Another Cochrane review of 8 trials examined the effectiveness of the symptomatic treatment of cough in children and adults with pertussis. There were many problems with the methodological quality of these trials, including small sample sizes and poor reporting of the methods. Diphenhydramine, pertussis immunoglobulin, corticosteroids and salbutamol were compared with placebo. There were no statistically significant differences in coughing paroxysms, mean number of whoops per 24 hours or in duration of hospital stay between these interventions and placebo.3
Extracorporeal circulatory life support has been used to maintain perfusion for patients with severe disease. The mortality of these patients is very high.4 No RCTs of the effectiveness of this intervention has been performed.
TABLE
Antibiotics for treatment and prophylaxis of pertussis in children and adults
| FIRST-LINE THERAPY | DOSAGE FOR CHILDREN | DOSAGE FOR ADULTS | COST* |
|---|---|---|---|
| Erythromycin | 40-50 mg/kg orally or intravenously in 4 divided doses for 14 days5,7 | 1–2 g orally or intravenously in 4 divided doses for 14 days5,7 | 56 tabs (500 mg), $16 (generic) |
| ALTERNATIVE THERAPY IF PATIENT DOESN’T TOLERATE ERYTHROMYCIN | |||
| Clarithromycin | 15-20 mg/per kg orally divided every 12 hours for 10-14 days7or 14-15 mg/kg orally divided every 12 hours for 7 days1,5 | 500 mg orally every 12 hours for 7 days5 | 20 tabs (500 mg), $78 28 tabs (500 mg), $109 (generic) |
| Azithromycin | 10-12 mg/kg orally as single daily dose for 5 to 7 days5,7or 10 mg/kg orally single daily dose for 3 days1 | 500 mg orally once, then 250 daily on days 2-55,7 | 5 tabs (500 mg), $75 7 tabs (500 mg), $105 (no generic) |
| Trimethoprim-sulfamethoxazole | 8 mg of TMP, 40 mg/kg SMX per kg orally divided every 12 hours for 14 days5,7 | 160 mg of TMP, 800 SMX orally (1 tab DS) every 12 hours for 14 days5,7 | 28 tabs $8 (generic) |
| All these therapies have gastrointestinal side effects and risk for hypersensitivity reactions. | |||
| *Approximate retail price for adult dose. Available at: http://www.drugstore.com. Accessed on June 28, 2005. | |||
Recommendations from others
The Centers for Disease Control and Prevention recommends erythromycin for 14 days as a first choice for the treatment and prophylaxis of pertussis. Antibiotics should be started no later than 3 weeks after the onset of cough. Trimethoprim-sulfamethoxazole can be used as an alternative treatment for patients who do not tolerate erythromycin. Prophylaxis is recommended for all household and close contacts if pertussis is highly suspected.5
The American Academy of Pediatrics recommends the use of azithromycin and clarithromycin as an alternative treatment for patients who do not tolerate erythromycin. 5
A national consensus conference on pertussis held in Canada recommended prophylaxis for household contacts of an infant aged <1 year, pregnant women during the third trimester, and for vulnerable individuals who have had face-to-face exposure, or have shared confined air for >1 hour.6
1. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis) (Cochrane Review). Cochrane Database Syst Rev 2005;(1):CD004404.
2. Langley JM, Halperin SA, Boucher FD, Smith B. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics 2004;114:e96-101.
3. Pillay V, Swingler G. Symptomatic treatment of the cough in whooping cough (Cochrane Review). Cochrane Database Syst Rev 2003;(4):CD003257.
4. Williams GD, Numa A, Sokol J, Tobias V, Duffy BJ. ECLS in pertussis: does it have a role? Intensive Care Med 1998;24:1089-1092.
5. Guris D. Treatment and Chemoprophylaxis. Guidelines for the Control of Pertussis Outbreaks. Atlanta, Ga: Centers for Disease Control and Prevention, 2000. Last updated January 2005. Available at: www.cdc.gov/nip/publications/pertussis/guide.htm. Accessed on November 14, 2005.
6. National consensus conference on pertussis Can Commun Dis Rep 2003;29(Suppl 3):S1-S33 (English), S1-S36 (French). Last updated June 5, 2003. Available at: www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/29s3/index.html. Accessed on November 14, 2005.
7. Pertussis Information for Physicians: Diagnostic and Treatment Criteria Texas Department of Health Pertussis Treatment Guidelines. TDH Immunization Division; 2004;Last updated January 26, 2004. Available at: www.tdh.state.tx.us/immunize/html/pert_physician_txt.htm. Accessed on November 14, 2005.
A short-term course of erythromycin, azithromycin, or clarithromycin is as effective as a long-term (2-week) erythromycin therapy in eradicating Bordetella pertussis from the nasopharynx (strength of recommendation [SOR]: A; based on one meta-analysis of randomized controlled trials [RCTs]). Evidence is insufficient to determine the benefit of antibiotic prophylaxis for pertussis contacts. However, due to high mortality and morbidity, prophylaxis is recommended for families who have an infant less than 6 months old (SOR: C; based on expert opinion).
Fewer doses and lower cost make compliance more likely
Marcia Warren, MD
Departments of Family and Community Medicine and Pediatrics, Baylor College of Medicine, Houston, Tex
I found this Clinical Inquiry on the treatment and prophylaxis of Bordetella pertussis invaluable as it addresses ease of dosing and cost, 2 things important in my pediatric community health practice with its inherent financial and social constraints. The alternatives suggested are easy to use and are as equally effective as the first-line therapy of erythromycin estolate, the long-term treatment recommended by the CDC and the AAP. These alternatives, clarithromycin and azithromycin, require either twice a day or a once a day dosing for 7 days or 3 days respectively, can be accommodated in busy households, thus promoting better compliance.
The cost of medication also relates to compliance. The cost difference between the first-line therapy and the alternative therapy is significant, and may be as much as $89. In an underinsured population, this out-of-pocket cost for the alternatives would prove prohibitive, resulting in decreased compliance. Where cost is not a great issue and concerns of compliance important, choosing the short-term treatment may be a preferable option. For the financially strapped, the 1-week regimen of erythromycin estolate would be preferable. The importance of counseling cannot be overstated in all dosing regimens, especially in those with a more difficult dosing schedule and in cases of prophylaxis in a household with an infant less than 6 months old.
Evidence summary
A 2005 Cochrane review of 11 RCTs and 1 quasi-randomized trial, with a total of 1720 adults and children, investigated several antibiotics for treatment and prophylaxis of pertussis. The outcome measures used to assess the efficacy of antibiotic treatment or prophylaxis vary between the trials and most of them did not report the immunization status of the participants. The Cochrane review included 1 meta-analysis of 3 studies with 252 participants, comparing azithromycin for 3 days, erythromycin estolate for 7 days, and clarithromycin for 7 days (short-term treatment) with erythromycin estolate for fourteen days (long-term treatment). The study showed equal efficacy in eradication of B pertussis from the nasopharynx of 99.2% to 97.7% (absolute risk reduction [ARR]=1.44%; 95% confidence interval [CI], –1.58 to 4.46). There were fewer side effects with the short-term treatment (32.1% vs 48.9%; ARR=16%; 95% CI, 7.84 to 25.84).1
A large, multicenter RCT of 477 children of 6 months to 16 years of age demonstrated that a 5-day treatment with azithromycin eradicated B pertussis from the nasopharynx as effectively as a 10-day course of erythromycin estolate.2 Similarly, trimethoprim/sulfamethoxazole proved as effective as erythromycin in eliminating B pertussis from the nasopharynx.
Although tetracycline and chloramphenicol are effective treatments for pertussis, they are not recommended because of their side effects.1 Six randomized trials failed to show any statistically significant difference between antibiotics and placebo on frequency and severity of cough or duration of pertussis disease.1 A randomized, placebo-controlled trial studied 300 household contacts of children with culture-positive pertussis. There was no statistically significant difference in either the frequency of pertussis disease or rate of positive cultures in household contacts between the erythromycin group (2.1%) and the placebo group (5.1%) (ARR=2.95%; 95% CI, –1.21 to 7.11).1
Another Cochrane review of 8 trials examined the effectiveness of the symptomatic treatment of cough in children and adults with pertussis. There were many problems with the methodological quality of these trials, including small sample sizes and poor reporting of the methods. Diphenhydramine, pertussis immunoglobulin, corticosteroids and salbutamol were compared with placebo. There were no statistically significant differences in coughing paroxysms, mean number of whoops per 24 hours or in duration of hospital stay between these interventions and placebo.3
Extracorporeal circulatory life support has been used to maintain perfusion for patients with severe disease. The mortality of these patients is very high.4 No RCTs of the effectiveness of this intervention has been performed.
TABLE
Antibiotics for treatment and prophylaxis of pertussis in children and adults
| FIRST-LINE THERAPY | DOSAGE FOR CHILDREN | DOSAGE FOR ADULTS | COST* |
|---|---|---|---|
| Erythromycin | 40-50 mg/kg orally or intravenously in 4 divided doses for 14 days5,7 | 1–2 g orally or intravenously in 4 divided doses for 14 days5,7 | 56 tabs (500 mg), $16 (generic) |
| ALTERNATIVE THERAPY IF PATIENT DOESN’T TOLERATE ERYTHROMYCIN | |||
| Clarithromycin | 15-20 mg/per kg orally divided every 12 hours for 10-14 days7or 14-15 mg/kg orally divided every 12 hours for 7 days1,5 | 500 mg orally every 12 hours for 7 days5 | 20 tabs (500 mg), $78 28 tabs (500 mg), $109 (generic) |
| Azithromycin | 10-12 mg/kg orally as single daily dose for 5 to 7 days5,7or 10 mg/kg orally single daily dose for 3 days1 | 500 mg orally once, then 250 daily on days 2-55,7 | 5 tabs (500 mg), $75 7 tabs (500 mg), $105 (no generic) |
| Trimethoprim-sulfamethoxazole | 8 mg of TMP, 40 mg/kg SMX per kg orally divided every 12 hours for 14 days5,7 | 160 mg of TMP, 800 SMX orally (1 tab DS) every 12 hours for 14 days5,7 | 28 tabs $8 (generic) |
| All these therapies have gastrointestinal side effects and risk for hypersensitivity reactions. | |||
| *Approximate retail price for adult dose. Available at: http://www.drugstore.com. Accessed on June 28, 2005. | |||
Recommendations from others
The Centers for Disease Control and Prevention recommends erythromycin for 14 days as a first choice for the treatment and prophylaxis of pertussis. Antibiotics should be started no later than 3 weeks after the onset of cough. Trimethoprim-sulfamethoxazole can be used as an alternative treatment for patients who do not tolerate erythromycin. Prophylaxis is recommended for all household and close contacts if pertussis is highly suspected.5
The American Academy of Pediatrics recommends the use of azithromycin and clarithromycin as an alternative treatment for patients who do not tolerate erythromycin. 5
A national consensus conference on pertussis held in Canada recommended prophylaxis for household contacts of an infant aged <1 year, pregnant women during the third trimester, and for vulnerable individuals who have had face-to-face exposure, or have shared confined air for >1 hour.6
A short-term course of erythromycin, azithromycin, or clarithromycin is as effective as a long-term (2-week) erythromycin therapy in eradicating Bordetella pertussis from the nasopharynx (strength of recommendation [SOR]: A; based on one meta-analysis of randomized controlled trials [RCTs]). Evidence is insufficient to determine the benefit of antibiotic prophylaxis for pertussis contacts. However, due to high mortality and morbidity, prophylaxis is recommended for families who have an infant less than 6 months old (SOR: C; based on expert opinion).
Fewer doses and lower cost make compliance more likely
Marcia Warren, MD
Departments of Family and Community Medicine and Pediatrics, Baylor College of Medicine, Houston, Tex
I found this Clinical Inquiry on the treatment and prophylaxis of Bordetella pertussis invaluable as it addresses ease of dosing and cost, 2 things important in my pediatric community health practice with its inherent financial and social constraints. The alternatives suggested are easy to use and are as equally effective as the first-line therapy of erythromycin estolate, the long-term treatment recommended by the CDC and the AAP. These alternatives, clarithromycin and azithromycin, require either twice a day or a once a day dosing for 7 days or 3 days respectively, can be accommodated in busy households, thus promoting better compliance.
The cost of medication also relates to compliance. The cost difference between the first-line therapy and the alternative therapy is significant, and may be as much as $89. In an underinsured population, this out-of-pocket cost for the alternatives would prove prohibitive, resulting in decreased compliance. Where cost is not a great issue and concerns of compliance important, choosing the short-term treatment may be a preferable option. For the financially strapped, the 1-week regimen of erythromycin estolate would be preferable. The importance of counseling cannot be overstated in all dosing regimens, especially in those with a more difficult dosing schedule and in cases of prophylaxis in a household with an infant less than 6 months old.
Evidence summary
A 2005 Cochrane review of 11 RCTs and 1 quasi-randomized trial, with a total of 1720 adults and children, investigated several antibiotics for treatment and prophylaxis of pertussis. The outcome measures used to assess the efficacy of antibiotic treatment or prophylaxis vary between the trials and most of them did not report the immunization status of the participants. The Cochrane review included 1 meta-analysis of 3 studies with 252 participants, comparing azithromycin for 3 days, erythromycin estolate for 7 days, and clarithromycin for 7 days (short-term treatment) with erythromycin estolate for fourteen days (long-term treatment). The study showed equal efficacy in eradication of B pertussis from the nasopharynx of 99.2% to 97.7% (absolute risk reduction [ARR]=1.44%; 95% confidence interval [CI], –1.58 to 4.46). There were fewer side effects with the short-term treatment (32.1% vs 48.9%; ARR=16%; 95% CI, 7.84 to 25.84).1
A large, multicenter RCT of 477 children of 6 months to 16 years of age demonstrated that a 5-day treatment with azithromycin eradicated B pertussis from the nasopharynx as effectively as a 10-day course of erythromycin estolate.2 Similarly, trimethoprim/sulfamethoxazole proved as effective as erythromycin in eliminating B pertussis from the nasopharynx.
Although tetracycline and chloramphenicol are effective treatments for pertussis, they are not recommended because of their side effects.1 Six randomized trials failed to show any statistically significant difference between antibiotics and placebo on frequency and severity of cough or duration of pertussis disease.1 A randomized, placebo-controlled trial studied 300 household contacts of children with culture-positive pertussis. There was no statistically significant difference in either the frequency of pertussis disease or rate of positive cultures in household contacts between the erythromycin group (2.1%) and the placebo group (5.1%) (ARR=2.95%; 95% CI, –1.21 to 7.11).1
Another Cochrane review of 8 trials examined the effectiveness of the symptomatic treatment of cough in children and adults with pertussis. There were many problems with the methodological quality of these trials, including small sample sizes and poor reporting of the methods. Diphenhydramine, pertussis immunoglobulin, corticosteroids and salbutamol were compared with placebo. There were no statistically significant differences in coughing paroxysms, mean number of whoops per 24 hours or in duration of hospital stay between these interventions and placebo.3
Extracorporeal circulatory life support has been used to maintain perfusion for patients with severe disease. The mortality of these patients is very high.4 No RCTs of the effectiveness of this intervention has been performed.
TABLE
Antibiotics for treatment and prophylaxis of pertussis in children and adults
| FIRST-LINE THERAPY | DOSAGE FOR CHILDREN | DOSAGE FOR ADULTS | COST* |
|---|---|---|---|
| Erythromycin | 40-50 mg/kg orally or intravenously in 4 divided doses for 14 days5,7 | 1–2 g orally or intravenously in 4 divided doses for 14 days5,7 | 56 tabs (500 mg), $16 (generic) |
| ALTERNATIVE THERAPY IF PATIENT DOESN’T TOLERATE ERYTHROMYCIN | |||
| Clarithromycin | 15-20 mg/per kg orally divided every 12 hours for 10-14 days7or 14-15 mg/kg orally divided every 12 hours for 7 days1,5 | 500 mg orally every 12 hours for 7 days5 | 20 tabs (500 mg), $78 28 tabs (500 mg), $109 (generic) |
| Azithromycin | 10-12 mg/kg orally as single daily dose for 5 to 7 days5,7or 10 mg/kg orally single daily dose for 3 days1 | 500 mg orally once, then 250 daily on days 2-55,7 | 5 tabs (500 mg), $75 7 tabs (500 mg), $105 (no generic) |
| Trimethoprim-sulfamethoxazole | 8 mg of TMP, 40 mg/kg SMX per kg orally divided every 12 hours for 14 days5,7 | 160 mg of TMP, 800 SMX orally (1 tab DS) every 12 hours for 14 days5,7 | 28 tabs $8 (generic) |
| All these therapies have gastrointestinal side effects and risk for hypersensitivity reactions. | |||
| *Approximate retail price for adult dose. Available at: http://www.drugstore.com. Accessed on June 28, 2005. | |||
Recommendations from others
The Centers for Disease Control and Prevention recommends erythromycin for 14 days as a first choice for the treatment and prophylaxis of pertussis. Antibiotics should be started no later than 3 weeks after the onset of cough. Trimethoprim-sulfamethoxazole can be used as an alternative treatment for patients who do not tolerate erythromycin. Prophylaxis is recommended for all household and close contacts if pertussis is highly suspected.5
The American Academy of Pediatrics recommends the use of azithromycin and clarithromycin as an alternative treatment for patients who do not tolerate erythromycin. 5
A national consensus conference on pertussis held in Canada recommended prophylaxis for household contacts of an infant aged <1 year, pregnant women during the third trimester, and for vulnerable individuals who have had face-to-face exposure, or have shared confined air for >1 hour.6
1. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis) (Cochrane Review). Cochrane Database Syst Rev 2005;(1):CD004404.
2. Langley JM, Halperin SA, Boucher FD, Smith B. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics 2004;114:e96-101.
