User login
Sexual activity alters the microbiome, with potential psychiatric implications
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
Will NAAT replace microscopy for the identification of organisms causing vaginitis?
Over the past 200 years, identification of the specific organism causing an infection has evolved from a reliance on patient history and physical examination to the use of microscopic examination of relevant biological samples to the rise of microbial culture and immunological testing as the gold standards for diagnosis. More recently, advances in nucleic acid testing have made nucleic acid amplification testing (NAAT) a primary method for identifying the specific organism causing an infection.
The evolution of the diagnosis of gonorrhea in clinical practice is a good example of the inexorable evolution of diagnostic techniques from physical examination to microscopic analysis to culture and finally to NAAT. Neiseer discovered Neisseria gonorrhea in 1879.1 In 19th century general medical practice gonorrhea was often diagnosed based on history and physical examination and sometimes microscopy was also utilized.2 In the mid-20th century, it was realized that culture was a superior approach to diagnosis of gonorrhea, and it became the gold standard for diagnosis in general practice.3 NAAT has now replaced culture as the gold standard for the diagnosis of gonorrhea because of its superior performance in clinical practice.4 It may now be time to consider using NAAT rather than microscopy and culture in general practice for the identification of specific microorganisms causing vaginitis.
Trichomoniasis
Vaginitis caused by Trichomonas vaginalis is characterized by a discharge that is foamy and green-yellow in color, with a vaginal pH that is >4.5. Microscopy of a vaginal specimen has low sensitivity, in the range of 50%, for detecting T vaginalis.5-7 There are many factors that make microscopy a poor approach to the diagnosis of T vaginalis, including the rapid decrease in protozoan motility once a vaginal specimen is placed on a glass slide and the similar size of non-motile T vaginalis and other cells in the vagina.
Given the low sensitivity of microscopy for the diagnosis of trichomoniasis, the American College of Obstetricians and Gynecologists (ACOG) recommends NAAT as a primary approach to test for T vaginalis, with culture or NAAT testing as alternative approaches.8 The Centers for Disease Control and Prevention (CDC) recommends that if a wet mount is negative for T vaginalis that NAAT should be utilized.9
In this 2-step testing process, the first step is to test the vaginal pH and perform a microscopic examination of a vaginal specimen for T vaginalis. If T vaginalis organisms are detected, the diagnosis of trichomoniasis is confirmed. If organisms are not detected the second step would be to send a vaginal or urine specimen for NAAT for T vaginalis or for culture. An advantage of NAAT over culture is that urine specimens can be used for diagnosis of T vaginalis while urine specimens are not suitable for culture because of low sensitivity. For patients diagnosed with trichomoniasis, the CDC recommends that testing be repeated in 3 months because of high rates of reinfection. NAAT would be an optimal test to use in this situation.
Continue to: Bacterial vaginosis and candidiasis...
Bacterial vaginosis and candidiasis
ACOG recommends using Amsel criteria or Nugent scoring of a specimen colorized with a Gram stain for the diagnosis of bacterial vaginosis and microscopy or culture for the diagnosis of candidiasis.8 Recent research reports that NAAT testing for bacterial vaginosis and candidiasis may be more sensitive than standard office-based approaches for detecting these two causes of vaginitis. In a study of approximately 1,740 patients with symptoms of vaginitis, vaginal specimens were analyzed using NAAT or standard office approaches to diagnosis.10 In this study the diagnostic gold standards were Nugent scoring with Amsel criteria to resolve intermediate Nugent scores for bacterial vaginosis and culture for Candida. The study demonstrated the superiority of NAAT testing over standard office approaches for the identification of the cause of the vaginitis. NAAT testing was reported to have superior sensitivity for diagnosing bacterial vaginosis compared with the original Amsel criteria (93% vs 76%, respectively (P <.0001), with similar respective specificities of 92% and 94% .10 NAAT testing also had superior sensitivity for diagnosing Candidiasis compared with microscopy after potassium hydroxide treatment of a vaginal specimen (91% vs 58%, respectively (P <.0001).10 NAAT testing also had superior specificity compared with microscopy after potassium hydroxide treatment of a vaginal specimen (94% vs 89%, respectively (P < .0005).10
In another study comparing NAAT with clinical diagnosis for 466 patients with symptoms of vaginitis, standard office approaches to the diagnosis of vaginitis resulted in the failure to identify the correct infection in a large number of cases. For the diagnosis of bacterial vaginosis, clinicians missed 42% of the cases identified by NAAT. For the diagnosis of Candida, clinicians missed 46% of the cases identified by NAAT. For T vaginalis diagnosis, clinicians missed 72% of the cases identified by NAAT. Clearly, this resulted in clinicians not treating many infections detected by NAAT.11
Continue to: One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis...
One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis
In a recent study, 1,471 patients with a symptom of vaginitis (abnormal vaginal discharge, itching or irritation, or odor) self-collected a vaginal swab and had a vaginal swab collected by a clinician.12 The swabs were placed in buffer and the samples were tested by NAAT using the BD Max system (Franklin Lakes, New Jersey) for the presence of nucleic acid sequences of the microorganisms responsible for the most common causes of vaginitis. In this cohort, using the clinician collected vaginal swabs for NAAT, the investigators reported the following pattern of detection of nucleic acid sequences: 36.1%, bacterial vaginosis pattern; 16.2%, Candida spp.; 1.6%, T vaginalis; 0.7%, Candida glabrata; and 0.1%, Candida krusei. Nucleic acid sequences of multiple organisms were detected in 21.7% of patients, including 13.9% with bacterial vaginosis pattern plus Candida spp., 4.9% with bacterial vaginosis pattern plus T vaginalis, 0.3% with Candida spp. plus T vaginalis, 0.2% with Candida spp. plus Candida glabrata, 0.2% with bacterial vaginosis pattern plus Candida glabrata, and 2.2% with all 3 organisms. A total of 23.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.
In another study of 1,491 patients with a symptom of vaginitis, clinician-collected vaginal swabs were tested by NAAT using the Aptima BV and Aptima Candida/Trichomonas systems (Hologic, Marlborough, Massachusetts) for the presence of nucleic acid sequences of microorganisms responsible for most cases of vaginitis.13 The investigators reported the following pattern of detection of nucleic acid sequences: 28.6%, bacterial vaginosis pattern; 14.2%, Candida spp.; 3%, T vaginalis; 1.9%, Candida glabrata.13 Nucleic acid sequences from multiple organisms were detected in 23.3% of patients. Nucleic acid sequences suggesting the presence of two different causes of vaginitis were detected among 20.8% of patients, including bacterial vaginosis plus Candida spp., 11.1%; bacterial vaginosis plus T vaginalis, 7.2%; Candida spp. plus T vaginalis, 1.0%; Candida spp. plus Candida glabrata, 0.9%; bacterial vaginosis plus Candida spp., 0.5%; Candida glabrata plus T vaginalis, 0.1%. Nucleic acid sequences suggesting the presence of 3 different causes of vaginitis were detected in 2.4% of patients, the most common being the combination of bacterial vaginosis plus Candida spp. plus T vaginalis, 1.7% and bacterial vaginosis plus Candida spp. plus Candida glabrata, 0.5%. Nucleic acid sequences suggesting the presence of 4 different causes of vaginitis were detected in 0.1% of patients. A total of 28.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.13
In clinical practice it is uncommon to see the diagnosis of multiple causes of vaginitis recorded in the medical record of a patient. This suggests that we are not effectively identifying the 20% of patients with multiple causes of vaginitis.
