User login
Taking the Next Step
As resident physicians, we find ourselves at the forefronts of medicine. While tackling the complexities of clinical medicine, all too soon we become entrenched in the bureaucracy of the medical system. We have come to accept realism and practicality over idealism. Shackled by the inefficiencies of a medical system in dire need of reform, we trek forward in hopes of a new future.
I remember the day quite clearly. My 30‐hour shift was nearing an end. It had not been a rough night, but covering both telemetry and coronary care unit services does take a toll on one's physical and mental well‐being. We were ready to discharge Mr. H, an indigent man recovering from a heart attack. I entered his room. He was all dressed. I explained all the important details and handed him a prescription. These medications are very important. Here is a taxi voucher to the pharmacy to get these medications.
He smiled, Thank you very much. You guys saved my life and I really appreciate it.
You are very welcome, I replied. In addition to the medications, it is very important that you follow up with your doctor within 1 to 2 weeks. Do you have any questions?
No problem. You guys saved my life. Thank you so much!
His eyes glistened with tears as he stared right through me, not hearing anything I had said. Mr. H, could you please wait a few more minutes? I will help you schedule a clinic visit.
I quickly located his clinic number at the county hospital. After 3 attempts, I reached a live person. I explained my situation: I am a doctor at a local hospital trying to help a patient setup a clinic appointment. She rattled off a slurry of information, clearly from a script, and left me with 5 different numbers to choose from (the main clinic line, an alternative number, a third number for the urgent advice nurse, one for new patients, and another for subspecialists). The main line was busy, and despite 3 additional attempts, this avenue ended in a recording advising me to leave a message. Unfortunately, my patient lived in an single resident occupancy (SRO) and did not have a phone, and thus leaving a message would not be helpful. I tried another number and the operator informed me that Mr. H was not in the system, and therefore, he would transfer me to the new patients department. The phone rang 5 times before the voicemail message answered, instructing me to leave a detailed message with my contact information. I hung up, and called the original operator back.
I'm sorry. Only the new patient department can schedule appointments for new patients. Here is the direct number. They must be busy. You can try again.
After several attempts, someone answered. My system shows that Mr. H has been assigned to the 7th Street Clinic. He's been seen there before, so you need to contact the main clinic line. I can only make appointments for new patients. She transferred me back to the first operator.
Oh yes, it looks like Mr. H is assigned to 7th Street Clinic. Please hold while I try to locate the next available appointment. He returns 3 minutes later. The next appointment I have is 4 months from now9:30 AM or 3:30 PM?
Is there anyway to schedule an earlier appointment? Mr. H has just recovered from a heart attack and needs to be seen sooner.
I'm sorry. This is the earliest appointment available. If he needs urgent care, he can go to the urgent care clinic Monday through Friday 7:30 AM to 6:00 PM. I persisted and he gave me the clinic's direct line. Sometimes, they may have earlier openings.
I called the 7th Street Clinic. The receptionist informed me that Mr. H was not in the system and she could not help me. I would need to speak with the new patient department. I just spoke with that department, and they were not able to schedule an appointment because their records show he has been seen at 7th Street Clinic in the past.
I'm sorry. He is not in our system. I can't schedule any appointments for him.
Again I went through all the previous numbers and spoke with the same 4 or 5 people, transferring me back and forth through this ridiculous maze, dodging voicemails and busy tones. One hour after I first began this endeavor, I finally succeeded in securing an appointment for Mr. H within 2 weeks as a new patient at a new clinic.
Why had this task been so difficult? Mr. H is the type of individual most at risk of falling through the cracks. Why is it that these individuals, homeless and indigent patients that lack social support, suffering already from countless barriers to health care access and resources because of their socioeconomic status continue to face such a horridly complex system of inefficiency and bureaucracy when trying to make a simple clinic appointment? How difficult and frustrating it was for me to accomplish this taskhow could I expect my patient living in an SRO without a phone to succeed?
Identifying barriers to health care access is the first step in addressing these issues. Previous studies have demonstrated that the majority of barriers to adequate follow‐up after a hospital visit occur among minority groups.17 Lower socioeconomic status is often associated with financial limitations from inability to take time off from work. Among immigrant cohorts, language and cultural barriers also play an important role in affecting follow‐up care. In addition to suboptimal follow‐up care, these barriers often lead to increased patient morbidity and increased rates of hospital readmission. For example, studies have reported that certain minority groups were more likely to receive no pain medication after bone fractures and were less likely to receive adequate analgesia for cancer‐related pain.1, 2, 7 In an attempt to address these issues, several studies have reported on multidisciplinary discharge planning interventions.810 One program in particular involved emergency departments providing free medication, transportation vouchers to and from the patient's primary care clinic, and telephone reminders to schedule follow‐up appointments.10 The implementation of these programs translated into improved primary care follow up, decreased hospital readmission rates, and decreased costs.
It is clear that our current health care system is wrought with inefficiencies that pose significant barriers to access by certain cohorts. The fact that minority groups and cohorts of the lowest socioeconomic status suffer most from these obstacles is concerning. Studies have shown that comprehensive programs to address these barriers including greater access to language interpreters and implementing a multidisciplinary approach to discharge planning improve patient outcomes. As we move forward in the ever‐evolving US medical system, there needs to be greater emphasis on preventative care. Education and resources to improve access to primary care physicians through identification of barriers and developing programs to address these issues is only the first step.
- ,.Effect of language barriers on follow‐up appointments after an emergency department visit.J Gen Intern Med.2000;15:256–264.
- ,,.Ethnicity as a risk factor for inadequate emergency department analgesia.JAMA.1993;269:1537–1539.
- ,.Differences in follow‐up visits between African‐American and White Medicaid children hospitalized with asthma.J Health Care Poor Underserved.1997;8:83–98.
- ,,.Predictors and barriers to timely medical follow‐up after cardiovascular disease risk factor screening according to race/ethnicity.J Natl Med Assoc.2008;100:534–539.
- ,.Nonprice barriers to ambulatory care after an emergency department visit.Ann Emerg Med.2008;51:607–613.
- ,,,,.Financial barriers to health care and outcomes after acute myocardial infarction.JAMA.2007;297:1063–1072.
- ,,,,.Pain and treatment of pain in minority patients with cancer: The Eastern Cooperative Oncology Group Minority Outpatient Pain Study.Ann Intern Med.1997;127:813–816.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders; a randomized clinical trial.JAMA.1999;281:613–620.
- .Approaches to help people with diabetes overcome barriers for improved health outcomes.Diabetes Educ.2008;34(1 Suppl):18S–24S.
- ,,, et al.A randomized, controlled trial of a simple emergency department intervention to improve the rate of primary care follow‐up for patients with acute asthma exacerbations.Ann Emerg Med.2001;38:115–122.
As resident physicians, we find ourselves at the forefronts of medicine. While tackling the complexities of clinical medicine, all too soon we become entrenched in the bureaucracy of the medical system. We have come to accept realism and practicality over idealism. Shackled by the inefficiencies of a medical system in dire need of reform, we trek forward in hopes of a new future.
I remember the day quite clearly. My 30‐hour shift was nearing an end. It had not been a rough night, but covering both telemetry and coronary care unit services does take a toll on one's physical and mental well‐being. We were ready to discharge Mr. H, an indigent man recovering from a heart attack. I entered his room. He was all dressed. I explained all the important details and handed him a prescription. These medications are very important. Here is a taxi voucher to the pharmacy to get these medications.
He smiled, Thank you very much. You guys saved my life and I really appreciate it.
You are very welcome, I replied. In addition to the medications, it is very important that you follow up with your doctor within 1 to 2 weeks. Do you have any questions?
No problem. You guys saved my life. Thank you so much!
His eyes glistened with tears as he stared right through me, not hearing anything I had said. Mr. H, could you please wait a few more minutes? I will help you schedule a clinic visit.
I quickly located his clinic number at the county hospital. After 3 attempts, I reached a live person. I explained my situation: I am a doctor at a local hospital trying to help a patient setup a clinic appointment. She rattled off a slurry of information, clearly from a script, and left me with 5 different numbers to choose from (the main clinic line, an alternative number, a third number for the urgent advice nurse, one for new patients, and another for subspecialists). The main line was busy, and despite 3 additional attempts, this avenue ended in a recording advising me to leave a message. Unfortunately, my patient lived in an single resident occupancy (SRO) and did not have a phone, and thus leaving a message would not be helpful. I tried another number and the operator informed me that Mr. H was not in the system, and therefore, he would transfer me to the new patients department. The phone rang 5 times before the voicemail message answered, instructing me to leave a detailed message with my contact information. I hung up, and called the original operator back.
I'm sorry. Only the new patient department can schedule appointments for new patients. Here is the direct number. They must be busy. You can try again.
After several attempts, someone answered. My system shows that Mr. H has been assigned to the 7th Street Clinic. He's been seen there before, so you need to contact the main clinic line. I can only make appointments for new patients. She transferred me back to the first operator.
Oh yes, it looks like Mr. H is assigned to 7th Street Clinic. Please hold while I try to locate the next available appointment. He returns 3 minutes later. The next appointment I have is 4 months from now9:30 AM or 3:30 PM?
Is there anyway to schedule an earlier appointment? Mr. H has just recovered from a heart attack and needs to be seen sooner.
I'm sorry. This is the earliest appointment available. If he needs urgent care, he can go to the urgent care clinic Monday through Friday 7:30 AM to 6:00 PM. I persisted and he gave me the clinic's direct line. Sometimes, they may have earlier openings.
I called the 7th Street Clinic. The receptionist informed me that Mr. H was not in the system and she could not help me. I would need to speak with the new patient department. I just spoke with that department, and they were not able to schedule an appointment because their records show he has been seen at 7th Street Clinic in the past.
I'm sorry. He is not in our system. I can't schedule any appointments for him.
Again I went through all the previous numbers and spoke with the same 4 or 5 people, transferring me back and forth through this ridiculous maze, dodging voicemails and busy tones. One hour after I first began this endeavor, I finally succeeded in securing an appointment for Mr. H within 2 weeks as a new patient at a new clinic.
Why had this task been so difficult? Mr. H is the type of individual most at risk of falling through the cracks. Why is it that these individuals, homeless and indigent patients that lack social support, suffering already from countless barriers to health care access and resources because of their socioeconomic status continue to face such a horridly complex system of inefficiency and bureaucracy when trying to make a simple clinic appointment? How difficult and frustrating it was for me to accomplish this taskhow could I expect my patient living in an SRO without a phone to succeed?
Identifying barriers to health care access is the first step in addressing these issues. Previous studies have demonstrated that the majority of barriers to adequate follow‐up after a hospital visit occur among minority groups.17 Lower socioeconomic status is often associated with financial limitations from inability to take time off from work. Among immigrant cohorts, language and cultural barriers also play an important role in affecting follow‐up care. In addition to suboptimal follow‐up care, these barriers often lead to increased patient morbidity and increased rates of hospital readmission. For example, studies have reported that certain minority groups were more likely to receive no pain medication after bone fractures and were less likely to receive adequate analgesia for cancer‐related pain.1, 2, 7 In an attempt to address these issues, several studies have reported on multidisciplinary discharge planning interventions.810 One program in particular involved emergency departments providing free medication, transportation vouchers to and from the patient's primary care clinic, and telephone reminders to schedule follow‐up appointments.10 The implementation of these programs translated into improved primary care follow up, decreased hospital readmission rates, and decreased costs.
It is clear that our current health care system is wrought with inefficiencies that pose significant barriers to access by certain cohorts. The fact that minority groups and cohorts of the lowest socioeconomic status suffer most from these obstacles is concerning. Studies have shown that comprehensive programs to address these barriers including greater access to language interpreters and implementing a multidisciplinary approach to discharge planning improve patient outcomes. As we move forward in the ever‐evolving US medical system, there needs to be greater emphasis on preventative care. Education and resources to improve access to primary care physicians through identification of barriers and developing programs to address these issues is only the first step.
As resident physicians, we find ourselves at the forefronts of medicine. While tackling the complexities of clinical medicine, all too soon we become entrenched in the bureaucracy of the medical system. We have come to accept realism and practicality over idealism. Shackled by the inefficiencies of a medical system in dire need of reform, we trek forward in hopes of a new future.
I remember the day quite clearly. My 30‐hour shift was nearing an end. It had not been a rough night, but covering both telemetry and coronary care unit services does take a toll on one's physical and mental well‐being. We were ready to discharge Mr. H, an indigent man recovering from a heart attack. I entered his room. He was all dressed. I explained all the important details and handed him a prescription. These medications are very important. Here is a taxi voucher to the pharmacy to get these medications.
He smiled, Thank you very much. You guys saved my life and I really appreciate it.
You are very welcome, I replied. In addition to the medications, it is very important that you follow up with your doctor within 1 to 2 weeks. Do you have any questions?
No problem. You guys saved my life. Thank you so much!
His eyes glistened with tears as he stared right through me, not hearing anything I had said. Mr. H, could you please wait a few more minutes? I will help you schedule a clinic visit.
I quickly located his clinic number at the county hospital. After 3 attempts, I reached a live person. I explained my situation: I am a doctor at a local hospital trying to help a patient setup a clinic appointment. She rattled off a slurry of information, clearly from a script, and left me with 5 different numbers to choose from (the main clinic line, an alternative number, a third number for the urgent advice nurse, one for new patients, and another for subspecialists). The main line was busy, and despite 3 additional attempts, this avenue ended in a recording advising me to leave a message. Unfortunately, my patient lived in an single resident occupancy (SRO) and did not have a phone, and thus leaving a message would not be helpful. I tried another number and the operator informed me that Mr. H was not in the system, and therefore, he would transfer me to the new patients department. The phone rang 5 times before the voicemail message answered, instructing me to leave a detailed message with my contact information. I hung up, and called the original operator back.
I'm sorry. Only the new patient department can schedule appointments for new patients. Here is the direct number. They must be busy. You can try again.
After several attempts, someone answered. My system shows that Mr. H has been assigned to the 7th Street Clinic. He's been seen there before, so you need to contact the main clinic line. I can only make appointments for new patients. She transferred me back to the first operator.
Oh yes, it looks like Mr. H is assigned to 7th Street Clinic. Please hold while I try to locate the next available appointment. He returns 3 minutes later. The next appointment I have is 4 months from now9:30 AM or 3:30 PM?
Is there anyway to schedule an earlier appointment? Mr. H has just recovered from a heart attack and needs to be seen sooner.
I'm sorry. This is the earliest appointment available. If he needs urgent care, he can go to the urgent care clinic Monday through Friday 7:30 AM to 6:00 PM. I persisted and he gave me the clinic's direct line. Sometimes, they may have earlier openings.
I called the 7th Street Clinic. The receptionist informed me that Mr. H was not in the system and she could not help me. I would need to speak with the new patient department. I just spoke with that department, and they were not able to schedule an appointment because their records show he has been seen at 7th Street Clinic in the past.
I'm sorry. He is not in our system. I can't schedule any appointments for him.
Again I went through all the previous numbers and spoke with the same 4 or 5 people, transferring me back and forth through this ridiculous maze, dodging voicemails and busy tones. One hour after I first began this endeavor, I finally succeeded in securing an appointment for Mr. H within 2 weeks as a new patient at a new clinic.
Why had this task been so difficult? Mr. H is the type of individual most at risk of falling through the cracks. Why is it that these individuals, homeless and indigent patients that lack social support, suffering already from countless barriers to health care access and resources because of their socioeconomic status continue to face such a horridly complex system of inefficiency and bureaucracy when trying to make a simple clinic appointment? How difficult and frustrating it was for me to accomplish this taskhow could I expect my patient living in an SRO without a phone to succeed?
Identifying barriers to health care access is the first step in addressing these issues. Previous studies have demonstrated that the majority of barriers to adequate follow‐up after a hospital visit occur among minority groups.17 Lower socioeconomic status is often associated with financial limitations from inability to take time off from work. Among immigrant cohorts, language and cultural barriers also play an important role in affecting follow‐up care. In addition to suboptimal follow‐up care, these barriers often lead to increased patient morbidity and increased rates of hospital readmission. For example, studies have reported that certain minority groups were more likely to receive no pain medication after bone fractures and were less likely to receive adequate analgesia for cancer‐related pain.1, 2, 7 In an attempt to address these issues, several studies have reported on multidisciplinary discharge planning interventions.810 One program in particular involved emergency departments providing free medication, transportation vouchers to and from the patient's primary care clinic, and telephone reminders to schedule follow‐up appointments.10 The implementation of these programs translated into improved primary care follow up, decreased hospital readmission rates, and decreased costs.
It is clear that our current health care system is wrought with inefficiencies that pose significant barriers to access by certain cohorts. The fact that minority groups and cohorts of the lowest socioeconomic status suffer most from these obstacles is concerning. Studies have shown that comprehensive programs to address these barriers including greater access to language interpreters and implementing a multidisciplinary approach to discharge planning improve patient outcomes. As we move forward in the ever‐evolving US medical system, there needs to be greater emphasis on preventative care. Education and resources to improve access to primary care physicians through identification of barriers and developing programs to address these issues is only the first step.
- ,.Effect of language barriers on follow‐up appointments after an emergency department visit.J Gen Intern Med.2000;15:256–264.
- ,,.Ethnicity as a risk factor for inadequate emergency department analgesia.JAMA.1993;269:1537–1539.
- ,.Differences in follow‐up visits between African‐American and White Medicaid children hospitalized with asthma.J Health Care Poor Underserved.1997;8:83–98.
- ,,.Predictors and barriers to timely medical follow‐up after cardiovascular disease risk factor screening according to race/ethnicity.J Natl Med Assoc.2008;100:534–539.
- ,.Nonprice barriers to ambulatory care after an emergency department visit.Ann Emerg Med.2008;51:607–613.
- ,,,,.Financial barriers to health care and outcomes after acute myocardial infarction.JAMA.2007;297:1063–1072.
- ,,,,.Pain and treatment of pain in minority patients with cancer: The Eastern Cooperative Oncology Group Minority Outpatient Pain Study.Ann Intern Med.1997;127:813–816.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders; a randomized clinical trial.JAMA.1999;281:613–620.
- .Approaches to help people with diabetes overcome barriers for improved health outcomes.Diabetes Educ.2008;34(1 Suppl):18S–24S.
- ,,, et al.A randomized, controlled trial of a simple emergency department intervention to improve the rate of primary care follow‐up for patients with acute asthma exacerbations.Ann Emerg Med.2001;38:115–122.
- ,.Effect of language barriers on follow‐up appointments after an emergency department visit.J Gen Intern Med.2000;15:256–264.
- ,,.Ethnicity as a risk factor for inadequate emergency department analgesia.JAMA.1993;269:1537–1539.
- ,.Differences in follow‐up visits between African‐American and White Medicaid children hospitalized with asthma.J Health Care Poor Underserved.1997;8:83–98.
- ,,.Predictors and barriers to timely medical follow‐up after cardiovascular disease risk factor screening according to race/ethnicity.J Natl Med Assoc.2008;100:534–539.
- ,.Nonprice barriers to ambulatory care after an emergency department visit.Ann Emerg Med.2008;51:607–613.
- ,,,,.Financial barriers to health care and outcomes after acute myocardial infarction.JAMA.2007;297:1063–1072.
- ,,,,.Pain and treatment of pain in minority patients with cancer: The Eastern Cooperative Oncology Group Minority Outpatient Pain Study.Ann Intern Med.1997;127:813–816.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders; a randomized clinical trial.JAMA.1999;281:613–620.
- .Approaches to help people with diabetes overcome barriers for improved health outcomes.Diabetes Educ.2008;34(1 Suppl):18S–24S.
- ,,, et al.A randomized, controlled trial of a simple emergency department intervention to improve the rate of primary care follow‐up for patients with acute asthma exacerbations.Ann Emerg Med.2001;38:115–122.
Timeliness of treatment is more important than choice of reperfusion therapy
Reperfusion therapy decreases morbidity and mortality rates in patients with ST-segment elevation myocardial infarction (MI). Primary percutaneous coronary intervention (PCI) is preferred over fibrinolytic therapy as a reperfusion strategy when the delay in the time to treatment is short and the patient presents to a high-volume, well-equipped center with expert interventional cardiologists.
Compared with fibrinolytic therapy in randomized clinical trials, primary PCI produces higher rates of infarct artery patency, complete reperfusion (grade 3 by the criteria of the Thrombolysis in Myocardial Infarction [TIMI] study), and access-site bleeding. It also produces lower rates of recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage, and death.1 If performed early and successfully, primary PCI also greatly decreases the rates of complications of ST-elevation MI that result from longer ischemic times or unsuccessful fibrinolytic therapy, allowing earlier hospital discharge and resumption of daily activities. Primary PCI is also the best reperfusion option in patients who present late after the onset of symptoms and in patients with cardiogenic shock, and it is the only option in patients who have contraindications to fibrinolytic therapy because of bleeding risk.
However, most hospitals do not have PCI capability. Two options at these hospitals are to transfer the patient to a PCI center quickly for primary PCI or to keep the patient on site and give fibrinolytic therapy, with its limitations. Earlier trials suggested that the transfer strategy was superior, but they had limitations: most patients received streptokinase, an inferior fibrinolytic agent, and door-to-door-to-balloon times were rapid, averaging only 95 minutes because of excellent logistical protocols and careful patient selection.2 Most importantly, rescue PCI and routine PCI were seldom performed in patients receiving fibrinolytics, so fibrinolytic therapy was being tested as monotherapy.
In the real world, however, treatment delays are much longer, and fibrinolytic therapy has evolved into a strategy that includes crossover to rescue PCI or routine PCI. Therefore, the initial trials of transfer for primary PCI do not reflect current practice. In fact, recent registry data suggest that prehospital fibrinolytic therapy followed by early angiography is superior to primary PCI because most patients can be treated within 2 hours of symptom onset; they also suggest that on-site fibrinolytic therapy followed by early angiography is equal in efficacy to primary PCI as long as rescue PCI and routine PCI can be performed.3,4
The most important modifiable predictor of outcome in ST-elevation MI is the time to treatment, a biological truth that continues to be supported by clinical evidence despite ideologic arguments by some interventional cardiology enthusiasts who claim that primary PCI is always superior to the fibrinolytic strategy, regardless of delays.
SURPRISINGLY, OUTCOMES WERE WORSE WITH FACILITATED PCI
It made sense, then, to conclude that the perfect strategy for hospitals without PCI capability would be a combined strategy of immediate fibrinolytic therapy to decrease the time delay associated with organizing PCI, and rapid transfer for immediate PCI to improve the limited reperfusion rates associated with fibrinolytic therapy.
Surprisingly, though, randomized trials found worse outcomes with this “facilitated PCI” strategy.5
Again, limitations in trial design might explain the lack of benefit in the trials. Inadequate anticoagulant and antiplatelet therapy were given to the fibrinolytic patients, and primary PCI patients had relatively short treatment delays, with many patients enrolled at hospitals with PCI capability.
PROGRESS IN REPERFUSION THERAPY
Great strides have been made in reperfusion therapy in recent years. Adjunctive therapy with clopidogrel (Plavix) and enoxaparin (Lovenox) has been shown to improve outcomes with fibrinolytic therapy. Bivalirudin (Angiomax) and stents have improved primary PCI’s performance. Reducing bleeding complications has become a clinical priority, with increasing emphasis on adjusting some drug doses according to renal function and using the radial artery for cardiac catheterization.
The American College of Cardiology initiative, “Door-to-Balloon (D2B): An Alliance for Quality,” focused much attention on organizing in-hospital systems of care for primary PCI, thus increasing the national rate of achieving a door-to-balloon time within 90 minutes from 50% to over 75% in patients who presented to hospitals with PCI capability.6
The American Heart Association has launched “Mission: Lifeline,” a national campaign to organize prehospital systems of care with their program,7 working within communities to address their unique needs, resources, and barriers to implementing systems of care for ST-elevation MI. The key aspect of this effort is to help geographic regions develop local solutions, an explicit recognition that there is no one-size-fits-all solution. Early triage by emergency medical services, rapid diagnosis with prehospital electrocardiography, destination and interhospital transfer protocols, and prehospital activation of the cardiac catheterization laboratory can greatly streamline emergency care and decrease treatment delays for primary PCI.
FOR OUTLYING HOSPITALS, A PHARMACOINVASIVE STRATEGY
So what about hospitals without PCI capability that cannot routinely transfer patients to a hospital with PCI capability within 90 minutes?
Lessons learned from the experiences with immediate PCI, rescue PCI, and facilitated PCI have evolved into the “pharmacoinvasive strategy.” Patients with ST-elevation MI are treated as rapidly as possible with a bolus of a fibrinolytic drug, eg, tenecteplase (TNKase) or reteplase (Retavase), and are also given aspirin, clopidogrel, and enoxaparin. Then, they are rapidly transferred to a PCI-capable hospital so that emergency PCI can be performed if reperfusion is not clinically apparent or if the patient develops pulmonary edema or cardiogenic shock. If the clinical signs suggest that reperfusion has been achieved (relief of chest pain, rapid resolution of ST-segment elevation, bursts of accelerated idioventricular rhythm), coronary angiography (and PCI, if indicated) can be performed within 3 to 24 hours of fibrinolytic therapy. This time frame allows the initial fibrinolytic effect to dissipate, while the antiplatelet and anticoagulant drugs achieve therapeutic levels.
Today, the goal is to treat every patient with the best reperfusion strategy available, given the limitations in resources and the geographic location of some centers, and to maximize the possibility of sustained patency of the infarct-related artery by implanting a stent, even if it takes several hours and transfer to another hospital to perform PCI.8 The pharmacoinvasive strategy of rapid administration of fibrinolytic therapy followed by PCI within 24 hours would be practical in most hospitals without PCI capability where treatment delays prohibit performance of primary PCI within 90 minutes of first medical contact.9
THE ‘STREAM’ TRIAL IS UNDER WAY
As proof of concept, the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial is enrolling 2,000 patients with ST-elevation MI presenting within 3 hours of symptom onset if primary PCI is not feasible within 60 minutes of first medical contact.10 Patients will be randomized to either of the following:
- Receive prehospital therapy with tenecteplase, aspirin, clopidogrel, and enoxaparin and undergo cardiac catheterization in 6 to 24 hours (or rescue PCI if reperfusion fails within 90 minutes of fibrinolysis)
- Undergo primary PCI performed according to local guidelines.
The primary measure of efficacy will be the composite rate of death, cardiogenic shock, heart failure, and reinfarction at 30 days. Measures of safety include the rates of ischemic stroke, intracranial hemorrhage, and major nonintracranial bleeding.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FASTMI). Circulation 2008; 118:268–276.
- Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155.
- Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367:579–588.
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008; 1:97–104.
- Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007; 116:217–230.
- Kushner FG, Hand M, Smith SC, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 120:2271–2306.
- Bates ER, Nallamothu BK. Commentary: the role of percutaneous coronary intervention in ST-segment-elevation myocardial infarction. Circulation 2008; 118:567–573.
- Armstrong PW, Gershlick A, Goldstein P, et al; STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J 2010; 160:30–35.
Reperfusion therapy decreases morbidity and mortality rates in patients with ST-segment elevation myocardial infarction (MI). Primary percutaneous coronary intervention (PCI) is preferred over fibrinolytic therapy as a reperfusion strategy when the delay in the time to treatment is short and the patient presents to a high-volume, well-equipped center with expert interventional cardiologists.
Compared with fibrinolytic therapy in randomized clinical trials, primary PCI produces higher rates of infarct artery patency, complete reperfusion (grade 3 by the criteria of the Thrombolysis in Myocardial Infarction [TIMI] study), and access-site bleeding. It also produces lower rates of recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage, and death.1 If performed early and successfully, primary PCI also greatly decreases the rates of complications of ST-elevation MI that result from longer ischemic times or unsuccessful fibrinolytic therapy, allowing earlier hospital discharge and resumption of daily activities. Primary PCI is also the best reperfusion option in patients who present late after the onset of symptoms and in patients with cardiogenic shock, and it is the only option in patients who have contraindications to fibrinolytic therapy because of bleeding risk.
However, most hospitals do not have PCI capability. Two options at these hospitals are to transfer the patient to a PCI center quickly for primary PCI or to keep the patient on site and give fibrinolytic therapy, with its limitations. Earlier trials suggested that the transfer strategy was superior, but they had limitations: most patients received streptokinase, an inferior fibrinolytic agent, and door-to-door-to-balloon times were rapid, averaging only 95 minutes because of excellent logistical protocols and careful patient selection.2 Most importantly, rescue PCI and routine PCI were seldom performed in patients receiving fibrinolytics, so fibrinolytic therapy was being tested as monotherapy.
In the real world, however, treatment delays are much longer, and fibrinolytic therapy has evolved into a strategy that includes crossover to rescue PCI or routine PCI. Therefore, the initial trials of transfer for primary PCI do not reflect current practice. In fact, recent registry data suggest that prehospital fibrinolytic therapy followed by early angiography is superior to primary PCI because most patients can be treated within 2 hours of symptom onset; they also suggest that on-site fibrinolytic therapy followed by early angiography is equal in efficacy to primary PCI as long as rescue PCI and routine PCI can be performed.3,4
The most important modifiable predictor of outcome in ST-elevation MI is the time to treatment, a biological truth that continues to be supported by clinical evidence despite ideologic arguments by some interventional cardiology enthusiasts who claim that primary PCI is always superior to the fibrinolytic strategy, regardless of delays.
SURPRISINGLY, OUTCOMES WERE WORSE WITH FACILITATED PCI
It made sense, then, to conclude that the perfect strategy for hospitals without PCI capability would be a combined strategy of immediate fibrinolytic therapy to decrease the time delay associated with organizing PCI, and rapid transfer for immediate PCI to improve the limited reperfusion rates associated with fibrinolytic therapy.
Surprisingly, though, randomized trials found worse outcomes with this “facilitated PCI” strategy.5
Again, limitations in trial design might explain the lack of benefit in the trials. Inadequate anticoagulant and antiplatelet therapy were given to the fibrinolytic patients, and primary PCI patients had relatively short treatment delays, with many patients enrolled at hospitals with PCI capability.
PROGRESS IN REPERFUSION THERAPY
Great strides have been made in reperfusion therapy in recent years. Adjunctive therapy with clopidogrel (Plavix) and enoxaparin (Lovenox) has been shown to improve outcomes with fibrinolytic therapy. Bivalirudin (Angiomax) and stents have improved primary PCI’s performance. Reducing bleeding complications has become a clinical priority, with increasing emphasis on adjusting some drug doses according to renal function and using the radial artery for cardiac catheterization.
The American College of Cardiology initiative, “Door-to-Balloon (D2B): An Alliance for Quality,” focused much attention on organizing in-hospital systems of care for primary PCI, thus increasing the national rate of achieving a door-to-balloon time within 90 minutes from 50% to over 75% in patients who presented to hospitals with PCI capability.6
The American Heart Association has launched “Mission: Lifeline,” a national campaign to organize prehospital systems of care with their program,7 working within communities to address their unique needs, resources, and barriers to implementing systems of care for ST-elevation MI. The key aspect of this effort is to help geographic regions develop local solutions, an explicit recognition that there is no one-size-fits-all solution. Early triage by emergency medical services, rapid diagnosis with prehospital electrocardiography, destination and interhospital transfer protocols, and prehospital activation of the cardiac catheterization laboratory can greatly streamline emergency care and decrease treatment delays for primary PCI.
FOR OUTLYING HOSPITALS, A PHARMACOINVASIVE STRATEGY
So what about hospitals without PCI capability that cannot routinely transfer patients to a hospital with PCI capability within 90 minutes?
Lessons learned from the experiences with immediate PCI, rescue PCI, and facilitated PCI have evolved into the “pharmacoinvasive strategy.” Patients with ST-elevation MI are treated as rapidly as possible with a bolus of a fibrinolytic drug, eg, tenecteplase (TNKase) or reteplase (Retavase), and are also given aspirin, clopidogrel, and enoxaparin. Then, they are rapidly transferred to a PCI-capable hospital so that emergency PCI can be performed if reperfusion is not clinically apparent or if the patient develops pulmonary edema or cardiogenic shock. If the clinical signs suggest that reperfusion has been achieved (relief of chest pain, rapid resolution of ST-segment elevation, bursts of accelerated idioventricular rhythm), coronary angiography (and PCI, if indicated) can be performed within 3 to 24 hours of fibrinolytic therapy. This time frame allows the initial fibrinolytic effect to dissipate, while the antiplatelet and anticoagulant drugs achieve therapeutic levels.
