User login
How should we evaluate a solitary pulmonary nodule found on chest x-ray?
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
How effective are leukotriene inhibitors for asthma in children?
Evidence on the use of leukotriene inhibitors in children is insufficient to permit conclusions regarding efficacy. Given the proven efficacy of inhaled corticosteroids in asthma management, leukotriene inhibitors should not replace inhaled corticosteroids for maintenance of asthma in children (strength of recommendation: B).
Current guidelines that list leukotriene inhibitors as a potential addition or alternative to corticosteroid therapy in children with asthma appear to be based on scant studies and extrapolation from adult research.
Evidence summary
Asthma is characterized by inflammation of the bronchial airways. Leukotrienes are potent mediators of inflammation and are believed to contribute significantly to the inflammatory pathophysiology of asthma. Leukotriene inhibitors interfere with leukotriene production or leukotriene receptors and thus inhibit inflammation.1
Leukotriene inhibitors are administered orally, a significant advantage over inhalation in the pediatric population. For children, the theoretical corticosteroid-sparing effect of leukotriene inhibitors is appealing but has not been demonstrated.
In January 2002, Cochrane reviewers identified 3 studies of leukotriene inhibitor use in children that met their quality criteria for meta-analysis. Unfortunately, recent changes in asthma classification terminology make it difficult to precisely translate past studies into current practice. Based on these studies, the Cochrane reviewers concluded there is insufficient evidence to support the use of leukotriene inhibitors in children as monotherapy or as an addition to corticosteroids.1,2
One randomized, double-blind crossover study of 279 children with corticosteroid-dependent (persistent) asthma compared montelukast 5 mg (Singulair) once a day plus inhaled budesonide 200 μg (Pulmicort) twice a day with placebo plus budesonide (Rhinocort). Each study period lasted only 4 weeks, starting after a 4-week run-in period. Montelukast modestly improved asthma control over placebo. Compared with the placebo period, montelukast decreased the average use of beta-agonists by 1 puff per day. Asthma exacerbation days decreased by about 1 per month during montelukast treatment. The effects of montelukast and placebo on forced expiratory volume in 1 second (FEV1), quality of life, and adverse events did not differ significantly.3
One randomized, open-label crossover study of 124 children with “mild” asthma found that montelukast provided equivalent control and superior patient and parent satisfaction when compared with inhaled corticosteroids. Outcomes assessed were FEV1, school and work loss, medical resource utilization, safety, and patient and parent satisfaction. Children entering this study were self-selected to extend participation from a previous larger study that did not meet Cochrane quality criteria for inclusion in meta-analysis. The authors acknowledge the potential for selection bias.4
A randomized, double-blind, placebo-controlled study of 338 patients aged 12 years to adult compared zafirlukast (Accolate) with fluticasone propionate (Flovent) for control of persistent asthma. This study concluded that fluticasone was superior for all clinical outcomes measured including symptom scores, albuterol use, nighttime awakenings pulmonary function, and number of exacerbations requiring oral corticosteroids. Pooling of adult and adolescent cases in this study limits generalized application of these results to pediatric practice.5
Recommendations from others
The National Asthma Education and Prevention Program6 and the Global Initiative for Asthma7 guidelines conclude that inhaled corticosteroid, at the lowest effective dose, is the preferred therapy for children of all ages with persistent asthma whether mild, moderate, or severe.
Both guidelines list leukotriene inhibitors as a potential adjunct to corticosteroids for moderate persistent asthma, as an alternative to corticosteroids plus long-acting beta2-agonist. The guidelines also list leukotriene inhibitors as an alternative treatment to inhaled corticosteroids for mild persistent asthma in patients aged >5 years. Montelukast (Singulair) is approved for use in children aged ≥12 months, zafirlukast (Accolate) is approved for children aged≥5 years, and zileuton (Zyflo) is approved only for children aged >12 years.
An inhaled corticosteroid controller should be the first step
Lawrence S. Slotnick, MD
Moses Cone Health System, Greensboro, NC
Until evidence supports a different conclusion, I think we should continue to follow current national and global guidelines. The most important concept in both is that once a child is diagnosed with persistent asthma, starting an inhaled corticosteroid controller should be the first step.
Leukotriene inhibitors should be considered as second or third choice as a controller. The main indications for using a leukotriene inhibitor are aspirin-sensitive, exerciseinduced, and nocturnal asthma. I would use a leukotriene inhibitor as a controller only if a patient could not comply with inhaled corticosteroids.
1. Ducharme F, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteriods for chronic asthma. Cochrane Database Syst Rev 2002;(1):CD003133.-
2. Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled coritcosteriods in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD002314-
3. Simons FE, Villa JR, Lee BW G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001;138:694-698.
4. Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclamethasone in 6- to 11- year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction and adherence with therapy. Curr Med Res Opin. 2001;17:96-104.
5. Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001;50:595-602.
6. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. 1997 (rev 2002). Available at: www.nhlbi.nih.gov/guidelines/asthma/. Accessed on March 5, 2004.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Rockville, Md: National Heart, Lung, and Blood Institute. 1995 (revised 2002). Available at: www.ginasthma.com/wr.html. Accessed on March 5, 2004.
Evidence on the use of leukotriene inhibitors in children is insufficient to permit conclusions regarding efficacy. Given the proven efficacy of inhaled corticosteroids in asthma management, leukotriene inhibitors should not replace inhaled corticosteroids for maintenance of asthma in children (strength of recommendation: B).
Current guidelines that list leukotriene inhibitors as a potential addition or alternative to corticosteroid therapy in children with asthma appear to be based on scant studies and extrapolation from adult research.
Evidence summary
Asthma is characterized by inflammation of the bronchial airways. Leukotrienes are potent mediators of inflammation and are believed to contribute significantly to the inflammatory pathophysiology of asthma. Leukotriene inhibitors interfere with leukotriene production or leukotriene receptors and thus inhibit inflammation.1
Leukotriene inhibitors are administered orally, a significant advantage over inhalation in the pediatric population. For children, the theoretical corticosteroid-sparing effect of leukotriene inhibitors is appealing but has not been demonstrated.
In January 2002, Cochrane reviewers identified 3 studies of leukotriene inhibitor use in children that met their quality criteria for meta-analysis. Unfortunately, recent changes in asthma classification terminology make it difficult to precisely translate past studies into current practice. Based on these studies, the Cochrane reviewers concluded there is insufficient evidence to support the use of leukotriene inhibitors in children as monotherapy or as an addition to corticosteroids.1,2
One randomized, double-blind crossover study of 279 children with corticosteroid-dependent (persistent) asthma compared montelukast 5 mg (Singulair) once a day plus inhaled budesonide 200 μg (Pulmicort) twice a day with placebo plus budesonide (Rhinocort). Each study period lasted only 4 weeks, starting after a 4-week run-in period. Montelukast modestly improved asthma control over placebo. Compared with the placebo period, montelukast decreased the average use of beta-agonists by 1 puff per day. Asthma exacerbation days decreased by about 1 per month during montelukast treatment. The effects of montelukast and placebo on forced expiratory volume in 1 second (FEV1), quality of life, and adverse events did not differ significantly.3
One randomized, open-label crossover study of 124 children with “mild” asthma found that montelukast provided equivalent control and superior patient and parent satisfaction when compared with inhaled corticosteroids. Outcomes assessed were FEV1, school and work loss, medical resource utilization, safety, and patient and parent satisfaction. Children entering this study were self-selected to extend participation from a previous larger study that did not meet Cochrane quality criteria for inclusion in meta-analysis. The authors acknowledge the potential for selection bias.4
A randomized, double-blind, placebo-controlled study of 338 patients aged 12 years to adult compared zafirlukast (Accolate) with fluticasone propionate (Flovent) for control of persistent asthma. This study concluded that fluticasone was superior for all clinical outcomes measured including symptom scores, albuterol use, nighttime awakenings pulmonary function, and number of exacerbations requiring oral corticosteroids. Pooling of adult and adolescent cases in this study limits generalized application of these results to pediatric practice.5
Recommendations from others
The National Asthma Education and Prevention Program6 and the Global Initiative for Asthma7 guidelines conclude that inhaled corticosteroid, at the lowest effective dose, is the preferred therapy for children of all ages with persistent asthma whether mild, moderate, or severe.
Both guidelines list leukotriene inhibitors as a potential adjunct to corticosteroids for moderate persistent asthma, as an alternative to corticosteroids plus long-acting beta2-agonist. The guidelines also list leukotriene inhibitors as an alternative treatment to inhaled corticosteroids for mild persistent asthma in patients aged >5 years. Montelukast (Singulair) is approved for use in children aged ≥12 months, zafirlukast (Accolate) is approved for children aged≥5 years, and zileuton (Zyflo) is approved only for children aged >12 years.
An inhaled corticosteroid controller should be the first step
Lawrence S. Slotnick, MD
Moses Cone Health System, Greensboro, NC
Until evidence supports a different conclusion, I think we should continue to follow current national and global guidelines. The most important concept in both is that once a child is diagnosed with persistent asthma, starting an inhaled corticosteroid controller should be the first step.
Leukotriene inhibitors should be considered as second or third choice as a controller. The main indications for using a leukotriene inhibitor are aspirin-sensitive, exerciseinduced, and nocturnal asthma. I would use a leukotriene inhibitor as a controller only if a patient could not comply with inhaled corticosteroids.
Evidence on the use of leukotriene inhibitors in children is insufficient to permit conclusions regarding efficacy. Given the proven efficacy of inhaled corticosteroids in asthma management, leukotriene inhibitors should not replace inhaled corticosteroids for maintenance of asthma in children (strength of recommendation: B).
Current guidelines that list leukotriene inhibitors as a potential addition or alternative to corticosteroid therapy in children with asthma appear to be based on scant studies and extrapolation from adult research.
Evidence summary
Asthma is characterized by inflammation of the bronchial airways. Leukotrienes are potent mediators of inflammation and are believed to contribute significantly to the inflammatory pathophysiology of asthma. Leukotriene inhibitors interfere with leukotriene production or leukotriene receptors and thus inhibit inflammation.1
Leukotriene inhibitors are administered orally, a significant advantage over inhalation in the pediatric population. For children, the theoretical corticosteroid-sparing effect of leukotriene inhibitors is appealing but has not been demonstrated.
In January 2002, Cochrane reviewers identified 3 studies of leukotriene inhibitor use in children that met their quality criteria for meta-analysis. Unfortunately, recent changes in asthma classification terminology make it difficult to precisely translate past studies into current practice. Based on these studies, the Cochrane reviewers concluded there is insufficient evidence to support the use of leukotriene inhibitors in children as monotherapy or as an addition to corticosteroids.1,2
One randomized, double-blind crossover study of 279 children with corticosteroid-dependent (persistent) asthma compared montelukast 5 mg (Singulair) once a day plus inhaled budesonide 200 μg (Pulmicort) twice a day with placebo plus budesonide (Rhinocort). Each study period lasted only 4 weeks, starting after a 4-week run-in period. Montelukast modestly improved asthma control over placebo. Compared with the placebo period, montelukast decreased the average use of beta-agonists by 1 puff per day. Asthma exacerbation days decreased by about 1 per month during montelukast treatment. The effects of montelukast and placebo on forced expiratory volume in 1 second (FEV1), quality of life, and adverse events did not differ significantly.3
One randomized, open-label crossover study of 124 children with “mild” asthma found that montelukast provided equivalent control and superior patient and parent satisfaction when compared with inhaled corticosteroids. Outcomes assessed were FEV1, school and work loss, medical resource utilization, safety, and patient and parent satisfaction. Children entering this study were self-selected to extend participation from a previous larger study that did not meet Cochrane quality criteria for inclusion in meta-analysis. The authors acknowledge the potential for selection bias.4
A randomized, double-blind, placebo-controlled study of 338 patients aged 12 years to adult compared zafirlukast (Accolate) with fluticasone propionate (Flovent) for control of persistent asthma. This study concluded that fluticasone was superior for all clinical outcomes measured including symptom scores, albuterol use, nighttime awakenings pulmonary function, and number of exacerbations requiring oral corticosteroids. Pooling of adult and adolescent cases in this study limits generalized application of these results to pediatric practice.5
Recommendations from others
The National Asthma Education and Prevention Program6 and the Global Initiative for Asthma7 guidelines conclude that inhaled corticosteroid, at the lowest effective dose, is the preferred therapy for children of all ages with persistent asthma whether mild, moderate, or severe.
Both guidelines list leukotriene inhibitors as a potential adjunct to corticosteroids for moderate persistent asthma, as an alternative to corticosteroids plus long-acting beta2-agonist. The guidelines also list leukotriene inhibitors as an alternative treatment to inhaled corticosteroids for mild persistent asthma in patients aged >5 years. Montelukast (Singulair) is approved for use in children aged ≥12 months, zafirlukast (Accolate) is approved for children aged≥5 years, and zileuton (Zyflo) is approved only for children aged >12 years.
An inhaled corticosteroid controller should be the first step
Lawrence S. Slotnick, MD
Moses Cone Health System, Greensboro, NC
Until evidence supports a different conclusion, I think we should continue to follow current national and global guidelines. The most important concept in both is that once a child is diagnosed with persistent asthma, starting an inhaled corticosteroid controller should be the first step.
Leukotriene inhibitors should be considered as second or third choice as a controller. The main indications for using a leukotriene inhibitor are aspirin-sensitive, exerciseinduced, and nocturnal asthma. I would use a leukotriene inhibitor as a controller only if a patient could not comply with inhaled corticosteroids.
1. Ducharme F, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteriods for chronic asthma. Cochrane Database Syst Rev 2002;(1):CD003133.-
2. Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled coritcosteriods in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD002314-
3. Simons FE, Villa JR, Lee BW G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001;138:694-698.
4. Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclamethasone in 6- to 11- year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction and adherence with therapy. Curr Med Res Opin. 2001;17:96-104.
5. Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001;50:595-602.
6. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. 1997 (rev 2002). Available at: www.nhlbi.nih.gov/guidelines/asthma/. Accessed on March 5, 2004.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Rockville, Md: National Heart, Lung, and Blood Institute. 1995 (revised 2002). Available at: www.ginasthma.com/wr.html. Accessed on March 5, 2004.
1. Ducharme F, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteriods for chronic asthma. Cochrane Database Syst Rev 2002;(1):CD003133.-
2. Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled coritcosteriods in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD002314-
3. Simons FE, Villa JR, Lee BW G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001;138:694-698.
4. Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclamethasone in 6- to 11- year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction and adherence with therapy. Curr Med Res Opin. 2001;17:96-104.
5. Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001;50:595-602.
6. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. 1997 (rev 2002). Available at: www.nhlbi.nih.gov/guidelines/asthma/. Accessed on March 5, 2004.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Rockville, Md: National Heart, Lung, and Blood Institute. 1995 (revised 2002). Available at: www.ginasthma.com/wr.html. Accessed on March 5, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Do systemic corticosteroids lessen symptoms in acute exacerbations of COPD?
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
Evidence-based answers from the Family Physicians Inquiries Network
How should we manage an acute exacerbation of COPD?
