User login
Editorial: Rules of Engagement
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
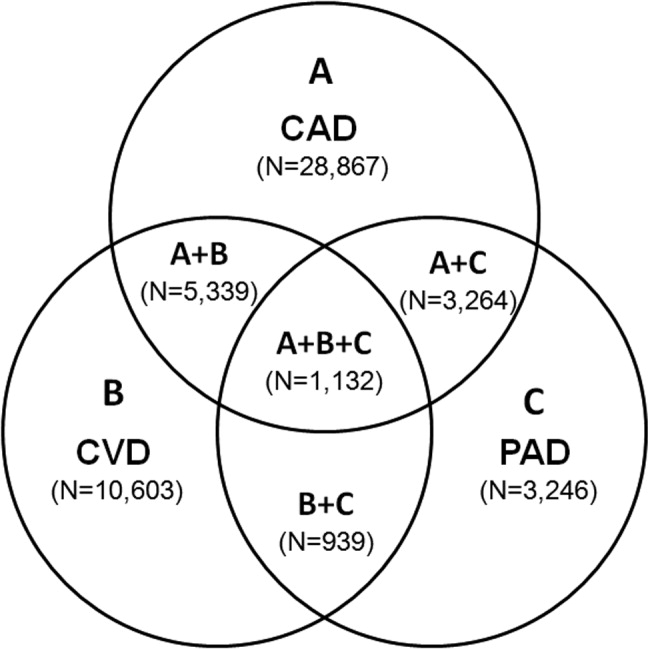
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712
ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.
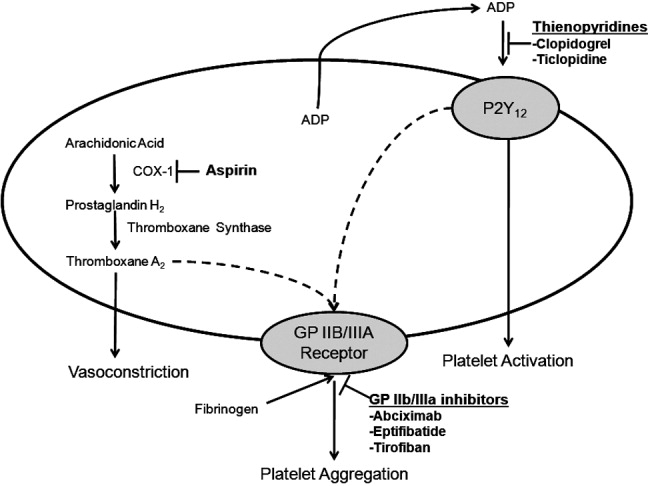
Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
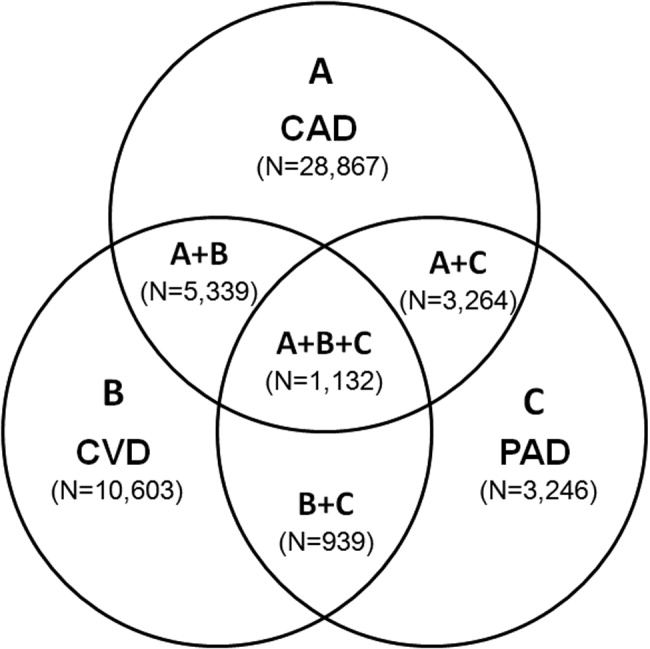
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712

ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.

Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712

ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.

Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
Editorial
We live in a moment of history where change is so speeded up that we begin to see the present only when it is already disappearing.
Two years ago we published the first issue of the Journal of Hospital Medicine and declared, Our goal is that JHM become the premier forum for peer‐reviewed research articles and evidence‐based reviews in the specialty of hospital medicine.1 That first issue was just one of many steps toward this ambition. At the completion of its first year, JHM was selected for indexing and inclusion in the National Library of Medicine's Medical Literature Analysis and Retrieval System Online (MEDLINE), the primary component of PubMed. Following this huge step, we welcomed a remarkable increase in submissions and will have exceeded 300 in our second year, an approximately 50% increase from our first year!
As important, JHM quickly became a valuable benefit of membership in the Society of Hospital Medicine, and the innumerable compliments received by the staff reflect the diligent efforts of a remarkable editorial staff and work by our reviewers. With profound gratitude we list on page 86 these 325 reviewers who donated their priceless time and expertise to enhancing the quality of the manuscripts. To handle the marked increase in submissions, we are expanding and modifying our editorial staff. Please welcome Sunil Kripalani (Vanderbilt) and Daniel Brotman (Johns Hopkins), who join our previous six associate editors and all eight will now serve as JHM's deputy editors. Seven new associate editors also join our team. Among them, Tom Baudendistel (California Pacific Medical Center, San Francisco), Eric Alper (UMass Memorial Health Care, Worcester), Brian Harte (Cleveland Clinic), and Rehan Qayyum (Johns Hopkins) will all focus on optimizing content for practicing hospitalists. Paul Aronowitz will continue to develop our Images section as an associate editor. Recognizing the growing number of pediatric hospitalists, Lisa Zauotis (Childrens Hospital of Philadelphia) and Erin Stucky (Children's Hospital San Diego) join JHM as the other 2 new associate editors. Finally, we welcome new Editorial Board members Mary C. Ottolini (Children's National Medical Center), Douglas Carlson (St. Louis Children's Hospital), and Daniel Rauch (NYU Children's Hospital). The welcome addition of these nationally recognized academicians prepares us for continued growth in manuscript submissions to JHM.
Although we could not excel without the editors, reviewers and our terrific new managing editor, Phaedra McGuinness, we would not survive without the authors who submit their manuscripts to JHMthey are responsible for the caliber of the journal, and we are immensely indebted to them. Originally, we hoped to include individuals involved in all aspects of hospital care,1 and fortunately this is now happening. Complementing hospitalists are nurses and pharmacists2 who recognize the importance of teamwork in the care of hospitalized patients. I encourage all members of the hospital care team to send us the results of their research, teaching, and quality improvement efforts.
As the specialty of hospital medicine continues to evolve, now with more than 20,000 hospitalists, JHM will develop with it. I am honored and grateful to collaborate with such a remarkable group of colleagues as we build the premier journal for the fastest growing specialty in the history of medicine in the United States. On to year 3!
P.S. Our tenuous hold on life confronted me this past Thanksgiving holiday. A fellow hospitalist and dear friend died unexpectedly. Two years before, he posted on the wall of the office shared with his colleagues the following quote:
What we do for ourselves fades, but what we do for another may be etched into eternity.
The smile and humanity of John Allen Garner (19632007) is etched into the lives of his family, many friends and colleagues, and innumerable grateful patients.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1:1–2.
- ,,, et al.ASHP–SHM joint statement on hospitalist–pharmacist collaboration.Am J Health‐Syst Pharm.2008;65:260–263.
We live in a moment of history where change is so speeded up that we begin to see the present only when it is already disappearing.
Two years ago we published the first issue of the Journal of Hospital Medicine and declared, Our goal is that JHM become the premier forum for peer‐reviewed research articles and evidence‐based reviews in the specialty of hospital medicine.1 That first issue was just one of many steps toward this ambition. At the completion of its first year, JHM was selected for indexing and inclusion in the National Library of Medicine's Medical Literature Analysis and Retrieval System Online (MEDLINE), the primary component of PubMed. Following this huge step, we welcomed a remarkable increase in submissions and will have exceeded 300 in our second year, an approximately 50% increase from our first year!
As important, JHM quickly became a valuable benefit of membership in the Society of Hospital Medicine, and the innumerable compliments received by the staff reflect the diligent efforts of a remarkable editorial staff and work by our reviewers. With profound gratitude we list on page 86 these 325 reviewers who donated their priceless time and expertise to enhancing the quality of the manuscripts. To handle the marked increase in submissions, we are expanding and modifying our editorial staff. Please welcome Sunil Kripalani (Vanderbilt) and Daniel Brotman (Johns Hopkins), who join our previous six associate editors and all eight will now serve as JHM's deputy editors. Seven new associate editors also join our team. Among them, Tom Baudendistel (California Pacific Medical Center, San Francisco), Eric Alper (UMass Memorial Health Care, Worcester), Brian Harte (Cleveland Clinic), and Rehan Qayyum (Johns Hopkins) will all focus on optimizing content for practicing hospitalists. Paul Aronowitz will continue to develop our Images section as an associate editor. Recognizing the growing number of pediatric hospitalists, Lisa Zauotis (Childrens Hospital of Philadelphia) and Erin Stucky (Children's Hospital San Diego) join JHM as the other 2 new associate editors. Finally, we welcome new Editorial Board members Mary C. Ottolini (Children's National Medical Center), Douglas Carlson (St. Louis Children's Hospital), and Daniel Rauch (NYU Children's Hospital). The welcome addition of these nationally recognized academicians prepares us for continued growth in manuscript submissions to JHM.
Although we could not excel without the editors, reviewers and our terrific new managing editor, Phaedra McGuinness, we would not survive without the authors who submit their manuscripts to JHMthey are responsible for the caliber of the journal, and we are immensely indebted to them. Originally, we hoped to include individuals involved in all aspects of hospital care,1 and fortunately this is now happening. Complementing hospitalists are nurses and pharmacists2 who recognize the importance of teamwork in the care of hospitalized patients. I encourage all members of the hospital care team to send us the results of their research, teaching, and quality improvement efforts.
As the specialty of hospital medicine continues to evolve, now with more than 20,000 hospitalists, JHM will develop with it. I am honored and grateful to collaborate with such a remarkable group of colleagues as we build the premier journal for the fastest growing specialty in the history of medicine in the United States. On to year 3!
P.S. Our tenuous hold on life confronted me this past Thanksgiving holiday. A fellow hospitalist and dear friend died unexpectedly. Two years before, he posted on the wall of the office shared with his colleagues the following quote:
What we do for ourselves fades, but what we do for another may be etched into eternity.
The smile and humanity of John Allen Garner (19632007) is etched into the lives of his family, many friends and colleagues, and innumerable grateful patients.
We live in a moment of history where change is so speeded up that we begin to see the present only when it is already disappearing.
Two years ago we published the first issue of the Journal of Hospital Medicine and declared, Our goal is that JHM become the premier forum for peer‐reviewed research articles and evidence‐based reviews in the specialty of hospital medicine.1 That first issue was just one of many steps toward this ambition. At the completion of its first year, JHM was selected for indexing and inclusion in the National Library of Medicine's Medical Literature Analysis and Retrieval System Online (MEDLINE), the primary component of PubMed. Following this huge step, we welcomed a remarkable increase in submissions and will have exceeded 300 in our second year, an approximately 50% increase from our first year!
As important, JHM quickly became a valuable benefit of membership in the Society of Hospital Medicine, and the innumerable compliments received by the staff reflect the diligent efforts of a remarkable editorial staff and work by our reviewers. With profound gratitude we list on page 86 these 325 reviewers who donated their priceless time and expertise to enhancing the quality of the manuscripts. To handle the marked increase in submissions, we are expanding and modifying our editorial staff. Please welcome Sunil Kripalani (Vanderbilt) and Daniel Brotman (Johns Hopkins), who join our previous six associate editors and all eight will now serve as JHM's deputy editors. Seven new associate editors also join our team. Among them, Tom Baudendistel (California Pacific Medical Center, San Francisco), Eric Alper (UMass Memorial Health Care, Worcester), Brian Harte (Cleveland Clinic), and Rehan Qayyum (Johns Hopkins) will all focus on optimizing content for practicing hospitalists. Paul Aronowitz will continue to develop our Images section as an associate editor. Recognizing the growing number of pediatric hospitalists, Lisa Zauotis (Childrens Hospital of Philadelphia) and Erin Stucky (Children's Hospital San Diego) join JHM as the other 2 new associate editors. Finally, we welcome new Editorial Board members Mary C. Ottolini (Children's National Medical Center), Douglas Carlson (St. Louis Children's Hospital), and Daniel Rauch (NYU Children's Hospital). The welcome addition of these nationally recognized academicians prepares us for continued growth in manuscript submissions to JHM.
Although we could not excel without the editors, reviewers and our terrific new managing editor, Phaedra McGuinness, we would not survive without the authors who submit their manuscripts to JHMthey are responsible for the caliber of the journal, and we are immensely indebted to them. Originally, we hoped to include individuals involved in all aspects of hospital care,1 and fortunately this is now happening. Complementing hospitalists are nurses and pharmacists2 who recognize the importance of teamwork in the care of hospitalized patients. I encourage all members of the hospital care team to send us the results of their research, teaching, and quality improvement efforts.
As the specialty of hospital medicine continues to evolve, now with more than 20,000 hospitalists, JHM will develop with it. I am honored and grateful to collaborate with such a remarkable group of colleagues as we build the premier journal for the fastest growing specialty in the history of medicine in the United States. On to year 3!
P.S. Our tenuous hold on life confronted me this past Thanksgiving holiday. A fellow hospitalist and dear friend died unexpectedly. Two years before, he posted on the wall of the office shared with his colleagues the following quote:
What we do for ourselves fades, but what we do for another may be etched into eternity.
The smile and humanity of John Allen Garner (19632007) is etched into the lives of his family, many friends and colleagues, and innumerable grateful patients.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1:1–2.
- ,,, et al.ASHP–SHM joint statement on hospitalist–pharmacist collaboration.Am J Health‐Syst Pharm.2008;65:260–263.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1:1–2.
- ,,, et al.ASHP–SHM joint statement on hospitalist–pharmacist collaboration.Am J Health‐Syst Pharm.2008;65:260–263.
Editorial
Autonomy is one of the most familiar principles in Western bioethics, whereas informed consent is probably its most practical expression.1 Autonomy's modern formulation was particularly shaped by political philosophers like John Locke (1632‐1704), who worried about the coercive powers of the state.2 As Lockean‐inspired governments evolved over the last 3 centuries, their legislatures became increasingly disposed to granting citizens an ever‐increasing number of individual rights and freedoms. In American medicine, that sensibility began to take a determinate shape early in the 20th century, such as in Judge Benjamin Cardozo's famous declaration in 1914 that:
Every human being of adult years and sound mind has a right to determine what shall be done with his body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.3
Another half century would be required, however, to agree on the informational content, or scope of disclosure, that would reasonably educate patients on what they would be consenting to. Precedent‐setting decisions in the 1960s and 1970s, such as in Natanson v. Kline4 and Canterbury v. Spence,5 ultimately held that informing a patient about a proposed clinical intervention must include an explanation as to why the intervention is recommended and what particular benefits might accrue from it. Most important, however, is informing the patient about any significant risks the intervention poses. Not associated with or pertaining to error or negligence, but rather understood as foreseeable complications or adverse events that could occur even if the standard of care was scrupulously followed, risk information must be imparted to decisionally able patients or their surrogates to honor their autonomy, or right of bodily ownership.6
The problem with determining whether a risk should be disclosed is that it is often reduced to a judgment call about a risk's severity and frequency. The common understanding is that risks whose severity and frequency are both extremely low need not be discussed. Risk disclosure becomes complex when either of these variables begins to increase, but even then, a significant likelihood of temporary headache or gastrointestinal upset associated with some treatment might not be mentioned. On the other hand, courts have awarded damages to plaintiffs who experienced the materialization of a 1 in 2500 chance of a serious but undisclosed risk.7 The ethical challenge in judging whether a particular risk needs to be disclosed involves the difficulty inherent in determining at what point in the comingling of risk severity and likelihood of materialization does disclosure become required.8
The article by Upadhyay et al. investigates a related facet about risk disclosure.9 For a long time, hospitals have exhibited inconsistent policies for securing informed consent for certain common but nevertheless risky procedures or treatments, especially those involving medications. Many hospitals, for example, would have staff members simply tell patients that they needed diuretics or thrombolytics, even though in certain instances, and especially with thrombolytic agents, the risk of a significant adverse event could well exceed some reasonable disclosure threshold (which is often set at 1%).8
The article by Upadhyay et al. suggests at least 3 issues meriting serious ethical consideration. The first is that the risk scenario primarily discussed in the articlea serious cerebral bleed from thrombolysis with a frequency of from 1% to 20%would most certainly require formal informed consent from patients. To the extent that hospitals recognize such risk scenarios but fail to secure informed consent, they are violating their patients' autonomous rights. The article by Upadhyay et al. is therefore a clarion call to these institutions to become more aggressive and conscientious in honoring their informed consent duties to patients.
