User login
Prior psychological distress tied to ‘long-COVID’ conditions
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.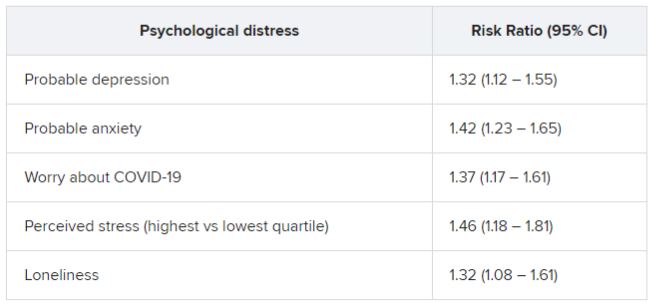
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Reporting Coronary Artery Calcium on Low-Dose Computed Tomography Impacts Statin Management in a Lung Cancer Screening Population
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
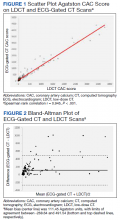
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Overall survival dips with vitamin D deficiency in melanoma
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
, according to research presented at the annual congress of the European Academy of Dermatology and Venereology.
Whereas the 5-year overall survival was 90% when vitamin D serum levels were above a 10 ng/mL threshold, it was 84% when levels fell below it. Notably, the gap in overall survival between those above and below the threshold appeared to widen as time went on.
The research adds to existing evidence that “vitamin D levels can play an important and independent role in patients’ survival outcomes,” study investigator Inés Gracia-Darder, MD, told this news organization. “The important application in clinical practice would be to know if vitamin D supplementation influences the survival of melanoma patients,” said Dr. Gracia-Darder, a clinical specialist in dermatology at the Hospital Universitari Son Espases, Mallorca, Spain.
Known association, but not much data
“It is not a new finding,” but there are limited data, especially in melanoma, said Julie De Smedt, MD, of KU Leuven, Belgium, who was asked to comment on the results. Other groups have shown, certainly for cancer in general, that vitamin D can have an effect on overall survival.
“Low levels of vitamin D are associated with the pathological parameters of the melanoma, such as the thickness of the tumor,” Dr. De Smedt said in an interview, indicating that it’s not just overall survival that might be affected.
“So we assume that also has an effect on melanoma-specific survival,” she added.
That assumption, however, is not supported by the data Dr. Gracia-Darder presented, as there was no difference in melanoma-specific survival among the two groups of patients that had been studied.
Retrospective cohort analysis
Vitamin D levels had been studied in 264 patients who were included in the retrospective cohort analysis. All had invasive melanomas, and all had been seen at the Hospital Clinic of Barcelona between January 1998 and June 2021. Their mean age was 57 years, and the median follow-up was 6.7 years.
For inclusion, all patients had to have had their vitamin D levels measured after being diagnosed with melanoma; those with a 25-hydroxyvitamin D3 serum level of less than 10 ng/mL were deemed to be vitamin D deficient, whereas those with levels of 10 ng/mL and above were deemed normal or insufficient.
A measurement less than 10 ng/mL is considered vitamin D deficiency, Dr. De Smedt said. “But there is a difference between countries, and there’s also a difference between societies,” noting the cut-off used in the lab where she works is 20 ng/mL. This makes it difficult to compare studies, she said.
Independent association with overall survival
Seasonal variation in vitamin D levels were considered as a possible confounding factor, but Dr. Gracia-Darder noted that there was a similar distribution of measurements taken between October to March and April to September.
Univariate and multivariate analyses established vitamin D deficiency as being independently associated with overall survival with hazard ratios of 2.34 and 2.45, respectively.
Other predictive factors were having a higher Breslow index, as well as older age and gender.
Time to recommend vitamin D supplementation?
So should patients with melanoma have their vitamin D levels routinely checked? And what about advising them to take vitamin D supplements?
“In our practice, we analyze the vitamin D levels of our patients,” Dr. Gracia-Darder said. Patients are told to limit their exposure to the sun because of their skin cancer, so they are very likely to become vitamin D deficient.
While dietary changes or supplements might be suggested, there’s no real evidence to support upping vitamin D levels to date, so “future prospective studies are needed,” Dr. Gracia-Darder added.
Such studies have already started, including one in Italy, one in Australia, and another study that Dr. De Smedt has been involved with for the past few years.
Called the ViDMe study, it’s a multicenter, randomized, double-blind trial in which patients are being given a high-dose oral vitamin D supplement or placebo once a month for at least 1 year. About 430 patients with a first cutaneous malignant melanoma have been included in the trial, which started in December 2012.
It is hoped that the results will show that the supplementation will have had a protective effect on the risk of relapse and that there will be a correlation between vitamin D levels in the blood and vitamin D receptor immunoreactivity in the tumor.
“The study is still blinded,” Dr. De Smedt said. “We will unblind in the coming months and then at the end of the year, maybe next year, we will have the results.”
The study reported by Dr. Gracia-Darder did not receive any specific funding. Dr. Gracia-Darder disclosed that the melanoma unit where the study was performed receives many grants and funds to carry out research. She reported no other relevant financial relationships. Dr. De Smedt had no relevant financial relationships. The ViDMe study is sponsored by the Universitaire Ziekenhuizen Leuven.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
NP-Led Suspicion of Cancer Clinic Improves Timeliness of Care for Veterans
Clinical Situation
Delays in diagnosis affect outcomes in veterans with cancer. Veterans sent into the community for suspected cancer frequently experience delays in diagnosis and treatment. This is further complicated by inappropriate workup, resulting in additional delays. Retaining veterans within the VA system for care and providing guidance to primary care providers (PCPs) to assist with expedited workup was an identified need. The Suspicion of Cancer Clinic (SOCC) was developed to address barriers to timely cancer diagnosis and care.
