User login
Children with tic disorders: How to match treatment with symptoms
Sammy, age 7, is referred to you by his pediatrician because of a 4-week history of frequent eye blinking. His parents say he blinks a lot when bored but very little when playing baseball. They recall that he also has intermittently sniffed and nodded his head over the last 12 months. Neither Sammy nor his friends seem to be bothered by the blinking. Except for the tics, Sammy’s physical and mental status exams are normal.
Since preschool, Sammy’s teachers have complained that his backpack and desk are always a mess. Sammy is well-meaning but forgetful in his chores at home. A paternal uncle has head-turning movements, counts his steps, and becomes distressed if books on his shelf are not in alphabetical order.
Tics, such as strong eye blinks or repetitive shoulder shrugs, can distress a child or his/her parents, but the conditions associated with tic disorders often are more problematic than the tic disorder itself. High rates of comorbid conditions are recognized in persons with Tourette syndrome, including:
- obsessive-compulsive disorder (OCD) in >80%1
- attention-deficit/hyperactivity disorder (ADHD) in ≤70%2
- anxiety disorders in 30%3
- rage, aggression, learning disabilities, and autism less commonly.
The strategy we recommend for managing tic disorders includes assessing tic severity, educating the family about the illness, determining whether a comorbid condition is present, and managing these conditions appropriately. Above all, we emphasize a risk-benefit analysis guided by the Hippocratic principle of “do no harm.”
Characteristics of tic disorders
You diagnose Sammy with Tourette syndrome because he meets DSM-IV-TR criteria of at least 2 motor tics and 1 vocal tic that have persisted for 1 year without more than a 3-month hiatus, with tic onset before age 18. Because tics may resemble other movement disorders, you rule out stereotypies, dystonia, chorea, ballism, and myoclonus (Table 1). You explain to his parents that Sammy’s condition is a heritable, neurobehavioral disorder that typically begins in childhood and is associated in families with OCD, ADHD, and autism spectrum disorders.
His parents ask about the difference between tics and other movements. You explain that eye-blinking tics—like other motor tics—appear as sudden, repetitive, stereotyped, nonrhythmic movements that involve discrete muscle groups. (View a video of a patient with tics.) Simple motor tics are focal movements involving 1 group of muscles, whereas complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements (Table 2).
Table 1
Features of 5 movement disorders that may resemble tics
| Tics | Stereotypies | Dystonia | Chorea | Ballism | Myoclonus |
|---|---|---|---|---|---|
| Sudden, repetitive, stereotyped, nonrhythmic movements or sounds | Patterned, nonpurposeful movement | Cocontraction of agonist and antagonist muscles, causing an abnormal twisting posture | Continuous, flowing, nonrhythmic, nonpurposeful movement | Forceful, flinging, large amplitude choreic movement | Sudden, quick, shock-like movement |
| Usually start after age 3 | Usually start before age 3 and resolve by adolescence | More common in adults | — | — | — |
| Decrease when focused; increase when stressed, anxious, fatigued, or bored | Occur when the child is excited | Worsens during motor tasks | Worsens during motor tasks | Worsens during motor tasks | — |
| Comorbid conditions include OCD and ADHD | Common in children with mental retardation or autism | — | Can occur after streptococcal infection | Can occur after streptococcal infection | — |
| Preceded by a premonitory urge or sensation | Possibly preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge |
| Temporarily suppressible | Suppressible | Not suppressible | Partially suppressible; can incorporate into semi-purposeful movements | Partially suppressible | Not suppressible |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder | |||||
Table 2
Characteristics of simple and complex motor and vocal tics*
| Simple tics | Complex tics |
|---|---|
| Eye blinking or eye rolling Nose, mouth, tongue, or facial grimaces (nose twitch, nasal flaring, chewing lip, teeth grinding, sticking out tongue, mouth stretching, lip licking) Head jerks or movements (neck stretching, touching chin to shoulder) Shoulder jerks/movements (shoulder shrugging, jerking a shoulder) Arm or hand movements (flexing or extending arms or fingers) Coughing Throat clearing, grunting Sniffing, snorting, shouting Humming | Jumping Spinning Touching objects or people Throwing objects Repeating others’ action (echopraxia) Obscene gestures (copropraxia) Repeating one’s own words (palilalia) Repeating what someone else said (echolalia) Obscene, inappropriate words (coprolalia) |
| *Simple tics are focal movements involving 1 group of muscles; complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements | |
Older children frequently describe a premonitory urge prior to the tic. Patients typically can suppress tics for a transient period of time, although during tic suppression they usually feel restless and anticipate performing their tic. The ultimate performance of the tic brings relief. Tic suppression also occurs during focused activity. Emotional stress, fatigue, illness, or boredom can exacerbate tics.
To begin monitoring Sammy’s clinical course, you administer 3 assessment tools described inTable 3. You explain to Sammy’s parents that these tests will be repeated yearly or when tics worsen. However, you tell his parents that these scores alone will not determine present or future clinical decisions, including treatments. You also recommend that they connect with support groups on the Tourette Syndrome Association (TSA) Web site.
CASE CONTINUED: Changes over time
Sammy’s parents appreciate your explanation and say they will share information from the TSA Web site with Sammy’s principal, teachers, and classmates. The family agrees to return in 6 months or sooner if the tics worsen.
By age 8, Sammy develops multiple tics: facial grimacing, looking upwards, punching movements, whistling, and throat clearing. He is slightly bothered by these tics, and his friends have asked him about them. He tells them he has Tourette syndrome, and that usually ends the questioning. He returns for a follow-up visit because his parents notice a dramatic increase in his tics after Sammy’s father loses his job.
Treatment options
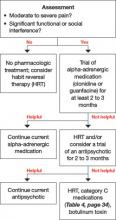
When deciding to treat a child’s tics, the first step is to determine whether to pursue a nonpharmacologic or pharmacologic approach (Algorithm). To tailor an approach most suited for an individual child, discuss with the family their feelings about therapy and medications. This information—along with tic severity—will help determine a treatment plan.
Behavior therapy and medication are management strategies; neither can cure a tic disorder. The most conservative approach to tic treatment is to:
- provide the child and family with basic guidelines for managing tics
- help alleviate environmental stress and other potential triggers.
Algorithm: Recommended treatment of tics in children and adolescents
CASE CONTINUED: A first intervention
You discuss treatment options with Sammy’s family, and they view medication as a last resort. Sammy does not seem to be bothered by his tics, and his parents do not wish to start him on daily medications. Given this situation, habit reversal therapy (HRT) is appropriate for Sammy because he is old enough to participate in HRT to reduce his tics.
HRT is an effective nonpharmacologic approach to help children with tics.4 Its 3 components are:
- awareness training
- competing response training
- social support.5
This simplified version of the original HRT can be completed in eight 1-hour sessions. Good candidates are patients who are cognitively mature enough to understand the therapy’s goals and compliant with frequent clinic visits. They also must practice the strategies at home.
It should not be difficult for psychiatrists to learn HRT—or refer to therapists who are willing to learn it—with the available instructional manual.
CASE CONTINUED: Practicing alternatives
You ask Sammy to imitate his tics. After helping him become more aware of his tics, you encourage him to develop a more socially appropriate movement to engage in whenever he feels the urge to punch. Sammy chooses to clench his fist in his pocket. He also learns to breathe in whenever he has an urge to whistle. you advise Sammy’s parents to reward his efforts to suppress the tics. He practices the strategies daily.
At age 12, Sammy returns to your office. He has begun to have frequent neck-jerking tics, which cause neck pain and daily headaches. He also is slapping his thigh and having frequent vocal tics characterized by loud shrieking. The vocal tics are disruptive in class, even though Sammy sits toward the back of the room. Sammy’s classmates tease him, and he is very frustrated.
Medication approach
The decision to start a medication for tics is complex. Scores from the YGTSS, PUTS, and GTS-QOL scales (Table 3) provide only a partial clinical picture. This decision should be reached after a detailed discussion with the family about benefits and risks of medications and ensuring that everyone’s expectations are reasonable.
A variety of medications are available to treat patients with tics (Table 4). No medication can completely eliminate tics, however, and many have substantial side effects. Before initiating medical treatment, consider 3 questions:
- Is moderate or severe pain involved?
- Is there significant functional interference?
- Is there significant social disruption despite efforts to optimize the social environment for the child?
Sammy’s frequent neck-jerking tics now cause chronic daily headaches, and his shrieking vocal tics are interfering with classroom activities, so we recommended a 3-month trial of guanfacine following the dosing schedule in Table 4.
Table 3
3 scales for assessing tic severity and impact on functioning
| Instrument | Purpose | Description | Design | Administration frequency |
|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | Assess tic severity | Review of motor and vocal tics. Rate number, frequency, intensity, complexity, and interference on a 5-point scale | Clinician-rated | Annual and as needed for increased tics |
| Premonitory Urge for Tics Scale (PUTS) | Detect the presence of unpleasant sensations that precedes tics | 10 questions | Self-report | Annual and as needed for increased tics |
| Gilles de la Tourette Syndrome Quality of Life Scale (GTS-QOL) | Measure quality of life | 27 questions, 4 subscales: psychological, physical, obsessional, and cognitive | Self-report | Annual and as needed for increased tics |
Table 4
Medications with evidence of tic-suppressing effects*
| Category A evidence | ||
|---|---|---|
| Medication | Starting dose | Target dose |
| Haloperidol | 0.25 to 0.5 mg/d | 1 to 4 mg/d |
| Pimozide | 0.5 to 1 mg/d | 2 to 8 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 1 to 3 mg/d |
| Category B evidence | ||
| Medication | Starting dose | Target dose |
| Fluphenazine | 0.5 to 1 mg/d | 1.5 to 10 mg/d |
| Ziprasidone | 5 to 10 mg/d | 10 to 80 mg/d |
| Clonidine | 0.025 to 0.05 mg/d | 0.1 to 0.3 mg/d |
| Guanfacine | 0.5 to 1 mg/d | 1 to 3 mg/d |
| Botulinum toxin | 30 to 300 units | |
| Category C evidence | ||
| Medication | Starting dose | Target dose |
| Olanzapine | 2.5 to 5 mg/d | 2.5 to 12.5 mg/d |
| Tetrabenazine | 25 mg/d | 37.5 to 150 mg/d |
| Baclofen | 10 mg/d | 40 to 60 mg/d |
| Nicotine patch | 7 mg/d | 7 to 21 mg/d |
| Mecamylamine | 2.5 mg/d | 2.5 to 7.5 mg/d |
| Flutamide | 250 mg/d | 750 mg/d |
| *Category A: supported by ≥2 placebo-controlled trials; category B: supported by 1 placebo-controlled trial; category C: supported by open-label study | ||
| Source: Reference 6 | ||
The first-line pharmacologic agent for tic suppression generally is an alpha-adrenergic medication, unless the tics are severe.6
Clonidine and guanfacine usually are started at low doses and increased gradually. Although not as effective as neuroleptics, alpha-adrenergics have a lower potential for side effects and are easier to use because no laboratory tests need to be monitored. Adverse effects associated with alpha-adrenergic medications include sedation, dry mouth, dizziness, headache, and rebound hypertension if discontinued abruptly.
If tics are causing pain, some clinicians prefer conservative measures such as heat or ice, massage, analgesics, relaxation therapy, and reassurance.
Second-line agents include typical and atypical antipsychotics. Haloperidol and pimozide have shown efficacy in reducing tics in placebo- controlled studies,7,8 as have risperidone (in 4 randomized controlled trials [RCTs]) and ziprasidone (in 1 RCT).9,10 The emergence of serious side effects is a risk for both typical and atypical antipsychotics (Table 5).
Table 5
Potential adverse effects of antipsychotic treatment in children*
| Adverse effect | Examples |
|---|---|
| Sedation | — |
| Acute dystonic reactions | Oculogyric crisis, torticollis |
| Appetite changes | Weight gain |
| Endocrine abnormalities | Amenorrhea, diabetes, galactorrhea, gynecomastia, hyperprolactinemia |
| Cognitive effects | Impaired concentration |
| Akathisia | Difficulty sitting still |
| ECG changes | Prolonged QT interval |
| Parkinsonism | Tremor, bradykinesia, rigidity, postural instability |
| Tardive syndrome | Orofacial dyskinesia, chorea, dystonia, myoclonus, tics |
| Neuroleptic malignant syndrome | Potentially fatal; consists of muscular rigidity, fever, autonomic dysfunction, labile blood pressure, sweating, urinary incontinence, fluctuating level of consciousness, leukocytosis, elevated serum creatine kinase |
| *Potential adverse effects are listed from most to least likely to occur | |
As part of your informed consent discussion, weigh the risk of side effects against the benefits of treatment. Point out to patients and their families that they can expect to see a decrease in tic frequency, but symptoms will not necessarily disappear with any medication. We tell our patients that with antipsychotics the best we can hope for is to reduce tic frequency by approximately one-half.6
When treating tics, start with 1 medication. However, if the tics are severe enough to require more than 1 medication, check for drug interactions.
Third-line agents. Agents that have not been tested in placebo-controlled trials can be considered third line; these are listed as category C (supported by open-label studies) in Table 4. Botulinum toxin injection has been found to be effective for motor and vocal tics.11,12 Botulinum toxin and implantation of deep brain stimulators13 are invasive options and generally are reserved for severe, treatment-resistant tics.
CASE CONTINUED: Managing antipsychotics
After trying guanfacine for 12 weeks, Sammy notices no tic reduction. His parents consent to a low dose of risperidone. you review with them the American Psychiatric Association (APA)/American Diabetes Association (ADA) guidelines14 for managing metabolic problems in patients treated with atypical antipsychotics.
As instructed in the APA/ADA guidelines, obtain baseline measurements and monitor for metabolic effects of antipsychotic therapy over time (Table 6). Sammy starts risperidone at 0.5 mg once daily. After 2 weeks, he notices a decrease in his tics. At the 3-month visit after starting risperidone, he is happy with his risperidone dose and does not want to increase it. He has gained 3 pounds, and you instruct him to eat a well-balanced diet and exercise routinely. At the 6-month visit, his tics are minimal and his weight has stabilized.
Table 6
Children receiving antipsychotics: monitoring recommendations
| Clinical information | Frequency |
|---|---|
| Family history | Initial visit |
| Weight | Baseline, monthly |
| Height | Baseline, monthly |
| BMI | Baseline, monthly |
| Waist circumference | Baseline, annually |
| Blood pressure | Baseline, 3 months after treatment starts, and annually thereafter |
| Fasting lipid profile | Baseline, every 3 months initially, then every 6 months thereafter |
| Fasting serum glucose | Baseline, every 3 months, then every 6 months thereafter |
| BMI: body mass index | |
| Source: References 14,16 | |
You recommend that Sammy remain on risperidone for another 3 months of stability and then begin to taper this medication. You review the risks and benefits of long-term treatment with risperidone, pointing out that it may lead to abnormal movements upon withdrawal, and explain that you typically do not treat children with antipsychotics for more than one year continuously.
CASE CONTINUED: Comorbid symptoms
Since starting 7th grade, Sammy has worried excessively about making mistakes. He spends 6 hours each night on homework, which he often does not turn in because of anxiety about not getting answers perfectly right. Classmates notice that Sammy taps the door 3 times when he comes into the classroom and that he steps over the black tiles in the hallway.
Consider the presence and impact of comorbid OCD or ADHD, which can impair children’s quality of life more than tics themselves.15 Assessment scales can help you make a diagnosis and monitor treatment.
If you suspect OCD, the clinician-rated Children’s Yale Brown Obsessive Compulsive Scale is the gold standard for describing the phenomenology and measuring symptom severity. Additional scales to measure symptoms’ impact on family life include the Leyton Obsessional Inventory—child version, Family Accommodation Scale for OCD, and Child OCD Impact Scale.
ADHD scales include the Conners Parent Rating Scale—Revised, Conners Teacher Rating Scale—Revised, Swanson, Nolan, and Pelham, or the Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales. Because ADHD symptoms must be present in more than 1 environment to meet diagnostic criteria, ask parents and teachers to complete the Conners or Vanderbilt scales.
In children who present with a tic disorder plus a comorbid condition, prioritize treatment by determining which symptoms interfere with the child’s ability to function at school, at home, and in the social arena. Children who require treatment for >1 disorder often are referred initially for cognitive-behavioral therapy for OCD symptoms while receiving pharmacologic treatment for ADHD and/or Tourette syndrome. When necessary, it is usually safe to combine antipsychotics, stimulants, and selective serotonin reuptake inhibitors, although medication interactions should be reviewed in each specific case.
Related resources
- Woods DW. Managing Tourette syndrome: a behavioral intervention for children and adults. Therapist guide. New York, NY: Oxford University Press; 2008.
- Tourette Syndrome Association. www.tsa-usa.org.
- International OCD Foundation. www.ocfoundation.org.
Drug brand names
- Baclofen • Lioresal
- Botulinum toxin • Botox, Myobloc
- Clomipramine • Anafranil
- Clonidine • Catapres
- Guanfacine • Tenex
- Fluphenazine • Prolixin
- Flutamide • Eulexin
- Haloperidol • Haldol
- Mecamylamine • Inversine
- Nicotine patch • NicoDerm
- Olanzapine • Zyprexa
- Pimozide • Orap
- Risperidone • Risperdal
- Tetrabenazine • Xenazine
- Ziprasidone • Geodon
Disclosures
Dr. Harris has received research support from the Translational Research Initiative at Cincinnati Children’s Hospital Medical Center.
Dr. Wu reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Robertson M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425-462.
2. Freeman R. For the Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a worldwide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):15-23.
3. Stefl M. Mental health needs associated with Tourette syndrome. Am J Public Health. 1984;74:1310-1313.
4. Deckersbach T, Rauch S, Buhlmann U, et al. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079-1090.
5. Woods DW, Miltenberger RG. Habit reversal: a review of applications and variations. J Behav Ther Exp Psychiatry. 1995;26:123-131.
6. Scahill L, Erenberg G, Berlin C, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
7. Shapiro E, Shapiro A, Fulop G, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. 1989;46:722-730.
8. Sallee F, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154:1057-1062.
9. Scahill L, Leckman J, Schultz R, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
10. Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
11. Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
12. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24(6):420-423.
13. Porta M, Sevello D, Sassi M, et al. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur Neurol. 2009;62(5):264-273.
14. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:1335-1342.
15. Bernard BA, Stebbins GT, Siegel S, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. 2009;24(7):1070-1073.
16. Understanding the risks of antipsychotic treatment in young people. Advice for managing side effects in children and teenagers. Harv Ment Health Lett. 2009;25(9):1-3.
Sammy, age 7, is referred to you by his pediatrician because of a 4-week history of frequent eye blinking. His parents say he blinks a lot when bored but very little when playing baseball. They recall that he also has intermittently sniffed and nodded his head over the last 12 months. Neither Sammy nor his friends seem to be bothered by the blinking. Except for the tics, Sammy’s physical and mental status exams are normal.
Since preschool, Sammy’s teachers have complained that his backpack and desk are always a mess. Sammy is well-meaning but forgetful in his chores at home. A paternal uncle has head-turning movements, counts his steps, and becomes distressed if books on his shelf are not in alphabetical order.
Tics, such as strong eye blinks or repetitive shoulder shrugs, can distress a child or his/her parents, but the conditions associated with tic disorders often are more problematic than the tic disorder itself. High rates of comorbid conditions are recognized in persons with Tourette syndrome, including:
- obsessive-compulsive disorder (OCD) in >80%1
- attention-deficit/hyperactivity disorder (ADHD) in ≤70%2
- anxiety disorders in 30%3
- rage, aggression, learning disabilities, and autism less commonly.
The strategy we recommend for managing tic disorders includes assessing tic severity, educating the family about the illness, determining whether a comorbid condition is present, and managing these conditions appropriately. Above all, we emphasize a risk-benefit analysis guided by the Hippocratic principle of “do no harm.”
Characteristics of tic disorders
You diagnose Sammy with Tourette syndrome because he meets DSM-IV-TR criteria of at least 2 motor tics and 1 vocal tic that have persisted for 1 year without more than a 3-month hiatus, with tic onset before age 18. Because tics may resemble other movement disorders, you rule out stereotypies, dystonia, chorea, ballism, and myoclonus (Table 1). You explain to his parents that Sammy’s condition is a heritable, neurobehavioral disorder that typically begins in childhood and is associated in families with OCD, ADHD, and autism spectrum disorders.
His parents ask about the difference between tics and other movements. You explain that eye-blinking tics—like other motor tics—appear as sudden, repetitive, stereotyped, nonrhythmic movements that involve discrete muscle groups. (View a video of a patient with tics.) Simple motor tics are focal movements involving 1 group of muscles, whereas complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements (Table 2).
Table 1
Features of 5 movement disorders that may resemble tics
| Tics | Stereotypies | Dystonia | Chorea | Ballism | Myoclonus |
|---|---|---|---|---|---|
| Sudden, repetitive, stereotyped, nonrhythmic movements or sounds | Patterned, nonpurposeful movement | Cocontraction of agonist and antagonist muscles, causing an abnormal twisting posture | Continuous, flowing, nonrhythmic, nonpurposeful movement | Forceful, flinging, large amplitude choreic movement | Sudden, quick, shock-like movement |
| Usually start after age 3 | Usually start before age 3 and resolve by adolescence | More common in adults | — | — | — |
| Decrease when focused; increase when stressed, anxious, fatigued, or bored | Occur when the child is excited | Worsens during motor tasks | Worsens during motor tasks | Worsens during motor tasks | — |
| Comorbid conditions include OCD and ADHD | Common in children with mental retardation or autism | — | Can occur after streptococcal infection | Can occur after streptococcal infection | — |
| Preceded by a premonitory urge or sensation | Possibly preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge |
| Temporarily suppressible | Suppressible | Not suppressible | Partially suppressible; can incorporate into semi-purposeful movements | Partially suppressible | Not suppressible |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder | |||||
Table 2
Characteristics of simple and complex motor and vocal tics*
| Simple tics | Complex tics |
|---|---|
| Eye blinking or eye rolling Nose, mouth, tongue, or facial grimaces (nose twitch, nasal flaring, chewing lip, teeth grinding, sticking out tongue, mouth stretching, lip licking) Head jerks or movements (neck stretching, touching chin to shoulder) Shoulder jerks/movements (shoulder shrugging, jerking a shoulder) Arm or hand movements (flexing or extending arms or fingers) Coughing Throat clearing, grunting Sniffing, snorting, shouting Humming | Jumping Spinning Touching objects or people Throwing objects Repeating others’ action (echopraxia) Obscene gestures (copropraxia) Repeating one’s own words (palilalia) Repeating what someone else said (echolalia) Obscene, inappropriate words (coprolalia) |
| *Simple tics are focal movements involving 1 group of muscles; complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements | |
Older children frequently describe a premonitory urge prior to the tic. Patients typically can suppress tics for a transient period of time, although during tic suppression they usually feel restless and anticipate performing their tic. The ultimate performance of the tic brings relief. Tic suppression also occurs during focused activity. Emotional stress, fatigue, illness, or boredom can exacerbate tics.
To begin monitoring Sammy’s clinical course, you administer 3 assessment tools described inTable 3. You explain to Sammy’s parents that these tests will be repeated yearly or when tics worsen. However, you tell his parents that these scores alone will not determine present or future clinical decisions, including treatments. You also recommend that they connect with support groups on the Tourette Syndrome Association (TSA) Web site.
CASE CONTINUED: Changes over time
Sammy’s parents appreciate your explanation and say they will share information from the TSA Web site with Sammy’s principal, teachers, and classmates. The family agrees to return in 6 months or sooner if the tics worsen.
By age 8, Sammy develops multiple tics: facial grimacing, looking upwards, punching movements, whistling, and throat clearing. He is slightly bothered by these tics, and his friends have asked him about them. He tells them he has Tourette syndrome, and that usually ends the questioning. He returns for a follow-up visit because his parents notice a dramatic increase in his tics after Sammy’s father loses his job.
Treatment options
When deciding to treat a child’s tics, the first step is to determine whether to pursue a nonpharmacologic or pharmacologic approach (Algorithm). To tailor an approach most suited for an individual child, discuss with the family their feelings about therapy and medications. This information—along with tic severity—will help determine a treatment plan.
Behavior therapy and medication are management strategies; neither can cure a tic disorder. The most conservative approach to tic treatment is to:
- provide the child and family with basic guidelines for managing tics
- help alleviate environmental stress and other potential triggers.
Algorithm: Recommended treatment of tics in children and adolescents
CASE CONTINUED: A first intervention
You discuss treatment options with Sammy’s family, and they view medication as a last resort. Sammy does not seem to be bothered by his tics, and his parents do not wish to start him on daily medications. Given this situation, habit reversal therapy (HRT) is appropriate for Sammy because he is old enough to participate in HRT to reduce his tics.
HRT is an effective nonpharmacologic approach to help children with tics.4 Its 3 components are:
- awareness training
- competing response training
- social support.5
This simplified version of the original HRT can be completed in eight 1-hour sessions. Good candidates are patients who are cognitively mature enough to understand the therapy’s goals and compliant with frequent clinic visits. They also must practice the strategies at home.
It should not be difficult for psychiatrists to learn HRT—or refer to therapists who are willing to learn it—with the available instructional manual.
CASE CONTINUED: Practicing alternatives
You ask Sammy to imitate his tics. After helping him become more aware of his tics, you encourage him to develop a more socially appropriate movement to engage in whenever he feels the urge to punch. Sammy chooses to clench his fist in his pocket. He also learns to breathe in whenever he has an urge to whistle. you advise Sammy’s parents to reward his efforts to suppress the tics. He practices the strategies daily.
At age 12, Sammy returns to your office. He has begun to have frequent neck-jerking tics, which cause neck pain and daily headaches. He also is slapping his thigh and having frequent vocal tics characterized by loud shrieking. The vocal tics are disruptive in class, even though Sammy sits toward the back of the room. Sammy’s classmates tease him, and he is very frustrated.
Medication approach
The decision to start a medication for tics is complex. Scores from the YGTSS, PUTS, and GTS-QOL scales (Table 3) provide only a partial clinical picture. This decision should be reached after a detailed discussion with the family about benefits and risks of medications and ensuring that everyone’s expectations are reasonable.
A variety of medications are available to treat patients with tics (Table 4). No medication can completely eliminate tics, however, and many have substantial side effects. Before initiating medical treatment, consider 3 questions:
- Is moderate or severe pain involved?
- Is there significant functional interference?
- Is there significant social disruption despite efforts to optimize the social environment for the child?
Sammy’s frequent neck-jerking tics now cause chronic daily headaches, and his shrieking vocal tics are interfering with classroom activities, so we recommended a 3-month trial of guanfacine following the dosing schedule in Table 4.
Table 3
3 scales for assessing tic severity and impact on functioning
| Instrument | Purpose | Description | Design | Administration frequency |
|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | Assess tic severity | Review of motor and vocal tics. Rate number, frequency, intensity, complexity, and interference on a 5-point scale | Clinician-rated | Annual and as needed for increased tics |
| Premonitory Urge for Tics Scale (PUTS) | Detect the presence of unpleasant sensations that precedes tics | 10 questions | Self-report | Annual and as needed for increased tics |
| Gilles de la Tourette Syndrome Quality of Life Scale (GTS-QOL) | Measure quality of life | 27 questions, 4 subscales: psychological, physical, obsessional, and cognitive | Self-report | Annual and as needed for increased tics |
Table 4
Medications with evidence of tic-suppressing effects*
| Category A evidence | ||
|---|---|---|
| Medication | Starting dose | Target dose |
| Haloperidol | 0.25 to 0.5 mg/d | 1 to 4 mg/d |
| Pimozide | 0.5 to 1 mg/d | 2 to 8 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 1 to 3 mg/d |
| Category B evidence | ||
| Medication | Starting dose | Target dose |
| Fluphenazine | 0.5 to 1 mg/d | 1.5 to 10 mg/d |
| Ziprasidone | 5 to 10 mg/d | 10 to 80 mg/d |
| Clonidine | 0.025 to 0.05 mg/d | 0.1 to 0.3 mg/d |
| Guanfacine | 0.5 to 1 mg/d | 1 to 3 mg/d |
| Botulinum toxin | 30 to 300 units | |
| Category C evidence | ||
| Medication | Starting dose | Target dose |
| Olanzapine | 2.5 to 5 mg/d | 2.5 to 12.5 mg/d |
| Tetrabenazine | 25 mg/d | 37.5 to 150 mg/d |
| Baclofen | 10 mg/d | 40 to 60 mg/d |
| Nicotine patch | 7 mg/d | 7 to 21 mg/d |
| Mecamylamine | 2.5 mg/d | 2.5 to 7.5 mg/d |
| Flutamide | 250 mg/d | 750 mg/d |
| *Category A: supported by ≥2 placebo-controlled trials; category B: supported by 1 placebo-controlled trial; category C: supported by open-label study | ||
| Source: Reference 6 | ||
The first-line pharmacologic agent for tic suppression generally is an alpha-adrenergic medication, unless the tics are severe.6
Clonidine and guanfacine usually are started at low doses and increased gradually. Although not as effective as neuroleptics, alpha-adrenergics have a lower potential for side effects and are easier to use because no laboratory tests need to be monitored. Adverse effects associated with alpha-adrenergic medications include sedation, dry mouth, dizziness, headache, and rebound hypertension if discontinued abruptly.
If tics are causing pain, some clinicians prefer conservative measures such as heat or ice, massage, analgesics, relaxation therapy, and reassurance.
Second-line agents include typical and atypical antipsychotics. Haloperidol and pimozide have shown efficacy in reducing tics in placebo- controlled studies,7,8 as have risperidone (in 4 randomized controlled trials [RCTs]) and ziprasidone (in 1 RCT).9,10 The emergence of serious side effects is a risk for both typical and atypical antipsychotics (Table 5).
Table 5
Potential adverse effects of antipsychotic treatment in children*
| Adverse effect | Examples |
|---|---|
| Sedation | — |
| Acute dystonic reactions | Oculogyric crisis, torticollis |
| Appetite changes | Weight gain |
| Endocrine abnormalities | Amenorrhea, diabetes, galactorrhea, gynecomastia, hyperprolactinemia |
| Cognitive effects | Impaired concentration |
| Akathisia | Difficulty sitting still |
| ECG changes | Prolonged QT interval |
| Parkinsonism | Tremor, bradykinesia, rigidity, postural instability |
| Tardive syndrome | Orofacial dyskinesia, chorea, dystonia, myoclonus, tics |
| Neuroleptic malignant syndrome | Potentially fatal; consists of muscular rigidity, fever, autonomic dysfunction, labile blood pressure, sweating, urinary incontinence, fluctuating level of consciousness, leukocytosis, elevated serum creatine kinase |
| *Potential adverse effects are listed from most to least likely to occur | |
As part of your informed consent discussion, weigh the risk of side effects against the benefits of treatment. Point out to patients and their families that they can expect to see a decrease in tic frequency, but symptoms will not necessarily disappear with any medication. We tell our patients that with antipsychotics the best we can hope for is to reduce tic frequency by approximately one-half.6
When treating tics, start with 1 medication. However, if the tics are severe enough to require more than 1 medication, check for drug interactions.
Third-line agents. Agents that have not been tested in placebo-controlled trials can be considered third line; these are listed as category C (supported by open-label studies) in Table 4. Botulinum toxin injection has been found to be effective for motor and vocal tics.11,12 Botulinum toxin and implantation of deep brain stimulators13 are invasive options and generally are reserved for severe, treatment-resistant tics.
CASE CONTINUED: Managing antipsychotics
After trying guanfacine for 12 weeks, Sammy notices no tic reduction. His parents consent to a low dose of risperidone. you review with them the American Psychiatric Association (APA)/American Diabetes Association (ADA) guidelines14 for managing metabolic problems in patients treated with atypical antipsychotics.
As instructed in the APA/ADA guidelines, obtain baseline measurements and monitor for metabolic effects of antipsychotic therapy over time (Table 6). Sammy starts risperidone at 0.5 mg once daily. After 2 weeks, he notices a decrease in his tics. At the 3-month visit after starting risperidone, he is happy with his risperidone dose and does not want to increase it. He has gained 3 pounds, and you instruct him to eat a well-balanced diet and exercise routinely. At the 6-month visit, his tics are minimal and his weight has stabilized.
Table 6
Children receiving antipsychotics: monitoring recommendations
| Clinical information | Frequency |
|---|---|
| Family history | Initial visit |
| Weight | Baseline, monthly |
| Height | Baseline, monthly |
| BMI | Baseline, monthly |
| Waist circumference | Baseline, annually |
| Blood pressure | Baseline, 3 months after treatment starts, and annually thereafter |
| Fasting lipid profile | Baseline, every 3 months initially, then every 6 months thereafter |
| Fasting serum glucose | Baseline, every 3 months, then every 6 months thereafter |
| BMI: body mass index | |
| Source: References 14,16 | |
You recommend that Sammy remain on risperidone for another 3 months of stability and then begin to taper this medication. You review the risks and benefits of long-term treatment with risperidone, pointing out that it may lead to abnormal movements upon withdrawal, and explain that you typically do not treat children with antipsychotics for more than one year continuously.
CASE CONTINUED: Comorbid symptoms
Since starting 7th grade, Sammy has worried excessively about making mistakes. He spends 6 hours each night on homework, which he often does not turn in because of anxiety about not getting answers perfectly right. Classmates notice that Sammy taps the door 3 times when he comes into the classroom and that he steps over the black tiles in the hallway.
Consider the presence and impact of comorbid OCD or ADHD, which can impair children’s quality of life more than tics themselves.15 Assessment scales can help you make a diagnosis and monitor treatment.
If you suspect OCD, the clinician-rated Children’s Yale Brown Obsessive Compulsive Scale is the gold standard for describing the phenomenology and measuring symptom severity. Additional scales to measure symptoms’ impact on family life include the Leyton Obsessional Inventory—child version, Family Accommodation Scale for OCD, and Child OCD Impact Scale.
ADHD scales include the Conners Parent Rating Scale—Revised, Conners Teacher Rating Scale—Revised, Swanson, Nolan, and Pelham, or the Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales. Because ADHD symptoms must be present in more than 1 environment to meet diagnostic criteria, ask parents and teachers to complete the Conners or Vanderbilt scales.
In children who present with a tic disorder plus a comorbid condition, prioritize treatment by determining which symptoms interfere with the child’s ability to function at school, at home, and in the social arena. Children who require treatment for >1 disorder often are referred initially for cognitive-behavioral therapy for OCD symptoms while receiving pharmacologic treatment for ADHD and/or Tourette syndrome. When necessary, it is usually safe to combine antipsychotics, stimulants, and selective serotonin reuptake inhibitors, although medication interactions should be reviewed in each specific case.
Related resources
- Woods DW. Managing Tourette syndrome: a behavioral intervention for children and adults. Therapist guide. New York, NY: Oxford University Press; 2008.