When multiple organisms that cause vaginitis are present, NAAT is superior to clinical evaluation for diagnosis
In a study of 1,264 patients with symptoms of vaginitis who had an identified microbial cause, more than 20% had multiple organisms detected by NAAT
Patient collection of a vaginal swab for NAAT
Multiple studies have reported that collection of a vaginal swab for NAAT by the patient or a clinician results in similar excellent test performance.4,12,13 This observation might catalyze the development of clinical protocols where patients with vaginitis could collect the swab for NAAT analysis, without needing to have a speculum examination by a clinician.
When collecting a vaginal specimen for NAAT it is important that no vaginal lubricants or creams contaminate the collection swab. Vaginal lubricants and creams may inhibit the polymerase chain reaction enzymes resulting in a false negative. The swab may be directly inserted into the vagina to collect the specimen or a speculum without a lubricant, except water can be used to facilitate specimen collection. To collect a specimen without a speculum the swab is inserted 2 inches into the vagina and rotated for 10 to 15 seconds.
What should clinicians do while waiting for a NAAT result?
A major problem with NAAT testing for vaginitis is that the results are not available at the initial patient visit, impacting the ability to make an immediate diagnosis and provide targeted antibiotic treatment. Given that bacterial vaginosis and Candida species are the most common causes of infectious vaginitis in many populations of gynecology patients, one approach is to initiate treatment with one dose of an oral antifungal agent and a multiday course of vaginal metronidazole. Once the NAAT test results are available, the treatment can be refined to specific infectious agents identified by the test, or the antibiotics can be discontinued if no relevant microorganisms are detected. Another approach would be to wait until the NAAT test is completed and then prescribe the appropriate antibiotic. My sense is that most patients would not favor this wait and see approach.
Barriers to the use of NAAT for vaginitis
A barrier to the use of NAAT for the diagnosis of vaginitis is that leading organizations do not currently recommend NAAT as a primary approach to diagnosis, favoring microscopy and measurement of vaginal pH.9 In addition, clinicians and patients may be rightfully concerned about the cost of NAAT, which can be substantial.
Vaginitis, especially when it is recurrent, can be stressful14 and have an impact on a patient’s quality of life15,16 and sexual health.17 Arguably, our current practice algorithms for diagnosing the cause of vaginitis are not optimized.18 Our failure to accurately diagnose the cause of vaginitis contributes to inappropriate antibiotic treatment and return visits because of inadequate initial treatment.18 We can improve and simplify our approach to the diagnosis of vaginitis by prioritizing the use of NAAT.19 In turn, reliably making the right diagnosis will result in the optimization of treatment. ●
- Jose PP, Vivekanandan V, Sobhanakumari K. Gonorrhea: Historical outlook. J Skin Sex Transm Dis. 2020;2:110-114.
- Bayly HW. The diagnosis and treatment of chronic gonorrhoea and its local complications. Br Med J. 1914;14:584-587.
- Stuart RD. The diagnosis and control of gonorrhoea by bacteriological cultures: with a preliminary report on a new method for transporting clinical material. Glasgow Med J. 1946;27:131-142.
- Wilson JD, Wallace HE, Loftus-Keeling M, et al. Swab-yourself trial with economic monitoring and testing for infections collectively (SYSTEMATIC): Part 2. A diagnostic accuracy and cost-effectiveness study comparing rectal, pharyngeal and urogenital samples analyzed individually, versus as a pooled specimen, for the diagnosis of gonorrhea and chlamydia. Clin Infect Dis. 2021;73:e3183-3193.
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new NAAT: association with ethnicity, symptoms and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312-316.
- Roth AM, Williams JA, Ly R. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398-400.
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infection. 2013;89:434-438.
- Vaginitis in nonpregnant patients. ACOG Practice Bulletin No 215. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e1-e17.
- Workowksi KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines 2021. MMWR. 2021;70:1-187.
- Schwebke JR, Gaydos CA, Hyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of vaginal panel assay compared with clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130:181-189.
- Schwebke JR, Taylor SN, Ackerman N, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginalis assays: results from a prospective multi-center study. J Clin Microbiol. 2020;58:e01643-19.
- Ehrstrom S, Kornfeld D, Rylander E. Perceived stress in women with recurrent vulvovaginal candidiasis. J Psychosomatic Obstet Gynecol. 2007;28:169-176.
- Abellea S, Guelfucci F, Wagner J, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis in Europe and the USA. Health Quality Life Outcomes. 2013;11:169.
- Fukazawa EI, Witkin SS, Robial R, et al. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch Gynecol Obstet. 2019;300:647-650.
- Giraldo PC, Polpeta NC, Juliato CT, et al. Evaluation of sexual function in Brazilian women with recurrent vulvovaginal candidiasis and localized provoked vulvodynia. J Sex Med. 2012;9:805-811.
- Hillier SL, Austin M, Macio I, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72:1538-1543.
- . Sobel JD. Syndromic treatment of women with vulvovaginal symptoms in the United States: a call to action. Clin Infect Dis. 2021;72:1544-1545.
Over the past 200 years, identification of the specific organism causing an infection has evolved from a reliance on patient history and physical examination to the use of microscopic examination of relevant biological samples to the rise of microbial culture and immunological testing as the gold standards for diagnosis. More recently, advances in nucleic acid testing have made nucleic acid amplification testing (NAAT) a primary method for identifying the specific organism causing an infection.
The evolution of the diagnosis of gonorrhea in clinical practice is a good example of the inexorable evolution of diagnostic techniques from physical examination to microscopic analysis to culture and finally to NAAT. Neiseer discovered Neisseria gonorrhea in 1879.1 In 19th century general medical practice gonorrhea was often diagnosed based on history and physical examination and sometimes microscopy was also utilized.2 In the mid-20th century, it was realized that culture was a superior approach to diagnosis of gonorrhea, and it became the gold standard for diagnosis in general practice.3 NAAT has now replaced culture as the gold standard for the diagnosis of gonorrhea because of its superior performance in clinical practice.4 It may now be time to consider using NAAT rather than microscopy and culture in general practice for the identification of specific microorganisms causing vaginitis.
Trichomoniasis
Vaginitis caused by Trichomonas vaginalis is characterized by a discharge that is foamy and green-yellow in color, with a vaginal pH that is >4.5. Microscopy of a vaginal specimen has low sensitivity, in the range of 50%, for detecting T vaginalis.5-7 There are many factors that make microscopy a poor approach to the diagnosis of T vaginalis, including the rapid decrease in protozoan motility once a vaginal specimen is placed on a glass slide and the similar size of non-motile T vaginalis and other cells in the vagina.