A second issue is that the patients surveyed in the study overwhelmingly desired risk disclosure. Notice that if a treatment's risk magnitude is such that it would normally obligate disclosure, the only factors that would preclude disclosure in nonemergent cases would be (1) if the patient was deemed judgmentally or psychologically impaired (and even then, next of kin or the patient's proxy would need to be contacted and informed) or (2) if the patient refused to hear a recitation of the risks (perhaps because it would cause him or her excessive anxiety).10 Otherwise, and as implied by the empirical findings reported in the article, disclosure in an instance like thrombolysis would not only be consistent with (and therefore obligated by) more familiar instances of disclosure such as occur in surgical interventions, it would also be consistent with patient centeredness, as indicated by the responses of the research participants themselves.
But a third issue raises a serious ethical complication. Many patients interviewed in this study also wanted informed consent (or at least wanted to provide permission) for seemingly banal medical interventions. Although respecting patient autonomy is an enduring tenet of medical ethics, it can be argued that it could be limited by other ethical constraints. If respecting a patient's autonomy becomes synonymous with an ethical obligation to disclose all potential risks of every possible treatment regardless of their likelihood or severity, the physician's time might be unreasonably compromised.11 For example, it seems fair to say that many physicians would think it ethically excessive or unreasonable to demand that busy hospitalists discuss the risks, benefits, alternatives, and likelihood of success before ordering intravenous furosemide, potassium supplementation, or routine phlebotomy.
In the general care of hospitalized patients, virtually all physicians will obtain specific, written informed consent prior to invasive procedures, but many might assume that consent for routine medical care has been secured during the consent documentation process of the patient's admission to hospital. Upadhyay et al.'s findings, however, make us question the extent to which consent on admission is ethically sufficient. If it is not, then we must ask what other opportunities exist for effecting patient‐centered explanations of proposed interventions without unduly compromising a health professional's duties and commitments during the workday.
A solution may consist in the way that artful communication skills are key to the physicianpatient relationship. The Accreditation Council on Graduate Medical Education outlines 6 core competencies that all resident physicians should attain during training. One core measure is communication skills: Residents must be able to demonstrate interpersonal and communication skills that result in effective information exchange and teaming with patients, their patients' families, and professional associates.12
Perhaps the individuals surveyed in this study would not require explicit informed consent from a physician if they enjoyed an appropriate number of informational exchanges with all their treating professionals. Their daily treatment plan with its attendant risks and benefits could be discussed in reasonable detail, their comprehension could be elicited through teach back, and their remaining concerns could be explored through empathic communication techniques. This process, which would fold informed consent into a more elaborate, transparent, and humanistically oriented sharing of information, might ease the tension over autonomy versus time constraints by spreading informational responsibilities throughout the health care system. Achieving that quality of informational exchange, however, will require a serious institutional and especially educational commitment in our undergraduate and graduate training programs because it is unlikely that most physicians or other health professionals would seek such skill development on their own.
- ,,.Clinical Ethics: a Practical Approach to Ethical Decisions in Clinical Medicine.6th ed.New York:McGraw‐Hill;2006.
- .Two Treatises of Government.Cambridge, UK:Cambridge University Press;1988.
- . Society of New York Hospital, 105 N.E. 92 (1914).
- ,350 P.2d1093 (1960).
- ,464 F.2d772 (1972).
- ,.Principles of Biomedical Ethics.5th ed.Oxford, UK:Oxford University Press;2001.
- ,286 A.2d647 (1971).
- .Informed Consent: A Guide for Health Care Providers.Rockville, MD:Aspen Systems Corporation;1981.
- ,,,,.Patients' predilections regarding informed consent for hospital treatments.J Hosp Med.2008;3:6–11.
- Council on Ethical and Judicial Affairs.Code of Medical Ethics: Current Opinions with Annotations.2002–2003 ed.Chicago, IL:AMA Press;2002:8.08.
- ,.Physicians' silent decisions: Because patient autonomy does not always come first.Am J Bioeth.2007;7:33–38.
- Available at http://www.acgme.org/outcome/comp/compFull.asp#4 (emphasis added). Accessed on November 6,2007.
Autonomy is one of the most familiar principles in Western bioethics, whereas informed consent is probably its most practical expression.1 Autonomy's modern formulation was particularly shaped by political philosophers like John Locke (1632‐1704), who worried about the coercive powers of the state.2 As Lockean‐inspired governments evolved over the last 3 centuries, their legislatures became increasingly disposed to granting citizens an ever‐increasing number of individual rights and freedoms. In American medicine, that sensibility began to take a determinate shape early in the 20th century, such as in Judge Benjamin Cardozo's famous declaration in 1914 that:
Every human being of adult years and sound mind has a right to determine what shall be done with his body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.3
Another half century would be required, however, to agree on the informational content, or scope of disclosure, that would reasonably educate patients on what they would be consenting to. Precedent‐setting decisions in the 1960s and 1970s, such as in Natanson v. Kline4 and Canterbury v. Spence,5 ultimately held that informing a patient about a proposed clinical intervention must include an explanation as to why the intervention is recommended and what particular benefits might accrue from it. Most important, however, is informing the patient about any significant risks the intervention poses. Not associated with or pertaining to error or negligence, but rather understood as foreseeable complications or adverse events that could occur even if the standard of care was scrupulously followed, risk information must be imparted to decisionally able patients or their surrogates to honor their autonomy, or right of bodily ownership.6
The problem with determining whether a risk should be disclosed is that it is often reduced to a judgment call about a risk's severity and frequency. The common understanding is that risks whose severity and frequency are both extremely low need not be discussed. Risk disclosure becomes complex when either of these variables begins to increase, but even then, a significant likelihood of temporary headache or gastrointestinal upset associated with some treatment might not be mentioned. On the other hand, courts have awarded damages to plaintiffs who experienced the materialization of a 1 in 2500 chance of a serious but undisclosed risk.7 The ethical challenge in judging whether a particular risk needs to be disclosed involves the difficulty inherent in determining at what point in the comingling of risk severity and likelihood of materialization does disclosure become required.8
The article by Upadhyay et al. investigates a related facet about risk disclosure.9 For a long time, hospitals have exhibited inconsistent policies for securing informed consent for certain common but nevertheless risky procedures or treatments, especially those involving medications. Many hospitals, for example, would have staff members simply tell patients that they needed diuretics or thrombolytics, even though in certain instances, and especially with thrombolytic agents, the risk of a significant adverse event could well exceed some reasonable disclosure threshold (which is often set at 1%).8
The article by Upadhyay et al. suggests at least 3 issues meriting serious ethical consideration. The first is that the risk scenario primarily discussed in the articlea serious cerebral bleed from thrombolysis with a frequency of from 1% to 20%would most certainly require formal informed consent from patients. To the extent that hospitals recognize such risk scenarios but fail to secure informed consent, they are violating their patients' autonomous rights. The article by Upadhyay et al. is therefore a clarion call to these institutions to become more aggressive and conscientious in honoring their informed consent duties to patients.
A second issue is that the patients surveyed in the study overwhelmingly desired risk disclosure. Notice that if a treatment's risk magnitude is such that it would normally obligate disclosure, the only factors that would preclude disclosure in nonemergent cases would be (1) if the patient was deemed judgmentally or psychologically impaired (and even then, next of kin or the patient's proxy would need to be contacted and informed) or (2) if the patient refused to hear a recitation of the risks (perhaps because it would cause him or her excessive anxiety).10 Otherwise, and as implied by the empirical findings reported in the article, disclosure in an instance like thrombolysis would not only be consistent with (and therefore obligated by) more familiar instances of disclosure such as occur in surgical interventions, it would also be consistent with patient centeredness, as indicated by the responses of the research participants themselves.
But a third issue raises a serious ethical complication. Many patients interviewed in this study also wanted informed consent (or at least wanted to provide permission) for seemingly banal medical interventions. Although respecting patient autonomy is an enduring tenet of medical ethics, it can be argued that it could be limited by other ethical constraints. If respecting a patient's autonomy becomes synonymous with an ethical obligation to disclose all potential risks of every possible treatment regardless of their likelihood or severity, the physician's time might be unreasonably compromised.11 For example, it seems fair to say that many physicians would think it ethically excessive or unreasonable to demand that busy hospitalists discuss the risks, benefits, alternatives, and likelihood of success before ordering intravenous furosemide, potassium supplementation, or routine phlebotomy.
In the general care of hospitalized patients, virtually all physicians will obtain specific, written informed consent prior to invasive procedures, but many might assume that consent for routine medical care has been secured during the consent documentation process of the patient's admission to hospital. Upadhyay et al.'s findings, however, make us question the extent to which consent on admission is ethically sufficient. If it is not, then we must ask what other opportunities exist for effecting patient‐centered explanations of proposed interventions without unduly compromising a health professional's duties and commitments during the workday.
A solution may consist in the way that artful communication skills are key to the physicianpatient relationship. The Accreditation Council on Graduate Medical Education outlines 6 core competencies that all resident physicians should attain during training. One core measure is communication skills: Residents must be able to demonstrate interpersonal and communication skills that result in effective information exchange and teaming with patients, their patients' families, and professional associates.12
Perhaps the individuals surveyed in this study would not require explicit informed consent from a physician if they enjoyed an appropriate number of informational exchanges with all their treating professionals. Their daily treatment plan with its attendant risks and benefits could be discussed in reasonable detail, their comprehension could be elicited through teach back, and their remaining concerns could be explored through empathic communication techniques. This process, which would fold informed consent into a more elaborate, transparent, and humanistically oriented sharing of information, might ease the tension over autonomy versus time constraints by spreading informational responsibilities throughout the health care system. Achieving that quality of informational exchange, however, will require a serious institutional and especially educational commitment in our undergraduate and graduate training programs because it is unlikely that most physicians or other health professionals would seek such skill development on their own.
Autonomy is one of the most familiar principles in Western bioethics, whereas informed consent is probably its most practical expression.1 Autonomy's modern formulation was particularly shaped by political philosophers like John Locke (1632‐1704), who worried about the coercive powers of the state.2 As Lockean‐inspired governments evolved over the last 3 centuries, their legislatures became increasingly disposed to granting citizens an ever‐increasing number of individual rights and freedoms. In American medicine, that sensibility began to take a determinate shape early in the 20th century, such as in Judge Benjamin Cardozo's famous declaration in 1914 that:
Every human being of adult years and sound mind has a right to determine what shall be done with his body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.3
Another half century would be required, however, to agree on the informational content, or scope of disclosure, that would reasonably educate patients on what they would be consenting to. Precedent‐setting decisions in the 1960s and 1970s, such as in Natanson v. Kline4 and Canterbury v. Spence,5 ultimately held that informing a patient about a proposed clinical intervention must include an explanation as to why the intervention is recommended and what particular benefits might accrue from it. Most important, however, is informing the patient about any significant risks the intervention poses. Not associated with or pertaining to error or negligence, but rather understood as foreseeable complications or adverse events that could occur even if the standard of care was scrupulously followed, risk information must be imparted to decisionally able patients or their surrogates to honor their autonomy, or right of bodily ownership.6
The problem with determining whether a risk should be disclosed is that it is often reduced to a judgment call about a risk's severity and frequency. The common understanding is that risks whose severity and frequency are both extremely low need not be discussed. Risk disclosure becomes complex when either of these variables begins to increase, but even then, a significant likelihood of temporary headache or gastrointestinal upset associated with some treatment might not be mentioned. On the other hand, courts have awarded damages to plaintiffs who experienced the materialization of a 1 in 2500 chance of a serious but undisclosed risk.7 The ethical challenge in judging whether a particular risk needs to be disclosed involves the difficulty inherent in determining at what point in the comingling of risk severity and likelihood of materialization does disclosure become required.8
The article by Upadhyay et al. investigates a related facet about risk disclosure.9 For a long time, hospitals have exhibited inconsistent policies for securing informed consent for certain common but nevertheless risky procedures or treatments, especially those involving medications. Many hospitals, for example, would have staff members simply tell patients that they needed diuretics or thrombolytics, even though in certain instances, and especially with thrombolytic agents, the risk of a significant adverse event could well exceed some reasonable disclosure threshold (which is often set at 1%).8
The article by Upadhyay et al. suggests at least 3 issues meriting serious ethical consideration. The first is that the risk scenario primarily discussed in the articlea serious cerebral bleed from thrombolysis with a frequency of from 1% to 20%would most certainly require formal informed consent from patients. To the extent that hospitals recognize such risk scenarios but fail to secure informed consent, they are violating their patients' autonomous rights. The article by Upadhyay et al. is therefore a clarion call to these institutions to become more aggressive and conscientious in honoring their informed consent duties to patients.
A second issue is that the patients surveyed in the study overwhelmingly desired risk disclosure. Notice that if a treatment's risk magnitude is such that it would normally obligate disclosure, the only factors that would preclude disclosure in nonemergent cases would be (1) if the patient was deemed judgmentally or psychologically impaired (and even then, next of kin or the patient's proxy would need to be contacted and informed) or (2) if the patient refused to hear a recitation of the risks (perhaps because it would cause him or her excessive anxiety).10 Otherwise, and as implied by the empirical findings reported in the article, disclosure in an instance like thrombolysis would not only be consistent with (and therefore obligated by) more familiar instances of disclosure such as occur in surgical interventions, it would also be consistent with patient centeredness, as indicated by the responses of the research participants themselves.
But a third issue raises a serious ethical complication. Many patients interviewed in this study also wanted informed consent (or at least wanted to provide permission) for seemingly banal medical interventions. Although respecting patient autonomy is an enduring tenet of medical ethics, it can be argued that it could be limited by other ethical constraints. If respecting a patient's autonomy becomes synonymous with an ethical obligation to disclose all potential risks of every possible treatment regardless of their likelihood or severity, the physician's time might be unreasonably compromised.11 For example, it seems fair to say that many physicians would think it ethically excessive or unreasonable to demand that busy hospitalists discuss the risks, benefits, alternatives, and likelihood of success before ordering intravenous furosemide, potassium supplementation, or routine phlebotomy.
In the general care of hospitalized patients, virtually all physicians will obtain specific, written informed consent prior to invasive procedures, but many might assume that consent for routine medical care has been secured during the consent documentation process of the patient's admission to hospital. Upadhyay et al.'s findings, however, make us question the extent to which consent on admission is ethically sufficient. If it is not, then we must ask what other opportunities exist for effecting patient‐centered explanations of proposed interventions without unduly compromising a health professional's duties and commitments during the workday.
A solution may consist in the way that artful communication skills are key to the physicianpatient relationship. The Accreditation Council on Graduate Medical Education outlines 6 core competencies that all resident physicians should attain during training. One core measure is communication skills: Residents must be able to demonstrate interpersonal and communication skills that result in effective information exchange and teaming with patients, their patients' families, and professional associates.12
Perhaps the individuals surveyed in this study would not require explicit informed consent from a physician if they enjoyed an appropriate number of informational exchanges with all their treating professionals. Their daily treatment plan with its attendant risks and benefits could be discussed in reasonable detail, their comprehension could be elicited through teach back, and their remaining concerns could be explored through empathic communication techniques. This process, which would fold informed consent into a more elaborate, transparent, and humanistically oriented sharing of information, might ease the tension over autonomy versus time constraints by spreading informational responsibilities throughout the health care system. Achieving that quality of informational exchange, however, will require a serious institutional and especially educational commitment in our undergraduate and graduate training programs because it is unlikely that most physicians or other health professionals would seek such skill development on their own.
- ,,.Clinical Ethics: a Practical Approach to Ethical Decisions in Clinical Medicine.6th ed.New York:McGraw‐Hill;2006.
- .Two Treatises of Government.Cambridge, UK:Cambridge University Press;1988.
- . Society of New York Hospital, 105 N.E. 92 (1914).
- ,350 P.2d1093 (1960).
- ,464 F.2d772 (1972).
- ,.Principles of Biomedical Ethics.5th ed.Oxford, UK:Oxford University Press;2001.
- ,286 A.2d647 (1971).
- .Informed Consent: A Guide for Health Care Providers.Rockville, MD:Aspen Systems Corporation;1981.
- ,,,,.Patients' predilections regarding informed consent for hospital treatments.J Hosp Med.2008;3:6–11.
- Council on Ethical and Judicial Affairs.Code of Medical Ethics: Current Opinions with Annotations.2002–2003 ed.Chicago, IL:AMA Press;2002:8.08.
- ,.Physicians' silent decisions: Because patient autonomy does not always come first.Am J Bioeth.2007;7:33–38.
- Available at http://www.acgme.org/outcome/comp/compFull.asp#4 (emphasis added). Accessed on November 6,2007.
- ,,.Clinical Ethics: a Practical Approach to Ethical Decisions in Clinical Medicine.6th ed.New York:McGraw‐Hill;2006.
