User login
Adiposis Dolorosa Pain Management
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
Systemic Epstein-Barr Virus–Positive T-cell Lymphoma of Childhood
Case Report
A 7-year-old Chinese boy presented with multiple painful oral and tongue ulcers of 2 weeks’ duration as well as acute onset of moderate to high fever (highest temperature, 39.3°C) for 5 days. The fever was reported to have run a relapsing course, accompanied by rigors but without convulsions or cognitive changes. At times, the patient had nasal congestion, nasal discharge, and cough. He also had a transient eruption on the back and hands as well as an indurated red nodule on the left forearm.
Before the patient was hospitalized, antibiotic therapy was administered by other physicians, but the condition of fever and oral ulcers did not improve. After the patient was hospitalized, new tender nodules emerged on the scalp, buttocks, and lower extremities. New ulcers also appeared on the palate.
History
Two months earlier, the patient had presented with a painful perioral skin ulcer that resolved after being treated as contagious eczema. Another dermatologist previously had considered a diagnosis of hand-foot-and-mouth disease.
The patient was born by normal spontaneous vaginal delivery, without abnormality. He was breastfed; feeding, growth, and the developmental history showed no abnormality. He was the family’s eldest child, with a healthy brother and sister. There was no history of familial illness. He received bacillus Calmette-Guérin and poliomyelitis vaccines after birth; the rest of the vaccine history was unclear. There was no history of immunologic abnormality.
Physical Examination
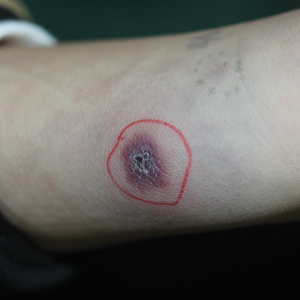
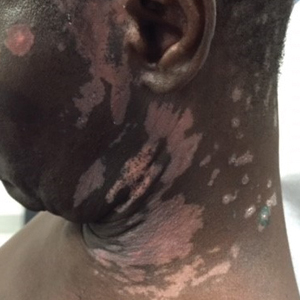
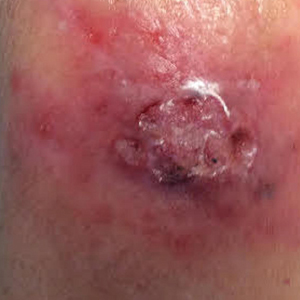
A 1.5×1.5-cm, warm, red nodule with a central black crust was noted on the left forearm (Figure 1A). Several similar lesions were noted on the buttocks, scalp, and lower extremities. Multiple ulcers, as large as 1 cm, were present on the tongue, palate, and left angle of the mouth (Figure 1B). The pharynx was congested, and the tonsils were mildly enlarged. Multiple enlarged, movable, nontender lymph nodes could be palpated in the cervical basins, axillae, and groin. No purpura or ecchymosis was detected.
Laboratory Results
Laboratory testing revealed a normal total white blood cell count (4.26×109/L [reference range, 4.0–12.0×109/L]), with normal neutrophils (1.36×109/L [reference range, 1.32–7.90×109/L]), lymphocytes (2.77×109/L [reference range, 1.20–6.00×109/L]), and monocytes (0.13×109/L [reference range, 0.08–0.80×109/L]); a mildly decreased hemoglobin level (115 g/L [reference range, 120–160 g/L]); a normal platelet count (102×109/L [reference range, 100–380×109/L]); an elevated lactate dehydrogenase level (614 U/L [reference range, 110–330 U/L]); an elevated α-hydroxybutyrate dehydrogenase level (483 U/L [reference range, 120–270 U/L]); elevated prothrombin time (15.3 s [reference range, 9–14 s]); elevated activated partial thromboplastin time (59.8 s [reference range, 20.6–39.6 s]); and an elevated D-dimer level (1.51 mg/L [reference range, <0.73 mg/L]). In addition, autoantibody testing revealed a positive antinuclear antibody titer of 1:320 and a strong positive anti–Ro-52 level.
The peripheral blood lymphocyte classification demonstrated a prominent elevated percentage of T lymphocytes, with predominantly CD8+ cells (CD3, 94.87%; CD8, 71.57%; CD4, 24.98%; CD4:CD8 ratio, 0.35) and a diminished percentage of B lymphocytes and natural killer (NK) cells. Epstein-Barr virus (EBV) antibody testing was positive for anti–viral capsid antigen (VCA) IgG and negative for anti-VCA IgM.
Smears of the ulcer on the tongue demonstrated gram-positive cocci, gram-negative bacilli, and diplococci. Culture of sputum showed methicillin-resistant Staphylococcus aureus. Inspection for acid-fast bacilli in sputum yielded negative results 3 times. A purified protein derivative skin test for Mycobacterium tuberculosis infection was negative.
Imaging and Other Studies
Computed tomography of the chest and abdomen demonstrated 2 nodular opacities on the lower right lung; spotted opacities on the upper right lung; floccular opacities on the rest area of the lung; mild pleural effusion; enlargement of lymph nodes on the mediastinum, the bilateral hilum of the lung, and mesentery; and hepatosplenomegaly. Electrocardiography showed sinus tachycardia. Nasal cavity endoscopy showed sinusitis. Fundus examination showed vasculopathy of the left retina. A colonoscopy showed normal mucosa.
Histopathology
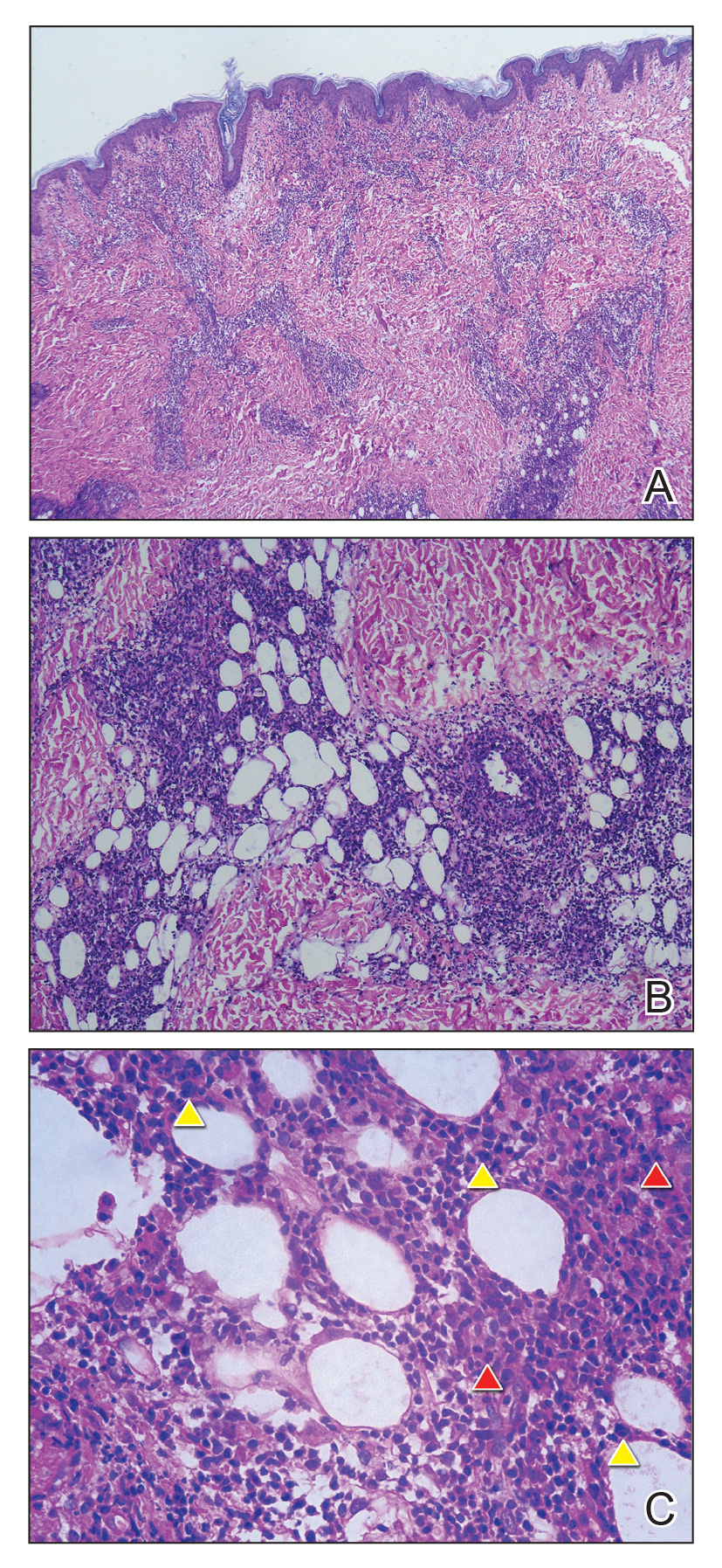
Biopsy of the nodule on the left arm showed dense, superficial to deep perivascular, periadnexal, perineural, and panniculitislike lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells (Figure 2). Infiltrative atypical lymphoid cells expressed CD3 and CD7 and were mostly CD8+, with a few CD4+ cells and most cells negative for CD5, CD20, CD30, CD56, and anaplastic lymphoma kinase. Cytotoxic markers granzyme B and T-cell intracellular antigen protein 1 were scattered positive. Immunostaining for Ki-67 protein highlighted an increased proliferative rate of 80% in malignant cells. In situ hybridization for EBV-encoded RNA (EBER) demonstrated EBV-positive atypical lymphoid cells (Figure 3). Analysis for T-cell receptor (TCR) γ gene rearrangement revealed a monoclonal pattern. Bone marrow aspirate showed proliferation of the 3 cell lines. The percentage of T lymphocytes was increased (20% of all nucleated cells). No hemophagocytic activity was found.
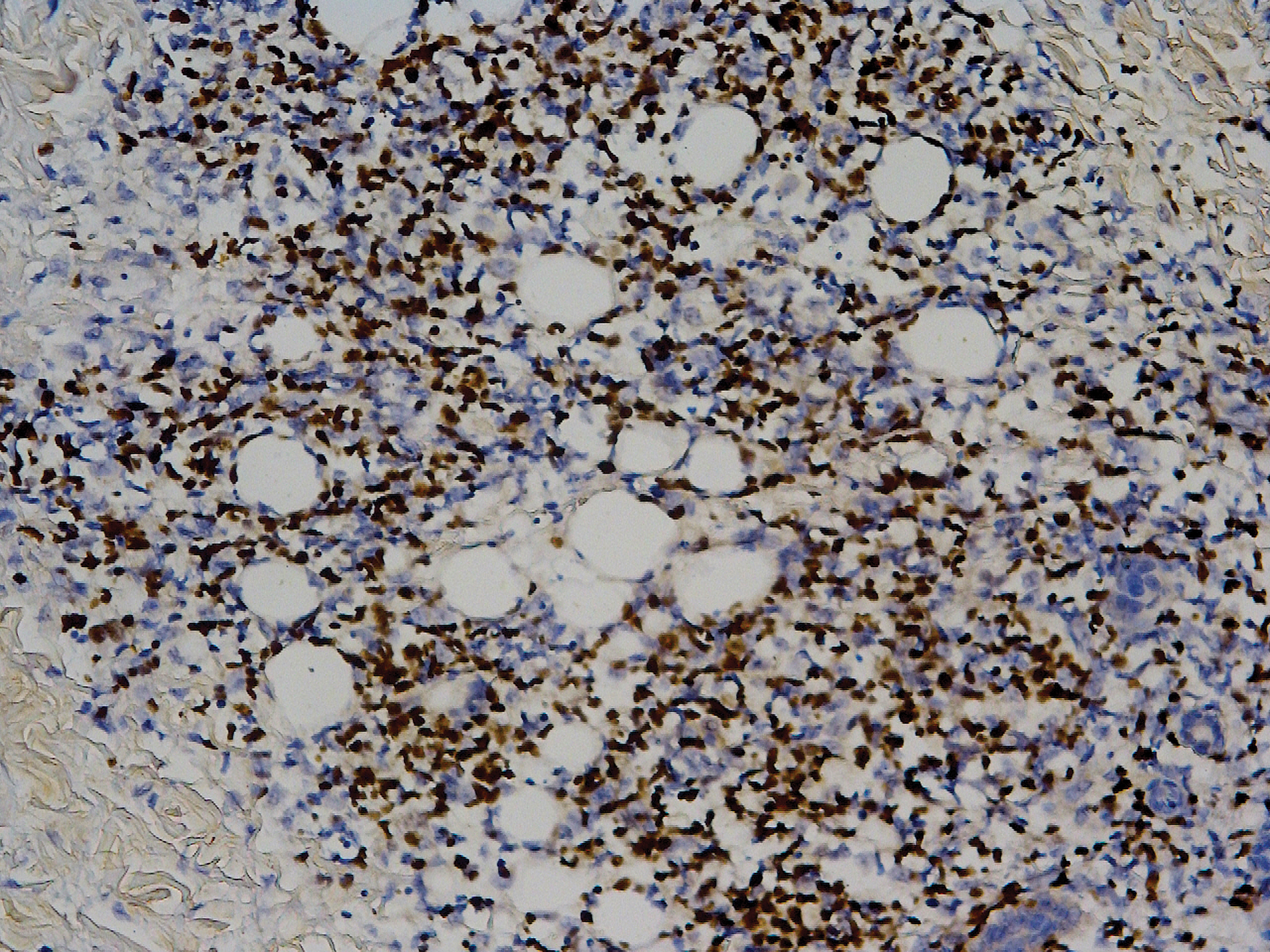
Diagnosis
A diagnosis of systemic EBV-positive T-cell lymphoma was made. Before the final diagnosis was made, the patient was treated by rheumatologists with antibiotics, antiviral drugs, nonsteroidal anti-inflammatory drugs, and other symptomatic treatments. Following antibiotic therapy, a sputum culture reverted to normal flora, the coagulation index (ie, prothrombin time, activated partial thromboplastin time) returned to normal, and the D-dimer level decreased to 1.19 mg/L.
The patient’s parents refused to accept chemotherapy for him. Instead, they chose herbal therapy only; 5 months later, they reported that all of his symptoms had resolved; however, the disease suddenly relapsed after another 7 months, with multiple skin nodules and fever. The patient died, even with chemotherapy in another hospital.
Comment
Prevalence and Presentation
Epstein-Barr virus is a ubiquitous γ-herpesvirus with tropism for B cells, affecting more than 90% of the adult population worldwide. In addition to infecting B cells, EBV is capable of infecting T and NK cells, leading to various EBV-related lymphoproliferative disorders (LPDs). The frequency and clinical presentation of infection varies based on the type of EBV-infected cells and the state of host immunity.1-3
Primary infection usually is asymptomatic and occurs early in life; when symptomatic, the disease usually presents as infectious mononucleosis (IM), characterized by polyclonal expansion of infected B cells and subsequent cytotoxic T-cell response. A diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against VCA, early antigens, and EBV nuclear antigen proteins.3,4
Associated LPDs
Although most symptoms associated with IM resolve within weeks or months, persistent or recurrent IM-like symptoms or even lasting disease occasionally occur, particularly in children and young adults. This complication is known as chronic active EBV infection (CAEBV), frequently associated with EBV-infected T-cell or NK-cell proliferation, especially in East Asian populations.3,5
Epstein-Barr virus–positive T-cell and NK-cell LPDs of childhood include CAEBV infection of T-cell and NK-cell types and systemic EBV-positive T-cell lymphoma of childhood. The former includes hydroa vacciniforme–like LPD and severe mosquito bite allergy.3
Systemic EBV-Positive T-cell Lymphoma of Childhood
This entity occurs not only in children but also in adolescents and young adults. A fulminant illness characterized by clonal proliferation of EBV-infected cytotoxic T cells, it can develop shortly after primary EBV infection or is linked to CAEBV infection. The disorder is rare and has a racial predilection for Asian (ie, Japanese, Chinese, Korean) populations and indigenous populations of Mexico and Central and South America.6-8
Complications
Systemic EBV-positive T-cell lymphoma of childhood is often complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. Other signs and symptoms include high fever, rash, jaundice, diarrhea, pancytopenia, and hepatosplenomegaly. The liver, spleen, lymph nodes, and bone marrow are commonly involved, and the disease can involve skin, the heart, and the lungs.9,10
Diagnosis
When systemic EBV-positive T-cell lymphoma occurs shortly after IM, serology shows low or absent anti-VCA IgM and positive anti-VCA IgG. Infiltrating T cells usually are small and lack cytologic atypia; however, cases with pleomorphic, medium to large lymphoid cells, irregular nuclei, and frequent mitoses have been described. Hemophagocytosis can be seen in the liver, spleen, and bone marrow.3,11
The most typical phenotype of systemic EBV-positive T-cell lymphoma is CD2+CD3+CD8+CD20−CD56−, with expression of the cytotoxic granules known as T-cell intracellular antigen 1 and granzyme B. Rare cases of CD4+ and mixed CD4+/CD8+ phenotypes have been described, usually in the setting of CAEBV infection.3,12 Neoplastic cells have monoclonally rearranged TCR-γ genes and consistent EBER positivity with in situ hybridization.13 A final diagnosis is based on a comprehensive analysis of clinical, morphological, immunohistochemical, and molecular biological aspects.
Clinical Course and Prognosis
Most patients with systemic EBV-positive T-cell lymphoma have an aggressive clinical course with high mortality. In a few cases, patients were reported to respond to a regimen of etoposide and dexamethasone, followed by allogeneic hematopoietic stem cell transplantation.3
In recognition of the aggressive clinical behavior and desire to clearly distinguish systemic EBV-positive T-cell lymphoma from CAEBV infection, the older term systemic EBV-positive T-cell LPD of childhood, which had been introduced in 2008 to the World Health Organization classification, was changed to systemic EBV-positive T-cell lymphoma of childhood in the revised 2016 World Health Organization classification.6,12 However, Kim et al14 reported a case with excellent response to corticosteroid administration, suggesting that systemic EBV-positive T-cell lymphoma of childhood may be more heterogeneous in terms of prognosis.
Our patient presented with acute IM-like symptoms, including high fever, tonsillar enlargement, lymphadenopathy, and hepatosplenomegaly, as well as uncommon oral ulcers and skin lesions, including indurated nodules. Histopathologic changes in the skin nodule, proliferation in bone marrow, immunohistochemical phenotype, and positivity of EBER and TCR-γ monoclonal rearrangement were all consistent with systemic EBV-positive T-cell lymphoma of childhood. The patient was positive for VCA IgG and negative for VCA IgM, compatible with systemic EBV-positive T-cell lymphoma of childhood occurring shortly after IM. Neither pancytopenia, hemophagocytic syndrome, nor multiorgan failure occurred during the course.
Differential Diagnosis
It is important to distinguish IM from systemic EBV-positive T-cell lymphoma of childhood and CAEBV infection. Detection of anti–VCA IgM in the early stage, its disappearance during the clinical course, and appearance of anti-EBV–determined nuclear antigen is useful to distinguish IM from the neoplasms, as systemic EBV-positive T-cell lymphoma of childhood is negative for anti-EBV–determined nuclear antigen. Carefully following the clinical course also is important.3,15
Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis can occur in association with systemic EBV-positive T-cell lymphoma of childhood and might represent a continuum of disease rather than distinct entities.14 The most useful marker for differentiating EBV-associated hemophagocytic lymphohistiocytosis and systemic EBV-positive T-cell lymphoma of childhood is an abnormal karyotype rather than molecular clonality.16
Outcome
Mortality risk in EBV-associated T-cell and NK-cell LPD is not primarily dependent on whether the lesion has progressed to lymphoma but instead is related to associated complications.17
Conclusion
Although systemic EBV-positive T-cell lymphoma of childhood is a rare disorder and has race predilection, dermatologists should be aware due to the aggressive clinical source and poor prognosis. Histopathology and in situ hybridization for EBER and TCR gene rearrangements are critical for final diagnosis. Although rare cases can show temporary resolution, the final outcome of this disease is not optimistic.
- Ameli F, Ghafourian F, Masir N. Systematic Epstein-Barr virus-positive T-cell lymphoproliferative disease presenting as a persistent fever and cough: a case report. J Med Case Rep. 2014;8:288.
- Kim HJ, Ko YH, Kim JE, et al. Epstein-Barr virus-associated lympho-proliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51:352-358.
- Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts [published online March 7, 2018]. Pathogens. doi:10.3390/pathogens7010028.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
- Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71.
- Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137-147.
- Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443-451.
- Chen G, Chen L, Qin X, et al. Systemic Epstein-Barr virus positive T-cell lymphoproliferative disease of childhood with hemophagocytic syndrome. Int J Clin Exp Pathol. 2014;7:7110-7113.
- Grywalska E, Rolinski J. Epstein-Barr virus-associated lymphomas. Semin Oncol. 2015;42:291-303.
- Huang W, Lv N, Ying J, et al. Clinicopathological characteristics of four cases of EBV positive T-cell lymphoproliferative disorders of childhood in China. Int J Clin Exp Pathol. 2014;7:4991-4999.
- Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011;5:218.
- Kim DH, Kim M, Kim Y, et al. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood with good response to steroid therapy. J Pediatr Hematol Oncol. 2017;39:e497-e500.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671-675.
- Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738-5749.
- Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53-63.
Case Report
A 7-year-old Chinese boy presented with multiple painful oral and tongue ulcers of 2 weeks’ duration as well as acute onset of moderate to high fever (highest temperature, 39.3°C) for 5 days. The fever was reported to have run a relapsing course, accompanied by rigors but without convulsions or cognitive changes. At times, the patient had nasal congestion, nasal discharge, and cough. He also had a transient eruption on the back and hands as well as an indurated red nodule on the left forearm.
Before the patient was hospitalized, antibiotic therapy was administered by other physicians, but the condition of fever and oral ulcers did not improve. After the patient was hospitalized, new tender nodules emerged on the scalp, buttocks, and lower extremities. New ulcers also appeared on the palate.
History
Two months earlier, the patient had presented with a painful perioral skin ulcer that resolved after being treated as contagious eczema. Another dermatologist previously had considered a diagnosis of hand-foot-and-mouth disease.
The patient was born by normal spontaneous vaginal delivery, without abnormality. He was breastfed; feeding, growth, and the developmental history showed no abnormality. He was the family’s eldest child, with a healthy brother and sister. There was no history of familial illness. He received bacillus Calmette-Guérin and poliomyelitis vaccines after birth; the rest of the vaccine history was unclear. There was no history of immunologic abnormality.
Physical Examination
A 1.5×1.5-cm, warm, red nodule with a central black crust was noted on the left forearm (Figure 1A). Several similar lesions were noted on the buttocks, scalp, and lower extremities. Multiple ulcers, as large as 1 cm, were present on the tongue, palate, and left angle of the mouth (Figure 1B). The pharynx was congested, and the tonsils were mildly enlarged. Multiple enlarged, movable, nontender lymph nodes could be palpated in the cervical basins, axillae, and groin. No purpura or ecchymosis was detected.