- Tourette Syndrome Association. www.tsa-usa.org.
- International OCD Foundation. www.ocfoundation.org.
Drug brand names
- Baclofen • Lioresal
- Botulinum toxin • Botox, Myobloc
- Clomipramine • Anafranil
- Clonidine • Catapres
- Guanfacine • Tenex
- Fluphenazine • Prolixin
- Flutamide • Eulexin
- Haloperidol • Haldol
- Mecamylamine • Inversine
- Nicotine patch • NicoDerm
- Olanzapine • Zyprexa
- Pimozide • Orap
- Risperidone • Risperdal
- Tetrabenazine • Xenazine
- Ziprasidone • Geodon
Disclosures
Dr. Harris has received research support from the Translational Research Initiative at Cincinnati Children’s Hospital Medical Center.
Dr. Wu reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Sammy, age 7, is referred to you by his pediatrician because of a 4-week history of frequent eye blinking. His parents say he blinks a lot when bored but very little when playing baseball. They recall that he also has intermittently sniffed and nodded his head over the last 12 months. Neither Sammy nor his friends seem to be bothered by the blinking. Except for the tics, Sammy’s physical and mental status exams are normal.
Since preschool, Sammy’s teachers have complained that his backpack and desk are always a mess. Sammy is well-meaning but forgetful in his chores at home. A paternal uncle has head-turning movements, counts his steps, and becomes distressed if books on his shelf are not in alphabetical order.
Tics, such as strong eye blinks or repetitive shoulder shrugs, can distress a child or his/her parents, but the conditions associated with tic disorders often are more problematic than the tic disorder itself. High rates of comorbid conditions are recognized in persons with Tourette syndrome, including:
- obsessive-compulsive disorder (OCD) in >80%1
- attention-deficit/hyperactivity disorder (ADHD) in ≤70%2
- anxiety disorders in 30%3
- rage, aggression, learning disabilities, and autism less commonly.
The strategy we recommend for managing tic disorders includes assessing tic severity, educating the family about the illness, determining whether a comorbid condition is present, and managing these conditions appropriately. Above all, we emphasize a risk-benefit analysis guided by the Hippocratic principle of “do no harm.”
Characteristics of tic disorders
You diagnose Sammy with Tourette syndrome because he meets DSM-IV-TR criteria of at least 2 motor tics and 1 vocal tic that have persisted for 1 year without more than a 3-month hiatus, with tic onset before age 18. Because tics may resemble other movement disorders, you rule out stereotypies, dystonia, chorea, ballism, and myoclonus (Table 1). You explain to his parents that Sammy’s condition is a heritable, neurobehavioral disorder that typically begins in childhood and is associated in families with OCD, ADHD, and autism spectrum disorders.
His parents ask about the difference between tics and other movements. You explain that eye-blinking tics—like other motor tics—appear as sudden, repetitive, stereotyped, nonrhythmic movements that involve discrete muscle groups. (View a video of a patient with tics.) Simple motor tics are focal movements involving 1 group of muscles, whereas complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements (Table 2).
Table 1
Features of 5 movement disorders that may resemble tics
| Tics | Stereotypies | Dystonia | Chorea | Ballism | Myoclonus |
|---|---|---|---|---|---|
| Sudden, repetitive, stereotyped, nonrhythmic movements or sounds | Patterned, nonpurposeful movement | Cocontraction of agonist and antagonist muscles, causing an abnormal twisting posture | Continuous, flowing, nonrhythmic, nonpurposeful movement | Forceful, flinging, large amplitude choreic movement | Sudden, quick, shock-like movement |
| Usually start after age 3 | Usually start before age 3 and resolve by adolescence | More common in adults | — | — | — |
| Decrease when focused; increase when stressed, anxious, fatigued, or bored | Occur when the child is excited | Worsens during motor tasks | Worsens during motor tasks | Worsens during motor tasks | — |
| Comorbid conditions include OCD and ADHD | Common in children with mental retardation or autism | — | Can occur after streptococcal infection | Can occur after streptococcal infection | — |
| Preceded by a premonitory urge or sensation | Possibly preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge |
| Temporarily suppressible | Suppressible | Not suppressible | Partially suppressible; can incorporate into semi-purposeful movements | Partially suppressible | Not suppressible |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder | |||||
Table 2
Characteristics of simple and complex motor and vocal tics*
| Simple tics | Complex tics |
|---|---|
| Eye blinking or eye rolling Nose, mouth, tongue, or facial grimaces (nose twitch, nasal flaring, chewing lip, teeth grinding, sticking out tongue, mouth stretching, lip licking) Head jerks or movements (neck stretching, touching chin to shoulder) Shoulder jerks/movements (shoulder shrugging, jerking a shoulder) Arm or hand movements (flexing or extending arms or fingers) Coughing Throat clearing, grunting Sniffing, snorting, shouting Humming | Jumping Spinning Touching objects or people Throwing objects Repeating others’ action (echopraxia) Obscene gestures (copropraxia) Repeating one’s own words (palilalia) Repeating what someone else said (echolalia) Obscene, inappropriate words (coprolalia) |
| *Simple tics are focal movements involving 1 group of muscles; complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements | |
Older children frequently describe a premonitory urge prior to the tic. Patients typically can suppress tics for a transient period of time, although during tic suppression they usually feel restless and anticipate performing their tic. The ultimate performance of the tic brings relief. Tic suppression also occurs during focused activity. Emotional stress, fatigue, illness, or boredom can exacerbate tics.
To begin monitoring Sammy’s clinical course, you administer 3 assessment tools described inTable 3. You explain to Sammy’s parents that these tests will be repeated yearly or when tics worsen. However, you tell his parents that these scores alone will not determine present or future clinical decisions, including treatments. You also recommend that they connect with support groups on the Tourette Syndrome Association (TSA) Web site.
CASE CONTINUED: Changes over time
Sammy’s parents appreciate your explanation and say they will share information from the TSA Web site with Sammy’s principal, teachers, and classmates. The family agrees to return in 6 months or sooner if the tics worsen.
By age 8, Sammy develops multiple tics: facial grimacing, looking upwards, punching movements, whistling, and throat clearing. He is slightly bothered by these tics, and his friends have asked him about them. He tells them he has Tourette syndrome, and that usually ends the questioning. He returns for a follow-up visit because his parents notice a dramatic increase in his tics after Sammy’s father loses his job.
Treatment options
When deciding to treat a child’s tics, the first step is to determine whether to pursue a nonpharmacologic or pharmacologic approach (Algorithm). To tailor an approach most suited for an individual child, discuss with the family their feelings about therapy and medications. This information—along with tic severity—will help determine a treatment plan.
Behavior therapy and medication are management strategies; neither can cure a tic disorder. The most conservative approach to tic treatment is to:
- provide the child and family with basic guidelines for managing tics
- help alleviate environmental stress and other potential triggers.
Algorithm: Recommended treatment of tics in children and adolescents
CASE CONTINUED: A first intervention
You discuss treatment options with Sammy’s family, and they view medication as a last resort. Sammy does not seem to be bothered by his tics, and his parents do not wish to start him on daily medications. Given this situation, habit reversal therapy (HRT) is appropriate for Sammy because he is old enough to participate in HRT to reduce his tics.
HRT is an effective nonpharmacologic approach to help children with tics.4 Its 3 components are:
- awareness training
- competing response training
- social support.5
This simplified version of the original HRT can be completed in eight 1-hour sessions. Good candidates are patients who are cognitively mature enough to understand the therapy’s goals and compliant with frequent clinic visits. They also must practice the strategies at home.
It should not be difficult for psychiatrists to learn HRT—or refer to therapists who are willing to learn it—with the available instructional manual.
CASE CONTINUED: Practicing alternatives
You ask Sammy to imitate his tics. After helping him become more aware of his tics, you encourage him to develop a more socially appropriate movement to engage in whenever he feels the urge to punch. Sammy chooses to clench his fist in his pocket. He also learns to breathe in whenever he has an urge to whistle. you advise Sammy’s parents to reward his efforts to suppress the tics. He practices the strategies daily.
At age 12, Sammy returns to your office. He has begun to have frequent neck-jerking tics, which cause neck pain and daily headaches. He also is slapping his thigh and having frequent vocal tics characterized by loud shrieking. The vocal tics are disruptive in class, even though Sammy sits toward the back of the room. Sammy’s classmates tease him, and he is very frustrated.
Medication approach
The decision to start a medication for tics is complex. Scores from the YGTSS, PUTS, and GTS-QOL scales (Table 3) provide only a partial clinical picture. This decision should be reached after a detailed discussion with the family about benefits and risks of medications and ensuring that everyone’s expectations are reasonable.
A variety of medications are available to treat patients with tics (Table 4). No medication can completely eliminate tics, however, and many have substantial side effects. Before initiating medical treatment, consider 3 questions:
- Is moderate or severe pain involved?
- Is there significant functional interference?
- Is there significant social disruption despite efforts to optimize the social environment for the child?
Sammy’s frequent neck-jerking tics now cause chronic daily headaches, and his shrieking vocal tics are interfering with classroom activities, so we recommended a 3-month trial of guanfacine following the dosing schedule in Table 4.
Table 3
3 scales for assessing tic severity and impact on functioning
| Instrument | Purpose | Description | Design | Administration frequency |
|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | Assess tic severity | Review of motor and vocal tics. Rate number, frequency, intensity, complexity, and interference on a 5-point scale | Clinician-rated | Annual and as needed for increased tics |
| Premonitory Urge for Tics Scale (PUTS) | Detect the presence of unpleasant sensations that precedes tics | 10 questions | Self-report | Annual and as needed for increased tics |
| Gilles de la Tourette Syndrome Quality of Life Scale (GTS-QOL) | Measure quality of life | 27 questions, 4 subscales: psychological, physical, obsessional, and cognitive | Self-report | Annual and as needed for increased tics |
Table 4
Medications with evidence of tic-suppressing effects*
| Category A evidence | ||
|---|---|---|
| Medication | Starting dose | Target dose |
| Haloperidol | 0.25 to 0.5 mg/d | 1 to 4 mg/d |
| Pimozide | 0.5 to 1 mg/d | 2 to 8 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 1 to 3 mg/d |
| Category B evidence | ||
| Medication | Starting dose | Target dose |
| Fluphenazine | 0.5 to 1 mg/d | 1.5 to 10 mg/d |
| Ziprasidone | 5 to 10 mg/d | 10 to 80 mg/d |
| Clonidine | 0.025 to 0.05 mg/d | 0.1 to 0.3 mg/d |
| Guanfacine | 0.5 to 1 mg/d | 1 to 3 mg/d |
| Botulinum toxin | 30 to 300 units | |
| Category C evidence | ||
| Medication | Starting dose | Target dose |
| Olanzapine | 2.5 to 5 mg/d | 2.5 to 12.5 mg/d |
| Tetrabenazine | 25 mg/d | 37.5 to 150 mg/d |
| Baclofen | 10 mg/d | 40 to 60 mg/d |
| Nicotine patch | 7 mg/d | 7 to 21 mg/d |
| Mecamylamine | 2.5 mg/d | 2.5 to 7.5 mg/d |
| Flutamide | 250 mg/d | 750 mg/d |
| *Category A: supported by ≥2 placebo-controlled trials; category B: supported by 1 placebo-controlled trial; category C: supported by open-label study | ||
| Source: Reference 6 | ||
The first-line pharmacologic agent for tic suppression generally is an alpha-adrenergic medication, unless the tics are severe.6
Clonidine and guanfacine usually are started at low doses and increased gradually. Although not as effective as neuroleptics, alpha-adrenergics have a lower potential for side effects and are easier to use because no laboratory tests need to be monitored. Adverse effects associated with alpha-adrenergic medications include sedation, dry mouth, dizziness, headache, and rebound hypertension if discontinued abruptly.
If tics are causing pain, some clinicians prefer conservative measures such as heat or ice, massage, analgesics, relaxation therapy, and reassurance.
Second-line agents include typical and atypical antipsychotics. Haloperidol and pimozide have shown efficacy in reducing tics in placebo- controlled studies,7,8 as have risperidone (in 4 randomized controlled trials [RCTs]) and ziprasidone (in 1 RCT).9,10 The emergence of serious side effects is a risk for both typical and atypical antipsychotics (Table 5).
Table 5
Potential adverse effects of antipsychotic treatment in children*
| Adverse effect | Examples |
|---|---|
| Sedation | — |
| Acute dystonic reactions | Oculogyric crisis, torticollis |
| Appetite changes | Weight gain |
| Endocrine abnormalities | Amenorrhea, diabetes, galactorrhea, gynecomastia, hyperprolactinemia |
| Cognitive effects | Impaired concentration |
| Akathisia | Difficulty sitting still |
| ECG changes | Prolonged QT interval |
| Parkinsonism | Tremor, bradykinesia, rigidity, postural instability |
| Tardive syndrome | Orofacial dyskinesia, chorea, dystonia, myoclonus, tics |
| Neuroleptic malignant syndrome | Potentially fatal; consists of muscular rigidity, fever, autonomic dysfunction, labile blood pressure, sweating, urinary incontinence, fluctuating level of consciousness, leukocytosis, elevated serum creatine kinase |
| *Potential adverse effects are listed from most to least likely to occur | |
As part of your informed consent discussion, weigh the risk of side effects against the benefits of treatment. Point out to patients and their families that they can expect to see a decrease in tic frequency, but symptoms will not necessarily disappear with any medication. We tell our patients that with antipsychotics the best we can hope for is to reduce tic frequency by approximately one-half.6
When treating tics, start with 1 medication. However, if the tics are severe enough to require more than 1 medication, check for drug interactions.
Third-line agents. Agents that have not been tested in placebo-controlled trials can be considered third line; these are listed as category C (supported by open-label studies) in Table 4. Botulinum toxin injection has been found to be effective for motor and vocal tics.11,12 Botulinum toxin and implantation of deep brain stimulators13 are invasive options and generally are reserved for severe, treatment-resistant tics.
CASE CONTINUED: Managing antipsychotics
After trying guanfacine for 12 weeks, Sammy notices no tic reduction. His parents consent to a low dose of risperidone. you review with them the American Psychiatric Association (APA)/American Diabetes Association (ADA) guidelines14 for managing metabolic problems in patients treated with atypical antipsychotics.
As instructed in the APA/ADA guidelines, obtain baseline measurements and monitor for metabolic effects of antipsychotic therapy over time (Table 6). Sammy starts risperidone at 0.5 mg once daily. After 2 weeks, he notices a decrease in his tics. At the 3-month visit after starting risperidone, he is happy with his risperidone dose and does not want to increase it. He has gained 3 pounds, and you instruct him to eat a well-balanced diet and exercise routinely. At the 6-month visit, his tics are minimal and his weight has stabilized.
Table 6
Children receiving antipsychotics: monitoring recommendations
| Clinical information | Frequency |
|---|---|
| Family history | Initial visit |
| Weight | Baseline, monthly |
| Height | Baseline, monthly |
| BMI | Baseline, monthly |
| Waist circumference | Baseline, annually |
| Blood pressure | Baseline, 3 months after treatment starts, and annually thereafter |
| Fasting lipid profile | Baseline, every 3 months initially, then every 6 months thereafter |
| Fasting serum glucose | Baseline, every 3 months, then every 6 months thereafter |
| BMI: body mass index | |
| Source: References 14,16 | |
You recommend that Sammy remain on risperidone for another 3 months of stability and then begin to taper this medication. You review the risks and benefits of long-term treatment with risperidone, pointing out that it may lead to abnormal movements upon withdrawal, and explain that you typically do not treat children with antipsychotics for more than one year continuously.
CASE CONTINUED: Comorbid symptoms
Since starting 7th grade, Sammy has worried excessively about making mistakes. He spends 6 hours each night on homework, which he often does not turn in because of anxiety about not getting answers perfectly right. Classmates notice that Sammy taps the door 3 times when he comes into the classroom and that he steps over the black tiles in the hallway.
Consider the presence and impact of comorbid OCD or ADHD, which can impair children’s quality of life more than tics themselves.15 Assessment scales can help you make a diagnosis and monitor treatment.
If you suspect OCD, the clinician-rated Children’s Yale Brown Obsessive Compulsive Scale is the gold standard for describing the phenomenology and measuring symptom severity. Additional scales to measure symptoms’ impact on family life include the Leyton Obsessional Inventory—child version, Family Accommodation Scale for OCD, and Child OCD Impact Scale.
ADHD scales include the Conners Parent Rating Scale—Revised, Conners Teacher Rating Scale—Revised, Swanson, Nolan, and Pelham, or the Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales. Because ADHD symptoms must be present in more than 1 environment to meet diagnostic criteria, ask parents and teachers to complete the Conners or Vanderbilt scales.
In children who present with a tic disorder plus a comorbid condition, prioritize treatment by determining which symptoms interfere with the child’s ability to function at school, at home, and in the social arena. Children who require treatment for >1 disorder often are referred initially for cognitive-behavioral therapy for OCD symptoms while receiving pharmacologic treatment for ADHD and/or Tourette syndrome. When necessary, it is usually safe to combine antipsychotics, stimulants, and selective serotonin reuptake inhibitors, although medication interactions should be reviewed in each specific case.
Related resources
- Woods DW. Managing Tourette syndrome: a behavioral intervention for children and adults. Therapist guide. New York, NY: Oxford University Press; 2008.
- Tourette Syndrome Association. www.tsa-usa.org.
- International OCD Foundation. www.ocfoundation.org.
Drug brand names
- Baclofen • Lioresal
- Botulinum toxin • Botox, Myobloc
- Clomipramine • Anafranil
- Clonidine • Catapres
- Guanfacine • Tenex
- Fluphenazine • Prolixin
- Flutamide • Eulexin
- Haloperidol • Haldol
- Mecamylamine • Inversine
- Nicotine patch • NicoDerm
- Olanzapine • Zyprexa
- Pimozide • Orap
- Risperidone • Risperdal
- Tetrabenazine • Xenazine
- Ziprasidone • Geodon
Disclosures
Dr. Harris has received research support from the Translational Research Initiative at Cincinnati Children’s Hospital Medical Center.
Dr. Wu reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Robertson M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425-462.
2. Freeman R. For the Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a worldwide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):15-23.
3. Stefl M. Mental health needs associated with Tourette syndrome. Am J Public Health. 1984;74:1310-1313.
4. Deckersbach T, Rauch S, Buhlmann U, et al. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079-1090.
5. Woods DW, Miltenberger RG. Habit reversal: a review of applications and variations. J Behav Ther Exp Psychiatry. 1995;26:123-131.
6. Scahill L, Erenberg G, Berlin C, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
7. Shapiro E, Shapiro A, Fulop G, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. 1989;46:722-730.
8. Sallee F, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154:1057-1062.
9. Scahill L, Leckman J, Schultz R, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
10. Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
11. Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
12. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24(6):420-423.
13. Porta M, Sevello D, Sassi M, et al. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur Neurol. 2009;62(5):264-273.
14. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:1335-1342.
15. Bernard BA, Stebbins GT, Siegel S, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. 2009;24(7):1070-1073.
16. Understanding the risks of antipsychotic treatment in young people. Advice for managing side effects in children and teenagers. Harv Ment Health Lett. 2009;25(9):1-3.
1. Robertson M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425-462.
2. Freeman R. For the Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a worldwide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):15-23.
3. Stefl M. Mental health needs associated with Tourette syndrome. Am J Public Health. 1984;74:1310-1313.
4. Deckersbach T, Rauch S, Buhlmann U, et al. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079-1090.
5. Woods DW, Miltenberger RG. Habit reversal: a review of applications and variations. J Behav Ther Exp Psychiatry. 1995;26:123-131.
6. Scahill L, Erenberg G, Berlin C, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
7. Shapiro E, Shapiro A, Fulop G, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. 1989;46:722-730.
8. Sallee F, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154:1057-1062.
9. Scahill L, Leckman J, Schultz R, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
10. Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
11. Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
12. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24(6):420-423.
13. Porta M, Sevello D, Sassi M, et al. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur Neurol. 2009;62(5):264-273.
14. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:1335-1342.
15. Bernard BA, Stebbins GT, Siegel S, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. 2009;24(7):1070-1073.
16. Understanding the risks of antipsychotic treatment in young people. Advice for managing side effects in children and teenagers. Harv Ment Health Lett. 2009;25(9):1-3.
Discontinuing an antidepressant?
Most psychiatrists have encountered patients who report distressing symptoms when they have forgotten to take their antidepressant for a few days or during changes in the medication regimen. A discontinuation syndrome can occur with almost any antidepressant, highlighting the need to slowly taper these medications when discontinuation is part of a treatment plan.
This article discusses antidepressant discontinuation syndrome (ADS) in a patient who experienced substantial distress after a rapid antidepressant taper in preparation for electroconvulsive therapy (ECT). My goal is to raise awareness of ADS, promote early detection of the syndrome, and address proper prevention and management strategies.
CASE REPORT: Feeling ‘worse than ever’
Mr. J, a 32-year-old tax accountant, is hospitalized for a major depressive episode (MDE) associated with deteriorating function and suicidal ideation. This second lifetime MDE started 8 months before his admission to an inpatient mood disorders unit.
Mr. J initially was treated with fluoxetine, up to 40 mg/d across 14 weeks, with good tolerability but no significant benefit. His psychiatrist switched Mr. J to bupropion but stopped it after 4 weeks because of side effects—including headaches, insomnia, and tremor—and limited antidepressant benefit. Venlafaxine XR was initiated next, at 150 mg/d within the first 2 weeks, increased to 225 mg/d at week 6, then titrated to 300 mg/d at week 10. After 10 weeks, aripiprazole, 5 mg/d, was added because Mr. J showed only partial, limited response to venlafaxine XR and this antipsychotic is indicated for adjunctive treatment of major depressive disorder.
Mr. J reported mild, transient restlessness but otherwise he tolerated the medications well, and he claimed excellent adherence. After 6 additional weeks of treatment, however, Mr. J was hospitalized because of persistent severely depressed mood, increasing suicidal ideation, and inability to function at work.
On admission, Mr. J is evaluated and agrees to ECT. To meet the ECT service’s protocol, venlafaxine XR is reduced to 150 mg/d for 2 days and then stopped when ECT is started. Aripiprazole is continued at 5 mg/d.
Mr. J tolerates the first ECT treatment well, but the morning before his second treatment he complains of feeling “worse than ever.” An agitated Mr. J reports dramatically intensified suicidal ideation—much more intrusive than before he was hospitalized. He also complains of diffuse muscle aches and cramps, runny nose, nausea, headache, and burning sensations in both arms and hands. He withdraws consent for ECT and returns to the mood disorders unit for ongoing treatment.
Could this be ADS?
Yes, it could. In this case, the inpatient psychiatrist and treatment team were lulled into a false sense of security by Mr. J’s history of few side effects with various treatments and medication changes. The ECT service wanted the patient off venlafaxine XR before beginning ECT, and the treatment team believed a quick taper would not cause discontinuation symptoms because Mr. J was taking an “extended-release” medication.
Within 72 hours, Mr. J went from taking 300 mg/d of venlafaxine XR to none. Within 2 days of cessation, he complained of symptoms that could characterize a discontinuation syndrome. A potential red herring in this case is that the patient complained of feeling worse after his first ECT treatment, and one might erroneously think the myalgias, headache, and other somatic symptoms were side effects of ECT and/or anesthesia.
Typical ADS symptoms
Nearly all antidepressant classes are associated with ADS. Symptoms vary from patient to patient but typically include the “FINISH” syndrome: flu-like symptoms, ###bold/bold###nsomnia, nausea, ###bold/bold###mbalance, sensory disturbances, and hyperarousal (anxiety/agitation) (Table 1).1
Adverse effects after stopping tricyclic antidepressants have been well documented. They may include FINISH syndrome features as well as cholinergic overdrive or “rebound” such as abdominal cramping and diarrhea.2-4 Reports of ADS after patients stopped selective serotonin reuptake inhibitors (SSRIs) emerged soon after these agents were introduced.5-7 Similarly, ADS has been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine,8-10 venlafaxine XR,11 and duloxetine.12 ADS symptoms are similar with SSRIs and SNRIs, generally without the anticholinergic effects associated with tricyclic antidepressant discontinuation.
Fewer reports of discontinuation syndrome exist for bupropion, mirtazapine, monoamine oxidase inhibitors (MAOIs), and nefazodone.13-17 Discontinuation-emergent syndromes with these non-SSRI/non-SNRI antidepressants tend to present differently. With MAOIs, for example, neuropsychiatric symptoms such as severe anxiety, agitation, pressured speech, sleeplessness or drowsiness, hallucinations, delirium, and paranoid psychosis can be prominent.17
The prevalence of ADS is unclear, and published estimates vary widely because of the lack of large controlled studies. ADS rates with SSRIs/SNRIs have been reported from as low as 0% for fluoxetine to higher rates for shorter half-life antidepressants:
- 2.2% with sertraline
- 14% with fluvoxamine
- 20% with paroxetine
- 30.8% with clomipramine.
These rates come from a retrospective case note review of patients who discontinued antidepressants under supervision.18 In a small cohort of outpatients being treated for major depressive disorder, stopping venlafaxine XR was associated with discontinuation symptoms for the next 3 days in 7 of 9 patients (78%), compared with 2 of 9 patients (22%) stopping placebo.11
Diagnostic criteria have been proposed for ADS associated with serotonin (5-HT) reuptake inhibitors.19-22 Proposed ADS definitions differ somewhat, but essentially 3 features guide the diagnosis:
- appearance of characteristic symptoms (Table 2)21,23
- timing of those symptoms, which usually emerge within 1 week of abrupt cessation or marked reduction of the antidepressant
- symptoms generally are mild, short-lived, self-limiting, and/or rapidly reversed by restarting the original antidepressant.
Evidence suggests shorter half-life antidepressants may be associated with the highest risk for ADS, but other risk factors remain presumptive (Table 3).
Table 1
FINISH: Symptoms of antidepressant discontinuation syndrome
| Flu-like symptoms |
| Insomnia |
| Nausea |
| Imbalance |
| Sensory disturbances |
| Hyperarousal (anxiety/agitation) |
| Source: Reference 1 |
Table 2
ADS symptoms can range across a variety of system clusters
| System cluster | Symptoms |
|---|---|
| Neurosensory | Vertigo, paresthesias, shock-like reactions, myalgias, numbness, sensitivity to sound, unusual visual sensations, ringing in the ears |
| Neuromotor | Tremor, myoclonus, ataxia/gait instability, visual changes, restless legs, problems with speech, tongue movements |
| Gastrointestinal | Nausea, vomiting, cramps/bloating, diarrhea, anorexia |
| Neuropsychiatric | Anxiety/panic, depression, mood swings, suicidal ideation, irritability, impulsivity, confusion, psychosis |
| Vasomotor | Diaphoresis, flushing, temperature intolerance |
| Other | Headache, insomnia, vivid dreams, nightmares, lethargy/fatigue, flu-like symptoms |
| ADS: antidepressant discontinuation syndrome | |
| Source: Construct suggested by Shelton,21 with additional symptoms added from other sources, including the discontinuation symptom checklist of Rosenbaum et al23 | |
Table 3
Possible patient risk factors for developing ADS*
| Abrupt antidepressant discontinuation |
| Shorter half-life antidepressants |
| Intermittent nonadherence/noncompliance |
| Interrupted treatment or use of ‘drug holiday’ |
| Specific antidepressant properties (such as potent [5-HT] receptor antagonism, cholinergic effects) |
| Younger patient age (including children and adolescents) |
| Female gender |
| Pregnancy |
| Neonate/breast-fed infant (mother on antidepressant therapy) |
| History of ADS |
| Vulnerability to depressive relapse |
| Duration of treatment (possible increased risk with more than 4 to 6 weeks of antidepressant exposure) |
| Switches to or between generic antidepressant formulations (related to variations in bioequivalence) |
| History of early adverse reactions when the antidepressant was initiated |
| ADS: antidepressant discontinuation syndrome |
| *Risk factors for ADS have not been rigorously studied in randomized controlled trials. Possible risk factors in this table were found in case reports |
What causes ADS?
Although the exact cause of ADS is unknown, the literature proposes several theories.
Because of the central serotonin system’s complex connections, acute reduction in synaptic serotonin when an SSRI or SNRI is abruptly or too quickly stopped may be the first in a cascade of steps affecting transmission of multiple monoamines. Parallels have been drawn between the phenomenon observed with rapid depletion of tryptophan—the essential amino acid precursor for the synthesis of 5-HT—and ADS seen with abrupt discontinuation of serotonergic antidepressants. This suggests that acute drops in neurotransmitter levels can precipitate neuropsychiatric and somatic manifestations of ADS.24
Patients’ uncomfortable symptoms likely are caused by the serotonin, norepinephrine, and cholinergic systems and their complex interactions.25 Individual genetic factors may influence patients’ vulnerability for ADS.
Managing ADS
Awareness and prevention. ADS can be misinterpreted as side effects of newly started treatment after an antidepressant is stopped. In Mr. J’s case, the appearance of muscle aches, headaches, and other ADS symptoms after ECT was started easily could have been perceived as adverse effects of ECT. Mr. J’s agitation and increased suicidal ideation could lead a clinician to mistakenly think that MDE was worsening because the antidepressant was stopped before ECT became effective. Being aware of ADS can prevent misdiagnosis and allow you to quickly identify the condition, manage the reversible syndrome, and continue with new treatment plan—in this case, ECT.
You can help prevent ADS by educating patients about the need to adhere to antidepressant regimens and to avoid missing doses. Consider ADS risk factors—particularly medications’ half-lives—before you start, change, or stop antidepressant therapy. Gradually taper all antidepressants being discontinued, with the possible exception of fluoxetine (which, including its active metabolite, has an elimination half-life of approximately 1 to 2 weeks).
Tapering antidepressants is more art than science because we have no controlled data to support any particular tapering regimen. Tailor the taper duration based on each patient’s response to sequential dosage reductions. Antidepressants with shorter half-lives—such as venlafaxine or paroxetine—may need to be tapered more slowly, perhaps by reducing the dosage by 25% every 4 to 6 weeks. If you plan to switch medications, this process may be expedited during a cross-taper to another antidepressant. You still may see discontinuation symptoms, however, depending on which new agent is chosen and which is being stopped.
Treating ADS. Appropriately recognizing ADS risk and slowly tapering antidepressants as needed usually prevents clinically significant distress associated with discontinuation. For some patients, however, ADS may be particularly severe or prolonged, or may emerge at the end of a slow taper.
Challenging cases may be more likely with paroxetine or venlafaxine—even the extended-release or controlled-release preparations. The elimination half-life of paroxetine is 15 to 20 hours, and the half-lives of venlafaxine and venlafaxine XR are 5 to 11 hours. Desvenlafaxine’s half-life is 11 hours, and product labeling of this enantiomer of racemic venlafaxine notes that discontinuation symptoms have occurred.26 ADS treatment depends on the severity of the reaction and whether or not further antidepressant therapy is necessary.
For mild ADS, reassurance and treatment focused on specific symptoms—such as sedative-hypnotics for insomnia or benzodiazepines for anxiety—may be all that is needed, because ADS tends to gradually resolve over an average of 10 days.27
For more severe ADS, or when ongoing antidepressant therapy is indicated, restarting the recently withdrawn antidepressant at the pre-ADS dosage typically resolves the syndrome within 24 hours. Then employ a slower, more cautious taper when next attempting to discontinue that antidepressant.
Another option. An alternate management strategy is to substitute fluoxetine to suppress ADS associated with shorter half-life SSRIs or SNRIs. Case reports18,20,28 suggest that fluoxetine, 5 to 20 mg/d, can be used to ameliorate venlafaxine-induced ADS. Fluoxetine can be tried as monotherapy for 1 to 2 weeks and then rapidly tapered or stopped. Others have suggested combination therapy, such as:
- restarting venlafaxine at the pre-ADS dose plus fluoxetine, 20 mg/d
- tapering venlafaxine by 50% every 5 days until stopped
- reducing fluoxetine 1 week later to 10 mg/d for 5 days
- then stopping fluoxetine.28
In general, SSRIs should not be co-administered with SNRIs long-term because of potential additive adverse effects such as serotonin syndrome. Combining fluoxetine with an SNRI such as venlafaxine for the purpose of tapering off venlafaxine and reducing ADS risk probably is safe, however, as long as the fluoxetine dose is low (5 to 20 mg) and SNRI reduction begins immediately, with a plan for complete tapering.
CASE CONTINUED: ECT treatment proceeds
Venlafaxine XR is not restarted to address Mr. J’s suspected ADS because of concerns about potential increased risk for cardiac events (asystole, prolonged bradycardia) during ECT with concomitant venlafaxine use.29,30 Fluoxetine, which rarely may prolong ECT-induced seizures, is deemed a safer choice and is started immediately at 20 mg/d.
Because of Mr. J’s other symptoms, we prescribe lorazepam, 0.5 mg bid, for anxiety for 2 days; increase aripiprazole to 5 mg bid for agitation; and add zolpidem, 10 mg at bedtime, for insomnia. The following day, Mr. J reports substantial relief from ADS symptoms, including myalgias, paresthesias, and suicidal ideation.21,23
His second ECT treatment is administered the next day, followed by a successful course of 9 treatments and partial remission of the MDE within 3 weeks. Fluoxetine is reduced to 10 mg/d one week into the ECT series, then discontinued one week later. No signs of emergent ADS are seen at discharge or 2-week outpatient follow-up. Mr. J achieves full remission with maintenance ECT plus bedtime doses of mirtazapine, 30 mg, and aripiprazole, 7.5 mg, across 6 months of follow-up care.
Related resources
- Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(suppl 4):27-30.
- American Family Physician. Patient handout on antidepressant discontinuation. www.aafp.org/afp/2006/0801/p457.html.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. See appendix for discontinuation-emergent signs and symptoms checklist.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Venlafaxine extended-release • Effexor XR
- Zolpidem • Ambien
Disclosure
Dr. Muzina reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
When Dr. Muzina submitted this article to Current Psychiatry, he was director, Center for Mood Disorders Treatment and Research, Cleveland Clinic Neurological Institute, Cleveland, OH.
1. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59(5):255.-
2. Ceccherini-Nelli A, Bardellini L, Cur A, et al. Antidepressant withdrawal: prospective findings. Am J Psychiatry. 1993;150(1):165.-
3. Dilsaver SC, Kronfol Z, Sackellares JC, et al. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983;3(3):157-164.
4. Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27(9):1068-1072.
5. Barr LC, Goodman WK, Price LH. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry. 1994;151(2):289.-
6. Frost L, Lal S. Shock-like sensations after discontinuation of selective serotonin reuptake inhibitors. Am J Psychiatry. 1995;152(5):810.-
7. Louie AK, Lannon RA, Ajari LJ. Withdrawal reaction after sertraline discontinuation. Am J Psychiatry. 1994;151(3):450-451.
