User login
Clinical Session
ELIZABETH BARLOW, MD, MPP, wants all hospitalists to know that upper-extremity DVT (UEDVT) is on the rise. Although most think of it “as a lesser entity,” Dr. Barlow told a jam-packed clinical-track session at HM10 the data show a higher rate of pulmonary em-bolism [PE] occurrence in UEDVT than was first thought. “So I think treating it seriously is important,” she said.
Dr. Barlow, a hospitalist at the University of Chicago Medical Center, outlined the case for greater attention to UEDVT during “Controversies in Anticoagu-lation and Thrombosis. “UEDVTs make up 1% to 4% of all DVTs in the U.S., and nearly 80% of UEDVT cases are provoked.
Much of the rise in—and controversy—UEDVT is due to the increased use of in-dwelling catheters, primarily how long to leave the catheter in place and when to remove it. “Judicious use of catheters is necessary. You should leave it in, if you need it,” Dr. Barlow said, adding that hospitalists should weigh the benefits and risks of PICC lines.
Some of Dr. Barlow’s key take-home points:
- Treat UEDVT seriously;
- Understand there is a higher rate of PE than previously thought;
- Insert central-vein catheters judiciously, and keep them in if you still need them;
- Manage the duration of therapy parallel to that of lower extremity DVT; and
- Routine thrombolytics use isn’t indicated at this time. HM10
ELIZABETH BARLOW, MD, MPP, wants all hospitalists to know that upper-extremity DVT (UEDVT) is on the rise. Although most think of it “as a lesser entity,” Dr. Barlow told a jam-packed clinical-track session at HM10 the data show a higher rate of pulmonary em-bolism [PE] occurrence in UEDVT than was first thought. “So I think treating it seriously is important,” she said.
Dr. Barlow, a hospitalist at the University of Chicago Medical Center, outlined the case for greater attention to UEDVT during “Controversies in Anticoagu-lation and Thrombosis. “UEDVTs make up 1% to 4% of all DVTs in the U.S., and nearly 80% of UEDVT cases are provoked.
Much of the rise in—and controversy—UEDVT is due to the increased use of in-dwelling catheters, primarily how long to leave the catheter in place and when to remove it. “Judicious use of catheters is necessary. You should leave it in, if you need it,” Dr. Barlow said, adding that hospitalists should weigh the benefits and risks of PICC lines.
Some of Dr. Barlow’s key take-home points:
- Treat UEDVT seriously;
- Understand there is a higher rate of PE than previously thought;
- Insert central-vein catheters judiciously, and keep them in if you still need them;
- Manage the duration of therapy parallel to that of lower extremity DVT; and
- Routine thrombolytics use isn’t indicated at this time. HM10
ELIZABETH BARLOW, MD, MPP, wants all hospitalists to know that upper-extremity DVT (UEDVT) is on the rise. Although most think of it “as a lesser entity,” Dr. Barlow told a jam-packed clinical-track session at HM10 the data show a higher rate of pulmonary em-bolism [PE] occurrence in UEDVT than was first thought. “So I think treating it seriously is important,” she said.
Dr. Barlow, a hospitalist at the University of Chicago Medical Center, outlined the case for greater attention to UEDVT during “Controversies in Anticoagu-lation and Thrombosis. “UEDVTs make up 1% to 4% of all DVTs in the U.S., and nearly 80% of UEDVT cases are provoked.
Much of the rise in—and controversy—UEDVT is due to the increased use of in-dwelling catheters, primarily how long to leave the catheter in place and when to remove it. “Judicious use of catheters is necessary. You should leave it in, if you need it,” Dr. Barlow said, adding that hospitalists should weigh the benefits and risks of PICC lines.
Some of Dr. Barlow’s key take-home points:
- Treat UEDVT seriously;
- Understand there is a higher rate of PE than previously thought;
- Insert central-vein catheters judiciously, and keep them in if you still need them;
- Manage the duration of therapy parallel to that of lower extremity DVT; and
- Routine thrombolytics use isn’t indicated at this time. HM10
What Is the Best Therapy for Acute Hepatic Encephalopathy?
Case
A 56-year-old man with a history of cirrhosis, complicated by esophageal varices and ongoing alcohol abuse, is admitted after his wife found him lethargic and disoriented in bed. His wife said he’d been increasingly irritable and agitated, with slurred speech, the past two days. On exam, he is somnolent but arousable; spider telangiectasias and asterixis are noted. Laboratory studies are consistent with chronic liver disease.
What is the best therapy for his acute hepatic encephalopathy?
Overview
Hepatic encephalopathy (HE) describes the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with liver dysfunction. The wide range of neuropsychiatric presentations led to the development of consensus HE classification terminology by the World Congress of Gastroenterology in 2002.
The primary tenet of all HE pathogenesis theories is firmly established: Nitrogenous substances derived from the gut adversely affect brain function. These compounds access the systemic circulation via decreased hepatic function or portal-systemic shunts. In the brain, they alter neurotransmission, which affects consciousness and behavior.
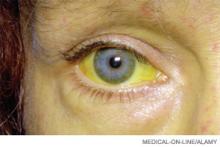
HE patients usually have advanced cirrhosis and, hence, many of the physical findings associated with severe hepatic dysfunction: muscle-wasting, jaundice, ascites, palmar erythema, edema, spider telangiectasias, and fetor hepaticus. Encephalopathy progresses from reversal of the sleep-wake cycle and mild mental status changes to irritability, confusion, and slurred speech.
Advanced neurologic features include asterixis or tongue fasciculations, bradykinesia, hyperreflexia, and ultimately coma. History and laboratory data can reveal a precipitating cause (see Table 2, p. 19). Measurement of ammonia concentration remains controversial. The value may be useful for monitoring the efficacy of ammonia-lowering therapy, but elevated levels are not required to make the diagnosis.
Multiple treatments have been used to manage HE, yet few well-designed randomized trials have assessed efficacy due to challenges inherent in measuring the wide range of neuropsychiatric presentations. Nonetheless, a critical appraisal of available data delineates a rational approach to therapy.
Review of the Data
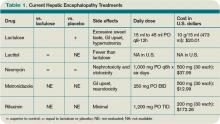
In addition to supportive care and the reversal of any precipitating factors, the treatment of acute HE is aimed at reducing or inhibiting intestinal ammonia production or increasing its removal (see Table 1, left).
Nonabsorbable disaccharides (NAD): Lactulose (beta-galactosidofructose) and lactitol (beta-galactosidosorbitol) are used as first-line agents for the treatment of HE and lead to symptomatic improvement in 67% to 87% of patients.1 They reduce the concentration of ammoniogenic substrates in the colonic lumen in two ways—first, by facilitating bacterial fermentation and secondary organic acid production (lowering colonic pH) and, second, by direct osmotic catharsis.
NAD are administered orally or via nasogastric tube at an initial dose of 45 ml, followed by repeated hourly doses until the patient has a bowel movement. For patients at risk of aspiration, NAD can be administered via enema (300 ml in 700 ml of water) every two hours as needed until mental function improves. Once the risk of aspiration is minimized, NAD can be administered orally and titrated to achieve two to three soft bowel movements daily (the usual oral dosage is 15 ml to 45 ml every eight to 12 hours).
Common side effects of NAD include an excessively sweet taste, flatulence, abdominal cramping, and electrolyte imbalance, particularly hypernatremia, which may further deteriorate mental status.
Als-Nielsen et al demonstrated in a systematic review that NAD were more effective than placebo in improving HE, but NAD had no significant benefit on mortality.1 However, the effect on HE no longer reached statistical significance when the analysis was confined to studies with the highest methodological quality. In a randomized, double-blind comparison, Morgan et al showed that lactitol was more tolerable than lactulose and produced fewer side effects.2 Lactitol is not currently available for use in the U.S.
Antibiotics: Certain oral antibiotics (e.g., neomycin, rifaximin, and metronidazole) reduce urease-producing intestinal bacteria, which results in decreased ammonia production and absorption through the gastrointestinal tract. Antibiotics generally are used in patients who do not tolerate NAD or who remain symptomatic despite NAD. The combined use of NAD and antibiotics is a subject of significant clinical relevance, though data are limited.
Neomycin is approved by the FDA for treatment of acute HE. It can be administered orally at a dose of 1,000 mg every six hours for up to six days. A randomized, controlled trial of neomycin versus placebo in 39 patients with acute HE demonstrated no significant difference in time to symptom improvement.3 Another study of 80 patients receiving neomycin and lactulose demonstrated no benefit against placebo, though some data suggest that the combination of lactulose and neomycin therapy might be more effective than either agent alone against placebo.4
Rifaximin was granted an orphan drug designation by the FDA for use in HE cases and has been compared with NAD. The recommended dose is 1,200 mg three times per day. It has minimal side effects and no reported drug interactions. A study of rifaximin versus lactitol administered for five to 10 days showed approximately 80% symptomatic improvement in both groups.5 Another trial demonstrated significantly greater improvement in blood ammonia concentrations, electroencephalographic (EEG) abnormalities, and mental status with rifaximin compared with lactulose.6 Studies comparing rifaximin and lactulose, either alone or in combination, have demonstrated that rifaximin is at least similar to lactulose, and in some cases superior in reversing encephalopathy, with better tolerability reported in the antibiotic group.7
Metronidazole is not approved by the FDA for the treatment of HE but has been evaluated. The recommended oral dose of metronidazole for chronic use is 250 mg twice per day. Prolonged administration of metronidazole can be associated with gastrointestinal disturbance and neurotoxicity. In a report of 11 HE patients with mild to moderate symptoms and seven chronically affected HE cirrhotic patients treated with metronidazole for one week, Morgan and colleagues showed metronidazole to be as effective as neomycin.8
Diet: Historically, patients with HE were placed on protein-restricted diets to reduce the production of intestinal ammonia. Recent evidence suggests that excessive restriction can raise serum ammonia levels as a result of reduced muscular ammonia metabolism. Furthermore, restricting protein intake worsens nutritional status and does not improve the outcome.9
In patients with established cirrhosis, the minimal daily dietary protein intake required to maintain nitrogen balance is 0.8 g/kg to 1.0 g/kg. At this time, a normoprotein diet for HE patients is considered the standard of care.
Other agents: L-ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, provides crucial substrates for glutamine and urea synthesis—key pathways in deammonation. In patients with cirrhosis and HE, oral LOLA reduces serum ammonia and improves clinical manifestations of HE, including EEG abnormalities.10 LOLA, however, is not available in the U.S.
Sodium benzoate might be beneficial in the treatment of acute HE; it increases urinary excretion of ammonia. A prospective, randomized, double-blind study of 74 patients with acute HE found that treatment with sodium benzoate 5 g twice daily, compared with lactulose, resulted in equivalent improvements in encephalopathy. There was no placebo group.11 Routine use has been limited due to concerns regarding sodium load and increased frequency of adverse gastrointestinal symptoms, particularly nausea.
Flumazenil, a short-acting benzodiazepine receptor antagonist, has been utilized on the basis of observed increases in benzodiazepine receptor activation among cirrhotic HE patients. In a systematic review of 12 controlled trials (765 patients), Als-Nielsen and colleagues found flumazenil to be associated with significant improvement.12 Flumazenil is not used routinely as an HE therapy because of significant side effects, namely seizures, nausea, vomiting, dizziness, and agitation.
Such therapies as L-carnitine, branched amino acids (BCAA), probiotics, bromocriptine, acarbose, and zinc are among the many experimental agents currently under evaluation. Few have been tested in clinical trials.
Back to the Case
Our patient has severe HE manifested by worsening somnolence. It is postulated that ongoing alcohol abuse led to medication nonadherence, precipitating his HE, but as HE has many causes, a complete workup for infection and metabolic derangement is performed. However, it is unrevealing.
The best initial action is the prescription of lactulose, the mainstay of HE therapy. Given concern for aspiration in patients with somnolence, a feeding tube is placed for administration. The lactulose dosage will be titrated to achieve two to three soft stools per day. If the patient remains symptomatic or develops significant side effects on lactulose, the addition of an antibiotic is recommended. Neomycin, a low-cost medicine approved by the FDA for HE treatment, is a good choice. The patient will be maintained on a normal protein diet.
Bottom Line
The first-line agents used to treat episodes of acute HE are the nonabsorbable disaccharides, lactulose or lactitol. TH
Dr. Shoeb is a resident in the Department of Medicine at the University of Washington in Seattle. Dr. Best is assistant professor of medicine in the Division of General Internal Medicine at the University of Washington.
References
- Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
- Morgan MY, Hawley KE. Lactitol v. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987; 7(6):1278-1284.
- Blanc P, Daurès JP, Liautard J, et al. Lactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trial. Gastroenterol Clin Biol. 1994;18(12):1063-1068.
- Mas A, Rodés J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-58.
- Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399-407.
- Massa P, Vallerino E, Dodero M. Treatment of hepatic encephalopathy with rifaximin: double blind, double dummy study versus lactulose. Eur J Clin Res. 1993;4:7-18.
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203-208.
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1-7.
- Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
- Poo JL, Gongora J, Sánchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006;5(4):281-288.
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16(16):138-144.
- Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798.
Case
A 56-year-old man with a history of cirrhosis, complicated by esophageal varices and ongoing alcohol abuse, is admitted after his wife found him lethargic and disoriented in bed. His wife said he’d been increasingly irritable and agitated, with slurred speech, the past two days. On exam, he is somnolent but arousable; spider telangiectasias and asterixis are noted. Laboratory studies are consistent with chronic liver disease.
What is the best therapy for his acute hepatic encephalopathy?
Overview
Hepatic encephalopathy (HE) describes the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with liver dysfunction. The wide range of neuropsychiatric presentations led to the development of consensus HE classification terminology by the World Congress of Gastroenterology in 2002.
The primary tenet of all HE pathogenesis theories is firmly established: Nitrogenous substances derived from the gut adversely affect brain function. These compounds access the systemic circulation via decreased hepatic function or portal-systemic shunts. In the brain, they alter neurotransmission, which affects consciousness and behavior.
HE patients usually have advanced cirrhosis and, hence, many of the physical findings associated with severe hepatic dysfunction: muscle-wasting, jaundice, ascites, palmar erythema, edema, spider telangiectasias, and fetor hepaticus. Encephalopathy progresses from reversal of the sleep-wake cycle and mild mental status changes to irritability, confusion, and slurred speech.
Advanced neurologic features include asterixis or tongue fasciculations, bradykinesia, hyperreflexia, and ultimately coma. History and laboratory data can reveal a precipitating cause (see Table 2, p. 19). Measurement of ammonia concentration remains controversial. The value may be useful for monitoring the efficacy of ammonia-lowering therapy, but elevated levels are not required to make the diagnosis.
Multiple treatments have been used to manage HE, yet few well-designed randomized trials have assessed efficacy due to challenges inherent in measuring the wide range of neuropsychiatric presentations. Nonetheless, a critical appraisal of available data delineates a rational approach to therapy.
Review of the Data
In addition to supportive care and the reversal of any precipitating factors, the treatment of acute HE is aimed at reducing or inhibiting intestinal ammonia production or increasing its removal (see Table 1, left).
Nonabsorbable disaccharides (NAD): Lactulose (beta-galactosidofructose) and lactitol (beta-galactosidosorbitol) are used as first-line agents for the treatment of HE and lead to symptomatic improvement in 67% to 87% of patients.1 They reduce the concentration of ammoniogenic substrates in the colonic lumen in two ways—first, by facilitating bacterial fermentation and secondary organic acid production (lowering colonic pH) and, second, by direct osmotic catharsis.
NAD are administered orally or via nasogastric tube at an initial dose of 45 ml, followed by repeated hourly doses until the patient has a bowel movement. For patients at risk of aspiration, NAD can be administered via enema (300 ml in 700 ml of water) every two hours as needed until mental function improves. Once the risk of aspiration is minimized, NAD can be administered orally and titrated to achieve two to three soft bowel movements daily (the usual oral dosage is 15 ml to 45 ml every eight to 12 hours).
Common side effects of NAD include an excessively sweet taste, flatulence, abdominal cramping, and electrolyte imbalance, particularly hypernatremia, which may further deteriorate mental status.
Als-Nielsen et al demonstrated in a systematic review that NAD were more effective than placebo in improving HE, but NAD had no significant benefit on mortality.1 However, the effect on HE no longer reached statistical significance when the analysis was confined to studies with the highest methodological quality. In a randomized, double-blind comparison, Morgan et al showed that lactitol was more tolerable than lactulose and produced fewer side effects.2 Lactitol is not currently available for use in the U.S.
Antibiotics: Certain oral antibiotics (e.g., neomycin, rifaximin, and metronidazole) reduce urease-producing intestinal bacteria, which results in decreased ammonia production and absorption through the gastrointestinal tract. Antibiotics generally are used in patients who do not tolerate NAD or who remain symptomatic despite NAD. The combined use of NAD and antibiotics is a subject of significant clinical relevance, though data are limited.
Neomycin is approved by the FDA for treatment of acute HE. It can be administered orally at a dose of 1,000 mg every six hours for up to six days. A randomized, controlled trial of neomycin versus placebo in 39 patients with acute HE demonstrated no significant difference in time to symptom improvement.3 Another study of 80 patients receiving neomycin and lactulose demonstrated no benefit against placebo, though some data suggest that the combination of lactulose and neomycin therapy might be more effective than either agent alone against placebo.4
Rifaximin was granted an orphan drug designation by the FDA for use in HE cases and has been compared with NAD. The recommended dose is 1,200 mg three times per day. It has minimal side effects and no reported drug interactions. A study of rifaximin versus lactitol administered for five to 10 days showed approximately 80% symptomatic improvement in both groups.5 Another trial demonstrated significantly greater improvement in blood ammonia concentrations, electroencephalographic (EEG) abnormalities, and mental status with rifaximin compared with lactulose.6 Studies comparing rifaximin and lactulose, either alone or in combination, have demonstrated that rifaximin is at least similar to lactulose, and in some cases superior in reversing encephalopathy, with better tolerability reported in the antibiotic group.7
Metronidazole is not approved by the FDA for the treatment of HE but has been evaluated. The recommended oral dose of metronidazole for chronic use is 250 mg twice per day. Prolonged administration of metronidazole can be associated with gastrointestinal disturbance and neurotoxicity. In a report of 11 HE patients with mild to moderate symptoms and seven chronically affected HE cirrhotic patients treated with metronidazole for one week, Morgan and colleagues showed metronidazole to be as effective as neomycin.8
Diet: Historically, patients with HE were placed on protein-restricted diets to reduce the production of intestinal ammonia. Recent evidence suggests that excessive restriction can raise serum ammonia levels as a result of reduced muscular ammonia metabolism. Furthermore, restricting protein intake worsens nutritional status and does not improve the outcome.9
In patients with established cirrhosis, the minimal daily dietary protein intake required to maintain nitrogen balance is 0.8 g/kg to 1.0 g/kg. At this time, a normoprotein diet for HE patients is considered the standard of care.
Other agents: L-ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, provides crucial substrates for glutamine and urea synthesis—key pathways in deammonation. In patients with cirrhosis and HE, oral LOLA reduces serum ammonia and improves clinical manifestations of HE, including EEG abnormalities.10 LOLA, however, is not available in the U.S.
Sodium benzoate might be beneficial in the treatment of acute HE; it increases urinary excretion of ammonia. A prospective, randomized, double-blind study of 74 patients with acute HE found that treatment with sodium benzoate 5 g twice daily, compared with lactulose, resulted in equivalent improvements in encephalopathy. There was no placebo group.11 Routine use has been limited due to concerns regarding sodium load and increased frequency of adverse gastrointestinal symptoms, particularly nausea.