Literature
Researched private sector models of rapid cancer diagnostic and suspicion clinics. Literature analyzed showed improved outcomes through reduction of diagnostic delay. Nurse practitioner (NP)-led clinics were determined to be effective in expediting diagnosis and reducing cancer care delays.
Intervention
The Suspicion of Cancer Clinic is a tele-clinic, staffed with a NP. Diagnostic consult for the NP to assume the workup upon discovery of high suspicion of cancer, or via non-visit consult (NVC) to provide diagnostic guidance are available to PCPs. Outreach and education were performed prior initial clinic launch and post-launch, when need for further clarification of role and scope of the clinic was identified, based on consult trends.
Outcomes/Implications
The SOCC received 133 consults between 9/1/2021 and 6/6/2022 for veterans ranging age 29-94 years. Of these consults, 25 were expedited, diagnostic workups, 47 were NVCs, eliminating unnecessary or incomplete workups, yielding 23 veterans diagnosed with one of 8 types cancer. An additional 34 consults were forwarded to other appropriate service, and 27 were not appropriate for clinic and cancelled. Further outreach and education resulted in a 55% decrease in inappropriate consults. The NP retained 10 veterans (50%) within the VA for diagnostics, who had planned to receive community workup, which is an average four-week delay to schedule in the community. The SOCC was developed utilizing existing staff. The tele-clinic relieves workspace burden. Veterans received timely and appropriate cancer workups, reducing diagnostic delays. PCPs received additional support and guidance. Veterans retained within the VA system is more cost-effective and avoids community care delays. NP-led suspicion/rapid diagnostic clinic effectively reduced care delays by immediate initiation of further diagnostics and appropriate utilization of resources.
References
Campbell C, Nowell A, Karagheusian K, Giroux J, Kiteley C, Martelli L, McQuestion M, Quinn M, Rowe Samadhin YP, Touw M, Moody L. Practical innovation: Advanced practice nurses in cancer care. Can Oncol Nurs J. 2020 Jan 1;30(1):9-15. doi:10.5737/23688076301915. PMID: 33119001; PMCID: PMC7585714.
Drudge-Coates L, Khati V, Ballesteros R, Martyn-Hemphill C, Brown C, Green J, Challacombe B, Muir G. A nurse practitioner model for the assessment of suspected prostate cancer referrals is safe, cost and time efficient. Ecancermedicalscience. 2019 Dec 18;13:994. doi:10.3332/ecancer.2019.994. PMID: 32010218; PMCID: PMC6974368.
Nixon S, Bezverbnaya K, Maganti M, Gullane P, Reedijk M, Kuruvilla J, Prica A, Kridel R, Kukreti V, Bennett S, Rogalla P, Delabie J, Pintilie M, Crump M. Evaluation of Lymphadenopathy and Suspected Lymphoma in a Lymphoma Rapid Diagnosis Clinic. JCO Oncol Pract. 2020 Jan;16(1):e29-e36. doi:10.1200/JOP.19.00202. Epub 2019 Oct 1. PMID: 31573831.
Vasilakis C, Forte P. Setting up a rapid diagnostic clinic for patients with vague symptoms of cancer: a mixed method process evaluation study. BMC Health Serv Res. 2021 Apr 17;21(1):357. doi: 10.1186/s12913-021-06360-0. PMID: 33865373; PMCID: PMC8052708.
Clinical Situation
Delays in diagnosis affect outcomes in veterans with cancer. Veterans sent into the community for suspected cancer frequently experience delays in diagnosis and treatment. This is further complicated by inappropriate workup, resulting in additional delays. Retaining veterans within the VA system for care and providing guidance to primary care providers (PCPs) to assist with expedited workup was an identified need. The Suspicion of Cancer Clinic (SOCC) was developed to address barriers to timely cancer diagnosis and care.
Literature
Researched private sector models of rapid cancer diagnostic and suspicion clinics. Literature analyzed showed improved outcomes through reduction of diagnostic delay. Nurse practitioner (NP)-led clinics were determined to be effective in expediting diagnosis and reducing cancer care delays.
Intervention
The Suspicion of Cancer Clinic is a tele-clinic, staffed with a NP. Diagnostic consult for the NP to assume the workup upon discovery of high suspicion of cancer, or via non-visit consult (NVC) to provide diagnostic guidance are available to PCPs. Outreach and education were performed prior initial clinic launch and post-launch, when need for further clarification of role and scope of the clinic was identified, based on consult trends.
Outcomes/Implications
The SOCC received 133 consults between 9/1/2021 and 6/6/2022 for veterans ranging age 29-94 years. Of these consults, 25 were expedited, diagnostic workups, 47 were NVCs, eliminating unnecessary or incomplete workups, yielding 23 veterans diagnosed with one of 8 types cancer. An additional 34 consults were forwarded to other appropriate service, and 27 were not appropriate for clinic and cancelled. Further outreach and education resulted in a 55% decrease in inappropriate consults. The NP retained 10 veterans (50%) within the VA for diagnostics, who had planned to receive community workup, which is an average four-week delay to schedule in the community. The SOCC was developed utilizing existing staff. The tele-clinic relieves workspace burden. Veterans received timely and appropriate cancer workups, reducing diagnostic delays. PCPs received additional support and guidance. Veterans retained within the VA system is more cost-effective and avoids community care delays. NP-led suspicion/rapid diagnostic clinic effectively reduced care delays by immediate initiation of further diagnostics and appropriate utilization of resources.
References
Campbell C, Nowell A, Karagheusian K, Giroux J, Kiteley C, Martelli L, McQuestion M, Quinn M, Rowe Samadhin YP, Touw M, Moody L. Practical innovation: Advanced practice nurses in cancer care. Can Oncol Nurs J. 2020 Jan 1;30(1):9-15. doi:10.5737/23688076301915. PMID: 33119001; PMCID: PMC7585714.
