User login
Healthcare Costs
Let's think about what we need to do ourselves. We have to acknowledge that orders we write drive up health care costs.1 AMA President, Nancy H. Nielsen, MD, PhD
As the most prominent providers of inpatient care, hospitalists should be aware that, of the total annual expenditures on US healthcare ($2.3 trillion in 2007),2 approximately one‐third goes to hospital‐based medical care, over one‐half of which (57%) is covered by public funds through Medicare and Medicaid3; this high cost of healthcare is increasingly being blamed for unnecessarily burdening our economy and preventing our industries from being globally competitive. I believe that the high proportion of spending on inpatient care places hospitalists firmly in the center of the debate on how to reduce healthcare costs. It is well known that the United States spends about twice as much per capita as other industrialized countries on healthcare,4 without evidence of superior health outcomes.5 However, it is also known that remarkable local and regional variations in healthcare spending also exist within the US, again, without evidence of superior health outcomes in the higher‐spending regions.6 Both of these observations suggest that we are spending many healthcare dollars on things that evidently do not improve the health of our patients. How much of this waste is administrative, operational, or clinical is debatable and remains the focus of growing national healthcare reform efforts.711 However, from the hospitalist perspective, we should be especially wary of providing so‐called flat‐of‐the‐curve medicine, that is, a level of intensity of care that provides no incremental health benefit.12 The purpose of this editorial is to challenge hospitalists to collectively examine how much of our inpatient spending is potentially unnecessary, and how we, as specialists in inpatient medicine, can assume a critical role in controlling healthcare costs.
To illustrate the issue, consider the following clinical scenario, managed in different ways by different hospitalists, with approximate costs itemized in Table 1. The patient is an elderly woman who presents to the emergency room with syncope occurring at church. The first hospitalist takes time to gather history from the patient, family, eyewitnesses, and the primary care physician, and requests a medication list and outside medical records, which reveal several recent and relevant cardiac and imaging studies. He performs a careful examination, discovers orthostatic hypotension, and his final diagnosis is syncope related to volume depletion from a recently added diuretic as well as a mild gastroenteritis. The patient is rehydrated and discharged home from the emergency room in the care of her family, and asked to hold her diuretic until seen by her family physician in 1 or 2 days. The second hospitalist receives the call from the emergency room and tells the staff to get the patient a telemetry bed. He sees the patient 2 hours later when she gets to the floor. The family has gone home and the mildly demented patient does not recall much of the event or her past medical history. The busy hospitalist constructs a broad differential diagnosis and writes some quick orders to evaluate the patient for possible stroke, seizure, pulmonary embolism, and cardiac ischemia or arrhythmia. He also asks cardiology and neurology to give an opinion. The testing is normal, and the patient is discharged with a cardiac event monitor and an outpatient tilt‐table test scheduled.
| Mrs. Syncope #1 | Cost | Mrs. Syncope #2 | Cost |
|---|---|---|---|
| |||
| Level 4 emergency room visit | $745 | Level 4 emergency room visit | $745 |
| Level 4 internal medicine consultation | $190 | Level 3 history and physical | $190 |
| Laboratory evaluation: CBC, CMP, cardiac panel, urinalysis, D‐dimer | $843 | ||
| EKG | $150 | ||
| Head CT | $1426 | ||
| Chest CT angiogram | $2120 | ||
| Brain MRI | $3388 | ||
| Echocardiogram | $687 | ||
| Carotid ultrasound | $911 | ||
| Level 4 neurology consult | $190 | ||
| Subsequent visits day 2, day 3 | $150 | ||
| EEG | $520 | ||
| Level 4 cardiology consult | $190 | ||
| Nuclear stress test | $1359 | ||
| Specialist subsequent visits | $150 | ||
| Telemetry bed, 3 days | $3453 | ||
| Discharge, low‐level | $90 | ||
| Cardiac event monitor | $421 | ||
| Tilt‐table test | $1766 | ||
| $935 | $18,749 | ||
Although the above scenarios purposely demonstrate 2 extremes of care, I suspect most readers would agree that each hospitalist has his or her own style of practice, and that these differences in style inevitably result in significant differences in the total cost of healthcare delivered. This variation in spending among individual physicians is perhaps more easily understood than the striking variations in healthcare spending seen when different states, regions, and hospitals are compared. For example, annual Medicare spending per beneficiary has varied widely from state to state, from $5436 in Iowa to $7995 in New York (in 2004), a 47% difference.13 Specific analysis of inpatient spending variations is presented in the Dartmouth Atlas of Health Care 2008, which reports healthcare spending in the last 2 years of life for patients with at least 1 chronic illness.14 While the average Medicare inpatient spending per capita for these patients was about $25,000, the state‐specific spending varied widely from $37,040 in New Jersey to $17,135 in Idaho. There was also significant variation in spending within individual states (ie, New York: Binghamton, $18,339; Manhattan, $57,000) and between similar types of hospitals (UCLA Medical Center, $63,900; Massachusetts General Hospital, $43,058). Yet there is no evidence that higher‐spending regions produce better health outcomes.6 Interestingly, the observed differences in spending within the US were primarily due to the volume and intensity of care, not the price of care, as has been seen in some comparisons of the US with other industrialized countries.8, 15 In overall Medicare expenditures, higher‐spending locations tended to have a more inpatient‐based and specialist‐oriented pattern of practice, with higher utilization of inpatient consultations, diagnostic testing, and minor procedures.6
Although the wide variation in spending observed is a bit baffling, the encouraging aspect of this data is that some places are apparently doing it right; that is, providing their patients with a much higher value per healthcare dollar. Ultimately, if the higher‐spending locations modeled the lower‐spending locations, we would have the potential to reduce overall healthcare costs by as much as 30% without harming health.9
What are the possible reasons that we are providing unnecessary care? There are both environment‐dependent and physician‐dependent reasons, which I will outline here. The first 3 reasons represent areas that would seem to require system‐wide change, whereas the remaining 7 reasons are perhaps more amenable to local and/or national hospitalist‐directed efforts.
-
Working in a litigious environment promotes unnecessary testing and consultations with the intent of reducing our exposure to malpractice liability, so‐called defensive medicine.16
-
A reimbursement system that is primarily fee‐for‐service encourages physicians to provide more care and involve more physicians in the care of each patient, with little or no incentive to spend less, a core problem that was recently highlighted in a public Society of Hospital Management (SHM) statement.17
-
The lack of integrated medical record systems promotes waste by leading to duplicate testing, simply because we cannot easily obtain old records to confirm whether tests were previously done. Interestingly, data from the Commonwealth Fund conclude that US physicians order duplicate diagnostic tests (a test repeated within 2 years) at more than twice the rate of Canada and the United Kingdom, while the nation with the lowest rate of duplicate testing, The Netherlands, has the highest rate of electronic medical record use (98%).18
-
Working with patients (or families) with high expectations who insist upon aggressive testing, treatment, and referral to specialists inflates spending, especially if associated with futile and expensive end‐of‐life care.
-
The involvement of one or more specialists may subsequently lead to even more aggressive care ordered by each specialist.
-
The availability and promotion of new technology (diagnostic testing, medical devices, etc.) may prompt us to make use of it simply because it is there, with or without evidence of a health benefit. Our natural curiosity or fascination with information, or our desire to do an overly complete evaluation, works against cost containment.
-
Local trends or traditions within our specific work environment, as suggested by the variability data, may have a strong influence on our individual practice. In such a setting, inadequate knowledge of the cost‐effectiveness of various tests and treatment options likely leads to unnecessary health care spending.
-
A hospitalist work environment in which a high patient load is carried will inevitably result in less time to gather a detailed history and obtain old records or other information that could help narrow a differential diagnosis and minimize unnecessary or duplicate testing.
-
Preventable readmissions resulting from inadequate coordination of care add cost,19 a phenomenon highly dependent on efficient information systems and proper physician‐physician communication.20
-
An overestimation of the need for inpatient evaluation and treatment (vs. outpatient) leads to unnecessary admissions and a longer average length‐of‐stay, each of which add dramatically to total healthcare costs. This is not only dependent on our individual threshold for admitting and discharging patients, but also on our efficiency in diagnosing and treating acute conditions. The fact that the average length‐of‐stay for congestive heart failure admissions, for example, ranges in different regions from 4.9 to 6.1 days (with costs of $9143 and $12,528, respectively)21 is enough to show that there is room for progress.
What joint efforts could be made to minimize unnecessary inpatient spending? The following are my personal opinions and suggestions (Table 2). Most importantly, I believe every physician deserves prompt and accurate feedback regarding their spending patterns, accompanied by valid comparisons to national and local standards, to demonstrate where they stand on the spectrum of healthcare spending. We are currently far behind other industries in our ability, as physicians, to evaluate what we are spending money on, how much, and why. If I knew, for example, that my spending was in the 95th percentile of all hospitalists in community hospitals similar to mine, I would be prompted to investigate where the differences were and why. In an informal survey of hospitalist colleagues, I found that the majority do not receive any data on the costs associated with their care, and are largely unaware of the actual cost of the inpatient tests they commonly order. Developing a secure, user‐friendly database of individual physician spending patterns relative to national and local standards could be a preliminary step, and would likely require a unified effort between government agencies, professional societies, hospitals, and the insurance industry. However, once available, the increased transparency and clarity of spending variations would hopefully prompt introspection and change. In the absence of hard data, however, individual self‐assessment on spending patterns could also be offered through the development of an online simulated case‐based examination in which a physician could gain a general idea of how his evaluation and treatment of a case scenario compares to his hospitalist colleagues, and to what degree each of his clinical decisions affects the overall cost of care. There are many excellent quality improvement tools offered through SHM but none that specifically address the cost of care.
| Spending Data | Guidelines | Patient Education | Advocacy | Professional Development | |
|---|---|---|---|---|---|
| |||||
| Defensive medicine | ✓ | ✓ | ✓✓ | ||
| Patient expectations | ✓ | ✓✓ | |||
| Specialist consultations | ✓ | ✓ | ✓ | ✓✓ | |
| Fee‐for‐service environment | ✓✓ | ||||
| Availability of technology | ✓✓ | ✓✓ | ✓ | ✓ | |
| Poor access to medical records | ✓✓ | ||||
| Local medical culture | ✓✓ | ✓✓ | ✓ | ||
| Insufficient knowledge of evidence‐based guidelines | ✓✓ | ✓ | ✓✓ | ||
| Lack of available value‐based data | ✓ | ✓✓ | |||
| High patient load | ✓ | ✓✓ | ✓ | ||
| Preventable readmissions from poor coordination | ✓ | ✓✓ | |||
| Overestimation of the need for inpatient care | ✓ | ✓✓ | ✓ | ✓✓ | |
Second, hospitalists need quick access to current evidence‐based guidelines regarding the true clinical value, or cost‐effectiveness, of testing and treatment for common inpatient conditions, including specific admission criteria. A single source or clearinghouse of guidelines, sponsored by SHM, may be particularly helpful, especially if it focuses on clarifying areas of highest variability in inpatient spending. In addition, I believe that, given the critically important interface between emergency medicine and hospital medicine, joint guidelines between the 2 groups would potentially be very helpful in controlling costs by limiting unnecessary admissions. Advocacy for comparative effectiveness research to establish validity in these guidelines will be fundamental22, 23; however, I suspect the common sense question: Will this added cost improve my patient's outcome? also needs to be applied more generously, since many individual clinical scenarios will not likely lend themselves to formal study. For discussion, some sample case scenarios are presented (Table 3).
| |
| An 82‐year‐old nursing home patient limited to a wheelchair due to severe osteoarthritis presents with new‐onset expressive aphasia and mild right‐sided hemiparesis. Head CT is negative for bleed, but shows an acute left middle cerebral artery infarct. | Would your stroke workup include an MRI/MRA of the brain, carotid ultrasound, echocardiogram, and neurology consultation? |
| A 68‐year‐old with known ischemic cardiomyopathy is admitted with a CHF exacerbation clearly due to medication noncompliance. The last echocardiogram was done 18 months ago and showed an ejection fraction of 20% with moderate to severe mitral regurgitation. | Would you order a repeat echocardiogram? Would you consult cardiology? |
| A 35‐year‐old construction worker presents with sharp chest pain that is partially reproducible on examination, and no other physical findings. Vital signs, EKG, and cardiac markers are normal. The patient had a negative stress test last year. However, his D‐dimer is slightly elevated. | Would you order a CT angiogram of the chest? If he had a normal one last month for the same symptoms, would you repeat it? In either case, would you admit him to the hospital? |
| A 42‐year‐old man presents with chest pain associated with recent cocaine use. His chest pain resolves in the emergency room and his repeat troponin is normal at 6 hours. | Would you order a nuclear stress test for the patient? Would your management change if a stress test was normal a year ago? Would you admit him? |
| A 58‐year‐old man admitted with community‐acquired pneumonia of the right lower lobe has improved clinically with empiric treatment. Before discharge, he asks for a repeat radiograph to make sure it is getting better. | Would you comply with the patient's request? |
| A 68‐year‐old woman who underwent left total knee arthroplasty 2 weeks ago presents with a left proximal DVT. She has no other symptoms and vitals are normal. She has no personal or family history of clotting. | Would you admit the patient to the hospital? Would you order a CT angiogram of the chest? Would you order a hypercoagulable workup? |
| A 43‐year‐old is admitted for atypical chest pain. Serial cardiac enzymes and nuclear stress test are negative. However, his transaminases are elevated at twice the normal upper limits. He takes a statin for dyslipidemia. | Would you order further laboratory tests or imaging to evaluate for hepatic disorders or discharge the patient? |
| A 63‐year‐old receiving chemotherapy for colon cancer with multiple liver metastases presents with new‐onset dyspnea and is found to have a large left‐sided pleural effusion on chest radiograph. You perform a thoracentesis and malignant cells are present. | Would you order a chest CT? Would you consult pulmonology and/or thoracic surgery (for chest tube and/or pleurodesis)? |
| A 78‐year‐old with severe oxygen‐dependent obstructive lung disease (FEV1 of 1.0 L) has a new 1‐cm nodule on his chest radiograph when admitted for a COPD exacerbation. | Would you order a chest CT? Would you arrange for a biopsy? Would you consult oncology or pulmonology? |
| A 45‐year‐old woke up with severe low‐back pain with right‐sided radiculitis after shoveling heavy snow yesterday. He is unable to walk due to pain, but no focal neurologic symptoms are identified on exam. | Would you order an MRI of the spine? Would you consult orthopedics? |
| A 68‐year‐old man on coumadin for chronic atrial fibrillation is incidentally found to have an INR of 6.5 in clinic. He is currently asymptomatic without evidence of bleeding and with normal vital signs. His hemoglobin is 10.1 compared to 10.8 last month. Digital rectal exam results in a hemoccult‐positive smear. | Would you admit him to the hospital? Would you give fresh frozen plasma? Would you consult gastroenterology? |
| A 58‐year old truck driver presents with acute PE, identified on CT angiogram. There is no previous history of DVT. The patient's arterial blood gas shows a pH of 7.45, pCO2 of 35 mmHg, and pO2 of 55 mmHg on room air. The heart rate is 75. | Would you order a lower extremity duplex to assess for DVT? Would you ask interventional radiology to place an IVC filter if a DVT was present? |
| A 26‐year‐old presents with fever, headache, and meningismus. Head CT is normal. | Would you perform a bedside spinal tap or send the patient for a fluoroscopically‐guided procedure in radiology? |
| A 68‐year‐old smoker presents with right‐sided pneumonia with a small parapneumonic effusion. He is afebrile after 24 hours of IV antibiotics and clinically feels much better. | Would you order a thoracentesis? If so, would you perform it bedside or send the patient to radiology for an ultrasound‐guided procedure? Would you consult a pulmonologist? |
| An 82‐year‐old severely demented nursing home resident who has required total care for the past few months presents with dehydration and a sodium of 158 after increasingly poor oral intake. No other illness is identified. | Would you begin IV fluids immediately and consider gastrostomy tube placement to maintain adequate hydration at the nursing home or would you contact family to discuss end‐of‐life care goals first? Would your management change if a UTI or pneumonia was diagnosed? |
Third, hospitalists could potentially benefit from the development of patient education materials, available through SHM, that address the cost‐effectiveness of common inpatient tests and treatments with the goal of decreasing patient demand for unnecessary testing. Education regarding advanced directives and end‐of‐life care decision‐making could be particularly valuable in minimizing futile care, as it is well‐documented that transitioning to palliative care as soon as it is appropriate reduces healthcare spending greatly during the end‐of‐life period.2427 At the same time, we need to be careful to reassure our patients that we are not trying to ration care, but are instead minimizing the risks and costs for them associated with unnecessary care. In my experience, most patients, if given appropriate time, attention, and education, are willing to accept the final recommendation of their physician.
Fourth, intensified federal and state advocacy in several areas could help reduce spending. For example, advocacy for medical liability reform may reduce the atmosphere of defensive medicine, although I suspect that because old habits die hard, it may take a full generation of decreased liability risk to actually change practice patterns. Advocacy for the development of a national, or at least more uniform, electronic medical record, may decrease duplicate testing and improve efficiency. Advocacy for value‐based reimbursement models may help dampen costs resulting from a predominantly fee‐for‐service environment.28
Fifth, and perhaps most fundamental to the future of our specialty, encouraging the broad professional development of hospitalists as a true specialists in inpatient medicine (based on the SHM Core Competencies,)29 could help minimize the unnecessary costs associated with specialist‐oriented care.6 With the desire to create, in the near future, a formal board‐certification in hospital medicine comes an obligation to develop broad knowledge and broad skill sets that are truly unique to our profession, whereas deferring to a specialist‐oriented pattern of care actually shrinks us down to something less than a traditional internist, rather than a unique entity.30 With our 24/7 focus on inpatient care, we should easily be able to demonstrate our superiority in safety, quality, and efficiency, all of which are closely linked to increased value per healthcare dollar. If, however, our focus is blurred by an overly productivity‐based practice, in which patient volume and procedures take precedence, we will not be able to claim any special value to the system.
Last, supporting efforts to improve coordination of care and transitions of care could reduce costs associated with unnecessary readmissions or posthospital complications. A recent policy statement from several professional societies, including SHM, highlights the importance of these transitions,20, 31 and within the past year, SHM has launched the successful Project BOOST (Better Outcomes for Older adults through Safe Transitions) to help in this effort.32
Unfortunately, there is an inherent problem with all of the above proposals: the assumption that physicians actually want to reduce healthcare spending. Since everyone who works in the medical industry benefits financially in some way from the current high levels of spending on healthcare, reducing spending is counterintuitive for many, and the incentives to spend more will likely persist until some form of spending targets or limits are set.33 Moreover, since physicians traditionally do not like to be told how to practice medicine, history would predict that, without attractive incentives, nothing will change. This is the fundamental and unfortunate dilemma that has apparently pushed us to the eleventh hour of a healthcare crisis.
Another concern with an extreme atmosphere of cost cutting is the risk of swinging too far in the opposite direction, focusing so intently on cost that we begin to compromise quality or access to care in order to achieve spending targets. Reassuringly, however, the data suggest that there is plenty of room for us to cut costs without harming health outcomes.
Despite these obstacles, during this historic time in US healthcare, I believe hospitalists have a unique and perhaps transient opportunity to demonstrate their singular commitment to rational healthcare spending and by doing so to gain significant influence in shaping the impending healthcare reforms. If we speak and act with one voice, with transparency, and with the proper data, we could be the first and only professional society to not only demonstrate our current pattern of spending, but also our potential for reducing spending and our plan on how to get there.
Acknowledgements
Judy Knight, MLS, provided valuable research and technical support.
- Medicare pay overhaul can no longer wait. American Medical News.2009. Available at: http://www.ama‐assn.org/amednews/2009/01/12/edsa0112.htm. Accessed July 2009.
- ,,, et al.Health spending projections through 2017: the baby‐boom generation is coming to Medicare.Health Aff (Millwood).2008;27(2):w145–w155.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:380.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:374.
- National Scorecard on U.S. Health System Performance, 2008 Chartpack.New York, NY:The Commonwealth Fund;2008:6.
- ,,,,,.The implications of regional variations in medicare spending. Part 1: The content, quality, and accessibility of care.Ann Intern Med.2003;138(4):273–287.
- ,,,.Waste in the U.S. health care system: a conceptual framework.Milbank Q.2008;86(4):629–659.
- ,,,.It's the prices, stupid: why the United States is so different from other countries.Health Aff (Millwood).2003;22(3):89–105.
- . Health Care and the budget: issues and challenges for reform.2007. Available at: http://www.cbo.gov/ftpdocs/82xx/doc8255/06–21‐HealthCareReform.pdf. Accessed July 2009.
- .Overtreated: Why Too Much Medicine Is Making Us Sicker and Poorer.1st ed.New York, NY:Bloomsbury;2007.
- ,,,. Slowing the growth of U.S. health care expensitures: what are the options?2007. Available at: http://www.commonwealthfund.org/publications/publications_show.htm?doc_id=449510. Accessed July 2009.
- .More variation in use of care, more flat‐of‐the‐curve medicine.Health Aff (Millwood).2004;(Suppl Web Exclusives):VAR104–VAR107.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:419.
- ,.Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008.Lebanon, NH:Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research;2008:25–32.
- ,.Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008.Lebanon, NH:Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research;2008:2–4.
- ,,.Effects of the medical liability system in Australia, the UK, and the USA.Lancet.2006;368(9531):240–246.
- Comments on the centers for Medicare and Medicaid services plan to transition to a Medicare value‐based purchasing program for physicians and other professional services.2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Issues_in_the_Spotlight12008:62,73.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,, et al.Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Gen Intern Med.2009;24(8):971–976.
- Hospitals like mine: 2006 national statistics.2006. Available at: http://www.hcupnet.ahrq.gov. Accessed July 2009.
- ,,.Evidence‐Based to Value‐Based Medicine.Chicago, IL:AMA Press;2005.
- Improved Availability of Comparative Effectiveness Information: An Essential Feature for a High‐Quality and Efficient United States Health Care System.Philadelphia, PA:American College of Physicians;2008.
- ,.Clinical practice. Palliative care.N Engl J Med.2004;350(25):2582–2590.
- ,,.The health economics of palliative care.Oncology (Williston Park).2002;16(6):801–808; discussion 808, 811–802.
- .Cost savings at the end of life. What do the data show?JAMA.1996;275(24):1907–1914.
- ,,, et al.Cost savings associated with US hospital palliative care consultation programs.Arch Intern Med.2008;168(16):1783–1790.
- ,,, et al.Toward a 21st‐century health care system: recommendations for health care reform.Ann Intern Med.2009;150(7):493–495.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- .The expanding or shrinking universe of the hospitalist.J Hosp Med.2008;3(4):288–291.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- Project BOOST.2009. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed Julyyear="2009"2009.
- ,,.The Obama administration's options for health care cost control: hope versus reality.Ann Intern Med.2009;150(7):485–489.
Let's think about what we need to do ourselves. We have to acknowledge that orders we write drive up health care costs.1 AMA President, Nancy H. Nielsen, MD, PhD
As the most prominent providers of inpatient care, hospitalists should be aware that, of the total annual expenditures on US healthcare ($2.3 trillion in 2007),2 approximately one‐third goes to hospital‐based medical care, over one‐half of which (57%) is covered by public funds through Medicare and Medicaid3; this high cost of healthcare is increasingly being blamed for unnecessarily burdening our economy and preventing our industries from being globally competitive. I believe that the high proportion of spending on inpatient care places hospitalists firmly in the center of the debate on how to reduce healthcare costs. It is well known that the United States spends about twice as much per capita as other industrialized countries on healthcare,4 without evidence of superior health outcomes.5 However, it is also known that remarkable local and regional variations in healthcare spending also exist within the US, again, without evidence of superior health outcomes in the higher‐spending regions.6 Both of these observations suggest that we are spending many healthcare dollars on things that evidently do not improve the health of our patients. How much of this waste is administrative, operational, or clinical is debatable and remains the focus of growing national healthcare reform efforts.711 However, from the hospitalist perspective, we should be especially wary of providing so‐called flat‐of‐the‐curve medicine, that is, a level of intensity of care that provides no incremental health benefit.12 The purpose of this editorial is to challenge hospitalists to collectively examine how much of our inpatient spending is potentially unnecessary, and how we, as specialists in inpatient medicine, can assume a critical role in controlling healthcare costs.
To illustrate the issue, consider the following clinical scenario, managed in different ways by different hospitalists, with approximate costs itemized in Table 1. The patient is an elderly woman who presents to the emergency room with syncope occurring at church. The first hospitalist takes time to gather history from the patient, family, eyewitnesses, and the primary care physician, and requests a medication list and outside medical records, which reveal several recent and relevant cardiac and imaging studies. He performs a careful examination, discovers orthostatic hypotension, and his final diagnosis is syncope related to volume depletion from a recently added diuretic as well as a mild gastroenteritis. The patient is rehydrated and discharged home from the emergency room in the care of her family, and asked to hold her diuretic until seen by her family physician in 1 or 2 days. The second hospitalist receives the call from the emergency room and tells the staff to get the patient a telemetry bed. He sees the patient 2 hours later when she gets to the floor. The family has gone home and the mildly demented patient does not recall much of the event or her past medical history. The busy hospitalist constructs a broad differential diagnosis and writes some quick orders to evaluate the patient for possible stroke, seizure, pulmonary embolism, and cardiac ischemia or arrhythmia. He also asks cardiology and neurology to give an opinion. The testing is normal, and the patient is discharged with a cardiac event monitor and an outpatient tilt‐table test scheduled.
| Mrs. Syncope #1 | Cost | Mrs. Syncope #2 | Cost |
|---|---|---|---|
| |||
| Level 4 emergency room visit | $745 | Level 4 emergency room visit | $745 |
| Level 4 internal medicine consultation | $190 | Level 3 history and physical | $190 |
| Laboratory evaluation: CBC, CMP, cardiac panel, urinalysis, D‐dimer | $843 | ||
| EKG | $150 | ||
| Head CT | $1426 | ||
| Chest CT angiogram | $2120 | ||
| Brain MRI | $3388 | ||
| Echocardiogram | $687 | ||
| Carotid ultrasound | $911 | ||
| Level 4 neurology consult | $190 | ||
| Subsequent visits day 2, day 3 | $150 | ||
| EEG | $520 | ||
| Level 4 cardiology consult | $190 | ||
| Nuclear stress test | $1359 | ||
| Specialist subsequent visits | $150 | ||
| Telemetry bed, 3 days | $3453 | ||
| Discharge, low‐level | $90 | ||
| Cardiac event monitor | $421 | ||
| Tilt‐table test | $1766 | ||
| $935 | $18,749 | ||
Although the above scenarios purposely demonstrate 2 extremes of care, I suspect most readers would agree that each hospitalist has his or her own style of practice, and that these differences in style inevitably result in significant differences in the total cost of healthcare delivered. This variation in spending among individual physicians is perhaps more easily understood than the striking variations in healthcare spending seen when different states, regions, and hospitals are compared. For example, annual Medicare spending per beneficiary has varied widely from state to state, from $5436 in Iowa to $7995 in New York (in 2004), a 47% difference.13 Specific analysis of inpatient spending variations is presented in the Dartmouth Atlas of Health Care 2008, which reports healthcare spending in the last 2 years of life for patients with at least 1 chronic illness.14 While the average Medicare inpatient spending per capita for these patients was about $25,000, the state‐specific spending varied widely from $37,040 in New Jersey to $17,135 in Idaho. There was also significant variation in spending within individual states (ie, New York: Binghamton, $18,339; Manhattan, $57,000) and between similar types of hospitals (UCLA Medical Center, $63,900; Massachusetts General Hospital, $43,058). Yet there is no evidence that higher‐spending regions produce better health outcomes.6 Interestingly, the observed differences in spending within the US were primarily due to the volume and intensity of care, not the price of care, as has been seen in some comparisons of the US with other industrialized countries.8, 15 In overall Medicare expenditures, higher‐spending locations tended to have a more inpatient‐based and specialist‐oriented pattern of practice, with higher utilization of inpatient consultations, diagnostic testing, and minor procedures.6
Although the wide variation in spending observed is a bit baffling, the encouraging aspect of this data is that some places are apparently doing it right; that is, providing their patients with a much higher value per healthcare dollar. Ultimately, if the higher‐spending locations modeled the lower‐spending locations, we would have the potential to reduce overall healthcare costs by as much as 30% without harming health.9
What are the possible reasons that we are providing unnecessary care? There are both environment‐dependent and physician‐dependent reasons, which I will outline here. The first 3 reasons represent areas that would seem to require system‐wide change, whereas the remaining 7 reasons are perhaps more amenable to local and/or national hospitalist‐directed efforts.
-
Working in a litigious environment promotes unnecessary testing and consultations with the intent of reducing our exposure to malpractice liability, so‐called defensive medicine.16
-
A reimbursement system that is primarily fee‐for‐service encourages physicians to provide more care and involve more physicians in the care of each patient, with little or no incentive to spend less, a core problem that was recently highlighted in a public Society of Hospital Management (SHM) statement.17
-
The lack of integrated medical record systems promotes waste by leading to duplicate testing, simply because we cannot easily obtain old records to confirm whether tests were previously done. Interestingly, data from the Commonwealth Fund conclude that US physicians order duplicate diagnostic tests (a test repeated within 2 years) at more than twice the rate of Canada and the United Kingdom, while the nation with the lowest rate of duplicate testing, The Netherlands, has the highest rate of electronic medical record use (98%).18
-
Working with patients (or families) with high expectations who insist upon aggressive testing, treatment, and referral to specialists inflates spending, especially if associated with futile and expensive end‐of‐life care.
-
The involvement of one or more specialists may subsequently lead to even more aggressive care ordered by each specialist.
-
The availability and promotion of new technology (diagnostic testing, medical devices, etc.) may prompt us to make use of it simply because it is there, with or without evidence of a health benefit. Our natural curiosity or fascination with information, or our desire to do an overly complete evaluation, works against cost containment.
-
Local trends or traditions within our specific work environment, as suggested by the variability data, may have a strong influence on our individual practice. In such a setting, inadequate knowledge of the cost‐effectiveness of various tests and treatment options likely leads to unnecessary health care spending.
-
A hospitalist work environment in which a high patient load is carried will inevitably result in less time to gather a detailed history and obtain old records or other information that could help narrow a differential diagnosis and minimize unnecessary or duplicate testing.
-
Preventable readmissions resulting from inadequate coordination of care add cost,19 a phenomenon highly dependent on efficient information systems and proper physician‐physician communication.20
-
An overestimation of the need for inpatient evaluation and treatment (vs. outpatient) leads to unnecessary admissions and a longer average length‐of‐stay, each of which add dramatically to total healthcare costs. This is not only dependent on our individual threshold for admitting and discharging patients, but also on our efficiency in diagnosing and treating acute conditions. The fact that the average length‐of‐stay for congestive heart failure admissions, for example, ranges in different regions from 4.9 to 6.1 days (with costs of $9143 and $12,528, respectively)21 is enough to show that there is room for progress.
What joint efforts could be made to minimize unnecessary inpatient spending? The following are my personal opinions and suggestions (Table 2). Most importantly, I believe every physician deserves prompt and accurate feedback regarding their spending patterns, accompanied by valid comparisons to national and local standards, to demonstrate where they stand on the spectrum of healthcare spending. We are currently far behind other industries in our ability, as physicians, to evaluate what we are spending money on, how much, and why. If I knew, for example, that my spending was in the 95th percentile of all hospitalists in community hospitals similar to mine, I would be prompted to investigate where the differences were and why. In an informal survey of hospitalist colleagues, I found that the majority do not receive any data on the costs associated with their care, and are largely unaware of the actual cost of the inpatient tests they commonly order. Developing a secure, user‐friendly database of individual physician spending patterns relative to national and local standards could be a preliminary step, and would likely require a unified effort between government agencies, professional societies, hospitals, and the insurance industry. However, once available, the increased transparency and clarity of spending variations would hopefully prompt introspection and change. In the absence of hard data, however, individual self‐assessment on spending patterns could also be offered through the development of an online simulated case‐based examination in which a physician could gain a general idea of how his evaluation and treatment of a case scenario compares to his hospitalist colleagues, and to what degree each of his clinical decisions affects the overall cost of care. There are many excellent quality improvement tools offered through SHM but none that specifically address the cost of care.
| Spending Data | Guidelines | Patient Education | Advocacy | Professional Development | |
|---|---|---|---|---|---|
| |||||
| Defensive medicine | ✓ | ✓ | ✓✓ | ||
| Patient expectations | ✓ | ✓✓ | |||
| Specialist consultations | ✓ | ✓ | ✓ | ✓✓ | |
| Fee‐for‐service environment | ✓✓ | ||||
| Availability of technology | ✓✓ | ✓✓ | ✓ | ✓ | |
| Poor access to medical records | ✓✓ | ||||
| Local medical culture | ✓✓ | ✓✓ | ✓ | ||
| Insufficient knowledge of evidence‐based guidelines | ✓✓ | ✓ | ✓✓ | ||
| Lack of available value‐based data | ✓ | ✓✓ | |||
| High patient load | ✓ | ✓✓ | ✓ | ||
| Preventable readmissions from poor coordination | ✓ | ✓✓ | |||
| Overestimation of the need for inpatient care | ✓ | ✓✓ | ✓ | ✓✓ | |
Second, hospitalists need quick access to current evidence‐based guidelines regarding the true clinical value, or cost‐effectiveness, of testing and treatment for common inpatient conditions, including specific admission criteria. A single source or clearinghouse of guidelines, sponsored by SHM, may be particularly helpful, especially if it focuses on clarifying areas of highest variability in inpatient spending. In addition, I believe that, given the critically important interface between emergency medicine and hospital medicine, joint guidelines between the 2 groups would potentially be very helpful in controlling costs by limiting unnecessary admissions. Advocacy for comparative effectiveness research to establish validity in these guidelines will be fundamental22, 23; however, I suspect the common sense question: Will this added cost improve my patient's outcome? also needs to be applied more generously, since many individual clinical scenarios will not likely lend themselves to formal study. For discussion, some sample case scenarios are presented (Table 3).
| |
| An 82‐year‐old nursing home patient limited to a wheelchair due to severe osteoarthritis presents with new‐onset expressive aphasia and mild right‐sided hemiparesis. Head CT is negative for bleed, but shows an acute left middle cerebral artery infarct. | Would your stroke workup include an MRI/MRA of the brain, carotid ultrasound, echocardiogram, and neurology consultation? |
| A 68‐year‐old with known ischemic cardiomyopathy is admitted with a CHF exacerbation clearly due to medication noncompliance. The last echocardiogram was done 18 months ago and showed an ejection fraction of 20% with moderate to severe mitral regurgitation. | Would you order a repeat echocardiogram? Would you consult cardiology? |
| A 35‐year‐old construction worker presents with sharp chest pain that is partially reproducible on examination, and no other physical findings. Vital signs, EKG, and cardiac markers are normal. The patient had a negative stress test last year. However, his D‐dimer is slightly elevated. | Would you order a CT angiogram of the chest? If he had a normal one last month for the same symptoms, would you repeat it? In either case, would you admit him to the hospital? |
| A 42‐year‐old man presents with chest pain associated with recent cocaine use. His chest pain resolves in the emergency room and his repeat troponin is normal at 6 hours. | Would you order a nuclear stress test for the patient? Would your management change if a stress test was normal a year ago? Would you admit him? |
| A 58‐year‐old man admitted with community‐acquired pneumonia of the right lower lobe has improved clinically with empiric treatment. Before discharge, he asks for a repeat radiograph to make sure it is getting better. | Would you comply with the patient's request? |
| A 68‐year‐old woman who underwent left total knee arthroplasty 2 weeks ago presents with a left proximal DVT. She has no other symptoms and vitals are normal. She has no personal or family history of clotting. | Would you admit the patient to the hospital? Would you order a CT angiogram of the chest? Would you order a hypercoagulable workup? |
| A 43‐year‐old is admitted for atypical chest pain. Serial cardiac enzymes and nuclear stress test are negative. However, his transaminases are elevated at twice the normal upper limits. He takes a statin for dyslipidemia. | Would you order further laboratory tests or imaging to evaluate for hepatic disorders or discharge the patient? |
| A 63‐year‐old receiving chemotherapy for colon cancer with multiple liver metastases presents with new‐onset dyspnea and is found to have a large left‐sided pleural effusion on chest radiograph. You perform a thoracentesis and malignant cells are present. | Would you order a chest CT? Would you consult pulmonology and/or thoracic surgery (for chest tube and/or pleurodesis)? |
| A 78‐year‐old with severe oxygen‐dependent obstructive lung disease (FEV1 of 1.0 L) has a new 1‐cm nodule on his chest radiograph when admitted for a COPD exacerbation. | Would you order a chest CT? Would you arrange for a biopsy? Would you consult oncology or pulmonology? |
| A 45‐year‐old woke up with severe low‐back pain with right‐sided radiculitis after shoveling heavy snow yesterday. He is unable to walk due to pain, but no focal neurologic symptoms are identified on exam. | Would you order an MRI of the spine? Would you consult orthopedics? |
| A 68‐year‐old man on coumadin for chronic atrial fibrillation is incidentally found to have an INR of 6.5 in clinic. He is currently asymptomatic without evidence of bleeding and with normal vital signs. His hemoglobin is 10.1 compared to 10.8 last month. Digital rectal exam results in a hemoccult‐positive smear. | Would you admit him to the hospital? Would you give fresh frozen plasma? Would you consult gastroenterology? |
| A 58‐year old truck driver presents with acute PE, identified on CT angiogram. There is no previous history of DVT. The patient's arterial blood gas shows a pH of 7.45, pCO2 of 35 mmHg, and pO2 of 55 mmHg on room air. The heart rate is 75. | Would you order a lower extremity duplex to assess for DVT? Would you ask interventional radiology to place an IVC filter if a DVT was present? |
| A 26‐year‐old presents with fever, headache, and meningismus. Head CT is normal. | Would you perform a bedside spinal tap or send the patient for a fluoroscopically‐guided procedure in radiology? |
| A 68‐year‐old smoker presents with right‐sided pneumonia with a small parapneumonic effusion. He is afebrile after 24 hours of IV antibiotics and clinically feels much better. | Would you order a thoracentesis? If so, would you perform it bedside or send the patient to radiology for an ultrasound‐guided procedure? Would you consult a pulmonologist? |
| An 82‐year‐old severely demented nursing home resident who has required total care for the past few months presents with dehydration and a sodium of 158 after increasingly poor oral intake. No other illness is identified. | Would you begin IV fluids immediately and consider gastrostomy tube placement to maintain adequate hydration at the nursing home or would you contact family to discuss end‐of‐life care goals first? Would your management change if a UTI or pneumonia was diagnosed? |
Third, hospitalists could potentially benefit from the development of patient education materials, available through SHM, that address the cost‐effectiveness of common inpatient tests and treatments with the goal of decreasing patient demand for unnecessary testing. Education regarding advanced directives and end‐of‐life care decision‐making could be particularly valuable in minimizing futile care, as it is well‐documented that transitioning to palliative care as soon as it is appropriate reduces healthcare spending greatly during the end‐of‐life period.2427 At the same time, we need to be careful to reassure our patients that we are not trying to ration care, but are instead minimizing the risks and costs for them associated with unnecessary care. In my experience, most patients, if given appropriate time, attention, and education, are willing to accept the final recommendation of their physician.