8. Benazzi F. Venlafaxine withdrawal symptoms. Can J Psychiatry. 1996;41(7):487.-
9. Farah A, Lauer TE. Possible venlafaxine withdrawal syndrome. Am J Psychiatry. 1996;153(4):576.-
10. Louie AK, Lannon RA, Kirsch MA, et al. Venlafaxine withdrawal reactions. Am J Psychiatry. 1996;153(12):1652.-
11. Fava M, Mulroy R, Alpert J, et al. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762.
12. Perahia DG, Kajdasz DK, Desaiah D, et al. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89(1-3):207-212.
13. Benazzi F. Mirtazapine withdrawal symptoms. Can J Psychiatry. 1998;43(5):525.-
14. Benazzi F. Nefazodone withdrawal symptoms. Can J Psychiatry. 1998;43(2):194-195.
15. Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4(2):78.-
16. Berigan TR, Harazin JS. Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry. 1999;1(2):50-51.
17. Dilsaver SC. Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1-7.
18. Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356-362.
19. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261.
20. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183-197.
21. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry. 2006;67(suppl 4):3-7.
22. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry. 1997;58(suppl 7):5-10.
23. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87.
24. Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22-26.
25. Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):8-13.
26. Wyeth. Desvenlafaxine (Pristiq) prescribing information. Available at: http://www.wyeth.com/content/showlabeling.asp?id=497. Accessed January 27, 2010.
27. Price JS, Waller PC, Wood SM, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757-763.
28. Benazzi F. SSRI discontinuation syndrome treated with fluoxetine. Int J Geriatr Psychiatry. 1998;13(6):421-422.
29. Agelink MM, Zeit T, Klieser E. Prolonged bradycardia complicates antidepressive treatment with venlafaxine and ECT. Br J Psychiatry. 1998;173:441.-
30. Gonzalez-Pinto A, Gutierrez M, Gonzalez N, et al. Efficacy and safety of venlafaxine-ECT combination in treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):206-209.
Most psychiatrists have encountered patients who report distressing symptoms when they have forgotten to take their antidepressant for a few days or during changes in the medication regimen. A discontinuation syndrome can occur with almost any antidepressant, highlighting the need to slowly taper these medications when discontinuation is part of a treatment plan.
This article discusses antidepressant discontinuation syndrome (ADS) in a patient who experienced substantial distress after a rapid antidepressant taper in preparation for electroconvulsive therapy (ECT). My goal is to raise awareness of ADS, promote early detection of the syndrome, and address proper prevention and management strategies.
CASE REPORT: Feeling ‘worse than ever’
Mr. J, a 32-year-old tax accountant, is hospitalized for a major depressive episode (MDE) associated with deteriorating function and suicidal ideation. This second lifetime MDE started 8 months before his admission to an inpatient mood disorders unit.
Mr. J initially was treated with fluoxetine, up to 40 mg/d across 14 weeks, with good tolerability but no significant benefit. His psychiatrist switched Mr. J to bupropion but stopped it after 4 weeks because of side effects—including headaches, insomnia, and tremor—and limited antidepressant benefit. Venlafaxine XR was initiated next, at 150 mg/d within the first 2 weeks, increased to 225 mg/d at week 6, then titrated to 300 mg/d at week 10. After 10 weeks, aripiprazole, 5 mg/d, was added because Mr. J showed only partial, limited response to venlafaxine XR and this antipsychotic is indicated for adjunctive treatment of major depressive disorder.
Mr. J reported mild, transient restlessness but otherwise he tolerated the medications well, and he claimed excellent adherence. After 6 additional weeks of treatment, however, Mr. J was hospitalized because of persistent severely depressed mood, increasing suicidal ideation, and inability to function at work.
On admission, Mr. J is evaluated and agrees to ECT. To meet the ECT service’s protocol, venlafaxine XR is reduced to 150 mg/d for 2 days and then stopped when ECT is started. Aripiprazole is continued at 5 mg/d.
Mr. J tolerates the first ECT treatment well, but the morning before his second treatment he complains of feeling “worse than ever.” An agitated Mr. J reports dramatically intensified suicidal ideation—much more intrusive than before he was hospitalized. He also complains of diffuse muscle aches and cramps, runny nose, nausea, headache, and burning sensations in both arms and hands. He withdraws consent for ECT and returns to the mood disorders unit for ongoing treatment.
Could this be ADS?
Yes, it could. In this case, the inpatient psychiatrist and treatment team were lulled into a false sense of security by Mr. J’s history of few side effects with various treatments and medication changes. The ECT service wanted the patient off venlafaxine XR before beginning ECT, and the treatment team believed a quick taper would not cause discontinuation symptoms because Mr. J was taking an “extended-release” medication.
Within 72 hours, Mr. J went from taking 300 mg/d of venlafaxine XR to none. Within 2 days of cessation, he complained of symptoms that could characterize a discontinuation syndrome. A potential red herring in this case is that the patient complained of feeling worse after his first ECT treatment, and one might erroneously think the myalgias, headache, and other somatic symptoms were side effects of ECT and/or anesthesia.
Typical ADS symptoms
Nearly all antidepressant classes are associated with ADS. Symptoms vary from patient to patient but typically include the “FINISH” syndrome: flu-like symptoms, ###bold/bold###nsomnia, nausea, ###bold/bold###mbalance, sensory disturbances, and hyperarousal (anxiety/agitation) (Table 1).1
Adverse effects after stopping tricyclic antidepressants have been well documented. They may include FINISH syndrome features as well as cholinergic overdrive or “rebound” such as abdominal cramping and diarrhea.2-4 Reports of ADS after patients stopped selective serotonin reuptake inhibitors (SSRIs) emerged soon after these agents were introduced.5-7 Similarly, ADS has been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine,8-10 venlafaxine XR,11 and duloxetine.12 ADS symptoms are similar with SSRIs and SNRIs, generally without the anticholinergic effects associated with tricyclic antidepressant discontinuation.
Fewer reports of discontinuation syndrome exist for bupropion, mirtazapine, monoamine oxidase inhibitors (MAOIs), and nefazodone.13-17 Discontinuation-emergent syndromes with these non-SSRI/non-SNRI antidepressants tend to present differently. With MAOIs, for example, neuropsychiatric symptoms such as severe anxiety, agitation, pressured speech, sleeplessness or drowsiness, hallucinations, delirium, and paranoid psychosis can be prominent.17
The prevalence of ADS is unclear, and published estimates vary widely because of the lack of large controlled studies. ADS rates with SSRIs/SNRIs have been reported from as low as 0% for fluoxetine to higher rates for shorter half-life antidepressants:
- 2.2% with sertraline
- 14% with fluvoxamine
- 20% with paroxetine
- 30.8% with clomipramine.
These rates come from a retrospective case note review of patients who discontinued antidepressants under supervision.18 In a small cohort of outpatients being treated for major depressive disorder, stopping venlafaxine XR was associated with discontinuation symptoms for the next 3 days in 7 of 9 patients (78%), compared with 2 of 9 patients (22%) stopping placebo.11
Diagnostic criteria have been proposed for ADS associated with serotonin (5-HT) reuptake inhibitors.19-22 Proposed ADS definitions differ somewhat, but essentially 3 features guide the diagnosis:
- appearance of characteristic symptoms (Table 2)21,23
- timing of those symptoms, which usually emerge within 1 week of abrupt cessation or marked reduction of the antidepressant
- symptoms generally are mild, short-lived, self-limiting, and/or rapidly reversed by restarting the original antidepressant.
Evidence suggests shorter half-life antidepressants may be associated with the highest risk for ADS, but other risk factors remain presumptive (Table 3).
Table 1
FINISH: Symptoms of antidepressant discontinuation syndrome
| Flu-like symptoms |
| Insomnia |
| Nausea |
| Imbalance |
| Sensory disturbances |
| Hyperarousal (anxiety/agitation) |
| Source: Reference 1 |
Table 2
ADS symptoms can range across a variety of system clusters
| System cluster | Symptoms |
|---|---|
| Neurosensory | Vertigo, paresthesias, shock-like reactions, myalgias, numbness, sensitivity to sound, unusual visual sensations, ringing in the ears |
| Neuromotor | Tremor, myoclonus, ataxia/gait instability, visual changes, restless legs, problems with speech, tongue movements |
| Gastrointestinal | Nausea, vomiting, cramps/bloating, diarrhea, anorexia |
| Neuropsychiatric | Anxiety/panic, depression, mood swings, suicidal ideation, irritability, impulsivity, confusion, psychosis |
| Vasomotor | Diaphoresis, flushing, temperature intolerance |
| Other | Headache, insomnia, vivid dreams, nightmares, lethargy/fatigue, flu-like symptoms |
| ADS: antidepressant discontinuation syndrome | |
| Source: Construct suggested by Shelton,21 with additional symptoms added from other sources, including the discontinuation symptom checklist of Rosenbaum et al23 | |
Table 3
Possible patient risk factors for developing ADS*
| Abrupt antidepressant discontinuation |
| Shorter half-life antidepressants |
| Intermittent nonadherence/noncompliance |
| Interrupted treatment or use of ‘drug holiday’ |
| Specific antidepressant properties (such as potent [5-HT] receptor antagonism, cholinergic effects) |
| Younger patient age (including children and adolescents) |
| Female gender |
| Pregnancy |
| Neonate/breast-fed infant (mother on antidepressant therapy) |
| History of ADS |
| Vulnerability to depressive relapse |
| Duration of treatment (possible increased risk with more than 4 to 6 weeks of antidepressant exposure) |
| Switches to or between generic antidepressant formulations (related to variations in bioequivalence) |
| History of early adverse reactions when the antidepressant was initiated |
| ADS: antidepressant discontinuation syndrome |
| *Risk factors for ADS have not been rigorously studied in randomized controlled trials. Possible risk factors in this table were found in case reports |
What causes ADS?
Although the exact cause of ADS is unknown, the literature proposes several theories.
Because of the central serotonin system’s complex connections, acute reduction in synaptic serotonin when an SSRI or SNRI is abruptly or too quickly stopped may be the first in a cascade of steps affecting transmission of multiple monoamines. Parallels have been drawn between the phenomenon observed with rapid depletion of tryptophan—the essential amino acid precursor for the synthesis of 5-HT—and ADS seen with abrupt discontinuation of serotonergic antidepressants. This suggests that acute drops in neurotransmitter levels can precipitate neuropsychiatric and somatic manifestations of ADS.24
Patients’ uncomfortable symptoms likely are caused by the serotonin, norepinephrine, and cholinergic systems and their complex interactions.25 Individual genetic factors may influence patients’ vulnerability for ADS.
Managing ADS
Awareness and prevention. ADS can be misinterpreted as side effects of newly started treatment after an antidepressant is stopped. In Mr. J’s case, the appearance of muscle aches, headaches, and other ADS symptoms after ECT was started easily could have been perceived as adverse effects of ECT. Mr. J’s agitation and increased suicidal ideation could lead a clinician to mistakenly think that MDE was worsening because the antidepressant was stopped before ECT became effective. Being aware of ADS can prevent misdiagnosis and allow you to quickly identify the condition, manage the reversible syndrome, and continue with new treatment plan—in this case, ECT.
You can help prevent ADS by educating patients about the need to adhere to antidepressant regimens and to avoid missing doses. Consider ADS risk factors—particularly medications’ half-lives—before you start, change, or stop antidepressant therapy. Gradually taper all antidepressants being discontinued, with the possible exception of fluoxetine (which, including its active metabolite, has an elimination half-life of approximately 1 to 2 weeks).
Tapering antidepressants is more art than science because we have no controlled data to support any particular tapering regimen. Tailor the taper duration based on each patient’s response to sequential dosage reductions. Antidepressants with shorter half-lives—such as venlafaxine or paroxetine—may need to be tapered more slowly, perhaps by reducing the dosage by 25% every 4 to 6 weeks. If you plan to switch medications, this process may be expedited during a cross-taper to another antidepressant. You still may see discontinuation symptoms, however, depending on which new agent is chosen and which is being stopped.
Treating ADS. Appropriately recognizing ADS risk and slowly tapering antidepressants as needed usually prevents clinically significant distress associated with discontinuation. For some patients, however, ADS may be particularly severe or prolonged, or may emerge at the end of a slow taper.
Challenging cases may be more likely with paroxetine or venlafaxine—even the extended-release or controlled-release preparations. The elimination half-life of paroxetine is 15 to 20 hours, and the half-lives of venlafaxine and venlafaxine XR are 5 to 11 hours. Desvenlafaxine’s half-life is 11 hours, and product labeling of this enantiomer of racemic venlafaxine notes that discontinuation symptoms have occurred.26 ADS treatment depends on the severity of the reaction and whether or not further antidepressant therapy is necessary.
For mild ADS, reassurance and treatment focused on specific symptoms—such as sedative-hypnotics for insomnia or benzodiazepines for anxiety—may be all that is needed, because ADS tends to gradually resolve over an average of 10 days.27
For more severe ADS, or when ongoing antidepressant therapy is indicated, restarting the recently withdrawn antidepressant at the pre-ADS dosage typically resolves the syndrome within 24 hours. Then employ a slower, more cautious taper when next attempting to discontinue that antidepressant.
Another option. An alternate management strategy is to substitute fluoxetine to suppress ADS associated with shorter half-life SSRIs or SNRIs. Case reports18,20,28 suggest that fluoxetine, 5 to 20 mg/d, can be used to ameliorate venlafaxine-induced ADS. Fluoxetine can be tried as monotherapy for 1 to 2 weeks and then rapidly tapered or stopped. Others have suggested combination therapy, such as:
- restarting venlafaxine at the pre-ADS dose plus fluoxetine, 20 mg/d
- tapering venlafaxine by 50% every 5 days until stopped
- reducing fluoxetine 1 week later to 10 mg/d for 5 days
- then stopping fluoxetine.28
In general, SSRIs should not be co-administered with SNRIs long-term because of potential additive adverse effects such as serotonin syndrome. Combining fluoxetine with an SNRI such as venlafaxine for the purpose of tapering off venlafaxine and reducing ADS risk probably is safe, however, as long as the fluoxetine dose is low (5 to 20 mg) and SNRI reduction begins immediately, with a plan for complete tapering.
CASE CONTINUED: ECT treatment proceeds
Venlafaxine XR is not restarted to address Mr. J’s suspected ADS because of concerns about potential increased risk for cardiac events (asystole, prolonged bradycardia) during ECT with concomitant venlafaxine use.29,30 Fluoxetine, which rarely may prolong ECT-induced seizures, is deemed a safer choice and is started immediately at 20 mg/d.
Because of Mr. J’s other symptoms, we prescribe lorazepam, 0.5 mg bid, for anxiety for 2 days; increase aripiprazole to 5 mg bid for agitation; and add zolpidem, 10 mg at bedtime, for insomnia. The following day, Mr. J reports substantial relief from ADS symptoms, including myalgias, paresthesias, and suicidal ideation.21,23
His second ECT treatment is administered the next day, followed by a successful course of 9 treatments and partial remission of the MDE within 3 weeks. Fluoxetine is reduced to 10 mg/d one week into the ECT series, then discontinued one week later. No signs of emergent ADS are seen at discharge or 2-week outpatient follow-up. Mr. J achieves full remission with maintenance ECT plus bedtime doses of mirtazapine, 30 mg, and aripiprazole, 7.5 mg, across 6 months of follow-up care.
Related resources
- Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(suppl 4):27-30.
- American Family Physician. Patient handout on antidepressant discontinuation. www.aafp.org/afp/2006/0801/p457.html.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. See appendix for discontinuation-emergent signs and symptoms checklist.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Venlafaxine extended-release • Effexor XR
- Zolpidem • Ambien
Disclosure
Dr. Muzina reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
When Dr. Muzina submitted this article to Current Psychiatry, he was director, Center for Mood Disorders Treatment and Research, Cleveland Clinic Neurological Institute, Cleveland, OH.
Most psychiatrists have encountered patients who report distressing symptoms when they have forgotten to take their antidepressant for a few days or during changes in the medication regimen. A discontinuation syndrome can occur with almost any antidepressant, highlighting the need to slowly taper these medications when discontinuation is part of a treatment plan.
This article discusses antidepressant discontinuation syndrome (ADS) in a patient who experienced substantial distress after a rapid antidepressant taper in preparation for electroconvulsive therapy (ECT). My goal is to raise awareness of ADS, promote early detection of the syndrome, and address proper prevention and management strategies.
CASE REPORT: Feeling ‘worse than ever’
Mr. J, a 32-year-old tax accountant, is hospitalized for a major depressive episode (MDE) associated with deteriorating function and suicidal ideation. This second lifetime MDE started 8 months before his admission to an inpatient mood disorders unit.
Mr. J initially was treated with fluoxetine, up to 40 mg/d across 14 weeks, with good tolerability but no significant benefit. His psychiatrist switched Mr. J to bupropion but stopped it after 4 weeks because of side effects—including headaches, insomnia, and tremor—and limited antidepressant benefit. Venlafaxine XR was initiated next, at 150 mg/d within the first 2 weeks, increased to 225 mg/d at week 6, then titrated to 300 mg/d at week 10. After 10 weeks, aripiprazole, 5 mg/d, was added because Mr. J showed only partial, limited response to venlafaxine XR and this antipsychotic is indicated for adjunctive treatment of major depressive disorder.
Mr. J reported mild, transient restlessness but otherwise he tolerated the medications well, and he claimed excellent adherence. After 6 additional weeks of treatment, however, Mr. J was hospitalized because of persistent severely depressed mood, increasing suicidal ideation, and inability to function at work.
On admission, Mr. J is evaluated and agrees to ECT. To meet the ECT service’s protocol, venlafaxine XR is reduced to 150 mg/d for 2 days and then stopped when ECT is started. Aripiprazole is continued at 5 mg/d.
Mr. J tolerates the first ECT treatment well, but the morning before his second treatment he complains of feeling “worse than ever.” An agitated Mr. J reports dramatically intensified suicidal ideation—much more intrusive than before he was hospitalized. He also complains of diffuse muscle aches and cramps, runny nose, nausea, headache, and burning sensations in both arms and hands. He withdraws consent for ECT and returns to the mood disorders unit for ongoing treatment.
Could this be ADS?
Yes, it could. In this case, the inpatient psychiatrist and treatment team were lulled into a false sense of security by Mr. J’s history of few side effects with various treatments and medication changes. The ECT service wanted the patient off venlafaxine XR before beginning ECT, and the treatment team believed a quick taper would not cause discontinuation symptoms because Mr. J was taking an “extended-release” medication.
Within 72 hours, Mr. J went from taking 300 mg/d of venlafaxine XR to none. Within 2 days of cessation, he complained of symptoms that could characterize a discontinuation syndrome. A potential red herring in this case is that the patient complained of feeling worse after his first ECT treatment, and one might erroneously think the myalgias, headache, and other somatic symptoms were side effects of ECT and/or anesthesia.
Typical ADS symptoms
Nearly all antidepressant classes are associated with ADS. Symptoms vary from patient to patient but typically include the “FINISH” syndrome: flu-like symptoms, ###bold/bold###nsomnia, nausea, ###bold/bold###mbalance, sensory disturbances, and hyperarousal (anxiety/agitation) (Table 1).1
Adverse effects after stopping tricyclic antidepressants have been well documented. They may include FINISH syndrome features as well as cholinergic overdrive or “rebound” such as abdominal cramping and diarrhea.2-4 Reports of ADS after patients stopped selective serotonin reuptake inhibitors (SSRIs) emerged soon after these agents were introduced.5-7 Similarly, ADS has been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine,8-10 venlafaxine XR,11 and duloxetine.12 ADS symptoms are similar with SSRIs and SNRIs, generally without the anticholinergic effects associated with tricyclic antidepressant discontinuation.
Fewer reports of discontinuation syndrome exist for bupropion, mirtazapine, monoamine oxidase inhibitors (MAOIs), and nefazodone.13-17 Discontinuation-emergent syndromes with these non-SSRI/non-SNRI antidepressants tend to present differently. With MAOIs, for example, neuropsychiatric symptoms such as severe anxiety, agitation, pressured speech, sleeplessness or drowsiness, hallucinations, delirium, and paranoid psychosis can be prominent.17
The prevalence of ADS is unclear, and published estimates vary widely because of the lack of large controlled studies. ADS rates with SSRIs/SNRIs have been reported from as low as 0% for fluoxetine to higher rates for shorter half-life antidepressants:
- 2.2% with sertraline
- 14% with fluvoxamine
- 20% with paroxetine
- 30.8% with clomipramine.
These rates come from a retrospective case note review of patients who discontinued antidepressants under supervision.18 In a small cohort of outpatients being treated for major depressive disorder, stopping venlafaxine XR was associated with discontinuation symptoms for the next 3 days in 7 of 9 patients (78%), compared with 2 of 9 patients (22%) stopping placebo.11
Diagnostic criteria have been proposed for ADS associated with serotonin (5-HT) reuptake inhibitors.19-22 Proposed ADS definitions differ somewhat, but essentially 3 features guide the diagnosis:
- appearance of characteristic symptoms (Table 2)21,23
- timing of those symptoms, which usually emerge within 1 week of abrupt cessation or marked reduction of the antidepressant
- symptoms generally are mild, short-lived, self-limiting, and/or rapidly reversed by restarting the original antidepressant.
Evidence suggests shorter half-life antidepressants may be associated with the highest risk for ADS, but other risk factors remain presumptive (Table 3).
Table 1
FINISH: Symptoms of antidepressant discontinuation syndrome
| Flu-like symptoms |
| Insomnia |
| Nausea |
| Imbalance |
| Sensory disturbances |
| Hyperarousal (anxiety/agitation) |
| Source: Reference 1 |
Table 2
ADS symptoms can range across a variety of system clusters
| System cluster | Symptoms |
|---|---|
| Neurosensory | Vertigo, paresthesias, shock-like reactions, myalgias, numbness, sensitivity to sound, unusual visual sensations, ringing in the ears |
| Neuromotor | Tremor, myoclonus, ataxia/gait instability, visual changes, restless legs, problems with speech, tongue movements |
| Gastrointestinal | Nausea, vomiting, cramps/bloating, diarrhea, anorexia |
| Neuropsychiatric | Anxiety/panic, depression, mood swings, suicidal ideation, irritability, impulsivity, confusion, psychosis |
| Vasomotor | Diaphoresis, flushing, temperature intolerance |
| Other | Headache, insomnia, vivid dreams, nightmares, lethargy/fatigue, flu-like symptoms |
| ADS: antidepressant discontinuation syndrome | |
| Source: Construct suggested by Shelton,21 with additional symptoms added from other sources, including the discontinuation symptom checklist of Rosenbaum et al23 | |
Table 3
Possible patient risk factors for developing ADS*
| Abrupt antidepressant discontinuation |
| Shorter half-life antidepressants |
| Intermittent nonadherence/noncompliance |
| Interrupted treatment or use of ‘drug holiday’ |
| Specific antidepressant properties (such as potent [5-HT] receptor antagonism, cholinergic effects) |
| Younger patient age (including children and adolescents) |
| Female gender |
| Pregnancy |
| Neonate/breast-fed infant (mother on antidepressant therapy) |
| History of ADS |
| Vulnerability to depressive relapse |
| Duration of treatment (possible increased risk with more than 4 to 6 weeks of antidepressant exposure) |
| Switches to or between generic antidepressant formulations (related to variations in bioequivalence) |
| History of early adverse reactions when the antidepressant was initiated |
| ADS: antidepressant discontinuation syndrome |
| *Risk factors for ADS have not been rigorously studied in randomized controlled trials. Possible risk factors in this table were found in case reports |
What causes ADS?
Although the exact cause of ADS is unknown, the literature proposes several theories.
Because of the central serotonin system’s complex connections, acute reduction in synaptic serotonin when an SSRI or SNRI is abruptly or too quickly stopped may be the first in a cascade of steps affecting transmission of multiple monoamines. Parallels have been drawn between the phenomenon observed with rapid depletion of tryptophan—the essential amino acid precursor for the synthesis of 5-HT—and ADS seen with abrupt discontinuation of serotonergic antidepressants. This suggests that acute drops in neurotransmitter levels can precipitate neuropsychiatric and somatic manifestations of ADS.24
Patients’ uncomfortable symptoms likely are caused by the serotonin, norepinephrine, and cholinergic systems and their complex interactions.25 Individual genetic factors may influence patients’ vulnerability for ADS.
Managing ADS
Awareness and prevention. ADS can be misinterpreted as side effects of newly started treatment after an antidepressant is stopped. In Mr. J’s case, the appearance of muscle aches, headaches, and other ADS symptoms after ECT was started easily could have been perceived as adverse effects of ECT. Mr. J’s agitation and increased suicidal ideation could lead a clinician to mistakenly think that MDE was worsening because the antidepressant was stopped before ECT became effective. Being aware of ADS can prevent misdiagnosis and allow you to quickly identify the condition, manage the reversible syndrome, and continue with new treatment plan—in this case, ECT.
You can help prevent ADS by educating patients about the need to adhere to antidepressant regimens and to avoid missing doses. Consider ADS risk factors—particularly medications’ half-lives—before you start, change, or stop antidepressant therapy. Gradually taper all antidepressants being discontinued, with the possible exception of fluoxetine (which, including its active metabolite, has an elimination half-life of approximately 1 to 2 weeks).
Tapering antidepressants is more art than science because we have no controlled data to support any particular tapering regimen. Tailor the taper duration based on each patient’s response to sequential dosage reductions. Antidepressants with shorter half-lives—such as venlafaxine or paroxetine—may need to be tapered more slowly, perhaps by reducing the dosage by 25% every 4 to 6 weeks. If you plan to switch medications, this process may be expedited during a cross-taper to another antidepressant. You still may see discontinuation symptoms, however, depending on which new agent is chosen and which is being stopped.
Treating ADS. Appropriately recognizing ADS risk and slowly tapering antidepressants as needed usually prevents clinically significant distress associated with discontinuation. For some patients, however, ADS may be particularly severe or prolonged, or may emerge at the end of a slow taper.
Challenging cases may be more likely with paroxetine or venlafaxine—even the extended-release or controlled-release preparations. The elimination half-life of paroxetine is 15 to 20 hours, and the half-lives of venlafaxine and venlafaxine XR are 5 to 11 hours. Desvenlafaxine’s half-life is 11 hours, and product labeling of this enantiomer of racemic venlafaxine notes that discontinuation symptoms have occurred.26 ADS treatment depends on the severity of the reaction and whether or not further antidepressant therapy is necessary.
For mild ADS, reassurance and treatment focused on specific symptoms—such as sedative-hypnotics for insomnia or benzodiazepines for anxiety—may be all that is needed, because ADS tends to gradually resolve over an average of 10 days.27
For more severe ADS, or when ongoing antidepressant therapy is indicated, restarting the recently withdrawn antidepressant at the pre-ADS dosage typically resolves the syndrome within 24 hours. Then employ a slower, more cautious taper when next attempting to discontinue that antidepressant.
Another option. An alternate management strategy is to substitute fluoxetine to suppress ADS associated with shorter half-life SSRIs or SNRIs. Case reports18,20,28 suggest that fluoxetine, 5 to 20 mg/d, can be used to ameliorate venlafaxine-induced ADS. Fluoxetine can be tried as monotherapy for 1 to 2 weeks and then rapidly tapered or stopped. Others have suggested combination therapy, such as:
- restarting venlafaxine at the pre-ADS dose plus fluoxetine, 20 mg/d
- tapering venlafaxine by 50% every 5 days until stopped
- reducing fluoxetine 1 week later to 10 mg/d for 5 days
- then stopping fluoxetine.28
In general, SSRIs should not be co-administered with SNRIs long-term because of potential additive adverse effects such as serotonin syndrome. Combining fluoxetine with an SNRI such as venlafaxine for the purpose of tapering off venlafaxine and reducing ADS risk probably is safe, however, as long as the fluoxetine dose is low (5 to 20 mg) and SNRI reduction begins immediately, with a plan for complete tapering.
CASE CONTINUED: ECT treatment proceeds
Venlafaxine XR is not restarted to address Mr. J’s suspected ADS because of concerns about potential increased risk for cardiac events (asystole, prolonged bradycardia) during ECT with concomitant venlafaxine use.29,30 Fluoxetine, which rarely may prolong ECT-induced seizures, is deemed a safer choice and is started immediately at 20 mg/d.
Because of Mr. J’s other symptoms, we prescribe lorazepam, 0.5 mg bid, for anxiety for 2 days; increase aripiprazole to 5 mg bid for agitation; and add zolpidem, 10 mg at bedtime, for insomnia. The following day, Mr. J reports substantial relief from ADS symptoms, including myalgias, paresthesias, and suicidal ideation.21,23
His second ECT treatment is administered the next day, followed by a successful course of 9 treatments and partial remission of the MDE within 3 weeks. Fluoxetine is reduced to 10 mg/d one week into the ECT series, then discontinued one week later. No signs of emergent ADS are seen at discharge or 2-week outpatient follow-up. Mr. J achieves full remission with maintenance ECT plus bedtime doses of mirtazapine, 30 mg, and aripiprazole, 7.5 mg, across 6 months of follow-up care.
Related resources
- Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(suppl 4):27-30.
- American Family Physician. Patient handout on antidepressant discontinuation. www.aafp.org/afp/2006/0801/p457.html.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. See appendix for discontinuation-emergent signs and symptoms checklist.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Venlafaxine extended-release • Effexor XR
- Zolpidem • Ambien
Disclosure
Dr. Muzina reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
When Dr. Muzina submitted this article to Current Psychiatry, he was director, Center for Mood Disorders Treatment and Research, Cleveland Clinic Neurological Institute, Cleveland, OH.
1. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59(5):255.-
2. Ceccherini-Nelli A, Bardellini L, Cur A, et al. Antidepressant withdrawal: prospective findings. Am J Psychiatry. 1993;150(1):165.-
3. Dilsaver SC, Kronfol Z, Sackellares JC, et al. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983;3(3):157-164.
4. Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27(9):1068-1072.
5. Barr LC, Goodman WK, Price LH. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry. 1994;151(2):289.-
6. Frost L, Lal S. Shock-like sensations after discontinuation of selective serotonin reuptake inhibitors. Am J Psychiatry. 1995;152(5):810.-
7. Louie AK, Lannon RA, Ajari LJ. Withdrawal reaction after sertraline discontinuation. Am J Psychiatry. 1994;151(3):450-451.
8. Benazzi F. Venlafaxine withdrawal symptoms. Can J Psychiatry. 1996;41(7):487.-
9. Farah A, Lauer TE. Possible venlafaxine withdrawal syndrome. Am J Psychiatry. 1996;153(4):576.-
10. Louie AK, Lannon RA, Kirsch MA, et al. Venlafaxine withdrawal reactions. Am J Psychiatry. 1996;153(12):1652.-
11. Fava M, Mulroy R, Alpert J, et al. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762.
12. Perahia DG, Kajdasz DK, Desaiah D, et al. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89(1-3):207-212.
13. Benazzi F. Mirtazapine withdrawal symptoms. Can J Psychiatry. 1998;43(5):525.-
14. Benazzi F. Nefazodone withdrawal symptoms. Can J Psychiatry. 1998;43(2):194-195.
15. Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4(2):78.-
16. Berigan TR, Harazin JS. Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry. 1999;1(2):50-51.
17. Dilsaver SC. Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1-7.
18. Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356-362.
19. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261.
20. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183-197.
21. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry. 2006;67(suppl 4):3-7.
22. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry. 1997;58(suppl 7):5-10.
23. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87.
24. Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22-26.
25. Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):8-13.
26. Wyeth. Desvenlafaxine (Pristiq) prescribing information. Available at: http://www.wyeth.com/content/showlabeling.asp?id=497. Accessed January 27, 2010.
27. Price JS, Waller PC, Wood SM, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757-763.
28. Benazzi F. SSRI discontinuation syndrome treated with fluoxetine. Int J Geriatr Psychiatry. 1998;13(6):421-422.
29. Agelink MM, Zeit T, Klieser E. Prolonged bradycardia complicates antidepressive treatment with venlafaxine and ECT. Br J Psychiatry. 1998;173:441.-
30. Gonzalez-Pinto A, Gutierrez M, Gonzalez N, et al. Efficacy and safety of venlafaxine-ECT combination in treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):206-209.
1. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59(5):255.-
2. Ceccherini-Nelli A, Bardellini L, Cur A, et al. Antidepressant withdrawal: prospective findings. Am J Psychiatry. 1993;150(1):165.-
3. Dilsaver SC, Kronfol Z, Sackellares JC, et al. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983;3(3):157-164.
4. Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27(9):1068-1072.
5. Barr LC, Goodman WK, Price LH. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry. 1994;151(2):289.-
6. Frost L, Lal S. Shock-like sensations after discontinuation of selective serotonin reuptake inhibitors. Am J Psychiatry. 1995;152(5):810.-
7. Louie AK, Lannon RA, Ajari LJ. Withdrawal reaction after sertraline discontinuation. Am J Psychiatry. 1994;151(3):450-451.
8. Benazzi F. Venlafaxine withdrawal symptoms. Can J Psychiatry. 1996;41(7):487.-
9. Farah A, Lauer TE. Possible venlafaxine withdrawal syndrome. Am J Psychiatry. 1996;153(4):576.-
10. Louie AK, Lannon RA, Kirsch MA, et al. Venlafaxine withdrawal reactions. Am J Psychiatry. 1996;153(12):1652.-
11. Fava M, Mulroy R, Alpert J, et al. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762.
12. Perahia DG, Kajdasz DK, Desaiah D, et al. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89(1-3):207-212.
13. Benazzi F. Mirtazapine withdrawal symptoms. Can J Psychiatry. 1998;43(5):525.-
14. Benazzi F. Nefazodone withdrawal symptoms. Can J Psychiatry. 1998;43(2):194-195.
15. Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4(2):78.-
16. Berigan TR, Harazin JS. Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry. 1999;1(2):50-51.
17. Dilsaver SC. Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1-7.
18. Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356-362.
19. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261.
20. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183-197.
21. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry. 2006;67(suppl 4):3-7.
22. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry. 1997;58(suppl 7):5-10.
23. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87.
24. Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22-26.
25. Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):8-13.
26. Wyeth. Desvenlafaxine (Pristiq) prescribing information. Available at: http://www.wyeth.com/content/showlabeling.asp?id=497. Accessed January 27, 2010.
27. Price JS, Waller PC, Wood SM, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757-763.
28. Benazzi F. SSRI discontinuation syndrome treated with fluoxetine. Int J Geriatr Psychiatry. 1998;13(6):421-422.
29. Agelink MM, Zeit T, Klieser E. Prolonged bradycardia complicates antidepressive treatment with venlafaxine and ECT. Br J Psychiatry. 1998;173:441.-
30. Gonzalez-Pinto A, Gutierrez M, Gonzalez N, et al. Efficacy and safety of venlafaxine-ECT combination in treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):206-209.