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
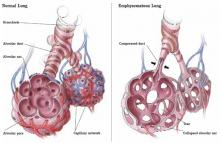
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
Nortriptyline effective for smoking cessation
ABSTRACT
BACKGROUND: Despite public health campaigns and recommendations from national organizations for clinicians to encourage smoking cessation, only 2.5% of all smokers succeed in abstaining for 1 year. Currently, bupropion (Zyban) is an antidepressant approved by the FDA as an aid in smoking cessation. This study evaluated the safety and efficacy of another antidepressant, nortriptyline (Pamelor), in smokers enrolled in a smoking support group.
POPULATION STUDIED: Patients were participating in a hospital-based smoking support group in Brazil. They were in good general health, aged 18 to 65 years, and smoked more than 15 cigarettes per day. Degree of nicotine dependence was determined by the Fagerstrom questionnaire. Patients were excluded if they were depressed as determined by the Beck Depression Inventory or had a history of other psychiatric syndromes, cardiovascular disease, glaucoma, urinary retention, thyroid disease, or epilepsy, or were pregnant or breast-feeding. Patients could not be receiving nicotine replacement therapy or have taken antidepressants, benzodiazepines, or antipsychotic agents during the past month. Of 144 patients who completed the study, most were women.
STUDY DESIGN AND VALIDITY: Patients were randomly assigned to receive either placebo or nortriptyline, increased at weekly intervals from 25 to 75 mg daily. Patients and clinicians were blinded to assigned treatment. During the 6-week treatment, all patients participated in weekly group therapy supervised by the same psychiatrist. Group therapy was based on cognitive-behavioral therapy. Follow-up abstinence rates were evaluated 6 months after the treatment period.
OUTCOMES MEASURED: Success was defined as cessation of smoking 1 week after the end of the treatment period. The rate of abstinence was also determined 6 months after the end of the study.
RESULTS: Patients receiving nortriptyline were less likely to report smoking in the week after the treatment period (55.9% vs 23.7% in the placebo group, P < .001, numbers needed to treat [NNT]=3). After 6 months of follow-up, 20.6% of patients receiving nortriptyline and 5.3% of patients receiving placebo reported to be free from nicotine use (P < .012, NNT=7). Patients most likely to respond had low nicotine dependence (Fagerstrom score less than 7 out of a possible 10) and were younger than 50 years.
Nortriptyline (Pamelor), in combination with weekly behavioral therapy, is effective in helping highly motivated smokers to quit. The medication may be an alternative for patients who cannot tolerate or do not benefit from bupropion. Given the high motivation of the group and the extensive behavioral therapy they also received, results are not likely to be as good in typical practice.
ABSTRACT
BACKGROUND: Despite public health campaigns and recommendations from national organizations for clinicians to encourage smoking cessation, only 2.5% of all smokers succeed in abstaining for 1 year. Currently, bupropion (Zyban) is an antidepressant approved by the FDA as an aid in smoking cessation. This study evaluated the safety and efficacy of another antidepressant, nortriptyline (Pamelor), in smokers enrolled in a smoking support group.
POPULATION STUDIED: Patients were participating in a hospital-based smoking support group in Brazil. They were in good general health, aged 18 to 65 years, and smoked more than 15 cigarettes per day. Degree of nicotine dependence was determined by the Fagerstrom questionnaire. Patients were excluded if they were depressed as determined by the Beck Depression Inventory or had a history of other psychiatric syndromes, cardiovascular disease, glaucoma, urinary retention, thyroid disease, or epilepsy, or were pregnant or breast-feeding. Patients could not be receiving nicotine replacement therapy or have taken antidepressants, benzodiazepines, or antipsychotic agents during the past month. Of 144 patients who completed the study, most were women.
STUDY DESIGN AND VALIDITY: Patients were randomly assigned to receive either placebo or nortriptyline, increased at weekly intervals from 25 to 75 mg daily. Patients and clinicians were blinded to assigned treatment. During the 6-week treatment, all patients participated in weekly group therapy supervised by the same psychiatrist. Group therapy was based on cognitive-behavioral therapy. Follow-up abstinence rates were evaluated 6 months after the treatment period.
OUTCOMES MEASURED: Success was defined as cessation of smoking 1 week after the end of the treatment period. The rate of abstinence was also determined 6 months after the end of the study.
RESULTS: Patients receiving nortriptyline were less likely to report smoking in the week after the treatment period (55.9% vs 23.7% in the placebo group, P < .001, numbers needed to treat [NNT]=3). After 6 months of follow-up, 20.6% of patients receiving nortriptyline and 5.3% of patients receiving placebo reported to be free from nicotine use (P < .012, NNT=7). Patients most likely to respond had low nicotine dependence (Fagerstrom score less than 7 out of a possible 10) and were younger than 50 years.
Nortriptyline (Pamelor), in combination with weekly behavioral therapy, is effective in helping highly motivated smokers to quit. The medication may be an alternative for patients who cannot tolerate or do not benefit from bupropion. Given the high motivation of the group and the extensive behavioral therapy they also received, results are not likely to be as good in typical practice.
ABSTRACT
BACKGROUND: Despite public health campaigns and recommendations from national organizations for clinicians to encourage smoking cessation, only 2.5% of all smokers succeed in abstaining for 1 year. Currently, bupropion (Zyban) is an antidepressant approved by the FDA as an aid in smoking cessation. This study evaluated the safety and efficacy of another antidepressant, nortriptyline (Pamelor), in smokers enrolled in a smoking support group.
POPULATION STUDIED: Patients were participating in a hospital-based smoking support group in Brazil. They were in good general health, aged 18 to 65 years, and smoked more than 15 cigarettes per day. Degree of nicotine dependence was determined by the Fagerstrom questionnaire. Patients were excluded if they were depressed as determined by the Beck Depression Inventory or had a history of other psychiatric syndromes, cardiovascular disease, glaucoma, urinary retention, thyroid disease, or epilepsy, or were pregnant or breast-feeding. Patients could not be receiving nicotine replacement therapy or have taken antidepressants, benzodiazepines, or antipsychotic agents during the past month. Of 144 patients who completed the study, most were women.
STUDY DESIGN AND VALIDITY: Patients were randomly assigned to receive either placebo or nortriptyline, increased at weekly intervals from 25 to 75 mg daily. Patients and clinicians were blinded to assigned treatment. During the 6-week treatment, all patients participated in weekly group therapy supervised by the same psychiatrist. Group therapy was based on cognitive-behavioral therapy. Follow-up abstinence rates were evaluated 6 months after the treatment period.
OUTCOMES MEASURED: Success was defined as cessation of smoking 1 week after the end of the treatment period. The rate of abstinence was also determined 6 months after the end of the study.
RESULTS: Patients receiving nortriptyline were less likely to report smoking in the week after the treatment period (55.9% vs 23.7% in the placebo group, P < .001, numbers needed to treat [NNT]=3). After 6 months of follow-up, 20.6% of patients receiving nortriptyline and 5.3% of patients receiving placebo reported to be free from nicotine use (P < .012, NNT=7). Patients most likely to respond had low nicotine dependence (Fagerstrom score less than 7 out of a possible 10) and were younger than 50 years.
Nortriptyline (Pamelor), in combination with weekly behavioral therapy, is effective in helping highly motivated smokers to quit. The medication may be an alternative for patients who cannot tolerate or do not benefit from bupropion. Given the high motivation of the group and the extensive behavioral therapy they also received, results are not likely to be as good in typical practice.
Vitamin E may worsen acute respiratory tract infections in the elderly
ABSTRACT
BACKGROUND: The geriatric population has a potentially increased risk of infectious diseases and related sequelae because of decreasing immunocompetency. Currently available trials assessing efficacy of multivitamins and minerals are limited and contradictory. In this study the authors compared whether daily supplementation with a multivitamin containing minerals, with or without vitamin E, or vitamin E alone, affected the incidence and severity of acute respiratory tract infections in elderly individuals.
POPULATION STUDIED: The study population included 652 noninstitutionalized men and women living in the Netherlands who were at least 60 years of age. Patients were excluded if they were taking immunosuppressive agents, anticoagulants that could interfere with vitamin K metabolism, or dietary supplements within the preceding 2 months. Additional exclusion criteria included a history of cancer, liver disease, or fat malabsorption within the preceding 5 years.
STUDY DESIGN AND VALIDITY: Allocation assignment was concealed in this randomized, placebo-controlled trial. Participants took either 2 capsules daily of a multivitamin and mineral complex; vitamin E (200 mg/dL α-tocopheryl acetate); multivitamin-mineral complex plus vitamin E; or placebo for a maximum of 15 months. Doses of multivitamins were at recommended daily allowance (RDA) levels and doses of minerals were 25% to 50% of RDA levels. Patients recorded signs and acute symptoms of respiratory tract infections using a diary. A study nurse confirmed possible respiratory tract infections based on predetermined definitions. Data analysis was performed on an intention-to-treat and per-protocol basis.
OUTCOMES MEASURED: The primary outcomes measured were incidence and severity of acute respiratory tract infections. Microbiology and serology testing for 9 common respiratory pathogens was preformed for a random subsample of symptomatic patients. Baseline and post-study plasma levels of α-tocopherol, ascorbic acid, retinol, and carotenoids were also measured.
RESULTS: After a median study duration of 441 days, 1024 episodes of acute respiratory tract infections were reported by 68% of the participants. Of the 74.4% of these reported to the study nurse, 99.2% were confirmed as respiratory tract infections. Of the 107 symptomatic episodes randomly selected for microbiological testing, 58% had a confirmed pathogen, the most common being rhinovirus (54%). When treatment groups were evaluated individually compared with placebo, results were similar for all aspects of incidence and severity of acute respiratory tract infection, except that more patients in the multivitamin-mineral treatment group experienced a significant reduction in activity restriction (34.8% vs 48.5%; P = .04; number needed to treat = 8). Participants taking either multivitamin-mineral or vitamin E supplementation did not have a decreased incidence rate ratio of acute respiratory tract infections, 0.95 (95% CI, 0.75–1.15) and 1.12 (95% CI, 0.88–1.25), respectively. Multivitamin-mineral supplementation had no effect on severity of infection, whereas vitamin E supplementation was associated with illnesses of significantly greater severity: median illness duration was 19 versus 14 days (P = .02); median number of symptoms was 6 versus 4 (P = .03); fever occurrence in 37% versus 25% (P = .009); and restriction of activity in 52% versus 41% (P = .02).
For the elderly living in noninstitutionalized settings, supplementation with multivitamins at RDA levels with minerals at 25% to 50% RDA levels exhibited no effect on incidence or severity of acute respiratory tract infections. However, vitamin E 200 mg daily adversely affected the severity, but not the incidence, of acute respiratory tract infections. Before altering current vitamin E prescribing patterns or counseling patients to discontinue use, these findings should be confirmed.
ABSTRACT
BACKGROUND: The geriatric population has a potentially increased risk of infectious diseases and related sequelae because of decreasing immunocompetency. Currently available trials assessing efficacy of multivitamins and minerals are limited and contradictory. In this study the authors compared whether daily supplementation with a multivitamin containing minerals, with or without vitamin E, or vitamin E alone, affected the incidence and severity of acute respiratory tract infections in elderly individuals.
POPULATION STUDIED: The study population included 652 noninstitutionalized men and women living in the Netherlands who were at least 60 years of age. Patients were excluded if they were taking immunosuppressive agents, anticoagulants that could interfere with vitamin K metabolism, or dietary supplements within the preceding 2 months. Additional exclusion criteria included a history of cancer, liver disease, or fat malabsorption within the preceding 5 years.
STUDY DESIGN AND VALIDITY: Allocation assignment was concealed in this randomized, placebo-controlled trial. Participants took either 2 capsules daily of a multivitamin and mineral complex; vitamin E (200 mg/dL α-tocopheryl acetate); multivitamin-mineral complex plus vitamin E; or placebo for a maximum of 15 months. Doses of multivitamins were at recommended daily allowance (RDA) levels and doses of minerals were 25% to 50% of RDA levels. Patients recorded signs and acute symptoms of respiratory tract infections using a diary. A study nurse confirmed possible respiratory tract infections based on predetermined definitions. Data analysis was performed on an intention-to-treat and per-protocol basis.
OUTCOMES MEASURED: The primary outcomes measured were incidence and severity of acute respiratory tract infections. Microbiology and serology testing for 9 common respiratory pathogens was preformed for a random subsample of symptomatic patients. Baseline and post-study plasma levels of α-tocopherol, ascorbic acid, retinol, and carotenoids were also measured.
RESULTS: After a median study duration of 441 days, 1024 episodes of acute respiratory tract infections were reported by 68% of the participants. Of the 74.4% of these reported to the study nurse, 99.2% were confirmed as respiratory tract infections. Of the 107 symptomatic episodes randomly selected for microbiological testing, 58% had a confirmed pathogen, the most common being rhinovirus (54%). When treatment groups were evaluated individually compared with placebo, results were similar for all aspects of incidence and severity of acute respiratory tract infection, except that more patients in the multivitamin-mineral treatment group experienced a significant reduction in activity restriction (34.8% vs 48.5%; P = .04; number needed to treat = 8). Participants taking either multivitamin-mineral or vitamin E supplementation did not have a decreased incidence rate ratio of acute respiratory tract infections, 0.95 (95% CI, 0.75–1.15) and 1.12 (95% CI, 0.88–1.25), respectively. Multivitamin-mineral supplementation had no effect on severity of infection, whereas vitamin E supplementation was associated with illnesses of significantly greater severity: median illness duration was 19 versus 14 days (P = .02); median number of symptoms was 6 versus 4 (P = .03); fever occurrence in 37% versus 25% (P = .009); and restriction of activity in 52% versus 41% (P = .02).
For the elderly living in noninstitutionalized settings, supplementation with multivitamins at RDA levels with minerals at 25% to 50% RDA levels exhibited no effect on incidence or severity of acute respiratory tract infections. However, vitamin E 200 mg daily adversely affected the severity, but not the incidence, of acute respiratory tract infections. Before altering current vitamin E prescribing patterns or counseling patients to discontinue use, these findings should be confirmed.
ABSTRACT
BACKGROUND: The geriatric population has a potentially increased risk of infectious diseases and related sequelae because of decreasing immunocompetency. Currently available trials assessing efficacy of multivitamins and minerals are limited and contradictory. In this study the authors compared whether daily supplementation with a multivitamin containing minerals, with or without vitamin E, or vitamin E alone, affected the incidence and severity of acute respiratory tract infections in elderly individuals.
POPULATION STUDIED: The study population included 652 noninstitutionalized men and women living in the Netherlands who were at least 60 years of age. Patients were excluded if they were taking immunosuppressive agents, anticoagulants that could interfere with vitamin K metabolism, or dietary supplements within the preceding 2 months. Additional exclusion criteria included a history of cancer, liver disease, or fat malabsorption within the preceding 5 years.
STUDY DESIGN AND VALIDITY: Allocation assignment was concealed in this randomized, placebo-controlled trial. Participants took either 2 capsules daily of a multivitamin and mineral complex; vitamin E (200 mg/dL α-tocopheryl acetate); multivitamin-mineral complex plus vitamin E; or placebo for a maximum of 15 months. Doses of multivitamins were at recommended daily allowance (RDA) levels and doses of minerals were 25% to 50% of RDA levels. Patients recorded signs and acute symptoms of respiratory tract infections using a diary. A study nurse confirmed possible respiratory tract infections based on predetermined definitions. Data analysis was performed on an intention-to-treat and per-protocol basis.