Flumazenil, a short-acting benzodiazepine receptor antagonist, has been utilized on the basis of observed increases in benzodiazepine receptor activation among cirrhotic HE patients. In a systematic review of 12 controlled trials (765 patients), Als-Nielsen and colleagues found flumazenil to be associated with significant improvement.12 Flumazenil is not used routinely as an HE therapy because of significant side effects, namely seizures, nausea, vomiting, dizziness, and agitation.
Such therapies as L-carnitine, branched amino acids (BCAA), probiotics, bromocriptine, acarbose, and zinc are among the many experimental agents currently under evaluation. Few have been tested in clinical trials.
Back to the Case
Our patient has severe HE manifested by worsening somnolence. It is postulated that ongoing alcohol abuse led to medication nonadherence, precipitating his HE, but as HE has many causes, a complete workup for infection and metabolic derangement is performed. However, it is unrevealing.
The best initial action is the prescription of lactulose, the mainstay of HE therapy. Given concern for aspiration in patients with somnolence, a feeding tube is placed for administration. The lactulose dosage will be titrated to achieve two to three soft stools per day. If the patient remains symptomatic or develops significant side effects on lactulose, the addition of an antibiotic is recommended. Neomycin, a low-cost medicine approved by the FDA for HE treatment, is a good choice. The patient will be maintained on a normal protein diet.
Bottom Line
The first-line agents used to treat episodes of acute HE are the nonabsorbable disaccharides, lactulose or lactitol. TH
Dr. Shoeb is a resident in the Department of Medicine at the University of Washington in Seattle. Dr. Best is assistant professor of medicine in the Division of General Internal Medicine at the University of Washington.
References
- Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
- Morgan MY, Hawley KE. Lactitol v. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987; 7(6):1278-1284.
- Blanc P, Daurès JP, Liautard J, et al. Lactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trial. Gastroenterol Clin Biol. 1994;18(12):1063-1068.
- Mas A, Rodés J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-58.
- Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399-407.
- Massa P, Vallerino E, Dodero M. Treatment of hepatic encephalopathy with rifaximin: double blind, double dummy study versus lactulose. Eur J Clin Res. 1993;4:7-18.
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203-208.
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1-7.
- Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
- Poo JL, Gongora J, Sánchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006;5(4):281-288.
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16(16):138-144.
- Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798.
Case
A 56-year-old man with a history of cirrhosis, complicated by esophageal varices and ongoing alcohol abuse, is admitted after his wife found him lethargic and disoriented in bed. His wife said he’d been increasingly irritable and agitated, with slurred speech, the past two days. On exam, he is somnolent but arousable; spider telangiectasias and asterixis are noted. Laboratory studies are consistent with chronic liver disease.
What is the best therapy for his acute hepatic encephalopathy?
Overview
Hepatic encephalopathy (HE) describes the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with liver dysfunction. The wide range of neuropsychiatric presentations led to the development of consensus HE classification terminology by the World Congress of Gastroenterology in 2002.
The primary tenet of all HE pathogenesis theories is firmly established: Nitrogenous substances derived from the gut adversely affect brain function. These compounds access the systemic circulation via decreased hepatic function or portal-systemic shunts. In the brain, they alter neurotransmission, which affects consciousness and behavior.
HE patients usually have advanced cirrhosis and, hence, many of the physical findings associated with severe hepatic dysfunction: muscle-wasting, jaundice, ascites, palmar erythema, edema, spider telangiectasias, and fetor hepaticus. Encephalopathy progresses from reversal of the sleep-wake cycle and mild mental status changes to irritability, confusion, and slurred speech.
Advanced neurologic features include asterixis or tongue fasciculations, bradykinesia, hyperreflexia, and ultimately coma. History and laboratory data can reveal a precipitating cause (see Table 2, p. 19). Measurement of ammonia concentration remains controversial. The value may be useful for monitoring the efficacy of ammonia-lowering therapy, but elevated levels are not required to make the diagnosis.
Multiple treatments have been used to manage HE, yet few well-designed randomized trials have assessed efficacy due to challenges inherent in measuring the wide range of neuropsychiatric presentations. Nonetheless, a critical appraisal of available data delineates a rational approach to therapy.
Review of the Data
In addition to supportive care and the reversal of any precipitating factors, the treatment of acute HE is aimed at reducing or inhibiting intestinal ammonia production or increasing its removal (see Table 1, left).
Nonabsorbable disaccharides (NAD): Lactulose (beta-galactosidofructose) and lactitol (beta-galactosidosorbitol) are used as first-line agents for the treatment of HE and lead to symptomatic improvement in 67% to 87% of patients.1 They reduce the concentration of ammoniogenic substrates in the colonic lumen in two ways—first, by facilitating bacterial fermentation and secondary organic acid production (lowering colonic pH) and, second, by direct osmotic catharsis.
NAD are administered orally or via nasogastric tube at an initial dose of 45 ml, followed by repeated hourly doses until the patient has a bowel movement. For patients at risk of aspiration, NAD can be administered via enema (300 ml in 700 ml of water) every two hours as needed until mental function improves. Once the risk of aspiration is minimized, NAD can be administered orally and titrated to achieve two to three soft bowel movements daily (the usual oral dosage is 15 ml to 45 ml every eight to 12 hours).
Common side effects of NAD include an excessively sweet taste, flatulence, abdominal cramping, and electrolyte imbalance, particularly hypernatremia, which may further deteriorate mental status.
Als-Nielsen et al demonstrated in a systematic review that NAD were more effective than placebo in improving HE, but NAD had no significant benefit on mortality.1 However, the effect on HE no longer reached statistical significance when the analysis was confined to studies with the highest methodological quality. In a randomized, double-blind comparison, Morgan et al showed that lactitol was more tolerable than lactulose and produced fewer side effects.2 Lactitol is not currently available for use in the U.S.
Antibiotics: Certain oral antibiotics (e.g., neomycin, rifaximin, and metronidazole) reduce urease-producing intestinal bacteria, which results in decreased ammonia production and absorption through the gastrointestinal tract. Antibiotics generally are used in patients who do not tolerate NAD or who remain symptomatic despite NAD. The combined use of NAD and antibiotics is a subject of significant clinical relevance, though data are limited.
Neomycin is approved by the FDA for treatment of acute HE. It can be administered orally at a dose of 1,000 mg every six hours for up to six days. A randomized, controlled trial of neomycin versus placebo in 39 patients with acute HE demonstrated no significant difference in time to symptom improvement.3 Another study of 80 patients receiving neomycin and lactulose demonstrated no benefit against placebo, though some data suggest that the combination of lactulose and neomycin therapy might be more effective than either agent alone against placebo.4
Rifaximin was granted an orphan drug designation by the FDA for use in HE cases and has been compared with NAD. The recommended dose is 1,200 mg three times per day. It has minimal side effects and no reported drug interactions. A study of rifaximin versus lactitol administered for five to 10 days showed approximately 80% symptomatic improvement in both groups.5 Another trial demonstrated significantly greater improvement in blood ammonia concentrations, electroencephalographic (EEG) abnormalities, and mental status with rifaximin compared with lactulose.6 Studies comparing rifaximin and lactulose, either alone or in combination, have demonstrated that rifaximin is at least similar to lactulose, and in some cases superior in reversing encephalopathy, with better tolerability reported in the antibiotic group.7
Metronidazole is not approved by the FDA for the treatment of HE but has been evaluated. The recommended oral dose of metronidazole for chronic use is 250 mg twice per day. Prolonged administration of metronidazole can be associated with gastrointestinal disturbance and neurotoxicity. In a report of 11 HE patients with mild to moderate symptoms and seven chronically affected HE cirrhotic patients treated with metronidazole for one week, Morgan and colleagues showed metronidazole to be as effective as neomycin.8
Diet: Historically, patients with HE were placed on protein-restricted diets to reduce the production of intestinal ammonia. Recent evidence suggests that excessive restriction can raise serum ammonia levels as a result of reduced muscular ammonia metabolism. Furthermore, restricting protein intake worsens nutritional status and does not improve the outcome.9
In patients with established cirrhosis, the minimal daily dietary protein intake required to maintain nitrogen balance is 0.8 g/kg to 1.0 g/kg. At this time, a normoprotein diet for HE patients is considered the standard of care.
Other agents: L-ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, provides crucial substrates for glutamine and urea synthesis—key pathways in deammonation. In patients with cirrhosis and HE, oral LOLA reduces serum ammonia and improves clinical manifestations of HE, including EEG abnormalities.10 LOLA, however, is not available in the U.S.
Sodium benzoate might be beneficial in the treatment of acute HE; it increases urinary excretion of ammonia. A prospective, randomized, double-blind study of 74 patients with acute HE found that treatment with sodium benzoate 5 g twice daily, compared with lactulose, resulted in equivalent improvements in encephalopathy. There was no placebo group.11 Routine use has been limited due to concerns regarding sodium load and increased frequency of adverse gastrointestinal symptoms, particularly nausea.
Flumazenil, a short-acting benzodiazepine receptor antagonist, has been utilized on the basis of observed increases in benzodiazepine receptor activation among cirrhotic HE patients. In a systematic review of 12 controlled trials (765 patients), Als-Nielsen and colleagues found flumazenil to be associated with significant improvement.12 Flumazenil is not used routinely as an HE therapy because of significant side effects, namely seizures, nausea, vomiting, dizziness, and agitation.
Such therapies as L-carnitine, branched amino acids (BCAA), probiotics, bromocriptine, acarbose, and zinc are among the many experimental agents currently under evaluation. Few have been tested in clinical trials.
Back to the Case
Our patient has severe HE manifested by worsening somnolence. It is postulated that ongoing alcohol abuse led to medication nonadherence, precipitating his HE, but as HE has many causes, a complete workup for infection and metabolic derangement is performed. However, it is unrevealing.
The best initial action is the prescription of lactulose, the mainstay of HE therapy. Given concern for aspiration in patients with somnolence, a feeding tube is placed for administration. The lactulose dosage will be titrated to achieve two to three soft stools per day. If the patient remains symptomatic or develops significant side effects on lactulose, the addition of an antibiotic is recommended. Neomycin, a low-cost medicine approved by the FDA for HE treatment, is a good choice. The patient will be maintained on a normal protein diet.
Bottom Line
The first-line agents used to treat episodes of acute HE are the nonabsorbable disaccharides, lactulose or lactitol. TH
Dr. Shoeb is a resident in the Department of Medicine at the University of Washington in Seattle. Dr. Best is assistant professor of medicine in the Division of General Internal Medicine at the University of Washington.
References
- Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
- Morgan MY, Hawley KE. Lactitol v. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987; 7(6):1278-1284.
- Blanc P, Daurès JP, Liautard J, et al. Lactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trial. Gastroenterol Clin Biol. 1994;18(12):1063-1068.
- Mas A, Rodés J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-58.
- Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399-407.
- Massa P, Vallerino E, Dodero M. Treatment of hepatic encephalopathy with rifaximin: double blind, double dummy study versus lactulose. Eur J Clin Res. 1993;4:7-18.
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203-208.
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1-7.
- Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
- Poo JL, Gongora J, Sánchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006;5(4):281-288.
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16(16):138-144.
- Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798.
Pediatric In the Literature
Clinical question: What is the incidence of apnea in infants hospitalized with respiratory syncytial virus (RSV) bronchiolitis?
Background: Apnea is a known and reported complication of RSV infection in infants. In clinical practice, this relationship could be the basis for admission despite a lack of symptoms that would otherwise necessitate hospitalization. The exact nature of this association remains unclear, specifically with respect to incidence and risk factors for apnea.
Study design: Systematic chart review.
Synopsis: A literature search was conducted using a combination of the terms “apnea” (or “apnoea”), “bronchiolitis,” “respiratory syncytial virus” and/or “lower respiratory tract infection.” Studies were included if they reported apnea rates for a consecutive cohort of hospitalized infants. Thirteen studies involving 5,575 patients were reviewed.
Rates of apnea ranged from 1.2% to 23.8%. Infants of younger, postconceptional age (≤44 weeks) and pre-term infants were at greater risk for apnea. Term infants without serious underlying illness appeared to have a <1% risk of apnea, based on the most recent studies.
A consistent finding of this review was the heterogeneity of the data in the included studies. Definitions of apnea varied, were broad, and included subjective criteria. Age stratification was infrequent. Inclusion and exclusion criteria were variable with respect to age cutoffs and relevant comorbidities. Future research will need to carefully delineate all of these potential confounding variables.
Bottom line: While rates of apnea in RSV bronchiolitis are difficult to quantify, there appears to be an association with younger, postconceptional age and pre-term birth.
Citation: Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. J Pediatr. 2009;155(5):728-733.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the incidence of apnea in infants hospitalized with respiratory syncytial virus (RSV) bronchiolitis?
Background: Apnea is a known and reported complication of RSV infection in infants. In clinical practice, this relationship could be the basis for admission despite a lack of symptoms that would otherwise necessitate hospitalization. The exact nature of this association remains unclear, specifically with respect to incidence and risk factors for apnea.
Study design: Systematic chart review.
Synopsis: A literature search was conducted using a combination of the terms “apnea” (or “apnoea”), “bronchiolitis,” “respiratory syncytial virus” and/or “lower respiratory tract infection.” Studies were included if they reported apnea rates for a consecutive cohort of hospitalized infants. Thirteen studies involving 5,575 patients were reviewed.
Rates of apnea ranged from 1.2% to 23.8%. Infants of younger, postconceptional age (≤44 weeks) and pre-term infants were at greater risk for apnea. Term infants without serious underlying illness appeared to have a <1% risk of apnea, based on the most recent studies.
A consistent finding of this review was the heterogeneity of the data in the included studies. Definitions of apnea varied, were broad, and included subjective criteria. Age stratification was infrequent. Inclusion and exclusion criteria were variable with respect to age cutoffs and relevant comorbidities. Future research will need to carefully delineate all of these potential confounding variables.
Bottom line: While rates of apnea in RSV bronchiolitis are difficult to quantify, there appears to be an association with younger, postconceptional age and pre-term birth.
Citation: Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. J Pediatr. 2009;155(5):728-733.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the incidence of apnea in infants hospitalized with respiratory syncytial virus (RSV) bronchiolitis?
Background: Apnea is a known and reported complication of RSV infection in infants. In clinical practice, this relationship could be the basis for admission despite a lack of symptoms that would otherwise necessitate hospitalization. The exact nature of this association remains unclear, specifically with respect to incidence and risk factors for apnea.
Study design: Systematic chart review.
Synopsis: A literature search was conducted using a combination of the terms “apnea” (or “apnoea”), “bronchiolitis,” “respiratory syncytial virus” and/or “lower respiratory tract infection.” Studies were included if they reported apnea rates for a consecutive cohort of hospitalized infants. Thirteen studies involving 5,575 patients were reviewed.
Rates of apnea ranged from 1.2% to 23.8%. Infants of younger, postconceptional age (≤44 weeks) and pre-term infants were at greater risk for apnea. Term infants without serious underlying illness appeared to have a <1% risk of apnea, based on the most recent studies.
A consistent finding of this review was the heterogeneity of the data in the included studies. Definitions of apnea varied, were broad, and included subjective criteria. Age stratification was infrequent. Inclusion and exclusion criteria were variable with respect to age cutoffs and relevant comorbidities. Future research will need to carefully delineate all of these potential confounding variables.
Bottom line: While rates of apnea in RSV bronchiolitis are difficult to quantify, there appears to be an association with younger, postconceptional age and pre-term birth.
Citation: Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. J Pediatr. 2009;155(5):728-733.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- Predictors of readmission for patients with CAP.
- High-dose statins vs. lipid-lowering therapy combinations
- Catheter retention and risks of reinfection in patients with coagulase-negative staph
- Stenting vs. medical management of renal-artery stenosis
- Dabigatran for VTE
- Surgical mask vs. N95 respirator for influenza prevention
- Hospitalization and the risk of long-term cognitive decline
- Maturation of rapid-response teams and outcomes
Commonly Available Clinical Variables Predict 30-Day Readmissions for Community-Acquired Pneumonia
Clinical question: What are the risk factors for 30-day readmission in patients hospitalized for community-acquired pneumonia (CAP)?
Background: CAP is a common admission diagnosis associated with significant morbidity, mortality, and resource utilization. While prior data suggested that patients who survive a hospitalization for CAP are particularly vulnerable to readmission, few studies have examined the risk factors for readmission in this population.
Study design: Prospective, observational study.
Setting: A 400-bed teaching hospital in northern Spain.
Synopsis: From 2003 to 2005, this study consecutively enrolled 1,117 patients who were discharged after hospitalization for CAP. Eighty-one patients (7.2%) were readmitted within 30 days of discharge; 29 (35.8%) of these patients were rehospitalized for pneumonia-related causes.
Variables associated with pneumonia-related rehospitalization were treatment failure (HR 2.9; 95% CI, 1.2-6.8) and one or more instability factors at hospital discharge—for example, vital-sign abnormalities or inability to take food or medications by mouth (HR 2.8; 95% CI, 1.3-6.2). Variables associated with readmission unrelated to pneumonia were age greater than 65 years (HR 4.5; 95% CI, 1.4-14.7), Charlson comorbidity index greater than 2 (HR 1.9; 95% CI, 1.0-3.4), and decompensated comorbidities during index hospitalization.
Patients with at least two of the above risk factors were at a significantly higher risk for 30-day hospital readmission (HR 3.37; 95% CI, 2.08-5.46).
Bottom line: The risk factors for readmission after hospitalization for CAP differed between the groups with readmissions related to pneumonia versus other causes. Patients at high risk for readmission can be identified using easily available clinical variables.
Citation: Capelastegui A, España Yandiola PP, Quintana JM, et al. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest. 2009;136(4): 1079-1085.
Combinations of Lipid-Lowering Agents No More Effective than High-Dose Statin Monotherapy
Clinical question: Is high-dose statin monotherapy better than combinations of lipid-lowering agents for dyslipidemia in adults at high risk for coronary artery disease?
Background: While current guidelines support the benefits of aggressive lipid targets, there is little to guide physicians as to the optimal strategy for attaining target lipid levels.
Study design: Systematic review.
Setting: North America, Europe, and Asia.
Synopsis: Very-low-strength evidence showed that statin-ezetimibe (two trials; N=439) and statin-fibrate (one trial; N=166) combinations did not reduce mortality more than high-dose statin monotherapy. No trial data were found comparing the effect of these two strategies on secondary endpoints, including myocardial infarction, stroke, or revascularization.
Two trials (N=295) suggested lower-target lipid levels were more often achieved with statin-ezetimibe combination therapy than with high-dose statin monotherapy (OR 7.21; 95% CI, 4.30-12.08).
Limitations of this systematic review include the small number of studies directly comparing the two strategies, the short duration of most of the studies included, the focus on surrogate outcomes, and the heterogeneity of the study populations’ risk for coronary artery disease. Few studies were available comparing combination therapies other than statin-ezetimibe.
Bottom line: Limited evidence suggests that the combination of a statin with another lipid-lowering agent does not improve clinical outcomes when compared with high-dose statin monotherapy. Low-quality evidence suggests that lower-target lipid levels were more often reached with statin-ezetimibe combination therapy than with high-dose statin monotherapy.
Citation: Sharma M, Ansari MT, Abou-Setta AM, et al. Systematic review: comparative effectiveness and harms of combination therapy and monotherapy for dyslipidemia. Ann Intern Med. 2009;151(9):622-630.
Catheter Retention in Catheter-Related Coagulase-Negative Staphylococcal Bacteremia Is a Significant Risk Factor for Recurrent Infection
Clinical question: Should central venous catheters (CVC) be removed in patients with coagulase-negative staphylococcal catheter-related bloodstream infections (CRBSI)?