Drudge-Coates L, Khati V, Ballesteros R, Martyn-Hemphill C, Brown C, Green J, Challacombe B, Muir G. A nurse practitioner model for the assessment of suspected prostate cancer referrals is safe, cost and time efficient. Ecancermedicalscience. 2019 Dec 18;13:994. doi:10.3332/ecancer.2019.994. PMID: 32010218; PMCID: PMC6974368.
Nixon S, Bezverbnaya K, Maganti M, Gullane P, Reedijk M, Kuruvilla J, Prica A, Kridel R, Kukreti V, Bennett S, Rogalla P, Delabie J, Pintilie M, Crump M. Evaluation of Lymphadenopathy and Suspected Lymphoma in a Lymphoma Rapid Diagnosis Clinic. JCO Oncol Pract. 2020 Jan;16(1):e29-e36. doi:10.1200/JOP.19.00202. Epub 2019 Oct 1. PMID: 31573831.
Vasilakis C, Forte P. Setting up a rapid diagnostic clinic for patients with vague symptoms of cancer: a mixed method process evaluation study. BMC Health Serv Res. 2021 Apr 17;21(1):357. doi: 10.1186/s12913-021-06360-0. PMID: 33865373; PMCID: PMC8052708.
Clinical Situation
Delays in diagnosis affect outcomes in veterans with cancer. Veterans sent into the community for suspected cancer frequently experience delays in diagnosis and treatment. This is further complicated by inappropriate workup, resulting in additional delays. Retaining veterans within the VA system for care and providing guidance to primary care providers (PCPs) to assist with expedited workup was an identified need. The Suspicion of Cancer Clinic (SOCC) was developed to address barriers to timely cancer diagnosis and care.
Literature
Researched private sector models of rapid cancer diagnostic and suspicion clinics. Literature analyzed showed improved outcomes through reduction of diagnostic delay. Nurse practitioner (NP)-led clinics were determined to be effective in expediting diagnosis and reducing cancer care delays.
Intervention
The Suspicion of Cancer Clinic is a tele-clinic, staffed with a NP. Diagnostic consult for the NP to assume the workup upon discovery of high suspicion of cancer, or via non-visit consult (NVC) to provide diagnostic guidance are available to PCPs. Outreach and education were performed prior initial clinic launch and post-launch, when need for further clarification of role and scope of the clinic was identified, based on consult trends.
Outcomes/Implications
The SOCC received 133 consults between 9/1/2021 and 6/6/2022 for veterans ranging age 29-94 years. Of these consults, 25 were expedited, diagnostic workups, 47 were NVCs, eliminating unnecessary or incomplete workups, yielding 23 veterans diagnosed with one of 8 types cancer. An additional 34 consults were forwarded to other appropriate service, and 27 were not appropriate for clinic and cancelled. Further outreach and education resulted in a 55% decrease in inappropriate consults. The NP retained 10 veterans (50%) within the VA for diagnostics, who had planned to receive community workup, which is an average four-week delay to schedule in the community. The SOCC was developed utilizing existing staff. The tele-clinic relieves workspace burden. Veterans received timely and appropriate cancer workups, reducing diagnostic delays. PCPs received additional support and guidance. Veterans retained within the VA system is more cost-effective and avoids community care delays. NP-led suspicion/rapid diagnostic clinic effectively reduced care delays by immediate initiation of further diagnostics and appropriate utilization of resources.
References
Campbell C, Nowell A, Karagheusian K, Giroux J, Kiteley C, Martelli L, McQuestion M, Quinn M, Rowe Samadhin YP, Touw M, Moody L. Practical innovation: Advanced practice nurses in cancer care. Can Oncol Nurs J. 2020 Jan 1;30(1):9-15. doi:10.5737/23688076301915. PMID: 33119001; PMCID: PMC7585714.
Drudge-Coates L, Khati V, Ballesteros R, Martyn-Hemphill C, Brown C, Green J, Challacombe B, Muir G. A nurse practitioner model for the assessment of suspected prostate cancer referrals is safe, cost and time efficient. Ecancermedicalscience. 2019 Dec 18;13:994. doi:10.3332/ecancer.2019.994. PMID: 32010218; PMCID: PMC6974368.
Nixon S, Bezverbnaya K, Maganti M, Gullane P, Reedijk M, Kuruvilla J, Prica A, Kridel R, Kukreti V, Bennett S, Rogalla P, Delabie J, Pintilie M, Crump M. Evaluation of Lymphadenopathy and Suspected Lymphoma in a Lymphoma Rapid Diagnosis Clinic. JCO Oncol Pract. 2020 Jan;16(1):e29-e36. doi:10.1200/JOP.19.00202. Epub 2019 Oct 1. PMID: 31573831.
Vasilakis C, Forte P. Setting up a rapid diagnostic clinic for patients with vague symptoms of cancer: a mixed method process evaluation study. BMC Health Serv Res. 2021 Apr 17;21(1):357. doi: 10.1186/s12913-021-06360-0. PMID: 33865373; PMCID: PMC8052708.
An Open-Label Feasibility and Acceptability Pilot of Hypnosis and Mindfulness Meditation for Cancer Pain in Veterans
Purpose
This was an open label trial to determine feasibility and acceptability of 2 complementary and integrative interventions (self-hypnosis [HYP] and mindfulness meditation [MM]) for pain in veterans undergoing treatment for head and neck cancer (HNC) at VA Puget Sound.
Background
HNC is associated with pain prior to and during treatment. HYP and MM have shown promise for procedural, acute, and chronic pain and may be a helpful addition to cancer treatment.