OUTCOMES MEASURED: The primary outcomes measured were incidence and severity of acute respiratory tract infections. Microbiology and serology testing for 9 common respiratory pathogens was preformed for a random subsample of symptomatic patients. Baseline and post-study plasma levels of α-tocopherol, ascorbic acid, retinol, and carotenoids were also measured.
RESULTS: After a median study duration of 441 days, 1024 episodes of acute respiratory tract infections were reported by 68% of the participants. Of the 74.4% of these reported to the study nurse, 99.2% were confirmed as respiratory tract infections. Of the 107 symptomatic episodes randomly selected for microbiological testing, 58% had a confirmed pathogen, the most common being rhinovirus (54%). When treatment groups were evaluated individually compared with placebo, results were similar for all aspects of incidence and severity of acute respiratory tract infection, except that more patients in the multivitamin-mineral treatment group experienced a significant reduction in activity restriction (34.8% vs 48.5%; P = .04; number needed to treat = 8). Participants taking either multivitamin-mineral or vitamin E supplementation did not have a decreased incidence rate ratio of acute respiratory tract infections, 0.95 (95% CI, 0.75–1.15) and 1.12 (95% CI, 0.88–1.25), respectively. Multivitamin-mineral supplementation had no effect on severity of infection, whereas vitamin E supplementation was associated with illnesses of significantly greater severity: median illness duration was 19 versus 14 days (P = .02); median number of symptoms was 6 versus 4 (P = .03); fever occurrence in 37% versus 25% (P = .009); and restriction of activity in 52% versus 41% (P = .02).
For the elderly living in noninstitutionalized settings, supplementation with multivitamins at RDA levels with minerals at 25% to 50% RDA levels exhibited no effect on incidence or severity of acute respiratory tract infections. However, vitamin E 200 mg daily adversely affected the severity, but not the incidence, of acute respiratory tract infections. Before altering current vitamin E prescribing patterns or counseling patients to discontinue use, these findings should be confirmed.
How accurate is the D-dimer assay in diagnosing pulmonary embolism?
ABSTRACT
BACKGROUND: The diagnosis of a pulmonary embolism is challenging because of the potentially severe consequences of missing the condition and imaging test results that are often equivocal. Clinical decisions may be aided by a test for D-dimer, a fibrin degradation product usually increased in the blood of patients with thromboembolic disease. Laboratory advances have led to more rapid and practical detection techniques, such as the enzyme-linked immunosorbent assay (ELISA) method examined by this meta-analysis.
POPULATION STUDIED: The 11 prospective studies included in this meta-analysis involved 2126 patients (aged 54 to 81 years), more than 98% of whom were outpatients presenting with symptoms and signs suspicious for a pulmonary embolism. There were more women than men in the study population.
STUDY DESIGN AND VALIDITY: MEDLINE and EMBASE searches identified relevant titles and abstracts of published articles from 1980 through 2000, and the researchers also searched for unpublished work, screened the reference lists of articles, and contacted authors to identify other studies. All of the included studies had to be original prospective investigations of the ELISA D-dimer test enrolling at least 80% outpatients. Independent reviewers screened articles for quality of reference standards and generalizability. Reference standards for the diagnosis of a pulmonary embolism included a high-probability ventilation/perfusion scan, positive computerized tomographic scan, or positive lower extremity imaging. Acceptable standards for a negative diagnosis were normal or very low probability ventilation/perfusion scan or the absence of a thromboembolic event for 3 months or more.
OUTCOMES MEASURED: The primary results were the pooled sensitivity and specificity of the ELISA D-dimer test in detecting a pulmonary embolism. The authors reported pooled estimates of sensitivity and specificity for various combinations of studies, based on the quality of the studies, method of ELISA testing, age of patients, comorbid conditions, and symptom duration.
RESULTS: Pooling all 11 studies led to an overall sensitivity of 0.95 (95% confidence interval [CI], 0.90–0.98) and a specificity of 0.45 (95% CI, 0.90–0.98). The 5 studies that used the most rigorous reference standard protocols yielded a sensitivity of 0.90 and a specificity of 0.40. A higher pooled accuracy was found in the group of 8 studies with the most typical spectrum of outpatients (sensitivity and specificity of 0.97 and 0.48, respectively). In general, the high sensitivity and low specificity of the ELISA D-dimer test will lead to the detection of a pulmonary embolism most of the time when the condition is present, but false-positive results are common. Several clinical scenarios may decrease the test’s accuracy. In the one small study of patients aged 70 years or older, the sensitivity was perfect (1.00) but the specificity was low (0.14). In another study examining patients whose symptoms lasted 4 days or more, the sensitivity fell to 0.73 and specificity was 0.33. Both of these studies suggest more consideration be given to other conditions, such as infection, cancer, or inflammatory arthritides, which could elevate D-dimer levels in these patient populations.
The ELISA D-dimer test gives relatively reliable information to rule out an acute pulmonary embolism in outpatients. A negative ELISA D-dimer result is particularly useful in ruling out a pulmonary embolism when the pretest probability of a pulmonary embolism is low, and a clinical assessment tool can help determine who fits such a low-probability profile.1 Patients with a higher pretest probability should probably still have an imaging test, considering the morbidity and mortality of a missed pulmonary embolism. Confirmatory imaging is also indicated in most instances when the D-dimer test is positive, due to the poor specificity of the test.
ABSTRACT
BACKGROUND: The diagnosis of a pulmonary embolism is challenging because of the potentially severe consequences of missing the condition and imaging test results that are often equivocal. Clinical decisions may be aided by a test for D-dimer, a fibrin degradation product usually increased in the blood of patients with thromboembolic disease. Laboratory advances have led to more rapid and practical detection techniques, such as the enzyme-linked immunosorbent assay (ELISA) method examined by this meta-analysis.
POPULATION STUDIED: The 11 prospective studies included in this meta-analysis involved 2126 patients (aged 54 to 81 years), more than 98% of whom were outpatients presenting with symptoms and signs suspicious for a pulmonary embolism. There were more women than men in the study population.
STUDY DESIGN AND VALIDITY: MEDLINE and EMBASE searches identified relevant titles and abstracts of published articles from 1980 through 2000, and the researchers also searched for unpublished work, screened the reference lists of articles, and contacted authors to identify other studies. All of the included studies had to be original prospective investigations of the ELISA D-dimer test enrolling at least 80% outpatients. Independent reviewers screened articles for quality of reference standards and generalizability. Reference standards for the diagnosis of a pulmonary embolism included a high-probability ventilation/perfusion scan, positive computerized tomographic scan, or positive lower extremity imaging. Acceptable standards for a negative diagnosis were normal or very low probability ventilation/perfusion scan or the absence of a thromboembolic event for 3 months or more.
OUTCOMES MEASURED: The primary results were the pooled sensitivity and specificity of the ELISA D-dimer test in detecting a pulmonary embolism. The authors reported pooled estimates of sensitivity and specificity for various combinations of studies, based on the quality of the studies, method of ELISA testing, age of patients, comorbid conditions, and symptom duration.
RESULTS: Pooling all 11 studies led to an overall sensitivity of 0.95 (95% confidence interval [CI], 0.90–0.98) and a specificity of 0.45 (95% CI, 0.90–0.98). The 5 studies that used the most rigorous reference standard protocols yielded a sensitivity of 0.90 and a specificity of 0.40. A higher pooled accuracy was found in the group of 8 studies with the most typical spectrum of outpatients (sensitivity and specificity of 0.97 and 0.48, respectively). In general, the high sensitivity and low specificity of the ELISA D-dimer test will lead to the detection of a pulmonary embolism most of the time when the condition is present, but false-positive results are common. Several clinical scenarios may decrease the test’s accuracy. In the one small study of patients aged 70 years or older, the sensitivity was perfect (1.00) but the specificity was low (0.14). In another study examining patients whose symptoms lasted 4 days or more, the sensitivity fell to 0.73 and specificity was 0.33. Both of these studies suggest more consideration be given to other conditions, such as infection, cancer, or inflammatory arthritides, which could elevate D-dimer levels in these patient populations.
The ELISA D-dimer test gives relatively reliable information to rule out an acute pulmonary embolism in outpatients. A negative ELISA D-dimer result is particularly useful in ruling out a pulmonary embolism when the pretest probability of a pulmonary embolism is low, and a clinical assessment tool can help determine who fits such a low-probability profile.1 Patients with a higher pretest probability should probably still have an imaging test, considering the morbidity and mortality of a missed pulmonary embolism. Confirmatory imaging is also indicated in most instances when the D-dimer test is positive, due to the poor specificity of the test.
ABSTRACT
BACKGROUND: The diagnosis of a pulmonary embolism is challenging because of the potentially severe consequences of missing the condition and imaging test results that are often equivocal. Clinical decisions may be aided by a test for D-dimer, a fibrin degradation product usually increased in the blood of patients with thromboembolic disease. Laboratory advances have led to more rapid and practical detection techniques, such as the enzyme-linked immunosorbent assay (ELISA) method examined by this meta-analysis.
POPULATION STUDIED: The 11 prospective studies included in this meta-analysis involved 2126 patients (aged 54 to 81 years), more than 98% of whom were outpatients presenting with symptoms and signs suspicious for a pulmonary embolism. There were more women than men in the study population.
STUDY DESIGN AND VALIDITY: MEDLINE and EMBASE searches identified relevant titles and abstracts of published articles from 1980 through 2000, and the researchers also searched for unpublished work, screened the reference lists of articles, and contacted authors to identify other studies. All of the included studies had to be original prospective investigations of the ELISA D-dimer test enrolling at least 80% outpatients. Independent reviewers screened articles for quality of reference standards and generalizability. Reference standards for the diagnosis of a pulmonary embolism included a high-probability ventilation/perfusion scan, positive computerized tomographic scan, or positive lower extremity imaging. Acceptable standards for a negative diagnosis were normal or very low probability ventilation/perfusion scan or the absence of a thromboembolic event for 3 months or more.
OUTCOMES MEASURED: The primary results were the pooled sensitivity and specificity of the ELISA D-dimer test in detecting a pulmonary embolism. The authors reported pooled estimates of sensitivity and specificity for various combinations of studies, based on the quality of the studies, method of ELISA testing, age of patients, comorbid conditions, and symptom duration.
RESULTS: Pooling all 11 studies led to an overall sensitivity of 0.95 (95% confidence interval [CI], 0.90–0.98) and a specificity of 0.45 (95% CI, 0.90–0.98). The 5 studies that used the most rigorous reference standard protocols yielded a sensitivity of 0.90 and a specificity of 0.40. A higher pooled accuracy was found in the group of 8 studies with the most typical spectrum of outpatients (sensitivity and specificity of 0.97 and 0.48, respectively). In general, the high sensitivity and low specificity of the ELISA D-dimer test will lead to the detection of a pulmonary embolism most of the time when the condition is present, but false-positive results are common. Several clinical scenarios may decrease the test’s accuracy. In the one small study of patients aged 70 years or older, the sensitivity was perfect (1.00) but the specificity was low (0.14). In another study examining patients whose symptoms lasted 4 days or more, the sensitivity fell to 0.73 and specificity was 0.33. Both of these studies suggest more consideration be given to other conditions, such as infection, cancer, or inflammatory arthritides, which could elevate D-dimer levels in these patient populations.
The ELISA D-dimer test gives relatively reliable information to rule out an acute pulmonary embolism in outpatients. A negative ELISA D-dimer result is particularly useful in ruling out a pulmonary embolism when the pretest probability of a pulmonary embolism is low, and a clinical assessment tool can help determine who fits such a low-probability profile.1 Patients with a higher pretest probability should probably still have an imaging test, considering the morbidity and mortality of a missed pulmonary embolism. Confirmatory imaging is also indicated in most instances when the D-dimer test is positive, due to the poor specificity of the test.
Do written action plans improve patient outcomes in asthma? An evidence-based analysis
- Most studies of asthma self-management do not permit retrospective isolation of the independent effects of a written action plan or peak flow meter use.
- Studies designed to isolate the effect of these self-care activities are generally underpowered or prone to systematic bias.
- Available evidence suggests that peak flow meters and written action plans do not have a large impact on outcomes when applied to the general population of asthmatics.
- These interventions are most likely to have beneficial effect when applied to selected populations, particularly patients with high baseline utilization.
Self-management skills are widely promoted by health plans and specialty societies with the expectation that they will improve care. The 1997 National Heart, Lung, and Blood Institute guidelines on treating asthma emphasize self-management,1 although they do not recommend specific programs. To maximize therapeutic effectiveness, it would be useful to know which components of patient self-management improve outcomes. Written action plans and peak flow meters are commonly used in asthma self-management programs. While these are simple, low-cost interventions for an individual, the aggregate cost for the entire population of asthmatics may be high.2
Much literature has accumulated on the effectiveness of providing asthma education alone and on programs that actively engage patients in their own care.Several systematic reviews have found that providing educational information alone has had little effect on asthma outcomes.3-5 There is evidence, though, that self-management activities are more effective than educational information alone. A recent Cochrane review of 24 trials found that self-management with regular practitioner review reduces hospi-talizations and emergency room visits.6 This review did not identify specific components contributing to improved outcomes. In contrast to the aforementioned studies on patient education, a large case-control study of children in the Kaiser Permanente System,7 found that written action plans were associated with lower rates of hospitalization and emergency room use. However, such observational studies often include confounding factors and are not sufficient to establish a cause-effect relationship between written action plans and improved outcomes.
We report on a systematic review that attempts to isolate the independent effect of a written action plan on asthma outcomes. We address two key questions:
- Compared with medical management alone, does the addition of a written asthma action plan (with or without peak flow meter use) improve outcomes?
- Compared with a written action plan based on symptoms, does a written action plan based on peak flow monitoring improve outcomes?
Methods
This study is part of a broader evidence report on the management of chronic asthma prepared for the Agency of Health Care Research and Quality8. Complete details of the methodology are available in the full report8 (http://www.ahcpr.gov/clinic/epcix.htm).
Literature search and study selection
We performed a comprehensive literature search from 1980 to August 2000 using MEDLINE, Embase, the Cochrane Library, and a hand search of recent bibliographies. The search was limited to full-length, peer-reviewed articles with an English abstract. Two independent reviewers carried out each step of study selection and data abstraction. Disagreements were resolved by consensus of the two reviewers or, if necessary, by the decision of a third reviewer.
Initial study selection was limited to comparative full-length reports or abstracts in peer-reviewed medical journals, with at least 25 evaluable children or adults per arm, treated for at least 12 weeks. Relevant comparisons included a written action plan and no written action plan; a written action plan based on peak flow readings and a written action plan based on symptoms. Study designs varied: clinical trials, cohort comparisons, case-control analyses, cross-sectional evaluations, and before-after comparisons. Specific components of the management plan had to be described.
Relevant outcomes included measures of inpatient and outpatient utilization, lung function, symptoms, rescue medication or oral steroid use, and quality of life. Outcomes of greatest interest were utilization parameters, as the goals of self-management usually focus on improving these outcomes.
These initial selection criteria yielded many studies that were confounded by multiple asthma management interventions and thus did not isolate the comparisons of interest. Therefore, the research team collectively determined the study design features that would best isolate the effects of written action plans and used them as new criteria in a second round of study selection. The studies thus selected satisfied 4 criteria: 1) randomization of patients; (2) delivery of the same interventions to experimental and control groups, except that the experimental group also received a written action plan; (3) delivery of the same interventions to experimental and control groups, except that one group received a written action plan based on peak flow meter readings, and the comparison group received a written action plan based on symptom monitoring; and 4) inclusion of a written action plan that met our specified definition.
