User login
The emotionally exhausted physician
CASE ‘I’m stuck’
Dr. D, age 48, an internist practicing general medicine who is a married mother of 2 teenagers, presents with emotional exhaustion. Her medical history is unremarkable other than hyperlipidemia controlled by diet. She describes an episode of reactive depressive symptoms and anxiety when in college, which was related to the stress of final exams, finances, and the dissolution of a long-standing relationship. Regardless, she functioned well and graduated summa cum laude. She says her current feelings of being “stuck” have gradually increased during the past 3 to 4 years. Although she describes being mildly anxious and dysphoric, she also said she feels like her “wheels are spinning” and that she doesn’t even seem to care. Dr. D had been a high achiever, yet says she feels like she isn’t getting anywhere at work or at home.
[polldaddy:10064977]
The authors’ observations
As psychiatrists in the business of diagnosis and treatment, we immediately considered common diagnoses, such as major depressive disorder and persistent depressive disorder. However, despite our training in pathology, we believe patients should be considered well until proven sick. This paradigm shift is in line with the definition of mental health per the World Health Organization: “A state of well-being in which every individual realizes his or her own potential, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to her or his community.”1
In Dr. D’s case, there was not enough information yet to fully support any of the diagnoses. She did not exhibit enough depressive symptoms to support a diagnosis of a depressive disorder. She said that she didn’t feel like she was getting anywhere at work or home. Yet there was no objective information that suggested impairment in functioning, which would preclude a diagnosis of adjustment disorder. At this point, we would support the “V code” diagnosis of phase of life problem, or even what is rarely seen in a psychiatric evaluation: “no diagnosis.”
EVALUATION Is work the problem?
We diligently conduct a thorough review of systems with Dr. D and confirm there is not enough information to diagnose a depressive disorder, anxiety disorder, or other psychiatric disorder. Collateral history suggests her teenagers are well-adjusted and doing well in high school, and she is well-respected at work with reportedly excellent performance ratings. We identify strongly with her and her situation.
Dr. D admits she is an idealist and half-jokingly says she entered into medicine “to save the world.” Yet she laments the long hours and finding herself mired in paperwork. She is barely making it to her kids’ school events and says she can’t believe her first child will be graduating within a year. She has had some particularly challenging patients recently, and although she is still diligent about their care, she is shocked she doesn’t seem to care as much about “solving the medical puzzle.” This came to light for her when a longtime patient observed that Dr. D didn’t seem herself and asked, “Are you all right, Doc?”
Dr. D is professionally dressed and has excellent grooming and hygiene. She looks tired, yet has a full affect, a witty conversational style, and responds appropriately to humor.
[polldaddy:10064980]
The authors’ observations
It can be difficult to know what to do next when there isn’t an established “playbook” for a problem without a diagnosis. We realized Dr. D was describing burnout, a syndrome of depersonalization (detached and not caring, to the point of viewing people as objects), emotional exhaustion, and low personal accomplishment that leads to decreased effectiveness at work.2 DSM-5 does not include “burnout” as a diagnosis3; however, if symptoms evolve to the point where they affect occupational or social functioning, burnout can be similar to adjustment disorder. Treatment with an antidepressant medication is not appropriate. It is possible that CBT might be helpful for many patients, yet there is no evidence that Dr. D has any cognitive distortions. Although we already had some collateral information, it is never wrong to gather additional collateral. However, because burnout is common, we may not need additional information. We could reassure her and send her on her way, but we want to add therapeutic value. We advocated exploring issues in her life and work related to meaning.
Continue to: Physician burnout
Physician burnout
Burnout is an alarmingly common problem among physicians that affects approximately half of psychiatrists. In 2014, 54.4% of physicians, and slightly less than 50% of psychiatrists, had at least 1 symptom of burnout.4 This was up from the 45.5% of physicians and a little more than 40% of psychiatrists who reported burnout in 2011,4 which suggests that as medicine continues to change, doctors may increasingly feel the brunt of this change. The rate of burnout is highest in front-line specialties (family medicine, general internal medicine, and emergency medicine) and lowest in preventive medicine.5 Physician burnout leads to real-world occupational issues, such as medical errors, poor relationships with coworkers and patients, decreased patient satisfaction, and medical malpractice suits.6,7
Even though burnout is clearly a concern for our colleagues, don’t expect them to proactively line up outside our offices. In a survey of 7,197 surgeons, 86.6% of respondents answered it was not important that “I have regular meetings with a psychologist/psychiatrist to discuss stress.”8 At the same time, the idea of meeting with a psychologist/psychiatrist was rated more highly by surgeons who were burnt out and found to be a factor independently associated with burnout.8 Perhaps we have some work to do in marketing our services in a way that welcomes our colleagues.
Although physician burnout has been a focus of recent studies, burnout in general has been studied for decades in other working populations. There are 2 useful models describing burnout:
- The job demand-control(-support) model suggests that individuals experience strain and subsequent ill effects when the demands of their job exceed the control they have,9 and social support from supervisors and colleagues can buffer the harmful effects of job strain.10
- The effort-reward imbalance model suggests that high-effort, low-reward occupational conditions are particularly stressful.11
Both models are simple, intuitive, and suggest solutions.
When engaging your physician colleagues about their burnout, remember that physicians are people, too, and have the same difficulties that everyone else does in successfully practicing healthy behaviors. As physicians, we have significant demands on our time that make it difficult to control our ability to eat, sleep, and exercise. In general, the food available where we work is not nutritious,12 half of us are overweight or obese,13 and working more than 40 hours per week increases the likelihood we’ll have a higher body mass index.14 We don’t sleep well, either—we get less sleep than the general population,15 and more sleep equates to less burnout.16 Regarding exercise, doctors who cannot prioritize exercise tend to have more burnout.17
Continue to: When evaluating a physician colleague for symptoms of burnout...
When evaluating a physician colleague for symptoms of burnout, start by assessing the basics: eating, sleeping, and moving. In Dr. D’s case, this also included taking a quick inventory of what demands were on her proverbial plate. Taking inventory of these demands (ie, demand-control[-support] model) may lead to new insights about what she can control. Prioritization is important to determine where efforts go (ie, effort-reward imbalance model). This is where your skills as a psychiatrist can especially help, as you explore values and bring trade-offs to light.
EVALUATION Permission not to be perfect
As we interview Dr. D, we realize she has some obsessive-compulsive personality traits that are mostly self-serving. She places a high value on being thorough and having elegant clinical notes. Yet this value competes with her desire to be efficient and get home on time to see her kids’ school events. You point this out to her and see if she can come up with some solutions. You also discuss with Dr. D the tension everyone feels between valuing career and valuing family and friends. You normalize her situation, and give her permission to pick something about which she will allow herself not to be perfect.
[polldaddy:10064981]
The authors’ observations
Since perfectionism is a common trait among physicians,18 failure doesn’t seem to be compatible with their DNA. We encourage other physicians to be scientific about their own lives, just as they are in the profession they have chosen. Physicians can delude themselves into thinking they can have it all, not recognizing that every choice has its cost. For example, a physician who decides that it’s okay to publish one fewer research paper this year might have more time to enjoy spending time with his or her children. In our work with physicians, we strive to normalize their experiences, helping them reframe their perfectionistic viewpoint to recognize that everyone struggles with work-life balance issues. We validate that physicians have difficult choices to make in finding what works for them, and we challenge and support them in exploring these choices.
Choosing where to put one’s efforts is also contingent upon the expected rewards. Sometime before the daily grind of our careers in medicine started, we had strong visions of what such careers would mean to us. We visualized the ideal of helping people and making a difference. Then, at some point, many of us took this for granted and forgot about the intrinsic rewards of our work. In a 2014-2015 survey of U.S. physicians across all specialties, only 64.6% of respondents who were highly burned out said they found their work personally rewarding. This is a sharp contrast to the 97.5% of respondents who were not burned out who reported that they found their work personally rewarding.19
As psychiatrists, we can challenge our physician colleagues to dare to dream again. We can help them rediscover the rewarding aspects of their work (ie, per effort-reward imbalance model) that drew them into medicine in the first place. This may include exploring their future legacy. How do they want to be remembered at retirement? Such consideration is linked to mental simulation and meaning in their lives.20 We guide our colleagues to reframe their current situation to see the myriad of choices they have based upon their own specific value system. If family and friends are currently taking priority over work, it also helps to reframe that working allows us to make a good living so we can fully enjoy that time spent with family and friends.
Continue to: If we do our jobs well...
If we do our jobs well, the next part is easy. We have them set specific short- and long-term goals related to their situation. This is something we do every day in our practices. It may help to make sure we’re using SMART (Specific, Measurable, Achievable, Relevant, Time-Bound), a well-known mnemonic used for goals (Table 121), and brush up on our motivational interviewing skills (see Related Resources). It is especially important to make sure our colleagues have goals that are relevant—the “R” in the SMART mnemonic—to their situation.

Finally, we do a better job of reaching our goals and engaging more at work and at home when we have good social support. For physicians, co-worker support has been found to be directly related to our well-being as well as buffering the negative effects of work demands.22 Furthermore, our colleagues are the most acceptable sources of support when we are faced with stressful situations.23 Thus, as psychiatrists, we can doubly help our physician patients by providing collegial support and doing our usual job of holding them accountable to their goals (Table 2).

OUTCOME Goal-setting, priorities, accountability
As we’re exploring goals with Dr. D, she makes a conscious decision to spend less time on documentation and start focusing on being present with her patients. She returns in 1 month to tell you time management is still a struggle, but her visit with you was instrumental in making her realize how important it was to get home on time for her kids’ activities. She says it greatly helped that you kept her accountable, yet also validated her struggles and gave her permission to design her life within the constraints of her situation and without the burden of having to be perfect at everything.
Bottom Line
We can best help our physician colleagues who are experiencing burnout by shifting our paradigm to a wellness model that focuses on helping them reach their potential and balance their professional and personal lives.
Related Resources
- Balch CM, Shanafelt TD. Combating stress and burnout in surgical practice: a review. Thorac Surg Clin. 2011;21(3):417-430.
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change, 2nd ed. New York, NY: The Guilford Press; 2002.
- Stanford Medicine WellMD. Stress & Burnout. https://wellmd.stanford.edu.
1. World Health Organization. Mental health: a state of well-being. http://www.who.int/features/factfiles/mental_health/en/. Updated August 2014. Accessed March 17, 2017.
2. Maslach C, Jackson S, Leiter M. Maslach burnout inventory manual, 3rd ed. Palo Alto, CA: Consulting Psychologists Press; 1996.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600-1613.
5. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385.
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519.
7. Balch CM, Shanafelt TD. Combating stress and burnout in surgical practice: a review. Adv Surg. 2010;44:29-47.
8. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US Surgeons. Ann Surg. 2012;255(4):625-633.
9. Karasek RA Jr. Job demands, job decision latitude, and mental strain: implications for job redesign. Adm Sci Q. 1979;24(2):285-308.
10. Johnson JV. Collective control: strategies for survival in the workplace. Int J Health Serv. 1989;19(3):469-480.
11. Siegrist J. Adverse health effects of high-effort/low-reward conditions. J Occup Health Psychol. 1996;1(1):27-41.
12. Lesser LI, Cohen DA, Brook RH. Changing eating habits for the medical profession. JAMA. 2012;308(10):983-984.
13. Bleich SN, Bennett WL, Gudzune KA, et al. Impact of physician BMI on obesity care and beliefs. Obesity (Silver Spring). 2012;20(5):999-1005.
14. Stanford FC, Durkin MW, Blair SN, et al. Determining levels of physical activity in attending physicians, resident and fellow physicians, and medical students in the USA. Br J Sports Med. 2012;46(5):360-364.
15. Tucker P, Bejerot E, Kecklund G, et al. Stress Research Institute at Stockholm University. Stress Research Report-doctors’ work hours in Sweden: their impact on sleep, health, work-family balance, patient care and thoughts about work. https://www.stressforskning.su.se/polopoly_fs/1.233341.1429526778!/menu/standard/file/sfr325.pdf. Accessed March 17, 2017.
16. Wisetborisut A, Angkurawaranon C, Jiraporncharoen W, et al. Shift work and burnout among health care workers. Occup Med (Lond). 2014;64(4):279-286.
17. Eckleberry-Hunt J, Lick D, Boura J, et al. An exploratory study of resident burnout and wellness. Acad Med. 2009;84(2):269-277.
18. Peters M, King J. Perfectionism in doctors. BMJ. 2012;344:e1674. doi: https://doi.org/10.1136/bmj.e1674.
19. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clin Proc. 2017;92(3):415-422.
20. Waytz A, Hershfield HE, Tamir DI. Neural and behavioral evidence for the role of mental simulation in meaning in life. J Pers Soc Psychol. 2015;108(2):336-355.
21. SMART Criteria. https://en.wikipedia.org/wiki/SMART_criteria. Accessed March 17, 2017.
22. Hu YY, Fix ML, Hevelone ND, et al. Physicians’ needs in coping with emotional stressors: the case for peer support. Arch Surg. 2012;147(3):212-217.
23. Wallace JE, Lemaire J. On physician well being–you’ll get by with a little help from your friends. Soc Sci Med. 2007;64(12):2565-2577.
CASE ‘I’m stuck’
Dr. D, age 48, an internist practicing general medicine who is a married mother of 2 teenagers, presents with emotional exhaustion. Her medical history is unremarkable other than hyperlipidemia controlled by diet. She describes an episode of reactive depressive symptoms and anxiety when in college, which was related to the stress of final exams, finances, and the dissolution of a long-standing relationship. Regardless, she functioned well and graduated summa cum laude. She says her current feelings of being “stuck” have gradually increased during the past 3 to 4 years. Although she describes being mildly anxious and dysphoric, she also said she feels like her “wheels are spinning” and that she doesn’t even seem to care. Dr. D had been a high achiever, yet says she feels like she isn’t getting anywhere at work or at home.
[polldaddy:10064977]
The authors’ observations
As psychiatrists in the business of diagnosis and treatment, we immediately considered common diagnoses, such as major depressive disorder and persistent depressive disorder. However, despite our training in pathology, we believe patients should be considered well until proven sick. This paradigm shift is in line with the definition of mental health per the World Health Organization: “A state of well-being in which every individual realizes his or her own potential, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to her or his community.”1
In Dr. D’s case, there was not enough information yet to fully support any of the diagnoses. She did not exhibit enough depressive symptoms to support a diagnosis of a depressive disorder. She said that she didn’t feel like she was getting anywhere at work or home. Yet there was no objective information that suggested impairment in functioning, which would preclude a diagnosis of adjustment disorder. At this point, we would support the “V code” diagnosis of phase of life problem, or even what is rarely seen in a psychiatric evaluation: “no diagnosis.”
EVALUATION Is work the problem?
We diligently conduct a thorough review of systems with Dr. D and confirm there is not enough information to diagnose a depressive disorder, anxiety disorder, or other psychiatric disorder. Collateral history suggests her teenagers are well-adjusted and doing well in high school, and she is well-respected at work with reportedly excellent performance ratings. We identify strongly with her and her situation.
Dr. D admits she is an idealist and half-jokingly says she entered into medicine “to save the world.” Yet she laments the long hours and finding herself mired in paperwork. She is barely making it to her kids’ school events and says she can’t believe her first child will be graduating within a year. She has had some particularly challenging patients recently, and although she is still diligent about their care, she is shocked she doesn’t seem to care as much about “solving the medical puzzle.” This came to light for her when a longtime patient observed that Dr. D didn’t seem herself and asked, “Are you all right, Doc?”
Dr. D is professionally dressed and has excellent grooming and hygiene. She looks tired, yet has a full affect, a witty conversational style, and responds appropriately to humor.
[polldaddy:10064980]
The authors’ observations
It can be difficult to know what to do next when there isn’t an established “playbook” for a problem without a diagnosis. We realized Dr. D was describing burnout, a syndrome of depersonalization (detached and not caring, to the point of viewing people as objects), emotional exhaustion, and low personal accomplishment that leads to decreased effectiveness at work.2 DSM-5 does not include “burnout” as a diagnosis3; however, if symptoms evolve to the point where they affect occupational or social functioning, burnout can be similar to adjustment disorder. Treatment with an antidepressant medication is not appropriate. It is possible that CBT might be helpful for many patients, yet there is no evidence that Dr. D has any cognitive distortions. Although we already had some collateral information, it is never wrong to gather additional collateral. However, because burnout is common, we may not need additional information. We could reassure her and send her on her way, but we want to add therapeutic value. We advocated exploring issues in her life and work related to meaning.
Continue to: Physician burnout
Physician burnout
Burnout is an alarmingly common problem among physicians that affects approximately half of psychiatrists. In 2014, 54.4% of physicians, and slightly less than 50% of psychiatrists, had at least 1 symptom of burnout.4 This was up from the 45.5% of physicians and a little more than 40% of psychiatrists who reported burnout in 2011,4 which suggests that as medicine continues to change, doctors may increasingly feel the brunt of this change. The rate of burnout is highest in front-line specialties (family medicine, general internal medicine, and emergency medicine) and lowest in preventive medicine.5 Physician burnout leads to real-world occupational issues, such as medical errors, poor relationships with coworkers and patients, decreased patient satisfaction, and medical malpractice suits.6,7
Even though burnout is clearly a concern for our colleagues, don’t expect them to proactively line up outside our offices. In a survey of 7,197 surgeons, 86.6% of respondents answered it was not important that “I have regular meetings with a psychologist/psychiatrist to discuss stress.”8 At the same time, the idea of meeting with a psychologist/psychiatrist was rated more highly by surgeons who were burnt out and found to be a factor independently associated with burnout.8 Perhaps we have some work to do in marketing our services in a way that welcomes our colleagues.
Although physician burnout has been a focus of recent studies, burnout in general has been studied for decades in other working populations. There are 2 useful models describing burnout:
- The job demand-control(-support) model suggests that individuals experience strain and subsequent ill effects when the demands of their job exceed the control they have,9 and social support from supervisors and colleagues can buffer the harmful effects of job strain.10
- The effort-reward imbalance model suggests that high-effort, low-reward occupational conditions are particularly stressful.11
Both models are simple, intuitive, and suggest solutions.
When engaging your physician colleagues about their burnout, remember that physicians are people, too, and have the same difficulties that everyone else does in successfully practicing healthy behaviors. As physicians, we have significant demands on our time that make it difficult to control our ability to eat, sleep, and exercise. In general, the food available where we work is not nutritious,12 half of us are overweight or obese,13 and working more than 40 hours per week increases the likelihood we’ll have a higher body mass index.14 We don’t sleep well, either—we get less sleep than the general population,15 and more sleep equates to less burnout.16 Regarding exercise, doctors who cannot prioritize exercise tend to have more burnout.17
Continue to: When evaluating a physician colleague for symptoms of burnout...
When evaluating a physician colleague for symptoms of burnout, start by assessing the basics: eating, sleeping, and moving. In Dr. D’s case, this also included taking a quick inventory of what demands were on her proverbial plate. Taking inventory of these demands (ie, demand-control[-support] model) may lead to new insights about what she can control. Prioritization is important to determine where efforts go (ie, effort-reward imbalance model). This is where your skills as a psychiatrist can especially help, as you explore values and bring trade-offs to light.
EVALUATION Permission not to be perfect
As we interview Dr. D, we realize she has some obsessive-compulsive personality traits that are mostly self-serving. She places a high value on being thorough and having elegant clinical notes. Yet this value competes with her desire to be efficient and get home on time to see her kids’ school events. You point this out to her and see if she can come up with some solutions. You also discuss with Dr. D the tension everyone feels between valuing career and valuing family and friends. You normalize her situation, and give her permission to pick something about which she will allow herself not to be perfect.
[polldaddy:10064981]
The authors’ observations
Since perfectionism is a common trait among physicians,18 failure doesn’t seem to be compatible with their DNA. We encourage other physicians to be scientific about their own lives, just as they are in the profession they have chosen. Physicians can delude themselves into thinking they can have it all, not recognizing that every choice has its cost. For example, a physician who decides that it’s okay to publish one fewer research paper this year might have more time to enjoy spending time with his or her children. In our work with physicians, we strive to normalize their experiences, helping them reframe their perfectionistic viewpoint to recognize that everyone struggles with work-life balance issues. We validate that physicians have difficult choices to make in finding what works for them, and we challenge and support them in exploring these choices.
Choosing where to put one’s efforts is also contingent upon the expected rewards. Sometime before the daily grind of our careers in medicine started, we had strong visions of what such careers would mean to us. We visualized the ideal of helping people and making a difference. Then, at some point, many of us took this for granted and forgot about the intrinsic rewards of our work. In a 2014-2015 survey of U.S. physicians across all specialties, only 64.6% of respondents who were highly burned out said they found their work personally rewarding. This is a sharp contrast to the 97.5% of respondents who were not burned out who reported that they found their work personally rewarding.19
As psychiatrists, we can challenge our physician colleagues to dare to dream again. We can help them rediscover the rewarding aspects of their work (ie, per effort-reward imbalance model) that drew them into medicine in the first place. This may include exploring their future legacy. How do they want to be remembered at retirement? Such consideration is linked to mental simulation and meaning in their lives.20 We guide our colleagues to reframe their current situation to see the myriad of choices they have based upon their own specific value system. If family and friends are currently taking priority over work, it also helps to reframe that working allows us to make a good living so we can fully enjoy that time spent with family and friends.
Continue to: If we do our jobs well...
If we do our jobs well, the next part is easy. We have them set specific short- and long-term goals related to their situation. This is something we do every day in our practices. It may help to make sure we’re using SMART (Specific, Measurable, Achievable, Relevant, Time-Bound), a well-known mnemonic used for goals (Table 121), and brush up on our motivational interviewing skills (see Related Resources). It is especially important to make sure our colleagues have goals that are relevant—the “R” in the SMART mnemonic—to their situation.

Finally, we do a better job of reaching our goals and engaging more at work and at home when we have good social support. For physicians, co-worker support has been found to be directly related to our well-being as well as buffering the negative effects of work demands.22 Furthermore, our colleagues are the most acceptable sources of support when we are faced with stressful situations.23 Thus, as psychiatrists, we can doubly help our physician patients by providing collegial support and doing our usual job of holding them accountable to their goals (Table 2).