Methods
All veterans initiating treatment during the study window (2018-2020) were offered study treatment in addition to usual care. After providing informed consent and hearing a brief description of the interventions, participants selected either the HYP or MM intervention or a control condition (ie, complete assessments but no intervention). Participants met with a study clinician who introduced the intervention and provided audio recordings and a workbook and instructed them to listen to the recordings as often as they deemed helpful. Participants completed survey assessments at baseline, week 4, and at study completion (8 weeks). Measures included patient-reported satisfaction and perceived treatment helpfulness, frequency of practice, and likeliness of using skills going forward.
Data Analysis
Descriptive statistics were computed for all measures collected. No statistical tests were conducted due to small sample size.
Results
Of the 15 veterans who enrolled, 7 selected HYP, 7 selected MM, none selected the control condition, and 1 withdrew prior to treatment selection. Of the 14 completers, 79% reported that their chosen treatment was helpful and that they practiced at least once a week; 71% reported that they are likely to use these skills going forward. No adverse events were reported.
Conclusions/Implications
Self-guided HYP and MM interventions can be administered feasibly and are highly acceptable to veterans undergoing HNC treatment in a VA setting. HYP or MM interventions are feasible to implement with little demand on resources, and that listening to recordings is acceptable and helpful for veterans with pain related to cancer and cancer treatment. Further research is warranted to formally evaluate the efficacy of these interventions in this population in a well-powered study.
Purpose
This was an open label trial to determine feasibility and acceptability of 2 complementary and integrative interventions (self-hypnosis [HYP] and mindfulness meditation [MM]) for pain in veterans undergoing treatment for head and neck cancer (HNC) at VA Puget Sound.
Background
HNC is associated with pain prior to and during treatment. HYP and MM have shown promise for procedural, acute, and chronic pain and may be a helpful addition to cancer treatment.
Methods
All veterans initiating treatment during the study window (2018-2020) were offered study treatment in addition to usual care. After providing informed consent and hearing a brief description of the interventions, participants selected either the HYP or MM intervention or a control condition (ie, complete assessments but no intervention). Participants met with a study clinician who introduced the intervention and provided audio recordings and a workbook and instructed them to listen to the recordings as often as they deemed helpful. Participants completed survey assessments at baseline, week 4, and at study completion (8 weeks). Measures included patient-reported satisfaction and perceived treatment helpfulness, frequency of practice, and likeliness of using skills going forward.
Data Analysis
Descriptive statistics were computed for all measures collected. No statistical tests were conducted due to small sample size.
Results
Of the 15 veterans who enrolled, 7 selected HYP, 7 selected MM, none selected the control condition, and 1 withdrew prior to treatment selection. Of the 14 completers, 79% reported that their chosen treatment was helpful and that they practiced at least once a week; 71% reported that they are likely to use these skills going forward. No adverse events were reported.
Conclusions/Implications
Self-guided HYP and MM interventions can be administered feasibly and are highly acceptable to veterans undergoing HNC treatment in a VA setting. HYP or MM interventions are feasible to implement with little demand on resources, and that listening to recordings is acceptable and helpful for veterans with pain related to cancer and cancer treatment. Further research is warranted to formally evaluate the efficacy of these interventions in this population in a well-powered study.
Purpose
This was an open label trial to determine feasibility and acceptability of 2 complementary and integrative interventions (self-hypnosis [HYP] and mindfulness meditation [MM]) for pain in veterans undergoing treatment for head and neck cancer (HNC) at VA Puget Sound.
Background
HNC is associated with pain prior to and during treatment. HYP and MM have shown promise for procedural, acute, and chronic pain and may be a helpful addition to cancer treatment.
Methods
All veterans initiating treatment during the study window (2018-2020) were offered study treatment in addition to usual care. After providing informed consent and hearing a brief description of the interventions, participants selected either the HYP or MM intervention or a control condition (ie, complete assessments but no intervention). Participants met with a study clinician who introduced the intervention and provided audio recordings and a workbook and instructed them to listen to the recordings as often as they deemed helpful. Participants completed survey assessments at baseline, week 4, and at study completion (8 weeks). Measures included patient-reported satisfaction and perceived treatment helpfulness, frequency of practice, and likeliness of using skills going forward.
Data Analysis
Descriptive statistics were computed for all measures collected. No statistical tests were conducted due to small sample size.
Results
Of the 15 veterans who enrolled, 7 selected HYP, 7 selected MM, none selected the control condition, and 1 withdrew prior to treatment selection. Of the 14 completers, 79% reported that their chosen treatment was helpful and that they practiced at least once a week; 71% reported that they are likely to use these skills going forward. No adverse events were reported.
Conclusions/Implications
Self-guided HYP and MM interventions can be administered feasibly and are highly acceptable to veterans undergoing HNC treatment in a VA setting. HYP or MM interventions are feasible to implement with little demand on resources, and that listening to recordings is acceptable and helpful for veterans with pain related to cancer and cancer treatment. Further research is warranted to formally evaluate the efficacy of these interventions in this population in a well-powered study.
The Use of Aromatherapy as a Complementary Alternative Medicine in the Management of Cancer-Related Pain
Purpose
To identify the effectiveness of aromatherapy as an adjunct in improving pain and overall sense of well-being among patients with cancer receiving hospice care.
Background
There is limited data available on the use of aromatherapy for pain management among patients with cancer receiving end-of-life care. This project identifies the benefits of aromatherapy in a population where it was not previously evaluated.
Methods
Patients with cancer who were admitted to the hospice unit of a local hospital within a large healthcare system for at least 24 hours and taking opioids for neoplasm related pain at least once a day were included in the study. Patients with allergy to essential oils, and those suffering from allergic rhinitis and common cold, and a history of asthma were excluded. Patients who were unable to consent for study participation were also excluded.