Given the low sensitivity of microscopy for the diagnosis of trichomoniasis, the American College of Obstetricians and Gynecologists (ACOG) recommends NAAT as a primary approach to test for T vaginalis, with culture or NAAT testing as alternative approaches.8 The Centers for Disease Control and Prevention (CDC) recommends that if a wet mount is negative for T vaginalis that NAAT should be utilized.9
In this 2-step testing process, the first step is to test the vaginal pH and perform a microscopic examination of a vaginal specimen for T vaginalis. If T vaginalis organisms are detected, the diagnosis of trichomoniasis is confirmed. If organisms are not detected the second step would be to send a vaginal or urine specimen for NAAT for T vaginalis or for culture. An advantage of NAAT over culture is that urine specimens can be used for diagnosis of T vaginalis while urine specimens are not suitable for culture because of low sensitivity. For patients diagnosed with trichomoniasis, the CDC recommends that testing be repeated in 3 months because of high rates of reinfection. NAAT would be an optimal test to use in this situation.
Continue to: Bacterial vaginosis and candidiasis...
Bacterial vaginosis and candidiasis
ACOG recommends using Amsel criteria or Nugent scoring of a specimen colorized with a Gram stain for the diagnosis of bacterial vaginosis and microscopy or culture for the diagnosis of candidiasis.8 Recent research reports that NAAT testing for bacterial vaginosis and candidiasis may be more sensitive than standard office-based approaches for detecting these two causes of vaginitis. In a study of approximately 1,740 patients with symptoms of vaginitis, vaginal specimens were analyzed using NAAT or standard office approaches to diagnosis.10 In this study the diagnostic gold standards were Nugent scoring with Amsel criteria to resolve intermediate Nugent scores for bacterial vaginosis and culture for Candida. The study demonstrated the superiority of NAAT testing over standard office approaches for the identification of the cause of the vaginitis. NAAT testing was reported to have superior sensitivity for diagnosing bacterial vaginosis compared with the original Amsel criteria (93% vs 76%, respectively (P <.0001), with similar respective specificities of 92% and 94% .10 NAAT testing also had superior sensitivity for diagnosing Candidiasis compared with microscopy after potassium hydroxide treatment of a vaginal specimen (91% vs 58%, respectively (P <.0001).10 NAAT testing also had superior specificity compared with microscopy after potassium hydroxide treatment of a vaginal specimen (94% vs 89%, respectively (P < .0005).10
In another study comparing NAAT with clinical diagnosis for 466 patients with symptoms of vaginitis, standard office approaches to the diagnosis of vaginitis resulted in the failure to identify the correct infection in a large number of cases. For the diagnosis of bacterial vaginosis, clinicians missed 42% of the cases identified by NAAT. For the diagnosis of Candida, clinicians missed 46% of the cases identified by NAAT. For T vaginalis diagnosis, clinicians missed 72% of the cases identified by NAAT. Clearly, this resulted in clinicians not treating many infections detected by NAAT.11
Continue to: One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis...
One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis
In a recent study, 1,471 patients with a symptom of vaginitis (abnormal vaginal discharge, itching or irritation, or odor) self-collected a vaginal swab and had a vaginal swab collected by a clinician.12 The swabs were placed in buffer and the samples were tested by NAAT using the BD Max system (Franklin Lakes, New Jersey) for the presence of nucleic acid sequences of the microorganisms responsible for the most common causes of vaginitis. In this cohort, using the clinician collected vaginal swabs for NAAT, the investigators reported the following pattern of detection of nucleic acid sequences: 36.1%, bacterial vaginosis pattern; 16.2%, Candida spp.; 1.6%, T vaginalis; 0.7%, Candida glabrata; and 0.1%, Candida krusei. Nucleic acid sequences of multiple organisms were detected in 21.7% of patients, including 13.9% with bacterial vaginosis pattern plus Candida spp., 4.9% with bacterial vaginosis pattern plus T vaginalis, 0.3% with Candida spp. plus T vaginalis, 0.2% with Candida spp. plus Candida glabrata, 0.2% with bacterial vaginosis pattern plus Candida glabrata, and 2.2% with all 3 organisms. A total of 23.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.
In another study of 1,491 patients with a symptom of vaginitis, clinician-collected vaginal swabs were tested by NAAT using the Aptima BV and Aptima Candida/Trichomonas systems (Hologic, Marlborough, Massachusetts) for the presence of nucleic acid sequences of microorganisms responsible for most cases of vaginitis.13 The investigators reported the following pattern of detection of nucleic acid sequences: 28.6%, bacterial vaginosis pattern; 14.2%, Candida spp.; 3%, T vaginalis; 1.9%, Candida glabrata.13 Nucleic acid sequences from multiple organisms were detected in 23.3% of patients. Nucleic acid sequences suggesting the presence of two different causes of vaginitis were detected among 20.8% of patients, including bacterial vaginosis plus Candida spp., 11.1%; bacterial vaginosis plus T vaginalis, 7.2%; Candida spp. plus T vaginalis, 1.0%; Candida spp. plus Candida glabrata, 0.9%; bacterial vaginosis plus Candida spp., 0.5%; Candida glabrata plus T vaginalis, 0.1%. Nucleic acid sequences suggesting the presence of 3 different causes of vaginitis were detected in 2.4% of patients, the most common being the combination of bacterial vaginosis plus Candida spp. plus T vaginalis, 1.7% and bacterial vaginosis plus Candida spp. plus Candida glabrata, 0.5%. Nucleic acid sequences suggesting the presence of 4 different causes of vaginitis were detected in 0.1% of patients. A total of 28.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.13
In clinical practice it is uncommon to see the diagnosis of multiple causes of vaginitis recorded in the medical record of a patient. This suggests that we are not effectively identifying the 20% of patients with multiple causes of vaginitis.
When multiple organisms that cause vaginitis are present, NAAT is superior to clinical evaluation for diagnosis
In a study of 1,264 patients with symptoms of vaginitis who had an identified microbial cause, more than 20% had multiple organisms detected by NAAT
Patient collection of a vaginal swab for NAAT
Multiple studies have reported that collection of a vaginal swab for NAAT by the patient or a clinician results in similar excellent test performance.4,12,13 This observation might catalyze the development of clinical protocols where patients with vaginitis could collect the swab for NAAT analysis, without needing to have a speculum examination by a clinician.
When collecting a vaginal specimen for NAAT it is important that no vaginal lubricants or creams contaminate the collection swab. Vaginal lubricants and creams may inhibit the polymerase chain reaction enzymes resulting in a false negative. The swab may be directly inserted into the vagina to collect the specimen or a speculum without a lubricant, except water can be used to facilitate specimen collection. To collect a specimen without a speculum the swab is inserted 2 inches into the vagina and rotated for 10 to 15 seconds.
What should clinicians do while waiting for a NAAT result?
A major problem with NAAT testing for vaginitis is that the results are not available at the initial patient visit, impacting the ability to make an immediate diagnosis and provide targeted antibiotic treatment. Given that bacterial vaginosis and Candida species are the most common causes of infectious vaginitis in many populations of gynecology patients, one approach is to initiate treatment with one dose of an oral antifungal agent and a multiday course of vaginal metronidazole. Once the NAAT test results are available, the treatment can be refined to specific infectious agents identified by the test, or the antibiotics can be discontinued if no relevant microorganisms are detected. Another approach would be to wait until the NAAT test is completed and then prescribe the appropriate antibiotic. My sense is that most patients would not favor this wait and see approach.