Today, the goal is to treat every patient with the best reperfusion strategy available, given the limitations in resources and the geographic location of some centers, and to maximize the possibility of sustained patency of the infarct-related artery by implanting a stent, even if it takes several hours and transfer to another hospital to perform PCI.8 The pharmacoinvasive strategy of rapid administration of fibrinolytic therapy followed by PCI within 24 hours would be practical in most hospitals without PCI capability where treatment delays prohibit performance of primary PCI within 90 minutes of first medical contact.9
THE ‘STREAM’ TRIAL IS UNDER WAY
As proof of concept, the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial is enrolling 2,000 patients with ST-elevation MI presenting within 3 hours of symptom onset if primary PCI is not feasible within 60 minutes of first medical contact.10 Patients will be randomized to either of the following:
- Receive prehospital therapy with tenecteplase, aspirin, clopidogrel, and enoxaparin and undergo cardiac catheterization in 6 to 24 hours (or rescue PCI if reperfusion fails within 90 minutes of fibrinolysis)
- Undergo primary PCI performed according to local guidelines.
The primary measure of efficacy will be the composite rate of death, cardiogenic shock, heart failure, and reinfarction at 30 days. Measures of safety include the rates of ischemic stroke, intracranial hemorrhage, and major nonintracranial bleeding.
Reperfusion therapy decreases morbidity and mortality rates in patients with ST-segment elevation myocardial infarction (MI). Primary percutaneous coronary intervention (PCI) is preferred over fibrinolytic therapy as a reperfusion strategy when the delay in the time to treatment is short and the patient presents to a high-volume, well-equipped center with expert interventional cardiologists.
Compared with fibrinolytic therapy in randomized clinical trials, primary PCI produces higher rates of infarct artery patency, complete reperfusion (grade 3 by the criteria of the Thrombolysis in Myocardial Infarction [TIMI] study), and access-site bleeding. It also produces lower rates of recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage, and death.1 If performed early and successfully, primary PCI also greatly decreases the rates of complications of ST-elevation MI that result from longer ischemic times or unsuccessful fibrinolytic therapy, allowing earlier hospital discharge and resumption of daily activities. Primary PCI is also the best reperfusion option in patients who present late after the onset of symptoms and in patients with cardiogenic shock, and it is the only option in patients who have contraindications to fibrinolytic therapy because of bleeding risk.
However, most hospitals do not have PCI capability. Two options at these hospitals are to transfer the patient to a PCI center quickly for primary PCI or to keep the patient on site and give fibrinolytic therapy, with its limitations. Earlier trials suggested that the transfer strategy was superior, but they had limitations: most patients received streptokinase, an inferior fibrinolytic agent, and door-to-door-to-balloon times were rapid, averaging only 95 minutes because of excellent logistical protocols and careful patient selection.2 Most importantly, rescue PCI and routine PCI were seldom performed in patients receiving fibrinolytics, so fibrinolytic therapy was being tested as monotherapy.
In the real world, however, treatment delays are much longer, and fibrinolytic therapy has evolved into a strategy that includes crossover to rescue PCI or routine PCI. Therefore, the initial trials of transfer for primary PCI do not reflect current practice. In fact, recent registry data suggest that prehospital fibrinolytic therapy followed by early angiography is superior to primary PCI because most patients can be treated within 2 hours of symptom onset; they also suggest that on-site fibrinolytic therapy followed by early angiography is equal in efficacy to primary PCI as long as rescue PCI and routine PCI can be performed.3,4
The most important modifiable predictor of outcome in ST-elevation MI is the time to treatment, a biological truth that continues to be supported by clinical evidence despite ideologic arguments by some interventional cardiology enthusiasts who claim that primary PCI is always superior to the fibrinolytic strategy, regardless of delays.
SURPRISINGLY, OUTCOMES WERE WORSE WITH FACILITATED PCI
It made sense, then, to conclude that the perfect strategy for hospitals without PCI capability would be a combined strategy of immediate fibrinolytic therapy to decrease the time delay associated with organizing PCI, and rapid transfer for immediate PCI to improve the limited reperfusion rates associated with fibrinolytic therapy.
Surprisingly, though, randomized trials found worse outcomes with this “facilitated PCI” strategy.5
Again, limitations in trial design might explain the lack of benefit in the trials. Inadequate anticoagulant and antiplatelet therapy were given to the fibrinolytic patients, and primary PCI patients had relatively short treatment delays, with many patients enrolled at hospitals with PCI capability.
PROGRESS IN REPERFUSION THERAPY
Great strides have been made in reperfusion therapy in recent years. Adjunctive therapy with clopidogrel (Plavix) and enoxaparin (Lovenox) has been shown to improve outcomes with fibrinolytic therapy. Bivalirudin (Angiomax) and stents have improved primary PCI’s performance. Reducing bleeding complications has become a clinical priority, with increasing emphasis on adjusting some drug doses according to renal function and using the radial artery for cardiac catheterization.
The American College of Cardiology initiative, “Door-to-Balloon (D2B): An Alliance for Quality,” focused much attention on organizing in-hospital systems of care for primary PCI, thus increasing the national rate of achieving a door-to-balloon time within 90 minutes from 50% to over 75% in patients who presented to hospitals with PCI capability.6
The American Heart Association has launched “Mission: Lifeline,” a national campaign to organize prehospital systems of care with their program,7 working within communities to address their unique needs, resources, and barriers to implementing systems of care for ST-elevation MI. The key aspect of this effort is to help geographic regions develop local solutions, an explicit recognition that there is no one-size-fits-all solution. Early triage by emergency medical services, rapid diagnosis with prehospital electrocardiography, destination and interhospital transfer protocols, and prehospital activation of the cardiac catheterization laboratory can greatly streamline emergency care and decrease treatment delays for primary PCI.
FOR OUTLYING HOSPITALS, A PHARMACOINVASIVE STRATEGY
So what about hospitals without PCI capability that cannot routinely transfer patients to a hospital with PCI capability within 90 minutes?
Lessons learned from the experiences with immediate PCI, rescue PCI, and facilitated PCI have evolved into the “pharmacoinvasive strategy.” Patients with ST-elevation MI are treated as rapidly as possible with a bolus of a fibrinolytic drug, eg, tenecteplase (TNKase) or reteplase (Retavase), and are also given aspirin, clopidogrel, and enoxaparin. Then, they are rapidly transferred to a PCI-capable hospital so that emergency PCI can be performed if reperfusion is not clinically apparent or if the patient develops pulmonary edema or cardiogenic shock. If the clinical signs suggest that reperfusion has been achieved (relief of chest pain, rapid resolution of ST-segment elevation, bursts of accelerated idioventricular rhythm), coronary angiography (and PCI, if indicated) can be performed within 3 to 24 hours of fibrinolytic therapy. This time frame allows the initial fibrinolytic effect to dissipate, while the antiplatelet and anticoagulant drugs achieve therapeutic levels.
Today, the goal is to treat every patient with the best reperfusion strategy available, given the limitations in resources and the geographic location of some centers, and to maximize the possibility of sustained patency of the infarct-related artery by implanting a stent, even if it takes several hours and transfer to another hospital to perform PCI.8 The pharmacoinvasive strategy of rapid administration of fibrinolytic therapy followed by PCI within 24 hours would be practical in most hospitals without PCI capability where treatment delays prohibit performance of primary PCI within 90 minutes of first medical contact.9
THE ‘STREAM’ TRIAL IS UNDER WAY
As proof of concept, the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial is enrolling 2,000 patients with ST-elevation MI presenting within 3 hours of symptom onset if primary PCI is not feasible within 60 minutes of first medical contact.10 Patients will be randomized to either of the following:
- Receive prehospital therapy with tenecteplase, aspirin, clopidogrel, and enoxaparin and undergo cardiac catheterization in 6 to 24 hours (or rescue PCI if reperfusion fails within 90 minutes of fibrinolysis)
- Undergo primary PCI performed according to local guidelines.
The primary measure of efficacy will be the composite rate of death, cardiogenic shock, heart failure, and reinfarction at 30 days. Measures of safety include the rates of ischemic stroke, intracranial hemorrhage, and major nonintracranial bleeding.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FASTMI). Circulation 2008; 118:268–276.
- Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155.
- Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367:579–588.
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008; 1:97–104.
- Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007; 116:217–230.
- Kushner FG, Hand M, Smith SC, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 120:2271–2306.
- Bates ER, Nallamothu BK. Commentary: the role of percutaneous coronary intervention in ST-segment-elevation myocardial infarction. Circulation 2008; 118:567–573.
- Armstrong PW, Gershlick A, Goldstein P, et al; STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J 2010; 160:30–35.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FASTMI). Circulation 2008; 118:268–276.
- Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155.
- Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367:579–588.
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008; 1:97–104.
- Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007; 116:217–230.
- Kushner FG, Hand M, Smith SC, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 120:2271–2306.
- Bates ER, Nallamothu BK. Commentary: the role of percutaneous coronary intervention in ST-segment-elevation myocardial infarction. Circulation 2008; 118:567–573.
- Armstrong PW, Gershlick A, Goldstein P, et al; STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J 2010; 160:30–35.
Short Title/Panesar
After another round of epinephrine, I started chest compressionsagain. Warm sweat seeped down my neck and back. In just 10 minutes, the 24‐bed pediatric intensive care unit (PICU) had become much smaller and more confined. Everyone funneled into this one room, this one bed. Beyond it was a blur of color and sound. I forced my strength onto Ricky, my 17‐year‐old patient with muscular dystrophy and end‐stage heart failure. He was admitted 1 week ago with respiratory distress, and he had only gotten worse since then.
Ricky's body, now cool and pale, was a blob of relaxed skin and loose bone beneath me. I straightened my arms, jutted the heel of my left hand over my right and pushed onto his chest. I pushed hard and fast, like I was taught; a dumb robotic motion again and again, trying to keep good position and form. I stared up at the monitor between every few compressions, looking at all the waveforms, anticipating, as if something on the screen was going to pop up suddenly and say EVERYTHING IS GOING TO BE OKAY. But it didn't.
I glanced beyond the bedside. There was a flurry of people bumping each other, asking for things, telling things, giving and receiving things. All of them were moving, but really had no place to go. This was the place. And in the far corner of the room, stood Mom and Dad.
In between the sink and recliner chair filled with clothes and books, they were the only people that stood still. Swollen feet planted in white socks. Their shoulders sagged.
I love you, I love you, Ricky. No, no, no. Mom and Dad kept on saying between sobs. I wondered if he could hear them. I wished he could hear them. Then I wished they would stop saying anything at all. I felt a rib give under my hands.
From the corner of my left eye, I saw Dad holding Mom upright by the wall. She wore red‐rimmed eyes and wet cheeks and took puttering breaths. Dad squeezed tissues in his hand, then into his eyes and nose and then back into his fist. They kept saying the words over and over, like they knew no other. I pushed harder and faster, but he didn't turn any pinker. Damn it. Damn it. There was plenty of noise, but above it all, I heard their voices. When I was told to hold compressions to check for a pulse, I stood still with my hands at my sides. I felt unnecessary. My precious little contribution to the commotion interrupted.
We all looked up at the monitor.
Stop, please stop. That's it, dad said, somewhere in the infinite pause. Mom still mumbled no over and over again. I turned to her and listened. I watched her pursed mouth and I imagined what it was Dad felt as he held her. Her body shook a Morse code into him, telling him it was time to give up. That it was over. That she couldn't take watching me pound away on her son's chest anymore. That it was okay to let Ricky rest. All the words that she couldn't find, or have the coordination to say, Dad translated for her.
He held her with a desperate grip, for a few moments longer. Maybe, the harder he squeezed, the more life he could push out of her, out of himself, and that effervescent pulse would find its way to their son's heart. But maybe all he could sense was her quiet internal whisper. And they told us again, as I remembered, just like all the soft conversations we had before in the back of the room, while Ricky slept, sedated on narcotics.
I put my fingers over his radial artery and closed my eyes.
Don't let Ricky feel pain, she said. It was the day we intubated him, only hours after he had been admitted.
Do what you can do, just don't Dad trailed off. He stared up at the ceiling and sighed. We listened to the gasp and hiss of the ventilator for a few more moments in silence.
We can try what you say, but no pain. We should know when to quitfor Ricky. Okay? Mom said, waiting for the tears as her nose moistened. She stared up at me.
Okay, I said. I nodded and stared back.
Dad squeezed her arm again, wrapped his around hers and massaged her. She had started to shake.
No pulse. I opened my eyes.
I stared at Ricky's face. His eyelids were half open, his lips were blue. No change on the monitor. I motioned to start compressions again.
Okay, Dad said.
Okay, stop! He's had enough. His dry lips and wet face moved and voiced the end. The room froze.
My muscles relaxed and I splayed my fingers wide, my way of showing that I was letting up. I watched myself lean back, unbelieving, and looked at the screen again. The rhythm drifted from pulseless electrical activity to asystole.
Mom and Dad simultaneously shut their eyes as if they saw something that we didn't and couldn't bear to see anymore. They opened them, looked at Ricky and slowly, as everyone stared, moved to the foot of the bed and began to rub his bare feet.
In subtle efficiency, the room was transformed. We turned off the monitors, we pushed out the carts and equipment, we picked up after ourselves, we dimmed the lights, we pulled the curtains and we left Mom and Dad with their son. Slow, deliberate whispers and motion now, it sounded empty without the background of rhythmic mechanical sounds. No dings, bleeps or rings; no pistons, suctions or pumps; only the occasional sound of tissue being ripped out of a box.
We sat with the family an hour later, in the conference room, to sign papers, for autopsy, for death certificates, for funeral preparations. Mom and Dad didn't know how to answer, and they drifted together to consider how they wanted Ricky's body cared for. Burial? Cremation?
I don't know, Mom said as she tried to fold the shredded tissues in her hand. He had a spinal rod placed his back years ago for his scoliosis. A titanium rod in his spine. Would that even burn? she asked.
We stared at each other, confused and unknowing.
We never thought of that, she said. She began to think out loud. If he were cremated, I don't think I'd want his spinal rod as a reminder. She cocked her head back. What a weird keepsake! She laughed, Where would I keep it? Above the fireplace? How odd. She rolled her eyes, and started to cry again.
After another round of epinephrine, I started chest compressionsagain. Warm sweat seeped down my neck and back. In just 10 minutes, the 24‐bed pediatric intensive care unit (PICU) had become much smaller and more confined. Everyone funneled into this one room, this one bed. Beyond it was a blur of color and sound. I forced my strength onto Ricky, my 17‐year‐old patient with muscular dystrophy and end‐stage heart failure. He was admitted 1 week ago with respiratory distress, and he had only gotten worse since then.
Ricky's body, now cool and pale, was a blob of relaxed skin and loose bone beneath me. I straightened my arms, jutted the heel of my left hand over my right and pushed onto his chest. I pushed hard and fast, like I was taught; a dumb robotic motion again and again, trying to keep good position and form. I stared up at the monitor between every few compressions, looking at all the waveforms, anticipating, as if something on the screen was going to pop up suddenly and say EVERYTHING IS GOING TO BE OKAY. But it didn't.
I glanced beyond the bedside. There was a flurry of people bumping each other, asking for things, telling things, giving and receiving things. All of them were moving, but really had no place to go. This was the place. And in the far corner of the room, stood Mom and Dad.
In between the sink and recliner chair filled with clothes and books, they were the only people that stood still. Swollen feet planted in white socks. Their shoulders sagged.
I love you, I love you, Ricky. No, no, no. Mom and Dad kept on saying between sobs. I wondered if he could hear them. I wished he could hear them. Then I wished they would stop saying anything at all. I felt a rib give under my hands.
From the corner of my left eye, I saw Dad holding Mom upright by the wall. She wore red‐rimmed eyes and wet cheeks and took puttering breaths. Dad squeezed tissues in his hand, then into his eyes and nose and then back into his fist. They kept saying the words over and over, like they knew no other. I pushed harder and faster, but he didn't turn any pinker. Damn it. Damn it. There was plenty of noise, but above it all, I heard their voices. When I was told to hold compressions to check for a pulse, I stood still with my hands at my sides. I felt unnecessary. My precious little contribution to the commotion interrupted.
We all looked up at the monitor.
Stop, please stop. That's it, dad said, somewhere in the infinite pause. Mom still mumbled no over and over again. I turned to her and listened. I watched her pursed mouth and I imagined what it was Dad felt as he held her. Her body shook a Morse code into him, telling him it was time to give up. That it was over. That she couldn't take watching me pound away on her son's chest anymore. That it was okay to let Ricky rest. All the words that she couldn't find, or have the coordination to say, Dad translated for her.
He held her with a desperate grip, for a few moments longer. Maybe, the harder he squeezed, the more life he could push out of her, out of himself, and that effervescent pulse would find its way to their son's heart. But maybe all he could sense was her quiet internal whisper. And they told us again, as I remembered, just like all the soft conversations we had before in the back of the room, while Ricky slept, sedated on narcotics.
I put my fingers over his radial artery and closed my eyes.
Don't let Ricky feel pain, she said. It was the day we intubated him, only hours after he had been admitted.
Do what you can do, just don't Dad trailed off. He stared up at the ceiling and sighed. We listened to the gasp and hiss of the ventilator for a few more moments in silence.
We can try what you say, but no pain. We should know when to quitfor Ricky. Okay? Mom said, waiting for the tears as her nose moistened. She stared up at me.
Okay, I said. I nodded and stared back.
Dad squeezed her arm again, wrapped his around hers and massaged her. She had started to shake.
No pulse. I opened my eyes.
I stared at Ricky's face. His eyelids were half open, his lips were blue. No change on the monitor. I motioned to start compressions again.
Okay, Dad said.
Okay, stop! He's had enough. His dry lips and wet face moved and voiced the end. The room froze.
My muscles relaxed and I splayed my fingers wide, my way of showing that I was letting up. I watched myself lean back, unbelieving, and looked at the screen again. The rhythm drifted from pulseless electrical activity to asystole.
Mom and Dad simultaneously shut their eyes as if they saw something that we didn't and couldn't bear to see anymore. They opened them, looked at Ricky and slowly, as everyone stared, moved to the foot of the bed and began to rub his bare feet.
In subtle efficiency, the room was transformed. We turned off the monitors, we pushed out the carts and equipment, we picked up after ourselves, we dimmed the lights, we pulled the curtains and we left Mom and Dad with their son. Slow, deliberate whispers and motion now, it sounded empty without the background of rhythmic mechanical sounds. No dings, bleeps or rings; no pistons, suctions or pumps; only the occasional sound of tissue being ripped out of a box.
We sat with the family an hour later, in the conference room, to sign papers, for autopsy, for death certificates, for funeral preparations. Mom and Dad didn't know how to answer, and they drifted together to consider how they wanted Ricky's body cared for. Burial? Cremation?
I don't know, Mom said as she tried to fold the shredded tissues in her hand. He had a spinal rod placed his back years ago for his scoliosis. A titanium rod in his spine. Would that even burn? she asked.
We stared at each other, confused and unknowing.
We never thought of that, she said. She began to think out loud. If he were cremated, I don't think I'd want his spinal rod as a reminder. She cocked her head back. What a weird keepsake! She laughed, Where would I keep it? Above the fireplace? How odd. She rolled her eyes, and started to cry again.
After another round of epinephrine, I started chest compressionsagain. Warm sweat seeped down my neck and back. In just 10 minutes, the 24‐bed pediatric intensive care unit (PICU) had become much smaller and more confined. Everyone funneled into this one room, this one bed. Beyond it was a blur of color and sound. I forced my strength onto Ricky, my 17‐year‐old patient with muscular dystrophy and end‐stage heart failure. He was admitted 1 week ago with respiratory distress, and he had only gotten worse since then.
Ricky's body, now cool and pale, was a blob of relaxed skin and loose bone beneath me. I straightened my arms, jutted the heel of my left hand over my right and pushed onto his chest. I pushed hard and fast, like I was taught; a dumb robotic motion again and again, trying to keep good position and form. I stared up at the monitor between every few compressions, looking at all the waveforms, anticipating, as if something on the screen was going to pop up suddenly and say EVERYTHING IS GOING TO BE OKAY. But it didn't.
I glanced beyond the bedside. There was a flurry of people bumping each other, asking for things, telling things, giving and receiving things. All of them were moving, but really had no place to go. This was the place. And in the far corner of the room, stood Mom and Dad.
In between the sink and recliner chair filled with clothes and books, they were the only people that stood still. Swollen feet planted in white socks. Their shoulders sagged.
I love you, I love you, Ricky. No, no, no. Mom and Dad kept on saying between sobs. I wondered if he could hear them. I wished he could hear them. Then I wished they would stop saying anything at all. I felt a rib give under my hands.
From the corner of my left eye, I saw Dad holding Mom upright by the wall. She wore red‐rimmed eyes and wet cheeks and took puttering breaths. Dad squeezed tissues in his hand, then into his eyes and nose and then back into his fist. They kept saying the words over and over, like they knew no other. I pushed harder and faster, but he didn't turn any pinker. Damn it. Damn it. There was plenty of noise, but above it all, I heard their voices. When I was told to hold compressions to check for a pulse, I stood still with my hands at my sides. I felt unnecessary. My precious little contribution to the commotion interrupted.
We all looked up at the monitor.
Stop, please stop. That's it, dad said, somewhere in the infinite pause. Mom still mumbled no over and over again. I turned to her and listened. I watched her pursed mouth and I imagined what it was Dad felt as he held her. Her body shook a Morse code into him, telling him it was time to give up. That it was over. That she couldn't take watching me pound away on her son's chest anymore. That it was okay to let Ricky rest. All the words that she couldn't find, or have the coordination to say, Dad translated for her.
He held her with a desperate grip, for a few moments longer. Maybe, the harder he squeezed, the more life he could push out of her, out of himself, and that effervescent pulse would find its way to their son's heart. But maybe all he could sense was her quiet internal whisper. And they told us again, as I remembered, just like all the soft conversations we had before in the back of the room, while Ricky slept, sedated on narcotics.
I put my fingers over his radial artery and closed my eyes.
Don't let Ricky feel pain, she said. It was the day we intubated him, only hours after he had been admitted.
Do what you can do, just don't Dad trailed off. He stared up at the ceiling and sighed. We listened to the gasp and hiss of the ventilator for a few more moments in silence.
We can try what you say, but no pain. We should know when to quitfor Ricky. Okay? Mom said, waiting for the tears as her nose moistened. She stared up at me.
Okay, I said. I nodded and stared back.
Dad squeezed her arm again, wrapped his around hers and massaged her. She had started to shake.
No pulse. I opened my eyes.
I stared at Ricky's face. His eyelids were half open, his lips were blue. No change on the monitor. I motioned to start compressions again.
Okay, Dad said.
Okay, stop! He's had enough. His dry lips and wet face moved and voiced the end. The room froze.
My muscles relaxed and I splayed my fingers wide, my way of showing that I was letting up. I watched myself lean back, unbelieving, and looked at the screen again. The rhythm drifted from pulseless electrical activity to asystole.
Mom and Dad simultaneously shut their eyes as if they saw something that we didn't and couldn't bear to see anymore. They opened them, looked at Ricky and slowly, as everyone stared, moved to the foot of the bed and began to rub his bare feet.
In subtle efficiency, the room was transformed. We turned off the monitors, we pushed out the carts and equipment, we picked up after ourselves, we dimmed the lights, we pulled the curtains and we left Mom and Dad with their son. Slow, deliberate whispers and motion now, it sounded empty without the background of rhythmic mechanical sounds. No dings, bleeps or rings; no pistons, suctions or pumps; only the occasional sound of tissue being ripped out of a box.
We sat with the family an hour later, in the conference room, to sign papers, for autopsy, for death certificates, for funeral preparations. Mom and Dad didn't know how to answer, and they drifted together to consider how they wanted Ricky's body cared for. Burial? Cremation?
I don't know, Mom said as she tried to fold the shredded tissues in her hand. He had a spinal rod placed his back years ago for his scoliosis. A titanium rod in his spine. Would that even burn? she asked.
We stared at each other, confused and unknowing.
We never thought of that, she said. She began to think out loud. If he were cremated, I don't think I'd want his spinal rod as a reminder. She cocked her head back. What a weird keepsake! She laughed, Where would I keep it? Above the fireplace? How odd. She rolled her eyes, and started to cry again.
Author Responsibilities and Disclosures
Since its founding in 2006,1 the editors of the Journal of Hospital Medicine (JHM), strongly supported the ethical guidelines and uniform requirements for manuscripts established by the International Committee of Medical Journal Editors (ICMJE).2 These guidelines require authors to verify that they have followed appropriate standards in the conduct of research, meet criteria for authorship, disclose potential conflicts of interest, and respect existing copyrights. With recent publication of editorials in leading medical journals affirming this responsibility for all authors submitting their scholarly work,38 the editors of the Journal echo the importance of following these ethical standards, and wish to update authors and readers on our policies related to authorship and plagiarism.
Disclosure of Competing Interests
Scientific publications commonly require that authors disclose relationships, financial or otherwise, with commercial entities that might have an interest in the subject matter of the article. Historically, biomedical journals varied in the content and format of the information they requested from authors,9 yielding inconsistent reporting by authors depending on the journal. Lack of clarity regarding what relationships authors should report contributed to this variable reporting. For example, an author might submit an article on headache management, and not believe it necessary to report honoraria received from pharmaceutical firms for giving lectures on antibiotic management of pneumonia. Thus, many believed that only funding related to the subject matter in a manuscript needed to be disclosed. While general advice has been to err on the side of disclosure, many authors hesitated to do so.
To clarify and standardize reporting requirements, the ICMJE recommended a uniform format for disclosure of competing interests,3 which was updated recently.10 The document, available online at
JHM strongly supports the ICMJE uniform requirements for manuscripts and has adopted the new form for disclosure of competing interests. Effective immediately, this documentation will be required for all types of manuscripts submitted to JHM. To help reduce the paperwork burden for authors, this documentation will be required only when authors are invited to revise and resubmit their work, after completion of the initial round of reviews. Typically at this stage, JHM also requests each author complete a Copyright Transfer Agreement (CTA). Thus, when a revision is requested by the Journal, we recommend that the corresponding author have each coauthor concurrently complete the CTA and disclosure of competing interests, and return all of the materials to JHM at the same time.
Criteria for Authorship
Authorship of scientific articles has important professional implications. In a field such as Hospital Medicine which explicitly values teamwork, it can sometimes be unclear which members of a team qualify for authorship on an article that may result from the group's work. The ICMJE provides the following guidance:2
-
Authorship credit should be based on (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
The ICMJE notes that authorship is not justified for individuals who simply obtain or provide funding, participate in data collection or general supervision of the research, or serve as head of the group. Members of the team who play roles such as these are more appropriately acknowledged, and their specific contributions noted. The corresponding author should obtain written permission as such acknowledgements may imply endorsement of the work or its conclusions.
Authors, too, should make note of their individual contributions to manuscripts submitted to JHM. The Journal will begin publishing these specific contributions with each article, as do other medical journals.11
Plagiarism
Perhaps the most serious ethical violation that journals confront is plagiarism of copyrighted work. In its 5 years, JHM has detected 4 episodes of plagiarism. Thankfully, the Committee on Publication Ethics (
We recognize that other journals have needed to handle similar episodes of plagiarism,1215 and that self‐plagiarism (recycling of one's own published text) is also a concern.16, 17 Many methods exist to detect these practices.18 One powerful approach gaining popularity among medical journals utilizes CrossCheck. The CrossCheck service has 2 components: (1) a large, full‐text database of scholarly work from leading publishers, maintained by CrossRef (
Any form of plagiarism is inexcusable, and, if detected, is immediately addressed. Additionally, any author who submits plagiarized work will be banned from submitting manuscripts to JHM in the future, and will not be allowed to serve the Journal as a reviewer or in any other capacity. Our notification in selected cases of the individual's supervisor or department chair may elicit additional adverse consequences.
Summary
As the Journal of Hospital Medicine continues to grow and evolve, we are extraordinarily grateful when authors choose to submit their scholarly work to us. But growth does not come without challenges and responsibilities, such as a requirement to uphold ethical standards of biomedical publishing. We believe that the uniform disclosure of competing interests, clear reporting of contributions for authorship, and monitoring for plagiarism will help JHM maintain the standards that its readership and contributing authors deserve. We look forward to your contributions during our next 5 years, and beyond.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1(1):1–2.
- International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals. Available at: http://www.icmje.org/. Accessed February 22,2010.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.N Engl J Med.2009;361(19):1896–1897.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.JAMA.2010;303(1):75–76.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.Ann Intern Med.2010;152(2):125–126.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.Lancet.2009;374(9699):1395–1396.
- , , , et al.Disclosure of competing interests.BMJ.2009;339:b4144.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.CMAJ.2009;181(9):565.
- , , , .Requirements and definitions in conflict of interest policies of medical journals.JAMA.2009;302(20):2230–2234.
- , , , , et al.Toward more uniform conflict disclosures ‐ the updated ICMJE conflict of interest reporting form.N Engl J Med.2010;363(2):188–189.
- , , , .Authorship criteria and disclosure of contributions: comparison of 3 general medical journals with different author contribution forms.JAMA.2004;292(1):86–88.
- , .Investigating allegations of scientific misconduct.BMJ.2005;331(7511):245–246.
- .Misconduct in medical research: does it exist in Britain?BMJ.1998;297:1531–1535.
- , .Research misconduct, retraction, and cleansing the medical literature: lessons from the Poehlman case.Annals of Internal Medicine.2006;144(8):609–613.
- .Report from the Scientific Integrity Advisor: issues arising in 2005 and 2006.Neurology.2007;68(21):1841–1842.
- Anonymous.Self‐plagiarism: unintentional, harmless, or fraud?Lancet.2009;374(9691):664.
- .Re‐using text from one's own previously published papers: an exploratory study of potential self‐plagiarism.Psychological Reports.2005;97(1):43–49.
- .Policing plagiarism.BMJ.2007;335(7627):963–964.
Since its founding in 2006,1 the editors of the Journal of Hospital Medicine (JHM), strongly supported the ethical guidelines and uniform requirements for manuscripts established by the International Committee of Medical Journal Editors (ICMJE).2 These guidelines require authors to verify that they have followed appropriate standards in the conduct of research, meet criteria for authorship, disclose potential conflicts of interest, and respect existing copyrights. With recent publication of editorials in leading medical journals affirming this responsibility for all authors submitting their scholarly work,38 the editors of the Journal echo the importance of following these ethical standards, and wish to update authors and readers on our policies related to authorship and plagiarism.
Disclosure of Competing Interests
Scientific publications commonly require that authors disclose relationships, financial or otherwise, with commercial entities that might have an interest in the subject matter of the article. Historically, biomedical journals varied in the content and format of the information they requested from authors,9 yielding inconsistent reporting by authors depending on the journal. Lack of clarity regarding what relationships authors should report contributed to this variable reporting. For example, an author might submit an article on headache management, and not believe it necessary to report honoraria received from pharmaceutical firms for giving lectures on antibiotic management of pneumonia. Thus, many believed that only funding related to the subject matter in a manuscript needed to be disclosed. While general advice has been to err on the side of disclosure, many authors hesitated to do so.
To clarify and standardize reporting requirements, the ICMJE recommended a uniform format for disclosure of competing interests,3 which was updated recently.10 The document, available online at
JHM strongly supports the ICMJE uniform requirements for manuscripts and has adopted the new form for disclosure of competing interests. Effective immediately, this documentation will be required for all types of manuscripts submitted to JHM. To help reduce the paperwork burden for authors, this documentation will be required only when authors are invited to revise and resubmit their work, after completion of the initial round of reviews. Typically at this stage, JHM also requests each author complete a Copyright Transfer Agreement (CTA). Thus, when a revision is requested by the Journal, we recommend that the corresponding author have each coauthor concurrently complete the CTA and disclosure of competing interests, and return all of the materials to JHM at the same time.
Criteria for Authorship
Authorship of scientific articles has important professional implications. In a field such as Hospital Medicine which explicitly values teamwork, it can sometimes be unclear which members of a team qualify for authorship on an article that may result from the group's work. The ICMJE provides the following guidance:2
-
Authorship credit should be based on (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
The ICMJE notes that authorship is not justified for individuals who simply obtain or provide funding, participate in data collection or general supervision of the research, or serve as head of the group. Members of the team who play roles such as these are more appropriately acknowledged, and their specific contributions noted. The corresponding author should obtain written permission as such acknowledgements may imply endorsement of the work or its conclusions.
Authors, too, should make note of their individual contributions to manuscripts submitted to JHM. The Journal will begin publishing these specific contributions with each article, as do other medical journals.11
Plagiarism
Perhaps the most serious ethical violation that journals confront is plagiarism of copyrighted work. In its 5 years, JHM has detected 4 episodes of plagiarism. Thankfully, the Committee on Publication Ethics (
We recognize that other journals have needed to handle similar episodes of plagiarism,1215 and that self‐plagiarism (recycling of one's own published text) is also a concern.16, 17 Many methods exist to detect these practices.18 One powerful approach gaining popularity among medical journals utilizes CrossCheck. The CrossCheck service has 2 components: (1) a large, full‐text database of scholarly work from leading publishers, maintained by CrossRef (
Any form of plagiarism is inexcusable, and, if detected, is immediately addressed. Additionally, any author who submits plagiarized work will be banned from submitting manuscripts to JHM in the future, and will not be allowed to serve the Journal as a reviewer or in any other capacity. Our notification in selected cases of the individual's supervisor or department chair may elicit additional adverse consequences.