OUTCOME Goal-setting, priorities, accountability
As we’re exploring goals with Dr. D, she makes a conscious decision to spend less time on documentation and start focusing on being present with her patients. She returns in 1 month to tell you time management is still a struggle, but her visit with you was instrumental in making her realize how important it was to get home on time for her kids’ activities. She says it greatly helped that you kept her accountable, yet also validated her struggles and gave her permission to design her life within the constraints of her situation and without the burden of having to be perfect at everything.
Bottom Line
We can best help our physician colleagues who are experiencing burnout by shifting our paradigm to a wellness model that focuses on helping them reach their potential and balance their professional and personal lives.
Related Resources
- Balch CM, Shanafelt TD. Combating stress and burnout in surgical practice: a review. Thorac Surg Clin. 2011;21(3):417-430.
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change, 2nd ed. New York, NY: The Guilford Press; 2002.
- Stanford Medicine WellMD. Stress & Burnout. https://wellmd.stanford.edu.
CASE ‘I’m stuck’
Dr. D, age 48, an internist practicing general medicine who is a married mother of 2 teenagers, presents with emotional exhaustion. Her medical history is unremarkable other than hyperlipidemia controlled by diet. She describes an episode of reactive depressive symptoms and anxiety when in college, which was related to the stress of final exams, finances, and the dissolution of a long-standing relationship. Regardless, she functioned well and graduated summa cum laude. She says her current feelings of being “stuck” have gradually increased during the past 3 to 4 years. Although she describes being mildly anxious and dysphoric, she also said she feels like her “wheels are spinning” and that she doesn’t even seem to care. Dr. D had been a high achiever, yet says she feels like she isn’t getting anywhere at work or at home.
[polldaddy:10064977]
The authors’ observations
As psychiatrists in the business of diagnosis and treatment, we immediately considered common diagnoses, such as major depressive disorder and persistent depressive disorder. However, despite our training in pathology, we believe patients should be considered well until proven sick. This paradigm shift is in line with the definition of mental health per the World Health Organization: “A state of well-being in which every individual realizes his or her own potential, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to her or his community.”1
In Dr. D’s case, there was not enough information yet to fully support any of the diagnoses. She did not exhibit enough depressive symptoms to support a diagnosis of a depressive disorder. She said that she didn’t feel like she was getting anywhere at work or home. Yet there was no objective information that suggested impairment in functioning, which would preclude a diagnosis of adjustment disorder. At this point, we would support the “V code” diagnosis of phase of life problem, or even what is rarely seen in a psychiatric evaluation: “no diagnosis.”
EVALUATION Is work the problem?
We diligently conduct a thorough review of systems with Dr. D and confirm there is not enough information to diagnose a depressive disorder, anxiety disorder, or other psychiatric disorder. Collateral history suggests her teenagers are well-adjusted and doing well in high school, and she is well-respected at work with reportedly excellent performance ratings. We identify strongly with her and her situation.
Dr. D admits she is an idealist and half-jokingly says she entered into medicine “to save the world.” Yet she laments the long hours and finding herself mired in paperwork. She is barely making it to her kids’ school events and says she can’t believe her first child will be graduating within a year. She has had some particularly challenging patients recently, and although she is still diligent about their care, she is shocked she doesn’t seem to care as much about “solving the medical puzzle.” This came to light for her when a longtime patient observed that Dr. D didn’t seem herself and asked, “Are you all right, Doc?”
Dr. D is professionally dressed and has excellent grooming and hygiene. She looks tired, yet has a full affect, a witty conversational style, and responds appropriately to humor.
[polldaddy:10064980]
The authors’ observations
It can be difficult to know what to do next when there isn’t an established “playbook” for a problem without a diagnosis. We realized Dr. D was describing burnout, a syndrome of depersonalization (detached and not caring, to the point of viewing people as objects), emotional exhaustion, and low personal accomplishment that leads to decreased effectiveness at work.2 DSM-5 does not include “burnout” as a diagnosis3; however, if symptoms evolve to the point where they affect occupational or social functioning, burnout can be similar to adjustment disorder. Treatment with an antidepressant medication is not appropriate. It is possible that CBT might be helpful for many patients, yet there is no evidence that Dr. D has any cognitive distortions. Although we already had some collateral information, it is never wrong to gather additional collateral. However, because burnout is common, we may not need additional information. We could reassure her and send her on her way, but we want to add therapeutic value. We advocated exploring issues in her life and work related to meaning.
Continue to: Physician burnout
Physician burnout
Burnout is an alarmingly common problem among physicians that affects approximately half of psychiatrists. In 2014, 54.4% of physicians, and slightly less than 50% of psychiatrists, had at least 1 symptom of burnout.4 This was up from the 45.5% of physicians and a little more than 40% of psychiatrists who reported burnout in 2011,4 which suggests that as medicine continues to change, doctors may increasingly feel the brunt of this change. The rate of burnout is highest in front-line specialties (family medicine, general internal medicine, and emergency medicine) and lowest in preventive medicine.5 Physician burnout leads to real-world occupational issues, such as medical errors, poor relationships with coworkers and patients, decreased patient satisfaction, and medical malpractice suits.6,7
Even though burnout is clearly a concern for our colleagues, don’t expect them to proactively line up outside our offices. In a survey of 7,197 surgeons, 86.6% of respondents answered it was not important that “I have regular meetings with a psychologist/psychiatrist to discuss stress.”8 At the same time, the idea of meeting with a psychologist/psychiatrist was rated more highly by surgeons who were burnt out and found to be a factor independently associated with burnout.8 Perhaps we have some work to do in marketing our services in a way that welcomes our colleagues.
Although physician burnout has been a focus of recent studies, burnout in general has been studied for decades in other working populations. There are 2 useful models describing burnout:
- The job demand-control(-support) model suggests that individuals experience strain and subsequent ill effects when the demands of their job exceed the control they have,9 and social support from supervisors and colleagues can buffer the harmful effects of job strain.10
- The effort-reward imbalance model suggests that high-effort, low-reward occupational conditions are particularly stressful.11
Both models are simple, intuitive, and suggest solutions.
When engaging your physician colleagues about their burnout, remember that physicians are people, too, and have the same difficulties that everyone else does in successfully practicing healthy behaviors. As physicians, we have significant demands on our time that make it difficult to control our ability to eat, sleep, and exercise. In general, the food available where we work is not nutritious,12 half of us are overweight or obese,13 and working more than 40 hours per week increases the likelihood we’ll have a higher body mass index.14 We don’t sleep well, either—we get less sleep than the general population,15 and more sleep equates to less burnout.16 Regarding exercise, doctors who cannot prioritize exercise tend to have more burnout.17
Continue to: When evaluating a physician colleague for symptoms of burnout...
When evaluating a physician colleague for symptoms of burnout, start by assessing the basics: eating, sleeping, and moving. In Dr. D’s case, this also included taking a quick inventory of what demands were on her proverbial plate. Taking inventory of these demands (ie, demand-control[-support] model) may lead to new insights about what she can control. Prioritization is important to determine where efforts go (ie, effort-reward imbalance model). This is where your skills as a psychiatrist can especially help, as you explore values and bring trade-offs to light.
EVALUATION Permission not to be perfect
As we interview Dr. D, we realize she has some obsessive-compulsive personality traits that are mostly self-serving. She places a high value on being thorough and having elegant clinical notes. Yet this value competes with her desire to be efficient and get home on time to see her kids’ school events. You point this out to her and see if she can come up with some solutions. You also discuss with Dr. D the tension everyone feels between valuing career and valuing family and friends. You normalize her situation, and give her permission to pick something about which she will allow herself not to be perfect.
[polldaddy:10064981]
The authors’ observations
Since perfectionism is a common trait among physicians,18 failure doesn’t seem to be compatible with their DNA. We encourage other physicians to be scientific about their own lives, just as they are in the profession they have chosen. Physicians can delude themselves into thinking they can have it all, not recognizing that every choice has its cost. For example, a physician who decides that it’s okay to publish one fewer research paper this year might have more time to enjoy spending time with his or her children. In our work with physicians, we strive to normalize their experiences, helping them reframe their perfectionistic viewpoint to recognize that everyone struggles with work-life balance issues. We validate that physicians have difficult choices to make in finding what works for them, and we challenge and support them in exploring these choices.
Choosing where to put one’s efforts is also contingent upon the expected rewards. Sometime before the daily grind of our careers in medicine started, we had strong visions of what such careers would mean to us. We visualized the ideal of helping people and making a difference. Then, at some point, many of us took this for granted and forgot about the intrinsic rewards of our work. In a 2014-2015 survey of U.S. physicians across all specialties, only 64.6% of respondents who were highly burned out said they found their work personally rewarding. This is a sharp contrast to the 97.5% of respondents who were not burned out who reported that they found their work personally rewarding.19
As psychiatrists, we can challenge our physician colleagues to dare to dream again. We can help them rediscover the rewarding aspects of their work (ie, per effort-reward imbalance model) that drew them into medicine in the first place. This may include exploring their future legacy. How do they want to be remembered at retirement? Such consideration is linked to mental simulation and meaning in their lives.20 We guide our colleagues to reframe their current situation to see the myriad of choices they have based upon their own specific value system. If family and friends are currently taking priority over work, it also helps to reframe that working allows us to make a good living so we can fully enjoy that time spent with family and friends.
Continue to: If we do our jobs well...
If we do our jobs well, the next part is easy. We have them set specific short- and long-term goals related to their situation. This is something we do every day in our practices. It may help to make sure we’re using SMART (Specific, Measurable, Achievable, Relevant, Time-Bound), a well-known mnemonic used for goals (Table 121), and brush up on our motivational interviewing skills (see Related Resources). It is especially important to make sure our colleagues have goals that are relevant—the “R” in the SMART mnemonic—to their situation.

Finally, we do a better job of reaching our goals and engaging more at work and at home when we have good social support. For physicians, co-worker support has been found to be directly related to our well-being as well as buffering the negative effects of work demands.22 Furthermore, our colleagues are the most acceptable sources of support when we are faced with stressful situations.23 Thus, as psychiatrists, we can doubly help our physician patients by providing collegial support and doing our usual job of holding them accountable to their goals (Table 2).