3. Pillay V, Swingler G. Symptomatic treatment of the cough in whooping cough (Cochrane Review). Cochrane Database Syst Rev 2003;(4):CD003257.
4. Williams GD, Numa A, Sokol J, Tobias V, Duffy BJ. ECLS in pertussis: does it have a role? Intensive Care Med 1998;24:1089-1092.
5. Guris D. Treatment and Chemoprophylaxis. Guidelines for the Control of Pertussis Outbreaks. Atlanta, Ga: Centers for Disease Control and Prevention, 2000. Last updated January 2005. Available at: www.cdc.gov/nip/publications/pertussis/guide.htm. Accessed on November 14, 2005.
6. National consensus conference on pertussis Can Commun Dis Rep 2003;29(Suppl 3):S1-S33 (English), S1-S36 (French). Last updated June 5, 2003. Available at: www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/29s3/index.html. Accessed on November 14, 2005.
7. Pertussis Information for Physicians: Diagnostic and Treatment Criteria Texas Department of Health Pertussis Treatment Guidelines. TDH Immunization Division; 2004;Last updated January 26, 2004. Available at: www.tdh.state.tx.us/immunize/html/pert_physician_txt.htm. Accessed on November 14, 2005.
1. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis) (Cochrane Review). Cochrane Database Syst Rev 2005;(1):CD004404.
2. Langley JM, Halperin SA, Boucher FD, Smith B. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics 2004;114:e96-101.
3. Pillay V, Swingler G. Symptomatic treatment of the cough in whooping cough (Cochrane Review). Cochrane Database Syst Rev 2003;(4):CD003257.
4. Williams GD, Numa A, Sokol J, Tobias V, Duffy BJ. ECLS in pertussis: does it have a role? Intensive Care Med 1998;24:1089-1092.
5. Guris D. Treatment and Chemoprophylaxis. Guidelines for the Control of Pertussis Outbreaks. Atlanta, Ga: Centers for Disease Control and Prevention, 2000. Last updated January 2005. Available at: www.cdc.gov/nip/publications/pertussis/guide.htm. Accessed on November 14, 2005.
6. National consensus conference on pertussis Can Commun Dis Rep 2003;29(Suppl 3):S1-S33 (English), S1-S36 (French). Last updated June 5, 2003. Available at: www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/29s3/index.html. Accessed on November 14, 2005.
7. Pertussis Information for Physicians: Diagnostic and Treatment Criteria Texas Department of Health Pertussis Treatment Guidelines. TDH Immunization Division; 2004;Last updated January 26, 2004. Available at: www.tdh.state.tx.us/immunize/html/pert_physician_txt.htm. Accessed on November 14, 2005.
Evidence-based answers from the Family Physicians Inquiries Network
Do beta-blockers worsen respiratory status for patients with COPD?
Patients with chronic obstructive pulmonary disease (COPD) who use cardioselective beta-blockers (beta1-blockers) do not experience a significant worsening of their short-term pulmonary status as measured by changes in forced expiratory volume in 1 second (FEV1), or by changes in patients’ self-reported symptoms. If such harmful effects do exist, they are likely to be less clinically important than the substantial proven benefits of beta-blockade for patients with concomitant cardiovascular disease (strength of recommendation: A, based on a high-quality meta-analysis of controlled trials).
Limited evidence suggests that most patients with congestive heart failure and COPD without reversible airflow obstruction tolerate carvedilol, which causes both nonselective beta- and alpha-adrenergic blockade (SOR: B, based on limited-quality cohort studies).
Evidence summary
In recent years, beta-blockers have been shown to substantially decrease mortality in patients with congestive heart failure, coronary heart disease, and hypertension. Patients with both cardiovascular disease and COPD, however, are much less likely to receive beta-blocker therapy than comparable patients without COPD. Clinicians may be fearful of using beta-blockers in these patients because of the possibility of worsening respiratory function from the potential side effect of bronchoconstriction.1
A 2004 meta-analysis synthesized the data of 19 clinical controlled trials that compared active therapy with either placebo or prior-to-treatment controls, assessing differences in FEV1, response to a beta2-agonist, and patient-reported respiratory symptoms.2 Trials included in the meta-analysis used cardioselective beta-blockers and evaluated either single-dose treatments or therapy of longer duration (2 days to 3.3 months). The authors concluded that patients with COPD who received cardioselective beta-blockers (such as metoprolol, atenolol, or bisoprolol) did not experience a statistically significant short-term deterioration in FEV1, worsening of COPD symptoms, or decreased responsiveness to beta2-agonists. The authors reported similar results for an analysis restricted to only those patients with severe COPD.
This meta-analysis was limited by the relatively small number of participants (N=141 in single-dose treatment studies; N=126 in studies of longer duration treatment) in the handful of eligible studies. Consequently, rare or minimally harmful effects could have gone undetected.
A retrospective analysis of a cohort study analyzed the tolerability of carvedilol, a nonselective beta- and alpha-adrenergic blocker, in patients with COPD who had been taking the medication for at least 3 months. Eighty-five percent of the 89 patients with COPD tolerated carvedilol. The authors of the study (which was funded by the manufacturer of carvedilol) did not state why the other 15% of patients did not tolerate carvedilol, nor did they mention whether the patients with COPD had reversible airflow obstruction.3
One of the sites that participated in this study subsequently published a smaller retrospective analysis of a cohort study that examined the outcomes of 31 patients with heart failure and COPD without reversible airflow obstruction who were started on carvedilol therapy. Over the 2.4 years that the patients were followed, 1 patient stopped taking carvedilol (mean dose 29 ± 19 mg daily) due to wheezing.4 Whether these 31 patients were also included in the larger study is unclear.
A 2004 narrative review article cited these 2 studies and concluded that carvedilol was well-tolerated in patients with COPD without reversible airflow obstruction, but no evidence exists regarding its tolerability in patients with reversible airflow obstruction.5
Recommendations from others
A 2002 evidence-based clinical guideline on the diagnosis and management of COPD reported that the use of cardioselective beta-blockers in patients with COPD did not significantly worsen respiratory status, citing a previous version of the meta-analysis reviewed above as its source of evidence.6 The American College of Cardiology and the American Heart Association recommended the cautious administration of low-dose, short-acting cardioselective beta-blockers for acute coronary syndrome in patients with COPD.7
A recent consensus workshop summary report issued by experts convened by the National Heart, Lung, and Blood Institute, cited continuing uncertainty regarding the use of beta-blockers for COPD patients with heart disease, and called for additional studies of management strategies for these often-coexisting conditions.8
Benefits outweigh risks for beta-blockade for patients with CV disease, comorbid COPD
It appears that the benefits outweigh the risks for the use of cardioselective beta-blocker therapy in patients with cardiovascular disease and comorbid COPD. Prudent management of these patients dictates that beta-blocker therapy should be initiated with a low-dose cardioselective beta-blocker, that the respiratory status of these patients should be monitored closely, and that any otherwise unexplained decline in respiratory status should warrant a reevaluation of the appropriateness of beta-blocker therapy.
1. Andrus MR, Holloway KP, Clark DB. Use of beta-blockers in patients with COPD. Ann Pharmacother 2004;38:142-145.
2. Salpeter SS, Ormiston T, Salpeter E, Poole P, Cates C. Cardioselective beta-blockers for chronic obstructive pulmonary disease (Cochrane Review). Cochrane Database Syst Rev2005 (1).
3. Krum H, Ninio D, Macdonald P. Baseline predictors of tolerability to carvedilol in patients with chronic heart failure. Heart 2000;84:615-619
4. Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002;21:1290-1295.
5. Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockage in patients with coexisting chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004;44:497-502.
6. Finnish Medical Society Duodecim. Chronic Obstructive Pulmonary Disease (COPD). Helsinki, Finland: Duodecim Medical; 2002.
7. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). 2002. Available at: www.acc.org/clinical/guidelines/unstable/unstable.pdf.
8. Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med 2003;167:1142-1149.
Patients with chronic obstructive pulmonary disease (COPD) who use cardioselective beta-blockers (beta1-blockers) do not experience a significant worsening of their short-term pulmonary status as measured by changes in forced expiratory volume in 1 second (FEV1), or by changes in patients’ self-reported symptoms. If such harmful effects do exist, they are likely to be less clinically important than the substantial proven benefits of beta-blockade for patients with concomitant cardiovascular disease (strength of recommendation: A, based on a high-quality meta-analysis of controlled trials).
Limited evidence suggests that most patients with congestive heart failure and COPD without reversible airflow obstruction tolerate carvedilol, which causes both nonselective beta- and alpha-adrenergic blockade (SOR: B, based on limited-quality cohort studies).
Evidence summary
In recent years, beta-blockers have been shown to substantially decrease mortality in patients with congestive heart failure, coronary heart disease, and hypertension. Patients with both cardiovascular disease and COPD, however, are much less likely to receive beta-blocker therapy than comparable patients without COPD. Clinicians may be fearful of using beta-blockers in these patients because of the possibility of worsening respiratory function from the potential side effect of bronchoconstriction.1
A 2004 meta-analysis synthesized the data of 19 clinical controlled trials that compared active therapy with either placebo or prior-to-treatment controls, assessing differences in FEV1, response to a beta2-agonist, and patient-reported respiratory symptoms.2 Trials included in the meta-analysis used cardioselective beta-blockers and evaluated either single-dose treatments or therapy of longer duration (2 days to 3.3 months). The authors concluded that patients with COPD who received cardioselective beta-blockers (such as metoprolol, atenolol, or bisoprolol) did not experience a statistically significant short-term deterioration in FEV1, worsening of COPD symptoms, or decreased responsiveness to beta2-agonists. The authors reported similar results for an analysis restricted to only those patients with severe COPD.
This meta-analysis was limited by the relatively small number of participants (N=141 in single-dose treatment studies; N=126 in studies of longer duration treatment) in the handful of eligible studies. Consequently, rare or minimally harmful effects could have gone undetected.
A retrospective analysis of a cohort study analyzed the tolerability of carvedilol, a nonselective beta- and alpha-adrenergic blocker, in patients with COPD who had been taking the medication for at least 3 months. Eighty-five percent of the 89 patients with COPD tolerated carvedilol. The authors of the study (which was funded by the manufacturer of carvedilol) did not state why the other 15% of patients did not tolerate carvedilol, nor did they mention whether the patients with COPD had reversible airflow obstruction.3
One of the sites that participated in this study subsequently published a smaller retrospective analysis of a cohort study that examined the outcomes of 31 patients with heart failure and COPD without reversible airflow obstruction who were started on carvedilol therapy. Over the 2.4 years that the patients were followed, 1 patient stopped taking carvedilol (mean dose 29 ± 19 mg daily) due to wheezing.4 Whether these 31 patients were also included in the larger study is unclear.
A 2004 narrative review article cited these 2 studies and concluded that carvedilol was well-tolerated in patients with COPD without reversible airflow obstruction, but no evidence exists regarding its tolerability in patients with reversible airflow obstruction.5
Recommendations from others
A 2002 evidence-based clinical guideline on the diagnosis and management of COPD reported that the use of cardioselective beta-blockers in patients with COPD did not significantly worsen respiratory status, citing a previous version of the meta-analysis reviewed above as its source of evidence.6 The American College of Cardiology and the American Heart Association recommended the cautious administration of low-dose, short-acting cardioselective beta-blockers for acute coronary syndrome in patients with COPD.7
A recent consensus workshop summary report issued by experts convened by the National Heart, Lung, and Blood Institute, cited continuing uncertainty regarding the use of beta-blockers for COPD patients with heart disease, and called for additional studies of management strategies for these often-coexisting conditions.8
Benefits outweigh risks for beta-blockade for patients with CV disease, comorbid COPD
It appears that the benefits outweigh the risks for the use of cardioselective beta-blocker therapy in patients with cardiovascular disease and comorbid COPD. Prudent management of these patients dictates that beta-blocker therapy should be initiated with a low-dose cardioselective beta-blocker, that the respiratory status of these patients should be monitored closely, and that any otherwise unexplained decline in respiratory status should warrant a reevaluation of the appropriateness of beta-blocker therapy.
Patients with chronic obstructive pulmonary disease (COPD) who use cardioselective beta-blockers (beta1-blockers) do not experience a significant worsening of their short-term pulmonary status as measured by changes in forced expiratory volume in 1 second (FEV1), or by changes in patients’ self-reported symptoms. If such harmful effects do exist, they are likely to be less clinically important than the substantial proven benefits of beta-blockade for patients with concomitant cardiovascular disease (strength of recommendation: A, based on a high-quality meta-analysis of controlled trials).
Limited evidence suggests that most patients with congestive heart failure and COPD without reversible airflow obstruction tolerate carvedilol, which causes both nonselective beta- and alpha-adrenergic blockade (SOR: B, based on limited-quality cohort studies).
Evidence summary
In recent years, beta-blockers have been shown to substantially decrease mortality in patients with congestive heart failure, coronary heart disease, and hypertension. Patients with both cardiovascular disease and COPD, however, are much less likely to receive beta-blocker therapy than comparable patients without COPD. Clinicians may be fearful of using beta-blockers in these patients because of the possibility of worsening respiratory function from the potential side effect of bronchoconstriction.1
A 2004 meta-analysis synthesized the data of 19 clinical controlled trials that compared active therapy with either placebo or prior-to-treatment controls, assessing differences in FEV1, response to a beta2-agonist, and patient-reported respiratory symptoms.2 Trials included in the meta-analysis used cardioselective beta-blockers and evaluated either single-dose treatments or therapy of longer duration (2 days to 3.3 months). The authors concluded that patients with COPD who received cardioselective beta-blockers (such as metoprolol, atenolol, or bisoprolol) did not experience a statistically significant short-term deterioration in FEV1, worsening of COPD symptoms, or decreased responsiveness to beta2-agonists. The authors reported similar results for an analysis restricted to only those patients with severe COPD.
This meta-analysis was limited by the relatively small number of participants (N=141 in single-dose treatment studies; N=126 in studies of longer duration treatment) in the handful of eligible studies. Consequently, rare or minimally harmful effects could have gone undetected.
A retrospective analysis of a cohort study analyzed the tolerability of carvedilol, a nonselective beta- and alpha-adrenergic blocker, in patients with COPD who had been taking the medication for at least 3 months. Eighty-five percent of the 89 patients with COPD tolerated carvedilol. The authors of the study (which was funded by the manufacturer of carvedilol) did not state why the other 15% of patients did not tolerate carvedilol, nor did they mention whether the patients with COPD had reversible airflow obstruction.3
One of the sites that participated in this study subsequently published a smaller retrospective analysis of a cohort study that examined the outcomes of 31 patients with heart failure and COPD without reversible airflow obstruction who were started on carvedilol therapy. Over the 2.4 years that the patients were followed, 1 patient stopped taking carvedilol (mean dose 29 ± 19 mg daily) due to wheezing.4 Whether these 31 patients were also included in the larger study is unclear.
A 2004 narrative review article cited these 2 studies and concluded that carvedilol was well-tolerated in patients with COPD without reversible airflow obstruction, but no evidence exists regarding its tolerability in patients with reversible airflow obstruction.5
Recommendations from others
A 2002 evidence-based clinical guideline on the diagnosis and management of COPD reported that the use of cardioselective beta-blockers in patients with COPD did not significantly worsen respiratory status, citing a previous version of the meta-analysis reviewed above as its source of evidence.6 The American College of Cardiology and the American Heart Association recommended the cautious administration of low-dose, short-acting cardioselective beta-blockers for acute coronary syndrome in patients with COPD.7
A recent consensus workshop summary report issued by experts convened by the National Heart, Lung, and Blood Institute, cited continuing uncertainty regarding the use of beta-blockers for COPD patients with heart disease, and called for additional studies of management strategies for these often-coexisting conditions.8
Benefits outweigh risks for beta-blockade for patients with CV disease, comorbid COPD
It appears that the benefits outweigh the risks for the use of cardioselective beta-blocker therapy in patients with cardiovascular disease and comorbid COPD. Prudent management of these patients dictates that beta-blocker therapy should be initiated with a low-dose cardioselective beta-blocker, that the respiratory status of these patients should be monitored closely, and that any otherwise unexplained decline in respiratory status should warrant a reevaluation of the appropriateness of beta-blocker therapy.
1. Andrus MR, Holloway KP, Clark DB. Use of beta-blockers in patients with COPD. Ann Pharmacother 2004;38:142-145.
2. Salpeter SS, Ormiston T, Salpeter E, Poole P, Cates C. Cardioselective beta-blockers for chronic obstructive pulmonary disease (Cochrane Review). Cochrane Database Syst Rev2005 (1).
3. Krum H, Ninio D, Macdonald P. Baseline predictors of tolerability to carvedilol in patients with chronic heart failure. Heart 2000;84:615-619
4. Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002;21:1290-1295.
5. Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockage in patients with coexisting chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004;44:497-502.
6. Finnish Medical Society Duodecim. Chronic Obstructive Pulmonary Disease (COPD). Helsinki, Finland: Duodecim Medical; 2002.
7. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). 2002. Available at: www.acc.org/clinical/guidelines/unstable/unstable.pdf.
8. Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med 2003;167:1142-1149.
1. Andrus MR, Holloway KP, Clark DB. Use of beta-blockers in patients with COPD. Ann Pharmacother 2004;38:142-145.
2. Salpeter SS, Ormiston T, Salpeter E, Poole P, Cates C. Cardioselective beta-blockers for chronic obstructive pulmonary disease (Cochrane Review). Cochrane Database Syst Rev2005 (1).
3. Krum H, Ninio D, Macdonald P. Baseline predictors of tolerability to carvedilol in patients with chronic heart failure. Heart 2000;84:615-619
4. Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002;21:1290-1295.
5. Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockage in patients with coexisting chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004;44:497-502.