Data Analysis
Retrospective chart analysis and surveys were used to collect the data. Univariate descriptive statistics were used for patient characteristics. A Wilcoxon Signed Rank Test was used to determine opioid use before and after aromatherapy. The t test was used to compare pain scores before and after aromatherapy. A 5-point Likert scale was used to evaluate how soothing the participants found the treatment to be. The Numeric Pain Intensity Scale was used for pain scores.
Results
There was a total of 40 participants, all of whom were male with an average age of 69 years. Pain scores before and after treatment were found to be statistically significant at an average of 5.15/10 vs 3.68/10, respectively. On a scale from 1-5 with 5 being the most soothing, there was an average rating of 3.87 among participants. There was not a statistically significant decline in opioid use from pre-treatment to post-treatment. Higher pain scores before intervention were associated with rating the lotion as more soothing.
Conclusions
The use of aromatherapy as a complement to opioids for cancer-related pain in the end-of-life was associated with an increase sense of well-being, resulted in lower pain scores and seems to have subjective comfort merit.
Implications
This study shows the potential benefits of using aromatherapy in end-of-life care among patients with cancer.
Purpose
To identify the effectiveness of aromatherapy as an adjunct in improving pain and overall sense of well-being among patients with cancer receiving hospice care.
Background
There is limited data available on the use of aromatherapy for pain management among patients with cancer receiving end-of-life care. This project identifies the benefits of aromatherapy in a population where it was not previously evaluated.
Methods
Patients with cancer who were admitted to the hospice unit of a local hospital within a large healthcare system for at least 24 hours and taking opioids for neoplasm related pain at least once a day were included in the study. Patients with allergy to essential oils, and those suffering from allergic rhinitis and common cold, and a history of asthma were excluded. Patients who were unable to consent for study participation were also excluded.
Data Analysis
Retrospective chart analysis and surveys were used to collect the data. Univariate descriptive statistics were used for patient characteristics. A Wilcoxon Signed Rank Test was used to determine opioid use before and after aromatherapy. The t test was used to compare pain scores before and after aromatherapy. A 5-point Likert scale was used to evaluate how soothing the participants found the treatment to be. The Numeric Pain Intensity Scale was used for pain scores.
Results
There was a total of 40 participants, all of whom were male with an average age of 69 years. Pain scores before and after treatment were found to be statistically significant at an average of 5.15/10 vs 3.68/10, respectively. On a scale from 1-5 with 5 being the most soothing, there was an average rating of 3.87 among participants. There was not a statistically significant decline in opioid use from pre-treatment to post-treatment. Higher pain scores before intervention were associated with rating the lotion as more soothing.
Conclusions
The use of aromatherapy as a complement to opioids for cancer-related pain in the end-of-life was associated with an increase sense of well-being, resulted in lower pain scores and seems to have subjective comfort merit.
Implications
This study shows the potential benefits of using aromatherapy in end-of-life care among patients with cancer.
Purpose
To identify the effectiveness of aromatherapy as an adjunct in improving pain and overall sense of well-being among patients with cancer receiving hospice care.
Background
There is limited data available on the use of aromatherapy for pain management among patients with cancer receiving end-of-life care. This project identifies the benefits of aromatherapy in a population where it was not previously evaluated.
Methods
Patients with cancer who were admitted to the hospice unit of a local hospital within a large healthcare system for at least 24 hours and taking opioids for neoplasm related pain at least once a day were included in the study. Patients with allergy to essential oils, and those suffering from allergic rhinitis and common cold, and a history of asthma were excluded. Patients who were unable to consent for study participation were also excluded.
Data Analysis
Retrospective chart analysis and surveys were used to collect the data. Univariate descriptive statistics were used for patient characteristics. A Wilcoxon Signed Rank Test was used to determine opioid use before and after aromatherapy. The t test was used to compare pain scores before and after aromatherapy. A 5-point Likert scale was used to evaluate how soothing the participants found the treatment to be. The Numeric Pain Intensity Scale was used for pain scores.
Results
There was a total of 40 participants, all of whom were male with an average age of 69 years. Pain scores before and after treatment were found to be statistically significant at an average of 5.15/10 vs 3.68/10, respectively. On a scale from 1-5 with 5 being the most soothing, there was an average rating of 3.87 among participants. There was not a statistically significant decline in opioid use from pre-treatment to post-treatment. Higher pain scores before intervention were associated with rating the lotion as more soothing.
Conclusions
The use of aromatherapy as a complement to opioids for cancer-related pain in the end-of-life was associated with an increase sense of well-being, resulted in lower pain scores and seems to have subjective comfort merit.
Implications
This study shows the potential benefits of using aromatherapy in end-of-life care among patients with cancer.
Palliative Care Disparities in Small Cell Carcinoma of the Prostate: An Analysis of the National Cancer Database
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Implementation of Clinical Triggers for Palliative Care Consultation on the Edward Hines Jr. VA Hematology/ Oncology Inpatient Service
Purpose
Hospitalized patients with advanced malignancies often have high symptom burden and poor quality of life, which are frequently under-recognized or under-treated. Accordingly, the integration of specialty palliative care (PC) in this population is imperative. Unfortunately, a sustainable referral model to capture patients for timely PC involvement is lacking. This quality improvement study evaluated the implementation of a clinical trigger-based referral process to PC for inpatients on the Hematology/Oncology (HO) service at Hines VA Hospital. Clinical outcomes studied included: Life-Sustaining Treatment (LST) note completion rates; measurement of overall survival at 3, 6, and 12 months; rate of re-hospitalization within 30 days; and venue of death and treating specialty of deceased patients.