Laboratory Results
Laboratory testing revealed a normal total white blood cell count (4.26×109/L [reference range, 4.0–12.0×109/L]), with normal neutrophils (1.36×109/L [reference range, 1.32–7.90×109/L]), lymphocytes (2.77×109/L [reference range, 1.20–6.00×109/L]), and monocytes (0.13×109/L [reference range, 0.08–0.80×109/L]); a mildly decreased hemoglobin level (115 g/L [reference range, 120–160 g/L]); a normal platelet count (102×109/L [reference range, 100–380×109/L]); an elevated lactate dehydrogenase level (614 U/L [reference range, 110–330 U/L]); an elevated α-hydroxybutyrate dehydrogenase level (483 U/L [reference range, 120–270 U/L]); elevated prothrombin time (15.3 s [reference range, 9–14 s]); elevated activated partial thromboplastin time (59.8 s [reference range, 20.6–39.6 s]); and an elevated D-dimer level (1.51 mg/L [reference range, <0.73 mg/L]). In addition, autoantibody testing revealed a positive antinuclear antibody titer of 1:320 and a strong positive anti–Ro-52 level.
The peripheral blood lymphocyte classification demonstrated a prominent elevated percentage of T lymphocytes, with predominantly CD8+ cells (CD3, 94.87%; CD8, 71.57%; CD4, 24.98%; CD4:CD8 ratio, 0.35) and a diminished percentage of B lymphocytes and natural killer (NK) cells. Epstein-Barr virus (EBV) antibody testing was positive for anti–viral capsid antigen (VCA) IgG and negative for anti-VCA IgM.
Smears of the ulcer on the tongue demonstrated gram-positive cocci, gram-negative bacilli, and diplococci. Culture of sputum showed methicillin-resistant Staphylococcus aureus. Inspection for acid-fast bacilli in sputum yielded negative results 3 times. A purified protein derivative skin test for Mycobacterium tuberculosis infection was negative.
Imaging and Other Studies
Computed tomography of the chest and abdomen demonstrated 2 nodular opacities on the lower right lung; spotted opacities on the upper right lung; floccular opacities on the rest area of the lung; mild pleural effusion; enlargement of lymph nodes on the mediastinum, the bilateral hilum of the lung, and mesentery; and hepatosplenomegaly. Electrocardiography showed sinus tachycardia. Nasal cavity endoscopy showed sinusitis. Fundus examination showed vasculopathy of the left retina. A colonoscopy showed normal mucosa.
Histopathology
Biopsy of the nodule on the left arm showed dense, superficial to deep perivascular, periadnexal, perineural, and panniculitislike lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells (Figure 2). Infiltrative atypical lymphoid cells expressed CD3 and CD7 and were mostly CD8+, with a few CD4+ cells and most cells negative for CD5, CD20, CD30, CD56, and anaplastic lymphoma kinase. Cytotoxic markers granzyme B and T-cell intracellular antigen protein 1 were scattered positive. Immunostaining for Ki-67 protein highlighted an increased proliferative rate of 80% in malignant cells. In situ hybridization for EBV-encoded RNA (EBER) demonstrated EBV-positive atypical lymphoid cells (Figure 3). Analysis for T-cell receptor (TCR) γ gene rearrangement revealed a monoclonal pattern. Bone marrow aspirate showed proliferation of the 3 cell lines. The percentage of T lymphocytes was increased (20% of all nucleated cells). No hemophagocytic activity was found.


Diagnosis
A diagnosis of systemic EBV-positive T-cell lymphoma was made. Before the final diagnosis was made, the patient was treated by rheumatologists with antibiotics, antiviral drugs, nonsteroidal anti-inflammatory drugs, and other symptomatic treatments. Following antibiotic therapy, a sputum culture reverted to normal flora, the coagulation index (ie, prothrombin time, activated partial thromboplastin time) returned to normal, and the D-dimer level decreased to 1.19 mg/L.
The patient’s parents refused to accept chemotherapy for him. Instead, they chose herbal therapy only; 5 months later, they reported that all of his symptoms had resolved; however, the disease suddenly relapsed after another 7 months, with multiple skin nodules and fever. The patient died, even with chemotherapy in another hospital.
Comment
Prevalence and Presentation
Epstein-Barr virus is a ubiquitous γ-herpesvirus with tropism for B cells, affecting more than 90% of the adult population worldwide. In addition to infecting B cells, EBV is capable of infecting T and NK cells, leading to various EBV-related lymphoproliferative disorders (LPDs). The frequency and clinical presentation of infection varies based on the type of EBV-infected cells and the state of host immunity.1-3
Primary infection usually is asymptomatic and occurs early in life; when symptomatic, the disease usually presents as infectious mononucleosis (IM), characterized by polyclonal expansion of infected B cells and subsequent cytotoxic T-cell response. A diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against VCA, early antigens, and EBV nuclear antigen proteins.3,4
Associated LPDs
Although most symptoms associated with IM resolve within weeks or months, persistent or recurrent IM-like symptoms or even lasting disease occasionally occur, particularly in children and young adults. This complication is known as chronic active EBV infection (CAEBV), frequently associated with EBV-infected T-cell or NK-cell proliferation, especially in East Asian populations.3,5
Epstein-Barr virus–positive T-cell and NK-cell LPDs of childhood include CAEBV infection of T-cell and NK-cell types and systemic EBV-positive T-cell lymphoma of childhood. The former includes hydroa vacciniforme–like LPD and severe mosquito bite allergy.3
Systemic EBV-Positive T-cell Lymphoma of Childhood
This entity occurs not only in children but also in adolescents and young adults. A fulminant illness characterized by clonal proliferation of EBV-infected cytotoxic T cells, it can develop shortly after primary EBV infection or is linked to CAEBV infection. The disorder is rare and has a racial predilection for Asian (ie, Japanese, Chinese, Korean) populations and indigenous populations of Mexico and Central and South America.6-8
Complications
Systemic EBV-positive T-cell lymphoma of childhood is often complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. Other signs and symptoms include high fever, rash, jaundice, diarrhea, pancytopenia, and hepatosplenomegaly. The liver, spleen, lymph nodes, and bone marrow are commonly involved, and the disease can involve skin, the heart, and the lungs.9,10
Diagnosis
When systemic EBV-positive T-cell lymphoma occurs shortly after IM, serology shows low or absent anti-VCA IgM and positive anti-VCA IgG. Infiltrating T cells usually are small and lack cytologic atypia; however, cases with pleomorphic, medium to large lymphoid cells, irregular nuclei, and frequent mitoses have been described. Hemophagocytosis can be seen in the liver, spleen, and bone marrow.3,11
The most typical phenotype of systemic EBV-positive T-cell lymphoma is CD2+CD3+CD8+CD20−CD56−, with expression of the cytotoxic granules known as T-cell intracellular antigen 1 and granzyme B. Rare cases of CD4+ and mixed CD4+/CD8+ phenotypes have been described, usually in the setting of CAEBV infection.3,12 Neoplastic cells have monoclonally rearranged TCR-γ genes and consistent EBER positivity with in situ hybridization.13 A final diagnosis is based on a comprehensive analysis of clinical, morphological, immunohistochemical, and molecular biological aspects.
Clinical Course and Prognosis
Most patients with systemic EBV-positive T-cell lymphoma have an aggressive clinical course with high mortality. In a few cases, patients were reported to respond to a regimen of etoposide and dexamethasone, followed by allogeneic hematopoietic stem cell transplantation.3
In recognition of the aggressive clinical behavior and desire to clearly distinguish systemic EBV-positive T-cell lymphoma from CAEBV infection, the older term systemic EBV-positive T-cell LPD of childhood, which had been introduced in 2008 to the World Health Organization classification, was changed to systemic EBV-positive T-cell lymphoma of childhood in the revised 2016 World Health Organization classification.6,12 However, Kim et al14 reported a case with excellent response to corticosteroid administration, suggesting that systemic EBV-positive T-cell lymphoma of childhood may be more heterogeneous in terms of prognosis.
Our patient presented with acute IM-like symptoms, including high fever, tonsillar enlargement, lymphadenopathy, and hepatosplenomegaly, as well as uncommon oral ulcers and skin lesions, including indurated nodules. Histopathologic changes in the skin nodule, proliferation in bone marrow, immunohistochemical phenotype, and positivity of EBER and TCR-γ monoclonal rearrangement were all consistent with systemic EBV-positive T-cell lymphoma of childhood. The patient was positive for VCA IgG and negative for VCA IgM, compatible with systemic EBV-positive T-cell lymphoma of childhood occurring shortly after IM. Neither pancytopenia, hemophagocytic syndrome, nor multiorgan failure occurred during the course.
Differential Diagnosis
It is important to distinguish IM from systemic EBV-positive T-cell lymphoma of childhood and CAEBV infection. Detection of anti–VCA IgM in the early stage, its disappearance during the clinical course, and appearance of anti-EBV–determined nuclear antigen is useful to distinguish IM from the neoplasms, as systemic EBV-positive T-cell lymphoma of childhood is negative for anti-EBV–determined nuclear antigen. Carefully following the clinical course also is important.3,15
Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis can occur in association with systemic EBV-positive T-cell lymphoma of childhood and might represent a continuum of disease rather than distinct entities.14 The most useful marker for differentiating EBV-associated hemophagocytic lymphohistiocytosis and systemic EBV-positive T-cell lymphoma of childhood is an abnormal karyotype rather than molecular clonality.16
Outcome
Mortality risk in EBV-associated T-cell and NK-cell LPD is not primarily dependent on whether the lesion has progressed to lymphoma but instead is related to associated complications.17
Conclusion
Although systemic EBV-positive T-cell lymphoma of childhood is a rare disorder and has race predilection, dermatologists should be aware due to the aggressive clinical source and poor prognosis. Histopathology and in situ hybridization for EBER and TCR gene rearrangements are critical for final diagnosis. Although rare cases can show temporary resolution, the final outcome of this disease is not optimistic.
Case Report
A 7-year-old Chinese boy presented with multiple painful oral and tongue ulcers of 2 weeks’ duration as well as acute onset of moderate to high fever (highest temperature, 39.3°C) for 5 days. The fever was reported to have run a relapsing course, accompanied by rigors but without convulsions or cognitive changes. At times, the patient had nasal congestion, nasal discharge, and cough. He also had a transient eruption on the back and hands as well as an indurated red nodule on the left forearm.
Before the patient was hospitalized, antibiotic therapy was administered by other physicians, but the condition of fever and oral ulcers did not improve. After the patient was hospitalized, new tender nodules emerged on the scalp, buttocks, and lower extremities. New ulcers also appeared on the palate.
History
Two months earlier, the patient had presented with a painful perioral skin ulcer that resolved after being treated as contagious eczema. Another dermatologist previously had considered a diagnosis of hand-foot-and-mouth disease.
The patient was born by normal spontaneous vaginal delivery, without abnormality. He was breastfed; feeding, growth, and the developmental history showed no abnormality. He was the family’s eldest child, with a healthy brother and sister. There was no history of familial illness. He received bacillus Calmette-Guérin and poliomyelitis vaccines after birth; the rest of the vaccine history was unclear. There was no history of immunologic abnormality.
Physical Examination
A 1.5×1.5-cm, warm, red nodule with a central black crust was noted on the left forearm (Figure 1A). Several similar lesions were noted on the buttocks, scalp, and lower extremities. Multiple ulcers, as large as 1 cm, were present on the tongue, palate, and left angle of the mouth (Figure 1B). The pharynx was congested, and the tonsils were mildly enlarged. Multiple enlarged, movable, nontender lymph nodes could be palpated in the cervical basins, axillae, and groin. No purpura or ecchymosis was detected.