Fourth, intensified federal and state advocacy in several areas could help reduce spending. For example, advocacy for medical liability reform may reduce the atmosphere of defensive medicine, although I suspect that because old habits die hard, it may take a full generation of decreased liability risk to actually change practice patterns. Advocacy for the development of a national, or at least more uniform, electronic medical record, may decrease duplicate testing and improve efficiency. Advocacy for value‐based reimbursement models may help dampen costs resulting from a predominantly fee‐for‐service environment.28
Fifth, and perhaps most fundamental to the future of our specialty, encouraging the broad professional development of hospitalists as a true specialists in inpatient medicine (based on the SHM Core Competencies,)29 could help minimize the unnecessary costs associated with specialist‐oriented care.6 With the desire to create, in the near future, a formal board‐certification in hospital medicine comes an obligation to develop broad knowledge and broad skill sets that are truly unique to our profession, whereas deferring to a specialist‐oriented pattern of care actually shrinks us down to something less than a traditional internist, rather than a unique entity.30 With our 24/7 focus on inpatient care, we should easily be able to demonstrate our superiority in safety, quality, and efficiency, all of which are closely linked to increased value per healthcare dollar. If, however, our focus is blurred by an overly productivity‐based practice, in which patient volume and procedures take precedence, we will not be able to claim any special value to the system.
Last, supporting efforts to improve coordination of care and transitions of care could reduce costs associated with unnecessary readmissions or posthospital complications. A recent policy statement from several professional societies, including SHM, highlights the importance of these transitions,20, 31 and within the past year, SHM has launched the successful Project BOOST (Better Outcomes for Older adults through Safe Transitions) to help in this effort.32
Unfortunately, there is an inherent problem with all of the above proposals: the assumption that physicians actually want to reduce healthcare spending. Since everyone who works in the medical industry benefits financially in some way from the current high levels of spending on healthcare, reducing spending is counterintuitive for many, and the incentives to spend more will likely persist until some form of spending targets or limits are set.33 Moreover, since physicians traditionally do not like to be told how to practice medicine, history would predict that, without attractive incentives, nothing will change. This is the fundamental and unfortunate dilemma that has apparently pushed us to the eleventh hour of a healthcare crisis.
Another concern with an extreme atmosphere of cost cutting is the risk of swinging too far in the opposite direction, focusing so intently on cost that we begin to compromise quality or access to care in order to achieve spending targets. Reassuringly, however, the data suggest that there is plenty of room for us to cut costs without harming health outcomes.
Despite these obstacles, during this historic time in US healthcare, I believe hospitalists have a unique and perhaps transient opportunity to demonstrate their singular commitment to rational healthcare spending and by doing so to gain significant influence in shaping the impending healthcare reforms. If we speak and act with one voice, with transparency, and with the proper data, we could be the first and only professional society to not only demonstrate our current pattern of spending, but also our potential for reducing spending and our plan on how to get there.
Acknowledgements
Judy Knight, MLS, provided valuable research and technical support.
Let's think about what we need to do ourselves. We have to acknowledge that orders we write drive up health care costs.1 AMA President, Nancy H. Nielsen, MD, PhD
As the most prominent providers of inpatient care, hospitalists should be aware that, of the total annual expenditures on US healthcare ($2.3 trillion in 2007),2 approximately one‐third goes to hospital‐based medical care, over one‐half of which (57%) is covered by public funds through Medicare and Medicaid3; this high cost of healthcare is increasingly being blamed for unnecessarily burdening our economy and preventing our industries from being globally competitive. I believe that the high proportion of spending on inpatient care places hospitalists firmly in the center of the debate on how to reduce healthcare costs. It is well known that the United States spends about twice as much per capita as other industrialized countries on healthcare,4 without evidence of superior health outcomes.5 However, it is also known that remarkable local and regional variations in healthcare spending also exist within the US, again, without evidence of superior health outcomes in the higher‐spending regions.6 Both of these observations suggest that we are spending many healthcare dollars on things that evidently do not improve the health of our patients. How much of this waste is administrative, operational, or clinical is debatable and remains the focus of growing national healthcare reform efforts.711 However, from the hospitalist perspective, we should be especially wary of providing so‐called flat‐of‐the‐curve medicine, that is, a level of intensity of care that provides no incremental health benefit.12 The purpose of this editorial is to challenge hospitalists to collectively examine how much of our inpatient spending is potentially unnecessary, and how we, as specialists in inpatient medicine, can assume a critical role in controlling healthcare costs.
To illustrate the issue, consider the following clinical scenario, managed in different ways by different hospitalists, with approximate costs itemized in Table 1. The patient is an elderly woman who presents to the emergency room with syncope occurring at church. The first hospitalist takes time to gather history from the patient, family, eyewitnesses, and the primary care physician, and requests a medication list and outside medical records, which reveal several recent and relevant cardiac and imaging studies. He performs a careful examination, discovers orthostatic hypotension, and his final diagnosis is syncope related to volume depletion from a recently added diuretic as well as a mild gastroenteritis. The patient is rehydrated and discharged home from the emergency room in the care of her family, and asked to hold her diuretic until seen by her family physician in 1 or 2 days. The second hospitalist receives the call from the emergency room and tells the staff to get the patient a telemetry bed. He sees the patient 2 hours later when she gets to the floor. The family has gone home and the mildly demented patient does not recall much of the event or her past medical history. The busy hospitalist constructs a broad differential diagnosis and writes some quick orders to evaluate the patient for possible stroke, seizure, pulmonary embolism, and cardiac ischemia or arrhythmia. He also asks cardiology and neurology to give an opinion. The testing is normal, and the patient is discharged with a cardiac event monitor and an outpatient tilt‐table test scheduled.
| Mrs. Syncope #1 | Cost | Mrs. Syncope #2 | Cost |
|---|---|---|---|
| |||
| Level 4 emergency room visit | $745 | Level 4 emergency room visit | $745 |
| Level 4 internal medicine consultation | $190 | Level 3 history and physical | $190 |
| Laboratory evaluation: CBC, CMP, cardiac panel, urinalysis, D‐dimer | $843 | ||
| EKG | $150 | ||
| Head CT | $1426 | ||
| Chest CT angiogram | $2120 | ||
| Brain MRI | $3388 | ||
| Echocardiogram | $687 | ||
| Carotid ultrasound | $911 | ||
| Level 4 neurology consult | $190 | ||
| Subsequent visits day 2, day 3 | $150 | ||
| EEG | $520 | ||
| Level 4 cardiology consult | $190 | ||
| Nuclear stress test | $1359 | ||
| Specialist subsequent visits | $150 | ||
| Telemetry bed, 3 days | $3453 | ||
| Discharge, low‐level | $90 | ||
| Cardiac event monitor | $421 | ||
| Tilt‐table test | $1766 | ||
| $935 | $18,749 | ||
Although the above scenarios purposely demonstrate 2 extremes of care, I suspect most readers would agree that each hospitalist has his or her own style of practice, and that these differences in style inevitably result in significant differences in the total cost of healthcare delivered. This variation in spending among individual physicians is perhaps more easily understood than the striking variations in healthcare spending seen when different states, regions, and hospitals are compared. For example, annual Medicare spending per beneficiary has varied widely from state to state, from $5436 in Iowa to $7995 in New York (in 2004), a 47% difference.13 Specific analysis of inpatient spending variations is presented in the Dartmouth Atlas of Health Care 2008, which reports healthcare spending in the last 2 years of life for patients with at least 1 chronic illness.14 While the average Medicare inpatient spending per capita for these patients was about $25,000, the state‐specific spending varied widely from $37,040 in New Jersey to $17,135 in Idaho. There was also significant variation in spending within individual states (ie, New York: Binghamton, $18,339; Manhattan, $57,000) and between similar types of hospitals (UCLA Medical Center, $63,900; Massachusetts General Hospital, $43,058). Yet there is no evidence that higher‐spending regions produce better health outcomes.6 Interestingly, the observed differences in spending within the US were primarily due to the volume and intensity of care, not the price of care, as has been seen in some comparisons of the US with other industrialized countries.8, 15 In overall Medicare expenditures, higher‐spending locations tended to have a more inpatient‐based and specialist‐oriented pattern of practice, with higher utilization of inpatient consultations, diagnostic testing, and minor procedures.6
Although the wide variation in spending observed is a bit baffling, the encouraging aspect of this data is that some places are apparently doing it right; that is, providing their patients with a much higher value per healthcare dollar. Ultimately, if the higher‐spending locations modeled the lower‐spending locations, we would have the potential to reduce overall healthcare costs by as much as 30% without harming health.9
What are the possible reasons that we are providing unnecessary care? There are both environment‐dependent and physician‐dependent reasons, which I will outline here. The first 3 reasons represent areas that would seem to require system‐wide change, whereas the remaining 7 reasons are perhaps more amenable to local and/or national hospitalist‐directed efforts.
-
Working in a litigious environment promotes unnecessary testing and consultations with the intent of reducing our exposure to malpractice liability, so‐called defensive medicine.16
-
A reimbursement system that is primarily fee‐for‐service encourages physicians to provide more care and involve more physicians in the care of each patient, with little or no incentive to spend less, a core problem that was recently highlighted in a public Society of Hospital Management (SHM) statement.17
-
The lack of integrated medical record systems promotes waste by leading to duplicate testing, simply because we cannot easily obtain old records to confirm whether tests were previously done. Interestingly, data from the Commonwealth Fund conclude that US physicians order duplicate diagnostic tests (a test repeated within 2 years) at more than twice the rate of Canada and the United Kingdom, while the nation with the lowest rate of duplicate testing, The Netherlands, has the highest rate of electronic medical record use (98%).18
-
Working with patients (or families) with high expectations who insist upon aggressive testing, treatment, and referral to specialists inflates spending, especially if associated with futile and expensive end‐of‐life care.
-
The involvement of one or more specialists may subsequently lead to even more aggressive care ordered by each specialist.
-
The availability and promotion of new technology (diagnostic testing, medical devices, etc.) may prompt us to make use of it simply because it is there, with or without evidence of a health benefit. Our natural curiosity or fascination with information, or our desire to do an overly complete evaluation, works against cost containment.
-
Local trends or traditions within our specific work environment, as suggested by the variability data, may have a strong influence on our individual practice. In such a setting, inadequate knowledge of the cost‐effectiveness of various tests and treatment options likely leads to unnecessary health care spending.
-
A hospitalist work environment in which a high patient load is carried will inevitably result in less time to gather a detailed history and obtain old records or other information that could help narrow a differential diagnosis and minimize unnecessary or duplicate testing.
-
Preventable readmissions resulting from inadequate coordination of care add cost,19 a phenomenon highly dependent on efficient information systems and proper physician‐physician communication.20
-
An overestimation of the need for inpatient evaluation and treatment (vs. outpatient) leads to unnecessary admissions and a longer average length‐of‐stay, each of which add dramatically to total healthcare costs. This is not only dependent on our individual threshold for admitting and discharging patients, but also on our efficiency in diagnosing and treating acute conditions. The fact that the average length‐of‐stay for congestive heart failure admissions, for example, ranges in different regions from 4.9 to 6.1 days (with costs of $9143 and $12,528, respectively)21 is enough to show that there is room for progress.
What joint efforts could be made to minimize unnecessary inpatient spending? The following are my personal opinions and suggestions (Table 2). Most importantly, I believe every physician deserves prompt and accurate feedback regarding their spending patterns, accompanied by valid comparisons to national and local standards, to demonstrate where they stand on the spectrum of healthcare spending. We are currently far behind other industries in our ability, as physicians, to evaluate what we are spending money on, how much, and why. If I knew, for example, that my spending was in the 95th percentile of all hospitalists in community hospitals similar to mine, I would be prompted to investigate where the differences were and why. In an informal survey of hospitalist colleagues, I found that the majority do not receive any data on the costs associated with their care, and are largely unaware of the actual cost of the inpatient tests they commonly order. Developing a secure, user‐friendly database of individual physician spending patterns relative to national and local standards could be a preliminary step, and would likely require a unified effort between government agencies, professional societies, hospitals, and the insurance industry. However, once available, the increased transparency and clarity of spending variations would hopefully prompt introspection and change. In the absence of hard data, however, individual self‐assessment on spending patterns could also be offered through the development of an online simulated case‐based examination in which a physician could gain a general idea of how his evaluation and treatment of a case scenario compares to his hospitalist colleagues, and to what degree each of his clinical decisions affects the overall cost of care. There are many excellent quality improvement tools offered through SHM but none that specifically address the cost of care.
| Spending Data | Guidelines | Patient Education | Advocacy | Professional Development | |
|---|---|---|---|---|---|
| |||||
| Defensive medicine | ✓ | ✓ | ✓✓ | ||
| Patient expectations | ✓ | ✓✓ | |||
| Specialist consultations | ✓ | ✓ | ✓ | ✓✓ | |
| Fee‐for‐service environment | ✓✓ | ||||
| Availability of technology | ✓✓ | ✓✓ | ✓ | ✓ | |
| Poor access to medical records | ✓✓ | ||||
| Local medical culture | ✓✓ | ✓✓ | ✓ | ||
| Insufficient knowledge of evidence‐based guidelines | ✓✓ | ✓ | ✓✓ | ||
| Lack of available value‐based data | ✓ | ✓✓ | |||
| High patient load | ✓ | ✓✓ | ✓ | ||
| Preventable readmissions from poor coordination | ✓ | ✓✓ | |||
| Overestimation of the need for inpatient care | ✓ | ✓✓ | ✓ | ✓✓ | |
Second, hospitalists need quick access to current evidence‐based guidelines regarding the true clinical value, or cost‐effectiveness, of testing and treatment for common inpatient conditions, including specific admission criteria. A single source or clearinghouse of guidelines, sponsored by SHM, may be particularly helpful, especially if it focuses on clarifying areas of highest variability in inpatient spending. In addition, I believe that, given the critically important interface between emergency medicine and hospital medicine, joint guidelines between the 2 groups would potentially be very helpful in controlling costs by limiting unnecessary admissions. Advocacy for comparative effectiveness research to establish validity in these guidelines will be fundamental22, 23; however, I suspect the common sense question: Will this added cost improve my patient's outcome? also needs to be applied more generously, since many individual clinical scenarios will not likely lend themselves to formal study. For discussion, some sample case scenarios are presented (Table 3).
| |
| An 82‐year‐old nursing home patient limited to a wheelchair due to severe osteoarthritis presents with new‐onset expressive aphasia and mild right‐sided hemiparesis. Head CT is negative for bleed, but shows an acute left middle cerebral artery infarct. | Would your stroke workup include an MRI/MRA of the brain, carotid ultrasound, echocardiogram, and neurology consultation? |
| A 68‐year‐old with known ischemic cardiomyopathy is admitted with a CHF exacerbation clearly due to medication noncompliance. The last echocardiogram was done 18 months ago and showed an ejection fraction of 20% with moderate to severe mitral regurgitation. | Would you order a repeat echocardiogram? Would you consult cardiology? |
| A 35‐year‐old construction worker presents with sharp chest pain that is partially reproducible on examination, and no other physical findings. Vital signs, EKG, and cardiac markers are normal. The patient had a negative stress test last year. However, his D‐dimer is slightly elevated. | Would you order a CT angiogram of the chest? If he had a normal one last month for the same symptoms, would you repeat it? In either case, would you admit him to the hospital? |
| A 42‐year‐old man presents with chest pain associated with recent cocaine use. His chest pain resolves in the emergency room and his repeat troponin is normal at 6 hours. | Would you order a nuclear stress test for the patient? Would your management change if a stress test was normal a year ago? Would you admit him? |
| A 58‐year‐old man admitted with community‐acquired pneumonia of the right lower lobe has improved clinically with empiric treatment. Before discharge, he asks for a repeat radiograph to make sure it is getting better. | Would you comply with the patient's request? |
| A 68‐year‐old woman who underwent left total knee arthroplasty 2 weeks ago presents with a left proximal DVT. She has no other symptoms and vitals are normal. She has no personal or family history of clotting. | Would you admit the patient to the hospital? Would you order a CT angiogram of the chest? Would you order a hypercoagulable workup? |
| A 43‐year‐old is admitted for atypical chest pain. Serial cardiac enzymes and nuclear stress test are negative. However, his transaminases are elevated at twice the normal upper limits. He takes a statin for dyslipidemia. | Would you order further laboratory tests or imaging to evaluate for hepatic disorders or discharge the patient? |
| A 63‐year‐old receiving chemotherapy for colon cancer with multiple liver metastases presents with new‐onset dyspnea and is found to have a large left‐sided pleural effusion on chest radiograph. You perform a thoracentesis and malignant cells are present. | Would you order a chest CT? Would you consult pulmonology and/or thoracic surgery (for chest tube and/or pleurodesis)? |
| A 78‐year‐old with severe oxygen‐dependent obstructive lung disease (FEV1 of 1.0 L) has a new 1‐cm nodule on his chest radiograph when admitted for a COPD exacerbation. | Would you order a chest CT? Would you arrange for a biopsy? Would you consult oncology or pulmonology? |
| A 45‐year‐old woke up with severe low‐back pain with right‐sided radiculitis after shoveling heavy snow yesterday. He is unable to walk due to pain, but no focal neurologic symptoms are identified on exam. | Would you order an MRI of the spine? Would you consult orthopedics? |
| A 68‐year‐old man on coumadin for chronic atrial fibrillation is incidentally found to have an INR of 6.5 in clinic. He is currently asymptomatic without evidence of bleeding and with normal vital signs. His hemoglobin is 10.1 compared to 10.8 last month. Digital rectal exam results in a hemoccult‐positive smear. | Would you admit him to the hospital? Would you give fresh frozen plasma? Would you consult gastroenterology? |
| A 58‐year old truck driver presents with acute PE, identified on CT angiogram. There is no previous history of DVT. The patient's arterial blood gas shows a pH of 7.45, pCO2 of 35 mmHg, and pO2 of 55 mmHg on room air. The heart rate is 75. | Would you order a lower extremity duplex to assess for DVT? Would you ask interventional radiology to place an IVC filter if a DVT was present? |
| A 26‐year‐old presents with fever, headache, and meningismus. Head CT is normal. | Would you perform a bedside spinal tap or send the patient for a fluoroscopically‐guided procedure in radiology? |
| A 68‐year‐old smoker presents with right‐sided pneumonia with a small parapneumonic effusion. He is afebrile after 24 hours of IV antibiotics and clinically feels much better. | Would you order a thoracentesis? If so, would you perform it bedside or send the patient to radiology for an ultrasound‐guided procedure? Would you consult a pulmonologist? |
| An 82‐year‐old severely demented nursing home resident who has required total care for the past few months presents with dehydration and a sodium of 158 after increasingly poor oral intake. No other illness is identified. | Would you begin IV fluids immediately and consider gastrostomy tube placement to maintain adequate hydration at the nursing home or would you contact family to discuss end‐of‐life care goals first? Would your management change if a UTI or pneumonia was diagnosed? |
Third, hospitalists could potentially benefit from the development of patient education materials, available through SHM, that address the cost‐effectiveness of common inpatient tests and treatments with the goal of decreasing patient demand for unnecessary testing. Education regarding advanced directives and end‐of‐life care decision‐making could be particularly valuable in minimizing futile care, as it is well‐documented that transitioning to palliative care as soon as it is appropriate reduces healthcare spending greatly during the end‐of‐life period.2427 At the same time, we need to be careful to reassure our patients that we are not trying to ration care, but are instead minimizing the risks and costs for them associated with unnecessary care. In my experience, most patients, if given appropriate time, attention, and education, are willing to accept the final recommendation of their physician.
Fourth, intensified federal and state advocacy in several areas could help reduce spending. For example, advocacy for medical liability reform may reduce the atmosphere of defensive medicine, although I suspect that because old habits die hard, it may take a full generation of decreased liability risk to actually change practice patterns. Advocacy for the development of a national, or at least more uniform, electronic medical record, may decrease duplicate testing and improve efficiency. Advocacy for value‐based reimbursement models may help dampen costs resulting from a predominantly fee‐for‐service environment.28
Fifth, and perhaps most fundamental to the future of our specialty, encouraging the broad professional development of hospitalists as a true specialists in inpatient medicine (based on the SHM Core Competencies,)29 could help minimize the unnecessary costs associated with specialist‐oriented care.6 With the desire to create, in the near future, a formal board‐certification in hospital medicine comes an obligation to develop broad knowledge and broad skill sets that are truly unique to our profession, whereas deferring to a specialist‐oriented pattern of care actually shrinks us down to something less than a traditional internist, rather than a unique entity.30 With our 24/7 focus on inpatient care, we should easily be able to demonstrate our superiority in safety, quality, and efficiency, all of which are closely linked to increased value per healthcare dollar. If, however, our focus is blurred by an overly productivity‐based practice, in which patient volume and procedures take precedence, we will not be able to claim any special value to the system.
Last, supporting efforts to improve coordination of care and transitions of care could reduce costs associated with unnecessary readmissions or posthospital complications. A recent policy statement from several professional societies, including SHM, highlights the importance of these transitions,20, 31 and within the past year, SHM has launched the successful Project BOOST (Better Outcomes for Older adults through Safe Transitions) to help in this effort.32
Unfortunately, there is an inherent problem with all of the above proposals: the assumption that physicians actually want to reduce healthcare spending. Since everyone who works in the medical industry benefits financially in some way from the current high levels of spending on healthcare, reducing spending is counterintuitive for many, and the incentives to spend more will likely persist until some form of spending targets or limits are set.33 Moreover, since physicians traditionally do not like to be told how to practice medicine, history would predict that, without attractive incentives, nothing will change. This is the fundamental and unfortunate dilemma that has apparently pushed us to the eleventh hour of a healthcare crisis.
Another concern with an extreme atmosphere of cost cutting is the risk of swinging too far in the opposite direction, focusing so intently on cost that we begin to compromise quality or access to care in order to achieve spending targets. Reassuringly, however, the data suggest that there is plenty of room for us to cut costs without harming health outcomes.
Despite these obstacles, during this historic time in US healthcare, I believe hospitalists have a unique and perhaps transient opportunity to demonstrate their singular commitment to rational healthcare spending and by doing so to gain significant influence in shaping the impending healthcare reforms. If we speak and act with one voice, with transparency, and with the proper data, we could be the first and only professional society to not only demonstrate our current pattern of spending, but also our potential for reducing spending and our plan on how to get there.
Acknowledgements
Judy Knight, MLS, provided valuable research and technical support.
- Medicare pay overhaul can no longer wait. American Medical News.2009. Available at: http://www.ama‐assn.org/amednews/2009/01/12/edsa0112.htm. Accessed July 2009.
- ,,, et al.Health spending projections through 2017: the baby‐boom generation is coming to Medicare.Health Aff (Millwood).2008;27(2):w145–w155.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:380.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:374.
- National Scorecard on U.S. Health System Performance, 2008 Chartpack.New York, NY:The Commonwealth Fund;2008:6.
- ,,,,,.The implications of regional variations in medicare spending. Part 1: The content, quality, and accessibility of care.Ann Intern Med.2003;138(4):273–287.
- ,,,.Waste in the U.S. health care system: a conceptual framework.Milbank Q.2008;86(4):629–659.
- ,,,.It's the prices, stupid: why the United States is so different from other countries.Health Aff (Millwood).2003;22(3):89–105.
- . Health Care and the budget: issues and challenges for reform.2007. Available at: http://www.cbo.gov/ftpdocs/82xx/doc8255/06–21‐HealthCareReform.pdf. Accessed July 2009.
- .Overtreated: Why Too Much Medicine Is Making Us Sicker and Poorer.1st ed.New York, NY:Bloomsbury;2007.
- ,,,. Slowing the growth of U.S. health care expensitures: what are the options?2007. Available at: http://www.commonwealthfund.org/publications/publications_show.htm?doc_id=449510. Accessed July 2009.
- .More variation in use of care, more flat‐of‐the‐curve medicine.Health Aff (Millwood).2004;(Suppl Web Exclusives):VAR104–VAR107.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:419.
- ,.Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008.Lebanon, NH:Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research;2008:25–32.
- ,.Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008.Lebanon, NH:Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research;2008:2–4.
- ,,.Effects of the medical liability system in Australia, the UK, and the USA.Lancet.2006;368(9531):240–246.
- Comments on the centers for Medicare and Medicaid services plan to transition to a Medicare value‐based purchasing program for physicians and other professional services.2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Issues_in_the_Spotlight12008:62,73.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,, et al.Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Gen Intern Med.2009;24(8):971–976.
- Hospitals like mine: 2006 national statistics.2006. Available at: http://www.hcupnet.ahrq.gov. Accessed July 2009.
- ,,.Evidence‐Based to Value‐Based Medicine.Chicago, IL:AMA Press;2005.
- Improved Availability of Comparative Effectiveness Information: An Essential Feature for a High‐Quality and Efficient United States Health Care System.Philadelphia, PA:American College of Physicians;2008.
- ,.Clinical practice. Palliative care.N Engl J Med.2004;350(25):2582–2590.
- ,,.The health economics of palliative care.Oncology (Williston Park).2002;16(6):801–808; discussion 808, 811–802.
- .Cost savings at the end of life. What do the data show?JAMA.1996;275(24):1907–1914.
- ,,, et al.Cost savings associated with US hospital palliative care consultation programs.Arch Intern Med.2008;168(16):1783–1790.
- ,,, et al.Toward a 21st‐century health care system: recommendations for health care reform.Ann Intern Med.2009;150(7):493–495.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- .The expanding or shrinking universe of the hospitalist.J Hosp Med.2008;3(4):288–291.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- Project BOOST.2009. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed Julyyear="2009"2009.
- ,,.The Obama administration's options for health care cost control: hope versus reality.Ann Intern Med.2009;150(7):485–489.
- Medicare pay overhaul can no longer wait. American Medical News.2009. Available at: http://www.ama‐assn.org/amednews/2009/01/12/edsa0112.htm. Accessed July 2009.
- ,,, et al.Health spending projections through 2017: the baby‐boom generation is coming to Medicare.Health Aff (Millwood).2008;27(2):w145–w155.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:380.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:374.
- National Scorecard on U.S. Health System Performance, 2008 Chartpack.New York, NY:The Commonwealth Fund;2008:6.
- ,,,,,.The implications of regional variations in medicare spending. Part 1: The content, quality, and accessibility of care.Ann Intern Med.2003;138(4):273–287.
- ,,,.Waste in the U.S. health care system: a conceptual framework.Milbank Q.2008;86(4):629–659.
- ,,,.It's the prices, stupid: why the United States is so different from other countries.Health Aff (Millwood).2003;22(3):89–105.
- . Health Care and the budget: issues and challenges for reform.2007. Available at: http://www.cbo.gov/ftpdocs/82xx/doc8255/06–21‐HealthCareReform.pdf. Accessed July 2009.
- .Overtreated: Why Too Much Medicine Is Making Us Sicker and Poorer.1st ed.New York, NY:Bloomsbury;2007.
- ,,,. Slowing the growth of U.S. health care expensitures: what are the options?2007. Available at: http://www.commonwealthfund.org/publications/publications_show.htm?doc_id=449510. Accessed July 2009.
- .More variation in use of care, more flat‐of‐the‐curve medicine.Health Aff (Millwood).2004;(Suppl Web Exclusives):VAR104–VAR107.
- Health, United States, 2007: Chartbook on Trends in the Health of Americans.Hyattsville, MD:National Center for Health Statistics;2007:419.
- ,.Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008.Lebanon, NH:Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research;2008:25–32.
- ,.Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008.Lebanon, NH:Dartmouth Institute for Health Policy and Clinical Practice, Center for Health Policy Research;2008:2–4.
- ,,.Effects of the medical liability system in Australia, the UK, and the USA.Lancet.2006;368(9531):240–246.
- Comments on the centers for Medicare and Medicaid services plan to transition to a Medicare value‐based purchasing program for physicians and other professional services.2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Issues_in_the_Spotlight12008:62,73.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,, et al.Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Gen Intern Med.2009;24(8):971–976.
- Hospitals like mine: 2006 national statistics.2006. Available at: http://www.hcupnet.ahrq.gov. Accessed July 2009.
- ,,.Evidence‐Based to Value‐Based Medicine.Chicago, IL:AMA Press;2005.
- Improved Availability of Comparative Effectiveness Information: An Essential Feature for a High‐Quality and Efficient United States Health Care System.Philadelphia, PA:American College of Physicians;2008.
- ,.Clinical practice. Palliative care.N Engl J Med.2004;350(25):2582–2590.
- ,,.The health economics of palliative care.Oncology (Williston Park).2002;16(6):801–808; discussion 808, 811–802.
- .Cost savings at the end of life. What do the data show?JAMA.1996;275(24):1907–1914.
- ,,, et al.Cost savings associated with US hospital palliative care consultation programs.Arch Intern Med.2008;168(16):1783–1790.
- ,,, et al.Toward a 21st‐century health care system: recommendations for health care reform.Ann Intern Med.2009;150(7):493–495.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- .The expanding or shrinking universe of the hospitalist.J Hosp Med.2008;3(4):288–291.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- Project BOOST.2009. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed Julyyear="2009"2009.
- ,,.The Obama administration's options for health care cost control: hope versus reality.Ann Intern Med.2009;150(7):485–489.
Hand‐Carried Ultrasound Use
Ultrasound, one of the most reliable diagnostic technologies in medicine, has a unique long‐term safety profile across a wide spectrum of applications. In line with the trend toward the miniaturization of many other technologies, increasingly sophisticated hand‐held or hand‐carried ultrasound (HCU) devices have become widely available. To date, the U.S. Food and Drug Administration (FDA) has approved more than 10 new‐generation portable (1.0‐4.5 kg) ultrasound devices, and a recent industry report projected that the HCU market will see revenues in excess of $1 billion by 2011.1
Although cardiovascular assessment remains its primary use, hospitalist physicians are increasingly turning to this technology for the localization of fluid and other abnormalities prior to paracentesis and thoracentesis. While there are other potential uses (eg, managing acute scrotal pain, diagnosing meniscal tears, measuring carotid intimal thickness), the higher‐quality studies of hospitalist‐physicians' use of HCU have focused on cardiovascular assessment. HCU confers a number of potential workflow‐related advantages, including coordinated point‐of‐care evaluation at short notice when formal ultrasound may be unavailable, as well as circumvention of the need to call on radiology or cardiology specialists.2 Even for experienced cardiologists, heart failure can be difficult to identify using any modality, and the clinical diagnosis of cardiovascular disease by hospital physicians has been documented as poor.3, 4 Thus, the addition of HCU to the palette of diagnostic and teaching tools available to frontline physicians potentially offers improvements over stethoscope‐assisted physical examination alone (including visual inspection, palpation, and auscultation), which has remained essentially unaltered for 150 years.57
Evidence Base for HCU Use by Hospitalists
The few primary studies on HCU use by hospitalists have focused on the potential utility of this technology as a valuable adjunct to the physical exam for the detection of cardiovascular disease (eg, asymptomatic left ventricular [LV] dysfunction, cardiomegaly, pericardial effusion) in the ambulatory or acute care setting.8, 9 Operation of HCU by hospitalists is not clearly indicated for the evaluation of valvular disease (eg, aortic and mitral regurgitation), in part due to the limited Doppler capabilities of the smaller devices.911 The risk of a gradual erosion of physical exam skills accompanying expansion of HCU use by hospitalists could itself become a potential disadvantage of a premature replacement of the stethoscope, since the results obtained by hospitalists performing a standard physical exam have been shown to be better than those obtained with HCU.8, 9
The lack of large, multicenter studies of HCU use by hospitalists leaves many questions unanswered, including whether or not the relatively low initial cost of an HCU device ($9,000‐$50,000) vs. that of a full‐sized hospital ultrasound system ($250,000) will eventually translate into overall cost‐effectiveness or actual patient‐centered benefit.10 While cautious advocates have insisted that HCU provides additive information in conjunction with the physical exam, this approach is not meant to serve as a substitute for standard echocardiography in patients requiring full evaluation in inpatient settings relevant for hospitalists.1114 Referral for additional testing or specialist opinionsand the associated costs incurredcannot necessarily be circumvented by hospitalist‐operated HCU.