Methods
House staff received a weekly email that included the clinical PC triggers. Admitted patients who met trigger criteria would prompt consultation to PC. Clinical triggers included: metastatic oncologic disease or relapsed hematologic disease; uncontrolled symptoms; > 2 unscheduled hospitalizations in the prior 30 days; and unscheduled hospitalizations lasting > 7 days.
Results
A total of 63 patients were admitted to the HO service between December 2020 through February 2021. Of those, 53 (84.1%) met at least 1 trigger and 36 (68%) received PC consultation. Of the patients that met trigger criteria and received a PC consult, 85.7% died with hospice compared to 44.4% in the group who did not receive a PC consult (P < .01). Nineteen (51.3%) died within 6 months of discharge compared to 7 (26.9%) who did not receive a PC consult (P = .08). Twelve (33.3%) had recurrent hospitalizations compared to 5 (29%) who did not receive a PC consult (P = .38), and 20 (55.6%) had a new or updated LST note compared to 2 (11.8%) who did not receive PC consultation (P < .01).
Conculsions
This study demonstrated the feasibility of implementing a trigger-based system for PC consultation in a veteran inpatient HO population. Notably, a large majority of HO inpatients met criteria for at least 1 PC trigger. No significant difference was found in overall survival at 6 months; however, patients who received PC consultation were more likely to receive hospice services at the end of life.
Purpose
Hospitalized patients with advanced malignancies often have high symptom burden and poor quality of life, which are frequently under-recognized or under-treated. Accordingly, the integration of specialty palliative care (PC) in this population is imperative. Unfortunately, a sustainable referral model to capture patients for timely PC involvement is lacking. This quality improvement study evaluated the implementation of a clinical trigger-based referral process to PC for inpatients on the Hematology/Oncology (HO) service at Hines VA Hospital. Clinical outcomes studied included: Life-Sustaining Treatment (LST) note completion rates; measurement of overall survival at 3, 6, and 12 months; rate of re-hospitalization within 30 days; and venue of death and treating specialty of deceased patients.
Methods
House staff received a weekly email that included the clinical PC triggers. Admitted patients who met trigger criteria would prompt consultation to PC. Clinical triggers included: metastatic oncologic disease or relapsed hematologic disease; uncontrolled symptoms; > 2 unscheduled hospitalizations in the prior 30 days; and unscheduled hospitalizations lasting > 7 days.
Results
A total of 63 patients were admitted to the HO service between December 2020 through February 2021. Of those, 53 (84.1%) met at least 1 trigger and 36 (68%) received PC consultation. Of the patients that met trigger criteria and received a PC consult, 85.7% died with hospice compared to 44.4% in the group who did not receive a PC consult (P < .01). Nineteen (51.3%) died within 6 months of discharge compared to 7 (26.9%) who did not receive a PC consult (P = .08). Twelve (33.3%) had recurrent hospitalizations compared to 5 (29%) who did not receive a PC consult (P = .38), and 20 (55.6%) had a new or updated LST note compared to 2 (11.8%) who did not receive PC consultation (P < .01).
Conculsions
This study demonstrated the feasibility of implementing a trigger-based system for PC consultation in a veteran inpatient HO population. Notably, a large majority of HO inpatients met criteria for at least 1 PC trigger. No significant difference was found in overall survival at 6 months; however, patients who received PC consultation were more likely to receive hospice services at the end of life.
Purpose
Hospitalized patients with advanced malignancies often have high symptom burden and poor quality of life, which are frequently under-recognized or under-treated. Accordingly, the integration of specialty palliative care (PC) in this population is imperative. Unfortunately, a sustainable referral model to capture patients for timely PC involvement is lacking. This quality improvement study evaluated the implementation of a clinical trigger-based referral process to PC for inpatients on the Hematology/Oncology (HO) service at Hines VA Hospital. Clinical outcomes studied included: Life-Sustaining Treatment (LST) note completion rates; measurement of overall survival at 3, 6, and 12 months; rate of re-hospitalization within 30 days; and venue of death and treating specialty of deceased patients.
Methods
House staff received a weekly email that included the clinical PC triggers. Admitted patients who met trigger criteria would prompt consultation to PC. Clinical triggers included: metastatic oncologic disease or relapsed hematologic disease; uncontrolled symptoms; > 2 unscheduled hospitalizations in the prior 30 days; and unscheduled hospitalizations lasting > 7 days.
Results
A total of 63 patients were admitted to the HO service between December 2020 through February 2021. Of those, 53 (84.1%) met at least 1 trigger and 36 (68%) received PC consultation. Of the patients that met trigger criteria and received a PC consult, 85.7% died with hospice compared to 44.4% in the group who did not receive a PC consult (P < .01). Nineteen (51.3%) died within 6 months of discharge compared to 7 (26.9%) who did not receive a PC consult (P = .08). Twelve (33.3%) had recurrent hospitalizations compared to 5 (29%) who did not receive a PC consult (P = .38), and 20 (55.6%) had a new or updated LST note compared to 2 (11.8%) who did not receive PC consultation (P < .01).
Conculsions
This study demonstrated the feasibility of implementing a trigger-based system for PC consultation in a veteran inpatient HO population. Notably, a large majority of HO inpatients met criteria for at least 1 PC trigger. No significant difference was found in overall survival at 6 months; however, patients who received PC consultation were more likely to receive hospice services at the end of life.
Hepatitis B Virus (HBV) Testing in Veterans Receiving Systemic Anticancer Treatment
Purpose
Examine hepatitis B virus (HBV) testing in veterans receiving systemic anticancer treatment (SACT) in the Veterans Health Administration (VHA).
Background
HBV reactivation is reported in patients with chronic (HB surface antigen, HBsAg, positive) or prior (HB core antibody, HBcAb, positive) HBV infection, who receive SACT. A recent American Society of Clinical Oncology provisional clinical opinion update recommended HBV screening for all patients prior to initiation of SACT (excluding hormonal therapy). HBV testing and the incidence of hepatitis in veterans receiving SACT in the VHA has not been reported.