Psychosis in women: Consider midlife medical and psychological triggers
Dr. I, a 48-year-old university professor, is brought to the ER by her husband because she has developed an irrational fear of being chased by Nazis. The fears have become increasingly bizarre, her husband reports. She believes her Nazi persecutors are bandaging their arms and using wheelchairs to pretend to be disabled. When out with her husband, Dr. I points to people in wheelchairs, convinced they are after her, will kill her, and are incensed because she left Germany—her country of birth. Her husband brought her to the ER when she started to hear her persecutors addressing her in German at night.
Psychoses of unknown cause usually begin in late adolescence or early adulthood. Less frequently the onset occurs in later adulthood (age ≥40). Late-onset psychosis is much more prevalent in women than in men for reasons that are imperfectly understood.
When you are evaluating a midlife woman with first onset of psychosis, don’t assume an illness of unknown cause (bipolar disorder or schizophrenia) until after you have done a comprehensive search for triggers of her psychotic symptoms. After age 40, women are more likely than men to develop psychosis because of gender-specific medical and psychological precipitants.
Predisposing factors for psychosis
Psychosis is an emergent quality of structural and chemical changes in the brain. As such, it can be expected to surface during:
• brain reorganization or transition (adolescence, senescence, brain trauma, stroke, starvation, inflammation, or brain tumor)
• change in brain chemistry (flux in gonadal, thyroid, or adrenal hormone levels; electrolyte imbalance; fever; exposure to chemical substances; immune response).
Psychological stress impacting the brain via stress hormones also can predispose a person to psychosis.
Because some individuals are more prone than others to develop psychosis during brain alteration, chemical and structural changes in the brain are assumed to interact with genetic propensities to influence gene expression. Once a psychotic event has occurred, it is thought to sensitize the brain so that subsequent events emerge more readily.1
Schizophrenia—though not the only illness in which psychosis plays a role—is a prototype for psychotic illness, and several reported sex differences in this disorder are worth noting.2 The incidence of schizophrenia is approximately the same in both sexes, but women show a later age of onset—a paradox in that the brain develops at a faster pace in females and theoretically should reach the threshold for the first appearance of schizophrenia earlier. Women also require lower doses of antipsychotic medication to recover from an acute psychotic episode and to maintain remission, at least before menopause.3,4 Both of these differences can be explained as an effect of estrogen on a) gene expression5 and b) liver enzymes that metabolize antipsychotics.6
The estrogen hypothesis. Women show a tendency toward premenstrual and postpartum exacerbation of symptoms when estrogen levels are relatively low. These clinical observations, confirmed by some but not all studies, have led to the hypothesis that estrogens are neuroprotective7 and also protect against psychosis.8
Estrogen withdrawal in specific brain cells may release a cascade of events that over time can increase the severity of psychotic and cognitive symptoms. The reason for suspecting such effects is based on what we know about estrogenic effects on neurotransmitter, cognitive, and stress-induction pathways, and—more fundamentally—on neuronal growth and atrophy.
According to the estrogen hypothesis, women are—to some degree—protected against schizophrenia by their relatively high gonadal estrogen production between puberty and and menopause. Women lose this protection with the onset of perimenopausal estrogen fluctuation and decline, accounting for their second peak of illness onset after age 45.
Epidemiologic studies showing a second peak of schizophrenia onset in women (but not men) around the age of menopause support this hypothesis.9,10 Longitudinal outcomes for schizophrenia—which are better in women than in men during late adolescence or early adulthood11—gradually even out after the first 15 years of illness, suggesting that women’s advantage is lost at a time approximating menopause (Box 1).
The question, then, becomes: Is it only because of estrogen loss after age 40 that women become more prone to develop a psychotic illness? Other differences between the sexes that may play roles include immune function, low iron stores, sleep sufficiency, thyroid function, exposure to toxic substances (including therapeutic drugs), societal pressures to be slim while aging (Table), and the experience of stress.12
CASE CONTINUED
Exhausted and confused
Dr. I is a well-groomed, handsome woman, but she hardly speaks when interviewed, looking frightened and somewhat bewildered. She has never had a mental health problem, nor has anyone in her family. She agrees to stay in the hospital but is not sure why. She has slept no more than 1 or 2 hours in the last several days.
Her early history is unremarkable. She did well in school. After earning a PhD at the University of Leipzig, she and her husband immigrated to Canada. Both are university professors. They never decided not to have children, but children hadn’t come. Her menstrual periods stopped 2 years before admission. The question about children is the only 1 that elicits emotion in Dr. I. When I ask about it, tears come to her eyes as she shakes her head.
Her husband reports that she has not been eating well and has, in the last year, started to drink more alcohol than usual—3 to 4 drinks of whiskey a night. She does not smoke cigarettes, and her health generally is good. She uses no medications. Her husband describes their marital relationship as very close, although it has become strained in recent weeks because of her unreasonable fears. He admits that their work is always stressful; competition is fierce, with more and more deadlines and less and less leisure time. The couple has few friends and no hobbies.
Late-onset psychosis symptoms
In late-onset psychosis (after age 45), men appear to suffer substantially milder symptoms and spend less time hospitalized than women.13 Women with late-onset schizophrenia have more severe positive symptoms than men and fewer negative symptoms.14,15 Overall, patients with late-onset schizophrenia have a lower prevalence of looseness of associations and negative symptoms than those with earlier onset.16,17
In addition, individuals with schizophrenia who become ill in middle age have been reported to:
• show better neuropsychological performance (particularly in learning and abstraction/cognitive flexibility) than those with early onset
• possibly have larger thalamic volumes
• respond to lower antipsychotic doses.18
Auditory and visual hallucinations frequently are observed in patients with comorbid late-onset schizophrenia and auditory and visual impairment.16 Palmer et al18 reported no difference in family history of schizophrenia between early and late onset, but this is controversial. Convert et al16 note that most studies reveal a lower lifetime risk of schizophrenia in first-degree relatives of patients with late-onset than early-onset schizophrenia.
CASE CONTINUED
Medical workup
Dr. I’s physical exam is unremarkable. Her thyroid is not enlarged; there are no breast lumps. On mental status exam, her mood is flat. She is preoccupied with fears of the Nazis. Routine blood tests show slight anemia; fasting glucose levels are within normal range.
I give Dr. I zopiclone, 7.5 mg, to help her sleep. The next day she keeps to herself, eats very little, and appears disinterested in her surroundings. Nursing staff report that she often seems frightened. Dr. I asks to use the ward phone to call Germany but is told that she cannot make long distance calls from that phone. This seems to disturb her.
Differential diagnosis
Sensory impairment, substance abuse, and metabolic changes have been implicated in the appearance of psychosis in later life. More specific to women than men, however, are medical and psychiatric precipitants. These include autoimmune disease (and its treatment) and psychiatric disorders, as well as thyroid dysfunction, self-induced starvation (anorexia nervosa) and diet aids, substance use and abuse, insomnia, and iron deficiency (Table).
Autoimmune disease and treatment. Nearly 80% of patients with autoimmune disease are women, and these disorders (as well as their treatment) can manifest as psychosis. Corticosteroids have a well-documented history of triggering psychotic symptoms, which are twice as likely in women than in men. The incidence of severe psychosis while taking oral prednisone ranges from 1.6% to 50% and averages 5.7%. The average daily dose of corticosteroids for patients who develop psychosis is 59.5 mg/d.
Corticosteroid creams absorbed through skin as well as inhaled and intranasal corticosteroids in their more potent formulations can have systemic effects, including psychosis. Nonsteroidal anti-inflammatory drugs such as ibuprofen also can trigger psychosis.19
Psychiatric disorders. Posttraumatic stress disorder with psychotic symptoms may overlap with categories such as psychogenic psychoses, hysterical psychoses, nonaffective remitting psychoses, acute brief psychoses, reactive psychoses, acute and transient psychoses, and bouffées délirantes (in France, the name for transient psychotic reactions).20 Consider these female-predominant conditions in the differential diagnosis, along with micropsychotic episodes in borderline personality disorder, in which the predominance of women is 3:1.
Medical treatment for depression and anxiety also can lead to psychotic symptoms through individual susceptibility to the action of specific drugs or through withdrawal effects.
Clinical assessment
Question all women presenting with psychosis about eating habits and diet pills, and check for hypokalemia and hypocalcemia to rule out starvation effects and reactions to stimulants. Also ask about inhalants, and examine for anemia and thyroid dysfunction. Consider all medications as having the potential to trigger psychotic symptoms.
A family history of illness is important, with a focus on autoimmune disorder and its treatment. A thorough psychiatric history is crucial and needs to include assessment of sleep, mood, and relationships with attachment figures. Do not assume illnesses of unknown cause (bipolar disorder or schizophrenia) until after a comprehensive search for precipitants of psychotic symptoms.
CASE CONTINUED
Guilty feelings
To address her delusions, I start Dr. I on risperidone, 2 mg at bedtime. She goes home for the weekend, and her husband reports that she slept throughout the visit. When she returns, she spends a lot of time in bed but is more communicative.
When I ask Dr. I whether she has called Germany, she says she called her recently widowed father. Dr. I begins to cry when talking of her mother, and tells the nurse she feels guilty for not visiting for the last few years. When her mother died 6 months ago, Dr. I had not seen her in 4 years.
Her fears remit with risperidone, maintained at 2 mg/d, but Dr. I remains depressed and responds slowly to treatment with citalopram, 20 mg/d, and supportive therapy. Her final diagnosis is mood disorder with psychotic features.
Treatment
When treating women with late-onset psychosis, remove all potential triggers and address underlying illness. Cognitive therapy targeting specific symptoms is useful; antipsychotics probably will be necessary. Age-related physiologic changes make older persons more sensitive to the therapeutic and toxic effects of antipsychotics.
Estrogen therapy? Women suffering from schizophrenia show significantly lower estrogen levels than the general population of women, and they experience first-onset or recurrence of a psychotic episode significantly more often in low estrogen phases of the cycle. Estrogens have therefore been postulated to constitute a protective factor against psychosis, which means perimenopause is an at-risk period.21 Although evidence is limited, preliminary studies have found beneficial effects from short-term, off-label use of estrogen therapy in women with psychotic illness (Box 2).
Because continuous use of estrogen plus progestin has been associated with an increased risk of adverse effects,22 off-label use of selective estrogen receptor modulators (SERMs) also is being investigated in women with schizophrenia. SERMs act as tissue-specific estrogen agonists and antagonists because they can either inhibit or enhance estrogen-induced activation of estrogen response element-containing genes.23
Wong et al24 used a crossover design to compare the SERM raloxifene with placebo as adjunctive treatment for 6 postmenopausal women with schizophrenia. Each woman received 8 weeks of raloxifene, 60 mg/d, and 8 weeks of placebo. Three began with placebo and 3 with raloxifene.
Verbal memory was measured weekly with the California Verbal Learning Test, using 5 memory trials, free and cued short-delay recall, and long-delay recall. At baseline, the participants had lower scores than older adults in the general population. Eight weeks of placebo improved scores somewhat, suggesting a practice effect. Eight weeks of raloxifene improved cognitive scores to a level similar to that of schizophrenia-free subjects. After 16 weeks, however, cognitive scores in the 2 groups were indistinguishable.
At present I do not recommend estrogen for women with late-onset schizophrenia because the risk is too high and raloxifene does not enter the brain sufficiently to be a valuable cognitive enhancer. Novel SERMs with more specific efficacy for improving cognitive function may prove useful in the future,25 however, as may phytoestrogens. Adjunctive hormone modulation is a promising area of gender-specific treatment for serious mental illness.26
CASE CONCLUSION
Gradually improving
Dr. I’s depression was triggered by her mother’s death and regrets about not visiting and not being a mother. The content of her delusions was related to her guilt about not having returned to Germany; the delusions were probably triggered by depression, alcohol intake, her relative hypoestrogenic state, stress at work, lack of social supports, and dependence on her husband.
Over the next few years, Dr. I is maintained on a low dose of risperidone (reduced from 2 mg/d to 1 mg/d) and citalopram (reduced from 20 mg/d to 10 mg/d). She becomes increasingly engaged in supportive dynamic therapy, and her symptoms gradually improve.
BOTTOM LINE
Psychosis onset in midlife is mostly a female phenomenon because a perimenopausal estrogen decline increases women’s susceptibility. Seek specific triggers such as medical illness or response to a drug before assuming an illness of unknown cause such as bipolar disorder or schizophrenia. Cognitive therapy targeting specific symptoms is useful; antipsychotics probably will be necessary.
Related Resources
• Women and psychosis: A guide for women and their families. Centre for Addiction and Mental Health. University of Toronto. www.camh.net/About_Addiction_Mental_ Health/Mental_Health_Information/Women_Psychosis.
• Seeman MV. Women and psychosis. www.medscape.com/ viewarticle/408912.
• Chattopadhyay S. Estrogen and schizophrenia: Any link? The Internet Journal of Mental Health. 2004;2(1). www.ispub. com/journal/the_internet_journal_of_mental_health.html.
Drug Brand Names
Citalopram • Celexa Prednisone • Deltasone,
Estradiol • Estrace, Orasone, others
Estrofem, others Raloxifene • Evista
Estradiol transdermal • Risperidone • Risperdal
Estraderm , Climara, others
Methylphenidate • Concerta,
Ritalin, others
Disclosure
Dr. Seeman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci Biobehav Rev. 2007;31:858-873.
2. Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27:107-112.
3. Seeman MV. Interaction of sex, age, and neuroleptic dose. Comp Psychiatry. 1983;24:125-128.
4. Usall J, Suarez D, Haro JM, and the SOHO Study Group. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153: 225-231.
5. Hare E, Glahn DC, Dassori A, et al. Heritability of age of onset of psychosis in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009 Apr 6 [Epub ahead of print].
6. Seeman MV. Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry. 2004;161:1324-1333.
7. Marin R, Guerra B, Alonso R, et al. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2:287-301.
8. Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185-194.
9. Castle DJ, Abel K, Takei N, et al. Gender differences in schizophrenia: hormonal effect or subtypes? Schizophr Bull. 1995;21:1-12.
10. Häfner H, an der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139-151.
11. Grossman LS, Harrow M, Rosen C, et al. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49:523-529.
12. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151-178.
13. Riecher-Rössler A, Löffler W, Munk-Jörgensen P. What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci. 1997;247:195-208.
14. Lindamer LA, Lohr JB, Harris MJ, et al. Gender-related clinical differences in older patients with schizophrenia. J Clin Psychiatry. 1999;60:61-67.
15. Seeman MV. Does menopause intensify symptoms in schizophrenia? In: Lewis-Hall F, Williams TS, Panetta JA, et al, eds. Psychiatric illness in women: emerging treatments and research. Arlington, VA: American Psychiatric Publishing, Inc.; 2002:239-248.
16. Convert H, Védie C, Paulin P. [Late-onset schizophrenia or chronic delusion]. Encephale. 2006;32:957-961.
17. Sato T, Bottlender R, Schröter A, et al. Psychopathology of early-onset versus late-onset schizophrenia revisited: an observation of 473 neuroleptic-naive patients before and after first-admission treatments. Schizophr Res. 2004;67:175-183.
18. Palmer BW, McClure FS, Jeste DV. Schizophrenia in late life: findings challenge traditional concepts. Harv Rev Psychiatry. 2001;9:51-58.
19. Weiss DB, Dyrud J, House RM, et al. Psychiatric manifestations of autoimmune disorders. Curr Treat Options Neurol. 2005;7:413-417.
20. Castagnini A, Bertelsen A, Munk-Jorgensen P, et al. The relationship of reactive psychosis and ICD-10 acute and transient psychotic disorders: evidence from a case register-based comparison. Psychopathology. 2007;40:47-53.
21. Huber TJ, Rollnik J, Wilhelms J, et al. Estradiol levels in psychotic disorders. Psychoneuroendocrinology. 2001;26: 27-35.
22. Heiss G, Wallace R, Anderson G, et al, for the WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036-1045.
23. Doncarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009 May 15 [Epub ahead of print].
24. Wong J, Seeman MV, Shapiro H. Case report: raloxifene in postmenopausal women with psychosis: preliminary findings. Am J Geriatr Psychiatry. 2003;11(6):697-698.
25. Ye L, Chan MY, Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009;302:73-80.
26. Kulkarni J, Gurvich C, Gilbert H, et al. Hormone modulation: a novel therapeutic approach for women with severe mental illness. Aust N Z J Psychiatry. 2008;42:83-88.
Dr. I, a 48-year-old university professor, is brought to the ER by her husband because she has developed an irrational fear of being chased by Nazis. The fears have become increasingly bizarre, her husband reports. She believes her Nazi persecutors are bandaging their arms and using wheelchairs to pretend to be disabled. When out with her husband, Dr. I points to people in wheelchairs, convinced they are after her, will kill her, and are incensed because she left Germany—her country of birth. Her husband brought her to the ER when she started to hear her persecutors addressing her in German at night.
Psychoses of unknown cause usually begin in late adolescence or early adulthood. Less frequently the onset occurs in later adulthood (age ≥40). Late-onset psychosis is much more prevalent in women than in men for reasons that are imperfectly understood.
When you are evaluating a midlife woman with first onset of psychosis, don’t assume an illness of unknown cause (bipolar disorder or schizophrenia) until after you have done a comprehensive search for triggers of her psychotic symptoms. After age 40, women are more likely than men to develop psychosis because of gender-specific medical and psychological precipitants.
Predisposing factors for psychosis
Psychosis is an emergent quality of structural and chemical changes in the brain. As such, it can be expected to surface during:
• brain reorganization or transition (adolescence, senescence, brain trauma, stroke, starvation, inflammation, or brain tumor)
• change in brain chemistry (flux in gonadal, thyroid, or adrenal hormone levels; electrolyte imbalance; fever; exposure to chemical substances; immune response).
Psychological stress impacting the brain via stress hormones also can predispose a person to psychosis.
Because some individuals are more prone than others to develop psychosis during brain alteration, chemical and structural changes in the brain are assumed to interact with genetic propensities to influence gene expression. Once a psychotic event has occurred, it is thought to sensitize the brain so that subsequent events emerge more readily.1
Schizophrenia—though not the only illness in which psychosis plays a role—is a prototype for psychotic illness, and several reported sex differences in this disorder are worth noting.2 The incidence of schizophrenia is approximately the same in both sexes, but women show a later age of onset—a paradox in that the brain develops at a faster pace in females and theoretically should reach the threshold for the first appearance of schizophrenia earlier. Women also require lower doses of antipsychotic medication to recover from an acute psychotic episode and to maintain remission, at least before menopause.3,4 Both of these differences can be explained as an effect of estrogen on a) gene expression5 and b) liver enzymes that metabolize antipsychotics.6
The estrogen hypothesis. Women show a tendency toward premenstrual and postpartum exacerbation of symptoms when estrogen levels are relatively low. These clinical observations, confirmed by some but not all studies, have led to the hypothesis that estrogens are neuroprotective7 and also protect against psychosis.8
Estrogen withdrawal in specific brain cells may release a cascade of events that over time can increase the severity of psychotic and cognitive symptoms. The reason for suspecting such effects is based on what we know about estrogenic effects on neurotransmitter, cognitive, and stress-induction pathways, and—more fundamentally—on neuronal growth and atrophy.
According to the estrogen hypothesis, women are—to some degree—protected against schizophrenia by their relatively high gonadal estrogen production between puberty and and menopause. Women lose this protection with the onset of perimenopausal estrogen fluctuation and decline, accounting for their second peak of illness onset after age 45.
Epidemiologic studies showing a second peak of schizophrenia onset in women (but not men) around the age of menopause support this hypothesis.9,10 Longitudinal outcomes for schizophrenia—which are better in women than in men during late adolescence or early adulthood11—gradually even out after the first 15 years of illness, suggesting that women’s advantage is lost at a time approximating menopause (Box 1).
The question, then, becomes: Is it only because of estrogen loss after age 40 that women become more prone to develop a psychotic illness? Other differences between the sexes that may play roles include immune function, low iron stores, sleep sufficiency, thyroid function, exposure to toxic substances (including therapeutic drugs), societal pressures to be slim while aging (Table), and the experience of stress.12
CASE CONTINUED
Exhausted and confused
Dr. I is a well-groomed, handsome woman, but she hardly speaks when interviewed, looking frightened and somewhat bewildered. She has never had a mental health problem, nor has anyone in her family. She agrees to stay in the hospital but is not sure why. She has slept no more than 1 or 2 hours in the last several days.
Her early history is unremarkable. She did well in school. After earning a PhD at the University of Leipzig, she and her husband immigrated to Canada. Both are university professors. They never decided not to have children, but children hadn’t come. Her menstrual periods stopped 2 years before admission. The question about children is the only 1 that elicits emotion in Dr. I. When I ask about it, tears come to her eyes as she shakes her head.
Her husband reports that she has not been eating well and has, in the last year, started to drink more alcohol than usual—3 to 4 drinks of whiskey a night. She does not smoke cigarettes, and her health generally is good. She uses no medications. Her husband describes their marital relationship as very close, although it has become strained in recent weeks because of her unreasonable fears. He admits that their work is always stressful; competition is fierce, with more and more deadlines and less and less leisure time. The couple has few friends and no hobbies.
Late-onset psychosis symptoms
In late-onset psychosis (after age 45), men appear to suffer substantially milder symptoms and spend less time hospitalized than women.13 Women with late-onset schizophrenia have more severe positive symptoms than men and fewer negative symptoms.14,15 Overall, patients with late-onset schizophrenia have a lower prevalence of looseness of associations and negative symptoms than those with earlier onset.16,17
In addition, individuals with schizophrenia who become ill in middle age have been reported to:
• show better neuropsychological performance (particularly in learning and abstraction/cognitive flexibility) than those with early onset
• possibly have larger thalamic volumes
• respond to lower antipsychotic doses.18
Auditory and visual hallucinations frequently are observed in patients with comorbid late-onset schizophrenia and auditory and visual impairment.16 Palmer et al18 reported no difference in family history of schizophrenia between early and late onset, but this is controversial. Convert et al16 note that most studies reveal a lower lifetime risk of schizophrenia in first-degree relatives of patients with late-onset than early-onset schizophrenia.
CASE CONTINUED
Medical workup
Dr. I’s physical exam is unremarkable. Her thyroid is not enlarged; there are no breast lumps. On mental status exam, her mood is flat. She is preoccupied with fears of the Nazis. Routine blood tests show slight anemia; fasting glucose levels are within normal range.
I give Dr. I zopiclone, 7.5 mg, to help her sleep. The next day she keeps to herself, eats very little, and appears disinterested in her surroundings. Nursing staff report that she often seems frightened. Dr. I asks to use the ward phone to call Germany but is told that she cannot make long distance calls from that phone. This seems to disturb her.
Differential diagnosis
Sensory impairment, substance abuse, and metabolic changes have been implicated in the appearance of psychosis in later life. More specific to women than men, however, are medical and psychiatric precipitants. These include autoimmune disease (and its treatment) and psychiatric disorders, as well as thyroid dysfunction, self-induced starvation (anorexia nervosa) and diet aids, substance use and abuse, insomnia, and iron deficiency (Table).
Autoimmune disease and treatment. Nearly 80% of patients with autoimmune disease are women, and these disorders (as well as their treatment) can manifest as psychosis. Corticosteroids have a well-documented history of triggering psychotic symptoms, which are twice as likely in women than in men. The incidence of severe psychosis while taking oral prednisone ranges from 1.6% to 50% and averages 5.7%. The average daily dose of corticosteroids for patients who develop psychosis is 59.5 mg/d.
Corticosteroid creams absorbed through skin as well as inhaled and intranasal corticosteroids in their more potent formulations can have systemic effects, including psychosis. Nonsteroidal anti-inflammatory drugs such as ibuprofen also can trigger psychosis.19
Psychiatric disorders. Posttraumatic stress disorder with psychotic symptoms may overlap with categories such as psychogenic psychoses, hysterical psychoses, nonaffective remitting psychoses, acute brief psychoses, reactive psychoses, acute and transient psychoses, and bouffées délirantes (in France, the name for transient psychotic reactions).20 Consider these female-predominant conditions in the differential diagnosis, along with micropsychotic episodes in borderline personality disorder, in which the predominance of women is 3:1.
Medical treatment for depression and anxiety also can lead to psychotic symptoms through individual susceptibility to the action of specific drugs or through withdrawal effects.
Clinical assessment
Question all women presenting with psychosis about eating habits and diet pills, and check for hypokalemia and hypocalcemia to rule out starvation effects and reactions to stimulants. Also ask about inhalants, and examine for anemia and thyroid dysfunction. Consider all medications as having the potential to trigger psychotic symptoms.
A family history of illness is important, with a focus on autoimmune disorder and its treatment. A thorough psychiatric history is crucial and needs to include assessment of sleep, mood, and relationships with attachment figures. Do not assume illnesses of unknown cause (bipolar disorder or schizophrenia) until after a comprehensive search for precipitants of psychotic symptoms.
CASE CONTINUED
Guilty feelings
To address her delusions, I start Dr. I on risperidone, 2 mg at bedtime. She goes home for the weekend, and her husband reports that she slept throughout the visit. When she returns, she spends a lot of time in bed but is more communicative.
When I ask Dr. I whether she has called Germany, she says she called her recently widowed father. Dr. I begins to cry when talking of her mother, and tells the nurse she feels guilty for not visiting for the last few years. When her mother died 6 months ago, Dr. I had not seen her in 4 years.
Her fears remit with risperidone, maintained at 2 mg/d, but Dr. I remains depressed and responds slowly to treatment with citalopram, 20 mg/d, and supportive therapy. Her final diagnosis is mood disorder with psychotic features.
Treatment
When treating women with late-onset psychosis, remove all potential triggers and address underlying illness. Cognitive therapy targeting specific symptoms is useful; antipsychotics probably will be necessary. Age-related physiologic changes make older persons more sensitive to the therapeutic and toxic effects of antipsychotics.
Estrogen therapy? Women suffering from schizophrenia show significantly lower estrogen levels than the general population of women, and they experience first-onset or recurrence of a psychotic episode significantly more often in low estrogen phases of the cycle. Estrogens have therefore been postulated to constitute a protective factor against psychosis, which means perimenopause is an at-risk period.21 Although evidence is limited, preliminary studies have found beneficial effects from short-term, off-label use of estrogen therapy in women with psychotic illness (Box 2).
Because continuous use of estrogen plus progestin has been associated with an increased risk of adverse effects,22 off-label use of selective estrogen receptor modulators (SERMs) also is being investigated in women with schizophrenia. SERMs act as tissue-specific estrogen agonists and antagonists because they can either inhibit or enhance estrogen-induced activation of estrogen response element-containing genes.23
Wong et al24 used a crossover design to compare the SERM raloxifene with placebo as adjunctive treatment for 6 postmenopausal women with schizophrenia. Each woman received 8 weeks of raloxifene, 60 mg/d, and 8 weeks of placebo. Three began with placebo and 3 with raloxifene.
Verbal memory was measured weekly with the California Verbal Learning Test, using 5 memory trials, free and cued short-delay recall, and long-delay recall. At baseline, the participants had lower scores than older adults in the general population. Eight weeks of placebo improved scores somewhat, suggesting a practice effect. Eight weeks of raloxifene improved cognitive scores to a level similar to that of schizophrenia-free subjects. After 16 weeks, however, cognitive scores in the 2 groups were indistinguishable.
At present I do not recommend estrogen for women with late-onset schizophrenia because the risk is too high and raloxifene does not enter the brain sufficiently to be a valuable cognitive enhancer. Novel SERMs with more specific efficacy for improving cognitive function may prove useful in the future,25 however, as may phytoestrogens. Adjunctive hormone modulation is a promising area of gender-specific treatment for serious mental illness.26
CASE CONCLUSION
Gradually improving
Dr. I’s depression was triggered by her mother’s death and regrets about not visiting and not being a mother. The content of her delusions was related to her guilt about not having returned to Germany; the delusions were probably triggered by depression, alcohol intake, her relative hypoestrogenic state, stress at work, lack of social supports, and dependence on her husband.
Over the next few years, Dr. I is maintained on a low dose of risperidone (reduced from 2 mg/d to 1 mg/d) and citalopram (reduced from 20 mg/d to 10 mg/d). She becomes increasingly engaged in supportive dynamic therapy, and her symptoms gradually improve.
BOTTOM LINE
Psychosis onset in midlife is mostly a female phenomenon because a perimenopausal estrogen decline increases women’s susceptibility. Seek specific triggers such as medical illness or response to a drug before assuming an illness of unknown cause such as bipolar disorder or schizophrenia. Cognitive therapy targeting specific symptoms is useful; antipsychotics probably will be necessary.
Related Resources
• Women and psychosis: A guide for women and their families. Centre for Addiction and Mental Health. University of Toronto. www.camh.net/About_Addiction_Mental_ Health/Mental_Health_Information/Women_Psychosis.
• Seeman MV. Women and psychosis. www.medscape.com/ viewarticle/408912.
• Chattopadhyay S. Estrogen and schizophrenia: Any link? The Internet Journal of Mental Health. 2004;2(1). www.ispub. com/journal/the_internet_journal_of_mental_health.html.
Drug Brand Names
Citalopram • Celexa Prednisone • Deltasone,
Estradiol • Estrace, Orasone, others
Estrofem, others Raloxifene • Evista
Estradiol transdermal • Risperidone • Risperdal
Estraderm , Climara, others
Methylphenidate • Concerta,
Ritalin, others
Disclosure
Dr. Seeman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. I, a 48-year-old university professor, is brought to the ER by her husband because she has developed an irrational fear of being chased by Nazis. The fears have become increasingly bizarre, her husband reports. She believes her Nazi persecutors are bandaging their arms and using wheelchairs to pretend to be disabled. When out with her husband, Dr. I points to people in wheelchairs, convinced they are after her, will kill her, and are incensed because she left Germany—her country of birth. Her husband brought her to the ER when she started to hear her persecutors addressing her in German at night.
Psychoses of unknown cause usually begin in late adolescence or early adulthood. Less frequently the onset occurs in later adulthood (age ≥40). Late-onset psychosis is much more prevalent in women than in men for reasons that are imperfectly understood.
When you are evaluating a midlife woman with first onset of psychosis, don’t assume an illness of unknown cause (bipolar disorder or schizophrenia) until after you have done a comprehensive search for triggers of her psychotic symptoms. After age 40, women are more likely than men to develop psychosis because of gender-specific medical and psychological precipitants.
Predisposing factors for psychosis
Psychosis is an emergent quality of structural and chemical changes in the brain. As such, it can be expected to surface during:
• brain reorganization or transition (adolescence, senescence, brain trauma, stroke, starvation, inflammation, or brain tumor)
• change in brain chemistry (flux in gonadal, thyroid, or adrenal hormone levels; electrolyte imbalance; fever; exposure to chemical substances; immune response).
Psychological stress impacting the brain via stress hormones also can predispose a person to psychosis.
Because some individuals are more prone than others to develop psychosis during brain alteration, chemical and structural changes in the brain are assumed to interact with genetic propensities to influence gene expression. Once a psychotic event has occurred, it is thought to sensitize the brain so that subsequent events emerge more readily.1
Schizophrenia—though not the only illness in which psychosis plays a role—is a prototype for psychotic illness, and several reported sex differences in this disorder are worth noting.2 The incidence of schizophrenia is approximately the same in both sexes, but women show a later age of onset—a paradox in that the brain develops at a faster pace in females and theoretically should reach the threshold for the first appearance of schizophrenia earlier. Women also require lower doses of antipsychotic medication to recover from an acute psychotic episode and to maintain remission, at least before menopause.3,4 Both of these differences can be explained as an effect of estrogen on a) gene expression5 and b) liver enzymes that metabolize antipsychotics.6
The estrogen hypothesis. Women show a tendency toward premenstrual and postpartum exacerbation of symptoms when estrogen levels are relatively low. These clinical observations, confirmed by some but not all studies, have led to the hypothesis that estrogens are neuroprotective7 and also protect against psychosis.8
Estrogen withdrawal in specific brain cells may release a cascade of events that over time can increase the severity of psychotic and cognitive symptoms. The reason for suspecting such effects is based on what we know about estrogenic effects on neurotransmitter, cognitive, and stress-induction pathways, and—more fundamentally—on neuronal growth and atrophy.
According to the estrogen hypothesis, women are—to some degree—protected against schizophrenia by their relatively high gonadal estrogen production between puberty and and menopause. Women lose this protection with the onset of perimenopausal estrogen fluctuation and decline, accounting for their second peak of illness onset after age 45.
Epidemiologic studies showing a second peak of schizophrenia onset in women (but not men) around the age of menopause support this hypothesis.9,10 Longitudinal outcomes for schizophrenia—which are better in women than in men during late adolescence or early adulthood11—gradually even out after the first 15 years of illness, suggesting that women’s advantage is lost at a time approximating menopause (Box 1).
The question, then, becomes: Is it only because of estrogen loss after age 40 that women become more prone to develop a psychotic illness? Other differences between the sexes that may play roles include immune function, low iron stores, sleep sufficiency, thyroid function, exposure to toxic substances (including therapeutic drugs), societal pressures to be slim while aging (Table), and the experience of stress.12
CASE CONTINUED
Exhausted and confused
Dr. I is a well-groomed, handsome woman, but she hardly speaks when interviewed, looking frightened and somewhat bewildered. She has never had a mental health problem, nor has anyone in her family. She agrees to stay in the hospital but is not sure why. She has slept no more than 1 or 2 hours in the last several days.
Her early history is unremarkable. She did well in school. After earning a PhD at the University of Leipzig, she and her husband immigrated to Canada. Both are university professors. They never decided not to have children, but children hadn’t come. Her menstrual periods stopped 2 years before admission. The question about children is the only 1 that elicits emotion in Dr. I. When I ask about it, tears come to her eyes as she shakes her head.
Her husband reports that she has not been eating well and has, in the last year, started to drink more alcohol than usual—3 to 4 drinks of whiskey a night. She does not smoke cigarettes, and her health generally is good. She uses no medications. Her husband describes their marital relationship as very close, although it has become strained in recent weeks because of her unreasonable fears. He admits that their work is always stressful; competition is fierce, with more and more deadlines and less and less leisure time. The couple has few friends and no hobbies.
Late-onset psychosis symptoms
In late-onset psychosis (after age 45), men appear to suffer substantially milder symptoms and spend less time hospitalized than women.13 Women with late-onset schizophrenia have more severe positive symptoms than men and fewer negative symptoms.14,15 Overall, patients with late-onset schizophrenia have a lower prevalence of looseness of associations and negative symptoms than those with earlier onset.16,17
In addition, individuals with schizophrenia who become ill in middle age have been reported to:
• show better neuropsychological performance (particularly in learning and abstraction/cognitive flexibility) than those with early onset
• possibly have larger thalamic volumes
• respond to lower antipsychotic doses.18
Auditory and visual hallucinations frequently are observed in patients with comorbid late-onset schizophrenia and auditory and visual impairment.16 Palmer et al18 reported no difference in family history of schizophrenia between early and late onset, but this is controversial. Convert et al16 note that most studies reveal a lower lifetime risk of schizophrenia in first-degree relatives of patients with late-onset than early-onset schizophrenia.