- .Two Treatises of Government.Cambridge, UK:Cambridge University Press;1988.
- . Society of New York Hospital, 105 N.E. 92 (1914).
- ,350 P.2d1093 (1960).
- ,464 F.2d772 (1972).
- ,.Principles of Biomedical Ethics.5th ed.Oxford, UK:Oxford University Press;2001.
- ,286 A.2d647 (1971).
- .Informed Consent: A Guide for Health Care Providers.Rockville, MD:Aspen Systems Corporation;1981.
- ,,,,.Patients' predilections regarding informed consent for hospital treatments.J Hosp Med.2008;3:6–11.
- Council on Ethical and Judicial Affairs.Code of Medical Ethics: Current Opinions with Annotations.2002–2003 ed.Chicago, IL:AMA Press;2002:8.08.
- ,.Physicians' silent decisions: Because patient autonomy does not always come first.Am J Bioeth.2007;7:33–38.
- Available at http://www.acgme.org/outcome/comp/compFull.asp#4 (emphasis added). Accessed on November 6,2007.
Gadolinium and nephrogenic systemic fibrosis: The evidence of things not seen
Now faith is the substance of things hoped for, the evidence of things not seen.
—HEBREWS 11:1
Since the first case appeared in 1997,1 nephrogenic systemic fibrosis (NSF) has been detected with increasing frequency in patients with chronic kidney disease. Recognition that this condition affects more than just the skin led to the change in its name from “nephrogenic fibrosing dermopathy” to “nephrogenic systemic fibrosis.”
In this issue, Issa and colleagues2 review this devastating new disease and discuss its association with gadolinium exposure.
NSF RESEMBLES OTHER FIBROSING DISORDERS
The clinical presentation of NSF most closely resembles that of scleromyxedema or scleroderma.1 However, the face is spared in patients with NSF except for yellow plaques on the sclerae, a frequent finding. Monoclonal gammopathy (which may be associated with scleromyxedema) and Raynaud’s phenomenon (which often is associated with scleroderma) usually are absent in NSF.3
A set of histologic findings differentiates NSF from other fibrosing disorders. Skin biopsy reveals fibrosis and elastosis, often with mucin deposition. If NSF is suspected, immunohistochemical stains for CD34, CD45RO, and type I procollagen should be performed to look for dermal spindle cells (presumably “circulating fibrocytes”) coexpressing these markers. Histiocytic cells and dermal dendrocytes expressing CD68 and factor XIIIa have also been described in NSF skin lesions, but other inflammatory cells usually are absent.4 However, the histologic changes of NSF are difficult to distinguish from those of scleromyxedema.5
Thus, as with scleroderma, the diagnosis of NSF remains clinical. Skin biopsy, even of an affected area, occasionally may yield non-diagnostic findings. Histologic findings serve to confirm the diagnosis of NSF in the appropriate clinical setting.
RISK FACTORS FOR NSF: POSSIBLE ASCERTAINMENT BIAS
Renal dysfunction
Because cases of NSF have been searched for only in patients with chronic kidney disease, reported cases have been found only in this patient population. A major limitation of most published case series is that cases have been gathered from among those with histologic confirmation of NSF, and “controls” have been gathered from the remainder of the population receiving dialysis treatment without confirmation by physical examination of the absence of cutaneous changes of NSF.
Most cases have been found in those with stage 5 chronic kidney disease (creatinine clearance < 15 mL/min or requiring dialysis). However, cases have been described in patients with stage 4 chronic kidney disease (creatinine clearance 15–29 mL/min) and, occasionally, in those with lesser degrees of impaired renal function.
Despite the ascertainment bias in identifying cases, this greater prevalence of NSF with lesser renal function suggests a role for renal dysfunction in the pathogenesis of NSF.
Gadolinium exposure
To date, nearly all patients who have developed NSF have had known exposure to gadolinium-containing contrast agents. Gadolinium has been found in tissue of patients with NSF,6,7 yielding the postulate that gadolinium drives tissue fibrosis.
More patients with chronic kidney disease who developed NSF had been exposed to gadodiamide (Omniscan) than to other gadolinium-containing contrast agents, leading to the hypothesis that less-stable gadolinium-chelate complexes release greater amounts of free gadolinium, which then deposits in tissue and triggers fibrosis. However, it has not yet been determined that the gadolinium deposited in tissue is in the free form and not bound to chelate. Furthermore, this attractive hypothesis must be tempered by the recognition that NSF also has developed after exposure to gadopentetate dimeglumine (Magnevist), a more stable gadolinium-chelate complex than gadodiamide.8 The greater number of patients who have developed NSF after gadodiamide exposure may reflect the relative use of these contrast agents in radiology practice.
It is important to be aware that gadolinium-containing contrast agents are used in more than just magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). Because gadolinium also blocks transmission of x-rays, radiologists occasionally have used gadolinium-containing contrast agents for angiography, venography, fistulography, and computed tomography in patients for whom use of iodinated contrast agents is contraindicated. Thus, a patient with chronic kidney disease may have received a gadolinium-containing contrast agent even if no magnetic resonance study had been performed.
Assessment of tissue gadolinium content may confirm prior exposure to a gadolinium-containing contrast agent if the patient does not recall having undergone an imaging study. In the one report that claims the development of NSF in two patients without prior gadolinium exposure, tissue was not assessed for gadolinium content.9
No study has yet been performed to assess the relative prevalence of NSF among patients with different stages of chronic kidney disease who have been exposed to gadolinium-containing contrast agents. Thus, it is impossible to ascertain a threshold of renal dysfunction above which the use of gadolinium-containing contrast agents might be safe.
In 90 patients with stage 5 chronic kidney disease, we found that 30% of those who previously had undergone gadolinium-enhanced imaging studies developed cutaneous changes of NSF; the relative risk of developing these skin changes after exposure to a gadolinium-containing contrast agent was 10.7 (95% confidence interval 1.5–6.9).8
Thus, it is essential that guidelines for the use of these contrast agents be formulated and implemented. Caution must be observed when administering a gadolinium-containing contrast agent to a patient with any degree of renal dysfunction. These patients must be informed of the possible risk of developing NSF, and appropriate follow-up must be conducted to assess for potential changes of NSF.
Other possible risk factors
Not all patients with chronic kidney disease who are exposed to gadolinium-containing contrast agents develop NSF: factors other than the degree of renal dysfunction must be involved in the pathogenesis of this condition.
Exposure to medications commonly taken by patients with chronic kidney disease, such as erythropoietin10 and iron supplements,11 has been suggested as a contributing factor. However, these medications are so widely used that this exposure is unlikely to explain why some patients develop NSF after receiving gadolinium-containing contrast agents and others do not.
Interestingly, lanthanum carbonate (Fosrenol) was approved by the US Food and Drug Administration in 2004 for use as a phosphate binder in patients with stage 5 chronic kidney disease. Since lanthanum and gadolinium both are rare earth metals of the lanthanide series, one might speculate that lanthanum deposition in tissue could produce similar changes or could potentiate those induced by gadolinium.
Future prospective case-control studies need to address risk factors for the development of NSF.
EFFECTIVE TREATMENT NEEDED
Because NSF imposes a markedly increased rate of death and devastating morbidity,8 efforts must be directed toward preventing its development and treating those who already are affected. So far, no treatment has been universally effective in reversing the fibrotic changes of NSF. Potentially effective therapeutic agents must be identified and studied in these patients.
Although performing hemodialysis promptly after the use of a gadolinium-containing contrast agent would appear to be a prudent clinical practice, there are no data to suggest that it is effective in preventing NSF. If free gadolinium disassociates from its chelate and deposits rapidly in tissue, it is unclear that hemodialysis could be performed soon enough to prevent this deposition. Furthermore, hemodialysis is not without associated potential risks and morbidity, especially in people with chronic kidney disease who are not already receiving hemodialysis. Thus, at present, avoiding the use of gadolinium-containing contrast agents in patients with chronic kidney disease appears to be the best preventive strategy.
A NAME CHANGE
Over the past decade, much has been learned about the clinical manifestations, course, and pathogenesis of NSF. However, the term “nephrogenic” in the name of this disease is misleading, in that this fibrosing disorder is not caused by the kidneys. Although some degree of renal dysfunction appears to be necessary for NSF to develop, the presence of gadolinium in tissue seems to drive fibrosis. Thus, it is time that “nephrogenic systemic fibrosis” be renamed more precisely as “gadolinium-associated systemic fibrosis” or “GASF.”
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356:1000–1001.
- Issa N, Poggio E, Fatica R, Patel R, Ruggieri PM, Heyka RJ. Nephrogenic systemic fibrosis and its association with gadolinium exposure during MRI. Cleve Clin J Med 2008; 75:95–111.
- Moschella SL, Kay J, Mackool BT, Liu V. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 35-2004. A 68-year-old man with end-stage renal disease and thickening of the skin. N Engl J Med 2004; 351:2219–2227.
- Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol 2001; 23:383–393.
- Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol 2005; 32:484–490.
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007; 56:21–26.
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007; 56:27–30.
- Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007; 56:3433–3441.
- Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant 2007; 7:2425–2432.
- Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high–dose erythropoietin therapy. Ann Intern Med 2006; 145:234–235.
- Swaminathan S, Horn TD, Pellowski D, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med 2007; 357:720–722.
Now faith is the substance of things hoped for, the evidence of things not seen.
—HEBREWS 11:1
Since the first case appeared in 1997,1 nephrogenic systemic fibrosis (NSF) has been detected with increasing frequency in patients with chronic kidney disease. Recognition that this condition affects more than just the skin led to the change in its name from “nephrogenic fibrosing dermopathy” to “nephrogenic systemic fibrosis.”
In this issue, Issa and colleagues2 review this devastating new disease and discuss its association with gadolinium exposure.
NSF RESEMBLES OTHER FIBROSING DISORDERS
The clinical presentation of NSF most closely resembles that of scleromyxedema or scleroderma.1 However, the face is spared in patients with NSF except for yellow plaques on the sclerae, a frequent finding. Monoclonal gammopathy (which may be associated with scleromyxedema) and Raynaud’s phenomenon (which often is associated with scleroderma) usually are absent in NSF.3
A set of histologic findings differentiates NSF from other fibrosing disorders. Skin biopsy reveals fibrosis and elastosis, often with mucin deposition. If NSF is suspected, immunohistochemical stains for CD34, CD45RO, and type I procollagen should be performed to look for dermal spindle cells (presumably “circulating fibrocytes”) coexpressing these markers. Histiocytic cells and dermal dendrocytes expressing CD68 and factor XIIIa have also been described in NSF skin lesions, but other inflammatory cells usually are absent.4 However, the histologic changes of NSF are difficult to distinguish from those of scleromyxedema.5
Thus, as with scleroderma, the diagnosis of NSF remains clinical. Skin biopsy, even of an affected area, occasionally may yield non-diagnostic findings. Histologic findings serve to confirm the diagnosis of NSF in the appropriate clinical setting.
RISK FACTORS FOR NSF: POSSIBLE ASCERTAINMENT BIAS
Renal dysfunction
Because cases of NSF have been searched for only in patients with chronic kidney disease, reported cases have been found only in this patient population. A major limitation of most published case series is that cases have been gathered from among those with histologic confirmation of NSF, and “controls” have been gathered from the remainder of the population receiving dialysis treatment without confirmation by physical examination of the absence of cutaneous changes of NSF.
Most cases have been found in those with stage 5 chronic kidney disease (creatinine clearance < 15 mL/min or requiring dialysis). However, cases have been described in patients with stage 4 chronic kidney disease (creatinine clearance 15–29 mL/min) and, occasionally, in those with lesser degrees of impaired renal function.
Despite the ascertainment bias in identifying cases, this greater prevalence of NSF with lesser renal function suggests a role for renal dysfunction in the pathogenesis of NSF.
Gadolinium exposure
To date, nearly all patients who have developed NSF have had known exposure to gadolinium-containing contrast agents. Gadolinium has been found in tissue of patients with NSF,6,7 yielding the postulate that gadolinium drives tissue fibrosis.
More patients with chronic kidney disease who developed NSF had been exposed to gadodiamide (Omniscan) than to other gadolinium-containing contrast agents, leading to the hypothesis that less-stable gadolinium-chelate complexes release greater amounts of free gadolinium, which then deposits in tissue and triggers fibrosis. However, it has not yet been determined that the gadolinium deposited in tissue is in the free form and not bound to chelate. Furthermore, this attractive hypothesis must be tempered by the recognition that NSF also has developed after exposure to gadopentetate dimeglumine (Magnevist), a more stable gadolinium-chelate complex than gadodiamide.8 The greater number of patients who have developed NSF after gadodiamide exposure may reflect the relative use of these contrast agents in radiology practice.
It is important to be aware that gadolinium-containing contrast agents are used in more than just magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). Because gadolinium also blocks transmission of x-rays, radiologists occasionally have used gadolinium-containing contrast agents for angiography, venography, fistulography, and computed tomography in patients for whom use of iodinated contrast agents is contraindicated. Thus, a patient with chronic kidney disease may have received a gadolinium-containing contrast agent even if no magnetic resonance study had been performed.
Assessment of tissue gadolinium content may confirm prior exposure to a gadolinium-containing contrast agent if the patient does not recall having undergone an imaging study. In the one report that claims the development of NSF in two patients without prior gadolinium exposure, tissue was not assessed for gadolinium content.9
No study has yet been performed to assess the relative prevalence of NSF among patients with different stages of chronic kidney disease who have been exposed to gadolinium-containing contrast agents. Thus, it is impossible to ascertain a threshold of renal dysfunction above which the use of gadolinium-containing contrast agents might be safe.
In 90 patients with stage 5 chronic kidney disease, we found that 30% of those who previously had undergone gadolinium-enhanced imaging studies developed cutaneous changes of NSF; the relative risk of developing these skin changes after exposure to a gadolinium-containing contrast agent was 10.7 (95% confidence interval 1.5–6.9).8
Thus, it is essential that guidelines for the use of these contrast agents be formulated and implemented. Caution must be observed when administering a gadolinium-containing contrast agent to a patient with any degree of renal dysfunction. These patients must be informed of the possible risk of developing NSF, and appropriate follow-up must be conducted to assess for potential changes of NSF.
Other possible risk factors
Not all patients with chronic kidney disease who are exposed to gadolinium-containing contrast agents develop NSF: factors other than the degree of renal dysfunction must be involved in the pathogenesis of this condition.
Exposure to medications commonly taken by patients with chronic kidney disease, such as erythropoietin10 and iron supplements,11 has been suggested as a contributing factor. However, these medications are so widely used that this exposure is unlikely to explain why some patients develop NSF after receiving gadolinium-containing contrast agents and others do not.
Interestingly, lanthanum carbonate (Fosrenol) was approved by the US Food and Drug Administration in 2004 for use as a phosphate binder in patients with stage 5 chronic kidney disease. Since lanthanum and gadolinium both are rare earth metals of the lanthanide series, one might speculate that lanthanum deposition in tissue could produce similar changes or could potentiate those induced by gadolinium.
Future prospective case-control studies need to address risk factors for the development of NSF.
EFFECTIVE TREATMENT NEEDED
Because NSF imposes a markedly increased rate of death and devastating morbidity,8 efforts must be directed toward preventing its development and treating those who already are affected. So far, no treatment has been universally effective in reversing the fibrotic changes of NSF. Potentially effective therapeutic agents must be identified and studied in these patients.
Although performing hemodialysis promptly after the use of a gadolinium-containing contrast agent would appear to be a prudent clinical practice, there are no data to suggest that it is effective in preventing NSF. If free gadolinium disassociates from its chelate and deposits rapidly in tissue, it is unclear that hemodialysis could be performed soon enough to prevent this deposition. Furthermore, hemodialysis is not without associated potential risks and morbidity, especially in people with chronic kidney disease who are not already receiving hemodialysis. Thus, at present, avoiding the use of gadolinium-containing contrast agents in patients with chronic kidney disease appears to be the best preventive strategy.
A NAME CHANGE
Over the past decade, much has been learned about the clinical manifestations, course, and pathogenesis of NSF. However, the term “nephrogenic” in the name of this disease is misleading, in that this fibrosing disorder is not caused by the kidneys. Although some degree of renal dysfunction appears to be necessary for NSF to develop, the presence of gadolinium in tissue seems to drive fibrosis. Thus, it is time that “nephrogenic systemic fibrosis” be renamed more precisely as “gadolinium-associated systemic fibrosis” or “GASF.”
Now faith is the substance of things hoped for, the evidence of things not seen.
—HEBREWS 11:1
Since the first case appeared in 1997,1 nephrogenic systemic fibrosis (NSF) has been detected with increasing frequency in patients with chronic kidney disease. Recognition that this condition affects more than just the skin led to the change in its name from “nephrogenic fibrosing dermopathy” to “nephrogenic systemic fibrosis.”
In this issue, Issa and colleagues2 review this devastating new disease and discuss its association with gadolinium exposure.