6. Finnish Medical Society Duodecim. Chronic Obstructive Pulmonary Disease (COPD). Helsinki, Finland: Duodecim Medical; 2002.
7. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). 2002. Available at: www.acc.org/clinical/guidelines/unstable/unstable.pdf.
8. Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med 2003;167:1142-1149.
Evidence-based answers from the Family Physicians Inquiries Network
Is nedocromil effective in preventing asthmatic attacks in patients with asthma?
Nedocromil (Tilade) is effective for the treatment of mild persistent asthma. It has not been shown to be effective in more severe forms of asthma for both children and adults. Although no studies looked specifically at exacerbation rates, multiple clinical and biologic outcomes (symptom scores, quality of life measures, bronchodilator use, forced expiratory flow in 1 second [FEV1], and peak expiratory flow rate [PEFR]) improved with nedocromil use compared with placebo.
The most effective dose for preventing exacerbations appears to be 4 mg (2 puffs) 4 times a day (SOR: A, multiple randomized controlled trials [RCTs] and meta-analyses). More severe forms of asthma respond better to inhaled steroids than to nedocromil (SOR: A, multiple RCTs). Nedocromil may allow some patients with severe asthma to use lower doses of inhaled steroids (SOR: C, conflicting RCTs). Nedocromil is also effective for the treatment of exercise-induced asthma (SOR: A, multiple RCTs and meta-analyses).
In general, about 50% to 70% of patients respond to nedocromil (SOR: A, multiple RCTs and meta-analyses). Unfortunately, which patients respond is not predictable from clinical parameters.1 Nedocromil is worth trying in mild persistent asthma, particularly for children where the parents are worried about the growth issues associated with inhaled steroids. Side effects (sore throat, nausea, and headache) are mild and infrequent. Maximal efficacy is usually seen after 6 to 8 weeks.
Evidence summary
A systematic review encompassing 127 trial centers and 4723 patients concluded that inhaled nedocromil was effective for a variety of patients with asthma. Significant improvements were noted in FEV1, PEFR, use of bronchodilators, symptom scores, and quality of life scores. The reviewers found nedocromil to be most effective for patients with moderate disease already taking bronchodilators,2 corresponding to the “mild persistent asthma” category ( Table ).
A contemporaneous European RCT, not included in the review, compared 4 mg of inhaled nedocromil 4 times daily with inhaled placebo among 209 asthmatic children for 12 weeks.3 After 8 weeks, they found a statistically significant reduction in total daily asthma symptom scores (50% nedocromil vs 9% placebo; P<.01). The proportion of parents and children rating treatment as moderately or very effective was 78% in the treatment group and 59% in the placebo group (number needed to treat [NNT]=5.2; P<.01); clinicians’ ratings were 73% for nedocromil and 50% for placebo (NNT=4.3; P<.01). The frequency of side effects—including nausea, headache, and sleepiness—did not reach statistical significance; however, the nedocromil group reported up to a 20% incidence of sore throat. Most of the studies reported no dropouts due to side effects.
When patients are already using inhaled steroids, the evidence is less clear whether nedocromil confers additional benefits, such as fewer exacerbations or lower inhaled steroid doses. Two small studies of patients either already on inhaled steroids4 or considered to be steroid-resistant5 found nonsignificant trends towards reductions in bronchodilator use, increased PEFR, increased FEV1, and improved quality of life. Although both studies were underpowered, the study on steroid-resistant asthma did find a statistically significant 20% improvement in PEFR and decreased bronchodilator use for 50% of patients at 8 and 12 weeks.
The inherent waxing and waning nature of asthma makes demonstrating benefits difficult. Furthermore, nedocromil tends to have an all-ornothing effect rather than a dose-response gradient. Unfortunately, none of these trials found useful predictors to help clinicians determine which patients respond.1,5
In a Cochrane Review, 20 RCTs involving 280 participants showed that 4 mg (2 puffs) of nedocromil inhaled 15 to 60 minutes prior to exercise significantly reduced the severity and duration of exercise-induced asthma for both adults and children. The maximum percentage fall in FEV1 improved significantly compared with placebo, with a weighted mean difference of 15.5% (95% confidence interval, 13.2–18.1). In addition, the time to complete recovery was shortened from 30 minutes with placebo to 10 minutes with nedocromil.6
TABLE
Classification of asthma
| Classification | Symptom frequency | Spirometry findings |
|---|---|---|
| Severe persistent | Continual symptoms | PEFR <60% Variability >30% |
| Moderate persistent | Daily symptoms, more than 1 night per week | PEFR >60% but <80% Variability >30% |
| Mild persistent | More than twice per week but less than daily; more than 2 nights per month | PEFR >80% Variability 20%–30% |
| Mild intermittent | Less than once per week; less than or equal to 2 nights per month | PEFR >80% Variability <20% |
| Source: Global Initiative for Asthma, National Heart, Lung and Blood Institute 2003.7 | ||
Recommendations from others
The Global Initiative for Asthma and the National Heart, Lung and Blood Institute Expert Panel Report list nedocromil as an option for the treatment of exercise-induced asthma and mild persistent asthma for adults and children. However, it is listed as a second choice to the use of inhaled steroids in the case of mild persistent asthma. It is not recommended for moderate or severe persistent asthma, or for mild intermittent asthma.7
Nedocromil and cromolyn sodium are safe but many patients do not respond
Ron Baldwin, MD
University of Wyoming Family Practice Residency at Casper
Inhaled nedocromil and cromolyn sodium have long been recognized as agents with an excellent safety profile. Unfortunately, as pointed about above, many patients do not respond to these agents. In addition, 4-times-daily dosing makes compliance difficult. Clinicians and parents must weigh the theoretical risk of inhaled corticosteroid-induced growth retardation with this potential differential in effectiveness.
1. Parish RC, Miller LJ. Nedocromil sodium. Ann Pharmacother 1993;27:599-606.
2. Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. Eur Respir J 1993;6:35-41.
3. Armenio L, Baldini G, Baldare M, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Arch Dis Child 1993;68:193-197.
4. O’Hickey SP, Rees PJ. High dose nedocromil sodium as an addition to inhaled corticosteroids in the treatment of asthma. Respir Med 1994;88:499-502.
5. Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid-resistant asthma: a randomized controlled trial. J Allergy Clin Immunol 1996;97:602-610.
6. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction (Cochrane Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
7. Global Strategy for Asthma Management and Prevention. Bethesda, Md: Global Initiative for Asthma, National Heart, Lung and Blood Institute; 2003.
Nedocromil (Tilade) is effective for the treatment of mild persistent asthma. It has not been shown to be effective in more severe forms of asthma for both children and adults. Although no studies looked specifically at exacerbation rates, multiple clinical and biologic outcomes (symptom scores, quality of life measures, bronchodilator use, forced expiratory flow in 1 second [FEV1], and peak expiratory flow rate [PEFR]) improved with nedocromil use compared with placebo.
The most effective dose for preventing exacerbations appears to be 4 mg (2 puffs) 4 times a day (SOR: A, multiple randomized controlled trials [RCTs] and meta-analyses). More severe forms of asthma respond better to inhaled steroids than to nedocromil (SOR: A, multiple RCTs). Nedocromil may allow some patients with severe asthma to use lower doses of inhaled steroids (SOR: C, conflicting RCTs). Nedocromil is also effective for the treatment of exercise-induced asthma (SOR: A, multiple RCTs and meta-analyses).
In general, about 50% to 70% of patients respond to nedocromil (SOR: A, multiple RCTs and meta-analyses). Unfortunately, which patients respond is not predictable from clinical parameters.1 Nedocromil is worth trying in mild persistent asthma, particularly for children where the parents are worried about the growth issues associated with inhaled steroids. Side effects (sore throat, nausea, and headache) are mild and infrequent. Maximal efficacy is usually seen after 6 to 8 weeks.
Evidence summary
A systematic review encompassing 127 trial centers and 4723 patients concluded that inhaled nedocromil was effective for a variety of patients with asthma. Significant improvements were noted in FEV1, PEFR, use of bronchodilators, symptom scores, and quality of life scores. The reviewers found nedocromil to be most effective for patients with moderate disease already taking bronchodilators,2 corresponding to the “mild persistent asthma” category ( Table ).
A contemporaneous European RCT, not included in the review, compared 4 mg of inhaled nedocromil 4 times daily with inhaled placebo among 209 asthmatic children for 12 weeks.3 After 8 weeks, they found a statistically significant reduction in total daily asthma symptom scores (50% nedocromil vs 9% placebo; P<.01). The proportion of parents and children rating treatment as moderately or very effective was 78% in the treatment group and 59% in the placebo group (number needed to treat [NNT]=5.2; P<.01); clinicians’ ratings were 73% for nedocromil and 50% for placebo (NNT=4.3; P<.01). The frequency of side effects—including nausea, headache, and sleepiness—did not reach statistical significance; however, the nedocromil group reported up to a 20% incidence of sore throat. Most of the studies reported no dropouts due to side effects.
When patients are already using inhaled steroids, the evidence is less clear whether nedocromil confers additional benefits, such as fewer exacerbations or lower inhaled steroid doses. Two small studies of patients either already on inhaled steroids4 or considered to be steroid-resistant5 found nonsignificant trends towards reductions in bronchodilator use, increased PEFR, increased FEV1, and improved quality of life. Although both studies were underpowered, the study on steroid-resistant asthma did find a statistically significant 20% improvement in PEFR and decreased bronchodilator use for 50% of patients at 8 and 12 weeks.
The inherent waxing and waning nature of asthma makes demonstrating benefits difficult. Furthermore, nedocromil tends to have an all-ornothing effect rather than a dose-response gradient. Unfortunately, none of these trials found useful predictors to help clinicians determine which patients respond.1,5
In a Cochrane Review, 20 RCTs involving 280 participants showed that 4 mg (2 puffs) of nedocromil inhaled 15 to 60 minutes prior to exercise significantly reduced the severity and duration of exercise-induced asthma for both adults and children. The maximum percentage fall in FEV1 improved significantly compared with placebo, with a weighted mean difference of 15.5% (95% confidence interval, 13.2–18.1). In addition, the time to complete recovery was shortened from 30 minutes with placebo to 10 minutes with nedocromil.6
TABLE
Classification of asthma
| Classification | Symptom frequency | Spirometry findings |
|---|---|---|
| Severe persistent | Continual symptoms | PEFR <60% Variability >30% |
| Moderate persistent | Daily symptoms, more than 1 night per week | PEFR >60% but <80% Variability >30% |
| Mild persistent | More than twice per week but less than daily; more than 2 nights per month | PEFR >80% Variability 20%–30% |
| Mild intermittent | Less than once per week; less than or equal to 2 nights per month | PEFR >80% Variability <20% |
| Source: Global Initiative for Asthma, National Heart, Lung and Blood Institute 2003.7 | ||
Recommendations from others
The Global Initiative for Asthma and the National Heart, Lung and Blood Institute Expert Panel Report list nedocromil as an option for the treatment of exercise-induced asthma and mild persistent asthma for adults and children. However, it is listed as a second choice to the use of inhaled steroids in the case of mild persistent asthma. It is not recommended for moderate or severe persistent asthma, or for mild intermittent asthma.7
Nedocromil and cromolyn sodium are safe but many patients do not respond
Ron Baldwin, MD
University of Wyoming Family Practice Residency at Casper
Inhaled nedocromil and cromolyn sodium have long been recognized as agents with an excellent safety profile. Unfortunately, as pointed about above, many patients do not respond to these agents. In addition, 4-times-daily dosing makes compliance difficult. Clinicians and parents must weigh the theoretical risk of inhaled corticosteroid-induced growth retardation with this potential differential in effectiveness.
Nedocromil (Tilade) is effective for the treatment of mild persistent asthma. It has not been shown to be effective in more severe forms of asthma for both children and adults. Although no studies looked specifically at exacerbation rates, multiple clinical and biologic outcomes (symptom scores, quality of life measures, bronchodilator use, forced expiratory flow in 1 second [FEV1], and peak expiratory flow rate [PEFR]) improved with nedocromil use compared with placebo.
The most effective dose for preventing exacerbations appears to be 4 mg (2 puffs) 4 times a day (SOR: A, multiple randomized controlled trials [RCTs] and meta-analyses). More severe forms of asthma respond better to inhaled steroids than to nedocromil (SOR: A, multiple RCTs). Nedocromil may allow some patients with severe asthma to use lower doses of inhaled steroids (SOR: C, conflicting RCTs). Nedocromil is also effective for the treatment of exercise-induced asthma (SOR: A, multiple RCTs and meta-analyses).
In general, about 50% to 70% of patients respond to nedocromil (SOR: A, multiple RCTs and meta-analyses). Unfortunately, which patients respond is not predictable from clinical parameters.1 Nedocromil is worth trying in mild persistent asthma, particularly for children where the parents are worried about the growth issues associated with inhaled steroids. Side effects (sore throat, nausea, and headache) are mild and infrequent. Maximal efficacy is usually seen after 6 to 8 weeks.
Evidence summary
A systematic review encompassing 127 trial centers and 4723 patients concluded that inhaled nedocromil was effective for a variety of patients with asthma. Significant improvements were noted in FEV1, PEFR, use of bronchodilators, symptom scores, and quality of life scores. The reviewers found nedocromil to be most effective for patients with moderate disease already taking bronchodilators,2 corresponding to the “mild persistent asthma” category ( Table ).
A contemporaneous European RCT, not included in the review, compared 4 mg of inhaled nedocromil 4 times daily with inhaled placebo among 209 asthmatic children for 12 weeks.3 After 8 weeks, they found a statistically significant reduction in total daily asthma symptom scores (50% nedocromil vs 9% placebo; P<.01). The proportion of parents and children rating treatment as moderately or very effective was 78% in the treatment group and 59% in the placebo group (number needed to treat [NNT]=5.2; P<.01); clinicians’ ratings were 73% for nedocromil and 50% for placebo (NNT=4.3; P<.01). The frequency of side effects—including nausea, headache, and sleepiness—did not reach statistical significance; however, the nedocromil group reported up to a 20% incidence of sore throat. Most of the studies reported no dropouts due to side effects.
When patients are already using inhaled steroids, the evidence is less clear whether nedocromil confers additional benefits, such as fewer exacerbations or lower inhaled steroid doses. Two small studies of patients either already on inhaled steroids4 or considered to be steroid-resistant5 found nonsignificant trends towards reductions in bronchodilator use, increased PEFR, increased FEV1, and improved quality of life. Although both studies were underpowered, the study on steroid-resistant asthma did find a statistically significant 20% improvement in PEFR and decreased bronchodilator use for 50% of patients at 8 and 12 weeks.
The inherent waxing and waning nature of asthma makes demonstrating benefits difficult. Furthermore, nedocromil tends to have an all-ornothing effect rather than a dose-response gradient. Unfortunately, none of these trials found useful predictors to help clinicians determine which patients respond.1,5
In a Cochrane Review, 20 RCTs involving 280 participants showed that 4 mg (2 puffs) of nedocromil inhaled 15 to 60 minutes prior to exercise significantly reduced the severity and duration of exercise-induced asthma for both adults and children. The maximum percentage fall in FEV1 improved significantly compared with placebo, with a weighted mean difference of 15.5% (95% confidence interval, 13.2–18.1). In addition, the time to complete recovery was shortened from 30 minutes with placebo to 10 minutes with nedocromil.6
TABLE
Classification of asthma
| Classification | Symptom frequency | Spirometry findings |
|---|---|---|
| Severe persistent | Continual symptoms | PEFR <60% Variability >30% |
| Moderate persistent | Daily symptoms, more than 1 night per week | PEFR >60% but <80% Variability >30% |
| Mild persistent | More than twice per week but less than daily; more than 2 nights per month | PEFR >80% Variability 20%–30% |
| Mild intermittent | Less than once per week; less than or equal to 2 nights per month | PEFR >80% Variability <20% |
| Source: Global Initiative for Asthma, National Heart, Lung and Blood Institute 2003.7 | ||
Recommendations from others
The Global Initiative for Asthma and the National Heart, Lung and Blood Institute Expert Panel Report list nedocromil as an option for the treatment of exercise-induced asthma and mild persistent asthma for adults and children. However, it is listed as a second choice to the use of inhaled steroids in the case of mild persistent asthma. It is not recommended for moderate or severe persistent asthma, or for mild intermittent asthma.7
Nedocromil and cromolyn sodium are safe but many patients do not respond
Ron Baldwin, MD
University of Wyoming Family Practice Residency at Casper
Inhaled nedocromil and cromolyn sodium have long been recognized as agents with an excellent safety profile. Unfortunately, as pointed about above, many patients do not respond to these agents. In addition, 4-times-daily dosing makes compliance difficult. Clinicians and parents must weigh the theoretical risk of inhaled corticosteroid-induced growth retardation with this potential differential in effectiveness.
1. Parish RC, Miller LJ. Nedocromil sodium. Ann Pharmacother 1993;27:599-606.
2. Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. Eur Respir J 1993;6:35-41.
3. Armenio L, Baldini G, Baldare M, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Arch Dis Child 1993;68:193-197.
4. O’Hickey SP, Rees PJ. High dose nedocromil sodium as an addition to inhaled corticosteroids in the treatment of asthma. Respir Med 1994;88:499-502.
5. Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid-resistant asthma: a randomized controlled trial. J Allergy Clin Immunol 1996;97:602-610.
6. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction (Cochrane Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
7. Global Strategy for Asthma Management and Prevention. Bethesda, Md: Global Initiative for Asthma, National Heart, Lung and Blood Institute; 2003.
1. Parish RC, Miller LJ. Nedocromil sodium. Ann Pharmacother 1993;27:599-606.
2. Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. Eur Respir J 1993;6:35-41.
3. Armenio L, Baldini G, Baldare M, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Arch Dis Child 1993;68:193-197.
4. O’Hickey SP, Rees PJ. High dose nedocromil sodium as an addition to inhaled corticosteroids in the treatment of asthma. Respir Med 1994;88:499-502.
5. Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid-resistant asthma: a randomized controlled trial. J Allergy Clin Immunol 1996;97:602-610.
6. Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise-induced bronchoconstriction (Cochrane Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
7. Global Strategy for Asthma Management and Prevention. Bethesda, Md: Global Initiative for Asthma, National Heart, Lung and Blood Institute; 2003.
Evidence-based answers from the Family Physicians Inquiries Network
What effect do inhaled steroids have on delaying the progression of COPD?
The annual rate of decline in forced expiratory volume for 1 second (FEV1) has been researchers’ gold standard as an objective measure for progression of chronic obstructive pulmonary disease (COPD). Inhaled corticosteroids (ICS) do not consistently have a statistically significant impact on FEV1 decline, and thus on the progression of COPD (strength of recommendation [SOR]: B, 2 conflicting meta-analyses and numerous conflicting randomized controlled trials). In those studies that did show improvements in FEV1 decline, the change does not appear to be clinically significant (7.7 to 9.0 mL/year).
These findings do not take into account the potential impact of ICS on such patient oriented outcomes as exacerbation rates, quality of life, outpatient visits, hospitalization, and mortality.
Evidence summary
No therapies are known to improve long-term lung function in COPD; the goal of disease-moderating therapy is therefore to slow the rate of decline compared with the expected rate. All of the studies reviewed used FEV1 as an objective measure of whether ICS reduce this rate of decline in lung function.
Two recent meta-analyses evaluating medium- to high-dose ICS effects on FEV1 decline provided conflicting results. One meta-analysis evaluated 8 controlled clinical trials lasting at least 2 years (n=3715) and found that, when compared with placebo, ICS significantly reduced the rate of FEV1 decline by 7.7 mL/year (P=.02) and that high-dose ICS had a greater effect of 9.9 mL/year (P=.01).1 Another meta-analysis of 6 randomized, placebo-controlled trials with a duration of at least 2 years (n=3571) found a nonsignificant trend in favor of ICS, with a difference in FEV1 decline of 5.31 mL/year (P=.08) between the ICS and placebo groups.2
The differences observed in these 2 meta-analyses may be explained by the authors using slightly different approximations to the standard error, applying slightly different statistical analytical methods, and using different inclusion criteria for trials. However, 5 of the trials in these reviews were the same. Both meta-analyses determined only rate of lung function decline and did not evaluate clinical outcomes.
A trial not included in the previously mentioned meta-analyses evaluated post-bronchodilator FEV1 decline in 48 patients with early signs and symptoms of COPD for 2 years.3 Subjects were assigned to medium-dose fluticasone propionate or placebo. Early initiation of ICS treatment did not affect the progressive deterioration of lung function as no modifying effect on annual FEV1 decline was observed, however, the study only had power to detect a 60-mL annual drop in FEV1.
Meta-analyses and trials evaluating COPD progression have focused on a disease-oriented outcome (the rate of FEV1 decline). However, patient-oriented outcomes such as exacerbation frequency, hospitalization, health-related quality of life, and mortality might be more important measures of successful therapy. Although such patient-oriented outcomes are not the focus of this review or the included meta-analyses, a few of the small randomized controlled trials included in these meta-analyses suggest that ICS may improve such patient-oriented outcomes. Notably, exacerbation rates significantly decreased by 25% (P=.026), and health status improved (P=.0043) among patients with moderate to severe COPD who were taking fluticasone compared with those taking placebo.4 In mild to moderate COPD, patients treated with triamcinolone had fewer respiratory symptoms (P=.005), fewer visits to a physician because of respiratory illness (P=.003), and improved airway reactivity (P=.02).5 Some systematic reviews and other randomized trials suggest that ICS have significant benefit on these patient outcomes.6
Recommendations from others
Scientists from the National Heart, Lung, and Blood Institute and the World Health Organization provided an update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2003.7 They reported that regular treatment with ICS does not modify the long-term decline of FEV1 in patients with COPD. However, they recommended treatment with ICS for symptomatic COPD patients with an FEV1 less than 50% of predicted (stage III: severe COPD and stage IV: very severe COPD) and repeated exacerbations (ie, 3 in the last 3 years). Guidelines from other countries also suggest that ICS do not affect the progression of COPD, but support the use of ICS for patients with severe COPD and repeated exacerbations.8-10
Smoking cessation a huge benefit to all COPD patients
Vincent Lo, MD
St. Elizabeth Family Medicine Residency Program, Utica, NY; SUNY Upstate Medical University, Syracuse
In adults aged more than 30 years old with COPD, the physiological abnormality is primarily an accelerated decline in the FEV1 from the normal rate of about 30 mL per year to nearly 60 mL per year. In patients with COPD, smoking cessation is the only proven means to slow down the progression of the disease, with up to a sustained 50% reduction in the rate of lung-function decline.
Therefore, it is imperative for family physicians to underscore the magnitude of the benefit of smoking cessation to all COPD patients and to emphasize the current evidence that inhaled corticosteroid has a limited impact in delaying the progression of the disease.
1. Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax 2003;58:937-941.
2. Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. A meta-analysis. Ann Intern Med 2003;138:969-973.
3. van Grunsven P, Schermer T, Akkermans R, et al. Short-and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med 2003;97:1303-1312.
4. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000;320:1297-1303.
5. The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902-1909.
6. Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301-2312.
7. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO workshop report. Bethesda, National Heart, Lung and Blood Institute, April 2001; update of the management sections, GOLD website (www.goldcopd.com). Accessed April 3, 2004.
8. Chronic Obstructive Pulmonary Disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59 Suppl 1:1-232.
9. McKenzie DK, Frith PA, Burdon JG, Town GI. The COPDX Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2003. Med J Aust 2003;178 Suppl:S7-S39.
10. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2003. Can Respir J 2003;10 Suppl A:11A-65A.
The annual rate of decline in forced expiratory volume for 1 second (FEV1) has been researchers’ gold standard as an objective measure for progression of chronic obstructive pulmonary disease (COPD). Inhaled corticosteroids (ICS) do not consistently have a statistically significant impact on FEV1 decline, and thus on the progression of COPD (strength of recommendation [SOR]: B, 2 conflicting meta-analyses and numerous conflicting randomized controlled trials). In those studies that did show improvements in FEV1 decline, the change does not appear to be clinically significant (7.7 to 9.0 mL/year).
These findings do not take into account the potential impact of ICS on such patient oriented outcomes as exacerbation rates, quality of life, outpatient visits, hospitalization, and mortality.
Evidence summary
No therapies are known to improve long-term lung function in COPD; the goal of disease-moderating therapy is therefore to slow the rate of decline compared with the expected rate. All of the studies reviewed used FEV1 as an objective measure of whether ICS reduce this rate of decline in lung function.
Two recent meta-analyses evaluating medium- to high-dose ICS effects on FEV1 decline provided conflicting results. One meta-analysis evaluated 8 controlled clinical trials lasting at least 2 years (n=3715) and found that, when compared with placebo, ICS significantly reduced the rate of FEV1 decline by 7.7 mL/year (P=.02) and that high-dose ICS had a greater effect of 9.9 mL/year (P=.01).1 Another meta-analysis of 6 randomized, placebo-controlled trials with a duration of at least 2 years (n=3571) found a nonsignificant trend in favor of ICS, with a difference in FEV1 decline of 5.31 mL/year (P=.08) between the ICS and placebo groups.2
The differences observed in these 2 meta-analyses may be explained by the authors using slightly different approximations to the standard error, applying slightly different statistical analytical methods, and using different inclusion criteria for trials. However, 5 of the trials in these reviews were the same. Both meta-analyses determined only rate of lung function decline and did not evaluate clinical outcomes.
A trial not included in the previously mentioned meta-analyses evaluated post-bronchodilator FEV1 decline in 48 patients with early signs and symptoms of COPD for 2 years.3 Subjects were assigned to medium-dose fluticasone propionate or placebo. Early initiation of ICS treatment did not affect the progressive deterioration of lung function as no modifying effect on annual FEV1 decline was observed, however, the study only had power to detect a 60-mL annual drop in FEV1.
Meta-analyses and trials evaluating COPD progression have focused on a disease-oriented outcome (the rate of FEV1 decline). However, patient-oriented outcomes such as exacerbation frequency, hospitalization, health-related quality of life, and mortality might be more important measures of successful therapy. Although such patient-oriented outcomes are not the focus of this review or the included meta-analyses, a few of the small randomized controlled trials included in these meta-analyses suggest that ICS may improve such patient-oriented outcomes. Notably, exacerbation rates significantly decreased by 25% (P=.026), and health status improved (P=.0043) among patients with moderate to severe COPD who were taking fluticasone compared with those taking placebo.4 In mild to moderate COPD, patients treated with triamcinolone had fewer respiratory symptoms (P=.005), fewer visits to a physician because of respiratory illness (P=.003), and improved airway reactivity (P=.02).5 Some systematic reviews and other randomized trials suggest that ICS have significant benefit on these patient outcomes.6
Recommendations from others
Scientists from the National Heart, Lung, and Blood Institute and the World Health Organization provided an update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2003.7 They reported that regular treatment with ICS does not modify the long-term decline of FEV1 in patients with COPD. However, they recommended treatment with ICS for symptomatic COPD patients with an FEV1 less than 50% of predicted (stage III: severe COPD and stage IV: very severe COPD) and repeated exacerbations (ie, 3 in the last 3 years). Guidelines from other countries also suggest that ICS do not affect the progression of COPD, but support the use of ICS for patients with severe COPD and repeated exacerbations.8-10
Smoking cessation a huge benefit to all COPD patients
Vincent Lo, MD
St. Elizabeth Family Medicine Residency Program, Utica, NY; SUNY Upstate Medical University, Syracuse
In adults aged more than 30 years old with COPD, the physiological abnormality is primarily an accelerated decline in the FEV1 from the normal rate of about 30 mL per year to nearly 60 mL per year. In patients with COPD, smoking cessation is the only proven means to slow down the progression of the disease, with up to a sustained 50% reduction in the rate of lung-function decline.
Therefore, it is imperative for family physicians to underscore the magnitude of the benefit of smoking cessation to all COPD patients and to emphasize the current evidence that inhaled corticosteroid has a limited impact in delaying the progression of the disease.
The annual rate of decline in forced expiratory volume for 1 second (FEV1) has been researchers’ gold standard as an objective measure for progression of chronic obstructive pulmonary disease (COPD). Inhaled corticosteroids (ICS) do not consistently have a statistically significant impact on FEV1 decline, and thus on the progression of COPD (strength of recommendation [SOR]: B, 2 conflicting meta-analyses and numerous conflicting randomized controlled trials). In those studies that did show improvements in FEV1 decline, the change does not appear to be clinically significant (7.7 to 9.0 mL/year).
These findings do not take into account the potential impact of ICS on such patient oriented outcomes as exacerbation rates, quality of life, outpatient visits, hospitalization, and mortality.
Evidence summary
No therapies are known to improve long-term lung function in COPD; the goal of disease-moderating therapy is therefore to slow the rate of decline compared with the expected rate. All of the studies reviewed used FEV1 as an objective measure of whether ICS reduce this rate of decline in lung function.
Two recent meta-analyses evaluating medium- to high-dose ICS effects on FEV1 decline provided conflicting results. One meta-analysis evaluated 8 controlled clinical trials lasting at least 2 years (n=3715) and found that, when compared with placebo, ICS significantly reduced the rate of FEV1 decline by 7.7 mL/year (P=.02) and that high-dose ICS had a greater effect of 9.9 mL/year (P=.01).1 Another meta-analysis of 6 randomized, placebo-controlled trials with a duration of at least 2 years (n=3571) found a nonsignificant trend in favor of ICS, with a difference in FEV1 decline of 5.31 mL/year (P=.08) between the ICS and placebo groups.2
The differences observed in these 2 meta-analyses may be explained by the authors using slightly different approximations to the standard error, applying slightly different statistical analytical methods, and using different inclusion criteria for trials. However, 5 of the trials in these reviews were the same. Both meta-analyses determined only rate of lung function decline and did not evaluate clinical outcomes.
A trial not included in the previously mentioned meta-analyses evaluated post-bronchodilator FEV1 decline in 48 patients with early signs and symptoms of COPD for 2 years.3 Subjects were assigned to medium-dose fluticasone propionate or placebo. Early initiation of ICS treatment did not affect the progressive deterioration of lung function as no modifying effect on annual FEV1 decline was observed, however, the study only had power to detect a 60-mL annual drop in FEV1.
Meta-analyses and trials evaluating COPD progression have focused on a disease-oriented outcome (the rate of FEV1 decline). However, patient-oriented outcomes such as exacerbation frequency, hospitalization, health-related quality of life, and mortality might be more important measures of successful therapy. Although such patient-oriented outcomes are not the focus of this review or the included meta-analyses, a few of the small randomized controlled trials included in these meta-analyses suggest that ICS may improve such patient-oriented outcomes. Notably, exacerbation rates significantly decreased by 25% (P=.026), and health status improved (P=.0043) among patients with moderate to severe COPD who were taking fluticasone compared with those taking placebo.4 In mild to moderate COPD, patients treated with triamcinolone had fewer respiratory symptoms (P=.005), fewer visits to a physician because of respiratory illness (P=.003), and improved airway reactivity (P=.02).5 Some systematic reviews and other randomized trials suggest that ICS have significant benefit on these patient outcomes.6
Recommendations from others
Scientists from the National Heart, Lung, and Blood Institute and the World Health Organization provided an update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2003.7 They reported that regular treatment with ICS does not modify the long-term decline of FEV1 in patients with COPD. However, they recommended treatment with ICS for symptomatic COPD patients with an FEV1 less than 50% of predicted (stage III: severe COPD and stage IV: very severe COPD) and repeated exacerbations (ie, 3 in the last 3 years). Guidelines from other countries also suggest that ICS do not affect the progression of COPD, but support the use of ICS for patients with severe COPD and repeated exacerbations.8-10
Smoking cessation a huge benefit to all COPD patients
Vincent Lo, MD
St. Elizabeth Family Medicine Residency Program, Utica, NY; SUNY Upstate Medical University, Syracuse
In adults aged more than 30 years old with COPD, the physiological abnormality is primarily an accelerated decline in the FEV1 from the normal rate of about 30 mL per year to nearly 60 mL per year. In patients with COPD, smoking cessation is the only proven means to slow down the progression of the disease, with up to a sustained 50% reduction in the rate of lung-function decline.
Therefore, it is imperative for family physicians to underscore the magnitude of the benefit of smoking cessation to all COPD patients and to emphasize the current evidence that inhaled corticosteroid has a limited impact in delaying the progression of the disease.
1. Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax 2003;58:937-941.
2. Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. A meta-analysis. Ann Intern Med 2003;138:969-973.
3. van Grunsven P, Schermer T, Akkermans R, et al. Short-and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med 2003;97:1303-1312.
4. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000;320:1297-1303.
5. The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902-1909.
6. Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301-2312.
7. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO workshop report. Bethesda, National Heart, Lung and Blood Institute, April 2001; update of the management sections, GOLD website (www.goldcopd.com). Accessed April 3, 2004.
8. Chronic Obstructive Pulmonary Disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59 Suppl 1:1-232.
9. McKenzie DK, Frith PA, Burdon JG, Town GI. The COPDX Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2003. Med J Aust 2003;178 Suppl:S7-S39.
10. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2003. Can Respir J 2003;10 Suppl A:11A-65A.
1. Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax 2003;58:937-941.
2. Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. A meta-analysis. Ann Intern Med 2003;138:969-973.
3. van Grunsven P, Schermer T, Akkermans R, et al. Short-and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med 2003;97:1303-1312.
4. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000;320:1297-1303.
5. The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902-1909.
6. Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301-2312.
7. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO workshop report. Bethesda, National Heart, Lung and Blood Institute, April 2001; update of the management sections, GOLD website (www.goldcopd.com). Accessed April 3, 2004.
8. Chronic Obstructive Pulmonary Disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59 Suppl 1:1-232.
9. McKenzie DK, Frith PA, Burdon JG, Town GI. The COPDX Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2003. Med J Aust 2003;178 Suppl:S7-S39.
10. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2003. Can Respir J 2003;10 Suppl A:11A-65A.
Evidence-based answers from the Family Physicians Inquiries Network
Changes in recommended treatments for mild and moderate asthma
- Every patient with persistent asthma, regardless of disease severity, should use a daily controller medication.
- Consider an inhaled corticosteroid (ICS) first when choosing controller medications for long-term treatment of mild, moderate, and severe persistent asthma in adults and children. Leukotriene modifiers, cromolyn, and nedocromil may be considered as alternative, not preferred, controller medications for patients with persistent asthma.
- Long-acting β2-adrenergic agonists should not be used as monotherapy.
- Long-term use of ICSs within labeled doses is safe for children in terms of growth, bone mineral density, and adrenal function; nonetheless, asthma should be monitored and ICS therapy stepped down to the lowest effective dose.
- Low-to medium-dose ICSs are not associated with the development of cataracts or glaucoma in children, but high cumulative lifetime doses may slightly increase the prevalence of cataracts in adults and elderly patients.
- ICSs are recommended for use in pregnant women with asthma; budesonide is the only ICS rated Pregnancy Category B.
Consider an adult with the following characteristics. To which disease severity would you assign this patient’s asthma?
- Forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥80%
- PEF variability 20%–30%
- Daytime symptoms less than once a day
- Nighttime symptoms more than 1 night a week.
This patient is said to have moderate persistent asthma based on nighttime symptoms. An accurate classification of a patient’s asthma is the foundation for selecting an appropriate treatment strategy.
In 2002 the National Asthma Education and Prevention Program (NAEPP) updated select topics1from its 1997 Guidelines for the Diagnosis and Management of Asthma.2 These evidence-based revisions to the stepwise approach to asthma management were made following a systematic review of the literature (see Search function).