Barriers to the use of NAAT for vaginitis
A barrier to the use of NAAT for the diagnosis of vaginitis is that leading organizations do not currently recommend NAAT as a primary approach to diagnosis, favoring microscopy and measurement of vaginal pH.9 In addition, clinicians and patients may be rightfully concerned about the cost of NAAT, which can be substantial.
Vaginitis, especially when it is recurrent, can be stressful14 and have an impact on a patient’s quality of life15,16 and sexual health.17 Arguably, our current practice algorithms for diagnosing the cause of vaginitis are not optimized.18 Our failure to accurately diagnose the cause of vaginitis contributes to inappropriate antibiotic treatment and return visits because of inadequate initial treatment.18 We can improve and simplify our approach to the diagnosis of vaginitis by prioritizing the use of NAAT.19 In turn, reliably making the right diagnosis will result in the optimization of treatment. ●
Over the past 200 years, identification of the specific organism causing an infection has evolved from a reliance on patient history and physical examination to the use of microscopic examination of relevant biological samples to the rise of microbial culture and immunological testing as the gold standards for diagnosis. More recently, advances in nucleic acid testing have made nucleic acid amplification testing (NAAT) a primary method for identifying the specific organism causing an infection.
The evolution of the diagnosis of gonorrhea in clinical practice is a good example of the inexorable evolution of diagnostic techniques from physical examination to microscopic analysis to culture and finally to NAAT. Neiseer discovered Neisseria gonorrhea in 1879.1 In 19th century general medical practice gonorrhea was often diagnosed based on history and physical examination and sometimes microscopy was also utilized.2 In the mid-20th century, it was realized that culture was a superior approach to diagnosis of gonorrhea, and it became the gold standard for diagnosis in general practice.3 NAAT has now replaced culture as the gold standard for the diagnosis of gonorrhea because of its superior performance in clinical practice.4 It may now be time to consider using NAAT rather than microscopy and culture in general practice for the identification of specific microorganisms causing vaginitis.
Trichomoniasis
Vaginitis caused by Trichomonas vaginalis is characterized by a discharge that is foamy and green-yellow in color, with a vaginal pH that is >4.5. Microscopy of a vaginal specimen has low sensitivity, in the range of 50%, for detecting T vaginalis.5-7 There are many factors that make microscopy a poor approach to the diagnosis of T vaginalis, including the rapid decrease in protozoan motility once a vaginal specimen is placed on a glass slide and the similar size of non-motile T vaginalis and other cells in the vagina.
Given the low sensitivity of microscopy for the diagnosis of trichomoniasis, the American College of Obstetricians and Gynecologists (ACOG) recommends NAAT as a primary approach to test for T vaginalis, with culture or NAAT testing as alternative approaches.8 The Centers for Disease Control and Prevention (CDC) recommends that if a wet mount is negative for T vaginalis that NAAT should be utilized.9
In this 2-step testing process, the first step is to test the vaginal pH and perform a microscopic examination of a vaginal specimen for T vaginalis. If T vaginalis organisms are detected, the diagnosis of trichomoniasis is confirmed. If organisms are not detected the second step would be to send a vaginal or urine specimen for NAAT for T vaginalis or for culture. An advantage of NAAT over culture is that urine specimens can be used for diagnosis of T vaginalis while urine specimens are not suitable for culture because of low sensitivity. For patients diagnosed with trichomoniasis, the CDC recommends that testing be repeated in 3 months because of high rates of reinfection. NAAT would be an optimal test to use in this situation.
Continue to: Bacterial vaginosis and candidiasis...
Bacterial vaginosis and candidiasis
ACOG recommends using Amsel criteria or Nugent scoring of a specimen colorized with a Gram stain for the diagnosis of bacterial vaginosis and microscopy or culture for the diagnosis of candidiasis.8 Recent research reports that NAAT testing for bacterial vaginosis and candidiasis may be more sensitive than standard office-based approaches for detecting these two causes of vaginitis. In a study of approximately 1,740 patients with symptoms of vaginitis, vaginal specimens were analyzed using NAAT or standard office approaches to diagnosis.10 In this study the diagnostic gold standards were Nugent scoring with Amsel criteria to resolve intermediate Nugent scores for bacterial vaginosis and culture for Candida. The study demonstrated the superiority of NAAT testing over standard office approaches for the identification of the cause of the vaginitis. NAAT testing was reported to have superior sensitivity for diagnosing bacterial vaginosis compared with the original Amsel criteria (93% vs 76%, respectively (P <.0001), with similar respective specificities of 92% and 94% .10 NAAT testing also had superior sensitivity for diagnosing Candidiasis compared with microscopy after potassium hydroxide treatment of a vaginal specimen (91% vs 58%, respectively (P <.0001).10 NAAT testing also had superior specificity compared with microscopy after potassium hydroxide treatment of a vaginal specimen (94% vs 89%, respectively (P < .0005).10
In another study comparing NAAT with clinical diagnosis for 466 patients with symptoms of vaginitis, standard office approaches to the diagnosis of vaginitis resulted in the failure to identify the correct infection in a large number of cases. For the diagnosis of bacterial vaginosis, clinicians missed 42% of the cases identified by NAAT. For the diagnosis of Candida, clinicians missed 46% of the cases identified by NAAT. For T vaginalis diagnosis, clinicians missed 72% of the cases identified by NAAT. Clearly, this resulted in clinicians not treating many infections detected by NAAT.11
Continue to: One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis...
One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis
In a recent study, 1,471 patients with a symptom of vaginitis (abnormal vaginal discharge, itching or irritation, or odor) self-collected a vaginal swab and had a vaginal swab collected by a clinician.12 The swabs were placed in buffer and the samples were tested by NAAT using the BD Max system (Franklin Lakes, New Jersey) for the presence of nucleic acid sequences of the microorganisms responsible for the most common causes of vaginitis. In this cohort, using the clinician collected vaginal swabs for NAAT, the investigators reported the following pattern of detection of nucleic acid sequences: 36.1%, bacterial vaginosis pattern; 16.2%, Candida spp.; 1.6%, T vaginalis; 0.7%, Candida glabrata; and 0.1%, Candida krusei. Nucleic acid sequences of multiple organisms were detected in 21.7% of patients, including 13.9% with bacterial vaginosis pattern plus Candida spp., 4.9% with bacterial vaginosis pattern plus T vaginalis, 0.3% with Candida spp. plus T vaginalis, 0.2% with Candida spp. plus Candida glabrata, 0.2% with bacterial vaginosis pattern plus Candida glabrata, and 2.2% with all 3 organisms. A total of 23.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.