NSF RESEMBLES OTHER FIBROSING DISORDERS
The clinical presentation of NSF most closely resembles that of scleromyxedema or scleroderma.1 However, the face is spared in patients with NSF except for yellow plaques on the sclerae, a frequent finding. Monoclonal gammopathy (which may be associated with scleromyxedema) and Raynaud’s phenomenon (which often is associated with scleroderma) usually are absent in NSF.3
A set of histologic findings differentiates NSF from other fibrosing disorders. Skin biopsy reveals fibrosis and elastosis, often with mucin deposition. If NSF is suspected, immunohistochemical stains for CD34, CD45RO, and type I procollagen should be performed to look for dermal spindle cells (presumably “circulating fibrocytes”) coexpressing these markers. Histiocytic cells and dermal dendrocytes expressing CD68 and factor XIIIa have also been described in NSF skin lesions, but other inflammatory cells usually are absent.4 However, the histologic changes of NSF are difficult to distinguish from those of scleromyxedema.5
Thus, as with scleroderma, the diagnosis of NSF remains clinical. Skin biopsy, even of an affected area, occasionally may yield non-diagnostic findings. Histologic findings serve to confirm the diagnosis of NSF in the appropriate clinical setting.
RISK FACTORS FOR NSF: POSSIBLE ASCERTAINMENT BIAS
Renal dysfunction
Because cases of NSF have been searched for only in patients with chronic kidney disease, reported cases have been found only in this patient population. A major limitation of most published case series is that cases have been gathered from among those with histologic confirmation of NSF, and “controls” have been gathered from the remainder of the population receiving dialysis treatment without confirmation by physical examination of the absence of cutaneous changes of NSF.
Most cases have been found in those with stage 5 chronic kidney disease (creatinine clearance < 15 mL/min or requiring dialysis). However, cases have been described in patients with stage 4 chronic kidney disease (creatinine clearance 15–29 mL/min) and, occasionally, in those with lesser degrees of impaired renal function.
Despite the ascertainment bias in identifying cases, this greater prevalence of NSF with lesser renal function suggests a role for renal dysfunction in the pathogenesis of NSF.
Gadolinium exposure
To date, nearly all patients who have developed NSF have had known exposure to gadolinium-containing contrast agents. Gadolinium has been found in tissue of patients with NSF,6,7 yielding the postulate that gadolinium drives tissue fibrosis.
More patients with chronic kidney disease who developed NSF had been exposed to gadodiamide (Omniscan) than to other gadolinium-containing contrast agents, leading to the hypothesis that less-stable gadolinium-chelate complexes release greater amounts of free gadolinium, which then deposits in tissue and triggers fibrosis. However, it has not yet been determined that the gadolinium deposited in tissue is in the free form and not bound to chelate. Furthermore, this attractive hypothesis must be tempered by the recognition that NSF also has developed after exposure to gadopentetate dimeglumine (Magnevist), a more stable gadolinium-chelate complex than gadodiamide.8 The greater number of patients who have developed NSF after gadodiamide exposure may reflect the relative use of these contrast agents in radiology practice.
It is important to be aware that gadolinium-containing contrast agents are used in more than just magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). Because gadolinium also blocks transmission of x-rays, radiologists occasionally have used gadolinium-containing contrast agents for angiography, venography, fistulography, and computed tomography in patients for whom use of iodinated contrast agents is contraindicated. Thus, a patient with chronic kidney disease may have received a gadolinium-containing contrast agent even if no magnetic resonance study had been performed.
Assessment of tissue gadolinium content may confirm prior exposure to a gadolinium-containing contrast agent if the patient does not recall having undergone an imaging study. In the one report that claims the development of NSF in two patients without prior gadolinium exposure, tissue was not assessed for gadolinium content.9
No study has yet been performed to assess the relative prevalence of NSF among patients with different stages of chronic kidney disease who have been exposed to gadolinium-containing contrast agents. Thus, it is impossible to ascertain a threshold of renal dysfunction above which the use of gadolinium-containing contrast agents might be safe.
In 90 patients with stage 5 chronic kidney disease, we found that 30% of those who previously had undergone gadolinium-enhanced imaging studies developed cutaneous changes of NSF; the relative risk of developing these skin changes after exposure to a gadolinium-containing contrast agent was 10.7 (95% confidence interval 1.5–6.9).8
Thus, it is essential that guidelines for the use of these contrast agents be formulated and implemented. Caution must be observed when administering a gadolinium-containing contrast agent to a patient with any degree of renal dysfunction. These patients must be informed of the possible risk of developing NSF, and appropriate follow-up must be conducted to assess for potential changes of NSF.
Other possible risk factors
Not all patients with chronic kidney disease who are exposed to gadolinium-containing contrast agents develop NSF: factors other than the degree of renal dysfunction must be involved in the pathogenesis of this condition.
Exposure to medications commonly taken by patients with chronic kidney disease, such as erythropoietin10 and iron supplements,11 has been suggested as a contributing factor. However, these medications are so widely used that this exposure is unlikely to explain why some patients develop NSF after receiving gadolinium-containing contrast agents and others do not.
Interestingly, lanthanum carbonate (Fosrenol) was approved by the US Food and Drug Administration in 2004 for use as a phosphate binder in patients with stage 5 chronic kidney disease. Since lanthanum and gadolinium both are rare earth metals of the lanthanide series, one might speculate that lanthanum deposition in tissue could produce similar changes or could potentiate those induced by gadolinium.
Future prospective case-control studies need to address risk factors for the development of NSF.
EFFECTIVE TREATMENT NEEDED
Because NSF imposes a markedly increased rate of death and devastating morbidity,8 efforts must be directed toward preventing its development and treating those who already are affected. So far, no treatment has been universally effective in reversing the fibrotic changes of NSF. Potentially effective therapeutic agents must be identified and studied in these patients.
Although performing hemodialysis promptly after the use of a gadolinium-containing contrast agent would appear to be a prudent clinical practice, there are no data to suggest that it is effective in preventing NSF. If free gadolinium disassociates from its chelate and deposits rapidly in tissue, it is unclear that hemodialysis could be performed soon enough to prevent this deposition. Furthermore, hemodialysis is not without associated potential risks and morbidity, especially in people with chronic kidney disease who are not already receiving hemodialysis. Thus, at present, avoiding the use of gadolinium-containing contrast agents in patients with chronic kidney disease appears to be the best preventive strategy.
A NAME CHANGE
Over the past decade, much has been learned about the clinical manifestations, course, and pathogenesis of NSF. However, the term “nephrogenic” in the name of this disease is misleading, in that this fibrosing disorder is not caused by the kidneys. Although some degree of renal dysfunction appears to be necessary for NSF to develop, the presence of gadolinium in tissue seems to drive fibrosis. Thus, it is time that “nephrogenic systemic fibrosis” be renamed more precisely as “gadolinium-associated systemic fibrosis” or “GASF.”
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356:1000–1001.
- Issa N, Poggio E, Fatica R, Patel R, Ruggieri PM, Heyka RJ. Nephrogenic systemic fibrosis and its association with gadolinium exposure during MRI. Cleve Clin J Med 2008; 75:95–111.
- Moschella SL, Kay J, Mackool BT, Liu V. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 35-2004. A 68-year-old man with end-stage renal disease and thickening of the skin. N Engl J Med 2004; 351:2219–2227.
- Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol 2001; 23:383–393.
- Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol 2005; 32:484–490.
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007; 56:21–26.
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007; 56:27–30.
- Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007; 56:3433–3441.
- Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant 2007; 7:2425–2432.
- Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high–dose erythropoietin therapy. Ann Intern Med 2006; 145:234–235.
- Swaminathan S, Horn TD, Pellowski D, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med 2007; 357:720–722.
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356:1000–1001.
- Issa N, Poggio E, Fatica R, Patel R, Ruggieri PM, Heyka RJ. Nephrogenic systemic fibrosis and its association with gadolinium exposure during MRI. Cleve Clin J Med 2008; 75:95–111.
- Moschella SL, Kay J, Mackool BT, Liu V. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 35-2004. A 68-year-old man with end-stage renal disease and thickening of the skin. N Engl J Med 2004; 351:2219–2227.
- Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol 2001; 23:383–393.
- Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol 2005; 32:484–490.
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007; 56:21–26.
- Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007; 56:27–30.
- Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007; 56:3433–3441.
- Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant 2007; 7:2425–2432.
- Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high–dose erythropoietin therapy. Ann Intern Med 2006; 145:234–235.
- Swaminathan S, Horn TD, Pellowski D, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med 2007; 357:720–722.
Prostate cancer: Too much dogma, not enough data
The article on prostate-specific antigen (PSA) testing from Drs. Jones and Klein1 in this issue of the Cleveland Clinic Journal of Medicine illustrates an important phenomenon in our recent approaches to management of prostate cancer: dogma often outweighs real data.
DOGMA 1: PSA ≤ 4 IS NORMAL AND PSA > 4 IS ABNORMAL
As Drs. Jones and Klein emphasize, a single PSA value does not necessarily indicate cancer is present or absent, although we should note that they are speaking predominantly of PSA values lower than 10 μg/L.
In reality, however, a confirmed blood PSA concentration of 100 μg/L is effectively diagnostic of prostate cancer, and I would be quite prepared to treat a patient for prostate cancer in an urgent setting (eg,spinal cord compression from sclerotic bone metastases) based on that confirmed PSA level without a tissue diagnosis. It is important to consider the costs and benefits of treatment and the impact of delay when making decisions of this type. In the setting of imminent spinal cord compression, the results of waiting for a diagnosis by conventional means (ie, by biopsy) are disappointing,2 and delay in care can be an important factor. Thus, we should not ignore the implications of a markedly raised PSA level when the clinical context is appropriate. The conundrum is determining at what cutoff the PSA allows that type of decision to be made without a tissue diagnosis.
DOGMA 2: PROSTATE SCREENING IS BENEFICIAL
An equally vexing issue is community-wide screening for prostate cancer. Screening is the assessment of symptom-free people in the general population for a particular disease, and for it to be successful, it must identify disease early in its course, and early identification of the disease must result in decreased morbidity of treatment or a reduced overall mortality rate.Current dogma is that prostate cancer screening is good for the community at large.
It seems intuitively sensible and logical tha tassessing healthy, symptom-free men for prostate cancer should be a good idea and should lead to earlier diagnosis and an increased chance of cure. The evidence in favor of routine screening includes “first principles,” common sense, the suggestion that death rates from prostate cancer have fallen in various countries since such approaches have been introduced, and the observation of stage migration (with a greater proportion of initial presentations with earlier-stage disease) in association with these screening exercises.
However, level-1 evidence to support this hypothesis is simply nonexistent—there have been no completed, well-designed randomized trials that demonstrate improved survival from the introduction of routine community screening for prostate cancer with digital rectal examination or PSA measurement. To know the true usefulness of community screening for prostate cancer, we must wait until the ongoing European randomized trial of screening is completed.
DOGMA 3: PROSTATE SCREENING IS WORKING
Although the concept of screening for prostate cancer is very appealing, we should not lose sight of the fact that absolute death rates from prostate cancer have fallen remarkably little in the United States since the introduction of our current screening techniques.
The absolute number of deaths from prostate cancer in the United States has hovered in the range of 26,000 to 30,000 per year since the 1980s, when PSA testing became widespread. In 1985, the American Cancer Society estimated that there were 25,500 deaths from prostate cancer3; in 2007, the estimate was 27,050 deaths,4 hardly a quantum leap forward!
In addition, even if one introduces changes in the incidence of prostate cancer and the aging of the community into the argument and thus increases the denominator for calculation of death rates, the diagnosis and treatment of prostate cancer have improved in many other ways besides screening, including better noninvasive imaging and staging techniques, refined methods for pathological classification, advances in surgery and radiotherapy, hormonal adjuvant therapy for locally advanced tumors, improved chemotherapy, and better support technologies. Thus, it is difficult to attribute any perceived major improvement only to screening.
DOGMA 4: SURGERY IS BETTER . . . OR . . .RADIOTHERAPY IS BETTER
One of the tantalizing dogmas of prostate cancer management is the myth that surgery is vastly superior to radiotherapy, or vice versa.
In reality, most of the comparisons of surgery vs radiotherapy constitute comparisons of apples and oranges—surgical staging vs clinical staging, careful case selection, historical comparison, or single-center vs collaborative group outcomes. Once again, few well-constructed randomized trials have attempted to address this question, and most have closed prematurely because of poor accrual. In fact, most clinicians evolve a case-based and intuition-based experience, which is colored to varying extents by their medical school teaching and the medical literature,5 and really believe in the dogma and opinions that they quote. When one takes a step back and considers the true long-term outcomes, balancing inaccuracies of definition and documentation of the side effects of treatment,6 the variables outlined above, and the heterogeneity of salvage therapy, it is hard to make a strong case that only one therapeutic option reigns supreme.
DOGMA 5: CHEMOTHERAPY NEVER WORKS
Similarly, the view prevailed for many years that cytotoxic chemotherapy had no role in the management of hormone-refractory prostate cancer. With improved clinical staging and assessment, the introduction of serial PSA measurement as a surrogate of response, better definition of the indices of quality of life, and the completion of large randomized trials, it has become clear that the use of chemotherapy improves quality of life,7 that survival can be prolonged by the use of cytotoxics drugs,8 and that it might even be worth testing the utility of chemotherapy in the adjuvant setting, in combination with hormonal therapy, as is done in locally advanced breast cancer.9
EVIDENCE-BASED MEDICINE: THE CURE FOR DOGMA
Ultimately, we have one major tool to help us resolve challenges to dogma, and it is neither rhetoric nor more dogma. Our ultimate weapon is data, and data are best gleaned from well-designed and well-supported randomized clinical trials.
Today, in the United States, fewer than 10% of patients with cancer enter structured clinical trials, reflecting the ennui of government, the medical profession, and patients themselves, as well as the downstream products of disbursement of dogma.10 As a community we need to address these issues for a broad range of medical conditions beyond cancer by using evidence gained from clinical trials, and by practicing evidence-based medicine.
- Jones JS, Klein E. Four no more: the ‘PSA cutoff era’ is over. Cleve Clin J Med 2008; 75:30–32.
- Rosenthal MA, Rosen D, Raghavan D, et al. Spinal cord compression in prostate cancer: A 10-year review. Br J Urol 1992; 69:530–532.
- Anonymous. Cancer statistics 1985. CA Cancer J Clin 1985; 35:19–35.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007. CA Cancer J Clin 2007; 57:43–66.
- Moore MJ, O’Sullivan B, Tannock IF. How expert physicians would wish to be treated if they had genitourinary cancer. J Clin Oncol 1988; 6:1736–1745.
- Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 2003; 21:3777–3784.
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14:1756–1764.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351:1513–1520.
- Flaig TW, Tangen CM, Hussain MHA, et al. Randomization reveals unexpected acute leukemias in SWOG prostate cancer trial. J Clin Oncol. In press.
- Raghavan D. An essay on rearranging the deck chairs: what’s wrong with the cancer trials system? Clin Cancer Res 2006; 12:1949–1950.
The article on prostate-specific antigen (PSA) testing from Drs. Jones and Klein1 in this issue of the Cleveland Clinic Journal of Medicine illustrates an important phenomenon in our recent approaches to management of prostate cancer: dogma often outweighs real data.
DOGMA 1: PSA ≤ 4 IS NORMAL AND PSA > 4 IS ABNORMAL
As Drs. Jones and Klein emphasize, a single PSA value does not necessarily indicate cancer is present or absent, although we should note that they are speaking predominantly of PSA values lower than 10 μg/L.
In reality, however, a confirmed blood PSA concentration of 100 μg/L is effectively diagnostic of prostate cancer, and I would be quite prepared to treat a patient for prostate cancer in an urgent setting (eg,spinal cord compression from sclerotic bone metastases) based on that confirmed PSA level without a tissue diagnosis. It is important to consider the costs and benefits of treatment and the impact of delay when making decisions of this type. In the setting of imminent spinal cord compression, the results of waiting for a diagnosis by conventional means (ie, by biopsy) are disappointing,2 and delay in care can be an important factor. Thus, we should not ignore the implications of a markedly raised PSA level when the clinical context is appropriate. The conundrum is determining at what cutoff the PSA allows that type of decision to be made without a tissue diagnosis.
DOGMA 2: PROSTATE SCREENING IS BENEFICIAL
An equally vexing issue is community-wide screening for prostate cancer. Screening is the assessment of symptom-free people in the general population for a particular disease, and for it to be successful, it must identify disease early in its course, and early identification of the disease must result in decreased morbidity of treatment or a reduced overall mortality rate.Current dogma is that prostate cancer screening is good for the community at large.
It seems intuitively sensible and logical tha tassessing healthy, symptom-free men for prostate cancer should be a good idea and should lead to earlier diagnosis and an increased chance of cure. The evidence in favor of routine screening includes “first principles,” common sense, the suggestion that death rates from prostate cancer have fallen in various countries since such approaches have been introduced, and the observation of stage migration (with a greater proportion of initial presentations with earlier-stage disease) in association with these screening exercises.