A comprehensive search of Medline and EMBASE databases was performed to identify controlled clinical studies relevant to each topic that were published (in English or foreign languages with English abstracts) from 1980 through August 2000. The search included studies published before 1980 if referenced in the post-1980 literature. Studies that did not include control groups were excluded, except for those reporting adverse effects of ICSs. Studies that met the study selection criteria established for each topic were included in a systematic review of the evidence. An expert panel reviewed the evidence, along with additional literature published since August 2000, and reached a consensus on whether the evidence supported 1997 guideline recommendations or indicated a need for revision. Writing committees were then assigned to developed position statements for each topic. The level of evidence for included studies was rated based on the system of Jadad and colleagues,3 where A = randomized controlled trials, rich body of data; B = randomized controlled trials, limited data; C = nonrandomized trials and observational studies; D = panel consensus judgment.
This article reviews the 2002 NAEPP recommendations for the use of controller medications for asthma, including:
- Relative effectiveness of inhaled corticosteroids (ICSs) versus other controller medications
- Safety of long-term ICS use in children
- Potential benefits of early ICS treatment.
We emphasize mild and moderate persistent asthma because the recommended treatments for these levels of severity have been most affected by the recent guideline changes. We also discuss a recent change by the US Food and Drug Administration (FDA) in its pregnancy category rating for an ICS.
2002 Stepwise approach to asthma management
New criteria for classifying asthma severity
The NAEPP classifies asthma severity according to symptoms and lung function in adults and children older than 5 years, and symptoms in children 5 years and younger.1 Persistent asthma is classified as mild, moderate, or severe according to the feature of greatest severity.
Asthma severity should be assigned according to symptoms before treatment.1 Because it is difficult to predict which infants and young children who wheeze with acute viral upper respiratory infection will go on to develop persistent asthma, new criteria have been detailed to help distinguish these children from those with transient wheeze (Table 1).1,4
TABLE 1
Criteria for children with intermittent wheeze
Infants and young children meeting these criteria should receive controller therapy for asthma:
|
AND presence of risk factors for development of persistent asthma:
|
Choosing pharmacologic treatment according to asthma classification
Quick-relief medications, which include the short-acting β2-agonists (SABAs), are taken as needed to promptly reverse acute airflow obstruction and relieve accompanying symptoms.2
Asthma controller medications (ie, ICSs, cromolyn sodium, long-acting β2-adrenergic-agonists [LABAs], leukotriene modifiers, nedocromil, and theophylline) are used daily to achieve and maintain long-term control of persistent asthma. All patients with persistent asthma, regardless of disease severity, should use a daily controller. Criteria for determining asthma severity and updated recommendations for the use of controller treatment in mild and moderate persistent asthma are presented in the Figure.3,5 Levels of evidence justifying NAEPP treatment recommendations are shown in Table 2.
For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma
Topics in the management of asthma in children
Recognizing the need for continual appraisal of the benefits and risks of asthma medications in children, the NAEPP Expert Panel considered new studies comparing the effectiveness of ICS monotherapy with that of as-needed SABAs and other controllers used as monotherapy in children with mild or moderate persistent asthma. In addition, the safety of long-term ICS use in children was evaluated based on vertical growth, bone mineral density, ocular toxicity, and adrenal suppression.
Effectiveness of ICSs compared with other asthma medications
Short-acting β2-adrenergic agonists. Eight studies met the eligibility criteria for evaluating the effectiveness of ICSs versus as-needed SABAs.6,10-16 Six studies (4 involving budesonide) in children 5 years and older showed that ICSs improve lung function and symptoms and reduce the need for emergency intervention compared with as-needed SABAs.1 Among all studies included in the NAEPP update, the Childhood Asthma Management Program (CAMP) Research Group Study,9 a placebo-controlled study of inhaled budesonide and nedocromil, contributed the most evidence. Studies with children 5 years and younger are limited to 2 small studies enrolling a total of 69 children.6,15 Consistent with studies of older children, these studies indicate that ICSs improve asthma control compared with as-needed SABAs.1
Cromolyn and nedocromil. Despite well-established safety profiles, cromolyn and nedocromil are no longer recommended as first-line therapy for children, even those with mild disease. New recommendations reflect the greater effectiveness of inhaled budesonide compared with nedocromil demonstrated in the CAMP study,10 and the lack of apparent benefit of cromolyn as maintenance treatment in childhood asthma reported by Tasche and colleagues in a systematic review of the literature.17
In the CAMP study, children 5 to 12 years of age receiving inhaled budesonide showed greater reductions in symptoms and albuterol use, lower rates of hospitalization and urgent care visits, and less need for additional asthma therapy and oral prednisone compared with placebo over 4 to 6 years of treatment.10 The marginal effectiveness of nedocromil demonstrated in the CAMP study mirrored that of cromolyn reported in the review of 24 randomized placebo-controlled studies by Tasche and colleagues.1,17
For children 5 years and younger, the NAEPP Expert Panel took into account 1 randomized placebo-controlled study conducted with children 2 to 5 years of age; it showed improvements in lung function, symptoms, and bronchial hyperre-activity with inhaled budesonide.9 Support for the new NAEPP recommendations preferring ICSs for preschool children is found in a more recent open-label study18 that showed greater symptom improvement and significantly lower rates of asthma exacerbations, urgent care visits, and oral prednisone use with budesonide inhalation suspension, compared with cromolyn sodium nebulizer solution (Intal Nebulizer Solution) in children 2 to 6 years of age with persistent asthma.
Leukotriene modifiers. The LTRAs zafir-lukast and montelukast are approved for use in children. According to the NAEPP Expert Panel, studies have shown only modest improvements in lung function and other asthma control outcomes with LTRA monotherapy in children as young as 6 and 2 years, respectively.1 Because studies comparing ICSs with LTRAs in children are lacking, findings of greater overall efficacy of ICSs in adults with persistent asthma have been extrapolated for use with children; clear superiority of ICSs versus LTRAs in most outcomes has resulted in the recommendation for ICSs as the preferred treatment for mild persistent asthma in children.
Long-acting β2-adrenergic agonists. There is no role for LABAs as monotherapy in asthma. No studies have compared the effectiveness of ICS versus LABA monotherapy in children younger than 5 years, and studies in older children have shown greater effectiveness of inhaled beclomethasone versus salmeterol.14,19 In the study by Verberne and colleagues, salmeterol monotherapy was associated with deterioration in FEV1.19 In a more recent study that included patients as young as 16 years, a switch from ICS to LABA treatment was associated with a significant increase in treatment failures and exacerbations.20
Theophylline. Only 1 study has compared outcomes with low-dose ICSs versus theophylline in adults and children.21 Although limited, the data support greater effectiveness of ICSs based on symptoms, bronchial hyperresponsiveness, and the need for β2-adrenergic agonists and oral corticosteroids.1
Safety of long-term ICS use in children
Systemic corticosteroids have the potential to suppress growth over the long term.2 Short-term growth studies with ICSs show an average reduction in growth velocity of 1 cm per year during the first year of treatment, but the CAMP study showed that initial reductions in growth velocity with inhaled budesonide were not maintained over a 4- to 6-year treatment period.1,10
Although catch-up growth was not observed in the CAMP study, Agertoft and Pedersen reported no effect of long-term treatment with inhaled budesonide (mean 9.2 years) on final adult height.22 Based on these long-term prospective studies of budesonide, showing only a transient reduction in growth velocity and attainment of expected final adult height, and retrospective studies including inhaled beclomethasone, the Expert Panel concluded that the ICS class is safe regarding growth effects.
According to the NAEPP Expert Panel, clinical study data for children monitored for up to 6 years strongly suggest that ICSs are safe when used at recommended doses (strength of recommendation: A).1 The panel could not rule out a potential cumulative effect of ICS use on some conditions, (eg, osteoporosis, cataracts, glaucoma) in adulthood, as sufficient long-term data are not available.
The panel did conclude that low- to medium-dose ICSs (Table 3) appear to have no serious adverse effects on bone mineral density in children.
Likewise, low- to medium-dose ICS use was not associated with the development of cataracts or glaucoma in children, although the potential for high cumulative lifetime doses of ICSs to slightly increase the prevalence of cataracts in adults and elderly patients was noted.
Strong evidence also indicates that ICS effects on adrenal function are usually clinically insignificant at low to medium doses; however, certain individuals may be at higher risk for hypothalam-ic pituitary adrenal axis effects while using conventional ICS doses.1
Although ICSs are safe when used within labeled dosing, it is still preferable to maintain doses at the lowest effective dose. In general, treatment should be reviewed every 1 to 6 months and doses reduced in a stepwise fashion when possible.1 For children showing a favorable response to treatment, a step down in dose should be considered, but not more frequently than every 3 months. If children show no clear response to treatment within 4 to 6 weeks, consider an alternative treatment or diagnosis.1
Safety of long-term ICS use in pregnant women
Uncontrolled asthma during pregnancy is associated with an increased risk of perinatal complications. 23 Since the consequences of not using asthma controllers during pregnancy can be worse than those with using them, daily controller treatment is recommended for all pregnant women with persistent asthma. 23
The American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology previously recommended cromolyn as the treatment of choice for pregnant women with mild persistent asthma. ICSs were recommended for patients whose asthma was inadequately controlled with cromolyn. 24 Beclomethasone and budesonide were the ICSs of choice for pregnant women and those who might become pregnant, with a preference for budesonide when high-dose therapy was indicated.24
These recommendations predate the 2002 NAEPP recommendations for ICSs as preferred therapy in mild persistent asthma and the 2004 NAEPP recommendations for ICSs as the first-choice controller therapy for mild persistent asthma during pregnancy. 25 Among ICSs, one (inhaled budesonide) has an FDA Pregnancy Category B rating based on studies showing no risk in pregnant women. 26,27 All other ICSs are rated Pregnancy Category C.
Based on current evidence, it seems reasonable to consider whether budesonide should now be the preferred therapy for mild persistent asthma during pregnancy.
Effects of early treatment on asthma progression
The potential for early ICS intervention to prevent progression of mild or moderate persistent asthma was evaluated solely with data from children enrolled in the CAMP study. 10 The NAEPP Expert Panel concluded that CAMP study data do not support a progressive decline in lung function in children aged 5 to 12 years with mild or moderate persistent asthma, but do suggest that lung function decline is influenced by age of asthma onset.
According to the panel, CAMP data suggest that most deficits in lung function growth due to childhood asthma occur during the first 3 years of life. Preliminary results of the recent START study (Inhaled Steroid Treatment As Regular Therapy in Early Asthma), 28 conducted with 7165 corticosteroidnaïve patients 5 to 66 years of age with recent onset mild persistent asthma, did show a decline in lung function in patients with mild persistent disease.
Although improvements in prebronchodilator and postbronchodilator FEV1 were significant after 3 years of treatment with inhaled budes-onide, differences from placebo in both outcomes were greatest after the first year. When patients with mild persistent disease inhaled budesonide once daily in addition to normal treatment within 2 years of asthma onset,28 they enjoyed considerable protection from severe and life-threatening asthma exacerbations and overall greater asthma control.
- Budesonide • Pulmicort
- Rhinocort Cromolyn • Intal
- Fluticasone • Flovent
- Formoterol • Foradil
- Montelukast • Singulair
- Nedocromil • Tilade
- Salmeterol • Servent
- Triamcinolone acetonide • Azmacort
- Zafirlukast • Accolate
Corresponding author
Gregory J. Redding, MD, Children’s Hospital and Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105-0371. E-mail: [email protected].
1. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics–2002. J Allergy Clin Immunol 2002;110(5 suppl):S141-S219.
2. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health; 1997. Publication 97;4051.-
3. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
4. Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403-1406.
5. Oxford Centre for Evidence-based Medicine Levels of Evidence Available atwww.cebm.net/levels_faq.asp . Accessed January 8, 2004.
6. Connett GJ, Warde C, Wooler E, Lenney W. Use of budes-onide in severe asthmatics aged 1–3 years. Arch Dis Child 1993;69:351-355.
7. de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996;98:14-20.
8. Bisgaard H, Gillies J, Groenewald M, Maden C, . for an International Study Group The effect of inhaled fluticas-one propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med 1999;160:126-131.
9. Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and metha-choline responsiveness in 2- to 5-year–old asthmatic children. Am J Respir Crit Care Med 2000;162:1500-1506.
10. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-1063.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373-381.
12. Hoekstra MO, Grol MH, Bouman K, Stijnen T, Koëter GH, Kauffman HF, et al. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med 1996;154:1039-1044.
13. Jónasson G, Carlsen K-H, Blomqvist P. Clinical efficacy of low-dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. Eur Respir J 1998;12:1099-1104.
14. Simons FER and the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-1665.
15. Storr J, Lenney CA, Lenney W. Nebulized beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986;61:270-273.
16. Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. and the Dutch Chronic Non-Specific Lung Disease Study Group Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547-554.
17. Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913-920.
18. Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002;109:866-872.
19. Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. and the Dutch Paediatric Asthma Study Group Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med 1998;158:213-219.
20. Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, , et al. for the Asthma Clinical Research Network of the National Heart Lung and Blood Institute. Long-acting 2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583-2593.
21. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. and the American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild or moderate persistent asthma. J Allergy Clin Immunol 1998;101:14-23.
22. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-1069.
23. National Asthma Education Program (NAEP). Report of the Working Group on Asthma and Pregnancy: Management of Asthma during Pregnancy. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health, 1993. NIH Publication No. 96-141593.
24. American College of Obstetricians and Gynecologists (ACOG) and the American College of Allergy, Asthmaand Immunology (ACAAI). The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
25. National Asthma Education and Prevention Program. NAEPP Expert Panel Report. Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment—Update 2004. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health. NIH Publication No. 04-5246. March 2004.
26. Källén B, Rydhstroem H, Äberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
27. Ericson A, Källén B. Use of drugs during pregnancy—unique Swedish registration method that can be improved. Information From the Swedish Medical Products Agency 1999;1:8-11.
28. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y-Z, Ohlsson SV, et al. for the START Investigators Group. Early intervention with budesonide in mild persistent asthma. Lancet 2003;361:1071-1076.
- Every patient with persistent asthma, regardless of disease severity, should use a daily controller medication.
- Consider an inhaled corticosteroid (ICS) first when choosing controller medications for long-term treatment of mild, moderate, and severe persistent asthma in adults and children. Leukotriene modifiers, cromolyn, and nedocromil may be considered as alternative, not preferred, controller medications for patients with persistent asthma.
- Long-acting β2-adrenergic agonists should not be used as monotherapy.
- Long-term use of ICSs within labeled doses is safe for children in terms of growth, bone mineral density, and adrenal function; nonetheless, asthma should be monitored and ICS therapy stepped down to the lowest effective dose.
- Low-to medium-dose ICSs are not associated with the development of cataracts or glaucoma in children, but high cumulative lifetime doses may slightly increase the prevalence of cataracts in adults and elderly patients.
- ICSs are recommended for use in pregnant women with asthma; budesonide is the only ICS rated Pregnancy Category B.
Consider an adult with the following characteristics. To which disease severity would you assign this patient’s asthma?
- Forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥80%
- PEF variability 20%–30%
- Daytime symptoms less than once a day
- Nighttime symptoms more than 1 night a week.
This patient is said to have moderate persistent asthma based on nighttime symptoms. An accurate classification of a patient’s asthma is the foundation for selecting an appropriate treatment strategy.
In 2002 the National Asthma Education and Prevention Program (NAEPP) updated select topics1from its 1997 Guidelines for the Diagnosis and Management of Asthma.2 These evidence-based revisions to the stepwise approach to asthma management were made following a systematic review of the literature (see Search function).
A comprehensive search of Medline and EMBASE databases was performed to identify controlled clinical studies relevant to each topic that were published (in English or foreign languages with English abstracts) from 1980 through August 2000. The search included studies published before 1980 if referenced in the post-1980 literature. Studies that did not include control groups were excluded, except for those reporting adverse effects of ICSs. Studies that met the study selection criteria established for each topic were included in a systematic review of the evidence. An expert panel reviewed the evidence, along with additional literature published since August 2000, and reached a consensus on whether the evidence supported 1997 guideline recommendations or indicated a need for revision. Writing committees were then assigned to developed position statements for each topic. The level of evidence for included studies was rated based on the system of Jadad and colleagues,3 where A = randomized controlled trials, rich body of data; B = randomized controlled trials, limited data; C = nonrandomized trials and observational studies; D = panel consensus judgment.
This article reviews the 2002 NAEPP recommendations for the use of controller medications for asthma, including:
- Relative effectiveness of inhaled corticosteroids (ICSs) versus other controller medications
- Safety of long-term ICS use in children
- Potential benefits of early ICS treatment.
We emphasize mild and moderate persistent asthma because the recommended treatments for these levels of severity have been most affected by the recent guideline changes. We also discuss a recent change by the US Food and Drug Administration (FDA) in its pregnancy category rating for an ICS.
2002 Stepwise approach to asthma management
New criteria for classifying asthma severity
The NAEPP classifies asthma severity according to symptoms and lung function in adults and children older than 5 years, and symptoms in children 5 years and younger.1 Persistent asthma is classified as mild, moderate, or severe according to the feature of greatest severity.