Summary
As the Journal of Hospital Medicine continues to grow and evolve, we are extraordinarily grateful when authors choose to submit their scholarly work to us. But growth does not come without challenges and responsibilities, such as a requirement to uphold ethical standards of biomedical publishing. We believe that the uniform disclosure of competing interests, clear reporting of contributions for authorship, and monitoring for plagiarism will help JHM maintain the standards that its readership and contributing authors deserve. We look forward to your contributions during our next 5 years, and beyond.
Since its founding in 2006,1 the editors of the Journal of Hospital Medicine (JHM), strongly supported the ethical guidelines and uniform requirements for manuscripts established by the International Committee of Medical Journal Editors (ICMJE).2 These guidelines require authors to verify that they have followed appropriate standards in the conduct of research, meet criteria for authorship, disclose potential conflicts of interest, and respect existing copyrights. With recent publication of editorials in leading medical journals affirming this responsibility for all authors submitting their scholarly work,38 the editors of the Journal echo the importance of following these ethical standards, and wish to update authors and readers on our policies related to authorship and plagiarism.
Disclosure of Competing Interests
Scientific publications commonly require that authors disclose relationships, financial or otherwise, with commercial entities that might have an interest in the subject matter of the article. Historically, biomedical journals varied in the content and format of the information they requested from authors,9 yielding inconsistent reporting by authors depending on the journal. Lack of clarity regarding what relationships authors should report contributed to this variable reporting. For example, an author might submit an article on headache management, and not believe it necessary to report honoraria received from pharmaceutical firms for giving lectures on antibiotic management of pneumonia. Thus, many believed that only funding related to the subject matter in a manuscript needed to be disclosed. While general advice has been to err on the side of disclosure, many authors hesitated to do so.
To clarify and standardize reporting requirements, the ICMJE recommended a uniform format for disclosure of competing interests,3 which was updated recently.10 The document, available online at
JHM strongly supports the ICMJE uniform requirements for manuscripts and has adopted the new form for disclosure of competing interests. Effective immediately, this documentation will be required for all types of manuscripts submitted to JHM. To help reduce the paperwork burden for authors, this documentation will be required only when authors are invited to revise and resubmit their work, after completion of the initial round of reviews. Typically at this stage, JHM also requests each author complete a Copyright Transfer Agreement (CTA). Thus, when a revision is requested by the Journal, we recommend that the corresponding author have each coauthor concurrently complete the CTA and disclosure of competing interests, and return all of the materials to JHM at the same time.
Criteria for Authorship
Authorship of scientific articles has important professional implications. In a field such as Hospital Medicine which explicitly values teamwork, it can sometimes be unclear which members of a team qualify for authorship on an article that may result from the group's work. The ICMJE provides the following guidance:2
-
Authorship credit should be based on (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
The ICMJE notes that authorship is not justified for individuals who simply obtain or provide funding, participate in data collection or general supervision of the research, or serve as head of the group. Members of the team who play roles such as these are more appropriately acknowledged, and their specific contributions noted. The corresponding author should obtain written permission as such acknowledgements may imply endorsement of the work or its conclusions.
Authors, too, should make note of their individual contributions to manuscripts submitted to JHM. The Journal will begin publishing these specific contributions with each article, as do other medical journals.11
Plagiarism
Perhaps the most serious ethical violation that journals confront is plagiarism of copyrighted work. In its 5 years, JHM has detected 4 episodes of plagiarism. Thankfully, the Committee on Publication Ethics (
We recognize that other journals have needed to handle similar episodes of plagiarism,1215 and that self‐plagiarism (recycling of one's own published text) is also a concern.16, 17 Many methods exist to detect these practices.18 One powerful approach gaining popularity among medical journals utilizes CrossCheck. The CrossCheck service has 2 components: (1) a large, full‐text database of scholarly work from leading publishers, maintained by CrossRef (
Any form of plagiarism is inexcusable, and, if detected, is immediately addressed. Additionally, any author who submits plagiarized work will be banned from submitting manuscripts to JHM in the future, and will not be allowed to serve the Journal as a reviewer or in any other capacity. Our notification in selected cases of the individual's supervisor or department chair may elicit additional adverse consequences.
Summary
As the Journal of Hospital Medicine continues to grow and evolve, we are extraordinarily grateful when authors choose to submit their scholarly work to us. But growth does not come without challenges and responsibilities, such as a requirement to uphold ethical standards of biomedical publishing. We believe that the uniform disclosure of competing interests, clear reporting of contributions for authorship, and monitoring for plagiarism will help JHM maintain the standards that its readership and contributing authors deserve. We look forward to your contributions during our next 5 years, and beyond.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1(1):1–2.
- International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals. Available at: http://www.icmje.org/. Accessed February 22,2010.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.N Engl J Med.2009;361(19):1896–1897.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.JAMA.2010;303(1):75–76.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.Ann Intern Med.2010;152(2):125–126.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.Lancet.2009;374(9699):1395–1396.
- , , , et al.Disclosure of competing interests.BMJ.2009;339:b4144.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.CMAJ.2009;181(9):565.
- , , , .Requirements and definitions in conflict of interest policies of medical journals.JAMA.2009;302(20):2230–2234.
- , , , , et al.Toward more uniform conflict disclosures ‐ the updated ICMJE conflict of interest reporting form.N Engl J Med.2010;363(2):188–189.
- , , , .Authorship criteria and disclosure of contributions: comparison of 3 general medical journals with different author contribution forms.JAMA.2004;292(1):86–88.
- , .Investigating allegations of scientific misconduct.BMJ.2005;331(7511):245–246.
- .Misconduct in medical research: does it exist in Britain?BMJ.1998;297:1531–1535.
- , .Research misconduct, retraction, and cleansing the medical literature: lessons from the Poehlman case.Annals of Internal Medicine.2006;144(8):609–613.
- .Report from the Scientific Integrity Advisor: issues arising in 2005 and 2006.Neurology.2007;68(21):1841–1842.
- Anonymous.Self‐plagiarism: unintentional, harmless, or fraud?Lancet.2009;374(9691):664.
- .Re‐using text from one's own previously published papers: an exploratory study of potential self‐plagiarism.Psychological Reports.2005;97(1):43–49.
- .Policing plagiarism.BMJ.2007;335(7627):963–964.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1(1):1–2.
- International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals. Available at: http://www.icmje.org/. Accessed February 22,2010.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.N Engl J Med.2009;361(19):1896–1897.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.JAMA.2010;303(1):75–76.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.Ann Intern Med.2010;152(2):125–126.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.Lancet.2009;374(9699):1395–1396.
- , , , et al.Disclosure of competing interests.BMJ.2009;339:b4144.
- , , , et al.Uniform format for disclosure of competing interests in ICMJE journals.CMAJ.2009;181(9):565.
- , , , .Requirements and definitions in conflict of interest policies of medical journals.JAMA.2009;302(20):2230–2234.
- , , , , et al.Toward more uniform conflict disclosures ‐ the updated ICMJE conflict of interest reporting form.N Engl J Med.2010;363(2):188–189.
- , , , .Authorship criteria and disclosure of contributions: comparison of 3 general medical journals with different author contribution forms.JAMA.2004;292(1):86–88.
- , .Investigating allegations of scientific misconduct.BMJ.2005;331(7511):245–246.
- .Misconduct in medical research: does it exist in Britain?BMJ.1998;297:1531–1535.
- , .Research misconduct, retraction, and cleansing the medical literature: lessons from the Poehlman case.Annals of Internal Medicine.2006;144(8):609–613.
- .Report from the Scientific Integrity Advisor: issues arising in 2005 and 2006.Neurology.2007;68(21):1841–1842.
- Anonymous.Self‐plagiarism: unintentional, harmless, or fraud?Lancet.2009;374(9691):664.
- .Re‐using text from one's own previously published papers: an exploratory study of potential self‐plagiarism.Psychological Reports.2005;97(1):43–49.
- .Policing plagiarism.BMJ.2007;335(7627):963–964.
Hospitalists: Lean leaders for hospitals
Unsustainable increases in health care costs mandate efforts at cost reduction.1 Such efforts necessitate enhanced productivity, especially given the specter of an aging population afflicted by a burgeoning chronic disease burden.2 Productivity is less a choice than an imperative forced upon hospitals and health systems as they attempt to address the competing requirements of diminished resources and increased demands. While the traditional mindset treats the goals of cost reduction and improving quality as tradeoffs, the methodology and philosophy known as Lean provides a proven approach for simultaneously improving both factors.3 Ideally, improved quality should lead to lower cost, and improved productivity should lead to better quality outcomes for patients.
This issue of the Journal of Hospital Medicine (JHM) describes multiple efforts to assess the activities of hospitalists and other hospital‐based physicians through use of time‐flow measurement.47 Understanding how health care workers spend their time and on which tasks that time is spent are essential steps toward applying Lean methodology at the point of care, or gembaa Japanese word that means the place where the work is actually done.8 At many health care institutions this gemba focus has not been integral to healthcare management models, and likely is a contributing factor to the cost and quality levels that exist today. The studies directly observing care delivery published in this issue of JHM provide invaluable lessons on how we might both improve productivity and quality of care delivery in the hospital. In this editorial, we review essential components of Lean methodology and propose how hospitalists and hospitals can benefit from its application.9
Value and Waste
In the Lean model, work and activity are broken down into the general categories of value and waste. The time and activities, as viewed from the customer's (ie, patient in the hospital) perspective, can also be categorized in a similar way. The goal in a Lean environment is to maximize value to the customer while reducing activities that are not value (ie, activities lacking value are waste).
Some define value as the simple mathematical equation of quality divided by cost.10 Better quality and/or lower cost means more value. A classical Lean definition of value requires three criteria to be met.11 First, the customer (patient) must be willing to pay for the given activity, directly or indirectly. When a hospitalist initiates care in the Emergency Department by placing admitting orders for a patient, the patient would view this activity as value because it progressed the care of the patient. However, if the patient is forced to wait 5 hours in the Emergency Room for an available inpatient bed while receiving minimal care, the patient may likely view that time as waste. Second, the activity must move the process forward toward the desired outcome in a meaningful way. Testing and exam activity that leads to a diagnosis would meet this criterion, while unnecessary CT scans might not. Third, the activity must be done properly the first time so as to minimize any rework, an important core quality component of the Lean approach.
All hospitalists perform activities that represent value and others that represent waste during their day. The nomenclature is not meant to be a value judgment on the clinician or their role. Lean provides a formal framework to describe waste in 8 key categories (Table 1), all meant to look at the system related elements of waste instead of the blaming of an individual.12 Common applications of Lean in healthcare focus on reducing waste to free up more time to deliver value, or to ensure that the value work is done at the highest possible level of quality. When hospitalists must take time to locate a colleague or a piece of information, that hunting and gathering time is waste. It distracts them from providing value. Too much waste within a fixed time period may lead to corners being cut or a lack of responsiveness to patient needsresulting in degradation in the quality of care and outcomes.
|
| Defects (correction, rework) |
| Overproduction |
| Transportation |
| Waiting |
| Inventory |
| Motion |
| Overprocessing |
| Human talent |
A simpler way of looking at activity for hospitalists and the care team often classifies any time spent in the patient room or at the bedside as direct value. This time can include clinical activities or time spent simply communicating with a patient and their families about their care or concerns. There may be activity in the room that could be considered waste (searching for information in the EMR), but proximity to patients is often considered valuable for other reasons. In the field of nursing, multiple studies in the past few years focused on identifying the percentage of time that nurses spend in patient rooms (consistently in the 3035% range across health systems and continents).13 The problem of waste is a long‐standing one in hospitals. In 1922 Henry Ford wrote, In the ordinary hospital the nurses must make useless steps. More of their time is spent in walking than in caring for the patient. [A hospital in Detroit] is designed to save steps we have tried to eliminate waste motion in the hospital.14
Activity outside of the patient room may be sometimes considered of indirect value, but this is often a gray area. Charting and medical decision‐making may benefit the patient and move the care process forward, and thus be of clear value. Yet, such activity may have questionable patient value if undertaken solely for billing or regulatory reasons. Effectively coordinating care between different members of the care team from both inside the hospital as well as beyond its walls does have value, but waste typically occurs when information is transferred incompletely or inaccurately.
Reducing waste often requires systemic changes to processes, workflow, and physical space. Motion (walking and searching) is a common form of waste in healthcare. Systemic Lean improvements might include changing the location of equipment and medication storage, or even patients.15 Uneven workloads often cannot be addressed by an individualthere must be a systemic effort to level workloads (the Lean term being heijunka), for example, leveling patient discharges throughout the day instead of doing them all in the late afternoon.
Lean also focuses on not wasting human talent or professional potential, often referred to in the literature as the eighth type of waste because it is missing from some Lean reference books.11 When hospitalists perform work that could be done by a midlevel provider (ie, physician assistant or nurse practitioner), or when a nurse performs work that could be done by a tech, the hospital wastes a scarce resource, human capital. Of note, changing these roles and responsibilities requires systemic effort rather than people just quitting a certain activity because it is below their pay grade; eg, it is better for the wrong person to be taking vital signs than to not have them documented at all.
Subject or Scientist
Toyota describes its management system, the Toyota Way, as having 2 equally important pillars: continuous improvement and what they call respect for humanity.16
If hospitals focus only on the improvement pillar, they run the risk of alienating the clinicians and staff members, undercutting any attempts at quality or productivity improvement. Respect for humanity is a much more sophisticated concept than just making employees happy in a superficial way. Respect, in a Lean sense, includes not robbing people of the opportunity to improve their own work. As participation increases the pride people feel in their work, more improvement resultsa virtuous cycle.17
Importantly, the Lean approach to quality improvement does not mirror the classical approach to improving productivity in a factory. Frederick Taylor (18561915) and Frank Gilbreth (18681924) are considered the fathers of Industrial Engineering, but their philosophy promulgated the belief that workers are not smart enough to participate in improvement.18 While they contributed a number of work analysis and process improvement methods that we use to this day, their philosophy is not one that fits with the respect for humanity principle of a modern professional workplace. Taylor believed a primary workplace problem was that people loafed and did not work hard enough; a seeming defect in their character as opposed to something that management should investigate and understand (for example, asking Why are people no longer motivated?).19 Taylor stood over workers, timing and watching their efforts, devising methods that workers should use to maximize their productivity. The term Taylorist is often used to describe this forced separation between working and thinking. The modern approach to Lean management draws more on the philosophy of Demingpeople want to do quality work, but the system gets in the way. The modern Lean approach emphasizes that every employee has 2 jobsboth to do the work and to improve it. The daily practice of kaizen, or continuous improvement, engages every employee in a problem‐solving dialogue with their leaders. In a Lean hospital, everybody deserves respect for their role, from a night‐time hospitalist to patient transporters, and all can play a role in process improvement.
Having research assistants shadow hospitalists could be done in a Taylorist or Deming way. Ideally, the role of a Lean improvement professional would be to teach those doing the work how to identify waste, allowing the hospitalists to develop and test their own improvements based on their existing professional knowledge combined with Lean principles. While the time‐flow studies published in this issue of JHM identified how the hospital system can be a barrier to hospitalist efficiency, this also potentially represents a wasted opportunity. Ideally, if the observers had been Lean improvement professionals they would not have just shadowed hospitalists without talking to or engaging them. They would have helped identify batching in a process or teaching the hospitalists why that practice is often not optimal. Future research should focus on applying this approach to time‐flow analysis in the hospital.
Simply putLean and process improvement techniques run the risk of being disrespectful, ineffective, and unsustainable when they are done to somebody, (the Taylor/Gilbreth approach) instead of utilized to both assess activities and glean learning from the front‐line staff. To be sustainable, effective, and respectful, hospitals should strive to truly engage in process improvement the people who are actually performing the work. Instead of efficiency experts, we need skilled coaches and mentors who can guide people towards generating their own improvements. Finally, when we have experts like Taylor or Gilbreth leading process improvement, those experts become a crutch and a bottleneck. Only by teaching the clinicians and staff members these skills, combined with patient focus and respect for humanity, can we begin moving a hospital's culture to one of true continuous improvementleading to better patient safety and quality, better access, lower costs, and better staff morale.
Conclusion
Hospitalists seem to be ideal leaders in efforts to generate ideas for improvement to remove waste from the health care system. Efficiency, value, and quality will be the mantra as we head into an era of healthcare where every action will be analyzed as to whether the action provides value to the patient. Hospitalists are well poised as Dr. Peter Pronovost recently stated. I think hospitalists' roles are going to go up dramatically, and I hope the field responds by making sure they put out people who have the skills to lead.20 Hospitalists experience and see waste in the processes of care. Yet, as Lord Kelvin is credited with the saying, If you can not measure it, you can not improve it and future time‐flow studies of hospitalists must take advantage of opportunities to also measure waste and not just document activity.
- .The cost implications of health care reform.N Engl J Med.2010 (in press).
- . The cost of inaction: the urgent need for health reform. Available at: http://www.healthreform.gov/reports/inaction/inactionreportprintmarch2009.pdf. Accessed May2010.
- .Writing the new playbook for U.S. health care: lessons from Wisconsin.Health Aff (Millwood).2009;28(5):1343–1350.
- , , , , .Systematic review of time studies evaluating physicians in the hospital setting.J Hosp Med.2010;5(6):353–359.
- , , , et al.Where did the day go?—A time‐motion study of hospitalists.J Hosp Med.2010;5(6):323–328.
- , , , , .Comparing Academic and Community‐Based Hospitalists.J Hosp Med.2010;5(6):349–352.
- , , , , .Hospitalist time usage and cyclicality: opportunities to improve efficiency.J Hosp Med.2010;5(6):329–334.
- , .Lean Lexicon: A Graphical Glossary for Lean Thinkers.Cambridge, MA:Lean Enterprise Institute;2003.
- , , , .Lean health care: what can hospitals learn from a world‐class automaker?J Hosp Med.2006;1(3):191–199.
- . Evaluating the Impact of Value‐Based Purchasing: A Guide for Purchasers. Available at: http://www.ahrq.gov/about/cods/valuebased/evalvbp1.htm. Accessed May2010.
- .Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction.New York:Productivity Press;2008.
- , .Lean Thinking.New York:Simon and Schuster;1996.
- , .Creating an environment for caring using lean principles of the Virginia Mason Production System.J Nurs Adm.2007;37(6):287–294.
- , .My Life and Work.Garden City, NY:Garden City Publishing;1922.
- , , , et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- .Toyota Production System: Beyond Large‐Scale Production.New York:Productivity Press;1988.
- .Out of the Crisis.Cambridge:Massachusetts Institute of Technology—Center of Advanced Educational Services;1982.
- .Frederick Taylor and Frank Gilbreth: competition in scientific management.Bus Hist Rev.1957;31(1):23–34.
- .The Principles of Scientific Management.Norwood, MA:The Plimpton Press;1911.
- .The Year Ahead.The hospitalist.2010;14(2):1,4–5.
Unsustainable increases in health care costs mandate efforts at cost reduction.1 Such efforts necessitate enhanced productivity, especially given the specter of an aging population afflicted by a burgeoning chronic disease burden.2 Productivity is less a choice than an imperative forced upon hospitals and health systems as they attempt to address the competing requirements of diminished resources and increased demands. While the traditional mindset treats the goals of cost reduction and improving quality as tradeoffs, the methodology and philosophy known as Lean provides a proven approach for simultaneously improving both factors.3 Ideally, improved quality should lead to lower cost, and improved productivity should lead to better quality outcomes for patients.
This issue of the Journal of Hospital Medicine (JHM) describes multiple efforts to assess the activities of hospitalists and other hospital‐based physicians through use of time‐flow measurement.47 Understanding how health care workers spend their time and on which tasks that time is spent are essential steps toward applying Lean methodology at the point of care, or gembaa Japanese word that means the place where the work is actually done.8 At many health care institutions this gemba focus has not been integral to healthcare management models, and likely is a contributing factor to the cost and quality levels that exist today. The studies directly observing care delivery published in this issue of JHM provide invaluable lessons on how we might both improve productivity and quality of care delivery in the hospital. In this editorial, we review essential components of Lean methodology and propose how hospitalists and hospitals can benefit from its application.9
Value and Waste
In the Lean model, work and activity are broken down into the general categories of value and waste. The time and activities, as viewed from the customer's (ie, patient in the hospital) perspective, can also be categorized in a similar way. The goal in a Lean environment is to maximize value to the customer while reducing activities that are not value (ie, activities lacking value are waste).
Some define value as the simple mathematical equation of quality divided by cost.10 Better quality and/or lower cost means more value. A classical Lean definition of value requires three criteria to be met.11 First, the customer (patient) must be willing to pay for the given activity, directly or indirectly. When a hospitalist initiates care in the Emergency Department by placing admitting orders for a patient, the patient would view this activity as value because it progressed the care of the patient. However, if the patient is forced to wait 5 hours in the Emergency Room for an available inpatient bed while receiving minimal care, the patient may likely view that time as waste. Second, the activity must move the process forward toward the desired outcome in a meaningful way. Testing and exam activity that leads to a diagnosis would meet this criterion, while unnecessary CT scans might not. Third, the activity must be done properly the first time so as to minimize any rework, an important core quality component of the Lean approach.
All hospitalists perform activities that represent value and others that represent waste during their day. The nomenclature is not meant to be a value judgment on the clinician or their role. Lean provides a formal framework to describe waste in 8 key categories (Table 1), all meant to look at the system related elements of waste instead of the blaming of an individual.12 Common applications of Lean in healthcare focus on reducing waste to free up more time to deliver value, or to ensure that the value work is done at the highest possible level of quality. When hospitalists must take time to locate a colleague or a piece of information, that hunting and gathering time is waste. It distracts them from providing value. Too much waste within a fixed time period may lead to corners being cut or a lack of responsiveness to patient needsresulting in degradation in the quality of care and outcomes.
|
| Defects (correction, rework) |
| Overproduction |
| Transportation |
| Waiting |
| Inventory |
| Motion |
| Overprocessing |
| Human talent |
A simpler way of looking at activity for hospitalists and the care team often classifies any time spent in the patient room or at the bedside as direct value. This time can include clinical activities or time spent simply communicating with a patient and their families about their care or concerns. There may be activity in the room that could be considered waste (searching for information in the EMR), but proximity to patients is often considered valuable for other reasons. In the field of nursing, multiple studies in the past few years focused on identifying the percentage of time that nurses spend in patient rooms (consistently in the 3035% range across health systems and continents).13 The problem of waste is a long‐standing one in hospitals. In 1922 Henry Ford wrote, In the ordinary hospital the nurses must make useless steps. More of their time is spent in walking than in caring for the patient. [A hospital in Detroit] is designed to save steps we have tried to eliminate waste motion in the hospital.14
Activity outside of the patient room may be sometimes considered of indirect value, but this is often a gray area. Charting and medical decision‐making may benefit the patient and move the care process forward, and thus be of clear value. Yet, such activity may have questionable patient value if undertaken solely for billing or regulatory reasons. Effectively coordinating care between different members of the care team from both inside the hospital as well as beyond its walls does have value, but waste typically occurs when information is transferred incompletely or inaccurately.
Reducing waste often requires systemic changes to processes, workflow, and physical space. Motion (walking and searching) is a common form of waste in healthcare. Systemic Lean improvements might include changing the location of equipment and medication storage, or even patients.15 Uneven workloads often cannot be addressed by an individualthere must be a systemic effort to level workloads (the Lean term being heijunka), for example, leveling patient discharges throughout the day instead of doing them all in the late afternoon.
Lean also focuses on not wasting human talent or professional potential, often referred to in the literature as the eighth type of waste because it is missing from some Lean reference books.11 When hospitalists perform work that could be done by a midlevel provider (ie, physician assistant or nurse practitioner), or when a nurse performs work that could be done by a tech, the hospital wastes a scarce resource, human capital. Of note, changing these roles and responsibilities requires systemic effort rather than people just quitting a certain activity because it is below their pay grade; eg, it is better for the wrong person to be taking vital signs than to not have them documented at all.
Subject or Scientist
Toyota describes its management system, the Toyota Way, as having 2 equally important pillars: continuous improvement and what they call respect for humanity.16
If hospitals focus only on the improvement pillar, they run the risk of alienating the clinicians and staff members, undercutting any attempts at quality or productivity improvement. Respect for humanity is a much more sophisticated concept than just making employees happy in a superficial way. Respect, in a Lean sense, includes not robbing people of the opportunity to improve their own work. As participation increases the pride people feel in their work, more improvement resultsa virtuous cycle.17
Importantly, the Lean approach to quality improvement does not mirror the classical approach to improving productivity in a factory. Frederick Taylor (18561915) and Frank Gilbreth (18681924) are considered the fathers of Industrial Engineering, but their philosophy promulgated the belief that workers are not smart enough to participate in improvement.18 While they contributed a number of work analysis and process improvement methods that we use to this day, their philosophy is not one that fits with the respect for humanity principle of a modern professional workplace. Taylor believed a primary workplace problem was that people loafed and did not work hard enough; a seeming defect in their character as opposed to something that management should investigate and understand (for example, asking Why are people no longer motivated?).19 Taylor stood over workers, timing and watching their efforts, devising methods that workers should use to maximize their productivity. The term Taylorist is often used to describe this forced separation between working and thinking. The modern approach to Lean management draws more on the philosophy of Demingpeople want to do quality work, but the system gets in the way. The modern Lean approach emphasizes that every employee has 2 jobsboth to do the work and to improve it. The daily practice of kaizen, or continuous improvement, engages every employee in a problem‐solving dialogue with their leaders. In a Lean hospital, everybody deserves respect for their role, from a night‐time hospitalist to patient transporters, and all can play a role in process improvement.
Having research assistants shadow hospitalists could be done in a Taylorist or Deming way. Ideally, the role of a Lean improvement professional would be to teach those doing the work how to identify waste, allowing the hospitalists to develop and test their own improvements based on their existing professional knowledge combined with Lean principles. While the time‐flow studies published in this issue of JHM identified how the hospital system can be a barrier to hospitalist efficiency, this also potentially represents a wasted opportunity. Ideally, if the observers had been Lean improvement professionals they would not have just shadowed hospitalists without talking to or engaging them. They would have helped identify batching in a process or teaching the hospitalists why that practice is often not optimal. Future research should focus on applying this approach to time‐flow analysis in the hospital.
Simply putLean and process improvement techniques run the risk of being disrespectful, ineffective, and unsustainable when they are done to somebody, (the Taylor/Gilbreth approach) instead of utilized to both assess activities and glean learning from the front‐line staff. To be sustainable, effective, and respectful, hospitals should strive to truly engage in process improvement the people who are actually performing the work. Instead of efficiency experts, we need skilled coaches and mentors who can guide people towards generating their own improvements. Finally, when we have experts like Taylor or Gilbreth leading process improvement, those experts become a crutch and a bottleneck. Only by teaching the clinicians and staff members these skills, combined with patient focus and respect for humanity, can we begin moving a hospital's culture to one of true continuous improvementleading to better patient safety and quality, better access, lower costs, and better staff morale.
Conclusion
Hospitalists seem to be ideal leaders in efforts to generate ideas for improvement to remove waste from the health care system. Efficiency, value, and quality will be the mantra as we head into an era of healthcare where every action will be analyzed as to whether the action provides value to the patient. Hospitalists are well poised as Dr. Peter Pronovost recently stated. I think hospitalists' roles are going to go up dramatically, and I hope the field responds by making sure they put out people who have the skills to lead.20 Hospitalists experience and see waste in the processes of care. Yet, as Lord Kelvin is credited with the saying, If you can not measure it, you can not improve it and future time‐flow studies of hospitalists must take advantage of opportunities to also measure waste and not just document activity.
Unsustainable increases in health care costs mandate efforts at cost reduction.1 Such efforts necessitate enhanced productivity, especially given the specter of an aging population afflicted by a burgeoning chronic disease burden.2 Productivity is less a choice than an imperative forced upon hospitals and health systems as they attempt to address the competing requirements of diminished resources and increased demands. While the traditional mindset treats the goals of cost reduction and improving quality as tradeoffs, the methodology and philosophy known as Lean provides a proven approach for simultaneously improving both factors.3 Ideally, improved quality should lead to lower cost, and improved productivity should lead to better quality outcomes for patients.
This issue of the Journal of Hospital Medicine (JHM) describes multiple efforts to assess the activities of hospitalists and other hospital‐based physicians through use of time‐flow measurement.47 Understanding how health care workers spend their time and on which tasks that time is spent are essential steps toward applying Lean methodology at the point of care, or gembaa Japanese word that means the place where the work is actually done.8 At many health care institutions this gemba focus has not been integral to healthcare management models, and likely is a contributing factor to the cost and quality levels that exist today. The studies directly observing care delivery published in this issue of JHM provide invaluable lessons on how we might both improve productivity and quality of care delivery in the hospital. In this editorial, we review essential components of Lean methodology and propose how hospitalists and hospitals can benefit from its application.9
Value and Waste
In the Lean model, work and activity are broken down into the general categories of value and waste. The time and activities, as viewed from the customer's (ie, patient in the hospital) perspective, can also be categorized in a similar way. The goal in a Lean environment is to maximize value to the customer while reducing activities that are not value (ie, activities lacking value are waste).
Some define value as the simple mathematical equation of quality divided by cost.10 Better quality and/or lower cost means more value. A classical Lean definition of value requires three criteria to be met.11 First, the customer (patient) must be willing to pay for the given activity, directly or indirectly. When a hospitalist initiates care in the Emergency Department by placing admitting orders for a patient, the patient would view this activity as value because it progressed the care of the patient. However, if the patient is forced to wait 5 hours in the Emergency Room for an available inpatient bed while receiving minimal care, the patient may likely view that time as waste. Second, the activity must move the process forward toward the desired outcome in a meaningful way. Testing and exam activity that leads to a diagnosis would meet this criterion, while unnecessary CT scans might not. Third, the activity must be done properly the first time so as to minimize any rework, an important core quality component of the Lean approach.
All hospitalists perform activities that represent value and others that represent waste during their day. The nomenclature is not meant to be a value judgment on the clinician or their role. Lean provides a formal framework to describe waste in 8 key categories (Table 1), all meant to look at the system related elements of waste instead of the blaming of an individual.12 Common applications of Lean in healthcare focus on reducing waste to free up more time to deliver value, or to ensure that the value work is done at the highest possible level of quality. When hospitalists must take time to locate a colleague or a piece of information, that hunting and gathering time is waste. It distracts them from providing value. Too much waste within a fixed time period may lead to corners being cut or a lack of responsiveness to patient needsresulting in degradation in the quality of care and outcomes.
|
| Defects (correction, rework) |
| Overproduction |
| Transportation |
| Waiting |
| Inventory |
| Motion |
| Overprocessing |
| Human talent |
A simpler way of looking at activity for hospitalists and the care team often classifies any time spent in the patient room or at the bedside as direct value. This time can include clinical activities or time spent simply communicating with a patient and their families about their care or concerns. There may be activity in the room that could be considered waste (searching for information in the EMR), but proximity to patients is often considered valuable for other reasons. In the field of nursing, multiple studies in the past few years focused on identifying the percentage of time that nurses spend in patient rooms (consistently in the 3035% range across health systems and continents).13 The problem of waste is a long‐standing one in hospitals. In 1922 Henry Ford wrote, In the ordinary hospital the nurses must make useless steps. More of their time is spent in walking than in caring for the patient. [A hospital in Detroit] is designed to save steps we have tried to eliminate waste motion in the hospital.14
Activity outside of the patient room may be sometimes considered of indirect value, but this is often a gray area. Charting and medical decision‐making may benefit the patient and move the care process forward, and thus be of clear value. Yet, such activity may have questionable patient value if undertaken solely for billing or regulatory reasons. Effectively coordinating care between different members of the care team from both inside the hospital as well as beyond its walls does have value, but waste typically occurs when information is transferred incompletely or inaccurately.
Reducing waste often requires systemic changes to processes, workflow, and physical space. Motion (walking and searching) is a common form of waste in healthcare. Systemic Lean improvements might include changing the location of equipment and medication storage, or even patients.15 Uneven workloads often cannot be addressed by an individualthere must be a systemic effort to level workloads (the Lean term being heijunka), for example, leveling patient discharges throughout the day instead of doing them all in the late afternoon.
Lean also focuses on not wasting human talent or professional potential, often referred to in the literature as the eighth type of waste because it is missing from some Lean reference books.11 When hospitalists perform work that could be done by a midlevel provider (ie, physician assistant or nurse practitioner), or when a nurse performs work that could be done by a tech, the hospital wastes a scarce resource, human capital. Of note, changing these roles and responsibilities requires systemic effort rather than people just quitting a certain activity because it is below their pay grade; eg, it is better for the wrong person to be taking vital signs than to not have them documented at all.