However, level-1 evidence to support this hypothesis is simply nonexistent—there have been no completed, well-designed randomized trials that demonstrate improved survival from the introduction of routine community screening for prostate cancer with digital rectal examination or PSA measurement. To know the true usefulness of community screening for prostate cancer, we must wait until the ongoing European randomized trial of screening is completed.
DOGMA 3: PROSTATE SCREENING IS WORKING
Although the concept of screening for prostate cancer is very appealing, we should not lose sight of the fact that absolute death rates from prostate cancer have fallen remarkably little in the United States since the introduction of our current screening techniques.
The absolute number of deaths from prostate cancer in the United States has hovered in the range of 26,000 to 30,000 per year since the 1980s, when PSA testing became widespread. In 1985, the American Cancer Society estimated that there were 25,500 deaths from prostate cancer3; in 2007, the estimate was 27,050 deaths,4 hardly a quantum leap forward!
In addition, even if one introduces changes in the incidence of prostate cancer and the aging of the community into the argument and thus increases the denominator for calculation of death rates, the diagnosis and treatment of prostate cancer have improved in many other ways besides screening, including better noninvasive imaging and staging techniques, refined methods for pathological classification, advances in surgery and radiotherapy, hormonal adjuvant therapy for locally advanced tumors, improved chemotherapy, and better support technologies. Thus, it is difficult to attribute any perceived major improvement only to screening.
DOGMA 4: SURGERY IS BETTER . . . OR . . .RADIOTHERAPY IS BETTER
One of the tantalizing dogmas of prostate cancer management is the myth that surgery is vastly superior to radiotherapy, or vice versa.
In reality, most of the comparisons of surgery vs radiotherapy constitute comparisons of apples and oranges—surgical staging vs clinical staging, careful case selection, historical comparison, or single-center vs collaborative group outcomes. Once again, few well-constructed randomized trials have attempted to address this question, and most have closed prematurely because of poor accrual. In fact, most clinicians evolve a case-based and intuition-based experience, which is colored to varying extents by their medical school teaching and the medical literature,5 and really believe in the dogma and opinions that they quote. When one takes a step back and considers the true long-term outcomes, balancing inaccuracies of definition and documentation of the side effects of treatment,6 the variables outlined above, and the heterogeneity of salvage therapy, it is hard to make a strong case that only one therapeutic option reigns supreme.
DOGMA 5: CHEMOTHERAPY NEVER WORKS
Similarly, the view prevailed for many years that cytotoxic chemotherapy had no role in the management of hormone-refractory prostate cancer. With improved clinical staging and assessment, the introduction of serial PSA measurement as a surrogate of response, better definition of the indices of quality of life, and the completion of large randomized trials, it has become clear that the use of chemotherapy improves quality of life,7 that survival can be prolonged by the use of cytotoxics drugs,8 and that it might even be worth testing the utility of chemotherapy in the adjuvant setting, in combination with hormonal therapy, as is done in locally advanced breast cancer.9
EVIDENCE-BASED MEDICINE: THE CURE FOR DOGMA
Ultimately, we have one major tool to help us resolve challenges to dogma, and it is neither rhetoric nor more dogma. Our ultimate weapon is data, and data are best gleaned from well-designed and well-supported randomized clinical trials.
Today, in the United States, fewer than 10% of patients with cancer enter structured clinical trials, reflecting the ennui of government, the medical profession, and patients themselves, as well as the downstream products of disbursement of dogma.10 As a community we need to address these issues for a broad range of medical conditions beyond cancer by using evidence gained from clinical trials, and by practicing evidence-based medicine.
The article on prostate-specific antigen (PSA) testing from Drs. Jones and Klein1 in this issue of the Cleveland Clinic Journal of Medicine illustrates an important phenomenon in our recent approaches to management of prostate cancer: dogma often outweighs real data.
DOGMA 1: PSA ≤ 4 IS NORMAL AND PSA > 4 IS ABNORMAL
As Drs. Jones and Klein emphasize, a single PSA value does not necessarily indicate cancer is present or absent, although we should note that they are speaking predominantly of PSA values lower than 10 μg/L.
In reality, however, a confirmed blood PSA concentration of 100 μg/L is effectively diagnostic of prostate cancer, and I would be quite prepared to treat a patient for prostate cancer in an urgent setting (eg,spinal cord compression from sclerotic bone metastases) based on that confirmed PSA level without a tissue diagnosis. It is important to consider the costs and benefits of treatment and the impact of delay when making decisions of this type. In the setting of imminent spinal cord compression, the results of waiting for a diagnosis by conventional means (ie, by biopsy) are disappointing,2 and delay in care can be an important factor. Thus, we should not ignore the implications of a markedly raised PSA level when the clinical context is appropriate. The conundrum is determining at what cutoff the PSA allows that type of decision to be made without a tissue diagnosis.
DOGMA 2: PROSTATE SCREENING IS BENEFICIAL
An equally vexing issue is community-wide screening for prostate cancer. Screening is the assessment of symptom-free people in the general population for a particular disease, and for it to be successful, it must identify disease early in its course, and early identification of the disease must result in decreased morbidity of treatment or a reduced overall mortality rate.Current dogma is that prostate cancer screening is good for the community at large.
It seems intuitively sensible and logical tha tassessing healthy, symptom-free men for prostate cancer should be a good idea and should lead to earlier diagnosis and an increased chance of cure. The evidence in favor of routine screening includes “first principles,” common sense, the suggestion that death rates from prostate cancer have fallen in various countries since such approaches have been introduced, and the observation of stage migration (with a greater proportion of initial presentations with earlier-stage disease) in association with these screening exercises.
However, level-1 evidence to support this hypothesis is simply nonexistent—there have been no completed, well-designed randomized trials that demonstrate improved survival from the introduction of routine community screening for prostate cancer with digital rectal examination or PSA measurement. To know the true usefulness of community screening for prostate cancer, we must wait until the ongoing European randomized trial of screening is completed.
DOGMA 3: PROSTATE SCREENING IS WORKING
Although the concept of screening for prostate cancer is very appealing, we should not lose sight of the fact that absolute death rates from prostate cancer have fallen remarkably little in the United States since the introduction of our current screening techniques.
The absolute number of deaths from prostate cancer in the United States has hovered in the range of 26,000 to 30,000 per year since the 1980s, when PSA testing became widespread. In 1985, the American Cancer Society estimated that there were 25,500 deaths from prostate cancer3; in 2007, the estimate was 27,050 deaths,4 hardly a quantum leap forward!
In addition, even if one introduces changes in the incidence of prostate cancer and the aging of the community into the argument and thus increases the denominator for calculation of death rates, the diagnosis and treatment of prostate cancer have improved in many other ways besides screening, including better noninvasive imaging and staging techniques, refined methods for pathological classification, advances in surgery and radiotherapy, hormonal adjuvant therapy for locally advanced tumors, improved chemotherapy, and better support technologies. Thus, it is difficult to attribute any perceived major improvement only to screening.
DOGMA 4: SURGERY IS BETTER . . . OR . . .RADIOTHERAPY IS BETTER
One of the tantalizing dogmas of prostate cancer management is the myth that surgery is vastly superior to radiotherapy, or vice versa.
In reality, most of the comparisons of surgery vs radiotherapy constitute comparisons of apples and oranges—surgical staging vs clinical staging, careful case selection, historical comparison, or single-center vs collaborative group outcomes. Once again, few well-constructed randomized trials have attempted to address this question, and most have closed prematurely because of poor accrual. In fact, most clinicians evolve a case-based and intuition-based experience, which is colored to varying extents by their medical school teaching and the medical literature,5 and really believe in the dogma and opinions that they quote. When one takes a step back and considers the true long-term outcomes, balancing inaccuracies of definition and documentation of the side effects of treatment,6 the variables outlined above, and the heterogeneity of salvage therapy, it is hard to make a strong case that only one therapeutic option reigns supreme.
DOGMA 5: CHEMOTHERAPY NEVER WORKS
Similarly, the view prevailed for many years that cytotoxic chemotherapy had no role in the management of hormone-refractory prostate cancer. With improved clinical staging and assessment, the introduction of serial PSA measurement as a surrogate of response, better definition of the indices of quality of life, and the completion of large randomized trials, it has become clear that the use of chemotherapy improves quality of life,7 that survival can be prolonged by the use of cytotoxics drugs,8 and that it might even be worth testing the utility of chemotherapy in the adjuvant setting, in combination with hormonal therapy, as is done in locally advanced breast cancer.9
EVIDENCE-BASED MEDICINE: THE CURE FOR DOGMA
Ultimately, we have one major tool to help us resolve challenges to dogma, and it is neither rhetoric nor more dogma. Our ultimate weapon is data, and data are best gleaned from well-designed and well-supported randomized clinical trials.
Today, in the United States, fewer than 10% of patients with cancer enter structured clinical trials, reflecting the ennui of government, the medical profession, and patients themselves, as well as the downstream products of disbursement of dogma.10 As a community we need to address these issues for a broad range of medical conditions beyond cancer by using evidence gained from clinical trials, and by practicing evidence-based medicine.
- Jones JS, Klein E. Four no more: the ‘PSA cutoff era’ is over. Cleve Clin J Med 2008; 75:30–32.
- Rosenthal MA, Rosen D, Raghavan D, et al. Spinal cord compression in prostate cancer: A 10-year review. Br J Urol 1992; 69:530–532.
- Anonymous. Cancer statistics 1985. CA Cancer J Clin 1985; 35:19–35.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007. CA Cancer J Clin 2007; 57:43–66.
- Moore MJ, O’Sullivan B, Tannock IF. How expert physicians would wish to be treated if they had genitourinary cancer. J Clin Oncol 1988; 6:1736–1745.
- Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 2003; 21:3777–3784.
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14:1756–1764.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351:1513–1520.
- Flaig TW, Tangen CM, Hussain MHA, et al. Randomization reveals unexpected acute leukemias in SWOG prostate cancer trial. J Clin Oncol. In press.
- Raghavan D. An essay on rearranging the deck chairs: what’s wrong with the cancer trials system? Clin Cancer Res 2006; 12:1949–1950.
- Jones JS, Klein E. Four no more: the ‘PSA cutoff era’ is over. Cleve Clin J Med 2008; 75:30–32.
- Rosenthal MA, Rosen D, Raghavan D, et al. Spinal cord compression in prostate cancer: A 10-year review. Br J Urol 1992; 69:530–532.
- Anonymous. Cancer statistics 1985. CA Cancer J Clin 1985; 35:19–35.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007. CA Cancer J Clin 2007; 57:43–66.
- Moore MJ, O’Sullivan B, Tannock IF. How expert physicians would wish to be treated if they had genitourinary cancer. J Clin Oncol 1988; 6:1736–1745.
- Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 2003; 21:3777–3784.
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14:1756–1764.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351:1513–1520.
- Flaig TW, Tangen CM, Hussain MHA, et al. Randomization reveals unexpected acute leukemias in SWOG prostate cancer trial. J Clin Oncol. In press.
- Raghavan D. An essay on rearranging the deck chairs: what’s wrong with the cancer trials system? Clin Cancer Res 2006; 12:1949–1950.
Acute aortic syndromes: Time to talk of many things
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
“The time has come,” the Walrus said,
“To talk of many things:
Of shoes—and ships—and sealing-wax—
Of cabbages—and kings—
And why the sea is boiling hot
And whether pigs have wings.”
—Lewis Carroll, The Walrus and the Carpenter (from Through the Looking-Glass and What Alice Found There, 1872).
Lewis Carroll's poem of 1872 is a useful starting point for identifying issues resulting from confusion over the variously described acute aortic syndromes—and, for oysters, the dangers of listening to walruses.
TALK OF MANY THINGS
In cases of aortic dissection (splitting or separation of the layers of the aortic wall), it is important to establish the type (ie, the location and extent) and class (ie, the structure) of the dissection, because these distinctions determine the treatment.1 Similarly, in cases of painful or leaking degenerative aneurysms, we need to know the location of the aneurysm and whether the presenting pain is from compression of surrounding tissue, particularly of the vertebral bodies, or from leakage.
The location and extent of an aortic dissection can be classified in three ways (see Figure 3 in Smith and Schoenhagen’s excellent review of the use of computed tomography [CT] in acute aortic syndromes in this issue of the Cleveland Clinic Journal of Medicine2):
- The DeBakey system (type I, II, or III)
- The Stanford system (type A or B)
- Distal or proximal to the left subclavian artery.
Of note, the DeBakey system does not include tears in the arch that extend distally without ascending involvement. The original Stanford system included arch tears with distal extension in type B; hence, type B excluded all patients without ascending involvement.
The importance of the extent of dissection is that most patients with Stanford type A or DeBakey type I or II dissections should undergo immediate surgery, as most of them would die without it. Surgery is also indicated for arch tears (non-DeBakey, original Stanford type B).
Because these classifications are somewhat confusing, the simplest approach is to note whether the dissection extends proximal or distal to the left subclavian artery, because proximal dissections need surgery and distal ones are first managed medically.
The classes of dissection also have bearing on treatment.1 These are:
- Class I—classic aortic dissection in the media with two lumens separated by a “flap” or septum
- Class II—intramural hematoma in the aortic wall from dissection in which the intimal tear cannot be imaged (these are nearly always found duringsurgery or autopsy)
- Class III—localized confined intimal tears without extensive undermining of the intima or flap formation. These are often seen with Marfan syndrome and can rupture or cause tamponade, as can any type of proximal dissection. The typical appearance is of a bulging bubble in the aortic wall.
- Class IV—penetrating atherosclerotic ulcers with localized dissections or wall hematomas, often with calcium at the base of a mushroom-shaped area of extraluminal contrast. Of note, the plane of dissection is often between the media and adventitia.
- Class V—iatrogenic or posttraumatic dissection.
All class I to class IV tears of the proximal aorta require surgery, whereas distal class IV and V tears may require either open or endovascular surgical intervention. Surgery is also indicated for patients with distal dissections who have severe narrowing of the true lumen, distal ischemia, uncontrolled pain, severe hypertension, or evidence of leaking, particularly with class IV tears.
In distal dissections that are subacute (2–6 week sold), the Investigation of Stent grafts in Patients With Type B Aortic Dissection (INSTEAD) trial found that inserting a stent prophylactically provided no benefit. Further-more, there is no proof that stenting is beneficial if the aortic dissection is chronic, ie, more than 6 weeks old.1,3–5
WHICH SHOE FITS?
There is no ideal procedure to detect dissection, although the trend is towards CT angiography, as Smith and Schoenhagen report.2 Although some investigators have optimistically estimated CT’s sensitivity and specificity as 100%, cardiovascular surgeons are well aware of both false-positive and false-negative CT studies. Thus, for emergency repairs of proximal dissections, transesophageal echocardiography should be done after intubation and before opening a patient’s chest if time allows. Magnetic resonance imaging, CT, and transesophageal echocardiography may all miss class III tears, but these are frequently evidenced by eccentric “bubbles”or “ballooning.”1
SHIPS
Patients with either acute aortic dissection or severe pain associated with degenerative aneurysms need to be “shipped” promptly to a tertiary medical center after diagnosis, since larger volumes of procedures appear to be associated with better outcomes.
SEALING WAX
Using current surgical methods, the aortic valve can be preserved during aortic dissection repair unless the valve is bicuspid or the patient has Marfan syndrome.1,3,4,6–8
Sealing wax in the form of biological glues, rather than for letters, is a new innovation. A caveat remains, however: we have seen patients who have required reoperation for false aneurysms or infection. Hence, glues should be used with caution.
CABBAGES
A dilemma is whether patients should undergo coronary catheterization (or CT angiography—a separate question) and subsequent coronary artery bypass grafting (CABG), if needed, at the time of aortic dissection repair. The problem is that approximately one-third of patients have coronary artery disease that may require CABG, but the delay for catheterization increases the risk of rupture or tamponade before surgery.
Indeed, 40% of patients with proximal dissections die immediately, and 1% to 3% die in the hour before surgery. The short-term (in-hospital and 30-day) mortality rates range from 3.4% (Cleveland Clinic 2006 data) to 25%, and of the survivors only about 50% area live 5 years after surgery.
Though dismal, the prognosis is improving. In 162 patients with aortic dissection and Marfan syndrome or connective tissue disorders who underwent surgery at Cleveland Clinic in the years 1978–2003, the 5-year survival rate in those with aortic dissection was 75% and the 10-year rate was 55%.7 In those without dissection, the 10-year survival rate was approximately 90% (P < .001).
KINGS
Noted personalities who have had aortic dissection include King George II of England (who died in 1760), Lucille Ball, Conway Twitty, Jan Larson, and most recently John Ritter. None of these famous people survived their aortic dissections. Indeed, dissection and diseases of the aorta or its branches cause between 43,000 and 47,000 deaths annually,9 more than from breast cancer, murders, or motor vehicle accidents. The main reason for these dismal statistics is that the disease is often misdiagnosed at the time of presentation.