In another study of 1,491 patients with a symptom of vaginitis, clinician-collected vaginal swabs were tested by NAAT using the Aptima BV and Aptima Candida/Trichomonas systems (Hologic, Marlborough, Massachusetts) for the presence of nucleic acid sequences of microorganisms responsible for most cases of vaginitis.13 The investigators reported the following pattern of detection of nucleic acid sequences: 28.6%, bacterial vaginosis pattern; 14.2%, Candida spp.; 3%, T vaginalis; 1.9%, Candida glabrata.13 Nucleic acid sequences from multiple organisms were detected in 23.3% of patients. Nucleic acid sequences suggesting the presence of two different causes of vaginitis were detected among 20.8% of patients, including bacterial vaginosis plus Candida spp., 11.1%; bacterial vaginosis plus T vaginalis, 7.2%; Candida spp. plus T vaginalis, 1.0%; Candida spp. plus Candida glabrata, 0.9%; bacterial vaginosis plus Candida spp., 0.5%; Candida glabrata plus T vaginalis, 0.1%. Nucleic acid sequences suggesting the presence of 3 different causes of vaginitis were detected in 2.4% of patients, the most common being the combination of bacterial vaginosis plus Candida spp. plus T vaginalis, 1.7% and bacterial vaginosis plus Candida spp. plus Candida glabrata, 0.5%. Nucleic acid sequences suggesting the presence of 4 different causes of vaginitis were detected in 0.1% of patients. A total of 28.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.13
In clinical practice it is uncommon to see the diagnosis of multiple causes of vaginitis recorded in the medical record of a patient. This suggests that we are not effectively identifying the 20% of patients with multiple causes of vaginitis.
When multiple organisms that cause vaginitis are present, NAAT is superior to clinical evaluation for diagnosis
In a study of 1,264 patients with symptoms of vaginitis who had an identified microbial cause, more than 20% had multiple organisms detected by NAAT
Patient collection of a vaginal swab for NAAT
Multiple studies have reported that collection of a vaginal swab for NAAT by the patient or a clinician results in similar excellent test performance.4,12,13 This observation might catalyze the development of clinical protocols where patients with vaginitis could collect the swab for NAAT analysis, without needing to have a speculum examination by a clinician.
When collecting a vaginal specimen for NAAT it is important that no vaginal lubricants or creams contaminate the collection swab. Vaginal lubricants and creams may inhibit the polymerase chain reaction enzymes resulting in a false negative. The swab may be directly inserted into the vagina to collect the specimen or a speculum without a lubricant, except water can be used to facilitate specimen collection. To collect a specimen without a speculum the swab is inserted 2 inches into the vagina and rotated for 10 to 15 seconds.
What should clinicians do while waiting for a NAAT result?
A major problem with NAAT testing for vaginitis is that the results are not available at the initial patient visit, impacting the ability to make an immediate diagnosis and provide targeted antibiotic treatment. Given that bacterial vaginosis and Candida species are the most common causes of infectious vaginitis in many populations of gynecology patients, one approach is to initiate treatment with one dose of an oral antifungal agent and a multiday course of vaginal metronidazole. Once the NAAT test results are available, the treatment can be refined to specific infectious agents identified by the test, or the antibiotics can be discontinued if no relevant microorganisms are detected. Another approach would be to wait until the NAAT test is completed and then prescribe the appropriate antibiotic. My sense is that most patients would not favor this wait and see approach.
Barriers to the use of NAAT for vaginitis
A barrier to the use of NAAT for the diagnosis of vaginitis is that leading organizations do not currently recommend NAAT as a primary approach to diagnosis, favoring microscopy and measurement of vaginal pH.9 In addition, clinicians and patients may be rightfully concerned about the cost of NAAT, which can be substantial.
Vaginitis, especially when it is recurrent, can be stressful14 and have an impact on a patient’s quality of life15,16 and sexual health.17 Arguably, our current practice algorithms for diagnosing the cause of vaginitis are not optimized.18 Our failure to accurately diagnose the cause of vaginitis contributes to inappropriate antibiotic treatment and return visits because of inadequate initial treatment.18 We can improve and simplify our approach to the diagnosis of vaginitis by prioritizing the use of NAAT.19 In turn, reliably making the right diagnosis will result in the optimization of treatment. ●
- Jose PP, Vivekanandan V, Sobhanakumari K. Gonorrhea: Historical outlook. J Skin Sex Transm Dis. 2020;2:110-114.
- Bayly HW. The diagnosis and treatment of chronic gonorrhoea and its local complications. Br Med J. 1914;14:584-587.
- Stuart RD. The diagnosis and control of gonorrhoea by bacteriological cultures: with a preliminary report on a new method for transporting clinical material. Glasgow Med J. 1946;27:131-142.
- Wilson JD, Wallace HE, Loftus-Keeling M, et al. Swab-yourself trial with economic monitoring and testing for infections collectively (SYSTEMATIC): Part 2. A diagnostic accuracy and cost-effectiveness study comparing rectal, pharyngeal and urogenital samples analyzed individually, versus as a pooled specimen, for the diagnosis of gonorrhea and chlamydia. Clin Infect Dis. 2021;73:e3183-3193.
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new NAAT: association with ethnicity, symptoms and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312-316.
- Roth AM, Williams JA, Ly R. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398-400.
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infection. 2013;89:434-438.
- Vaginitis in nonpregnant patients. ACOG Practice Bulletin No 215. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e1-e17.
- Workowksi KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines 2021. MMWR. 2021;70:1-187.
- Schwebke JR, Gaydos CA, Hyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of vaginal panel assay compared with clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130:181-189.
- Schwebke JR, Taylor SN, Ackerman N, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginalis assays: results from a prospective multi-center study. J Clin Microbiol. 2020;58:e01643-19.
- Ehrstrom S, Kornfeld D, Rylander E. Perceived stress in women with recurrent vulvovaginal candidiasis. J Psychosomatic Obstet Gynecol. 2007;28:169-176.
- Abellea S, Guelfucci F, Wagner J, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis in Europe and the USA. Health Quality Life Outcomes. 2013;11:169.
- Fukazawa EI, Witkin SS, Robial R, et al. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch Gynecol Obstet. 2019;300:647-650.
- Giraldo PC, Polpeta NC, Juliato CT, et al. Evaluation of sexual function in Brazilian women with recurrent vulvovaginal candidiasis and localized provoked vulvodynia. J Sex Med. 2012;9:805-811.
- Hillier SL, Austin M, Macio I, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72:1538-1543.
- . Sobel JD. Syndromic treatment of women with vulvovaginal symptoms in the United States: a call to action. Clin Infect Dis. 2021;72:1544-1545.
- Jose PP, Vivekanandan V, Sobhanakumari K. Gonorrhea: Historical outlook. J Skin Sex Transm Dis. 2020;2:110-114.
- Bayly HW. The diagnosis and treatment of chronic gonorrhoea and its local complications. Br Med J. 1914;14:584-587.
- Stuart RD. The diagnosis and control of gonorrhoea by bacteriological cultures: with a preliminary report on a new method for transporting clinical material. Glasgow Med J. 1946;27:131-142.
- Wilson JD, Wallace HE, Loftus-Keeling M, et al. Swab-yourself trial with economic monitoring and testing for infections collectively (SYSTEMATIC): Part 2. A diagnostic accuracy and cost-effectiveness study comparing rectal, pharyngeal and urogenital samples analyzed individually, versus as a pooled specimen, for the diagnosis of gonorrhea and chlamydia. Clin Infect Dis. 2021;73:e3183-3193.