OUTCOME Goal-setting, priorities, accountability
As we’re exploring goals with Dr. D, she makes a conscious decision to spend less time on documentation and start focusing on being present with her patients. She returns in 1 month to tell you time management is still a struggle, but her visit with you was instrumental in making her realize how important it was to get home on time for her kids’ activities. She says it greatly helped that you kept her accountable, yet also validated her struggles and gave her permission to design her life within the constraints of her situation and without the burden of having to be perfect at everything.
Bottom Line
We can best help our physician colleagues who are experiencing burnout by shifting our paradigm to a wellness model that focuses on helping them reach their potential and balance their professional and personal lives.
Related Resources
- Balch CM, Shanafelt TD. Combating stress and burnout in surgical practice: a review. Thorac Surg Clin. 2011;21(3):417-430.
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change, 2nd ed. New York, NY: The Guilford Press; 2002.
- Stanford Medicine WellMD. Stress & Burnout. https://wellmd.stanford.edu.
1. World Health Organization. Mental health: a state of well-being. http://www.who.int/features/factfiles/mental_health/en/. Updated August 2014. Accessed March 17, 2017.
2. Maslach C, Jackson S, Leiter M. Maslach burnout inventory manual, 3rd ed. Palo Alto, CA: Consulting Psychologists Press; 1996.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600-1613.
5. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385.
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519.
7. Balch CM, Shanafelt TD. Combating stress and burnout in surgical practice: a review. Adv Surg. 2010;44:29-47.
8. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US Surgeons. Ann Surg. 2012;255(4):625-633.
9. Karasek RA Jr. Job demands, job decision latitude, and mental strain: implications for job redesign. Adm Sci Q. 1979;24(2):285-308.
10. Johnson JV. Collective control: strategies for survival in the workplace. Int J Health Serv. 1989;19(3):469-480.
11. Siegrist J. Adverse health effects of high-effort/low-reward conditions. J Occup Health Psychol. 1996;1(1):27-41.
12. Lesser LI, Cohen DA, Brook RH. Changing eating habits for the medical profession. JAMA. 2012;308(10):983-984.
13. Bleich SN, Bennett WL, Gudzune KA, et al. Impact of physician BMI on obesity care and beliefs. Obesity (Silver Spring). 2012;20(5):999-1005.
14. Stanford FC, Durkin MW, Blair SN, et al. Determining levels of physical activity in attending physicians, resident and fellow physicians, and medical students in the USA. Br J Sports Med. 2012;46(5):360-364.
15. Tucker P, Bejerot E, Kecklund G, et al. Stress Research Institute at Stockholm University. Stress Research Report-doctors’ work hours in Sweden: their impact on sleep, health, work-family balance, patient care and thoughts about work. https://www.stressforskning.su.se/polopoly_fs/1.233341.1429526778!/menu/standard/file/sfr325.pdf. Accessed March 17, 2017.
16. Wisetborisut A, Angkurawaranon C, Jiraporncharoen W, et al. Shift work and burnout among health care workers. Occup Med (Lond). 2014;64(4):279-286.
17. Eckleberry-Hunt J, Lick D, Boura J, et al. An exploratory study of resident burnout and wellness. Acad Med. 2009;84(2):269-277.
18. Peters M, King J. Perfectionism in doctors. BMJ. 2012;344:e1674. doi: https://doi.org/10.1136/bmj.e1674.
19. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clin Proc. 2017;92(3):415-422.
20. Waytz A, Hershfield HE, Tamir DI. Neural and behavioral evidence for the role of mental simulation in meaning in life. J Pers Soc Psychol. 2015;108(2):336-355.
21. SMART Criteria. https://en.wikipedia.org/wiki/SMART_criteria. Accessed March 17, 2017.
22. Hu YY, Fix ML, Hevelone ND, et al. Physicians’ needs in coping with emotional stressors: the case for peer support. Arch Surg. 2012;147(3):212-217.
23. Wallace JE, Lemaire J. On physician well being–you’ll get by with a little help from your friends. Soc Sci Med. 2007;64(12):2565-2577.
1. World Health Organization. Mental health: a state of well-being. http://www.who.int/features/factfiles/mental_health/en/. Updated August 2014. Accessed March 17, 2017.
2. Maslach C, Jackson S, Leiter M. Maslach burnout inventory manual, 3rd ed. Palo Alto, CA: Consulting Psychologists Press; 1996.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600-1613.
5. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377-1385.
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519.
7. Balch CM, Shanafelt TD. Combating stress and burnout in surgical practice: a review. Adv Surg. 2010;44:29-47.
8. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US Surgeons. Ann Surg. 2012;255(4):625-633.
9. Karasek RA Jr. Job demands, job decision latitude, and mental strain: implications for job redesign. Adm Sci Q. 1979;24(2):285-308.
10. Johnson JV. Collective control: strategies for survival in the workplace. Int J Health Serv. 1989;19(3):469-480.
11. Siegrist J. Adverse health effects of high-effort/low-reward conditions. J Occup Health Psychol. 1996;1(1):27-41.
12. Lesser LI, Cohen DA, Brook RH. Changing eating habits for the medical profession. JAMA. 2012;308(10):983-984.
13. Bleich SN, Bennett WL, Gudzune KA, et al. Impact of physician BMI on obesity care and beliefs. Obesity (Silver Spring). 2012;20(5):999-1005.
14. Stanford FC, Durkin MW, Blair SN, et al. Determining levels of physical activity in attending physicians, resident and fellow physicians, and medical students in the USA. Br J Sports Med. 2012;46(5):360-364.
15. Tucker P, Bejerot E, Kecklund G, et al. Stress Research Institute at Stockholm University. Stress Research Report-doctors’ work hours in Sweden: their impact on sleep, health, work-family balance, patient care and thoughts about work. https://www.stressforskning.su.se/polopoly_fs/1.233341.1429526778!/menu/standard/file/sfr325.pdf. Accessed March 17, 2017.
16. Wisetborisut A, Angkurawaranon C, Jiraporncharoen W, et al. Shift work and burnout among health care workers. Occup Med (Lond). 2014;64(4):279-286.
17. Eckleberry-Hunt J, Lick D, Boura J, et al. An exploratory study of resident burnout and wellness. Acad Med. 2009;84(2):269-277.
18. Peters M, King J. Perfectionism in doctors. BMJ. 2012;344:e1674. doi: https://doi.org/10.1136/bmj.e1674.
19. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clin Proc. 2017;92(3):415-422.
20. Waytz A, Hershfield HE, Tamir DI. Neural and behavioral evidence for the role of mental simulation in meaning in life. J Pers Soc Psychol. 2015;108(2):336-355.
21. SMART Criteria. https://en.wikipedia.org/wiki/SMART_criteria. Accessed March 17, 2017.
22. Hu YY, Fix ML, Hevelone ND, et al. Physicians’ needs in coping with emotional stressors: the case for peer support. Arch Surg. 2012;147(3):212-217.
23. Wallace JE, Lemaire J. On physician well being–you’ll get by with a little help from your friends. Soc Sci Med. 2007;64(12):2565-2577.
Bugs on her skin—but nobody else sees them
CASE Scratching, anxious, and hopeless
Ms. L, age 74, who is paraplegic and uses a wheelchair, presents to our hospital’s emergency department (ED) accompanied by staff from the nursing home where she resides. She reports that she can feel and see bugs crawling all over her skin, biting
Ms. L experiences generalized pruritus with excoriations scattered over her upper and lower extremities and her trunk. She copes with the pruritus by scratching. She reports that the bugs are present throughout the day and are worse at night when she tries to go to bed. Nothing she does provides relief from the infestation. Earlier, at the nursing home, Ms. L had obtained a detergent powder and used it in an attempt to purge the bugs. She now has large swaths of irritated skin, mostly on her lower back and perineal region.
She says the bug infestation became unbearable 3 weeks ago, but she can’t identify any precipitants for her symptoms. Ms. L reports that the impact of the bugs on her daily activity, sleep, and quality of life is enormous. Despite her complaints, neither the nursing home staff nor the ED staff can find any evidence of bugs on Ms. L’s clothes or skin.
Because Ms. L resorted to such drastic measures in her attempt to rid her body of the bugs, she is considered a safety risk and is admitted to the psychiatric unit, although she vehemently denies any intention to harm herself.
On the psychiatric unit, Ms. L states that the infestation began approximately 2 years ago. She began to experience severe worsening of her symptoms a few weeks before presenting to the ED.
During evaluation, Ms. L is alert and oriented to person, place, and situation. She is also quite cooperative but guarded in describing her infestation. There is some degree of suspiciousness and paranoia with regards to her infestation; she is very sensitive to how the clinical staff respond to her condition. She appears worried, and exhibits anxiety, sadness, hopelessness, and tearfulness. Her thought process is goal-directed, but preoccupied by the bugs.
[polldaddy:10064801]
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis is a rare disorder that is defined by an individual having a fixed, false belief that he or she is being infected or grossly invaded by a living organism. Karl A. Ekbom, a Swedish neurologist, was the first practitioner to definitively describe this affliction in 1938.1
Primary delusional parasitosis is a disease defined by this single psychotic symptom without other classic symptoms of schizophrenia; this single symptom cannot be attributed to the effects of substance abuse or a medical condition. Many affected patients remain functional in their daily lives; only a minority of patients experience delusions that interfere with usual activity.2 Secondary delusional parasitosis is a symptom of another psychiatric or medical disease.
Morgellons disease is characterized by symptoms similar to primary delusional parasitosis, but symptoms of this condition also include the delusional belief that inanimate objects, usually fibers, are in the skin as well as the parasites.3
A population-based study among individuals living in Olmsted County, Minnesota from 1976 to 2010 found that the incidence of delusional infestation was 1.9 cases (95% confidence interval, 1.5 to 2.4) per 100,000 person-years.4 In a retrospective study of 147 patients with delusional parasitosis, 33% of these patients described themselves as disabled, 28% were retired, and 26% were employed.5 In this study, the mean age of diagnosis was 57, with a female-to-male ratio of 2.89:1.5
Continue to: HISTORY Prior psychiatric hospitalization
HISTORY Prior psychiatric hospitalization
Ms. L, who is divorced and retired, lives in a nursing home and has no pets, no exposure to scabies, no recent travel, no allergies, and no difficulty with her hygiene except at the peak of her illness. She denies any alcohol or illicit drug use but reports a 6 pack year history of smoking. She has a son, 2 grandchildren, and 2 great grandchildren who all live in town and see her regularly. She reports no history of arrests or legal problems.
Ms. L has a history of depression and anxiety that culminated in a “nervous breakdown” in 1985 with a brief stay in a psychiatric hospital. She reports that she had seen a therapist for 6 years as part of her treatment following that event. During her hospitalization, she was treated with a tricyclic antidepressant and received electroconvulsive therapy. She denies being suicidal during the incident in 1985 or at any point in time before or since then. She now takes venlafaxine, 75 mg/d, for depression and anxiety.
Ms. L’s paraplegia resulted from her sixth corrective surgery for scoliosis, which occurred 6 years ago. She has had chronic pain since this surgery. Her medical history also includes hypertension, atrial fibrillation, mild neurocognitive changes, and gastroesophageal reflux disease.
EVALUATION Skin examination, blood analysis normal
On admission, Ms. L undergoes a skin examination, which yields no evidence consistent with infestation with Pediculus humanus corporis (body louse) or Sarcoptes scabiei (scabies).6 Blood analysis shows no iron deficiency, renal failure, hyperbilirubinemia, or eosinophilia. In the ED, the medical team examines Ms. L and explores other medical and dermatological causes of her condition. Because dermatological causes had been ruled out before Ms. L was admitted to the inpatient psychiatric unit, no dermatology consult is requested.
Continue to: TREATMENT A first-generation antipsychotic
TREATMENT A first-generation antipsychotic
When Ms. L is admitted to the psychiatric unit, she is started
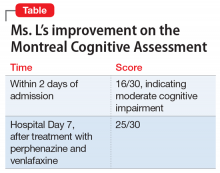
During the week, Ms. L’s perphenazine is titrated up to 24 mg twice daily and venlafaxine is titrated to 150 mg/d. A Montreal Cognitive Assessment (MoCA) is performed within the first 2 days of admission and she scores 16/30, indicating moderate cognitive impairment. On Friday, the attending physician explains that her medications should start to have therapeutic effect. During this time, this clinician engages in cognitive restructuring by providing validation of Ms. L’s suffering, verbal support, and medication compliance counseling. At this time, the treating team also suggests to Ms. L that she should expect the activity and effects of the bugs to dissipate. She is receptive to this suggestion. She also participates in the milieu, including unit activities, but is limited in her ability to engage in group therapy due to the intensity of her illness.
Throughout the weekend, the on-call physician also engages Ms. L and reports minor improvement.
OUTCOME Significant relief
On re-evaluation Monday morning—almost a week after Ms. L had been admitted to the inpatient psychiatric unit—she has achieved significant relief from her delusions. She says that she has no idea where the bugs have gone. Ms. L appears to be a completely different person. She no longer appears guarded. The suspiciousness, paranoia, hopelessness, and negative outlook she previously experienced have significantly diminished. Her MoCA score improves to 25/30, indicating no cognitive impairment (Table). She is discharged after a 7-night stay on the inpatient psychiatric unit.
Continue to: The authors' observations
The authors’ observations
During one of the clinical multidisciplinary treatment team meetings held for Ms. L, it was initially estimated that it would take at least 2 weeks for the delusional parasitosis to significantly respond to antipsychotic therapy. However, it is our professional opinion that the applied cognitive restructuring, with validation of her suffering, verbal support, and medication adherence counseling, expedited her recovery. This coincided with the aggressive titration of her antipsychotic and antidepressant, although the treatment team’s acknowledgment of Ms. L’s misery appeared to lower her guard and make her more susceptible to the power of cognitive restructuring. The efforts to validate the patient’s feelings and decrease hopelessness by telling her that the medication would make the bugs go away appeared to be the tipping point for her recovery. Patients with primary delusional parasitosis often are guarded and may feel alone in their predicament when they are met with perplexed responses from individuals with whom they discuss their symptoms. Compared with patients with schizophrenia, patients with delusional parasitosis maintain normal cognitive functioning, which may give them the insight to understand how their experience may be perceived as incompatible with reality.7 This understanding, coupled with some perceived helplessness, can lead a patient to fear having a severe mental decompensation, which can contribute to a delayed or complicated recovery.
The cognitive process described above might have been responsible for the difference in Ms. L’s MoCA scores because her performance in the initial test was hindered by her constant obsession with the bugs, which made her distracted during the test. By the time she responded to treatment, she gained significant clarity of thought, which enabled her to perform optimally in the test.
The difficulty in treating patients with delusional parasitosis may be further affected by lack of insight, and the fact that they often do not present to a psychiatrist for treatment in a timely manner because their delusion is impregnable and presents them with an alternate reality. These patients are more likely to seek out primary care physicians, dermatologists, infectious disease doctors, and entomologists because of the fervor of their delusion and the intensity of their discomfort. Because of this, a collaboration between these providers would likely lead to improved care and treatment acceptance for patients with delusional parasitosis.
Antipsychotics are the preferred medication for treating delusional parasitosis, and the literature supports their use for this purpose.6,8 The overall response rate is 60% to 100%.6 Previously, in small placebo-controlled trials, the first-generation antipsychotic (FGA) pimozide was considered first-line treatment for this disease.6 However, this antipsychotic is no longer favored because evidence is mounting that other FGAs result in comparable response rates with fewer tolerability issues.8,9
The bulk of data on the use of antipsychotics for treating delusional parasitosis comes from retrospective case reports and case series.6 Multiple antipsychotics have been shown to be effective in treating delusional parasitosis, including both FGAs and second-generation antipsychotics (SGAs).6,10 Published case reports and series have shown the effectiveness of the FGAs
Continue to: The SGAs risperidone, olanzapine, aripiprazole...
The SGAs
When selecting antipsychotic therapy for a patient diagnosed with delusional parasitosis, consider patient-specific factors, such as age, medication history, comorbidities, and the adverse-effect profile of the medication(s). These medications should be started at a low dose and titrated based on efficacy and safety. The optimal duration of therapy varies by patient. Patients should continually be assessed for possible treatment discontinuation, although if therapy is tapered off, patients need to be closely monitored for possible relapse or recurrence of symptoms.
Ms. L received perphenazine titrated up to 24 mg/d for the treatment of delusional parasitosis. The maximum dose used for Ms. L was higher than those used in previous reports, although she appeared to tolerate the medication well and respond rapidly. Her symptoms showed improvement within 1 week. Importantly, in published case reports, patients have been resistant to the use of psychotropic medications without other treatment modalities (eg, psychotherapy, various behavioral approaches). We conclude that Ms. L’s response was attributable to the use of the combination of psychotherapeutic techniques and the effectiveness of perphenazine and venlafaxine.
Bottom Line
Managing patients with primary delusional parasitosis can be challenging due to the fixed nature of the delusion. A combination of antipsychotics and psychotherapeutic techniques can benefit some patients. The optimal duration of treatment varies by patient.
Related Resource
- Trenton A, Pansare N, Tobia A, et al. Delusional parasitosis on the psychiatric consultation service-a longitudinal perspective: case study. BJPsych Open. 2017;3(3):154-158.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Paliperidone • Invega
Paliperidone palmitate • Invega Sustenna
Perphenazine • Trilafon
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Ekbom KA. Der präsenile dermatozoenwahn [in Swedish]. Acta Psychiatr Neurol Scand. 1938;13(3):227-259.
2. Lynch PJ. Delusions of parasitosis. Semin Dermatol. 1993;12(1):39-45.
3. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71-90.
4. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976–2010. Br J Dermatol. 2014;170(5):1130-1135.
5. Foster AA, Hylwa SA, Bury JE, et al. Delusional infestation: clinical presentation in 147 patients seen at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1-e10.
6. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of primary delusional parasitosis: systematic review. Br J Psychiatry. 2007;191(3):198-205.
7. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690-732.
8. Mercan S, Altunay IK, Taskintuna N, et al. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Intl J Psychiatry Med. 2007:37(1):29-37.
9. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246.
10. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis. J Clin Psychopharmacol. 2008;28(5):500-508.
11. Boggild AK, Nicks BA, Yen L, et al. Delusional parasitosis: six-year experience with 23 consecutive cases at an academic medical center. Int J Infect Dis. 2010;14(4):e317-e321.
CASE Scratching, anxious, and hopeless
Ms. L, age 74, who is paraplegic and uses a wheelchair, presents to our hospital’s emergency department (ED) accompanied by staff from the nursing home where she resides. She reports that she can feel and see bugs crawling all over her skin, biting
Ms. L experiences generalized pruritus with excoriations scattered over her upper and lower extremities and her trunk. She copes with the pruritus by scratching. She reports that the bugs are present throughout the day and are worse at night when she tries to go to bed. Nothing she does provides relief from the infestation. Earlier, at the nursing home, Ms. L had obtained a detergent powder and used it in an attempt to purge the bugs. She now has large swaths of irritated skin, mostly on her lower back and perineal region.
She says the bug infestation became unbearable 3 weeks ago, but she can’t identify any precipitants for her symptoms. Ms. L reports that the impact of the bugs on her daily activity, sleep, and quality of life is enormous. Despite her complaints, neither the nursing home staff nor the ED staff can find any evidence of bugs on Ms. L’s clothes or skin.
Because Ms. L resorted to such drastic measures in her attempt to rid her body of the bugs, she is considered a safety risk and is admitted to the psychiatric unit, although she vehemently denies any intention to harm herself.
On the psychiatric unit, Ms. L states that the infestation began approximately 2 years ago. She began to experience severe worsening of her symptoms a few weeks before presenting to the ED.
During evaluation, Ms. L is alert and oriented to person, place, and situation. She is also quite cooperative but guarded in describing her infestation. There is some degree of suspiciousness and paranoia with regards to her infestation; she is very sensitive to how the clinical staff respond to her condition. She appears worried, and exhibits anxiety, sadness, hopelessness, and tearfulness. Her thought process is goal-directed, but preoccupied by the bugs.
[polldaddy:10064801]
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis is a rare disorder that is defined by an individual having a fixed, false belief that he or she is being infected or grossly invaded by a living organism. Karl A. Ekbom, a Swedish neurologist, was the first practitioner to definitively describe this affliction in 1938.1
Primary delusional parasitosis is a disease defined by this single psychotic symptom without other classic symptoms of schizophrenia; this single symptom cannot be attributed to the effects of substance abuse or a medical condition. Many affected patients remain functional in their daily lives; only a minority of patients experience delusions that interfere with usual activity.2 Secondary delusional parasitosis is a symptom of another psychiatric or medical disease.
Morgellons disease is characterized by symptoms similar to primary delusional parasitosis, but symptoms of this condition also include the delusional belief that inanimate objects, usually fibers, are in the skin as well as the parasites.3
A population-based study among individuals living in Olmsted County, Minnesota from 1976 to 2010 found that the incidence of delusional infestation was 1.9 cases (95% confidence interval, 1.5 to 2.4) per 100,000 person-years.4 In a retrospective study of 147 patients with delusional parasitosis, 33% of these patients described themselves as disabled, 28% were retired, and 26% were employed.5 In this study, the mean age of diagnosis was 57, with a female-to-male ratio of 2.89:1.5
Continue to: HISTORY Prior psychiatric hospitalization
HISTORY Prior psychiatric hospitalization
Ms. L, who is divorced and retired, lives in a nursing home and has no pets, no exposure to scabies, no recent travel, no allergies, and no difficulty with her hygiene except at the peak of her illness. She denies any alcohol or illicit drug use but reports a 6 pack year history of smoking. She has a son, 2 grandchildren, and 2 great grandchildren who all live in town and see her regularly. She reports no history of arrests or legal problems.
Ms. L has a history of depression and anxiety that culminated in a “nervous breakdown” in 1985 with a brief stay in a psychiatric hospital. She reports that she had seen a therapist for 6 years as part of her treatment following that event. During her hospitalization, she was treated with a tricyclic antidepressant and received electroconvulsive therapy. She denies being suicidal during the incident in 1985 or at any point in time before or since then. She now takes venlafaxine, 75 mg/d, for depression and anxiety.
Ms. L’s paraplegia resulted from her sixth corrective surgery for scoliosis, which occurred 6 years ago. She has had chronic pain since this surgery. Her medical history also includes hypertension, atrial fibrillation, mild neurocognitive changes, and gastroesophageal reflux disease.
EVALUATION Skin examination, blood analysis normal
On admission, Ms. L undergoes a skin examination, which yields no evidence consistent with infestation with Pediculus humanus corporis (body louse) or Sarcoptes scabiei (scabies).6 Blood analysis shows no iron deficiency, renal failure, hyperbilirubinemia, or eosinophilia. In the ED, the medical team examines Ms. L and explores other medical and dermatological causes of her condition. Because dermatological causes had been ruled out before Ms. L was admitted to the inpatient psychiatric unit, no dermatology consult is requested.
Continue to: TREATMENT A first-generation antipsychotic
TREATMENT A first-generation antipsychotic
When Ms. L is admitted to the psychiatric unit, she is started
During the week, Ms. L’s perphenazine is titrated up to 24 mg twice daily and venlafaxine is titrated to 150 mg/d. A Montreal Cognitive Assessment (MoCA) is performed within the first 2 days of admission and she scores 16/30, indicating moderate cognitive impairment. On Friday, the attending physician explains that her medications should start to have therapeutic effect. During this time, this clinician engages in cognitive restructuring by providing validation of Ms. L’s suffering, verbal support, and medication compliance counseling. At this time, the treating team also suggests to Ms. L that she should expect the activity and effects of the bugs to dissipate. She is receptive to this suggestion. She also participates in the milieu, including unit activities, but is limited in her ability to engage in group therapy due to the intensity of her illness.
Throughout the weekend, the on-call physician also engages Ms. L and reports minor improvement.
OUTCOME Significant relief
On re-evaluation Monday morning—almost a week after Ms. L had been admitted to the inpatient psychiatric unit—she has achieved significant relief from her delusions. She says that she has no idea where the bugs have gone. Ms. L appears to be a completely different person. She no longer appears guarded. The suspiciousness, paranoia, hopelessness, and negative outlook she previously experienced have significantly diminished. Her MoCA score improves to 25/30, indicating no cognitive impairment (Table). She is discharged after a 7-night stay on the inpatient psychiatric unit.
Continue to: The authors' observations
The authors’ observations
During one of the clinical multidisciplinary treatment team meetings held for Ms. L, it was initially estimated that it would take at least 2 weeks for the delusional parasitosis to significantly respond to antipsychotic therapy. However, it is our professional opinion that the applied cognitive restructuring, with validation of her suffering, verbal support, and medication adherence counseling, expedited her recovery. This coincided with the aggressive titration of her antipsychotic and antidepressant, although the treatment team’s acknowledgment of Ms. L’s misery appeared to lower her guard and make her more susceptible to the power of cognitive restructuring. The efforts to validate the patient’s feelings and decrease hopelessness by telling her that the medication would make the bugs go away appeared to be the tipping point for her recovery. Patients with primary delusional parasitosis often are guarded and may feel alone in their predicament when they are met with perplexed responses from individuals with whom they discuss their symptoms. Compared with patients with schizophrenia, patients with delusional parasitosis maintain normal cognitive functioning, which may give them the insight to understand how their experience may be perceived as incompatible with reality.7 This understanding, coupled with some perceived helplessness, can lead a patient to fear having a severe mental decompensation, which can contribute to a delayed or complicated recovery.
The cognitive process described above might have been responsible for the difference in Ms. L’s MoCA scores because her performance in the initial test was hindered by her constant obsession with the bugs, which made her distracted during the test. By the time she responded to treatment, she gained significant clarity of thought, which enabled her to perform optimally in the test.
The difficulty in treating patients with delusional parasitosis may be further affected by lack of insight, and the fact that they often do not present to a psychiatrist for treatment in a timely manner because their delusion is impregnable and presents them with an alternate reality. These patients are more likely to seek out primary care physicians, dermatologists, infectious disease doctors, and entomologists because of the fervor of their delusion and the intensity of their discomfort. Because of this, a collaboration between these providers would likely lead to improved care and treatment acceptance for patients with delusional parasitosis.
Antipsychotics are the preferred medication for treating delusional parasitosis, and the literature supports their use for this purpose.6,8 The overall response rate is 60% to 100%.6 Previously, in small placebo-controlled trials, the first-generation antipsychotic (FGA) pimozide was considered first-line treatment for this disease.6 However, this antipsychotic is no longer favored because evidence is mounting that other FGAs result in comparable response rates with fewer tolerability issues.8,9
The bulk of data on the use of antipsychotics for treating delusional parasitosis comes from retrospective case reports and case series.6 Multiple antipsychotics have been shown to be effective in treating delusional parasitosis, including both FGAs and second-generation antipsychotics (SGAs).6,10 Published case reports and series have shown the effectiveness of the FGAs
Continue to: The SGAs risperidone, olanzapine, aripiprazole...
The SGAs
When selecting antipsychotic therapy for a patient diagnosed with delusional parasitosis, consider patient-specific factors, such as age, medication history, comorbidities, and the adverse-effect profile of the medication(s). These medications should be started at a low dose and titrated based on efficacy and safety. The optimal duration of therapy varies by patient. Patients should continually be assessed for possible treatment discontinuation, although if therapy is tapered off, patients need to be closely monitored for possible relapse or recurrence of symptoms.
Ms. L received perphenazine titrated up to 24 mg/d for the treatment of delusional parasitosis. The maximum dose used for Ms. L was higher than those used in previous reports, although she appeared to tolerate the medication well and respond rapidly. Her symptoms showed improvement within 1 week. Importantly, in published case reports, patients have been resistant to the use of psychotropic medications without other treatment modalities (eg, psychotherapy, various behavioral approaches). We conclude that Ms. L’s response was attributable to the use of the combination of psychotherapeutic techniques and the effectiveness of perphenazine and venlafaxine.
Bottom Line
Managing patients with primary delusional parasitosis can be challenging due to the fixed nature of the delusion. A combination of antipsychotics and psychotherapeutic techniques can benefit some patients. The optimal duration of treatment varies by patient.
Related Resource
- Trenton A, Pansare N, Tobia A, et al. Delusional parasitosis on the psychiatric consultation service-a longitudinal perspective: case study. BJPsych Open. 2017;3(3):154-158.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Paliperidone • Invega
Paliperidone palmitate • Invega Sustenna
Perphenazine • Trilafon
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
Ziprasidone • Geodon
CASE Scratching, anxious, and hopeless
Ms. L, age 74, who is paraplegic and uses a wheelchair, presents to our hospital’s emergency department (ED) accompanied by staff from the nursing home where she resides. She reports that she can feel and see bugs crawling all over her skin, biting
Ms. L experiences generalized pruritus with excoriations scattered over her upper and lower extremities and her trunk. She copes with the pruritus by scratching. She reports that the bugs are present throughout the day and are worse at night when she tries to go to bed. Nothing she does provides relief from the infestation. Earlier, at the nursing home, Ms. L had obtained a detergent powder and used it in an attempt to purge the bugs. She now has large swaths of irritated skin, mostly on her lower back and perineal region.
She says the bug infestation became unbearable 3 weeks ago, but she can’t identify any precipitants for her symptoms. Ms. L reports that the impact of the bugs on her daily activity, sleep, and quality of life is enormous. Despite her complaints, neither the nursing home staff nor the ED staff can find any evidence of bugs on Ms. L’s clothes or skin.
Because Ms. L resorted to such drastic measures in her attempt to rid her body of the bugs, she is considered a safety risk and is admitted to the psychiatric unit, although she vehemently denies any intention to harm herself.
On the psychiatric unit, Ms. L states that the infestation began approximately 2 years ago. She began to experience severe worsening of her symptoms a few weeks before presenting to the ED.
During evaluation, Ms. L is alert and oriented to person, place, and situation. She is also quite cooperative but guarded in describing her infestation. There is some degree of suspiciousness and paranoia with regards to her infestation; she is very sensitive to how the clinical staff respond to her condition. She appears worried, and exhibits anxiety, sadness, hopelessness, and tearfulness. Her thought process is goal-directed, but preoccupied by the bugs.
[polldaddy:10064801]
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis is a rare disorder that is defined by an individual having a fixed, false belief that he or she is being infected or grossly invaded by a living organism. Karl A. Ekbom, a Swedish neurologist, was the first practitioner to definitively describe this affliction in 1938.1
Primary delusional parasitosis is a disease defined by this single psychotic symptom without other classic symptoms of schizophrenia; this single symptom cannot be attributed to the effects of substance abuse or a medical condition. Many affected patients remain functional in their daily lives; only a minority of patients experience delusions that interfere with usual activity.2 Secondary delusional parasitosis is a symptom of another psychiatric or medical disease.
Morgellons disease is characterized by symptoms similar to primary delusional parasitosis, but symptoms of this condition also include the delusional belief that inanimate objects, usually fibers, are in the skin as well as the parasites.3
A population-based study among individuals living in Olmsted County, Minnesota from 1976 to 2010 found that the incidence of delusional infestation was 1.9 cases (95% confidence interval, 1.5 to 2.4) per 100,000 person-years.4 In a retrospective study of 147 patients with delusional parasitosis, 33% of these patients described themselves as disabled, 28% were retired, and 26% were employed.5 In this study, the mean age of diagnosis was 57, with a female-to-male ratio of 2.89:1.5
Continue to: HISTORY Prior psychiatric hospitalization
HISTORY Prior psychiatric hospitalization
Ms. L, who is divorced and retired, lives in a nursing home and has no pets, no exposure to scabies, no recent travel, no allergies, and no difficulty with her hygiene except at the peak of her illness. She denies any alcohol or illicit drug use but reports a 6 pack year history of smoking. She has a son, 2 grandchildren, and 2 great grandchildren who all live in town and see her regularly. She reports no history of arrests or legal problems.
Ms. L has a history of depression and anxiety that culminated in a “nervous breakdown” in 1985 with a brief stay in a psychiatric hospital. She reports that she had seen a therapist for 6 years as part of her treatment following that event. During her hospitalization, she was treated with a tricyclic antidepressant and received electroconvulsive therapy. She denies being suicidal during the incident in 1985 or at any point in time before or since then. She now takes venlafaxine, 75 mg/d, for depression and anxiety.
Ms. L’s paraplegia resulted from her sixth corrective surgery for scoliosis, which occurred 6 years ago. She has had chronic pain since this surgery. Her medical history also includes hypertension, atrial fibrillation, mild neurocognitive changes, and gastroesophageal reflux disease.
EVALUATION Skin examination, blood analysis normal
On admission, Ms. L undergoes a skin examination, which yields no evidence consistent with infestation with Pediculus humanus corporis (body louse) or Sarcoptes scabiei (scabies).6 Blood analysis shows no iron deficiency, renal failure, hyperbilirubinemia, or eosinophilia. In the ED, the medical team examines Ms. L and explores other medical and dermatological causes of her condition. Because dermatological causes had been ruled out before Ms. L was admitted to the inpatient psychiatric unit, no dermatology consult is requested.
Continue to: TREATMENT A first-generation antipsychotic
TREATMENT A first-generation antipsychotic
When Ms. L is admitted to the psychiatric unit, she is started
During the week, Ms. L’s perphenazine is titrated up to 24 mg twice daily and venlafaxine is titrated to 150 mg/d. A Montreal Cognitive Assessment (MoCA) is performed within the first 2 days of admission and she scores 16/30, indicating moderate cognitive impairment. On Friday, the attending physician explains that her medications should start to have therapeutic effect. During this time, this clinician engages in cognitive restructuring by providing validation of Ms. L’s suffering, verbal support, and medication compliance counseling. At this time, the treating team also suggests to Ms. L that she should expect the activity and effects of the bugs to dissipate. She is receptive to this suggestion. She also participates in the milieu, including unit activities, but is limited in her ability to engage in group therapy due to the intensity of her illness.
Throughout the weekend, the on-call physician also engages Ms. L and reports minor improvement.
OUTCOME Significant relief
On re-evaluation Monday morning—almost a week after Ms. L had been admitted to the inpatient psychiatric unit—she has achieved significant relief from her delusions. She says that she has no idea where the bugs have gone. Ms. L appears to be a completely different person. She no longer appears guarded. The suspiciousness, paranoia, hopelessness, and negative outlook she previously experienced have significantly diminished. Her MoCA score improves to 25/30, indicating no cognitive impairment (Table). She is discharged after a 7-night stay on the inpatient psychiatric unit.
Continue to: The authors' observations
The authors’ observations
During one of the clinical multidisciplinary treatment team meetings held for Ms. L, it was initially estimated that it would take at least 2 weeks for the delusional parasitosis to significantly respond to antipsychotic therapy. However, it is our professional opinion that the applied cognitive restructuring, with validation of her suffering, verbal support, and medication adherence counseling, expedited her recovery. This coincided with the aggressive titration of her antipsychotic and antidepressant, although the treatment team’s acknowledgment of Ms. L’s misery appeared to lower her guard and make her more susceptible to the power of cognitive restructuring. The efforts to validate the patient’s feelings and decrease hopelessness by telling her that the medication would make the bugs go away appeared to be the tipping point for her recovery. Patients with primary delusional parasitosis often are guarded and may feel alone in their predicament when they are met with perplexed responses from individuals with whom they discuss their symptoms. Compared with patients with schizophrenia, patients with delusional parasitosis maintain normal cognitive functioning, which may give them the insight to understand how their experience may be perceived as incompatible with reality.7 This understanding, coupled with some perceived helplessness, can lead a patient to fear having a severe mental decompensation, which can contribute to a delayed or complicated recovery.
The cognitive process described above might have been responsible for the difference in Ms. L’s MoCA scores because her performance in the initial test was hindered by her constant obsession with the bugs, which made her distracted during the test. By the time she responded to treatment, she gained significant clarity of thought, which enabled her to perform optimally in the test.
The difficulty in treating patients with delusional parasitosis may be further affected by lack of insight, and the fact that they often do not present to a psychiatrist for treatment in a timely manner because their delusion is impregnable and presents them with an alternate reality. These patients are more likely to seek out primary care physicians, dermatologists, infectious disease doctors, and entomologists because of the fervor of their delusion and the intensity of their discomfort. Because of this, a collaboration between these providers would likely lead to improved care and treatment acceptance for patients with delusional parasitosis.
Antipsychotics are the preferred medication for treating delusional parasitosis, and the literature supports their use for this purpose.6,8 The overall response rate is 60% to 100%.6 Previously, in small placebo-controlled trials, the first-generation antipsychotic (FGA) pimozide was considered first-line treatment for this disease.6 However, this antipsychotic is no longer favored because evidence is mounting that other FGAs result in comparable response rates with fewer tolerability issues.8,9
The bulk of data on the use of antipsychotics for treating delusional parasitosis comes from retrospective case reports and case series.6 Multiple antipsychotics have been shown to be effective in treating delusional parasitosis, including both FGAs and second-generation antipsychotics (SGAs).6,10 Published case reports and series have shown the effectiveness of the FGAs
Continue to: The SGAs risperidone, olanzapine, aripiprazole...
The SGAs
When selecting antipsychotic therapy for a patient diagnosed with delusional parasitosis, consider patient-specific factors, such as age, medication history, comorbidities, and the adverse-effect profile of the medication(s). These medications should be started at a low dose and titrated based on efficacy and safety. The optimal duration of therapy varies by patient. Patients should continually be assessed for possible treatment discontinuation, although if therapy is tapered off, patients need to be closely monitored for possible relapse or recurrence of symptoms.
Ms. L received perphenazine titrated up to 24 mg/d for the treatment of delusional parasitosis. The maximum dose used for Ms. L was higher than those used in previous reports, although she appeared to tolerate the medication well and respond rapidly. Her symptoms showed improvement within 1 week. Importantly, in published case reports, patients have been resistant to the use of psychotropic medications without other treatment modalities (eg, psychotherapy, various behavioral approaches). We conclude that Ms. L’s response was attributable to the use of the combination of psychotherapeutic techniques and the effectiveness of perphenazine and venlafaxine.
Bottom Line
Managing patients with primary delusional parasitosis can be challenging due to the fixed nature of the delusion. A combination of antipsychotics and psychotherapeutic techniques can benefit some patients. The optimal duration of treatment varies by patient.
Related Resource
- Trenton A, Pansare N, Tobia A, et al. Delusional parasitosis on the psychiatric consultation service-a longitudinal perspective: case study. BJPsych Open. 2017;3(3):154-158.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Paliperidone • Invega
Paliperidone palmitate • Invega Sustenna
Perphenazine • Trilafon
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Ekbom KA. Der präsenile dermatozoenwahn [in Swedish]. Acta Psychiatr Neurol Scand. 1938;13(3):227-259.
2. Lynch PJ. Delusions of parasitosis. Semin Dermatol. 1993;12(1):39-45.
3. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71-90.
4. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976–2010. Br J Dermatol. 2014;170(5):1130-1135.
5. Foster AA, Hylwa SA, Bury JE, et al. Delusional infestation: clinical presentation in 147 patients seen at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1-e10.
6. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of primary delusional parasitosis: systematic review. Br J Psychiatry. 2007;191(3):198-205.
7. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690-732.
8. Mercan S, Altunay IK, Taskintuna N, et al. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Intl J Psychiatry Med. 2007:37(1):29-37.
9. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246.
10. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis. J Clin Psychopharmacol. 2008;28(5):500-508.
11. Boggild AK, Nicks BA, Yen L, et al. Delusional parasitosis: six-year experience with 23 consecutive cases at an academic medical center. Int J Infect Dis. 2010;14(4):e317-e321.
1. Ekbom KA. Der präsenile dermatozoenwahn [in Swedish]. Acta Psychiatr Neurol Scand. 1938;13(3):227-259.
2. Lynch PJ. Delusions of parasitosis. Semin Dermatol. 1993;12(1):39-45.
3. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71-90.
4. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976–2010. Br J Dermatol. 2014;170(5):1130-1135.
5. Foster AA, Hylwa SA, Bury JE, et al. Delusional infestation: clinical presentation in 147 patients seen at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1-e10.
6. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of primary delusional parasitosis: systematic review. Br J Psychiatry. 2007;191(3):198-205.
7. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690-732.
8. Mercan S, Altunay IK, Taskintuna N, et al. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Intl J Psychiatry Med. 2007:37(1):29-37.
9. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246.
10. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis. J Clin Psychopharmacol. 2008;28(5):500-508.
11. Boggild AK, Nicks BA, Yen L, et al. Delusional parasitosis: six-year experience with 23 consecutive cases at an academic medical center. Int J Infect Dis. 2010;14(4):e317-e321.
Is this adolescent suicidal? Challenges in pediatric inpatient consultation-liaison
CASE Attempted suicide?
Ms. S, a 16-year-old Yemeni-American girl, is brought to the emergency department (ED) by her mother and brother after ingesting an overdose of painkillers and fainting. During the initial evaluation, Ms. S says she had in the past attempted suicide by knife. The medical team suspects that the current overdose is a suicide attempt, and they call the consultation-liaison (C-L) psychiatry/psychology team. Ms. S’s brother strongly denies that his sister had previously attempted suicide, stating, “She’s from a good family, and she is smart. She cannot feel that way.” He also requests the name of the clinician who documented this information in the medical record.
During the consultation, Ms. S reports that the previous morning, she developed strong abdominal pain and discovered that she was menstruating for the first time. She explains that she did not understand what was happening to her and that no one had discussed menstruation with her before. Ms. S took her mother’s opioid pain medication. Ms. S reports she took one pill, but when it did not immediately alleviate her pain, she ingested several more. After this, Ms. S says she went to play with her siblings, but gradually became dizzy and confused, and informed her sister and mother of this. The family was fasting in observance of Ramadan, and as they walked toward the mosque, Ms. S fainted, which prompted her family to bring her to the ED.
During the C-L consultation, Ms. S’s brother, who speaks English, is present, as is her mother, who speaks only Arabic and thus needs a phone interpreter. As the C-L team asks Ms. S a question, it is translated to her mother, and then Ms. S’s response is also translated, and then finally, the mother shares her own response. At times, her brother provides translation. Ms. S speaks in English, but often asks for the translation of words or questions.
Ms. S reports that she and her family emigrated from Yemen to the United States 9 months ago. Ms. S says that she enjoys school and is doing well academically. She denies experiencing any anxiety, worry, or stress related to her life in Yemen, her move to a new country, her parents’ health, school, or other domains. Ms. S also denies any history of depressive episodes or previous suicidal ideation, intention, or attempt, which contradicts her endorsement of a previous suicide attempt to one clinician when she was initially evaluated.
[polldaddy:10040204]
Continue to: The authors' observation
The authors’ observation
The C-L team determined that Ms. S did not meet criteria for major depressive disorder. She did not endorse current feelings of depression and denied anhedonia and other associated symptoms included in DSM-5 criteria for major depressive disorder or adjustment disorder with depressed mood (Table 11).
Although Ms. S and her family recently emigrated from Yemen, she did not report any symptoms consistent with an adjustment disorder with depression. Further,
Accurate case conceptualization and diagnosis is particularly crucial in C-L services, where there is an urgency for clinical decision-making after an initial evaluation without the luxury of amending conceptualization in follow-up sessions. Providing a diagnosis for which a patient does not fully or accurately meet the criteria can have deleterious effects. An inaccurate diagnosis for Ms. S would have unnecessarily added the perceived stigma of a mental disorder to her medical record. Additionally, misdiagnosing or pathologizing a natural process of acculturation could have led to inappropriate or even harmful treatment.
The C-L team evaluated alternative explanations for Ms. S’s statements that suggested she was suicidal. First, they considered her mental status at the time she presented to the ED. An overdose of opioids alters mental status. Complicating reversal of opioid overdose is that some opioids have longer half-lives than naloxone, an opioid antagonist, so the individual can become reintoxicated. Similarly, some opioids are more potent and difficult to reverse.2 An altered mental status may have limited Ms. S’s ability to comprehend and answer questions accurately when she first presented to the ED.
Continue to: Cultural factors and the clinical evaluation
Cultural factors and the clinical evaluation
Next, the C-L team considered Ms. S’s clinical picture as it related to her cultural background. Cultural factors interact with the clinical evaluation in a complex manner, influencing the way patients approach the encounter, the symptoms they report, and the language they use to describe their experiences. While these variables are thoroughly evaluated during comprehensive psychological assessments, within the inpatient consultation service, the goal for pediatric C-L clinicians is to conduct a focused assessment to answer specific and critically important questions about a youth’s psychological functioning. Thus, the fundamental challenge of inpatient consultation is to answer the referral question in a brief period and in a culturally informed manner, to appraise the referring medical team about the relevant clinical and cultural issues, with the goal of ethical and clinically sound decision-making.
The C-L team considered key cultural factors in its assessment of Ms. S (Table 31). Several issues were of concern. First, language is often cited as the top barrier to health care access by Arab Americans, even by those with competency in English.3
Experts in culture argue that even with access to interpreters, many words and phrases lack direct translation, and their implicit meaning may be difficult to reveal. Additionally, at times more significance is placed on nonverbal cues and unspoken expectations.4 This can create barriers to communication with clinicians, especially in the context of an inpatient psychiatric consultation, when thorough understanding of an adolescent and family often needs to occur in a single encounter, and clinicians may not appreciate the subtle nuances of nonverbal communication.
The language barrier also may have influenced Ms. S’s initial endorsement of a previous suicide attempt by knife because the medical staff first interviewed Ms. S without an interpreter. For instance, many medical and psychosocial providers probe patients regarding suicidality with questions such as “Have you ever hurt yourself?” or “Have you ever tried to hurt yourself?” It is possible that in another language, an individual might interpret that question as, “Have you ever gotten hurt?” This interpretation completely alters the meaning of the question and eliminates intention or motivation to harm oneself. Language ambiguity and lack of shared cultural understanding may have influenced Ms. S’s interpretation of and response to such questions. Ms. S and her family were perplexed by the C-L team’s reference to the knife and continued to deny the incident.
Continue to: Cultural attitudes to puberty
Cultural attitudes to puberty
Cultures vary with respect to education of sensitive topics such as puberty. The medical providers assumed that Ms. S was informed about the onset of menses. Therefore, they could not consider the strong impact of such an event on an unsuspecting adolescent. Many adolescent girls in Yemen have poor health and lack menstruation-related knowledge, and many are “prescribed” medications by their mothers without contacting a physician.5 Ms. S reported to the C-L team that no one from her family had discussed menstruation with her. She reported that since arriving at the hospital, nurses had educated her about menstruation, and that she was no longer afraid. She also noted that if she experienced such pain again, she would go to the hospital or “just deal with it.”
Family identification and attitudes toward mental health
Ms. S’s strong identification with her family and attitudes toward mental health may have limited what she chose to disclose regarding her experiences of loss related to leaving her country of origin, adjustment, and acculturation to the new environment, as well as feelings of sadness. Family has a central and critical role in Arab cultures. Commitment to a family’s well-being and enhancement of honor and status is highly valued and encouraged.4 Conversely, being concerned with individual needs may be a source of guilt and feelings of betraying the family.6 Arab Americans tend not to discuss personal problems with people outside their extended family, including counselors and therapists, partly because of cultural stigma against mental illness7,8 and partly because revealing family problems to strangers (ie, clinicians) may be considered a cultural taboo9 and a threat to family honor.10 Although Ms. S was interviewed privately when she first came to the ED and also during the psychiatric consultation, the stigma of psychiatric problems11 and possible concerns about protecting her family’s name may have influenced her readiness to reveal intimate information to “strangers.”
Additionally, family statements that appeared to imply negative beliefs about mental health would have strongly deterred Ms. S from expressing any psychological concerns. For example, Ms. S’s brother took offense when the C-L team said it was evaluating his sister because she had said she had previously attempted suicide.
The tenets of Islam may have provided a framework through which Ms. S interprets emotional concerns and may have defined her explanatory models of psychological stress. For instance, it is not uncommon among American Muslims to view mental health problems as rising from “loss of faith in God,”9 and suicidal ideation may not be disclosed because suicide is forbidden in Islam.12 Therefore, it might be particularly difficult to assess suicidal ideation in a patient who is Muslim, especially those who are less acculturated to Western culture.13
Continue to: Directly asking Ms. S...
Directly asking Ms. S if she had thoughts of harming herself may have been too frightening or guilt-provoking for an adolescent with her background. Asking about passive expression of suicidal ideation would have been more culturally appropriate. For example, asking, “Do you wish that God would let you die?”12 may have elicited more meaningful clinical information about Ms. S’s emotional state and possibly suicide risk.
Furthermore, Ms. S’s identification of coping strategies (ie, “just deal with it”) may have sounded limited to a Western clinician, but this may have been consistent with cultural norms of emotional expression of limiting complaints.4 Also, among Arab Americans, psychiatric symptoms often are expressed through somatization.7,14 Expressing psychological pain through physical symptoms appears protective against public stigma. Public image and opinion is important, and behaviors that would reflect well to others are dictated by the family. These attitudes, beliefs, and values likely impact how Ms. S presented her psychological concerns.
[polldaddy:10040206]
The authors’ observations
Although inpatient hospitalization was initially considered, it was not pursued due to denial of past and current suicidal ideation or suicide attempts, the lack of comorbidity, age-appropriate functioning, and a supportive family environment. Similarly, due to the absence of acute psychiatric symptoms, partial hospitalization was not pursued. The C-L team evaluated treatment options with extreme caution and sensitivity because recommending the wrong treatment option could have deleterious effects on Ms. S and her family’s life. If inpatient hospitalization had been pursued, it could have likely caused the family unnecessary suffering and could have negatively affected familial relationships. Strong feelings of shame, betrayal, and guilt would be intensified, impairing the family’s cohesion, removing environmental and family supports, and putting Ms. S at further risk of developing more severe symptoms of low mood.
Although there were significant concerns about making the wrong recommendation to the family, the C-L team’s highest priority was Ms. S’s safety. Despite cultural concerns, the team would have recommended hospitalization if Ms. S’s clinical picture had warranted this decision.
Continue to: OUTCOME Culturally-appropriate outpatient therapy
OUTCOME Culturally-appropriate outpatient therapy
Due to the lack of substantial evidence of apparent risk for self-harm, the presence of a supportive family, and Ms. S’s high academic performance and future orientation, the C-L team concludes that Ms. S’s concerns were most likely the result of the challenges of acculturation related to the language barrier and a lack of health knowledge. However, the C-L team remains cautious that Ms. S may have minimized or denied her mental health concerns due to various cultural factors. The team recommends that Ms. S seek outpatient psychotherapy from a clinician who specializes in working with Arab American individuals and families in their native language. The C-L team communicates these conclusions to the medical team verbally and in writing.
The authors’ observations
Cultural issues experienced during this consultation may not generalize to other Arab American adolescents and their families because there is diversity even within groups that share common cultural characteristics. Nevertheless, this case underscores the challenge of accurately assessing suicide risk, and making a differential diagnosis in the presence of complex cultural data and the dilemmas clinicians may encounter when attempting to answer important referral questions such as, “Is this adolescent suicidal and in need of psychiatric hospitalization?”
Bottom Line
Cultural factors and attitudes toward mental health and language barriers may play a large role in how patients answer clinical questions. Cultural issues may add a level of intricacy not easily resolved within the restrictions of an inpatient setting, and this complexity may influence clinical judgment, recommendations, and possibly health outcomes. Culturally appropriate psychotherapy is key for patients experiencing difficulty with acculturation.
Related Resources
- Adam B. Caring for Muslim patients: Understanding cultural and religious factors. Current Psychiatry. 2017;16(12):56-57.
- Nassar-McMillan SC, Hakim-Larson J. Counseling considerations among Arab Americans. Journal of Counseling & Development. 2003;81(2):150-159.
- Sue DW. Multidimensional facets of cultural competence. The Counseling Psychologist. 2001;29(6):790-821.
Drug Brand Name
Naloxone • Narcan
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Meehan TJ, Bryant SM, Aks SE. Drugs of abuse: the highs and lows of altered mental states in the emergency department. Emerg Med Clin North Am. 2010;28(3):663-682.
3. Shah SM, Ayash C, Pharaon NA, et al. Arab American immigrants in New York: health care and cancer knowledge, attitudes, and beliefs. J Immigr Minor Health. 2008;10(5):429-436.
4. Budman CL, Lipson JG, Meleis AI. The cultural consultant in mental health care: the case of an Arab adolescent. Am J Orthopsychiatry. 1992; 62(3):359-370.
5. Mohamed EM, Mohamed AG, Al-Ajeal Ly. Knowledge, beliefs and practices regarding menstruation among adolescent schoolgirls in Seiyun City, Yemen. Al-Azhar Assiut Medical Journal. 2011;9(3):67-86.