CASE CONTINUED
Medical workup
Dr. I’s physical exam is unremarkable. Her thyroid is not enlarged; there are no breast lumps. On mental status exam, her mood is flat. She is preoccupied with fears of the Nazis. Routine blood tests show slight anemia; fasting glucose levels are within normal range.
I give Dr. I zopiclone, 7.5 mg, to help her sleep. The next day she keeps to herself, eats very little, and appears disinterested in her surroundings. Nursing staff report that she often seems frightened. Dr. I asks to use the ward phone to call Germany but is told that she cannot make long distance calls from that phone. This seems to disturb her.
Differential diagnosis
Sensory impairment, substance abuse, and metabolic changes have been implicated in the appearance of psychosis in later life. More specific to women than men, however, are medical and psychiatric precipitants. These include autoimmune disease (and its treatment) and psychiatric disorders, as well as thyroid dysfunction, self-induced starvation (anorexia nervosa) and diet aids, substance use and abuse, insomnia, and iron deficiency (Table).
Autoimmune disease and treatment. Nearly 80% of patients with autoimmune disease are women, and these disorders (as well as their treatment) can manifest as psychosis. Corticosteroids have a well-documented history of triggering psychotic symptoms, which are twice as likely in women than in men. The incidence of severe psychosis while taking oral prednisone ranges from 1.6% to 50% and averages 5.7%. The average daily dose of corticosteroids for patients who develop psychosis is 59.5 mg/d.
Corticosteroid creams absorbed through skin as well as inhaled and intranasal corticosteroids in their more potent formulations can have systemic effects, including psychosis. Nonsteroidal anti-inflammatory drugs such as ibuprofen also can trigger psychosis.19
Psychiatric disorders. Posttraumatic stress disorder with psychotic symptoms may overlap with categories such as psychogenic psychoses, hysterical psychoses, nonaffective remitting psychoses, acute brief psychoses, reactive psychoses, acute and transient psychoses, and bouffées délirantes (in France, the name for transient psychotic reactions).20 Consider these female-predominant conditions in the differential diagnosis, along with micropsychotic episodes in borderline personality disorder, in which the predominance of women is 3:1.
Medical treatment for depression and anxiety also can lead to psychotic symptoms through individual susceptibility to the action of specific drugs or through withdrawal effects.
Clinical assessment
Question all women presenting with psychosis about eating habits and diet pills, and check for hypokalemia and hypocalcemia to rule out starvation effects and reactions to stimulants. Also ask about inhalants, and examine for anemia and thyroid dysfunction. Consider all medications as having the potential to trigger psychotic symptoms.
A family history of illness is important, with a focus on autoimmune disorder and its treatment. A thorough psychiatric history is crucial and needs to include assessment of sleep, mood, and relationships with attachment figures. Do not assume illnesses of unknown cause (bipolar disorder or schizophrenia) until after a comprehensive search for precipitants of psychotic symptoms.
CASE CONTINUED
Guilty feelings
To address her delusions, I start Dr. I on risperidone, 2 mg at bedtime. She goes home for the weekend, and her husband reports that she slept throughout the visit. When she returns, she spends a lot of time in bed but is more communicative.
When I ask Dr. I whether she has called Germany, she says she called her recently widowed father. Dr. I begins to cry when talking of her mother, and tells the nurse she feels guilty for not visiting for the last few years. When her mother died 6 months ago, Dr. I had not seen her in 4 years.
Her fears remit with risperidone, maintained at 2 mg/d, but Dr. I remains depressed and responds slowly to treatment with citalopram, 20 mg/d, and supportive therapy. Her final diagnosis is mood disorder with psychotic features.
Treatment
When treating women with late-onset psychosis, remove all potential triggers and address underlying illness. Cognitive therapy targeting specific symptoms is useful; antipsychotics probably will be necessary. Age-related physiologic changes make older persons more sensitive to the therapeutic and toxic effects of antipsychotics.
Estrogen therapy? Women suffering from schizophrenia show significantly lower estrogen levels than the general population of women, and they experience first-onset or recurrence of a psychotic episode significantly more often in low estrogen phases of the cycle. Estrogens have therefore been postulated to constitute a protective factor against psychosis, which means perimenopause is an at-risk period.21 Although evidence is limited, preliminary studies have found beneficial effects from short-term, off-label use of estrogen therapy in women with psychotic illness (Box 2).
Because continuous use of estrogen plus progestin has been associated with an increased risk of adverse effects,22 off-label use of selective estrogen receptor modulators (SERMs) also is being investigated in women with schizophrenia. SERMs act as tissue-specific estrogen agonists and antagonists because they can either inhibit or enhance estrogen-induced activation of estrogen response element-containing genes.23
Wong et al24 used a crossover design to compare the SERM raloxifene with placebo as adjunctive treatment for 6 postmenopausal women with schizophrenia. Each woman received 8 weeks of raloxifene, 60 mg/d, and 8 weeks of placebo. Three began with placebo and 3 with raloxifene.
Verbal memory was measured weekly with the California Verbal Learning Test, using 5 memory trials, free and cued short-delay recall, and long-delay recall. At baseline, the participants had lower scores than older adults in the general population. Eight weeks of placebo improved scores somewhat, suggesting a practice effect. Eight weeks of raloxifene improved cognitive scores to a level similar to that of schizophrenia-free subjects. After 16 weeks, however, cognitive scores in the 2 groups were indistinguishable.
At present I do not recommend estrogen for women with late-onset schizophrenia because the risk is too high and raloxifene does not enter the brain sufficiently to be a valuable cognitive enhancer. Novel SERMs with more specific efficacy for improving cognitive function may prove useful in the future,25 however, as may phytoestrogens. Adjunctive hormone modulation is a promising area of gender-specific treatment for serious mental illness.26
CASE CONCLUSION
Gradually improving
Dr. I’s depression was triggered by her mother’s death and regrets about not visiting and not being a mother. The content of her delusions was related to her guilt about not having returned to Germany; the delusions were probably triggered by depression, alcohol intake, her relative hypoestrogenic state, stress at work, lack of social supports, and dependence on her husband.
Over the next few years, Dr. I is maintained on a low dose of risperidone (reduced from 2 mg/d to 1 mg/d) and citalopram (reduced from 20 mg/d to 10 mg/d). She becomes increasingly engaged in supportive dynamic therapy, and her symptoms gradually improve.
BOTTOM LINE
Psychosis onset in midlife is mostly a female phenomenon because a perimenopausal estrogen decline increases women’s susceptibility. Seek specific triggers such as medical illness or response to a drug before assuming an illness of unknown cause such as bipolar disorder or schizophrenia. Cognitive therapy targeting specific symptoms is useful; antipsychotics probably will be necessary.
Related Resources
• Women and psychosis: A guide for women and their families. Centre for Addiction and Mental Health. University of Toronto. www.camh.net/About_Addiction_Mental_ Health/Mental_Health_Information/Women_Psychosis.
• Seeman MV. Women and psychosis. www.medscape.com/ viewarticle/408912.
• Chattopadhyay S. Estrogen and schizophrenia: Any link? The Internet Journal of Mental Health. 2004;2(1). www.ispub. com/journal/the_internet_journal_of_mental_health.html.
Drug Brand Names
Citalopram • Celexa Prednisone • Deltasone,
Estradiol • Estrace, Orasone, others
Estrofem, others Raloxifene • Evista
Estradiol transdermal • Risperidone • Risperdal
Estraderm , Climara, others
Methylphenidate • Concerta,
Ritalin, others
Disclosure
Dr. Seeman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci Biobehav Rev. 2007;31:858-873.
2. Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27:107-112.
3. Seeman MV. Interaction of sex, age, and neuroleptic dose. Comp Psychiatry. 1983;24:125-128.
4. Usall J, Suarez D, Haro JM, and the SOHO Study Group. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153: 225-231.
5. Hare E, Glahn DC, Dassori A, et al. Heritability of age of onset of psychosis in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009 Apr 6 [Epub ahead of print].
6. Seeman MV. Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry. 2004;161:1324-1333.
7. Marin R, Guerra B, Alonso R, et al. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2:287-301.
8. Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185-194.
9. Castle DJ, Abel K, Takei N, et al. Gender differences in schizophrenia: hormonal effect or subtypes? Schizophr Bull. 1995;21:1-12.
10. Häfner H, an der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139-151.
11. Grossman LS, Harrow M, Rosen C, et al. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49:523-529.
12. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151-178.
13. Riecher-Rössler A, Löffler W, Munk-Jörgensen P. What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci. 1997;247:195-208.
14. Lindamer LA, Lohr JB, Harris MJ, et al. Gender-related clinical differences in older patients with schizophrenia. J Clin Psychiatry. 1999;60:61-67.
15. Seeman MV. Does menopause intensify symptoms in schizophrenia? In: Lewis-Hall F, Williams TS, Panetta JA, et al, eds. Psychiatric illness in women: emerging treatments and research. Arlington, VA: American Psychiatric Publishing, Inc.; 2002:239-248.
16. Convert H, Védie C, Paulin P. [Late-onset schizophrenia or chronic delusion]. Encephale. 2006;32:957-961.
17. Sato T, Bottlender R, Schröter A, et al. Psychopathology of early-onset versus late-onset schizophrenia revisited: an observation of 473 neuroleptic-naive patients before and after first-admission treatments. Schizophr Res. 2004;67:175-183.
18. Palmer BW, McClure FS, Jeste DV. Schizophrenia in late life: findings challenge traditional concepts. Harv Rev Psychiatry. 2001;9:51-58.
19. Weiss DB, Dyrud J, House RM, et al. Psychiatric manifestations of autoimmune disorders. Curr Treat Options Neurol. 2005;7:413-417.
20. Castagnini A, Bertelsen A, Munk-Jorgensen P, et al. The relationship of reactive psychosis and ICD-10 acute and transient psychotic disorders: evidence from a case register-based comparison. Psychopathology. 2007;40:47-53.
21. Huber TJ, Rollnik J, Wilhelms J, et al. Estradiol levels in psychotic disorders. Psychoneuroendocrinology. 2001;26: 27-35.
22. Heiss G, Wallace R, Anderson G, et al, for the WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036-1045.
23. Doncarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009 May 15 [Epub ahead of print].
24. Wong J, Seeman MV, Shapiro H. Case report: raloxifene in postmenopausal women with psychosis: preliminary findings. Am J Geriatr Psychiatry. 2003;11(6):697-698.
25. Ye L, Chan MY, Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009;302:73-80.
26. Kulkarni J, Gurvich C, Gilbert H, et al. Hormone modulation: a novel therapeutic approach for women with severe mental illness. Aust N Z J Psychiatry. 2008;42:83-88.
1. Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci Biobehav Rev. 2007;31:858-873.
2. Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27:107-112.
3. Seeman MV. Interaction of sex, age, and neuroleptic dose. Comp Psychiatry. 1983;24:125-128.
4. Usall J, Suarez D, Haro JM, and the SOHO Study Group. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153: 225-231.
5. Hare E, Glahn DC, Dassori A, et al. Heritability of age of onset of psychosis in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009 Apr 6 [Epub ahead of print].
6. Seeman MV. Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry. 2004;161:1324-1333.
7. Marin R, Guerra B, Alonso R, et al. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2:287-301.
8. Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185-194.
9. Castle DJ, Abel K, Takei N, et al. Gender differences in schizophrenia: hormonal effect or subtypes? Schizophr Bull. 1995;21:1-12.
10. Häfner H, an der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139-151.
11. Grossman LS, Harrow M, Rosen C, et al. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49:523-529.
12. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151-178.
13. Riecher-Rössler A, Löffler W, Munk-Jörgensen P. What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci. 1997;247:195-208.
14. Lindamer LA, Lohr JB, Harris MJ, et al. Gender-related clinical differences in older patients with schizophrenia. J Clin Psychiatry. 1999;60:61-67.
15. Seeman MV. Does menopause intensify symptoms in schizophrenia? In: Lewis-Hall F, Williams TS, Panetta JA, et al, eds. Psychiatric illness in women: emerging treatments and research. Arlington, VA: American Psychiatric Publishing, Inc.; 2002:239-248.
16. Convert H, Védie C, Paulin P. [Late-onset schizophrenia or chronic delusion]. Encephale. 2006;32:957-961.
17. Sato T, Bottlender R, Schröter A, et al. Psychopathology of early-onset versus late-onset schizophrenia revisited: an observation of 473 neuroleptic-naive patients before and after first-admission treatments. Schizophr Res. 2004;67:175-183.
18. Palmer BW, McClure FS, Jeste DV. Schizophrenia in late life: findings challenge traditional concepts. Harv Rev Psychiatry. 2001;9:51-58.
19. Weiss DB, Dyrud J, House RM, et al. Psychiatric manifestations of autoimmune disorders. Curr Treat Options Neurol. 2005;7:413-417.
20. Castagnini A, Bertelsen A, Munk-Jorgensen P, et al. The relationship of reactive psychosis and ICD-10 acute and transient psychotic disorders: evidence from a case register-based comparison. Psychopathology. 2007;40:47-53.
21. Huber TJ, Rollnik J, Wilhelms J, et al. Estradiol levels in psychotic disorders. Psychoneuroendocrinology. 2001;26: 27-35.
22. Heiss G, Wallace R, Anderson G, et al, for the WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036-1045.
23. Doncarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009 May 15 [Epub ahead of print].
24. Wong J, Seeman MV, Shapiro H. Case report: raloxifene in postmenopausal women with psychosis: preliminary findings. Am J Geriatr Psychiatry. 2003;11(6):697-698.
25. Ye L, Chan MY, Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009;302:73-80.
26. Kulkarni J, Gurvich C, Gilbert H, et al. Hormone modulation: a novel therapeutic approach for women with severe mental illness. Aust N Z J Psychiatry. 2008;42:83-88.
SAD: Is seasonal affective disorder a bipolar variant?
Ms. S, age 24, is referred to our team in early December by her primary care physician for “fatigue.” The patient describes going to bed and falling asleep before 9:30 these winter evenings, whereas in summer she went to bed at 11 PM. She craves bread, pasta, and sweets and reports increased appetite in winter compared with summer. Her mood is low, and she misses warm-weather activities of gardening and walking. Fatigue and difficulty concentrating are causing her problems at work and school.
Her history reveals mood elevation in spring as days become longer, with a clear change at approximately March 10 to 20. She reports “spring fever” and feeling “great” last year as soon as daylight saving time began. She slept only 3 hours a night and had a burst of ideas to expand her small business. She threw herself into her work, feeling she was making up for lost time and productivity. She also admits to making a large, misguided business investment during that time.
Upon questioning, she recalls that the previous spring she argued with her father and threw a cup of hot tea at him. When interviewed, Ms. S’s mother describes her daughter at that time as having “a very short fuse,” speaking loud and fast, staying up late at night, and looking as though she was not herself.
Seasonal affective disorder (SAD) is an umbrella term for mood disorders that follow a seasonal pattern of recurrence. Bipolar I disorder (BD I) or bipolar II disorder (BD II) with seasonal pattern (BD SP) is the DSM-IV-TR diagnosis for persons with depressive episodes in the fall or winter and mania (BD I) or hypomania (BD II) in spring or summer ( Table 1 ).1
This article compares BD SP with major depressive disorder with seasonal pattern (MDD SP), in which depressive episodes usually occur in fall or winter and fully remit in spring or summer.1 Rather than being categorically distinct from each other, BD SP and MDD SP may represent extreme variants on a seasonal depression continuum from unipolar to bipolar.
Table 1
DSM-IV-TR criteria for seasonal pattern specifier*
| A | A regular pattern of major depressive episodes (MDEs) at a particular time of year (such as fall and/or winter) |
| B | Full remission or change to mania or hypomania at a particular time of year (such as spring or summer) |
| C | 2 seasonal MDEs that followed the pattern described in (A) and (B) occurred in the past 2 years (and no nonseasonal MDEs) |
| D | Seasonal MDEs substantially outnumber nonseasonal MDEs across the lifespan |
Cases do not meet criteria if:
| |
| *Can be applied to a pattern of major depressive episodes in bipolar I disorder, bipolar II disorder, or major depressive disorder | |
| Source: Adapted from reference 1 | |
Overlap of MDD SP and BD SP
The seasonal pattern specifier can be applied to a diagnosis of MDD, BD I, or BD II.1 Seasonality-focused assessments, described below, can help characterize seasonal patterns that do not meet full SP criteria but may deserve clinical attention.
Symptom presentation. MDD SP and BD SP share similar atypical depressive symptom presentations and seasonal recurrence patterns ( Box 1 ). Hypersomnia, hyperphagia, and psychomotor retardation are more prevalent in major depressive episodes of bipolar disorders and SAD than in unipolar or nonseasonal mood disorders.2-4 Individuals with SAD also report fatigue and decreased physical activity,3 both of which are characteristic of bipolar depressive episodes.5
Although psychosis and psychiatric hospitalizations are more common in BD I than unipolar disorders,6 individuals with BD SP are less likely to report psychosis than those with nonseasonal BD.7 Another study found that BD SP patients reported a higher rate of psychiatric hospitalizations than MDD SP patients (28% vs 9.4%).6
Recurrence pattern. Major depressive episodes are highly recurrent in both MDD and BD, with or without a seasonal pattern. Approximately 75% of individuals with MDD experience ≥1 recurrence (mean, 10.8 episodes);8 MDD SP patients report a mean of 13.4 episodes.9 The mean lifetime episodes in BD SP is 20.74, compared with 11.67 in nonseasonal BD.7
Cassidy and Carroll10 measured the frequency of mood episodes in 304 BD patients not assessed for seasonality. Manic episodes peaked in early spring, mixed episodes peaked in late summer or fall, and depressive episodes peaked in fall-winter.
Irregular rhythm. Both BD and MDD SP involve irregularities in daily or circadian rhythms, such as changes in the timing of sleep, melatonin release, and body temperature.3,5,11 Circadian phase delays—in which internal rhythms lag behind the sleep cycle—are correlated with symptom severity in BD12 and are implicated in the core pathology of BD13 ( Box 2 ). In BD, life events that change social rhythms may disrupt circadian rhythms, triggering mood episodes.5
Etiologic hypotheses for both BD and SAD propose that an external event (life stress in BD; decreased photoperiod in SAD) leads to circadian dysregulation and, in turn, mood episodes. Circadian-related hypotheses for SAD and BD are supported by evidence showing efficacy of treatments that manipulate behavioral and circadian rhythms.
from unipolar to bipolar?
Seasonality refers to the degree of seasonal changes in behavior and mood within an individual. Seasonality scores are normally distributed,a suggesting that seasonality may be continuous in the general population—with some individuals meeting criteria for a seasonal mood disorder:
- A seasonal pattern is reported by approximately 10% to 20% of depressed outpatients with recurrent mood disorders and an estimated 15% to 22% of individuals with bipolar disorder (BD).b
- Persons with BD—seasonal or not—report greater seasonality compared with those with major depressive disorder (MDD).c
Among individuals with seasonal affective disorder, the course is bipolar in an estimated 12% to 22% and unipolar in 78% to 88%.d These estimates may reflect underdiagnosis of BD with seasonal pattern because hypomania is difficult to diagnose retrospectively.e
The bipolar-unipolar continuum includes (in order): BD I, BD II, bipolar disorder not otherwise specified, cyclothymia, bipolar spectrum disorder, and MDD.f In examining the validity of the bipolar spectrum model, Phelps et alg noted:
- At least 3 studies found that all symptoms reported by individuals with unipolar and bipolar diagnoses approach a normal distribution, rather than a bimodal distribution separating unipolar from bipolar symptom profiles.
- Data from 2 population-based studies indicate that subthreshold hypomanic symptoms are more common than and cause as much impairment as symptoms meeting criteria for BD II or I.
Some individuals who meet criteria for MDD with seasonal pattern have summertime periods of transient hypomania and hyperthymia (hypomanic-like periods without clinically significant impairment).h This suggests that the bipolar continuum also may exist among individuals with seasonal pattern mood disorders.
Source: Access reference citations here
Etiologic hypotheses of seasonal affective disorder (SAD) include:
- photoperiodic hypothesis (shorter winter days cause SAD,a perhaps mediated by a summer vs winter difference in duration of nightly melatonin release)b
- phase shift hypothesis (less available light in winter may lead to an inability to synchronize circadian rhythms with sleep/wake rhythms).c
Some case studies of rapid-cycling bipolar disorder (BD) suggest that mood is correlated with daily hours of sunshine and light therapy is antidepressant. Rapid-cycling patients may be hypersensitive to day-to-day changes in photoperiod, analogous to mood changes in response to changes in photoperiod across the seasons in SAD.d
Circadian phase delays—in which internal rhythms lag behind the sleep cycle—are correlated with symptom severity in BDe and are implicated in the core pathology of BD.f Phase delays also are present in some individuals with SAD and are associated with severity and treatment response.g Preliminary evidence suggests that variation in circadian clock genes is related to both BDf,h and SAD.i
Source: Access reference citations here
CASE CONTINUED: Seasonal pattern revealed
Ms. S was aware that she is vulnerable to depressive episodes in fall and winter but unaware of a pattern of hypomanic/manic episodes in spring and summer. Her family psychiatric history includes a sister diagnosed with BD I (with no seasonal specifier), and a maternal aunt who has attempted suicide several times.
Ms. S agrees to an assessment plan including a diagnostic interview, interviews measuring symptom severity and pattern of recurrence, routine laboratory examination, and self-report questionnaires. These show that she meets DSM-IV-TR criteria for BD I, depressed, moderate, with seasonal pattern.
Her assessment scores are 28 on the Structured Interview Guide for HDRS-seasonal affective disorder version (SIGH-SAD), 17 on the Hamilton Depression Rating Scale (HDRS), and 11 on the atypical subscale. The HDRS and atypical subscale are components of the SIGH-SAD reflecting typical (eg, insomnia, loss of appetite, etc.) and atypical (eg, hypersomnia, increased appetite, etc.) depression symptoms, respectively. Ms. S’s scores exceed the threshold scores defining a BD SP episode (>20 SIGH-SAD + >10 HDRS + >5 atypical subscale14 ). Data from self-report questionnaires corroborate this assessment.
We plan to administer the Hypomania Interview Guide (including Hyperthymia) for Seasonal Affective Disorder (HIGH-SAD) during treatment and the following spring to monitor prospectively for hypomanic symptoms.
Assessment tools
After complete assessment for mood episodes and mood disorders based on DSM-IV-TR, an additional assessment for bipolarity and seasonality may be helpful.1
Screen for bipolarity in patients with SAD to avoid triggering mania or hypomania during treatment. Useful tools include:
- HIGH-SAD15
- the National Institutes of Health Life Chart Method to establish a recurrent pattern of mood episodes and track treatment efficacy16
- assessments that characterize sub-threshold bipolar symptoms, such as the Bipolar Spectrum Diagnostic Scale17 ( see Box 3 ) and the Bipolarity Index.18
Also obtain collateral reports from significant others, review patient records, and use the same mania and hypomania scales for prospective assessment as the next spring approaches.6
Assess seasonality in patients with BD to improve diagnosis and treatment. Characterizing a seasonal pattern may allow you and your patient to predict episodes and treat proactively. Commonly used assessments include the SIGH-SAD and the Structured Clinical Interview for DSM Disorders (SCID) seasonal pattern specifier module.19
The SIGH-SAD measures symptom severity and provides recovery criteria based on changes in scores during treatment. Response is defined as a 50% reduction in symptoms; remission is >50% improvement in SIGH-SAD + HDRS <7 + atypical <7 or HDRS <2 + atypical <10.14
CASE CONTINUED: Treatment begins
Considering Ms. S’s diagnosis of BD I SP and the risk of precipitating mania with light treatment, we recommend starting treatment with a mood stabilizer. We narrow our options to those that have a direct antidepressant effect, with the hope that this may reduce the need for future antidepressant medications. For patients diagnosed with BD II SP, we could consider a regimen without mood stabilizers.
We offer Ms. S lithium, a first-line mood stabilizer with evidence of usefulness in treatment before chronotherapeutic interventions and in preventing suicidal behavior. However, Ms. S prefers our second option, lamotrigine, because she is concerned about lithium’s side effects and required blood draws to check drug levels as well as thyroid and kidney status.
Despite causing some initial drowsiness, her lamotrigine dosage is successfully titrated after 2 weeks of treatment to 300 mg/d, without side effects. Only then do we initiate light treatment, which Ms. S wishes to try before antidepressant medications. She also begins sessions with a therapist trained in cognitive-behavioral therapy (CBT) for SAD. (For details of this comprehensive treatment, see Box 4 )
Treating bipolar variant of SAD
Significant differences exist in the clinical management of BD SP and MDD SP, despite their commonalities ( Table 2 ). BD SP treatment remains distinct because of the risk of switching with the use of light therapy or antidepressants and the importance of mood stabilizers, especially in BD I.
Consensus guidelines for treating SAD recommend mood stabilizers and close monitoring during light therapy for patients with BD SP ( Table 3 ).2 Therapeutic sleep deprivation can quickly reverse depression during hospitalization but is not used often or recommended for outpatient treatment.20
Light therapy. A small body of evidence suggests that depressive symptoms in BD SP improve with bright light therapy, a treatment with demonstrated efficacy in MDD SP.21 No differences in response have been reported between light therapy for winter depressive episodes among individuals with BD SP or MDD SP.22-24 Light therapy may increase the risk of switching to mania/hypomania in patients with BD SP, however. Clinical supervision is imperative, even for patients thought to have MDD SP, because of the risk of undiagnosed BD.
Regular monitoring by a physician is indicated for individuals taking medications or remedies with photosensitizing effects (such as lithium, thioridazine, or St. John’s wort). An ophthalmologist consultation and monitoring is necessary for patients with preexisting eye problems, those taking photosensitizing medications, and those who develop eye problems during light treatment.2,6
The recommended starting dose for light therapy in MDD SP is 30 minutes daily in the early morning, but this dose may be too high for individuals with BD SP.25 To minimize the risk of switching, begin light therapy at 5 to 10 minutes daily and slowly increase while monitoring the clinical effect (see Related Resources , for more information about light therapy for affective disorders).
Pharmacotherapy. Pharmacologic treatments have not been studied for effectiveness in BD SP, and we hesitate to provide specific recommendations. Effective treatments may include those used for nonseasonal MDD, nonseasonal BD, and MDD SP. When using any medication for BD SP, weigh the risk of switching states against the potential beneficial effects.2
Year-round mood-stabilizer treatment is indicated to minimize the risk of mood episodes in BD SP, especially in patients with BD I. When treating SAD, mood stabilizers with antidepressant effects—such as lamotrigine or lithium (for maintenance), and quetiapine or aripiprazole (for acute treatment)—are preferable to agents without an antidepressant effect in monotherapy. More-sedating mood stabilizers (such as valproate or carbamazepine) likely would not be as beneficial as less-sedating agents, considering that patients with SAD frequently experience fatigue.
Because of the lack of adequate clinical trials of treatments for BD SP, we suggest that clinicians choose medications and follow algorithms relevant to BD without a seasonal specifier. Use similar schedules and dosages, with individual tailoring.5,26
Antidepressants that have shown efficacy in MDD SP include fluoxetine, bupropion, citalopram, and sertraline.6,9,27-29 For patients with BD SP, we initiate antidepressants when:
- light treatment fails
- the patient is unable to travel to the south
- light treatment is not available (often because patients cannot afford the cost, which is not covered by insurance)
- patient lacks time for light treatment.
An additional important consideration is history of response (such as a patient who did not respond well to light therapy in the past but responded very well to a particular antidepressant).
No studies have compared antidepressant classes or individual medications for MDD SP. Clinical wisdom is to base the antidepressant choice, dosages, decision points of when to switch, and schedule of switching (cross-tapering) on individual patients’ symptom clusters and comorbid conditions as well as the medication’s side effects.
Prophylactic treatment with bupropion would seem an appropriate initial choice for a prototypical SAD patient, considering this medication’s more activating effects and FDA approval for SAD treatment.9 For the minority of patients with SAD who present with agitation and increased sleepiness, a slightly sedating selective serotonin reuptake inhibitor such as citalopram would make more sense as a first-line treatment. Finally, we would recommend sertraline for patients with marked anxiety—especially panic attacks or obsessive-compulsive symptoms—but without insomnia.
For specific dosages, rely on the literature of treating nonseasonal depression (unipolar or bipolar). It is important to define decision-making points for dosage increases, augmentation, switching to another antidepressant, and cross-tapering, similar to how you would address a nonseasonal depression, typical or atypical.
In our view, treating a patient with BD I SP with an antidepressant alone—without a mood stabilizer—is almost always wrong. For BD II SP we leave it to the clinician to decide, based on individual patients, clinical experience, and ideally in consultation with a peer.
Seasonal dosages. You may wish to seasonally vary medications and dosages for patients with BD SP. Although no strong evidence exists, we recommend 2 options:
- Consider increasing mood-stabilizing medication in spring and summer, with a reduction (but no tapering for BD I) in fall and winter.
- Consider a complete antidepressant taper 2 weeks after daylight saving time begins in spring; taper under increased observation and not faster than 6 weeks, with close attention to emerging symptoms of depression or antidepressant withdrawal.
We do not taper antidepressants before daylight saving time, and we always consider additional stressors, losses, and challenges in our patients’ lives before tapering antidepressants in spring or summer. We also assess and monitor compliance.
Psychotherapy. Referral can be made to clinicians trained in CBT for patients with a seasonal pattern and interpersonal and social rhythm therapy (IPSRT) for BD. Integrative models for SAD and BD propose that psychological and biologic vulnerability factors interact with environmental events (such as winter season or disruption of daily routine) to trigger mood episodes.
CBT adapted for SAD targets mal-adaptive thinking and behavioral disengagement through cognitive therapy and behavioral activation to counteract SAD symptoms.30,31 Preliminary trials by our group suggest that CBT for MDD SP is an effective acute treatment30 and may prevent future episodes.32
IPSRT is an adaptation of interpersonal psychotherapy that aims to stabilize social relationships and rhythms in BD.33 IPSRT posits that irregularity in daily routines leads to circadian dysregulation, precipitating mood episodes in persons vulnerable to BD.34 The degree of regularity in social rhythms achieved in IPSRT is associated with reduced likelihood of recurrence post-treatment.34 If stabilizing social rhythms has a similar effect of regulating circadian rhythms in SAD, IPSRT may be effective in treating BD SP.
Table 2
Physiopathologic findings and clinical management for SAD vs BD
| SAD | BD | |
|---|---|---|
| Differences | May be unipolar or bipolar Defined by seasonality Light therapy and antidepressants indicated | Increased risk of psychosis and psychiatric hospitalization Most BD is not seasonal Mood stabilizers indicated Risk of switching states with light therapy and antidepressants |
| Similarities | Atypical depressive symptom presentation Highly recurrent Predictable season of recurrence allows proactive treatment Assess for mania and hypomania in both disorders Light therapy requires clinical supervision Psychotherapy may be beneficial | |
| BD: bipolar disorder; SAD: seasonal affective disorder | ||
Table 3
Recommended treatment for bipolar disorder with seasonal pattern
| Treatment | Recommendation |
|---|---|
| Mood-stabilizing medications | Maintain year-round, especially in patients with BP I |
| Antidepressants | Consider those with efficacy in unipolar SAD or nonseasonal bipolar depression |
| Light therapy | Initiate for 5 to 10 min/day for bipolar depressive episodes in patients receiving mood stabilizers or atypical antipsychotics; slowly increase duration while monitoring mood, sleep, and side effects to manage risk of hypomanic or manic switch |
| Psychotherapy | Consider CBT or interpersonal and social rhythm therapy to help manage symptoms and reduce episode recurrence |
| BP I: bipolar disorder type I; CBT: cognitive-behavioral therapy; SAD: seasonal affective disorder | |
CASE CONCLUSION: Ongoing treatment required
After several months of light therapy, Ms. S begins to feel better and reports having more energy. We taper her light therapy to 10 minutes daily in the morning from late February until 1 week after daylight saving time begins in mid-March. Weekly phone calls during this transition screen for signs of hypomania or mania. Lamotrigine is effective in preventing switches in spring.
Future plans include monitoring for hypomania through summer and possibly reinitiating light therapy in fall or winter. Because approximately one-half of individuals who undergo CBT for SAD do not experience another episode the winter after treatment, light therapy will be initiated only if depressive symptoms emerge. A booster session is scheduled with Ms. S’s CBT therapist in early fall to reinforce relapse prevention skills.
Antidepressant therapy will be recommended if full treatment response is not maintained with light therapy and continued use of CBT skills for SAD. During sessions, we emphasize compliance with lamotrigine. On several occasions Ms. S questions the need for ongoing therapy, but with education about the potential effects of mania she agrees to continue treatment as indicated.
Seasonality screening tools
- Seasonal Pattern module of the Structured Clinical Interview for DSM Disorders (SCID). www.scid4.org/faq/clinician_version.html.
- Hypomania Interview Guide for Seasonal Affective Disorder (HIGH-SAD). www.chronotherapeutics.org/Tools_ENG.html.
- National Institutes of Health Life Chart Method. www.bipolarnews.org/Clinician%20Life%20Charting.htm.
- Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD). www.chronotherapeutics.org/Tools_ENG.html.
Bipolarity screening tools
- Bipolar Spectrum Diagnostic Scale. Click here to download.
- Bipolarity Index. http://psycheducation.org/depression/STEPBipolarityIndex.htm.
Light therapy
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders: a clinician’s manual for light and wake therapy. Basel, Switzerland: S. Karger AG; 2009.
- Wirz-Justice A, Benedetti F, Berger M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
Psychotherapy
- Rohan KJ. Coping with the seasons: a cognitive-behavioral approach to seasonal affective disorder (therapist guide). New York, NY: Oxford University Press; 2008.
- Frank E. Treating bipolar disorder: a clinician’s guide to interpersonal and social rhythm therapy. New York, NY: Guilford Press, Inc.; 2005.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin XL
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Valproate • Depakote
Disclosures
Drs. Roecklein and Rohan report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Postolache received an investigator-initiated award from The LiteBook Company (Vancouver, Canada) via the Society for Light Treatment and Biological Rhythms, as well as research support from Apollo Health (Salt Lake City, UT).
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lam RW, Levitt AJ. eds. Clinical guidelines for the treatment of seasonal affective disorder. Vancouver, BC: Clinical and Academic Publishing; 1999.
3. Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72-80.
4. Michalak EE, Wilkinson C, Hood K, et al. Seasonal and nonseasonal depression: how do they differ? Symptom profile, clinical and family history in a general population sample. J Affect Disord. 2002;69(1-3):185-192.
5. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
6. Sohn CH, Lam RW. Treatment of seasonal affective disorder: unipolar versus bipolar differences. Curr Psychiatry Rep. 2004;6(6):478-485.