A written action plan, by our definition, had two components: an algorithm that identified specific clinical indicators signaling the need for adjustments in medication; and specific instructions on how to adjust medications in response to such indicators. Many publications lacked sufficient detail on the written plan, so a brief survey was sent to the primary author of each of the 36 studies. If no response was obtained (36%), the article was excluded only when it was clear from the publication that our definition was not met.
Assessment of study quality
High-quality studies were randomized controlled trials that met the 3 domains of study quality that have been demonstrated empirically to impact effect size: concealment of treatment allocation; double-blinding; and minimization of exclusion bias.9,10 However, we doubted the feasibility of double-blinding a written asthma plan intervention, and so relaxed this requirement. We considered exclusion bias to be minimized when a study either reported intent-to-treat analysis or excluded fewer than 10% of subjects from analysis, with the ratio of subjects excluded from each arm being less than 2:1.
To more fully evaluate study design issues that may be particularly important in asthma research,11,12 we constructed asthma-specific quality indicators in consultation with an expert panel. Controls for potential confounders of treatment effect included establishing reversibility of airway obstruction, controlling for other medication use, reporting compliance, and addressing seasonality. In addition, a priori reporting of power calculations and accounting for exclusions and withdrawals were judged to be study quality characteristics pertinent to this body of evidence.
Data analysis
We constructed evidence tables for the outcomes of interest, and performed a qualitative synthesis of the data. Meta-analysis was not appropriate due to wide discrepancies in the patient populations studied, the interventions employed, and measurement and reporting of outcomes.
Results
Our literature search yielded a total of 4578 citations. Of these, 36 studies met the initial selection criteria. Many of these qualifying studies, however, were confounded by multiple asthma management interventions applied inconsistently across treatment arms. For example, a common confounder was review of and change in long-term medication use in the treatment group, but not in the control group. This necessitated a refinement in our selection criteria to focus on studies that largely isolated the effect of written action plans.13-21 This step yielded a final evidence base of 9 randomized controlled trials with a total enrollment of 1501 patients.
Table 1 summarizes the characteristics, interventions, and outcomes of the 9 studies. Two studies were 3-arm trials,16,17 which raised the total number of comparisons among the 9 studies to 11. The largest study was the Grampian Asthma Study of Integrated Care (n=569),14 a community study conducted in the UK. Enrollment in the other 8 studies ranged from 43 to 64 patients per arm. Treatment duration ranged from 24 to 52 weeks.
None of the studies met our definition of high quality. In fact, no study met any of the generic quality criteria—none was blinded, none described concealment of allocation, and all excluded more than 10% of subjects. Furthermore, none reported an intention-to-treat analysis. Thus these trials were prone to withdrawal bias as well as overestimation of treatment effect due to lack of allocation concealment.
No study met the majority of asthma-specific indicators (Table 1). Of the 9 studies, only 5 met any asthma-specific indicator. Three reported prospective power calculations,13-15 but 2 of these substantially overestimated the expected effect.13,15 Two studies established reversibility;14,17 2 controlled for other medication use;13,15 and 2 reported compliance.17,21 Thus, the studies were also prone to a type II error (failing to detect a true effect) and to potential confounding of outcomes.
We performed sample power calculations for hospitalizations (Table 2), derived from baseline rates reported in 4 studies14,16-18 and standard deviations reported in 2.14,17 A study with 250 patients per arm could detect a reduction of 50% or more in hospitalization, given a control rate of at least 0.2 hospitalizations/patient/year. In actuality, GRASSIC,14 which is the largest available trial (N=569), had baseline hospitalization rates of 0.12 and 0.13. With this baseline rate, over 700 patients per arm are required, higher than the actual enrollment in GRASSIC. The other studies in this review would be adequately powered to detect a 50% difference only in the setting of even higher baseline utilization (eg, 0.30 hospitalizations/patient/year).
Table 3 displays utilization outcomes for the 11 comparisons in the 9 trials. In 5 studies (N=1019), medical management with a written action plan was compared with medical management without a written action plan.13-17 Two trials (N=185) compared a peak flow meter plus a written action plan with a peak flow meter and no written action plan18,19 In 4 studies (N=393), a written action plan based on peak flow monitoring was compared with a written action plan based on symptoms.
TABLE 1
Study characteristics
| Study | Patient popultation | Study Arms | Intervention components | Outcomes reported | Asthma quality indicators met |
|---|---|---|---|---|---|
| Optimal medical management vs. optimal medical management + PFM action plan | |||||
| Jones 199514 | Inclusions: patients using ICS | Usual care | SxD, FU | Ut, LF, Sx | Pow, Med |
| Exclusions: patients on oral steroids or using peak flow meters at home | PFM action plan | AP, PF, SxD, FU | |||
| Mean age: 29.5 years | |||||
| Severity level: Mild–moderate | |||||
| Drummond 1994 (GRASSIC)15 | Inclusion: FEV1 reversibility 20% or greater | Usual care | FU | Ut, LF, Med Ex | Pow, Rev |
| Exclusions: patients who already owned a PFM | PFM action plan | AP, PF, FU | |||
| Mean age: 50.8 years | |||||
| Severity level: Mild–severe | |||||
| Ayres 199516 | Inclusions: maximum PEF variability, 0.15%; minimum nights/week with symptoms, 3; minimum use of ICS or sodium cromoglycate, 3 months | Usual care | SxD, FU | LF, Sx, Ex | Pow, Med |
| Mean age: 45 years | PFM action plan | AP, PF, SxD, FU | |||
| Severity level: Moderate–severe | |||||
| Cowie 199713 | Inclusions: treatment for an exacerbation of asthma in an ER asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Usual care | Ed, SxD, FU | Ut, PF, Med, Ex | None |
| Mean age: 37.8 years | PFM action plan | AP, PF, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Cote 199717 | Inclusions: FEV1postbronchodilator 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Usual care | Ed | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: patients having previously taken an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Mean age: 36.5 years Severity level: Mild | |||||
| Usual care + PFM use alone vs. usual care + PFM action plan | |||||
| Ignacio-Garcia 199518 | Inclusions: patients from outpatient asthma clinic with asthma for 2 years | Usual care + PFM | PF, SxD, FU | Ut, LF, Med | None |
| Mean age: 41.9 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Charlton 199419 | Inclusion: patients with inpatient or outpatient visit for asthma | Usual care + PFM | PF, Ed, SxD, FU | Ut, Sx, Med, Ex | None |
| Mean age: 6.5 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–moderate | |||||
| PFM action plan vs. Symptom action plan | |||||
| Turner 199820 | Inclusions: Maximum methacholine PC20, 7.9; using ICS | Symptom action plan | AP, Ed, SxD, Cn BM, EM | Ut, LF, Sx, Med | Exc, Com |
| Exclusions: previous PFM use; significant comorbid conditions | PFM action plan | PF, AP, Ed, SxD, Cn BM, EM | |||
| Mean age: 34.1 years | |||||
| Severity level: Mild–severe | |||||
| Charlton 199021 | Inclusions: patients on repeat prescribing register | Symptom action plan | AP, Ed, FU | Ut, Med | None |
| Mean age: NR | PFM action plan | PF, AP, Ed, FU, Cn | Ut, PF, Med, Ex | None | |
| Severity level: Mild–severe (?) | |||||
| Cowie 199716 | Inclusions: treatment for an exacerbation of asthma in an ER, or asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Symptom action plan | AP, Ed, SxD, FU | ||
| PFM action plan | AP, PF, Ed, SxD, FU | ||||
| Cote 199717 | Inclusions: FEV1postbronchodilator, 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Symptom action plan | Ed, AP | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: previous enrollment in an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Eligibility criteria: ICS = inhaled corticosteroid; FEV1 = forced expiratory volume in 1 second; PEF = peak expiratory flow; PFM = peak flow meter; ER = emergency room; PC20 = 20% fall in FEV1 Intervention components: PF = Peak flow meter; AP = Written Action Plan; Ed = Education; SxD = Symptom diary; FU = Follow-up visits; Cn = Counseling; BM = Behavior modification; EM = Environmental modification | |||||
| Outcomes: Ut = Utilization measures; LF= Lung function measurements; Sx = Symptom=based measurements; Med = Medication use; Ex = Exacerbations of asth ma Asthma Quality Indicators: Exc = Accounted for excluded patients; Pow = Reported power calculations; Rev = Established reversibility of airway obstruction; Med = Controlled for other medication use; Com = Reported compliance; Sea = Addressed seasonality. | |||||
TABLE 2
Power calculations for hospitalizations per patient per year
| Assumed control mean | Possible treatment mean | % decrease | N needed per study arm |
|---|---|---|---|
| 0.10 | 0.075 | 25 | 3077 |
| 0.10 | 0.05 | 50 | 770 |
| 0.10 | 0.025 | 75 | 342 |
| 0.20 | 0.015 | 25 | 770 |
| 0.20 | 0.10 | 50 | 193 |
| 0.20 | 0.05 | 75 | 86 |
| 0.30 | 0.225 | 25 | 342 |
| 0.30 | 0.15 | 50 | 86 |
| 0.30 | 0.075 | 75 | 38 |
| Studies were identified that contained baseline rates on hospitalizations/patient/year, or information that allowed calculation of this parameter (Drummond, Abdalla, Beattie et al., 1994; Cote, Cartier, Robichaud et al. 1997; Cowie, Revitt, Underwood et al., 1997; Ignacio-Garcia and Gonzalez-Santos, 1995). Baseline rates of hospitalization varied in these studies from 0.04-0.29/patient/year. Standard deviations for this outcome were available only in two studies; Cote, Cartier, Robichaud et al. (1997) reported an SD of 0.30 for this variable, and an SD of 0.35 was calculated from the confidence intervals reported in GRASSIC (Drummond, Abdalla, Beattie et al., 1994). For the calculations, the more conservative 0.35 estimate for SD was used. | |||
| Number of patients per study arm were estimated for 80 percent power at the 5 percent significance level using control arm means of 0.10, 0.20, and 0.30 hospitalizations/patient/year. The expected reduction in this variable was tested along a spectrum from 25-75 percent. | |||
Written action plan versus no written action plan
All 5 studies used a peak flow meter based written action plan. All reported utilization outcomes, but the types and units of measurement were not consistent across studies (Table 2). Additionally, 4 studies reported on symptoms,13-16 and 3 reported lung function outcomes.13-15
With one notable exception, there were no statistically significant differences in outcomes among groups. Cowie et al16 reported an 11-fold decrease in total emergency room visits for the group using a peak-flow action plan (5 vs 55, P = .02), and also reported a reduction in hospitalizations of a similar magnitude (2 vs 12) that did not reach statistical significance. However, this study suffers from notable flaws that diminish confidence in the results. It is a post-intervention comparison among groups, which does not compare change from baseline, or incorporate baseline values as covariates in the analysis. Moreover baseline utilization data were provided by patient recall and not corroborated by medical records. There was a substantially larger variability in the baseline utilization rates for the peak flow group compared with the control group. This suggests that a subset of very high frequency users may have been over-represented in the peak flow group, and the reduction in emergency room visits may be concentrated in this subset.
Peak-flow meter-based written action plan versus peak flow meter with no written action plan
Two studies18,19 addressed the independent effect of a written action plan when added to peak flow self-monitoring (Table 3). Charlton19 reported no significant group differences for main outcomes, while Ignacio-Garcia18 reported large and statistically significant differences in most of the outcomes, favoring the group that used the written action plan.
The Ignacio-Garcia study, however, suffers from notable flaws suggesting the results may be attributable to bias. The sole participating physician, not blinded to treatment assignment, was highly involved in all phases of patient assessment, monitoring, and treatment. There was evidence of baseline differences between the two groups. A total of 25% of patients were withdrawn after randomization, and an unexplained decline in lung function occurred in the control group. Thus, the potential for selection bias, withdrawal bias, and ascertainment bias limits confidence in the results of this study
Symptom-based written action plan compared with peak flow-based written action plan
In 4 studies,16,17,20,21 reported outcomes were generally equivalent between groups and comparisons were not statistically significant, with one exception (Table 3). The 3-arm study by Cowie et al16 reported a striking reduction in the total number of emergency room visits with a peak flow meter-based written action plan compared with a symptom-based written action plan (5 versus 45, P
Discussion
The objective of this systematic review was to assess the independent effects of 2 specific components commonly included in asthma self-management plans—a written action plan and a peak flow meter. Few studies, however, are designed to permit reviewers to isolate the effects of these components. Moreover, the studies we reviewed did not clearly identify the population expected to benefit from interventions or specify the primary outcomes of interest; nor was the level of clinically meaningful improvement prospectively defined.
Most of the trials we reviewed, including the largest community study of 569 patients, did not demonstrate improved outcomes. The 2 trials that reported statistically significant results favoring a peak flow-based written action plan suffer from notable flaws suggesting the results may be attributable to bias. In the other 7 trials, there was little difference in outcomes between groups. However, these studies had insufficient power to detect group differences or confidently conclude equivalence between groups.
Thus, available evidence is insufficient to demonstrate that asthma outcomes are improved by use of a written asthma action plan, with or without peak flow monitoring. While this body of literature does not establish that these interventions are ineffective, it suggests they will not have a large effect on outcomes when applied to the general asthmatic population. The application of written action plans to all asthmatics indiscriminately may be a wasteful use of resources. This systematic review also questions the validity of written action plans as an indicator of asthma quality of care, or as a means to achieve quality improvement.
This analysis also highlights several obstacles to assessing the effects of disease management interventions. First, while the impact of whole intervention programs can be evaluated in controlled trials, it may be unfeasible to isolate each component of such programs and subject it to a rigorous analysis. Furthermore, as a behavioral intervention, the general principle of engaging patients in self-management may be more important that the specific components of these programs. Finally, regarding the optimization of medications (most obviously initiation of inhaled steroids) the impact of written action plans is likely to be relatively small, particularly on lung function or symptom control.
Future clinical trials should be done selectively, aimed at producing rigorous results that can improve the effectiveness of self-management interventions. Further study is warranted for specific subpopulations, such as those with higher baseline severity of illness or those with high baseline utilization rates. Available data suggest that, if there is benefit to be gained from self-management interventions, it will most likely be seen among these patients. Specific components of self-management that might be tested individually are those that are relatively high-cost, resource intensive, or risky for the patient.
Existing trials have tended to over-estimate the effects of action plan-based interventions, thus having invested resources for results inadequate for optimizing self-management strategies. Careful consideration needs to be taken in future trials to realistically estimate the expected impact of each intervention, and to specify the primary outcomes of interest and their baseline frequencies. Future trials should be large enough to detect a difference if one exists, or to confidently conclude that the intervention is ineffective.
Attention to these principles will help to advance our knowledge in this area most efficiently and to ultimately improve the quality of care for the entire population of patients with asthma.
· Acknowledgments ·
We acknowledge Kathleen Ziegler, Pharm.D, and Claudia Bonnell, RN, MSL, for their assistance in the research and preparation of this manuscript.
1. National Heart, Lung and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4051.
2. Ruffin RE, Pierce RJ. Peak flow monitoring—which asthmatics, when, and how? Aust N Z J Med 1994;24:519-20.
3. Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nursing Health 1996;19:367-76.