6. Gorkin M, Masalha S, Yatziv G. Psychotherapy of Israeli-Arab patients: some cultural considerations. Journal of Psychoanalytic Anthropology. 1985;8(4);215-230.
7. Gearing RE, MacKenzie MJ, Ibrahim RW, et al. Stigma and mental health treatment of adolescents with depression in Jordan. Community Ment Health J. 2015;51(1):111-117.
8. Timimi SB. Adolescence in immigrant Arab families. Psychotherapy: theory, research, practice, training. 1995;32(1):141-149.
9. Ahmed S and Reddy LA. Understanding the mental health needs of American Muslims: recommendations and considerations for practice. Journal of Multicultural Counseling and Development. 2007;35(4):207-218.
10. Abudabbeh N, Nydell MK. Transcultural counseling and Arab Americans. In: McFadden J, ed. Transcultural counseling: bilateral and international perspectives. Alexandria, VA. American Counseling Association. 1993:261-284.
11. Erickson CD, al-Timimi NR. Providing mental health services to Arab Americans: recommendations and considerations. Cultur Divers Ethnic Minor Psychol. 2001;7(4):308-327.
12. Ali SR, Liu WM, Humedian M. Islam 101: understanding the religion and therapy implications. Prof Psychol Res Pr. 2004;35(6):635-642.
13. Hedayat-Diba Z. Psychotherapy with Muslims. In: Richards PS, Bergin AE, eds. Handbook of psychotherapy and religious diversity, 2nd ed. Washington, DC: Amercian Psychological Association. 2000:289-314.
14. Al-Krenawi A. Mental health practice in Arab countries. Curr Opin in Psychiatry. 2005;18(5):560-564.
CASE Attempted suicide?
Ms. S, a 16-year-old Yemeni-American girl, is brought to the emergency department (ED) by her mother and brother after ingesting an overdose of painkillers and fainting. During the initial evaluation, Ms. S says she had in the past attempted suicide by knife. The medical team suspects that the current overdose is a suicide attempt, and they call the consultation-liaison (C-L) psychiatry/psychology team. Ms. S’s brother strongly denies that his sister had previously attempted suicide, stating, “She’s from a good family, and she is smart. She cannot feel that way.” He also requests the name of the clinician who documented this information in the medical record.
During the consultation, Ms. S reports that the previous morning, she developed strong abdominal pain and discovered that she was menstruating for the first time. She explains that she did not understand what was happening to her and that no one had discussed menstruation with her before. Ms. S took her mother’s opioid pain medication. Ms. S reports she took one pill, but when it did not immediately alleviate her pain, she ingested several more. After this, Ms. S says she went to play with her siblings, but gradually became dizzy and confused, and informed her sister and mother of this. The family was fasting in observance of Ramadan, and as they walked toward the mosque, Ms. S fainted, which prompted her family to bring her to the ED.
During the C-L consultation, Ms. S’s brother, who speaks English, is present, as is her mother, who speaks only Arabic and thus needs a phone interpreter. As the C-L team asks Ms. S a question, it is translated to her mother, and then Ms. S’s response is also translated, and then finally, the mother shares her own response. At times, her brother provides translation. Ms. S speaks in English, but often asks for the translation of words or questions.
Ms. S reports that she and her family emigrated from Yemen to the United States 9 months ago. Ms. S says that she enjoys school and is doing well academically. She denies experiencing any anxiety, worry, or stress related to her life in Yemen, her move to a new country, her parents’ health, school, or other domains. Ms. S also denies any history of depressive episodes or previous suicidal ideation, intention, or attempt, which contradicts her endorsement of a previous suicide attempt to one clinician when she was initially evaluated.
[polldaddy:10040204]
Continue to: The authors' observation
The authors’ observation
The C-L team determined that Ms. S did not meet criteria for major depressive disorder. She did not endorse current feelings of depression and denied anhedonia and other associated symptoms included in DSM-5 criteria for major depressive disorder or adjustment disorder with depressed mood (Table 11).
Although Ms. S and her family recently emigrated from Yemen, she did not report any symptoms consistent with an adjustment disorder with depression. Further,
Accurate case conceptualization and diagnosis is particularly crucial in C-L services, where there is an urgency for clinical decision-making after an initial evaluation without the luxury of amending conceptualization in follow-up sessions. Providing a diagnosis for which a patient does not fully or accurately meet the criteria can have deleterious effects. An inaccurate diagnosis for Ms. S would have unnecessarily added the perceived stigma of a mental disorder to her medical record. Additionally, misdiagnosing or pathologizing a natural process of acculturation could have led to inappropriate or even harmful treatment.
The C-L team evaluated alternative explanations for Ms. S’s statements that suggested she was suicidal. First, they considered her mental status at the time she presented to the ED. An overdose of opioids alters mental status. Complicating reversal of opioid overdose is that some opioids have longer half-lives than naloxone, an opioid antagonist, so the individual can become reintoxicated. Similarly, some opioids are more potent and difficult to reverse.2 An altered mental status may have limited Ms. S’s ability to comprehend and answer questions accurately when she first presented to the ED.
Continue to: Cultural factors and the clinical evaluation
Cultural factors and the clinical evaluation
Next, the C-L team considered Ms. S’s clinical picture as it related to her cultural background. Cultural factors interact with the clinical evaluation in a complex manner, influencing the way patients approach the encounter, the symptoms they report, and the language they use to describe their experiences. While these variables are thoroughly evaluated during comprehensive psychological assessments, within the inpatient consultation service, the goal for pediatric C-L clinicians is to conduct a focused assessment to answer specific and critically important questions about a youth’s psychological functioning. Thus, the fundamental challenge of inpatient consultation is to answer the referral question in a brief period and in a culturally informed manner, to appraise the referring medical team about the relevant clinical and cultural issues, with the goal of ethical and clinically sound decision-making.
The C-L team considered key cultural factors in its assessment of Ms. S (Table 31). Several issues were of concern. First, language is often cited as the top barrier to health care access by Arab Americans, even by those with competency in English.3
Experts in culture argue that even with access to interpreters, many words and phrases lack direct translation, and their implicit meaning may be difficult to reveal. Additionally, at times more significance is placed on nonverbal cues and unspoken expectations.4 This can create barriers to communication with clinicians, especially in the context of an inpatient psychiatric consultation, when thorough understanding of an adolescent and family often needs to occur in a single encounter, and clinicians may not appreciate the subtle nuances of nonverbal communication.
The language barrier also may have influenced Ms. S’s initial endorsement of a previous suicide attempt by knife because the medical staff first interviewed Ms. S without an interpreter. For instance, many medical and psychosocial providers probe patients regarding suicidality with questions such as “Have you ever hurt yourself?” or “Have you ever tried to hurt yourself?” It is possible that in another language, an individual might interpret that question as, “Have you ever gotten hurt?” This interpretation completely alters the meaning of the question and eliminates intention or motivation to harm oneself. Language ambiguity and lack of shared cultural understanding may have influenced Ms. S’s interpretation of and response to such questions. Ms. S and her family were perplexed by the C-L team’s reference to the knife and continued to deny the incident.
Continue to: Cultural attitudes to puberty
Cultural attitudes to puberty
Cultures vary with respect to education of sensitive topics such as puberty. The medical providers assumed that Ms. S was informed about the onset of menses. Therefore, they could not consider the strong impact of such an event on an unsuspecting adolescent. Many adolescent girls in Yemen have poor health and lack menstruation-related knowledge, and many are “prescribed” medications by their mothers without contacting a physician.5 Ms. S reported to the C-L team that no one from her family had discussed menstruation with her. She reported that since arriving at the hospital, nurses had educated her about menstruation, and that she was no longer afraid. She also noted that if she experienced such pain again, she would go to the hospital or “just deal with it.”
Family identification and attitudes toward mental health
Ms. S’s strong identification with her family and attitudes toward mental health may have limited what she chose to disclose regarding her experiences of loss related to leaving her country of origin, adjustment, and acculturation to the new environment, as well as feelings of sadness. Family has a central and critical role in Arab cultures. Commitment to a family’s well-being and enhancement of honor and status is highly valued and encouraged.4 Conversely, being concerned with individual needs may be a source of guilt and feelings of betraying the family.6 Arab Americans tend not to discuss personal problems with people outside their extended family, including counselors and therapists, partly because of cultural stigma against mental illness7,8 and partly because revealing family problems to strangers (ie, clinicians) may be considered a cultural taboo9 and a threat to family honor.10 Although Ms. S was interviewed privately when she first came to the ED and also during the psychiatric consultation, the stigma of psychiatric problems11 and possible concerns about protecting her family’s name may have influenced her readiness to reveal intimate information to “strangers.”
Additionally, family statements that appeared to imply negative beliefs about mental health would have strongly deterred Ms. S from expressing any psychological concerns. For example, Ms. S’s brother took offense when the C-L team said it was evaluating his sister because she had said she had previously attempted suicide.
The tenets of Islam may have provided a framework through which Ms. S interprets emotional concerns and may have defined her explanatory models of psychological stress. For instance, it is not uncommon among American Muslims to view mental health problems as rising from “loss of faith in God,”9 and suicidal ideation may not be disclosed because suicide is forbidden in Islam.12 Therefore, it might be particularly difficult to assess suicidal ideation in a patient who is Muslim, especially those who are less acculturated to Western culture.13
Continue to: Directly asking Ms. S...
Directly asking Ms. S if she had thoughts of harming herself may have been too frightening or guilt-provoking for an adolescent with her background. Asking about passive expression of suicidal ideation would have been more culturally appropriate. For example, asking, “Do you wish that God would let you die?”12 may have elicited more meaningful clinical information about Ms. S’s emotional state and possibly suicide risk.
Furthermore, Ms. S’s identification of coping strategies (ie, “just deal with it”) may have sounded limited to a Western clinician, but this may have been consistent with cultural norms of emotional expression of limiting complaints.4 Also, among Arab Americans, psychiatric symptoms often are expressed through somatization.7,14 Expressing psychological pain through physical symptoms appears protective against public stigma. Public image and opinion is important, and behaviors that would reflect well to others are dictated by the family. These attitudes, beliefs, and values likely impact how Ms. S presented her psychological concerns.
[polldaddy:10040206]
The authors’ observations
Although inpatient hospitalization was initially considered, it was not pursued due to denial of past and current suicidal ideation or suicide attempts, the lack of comorbidity, age-appropriate functioning, and a supportive family environment. Similarly, due to the absence of acute psychiatric symptoms, partial hospitalization was not pursued. The C-L team evaluated treatment options with extreme caution and sensitivity because recommending the wrong treatment option could have deleterious effects on Ms. S and her family’s life. If inpatient hospitalization had been pursued, it could have likely caused the family unnecessary suffering and could have negatively affected familial relationships. Strong feelings of shame, betrayal, and guilt would be intensified, impairing the family’s cohesion, removing environmental and family supports, and putting Ms. S at further risk of developing more severe symptoms of low mood.
Although there were significant concerns about making the wrong recommendation to the family, the C-L team’s highest priority was Ms. S’s safety. Despite cultural concerns, the team would have recommended hospitalization if Ms. S’s clinical picture had warranted this decision.
Continue to: OUTCOME Culturally-appropriate outpatient therapy
OUTCOME Culturally-appropriate outpatient therapy
Due to the lack of substantial evidence of apparent risk for self-harm, the presence of a supportive family, and Ms. S’s high academic performance and future orientation, the C-L team concludes that Ms. S’s concerns were most likely the result of the challenges of acculturation related to the language barrier and a lack of health knowledge. However, the C-L team remains cautious that Ms. S may have minimized or denied her mental health concerns due to various cultural factors. The team recommends that Ms. S seek outpatient psychotherapy from a clinician who specializes in working with Arab American individuals and families in their native language. The C-L team communicates these conclusions to the medical team verbally and in writing.
The authors’ observations
Cultural issues experienced during this consultation may not generalize to other Arab American adolescents and their families because there is diversity even within groups that share common cultural characteristics. Nevertheless, this case underscores the challenge of accurately assessing suicide risk, and making a differential diagnosis in the presence of complex cultural data and the dilemmas clinicians may encounter when attempting to answer important referral questions such as, “Is this adolescent suicidal and in need of psychiatric hospitalization?”
Bottom Line
Cultural factors and attitudes toward mental health and language barriers may play a large role in how patients answer clinical questions. Cultural issues may add a level of intricacy not easily resolved within the restrictions of an inpatient setting, and this complexity may influence clinical judgment, recommendations, and possibly health outcomes. Culturally appropriate psychotherapy is key for patients experiencing difficulty with acculturation.
Related Resources
- Adam B. Caring for Muslim patients: Understanding cultural and religious factors. Current Psychiatry. 2017;16(12):56-57.
- Nassar-McMillan SC, Hakim-Larson J. Counseling considerations among Arab Americans. Journal of Counseling & Development. 2003;81(2):150-159.
- Sue DW. Multidimensional facets of cultural competence. The Counseling Psychologist. 2001;29(6):790-821.
Drug Brand Name
Naloxone • Narcan
CASE Attempted suicide?
Ms. S, a 16-year-old Yemeni-American girl, is brought to the emergency department (ED) by her mother and brother after ingesting an overdose of painkillers and fainting. During the initial evaluation, Ms. S says she had in the past attempted suicide by knife. The medical team suspects that the current overdose is a suicide attempt, and they call the consultation-liaison (C-L) psychiatry/psychology team. Ms. S’s brother strongly denies that his sister had previously attempted suicide, stating, “She’s from a good family, and she is smart. She cannot feel that way.” He also requests the name of the clinician who documented this information in the medical record.
During the consultation, Ms. S reports that the previous morning, she developed strong abdominal pain and discovered that she was menstruating for the first time. She explains that she did not understand what was happening to her and that no one had discussed menstruation with her before. Ms. S took her mother’s opioid pain medication. Ms. S reports she took one pill, but when it did not immediately alleviate her pain, she ingested several more. After this, Ms. S says she went to play with her siblings, but gradually became dizzy and confused, and informed her sister and mother of this. The family was fasting in observance of Ramadan, and as they walked toward the mosque, Ms. S fainted, which prompted her family to bring her to the ED.
During the C-L consultation, Ms. S’s brother, who speaks English, is present, as is her mother, who speaks only Arabic and thus needs a phone interpreter. As the C-L team asks Ms. S a question, it is translated to her mother, and then Ms. S’s response is also translated, and then finally, the mother shares her own response. At times, her brother provides translation. Ms. S speaks in English, but often asks for the translation of words or questions.
Ms. S reports that she and her family emigrated from Yemen to the United States 9 months ago. Ms. S says that she enjoys school and is doing well academically. She denies experiencing any anxiety, worry, or stress related to her life in Yemen, her move to a new country, her parents’ health, school, or other domains. Ms. S also denies any history of depressive episodes or previous suicidal ideation, intention, or attempt, which contradicts her endorsement of a previous suicide attempt to one clinician when she was initially evaluated.
[polldaddy:10040204]
Continue to: The authors' observation
The authors’ observation
The C-L team determined that Ms. S did not meet criteria for major depressive disorder. She did not endorse current feelings of depression and denied anhedonia and other associated symptoms included in DSM-5 criteria for major depressive disorder or adjustment disorder with depressed mood (Table 11).
Although Ms. S and her family recently emigrated from Yemen, she did not report any symptoms consistent with an adjustment disorder with depression. Further,
Accurate case conceptualization and diagnosis is particularly crucial in C-L services, where there is an urgency for clinical decision-making after an initial evaluation without the luxury of amending conceptualization in follow-up sessions. Providing a diagnosis for which a patient does not fully or accurately meet the criteria can have deleterious effects. An inaccurate diagnosis for Ms. S would have unnecessarily added the perceived stigma of a mental disorder to her medical record. Additionally, misdiagnosing or pathologizing a natural process of acculturation could have led to inappropriate or even harmful treatment.
The C-L team evaluated alternative explanations for Ms. S’s statements that suggested she was suicidal. First, they considered her mental status at the time she presented to the ED. An overdose of opioids alters mental status. Complicating reversal of opioid overdose is that some opioids have longer half-lives than naloxone, an opioid antagonist, so the individual can become reintoxicated. Similarly, some opioids are more potent and difficult to reverse.2 An altered mental status may have limited Ms. S’s ability to comprehend and answer questions accurately when she first presented to the ED.
Continue to: Cultural factors and the clinical evaluation
Cultural factors and the clinical evaluation
Next, the C-L team considered Ms. S’s clinical picture as it related to her cultural background. Cultural factors interact with the clinical evaluation in a complex manner, influencing the way patients approach the encounter, the symptoms they report, and the language they use to describe their experiences. While these variables are thoroughly evaluated during comprehensive psychological assessments, within the inpatient consultation service, the goal for pediatric C-L clinicians is to conduct a focused assessment to answer specific and critically important questions about a youth’s psychological functioning. Thus, the fundamental challenge of inpatient consultation is to answer the referral question in a brief period and in a culturally informed manner, to appraise the referring medical team about the relevant clinical and cultural issues, with the goal of ethical and clinically sound decision-making.
The C-L team considered key cultural factors in its assessment of Ms. S (Table 31). Several issues were of concern. First, language is often cited as the top barrier to health care access by Arab Americans, even by those with competency in English.3
Experts in culture argue that even with access to interpreters, many words and phrases lack direct translation, and their implicit meaning may be difficult to reveal. Additionally, at times more significance is placed on nonverbal cues and unspoken expectations.4 This can create barriers to communication with clinicians, especially in the context of an inpatient psychiatric consultation, when thorough understanding of an adolescent and family often needs to occur in a single encounter, and clinicians may not appreciate the subtle nuances of nonverbal communication.
The language barrier also may have influenced Ms. S’s initial endorsement of a previous suicide attempt by knife because the medical staff first interviewed Ms. S without an interpreter. For instance, many medical and psychosocial providers probe patients regarding suicidality with questions such as “Have you ever hurt yourself?” or “Have you ever tried to hurt yourself?” It is possible that in another language, an individual might interpret that question as, “Have you ever gotten hurt?” This interpretation completely alters the meaning of the question and eliminates intention or motivation to harm oneself. Language ambiguity and lack of shared cultural understanding may have influenced Ms. S’s interpretation of and response to such questions. Ms. S and her family were perplexed by the C-L team’s reference to the knife and continued to deny the incident.
Continue to: Cultural attitudes to puberty
Cultural attitudes to puberty
Cultures vary with respect to education of sensitive topics such as puberty. The medical providers assumed that Ms. S was informed about the onset of menses. Therefore, they could not consider the strong impact of such an event on an unsuspecting adolescent. Many adolescent girls in Yemen have poor health and lack menstruation-related knowledge, and many are “prescribed” medications by their mothers without contacting a physician.5 Ms. S reported to the C-L team that no one from her family had discussed menstruation with her. She reported that since arriving at the hospital, nurses had educated her about menstruation, and that she was no longer afraid. She also noted that if she experienced such pain again, she would go to the hospital or “just deal with it.”
Family identification and attitudes toward mental health
Ms. S’s strong identification with her family and attitudes toward mental health may have limited what she chose to disclose regarding her experiences of loss related to leaving her country of origin, adjustment, and acculturation to the new environment, as well as feelings of sadness. Family has a central and critical role in Arab cultures. Commitment to a family’s well-being and enhancement of honor and status is highly valued and encouraged.4 Conversely, being concerned with individual needs may be a source of guilt and feelings of betraying the family.6 Arab Americans tend not to discuss personal problems with people outside their extended family, including counselors and therapists, partly because of cultural stigma against mental illness7,8 and partly because revealing family problems to strangers (ie, clinicians) may be considered a cultural taboo9 and a threat to family honor.10 Although Ms. S was interviewed privately when she first came to the ED and also during the psychiatric consultation, the stigma of psychiatric problems11 and possible concerns about protecting her family’s name may have influenced her readiness to reveal intimate information to “strangers.”
Additionally, family statements that appeared to imply negative beliefs about mental health would have strongly deterred Ms. S from expressing any psychological concerns. For example, Ms. S’s brother took offense when the C-L team said it was evaluating his sister because she had said she had previously attempted suicide.
The tenets of Islam may have provided a framework through which Ms. S interprets emotional concerns and may have defined her explanatory models of psychological stress. For instance, it is not uncommon among American Muslims to view mental health problems as rising from “loss of faith in God,”9 and suicidal ideation may not be disclosed because suicide is forbidden in Islam.12 Therefore, it might be particularly difficult to assess suicidal ideation in a patient who is Muslim, especially those who are less acculturated to Western culture.13
Continue to: Directly asking Ms. S...
Directly asking Ms. S if she had thoughts of harming herself may have been too frightening or guilt-provoking for an adolescent with her background. Asking about passive expression of suicidal ideation would have been more culturally appropriate. For example, asking, “Do you wish that God would let you die?”12 may have elicited more meaningful clinical information about Ms. S’s emotional state and possibly suicide risk.
Furthermore, Ms. S’s identification of coping strategies (ie, “just deal with it”) may have sounded limited to a Western clinician, but this may have been consistent with cultural norms of emotional expression of limiting complaints.4 Also, among Arab Americans, psychiatric symptoms often are expressed through somatization.7,14 Expressing psychological pain through physical symptoms appears protective against public stigma. Public image and opinion is important, and behaviors that would reflect well to others are dictated by the family. These attitudes, beliefs, and values likely impact how Ms. S presented her psychological concerns.
[polldaddy:10040206]
The authors’ observations
Although inpatient hospitalization was initially considered, it was not pursued due to denial of past and current suicidal ideation or suicide attempts, the lack of comorbidity, age-appropriate functioning, and a supportive family environment. Similarly, due to the absence of acute psychiatric symptoms, partial hospitalization was not pursued. The C-L team evaluated treatment options with extreme caution and sensitivity because recommending the wrong treatment option could have deleterious effects on Ms. S and her family’s life. If inpatient hospitalization had been pursued, it could have likely caused the family unnecessary suffering and could have negatively affected familial relationships. Strong feelings of shame, betrayal, and guilt would be intensified, impairing the family’s cohesion, removing environmental and family supports, and putting Ms. S at further risk of developing more severe symptoms of low mood.
Although there were significant concerns about making the wrong recommendation to the family, the C-L team’s highest priority was Ms. S’s safety. Despite cultural concerns, the team would have recommended hospitalization if Ms. S’s clinical picture had warranted this decision.
Continue to: OUTCOME Culturally-appropriate outpatient therapy
OUTCOME Culturally-appropriate outpatient therapy
Due to the lack of substantial evidence of apparent risk for self-harm, the presence of a supportive family, and Ms. S’s high academic performance and future orientation, the C-L team concludes that Ms. S’s concerns were most likely the result of the challenges of acculturation related to the language barrier and a lack of health knowledge. However, the C-L team remains cautious that Ms. S may have minimized or denied her mental health concerns due to various cultural factors. The team recommends that Ms. S seek outpatient psychotherapy from a clinician who specializes in working with Arab American individuals and families in their native language. The C-L team communicates these conclusions to the medical team verbally and in writing.
The authors’ observations
Cultural issues experienced during this consultation may not generalize to other Arab American adolescents and their families because there is diversity even within groups that share common cultural characteristics. Nevertheless, this case underscores the challenge of accurately assessing suicide risk, and making a differential diagnosis in the presence of complex cultural data and the dilemmas clinicians may encounter when attempting to answer important referral questions such as, “Is this adolescent suicidal and in need of psychiatric hospitalization?”
Bottom Line
Cultural factors and attitudes toward mental health and language barriers may play a large role in how patients answer clinical questions. Cultural issues may add a level of intricacy not easily resolved within the restrictions of an inpatient setting, and this complexity may influence clinical judgment, recommendations, and possibly health outcomes. Culturally appropriate psychotherapy is key for patients experiencing difficulty with acculturation.
Related Resources
- Adam B. Caring for Muslim patients: Understanding cultural and religious factors. Current Psychiatry. 2017;16(12):56-57.
- Nassar-McMillan SC, Hakim-Larson J. Counseling considerations among Arab Americans. Journal of Counseling & Development. 2003;81(2):150-159.
- Sue DW. Multidimensional facets of cultural competence. The Counseling Psychologist. 2001;29(6):790-821.
Drug Brand Name
Naloxone • Narcan
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Meehan TJ, Bryant SM, Aks SE. Drugs of abuse: the highs and lows of altered mental states in the emergency department. Emerg Med Clin North Am. 2010;28(3):663-682.
3. Shah SM, Ayash C, Pharaon NA, et al. Arab American immigrants in New York: health care and cancer knowledge, attitudes, and beliefs. J Immigr Minor Health. 2008;10(5):429-436.
4. Budman CL, Lipson JG, Meleis AI. The cultural consultant in mental health care: the case of an Arab adolescent. Am J Orthopsychiatry. 1992; 62(3):359-370.
5. Mohamed EM, Mohamed AG, Al-Ajeal Ly. Knowledge, beliefs and practices regarding menstruation among adolescent schoolgirls in Seiyun City, Yemen. Al-Azhar Assiut Medical Journal. 2011;9(3):67-86.
6. Gorkin M, Masalha S, Yatziv G. Psychotherapy of Israeli-Arab patients: some cultural considerations. Journal of Psychoanalytic Anthropology. 1985;8(4);215-230.
7. Gearing RE, MacKenzie MJ, Ibrahim RW, et al. Stigma and mental health treatment of adolescents with depression in Jordan. Community Ment Health J. 2015;51(1):111-117.
8. Timimi SB. Adolescence in immigrant Arab families. Psychotherapy: theory, research, practice, training. 1995;32(1):141-149.
9. Ahmed S and Reddy LA. Understanding the mental health needs of American Muslims: recommendations and considerations for practice. Journal of Multicultural Counseling and Development. 2007;35(4):207-218.
10. Abudabbeh N, Nydell MK. Transcultural counseling and Arab Americans. In: McFadden J, ed. Transcultural counseling: bilateral and international perspectives. Alexandria, VA. American Counseling Association. 1993:261-284.
11. Erickson CD, al-Timimi NR. Providing mental health services to Arab Americans: recommendations and considerations. Cultur Divers Ethnic Minor Psychol. 2001;7(4):308-327.
12. Ali SR, Liu WM, Humedian M. Islam 101: understanding the religion and therapy implications. Prof Psychol Res Pr. 2004;35(6):635-642.
13. Hedayat-Diba Z. Psychotherapy with Muslims. In: Richards PS, Bergin AE, eds. Handbook of psychotherapy and religious diversity, 2nd ed. Washington, DC: Amercian Psychological Association. 2000:289-314.
14. Al-Krenawi A. Mental health practice in Arab countries. Curr Opin in Psychiatry. 2005;18(5):560-564.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Meehan TJ, Bryant SM, Aks SE. Drugs of abuse: the highs and lows of altered mental states in the emergency department. Emerg Med Clin North Am. 2010;28(3):663-682.
3. Shah SM, Ayash C, Pharaon NA, et al. Arab American immigrants in New York: health care and cancer knowledge, attitudes, and beliefs. J Immigr Minor Health. 2008;10(5):429-436.
4. Budman CL, Lipson JG, Meleis AI. The cultural consultant in mental health care: the case of an Arab adolescent. Am J Orthopsychiatry. 1992; 62(3):359-370.
5. Mohamed EM, Mohamed AG, Al-Ajeal Ly. Knowledge, beliefs and practices regarding menstruation among adolescent schoolgirls in Seiyun City, Yemen. Al-Azhar Assiut Medical Journal. 2011;9(3):67-86.
6. Gorkin M, Masalha S, Yatziv G. Psychotherapy of Israeli-Arab patients: some cultural considerations. Journal of Psychoanalytic Anthropology. 1985;8(4);215-230.
7. Gearing RE, MacKenzie MJ, Ibrahim RW, et al. Stigma and mental health treatment of adolescents with depression in Jordan. Community Ment Health J. 2015;51(1):111-117.
8. Timimi SB. Adolescence in immigrant Arab families. Psychotherapy: theory, research, practice, training. 1995;32(1):141-149.
9. Ahmed S and Reddy LA. Understanding the mental health needs of American Muslims: recommendations and considerations for practice. Journal of Multicultural Counseling and Development. 2007;35(4):207-218.
10. Abudabbeh N, Nydell MK. Transcultural counseling and Arab Americans. In: McFadden J, ed. Transcultural counseling: bilateral and international perspectives. Alexandria, VA. American Counseling Association. 1993:261-284.
11. Erickson CD, al-Timimi NR. Providing mental health services to Arab Americans: recommendations and considerations. Cultur Divers Ethnic Minor Psychol. 2001;7(4):308-327.
12. Ali SR, Liu WM, Humedian M. Islam 101: understanding the religion and therapy implications. Prof Psychol Res Pr. 2004;35(6):635-642.
13. Hedayat-Diba Z. Psychotherapy with Muslims. In: Richards PS, Bergin AE, eds. Handbook of psychotherapy and religious diversity, 2nd ed. Washington, DC: Amercian Psychological Association. 2000:289-314.
14. Al-Krenawi A. Mental health practice in Arab countries. Curr Opin in Psychiatry. 2005;18(5):560-564.
Sudden-onset memory problems, visual hallucinations, and odd behaviors
CASE A rapid decline
Ms. D, age 62, presents to a psychiatric emergency room (ER) after experiencing visual hallucinations, exhibiting odd behaviors, and having memory problems. On interview, she is disoriented, distractible, tearful, and tangential. She plays with her shirt and glasses, and occasionally shouts. She perseverates on “the aerialists,” acrobatic children she has been seeing in her apartment. She becomes distressed and shouts, “I would love to just get them!”
Ms. D is unable to provide an account of her history. Collateral information is obtained from her daughter, who has brought Ms. D to the ER for evaluation. She reports that her mother has no relevant medical or psychiatric history, and does not take any medications, except a mixture of Chinese herbs that she brews into a tea.
Ms. D’s daughter says that her mother began to deteriorate 5 months ago, after she traveled to California to care for her sister, who was seriously ill and passed away. After Ms. D returned, she would cry frequently. She also appeared “spaced out,” complained of feeling dizzy, and frequently misplaced belongings. Three months before presenting to the ER, she began to experience weakness, fatigue, and difficulty walking. Her daughter became more worried 2 months ago, when Ms. D began sleeping with her purse and hiding her belongings around their house. When asked about these odd behaviors, Ms. D claimed that “the aerialists” were climbing through her windows at night and stealing her things.
A week before seeking treatment at the ER, Ms. D’s daughter had taken her to a neurologist at another facility for clinical evaluation. An MRI of the brain showed minimal dilation in the subarachnoid space and a focal 1 cm lipoma in the anterior falx cerebri, but was otherwise unremarkable. However, Ms. D’s symptoms continued to worsen, and began to interfere with her ability to care for herself.
The team in the psychiatric ER attributes Ms. D’s symptoms to a severe, psychotic depressive episode. They admit her to the psychiatric inpatient unit for further evaluation.
[polldaddy:10012742]
Continue to: The authors' observations
The authors’ observations
Ms. D was plagued by several mood and psychotic symptoms. Such symptoms can arise from many different psychiatric or organic etiologies. In Ms. D’s case, several aspects of her presentation suggest that her illness was psychiatric. The severe illness of a beloved family member is a significant stressor that could cause a great deal of grief and devastation, possibly leading to depression. Indeed, Ms. D’s daughter noticed that her mother was crying frequently, which is consistent with grief or depression.
Memory problems, which might manifest as misplacing belongings, can also indicate a depressive illness, especially in older patients. Moreover, impaired concentration, which can cause one to appear “spaced out” or distractible, is a core symptom of major depressive disorder. Sadness and grief also can be appropriate during bereavement and in response to significant losses. Therefore, in Ms. D’s case, it is possible her frequent crying, “spaced out” appearance, and other mood symptoms she experienced immediately after caring for her sister were an appropriate response to her sister’s illness and death.
However, other aspects of Ms. D’s presentation suggested an organic etiology. Her rapid deterioration and symptom onset relatively late in life were consistent with dementia and malignancy. Her complaint of feeling dizzy suggested a neurologic process was affecting her vestibular system. Finally, while psychiatric disorders can certainly cause visual hallucinations, they occur in only a small percentage of cases.1 Visual hallucinations are commonly associated with delirium, intoxication, and neurologic illness.
Continue to: EVALUATION Severe impairment
EVALUATION Severe impairment
On the psychiatric inpatient unit, Ms. D remains unable to give a coherent account of her illness or recent events. During interviews, she abruptly shifts from laughing to crying for no apparent reason. While answering questions, her responses trail off and she appears to forget what she had been saying. However, she continues to speak at length about “the aerialists,” stating that she sees them living in her wardrobe and jumping from rooftop to rooftop in her neighborhood.
A mental status examination finds evidence of severe cognitive impairment. Ms. D is unable to identify the correct date, time, or place, and appears surprised when told she is in a hospital. She can repeat the names of 3 objects but cannot recall them a few minutes later. Finally, she scores a 14 on the Mini-Mental State Examination (MMSE) and a 5 on the Montreal Cognitive Assessment (MoCA), indicating severe impairment.
On the unit, Ms. D cannot remember the location of her room or bathroom, and even when given directions, she needs to be escorted to her destination. Her gait is unsteady and wide-spaced, and she walks on her toes at times. When food is placed before her, she needs to be shown how to take the lids off containers, pick up utensils, and start eating.
All laboratory results are unremarkable, including a complete blood count, basic metabolic panel, liver function tests, gamma-glutamyl transpeptidase, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, methylmalonic acid, homocysteine, folate, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rapid plasma reagin, human immunodeficiency virus, and Lyme titers. The team also considers Ms. D’s history of herbal medicine use, because herbal mixtures can contain heavy metals and other contaminants. However, all toxicology results are normal, including arsenic, mercury, lead, copper, and zinc.
To address her symptoms, Ms. D is given risperidone, 0.5 mg twice a day, and donepezil, 5 mg/d.
[polldaddy:10012743]
Continue to: The authors' observations
The authors’ observations
Despite her persistent psychiatric symptoms, Ms. D had several neurologic symptoms that warranted further investigation. Her abrupt shifts from laughter to tears for no apparent reason were consistent with pseudobulbar affect. Her inability to remember how to use utensils during meals was consistent with apraxia. Finally, her abnormal gait raised concern for a process affecting her motor system.
OUTCOME A rare disorder
Given the psychiatry team’s suspicions for a neurologic etiology of Ms. D’s symptoms, an MRI of her brain is repeated. The results are notable for abnormal restricted diffusion in the caudate and putamen bilaterally, which is consistent with Creutzfeldt-Jakob disease (CJD). EEG shows moderate diffuse cerebral dysfunction, frontal intermittent delta activity, and diffuse cortical hyperexcitability, consistent with early- to mid-onset prion disease. Upon evaluation by the neurology team, Ms. D appears fearful, suspicious, and disorganized, but her examination does not reveal additional significant sensorimotor findings.
Ms. D is transferred to the neurology service for further workup and management. A lumbar puncture is positive for real-time quaking-induced conversion (RT-QuIC) and 14-3-3 protein with elevated tau proteins; these findings also are consistent with CJD. She develops transaminitis, with an alanine transaminase (ALT) of 127 and aspartate transaminase (AST) of 355, and a malignancy is suspected. However, CT scans of the chest, abdomen, and pelvis show no evidence of malignancy, and an extensive gastrointestinal workup is unremarkable, including anti-smooth muscle antibodies, anti-liver-kidney microsomal antibody, antimitochondrial antibodies, gliadin antibody, alpha-1 antitrypsin, liver/kidney microsomal antibody, and hepatitis serologies. While on the neurology service, risperidone and donepezil are discontinued because
After discontinuing these medications, she is evaluated by the psychiatry consult team for mood lability. The psychiatry consult team recommends quetiapine, which is later started at 25 mg nightly at bedtime.
Clinically, Ms. D’s mental status continues to deteriorate. She becomes nonverbal and minimally able to follow commands. She is ultimately discharged to an inpatient hospice for end-of-life care and the team recommends that she continue with quetiapine once there.
Continue to: The authors' observations
The authors’ observations
CJD is a rare, rapidly progressive, fatal form of dementia. In the United States, the incidence is approximately 1 to 1.5 cases per 1 million people each year.2 There are various forms of the disease. Sporadic CJD is the most common, representing 85% of cases.3 Sporadic CJD typically occurs in patients in their 60s and quickly leads to death—50% of patients die within 5 months, and 90% of patients die within 1 year.2,3 The illness is hypothesized to arise from the production of misfolded prion proteins, ultimately leading to vacuolation, neuronal loss, and the spongiform appearance characteristic of CJD.3,4
Psychiatric symptoms have long been acknowledged as a feature of CJD. Recent data indicates that psychiatric symptoms occur in 90% to 92% of cases.5,6 Sleep disturbances and depressive symptoms, including vegetative symptoms, anhedonia, and tearfulness, appear to be most common.5 Psychotic symptoms occur in approximately 42% of cases and may include persecutory and paranoid delusions, as well as an array of vivid auditory, visual, and tactile hallucinations.5,7
There is also evidence that psychiatric symptoms may be an early marker of CJD.5,8 A Mayo Clinic study found that psychiatric symptoms occurred within the prodromal phase of CJD in 26% of cases, and psychiatric symptoms occurred within the first 100 days of illness in 86% of cases.5
Case reports have described patients with CJD who initially presented with depression, psychosis, and other psychiatric symptoms.9-11 Interestingly, there have been cases with only psychiatric symptoms, and no neurologic symptoms until relatively late in the illness.10,11 Several patients with CJD have been evaluated in psychiatric ERs, admitted to psychiatric hospitals, and treated with psychiatric medications and ECT.5,9 In one study, 44% of CJD cases were misdiagnosed as “psychiatric patients” due to the prominence of their psychiatric symptomatology.8
Continue to: Making the diagnosis in psychiatric settings
Making the diagnosis in psychiatric settings. Often, the most difficult aspect of CJD is making the diagnosis.3,12 Sporadic CJD in particular can vary widely in its clinical presentation.3 The core clinical feature of CJD is rapidly progressive dementia, so suspect CJD in these patients. However, CJD can be difficult to distinguish from other rapidly progressive dementias, such as autoimmune and paraneoplastic encephalopathies.2,3 The presence of neurologic features, specifically myoclonus, akinetic mutism, and visual, cerebellar, and extrapyramidal symptoms, should also be considered a red flag for the disorder3 (Table).
Finally, positive findings on MRI, EEG, or CSF assay can indicate a probable diagnosis of CJD.13 MRI, particularly diffusion weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), is recognized as the most studied, sensitive, and overall useful neuroimaging modality for detecting CJD.2,3,12 Although the appearance of CJD on MRI can vary widely, asymmetric hyperintensities in ≥3 cortical gyri, particularly in the frontal and parietal lobes, provide strong evidence of CJD and are observed in 80% to 81% of cases.4,12 Asymmetric hyperintensities in the basal ganglia, particularly the caudate and rostral putamen, are observed in 69% to 70% of cases.4,12,13
EEG and CSF assay also can be useful for making the diagnosis. While diffuse slowing and frontal rhythmic delta activity appear early in the course of CJD, periodic sharp wave complexes emerge later in the illness.4 However, EEG findings are not diagnostic, because periodic sharp wave complexes are seen in only two-thirds of CJD cases and also occur in other neurologic illnesses.3,4 In recent years, lumbar puncture with subsequent CSF testing has become increasingly useful in detecting the illness. The presence of the 14-3-3 protein and tau protein is highly sensitive, although not specific, for CJD.3 A definite diagnosis of CJD requires discovery of the misfolded prion proteins, such as by RT-QuIC or brain biopsy.2,3,13
Management of CJD in psychiatric patients. CJD is an invariably fatal disease for which there is no effective cure or disease modifying treatment.2 Therefore, supportive therapies are the mainstay of care. Psychotropic medications can be used to provide symptom relief. While the sleep disturbances, anxiety, and agitation/hallucinations associated with CJD appear to respond well to hypnotic, anxiolytic, and antipsychotic medications, respectively, antidepressants and mood-stabilizing medications appear to have little benefit for patients with CJD.5 During the final stages of the disease, patients may suffer from akinetic mutism and inability to swallow, which often leads to aspiration pneumonia.14 Patients should also be offered end-of-life counseling, planning, and care, and provided with other comfort measures wherever possible (Figure).
Continue to: Bottom Line
Bottom Line
Patients with Creutzfeldt-Jakob disease (CJD) may present to psychiatric settings, particularly to a psychiatric emergency room. Consider CJD as a possible etiology in patients with rapidly progressive dementia, depression, and psychosis. CJD is invariably fatal and there is no effective disease-modifying treatment. Supportive therapies are the mainstay of care.
Related Resources
- National Institute of Neurological Disorders and Stroke. Creutzfeldt-Jakob disease fact sheet. http://www.ninds.nih.gov/disorders/cjd/detail_cjd.htm.
- Centers for Disease Control and Prevention. Creutzfeldt-Jakob disease, classic (CJD). http://www.cdc.gov/prions/cjd.
Drug Brand Names
Donepezil • Aricept
Risperidone • Risperdal
Quetiapine • Seroquel
1. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am. 1999;22(1):159-172.
2. Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mo Med. 2013;110(5):422-428.
3. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
4. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
5. Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17(4):489-495.
6. Krasnianski A, Bohling GT, Harden M, et al. Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. J Clin Psychiatry. 2015;76(9):1209-1215.
7. Javed Q, Alam F, Krishna S, et al. An unusual case of sporadic Creutzfeldt-Jakob disease (CJD). BMJ Case Rep. 2010;pii: bcr1220092576. doi:10.1136/bcr.12.2009.2576.
8. Abudy A, Juven-Wetzler A, Zohar J. The different faces of Creutzfeldt-Jacob disease CJD in psychiatry. Gen Hosp Psychiatry. 2014;36(3):245-248.
9. Jiang TT, Moses H, Gordon H, et al. Sporadic Creutzfeldt-Jakob disease presenting as major depression. South Med J. 1999;92(8):807-808.
10. Ali R, Baborie A, Larner AJ et al. Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci. 2013;25(4):335-338.
11. Gençer AG, Pelin Z, Küçükali CI., et al. Creutzfeldt-Jakob disease. Psychogeriatrics. 2011;11(2):119-124.
12. Caobelli F, Cobelli M, Pizzocaro C, et al. The role of neuroimaging in evaluating patients affected by Creutzfeldt-Jakob disease: a systematic review of the literature. J Neuroimaging. 2015;25(1):2-13.
13. Centers for Disease Control and Prevention. CDC's diagnostic criteria for Creutzfeldt-Jakob disease, 2010. http://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Updated February 11, 2015. Accessed August 2, 2016.
14. Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5(1):43-46.
CASE A rapid decline
Ms. D, age 62, presents to a psychiatric emergency room (ER) after experiencing visual hallucinations, exhibiting odd behaviors, and having memory problems. On interview, she is disoriented, distractible, tearful, and tangential. She plays with her shirt and glasses, and occasionally shouts. She perseverates on “the aerialists,” acrobatic children she has been seeing in her apartment. She becomes distressed and shouts, “I would love to just get them!”
Ms. D is unable to provide an account of her history. Collateral information is obtained from her daughter, who has brought Ms. D to the ER for evaluation. She reports that her mother has no relevant medical or psychiatric history, and does not take any medications, except a mixture of Chinese herbs that she brews into a tea.
Ms. D’s daughter says that her mother began to deteriorate 5 months ago, after she traveled to California to care for her sister, who was seriously ill and passed away. After Ms. D returned, she would cry frequently. She also appeared “spaced out,” complained of feeling dizzy, and frequently misplaced belongings. Three months before presenting to the ER, she began to experience weakness, fatigue, and difficulty walking. Her daughter became more worried 2 months ago, when Ms. D began sleeping with her purse and hiding her belongings around their house. When asked about these odd behaviors, Ms. D claimed that “the aerialists” were climbing through her windows at night and stealing her things.
A week before seeking treatment at the ER, Ms. D’s daughter had taken her to a neurologist at another facility for clinical evaluation. An MRI of the brain showed minimal dilation in the subarachnoid space and a focal 1 cm lipoma in the anterior falx cerebri, but was otherwise unremarkable. However, Ms. D’s symptoms continued to worsen, and began to interfere with her ability to care for herself.
The team in the psychiatric ER attributes Ms. D’s symptoms to a severe, psychotic depressive episode. They admit her to the psychiatric inpatient unit for further evaluation.
[polldaddy:10012742]
Continue to: The authors' observations
The authors’ observations
Ms. D was plagued by several mood and psychotic symptoms. Such symptoms can arise from many different psychiatric or organic etiologies. In Ms. D’s case, several aspects of her presentation suggest that her illness was psychiatric. The severe illness of a beloved family member is a significant stressor that could cause a great deal of grief and devastation, possibly leading to depression. Indeed, Ms. D’s daughter noticed that her mother was crying frequently, which is consistent with grief or depression.
Memory problems, which might manifest as misplacing belongings, can also indicate a depressive illness, especially in older patients. Moreover, impaired concentration, which can cause one to appear “spaced out” or distractible, is a core symptom of major depressive disorder. Sadness and grief also can be appropriate during bereavement and in response to significant losses. Therefore, in Ms. D’s case, it is possible her frequent crying, “spaced out” appearance, and other mood symptoms she experienced immediately after caring for her sister were an appropriate response to her sister’s illness and death.
However, other aspects of Ms. D’s presentation suggested an organic etiology. Her rapid deterioration and symptom onset relatively late in life were consistent with dementia and malignancy. Her complaint of feeling dizzy suggested a neurologic process was affecting her vestibular system. Finally, while psychiatric disorders can certainly cause visual hallucinations, they occur in only a small percentage of cases.1 Visual hallucinations are commonly associated with delirium, intoxication, and neurologic illness.
Continue to: EVALUATION Severe impairment
EVALUATION Severe impairment
On the psychiatric inpatient unit, Ms. D remains unable to give a coherent account of her illness or recent events. During interviews, she abruptly shifts from laughing to crying for no apparent reason. While answering questions, her responses trail off and she appears to forget what she had been saying. However, she continues to speak at length about “the aerialists,” stating that she sees them living in her wardrobe and jumping from rooftop to rooftop in her neighborhood.
A mental status examination finds evidence of severe cognitive impairment. Ms. D is unable to identify the correct date, time, or place, and appears surprised when told she is in a hospital. She can repeat the names of 3 objects but cannot recall them a few minutes later. Finally, she scores a 14 on the Mini-Mental State Examination (MMSE) and a 5 on the Montreal Cognitive Assessment (MoCA), indicating severe impairment.
On the unit, Ms. D cannot remember the location of her room or bathroom, and even when given directions, she needs to be escorted to her destination. Her gait is unsteady and wide-spaced, and she walks on her toes at times. When food is placed before her, she needs to be shown how to take the lids off containers, pick up utensils, and start eating.
All laboratory results are unremarkable, including a complete blood count, basic metabolic panel, liver function tests, gamma-glutamyl transpeptidase, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, methylmalonic acid, homocysteine, folate, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rapid plasma reagin, human immunodeficiency virus, and Lyme titers. The team also considers Ms. D’s history of herbal medicine use, because herbal mixtures can contain heavy metals and other contaminants. However, all toxicology results are normal, including arsenic, mercury, lead, copper, and zinc.
To address her symptoms, Ms. D is given risperidone, 0.5 mg twice a day, and donepezil, 5 mg/d.
[polldaddy:10012743]
Continue to: The authors' observations
The authors’ observations
Despite her persistent psychiatric symptoms, Ms. D had several neurologic symptoms that warranted further investigation. Her abrupt shifts from laughter to tears for no apparent reason were consistent with pseudobulbar affect. Her inability to remember how to use utensils during meals was consistent with apraxia. Finally, her abnormal gait raised concern for a process affecting her motor system.
OUTCOME A rare disorder
Given the psychiatry team’s suspicions for a neurologic etiology of Ms. D’s symptoms, an MRI of her brain is repeated. The results are notable for abnormal restricted diffusion in the caudate and putamen bilaterally, which is consistent with Creutzfeldt-Jakob disease (CJD). EEG shows moderate diffuse cerebral dysfunction, frontal intermittent delta activity, and diffuse cortical hyperexcitability, consistent with early- to mid-onset prion disease. Upon evaluation by the neurology team, Ms. D appears fearful, suspicious, and disorganized, but her examination does not reveal additional significant sensorimotor findings.
Ms. D is transferred to the neurology service for further workup and management. A lumbar puncture is positive for real-time quaking-induced conversion (RT-QuIC) and 14-3-3 protein with elevated tau proteins; these findings also are consistent with CJD. She develops transaminitis, with an alanine transaminase (ALT) of 127 and aspartate transaminase (AST) of 355, and a malignancy is suspected. However, CT scans of the chest, abdomen, and pelvis show no evidence of malignancy, and an extensive gastrointestinal workup is unremarkable, including anti-smooth muscle antibodies, anti-liver-kidney microsomal antibody, antimitochondrial antibodies, gliadin antibody, alpha-1 antitrypsin, liver/kidney microsomal antibody, and hepatitis serologies. While on the neurology service, risperidone and donepezil are discontinued because
After discontinuing these medications, she is evaluated by the psychiatry consult team for mood lability. The psychiatry consult team recommends quetiapine, which is later started at 25 mg nightly at bedtime.
Clinically, Ms. D’s mental status continues to deteriorate. She becomes nonverbal and minimally able to follow commands. She is ultimately discharged to an inpatient hospice for end-of-life care and the team recommends that she continue with quetiapine once there.
Continue to: The authors' observations
The authors’ observations
CJD is a rare, rapidly progressive, fatal form of dementia. In the United States, the incidence is approximately 1 to 1.5 cases per 1 million people each year.2 There are various forms of the disease. Sporadic CJD is the most common, representing 85% of cases.3 Sporadic CJD typically occurs in patients in their 60s and quickly leads to death—50% of patients die within 5 months, and 90% of patients die within 1 year.2,3 The illness is hypothesized to arise from the production of misfolded prion proteins, ultimately leading to vacuolation, neuronal loss, and the spongiform appearance characteristic of CJD.3,4
Psychiatric symptoms have long been acknowledged as a feature of CJD. Recent data indicates that psychiatric symptoms occur in 90% to 92% of cases.5,6 Sleep disturbances and depressive symptoms, including vegetative symptoms, anhedonia, and tearfulness, appear to be most common.5 Psychotic symptoms occur in approximately 42% of cases and may include persecutory and paranoid delusions, as well as an array of vivid auditory, visual, and tactile hallucinations.5,7
There is also evidence that psychiatric symptoms may be an early marker of CJD.5,8 A Mayo Clinic study found that psychiatric symptoms occurred within the prodromal phase of CJD in 26% of cases, and psychiatric symptoms occurred within the first 100 days of illness in 86% of cases.5
Case reports have described patients with CJD who initially presented with depression, psychosis, and other psychiatric symptoms.9-11 Interestingly, there have been cases with only psychiatric symptoms, and no neurologic symptoms until relatively late in the illness.10,11 Several patients with CJD have been evaluated in psychiatric ERs, admitted to psychiatric hospitals, and treated with psychiatric medications and ECT.5,9 In one study, 44% of CJD cases were misdiagnosed as “psychiatric patients” due to the prominence of their psychiatric symptomatology.8
Continue to: Making the diagnosis in psychiatric settings
Making the diagnosis in psychiatric settings. Often, the most difficult aspect of CJD is making the diagnosis.3,12 Sporadic CJD in particular can vary widely in its clinical presentation.3 The core clinical feature of CJD is rapidly progressive dementia, so suspect CJD in these patients. However, CJD can be difficult to distinguish from other rapidly progressive dementias, such as autoimmune and paraneoplastic encephalopathies.2,3 The presence of neurologic features, specifically myoclonus, akinetic mutism, and visual, cerebellar, and extrapyramidal symptoms, should also be considered a red flag for the disorder3 (Table).
Finally, positive findings on MRI, EEG, or CSF assay can indicate a probable diagnosis of CJD.13 MRI, particularly diffusion weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), is recognized as the most studied, sensitive, and overall useful neuroimaging modality for detecting CJD.2,3,12 Although the appearance of CJD on MRI can vary widely, asymmetric hyperintensities in ≥3 cortical gyri, particularly in the frontal and parietal lobes, provide strong evidence of CJD and are observed in 80% to 81% of cases.4,12 Asymmetric hyperintensities in the basal ganglia, particularly the caudate and rostral putamen, are observed in 69% to 70% of cases.4,12,13
EEG and CSF assay also can be useful for making the diagnosis. While diffuse slowing and frontal rhythmic delta activity appear early in the course of CJD, periodic sharp wave complexes emerge later in the illness.4 However, EEG findings are not diagnostic, because periodic sharp wave complexes are seen in only two-thirds of CJD cases and also occur in other neurologic illnesses.3,4 In recent years, lumbar puncture with subsequent CSF testing has become increasingly useful in detecting the illness. The presence of the 14-3-3 protein and tau protein is highly sensitive, although not specific, for CJD.3 A definite diagnosis of CJD requires discovery of the misfolded prion proteins, such as by RT-QuIC or brain biopsy.2,3,13
Management of CJD in psychiatric patients. CJD is an invariably fatal disease for which there is no effective cure or disease modifying treatment.2 Therefore, supportive therapies are the mainstay of care. Psychotropic medications can be used to provide symptom relief. While the sleep disturbances, anxiety, and agitation/hallucinations associated with CJD appear to respond well to hypnotic, anxiolytic, and antipsychotic medications, respectively, antidepressants and mood-stabilizing medications appear to have little benefit for patients with CJD.5 During the final stages of the disease, patients may suffer from akinetic mutism and inability to swallow, which often leads to aspiration pneumonia.14 Patients should also be offered end-of-life counseling, planning, and care, and provided with other comfort measures wherever possible (Figure).