A major problem with the HCU literature in general is its lack of standardization betweenand withinstudies, which renders it nearly impossible to generalize findings about important clinical outcomes, patient satisfaction, quality‐of‐life, symptoms, physical functioning, and morbidity and mortality. There are a preponderance of underpowered, methodologically inconsistent, single‐center case series that do not evaluate diagnostic accuracy in terms of patient outcomes. For example, although one study did find a modest (22‐29%) reduction in department workload with HCU, the authors omitted important information regarding blinding, and no power calculations were reported; thus, it was not possible to ascertain whether or not the reported results were due to the intervention or to chance.15 There clearly remains a need to convincingly demonstrate that patient care, shortening of length of stay, long‐term prognosis, or potential financial savings could occur with use of these devices by hospitalists.5 The process of device acquisition and resource allocation is, at least in part, based on accumulated evidence from studies that have ill‐defined relevant outcomes (eg, left ventricular function). However, even if such outcomes were to be more closely examined, medical decision‐making would still suffer from discrepant findings due to numerous differences in study design, including parameters involving patient population and selection, setting (eg, echocardiography laboratory vs. critical care unit), provider background, and specific device(s) used.
Training Issues
Hospitalist proficiency across HCU imaging skills (ie, acquisition, measurement, interpretation) has been found to be inconsistent.9 Endorsement and expansion of hospitalist use of HCU may to some extent reflect an overgeneralization from disparate comparative studies showing moderate success obtained with HCU (vs. physical exam) by other practitioner groups such as medical students and fellows with limited experience.16, 17 Whereas in 2005, Hellmann et al.18 concluded that medical residents with minimal training can learn to perform some of the basic functions of HCU with reasonable accuracy, Martin et al.8, 9 (in 2007 and 2009) reported conflicting results from a study of hospitalists trained at the same institution.
Concern about switching from standard to nonstandard HCU operators is raised by studies in which specialized operators (eg, echocardiography technicians) obtained better results than hospitalists using these devices.8, 9 In 2004, Borges et al.19 reported the results of 315 patients referred to specialists at a cardiology clinic for preoperative assessment prior to noncardiac surgery; the results (94.8% and 96.7% agreement with standard echocardiography on the main echocardiographic finding and detection of valve disease, respectively) were attributed to the fact that experienced cardiologists were working under ideal conditions using only the most advanced HCU devices with Doppler as well as harmonic imaging capabilities. Likewise, in 2004, Tsutsui et al.20 studied 44 consecutive hospitalized patients who underwent comprehensive echocardiography and bedside HCU. They reported that hemodynamic assessment by HCU was poor, even when performed by practitioners with relatively high levels of training.20 In 2003, DeCara et al.12 performed standard echocardiography on 300 adult inpatients referred for imaging, and concluded that standardized training, competency testing, and quality assurance guidelines need to be established before these devices can be utilized for clinical decision‐making by physicians without formal training in echocardiography. Although there have been numerous calls for training guidelines, it has not yet been determined how much training would be optimalor even necessaryfor professionals of each subspecialty to achieve levels of accuracy that are acceptable. Furthermore, it is well known that skill level declines unless a technique is regularly reinforced with practice, and therefore, recertification or procedure volume standards should be established.
The issue of potential harm needs to be raised, if hospitalists with access to HCU are indeed less accurate in their diagnoses than trained cardiologists interpreting images acquired by an established alternative such as echocardiography. False negatives can lead to delayed treatment, and false positives to unwarranted treatment. Given that the treatment effects of HCU use by hospitalists have not been closely scrutinized, the expansion of such use appears unwarranted, at least until further randomized studies with well‐defined outcomes have been conducted. Although the HCU devices themselves have a good safety profile, their potential benefits and harms (eg, possibility of increased nosocomial infection) will ultimately reflect operator skill and their impact on patient management relative to the gold‐standard diagnostic modalities for which there is abundant evidence of safety and efficacy.21
Premarketing and Postmarketing Concerns
The controversy regarding hospitalist use of HCU exposes gaps in the FDA approval process for medical devices, which are subjected to much less rigorous scrutiny during the premarketing approval process than pharmaceuticals.22 Moreover, the aggressive marketing of newly approved devices (and drugs) can drive medically unwarranted overuse, or indication creep, which justifies calls for the establishment of rigorous standards of clinical relevance and practice.23, 24 While the available literature on HCU operation by hospitalists is focused on cardiovascular indications for the technology, hospital medicine physicians are increasingly using HCU to guide paracentesis and thoracentesis. Given how commonplace the expansion of such practices has become, it is noteworthy that HCU operation by hospitalists has not yet been evaluated and endorsed in larger, controlled trials demonstrating appropriate outcomes.25
Across all fields of medicine, the transition from traditional to newer modalities remains a slippery slope in terms of demonstration of persuasive evidence of patient‐centered benefit.26 Fascination with emerging technologies (so‐called gizmo idolatry) and increased reimbursement potential threaten to distract patients and their providers from legitimate concerns about how medical device manufacturers and for‐profit corporations increasingly influence device acquisition and clinical practice.2731 While we lack strong evidence demonstrating that diagnostic tests such as HCU are beneficial when performed by hospitalists, the expanded use of these handy new devices by hospitalists is simultaneously generating increased incidental and equivocal findings, which in turn render it necessary to go back and perform secondary verification studies by specialists using older, gold‐standard modalities. This vicious cycle, coupled with the current lack of evidence, will continue to degrade confidence in the initiation of either acute or chronic treatment on the basis of HCU results obtained by hospitalist physicians.
Eventually, the increased use of HCU by hospitalists might lead to demonstrations of improved hospital workflow management, but it may just as easily represent another new coupling of technology and practitioner that prematurely becomes the standard of care in the absence of any demonstration of added value. The initially enthusiastic application of pulmonary artery catheters (PACs) serves as a cautionary tale in which the acquisition of additional clinical data did not necessarily lead to improved clinical outcomes: whereas PACs did enhance the clinical understanding of hemodynamics, they were not associated with an overall advantage in terms of mortality, length of hospital stay, or cost.3235 Ultimately, more information is not necessarily better information. Although new medical technologies can produce extremely useful diagnostic results that aid in the management of critically ill patients, poor data interpretation resulting from lack of targeted training and experience can nullify point‐of‐care advantages, and perhaps lead to excess morbidity and mortality.14 In clinical practice, it is generally best to avoid reliance on assumptions of added value in lieu of demonstrations of the same.
Conclusions
Hospital practitioners should not yet put away their stethoscopes. New technologies such as HCU need to be embraced in parallel with accumulating evidence of benefit. In the hands of hospitalists, the smaller HCU devices may very well prove handy, but at present, the literature simply does not support the use of HCU by hospitalist physicians.
- Hand‐Carried Ultrasound—Reshaping the ultrasound marketplace. Available at: http://www.sonoworld.com/NewsStories/NewsStories.aspx?ID= 450. Accessed August2009.
- ,,.A new narrative for hospitalists.J Hosp Med.2009;4(4):207–208.
- .Can heart failure be diagnosed in primary care?BMJ.2000;321(7255):188–189.
- ,,.Evidence of inadequate investigation and treatment of patients with heart failure.Br Heart J.1994;71(6):584–587.
- .Utility of hand‐carried ultrasound for consultative cardiology.Echocardiography.2003;20(5):463–469.
- .Tomorrow's stethoscope: the hand‐held ultrasound device?J S C Med Assoc.2006;102(10):345.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,.Should a hand‐carried ultrasound machine become standard equipment for every internist?Am J Med.2009;122(1):1–3.
- ,,,.How useful is hand‐carried bedside echocardiography in critically ill patients?J Am Coll Cardiol.2001;37(8):2019–2022.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4(2):141–147.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20(5):471–476.
- .Specific skill set and goals of focused echocardiography for critical care clinicians.Crit Care Med.2007;35(5 suppl):S144–S149.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,,,.Diagnostic accuracy of new handheld echocardiography with Doppler and harmonic imaging properties.J Am Soc Echocardiogr.2004;17(3):234–238.
- ,,,,,.Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography.Cardiovasc Ultrasound.2004;2:24.
- ,,Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology.J Am Soc Echocardiogr.2004;17(1):50–55.
- ,,,.Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment.J Gen Intern Med.2008;23(suppl 1):57–63.
- ,,.Newly approved does not always mean new and improved.JAMA.2008;299(13):1598–1600.
- ,.Indication creep: physician beware.CMAJ.2007;177(7):697,699.
- ,,,.Ultrasound‐guided interventional radiology in critical care.Crit Care Med.2007;35(5 suppl):S186–S197.
- ,.Pay now, benefits may follow—the case of cardiac computed tomographic angiography.N Engl J Med.2008;359(22):2309–2311.
- ,.Gizmo idolatry.JAMA.2008;299(15):1830–1832.
- .Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Impugning the integrity of medical science: the adverse effects of industry influence.JAMA.2008;299(15):1833–1835.
- ,,, et al.The 2007 ABJS Marshall Urist Award: the impact of direct‐to‐consumer advertising in orthopaedics.Clin Orthop Relat Res.2007;458:202–219.
- ,.Direct to consumer advertising in healthcare: history, benefits, and concerns.Clin Orthop Relat Res.2007;457:96–104.
- Connors,,, et al.The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators.JAMA.1996;276(11):889–897.
- ,,, et al.Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC‐Man): a randomised controlled trial.Lancet.2005;366(9484):472–477.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial.JAMA.2003;290(20):2713–2720.
Ultrasound, one of the most reliable diagnostic technologies in medicine, has a unique long‐term safety profile across a wide spectrum of applications. In line with the trend toward the miniaturization of many other technologies, increasingly sophisticated hand‐held or hand‐carried ultrasound (HCU) devices have become widely available. To date, the U.S. Food and Drug Administration (FDA) has approved more than 10 new‐generation portable (1.0‐4.5 kg) ultrasound devices, and a recent industry report projected that the HCU market will see revenues in excess of $1 billion by 2011.1
Although cardiovascular assessment remains its primary use, hospitalist physicians are increasingly turning to this technology for the localization of fluid and other abnormalities prior to paracentesis and thoracentesis. While there are other potential uses (eg, managing acute scrotal pain, diagnosing meniscal tears, measuring carotid intimal thickness), the higher‐quality studies of hospitalist‐physicians' use of HCU have focused on cardiovascular assessment. HCU confers a number of potential workflow‐related advantages, including coordinated point‐of‐care evaluation at short notice when formal ultrasound may be unavailable, as well as circumvention of the need to call on radiology or cardiology specialists.2 Even for experienced cardiologists, heart failure can be difficult to identify using any modality, and the clinical diagnosis of cardiovascular disease by hospital physicians has been documented as poor.3, 4 Thus, the addition of HCU to the palette of diagnostic and teaching tools available to frontline physicians potentially offers improvements over stethoscope‐assisted physical examination alone (including visual inspection, palpation, and auscultation), which has remained essentially unaltered for 150 years.57
Evidence Base for HCU Use by Hospitalists
The few primary studies on HCU use by hospitalists have focused on the potential utility of this technology as a valuable adjunct to the physical exam for the detection of cardiovascular disease (eg, asymptomatic left ventricular [LV] dysfunction, cardiomegaly, pericardial effusion) in the ambulatory or acute care setting.8, 9 Operation of HCU by hospitalists is not clearly indicated for the evaluation of valvular disease (eg, aortic and mitral regurgitation), in part due to the limited Doppler capabilities of the smaller devices.911 The risk of a gradual erosion of physical exam skills accompanying expansion of HCU use by hospitalists could itself become a potential disadvantage of a premature replacement of the stethoscope, since the results obtained by hospitalists performing a standard physical exam have been shown to be better than those obtained with HCU.8, 9
The lack of large, multicenter studies of HCU use by hospitalists leaves many questions unanswered, including whether or not the relatively low initial cost of an HCU device ($9,000‐$50,000) vs. that of a full‐sized hospital ultrasound system ($250,000) will eventually translate into overall cost‐effectiveness or actual patient‐centered benefit.10 While cautious advocates have insisted that HCU provides additive information in conjunction with the physical exam, this approach is not meant to serve as a substitute for standard echocardiography in patients requiring full evaluation in inpatient settings relevant for hospitalists.1114 Referral for additional testing or specialist opinionsand the associated costs incurredcannot necessarily be circumvented by hospitalist‐operated HCU.
A major problem with the HCU literature in general is its lack of standardization betweenand withinstudies, which renders it nearly impossible to generalize findings about important clinical outcomes, patient satisfaction, quality‐of‐life, symptoms, physical functioning, and morbidity and mortality. There are a preponderance of underpowered, methodologically inconsistent, single‐center case series that do not evaluate diagnostic accuracy in terms of patient outcomes. For example, although one study did find a modest (22‐29%) reduction in department workload with HCU, the authors omitted important information regarding blinding, and no power calculations were reported; thus, it was not possible to ascertain whether or not the reported results were due to the intervention or to chance.15 There clearly remains a need to convincingly demonstrate that patient care, shortening of length of stay, long‐term prognosis, or potential financial savings could occur with use of these devices by hospitalists.5 The process of device acquisition and resource allocation is, at least in part, based on accumulated evidence from studies that have ill‐defined relevant outcomes (eg, left ventricular function). However, even if such outcomes were to be more closely examined, medical decision‐making would still suffer from discrepant findings due to numerous differences in study design, including parameters involving patient population and selection, setting (eg, echocardiography laboratory vs. critical care unit), provider background, and specific device(s) used.
Training Issues
Hospitalist proficiency across HCU imaging skills (ie, acquisition, measurement, interpretation) has been found to be inconsistent.9 Endorsement and expansion of hospitalist use of HCU may to some extent reflect an overgeneralization from disparate comparative studies showing moderate success obtained with HCU (vs. physical exam) by other practitioner groups such as medical students and fellows with limited experience.16, 17 Whereas in 2005, Hellmann et al.18 concluded that medical residents with minimal training can learn to perform some of the basic functions of HCU with reasonable accuracy, Martin et al.8, 9 (in 2007 and 2009) reported conflicting results from a study of hospitalists trained at the same institution.
Concern about switching from standard to nonstandard HCU operators is raised by studies in which specialized operators (eg, echocardiography technicians) obtained better results than hospitalists using these devices.8, 9 In 2004, Borges et al.19 reported the results of 315 patients referred to specialists at a cardiology clinic for preoperative assessment prior to noncardiac surgery; the results (94.8% and 96.7% agreement with standard echocardiography on the main echocardiographic finding and detection of valve disease, respectively) were attributed to the fact that experienced cardiologists were working under ideal conditions using only the most advanced HCU devices with Doppler as well as harmonic imaging capabilities. Likewise, in 2004, Tsutsui et al.20 studied 44 consecutive hospitalized patients who underwent comprehensive echocardiography and bedside HCU. They reported that hemodynamic assessment by HCU was poor, even when performed by practitioners with relatively high levels of training.20 In 2003, DeCara et al.12 performed standard echocardiography on 300 adult inpatients referred for imaging, and concluded that standardized training, competency testing, and quality assurance guidelines need to be established before these devices can be utilized for clinical decision‐making by physicians without formal training in echocardiography. Although there have been numerous calls for training guidelines, it has not yet been determined how much training would be optimalor even necessaryfor professionals of each subspecialty to achieve levels of accuracy that are acceptable. Furthermore, it is well known that skill level declines unless a technique is regularly reinforced with practice, and therefore, recertification or procedure volume standards should be established.
The issue of potential harm needs to be raised, if hospitalists with access to HCU are indeed less accurate in their diagnoses than trained cardiologists interpreting images acquired by an established alternative such as echocardiography. False negatives can lead to delayed treatment, and false positives to unwarranted treatment. Given that the treatment effects of HCU use by hospitalists have not been closely scrutinized, the expansion of such use appears unwarranted, at least until further randomized studies with well‐defined outcomes have been conducted. Although the HCU devices themselves have a good safety profile, their potential benefits and harms (eg, possibility of increased nosocomial infection) will ultimately reflect operator skill and their impact on patient management relative to the gold‐standard diagnostic modalities for which there is abundant evidence of safety and efficacy.21
Premarketing and Postmarketing Concerns
The controversy regarding hospitalist use of HCU exposes gaps in the FDA approval process for medical devices, which are subjected to much less rigorous scrutiny during the premarketing approval process than pharmaceuticals.22 Moreover, the aggressive marketing of newly approved devices (and drugs) can drive medically unwarranted overuse, or indication creep, which justifies calls for the establishment of rigorous standards of clinical relevance and practice.23, 24 While the available literature on HCU operation by hospitalists is focused on cardiovascular indications for the technology, hospital medicine physicians are increasingly using HCU to guide paracentesis and thoracentesis. Given how commonplace the expansion of such practices has become, it is noteworthy that HCU operation by hospitalists has not yet been evaluated and endorsed in larger, controlled trials demonstrating appropriate outcomes.25
Across all fields of medicine, the transition from traditional to newer modalities remains a slippery slope in terms of demonstration of persuasive evidence of patient‐centered benefit.26 Fascination with emerging technologies (so‐called gizmo idolatry) and increased reimbursement potential threaten to distract patients and their providers from legitimate concerns about how medical device manufacturers and for‐profit corporations increasingly influence device acquisition and clinical practice.2731 While we lack strong evidence demonstrating that diagnostic tests such as HCU are beneficial when performed by hospitalists, the expanded use of these handy new devices by hospitalists is simultaneously generating increased incidental and equivocal findings, which in turn render it necessary to go back and perform secondary verification studies by specialists using older, gold‐standard modalities. This vicious cycle, coupled with the current lack of evidence, will continue to degrade confidence in the initiation of either acute or chronic treatment on the basis of HCU results obtained by hospitalist physicians.
Eventually, the increased use of HCU by hospitalists might lead to demonstrations of improved hospital workflow management, but it may just as easily represent another new coupling of technology and practitioner that prematurely becomes the standard of care in the absence of any demonstration of added value. The initially enthusiastic application of pulmonary artery catheters (PACs) serves as a cautionary tale in which the acquisition of additional clinical data did not necessarily lead to improved clinical outcomes: whereas PACs did enhance the clinical understanding of hemodynamics, they were not associated with an overall advantage in terms of mortality, length of hospital stay, or cost.3235 Ultimately, more information is not necessarily better information. Although new medical technologies can produce extremely useful diagnostic results that aid in the management of critically ill patients, poor data interpretation resulting from lack of targeted training and experience can nullify point‐of‐care advantages, and perhaps lead to excess morbidity and mortality.14 In clinical practice, it is generally best to avoid reliance on assumptions of added value in lieu of demonstrations of the same.
Conclusions
Hospital practitioners should not yet put away their stethoscopes. New technologies such as HCU need to be embraced in parallel with accumulating evidence of benefit. In the hands of hospitalists, the smaller HCU devices may very well prove handy, but at present, the literature simply does not support the use of HCU by hospitalist physicians.
Ultrasound, one of the most reliable diagnostic technologies in medicine, has a unique long‐term safety profile across a wide spectrum of applications. In line with the trend toward the miniaturization of many other technologies, increasingly sophisticated hand‐held or hand‐carried ultrasound (HCU) devices have become widely available. To date, the U.S. Food and Drug Administration (FDA) has approved more than 10 new‐generation portable (1.0‐4.5 kg) ultrasound devices, and a recent industry report projected that the HCU market will see revenues in excess of $1 billion by 2011.1
Although cardiovascular assessment remains its primary use, hospitalist physicians are increasingly turning to this technology for the localization of fluid and other abnormalities prior to paracentesis and thoracentesis. While there are other potential uses (eg, managing acute scrotal pain, diagnosing meniscal tears, measuring carotid intimal thickness), the higher‐quality studies of hospitalist‐physicians' use of HCU have focused on cardiovascular assessment. HCU confers a number of potential workflow‐related advantages, including coordinated point‐of‐care evaluation at short notice when formal ultrasound may be unavailable, as well as circumvention of the need to call on radiology or cardiology specialists.2 Even for experienced cardiologists, heart failure can be difficult to identify using any modality, and the clinical diagnosis of cardiovascular disease by hospital physicians has been documented as poor.3, 4 Thus, the addition of HCU to the palette of diagnostic and teaching tools available to frontline physicians potentially offers improvements over stethoscope‐assisted physical examination alone (including visual inspection, palpation, and auscultation), which has remained essentially unaltered for 150 years.57
Evidence Base for HCU Use by Hospitalists
The few primary studies on HCU use by hospitalists have focused on the potential utility of this technology as a valuable adjunct to the physical exam for the detection of cardiovascular disease (eg, asymptomatic left ventricular [LV] dysfunction, cardiomegaly, pericardial effusion) in the ambulatory or acute care setting.8, 9 Operation of HCU by hospitalists is not clearly indicated for the evaluation of valvular disease (eg, aortic and mitral regurgitation), in part due to the limited Doppler capabilities of the smaller devices.911 The risk of a gradual erosion of physical exam skills accompanying expansion of HCU use by hospitalists could itself become a potential disadvantage of a premature replacement of the stethoscope, since the results obtained by hospitalists performing a standard physical exam have been shown to be better than those obtained with HCU.8, 9
The lack of large, multicenter studies of HCU use by hospitalists leaves many questions unanswered, including whether or not the relatively low initial cost of an HCU device ($9,000‐$50,000) vs. that of a full‐sized hospital ultrasound system ($250,000) will eventually translate into overall cost‐effectiveness or actual patient‐centered benefit.10 While cautious advocates have insisted that HCU provides additive information in conjunction with the physical exam, this approach is not meant to serve as a substitute for standard echocardiography in patients requiring full evaluation in inpatient settings relevant for hospitalists.1114 Referral for additional testing or specialist opinionsand the associated costs incurredcannot necessarily be circumvented by hospitalist‐operated HCU.
A major problem with the HCU literature in general is its lack of standardization betweenand withinstudies, which renders it nearly impossible to generalize findings about important clinical outcomes, patient satisfaction, quality‐of‐life, symptoms, physical functioning, and morbidity and mortality. There are a preponderance of underpowered, methodologically inconsistent, single‐center case series that do not evaluate diagnostic accuracy in terms of patient outcomes. For example, although one study did find a modest (22‐29%) reduction in department workload with HCU, the authors omitted important information regarding blinding, and no power calculations were reported; thus, it was not possible to ascertain whether or not the reported results were due to the intervention or to chance.15 There clearly remains a need to convincingly demonstrate that patient care, shortening of length of stay, long‐term prognosis, or potential financial savings could occur with use of these devices by hospitalists.5 The process of device acquisition and resource allocation is, at least in part, based on accumulated evidence from studies that have ill‐defined relevant outcomes (eg, left ventricular function). However, even if such outcomes were to be more closely examined, medical decision‐making would still suffer from discrepant findings due to numerous differences in study design, including parameters involving patient population and selection, setting (eg, echocardiography laboratory vs. critical care unit), provider background, and specific device(s) used.
Training Issues
Hospitalist proficiency across HCU imaging skills (ie, acquisition, measurement, interpretation) has been found to be inconsistent.9 Endorsement and expansion of hospitalist use of HCU may to some extent reflect an overgeneralization from disparate comparative studies showing moderate success obtained with HCU (vs. physical exam) by other practitioner groups such as medical students and fellows with limited experience.16, 17 Whereas in 2005, Hellmann et al.18 concluded that medical residents with minimal training can learn to perform some of the basic functions of HCU with reasonable accuracy, Martin et al.8, 9 (in 2007 and 2009) reported conflicting results from a study of hospitalists trained at the same institution.
Concern about switching from standard to nonstandard HCU operators is raised by studies in which specialized operators (eg, echocardiography technicians) obtained better results than hospitalists using these devices.8, 9 In 2004, Borges et al.19 reported the results of 315 patients referred to specialists at a cardiology clinic for preoperative assessment prior to noncardiac surgery; the results (94.8% and 96.7% agreement with standard echocardiography on the main echocardiographic finding and detection of valve disease, respectively) were attributed to the fact that experienced cardiologists were working under ideal conditions using only the most advanced HCU devices with Doppler as well as harmonic imaging capabilities. Likewise, in 2004, Tsutsui et al.20 studied 44 consecutive hospitalized patients who underwent comprehensive echocardiography and bedside HCU. They reported that hemodynamic assessment by HCU was poor, even when performed by practitioners with relatively high levels of training.20 In 2003, DeCara et al.12 performed standard echocardiography on 300 adult inpatients referred for imaging, and concluded that standardized training, competency testing, and quality assurance guidelines need to be established before these devices can be utilized for clinical decision‐making by physicians without formal training in echocardiography. Although there have been numerous calls for training guidelines, it has not yet been determined how much training would be optimalor even necessaryfor professionals of each subspecialty to achieve levels of accuracy that are acceptable. Furthermore, it is well known that skill level declines unless a technique is regularly reinforced with practice, and therefore, recertification or procedure volume standards should be established.
The issue of potential harm needs to be raised, if hospitalists with access to HCU are indeed less accurate in their diagnoses than trained cardiologists interpreting images acquired by an established alternative such as echocardiography. False negatives can lead to delayed treatment, and false positives to unwarranted treatment. Given that the treatment effects of HCU use by hospitalists have not been closely scrutinized, the expansion of such use appears unwarranted, at least until further randomized studies with well‐defined outcomes have been conducted. Although the HCU devices themselves have a good safety profile, their potential benefits and harms (eg, possibility of increased nosocomial infection) will ultimately reflect operator skill and their impact on patient management relative to the gold‐standard diagnostic modalities for which there is abundant evidence of safety and efficacy.21
Premarketing and Postmarketing Concerns
The controversy regarding hospitalist use of HCU exposes gaps in the FDA approval process for medical devices, which are subjected to much less rigorous scrutiny during the premarketing approval process than pharmaceuticals.22 Moreover, the aggressive marketing of newly approved devices (and drugs) can drive medically unwarranted overuse, or indication creep, which justifies calls for the establishment of rigorous standards of clinical relevance and practice.23, 24 While the available literature on HCU operation by hospitalists is focused on cardiovascular indications for the technology, hospital medicine physicians are increasingly using HCU to guide paracentesis and thoracentesis. Given how commonplace the expansion of such practices has become, it is noteworthy that HCU operation by hospitalists has not yet been evaluated and endorsed in larger, controlled trials demonstrating appropriate outcomes.25
Across all fields of medicine, the transition from traditional to newer modalities remains a slippery slope in terms of demonstration of persuasive evidence of patient‐centered benefit.26 Fascination with emerging technologies (so‐called gizmo idolatry) and increased reimbursement potential threaten to distract patients and their providers from legitimate concerns about how medical device manufacturers and for‐profit corporations increasingly influence device acquisition and clinical practice.2731 While we lack strong evidence demonstrating that diagnostic tests such as HCU are beneficial when performed by hospitalists, the expanded use of these handy new devices by hospitalists is simultaneously generating increased incidental and equivocal findings, which in turn render it necessary to go back and perform secondary verification studies by specialists using older, gold‐standard modalities. This vicious cycle, coupled with the current lack of evidence, will continue to degrade confidence in the initiation of either acute or chronic treatment on the basis of HCU results obtained by hospitalist physicians.
Eventually, the increased use of HCU by hospitalists might lead to demonstrations of improved hospital workflow management, but it may just as easily represent another new coupling of technology and practitioner that prematurely becomes the standard of care in the absence of any demonstration of added value. The initially enthusiastic application of pulmonary artery catheters (PACs) serves as a cautionary tale in which the acquisition of additional clinical data did not necessarily lead to improved clinical outcomes: whereas PACs did enhance the clinical understanding of hemodynamics, they were not associated with an overall advantage in terms of mortality, length of hospital stay, or cost.3235 Ultimately, more information is not necessarily better information. Although new medical technologies can produce extremely useful diagnostic results that aid in the management of critically ill patients, poor data interpretation resulting from lack of targeted training and experience can nullify point‐of‐care advantages, and perhaps lead to excess morbidity and mortality.14 In clinical practice, it is generally best to avoid reliance on assumptions of added value in lieu of demonstrations of the same.
Conclusions
Hospital practitioners should not yet put away their stethoscopes. New technologies such as HCU need to be embraced in parallel with accumulating evidence of benefit. In the hands of hospitalists, the smaller HCU devices may very well prove handy, but at present, the literature simply does not support the use of HCU by hospitalist physicians.
- Hand‐Carried Ultrasound—Reshaping the ultrasound marketplace. Available at: http://www.sonoworld.com/NewsStories/NewsStories.aspx?ID= 450. Accessed August2009.
- ,,.A new narrative for hospitalists.J Hosp Med.2009;4(4):207–208.
- .Can heart failure be diagnosed in primary care?BMJ.2000;321(7255):188–189.
- ,,.Evidence of inadequate investigation and treatment of patients with heart failure.Br Heart J.1994;71(6):584–587.
- .Utility of hand‐carried ultrasound for consultative cardiology.Echocardiography.2003;20(5):463–469.
- .Tomorrow's stethoscope: the hand‐held ultrasound device?J S C Med Assoc.2006;102(10):345.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,.Should a hand‐carried ultrasound machine become standard equipment for every internist?Am J Med.2009;122(1):1–3.
- ,,,.How useful is hand‐carried bedside echocardiography in critically ill patients?J Am Coll Cardiol.2001;37(8):2019–2022.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4(2):141–147.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20(5):471–476.
- .Specific skill set and goals of focused echocardiography for critical care clinicians.Crit Care Med.2007;35(5 suppl):S144–S149.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,,,.Diagnostic accuracy of new handheld echocardiography with Doppler and harmonic imaging properties.J Am Soc Echocardiogr.2004;17(3):234–238.
- ,,,,,.Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography.Cardiovasc Ultrasound.2004;2:24.
- ,,Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology.J Am Soc Echocardiogr.2004;17(1):50–55.
- ,,,.Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment.J Gen Intern Med.2008;23(suppl 1):57–63.
- ,,.Newly approved does not always mean new and improved.JAMA.2008;299(13):1598–1600.
- ,.Indication creep: physician beware.CMAJ.2007;177(7):697,699.
- ,,,.Ultrasound‐guided interventional radiology in critical care.Crit Care Med.2007;35(5 suppl):S186–S197.
- ,.Pay now, benefits may follow—the case of cardiac computed tomographic angiography.N Engl J Med.2008;359(22):2309–2311.
- ,.Gizmo idolatry.JAMA.2008;299(15):1830–1832.
- .Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Impugning the integrity of medical science: the adverse effects of industry influence.JAMA.2008;299(15):1833–1835.
- ,,, et al.The 2007 ABJS Marshall Urist Award: the impact of direct‐to‐consumer advertising in orthopaedics.Clin Orthop Relat Res.2007;458:202–219.
- ,.Direct to consumer advertising in healthcare: history, benefits, and concerns.Clin Orthop Relat Res.2007;457:96–104.
- Connors,,, et al.The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators.JAMA.1996;276(11):889–897.
- ,,, et al.Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC‐Man): a randomised controlled trial.Lancet.2005;366(9484):472–477.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial.JAMA.2003;290(20):2713–2720.
- Hand‐Carried Ultrasound—Reshaping the ultrasound marketplace. Available at: http://www.sonoworld.com/NewsStories/NewsStories.aspx?ID= 450. Accessed August2009.
- ,,.A new narrative for hospitalists.J Hosp Med.2009;4(4):207–208.
- .Can heart failure be diagnosed in primary care?BMJ.2000;321(7255):188–189.
- ,,.Evidence of inadequate investigation and treatment of patients with heart failure.Br Heart J.1994;71(6):584–587.
- .Utility of hand‐carried ultrasound for consultative cardiology.Echocardiography.2003;20(5):463–469.
- .Tomorrow's stethoscope: the hand‐held ultrasound device?J S C Med Assoc.2006;102(10):345.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,.Should a hand‐carried ultrasound machine become standard equipment for every internist?Am J Med.2009;122(1):1–3.
- ,,,.How useful is hand‐carried bedside echocardiography in critically ill patients?J Am Coll Cardiol.2001;37(8):2019–2022.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4(2):141–147.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20(5):471–476.
- .Specific skill set and goals of focused echocardiography for critical care clinicians.Crit Care Med.2007;35(5 suppl):S144–S149.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,,,.Diagnostic accuracy of new handheld echocardiography with Doppler and harmonic imaging properties.J Am Soc Echocardiogr.2004;17(3):234–238.
- ,,,,,.Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography.Cardiovasc Ultrasound.2004;2:24.
- ,,Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology.J Am Soc Echocardiogr.2004;17(1):50–55.
- ,,,.Who is responsible for evaluating the safety and effectiveness of medical devices? The role of independent technology assessment.J Gen Intern Med.2008;23(suppl 1):57–63.
- ,,.Newly approved does not always mean new and improved.JAMA.2008;299(13):1598–1600.
- ,.Indication creep: physician beware.CMAJ.2007;177(7):697,699.
- ,,,.Ultrasound‐guided interventional radiology in critical care.Crit Care Med.2007;35(5 suppl):S186–S197.
- ,.Pay now, benefits may follow—the case of cardiac computed tomographic angiography.N Engl J Med.2008;359(22):2309–2311.
- ,.Gizmo idolatry.JAMA.2008;299(15):1830–1832.
- .Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement.J Hosp Med.2008;3(5):398–402.
- ,.Impugning the integrity of medical science: the adverse effects of industry influence.JAMA.2008;299(15):1833–1835.
- ,,, et al.The 2007 ABJS Marshall Urist Award: the impact of direct‐to‐consumer advertising in orthopaedics.Clin Orthop Relat Res.2007;458:202–219.
- ,.Direct to consumer advertising in healthcare: history, benefits, and concerns.Clin Orthop Relat Res.2007;457:96–104.
- Connors,,, et al.The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators.JAMA.1996;276(11):889–897.
- ,,, et al.Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC‐Man): a randomised controlled trial.Lancet.2005;366(9484):472–477.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial.JAMA.2003;290(20):2713–2720.
Hospitalist Use of HCU
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.
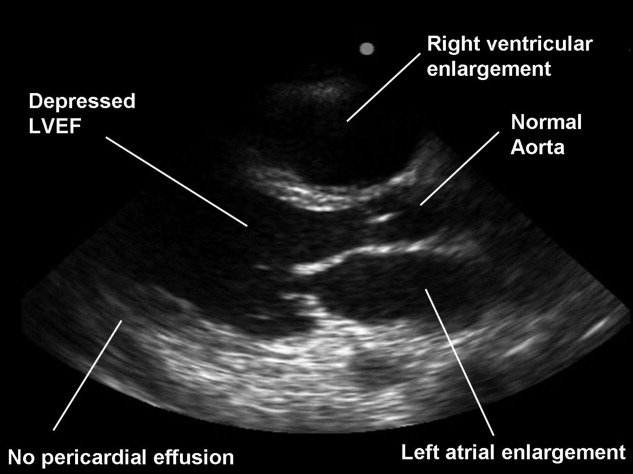
Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.

Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.

Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
Short Title
The United States spends more on healthcare than any country in the world, and it is widely believed that the Nation could spend less while achieving comparable or better outcomes. The recent debate over healthcare reform in the United States, the large Federal budget deficit in the context of the current economic recession, and the prospect of widening gaps in Medicare funding with the increasing entry of baby boomers into old age suggest that the issue of healthcare cost will remain intense for many years to come. What roles hospitalists will play in the nation's struggle to control health care costs remain to be seen. Six papers in this issue of the Journal of Hospital Medicine discuss issues related to costs, and reflect several of the ways in which hospital medicine can contribute to understanding, and ultimately, controlling healthcare costs.