Subject or Scientist
Toyota describes its management system, the Toyota Way, as having 2 equally important pillars: continuous improvement and what they call respect for humanity.16
If hospitals focus only on the improvement pillar, they run the risk of alienating the clinicians and staff members, undercutting any attempts at quality or productivity improvement. Respect for humanity is a much more sophisticated concept than just making employees happy in a superficial way. Respect, in a Lean sense, includes not robbing people of the opportunity to improve their own work. As participation increases the pride people feel in their work, more improvement resultsa virtuous cycle.17
Importantly, the Lean approach to quality improvement does not mirror the classical approach to improving productivity in a factory. Frederick Taylor (18561915) and Frank Gilbreth (18681924) are considered the fathers of Industrial Engineering, but their philosophy promulgated the belief that workers are not smart enough to participate in improvement.18 While they contributed a number of work analysis and process improvement methods that we use to this day, their philosophy is not one that fits with the respect for humanity principle of a modern professional workplace. Taylor believed a primary workplace problem was that people loafed and did not work hard enough; a seeming defect in their character as opposed to something that management should investigate and understand (for example, asking Why are people no longer motivated?).19 Taylor stood over workers, timing and watching their efforts, devising methods that workers should use to maximize their productivity. The term Taylorist is often used to describe this forced separation between working and thinking. The modern approach to Lean management draws more on the philosophy of Demingpeople want to do quality work, but the system gets in the way. The modern Lean approach emphasizes that every employee has 2 jobsboth to do the work and to improve it. The daily practice of kaizen, or continuous improvement, engages every employee in a problem‐solving dialogue with their leaders. In a Lean hospital, everybody deserves respect for their role, from a night‐time hospitalist to patient transporters, and all can play a role in process improvement.
Having research assistants shadow hospitalists could be done in a Taylorist or Deming way. Ideally, the role of a Lean improvement professional would be to teach those doing the work how to identify waste, allowing the hospitalists to develop and test their own improvements based on their existing professional knowledge combined with Lean principles. While the time‐flow studies published in this issue of JHM identified how the hospital system can be a barrier to hospitalist efficiency, this also potentially represents a wasted opportunity. Ideally, if the observers had been Lean improvement professionals they would not have just shadowed hospitalists without talking to or engaging them. They would have helped identify batching in a process or teaching the hospitalists why that practice is often not optimal. Future research should focus on applying this approach to time‐flow analysis in the hospital.
Simply putLean and process improvement techniques run the risk of being disrespectful, ineffective, and unsustainable when they are done to somebody, (the Taylor/Gilbreth approach) instead of utilized to both assess activities and glean learning from the front‐line staff. To be sustainable, effective, and respectful, hospitals should strive to truly engage in process improvement the people who are actually performing the work. Instead of efficiency experts, we need skilled coaches and mentors who can guide people towards generating their own improvements. Finally, when we have experts like Taylor or Gilbreth leading process improvement, those experts become a crutch and a bottleneck. Only by teaching the clinicians and staff members these skills, combined with patient focus and respect for humanity, can we begin moving a hospital's culture to one of true continuous improvementleading to better patient safety and quality, better access, lower costs, and better staff morale.
Conclusion
Hospitalists seem to be ideal leaders in efforts to generate ideas for improvement to remove waste from the health care system. Efficiency, value, and quality will be the mantra as we head into an era of healthcare where every action will be analyzed as to whether the action provides value to the patient. Hospitalists are well poised as Dr. Peter Pronovost recently stated. I think hospitalists' roles are going to go up dramatically, and I hope the field responds by making sure they put out people who have the skills to lead.20 Hospitalists experience and see waste in the processes of care. Yet, as Lord Kelvin is credited with the saying, If you can not measure it, you can not improve it and future time‐flow studies of hospitalists must take advantage of opportunities to also measure waste and not just document activity.
- .The cost implications of health care reform.N Engl J Med.2010 (in press).
- . The cost of inaction: the urgent need for health reform. Available at: http://www.healthreform.gov/reports/inaction/inactionreportprintmarch2009.pdf. Accessed May2010.
- .Writing the new playbook for U.S. health care: lessons from Wisconsin.Health Aff (Millwood).2009;28(5):1343–1350.
- , , , , .Systematic review of time studies evaluating physicians in the hospital setting.J Hosp Med.2010;5(6):353–359.
- , , , et al.Where did the day go?—A time‐motion study of hospitalists.J Hosp Med.2010;5(6):323–328.
- , , , , .Comparing Academic and Community‐Based Hospitalists.J Hosp Med.2010;5(6):349–352.
- , , , , .Hospitalist time usage and cyclicality: opportunities to improve efficiency.J Hosp Med.2010;5(6):329–334.
- , .Lean Lexicon: A Graphical Glossary for Lean Thinkers.Cambridge, MA:Lean Enterprise Institute;2003.
- , , , .Lean health care: what can hospitals learn from a world‐class automaker?J Hosp Med.2006;1(3):191–199.
- . Evaluating the Impact of Value‐Based Purchasing: A Guide for Purchasers. Available at: http://www.ahrq.gov/about/cods/valuebased/evalvbp1.htm. Accessed May2010.
- .Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction.New York:Productivity Press;2008.
- , .Lean Thinking.New York:Simon and Schuster;1996.
- , .Creating an environment for caring using lean principles of the Virginia Mason Production System.J Nurs Adm.2007;37(6):287–294.
- , .My Life and Work.Garden City, NY:Garden City Publishing;1922.
- , , , et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- .Toyota Production System: Beyond Large‐Scale Production.New York:Productivity Press;1988.
- .Out of the Crisis.Cambridge:Massachusetts Institute of Technology—Center of Advanced Educational Services;1982.
- .Frederick Taylor and Frank Gilbreth: competition in scientific management.Bus Hist Rev.1957;31(1):23–34.
- .The Principles of Scientific Management.Norwood, MA:The Plimpton Press;1911.
- .The Year Ahead.The hospitalist.2010;14(2):1,4–5.
- .The cost implications of health care reform.N Engl J Med.2010 (in press).
- . The cost of inaction: the urgent need for health reform. Available at: http://www.healthreform.gov/reports/inaction/inactionreportprintmarch2009.pdf. Accessed May2010.
- .Writing the new playbook for U.S. health care: lessons from Wisconsin.Health Aff (Millwood).2009;28(5):1343–1350.
- , , , , .Systematic review of time studies evaluating physicians in the hospital setting.J Hosp Med.2010;5(6):353–359.
- , , , et al.Where did the day go?—A time‐motion study of hospitalists.J Hosp Med.2010;5(6):323–328.
- , , , , .Comparing Academic and Community‐Based Hospitalists.J Hosp Med.2010;5(6):349–352.
- , , , , .Hospitalist time usage and cyclicality: opportunities to improve efficiency.J Hosp Med.2010;5(6):329–334.
- , .Lean Lexicon: A Graphical Glossary for Lean Thinkers.Cambridge, MA:Lean Enterprise Institute;2003.
- , , , .Lean health care: what can hospitals learn from a world‐class automaker?J Hosp Med.2006;1(3):191–199.
- . Evaluating the Impact of Value‐Based Purchasing: A Guide for Purchasers. Available at: http://www.ahrq.gov/about/cods/valuebased/evalvbp1.htm. Accessed May2010.
- .Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction.New York:Productivity Press;2008.
- , .Lean Thinking.New York:Simon and Schuster;1996.
- , .Creating an environment for caring using lean principles of the Virginia Mason Production System.J Nurs Adm.2007;37(6):287–294.
- , .My Life and Work.Garden City, NY:Garden City Publishing;1922.
- , , , et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- .Toyota Production System: Beyond Large‐Scale Production.New York:Productivity Press;1988.
- .Out of the Crisis.Cambridge:Massachusetts Institute of Technology—Center of Advanced Educational Services;1982.
- .Frederick Taylor and Frank Gilbreth: competition in scientific management.Bus Hist Rev.1957;31(1):23–34.
- .The Principles of Scientific Management.Norwood, MA:The Plimpton Press;1911.
- .The Year Ahead.The hospitalist.2010;14(2):1,4–5.
A Small Kindness
On August 6, 2009, my vigorously healthy 59‐year‐old brother‐in‐law, a beloved husband and father of 2 sons, developed mild right hand clumsiness and slight slurring of speech. This led to a primary care visit and the symptoms were originally felt to be related to working long hours and stress. The symptoms failed to improve and on August 11th, an magnetic resonance imaging (MRI) made a shocking discovery, my brother‐in‐law had a brain tumor. He was seen in the Neurosurgery department of a major academic center the following day and on August 13th he underwent resection of the majority of the tumor with a pathologic finding of grade IV glioblastoma multiforme (GBM).
During this initial admission, a consultation was obtained from a neuro‐oncologist who would then become the principle director of his care. My brother‐in‐law was crystal clear with his physicians; he wanted honest information about what to expect. From the outset he understood this was an incurable disease, but hoped that with aggressive treatment, he could live for months, possibly even years. He started chemotherapy almost immediately and in early October started a 6‐week course of daily radiation. The early weeks went relatively well. He saw his oncologist regularly and stayed in close contact with his oncology nurse. With his engineering background and attention to details, he followed his physician's instructions to the letter; however, despite his very best efforts at compliance and working intensively with physical therapy he was becoming progressively weaker.
In early November, a follow‐up MRI appeared to show progression of tumor and on November 12th, a second resection was undertaken. Pathologically, this seemed successful with removal of tumor bulk, but he was left even weaker, particularly on the right side. His symptoms were managed with dexamethasone; however, aphasia and hemiparesis would always reemerge with attempts to taper the drug and his functional status was too poor to allow for further chemotherapy. As his communication ability was becoming more limited, my sister‐in‐law was increasingly becoming his spokesperson at doctor visits and with phone updates to his oncology nurse.
On January 21, 2010 after working with a home physical therapist, he was making a transfer and became nonresponsive. Paramedics were called, arriving within minutes, but he was found pulseless. Despite a heroic resuscitative effort, he was pronounced dead at a nearby hospital a short time thereafter. A postmortem was not performed and the presumptive cause of death was pulmonary embolism.
His funeral was January 29th.
My brother‐in‐law and I lived 1600 miles apart and saw each other on only the rarest occasions. When the diagnosis was made, my role changed as I am the only relative within the extended family with medical expertise. Questions were directed to me via e‐mails and I did my best using UpToDate and other resources to learn about GBM and relay this information back to the family.
Recently, and in a vicarious way, I was becoming more and more deeply involved. Using the best descriptions of functional status that I could extract from e‐mail and phone calls, I estimated his Karnofsky Performance Score as being fairly poor. By way of my e‐mails to him and his wife, I was just beginning, ever so gently, to touch on the subject of hospice care at the time of his sudden death.
At the visitation and reception following the funeral, I think I was seen as more than a distant brother‐in‐law; I was also seen as a surrogate for the medical profession and for his doctors in particular. One message that I got loud and clear, from more than 1 family member was the devastated, abandoned feeling that was emerging in the 8 days since his death. On the morning following his death, my sister‐in‐law called his physician's office and told his oncology nurse that her husband had died suddenly and unexpectedly the night before. The nurse expressed sympathy and indicated she would relay this information to his doctor and she would personally call back. As of the time I left to return home, that was the last communication any family member had received from anyone involved in his care.
Although the outpouring of community support and sympathy was powerful and touching, not a single condolence card or phone call came from his doctor. I was shocked at the suddenness of his death, but I was also shocked at the complete absence of any communication, any acknowledgement of his courageous struggle against a terrible illness, or of his family's depth of caring and love over these last few months.
I am reminded of an essay by Gregory Kane, MD in CHEST,1 in which he describes a disturbing personal encounter with the following;
In a personal and memorable patient encounter, I sat and listened while a tearful patient cried at having received no contact from the physician who treated her husband for metastatic lung cancer for a treatment duration of 9 months. As I struggled to comprehend her sense of pain and abandonment, I considered offering as possible explanation that the physician may not have been on call at the time of the death and may have mistakenly believed that his partner had offered such a gesture verbally. Before I could respond, however, my patient added that her veterinarian had sent a card when the family dog died. I was speechless.1
As Hospitalists, we are gifted and privileged to work closely with patients and their loved ones struggling with the existential and eternal questions of life and death. As we can all well attest from 6 PM family meetings, the unit‐of‐care extends beyond the patient and certainly includes the loving and caring members of the patient's family and close support system. If we fail to acknowledge a family's bereavement, we run the risk of unintentionally communicating the message that the patient was not important, that their suffering did not matter or that the crushing grief the family may be bearing is somehow insignificant compared to our busy schedules.
I have asked you to join me on this short journey my brother‐in‐law has taken these last few months in the interest of raising awareness. There are many occasions when a verbal, bedside expression of condolence is very appropriate and completely adequate. There are other times when a condolence letter will better facilitate the closure of the physician‐patient‐family relationship. This may be intimidating, both the extra work involved and especially the challenge of not knowing what to say. The article quoted above by Dr. Kane is an excellent resource for guidance concerning content, style, and other writing considerations. Using examples, such as a letter from Abraham Lincoln to a girl whose father died in the Civil War, he shows that this essential communication does not have to be lengthy or difficult. Another excellent resource can be found in the New England Journal of Medicine; The Doctor's Letter of Condolence.2 See Table 1 for suggestions to help with bereavement communications.
| Handwritten on a card or stationary. |
| Timely (within 1‐2 weeks of death). |
| Use sensitive, caring language and avoid clich. |
| Acknowledge the family's grief and loss. |
| Acknowledge the patient's courage or other qualities. |
| Mention the privilege it has been to work with the patient. |
| Mention your appreciation of the family's caring. |
| Avoid sincerely yours and end on a personal note such as suggesting your thoughts are with them at this most difficult time. |
We are the profession of Hospital Medicine. It is our knowledge, our hope, our compassion, our experience and judgment that often directs care at the end‐of‐life for many of our patients. We are teammates along with the primary care provider, subspecialty consultants, palliative care specialists and other members of the care team. We have a professional obligation to extend a thoughtful condolence to surviving family members and to contact other members of the care team so that they too may have this opportunity. The responsibility for the final closure rests with us and within this responsibility is a powerful fulfillment of the promise of the practice of medicine.
The condolence note is a small kindness, a part of the art of medicine, a part of our humanness and essential to our vision of patient‐centered hospital care.
- .A Dying Art?: The Doctor's Letter of Condolence.Chest.2007;131(4):1245–1247. Permission for use obtained by direct communication with the author on January 31, 2010.
- ,,.The doctor's letter of condolence.N Engl J Med.2001;344(15):1162–1163.
On August 6, 2009, my vigorously healthy 59‐year‐old brother‐in‐law, a beloved husband and father of 2 sons, developed mild right hand clumsiness and slight slurring of speech. This led to a primary care visit and the symptoms were originally felt to be related to working long hours and stress. The symptoms failed to improve and on August 11th, an magnetic resonance imaging (MRI) made a shocking discovery, my brother‐in‐law had a brain tumor. He was seen in the Neurosurgery department of a major academic center the following day and on August 13th he underwent resection of the majority of the tumor with a pathologic finding of grade IV glioblastoma multiforme (GBM).
During this initial admission, a consultation was obtained from a neuro‐oncologist who would then become the principle director of his care. My brother‐in‐law was crystal clear with his physicians; he wanted honest information about what to expect. From the outset he understood this was an incurable disease, but hoped that with aggressive treatment, he could live for months, possibly even years. He started chemotherapy almost immediately and in early October started a 6‐week course of daily radiation. The early weeks went relatively well. He saw his oncologist regularly and stayed in close contact with his oncology nurse. With his engineering background and attention to details, he followed his physician's instructions to the letter; however, despite his very best efforts at compliance and working intensively with physical therapy he was becoming progressively weaker.
In early November, a follow‐up MRI appeared to show progression of tumor and on November 12th, a second resection was undertaken. Pathologically, this seemed successful with removal of tumor bulk, but he was left even weaker, particularly on the right side. His symptoms were managed with dexamethasone; however, aphasia and hemiparesis would always reemerge with attempts to taper the drug and his functional status was too poor to allow for further chemotherapy. As his communication ability was becoming more limited, my sister‐in‐law was increasingly becoming his spokesperson at doctor visits and with phone updates to his oncology nurse.
On January 21, 2010 after working with a home physical therapist, he was making a transfer and became nonresponsive. Paramedics were called, arriving within minutes, but he was found pulseless. Despite a heroic resuscitative effort, he was pronounced dead at a nearby hospital a short time thereafter. A postmortem was not performed and the presumptive cause of death was pulmonary embolism.
His funeral was January 29th.
My brother‐in‐law and I lived 1600 miles apart and saw each other on only the rarest occasions. When the diagnosis was made, my role changed as I am the only relative within the extended family with medical expertise. Questions were directed to me via e‐mails and I did my best using UpToDate and other resources to learn about GBM and relay this information back to the family.
Recently, and in a vicarious way, I was becoming more and more deeply involved. Using the best descriptions of functional status that I could extract from e‐mail and phone calls, I estimated his Karnofsky Performance Score as being fairly poor. By way of my e‐mails to him and his wife, I was just beginning, ever so gently, to touch on the subject of hospice care at the time of his sudden death.
At the visitation and reception following the funeral, I think I was seen as more than a distant brother‐in‐law; I was also seen as a surrogate for the medical profession and for his doctors in particular. One message that I got loud and clear, from more than 1 family member was the devastated, abandoned feeling that was emerging in the 8 days since his death. On the morning following his death, my sister‐in‐law called his physician's office and told his oncology nurse that her husband had died suddenly and unexpectedly the night before. The nurse expressed sympathy and indicated she would relay this information to his doctor and she would personally call back. As of the time I left to return home, that was the last communication any family member had received from anyone involved in his care.
Although the outpouring of community support and sympathy was powerful and touching, not a single condolence card or phone call came from his doctor. I was shocked at the suddenness of his death, but I was also shocked at the complete absence of any communication, any acknowledgement of his courageous struggle against a terrible illness, or of his family's depth of caring and love over these last few months.
I am reminded of an essay by Gregory Kane, MD in CHEST,1 in which he describes a disturbing personal encounter with the following;
In a personal and memorable patient encounter, I sat and listened while a tearful patient cried at having received no contact from the physician who treated her husband for metastatic lung cancer for a treatment duration of 9 months. As I struggled to comprehend her sense of pain and abandonment, I considered offering as possible explanation that the physician may not have been on call at the time of the death and may have mistakenly believed that his partner had offered such a gesture verbally. Before I could respond, however, my patient added that her veterinarian had sent a card when the family dog died. I was speechless.1
As Hospitalists, we are gifted and privileged to work closely with patients and their loved ones struggling with the existential and eternal questions of life and death. As we can all well attest from 6 PM family meetings, the unit‐of‐care extends beyond the patient and certainly includes the loving and caring members of the patient's family and close support system. If we fail to acknowledge a family's bereavement, we run the risk of unintentionally communicating the message that the patient was not important, that their suffering did not matter or that the crushing grief the family may be bearing is somehow insignificant compared to our busy schedules.
I have asked you to join me on this short journey my brother‐in‐law has taken these last few months in the interest of raising awareness. There are many occasions when a verbal, bedside expression of condolence is very appropriate and completely adequate. There are other times when a condolence letter will better facilitate the closure of the physician‐patient‐family relationship. This may be intimidating, both the extra work involved and especially the challenge of not knowing what to say. The article quoted above by Dr. Kane is an excellent resource for guidance concerning content, style, and other writing considerations. Using examples, such as a letter from Abraham Lincoln to a girl whose father died in the Civil War, he shows that this essential communication does not have to be lengthy or difficult. Another excellent resource can be found in the New England Journal of Medicine; The Doctor's Letter of Condolence.2 See Table 1 for suggestions to help with bereavement communications.
| Handwritten on a card or stationary. |
| Timely (within 1‐2 weeks of death). |
| Use sensitive, caring language and avoid clich. |
| Acknowledge the family's grief and loss. |
| Acknowledge the patient's courage or other qualities. |
| Mention the privilege it has been to work with the patient. |
| Mention your appreciation of the family's caring. |
| Avoid sincerely yours and end on a personal note such as suggesting your thoughts are with them at this most difficult time. |
We are the profession of Hospital Medicine. It is our knowledge, our hope, our compassion, our experience and judgment that often directs care at the end‐of‐life for many of our patients. We are teammates along with the primary care provider, subspecialty consultants, palliative care specialists and other members of the care team. We have a professional obligation to extend a thoughtful condolence to surviving family members and to contact other members of the care team so that they too may have this opportunity. The responsibility for the final closure rests with us and within this responsibility is a powerful fulfillment of the promise of the practice of medicine.
The condolence note is a small kindness, a part of the art of medicine, a part of our humanness and essential to our vision of patient‐centered hospital care.
On August 6, 2009, my vigorously healthy 59‐year‐old brother‐in‐law, a beloved husband and father of 2 sons, developed mild right hand clumsiness and slight slurring of speech. This led to a primary care visit and the symptoms were originally felt to be related to working long hours and stress. The symptoms failed to improve and on August 11th, an magnetic resonance imaging (MRI) made a shocking discovery, my brother‐in‐law had a brain tumor. He was seen in the Neurosurgery department of a major academic center the following day and on August 13th he underwent resection of the majority of the tumor with a pathologic finding of grade IV glioblastoma multiforme (GBM).
During this initial admission, a consultation was obtained from a neuro‐oncologist who would then become the principle director of his care. My brother‐in‐law was crystal clear with his physicians; he wanted honest information about what to expect. From the outset he understood this was an incurable disease, but hoped that with aggressive treatment, he could live for months, possibly even years. He started chemotherapy almost immediately and in early October started a 6‐week course of daily radiation. The early weeks went relatively well. He saw his oncologist regularly and stayed in close contact with his oncology nurse. With his engineering background and attention to details, he followed his physician's instructions to the letter; however, despite his very best efforts at compliance and working intensively with physical therapy he was becoming progressively weaker.
In early November, a follow‐up MRI appeared to show progression of tumor and on November 12th, a second resection was undertaken. Pathologically, this seemed successful with removal of tumor bulk, but he was left even weaker, particularly on the right side. His symptoms were managed with dexamethasone; however, aphasia and hemiparesis would always reemerge with attempts to taper the drug and his functional status was too poor to allow for further chemotherapy. As his communication ability was becoming more limited, my sister‐in‐law was increasingly becoming his spokesperson at doctor visits and with phone updates to his oncology nurse.
On January 21, 2010 after working with a home physical therapist, he was making a transfer and became nonresponsive. Paramedics were called, arriving within minutes, but he was found pulseless. Despite a heroic resuscitative effort, he was pronounced dead at a nearby hospital a short time thereafter. A postmortem was not performed and the presumptive cause of death was pulmonary embolism.
His funeral was January 29th.
My brother‐in‐law and I lived 1600 miles apart and saw each other on only the rarest occasions. When the diagnosis was made, my role changed as I am the only relative within the extended family with medical expertise. Questions were directed to me via e‐mails and I did my best using UpToDate and other resources to learn about GBM and relay this information back to the family.
Recently, and in a vicarious way, I was becoming more and more deeply involved. Using the best descriptions of functional status that I could extract from e‐mail and phone calls, I estimated his Karnofsky Performance Score as being fairly poor. By way of my e‐mails to him and his wife, I was just beginning, ever so gently, to touch on the subject of hospice care at the time of his sudden death.
At the visitation and reception following the funeral, I think I was seen as more than a distant brother‐in‐law; I was also seen as a surrogate for the medical profession and for his doctors in particular. One message that I got loud and clear, from more than 1 family member was the devastated, abandoned feeling that was emerging in the 8 days since his death. On the morning following his death, my sister‐in‐law called his physician's office and told his oncology nurse that her husband had died suddenly and unexpectedly the night before. The nurse expressed sympathy and indicated she would relay this information to his doctor and she would personally call back. As of the time I left to return home, that was the last communication any family member had received from anyone involved in his care.
Although the outpouring of community support and sympathy was powerful and touching, not a single condolence card or phone call came from his doctor. I was shocked at the suddenness of his death, but I was also shocked at the complete absence of any communication, any acknowledgement of his courageous struggle against a terrible illness, or of his family's depth of caring and love over these last few months.
I am reminded of an essay by Gregory Kane, MD in CHEST,1 in which he describes a disturbing personal encounter with the following;
In a personal and memorable patient encounter, I sat and listened while a tearful patient cried at having received no contact from the physician who treated her husband for metastatic lung cancer for a treatment duration of 9 months. As I struggled to comprehend her sense of pain and abandonment, I considered offering as possible explanation that the physician may not have been on call at the time of the death and may have mistakenly believed that his partner had offered such a gesture verbally. Before I could respond, however, my patient added that her veterinarian had sent a card when the family dog died. I was speechless.1
As Hospitalists, we are gifted and privileged to work closely with patients and their loved ones struggling with the existential and eternal questions of life and death. As we can all well attest from 6 PM family meetings, the unit‐of‐care extends beyond the patient and certainly includes the loving and caring members of the patient's family and close support system. If we fail to acknowledge a family's bereavement, we run the risk of unintentionally communicating the message that the patient was not important, that their suffering did not matter or that the crushing grief the family may be bearing is somehow insignificant compared to our busy schedules.
I have asked you to join me on this short journey my brother‐in‐law has taken these last few months in the interest of raising awareness. There are many occasions when a verbal, bedside expression of condolence is very appropriate and completely adequate. There are other times when a condolence letter will better facilitate the closure of the physician‐patient‐family relationship. This may be intimidating, both the extra work involved and especially the challenge of not knowing what to say. The article quoted above by Dr. Kane is an excellent resource for guidance concerning content, style, and other writing considerations. Using examples, such as a letter from Abraham Lincoln to a girl whose father died in the Civil War, he shows that this essential communication does not have to be lengthy or difficult. Another excellent resource can be found in the New England Journal of Medicine; The Doctor's Letter of Condolence.2 See Table 1 for suggestions to help with bereavement communications.
| Handwritten on a card or stationary. |
| Timely (within 1‐2 weeks of death). |
| Use sensitive, caring language and avoid clich. |
| Acknowledge the family's grief and loss. |
| Acknowledge the patient's courage or other qualities. |
| Mention the privilege it has been to work with the patient. |
| Mention your appreciation of the family's caring. |
| Avoid sincerely yours and end on a personal note such as suggesting your thoughts are with them at this most difficult time. |
We are the profession of Hospital Medicine. It is our knowledge, our hope, our compassion, our experience and judgment that often directs care at the end‐of‐life for many of our patients. We are teammates along with the primary care provider, subspecialty consultants, palliative care specialists and other members of the care team. We have a professional obligation to extend a thoughtful condolence to surviving family members and to contact other members of the care team so that they too may have this opportunity. The responsibility for the final closure rests with us and within this responsibility is a powerful fulfillment of the promise of the practice of medicine.
The condolence note is a small kindness, a part of the art of medicine, a part of our humanness and essential to our vision of patient‐centered hospital care.
- .A Dying Art?: The Doctor's Letter of Condolence.Chest.2007;131(4):1245–1247. Permission for use obtained by direct communication with the author on January 31, 2010.
- ,,.The doctor's letter of condolence.N Engl J Med.2001;344(15):1162–1163.
- .A Dying Art?: The Doctor's Letter of Condolence.Chest.2007;131(4):1245–1247. Permission for use obtained by direct communication with the author on January 31, 2010.
- ,,.The doctor's letter of condolence.N Engl J Med.2001;344(15):1162–1163.
CER and Hospital Medicine
The topic of comparative effectiveness research (CER) has recently gained prominence within the context of the national focus on health reform. This article provides a brief overview and history of CER, and discusses the implications of CER for hospitalists in each of four major career roles: research, clinical practice, education and training, and hospital leadership. Both medical journals and lay media have produced a flurry of articles recently on a variety of health reform subjects. One topic that has achieved prominence within this growing body of literature is comparative effectiveness research (CER). For many hospitalists, this particular brand of research may be unfamiliar. As discussions about CER priorities, the controversy surrounding CER, and even the definition of CER gain visibility, hospitalists may be left wondering, What exactly is CER and what does it mean for me?
Until recently no common definition for CER existed, and the very concept was identified only in relatively narrow policy and research circles. However, CER is not a new idea. Its ancestor is the notion of medical technology assessment (MTA), which garnered enthusiasm and support in the 1970s. In 1978, Congress established the National Center for Health Technology Assessment (which, over time, evolved into the Agency for Healthcare Research and Quality [AHRQ]), whose charge was to coordinate efforts within the government to assess the safety, efficacy, effectiveness, and cost‐effectiveness of medical technologies. The recognition of a need for technology assessment at that time is mirrored by the widespread interest in CER seen today. Part of the reason that MTA did not take hold is that then, as now, this type of evaluation is challenging and time consuming, requiring large, well‐designed effectiveness studies. These studies require rigorous methods, typically long‐term follow‐up, and acceptance via editors and the medical literature that effectiveness is as important as efficacy demonstrated in a randomized trial. With the spread of antiregulatory sentiment and the lack of an economic imperative to reduce costs, the national focus on technology assessment waned. The current economic crisis has refocused the government and private sector on the soaring cost of health care and the need to improve quality, and the stimulus package passed in February of 2009 placed CER once again in the forefront. The American Recovery and Reinvestment Act (ARRA) of 2009 allocated $1.1 billion for CER.1 On June 30, 2009, 2 reports delineating the strategy and priorities for CER were released. The report from the ARRA‐mandated Federal Coordinating Council (FCC) for CER includes a broad definition of CER and outlines a high‐level strategic framework for priorities and investments in CER.2 Simultaneously, the report from the Institute of Medicine (IOM) lists 100 priority research topics, and gives 10 general recommendations for the CER enterprise going forward.3
So what is CER and why is it important? How is it different from standard research that hospitalists use every day to inform their clinical decision‐making? Unfortunately, patients and providers confront medical decisions daily that are not evidence based. All too frequently it is unclear what therapeutic option works best for which patient under which circumstances. For example, what is the best inpatient diabetes management strategy for an African American woman with multiple medical problems? What is the best discharge process for an elderly man with heart disease in order to prevent readmission? CER seeks to fill the gaps in evidence needed by patients and clinicians in order to make appropriate medical decisions. It differs from standard efficacy research in that it compares interventions or management strategies in real world settings, allows identification of effectiveness in patient subgroups, and is more patient‐centered, focusing on the decisions confronting patients and their physicians. The following definition of CER was developed by the FCC for CER:
CER is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in real world settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence‐based information to patients, clinicians, and other decision‐makers, responding to their expressed needs about which interventions are most effective for which patients under specific circumstances.
-
To provide this information, CER must assess a comprehensive array of health‐related outcomes for diverse patient populations and sub‐groups.
-
Defined interventions compared may include medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies.
-
This research necessitates the development, expansion and use of a variety of data sources and methods to assess comparative effectiveness and actively disseminate the results.
While CER is an evolving field requiring continued methodological development (such as enhancement of methods for practical, or pragmatic trials and complex analyses of large, linked databases), examples of rigorous comparative studies do exist. The Veterans Administration (VA) COURAGE trial compared optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) for patients with stable coronary disease, finding that PCI did not reduce the risk of death or cardiovascular events compared to OMT alone.4 Another example is the Diabetes Prevention Program which compared placebo, metformin, and a lifestyle modification program to prevent or delay the onset of type 2 diabetes. This study famously showed that lifestyle modification was more effective than metformin or placebo in reducing the incidence of diabetes.5
CER holds the promise of significantly improving the health of Americans through the ability to target treatments and other interventions to individual patients. As noted by the FCC, CER can allow for the delivery of the right treatment to the right patient at the right time2 even as the field continues to evolve. To quote Fineberg and Hiatt6 in describing technology assessment in 1979, we cannot expect CER to lead to perfect decisions, but we can expect even imperfect methods to facilitate better informed decisions than would otherwise be possible.
CER has important implications for hospitalists in all roles and settings. As the field of hospital medicine has grown, hospitalists have increasingly assumed more responsibilities than just patient care. In academic and community hospitals, hospitalists take on leadership roles, particularly in quality improvement (QI) and patient safety, and educational roles in the training of housestaff, medical students, and physician extenders. The last several years have also seen a significant increase in hospitalists participating in research. The relevance of CER to each of these 4 major activities is described below and in the accompanying Table 1.
| Primary Role | Potential Implications of CER |
|---|---|
| |
| Research | New availability of funds for hospital‐based CER |
| Enhanced data infrastructure to conduct CER | |
| Opportunity to apply CER to issues unique to hospital medicine | |
| Opportunity to develop methodologic skills | |
| Clinical practice | End users of CER evidence |
| Responsibility for translation of CER into practice | |
| Targets of Federal and non‐Federal dissemination efforts | |
| Education and training | Development of a workforce to conduct hospital‐based CER |
| Responsibility for teaching physician and nonphysician trainees about CER concepts and review of CER literature | |
| Hospital leadership | Direct hospital‐wide efforts to implement emerging CER evidence into practice through a multidisciplinary approach |
| Education and empowerment of clinician and nonclinician staff to translate CER information into practice | |
Hospitalists and Research
Many comparative effectiveness questions about clinical care, processes of care, and quality of care within the inpatient setting are in need of answers. Hospitalist researchers have the opportunity to make a significant impact on care by pursuing answers to questions that are unique to the field of hospital medicine. With the new availability of funds for CER, now is the time to address many of these questions head‐on. For example, there is a lack of evidence about best practices for a large number of inpatient acute conditions. What is the best strategy to manage acute hospital delirium in an elderly patient? What is the best approach to treating acute pain in an elderly woman on multiple medications? Overwhelmingly the patients that hospitalists care for are elderly and/or have multiple chronic conditions, including children with special health care needs. Many are from racial or ethnic minority backgrounds. These subgroups of patients have been historically under‐represented in clinical trials, yet represent exactly the priority populations that the Federal CER effort targets. The field of hospital medicine can be transformed with a substantial investment in research to address common inpatient clinical conditions in real world settings focused on the kinds of patients hospitalists actually care for.