BOILING SEA
Careful studies from Olmsted County, Minnesota,10 have shown a tripling of the incidence of aortic disease, particularly in women, even though the rate of deaths from coronary artery disease has been decreasing. Furthermore, Olsson et al11 report that the incidence of aortic dissection in men in Sweden increased to approximately16 per 100,000 per year from 1987 to 2002, a 52% increase. The aging of the population must play a large role, but other factors may exist that are not well understood or defined and require further research.
PIGS HAVE WINGS
Will it be possible to overcome this rising problem? The answer is a definite yes. The results of aortic surgery have never been better. Many new innovations are available, such as aortic root preservation and endovascular stenting procedures. It may be possible to slow the growth of or prevent some aneurysms and aortic dissections, particularly with beta-blockers and, potentially, with losartan (Cozaar) for Marfan syndrome patients.
One of the keys to preventing aortic catastrophes and aortic dissection is to repair aortic aneurysms. The threshold for surgery, however, depends on a surgeon’s experience and results, the underlying pathology, and the aortic size.
We observed that 12.5% of dissections in patients with bicuspid valves and 15% of those in patients with Marfan syndrome were in aortas smaller than 5.0 cm in diameter, that aortic dissection occurred at smaller diameters in shorter patients, and that the risk of dissection increased exponentially with the size of the aorta. Subsequently, we found that a better measure of risk is the maximal aortic cross-sectional area in cm2 divided by the patient’s height in meters; if this ratio exceeds 10, then surgery is recommended.12
Results of surgery are good in experienced hands. In patients who undergo surgical repair of bicuspid aortic valves with or without concurrent repair of the ascending aorta (mostly in patients with an aortic cross-section-to-height ratio > 10), the perioperative mortality rate is about 1.0% for both groups, and at 10 years about 98% of patients are free from re-operation on the aorta and more than 90% are free from re-operation on the aortic valve.8 This is important because these are typically young patients who would do better without biological replacement valves (which are not very durable) or mechanical valves (which necessitate lifelong anticoagulation). Results are also good in surgery of the aortic arch and even better in patients with tricuspid aortic valves.4,6,8
Increasingly, in patients at high risk, we are inserting thoracic, abdominal, and thoracoabdominal stent grafts, with excellent early results. An even newer innovation is to replace the aortic valve in high-risk patients via a transcatheter balloon-expandable valve stent inserted through the groin or left ventricular apex.
These treatment innovations have been big strides, but aortic disease continues to increase. Indeed, our volume of thoracic aortic surgery at Cleveland Clinic increased from 190 procedures in 1999 to 717 in 2006. Early detection—before acute emergency surgery is required, with its concomitant high risk of death—is the key to successful surgical outcome and long-term survival.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
- Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation 1999; 99:1331–1336.
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75:7–24.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002; 74:2040–2046.
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004; 78:109–116.
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005; 112:2619–2626.
- Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg 2003; 76:1751–1753.
- Svensson LG, Blackstone EH, Feng J, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg 2007; 83:1067–1074.
- Svensson LG, Blackstone EH, Cosgrove DM 3rd. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Rodriguez ER. Aortic organ disease epidemic, and why do balloons pop? Circulation 2005; 112:1082–1084.
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280:1926–1929.
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection inpatients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–893.
Handoffs
Editorial / Kutner
Communication, palliative care, and patient safety have been identified by the Society of Hospital Medicine as core competencies in hospital medicine. Effective communication is recognized as being central to the role of the hospitalist to promote efficient, safe, and high quality care.1 Hospitalists are increasingly recognized as having a central role in initiatives to improve palliative care for hospitalized patients and their families24 and have a vital role in leading and participating in interventions to mitigate system and process failures that affect patient safety.1 The obligation of the hospitalist to assure safe, quality care for hospitalized people with advanced illness extends from direct patient care to advocacy for systems that facilitate the provision of such care.
Four articles in this issue of the Journal of Hospital Medicine provide complementary perspectives on these crucial roles of the hospitalist. Cherlin and colleagues describe findings from a survey of hospitalists and medical residents regarding their knowledge, attitudes, and practices relative to caring for patients with terminal illness. The article identifies misperceptions related to core components of quality palliative care: pain and symptom control, hospice eligibility, and communication about prognosis and hospice and palliative care.5 Although this study was conducted at only a single academic medical center and certainly deserves to be repeated in an expanded and more representative sample, it clearly identifies deficits in core components of quality care for persons with advanced illness. The article by Minichiello and colleagues provides practical guidance and resources for addressing one of the deficits identified: communicating a poor prognosis, or bad news.6
Pain and symptom management and communication are commonly recognized aspects of quality care for persons with advanced illness. Less often appreciated are the significant threats to patient safety and medical errors that occur in the care of this vulnerable population.79 Potential errors include failure of a planned action to be completed as intended (ie, not following advance directives) and failure to treat symptoms adequately. The original research article and accompanying images discussion by Sehgal and colleagues serve as a call to action to both recognize and address the potentially significant patient safety issue related to the use of color‐coded wristbands, particularly variation in color used by different hospitals to designate do not resuscitate status.10, 11 What is exciting about this sequence of articles is that they describe opportunities for improvement and provide potential solutions. We have to be aware that there is a problem in order to initiate change. Hospitalists are in an a prime position to both identify these potential critical issues and effect the necessary changes to facilitate our ability to provide safe, effective care to our patients with advanced illness.
Palliative care is increasingly being accepted as a means for improving care for persons with advanced illness. The National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, released in 2004, was endorsed by the National Quality Forum and incorporated into its Framework for Hospice and Palliative Care in 2007.12, 13 The Joint Commission (TJC; previously known as JCAHO) is developing a Health Care Services Certification Program for palliative care services modeled on existing programs for diabetes and stroke care, to take effect in 2008.14 Newsweek featured palliative care in its August 2006 issue focused on Fixing America's Hospitals.15 US News and World Report has included hospice and palliative care indicators in its ranking of America's Best Hospitals since 2002.16 There has been significant recent growth in hospital‐based palliative care programs, with 1250 hospitals reporting palliative care programs in 2005, an increase of almost 100% over 2000. Seventy percent of U.S. hospitals with more than 250 beds report having a palliative care program.17
Although hospital‐based palliative care programs are increasing, it is the obligation of all hospitalists who care for an ill, often elderly population to assure that all hospitalized patients with advanced illness receive safe, quality care while hospitalized. This includes avoiding medical errors such as inappropriate resuscitation attempts because of miscommunication of do‐not‐resuscitate orders or advance directives, as well as minimizing distress, maximizing comfort, and addressing informational and psychosocial support needs. As evidenced by the 4 articles in this issue of the Journal of Hospital Medicine, we need to make safe, effective care for people with advanced illness a priority, then implement appropriate training and education and create systems that assure delivery of quality care.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:1–67.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.Am J Med.2001;111:10S–14S.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- .Palliative Care in Hospitals.J Hosp Med.2006;1:21–28.
- ,,,,,.Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting.J Hosp Med.2007;2:357–365.
- ,, andBreaking bad news: a practical approach for the hospitalist.J Hosp Med.2007;2:415–421.
- ,.Patients with eventually fatal chronic illness: their importance within a national research agenda on improving patient safety and reducing medical errors.J Palliat Med.2001;4:325–332.
- ,.Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering.Ann Intern Med.2003;138:812–818.
- ,.Mortality as a measure of quality: implications for palliative and end‐of‐life care.JAMA.2007;298:802–804.
- ,.Color‐coded wristbands: promoting safety or confusion?J Hosp Med.2007;2:445.
- ,.Identification of inpatient DNR status: a safety hazard begging for standardization.J Hosp Med.2007;2:366–371.
- National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. Available at: http://www.nationalconsensusproject.org. Accessed August 26,2007.
- National Quality Forum. Available at: Available at: http://www.qualityforum.org. Accessed August 25,2007.
- Joint Commission for Accreditation of Health Care Organizations. Available at: http://www.jointcommission.org. Accessed August 26,2007.
- .Special Care at the End of Life.Newsweek. October 16,2006. Available at: http://www.msnbc.msn.com/id/15175919/site/newsweek/page/0/. Accessed September 22,year="2007"2007.
- U.S. News and World Report America's Best Hospitals 2007 Methodology. Available at: http://health.usnews.com/usnews/health/best‐hospitals/methodology_report.pdf. Accessed September 22,2007.
- Center to Advance Palliative Care (CAPC). Available at: http://www.capc.org. Accessed August 26,2007.
Communication, palliative care, and patient safety have been identified by the Society of Hospital Medicine as core competencies in hospital medicine. Effective communication is recognized as being central to the role of the hospitalist to promote efficient, safe, and high quality care.1 Hospitalists are increasingly recognized as having a central role in initiatives to improve palliative care for hospitalized patients and their families24 and have a vital role in leading and participating in interventions to mitigate system and process failures that affect patient safety.1 The obligation of the hospitalist to assure safe, quality care for hospitalized people with advanced illness extends from direct patient care to advocacy for systems that facilitate the provision of such care.
Four articles in this issue of the Journal of Hospital Medicine provide complementary perspectives on these crucial roles of the hospitalist. Cherlin and colleagues describe findings from a survey of hospitalists and medical residents regarding their knowledge, attitudes, and practices relative to caring for patients with terminal illness. The article identifies misperceptions related to core components of quality palliative care: pain and symptom control, hospice eligibility, and communication about prognosis and hospice and palliative care.5 Although this study was conducted at only a single academic medical center and certainly deserves to be repeated in an expanded and more representative sample, it clearly identifies deficits in core components of quality care for persons with advanced illness. The article by Minichiello and colleagues provides practical guidance and resources for addressing one of the deficits identified: communicating a poor prognosis, or bad news.6
Pain and symptom management and communication are commonly recognized aspects of quality care for persons with advanced illness. Less often appreciated are the significant threats to patient safety and medical errors that occur in the care of this vulnerable population.79 Potential errors include failure of a planned action to be completed as intended (ie, not following advance directives) and failure to treat symptoms adequately. The original research article and accompanying images discussion by Sehgal and colleagues serve as a call to action to both recognize and address the potentially significant patient safety issue related to the use of color‐coded wristbands, particularly variation in color used by different hospitals to designate do not resuscitate status.10, 11 What is exciting about this sequence of articles is that they describe opportunities for improvement and provide potential solutions. We have to be aware that there is a problem in order to initiate change. Hospitalists are in an a prime position to both identify these potential critical issues and effect the necessary changes to facilitate our ability to provide safe, effective care to our patients with advanced illness.
Palliative care is increasingly being accepted as a means for improving care for persons with advanced illness. The National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, released in 2004, was endorsed by the National Quality Forum and incorporated into its Framework for Hospice and Palliative Care in 2007.12, 13 The Joint Commission (TJC; previously known as JCAHO) is developing a Health Care Services Certification Program for palliative care services modeled on existing programs for diabetes and stroke care, to take effect in 2008.14 Newsweek featured palliative care in its August 2006 issue focused on Fixing America's Hospitals.15 US News and World Report has included hospice and palliative care indicators in its ranking of America's Best Hospitals since 2002.16 There has been significant recent growth in hospital‐based palliative care programs, with 1250 hospitals reporting palliative care programs in 2005, an increase of almost 100% over 2000. Seventy percent of U.S. hospitals with more than 250 beds report having a palliative care program.17
Although hospital‐based palliative care programs are increasing, it is the obligation of all hospitalists who care for an ill, often elderly population to assure that all hospitalized patients with advanced illness receive safe, quality care while hospitalized. This includes avoiding medical errors such as inappropriate resuscitation attempts because of miscommunication of do‐not‐resuscitate orders or advance directives, as well as minimizing distress, maximizing comfort, and addressing informational and psychosocial support needs. As evidenced by the 4 articles in this issue of the Journal of Hospital Medicine, we need to make safe, effective care for people with advanced illness a priority, then implement appropriate training and education and create systems that assure delivery of quality care.
Communication, palliative care, and patient safety have been identified by the Society of Hospital Medicine as core competencies in hospital medicine. Effective communication is recognized as being central to the role of the hospitalist to promote efficient, safe, and high quality care.1 Hospitalists are increasingly recognized as having a central role in initiatives to improve palliative care for hospitalized patients and their families24 and have a vital role in leading and participating in interventions to mitigate system and process failures that affect patient safety.1 The obligation of the hospitalist to assure safe, quality care for hospitalized people with advanced illness extends from direct patient care to advocacy for systems that facilitate the provision of such care.
Four articles in this issue of the Journal of Hospital Medicine provide complementary perspectives on these crucial roles of the hospitalist. Cherlin and colleagues describe findings from a survey of hospitalists and medical residents regarding their knowledge, attitudes, and practices relative to caring for patients with terminal illness. The article identifies misperceptions related to core components of quality palliative care: pain and symptom control, hospice eligibility, and communication about prognosis and hospice and palliative care.5 Although this study was conducted at only a single academic medical center and certainly deserves to be repeated in an expanded and more representative sample, it clearly identifies deficits in core components of quality care for persons with advanced illness. The article by Minichiello and colleagues provides practical guidance and resources for addressing one of the deficits identified: communicating a poor prognosis, or bad news.6
Pain and symptom management and communication are commonly recognized aspects of quality care for persons with advanced illness. Less often appreciated are the significant threats to patient safety and medical errors that occur in the care of this vulnerable population.79 Potential errors include failure of a planned action to be completed as intended (ie, not following advance directives) and failure to treat symptoms adequately. The original research article and accompanying images discussion by Sehgal and colleagues serve as a call to action to both recognize and address the potentially significant patient safety issue related to the use of color‐coded wristbands, particularly variation in color used by different hospitals to designate do not resuscitate status.10, 11 What is exciting about this sequence of articles is that they describe opportunities for improvement and provide potential solutions. We have to be aware that there is a problem in order to initiate change. Hospitalists are in an a prime position to both identify these potential critical issues and effect the necessary changes to facilitate our ability to provide safe, effective care to our patients with advanced illness.
Palliative care is increasingly being accepted as a means for improving care for persons with advanced illness. The National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, released in 2004, was endorsed by the National Quality Forum and incorporated into its Framework for Hospice and Palliative Care in 2007.12, 13 The Joint Commission (TJC; previously known as JCAHO) is developing a Health Care Services Certification Program for palliative care services modeled on existing programs for diabetes and stroke care, to take effect in 2008.14 Newsweek featured palliative care in its August 2006 issue focused on Fixing America's Hospitals.15 US News and World Report has included hospice and palliative care indicators in its ranking of America's Best Hospitals since 2002.16 There has been significant recent growth in hospital‐based palliative care programs, with 1250 hospitals reporting palliative care programs in 2005, an increase of almost 100% over 2000. Seventy percent of U.S. hospitals with more than 250 beds report having a palliative care program.17
Although hospital‐based palliative care programs are increasing, it is the obligation of all hospitalists who care for an ill, often elderly population to assure that all hospitalized patients with advanced illness receive safe, quality care while hospitalized. This includes avoiding medical errors such as inappropriate resuscitation attempts because of miscommunication of do‐not‐resuscitate orders or advance directives, as well as minimizing distress, maximizing comfort, and addressing informational and psychosocial support needs. As evidenced by the 4 articles in this issue of the Journal of Hospital Medicine, we need to make safe, effective care for people with advanced illness a priority, then implement appropriate training and education and create systems that assure delivery of quality care.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:1–67.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.Am J Med.2001;111:10S–14S.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- .Palliative Care in Hospitals.J Hosp Med.2006;1:21–28.
- ,,,,,.Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting.J Hosp Med.2007;2:357–365.
- ,, andBreaking bad news: a practical approach for the hospitalist.J Hosp Med.2007;2:415–421.
- ,.Patients with eventually fatal chronic illness: their importance within a national research agenda on improving patient safety and reducing medical errors.J Palliat Med.2001;4:325–332.
- ,.Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering.Ann Intern Med.2003;138:812–818.
- ,.Mortality as a measure of quality: implications for palliative and end‐of‐life care.JAMA.2007;298:802–804.
- ,.Color‐coded wristbands: promoting safety or confusion?J Hosp Med.2007;2:445.
- ,.Identification of inpatient DNR status: a safety hazard begging for standardization.J Hosp Med.2007;2:366–371.
- National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. Available at: http://www.nationalconsensusproject.org. Accessed August 26,2007.
- National Quality Forum. Available at: Available at: http://www.qualityforum.org. Accessed August 25,2007.
- Joint Commission for Accreditation of Health Care Organizations. Available at: http://www.jointcommission.org. Accessed August 26,2007.
- .Special Care at the End of Life.Newsweek. October 16,2006. Available at: http://www.msnbc.msn.com/id/15175919/site/newsweek/page/0/. Accessed September 22,year="2007"2007.