Two papers, one by Whelan et al.1 examining the costs associated with upper vs. lower GI bleeding and one by Lorch et al.2 examining the costs associated with herpex simplex virus (HSV) infections among neonates with and without congenital abnormalities, focus on epidemiologic determinants of healthcare costs. Such studies can identify subgroups of patients with high costs who may be logical targets for efforts to control costs. One tension in the use of such analyses to control cost is that total cost for any patient group is the product of both the cost per patient and the number of patients falling into each group. In the case of gastrointestinal (GI) bleeding, the surprise compared to past reports is that lower GI bleeding is about as common among hospitalized patients as upper GI bleeding. This may be because pharmacotherapy for conditions that cause upper GI bleeding is reducing the rate at which disease progresses to the point where hospitalization is required. The importance of prevalence is reinforced even in the findings about HSV infection, where despite 2‐ to 3‐fold higher average costs among babies with HSV who have congenital abnormalities, the fact that 90% of babies hospitalized with HSV lack congenital abnormalities implies that the clear majority of costs are due to babies without congenital abnormalities. In seeking strategies to control costs, it is important to pay attention to both the prevalence and cost per case of specific conditions. Because hospitalists are generalist physicians who typically care for few patients with any given diagnosis, the importance of prevalence implies that disease‐specific efforts to control costs may produce smaller total gains than those that cross diseases, such as efforts to improve communication between inpatient and outpatient physicians.
Moreover, the presence of high costs for some condition does not, of course, imply that effective interventions exist to reduce those costs. Two other papers, one by Mudge et al.3 examining a disease management program for heart failure, and one by Go et al.4 examining the effects of hospitalists on the costs of hospitalization for GI bleeding, reinforce the idea that interventions to reduce hospital costs are not always as effective as hoped. Even worse, efforts to control costs can have unintended effects, such as the delays in antimicrobial administration with antimicrobial approval policies that are reported by Winters et al.5 These studies also illustrate that analyses of the effectiveness of interventions can be performed using a variety of experimental designs (eg, the before/after comparison used by Mudge et al,3 and the natural experiments based on assignment of patients to physicians based on day of admission used by Go et al.4 or based on time of day used by Winters et al.)5 The role of hospitalists as clinical leaders in hospitals often places them in positions to design and execute experiments, but the role of hospitalists as astute clinicians who can recognize the presence of natural experiments in their clinical environment can be every bit as powerful in producing valid research designs.
As society seeks strategies to control healthcare costs in the years ahead, it will almost certainly turn to the same general strategies that have been used in the past: bundling services into fixed payments for a prospectively defined episode of care, asking patients to pay more of the costs of care, and simply not paying for, or paying less for, any given type of care. Hospitalists already have dealt with many of these approaches in one form or another. Medicare's prospective payment system and the payment of fixed annual fees for the care of patients in health maintenance organizations have given all hospitalists some exposure to the pressure for lower hospital resources use under prospective payment systems. Proposals for demonstration projects within healthcare reform to study the effects of bundling inpatient and outpatient care or even hospital and professional fees suggest that hospitalists may need to be open to new incentive structures in the years to come. For example, reduced incentives for rapid discharge if costs pushed into the outpatient setting are borne by the hospital, there may be co‐management models if professional and hospital fees are bundled. Increases in patient copayments may also play some role in healthcare reform, and the paper by Ross et al.6 should be a reminder to hospitalists that we may do our patients a great disservice if we fail to recognize the effects of our decisions on their out‐of‐pocket costs. Indeed, while doctors and patient both recognize the importance of discussing out‐of‐pocket costs, they both agree that these discussions rarely occur.7 That such discussions are not reimbursed explicitly suggests one of the many challenges of controlling healthcare costs; if physician payments are decreased to control costs and physicians respond by attempting to see even more patients in any given time period, discussions of important but less urgent issues such as out‐of‐pocket costs seem likely to be reduced. Such dilemmas arise frequently as the healthcare system devises increasingly complex approaches to the control of costs and suggest to many that fundamental reform of the payment and delivery system with greater reliance on integrated health systems paid through full capitation will eventually need to become the nation's approach to healthcare cost containment.8
- ,., et al.Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization.J Hosp Med.2010;5(3):140–146.
- ,,.Impact of congenital anomalies and treatment location on clinical outcomes and health resource use in infants hospitalized with herpes simplex virus.J Hosp Med.2010;5(3):154–158.
- ,,, et al.The paradox of readmission: effect of a quality improvement programme in hospitalised patients with heart failure.J Hosp Med.2010;5:147–152.
- ,,, et al.Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)?J Hosp Med.2010;5(3):138–138.
- ,,.Impact of a restrictive antimicrobial policy on the process and timing of antimicrobial administration.J Hosp Med.2010;5(2):E41–E45.
- ,.Reducing patient financial liability for hospitalizations: the physician role.J Hosp Med.2010;5(3):159–161.
- ,,.Patient‐physician communication about out‐of‐pocket costs.JAMA.2003;290(7):953–958.
- ,,, et al.Toward a 21st‐century health care system: recommendations for health care reform.Ann Intern Med.2009;150:493–495.
The United States spends more on healthcare than any country in the world, and it is widely believed that the Nation could spend less while achieving comparable or better outcomes. The recent debate over healthcare reform in the United States, the large Federal budget deficit in the context of the current economic recession, and the prospect of widening gaps in Medicare funding with the increasing entry of baby boomers into old age suggest that the issue of healthcare cost will remain intense for many years to come. What roles hospitalists will play in the nation's struggle to control health care costs remain to be seen. Six papers in this issue of the Journal of Hospital Medicine discuss issues related to costs, and reflect several of the ways in which hospital medicine can contribute to understanding, and ultimately, controlling healthcare costs.
Two papers, one by Whelan et al.1 examining the costs associated with upper vs. lower GI bleeding and one by Lorch et al.2 examining the costs associated with herpex simplex virus (HSV) infections among neonates with and without congenital abnormalities, focus on epidemiologic determinants of healthcare costs. Such studies can identify subgroups of patients with high costs who may be logical targets for efforts to control costs. One tension in the use of such analyses to control cost is that total cost for any patient group is the product of both the cost per patient and the number of patients falling into each group. In the case of gastrointestinal (GI) bleeding, the surprise compared to past reports is that lower GI bleeding is about as common among hospitalized patients as upper GI bleeding. This may be because pharmacotherapy for conditions that cause upper GI bleeding is reducing the rate at which disease progresses to the point where hospitalization is required. The importance of prevalence is reinforced even in the findings about HSV infection, where despite 2‐ to 3‐fold higher average costs among babies with HSV who have congenital abnormalities, the fact that 90% of babies hospitalized with HSV lack congenital abnormalities implies that the clear majority of costs are due to babies without congenital abnormalities. In seeking strategies to control costs, it is important to pay attention to both the prevalence and cost per case of specific conditions. Because hospitalists are generalist physicians who typically care for few patients with any given diagnosis, the importance of prevalence implies that disease‐specific efforts to control costs may produce smaller total gains than those that cross diseases, such as efforts to improve communication between inpatient and outpatient physicians.
Moreover, the presence of high costs for some condition does not, of course, imply that effective interventions exist to reduce those costs. Two other papers, one by Mudge et al.3 examining a disease management program for heart failure, and one by Go et al.4 examining the effects of hospitalists on the costs of hospitalization for GI bleeding, reinforce the idea that interventions to reduce hospital costs are not always as effective as hoped. Even worse, efforts to control costs can have unintended effects, such as the delays in antimicrobial administration with antimicrobial approval policies that are reported by Winters et al.5 These studies also illustrate that analyses of the effectiveness of interventions can be performed using a variety of experimental designs (eg, the before/after comparison used by Mudge et al,3 and the natural experiments based on assignment of patients to physicians based on day of admission used by Go et al.4 or based on time of day used by Winters et al.)5 The role of hospitalists as clinical leaders in hospitals often places them in positions to design and execute experiments, but the role of hospitalists as astute clinicians who can recognize the presence of natural experiments in their clinical environment can be every bit as powerful in producing valid research designs.
As society seeks strategies to control healthcare costs in the years ahead, it will almost certainly turn to the same general strategies that have been used in the past: bundling services into fixed payments for a prospectively defined episode of care, asking patients to pay more of the costs of care, and simply not paying for, or paying less for, any given type of care. Hospitalists already have dealt with many of these approaches in one form or another. Medicare's prospective payment system and the payment of fixed annual fees for the care of patients in health maintenance organizations have given all hospitalists some exposure to the pressure for lower hospital resources use under prospective payment systems. Proposals for demonstration projects within healthcare reform to study the effects of bundling inpatient and outpatient care or even hospital and professional fees suggest that hospitalists may need to be open to new incentive structures in the years to come. For example, reduced incentives for rapid discharge if costs pushed into the outpatient setting are borne by the hospital, there may be co‐management models if professional and hospital fees are bundled. Increases in patient copayments may also play some role in healthcare reform, and the paper by Ross et al.6 should be a reminder to hospitalists that we may do our patients a great disservice if we fail to recognize the effects of our decisions on their out‐of‐pocket costs. Indeed, while doctors and patient both recognize the importance of discussing out‐of‐pocket costs, they both agree that these discussions rarely occur.7 That such discussions are not reimbursed explicitly suggests one of the many challenges of controlling healthcare costs; if physician payments are decreased to control costs and physicians respond by attempting to see even more patients in any given time period, discussions of important but less urgent issues such as out‐of‐pocket costs seem likely to be reduced. Such dilemmas arise frequently as the healthcare system devises increasingly complex approaches to the control of costs and suggest to many that fundamental reform of the payment and delivery system with greater reliance on integrated health systems paid through full capitation will eventually need to become the nation's approach to healthcare cost containment.8
The United States spends more on healthcare than any country in the world, and it is widely believed that the Nation could spend less while achieving comparable or better outcomes. The recent debate over healthcare reform in the United States, the large Federal budget deficit in the context of the current economic recession, and the prospect of widening gaps in Medicare funding with the increasing entry of baby boomers into old age suggest that the issue of healthcare cost will remain intense for many years to come. What roles hospitalists will play in the nation's struggle to control health care costs remain to be seen. Six papers in this issue of the Journal of Hospital Medicine discuss issues related to costs, and reflect several of the ways in which hospital medicine can contribute to understanding, and ultimately, controlling healthcare costs.
Two papers, one by Whelan et al.1 examining the costs associated with upper vs. lower GI bleeding and one by Lorch et al.2 examining the costs associated with herpex simplex virus (HSV) infections among neonates with and without congenital abnormalities, focus on epidemiologic determinants of healthcare costs. Such studies can identify subgroups of patients with high costs who may be logical targets for efforts to control costs. One tension in the use of such analyses to control cost is that total cost for any patient group is the product of both the cost per patient and the number of patients falling into each group. In the case of gastrointestinal (GI) bleeding, the surprise compared to past reports is that lower GI bleeding is about as common among hospitalized patients as upper GI bleeding. This may be because pharmacotherapy for conditions that cause upper GI bleeding is reducing the rate at which disease progresses to the point where hospitalization is required. The importance of prevalence is reinforced even in the findings about HSV infection, where despite 2‐ to 3‐fold higher average costs among babies with HSV who have congenital abnormalities, the fact that 90% of babies hospitalized with HSV lack congenital abnormalities implies that the clear majority of costs are due to babies without congenital abnormalities. In seeking strategies to control costs, it is important to pay attention to both the prevalence and cost per case of specific conditions. Because hospitalists are generalist physicians who typically care for few patients with any given diagnosis, the importance of prevalence implies that disease‐specific efforts to control costs may produce smaller total gains than those that cross diseases, such as efforts to improve communication between inpatient and outpatient physicians.
Moreover, the presence of high costs for some condition does not, of course, imply that effective interventions exist to reduce those costs. Two other papers, one by Mudge et al.3 examining a disease management program for heart failure, and one by Go et al.4 examining the effects of hospitalists on the costs of hospitalization for GI bleeding, reinforce the idea that interventions to reduce hospital costs are not always as effective as hoped. Even worse, efforts to control costs can have unintended effects, such as the delays in antimicrobial administration with antimicrobial approval policies that are reported by Winters et al.5 These studies also illustrate that analyses of the effectiveness of interventions can be performed using a variety of experimental designs (eg, the before/after comparison used by Mudge et al,3 and the natural experiments based on assignment of patients to physicians based on day of admission used by Go et al.4 or based on time of day used by Winters et al.)5 The role of hospitalists as clinical leaders in hospitals often places them in positions to design and execute experiments, but the role of hospitalists as astute clinicians who can recognize the presence of natural experiments in their clinical environment can be every bit as powerful in producing valid research designs.
As society seeks strategies to control healthcare costs in the years ahead, it will almost certainly turn to the same general strategies that have been used in the past: bundling services into fixed payments for a prospectively defined episode of care, asking patients to pay more of the costs of care, and simply not paying for, or paying less for, any given type of care. Hospitalists already have dealt with many of these approaches in one form or another. Medicare's prospective payment system and the payment of fixed annual fees for the care of patients in health maintenance organizations have given all hospitalists some exposure to the pressure for lower hospital resources use under prospective payment systems. Proposals for demonstration projects within healthcare reform to study the effects of bundling inpatient and outpatient care or even hospital and professional fees suggest that hospitalists may need to be open to new incentive structures in the years to come. For example, reduced incentives for rapid discharge if costs pushed into the outpatient setting are borne by the hospital, there may be co‐management models if professional and hospital fees are bundled. Increases in patient copayments may also play some role in healthcare reform, and the paper by Ross et al.6 should be a reminder to hospitalists that we may do our patients a great disservice if we fail to recognize the effects of our decisions on their out‐of‐pocket costs. Indeed, while doctors and patient both recognize the importance of discussing out‐of‐pocket costs, they both agree that these discussions rarely occur.7 That such discussions are not reimbursed explicitly suggests one of the many challenges of controlling healthcare costs; if physician payments are decreased to control costs and physicians respond by attempting to see even more patients in any given time period, discussions of important but less urgent issues such as out‐of‐pocket costs seem likely to be reduced. Such dilemmas arise frequently as the healthcare system devises increasingly complex approaches to the control of costs and suggest to many that fundamental reform of the payment and delivery system with greater reliance on integrated health systems paid through full capitation will eventually need to become the nation's approach to healthcare cost containment.8
- ,., et al.Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization.J Hosp Med.2010;5(3):140–146.
- ,,.Impact of congenital anomalies and treatment location on clinical outcomes and health resource use in infants hospitalized with herpes simplex virus.J Hosp Med.2010;5(3):154–158.
- ,,, et al.The paradox of readmission: effect of a quality improvement programme in hospitalised patients with heart failure.J Hosp Med.2010;5:147–152.
- ,,, et al.Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)?J Hosp Med.2010;5(3):138–138.
- ,,.Impact of a restrictive antimicrobial policy on the process and timing of antimicrobial administration.J Hosp Med.2010;5(2):E41–E45.
- ,.Reducing patient financial liability for hospitalizations: the physician role.J Hosp Med.2010;5(3):159–161.
- ,,.Patient‐physician communication about out‐of‐pocket costs.JAMA.2003;290(7):953–958.
- ,,, et al.Toward a 21st‐century health care system: recommendations for health care reform.Ann Intern Med.2009;150:493–495.
- ,., et al.Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization.J Hosp Med.2010;5(3):140–146.
- ,,.Impact of congenital anomalies and treatment location on clinical outcomes and health resource use in infants hospitalized with herpes simplex virus.J Hosp Med.2010;5(3):154–158.
- ,,, et al.The paradox of readmission: effect of a quality improvement programme in hospitalised patients with heart failure.J Hosp Med.2010;5:147–152.
- ,,, et al.Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)?J Hosp Med.2010;5(3):138–138.
- ,,.Impact of a restrictive antimicrobial policy on the process and timing of antimicrobial administration.J Hosp Med.2010;5(2):E41–E45.
- ,.Reducing patient financial liability for hospitalizations: the physician role.J Hosp Med.2010;5(3):159–161.
- ,,.Patient‐physician communication about out‐of‐pocket costs.JAMA.2003;290(7):953–958.
- ,,, et al.Toward a 21st‐century health care system: recommendations for health care reform.Ann Intern Med.2009;150:493–495.
Stenting for atherosclerotic renal artery stenosis: One poorly designed trial after another
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
Wearing White—Right or Wrong
Physicians wear white coats. In many medical centers, the length of one's coat grows with seniority; medical students, interns, and residents wear short white coats, while attending staff graduate to long white coats with their names embroidered on the front. This traditional uniform serves a similar role to the stripes on a military sleeve. That is, by examining the length of a person's coat, a nurse or other hospital employee can rapidly determine the seniority, and theoretically the increased medical knowledge, of the person inside.
Of course, it isn't quite this simple. In many places, all residents wear long coats (with embroidered names). In other hospitals, attending staff wear short coats. The problem of distinguishing between a medical student, resident, and attending physician can be quite vexing, particularly after the summer months when the anxiety visible on the faces of new house staff and third‐year medical students fades away into the more seasoned look of fatigue and malaise. As hospital‐based practitioners, what are we to do?
To be certain there are clues one can use to identify one's rank in the medical profession. Often medical students wear a medical school pin bestowed upon them at a robing ceremony or other coming‐of‐age festivity to mark the transition to the clinical training years.1 (Little do they know that the primary utility of such adornments is to mark them, as though with a scarlet letter, for easy identification when one wishes to pimp the medical team.)2 Similarly, one can look for an arm patch. Just as a mother hen has the need to identify her own chicks, so too is it helpful for the residency director to have each of his or her own residents easily identifiable by the residency patch emblazoned upon the arm of their (long) white coat. The patch test can fail, though, for too often the residency emblem is merely a simple modification of the hospital or medical center logo, and thus not easily distinguishable.
When all else fails, of course, you can look to the pockets. As one's medical knowledge and training rank increases, the number of papers, pens, and instruments in the pockets of the white coat decreases. This inverse relationship provides some increased ability to identify medical students and junior house staff. For example, a short white coat, busting with oversized index cards held together by a large metal ring, several tattered and folded journal articles, and a worn spiral‐bound text suggests a medical student or intern. If they have an electrocardiogram (ECG), calipers, and tuning fork visible, safe bet is you've found a medical student. Conversely, if the white coat sports several free pens (with medication logos embossed on their stems) and has more than a few stains, chances are you've spotted an intern.
Unfortunately, none of this is of much utility in identifying a physician, as the initial premise that physicians wear white coats may be false. The problem is 2‐fold. First of all, not all physicians wear white coats (or any coat for that matter), and, many nonphysicians wear white coats. Commonly, phlebotomists, pharmacists, and respiratory technicians wear white coats; long white coats, in fact. So do nutritionists, speech pathologists, the clerk at the radiology file room, and even the cashiers in the cafeteria. And why not? Each of these persons has an important role to play in the operation of a modern medical center. The long white coat serves as professional uniform to identify the wearer as contributing to the mission of the medical center. It engenders confidence and suggests cleanliness and purity.
The problem remains, however, what should I, as an attending physician wear? You see, patients encounter so many persons in their visits to the hospital that it is not clear who their doctor is. (It's not statistically likely to be the man in a long white coat.) Being able to identify the attending physician is important. The attending physician is ultimately responsible for the patient's care.
There are many guidelines for how to dress. For example, don't wear white after Labor Day. The pleats on a cummerbund point so that the folds open pointing up (apparently it was originally meant to serve as a ticket holder, perhaps in the days before pockets?). Match your belt to your shoes. The list goes on. However, physicians are not commonly associated with natty attire, so fashion help for the MD is hard to find, though nonetheless necessary.
Even wearing white is questionable. White robes have been associated with purity and sanctity for centuries. Religious leaders have donned white for spiritual cleansing of their communities on the most holy of days. I like the idea of appearing pure and holy. Of course, I don't want to mislead anybody, either.
Brides have traditionally worn white dresses. But, while many believe this is to convey a sense of premarital purity, more likely the tradition stems from an ancient Roman practice of wearing white as a symbol of joyous celebration. Interestingly, in some parts of Asia, people at a funeral wear white while the deceased is dressed in red.
In some environments, the culture of the medical center prescribes appropriate apparel. The Mayo Clinic in Minnesota, for example, has had a tradition of having their physician staff dress in business attire. By this, they mean conservative sport coats or suits with ties for men, and similar appropriate women's business clothing. As noted by Leonard L. Berry and Neeli Bendapudi in their article Why Docs Don't Wear White Coats or Polo Shirts at the Mayo Clinic3 in the Harvard Business School publication Working Knowledge for Business Leaders, while some may consider this semiformal business dress pretentious, it should be considered no more pretentious than, say, the dress code for airline pilots. Airline passengers don't want to see their pilot in a polo shirt, and patients feel the same way about their doctors. The business attire is a uniform; it's a visible clue that communicates respect to patients and their families. But, isn't a white coat a uniform that conveys respect? Perhaps a white coat isn't safe if the physician wants to stand out to oncoming traffic in his snowy Minnesota environs.
Besides, is it true that patients don't want their physician to wear a polo shirt? Perhaps casual dress will break down 1 of the patient‐doctor barriers to communication and allow for improved comfort with greater honesty in patientphysician interactions? Prior to designing a prospective controlled randomized trial to answer this question myself, I reviewed the available literature.
It turns out, that an evaluation of polo‐shirt‐wearing physicians has been carried out. Drs. Barrett and Booth4 from the Birmingham Maternity Hospital in England questioned 203 groups of parents and children (406 individuals) about various levels of physician dress. Seventy percent of participants thought that how the doctor dressed was important. Among children, a male physician in a polo shirt was considered more friendly and gentle than the male physician in a white coat (who did get points for being more competent and more concerned). Women physicians in T‐shirts were also though to be friendlier than if in a white coat, but similarly less competent. Parents were more likely to prefer casually‐dressed physicians and were poor at predicting what their children would want.
So, a polo shirt makes me look friendly, gentle, and less competent? What about a polo shirt under a white coatis that the whole package? What about business attire, as per the Mayo Clinic requirements? These questions remain unanswered. So, back to the literature.
In the Journal of the American Medical Association, in 1987, Dunn et al.5 evaluated 200 medical patients in Boston and San Francisco regarding their preference for physician dress. Sixty‐five percent believed physicians should wear a white coat, 27% said no tennis shoes, one‐half said no blue jeans, and about one‐third thought male physicians should wear ties and female physicians should be in a skirt or dress. I suppose the conclusion here is to wear a white coat with or without jeans and most of the time without a tie, though almost always with proper shoes, or at least without tennis shoes. (I practice in Boston and trained partly in San Francisco so this advice seems particularly valid for me.)
It may be more obvious what to do in Japan. Ikusaka et al.6 evaluated the experience of patients at a university clinic seen by a consulting physician in either a white coat, or private clothes. To me, private clothes imply pajamas and a bathrobe, but we must assume this means some form of professional dress lacking a white coat. It turns out that 71% of the Japanese patients seeing a doctor in a white coat preferred a white coat, though more patients seeing a physician in a white coat (vs. private clothes) felt tense during their consultation. The researchers stress that the presence of a white coat did not increase satisfaction with the consultation. They conclude, that while patients may say they prefer a white coat, maybe it would be better not to wear one since it makes patients feel tense.
In addition to feeling tense, white coats may cause hypertension. The phenomenon of elevated blood pressure when in the presence of the physician (or other hospital staff in a white coat) has been long documented. This white coat hypertension can be found in more than 15% of the population who have a measured blood pressure in the office of over 140/90 mmHg with normal daytime mean ambulatory blood pressure readings (when not around a white‐coat‐wearing stress‐inducing medical worker).7 Older adults, females, and nonsmokers were more likely to have white coat hypertension than other persons.
And yet, older adults prefer white coats. The Japanese study (Ikusaka et al.6) concluded that elderly patients prefer a white coat to other attire. Similarly, a study from the Royal Free Hospital, London, showed that white coats were twice as popular with patients as they were with physicians.8 Specifically, patients found the white coats made doctors easier to identify. In an article by the British Broadcasting Corporation (BBC) on the subject, it was noted that the elderly largely preferred physicians in white coats, while children preferred physicians without white coats. British children must prefer a friendly doctor to a competent one.
The article further suggests that only 1 in 8 physicians wears a white coat, complaining that they are too hot and uncomfortable, and may carry the risk of transmitting infections. The white coat, the symbol of cleanliness and purity, a source of infection? To add hypocrisy to the equation, one‐half of physicians who thought white coats should be worn admitted to never actually wearing a white coat. In fact, only 7 of 86 physicians surveyed wore their white coat on a daily basis. The BBC goes on to note that in Australia, the white coat is gaining momentum as there seems to be a movement towards rediscovering the white coat as a symbol of purpose and pride as a profession.8
Really? Let's consult the literature! According to Dr. D.A. Watson,9 White coats have largely disappeared from Australian teaching hospitals and the majority of junior doctors in Australia oppose the wearing of white coats. In a survey of 337 junior medical officers, only 16% preferred to wear a white coat. Peer pressure seems to have something to do with this, as 70% say they don't wear a white coat because nobody else wears a white coat. This is indeed a compelling argument.
Of course, a better argument against wearing a white coat may be that it causes tension (at least in Japanese patients) and may cause white‐coat‐hypertension, resulting in the inappropriate diagnosis and treatment of elevated blood pressure. In Australia, however, it seems that wearing a white coat may make patients too relaxed. Wing et al.10 noted that in 21% to 45% of elderly patients, blood pressure was atypically low when checked in the physician's office as compared to mean ambulatory blood pressure. This reverse‐white‐coat‐hypertension could result in the omission of necessary blood pressure treatment.
In the United States, residentsour own version of the Australian junior medical officercommonly wear scrubs covered by a white coat. Scrub clothes are typically available without charge from the hospital, limit the amount of laundry a busy resident needs to do, and can be put on with little concern as to pressed pleats or matching colors. The overlying white coat adds a moderate degree of formality to what could otherwise be mistaken as pajamas, while providing convenient pockets for the aforementioned papers and miscellaneous equipment and souvenirs. This outfit is likely one of practicality rather than a desire to be most appealing to patients. But, what do you know? It seems that patients prefer their resident physicians to dress this way. Dr. A. Cha et al.11 at the Northeastern Ohio College of Medicine found that patients in an obstetrics and gynecology clinic overall felt that resident physicians dressed in surgical scrubs with a white coat made them feel more comfortable and confident than if dressed otherwise. On the question of a white coat specifically, the majority had no preference that their physician wear one.
So, it's very much unclear whether a white coat is a tension‐causing, blood‐pressure‐elevating, infection risk or a competence‐implying blood‐pressure‐lowering way to identify a physician. As a result, the jury is still out on whether physicians should wear white.
One thing I do know, however, is that patients shouldn't wear white. But they do. As an OtolaryngologistHead and Neck Surgeon, my clinical practice is split between surgical procedures and office visits. Commonly, patients with sinus or nasal surgery will require some form of cotton gauze or foam material within the nose in order to tamponade bleeding. Similarly, patients presenting to the emergency department or urgent care center with epistaxis may have their noses packed and then be told to see an otolaryngologist (eg, me) in 3 or 4 days to have the packing removed. I also commonly remove facial skin lesions, biopsy tongue masses, reduce nasal fractures, and otherwise engage in activities with an above average propensity to result in a mess. More often than happenstance will allow, patients come to see me for such visits in their white Sunday best. I truly care for my patients. I respect them as individuals and desire to do no harm. This includes not staining their shirts, ties, or pants. However, there is no amount of blue towels or gauze pads than can keep a white shirt clean when you have that first sneeze after removal of your nasal packing.12
So what is it that makes so many patients come to the office in a white shirt? Perhaps patients subconsciously associate healthcare with the color white since their doctors wear white coats and the nurses wear starched white dresses with tight white folded caps on top of their head. I've never seen a nurse in a white dress and hat, but believe television programs have shown this in the past.
Maybe the answer lies in an adaptation of data from another Australian study. In a survey of 180 oncology patients about white coats on physicians, the most common argument against wearing the white coat was that it represented a barrier between the physician and the patient.13 However, it is indeed the sane patient who desires to have a barrier between their physician and the removal of nasal packing, a skin lesion, or a tongue mass. Another possibility is that as fewer and fewer physicians wear white, patients are gravitating toward this color as a way of distinguishing themselves from the medical staff. Perhaps a person dressed in white is less likely to be grabbed in the hallway with a Doctor come quick! and more likely to be allowed to just sit peacefully in the waiting room with a 1997 issue of Ladies Home Journal or Senior Fisherman magazine.
Of course, perhaps patients believe the fashion experts who expound that white goes with everything. If so, they soon learn that white doesn't go so well with blotchy splattered red.
The truth is, I don't want my patients to wear white. Between making certain I project myself as approachable and easy to speak with, remembering to cover all the appropriate irrelevant parts of the history and physical to comply with billing requirements, entering data in our easy‐to‐navigate electronic medical record, and attempting to both diagnose the problem and discern an acceptable and effective treatment, the last thing I need is to worry about staining patient shirts! I believe that this phenomenon is widespread among medical practitioners and should be called white‐shirt‐hypertension.
Conclusion
After reviewing the available literature, I've determined that patients should not wear white. However, I'm still not certain how I should dress. For now, I'll stick with whatever is clean and professional and make sure my belt and shoes match. I may or may not wear a tie, since another study showed that 30% of patients believed their physician wore a tie even if they didn't.14 Of course, I'll put on the white coat for the semiannual meeting with the chairman, or if I'm having something messy for lunch and wore a tie that day.
I'll also wear my name badge. It says I'm an attending physician and not a resident. It opens doors around the hospital. Literally. It's got a magnetic stripe.
- .Deconstructing the white coat.Ann Intern Med.1998;129:740–742.
- .The art of pimping.JAMA.1989;262(1):89–90.
- ,. “Why Docs don't wear white coats or Polo Shirts at the Mayo Clinic” working knowledge for business leaders. Available at:http://hbswk.hbs.edu/pubitem.jhtml?id=3380309(6970):1710–1712.
- ,,,,.Patient and house officer attitudes on physician attire and etiquette.JAMA.1987;257(1):65–68.
- ,,, et al.Patients' attitude toward consultations by a physician without a white coat in Japan.Intern Med.1999;38(7):533–536.
- ,,, et al.Determinants of white‐coat hypertension.Blood Press Monit.2004;9(6):307–309.
- Doctors ‘should wear white coats.’ BBC news, Thursday, 13 May, 2004. Available at:http://news.bbc.co.uk/2/hi/health/3706783.stm. Accessed May2009.
- .What do Australian junior doctors think of white coats?Med Educ.2002;36(12):1209–1213.
- ,,, ,;ANBP2 Management Committee and Investigators.Second Australian National Blood Pressure Study. ‘Reverse white‐coat hypertension’ in older hypertensives.J Hypertens.2002;20(4):639–644.
- ,,,.Resident physician attire: does it make a difference to our patients?Am J Obstet Gynecol.2004;190(5):1484–1488.
- . Personal communication.1994–2008.
- .Should doctors wear white coats?Med J Aust.2001;174:343–344.
- ,,,,.Does wearing a necktie influence patient perceptions of emergency department care?J Emerg Med.1998;16(4):541–543.
Physicians wear white coats. In many medical centers, the length of one's coat grows with seniority; medical students, interns, and residents wear short white coats, while attending staff graduate to long white coats with their names embroidered on the front. This traditional uniform serves a similar role to the stripes on a military sleeve. That is, by examining the length of a person's coat, a nurse or other hospital employee can rapidly determine the seniority, and theoretically the increased medical knowledge, of the person inside.
Of course, it isn't quite this simple. In many places, all residents wear long coats (with embroidered names). In other hospitals, attending staff wear short coats. The problem of distinguishing between a medical student, resident, and attending physician can be quite vexing, particularly after the summer months when the anxiety visible on the faces of new house staff and third‐year medical students fades away into the more seasoned look of fatigue and malaise. As hospital‐based practitioners, what are we to do?
To be certain there are clues one can use to identify one's rank in the medical profession. Often medical students wear a medical school pin bestowed upon them at a robing ceremony or other coming‐of‐age festivity to mark the transition to the clinical training years.1 (Little do they know that the primary utility of such adornments is to mark them, as though with a scarlet letter, for easy identification when one wishes to pimp the medical team.)2 Similarly, one can look for an arm patch. Just as a mother hen has the need to identify her own chicks, so too is it helpful for the residency director to have each of his or her own residents easily identifiable by the residency patch emblazoned upon the arm of their (long) white coat. The patch test can fail, though, for too often the residency emblem is merely a simple modification of the hospital or medical center logo, and thus not easily distinguishable.
When all else fails, of course, you can look to the pockets. As one's medical knowledge and training rank increases, the number of papers, pens, and instruments in the pockets of the white coat decreases. This inverse relationship provides some increased ability to identify medical students and junior house staff. For example, a short white coat, busting with oversized index cards held together by a large metal ring, several tattered and folded journal articles, and a worn spiral‐bound text suggests a medical student or intern. If they have an electrocardiogram (ECG), calipers, and tuning fork visible, safe bet is you've found a medical student. Conversely, if the white coat sports several free pens (with medication logos embossed on their stems) and has more than a few stains, chances are you've spotted an intern.
Unfortunately, none of this is of much utility in identifying a physician, as the initial premise that physicians wear white coats may be false. The problem is 2‐fold. First of all, not all physicians wear white coats (or any coat for that matter), and, many nonphysicians wear white coats. Commonly, phlebotomists, pharmacists, and respiratory technicians wear white coats; long white coats, in fact. So do nutritionists, speech pathologists, the clerk at the radiology file room, and even the cashiers in the cafeteria. And why not? Each of these persons has an important role to play in the operation of a modern medical center. The long white coat serves as professional uniform to identify the wearer as contributing to the mission of the medical center. It engenders confidence and suggests cleanliness and purity.
The problem remains, however, what should I, as an attending physician wear? You see, patients encounter so many persons in their visits to the hospital that it is not clear who their doctor is. (It's not statistically likely to be the man in a long white coat.) Being able to identify the attending physician is important. The attending physician is ultimately responsible for the patient's care.
There are many guidelines for how to dress. For example, don't wear white after Labor Day. The pleats on a cummerbund point so that the folds open pointing up (apparently it was originally meant to serve as a ticket holder, perhaps in the days before pockets?). Match your belt to your shoes. The list goes on. However, physicians are not commonly associated with natty attire, so fashion help for the MD is hard to find, though nonetheless necessary.
Even wearing white is questionable. White robes have been associated with purity and sanctity for centuries. Religious leaders have donned white for spiritual cleansing of their communities on the most holy of days. I like the idea of appearing pure and holy. Of course, I don't want to mislead anybody, either.
Brides have traditionally worn white dresses. But, while many believe this is to convey a sense of premarital purity, more likely the tradition stems from an ancient Roman practice of wearing white as a symbol of joyous celebration. Interestingly, in some parts of Asia, people at a funeral wear white while the deceased is dressed in red.
In some environments, the culture of the medical center prescribes appropriate apparel. The Mayo Clinic in Minnesota, for example, has had a tradition of having their physician staff dress in business attire. By this, they mean conservative sport coats or suits with ties for men, and similar appropriate women's business clothing. As noted by Leonard L. Berry and Neeli Bendapudi in their article Why Docs Don't Wear White Coats or Polo Shirts at the Mayo Clinic3 in the Harvard Business School publication Working Knowledge for Business Leaders, while some may consider this semiformal business dress pretentious, it should be considered no more pretentious than, say, the dress code for airline pilots. Airline passengers don't want to see their pilot in a polo shirt, and patients feel the same way about their doctors. The business attire is a uniform; it's a visible clue that communicates respect to patients and their families. But, isn't a white coat a uniform that conveys respect? Perhaps a white coat isn't safe if the physician wants to stand out to oncoming traffic in his snowy Minnesota environs.
Besides, is it true that patients don't want their physician to wear a polo shirt? Perhaps casual dress will break down 1 of the patient‐doctor barriers to communication and allow for improved comfort with greater honesty in patientphysician interactions? Prior to designing a prospective controlled randomized trial to answer this question myself, I reviewed the available literature.