One of the most vexing and frustrating care delivery issues for hospitalists, clinicians and researchers alike, is the discharge process. This problem received increased attention after a recent article highlighted the high rate of readmissions in the Medicare population.7 Research on the discharge process has grown substantially in recent years, and has become an area of intense focus and attention for hospitalists, nurses, researchers, hospital administrators and policymakers. Without question, hospitalists are uniquely poised to conduct research on this critically important topic, and CER is an ideal vehicle for moving this field forward. In collaboration with nurses, primary care physicians, pharmacists, case managers and others, hospitalists should take advantage of the Federal investment in studying care delivery systems interventions, and develop innovative methods and strategies for studying and improving this crucial transition in care. CER is also applicable to other care transitions, including the admission process, transitions within the hospital, and discharge to nursing facilities. Other examples of comparative effectiveness topics that hospitalist researchers are particularly suited for include comparing methods for implementing inpatient treatment protocols or clinical pathways, comparison of health information technology (IT) systems to reduce medical error, and QI approaches.
What are the methodologies that hospitalists should use to conduct CER? While randomized pragmatic real world trials are appealing, this method may not always be practical. Other methodologies are available for rigorous use, including cohort studies, comparative QI interventions, clustered and factorial design, systematic reviews, and analysis of registries, administrative claims, or other databases. Databases currently available for analysis on priority populations and subgroups are limited, and include the VA and Medicare databases. To address this need, one of the primary Federal investments in CER is for the enhancement and expansion of data infrastructure. Data infrastructure tools that are likely to be available to hospitalist researchers for CER include expanded longitudinal administrative claims databases with linkages to electronic health records (EHRs), expanded patient registries with linkages to other forms of data, and distributed data networks that are populated by EHRs in provider and practice settings. Hospitalist researchers should take advantage of these resources as they become available, as they have tremendous potential to inform decision‐making for providers and patients alike.
Hospitalists and Clinical Practice
As with all providers, hospitalists will be end‐users of CER evidence, and will have the responsibility of translating new knowledge into practice. This process will not be easy. How are hospitalists to reliably access and incorporate new comparative effectiveness information into their daily practice? How should they deal with some of the potential unintended consequences of CER, such as information overload or conflicting evidence? While hospitalists have a professional responsibility to search for and apply CER findings, the future development of CER‐based practice guidelines will encourage evidence translation. The development of a common platform for the dissemination of CER relevant to hospitalists would significantly enhance the uptake of new evidence by practicing hospitalists and other hospital‐based providers such as physician assistants or nurse practitioners. Medical societies such as the Society of Hospital Medicine and the American Academy of Pediatrics should consider developing committees for CER and leading coordinated educational efforts specifically focused on CER results through publications and presentations at local, regional, and national meetings. In addition, other dissemination tools for CER will soon emerge and existing tools will be enhanced, such as the Effective Health Care Program and Eisenberg Center housed at the AHRQ. The coming years will see an expansion of these and other dissemination efforts to both providers and patients, and hospitalists must be vigilant about accessing these resources and integrating comparative effectiveness evidence into practice. As Federal dissemination efforts to consumers spread, patients will increasingly expect physicians to discuss comparative effectiveness evidence in describing options for their individual health needs. Finally, a key lever for translating CER into practice will be payment models that place accountability for performance on physicians and hospitals, with a significant proportion of payment based on the delivery of high quality, efficient care.
Education and Training
Investment in the training and development of a skilled workforce to conduct CER is an important priority. Hospitalist researchers should take advantage of education and training programs to support the development of methodologies and skills for conducting CER that will become available. These programs will enable hospitalists to learn such skills as the use of the newly enhanced data infrastructure discussed above. The national investment in human and scientific capital for CER can promote the training of a corps of hospitalist researchers focused on this research which, in turn, could support the growth of the academic hospitalist field. Hospitalists who have responsibilities in medical education and residency training programs should take the lead in teaching CER concepts that are relevant to inpatient care. They will need to train the next generation of medical students and residents to read and understand comparative effectiveness literature and its application in clinical practice. Hospitalist educators are also best positioned to teach medical trainees comparative effectiveness evidence about inpatient QI methods and care processes.
Hospital Leadership
As front‐line providers and team leaders, hospitalists are well placed to direct the efforts within their hospitals to implement new CER evidence. For example, suppose new comparative effectiveness evidence about best practices for the discharge process for community‐dwelling older adults with multiple chronic conditions were to emerge. Hospitalists could lead efforts within their hospital to establish a multidisciplinary team to address this development, create standard protocols for implementing the new discharge process that align with their hospital's unique systems and organizational structure, advocate for necessary resources for the team to accomplish the goal of safely discharging these patients, ensure a method to track outcomes such as readmissions once the new discharge process is implemented, and provide data feedback to the team, hospital staff, and administrative leadership of the hospital. All of these activities should include a variety of disciplines working together, but as physician leaders, hospitalists can take the initiative to spearhead these endeavors. The inpatient setting is one that requires teamwork and coordination, and as team leaders, hospitalists can strongly influence the spread and adoption of CER results. Similarly, hospitalists are in a position to affect this dissemination and translation process by actively educating and empowering other clinicians and hospital staff within their local environment. Finally, as hospitalists increasingly take on leadership roles in QI departments and as chief medical officers within both community and university‐affiliated hospitals8, they are in a unique position to lead efforts to implement CER‐based QI activities. These may range from the implementation of IT functions to reduce medical error to strategies to reduce hospital‐acquired infections or falls.
Conclusion
As a result of the stimulus funds directed towards CER, the coming years will see a vast increase in the generation of comparative effectiveness evidence and the application of that evidence into practice.9 The national CER endeavor is particularly germane to the field of hospital medicine, as uncertainty about best practices is common, and the patients hospitalists serve represent priority populations for CER investments. Hospitalists can play a central role in both generating CER and implementing its findings in settings in which patients are highly vulnerable, and existing information is insufficient. In addition to clinical questions, hospitalist researchers are particularly suited to answering important questions about quality of care and inpatient processes such as transitions of care and care coordination. Having evidence on the best practices for care transitions or strategies to reduce medical error, for example, could have a significant impact on patient outcomes, quality of life, and cost of care. However, none of this new evidence will be of any value if it is not used by front‐line providers.10 Practicing hospitalists should lead efforts within their hospital to disseminate new CER findings to their hospitalist and non‐hospitalist colleagues, and to leverage their position as hospital and team leaders to implement inpatient‐based CER findings. All of these combined efforts have the potential to significantly move the field of hospital medicine forward, with the end result being improved health and better outcomes for patients.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills356:1503–1516.
- ,,, et al.Reduction in the Incidence of Type 2 diabetes with lifestyle intervention or metformin.N Engl J Med.2002;346:393–403.
- ,,Evaluation of medical practices: the case for technology assessment.N Engl J Med.1979;301:1086–1091.
- ,,,Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360:1418–1428.
- 2005–2006 Society of Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2361:328–330.
- ,,Transformation of health care at the front line.JAMA.2009;301:763–765.
The topic of comparative effectiveness research (CER) has recently gained prominence within the context of the national focus on health reform. This article provides a brief overview and history of CER, and discusses the implications of CER for hospitalists in each of four major career roles: research, clinical practice, education and training, and hospital leadership. Both medical journals and lay media have produced a flurry of articles recently on a variety of health reform subjects. One topic that has achieved prominence within this growing body of literature is comparative effectiveness research (CER). For many hospitalists, this particular brand of research may be unfamiliar. As discussions about CER priorities, the controversy surrounding CER, and even the definition of CER gain visibility, hospitalists may be left wondering, What exactly is CER and what does it mean for me?
Until recently no common definition for CER existed, and the very concept was identified only in relatively narrow policy and research circles. However, CER is not a new idea. Its ancestor is the notion of medical technology assessment (MTA), which garnered enthusiasm and support in the 1970s. In 1978, Congress established the National Center for Health Technology Assessment (which, over time, evolved into the Agency for Healthcare Research and Quality [AHRQ]), whose charge was to coordinate efforts within the government to assess the safety, efficacy, effectiveness, and cost‐effectiveness of medical technologies. The recognition of a need for technology assessment at that time is mirrored by the widespread interest in CER seen today. Part of the reason that MTA did not take hold is that then, as now, this type of evaluation is challenging and time consuming, requiring large, well‐designed effectiveness studies. These studies require rigorous methods, typically long‐term follow‐up, and acceptance via editors and the medical literature that effectiveness is as important as efficacy demonstrated in a randomized trial. With the spread of antiregulatory sentiment and the lack of an economic imperative to reduce costs, the national focus on technology assessment waned. The current economic crisis has refocused the government and private sector on the soaring cost of health care and the need to improve quality, and the stimulus package passed in February of 2009 placed CER once again in the forefront. The American Recovery and Reinvestment Act (ARRA) of 2009 allocated $1.1 billion for CER.1 On June 30, 2009, 2 reports delineating the strategy and priorities for CER were released. The report from the ARRA‐mandated Federal Coordinating Council (FCC) for CER includes a broad definition of CER and outlines a high‐level strategic framework for priorities and investments in CER.2 Simultaneously, the report from the Institute of Medicine (IOM) lists 100 priority research topics, and gives 10 general recommendations for the CER enterprise going forward.3
So what is CER and why is it important? How is it different from standard research that hospitalists use every day to inform their clinical decision‐making? Unfortunately, patients and providers confront medical decisions daily that are not evidence based. All too frequently it is unclear what therapeutic option works best for which patient under which circumstances. For example, what is the best inpatient diabetes management strategy for an African American woman with multiple medical problems? What is the best discharge process for an elderly man with heart disease in order to prevent readmission? CER seeks to fill the gaps in evidence needed by patients and clinicians in order to make appropriate medical decisions. It differs from standard efficacy research in that it compares interventions or management strategies in real world settings, allows identification of effectiveness in patient subgroups, and is more patient‐centered, focusing on the decisions confronting patients and their physicians. The following definition of CER was developed by the FCC for CER:
CER is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in real world settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence‐based information to patients, clinicians, and other decision‐makers, responding to their expressed needs about which interventions are most effective for which patients under specific circumstances.
-
To provide this information, CER must assess a comprehensive array of health‐related outcomes for diverse patient populations and sub‐groups.
-
Defined interventions compared may include medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies.
-
This research necessitates the development, expansion and use of a variety of data sources and methods to assess comparative effectiveness and actively disseminate the results.
While CER is an evolving field requiring continued methodological development (such as enhancement of methods for practical, or pragmatic trials and complex analyses of large, linked databases), examples of rigorous comparative studies do exist. The Veterans Administration (VA) COURAGE trial compared optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) for patients with stable coronary disease, finding that PCI did not reduce the risk of death or cardiovascular events compared to OMT alone.4 Another example is the Diabetes Prevention Program which compared placebo, metformin, and a lifestyle modification program to prevent or delay the onset of type 2 diabetes. This study famously showed that lifestyle modification was more effective than metformin or placebo in reducing the incidence of diabetes.5
CER holds the promise of significantly improving the health of Americans through the ability to target treatments and other interventions to individual patients. As noted by the FCC, CER can allow for the delivery of the right treatment to the right patient at the right time2 even as the field continues to evolve. To quote Fineberg and Hiatt6 in describing technology assessment in 1979, we cannot expect CER to lead to perfect decisions, but we can expect even imperfect methods to facilitate better informed decisions than would otherwise be possible.
CER has important implications for hospitalists in all roles and settings. As the field of hospital medicine has grown, hospitalists have increasingly assumed more responsibilities than just patient care. In academic and community hospitals, hospitalists take on leadership roles, particularly in quality improvement (QI) and patient safety, and educational roles in the training of housestaff, medical students, and physician extenders. The last several years have also seen a significant increase in hospitalists participating in research. The relevance of CER to each of these 4 major activities is described below and in the accompanying Table 1.
| Primary Role | Potential Implications of CER |
|---|---|
| |
| Research | New availability of funds for hospital‐based CER |
| Enhanced data infrastructure to conduct CER | |
| Opportunity to apply CER to issues unique to hospital medicine | |
| Opportunity to develop methodologic skills | |
| Clinical practice | End users of CER evidence |
| Responsibility for translation of CER into practice | |
| Targets of Federal and non‐Federal dissemination efforts | |
| Education and training | Development of a workforce to conduct hospital‐based CER |
| Responsibility for teaching physician and nonphysician trainees about CER concepts and review of CER literature | |
| Hospital leadership | Direct hospital‐wide efforts to implement emerging CER evidence into practice through a multidisciplinary approach |
| Education and empowerment of clinician and nonclinician staff to translate CER information into practice | |
Hospitalists and Research
Many comparative effectiveness questions about clinical care, processes of care, and quality of care within the inpatient setting are in need of answers. Hospitalist researchers have the opportunity to make a significant impact on care by pursuing answers to questions that are unique to the field of hospital medicine. With the new availability of funds for CER, now is the time to address many of these questions head‐on. For example, there is a lack of evidence about best practices for a large number of inpatient acute conditions. What is the best strategy to manage acute hospital delirium in an elderly patient? What is the best approach to treating acute pain in an elderly woman on multiple medications? Overwhelmingly the patients that hospitalists care for are elderly and/or have multiple chronic conditions, including children with special health care needs. Many are from racial or ethnic minority backgrounds. These subgroups of patients have been historically under‐represented in clinical trials, yet represent exactly the priority populations that the Federal CER effort targets. The field of hospital medicine can be transformed with a substantial investment in research to address common inpatient clinical conditions in real world settings focused on the kinds of patients hospitalists actually care for.
One of the most vexing and frustrating care delivery issues for hospitalists, clinicians and researchers alike, is the discharge process. This problem received increased attention after a recent article highlighted the high rate of readmissions in the Medicare population.7 Research on the discharge process has grown substantially in recent years, and has become an area of intense focus and attention for hospitalists, nurses, researchers, hospital administrators and policymakers. Without question, hospitalists are uniquely poised to conduct research on this critically important topic, and CER is an ideal vehicle for moving this field forward. In collaboration with nurses, primary care physicians, pharmacists, case managers and others, hospitalists should take advantage of the Federal investment in studying care delivery systems interventions, and develop innovative methods and strategies for studying and improving this crucial transition in care. CER is also applicable to other care transitions, including the admission process, transitions within the hospital, and discharge to nursing facilities. Other examples of comparative effectiveness topics that hospitalist researchers are particularly suited for include comparing methods for implementing inpatient treatment protocols or clinical pathways, comparison of health information technology (IT) systems to reduce medical error, and QI approaches.
What are the methodologies that hospitalists should use to conduct CER? While randomized pragmatic real world trials are appealing, this method may not always be practical. Other methodologies are available for rigorous use, including cohort studies, comparative QI interventions, clustered and factorial design, systematic reviews, and analysis of registries, administrative claims, or other databases. Databases currently available for analysis on priority populations and subgroups are limited, and include the VA and Medicare databases. To address this need, one of the primary Federal investments in CER is for the enhancement and expansion of data infrastructure. Data infrastructure tools that are likely to be available to hospitalist researchers for CER include expanded longitudinal administrative claims databases with linkages to electronic health records (EHRs), expanded patient registries with linkages to other forms of data, and distributed data networks that are populated by EHRs in provider and practice settings. Hospitalist researchers should take advantage of these resources as they become available, as they have tremendous potential to inform decision‐making for providers and patients alike.
Hospitalists and Clinical Practice
As with all providers, hospitalists will be end‐users of CER evidence, and will have the responsibility of translating new knowledge into practice. This process will not be easy. How are hospitalists to reliably access and incorporate new comparative effectiveness information into their daily practice? How should they deal with some of the potential unintended consequences of CER, such as information overload or conflicting evidence? While hospitalists have a professional responsibility to search for and apply CER findings, the future development of CER‐based practice guidelines will encourage evidence translation. The development of a common platform for the dissemination of CER relevant to hospitalists would significantly enhance the uptake of new evidence by practicing hospitalists and other hospital‐based providers such as physician assistants or nurse practitioners. Medical societies such as the Society of Hospital Medicine and the American Academy of Pediatrics should consider developing committees for CER and leading coordinated educational efforts specifically focused on CER results through publications and presentations at local, regional, and national meetings. In addition, other dissemination tools for CER will soon emerge and existing tools will be enhanced, such as the Effective Health Care Program and Eisenberg Center housed at the AHRQ. The coming years will see an expansion of these and other dissemination efforts to both providers and patients, and hospitalists must be vigilant about accessing these resources and integrating comparative effectiveness evidence into practice. As Federal dissemination efforts to consumers spread, patients will increasingly expect physicians to discuss comparative effectiveness evidence in describing options for their individual health needs. Finally, a key lever for translating CER into practice will be payment models that place accountability for performance on physicians and hospitals, with a significant proportion of payment based on the delivery of high quality, efficient care.
Education and Training
Investment in the training and development of a skilled workforce to conduct CER is an important priority. Hospitalist researchers should take advantage of education and training programs to support the development of methodologies and skills for conducting CER that will become available. These programs will enable hospitalists to learn such skills as the use of the newly enhanced data infrastructure discussed above. The national investment in human and scientific capital for CER can promote the training of a corps of hospitalist researchers focused on this research which, in turn, could support the growth of the academic hospitalist field. Hospitalists who have responsibilities in medical education and residency training programs should take the lead in teaching CER concepts that are relevant to inpatient care. They will need to train the next generation of medical students and residents to read and understand comparative effectiveness literature and its application in clinical practice. Hospitalist educators are also best positioned to teach medical trainees comparative effectiveness evidence about inpatient QI methods and care processes.
Hospital Leadership
As front‐line providers and team leaders, hospitalists are well placed to direct the efforts within their hospitals to implement new CER evidence. For example, suppose new comparative effectiveness evidence about best practices for the discharge process for community‐dwelling older adults with multiple chronic conditions were to emerge. Hospitalists could lead efforts within their hospital to establish a multidisciplinary team to address this development, create standard protocols for implementing the new discharge process that align with their hospital's unique systems and organizational structure, advocate for necessary resources for the team to accomplish the goal of safely discharging these patients, ensure a method to track outcomes such as readmissions once the new discharge process is implemented, and provide data feedback to the team, hospital staff, and administrative leadership of the hospital. All of these activities should include a variety of disciplines working together, but as physician leaders, hospitalists can take the initiative to spearhead these endeavors. The inpatient setting is one that requires teamwork and coordination, and as team leaders, hospitalists can strongly influence the spread and adoption of CER results. Similarly, hospitalists are in a position to affect this dissemination and translation process by actively educating and empowering other clinicians and hospital staff within their local environment. Finally, as hospitalists increasingly take on leadership roles in QI departments and as chief medical officers within both community and university‐affiliated hospitals8, they are in a unique position to lead efforts to implement CER‐based QI activities. These may range from the implementation of IT functions to reduce medical error to strategies to reduce hospital‐acquired infections or falls.
Conclusion
As a result of the stimulus funds directed towards CER, the coming years will see a vast increase in the generation of comparative effectiveness evidence and the application of that evidence into practice.9 The national CER endeavor is particularly germane to the field of hospital medicine, as uncertainty about best practices is common, and the patients hospitalists serve represent priority populations for CER investments. Hospitalists can play a central role in both generating CER and implementing its findings in settings in which patients are highly vulnerable, and existing information is insufficient. In addition to clinical questions, hospitalist researchers are particularly suited to answering important questions about quality of care and inpatient processes such as transitions of care and care coordination. Having evidence on the best practices for care transitions or strategies to reduce medical error, for example, could have a significant impact on patient outcomes, quality of life, and cost of care. However, none of this new evidence will be of any value if it is not used by front‐line providers.10 Practicing hospitalists should lead efforts within their hospital to disseminate new CER findings to their hospitalist and non‐hospitalist colleagues, and to leverage their position as hospital and team leaders to implement inpatient‐based CER findings. All of these combined efforts have the potential to significantly move the field of hospital medicine forward, with the end result being improved health and better outcomes for patients.
The topic of comparative effectiveness research (CER) has recently gained prominence within the context of the national focus on health reform. This article provides a brief overview and history of CER, and discusses the implications of CER for hospitalists in each of four major career roles: research, clinical practice, education and training, and hospital leadership. Both medical journals and lay media have produced a flurry of articles recently on a variety of health reform subjects. One topic that has achieved prominence within this growing body of literature is comparative effectiveness research (CER). For many hospitalists, this particular brand of research may be unfamiliar. As discussions about CER priorities, the controversy surrounding CER, and even the definition of CER gain visibility, hospitalists may be left wondering, What exactly is CER and what does it mean for me?
Until recently no common definition for CER existed, and the very concept was identified only in relatively narrow policy and research circles. However, CER is not a new idea. Its ancestor is the notion of medical technology assessment (MTA), which garnered enthusiasm and support in the 1970s. In 1978, Congress established the National Center for Health Technology Assessment (which, over time, evolved into the Agency for Healthcare Research and Quality [AHRQ]), whose charge was to coordinate efforts within the government to assess the safety, efficacy, effectiveness, and cost‐effectiveness of medical technologies. The recognition of a need for technology assessment at that time is mirrored by the widespread interest in CER seen today. Part of the reason that MTA did not take hold is that then, as now, this type of evaluation is challenging and time consuming, requiring large, well‐designed effectiveness studies. These studies require rigorous methods, typically long‐term follow‐up, and acceptance via editors and the medical literature that effectiveness is as important as efficacy demonstrated in a randomized trial. With the spread of antiregulatory sentiment and the lack of an economic imperative to reduce costs, the national focus on technology assessment waned. The current economic crisis has refocused the government and private sector on the soaring cost of health care and the need to improve quality, and the stimulus package passed in February of 2009 placed CER once again in the forefront. The American Recovery and Reinvestment Act (ARRA) of 2009 allocated $1.1 billion for CER.1 On June 30, 2009, 2 reports delineating the strategy and priorities for CER were released. The report from the ARRA‐mandated Federal Coordinating Council (FCC) for CER includes a broad definition of CER and outlines a high‐level strategic framework for priorities and investments in CER.2 Simultaneously, the report from the Institute of Medicine (IOM) lists 100 priority research topics, and gives 10 general recommendations for the CER enterprise going forward.3
So what is CER and why is it important? How is it different from standard research that hospitalists use every day to inform their clinical decision‐making? Unfortunately, patients and providers confront medical decisions daily that are not evidence based. All too frequently it is unclear what therapeutic option works best for which patient under which circumstances. For example, what is the best inpatient diabetes management strategy for an African American woman with multiple medical problems? What is the best discharge process for an elderly man with heart disease in order to prevent readmission? CER seeks to fill the gaps in evidence needed by patients and clinicians in order to make appropriate medical decisions. It differs from standard efficacy research in that it compares interventions or management strategies in real world settings, allows identification of effectiveness in patient subgroups, and is more patient‐centered, focusing on the decisions confronting patients and their physicians. The following definition of CER was developed by the FCC for CER:
CER is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in real world settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence‐based information to patients, clinicians, and other decision‐makers, responding to their expressed needs about which interventions are most effective for which patients under specific circumstances.
-
To provide this information, CER must assess a comprehensive array of health‐related outcomes for diverse patient populations and sub‐groups.
-
Defined interventions compared may include medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies.
-
This research necessitates the development, expansion and use of a variety of data sources and methods to assess comparative effectiveness and actively disseminate the results.
While CER is an evolving field requiring continued methodological development (such as enhancement of methods for practical, or pragmatic trials and complex analyses of large, linked databases), examples of rigorous comparative studies do exist. The Veterans Administration (VA) COURAGE trial compared optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) for patients with stable coronary disease, finding that PCI did not reduce the risk of death or cardiovascular events compared to OMT alone.4 Another example is the Diabetes Prevention Program which compared placebo, metformin, and a lifestyle modification program to prevent or delay the onset of type 2 diabetes. This study famously showed that lifestyle modification was more effective than metformin or placebo in reducing the incidence of diabetes.5
CER holds the promise of significantly improving the health of Americans through the ability to target treatments and other interventions to individual patients. As noted by the FCC, CER can allow for the delivery of the right treatment to the right patient at the right time2 even as the field continues to evolve. To quote Fineberg and Hiatt6 in describing technology assessment in 1979, we cannot expect CER to lead to perfect decisions, but we can expect even imperfect methods to facilitate better informed decisions than would otherwise be possible.
CER has important implications for hospitalists in all roles and settings. As the field of hospital medicine has grown, hospitalists have increasingly assumed more responsibilities than just patient care. In academic and community hospitals, hospitalists take on leadership roles, particularly in quality improvement (QI) and patient safety, and educational roles in the training of housestaff, medical students, and physician extenders. The last several years have also seen a significant increase in hospitalists participating in research. The relevance of CER to each of these 4 major activities is described below and in the accompanying Table 1.
| Primary Role | Potential Implications of CER |
|---|---|
| |
| Research | New availability of funds for hospital‐based CER |
| Enhanced data infrastructure to conduct CER | |
| Opportunity to apply CER to issues unique to hospital medicine | |
| Opportunity to develop methodologic skills | |
| Clinical practice | End users of CER evidence |
| Responsibility for translation of CER into practice | |
| Targets of Federal and non‐Federal dissemination efforts | |
| Education and training | Development of a workforce to conduct hospital‐based CER |
| Responsibility for teaching physician and nonphysician trainees about CER concepts and review of CER literature | |
| Hospital leadership | Direct hospital‐wide efforts to implement emerging CER evidence into practice through a multidisciplinary approach |
| Education and empowerment of clinician and nonclinician staff to translate CER information into practice | |
Hospitalists and Research
Many comparative effectiveness questions about clinical care, processes of care, and quality of care within the inpatient setting are in need of answers. Hospitalist researchers have the opportunity to make a significant impact on care by pursuing answers to questions that are unique to the field of hospital medicine. With the new availability of funds for CER, now is the time to address many of these questions head‐on. For example, there is a lack of evidence about best practices for a large number of inpatient acute conditions. What is the best strategy to manage acute hospital delirium in an elderly patient? What is the best approach to treating acute pain in an elderly woman on multiple medications? Overwhelmingly the patients that hospitalists care for are elderly and/or have multiple chronic conditions, including children with special health care needs. Many are from racial or ethnic minority backgrounds. These subgroups of patients have been historically under‐represented in clinical trials, yet represent exactly the priority populations that the Federal CER effort targets. The field of hospital medicine can be transformed with a substantial investment in research to address common inpatient clinical conditions in real world settings focused on the kinds of patients hospitalists actually care for.
One of the most vexing and frustrating care delivery issues for hospitalists, clinicians and researchers alike, is the discharge process. This problem received increased attention after a recent article highlighted the high rate of readmissions in the Medicare population.7 Research on the discharge process has grown substantially in recent years, and has become an area of intense focus and attention for hospitalists, nurses, researchers, hospital administrators and policymakers. Without question, hospitalists are uniquely poised to conduct research on this critically important topic, and CER is an ideal vehicle for moving this field forward. In collaboration with nurses, primary care physicians, pharmacists, case managers and others, hospitalists should take advantage of the Federal investment in studying care delivery systems interventions, and develop innovative methods and strategies for studying and improving this crucial transition in care. CER is also applicable to other care transitions, including the admission process, transitions within the hospital, and discharge to nursing facilities. Other examples of comparative effectiveness topics that hospitalist researchers are particularly suited for include comparing methods for implementing inpatient treatment protocols or clinical pathways, comparison of health information technology (IT) systems to reduce medical error, and QI approaches.
What are the methodologies that hospitalists should use to conduct CER? While randomized pragmatic real world trials are appealing, this method may not always be practical. Other methodologies are available for rigorous use, including cohort studies, comparative QI interventions, clustered and factorial design, systematic reviews, and analysis of registries, administrative claims, or other databases. Databases currently available for analysis on priority populations and subgroups are limited, and include the VA and Medicare databases. To address this need, one of the primary Federal investments in CER is for the enhancement and expansion of data infrastructure. Data infrastructure tools that are likely to be available to hospitalist researchers for CER include expanded longitudinal administrative claims databases with linkages to electronic health records (EHRs), expanded patient registries with linkages to other forms of data, and distributed data networks that are populated by EHRs in provider and practice settings. Hospitalist researchers should take advantage of these resources as they become available, as they have tremendous potential to inform decision‐making for providers and patients alike.
Hospitalists and Clinical Practice
As with all providers, hospitalists will be end‐users of CER evidence, and will have the responsibility of translating new knowledge into practice. This process will not be easy. How are hospitalists to reliably access and incorporate new comparative effectiveness information into their daily practice? How should they deal with some of the potential unintended consequences of CER, such as information overload or conflicting evidence? While hospitalists have a professional responsibility to search for and apply CER findings, the future development of CER‐based practice guidelines will encourage evidence translation. The development of a common platform for the dissemination of CER relevant to hospitalists would significantly enhance the uptake of new evidence by practicing hospitalists and other hospital‐based providers such as physician assistants or nurse practitioners. Medical societies such as the Society of Hospital Medicine and the American Academy of Pediatrics should consider developing committees for CER and leading coordinated educational efforts specifically focused on CER results through publications and presentations at local, regional, and national meetings. In addition, other dissemination tools for CER will soon emerge and existing tools will be enhanced, such as the Effective Health Care Program and Eisenberg Center housed at the AHRQ. The coming years will see an expansion of these and other dissemination efforts to both providers and patients, and hospitalists must be vigilant about accessing these resources and integrating comparative effectiveness evidence into practice. As Federal dissemination efforts to consumers spread, patients will increasingly expect physicians to discuss comparative effectiveness evidence in describing options for their individual health needs. Finally, a key lever for translating CER into practice will be payment models that place accountability for performance on physicians and hospitals, with a significant proportion of payment based on the delivery of high quality, efficient care.
Education and Training
Investment in the training and development of a skilled workforce to conduct CER is an important priority. Hospitalist researchers should take advantage of education and training programs to support the development of methodologies and skills for conducting CER that will become available. These programs will enable hospitalists to learn such skills as the use of the newly enhanced data infrastructure discussed above. The national investment in human and scientific capital for CER can promote the training of a corps of hospitalist researchers focused on this research which, in turn, could support the growth of the academic hospitalist field. Hospitalists who have responsibilities in medical education and residency training programs should take the lead in teaching CER concepts that are relevant to inpatient care. They will need to train the next generation of medical students and residents to read and understand comparative effectiveness literature and its application in clinical practice. Hospitalist educators are also best positioned to teach medical trainees comparative effectiveness evidence about inpatient QI methods and care processes.
Hospital Leadership
As front‐line providers and team leaders, hospitalists are well placed to direct the efforts within their hospitals to implement new CER evidence. For example, suppose new comparative effectiveness evidence about best practices for the discharge process for community‐dwelling older adults with multiple chronic conditions were to emerge. Hospitalists could lead efforts within their hospital to establish a multidisciplinary team to address this development, create standard protocols for implementing the new discharge process that align with their hospital's unique systems and organizational structure, advocate for necessary resources for the team to accomplish the goal of safely discharging these patients, ensure a method to track outcomes such as readmissions once the new discharge process is implemented, and provide data feedback to the team, hospital staff, and administrative leadership of the hospital. All of these activities should include a variety of disciplines working together, but as physician leaders, hospitalists can take the initiative to spearhead these endeavors. The inpatient setting is one that requires teamwork and coordination, and as team leaders, hospitalists can strongly influence the spread and adoption of CER results. Similarly, hospitalists are in a position to affect this dissemination and translation process by actively educating and empowering other clinicians and hospital staff within their local environment. Finally, as hospitalists increasingly take on leadership roles in QI departments and as chief medical officers within both community and university‐affiliated hospitals8, they are in a unique position to lead efforts to implement CER‐based QI activities. These may range from the implementation of IT functions to reduce medical error to strategies to reduce hospital‐acquired infections or falls.