Background: Current guidelines for the management of coagulase-negative staphylococcal CRBSI do not recommend routine removal of the CVC, but are based on studies that did not use a strict definition of coagulase-negative staphylococcal CRBSI. Additionally, the studies did not look explicitly at the risk of recurrent infection.
Study design: Retrospective chart review.
Setting: Single academic medical center.
Synopsis: The study retrospectively evaluated 188 patients with coagulase-negative staphylococcal CRBSI. Immediate resolution of the infection was not influenced by the management of the CVC (retention vs. removal or exchange). However, using the multiple logistic regression technique, patients with catheter retention were found to be 6.6 times (95% CI, 1.8-23.9 times) more likely to have recurrence compared with those patients whose catheter was removed or exchanged.
Bottom line: While CVC management does not appear to have an impact on the acute resolution of infection, catheter retention is a significant risk factor for recurrent bacteremia.
Citation: Raad I, Kassar R, Ghannam D, Chaftari AM, Hachem R, Jiang Y. Management of the catheter in documented catheter-related coagulase-negative staphylococcal bacteremia: remove or retain? Clin Infect Dis. 2009;49(8):1187-1194.
Revascularization Offers No Benefit over Medical Therapy for Renal-Artery Stenosis
Clinical question: Does revascularization plus medical therapy compared with medical therapy alone improve outcomes in patients with renal-artery stenosis?
Background: Renal-artery stenosis is associated with significant hypertension and renal dysfunction. Revascularization for atherosclerotic renal-artery stenosis can improve artery patency, but it remains unclear if it provides clinical benefit in terms of preserving renal function or reducing overall mortality.
Study design: Randomized, controlled trial.
Setting: Fifty-seven outpatient sites in the United Kingdom, Australia, and New Zealand.
Synopsis: The study randomized 806 patients with renal-artery stenosis to receive either medical therapy alone (N=403) or medical management plus endovascular revascularization (N=403).
The majority of the patients who underwent revascularization (95%) received a stent.
The data show no significant difference between the two groups in the rate of progression of renal dysfunction, systolic blood pressure, rates of adverse renal and cardiovascular events, and overall survival. Of the 359 patients who underwent revascularization, 23 (6%) experienced serious complications from the procedure, including two deaths and three cases of amputated toes or limbs.
The primary limitation of this trial is the population studied. The trial only included subjects for whom revascularization offered uncertain clinical benefits, according to their doctor. Those subjects for whom revascularization offered certain clinical benefits, as noted by their primary-care physician (PCP), were excluded from the study. Examples include patients presenting with rapidly progressive renal dysfunction or pulmonary edema thought to be a result of renal-artery stenosis.
Bottom line: Revascularization provides no benefit to most patients with renal-artery stenosis, and is associated with some risk.
Citation: ASTRAL investigators, Wheatley K, Ives N, et al. Revascularization versus medical therapy for renal-artery stenosis. N Eng J Med. 2009;361(20):1953-1962.
Dabigatran as Effective as Warfarin in Treatment of Acute VTE
Clinical question: Is dabigatran a safe and effective alternative to warfarin for treatment of acute VTE?
Background: Parenteral anticoagulation followed by warfarin is the standard of care for acute VTE. Warfarin requires frequent monitoring and has numerous drug and food interactions. Dabigatran, which the FDA has yet to approve for use in the U.S., is an oral direct thrombin inhibitor that does not require laboratory monitoring. The role of dabigatran in acute VTE has not been evaluated.
Study design: Randomized, double-blind, noninferiority trial.
Setting: Two hundred twenty-two clinical centers in 29 countries.
Synopsis: This study randomized 2,564 patients with documented VTE (either DVT or pulmonary embolism [PE]) to receive dabigatran 150mg twice daily or warfarin after at least five days of a parenteral anticoagulant. Warfarin was dose-adjusted to an INR goal of 2.0-3.0. The primary outcome was incidence of recurrent VTE and related deaths at six months.
A total of 2.4% of patients assigned to dabigatran and 2.1% of patients assigned to warfarin had recurrent VTE (HR 1.10; 95% CI, 0.8-1.5), which met criteria for noninferiority. Major bleeding occurred in 1.6% of patients assigned to dabigatran and 1.9% assigned to warfarin (HR 0.82; 95% CI, 0.45-1.48). There was no difference between groups in overall adverse effects. Discontinuation due to adverse events was 9% with dabigatran compared with 6.8% with warfarin (P=0.05). Dyspepsia was more common with dabigatran (P<0.001).
Bottom line: Following parenteral anticoagulation, dabigatran is a safe and effective alternative to warfarin for the treatment of acute VTE and does not require therapeutic monitoring.
Citation: Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352.
Surgical Masks as Effective as N95 Respirators for Preventing Influenza
Clinical question: How effective are surgical masks compared with N95 respirators in protecting healthcare workers against influenza?
Background: Evidence surrounding the effectiveness of the surgical mask compared with the N95 respirator for protecting healthcare workers against influenza is sparse.
Study design: Randomized, controlled trial.
Setting: Eight hospitals in Ontario.
Synopsis: The study looked at 446 nurses working in EDs, medical units, and pediatric units randomized to use either a fit-tested N95 respirator or a surgical mask when caring for patients with febrile respiratory illness during the 2008-2009 flu season. The primary outcome measured was laboratory-confirmed influenza. Only a minority of the study participants (30% in the surgical mask group; 28% in the respirator group) received the influenza vaccine during the study year.
Influenza infection occurred with similar incidence in both the surgical-mask and N95 respirator groups (23.6% vs. 22.9%). A two-week audit period demonstrated solid adherence to the assigned respiratory protection device in both groups (11 out of 11 nurses were compliant in the surgical-mask group; six out of seven nurses were compliant in the respirator group).
The major limitation of this study is that it cannot be extrapolated to other settings where there is a high risk for aerosolization, such as intubation or bronchoscopy, where N95 respirators may be more effective than surgical masks.
Bottom line: Surgical masks are as effective as fit-tested N95 respirators in protecting healthcare workers against influenza in most settings.
Citation: Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs. N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302 (17):1865-1871.
Neither Major Illness Nor Noncardiac Surgery Associated with Long-Term Cognitive Decline in Older Patients
Clinical question: Is there a measurable and lasting cognitive decline in older adults following noncardiac surgery or major illness?
Background: Despite limited evidence, there is some concern that elderly patients are susceptible to significant, long-term deterioration in mental function following surgery or a major illness. Prior studies often have been limited by lack of information about the trajectory of surgical patients’ cognitive status before surgery and lack of relevant control groups.
Study design: Retrospective, cohort study.
Setting: Single outpatient research center.
Synopsis: The Alzheimer’s Disease Research Center (ADRC) at the University of Washington in St. Louis continually enrolls research subjects without regard to their baseline cognitive function and provides annual assessment of cognitive functioning.
From the ADRC database, 575 eligible research participants were identified. Of these, 361 had very mild or mild dementia at enrollment, and 214 had no dementia. Participants were then categorized into three groups: those who had undergone noncardiac surgery (N=180); those who had been admitted to the hospital with a major illness (N=119); and those who had experienced neither surgery nor major illness (N=276).
Cognitive trajectory did not differ between the three groups, although participants with baseline dementia declined more rapidly than participants without dementia. Although 23% of patients without dementia developed detectable evidence of dementia during the study period, this outcome was not more common following surgery or major illness.
As participants were assessed annually, this study does not address the issue of post-operative delirium or early cognitive impairment following surgery.
Bottom line: There is no evidence for a long-term effect on cognitive function independently attributable to noncardiac surgery or major illness.
Citation: Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964-970.
Rapid-Response System Maturation Decreases Delays in Emergency Team Activation
Clinical question: Does the maturation of a rapid-response system (RRS) improve performance by decreasing delays in medical emergency team (MET) activation?
Background: RRSs have been widely embraced as a possible means to reduce inpatient cardiopulmonary arrests and unplanned ICU admissions. Assessment of RRSs early in their implementation might underestimate their long-term efficacy. Whether the use and performance of RRSs improve as they mature is currently unknown.
Study design: Observational, cohort study.
Setting: Single tertiary-care hospital.
Synopsis: A recent cohort of 200 patients receiving MET review was prospectively compared with a control cohort of 400 patients receiving an MET review five years earlier, at the start of RRS implementation. Information obtained on the two cohorts included demographics, timing of MET activation in relation to the first documented MET review criterion (activation delay), and patient outcomes.
Fewer patients in the recent cohort had delayed MET activation (22.0% vs. 40.3%). The recent cohort also was independently associated with a decreased risk of delayed activation (OR 0.45; 95% C.I., 0.30-0.67) and ICU admission (OR 0.5; 95% C.I., 0.32-0.78). Delayed MET activation independently was associated with greater risk of unplanned ICU admission (OR 1.79; 95% C.I., 1.33-2.93) and hospital mortality (OR 2.18; 95% C.I., 1.42-3.33).
The study is limited by its observational nature, and thus the association between greater delay and unfavorable outcomes should not infer causality.
Bottom line: The maturation of a RRS decreases delays in MET activation. RRSs might need to mature before their full impact is felt.
Citation: Calzavacca P, Licari E, Tee A, et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow-up study. Resuscitation. 2010;81(1):31-35. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Predictors of readmission for patients with CAP.
- High-dose statins vs. lipid-lowering therapy combinations
- Catheter retention and risks of reinfection in patients with coagulase-negative staph
- Stenting vs. medical management of renal-artery stenosis
- Dabigatran for VTE
- Surgical mask vs. N95 respirator for influenza prevention
- Hospitalization and the risk of long-term cognitive decline
- Maturation of rapid-response teams and outcomes
Commonly Available Clinical Variables Predict 30-Day Readmissions for Community-Acquired Pneumonia
Clinical question: What are the risk factors for 30-day readmission in patients hospitalized for community-acquired pneumonia (CAP)?
Background: CAP is a common admission diagnosis associated with significant morbidity, mortality, and resource utilization. While prior data suggested that patients who survive a hospitalization for CAP are particularly vulnerable to readmission, few studies have examined the risk factors for readmission in this population.
Study design: Prospective, observational study.
Setting: A 400-bed teaching hospital in northern Spain.
Synopsis: From 2003 to 2005, this study consecutively enrolled 1,117 patients who were discharged after hospitalization for CAP. Eighty-one patients (7.2%) were readmitted within 30 days of discharge; 29 (35.8%) of these patients were rehospitalized for pneumonia-related causes.
Variables associated with pneumonia-related rehospitalization were treatment failure (HR 2.9; 95% CI, 1.2-6.8) and one or more instability factors at hospital discharge—for example, vital-sign abnormalities or inability to take food or medications by mouth (HR 2.8; 95% CI, 1.3-6.2). Variables associated with readmission unrelated to pneumonia were age greater than 65 years (HR 4.5; 95% CI, 1.4-14.7), Charlson comorbidity index greater than 2 (HR 1.9; 95% CI, 1.0-3.4), and decompensated comorbidities during index hospitalization.
Patients with at least two of the above risk factors were at a significantly higher risk for 30-day hospital readmission (HR 3.37; 95% CI, 2.08-5.46).
Bottom line: The risk factors for readmission after hospitalization for CAP differed between the groups with readmissions related to pneumonia versus other causes. Patients at high risk for readmission can be identified using easily available clinical variables.
Citation: Capelastegui A, España Yandiola PP, Quintana JM, et al. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest. 2009;136(4): 1079-1085.
Combinations of Lipid-Lowering Agents No More Effective than High-Dose Statin Monotherapy
Clinical question: Is high-dose statin monotherapy better than combinations of lipid-lowering agents for dyslipidemia in adults at high risk for coronary artery disease?
Background: While current guidelines support the benefits of aggressive lipid targets, there is little to guide physicians as to the optimal strategy for attaining target lipid levels.
Study design: Systematic review.
Setting: North America, Europe, and Asia.
Synopsis: Very-low-strength evidence showed that statin-ezetimibe (two trials; N=439) and statin-fibrate (one trial; N=166) combinations did not reduce mortality more than high-dose statin monotherapy. No trial data were found comparing the effect of these two strategies on secondary endpoints, including myocardial infarction, stroke, or revascularization.
Two trials (N=295) suggested lower-target lipid levels were more often achieved with statin-ezetimibe combination therapy than with high-dose statin monotherapy (OR 7.21; 95% CI, 4.30-12.08).
Limitations of this systematic review include the small number of studies directly comparing the two strategies, the short duration of most of the studies included, the focus on surrogate outcomes, and the heterogeneity of the study populations’ risk for coronary artery disease. Few studies were available comparing combination therapies other than statin-ezetimibe.
Bottom line: Limited evidence suggests that the combination of a statin with another lipid-lowering agent does not improve clinical outcomes when compared with high-dose statin monotherapy. Low-quality evidence suggests that lower-target lipid levels were more often reached with statin-ezetimibe combination therapy than with high-dose statin monotherapy.
Citation: Sharma M, Ansari MT, Abou-Setta AM, et al. Systematic review: comparative effectiveness and harms of combination therapy and monotherapy for dyslipidemia. Ann Intern Med. 2009;151(9):622-630.
Catheter Retention in Catheter-Related Coagulase-Negative Staphylococcal Bacteremia Is a Significant Risk Factor for Recurrent Infection
Clinical question: Should central venous catheters (CVC) be removed in patients with coagulase-negative staphylococcal catheter-related bloodstream infections (CRBSI)?
Background: Current guidelines for the management of coagulase-negative staphylococcal CRBSI do not recommend routine removal of the CVC, but are based on studies that did not use a strict definition of coagulase-negative staphylococcal CRBSI. Additionally, the studies did not look explicitly at the risk of recurrent infection.
Study design: Retrospective chart review.
Setting: Single academic medical center.
Synopsis: The study retrospectively evaluated 188 patients with coagulase-negative staphylococcal CRBSI. Immediate resolution of the infection was not influenced by the management of the CVC (retention vs. removal or exchange). However, using the multiple logistic regression technique, patients with catheter retention were found to be 6.6 times (95% CI, 1.8-23.9 times) more likely to have recurrence compared with those patients whose catheter was removed or exchanged.
Bottom line: While CVC management does not appear to have an impact on the acute resolution of infection, catheter retention is a significant risk factor for recurrent bacteremia.
Citation: Raad I, Kassar R, Ghannam D, Chaftari AM, Hachem R, Jiang Y. Management of the catheter in documented catheter-related coagulase-negative staphylococcal bacteremia: remove or retain? Clin Infect Dis. 2009;49(8):1187-1194.
Revascularization Offers No Benefit over Medical Therapy for Renal-Artery Stenosis
Clinical question: Does revascularization plus medical therapy compared with medical therapy alone improve outcomes in patients with renal-artery stenosis?
Background: Renal-artery stenosis is associated with significant hypertension and renal dysfunction. Revascularization for atherosclerotic renal-artery stenosis can improve artery patency, but it remains unclear if it provides clinical benefit in terms of preserving renal function or reducing overall mortality.
Study design: Randomized, controlled trial.
Setting: Fifty-seven outpatient sites in the United Kingdom, Australia, and New Zealand.
Synopsis: The study randomized 806 patients with renal-artery stenosis to receive either medical therapy alone (N=403) or medical management plus endovascular revascularization (N=403).
The majority of the patients who underwent revascularization (95%) received a stent.
The data show no significant difference between the two groups in the rate of progression of renal dysfunction, systolic blood pressure, rates of adverse renal and cardiovascular events, and overall survival. Of the 359 patients who underwent revascularization, 23 (6%) experienced serious complications from the procedure, including two deaths and three cases of amputated toes or limbs.
The primary limitation of this trial is the population studied. The trial only included subjects for whom revascularization offered uncertain clinical benefits, according to their doctor. Those subjects for whom revascularization offered certain clinical benefits, as noted by their primary-care physician (PCP), were excluded from the study. Examples include patients presenting with rapidly progressive renal dysfunction or pulmonary edema thought to be a result of renal-artery stenosis.
Bottom line: Revascularization provides no benefit to most patients with renal-artery stenosis, and is associated with some risk.
Citation: ASTRAL investigators, Wheatley K, Ives N, et al. Revascularization versus medical therapy for renal-artery stenosis. N Eng J Med. 2009;361(20):1953-1962.
Dabigatran as Effective as Warfarin in Treatment of Acute VTE
Clinical question: Is dabigatran a safe and effective alternative to warfarin for treatment of acute VTE?
Background: Parenteral anticoagulation followed by warfarin is the standard of care for acute VTE. Warfarin requires frequent monitoring and has numerous drug and food interactions. Dabigatran, which the FDA has yet to approve for use in the U.S., is an oral direct thrombin inhibitor that does not require laboratory monitoring. The role of dabigatran in acute VTE has not been evaluated.
Study design: Randomized, double-blind, noninferiority trial.
Setting: Two hundred twenty-two clinical centers in 29 countries.
Synopsis: This study randomized 2,564 patients with documented VTE (either DVT or pulmonary embolism [PE]) to receive dabigatran 150mg twice daily or warfarin after at least five days of a parenteral anticoagulant. Warfarin was dose-adjusted to an INR goal of 2.0-3.0. The primary outcome was incidence of recurrent VTE and related deaths at six months.
A total of 2.4% of patients assigned to dabigatran and 2.1% of patients assigned to warfarin had recurrent VTE (HR 1.10; 95% CI, 0.8-1.5), which met criteria for noninferiority. Major bleeding occurred in 1.6% of patients assigned to dabigatran and 1.9% assigned to warfarin (HR 0.82; 95% CI, 0.45-1.48). There was no difference between groups in overall adverse effects. Discontinuation due to adverse events was 9% with dabigatran compared with 6.8% with warfarin (P=0.05). Dyspepsia was more common with dabigatran (P<0.001).
Bottom line: Following parenteral anticoagulation, dabigatran is a safe and effective alternative to warfarin for the treatment of acute VTE and does not require therapeutic monitoring.
Citation: Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352.
Surgical Masks as Effective as N95 Respirators for Preventing Influenza
Clinical question: How effective are surgical masks compared with N95 respirators in protecting healthcare workers against influenza?
Background: Evidence surrounding the effectiveness of the surgical mask compared with the N95 respirator for protecting healthcare workers against influenza is sparse.
Study design: Randomized, controlled trial.
Setting: Eight hospitals in Ontario.
Synopsis: The study looked at 446 nurses working in EDs, medical units, and pediatric units randomized to use either a fit-tested N95 respirator or a surgical mask when caring for patients with febrile respiratory illness during the 2008-2009 flu season. The primary outcome measured was laboratory-confirmed influenza. Only a minority of the study participants (30% in the surgical mask group; 28% in the respirator group) received the influenza vaccine during the study year.
Influenza infection occurred with similar incidence in both the surgical-mask and N95 respirator groups (23.6% vs. 22.9%). A two-week audit period demonstrated solid adherence to the assigned respiratory protection device in both groups (11 out of 11 nurses were compliant in the surgical-mask group; six out of seven nurses were compliant in the respirator group).
The major limitation of this study is that it cannot be extrapolated to other settings where there is a high risk for aerosolization, such as intubation or bronchoscopy, where N95 respirators may be more effective than surgical masks.
Bottom line: Surgical masks are as effective as fit-tested N95 respirators in protecting healthcare workers against influenza in most settings.
Citation: Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs. N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302 (17):1865-1871.
Neither Major Illness Nor Noncardiac Surgery Associated with Long-Term Cognitive Decline in Older Patients
Clinical question: Is there a measurable and lasting cognitive decline in older adults following noncardiac surgery or major illness?
Background: Despite limited evidence, there is some concern that elderly patients are susceptible to significant, long-term deterioration in mental function following surgery or a major illness. Prior studies often have been limited by lack of information about the trajectory of surgical patients’ cognitive status before surgery and lack of relevant control groups.
Study design: Retrospective, cohort study.