Methods/Data Analysis
VHA EHR data were used to identify veterans receiving SACT (01/2010-12/2021). Testing for HBsAg, HBcAb and alanine aminotransferase (ALT) was extracted. Patients known to have HBV or elevated ALT prior to first SACT, and those receiving anti-CD20 were excluded. Patients were followed until two years after the last SACT or 12/2021, whichever occurred first. Patients receiving intravenous SACT and those receiving oral SACT are described separately.
Results
Between 2010 and 2021, 215,395 veterans received an intravenous SACT, while 80,752 veterans received an oral SACT. Of patients treated with an SACT, 80% had no evidence of HBsAg or HBcAb testing prior to treatment initiation, and 8-12% experienced at least one elevated ALT between treatment initiation and two years after the last SACT. There was no evidence of increased ALT elevation in patients who were not tested compared to those that were tested prior to treatment initiation. In patients with at least one ALT elevation, approximately 30% were tested for HBV and of these, 3% tested positive.
Conclusions/Implications
Most veterans receiving SACT are not tested for HBV prior to treatment initiation, and do not experience elevated ALTs. In patients with elevated ALT during or subsequent to SACT, the majority are not tested for HBV. Veterans that are tested reveal an HBV prevalence of about 10%. Our results suggest that HBV testing prior to SACT initiation should not be at the expense of delaying treatment, given the magnitude of proposed change from current practice and the anticipated low probability of benefit.
Purpose
Examine hepatitis B virus (HBV) testing in veterans receiving systemic anticancer treatment (SACT) in the Veterans Health Administration (VHA).
Background
HBV reactivation is reported in patients with chronic (HB surface antigen, HBsAg, positive) or prior (HB core antibody, HBcAb, positive) HBV infection, who receive SACT. A recent American Society of Clinical Oncology provisional clinical opinion update recommended HBV screening for all patients prior to initiation of SACT (excluding hormonal therapy). HBV testing and the incidence of hepatitis in veterans receiving SACT in the VHA has not been reported.
Methods/Data Analysis
VHA EHR data were used to identify veterans receiving SACT (01/2010-12/2021). Testing for HBsAg, HBcAb and alanine aminotransferase (ALT) was extracted. Patients known to have HBV or elevated ALT prior to first SACT, and those receiving anti-CD20 were excluded. Patients were followed until two years after the last SACT or 12/2021, whichever occurred first. Patients receiving intravenous SACT and those receiving oral SACT are described separately.
Results
Between 2010 and 2021, 215,395 veterans received an intravenous SACT, while 80,752 veterans received an oral SACT. Of patients treated with an SACT, 80% had no evidence of HBsAg or HBcAb testing prior to treatment initiation, and 8-12% experienced at least one elevated ALT between treatment initiation and two years after the last SACT. There was no evidence of increased ALT elevation in patients who were not tested compared to those that were tested prior to treatment initiation. In patients with at least one ALT elevation, approximately 30% were tested for HBV and of these, 3% tested positive.
Conclusions/Implications
Most veterans receiving SACT are not tested for HBV prior to treatment initiation, and do not experience elevated ALTs. In patients with elevated ALT during or subsequent to SACT, the majority are not tested for HBV. Veterans that are tested reveal an HBV prevalence of about 10%. Our results suggest that HBV testing prior to SACT initiation should not be at the expense of delaying treatment, given the magnitude of proposed change from current practice and the anticipated low probability of benefit.
Purpose
Examine hepatitis B virus (HBV) testing in veterans receiving systemic anticancer treatment (SACT) in the Veterans Health Administration (VHA).
Background
HBV reactivation is reported in patients with chronic (HB surface antigen, HBsAg, positive) or prior (HB core antibody, HBcAb, positive) HBV infection, who receive SACT. A recent American Society of Clinical Oncology provisional clinical opinion update recommended HBV screening for all patients prior to initiation of SACT (excluding hormonal therapy). HBV testing and the incidence of hepatitis in veterans receiving SACT in the VHA has not been reported.
Methods/Data Analysis
VHA EHR data were used to identify veterans receiving SACT (01/2010-12/2021). Testing for HBsAg, HBcAb and alanine aminotransferase (ALT) was extracted. Patients known to have HBV or elevated ALT prior to first SACT, and those receiving anti-CD20 were excluded. Patients were followed until two years after the last SACT or 12/2021, whichever occurred first. Patients receiving intravenous SACT and those receiving oral SACT are described separately.
Results
Between 2010 and 2021, 215,395 veterans received an intravenous SACT, while 80,752 veterans received an oral SACT. Of patients treated with an SACT, 80% had no evidence of HBsAg or HBcAb testing prior to treatment initiation, and 8-12% experienced at least one elevated ALT between treatment initiation and two years after the last SACT. There was no evidence of increased ALT elevation in patients who were not tested compared to those that were tested prior to treatment initiation. In patients with at least one ALT elevation, approximately 30% were tested for HBV and of these, 3% tested positive.
Conclusions/Implications
Most veterans receiving SACT are not tested for HBV prior to treatment initiation, and do not experience elevated ALTs. In patients with elevated ALT during or subsequent to SACT, the majority are not tested for HBV. Veterans that are tested reveal an HBV prevalence of about 10%. Our results suggest that HBV testing prior to SACT initiation should not be at the expense of delaying treatment, given the magnitude of proposed change from current practice and the anticipated low probability of benefit.