Conclusion
As a result of the stimulus funds directed towards CER, the coming years will see a vast increase in the generation of comparative effectiveness evidence and the application of that evidence into practice.9 The national CER endeavor is particularly germane to the field of hospital medicine, as uncertainty about best practices is common, and the patients hospitalists serve represent priority populations for CER investments. Hospitalists can play a central role in both generating CER and implementing its findings in settings in which patients are highly vulnerable, and existing information is insufficient. In addition to clinical questions, hospitalist researchers are particularly suited to answering important questions about quality of care and inpatient processes such as transitions of care and care coordination. Having evidence on the best practices for care transitions or strategies to reduce medical error, for example, could have a significant impact on patient outcomes, quality of life, and cost of care. However, none of this new evidence will be of any value if it is not used by front‐line providers.10 Practicing hospitalists should lead efforts within their hospital to disseminate new CER findings to their hospitalist and non‐hospitalist colleagues, and to leverage their position as hospital and team leaders to implement inpatient‐based CER findings. All of these combined efforts have the potential to significantly move the field of hospital medicine forward, with the end result being improved health and better outcomes for patients.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills356:1503–1516.
- ,,, et al.Reduction in the Incidence of Type 2 diabetes with lifestyle intervention or metformin.N Engl J Med.2002;346:393–403.
- ,,Evaluation of medical practices: the case for technology assessment.N Engl J Med.1979;301:1086–1091.
- ,,,Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360:1418–1428.
- 2005–2006 Society of Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2361:328–330.
- ,,Transformation of health care at the front line.JAMA.2009;301:763–765.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills356:1503–1516.
- ,,, et al.Reduction in the Incidence of Type 2 diabetes with lifestyle intervention or metformin.N Engl J Med.2002;346:393–403.
- ,,Evaluation of medical practices: the case for technology assessment.N Engl J Med.1979;301:1086–1091.
- ,,,Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360:1418–1428.
- 2005–2006 Society of Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2361:328–330.
- ,,Transformation of health care at the front line.JAMA.2009;301:763–765.
Postcards from Our Students
During their junior medicine rotation, our students are asked to post to Blackboard (an online student forum) an anonymous essay about an issue of professionalism or ethics, either inspiring or troubling. In many ways, these vignettes are like postcards, written by visitors describing foreign cultures and norms. They represent a way for the students to debrief, but also provide an opportunity for us, as faculty, to reflect upon the way we practice and teach medicine. Many postingslike postcards from exotic or historic placesare inspiring stories of residents and faculty extending themselves for their patients. Unfortunately, unlike typical postcards, there are also essays that are troubling or provoking and challenge us to consider how we could improve the professional and ethical environment on our teams.
In order to begin a learning process with our faculty and housestaff, we have presented a number of these anonymous essays at both faculty and housestaff Department of Medicine conferences as well as our monthly hospital Ethics conference. The goal of these conferences was to gather as a moral community to reflect on our students' experience and consider ways in which our day to day practice as attendings could be informed by what they tell us. In addition, the junior medicine site directors have a session each quarter with their junior students to review some of the most significant issues brought up by their essays.
Practically, these vignettes and conferences serve three main purposes:
-
Raising Awareness: Many professional issues noted by our students occur under the radar. Attendings are often unaware of the issues of professionalism and/or ethics confronting our students and housestaff.
-
Exploring Attitudes: Some attending may underemphasize the importance of specific issues of professionalism and/or ethics. Open discussions at faculty or resident conferences create opportunities for individuals to reflect upon their own reactions and for the group to create a norm.
-
Sharing Skills: It is difficult to learn the practice of professionalism and ethics from a book. Skill in this area is gained primarily by experience. Conferences provide an excellent forum for seasoned physicians to share wisdom with less experienced physicians. In addition, important teaching points can be made: Students should not deliver bad news alone. Errors should be disclosed.
Following are 3 of the essays we presented, along with brief commentaries. At the end, we provide practical suggestions for individual attendings to improve the professional climate on their teams.
The Hospital Didn't Wait
Code. On 12, the surgical wards floor. Elise sprinted to the stairwell, dashed up to 12, and ran to the corner room as fast as she could. She could see the room before she got there. Instinctively, she started reviewing the steps she had memorized so many months ago. But when she finally arrived at the patient's bathroom, her thought process came to a jolting halt as she came upon the gruesome scene.
The 76‐year‐old patient had hanged himself with the cinching rope from his garment bag, and now dangled suspended from a high towel rack against the wall. Nurses from the floor started to file in, and without losing a beat Elise barked commands. Together they brought the man's body down to the floor, laid him on his back, and stripped off his hospital gown. Elise was in charge; deliberately but forcefully, she ordered a nurse to retrieve a defibrillator, and had another resident check for a pulse. There was none. Anesthesiology was here. Quickly and expertly, they shoved a plastic tube down his throat and began ventilation. The nurse placed on the electrodes between chest compressions then called to clear the body. Airway stepped back. The chest pumper stepped back. The body lurched forward as the defibrillator issued a long beep and discharged. Still no pulse. The cycle repeated.
Finally, Elise called a stop. Time of death, 19:37. By now there were about 20 people crammed in the patient room, all of whom had a separate role during the code. Some stayed behind, while the rest left to return to their interrupted work. The medical student didn't know what to think as he returned to the team room. His jaw was sorehad he been clenching it the whole time?and as he brought his hand up to rub his face, he saw that his knuckles were bloody. Somehow he had scraped them during the code. As he logged back into the computer to finish off his evening notes, he knew that he wouldn't have time to reflect until hours later when he returned home. Codes happened all the time. There was still work to be done in the hospital, and the hospital didn't wait.
The room had already been assigned to a patient waiting in the Emergency Department downstairs. That patient would be here in a few minutes. The hospital didn't wait.
When we presented this case in our conferences, there was universal agreement that such a traumatic event merits, even demands, team debriefing and processing. But in the real life aftermath of this traumatic event, the take‐home message for the medical student was that the hospital didn't wait for such discussions. We know this is not unique to our institution. In a study of 32 medical students who were asked to reflect on their most memorable patient death,1 debriefing sessions were rare and many students felt inadequately supported. While experienced clinicians may be accustomed to seeing patients die, students are new to the culture of the hospital, and have not had the chance to develop the defense mechanisms necessary to cope with this sort of experience. Angoff2 writes, As medical educators, we ought to ask our students how they are coping with long hours, fatigue, illness, suffering, and death. We ought to model and commend compassion and react to the deep feelings of our students in the same way we would teach them to react to the deep feelings of their patients.
I Told a Man Today That He Had Brain Cancer
The resident, intern, and I were huddled together in our team room when the report came back on the computer. New 3.5 2.3 1.7 cm contrast‐enhancing lesion seen anterior to genu of corpus callosum. Concerning for metastatic focus vs. lymphoma. Advise follow‐up. It wasn't unexpected but we had nevertheless been hoping for better.
The three of us went into his room and I was waiting to see how my resident would deliver the bad news, but she didn't. She simply said that we were continuing to do imaging studies and that a neurology team would be in touch. There were probably several reasons why she didn't tell him: not enough time, not her responsibility, or maybe she was just uncomfortable with it. Whatever the case, we left the room with my patient still oblivious to the awful mass now tangled in his head.
If my resident was taking a pass on this conversation, I knew it fell to me he needed to hear it from his primary team. I came back after rounds alone, sat down next to his bed, and told him that his MRI results had come back, and that I had unfortunate news.
I told him that the images showed that his lung cancer had spread to his brain.
I paused to give him a chance to let it sink in. He turned away and looked up at the ceiling.
Where is it? How big is it?
What now?
Reflecting on this case, our audiences were disturbed that a student would attempt this difficult conversation alone, while recognizing that the student clearly felt a sense of responsibility and desire to help his patient by sharing important information. We talked about how students may erroneously pick up a message that the team member who has spent the most time with a patient is the most obvious choice to have difficult conversations. We also noted that, unfortunately, sometimes students are directly asked by their team to shoulder this responsibility on their own. In this painful account, there is no mention of preparation, supervision, or support for the student before or after the encounter. The student perceived (rightly or wrongly) that the team leaders lacked comfort or skill to deliver the bad news, and stepped in. It is possible that the attending lacked the skill and ability to model an interaction, but more likely the deficit was in awareness and attitude. It is unlikely the attending knew that the student had this conversation alone. One of the major reasons we present these vignettes is to make attendings and housestaff more aware of issues that occur under their radar so that they can take preventative action. However, once the resident or attending found out that the student had this conversation alone, the student should be pulled aside for a 1:1 discussion. At the end of the day, the student should know that it was inappropriate to attempt this conversation alone
Rosenbaum3 reviewed a number of strategies to teach the skill of delivering bad news, from lecture and small group discussions to role play and standardized patients. When asked, students cited role‐modeling as the best way to learn how to deliver bad news.4 Observation of a veteran clinician provides a firm foundation for learning; but that is not enough. Unfortunately, we know from the literature (and our student vignettes suggest) that students and residents are unprepared to carry out these conversations properly, either because of misguided attitudes, lack of experience, or inadequate training.57 We conceptualize engaging in difficult conversations as a procedure, demanding a skill set. Mere observation of an expert executing this procedure is only a beginning. With any other skill, from successful completion of a lumbar puncture to initiating cardiopulmonary resuscitation (CPR), a student would never conclude that knowing the patient the best sufficiently credentials the student to undertake these procedures. We maintain that a difficult conversationbe it breaking bad news, discussing end‐of‐life care preferences, code status discussions, or prognosisis a clinical intervention, like any other procedure in medicine. If performed with skill and caution, it can bring about a stronger therapeutic relationship and increased support for the patient; if performed clumsily, it can lead to unintended adverse outcomes, including misunderstanding, mistrust, anxiety, and anger.
A Decimal Point Got Misplaced
On palliative care, I had a 90 year‐old man with end stage lung CA that presented to the ED with increasing SOB. The resident decided that giving him some morphine would be a good solution but was worried that too much would push him over the edge. He was thin; his O2 sats weren't that good After some discussion it was decided that 2.5 mg should be the starting amount. Unfortunately, when the note was written a decimal point got misplaced and he got 25 mg as a first dose. He ended up very sedated for most of the day but his breathing was ok.
The mistake was not discussed with the patient or the patient's family. While it did not cause any lasting harm, I wondered if telling the patient/patient's family that an error had been made would have been more ethically sound.
When we presented this case in our conferences, there was little controversy about whether the error should have been disclosed. The discussion did provide reinforcement for doing a simple but difficult task. Our analysis is that the nondiscussion of this error reflects a deficit in attitude and possibly skill. The team was aware of the error, but the resident and attending did not take the opportunity to disclose an error. They should have. We do not know whether the attending or resident felt unprepared to discuss this or were simply unimpressed with the adverse event. We do get the sense that the student did not feel comfortable raising the issue with the team. As such, it was a missed opportunity to seek help from any number of hospital resources and find encouragement to take on difficult encounters.
Much has been written about apologies.810 Disclosing errors and apologizing is the ethical standard, and many of our institutions have made it policy. Yet in the moment, it is embarrassing, anxiety provoking, and our concern about litigation looms large. Learning to do the right thing begins, perhaps with lectures and standardized patients, but only when students see it modeled by our housestaff and faculty, does it take root for good.
Our housestaff are quite good at managing medical issues, but they may still need help in creating the appropriate environment for professional learning and growth. This is 1 of the most important contributions an attending can make. We have emphasized that faculty have an important role to play in the area of professional development, reinforcing the rudimentary information preclinical students are presented with in the classroom and processing experiences residents are exposed to on a regular basis. If the hospital doesn't wait, then it is the attending physician's job to create the space and time for trainees to think about what is happening and ask if it could have been done better.
A number of seasoned clinical teachers have written about ways to improve teaching on the wards.11 Below, we will add to that discussion by considering practical ways to enhance learning about professionalism and ethics (see Table 1). Note should be made that while we focus on specific behaviors and activities, underlying all is the importance of availability, presence, and intention. Like all good teaching, these activities require planning and effort.
| Attending Activity | Examples |
|---|---|
| Creating an Open Climate | |
| Breaking Communication Barriers | Setting aside time for introductions and team building exercises at the beginning of a rotation, with attending participating equally with residents and students |
| Emphasizing attending availability to discuss or review problems of any kind | |
| Setting Clear Expectations | Emphasizing the importance of patient‐clinician or family‐clinician communication from the outset |
| Devoting some attending rounds to Difficult Conversations (e.g., breaking bad news or code status discussions) | |
| Explicitly stating that no ethical question is a stupid question and providing positive feedback for raising such questions for the team | |
| Regular Check‐ins | Establishing team communication rounds: 10 minutes every day to review a good, bad, or awkward interaction from the past day (e.g., family meeting, DNR discussion) |
| Setting aside time on rounds or during attending teaching sessions to explore the team's or an individual's emotional responses to a patient's death or deterioration | |
| Writing exercises that focus on our reactions to challenging situations that are shared with the group | |
| Supervision and Modeling | |
| Planning | Clarifying an agenda and practicing key phrases for a family meeting with the resident prior to meeting the family |
| Anticipating which patients may require a code status discussion and discussing a game plan on rounds | |
| Modeling | Students observe the attending facilitate a family meeting |
| Residents observe the attending apologizing for an error, no matter how small | |
| Attending thinks about an interpersonal conflict out loud and models asking patient‐relations for help | |
| Debriefing | Reviewing a family meeting with and giving feedback to the resident who facilitated |
| Reviewing a challenging code status discussion as a team |
Creating an Open Climate
The medical team, of which the attending, residents, and students are all a part, should not only be a unit that provides excellent medical care to its patients, but should also create a culture of continuous learning and improvement. As such, it is important to create a safe atmosphere where teachers are invested in the growth of their learners and learners feel free to question the prevailing logic and practice, including issues of professionalism and ethics. As Malcolm Gladwell12 describes in Outliers, Korean Air jets were crashing because subordinates were afraid to question their superiors. Once that culture changed, Korean Air safety improved dramatically. Similarly, breaking down some of the hierarchical barriers should improve the culture of a medical team. We typically make an effort to get to know our students and residents on a more personal basis: where they are from, who is in their family, what was their major, what are their interests outside of medicine, and what has been surprising to them in their training so far. Whether we set aside time when we first meet or e‐mail our questions before the first day, we aim for this to be 1 of the first team activities. We also share our own stories, making clear that the attending is part of the team, and not just an evaluating supervisor.
Vignette 1 describes the student's trauma of witnessing a code and the inability to process the event with anyone afterward. Failed resuscitation attempts are the most dramatic examples, but even expected deaths, nonfatal adverse events, and conflict between patients and providers may be traumatic for new trainees inexperienced with the reality of medicine. Attendings should be aware of these potentially traumatic events and make time to check in with the team members about how they are dealing with their emotions. Taking time on attending rounds, for example, allows the attending to not only model reflective practice and self‐care, but also elevates team support to a place traditionally reserved for discussions about diagnosis and treatment.
Supervision and Modeling
Vignettes 2 and 3 center around challenging communication tasks that require special training, including instruction, modeling, feedback, and practice. Unfortunately, as some of our student accounts document, many teaching opportunities are missed. As attendings, our duties include being aware of these opportunities, and being prepared to model competent patientor familydoctor interactions. Emphasizing the importance of the doctor‐patient relationship is in fact one of the key skills of an effective attending role model.13
When opportunities arise for any potentially difficult conversation, we make every effort to identify the issue, prebrief with the team about how to conduct the discussion, and either offer to model the conversation or be present to observe and provide feedback and debriefing afterwards. For example, by asking about all DNR discussions had with our patients, we gain insight into the skill level of our housestaff. As important, the housestaff understand that we believe that these conversations are vital to review during formal rounds, with the same attention we give to chest pain and electrocardiograms (ECGs).
Two key skills that develop with experience are the ability to know the limits of one's knowledge and to know when to ask for help. We try to be open about naming those limits and thinking about the other members of the larger healthcare team that may provide insight, skill, and expertise. We are used to doing this with medical questions (eg, asking the gastroenterology consult team to locate a source of bleeding). Asking our risk management, patient‐relations, or ethics services to assist with a difficult communication task or conflict with a family is no different, and often something the housestaff may not readily do.
We are grateful to our students and their postcards for the snapshots of our local medical culture. While we are gratified to read of excellent role modeling, we are also disappointed to read of situations which have left our students confused, demoralized and cynical. But if these exercises are to reach their full potential, they should tell us about where we would like to go, in addition to where we have been. We believe that our conferences have stimulated our faculty and housestaff to reflect on the professionalism lessons they are teaching. Reading the student postings has definitely affected our approach to teaching professionalism. They reinforce what every parent and educator knows: when it comes to teaching professionalism, communication and ethics, what matters most is the behavior of the teacher. Our words mean little if our actions do not live out what we espouse.
Acknowledgements
We are grateful for Michael Chan and his classmates from the NUFSM class of 2010 for their thoughtful essays. David Neely, Director of Undergraduate Education, Department of Medicine, Eytan Szmuilowicz, Palliative Medicine. Kathy Neely, Chairman of NMH Ethics Committee. Co‐director of Patient, Physician and Society.
- .This is just too awful; I just can't believe I experienced that.Acad Med.2005;80(7):634–640.
- .A piece of my mind.JAMA.2001;286(9):1017–1018.
- .Teaching medical students and residents skills for delivering bad news: a review of strategies.Acad Med.2004;79(2):107–117.
- .Third‐year medical students' experiences with dying patients during the internal medicine clerkship: a qualitative study of the informal curriculum.Acad Med.2005;80(7):641–647.
- ,,.How do medical residents discuss resuscitation with patients? Official journal of the Society for Research and Education in Primary Care Internal Medicine.J Gen Intern Med.1995;10(8):436–442.
- ,,.See one, do one, teach one? House staff experience discussing do‐not‐resuscitate orders.Arch Intern Med.1996;156(12):1285–1289.
- ,,,.Residents' end‐of‐life decision making with adult hospitalized patients: a review of the literature.Acad Med.2005:80(7)622–633.
- .Physician error and disclosure.Clin Obstet Gynecol.2008;51(4):700–708.
- .Revealing medical errors to your patients.Chest.2009;133:1064–1065.
- .Apology in medical practice.JAMA.2006;296:1401–1404.
- .What if Osler were one of us? Inpatient teaching today.J Gen Intern Med.1997;12(Suppl 2):S41–S48.
- Malcolm Gladwell.Outliers.New York:Little, Brown, Co.,2008: p.177–223.
- ,,,,.Attributes of excellent attending‐physician role models.N Engl J Med.1998;339(27):1986–1993.
During their junior medicine rotation, our students are asked to post to Blackboard (an online student forum) an anonymous essay about an issue of professionalism or ethics, either inspiring or troubling. In many ways, these vignettes are like postcards, written by visitors describing foreign cultures and norms. They represent a way for the students to debrief, but also provide an opportunity for us, as faculty, to reflect upon the way we practice and teach medicine. Many postingslike postcards from exotic or historic placesare inspiring stories of residents and faculty extending themselves for their patients. Unfortunately, unlike typical postcards, there are also essays that are troubling or provoking and challenge us to consider how we could improve the professional and ethical environment on our teams.
In order to begin a learning process with our faculty and housestaff, we have presented a number of these anonymous essays at both faculty and housestaff Department of Medicine conferences as well as our monthly hospital Ethics conference. The goal of these conferences was to gather as a moral community to reflect on our students' experience and consider ways in which our day to day practice as attendings could be informed by what they tell us. In addition, the junior medicine site directors have a session each quarter with their junior students to review some of the most significant issues brought up by their essays.
Practically, these vignettes and conferences serve three main purposes:
-
Raising Awareness: Many professional issues noted by our students occur under the radar. Attendings are often unaware of the issues of professionalism and/or ethics confronting our students and housestaff.
-
Exploring Attitudes: Some attending may underemphasize the importance of specific issues of professionalism and/or ethics. Open discussions at faculty or resident conferences create opportunities for individuals to reflect upon their own reactions and for the group to create a norm.
-
Sharing Skills: It is difficult to learn the practice of professionalism and ethics from a book. Skill in this area is gained primarily by experience. Conferences provide an excellent forum for seasoned physicians to share wisdom with less experienced physicians. In addition, important teaching points can be made: Students should not deliver bad news alone. Errors should be disclosed.
Following are 3 of the essays we presented, along with brief commentaries. At the end, we provide practical suggestions for individual attendings to improve the professional climate on their teams.
The Hospital Didn't Wait
Code. On 12, the surgical wards floor. Elise sprinted to the stairwell, dashed up to 12, and ran to the corner room as fast as she could. She could see the room before she got there. Instinctively, she started reviewing the steps she had memorized so many months ago. But when she finally arrived at the patient's bathroom, her thought process came to a jolting halt as she came upon the gruesome scene.
The 76‐year‐old patient had hanged himself with the cinching rope from his garment bag, and now dangled suspended from a high towel rack against the wall. Nurses from the floor started to file in, and without losing a beat Elise barked commands. Together they brought the man's body down to the floor, laid him on his back, and stripped off his hospital gown. Elise was in charge; deliberately but forcefully, she ordered a nurse to retrieve a defibrillator, and had another resident check for a pulse. There was none. Anesthesiology was here. Quickly and expertly, they shoved a plastic tube down his throat and began ventilation. The nurse placed on the electrodes between chest compressions then called to clear the body. Airway stepped back. The chest pumper stepped back. The body lurched forward as the defibrillator issued a long beep and discharged. Still no pulse. The cycle repeated.
Finally, Elise called a stop. Time of death, 19:37. By now there were about 20 people crammed in the patient room, all of whom had a separate role during the code. Some stayed behind, while the rest left to return to their interrupted work. The medical student didn't know what to think as he returned to the team room. His jaw was sorehad he been clenching it the whole time?and as he brought his hand up to rub his face, he saw that his knuckles were bloody. Somehow he had scraped them during the code. As he logged back into the computer to finish off his evening notes, he knew that he wouldn't have time to reflect until hours later when he returned home. Codes happened all the time. There was still work to be done in the hospital, and the hospital didn't wait.
The room had already been assigned to a patient waiting in the Emergency Department downstairs. That patient would be here in a few minutes. The hospital didn't wait.
When we presented this case in our conferences, there was universal agreement that such a traumatic event merits, even demands, team debriefing and processing. But in the real life aftermath of this traumatic event, the take‐home message for the medical student was that the hospital didn't wait for such discussions. We know this is not unique to our institution. In a study of 32 medical students who were asked to reflect on their most memorable patient death,1 debriefing sessions were rare and many students felt inadequately supported. While experienced clinicians may be accustomed to seeing patients die, students are new to the culture of the hospital, and have not had the chance to develop the defense mechanisms necessary to cope with this sort of experience. Angoff2 writes, As medical educators, we ought to ask our students how they are coping with long hours, fatigue, illness, suffering, and death. We ought to model and commend compassion and react to the deep feelings of our students in the same way we would teach them to react to the deep feelings of their patients.
I Told a Man Today That He Had Brain Cancer
The resident, intern, and I were huddled together in our team room when the report came back on the computer. New 3.5 2.3 1.7 cm contrast‐enhancing lesion seen anterior to genu of corpus callosum. Concerning for metastatic focus vs. lymphoma. Advise follow‐up. It wasn't unexpected but we had nevertheless been hoping for better.
The three of us went into his room and I was waiting to see how my resident would deliver the bad news, but she didn't. She simply said that we were continuing to do imaging studies and that a neurology team would be in touch. There were probably several reasons why she didn't tell him: not enough time, not her responsibility, or maybe she was just uncomfortable with it. Whatever the case, we left the room with my patient still oblivious to the awful mass now tangled in his head.
If my resident was taking a pass on this conversation, I knew it fell to me he needed to hear it from his primary team. I came back after rounds alone, sat down next to his bed, and told him that his MRI results had come back, and that I had unfortunate news.
I told him that the images showed that his lung cancer had spread to his brain.
I paused to give him a chance to let it sink in. He turned away and looked up at the ceiling.
Where is it? How big is it?
What now?
Reflecting on this case, our audiences were disturbed that a student would attempt this difficult conversation alone, while recognizing that the student clearly felt a sense of responsibility and desire to help his patient by sharing important information. We talked about how students may erroneously pick up a message that the team member who has spent the most time with a patient is the most obvious choice to have difficult conversations. We also noted that, unfortunately, sometimes students are directly asked by their team to shoulder this responsibility on their own. In this painful account, there is no mention of preparation, supervision, or support for the student before or after the encounter. The student perceived (rightly or wrongly) that the team leaders lacked comfort or skill to deliver the bad news, and stepped in. It is possible that the attending lacked the skill and ability to model an interaction, but more likely the deficit was in awareness and attitude. It is unlikely the attending knew that the student had this conversation alone. One of the major reasons we present these vignettes is to make attendings and housestaff more aware of issues that occur under their radar so that they can take preventative action. However, once the resident or attending found out that the student had this conversation alone, the student should be pulled aside for a 1:1 discussion. At the end of the day, the student should know that it was inappropriate to attempt this conversation alone
Rosenbaum3 reviewed a number of strategies to teach the skill of delivering bad news, from lecture and small group discussions to role play and standardized patients. When asked, students cited role‐modeling as the best way to learn how to deliver bad news.4 Observation of a veteran clinician provides a firm foundation for learning; but that is not enough. Unfortunately, we know from the literature (and our student vignettes suggest) that students and residents are unprepared to carry out these conversations properly, either because of misguided attitudes, lack of experience, or inadequate training.57 We conceptualize engaging in difficult conversations as a procedure, demanding a skill set. Mere observation of an expert executing this procedure is only a beginning. With any other skill, from successful completion of a lumbar puncture to initiating cardiopulmonary resuscitation (CPR), a student would never conclude that knowing the patient the best sufficiently credentials the student to undertake these procedures. We maintain that a difficult conversationbe it breaking bad news, discussing end‐of‐life care preferences, code status discussions, or prognosisis a clinical intervention, like any other procedure in medicine. If performed with skill and caution, it can bring about a stronger therapeutic relationship and increased support for the patient; if performed clumsily, it can lead to unintended adverse outcomes, including misunderstanding, mistrust, anxiety, and anger.
A Decimal Point Got Misplaced
On palliative care, I had a 90 year‐old man with end stage lung CA that presented to the ED with increasing SOB. The resident decided that giving him some morphine would be a good solution but was worried that too much would push him over the edge. He was thin; his O2 sats weren't that good After some discussion it was decided that 2.5 mg should be the starting amount. Unfortunately, when the note was written a decimal point got misplaced and he got 25 mg as a first dose. He ended up very sedated for most of the day but his breathing was ok.
The mistake was not discussed with the patient or the patient's family. While it did not cause any lasting harm, I wondered if telling the patient/patient's family that an error had been made would have been more ethically sound.
When we presented this case in our conferences, there was little controversy about whether the error should have been disclosed. The discussion did provide reinforcement for doing a simple but difficult task. Our analysis is that the nondiscussion of this error reflects a deficit in attitude and possibly skill. The team was aware of the error, but the resident and attending did not take the opportunity to disclose an error. They should have. We do not know whether the attending or resident felt unprepared to discuss this or were simply unimpressed with the adverse event. We do get the sense that the student did not feel comfortable raising the issue with the team. As such, it was a missed opportunity to seek help from any number of hospital resources and find encouragement to take on difficult encounters.
Much has been written about apologies.810 Disclosing errors and apologizing is the ethical standard, and many of our institutions have made it policy. Yet in the moment, it is embarrassing, anxiety provoking, and our concern about litigation looms large. Learning to do the right thing begins, perhaps with lectures and standardized patients, but only when students see it modeled by our housestaff and faculty, does it take root for good.
Our housestaff are quite good at managing medical issues, but they may still need help in creating the appropriate environment for professional learning and growth. This is 1 of the most important contributions an attending can make. We have emphasized that faculty have an important role to play in the area of professional development, reinforcing the rudimentary information preclinical students are presented with in the classroom and processing experiences residents are exposed to on a regular basis. If the hospital doesn't wait, then it is the attending physician's job to create the space and time for trainees to think about what is happening and ask if it could have been done better.
A number of seasoned clinical teachers have written about ways to improve teaching on the wards.11 Below, we will add to that discussion by considering practical ways to enhance learning about professionalism and ethics (see Table 1). Note should be made that while we focus on specific behaviors and activities, underlying all is the importance of availability, presence, and intention. Like all good teaching, these activities require planning and effort.
| Attending Activity | Examples |
|---|---|
| Creating an Open Climate | |
| Breaking Communication Barriers | Setting aside time for introductions and team building exercises at the beginning of a rotation, with attending participating equally with residents and students |
| Emphasizing attending availability to discuss or review problems of any kind | |
| Setting Clear Expectations | Emphasizing the importance of patient‐clinician or family‐clinician communication from the outset |
| Devoting some attending rounds to Difficult Conversations (e.g., breaking bad news or code status discussions) | |
| Explicitly stating that no ethical question is a stupid question and providing positive feedback for raising such questions for the team | |
| Regular Check‐ins | Establishing team communication rounds: 10 minutes every day to review a good, bad, or awkward interaction from the past day (e.g., family meeting, DNR discussion) |
| Setting aside time on rounds or during attending teaching sessions to explore the team's or an individual's emotional responses to a patient's death or deterioration | |
| Writing exercises that focus on our reactions to challenging situations that are shared with the group | |
| Supervision and Modeling | |
| Planning | Clarifying an agenda and practicing key phrases for a family meeting with the resident prior to meeting the family |
| Anticipating which patients may require a code status discussion and discussing a game plan on rounds | |
| Modeling | Students observe the attending facilitate a family meeting |
| Residents observe the attending apologizing for an error, no matter how small | |
| Attending thinks about an interpersonal conflict out loud and models asking patient‐relations for help | |
| Debriefing | Reviewing a family meeting with and giving feedback to the resident who facilitated |
| Reviewing a challenging code status discussion as a team |
Creating an Open Climate
The medical team, of which the attending, residents, and students are all a part, should not only be a unit that provides excellent medical care to its patients, but should also create a culture of continuous learning and improvement. As such, it is important to create a safe atmosphere where teachers are invested in the growth of their learners and learners feel free to question the prevailing logic and practice, including issues of professionalism and ethics. As Malcolm Gladwell12 describes in Outliers, Korean Air jets were crashing because subordinates were afraid to question their superiors. Once that culture changed, Korean Air safety improved dramatically. Similarly, breaking down some of the hierarchical barriers should improve the culture of a medical team. We typically make an effort to get to know our students and residents on a more personal basis: where they are from, who is in their family, what was their major, what are their interests outside of medicine, and what has been surprising to them in their training so far. Whether we set aside time when we first meet or e‐mail our questions before the first day, we aim for this to be 1 of the first team activities. We also share our own stories, making clear that the attending is part of the team, and not just an evaluating supervisor.
Vignette 1 describes the student's trauma of witnessing a code and the inability to process the event with anyone afterward. Failed resuscitation attempts are the most dramatic examples, but even expected deaths, nonfatal adverse events, and conflict between patients and providers may be traumatic for new trainees inexperienced with the reality of medicine. Attendings should be aware of these potentially traumatic events and make time to check in with the team members about how they are dealing with their emotions. Taking time on attending rounds, for example, allows the attending to not only model reflective practice and self‐care, but also elevates team support to a place traditionally reserved for discussions about diagnosis and treatment.
Supervision and Modeling
Vignettes 2 and 3 center around challenging communication tasks that require special training, including instruction, modeling, feedback, and practice. Unfortunately, as some of our student accounts document, many teaching opportunities are missed. As attendings, our duties include being aware of these opportunities, and being prepared to model competent patientor familydoctor interactions. Emphasizing the importance of the doctor‐patient relationship is in fact one of the key skills of an effective attending role model.13
When opportunities arise for any potentially difficult conversation, we make every effort to identify the issue, prebrief with the team about how to conduct the discussion, and either offer to model the conversation or be present to observe and provide feedback and debriefing afterwards. For example, by asking about all DNR discussions had with our patients, we gain insight into the skill level of our housestaff. As important, the housestaff understand that we believe that these conversations are vital to review during formal rounds, with the same attention we give to chest pain and electrocardiograms (ECGs).
Two key skills that develop with experience are the ability to know the limits of one's knowledge and to know when to ask for help. We try to be open about naming those limits and thinking about the other members of the larger healthcare team that may provide insight, skill, and expertise. We are used to doing this with medical questions (eg, asking the gastroenterology consult team to locate a source of bleeding). Asking our risk management, patient‐relations, or ethics services to assist with a difficult communication task or conflict with a family is no different, and often something the housestaff may not readily do.
We are grateful to our students and their postcards for the snapshots of our local medical culture. While we are gratified to read of excellent role modeling, we are also disappointed to read of situations which have left our students confused, demoralized and cynical. But if these exercises are to reach their full potential, they should tell us about where we would like to go, in addition to where we have been. We believe that our conferences have stimulated our faculty and housestaff to reflect on the professionalism lessons they are teaching. Reading the student postings has definitely affected our approach to teaching professionalism. They reinforce what every parent and educator knows: when it comes to teaching professionalism, communication and ethics, what matters most is the behavior of the teacher. Our words mean little if our actions do not live out what we espouse.