Setting: Single outpatient research center.
Synopsis: The Alzheimer’s Disease Research Center (ADRC) at the University of Washington in St. Louis continually enrolls research subjects without regard to their baseline cognitive function and provides annual assessment of cognitive functioning.
From the ADRC database, 575 eligible research participants were identified. Of these, 361 had very mild or mild dementia at enrollment, and 214 had no dementia. Participants were then categorized into three groups: those who had undergone noncardiac surgery (N=180); those who had been admitted to the hospital with a major illness (N=119); and those who had experienced neither surgery nor major illness (N=276).
Cognitive trajectory did not differ between the three groups, although participants with baseline dementia declined more rapidly than participants without dementia. Although 23% of patients without dementia developed detectable evidence of dementia during the study period, this outcome was not more common following surgery or major illness.
As participants were assessed annually, this study does not address the issue of post-operative delirium or early cognitive impairment following surgery.
Bottom line: There is no evidence for a long-term effect on cognitive function independently attributable to noncardiac surgery or major illness.
Citation: Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964-970.
Rapid-Response System Maturation Decreases Delays in Emergency Team Activation
Clinical question: Does the maturation of a rapid-response system (RRS) improve performance by decreasing delays in medical emergency team (MET) activation?
Background: RRSs have been widely embraced as a possible means to reduce inpatient cardiopulmonary arrests and unplanned ICU admissions. Assessment of RRSs early in their implementation might underestimate their long-term efficacy. Whether the use and performance of RRSs improve as they mature is currently unknown.
Study design: Observational, cohort study.
Setting: Single tertiary-care hospital.
Synopsis: A recent cohort of 200 patients receiving MET review was prospectively compared with a control cohort of 400 patients receiving an MET review five years earlier, at the start of RRS implementation. Information obtained on the two cohorts included demographics, timing of MET activation in relation to the first documented MET review criterion (activation delay), and patient outcomes.
Fewer patients in the recent cohort had delayed MET activation (22.0% vs. 40.3%). The recent cohort also was independently associated with a decreased risk of delayed activation (OR 0.45; 95% C.I., 0.30-0.67) and ICU admission (OR 0.5; 95% C.I., 0.32-0.78). Delayed MET activation independently was associated with greater risk of unplanned ICU admission (OR 1.79; 95% C.I., 1.33-2.93) and hospital mortality (OR 2.18; 95% C.I., 1.42-3.33).
The study is limited by its observational nature, and thus the association between greater delay and unfavorable outcomes should not infer causality.
Bottom line: The maturation of a RRS decreases delays in MET activation. RRSs might need to mature before their full impact is felt.
Citation: Calzavacca P, Licari E, Tee A, et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow-up study. Resuscitation. 2010;81(1):31-35. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Predictors of readmission for patients with CAP.
- High-dose statins vs. lipid-lowering therapy combinations
- Catheter retention and risks of reinfection in patients with coagulase-negative staph
- Stenting vs. medical management of renal-artery stenosis
- Dabigatran for VTE
- Surgical mask vs. N95 respirator for influenza prevention
- Hospitalization and the risk of long-term cognitive decline
- Maturation of rapid-response teams and outcomes
Commonly Available Clinical Variables Predict 30-Day Readmissions for Community-Acquired Pneumonia
Clinical question: What are the risk factors for 30-day readmission in patients hospitalized for community-acquired pneumonia (CAP)?
Background: CAP is a common admission diagnosis associated with significant morbidity, mortality, and resource utilization. While prior data suggested that patients who survive a hospitalization for CAP are particularly vulnerable to readmission, few studies have examined the risk factors for readmission in this population.
Study design: Prospective, observational study.
Setting: A 400-bed teaching hospital in northern Spain.
Synopsis: From 2003 to 2005, this study consecutively enrolled 1,117 patients who were discharged after hospitalization for CAP. Eighty-one patients (7.2%) were readmitted within 30 days of discharge; 29 (35.8%) of these patients were rehospitalized for pneumonia-related causes.
Variables associated with pneumonia-related rehospitalization were treatment failure (HR 2.9; 95% CI, 1.2-6.8) and one or more instability factors at hospital discharge—for example, vital-sign abnormalities or inability to take food or medications by mouth (HR 2.8; 95% CI, 1.3-6.2). Variables associated with readmission unrelated to pneumonia were age greater than 65 years (HR 4.5; 95% CI, 1.4-14.7), Charlson comorbidity index greater than 2 (HR 1.9; 95% CI, 1.0-3.4), and decompensated comorbidities during index hospitalization.
Patients with at least two of the above risk factors were at a significantly higher risk for 30-day hospital readmission (HR 3.37; 95% CI, 2.08-5.46).
Bottom line: The risk factors for readmission after hospitalization for CAP differed between the groups with readmissions related to pneumonia versus other causes. Patients at high risk for readmission can be identified using easily available clinical variables.
Citation: Capelastegui A, España Yandiola PP, Quintana JM, et al. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest. 2009;136(4): 1079-1085.
Combinations of Lipid-Lowering Agents No More Effective than High-Dose Statin Monotherapy
Clinical question: Is high-dose statin monotherapy better than combinations of lipid-lowering agents for dyslipidemia in adults at high risk for coronary artery disease?
Background: While current guidelines support the benefits of aggressive lipid targets, there is little to guide physicians as to the optimal strategy for attaining target lipid levels.
Study design: Systematic review.
Setting: North America, Europe, and Asia.
Synopsis: Very-low-strength evidence showed that statin-ezetimibe (two trials; N=439) and statin-fibrate (one trial; N=166) combinations did not reduce mortality more than high-dose statin monotherapy. No trial data were found comparing the effect of these two strategies on secondary endpoints, including myocardial infarction, stroke, or revascularization.
Two trials (N=295) suggested lower-target lipid levels were more often achieved with statin-ezetimibe combination therapy than with high-dose statin monotherapy (OR 7.21; 95% CI, 4.30-12.08).
Limitations of this systematic review include the small number of studies directly comparing the two strategies, the short duration of most of the studies included, the focus on surrogate outcomes, and the heterogeneity of the study populations’ risk for coronary artery disease. Few studies were available comparing combination therapies other than statin-ezetimibe.
Bottom line: Limited evidence suggests that the combination of a statin with another lipid-lowering agent does not improve clinical outcomes when compared with high-dose statin monotherapy. Low-quality evidence suggests that lower-target lipid levels were more often reached with statin-ezetimibe combination therapy than with high-dose statin monotherapy.
Citation: Sharma M, Ansari MT, Abou-Setta AM, et al. Systematic review: comparative effectiveness and harms of combination therapy and monotherapy for dyslipidemia. Ann Intern Med. 2009;151(9):622-630.
Catheter Retention in Catheter-Related Coagulase-Negative Staphylococcal Bacteremia Is a Significant Risk Factor for Recurrent Infection
Clinical question: Should central venous catheters (CVC) be removed in patients with coagulase-negative staphylococcal catheter-related bloodstream infections (CRBSI)?
Background: Current guidelines for the management of coagulase-negative staphylococcal CRBSI do not recommend routine removal of the CVC, but are based on studies that did not use a strict definition of coagulase-negative staphylococcal CRBSI. Additionally, the studies did not look explicitly at the risk of recurrent infection.
Study design: Retrospective chart review.
Setting: Single academic medical center.
Synopsis: The study retrospectively evaluated 188 patients with coagulase-negative staphylococcal CRBSI. Immediate resolution of the infection was not influenced by the management of the CVC (retention vs. removal or exchange). However, using the multiple logistic regression technique, patients with catheter retention were found to be 6.6 times (95% CI, 1.8-23.9 times) more likely to have recurrence compared with those patients whose catheter was removed or exchanged.
Bottom line: While CVC management does not appear to have an impact on the acute resolution of infection, catheter retention is a significant risk factor for recurrent bacteremia.
Citation: Raad I, Kassar R, Ghannam D, Chaftari AM, Hachem R, Jiang Y. Management of the catheter in documented catheter-related coagulase-negative staphylococcal bacteremia: remove or retain? Clin Infect Dis. 2009;49(8):1187-1194.
Revascularization Offers No Benefit over Medical Therapy for Renal-Artery Stenosis
Clinical question: Does revascularization plus medical therapy compared with medical therapy alone improve outcomes in patients with renal-artery stenosis?
Background: Renal-artery stenosis is associated with significant hypertension and renal dysfunction. Revascularization for atherosclerotic renal-artery stenosis can improve artery patency, but it remains unclear if it provides clinical benefit in terms of preserving renal function or reducing overall mortality.
Study design: Randomized, controlled trial.
Setting: Fifty-seven outpatient sites in the United Kingdom, Australia, and New Zealand.
Synopsis: The study randomized 806 patients with renal-artery stenosis to receive either medical therapy alone (N=403) or medical management plus endovascular revascularization (N=403).
The majority of the patients who underwent revascularization (95%) received a stent.
The data show no significant difference between the two groups in the rate of progression of renal dysfunction, systolic blood pressure, rates of adverse renal and cardiovascular events, and overall survival. Of the 359 patients who underwent revascularization, 23 (6%) experienced serious complications from the procedure, including two deaths and three cases of amputated toes or limbs.
The primary limitation of this trial is the population studied. The trial only included subjects for whom revascularization offered uncertain clinical benefits, according to their doctor. Those subjects for whom revascularization offered certain clinical benefits, as noted by their primary-care physician (PCP), were excluded from the study. Examples include patients presenting with rapidly progressive renal dysfunction or pulmonary edema thought to be a result of renal-artery stenosis.
Bottom line: Revascularization provides no benefit to most patients with renal-artery stenosis, and is associated with some risk.
Citation: ASTRAL investigators, Wheatley K, Ives N, et al. Revascularization versus medical therapy for renal-artery stenosis. N Eng J Med. 2009;361(20):1953-1962.
Dabigatran as Effective as Warfarin in Treatment of Acute VTE
Clinical question: Is dabigatran a safe and effective alternative to warfarin for treatment of acute VTE?
Background: Parenteral anticoagulation followed by warfarin is the standard of care for acute VTE. Warfarin requires frequent monitoring and has numerous drug and food interactions. Dabigatran, which the FDA has yet to approve for use in the U.S., is an oral direct thrombin inhibitor that does not require laboratory monitoring. The role of dabigatran in acute VTE has not been evaluated.
Study design: Randomized, double-blind, noninferiority trial.
Setting: Two hundred twenty-two clinical centers in 29 countries.
Synopsis: This study randomized 2,564 patients with documented VTE (either DVT or pulmonary embolism [PE]) to receive dabigatran 150mg twice daily or warfarin after at least five days of a parenteral anticoagulant. Warfarin was dose-adjusted to an INR goal of 2.0-3.0. The primary outcome was incidence of recurrent VTE and related deaths at six months.
A total of 2.4% of patients assigned to dabigatran and 2.1% of patients assigned to warfarin had recurrent VTE (HR 1.10; 95% CI, 0.8-1.5), which met criteria for noninferiority. Major bleeding occurred in 1.6% of patients assigned to dabigatran and 1.9% assigned to warfarin (HR 0.82; 95% CI, 0.45-1.48). There was no difference between groups in overall adverse effects. Discontinuation due to adverse events was 9% with dabigatran compared with 6.8% with warfarin (P=0.05). Dyspepsia was more common with dabigatran (P<0.001).
Bottom line: Following parenteral anticoagulation, dabigatran is a safe and effective alternative to warfarin for the treatment of acute VTE and does not require therapeutic monitoring.
Citation: Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352.
Surgical Masks as Effective as N95 Respirators for Preventing Influenza
Clinical question: How effective are surgical masks compared with N95 respirators in protecting healthcare workers against influenza?
Background: Evidence surrounding the effectiveness of the surgical mask compared with the N95 respirator for protecting healthcare workers against influenza is sparse.
Study design: Randomized, controlled trial.
Setting: Eight hospitals in Ontario.
Synopsis: The study looked at 446 nurses working in EDs, medical units, and pediatric units randomized to use either a fit-tested N95 respirator or a surgical mask when caring for patients with febrile respiratory illness during the 2008-2009 flu season. The primary outcome measured was laboratory-confirmed influenza. Only a minority of the study participants (30% in the surgical mask group; 28% in the respirator group) received the influenza vaccine during the study year.
Influenza infection occurred with similar incidence in both the surgical-mask and N95 respirator groups (23.6% vs. 22.9%). A two-week audit period demonstrated solid adherence to the assigned respiratory protection device in both groups (11 out of 11 nurses were compliant in the surgical-mask group; six out of seven nurses were compliant in the respirator group).
The major limitation of this study is that it cannot be extrapolated to other settings where there is a high risk for aerosolization, such as intubation or bronchoscopy, where N95 respirators may be more effective than surgical masks.
Bottom line: Surgical masks are as effective as fit-tested N95 respirators in protecting healthcare workers against influenza in most settings.
Citation: Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs. N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302 (17):1865-1871.
Neither Major Illness Nor Noncardiac Surgery Associated with Long-Term Cognitive Decline in Older Patients
Clinical question: Is there a measurable and lasting cognitive decline in older adults following noncardiac surgery or major illness?
Background: Despite limited evidence, there is some concern that elderly patients are susceptible to significant, long-term deterioration in mental function following surgery or a major illness. Prior studies often have been limited by lack of information about the trajectory of surgical patients’ cognitive status before surgery and lack of relevant control groups.
Study design: Retrospective, cohort study.
Setting: Single outpatient research center.
Synopsis: The Alzheimer’s Disease Research Center (ADRC) at the University of Washington in St. Louis continually enrolls research subjects without regard to their baseline cognitive function and provides annual assessment of cognitive functioning.
From the ADRC database, 575 eligible research participants were identified. Of these, 361 had very mild or mild dementia at enrollment, and 214 had no dementia. Participants were then categorized into three groups: those who had undergone noncardiac surgery (N=180); those who had been admitted to the hospital with a major illness (N=119); and those who had experienced neither surgery nor major illness (N=276).
Cognitive trajectory did not differ between the three groups, although participants with baseline dementia declined more rapidly than participants without dementia. Although 23% of patients without dementia developed detectable evidence of dementia during the study period, this outcome was not more common following surgery or major illness.
As participants were assessed annually, this study does not address the issue of post-operative delirium or early cognitive impairment following surgery.
Bottom line: There is no evidence for a long-term effect on cognitive function independently attributable to noncardiac surgery or major illness.
Citation: Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964-970.
Rapid-Response System Maturation Decreases Delays in Emergency Team Activation
Clinical question: Does the maturation of a rapid-response system (RRS) improve performance by decreasing delays in medical emergency team (MET) activation?
Background: RRSs have been widely embraced as a possible means to reduce inpatient cardiopulmonary arrests and unplanned ICU admissions. Assessment of RRSs early in their implementation might underestimate their long-term efficacy. Whether the use and performance of RRSs improve as they mature is currently unknown.
Study design: Observational, cohort study.
Setting: Single tertiary-care hospital.
Synopsis: A recent cohort of 200 patients receiving MET review was prospectively compared with a control cohort of 400 patients receiving an MET review five years earlier, at the start of RRS implementation. Information obtained on the two cohorts included demographics, timing of MET activation in relation to the first documented MET review criterion (activation delay), and patient outcomes.
Fewer patients in the recent cohort had delayed MET activation (22.0% vs. 40.3%). The recent cohort also was independently associated with a decreased risk of delayed activation (OR 0.45; 95% C.I., 0.30-0.67) and ICU admission (OR 0.5; 95% C.I., 0.32-0.78). Delayed MET activation independently was associated with greater risk of unplanned ICU admission (OR 1.79; 95% C.I., 1.33-2.93) and hospital mortality (OR 2.18; 95% C.I., 1.42-3.33).
The study is limited by its observational nature, and thus the association between greater delay and unfavorable outcomes should not infer causality.
Bottom line: The maturation of a RRS decreases delays in MET activation. RRSs might need to mature before their full impact is felt.
Citation: Calzavacca P, Licari E, Tee A, et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow-up study. Resuscitation. 2010;81(1):31-35. TH
Hospitalists in Haiti
The patient had a number of wounds to her battered body, but her most pressing question was how to stanch the flow of milk from her breasts, recalls Lisa Luly-Rivera, MD. The woman was in an endless line of people Dr. Luly-Rivera, a hospitalist at the University of Miami (Fla.) Hospital, cared for during a five-day medical volunteer mission to Haiti in the aftermath of the January earthquake that devastated much of the country.
“She had lost everything, including her seven-month-old baby, who she watched die in the earthquake. She was still lactating and wanted to know how to get the milk to stop,” Dr. Luly-Rivera says. “I heard story after story after story like this. For me, it was emotionally jarring.”
A Haitian-American who has extended-family members in Haiti who survived the Jan. 12 earthquake, Dr. Luly-Rivera leaped at the chance to participate in the medical relief effort organized by the university’s Miller School of Medicine in conjunction with Project Medishare and Jackson Memorial Hospital in Miami. But soon after arriving in the Haitian capital of Port-au-Prince on Jan. 20 and witnessing the magnitude of human suffering there, she second-guessed her decision, wondering if she was emotionally strong enough to deal with such tragedy.
She wasn’t the only one with reservations. Some at the University of Miami Hospital were skeptical that hospitalists could help the situation in Haiti. They questioned why she and her colleagues were included on the volunteer team, Dr. Luly-Rivera says. Ultimately, she proved herself—and the doubters—wrong.
“As internists, we were very valuable there,” says Dr. Luly-Rivera, who logged long hours treating patients and listening to their stories.
Determined to do their part to help survivors of the earthquake, hospitalists across the country joined a surge of American medical personnel in Haiti. Once there, they faced a severely traumatized populace (the Haitian government estimates more than 215,000 were killed and 300,000 injured in the quake), a crippled hospital infrastructure, and a debilitated public health system that had failed even before the earthquake to provide adequate sanitation, vaccinations, infectious-disease control, and basic primary care.
“If Haiti wasn’t chronically poor, if it hadn’t suffered for so long outside of the eye of the world community, then the devastation would have never been so great,” says Sriram Shamasunder, MD, a hospitalist and assistant clinical professor at the University of California at San Francisco’s Department of Medicine who volunteered in the relief effort with the Boston-based nonprofit group Partners in Health. “The house that crumbled is the one chronic poverty built.”
Worthy Cause, Unimaginable Conditions
Mario A. Reyes, MD, FHM, director of the Division of Pediatric Hospital Medicine at Miami Children’s Hospital, shakes his head when he thinks of the conditions in Haiti, one of the poorest nations in the Western Hemisphere. “This is how unfair the world is, that you can fly one and a half hours from a country of such plenty to a country with so much poverty,” says Dr. Reyes, who made his third trip to the island nation in as many years. “Once you go the first time, you feel a connection to the country and the people. It’s a sense of duty to help a very poor neighbor.”
This time, Dr. Reyes and colleague Andrea Maggioni, MD, organized the 75-cot pediatric unit of a 250-bed tent hospital that the University of Miami opened Jan. 21 at the airport in Port-au-Prince in collaboration with Jackson Memorial Hospital and Miami-based Project Medishare, a nonprofit organization founded by doctors from the University of Miami’s medical school in an effort to bring quality healthcare and development services to Haiti.
“There were a few general pediatricians there. They relied on us to lead the way,” Dr. Reyes says. “When I got to the pediatric tent, I saw so many kids screaming at the same time, some with bones sticking out of their body. There’s nothing more gut-wrenching than that. I spent the first night giving morphine and antibiotics like lollipops.”