Continue to: Bottom Line
Bottom Line
Patients with Creutzfeldt-Jakob disease (CJD) may present to psychiatric settings, particularly to a psychiatric emergency room. Consider CJD as a possible etiology in patients with rapidly progressive dementia, depression, and psychosis. CJD is invariably fatal and there is no effective disease-modifying treatment. Supportive therapies are the mainstay of care.
Related Resources
- National Institute of Neurological Disorders and Stroke. Creutzfeldt-Jakob disease fact sheet. http://www.ninds.nih.gov/disorders/cjd/detail_cjd.htm.
- Centers for Disease Control and Prevention. Creutzfeldt-Jakob disease, classic (CJD). http://www.cdc.gov/prions/cjd.
Drug Brand Names
Donepezil • Aricept
Risperidone • Risperdal
Quetiapine • Seroquel
CASE A rapid decline
Ms. D, age 62, presents to a psychiatric emergency room (ER) after experiencing visual hallucinations, exhibiting odd behaviors, and having memory problems. On interview, she is disoriented, distractible, tearful, and tangential. She plays with her shirt and glasses, and occasionally shouts. She perseverates on “the aerialists,” acrobatic children she has been seeing in her apartment. She becomes distressed and shouts, “I would love to just get them!”
Ms. D is unable to provide an account of her history. Collateral information is obtained from her daughter, who has brought Ms. D to the ER for evaluation. She reports that her mother has no relevant medical or psychiatric history, and does not take any medications, except a mixture of Chinese herbs that she brews into a tea.
Ms. D’s daughter says that her mother began to deteriorate 5 months ago, after she traveled to California to care for her sister, who was seriously ill and passed away. After Ms. D returned, she would cry frequently. She also appeared “spaced out,” complained of feeling dizzy, and frequently misplaced belongings. Three months before presenting to the ER, she began to experience weakness, fatigue, and difficulty walking. Her daughter became more worried 2 months ago, when Ms. D began sleeping with her purse and hiding her belongings around their house. When asked about these odd behaviors, Ms. D claimed that “the aerialists” were climbing through her windows at night and stealing her things.
A week before seeking treatment at the ER, Ms. D’s daughter had taken her to a neurologist at another facility for clinical evaluation. An MRI of the brain showed minimal dilation in the subarachnoid space and a focal 1 cm lipoma in the anterior falx cerebri, but was otherwise unremarkable. However, Ms. D’s symptoms continued to worsen, and began to interfere with her ability to care for herself.
The team in the psychiatric ER attributes Ms. D’s symptoms to a severe, psychotic depressive episode. They admit her to the psychiatric inpatient unit for further evaluation.
[polldaddy:10012742]
Continue to: The authors' observations
The authors’ observations
Ms. D was plagued by several mood and psychotic symptoms. Such symptoms can arise from many different psychiatric or organic etiologies. In Ms. D’s case, several aspects of her presentation suggest that her illness was psychiatric. The severe illness of a beloved family member is a significant stressor that could cause a great deal of grief and devastation, possibly leading to depression. Indeed, Ms. D’s daughter noticed that her mother was crying frequently, which is consistent with grief or depression.
Memory problems, which might manifest as misplacing belongings, can also indicate a depressive illness, especially in older patients. Moreover, impaired concentration, which can cause one to appear “spaced out” or distractible, is a core symptom of major depressive disorder. Sadness and grief also can be appropriate during bereavement and in response to significant losses. Therefore, in Ms. D’s case, it is possible her frequent crying, “spaced out” appearance, and other mood symptoms she experienced immediately after caring for her sister were an appropriate response to her sister’s illness and death.
However, other aspects of Ms. D’s presentation suggested an organic etiology. Her rapid deterioration and symptom onset relatively late in life were consistent with dementia and malignancy. Her complaint of feeling dizzy suggested a neurologic process was affecting her vestibular system. Finally, while psychiatric disorders can certainly cause visual hallucinations, they occur in only a small percentage of cases.1 Visual hallucinations are commonly associated with delirium, intoxication, and neurologic illness.
Continue to: EVALUATION Severe impairment
EVALUATION Severe impairment
On the psychiatric inpatient unit, Ms. D remains unable to give a coherent account of her illness or recent events. During interviews, she abruptly shifts from laughing to crying for no apparent reason. While answering questions, her responses trail off and she appears to forget what she had been saying. However, she continues to speak at length about “the aerialists,” stating that she sees them living in her wardrobe and jumping from rooftop to rooftop in her neighborhood.
A mental status examination finds evidence of severe cognitive impairment. Ms. D is unable to identify the correct date, time, or place, and appears surprised when told she is in a hospital. She can repeat the names of 3 objects but cannot recall them a few minutes later. Finally, she scores a 14 on the Mini-Mental State Examination (MMSE) and a 5 on the Montreal Cognitive Assessment (MoCA), indicating severe impairment.
On the unit, Ms. D cannot remember the location of her room or bathroom, and even when given directions, she needs to be escorted to her destination. Her gait is unsteady and wide-spaced, and she walks on her toes at times. When food is placed before her, she needs to be shown how to take the lids off containers, pick up utensils, and start eating.
All laboratory results are unremarkable, including a complete blood count, basic metabolic panel, liver function tests, gamma-glutamyl transpeptidase, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, methylmalonic acid, homocysteine, folate, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rapid plasma reagin, human immunodeficiency virus, and Lyme titers. The team also considers Ms. D’s history of herbal medicine use, because herbal mixtures can contain heavy metals and other contaminants. However, all toxicology results are normal, including arsenic, mercury, lead, copper, and zinc.
To address her symptoms, Ms. D is given risperidone, 0.5 mg twice a day, and donepezil, 5 mg/d.
[polldaddy:10012743]
Continue to: The authors' observations
The authors’ observations
Despite her persistent psychiatric symptoms, Ms. D had several neurologic symptoms that warranted further investigation. Her abrupt shifts from laughter to tears for no apparent reason were consistent with pseudobulbar affect. Her inability to remember how to use utensils during meals was consistent with apraxia. Finally, her abnormal gait raised concern for a process affecting her motor system.
OUTCOME A rare disorder
Given the psychiatry team’s suspicions for a neurologic etiology of Ms. D’s symptoms, an MRI of her brain is repeated. The results are notable for abnormal restricted diffusion in the caudate and putamen bilaterally, which is consistent with Creutzfeldt-Jakob disease (CJD). EEG shows moderate diffuse cerebral dysfunction, frontal intermittent delta activity, and diffuse cortical hyperexcitability, consistent with early- to mid-onset prion disease. Upon evaluation by the neurology team, Ms. D appears fearful, suspicious, and disorganized, but her examination does not reveal additional significant sensorimotor findings.
Ms. D is transferred to the neurology service for further workup and management. A lumbar puncture is positive for real-time quaking-induced conversion (RT-QuIC) and 14-3-3 protein with elevated tau proteins; these findings also are consistent with CJD. She develops transaminitis, with an alanine transaminase (ALT) of 127 and aspartate transaminase (AST) of 355, and a malignancy is suspected. However, CT scans of the chest, abdomen, and pelvis show no evidence of malignancy, and an extensive gastrointestinal workup is unremarkable, including anti-smooth muscle antibodies, anti-liver-kidney microsomal antibody, antimitochondrial antibodies, gliadin antibody, alpha-1 antitrypsin, liver/kidney microsomal antibody, and hepatitis serologies. While on the neurology service, risperidone and donepezil are discontinued because
After discontinuing these medications, she is evaluated by the psychiatry consult team for mood lability. The psychiatry consult team recommends quetiapine, which is later started at 25 mg nightly at bedtime.
Clinically, Ms. D’s mental status continues to deteriorate. She becomes nonverbal and minimally able to follow commands. She is ultimately discharged to an inpatient hospice for end-of-life care and the team recommends that she continue with quetiapine once there.
Continue to: The authors' observations
The authors’ observations
CJD is a rare, rapidly progressive, fatal form of dementia. In the United States, the incidence is approximately 1 to 1.5 cases per 1 million people each year.2 There are various forms of the disease. Sporadic CJD is the most common, representing 85% of cases.3 Sporadic CJD typically occurs in patients in their 60s and quickly leads to death—50% of patients die within 5 months, and 90% of patients die within 1 year.2,3 The illness is hypothesized to arise from the production of misfolded prion proteins, ultimately leading to vacuolation, neuronal loss, and the spongiform appearance characteristic of CJD.3,4
Psychiatric symptoms have long been acknowledged as a feature of CJD. Recent data indicates that psychiatric symptoms occur in 90% to 92% of cases.5,6 Sleep disturbances and depressive symptoms, including vegetative symptoms, anhedonia, and tearfulness, appear to be most common.5 Psychotic symptoms occur in approximately 42% of cases and may include persecutory and paranoid delusions, as well as an array of vivid auditory, visual, and tactile hallucinations.5,7
There is also evidence that psychiatric symptoms may be an early marker of CJD.5,8 A Mayo Clinic study found that psychiatric symptoms occurred within the prodromal phase of CJD in 26% of cases, and psychiatric symptoms occurred within the first 100 days of illness in 86% of cases.5
Case reports have described patients with CJD who initially presented with depression, psychosis, and other psychiatric symptoms.9-11 Interestingly, there have been cases with only psychiatric symptoms, and no neurologic symptoms until relatively late in the illness.10,11 Several patients with CJD have been evaluated in psychiatric ERs, admitted to psychiatric hospitals, and treated with psychiatric medications and ECT.5,9 In one study, 44% of CJD cases were misdiagnosed as “psychiatric patients” due to the prominence of their psychiatric symptomatology.8
Continue to: Making the diagnosis in psychiatric settings
Making the diagnosis in psychiatric settings. Often, the most difficult aspect of CJD is making the diagnosis.3,12 Sporadic CJD in particular can vary widely in its clinical presentation.3 The core clinical feature of CJD is rapidly progressive dementia, so suspect CJD in these patients. However, CJD can be difficult to distinguish from other rapidly progressive dementias, such as autoimmune and paraneoplastic encephalopathies.2,3 The presence of neurologic features, specifically myoclonus, akinetic mutism, and visual, cerebellar, and extrapyramidal symptoms, should also be considered a red flag for the disorder3 (Table).
Finally, positive findings on MRI, EEG, or CSF assay can indicate a probable diagnosis of CJD.13 MRI, particularly diffusion weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), is recognized as the most studied, sensitive, and overall useful neuroimaging modality for detecting CJD.2,3,12 Although the appearance of CJD on MRI can vary widely, asymmetric hyperintensities in ≥3 cortical gyri, particularly in the frontal and parietal lobes, provide strong evidence of CJD and are observed in 80% to 81% of cases.4,12 Asymmetric hyperintensities in the basal ganglia, particularly the caudate and rostral putamen, are observed in 69% to 70% of cases.4,12,13
EEG and CSF assay also can be useful for making the diagnosis. While diffuse slowing and frontal rhythmic delta activity appear early in the course of CJD, periodic sharp wave complexes emerge later in the illness.4 However, EEG findings are not diagnostic, because periodic sharp wave complexes are seen in only two-thirds of CJD cases and also occur in other neurologic illnesses.3,4 In recent years, lumbar puncture with subsequent CSF testing has become increasingly useful in detecting the illness. The presence of the 14-3-3 protein and tau protein is highly sensitive, although not specific, for CJD.3 A definite diagnosis of CJD requires discovery of the misfolded prion proteins, such as by RT-QuIC or brain biopsy.2,3,13
Management of CJD in psychiatric patients. CJD is an invariably fatal disease for which there is no effective cure or disease modifying treatment.2 Therefore, supportive therapies are the mainstay of care. Psychotropic medications can be used to provide symptom relief. While the sleep disturbances, anxiety, and agitation/hallucinations associated with CJD appear to respond well to hypnotic, anxiolytic, and antipsychotic medications, respectively, antidepressants and mood-stabilizing medications appear to have little benefit for patients with CJD.5 During the final stages of the disease, patients may suffer from akinetic mutism and inability to swallow, which often leads to aspiration pneumonia.14 Patients should also be offered end-of-life counseling, planning, and care, and provided with other comfort measures wherever possible (Figure).