- U.S. News and World Report America's Best Hospitals 2007 Methodology. Available at: http://health.usnews.com/usnews/health/best‐hospitals/methodology_report.pdf. Accessed September 22,2007.
- Center to Advance Palliative Care (CAPC). Available at: http://www.capc.org. Accessed August 26,2007.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:1–67.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.Am J Med.2001;111:10S–14S.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- .Palliative Care in Hospitals.J Hosp Med.2006;1:21–28.
- ,,,,,.Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting.J Hosp Med.2007;2:357–365.
- ,, andBreaking bad news: a practical approach for the hospitalist.J Hosp Med.2007;2:415–421.
- ,.Patients with eventually fatal chronic illness: their importance within a national research agenda on improving patient safety and reducing medical errors.J Palliat Med.2001;4:325–332.
- ,.Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering.Ann Intern Med.2003;138:812–818.
- ,.Mortality as a measure of quality: implications for palliative and end‐of‐life care.JAMA.2007;298:802–804.
- ,.Color‐coded wristbands: promoting safety or confusion?J Hosp Med.2007;2:445.
- ,.Identification of inpatient DNR status: a safety hazard begging for standardization.J Hosp Med.2007;2:366–371.
- National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. Available at: http://www.nationalconsensusproject.org. Accessed August 26,2007.
- National Quality Forum. Available at: Available at: http://www.qualityforum.org. Accessed August 25,2007.
- Joint Commission for Accreditation of Health Care Organizations. Available at: http://www.jointcommission.org. Accessed August 26,2007.
- .Special Care at the End of Life.Newsweek. October 16,2006. Available at: http://www.msnbc.msn.com/id/15175919/site/newsweek/page/0/. Accessed September 22,year="2007"2007.
- U.S. News and World Report America's Best Hospitals 2007 Methodology. Available at: http://health.usnews.com/usnews/health/best‐hospitals/methodology_report.pdf. Accessed September 22,2007.
- Center to Advance Palliative Care (CAPC). Available at: http://www.capc.org. Accessed August 26,2007.
Editorial
As a hospital practitioner, you have undoubtedly experienced the frustration of witnessing how easily the excellent care you provide can unravel as the patient goes out the door. Patients are admitted acutely ill, and largely attributed to your clinical acumen, they are discharged tuned up and stable to return home. Days later, however, you may learn that your best‐laid discharge plans were not properly executed, and the patient returned with yet another exacerbation. Clearly this scenario represents a major setback for the patient and family caregivers. Possibly dismissed as another episode of patient noncompliance, such readmissions are now being recognized as system failures and reflect a discharge process that has been described as random events connected to highly variable actions with only a remote possibility of meeting implied expectations (Roger Resar, MD, Senior Fellow, Institute for Healthcare Improvement).
Once an area that received relatively little attention, transitions out of the hospital has been identified as a priority area in need of action by a confluence of recent research and national activities. Recognizing the expanding evidence for lapses in quality and safety, many esteemed organizations, including the Joint Commission, the Centers for Medicare and Medicaid Services and their accompanying Quality Improvement Organizations, the Institute for Healthcare Improvement, the Institute of Medicine, National Quality Forum, the Medicare Payment Advisory Committee, the American Board of Internal Medicine Foundation, the National Transitions of Care Coalition, the American College of Physicians, the Society for General Medicine, and the Society for Hospital Medicine, are currently focusing their efforts on how to optimize transitions. All have articulated the need for further clinical investigation that can offer greater insight into the nature of the problems that arise during this vulnerable period and what the potential solutions are.
In this edition of the Journal of Hospital Medicine, 3 teams of investigators have responded to this need, making timely, important, and unique contributions to advance the field.13 Specifically, each of these articles further raises awareness that a patient's transition out of the hospital often unfolds quickly in a fast‐paced, chaotic manner, placing many competing demands on clinicians, patients, and family caregivers. Not surprisingly, such competing demands can contribute to deficits in quality and safety. The authors of these studies all directly identify the central role of communication among clinicians as well as between patients and clinicians in ensuring successful handoffs, further affirming the Joint Commission's finding that inadequate communication is the leading cause of sentinel events.4 In this respect, communication is more than simply the transfer of information; rather, it involves the need to ensure comprehension and provide an opportunity to have a 2‐way dialogue. Importantly, these articles share a common approach in fostering our understanding of the perspective of patients and family caregivers with a particular focus on disadvantaged populations.
Kripalani and colleagues conducted a comprehensive review of the state of the science for those key hospital discharge issues that pertain to hospitalists. They identified a number of challenges including communication between hospital‐ and ambulatory‐based clinicians, medication reconciliation, timely hospital follow‐up, and engaging patients in self‐care. For each of these priority areas, the authors provide pragmatic recommendations for improving care that could be implemented within the current state of practice, either individually or as a bundle of interventions.
Recognizing that patients are often the only common thread across different sites of care, Strunin and colleagues demonstrate the value of including the voice of the patient in helping clinicians to understand the challenges and larger context in which they attempt to follow through with discharge instructions. Strunin et al. found that among a range of factors that contribute to adherence problems, many were nonmedical. Fortunately, a number of these are modifiable and point to the need to both prioritize recommendations to patients and to simplify them whenever possible. The authors' findings resonate with a growing literature that examines the hospital discharge process from the patient's perspective.510
Flacker and colleagues surveyed older patients to gain greater insights into their information needs at discharge. From a process standpoint, they demonstrated that a posthospital survey was feasible and acceptable to older patients. In and of itself, this finding has important implications in the context of national efforts aimed at implementing performance measurement and accompanying public reporting. It also may reflect patients' eagerness to be contacted after discharge; hospitalization is a major event in patients' lives, and attentive follow‐up is appreciated. The authors found that more than half of patients did not recall being asked about how they would care for themselves on returning home from the hospital. Although there may be a variety of explanations for this finding, it nevertheless points to an immediate area for intervention.
Collectively, these 3 articles set the stage for a proposed clinical investigation agenda aimed at optimizing transitions out of the hospital (see Table 1).
| 1. Greater recognition of the integral role of family caregivers |
| 2. Empirically define the appropriate follow‐up interval |
| 3. Define physician accountability for patients referred to home health on hospital discharge |
| 4. Delineate the role of the hospitalist in the advanced medical home |
| 5. Develop the ability to examine episodes of care |
These 5 recommendations have the potential to ensure that the gains patients make in our hospitals are maintained long after discharge.
-
Greater Recognition for the Integral Role of Family Caregivers. The patient and family caregivers should be integrated into health care professionals' efforts to improve care coordination across settings. Family caregivers have been silent partners in health care delivery, functioning as de facto care coordinators. During care handoffs, family caregivers make important contributions to ensuring quality, safety, and adherence to patient preferences; their role needs to be formally recognized and supported. An important initial step would be standardizing the approach to defining the types and intensity of the roles family caregivers play to facilitate improved communication. One proposed working definition is the FACED classification developed by one of the authors (E.C.). Modeled after the TNM system used in cancer, each letter of FACED refers to a different contribution made by a family caregiver: F = Financial; A = Advocacy; C = Care coordination; E = Emotional support; and D = Direct care provision. A simple numeric rating system could be developed whereby 0 = does not contribute in this area and 3 = makes significant contribution to this area. Such a straightforward approach would readily inform all members of the health care team about the caregivers' roles and capabilities and how they can optimally collaborate in the care plan.
-
Empirically Define Appropriate Follow‐Up Interval. At present, patients are given rather arbitrary and generic instructions for when to obtain follow‐up with their outpatient primary care physician or specialist. Surgical patients are often instructed to follow up with their surgeon, and yet most of the readmissions of these patients are attributable to medical conditions (personal communication, Steven Jencks, MD, Centers for Medicare and Medicaid Services). Furthermore, a significant number of discharged medical and surgical patients are readmitted to the hospital within 30 days without any outpatient contact with a health professional. One may envision an evidence‐based tiered approach whereby patients are assigned a hospital readmission risk score at the time of discharge that then determines the timing of their follow‐up appointment. Using this framework, the highest‐risk patients may be encouraged to receive follow‐up within 2472 hours, whereas lower‐risk patients may be able to wait 1421 days. Of course, there will need to be sufficient access to outpatient physicians, who will need to be available, to ensure the success of this strategy.
-
Define Physician Accountability for Patients Referred to Home Health on Hospital Discharge. Communication problems between the hospital and the home health care agency are a source of aggravation for both parties, not to mention patients. Typically, a hospitalist provides the initial order for services and then expects subsequent home care coordination to be managed by an outpatient physician. Unfortunately, in some cases the patient may not have an outpatient physician or the patient's primary physician may be unaware of the recent hospitalization and thus unwilling to assume management of an unfamiliar care plan. As a result, home care nurses often cannot identify a physician to respond to their questions or concerns. At the center of this problem lies a lack of understanding of where the responsibility of the ordering hospitalist ends and the outpatient physician assuming care begins. Recognizing the profound costs of failed home health care leading to hospital readmission, the nation's Quality Improvement Organizations launched a national campaign in 2006 to address this problem. Hospitalists should engage in this effort and not punt the entire responsibility to home health agenciesimagine if hospitals and hospitalists were financially penalized if a patient was readmitted.
-
Delineate the Role of the Hospitalist in the Advanced Medical Home. Modeled after a concept with origins in pediatrics, the American College of Physicians and American Academy of Family Physicians are promoting the advanced medical home as a new care model that aims to provide comprehensive ambulatory care with an explicit focus on care coordination.11 The Centers for Medicare and Medicaid Services is planning to initiate a demonstration of this approach. What has not been adequately underscored is how the advanced medical home will communicate essential clinical information with the hospitalist and what, if any, will be the role of the hospitalist in relation to a patient's medical home? Ideally, the medical home approach will alleviate many of the current access problems that impede timely follow‐up.
-
Develop Ability to Examine Episodes of Care. Patients with complex conditions often require care from different practitioners in multiple settings. From the vantage point of health care professionals, these may appear to occur as merely a string of individual interactions, including hospital admissions and discharges. However from the patient's perspective, the experience is more appropriately characterized as a journey across an aggregated episode of care. The National Quality Forum is currently exploring how to measure quality of care delivered across such an episode of care. Additionally, the Centers for Medicare and Medicaid Services is developing a new assessment tool that will transcend acute and postacute care settings, the Continuity Assessment Record and Evaluation (CARE). This tool will potentially enhance our ability to measure care across a predefined episode. Measurement can further pave the way for payment reform designed to align incentives toward higher‐quality care transitions. Currently, professional fees for coronary artery bypass grafting surgery are bundled across an episode, including hospital and posthospital care settings. Extending this approach to a wider array of conditions and services could encourage new perspectives on the timing of discharge and the use of posthospital care venues. For example, under bundled payment, incentives might support a plan to keep a patient in the hospital an extra 1 or 2 days in order to obviate a transfer to a skilled nursing facility and the concomitant risks of transfer‐related problems. Further, bundled payment may allow for the provision of additional services not currently covered, including transportation, as identified by Strunin and colleagues.3
Hospitalists are well positioned to offer leadership in these high‐leverage areas and thereby make a unique contribution to the quality and safety of care transitions. By so doing, they are poised to reaffirm their professionalism,12 ensuring that the excellent care that they provide in the hospital is sustained well into the future.
- ,,.Discharge information and older patients: do they get what they need?J Hosp Med.2007;2:291–296.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,.Understanding rehospitalization risk: can the hospital discharge be modified to impact recurrent hospitalization.J Hosp Med.2007;2:297–304.
- The Joint Commission. Sentinel event statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed August 7,2007.
- ,,.Patients' and caregivers' transition from hospital to home: needs and recommendations.Home Health Care Serv Q.1998;17:27–48.
- ,,,,.Carepartner experiences with hospital care.Med Care.1999;37:33–38.
- ,.Understanding coordination of care from the consumer's perspective in a regional health system.Health Serv Res.2002;37:1031–1054.
- ,,, et al.Development and testing of a measure designed to assess the quality of care transitions.Int J Integrated Care.2002;2:e02.
- .Rough crossings: family caregivers odysseys through the health care system.New York:United Hospital Fund of New York;1998.
- ,.Facilitating the transition out of the hospital. In:Gerteis M,Edgman‐Levitan S,Daley J,Delbanco T, editors.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco:Jossey‐Bass;1993:204–223.
- ,,American College of Physicians. The advanced medical home: a patient‐centered, physician‐guided model of health care. p. 1–22. http://www.acponline.org/hpp/adv_med.pdf Accessed August 7,2007.
- Project of the ABIM Foundation A‐AFaEFoIM.Medical professionalism in the new millennium: a physician charter.Ann Intern Med.2002;136:243–246.
As a hospital practitioner, you have undoubtedly experienced the frustration of witnessing how easily the excellent care you provide can unravel as the patient goes out the door. Patients are admitted acutely ill, and largely attributed to your clinical acumen, they are discharged tuned up and stable to return home. Days later, however, you may learn that your best‐laid discharge plans were not properly executed, and the patient returned with yet another exacerbation. Clearly this scenario represents a major setback for the patient and family caregivers. Possibly dismissed as another episode of patient noncompliance, such readmissions are now being recognized as system failures and reflect a discharge process that has been described as random events connected to highly variable actions with only a remote possibility of meeting implied expectations (Roger Resar, MD, Senior Fellow, Institute for Healthcare Improvement).
Once an area that received relatively little attention, transitions out of the hospital has been identified as a priority area in need of action by a confluence of recent research and national activities. Recognizing the expanding evidence for lapses in quality and safety, many esteemed organizations, including the Joint Commission, the Centers for Medicare and Medicaid Services and their accompanying Quality Improvement Organizations, the Institute for Healthcare Improvement, the Institute of Medicine, National Quality Forum, the Medicare Payment Advisory Committee, the American Board of Internal Medicine Foundation, the National Transitions of Care Coalition, the American College of Physicians, the Society for General Medicine, and the Society for Hospital Medicine, are currently focusing their efforts on how to optimize transitions. All have articulated the need for further clinical investigation that can offer greater insight into the nature of the problems that arise during this vulnerable period and what the potential solutions are.
In this edition of the Journal of Hospital Medicine, 3 teams of investigators have responded to this need, making timely, important, and unique contributions to advance the field.13 Specifically, each of these articles further raises awareness that a patient's transition out of the hospital often unfolds quickly in a fast‐paced, chaotic manner, placing many competing demands on clinicians, patients, and family caregivers. Not surprisingly, such competing demands can contribute to deficits in quality and safety. The authors of these studies all directly identify the central role of communication among clinicians as well as between patients and clinicians in ensuring successful handoffs, further affirming the Joint Commission's finding that inadequate communication is the leading cause of sentinel events.4 In this respect, communication is more than simply the transfer of information; rather, it involves the need to ensure comprehension and provide an opportunity to have a 2‐way dialogue. Importantly, these articles share a common approach in fostering our understanding of the perspective of patients and family caregivers with a particular focus on disadvantaged populations.
Kripalani and colleagues conducted a comprehensive review of the state of the science for those key hospital discharge issues that pertain to hospitalists. They identified a number of challenges including communication between hospital‐ and ambulatory‐based clinicians, medication reconciliation, timely hospital follow‐up, and engaging patients in self‐care. For each of these priority areas, the authors provide pragmatic recommendations for improving care that could be implemented within the current state of practice, either individually or as a bundle of interventions.
Recognizing that patients are often the only common thread across different sites of care, Strunin and colleagues demonstrate the value of including the voice of the patient in helping clinicians to understand the challenges and larger context in which they attempt to follow through with discharge instructions. Strunin et al. found that among a range of factors that contribute to adherence problems, many were nonmedical. Fortunately, a number of these are modifiable and point to the need to both prioritize recommendations to patients and to simplify them whenever possible. The authors' findings resonate with a growing literature that examines the hospital discharge process from the patient's perspective.510
Flacker and colleagues surveyed older patients to gain greater insights into their information needs at discharge. From a process standpoint, they demonstrated that a posthospital survey was feasible and acceptable to older patients. In and of itself, this finding has important implications in the context of national efforts aimed at implementing performance measurement and accompanying public reporting. It also may reflect patients' eagerness to be contacted after discharge; hospitalization is a major event in patients' lives, and attentive follow‐up is appreciated. The authors found that more than half of patients did not recall being asked about how they would care for themselves on returning home from the hospital. Although there may be a variety of explanations for this finding, it nevertheless points to an immediate area for intervention.
Collectively, these 3 articles set the stage for a proposed clinical investigation agenda aimed at optimizing transitions out of the hospital (see Table 1).
| 1. Greater recognition of the integral role of family caregivers |
| 2. Empirically define the appropriate follow‐up interval |
| 3. Define physician accountability for patients referred to home health on hospital discharge |
| 4. Delineate the role of the hospitalist in the advanced medical home |
| 5. Develop the ability to examine episodes of care |
These 5 recommendations have the potential to ensure that the gains patients make in our hospitals are maintained long after discharge.