Laboratory Results
Laboratory testing revealed a normal total white blood cell count (4.26×109/L [reference range, 4.0–12.0×109/L]), with normal neutrophils (1.36×109/L [reference range, 1.32–7.90×109/L]), lymphocytes (2.77×109/L [reference range, 1.20–6.00×109/L]), and monocytes (0.13×109/L [reference range, 0.08–0.80×109/L]); a mildly decreased hemoglobin level (115 g/L [reference range, 120–160 g/L]); a normal platelet count (102×109/L [reference range, 100–380×109/L]); an elevated lactate dehydrogenase level (614 U/L [reference range, 110–330 U/L]); an elevated α-hydroxybutyrate dehydrogenase level (483 U/L [reference range, 120–270 U/L]); elevated prothrombin time (15.3 s [reference range, 9–14 s]); elevated activated partial thromboplastin time (59.8 s [reference range, 20.6–39.6 s]); and an elevated D-dimer level (1.51 mg/L [reference range, <0.73 mg/L]). In addition, autoantibody testing revealed a positive antinuclear antibody titer of 1:320 and a strong positive anti–Ro-52 level.
The peripheral blood lymphocyte classification demonstrated a prominent elevated percentage of T lymphocytes, with predominantly CD8+ cells (CD3, 94.87%; CD8, 71.57%; CD4, 24.98%; CD4:CD8 ratio, 0.35) and a diminished percentage of B lymphocytes and natural killer (NK) cells. Epstein-Barr virus (EBV) antibody testing was positive for anti–viral capsid antigen (VCA) IgG and negative for anti-VCA IgM.
Smears of the ulcer on the tongue demonstrated gram-positive cocci, gram-negative bacilli, and diplococci. Culture of sputum showed methicillin-resistant Staphylococcus aureus. Inspection for acid-fast bacilli in sputum yielded negative results 3 times. A purified protein derivative skin test for Mycobacterium tuberculosis infection was negative.
Imaging and Other Studies
Computed tomography of the chest and abdomen demonstrated 2 nodular opacities on the lower right lung; spotted opacities on the upper right lung; floccular opacities on the rest area of the lung; mild pleural effusion; enlargement of lymph nodes on the mediastinum, the bilateral hilum of the lung, and mesentery; and hepatosplenomegaly. Electrocardiography showed sinus tachycardia. Nasal cavity endoscopy showed sinusitis. Fundus examination showed vasculopathy of the left retina. A colonoscopy showed normal mucosa.
Histopathology
Biopsy of the nodule on the left arm showed dense, superficial to deep perivascular, periadnexal, perineural, and panniculitislike lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells (Figure 2). Infiltrative atypical lymphoid cells expressed CD3 and CD7 and were mostly CD8+, with a few CD4+ cells and most cells negative for CD5, CD20, CD30, CD56, and anaplastic lymphoma kinase. Cytotoxic markers granzyme B and T-cell intracellular antigen protein 1 were scattered positive. Immunostaining for Ki-67 protein highlighted an increased proliferative rate of 80% in malignant cells. In situ hybridization for EBV-encoded RNA (EBER) demonstrated EBV-positive atypical lymphoid cells (Figure 3). Analysis for T-cell receptor (TCR) γ gene rearrangement revealed a monoclonal pattern. Bone marrow aspirate showed proliferation of the 3 cell lines. The percentage of T lymphocytes was increased (20% of all nucleated cells). No hemophagocytic activity was found.


Diagnosis
A diagnosis of systemic EBV-positive T-cell lymphoma was made. Before the final diagnosis was made, the patient was treated by rheumatologists with antibiotics, antiviral drugs, nonsteroidal anti-inflammatory drugs, and other symptomatic treatments. Following antibiotic therapy, a sputum culture reverted to normal flora, the coagulation index (ie, prothrombin time, activated partial thromboplastin time) returned to normal, and the D-dimer level decreased to 1.19 mg/L.
The patient’s parents refused to accept chemotherapy for him. Instead, they chose herbal therapy only; 5 months later, they reported that all of his symptoms had resolved; however, the disease suddenly relapsed after another 7 months, with multiple skin nodules and fever. The patient died, even with chemotherapy in another hospital.
Comment
Prevalence and Presentation
Epstein-Barr virus is a ubiquitous γ-herpesvirus with tropism for B cells, affecting more than 90% of the adult population worldwide. In addition to infecting B cells, EBV is capable of infecting T and NK cells, leading to various EBV-related lymphoproliferative disorders (LPDs). The frequency and clinical presentation of infection varies based on the type of EBV-infected cells and the state of host immunity.1-3
Primary infection usually is asymptomatic and occurs early in life; when symptomatic, the disease usually presents as infectious mononucleosis (IM), characterized by polyclonal expansion of infected B cells and subsequent cytotoxic T-cell response. A diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against VCA, early antigens, and EBV nuclear antigen proteins.3,4
Associated LPDs
Although most symptoms associated with IM resolve within weeks or months, persistent or recurrent IM-like symptoms or even lasting disease occasionally occur, particularly in children and young adults. This complication is known as chronic active EBV infection (CAEBV), frequently associated with EBV-infected T-cell or NK-cell proliferation, especially in East Asian populations.3,5
Epstein-Barr virus–positive T-cell and NK-cell LPDs of childhood include CAEBV infection of T-cell and NK-cell types and systemic EBV-positive T-cell lymphoma of childhood. The former includes hydroa vacciniforme–like LPD and severe mosquito bite allergy.3
Systemic EBV-Positive T-cell Lymphoma of Childhood
This entity occurs not only in children but also in adolescents and young adults. A fulminant illness characterized by clonal proliferation of EBV-infected cytotoxic T cells, it can develop shortly after primary EBV infection or is linked to CAEBV infection. The disorder is rare and has a racial predilection for Asian (ie, Japanese, Chinese, Korean) populations and indigenous populations of Mexico and Central and South America.6-8
Complications
Systemic EBV-positive T-cell lymphoma of childhood is often complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. Other signs and symptoms include high fever, rash, jaundice, diarrhea, pancytopenia, and hepatosplenomegaly. The liver, spleen, lymph nodes, and bone marrow are commonly involved, and the disease can involve skin, the heart, and the lungs.9,10
Diagnosis
When systemic EBV-positive T-cell lymphoma occurs shortly after IM, serology shows low or absent anti-VCA IgM and positive anti-VCA IgG. Infiltrating T cells usually are small and lack cytologic atypia; however, cases with pleomorphic, medium to large lymphoid cells, irregular nuclei, and frequent mitoses have been described. Hemophagocytosis can be seen in the liver, spleen, and bone marrow.3,11
The most typical phenotype of systemic EBV-positive T-cell lymphoma is CD2+CD3+CD8+CD20−CD56−, with expression of the cytotoxic granules known as T-cell intracellular antigen 1 and granzyme B. Rare cases of CD4+ and mixed CD4+/CD8+ phenotypes have been described, usually in the setting of CAEBV infection.3,12 Neoplastic cells have monoclonally rearranged TCR-γ genes and consistent EBER positivity with in situ hybridization.13 A final diagnosis is based on a comprehensive analysis of clinical, morphological, immunohistochemical, and molecular biological aspects.
Clinical Course and Prognosis
Most patients with systemic EBV-positive T-cell lymphoma have an aggressive clinical course with high mortality. In a few cases, patients were reported to respond to a regimen of etoposide and dexamethasone, followed by allogeneic hematopoietic stem cell transplantation.3
In recognition of the aggressive clinical behavior and desire to clearly distinguish systemic EBV-positive T-cell lymphoma from CAEBV infection, the older term systemic EBV-positive T-cell LPD of childhood, which had been introduced in 2008 to the World Health Organization classification, was changed to systemic EBV-positive T-cell lymphoma of childhood in the revised 2016 World Health Organization classification.6,12 However, Kim et al14 reported a case with excellent response to corticosteroid administration, suggesting that systemic EBV-positive T-cell lymphoma of childhood may be more heterogeneous in terms of prognosis.
Our patient presented with acute IM-like symptoms, including high fever, tonsillar enlargement, lymphadenopathy, and hepatosplenomegaly, as well as uncommon oral ulcers and skin lesions, including indurated nodules. Histopathologic changes in the skin nodule, proliferation in bone marrow, immunohistochemical phenotype, and positivity of EBER and TCR-γ monoclonal rearrangement were all consistent with systemic EBV-positive T-cell lymphoma of childhood. The patient was positive for VCA IgG and negative for VCA IgM, compatible with systemic EBV-positive T-cell lymphoma of childhood occurring shortly after IM. Neither pancytopenia, hemophagocytic syndrome, nor multiorgan failure occurred during the course.
Differential Diagnosis
It is important to distinguish IM from systemic EBV-positive T-cell lymphoma of childhood and CAEBV infection. Detection of anti–VCA IgM in the early stage, its disappearance during the clinical course, and appearance of anti-EBV–determined nuclear antigen is useful to distinguish IM from the neoplasms, as systemic EBV-positive T-cell lymphoma of childhood is negative for anti-EBV–determined nuclear antigen. Carefully following the clinical course also is important.3,15
Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis can occur in association with systemic EBV-positive T-cell lymphoma of childhood and might represent a continuum of disease rather than distinct entities.14 The most useful marker for differentiating EBV-associated hemophagocytic lymphohistiocytosis and systemic EBV-positive T-cell lymphoma of childhood is an abnormal karyotype rather than molecular clonality.16
Outcome
Mortality risk in EBV-associated T-cell and NK-cell LPD is not primarily dependent on whether the lesion has progressed to lymphoma but instead is related to associated complications.17
Conclusion
Although systemic EBV-positive T-cell lymphoma of childhood is a rare disorder and has race predilection, dermatologists should be aware due to the aggressive clinical source and poor prognosis. Histopathology and in situ hybridization for EBER and TCR gene rearrangements are critical for final diagnosis. Although rare cases can show temporary resolution, the final outcome of this disease is not optimistic.
- Ameli F, Ghafourian F, Masir N. Systematic Epstein-Barr virus-positive T-cell lymphoproliferative disease presenting as a persistent fever and cough: a case report. J Med Case Rep. 2014;8:288.
- Kim HJ, Ko YH, Kim JE, et al. Epstein-Barr virus-associated lympho-proliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51:352-358.
- Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts [published online March 7, 2018]. Pathogens. doi:10.3390/pathogens7010028.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
- Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71.
- Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137-147.
- Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443-451.
- Chen G, Chen L, Qin X, et al. Systemic Epstein-Barr virus positive T-cell lymphoproliferative disease of childhood with hemophagocytic syndrome. Int J Clin Exp Pathol. 2014;7:7110-7113.
- Grywalska E, Rolinski J. Epstein-Barr virus-associated lymphomas. Semin Oncol. 2015;42:291-303.
- Huang W, Lv N, Ying J, et al. Clinicopathological characteristics of four cases of EBV positive T-cell lymphoproliferative disorders of childhood in China. Int J Clin Exp Pathol. 2014;7:4991-4999.
- Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011;5:218.
- Kim DH, Kim M, Kim Y, et al. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood with good response to steroid therapy. J Pediatr Hematol Oncol. 2017;39:e497-e500.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671-675.
- Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738-5749.
- Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53-63.
- Ameli F, Ghafourian F, Masir N. Systematic Epstein-Barr virus-positive T-cell lymphoproliferative disease presenting as a persistent fever and cough: a case report. J Med Case Rep. 2014;8:288.
- Kim HJ, Ko YH, Kim JE, et al. Epstein-Barr virus-associated lympho-proliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51:352-358.
- Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts [published online March 7, 2018]. Pathogens. doi:10.3390/pathogens7010028.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
- Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71.
- Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137-147.
- Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443-451.
- Chen G, Chen L, Qin X, et al. Systemic Epstein-Barr virus positive T-cell lymphoproliferative disease of childhood with hemophagocytic syndrome. Int J Clin Exp Pathol. 2014;7:7110-7113.
- Grywalska E, Rolinski J. Epstein-Barr virus-associated lymphomas. Semin Oncol. 2015;42:291-303.
- Huang W, Lv N, Ying J, et al. Clinicopathological characteristics of four cases of EBV positive T-cell lymphoproliferative disorders of childhood in China. Int J Clin Exp Pathol. 2014;7:4991-4999.
- Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011;5:218.
- Kim DH, Kim M, Kim Y, et al. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood with good response to steroid therapy. J Pediatr Hematol Oncol. 2017;39:e497-e500.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671-675.
- Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738-5749.
- Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53-63.
Practice Points
- Systemic Epstein-Barr virus (EBV)–positive T-cell lymphoma of childhood is a fulminant illness with a predilection for Asians and indigenous populations from Mexico and Central and South America. In most patients, the disease has an aggressive clinical course with high mortality.
- The disease often is complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. When these severe complications are absent, the prognosis might be better.
- In situ hybridization for EBV-encoded RNA and for T-cell receptor gene rearrangements is an important tool to establish the diagnosis as well as for treatment options and predicting the prognosis.
Seborrhea Herpeticum: Cutaneous Herpes Simplex Virus Infection Within Infantile Seborrheic Dermatitis
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.
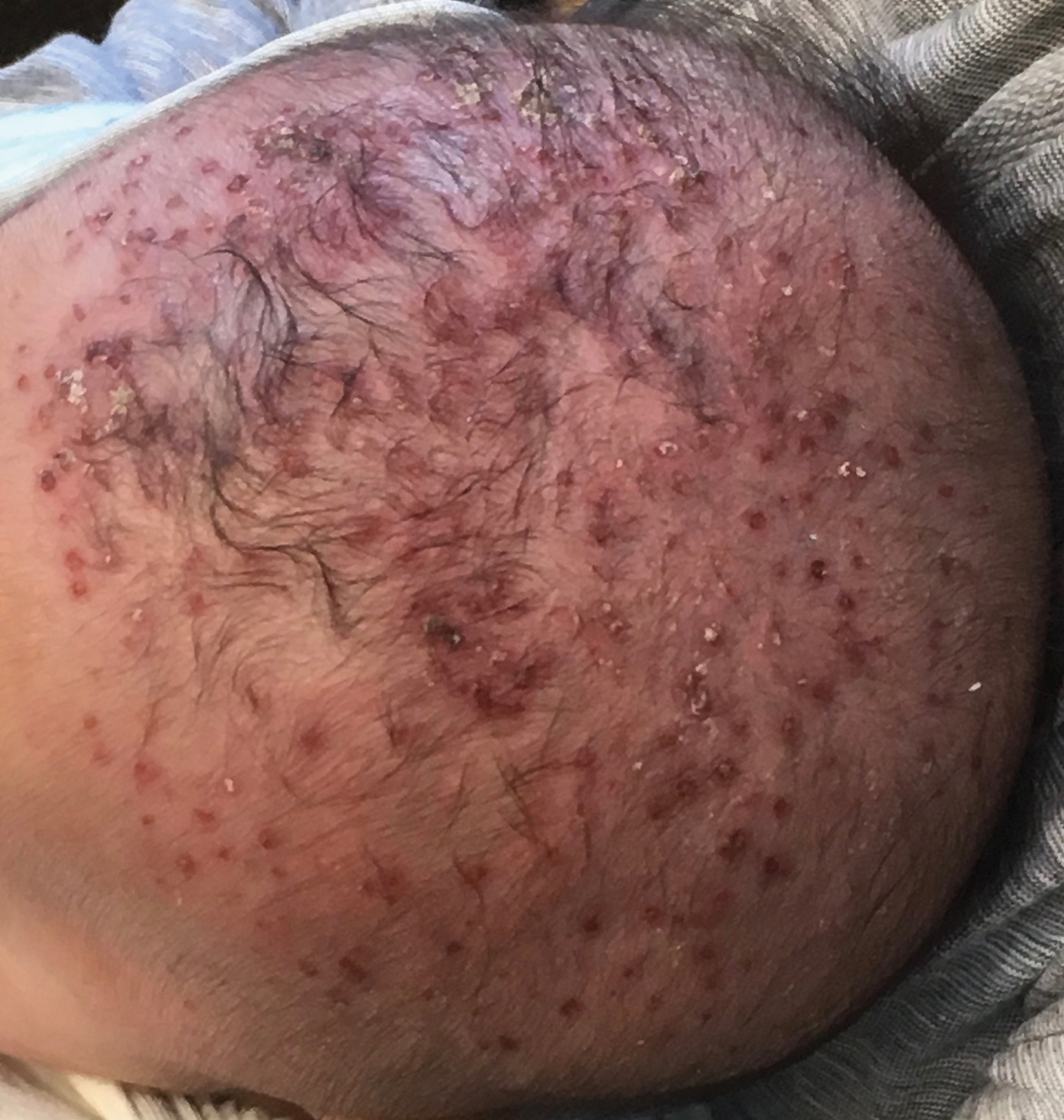
Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Practice Points
- Cutaneous herpes simplex virus may present in a seborrheic distribution within infantile seborrheic dermatitis, suggesting underlying dysfunction secondary to seborrheic dermatitis.
- Treatment of seborrhea herpeticum involves antiviral therapy to treat the secondary viral infection and gentle skin care precautions for the primary condition.
Secondary Syphilis Mimicking Molluscum Contagiosum in the Beard Area of an AIDS Patient
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.