Acknowledgements
We are grateful for Michael Chan and his classmates from the NUFSM class of 2010 for their thoughtful essays. David Neely, Director of Undergraduate Education, Department of Medicine, Eytan Szmuilowicz, Palliative Medicine. Kathy Neely, Chairman of NMH Ethics Committee. Co‐director of Patient, Physician and Society.
During their junior medicine rotation, our students are asked to post to Blackboard (an online student forum) an anonymous essay about an issue of professionalism or ethics, either inspiring or troubling. In many ways, these vignettes are like postcards, written by visitors describing foreign cultures and norms. They represent a way for the students to debrief, but also provide an opportunity for us, as faculty, to reflect upon the way we practice and teach medicine. Many postingslike postcards from exotic or historic placesare inspiring stories of residents and faculty extending themselves for their patients. Unfortunately, unlike typical postcards, there are also essays that are troubling or provoking and challenge us to consider how we could improve the professional and ethical environment on our teams.
In order to begin a learning process with our faculty and housestaff, we have presented a number of these anonymous essays at both faculty and housestaff Department of Medicine conferences as well as our monthly hospital Ethics conference. The goal of these conferences was to gather as a moral community to reflect on our students' experience and consider ways in which our day to day practice as attendings could be informed by what they tell us. In addition, the junior medicine site directors have a session each quarter with their junior students to review some of the most significant issues brought up by their essays.
Practically, these vignettes and conferences serve three main purposes:
-
Raising Awareness: Many professional issues noted by our students occur under the radar. Attendings are often unaware of the issues of professionalism and/or ethics confronting our students and housestaff.
-
Exploring Attitudes: Some attending may underemphasize the importance of specific issues of professionalism and/or ethics. Open discussions at faculty or resident conferences create opportunities for individuals to reflect upon their own reactions and for the group to create a norm.
-
Sharing Skills: It is difficult to learn the practice of professionalism and ethics from a book. Skill in this area is gained primarily by experience. Conferences provide an excellent forum for seasoned physicians to share wisdom with less experienced physicians. In addition, important teaching points can be made: Students should not deliver bad news alone. Errors should be disclosed.
Following are 3 of the essays we presented, along with brief commentaries. At the end, we provide practical suggestions for individual attendings to improve the professional climate on their teams.
The Hospital Didn't Wait
Code. On 12, the surgical wards floor. Elise sprinted to the stairwell, dashed up to 12, and ran to the corner room as fast as she could. She could see the room before she got there. Instinctively, she started reviewing the steps she had memorized so many months ago. But when she finally arrived at the patient's bathroom, her thought process came to a jolting halt as she came upon the gruesome scene.
The 76‐year‐old patient had hanged himself with the cinching rope from his garment bag, and now dangled suspended from a high towel rack against the wall. Nurses from the floor started to file in, and without losing a beat Elise barked commands. Together they brought the man's body down to the floor, laid him on his back, and stripped off his hospital gown. Elise was in charge; deliberately but forcefully, she ordered a nurse to retrieve a defibrillator, and had another resident check for a pulse. There was none. Anesthesiology was here. Quickly and expertly, they shoved a plastic tube down his throat and began ventilation. The nurse placed on the electrodes between chest compressions then called to clear the body. Airway stepped back. The chest pumper stepped back. The body lurched forward as the defibrillator issued a long beep and discharged. Still no pulse. The cycle repeated.
Finally, Elise called a stop. Time of death, 19:37. By now there were about 20 people crammed in the patient room, all of whom had a separate role during the code. Some stayed behind, while the rest left to return to their interrupted work. The medical student didn't know what to think as he returned to the team room. His jaw was sorehad he been clenching it the whole time?and as he brought his hand up to rub his face, he saw that his knuckles were bloody. Somehow he had scraped them during the code. As he logged back into the computer to finish off his evening notes, he knew that he wouldn't have time to reflect until hours later when he returned home. Codes happened all the time. There was still work to be done in the hospital, and the hospital didn't wait.
The room had already been assigned to a patient waiting in the Emergency Department downstairs. That patient would be here in a few minutes. The hospital didn't wait.
When we presented this case in our conferences, there was universal agreement that such a traumatic event merits, even demands, team debriefing and processing. But in the real life aftermath of this traumatic event, the take‐home message for the medical student was that the hospital didn't wait for such discussions. We know this is not unique to our institution. In a study of 32 medical students who were asked to reflect on their most memorable patient death,1 debriefing sessions were rare and many students felt inadequately supported. While experienced clinicians may be accustomed to seeing patients die, students are new to the culture of the hospital, and have not had the chance to develop the defense mechanisms necessary to cope with this sort of experience. Angoff2 writes, As medical educators, we ought to ask our students how they are coping with long hours, fatigue, illness, suffering, and death. We ought to model and commend compassion and react to the deep feelings of our students in the same way we would teach them to react to the deep feelings of their patients.
I Told a Man Today That He Had Brain Cancer
The resident, intern, and I were huddled together in our team room when the report came back on the computer. New 3.5 2.3 1.7 cm contrast‐enhancing lesion seen anterior to genu of corpus callosum. Concerning for metastatic focus vs. lymphoma. Advise follow‐up. It wasn't unexpected but we had nevertheless been hoping for better.
The three of us went into his room and I was waiting to see how my resident would deliver the bad news, but she didn't. She simply said that we were continuing to do imaging studies and that a neurology team would be in touch. There were probably several reasons why she didn't tell him: not enough time, not her responsibility, or maybe she was just uncomfortable with it. Whatever the case, we left the room with my patient still oblivious to the awful mass now tangled in his head.
If my resident was taking a pass on this conversation, I knew it fell to me he needed to hear it from his primary team. I came back after rounds alone, sat down next to his bed, and told him that his MRI results had come back, and that I had unfortunate news.
I told him that the images showed that his lung cancer had spread to his brain.
I paused to give him a chance to let it sink in. He turned away and looked up at the ceiling.
Where is it? How big is it?
What now?
Reflecting on this case, our audiences were disturbed that a student would attempt this difficult conversation alone, while recognizing that the student clearly felt a sense of responsibility and desire to help his patient by sharing important information. We talked about how students may erroneously pick up a message that the team member who has spent the most time with a patient is the most obvious choice to have difficult conversations. We also noted that, unfortunately, sometimes students are directly asked by their team to shoulder this responsibility on their own. In this painful account, there is no mention of preparation, supervision, or support for the student before or after the encounter. The student perceived (rightly or wrongly) that the team leaders lacked comfort or skill to deliver the bad news, and stepped in. It is possible that the attending lacked the skill and ability to model an interaction, but more likely the deficit was in awareness and attitude. It is unlikely the attending knew that the student had this conversation alone. One of the major reasons we present these vignettes is to make attendings and housestaff more aware of issues that occur under their radar so that they can take preventative action. However, once the resident or attending found out that the student had this conversation alone, the student should be pulled aside for a 1:1 discussion. At the end of the day, the student should know that it was inappropriate to attempt this conversation alone
Rosenbaum3 reviewed a number of strategies to teach the skill of delivering bad news, from lecture and small group discussions to role play and standardized patients. When asked, students cited role‐modeling as the best way to learn how to deliver bad news.4 Observation of a veteran clinician provides a firm foundation for learning; but that is not enough. Unfortunately, we know from the literature (and our student vignettes suggest) that students and residents are unprepared to carry out these conversations properly, either because of misguided attitudes, lack of experience, or inadequate training.57 We conceptualize engaging in difficult conversations as a procedure, demanding a skill set. Mere observation of an expert executing this procedure is only a beginning. With any other skill, from successful completion of a lumbar puncture to initiating cardiopulmonary resuscitation (CPR), a student would never conclude that knowing the patient the best sufficiently credentials the student to undertake these procedures. We maintain that a difficult conversationbe it breaking bad news, discussing end‐of‐life care preferences, code status discussions, or prognosisis a clinical intervention, like any other procedure in medicine. If performed with skill and caution, it can bring about a stronger therapeutic relationship and increased support for the patient; if performed clumsily, it can lead to unintended adverse outcomes, including misunderstanding, mistrust, anxiety, and anger.
A Decimal Point Got Misplaced
On palliative care, I had a 90 year‐old man with end stage lung CA that presented to the ED with increasing SOB. The resident decided that giving him some morphine would be a good solution but was worried that too much would push him over the edge. He was thin; his O2 sats weren't that good After some discussion it was decided that 2.5 mg should be the starting amount. Unfortunately, when the note was written a decimal point got misplaced and he got 25 mg as a first dose. He ended up very sedated for most of the day but his breathing was ok.
The mistake was not discussed with the patient or the patient's family. While it did not cause any lasting harm, I wondered if telling the patient/patient's family that an error had been made would have been more ethically sound.
When we presented this case in our conferences, there was little controversy about whether the error should have been disclosed. The discussion did provide reinforcement for doing a simple but difficult task. Our analysis is that the nondiscussion of this error reflects a deficit in attitude and possibly skill. The team was aware of the error, but the resident and attending did not take the opportunity to disclose an error. They should have. We do not know whether the attending or resident felt unprepared to discuss this or were simply unimpressed with the adverse event. We do get the sense that the student did not feel comfortable raising the issue with the team. As such, it was a missed opportunity to seek help from any number of hospital resources and find encouragement to take on difficult encounters.
Much has been written about apologies.810 Disclosing errors and apologizing is the ethical standard, and many of our institutions have made it policy. Yet in the moment, it is embarrassing, anxiety provoking, and our concern about litigation looms large. Learning to do the right thing begins, perhaps with lectures and standardized patients, but only when students see it modeled by our housestaff and faculty, does it take root for good.
Our housestaff are quite good at managing medical issues, but they may still need help in creating the appropriate environment for professional learning and growth. This is 1 of the most important contributions an attending can make. We have emphasized that faculty have an important role to play in the area of professional development, reinforcing the rudimentary information preclinical students are presented with in the classroom and processing experiences residents are exposed to on a regular basis. If the hospital doesn't wait, then it is the attending physician's job to create the space and time for trainees to think about what is happening and ask if it could have been done better.
A number of seasoned clinical teachers have written about ways to improve teaching on the wards.11 Below, we will add to that discussion by considering practical ways to enhance learning about professionalism and ethics (see Table 1). Note should be made that while we focus on specific behaviors and activities, underlying all is the importance of availability, presence, and intention. Like all good teaching, these activities require planning and effort.
| Attending Activity | Examples |
|---|---|
| Creating an Open Climate | |
| Breaking Communication Barriers | Setting aside time for introductions and team building exercises at the beginning of a rotation, with attending participating equally with residents and students |
| Emphasizing attending availability to discuss or review problems of any kind | |
| Setting Clear Expectations | Emphasizing the importance of patient‐clinician or family‐clinician communication from the outset |
| Devoting some attending rounds to Difficult Conversations (e.g., breaking bad news or code status discussions) | |
| Explicitly stating that no ethical question is a stupid question and providing positive feedback for raising such questions for the team | |
| Regular Check‐ins | Establishing team communication rounds: 10 minutes every day to review a good, bad, or awkward interaction from the past day (e.g., family meeting, DNR discussion) |
| Setting aside time on rounds or during attending teaching sessions to explore the team's or an individual's emotional responses to a patient's death or deterioration | |
| Writing exercises that focus on our reactions to challenging situations that are shared with the group | |
| Supervision and Modeling | |
| Planning | Clarifying an agenda and practicing key phrases for a family meeting with the resident prior to meeting the family |
| Anticipating which patients may require a code status discussion and discussing a game plan on rounds | |
| Modeling | Students observe the attending facilitate a family meeting |
| Residents observe the attending apologizing for an error, no matter how small | |
| Attending thinks about an interpersonal conflict out loud and models asking patient‐relations for help | |
| Debriefing | Reviewing a family meeting with and giving feedback to the resident who facilitated |
| Reviewing a challenging code status discussion as a team |
Creating an Open Climate
The medical team, of which the attending, residents, and students are all a part, should not only be a unit that provides excellent medical care to its patients, but should also create a culture of continuous learning and improvement. As such, it is important to create a safe atmosphere where teachers are invested in the growth of their learners and learners feel free to question the prevailing logic and practice, including issues of professionalism and ethics. As Malcolm Gladwell12 describes in Outliers, Korean Air jets were crashing because subordinates were afraid to question their superiors. Once that culture changed, Korean Air safety improved dramatically. Similarly, breaking down some of the hierarchical barriers should improve the culture of a medical team. We typically make an effort to get to know our students and residents on a more personal basis: where they are from, who is in their family, what was their major, what are their interests outside of medicine, and what has been surprising to them in their training so far. Whether we set aside time when we first meet or e‐mail our questions before the first day, we aim for this to be 1 of the first team activities. We also share our own stories, making clear that the attending is part of the team, and not just an evaluating supervisor.
Vignette 1 describes the student's trauma of witnessing a code and the inability to process the event with anyone afterward. Failed resuscitation attempts are the most dramatic examples, but even expected deaths, nonfatal adverse events, and conflict between patients and providers may be traumatic for new trainees inexperienced with the reality of medicine. Attendings should be aware of these potentially traumatic events and make time to check in with the team members about how they are dealing with their emotions. Taking time on attending rounds, for example, allows the attending to not only model reflective practice and self‐care, but also elevates team support to a place traditionally reserved for discussions about diagnosis and treatment.
Supervision and Modeling
Vignettes 2 and 3 center around challenging communication tasks that require special training, including instruction, modeling, feedback, and practice. Unfortunately, as some of our student accounts document, many teaching opportunities are missed. As attendings, our duties include being aware of these opportunities, and being prepared to model competent patientor familydoctor interactions. Emphasizing the importance of the doctor‐patient relationship is in fact one of the key skills of an effective attending role model.13
When opportunities arise for any potentially difficult conversation, we make every effort to identify the issue, prebrief with the team about how to conduct the discussion, and either offer to model the conversation or be present to observe and provide feedback and debriefing afterwards. For example, by asking about all DNR discussions had with our patients, we gain insight into the skill level of our housestaff. As important, the housestaff understand that we believe that these conversations are vital to review during formal rounds, with the same attention we give to chest pain and electrocardiograms (ECGs).
Two key skills that develop with experience are the ability to know the limits of one's knowledge and to know when to ask for help. We try to be open about naming those limits and thinking about the other members of the larger healthcare team that may provide insight, skill, and expertise. We are used to doing this with medical questions (eg, asking the gastroenterology consult team to locate a source of bleeding). Asking our risk management, patient‐relations, or ethics services to assist with a difficult communication task or conflict with a family is no different, and often something the housestaff may not readily do.
We are grateful to our students and their postcards for the snapshots of our local medical culture. While we are gratified to read of excellent role modeling, we are also disappointed to read of situations which have left our students confused, demoralized and cynical. But if these exercises are to reach their full potential, they should tell us about where we would like to go, in addition to where we have been. We believe that our conferences have stimulated our faculty and housestaff to reflect on the professionalism lessons they are teaching. Reading the student postings has definitely affected our approach to teaching professionalism. They reinforce what every parent and educator knows: when it comes to teaching professionalism, communication and ethics, what matters most is the behavior of the teacher. Our words mean little if our actions do not live out what we espouse.
Acknowledgements
We are grateful for Michael Chan and his classmates from the NUFSM class of 2010 for their thoughtful essays. David Neely, Director of Undergraduate Education, Department of Medicine, Eytan Szmuilowicz, Palliative Medicine. Kathy Neely, Chairman of NMH Ethics Committee. Co‐director of Patient, Physician and Society.
- .This is just too awful; I just can't believe I experienced that.Acad Med.2005;80(7):634–640.
- .A piece of my mind.JAMA.2001;286(9):1017–1018.
- .Teaching medical students and residents skills for delivering bad news: a review of strategies.Acad Med.2004;79(2):107–117.
- .Third‐year medical students' experiences with dying patients during the internal medicine clerkship: a qualitative study of the informal curriculum.Acad Med.2005;80(7):641–647.
- ,,.How do medical residents discuss resuscitation with patients? Official journal of the Society for Research and Education in Primary Care Internal Medicine.J Gen Intern Med.1995;10(8):436–442.
- ,,.See one, do one, teach one? House staff experience discussing do‐not‐resuscitate orders.Arch Intern Med.1996;156(12):1285–1289.
- ,,,.Residents' end‐of‐life decision making with adult hospitalized patients: a review of the literature.Acad Med.2005:80(7)622–633.
- .Physician error and disclosure.Clin Obstet Gynecol.2008;51(4):700–708.
- .Revealing medical errors to your patients.Chest.2009;133:1064–1065.
- .Apology in medical practice.JAMA.2006;296:1401–1404.
- .What if Osler were one of us? Inpatient teaching today.J Gen Intern Med.1997;12(Suppl 2):S41–S48.
- Malcolm Gladwell.Outliers.New York:Little, Brown, Co.,2008: p.177–223.
- ,,,,.Attributes of excellent attending‐physician role models.N Engl J Med.1998;339(27):1986–1993.
- .This is just too awful; I just can't believe I experienced that.Acad Med.2005;80(7):634–640.
- .A piece of my mind.JAMA.2001;286(9):1017–1018.
- .Teaching medical students and residents skills for delivering bad news: a review of strategies.Acad Med.2004;79(2):107–117.
- .Third‐year medical students' experiences with dying patients during the internal medicine clerkship: a qualitative study of the informal curriculum.Acad Med.2005;80(7):641–647.
- ,,.How do medical residents discuss resuscitation with patients? Official journal of the Society for Research and Education in Primary Care Internal Medicine.J Gen Intern Med.1995;10(8):436–442.
- ,,.See one, do one, teach one? House staff experience discussing do‐not‐resuscitate orders.Arch Intern Med.1996;156(12):1285–1289.
- ,,,.Residents' end‐of‐life decision making with adult hospitalized patients: a review of the literature.Acad Med.2005:80(7)622–633.
- .Physician error and disclosure.Clin Obstet Gynecol.2008;51(4):700–708.
- .Revealing medical errors to your patients.Chest.2009;133:1064–1065.
- .Apology in medical practice.JAMA.2006;296:1401–1404.
- .What if Osler were one of us? Inpatient teaching today.J Gen Intern Med.1997;12(Suppl 2):S41–S48.
- Malcolm Gladwell.Outliers.New York:Little, Brown, Co.,2008: p.177–223.
- ,,,,.Attributes of excellent attending‐physician role models.N Engl J Med.1998;339(27):1986–1993.
Hope
Hello Mrs. K, I'm Dr. Baru, I said. I squeezed the hand limply resting on her cover with my own gloved one.
The room was quiet except for the sighing of her ventilator in the background. She had a broad round face with high cheek bones. Her skin was wrinkle‐free except around the eyes and the corners of her mouth. She breathed peacefully through her tracheostomy. She slowly nodded her head when I squeezed her hand and her blue eyes shone as she smiled broadly. Her son stood at my side, his mouth set in a straight line his eyes gazing intently at his mom. His handshake was firm and brisk, a single downstroke.
I was called in as the Palliative Care consultant. She's in denial, I was told. There's nothing more that we can do for her here. She had already been in the hospital for 2 months but this was the first time I was meeting her and her son. With the help of the Polish interpreter and her son, who had become adept at reading her lips and translating her breathy rasps, I began to sift through all of the information that they had been told, all of the information they had gathered on their own, and what they understood.
Mrs. K was 59 years old. She'd given up her job as a kindergarten teacher and come from Poland 3 years ago to help care for her first grandchild. Two years later, her daughter‐in‐law delivered 2 more grandchildren: twins. Just weeks after the delivery, Mrs. K was diagnosed with multiple myeloma. She was told that her prognosis was good and that, with chemotherapy, she had years to live. She thought about returning home but she felt fine. Though she didn't yet qualify for any kind of insurance, she was getting good care in our County health system. Besides, her grandchildren meant everything to her and her son and daughter‐in‐law needed her now more than ever.
Five months into her treatment she developed pain in her neck and started noticing numbness in her hands. She immediately went to the hospital where she was found to have a tumor in her cervical spine. Despite early radiation and surgery, she was completely paralyzed and dependent on mechanical ventilation within a week. In a matter of days she had been torn from life as she knew it.
It became clear during our conversation that, though Mrs. K was not physically uncomfortable, being confined to the hospital was difficult for her. Her grandchildren couldn't visit because she was on contact precautions. She missed them deeply. She missed sitting on her porch drinking her morning coffee. Unable to move her head, she spent most of her day staring at the ceiling or at the TV watching shows in a language she couldn't understand. She had met countless doctors, nurses, and medical personnel, endured multiple complications, including a pulseless arrest, and had been placed in 3 different ICUs. Yet she was unwavering in her desire to remain on the ventilator and continue doing everything.
Both she and her son expected that the treatments she had been getting would help get her off the ventilator so that she could go home. I struggled to balance their hopes with the information at hand, exploring realistic goals.
Could she go to a nursing home and wait and see if she will recover? I know they have these for people that are on ventilators, her son asked.
This was not going to be possible given her disease. Her paralysis was complete and permanent. She would not recover the ability to breathe on her own. Besides, without insurance they would have to pay for these services. It was not realistic to hope for this.
Could we fly her back to Poland so that she can die and be buried there?
She was not stable enough to fly without medical assistance. Furthermore, she would need to be in contact isolation given the virulence of her uniquely resistant bacteria. The cost to arrange an air ambulance was exorbitant and unaffordable on her son's electrician salary. It was not realistic to hope for this.
Well, if she can't go back to Poland and can't get off of the ventilator, could we set up a ventilator at home so that we can care for her there until she dies?
I explained that this would require help from medical personnel trained in ventilator management. They would also need assistance from individuals trained in palliative care to insure that Mrs. K had adequate nursing and symptom relief, particularly in the event of a medical emergency or a complication with the ventilator. The family would be required to pay for these services out of pocket. After spending hours contacting hospice agencies, medical suppliers, nursing agencies, friends of the family, and community organizations it became clear that even though the family was willing to bankrupt themselves for their mother's care there was not a safe, affordable solution. It was not realistic to hope for this.
What can you do for me? Mrs. K asked.
My tongue sat numbly in my mouth. I felt a tide of shame and sorrow rising. Nothing! I thought, knowing not to say it. At times like this, we often fall back on training; I tried to force her into the round hole.
We can care for you here. We can insure that you are as comfortable as possible for whatever time you have left. We can shift our focus from life‐prolonging treatments to those that are purely focused on your comfort. We can stop those treatments that will interrupt the time that you spend with your family, and try to give you and your family as much space as possible to be together here in the ICU. As your death approaches we would work to keep you as comfortable and peaceful as possible and to allow your family to be with you here at your bedside, but we wouldn't try to prolong the dying process.
I don't WANT to die. I WANT to go home, I WANT to smell fresh air. I'm willing to take risks with my life for that, but not otherwise.
My impotence, my inability to give this woman any of the things that she was hoping for was overwhelming. Her suffering was overwhelming. I was unable to find a thread of hope in her world. I was failing my patient.
Feeling a sense of despair, I told her, I cannot help you breathe on your own or smell the fresh air or be with your grandchildren. None of those goals are realistic. I don't know what I can do for you, but I would like to continue to see you. I want to come back tomorrow.
I hope you do, she said, her blue eyes shining as she smiled softly.
I was struck by the words. Though my loftier goals had been frustrated, I realized that my efforts and my presence at her bedside alone were easing her sufferingthis is something that we all hope for.
Hello Mrs. K, I'm Dr. Baru, I said. I squeezed the hand limply resting on her cover with my own gloved one.
The room was quiet except for the sighing of her ventilator in the background. She had a broad round face with high cheek bones. Her skin was wrinkle‐free except around the eyes and the corners of her mouth. She breathed peacefully through her tracheostomy. She slowly nodded her head when I squeezed her hand and her blue eyes shone as she smiled broadly. Her son stood at my side, his mouth set in a straight line his eyes gazing intently at his mom. His handshake was firm and brisk, a single downstroke.
I was called in as the Palliative Care consultant. She's in denial, I was told. There's nothing more that we can do for her here. She had already been in the hospital for 2 months but this was the first time I was meeting her and her son. With the help of the Polish interpreter and her son, who had become adept at reading her lips and translating her breathy rasps, I began to sift through all of the information that they had been told, all of the information they had gathered on their own, and what they understood.
Mrs. K was 59 years old. She'd given up her job as a kindergarten teacher and come from Poland 3 years ago to help care for her first grandchild. Two years later, her daughter‐in‐law delivered 2 more grandchildren: twins. Just weeks after the delivery, Mrs. K was diagnosed with multiple myeloma. She was told that her prognosis was good and that, with chemotherapy, she had years to live. She thought about returning home but she felt fine. Though she didn't yet qualify for any kind of insurance, she was getting good care in our County health system. Besides, her grandchildren meant everything to her and her son and daughter‐in‐law needed her now more than ever.
Five months into her treatment she developed pain in her neck and started noticing numbness in her hands. She immediately went to the hospital where she was found to have a tumor in her cervical spine. Despite early radiation and surgery, she was completely paralyzed and dependent on mechanical ventilation within a week. In a matter of days she had been torn from life as she knew it.
It became clear during our conversation that, though Mrs. K was not physically uncomfortable, being confined to the hospital was difficult for her. Her grandchildren couldn't visit because she was on contact precautions. She missed them deeply. She missed sitting on her porch drinking her morning coffee. Unable to move her head, she spent most of her day staring at the ceiling or at the TV watching shows in a language she couldn't understand. She had met countless doctors, nurses, and medical personnel, endured multiple complications, including a pulseless arrest, and had been placed in 3 different ICUs. Yet she was unwavering in her desire to remain on the ventilator and continue doing everything.
Both she and her son expected that the treatments she had been getting would help get her off the ventilator so that she could go home. I struggled to balance their hopes with the information at hand, exploring realistic goals.
Could she go to a nursing home and wait and see if she will recover? I know they have these for people that are on ventilators, her son asked.
This was not going to be possible given her disease. Her paralysis was complete and permanent. She would not recover the ability to breathe on her own. Besides, without insurance they would have to pay for these services. It was not realistic to hope for this.
Could we fly her back to Poland so that she can die and be buried there?
She was not stable enough to fly without medical assistance. Furthermore, she would need to be in contact isolation given the virulence of her uniquely resistant bacteria. The cost to arrange an air ambulance was exorbitant and unaffordable on her son's electrician salary. It was not realistic to hope for this.
Well, if she can't go back to Poland and can't get off of the ventilator, could we set up a ventilator at home so that we can care for her there until she dies?
I explained that this would require help from medical personnel trained in ventilator management. They would also need assistance from individuals trained in palliative care to insure that Mrs. K had adequate nursing and symptom relief, particularly in the event of a medical emergency or a complication with the ventilator. The family would be required to pay for these services out of pocket. After spending hours contacting hospice agencies, medical suppliers, nursing agencies, friends of the family, and community organizations it became clear that even though the family was willing to bankrupt themselves for their mother's care there was not a safe, affordable solution. It was not realistic to hope for this.
What can you do for me? Mrs. K asked.
My tongue sat numbly in my mouth. I felt a tide of shame and sorrow rising. Nothing! I thought, knowing not to say it. At times like this, we often fall back on training; I tried to force her into the round hole.
We can care for you here. We can insure that you are as comfortable as possible for whatever time you have left. We can shift our focus from life‐prolonging treatments to those that are purely focused on your comfort. We can stop those treatments that will interrupt the time that you spend with your family, and try to give you and your family as much space as possible to be together here in the ICU. As your death approaches we would work to keep you as comfortable and peaceful as possible and to allow your family to be with you here at your bedside, but we wouldn't try to prolong the dying process.
I don't WANT to die. I WANT to go home, I WANT to smell fresh air. I'm willing to take risks with my life for that, but not otherwise.
My impotence, my inability to give this woman any of the things that she was hoping for was overwhelming. Her suffering was overwhelming. I was unable to find a thread of hope in her world. I was failing my patient.
Feeling a sense of despair, I told her, I cannot help you breathe on your own or smell the fresh air or be with your grandchildren. None of those goals are realistic. I don't know what I can do for you, but I would like to continue to see you. I want to come back tomorrow.
I hope you do, she said, her blue eyes shining as she smiled softly.
I was struck by the words. Though my loftier goals had been frustrated, I realized that my efforts and my presence at her bedside alone were easing her sufferingthis is something that we all hope for.
Hello Mrs. K, I'm Dr. Baru, I said. I squeezed the hand limply resting on her cover with my own gloved one.
The room was quiet except for the sighing of her ventilator in the background. She had a broad round face with high cheek bones. Her skin was wrinkle‐free except around the eyes and the corners of her mouth. She breathed peacefully through her tracheostomy. She slowly nodded her head when I squeezed her hand and her blue eyes shone as she smiled broadly. Her son stood at my side, his mouth set in a straight line his eyes gazing intently at his mom. His handshake was firm and brisk, a single downstroke.
I was called in as the Palliative Care consultant. She's in denial, I was told. There's nothing more that we can do for her here. She had already been in the hospital for 2 months but this was the first time I was meeting her and her son. With the help of the Polish interpreter and her son, who had become adept at reading her lips and translating her breathy rasps, I began to sift through all of the information that they had been told, all of the information they had gathered on their own, and what they understood.
Mrs. K was 59 years old. She'd given up her job as a kindergarten teacher and come from Poland 3 years ago to help care for her first grandchild. Two years later, her daughter‐in‐law delivered 2 more grandchildren: twins. Just weeks after the delivery, Mrs. K was diagnosed with multiple myeloma. She was told that her prognosis was good and that, with chemotherapy, she had years to live. She thought about returning home but she felt fine. Though she didn't yet qualify for any kind of insurance, she was getting good care in our County health system. Besides, her grandchildren meant everything to her and her son and daughter‐in‐law needed her now more than ever.
Five months into her treatment she developed pain in her neck and started noticing numbness in her hands. She immediately went to the hospital where she was found to have a tumor in her cervical spine. Despite early radiation and surgery, she was completely paralyzed and dependent on mechanical ventilation within a week. In a matter of days she had been torn from life as she knew it.
It became clear during our conversation that, though Mrs. K was not physically uncomfortable, being confined to the hospital was difficult for her. Her grandchildren couldn't visit because she was on contact precautions. She missed them deeply. She missed sitting on her porch drinking her morning coffee. Unable to move her head, she spent most of her day staring at the ceiling or at the TV watching shows in a language she couldn't understand. She had met countless doctors, nurses, and medical personnel, endured multiple complications, including a pulseless arrest, and had been placed in 3 different ICUs. Yet she was unwavering in her desire to remain on the ventilator and continue doing everything.
Both she and her son expected that the treatments she had been getting would help get her off the ventilator so that she could go home. I struggled to balance their hopes with the information at hand, exploring realistic goals.
Could she go to a nursing home and wait and see if she will recover? I know they have these for people that are on ventilators, her son asked.
This was not going to be possible given her disease. Her paralysis was complete and permanent. She would not recover the ability to breathe on her own. Besides, without insurance they would have to pay for these services. It was not realistic to hope for this.
Could we fly her back to Poland so that she can die and be buried there?
She was not stable enough to fly without medical assistance. Furthermore, she would need to be in contact isolation given the virulence of her uniquely resistant bacteria. The cost to arrange an air ambulance was exorbitant and unaffordable on her son's electrician salary. It was not realistic to hope for this.
Well, if she can't go back to Poland and can't get off of the ventilator, could we set up a ventilator at home so that we can care for her there until she dies?
I explained that this would require help from medical personnel trained in ventilator management. They would also need assistance from individuals trained in palliative care to insure that Mrs. K had adequate nursing and symptom relief, particularly in the event of a medical emergency or a complication with the ventilator. The family would be required to pay for these services out of pocket. After spending hours contacting hospice agencies, medical suppliers, nursing agencies, friends of the family, and community organizations it became clear that even though the family was willing to bankrupt themselves for their mother's care there was not a safe, affordable solution. It was not realistic to hope for this.
What can you do for me? Mrs. K asked.
My tongue sat numbly in my mouth. I felt a tide of shame and sorrow rising. Nothing! I thought, knowing not to say it. At times like this, we often fall back on training; I tried to force her into the round hole.
We can care for you here. We can insure that you are as comfortable as possible for whatever time you have left. We can shift our focus from life‐prolonging treatments to those that are purely focused on your comfort. We can stop those treatments that will interrupt the time that you spend with your family, and try to give you and your family as much space as possible to be together here in the ICU. As your death approaches we would work to keep you as comfortable and peaceful as possible and to allow your family to be with you here at your bedside, but we wouldn't try to prolong the dying process.
I don't WANT to die. I WANT to go home, I WANT to smell fresh air. I'm willing to take risks with my life for that, but not otherwise.
My impotence, my inability to give this woman any of the things that she was hoping for was overwhelming. Her suffering was overwhelming. I was unable to find a thread of hope in her world. I was failing my patient.