7. Goikolea JM, Colom F, Martinez-Aran A, et al. Clinical and prognostic implications of seasonal pattern in bipolar disorder: a 10-year follow-up of 302 patients. Psychol Med. 2007;37:1595-1599.
8. Kessler RC, Zhao S, Blazer DG, et al. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45(1-2):19-30.
9. Modell JG, Rosenthal NE, Harriett AE, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry. 2005;58:658-667.
10. Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002;68:25-31.
11. Shin K, Schaffer A, Levitt AJ, et al. Seasonality in a community sample of bipolar, unipolar and control subjects. J Affect Disord. 2005;86:19-25.
12. Wood J, Birmaher B, Axelson D, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166(2-3):201-209.
13. Soreca I, Frank E, Kupfer DJ. The phenomenology of bipolar disorder: what drives the high rate of medical burden and determines long-term prognosis? Depress Anxiety. 2009;26(1):73-82.
14. Terman M, Terman J, Rafferty B. Experimental design and measures of success in the treatment of winter depression by bright light. Psychopharmacol Bull. 1990;26(4):505-510.
15. Goel N, Terman M, Terman JS, et al. Summer mood in winter depressives: validation of a structured interview. Depress Anxiety. 1999;9:83-91.
16. Denicoff KD, Leverich GS, Nolen WA, et al. Validation of the prospective NIMH-Life-Chart Method (NIMH-LCM-p) for longitudinal assessment of bipolar illness. Psychol Med. 2000;30:1391-1397.
17. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity of a new Bipolar Spectrum Diagnostic Scale. J Affect Disord. 2005;84:273-277.
18. Phelps J, Angst J, Katzow J, et al. Validity and utility of bipolar spectrum models. Bipolar Disord. 2008;10:179-193.
19. Williams JB, Link MJ, Rosenthal NE, et al. Structured Interview Guide for the Hamilton Depression Rating Scale - Seasonal Affective Disorder Version (SIGH-SAD). New York, NY: New York State Psychiatric Institute; 1992.
20. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298-301.
21. Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162(4):656-662.
22. Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry. 1998;55(10):875-882.
23. Eastman CI, Young MA, Fogg LF, et al. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55(10):883-889.
24. Lewy AJ, Bauer VK, Cutler NL, et al. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55(10):890-896.
25. Sit D, Wisner KL, Hanusa BH, et al. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918-927.
26. Goodwin GM. Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2009;23(4):346-388.
27. Lam RW, Levitt AJ, Levitan RD, et al. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry. 2006;163:805-812.
28. Pjrek E, Konstantinidis A, Assem-Hilger E, et al. Therapeutic effects of escitalopram and reboxetine in seasonal affective disorder: a pooled analysis. J Psychiatr Res. 2009;43(8):792-797.
29. Moscovitch A, Blashko CA, Eagles JM, et al. A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berl). 2004;171(4):390-397.
30. Rohan KJ, Roecklein KA, Tierney Lindsey K, et al. A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. J Consult Clin Psychol. 2007;75(3):489-500.
31. Rohan KJ. Coping with the seasons: a cognitive-behavioral approach to seasonal affective disorder. Therapist guide. New York, NY: Oxford University Press; 2008.
32. Rohan KJ, Roecklein KA, Lacy TJ, et al. Winter depression recurrence one year after cognitive-behavioral therapy, light therapy, or combination treatment. Behav Ther. 2009;40(3):225-238.
33. Frank E, Kupfer DJ, Ehlers CL, et al. Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behavior Therapist. 1994;17:143-149.
34. Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996-1004.
Ms. S, age 24, is referred to our team in early December by her primary care physician for “fatigue.” The patient describes going to bed and falling asleep before 9:30 these winter evenings, whereas in summer she went to bed at 11 PM. She craves bread, pasta, and sweets and reports increased appetite in winter compared with summer. Her mood is low, and she misses warm-weather activities of gardening and walking. Fatigue and difficulty concentrating are causing her problems at work and school.
Her history reveals mood elevation in spring as days become longer, with a clear change at approximately March 10 to 20. She reports “spring fever” and feeling “great” last year as soon as daylight saving time began. She slept only 3 hours a night and had a burst of ideas to expand her small business. She threw herself into her work, feeling she was making up for lost time and productivity. She also admits to making a large, misguided business investment during that time.
Upon questioning, she recalls that the previous spring she argued with her father and threw a cup of hot tea at him. When interviewed, Ms. S’s mother describes her daughter at that time as having “a very short fuse,” speaking loud and fast, staying up late at night, and looking as though she was not herself.
Seasonal affective disorder (SAD) is an umbrella term for mood disorders that follow a seasonal pattern of recurrence. Bipolar I disorder (BD I) or bipolar II disorder (BD II) with seasonal pattern (BD SP) is the DSM-IV-TR diagnosis for persons with depressive episodes in the fall or winter and mania (BD I) or hypomania (BD II) in spring or summer ( Table 1 ).1
This article compares BD SP with major depressive disorder with seasonal pattern (MDD SP), in which depressive episodes usually occur in fall or winter and fully remit in spring or summer.1 Rather than being categorically distinct from each other, BD SP and MDD SP may represent extreme variants on a seasonal depression continuum from unipolar to bipolar.
Table 1
DSM-IV-TR criteria for seasonal pattern specifier*
| A | A regular pattern of major depressive episodes (MDEs) at a particular time of year (such as fall and/or winter) |
| B | Full remission or change to mania or hypomania at a particular time of year (such as spring or summer) |
| C | 2 seasonal MDEs that followed the pattern described in (A) and (B) occurred in the past 2 years (and no nonseasonal MDEs) |
| D | Seasonal MDEs substantially outnumber nonseasonal MDEs across the lifespan |
Cases do not meet criteria if:
| |
| *Can be applied to a pattern of major depressive episodes in bipolar I disorder, bipolar II disorder, or major depressive disorder | |
| Source: Adapted from reference 1 | |
Overlap of MDD SP and BD SP
The seasonal pattern specifier can be applied to a diagnosis of MDD, BD I, or BD II.1 Seasonality-focused assessments, described below, can help characterize seasonal patterns that do not meet full SP criteria but may deserve clinical attention.
Symptom presentation. MDD SP and BD SP share similar atypical depressive symptom presentations and seasonal recurrence patterns ( Box 1 ). Hypersomnia, hyperphagia, and psychomotor retardation are more prevalent in major depressive episodes of bipolar disorders and SAD than in unipolar or nonseasonal mood disorders.2-4 Individuals with SAD also report fatigue and decreased physical activity,3 both of which are characteristic of bipolar depressive episodes.5
Although psychosis and psychiatric hospitalizations are more common in BD I than unipolar disorders,6 individuals with BD SP are less likely to report psychosis than those with nonseasonal BD.7 Another study found that BD SP patients reported a higher rate of psychiatric hospitalizations than MDD SP patients (28% vs 9.4%).6
Recurrence pattern. Major depressive episodes are highly recurrent in both MDD and BD, with or without a seasonal pattern. Approximately 75% of individuals with MDD experience ≥1 recurrence (mean, 10.8 episodes);8 MDD SP patients report a mean of 13.4 episodes.9 The mean lifetime episodes in BD SP is 20.74, compared with 11.67 in nonseasonal BD.7
Cassidy and Carroll10 measured the frequency of mood episodes in 304 BD patients not assessed for seasonality. Manic episodes peaked in early spring, mixed episodes peaked in late summer or fall, and depressive episodes peaked in fall-winter.
Irregular rhythm. Both BD and MDD SP involve irregularities in daily or circadian rhythms, such as changes in the timing of sleep, melatonin release, and body temperature.3,5,11 Circadian phase delays—in which internal rhythms lag behind the sleep cycle—are correlated with symptom severity in BD12 and are implicated in the core pathology of BD13 ( Box 2 ). In BD, life events that change social rhythms may disrupt circadian rhythms, triggering mood episodes.5
Etiologic hypotheses for both BD and SAD propose that an external event (life stress in BD; decreased photoperiod in SAD) leads to circadian dysregulation and, in turn, mood episodes. Circadian-related hypotheses for SAD and BD are supported by evidence showing efficacy of treatments that manipulate behavioral and circadian rhythms.
from unipolar to bipolar?
Seasonality refers to the degree of seasonal changes in behavior and mood within an individual. Seasonality scores are normally distributed,a suggesting that seasonality may be continuous in the general population—with some individuals meeting criteria for a seasonal mood disorder:
- A seasonal pattern is reported by approximately 10% to 20% of depressed outpatients with recurrent mood disorders and an estimated 15% to 22% of individuals with bipolar disorder (BD).b
- Persons with BD—seasonal or not—report greater seasonality compared with those with major depressive disorder (MDD).c
Among individuals with seasonal affective disorder, the course is bipolar in an estimated 12% to 22% and unipolar in 78% to 88%.d These estimates may reflect underdiagnosis of BD with seasonal pattern because hypomania is difficult to diagnose retrospectively.e
The bipolar-unipolar continuum includes (in order): BD I, BD II, bipolar disorder not otherwise specified, cyclothymia, bipolar spectrum disorder, and MDD.f In examining the validity of the bipolar spectrum model, Phelps et alg noted:
- At least 3 studies found that all symptoms reported by individuals with unipolar and bipolar diagnoses approach a normal distribution, rather than a bimodal distribution separating unipolar from bipolar symptom profiles.
- Data from 2 population-based studies indicate that subthreshold hypomanic symptoms are more common than and cause as much impairment as symptoms meeting criteria for BD II or I.
Some individuals who meet criteria for MDD with seasonal pattern have summertime periods of transient hypomania and hyperthymia (hypomanic-like periods without clinically significant impairment).h This suggests that the bipolar continuum also may exist among individuals with seasonal pattern mood disorders.
Source: Access reference citations here
Etiologic hypotheses of seasonal affective disorder (SAD) include:
- photoperiodic hypothesis (shorter winter days cause SAD,a perhaps mediated by a summer vs winter difference in duration of nightly melatonin release)b
- phase shift hypothesis (less available light in winter may lead to an inability to synchronize circadian rhythms with sleep/wake rhythms).c
Some case studies of rapid-cycling bipolar disorder (BD) suggest that mood is correlated with daily hours of sunshine and light therapy is antidepressant. Rapid-cycling patients may be hypersensitive to day-to-day changes in photoperiod, analogous to mood changes in response to changes in photoperiod across the seasons in SAD.d
Circadian phase delays—in which internal rhythms lag behind the sleep cycle—are correlated with symptom severity in BDe and are implicated in the core pathology of BD.f Phase delays also are present in some individuals with SAD and are associated with severity and treatment response.g Preliminary evidence suggests that variation in circadian clock genes is related to both BDf,h and SAD.i
Source: Access reference citations here
CASE CONTINUED: Seasonal pattern revealed
Ms. S was aware that she is vulnerable to depressive episodes in fall and winter but unaware of a pattern of hypomanic/manic episodes in spring and summer. Her family psychiatric history includes a sister diagnosed with BD I (with no seasonal specifier), and a maternal aunt who has attempted suicide several times.
Ms. S agrees to an assessment plan including a diagnostic interview, interviews measuring symptom severity and pattern of recurrence, routine laboratory examination, and self-report questionnaires. These show that she meets DSM-IV-TR criteria for BD I, depressed, moderate, with seasonal pattern.
Her assessment scores are 28 on the Structured Interview Guide for HDRS-seasonal affective disorder version (SIGH-SAD), 17 on the Hamilton Depression Rating Scale (HDRS), and 11 on the atypical subscale. The HDRS and atypical subscale are components of the SIGH-SAD reflecting typical (eg, insomnia, loss of appetite, etc.) and atypical (eg, hypersomnia, increased appetite, etc.) depression symptoms, respectively. Ms. S’s scores exceed the threshold scores defining a BD SP episode (>20 SIGH-SAD + >10 HDRS + >5 atypical subscale14 ). Data from self-report questionnaires corroborate this assessment.
We plan to administer the Hypomania Interview Guide (including Hyperthymia) for Seasonal Affective Disorder (HIGH-SAD) during treatment and the following spring to monitor prospectively for hypomanic symptoms.
Assessment tools
After complete assessment for mood episodes and mood disorders based on DSM-IV-TR, an additional assessment for bipolarity and seasonality may be helpful.1
Screen for bipolarity in patients with SAD to avoid triggering mania or hypomania during treatment. Useful tools include:
- HIGH-SAD15
- the National Institutes of Health Life Chart Method to establish a recurrent pattern of mood episodes and track treatment efficacy16
- assessments that characterize sub-threshold bipolar symptoms, such as the Bipolar Spectrum Diagnostic Scale17 ( see Box 3 ) and the Bipolarity Index.18
Also obtain collateral reports from significant others, review patient records, and use the same mania and hypomania scales for prospective assessment as the next spring approaches.6
Assess seasonality in patients with BD to improve diagnosis and treatment. Characterizing a seasonal pattern may allow you and your patient to predict episodes and treat proactively. Commonly used assessments include the SIGH-SAD and the Structured Clinical Interview for DSM Disorders (SCID) seasonal pattern specifier module.19
The SIGH-SAD measures symptom severity and provides recovery criteria based on changes in scores during treatment. Response is defined as a 50% reduction in symptoms; remission is >50% improvement in SIGH-SAD + HDRS <7 + atypical <7 or HDRS <2 + atypical <10.14
CASE CONTINUED: Treatment begins
Considering Ms. S’s diagnosis of BD I SP and the risk of precipitating mania with light treatment, we recommend starting treatment with a mood stabilizer. We narrow our options to those that have a direct antidepressant effect, with the hope that this may reduce the need for future antidepressant medications. For patients diagnosed with BD II SP, we could consider a regimen without mood stabilizers.
We offer Ms. S lithium, a first-line mood stabilizer with evidence of usefulness in treatment before chronotherapeutic interventions and in preventing suicidal behavior. However, Ms. S prefers our second option, lamotrigine, because she is concerned about lithium’s side effects and required blood draws to check drug levels as well as thyroid and kidney status.
Despite causing some initial drowsiness, her lamotrigine dosage is successfully titrated after 2 weeks of treatment to 300 mg/d, without side effects. Only then do we initiate light treatment, which Ms. S wishes to try before antidepressant medications. She also begins sessions with a therapist trained in cognitive-behavioral therapy (CBT) for SAD. (For details of this comprehensive treatment, see Box 4 )
Treating bipolar variant of SAD
Significant differences exist in the clinical management of BD SP and MDD SP, despite their commonalities ( Table 2 ). BD SP treatment remains distinct because of the risk of switching with the use of light therapy or antidepressants and the importance of mood stabilizers, especially in BD I.
Consensus guidelines for treating SAD recommend mood stabilizers and close monitoring during light therapy for patients with BD SP ( Table 3 ).2 Therapeutic sleep deprivation can quickly reverse depression during hospitalization but is not used often or recommended for outpatient treatment.20
Light therapy. A small body of evidence suggests that depressive symptoms in BD SP improve with bright light therapy, a treatment with demonstrated efficacy in MDD SP.21 No differences in response have been reported between light therapy for winter depressive episodes among individuals with BD SP or MDD SP.22-24 Light therapy may increase the risk of switching to mania/hypomania in patients with BD SP, however. Clinical supervision is imperative, even for patients thought to have MDD SP, because of the risk of undiagnosed BD.
Regular monitoring by a physician is indicated for individuals taking medications or remedies with photosensitizing effects (such as lithium, thioridazine, or St. John’s wort). An ophthalmologist consultation and monitoring is necessary for patients with preexisting eye problems, those taking photosensitizing medications, and those who develop eye problems during light treatment.2,6
The recommended starting dose for light therapy in MDD SP is 30 minutes daily in the early morning, but this dose may be too high for individuals with BD SP.25 To minimize the risk of switching, begin light therapy at 5 to 10 minutes daily and slowly increase while monitoring the clinical effect (see Related Resources , for more information about light therapy for affective disorders).
Pharmacotherapy. Pharmacologic treatments have not been studied for effectiveness in BD SP, and we hesitate to provide specific recommendations. Effective treatments may include those used for nonseasonal MDD, nonseasonal BD, and MDD SP. When using any medication for BD SP, weigh the risk of switching states against the potential beneficial effects.2
Year-round mood-stabilizer treatment is indicated to minimize the risk of mood episodes in BD SP, especially in patients with BD I. When treating SAD, mood stabilizers with antidepressant effects—such as lamotrigine or lithium (for maintenance), and quetiapine or aripiprazole (for acute treatment)—are preferable to agents without an antidepressant effect in monotherapy. More-sedating mood stabilizers (such as valproate or carbamazepine) likely would not be as beneficial as less-sedating agents, considering that patients with SAD frequently experience fatigue.
Because of the lack of adequate clinical trials of treatments for BD SP, we suggest that clinicians choose medications and follow algorithms relevant to BD without a seasonal specifier. Use similar schedules and dosages, with individual tailoring.5,26
Antidepressants that have shown efficacy in MDD SP include fluoxetine, bupropion, citalopram, and sertraline.6,9,27-29 For patients with BD SP, we initiate antidepressants when:
- light treatment fails
- the patient is unable to travel to the south
- light treatment is not available (often because patients cannot afford the cost, which is not covered by insurance)
- patient lacks time for light treatment.
An additional important consideration is history of response (such as a patient who did not respond well to light therapy in the past but responded very well to a particular antidepressant).
No studies have compared antidepressant classes or individual medications for MDD SP. Clinical wisdom is to base the antidepressant choice, dosages, decision points of when to switch, and schedule of switching (cross-tapering) on individual patients’ symptom clusters and comorbid conditions as well as the medication’s side effects.
Prophylactic treatment with bupropion would seem an appropriate initial choice for a prototypical SAD patient, considering this medication’s more activating effects and FDA approval for SAD treatment.9 For the minority of patients with SAD who present with agitation and increased sleepiness, a slightly sedating selective serotonin reuptake inhibitor such as citalopram would make more sense as a first-line treatment. Finally, we would recommend sertraline for patients with marked anxiety—especially panic attacks or obsessive-compulsive symptoms—but without insomnia.
For specific dosages, rely on the literature of treating nonseasonal depression (unipolar or bipolar). It is important to define decision-making points for dosage increases, augmentation, switching to another antidepressant, and cross-tapering, similar to how you would address a nonseasonal depression, typical or atypical.
In our view, treating a patient with BD I SP with an antidepressant alone—without a mood stabilizer—is almost always wrong. For BD II SP we leave it to the clinician to decide, based on individual patients, clinical experience, and ideally in consultation with a peer.
Seasonal dosages. You may wish to seasonally vary medications and dosages for patients with BD SP. Although no strong evidence exists, we recommend 2 options:
- Consider increasing mood-stabilizing medication in spring and summer, with a reduction (but no tapering for BD I) in fall and winter.
- Consider a complete antidepressant taper 2 weeks after daylight saving time begins in spring; taper under increased observation and not faster than 6 weeks, with close attention to emerging symptoms of depression or antidepressant withdrawal.
We do not taper antidepressants before daylight saving time, and we always consider additional stressors, losses, and challenges in our patients’ lives before tapering antidepressants in spring or summer. We also assess and monitor compliance.
Psychotherapy. Referral can be made to clinicians trained in CBT for patients with a seasonal pattern and interpersonal and social rhythm therapy (IPSRT) for BD. Integrative models for SAD and BD propose that psychological and biologic vulnerability factors interact with environmental events (such as winter season or disruption of daily routine) to trigger mood episodes.
CBT adapted for SAD targets mal-adaptive thinking and behavioral disengagement through cognitive therapy and behavioral activation to counteract SAD symptoms.30,31 Preliminary trials by our group suggest that CBT for MDD SP is an effective acute treatment30 and may prevent future episodes.32
IPSRT is an adaptation of interpersonal psychotherapy that aims to stabilize social relationships and rhythms in BD.33 IPSRT posits that irregularity in daily routines leads to circadian dysregulation, precipitating mood episodes in persons vulnerable to BD.34 The degree of regularity in social rhythms achieved in IPSRT is associated with reduced likelihood of recurrence post-treatment.34 If stabilizing social rhythms has a similar effect of regulating circadian rhythms in SAD, IPSRT may be effective in treating BD SP.
Table 2
Physiopathologic findings and clinical management for SAD vs BD
| SAD | BD | |
|---|---|---|
| Differences | May be unipolar or bipolar Defined by seasonality Light therapy and antidepressants indicated | Increased risk of psychosis and psychiatric hospitalization Most BD is not seasonal Mood stabilizers indicated Risk of switching states with light therapy and antidepressants |
| Similarities | Atypical depressive symptom presentation Highly recurrent Predictable season of recurrence allows proactive treatment Assess for mania and hypomania in both disorders Light therapy requires clinical supervision Psychotherapy may be beneficial | |
| BD: bipolar disorder; SAD: seasonal affective disorder | ||
Table 3
Recommended treatment for bipolar disorder with seasonal pattern
| Treatment | Recommendation |
|---|---|
| Mood-stabilizing medications | Maintain year-round, especially in patients with BP I |
| Antidepressants | Consider those with efficacy in unipolar SAD or nonseasonal bipolar depression |
| Light therapy | Initiate for 5 to 10 min/day for bipolar depressive episodes in patients receiving mood stabilizers or atypical antipsychotics; slowly increase duration while monitoring mood, sleep, and side effects to manage risk of hypomanic or manic switch |
| Psychotherapy | Consider CBT or interpersonal and social rhythm therapy to help manage symptoms and reduce episode recurrence |
| BP I: bipolar disorder type I; CBT: cognitive-behavioral therapy; SAD: seasonal affective disorder | |
CASE CONCLUSION: Ongoing treatment required
After several months of light therapy, Ms. S begins to feel better and reports having more energy. We taper her light therapy to 10 minutes daily in the morning from late February until 1 week after daylight saving time begins in mid-March. Weekly phone calls during this transition screen for signs of hypomania or mania. Lamotrigine is effective in preventing switches in spring.
Future plans include monitoring for hypomania through summer and possibly reinitiating light therapy in fall or winter. Because approximately one-half of individuals who undergo CBT for SAD do not experience another episode the winter after treatment, light therapy will be initiated only if depressive symptoms emerge. A booster session is scheduled with Ms. S’s CBT therapist in early fall to reinforce relapse prevention skills.
Antidepressant therapy will be recommended if full treatment response is not maintained with light therapy and continued use of CBT skills for SAD. During sessions, we emphasize compliance with lamotrigine. On several occasions Ms. S questions the need for ongoing therapy, but with education about the potential effects of mania she agrees to continue treatment as indicated.
Seasonality screening tools
- Seasonal Pattern module of the Structured Clinical Interview for DSM Disorders (SCID). www.scid4.org/faq/clinician_version.html.
- Hypomania Interview Guide for Seasonal Affective Disorder (HIGH-SAD). www.chronotherapeutics.org/Tools_ENG.html.
- National Institutes of Health Life Chart Method. www.bipolarnews.org/Clinician%20Life%20Charting.htm.
- Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD). www.chronotherapeutics.org/Tools_ENG.html.
Bipolarity screening tools
- Bipolar Spectrum Diagnostic Scale. Click here to download.
- Bipolarity Index. http://psycheducation.org/depression/STEPBipolarityIndex.htm.
Light therapy
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders: a clinician’s manual for light and wake therapy. Basel, Switzerland: S. Karger AG; 2009.
- Wirz-Justice A, Benedetti F, Berger M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
Psychotherapy
- Rohan KJ. Coping with the seasons: a cognitive-behavioral approach to seasonal affective disorder (therapist guide). New York, NY: Oxford University Press; 2008.
- Frank E. Treating bipolar disorder: a clinician’s guide to interpersonal and social rhythm therapy. New York, NY: Guilford Press, Inc.; 2005.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin XL
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Valproate • Depakote
Disclosures
Drs. Roecklein and Rohan report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Postolache received an investigator-initiated award from The LiteBook Company (Vancouver, Canada) via the Society for Light Treatment and Biological Rhythms, as well as research support from Apollo Health (Salt Lake City, UT).
Ms. S, age 24, is referred to our team in early December by her primary care physician for “fatigue.” The patient describes going to bed and falling asleep before 9:30 these winter evenings, whereas in summer she went to bed at 11 PM. She craves bread, pasta, and sweets and reports increased appetite in winter compared with summer. Her mood is low, and she misses warm-weather activities of gardening and walking. Fatigue and difficulty concentrating are causing her problems at work and school.
Her history reveals mood elevation in spring as days become longer, with a clear change at approximately March 10 to 20. She reports “spring fever” and feeling “great” last year as soon as daylight saving time began. She slept only 3 hours a night and had a burst of ideas to expand her small business. She threw herself into her work, feeling she was making up for lost time and productivity. She also admits to making a large, misguided business investment during that time.
Upon questioning, she recalls that the previous spring she argued with her father and threw a cup of hot tea at him. When interviewed, Ms. S’s mother describes her daughter at that time as having “a very short fuse,” speaking loud and fast, staying up late at night, and looking as though she was not herself.
Seasonal affective disorder (SAD) is an umbrella term for mood disorders that follow a seasonal pattern of recurrence. Bipolar I disorder (BD I) or bipolar II disorder (BD II) with seasonal pattern (BD SP) is the DSM-IV-TR diagnosis for persons with depressive episodes in the fall or winter and mania (BD I) or hypomania (BD II) in spring or summer ( Table 1 ).1
This article compares BD SP with major depressive disorder with seasonal pattern (MDD SP), in which depressive episodes usually occur in fall or winter and fully remit in spring or summer.1 Rather than being categorically distinct from each other, BD SP and MDD SP may represent extreme variants on a seasonal depression continuum from unipolar to bipolar.
Table 1
DSM-IV-TR criteria for seasonal pattern specifier*
| A | A regular pattern of major depressive episodes (MDEs) at a particular time of year (such as fall and/or winter) |
| B | Full remission or change to mania or hypomania at a particular time of year (such as spring or summer) |
| C | 2 seasonal MDEs that followed the pattern described in (A) and (B) occurred in the past 2 years (and no nonseasonal MDEs) |
| D | Seasonal MDEs substantially outnumber nonseasonal MDEs across the lifespan |
Cases do not meet criteria if:
| |
| *Can be applied to a pattern of major depressive episodes in bipolar I disorder, bipolar II disorder, or major depressive disorder | |
| Source: Adapted from reference 1 | |
Overlap of MDD SP and BD SP
The seasonal pattern specifier can be applied to a diagnosis of MDD, BD I, or BD II.1 Seasonality-focused assessments, described below, can help characterize seasonal patterns that do not meet full SP criteria but may deserve clinical attention.
Symptom presentation. MDD SP and BD SP share similar atypical depressive symptom presentations and seasonal recurrence patterns ( Box 1 ). Hypersomnia, hyperphagia, and psychomotor retardation are more prevalent in major depressive episodes of bipolar disorders and SAD than in unipolar or nonseasonal mood disorders.2-4 Individuals with SAD also report fatigue and decreased physical activity,3 both of which are characteristic of bipolar depressive episodes.5
Although psychosis and psychiatric hospitalizations are more common in BD I than unipolar disorders,6 individuals with BD SP are less likely to report psychosis than those with nonseasonal BD.7 Another study found that BD SP patients reported a higher rate of psychiatric hospitalizations than MDD SP patients (28% vs 9.4%).6
Recurrence pattern. Major depressive episodes are highly recurrent in both MDD and BD, with or without a seasonal pattern. Approximately 75% of individuals with MDD experience ≥1 recurrence (mean, 10.8 episodes);8 MDD SP patients report a mean of 13.4 episodes.9 The mean lifetime episodes in BD SP is 20.74, compared with 11.67 in nonseasonal BD.7
Cassidy and Carroll10 measured the frequency of mood episodes in 304 BD patients not assessed for seasonality. Manic episodes peaked in early spring, mixed episodes peaked in late summer or fall, and depressive episodes peaked in fall-winter.
Irregular rhythm. Both BD and MDD SP involve irregularities in daily or circadian rhythms, such as changes in the timing of sleep, melatonin release, and body temperature.3,5,11 Circadian phase delays—in which internal rhythms lag behind the sleep cycle—are correlated with symptom severity in BD12 and are implicated in the core pathology of BD13 ( Box 2 ). In BD, life events that change social rhythms may disrupt circadian rhythms, triggering mood episodes.5
Etiologic hypotheses for both BD and SAD propose that an external event (life stress in BD; decreased photoperiod in SAD) leads to circadian dysregulation and, in turn, mood episodes. Circadian-related hypotheses for SAD and BD are supported by evidence showing efficacy of treatments that manipulate behavioral and circadian rhythms.
from unipolar to bipolar?
Seasonality refers to the degree of seasonal changes in behavior and mood within an individual. Seasonality scores are normally distributed,a suggesting that seasonality may be continuous in the general population—with some individuals meeting criteria for a seasonal mood disorder:
- A seasonal pattern is reported by approximately 10% to 20% of depressed outpatients with recurrent mood disorders and an estimated 15% to 22% of individuals with bipolar disorder (BD).b
- Persons with BD—seasonal or not—report greater seasonality compared with those with major depressive disorder (MDD).c
Among individuals with seasonal affective disorder, the course is bipolar in an estimated 12% to 22% and unipolar in 78% to 88%.d These estimates may reflect underdiagnosis of BD with seasonal pattern because hypomania is difficult to diagnose retrospectively.e
The bipolar-unipolar continuum includes (in order): BD I, BD II, bipolar disorder not otherwise specified, cyclothymia, bipolar spectrum disorder, and MDD.f In examining the validity of the bipolar spectrum model, Phelps et alg noted:
- At least 3 studies found that all symptoms reported by individuals with unipolar and bipolar diagnoses approach a normal distribution, rather than a bimodal distribution separating unipolar from bipolar symptom profiles.
- Data from 2 population-based studies indicate that subthreshold hypomanic symptoms are more common than and cause as much impairment as symptoms meeting criteria for BD II or I.
Some individuals who meet criteria for MDD with seasonal pattern have summertime periods of transient hypomania and hyperthymia (hypomanic-like periods without clinically significant impairment).h This suggests that the bipolar continuum also may exist among individuals with seasonal pattern mood disorders.
Source: Access reference citations here
Etiologic hypotheses of seasonal affective disorder (SAD) include:
- photoperiodic hypothesis (shorter winter days cause SAD,a perhaps mediated by a summer vs winter difference in duration of nightly melatonin release)b
- phase shift hypothesis (less available light in winter may lead to an inability to synchronize circadian rhythms with sleep/wake rhythms).c
Some case studies of rapid-cycling bipolar disorder (BD) suggest that mood is correlated with daily hours of sunshine and light therapy is antidepressant. Rapid-cycling patients may be hypersensitive to day-to-day changes in photoperiod, analogous to mood changes in response to changes in photoperiod across the seasons in SAD.d
Circadian phase delays—in which internal rhythms lag behind the sleep cycle—are correlated with symptom severity in BDe and are implicated in the core pathology of BD.f Phase delays also are present in some individuals with SAD and are associated with severity and treatment response.g Preliminary evidence suggests that variation in circadian clock genes is related to both BDf,h and SAD.i
Source: Access reference citations here
CASE CONTINUED: Seasonal pattern revealed
Ms. S was aware that she is vulnerable to depressive episodes in fall and winter but unaware of a pattern of hypomanic/manic episodes in spring and summer. Her family psychiatric history includes a sister diagnosed with BD I (with no seasonal specifier), and a maternal aunt who has attempted suicide several times.
Ms. S agrees to an assessment plan including a diagnostic interview, interviews measuring symptom severity and pattern of recurrence, routine laboratory examination, and self-report questionnaires. These show that she meets DSM-IV-TR criteria for BD I, depressed, moderate, with seasonal pattern.
Her assessment scores are 28 on the Structured Interview Guide for HDRS-seasonal affective disorder version (SIGH-SAD), 17 on the Hamilton Depression Rating Scale (HDRS), and 11 on the atypical subscale. The HDRS and atypical subscale are components of the SIGH-SAD reflecting typical (eg, insomnia, loss of appetite, etc.) and atypical (eg, hypersomnia, increased appetite, etc.) depression symptoms, respectively. Ms. S’s scores exceed the threshold scores defining a BD SP episode (>20 SIGH-SAD + >10 HDRS + >5 atypical subscale14 ). Data from self-report questionnaires corroborate this assessment.
We plan to administer the Hypomania Interview Guide (including Hyperthymia) for Seasonal Affective Disorder (HIGH-SAD) during treatment and the following spring to monitor prospectively for hypomanic symptoms.
Assessment tools
After complete assessment for mood episodes and mood disorders based on DSM-IV-TR, an additional assessment for bipolarity and seasonality may be helpful.1
Screen for bipolarity in patients with SAD to avoid triggering mania or hypomania during treatment. Useful tools include:
- HIGH-SAD15
- the National Institutes of Health Life Chart Method to establish a recurrent pattern of mood episodes and track treatment efficacy16
- assessments that characterize sub-threshold bipolar symptoms, such as the Bipolar Spectrum Diagnostic Scale17 ( see Box 3 ) and the Bipolarity Index.18
Also obtain collateral reports from significant others, review patient records, and use the same mania and hypomania scales for prospective assessment as the next spring approaches.6
Assess seasonality in patients with BD to improve diagnosis and treatment. Characterizing a seasonal pattern may allow you and your patient to predict episodes and treat proactively. Commonly used assessments include the SIGH-SAD and the Structured Clinical Interview for DSM Disorders (SCID) seasonal pattern specifier module.19
The SIGH-SAD measures symptom severity and provides recovery criteria based on changes in scores during treatment. Response is defined as a 50% reduction in symptoms; remission is >50% improvement in SIGH-SAD + HDRS <7 + atypical <7 or HDRS <2 + atypical <10.14
CASE CONTINUED: Treatment begins
Considering Ms. S’s diagnosis of BD I SP and the risk of precipitating mania with light treatment, we recommend starting treatment with a mood stabilizer. We narrow our options to those that have a direct antidepressant effect, with the hope that this may reduce the need for future antidepressant medications. For patients diagnosed with BD II SP, we could consider a regimen without mood stabilizers.
We offer Ms. S lithium, a first-line mood stabilizer with evidence of usefulness in treatment before chronotherapeutic interventions and in preventing suicidal behavior. However, Ms. S prefers our second option, lamotrigine, because she is concerned about lithium’s side effects and required blood draws to check drug levels as well as thyroid and kidney status.
Despite causing some initial drowsiness, her lamotrigine dosage is successfully titrated after 2 weeks of treatment to 300 mg/d, without side effects. Only then do we initiate light treatment, which Ms. S wishes to try before antidepressant medications. She also begins sessions with a therapist trained in cognitive-behavioral therapy (CBT) for SAD. (For details of this comprehensive treatment, see Box 4 )
Treating bipolar variant of SAD
Significant differences exist in the clinical management of BD SP and MDD SP, despite their commonalities ( Table 2 ). BD SP treatment remains distinct because of the risk of switching with the use of light therapy or antidepressants and the importance of mood stabilizers, especially in BD I.