A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).

Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.

A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).

Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
Practice Points
- Recognize typical and atypical presentations of secondary syphilis (SS).
- This case reinforces the importance of cutaneous biopsies in immunocompromised patients.
- Consider the possibility of autoinoculation in SS.
Larval Tick Infestation Causing an Eruption of Pruritic Papules and Pustules
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.
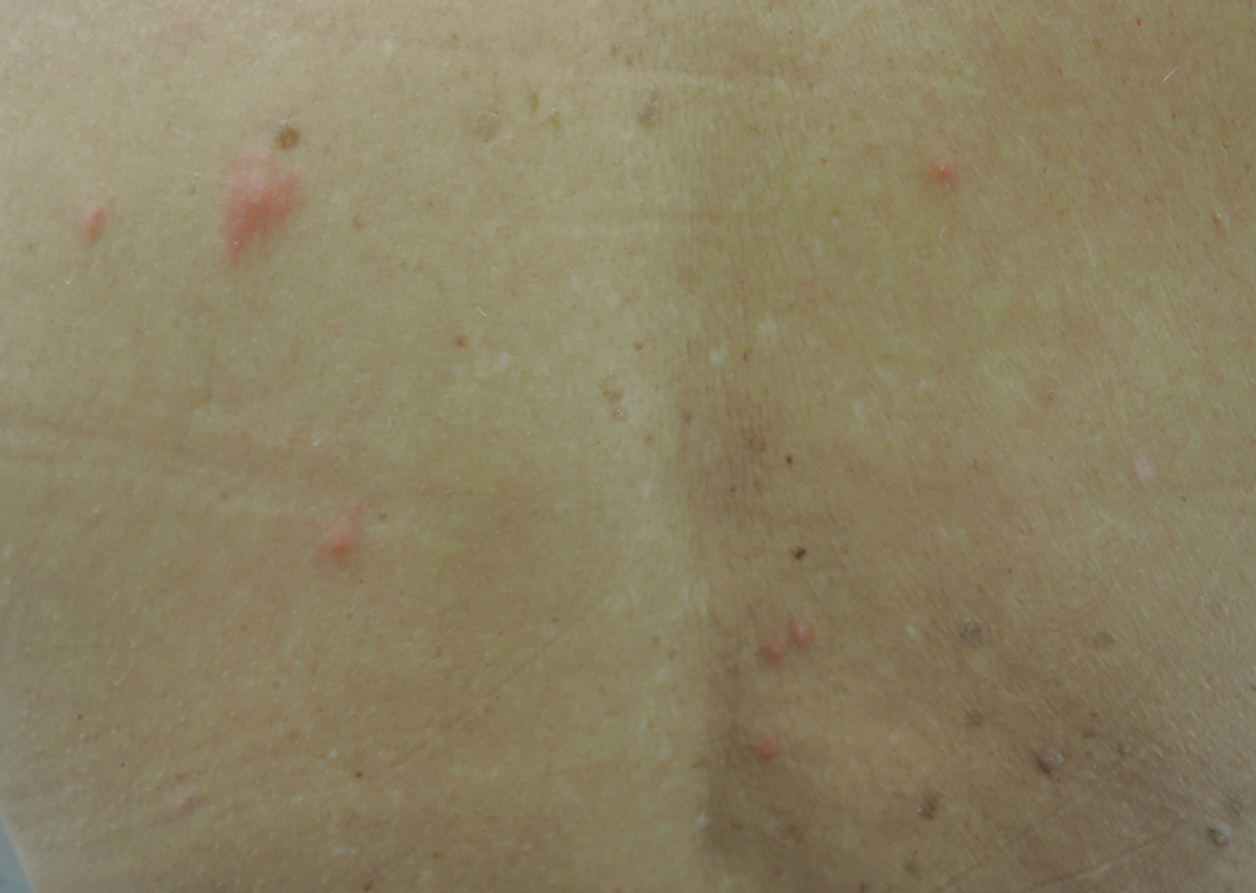


Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.
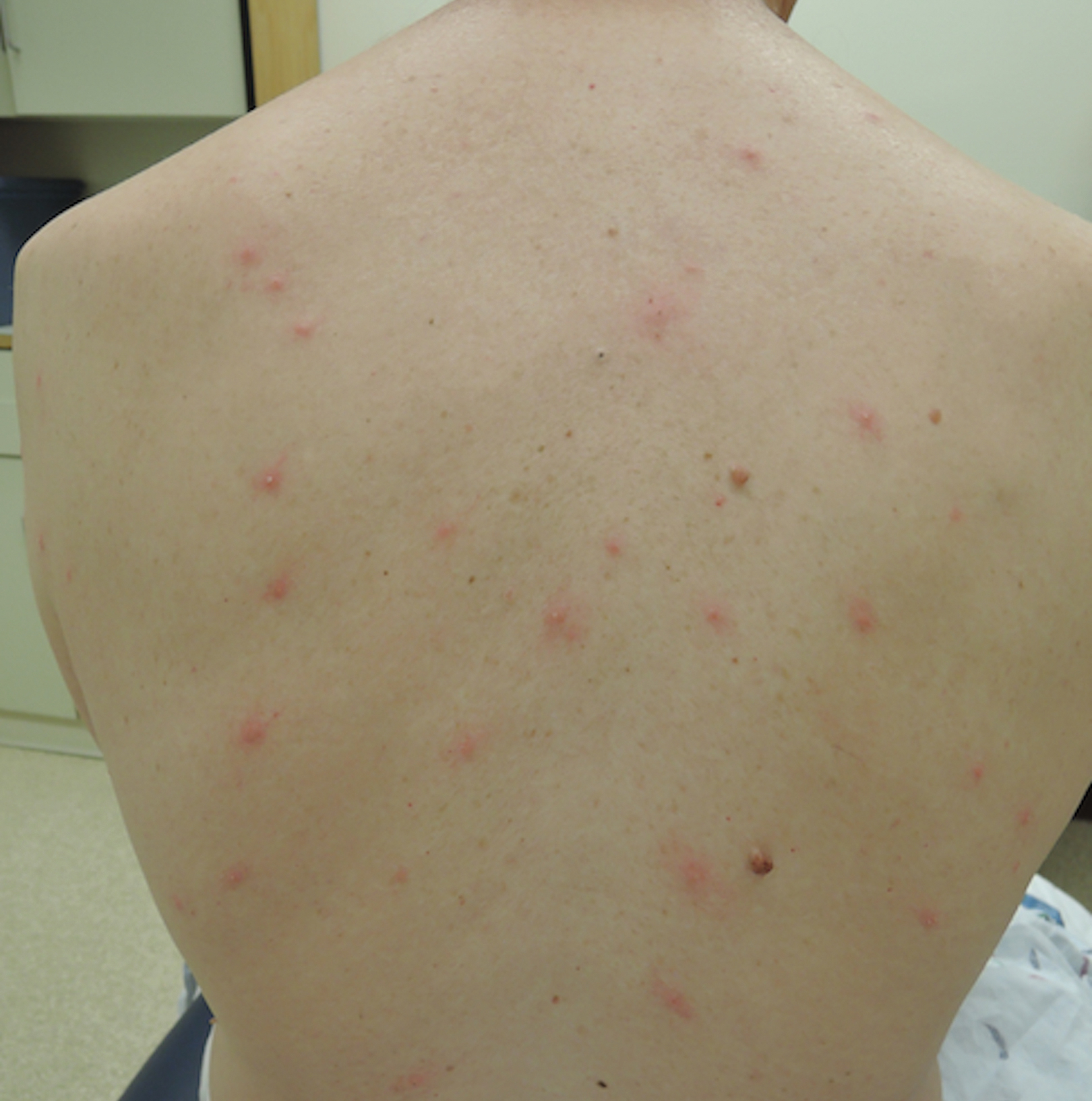
Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.



Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.

Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.



Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.

Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
Practice Points
- Larval (“seed”) ticks can attack in droves, causing a widespread rash consisting of pruritic erythematous papules and pustules.
- Tiny black dots can be seen in some papules, which are the seed ticks themselves. Careful dermoscopic examination is critical to avoid easy misdiagnosis as hemorrhagic crust.
- We encourage providers to include larval tick infestation in the differential for eruptive pruritic papules and pustules with a history of outdoor exposure, especially during the summer months.
20-year-old male college basketball prospect • wrist pain after falling on wrist • normal ROM • pain with active/passive wrist extension • Dx?
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.
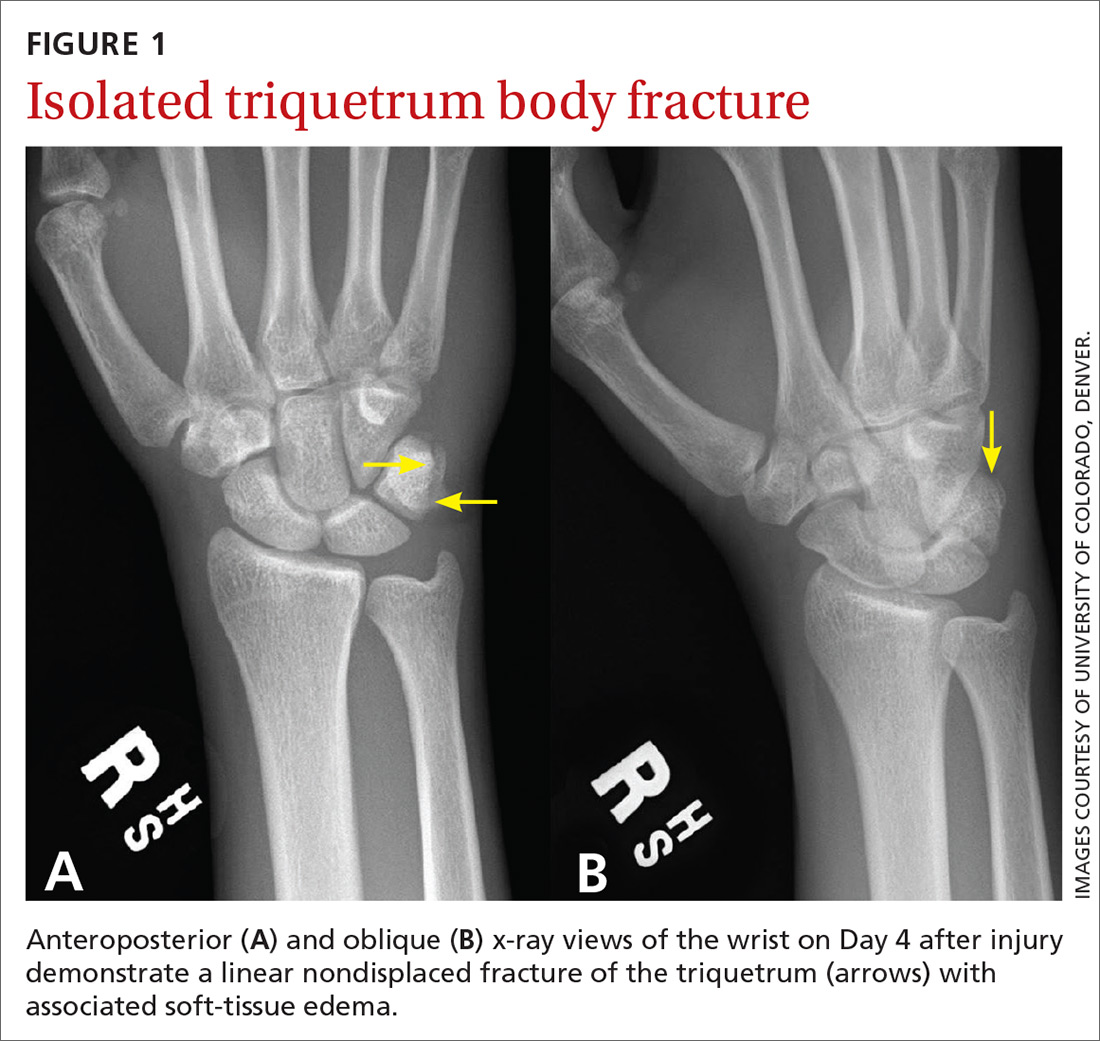
At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.
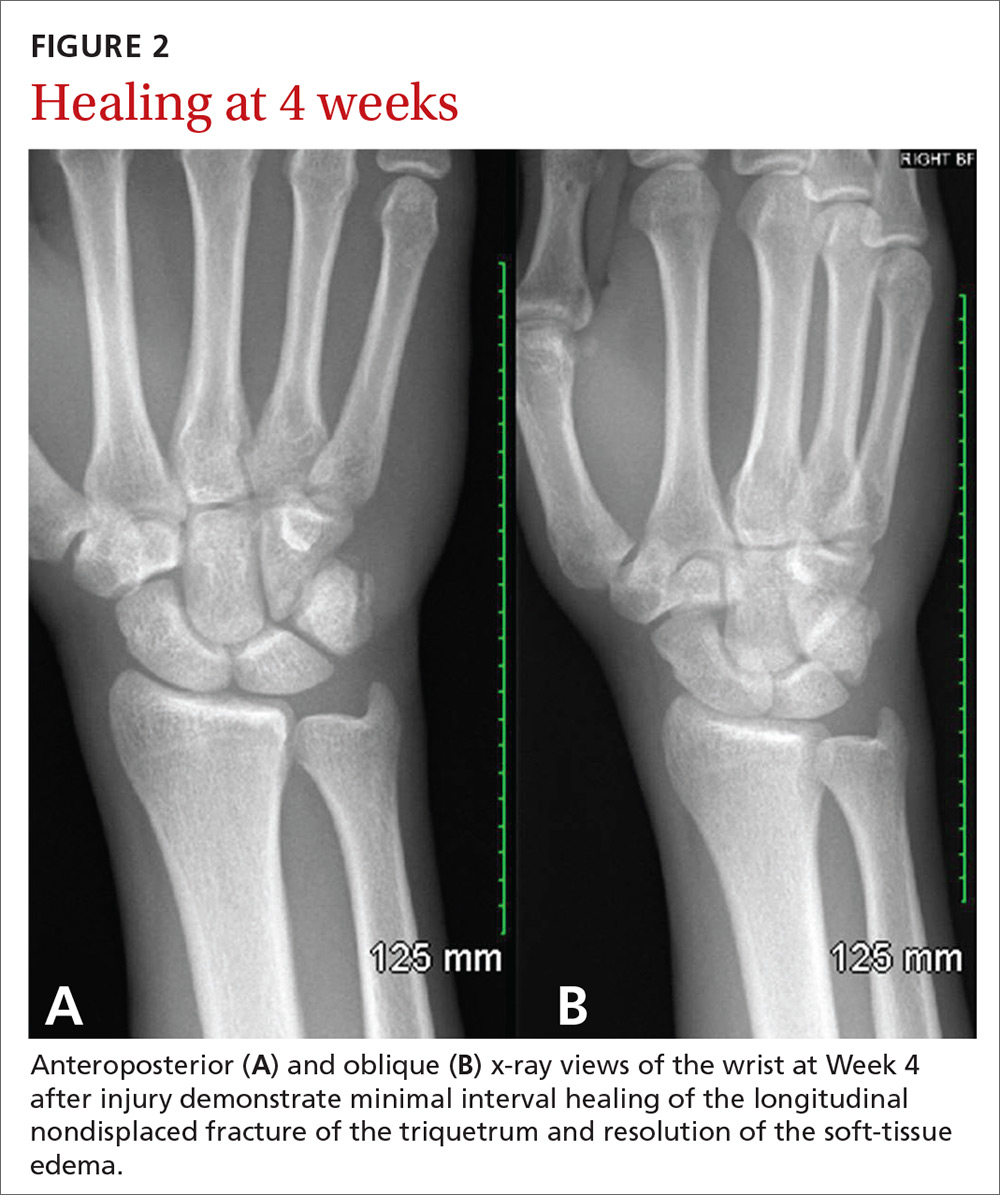
DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9

Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.