Asthma severity should be assigned according to symptoms before treatment.1 Because it is difficult to predict which infants and young children who wheeze with acute viral upper respiratory infection will go on to develop persistent asthma, new criteria have been detailed to help distinguish these children from those with transient wheeze (Table 1).1,4
TABLE 1
Criteria for children with intermittent wheeze
Infants and young children meeting these criteria should receive controller therapy for asthma:
|
AND presence of risk factors for development of persistent asthma:
|
Choosing pharmacologic treatment according to asthma classification
Quick-relief medications, which include the short-acting β2-agonists (SABAs), are taken as needed to promptly reverse acute airflow obstruction and relieve accompanying symptoms.2
Asthma controller medications (ie, ICSs, cromolyn sodium, long-acting β2-adrenergic-agonists [LABAs], leukotriene modifiers, nedocromil, and theophylline) are used daily to achieve and maintain long-term control of persistent asthma. All patients with persistent asthma, regardless of disease severity, should use a daily controller. Criteria for determining asthma severity and updated recommendations for the use of controller treatment in mild and moderate persistent asthma are presented in the Figure.3,5 Levels of evidence justifying NAEPP treatment recommendations are shown in Table 2.
For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma
Topics in the management of asthma in children
Recognizing the need for continual appraisal of the benefits and risks of asthma medications in children, the NAEPP Expert Panel considered new studies comparing the effectiveness of ICS monotherapy with that of as-needed SABAs and other controllers used as monotherapy in children with mild or moderate persistent asthma. In addition, the safety of long-term ICS use in children was evaluated based on vertical growth, bone mineral density, ocular toxicity, and adrenal suppression.
Effectiveness of ICSs compared with other asthma medications
Short-acting β2-adrenergic agonists. Eight studies met the eligibility criteria for evaluating the effectiveness of ICSs versus as-needed SABAs.6,10-16 Six studies (4 involving budesonide) in children 5 years and older showed that ICSs improve lung function and symptoms and reduce the need for emergency intervention compared with as-needed SABAs.1 Among all studies included in the NAEPP update, the Childhood Asthma Management Program (CAMP) Research Group Study,9 a placebo-controlled study of inhaled budesonide and nedocromil, contributed the most evidence. Studies with children 5 years and younger are limited to 2 small studies enrolling a total of 69 children.6,15 Consistent with studies of older children, these studies indicate that ICSs improve asthma control compared with as-needed SABAs.1
Cromolyn and nedocromil. Despite well-established safety profiles, cromolyn and nedocromil are no longer recommended as first-line therapy for children, even those with mild disease. New recommendations reflect the greater effectiveness of inhaled budesonide compared with nedocromil demonstrated in the CAMP study,10 and the lack of apparent benefit of cromolyn as maintenance treatment in childhood asthma reported by Tasche and colleagues in a systematic review of the literature.17
In the CAMP study, children 5 to 12 years of age receiving inhaled budesonide showed greater reductions in symptoms and albuterol use, lower rates of hospitalization and urgent care visits, and less need for additional asthma therapy and oral prednisone compared with placebo over 4 to 6 years of treatment.10 The marginal effectiveness of nedocromil demonstrated in the CAMP study mirrored that of cromolyn reported in the review of 24 randomized placebo-controlled studies by Tasche and colleagues.1,17
For children 5 years and younger, the NAEPP Expert Panel took into account 1 randomized placebo-controlled study conducted with children 2 to 5 years of age; it showed improvements in lung function, symptoms, and bronchial hyperre-activity with inhaled budesonide.9 Support for the new NAEPP recommendations preferring ICSs for preschool children is found in a more recent open-label study18 that showed greater symptom improvement and significantly lower rates of asthma exacerbations, urgent care visits, and oral prednisone use with budesonide inhalation suspension, compared with cromolyn sodium nebulizer solution (Intal Nebulizer Solution) in children 2 to 6 years of age with persistent asthma.
Leukotriene modifiers. The LTRAs zafir-lukast and montelukast are approved for use in children. According to the NAEPP Expert Panel, studies have shown only modest improvements in lung function and other asthma control outcomes with LTRA monotherapy in children as young as 6 and 2 years, respectively.1 Because studies comparing ICSs with LTRAs in children are lacking, findings of greater overall efficacy of ICSs in adults with persistent asthma have been extrapolated for use with children; clear superiority of ICSs versus LTRAs in most outcomes has resulted in the recommendation for ICSs as the preferred treatment for mild persistent asthma in children.
Long-acting β2-adrenergic agonists. There is no role for LABAs as monotherapy in asthma. No studies have compared the effectiveness of ICS versus LABA monotherapy in children younger than 5 years, and studies in older children have shown greater effectiveness of inhaled beclomethasone versus salmeterol.14,19 In the study by Verberne and colleagues, salmeterol monotherapy was associated with deterioration in FEV1.19 In a more recent study that included patients as young as 16 years, a switch from ICS to LABA treatment was associated with a significant increase in treatment failures and exacerbations.20
Theophylline. Only 1 study has compared outcomes with low-dose ICSs versus theophylline in adults and children.21 Although limited, the data support greater effectiveness of ICSs based on symptoms, bronchial hyperresponsiveness, and the need for β2-adrenergic agonists and oral corticosteroids.1
Safety of long-term ICS use in children
Systemic corticosteroids have the potential to suppress growth over the long term.2 Short-term growth studies with ICSs show an average reduction in growth velocity of 1 cm per year during the first year of treatment, but the CAMP study showed that initial reductions in growth velocity with inhaled budesonide were not maintained over a 4- to 6-year treatment period.1,10
Although catch-up growth was not observed in the CAMP study, Agertoft and Pedersen reported no effect of long-term treatment with inhaled budesonide (mean 9.2 years) on final adult height.22 Based on these long-term prospective studies of budesonide, showing only a transient reduction in growth velocity and attainment of expected final adult height, and retrospective studies including inhaled beclomethasone, the Expert Panel concluded that the ICS class is safe regarding growth effects.
According to the NAEPP Expert Panel, clinical study data for children monitored for up to 6 years strongly suggest that ICSs are safe when used at recommended doses (strength of recommendation: A).1 The panel could not rule out a potential cumulative effect of ICS use on some conditions, (eg, osteoporosis, cataracts, glaucoma) in adulthood, as sufficient long-term data are not available.
The panel did conclude that low- to medium-dose ICSs (Table 3) appear to have no serious adverse effects on bone mineral density in children.
Likewise, low- to medium-dose ICS use was not associated with the development of cataracts or glaucoma in children, although the potential for high cumulative lifetime doses of ICSs to slightly increase the prevalence of cataracts in adults and elderly patients was noted.
Strong evidence also indicates that ICS effects on adrenal function are usually clinically insignificant at low to medium doses; however, certain individuals may be at higher risk for hypothalam-ic pituitary adrenal axis effects while using conventional ICS doses.1
Although ICSs are safe when used within labeled dosing, it is still preferable to maintain doses at the lowest effective dose. In general, treatment should be reviewed every 1 to 6 months and doses reduced in a stepwise fashion when possible.1 For children showing a favorable response to treatment, a step down in dose should be considered, but not more frequently than every 3 months. If children show no clear response to treatment within 4 to 6 weeks, consider an alternative treatment or diagnosis.1
Safety of long-term ICS use in pregnant women
Uncontrolled asthma during pregnancy is associated with an increased risk of perinatal complications. 23 Since the consequences of not using asthma controllers during pregnancy can be worse than those with using them, daily controller treatment is recommended for all pregnant women with persistent asthma. 23
The American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology previously recommended cromolyn as the treatment of choice for pregnant women with mild persistent asthma. ICSs were recommended for patients whose asthma was inadequately controlled with cromolyn. 24 Beclomethasone and budesonide were the ICSs of choice for pregnant women and those who might become pregnant, with a preference for budesonide when high-dose therapy was indicated.24
These recommendations predate the 2002 NAEPP recommendations for ICSs as preferred therapy in mild persistent asthma and the 2004 NAEPP recommendations for ICSs as the first-choice controller therapy for mild persistent asthma during pregnancy. 25 Among ICSs, one (inhaled budesonide) has an FDA Pregnancy Category B rating based on studies showing no risk in pregnant women. 26,27 All other ICSs are rated Pregnancy Category C.
Based on current evidence, it seems reasonable to consider whether budesonide should now be the preferred therapy for mild persistent asthma during pregnancy.
Effects of early treatment on asthma progression
The potential for early ICS intervention to prevent progression of mild or moderate persistent asthma was evaluated solely with data from children enrolled in the CAMP study. 10 The NAEPP Expert Panel concluded that CAMP study data do not support a progressive decline in lung function in children aged 5 to 12 years with mild or moderate persistent asthma, but do suggest that lung function decline is influenced by age of asthma onset.
According to the panel, CAMP data suggest that most deficits in lung function growth due to childhood asthma occur during the first 3 years of life. Preliminary results of the recent START study (Inhaled Steroid Treatment As Regular Therapy in Early Asthma), 28 conducted with 7165 corticosteroidnaïve patients 5 to 66 years of age with recent onset mild persistent asthma, did show a decline in lung function in patients with mild persistent disease.
Although improvements in prebronchodilator and postbronchodilator FEV1 were significant after 3 years of treatment with inhaled budes-onide, differences from placebo in both outcomes were greatest after the first year. When patients with mild persistent disease inhaled budesonide once daily in addition to normal treatment within 2 years of asthma onset,28 they enjoyed considerable protection from severe and life-threatening asthma exacerbations and overall greater asthma control.
- Budesonide • Pulmicort
- Rhinocort Cromolyn • Intal
- Fluticasone • Flovent
- Formoterol • Foradil
- Montelukast • Singulair
- Nedocromil • Tilade
- Salmeterol • Servent
- Triamcinolone acetonide • Azmacort
- Zafirlukast • Accolate
Corresponding author
Gregory J. Redding, MD, Children’s Hospital and Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105-0371. E-mail: [email protected].
- Every patient with persistent asthma, regardless of disease severity, should use a daily controller medication.
- Consider an inhaled corticosteroid (ICS) first when choosing controller medications for long-term treatment of mild, moderate, and severe persistent asthma in adults and children. Leukotriene modifiers, cromolyn, and nedocromil may be considered as alternative, not preferred, controller medications for patients with persistent asthma.
- Long-acting β2-adrenergic agonists should not be used as monotherapy.
- Long-term use of ICSs within labeled doses is safe for children in terms of growth, bone mineral density, and adrenal function; nonetheless, asthma should be monitored and ICS therapy stepped down to the lowest effective dose.
- Low-to medium-dose ICSs are not associated with the development of cataracts or glaucoma in children, but high cumulative lifetime doses may slightly increase the prevalence of cataracts in adults and elderly patients.
- ICSs are recommended for use in pregnant women with asthma; budesonide is the only ICS rated Pregnancy Category B.
Consider an adult with the following characteristics. To which disease severity would you assign this patient’s asthma?
- Forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥80%
- PEF variability 20%–30%
- Daytime symptoms less than once a day
- Nighttime symptoms more than 1 night a week.
This patient is said to have moderate persistent asthma based on nighttime symptoms. An accurate classification of a patient’s asthma is the foundation for selecting an appropriate treatment strategy.
In 2002 the National Asthma Education and Prevention Program (NAEPP) updated select topics1from its 1997 Guidelines for the Diagnosis and Management of Asthma.2 These evidence-based revisions to the stepwise approach to asthma management were made following a systematic review of the literature (see Search function).
A comprehensive search of Medline and EMBASE databases was performed to identify controlled clinical studies relevant to each topic that were published (in English or foreign languages with English abstracts) from 1980 through August 2000. The search included studies published before 1980 if referenced in the post-1980 literature. Studies that did not include control groups were excluded, except for those reporting adverse effects of ICSs. Studies that met the study selection criteria established for each topic were included in a systematic review of the evidence. An expert panel reviewed the evidence, along with additional literature published since August 2000, and reached a consensus on whether the evidence supported 1997 guideline recommendations or indicated a need for revision. Writing committees were then assigned to developed position statements for each topic. The level of evidence for included studies was rated based on the system of Jadad and colleagues,3 where A = randomized controlled trials, rich body of data; B = randomized controlled trials, limited data; C = nonrandomized trials and observational studies; D = panel consensus judgment.
This article reviews the 2002 NAEPP recommendations for the use of controller medications for asthma, including:
- Relative effectiveness of inhaled corticosteroids (ICSs) versus other controller medications
- Safety of long-term ICS use in children
- Potential benefits of early ICS treatment.
We emphasize mild and moderate persistent asthma because the recommended treatments for these levels of severity have been most affected by the recent guideline changes. We also discuss a recent change by the US Food and Drug Administration (FDA) in its pregnancy category rating for an ICS.
2002 Stepwise approach to asthma management
New criteria for classifying asthma severity
The NAEPP classifies asthma severity according to symptoms and lung function in adults and children older than 5 years, and symptoms in children 5 years and younger.1 Persistent asthma is classified as mild, moderate, or severe according to the feature of greatest severity.
Asthma severity should be assigned according to symptoms before treatment.1 Because it is difficult to predict which infants and young children who wheeze with acute viral upper respiratory infection will go on to develop persistent asthma, new criteria have been detailed to help distinguish these children from those with transient wheeze (Table 1).1,4
TABLE 1
Criteria for children with intermittent wheeze
Infants and young children meeting these criteria should receive controller therapy for asthma:
|
AND presence of risk factors for development of persistent asthma:
|
Choosing pharmacologic treatment according to asthma classification
Quick-relief medications, which include the short-acting β2-agonists (SABAs), are taken as needed to promptly reverse acute airflow obstruction and relieve accompanying symptoms.2
Asthma controller medications (ie, ICSs, cromolyn sodium, long-acting β2-adrenergic-agonists [LABAs], leukotriene modifiers, nedocromil, and theophylline) are used daily to achieve and maintain long-term control of persistent asthma. All patients with persistent asthma, regardless of disease severity, should use a daily controller. Criteria for determining asthma severity and updated recommendations for the use of controller treatment in mild and moderate persistent asthma are presented in the Figure.3,5 Levels of evidence justifying NAEPP treatment recommendations are shown in Table 2.
For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma
Topics in the management of asthma in children
Recognizing the need for continual appraisal of the benefits and risks of asthma medications in children, the NAEPP Expert Panel considered new studies comparing the effectiveness of ICS monotherapy with that of as-needed SABAs and other controllers used as monotherapy in children with mild or moderate persistent asthma. In addition, the safety of long-term ICS use in children was evaluated based on vertical growth, bone mineral density, ocular toxicity, and adrenal suppression.
Effectiveness of ICSs compared with other asthma medications
Short-acting β2-adrenergic agonists. Eight studies met the eligibility criteria for evaluating the effectiveness of ICSs versus as-needed SABAs.6,10-16 Six studies (4 involving budesonide) in children 5 years and older showed that ICSs improve lung function and symptoms and reduce the need for emergency intervention compared with as-needed SABAs.1 Among all studies included in the NAEPP update, the Childhood Asthma Management Program (CAMP) Research Group Study,9 a placebo-controlled study of inhaled budesonide and nedocromil, contributed the most evidence. Studies with children 5 years and younger are limited to 2 small studies enrolling a total of 69 children.6,15 Consistent with studies of older children, these studies indicate that ICSs improve asthma control compared with as-needed SABAs.1
Cromolyn and nedocromil. Despite well-established safety profiles, cromolyn and nedocromil are no longer recommended as first-line therapy for children, even those with mild disease. New recommendations reflect the greater effectiveness of inhaled budesonide compared with nedocromil demonstrated in the CAMP study,10 and the lack of apparent benefit of cromolyn as maintenance treatment in childhood asthma reported by Tasche and colleagues in a systematic review of the literature.17
In the CAMP study, children 5 to 12 years of age receiving inhaled budesonide showed greater reductions in symptoms and albuterol use, lower rates of hospitalization and urgent care visits, and less need for additional asthma therapy and oral prednisone compared with placebo over 4 to 6 years of treatment.10 The marginal effectiveness of nedocromil demonstrated in the CAMP study mirrored that of cromolyn reported in the review of 24 randomized placebo-controlled studies by Tasche and colleagues.1,17
For children 5 years and younger, the NAEPP Expert Panel took into account 1 randomized placebo-controlled study conducted with children 2 to 5 years of age; it showed improvements in lung function, symptoms, and bronchial hyperre-activity with inhaled budesonide.9 Support for the new NAEPP recommendations preferring ICSs for preschool children is found in a more recent open-label study18 that showed greater symptom improvement and significantly lower rates of asthma exacerbations, urgent care visits, and oral prednisone use with budesonide inhalation suspension, compared with cromolyn sodium nebulizer solution (Intal Nebulizer Solution) in children 2 to 6 years of age with persistent asthma.
Leukotriene modifiers. The LTRAs zafir-lukast and montelukast are approved for use in children. According to the NAEPP Expert Panel, studies have shown only modest improvements in lung function and other asthma control outcomes with LTRA monotherapy in children as young as 6 and 2 years, respectively.1 Because studies comparing ICSs with LTRAs in children are lacking, findings of greater overall efficacy of ICSs in adults with persistent asthma have been extrapolated for use with children; clear superiority of ICSs versus LTRAs in most outcomes has resulted in the recommendation for ICSs as the preferred treatment for mild persistent asthma in children.
Long-acting β2-adrenergic agonists. There is no role for LABAs as monotherapy in asthma. No studies have compared the effectiveness of ICS versus LABA monotherapy in children younger than 5 years, and studies in older children have shown greater effectiveness of inhaled beclomethasone versus salmeterol.14,19 In the study by Verberne and colleagues, salmeterol monotherapy was associated with deterioration in FEV1.19 In a more recent study that included patients as young as 16 years, a switch from ICS to LABA treatment was associated with a significant increase in treatment failures and exacerbations.20
Theophylline. Only 1 study has compared outcomes with low-dose ICSs versus theophylline in adults and children.21 Although limited, the data support greater effectiveness of ICSs based on symptoms, bronchial hyperresponsiveness, and the need for β2-adrenergic agonists and oral corticosteroids.1
Safety of long-term ICS use in children
Systemic corticosteroids have the potential to suppress growth over the long term.2 Short-term growth studies with ICSs show an average reduction in growth velocity of 1 cm per year during the first year of treatment, but the CAMP study showed that initial reductions in growth velocity with inhaled budesonide were not maintained over a 4- to 6-year treatment period.1,10
Although catch-up growth was not observed in the CAMP study, Agertoft and Pedersen reported no effect of long-term treatment with inhaled budesonide (mean 9.2 years) on final adult height.22 Based on these long-term prospective studies of budesonide, showing only a transient reduction in growth velocity and attainment of expected final adult height, and retrospective studies including inhaled beclomethasone, the Expert Panel concluded that the ICS class is safe regarding growth effects.