4. Bernard-Bonnin AC, Stachenko S, Bonin D, et al. Self-management teaching programs and morbidity of pediatric asthma: a meta-analysis. J Allergy Clin Immunol 1995;95(1 Pt 1):34-41.
5. Gibson PG, Coughlan J, Wilson AJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000a;(2):CD001005.-
6. Gibson PG, Coughlan J, Wilson AJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000b (2):CD001117.-
7. Lieu TA, Quesenberry CP, Jr, Capra AM, et al. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics 1997;100(3 Pt 1):334-41.
8. Lefevre F, Piper M, Mark D, et al. Management of Chronic Asthma. AHRQ evidence report, contract number 290-97-001-5, 2001, http://www.ahcpr.gov/clinic/epcix.htm.
9. Mulrow CD, Oxman AD, editors. Cochrane Collaboration Handbook. Available in the Cochrane Library [database on disk and CD-ROM]. The Cochrane Collaboration; Issue 1. Oxford: Update Software; 1997.
10. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12.
11. Berlin JA, Rennie D. Measuring the quality of trials: the quality of the quality scales. JAMA 1999;282(11):1083-5.
12. Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11):1054-60.
13. Jones KP, Mullee MA, Middleton M, et al. Peak flow based asthma self-management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851-7.
14. Drummond N, Abdalla M, Beattie JAG, et al. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care GRASSIC). BMJ 1994 Feb. 26;308(6928):564-7.
15. Ayres JG, Campbell LM. A controlled assessment of an asthma self-management plan involving a budesonide dose regimen. OPTIONS Research Group. Eur Respir J 1996;886-92.
16. Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534-8.
17. Cote J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155(5):1509-14.
18. Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med 1995;151(2 Pt 1):353-9.
19. Charlton I, Antoniou AG, Atkinson J, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Arch Dis Child 1994;70(4):313-8.
20. Turner MO, Taylor D, Bennett R, et al. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med 1998;157(2):540-6.
21. Charlton I, Charlton G, Broomfield J, et al. Evaluation of peak flow and symptoms only self-management plans for control of asthma in general practice. BMJ 1990;301(6765):1355-9.
- Most studies of asthma self-management do not permit retrospective isolation of the independent effects of a written action plan or peak flow meter use.
- Studies designed to isolate the effect of these self-care activities are generally underpowered or prone to systematic bias.
- Available evidence suggests that peak flow meters and written action plans do not have a large impact on outcomes when applied to the general population of asthmatics.
- These interventions are most likely to have beneficial effect when applied to selected populations, particularly patients with high baseline utilization.
Self-management skills are widely promoted by health plans and specialty societies with the expectation that they will improve care. The 1997 National Heart, Lung, and Blood Institute guidelines on treating asthma emphasize self-management,1 although they do not recommend specific programs. To maximize therapeutic effectiveness, it would be useful to know which components of patient self-management improve outcomes. Written action plans and peak flow meters are commonly used in asthma self-management programs. While these are simple, low-cost interventions for an individual, the aggregate cost for the entire population of asthmatics may be high.2
Much literature has accumulated on the effectiveness of providing asthma education alone and on programs that actively engage patients in their own care.Several systematic reviews have found that providing educational information alone has had little effect on asthma outcomes.3-5 There is evidence, though, that self-management activities are more effective than educational information alone. A recent Cochrane review of 24 trials found that self-management with regular practitioner review reduces hospi-talizations and emergency room visits.6 This review did not identify specific components contributing to improved outcomes. In contrast to the aforementioned studies on patient education, a large case-control study of children in the Kaiser Permanente System,7 found that written action plans were associated with lower rates of hospitalization and emergency room use. However, such observational studies often include confounding factors and are not sufficient to establish a cause-effect relationship between written action plans and improved outcomes.
We report on a systematic review that attempts to isolate the independent effect of a written action plan on asthma outcomes. We address two key questions:
- Compared with medical management alone, does the addition of a written asthma action plan (with or without peak flow meter use) improve outcomes?
- Compared with a written action plan based on symptoms, does a written action plan based on peak flow monitoring improve outcomes?
Methods
This study is part of a broader evidence report on the management of chronic asthma prepared for the Agency of Health Care Research and Quality8. Complete details of the methodology are available in the full report8 (http://www.ahcpr.gov/clinic/epcix.htm).
Literature search and study selection
We performed a comprehensive literature search from 1980 to August 2000 using MEDLINE, Embase, the Cochrane Library, and a hand search of recent bibliographies. The search was limited to full-length, peer-reviewed articles with an English abstract. Two independent reviewers carried out each step of study selection and data abstraction. Disagreements were resolved by consensus of the two reviewers or, if necessary, by the decision of a third reviewer.
Initial study selection was limited to comparative full-length reports or abstracts in peer-reviewed medical journals, with at least 25 evaluable children or adults per arm, treated for at least 12 weeks. Relevant comparisons included a written action plan and no written action plan; a written action plan based on peak flow readings and a written action plan based on symptoms. Study designs varied: clinical trials, cohort comparisons, case-control analyses, cross-sectional evaluations, and before-after comparisons. Specific components of the management plan had to be described.
Relevant outcomes included measures of inpatient and outpatient utilization, lung function, symptoms, rescue medication or oral steroid use, and quality of life. Outcomes of greatest interest were utilization parameters, as the goals of self-management usually focus on improving these outcomes.
These initial selection criteria yielded many studies that were confounded by multiple asthma management interventions and thus did not isolate the comparisons of interest. Therefore, the research team collectively determined the study design features that would best isolate the effects of written action plans and used them as new criteria in a second round of study selection. The studies thus selected satisfied 4 criteria: 1) randomization of patients; (2) delivery of the same interventions to experimental and control groups, except that the experimental group also received a written action plan; (3) delivery of the same interventions to experimental and control groups, except that one group received a written action plan based on peak flow meter readings, and the comparison group received a written action plan based on symptom monitoring; and 4) inclusion of a written action plan that met our specified definition.
A written action plan, by our definition, had two components: an algorithm that identified specific clinical indicators signaling the need for adjustments in medication; and specific instructions on how to adjust medications in response to such indicators. Many publications lacked sufficient detail on the written plan, so a brief survey was sent to the primary author of each of the 36 studies. If no response was obtained (36%), the article was excluded only when it was clear from the publication that our definition was not met.
Assessment of study quality
High-quality studies were randomized controlled trials that met the 3 domains of study quality that have been demonstrated empirically to impact effect size: concealment of treatment allocation; double-blinding; and minimization of exclusion bias.9,10 However, we doubted the feasibility of double-blinding a written asthma plan intervention, and so relaxed this requirement. We considered exclusion bias to be minimized when a study either reported intent-to-treat analysis or excluded fewer than 10% of subjects from analysis, with the ratio of subjects excluded from each arm being less than 2:1.
To more fully evaluate study design issues that may be particularly important in asthma research,11,12 we constructed asthma-specific quality indicators in consultation with an expert panel. Controls for potential confounders of treatment effect included establishing reversibility of airway obstruction, controlling for other medication use, reporting compliance, and addressing seasonality. In addition, a priori reporting of power calculations and accounting for exclusions and withdrawals were judged to be study quality characteristics pertinent to this body of evidence.
Data analysis
We constructed evidence tables for the outcomes of interest, and performed a qualitative synthesis of the data. Meta-analysis was not appropriate due to wide discrepancies in the patient populations studied, the interventions employed, and measurement and reporting of outcomes.
Results
Our literature search yielded a total of 4578 citations. Of these, 36 studies met the initial selection criteria. Many of these qualifying studies, however, were confounded by multiple asthma management interventions applied inconsistently across treatment arms. For example, a common confounder was review of and change in long-term medication use in the treatment group, but not in the control group. This necessitated a refinement in our selection criteria to focus on studies that largely isolated the effect of written action plans.13-21 This step yielded a final evidence base of 9 randomized controlled trials with a total enrollment of 1501 patients.
Table 1 summarizes the characteristics, interventions, and outcomes of the 9 studies. Two studies were 3-arm trials,16,17 which raised the total number of comparisons among the 9 studies to 11. The largest study was the Grampian Asthma Study of Integrated Care (n=569),14 a community study conducted in the UK. Enrollment in the other 8 studies ranged from 43 to 64 patients per arm. Treatment duration ranged from 24 to 52 weeks.
None of the studies met our definition of high quality. In fact, no study met any of the generic quality criteria—none was blinded, none described concealment of allocation, and all excluded more than 10% of subjects. Furthermore, none reported an intention-to-treat analysis. Thus these trials were prone to withdrawal bias as well as overestimation of treatment effect due to lack of allocation concealment.
No study met the majority of asthma-specific indicators (Table 1). Of the 9 studies, only 5 met any asthma-specific indicator. Three reported prospective power calculations,13-15 but 2 of these substantially overestimated the expected effect.13,15 Two studies established reversibility;14,17 2 controlled for other medication use;13,15 and 2 reported compliance.17,21 Thus, the studies were also prone to a type II error (failing to detect a true effect) and to potential confounding of outcomes.
We performed sample power calculations for hospitalizations (Table 2), derived from baseline rates reported in 4 studies14,16-18 and standard deviations reported in 2.14,17 A study with 250 patients per arm could detect a reduction of 50% or more in hospitalization, given a control rate of at least 0.2 hospitalizations/patient/year. In actuality, GRASSIC,14 which is the largest available trial (N=569), had baseline hospitalization rates of 0.12 and 0.13. With this baseline rate, over 700 patients per arm are required, higher than the actual enrollment in GRASSIC. The other studies in this review would be adequately powered to detect a 50% difference only in the setting of even higher baseline utilization (eg, 0.30 hospitalizations/patient/year).
Table 3 displays utilization outcomes for the 11 comparisons in the 9 trials. In 5 studies (N=1019), medical management with a written action plan was compared with medical management without a written action plan.13-17 Two trials (N=185) compared a peak flow meter plus a written action plan with a peak flow meter and no written action plan18,19 In 4 studies (N=393), a written action plan based on peak flow monitoring was compared with a written action plan based on symptoms.
TABLE 1
Study characteristics
| Study | Patient popultation | Study Arms | Intervention components | Outcomes reported | Asthma quality indicators met |
|---|---|---|---|---|---|
| Optimal medical management vs. optimal medical management + PFM action plan | |||||
| Jones 199514 | Inclusions: patients using ICS | Usual care | SxD, FU | Ut, LF, Sx | Pow, Med |
| Exclusions: patients on oral steroids or using peak flow meters at home | PFM action plan | AP, PF, SxD, FU | |||
| Mean age: 29.5 years | |||||
| Severity level: Mild–moderate | |||||
| Drummond 1994 (GRASSIC)15 | Inclusion: FEV1 reversibility 20% or greater | Usual care | FU | Ut, LF, Med Ex | Pow, Rev |
| Exclusions: patients who already owned a PFM | PFM action plan | AP, PF, FU | |||
| Mean age: 50.8 years | |||||
| Severity level: Mild–severe | |||||
| Ayres 199516 | Inclusions: maximum PEF variability, 0.15%; minimum nights/week with symptoms, 3; minimum use of ICS or sodium cromoglycate, 3 months | Usual care | SxD, FU | LF, Sx, Ex | Pow, Med |
| Mean age: 45 years | PFM action plan | AP, PF, SxD, FU | |||
| Severity level: Moderate–severe | |||||
| Cowie 199713 | Inclusions: treatment for an exacerbation of asthma in an ER asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Usual care | Ed, SxD, FU | Ut, PF, Med, Ex | None |
| Mean age: 37.8 years | PFM action plan | AP, PF, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Cote 199717 | Inclusions: FEV1postbronchodilator 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Usual care | Ed | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: patients having previously taken an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Mean age: 36.5 years Severity level: Mild | |||||
| Usual care + PFM use alone vs. usual care + PFM action plan | |||||
| Ignacio-Garcia 199518 | Inclusions: patients from outpatient asthma clinic with asthma for 2 years | Usual care + PFM | PF, SxD, FU | Ut, LF, Med | None |
| Mean age: 41.9 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Charlton 199419 | Inclusion: patients with inpatient or outpatient visit for asthma | Usual care + PFM | PF, Ed, SxD, FU | Ut, Sx, Med, Ex | None |
| Mean age: 6.5 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–moderate | |||||
| PFM action plan vs. Symptom action plan | |||||
| Turner 199820 | Inclusions: Maximum methacholine PC20, 7.9; using ICS | Symptom action plan | AP, Ed, SxD, Cn BM, EM | Ut, LF, Sx, Med | Exc, Com |
| Exclusions: previous PFM use; significant comorbid conditions | PFM action plan | PF, AP, Ed, SxD, Cn BM, EM | |||
| Mean age: 34.1 years | |||||
| Severity level: Mild–severe | |||||
| Charlton 199021 | Inclusions: patients on repeat prescribing register | Symptom action plan | AP, Ed, FU | Ut, Med | None |
| Mean age: NR | PFM action plan | PF, AP, Ed, FU, Cn | Ut, PF, Med, Ex | None | |
| Severity level: Mild–severe (?) | |||||
| Cowie 199716 | Inclusions: treatment for an exacerbation of asthma in an ER, or asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Symptom action plan | AP, Ed, SxD, FU | ||
| PFM action plan | AP, PF, Ed, SxD, FU | ||||
| Cote 199717 | Inclusions: FEV1postbronchodilator, 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Symptom action plan | Ed, AP | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: previous enrollment in an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Eligibility criteria: ICS = inhaled corticosteroid; FEV1 = forced expiratory volume in 1 second; PEF = peak expiratory flow; PFM = peak flow meter; ER = emergency room; PC20 = 20% fall in FEV1 Intervention components: PF = Peak flow meter; AP = Written Action Plan; Ed = Education; SxD = Symptom diary; FU = Follow-up visits; Cn = Counseling; BM = Behavior modification; EM = Environmental modification | |||||
| Outcomes: Ut = Utilization measures; LF= Lung function measurements; Sx = Symptom=based measurements; Med = Medication use; Ex = Exacerbations of asth ma Asthma Quality Indicators: Exc = Accounted for excluded patients; Pow = Reported power calculations; Rev = Established reversibility of airway obstruction; Med = Controlled for other medication use; Com = Reported compliance; Sea = Addressed seasonality. | |||||
TABLE 2
Power calculations for hospitalizations per patient per year
| Assumed control mean | Possible treatment mean | % decrease | N needed per study arm |
|---|---|---|---|
| 0.10 | 0.075 | 25 | 3077 |
| 0.10 | 0.05 | 50 | 770 |
| 0.10 | 0.025 | 75 | 342 |
| 0.20 | 0.015 | 25 | 770 |
| 0.20 | 0.10 | 50 | 193 |
| 0.20 | 0.05 | 75 | 86 |
| 0.30 | 0.225 | 25 | 342 |
| 0.30 | 0.15 | 50 | 86 |
| 0.30 | 0.075 | 75 | 38 |
| Studies were identified that contained baseline rates on hospitalizations/patient/year, or information that allowed calculation of this parameter (Drummond, Abdalla, Beattie et al., 1994; Cote, Cartier, Robichaud et al. 1997; Cowie, Revitt, Underwood et al., 1997; Ignacio-Garcia and Gonzalez-Santos, 1995). Baseline rates of hospitalization varied in these studies from 0.04-0.29/patient/year. Standard deviations for this outcome were available only in two studies; Cote, Cartier, Robichaud et al. (1997) reported an SD of 0.30 for this variable, and an SD of 0.35 was calculated from the confidence intervals reported in GRASSIC (Drummond, Abdalla, Beattie et al., 1994). For the calculations, the more conservative 0.35 estimate for SD was used. | |||
| Number of patients per study arm were estimated for 80 percent power at the 5 percent significance level using control arm means of 0.10, 0.20, and 0.30 hospitalizations/patient/year. The expected reduction in this variable was tested along a spectrum from 25-75 percent. | |||
Written action plan versus no written action plan
All 5 studies used a peak flow meter based written action plan. All reported utilization outcomes, but the types and units of measurement were not consistent across studies (Table 2). Additionally, 4 studies reported on symptoms,13-16 and 3 reported lung function outcomes.13-15
With one notable exception, there were no statistically significant differences in outcomes among groups. Cowie et al16 reported an 11-fold decrease in total emergency room visits for the group using a peak-flow action plan (5 vs 55, P = .02), and also reported a reduction in hospitalizations of a similar magnitude (2 vs 12) that did not reach statistical significance. However, this study suffers from notable flaws that diminish confidence in the results. It is a post-intervention comparison among groups, which does not compare change from baseline, or incorporate baseline values as covariates in the analysis. Moreover baseline utilization data were provided by patient recall and not corroborated by medical records. There was a substantially larger variability in the baseline utilization rates for the peak flow group compared with the control group. This suggests that a subset of very high frequency users may have been over-represented in the peak flow group, and the reduction in emergency room visits may be concentrated in this subset.