At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.

DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9

Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.

At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.

DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9

Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
Guttate Psoriasis Following Presumed Coxsackievirus A
There are 4 variants of psoriasis: plaque, guttate, pustular, and erythroderma (in order of prevalence).2 Guttate psoriasis is characterized by small, 2- to 10-mm, raindroplike lesions on the skin.1 It accounts for approximately 2% of total psoriasis cases and is commonly triggered by group A streptococcal pharyngitis or tonsillitis.3,4
Hand-foot-and-mouth disease (HFMD) is an illness most commonly caused by a coxsackievirus A infection but also can be caused by other enteroviruses.5,6 Coxsackievirus is a serotype of the Enterovirus species within the Picornaviridae family.7 Hand-foot-and-mouth disease is characterized by a brief fever and vesicular rashes on the palms, soles, or buttocks, as well as oropharyngeal ulcers.8 Typically, the rash is benign and short-lived.9 In rare cases, neurologic complications develop. There have been no reported cases of guttate psoriasis following a coxsackievirus A infection.
The involvement of coxsackievirus B in the etiopathogenesis of psoriasis has been previously reported.10 We report the case of guttate psoriasis following presumed coxsackievirus A HFMD.
Case Report
A 56-year-old woman presented with a vesicular rash on the hands, feet, and lips. The patient reported having a sore throat that started around the same time that the rash developed. The severity of the sore throat was rated as moderate. No fever was reported. One day prior, the patient’s primary care physician prescribed a tapered course of prednisone for the rash. The patient reported a medical history of herpes zoster virus, sunburn, and genital herpes. She was taking clonazepam and had a known allergy to penicillin.
Physical examination revealed erythematous vesicular and papular lesions on the extensor surfaces of the hands and feet. Vesicles also were noted on the vermilion border of the lip. Examination of the patient’s mouth showed blisters and shallow ulcerations in the oral cavity. A clinical diagnosis of coxsackievirus A HFMD was made, and the treatment plan included triamcinolone acetonide ointment 0.025% applied twice daily for 2 weeks and oral valacyclovir hydrochloride 1 g taken 3 times daily for 7 days. A topical emollient also was recommended for the lips when necessary. The lesions all resolved within a 2-week period with no sequela.
The patient returned 1 month later, citing newer red abdominal skin lesions. Fever was denied. She reported that both prescribed treatments had not been helping for the newer lesions. She noticed similar lesions on the groin and brought them to the attention of her gynecologist. Physical examination revealed salmon pink papules and plaques with silvery scaling involving the abdomen, bilateral upper extremities and ears, and scalp. The patient was then clinically diagnosed with guttate psoriasis. A shave biopsy of a representative lesion on the abdomen was performed. The treatment plan included betamethasone dipropionate cream 0.05% applied twice daily for 2 weeks, clobetasol propionate solution 0.05% applied twice daily for 14 days (for the scalp), and hydrocortisone valerate cream 0.2% applied twice daily for 14 days (for the groin).
The skin biopsy shown in the Figure was received in 10% buffered formalin, measuring 5×4×1 mm of skin. Sections showed an acanthotic epidermis with foci of spongiosis and hypergranulosis covered by mounds of parakeratosis infiltrated by neutrophils. Superficial perivascular and interstitial lymphocytic inflammation was present. Tortuous blood vessels within the papillary dermis also were present. Results showed psoriasiform dermatitis with mild spongiosis. Periodic acid–Schiff stain did not reveal any fungal organisms. These findings were consistent with a diagnosis of guttate psoriasis.
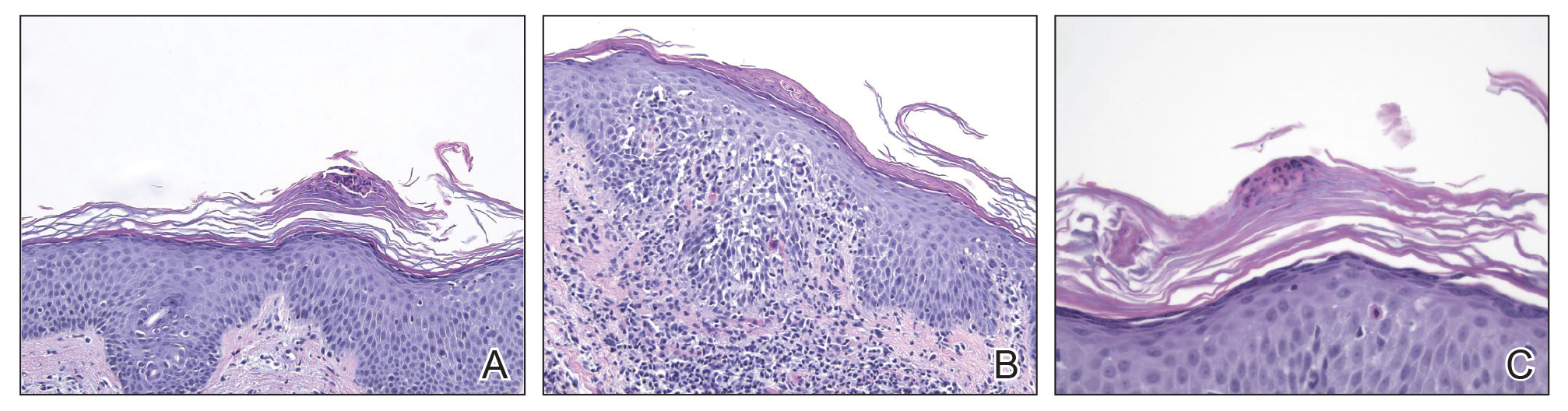
The patient then returned 1 month later mentioning continued flare-ups of the scalp as well as newer patches on the arms and hands that were less eruptive and faded more quickly. The plaques in the groin area had resolved. Physical examination showed fewer pink papules and plaques with silvery scaling on the abdomen, bilateral upper extremities and ears, and scalp. Topical medications were continued, and possible apremilast therapy for the psoriasis was discussed.
Comment
Enterovirus-derived HFMD likely is caused by coxsackie-virus A. Current evidence supports the theory that guttate psoriasis can be environmentally triggered in genetically susceptible individuals, often but not exclusively by a streptococcal infection. The causative agent elicits a T-cell–mediated reaction leading to increased type 1 helper T cells, IFN-γ, and IL-2 cytokine levels. HLA-Cw∗0602–positive patients are considered genetically susceptible and more likely to develop guttate psoriasis following an environmental trigger. Based on the coincidence in timing of both diagnoses, this reported case of guttate psoriasis may have been triggered by a coxsackievirus A infection.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;1:79-82.
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? an immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326-332.
- Telfer N, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Seitsonen J, Shakeel S, Susi P, et al. Structural analysis of coxsackievirus A7 reveals conformational changes associated with uncoating. J Virol. 2012;86:7207-7215.
- Wu Y, Yeo A, Phoon M, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Tesini BL. Hand-foot-and-mouth-disease (HFMD). May 2018. https://www.msdmanuals.com/professional/infectious-diseases/enteroviruses/hand-foot-and-mouth-disease-hfmd. Accessed September 25, 2019.
- Korzhova TP, Shyrobokov VP, Koliadenko VH, et al. Coxsackie B viral infection in the etiology and clinical pathogenesis of psoriasis [in Ukrainian]. Lik Sprava. 2001:54-58.
There are 4 variants of psoriasis: plaque, guttate, pustular, and erythroderma (in order of prevalence).2 Guttate psoriasis is characterized by small, 2- to 10-mm, raindroplike lesions on the skin.1 It accounts for approximately 2% of total psoriasis cases and is commonly triggered by group A streptococcal pharyngitis or tonsillitis.3,4
Hand-foot-and-mouth disease (HFMD) is an illness most commonly caused by a coxsackievirus A infection but also can be caused by other enteroviruses.5,6 Coxsackievirus is a serotype of the Enterovirus species within the Picornaviridae family.7 Hand-foot-and-mouth disease is characterized by a brief fever and vesicular rashes on the palms, soles, or buttocks, as well as oropharyngeal ulcers.8 Typically, the rash is benign and short-lived.9 In rare cases, neurologic complications develop. There have been no reported cases of guttate psoriasis following a coxsackievirus A infection.
The involvement of coxsackievirus B in the etiopathogenesis of psoriasis has been previously reported.10 We report the case of guttate psoriasis following presumed coxsackievirus A HFMD.
Case Report
A 56-year-old woman presented with a vesicular rash on the hands, feet, and lips. The patient reported having a sore throat that started around the same time that the rash developed. The severity of the sore throat was rated as moderate. No fever was reported. One day prior, the patient’s primary care physician prescribed a tapered course of prednisone for the rash. The patient reported a medical history of herpes zoster virus, sunburn, and genital herpes. She was taking clonazepam and had a known allergy to penicillin.
Physical examination revealed erythematous vesicular and papular lesions on the extensor surfaces of the hands and feet. Vesicles also were noted on the vermilion border of the lip. Examination of the patient’s mouth showed blisters and shallow ulcerations in the oral cavity. A clinical diagnosis of coxsackievirus A HFMD was made, and the treatment plan included triamcinolone acetonide ointment 0.025% applied twice daily for 2 weeks and oral valacyclovir hydrochloride 1 g taken 3 times daily for 7 days. A topical emollient also was recommended for the lips when necessary. The lesions all resolved within a 2-week period with no sequela.
The patient returned 1 month later, citing newer red abdominal skin lesions. Fever was denied. She reported that both prescribed treatments had not been helping for the newer lesions. She noticed similar lesions on the groin and brought them to the attention of her gynecologist. Physical examination revealed salmon pink papules and plaques with silvery scaling involving the abdomen, bilateral upper extremities and ears, and scalp. The patient was then clinically diagnosed with guttate psoriasis. A shave biopsy of a representative lesion on the abdomen was performed. The treatment plan included betamethasone dipropionate cream 0.05% applied twice daily for 2 weeks, clobetasol propionate solution 0.05% applied twice daily for 14 days (for the scalp), and hydrocortisone valerate cream 0.2% applied twice daily for 14 days (for the groin).
The skin biopsy shown in the Figure was received in 10% buffered formalin, measuring 5×4×1 mm of skin. Sections showed an acanthotic epidermis with foci of spongiosis and hypergranulosis covered by mounds of parakeratosis infiltrated by neutrophils. Superficial perivascular and interstitial lymphocytic inflammation was present. Tortuous blood vessels within the papillary dermis also were present. Results showed psoriasiform dermatitis with mild spongiosis. Periodic acid–Schiff stain did not reveal any fungal organisms. These findings were consistent with a diagnosis of guttate psoriasis.

The patient then returned 1 month later mentioning continued flare-ups of the scalp as well as newer patches on the arms and hands that were less eruptive and faded more quickly. The plaques in the groin area had resolved. Physical examination showed fewer pink papules and plaques with silvery scaling on the abdomen, bilateral upper extremities and ears, and scalp. Topical medications were continued, and possible apremilast therapy for the psoriasis was discussed.
Comment
Enterovirus-derived HFMD likely is caused by coxsackie-virus A. Current evidence supports the theory that guttate psoriasis can be environmentally triggered in genetically susceptible individuals, often but not exclusively by a streptococcal infection. The causative agent elicits a T-cell–mediated reaction leading to increased type 1 helper T cells, IFN-γ, and IL-2 cytokine levels. HLA-Cw∗0602–positive patients are considered genetically susceptible and more likely to develop guttate psoriasis following an environmental trigger. Based on the coincidence in timing of both diagnoses, this reported case of guttate psoriasis may have been triggered by a coxsackievirus A infection.
There are 4 variants of psoriasis: plaque, guttate, pustular, and erythroderma (in order of prevalence).2 Guttate psoriasis is characterized by small, 2- to 10-mm, raindroplike lesions on the skin.1 It accounts for approximately 2% of total psoriasis cases and is commonly triggered by group A streptococcal pharyngitis or tonsillitis.3,4
Hand-foot-and-mouth disease (HFMD) is an illness most commonly caused by a coxsackievirus A infection but also can be caused by other enteroviruses.5,6 Coxsackievirus is a serotype of the Enterovirus species within the Picornaviridae family.7 Hand-foot-and-mouth disease is characterized by a brief fever and vesicular rashes on the palms, soles, or buttocks, as well as oropharyngeal ulcers.8 Typically, the rash is benign and short-lived.9 In rare cases, neurologic complications develop. There have been no reported cases of guttate psoriasis following a coxsackievirus A infection.
The involvement of coxsackievirus B in the etiopathogenesis of psoriasis has been previously reported.10 We report the case of guttate psoriasis following presumed coxsackievirus A HFMD.
Case Report
A 56-year-old woman presented with a vesicular rash on the hands, feet, and lips. The patient reported having a sore throat that started around the same time that the rash developed. The severity of the sore throat was rated as moderate. No fever was reported. One day prior, the patient’s primary care physician prescribed a tapered course of prednisone for the rash. The patient reported a medical history of herpes zoster virus, sunburn, and genital herpes. She was taking clonazepam and had a known allergy to penicillin.
Physical examination revealed erythematous vesicular and papular lesions on the extensor surfaces of the hands and feet. Vesicles also were noted on the vermilion border of the lip. Examination of the patient’s mouth showed blisters and shallow ulcerations in the oral cavity. A clinical diagnosis of coxsackievirus A HFMD was made, and the treatment plan included triamcinolone acetonide ointment 0.025% applied twice daily for 2 weeks and oral valacyclovir hydrochloride 1 g taken 3 times daily for 7 days. A topical emollient also was recommended for the lips when necessary. The lesions all resolved within a 2-week period with no sequela.
The patient returned 1 month later, citing newer red abdominal skin lesions. Fever was denied. She reported that both prescribed treatments had not been helping for the newer lesions. She noticed similar lesions on the groin and brought them to the attention of her gynecologist. Physical examination revealed salmon pink papules and plaques with silvery scaling involving the abdomen, bilateral upper extremities and ears, and scalp. The patient was then clinically diagnosed with guttate psoriasis. A shave biopsy of a representative lesion on the abdomen was performed. The treatment plan included betamethasone dipropionate cream 0.05% applied twice daily for 2 weeks, clobetasol propionate solution 0.05% applied twice daily for 14 days (for the scalp), and hydrocortisone valerate cream 0.2% applied twice daily for 14 days (for the groin).
The skin biopsy shown in the Figure was received in 10% buffered formalin, measuring 5×4×1 mm of skin. Sections showed an acanthotic epidermis with foci of spongiosis and hypergranulosis covered by mounds of parakeratosis infiltrated by neutrophils. Superficial perivascular and interstitial lymphocytic inflammation was present. Tortuous blood vessels within the papillary dermis also were present. Results showed psoriasiform dermatitis with mild spongiosis. Periodic acid–Schiff stain did not reveal any fungal organisms. These findings were consistent with a diagnosis of guttate psoriasis.