Feeling a sense of despair, I told her, I cannot help you breathe on your own or smell the fresh air or be with your grandchildren. None of those goals are realistic. I don't know what I can do for you, but I would like to continue to see you. I want to come back tomorrow.
I hope you do, she said, her blue eyes shining as she smiled softly.
I was struck by the words. Though my loftier goals had been frustrated, I realized that my efforts and my presence at her bedside alone were easing her sufferingthis is something that we all hope for.
The New Vocabulary of Healthcare Reform
On March 21, 2010, the United States Congress passed the most comprehensive healthcare reform bill since the formation of Medicare. The legislation's greatest impact will be to improve access for nearly 50 million Americans who are presently uninsured. Yet the bill does little to tackle the fundamental problems of the payment and delivery systemsproblems that have resulted in major quality gaps, large numbers of medical errors, fragmented care, and backbreaking costs.
While these tough questions were mostly kicked down the road, the debate did bring many of the key questions and potential solutions into high relief. Our political leaders, pundits, and health policy scholars introduced or popularized a number of terms during the healthcare debates of 2009‐2010 (Table 1). I will attempt to place them in context and discuss their implications for future healthcare reform efforts.
|
| Value‐based purchasing |
| Bending the cost curve |
| Comparative effectiveness research (see also NICE) |
| Dartmouth atlas (see also McAllen, Texas) |
| Death panels (see also rationing) |
| Bundled payments |
| Accountable care organizations (see also Mayo Clinic, Cleveland Clinic, Geisinger; replaces HMOs) |
Some Context for the Healthcare Reform Debate
In our capitalistic economy, we make most purchases based on considerations of value: quality divided by cost. There are few among us wealthy enough to always buy the best product, or cheap enough to always buy the least expensive. Instead, we try to determine value when we purchase a restaurant meal, a house, or a vacation.
Healthcare has traditionally been the major exception to this rule, both because healthcare insurance has partly insulated consumers (patients or their proxies) from the cost consequences of their decisions, and because it is so difficult to determine the quality of healthcare. But, over the past 10 to 15 years, problems with both the numerator and denominator of this equation have created widespread recognition of the need for change.
In the numerator, we now appreciate that there are nearly 100,000 deaths per year from medical mistakes;1 that we deliver evidence‐based care only about half the time,2 and that our healthcare system is extraordinarily fragmented and chaotic. We also know that there are more than 40 million people without healthcare insurance, a uniquely American problem, since other industrialized countries manage to guarantee coverage.
This is the fundamental conundrum that needs to be addressed by healthcare reform: we have a system that produces surprisingly low‐quality, unreliable care at an exorbitant and ever‐increasing cost, and does so while leaving more than 1 out of 8 citizens without coverage. Although government is a large payer (through Medicare, Medicaid, the Veterans Affairs [VA] programs and others), most Americans receive healthcare coverage as an employee benefit; a smaller number pay for health insurance themselves. The government has a key role even in these nongovernment‐sponsored payment systems, by providing tax breaks for healthcare coverage, creating a regulatory framework, and often defining the market through its actions in its public programs.
The end result is that all the involved partiesgovernments, businesses, providers, and patientsare crying out for change. An observer of this situation feels compelled to invoke the popular version of Stein's Law: if a trend can't continue, it won't.
Bending the Cost Curve
Everyone is now familiar with the scary trends (such as in Figure 1) demonstrating the unsustainable rate of healthcare inflation in the US, trends that are projected to lead to the insolvency of the Medicare Trust Fund within a decade. The term bending the cost curve implies that our solvency depends not on lowering total costs (a political impossibility), but rather on simply decreasing the rate of rise. There are only so many ways to do this.
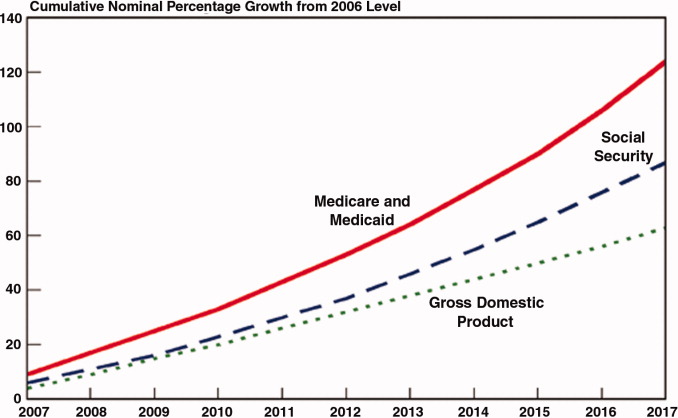
The most attractive, of course, is to stop providing expensive care that adds no or little value in terms of patient outcomes. The term comparative effectiveness research (CER) emerged over the past few years to describe research that pits one approach against another (or, presumably, against no treatment) on both outcomes and cost.3 Obviously, one would favor the less expensive treatment if the efficacy were equal. However, the more common (and politically fraught) question is whether a more expensive but slightly better approach is worth its additional cost.
This, of course, makes complete sense in a world of limited resources, and some countries, mostly notably the United Kingdom, are using CER to inform healthcare coverage decisions. In the United Kingdom, the research is analyzed, and coverage recommendations made, by an organization called the National Institute for Health and Clinical Excellence (NICE).4 While NICE appears to be working well, all signs indicate that the US political system is not ready for such an approach. In fact, although Medicare generally supports CER, most of the healthcare reform proposals considered by Congress explicitly prohibited Medicare from using CER results to influence payment decisions.
If an overall CER approach is too politically difficult for the US, how about focusing on 1 small segment of healthcare: expensive care at the end of life? Over the past 30 years, a group of Dartmouth researchers has examined the costs and quality of care across the entire country, demonstrating a ubiquitous pattern of highly variable costs (varying up to 2‐fold) that is unassociated with quality and outcomes (and sometimes even inversely associated).5 The findings, well known among healthcare researchers but relatively unknown by the public until recently, were brought to public attention by a 2009 New Yorker article that made the border town of McAllen, Texas the poster child for a medical culture that produces high costs without comparable benefits.6 The Dartmouth researchers, who publish their data in a document known as The Dartmouth Atlas, have found striking variations in care at the end of life. For example, even among academic medical centers (which presumably have similarly sick patient populations), the number of hospital days in the last 6 months of life varies strikingly: patients at New York University average 27.1 days, whereas those in my hospital average 11.5 days.7
So promoting better end‐of‐life carebeing sure that patients are aware of their options and that high‐quality palliative care is availableseemed like an obvious solution to part of our cost‐quality conundrum. Some early drafts of reform bills in Congress contained provisions to pay for physicians' time to discuss end‐of‐life options. This, of course, was caricaturized into the now famous Death Panelsproving that American political discourse is not yet mature enough to support realistic discussions about difficult subjects.8
It seems like having payers (government, insurance companies) make formal decisions about which services to cover (ie, rationing) is too hard. Is there another way to force these tough choices but do so without creating a political piata?
Encircling a Population
Rather than explicitly rationing care (using CER results, for example), another way of constraining costs is to place a population of patients on a fixed budget. There is evidence that provider organizations, working within such a budget (structured in a way that permits the providers to pocket any savings), are able to reorganize and change their practice style in a way that can cut costs.9 In the 1990s, we conducted a national experiment by promoting managed care, working through integrated delivery systems called Health Maintenance Organizations (HMOs) that received fixed, capitated payments for every patient. And, in fact, these organizations did cut overall costs.
The problem was that patients neither liked HMOs nor trusted that they were acting in their best interests. Ultimately, managed care became a less important delivery mechanism, and even patients who remained in HMOs had fewer constraints on their choices. Of course, the softening of the managed care market resulted in an uptick in healthcare inflation, contributing to our present predicament.
The concept of fixed payments has resurfaced, but with some modern twists. It appears that organizations that perform best on the Dartmouth measures (namely, they provide high quality care at lower costs) are generally large delivery systems with advanced information technology, strong primary care infrastructures, andprobably most importantlytight integration between physicians and the rest of the organization. During the healthcare debate, the organizations that received the most attention were the Mayo and Cleveland Clinics and the Geisinger system in central Pennsylvania. The problem is that the defining characteristic of these organizations (and others like them) is that they have been at this business of integrated care for more than 50 years! Can the model be emulated?
Two main policy changes have been promoted to try to achieve this integration: one is a change in payment structure, the other a change in organization. The first is known as bundlingin which multiple providers are reimbursed a single sum for all the care related to an episode of illness (such as a hospitalization and a 60‐ or 90‐day period afterwards). You will recognize this as a new form of capitation, but, rather than covering all of a patient's care, a more circumscribed version, focusing on a single illness or procedure. There is some evidence that bundling does reduce costs and may improve quality, by forcing hospitals, post‐acute care facilities, and doctors into collaborative arrangements (both to deliver care and, just as complex, to split the single payment without undue acrimony).10 Fisher et al.11 have promoted a new structure to deliver this kind of bundled care more effectively: The Accountable Care Organization (ACO), which is best thought of as a less ambitious, and potentially more virtual, incarnation of the HMO.
Interestingly, while many healthcare organizations have struggled to remake themselves in Mayo's image in preparation for upcoming pressures to form ACOs, some organizations with hospitalist programs need look no further than these programs to chart a course toward more effective physician‐hospital integration.12 Why? The majority of US hospitals now have hospitalists, and virtually all hospitalist programs receive support payments from their hospital (a sizable minority are on salary from the hospital). Hospitalists recognize that part of their value equation (which justifies the hospital support dollars) is that they help the hospital deliver higher quality care more efficiently. Because of this relationship, a well‐functioning hospitalist program can assume many of the attributes of an ACO, even in organizations with otherwise challenging physician‐hospital relations. It may be that hospitals and doctors need not look to Rochester, Minnesota or Danville, Pennsylvania for positive examples of physician‐hospital integration, but simply to their own local hospitalist groups.
The Bottom Line
While proponents of the Obama reform plan celebrate its passage, virtually all experts agree that it left fundamental problems with the healthcare system unaddressed. Although the 20092010 debate did not solve these problems, the new vocabulary introduced during the debateboth reasonable policy ideas like bundling and ACOs and cynical caricatures like death panelsare here to stay. Understanding these terms and the context that shaped them will be critical for hospitalists and other stakeholders interested in the future of the American healthcare system.
- ,,, eds.To Err is Human: Building a Safer Health System.Washington DC:Committee on Quality of Health Care in America, Institute of Medicine. National Academy Press,2000.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348:2635–2645.
- ,.Health care reform and the need for comparative‐effectiveness research.N Engl J Med.2010;362:e6.
- .Saying no isn't NICE—the travails of Britain's National Institute for health and clinical excellence.N Engl J Med.2008;359:1977–1981.
- .Wrestling with variation: an interview with Jack Wennberg [interviewed by Fitzhugh Mullan].Health Aff (Millwood).2004; Suppl Web Exclsives:VAR73–80.
- .The cost conundrum. What a Texas town can teach us about health care.The New Yorker2009. Available at: http://www.newyorker. com/reporting/2009/06/01/090601fa_fact_gawande. Accessed February 2010.
- .Researchers find huge variations in end‐of‐life treatment.New York Times.2008. Available at: http://www.nytimes.com/2008/04/07/health/policy/07care.html?_r=1. Accessed February 2010.
- .Ending end‐of‐life phobia—a prescription for enlightened health care reform.N Engl J Med.2009. Available at: http://healthcarere form.nejm.org/?p=2580. Accessed February 2010.
- ,.The RAND Health Insurance Experiment and HMOs.Med Care.1990;28:191–200.
- ,,.Cost savings and physician responses to global bundled payments for Medicare heart bypass surgery.Health Care Fin Rev.1997;19:41–57.
- ,,,.Creating accountable care organizations: the extended hospital medical staff.Health Aff (Millwood).2007;26(1):w44–w57.
- . Hospitalists: a little slice of Mayo. Available at: http://community.the‐hospitalist.org/blogs/wachters_world/archive/2009/08/30/hospitalists‐a‐little‐slice‐of‐mayo.aspx. Accessed February 2010.
On March 21, 2010, the United States Congress passed the most comprehensive healthcare reform bill since the formation of Medicare. The legislation's greatest impact will be to improve access for nearly 50 million Americans who are presently uninsured. Yet the bill does little to tackle the fundamental problems of the payment and delivery systemsproblems that have resulted in major quality gaps, large numbers of medical errors, fragmented care, and backbreaking costs.
While these tough questions were mostly kicked down the road, the debate did bring many of the key questions and potential solutions into high relief. Our political leaders, pundits, and health policy scholars introduced or popularized a number of terms during the healthcare debates of 2009‐2010 (Table 1). I will attempt to place them in context and discuss their implications for future healthcare reform efforts.
|
| Value‐based purchasing |
| Bending the cost curve |
| Comparative effectiveness research (see also NICE) |
| Dartmouth atlas (see also McAllen, Texas) |
| Death panels (see also rationing) |
| Bundled payments |
| Accountable care organizations (see also Mayo Clinic, Cleveland Clinic, Geisinger; replaces HMOs) |
Some Context for the Healthcare Reform Debate
In our capitalistic economy, we make most purchases based on considerations of value: quality divided by cost. There are few among us wealthy enough to always buy the best product, or cheap enough to always buy the least expensive. Instead, we try to determine value when we purchase a restaurant meal, a house, or a vacation.
Healthcare has traditionally been the major exception to this rule, both because healthcare insurance has partly insulated consumers (patients or their proxies) from the cost consequences of their decisions, and because it is so difficult to determine the quality of healthcare. But, over the past 10 to 15 years, problems with both the numerator and denominator of this equation have created widespread recognition of the need for change.
In the numerator, we now appreciate that there are nearly 100,000 deaths per year from medical mistakes;1 that we deliver evidence‐based care only about half the time,2 and that our healthcare system is extraordinarily fragmented and chaotic. We also know that there are more than 40 million people without healthcare insurance, a uniquely American problem, since other industrialized countries manage to guarantee coverage.
This is the fundamental conundrum that needs to be addressed by healthcare reform: we have a system that produces surprisingly low‐quality, unreliable care at an exorbitant and ever‐increasing cost, and does so while leaving more than 1 out of 8 citizens without coverage. Although government is a large payer (through Medicare, Medicaid, the Veterans Affairs [VA] programs and others), most Americans receive healthcare coverage as an employee benefit; a smaller number pay for health insurance themselves. The government has a key role even in these nongovernment‐sponsored payment systems, by providing tax breaks for healthcare coverage, creating a regulatory framework, and often defining the market through its actions in its public programs.
The end result is that all the involved partiesgovernments, businesses, providers, and patientsare crying out for change. An observer of this situation feels compelled to invoke the popular version of Stein's Law: if a trend can't continue, it won't.
Bending the Cost Curve
Everyone is now familiar with the scary trends (such as in Figure 1) demonstrating the unsustainable rate of healthcare inflation in the US, trends that are projected to lead to the insolvency of the Medicare Trust Fund within a decade. The term bending the cost curve implies that our solvency depends not on lowering total costs (a political impossibility), but rather on simply decreasing the rate of rise. There are only so many ways to do this.

The most attractive, of course, is to stop providing expensive care that adds no or little value in terms of patient outcomes. The term comparative effectiveness research (CER) emerged over the past few years to describe research that pits one approach against another (or, presumably, against no treatment) on both outcomes and cost.3 Obviously, one would favor the less expensive treatment if the efficacy were equal. However, the more common (and politically fraught) question is whether a more expensive but slightly better approach is worth its additional cost.
This, of course, makes complete sense in a world of limited resources, and some countries, mostly notably the United Kingdom, are using CER to inform healthcare coverage decisions. In the United Kingdom, the research is analyzed, and coverage recommendations made, by an organization called the National Institute for Health and Clinical Excellence (NICE).4 While NICE appears to be working well, all signs indicate that the US political system is not ready for such an approach. In fact, although Medicare generally supports CER, most of the healthcare reform proposals considered by Congress explicitly prohibited Medicare from using CER results to influence payment decisions.
If an overall CER approach is too politically difficult for the US, how about focusing on 1 small segment of healthcare: expensive care at the end of life? Over the past 30 years, a group of Dartmouth researchers has examined the costs and quality of care across the entire country, demonstrating a ubiquitous pattern of highly variable costs (varying up to 2‐fold) that is unassociated with quality and outcomes (and sometimes even inversely associated).5 The findings, well known among healthcare researchers but relatively unknown by the public until recently, were brought to public attention by a 2009 New Yorker article that made the border town of McAllen, Texas the poster child for a medical culture that produces high costs without comparable benefits.6 The Dartmouth researchers, who publish their data in a document known as The Dartmouth Atlas, have found striking variations in care at the end of life. For example, even among academic medical centers (which presumably have similarly sick patient populations), the number of hospital days in the last 6 months of life varies strikingly: patients at New York University average 27.1 days, whereas those in my hospital average 11.5 days.7
So promoting better end‐of‐life carebeing sure that patients are aware of their options and that high‐quality palliative care is availableseemed like an obvious solution to part of our cost‐quality conundrum. Some early drafts of reform bills in Congress contained provisions to pay for physicians' time to discuss end‐of‐life options. This, of course, was caricaturized into the now famous Death Panelsproving that American political discourse is not yet mature enough to support realistic discussions about difficult subjects.8
It seems like having payers (government, insurance companies) make formal decisions about which services to cover (ie, rationing) is too hard. Is there another way to force these tough choices but do so without creating a political piata?
Encircling a Population
Rather than explicitly rationing care (using CER results, for example), another way of constraining costs is to place a population of patients on a fixed budget. There is evidence that provider organizations, working within such a budget (structured in a way that permits the providers to pocket any savings), are able to reorganize and change their practice style in a way that can cut costs.9 In the 1990s, we conducted a national experiment by promoting managed care, working through integrated delivery systems called Health Maintenance Organizations (HMOs) that received fixed, capitated payments for every patient. And, in fact, these organizations did cut overall costs.
The problem was that patients neither liked HMOs nor trusted that they were acting in their best interests. Ultimately, managed care became a less important delivery mechanism, and even patients who remained in HMOs had fewer constraints on their choices. Of course, the softening of the managed care market resulted in an uptick in healthcare inflation, contributing to our present predicament.
The concept of fixed payments has resurfaced, but with some modern twists. It appears that organizations that perform best on the Dartmouth measures (namely, they provide high quality care at lower costs) are generally large delivery systems with advanced information technology, strong primary care infrastructures, andprobably most importantlytight integration between physicians and the rest of the organization. During the healthcare debate, the organizations that received the most attention were the Mayo and Cleveland Clinics and the Geisinger system in central Pennsylvania. The problem is that the defining characteristic of these organizations (and others like them) is that they have been at this business of integrated care for more than 50 years! Can the model be emulated?
Two main policy changes have been promoted to try to achieve this integration: one is a change in payment structure, the other a change in organization. The first is known as bundlingin which multiple providers are reimbursed a single sum for all the care related to an episode of illness (such as a hospitalization and a 60‐ or 90‐day period afterwards). You will recognize this as a new form of capitation, but, rather than covering all of a patient's care, a more circumscribed version, focusing on a single illness or procedure. There is some evidence that bundling does reduce costs and may improve quality, by forcing hospitals, post‐acute care facilities, and doctors into collaborative arrangements (both to deliver care and, just as complex, to split the single payment without undue acrimony).10 Fisher et al.11 have promoted a new structure to deliver this kind of bundled care more effectively: The Accountable Care Organization (ACO), which is best thought of as a less ambitious, and potentially more virtual, incarnation of the HMO.
Interestingly, while many healthcare organizations have struggled to remake themselves in Mayo's image in preparation for upcoming pressures to form ACOs, some organizations with hospitalist programs need look no further than these programs to chart a course toward more effective physician‐hospital integration.12 Why? The majority of US hospitals now have hospitalists, and virtually all hospitalist programs receive support payments from their hospital (a sizable minority are on salary from the hospital). Hospitalists recognize that part of their value equation (which justifies the hospital support dollars) is that they help the hospital deliver higher quality care more efficiently. Because of this relationship, a well‐functioning hospitalist program can assume many of the attributes of an ACO, even in organizations with otherwise challenging physician‐hospital relations. It may be that hospitals and doctors need not look to Rochester, Minnesota or Danville, Pennsylvania for positive examples of physician‐hospital integration, but simply to their own local hospitalist groups.
The Bottom Line
While proponents of the Obama reform plan celebrate its passage, virtually all experts agree that it left fundamental problems with the healthcare system unaddressed. Although the 20092010 debate did not solve these problems, the new vocabulary introduced during the debateboth reasonable policy ideas like bundling and ACOs and cynical caricatures like death panelsare here to stay. Understanding these terms and the context that shaped them will be critical for hospitalists and other stakeholders interested in the future of the American healthcare system.
On March 21, 2010, the United States Congress passed the most comprehensive healthcare reform bill since the formation of Medicare. The legislation's greatest impact will be to improve access for nearly 50 million Americans who are presently uninsured. Yet the bill does little to tackle the fundamental problems of the payment and delivery systemsproblems that have resulted in major quality gaps, large numbers of medical errors, fragmented care, and backbreaking costs.
While these tough questions were mostly kicked down the road, the debate did bring many of the key questions and potential solutions into high relief. Our political leaders, pundits, and health policy scholars introduced or popularized a number of terms during the healthcare debates of 2009‐2010 (Table 1). I will attempt to place them in context and discuss their implications for future healthcare reform efforts.
|
| Value‐based purchasing |
| Bending the cost curve |
| Comparative effectiveness research (see also NICE) |
| Dartmouth atlas (see also McAllen, Texas) |
| Death panels (see also rationing) |
| Bundled payments |
| Accountable care organizations (see also Mayo Clinic, Cleveland Clinic, Geisinger; replaces HMOs) |
Some Context for the Healthcare Reform Debate
In our capitalistic economy, we make most purchases based on considerations of value: quality divided by cost. There are few among us wealthy enough to always buy the best product, or cheap enough to always buy the least expensive. Instead, we try to determine value when we purchase a restaurant meal, a house, or a vacation.
Healthcare has traditionally been the major exception to this rule, both because healthcare insurance has partly insulated consumers (patients or their proxies) from the cost consequences of their decisions, and because it is so difficult to determine the quality of healthcare. But, over the past 10 to 15 years, problems with both the numerator and denominator of this equation have created widespread recognition of the need for change.
In the numerator, we now appreciate that there are nearly 100,000 deaths per year from medical mistakes;1 that we deliver evidence‐based care only about half the time,2 and that our healthcare system is extraordinarily fragmented and chaotic. We also know that there are more than 40 million people without healthcare insurance, a uniquely American problem, since other industrialized countries manage to guarantee coverage.
This is the fundamental conundrum that needs to be addressed by healthcare reform: we have a system that produces surprisingly low‐quality, unreliable care at an exorbitant and ever‐increasing cost, and does so while leaving more than 1 out of 8 citizens without coverage. Although government is a large payer (through Medicare, Medicaid, the Veterans Affairs [VA] programs and others), most Americans receive healthcare coverage as an employee benefit; a smaller number pay for health insurance themselves. The government has a key role even in these nongovernment‐sponsored payment systems, by providing tax breaks for healthcare coverage, creating a regulatory framework, and often defining the market through its actions in its public programs.
The end result is that all the involved partiesgovernments, businesses, providers, and patientsare crying out for change. An observer of this situation feels compelled to invoke the popular version of Stein's Law: if a trend can't continue, it won't.
Bending the Cost Curve
Everyone is now familiar with the scary trends (such as in Figure 1) demonstrating the unsustainable rate of healthcare inflation in the US, trends that are projected to lead to the insolvency of the Medicare Trust Fund within a decade. The term bending the cost curve implies that our solvency depends not on lowering total costs (a political impossibility), but rather on simply decreasing the rate of rise. There are only so many ways to do this.

The most attractive, of course, is to stop providing expensive care that adds no or little value in terms of patient outcomes. The term comparative effectiveness research (CER) emerged over the past few years to describe research that pits one approach against another (or, presumably, against no treatment) on both outcomes and cost.3 Obviously, one would favor the less expensive treatment if the efficacy were equal. However, the more common (and politically fraught) question is whether a more expensive but slightly better approach is worth its additional cost.
This, of course, makes complete sense in a world of limited resources, and some countries, mostly notably the United Kingdom, are using CER to inform healthcare coverage decisions. In the United Kingdom, the research is analyzed, and coverage recommendations made, by an organization called the National Institute for Health and Clinical Excellence (NICE).4 While NICE appears to be working well, all signs indicate that the US political system is not ready for such an approach. In fact, although Medicare generally supports CER, most of the healthcare reform proposals considered by Congress explicitly prohibited Medicare from using CER results to influence payment decisions.
If an overall CER approach is too politically difficult for the US, how about focusing on 1 small segment of healthcare: expensive care at the end of life? Over the past 30 years, a group of Dartmouth researchers has examined the costs and quality of care across the entire country, demonstrating a ubiquitous pattern of highly variable costs (varying up to 2‐fold) that is unassociated with quality and outcomes (and sometimes even inversely associated).5 The findings, well known among healthcare researchers but relatively unknown by the public until recently, were brought to public attention by a 2009 New Yorker article that made the border town of McAllen, Texas the poster child for a medical culture that produces high costs without comparable benefits.6 The Dartmouth researchers, who publish their data in a document known as The Dartmouth Atlas, have found striking variations in care at the end of life. For example, even among academic medical centers (which presumably have similarly sick patient populations), the number of hospital days in the last 6 months of life varies strikingly: patients at New York University average 27.1 days, whereas those in my hospital average 11.5 days.7
So promoting better end‐of‐life carebeing sure that patients are aware of their options and that high‐quality palliative care is availableseemed like an obvious solution to part of our cost‐quality conundrum. Some early drafts of reform bills in Congress contained provisions to pay for physicians' time to discuss end‐of‐life options. This, of course, was caricaturized into the now famous Death Panelsproving that American political discourse is not yet mature enough to support realistic discussions about difficult subjects.8
It seems like having payers (government, insurance companies) make formal decisions about which services to cover (ie, rationing) is too hard. Is there another way to force these tough choices but do so without creating a political piata?
Encircling a Population
Rather than explicitly rationing care (using CER results, for example), another way of constraining costs is to place a population of patients on a fixed budget. There is evidence that provider organizations, working within such a budget (structured in a way that permits the providers to pocket any savings), are able to reorganize and change their practice style in a way that can cut costs.9 In the 1990s, we conducted a national experiment by promoting managed care, working through integrated delivery systems called Health Maintenance Organizations (HMOs) that received fixed, capitated payments for every patient. And, in fact, these organizations did cut overall costs.
The problem was that patients neither liked HMOs nor trusted that they were acting in their best interests. Ultimately, managed care became a less important delivery mechanism, and even patients who remained in HMOs had fewer constraints on their choices. Of course, the softening of the managed care market resulted in an uptick in healthcare inflation, contributing to our present predicament.
The concept of fixed payments has resurfaced, but with some modern twists. It appears that organizations that perform best on the Dartmouth measures (namely, they provide high quality care at lower costs) are generally large delivery systems with advanced information technology, strong primary care infrastructures, andprobably most importantlytight integration between physicians and the rest of the organization. During the healthcare debate, the organizations that received the most attention were the Mayo and Cleveland Clinics and the Geisinger system in central Pennsylvania. The problem is that the defining characteristic of these organizations (and others like them) is that they have been at this business of integrated care for more than 50 years! Can the model be emulated?
Two main policy changes have been promoted to try to achieve this integration: one is a change in payment structure, the other a change in organization. The first is known as bundlingin which multiple providers are reimbursed a single sum for all the care related to an episode of illness (such as a hospitalization and a 60‐ or 90‐day period afterwards). You will recognize this as a new form of capitation, but, rather than covering all of a patient's care, a more circumscribed version, focusing on a single illness or procedure. There is some evidence that bundling does reduce costs and may improve quality, by forcing hospitals, post‐acute care facilities, and doctors into collaborative arrangements (both to deliver care and, just as complex, to split the single payment without undue acrimony).10 Fisher et al.11 have promoted a new structure to deliver this kind of bundled care more effectively: The Accountable Care Organization (ACO), which is best thought of as a less ambitious, and potentially more virtual, incarnation of the HMO.
Interestingly, while many healthcare organizations have struggled to remake themselves in Mayo's image in preparation for upcoming pressures to form ACOs, some organizations with hospitalist programs need look no further than these programs to chart a course toward more effective physician‐hospital integration.12 Why? The majority of US hospitals now have hospitalists, and virtually all hospitalist programs receive support payments from their hospital (a sizable minority are on salary from the hospital). Hospitalists recognize that part of their value equation (which justifies the hospital support dollars) is that they help the hospital deliver higher quality care more efficiently. Because of this relationship, a well‐functioning hospitalist program can assume many of the attributes of an ACO, even in organizations with otherwise challenging physician‐hospital relations. It may be that hospitals and doctors need not look to Rochester, Minnesota or Danville, Pennsylvania for positive examples of physician‐hospital integration, but simply to their own local hospitalist groups.
The Bottom Line
While proponents of the Obama reform plan celebrate its passage, virtually all experts agree that it left fundamental problems with the healthcare system unaddressed. Although the 20092010 debate did not solve these problems, the new vocabulary introduced during the debateboth reasonable policy ideas like bundling and ACOs and cynical caricatures like death panelsare here to stay. Understanding these terms and the context that shaped them will be critical for hospitalists and other stakeholders interested in the future of the American healthcare system.
- ,,, eds.To Err is Human: Building a Safer Health System.Washington DC:Committee on Quality of Health Care in America, Institute of Medicine. National Academy Press,2000.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348:2635–2645.
- ,.Health care reform and the need for comparative‐effectiveness research.N Engl J Med.2010;362:e6.
- .Saying no isn't NICE—the travails of Britain's National Institute for health and clinical excellence.N Engl J Med.2008;359:1977–1981.
- .Wrestling with variation: an interview with Jack Wennberg [interviewed by Fitzhugh Mullan].Health Aff (Millwood).2004; Suppl Web Exclsives:VAR73–80.
- .The cost conundrum. What a Texas town can teach us about health care.The New Yorker2009. Available at: http://www.newyorker. com/reporting/2009/06/01/090601fa_fact_gawande. Accessed February 2010.
- .Researchers find huge variations in end‐of‐life treatment.New York Times.2008. Available at: http://www.nytimes.com/2008/04/07/health/policy/07care.html?_r=1. Accessed February 2010.
- .Ending end‐of‐life phobia—a prescription for enlightened health care reform.N Engl J Med.2009. Available at: http://healthcarere form.nejm.org/?p=2580. Accessed February 2010.
- ,.The RAND Health Insurance Experiment and HMOs.Med Care.1990;28:191–200.
- ,,.Cost savings and physician responses to global bundled payments for Medicare heart bypass surgery.Health Care Fin Rev.1997;19:41–57.
- ,,,.Creating accountable care organizations: the extended hospital medical staff.Health Aff (Millwood).2007;26(1):w44–w57.
- . Hospitalists: a little slice of Mayo. Available at: http://community.the‐hospitalist.org/blogs/wachters_world/archive/2009/08/30/hospitalists‐a‐little‐slice‐of‐mayo.aspx. Accessed February 2010.
- ,,, eds.To Err is Human: Building a Safer Health System.Washington DC:Committee on Quality of Health Care in America, Institute of Medicine. National Academy Press,2000.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348:2635–2645.
- ,.Health care reform and the need for comparative‐effectiveness research.N Engl J Med.2010;362:e6.
- .Saying no isn't NICE—the travails of Britain's National Institute for health and clinical excellence.N Engl J Med.2008;359:1977–1981.
- .Wrestling with variation: an interview with Jack Wennberg [interviewed by Fitzhugh Mullan].Health Aff (Millwood).2004; Suppl Web Exclsives:VAR73–80.
- .The cost conundrum. What a Texas town can teach us about health care.The New Yorker2009. Available at: http://www.newyorker. com/reporting/2009/06/01/090601fa_fact_gawande. Accessed February 2010.
- .Researchers find huge variations in end‐of‐life treatment.New York Times.2008. Available at: http://www.nytimes.com/2008/04/07/health/policy/07care.html?_r=1. Accessed February 2010.
- .Ending end‐of‐life phobia—a prescription for enlightened health care reform.N Engl J Med.2009. Available at: http://healthcarere form.nejm.org/?p=2580. Accessed February 2010.
- ,.The RAND Health Insurance Experiment and HMOs.Med Care.1990;28:191–200.
- ,,.Cost savings and physician responses to global bundled payments for Medicare heart bypass surgery.Health Care Fin Rev.1997;19:41–57.
- ,,,.Creating accountable care organizations: the extended hospital medical staff.Health Aff (Millwood).2007;26(1):w44–w57.
- . Hospitalists: a little slice of Mayo. Available at: http://community.the‐hospitalist.org/blogs/wachters_world/archive/2009/08/30/hospitalists‐a‐little‐slice‐of‐mayo.aspx. Accessed February 2010.