Before the tent hospital—four tents in all, one for supplies, one for volunteers to sleep in, and two for patients—was set up at the airport, doctors from the University of Miami and its partnering organizations treated adult and pediatric patients at a facility in the United Nations compound in Port-au-Prince. It was utter chaos, according to Amir Jaffer, MD, FHM, chief of the Division of Hospital Medicine and an associate professor of medicine at the Miller School of Medicine. He described earthquake survivors walking around in a daze amidst the rubble, and huge numbers of people searching for food and water.
Same Work, Makeshift Surroundings
Drawing on his HM experience, Dr. Jaffer helped orchestrate the transfer of approximately 140 patients from the makeshift U.N. hospital to the university’s tent hospital a couple of miles away. He also helped lead the effort to organize patients once they arrived at the new facility, which featured a supply tent, staff sleeping tent, medical tent, and surgical tent with four operating rooms. Each patient received a medical wristband and medical record number, and had their medical care charted.
An ICU was set up for those patients who were in more serious condition, and severely ill and injured patients were airlifted to medical centers in Florida and the USNS Comfort, a U.S. Navy ship dispatched to Haiti to provide full hospital service to earthquake survivors. The tent hospital had nearly 250 patients by the end of his five-day trip, Dr. Jaffer says.
Hospitalists administered IV fluids, prescribed antibiotics and pain medication, treated infected wounds, managed patients with dehydration, gastroenteritis, and tetanus, and triaged patients. “Many patients had splints placed in the field, and we would do X-rays to confirm the diagnosis. Patients were being casted right after diagnosis,” Dr. Jaffer says.
Outside the Capital
Hospitalists volunteering with Partners in Health (PIH) were tasked with maximizing the time the surgical team could spend in the OR by assessing incoming patients, triaging cases, providing post-op care, monitoring for development of medical issues related to trauma, and ensuring that every patient was seen daily, says Jonathan Crocker, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston.
Dr. Crocker arrived in Haiti four days after the earthquake and was sent to Clinique Bon Saveur, a hospital in Cange, a town located two hours outside the capital on the country’s Central Plateau. The hospital is one of 10 health facilities run by Zamni Lasante, PIH’s sister organization in Haiti. Dr. Shamasunder, of UC San Francisco, arrived in the country a few days later and was stationed at St. Marc Hospital, on the west coast of the island, about 60 miles from Port-au-Prince.
At St. Marc’s, conditions were “chaotic but functioning, bare-bones but a work in progress,” as Haitian doctors began returning to work and Creole-speaking nurses from the U.S. reached the hospital, Dr. Shamasunder explains. PIH volunteers coordinated with teams from Canada and Nepal to provide the best possible medical care to patients dealing with sepsis, serious wounds, and heart failure.
Hundreds of patients, many with multiple injuries, had been streaming into Clinique Bon Saveur since the day the earthquake struck. When Dr. Crocker arrived, the hospital was overcrowded, spilling into makeshift wards that had been set up in a church and a nearby school.
“As a hospitalist, my first concern upon arrival was anticipating the likely medical complications we would encounter with a large population of patients having experienced physical trauma,” Dr. Crocker says. “These complications included, namely, DVT and PE events, compartment syndrome, rhabdomyolysis with renal failure, hyperkalemia, wound infection, and sepsis.”
After speaking with their Haitian colleagues, PIH volunteers placed all adult patients at Clinique Bon Saveur on heparin prophylaxis. They also instituted a standard antibiotic regimen for all patients with open fractures, ensured patients received tetanus shots, and made it a priority to see every patient daily in an effort to prevent compartment syndrome and complications from rhabdomyolysis.
“As we identified more patients with acute renal failure, we moved into active screening with ‘creatinine rounds,’ where we performed BUN/Cr checks on any patient suspected of having suffered major crush injuries,” says Dr. Crocker, who used a portable ultrasound to assess patients for suspected lower-extremity DVTs. “As a team, we made a daily A, B, and C priority list for patients in need of surgeries available at the hospital, and a list of patients with injuries too complex for our surgical teams requiring transfer.”
Resume Expansion
Back at the University of Miami’s tent facility, hospitalists were chipping in wherever help was needed. “I cleaned rooms, I took out the trash, I swept floors, I dispensed medicine from the pharmacy. I just did everything,” Dr. Luly-Rivera says. “You have to go with an open mind and be prepared to do things outside your own discipline.”
Volunteers must be prepared to deal with difficult patients who are under considerable stress over their present and future situations, Dr. Luly-Rivera explains. She worries about what is to come for a country that’s ill-equipped to handle so many physically disabled people. For years, there will be a pressing need for orthopedic surgeons and physical and occupational therapists, she says.
Earthquake survivors also will need help in coping with the psychological trauma they’ve endured, says Dr. Reyes, who frequently played the role of hospital clown in the tent facility’s pediatric ward—just to help the children to laugh a bit.
“These kids are fully traumatized. They don’t want to go inside buildings because they’re afraid they will collapse,” he says. “There’s a high percentage of them who lost at least one parent in the disaster. When you go to discharge them, many don’t have a home to go to. You just feel tremendous sadness.”
Emotional Connection
The sorrow intensified when Dr. Reyes returned to work after returning from his trip to Haiti. “You can barely eat because you have a knot in your throat,” he says.
Upon her return to Miami, Dr. Luly-Rivera spent almost every spare minute watching news coverage on television and reading about the relief effort online. It was difficult for her to concentrate when working, she admits.
“It wasn’t that I felt the patients here didn’t need me,” she says. “It’s just that my mind was still in Haiti and thinking about my patients there. I had to let it go.”
Feelings of sadness and grief are common reactions to witnessing acute injuries and loss of life, says Dr. Jaffer. Some people react by refusing to leave until the work is done, or returning to the relief effort before they are ready.
—Mario Reyes, MD, FHM, director, Division of Pediatric Hospital Medicine, Miami Children’s Hospital
“Medical volunteerism shows you there is life beyond what you do in your workplace. It allows you to bridge the gap between your job and people who are less fortunate. The experience can be invigorating, but it can also be stress-inducing and lead to depression,” Dr. Jaffer says. “It’s always good to have someone you pair up with to monitor your stress level.”
After taking time to decompress, Drs. Luly-Rivera and Reyes plan to return to Haiti. They hope healthcare workers from all parts of the U.S. will continue to volunteer in the months ahead. Haiti’s weighty issues demand that non-governmental organizations (NGOs) working in the country stay and better coordinate their efforts, Dr. Reyes says.
“Ultimately, it is going to be important for any group present in Haiti to work to support the Haitian medical community,” Dr. Crocker adds. “The long-term recovery and rehabilitation of so many thousands of patients will be possible only through a robust, functional, public healthcare delivery system.”
It remains to be seen how many NGOs and volunteers will still be in Haiti a few months from now, the hospitalists said.
It’s always a concern that the attention of the global community may shift away from Haiti when the next calamity strikes in another part of the world, Dr. Jaffer notes. If the focus stays on Haiti as it rebuilds, then possibly some good will come out of the earthquake, Dr. Luly-Rivera says. But if NGOs begin to leave in the short term, the quake would only be the latest setback for one of the world’s poorest and most underdeveloped countries.
Even if the latter were to happen, Dr. Luly-Rivera still says she and other volunteers make a difference. “I’m still glad I went,” she says. “The people were so thankful.”
“You see the best of the American people there,” Dr. Reyes adds. “It’s encouraging and uplifting. It brings back faith in the medical profession and faith in people.” TH
Lisa Ryan is a freelance writer based in New Jersey.
The patient had a number of wounds to her battered body, but her most pressing question was how to stanch the flow of milk from her breasts, recalls Lisa Luly-Rivera, MD. The woman was in an endless line of people Dr. Luly-Rivera, a hospitalist at the University of Miami (Fla.) Hospital, cared for during a five-day medical volunteer mission to Haiti in the aftermath of the January earthquake that devastated much of the country.
“She had lost everything, including her seven-month-old baby, who she watched die in the earthquake. She was still lactating and wanted to know how to get the milk to stop,” Dr. Luly-Rivera says. “I heard story after story after story like this. For me, it was emotionally jarring.”
A Haitian-American who has extended-family members in Haiti who survived the Jan. 12 earthquake, Dr. Luly-Rivera leaped at the chance to participate in the medical relief effort organized by the university’s Miller School of Medicine in conjunction with Project Medishare and Jackson Memorial Hospital in Miami. But soon after arriving in the Haitian capital of Port-au-Prince on Jan. 20 and witnessing the magnitude of human suffering there, she second-guessed her decision, wondering if she was emotionally strong enough to deal with such tragedy.
She wasn’t the only one with reservations. Some at the University of Miami Hospital were skeptical that hospitalists could help the situation in Haiti. They questioned why she and her colleagues were included on the volunteer team, Dr. Luly-Rivera says. Ultimately, she proved herself—and the doubters—wrong.
“As internists, we were very valuable there,” says Dr. Luly-Rivera, who logged long hours treating patients and listening to their stories.
Determined to do their part to help survivors of the earthquake, hospitalists across the country joined a surge of American medical personnel in Haiti. Once there, they faced a severely traumatized populace (the Haitian government estimates more than 215,000 were killed and 300,000 injured in the quake), a crippled hospital infrastructure, and a debilitated public health system that had failed even before the earthquake to provide adequate sanitation, vaccinations, infectious-disease control, and basic primary care.
“If Haiti wasn’t chronically poor, if it hadn’t suffered for so long outside of the eye of the world community, then the devastation would have never been so great,” says Sriram Shamasunder, MD, a hospitalist and assistant clinical professor at the University of California at San Francisco’s Department of Medicine who volunteered in the relief effort with the Boston-based nonprofit group Partners in Health. “The house that crumbled is the one chronic poverty built.”
Worthy Cause, Unimaginable Conditions
Mario A. Reyes, MD, FHM, director of the Division of Pediatric Hospital Medicine at Miami Children’s Hospital, shakes his head when he thinks of the conditions in Haiti, one of the poorest nations in the Western Hemisphere. “This is how unfair the world is, that you can fly one and a half hours from a country of such plenty to a country with so much poverty,” says Dr. Reyes, who made his third trip to the island nation in as many years. “Once you go the first time, you feel a connection to the country and the people. It’s a sense of duty to help a very poor neighbor.”
This time, Dr. Reyes and colleague Andrea Maggioni, MD, organized the 75-cot pediatric unit of a 250-bed tent hospital that the University of Miami opened Jan. 21 at the airport in Port-au-Prince in collaboration with Jackson Memorial Hospital and Miami-based Project Medishare, a nonprofit organization founded by doctors from the University of Miami’s medical school in an effort to bring quality healthcare and development services to Haiti.
“There were a few general pediatricians there. They relied on us to lead the way,” Dr. Reyes says. “When I got to the pediatric tent, I saw so many kids screaming at the same time, some with bones sticking out of their body. There’s nothing more gut-wrenching than that. I spent the first night giving morphine and antibiotics like lollipops.”
Before the tent hospital—four tents in all, one for supplies, one for volunteers to sleep in, and two for patients—was set up at the airport, doctors from the University of Miami and its partnering organizations treated adult and pediatric patients at a facility in the United Nations compound in Port-au-Prince. It was utter chaos, according to Amir Jaffer, MD, FHM, chief of the Division of Hospital Medicine and an associate professor of medicine at the Miller School of Medicine. He described earthquake survivors walking around in a daze amidst the rubble, and huge numbers of people searching for food and water.
Same Work, Makeshift Surroundings
Drawing on his HM experience, Dr. Jaffer helped orchestrate the transfer of approximately 140 patients from the makeshift U.N. hospital to the university’s tent hospital a couple of miles away. He also helped lead the effort to organize patients once they arrived at the new facility, which featured a supply tent, staff sleeping tent, medical tent, and surgical tent with four operating rooms. Each patient received a medical wristband and medical record number, and had their medical care charted.
An ICU was set up for those patients who were in more serious condition, and severely ill and injured patients were airlifted to medical centers in Florida and the USNS Comfort, a U.S. Navy ship dispatched to Haiti to provide full hospital service to earthquake survivors. The tent hospital had nearly 250 patients by the end of his five-day trip, Dr. Jaffer says.
Hospitalists administered IV fluids, prescribed antibiotics and pain medication, treated infected wounds, managed patients with dehydration, gastroenteritis, and tetanus, and triaged patients. “Many patients had splints placed in the field, and we would do X-rays to confirm the diagnosis. Patients were being casted right after diagnosis,” Dr. Jaffer says.
Outside the Capital
Hospitalists volunteering with Partners in Health (PIH) were tasked with maximizing the time the surgical team could spend in the OR by assessing incoming patients, triaging cases, providing post-op care, monitoring for development of medical issues related to trauma, and ensuring that every patient was seen daily, says Jonathan Crocker, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston.
Dr. Crocker arrived in Haiti four days after the earthquake and was sent to Clinique Bon Saveur, a hospital in Cange, a town located two hours outside the capital on the country’s Central Plateau. The hospital is one of 10 health facilities run by Zamni Lasante, PIH’s sister organization in Haiti. Dr. Shamasunder, of UC San Francisco, arrived in the country a few days later and was stationed at St. Marc Hospital, on the west coast of the island, about 60 miles from Port-au-Prince.
At St. Marc’s, conditions were “chaotic but functioning, bare-bones but a work in progress,” as Haitian doctors began returning to work and Creole-speaking nurses from the U.S. reached the hospital, Dr. Shamasunder explains. PIH volunteers coordinated with teams from Canada and Nepal to provide the best possible medical care to patients dealing with sepsis, serious wounds, and heart failure.
Hundreds of patients, many with multiple injuries, had been streaming into Clinique Bon Saveur since the day the earthquake struck. When Dr. Crocker arrived, the hospital was overcrowded, spilling into makeshift wards that had been set up in a church and a nearby school.
“As a hospitalist, my first concern upon arrival was anticipating the likely medical complications we would encounter with a large population of patients having experienced physical trauma,” Dr. Crocker says. “These complications included, namely, DVT and PE events, compartment syndrome, rhabdomyolysis with renal failure, hyperkalemia, wound infection, and sepsis.”
After speaking with their Haitian colleagues, PIH volunteers placed all adult patients at Clinique Bon Saveur on heparin prophylaxis. They also instituted a standard antibiotic regimen for all patients with open fractures, ensured patients received tetanus shots, and made it a priority to see every patient daily in an effort to prevent compartment syndrome and complications from rhabdomyolysis.
“As we identified more patients with acute renal failure, we moved into active screening with ‘creatinine rounds,’ where we performed BUN/Cr checks on any patient suspected of having suffered major crush injuries,” says Dr. Crocker, who used a portable ultrasound to assess patients for suspected lower-extremity DVTs. “As a team, we made a daily A, B, and C priority list for patients in need of surgeries available at the hospital, and a list of patients with injuries too complex for our surgical teams requiring transfer.”
Resume Expansion
Back at the University of Miami’s tent facility, hospitalists were chipping in wherever help was needed. “I cleaned rooms, I took out the trash, I swept floors, I dispensed medicine from the pharmacy. I just did everything,” Dr. Luly-Rivera says. “You have to go with an open mind and be prepared to do things outside your own discipline.”
Volunteers must be prepared to deal with difficult patients who are under considerable stress over their present and future situations, Dr. Luly-Rivera explains. She worries about what is to come for a country that’s ill-equipped to handle so many physically disabled people. For years, there will be a pressing need for orthopedic surgeons and physical and occupational therapists, she says.
Earthquake survivors also will need help in coping with the psychological trauma they’ve endured, says Dr. Reyes, who frequently played the role of hospital clown in the tent facility’s pediatric ward—just to help the children to laugh a bit.
“These kids are fully traumatized. They don’t want to go inside buildings because they’re afraid they will collapse,” he says. “There’s a high percentage of them who lost at least one parent in the disaster. When you go to discharge them, many don’t have a home to go to. You just feel tremendous sadness.”
Emotional Connection
The sorrow intensified when Dr. Reyes returned to work after returning from his trip to Haiti. “You can barely eat because you have a knot in your throat,” he says.
Upon her return to Miami, Dr. Luly-Rivera spent almost every spare minute watching news coverage on television and reading about the relief effort online. It was difficult for her to concentrate when working, she admits.
“It wasn’t that I felt the patients here didn’t need me,” she says. “It’s just that my mind was still in Haiti and thinking about my patients there. I had to let it go.”
Feelings of sadness and grief are common reactions to witnessing acute injuries and loss of life, says Dr. Jaffer. Some people react by refusing to leave until the work is done, or returning to the relief effort before they are ready.
—Mario Reyes, MD, FHM, director, Division of Pediatric Hospital Medicine, Miami Children’s Hospital
“Medical volunteerism shows you there is life beyond what you do in your workplace. It allows you to bridge the gap between your job and people who are less fortunate. The experience can be invigorating, but it can also be stress-inducing and lead to depression,” Dr. Jaffer says. “It’s always good to have someone you pair up with to monitor your stress level.”
After taking time to decompress, Drs. Luly-Rivera and Reyes plan to return to Haiti. They hope healthcare workers from all parts of the U.S. will continue to volunteer in the months ahead. Haiti’s weighty issues demand that non-governmental organizations (NGOs) working in the country stay and better coordinate their efforts, Dr. Reyes says.
“Ultimately, it is going to be important for any group present in Haiti to work to support the Haitian medical community,” Dr. Crocker adds. “The long-term recovery and rehabilitation of so many thousands of patients will be possible only through a robust, functional, public healthcare delivery system.”
It remains to be seen how many NGOs and volunteers will still be in Haiti a few months from now, the hospitalists said.
It’s always a concern that the attention of the global community may shift away from Haiti when the next calamity strikes in another part of the world, Dr. Jaffer notes. If the focus stays on Haiti as it rebuilds, then possibly some good will come out of the earthquake, Dr. Luly-Rivera says. But if NGOs begin to leave in the short term, the quake would only be the latest setback for one of the world’s poorest and most underdeveloped countries.
Even if the latter were to happen, Dr. Luly-Rivera still says she and other volunteers make a difference. “I’m still glad I went,” she says. “The people were so thankful.”
“You see the best of the American people there,” Dr. Reyes adds. “It’s encouraging and uplifting. It brings back faith in the medical profession and faith in people.” TH
Lisa Ryan is a freelance writer based in New Jersey.
The patient had a number of wounds to her battered body, but her most pressing question was how to stanch the flow of milk from her breasts, recalls Lisa Luly-Rivera, MD. The woman was in an endless line of people Dr. Luly-Rivera, a hospitalist at the University of Miami (Fla.) Hospital, cared for during a five-day medical volunteer mission to Haiti in the aftermath of the January earthquake that devastated much of the country.
“She had lost everything, including her seven-month-old baby, who she watched die in the earthquake. She was still lactating and wanted to know how to get the milk to stop,” Dr. Luly-Rivera says. “I heard story after story after story like this. For me, it was emotionally jarring.”
A Haitian-American who has extended-family members in Haiti who survived the Jan. 12 earthquake, Dr. Luly-Rivera leaped at the chance to participate in the medical relief effort organized by the university’s Miller School of Medicine in conjunction with Project Medishare and Jackson Memorial Hospital in Miami. But soon after arriving in the Haitian capital of Port-au-Prince on Jan. 20 and witnessing the magnitude of human suffering there, she second-guessed her decision, wondering if she was emotionally strong enough to deal with such tragedy.
She wasn’t the only one with reservations. Some at the University of Miami Hospital were skeptical that hospitalists could help the situation in Haiti. They questioned why she and her colleagues were included on the volunteer team, Dr. Luly-Rivera says. Ultimately, she proved herself—and the doubters—wrong.
“As internists, we were very valuable there,” says Dr. Luly-Rivera, who logged long hours treating patients and listening to their stories.