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new NAAT: association with ethnicity, symptoms and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312-316.
- Roth AM, Williams JA, Ly R. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398-400.
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infection. 2013;89:434-438.
- Vaginitis in nonpregnant patients. ACOG Practice Bulletin No 215. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e1-e17.
- Workowksi KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines 2021. MMWR. 2021;70:1-187.
- Schwebke JR, Gaydos CA, Hyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of vaginal panel assay compared with clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130:181-189.
- Schwebke JR, Taylor SN, Ackerman N, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginalis assays: results from a prospective multi-center study. J Clin Microbiol. 2020;58:e01643-19.
- Ehrstrom S, Kornfeld D, Rylander E. Perceived stress in women with recurrent vulvovaginal candidiasis. J Psychosomatic Obstet Gynecol. 2007;28:169-176.
- Abellea S, Guelfucci F, Wagner J, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis in Europe and the USA. Health Quality Life Outcomes. 2013;11:169.
- Fukazawa EI, Witkin SS, Robial R, et al. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch Gynecol Obstet. 2019;300:647-650.
- Giraldo PC, Polpeta NC, Juliato CT, et al. Evaluation of sexual function in Brazilian women with recurrent vulvovaginal candidiasis and localized provoked vulvodynia. J Sex Med. 2012;9:805-811.
- Hillier SL, Austin M, Macio I, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72:1538-1543.
- . Sobel JD. Syndromic treatment of women with vulvovaginal symptoms in the United States: a call to action. Clin Infect Dis. 2021;72:1544-1545.
Beyond diabetes: The beneficial uses of metformin in psychiatry
Metabolic dysregulation is quite common among psychiatric patients, especially those with psychotic or mood disorders. Obesity, diabetes, and dyslipidemia can be present at the onset of the illness, or as an iatrogenic complication. This often leads to premature mortality due to elevated cardiovascular and cerebrovascular risks.
Enter metformin. It is the most widely used hypoglycemic agent for type 2 diabetes (T2D), and it is frequently used by psychiatric clinicians. Discovered in 1922 and developed in France in the 1950s, metformin was approved for use in the United States in 1995, 3 decades after its launch in Europe. Its original trade name in the United States was Glucophage, and it is currently available from several companies in generic form. It is included on the World Health Organization list of essential medications.
T2D is currently an epidemic across the general populations globally, especially in the United States, where approximately 95% of the 37 million individuals with diabetes have been diagnosed with T2D.1 This is 300% higher than the prevalence in the 1970s. No wonder metformin is one of the most often-used drugs in all of medicine, and a staple in primary care and psychiatry. It has helped countless patients avoid the multisystem hazards of insulin resistance, which is the root cause of T2D.
Metformin exerts its hypoglycemic effects by:
- decreasing glucose production from the liver
- increasing insulin receptors’ sensitivity in various body tissues
- increasing secretion of growth differentiating factor, which reduces appetite and calorie intake.
In 2017, the American College of Physicians updated its guidelines to adopt metformin as the first-line treatment for T2D, especially because the class of sulfonylureas were associated with a more than 5-fold higher risk of severe low blood sugar events compared with metformin.2 In addition, metformin causes weight loss, while sulfonylureas are associated with weight gain. Metformin is particularly useful in gestational diabetes, where babies are born with less visceral fat and are less prone to insulin resistance later in life as adults.
The adverse effects of metformin are dose-related and mostly gastrointestinal (GI), including nausea, vomiting, cramps, diarrhea, and flatulence. Gradual titration or using the extended-release formulation can lower or avert GI discomfort. Metformin should not be used in patients with severe kidney or liver disease. With long-term use, metformin can cause malabsorption and eventual deficiency of vitamin B12.
The metabolic benefits of metformin listed below are why psychiatrists use it in clinical practice. However, this medication has several benefits that go beyond metabolic disorders. Clinicians should be aware of all of the following salutary physical and mental effects of metformin.
Metabolic benefits
- Decreasing glucose dysregulation with the use of clozapine and other antipsychotics.3
- Decreasing weight, body mass index, and waist circumference with the use of clozapine.4
- Decreasing triglycerides and total cholesterol.5
- Mitigating clozapine-induced obesity, especially if used prophylactically.6
- Lowering antipsychotic-induced weight gain.7
Continue on to: Nonmetabolic benefits...
Nonmetabolic benefits
- Lowering elevated serum prolactin levels to avert sexual dysfunction.8-10
- Increasing the production of neurons by inducing neurogenesis.11,12
- Activating the cerebral cortex to blunt the adverse effects of clozapine (such as deterioration of motivation, attention, cognition, and behavior) and increasing the activity of the dopamine D1 receptor, which is believed to be involved with cognition in schizophrenia.13
- Reducing the symptoms of anxiety and depression by increasing serotonin activity and hippocampal concentration of serotonin.14
- Decreasing the depressive symptoms known to be associated with uncontrolled diabetes.15
- Improving insulin resistance associated with polycystic ovary syndrome and helping with infertility.16
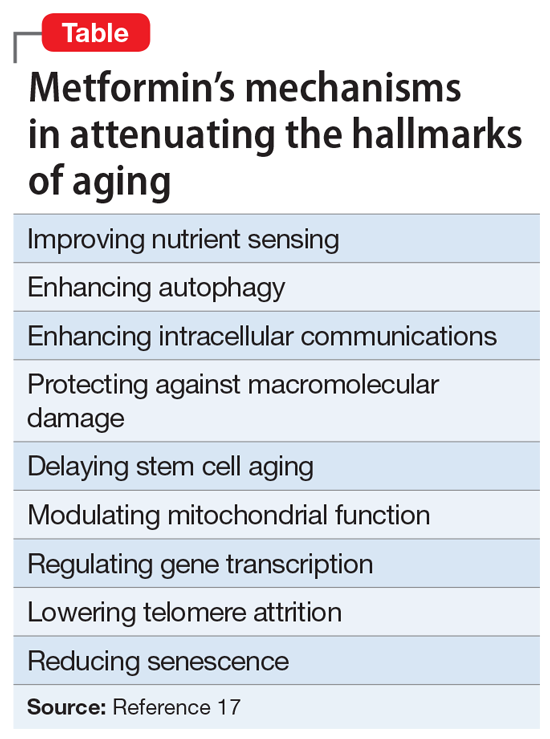
- Exerting multiple anti-aging effects (Table17). Metformin reduces several hallmarks of aging and may increase longevity.17
- Lowering the risks of cancer, dementia, and mortality in patients with and without diabetes18 due to its anti-aging effects. Scientists are actively studying metformin’s anti-aging effects and trying to develop drugs with similar effects.
- Counteracting inflammatory bowel disease, osteoporosis, neurodegeneration, inflammation, frailty, and senescence.19
Metformin may sound like a wonder drug or panacea, but most of its multiple beneficial effects have been reported and replicated. Its therapeutic effects on obesity, diabetes, and dyslipidemia can prevent early mortality, but its anti-aging effects are also important and may help reduce premature mortality, which is common in psychiatric patients.20 So, the question arises: At some point, will metformin be used for persons not afflicted by diabetes or metabolic syndrome? For now, psychiatrists should continue to use it on label, but in the future, our patients may benefit from its “fringe benefits.”