Peak-flow meter-based written action plan versus peak flow meter with no written action plan
Two studies18,19 addressed the independent effect of a written action plan when added to peak flow self-monitoring (Table 3). Charlton19 reported no significant group differences for main outcomes, while Ignacio-Garcia18 reported large and statistically significant differences in most of the outcomes, favoring the group that used the written action plan.
The Ignacio-Garcia study, however, suffers from notable flaws suggesting the results may be attributable to bias. The sole participating physician, not blinded to treatment assignment, was highly involved in all phases of patient assessment, monitoring, and treatment. There was evidence of baseline differences between the two groups. A total of 25% of patients were withdrawn after randomization, and an unexplained decline in lung function occurred in the control group. Thus, the potential for selection bias, withdrawal bias, and ascertainment bias limits confidence in the results of this study
Symptom-based written action plan compared with peak flow-based written action plan
In 4 studies,16,17,20,21 reported outcomes were generally equivalent between groups and comparisons were not statistically significant, with one exception (Table 3). The 3-arm study by Cowie et al16 reported a striking reduction in the total number of emergency room visits with a peak flow meter-based written action plan compared with a symptom-based written action plan (5 versus 45, P
Discussion
The objective of this systematic review was to assess the independent effects of 2 specific components commonly included in asthma self-management plans—a written action plan and a peak flow meter. Few studies, however, are designed to permit reviewers to isolate the effects of these components. Moreover, the studies we reviewed did not clearly identify the population expected to benefit from interventions or specify the primary outcomes of interest; nor was the level of clinically meaningful improvement prospectively defined.
Most of the trials we reviewed, including the largest community study of 569 patients, did not demonstrate improved outcomes. The 2 trials that reported statistically significant results favoring a peak flow-based written action plan suffer from notable flaws suggesting the results may be attributable to bias. In the other 7 trials, there was little difference in outcomes between groups. However, these studies had insufficient power to detect group differences or confidently conclude equivalence between groups.
Thus, available evidence is insufficient to demonstrate that asthma outcomes are improved by use of a written asthma action plan, with or without peak flow monitoring. While this body of literature does not establish that these interventions are ineffective, it suggests they will not have a large effect on outcomes when applied to the general asthmatic population. The application of written action plans to all asthmatics indiscriminately may be a wasteful use of resources. This systematic review also questions the validity of written action plans as an indicator of asthma quality of care, or as a means to achieve quality improvement.
This analysis also highlights several obstacles to assessing the effects of disease management interventions. First, while the impact of whole intervention programs can be evaluated in controlled trials, it may be unfeasible to isolate each component of such programs and subject it to a rigorous analysis. Furthermore, as a behavioral intervention, the general principle of engaging patients in self-management may be more important that the specific components of these programs. Finally, regarding the optimization of medications (most obviously initiation of inhaled steroids) the impact of written action plans is likely to be relatively small, particularly on lung function or symptom control.
Future clinical trials should be done selectively, aimed at producing rigorous results that can improve the effectiveness of self-management interventions. Further study is warranted for specific subpopulations, such as those with higher baseline severity of illness or those with high baseline utilization rates. Available data suggest that, if there is benefit to be gained from self-management interventions, it will most likely be seen among these patients. Specific components of self-management that might be tested individually are those that are relatively high-cost, resource intensive, or risky for the patient.
Existing trials have tended to over-estimate the effects of action plan-based interventions, thus having invested resources for results inadequate for optimizing self-management strategies. Careful consideration needs to be taken in future trials to realistically estimate the expected impact of each intervention, and to specify the primary outcomes of interest and their baseline frequencies. Future trials should be large enough to detect a difference if one exists, or to confidently conclude that the intervention is ineffective.
Attention to these principles will help to advance our knowledge in this area most efficiently and to ultimately improve the quality of care for the entire population of patients with asthma.
· Acknowledgments ·
We acknowledge Kathleen Ziegler, Pharm.D, and Claudia Bonnell, RN, MSL, for their assistance in the research and preparation of this manuscript.
- Most studies of asthma self-management do not permit retrospective isolation of the independent effects of a written action plan or peak flow meter use.
- Studies designed to isolate the effect of these self-care activities are generally underpowered or prone to systematic bias.
- Available evidence suggests that peak flow meters and written action plans do not have a large impact on outcomes when applied to the general population of asthmatics.
- These interventions are most likely to have beneficial effect when applied to selected populations, particularly patients with high baseline utilization.
Self-management skills are widely promoted by health plans and specialty societies with the expectation that they will improve care. The 1997 National Heart, Lung, and Blood Institute guidelines on treating asthma emphasize self-management,1 although they do not recommend specific programs. To maximize therapeutic effectiveness, it would be useful to know which components of patient self-management improve outcomes. Written action plans and peak flow meters are commonly used in asthma self-management programs. While these are simple, low-cost interventions for an individual, the aggregate cost for the entire population of asthmatics may be high.2
Much literature has accumulated on the effectiveness of providing asthma education alone and on programs that actively engage patients in their own care.Several systematic reviews have found that providing educational information alone has had little effect on asthma outcomes.3-5 There is evidence, though, that self-management activities are more effective than educational information alone. A recent Cochrane review of 24 trials found that self-management with regular practitioner review reduces hospi-talizations and emergency room visits.6 This review did not identify specific components contributing to improved outcomes. In contrast to the aforementioned studies on patient education, a large case-control study of children in the Kaiser Permanente System,7 found that written action plans were associated with lower rates of hospitalization and emergency room use. However, such observational studies often include confounding factors and are not sufficient to establish a cause-effect relationship between written action plans and improved outcomes.
We report on a systematic review that attempts to isolate the independent effect of a written action plan on asthma outcomes. We address two key questions:
- Compared with medical management alone, does the addition of a written asthma action plan (with or without peak flow meter use) improve outcomes?
- Compared with a written action plan based on symptoms, does a written action plan based on peak flow monitoring improve outcomes?
Methods
This study is part of a broader evidence report on the management of chronic asthma prepared for the Agency of Health Care Research and Quality8. Complete details of the methodology are available in the full report8 (http://www.ahcpr.gov/clinic/epcix.htm).
Literature search and study selection
We performed a comprehensive literature search from 1980 to August 2000 using MEDLINE, Embase, the Cochrane Library, and a hand search of recent bibliographies. The search was limited to full-length, peer-reviewed articles with an English abstract. Two independent reviewers carried out each step of study selection and data abstraction. Disagreements were resolved by consensus of the two reviewers or, if necessary, by the decision of a third reviewer.
Initial study selection was limited to comparative full-length reports or abstracts in peer-reviewed medical journals, with at least 25 evaluable children or adults per arm, treated for at least 12 weeks. Relevant comparisons included a written action plan and no written action plan; a written action plan based on peak flow readings and a written action plan based on symptoms. Study designs varied: clinical trials, cohort comparisons, case-control analyses, cross-sectional evaluations, and before-after comparisons. Specific components of the management plan had to be described.
Relevant outcomes included measures of inpatient and outpatient utilization, lung function, symptoms, rescue medication or oral steroid use, and quality of life. Outcomes of greatest interest were utilization parameters, as the goals of self-management usually focus on improving these outcomes.
These initial selection criteria yielded many studies that were confounded by multiple asthma management interventions and thus did not isolate the comparisons of interest. Therefore, the research team collectively determined the study design features that would best isolate the effects of written action plans and used them as new criteria in a second round of study selection. The studies thus selected satisfied 4 criteria: 1) randomization of patients; (2) delivery of the same interventions to experimental and control groups, except that the experimental group also received a written action plan; (3) delivery of the same interventions to experimental and control groups, except that one group received a written action plan based on peak flow meter readings, and the comparison group received a written action plan based on symptom monitoring; and 4) inclusion of a written action plan that met our specified definition.
A written action plan, by our definition, had two components: an algorithm that identified specific clinical indicators signaling the need for adjustments in medication; and specific instructions on how to adjust medications in response to such indicators. Many publications lacked sufficient detail on the written plan, so a brief survey was sent to the primary author of each of the 36 studies. If no response was obtained (36%), the article was excluded only when it was clear from the publication that our definition was not met.
Assessment of study quality
High-quality studies were randomized controlled trials that met the 3 domains of study quality that have been demonstrated empirically to impact effect size: concealment of treatment allocation; double-blinding; and minimization of exclusion bias.9,10 However, we doubted the feasibility of double-blinding a written asthma plan intervention, and so relaxed this requirement. We considered exclusion bias to be minimized when a study either reported intent-to-treat analysis or excluded fewer than 10% of subjects from analysis, with the ratio of subjects excluded from each arm being less than 2:1.
To more fully evaluate study design issues that may be particularly important in asthma research,11,12 we constructed asthma-specific quality indicators in consultation with an expert panel. Controls for potential confounders of treatment effect included establishing reversibility of airway obstruction, controlling for other medication use, reporting compliance, and addressing seasonality. In addition, a priori reporting of power calculations and accounting for exclusions and withdrawals were judged to be study quality characteristics pertinent to this body of evidence.
Data analysis
We constructed evidence tables for the outcomes of interest, and performed a qualitative synthesis of the data. Meta-analysis was not appropriate due to wide discrepancies in the patient populations studied, the interventions employed, and measurement and reporting of outcomes.
Results
Our literature search yielded a total of 4578 citations. Of these, 36 studies met the initial selection criteria. Many of these qualifying studies, however, were confounded by multiple asthma management interventions applied inconsistently across treatment arms. For example, a common confounder was review of and change in long-term medication use in the treatment group, but not in the control group. This necessitated a refinement in our selection criteria to focus on studies that largely isolated the effect of written action plans.13-21 This step yielded a final evidence base of 9 randomized controlled trials with a total enrollment of 1501 patients.
Table 1 summarizes the characteristics, interventions, and outcomes of the 9 studies. Two studies were 3-arm trials,16,17 which raised the total number of comparisons among the 9 studies to 11. The largest study was the Grampian Asthma Study of Integrated Care (n=569),14 a community study conducted in the UK. Enrollment in the other 8 studies ranged from 43 to 64 patients per arm. Treatment duration ranged from 24 to 52 weeks.
None of the studies met our definition of high quality. In fact, no study met any of the generic quality criteria—none was blinded, none described concealment of allocation, and all excluded more than 10% of subjects. Furthermore, none reported an intention-to-treat analysis. Thus these trials were prone to withdrawal bias as well as overestimation of treatment effect due to lack of allocation concealment.
No study met the majority of asthma-specific indicators (Table 1). Of the 9 studies, only 5 met any asthma-specific indicator. Three reported prospective power calculations,13-15 but 2 of these substantially overestimated the expected effect.13,15 Two studies established reversibility;14,17 2 controlled for other medication use;13,15 and 2 reported compliance.17,21 Thus, the studies were also prone to a type II error (failing to detect a true effect) and to potential confounding of outcomes.
We performed sample power calculations for hospitalizations (Table 2), derived from baseline rates reported in 4 studies14,16-18 and standard deviations reported in 2.14,17 A study with 250 patients per arm could detect a reduction of 50% or more in hospitalization, given a control rate of at least 0.2 hospitalizations/patient/year. In actuality, GRASSIC,14 which is the largest available trial (N=569), had baseline hospitalization rates of 0.12 and 0.13. With this baseline rate, over 700 patients per arm are required, higher than the actual enrollment in GRASSIC. The other studies in this review would be adequately powered to detect a 50% difference only in the setting of even higher baseline utilization (eg, 0.30 hospitalizations/patient/year).
Table 3 displays utilization outcomes for the 11 comparisons in the 9 trials. In 5 studies (N=1019), medical management with a written action plan was compared with medical management without a written action plan.13-17 Two trials (N=185) compared a peak flow meter plus a written action plan with a peak flow meter and no written action plan18,19 In 4 studies (N=393), a written action plan based on peak flow monitoring was compared with a written action plan based on symptoms.