It turns out, that an evaluation of polo‐shirt‐wearing physicians has been carried out. Drs. Barrett and Booth4 from the Birmingham Maternity Hospital in England questioned 203 groups of parents and children (406 individuals) about various levels of physician dress. Seventy percent of participants thought that how the doctor dressed was important. Among children, a male physician in a polo shirt was considered more friendly and gentle than the male physician in a white coat (who did get points for being more competent and more concerned). Women physicians in T‐shirts were also though to be friendlier than if in a white coat, but similarly less competent. Parents were more likely to prefer casually‐dressed physicians and were poor at predicting what their children would want.
So, a polo shirt makes me look friendly, gentle, and less competent? What about a polo shirt under a white coatis that the whole package? What about business attire, as per the Mayo Clinic requirements? These questions remain unanswered. So, back to the literature.
In the Journal of the American Medical Association, in 1987, Dunn et al.5 evaluated 200 medical patients in Boston and San Francisco regarding their preference for physician dress. Sixty‐five percent believed physicians should wear a white coat, 27% said no tennis shoes, one‐half said no blue jeans, and about one‐third thought male physicians should wear ties and female physicians should be in a skirt or dress. I suppose the conclusion here is to wear a white coat with or without jeans and most of the time without a tie, though almost always with proper shoes, or at least without tennis shoes. (I practice in Boston and trained partly in San Francisco so this advice seems particularly valid for me.)
It may be more obvious what to do in Japan. Ikusaka et al.6 evaluated the experience of patients at a university clinic seen by a consulting physician in either a white coat, or private clothes. To me, private clothes imply pajamas and a bathrobe, but we must assume this means some form of professional dress lacking a white coat. It turns out that 71% of the Japanese patients seeing a doctor in a white coat preferred a white coat, though more patients seeing a physician in a white coat (vs. private clothes) felt tense during their consultation. The researchers stress that the presence of a white coat did not increase satisfaction with the consultation. They conclude, that while patients may say they prefer a white coat, maybe it would be better not to wear one since it makes patients feel tense.
In addition to feeling tense, white coats may cause hypertension. The phenomenon of elevated blood pressure when in the presence of the physician (or other hospital staff in a white coat) has been long documented. This white coat hypertension can be found in more than 15% of the population who have a measured blood pressure in the office of over 140/90 mmHg with normal daytime mean ambulatory blood pressure readings (when not around a white‐coat‐wearing stress‐inducing medical worker).7 Older adults, females, and nonsmokers were more likely to have white coat hypertension than other persons.
And yet, older adults prefer white coats. The Japanese study (Ikusaka et al.6) concluded that elderly patients prefer a white coat to other attire. Similarly, a study from the Royal Free Hospital, London, showed that white coats were twice as popular with patients as they were with physicians.8 Specifically, patients found the white coats made doctors easier to identify. In an article by the British Broadcasting Corporation (BBC) on the subject, it was noted that the elderly largely preferred physicians in white coats, while children preferred physicians without white coats. British children must prefer a friendly doctor to a competent one.
The article further suggests that only 1 in 8 physicians wears a white coat, complaining that they are too hot and uncomfortable, and may carry the risk of transmitting infections. The white coat, the symbol of cleanliness and purity, a source of infection? To add hypocrisy to the equation, one‐half of physicians who thought white coats should be worn admitted to never actually wearing a white coat. In fact, only 7 of 86 physicians surveyed wore their white coat on a daily basis. The BBC goes on to note that in Australia, the white coat is gaining momentum as there seems to be a movement towards rediscovering the white coat as a symbol of purpose and pride as a profession.8
Really? Let's consult the literature! According to Dr. D.A. Watson,9 White coats have largely disappeared from Australian teaching hospitals and the majority of junior doctors in Australia oppose the wearing of white coats. In a survey of 337 junior medical officers, only 16% preferred to wear a white coat. Peer pressure seems to have something to do with this, as 70% say they don't wear a white coat because nobody else wears a white coat. This is indeed a compelling argument.
Of course, a better argument against wearing a white coat may be that it causes tension (at least in Japanese patients) and may cause white‐coat‐hypertension, resulting in the inappropriate diagnosis and treatment of elevated blood pressure. In Australia, however, it seems that wearing a white coat may make patients too relaxed. Wing et al.10 noted that in 21% to 45% of elderly patients, blood pressure was atypically low when checked in the physician's office as compared to mean ambulatory blood pressure. This reverse‐white‐coat‐hypertension could result in the omission of necessary blood pressure treatment.
In the United States, residentsour own version of the Australian junior medical officercommonly wear scrubs covered by a white coat. Scrub clothes are typically available without charge from the hospital, limit the amount of laundry a busy resident needs to do, and can be put on with little concern as to pressed pleats or matching colors. The overlying white coat adds a moderate degree of formality to what could otherwise be mistaken as pajamas, while providing convenient pockets for the aforementioned papers and miscellaneous equipment and souvenirs. This outfit is likely one of practicality rather than a desire to be most appealing to patients. But, what do you know? It seems that patients prefer their resident physicians to dress this way. Dr. A. Cha et al.11 at the Northeastern Ohio College of Medicine found that patients in an obstetrics and gynecology clinic overall felt that resident physicians dressed in surgical scrubs with a white coat made them feel more comfortable and confident than if dressed otherwise. On the question of a white coat specifically, the majority had no preference that their physician wear one.
So, it's very much unclear whether a white coat is a tension‐causing, blood‐pressure‐elevating, infection risk or a competence‐implying blood‐pressure‐lowering way to identify a physician. As a result, the jury is still out on whether physicians should wear white.
One thing I do know, however, is that patients shouldn't wear white. But they do. As an OtolaryngologistHead and Neck Surgeon, my clinical practice is split between surgical procedures and office visits. Commonly, patients with sinus or nasal surgery will require some form of cotton gauze or foam material within the nose in order to tamponade bleeding. Similarly, patients presenting to the emergency department or urgent care center with epistaxis may have their noses packed and then be told to see an otolaryngologist (eg, me) in 3 or 4 days to have the packing removed. I also commonly remove facial skin lesions, biopsy tongue masses, reduce nasal fractures, and otherwise engage in activities with an above average propensity to result in a mess. More often than happenstance will allow, patients come to see me for such visits in their white Sunday best. I truly care for my patients. I respect them as individuals and desire to do no harm. This includes not staining their shirts, ties, or pants. However, there is no amount of blue towels or gauze pads than can keep a white shirt clean when you have that first sneeze after removal of your nasal packing.12
So what is it that makes so many patients come to the office in a white shirt? Perhaps patients subconsciously associate healthcare with the color white since their doctors wear white coats and the nurses wear starched white dresses with tight white folded caps on top of their head. I've never seen a nurse in a white dress and hat, but believe television programs have shown this in the past.
Maybe the answer lies in an adaptation of data from another Australian study. In a survey of 180 oncology patients about white coats on physicians, the most common argument against wearing the white coat was that it represented a barrier between the physician and the patient.13 However, it is indeed the sane patient who desires to have a barrier between their physician and the removal of nasal packing, a skin lesion, or a tongue mass. Another possibility is that as fewer and fewer physicians wear white, patients are gravitating toward this color as a way of distinguishing themselves from the medical staff. Perhaps a person dressed in white is less likely to be grabbed in the hallway with a Doctor come quick! and more likely to be allowed to just sit peacefully in the waiting room with a 1997 issue of Ladies Home Journal or Senior Fisherman magazine.
Of course, perhaps patients believe the fashion experts who expound that white goes with everything. If so, they soon learn that white doesn't go so well with blotchy splattered red.
The truth is, I don't want my patients to wear white. Between making certain I project myself as approachable and easy to speak with, remembering to cover all the appropriate irrelevant parts of the history and physical to comply with billing requirements, entering data in our easy‐to‐navigate electronic medical record, and attempting to both diagnose the problem and discern an acceptable and effective treatment, the last thing I need is to worry about staining patient shirts! I believe that this phenomenon is widespread among medical practitioners and should be called white‐shirt‐hypertension.
Conclusion
After reviewing the available literature, I've determined that patients should not wear white. However, I'm still not certain how I should dress. For now, I'll stick with whatever is clean and professional and make sure my belt and shoes match. I may or may not wear a tie, since another study showed that 30% of patients believed their physician wore a tie even if they didn't.14 Of course, I'll put on the white coat for the semiannual meeting with the chairman, or if I'm having something messy for lunch and wore a tie that day.
I'll also wear my name badge. It says I'm an attending physician and not a resident. It opens doors around the hospital. Literally. It's got a magnetic stripe.
Physicians wear white coats. In many medical centers, the length of one's coat grows with seniority; medical students, interns, and residents wear short white coats, while attending staff graduate to long white coats with their names embroidered on the front. This traditional uniform serves a similar role to the stripes on a military sleeve. That is, by examining the length of a person's coat, a nurse or other hospital employee can rapidly determine the seniority, and theoretically the increased medical knowledge, of the person inside.
Of course, it isn't quite this simple. In many places, all residents wear long coats (with embroidered names). In other hospitals, attending staff wear short coats. The problem of distinguishing between a medical student, resident, and attending physician can be quite vexing, particularly after the summer months when the anxiety visible on the faces of new house staff and third‐year medical students fades away into the more seasoned look of fatigue and malaise. As hospital‐based practitioners, what are we to do?
To be certain there are clues one can use to identify one's rank in the medical profession. Often medical students wear a medical school pin bestowed upon them at a robing ceremony or other coming‐of‐age festivity to mark the transition to the clinical training years.1 (Little do they know that the primary utility of such adornments is to mark them, as though with a scarlet letter, for easy identification when one wishes to pimp the medical team.)2 Similarly, one can look for an arm patch. Just as a mother hen has the need to identify her own chicks, so too is it helpful for the residency director to have each of his or her own residents easily identifiable by the residency patch emblazoned upon the arm of their (long) white coat. The patch test can fail, though, for too often the residency emblem is merely a simple modification of the hospital or medical center logo, and thus not easily distinguishable.
When all else fails, of course, you can look to the pockets. As one's medical knowledge and training rank increases, the number of papers, pens, and instruments in the pockets of the white coat decreases. This inverse relationship provides some increased ability to identify medical students and junior house staff. For example, a short white coat, busting with oversized index cards held together by a large metal ring, several tattered and folded journal articles, and a worn spiral‐bound text suggests a medical student or intern. If they have an electrocardiogram (ECG), calipers, and tuning fork visible, safe bet is you've found a medical student. Conversely, if the white coat sports several free pens (with medication logos embossed on their stems) and has more than a few stains, chances are you've spotted an intern.
Unfortunately, none of this is of much utility in identifying a physician, as the initial premise that physicians wear white coats may be false. The problem is 2‐fold. First of all, not all physicians wear white coats (or any coat for that matter), and, many nonphysicians wear white coats. Commonly, phlebotomists, pharmacists, and respiratory technicians wear white coats; long white coats, in fact. So do nutritionists, speech pathologists, the clerk at the radiology file room, and even the cashiers in the cafeteria. And why not? Each of these persons has an important role to play in the operation of a modern medical center. The long white coat serves as professional uniform to identify the wearer as contributing to the mission of the medical center. It engenders confidence and suggests cleanliness and purity.
The problem remains, however, what should I, as an attending physician wear? You see, patients encounter so many persons in their visits to the hospital that it is not clear who their doctor is. (It's not statistically likely to be the man in a long white coat.) Being able to identify the attending physician is important. The attending physician is ultimately responsible for the patient's care.
There are many guidelines for how to dress. For example, don't wear white after Labor Day. The pleats on a cummerbund point so that the folds open pointing up (apparently it was originally meant to serve as a ticket holder, perhaps in the days before pockets?). Match your belt to your shoes. The list goes on. However, physicians are not commonly associated with natty attire, so fashion help for the MD is hard to find, though nonetheless necessary.
Even wearing white is questionable. White robes have been associated with purity and sanctity for centuries. Religious leaders have donned white for spiritual cleansing of their communities on the most holy of days. I like the idea of appearing pure and holy. Of course, I don't want to mislead anybody, either.
Brides have traditionally worn white dresses. But, while many believe this is to convey a sense of premarital purity, more likely the tradition stems from an ancient Roman practice of wearing white as a symbol of joyous celebration. Interestingly, in some parts of Asia, people at a funeral wear white while the deceased is dressed in red.
In some environments, the culture of the medical center prescribes appropriate apparel. The Mayo Clinic in Minnesota, for example, has had a tradition of having their physician staff dress in business attire. By this, they mean conservative sport coats or suits with ties for men, and similar appropriate women's business clothing. As noted by Leonard L. Berry and Neeli Bendapudi in their article Why Docs Don't Wear White Coats or Polo Shirts at the Mayo Clinic3 in the Harvard Business School publication Working Knowledge for Business Leaders, while some may consider this semiformal business dress pretentious, it should be considered no more pretentious than, say, the dress code for airline pilots. Airline passengers don't want to see their pilot in a polo shirt, and patients feel the same way about their doctors. The business attire is a uniform; it's a visible clue that communicates respect to patients and their families. But, isn't a white coat a uniform that conveys respect? Perhaps a white coat isn't safe if the physician wants to stand out to oncoming traffic in his snowy Minnesota environs.
Besides, is it true that patients don't want their physician to wear a polo shirt? Perhaps casual dress will break down 1 of the patient‐doctor barriers to communication and allow for improved comfort with greater honesty in patientphysician interactions? Prior to designing a prospective controlled randomized trial to answer this question myself, I reviewed the available literature.
It turns out, that an evaluation of polo‐shirt‐wearing physicians has been carried out. Drs. Barrett and Booth4 from the Birmingham Maternity Hospital in England questioned 203 groups of parents and children (406 individuals) about various levels of physician dress. Seventy percent of participants thought that how the doctor dressed was important. Among children, a male physician in a polo shirt was considered more friendly and gentle than the male physician in a white coat (who did get points for being more competent and more concerned). Women physicians in T‐shirts were also though to be friendlier than if in a white coat, but similarly less competent. Parents were more likely to prefer casually‐dressed physicians and were poor at predicting what their children would want.
So, a polo shirt makes me look friendly, gentle, and less competent? What about a polo shirt under a white coatis that the whole package? What about business attire, as per the Mayo Clinic requirements? These questions remain unanswered. So, back to the literature.
In the Journal of the American Medical Association, in 1987, Dunn et al.5 evaluated 200 medical patients in Boston and San Francisco regarding their preference for physician dress. Sixty‐five percent believed physicians should wear a white coat, 27% said no tennis shoes, one‐half said no blue jeans, and about one‐third thought male physicians should wear ties and female physicians should be in a skirt or dress. I suppose the conclusion here is to wear a white coat with or without jeans and most of the time without a tie, though almost always with proper shoes, or at least without tennis shoes. (I practice in Boston and trained partly in San Francisco so this advice seems particularly valid for me.)
It may be more obvious what to do in Japan. Ikusaka et al.6 evaluated the experience of patients at a university clinic seen by a consulting physician in either a white coat, or private clothes. To me, private clothes imply pajamas and a bathrobe, but we must assume this means some form of professional dress lacking a white coat. It turns out that 71% of the Japanese patients seeing a doctor in a white coat preferred a white coat, though more patients seeing a physician in a white coat (vs. private clothes) felt tense during their consultation. The researchers stress that the presence of a white coat did not increase satisfaction with the consultation. They conclude, that while patients may say they prefer a white coat, maybe it would be better not to wear one since it makes patients feel tense.
In addition to feeling tense, white coats may cause hypertension. The phenomenon of elevated blood pressure when in the presence of the physician (or other hospital staff in a white coat) has been long documented. This white coat hypertension can be found in more than 15% of the population who have a measured blood pressure in the office of over 140/90 mmHg with normal daytime mean ambulatory blood pressure readings (when not around a white‐coat‐wearing stress‐inducing medical worker).7 Older adults, females, and nonsmokers were more likely to have white coat hypertension than other persons.
And yet, older adults prefer white coats. The Japanese study (Ikusaka et al.6) concluded that elderly patients prefer a white coat to other attire. Similarly, a study from the Royal Free Hospital, London, showed that white coats were twice as popular with patients as they were with physicians.8 Specifically, patients found the white coats made doctors easier to identify. In an article by the British Broadcasting Corporation (BBC) on the subject, it was noted that the elderly largely preferred physicians in white coats, while children preferred physicians without white coats. British children must prefer a friendly doctor to a competent one.
The article further suggests that only 1 in 8 physicians wears a white coat, complaining that they are too hot and uncomfortable, and may carry the risk of transmitting infections. The white coat, the symbol of cleanliness and purity, a source of infection? To add hypocrisy to the equation, one‐half of physicians who thought white coats should be worn admitted to never actually wearing a white coat. In fact, only 7 of 86 physicians surveyed wore their white coat on a daily basis. The BBC goes on to note that in Australia, the white coat is gaining momentum as there seems to be a movement towards rediscovering the white coat as a symbol of purpose and pride as a profession.8
Really? Let's consult the literature! According to Dr. D.A. Watson,9 White coats have largely disappeared from Australian teaching hospitals and the majority of junior doctors in Australia oppose the wearing of white coats. In a survey of 337 junior medical officers, only 16% preferred to wear a white coat. Peer pressure seems to have something to do with this, as 70% say they don't wear a white coat because nobody else wears a white coat. This is indeed a compelling argument.
Of course, a better argument against wearing a white coat may be that it causes tension (at least in Japanese patients) and may cause white‐coat‐hypertension, resulting in the inappropriate diagnosis and treatment of elevated blood pressure. In Australia, however, it seems that wearing a white coat may make patients too relaxed. Wing et al.10 noted that in 21% to 45% of elderly patients, blood pressure was atypically low when checked in the physician's office as compared to mean ambulatory blood pressure. This reverse‐white‐coat‐hypertension could result in the omission of necessary blood pressure treatment.
In the United States, residentsour own version of the Australian junior medical officercommonly wear scrubs covered by a white coat. Scrub clothes are typically available without charge from the hospital, limit the amount of laundry a busy resident needs to do, and can be put on with little concern as to pressed pleats or matching colors. The overlying white coat adds a moderate degree of formality to what could otherwise be mistaken as pajamas, while providing convenient pockets for the aforementioned papers and miscellaneous equipment and souvenirs. This outfit is likely one of practicality rather than a desire to be most appealing to patients. But, what do you know? It seems that patients prefer their resident physicians to dress this way. Dr. A. Cha et al.11 at the Northeastern Ohio College of Medicine found that patients in an obstetrics and gynecology clinic overall felt that resident physicians dressed in surgical scrubs with a white coat made them feel more comfortable and confident than if dressed otherwise. On the question of a white coat specifically, the majority had no preference that their physician wear one.
So, it's very much unclear whether a white coat is a tension‐causing, blood‐pressure‐elevating, infection risk or a competence‐implying blood‐pressure‐lowering way to identify a physician. As a result, the jury is still out on whether physicians should wear white.
One thing I do know, however, is that patients shouldn't wear white. But they do. As an OtolaryngologistHead and Neck Surgeon, my clinical practice is split between surgical procedures and office visits. Commonly, patients with sinus or nasal surgery will require some form of cotton gauze or foam material within the nose in order to tamponade bleeding. Similarly, patients presenting to the emergency department or urgent care center with epistaxis may have their noses packed and then be told to see an otolaryngologist (eg, me) in 3 or 4 days to have the packing removed. I also commonly remove facial skin lesions, biopsy tongue masses, reduce nasal fractures, and otherwise engage in activities with an above average propensity to result in a mess. More often than happenstance will allow, patients come to see me for such visits in their white Sunday best. I truly care for my patients. I respect them as individuals and desire to do no harm. This includes not staining their shirts, ties, or pants. However, there is no amount of blue towels or gauze pads than can keep a white shirt clean when you have that first sneeze after removal of your nasal packing.12
So what is it that makes so many patients come to the office in a white shirt? Perhaps patients subconsciously associate healthcare with the color white since their doctors wear white coats and the nurses wear starched white dresses with tight white folded caps on top of their head. I've never seen a nurse in a white dress and hat, but believe television programs have shown this in the past.
Maybe the answer lies in an adaptation of data from another Australian study. In a survey of 180 oncology patients about white coats on physicians, the most common argument against wearing the white coat was that it represented a barrier between the physician and the patient.13 However, it is indeed the sane patient who desires to have a barrier between their physician and the removal of nasal packing, a skin lesion, or a tongue mass. Another possibility is that as fewer and fewer physicians wear white, patients are gravitating toward this color as a way of distinguishing themselves from the medical staff. Perhaps a person dressed in white is less likely to be grabbed in the hallway with a Doctor come quick! and more likely to be allowed to just sit peacefully in the waiting room with a 1997 issue of Ladies Home Journal or Senior Fisherman magazine.
Of course, perhaps patients believe the fashion experts who expound that white goes with everything. If so, they soon learn that white doesn't go so well with blotchy splattered red.
The truth is, I don't want my patients to wear white. Between making certain I project myself as approachable and easy to speak with, remembering to cover all the appropriate irrelevant parts of the history and physical to comply with billing requirements, entering data in our easy‐to‐navigate electronic medical record, and attempting to both diagnose the problem and discern an acceptable and effective treatment, the last thing I need is to worry about staining patient shirts! I believe that this phenomenon is widespread among medical practitioners and should be called white‐shirt‐hypertension.
Conclusion
After reviewing the available literature, I've determined that patients should not wear white. However, I'm still not certain how I should dress. For now, I'll stick with whatever is clean and professional and make sure my belt and shoes match. I may or may not wear a tie, since another study showed that 30% of patients believed their physician wore a tie even if they didn't.14 Of course, I'll put on the white coat for the semiannual meeting with the chairman, or if I'm having something messy for lunch and wore a tie that day.
I'll also wear my name badge. It says I'm an attending physician and not a resident. It opens doors around the hospital. Literally. It's got a magnetic stripe.
- .Deconstructing the white coat.Ann Intern Med.1998;129:740–742.
- .The art of pimping.JAMA.1989;262(1):89–90.
- ,. “Why Docs don't wear white coats or Polo Shirts at the Mayo Clinic” working knowledge for business leaders. Available at:http://hbswk.hbs.edu/pubitem.jhtml?id=3380309(6970):1710–1712.
- ,,,,.Patient and house officer attitudes on physician attire and etiquette.JAMA.1987;257(1):65–68.
- ,,, et al.Patients' attitude toward consultations by a physician without a white coat in Japan.Intern Med.1999;38(7):533–536.
- ,,, et al.Determinants of white‐coat hypertension.Blood Press Monit.2004;9(6):307–309.
- Doctors ‘should wear white coats.’ BBC news, Thursday, 13 May, 2004. Available at:http://news.bbc.co.uk/2/hi/health/3706783.stm. Accessed May2009.
- .What do Australian junior doctors think of white coats?Med Educ.2002;36(12):1209–1213.
- ,,, ,;ANBP2 Management Committee and Investigators.Second Australian National Blood Pressure Study. ‘Reverse white‐coat hypertension’ in older hypertensives.J Hypertens.2002;20(4):639–644.
- ,,,.Resident physician attire: does it make a difference to our patients?Am J Obstet Gynecol.2004;190(5):1484–1488.
- . Personal communication.1994–2008.
- .Should doctors wear white coats?Med J Aust.2001;174:343–344.
- ,,,,.Does wearing a necktie influence patient perceptions of emergency department care?J Emerg Med.1998;16(4):541–543.
- .Deconstructing the white coat.Ann Intern Med.1998;129:740–742.
- .The art of pimping.JAMA.1989;262(1):89–90.
- ,. “Why Docs don't wear white coats or Polo Shirts at the Mayo Clinic” working knowledge for business leaders. Available at:http://hbswk.hbs.edu/pubitem.jhtml?id=3380309(6970):1710–1712.
- ,,,,.Patient and house officer attitudes on physician attire and etiquette.JAMA.1987;257(1):65–68.
- ,,, et al.Patients' attitude toward consultations by a physician without a white coat in Japan.Intern Med.1999;38(7):533–536.
- ,,, et al.Determinants of white‐coat hypertension.Blood Press Monit.2004;9(6):307–309.
- Doctors ‘should wear white coats.’ BBC news, Thursday, 13 May, 2004. Available at:http://news.bbc.co.uk/2/hi/health/3706783.stm. Accessed May2009.
- .What do Australian junior doctors think of white coats?Med Educ.2002;36(12):1209–1213.
- ,,, ,;ANBP2 Management Committee and Investigators.Second Australian National Blood Pressure Study. ‘Reverse white‐coat hypertension’ in older hypertensives.J Hypertens.2002;20(4):639–644.
- ,,,.Resident physician attire: does it make a difference to our patients?Am J Obstet Gynecol.2004;190(5):1484–1488.
- . Personal communication.1994–2008.
- .Should doctors wear white coats?Med J Aust.2001;174:343–344.
- ,,,,.Does wearing a necktie influence patient perceptions of emergency department care?J Emerg Med.1998;16(4):541–543.
Nonphysician Providers
The current state of our profession is that the US population is aging rapidly, requiring ever more healthcare, and there is a stagnant number of physicians to care for them. The question of who will care for our aging population has been raised over and over in the past decade but the question is worth repeating. As our country continues to deliver state‐of‐the‐art medical care, it is slow to embrace the notion that in order for it to continue, it will need to incorporate the professions of advanced practice nurses and physician assistants. Without these nonphysician providers our medical community will not be able to reach the patients we have sworn to treat.
The percent of the US population age >65 years is projected to increase from 12.4% in 2000 to 19.6% in 2030. The number of persons age >65 years is expected to increase from approximately 35 million in 2000 to an estimated 71 million in 2030, and the number of persons age >80 years is expected to increase from 9.3 million in 2000 to 19.5 million in 2030.1 Our aging America is also coupled with a growing physician shortage. In its report entitled Physician Workforce Policy Guidelines for the United States, 2000‐2020, the Council on Graduate Medical Education recommended increasing the number of medical school graduates by 3000 per year by the year 2015 to meet the increasing need.2 Given the current trend of decreasing physician reimbursement coupled with the average medical school debt of $139,517,3 it is doubtful that the extra 3000 physicians needed to graduate in 2015 will actually ever do so. Despite this possible additional physician workforce, there still stands to be enormous need for the nonphysician provider with our rapidly expanding senior population.
Our nation's hospitals are by no means spared from our aging population or physician shortage. In fact, they are likely to be the hardest hit. Hospitalists are already feeling the pressure of an overstressed workforce coupled with increasing patient volume.4 There is a growing body of evidence supporting the successful collaboration between hospitalists and nurse practitioners (NPs)/physician assistants (PAs) (collectively, nonphysician providers [NPPs]). No longer are NPPs only working in outpatient practices or in the operating room, but they are actively involved with inpatient medical units improving our Hospital Medicine (HM) specialty. According to Myers et al.,5 the hospitalist NP model improved program finances and increased physician and resident satisfaction. In order for Hospital Medicine to create increasing value for its parent hospital or to the community it serves, NPPs will need increased integration into our care model for improved overall efficiency. We focus herein on the advantages and potential benefits of NPPs relating to their varied roles within HM.
Scope of Practice
The scope of practice of NPPs is regulated by each individual state board of registration. However, differences from state to state are usually minor and general statements on the practice scope of PAs and NPs can be made.
PAs
PAs practice under the supervision of a physician. PAs are trained in programs affiliated with medical schools and according to the medical model of care that emphasizes diagnosis and treatment. Most PAs graduate with a masters of science degree. According to the American Association of Physician Assistants (AAPA), the scope of practice is guided by state law, facility policy, and delegatory decisions made by the supervising physician.6 Prior experience and training should be the framework for scope of practice decisions. All 50 states allow PAs to prescribe with some oversight and restriction of schedule 2 controlled substances or by using a state formulary. The AAPA embraces the concept of the physician as the captain of the healthcare team and sees the PA role as entirely complementary to the care provided by physicians.7 This means that PAs, under an individual supervision agreement, can prescribe medicines, order and interpret tests, diagnose, and treat patients just as a physician would.
Advanced Practice Nurses
Advanced practice nurses (APNs) are trained under the nursing model and generally have some years of nursing experience before they pursue an entry‐level masters of science degree to become an APN. APNs can be divided into two categories: Clinical nurse specialists, who generally focus on patient and institutional education and are considered experts in nursing practice, and NPs, who have a focus on diagnosis and treatment of medical conditions. A clinical nurse specialist does not have prescriptive training or authority. NP training can be general (adult or family) or specific (eg, acute care, geriatric, pediatric, psychiatric). The American Association of Colleges of Nursing (AACN) has recommended that the entry level of all new NPs should be a clinical doctorate of nursing practice. Although controversial, many colleges have embraced this recommendation and are opening clinical doctorate‐level programs.8 Although some states allow NPs to practice independently, most NPs have a practice agreement with a collaborating physician that delineates the degree of supervision. Generally, the NP's scope of practice is identical to PAs and includes the above‐mentioned activities as proscribed by state regulations and facility bylaws. As with PAs, their prior experience and training should be the most important determinant of their scope of practice in a new position.
Potential Benefits of NPPs
Continuity
If a nonacademic hospitalist program has high yearly turnover due to use of recent medical graduates who are planning to do fellowships, NPPs can provide much needed stability and facilitate orientation of new physicians to the hospital. NPPs who work in academic settings can also provide increased continuity for patients and hospital staff. Residents, fellows, and attendings have certain rotational cycles on each medical service. NPPs generally do not rotate and can be the anchor of a medical team for patients and ancillary staff. Utilizing NPPs as liaisons between the hospitalist team and other members of the care team (eg, nurses, case managers, therapists, and administration) provides continuity for these groups and a central person who can help to facilitate change.
Quality Measures
NPPs can play an important role in hospital compliance with internal hospital or insurance provider quality initiatives. Surveillance of patients and charts for compliance with core measures, infection control, and prevention of complications are within the scope of practice of NPPs and can be incorporated into job descriptions. NPs and PAs will have the added responsibility of not only leading these surveillance teams but also in the correction of outliers given their prescriptive abilities. This will become an increasingly important task as reimbursement for preventable complications is curtailed. Additionally, the development and implementation of clinical pathways can be a focus of the NPP role to standardize and enhance quality of care.
Multidisciplinary Team Approach
Multidisciplinary teams that consist of NPPs, physicians, nurses, and therapists have been shown to increase communication and collaboration between participants.9 Mary Naylor, a Professor of Nursing at the University of Pennsylvania, has authored multiple articles and studies which examine the benefit of a multidisciplinary team that includes APNs with hospitalized patients. She has found that involving APNs in patient care, discharges, and routine follow‐up after discharge led to longer time to readmissions and decreased healthcare costs.1012 Furthermore, a nonteaching group consisting of NPPs, fellows, and attendings at the Mayo Clinic found increased physician satisfaction, shorter length of stay (LOS), and increased efficiency for their patients.13 A study done at JFK Medical Center in Florida noted that a collaborative practice which included unit‐based NPs serving in the dual role of NP and clinical nurse specialist increased patient satisfaction and improved patient outcomes.14
Financial Advantages
Efficiency and quality care are the cornerstones of HM. The partnership of NPPs within the specialty is creating even better performance. Models incorporating NPPs in the Hospitalist team approach are continuing to drive efficiency. Cowan et al.15 demonstrated that a multidisciplinary team, including nurse practitioners, decreased LOS from 6.01 to 5.0 and a reduced cost by $1,591 per patient. It is this team approach that will lift our specialty to be the model of care for all future hospital practice.
Another factor in determining the fiscal advantage of NPPs is salary and medical liability comparison. According to the 2007 Society of Hospital Medicine (SHM) Survey, the average hospitalist salary is approaching $190,000, compared to an average NP earning $87,000 and PA earning $84,500.4 Furthermore, the average internal medicine malpractice payment for physicians ranges from $14,237 to $68,867.16 In comparison, the average malpractice insurance premium for NPPs varies from state to state but is approximately $800 to $2000 per year.17, 18 With increasing fiscal scrutiny from hospitals, HM groups (HMGs) will need to include NPPs to be fiscally stable.
Models of Care
There are many models for NPP roles in hospital medicine groups. Some groups use NPPs in the same role as physicians. They perform admissions, rounding, and discharges with varying degrees of oversight by physicians. Other groups use NPPs for a more limited role, such as exclusively performing histories and physicals in the emergency department or handling discharges on the wards. It is important to take into account the preferences and expectations of NPPs when designing job descriptions. While some NPPs may like the fast pace and quick turnover of admissions and discharges, others may prefer to follow patients throughout their hospital stay. The quality of handoffs is crucial if the former model is used, just as it is with physicians in this more truncated role. An NPP who works in a nonacademic model will likely have more autonomy and control over patient care decisions. An NPP role in the teaching service of an academic hospital is likely to be more collaborative and focus more on quality initiatives, patient teaching, and communication. It is crucial to design an NPP model that is sustainable with very strong support of management once the NPP is hired and orientated.
Registered Nurses And Hospital Medicine
Patient handoffs and communication are one of the most challenging aspects of an HMG. There is an increasing movement, throughout the country, to incorporate registered nurses (RNs) into daily workflow. The RN on the HM team can serve to augment the communication and workflow process. A highly motivated and organized registered nurse can help to improve overall provider's workflow efficiency. Communication to primary care physician and collecting ancillary medical information can allow the provider to treat more patients in a given shift and decrease the liability risk from lack of information. As HM organizations and hospitals become more financially bound, HMGs will need to become more efficient at time management and a dedicated RN can help smooth that process.
Potential Unintended Side Effects
Obviously, integration of NPPs can be a disaster for an HMG if not handled properly. Most hospitalists have heard of an integration of NPP into a group that was an unqualified failure. NPPs can feel unsupported, poorly oriented to the job, or thrown into a situation that is over their heads. Before an NPP is hired into an HMG, there needs to be a thorough examination of the rationale behind the decision and assessment of the hospital culture that will be the host of the new NPP. What does the HMG need for support? Are they looking for a short‐term fix for increased volume or a long‐term strategy to build a multidisciplinary team? Does the hospital culture see NPPs as poorly qualified to act as hospitalists or uniquely qualified to address shortcomings of the program? A clear job description should be the first step in determining what the NPP is expected to do. This can then be shared with the hospital leadership in advance to promote buy‐in. The second step is finding an NPP that fits the goals of the program. A new NPP, by virtue of the fact that they have less clinical hours in training than a physician hospitalist, will need more support and a longer orientation. NPPs who have experience in hospital medicine will have a much shorter orientation. A stepwise approach to orientation can be helpful in assessing skill level of new hires. These NPPs can be initially paired with an enthusiastic physician to provide support and assessment of existing skills. A gradual increase in independence can provide assurance that the NPP is qualified to provide care and gives many opportunities for reevaluation of the NPP. Clear expectations and constructive feedback should ultimately lead to a degree of comfort within the HMG, hospital, and the NPPs themselves.
Conclusions
It is clear that our healthcare system will need a very different approach to the economic problems it is facing. Standardization of care, integrated medical records, and expanded and universal resource utilization will drive the next generation of healthcare providers. The model of a private physician working alone under the direction of only his or her own medical knowledge is a thing of the past. Just as the HM specialty has grown from 300 in 1996 to more than 20,000 in 2008, so shall the integration of NPPs grow into our healthcare fabric.
- Centers for Disease Control and Prevention (CDC). Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52(6):101–104, 106.
- Council on Graduate Medical Education. Physician Workforce Policy Guidelines for the U.S. for 2000‐2020. Rockville, MD: U.S. Department of Health and Human Services;2005.
- American Medical Association. Medical Student Section. Advocacy and Policy. Medical Student Debt. Available at: http://www.ama‐assn.org/ama/pub/category/5349.html. Accessed June 2009.