-
Greater Recognition for the Integral Role of Family Caregivers. The patient and family caregivers should be integrated into health care professionals' efforts to improve care coordination across settings. Family caregivers have been silent partners in health care delivery, functioning as de facto care coordinators. During care handoffs, family caregivers make important contributions to ensuring quality, safety, and adherence to patient preferences; their role needs to be formally recognized and supported. An important initial step would be standardizing the approach to defining the types and intensity of the roles family caregivers play to facilitate improved communication. One proposed working definition is the FACED classification developed by one of the authors (E.C.). Modeled after the TNM system used in cancer, each letter of FACED refers to a different contribution made by a family caregiver: F = Financial; A = Advocacy; C = Care coordination; E = Emotional support; and D = Direct care provision. A simple numeric rating system could be developed whereby 0 = does not contribute in this area and 3 = makes significant contribution to this area. Such a straightforward approach would readily inform all members of the health care team about the caregivers' roles and capabilities and how they can optimally collaborate in the care plan.
-
Empirically Define Appropriate Follow‐Up Interval. At present, patients are given rather arbitrary and generic instructions for when to obtain follow‐up with their outpatient primary care physician or specialist. Surgical patients are often instructed to follow up with their surgeon, and yet most of the readmissions of these patients are attributable to medical conditions (personal communication, Steven Jencks, MD, Centers for Medicare and Medicaid Services). Furthermore, a significant number of discharged medical and surgical patients are readmitted to the hospital within 30 days without any outpatient contact with a health professional. One may envision an evidence‐based tiered approach whereby patients are assigned a hospital readmission risk score at the time of discharge that then determines the timing of their follow‐up appointment. Using this framework, the highest‐risk patients may be encouraged to receive follow‐up within 2472 hours, whereas lower‐risk patients may be able to wait 1421 days. Of course, there will need to be sufficient access to outpatient physicians, who will need to be available, to ensure the success of this strategy.
-
Define Physician Accountability for Patients Referred to Home Health on Hospital Discharge. Communication problems between the hospital and the home health care agency are a source of aggravation for both parties, not to mention patients. Typically, a hospitalist provides the initial order for services and then expects subsequent home care coordination to be managed by an outpatient physician. Unfortunately, in some cases the patient may not have an outpatient physician or the patient's primary physician may be unaware of the recent hospitalization and thus unwilling to assume management of an unfamiliar care plan. As a result, home care nurses often cannot identify a physician to respond to their questions or concerns. At the center of this problem lies a lack of understanding of where the responsibility of the ordering hospitalist ends and the outpatient physician assuming care begins. Recognizing the profound costs of failed home health care leading to hospital readmission, the nation's Quality Improvement Organizations launched a national campaign in 2006 to address this problem. Hospitalists should engage in this effort and not punt the entire responsibility to home health agenciesimagine if hospitals and hospitalists were financially penalized if a patient was readmitted.
-
Delineate the Role of the Hospitalist in the Advanced Medical Home. Modeled after a concept with origins in pediatrics, the American College of Physicians and American Academy of Family Physicians are promoting the advanced medical home as a new care model that aims to provide comprehensive ambulatory care with an explicit focus on care coordination.11 The Centers for Medicare and Medicaid Services is planning to initiate a demonstration of this approach. What has not been adequately underscored is how the advanced medical home will communicate essential clinical information with the hospitalist and what, if any, will be the role of the hospitalist in relation to a patient's medical home? Ideally, the medical home approach will alleviate many of the current access problems that impede timely follow‐up.
-
Develop Ability to Examine Episodes of Care. Patients with complex conditions often require care from different practitioners in multiple settings. From the vantage point of health care professionals, these may appear to occur as merely a string of individual interactions, including hospital admissions and discharges. However from the patient's perspective, the experience is more appropriately characterized as a journey across an aggregated episode of care. The National Quality Forum is currently exploring how to measure quality of care delivered across such an episode of care. Additionally, the Centers for Medicare and Medicaid Services is developing a new assessment tool that will transcend acute and postacute care settings, the Continuity Assessment Record and Evaluation (CARE). This tool will potentially enhance our ability to measure care across a predefined episode. Measurement can further pave the way for payment reform designed to align incentives toward higher‐quality care transitions. Currently, professional fees for coronary artery bypass grafting surgery are bundled across an episode, including hospital and posthospital care settings. Extending this approach to a wider array of conditions and services could encourage new perspectives on the timing of discharge and the use of posthospital care venues. For example, under bundled payment, incentives might support a plan to keep a patient in the hospital an extra 1 or 2 days in order to obviate a transfer to a skilled nursing facility and the concomitant risks of transfer‐related problems. Further, bundled payment may allow for the provision of additional services not currently covered, including transportation, as identified by Strunin and colleagues.3
Hospitalists are well positioned to offer leadership in these high‐leverage areas and thereby make a unique contribution to the quality and safety of care transitions. By so doing, they are poised to reaffirm their professionalism,12 ensuring that the excellent care that they provide in the hospital is sustained well into the future.
As a hospital practitioner, you have undoubtedly experienced the frustration of witnessing how easily the excellent care you provide can unravel as the patient goes out the door. Patients are admitted acutely ill, and largely attributed to your clinical acumen, they are discharged tuned up and stable to return home. Days later, however, you may learn that your best‐laid discharge plans were not properly executed, and the patient returned with yet another exacerbation. Clearly this scenario represents a major setback for the patient and family caregivers. Possibly dismissed as another episode of patient noncompliance, such readmissions are now being recognized as system failures and reflect a discharge process that has been described as random events connected to highly variable actions with only a remote possibility of meeting implied expectations (Roger Resar, MD, Senior Fellow, Institute for Healthcare Improvement).
Once an area that received relatively little attention, transitions out of the hospital has been identified as a priority area in need of action by a confluence of recent research and national activities. Recognizing the expanding evidence for lapses in quality and safety, many esteemed organizations, including the Joint Commission, the Centers for Medicare and Medicaid Services and their accompanying Quality Improvement Organizations, the Institute for Healthcare Improvement, the Institute of Medicine, National Quality Forum, the Medicare Payment Advisory Committee, the American Board of Internal Medicine Foundation, the National Transitions of Care Coalition, the American College of Physicians, the Society for General Medicine, and the Society for Hospital Medicine, are currently focusing their efforts on how to optimize transitions. All have articulated the need for further clinical investigation that can offer greater insight into the nature of the problems that arise during this vulnerable period and what the potential solutions are.
In this edition of the Journal of Hospital Medicine, 3 teams of investigators have responded to this need, making timely, important, and unique contributions to advance the field.13 Specifically, each of these articles further raises awareness that a patient's transition out of the hospital often unfolds quickly in a fast‐paced, chaotic manner, placing many competing demands on clinicians, patients, and family caregivers. Not surprisingly, such competing demands can contribute to deficits in quality and safety. The authors of these studies all directly identify the central role of communication among clinicians as well as between patients and clinicians in ensuring successful handoffs, further affirming the Joint Commission's finding that inadequate communication is the leading cause of sentinel events.4 In this respect, communication is more than simply the transfer of information; rather, it involves the need to ensure comprehension and provide an opportunity to have a 2‐way dialogue. Importantly, these articles share a common approach in fostering our understanding of the perspective of patients and family caregivers with a particular focus on disadvantaged populations.
Kripalani and colleagues conducted a comprehensive review of the state of the science for those key hospital discharge issues that pertain to hospitalists. They identified a number of challenges including communication between hospital‐ and ambulatory‐based clinicians, medication reconciliation, timely hospital follow‐up, and engaging patients in self‐care. For each of these priority areas, the authors provide pragmatic recommendations for improving care that could be implemented within the current state of practice, either individually or as a bundle of interventions.
Recognizing that patients are often the only common thread across different sites of care, Strunin and colleagues demonstrate the value of including the voice of the patient in helping clinicians to understand the challenges and larger context in which they attempt to follow through with discharge instructions. Strunin et al. found that among a range of factors that contribute to adherence problems, many were nonmedical. Fortunately, a number of these are modifiable and point to the need to both prioritize recommendations to patients and to simplify them whenever possible. The authors' findings resonate with a growing literature that examines the hospital discharge process from the patient's perspective.510
Flacker and colleagues surveyed older patients to gain greater insights into their information needs at discharge. From a process standpoint, they demonstrated that a posthospital survey was feasible and acceptable to older patients. In and of itself, this finding has important implications in the context of national efforts aimed at implementing performance measurement and accompanying public reporting. It also may reflect patients' eagerness to be contacted after discharge; hospitalization is a major event in patients' lives, and attentive follow‐up is appreciated. The authors found that more than half of patients did not recall being asked about how they would care for themselves on returning home from the hospital. Although there may be a variety of explanations for this finding, it nevertheless points to an immediate area for intervention.
Collectively, these 3 articles set the stage for a proposed clinical investigation agenda aimed at optimizing transitions out of the hospital (see Table 1).
| 1. Greater recognition of the integral role of family caregivers |
| 2. Empirically define the appropriate follow‐up interval |
| 3. Define physician accountability for patients referred to home health on hospital discharge |
| 4. Delineate the role of the hospitalist in the advanced medical home |
| 5. Develop the ability to examine episodes of care |
These 5 recommendations have the potential to ensure that the gains patients make in our hospitals are maintained long after discharge.
-
Greater Recognition for the Integral Role of Family Caregivers. The patient and family caregivers should be integrated into health care professionals' efforts to improve care coordination across settings. Family caregivers have been silent partners in health care delivery, functioning as de facto care coordinators. During care handoffs, family caregivers make important contributions to ensuring quality, safety, and adherence to patient preferences; their role needs to be formally recognized and supported. An important initial step would be standardizing the approach to defining the types and intensity of the roles family caregivers play to facilitate improved communication. One proposed working definition is the FACED classification developed by one of the authors (E.C.). Modeled after the TNM system used in cancer, each letter of FACED refers to a different contribution made by a family caregiver: F = Financial; A = Advocacy; C = Care coordination; E = Emotional support; and D = Direct care provision. A simple numeric rating system could be developed whereby 0 = does not contribute in this area and 3 = makes significant contribution to this area. Such a straightforward approach would readily inform all members of the health care team about the caregivers' roles and capabilities and how they can optimally collaborate in the care plan.
-
Empirically Define Appropriate Follow‐Up Interval. At present, patients are given rather arbitrary and generic instructions for when to obtain follow‐up with their outpatient primary care physician or specialist. Surgical patients are often instructed to follow up with their surgeon, and yet most of the readmissions of these patients are attributable to medical conditions (personal communication, Steven Jencks, MD, Centers for Medicare and Medicaid Services). Furthermore, a significant number of discharged medical and surgical patients are readmitted to the hospital within 30 days without any outpatient contact with a health professional. One may envision an evidence‐based tiered approach whereby patients are assigned a hospital readmission risk score at the time of discharge that then determines the timing of their follow‐up appointment. Using this framework, the highest‐risk patients may be encouraged to receive follow‐up within 2472 hours, whereas lower‐risk patients may be able to wait 1421 days. Of course, there will need to be sufficient access to outpatient physicians, who will need to be available, to ensure the success of this strategy.
-
Define Physician Accountability for Patients Referred to Home Health on Hospital Discharge. Communication problems between the hospital and the home health care agency are a source of aggravation for both parties, not to mention patients. Typically, a hospitalist provides the initial order for services and then expects subsequent home care coordination to be managed by an outpatient physician. Unfortunately, in some cases the patient may not have an outpatient physician or the patient's primary physician may be unaware of the recent hospitalization and thus unwilling to assume management of an unfamiliar care plan. As a result, home care nurses often cannot identify a physician to respond to their questions or concerns. At the center of this problem lies a lack of understanding of where the responsibility of the ordering hospitalist ends and the outpatient physician assuming care begins. Recognizing the profound costs of failed home health care leading to hospital readmission, the nation's Quality Improvement Organizations launched a national campaign in 2006 to address this problem. Hospitalists should engage in this effort and not punt the entire responsibility to home health agenciesimagine if hospitals and hospitalists were financially penalized if a patient was readmitted.
-
Delineate the Role of the Hospitalist in the Advanced Medical Home. Modeled after a concept with origins in pediatrics, the American College of Physicians and American Academy of Family Physicians are promoting the advanced medical home as a new care model that aims to provide comprehensive ambulatory care with an explicit focus on care coordination.11 The Centers for Medicare and Medicaid Services is planning to initiate a demonstration of this approach. What has not been adequately underscored is how the advanced medical home will communicate essential clinical information with the hospitalist and what, if any, will be the role of the hospitalist in relation to a patient's medical home? Ideally, the medical home approach will alleviate many of the current access problems that impede timely follow‐up.
-
Develop Ability to Examine Episodes of Care. Patients with complex conditions often require care from different practitioners in multiple settings. From the vantage point of health care professionals, these may appear to occur as merely a string of individual interactions, including hospital admissions and discharges. However from the patient's perspective, the experience is more appropriately characterized as a journey across an aggregated episode of care. The National Quality Forum is currently exploring how to measure quality of care delivered across such an episode of care. Additionally, the Centers for Medicare and Medicaid Services is developing a new assessment tool that will transcend acute and postacute care settings, the Continuity Assessment Record and Evaluation (CARE). This tool will potentially enhance our ability to measure care across a predefined episode. Measurement can further pave the way for payment reform designed to align incentives toward higher‐quality care transitions. Currently, professional fees for coronary artery bypass grafting surgery are bundled across an episode, including hospital and posthospital care settings. Extending this approach to a wider array of conditions and services could encourage new perspectives on the timing of discharge and the use of posthospital care venues. For example, under bundled payment, incentives might support a plan to keep a patient in the hospital an extra 1 or 2 days in order to obviate a transfer to a skilled nursing facility and the concomitant risks of transfer‐related problems. Further, bundled payment may allow for the provision of additional services not currently covered, including transportation, as identified by Strunin and colleagues.3
Hospitalists are well positioned to offer leadership in these high‐leverage areas and thereby make a unique contribution to the quality and safety of care transitions. By so doing, they are poised to reaffirm their professionalism,12 ensuring that the excellent care that they provide in the hospital is sustained well into the future.
- ,,.Discharge information and older patients: do they get what they need?J Hosp Med.2007;2:291–296.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,.Understanding rehospitalization risk: can the hospital discharge be modified to impact recurrent hospitalization.J Hosp Med.2007;2:297–304.
- The Joint Commission. Sentinel event statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed August 7,2007.
- ,,.Patients' and caregivers' transition from hospital to home: needs and recommendations.Home Health Care Serv Q.1998;17:27–48.
- ,,,,.Carepartner experiences with hospital care.Med Care.1999;37:33–38.
- ,.Understanding coordination of care from the consumer's perspective in a regional health system.Health Serv Res.2002;37:1031–1054.
- ,,, et al.Development and testing of a measure designed to assess the quality of care transitions.Int J Integrated Care.2002;2:e02.
- .Rough crossings: family caregivers odysseys through the health care system.New York:United Hospital Fund of New York;1998.
- ,.Facilitating the transition out of the hospital. In:Gerteis M,Edgman‐Levitan S,Daley J,Delbanco T, editors.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco:Jossey‐Bass;1993:204–223.
- ,,American College of Physicians. The advanced medical home: a patient‐centered, physician‐guided model of health care. p. 1–22. http://www.acponline.org/hpp/adv_med.pdf Accessed August 7,2007.
- Project of the ABIM Foundation A‐AFaEFoIM.Medical professionalism in the new millennium: a physician charter.Ann Intern Med.2002;136:243–246.
- ,,.Discharge information and older patients: do they get what they need?J Hosp Med.2007;2:291–296.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,.Understanding rehospitalization risk: can the hospital discharge be modified to impact recurrent hospitalization.J Hosp Med.2007;2:297–304.
- The Joint Commission. Sentinel event statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed August 7,2007.
- ,,.Patients' and caregivers' transition from hospital to home: needs and recommendations.Home Health Care Serv Q.1998;17:27–48.
- ,,,,.Carepartner experiences with hospital care.Med Care.1999;37:33–38.
- ,.Understanding coordination of care from the consumer's perspective in a regional health system.Health Serv Res.2002;37:1031–1054.
- ,,, et al.Development and testing of a measure designed to assess the quality of care transitions.Int J Integrated Care.2002;2:e02.
- .Rough crossings: family caregivers odysseys through the health care system.New York:United Hospital Fund of New York;1998.
- ,.Facilitating the transition out of the hospital. In:Gerteis M,Edgman‐Levitan S,Daley J,Delbanco T, editors.Through the Patient's Eyes: Understanding and Promoting Patient‐Centered Care.San Francisco:Jossey‐Bass;1993:204–223.
- ,,American College of Physicians. The advanced medical home: a patient‐centered, physician‐guided model of health care. p. 1–22. http://www.acponline.org/hpp/adv_med.pdf Accessed August 7,2007.
- Project of the ABIM Foundation A‐AFaEFoIM.Medical professionalism in the new millennium: a physician charter.Ann Intern Med.2002;136:243–246.