1. Centers for Disease Control and Prevention. Type 2 diabetes. Accessed January 28, 2022. https://www.cdc.gov/diabetes/basics/type2.html
2. Qaseem A, Barry MJ, Humphrey LL, et al; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
3. Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219.
4. Hebrani P, Manteghi AA, Behdani F, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364-371.
5. Jiang WL, Cai DB, Yin F, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. 2020;10(1):117.
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208
7. de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
8. Zheng W, Yang XH, Cai DB, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31(5):625-631.
9. Krysiak R, Kowalcze K, Szkrobka W, et al. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. 2016;30:94-98.
10. Bo QJ, Wang ZM, Li XB, et al. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. 2016;237:257-263.
11. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23-35.
12. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988-995.
13. Horvath G, Kis G, Kekesi G, et al. Interaction of clozapine with metformin in a schizophrenia rat model. Sci Rep. 2021;11(1):16862.
14. Zemdegs J, Martin H, Pintana H, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39(30):5935-5948.
15. B˘adescu SV, T˘ataru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120-125.
16. Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72-75.
17. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30.
18. Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44.
19. Ala M, Ala M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS Pharmacol Transl Sci. 2021;4(6):1747-1770.
20. Nasrallah HA. Premature mortality across most psychiatric disorders. Current Psychiatry. 2019;8(10):9-10,12,34.
Metabolic dysregulation is quite common among psychiatric patients, especially those with psychotic or mood disorders. Obesity, diabetes, and dyslipidemia can be present at the onset of the illness, or as an iatrogenic complication. This often leads to premature mortality due to elevated cardiovascular and cerebrovascular risks.
Enter metformin. It is the most widely used hypoglycemic agent for type 2 diabetes (T2D), and it is frequently used by psychiatric clinicians. Discovered in 1922 and developed in France in the 1950s, metformin was approved for use in the United States in 1995, 3 decades after its launch in Europe. Its original trade name in the United States was Glucophage, and it is currently available from several companies in generic form. It is included on the World Health Organization list of essential medications.
T2D is currently an epidemic across the general populations globally, especially in the United States, where approximately 95% of the 37 million individuals with diabetes have been diagnosed with T2D.1 This is 300% higher than the prevalence in the 1970s. No wonder metformin is one of the most often-used drugs in all of medicine, and a staple in primary care and psychiatry. It has helped countless patients avoid the multisystem hazards of insulin resistance, which is the root cause of T2D.
Metformin exerts its hypoglycemic effects by:
- decreasing glucose production from the liver
- increasing insulin receptors’ sensitivity in various body tissues
- increasing secretion of growth differentiating factor, which reduces appetite and calorie intake.
In 2017, the American College of Physicians updated its guidelines to adopt metformin as the first-line treatment for T2D, especially because the class of sulfonylureas were associated with a more than 5-fold higher risk of severe low blood sugar events compared with metformin.2 In addition, metformin causes weight loss, while sulfonylureas are associated with weight gain. Metformin is particularly useful in gestational diabetes, where babies are born with less visceral fat and are less prone to insulin resistance later in life as adults.
The adverse effects of metformin are dose-related and mostly gastrointestinal (GI), including nausea, vomiting, cramps, diarrhea, and flatulence. Gradual titration or using the extended-release formulation can lower or avert GI discomfort. Metformin should not be used in patients with severe kidney or liver disease. With long-term use, metformin can cause malabsorption and eventual deficiency of vitamin B12.
The metabolic benefits of metformin listed below are why psychiatrists use it in clinical practice. However, this medication has several benefits that go beyond metabolic disorders. Clinicians should be aware of all of the following salutary physical and mental effects of metformin.
Metabolic benefits
- Decreasing glucose dysregulation with the use of clozapine and other antipsychotics.3
- Decreasing weight, body mass index, and waist circumference with the use of clozapine.4
- Decreasing triglycerides and total cholesterol.5
- Mitigating clozapine-induced obesity, especially if used prophylactically.6
- Lowering antipsychotic-induced weight gain.7
Continue on to: Nonmetabolic benefits...
Nonmetabolic benefits
- Lowering elevated serum prolactin levels to avert sexual dysfunction.8-10
- Increasing the production of neurons by inducing neurogenesis.11,12
- Activating the cerebral cortex to blunt the adverse effects of clozapine (such as deterioration of motivation, attention, cognition, and behavior) and increasing the activity of the dopamine D1 receptor, which is believed to be involved with cognition in schizophrenia.13
- Reducing the symptoms of anxiety and depression by increasing serotonin activity and hippocampal concentration of serotonin.14
- Decreasing the depressive symptoms known to be associated with uncontrolled diabetes.15
- Improving insulin resistance associated with polycystic ovary syndrome and helping with infertility.16
- Exerting multiple anti-aging effects (Table17). Metformin reduces several hallmarks of aging and may increase longevity.17
- Lowering the risks of cancer, dementia, and mortality in patients with and without diabetes18 due to its anti-aging effects. Scientists are actively studying metformin’s anti-aging effects and trying to develop drugs with similar effects.
- Counteracting inflammatory bowel disease, osteoporosis, neurodegeneration, inflammation, frailty, and senescence.19

Metformin may sound like a wonder drug or panacea, but most of its multiple beneficial effects have been reported and replicated. Its therapeutic effects on obesity, diabetes, and dyslipidemia can prevent early mortality, but its anti-aging effects are also important and may help reduce premature mortality, which is common in psychiatric patients.20 So, the question arises: At some point, will metformin be used for persons not afflicted by diabetes or metabolic syndrome? For now, psychiatrists should continue to use it on label, but in the future, our patients may benefit from its “fringe benefits.”
Metabolic dysregulation is quite common among psychiatric patients, especially those with psychotic or mood disorders. Obesity, diabetes, and dyslipidemia can be present at the onset of the illness, or as an iatrogenic complication. This often leads to premature mortality due to elevated cardiovascular and cerebrovascular risks.
Enter metformin. It is the most widely used hypoglycemic agent for type 2 diabetes (T2D), and it is frequently used by psychiatric clinicians. Discovered in 1922 and developed in France in the 1950s, metformin was approved for use in the United States in 1995, 3 decades after its launch in Europe. Its original trade name in the United States was Glucophage, and it is currently available from several companies in generic form. It is included on the World Health Organization list of essential medications.
T2D is currently an epidemic across the general populations globally, especially in the United States, where approximately 95% of the 37 million individuals with diabetes have been diagnosed with T2D.1 This is 300% higher than the prevalence in the 1970s. No wonder metformin is one of the most often-used drugs in all of medicine, and a staple in primary care and psychiatry. It has helped countless patients avoid the multisystem hazards of insulin resistance, which is the root cause of T2D.
Metformin exerts its hypoglycemic effects by:
- decreasing glucose production from the liver
- increasing insulin receptors’ sensitivity in various body tissues
- increasing secretion of growth differentiating factor, which reduces appetite and calorie intake.