The Effect of Adjuvant Therapy Type on Survival for Patients With Sage II Osteosarcoma
Background
Osteosarcoma is an aggressive bone malignancy often treated with surgery. On diagnosis, the majority of patients present with stage II disease. The objective of this study was to compare differences in survival between three commonly used adjuvant therapies: chemotherapy, radiation, and combined chemoradiation therapy in patients presenting with stage II disease.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with osteosarcoma from 2004 to 2018 using the ICD-O-3 histology codes 9180-9187. Patients with stage II disease and who had undergone a surgical procedure at the primary site were identified. Patients were grouped by the receipt of adjuvant chemotherapy, radiation, or chemoradiation. Descriptive statistics and Kaplan-Meier curves were used to measure survival in these patients. One way ANOVA and chi-square analyses were used to evaluate differences among treatment groups. Data were analyzed using SPSS and statistical significance was set at P = .05.
Results
Of 9955 patients in the NCDB diagnosed with osteosarcoma, 4378 (44%) presented with stage II disease. 710 (17.9%) of these surgical patients received additional adjuvant therapy. 66.0% received chemotherapy, 24.4% received radiation, and 9.57% received combined chemoradiation. Adjuvant chemotherapy had the longest median survival time of 91.7 months. Median survival for adjuvant radiotherapy was 48 months and combined chemoradiation was 50.5 months. On log-rank pairwise comparison, the difference in survival between adjuvant chemotherapy and adjuvant chemoradiation was found to be statistically significant. (P < .001). Patients receiving adjuvant chemotherapy were more likely to be younger and have private insurance. (P < .05) Conversely, patients receiving adjuvant radiation were more likely to be older and have Medicare. (P < .05). No significant differences were seen among patient race, sex, income, or Charleson-Deyo comorbidity score.
Conclusions
This study showed that patients with stage II osteosarcoma who receive adjuvant chemotherapy experience improved median survival in comparison to patients who receive adjuvant radiation. This is an important clinical finding, which should guide future treatment. However, further investigation is required to identify patient and treatment specific factors, which are contributing to mortality.
Background
Osteosarcoma is an aggressive bone malignancy often treated with surgery. On diagnosis, the majority of patients present with stage II disease. The objective of this study was to compare differences in survival between three commonly used adjuvant therapies: chemotherapy, radiation, and combined chemoradiation therapy in patients presenting with stage II disease.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with osteosarcoma from 2004 to 2018 using the ICD-O-3 histology codes 9180-9187. Patients with stage II disease and who had undergone a surgical procedure at the primary site were identified. Patients were grouped by the receipt of adjuvant chemotherapy, radiation, or chemoradiation. Descriptive statistics and Kaplan-Meier curves were used to measure survival in these patients. One way ANOVA and chi-square analyses were used to evaluate differences among treatment groups. Data were analyzed using SPSS and statistical significance was set at P = .05.
Results
Of 9955 patients in the NCDB diagnosed with osteosarcoma, 4378 (44%) presented with stage II disease. 710 (17.9%) of these surgical patients received additional adjuvant therapy. 66.0% received chemotherapy, 24.4% received radiation, and 9.57% received combined chemoradiation. Adjuvant chemotherapy had the longest median survival time of 91.7 months. Median survival for adjuvant radiotherapy was 48 months and combined chemoradiation was 50.5 months. On log-rank pairwise comparison, the difference in survival between adjuvant chemotherapy and adjuvant chemoradiation was found to be statistically significant. (P < .001). Patients receiving adjuvant chemotherapy were more likely to be younger and have private insurance. (P < .05) Conversely, patients receiving adjuvant radiation were more likely to be older and have Medicare. (P < .05). No significant differences were seen among patient race, sex, income, or Charleson-Deyo comorbidity score.
Conclusions
This study showed that patients with stage II osteosarcoma who receive adjuvant chemotherapy experience improved median survival in comparison to patients who receive adjuvant radiation. This is an important clinical finding, which should guide future treatment. However, further investigation is required to identify patient and treatment specific factors, which are contributing to mortality.
Background
Osteosarcoma is an aggressive bone malignancy often treated with surgery. On diagnosis, the majority of patients present with stage II disease. The objective of this study was to compare differences in survival between three commonly used adjuvant therapies: chemotherapy, radiation, and combined chemoradiation therapy in patients presenting with stage II disease.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with osteosarcoma from 2004 to 2018 using the ICD-O-3 histology codes 9180-9187. Patients with stage II disease and who had undergone a surgical procedure at the primary site were identified. Patients were grouped by the receipt of adjuvant chemotherapy, radiation, or chemoradiation. Descriptive statistics and Kaplan-Meier curves were used to measure survival in these patients. One way ANOVA and chi-square analyses were used to evaluate differences among treatment groups. Data were analyzed using SPSS and statistical significance was set at P = .05.
Results
Of 9955 patients in the NCDB diagnosed with osteosarcoma, 4378 (44%) presented with stage II disease. 710 (17.9%) of these surgical patients received additional adjuvant therapy. 66.0% received chemotherapy, 24.4% received radiation, and 9.57% received combined chemoradiation. Adjuvant chemotherapy had the longest median survival time of 91.7 months. Median survival for adjuvant radiotherapy was 48 months and combined chemoradiation was 50.5 months. On log-rank pairwise comparison, the difference in survival between adjuvant chemotherapy and adjuvant chemoradiation was found to be statistically significant. (P < .001). Patients receiving adjuvant chemotherapy were more likely to be younger and have private insurance. (P < .05) Conversely, patients receiving adjuvant radiation were more likely to be older and have Medicare. (P < .05). No significant differences were seen among patient race, sex, income, or Charleson-Deyo comorbidity score.
Conclusions
This study showed that patients with stage II osteosarcoma who receive adjuvant chemotherapy experience improved median survival in comparison to patients who receive adjuvant radiation. This is an important clinical finding, which should guide future treatment. However, further investigation is required to identify patient and treatment specific factors, which are contributing to mortality.