TABLE 1
Study characteristics
| Study | Patient popultation | Study Arms | Intervention components | Outcomes reported | Asthma quality indicators met |
|---|---|---|---|---|---|
| Optimal medical management vs. optimal medical management + PFM action plan | |||||
| Jones 199514 | Inclusions: patients using ICS | Usual care | SxD, FU | Ut, LF, Sx | Pow, Med |
| Exclusions: patients on oral steroids or using peak flow meters at home | PFM action plan | AP, PF, SxD, FU | |||
| Mean age: 29.5 years | |||||
| Severity level: Mild–moderate | |||||
| Drummond 1994 (GRASSIC)15 | Inclusion: FEV1 reversibility 20% or greater | Usual care | FU | Ut, LF, Med Ex | Pow, Rev |
| Exclusions: patients who already owned a PFM | PFM action plan | AP, PF, FU | |||
| Mean age: 50.8 years | |||||
| Severity level: Mild–severe | |||||
| Ayres 199516 | Inclusions: maximum PEF variability, 0.15%; minimum nights/week with symptoms, 3; minimum use of ICS or sodium cromoglycate, 3 months | Usual care | SxD, FU | LF, Sx, Ex | Pow, Med |
| Mean age: 45 years | PFM action plan | AP, PF, SxD, FU | |||
| Severity level: Moderate–severe | |||||
| Cowie 199713 | Inclusions: treatment for an exacerbation of asthma in an ER asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Usual care | Ed, SxD, FU | Ut, PF, Med, Ex | None |
| Mean age: 37.8 years | PFM action plan | AP, PF, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Cote 199717 | Inclusions: FEV1postbronchodilator 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Usual care | Ed | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: patients having previously taken an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Mean age: 36.5 years Severity level: Mild | |||||
| Usual care + PFM use alone vs. usual care + PFM action plan | |||||
| Ignacio-Garcia 199518 | Inclusions: patients from outpatient asthma clinic with asthma for 2 years | Usual care + PFM | PF, SxD, FU | Ut, LF, Med | None |
| Mean age: 41.9 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Charlton 199419 | Inclusion: patients with inpatient or outpatient visit for asthma | Usual care + PFM | PF, Ed, SxD, FU | Ut, Sx, Med, Ex | None |
| Mean age: 6.5 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–moderate | |||||
| PFM action plan vs. Symptom action plan | |||||
| Turner 199820 | Inclusions: Maximum methacholine PC20, 7.9; using ICS | Symptom action plan | AP, Ed, SxD, Cn BM, EM | Ut, LF, Sx, Med | Exc, Com |
| Exclusions: previous PFM use; significant comorbid conditions | PFM action plan | PF, AP, Ed, SxD, Cn BM, EM | |||
| Mean age: 34.1 years | |||||
| Severity level: Mild–severe | |||||
| Charlton 199021 | Inclusions: patients on repeat prescribing register | Symptom action plan | AP, Ed, FU | Ut, Med | None |
| Mean age: NR | PFM action plan | PF, AP, Ed, FU, Cn | Ut, PF, Med, Ex | None | |
| Severity level: Mild–severe (?) | |||||
| Cowie 199716 | Inclusions: treatment for an exacerbation of asthma in an ER, or asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Symptom action plan | AP, Ed, SxD, FU | ||
| PFM action plan | AP, PF, Ed, SxD, FU | ||||
| Cote 199717 | Inclusions: FEV1postbronchodilator, 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Symptom action plan | Ed, AP | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: previous enrollment in an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Eligibility criteria: ICS = inhaled corticosteroid; FEV1 = forced expiratory volume in 1 second; PEF = peak expiratory flow; PFM = peak flow meter; ER = emergency room; PC20 = 20% fall in FEV1 Intervention components: PF = Peak flow meter; AP = Written Action Plan; Ed = Education; SxD = Symptom diary; FU = Follow-up visits; Cn = Counseling; BM = Behavior modification; EM = Environmental modification | |||||
| Outcomes: Ut = Utilization measures; LF= Lung function measurements; Sx = Symptom=based measurements; Med = Medication use; Ex = Exacerbations of asth ma Asthma Quality Indicators: Exc = Accounted for excluded patients; Pow = Reported power calculations; Rev = Established reversibility of airway obstruction; Med = Controlled for other medication use; Com = Reported compliance; Sea = Addressed seasonality. | |||||
TABLE 2
Power calculations for hospitalizations per patient per year
| Assumed control mean | Possible treatment mean | % decrease | N needed per study arm |
|---|---|---|---|
| 0.10 | 0.075 | 25 | 3077 |
| 0.10 | 0.05 | 50 | 770 |
| 0.10 | 0.025 | 75 | 342 |
| 0.20 | 0.015 | 25 | 770 |
| 0.20 | 0.10 | 50 | 193 |
| 0.20 | 0.05 | 75 | 86 |
| 0.30 | 0.225 | 25 | 342 |
| 0.30 | 0.15 | 50 | 86 |
| 0.30 | 0.075 | 75 | 38 |
| Studies were identified that contained baseline rates on hospitalizations/patient/year, or information that allowed calculation of this parameter (Drummond, Abdalla, Beattie et al., 1994; Cote, Cartier, Robichaud et al. 1997; Cowie, Revitt, Underwood et al., 1997; Ignacio-Garcia and Gonzalez-Santos, 1995). Baseline rates of hospitalization varied in these studies from 0.04-0.29/patient/year. Standard deviations for this outcome were available only in two studies; Cote, Cartier, Robichaud et al. (1997) reported an SD of 0.30 for this variable, and an SD of 0.35 was calculated from the confidence intervals reported in GRASSIC (Drummond, Abdalla, Beattie et al., 1994). For the calculations, the more conservative 0.35 estimate for SD was used. | |||
| Number of patients per study arm were estimated for 80 percent power at the 5 percent significance level using control arm means of 0.10, 0.20, and 0.30 hospitalizations/patient/year. The expected reduction in this variable was tested along a spectrum from 25-75 percent. | |||
Written action plan versus no written action plan
All 5 studies used a peak flow meter based written action plan. All reported utilization outcomes, but the types and units of measurement were not consistent across studies (Table 2). Additionally, 4 studies reported on symptoms,13-16 and 3 reported lung function outcomes.13-15
With one notable exception, there were no statistically significant differences in outcomes among groups. Cowie et al16 reported an 11-fold decrease in total emergency room visits for the group using a peak-flow action plan (5 vs 55, P = .02), and also reported a reduction in hospitalizations of a similar magnitude (2 vs 12) that did not reach statistical significance. However, this study suffers from notable flaws that diminish confidence in the results. It is a post-intervention comparison among groups, which does not compare change from baseline, or incorporate baseline values as covariates in the analysis. Moreover baseline utilization data were provided by patient recall and not corroborated by medical records. There was a substantially larger variability in the baseline utilization rates for the peak flow group compared with the control group. This suggests that a subset of very high frequency users may have been over-represented in the peak flow group, and the reduction in emergency room visits may be concentrated in this subset.
Peak-flow meter-based written action plan versus peak flow meter with no written action plan
Two studies18,19 addressed the independent effect of a written action plan when added to peak flow self-monitoring (Table 3). Charlton19 reported no significant group differences for main outcomes, while Ignacio-Garcia18 reported large and statistically significant differences in most of the outcomes, favoring the group that used the written action plan.
The Ignacio-Garcia study, however, suffers from notable flaws suggesting the results may be attributable to bias. The sole participating physician, not blinded to treatment assignment, was highly involved in all phases of patient assessment, monitoring, and treatment. There was evidence of baseline differences between the two groups. A total of 25% of patients were withdrawn after randomization, and an unexplained decline in lung function occurred in the control group. Thus, the potential for selection bias, withdrawal bias, and ascertainment bias limits confidence in the results of this study
Symptom-based written action plan compared with peak flow-based written action plan
In 4 studies,16,17,20,21 reported outcomes were generally equivalent between groups and comparisons were not statistically significant, with one exception (Table 3). The 3-arm study by Cowie et al16 reported a striking reduction in the total number of emergency room visits with a peak flow meter-based written action plan compared with a symptom-based written action plan (5 versus 45, P
Discussion
The objective of this systematic review was to assess the independent effects of 2 specific components commonly included in asthma self-management plans—a written action plan and a peak flow meter. Few studies, however, are designed to permit reviewers to isolate the effects of these components. Moreover, the studies we reviewed did not clearly identify the population expected to benefit from interventions or specify the primary outcomes of interest; nor was the level of clinically meaningful improvement prospectively defined.
Most of the trials we reviewed, including the largest community study of 569 patients, did not demonstrate improved outcomes. The 2 trials that reported statistically significant results favoring a peak flow-based written action plan suffer from notable flaws suggesting the results may be attributable to bias. In the other 7 trials, there was little difference in outcomes between groups. However, these studies had insufficient power to detect group differences or confidently conclude equivalence between groups.
Thus, available evidence is insufficient to demonstrate that asthma outcomes are improved by use of a written asthma action plan, with or without peak flow monitoring. While this body of literature does not establish that these interventions are ineffective, it suggests they will not have a large effect on outcomes when applied to the general asthmatic population. The application of written action plans to all asthmatics indiscriminately may be a wasteful use of resources. This systematic review also questions the validity of written action plans as an indicator of asthma quality of care, or as a means to achieve quality improvement.
This analysis also highlights several obstacles to assessing the effects of disease management interventions. First, while the impact of whole intervention programs can be evaluated in controlled trials, it may be unfeasible to isolate each component of such programs and subject it to a rigorous analysis. Furthermore, as a behavioral intervention, the general principle of engaging patients in self-management may be more important that the specific components of these programs. Finally, regarding the optimization of medications (most obviously initiation of inhaled steroids) the impact of written action plans is likely to be relatively small, particularly on lung function or symptom control.
Future clinical trials should be done selectively, aimed at producing rigorous results that can improve the effectiveness of self-management interventions. Further study is warranted for specific subpopulations, such as those with higher baseline severity of illness or those with high baseline utilization rates. Available data suggest that, if there is benefit to be gained from self-management interventions, it will most likely be seen among these patients. Specific components of self-management that might be tested individually are those that are relatively high-cost, resource intensive, or risky for the patient.
Existing trials have tended to over-estimate the effects of action plan-based interventions, thus having invested resources for results inadequate for optimizing self-management strategies. Careful consideration needs to be taken in future trials to realistically estimate the expected impact of each intervention, and to specify the primary outcomes of interest and their baseline frequencies. Future trials should be large enough to detect a difference if one exists, or to confidently conclude that the intervention is ineffective.
Attention to these principles will help to advance our knowledge in this area most efficiently and to ultimately improve the quality of care for the entire population of patients with asthma.
· Acknowledgments ·
We acknowledge Kathleen Ziegler, Pharm.D, and Claudia Bonnell, RN, MSL, for their assistance in the research and preparation of this manuscript.
1. National Heart, Lung and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4051.
2. Ruffin RE, Pierce RJ. Peak flow monitoring—which asthmatics, when, and how? Aust N Z J Med 1994;24:519-20.
3. Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nursing Health 1996;19:367-76.
4. Bernard-Bonnin AC, Stachenko S, Bonin D, et al. Self-management teaching programs and morbidity of pediatric asthma: a meta-analysis. J Allergy Clin Immunol 1995;95(1 Pt 1):34-41.
5. Gibson PG, Coughlan J, Wilson AJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000a;(2):CD001005.-
6. Gibson PG, Coughlan J, Wilson AJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000b (2):CD001117.-
7. Lieu TA, Quesenberry CP, Jr, Capra AM, et al. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics 1997;100(3 Pt 1):334-41.
8. Lefevre F, Piper M, Mark D, et al. Management of Chronic Asthma. AHRQ evidence report, contract number 290-97-001-5, 2001, http://www.ahcpr.gov/clinic/epcix.htm.
9. Mulrow CD, Oxman AD, editors. Cochrane Collaboration Handbook. Available in the Cochrane Library [database on disk and CD-ROM]. The Cochrane Collaboration; Issue 1. Oxford: Update Software; 1997.
10. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12.
11. Berlin JA, Rennie D. Measuring the quality of trials: the quality of the quality scales. JAMA 1999;282(11):1083-5.
12. Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11):1054-60.
13. Jones KP, Mullee MA, Middleton M, et al. Peak flow based asthma self-management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851-7.
14. Drummond N, Abdalla M, Beattie JAG, et al. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care GRASSIC). BMJ 1994 Feb. 26;308(6928):564-7.
15. Ayres JG, Campbell LM. A controlled assessment of an asthma self-management plan involving a budesonide dose regimen. OPTIONS Research Group. Eur Respir J 1996;886-92.
16. Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534-8.
17. Cote J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155(5):1509-14.
18. Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med 1995;151(2 Pt 1):353-9.
19. Charlton I, Antoniou AG, Atkinson J, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Arch Dis Child 1994;70(4):313-8.
20. Turner MO, Taylor D, Bennett R, et al. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med 1998;157(2):540-6.
21. Charlton I, Charlton G, Broomfield J, et al. Evaluation of peak flow and symptoms only self-management plans for control of asthma in general practice. BMJ 1990;301(6765):1355-9.
1. National Heart, Lung and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4051.
2. Ruffin RE, Pierce RJ. Peak flow monitoring—which asthmatics, when, and how? Aust N Z J Med 1994;24:519-20.
3. Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nursing Health 1996;19:367-76.
4. Bernard-Bonnin AC, Stachenko S, Bonin D, et al. Self-management teaching programs and morbidity of pediatric asthma: a meta-analysis. J Allergy Clin Immunol 1995;95(1 Pt 1):34-41.
5. Gibson PG, Coughlan J, Wilson AJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000a;(2):CD001005.-
6. Gibson PG, Coughlan J, Wilson AJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000b (2):CD001117.-
7. Lieu TA, Quesenberry CP, Jr, Capra AM, et al. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics 1997;100(3 Pt 1):334-41.
8. Lefevre F, Piper M, Mark D, et al. Management of Chronic Asthma. AHRQ evidence report, contract number 290-97-001-5, 2001, http://www.ahcpr.gov/clinic/epcix.htm.
9. Mulrow CD, Oxman AD, editors. Cochrane Collaboration Handbook. Available in the Cochrane Library [database on disk and CD-ROM]. The Cochrane Collaboration; Issue 1. Oxford: Update Software; 1997.
10. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12.
11. Berlin JA, Rennie D. Measuring the quality of trials: the quality of the quality scales. JAMA 1999;282(11):1083-5.
12. Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11):1054-60.
13. Jones KP, Mullee MA, Middleton M, et al. Peak flow based asthma self-management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851-7.
14. Drummond N, Abdalla M, Beattie JAG, et al. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care GRASSIC). BMJ 1994 Feb. 26;308(6928):564-7.
15. Ayres JG, Campbell LM. A controlled assessment of an asthma self-management plan involving a budesonide dose regimen. OPTIONS Research Group. Eur Respir J 1996;886-92.
16. Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534-8.
17. Cote J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155(5):1509-14.
18. Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med 1995;151(2 Pt 1):353-9.
19. Charlton I, Antoniou AG, Atkinson J, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Arch Dis Child 1994;70(4):313-8.
20. Turner MO, Taylor D, Bennett R, et al. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med 1998;157(2):540-6.
21. Charlton I, Charlton G, Broomfield J, et al. Evaluation of peak flow and symptoms only self-management plans for control of asthma in general practice. BMJ 1990;301(6765):1355-9.
Inhaled salmeterol prevents high-altitude pulmonary edema
ABSTRACT
BACKGROUND: High-altitude pulmonary edema (HAPE) is a life-threatening manifestation of high-altitude illness. Although conventional medications such as acetazolamide and dexamethasone can prevent acute mountain sickness (a more common and less severe stage of high-altitude illness). Dexamethasone is known to be ineffective and acetazolamide has not been studied specifically for HAPE.1 Beta-agonists may decrease HAPE by promoting the clearance of alveolar fluid and thus relieving pulmonary edema and alveolar hypoxia. This study investigated the use of salmeterol to prevent HAPE in climbers at high risk for this condition.
POPULATION STUDIED: The investigators studied 37 mountaineers who had a history of HAPE (average of 2 previous episodes per subject). Most subjects were men, and the average age was 48 years. Baseline demographics were similar between groups. The population was appropriate for the condition being studied, although these men were at much higher risk for HAPE than the average recreational mountain climber.
STUDY DESIGN AND VALIDITY: This study was double-blind, randomized, and placebo controlled. Starting the day before ascent, the climbers inhaled either salmeterol 125 μg (about 3 times the normal asthma dosage) or placebo every 12 hours via metered-dose inhaler with spacer. They ascended (via cable car and mountaineering) from 1130 m to a high-altitude (4559 m) research laboratory in Italy over a period of 22 hours. Investigators then observed the subjects over a period of 2 days and nights for clinical and laboratory signs of HAPE and acute mountain sickness. Participants who developed symptoms of HAPE were evacuated to low altitude.
OUTCOMES MEASURED: The major patient-oriented end point was clinical and radiographic evidence of pulmonary edema. Investigators recorded Lake Louise Acute Mountain Sickness scores, arterial oxygen saturations, and carbon dioxide and oxygen arterial partial pressures. They also compared chest radiographs obtained at the high-altitude laboratory.