The patient then returned 1 month later mentioning continued flare-ups of the scalp as well as newer patches on the arms and hands that were less eruptive and faded more quickly. The plaques in the groin area had resolved. Physical examination showed fewer pink papules and plaques with silvery scaling on the abdomen, bilateral upper extremities and ears, and scalp. Topical medications were continued, and possible apremilast therapy for the psoriasis was discussed.
Comment
Enterovirus-derived HFMD likely is caused by coxsackie-virus A. Current evidence supports the theory that guttate psoriasis can be environmentally triggered in genetically susceptible individuals, often but not exclusively by a streptococcal infection. The causative agent elicits a T-cell–mediated reaction leading to increased type 1 helper T cells, IFN-γ, and IL-2 cytokine levels. HLA-Cw∗0602–positive patients are considered genetically susceptible and more likely to develop guttate psoriasis following an environmental trigger. Based on the coincidence in timing of both diagnoses, this reported case of guttate psoriasis may have been triggered by a coxsackievirus A infection.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;1:79-82.
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? an immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326-332.
- Telfer N, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Seitsonen J, Shakeel S, Susi P, et al. Structural analysis of coxsackievirus A7 reveals conformational changes associated with uncoating. J Virol. 2012;86:7207-7215.
- Wu Y, Yeo A, Phoon M, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Tesini BL. Hand-foot-and-mouth-disease (HFMD). May 2018. https://www.msdmanuals.com/professional/infectious-diseases/enteroviruses/hand-foot-and-mouth-disease-hfmd. Accessed September 25, 2019.
- Korzhova TP, Shyrobokov VP, Koliadenko VH, et al. Coxsackie B viral infection in the etiology and clinical pathogenesis of psoriasis [in Ukrainian]. Lik Sprava. 2001:54-58.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;1:79-82.
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? an immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326-332.
- Telfer N, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Seitsonen J, Shakeel S, Susi P, et al. Structural analysis of coxsackievirus A7 reveals conformational changes associated with uncoating. J Virol. 2012;86:7207-7215.
- Wu Y, Yeo A, Phoon M, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Tesini BL. Hand-foot-and-mouth-disease (HFMD). May 2018. https://www.msdmanuals.com/professional/infectious-diseases/enteroviruses/hand-foot-and-mouth-disease-hfmd. Accessed September 25, 2019.
- Korzhova TP, Shyrobokov VP, Koliadenko VH, et al. Coxsackie B viral infection in the etiology and clinical pathogenesis of psoriasis [in Ukrainian]. Lik Sprava. 2001:54-58.
Practice Points
- Inquire about any illnesses preceding derma-tologic diseases.
- There may be additional microbial triggers for dermatologic diseases.
Photolichenoid Dermatitis: A Presenting Sign of Human Immunodeficiency Virus
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.
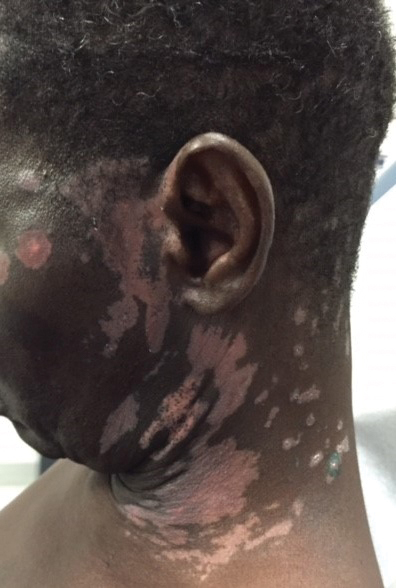
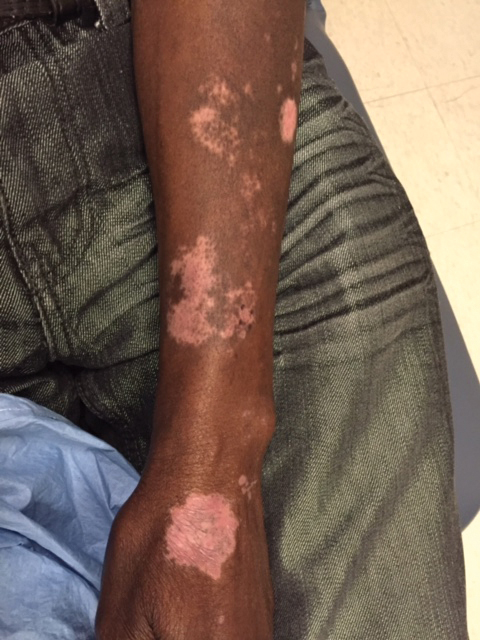
A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.
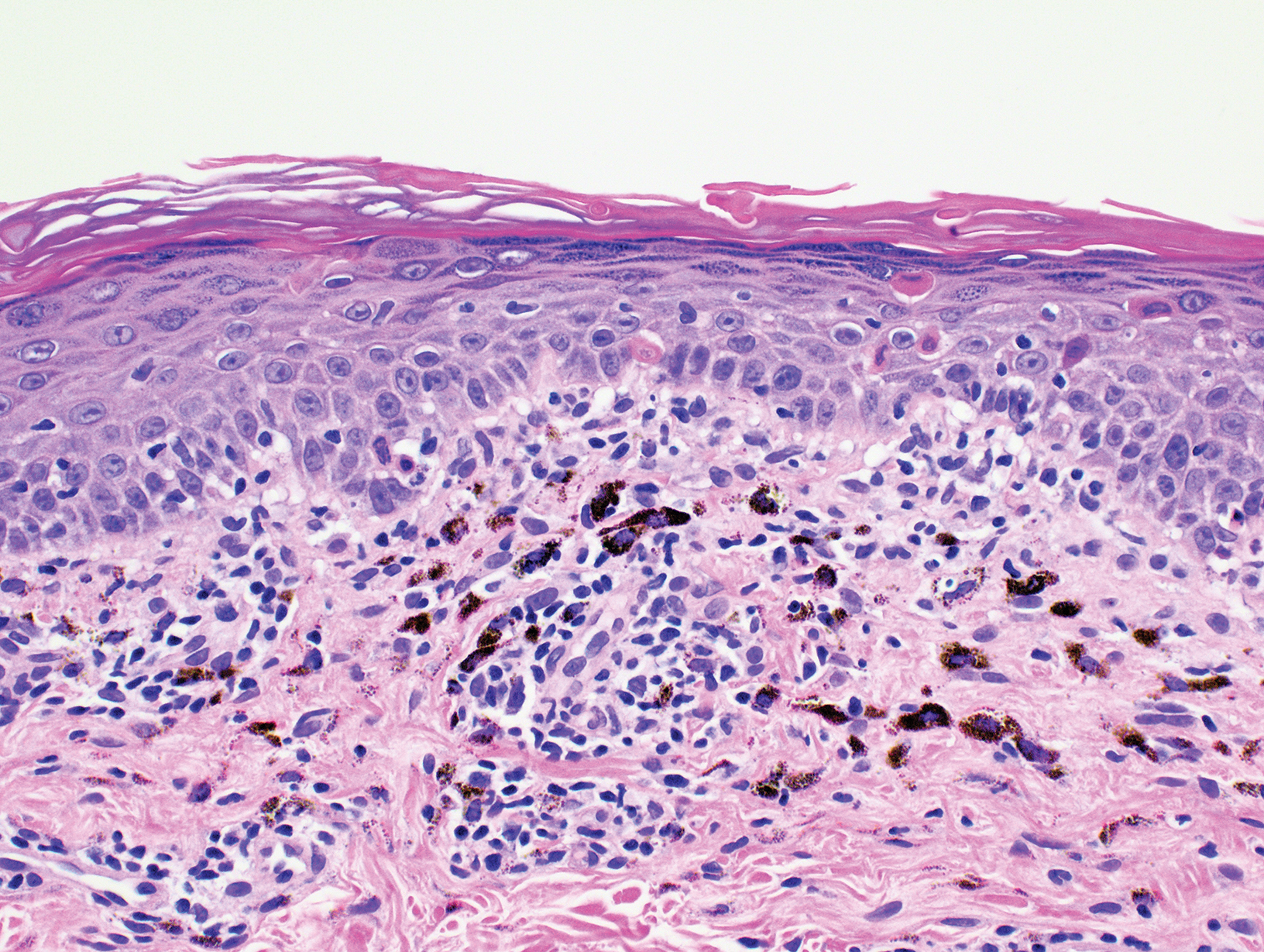
Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.


A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.

Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.


A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.

Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
Practice Points
- There are few reports in the literature of human immunodeficiency virus (HIV) presenting as a photolichenoid eruption.
- We report the case of a 62-year-old African man who presented with a new-onset photodistributed eruption and was subsequently diagnosed with HIV.
- This case supports testing for HIV in patients with a similar clinical presentation.
Cutaneous Mycobacterium haemophilum Infection Involving the Upper Extremities: Diagnosis and Management Guidelines
Infection with Mycobacterium haemophilum, a rare, slow-growing organism, most commonly presents as ulcerating cutaneous lesions and subcutaneous nodules in immunocompromised adults.1 The most common clinical presentation in adults includes cutaneous lesions, nodules, cysts, and papules, with signs and symptoms of erythema, pain, pruritus, and drainage.2 Disseminated disease states of septic arthritis, pulmonary infiltration, and osteomyelitis, though life-threatening, are less common manifestations reported in highly immunocompromised persons.3
Infection with M haemophilum presents a challenge to the dermatology community because it is infrequently suspected and misidentified, resulting in delayed diagnosis. Additionally, M haemophilum is an extremely fastidious organism that requires heme-supplemented culture media and a carefully regulated low temperature for many consecutive weeks to yield valid culture results.1 These features contribute to complications and delays in diagnosis of an already overlooked source of infection.
We discuss the clinical presentation, diagnosis, and treatment of 3 unusual cases of cutaneous M haemophilum infection involving the upper arms. The findings in these cases highlight the challenges inherent in diagnosis as well as the obstacles that arise in providing effective, long-term treatment of this infection.
Case Reports
Patient 1
A 69-year-old woman with a medical history of a single functioning kidney and moderate psoriasis managed with low-dosage methotrexate presented with an erythematous nonhealing wound on the left forearm that developed after she was scratched by a dog. The pustules, appearing as bright red, tender, warm abscesses, had been present for 3 months and were distributed on the left proximal and distal dorsal forearm (Figure 1A). The patient reported no recent travel, sick contacts, allergies, or new medications.
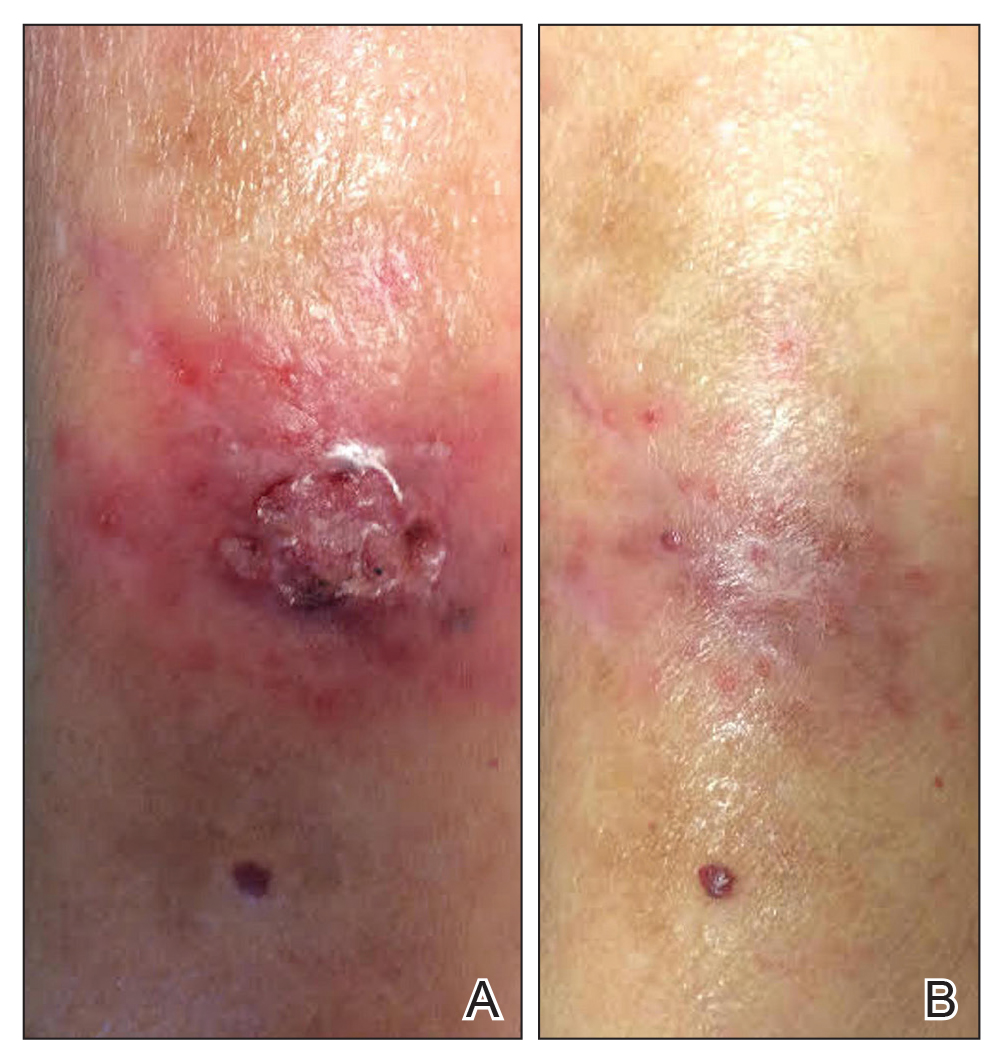
A shave biopsy was initially obtained. Swab specimens were sent for bacterial, fungal, and mycobacterial culture following discontinuation of methotrexate. Initial histopathologic analysis revealed aggregates of histiocytes and multinucleated giant cells within the dermis, surrounded by infiltrates of lymphocytes and neutrophils (Figure 2), consistent with a dermal noncaseating granulomatosis. Acid-fast bacilli (AFB), periodic acid–Schiff, Gram, and Grocott-Gomori methenamine-silver stains were negative for pathogenic microorganisms. There was no evidence of vasculitis.
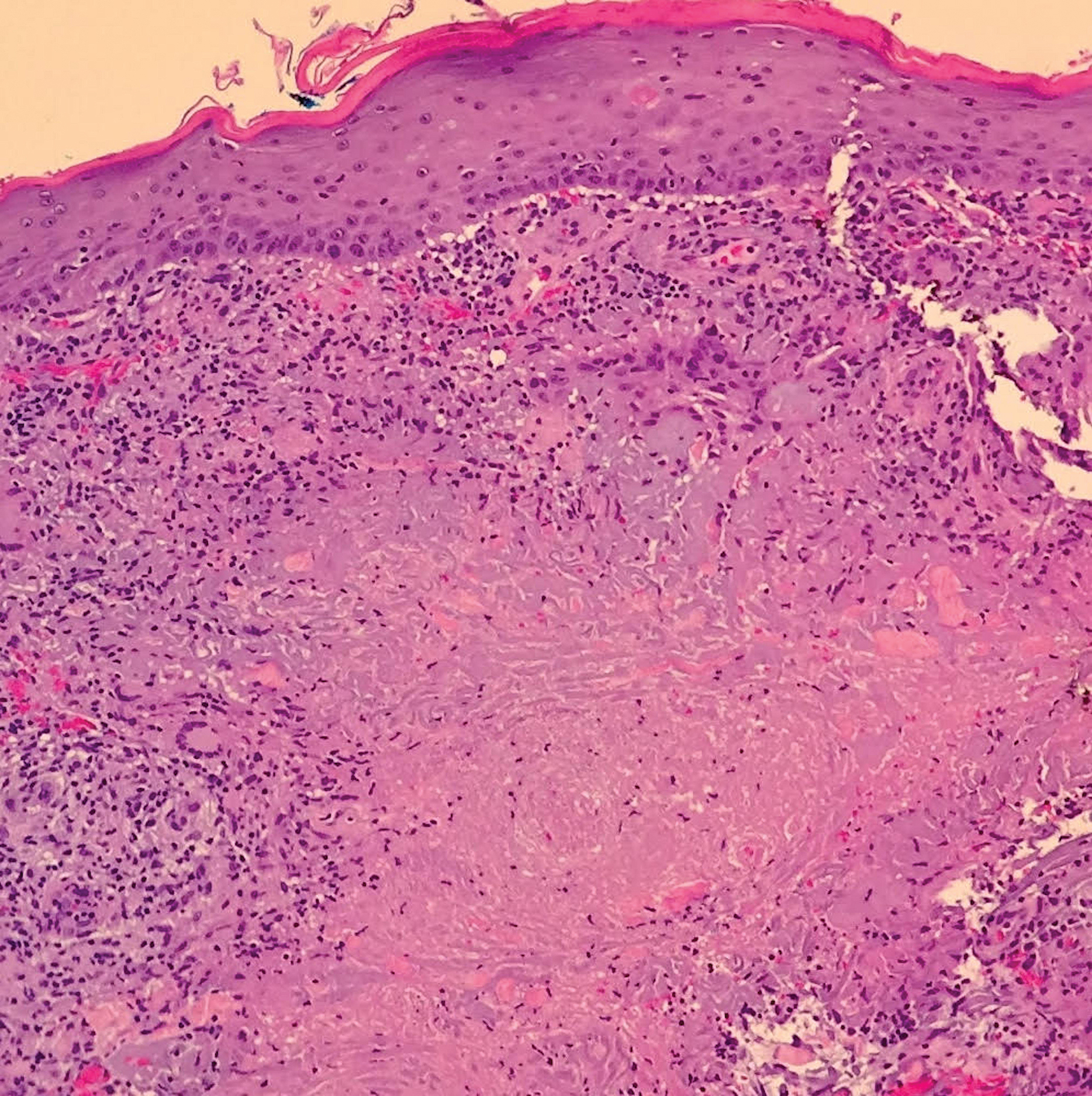
Despite negative special stains, an infectious cause was still suspected. Oral doxycycline monohydrate 100 mg twice daily, oral fluconazole 200 mg daily, and econazole cream 1% were prescribed because of concern for mycobacterial infection and initial growth of Candida parapsilosis in the swab culture.
A punch biopsy also was performed at this time for both repeat histopathologic analysis and tissue culture. Follow-up appointments were scheduled every 2 weeks. Staining by AFB of the repeat histopathologic specimen was negative.
The patient demonstrated symptomatic and aesthetic improvement (Figure 1B) during consecutive regular follow-up appointments while culture results were pending. No lesions appeared above the left elbow and she had no lymphadenopathy. Results of blood chemistry analyses and complete blood cell count throughout follow-up were normal.
The final tissue culture report obtained 7 weeks after initial presentation showed growth of M haemophilum despite a negative smear. The swab culture that initially was taken did not grow pathogenic organisms.
The patient was referred to an infectious disease specialist who confirmed that the atypical mycobacterial infection likely was the main source of the cutaneous lesions. She was instructed to continue econazole cream 1% and was given prescriptions for clarithromycin 500 mg twice daily, ciprofloxacin 500 mg twice daily, and rifampin 300 mg twice daily for a total duration of 12 to 18 months. The patient has remained on this triple-drug regimen and demonstrated improvement in the lesions. She has been off methotrexate while on antibiotic therapy.
Patient 2
A 79-year-old man with a medical history of chronic lymphocytic leukemia, basal cell carcinoma, and squamous cell carcinoma presented with a nonhealing, painful, red lesion on the left forearm of 1 week’s duration. Physical examination revealed a violaceous nontender plaque with erosions and desquamation that was initially diagnosed as a carbuncle. The patient reported a similar eruption on the right foot that was successfully treated with silver sulfadiazine by another physician.
Biopsy was performed by the shave method for histologic analysis and tissue culture. Doxycycline 100 mg twice daily was prescribed because of high suspicion of infection. Histologic findings revealed granulomatous inflammation with pseudoepitheliomatous hyperplasia, reported as squamous cell carcinoma. A second opinion confirmed suspicion of an infectious process; the patient remained on doxycycline. During follow-up, the lesion progressed to a 5-cm plaque studded with pustules and satellite papules. Multiple additional tissue cultures were performed over 2 months until “light growth” of M haemophilum was reported.
The patient showed minimal improvement on tetracycline antibiotics. His condition was complicated by a photosensitivity reaction to doxycycline on the left and right forearms, hands, and nose. Consequently, triamcinolone was prescribed, doxycycline was discontinued, and minocycline 100 mg twice daily and ciprofloxacin 500 mg twice daily were prescribed.
Nine months after initial presentation, the lesions were still present but remarkably improved. The antibiotic regimen was discontinued after 11 months.
Patient 3
A 77-year-old woman with a history of rheumatoid arthritis treated with methotrexate and abatacept as well as cutaneous T-cell lymphoma treated with narrowband UVB radiation presented to the emergency department with fever and an inflamed right forearm (Figure 3A). Initial bacterial cultures of the wound and blood were negative.
The patient was treated with vancomycin and discharged on cephalexin once she became afebrile. She was seen at our office the next week for further evaluation. We recommended that she discontinue all immunosuppressant medications. A 4-mm tissue biopsy for hematoxylin and eosin staining and a separate 4-mm punch biopsy for culture were performed while she was taking cephalexin. Histopathologic analysis revealed numerous neutrophilic abscesses; however, Gram, AFB, and fungal stains were negative.
Arm edema and pustules slowly resolved, but the eschar and verrucous plaques continued to slowly progress while the patient was off immunosuppression. She was kept off antibiotics until mycobacterial culture was positive at 4 weeks, at which time she was placed on doxycycline and clarithromycin. Final identification of M haemophilum was made at 6 weeks; consequently, doxycycline was discontinued and she was referred to infectious disease for multidrug therapy. She remained afebrile during the entire 6 weeks until cultures were final.
While immunosuppressants were discontinued and clarithromycin was administered, the plaque changed from an edematous pustular dermatitis to a verrucous crusted plaque. Neither epitrochlear nor axillary lymphadenopathy was noted during the treatment period. The infectious disease specialist prescribed azithromycin, ethambutol, and rifampin, which produced marked improvement (Figure 3B). The patient has remained off immunosuppressive therapy while on antibiotics.
Comment
Clinical Presentation and Diagnosis
Mycobacterium haemophilum is a rare infectious organism that affects primarily immunocompromised adults but also has been identified in immunocompetent adults and pediatric patients.2 Commonly affected immunosuppressed groups include solid organ transplant recipients, bone marrow transplant recipients, human immunodeficiency virus–positive patients, and patients with rheumatoid arthritis.
The infection typically presents as small violaceous papules and pustules that become painful and erythematous, with progression and draining ulceration in later stages.2 In our cases, all lesions tended to evolve into a verrucous plaque that slowly resolved with antibiotic therapy.
Due to the rarity of this infection, the initial differential diagnosis can include infection with other mycobacteria, Sporothrix, Staphylococcus aureus, and other fungal pathogens. Misdiagnosis is a common obstacle in the treatment of M haemophilum due to its rarity, often negative AFB stains, and slow growth on culture media; therefore, tissue culture is essential to successful diagnosis and management. The natural reservoir of M haemophilum is unknown, but infection has been associated with contaminated water sources.1 In one case (patient 1), symptoms developed after a dog scratch; the other 2 patients were unaware of injury to the skin.Laboratory diagnosis of M haemophilum is inherently difficult and protracted. The species is a highly fastidious and slow-growing Mycobacterium that requires cooler (30°C) incubation for many weeks on agar medium enriched with hemin or ferric ammonium citrate to obtain valid growth.1 To secure timely diagnosis, the organism’s slow agar growth warrants immediate tissue culture and biopsy when an immunocompromised patient presents with clinical features of atypical infection of an extremity. Mycobacterium haemophilum infection likely is underreported because of these difficulties in diagnosis.
Management
Although there are no standard guidelines for antibiotic treatment of M haemophilum, the current literature recommends triple-drug therapy with clarithromycin, ciprofloxacin, and rifamycin for at least 12 to 24 months.2
Upon clinical suspicion of an atypical Mycobacterium, we recommend a macrolide antibiotic over doxycycline, however, because this class of agents maintains broad coverage while being more specific for atypical mycobacteria. Although an atypical Mycobacterium was suspected early in the presentation in our cases, we discourage immediate use of triple-agent antibiotic therapy until laboratory evidence is procured to minimize antibiotic overuse in patients who do not have a final diagnosis. Single-agent therapy for prolonged treatment is discouraged for atypical mycobacterial infections because of the high risk of antibiotic resistance. Therapy should be tailored to the needs of the individual based on the extent of dissemination of disease and the severity of immunosuppression.1,2
Additionally, underlying disease that results in immunosuppression might necessitate treatment reevaluation (as occurred in our cases) requiring cessation of immunosuppressive drugs, extended careful monitoring, and pharmacotherapeutic readjustment through the course of treatment. The degree to which antibiotics contribute to eradication of M haemophilum is unknown; therefore, it is recommended that long-term antibiotic use and treatment aimed at recovering the immunocompromised state (eg, highly active antiretroviral therapy in a patient with AIDS) be implemented.2
Conclusion
Our 3 cases of M haemophilum infection involved the upper extremities of immunosuppressed patients older than 65 years. This propensity to affect the upper extremities could possibly be due to the lower temperature required for growth of M haemophilum. Initial histopathologic study showed granulomatous and neutrophilic infiltrates, yet histopathologic specimens from all 3 patients failed to display positive AFB staining, which delayed the initial antibiotic choice. In all cases, diagnosis was made by tissue culture after swab culture failed to grow the pathogen. Furthermore, the 3 cases took approximately 6 weeks to achieve final identification of the organism. Neither clinical lymphadenopathy nor systemic spread was noted in our patients; immunosuppression was discontinued when possible.
Mycobacterium haemophilum is an uncommon but potentially life-threatening infection that should be suspected in immunocompromised adults who present with atypical cellulitis of the extremities. The ultimate diagnosis often is delayed because the organism grows slowly (as long as 8 weeks) in tissue culture. For that reason, empiric antibiotic treatment, including a macrolide, should be considered in patients with disseminated or severe infection or critical immunosuppression and in those who do not demonstrate improvement in symptoms once immunosuppressants are withheld. A prolonged course of multiple-drug antibiotic therapy has proved to be effective for treating cutaneous infection with M haemophilum.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24:701-717.
- Tangkosakul T, Hongmanee P, Malathum K. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a tertiary hospital in Bangkok, Thailand: under-reported/under-recognized infection. JMM Case Rep. 2014;1:E002618.
- Sabeti S, Pourabdollah Tootkaboni M, Abdolahi M, et al. Mycobacterium haemophilum: a report of cutaneous infection in a patient with end-stage renal disease. Int J Mycobacteriol. 2016;5(suppl 1):S236.
Infection with Mycobacterium haemophilum, a rare, slow-growing organism, most commonly presents as ulcerating cutaneous lesions and subcutaneous nodules in immunocompromised adults.1 The most common clinical presentation in adults includes cutaneous lesions, nodules, cysts, and papules, with signs and symptoms of erythema, pain, pruritus, and drainage.2 Disseminated disease states of septic arthritis, pulmonary infiltration, and osteomyelitis, though life-threatening, are less common manifestations reported in highly immunocompromised persons.3
Infection with M haemophilum presents a challenge to the dermatology community because it is infrequently suspected and misidentified, resulting in delayed diagnosis. Additionally, M haemophilum is an extremely fastidious organism that requires heme-supplemented culture media and a carefully regulated low temperature for many consecutive weeks to yield valid culture results.1 These features contribute to complications and delays in diagnosis of an already overlooked source of infection.
We discuss the clinical presentation, diagnosis, and treatment of 3 unusual cases of cutaneous M haemophilum infection involving the upper arms. The findings in these cases highlight the challenges inherent in diagnosis as well as the obstacles that arise in providing effective, long-term treatment of this infection.
Case Reports
Patient 1
A 69-year-old woman with a medical history of a single functioning kidney and moderate psoriasis managed with low-dosage methotrexate presented with an erythematous nonhealing wound on the left forearm that developed after she was scratched by a dog. The pustules, appearing as bright red, tender, warm abscesses, had been present for 3 months and were distributed on the left proximal and distal dorsal forearm (Figure 1A). The patient reported no recent travel, sick contacts, allergies, or new medications.

A shave biopsy was initially obtained. Swab specimens were sent for bacterial, fungal, and mycobacterial culture following discontinuation of methotrexate. Initial histopathologic analysis revealed aggregates of histiocytes and multinucleated giant cells within the dermis, surrounded by infiltrates of lymphocytes and neutrophils (Figure 2), consistent with a dermal noncaseating granulomatosis. Acid-fast bacilli (AFB), periodic acid–Schiff, Gram, and Grocott-Gomori methenamine-silver stains were negative for pathogenic microorganisms. There was no evidence of vasculitis.

Despite negative special stains, an infectious cause was still suspected. Oral doxycycline monohydrate 100 mg twice daily, oral fluconazole 200 mg daily, and econazole cream 1% were prescribed because of concern for mycobacterial infection and initial growth of Candida parapsilosis in the swab culture.
A punch biopsy also was performed at this time for both repeat histopathologic analysis and tissue culture. Follow-up appointments were scheduled every 2 weeks. Staining by AFB of the repeat histopathologic specimen was negative.
The patient demonstrated symptomatic and aesthetic improvement (Figure 1B) during consecutive regular follow-up appointments while culture results were pending. No lesions appeared above the left elbow and she had no lymphadenopathy. Results of blood chemistry analyses and complete blood cell count throughout follow-up were normal.
The final tissue culture report obtained 7 weeks after initial presentation showed growth of M haemophilum despite a negative smear. The swab culture that initially was taken did not grow pathogenic organisms.
The patient was referred to an infectious disease specialist who confirmed that the atypical mycobacterial infection likely was the main source of the cutaneous lesions. She was instructed to continue econazole cream 1% and was given prescriptions for clarithromycin 500 mg twice daily, ciprofloxacin 500 mg twice daily, and rifampin 300 mg twice daily for a total duration of 12 to 18 months. The patient has remained on this triple-drug regimen and demonstrated improvement in the lesions. She has been off methotrexate while on antibiotic therapy.
Patient 2
A 79-year-old man with a medical history of chronic lymphocytic leukemia, basal cell carcinoma, and squamous cell carcinoma presented with a nonhealing, painful, red lesion on the left forearm of 1 week’s duration. Physical examination revealed a violaceous nontender plaque with erosions and desquamation that was initially diagnosed as a carbuncle. The patient reported a similar eruption on the right foot that was successfully treated with silver sulfadiazine by another physician.
Biopsy was performed by the shave method for histologic analysis and tissue culture. Doxycycline 100 mg twice daily was prescribed because of high suspicion of infection. Histologic findings revealed granulomatous inflammation with pseudoepitheliomatous hyperplasia, reported as squamous cell carcinoma. A second opinion confirmed suspicion of an infectious process; the patient remained on doxycycline. During follow-up, the lesion progressed to a 5-cm plaque studded with pustules and satellite papules. Multiple additional tissue cultures were performed over 2 months until “light growth” of M haemophilum was reported.
The patient showed minimal improvement on tetracycline antibiotics. His condition was complicated by a photosensitivity reaction to doxycycline on the left and right forearms, hands, and nose. Consequently, triamcinolone was prescribed, doxycycline was discontinued, and minocycline 100 mg twice daily and ciprofloxacin 500 mg twice daily were prescribed.
Nine months after initial presentation, the lesions were still present but remarkably improved. The antibiotic regimen was discontinued after 11 months.
Patient 3
A 77-year-old woman with a history of rheumatoid arthritis treated with methotrexate and abatacept as well as cutaneous T-cell lymphoma treated with narrowband UVB radiation presented to the emergency department with fever and an inflamed right forearm (Figure 3A). Initial bacterial cultures of the wound and blood were negative.