Consensus guidelines for treating SAD recommend mood stabilizers and close monitoring during light therapy for patients with BD SP ( Table 3 ).2 Therapeutic sleep deprivation can quickly reverse depression during hospitalization but is not used often or recommended for outpatient treatment.20
Light therapy. A small body of evidence suggests that depressive symptoms in BD SP improve with bright light therapy, a treatment with demonstrated efficacy in MDD SP.21 No differences in response have been reported between light therapy for winter depressive episodes among individuals with BD SP or MDD SP.22-24 Light therapy may increase the risk of switching to mania/hypomania in patients with BD SP, however. Clinical supervision is imperative, even for patients thought to have MDD SP, because of the risk of undiagnosed BD.
Regular monitoring by a physician is indicated for individuals taking medications or remedies with photosensitizing effects (such as lithium, thioridazine, or St. John’s wort). An ophthalmologist consultation and monitoring is necessary for patients with preexisting eye problems, those taking photosensitizing medications, and those who develop eye problems during light treatment.2,6
The recommended starting dose for light therapy in MDD SP is 30 minutes daily in the early morning, but this dose may be too high for individuals with BD SP.25 To minimize the risk of switching, begin light therapy at 5 to 10 minutes daily and slowly increase while monitoring the clinical effect (see Related Resources , for more information about light therapy for affective disorders).
Pharmacotherapy. Pharmacologic treatments have not been studied for effectiveness in BD SP, and we hesitate to provide specific recommendations. Effective treatments may include those used for nonseasonal MDD, nonseasonal BD, and MDD SP. When using any medication for BD SP, weigh the risk of switching states against the potential beneficial effects.2
Year-round mood-stabilizer treatment is indicated to minimize the risk of mood episodes in BD SP, especially in patients with BD I. When treating SAD, mood stabilizers with antidepressant effects—such as lamotrigine or lithium (for maintenance), and quetiapine or aripiprazole (for acute treatment)—are preferable to agents without an antidepressant effect in monotherapy. More-sedating mood stabilizers (such as valproate or carbamazepine) likely would not be as beneficial as less-sedating agents, considering that patients with SAD frequently experience fatigue.
Because of the lack of adequate clinical trials of treatments for BD SP, we suggest that clinicians choose medications and follow algorithms relevant to BD without a seasonal specifier. Use similar schedules and dosages, with individual tailoring.5,26
Antidepressants that have shown efficacy in MDD SP include fluoxetine, bupropion, citalopram, and sertraline.6,9,27-29 For patients with BD SP, we initiate antidepressants when:
- light treatment fails
- the patient is unable to travel to the south
- light treatment is not available (often because patients cannot afford the cost, which is not covered by insurance)
- patient lacks time for light treatment.
An additional important consideration is history of response (such as a patient who did not respond well to light therapy in the past but responded very well to a particular antidepressant).
No studies have compared antidepressant classes or individual medications for MDD SP. Clinical wisdom is to base the antidepressant choice, dosages, decision points of when to switch, and schedule of switching (cross-tapering) on individual patients’ symptom clusters and comorbid conditions as well as the medication’s side effects.
Prophylactic treatment with bupropion would seem an appropriate initial choice for a prototypical SAD patient, considering this medication’s more activating effects and FDA approval for SAD treatment.9 For the minority of patients with SAD who present with agitation and increased sleepiness, a slightly sedating selective serotonin reuptake inhibitor such as citalopram would make more sense as a first-line treatment. Finally, we would recommend sertraline for patients with marked anxiety—especially panic attacks or obsessive-compulsive symptoms—but without insomnia.
For specific dosages, rely on the literature of treating nonseasonal depression (unipolar or bipolar). It is important to define decision-making points for dosage increases, augmentation, switching to another antidepressant, and cross-tapering, similar to how you would address a nonseasonal depression, typical or atypical.
In our view, treating a patient with BD I SP with an antidepressant alone—without a mood stabilizer—is almost always wrong. For BD II SP we leave it to the clinician to decide, based on individual patients, clinical experience, and ideally in consultation with a peer.
Seasonal dosages. You may wish to seasonally vary medications and dosages for patients with BD SP. Although no strong evidence exists, we recommend 2 options:
- Consider increasing mood-stabilizing medication in spring and summer, with a reduction (but no tapering for BD I) in fall and winter.
- Consider a complete antidepressant taper 2 weeks after daylight saving time begins in spring; taper under increased observation and not faster than 6 weeks, with close attention to emerging symptoms of depression or antidepressant withdrawal.
We do not taper antidepressants before daylight saving time, and we always consider additional stressors, losses, and challenges in our patients’ lives before tapering antidepressants in spring or summer. We also assess and monitor compliance.
Psychotherapy. Referral can be made to clinicians trained in CBT for patients with a seasonal pattern and interpersonal and social rhythm therapy (IPSRT) for BD. Integrative models for SAD and BD propose that psychological and biologic vulnerability factors interact with environmental events (such as winter season or disruption of daily routine) to trigger mood episodes.
CBT adapted for SAD targets mal-adaptive thinking and behavioral disengagement through cognitive therapy and behavioral activation to counteract SAD symptoms.30,31 Preliminary trials by our group suggest that CBT for MDD SP is an effective acute treatment30 and may prevent future episodes.32
IPSRT is an adaptation of interpersonal psychotherapy that aims to stabilize social relationships and rhythms in BD.33 IPSRT posits that irregularity in daily routines leads to circadian dysregulation, precipitating mood episodes in persons vulnerable to BD.34 The degree of regularity in social rhythms achieved in IPSRT is associated with reduced likelihood of recurrence post-treatment.34 If stabilizing social rhythms has a similar effect of regulating circadian rhythms in SAD, IPSRT may be effective in treating BD SP.
Table 2
Physiopathologic findings and clinical management for SAD vs BD
| SAD | BD | |
|---|---|---|
| Differences | May be unipolar or bipolar Defined by seasonality Light therapy and antidepressants indicated | Increased risk of psychosis and psychiatric hospitalization Most BD is not seasonal Mood stabilizers indicated Risk of switching states with light therapy and antidepressants |
| Similarities | Atypical depressive symptom presentation Highly recurrent Predictable season of recurrence allows proactive treatment Assess for mania and hypomania in both disorders Light therapy requires clinical supervision Psychotherapy may be beneficial | |
| BD: bipolar disorder; SAD: seasonal affective disorder | ||
Table 3
Recommended treatment for bipolar disorder with seasonal pattern
| Treatment | Recommendation |
|---|---|
| Mood-stabilizing medications | Maintain year-round, especially in patients with BP I |
| Antidepressants | Consider those with efficacy in unipolar SAD or nonseasonal bipolar depression |
| Light therapy | Initiate for 5 to 10 min/day for bipolar depressive episodes in patients receiving mood stabilizers or atypical antipsychotics; slowly increase duration while monitoring mood, sleep, and side effects to manage risk of hypomanic or manic switch |
| Psychotherapy | Consider CBT or interpersonal and social rhythm therapy to help manage symptoms and reduce episode recurrence |
| BP I: bipolar disorder type I; CBT: cognitive-behavioral therapy; SAD: seasonal affective disorder | |
CASE CONCLUSION: Ongoing treatment required
After several months of light therapy, Ms. S begins to feel better and reports having more energy. We taper her light therapy to 10 minutes daily in the morning from late February until 1 week after daylight saving time begins in mid-March. Weekly phone calls during this transition screen for signs of hypomania or mania. Lamotrigine is effective in preventing switches in spring.
Future plans include monitoring for hypomania through summer and possibly reinitiating light therapy in fall or winter. Because approximately one-half of individuals who undergo CBT for SAD do not experience another episode the winter after treatment, light therapy will be initiated only if depressive symptoms emerge. A booster session is scheduled with Ms. S’s CBT therapist in early fall to reinforce relapse prevention skills.
Antidepressant therapy will be recommended if full treatment response is not maintained with light therapy and continued use of CBT skills for SAD. During sessions, we emphasize compliance with lamotrigine. On several occasions Ms. S questions the need for ongoing therapy, but with education about the potential effects of mania she agrees to continue treatment as indicated.
Seasonality screening tools
- Seasonal Pattern module of the Structured Clinical Interview for DSM Disorders (SCID). www.scid4.org/faq/clinician_version.html.
- Hypomania Interview Guide for Seasonal Affective Disorder (HIGH-SAD). www.chronotherapeutics.org/Tools_ENG.html.
- National Institutes of Health Life Chart Method. www.bipolarnews.org/Clinician%20Life%20Charting.htm.
- Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD). www.chronotherapeutics.org/Tools_ENG.html.
Bipolarity screening tools
- Bipolar Spectrum Diagnostic Scale. Click here to download.
- Bipolarity Index. http://psycheducation.org/depression/STEPBipolarityIndex.htm.
Light therapy
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders: a clinician’s manual for light and wake therapy. Basel, Switzerland: S. Karger AG; 2009.
- Wirz-Justice A, Benedetti F, Berger M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
Psychotherapy
- Rohan KJ. Coping with the seasons: a cognitive-behavioral approach to seasonal affective disorder (therapist guide). New York, NY: Oxford University Press; 2008.
- Frank E. Treating bipolar disorder: a clinician’s guide to interpersonal and social rhythm therapy. New York, NY: Guilford Press, Inc.; 2005.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin XL
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Valproate • Depakote
Disclosures
Drs. Roecklein and Rohan report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Postolache received an investigator-initiated award from The LiteBook Company (Vancouver, Canada) via the Society for Light Treatment and Biological Rhythms, as well as research support from Apollo Health (Salt Lake City, UT).
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lam RW, Levitt AJ. eds. Clinical guidelines for the treatment of seasonal affective disorder. Vancouver, BC: Clinical and Academic Publishing; 1999.
3. Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72-80.
4. Michalak EE, Wilkinson C, Hood K, et al. Seasonal and nonseasonal depression: how do they differ? Symptom profile, clinical and family history in a general population sample. J Affect Disord. 2002;69(1-3):185-192.
5. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
6. Sohn CH, Lam RW. Treatment of seasonal affective disorder: unipolar versus bipolar differences. Curr Psychiatry Rep. 2004;6(6):478-485.
7. Goikolea JM, Colom F, Martinez-Aran A, et al. Clinical and prognostic implications of seasonal pattern in bipolar disorder: a 10-year follow-up of 302 patients. Psychol Med. 2007;37:1595-1599.
8. Kessler RC, Zhao S, Blazer DG, et al. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45(1-2):19-30.
9. Modell JG, Rosenthal NE, Harriett AE, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry. 2005;58:658-667.
10. Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002;68:25-31.
11. Shin K, Schaffer A, Levitt AJ, et al. Seasonality in a community sample of bipolar, unipolar and control subjects. J Affect Disord. 2005;86:19-25.
12. Wood J, Birmaher B, Axelson D, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166(2-3):201-209.
13. Soreca I, Frank E, Kupfer DJ. The phenomenology of bipolar disorder: what drives the high rate of medical burden and determines long-term prognosis? Depress Anxiety. 2009;26(1):73-82.
14. Terman M, Terman J, Rafferty B. Experimental design and measures of success in the treatment of winter depression by bright light. Psychopharmacol Bull. 1990;26(4):505-510.
15. Goel N, Terman M, Terman JS, et al. Summer mood in winter depressives: validation of a structured interview. Depress Anxiety. 1999;9:83-91.
16. Denicoff KD, Leverich GS, Nolen WA, et al. Validation of the prospective NIMH-Life-Chart Method (NIMH-LCM-p) for longitudinal assessment of bipolar illness. Psychol Med. 2000;30:1391-1397.
17. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity of a new Bipolar Spectrum Diagnostic Scale. J Affect Disord. 2005;84:273-277.
18. Phelps J, Angst J, Katzow J, et al. Validity and utility of bipolar spectrum models. Bipolar Disord. 2008;10:179-193.
19. Williams JB, Link MJ, Rosenthal NE, et al. Structured Interview Guide for the Hamilton Depression Rating Scale - Seasonal Affective Disorder Version (SIGH-SAD). New York, NY: New York State Psychiatric Institute; 1992.
20. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298-301.
21. Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162(4):656-662.
22. Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry. 1998;55(10):875-882.
23. Eastman CI, Young MA, Fogg LF, et al. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55(10):883-889.
24. Lewy AJ, Bauer VK, Cutler NL, et al. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55(10):890-896.
25. Sit D, Wisner KL, Hanusa BH, et al. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918-927.
26. Goodwin GM. Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2009;23(4):346-388.
27. Lam RW, Levitt AJ, Levitan RD, et al. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry. 2006;163:805-812.
28. Pjrek E, Konstantinidis A, Assem-Hilger E, et al. Therapeutic effects of escitalopram and reboxetine in seasonal affective disorder: a pooled analysis. J Psychiatr Res. 2009;43(8):792-797.
29. Moscovitch A, Blashko CA, Eagles JM, et al. A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berl). 2004;171(4):390-397.
30. Rohan KJ, Roecklein KA, Tierney Lindsey K, et al. A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. J Consult Clin Psychol. 2007;75(3):489-500.
31. Rohan KJ. Coping with the seasons: a cognitive-behavioral approach to seasonal affective disorder. Therapist guide. New York, NY: Oxford University Press; 2008.
32. Rohan KJ, Roecklein KA, Lacy TJ, et al. Winter depression recurrence one year after cognitive-behavioral therapy, light therapy, or combination treatment. Behav Ther. 2009;40(3):225-238.
33. Frank E, Kupfer DJ, Ehlers CL, et al. Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behavior Therapist. 1994;17:143-149.
34. Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996-1004.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lam RW, Levitt AJ. eds. Clinical guidelines for the treatment of seasonal affective disorder. Vancouver, BC: Clinical and Academic Publishing; 1999.
3. Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72-80.
4. Michalak EE, Wilkinson C, Hood K, et al. Seasonal and nonseasonal depression: how do they differ? Symptom profile, clinical and family history in a general population sample. J Affect Disord. 2002;69(1-3):185-192.
5. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
6. Sohn CH, Lam RW. Treatment of seasonal affective disorder: unipolar versus bipolar differences. Curr Psychiatry Rep. 2004;6(6):478-485.
7. Goikolea JM, Colom F, Martinez-Aran A, et al. Clinical and prognostic implications of seasonal pattern in bipolar disorder: a 10-year follow-up of 302 patients. Psychol Med. 2007;37:1595-1599.
8. Kessler RC, Zhao S, Blazer DG, et al. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45(1-2):19-30.
9. Modell JG, Rosenthal NE, Harriett AE, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry. 2005;58:658-667.
10. Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002;68:25-31.
11. Shin K, Schaffer A, Levitt AJ, et al. Seasonality in a community sample of bipolar, unipolar and control subjects. J Affect Disord. 2005;86:19-25.
12. Wood J, Birmaher B, Axelson D, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166(2-3):201-209.
13. Soreca I, Frank E, Kupfer DJ. The phenomenology of bipolar disorder: what drives the high rate of medical burden and determines long-term prognosis? Depress Anxiety. 2009;26(1):73-82.
14. Terman M, Terman J, Rafferty B. Experimental design and measures of success in the treatment of winter depression by bright light. Psychopharmacol Bull. 1990;26(4):505-510.
15. Goel N, Terman M, Terman JS, et al. Summer mood in winter depressives: validation of a structured interview. Depress Anxiety. 1999;9:83-91.
16. Denicoff KD, Leverich GS, Nolen WA, et al. Validation of the prospective NIMH-Life-Chart Method (NIMH-LCM-p) for longitudinal assessment of bipolar illness. Psychol Med. 2000;30:1391-1397.
17. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity of a new Bipolar Spectrum Diagnostic Scale. J Affect Disord. 2005;84:273-277.
18. Phelps J, Angst J, Katzow J, et al. Validity and utility of bipolar spectrum models. Bipolar Disord. 2008;10:179-193.
19. Williams JB, Link MJ, Rosenthal NE, et al. Structured Interview Guide for the Hamilton Depression Rating Scale - Seasonal Affective Disorder Version (SIGH-SAD). New York, NY: New York State Psychiatric Institute; 1992.
20. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298-301.
21. Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162(4):656-662.
22. Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry. 1998;55(10):875-882.
23. Eastman CI, Young MA, Fogg LF, et al. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55(10):883-889.
24. Lewy AJ, Bauer VK, Cutler NL, et al. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55(10):890-896.
25. Sit D, Wisner KL, Hanusa BH, et al. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918-927.
26. Goodwin GM. Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2009;23(4):346-388.
27. Lam RW, Levitt AJ, Levitan RD, et al. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry. 2006;163:805-812.
28. Pjrek E, Konstantinidis A, Assem-Hilger E, et al. Therapeutic effects of escitalopram and reboxetine in seasonal affective disorder: a pooled analysis. J Psychiatr Res. 2009;43(8):792-797.
29. Moscovitch A, Blashko CA, Eagles JM, et al. A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology (Berl). 2004;171(4):390-397.
30. Rohan KJ, Roecklein KA, Tierney Lindsey K, et al. A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. J Consult Clin Psychol. 2007;75(3):489-500.
31. Rohan KJ. Coping with the seasons: a cognitive-behavioral approach to seasonal affective disorder. Therapist guide. New York, NY: Oxford University Press; 2008.
32. Rohan KJ, Roecklein KA, Lacy TJ, et al. Winter depression recurrence one year after cognitive-behavioral therapy, light therapy, or combination treatment. Behav Ther. 2009;40(3):225-238.
33. Frank E, Kupfer DJ, Ehlers CL, et al. Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behavior Therapist. 1994;17:143-149.
34. Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996-1004.
Managing medication and alcohol misuse by your older patients
As the eldest post-World War II “baby boomers” turn 64 this year, relaxed social attitudes about substance use during their lifetimes may predict an increasing risk for substance use disorders (SUDs) in older Americans.1 This presents challenges for psychiatric clinicians:
- Common screening tools used for younger patients might not adequately diagnose SUDs in patients clinically defined as elderly (age ≥65).
- DSM-IV-TR’s definition of substance use as causing clinically significant impairment or distress—such as occupational difficulties, legal problems, or decreased participation in social activities—might not apply to older patients, or these problems could be caused by other factors in older individuals.2
This article describes screening and treatment approaches shown to be most effective for identifying and managing primary SUDs in older patients. Our goal is to help you ask the right questions and provide appropriate care.
Phase-of-life issues
Most older adults have a primary care physician, but their SUDs often go unrecognized.3 Clinicians and family members might hesitate to ask about substance use or prescription medication misuse, and complications—such as falls or cognitive impairment—may be misattributed to normal aging. Thus, SUD screening of older individuals referred for psychiatric care is important.
Older adults respond with higher adherence rates when SUD treatment addresses age-specific issues—such as recent losses, medical problems, and challenges of keeping scheduled appointments or multiple providers/referrals. A combination of psychosocial and biologic treatments may be most beneficial. Although outcomes vary, some evidence indicates that age-specific programs for older alcoholics significantly improve abstinence rates at 6 and 12 months, compared with mixed-age programs4 ( see Related Resources ).
We recommend that you incorporate phase-of-life considerations at all stages of treatment. These include:
- education regarding lowered alcohol intake recommendations
- assessment tools that use criteria relevant to older adults
- treatment interventions that involve age-specific groups and programming.
Screening tools
In a routine office visit, a sensible approach is to screen for alcohol, tobacco, and prescription medication misuse. First-line screening tools for alcohol abuse include the AUDIT-5, CAGE, or MAST-G ( Table 1 ), accompanied by questions about medication side effects and observation of behavioral signs of medication misuse.
Alcohol use disorders. The spectrum of alcohol use disorders includes heavy drinking, hazardous use, harmful use, abuse, and dependence ( Table 2 ). Taking into account older adults’ physiology—these individuals have slower metabolism and smaller volume of distribution—National Institute on Alcohol Abuse and Alcoholism (NIAAA) alcohol consumption guidelines for the elderly differ from those for younger adults.
NIAAA guidelines for the elderly define hazardous use as >3 drinks in 1 sitting or >7 drinks in 1 week for both men and women. This is in comparison with guidelines for younger adults that define hazardous use as >5 drinks in 1 sitting (or >2 drinks/day) for men and >3 drinks in 1 sitting (or >1 drink/day) for women. The NIAAA recommendation considers a standard drink to be 12 oz of beer, 5 oz of wine, or 1.5 oz of distilled spirits, each drink containing approximately 0.5 oz of alcohol.5
Not all screening tools developed to assess alcohol use have been studied extensively in older cohorts,6 and some might not be useful in certain populations.7 The CAGE screening tool, for example—although easy to administer and widely studied—has low sensitivity in psychiatric populations, does not address past vs current drinking problems, and does not distinguish age-specific criteria for problem drinking.
Consider using instruments specific to an older individual’s comorbidities:
- the AUDIT-5 is appropriate for an older patient with psychiatric illness
- the ARPS (or the shorter shARPS) for an older individual with medical problems is likely to improve the rate of identifying problem drinkers.
Table 1
Comparing screening tools for alcohol use disorders in the elderly
| Screening tool | Characteristics | Clinical usefulness |
|---|---|---|
| CAGE | 4 items; self-report; most widely used/studied alcohol use screen; specificity > sensitivity | First-line; most useful if goal is to identify alcohol dependence; may miss misuse or hazardous use |
| AUDIT-5 | 5 items; self-report; specificity > sensitivity; a shortened version of the 10-item AUDIT | First-line; helpful for identifying hazardous use; sensitive for a broader spectrum of alcohol misuse than CAGE |
| MAST-G | 22-item yes/no self-report; questions specific to elderly | First-line; designed to identify a population that drinks less than heavy drinkers |
| SMAST-G | 10 items; shorter version of MAST-G with similar characteristics | Less sensitive and specific than MAST-G; may be useful when time is limited |
| Cyr-Wartman | 2-question screen (“Have you ever had a drinking problem?” “When was your last drink?”); specificity > sensitivity | Use for brief screening; follow up with more thorough screening in case of positive response |
| ARPS/shARPS | 18 items in ARPS (shARPS is shorter); self-report; classifies patients as nonhazardous, hazardous, or harmful drinkers; good sensitivity | Focuses on relationship of alcohol and medical problems, medication use, and functional status |
| ARPS/shARPS: Alcohol-Related Problems Survey/short version of ARPS; AUDIT-5: Alcohol Use Disorders Identification Test, 5-item version; CAGE: Cut down, Annoyed, Guilty, Eye opener; MAST-G: Michigan Alcoholism Screening Test—Geriatric version; SMAST-G: shorter version of MAST-G | ||
Table 2
Spectrum of alcohol use disorders: Heavy drinking to dependence
| Term | Definition | Recommended intervention for patients age ≥65 |
|---|---|---|
| Heavy drinking | >1 drink/day | Brief alcohol intervention |
| Hazardous use | >3 drinks in 1 sitting or >7 drinks/week; places patient at risk for adverse consequences | Brief alcohol intervention |
| Harmful use | Greater than hazardous use, with evidence of negative physical or psychological consequences | Brief alcohol intervention |
| Abuse | Signs of increasing use or decreasing functioning, including engaging in fewer activities, preoccupation with substance, continued use despite adverse consequences | Brief interventions (advise to cut down, educate regarding deleterious effects, and consider referral to substance abuse specialist for evaluation) |
| Dependence | Clear interference with daily function (such as increased falls, otherwise unexplained cognitive impairment); unsuccessful quit attempts; continued use despite adverse consequences | Refer to substance abuse specialist for treatment, including detoxification and age-specific rehabilitation program |
Drug abuse or medication misuse. Illegal drug use is relatively rare in the geriatric population,8 although the rates in patients age 50 to 59 increased from 2.7% in 2002 to 5.0% in 2007.9 In part this may reflect a higher lifetime use of illicit drugs by the baby boomers compared with previous generations.
Evidence also suggests an increasing risk for misuse and abuse of prescription drugs. One factor associated with this risk is medical exposure to prescription drugs with abuse potential. Among older adults in the United States:
- 10% are taking sedative-hypnotic medications
- 15% have been prescribed an opioid-analgesic medication.10
Other factors associated with prescription medication misuse and abuse by older adults include female sex, social isolation, history of substance use or psychiatric disorder, polypharmacy, and chronic medical problems.11
Very few screening instruments detect illicit drug use or prescription medication abuse. To screen older patients, ask about the drugs they are using (prescription and nonprescription), ask about side effects, and look for behavioral signs of medication misuse ( Table 3 ).12,13
Laboratory tests for alcohol’s metabolic effects can identify biologic markers of alcohol use disorders. An elevated mean corpuscular volume (MCV) or gamma-glutamyl transpeptidase (GGT) above the upper normal value can indicate possible problem drinking, even without considering total alcohol intake. Normal lab values are the same for older and younger adults.
Evidence suggests a poor association between findings of the CAGE questionnaire and MCV and GGT tests. Di Bari et al14 reported that biologic markers help identify older drinkers with compromised health status independent of a positive CAGE. This suggests that using a combination of tools to screen for psychosocial and biologic consequences could be more accurate than a single instrument in identifying older individuals with alcohol use disorders.14 We often use a GGT and MCV, along with the CAGE and the AUDIT-5 or SMAST-G.
Tobacco use. Smoking rates decrease with age, but this trend may reflect early mortality among tobacco users. Nicotine dependence remains a significant public health issue among the 7% to 9% of adults age ≥65 who smoke.15 An estimated 70% of all smokers want to quit, and 46% make an attempt each year.11
The single most important step in addressing tobacco use and dependence is screening. After asking about tobacco use and assessing the patient’s willingness to quit, you can provide appropriate interventions.16
Table 3
Behavioral signs of medication misuse by elderly patients
| Excessive worry about whether the medications are working |
| Strong attachment to a particular psychoactive medication |
| Resisting cessation or decreased doses of a prescribed psychoactive drug |
| Excessive anxiety about the supply and timing of medications |
| Decline in hygiene or grooming |
| Daytime sleeping |
| Medical symptoms such as fatigue, weight loss, or insomnia |
| Psychiatric symptoms such as irritability, memory problems, or depression |
| Source: References 12,13 |
Treatment options
General treatment options to consider for older patients with SUDs include a brief outpatient intervention, referral to a substance abuse specialist or inpatient treatment, and appropriate pharmacotherapy ( Table 4 ).
Brief interventions vary from relatively unstructured interactions in a physician’s office to more formal therapy. Components of these interventions include expression of concern, assessment and feedback, and direct advice. For older patients with SUDs, psychosocial approaches can improve treatment outcomes. One useful example—designed for alcohol use disorders—is the BRENDA model ( Table 5 ). Any trained health care staff member can administer this model, which is standardized with a comprehensive manual.17
Several brief intervention trials—including Project Guiding Older Adult Lifestyles (GOAL), the Health Profile Project, and the Staying Healthy Project—found that brief intervention results in significantly decreased alcohol consumption, sometimes even at 12-month follow-up.18 These trials were conducted in primary care settings, but brief interventions likely would be effective in psychiatric practice as well. Project GOAL included two 10- to 15-minute sessions with a physician scheduled 1 month apart and a follow-up phone call 2 weeks after each visit. The Health Profile Project consisted of a single motivational enhancement session.19
When to refer. Severe cases may require evaluation by a substance abuse specialist of the need for detoxification from alcohol, benzodiazepines, or opioids. Referral is appropriate if the patient has:
- a history of complicated withdrawal, including withdrawal seizures or delirium tremens
- complicated underlying medical conditions, such as severe coronary artery disease, uncontrolled hypertension, or uncontrolled diabetes.
Because of age-related physiologic changes, the older population is at risk for a more protracted withdrawal with more severe symptoms, compared with younger patients.20 Specialized care may include detoxification (outpatient or inpatient, depending on withdrawal symptom severity), day hospital program, or—in the case of a patient with a long history of substance use and multiple relapses—a longer-term residential program.
Table 4
Recommended treatments for substance use disorders in the elderly
| Disorder | Treatment |
|---|---|
| Hazardous use | Assess for withdrawal symptoms; brief intervention |
| Alcohol dependence | Assess for withdrawal symptoms; Alcoholics Anonymous; use of BRENDA model ( Table 5 ); pharmacotherapy (naltrexone, acamprosate); structured rehabilitation program with age-appropriate programming |
| Prescription medication misuse* | Assess for withdrawal symptoms; taper off medication (slowly and gradually); buprenorphine detoxification; brief intervention |
| Opioid dependence | Appropriate detoxification; drug-free trial; harm reduction approach with methadone or buprenorphine; age-appropriate psychosocial groups; Narcotics Anonymous |
| *Sedative-hypnotic and opioid pain medications (such as oxycodone HCl) | |
Table 5
The BRENDA model:
A brief psychosocial intervention for alcohol use disorders*
| Biopsychosocial evaluation |
| Reporting the assessment to the patient |
| Empathy |
| Needs identification |
| Direct advice |
| Assessment of patient reaction to the advice |
| *Any trained health care staff member can administer this model, which is standardized with a comprehensive manual |
| Source: Reference 17 |
Pharmacotherapy
Pharmacotherapy is an important component in the treatment of older adults with SUDs. Other elements include psychosocial interventions, brief interventions, cognitive-behavioral therapies, and supportive programs such as Alcoholics Anonymous or Narcotics Anonymous. Randomized controlled trials on the use of medications for SUDs in older patients are limited. As with any other medication trial in the elderly, start with the lowest possible dose and titrate slowly to treatment effect.
Alcohol use disorders. In our experience, naltrexone—an opioid antagonist—is the first-line agent to consider for alcohol dependence in older patients ( Table 4 ). Oslin et al21 found naltrexone, 50 mg/d, to be well-tolerated and effective in decreasing rates of relapse to heavy drinking in older adults.
Because of its potential hepatotoxic effects, use naltrexone with caution in patients with hepatic impairment. We recommend baseline liver function tests, with repeat testing in 3 to 6 months. Severe liver disease would be a contraindication for naltrexone, but consider risk vs benefit in individual patients.
Acamprosate—a glutamatergic medication—has been studied and approved for treating alcohol dependence in adults, although no study has specifically examined its use in elderly patients. Acamprosate may offer an alternative for patients with severe liver disease or those who can’t tolerate naltrexone.
Disulfiram is rarely used in the elderly because of potential risks of hypotension and cardiovascular adverse effects in a disulfiram-ethanol reaction. Topiramate—an anticonvulsant that potentiates gamma-aminobutyric acid—has shown benefit in treating initiation of abstinence from alcohol.22 It is an incompatible treatment for the elderly, however, because it may cause cognitive impairment.
Sedative-hypnotic misuse. The goal in treating patients who misuse sedatives or hypnotics is detoxification, which usually is addressed with a gradual and slow taper under controlled supervision in the outpatient setting.
Opioid dependence. Treatment options for opioid dependence are the same whether older patients are misusing prescription opioids or illicit ones such as heroin. Naltrexone, methadone, and buprenorphine/naloxone have been widely studied and used in younger adults but only minimally in the elderly.
Studies conducted in methadone maintenance clinics have found positive results when older patients are treated for opioid dependence:
- patients age ≥55 may have fewer problems and better outcomes with opioid treatment than younger patients23
- older age is 1 of only 2 variables (the other is no criminal justice involvement) found to be positively associated with longer duration in treatment.24
Older individuals are more sensitive than younger adults to the sedation and respiratory depression of opioids. Buprenorphine is the only opioid with a ceiling effect for respiratory depression, and it does not have an increased half-life in the elderly as do other opioids.25
Other potential side effects of these medications include urinary retention—particularly in elderly males with prostatic hyperplasia—constipation, and movement disorders.
Despite potential side effects, we find that opioid dependence is more successfully treated with agonist or partial agonist therapy than with blocking agents. Buprenorphine and methadone address urges and cravings to use opioids, resulting in greater treatment retention and longer abstinence. Buprenorphine treatment is available in office-based practices of physicians who have received training and certification.
Smoking cessation. Pharmacotherapy and brief treatment interventions can be effective and should be offered to the older smoker. Nicotine replacement therapy, bupropion, varenicline, and nortriptyline help improve quit rates in younger adults, but studies of these agents in older adults are limited.
If monotherapy fails, try combining shorter-acting nicotine replacement therapy with longer-acting agents such as bupropion or varenicline. To our knowledge no dosing adjustment is necessary for the elderly, although we recommend low starting doses with gradual titration.
Some literature suggests nortriptyline as a second-line smoking cessation agent in the elderly. We do not recommend nortriptyline for smoking cessation in this population, however, because of tricyclic antidepressants’ cardiac effects.
- Schultz SK, Arndt S, Liesved J. Locations of facilities with special programs for older substance abuse clients in the U.S. Int J Geriatr Psychiatry. 2003;18(9):839-843.
- National Association of Addiction Treatment Providers. www.naatp.org.
- National Association of State Alcohol/Drug Abuse Directors. www.nasadad.org.
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
- American Academy of Addiction Psychiatry. www.aaap.org.
Drug brand names
- Acamprosate • Campral
- Buprenorphine/naloxone • Suboxone
- Bupropion • Zyban
- Disulfiram • Antabuse
- Methadone • Dolophine, Methadose
- Naltrexone • ReVia
- Nortriptyline • Aventyl, Pamelor
- Oxycodone • OxyContin, Roxicodone, others
- Topiramate • Topamax
- Varenicline • Chantix
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Patterson TL, Jeste DV. The potential impact of the baby boom generation on substance abuse among elderly persons. Psychiatr Serv. 1999;50(9):1184-1188.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Oslin D, Pettinati H, Volpicelli J. Alcoholism treatment adherence. Older age predicts better adherence and drinking outcomes. Am J Geriatr Psychiatry. 2002;10(6):740-747.
4. Kashner TM, Rodell DI, Ogden SR, et al. Outcomes and costs of two VA inpatient treatment programs for older alcoholic patients. Hosp Community Psychiatry. 1992;43:985-989.
5. Alcohol and aging. Alcohol Alert, issue 40; April 1998. National Institute on Alcohol Abuse and Alcoholism. National Institutes of Health. Available at: http://pubs.niaaa.nih.gov/publications/aa40.htm. Accessed November 19, 2009.
6. Cyr MG, Wartman SA. The effectiveness of routine screening questions in the detection of alcoholism. JAMA. 1988;259(1):51-54.
7. O’Connell H, Chin AV, Hamilton F, et al. A systematic review of the utility of self-report alcohol screening instruments in the elderly. Int J Geriatr Psychiatry. 2004;19:1074-1086.
8. Simoni-Wastila L, Yang HK. Psychoactive drug abuse in older adults. Am J Geriatr Pharmacother. 2006;4:380-394.
9. Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2008. DHHS Publication SMA08-4343, NSDUH Series H-34.
10. Simoni-Wastila L, Zuckerman IH, Singhal PK, et al. National estimates of exposure to prescription drugs with addiction potential in community-dwelling elders. Subst Abus. 2005;26:33-42.
11. Reducing tobacco use: a report of the Surgeon General. Office of the Surgeon General. Public Health Service. U.S. Department of Health and Human Services. August 9, 2000. Available at: http://www.surgeongeneral.gov/library/tobacco_use. Accessed November 19, 2009.