Continue to: Bottom Line
Bottom Line
Patients with Creutzfeldt-Jakob disease (CJD) may present to psychiatric settings, particularly to a psychiatric emergency room. Consider CJD as a possible etiology in patients with rapidly progressive dementia, depression, and psychosis. CJD is invariably fatal and there is no effective disease-modifying treatment. Supportive therapies are the mainstay of care.
Related Resources
- National Institute of Neurological Disorders and Stroke. Creutzfeldt-Jakob disease fact sheet. http://www.ninds.nih.gov/disorders/cjd/detail_cjd.htm.
- Centers for Disease Control and Prevention. Creutzfeldt-Jakob disease, classic (CJD). http://www.cdc.gov/prions/cjd.
Drug Brand Names
Donepezil • Aricept
Risperidone • Risperdal
Quetiapine • Seroquel
1. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am. 1999;22(1):159-172.
2. Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mo Med. 2013;110(5):422-428.
3. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
4. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
5. Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17(4):489-495.
6. Krasnianski A, Bohling GT, Harden M, et al. Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. J Clin Psychiatry. 2015;76(9):1209-1215.
7. Javed Q, Alam F, Krishna S, et al. An unusual case of sporadic Creutzfeldt-Jakob disease (CJD). BMJ Case Rep. 2010;pii: bcr1220092576. doi:10.1136/bcr.12.2009.2576.
8. Abudy A, Juven-Wetzler A, Zohar J. The different faces of Creutzfeldt-Jacob disease CJD in psychiatry. Gen Hosp Psychiatry. 2014;36(3):245-248.
9. Jiang TT, Moses H, Gordon H, et al. Sporadic Creutzfeldt-Jakob disease presenting as major depression. South Med J. 1999;92(8):807-808.
10. Ali R, Baborie A, Larner AJ et al. Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci. 2013;25(4):335-338.
11. Gençer AG, Pelin Z, Küçükali CI., et al. Creutzfeldt-Jakob disease. Psychogeriatrics. 2011;11(2):119-124.
12. Caobelli F, Cobelli M, Pizzocaro C, et al. The role of neuroimaging in evaluating patients affected by Creutzfeldt-Jakob disease: a systematic review of the literature. J Neuroimaging. 2015;25(1):2-13.
13. Centers for Disease Control and Prevention. CDC's diagnostic criteria for Creutzfeldt-Jakob disease, 2010. http://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Updated February 11, 2015. Accessed August 2, 2016.
14. Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5(1):43-46.
1. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am. 1999;22(1):159-172.
2. Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mo Med. 2013;110(5):422-428.
3. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
4. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
5. Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17(4):489-495.
6. Krasnianski A, Bohling GT, Harden M, et al. Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. J Clin Psychiatry. 2015;76(9):1209-1215.
7. Javed Q, Alam F, Krishna S, et al. An unusual case of sporadic Creutzfeldt-Jakob disease (CJD). BMJ Case Rep. 2010;pii: bcr1220092576. doi:10.1136/bcr.12.2009.2576.
8. Abudy A, Juven-Wetzler A, Zohar J. The different faces of Creutzfeldt-Jacob disease CJD in psychiatry. Gen Hosp Psychiatry. 2014;36(3):245-248.
9. Jiang TT, Moses H, Gordon H, et al. Sporadic Creutzfeldt-Jakob disease presenting as major depression. South Med J. 1999;92(8):807-808.
10. Ali R, Baborie A, Larner AJ et al. Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci. 2013;25(4):335-338.
11. Gençer AG, Pelin Z, Küçükali CI., et al. Creutzfeldt-Jakob disease. Psychogeriatrics. 2011;11(2):119-124.
12. Caobelli F, Cobelli M, Pizzocaro C, et al. The role of neuroimaging in evaluating patients affected by Creutzfeldt-Jakob disease: a systematic review of the literature. J Neuroimaging. 2015;25(1):2-13.
13. Centers for Disease Control and Prevention. CDC's diagnostic criteria for Creutzfeldt-Jakob disease, 2010. http://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Updated February 11, 2015. Accessed August 2, 2016.
14. Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5(1):43-46.
Aggressive outbursts and emotional lability in a 16-year-old boy
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7
PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.
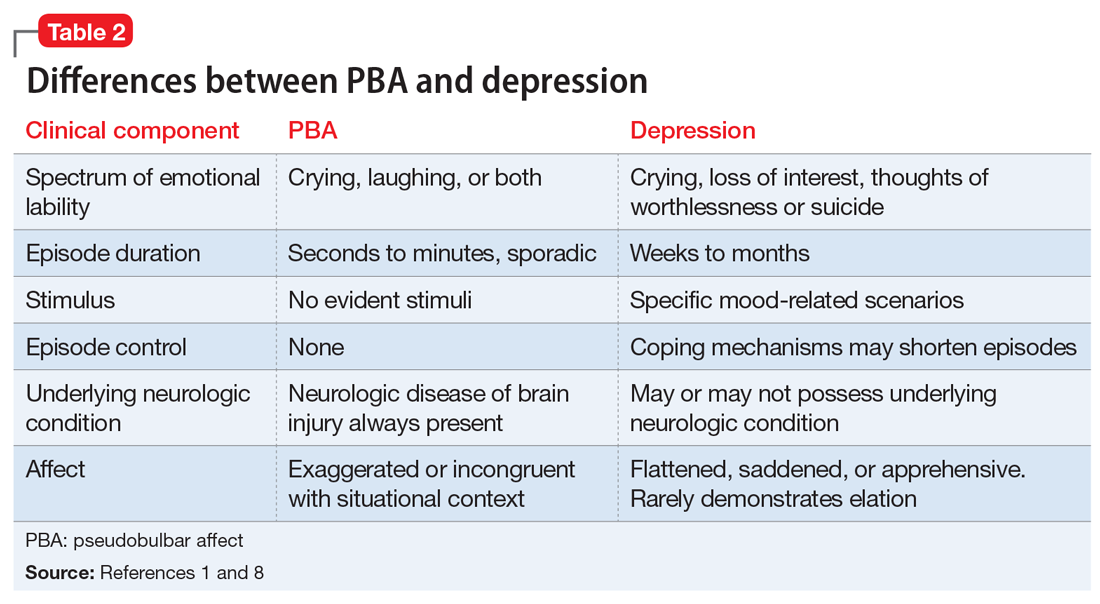
Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7

PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.

Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7

PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.

Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
Visual hallucinations and severe anxiety in the ICU after surgery
CASE Anxiety in the ICU
Mr. B, age 42, an African American man, is admitted to the inpatient medical unit for surgical treatment of peritoneal carcinomatosis with pelvic exenteration. He has a history of metastatic rectal cancer, chronic pain, and hypertension, but no psychiatric history. Mr. B’s postsurgical hospital stay is complicated by treatment-resistant tachycardia and hypertension, and he requires a lengthy stay in the ICU. In the ICU, Mr. B reports having visual hallucinations where he sees an individual placing a drug in his IV line. Additionally, he reports severe anxiety related to this experience. His anxiety and visual hallucinations are treated with coadministration of IV lorazepam, diphenhydramine, and haloperidol. These medications resolve the hallucinations, but his anxiety worsens and he becomes restless. He receives additional doses of IV haloperidol administered in 5 mg increments and reaching a cumulative 12-hour dose of 50 mg. Mr. B continues to report anxiety, so the psychiatry consultation-liaison (C-L) service is called.
[polldaddy:9970907]
The authors’ observations
Determining the cause of Mr. B’s anxiety is challenging because of his prolonged medical course, com
From a medical perspective, in a post-surgical patient treated in the ICU, the consulting practitioner must pay particular attention to delirium. ICU delirium is common—one report indicated that it occurs in 32.3% of ICU patients1—and frequently confused with psychiatric morbidity.2 Identifying delirium as the cause of impairment is important because delirium has potentially modifiable underlying etiologies. Symptomatically, delirium presents as impairment and fluctuation in attention, awareness, and at least one other cognitive domain, with a clear indication that the impairment occurred over a short period of time and represents a departure from baseline.3 In Mr. B’s case, these symptoms have not been excluded and should be considered by the C-L psychiatrists.
In addition to delirium, the C-L team must consider psychiatric comorbidity. Mr. B has no psychiatric history and a sudden first occurrence of hallucinations; therefore, it is unlikely that he has developed a primary psychotic disorder. Because he reported his symptoms had been present only for several days, he would not meet criteria for schizophrenia, which according to DSM-5 criteria require at least 1 month of ≥2 symptoms (including delusions, hallucinations, disorganized speech, disorganized behavior, or negative symptoms) and 6 months of declining function.3 However, although it is improbable, the C-L team must consider a primary psychotic illness, particularly given the potential devastating consequence of being misdiagnosed and mismanaged for an alternative illness. Unlike psychotic disorders, anxiety disorders are significantly more prevalent in the U.S. general population than primary psychotic disorders.4 Furthermore, the prevalence of anxiety disorders increases in the ICU setting; one study found that up to 61% of ICU patients setting experience “anxiety features.”5 Therefore, anxiety disorders and stress disorders should be considered in ICU patients who exhibit psychiatric symptoms.
Clinicians also should consider medication-induced adverse effects. In the ICU, patients are frequently managed on multiple medications, which increase their risk of developing adverse effects and adverse reactions.6 One potential consequence of polypharmacy is delirium, which remains a relevant potential diagnosis for Mr. B.7 Alternative consequences vary by medication and their respective pharmacodynamics. We take into consideration Mr. B’s exposure to high doses of the high-potency antipsychotic agent, haloperidol. Exposure to haloperidol can result in extrapyramidal symptoms, including akathisia,8,9,10 and the rare, but potentially fatal, NMS.11 These reactions can often be distinguished by taking a thorough history and a physical evaluation. In the case of akathisia, the clinician should look for medication exposure, titration, or taper. Most commonly, akathisia occurs secondary to antipsychotic exposure,12 followed by the onset of a combination of subjective symptoms, such as restlessness, anxiety, and irritability, and an objective symptom of increased motor activity.3 NMS, on the other hand, is distinguished by symptoms that include hyperthermia (>38ºC), diaphoresis, severe rigidity, urinary incontinence, vital instability, alterations in mental health status, and elevations in creatine kinase greater than 4-fold the upper limit, usually in the setting of treatment with antipsychotics.3 Nearly all cases of NMS occur within the first 30 days of antipsychotic exposure.3 While, overtly, NMS may appear to be less subtle than akathisia, clinicians should still be weary to rule out this admittedly rare, though potentially lethal diagnosis, especially in an ICU patient, where the diagnosis can be muddied by medical comorbidities that may mask the syndrome.
Continue to: EVALUATION Focus on akathisia
EVALUATION Focus on akathisia
On interview by the C-L team, Mr. B is visibly restless, moving all 4 extremities. He reports increased anxiety and irritability over the past 2 to 3 days. Mr. B states that he is aware of his increased motor movements and can briefly suppress them. However, after several seconds, he again begins spontaneously fidgeting, moving all 4 extremities and shifting from side to side in bed, saying, “I just feel anxious.” He denies having visual hallucinations, and says that the previous hallucinations had spontaneously presented and remitted after surgery. He denies the use of psychotropics for mental illness, prior similar symptoms to this presentation, a family history of mental illness, recent illicit substance use, or excessive alcohol use prior to presentation. This history is corroborated by collateral information from his brother, who was present in the ICU. On physical examination, Mr. B is afebrile and his vital signs are within normal limits. He does not have muscular rigidity or neck dystonia. His laboratory values, including complete blood count, electrolytes, liver function tests, and creatine phosphokinase, are within normal limits.
His medication administration record includes 46 standing agents, 16 “as-needed” agents, and 8 infusions. Several of the standing agents had psychotropic properties; however, the most salient were several opioids, ketamine, midazolam, lorazepam, dexamethasone, haloperidol, and olanzapine.
[polldaddy:9970908]
The authors’ observations
We determined that the most likely diagnosis for Mr. B’s symptoms was medication-induced akathisia secondary to haloperidol. Akathisia, coined by Haskovec in 1901,12,13 is from Greek, meaning an “inability to sit.”12 DSM-5 describes 2 forms of akathisia: medication-induced acute akathisia, and tardive akathisia.3 In the literature, others have described additional classifications, including chronic akathisia, withdrawal akathisia, and pseudoakathisia (Table 13,14-17). In Mr. B’s case, given his sudden development of symptoms and their direct chronologic relationship to antipsychotic treatment, and his combined subjective and objective symptoms, we believed that Mr. B’s symptoms were consistent with medication-induced acute akathisia (MIA). The identification and treatment of this clinical entity is important for several reasons, including reducing patient morbidity and maximizing patient comfort. Additionally, because akathisia has been associated with poor medication adherence, increased agitation/aggression, increased suicidality, and the eventual development of tardive dyskinesia,18 it is a relevant prognostic consideration when deciding to treat a patient with antipsychotics.
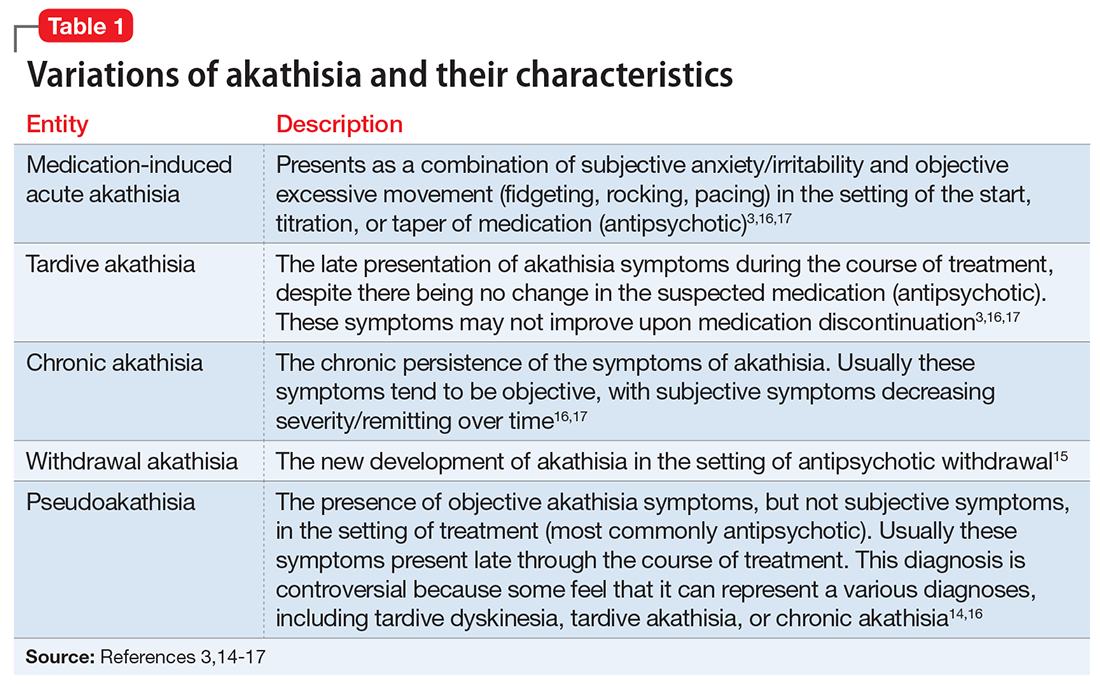
Pathophysiologically, we have yet to fully shed light on the exact underpinnings of akathisia. Much of our present knowledge stems from patient response to pharmacologic agents. While dopamine blockade has been linked to akathisia, the exact mechanism is not completely understood. Previous theories linking nigrostriatal pathways have been expanded to include mesocortical and mesolimbic considerations.12,17,18 Similarly surmised from medication effects, the transmitters y-aminobutyric acid, serotonin/5-hydroxytryptamine (5-HT), norepinephrine, and acetylcholine also have been linked to this syndrome, though as of yet, exact gross pathophysiologic mechanisms have not been fully elucidated.12 More recently, Stahl and Loonen19 described a novel mechanism by which they link the shell of the nucleus accumbens to akathisia. In their report, they indicate that the potential reduction in dopaminergic activity, secondary to antipsychotic administration, can result in compensatory noradrenergic activation of the locus coeruleus.19 The increased noradrenergic activity results in the downstream activation of the shell of the nucleus accumbens.19 The activation of the nucleus accumbens shell, which has been linked to unconditioned feeding and fear behavior, can then result in a cascade of effects that would phenotypically present as the syndrome we recognize to be akathisia.19
Numerous etiologies have been linked to MIA. Of these, high-potency antipsychotics are believed to remain the greatest risk factor for akathisia,18 although atypical antipsychotics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, have been linked to the disorder.18,19
Continue to: Regarding antipsychotics...
Regarding antipsychotics, risk factors for akathisia include drug potency, dose, and rapidity of titration.20 All of these factors were relevant in our patient’s case. Risk across antipsychotic classes is not well understood; few head-to-head studies have comparing antipsychotics. However, general estimates suggest a 15% to 40% prevalence in patients exposed to typical antipsychotics, as compared with 0% to 12% exposed to atypical antipsychotics.8 The literature-reported difference in risk, as well as our patient’s comparative difference in exposure to large doses of haloperidol (50 mg) as compared with 1 dose of olanzapine (5 mg), led us to believe his akathisia developed primarily due to his exposure to haloperidol. Conclusively linking his symptoms to haloperidol alone, however, is not possible, and we did consider that olanzapine may in fact have had some effect in worsening Mr. B’s akathisia.
[polldaddy:9970909]
The authors’ observations
While there are reports on the efficacy of various agents in the treatment of akathisia, the most commonly evaluated agents are propranolol, anticholinergics, and benzodiazepines.17, 21
Propranolol is a nonselective beta-adrenergic blocker with numerous indications.17 Despite a 2004 Cochrane Review indicating that there is no evidence in support of central acting beta-blockers for treating akathisia,22 propranolol is not yet recognized as an appropriate treatment.17 The reason for this discrepancy is likely due to the Cochrane Review’s restrictive inclusion criteria, which prevented the analysis of much of the literature.22 In fact, several reports cite evidence for the treatment efficacy of propranolol17 and, to date, some reports continue to advocate for its use as a first-line agent in the treatment of akathisia. Admittedly, besides the Cochrane Review,22 other reports have found propranolol to be ineffective for treating akathisia,23 although these tend to be limited by their population size and generalizability.
As with propranolol, a 2006 Cochrane Review found “no reliable evidence to support or refute” using anticholinergic agents in the treatment of akathisia.24 We suspect that the review’s findings were likely secondary to its strict inclusion criteria.24 In fact, several reports support using anticholinergic agents for treating akathisia.25 Here we focus on benztropine and diphenhydramine.
Two reviews—Blaisdell26 (1994) and Poyurovsky27 (2010)—suggest modest benefits from benztropine, primarily in patients with comorbid Parkinson’s disease. Despite these benefits, head-to-head trials seem to either point to the superiority of propranolol or to no difference between these agents for treating akathisia.28,29 In a review, we only found 1 trial demonstrating benztropine’s superiority over propranolol,23 but this trial was constrained by its small population (6 patients). Therefore, the data suggest that, when indicated, clinicians should lean towards using propranolol for treating akathisia.
Continue to: Diphenhydramine, a first-generation antihistamine...
Diphenhydramine, a first-generation antihistamine with antimuscarinic properties, has been studied for its efficacy in treating metoclopramide-induced akathisia in the emergency setting.30 There are several reports on the efficacy of this agent, including a large randomized study involving 281 patients that found it effective for preventing metoclopramide-induced akathisia.30 Another head-to-head trial reported the benefit of the diphenhydramine vs midazolam.31 Both agents were efficacious for treating akathisia; however, midazolam had a more rapid onset. Despite these positive reports, double-blind trials have found diphenhydramine to be ineffective,17 which suggests propranolol should be the first-line agent, assuming it is not contraindicated.
Benzodiazepines have also been found to be efficacious for treating akathisia. A 1999 Cochrane Review included 2 randomized controlled trials that assessed the efficacy of clonazepam vs placebo for treating akathisia.32 It found evidence of benefit for clonazepam, but questioned the generalizability of these studies.32 This review did not include several other reports that suggest benefits of other benzodiazepines for treating akathisia. Other than clonazepam, reports suggest benefit for diazepam, lorazepam, and midazolam for treating akathisia.17 Despite this evidence and the findings from this Cochrane Review, the literature does not appear to point to clear dominance of these agents over propranolol. Given the safety concerns when prescribing benzodiazepines, it would be prudent to utilize propranolol as a first-line agent for treating akathisia.
Finally, other reports have cited treatment efficacy linked to serotonin 2A receptor (5-HT2A) antagonists (mianserin, mirtazapine, and trazodone), clonidine, gabapentin, amantadine, and other agents.17 If treatment with propranolol is ineffective or contraindicated, clinicians should utilize their clinical judgement in deciding on the use of one agent over another.
OUTCOME Complete resolution
Haloperidol is discontinued and diphenhydramine, 50 mg IV, is administered. (Diphenhydramine was used instead of propranolol due to immediacy of availability.) Most of Mr. B’s signs and symptoms resolve on a repeat interview 3 hours later. He receives another dose of diphenhydramine, 25 mg IV, for persistent mild irritability. By Day 2 of follow-up, his symptoms completely resolve as measured on the Barnes Akathisia Scale33 (Table 2).
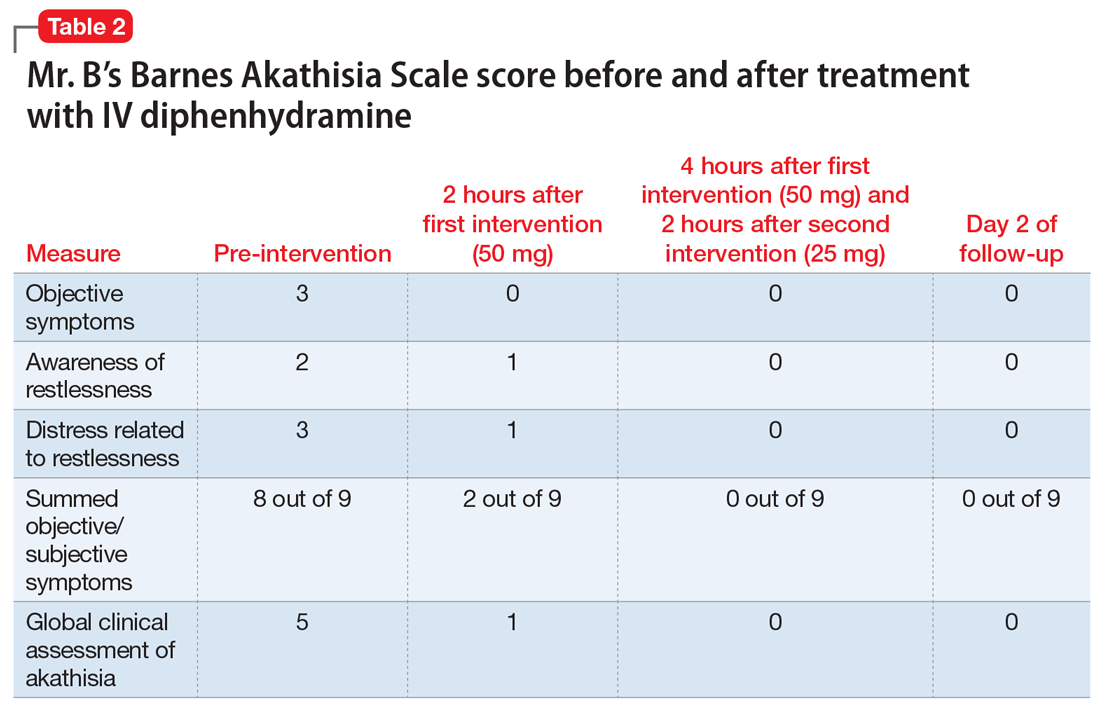
Continue to: Bottom Line
Bottom Line
Akathisia is an elusive adverse effect of antipsychotics and can be misdiagnosed as anxiety. Close consideration should be given to potential medical, psychiatric, and drug-related etiologies in patients who have a prolonged medical course, comorbidities, and exposure to multiple pharmacologic agents.
Related Resources
- Factor SA, Leffler JB, Murray CF. Drug-induced movement disorders: a clinical review. Medscape. http://www.medscape.org/viewarticle/586881.
- Marder S, Stroup TS. Pharmacotherapy for schizophrenia: side effect management. UpToDate. https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-side-effect-management.
Drug Brand Names
Amantadine • Symmetrel
Benztropine • Cogentin
Clonazepam • Klonopin
Clonidine • Catapres
Dexamethasone • Decadron
Diazepam • Valium
Diphenhydramine • Benadryl
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Metoclopramide • Reglan
Mianserin • Tolvon
Midazolam • Versed
Mirtazapine • Remeron
Olanzapine • Zyprexa
Propranolol • Inderal
Rivastigmine • Exelon
Trazodone • Oleptro
1. Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: an overview. Ann Intensive Care. 2012;2:49.
2. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. National Alliance on Mental Illness. Mental health by the numbers. https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed March 4, 2018.
5. Jacka MJ, Mitchell N, Perez-Parada J. Incidence and prevalence of anxiety, depression, and post-traumatic stress disorder among critical care patients, families, and practitioners. J Anest & Inten Care Med. 2016;1(1):55555. doi: 10.19080/JAICM.2016.01.555555.
6. Reis AM, Cassiani SH. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625-632.
7. Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33(2):189-203.
8. Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127-148.
9. Van Putten T, Marder SR. Toward a more reliable diagnosis of akathisia. Arch Gen Psychiatry. 1986;43(10):1015-1016.
10. Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463-465.
11. Naganuma H, Fujii I. Incidence and risk factors in neuroleptic malignant syndrome. Acta Psychiatr Scand. 1994;90(6):424-426.
12. Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77-84.
13. Brune M, Sachdev, PS. Ladislav Haskovec and 100 years of akathisia. American Journal of Psychiatry. 2002;159(5):727-727.
14. Havaki-Kontaxaki BJ, Kontaxakis VP, Christodoulou GN. Prevalence and characteristics of patients with pseudoakathisia. Eur Neuropsychopharmacol. 2000;10(5):333-336.
15. Lang AE. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9(2):188-192.
16. Sachdev P. The epidemiology of drug-induced akathisia: Part II. Chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461.
17. Kern DS, Lang AE. Acute akathisia. In: Friedman JH, ed. Medication-induced movement disorders. Cambridge, United Kingdom: Cambridge University Press; 2015:12-24.
18. Adler LA, Angrist B, Reiter S, et al. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl). 1989;97(1):1-11.
19. Stahl SM, Loonen AJM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
20. Sachdev P, Kruk J. Clinical characteristics and predisposing factors in acute drug-induced akathisia. Arch Gen Psychiatry. 1994;51(12):963-974.
21. Laoutidis ZG, Luckhaus C. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(5):823-832.
22. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
23. Sachdev P, Loneragan C. Intravenous benztropine and propranolol challenges in acute neuroleptic-induced akathisia. Clin Neuropharmacol. 1993;16(4):324-331.
24. Lima AR, Weiser KV, Bacaltchuk J, et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(1):CD003727.
25. Fleischhacker WW, Roth SD, Kane JM. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol. 1990;10(1):12-21.
26. Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139-146.
27. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
28. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
29. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Bender B, Friedman B, Davitt M, et al. 118: metoclopramide in the emergency department: a randomized factorial design study to determine the influence of dose and diphenhydramine on akathisia. Ann of Emerg Med. 2008;52(4):S78.
31. Parlak I, Erdur B, Parlak M, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715-721.
32. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 1999;(4):CD001950.
33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676..
CASE Anxiety in the ICU
Mr. B, age 42, an African American man, is admitted to the inpatient medical unit for surgical treatment of peritoneal carcinomatosis with pelvic exenteration. He has a history of metastatic rectal cancer, chronic pain, and hypertension, but no psychiatric history. Mr. B’s postsurgical hospital stay is complicated by treatment-resistant tachycardia and hypertension, and he requires a lengthy stay in the ICU. In the ICU, Mr. B reports having visual hallucinations where he sees an individual placing a drug in his IV line. Additionally, he reports severe anxiety related to this experience. His anxiety and visual hallucinations are treated with coadministration of IV lorazepam, diphenhydramine, and haloperidol. These medications resolve the hallucinations, but his anxiety worsens and he becomes restless. He receives additional doses of IV haloperidol administered in 5 mg increments and reaching a cumulative 12-hour dose of 50 mg. Mr. B continues to report anxiety, so the psychiatry consultation-liaison (C-L) service is called.
[polldaddy:9970907]
The authors’ observations
Determining the cause of Mr. B’s anxiety is challenging because of his prolonged medical course, com
From a medical perspective, in a post-surgical patient treated in the ICU, the consulting practitioner must pay particular attention to delirium. ICU delirium is common—one report indicated that it occurs in 32.3% of ICU patients1—and frequently confused with psychiatric morbidity.2 Identifying delirium as the cause of impairment is important because delirium has potentially modifiable underlying etiologies. Symptomatically, delirium presents as impairment and fluctuation in attention, awareness, and at least one other cognitive domain, with a clear indication that the impairment occurred over a short period of time and represents a departure from baseline.3 In Mr. B’s case, these symptoms have not been excluded and should be considered by the C-L psychiatrists.
In addition to delirium, the C-L team must consider psychiatric comorbidity. Mr. B has no psychiatric history and a sudden first occurrence of hallucinations; therefore, it is unlikely that he has developed a primary psychotic disorder. Because he reported his symptoms had been present only for several days, he would not meet criteria for schizophrenia, which according to DSM-5 criteria require at least 1 month of ≥2 symptoms (including delusions, hallucinations, disorganized speech, disorganized behavior, or negative symptoms) and 6 months of declining function.3 However, although it is improbable, the C-L team must consider a primary psychotic illness, particularly given the potential devastating consequence of being misdiagnosed and mismanaged for an alternative illness. Unlike psychotic disorders, anxiety disorders are significantly more prevalent in the U.S. general population than primary psychotic disorders.4 Furthermore, the prevalence of anxiety disorders increases in the ICU setting; one study found that up to 61% of ICU patients setting experience “anxiety features.”5 Therefore, anxiety disorders and stress disorders should be considered in ICU patients who exhibit psychiatric symptoms.
Clinicians also should consider medication-induced adverse effects. In the ICU, patients are frequently managed on multiple medications, which increase their risk of developing adverse effects and adverse reactions.6 One potential consequence of polypharmacy is delirium, which remains a relevant potential diagnosis for Mr. B.7 Alternative consequences vary by medication and their respective pharmacodynamics. We take into consideration Mr. B’s exposure to high doses of the high-potency antipsychotic agent, haloperidol. Exposure to haloperidol can result in extrapyramidal symptoms, including akathisia,8,9,10 and the rare, but potentially fatal, NMS.11 These reactions can often be distinguished by taking a thorough history and a physical evaluation. In the case of akathisia, the clinician should look for medication exposure, titration, or taper. Most commonly, akathisia occurs secondary to antipsychotic exposure,12 followed by the onset of a combination of subjective symptoms, such as restlessness, anxiety, and irritability, and an objective symptom of increased motor activity.3 NMS, on the other hand, is distinguished by symptoms that include hyperthermia (>38ºC), diaphoresis, severe rigidity, urinary incontinence, vital instability, alterations in mental health status, and elevations in creatine kinase greater than 4-fold the upper limit, usually in the setting of treatment with antipsychotics.3 Nearly all cases of NMS occur within the first 30 days of antipsychotic exposure.3 While, overtly, NMS may appear to be less subtle than akathisia, clinicians should still be weary to rule out this admittedly rare, though potentially lethal diagnosis, especially in an ICU patient, where the diagnosis can be muddied by medical comorbidities that may mask the syndrome.
Continue to: EVALUATION Focus on akathisia
EVALUATION Focus on akathisia
On interview by the C-L team, Mr. B is visibly restless, moving all 4 extremities. He reports increased anxiety and irritability over the past 2 to 3 days. Mr. B states that he is aware of his increased motor movements and can briefly suppress them. However, after several seconds, he again begins spontaneously fidgeting, moving all 4 extremities and shifting from side to side in bed, saying, “I just feel anxious.” He denies having visual hallucinations, and says that the previous hallucinations had spontaneously presented and remitted after surgery. He denies the use of psychotropics for mental illness, prior similar symptoms to this presentation, a family history of mental illness, recent illicit substance use, or excessive alcohol use prior to presentation. This history is corroborated by collateral information from his brother, who was present in the ICU. On physical examination, Mr. B is afebrile and his vital signs are within normal limits. He does not have muscular rigidity or neck dystonia. His laboratory values, including complete blood count, electrolytes, liver function tests, and creatine phosphokinase, are within normal limits.
His medication administration record includes 46 standing agents, 16 “as-needed” agents, and 8 infusions. Several of the standing agents had psychotropic properties; however, the most salient were several opioids, ketamine, midazolam, lorazepam, dexamethasone, haloperidol, and olanzapine.
[polldaddy:9970908]
The authors’ observations
We determined that the most likely diagnosis for Mr. B’s symptoms was medication-induced akathisia secondary to haloperidol. Akathisia, coined by Haskovec in 1901,12,13 is from Greek, meaning an “inability to sit.”12 DSM-5 describes 2 forms of akathisia: medication-induced acute akathisia, and tardive akathisia.3 In the literature, others have described additional classifications, including chronic akathisia, withdrawal akathisia, and pseudoakathisia (Table 13,14-17). In Mr. B’s case, given his sudden development of symptoms and their direct chronologic relationship to antipsychotic treatment, and his combined subjective and objective symptoms, we believed that Mr. B’s symptoms were consistent with medication-induced acute akathisia (MIA). The identification and treatment of this clinical entity is important for several reasons, including reducing patient morbidity and maximizing patient comfort. Additionally, because akathisia has been associated with poor medication adherence, increased agitation/aggression, increased suicidality, and the eventual development of tardive dyskinesia,18 it is a relevant prognostic consideration when deciding to treat a patient with antipsychotics.