Determined to do their part to help survivors of the earthquake, hospitalists across the country joined a surge of American medical personnel in Haiti. Once there, they faced a severely traumatized populace (the Haitian government estimates more than 215,000 were killed and 300,000 injured in the quake), a crippled hospital infrastructure, and a debilitated public health system that had failed even before the earthquake to provide adequate sanitation, vaccinations, infectious-disease control, and basic primary care.
“If Haiti wasn’t chronically poor, if it hadn’t suffered for so long outside of the eye of the world community, then the devastation would have never been so great,” says Sriram Shamasunder, MD, a hospitalist and assistant clinical professor at the University of California at San Francisco’s Department of Medicine who volunteered in the relief effort with the Boston-based nonprofit group Partners in Health. “The house that crumbled is the one chronic poverty built.”
Worthy Cause, Unimaginable Conditions
Mario A. Reyes, MD, FHM, director of the Division of Pediatric Hospital Medicine at Miami Children’s Hospital, shakes his head when he thinks of the conditions in Haiti, one of the poorest nations in the Western Hemisphere. “This is how unfair the world is, that you can fly one and a half hours from a country of such plenty to a country with so much poverty,” says Dr. Reyes, who made his third trip to the island nation in as many years. “Once you go the first time, you feel a connection to the country and the people. It’s a sense of duty to help a very poor neighbor.”
This time, Dr. Reyes and colleague Andrea Maggioni, MD, organized the 75-cot pediatric unit of a 250-bed tent hospital that the University of Miami opened Jan. 21 at the airport in Port-au-Prince in collaboration with Jackson Memorial Hospital and Miami-based Project Medishare, a nonprofit organization founded by doctors from the University of Miami’s medical school in an effort to bring quality healthcare and development services to Haiti.
“There were a few general pediatricians there. They relied on us to lead the way,” Dr. Reyes says. “When I got to the pediatric tent, I saw so many kids screaming at the same time, some with bones sticking out of their body. There’s nothing more gut-wrenching than that. I spent the first night giving morphine and antibiotics like lollipops.”
Before the tent hospital—four tents in all, one for supplies, one for volunteers to sleep in, and two for patients—was set up at the airport, doctors from the University of Miami and its partnering organizations treated adult and pediatric patients at a facility in the United Nations compound in Port-au-Prince. It was utter chaos, according to Amir Jaffer, MD, FHM, chief of the Division of Hospital Medicine and an associate professor of medicine at the Miller School of Medicine. He described earthquake survivors walking around in a daze amidst the rubble, and huge numbers of people searching for food and water.
Same Work, Makeshift Surroundings
Drawing on his HM experience, Dr. Jaffer helped orchestrate the transfer of approximately 140 patients from the makeshift U.N. hospital to the university’s tent hospital a couple of miles away. He also helped lead the effort to organize patients once they arrived at the new facility, which featured a supply tent, staff sleeping tent, medical tent, and surgical tent with four operating rooms. Each patient received a medical wristband and medical record number, and had their medical care charted.
An ICU was set up for those patients who were in more serious condition, and severely ill and injured patients were airlifted to medical centers in Florida and the USNS Comfort, a U.S. Navy ship dispatched to Haiti to provide full hospital service to earthquake survivors. The tent hospital had nearly 250 patients by the end of his five-day trip, Dr. Jaffer says.
Hospitalists administered IV fluids, prescribed antibiotics and pain medication, treated infected wounds, managed patients with dehydration, gastroenteritis, and tetanus, and triaged patients. “Many patients had splints placed in the field, and we would do X-rays to confirm the diagnosis. Patients were being casted right after diagnosis,” Dr. Jaffer says.
Outside the Capital
Hospitalists volunteering with Partners in Health (PIH) were tasked with maximizing the time the surgical team could spend in the OR by assessing incoming patients, triaging cases, providing post-op care, monitoring for development of medical issues related to trauma, and ensuring that every patient was seen daily, says Jonathan Crocker, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston.
Dr. Crocker arrived in Haiti four days after the earthquake and was sent to Clinique Bon Saveur, a hospital in Cange, a town located two hours outside the capital on the country’s Central Plateau. The hospital is one of 10 health facilities run by Zamni Lasante, PIH’s sister organization in Haiti. Dr. Shamasunder, of UC San Francisco, arrived in the country a few days later and was stationed at St. Marc Hospital, on the west coast of the island, about 60 miles from Port-au-Prince.
At St. Marc’s, conditions were “chaotic but functioning, bare-bones but a work in progress,” as Haitian doctors began returning to work and Creole-speaking nurses from the U.S. reached the hospital, Dr. Shamasunder explains. PIH volunteers coordinated with teams from Canada and Nepal to provide the best possible medical care to patients dealing with sepsis, serious wounds, and heart failure.
Hundreds of patients, many with multiple injuries, had been streaming into Clinique Bon Saveur since the day the earthquake struck. When Dr. Crocker arrived, the hospital was overcrowded, spilling into makeshift wards that had been set up in a church and a nearby school.
“As a hospitalist, my first concern upon arrival was anticipating the likely medical complications we would encounter with a large population of patients having experienced physical trauma,” Dr. Crocker says. “These complications included, namely, DVT and PE events, compartment syndrome, rhabdomyolysis with renal failure, hyperkalemia, wound infection, and sepsis.”
After speaking with their Haitian colleagues, PIH volunteers placed all adult patients at Clinique Bon Saveur on heparin prophylaxis. They also instituted a standard antibiotic regimen for all patients with open fractures, ensured patients received tetanus shots, and made it a priority to see every patient daily in an effort to prevent compartment syndrome and complications from rhabdomyolysis.
“As we identified more patients with acute renal failure, we moved into active screening with ‘creatinine rounds,’ where we performed BUN/Cr checks on any patient suspected of having suffered major crush injuries,” says Dr. Crocker, who used a portable ultrasound to assess patients for suspected lower-extremity DVTs. “As a team, we made a daily A, B, and C priority list for patients in need of surgeries available at the hospital, and a list of patients with injuries too complex for our surgical teams requiring transfer.”
Resume Expansion
Back at the University of Miami’s tent facility, hospitalists were chipping in wherever help was needed. “I cleaned rooms, I took out the trash, I swept floors, I dispensed medicine from the pharmacy. I just did everything,” Dr. Luly-Rivera says. “You have to go with an open mind and be prepared to do things outside your own discipline.”
Volunteers must be prepared to deal with difficult patients who are under considerable stress over their present and future situations, Dr. Luly-Rivera explains. She worries about what is to come for a country that’s ill-equipped to handle so many physically disabled people. For years, there will be a pressing need for orthopedic surgeons and physical and occupational therapists, she says.
Earthquake survivors also will need help in coping with the psychological trauma they’ve endured, says Dr. Reyes, who frequently played the role of hospital clown in the tent facility’s pediatric ward—just to help the children to laugh a bit.
“These kids are fully traumatized. They don’t want to go inside buildings because they’re afraid they will collapse,” he says. “There’s a high percentage of them who lost at least one parent in the disaster. When you go to discharge them, many don’t have a home to go to. You just feel tremendous sadness.”
Emotional Connection
The sorrow intensified when Dr. Reyes returned to work after returning from his trip to Haiti. “You can barely eat because you have a knot in your throat,” he says.
Upon her return to Miami, Dr. Luly-Rivera spent almost every spare minute watching news coverage on television and reading about the relief effort online. It was difficult for her to concentrate when working, she admits.
“It wasn’t that I felt the patients here didn’t need me,” she says. “It’s just that my mind was still in Haiti and thinking about my patients there. I had to let it go.”
Feelings of sadness and grief are common reactions to witnessing acute injuries and loss of life, says Dr. Jaffer. Some people react by refusing to leave until the work is done, or returning to the relief effort before they are ready.
—Mario Reyes, MD, FHM, director, Division of Pediatric Hospital Medicine, Miami Children’s Hospital
“Medical volunteerism shows you there is life beyond what you do in your workplace. It allows you to bridge the gap between your job and people who are less fortunate. The experience can be invigorating, but it can also be stress-inducing and lead to depression,” Dr. Jaffer says. “It’s always good to have someone you pair up with to monitor your stress level.”
After taking time to decompress, Drs. Luly-Rivera and Reyes plan to return to Haiti. They hope healthcare workers from all parts of the U.S. will continue to volunteer in the months ahead. Haiti’s weighty issues demand that non-governmental organizations (NGOs) working in the country stay and better coordinate their efforts, Dr. Reyes says.
“Ultimately, it is going to be important for any group present in Haiti to work to support the Haitian medical community,” Dr. Crocker adds. “The long-term recovery and rehabilitation of so many thousands of patients will be possible only through a robust, functional, public healthcare delivery system.”
It remains to be seen how many NGOs and volunteers will still be in Haiti a few months from now, the hospitalists said.
It’s always a concern that the attention of the global community may shift away from Haiti when the next calamity strikes in another part of the world, Dr. Jaffer notes. If the focus stays on Haiti as it rebuilds, then possibly some good will come out of the earthquake, Dr. Luly-Rivera says. But if NGOs begin to leave in the short term, the quake would only be the latest setback for one of the world’s poorest and most underdeveloped countries.
Even if the latter were to happen, Dr. Luly-Rivera still says she and other volunteers make a difference. “I’m still glad I went,” she says. “The people were so thankful.”
“You see the best of the American people there,” Dr. Reyes adds. “It’s encouraging and uplifting. It brings back faith in the medical profession and faith in people.” TH
Lisa Ryan is a freelance writer based in New Jersey.
In sight but out of mind
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).
Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
QBs vs. Hospitalists
O K, I’ll admit it: I like football. Call me a Neanderthal, but there is nothing quite like an afternoon with friends watching a tightly contested game of titans battling it out on the gridiron. Back in January, I enjoyed that glorious weekend in which the NFC and AFC crown their respective champions, each sending a team of combatants to the Super Bowl.
Fully enjoying the Sunday afternoon of ambrosia requires tons of preparation. Practically speaking, this means clearing my schedule of such clutter as child-rearing and housekeeping, along with dispatching my wife to the store minutes before my friends arrive to procure a second-chin’s worth of kettle chips and a potomaniac’s quantity of cheap beer. Then I settle into the butt-dented comfort of my overworked couch, where I’m surrounded by a rowdy pack of friends.
During hour three of the pre-game analysis, I can’t help but notice that my lovely wife, neither a fan of football or my friends spilling beer on her couch, has contracted a nasty case of the angry stink-eye, which she wields like a laser beam through my skull. I ponder the cost that all of this revelry, last-minute dispatching, and spilled beer will have on my marriage. Concluding that I indeed have at least three paws in the doghouse, I reflect on the facts that a) my wife is a saint; b) she reads this column—honey, read point “a” again; and c) Valentine’s Day is right around the corner.
Oh, well. The game must go on, and right now, it’s all about the NFL—hard-hitting, back-and-forth, in-your-face, smash-mouth action. Unbeatable. Unbeatable, that is, until you realize that a typical football game contains a lot of things, except for much actual football.
The Facts on Football
A recent Wall Street Journal analysis of NFL playoff games reported that the typical football game consists of many things, but not much real action. In fact, the average three-plus-hour telecast consists of just 10 minutes and 43 seconds of play. After subtracting about an hour of commercials, the rest of an average telecast consists of such things as players standing around (67 minutes), replays (17 minutes), and, of course, the all-important shots of cheerleaders—which is allotted, remarkably, only three seconds per game. Seems like more.
In percentage terms, the pie is doled out this way: standing around (58.5%), replays (14.5%), playing time (9.4%), coach shots (4.9%), sideline player shots (3.4%), referee shots (2.4%), crowd shots (0.9%), and other miscellany, such as footage of owners in their high-priced luxury suites (0.3%), the kicker warming up (0.2%), and, of course, cheerleaders (0.1%).
While this level of inaction has an enabling effect on convivial taunting, bet-brokering, and beer runs, it is, to be frank, a laughably low amount of action. How can an entire industry be built on such a level of inactivity? It’s a great question—one that induces a momentary chuckle until I consider how I spend much of my clinical days.
Inactivity in the Workplace
A 2006 paper in the Journal of Hospital Medicine tackled the issue of hospitalist workflow.1 Researchers followed 10 academic hospitalists through various parts of a routine day, all the while measuring to the minute how they spent their time. What they found would be as astounding to hospital outsiders as the NFL data, should anyone ever find themselves so deep in the boredom pit to be watching a hospitalist make rounds.
Which made me wonder: What would Brett Favre, the Minnesota Vikings’ future Hall of Fame quarterback, think if he were watching me ply my trade? Which led me to further wonder how far afield Brett Favre’s life would have to be derailed for him to watch me round. Finally, it left me wondering why I don’t spend my time wondering about more productive things.
Anyway, if Brett were to watch one of us, this is what he’d see: The average hospitalist in this study spent only 18% of their time in direct-patient-care activities, including taking a patient history, examining a patient, and meeting with a patient’s family. Eighteen percent! Isn’t seeing patients why I became a doctor?
While it’s more time than Brett Favre spends slinging the pigskin, it’s still an astonishingly low amount of time actually working with patients. Then there’s the “indirect patient care” category (e.g., reviewing tests, writing notes, making orders), where we spend 69% of our day. This is our time in the huddle, so to speak: lots of planning, little action. Further, these academic hospitalists spent 4% of their time on personal activities (e.g., lunch, bathroom breaks), and 3% of their time in each of the following endeavors: professional development (learning, conferences), teaching, and traveling from floor to floor seeing patients.
Another revealing find was that the average hospitalist spent 6% of their time paging other physicians and 7% returning pages from others (the average hospitalist received 3.4 pages an hour). That’s 13% of the day spent on the phone, or waiting for a phone to ring. That’s about 1.5 hours of a typical 12-hour shift. Over the course of a year, that equates to about 300 hours of time (25 shifts) spent in the paging process. If we could find a way to totally remove the paging process from hospital communication, the average hospitalist could accomplish the same amount of work they do now, and take an additional 20-25 days off per year. Perhaps we should wear high-tech helmets—you know, the kind quarterbacks like Brett Favre use to communicate with his coaches on the sidelines.
Efficient Solutions
Before my hospitalists hit me up for wireless communication devices and an extra three weeks of vacation, understand that much of the paging downtime likely is used for multitasking. In fact, in the study, 21% of a hospitalist’s time was spent working on more than one endeavor. Still, my experience tells me that there is a lot of time lost in the paging vortex.
Furthermore, the 3% of time hospitalists spent walking to other floors, 5% spent on discharge paperwork, and 1% of time spent on routine clerical work (did the researchers inadvertently report 1% instead of 10%?) adds up to nearly a tenth of the day that is either wasted, could be automated, or could be completed by ancillary staff.
To be clear, this happens through no fault of individual hospitalists. Rather, it results from the inefficiency of hospital care systems. And if we endeavor to enhance the revenue, efficiency, and satisfaction of our providers, we need to re-engineer our systems to alter that vast expanse of time spent on inactivity and inefficiency. This means adopting new modes of communication, moving toward geographic rounds, and generally retooling our operational inefficiencies.
Short of that, we risk becoming as idle as the NFL—without the cheerleaders. TH
Dr. Glasheen is The Hospitalist’s physician editor.
Reference
- O’Leary KJ, Liebovitz DM, Baker DW. How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93.
O K, I’ll admit it: I like football. Call me a Neanderthal, but there is nothing quite like an afternoon with friends watching a tightly contested game of titans battling it out on the gridiron. Back in January, I enjoyed that glorious weekend in which the NFC and AFC crown their respective champions, each sending a team of combatants to the Super Bowl.
Fully enjoying the Sunday afternoon of ambrosia requires tons of preparation. Practically speaking, this means clearing my schedule of such clutter as child-rearing and housekeeping, along with dispatching my wife to the store minutes before my friends arrive to procure a second-chin’s worth of kettle chips and a potomaniac’s quantity of cheap beer. Then I settle into the butt-dented comfort of my overworked couch, where I’m surrounded by a rowdy pack of friends.
During hour three of the pre-game analysis, I can’t help but notice that my lovely wife, neither a fan of football or my friends spilling beer on her couch, has contracted a nasty case of the angry stink-eye, which she wields like a laser beam through my skull. I ponder the cost that all of this revelry, last-minute dispatching, and spilled beer will have on my marriage. Concluding that I indeed have at least three paws in the doghouse, I reflect on the facts that a) my wife is a saint; b) she reads this column—honey, read point “a” again; and c) Valentine’s Day is right around the corner.
Oh, well. The game must go on, and right now, it’s all about the NFL—hard-hitting, back-and-forth, in-your-face, smash-mouth action. Unbeatable. Unbeatable, that is, until you realize that a typical football game contains a lot of things, except for much actual football.
The Facts on Football
A recent Wall Street Journal analysis of NFL playoff games reported that the typical football game consists of many things, but not much real action. In fact, the average three-plus-hour telecast consists of just 10 minutes and 43 seconds of play. After subtracting about an hour of commercials, the rest of an average telecast consists of such things as players standing around (67 minutes), replays (17 minutes), and, of course, the all-important shots of cheerleaders—which is allotted, remarkably, only three seconds per game. Seems like more.
In percentage terms, the pie is doled out this way: standing around (58.5%), replays (14.5%), playing time (9.4%), coach shots (4.9%), sideline player shots (3.4%), referee shots (2.4%), crowd shots (0.9%), and other miscellany, such as footage of owners in their high-priced luxury suites (0.3%), the kicker warming up (0.2%), and, of course, cheerleaders (0.1%).
While this level of inaction has an enabling effect on convivial taunting, bet-brokering, and beer runs, it is, to be frank, a laughably low amount of action. How can an entire industry be built on such a level of inactivity? It’s a great question—one that induces a momentary chuckle until I consider how I spend much of my clinical days.
Inactivity in the Workplace
A 2006 paper in the Journal of Hospital Medicine tackled the issue of hospitalist workflow.1 Researchers followed 10 academic hospitalists through various parts of a routine day, all the while measuring to the minute how they spent their time. What they found would be as astounding to hospital outsiders as the NFL data, should anyone ever find themselves so deep in the boredom pit to be watching a hospitalist make rounds.
Which made me wonder: What would Brett Favre, the Minnesota Vikings’ future Hall of Fame quarterback, think if he were watching me ply my trade? Which led me to further wonder how far afield Brett Favre’s life would have to be derailed for him to watch me round. Finally, it left me wondering why I don’t spend my time wondering about more productive things.
Anyway, if Brett were to watch one of us, this is what he’d see: The average hospitalist in this study spent only 18% of their time in direct-patient-care activities, including taking a patient history, examining a patient, and meeting with a patient’s family. Eighteen percent! Isn’t seeing patients why I became a doctor?
While it’s more time than Brett Favre spends slinging the pigskin, it’s still an astonishingly low amount of time actually working with patients. Then there’s the “indirect patient care” category (e.g., reviewing tests, writing notes, making orders), where we spend 69% of our day. This is our time in the huddle, so to speak: lots of planning, little action. Further, these academic hospitalists spent 4% of their time on personal activities (e.g., lunch, bathroom breaks), and 3% of their time in each of the following endeavors: professional development (learning, conferences), teaching, and traveling from floor to floor seeing patients.
Another revealing find was that the average hospitalist spent 6% of their time paging other physicians and 7% returning pages from others (the average hospitalist received 3.4 pages an hour). That’s 13% of the day spent on the phone, or waiting for a phone to ring. That’s about 1.5 hours of a typical 12-hour shift. Over the course of a year, that equates to about 300 hours of time (25 shifts) spent in the paging process. If we could find a way to totally remove the paging process from hospital communication, the average hospitalist could accomplish the same amount of work they do now, and take an additional 20-25 days off per year. Perhaps we should wear high-tech helmets—you know, the kind quarterbacks like Brett Favre use to communicate with his coaches on the sidelines.