- Society of Hospital Medicine (SHM). 2007‐2008 SHM Survey: State of the Hospital Medicine Movement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2
The current state of our profession is that the US population is aging rapidly, requiring ever more healthcare, and there is a stagnant number of physicians to care for them. The question of who will care for our aging population has been raised over and over in the past decade but the question is worth repeating. As our country continues to deliver state‐of‐the‐art medical care, it is slow to embrace the notion that in order for it to continue, it will need to incorporate the professions of advanced practice nurses and physician assistants. Without these nonphysician providers our medical community will not be able to reach the patients we have sworn to treat.
The percent of the US population age >65 years is projected to increase from 12.4% in 2000 to 19.6% in 2030. The number of persons age >65 years is expected to increase from approximately 35 million in 2000 to an estimated 71 million in 2030, and the number of persons age >80 years is expected to increase from 9.3 million in 2000 to 19.5 million in 2030.1 Our aging America is also coupled with a growing physician shortage. In its report entitled Physician Workforce Policy Guidelines for the United States, 2000‐2020, the Council on Graduate Medical Education recommended increasing the number of medical school graduates by 3000 per year by the year 2015 to meet the increasing need.2 Given the current trend of decreasing physician reimbursement coupled with the average medical school debt of $139,517,3 it is doubtful that the extra 3000 physicians needed to graduate in 2015 will actually ever do so. Despite this possible additional physician workforce, there still stands to be enormous need for the nonphysician provider with our rapidly expanding senior population.
Our nation's hospitals are by no means spared from our aging population or physician shortage. In fact, they are likely to be the hardest hit. Hospitalists are already feeling the pressure of an overstressed workforce coupled with increasing patient volume.4 There is a growing body of evidence supporting the successful collaboration between hospitalists and nurse practitioners (NPs)/physician assistants (PAs) (collectively, nonphysician providers [NPPs]). No longer are NPPs only working in outpatient practices or in the operating room, but they are actively involved with inpatient medical units improving our Hospital Medicine (HM) specialty. According to Myers et al.,5 the hospitalist NP model improved program finances and increased physician and resident satisfaction. In order for Hospital Medicine to create increasing value for its parent hospital or to the community it serves, NPPs will need increased integration into our care model for improved overall efficiency. We focus herein on the advantages and potential benefits of NPPs relating to their varied roles within HM.
Scope of Practice
The scope of practice of NPPs is regulated by each individual state board of registration. However, differences from state to state are usually minor and general statements on the practice scope of PAs and NPs can be made.
PAs
PAs practice under the supervision of a physician. PAs are trained in programs affiliated with medical schools and according to the medical model of care that emphasizes diagnosis and treatment. Most PAs graduate with a masters of science degree. According to the American Association of Physician Assistants (AAPA), the scope of practice is guided by state law, facility policy, and delegatory decisions made by the supervising physician.6 Prior experience and training should be the framework for scope of practice decisions. All 50 states allow PAs to prescribe with some oversight and restriction of schedule 2 controlled substances or by using a state formulary. The AAPA embraces the concept of the physician as the captain of the healthcare team and sees the PA role as entirely complementary to the care provided by physicians.7 This means that PAs, under an individual supervision agreement, can prescribe medicines, order and interpret tests, diagnose, and treat patients just as a physician would.
Advanced Practice Nurses
Advanced practice nurses (APNs) are trained under the nursing model and generally have some years of nursing experience before they pursue an entry‐level masters of science degree to become an APN. APNs can be divided into two categories: Clinical nurse specialists, who generally focus on patient and institutional education and are considered experts in nursing practice, and NPs, who have a focus on diagnosis and treatment of medical conditions. A clinical nurse specialist does not have prescriptive training or authority. NP training can be general (adult or family) or specific (eg, acute care, geriatric, pediatric, psychiatric). The American Association of Colleges of Nursing (AACN) has recommended that the entry level of all new NPs should be a clinical doctorate of nursing practice. Although controversial, many colleges have embraced this recommendation and are opening clinical doctorate‐level programs.8 Although some states allow NPs to practice independently, most NPs have a practice agreement with a collaborating physician that delineates the degree of supervision. Generally, the NP's scope of practice is identical to PAs and includes the above‐mentioned activities as proscribed by state regulations and facility bylaws. As with PAs, their prior experience and training should be the most important determinant of their scope of practice in a new position.
Potential Benefits of NPPs
Continuity
If a nonacademic hospitalist program has high yearly turnover due to use of recent medical graduates who are planning to do fellowships, NPPs can provide much needed stability and facilitate orientation of new physicians to the hospital. NPPs who work in academic settings can also provide increased continuity for patients and hospital staff. Residents, fellows, and attendings have certain rotational cycles on each medical service. NPPs generally do not rotate and can be the anchor of a medical team for patients and ancillary staff. Utilizing NPPs as liaisons between the hospitalist team and other members of the care team (eg, nurses, case managers, therapists, and administration) provides continuity for these groups and a central person who can help to facilitate change.
Quality Measures
NPPs can play an important role in hospital compliance with internal hospital or insurance provider quality initiatives. Surveillance of patients and charts for compliance with core measures, infection control, and prevention of complications are within the scope of practice of NPPs and can be incorporated into job descriptions. NPs and PAs will have the added responsibility of not only leading these surveillance teams but also in the correction of outliers given their prescriptive abilities. This will become an increasingly important task as reimbursement for preventable complications is curtailed. Additionally, the development and implementation of clinical pathways can be a focus of the NPP role to standardize and enhance quality of care.
Multidisciplinary Team Approach
Multidisciplinary teams that consist of NPPs, physicians, nurses, and therapists have been shown to increase communication and collaboration between participants.9 Mary Naylor, a Professor of Nursing at the University of Pennsylvania, has authored multiple articles and studies which examine the benefit of a multidisciplinary team that includes APNs with hospitalized patients. She has found that involving APNs in patient care, discharges, and routine follow‐up after discharge led to longer time to readmissions and decreased healthcare costs.1012 Furthermore, a nonteaching group consisting of NPPs, fellows, and attendings at the Mayo Clinic found increased physician satisfaction, shorter length of stay (LOS), and increased efficiency for their patients.13 A study done at JFK Medical Center in Florida noted that a collaborative practice which included unit‐based NPs serving in the dual role of NP and clinical nurse specialist increased patient satisfaction and improved patient outcomes.14
Financial Advantages
Efficiency and quality care are the cornerstones of HM. The partnership of NPPs within the specialty is creating even better performance. Models incorporating NPPs in the Hospitalist team approach are continuing to drive efficiency. Cowan et al.15 demonstrated that a multidisciplinary team, including nurse practitioners, decreased LOS from 6.01 to 5.0 and a reduced cost by $1,591 per patient. It is this team approach that will lift our specialty to be the model of care for all future hospital practice.
Another factor in determining the fiscal advantage of NPPs is salary and medical liability comparison. According to the 2007 Society of Hospital Medicine (SHM) Survey, the average hospitalist salary is approaching $190,000, compared to an average NP earning $87,000 and PA earning $84,500.4 Furthermore, the average internal medicine malpractice payment for physicians ranges from $14,237 to $68,867.16 In comparison, the average malpractice insurance premium for NPPs varies from state to state but is approximately $800 to $2000 per year.17, 18 With increasing fiscal scrutiny from hospitals, HM groups (HMGs) will need to include NPPs to be fiscally stable.
Models of Care
There are many models for NPP roles in hospital medicine groups. Some groups use NPPs in the same role as physicians. They perform admissions, rounding, and discharges with varying degrees of oversight by physicians. Other groups use NPPs for a more limited role, such as exclusively performing histories and physicals in the emergency department or handling discharges on the wards. It is important to take into account the preferences and expectations of NPPs when designing job descriptions. While some NPPs may like the fast pace and quick turnover of admissions and discharges, others may prefer to follow patients throughout their hospital stay. The quality of handoffs is crucial if the former model is used, just as it is with physicians in this more truncated role. An NPP who works in a nonacademic model will likely have more autonomy and control over patient care decisions. An NPP role in the teaching service of an academic hospital is likely to be more collaborative and focus more on quality initiatives, patient teaching, and communication. It is crucial to design an NPP model that is sustainable with very strong support of management once the NPP is hired and orientated.
Registered Nurses And Hospital Medicine
Patient handoffs and communication are one of the most challenging aspects of an HMG. There is an increasing movement, throughout the country, to incorporate registered nurses (RNs) into daily workflow. The RN on the HM team can serve to augment the communication and workflow process. A highly motivated and organized registered nurse can help to improve overall provider's workflow efficiency. Communication to primary care physician and collecting ancillary medical information can allow the provider to treat more patients in a given shift and decrease the liability risk from lack of information. As HM organizations and hospitals become more financially bound, HMGs will need to become more efficient at time management and a dedicated RN can help smooth that process.
Potential Unintended Side Effects
Obviously, integration of NPPs can be a disaster for an HMG if not handled properly. Most hospitalists have heard of an integration of NPP into a group that was an unqualified failure. NPPs can feel unsupported, poorly oriented to the job, or thrown into a situation that is over their heads. Before an NPP is hired into an HMG, there needs to be a thorough examination of the rationale behind the decision and assessment of the hospital culture that will be the host of the new NPP. What does the HMG need for support? Are they looking for a short‐term fix for increased volume or a long‐term strategy to build a multidisciplinary team? Does the hospital culture see NPPs as poorly qualified to act as hospitalists or uniquely qualified to address shortcomings of the program? A clear job description should be the first step in determining what the NPP is expected to do. This can then be shared with the hospital leadership in advance to promote buy‐in. The second step is finding an NPP that fits the goals of the program. A new NPP, by virtue of the fact that they have less clinical hours in training than a physician hospitalist, will need more support and a longer orientation. NPPs who have experience in hospital medicine will have a much shorter orientation. A stepwise approach to orientation can be helpful in assessing skill level of new hires. These NPPs can be initially paired with an enthusiastic physician to provide support and assessment of existing skills. A gradual increase in independence can provide assurance that the NPP is qualified to provide care and gives many opportunities for reevaluation of the NPP. Clear expectations and constructive feedback should ultimately lead to a degree of comfort within the HMG, hospital, and the NPPs themselves.
Conclusions
It is clear that our healthcare system will need a very different approach to the economic problems it is facing. Standardization of care, integrated medical records, and expanded and universal resource utilization will drive the next generation of healthcare providers. The model of a private physician working alone under the direction of only his or her own medical knowledge is a thing of the past. Just as the HM specialty has grown from 300 in 1996 to more than 20,000 in 2008, so shall the integration of NPPs grow into our healthcare fabric.
The current state of our profession is that the US population is aging rapidly, requiring ever more healthcare, and there is a stagnant number of physicians to care for them. The question of who will care for our aging population has been raised over and over in the past decade but the question is worth repeating. As our country continues to deliver state‐of‐the‐art medical care, it is slow to embrace the notion that in order for it to continue, it will need to incorporate the professions of advanced practice nurses and physician assistants. Without these nonphysician providers our medical community will not be able to reach the patients we have sworn to treat.
The percent of the US population age >65 years is projected to increase from 12.4% in 2000 to 19.6% in 2030. The number of persons age >65 years is expected to increase from approximately 35 million in 2000 to an estimated 71 million in 2030, and the number of persons age >80 years is expected to increase from 9.3 million in 2000 to 19.5 million in 2030.1 Our aging America is also coupled with a growing physician shortage. In its report entitled Physician Workforce Policy Guidelines for the United States, 2000‐2020, the Council on Graduate Medical Education recommended increasing the number of medical school graduates by 3000 per year by the year 2015 to meet the increasing need.2 Given the current trend of decreasing physician reimbursement coupled with the average medical school debt of $139,517,3 it is doubtful that the extra 3000 physicians needed to graduate in 2015 will actually ever do so. Despite this possible additional physician workforce, there still stands to be enormous need for the nonphysician provider with our rapidly expanding senior population.
Our nation's hospitals are by no means spared from our aging population or physician shortage. In fact, they are likely to be the hardest hit. Hospitalists are already feeling the pressure of an overstressed workforce coupled with increasing patient volume.4 There is a growing body of evidence supporting the successful collaboration between hospitalists and nurse practitioners (NPs)/physician assistants (PAs) (collectively, nonphysician providers [NPPs]). No longer are NPPs only working in outpatient practices or in the operating room, but they are actively involved with inpatient medical units improving our Hospital Medicine (HM) specialty. According to Myers et al.,5 the hospitalist NP model improved program finances and increased physician and resident satisfaction. In order for Hospital Medicine to create increasing value for its parent hospital or to the community it serves, NPPs will need increased integration into our care model for improved overall efficiency. We focus herein on the advantages and potential benefits of NPPs relating to their varied roles within HM.
Scope of Practice
The scope of practice of NPPs is regulated by each individual state board of registration. However, differences from state to state are usually minor and general statements on the practice scope of PAs and NPs can be made.
PAs
PAs practice under the supervision of a physician. PAs are trained in programs affiliated with medical schools and according to the medical model of care that emphasizes diagnosis and treatment. Most PAs graduate with a masters of science degree. According to the American Association of Physician Assistants (AAPA), the scope of practice is guided by state law, facility policy, and delegatory decisions made by the supervising physician.6 Prior experience and training should be the framework for scope of practice decisions. All 50 states allow PAs to prescribe with some oversight and restriction of schedule 2 controlled substances or by using a state formulary. The AAPA embraces the concept of the physician as the captain of the healthcare team and sees the PA role as entirely complementary to the care provided by physicians.7 This means that PAs, under an individual supervision agreement, can prescribe medicines, order and interpret tests, diagnose, and treat patients just as a physician would.
Advanced Practice Nurses
Advanced practice nurses (APNs) are trained under the nursing model and generally have some years of nursing experience before they pursue an entry‐level masters of science degree to become an APN. APNs can be divided into two categories: Clinical nurse specialists, who generally focus on patient and institutional education and are considered experts in nursing practice, and NPs, who have a focus on diagnosis and treatment of medical conditions. A clinical nurse specialist does not have prescriptive training or authority. NP training can be general (adult or family) or specific (eg, acute care, geriatric, pediatric, psychiatric). The American Association of Colleges of Nursing (AACN) has recommended that the entry level of all new NPs should be a clinical doctorate of nursing practice. Although controversial, many colleges have embraced this recommendation and are opening clinical doctorate‐level programs.8 Although some states allow NPs to practice independently, most NPs have a practice agreement with a collaborating physician that delineates the degree of supervision. Generally, the NP's scope of practice is identical to PAs and includes the above‐mentioned activities as proscribed by state regulations and facility bylaws. As with PAs, their prior experience and training should be the most important determinant of their scope of practice in a new position.
Potential Benefits of NPPs
Continuity
If a nonacademic hospitalist program has high yearly turnover due to use of recent medical graduates who are planning to do fellowships, NPPs can provide much needed stability and facilitate orientation of new physicians to the hospital. NPPs who work in academic settings can also provide increased continuity for patients and hospital staff. Residents, fellows, and attendings have certain rotational cycles on each medical service. NPPs generally do not rotate and can be the anchor of a medical team for patients and ancillary staff. Utilizing NPPs as liaisons between the hospitalist team and other members of the care team (eg, nurses, case managers, therapists, and administration) provides continuity for these groups and a central person who can help to facilitate change.
Quality Measures
NPPs can play an important role in hospital compliance with internal hospital or insurance provider quality initiatives. Surveillance of patients and charts for compliance with core measures, infection control, and prevention of complications are within the scope of practice of NPPs and can be incorporated into job descriptions. NPs and PAs will have the added responsibility of not only leading these surveillance teams but also in the correction of outliers given their prescriptive abilities. This will become an increasingly important task as reimbursement for preventable complications is curtailed. Additionally, the development and implementation of clinical pathways can be a focus of the NPP role to standardize and enhance quality of care.
Multidisciplinary Team Approach
Multidisciplinary teams that consist of NPPs, physicians, nurses, and therapists have been shown to increase communication and collaboration between participants.9 Mary Naylor, a Professor of Nursing at the University of Pennsylvania, has authored multiple articles and studies which examine the benefit of a multidisciplinary team that includes APNs with hospitalized patients. She has found that involving APNs in patient care, discharges, and routine follow‐up after discharge led to longer time to readmissions and decreased healthcare costs.1012 Furthermore, a nonteaching group consisting of NPPs, fellows, and attendings at the Mayo Clinic found increased physician satisfaction, shorter length of stay (LOS), and increased efficiency for their patients.13 A study done at JFK Medical Center in Florida noted that a collaborative practice which included unit‐based NPs serving in the dual role of NP and clinical nurse specialist increased patient satisfaction and improved patient outcomes.14
Financial Advantages
Efficiency and quality care are the cornerstones of HM. The partnership of NPPs within the specialty is creating even better performance. Models incorporating NPPs in the Hospitalist team approach are continuing to drive efficiency. Cowan et al.15 demonstrated that a multidisciplinary team, including nurse practitioners, decreased LOS from 6.01 to 5.0 and a reduced cost by $1,591 per patient. It is this team approach that will lift our specialty to be the model of care for all future hospital practice.
Another factor in determining the fiscal advantage of NPPs is salary and medical liability comparison. According to the 2007 Society of Hospital Medicine (SHM) Survey, the average hospitalist salary is approaching $190,000, compared to an average NP earning $87,000 and PA earning $84,500.4 Furthermore, the average internal medicine malpractice payment for physicians ranges from $14,237 to $68,867.16 In comparison, the average malpractice insurance premium for NPPs varies from state to state but is approximately $800 to $2000 per year.17, 18 With increasing fiscal scrutiny from hospitals, HM groups (HMGs) will need to include NPPs to be fiscally stable.
Models of Care
There are many models for NPP roles in hospital medicine groups. Some groups use NPPs in the same role as physicians. They perform admissions, rounding, and discharges with varying degrees of oversight by physicians. Other groups use NPPs for a more limited role, such as exclusively performing histories and physicals in the emergency department or handling discharges on the wards. It is important to take into account the preferences and expectations of NPPs when designing job descriptions. While some NPPs may like the fast pace and quick turnover of admissions and discharges, others may prefer to follow patients throughout their hospital stay. The quality of handoffs is crucial if the former model is used, just as it is with physicians in this more truncated role. An NPP who works in a nonacademic model will likely have more autonomy and control over patient care decisions. An NPP role in the teaching service of an academic hospital is likely to be more collaborative and focus more on quality initiatives, patient teaching, and communication. It is crucial to design an NPP model that is sustainable with very strong support of management once the NPP is hired and orientated.
Registered Nurses And Hospital Medicine
Patient handoffs and communication are one of the most challenging aspects of an HMG. There is an increasing movement, throughout the country, to incorporate registered nurses (RNs) into daily workflow. The RN on the HM team can serve to augment the communication and workflow process. A highly motivated and organized registered nurse can help to improve overall provider's workflow efficiency. Communication to primary care physician and collecting ancillary medical information can allow the provider to treat more patients in a given shift and decrease the liability risk from lack of information. As HM organizations and hospitals become more financially bound, HMGs will need to become more efficient at time management and a dedicated RN can help smooth that process.
Potential Unintended Side Effects
Obviously, integration of NPPs can be a disaster for an HMG if not handled properly. Most hospitalists have heard of an integration of NPP into a group that was an unqualified failure. NPPs can feel unsupported, poorly oriented to the job, or thrown into a situation that is over their heads. Before an NPP is hired into an HMG, there needs to be a thorough examination of the rationale behind the decision and assessment of the hospital culture that will be the host of the new NPP. What does the HMG need for support? Are they looking for a short‐term fix for increased volume or a long‐term strategy to build a multidisciplinary team? Does the hospital culture see NPPs as poorly qualified to act as hospitalists or uniquely qualified to address shortcomings of the program? A clear job description should be the first step in determining what the NPP is expected to do. This can then be shared with the hospital leadership in advance to promote buy‐in. The second step is finding an NPP that fits the goals of the program. A new NPP, by virtue of the fact that they have less clinical hours in training than a physician hospitalist, will need more support and a longer orientation. NPPs who have experience in hospital medicine will have a much shorter orientation. A stepwise approach to orientation can be helpful in assessing skill level of new hires. These NPPs can be initially paired with an enthusiastic physician to provide support and assessment of existing skills. A gradual increase in independence can provide assurance that the NPP is qualified to provide care and gives many opportunities for reevaluation of the NPP. Clear expectations and constructive feedback should ultimately lead to a degree of comfort within the HMG, hospital, and the NPPs themselves.
Conclusions
It is clear that our healthcare system will need a very different approach to the economic problems it is facing. Standardization of care, integrated medical records, and expanded and universal resource utilization will drive the next generation of healthcare providers. The model of a private physician working alone under the direction of only his or her own medical knowledge is a thing of the past. Just as the HM specialty has grown from 300 in 1996 to more than 20,000 in 2008, so shall the integration of NPPs grow into our healthcare fabric.
- Centers for Disease Control and Prevention (CDC). Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52(6):101–104, 106.
- Council on Graduate Medical Education. Physician Workforce Policy Guidelines for the U.S. for 2000‐2020. Rockville, MD: U.S. Department of Health and Human Services;2005.
- American Medical Association. Medical Student Section. Advocacy and Policy. Medical Student Debt. Available at: http://www.ama‐assn.org/ama/pub/category/5349.html. Accessed June 2009.
- Society of Hospital Medicine (SHM). 2007‐2008 SHM Survey: State of the Hospital Medicine Movement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2
- Centers for Disease Control and Prevention (CDC). Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52(6):101–104, 106.
- Council on Graduate Medical Education. Physician Workforce Policy Guidelines for the U.S. for 2000‐2020. Rockville, MD: U.S. Department of Health and Human Services;2005.
- American Medical Association. Medical Student Section. Advocacy and Policy. Medical Student Debt. Available at: http://www.ama‐assn.org/ama/pub/category/5349.html. Accessed June 2009.
- Society of Hospital Medicine (SHM). 2007‐2008 SHM Survey: State of the Hospital Medicine Movement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2
Nonphysicians in Hospital Medicine
Ford and Britting's1 editorial in this month's Journal of Hospital Medicine raises important questions concerning the use of nonphysician providers in hospital medicine. They focus primarily on the use of mid‐level providers (MLPs), namely physician‐assistants (PAs) and nurse practitioners (NPs), as a potential solution to the current physician workforce shortages in our field. While we acknowledge the challenges of meeting workforce needs, we also believe that much is unknown about the optimal use of MLPs on inpatient general medicine services and it is premature to tout MLPs as the solution to hospital medicine staffing problems. This is especially true in those hospitals where hospitalists care for complex, general medical patients with a wide variety of medical conditions, a trend that is especially common in academic medical centers.2
This article discusses the current literature, our own experiences with MLPs, and suggests some future initiatives that might help better integrate MLPs into hospital medicine.
The Literature on MLPs in Inpatient Venues
The existing literature on the use of MLPs in inpatient venues is quite limited, and a recent review, while suggesting that the existing literature does describe benefits of MLPs in the inpatient setting, also states that the overall quality of the evidence is quite poor and that many studies suffer from significant limitations, including small populations, limited patient mixes, use of selected settings, and short durations of outcome assessment.3
Ford and Britting,1 in their article, cite several studies46 as evidence that a MLP model of care either improved outcomes or provided cost benefits. Each of these studies has important limitations that are worth examining.
The study by Myers et al.4 described the use of MLPs in a chest pain unit. NPs partnered with hospitalists to care for a low‐acuity chest pain population. In addition, 5 NPs only staffed the unit during daytime weekday hours. Off‐hour and weekend staffing was accomplished through the use of resident physicians. Notably, the work suggests the service only admitted 113 low‐risk patients over 10 months. The service was staffed by 3 full‐time equivalent (FTE) NPs in addition to involving hospitalists during the day. It is not surprising, given the extremely low volume of patients coupled with a daytime‐only focus, that this service showed efficiency gains. In addition, given the service was only staffed by NPs 40 hours a week and by resident physicians on nights and weekends, the true cost of such an intervention needs to take into account the full cost of 24/7 coverage. In addition, the model of using residents to cover nonteaching patients is no longer permitted by the current Accreditation Council for Graduate Medical Education (ACGME) Internal Medicine Residency Requirements7 and thus implementation of a model such as this in 2009 would require alternative means of nighttime coverage.
The study by Nishimura et al.,5 also describing the use of MLPs in cardiovascular care, has important caveats that make full assessment of the model impossible. The model describes the implementation of a care team consisting of an attending, a fellow, and MLPs to replace a traditional teaching team of an attending, senior resident, and 2 interns. The study states that the model resulted in a lower length of stay (LOS) and lower costs per case. Importantly, the new MLP‐based team only admitted during the hours of 7 AM to 2 PM. The study does not fully describe the number of MLPs required nor does it fully describe the role of cardiovascular fellows in the model. The study does state that the cost savings offset the cost of the MLPs but it is not clear if this cost analysis took into account the cost of the fellow's daytime involvement or if it measured attending time required before and after the implementation of the new model. In addition, this model presumes the availability of other services to admit patients during afternoon and nighttime hours and so may not be generalizable to other settings.
The final study by Cowan et al.6 describes the addition of a NP, a hospitalist medical director, and daily multidisciplinary rounds to a traditional teaching service model. Importantly, the NP was not involved in the admission process nor were they the primary providers for day‐to‐day medical care but rather they focused on implementation of care protocols, multidisciplinary coordination of care and discharge planning, and postdischarge follow‐up. In addition, the NP worked only weekdays for about 40 hours a week. It is not surprising that adding multiple additional resources to existing care models might provide benefits but this does not address any issues in terms of the workforce since the care in this model required a higher total input of providers than the usual care model being studied. Cost savings from such a model may make it cost‐effective but it does not represent a workforce solution.
There have been other studies examining the use of MLPs in the inpatient setting in internal medicine. Some of these studies have suggested that MLP‐based models result in equivalent outcomes and efficiency810 to traditional teaching or nonteaching physician‐only models. There are 2 important caveats, however, that must be considered. The total resources required for such models may be quite high, especially taking into account the costs of 24/7 coverage and physician backup of the MLPs, and most importantly there is almost no literature that robustly examines ultimate clinical outcomes in these models. We do note that a recent study11 did show a lower inpatient mortality rate over a 2‐year period of time after substituting a PA‐hospitalist model for a traditional academic medicine residency model in a community hospital. Importantly, however, the new model also added 24/7 hospitalist physicians and night and weekend intensivists that were not present in the prior residency‐based model. Thus, the lower mortality rate could be attributed to the addition of hospitalists or the more robust in‐house physician coverage during off‐hours rather than the use of MLPs.
Notably, while the evidence base in internal medicine is not robust, many studies have described successful use of MLPs in non‐internal medicine inpatient settings.1214 The reasons for this success is debatable, but it may be that MLPs are more successful in settings where the care is either more protocol‐driven or where there is less diagnostic and therapeutic complexity.
Recent Experiences with MLPs in Academic Hospital Medicine
Given the paucity of data, it is clear that further research is needed on the role of MLPs in hospital medicine. While waiting for such evidence to appear, it may be worthwhile to reflect on the recent experience of 3 major medical centers. A recent article described 5 hospitalist models at major academic medical centers across the country. Two of the institutions described at the time (University of Michigan Health System, Ann Arbor, MI; and Brigham and Women's Hospital, Boston, MA) utilized MLPs as a major element of their staffing of nonresident hospitalist services while another (University of California, San Francisco [UCSF] Medical Center at Mt. Zion, San Francisco, CA) had previously used MLPs as part of its model but phased them out about 1 year prior to publication of the article.2 The model used by the Brigham and Women's Hospital was later described in more detail in a subsequent publication.8 Recently 1 of these institutions (Michigan) has chosen to phase out MLPs. At Michigan, a 4‐year experience with PAs on a general‐medicine focused hospitalist service eventually led to the conclusion that continued use of PAs was not cost‐effective. Significant barriers to success included a steep learning curve and the significant time required before PAs developed sufficient autonomy and efficiency in caring for a highly complex heterogeneous patient population. In the Michigan experience, PAs took up to 2 years to attain a significant level of autonomy and efficiency and even then some PAs still required a significant amount of physician oversight. Similar concerns at UCSF Mt. Zion led to the elimination of their MLP program as well. At Brigham and Women's, the MLP service continues but has required additional hospitalist staffing due to difficulties recruiting qualified MLPs with appropriate inpatient experience. In all cases, the models were challenged by high costs and the difficulty of developing MLPs to attain the level of autonomy and efficiency needed to justify their continued use. A key point is that in each institution, MLPs continue to play an important role in some specialty inpatient areas such as Hematology/Oncology and Bone Marrow Transplant, which is where MLPs have traditionally found their niche in inpatient Internal Medicine. These focus shops allow MLPs to develop a niche and expertise in a specialized area, where they may become more autonomous and efficient than house staff. Thus these settings may be more appropriate for MLPs than a heterogeneous general medicine inpatient setting.
Reviewing the Financial Case
In their article, Ford and Britting1 cite potential financial advantages for the use of MLPs in hospital medicine by comparing the relative salaries of MLPs to Hospitalists. What was missing in their analysis was the relative productivity of the 2 types of providers. We do have some limited data from the Society of Hospital Medicine (SHM) annual survey that looks at MLPs in hospital medicine but, again, the number of respondents for most data elements is less than 70, making generalizability difficult. Nonetheless, the data suggest that MLPs in hospital medicine average about 60% to 75% of the productivity of a physician when measured by encounters, although there is wide variability depending on the employment model (academic vs. multispecialty group).15 Importantly, the existing data do not provide any measure of how much physician input is provided to these MLPs but we suspect that in most models there is some physician time and input. If we presume that the MLPs bill independently and collect 85% of the physician fee schedule for a Medicare population, then collections would be about 50% to 65% of a typical physician. Given that median total compensation including benefits from the SHM survey was $120,000 for MLPs and $216,000 for physiciansabout a 55% ratiothis would argue for potential financial neutrality when substituting MLPs for physicians in a 2:1 ratio but only if we presume they require no physician supervision, which in our own experience is not likely in a general medicine population. In an alternative model, in which the physician sees every patient with the MLP and the physician bills, one would need to see roughly 50% more patients to achieve a financially neutral situation. In our experience at our own institutions, this level of increased productivity was not achievable. It is important to note that our figures are median compensation and benefit cost figures and local markets vary widely. We know that in major east and west coast cities MLPs may command far higher salaries while early career hospitalist physicians may be paid somewhat less than the reported medians. Recent market changes have significantly pressured MLP salaries,15, 16 further impacting the financial equation and perhaps tilting it farther against a financial benefit for MLPs. Furthermore, night coverage for MLP services should always be considered in a financial analysis and is not captured in this simple analysis.
Next Steps
Given the current shortage of physicians, we imagine that many hospitalist groups will consider the use of MLPs as a solution to the current workforce issues. However, data on how best to utilize MLPs and the true impact on both the cost and quality of such models is lacking. In addition to urging increased publication and dissemination of existing experiences with NP and PAs, we strongly suggest that groups considering starting a MLP model do so in a way which would facilitate robust analysis and comparison of the model with alternatives. We also suggest that SHM consider the following: modifying its biennial survey to better capture the nuances of MLP productivity (such as assessing the amount of physician input and supervision required); targeting MLPs so as to increase the number of respondents; and doing an additional survey to capture demographics and basic data on existing MLP models given the lack of published literature.
In addition to gathering more data on effective models, a critical gap that we have identified is the development of models for the training and development of MLPs interested in hospital medicine. It would be a mistake to believe that MLPs could function in a manner similar to residency‐trained physicians if they do not undergo similar training. NP/PA programs generally do not have a significant inpatient internal medicine focus and so newly minted graduates often lack the skills needed to succeed in hospital medicine.17 Some hospitalist programs train their MLPs on the job, but many programs cannot afford the amount of time and effort required to do this on their own. There are a small number of advanced training options for MLPs in hospital medicine18 but it is not likely such models will proliferate given the inherent opportunity costs that exist for extended training in the current competitive job market for MLPs. Instead we think that very motivated hospital medicine groups may develop training relationships with PA and NP schools in an effort to train their own. In addition, national initiatives such as the Hospital Medicine Boot Camp for NPs and PAs, which is cosponsored by SHM, the American Association of Physician Assistants (AAPA), and the American Academy of Nurse Practitioners (AANP),19 can help fill the educational needs for MLPs who are already in practice.
Conclusions
While some literature exists that suggests that MLPs can successfully be used in the inpatient internal medicine setting, it is important to note that the evidence is quite limited and cannot be generalized across all care settings and patient populations. There is an urgent need to gather more data and share our collective experiences to better inform our decision‐making before we state that MLPs are the solution to workforce shortages in hospital medicine. In addition, existing data and experience suggest that MLPs may not be a cost‐effective workforce solution for complex general medical patients who require significant physician input. We believe that redesigning the clinical training of MLPs to focus on inpatient skills may hold promise and encourage interested parties to consider developing partnerships with MLP training programs and hospital medicine groups, as a way to build a more robust and successful hospital medicine MLP workforce.
- ,.Nonphysician providers in the hospitalist model: a prescription for change and a warning about unintended side effects.J Hosp Med.2010;5:99–102.
- ,,,,.Non‐housestaff medicine services in academic medical centers: models and challenges.J Hosp Med.2008;3:247–255.
- ,,.Nurse practitioners and physician assistants in the intensive care unit: an evidence‐based review.Crit Care Med.2008;36:2888–2897.
- ,,.Improving resource utilization in a teaching hospital: development of a nonteaching service for chest pain admissions.Acad Med.2006;81:432–435.
- ,,,,,.A nonresident cardiovascular inpatient service improves residents' experiences in an academic medical center: a new model to meet the challenges of the new millennium.Acad Med.2004;79;426–431.
- .The effect of a multidisciplinary hospitalist/physician and advance practice nurse collaboration on hospital care.J Nurs Adm.2006;36:79–85.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Residency Education in Internal Medicine. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140_internal_ medicine_07012009.pdf. Accessed July2009.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3:361–368.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–38.
- ,,,, et al.Outcomes‐based trial of an inpatient nurse practitioner service for general medical patients.J Eval Clin Pract.2001;7:21–33.
- ,.Replacing an academic internal medicine residency program with a physician assistant‐hospitalist model: a comparative analysis study.Am J Med Qual.2009;2:132–139.
- ,,,,.Integrating midlevel practitioners into a teaching service.Am J Surg.2006;1:119–124.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,.Physician assistants in cardiothoracic surgery: a 30‐year experience in a university center.Ann Thorac Surg.2006;1:195–199.
- 2007–2008 Society of Hospital Medicine Bi‐Annual Survey: the Authoritative Source on the State of the Hospital Medicine Movement.Philadelphia:Society of Hospital Medicine;2008.
- American Association of Physician Assistants. Physician Assistant Income. Available at: http://www.aapa.org/images/stories/iu08incchange. pdf. Accessed July2009.
- Accreditation Review Commission on Education for the Physician Assistant. Accreditation Standards for Physician Assistant Education, 3rd ed. Available at: http://www.arcpa.org/Standards/3rdeditionwithPDchangesandregionals4.24.08a.pdf. Accessed July2009.
- Association of Postgraduate PA Programs. Postgraduate PA Program Listing by State. Available at: http://www.appap.org/index1.html. Accessed July2009.
- American Association of Physician Assistants. Adult Hospitalist Physician Assistant and Nurse Practitioner Boot Camp. Available at: http://www. aapa.org/component/content/article/23‐‐general‐/673‐adult‐hospitalist‐physician‐assistant‐and‐nurse‐practitioner‐boot‐camp. Accessed July2009.
Ford and Britting's1 editorial in this month's Journal of Hospital Medicine raises important questions concerning the use of nonphysician providers in hospital medicine. They focus primarily on the use of mid‐level providers (MLPs), namely physician‐assistants (PAs) and nurse practitioners (NPs), as a potential solution to the current physician workforce shortages in our field. While we acknowledge the challenges of meeting workforce needs, we also believe that much is unknown about the optimal use of MLPs on inpatient general medicine services and it is premature to tout MLPs as the solution to hospital medicine staffing problems. This is especially true in those hospitals where hospitalists care for complex, general medical patients with a wide variety of medical conditions, a trend that is especially common in academic medical centers.2
This article discusses the current literature, our own experiences with MLPs, and suggests some future initiatives that might help better integrate MLPs into hospital medicine.