Pathophysiologically, we have yet to fully shed light on the exact underpinnings of akathisia. Much of our present knowledge stems from patient response to pharmacologic agents. While dopamine blockade has been linked to akathisia, the exact mechanism is not completely understood. Previous theories linking nigrostriatal pathways have been expanded to include mesocortical and mesolimbic considerations.12,17,18 Similarly surmised from medication effects, the transmitters y-aminobutyric acid, serotonin/5-hydroxytryptamine (5-HT), norepinephrine, and acetylcholine also have been linked to this syndrome, though as of yet, exact gross pathophysiologic mechanisms have not been fully elucidated.12 More recently, Stahl and Loonen19 described a novel mechanism by which they link the shell of the nucleus accumbens to akathisia. In their report, they indicate that the potential reduction in dopaminergic activity, secondary to antipsychotic administration, can result in compensatory noradrenergic activation of the locus coeruleus.19 The increased noradrenergic activity results in the downstream activation of the shell of the nucleus accumbens.19 The activation of the nucleus accumbens shell, which has been linked to unconditioned feeding and fear behavior, can then result in a cascade of effects that would phenotypically present as the syndrome we recognize to be akathisia.19
Numerous etiologies have been linked to MIA. Of these, high-potency antipsychotics are believed to remain the greatest risk factor for akathisia,18 although atypical antipsychotics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, have been linked to the disorder.18,19
Continue to: Regarding antipsychotics...
Regarding antipsychotics, risk factors for akathisia include drug potency, dose, and rapidity of titration.20 All of these factors were relevant in our patient’s case. Risk across antipsychotic classes is not well understood; few head-to-head studies have comparing antipsychotics. However, general estimates suggest a 15% to 40% prevalence in patients exposed to typical antipsychotics, as compared with 0% to 12% exposed to atypical antipsychotics.8 The literature-reported difference in risk, as well as our patient’s comparative difference in exposure to large doses of haloperidol (50 mg) as compared with 1 dose of olanzapine (5 mg), led us to believe his akathisia developed primarily due to his exposure to haloperidol. Conclusively linking his symptoms to haloperidol alone, however, is not possible, and we did consider that olanzapine may in fact have had some effect in worsening Mr. B’s akathisia.
[polldaddy:9970909]
The authors’ observations
While there are reports on the efficacy of various agents in the treatment of akathisia, the most commonly evaluated agents are propranolol, anticholinergics, and benzodiazepines.17, 21
Propranolol is a nonselective beta-adrenergic blocker with numerous indications.17 Despite a 2004 Cochrane Review indicating that there is no evidence in support of central acting beta-blockers for treating akathisia,22 propranolol is not yet recognized as an appropriate treatment.17 The reason for this discrepancy is likely due to the Cochrane Review’s restrictive inclusion criteria, which prevented the analysis of much of the literature.22 In fact, several reports cite evidence for the treatment efficacy of propranolol17 and, to date, some reports continue to advocate for its use as a first-line agent in the treatment of akathisia. Admittedly, besides the Cochrane Review,22 other reports have found propranolol to be ineffective for treating akathisia,23 although these tend to be limited by their population size and generalizability.
As with propranolol, a 2006 Cochrane Review found “no reliable evidence to support or refute” using anticholinergic agents in the treatment of akathisia.24 We suspect that the review’s findings were likely secondary to its strict inclusion criteria.24 In fact, several reports support using anticholinergic agents for treating akathisia.25 Here we focus on benztropine and diphenhydramine.
Two reviews—Blaisdell26 (1994) and Poyurovsky27 (2010)—suggest modest benefits from benztropine, primarily in patients with comorbid Parkinson’s disease. Despite these benefits, head-to-head trials seem to either point to the superiority of propranolol or to no difference between these agents for treating akathisia.28,29 In a review, we only found 1 trial demonstrating benztropine’s superiority over propranolol,23 but this trial was constrained by its small population (6 patients). Therefore, the data suggest that, when indicated, clinicians should lean towards using propranolol for treating akathisia.
Continue to: Diphenhydramine, a first-generation antihistamine...
Diphenhydramine, a first-generation antihistamine with antimuscarinic properties, has been studied for its efficacy in treating metoclopramide-induced akathisia in the emergency setting.30 There are several reports on the efficacy of this agent, including a large randomized study involving 281 patients that found it effective for preventing metoclopramide-induced akathisia.30 Another head-to-head trial reported the benefit of the diphenhydramine vs midazolam.31 Both agents were efficacious for treating akathisia; however, midazolam had a more rapid onset. Despite these positive reports, double-blind trials have found diphenhydramine to be ineffective,17 which suggests propranolol should be the first-line agent, assuming it is not contraindicated.
Benzodiazepines have also been found to be efficacious for treating akathisia. A 1999 Cochrane Review included 2 randomized controlled trials that assessed the efficacy of clonazepam vs placebo for treating akathisia.32 It found evidence of benefit for clonazepam, but questioned the generalizability of these studies.32 This review did not include several other reports that suggest benefits of other benzodiazepines for treating akathisia. Other than clonazepam, reports suggest benefit for diazepam, lorazepam, and midazolam for treating akathisia.17 Despite this evidence and the findings from this Cochrane Review, the literature does not appear to point to clear dominance of these agents over propranolol. Given the safety concerns when prescribing benzodiazepines, it would be prudent to utilize propranolol as a first-line agent for treating akathisia.
Finally, other reports have cited treatment efficacy linked to serotonin 2A receptor (5-HT2A) antagonists (mianserin, mirtazapine, and trazodone), clonidine, gabapentin, amantadine, and other agents.17 If treatment with propranolol is ineffective or contraindicated, clinicians should utilize their clinical judgement in deciding on the use of one agent over another.
OUTCOME Complete resolution
Haloperidol is discontinued and diphenhydramine, 50 mg IV, is administered. (Diphenhydramine was used instead of propranolol due to immediacy of availability.) Most of Mr. B’s signs and symptoms resolve on a repeat interview 3 hours later. He receives another dose of diphenhydramine, 25 mg IV, for persistent mild irritability. By Day 2 of follow-up, his symptoms completely resolve as measured on the Barnes Akathisia Scale33 (Table 2).

Continue to: Bottom Line
Bottom Line
Akathisia is an elusive adverse effect of antipsychotics and can be misdiagnosed as anxiety. Close consideration should be given to potential medical, psychiatric, and drug-related etiologies in patients who have a prolonged medical course, comorbidities, and exposure to multiple pharmacologic agents.
Related Resources
- Factor SA, Leffler JB, Murray CF. Drug-induced movement disorders: a clinical review. Medscape. http://www.medscape.org/viewarticle/586881.
- Marder S, Stroup TS. Pharmacotherapy for schizophrenia: side effect management. UpToDate. https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-side-effect-management.
Drug Brand Names
Amantadine • Symmetrel
Benztropine • Cogentin
Clonazepam • Klonopin
Clonidine • Catapres
Dexamethasone • Decadron
Diazepam • Valium
Diphenhydramine • Benadryl
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Metoclopramide • Reglan
Mianserin • Tolvon
Midazolam • Versed
Mirtazapine • Remeron
Olanzapine • Zyprexa
Propranolol • Inderal
Rivastigmine • Exelon
Trazodone • Oleptro
CASE Anxiety in the ICU
Mr. B, age 42, an African American man, is admitted to the inpatient medical unit for surgical treatment of peritoneal carcinomatosis with pelvic exenteration. He has a history of metastatic rectal cancer, chronic pain, and hypertension, but no psychiatric history. Mr. B’s postsurgical hospital stay is complicated by treatment-resistant tachycardia and hypertension, and he requires a lengthy stay in the ICU. In the ICU, Mr. B reports having visual hallucinations where he sees an individual placing a drug in his IV line. Additionally, he reports severe anxiety related to this experience. His anxiety and visual hallucinations are treated with coadministration of IV lorazepam, diphenhydramine, and haloperidol. These medications resolve the hallucinations, but his anxiety worsens and he becomes restless. He receives additional doses of IV haloperidol administered in 5 mg increments and reaching a cumulative 12-hour dose of 50 mg. Mr. B continues to report anxiety, so the psychiatry consultation-liaison (C-L) service is called.
[polldaddy:9970907]
The authors’ observations
Determining the cause of Mr. B’s anxiety is challenging because of his prolonged medical course, com
From a medical perspective, in a post-surgical patient treated in the ICU, the consulting practitioner must pay particular attention to delirium. ICU delirium is common—one report indicated that it occurs in 32.3% of ICU patients1—and frequently confused with psychiatric morbidity.2 Identifying delirium as the cause of impairment is important because delirium has potentially modifiable underlying etiologies. Symptomatically, delirium presents as impairment and fluctuation in attention, awareness, and at least one other cognitive domain, with a clear indication that the impairment occurred over a short period of time and represents a departure from baseline.3 In Mr. B’s case, these symptoms have not been excluded and should be considered by the C-L psychiatrists.
In addition to delirium, the C-L team must consider psychiatric comorbidity. Mr. B has no psychiatric history and a sudden first occurrence of hallucinations; therefore, it is unlikely that he has developed a primary psychotic disorder. Because he reported his symptoms had been present only for several days, he would not meet criteria for schizophrenia, which according to DSM-5 criteria require at least 1 month of ≥2 symptoms (including delusions, hallucinations, disorganized speech, disorganized behavior, or negative symptoms) and 6 months of declining function.3 However, although it is improbable, the C-L team must consider a primary psychotic illness, particularly given the potential devastating consequence of being misdiagnosed and mismanaged for an alternative illness. Unlike psychotic disorders, anxiety disorders are significantly more prevalent in the U.S. general population than primary psychotic disorders.4 Furthermore, the prevalence of anxiety disorders increases in the ICU setting; one study found that up to 61% of ICU patients setting experience “anxiety features.”5 Therefore, anxiety disorders and stress disorders should be considered in ICU patients who exhibit psychiatric symptoms.
Clinicians also should consider medication-induced adverse effects. In the ICU, patients are frequently managed on multiple medications, which increase their risk of developing adverse effects and adverse reactions.6 One potential consequence of polypharmacy is delirium, which remains a relevant potential diagnosis for Mr. B.7 Alternative consequences vary by medication and their respective pharmacodynamics. We take into consideration Mr. B’s exposure to high doses of the high-potency antipsychotic agent, haloperidol. Exposure to haloperidol can result in extrapyramidal symptoms, including akathisia,8,9,10 and the rare, but potentially fatal, NMS.11 These reactions can often be distinguished by taking a thorough history and a physical evaluation. In the case of akathisia, the clinician should look for medication exposure, titration, or taper. Most commonly, akathisia occurs secondary to antipsychotic exposure,12 followed by the onset of a combination of subjective symptoms, such as restlessness, anxiety, and irritability, and an objective symptom of increased motor activity.3 NMS, on the other hand, is distinguished by symptoms that include hyperthermia (>38ºC), diaphoresis, severe rigidity, urinary incontinence, vital instability, alterations in mental health status, and elevations in creatine kinase greater than 4-fold the upper limit, usually in the setting of treatment with antipsychotics.3 Nearly all cases of NMS occur within the first 30 days of antipsychotic exposure.3 While, overtly, NMS may appear to be less subtle than akathisia, clinicians should still be weary to rule out this admittedly rare, though potentially lethal diagnosis, especially in an ICU patient, where the diagnosis can be muddied by medical comorbidities that may mask the syndrome.
Continue to: EVALUATION Focus on akathisia
EVALUATION Focus on akathisia
On interview by the C-L team, Mr. B is visibly restless, moving all 4 extremities. He reports increased anxiety and irritability over the past 2 to 3 days. Mr. B states that he is aware of his increased motor movements and can briefly suppress them. However, after several seconds, he again begins spontaneously fidgeting, moving all 4 extremities and shifting from side to side in bed, saying, “I just feel anxious.” He denies having visual hallucinations, and says that the previous hallucinations had spontaneously presented and remitted after surgery. He denies the use of psychotropics for mental illness, prior similar symptoms to this presentation, a family history of mental illness, recent illicit substance use, or excessive alcohol use prior to presentation. This history is corroborated by collateral information from his brother, who was present in the ICU. On physical examination, Mr. B is afebrile and his vital signs are within normal limits. He does not have muscular rigidity or neck dystonia. His laboratory values, including complete blood count, electrolytes, liver function tests, and creatine phosphokinase, are within normal limits.
His medication administration record includes 46 standing agents, 16 “as-needed” agents, and 8 infusions. Several of the standing agents had psychotropic properties; however, the most salient were several opioids, ketamine, midazolam, lorazepam, dexamethasone, haloperidol, and olanzapine.
[polldaddy:9970908]
The authors’ observations
We determined that the most likely diagnosis for Mr. B’s symptoms was medication-induced akathisia secondary to haloperidol. Akathisia, coined by Haskovec in 1901,12,13 is from Greek, meaning an “inability to sit.”12 DSM-5 describes 2 forms of akathisia: medication-induced acute akathisia, and tardive akathisia.3 In the literature, others have described additional classifications, including chronic akathisia, withdrawal akathisia, and pseudoakathisia (Table 13,14-17). In Mr. B’s case, given his sudden development of symptoms and their direct chronologic relationship to antipsychotic treatment, and his combined subjective and objective symptoms, we believed that Mr. B’s symptoms were consistent with medication-induced acute akathisia (MIA). The identification and treatment of this clinical entity is important for several reasons, including reducing patient morbidity and maximizing patient comfort. Additionally, because akathisia has been associated with poor medication adherence, increased agitation/aggression, increased suicidality, and the eventual development of tardive dyskinesia,18 it is a relevant prognostic consideration when deciding to treat a patient with antipsychotics.

Pathophysiologically, we have yet to fully shed light on the exact underpinnings of akathisia. Much of our present knowledge stems from patient response to pharmacologic agents. While dopamine blockade has been linked to akathisia, the exact mechanism is not completely understood. Previous theories linking nigrostriatal pathways have been expanded to include mesocortical and mesolimbic considerations.12,17,18 Similarly surmised from medication effects, the transmitters y-aminobutyric acid, serotonin/5-hydroxytryptamine (5-HT), norepinephrine, and acetylcholine also have been linked to this syndrome, though as of yet, exact gross pathophysiologic mechanisms have not been fully elucidated.12 More recently, Stahl and Loonen19 described a novel mechanism by which they link the shell of the nucleus accumbens to akathisia. In their report, they indicate that the potential reduction in dopaminergic activity, secondary to antipsychotic administration, can result in compensatory noradrenergic activation of the locus coeruleus.19 The increased noradrenergic activity results in the downstream activation of the shell of the nucleus accumbens.19 The activation of the nucleus accumbens shell, which has been linked to unconditioned feeding and fear behavior, can then result in a cascade of effects that would phenotypically present as the syndrome we recognize to be akathisia.19
Numerous etiologies have been linked to MIA. Of these, high-potency antipsychotics are believed to remain the greatest risk factor for akathisia,18 although atypical antipsychotics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, have been linked to the disorder.18,19
Continue to: Regarding antipsychotics...
Regarding antipsychotics, risk factors for akathisia include drug potency, dose, and rapidity of titration.20 All of these factors were relevant in our patient’s case. Risk across antipsychotic classes is not well understood; few head-to-head studies have comparing antipsychotics. However, general estimates suggest a 15% to 40% prevalence in patients exposed to typical antipsychotics, as compared with 0% to 12% exposed to atypical antipsychotics.8 The literature-reported difference in risk, as well as our patient’s comparative difference in exposure to large doses of haloperidol (50 mg) as compared with 1 dose of olanzapine (5 mg), led us to believe his akathisia developed primarily due to his exposure to haloperidol. Conclusively linking his symptoms to haloperidol alone, however, is not possible, and we did consider that olanzapine may in fact have had some effect in worsening Mr. B’s akathisia.
[polldaddy:9970909]
The authors’ observations
While there are reports on the efficacy of various agents in the treatment of akathisia, the most commonly evaluated agents are propranolol, anticholinergics, and benzodiazepines.17, 21
Propranolol is a nonselective beta-adrenergic blocker with numerous indications.17 Despite a 2004 Cochrane Review indicating that there is no evidence in support of central acting beta-blockers for treating akathisia,22 propranolol is not yet recognized as an appropriate treatment.17 The reason for this discrepancy is likely due to the Cochrane Review’s restrictive inclusion criteria, which prevented the analysis of much of the literature.22 In fact, several reports cite evidence for the treatment efficacy of propranolol17 and, to date, some reports continue to advocate for its use as a first-line agent in the treatment of akathisia. Admittedly, besides the Cochrane Review,22 other reports have found propranolol to be ineffective for treating akathisia,23 although these tend to be limited by their population size and generalizability.
As with propranolol, a 2006 Cochrane Review found “no reliable evidence to support or refute” using anticholinergic agents in the treatment of akathisia.24 We suspect that the review’s findings were likely secondary to its strict inclusion criteria.24 In fact, several reports support using anticholinergic agents for treating akathisia.25 Here we focus on benztropine and diphenhydramine.
Two reviews—Blaisdell26 (1994) and Poyurovsky27 (2010)—suggest modest benefits from benztropine, primarily in patients with comorbid Parkinson’s disease. Despite these benefits, head-to-head trials seem to either point to the superiority of propranolol or to no difference between these agents for treating akathisia.28,29 In a review, we only found 1 trial demonstrating benztropine’s superiority over propranolol,23 but this trial was constrained by its small population (6 patients). Therefore, the data suggest that, when indicated, clinicians should lean towards using propranolol for treating akathisia.
Continue to: Diphenhydramine, a first-generation antihistamine...
Diphenhydramine, a first-generation antihistamine with antimuscarinic properties, has been studied for its efficacy in treating metoclopramide-induced akathisia in the emergency setting.30 There are several reports on the efficacy of this agent, including a large randomized study involving 281 patients that found it effective for preventing metoclopramide-induced akathisia.30 Another head-to-head trial reported the benefit of the diphenhydramine vs midazolam.31 Both agents were efficacious for treating akathisia; however, midazolam had a more rapid onset. Despite these positive reports, double-blind trials have found diphenhydramine to be ineffective,17 which suggests propranolol should be the first-line agent, assuming it is not contraindicated.
Benzodiazepines have also been found to be efficacious for treating akathisia. A 1999 Cochrane Review included 2 randomized controlled trials that assessed the efficacy of clonazepam vs placebo for treating akathisia.32 It found evidence of benefit for clonazepam, but questioned the generalizability of these studies.32 This review did not include several other reports that suggest benefits of other benzodiazepines for treating akathisia. Other than clonazepam, reports suggest benefit for diazepam, lorazepam, and midazolam for treating akathisia.17 Despite this evidence and the findings from this Cochrane Review, the literature does not appear to point to clear dominance of these agents over propranolol. Given the safety concerns when prescribing benzodiazepines, it would be prudent to utilize propranolol as a first-line agent for treating akathisia.
Finally, other reports have cited treatment efficacy linked to serotonin 2A receptor (5-HT2A) antagonists (mianserin, mirtazapine, and trazodone), clonidine, gabapentin, amantadine, and other agents.17 If treatment with propranolol is ineffective or contraindicated, clinicians should utilize their clinical judgement in deciding on the use of one agent over another.
OUTCOME Complete resolution
Haloperidol is discontinued and diphenhydramine, 50 mg IV, is administered. (Diphenhydramine was used instead of propranolol due to immediacy of availability.) Most of Mr. B’s signs and symptoms resolve on a repeat interview 3 hours later. He receives another dose of diphenhydramine, 25 mg IV, for persistent mild irritability. By Day 2 of follow-up, his symptoms completely resolve as measured on the Barnes Akathisia Scale33 (Table 2).

Continue to: Bottom Line
Bottom Line
Akathisia is an elusive adverse effect of antipsychotics and can be misdiagnosed as anxiety. Close consideration should be given to potential medical, psychiatric, and drug-related etiologies in patients who have a prolonged medical course, comorbidities, and exposure to multiple pharmacologic agents.
Related Resources
- Factor SA, Leffler JB, Murray CF. Drug-induced movement disorders: a clinical review. Medscape. http://www.medscape.org/viewarticle/586881.
- Marder S, Stroup TS. Pharmacotherapy for schizophrenia: side effect management. UpToDate. https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-side-effect-management.
Drug Brand Names
Amantadine • Symmetrel
Benztropine • Cogentin
Clonazepam • Klonopin
Clonidine • Catapres
Dexamethasone • Decadron
Diazepam • Valium
Diphenhydramine • Benadryl
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Metoclopramide • Reglan
Mianserin • Tolvon
Midazolam • Versed
Mirtazapine • Remeron
Olanzapine • Zyprexa
Propranolol • Inderal
Rivastigmine • Exelon
Trazodone • Oleptro
1. Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: an overview. Ann Intensive Care. 2012;2:49.
2. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. National Alliance on Mental Illness. Mental health by the numbers. https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed March 4, 2018.
5. Jacka MJ, Mitchell N, Perez-Parada J. Incidence and prevalence of anxiety, depression, and post-traumatic stress disorder among critical care patients, families, and practitioners. J Anest & Inten Care Med. 2016;1(1):55555. doi: 10.19080/JAICM.2016.01.555555.
6. Reis AM, Cassiani SH. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625-632.
7. Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33(2):189-203.
8. Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127-148.
9. Van Putten T, Marder SR. Toward a more reliable diagnosis of akathisia. Arch Gen Psychiatry. 1986;43(10):1015-1016.
10. Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463-465.
11. Naganuma H, Fujii I. Incidence and risk factors in neuroleptic malignant syndrome. Acta Psychiatr Scand. 1994;90(6):424-426.
12. Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77-84.
13. Brune M, Sachdev, PS. Ladislav Haskovec and 100 years of akathisia. American Journal of Psychiatry. 2002;159(5):727-727.
14. Havaki-Kontaxaki BJ, Kontaxakis VP, Christodoulou GN. Prevalence and characteristics of patients with pseudoakathisia. Eur Neuropsychopharmacol. 2000;10(5):333-336.
15. Lang AE. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9(2):188-192.
16. Sachdev P. The epidemiology of drug-induced akathisia: Part II. Chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461.
17. Kern DS, Lang AE. Acute akathisia. In: Friedman JH, ed. Medication-induced movement disorders. Cambridge, United Kingdom: Cambridge University Press; 2015:12-24.
18. Adler LA, Angrist B, Reiter S, et al. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl). 1989;97(1):1-11.
19. Stahl SM, Loonen AJM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
20. Sachdev P, Kruk J. Clinical characteristics and predisposing factors in acute drug-induced akathisia. Arch Gen Psychiatry. 1994;51(12):963-974.
21. Laoutidis ZG, Luckhaus C. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(5):823-832.
22. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
23. Sachdev P, Loneragan C. Intravenous benztropine and propranolol challenges in acute neuroleptic-induced akathisia. Clin Neuropharmacol. 1993;16(4):324-331.
24. Lima AR, Weiser KV, Bacaltchuk J, et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(1):CD003727.
25. Fleischhacker WW, Roth SD, Kane JM. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol. 1990;10(1):12-21.
26. Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139-146.
27. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
28. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
29. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Bender B, Friedman B, Davitt M, et al. 118: metoclopramide in the emergency department: a randomized factorial design study to determine the influence of dose and diphenhydramine on akathisia. Ann of Emerg Med. 2008;52(4):S78.
31. Parlak I, Erdur B, Parlak M, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715-721.
32. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 1999;(4):CD001950.
33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676..
1. Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: an overview. Ann Intensive Care. 2012;2:49.
2. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. National Alliance on Mental Illness. Mental health by the numbers. https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed March 4, 2018.
5. Jacka MJ, Mitchell N, Perez-Parada J. Incidence and prevalence of anxiety, depression, and post-traumatic stress disorder among critical care patients, families, and practitioners. J Anest & Inten Care Med. 2016;1(1):55555. doi: 10.19080/JAICM.2016.01.555555.
6. Reis AM, Cassiani SH. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625-632.
7. Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33(2):189-203.
8. Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127-148.
9. Van Putten T, Marder SR. Toward a more reliable diagnosis of akathisia. Arch Gen Psychiatry. 1986;43(10):1015-1016.
10. Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463-465.
11. Naganuma H, Fujii I. Incidence and risk factors in neuroleptic malignant syndrome. Acta Psychiatr Scand. 1994;90(6):424-426.
12. Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77-84.
13. Brune M, Sachdev, PS. Ladislav Haskovec and 100 years of akathisia. American Journal of Psychiatry. 2002;159(5):727-727.
14. Havaki-Kontaxaki BJ, Kontaxakis VP, Christodoulou GN. Prevalence and characteristics of patients with pseudoakathisia. Eur Neuropsychopharmacol. 2000;10(5):333-336.
15. Lang AE. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9(2):188-192.
16. Sachdev P. The epidemiology of drug-induced akathisia: Part II. Chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461.
17. Kern DS, Lang AE. Acute akathisia. In: Friedman JH, ed. Medication-induced movement disorders. Cambridge, United Kingdom: Cambridge University Press; 2015:12-24.
18. Adler LA, Angrist B, Reiter S, et al. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl). 1989;97(1):1-11.
19. Stahl SM, Loonen AJM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
20. Sachdev P, Kruk J. Clinical characteristics and predisposing factors in acute drug-induced akathisia. Arch Gen Psychiatry. 1994;51(12):963-974.
21. Laoutidis ZG, Luckhaus C. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(5):823-832.
22. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
23. Sachdev P, Loneragan C. Intravenous benztropine and propranolol challenges in acute neuroleptic-induced akathisia. Clin Neuropharmacol. 1993;16(4):324-331.
24. Lima AR, Weiser KV, Bacaltchuk J, et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(1):CD003727.
25. Fleischhacker WW, Roth SD, Kane JM. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol. 1990;10(1):12-21.
26. Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139-146.
27. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
28. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
29. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Bender B, Friedman B, Davitt M, et al. 118: metoclopramide in the emergency department: a randomized factorial design study to determine the influence of dose and diphenhydramine on akathisia. Ann of Emerg Med. 2008;52(4):S78.
31. Parlak I, Erdur B, Parlak M, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715-721.
32. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 1999;(4):CD001950.
33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676..