Efficient Solutions
Before my hospitalists hit me up for wireless communication devices and an extra three weeks of vacation, understand that much of the paging downtime likely is used for multitasking. In fact, in the study, 21% of a hospitalist’s time was spent working on more than one endeavor. Still, my experience tells me that there is a lot of time lost in the paging vortex.
Furthermore, the 3% of time hospitalists spent walking to other floors, 5% spent on discharge paperwork, and 1% of time spent on routine clerical work (did the researchers inadvertently report 1% instead of 10%?) adds up to nearly a tenth of the day that is either wasted, could be automated, or could be completed by ancillary staff.
To be clear, this happens through no fault of individual hospitalists. Rather, it results from the inefficiency of hospital care systems. And if we endeavor to enhance the revenue, efficiency, and satisfaction of our providers, we need to re-engineer our systems to alter that vast expanse of time spent on inactivity and inefficiency. This means adopting new modes of communication, moving toward geographic rounds, and generally retooling our operational inefficiencies.
Short of that, we risk becoming as idle as the NFL—without the cheerleaders. TH
Dr. Glasheen is The Hospitalist’s physician editor.
Reference
- O’Leary KJ, Liebovitz DM, Baker DW. How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93.
O K, I’ll admit it: I like football. Call me a Neanderthal, but there is nothing quite like an afternoon with friends watching a tightly contested game of titans battling it out on the gridiron. Back in January, I enjoyed that glorious weekend in which the NFC and AFC crown their respective champions, each sending a team of combatants to the Super Bowl.
Fully enjoying the Sunday afternoon of ambrosia requires tons of preparation. Practically speaking, this means clearing my schedule of such clutter as child-rearing and housekeeping, along with dispatching my wife to the store minutes before my friends arrive to procure a second-chin’s worth of kettle chips and a potomaniac’s quantity of cheap beer. Then I settle into the butt-dented comfort of my overworked couch, where I’m surrounded by a rowdy pack of friends.
During hour three of the pre-game analysis, I can’t help but notice that my lovely wife, neither a fan of football or my friends spilling beer on her couch, has contracted a nasty case of the angry stink-eye, which she wields like a laser beam through my skull. I ponder the cost that all of this revelry, last-minute dispatching, and spilled beer will have on my marriage. Concluding that I indeed have at least three paws in the doghouse, I reflect on the facts that a) my wife is a saint; b) she reads this column—honey, read point “a” again; and c) Valentine’s Day is right around the corner.
Oh, well. The game must go on, and right now, it’s all about the NFL—hard-hitting, back-and-forth, in-your-face, smash-mouth action. Unbeatable. Unbeatable, that is, until you realize that a typical football game contains a lot of things, except for much actual football.
The Facts on Football
A recent Wall Street Journal analysis of NFL playoff games reported that the typical football game consists of many things, but not much real action. In fact, the average three-plus-hour telecast consists of just 10 minutes and 43 seconds of play. After subtracting about an hour of commercials, the rest of an average telecast consists of such things as players standing around (67 minutes), replays (17 minutes), and, of course, the all-important shots of cheerleaders—which is allotted, remarkably, only three seconds per game. Seems like more.
In percentage terms, the pie is doled out this way: standing around (58.5%), replays (14.5%), playing time (9.4%), coach shots (4.9%), sideline player shots (3.4%), referee shots (2.4%), crowd shots (0.9%), and other miscellany, such as footage of owners in their high-priced luxury suites (0.3%), the kicker warming up (0.2%), and, of course, cheerleaders (0.1%).
While this level of inaction has an enabling effect on convivial taunting, bet-brokering, and beer runs, it is, to be frank, a laughably low amount of action. How can an entire industry be built on such a level of inactivity? It’s a great question—one that induces a momentary chuckle until I consider how I spend much of my clinical days.
Inactivity in the Workplace
A 2006 paper in the Journal of Hospital Medicine tackled the issue of hospitalist workflow.1 Researchers followed 10 academic hospitalists through various parts of a routine day, all the while measuring to the minute how they spent their time. What they found would be as astounding to hospital outsiders as the NFL data, should anyone ever find themselves so deep in the boredom pit to be watching a hospitalist make rounds.
Which made me wonder: What would Brett Favre, the Minnesota Vikings’ future Hall of Fame quarterback, think if he were watching me ply my trade? Which led me to further wonder how far afield Brett Favre’s life would have to be derailed for him to watch me round. Finally, it left me wondering why I don’t spend my time wondering about more productive things.
Anyway, if Brett were to watch one of us, this is what he’d see: The average hospitalist in this study spent only 18% of their time in direct-patient-care activities, including taking a patient history, examining a patient, and meeting with a patient’s family. Eighteen percent! Isn’t seeing patients why I became a doctor?
While it’s more time than Brett Favre spends slinging the pigskin, it’s still an astonishingly low amount of time actually working with patients. Then there’s the “indirect patient care” category (e.g., reviewing tests, writing notes, making orders), where we spend 69% of our day. This is our time in the huddle, so to speak: lots of planning, little action. Further, these academic hospitalists spent 4% of their time on personal activities (e.g., lunch, bathroom breaks), and 3% of their time in each of the following endeavors: professional development (learning, conferences), teaching, and traveling from floor to floor seeing patients.
Another revealing find was that the average hospitalist spent 6% of their time paging other physicians and 7% returning pages from others (the average hospitalist received 3.4 pages an hour). That’s 13% of the day spent on the phone, or waiting for a phone to ring. That’s about 1.5 hours of a typical 12-hour shift. Over the course of a year, that equates to about 300 hours of time (25 shifts) spent in the paging process. If we could find a way to totally remove the paging process from hospital communication, the average hospitalist could accomplish the same amount of work they do now, and take an additional 20-25 days off per year. Perhaps we should wear high-tech helmets—you know, the kind quarterbacks like Brett Favre use to communicate with his coaches on the sidelines.
Efficient Solutions
Before my hospitalists hit me up for wireless communication devices and an extra three weeks of vacation, understand that much of the paging downtime likely is used for multitasking. In fact, in the study, 21% of a hospitalist’s time was spent working on more than one endeavor. Still, my experience tells me that there is a lot of time lost in the paging vortex.
Furthermore, the 3% of time hospitalists spent walking to other floors, 5% spent on discharge paperwork, and 1% of time spent on routine clerical work (did the researchers inadvertently report 1% instead of 10%?) adds up to nearly a tenth of the day that is either wasted, could be automated, or could be completed by ancillary staff.
To be clear, this happens through no fault of individual hospitalists. Rather, it results from the inefficiency of hospital care systems. And if we endeavor to enhance the revenue, efficiency, and satisfaction of our providers, we need to re-engineer our systems to alter that vast expanse of time spent on inactivity and inefficiency. This means adopting new modes of communication, moving toward geographic rounds, and generally retooling our operational inefficiencies.
Short of that, we risk becoming as idle as the NFL—without the cheerleaders. TH
Dr. Glasheen is The Hospitalist’s physician editor.
Reference
- O’Leary KJ, Liebovitz DM, Baker DW. How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93.
Should Hospitalists Who Fail to Provide a Standard of Care Be Paid for Subsequent Care?
A 72-year-old male with a history of CHF is admitted for elective total hip arthroplasty. On postoperative day one, he develops dyspnea and hypoxia, and is diagnosed with acute pulmonary edema by the hospitalist co-managing his care. Furosemide is prescribed, and he improves, and by day four is ready for discharge following another dose of diuretics. Overnight, he develops acute onset of shortness of breath and is diagnosed with a pulmonary embolism (PE). Under new regulations, the hospital will not be reimbursed for the extra cost associated with subsequent patient care. Should the hospitalist be paid for the subsequent care?
PRO
Nonpayment won’t improve quality or significantly decrease costs
The real essence of the question raised in the clinical case above is “Should doctors profit from errors?” The answer might be “It’s better than the alternative.” Allow me to explain. There essentially are two reasons to withhold payment in this scenario: one, as a mechanism for promoting quality; two, as a mechanism for decreasing costs to the payor.
The quality argument assumes the physician will deliver higher-quality care (i.e., prescribe chemical thromboprophylaxis) if a threat of nonpayment exists. This concept is simply hogwash. If expensive medical malpractice threats fail as quality-improvement (QI) mechanisms, it is absurd to think withholding a few subsequent-care charges will generate better results.
The key issue is the type of error involved. As defined by Lucien Leape, MD, in his celebrated 1994 article on medical errors, “mistakes” reflect failures during attentional behaviors, or incorrect choices.1 “Slips” reflect lapses in concentration. “Slips occur in the face of competing sensory or emotional distractions, fatigue, and stress,” and “reducing the risk of slips requires attention to the designs of protocols, devices, and work environments.”
Misjudging the type of error—in this case, a slip (find me a hospitalist who doesn’t know total hip arthroplasty requires thrombophrophylaxis)—and misapplying corrective actions will have little to no effect on outcomes. Thus, pay-withholding schemes can have a negative net effect by diverting resources from QI projects that truly improve patient outcomes.
Withholding payment in this case generates approximately $160 in direct savings to the payor (assuming Medicare payments for one 99233 and two 99232 subsequent care visits), yet the operational costs are not negligible and must be factored into the equation. The payor needs to first determine who is truly at fault: the hospitalist or the orthopedic surgeon. Answering that question requires the payor to review the co-management agreement, perhaps aided by an attorney. That’s a costly endeavor.
For the sake of argument, let’s assume in this case the hospitalist is at fault. The next step is determining if the hospitalist who failed to prescribe prophylaxis prior to the PE is the same hospitalist caring for the patient after the PE. It is inappropriate to withhold payment to hospitalist A if hospitalist B made the error. Again, significant manpower will be required to determine fault, as this is not information one finds on a UB-04 claim form submitted to Medicare.
Further eroding the $160 savings is the cost of determining whether a contraindication exists: Bleeding ulcer? Subdural hematoma? Heparinoid allergy? Let us not forget the additional costs in copying, shipping, warehousing, and eventual shredding of the records. One can readily see that the operational costs can quickly negate the $160 anticipated savings. In fact, it’s likely a negative return on investment.
Clearly, withholding payment in this scenario is an ineffective mechanism for improving quality or decreasing cost. I am not generally a proponent of rewarding failure, and perhaps as we usher in a new era of healthcare reform, the system will be redesigned in such a way that better aligns quality and cost-control measures. However, under the current system, payment denial as outlined above likely does more harm than good.
CON
Withhold payment when medical errors are easily identifiable
When I first learned of the proposal to withhold Medicare payment for hospital-acquired conditions (HACs), I had mixed emotions. On the one hand, I firmly believe that physicians should be accountable for their work; on the other hand, this policy seems to conflict sharply with the “no blame” mantra that has been prevalent in patient safety for more than a decade.2 More recently, though, many have argued for balancing the pursuit of system fixes for quality and patient-safety issues with the development of a culture of accountability.3
In theory, the HACs should meet the following criteria: They should be high-cost conditions, high-volume conditions, or both; they should be identifiable through ICD-9-CM coding as complicating conditions (CCs) or major complicating conditions (MCCs) that result in a higher-paying MS-DRG; and they should be reasonably preventable through the application of evidence-based guidelines. Some HACs are jaw-dropping lapses in care (e.g., leaving foreign bodies in during surgery). Other HACs seem to me to be much less preventable, especially fall injuries and catheter-associated urinary tract infections (UTIs). Several experts have written eloquently regarding the limitations of these new measures, particularly emphasizing the potential for increased administrative burden on hospitals and the potential for unintended consequences.4,5
However, in the case described above involving a hospitalist, I have no reservations in limiting payment to the provider. To me, failing to prescribe VTE prophylaxis in an elderly, immobilized, post-op hip replacement patient with a CHF exacerbation is the hospitalist’s equivalent to a surgeon leaving behind a sponge in an appendectomy. It also meets the elements outlined in the HAC withholding program:
- It is high-cost. The 2007 MS-DRG payment for elective hip arthroplasty was $9,863, but adding an MCC increased that cost by one-third.6
- It is readily identifiable, though one concern might be that hospitals would perform unnecessary pre-operative testing to identify asymptomatic DVT, incurring increased testing and treatment costs and increasing the incidence of bleeding complications.
- It is very preventable. Without thromboprophylaxis, 40% to 60% of hip arthroplasty patients will develop an asymptomatic DVT, and 1 in 300 will die from a PE. However, such fatal events are exceedingly rare with appropriate prevention.7
Ultimately, I think a policy of nonpayment for this case keeps with the culture of accountability we need to foster in healthcare. The financial implications of nonpayment will drive hospital innovation and force the hospital to police provider behavior in more effective ways. This is likely to be a painful process, similar to the tribulations experienced with implementing pay-for-performance programs. The Centers for Medicare and Medicaid Services (CMS) needs to be flexible in adding—and removing—new HACs based on good evidence.
Regardless, the goal of achieving a safer, more effective healthcare system remains.
References
- Leape LL. Error in medicine. JAMA. 1994;272(23):1851-1857.
- Institute of Medicine. To Err Is Human: Building a Safer Healthcare System. Washington, D.C.: National Academies Press; 2000.
- Wachter RM, Pronovost PJ. Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361:1401-1406.
- Saint S, Meddings JA, Calfee D, Kowalski CP, Krein SL. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877-884.
- Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390-2393.
- Wachter RM, Foster NE, Dudley RA. Medicare’s decision to withhold payment for hospital errors: the devil is in the det. Jt Comm J Qual Patient Saf. 2008;34(2):116-123.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S-453S.
The opinions expressed herein are those of the authors and do not represent those of SHM or The Hospitalist.
A 72-year-old male with a history of CHF is admitted for elective total hip arthroplasty. On postoperative day one, he develops dyspnea and hypoxia, and is diagnosed with acute pulmonary edema by the hospitalist co-managing his care. Furosemide is prescribed, and he improves, and by day four is ready for discharge following another dose of diuretics. Overnight, he develops acute onset of shortness of breath and is diagnosed with a pulmonary embolism (PE). Under new regulations, the hospital will not be reimbursed for the extra cost associated with subsequent patient care. Should the hospitalist be paid for the subsequent care?
PRO
Nonpayment won’t improve quality or significantly decrease costs
The real essence of the question raised in the clinical case above is “Should doctors profit from errors?” The answer might be “It’s better than the alternative.” Allow me to explain. There essentially are two reasons to withhold payment in this scenario: one, as a mechanism for promoting quality; two, as a mechanism for decreasing costs to the payor.
The quality argument assumes the physician will deliver higher-quality care (i.e., prescribe chemical thromboprophylaxis) if a threat of nonpayment exists. This concept is simply hogwash. If expensive medical malpractice threats fail as quality-improvement (QI) mechanisms, it is absurd to think withholding a few subsequent-care charges will generate better results.
The key issue is the type of error involved. As defined by Lucien Leape, MD, in his celebrated 1994 article on medical errors, “mistakes” reflect failures during attentional behaviors, or incorrect choices.1 “Slips” reflect lapses in concentration. “Slips occur in the face of competing sensory or emotional distractions, fatigue, and stress,” and “reducing the risk of slips requires attention to the designs of protocols, devices, and work environments.”
Misjudging the type of error—in this case, a slip (find me a hospitalist who doesn’t know total hip arthroplasty requires thrombophrophylaxis)—and misapplying corrective actions will have little to no effect on outcomes. Thus, pay-withholding schemes can have a negative net effect by diverting resources from QI projects that truly improve patient outcomes.
Withholding payment in this case generates approximately $160 in direct savings to the payor (assuming Medicare payments for one 99233 and two 99232 subsequent care visits), yet the operational costs are not negligible and must be factored into the equation. The payor needs to first determine who is truly at fault: the hospitalist or the orthopedic surgeon. Answering that question requires the payor to review the co-management agreement, perhaps aided by an attorney. That’s a costly endeavor.
For the sake of argument, let’s assume in this case the hospitalist is at fault. The next step is determining if the hospitalist who failed to prescribe prophylaxis prior to the PE is the same hospitalist caring for the patient after the PE. It is inappropriate to withhold payment to hospitalist A if hospitalist B made the error. Again, significant manpower will be required to determine fault, as this is not information one finds on a UB-04 claim form submitted to Medicare.
Further eroding the $160 savings is the cost of determining whether a contraindication exists: Bleeding ulcer? Subdural hematoma? Heparinoid allergy? Let us not forget the additional costs in copying, shipping, warehousing, and eventual shredding of the records. One can readily see that the operational costs can quickly negate the $160 anticipated savings. In fact, it’s likely a negative return on investment.
Clearly, withholding payment in this scenario is an ineffective mechanism for improving quality or decreasing cost. I am not generally a proponent of rewarding failure, and perhaps as we usher in a new era of healthcare reform, the system will be redesigned in such a way that better aligns quality and cost-control measures. However, under the current system, payment denial as outlined above likely does more harm than good.
CON
Withhold payment when medical errors are easily identifiable
When I first learned of the proposal to withhold Medicare payment for hospital-acquired conditions (HACs), I had mixed emotions. On the one hand, I firmly believe that physicians should be accountable for their work; on the other hand, this policy seems to conflict sharply with the “no blame” mantra that has been prevalent in patient safety for more than a decade.2 More recently, though, many have argued for balancing the pursuit of system fixes for quality and patient-safety issues with the development of a culture of accountability.3
In theory, the HACs should meet the following criteria: They should be high-cost conditions, high-volume conditions, or both; they should be identifiable through ICD-9-CM coding as complicating conditions (CCs) or major complicating conditions (MCCs) that result in a higher-paying MS-DRG; and they should be reasonably preventable through the application of evidence-based guidelines. Some HACs are jaw-dropping lapses in care (e.g., leaving foreign bodies in during surgery). Other HACs seem to me to be much less preventable, especially fall injuries and catheter-associated urinary tract infections (UTIs). Several experts have written eloquently regarding the limitations of these new measures, particularly emphasizing the potential for increased administrative burden on hospitals and the potential for unintended consequences.4,5
However, in the case described above involving a hospitalist, I have no reservations in limiting payment to the provider. To me, failing to prescribe VTE prophylaxis in an elderly, immobilized, post-op hip replacement patient with a CHF exacerbation is the hospitalist’s equivalent to a surgeon leaving behind a sponge in an appendectomy. It also meets the elements outlined in the HAC withholding program:
- It is high-cost. The 2007 MS-DRG payment for elective hip arthroplasty was $9,863, but adding an MCC increased that cost by one-third.6
- It is readily identifiable, though one concern might be that hospitals would perform unnecessary pre-operative testing to identify asymptomatic DVT, incurring increased testing and treatment costs and increasing the incidence of bleeding complications.
- It is very preventable. Without thromboprophylaxis, 40% to 60% of hip arthroplasty patients will develop an asymptomatic DVT, and 1 in 300 will die from a PE. However, such fatal events are exceedingly rare with appropriate prevention.7
Ultimately, I think a policy of nonpayment for this case keeps with the culture of accountability we need to foster in healthcare. The financial implications of nonpayment will drive hospital innovation and force the hospital to police provider behavior in more effective ways. This is likely to be a painful process, similar to the tribulations experienced with implementing pay-for-performance programs. The Centers for Medicare and Medicaid Services (CMS) needs to be flexible in adding—and removing—new HACs based on good evidence.
Regardless, the goal of achieving a safer, more effective healthcare system remains.
References
- Leape LL. Error in medicine. JAMA. 1994;272(23):1851-1857.
- Institute of Medicine. To Err Is Human: Building a Safer Healthcare System. Washington, D.C.: National Academies Press; 2000.
- Wachter RM, Pronovost PJ. Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361:1401-1406.
- Saint S, Meddings JA, Calfee D, Kowalski CP, Krein SL. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877-884.
- Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390-2393.