The Literature on MLPs in Inpatient Venues
The existing literature on the use of MLPs in inpatient venues is quite limited, and a recent review, while suggesting that the existing literature does describe benefits of MLPs in the inpatient setting, also states that the overall quality of the evidence is quite poor and that many studies suffer from significant limitations, including small populations, limited patient mixes, use of selected settings, and short durations of outcome assessment.3
Ford and Britting,1 in their article, cite several studies46 as evidence that a MLP model of care either improved outcomes or provided cost benefits. Each of these studies has important limitations that are worth examining.
The study by Myers et al.4 described the use of MLPs in a chest pain unit. NPs partnered with hospitalists to care for a low‐acuity chest pain population. In addition, 5 NPs only staffed the unit during daytime weekday hours. Off‐hour and weekend staffing was accomplished through the use of resident physicians. Notably, the work suggests the service only admitted 113 low‐risk patients over 10 months. The service was staffed by 3 full‐time equivalent (FTE) NPs in addition to involving hospitalists during the day. It is not surprising, given the extremely low volume of patients coupled with a daytime‐only focus, that this service showed efficiency gains. In addition, given the service was only staffed by NPs 40 hours a week and by resident physicians on nights and weekends, the true cost of such an intervention needs to take into account the full cost of 24/7 coverage. In addition, the model of using residents to cover nonteaching patients is no longer permitted by the current Accreditation Council for Graduate Medical Education (ACGME) Internal Medicine Residency Requirements7 and thus implementation of a model such as this in 2009 would require alternative means of nighttime coverage.
The study by Nishimura et al.,5 also describing the use of MLPs in cardiovascular care, has important caveats that make full assessment of the model impossible. The model describes the implementation of a care team consisting of an attending, a fellow, and MLPs to replace a traditional teaching team of an attending, senior resident, and 2 interns. The study states that the model resulted in a lower length of stay (LOS) and lower costs per case. Importantly, the new MLP‐based team only admitted during the hours of 7 AM to 2 PM. The study does not fully describe the number of MLPs required nor does it fully describe the role of cardiovascular fellows in the model. The study does state that the cost savings offset the cost of the MLPs but it is not clear if this cost analysis took into account the cost of the fellow's daytime involvement or if it measured attending time required before and after the implementation of the new model. In addition, this model presumes the availability of other services to admit patients during afternoon and nighttime hours and so may not be generalizable to other settings.
The final study by Cowan et al.6 describes the addition of a NP, a hospitalist medical director, and daily multidisciplinary rounds to a traditional teaching service model. Importantly, the NP was not involved in the admission process nor were they the primary providers for day‐to‐day medical care but rather they focused on implementation of care protocols, multidisciplinary coordination of care and discharge planning, and postdischarge follow‐up. In addition, the NP worked only weekdays for about 40 hours a week. It is not surprising that adding multiple additional resources to existing care models might provide benefits but this does not address any issues in terms of the workforce since the care in this model required a higher total input of providers than the usual care model being studied. Cost savings from such a model may make it cost‐effective but it does not represent a workforce solution.
There have been other studies examining the use of MLPs in the inpatient setting in internal medicine. Some of these studies have suggested that MLP‐based models result in equivalent outcomes and efficiency810 to traditional teaching or nonteaching physician‐only models. There are 2 important caveats, however, that must be considered. The total resources required for such models may be quite high, especially taking into account the costs of 24/7 coverage and physician backup of the MLPs, and most importantly there is almost no literature that robustly examines ultimate clinical outcomes in these models. We do note that a recent study11 did show a lower inpatient mortality rate over a 2‐year period of time after substituting a PA‐hospitalist model for a traditional academic medicine residency model in a community hospital. Importantly, however, the new model also added 24/7 hospitalist physicians and night and weekend intensivists that were not present in the prior residency‐based model. Thus, the lower mortality rate could be attributed to the addition of hospitalists or the more robust in‐house physician coverage during off‐hours rather than the use of MLPs.
Notably, while the evidence base in internal medicine is not robust, many studies have described successful use of MLPs in non‐internal medicine inpatient settings.1214 The reasons for this success is debatable, but it may be that MLPs are more successful in settings where the care is either more protocol‐driven or where there is less diagnostic and therapeutic complexity.
Recent Experiences with MLPs in Academic Hospital Medicine
Given the paucity of data, it is clear that further research is needed on the role of MLPs in hospital medicine. While waiting for such evidence to appear, it may be worthwhile to reflect on the recent experience of 3 major medical centers. A recent article described 5 hospitalist models at major academic medical centers across the country. Two of the institutions described at the time (University of Michigan Health System, Ann Arbor, MI; and Brigham and Women's Hospital, Boston, MA) utilized MLPs as a major element of their staffing of nonresident hospitalist services while another (University of California, San Francisco [UCSF] Medical Center at Mt. Zion, San Francisco, CA) had previously used MLPs as part of its model but phased them out about 1 year prior to publication of the article.2 The model used by the Brigham and Women's Hospital was later described in more detail in a subsequent publication.8 Recently 1 of these institutions (Michigan) has chosen to phase out MLPs. At Michigan, a 4‐year experience with PAs on a general‐medicine focused hospitalist service eventually led to the conclusion that continued use of PAs was not cost‐effective. Significant barriers to success included a steep learning curve and the significant time required before PAs developed sufficient autonomy and efficiency in caring for a highly complex heterogeneous patient population. In the Michigan experience, PAs took up to 2 years to attain a significant level of autonomy and efficiency and even then some PAs still required a significant amount of physician oversight. Similar concerns at UCSF Mt. Zion led to the elimination of their MLP program as well. At Brigham and Women's, the MLP service continues but has required additional hospitalist staffing due to difficulties recruiting qualified MLPs with appropriate inpatient experience. In all cases, the models were challenged by high costs and the difficulty of developing MLPs to attain the level of autonomy and efficiency needed to justify their continued use. A key point is that in each institution, MLPs continue to play an important role in some specialty inpatient areas such as Hematology/Oncology and Bone Marrow Transplant, which is where MLPs have traditionally found their niche in inpatient Internal Medicine. These focus shops allow MLPs to develop a niche and expertise in a specialized area, where they may become more autonomous and efficient than house staff. Thus these settings may be more appropriate for MLPs than a heterogeneous general medicine inpatient setting.
Reviewing the Financial Case
In their article, Ford and Britting1 cite potential financial advantages for the use of MLPs in hospital medicine by comparing the relative salaries of MLPs to Hospitalists. What was missing in their analysis was the relative productivity of the 2 types of providers. We do have some limited data from the Society of Hospital Medicine (SHM) annual survey that looks at MLPs in hospital medicine but, again, the number of respondents for most data elements is less than 70, making generalizability difficult. Nonetheless, the data suggest that MLPs in hospital medicine average about 60% to 75% of the productivity of a physician when measured by encounters, although there is wide variability depending on the employment model (academic vs. multispecialty group).15 Importantly, the existing data do not provide any measure of how much physician input is provided to these MLPs but we suspect that in most models there is some physician time and input. If we presume that the MLPs bill independently and collect 85% of the physician fee schedule for a Medicare population, then collections would be about 50% to 65% of a typical physician. Given that median total compensation including benefits from the SHM survey was $120,000 for MLPs and $216,000 for physiciansabout a 55% ratiothis would argue for potential financial neutrality when substituting MLPs for physicians in a 2:1 ratio but only if we presume they require no physician supervision, which in our own experience is not likely in a general medicine population. In an alternative model, in which the physician sees every patient with the MLP and the physician bills, one would need to see roughly 50% more patients to achieve a financially neutral situation. In our experience at our own institutions, this level of increased productivity was not achievable. It is important to note that our figures are median compensation and benefit cost figures and local markets vary widely. We know that in major east and west coast cities MLPs may command far higher salaries while early career hospitalist physicians may be paid somewhat less than the reported medians. Recent market changes have significantly pressured MLP salaries,15, 16 further impacting the financial equation and perhaps tilting it farther against a financial benefit for MLPs. Furthermore, night coverage for MLP services should always be considered in a financial analysis and is not captured in this simple analysis.
Next Steps
Given the current shortage of physicians, we imagine that many hospitalist groups will consider the use of MLPs as a solution to the current workforce issues. However, data on how best to utilize MLPs and the true impact on both the cost and quality of such models is lacking. In addition to urging increased publication and dissemination of existing experiences with NP and PAs, we strongly suggest that groups considering starting a MLP model do so in a way which would facilitate robust analysis and comparison of the model with alternatives. We also suggest that SHM consider the following: modifying its biennial survey to better capture the nuances of MLP productivity (such as assessing the amount of physician input and supervision required); targeting MLPs so as to increase the number of respondents; and doing an additional survey to capture demographics and basic data on existing MLP models given the lack of published literature.
In addition to gathering more data on effective models, a critical gap that we have identified is the development of models for the training and development of MLPs interested in hospital medicine. It would be a mistake to believe that MLPs could function in a manner similar to residency‐trained physicians if they do not undergo similar training. NP/PA programs generally do not have a significant inpatient internal medicine focus and so newly minted graduates often lack the skills needed to succeed in hospital medicine.17 Some hospitalist programs train their MLPs on the job, but many programs cannot afford the amount of time and effort required to do this on their own. There are a small number of advanced training options for MLPs in hospital medicine18 but it is not likely such models will proliferate given the inherent opportunity costs that exist for extended training in the current competitive job market for MLPs. Instead we think that very motivated hospital medicine groups may develop training relationships with PA and NP schools in an effort to train their own. In addition, national initiatives such as the Hospital Medicine Boot Camp for NPs and PAs, which is cosponsored by SHM, the American Association of Physician Assistants (AAPA), and the American Academy of Nurse Practitioners (AANP),19 can help fill the educational needs for MLPs who are already in practice.
Conclusions
While some literature exists that suggests that MLPs can successfully be used in the inpatient internal medicine setting, it is important to note that the evidence is quite limited and cannot be generalized across all care settings and patient populations. There is an urgent need to gather more data and share our collective experiences to better inform our decision‐making before we state that MLPs are the solution to workforce shortages in hospital medicine. In addition, existing data and experience suggest that MLPs may not be a cost‐effective workforce solution for complex general medical patients who require significant physician input. We believe that redesigning the clinical training of MLPs to focus on inpatient skills may hold promise and encourage interested parties to consider developing partnerships with MLP training programs and hospital medicine groups, as a way to build a more robust and successful hospital medicine MLP workforce.
Ford and Britting's1 editorial in this month's Journal of Hospital Medicine raises important questions concerning the use of nonphysician providers in hospital medicine. They focus primarily on the use of mid‐level providers (MLPs), namely physician‐assistants (PAs) and nurse practitioners (NPs), as a potential solution to the current physician workforce shortages in our field. While we acknowledge the challenges of meeting workforce needs, we also believe that much is unknown about the optimal use of MLPs on inpatient general medicine services and it is premature to tout MLPs as the solution to hospital medicine staffing problems. This is especially true in those hospitals where hospitalists care for complex, general medical patients with a wide variety of medical conditions, a trend that is especially common in academic medical centers.2
This article discusses the current literature, our own experiences with MLPs, and suggests some future initiatives that might help better integrate MLPs into hospital medicine.
The Literature on MLPs in Inpatient Venues
The existing literature on the use of MLPs in inpatient venues is quite limited, and a recent review, while suggesting that the existing literature does describe benefits of MLPs in the inpatient setting, also states that the overall quality of the evidence is quite poor and that many studies suffer from significant limitations, including small populations, limited patient mixes, use of selected settings, and short durations of outcome assessment.3
Ford and Britting,1 in their article, cite several studies46 as evidence that a MLP model of care either improved outcomes or provided cost benefits. Each of these studies has important limitations that are worth examining.
The study by Myers et al.4 described the use of MLPs in a chest pain unit. NPs partnered with hospitalists to care for a low‐acuity chest pain population. In addition, 5 NPs only staffed the unit during daytime weekday hours. Off‐hour and weekend staffing was accomplished through the use of resident physicians. Notably, the work suggests the service only admitted 113 low‐risk patients over 10 months. The service was staffed by 3 full‐time equivalent (FTE) NPs in addition to involving hospitalists during the day. It is not surprising, given the extremely low volume of patients coupled with a daytime‐only focus, that this service showed efficiency gains. In addition, given the service was only staffed by NPs 40 hours a week and by resident physicians on nights and weekends, the true cost of such an intervention needs to take into account the full cost of 24/7 coverage. In addition, the model of using residents to cover nonteaching patients is no longer permitted by the current Accreditation Council for Graduate Medical Education (ACGME) Internal Medicine Residency Requirements7 and thus implementation of a model such as this in 2009 would require alternative means of nighttime coverage.
The study by Nishimura et al.,5 also describing the use of MLPs in cardiovascular care, has important caveats that make full assessment of the model impossible. The model describes the implementation of a care team consisting of an attending, a fellow, and MLPs to replace a traditional teaching team of an attending, senior resident, and 2 interns. The study states that the model resulted in a lower length of stay (LOS) and lower costs per case. Importantly, the new MLP‐based team only admitted during the hours of 7 AM to 2 PM. The study does not fully describe the number of MLPs required nor does it fully describe the role of cardiovascular fellows in the model. The study does state that the cost savings offset the cost of the MLPs but it is not clear if this cost analysis took into account the cost of the fellow's daytime involvement or if it measured attending time required before and after the implementation of the new model. In addition, this model presumes the availability of other services to admit patients during afternoon and nighttime hours and so may not be generalizable to other settings.
The final study by Cowan et al.6 describes the addition of a NP, a hospitalist medical director, and daily multidisciplinary rounds to a traditional teaching service model. Importantly, the NP was not involved in the admission process nor were they the primary providers for day‐to‐day medical care but rather they focused on implementation of care protocols, multidisciplinary coordination of care and discharge planning, and postdischarge follow‐up. In addition, the NP worked only weekdays for about 40 hours a week. It is not surprising that adding multiple additional resources to existing care models might provide benefits but this does not address any issues in terms of the workforce since the care in this model required a higher total input of providers than the usual care model being studied. Cost savings from such a model may make it cost‐effective but it does not represent a workforce solution.
There have been other studies examining the use of MLPs in the inpatient setting in internal medicine. Some of these studies have suggested that MLP‐based models result in equivalent outcomes and efficiency810 to traditional teaching or nonteaching physician‐only models. There are 2 important caveats, however, that must be considered. The total resources required for such models may be quite high, especially taking into account the costs of 24/7 coverage and physician backup of the MLPs, and most importantly there is almost no literature that robustly examines ultimate clinical outcomes in these models. We do note that a recent study11 did show a lower inpatient mortality rate over a 2‐year period of time after substituting a PA‐hospitalist model for a traditional academic medicine residency model in a community hospital. Importantly, however, the new model also added 24/7 hospitalist physicians and night and weekend intensivists that were not present in the prior residency‐based model. Thus, the lower mortality rate could be attributed to the addition of hospitalists or the more robust in‐house physician coverage during off‐hours rather than the use of MLPs.
Notably, while the evidence base in internal medicine is not robust, many studies have described successful use of MLPs in non‐internal medicine inpatient settings.1214 The reasons for this success is debatable, but it may be that MLPs are more successful in settings where the care is either more protocol‐driven or where there is less diagnostic and therapeutic complexity.
Recent Experiences with MLPs in Academic Hospital Medicine
Given the paucity of data, it is clear that further research is needed on the role of MLPs in hospital medicine. While waiting for such evidence to appear, it may be worthwhile to reflect on the recent experience of 3 major medical centers. A recent article described 5 hospitalist models at major academic medical centers across the country. Two of the institutions described at the time (University of Michigan Health System, Ann Arbor, MI; and Brigham and Women's Hospital, Boston, MA) utilized MLPs as a major element of their staffing of nonresident hospitalist services while another (University of California, San Francisco [UCSF] Medical Center at Mt. Zion, San Francisco, CA) had previously used MLPs as part of its model but phased them out about 1 year prior to publication of the article.2 The model used by the Brigham and Women's Hospital was later described in more detail in a subsequent publication.8 Recently 1 of these institutions (Michigan) has chosen to phase out MLPs. At Michigan, a 4‐year experience with PAs on a general‐medicine focused hospitalist service eventually led to the conclusion that continued use of PAs was not cost‐effective. Significant barriers to success included a steep learning curve and the significant time required before PAs developed sufficient autonomy and efficiency in caring for a highly complex heterogeneous patient population. In the Michigan experience, PAs took up to 2 years to attain a significant level of autonomy and efficiency and even then some PAs still required a significant amount of physician oversight. Similar concerns at UCSF Mt. Zion led to the elimination of their MLP program as well. At Brigham and Women's, the MLP service continues but has required additional hospitalist staffing due to difficulties recruiting qualified MLPs with appropriate inpatient experience. In all cases, the models were challenged by high costs and the difficulty of developing MLPs to attain the level of autonomy and efficiency needed to justify their continued use. A key point is that in each institution, MLPs continue to play an important role in some specialty inpatient areas such as Hematology/Oncology and Bone Marrow Transplant, which is where MLPs have traditionally found their niche in inpatient Internal Medicine. These focus shops allow MLPs to develop a niche and expertise in a specialized area, where they may become more autonomous and efficient than house staff. Thus these settings may be more appropriate for MLPs than a heterogeneous general medicine inpatient setting.
Reviewing the Financial Case
In their article, Ford and Britting1 cite potential financial advantages for the use of MLPs in hospital medicine by comparing the relative salaries of MLPs to Hospitalists. What was missing in their analysis was the relative productivity of the 2 types of providers. We do have some limited data from the Society of Hospital Medicine (SHM) annual survey that looks at MLPs in hospital medicine but, again, the number of respondents for most data elements is less than 70, making generalizability difficult. Nonetheless, the data suggest that MLPs in hospital medicine average about 60% to 75% of the productivity of a physician when measured by encounters, although there is wide variability depending on the employment model (academic vs. multispecialty group).15 Importantly, the existing data do not provide any measure of how much physician input is provided to these MLPs but we suspect that in most models there is some physician time and input. If we presume that the MLPs bill independently and collect 85% of the physician fee schedule for a Medicare population, then collections would be about 50% to 65% of a typical physician. Given that median total compensation including benefits from the SHM survey was $120,000 for MLPs and $216,000 for physiciansabout a 55% ratiothis would argue for potential financial neutrality when substituting MLPs for physicians in a 2:1 ratio but only if we presume they require no physician supervision, which in our own experience is not likely in a general medicine population. In an alternative model, in which the physician sees every patient with the MLP and the physician bills, one would need to see roughly 50% more patients to achieve a financially neutral situation. In our experience at our own institutions, this level of increased productivity was not achievable. It is important to note that our figures are median compensation and benefit cost figures and local markets vary widely. We know that in major east and west coast cities MLPs may command far higher salaries while early career hospitalist physicians may be paid somewhat less than the reported medians. Recent market changes have significantly pressured MLP salaries,15, 16 further impacting the financial equation and perhaps tilting it farther against a financial benefit for MLPs. Furthermore, night coverage for MLP services should always be considered in a financial analysis and is not captured in this simple analysis.
Next Steps
Given the current shortage of physicians, we imagine that many hospitalist groups will consider the use of MLPs as a solution to the current workforce issues. However, data on how best to utilize MLPs and the true impact on both the cost and quality of such models is lacking. In addition to urging increased publication and dissemination of existing experiences with NP and PAs, we strongly suggest that groups considering starting a MLP model do so in a way which would facilitate robust analysis and comparison of the model with alternatives. We also suggest that SHM consider the following: modifying its biennial survey to better capture the nuances of MLP productivity (such as assessing the amount of physician input and supervision required); targeting MLPs so as to increase the number of respondents; and doing an additional survey to capture demographics and basic data on existing MLP models given the lack of published literature.
In addition to gathering more data on effective models, a critical gap that we have identified is the development of models for the training and development of MLPs interested in hospital medicine. It would be a mistake to believe that MLPs could function in a manner similar to residency‐trained physicians if they do not undergo similar training. NP/PA programs generally do not have a significant inpatient internal medicine focus and so newly minted graduates often lack the skills needed to succeed in hospital medicine.17 Some hospitalist programs train their MLPs on the job, but many programs cannot afford the amount of time and effort required to do this on their own. There are a small number of advanced training options for MLPs in hospital medicine18 but it is not likely such models will proliferate given the inherent opportunity costs that exist for extended training in the current competitive job market for MLPs. Instead we think that very motivated hospital medicine groups may develop training relationships with PA and NP schools in an effort to train their own. In addition, national initiatives such as the Hospital Medicine Boot Camp for NPs and PAs, which is cosponsored by SHM, the American Association of Physician Assistants (AAPA), and the American Academy of Nurse Practitioners (AANP),19 can help fill the educational needs for MLPs who are already in practice.
Conclusions
While some literature exists that suggests that MLPs can successfully be used in the inpatient internal medicine setting, it is important to note that the evidence is quite limited and cannot be generalized across all care settings and patient populations. There is an urgent need to gather more data and share our collective experiences to better inform our decision‐making before we state that MLPs are the solution to workforce shortages in hospital medicine. In addition, existing data and experience suggest that MLPs may not be a cost‐effective workforce solution for complex general medical patients who require significant physician input. We believe that redesigning the clinical training of MLPs to focus on inpatient skills may hold promise and encourage interested parties to consider developing partnerships with MLP training programs and hospital medicine groups, as a way to build a more robust and successful hospital medicine MLP workforce.
- ,.Nonphysician providers in the hospitalist model: a prescription for change and a warning about unintended side effects.J Hosp Med.2010;5:99–102.
- ,,,,.Non‐housestaff medicine services in academic medical centers: models and challenges.J Hosp Med.2008;3:247–255.
- ,,.Nurse practitioners and physician assistants in the intensive care unit: an evidence‐based review.Crit Care Med.2008;36:2888–2897.
- ,,.Improving resource utilization in a teaching hospital: development of a nonteaching service for chest pain admissions.Acad Med.2006;81:432–435.
- ,,,,,.A nonresident cardiovascular inpatient service improves residents' experiences in an academic medical center: a new model to meet the challenges of the new millennium.Acad Med.2004;79;426–431.
- .The effect of a multidisciplinary hospitalist/physician and advance practice nurse collaboration on hospital care.J Nurs Adm.2006;36:79–85.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Residency Education in Internal Medicine. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140_internal_ medicine_07012009.pdf. Accessed July2009.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3:361–368.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–38.
- ,,,, et al.Outcomes‐based trial of an inpatient nurse practitioner service for general medical patients.J Eval Clin Pract.2001;7:21–33.
- ,.Replacing an academic internal medicine residency program with a physician assistant‐hospitalist model: a comparative analysis study.Am J Med Qual.2009;2:132–139.
- ,,,,.Integrating midlevel practitioners into a teaching service.Am J Surg.2006;1:119–124.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,.Physician assistants in cardiothoracic surgery: a 30‐year experience in a university center.Ann Thorac Surg.2006;1:195–199.
- 2007–2008 Society of Hospital Medicine Bi‐Annual Survey: the Authoritative Source on the State of the Hospital Medicine Movement.Philadelphia:Society of Hospital Medicine;2008.
- American Association of Physician Assistants. Physician Assistant Income. Available at: http://www.aapa.org/images/stories/iu08incchange. pdf. Accessed July2009.
- Accreditation Review Commission on Education for the Physician Assistant. Accreditation Standards for Physician Assistant Education, 3rd ed. Available at: http://www.arcpa.org/Standards/3rdeditionwithPDchangesandregionals4.24.08a.pdf. Accessed July2009.
- Association of Postgraduate PA Programs. Postgraduate PA Program Listing by State. Available at: http://www.appap.org/index1.html. Accessed July2009.
- American Association of Physician Assistants. Adult Hospitalist Physician Assistant and Nurse Practitioner Boot Camp. Available at: http://www. aapa.org/component/content/article/23‐‐general‐/673‐adult‐hospitalist‐physician‐assistant‐and‐nurse‐practitioner‐boot‐camp. Accessed July2009.
- ,.Nonphysician providers in the hospitalist model: a prescription for change and a warning about unintended side effects.J Hosp Med.2010;5:99–102.
- ,,,,.Non‐housestaff medicine services in academic medical centers: models and challenges.J Hosp Med.2008;3:247–255.
- ,,.Nurse practitioners and physician assistants in the intensive care unit: an evidence‐based review.Crit Care Med.2008;36:2888–2897.
- ,,.Improving resource utilization in a teaching hospital: development of a nonteaching service for chest pain admissions.Acad Med.2006;81:432–435.
- ,,,,,.A nonresident cardiovascular inpatient service improves residents' experiences in an academic medical center: a new model to meet the challenges of the new millennium.Acad Med.2004;79;426–431.
- .The effect of a multidisciplinary hospitalist/physician and advance practice nurse collaboration on hospital care.J Nurs Adm.2006;36:79–85.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Residency Education in Internal Medicine. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140_internal_ medicine_07012009.pdf. Accessed July2009.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3:361–368.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–38.
- ,,,, et al.Outcomes‐based trial of an inpatient nurse practitioner service for general medical patients.J Eval Clin Pract.2001;7:21–33.
- ,.Replacing an academic internal medicine residency program with a physician assistant‐hospitalist model: a comparative analysis study.Am J Med Qual.2009;2:132–139.
- ,,,,.Integrating midlevel practitioners into a teaching service.Am J Surg.2006;1:119–124.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,.Physician assistants in cardiothoracic surgery: a 30‐year experience in a university center.Ann Thorac Surg.2006;1:195–199.
- 2007–2008 Society of Hospital Medicine Bi‐Annual Survey: the Authoritative Source on the State of the Hospital Medicine Movement.Philadelphia:Society of Hospital Medicine;2008.
- American Association of Physician Assistants. Physician Assistant Income. Available at: http://www.aapa.org/images/stories/iu08incchange. pdf. Accessed July2009.
- Accreditation Review Commission on Education for the Physician Assistant. Accreditation Standards for Physician Assistant Education, 3rd ed. Available at: http://www.arcpa.org/Standards/3rdeditionwithPDchangesandregionals4.24.08a.pdf. Accessed July2009.
- Association of Postgraduate PA Programs. Postgraduate PA Program Listing by State. Available at: http://www.appap.org/index1.html. Accessed July2009.
- American Association of Physician Assistants. Adult Hospitalist Physician Assistant and Nurse Practitioner Boot Camp. Available at: http://www. aapa.org/component/content/article/23‐‐general‐/673‐adult‐hospitalist‐physician‐assistant‐and‐nurse‐practitioner‐boot‐camp. Accessed July2009.
Hospitalists and Intensivists
A looming gap in the supply of intensivists prompted the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Society of Critical Care Medicine (SCCM) to publish a report in 2000 by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). This study predicted that beginning in 2007 a shortfall would become apparent and steadily increase to 22% by 2020 and to 35% by 2030. Subsequent reports have reiterated those projections, including a report to Congress in 2006 by the U.S. Department of Health and Human Services/Health Resources and Services Administration.14
The concern regarding the shortage of intensivists has been increased by the growing evidence that supports improved critical care outcomesespecially decreased intensive care unit (ICU) and hospital mortalitywith intensivist staffing of ICUs.5, 6 Based on this data and on recommendations from the Society of Critical Care Medicine, the Leapfrog Group made onsite, high‐intensity ICU staffing with intensivists 1 of their 4 leaps.7 A paper by Pronovost et al.8 published in 2001, however, noted that in order for all ICUs in the United States to meet the Leapfrog ICU Physician Staffing (IPS) standard, the number of intensivists would need to increase by a factor of 2.6. Interestingly, a retrospective study published in the Annals of Internal Medicine in June of 2008 by Levy et al.9 suggested that mortality rates may actually be higher in intensivist‐staffed ICUs. An accompanying editorial raised concerns about limitations of the study design, but endorsed Levy's recommendation that more carefully designed, prospective studies were needed; (ie, we still are not certain as to optimal physician staffing for the care of patients requiring the sophisticated treatment available only in an ICU.)10
The health policy challenge, however, remains clear: while there is basic consensus that care of critically ill patients by intensivists improves outcomes, the reality is that the shortage of intensivists in the United States as predicted by the COMPACCS report will only increase, leading some to refer to this as a healthcare crisis. Two major task forces attempted to address this situation, resulting in the publication of the 2004 Framing Options for Critical Care in the United States (FOCCUS) report, The Critical Care Medicine Crisis: A Call for Federal ActionA White Paper from the Critical Care Professional Societies; and the 2007 Prioritizing the Organization and Management of Intensive Care Services in the Unites States (PrOMIS) Conference Report.11, 12 Both reports made specific recommendations including, for example, development of uniform standards for accreditation of institutional critical care capacity, identification and endorsement of core competencies in critical care, investment in health services research, the use of uniform protocols for ICU care, leverage of information technology to promote standardization and improve efficiency, and the development of incentives to attract healthcare professionals to critical care medicine.
A Possible Solution: The Role of Hospitalists
Multiple important efforts are already underway to increase the competency of professionals providing critical care services including the Society of Critical Care's Fundamentals in Critical Care Support (FCCS) program. Additionally, physician assistants and nurse practitioners are playing an increasingly important role as members of critical care services. As another component of this collaborative effort, the PrOMIS Report noted the potential impact of hospitalists in addressing this crisis.
As early as 1999, surveys revealed that as many as 35% of hospitalists were providing critical care services.13 According to the 2005/2006 Society of Hospital Medicine (SHM) National Survey, that number has increased to 75% with a low of 66% in the eastern United States and a high of 84% in the western United States. In community hospitals, 87% of hospitalists care for patients in the ICU, and 30% provide critical care services in academic medical centers.13 While there is some research14, 15 and many anecdotal reports that suggest hospitalists perform well in the ICU, there is, unfortunately, little data addressing outcomes for patients cared for by hospitalists. The results from a prospective, severity‐adjusted study from the Emory University Section of Hospital Medicine and the Division of Pulmonary/Critical Care Medicine examining outcomes for critical care patients cared for by hospitalists with criteria for Pulmonary/Intensivist consults vs. patients cared for by the Pulmonary/Critical Care Medical ICU Service await peer‐review publication.
Despite the lack of outcome data regarding adult hospitalists, it is clear that by default they are already providing a significant proportion of critical care services across the healthcare system, including in tertiary care centers. The two primary models of care include: (1) hospitalists serving as the primary provider without critical care consultant services and (2) comanagement of patients where intensivists and hospitalists collaborate. These collaborative models involve hospitalists actively co‐managing critical care patients along with intensivists or hospitalists managing less critically ill patients with intensivist consultation when indicated. In hospitals lacking intensivists, hospitalists often manage critically ill patients either with intensivist phone consultation, or with the intent to stabilize and transfer. Electronic ICUs are another expanding model of care that provide intensivist support to hospitalists and other primary care providersdecreasing ICU length of stay and severity‐adjusted ICU mortality.16 There are now 40 electronic ICU programs in the United States, and that number continues to grow.
In 2003, there were approximately 10,000 hospitalists in the United States,17 and recent data from an American Hospital Association survey indicates that the number has grown to about 28,000 in 2009. Recent research also documents that hospitalists are soon likely to care for the majority of elderly hospitalized patients in America.18 Aware that the number of intensivists is unlikely to change significantly over the next 25 years the question is no longer if hospitalists should be in the ICU; rather, the question is how to assure quality and improved clinical outcomes through enhanced collaboration between Hospital Medicine and Critical Care Medicine.
Recommendations
There are 3 steps that should be taken urgently to meet this challenge:
-
Per the recommendation of the FOCCUS Report and the PrOMIS Conference Report, uniform protocols for intensive care treatmentmany of which already exist but are not used consistentlyshould be identified and implemented across all ICUs regardless of the level or certification of the provider.
-
Also per the PrOMIS Report, a process for certification of physicians providing critical care services should be established by the appropriate governing bodies, including the Society for Critical Care Medicine, the Society of Hospital Medicine, and the American Thoracic Society, among others. While the PrOMIS Report called for cross‐training of hospital‐based providers to provide intensive care services in lower tier hospitals, a more realistic recommendation given current involvement of hospitalists in the provision of critical care services in secondary and tertiary centers is a competency‐assurance process that includes hospitalists practicing at all levels. This would not be equivalent to board certification, but would be based on a rigorous, comprehensive education and skills training process leading to recognition that would distinguish the recipient as having competencies beyond those obtained in internal medicine residency training. Models for certification could include 4‐month onsite training or a distance learning curriculum with regular blocks of onsite training. Another strategy might be for appropriate governing bodies to establish basic criteria for competency that would then be provided by individual institutions. Emory University, for example, has developed a pilot program incorporating significant components from the European Society for Critical Care Medicine's Syllabus for Competency Based Training in Intensive Care Medicine in Europe.19 Other institutions are also exploring the creation of certification/competency programs. Minimally, and prior to any decision about establishing formal criteria, institutions could identify designated hospitalists within groups who have particular interest and ability in the critical care setting. These providers, based on models already in place at sites across the United States, could, as an example, be required to spend a minimum of 50% of their clinical time in the ICU and to complete 10 to 20 hours of critical care continuing medical education (CME) per year. One strategy to address this issue and develop clear consensus and guidelines would be to convene the often discussed PrOMIS II working group.
-
Per both the FOCCUS Report and the PrOMIS Report as well as a number of other publications,19 health services research in ICU care should be identified, funded, and implemented. A major focus of this effort should be the evaluation of clinical outcomes for ICU patients cared for by hospitalists. This research is needed for at least 2 reasons:
-
As noted, there is little research that has assessed hospitalists' impact on outcomes of ICU patients. Hospitalists are already caring for patients in ICUs across the United States and given the research that has identified the outcomes benefit provided by intensivists, it is important to know objectively if hospitalists have similar levels of performance.
-
An increasing number of hospitals and healthcare systems are now committed to achieving the Leapfrog IPS standard‐a challenge for many because of the difficulty with recruiting intensivists. If new research reveals that hospitalists with board certification in Internal Medicine, and more specifically with additional competency training in critical care, also improve outcomes in the ICU then it may be possible for Leapfrog to revise the criteria for meeting the IPS standard.
Summary
As discussed in a number of publications,20 including an article from the Mayo Clinic in the April 2009 edition of Chest entitled, Physicians Staffing Models and Patient Safety in the ICU,21 along with an accompanying editorial, Should Intensive Care Medicine Itself Be on the Critical List,22 creative and realistic solutions are urgently needed to address the crisis in critical care in the U.S. Collaborative efforts between Critical Care Medicine and Hospital Medicine to meet this challenge benefit all involved:
-
Intensivists will continue to direct tertiary care units and/or co‐manage patients in tertiary and secondary care centers with Hospitalists.
-
Hospitalists will benefit by having the opportunity to secure critical care competency training and by having their appropriate role in the ICU defined.
-
All secondary and tertiary care institutions will have a realistic opportunity to meet Leapfrog IPS criteria and therefore benefit from the potential decreased length of stay (LOS), decreased mortality, and improved quality.
-
Patients benefit by receiving uniform, evidence‐based, protocol‐driven care.
There is now a need and an opportunity for ACCP, SCCM, ATS, and the American Association of Critical Care Nurses (ACCN), to expand the important work they have already begun through the Critical Care Workforce Partnership. The Partnership should join with the SHM to take the lead in supporting and promoting this collaborative relationship between intensivists and hospitalists: aware that in the final analysis, it is the patients we serve who will benefit the most.
- ,,, et al.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284:2762–2770.
- ,,.The critical care professional societies address the critical care crisis in the united states.Chest.2004;125:1512–1513.
- ,,, et al.The critical care crisis in the United States; a report from the profession.Chest.2004;125:1514–1517.