The patient was treated with vancomycin and discharged on cephalexin once she became afebrile. She was seen at our office the next week for further evaluation. We recommended that she discontinue all immunosuppressant medications. A 4-mm tissue biopsy for hematoxylin and eosin staining and a separate 4-mm punch biopsy for culture were performed while she was taking cephalexin. Histopathologic analysis revealed numerous neutrophilic abscesses; however, Gram, AFB, and fungal stains were negative.
Arm edema and pustules slowly resolved, but the eschar and verrucous plaques continued to slowly progress while the patient was off immunosuppression. She was kept off antibiotics until mycobacterial culture was positive at 4 weeks, at which time she was placed on doxycycline and clarithromycin. Final identification of M haemophilum was made at 6 weeks; consequently, doxycycline was discontinued and she was referred to infectious disease for multidrug therapy. She remained afebrile during the entire 6 weeks until cultures were final.
While immunosuppressants were discontinued and clarithromycin was administered, the plaque changed from an edematous pustular dermatitis to a verrucous crusted plaque. Neither epitrochlear nor axillary lymphadenopathy was noted during the treatment period. The infectious disease specialist prescribed azithromycin, ethambutol, and rifampin, which produced marked improvement (Figure 3B). The patient has remained off immunosuppressive therapy while on antibiotics.
Comment
Clinical Presentation and Diagnosis
Mycobacterium haemophilum is a rare infectious organism that affects primarily immunocompromised adults but also has been identified in immunocompetent adults and pediatric patients.2 Commonly affected immunosuppressed groups include solid organ transplant recipients, bone marrow transplant recipients, human immunodeficiency virus–positive patients, and patients with rheumatoid arthritis.
The infection typically presents as small violaceous papules and pustules that become painful and erythematous, with progression and draining ulceration in later stages.2 In our cases, all lesions tended to evolve into a verrucous plaque that slowly resolved with antibiotic therapy.
Due to the rarity of this infection, the initial differential diagnosis can include infection with other mycobacteria, Sporothrix, Staphylococcus aureus, and other fungal pathogens. Misdiagnosis is a common obstacle in the treatment of M haemophilum due to its rarity, often negative AFB stains, and slow growth on culture media; therefore, tissue culture is essential to successful diagnosis and management. The natural reservoir of M haemophilum is unknown, but infection has been associated with contaminated water sources.1 In one case (patient 1), symptoms developed after a dog scratch; the other 2 patients were unaware of injury to the skin.Laboratory diagnosis of M haemophilum is inherently difficult and protracted. The species is a highly fastidious and slow-growing Mycobacterium that requires cooler (30°C) incubation for many weeks on agar medium enriched with hemin or ferric ammonium citrate to obtain valid growth.1 To secure timely diagnosis, the organism’s slow agar growth warrants immediate tissue culture and biopsy when an immunocompromised patient presents with clinical features of atypical infection of an extremity. Mycobacterium haemophilum infection likely is underreported because of these difficulties in diagnosis.
Management
Although there are no standard guidelines for antibiotic treatment of M haemophilum, the current literature recommends triple-drug therapy with clarithromycin, ciprofloxacin, and rifamycin for at least 12 to 24 months.2
Upon clinical suspicion of an atypical Mycobacterium, we recommend a macrolide antibiotic over doxycycline, however, because this class of agents maintains broad coverage while being more specific for atypical mycobacteria. Although an atypical Mycobacterium was suspected early in the presentation in our cases, we discourage immediate use of triple-agent antibiotic therapy until laboratory evidence is procured to minimize antibiotic overuse in patients who do not have a final diagnosis. Single-agent therapy for prolonged treatment is discouraged for atypical mycobacterial infections because of the high risk of antibiotic resistance. Therapy should be tailored to the needs of the individual based on the extent of dissemination of disease and the severity of immunosuppression.1,2
Additionally, underlying disease that results in immunosuppression might necessitate treatment reevaluation (as occurred in our cases) requiring cessation of immunosuppressive drugs, extended careful monitoring, and pharmacotherapeutic readjustment through the course of treatment. The degree to which antibiotics contribute to eradication of M haemophilum is unknown; therefore, it is recommended that long-term antibiotic use and treatment aimed at recovering the immunocompromised state (eg, highly active antiretroviral therapy in a patient with AIDS) be implemented.2
Conclusion
Our 3 cases of M haemophilum infection involved the upper extremities of immunosuppressed patients older than 65 years. This propensity to affect the upper extremities could possibly be due to the lower temperature required for growth of M haemophilum. Initial histopathologic study showed granulomatous and neutrophilic infiltrates, yet histopathologic specimens from all 3 patients failed to display positive AFB staining, which delayed the initial antibiotic choice. In all cases, diagnosis was made by tissue culture after swab culture failed to grow the pathogen. Furthermore, the 3 cases took approximately 6 weeks to achieve final identification of the organism. Neither clinical lymphadenopathy nor systemic spread was noted in our patients; immunosuppression was discontinued when possible.
Mycobacterium haemophilum is an uncommon but potentially life-threatening infection that should be suspected in immunocompromised adults who present with atypical cellulitis of the extremities. The ultimate diagnosis often is delayed because the organism grows slowly (as long as 8 weeks) in tissue culture. For that reason, empiric antibiotic treatment, including a macrolide, should be considered in patients with disseminated or severe infection or critical immunosuppression and in those who do not demonstrate improvement in symptoms once immunosuppressants are withheld. A prolonged course of multiple-drug antibiotic therapy has proved to be effective for treating cutaneous infection with M haemophilum.
Infection with Mycobacterium haemophilum, a rare, slow-growing organism, most commonly presents as ulcerating cutaneous lesions and subcutaneous nodules in immunocompromised adults.1 The most common clinical presentation in adults includes cutaneous lesions, nodules, cysts, and papules, with signs and symptoms of erythema, pain, pruritus, and drainage.2 Disseminated disease states of septic arthritis, pulmonary infiltration, and osteomyelitis, though life-threatening, are less common manifestations reported in highly immunocompromised persons.3
Infection with M haemophilum presents a challenge to the dermatology community because it is infrequently suspected and misidentified, resulting in delayed diagnosis. Additionally, M haemophilum is an extremely fastidious organism that requires heme-supplemented culture media and a carefully regulated low temperature for many consecutive weeks to yield valid culture results.1 These features contribute to complications and delays in diagnosis of an already overlooked source of infection.
We discuss the clinical presentation, diagnosis, and treatment of 3 unusual cases of cutaneous M haemophilum infection involving the upper arms. The findings in these cases highlight the challenges inherent in diagnosis as well as the obstacles that arise in providing effective, long-term treatment of this infection.
Case Reports
Patient 1
A 69-year-old woman with a medical history of a single functioning kidney and moderate psoriasis managed with low-dosage methotrexate presented with an erythematous nonhealing wound on the left forearm that developed after she was scratched by a dog. The pustules, appearing as bright red, tender, warm abscesses, had been present for 3 months and were distributed on the left proximal and distal dorsal forearm (Figure 1A). The patient reported no recent travel, sick contacts, allergies, or new medications.

A shave biopsy was initially obtained. Swab specimens were sent for bacterial, fungal, and mycobacterial culture following discontinuation of methotrexate. Initial histopathologic analysis revealed aggregates of histiocytes and multinucleated giant cells within the dermis, surrounded by infiltrates of lymphocytes and neutrophils (Figure 2), consistent with a dermal noncaseating granulomatosis. Acid-fast bacilli (AFB), periodic acid–Schiff, Gram, and Grocott-Gomori methenamine-silver stains were negative for pathogenic microorganisms. There was no evidence of vasculitis.

Despite negative special stains, an infectious cause was still suspected. Oral doxycycline monohydrate 100 mg twice daily, oral fluconazole 200 mg daily, and econazole cream 1% were prescribed because of concern for mycobacterial infection and initial growth of Candida parapsilosis in the swab culture.
A punch biopsy also was performed at this time for both repeat histopathologic analysis and tissue culture. Follow-up appointments were scheduled every 2 weeks. Staining by AFB of the repeat histopathologic specimen was negative.
The patient demonstrated symptomatic and aesthetic improvement (Figure 1B) during consecutive regular follow-up appointments while culture results were pending. No lesions appeared above the left elbow and she had no lymphadenopathy. Results of blood chemistry analyses and complete blood cell count throughout follow-up were normal.
The final tissue culture report obtained 7 weeks after initial presentation showed growth of M haemophilum despite a negative smear. The swab culture that initially was taken did not grow pathogenic organisms.
The patient was referred to an infectious disease specialist who confirmed that the atypical mycobacterial infection likely was the main source of the cutaneous lesions. She was instructed to continue econazole cream 1% and was given prescriptions for clarithromycin 500 mg twice daily, ciprofloxacin 500 mg twice daily, and rifampin 300 mg twice daily for a total duration of 12 to 18 months. The patient has remained on this triple-drug regimen and demonstrated improvement in the lesions. She has been off methotrexate while on antibiotic therapy.
Patient 2
A 79-year-old man with a medical history of chronic lymphocytic leukemia, basal cell carcinoma, and squamous cell carcinoma presented with a nonhealing, painful, red lesion on the left forearm of 1 week’s duration. Physical examination revealed a violaceous nontender plaque with erosions and desquamation that was initially diagnosed as a carbuncle. The patient reported a similar eruption on the right foot that was successfully treated with silver sulfadiazine by another physician.
Biopsy was performed by the shave method for histologic analysis and tissue culture. Doxycycline 100 mg twice daily was prescribed because of high suspicion of infection. Histologic findings revealed granulomatous inflammation with pseudoepitheliomatous hyperplasia, reported as squamous cell carcinoma. A second opinion confirmed suspicion of an infectious process; the patient remained on doxycycline. During follow-up, the lesion progressed to a 5-cm plaque studded with pustules and satellite papules. Multiple additional tissue cultures were performed over 2 months until “light growth” of M haemophilum was reported.
The patient showed minimal improvement on tetracycline antibiotics. His condition was complicated by a photosensitivity reaction to doxycycline on the left and right forearms, hands, and nose. Consequently, triamcinolone was prescribed, doxycycline was discontinued, and minocycline 100 mg twice daily and ciprofloxacin 500 mg twice daily were prescribed.
Nine months after initial presentation, the lesions were still present but remarkably improved. The antibiotic regimen was discontinued after 11 months.
Patient 3
A 77-year-old woman with a history of rheumatoid arthritis treated with methotrexate and abatacept as well as cutaneous T-cell lymphoma treated with narrowband UVB radiation presented to the emergency department with fever and an inflamed right forearm (Figure 3A). Initial bacterial cultures of the wound and blood were negative.

The patient was treated with vancomycin and discharged on cephalexin once she became afebrile. She was seen at our office the next week for further evaluation. We recommended that she discontinue all immunosuppressant medications. A 4-mm tissue biopsy for hematoxylin and eosin staining and a separate 4-mm punch biopsy for culture were performed while she was taking cephalexin. Histopathologic analysis revealed numerous neutrophilic abscesses; however, Gram, AFB, and fungal stains were negative.
Arm edema and pustules slowly resolved, but the eschar and verrucous plaques continued to slowly progress while the patient was off immunosuppression. She was kept off antibiotics until mycobacterial culture was positive at 4 weeks, at which time she was placed on doxycycline and clarithromycin. Final identification of M haemophilum was made at 6 weeks; consequently, doxycycline was discontinued and she was referred to infectious disease for multidrug therapy. She remained afebrile during the entire 6 weeks until cultures were final.
While immunosuppressants were discontinued and clarithromycin was administered, the plaque changed from an edematous pustular dermatitis to a verrucous crusted plaque. Neither epitrochlear nor axillary lymphadenopathy was noted during the treatment period. The infectious disease specialist prescribed azithromycin, ethambutol, and rifampin, which produced marked improvement (Figure 3B). The patient has remained off immunosuppressive therapy while on antibiotics.
Comment
Clinical Presentation and Diagnosis
Mycobacterium haemophilum is a rare infectious organism that affects primarily immunocompromised adults but also has been identified in immunocompetent adults and pediatric patients.2 Commonly affected immunosuppressed groups include solid organ transplant recipients, bone marrow transplant recipients, human immunodeficiency virus–positive patients, and patients with rheumatoid arthritis.
The infection typically presents as small violaceous papules and pustules that become painful and erythematous, with progression and draining ulceration in later stages.2 In our cases, all lesions tended to evolve into a verrucous plaque that slowly resolved with antibiotic therapy.
Due to the rarity of this infection, the initial differential diagnosis can include infection with other mycobacteria, Sporothrix, Staphylococcus aureus, and other fungal pathogens. Misdiagnosis is a common obstacle in the treatment of M haemophilum due to its rarity, often negative AFB stains, and slow growth on culture media; therefore, tissue culture is essential to successful diagnosis and management. The natural reservoir of M haemophilum is unknown, but infection has been associated with contaminated water sources.1 In one case (patient 1), symptoms developed after a dog scratch; the other 2 patients were unaware of injury to the skin.Laboratory diagnosis of M haemophilum is inherently difficult and protracted. The species is a highly fastidious and slow-growing Mycobacterium that requires cooler (30°C) incubation for many weeks on agar medium enriched with hemin or ferric ammonium citrate to obtain valid growth.1 To secure timely diagnosis, the organism’s slow agar growth warrants immediate tissue culture and biopsy when an immunocompromised patient presents with clinical features of atypical infection of an extremity. Mycobacterium haemophilum infection likely is underreported because of these difficulties in diagnosis.
Management
Although there are no standard guidelines for antibiotic treatment of M haemophilum, the current literature recommends triple-drug therapy with clarithromycin, ciprofloxacin, and rifamycin for at least 12 to 24 months.2
Upon clinical suspicion of an atypical Mycobacterium, we recommend a macrolide antibiotic over doxycycline, however, because this class of agents maintains broad coverage while being more specific for atypical mycobacteria. Although an atypical Mycobacterium was suspected early in the presentation in our cases, we discourage immediate use of triple-agent antibiotic therapy until laboratory evidence is procured to minimize antibiotic overuse in patients who do not have a final diagnosis. Single-agent therapy for prolonged treatment is discouraged for atypical mycobacterial infections because of the high risk of antibiotic resistance. Therapy should be tailored to the needs of the individual based on the extent of dissemination of disease and the severity of immunosuppression.1,2
Additionally, underlying disease that results in immunosuppression might necessitate treatment reevaluation (as occurred in our cases) requiring cessation of immunosuppressive drugs, extended careful monitoring, and pharmacotherapeutic readjustment through the course of treatment. The degree to which antibiotics contribute to eradication of M haemophilum is unknown; therefore, it is recommended that long-term antibiotic use and treatment aimed at recovering the immunocompromised state (eg, highly active antiretroviral therapy in a patient with AIDS) be implemented.2
Conclusion
Our 3 cases of M haemophilum infection involved the upper extremities of immunosuppressed patients older than 65 years. This propensity to affect the upper extremities could possibly be due to the lower temperature required for growth of M haemophilum. Initial histopathologic study showed granulomatous and neutrophilic infiltrates, yet histopathologic specimens from all 3 patients failed to display positive AFB staining, which delayed the initial antibiotic choice. In all cases, diagnosis was made by tissue culture after swab culture failed to grow the pathogen. Furthermore, the 3 cases took approximately 6 weeks to achieve final identification of the organism. Neither clinical lymphadenopathy nor systemic spread was noted in our patients; immunosuppression was discontinued when possible.
Mycobacterium haemophilum is an uncommon but potentially life-threatening infection that should be suspected in immunocompromised adults who present with atypical cellulitis of the extremities. The ultimate diagnosis often is delayed because the organism grows slowly (as long as 8 weeks) in tissue culture. For that reason, empiric antibiotic treatment, including a macrolide, should be considered in patients with disseminated or severe infection or critical immunosuppression and in those who do not demonstrate improvement in symptoms once immunosuppressants are withheld. A prolonged course of multiple-drug antibiotic therapy has proved to be effective for treating cutaneous infection with M haemophilum.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24:701-717.
- Tangkosakul T, Hongmanee P, Malathum K. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a tertiary hospital in Bangkok, Thailand: under-reported/under-recognized infection. JMM Case Rep. 2014;1:E002618.
- Sabeti S, Pourabdollah Tootkaboni M, Abdolahi M, et al. Mycobacterium haemophilum: a report of cutaneous infection in a patient with end-stage renal disease. Int J Mycobacteriol. 2016;5(suppl 1):S236.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24:701-717.
- Tangkosakul T, Hongmanee P, Malathum K. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a tertiary hospital in Bangkok, Thailand: under-reported/under-recognized infection. JMM Case Rep. 2014;1:E002618.
- Sabeti S, Pourabdollah Tootkaboni M, Abdolahi M, et al. Mycobacterium haemophilum: a report of cutaneous infection in a patient with end-stage renal disease. Int J Mycobacteriol. 2016;5(suppl 1):S236.
Practice Points
- Mycobacterium haemophilum infections typically occur on the extremities of immunosuppressed patients.
- Acid-fast bacilli staining may be negative.
- Mycobacterial cultures may take up to 6 weeks for growth.
- Prolonged triple-antibiotic therapy and lowering of immunosuppression is ideal treatment.