According to the NAEPP Expert Panel, clinical study data for children monitored for up to 6 years strongly suggest that ICSs are safe when used at recommended doses (strength of recommendation: A).1 The panel could not rule out a potential cumulative effect of ICS use on some conditions, (eg, osteoporosis, cataracts, glaucoma) in adulthood, as sufficient long-term data are not available.
The panel did conclude that low- to medium-dose ICSs (Table 3) appear to have no serious adverse effects on bone mineral density in children.
Likewise, low- to medium-dose ICS use was not associated with the development of cataracts or glaucoma in children, although the potential for high cumulative lifetime doses of ICSs to slightly increase the prevalence of cataracts in adults and elderly patients was noted.
Strong evidence also indicates that ICS effects on adrenal function are usually clinically insignificant at low to medium doses; however, certain individuals may be at higher risk for hypothalam-ic pituitary adrenal axis effects while using conventional ICS doses.1
Although ICSs are safe when used within labeled dosing, it is still preferable to maintain doses at the lowest effective dose. In general, treatment should be reviewed every 1 to 6 months and doses reduced in a stepwise fashion when possible.1 For children showing a favorable response to treatment, a step down in dose should be considered, but not more frequently than every 3 months. If children show no clear response to treatment within 4 to 6 weeks, consider an alternative treatment or diagnosis.1
Safety of long-term ICS use in pregnant women
Uncontrolled asthma during pregnancy is associated with an increased risk of perinatal complications. 23 Since the consequences of not using asthma controllers during pregnancy can be worse than those with using them, daily controller treatment is recommended for all pregnant women with persistent asthma. 23
The American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology previously recommended cromolyn as the treatment of choice for pregnant women with mild persistent asthma. ICSs were recommended for patients whose asthma was inadequately controlled with cromolyn. 24 Beclomethasone and budesonide were the ICSs of choice for pregnant women and those who might become pregnant, with a preference for budesonide when high-dose therapy was indicated.24
These recommendations predate the 2002 NAEPP recommendations for ICSs as preferred therapy in mild persistent asthma and the 2004 NAEPP recommendations for ICSs as the first-choice controller therapy for mild persistent asthma during pregnancy. 25 Among ICSs, one (inhaled budesonide) has an FDA Pregnancy Category B rating based on studies showing no risk in pregnant women. 26,27 All other ICSs are rated Pregnancy Category C.
Based on current evidence, it seems reasonable to consider whether budesonide should now be the preferred therapy for mild persistent asthma during pregnancy.
Effects of early treatment on asthma progression
The potential for early ICS intervention to prevent progression of mild or moderate persistent asthma was evaluated solely with data from children enrolled in the CAMP study. 10 The NAEPP Expert Panel concluded that CAMP study data do not support a progressive decline in lung function in children aged 5 to 12 years with mild or moderate persistent asthma, but do suggest that lung function decline is influenced by age of asthma onset.
According to the panel, CAMP data suggest that most deficits in lung function growth due to childhood asthma occur during the first 3 years of life. Preliminary results of the recent START study (Inhaled Steroid Treatment As Regular Therapy in Early Asthma), 28 conducted with 7165 corticosteroidnaïve patients 5 to 66 years of age with recent onset mild persistent asthma, did show a decline in lung function in patients with mild persistent disease.
Although improvements in prebronchodilator and postbronchodilator FEV1 were significant after 3 years of treatment with inhaled budes-onide, differences from placebo in both outcomes were greatest after the first year. When patients with mild persistent disease inhaled budesonide once daily in addition to normal treatment within 2 years of asthma onset,28 they enjoyed considerable protection from severe and life-threatening asthma exacerbations and overall greater asthma control.
- Budesonide • Pulmicort
- Rhinocort Cromolyn • Intal
- Fluticasone • Flovent
- Formoterol • Foradil
- Montelukast • Singulair
- Nedocromil • Tilade
- Salmeterol • Servent
- Triamcinolone acetonide • Azmacort
- Zafirlukast • Accolate
Corresponding author
Gregory J. Redding, MD, Children’s Hospital and Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105-0371. E-mail: [email protected].
1. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics–2002. J Allergy Clin Immunol 2002;110(5 suppl):S141-S219.
2. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health; 1997. Publication 97;4051.-
3. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
4. Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403-1406.
5. Oxford Centre for Evidence-based Medicine Levels of Evidence Available atwww.cebm.net/levels_faq.asp . Accessed January 8, 2004.
6. Connett GJ, Warde C, Wooler E, Lenney W. Use of budes-onide in severe asthmatics aged 1–3 years. Arch Dis Child 1993;69:351-355.
7. de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996;98:14-20.
8. Bisgaard H, Gillies J, Groenewald M, Maden C, . for an International Study Group The effect of inhaled fluticas-one propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med 1999;160:126-131.
9. Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and metha-choline responsiveness in 2- to 5-year–old asthmatic children. Am J Respir Crit Care Med 2000;162:1500-1506.
10. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-1063.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373-381.
12. Hoekstra MO, Grol MH, Bouman K, Stijnen T, Koëter GH, Kauffman HF, et al. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med 1996;154:1039-1044.
13. Jónasson G, Carlsen K-H, Blomqvist P. Clinical efficacy of low-dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. Eur Respir J 1998;12:1099-1104.
14. Simons FER and the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-1665.
15. Storr J, Lenney CA, Lenney W. Nebulized beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986;61:270-273.
16. Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. and the Dutch Chronic Non-Specific Lung Disease Study Group Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547-554.
17. Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913-920.
18. Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002;109:866-872.
19. Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. and the Dutch Paediatric Asthma Study Group Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med 1998;158:213-219.
20. Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, , et al. for the Asthma Clinical Research Network of the National Heart Lung and Blood Institute. Long-acting 2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583-2593.
21. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. and the American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild or moderate persistent asthma. J Allergy Clin Immunol 1998;101:14-23.
22. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-1069.
23. National Asthma Education Program (NAEP). Report of the Working Group on Asthma and Pregnancy: Management of Asthma during Pregnancy. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health, 1993. NIH Publication No. 96-141593.
24. American College of Obstetricians and Gynecologists (ACOG) and the American College of Allergy, Asthmaand Immunology (ACAAI). The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
25. National Asthma Education and Prevention Program. NAEPP Expert Panel Report. Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment—Update 2004. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health. NIH Publication No. 04-5246. March 2004.
26. Källén B, Rydhstroem H, Äberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
27. Ericson A, Källén B. Use of drugs during pregnancy—unique Swedish registration method that can be improved. Information From the Swedish Medical Products Agency 1999;1:8-11.
28. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y-Z, Ohlsson SV, et al. for the START Investigators Group. Early intervention with budesonide in mild persistent asthma. Lancet 2003;361:1071-1076.
1. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics–2002. J Allergy Clin Immunol 2002;110(5 suppl):S141-S219.
2. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health; 1997. Publication 97;4051.-
3. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
4. Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403-1406.
5. Oxford Centre for Evidence-based Medicine Levels of Evidence Available atwww.cebm.net/levels_faq.asp . Accessed January 8, 2004.
6. Connett GJ, Warde C, Wooler E, Lenney W. Use of budes-onide in severe asthmatics aged 1–3 years. Arch Dis Child 1993;69:351-355.
7. de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996;98:14-20.
8. Bisgaard H, Gillies J, Groenewald M, Maden C, . for an International Study Group The effect of inhaled fluticas-one propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med 1999;160:126-131.
9. Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and metha-choline responsiveness in 2- to 5-year–old asthmatic children. Am J Respir Crit Care Med 2000;162:1500-1506.
10. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-1063.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373-381.
12. Hoekstra MO, Grol MH, Bouman K, Stijnen T, Koëter GH, Kauffman HF, et al. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med 1996;154:1039-1044.
13. Jónasson G, Carlsen K-H, Blomqvist P. Clinical efficacy of low-dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. Eur Respir J 1998;12:1099-1104.
14. Simons FER and the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-1665.
15. Storr J, Lenney CA, Lenney W. Nebulized beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986;61:270-273.
16. Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. and the Dutch Chronic Non-Specific Lung Disease Study Group Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547-554.
17. Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913-920.
18. Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002;109:866-872.
19. Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. and the Dutch Paediatric Asthma Study Group Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med 1998;158:213-219.
20. Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, , et al. for the Asthma Clinical Research Network of the National Heart Lung and Blood Institute. Long-acting 2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583-2593.
21. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. and the American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild or moderate persistent asthma. J Allergy Clin Immunol 1998;101:14-23.
22. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-1069.
23. National Asthma Education Program (NAEP). Report of the Working Group on Asthma and Pregnancy: Management of Asthma during Pregnancy. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health, 1993. NIH Publication No. 96-141593.
24. American College of Obstetricians and Gynecologists (ACOG) and the American College of Allergy, Asthmaand Immunology (ACAAI). The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
25. National Asthma Education and Prevention Program. NAEPP Expert Panel Report. Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment—Update 2004. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health. NIH Publication No. 04-5246. March 2004.
26. Källén B, Rydhstroem H, Äberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
27. Ericson A, Källén B. Use of drugs during pregnancy—unique Swedish registration method that can be improved. Information From the Swedish Medical Products Agency 1999;1:8-11.
28. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y-Z, Ohlsson SV, et al. for the START Investigators Group. Early intervention with budesonide in mild persistent asthma. Lancet 2003;361:1071-1076.
Do inhaled beta-agonists control cough in URIs or acute bronchitis?
Patients who receive inhaled beta-agonists for cough due to acute upper respiratory infections (URI) are just as likely to report a productive cough at 7 days compared with patients treated with placebo (strength of recommendation [SOR]: A, based on a systematic review).
One trial, however, showed a reduction in overall cough at 7 days (number needed to treat [NNT]=3, SOR: B, a small randomized controlled trial), and another trial found a reduction in overall symptom score in smokers and those with wheezing on initial exam (SOR: B, based on a small randomized controlled trial).
Evidence summary
No studies of inhaled beta-agonists have been conducted with patients who have an explicit diagnosis of acute cough due to URI. While some clinicians feel a distinction between URI and acute bronchitis should be made, there is significant overlap between these diagnoses in clinical practice, as well as in the available studies.
A systematic review looking at beta-agonists for acute bronchitis included the clinical diagnoses of both acute bronchitis and acute cough because a standard definition of bronchitis is lacking.1 Only two trials in this review examined inhaled beta-agonists. When results from these trials were combined for the outcome of productive cough at 7 days, inhaled beta-agonists showed no benefit. However, the authors note that details of the individual trials may help to clarify the effect of inhaled beta-agonists.
One trial, a randomized controlled trial of adult patients with acute bronchitis in 2 community-based family practices, compared 23 patients receiving albuterolin a multidose inhaler (MDI) with 23 patients receiving placebo inhaler.2 Patients were also randomized to receive erythromycin or placebo tablets. Patients with pneumonia or a history of asthma or chronic obstructive pulmonary disease (COPD) were excluded. At 7 days, 61% of patients in the albuterol group reported cough compared with 91% in the control group (P=.02, NNT=3). No statistically significant difference was seen in productive cough or night cough. Smokers responded to inhaled albuterol similarly to nonsmokers. Erythromycin had no effect on cough and side effects were similar among all groups.
The other trial was a randomized controlled trial of 80 adults with cough due to acute respiratory infection; it compared fenoterol aerosol 4 times daily with placebo.3 Inhaled fenoterol is not available in the US but is similar to albuterol. This study showed no difference in cough at 7 days (relative risk [RR]=0.83; 95% confidence interval [CI], 0.52–1.30). In a subgroup analysis, however, smokers and those wheezing on initial exam had lower overall symptom scores when treated with fenoterol.
Recommendations from others
We were unable to find any guidelines on the use of albuterol via MDI for cough from bronchitis or URIs.
Inhaled beta-agonists may aid symptoms; other outcomes may not be improved
Even without a history of lung disease, patients presenting with cough due to acute respiratory illness and with evidence of airflow obstruction (wheezing) appear to receive symptom relief from inhaled beta-agonists. Smokers may be another subgroup who benefit from treatment. However, important patient-oriented outcomes (such as reduced need for over-the-counter medicines, general well being, and return to work) do not improve. If using inhaled albuterol to treat acute cough in practice, one must also consider the financial costs and adverse effects associated with treatment.
1. Smucny J, Flynn C, Becker L, Glazier R. Beta2-agonists for acute bronchitis (Cochrane Review). In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-440.
3. Melbye H, Aasebo U, Straume B. Symptomatic effect of inhaled fenoterol in acute bronchitis: a placebo controlled double-blind study. Fam Pract 1991;8:216-222.
Patients who receive inhaled beta-agonists for cough due to acute upper respiratory infections (URI) are just as likely to report a productive cough at 7 days compared with patients treated with placebo (strength of recommendation [SOR]: A, based on a systematic review).
One trial, however, showed a reduction in overall cough at 7 days (number needed to treat [NNT]=3, SOR: B, a small randomized controlled trial), and another trial found a reduction in overall symptom score in smokers and those with wheezing on initial exam (SOR: B, based on a small randomized controlled trial).
Evidence summary
No studies of inhaled beta-agonists have been conducted with patients who have an explicit diagnosis of acute cough due to URI. While some clinicians feel a distinction between URI and acute bronchitis should be made, there is significant overlap between these diagnoses in clinical practice, as well as in the available studies.
A systematic review looking at beta-agonists for acute bronchitis included the clinical diagnoses of both acute bronchitis and acute cough because a standard definition of bronchitis is lacking.1 Only two trials in this review examined inhaled beta-agonists. When results from these trials were combined for the outcome of productive cough at 7 days, inhaled beta-agonists showed no benefit. However, the authors note that details of the individual trials may help to clarify the effect of inhaled beta-agonists.
One trial, a randomized controlled trial of adult patients with acute bronchitis in 2 community-based family practices, compared 23 patients receiving albuterolin a multidose inhaler (MDI) with 23 patients receiving placebo inhaler.2 Patients were also randomized to receive erythromycin or placebo tablets. Patients with pneumonia or a history of asthma or chronic obstructive pulmonary disease (COPD) were excluded. At 7 days, 61% of patients in the albuterol group reported cough compared with 91% in the control group (P=.02, NNT=3). No statistically significant difference was seen in productive cough or night cough. Smokers responded to inhaled albuterol similarly to nonsmokers. Erythromycin had no effect on cough and side effects were similar among all groups.
The other trial was a randomized controlled trial of 80 adults with cough due to acute respiratory infection; it compared fenoterol aerosol 4 times daily with placebo.3 Inhaled fenoterol is not available in the US but is similar to albuterol. This study showed no difference in cough at 7 days (relative risk [RR]=0.83; 95% confidence interval [CI], 0.52–1.30). In a subgroup analysis, however, smokers and those wheezing on initial exam had lower overall symptom scores when treated with fenoterol.
Recommendations from others
We were unable to find any guidelines on the use of albuterol via MDI for cough from bronchitis or URIs.
Inhaled beta-agonists may aid symptoms; other outcomes may not be improved
Even without a history of lung disease, patients presenting with cough due to acute respiratory illness and with evidence of airflow obstruction (wheezing) appear to receive symptom relief from inhaled beta-agonists. Smokers may be another subgroup who benefit from treatment. However, important patient-oriented outcomes (such as reduced need for over-the-counter medicines, general well being, and return to work) do not improve. If using inhaled albuterol to treat acute cough in practice, one must also consider the financial costs and adverse effects associated with treatment.
Patients who receive inhaled beta-agonists for cough due to acute upper respiratory infections (URI) are just as likely to report a productive cough at 7 days compared with patients treated with placebo (strength of recommendation [SOR]: A, based on a systematic review).
One trial, however, showed a reduction in overall cough at 7 days (number needed to treat [NNT]=3, SOR: B, a small randomized controlled trial), and another trial found a reduction in overall symptom score in smokers and those with wheezing on initial exam (SOR: B, based on a small randomized controlled trial).
Evidence summary
No studies of inhaled beta-agonists have been conducted with patients who have an explicit diagnosis of acute cough due to URI. While some clinicians feel a distinction between URI and acute bronchitis should be made, there is significant overlap between these diagnoses in clinical practice, as well as in the available studies.
A systematic review looking at beta-agonists for acute bronchitis included the clinical diagnoses of both acute bronchitis and acute cough because a standard definition of bronchitis is lacking.1 Only two trials in this review examined inhaled beta-agonists. When results from these trials were combined for the outcome of productive cough at 7 days, inhaled beta-agonists showed no benefit. However, the authors note that details of the individual trials may help to clarify the effect of inhaled beta-agonists.
One trial, a randomized controlled trial of adult patients with acute bronchitis in 2 community-based family practices, compared 23 patients receiving albuterolin a multidose inhaler (MDI) with 23 patients receiving placebo inhaler.2 Patients were also randomized to receive erythromycin or placebo tablets. Patients with pneumonia or a history of asthma or chronic obstructive pulmonary disease (COPD) were excluded. At 7 days, 61% of patients in the albuterol group reported cough compared with 91% in the control group (P=.02, NNT=3). No statistically significant difference was seen in productive cough or night cough. Smokers responded to inhaled albuterol similarly to nonsmokers. Erythromycin had no effect on cough and side effects were similar among all groups.