RESULTS: The incidence of pulmonary edema was less in the salmeterol group than with placebo (74% vs 33%; P=.02; numbers needed to treat=2.5). Lake Louise Acute Mountain Sickness scores were significantly better in the salmeterol group than in the placebo group (5.8 vs 11.5 out of a possible 24; P < .001). Chest radiographs, arterial oxygen saturations, and oxygen arterial partial pressures were also significantly improved with salmeterol.
Inhaled salmeterol decreases the incidence of HAPE in climbers with previous episodes of this condition. Nifedipine is the only other drug specifically shown to prevent HAPE2; although both the nifedipine study and the current salmeterol study were small, the 2 drugs appear roughly comparable in efficacy. It is unclear whether salmeterol would be effective for preventing more common and less severe stages of high-altitude illness (eg, acute mountain sickness), or whether the drug would be worthwhile in persons without a history of HAPE. Because of established efficacy in preventing acute mountain sickness, acetazolamide or dexamethasone should remain first-line agents for prevention of high-altitude illness in most climbers, with salmeterol or nifedipine added for individuals at high risk of HAPE.
ABSTRACT
BACKGROUND: High-altitude pulmonary edema (HAPE) is a life-threatening manifestation of high-altitude illness. Although conventional medications such as acetazolamide and dexamethasone can prevent acute mountain sickness (a more common and less severe stage of high-altitude illness). Dexamethasone is known to be ineffective and acetazolamide has not been studied specifically for HAPE.1 Beta-agonists may decrease HAPE by promoting the clearance of alveolar fluid and thus relieving pulmonary edema and alveolar hypoxia. This study investigated the use of salmeterol to prevent HAPE in climbers at high risk for this condition.
POPULATION STUDIED: The investigators studied 37 mountaineers who had a history of HAPE (average of 2 previous episodes per subject). Most subjects were men, and the average age was 48 years. Baseline demographics were similar between groups. The population was appropriate for the condition being studied, although these men were at much higher risk for HAPE than the average recreational mountain climber.
STUDY DESIGN AND VALIDITY: This study was double-blind, randomized, and placebo controlled. Starting the day before ascent, the climbers inhaled either salmeterol 125 μg (about 3 times the normal asthma dosage) or placebo every 12 hours via metered-dose inhaler with spacer. They ascended (via cable car and mountaineering) from 1130 m to a high-altitude (4559 m) research laboratory in Italy over a period of 22 hours. Investigators then observed the subjects over a period of 2 days and nights for clinical and laboratory signs of HAPE and acute mountain sickness. Participants who developed symptoms of HAPE were evacuated to low altitude.
OUTCOMES MEASURED: The major patient-oriented end point was clinical and radiographic evidence of pulmonary edema. Investigators recorded Lake Louise Acute Mountain Sickness scores, arterial oxygen saturations, and carbon dioxide and oxygen arterial partial pressures. They also compared chest radiographs obtained at the high-altitude laboratory.
RESULTS: The incidence of pulmonary edema was less in the salmeterol group than with placebo (74% vs 33%; P=.02; numbers needed to treat=2.5). Lake Louise Acute Mountain Sickness scores were significantly better in the salmeterol group than in the placebo group (5.8 vs 11.5 out of a possible 24; P < .001). Chest radiographs, arterial oxygen saturations, and oxygen arterial partial pressures were also significantly improved with salmeterol.
Inhaled salmeterol decreases the incidence of HAPE in climbers with previous episodes of this condition. Nifedipine is the only other drug specifically shown to prevent HAPE2; although both the nifedipine study and the current salmeterol study were small, the 2 drugs appear roughly comparable in efficacy. It is unclear whether salmeterol would be effective for preventing more common and less severe stages of high-altitude illness (eg, acute mountain sickness), or whether the drug would be worthwhile in persons without a history of HAPE. Because of established efficacy in preventing acute mountain sickness, acetazolamide or dexamethasone should remain first-line agents for prevention of high-altitude illness in most climbers, with salmeterol or nifedipine added for individuals at high risk of HAPE.
ABSTRACT
BACKGROUND: High-altitude pulmonary edema (HAPE) is a life-threatening manifestation of high-altitude illness. Although conventional medications such as acetazolamide and dexamethasone can prevent acute mountain sickness (a more common and less severe stage of high-altitude illness). Dexamethasone is known to be ineffective and acetazolamide has not been studied specifically for HAPE.1 Beta-agonists may decrease HAPE by promoting the clearance of alveolar fluid and thus relieving pulmonary edema and alveolar hypoxia. This study investigated the use of salmeterol to prevent HAPE in climbers at high risk for this condition.
POPULATION STUDIED: The investigators studied 37 mountaineers who had a history of HAPE (average of 2 previous episodes per subject). Most subjects were men, and the average age was 48 years. Baseline demographics were similar between groups. The population was appropriate for the condition being studied, although these men were at much higher risk for HAPE than the average recreational mountain climber.
STUDY DESIGN AND VALIDITY: This study was double-blind, randomized, and placebo controlled. Starting the day before ascent, the climbers inhaled either salmeterol 125 μg (about 3 times the normal asthma dosage) or placebo every 12 hours via metered-dose inhaler with spacer. They ascended (via cable car and mountaineering) from 1130 m to a high-altitude (4559 m) research laboratory in Italy over a period of 22 hours. Investigators then observed the subjects over a period of 2 days and nights for clinical and laboratory signs of HAPE and acute mountain sickness. Participants who developed symptoms of HAPE were evacuated to low altitude.
OUTCOMES MEASURED: The major patient-oriented end point was clinical and radiographic evidence of pulmonary edema. Investigators recorded Lake Louise Acute Mountain Sickness scores, arterial oxygen saturations, and carbon dioxide and oxygen arterial partial pressures. They also compared chest radiographs obtained at the high-altitude laboratory.
RESULTS: The incidence of pulmonary edema was less in the salmeterol group than with placebo (74% vs 33%; P=.02; numbers needed to treat=2.5). Lake Louise Acute Mountain Sickness scores were significantly better in the salmeterol group than in the placebo group (5.8 vs 11.5 out of a possible 24; P < .001). Chest radiographs, arterial oxygen saturations, and oxygen arterial partial pressures were also significantly improved with salmeterol.
Inhaled salmeterol decreases the incidence of HAPE in climbers with previous episodes of this condition. Nifedipine is the only other drug specifically shown to prevent HAPE2; although both the nifedipine study and the current salmeterol study were small, the 2 drugs appear roughly comparable in efficacy. It is unclear whether salmeterol would be effective for preventing more common and less severe stages of high-altitude illness (eg, acute mountain sickness), or whether the drug would be worthwhile in persons without a history of HAPE. Because of established efficacy in preventing acute mountain sickness, acetazolamide or dexamethasone should remain first-line agents for prevention of high-altitude illness in most climbers, with salmeterol or nifedipine added for individuals at high risk of HAPE.
Azithromycin no more effective than vitamin C for acute bronchitis
ABSTRACT
BACKGROUND: The results of studies evaluating the effectiveness of antibiotic treatment for acute bronchitis are conflicting, some with uncertain reliability and validity. Although most studies of antibiotics have focused on cure of disease or reduction in symptoms, this study tested whether patients with acute bronchitis who were treated with azithromycin experienced greater improvements in health-related quality of life than those treated with vitamin C. The authors chose to compare azithromycin with vitamin C instead of traditional placebo because they believed potential patients might refuse to participate in the study if there was a chance they would receive a placebo. Evidence has shown that vitamin C at the doses used in this study is ineffective in the treatment of acute bronchitis or other respiratory illnesses, making the vitamin a reasonable placebo for this study.1
POPULATION STUDIED: The authors studied 220 adults with cough lasting 2–14 days who were diagnosed with acute bronchitis after presenting to an ambulatory screening clinic in Chicago, Illinois. Patients were excluded if they had any underlying lung disorder, clinical characteristics of pneumonia, antibiotic treatment within the previous 2 weeks, pregnancy, steroid treatment, or had been started on an angiotensin-converting enzyme inhibitor within the previous 4 weeks.
STUDY DESIGN AND VALIDITY: This study was a randomized, double-blinded, controlled trial with concealed allocation. Patients were randomized to receive a total of 1.5 g of either azithromycin or vitamin C over 5 days (500 mg on the first day, then 250 mg/day for 4 more days). All patients also received symptomatic care with dex-tromethorphan and an albuterol inhaler with a spacer. Trained research assistants interviewed patients on enrollment in the study to assess their baseline health-related quality of life. The interview, consisting of 22 questions adapted from similar instruments developed at McMaster University, was repeated on days 3 and 7. For each of the questions, patients were asked to rate how troubled they had been during the previous few days as a result of their bronchitis symptoms on a 7-point scale. Follow-up was for 7 days from the beginning of the study and was 85.9% complete. Analysis was by intention to treat.
OUTCOMES MEASURED: The primary outcome measured was health-related quality of life on day 7 of follow-up. Secondary end points were return to usual daily activities at follow-up and adverse effects.
RESULTS: The adjusted difference in health-related quality of life between the patients taking azithromycin and those taking vitamin C was not significant on day 7 of the study (difference = 0.03; 95% confidence interval [CI], –0.20 to 0.26). Overall, 89% of patients in both groups returned to work by day 7 (difference = 0.5%; 95% CI, –10% to 9%). No difference was noted in the fre treat acute bronchitis in otherwise healthy adults.
Azithromycin is no more effective than vitamin C in treating acute bronchitis in healthy adults. Given the evidence that treatment with vitamin C is not effective in respiratory illnesses, azithromycin appears equally ineffective. With increasing health care costs and rising concerns about antibiotic resistance, azithromycin, and probably other antibiotics, should not be used to treat acute bronchitis in otherwise healthy adults.
ABSTRACT
BACKGROUND: The results of studies evaluating the effectiveness of antibiotic treatment for acute bronchitis are conflicting, some with uncertain reliability and validity. Although most studies of antibiotics have focused on cure of disease or reduction in symptoms, this study tested whether patients with acute bronchitis who were treated with azithromycin experienced greater improvements in health-related quality of life than those treated with vitamin C. The authors chose to compare azithromycin with vitamin C instead of traditional placebo because they believed potential patients might refuse to participate in the study if there was a chance they would receive a placebo. Evidence has shown that vitamin C at the doses used in this study is ineffective in the treatment of acute bronchitis or other respiratory illnesses, making the vitamin a reasonable placebo for this study.1
POPULATION STUDIED: The authors studied 220 adults with cough lasting 2–14 days who were diagnosed with acute bronchitis after presenting to an ambulatory screening clinic in Chicago, Illinois. Patients were excluded if they had any underlying lung disorder, clinical characteristics of pneumonia, antibiotic treatment within the previous 2 weeks, pregnancy, steroid treatment, or had been started on an angiotensin-converting enzyme inhibitor within the previous 4 weeks.
STUDY DESIGN AND VALIDITY: This study was a randomized, double-blinded, controlled trial with concealed allocation. Patients were randomized to receive a total of 1.5 g of either azithromycin or vitamin C over 5 days (500 mg on the first day, then 250 mg/day for 4 more days). All patients also received symptomatic care with dex-tromethorphan and an albuterol inhaler with a spacer. Trained research assistants interviewed patients on enrollment in the study to assess their baseline health-related quality of life. The interview, consisting of 22 questions adapted from similar instruments developed at McMaster University, was repeated on days 3 and 7. For each of the questions, patients were asked to rate how troubled they had been during the previous few days as a result of their bronchitis symptoms on a 7-point scale. Follow-up was for 7 days from the beginning of the study and was 85.9% complete. Analysis was by intention to treat.
OUTCOMES MEASURED: The primary outcome measured was health-related quality of life on day 7 of follow-up. Secondary end points were return to usual daily activities at follow-up and adverse effects.
RESULTS: The adjusted difference in health-related quality of life between the patients taking azithromycin and those taking vitamin C was not significant on day 7 of the study (difference = 0.03; 95% confidence interval [CI], –0.20 to 0.26). Overall, 89% of patients in both groups returned to work by day 7 (difference = 0.5%; 95% CI, –10% to 9%). No difference was noted in the fre treat acute bronchitis in otherwise healthy adults.
Azithromycin is no more effective than vitamin C in treating acute bronchitis in healthy adults. Given the evidence that treatment with vitamin C is not effective in respiratory illnesses, azithromycin appears equally ineffective. With increasing health care costs and rising concerns about antibiotic resistance, azithromycin, and probably other antibiotics, should not be used to treat acute bronchitis in otherwise healthy adults.
ABSTRACT
BACKGROUND: The results of studies evaluating the effectiveness of antibiotic treatment for acute bronchitis are conflicting, some with uncertain reliability and validity. Although most studies of antibiotics have focused on cure of disease or reduction in symptoms, this study tested whether patients with acute bronchitis who were treated with azithromycin experienced greater improvements in health-related quality of life than those treated with vitamin C. The authors chose to compare azithromycin with vitamin C instead of traditional placebo because they believed potential patients might refuse to participate in the study if there was a chance they would receive a placebo. Evidence has shown that vitamin C at the doses used in this study is ineffective in the treatment of acute bronchitis or other respiratory illnesses, making the vitamin a reasonable placebo for this study.1
POPULATION STUDIED: The authors studied 220 adults with cough lasting 2–14 days who were diagnosed with acute bronchitis after presenting to an ambulatory screening clinic in Chicago, Illinois. Patients were excluded if they had any underlying lung disorder, clinical characteristics of pneumonia, antibiotic treatment within the previous 2 weeks, pregnancy, steroid treatment, or had been started on an angiotensin-converting enzyme inhibitor within the previous 4 weeks.
STUDY DESIGN AND VALIDITY: This study was a randomized, double-blinded, controlled trial with concealed allocation. Patients were randomized to receive a total of 1.5 g of either azithromycin or vitamin C over 5 days (500 mg on the first day, then 250 mg/day for 4 more days). All patients also received symptomatic care with dex-tromethorphan and an albuterol inhaler with a spacer. Trained research assistants interviewed patients on enrollment in the study to assess their baseline health-related quality of life. The interview, consisting of 22 questions adapted from similar instruments developed at McMaster University, was repeated on days 3 and 7. For each of the questions, patients were asked to rate how troubled they had been during the previous few days as a result of their bronchitis symptoms on a 7-point scale. Follow-up was for 7 days from the beginning of the study and was 85.9% complete. Analysis was by intention to treat.
OUTCOMES MEASURED: The primary outcome measured was health-related quality of life on day 7 of follow-up. Secondary end points were return to usual daily activities at follow-up and adverse effects.
RESULTS: The adjusted difference in health-related quality of life between the patients taking azithromycin and those taking vitamin C was not significant on day 7 of the study (difference = 0.03; 95% confidence interval [CI], –0.20 to 0.26). Overall, 89% of patients in both groups returned to work by day 7 (difference = 0.5%; 95% CI, –10% to 9%). No difference was noted in the fre treat acute bronchitis in otherwise healthy adults.
Azithromycin is no more effective than vitamin C in treating acute bronchitis in healthy adults. Given the evidence that treatment with vitamin C is not effective in respiratory illnesses, azithromycin appears equally ineffective. With increasing health care costs and rising concerns about antibiotic resistance, azithromycin, and probably other antibiotics, should not be used to treat acute bronchitis in otherwise healthy adults.