- Wachter RM, Foster NE, Dudley RA. Medicare’s decision to withhold payment for hospital errors: the devil is in the det. Jt Comm J Qual Patient Saf. 2008;34(2):116-123.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S-453S.
The opinions expressed herein are those of the authors and do not represent those of SHM or The Hospitalist.
A 72-year-old male with a history of CHF is admitted for elective total hip arthroplasty. On postoperative day one, he develops dyspnea and hypoxia, and is diagnosed with acute pulmonary edema by the hospitalist co-managing his care. Furosemide is prescribed, and he improves, and by day four is ready for discharge following another dose of diuretics. Overnight, he develops acute onset of shortness of breath and is diagnosed with a pulmonary embolism (PE). Under new regulations, the hospital will not be reimbursed for the extra cost associated with subsequent patient care. Should the hospitalist be paid for the subsequent care?
PRO
Nonpayment won’t improve quality or significantly decrease costs
The real essence of the question raised in the clinical case above is “Should doctors profit from errors?” The answer might be “It’s better than the alternative.” Allow me to explain. There essentially are two reasons to withhold payment in this scenario: one, as a mechanism for promoting quality; two, as a mechanism for decreasing costs to the payor.
The quality argument assumes the physician will deliver higher-quality care (i.e., prescribe chemical thromboprophylaxis) if a threat of nonpayment exists. This concept is simply hogwash. If expensive medical malpractice threats fail as quality-improvement (QI) mechanisms, it is absurd to think withholding a few subsequent-care charges will generate better results.
The key issue is the type of error involved. As defined by Lucien Leape, MD, in his celebrated 1994 article on medical errors, “mistakes” reflect failures during attentional behaviors, or incorrect choices.1 “Slips” reflect lapses in concentration. “Slips occur in the face of competing sensory or emotional distractions, fatigue, and stress,” and “reducing the risk of slips requires attention to the designs of protocols, devices, and work environments.”
Misjudging the type of error—in this case, a slip (find me a hospitalist who doesn’t know total hip arthroplasty requires thrombophrophylaxis)—and misapplying corrective actions will have little to no effect on outcomes. Thus, pay-withholding schemes can have a negative net effect by diverting resources from QI projects that truly improve patient outcomes.
Withholding payment in this case generates approximately $160 in direct savings to the payor (assuming Medicare payments for one 99233 and two 99232 subsequent care visits), yet the operational costs are not negligible and must be factored into the equation. The payor needs to first determine who is truly at fault: the hospitalist or the orthopedic surgeon. Answering that question requires the payor to review the co-management agreement, perhaps aided by an attorney. That’s a costly endeavor.
For the sake of argument, let’s assume in this case the hospitalist is at fault. The next step is determining if the hospitalist who failed to prescribe prophylaxis prior to the PE is the same hospitalist caring for the patient after the PE. It is inappropriate to withhold payment to hospitalist A if hospitalist B made the error. Again, significant manpower will be required to determine fault, as this is not information one finds on a UB-04 claim form submitted to Medicare.
Further eroding the $160 savings is the cost of determining whether a contraindication exists: Bleeding ulcer? Subdural hematoma? Heparinoid allergy? Let us not forget the additional costs in copying, shipping, warehousing, and eventual shredding of the records. One can readily see that the operational costs can quickly negate the $160 anticipated savings. In fact, it’s likely a negative return on investment.
Clearly, withholding payment in this scenario is an ineffective mechanism for improving quality or decreasing cost. I am not generally a proponent of rewarding failure, and perhaps as we usher in a new era of healthcare reform, the system will be redesigned in such a way that better aligns quality and cost-control measures. However, under the current system, payment denial as outlined above likely does more harm than good.
CON
Withhold payment when medical errors are easily identifiable
When I first learned of the proposal to withhold Medicare payment for hospital-acquired conditions (HACs), I had mixed emotions. On the one hand, I firmly believe that physicians should be accountable for their work; on the other hand, this policy seems to conflict sharply with the “no blame” mantra that has been prevalent in patient safety for more than a decade.2 More recently, though, many have argued for balancing the pursuit of system fixes for quality and patient-safety issues with the development of a culture of accountability.3
In theory, the HACs should meet the following criteria: They should be high-cost conditions, high-volume conditions, or both; they should be identifiable through ICD-9-CM coding as complicating conditions (CCs) or major complicating conditions (MCCs) that result in a higher-paying MS-DRG; and they should be reasonably preventable through the application of evidence-based guidelines. Some HACs are jaw-dropping lapses in care (e.g., leaving foreign bodies in during surgery). Other HACs seem to me to be much less preventable, especially fall injuries and catheter-associated urinary tract infections (UTIs). Several experts have written eloquently regarding the limitations of these new measures, particularly emphasizing the potential for increased administrative burden on hospitals and the potential for unintended consequences.4,5
However, in the case described above involving a hospitalist, I have no reservations in limiting payment to the provider. To me, failing to prescribe VTE prophylaxis in an elderly, immobilized, post-op hip replacement patient with a CHF exacerbation is the hospitalist’s equivalent to a surgeon leaving behind a sponge in an appendectomy. It also meets the elements outlined in the HAC withholding program:
- It is high-cost. The 2007 MS-DRG payment for elective hip arthroplasty was $9,863, but adding an MCC increased that cost by one-third.6
- It is readily identifiable, though one concern might be that hospitals would perform unnecessary pre-operative testing to identify asymptomatic DVT, incurring increased testing and treatment costs and increasing the incidence of bleeding complications.
- It is very preventable. Without thromboprophylaxis, 40% to 60% of hip arthroplasty patients will develop an asymptomatic DVT, and 1 in 300 will die from a PE. However, such fatal events are exceedingly rare with appropriate prevention.7
Ultimately, I think a policy of nonpayment for this case keeps with the culture of accountability we need to foster in healthcare. The financial implications of nonpayment will drive hospital innovation and force the hospital to police provider behavior in more effective ways. This is likely to be a painful process, similar to the tribulations experienced with implementing pay-for-performance programs. The Centers for Medicare and Medicaid Services (CMS) needs to be flexible in adding—and removing—new HACs based on good evidence.
Regardless, the goal of achieving a safer, more effective healthcare system remains.
References
- Leape LL. Error in medicine. JAMA. 1994;272(23):1851-1857.
- Institute of Medicine. To Err Is Human: Building a Safer Healthcare System. Washington, D.C.: National Academies Press; 2000.
- Wachter RM, Pronovost PJ. Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361:1401-1406.
- Saint S, Meddings JA, Calfee D, Kowalski CP, Krein SL. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877-884.
- Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390-2393.
- Wachter RM, Foster NE, Dudley RA. Medicare’s decision to withhold payment for hospital errors: the devil is in the det. Jt Comm J Qual Patient Saf. 2008;34(2):116-123.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S-453S.
The opinions expressed herein are those of the authors and do not represent those of SHM or The Hospitalist.
Group Leaders Can Shift the HM Negotiation Paradigm
Whether hospitalists like it or not, the art of negotiation has a significant impact on their daily activities. Negotiations take place with consultants over what the perceived optimal plan of care should be. Discussions are held with patients on how best to overcome the social, financial, and psychological barriers that may impede their health. Hospitalists negotiate with administrators over schedules, benefits, and responsibilities.
Quite frequently, negotiation is viewed as a process where one party wins and the other loses, a zero-sum game, like chess. The spoils may be financial (e.g., better reimbursements) or they may be cognitive (e.g., success in convincing someone of your particular viewpoint). Significant value that could potentially benefit both parties may be lost if the negotiation is approached with a win-loss mentality. However, with proper preparation and insight, a hospitalist can create value in a negotiation that otherwise may be lost by shifting their negotiation paradigm to a collaborative strategy.
A collaborative strategy is when the relationship—and not just the outcome—is important. This would apply to most negotiations that hospitalists take part in.
A significant part of this strategy involves listening and allowing the other side to divulge their interests and positions. Information must flow freely. Once the problem is identified, it must then be detailed further, ensuring both parties understand each other.
Only once both party’s issues are presented can an alternative solution be contemplated that will be win-win in nature. The parties then must both agree to choose that solution and move forward.
The optimal result is that the chosen solution appeases both parties and has a greater total value than if both sides were solely vying for their own interests.
Riyad Fares, MD,
hospitalist,
Adventist Hospital, Portland, Ore.
Whether hospitalists like it or not, the art of negotiation has a significant impact on their daily activities. Negotiations take place with consultants over what the perceived optimal plan of care should be. Discussions are held with patients on how best to overcome the social, financial, and psychological barriers that may impede their health. Hospitalists negotiate with administrators over schedules, benefits, and responsibilities.
Quite frequently, negotiation is viewed as a process where one party wins and the other loses, a zero-sum game, like chess. The spoils may be financial (e.g., better reimbursements) or they may be cognitive (e.g., success in convincing someone of your particular viewpoint). Significant value that could potentially benefit both parties may be lost if the negotiation is approached with a win-loss mentality. However, with proper preparation and insight, a hospitalist can create value in a negotiation that otherwise may be lost by shifting their negotiation paradigm to a collaborative strategy.
A collaborative strategy is when the relationship—and not just the outcome—is important. This would apply to most negotiations that hospitalists take part in.
A significant part of this strategy involves listening and allowing the other side to divulge their interests and positions. Information must flow freely. Once the problem is identified, it must then be detailed further, ensuring both parties understand each other.
Only once both party’s issues are presented can an alternative solution be contemplated that will be win-win in nature. The parties then must both agree to choose that solution and move forward.
The optimal result is that the chosen solution appeases both parties and has a greater total value than if both sides were solely vying for their own interests.
Riyad Fares, MD,
hospitalist,
Adventist Hospital, Portland, Ore.
Whether hospitalists like it or not, the art of negotiation has a significant impact on their daily activities. Negotiations take place with consultants over what the perceived optimal plan of care should be. Discussions are held with patients on how best to overcome the social, financial, and psychological barriers that may impede their health. Hospitalists negotiate with administrators over schedules, benefits, and responsibilities.
Quite frequently, negotiation is viewed as a process where one party wins and the other loses, a zero-sum game, like chess. The spoils may be financial (e.g., better reimbursements) or they may be cognitive (e.g., success in convincing someone of your particular viewpoint). Significant value that could potentially benefit both parties may be lost if the negotiation is approached with a win-loss mentality. However, with proper preparation and insight, a hospitalist can create value in a negotiation that otherwise may be lost by shifting their negotiation paradigm to a collaborative strategy.
A collaborative strategy is when the relationship—and not just the outcome—is important. This would apply to most negotiations that hospitalists take part in.
A significant part of this strategy involves listening and allowing the other side to divulge their interests and positions. Information must flow freely. Once the problem is identified, it must then be detailed further, ensuring both parties understand each other.
Only once both party’s issues are presented can an alternative solution be contemplated that will be win-win in nature. The parties then must both agree to choose that solution and move forward.
The optimal result is that the chosen solution appeases both parties and has a greater total value than if both sides were solely vying for their own interests.
Riyad Fares, MD,
hospitalist,
Adventist Hospital, Portland, Ore.
Transitions of Care Integral to HM Patient Care
Transitions of Care Integral to HM Patient Care
I just finished my internal-medicine training and started a job as a hospitalist. We are a new hospitalist group, and I have been told that “transitions of care” is important to HM groups. I understand that getting information back to the patients’ primary-care physicians (PCPs) is important, but I am worried that I don’t have the whole picture. Is there something I am missing?
E. Parkhurst, MD
Tampa, Fla.
Dr. Hospitalist responds: Congrats on your new job. I am pleased to hear that you are motivated to learn more about transitions of care. It is important to hospitalist groups, but even more important to patients. I suspect your instincts are correct. You have an idea of what is meant by “transitions of care,” but probably do not appreciate all the nuances of the term. I certainly did not when I came out of training many years ago.
Transitions of care is a critical aspect of every patient’s care, and thus should be important to every healthcare provider. Our job is to care for the hospitalized patient and help them navigate through the complex systems of the hospital. How well we guide the patients through these transitions is reflected in their outcomes.
What is the definition of “transitions of care”? I find it useful to think about the patient’s journey when the decision is made to hospitalize the patient. When the patient is hospitalized, it is easy to recognize that the patient’s physical location is different; some, if not all, of the patient’s providers are different, too. The patient might have the same PCP caring for them in the hospital, but the nurses are different. The contrast is more evident if all of the patient’s providers are different. The ED is the point of entry for most patients. This is another location with another group of providers who do not have knowledge of all of the patient’s medical issues.
The hospital discharge is another inevitable transition. Most patients go home, but some will go to another healthcare facility (e.g., rehabilitation hospital) with another group of providers.
As you can see, the admission and discharge from the hospital involves multiple transitions. But multiple transitions also occur within the hospital. The patient could move from the general medical ward to the ICU and back; the patient might spend time in the surgical suite or operating room. Many patients go to radiology or other parts of the hospital for testing or procedures. At each location, the patient has a new group of providers.
But even if a physical location does not change, there could be a transition in care. During the day, one hospitalist or nurse might care for the patient. At night, another group of doctors and nurses are responsible for the patient’s care. Information must be transmitted and received between all of the parties at each transition in order for the appropriate care to proceed.
Effective transitions can improve provider efficiency. Think about how much easier it is to care for a patient whose care you assume when you have a clear understanding of the patient’s issues. Minimizing medical errors and increasing effective communication can reduce medical and legal risks. Effective transitions also minimize the length of hospital stay for the patient and minimize the risk of unnecessary readmission to the hospital. These can result in enhanced financial outcomes.
I think the key to effective and safe transitions of care is to create a mutually-agreed-upon process of communication and a level of expectation from all providers to carry out their role in the process. This is always easier said than done. In fact, the lack of an agreed-upon process often is a common barrier to effective transitions of care. Each participant’s role in the patient’s transitions might compete with another set of agendas.
As you can see, transitions of care is a complex topic, and I have only briefly reviewed it here. For more information, visit www.hospitalmedicine.org/boost. TH
Image Source: AMANE KANEKO
Transitions of Care Integral to HM Patient Care
I just finished my internal-medicine training and started a job as a hospitalist. We are a new hospitalist group, and I have been told that “transitions of care” is important to HM groups. I understand that getting information back to the patients’ primary-care physicians (PCPs) is important, but I am worried that I don’t have the whole picture. Is there something I am missing?
E. Parkhurst, MD
Tampa, Fla.
Dr. Hospitalist responds: Congrats on your new job. I am pleased to hear that you are motivated to learn more about transitions of care. It is important to hospitalist groups, but even more important to patients. I suspect your instincts are correct. You have an idea of what is meant by “transitions of care,” but probably do not appreciate all the nuances of the term. I certainly did not when I came out of training many years ago.
Transitions of care is a critical aspect of every patient’s care, and thus should be important to every healthcare provider. Our job is to care for the hospitalized patient and help them navigate through the complex systems of the hospital. How well we guide the patients through these transitions is reflected in their outcomes.
What is the definition of “transitions of care”? I find it useful to think about the patient’s journey when the decision is made to hospitalize the patient. When the patient is hospitalized, it is easy to recognize that the patient’s physical location is different; some, if not all, of the patient’s providers are different, too. The patient might have the same PCP caring for them in the hospital, but the nurses are different. The contrast is more evident if all of the patient’s providers are different. The ED is the point of entry for most patients. This is another location with another group of providers who do not have knowledge of all of the patient’s medical issues.
The hospital discharge is another inevitable transition. Most patients go home, but some will go to another healthcare facility (e.g., rehabilitation hospital) with another group of providers.
As you can see, the admission and discharge from the hospital involves multiple transitions. But multiple transitions also occur within the hospital. The patient could move from the general medical ward to the ICU and back; the patient might spend time in the surgical suite or operating room. Many patients go to radiology or other parts of the hospital for testing or procedures. At each location, the patient has a new group of providers.
But even if a physical location does not change, there could be a transition in care. During the day, one hospitalist or nurse might care for the patient. At night, another group of doctors and nurses are responsible for the patient’s care. Information must be transmitted and received between all of the parties at each transition in order for the appropriate care to proceed.
Effective transitions can improve provider efficiency. Think about how much easier it is to care for a patient whose care you assume when you have a clear understanding of the patient’s issues. Minimizing medical errors and increasing effective communication can reduce medical and legal risks. Effective transitions also minimize the length of hospital stay for the patient and minimize the risk of unnecessary readmission to the hospital. These can result in enhanced financial outcomes.
I think the key to effective and safe transitions of care is to create a mutually-agreed-upon process of communication and a level of expectation from all providers to carry out their role in the process. This is always easier said than done. In fact, the lack of an agreed-upon process often is a common barrier to effective transitions of care. Each participant’s role in the patient’s transitions might compete with another set of agendas.
As you can see, transitions of care is a complex topic, and I have only briefly reviewed it here. For more information, visit www.hospitalmedicine.org/boost. TH
Image Source: AMANE KANEKO
Transitions of Care Integral to HM Patient Care
I just finished my internal-medicine training and started a job as a hospitalist. We are a new hospitalist group, and I have been told that “transitions of care” is important to HM groups. I understand that getting information back to the patients’ primary-care physicians (PCPs) is important, but I am worried that I don’t have the whole picture. Is there something I am missing?
E. Parkhurst, MD
Tampa, Fla.
Dr. Hospitalist responds: Congrats on your new job. I am pleased to hear that you are motivated to learn more about transitions of care. It is important to hospitalist groups, but even more important to patients. I suspect your instincts are correct. You have an idea of what is meant by “transitions of care,” but probably do not appreciate all the nuances of the term. I certainly did not when I came out of training many years ago.
Transitions of care is a critical aspect of every patient’s care, and thus should be important to every healthcare provider. Our job is to care for the hospitalized patient and help them navigate through the complex systems of the hospital. How well we guide the patients through these transitions is reflected in their outcomes.
What is the definition of “transitions of care”? I find it useful to think about the patient’s journey when the decision is made to hospitalize the patient. When the patient is hospitalized, it is easy to recognize that the patient’s physical location is different; some, if not all, of the patient’s providers are different, too. The patient might have the same PCP caring for them in the hospital, but the nurses are different. The contrast is more evident if all of the patient’s providers are different. The ED is the point of entry for most patients. This is another location with another group of providers who do not have knowledge of all of the patient’s medical issues.
The hospital discharge is another inevitable transition. Most patients go home, but some will go to another healthcare facility (e.g., rehabilitation hospital) with another group of providers.
As you can see, the admission and discharge from the hospital involves multiple transitions. But multiple transitions also occur within the hospital. The patient could move from the general medical ward to the ICU and back; the patient might spend time in the surgical suite or operating room. Many patients go to radiology or other parts of the hospital for testing or procedures. At each location, the patient has a new group of providers.
But even if a physical location does not change, there could be a transition in care. During the day, one hospitalist or nurse might care for the patient. At night, another group of doctors and nurses are responsible for the patient’s care. Information must be transmitted and received between all of the parties at each transition in order for the appropriate care to proceed.
Effective transitions can improve provider efficiency. Think about how much easier it is to care for a patient whose care you assume when you have a clear understanding of the patient’s issues. Minimizing medical errors and increasing effective communication can reduce medical and legal risks. Effective transitions also minimize the length of hospital stay for the patient and minimize the risk of unnecessary readmission to the hospital. These can result in enhanced financial outcomes.
I think the key to effective and safe transitions of care is to create a mutually-agreed-upon process of communication and a level of expectation from all providers to carry out their role in the process. This is always easier said than done. In fact, the lack of an agreed-upon process often is a common barrier to effective transitions of care. Each participant’s role in the patient’s transitions might compete with another set of agendas.
As you can see, transitions of care is a complex topic, and I have only briefly reviewed it here. For more information, visit www.hospitalmedicine.org/boost. TH
Image Source: AMANE KANEKO