The other trial was a randomized controlled trial of 80 adults with cough due to acute respiratory infection; it compared fenoterol aerosol 4 times daily with placebo.3 Inhaled fenoterol is not available in the US but is similar to albuterol. This study showed no difference in cough at 7 days (relative risk [RR]=0.83; 95% confidence interval [CI], 0.52–1.30). In a subgroup analysis, however, smokers and those wheezing on initial exam had lower overall symptom scores when treated with fenoterol.
Recommendations from others
We were unable to find any guidelines on the use of albuterol via MDI for cough from bronchitis or URIs.
Inhaled beta-agonists may aid symptoms; other outcomes may not be improved
Even without a history of lung disease, patients presenting with cough due to acute respiratory illness and with evidence of airflow obstruction (wheezing) appear to receive symptom relief from inhaled beta-agonists. Smokers may be another subgroup who benefit from treatment. However, important patient-oriented outcomes (such as reduced need for over-the-counter medicines, general well being, and return to work) do not improve. If using inhaled albuterol to treat acute cough in practice, one must also consider the financial costs and adverse effects associated with treatment.
1. Smucny J, Flynn C, Becker L, Glazier R. Beta2-agonists for acute bronchitis (Cochrane Review). In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-440.
3. Melbye H, Aasebo U, Straume B. Symptomatic effect of inhaled fenoterol in acute bronchitis: a placebo controlled double-blind study. Fam Pract 1991;8:216-222.
1. Smucny J, Flynn C, Becker L, Glazier R. Beta2-agonists for acute bronchitis (Cochrane Review). In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-440.
3. Melbye H, Aasebo U, Straume B. Symptomatic effect of inhaled fenoterol in acute bronchitis: a placebo controlled double-blind study. Fam Pract 1991;8:216-222.
Evidence-based answers from the Family Physicians Inquiries Network
Are beta-2-agonists or anticholinergics more effective for treating COPD?
Both β2-agonists and anticholinergics appear to improve symptoms for patients with chronic obstructive pulmonary disease (COPD). Recent research indicates that adding a long-acting anti-cholinergic to a β2-agonist may improve quality of life for patients with stable COPD more than the use of β2-agonists alone.
Both drug classes increase exercise capacity and alleviate symptoms of COPD, although neither alters disease progression (strength of recommendation [SOR]: A). Combination therapy can lead to greater improvements in forced expiratory volume in 1 second (FEV1) than either drug alone (SOR: A). However, until recently there were no convincing direct head-to-head comparisons of the 2 classes, and it is unclear whether this difference is clinically significant.
Evidence summary
A review of 33 double-blind randomized placebo-controlled studies showed a significant effect of bronchodilator therapy on exercise capacity in COPD patients in about one half of studies. Anticholinergic agents had significant beneficial effects in the majority, and these effects tended to be somewhat dose-dependent. Short-acting β2-agonists improved exercise capacity in more than two thirds of the studies, but long-acting agents led to mixed outcomes. The researchers identified no superior agent between the 2 classes, citing a lack of adequate studies making a direct comparison.1
A recent Cochrane Review comparing the short-term effects of ipratropium to β2-agonists in changes in FEV1and arterial oxygen pressure (PaO2) concluded there was no evidence that the degree of bronchodilation from ipratropium was greater than that from short-acting β2-agonists.2Subjective endpoints such as dyspnea and quality of life were not assessed, and neither of the above reviews included studies focusing on long-term outcomes.
A 12-week double-blind, double-placebo-controlled parallel group study published in 2000 followed 144 patients (age 64 ± 7 years with a FEV1of 44 ±11% predicted) randomized to receive salmeterol 50 μg twice daily alone, salmeterol 50 μg twice daily plus ipratropium 40 μg 4 times daily, or placebo. Patients were assessed for changes in FEV1, daytime symptom scores, specific airway conductance, and the need for rescue medication. The study demonstrated a significant benefit from the addition of ipratropium to salmeterol in terms of reduction of airway obstruction, but not in symptom control or need for rescue medication.3 However, no patients were randomized to receive ipratropium alone, so comparison of the relative contribution of the 2 classes is limited.
A 6-month, randomized double-blind placebo-controlled study evaluating the efficacy of salmeterol 50 μg twice daily vs tiotropium (a new long-acting inhaled anticholinergic) 18 μg once daily was published in 2002. Endpoints in 623 patients were assessed using 12-hour spirometric performance, transition dyspnea index (TDI), and the St. George Respiratory Questionnaire (SGRQ). (SGRQ is a validated disease-specific instrument designed to measure impact on overall health, daily life, and perceived well-being. It measures activity limitations, symptoms, and psychosocial impact.) Tiotropium showed superiority over salmeterol in all endpoints assessed (0.14 L increase in morning FEV1vs 0.09 L, 1.02 U improvement in TDI score vs 0.24, and –5.14 U improvement of SGRQ total score from baseline vs –3.54). However, it should be noted that a difference of 1 on the TDI score was necessary to suggest a clinical benefit. While the overall difference in SGQR between tiotropium and salmeterol did not reach statistical significance, the proportion of patients in the tiotropium group that reached the clinically significant threshold of 4 units improvement in SGRQ score was significantly higher than in the salmeterol group (51% vs 40%; P<.05).4
In a similar study in 2003, 1207 patients were randomized to receive the above doses of salmeterol, tiotropium, or placebo. Over the course of 6 months, tiotropium was associated with a significant delay in onset of the first exacerbation compared with placebo, and overall it led to the fewest exacerbations per patient-year. Fewer hospital admissions were also demonstrated in the tiotropium group per patient-year, and the number of days that patients were unable to perform usual activities was lowest for the tiotropium group. Again, improvement in TDI and SGRQ scores was significantly greater with tiotropium than placebo. In almost all outcomes, the salmeterol results were intermediate between those of tiotropium and placebo, and were not statistically different from placebo.5
Recommendations from others
The GOLD (Global Strategy for the Diagnosis, Management, and Prevention of COPD) Report states that the choice between β2-agonist, anti-cholinergics, or combination therapy depends on the availability and the response of a given patient in terms of symptom relief and side effects. The 2003 GOLD Workshop Report update further recommends the use of regular treatment with long-acting bronchodilators, including tiotropium, rather than short-acting bronchodilators for moderate-to-severe COPD.6
A separate report for the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians—American Society of Internal Medicine states that both are beneficial for management of acute exacerbations, but that anticholinergics should be considered first because they are associated with fewer and more benign side effects.7
Patient response and tolerance of side effects determine which drug class to use
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Although recent national guidelines for the management of COPD, such as the GOLD report, give more cohesiveness to treatment strategies for patients with COPD, there is still room for tailoring a treatment approach. I find that when choosing between beta-agonists and anticholinergics, patient response and tolerability of side effects determine what I use.
This Clinical Inquiry supports my clinical impression that neither class of drug is significantly superior to the other in regards to COPD outcome measures. In my experience, when neither drug offers a clear advantage, factors affecting compliance and tolerability tend to determine how effective it is for my patients. Therefore, a trial of either class seems reasonable at first and follow-up determines what is used in the long run.
1. Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608.
2. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library, Issue 1,2004. Chichester, UK: John Wiley, Ltd; 2003.
3. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000;15:878-885.
4. Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55.
5. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404.
6. Fabbri LM, Hurd SS. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
7. Snow V, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
Both β2-agonists and anticholinergics appear to improve symptoms for patients with chronic obstructive pulmonary disease (COPD). Recent research indicates that adding a long-acting anti-cholinergic to a β2-agonist may improve quality of life for patients with stable COPD more than the use of β2-agonists alone.
Both drug classes increase exercise capacity and alleviate symptoms of COPD, although neither alters disease progression (strength of recommendation [SOR]: A). Combination therapy can lead to greater improvements in forced expiratory volume in 1 second (FEV1) than either drug alone (SOR: A). However, until recently there were no convincing direct head-to-head comparisons of the 2 classes, and it is unclear whether this difference is clinically significant.
Evidence summary
A review of 33 double-blind randomized placebo-controlled studies showed a significant effect of bronchodilator therapy on exercise capacity in COPD patients in about one half of studies. Anticholinergic agents had significant beneficial effects in the majority, and these effects tended to be somewhat dose-dependent. Short-acting β2-agonists improved exercise capacity in more than two thirds of the studies, but long-acting agents led to mixed outcomes. The researchers identified no superior agent between the 2 classes, citing a lack of adequate studies making a direct comparison.1
A recent Cochrane Review comparing the short-term effects of ipratropium to β2-agonists in changes in FEV1and arterial oxygen pressure (PaO2) concluded there was no evidence that the degree of bronchodilation from ipratropium was greater than that from short-acting β2-agonists.2Subjective endpoints such as dyspnea and quality of life were not assessed, and neither of the above reviews included studies focusing on long-term outcomes.
A 12-week double-blind, double-placebo-controlled parallel group study published in 2000 followed 144 patients (age 64 ± 7 years with a FEV1of 44 ±11% predicted) randomized to receive salmeterol 50 μg twice daily alone, salmeterol 50 μg twice daily plus ipratropium 40 μg 4 times daily, or placebo. Patients were assessed for changes in FEV1, daytime symptom scores, specific airway conductance, and the need for rescue medication. The study demonstrated a significant benefit from the addition of ipratropium to salmeterol in terms of reduction of airway obstruction, but not in symptom control or need for rescue medication.3 However, no patients were randomized to receive ipratropium alone, so comparison of the relative contribution of the 2 classes is limited.
A 6-month, randomized double-blind placebo-controlled study evaluating the efficacy of salmeterol 50 μg twice daily vs tiotropium (a new long-acting inhaled anticholinergic) 18 μg once daily was published in 2002. Endpoints in 623 patients were assessed using 12-hour spirometric performance, transition dyspnea index (TDI), and the St. George Respiratory Questionnaire (SGRQ). (SGRQ is a validated disease-specific instrument designed to measure impact on overall health, daily life, and perceived well-being. It measures activity limitations, symptoms, and psychosocial impact.) Tiotropium showed superiority over salmeterol in all endpoints assessed (0.14 L increase in morning FEV1vs 0.09 L, 1.02 U improvement in TDI score vs 0.24, and –5.14 U improvement of SGRQ total score from baseline vs –3.54). However, it should be noted that a difference of 1 on the TDI score was necessary to suggest a clinical benefit. While the overall difference in SGQR between tiotropium and salmeterol did not reach statistical significance, the proportion of patients in the tiotropium group that reached the clinically significant threshold of 4 units improvement in SGRQ score was significantly higher than in the salmeterol group (51% vs 40%; P<.05).4
In a similar study in 2003, 1207 patients were randomized to receive the above doses of salmeterol, tiotropium, or placebo. Over the course of 6 months, tiotropium was associated with a significant delay in onset of the first exacerbation compared with placebo, and overall it led to the fewest exacerbations per patient-year. Fewer hospital admissions were also demonstrated in the tiotropium group per patient-year, and the number of days that patients were unable to perform usual activities was lowest for the tiotropium group. Again, improvement in TDI and SGRQ scores was significantly greater with tiotropium than placebo. In almost all outcomes, the salmeterol results were intermediate between those of tiotropium and placebo, and were not statistically different from placebo.5
Recommendations from others
The GOLD (Global Strategy for the Diagnosis, Management, and Prevention of COPD) Report states that the choice between β2-agonist, anti-cholinergics, or combination therapy depends on the availability and the response of a given patient in terms of symptom relief and side effects. The 2003 GOLD Workshop Report update further recommends the use of regular treatment with long-acting bronchodilators, including tiotropium, rather than short-acting bronchodilators for moderate-to-severe COPD.6
A separate report for the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians—American Society of Internal Medicine states that both are beneficial for management of acute exacerbations, but that anticholinergics should be considered first because they are associated with fewer and more benign side effects.7
Patient response and tolerance of side effects determine which drug class to use
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Although recent national guidelines for the management of COPD, such as the GOLD report, give more cohesiveness to treatment strategies for patients with COPD, there is still room for tailoring a treatment approach. I find that when choosing between beta-agonists and anticholinergics, patient response and tolerability of side effects determine what I use.
This Clinical Inquiry supports my clinical impression that neither class of drug is significantly superior to the other in regards to COPD outcome measures. In my experience, when neither drug offers a clear advantage, factors affecting compliance and tolerability tend to determine how effective it is for my patients. Therefore, a trial of either class seems reasonable at first and follow-up determines what is used in the long run.
Both β2-agonists and anticholinergics appear to improve symptoms for patients with chronic obstructive pulmonary disease (COPD). Recent research indicates that adding a long-acting anti-cholinergic to a β2-agonist may improve quality of life for patients with stable COPD more than the use of β2-agonists alone.
Both drug classes increase exercise capacity and alleviate symptoms of COPD, although neither alters disease progression (strength of recommendation [SOR]: A). Combination therapy can lead to greater improvements in forced expiratory volume in 1 second (FEV1) than either drug alone (SOR: A). However, until recently there were no convincing direct head-to-head comparisons of the 2 classes, and it is unclear whether this difference is clinically significant.
Evidence summary
A review of 33 double-blind randomized placebo-controlled studies showed a significant effect of bronchodilator therapy on exercise capacity in COPD patients in about one half of studies. Anticholinergic agents had significant beneficial effects in the majority, and these effects tended to be somewhat dose-dependent. Short-acting β2-agonists improved exercise capacity in more than two thirds of the studies, but long-acting agents led to mixed outcomes. The researchers identified no superior agent between the 2 classes, citing a lack of adequate studies making a direct comparison.1
A recent Cochrane Review comparing the short-term effects of ipratropium to β2-agonists in changes in FEV1and arterial oxygen pressure (PaO2) concluded there was no evidence that the degree of bronchodilation from ipratropium was greater than that from short-acting β2-agonists.2Subjective endpoints such as dyspnea and quality of life were not assessed, and neither of the above reviews included studies focusing on long-term outcomes.
A 12-week double-blind, double-placebo-controlled parallel group study published in 2000 followed 144 patients (age 64 ± 7 years with a FEV1of 44 ±11% predicted) randomized to receive salmeterol 50 μg twice daily alone, salmeterol 50 μg twice daily plus ipratropium 40 μg 4 times daily, or placebo. Patients were assessed for changes in FEV1, daytime symptom scores, specific airway conductance, and the need for rescue medication. The study demonstrated a significant benefit from the addition of ipratropium to salmeterol in terms of reduction of airway obstruction, but not in symptom control or need for rescue medication.3 However, no patients were randomized to receive ipratropium alone, so comparison of the relative contribution of the 2 classes is limited.
A 6-month, randomized double-blind placebo-controlled study evaluating the efficacy of salmeterol 50 μg twice daily vs tiotropium (a new long-acting inhaled anticholinergic) 18 μg once daily was published in 2002. Endpoints in 623 patients were assessed using 12-hour spirometric performance, transition dyspnea index (TDI), and the St. George Respiratory Questionnaire (SGRQ). (SGRQ is a validated disease-specific instrument designed to measure impact on overall health, daily life, and perceived well-being. It measures activity limitations, symptoms, and psychosocial impact.) Tiotropium showed superiority over salmeterol in all endpoints assessed (0.14 L increase in morning FEV1vs 0.09 L, 1.02 U improvement in TDI score vs 0.24, and –5.14 U improvement of SGRQ total score from baseline vs –3.54). However, it should be noted that a difference of 1 on the TDI score was necessary to suggest a clinical benefit. While the overall difference in SGQR between tiotropium and salmeterol did not reach statistical significance, the proportion of patients in the tiotropium group that reached the clinically significant threshold of 4 units improvement in SGRQ score was significantly higher than in the salmeterol group (51% vs 40%; P<.05).4
In a similar study in 2003, 1207 patients were randomized to receive the above doses of salmeterol, tiotropium, or placebo. Over the course of 6 months, tiotropium was associated with a significant delay in onset of the first exacerbation compared with placebo, and overall it led to the fewest exacerbations per patient-year. Fewer hospital admissions were also demonstrated in the tiotropium group per patient-year, and the number of days that patients were unable to perform usual activities was lowest for the tiotropium group. Again, improvement in TDI and SGRQ scores was significantly greater with tiotropium than placebo. In almost all outcomes, the salmeterol results were intermediate between those of tiotropium and placebo, and were not statistically different from placebo.5
Recommendations from others
The GOLD (Global Strategy for the Diagnosis, Management, and Prevention of COPD) Report states that the choice between β2-agonist, anti-cholinergics, or combination therapy depends on the availability and the response of a given patient in terms of symptom relief and side effects. The 2003 GOLD Workshop Report update further recommends the use of regular treatment with long-acting bronchodilators, including tiotropium, rather than short-acting bronchodilators for moderate-to-severe COPD.6
A separate report for the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians—American Society of Internal Medicine states that both are beneficial for management of acute exacerbations, but that anticholinergics should be considered first because they are associated with fewer and more benign side effects.7
Patient response and tolerance of side effects determine which drug class to use
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Although recent national guidelines for the management of COPD, such as the GOLD report, give more cohesiveness to treatment strategies for patients with COPD, there is still room for tailoring a treatment approach. I find that when choosing between beta-agonists and anticholinergics, patient response and tolerability of side effects determine what I use.
This Clinical Inquiry supports my clinical impression that neither class of drug is significantly superior to the other in regards to COPD outcome measures. In my experience, when neither drug offers a clear advantage, factors affecting compliance and tolerability tend to determine how effective it is for my patients. Therefore, a trial of either class seems reasonable at first and follow-up determines what is used in the long run.
1. Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608.
2. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library, Issue 1,2004. Chichester, UK: John Wiley, Ltd; 2003.
3. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000;15:878-885.
4. Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55.
5. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404.
6. Fabbri LM, Hurd SS. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
7. Snow V, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
1. Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608.
2. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library, Issue 1,2004. Chichester, UK: John Wiley, Ltd; 2003.
3. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000;15:878-885.
4. Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55.
5. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404.
6. Fabbri LM, Hurd SS. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
7. Snow V, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
Evidence-based answers from the Family Physicians Inquiries Network