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Report to Congress: the critical care workforce; a study of the supply and demand for critical care physicians. Senate Report 108–181. May2006.
- ,,, et al.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002:288:2151–2162.
- ,,.Do intensivists in ICU improve outcome? Best practice and research.Best Pract Res Clin Anaesthesiol.2005;19:125–135.
- The Leap Frog Group website. Available at: http://www.leapfroggroup.org. Accessed July2009.
- ,,.Impact of critical care physician workforce for intensive care unit physician staffing.Curr Opin Crit Care.2001;7:456–459.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–810.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.The critical care medicine crisis: a call for federal action; a white paper from the critical care professional societies.Chest.2004;125:1518–1521.
- ,,, et al.Prioritizing the organization and management of intensive care services in the Unites States: the PrOMIS conference.Crit Care Med.2007;35:1103–1111.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- .Integrating hospitalists into the pediatric intensive care unit.Crit Care Med.2003;32:813–816.
- ,,, et al.Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system.SD Med2006;59(9):391–393.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- CoBaTrICE Syllabus (Competency‐Based Training in Intensive Care Medicine in Europe), Version 1.0.Brussels:European Society of Intensive Care Medicine;2006.
- .Caring for the critically ill. patient challenges and opportunities.JAMA.2007;298(4):456–458.
- ,.Physician staffing models and patient safety in the ICU.Chest.2009;135:1038–1044.
- ,.Should intensive care medicine itself be on the critical list.Chest.2009;135:892–894.
A looming gap in the supply of intensivists prompted the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Society of Critical Care Medicine (SCCM) to publish a report in 2000 by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). This study predicted that beginning in 2007 a shortfall would become apparent and steadily increase to 22% by 2020 and to 35% by 2030. Subsequent reports have reiterated those projections, including a report to Congress in 2006 by the U.S. Department of Health and Human Services/Health Resources and Services Administration.14
The concern regarding the shortage of intensivists has been increased by the growing evidence that supports improved critical care outcomesespecially decreased intensive care unit (ICU) and hospital mortalitywith intensivist staffing of ICUs.5, 6 Based on this data and on recommendations from the Society of Critical Care Medicine, the Leapfrog Group made onsite, high‐intensity ICU staffing with intensivists 1 of their 4 leaps.7 A paper by Pronovost et al.8 published in 2001, however, noted that in order for all ICUs in the United States to meet the Leapfrog ICU Physician Staffing (IPS) standard, the number of intensivists would need to increase by a factor of 2.6. Interestingly, a retrospective study published in the Annals of Internal Medicine in June of 2008 by Levy et al.9 suggested that mortality rates may actually be higher in intensivist‐staffed ICUs. An accompanying editorial raised concerns about limitations of the study design, but endorsed Levy's recommendation that more carefully designed, prospective studies were needed; (ie, we still are not certain as to optimal physician staffing for the care of patients requiring the sophisticated treatment available only in an ICU.)10
The health policy challenge, however, remains clear: while there is basic consensus that care of critically ill patients by intensivists improves outcomes, the reality is that the shortage of intensivists in the United States as predicted by the COMPACCS report will only increase, leading some to refer to this as a healthcare crisis. Two major task forces attempted to address this situation, resulting in the publication of the 2004 Framing Options for Critical Care in the United States (FOCCUS) report, The Critical Care Medicine Crisis: A Call for Federal ActionA White Paper from the Critical Care Professional Societies; and the 2007 Prioritizing the Organization and Management of Intensive Care Services in the Unites States (PrOMIS) Conference Report.11, 12 Both reports made specific recommendations including, for example, development of uniform standards for accreditation of institutional critical care capacity, identification and endorsement of core competencies in critical care, investment in health services research, the use of uniform protocols for ICU care, leverage of information technology to promote standardization and improve efficiency, and the development of incentives to attract healthcare professionals to critical care medicine.
A Possible Solution: The Role of Hospitalists
Multiple important efforts are already underway to increase the competency of professionals providing critical care services including the Society of Critical Care's Fundamentals in Critical Care Support (FCCS) program. Additionally, physician assistants and nurse practitioners are playing an increasingly important role as members of critical care services. As another component of this collaborative effort, the PrOMIS Report noted the potential impact of hospitalists in addressing this crisis.
As early as 1999, surveys revealed that as many as 35% of hospitalists were providing critical care services.13 According to the 2005/2006 Society of Hospital Medicine (SHM) National Survey, that number has increased to 75% with a low of 66% in the eastern United States and a high of 84% in the western United States. In community hospitals, 87% of hospitalists care for patients in the ICU, and 30% provide critical care services in academic medical centers.13 While there is some research14, 15 and many anecdotal reports that suggest hospitalists perform well in the ICU, there is, unfortunately, little data addressing outcomes for patients cared for by hospitalists. The results from a prospective, severity‐adjusted study from the Emory University Section of Hospital Medicine and the Division of Pulmonary/Critical Care Medicine examining outcomes for critical care patients cared for by hospitalists with criteria for Pulmonary/Intensivist consults vs. patients cared for by the Pulmonary/Critical Care Medical ICU Service await peer‐review publication.
Despite the lack of outcome data regarding adult hospitalists, it is clear that by default they are already providing a significant proportion of critical care services across the healthcare system, including in tertiary care centers. The two primary models of care include: (1) hospitalists serving as the primary provider without critical care consultant services and (2) comanagement of patients where intensivists and hospitalists collaborate. These collaborative models involve hospitalists actively co‐managing critical care patients along with intensivists or hospitalists managing less critically ill patients with intensivist consultation when indicated. In hospitals lacking intensivists, hospitalists often manage critically ill patients either with intensivist phone consultation, or with the intent to stabilize and transfer. Electronic ICUs are another expanding model of care that provide intensivist support to hospitalists and other primary care providersdecreasing ICU length of stay and severity‐adjusted ICU mortality.16 There are now 40 electronic ICU programs in the United States, and that number continues to grow.
In 2003, there were approximately 10,000 hospitalists in the United States,17 and recent data from an American Hospital Association survey indicates that the number has grown to about 28,000 in 2009. Recent research also documents that hospitalists are soon likely to care for the majority of elderly hospitalized patients in America.18 Aware that the number of intensivists is unlikely to change significantly over the next 25 years the question is no longer if hospitalists should be in the ICU; rather, the question is how to assure quality and improved clinical outcomes through enhanced collaboration between Hospital Medicine and Critical Care Medicine.
Recommendations
There are 3 steps that should be taken urgently to meet this challenge:
-
Per the recommendation of the FOCCUS Report and the PrOMIS Conference Report, uniform protocols for intensive care treatmentmany of which already exist but are not used consistentlyshould be identified and implemented across all ICUs regardless of the level or certification of the provider.
-
Also per the PrOMIS Report, a process for certification of physicians providing critical care services should be established by the appropriate governing bodies, including the Society for Critical Care Medicine, the Society of Hospital Medicine, and the American Thoracic Society, among others. While the PrOMIS Report called for cross‐training of hospital‐based providers to provide intensive care services in lower tier hospitals, a more realistic recommendation given current involvement of hospitalists in the provision of critical care services in secondary and tertiary centers is a competency‐assurance process that includes hospitalists practicing at all levels. This would not be equivalent to board certification, but would be based on a rigorous, comprehensive education and skills training process leading to recognition that would distinguish the recipient as having competencies beyond those obtained in internal medicine residency training. Models for certification could include 4‐month onsite training or a distance learning curriculum with regular blocks of onsite training. Another strategy might be for appropriate governing bodies to establish basic criteria for competency that would then be provided by individual institutions. Emory University, for example, has developed a pilot program incorporating significant components from the European Society for Critical Care Medicine's Syllabus for Competency Based Training in Intensive Care Medicine in Europe.19 Other institutions are also exploring the creation of certification/competency programs. Minimally, and prior to any decision about establishing formal criteria, institutions could identify designated hospitalists within groups who have particular interest and ability in the critical care setting. These providers, based on models already in place at sites across the United States, could, as an example, be required to spend a minimum of 50% of their clinical time in the ICU and to complete 10 to 20 hours of critical care continuing medical education (CME) per year. One strategy to address this issue and develop clear consensus and guidelines would be to convene the often discussed PrOMIS II working group.
-
Per both the FOCCUS Report and the PrOMIS Report as well as a number of other publications,19 health services research in ICU care should be identified, funded, and implemented. A major focus of this effort should be the evaluation of clinical outcomes for ICU patients cared for by hospitalists. This research is needed for at least 2 reasons:
-
As noted, there is little research that has assessed hospitalists' impact on outcomes of ICU patients. Hospitalists are already caring for patients in ICUs across the United States and given the research that has identified the outcomes benefit provided by intensivists, it is important to know objectively if hospitalists have similar levels of performance.
-
An increasing number of hospitals and healthcare systems are now committed to achieving the Leapfrog IPS standard‐a challenge for many because of the difficulty with recruiting intensivists. If new research reveals that hospitalists with board certification in Internal Medicine, and more specifically with additional competency training in critical care, also improve outcomes in the ICU then it may be possible for Leapfrog to revise the criteria for meeting the IPS standard.
Summary
As discussed in a number of publications,20 including an article from the Mayo Clinic in the April 2009 edition of Chest entitled, Physicians Staffing Models and Patient Safety in the ICU,21 along with an accompanying editorial, Should Intensive Care Medicine Itself Be on the Critical List,22 creative and realistic solutions are urgently needed to address the crisis in critical care in the U.S. Collaborative efforts between Critical Care Medicine and Hospital Medicine to meet this challenge benefit all involved:
-
Intensivists will continue to direct tertiary care units and/or co‐manage patients in tertiary and secondary care centers with Hospitalists.
-
Hospitalists will benefit by having the opportunity to secure critical care competency training and by having their appropriate role in the ICU defined.
-
All secondary and tertiary care institutions will have a realistic opportunity to meet Leapfrog IPS criteria and therefore benefit from the potential decreased length of stay (LOS), decreased mortality, and improved quality.
-
Patients benefit by receiving uniform, evidence‐based, protocol‐driven care.
There is now a need and an opportunity for ACCP, SCCM, ATS, and the American Association of Critical Care Nurses (ACCN), to expand the important work they have already begun through the Critical Care Workforce Partnership. The Partnership should join with the SHM to take the lead in supporting and promoting this collaborative relationship between intensivists and hospitalists: aware that in the final analysis, it is the patients we serve who will benefit the most.
A looming gap in the supply of intensivists prompted the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Society of Critical Care Medicine (SCCM) to publish a report in 2000 by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). This study predicted that beginning in 2007 a shortfall would become apparent and steadily increase to 22% by 2020 and to 35% by 2030. Subsequent reports have reiterated those projections, including a report to Congress in 2006 by the U.S. Department of Health and Human Services/Health Resources and Services Administration.14
The concern regarding the shortage of intensivists has been increased by the growing evidence that supports improved critical care outcomesespecially decreased intensive care unit (ICU) and hospital mortalitywith intensivist staffing of ICUs.5, 6 Based on this data and on recommendations from the Society of Critical Care Medicine, the Leapfrog Group made onsite, high‐intensity ICU staffing with intensivists 1 of their 4 leaps.7 A paper by Pronovost et al.8 published in 2001, however, noted that in order for all ICUs in the United States to meet the Leapfrog ICU Physician Staffing (IPS) standard, the number of intensivists would need to increase by a factor of 2.6. Interestingly, a retrospective study published in the Annals of Internal Medicine in June of 2008 by Levy et al.9 suggested that mortality rates may actually be higher in intensivist‐staffed ICUs. An accompanying editorial raised concerns about limitations of the study design, but endorsed Levy's recommendation that more carefully designed, prospective studies were needed; (ie, we still are not certain as to optimal physician staffing for the care of patients requiring the sophisticated treatment available only in an ICU.)10
The health policy challenge, however, remains clear: while there is basic consensus that care of critically ill patients by intensivists improves outcomes, the reality is that the shortage of intensivists in the United States as predicted by the COMPACCS report will only increase, leading some to refer to this as a healthcare crisis. Two major task forces attempted to address this situation, resulting in the publication of the 2004 Framing Options for Critical Care in the United States (FOCCUS) report, The Critical Care Medicine Crisis: A Call for Federal ActionA White Paper from the Critical Care Professional Societies; and the 2007 Prioritizing the Organization and Management of Intensive Care Services in the Unites States (PrOMIS) Conference Report.11, 12 Both reports made specific recommendations including, for example, development of uniform standards for accreditation of institutional critical care capacity, identification and endorsement of core competencies in critical care, investment in health services research, the use of uniform protocols for ICU care, leverage of information technology to promote standardization and improve efficiency, and the development of incentives to attract healthcare professionals to critical care medicine.
A Possible Solution: The Role of Hospitalists
Multiple important efforts are already underway to increase the competency of professionals providing critical care services including the Society of Critical Care's Fundamentals in Critical Care Support (FCCS) program. Additionally, physician assistants and nurse practitioners are playing an increasingly important role as members of critical care services. As another component of this collaborative effort, the PrOMIS Report noted the potential impact of hospitalists in addressing this crisis.
As early as 1999, surveys revealed that as many as 35% of hospitalists were providing critical care services.13 According to the 2005/2006 Society of Hospital Medicine (SHM) National Survey, that number has increased to 75% with a low of 66% in the eastern United States and a high of 84% in the western United States. In community hospitals, 87% of hospitalists care for patients in the ICU, and 30% provide critical care services in academic medical centers.13 While there is some research14, 15 and many anecdotal reports that suggest hospitalists perform well in the ICU, there is, unfortunately, little data addressing outcomes for patients cared for by hospitalists. The results from a prospective, severity‐adjusted study from the Emory University Section of Hospital Medicine and the Division of Pulmonary/Critical Care Medicine examining outcomes for critical care patients cared for by hospitalists with criteria for Pulmonary/Intensivist consults vs. patients cared for by the Pulmonary/Critical Care Medical ICU Service await peer‐review publication.
Despite the lack of outcome data regarding adult hospitalists, it is clear that by default they are already providing a significant proportion of critical care services across the healthcare system, including in tertiary care centers. The two primary models of care include: (1) hospitalists serving as the primary provider without critical care consultant services and (2) comanagement of patients where intensivists and hospitalists collaborate. These collaborative models involve hospitalists actively co‐managing critical care patients along with intensivists or hospitalists managing less critically ill patients with intensivist consultation when indicated. In hospitals lacking intensivists, hospitalists often manage critically ill patients either with intensivist phone consultation, or with the intent to stabilize and transfer. Electronic ICUs are another expanding model of care that provide intensivist support to hospitalists and other primary care providersdecreasing ICU length of stay and severity‐adjusted ICU mortality.16 There are now 40 electronic ICU programs in the United States, and that number continues to grow.
In 2003, there were approximately 10,000 hospitalists in the United States,17 and recent data from an American Hospital Association survey indicates that the number has grown to about 28,000 in 2009. Recent research also documents that hospitalists are soon likely to care for the majority of elderly hospitalized patients in America.18 Aware that the number of intensivists is unlikely to change significantly over the next 25 years the question is no longer if hospitalists should be in the ICU; rather, the question is how to assure quality and improved clinical outcomes through enhanced collaboration between Hospital Medicine and Critical Care Medicine.
Recommendations
There are 3 steps that should be taken urgently to meet this challenge:
-
Per the recommendation of the FOCCUS Report and the PrOMIS Conference Report, uniform protocols for intensive care treatmentmany of which already exist but are not used consistentlyshould be identified and implemented across all ICUs regardless of the level or certification of the provider.
-
Also per the PrOMIS Report, a process for certification of physicians providing critical care services should be established by the appropriate governing bodies, including the Society for Critical Care Medicine, the Society of Hospital Medicine, and the American Thoracic Society, among others. While the PrOMIS Report called for cross‐training of hospital‐based providers to provide intensive care services in lower tier hospitals, a more realistic recommendation given current involvement of hospitalists in the provision of critical care services in secondary and tertiary centers is a competency‐assurance process that includes hospitalists practicing at all levels. This would not be equivalent to board certification, but would be based on a rigorous, comprehensive education and skills training process leading to recognition that would distinguish the recipient as having competencies beyond those obtained in internal medicine residency training. Models for certification could include 4‐month onsite training or a distance learning curriculum with regular blocks of onsite training. Another strategy might be for appropriate governing bodies to establish basic criteria for competency that would then be provided by individual institutions. Emory University, for example, has developed a pilot program incorporating significant components from the European Society for Critical Care Medicine's Syllabus for Competency Based Training in Intensive Care Medicine in Europe.19 Other institutions are also exploring the creation of certification/competency programs. Minimally, and prior to any decision about establishing formal criteria, institutions could identify designated hospitalists within groups who have particular interest and ability in the critical care setting. These providers, based on models already in place at sites across the United States, could, as an example, be required to spend a minimum of 50% of their clinical time in the ICU and to complete 10 to 20 hours of critical care continuing medical education (CME) per year. One strategy to address this issue and develop clear consensus and guidelines would be to convene the often discussed PrOMIS II working group.
-
Per both the FOCCUS Report and the PrOMIS Report as well as a number of other publications,19 health services research in ICU care should be identified, funded, and implemented. A major focus of this effort should be the evaluation of clinical outcomes for ICU patients cared for by hospitalists. This research is needed for at least 2 reasons:
-
As noted, there is little research that has assessed hospitalists' impact on outcomes of ICU patients. Hospitalists are already caring for patients in ICUs across the United States and given the research that has identified the outcomes benefit provided by intensivists, it is important to know objectively if hospitalists have similar levels of performance.
-
An increasing number of hospitals and healthcare systems are now committed to achieving the Leapfrog IPS standard‐a challenge for many because of the difficulty with recruiting intensivists. If new research reveals that hospitalists with board certification in Internal Medicine, and more specifically with additional competency training in critical care, also improve outcomes in the ICU then it may be possible for Leapfrog to revise the criteria for meeting the IPS standard.
Summary
As discussed in a number of publications,20 including an article from the Mayo Clinic in the April 2009 edition of Chest entitled, Physicians Staffing Models and Patient Safety in the ICU,21 along with an accompanying editorial, Should Intensive Care Medicine Itself Be on the Critical List,22 creative and realistic solutions are urgently needed to address the crisis in critical care in the U.S. Collaborative efforts between Critical Care Medicine and Hospital Medicine to meet this challenge benefit all involved:
-
Intensivists will continue to direct tertiary care units and/or co‐manage patients in tertiary and secondary care centers with Hospitalists.
-
Hospitalists will benefit by having the opportunity to secure critical care competency training and by having their appropriate role in the ICU defined.
-
All secondary and tertiary care institutions will have a realistic opportunity to meet Leapfrog IPS criteria and therefore benefit from the potential decreased length of stay (LOS), decreased mortality, and improved quality.
-
Patients benefit by receiving uniform, evidence‐based, protocol‐driven care.
There is now a need and an opportunity for ACCP, SCCM, ATS, and the American Association of Critical Care Nurses (ACCN), to expand the important work they have already begun through the Critical Care Workforce Partnership. The Partnership should join with the SHM to take the lead in supporting and promoting this collaborative relationship between intensivists and hospitalists: aware that in the final analysis, it is the patients we serve who will benefit the most.
- ,,, et al.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284:2762–2770.
- ,,.The critical care professional societies address the critical care crisis in the united states.Chest.2004;125:1512–1513.
- ,,, et al.The critical care crisis in the United States; a report from the profession.Chest.2004;125:1514–1517.
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Report to Congress: the critical care workforce; a study of the supply and demand for critical care physicians. Senate Report 108–181. May2006.
- ,,, et al.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002:288:2151–2162.
- ,,.Do intensivists in ICU improve outcome? Best practice and research.Best Pract Res Clin Anaesthesiol.2005;19:125–135.
- The Leap Frog Group website. Available at: http://www.leapfroggroup.org. Accessed July2009.
- ,,.Impact of critical care physician workforce for intensive care unit physician staffing.Curr Opin Crit Care.2001;7:456–459.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–810.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.The critical care medicine crisis: a call for federal action; a white paper from the critical care professional societies.Chest.2004;125:1518–1521.
- ,,, et al.Prioritizing the organization and management of intensive care services in the Unites States: the PrOMIS conference.Crit Care Med.2007;35:1103–1111.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- .Integrating hospitalists into the pediatric intensive care unit.Crit Care Med.2003;32:813–816.
- ,,, et al.Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system.SD Med2006;59(9):391–393.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- CoBaTrICE Syllabus (Competency‐Based Training in Intensive Care Medicine in Europe), Version 1.0.Brussels:European Society of Intensive Care Medicine;2006.
- .Caring for the critically ill. patient challenges and opportunities.JAMA.2007;298(4):456–458.
- ,.Physician staffing models and patient safety in the ICU.Chest.2009;135:1038–1044.
- ,.Should intensive care medicine itself be on the critical list.Chest.2009;135:892–894.
- ,,, et al.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284:2762–2770.
- ,,.The critical care professional societies address the critical care crisis in the united states.Chest.2004;125:1512–1513.
- ,,, et al.The critical care crisis in the United States; a report from the profession.Chest.2004;125:1514–1517.
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Report to Congress: the critical care workforce; a study of the supply and demand for critical care physicians. Senate Report 108–181. May2006.
- ,,, et al.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002:288:2151–2162.
- ,,.Do intensivists in ICU improve outcome? Best practice and research.Best Pract Res Clin Anaesthesiol.2005;19:125–135.
- The Leap Frog Group website. Available at: http://www.leapfroggroup.org. Accessed July2009.
- ,,.Impact of critical care physician workforce for intensive care unit physician staffing.Curr Opin Crit Care.2001;7:456–459.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–810.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.The critical care medicine crisis: a call for federal action; a white paper from the critical care professional societies.Chest.2004;125:1518–1521.
- ,,, et al.Prioritizing the organization and management of intensive care services in the Unites States: the PrOMIS conference.Crit Care Med.2007;35:1103–1111.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- .Integrating hospitalists into the pediatric intensive care unit.Crit Care Med.2003;32:813–816.
- ,,, et al.Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system.SD Med2006;59(9):391–393.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- CoBaTrICE Syllabus (Competency‐Based Training in Intensive Care Medicine in Europe), Version 1.0.Brussels:European Society of Intensive Care Medicine;2006.
- .Caring for the critically ill. patient challenges and opportunities.JAMA.2007;298(4):456–458.
- ,.Physician staffing models and patient safety in the ICU.Chest.2009;135:1038–1044.
- ,.Should intensive care medicine itself be on the critical list.Chest.2009;135:892–894.
Activities of Daily Living
Tears well up in my eyes. Not from pain, but frustration.
I pleaded with my 3‐year‐olds to come to me, so I could help them wash and dress for the day. Ordinarily, I would have come into their room, leaned over their beds, and whispered good morning into their ears.
Four days after surgery, I wasn't able to do that. Not without the crutches. Even with the crutches, I was moving slowly. I scooted up, put a few pillows behind my back, and carefully lifted up my leg. Putting on the knee immobilizer would take too long; I would only be crossing the hall.
Weighing less than 800 g, the immobilizer is an adjustable aluminum frame attached to foam rubber and 4 wide Velcro straps. It is a device of torment. I can never seem to find the proper fit. If it is too tight, my leg hurts and starts turning blue. When it is too loose, it pulls on my incision, multiplying the pain. You put it on while sitting, but you only discover whether it is too tight or too loose when you stand up.
With one foot on the floor at the edge of the bed, I took one crutch in hand, shifted to the left, and grabbed for the other crutch. I squeezed the handgrips of the crutches tightly and pulled myself up.
Put your weight on your hands, NOT your armpits, the instructions said. Easier said than done, because my armpits were now sore too.
Keep your crutches even with each other. I tried to remember to make an equilateral triangle with my good foot and 2 crutches, but the instructions only seemed to account for movement in a straight line.
Keep your elbows slightly bent and close to your sides to help keep the crutches under your arm. I am trying that too.
Lock your elbows. This instruction contradicts the previous one. Which should I follow?
Place your crutches 2 to 3 inches outside of each foot. How do I do this and keep my elbows close to my sides?
Swing your injured leg through first. But not too much, or I'll pole vault across the room.
After 3 steps, my leg started to throb, and my quadriceps went into a spasm. I needed to sit down, immediately.
Non‐weight‐bearing for next 4 weeks, was written on the doctor's note. Four weeks feels like an eternity. If my heel or toe even touched the ground, I felt an immediate throb of pain in my knee.
How do my orthopedic patients do it: those with broken hips and bad or broken knees, with hip replacements, or knee replacements? I was sensitive to their pain and could optimize control. Postoperatively, I could support gastrointestinal and other organ systems. I made sure that the basic weight‐bearing order was correct. I carefully followed the physical and/or occupational therapy recommendation for home, rehabilitation, or a nursing home. I spoke with families. Yet I did not dwell on activities of daily living nor how my patients felt to be dependent on others for simple things they needed. Some patients were non‐weight‐bearing for weeks, some for months, others never walked again. How can one deeply understand it if one has never experienced it?
According to Centers for Disease Control and Prevention (CDC) statistics,1 unintentional injuries are the leading cause of death between the ages of 1 and 44 years. Accidents are again the leading cause of death after age 75 years. More startling is the fact that these numbers have not been significantly reduced since these data were first collected. Nor do these numbers reveal the number of those debilitated, but still living. In my case, I was not playing basketball, water skiing, or rock climbing: I slipped and fell while stepping into a wading pool with my children, severing ligaments, tearing meniscus, and creating a hairline fracture of my tibia.
Expect to move slowly with the crutches. Yes, I am moving very slowly, only 1 to 2 feet with each stride. If I take a longer stride, I lose my balance.
Learn to sit down with the crutches. Learn to stand up. Learn to get into a car.
Learn to go up the stairs. Learn to go down the stairs.
Avoid wet surfaces. Otherwise you'll start skating and reinjure that knee.
It was effortless to think of patients and colleagues of mine. Mr. S., how do you do it? At age 30 years, he was quadriplegic from falling from a tree as a teenager. Mr. D, how about you, in a wheelchair, after being hit by a car and multiple postoperative leg infections and amputations: living in hotels, with estranged family and no social support. How did you do it, while we nagged you about controlling your glucose. Dr. J? The sadness fills my heart. At only 60 years old, my wonderful professor now made rounds in an electric wheelchair, the victim of amyotrophic lateral sclerosis (ALS). My fate, in comparison, is fortunate; my immobility only temporary. I can still think. And talk. And use a phone. And eat, as well as write this essay. And, best of all, read stories to my children. Be careful about this leg, I remind them. Don't come too close!
Maybe what life is trying to tell you is, slow down. In fact, it's forcing you to do that, a colleague and friend said. I had prided myself in thorough and compassionate doctoring. Now, having been a patient on crutches, I have a greater understanding of how limits to mobility can impact daily living on my patients after I discharge them. And the mundane subject of accident prevention has gained a new urgency.
- ,,,.Deaths: final data for 2005.Natl Vital Stat Rep.2008;56(10):1–120.
Tears well up in my eyes. Not from pain, but frustration.
I pleaded with my 3‐year‐olds to come to me, so I could help them wash and dress for the day. Ordinarily, I would have come into their room, leaned over their beds, and whispered good morning into their ears.
Four days after surgery, I wasn't able to do that. Not without the crutches. Even with the crutches, I was moving slowly. I scooted up, put a few pillows behind my back, and carefully lifted up my leg. Putting on the knee immobilizer would take too long; I would only be crossing the hall.
Weighing less than 800 g, the immobilizer is an adjustable aluminum frame attached to foam rubber and 4 wide Velcro straps. It is a device of torment. I can never seem to find the proper fit. If it is too tight, my leg hurts and starts turning blue. When it is too loose, it pulls on my incision, multiplying the pain. You put it on while sitting, but you only discover whether it is too tight or too loose when you stand up.
With one foot on the floor at the edge of the bed, I took one crutch in hand, shifted to the left, and grabbed for the other crutch. I squeezed the handgrips of the crutches tightly and pulled myself up.
Put your weight on your hands, NOT your armpits, the instructions said. Easier said than done, because my armpits were now sore too.
Keep your crutches even with each other. I tried to remember to make an equilateral triangle with my good foot and 2 crutches, but the instructions only seemed to account for movement in a straight line.
Keep your elbows slightly bent and close to your sides to help keep the crutches under your arm. I am trying that too.
Lock your elbows. This instruction contradicts the previous one. Which should I follow?
Place your crutches 2 to 3 inches outside of each foot. How do I do this and keep my elbows close to my sides?
Swing your injured leg through first. But not too much, or I'll pole vault across the room.
After 3 steps, my leg started to throb, and my quadriceps went into a spasm. I needed to sit down, immediately.
Non‐weight‐bearing for next 4 weeks, was written on the doctor's note. Four weeks feels like an eternity. If my heel or toe even touched the ground, I felt an immediate throb of pain in my knee.
How do my orthopedic patients do it: those with broken hips and bad or broken knees, with hip replacements, or knee replacements? I was sensitive to their pain and could optimize control. Postoperatively, I could support gastrointestinal and other organ systems. I made sure that the basic weight‐bearing order was correct. I carefully followed the physical and/or occupational therapy recommendation for home, rehabilitation, or a nursing home. I spoke with families. Yet I did not dwell on activities of daily living nor how my patients felt to be dependent on others for simple things they needed. Some patients were non‐weight‐bearing for weeks, some for months, others never walked again. How can one deeply understand it if one has never experienced it?
According to Centers for Disease Control and Prevention (CDC) statistics,1 unintentional injuries are the leading cause of death between the ages of 1 and 44 years. Accidents are again the leading cause of death after age 75 years. More startling is the fact that these numbers have not been significantly reduced since these data were first collected. Nor do these numbers reveal the number of those debilitated, but still living. In my case, I was not playing basketball, water skiing, or rock climbing: I slipped and fell while stepping into a wading pool with my children, severing ligaments, tearing meniscus, and creating a hairline fracture of my tibia.
Expect to move slowly with the crutches. Yes, I am moving very slowly, only 1 to 2 feet with each stride. If I take a longer stride, I lose my balance.
Learn to sit down with the crutches. Learn to stand up. Learn to get into a car.
Learn to go up the stairs. Learn to go down the stairs.
Avoid wet surfaces. Otherwise you'll start skating and reinjure that knee.
It was effortless to think of patients and colleagues of mine. Mr. S., how do you do it? At age 30 years, he was quadriplegic from falling from a tree as a teenager. Mr. D, how about you, in a wheelchair, after being hit by a car and multiple postoperative leg infections and amputations: living in hotels, with estranged family and no social support. How did you do it, while we nagged you about controlling your glucose. Dr. J? The sadness fills my heart. At only 60 years old, my wonderful professor now made rounds in an electric wheelchair, the victim of amyotrophic lateral sclerosis (ALS). My fate, in comparison, is fortunate; my immobility only temporary. I can still think. And talk. And use a phone. And eat, as well as write this essay. And, best of all, read stories to my children. Be careful about this leg, I remind them. Don't come too close!
Maybe what life is trying to tell you is, slow down. In fact, it's forcing you to do that, a colleague and friend said. I had prided myself in thorough and compassionate doctoring. Now, having been a patient on crutches, I have a greater understanding of how limits to mobility can impact daily living on my patients after I discharge them. And the mundane subject of accident prevention has gained a new urgency.
Tears well up in my eyes. Not from pain, but frustration.
I pleaded with my 3‐year‐olds to come to me, so I could help them wash and dress for the day. Ordinarily, I would have come into their room, leaned over their beds, and whispered good morning into their ears.
Four days after surgery, I wasn't able to do that. Not without the crutches. Even with the crutches, I was moving slowly. I scooted up, put a few pillows behind my back, and carefully lifted up my leg. Putting on the knee immobilizer would take too long; I would only be crossing the hall.
Weighing less than 800 g, the immobilizer is an adjustable aluminum frame attached to foam rubber and 4 wide Velcro straps. It is a device of torment. I can never seem to find the proper fit. If it is too tight, my leg hurts and starts turning blue. When it is too loose, it pulls on my incision, multiplying the pain. You put it on while sitting, but you only discover whether it is too tight or too loose when you stand up.
With one foot on the floor at the edge of the bed, I took one crutch in hand, shifted to the left, and grabbed for the other crutch. I squeezed the handgrips of the crutches tightly and pulled myself up.
Put your weight on your hands, NOT your armpits, the instructions said. Easier said than done, because my armpits were now sore too.
Keep your crutches even with each other. I tried to remember to make an equilateral triangle with my good foot and 2 crutches, but the instructions only seemed to account for movement in a straight line.
Keep your elbows slightly bent and close to your sides to help keep the crutches under your arm. I am trying that too.
Lock your elbows. This instruction contradicts the previous one. Which should I follow?
Place your crutches 2 to 3 inches outside of each foot. How do I do this and keep my elbows close to my sides?
Swing your injured leg through first. But not too much, or I'll pole vault across the room.
After 3 steps, my leg started to throb, and my quadriceps went into a spasm. I needed to sit down, immediately.
Non‐weight‐bearing for next 4 weeks, was written on the doctor's note. Four weeks feels like an eternity. If my heel or toe even touched the ground, I felt an immediate throb of pain in my knee.
How do my orthopedic patients do it: those with broken hips and bad or broken knees, with hip replacements, or knee replacements? I was sensitive to their pain and could optimize control. Postoperatively, I could support gastrointestinal and other organ systems. I made sure that the basic weight‐bearing order was correct. I carefully followed the physical and/or occupational therapy recommendation for home, rehabilitation, or a nursing home. I spoke with families. Yet I did not dwell on activities of daily living nor how my patients felt to be dependent on others for simple things they needed. Some patients were non‐weight‐bearing for weeks, some for months, others never walked again. How can one deeply understand it if one has never experienced it?
According to Centers for Disease Control and Prevention (CDC) statistics,1 unintentional injuries are the leading cause of death between the ages of 1 and 44 years. Accidents are again the leading cause of death after age 75 years. More startling is the fact that these numbers have not been significantly reduced since these data were first collected. Nor do these numbers reveal the number of those debilitated, but still living. In my case, I was not playing basketball, water skiing, or rock climbing: I slipped and fell while stepping into a wading pool with my children, severing ligaments, tearing meniscus, and creating a hairline fracture of my tibia.
Expect to move slowly with the crutches. Yes, I am moving very slowly, only 1 to 2 feet with each stride. If I take a longer stride, I lose my balance.
Learn to sit down with the crutches. Learn to stand up. Learn to get into a car.
Learn to go up the stairs. Learn to go down the stairs.
Avoid wet surfaces. Otherwise you'll start skating and reinjure that knee.
It was effortless to think of patients and colleagues of mine. Mr. S., how do you do it? At age 30 years, he was quadriplegic from falling from a tree as a teenager. Mr. D, how about you, in a wheelchair, after being hit by a car and multiple postoperative leg infections and amputations: living in hotels, with estranged family and no social support. How did you do it, while we nagged you about controlling your glucose. Dr. J? The sadness fills my heart. At only 60 years old, my wonderful professor now made rounds in an electric wheelchair, the victim of amyotrophic lateral sclerosis (ALS). My fate, in comparison, is fortunate; my immobility only temporary. I can still think. And talk. And use a phone. And eat, as well as write this essay. And, best of all, read stories to my children. Be careful about this leg, I remind them. Don't come too close!
Maybe what life is trying to tell you is, slow down. In fact, it's forcing you to do that, a colleague and friend said. I had prided myself in thorough and compassionate doctoring. Now, having been a patient on crutches, I have a greater understanding of how limits to mobility can impact daily living on my patients after I discharge them. And the mundane subject of accident prevention has gained a new urgency.
- ,,,.Deaths: final data for 2005.Natl Vital Stat Rep.2008;56(10):1–120.
- ,,,.Deaths: final data for 2005.Natl Vital Stat Rep.2008;56(10):1–120.