In 2017, the American College of Physicians updated its guidelines to adopt metformin as the first-line treatment for T2D, especially because the class of sulfonylureas were associated with a more than 5-fold higher risk of severe low blood sugar events compared with metformin.2 In addition, metformin causes weight loss, while sulfonylureas are associated with weight gain. Metformin is particularly useful in gestational diabetes, where babies are born with less visceral fat and are less prone to insulin resistance later in life as adults.
The adverse effects of metformin are dose-related and mostly gastrointestinal (GI), including nausea, vomiting, cramps, diarrhea, and flatulence. Gradual titration or using the extended-release formulation can lower or avert GI discomfort. Metformin should not be used in patients with severe kidney or liver disease. With long-term use, metformin can cause malabsorption and eventual deficiency of vitamin B12.
The metabolic benefits of metformin listed below are why psychiatrists use it in clinical practice. However, this medication has several benefits that go beyond metabolic disorders. Clinicians should be aware of all of the following salutary physical and mental effects of metformin.
Metabolic benefits
- Decreasing glucose dysregulation with the use of clozapine and other antipsychotics.3
- Decreasing weight, body mass index, and waist circumference with the use of clozapine.4
- Decreasing triglycerides and total cholesterol.5
- Mitigating clozapine-induced obesity, especially if used prophylactically.6
- Lowering antipsychotic-induced weight gain.7
Continue on to: Nonmetabolic benefits...
Nonmetabolic benefits
- Lowering elevated serum prolactin levels to avert sexual dysfunction.8-10
- Increasing the production of neurons by inducing neurogenesis.11,12
- Activating the cerebral cortex to blunt the adverse effects of clozapine (such as deterioration of motivation, attention, cognition, and behavior) and increasing the activity of the dopamine D1 receptor, which is believed to be involved with cognition in schizophrenia.13
- Reducing the symptoms of anxiety and depression by increasing serotonin activity and hippocampal concentration of serotonin.14
- Decreasing the depressive symptoms known to be associated with uncontrolled diabetes.15
- Improving insulin resistance associated with polycystic ovary syndrome and helping with infertility.16
- Exerting multiple anti-aging effects (Table17). Metformin reduces several hallmarks of aging and may increase longevity.17
- Lowering the risks of cancer, dementia, and mortality in patients with and without diabetes18 due to its anti-aging effects. Scientists are actively studying metformin’s anti-aging effects and trying to develop drugs with similar effects.
- Counteracting inflammatory bowel disease, osteoporosis, neurodegeneration, inflammation, frailty, and senescence.19

Metformin may sound like a wonder drug or panacea, but most of its multiple beneficial effects have been reported and replicated. Its therapeutic effects on obesity, diabetes, and dyslipidemia can prevent early mortality, but its anti-aging effects are also important and may help reduce premature mortality, which is common in psychiatric patients.20 So, the question arises: At some point, will metformin be used for persons not afflicted by diabetes or metabolic syndrome? For now, psychiatrists should continue to use it on label, but in the future, our patients may benefit from its “fringe benefits.”
1. Centers for Disease Control and Prevention. Type 2 diabetes. Accessed January 28, 2022. https://www.cdc.gov/diabetes/basics/type2.html
2. Qaseem A, Barry MJ, Humphrey LL, et al; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
3. Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219.
4. Hebrani P, Manteghi AA, Behdani F, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364-371.
5. Jiang WL, Cai DB, Yin F, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. 2020;10(1):117.
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208
7. de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
8. Zheng W, Yang XH, Cai DB, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31(5):625-631.
9. Krysiak R, Kowalcze K, Szkrobka W, et al. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. 2016;30:94-98.
10. Bo QJ, Wang ZM, Li XB, et al. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. 2016;237:257-263.
11. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23-35.
12. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988-995.
13. Horvath G, Kis G, Kekesi G, et al. Interaction of clozapine with metformin in a schizophrenia rat model. Sci Rep. 2021;11(1):16862.
14. Zemdegs J, Martin H, Pintana H, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39(30):5935-5948.
15. B˘adescu SV, T˘ataru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120-125.
16. Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72-75.
17. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30.
18. Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44.
19. Ala M, Ala M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS Pharmacol Transl Sci. 2021;4(6):1747-1770.
20. Nasrallah HA. Premature mortality across most psychiatric disorders. Current Psychiatry. 2019;8(10):9-10,12,34.
1. Centers for Disease Control and Prevention. Type 2 diabetes. Accessed January 28, 2022. https://www.cdc.gov/diabetes/basics/type2.html
2. Qaseem A, Barry MJ, Humphrey LL, et al; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
3. Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219.
4. Hebrani P, Manteghi AA, Behdani F, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364-371.
5. Jiang WL, Cai DB, Yin F, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. 2020;10(1):117.
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208
7. de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
8. Zheng W, Yang XH, Cai DB, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31(5):625-631.
9. Krysiak R, Kowalcze K, Szkrobka W, et al. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. 2016;30:94-98.
10. Bo QJ, Wang ZM, Li XB, et al. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. 2016;237:257-263.
11. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23-35.
12. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988-995.
13. Horvath G, Kis G, Kekesi G, et al. Interaction of clozapine with metformin in a schizophrenia rat model. Sci Rep. 2021;11(1):16862.
14. Zemdegs J, Martin H, Pintana H, et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J Neurosci. 2019;39(30):5935-5948.
15. B˘adescu SV, T˘ataru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120-125.
16. Erensoy H, Niafar M, Ghafarzadeh S, et al. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72-75.
17. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30.
18. Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44.
19. Ala M, Ala M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS Pharmacol Transl Sci. 2021;4(6):1747-1770.
20. Nasrallah HA. Premature mortality across most psychiatric disorders. Current Psychiatry. 2019;8(10):9-10,12,34.
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.
Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
It’s time for moonshot thinking in psychiatry
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
Individualize the duration of postpartum magnesium treatment for patients with preeclampsia to best balance the benefits and harms of treatment
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15
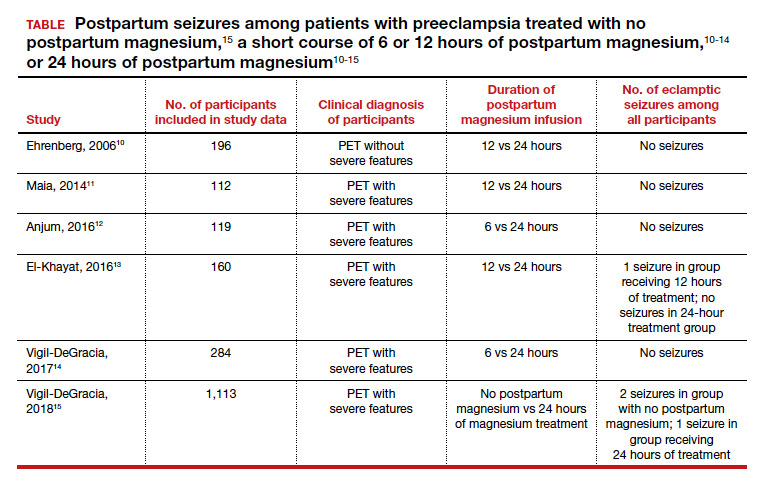
Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.