12. Blow FC. Substance abuse among older adults. Treatment improvement protocol (TIP) series 26. Substance Abuse and Mental Health Services Administration. Public Health Service. U.S. Department of Health and Human Services. Rockville, MD: Center for Substance Abuse Treatment; June 1998. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hssamhsatip&part=A48302. Accessed November 19, 2009.
13. Finfgeld-Connett DL. Treatment of substance misuse in older women: using a brief intervention model. J Gerontol Nurs. 2004;30(8):30-37.
14. Di Bari M, Silvestrini G, Chiarlone M, et al. Features of excessive alcohol drinking in older adults distinctively captured by behavioral and biological screening instruments. An epidemiological study. J Clin Epidemiol. 2002;55:41-47.
15. Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2007. MMWR. 2008;57(45):1221-1226.
16. Treating tobacco use and dependence: 2008 update. Office of the Surgeon General. Public Health Service. U.S. Department of Health and Human Services. Available at: http://www.surgeongeneral.gov/tobacco. Accessed November 19, 2009.
17. Starosta A, Leeman R, Volpicelli J. The BRENDA model: integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. J Psychiatr Pract. 2006;12(2):80-89.
18. Fleming M, Manuwell L, Barry K, et al. Brief physician advice for alcohol problems in older adults: a randomized community-based trial. J Fam Pract. 1999;48(5):378-384.
19. Barry KL, Blow FC, Cullinane P, et al. The effectiveness of implementing a brief alcohol intervention with older adults in community settings. Washington, DC: National Council on Aging; 2006.
20. Oslin D. Evidence-based treatment of geriatric substance abuse. Psychiatr Clin North Am. 2005;28:897-911.
21. Oslin D, Liberto JG, O’Brien J, et al. Naltrexone as an adjunctive treatment for older patients with alcohol dependence. Am J Geriatr Psychiatry. 1997;5:324-332.
22. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treatment of alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-1651.
23. Firoz S, Carlson G. Characteristics and treatment outcome of older methadone-maintenance patients. Am J Geriatr Psychiatry. 2004;12(5):539-541.
24. Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51-60.
25. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287-313.
As the eldest post-World War II “baby boomers” turn 64 this year, relaxed social attitudes about substance use during their lifetimes may predict an increasing risk for substance use disorders (SUDs) in older Americans.1 This presents challenges for psychiatric clinicians:
- Common screening tools used for younger patients might not adequately diagnose SUDs in patients clinically defined as elderly (age ≥65).
- DSM-IV-TR’s definition of substance use as causing clinically significant impairment or distress—such as occupational difficulties, legal problems, or decreased participation in social activities—might not apply to older patients, or these problems could be caused by other factors in older individuals.2
This article describes screening and treatment approaches shown to be most effective for identifying and managing primary SUDs in older patients. Our goal is to help you ask the right questions and provide appropriate care.
Phase-of-life issues
Most older adults have a primary care physician, but their SUDs often go unrecognized.3 Clinicians and family members might hesitate to ask about substance use or prescription medication misuse, and complications—such as falls or cognitive impairment—may be misattributed to normal aging. Thus, SUD screening of older individuals referred for psychiatric care is important.
Older adults respond with higher adherence rates when SUD treatment addresses age-specific issues—such as recent losses, medical problems, and challenges of keeping scheduled appointments or multiple providers/referrals. A combination of psychosocial and biologic treatments may be most beneficial. Although outcomes vary, some evidence indicates that age-specific programs for older alcoholics significantly improve abstinence rates at 6 and 12 months, compared with mixed-age programs4 ( see Related Resources ).
We recommend that you incorporate phase-of-life considerations at all stages of treatment. These include:
- education regarding lowered alcohol intake recommendations
- assessment tools that use criteria relevant to older adults
- treatment interventions that involve age-specific groups and programming.
Screening tools
In a routine office visit, a sensible approach is to screen for alcohol, tobacco, and prescription medication misuse. First-line screening tools for alcohol abuse include the AUDIT-5, CAGE, or MAST-G ( Table 1 ), accompanied by questions about medication side effects and observation of behavioral signs of medication misuse.
Alcohol use disorders. The spectrum of alcohol use disorders includes heavy drinking, hazardous use, harmful use, abuse, and dependence ( Table 2 ). Taking into account older adults’ physiology—these individuals have slower metabolism and smaller volume of distribution—National Institute on Alcohol Abuse and Alcoholism (NIAAA) alcohol consumption guidelines for the elderly differ from those for younger adults.
NIAAA guidelines for the elderly define hazardous use as >3 drinks in 1 sitting or >7 drinks in 1 week for both men and women. This is in comparison with guidelines for younger adults that define hazardous use as >5 drinks in 1 sitting (or >2 drinks/day) for men and >3 drinks in 1 sitting (or >1 drink/day) for women. The NIAAA recommendation considers a standard drink to be 12 oz of beer, 5 oz of wine, or 1.5 oz of distilled spirits, each drink containing approximately 0.5 oz of alcohol.5
Not all screening tools developed to assess alcohol use have been studied extensively in older cohorts,6 and some might not be useful in certain populations.7 The CAGE screening tool, for example—although easy to administer and widely studied—has low sensitivity in psychiatric populations, does not address past vs current drinking problems, and does not distinguish age-specific criteria for problem drinking.
Consider using instruments specific to an older individual’s comorbidities:
- the AUDIT-5 is appropriate for an older patient with psychiatric illness
- the ARPS (or the shorter shARPS) for an older individual with medical problems is likely to improve the rate of identifying problem drinkers.
Table 1
Comparing screening tools for alcohol use disorders in the elderly
| Screening tool | Characteristics | Clinical usefulness |
|---|---|---|
| CAGE | 4 items; self-report; most widely used/studied alcohol use screen; specificity > sensitivity | First-line; most useful if goal is to identify alcohol dependence; may miss misuse or hazardous use |
| AUDIT-5 | 5 items; self-report; specificity > sensitivity; a shortened version of the 10-item AUDIT | First-line; helpful for identifying hazardous use; sensitive for a broader spectrum of alcohol misuse than CAGE |
| MAST-G | 22-item yes/no self-report; questions specific to elderly | First-line; designed to identify a population that drinks less than heavy drinkers |
| SMAST-G | 10 items; shorter version of MAST-G with similar characteristics | Less sensitive and specific than MAST-G; may be useful when time is limited |
| Cyr-Wartman | 2-question screen (“Have you ever had a drinking problem?” “When was your last drink?”); specificity > sensitivity | Use for brief screening; follow up with more thorough screening in case of positive response |
| ARPS/shARPS | 18 items in ARPS (shARPS is shorter); self-report; classifies patients as nonhazardous, hazardous, or harmful drinkers; good sensitivity | Focuses on relationship of alcohol and medical problems, medication use, and functional status |
| ARPS/shARPS: Alcohol-Related Problems Survey/short version of ARPS; AUDIT-5: Alcohol Use Disorders Identification Test, 5-item version; CAGE: Cut down, Annoyed, Guilty, Eye opener; MAST-G: Michigan Alcoholism Screening Test—Geriatric version; SMAST-G: shorter version of MAST-G | ||
Table 2
Spectrum of alcohol use disorders: Heavy drinking to dependence
| Term | Definition | Recommended intervention for patients age ≥65 |
|---|---|---|
| Heavy drinking | >1 drink/day | Brief alcohol intervention |
| Hazardous use | >3 drinks in 1 sitting or >7 drinks/week; places patient at risk for adverse consequences | Brief alcohol intervention |
| Harmful use | Greater than hazardous use, with evidence of negative physical or psychological consequences | Brief alcohol intervention |
| Abuse | Signs of increasing use or decreasing functioning, including engaging in fewer activities, preoccupation with substance, continued use despite adverse consequences | Brief interventions (advise to cut down, educate regarding deleterious effects, and consider referral to substance abuse specialist for evaluation) |
| Dependence | Clear interference with daily function (such as increased falls, otherwise unexplained cognitive impairment); unsuccessful quit attempts; continued use despite adverse consequences | Refer to substance abuse specialist for treatment, including detoxification and age-specific rehabilitation program |
Drug abuse or medication misuse. Illegal drug use is relatively rare in the geriatric population,8 although the rates in patients age 50 to 59 increased from 2.7% in 2002 to 5.0% in 2007.9 In part this may reflect a higher lifetime use of illicit drugs by the baby boomers compared with previous generations.
Evidence also suggests an increasing risk for misuse and abuse of prescription drugs. One factor associated with this risk is medical exposure to prescription drugs with abuse potential. Among older adults in the United States:
- 10% are taking sedative-hypnotic medications
- 15% have been prescribed an opioid-analgesic medication.10
Other factors associated with prescription medication misuse and abuse by older adults include female sex, social isolation, history of substance use or psychiatric disorder, polypharmacy, and chronic medical problems.11
Very few screening instruments detect illicit drug use or prescription medication abuse. To screen older patients, ask about the drugs they are using (prescription and nonprescription), ask about side effects, and look for behavioral signs of medication misuse ( Table 3 ).12,13
Laboratory tests for alcohol’s metabolic effects can identify biologic markers of alcohol use disorders. An elevated mean corpuscular volume (MCV) or gamma-glutamyl transpeptidase (GGT) above the upper normal value can indicate possible problem drinking, even without considering total alcohol intake. Normal lab values are the same for older and younger adults.
Evidence suggests a poor association between findings of the CAGE questionnaire and MCV and GGT tests. Di Bari et al14 reported that biologic markers help identify older drinkers with compromised health status independent of a positive CAGE. This suggests that using a combination of tools to screen for psychosocial and biologic consequences could be more accurate than a single instrument in identifying older individuals with alcohol use disorders.14 We often use a GGT and MCV, along with the CAGE and the AUDIT-5 or SMAST-G.
Tobacco use. Smoking rates decrease with age, but this trend may reflect early mortality among tobacco users. Nicotine dependence remains a significant public health issue among the 7% to 9% of adults age ≥65 who smoke.15 An estimated 70% of all smokers want to quit, and 46% make an attempt each year.11
The single most important step in addressing tobacco use and dependence is screening. After asking about tobacco use and assessing the patient’s willingness to quit, you can provide appropriate interventions.16
Table 3
Behavioral signs of medication misuse by elderly patients
| Excessive worry about whether the medications are working |
| Strong attachment to a particular psychoactive medication |
| Resisting cessation or decreased doses of a prescribed psychoactive drug |
| Excessive anxiety about the supply and timing of medications |
| Decline in hygiene or grooming |
| Daytime sleeping |
| Medical symptoms such as fatigue, weight loss, or insomnia |
| Psychiatric symptoms such as irritability, memory problems, or depression |
| Source: References 12,13 |
Treatment options
General treatment options to consider for older patients with SUDs include a brief outpatient intervention, referral to a substance abuse specialist or inpatient treatment, and appropriate pharmacotherapy ( Table 4 ).
Brief interventions vary from relatively unstructured interactions in a physician’s office to more formal therapy. Components of these interventions include expression of concern, assessment and feedback, and direct advice. For older patients with SUDs, psychosocial approaches can improve treatment outcomes. One useful example—designed for alcohol use disorders—is the BRENDA model ( Table 5 ). Any trained health care staff member can administer this model, which is standardized with a comprehensive manual.17
Several brief intervention trials—including Project Guiding Older Adult Lifestyles (GOAL), the Health Profile Project, and the Staying Healthy Project—found that brief intervention results in significantly decreased alcohol consumption, sometimes even at 12-month follow-up.18 These trials were conducted in primary care settings, but brief interventions likely would be effective in psychiatric practice as well. Project GOAL included two 10- to 15-minute sessions with a physician scheduled 1 month apart and a follow-up phone call 2 weeks after each visit. The Health Profile Project consisted of a single motivational enhancement session.19
When to refer. Severe cases may require evaluation by a substance abuse specialist of the need for detoxification from alcohol, benzodiazepines, or opioids. Referral is appropriate if the patient has:
- a history of complicated withdrawal, including withdrawal seizures or delirium tremens
- complicated underlying medical conditions, such as severe coronary artery disease, uncontrolled hypertension, or uncontrolled diabetes.
Because of age-related physiologic changes, the older population is at risk for a more protracted withdrawal with more severe symptoms, compared with younger patients.20 Specialized care may include detoxification (outpatient or inpatient, depending on withdrawal symptom severity), day hospital program, or—in the case of a patient with a long history of substance use and multiple relapses—a longer-term residential program.
Table 4
Recommended treatments for substance use disorders in the elderly
| Disorder | Treatment |
|---|---|
| Hazardous use | Assess for withdrawal symptoms; brief intervention |
| Alcohol dependence | Assess for withdrawal symptoms; Alcoholics Anonymous; use of BRENDA model ( Table 5 ); pharmacotherapy (naltrexone, acamprosate); structured rehabilitation program with age-appropriate programming |
| Prescription medication misuse* | Assess for withdrawal symptoms; taper off medication (slowly and gradually); buprenorphine detoxification; brief intervention |
| Opioid dependence | Appropriate detoxification; drug-free trial; harm reduction approach with methadone or buprenorphine; age-appropriate psychosocial groups; Narcotics Anonymous |
| *Sedative-hypnotic and opioid pain medications (such as oxycodone HCl) | |
Table 5
The BRENDA model:
A brief psychosocial intervention for alcohol use disorders*
| Biopsychosocial evaluation |
| Reporting the assessment to the patient |
| Empathy |
| Needs identification |
| Direct advice |
| Assessment of patient reaction to the advice |
| *Any trained health care staff member can administer this model, which is standardized with a comprehensive manual |
| Source: Reference 17 |
Pharmacotherapy
Pharmacotherapy is an important component in the treatment of older adults with SUDs. Other elements include psychosocial interventions, brief interventions, cognitive-behavioral therapies, and supportive programs such as Alcoholics Anonymous or Narcotics Anonymous. Randomized controlled trials on the use of medications for SUDs in older patients are limited. As with any other medication trial in the elderly, start with the lowest possible dose and titrate slowly to treatment effect.
Alcohol use disorders. In our experience, naltrexone—an opioid antagonist—is the first-line agent to consider for alcohol dependence in older patients ( Table 4 ). Oslin et al21 found naltrexone, 50 mg/d, to be well-tolerated and effective in decreasing rates of relapse to heavy drinking in older adults.
Because of its potential hepatotoxic effects, use naltrexone with caution in patients with hepatic impairment. We recommend baseline liver function tests, with repeat testing in 3 to 6 months. Severe liver disease would be a contraindication for naltrexone, but consider risk vs benefit in individual patients.
Acamprosate—a glutamatergic medication—has been studied and approved for treating alcohol dependence in adults, although no study has specifically examined its use in elderly patients. Acamprosate may offer an alternative for patients with severe liver disease or those who can’t tolerate naltrexone.
Disulfiram is rarely used in the elderly because of potential risks of hypotension and cardiovascular adverse effects in a disulfiram-ethanol reaction. Topiramate—an anticonvulsant that potentiates gamma-aminobutyric acid—has shown benefit in treating initiation of abstinence from alcohol.22 It is an incompatible treatment for the elderly, however, because it may cause cognitive impairment.
Sedative-hypnotic misuse. The goal in treating patients who misuse sedatives or hypnotics is detoxification, which usually is addressed with a gradual and slow taper under controlled supervision in the outpatient setting.
Opioid dependence. Treatment options for opioid dependence are the same whether older patients are misusing prescription opioids or illicit ones such as heroin. Naltrexone, methadone, and buprenorphine/naloxone have been widely studied and used in younger adults but only minimally in the elderly.
Studies conducted in methadone maintenance clinics have found positive results when older patients are treated for opioid dependence:
- patients age ≥55 may have fewer problems and better outcomes with opioid treatment than younger patients23
- older age is 1 of only 2 variables (the other is no criminal justice involvement) found to be positively associated with longer duration in treatment.24
Older individuals are more sensitive than younger adults to the sedation and respiratory depression of opioids. Buprenorphine is the only opioid with a ceiling effect for respiratory depression, and it does not have an increased half-life in the elderly as do other opioids.25
Other potential side effects of these medications include urinary retention—particularly in elderly males with prostatic hyperplasia—constipation, and movement disorders.
Despite potential side effects, we find that opioid dependence is more successfully treated with agonist or partial agonist therapy than with blocking agents. Buprenorphine and methadone address urges and cravings to use opioids, resulting in greater treatment retention and longer abstinence. Buprenorphine treatment is available in office-based practices of physicians who have received training and certification.
Smoking cessation. Pharmacotherapy and brief treatment interventions can be effective and should be offered to the older smoker. Nicotine replacement therapy, bupropion, varenicline, and nortriptyline help improve quit rates in younger adults, but studies of these agents in older adults are limited.
If monotherapy fails, try combining shorter-acting nicotine replacement therapy with longer-acting agents such as bupropion or varenicline. To our knowledge no dosing adjustment is necessary for the elderly, although we recommend low starting doses with gradual titration.
Some literature suggests nortriptyline as a second-line smoking cessation agent in the elderly. We do not recommend nortriptyline for smoking cessation in this population, however, because of tricyclic antidepressants’ cardiac effects.
- Schultz SK, Arndt S, Liesved J. Locations of facilities with special programs for older substance abuse clients in the U.S. Int J Geriatr Psychiatry. 2003;18(9):839-843.
- National Association of Addiction Treatment Providers. www.naatp.org.
- National Association of State Alcohol/Drug Abuse Directors. www.nasadad.org.
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
- American Academy of Addiction Psychiatry. www.aaap.org.
Drug brand names
- Acamprosate • Campral
- Buprenorphine/naloxone • Suboxone
- Bupropion • Zyban
- Disulfiram • Antabuse
- Methadone • Dolophine, Methadose
- Naltrexone • ReVia
- Nortriptyline • Aventyl, Pamelor
- Oxycodone • OxyContin, Roxicodone, others
- Topiramate • Topamax
- Varenicline • Chantix
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
As the eldest post-World War II “baby boomers” turn 64 this year, relaxed social attitudes about substance use during their lifetimes may predict an increasing risk for substance use disorders (SUDs) in older Americans.1 This presents challenges for psychiatric clinicians:
- Common screening tools used for younger patients might not adequately diagnose SUDs in patients clinically defined as elderly (age ≥65).
- DSM-IV-TR’s definition of substance use as causing clinically significant impairment or distress—such as occupational difficulties, legal problems, or decreased participation in social activities—might not apply to older patients, or these problems could be caused by other factors in older individuals.2
This article describes screening and treatment approaches shown to be most effective for identifying and managing primary SUDs in older patients. Our goal is to help you ask the right questions and provide appropriate care.
Phase-of-life issues
Most older adults have a primary care physician, but their SUDs often go unrecognized.3 Clinicians and family members might hesitate to ask about substance use or prescription medication misuse, and complications—such as falls or cognitive impairment—may be misattributed to normal aging. Thus, SUD screening of older individuals referred for psychiatric care is important.
Older adults respond with higher adherence rates when SUD treatment addresses age-specific issues—such as recent losses, medical problems, and challenges of keeping scheduled appointments or multiple providers/referrals. A combination of psychosocial and biologic treatments may be most beneficial. Although outcomes vary, some evidence indicates that age-specific programs for older alcoholics significantly improve abstinence rates at 6 and 12 months, compared with mixed-age programs4 ( see Related Resources ).
We recommend that you incorporate phase-of-life considerations at all stages of treatment. These include:
- education regarding lowered alcohol intake recommendations
- assessment tools that use criteria relevant to older adults
- treatment interventions that involve age-specific groups and programming.
Screening tools
In a routine office visit, a sensible approach is to screen for alcohol, tobacco, and prescription medication misuse. First-line screening tools for alcohol abuse include the AUDIT-5, CAGE, or MAST-G ( Table 1 ), accompanied by questions about medication side effects and observation of behavioral signs of medication misuse.
Alcohol use disorders. The spectrum of alcohol use disorders includes heavy drinking, hazardous use, harmful use, abuse, and dependence ( Table 2 ). Taking into account older adults’ physiology—these individuals have slower metabolism and smaller volume of distribution—National Institute on Alcohol Abuse and Alcoholism (NIAAA) alcohol consumption guidelines for the elderly differ from those for younger adults.
NIAAA guidelines for the elderly define hazardous use as >3 drinks in 1 sitting or >7 drinks in 1 week for both men and women. This is in comparison with guidelines for younger adults that define hazardous use as >5 drinks in 1 sitting (or >2 drinks/day) for men and >3 drinks in 1 sitting (or >1 drink/day) for women. The NIAAA recommendation considers a standard drink to be 12 oz of beer, 5 oz of wine, or 1.5 oz of distilled spirits, each drink containing approximately 0.5 oz of alcohol.5
Not all screening tools developed to assess alcohol use have been studied extensively in older cohorts,6 and some might not be useful in certain populations.7 The CAGE screening tool, for example—although easy to administer and widely studied—has low sensitivity in psychiatric populations, does not address past vs current drinking problems, and does not distinguish age-specific criteria for problem drinking.
Consider using instruments specific to an older individual’s comorbidities:
- the AUDIT-5 is appropriate for an older patient with psychiatric illness
- the ARPS (or the shorter shARPS) for an older individual with medical problems is likely to improve the rate of identifying problem drinkers.
Table 1
Comparing screening tools for alcohol use disorders in the elderly
| Screening tool | Characteristics | Clinical usefulness |
|---|---|---|
| CAGE | 4 items; self-report; most widely used/studied alcohol use screen; specificity > sensitivity | First-line; most useful if goal is to identify alcohol dependence; may miss misuse or hazardous use |
| AUDIT-5 | 5 items; self-report; specificity > sensitivity; a shortened version of the 10-item AUDIT | First-line; helpful for identifying hazardous use; sensitive for a broader spectrum of alcohol misuse than CAGE |
| MAST-G | 22-item yes/no self-report; questions specific to elderly | First-line; designed to identify a population that drinks less than heavy drinkers |
| SMAST-G | 10 items; shorter version of MAST-G with similar characteristics | Less sensitive and specific than MAST-G; may be useful when time is limited |
| Cyr-Wartman | 2-question screen (“Have you ever had a drinking problem?” “When was your last drink?”); specificity > sensitivity | Use for brief screening; follow up with more thorough screening in case of positive response |
| ARPS/shARPS | 18 items in ARPS (shARPS is shorter); self-report; classifies patients as nonhazardous, hazardous, or harmful drinkers; good sensitivity | Focuses on relationship of alcohol and medical problems, medication use, and functional status |
| ARPS/shARPS: Alcohol-Related Problems Survey/short version of ARPS; AUDIT-5: Alcohol Use Disorders Identification Test, 5-item version; CAGE: Cut down, Annoyed, Guilty, Eye opener; MAST-G: Michigan Alcoholism Screening Test—Geriatric version; SMAST-G: shorter version of MAST-G | ||
Table 2
Spectrum of alcohol use disorders: Heavy drinking to dependence
| Term | Definition | Recommended intervention for patients age ≥65 |
|---|---|---|
| Heavy drinking | >1 drink/day | Brief alcohol intervention |
| Hazardous use | >3 drinks in 1 sitting or >7 drinks/week; places patient at risk for adverse consequences | Brief alcohol intervention |
| Harmful use | Greater than hazardous use, with evidence of negative physical or psychological consequences | Brief alcohol intervention |
| Abuse | Signs of increasing use or decreasing functioning, including engaging in fewer activities, preoccupation with substance, continued use despite adverse consequences | Brief interventions (advise to cut down, educate regarding deleterious effects, and consider referral to substance abuse specialist for evaluation) |
| Dependence | Clear interference with daily function (such as increased falls, otherwise unexplained cognitive impairment); unsuccessful quit attempts; continued use despite adverse consequences | Refer to substance abuse specialist for treatment, including detoxification and age-specific rehabilitation program |
Drug abuse or medication misuse. Illegal drug use is relatively rare in the geriatric population,8 although the rates in patients age 50 to 59 increased from 2.7% in 2002 to 5.0% in 2007.9 In part this may reflect a higher lifetime use of illicit drugs by the baby boomers compared with previous generations.
Evidence also suggests an increasing risk for misuse and abuse of prescription drugs. One factor associated with this risk is medical exposure to prescription drugs with abuse potential. Among older adults in the United States:
- 10% are taking sedative-hypnotic medications
- 15% have been prescribed an opioid-analgesic medication.10
Other factors associated with prescription medication misuse and abuse by older adults include female sex, social isolation, history of substance use or psychiatric disorder, polypharmacy, and chronic medical problems.11
Very few screening instruments detect illicit drug use or prescription medication abuse. To screen older patients, ask about the drugs they are using (prescription and nonprescription), ask about side effects, and look for behavioral signs of medication misuse ( Table 3 ).12,13
Laboratory tests for alcohol’s metabolic effects can identify biologic markers of alcohol use disorders. An elevated mean corpuscular volume (MCV) or gamma-glutamyl transpeptidase (GGT) above the upper normal value can indicate possible problem drinking, even without considering total alcohol intake. Normal lab values are the same for older and younger adults.
Evidence suggests a poor association between findings of the CAGE questionnaire and MCV and GGT tests. Di Bari et al14 reported that biologic markers help identify older drinkers with compromised health status independent of a positive CAGE. This suggests that using a combination of tools to screen for psychosocial and biologic consequences could be more accurate than a single instrument in identifying older individuals with alcohol use disorders.14 We often use a GGT and MCV, along with the CAGE and the AUDIT-5 or SMAST-G.
Tobacco use. Smoking rates decrease with age, but this trend may reflect early mortality among tobacco users. Nicotine dependence remains a significant public health issue among the 7% to 9% of adults age ≥65 who smoke.15 An estimated 70% of all smokers want to quit, and 46% make an attempt each year.11
The single most important step in addressing tobacco use and dependence is screening. After asking about tobacco use and assessing the patient’s willingness to quit, you can provide appropriate interventions.16
Table 3
Behavioral signs of medication misuse by elderly patients
| Excessive worry about whether the medications are working |
| Strong attachment to a particular psychoactive medication |
| Resisting cessation or decreased doses of a prescribed psychoactive drug |
| Excessive anxiety about the supply and timing of medications |
| Decline in hygiene or grooming |
| Daytime sleeping |
| Medical symptoms such as fatigue, weight loss, or insomnia |
| Psychiatric symptoms such as irritability, memory problems, or depression |
| Source: References 12,13 |
Treatment options
General treatment options to consider for older patients with SUDs include a brief outpatient intervention, referral to a substance abuse specialist or inpatient treatment, and appropriate pharmacotherapy ( Table 4 ).
Brief interventions vary from relatively unstructured interactions in a physician’s office to more formal therapy. Components of these interventions include expression of concern, assessment and feedback, and direct advice. For older patients with SUDs, psychosocial approaches can improve treatment outcomes. One useful example—designed for alcohol use disorders—is the BRENDA model ( Table 5 ). Any trained health care staff member can administer this model, which is standardized with a comprehensive manual.17
Several brief intervention trials—including Project Guiding Older Adult Lifestyles (GOAL), the Health Profile Project, and the Staying Healthy Project—found that brief intervention results in significantly decreased alcohol consumption, sometimes even at 12-month follow-up.18 These trials were conducted in primary care settings, but brief interventions likely would be effective in psychiatric practice as well. Project GOAL included two 10- to 15-minute sessions with a physician scheduled 1 month apart and a follow-up phone call 2 weeks after each visit. The Health Profile Project consisted of a single motivational enhancement session.19
When to refer. Severe cases may require evaluation by a substance abuse specialist of the need for detoxification from alcohol, benzodiazepines, or opioids. Referral is appropriate if the patient has:
- a history of complicated withdrawal, including withdrawal seizures or delirium tremens
- complicated underlying medical conditions, such as severe coronary artery disease, uncontrolled hypertension, or uncontrolled diabetes.
Because of age-related physiologic changes, the older population is at risk for a more protracted withdrawal with more severe symptoms, compared with younger patients.20 Specialized care may include detoxification (outpatient or inpatient, depending on withdrawal symptom severity), day hospital program, or—in the case of a patient with a long history of substance use and multiple relapses—a longer-term residential program.
Table 4
Recommended treatments for substance use disorders in the elderly
| Disorder | Treatment |
|---|---|
| Hazardous use | Assess for withdrawal symptoms; brief intervention |
| Alcohol dependence | Assess for withdrawal symptoms; Alcoholics Anonymous; use of BRENDA model ( Table 5 ); pharmacotherapy (naltrexone, acamprosate); structured rehabilitation program with age-appropriate programming |
| Prescription medication misuse* | Assess for withdrawal symptoms; taper off medication (slowly and gradually); buprenorphine detoxification; brief intervention |
| Opioid dependence | Appropriate detoxification; drug-free trial; harm reduction approach with methadone or buprenorphine; age-appropriate psychosocial groups; Narcotics Anonymous |
| *Sedative-hypnotic and opioid pain medications (such as oxycodone HCl) | |
Table 5
The BRENDA model:
A brief psychosocial intervention for alcohol use disorders*
| Biopsychosocial evaluation |
| Reporting the assessment to the patient |
| Empathy |
| Needs identification |
| Direct advice |
| Assessment of patient reaction to the advice |
| *Any trained health care staff member can administer this model, which is standardized with a comprehensive manual |
| Source: Reference 17 |
Pharmacotherapy
Pharmacotherapy is an important component in the treatment of older adults with SUDs. Other elements include psychosocial interventions, brief interventions, cognitive-behavioral therapies, and supportive programs such as Alcoholics Anonymous or Narcotics Anonymous. Randomized controlled trials on the use of medications for SUDs in older patients are limited. As with any other medication trial in the elderly, start with the lowest possible dose and titrate slowly to treatment effect.
Alcohol use disorders. In our experience, naltrexone—an opioid antagonist—is the first-line agent to consider for alcohol dependence in older patients ( Table 4 ). Oslin et al21 found naltrexone, 50 mg/d, to be well-tolerated and effective in decreasing rates of relapse to heavy drinking in older adults.
Because of its potential hepatotoxic effects, use naltrexone with caution in patients with hepatic impairment. We recommend baseline liver function tests, with repeat testing in 3 to 6 months. Severe liver disease would be a contraindication for naltrexone, but consider risk vs benefit in individual patients.
Acamprosate—a glutamatergic medication—has been studied and approved for treating alcohol dependence in adults, although no study has specifically examined its use in elderly patients. Acamprosate may offer an alternative for patients with severe liver disease or those who can’t tolerate naltrexone.
Disulfiram is rarely used in the elderly because of potential risks of hypotension and cardiovascular adverse effects in a disulfiram-ethanol reaction. Topiramate—an anticonvulsant that potentiates gamma-aminobutyric acid—has shown benefit in treating initiation of abstinence from alcohol.22 It is an incompatible treatment for the elderly, however, because it may cause cognitive impairment.
Sedative-hypnotic misuse. The goal in treating patients who misuse sedatives or hypnotics is detoxification, which usually is addressed with a gradual and slow taper under controlled supervision in the outpatient setting.
Opioid dependence. Treatment options for opioid dependence are the same whether older patients are misusing prescription opioids or illicit ones such as heroin. Naltrexone, methadone, and buprenorphine/naloxone have been widely studied and used in younger adults but only minimally in the elderly.
Studies conducted in methadone maintenance clinics have found positive results when older patients are treated for opioid dependence:
- patients age ≥55 may have fewer problems and better outcomes with opioid treatment than younger patients23
- older age is 1 of only 2 variables (the other is no criminal justice involvement) found to be positively associated with longer duration in treatment.24
Older individuals are more sensitive than younger adults to the sedation and respiratory depression of opioids. Buprenorphine is the only opioid with a ceiling effect for respiratory depression, and it does not have an increased half-life in the elderly as do other opioids.25
Other potential side effects of these medications include urinary retention—particularly in elderly males with prostatic hyperplasia—constipation, and movement disorders.
Despite potential side effects, we find that opioid dependence is more successfully treated with agonist or partial agonist therapy than with blocking agents. Buprenorphine and methadone address urges and cravings to use opioids, resulting in greater treatment retention and longer abstinence. Buprenorphine treatment is available in office-based practices of physicians who have received training and certification.
Smoking cessation. Pharmacotherapy and brief treatment interventions can be effective and should be offered to the older smoker. Nicotine replacement therapy, bupropion, varenicline, and nortriptyline help improve quit rates in younger adults, but studies of these agents in older adults are limited.
If monotherapy fails, try combining shorter-acting nicotine replacement therapy with longer-acting agents such as bupropion or varenicline. To our knowledge no dosing adjustment is necessary for the elderly, although we recommend low starting doses with gradual titration.
Some literature suggests nortriptyline as a second-line smoking cessation agent in the elderly. We do not recommend nortriptyline for smoking cessation in this population, however, because of tricyclic antidepressants’ cardiac effects.
- Schultz SK, Arndt S, Liesved J. Locations of facilities with special programs for older substance abuse clients in the U.S. Int J Geriatr Psychiatry. 2003;18(9):839-843.
- National Association of Addiction Treatment Providers. www.naatp.org.
- National Association of State Alcohol/Drug Abuse Directors. www.nasadad.org.
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
- American Academy of Addiction Psychiatry. www.aaap.org.
Drug brand names
- Acamprosate • Campral
- Buprenorphine/naloxone • Suboxone
- Bupropion • Zyban
- Disulfiram • Antabuse
- Methadone • Dolophine, Methadose
- Naltrexone • ReVia
- Nortriptyline • Aventyl, Pamelor
- Oxycodone • OxyContin, Roxicodone, others
- Topiramate • Topamax
- Varenicline • Chantix
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Patterson TL, Jeste DV. The potential impact of the baby boom generation on substance abuse among elderly persons. Psychiatr Serv. 1999;50(9):1184-1188.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Oslin D, Pettinati H, Volpicelli J. Alcoholism treatment adherence. Older age predicts better adherence and drinking outcomes. Am J Geriatr Psychiatry. 2002;10(6):740-747.
4. Kashner TM, Rodell DI, Ogden SR, et al. Outcomes and costs of two VA inpatient treatment programs for older alcoholic patients. Hosp Community Psychiatry. 1992;43:985-989.
5. Alcohol and aging. Alcohol Alert, issue 40; April 1998. National Institute on Alcohol Abuse and Alcoholism. National Institutes of Health. Available at: http://pubs.niaaa.nih.gov/publications/aa40.htm. Accessed November 19, 2009.
6. Cyr MG, Wartman SA. The effectiveness of routine screening questions in the detection of alcoholism. JAMA. 1988;259(1):51-54.
7. O’Connell H, Chin AV, Hamilton F, et al. A systematic review of the utility of self-report alcohol screening instruments in the elderly. Int J Geriatr Psychiatry. 2004;19:1074-1086.
8. Simoni-Wastila L, Yang HK. Psychoactive drug abuse in older adults. Am J Geriatr Pharmacother. 2006;4:380-394.
9. Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2008. DHHS Publication SMA08-4343, NSDUH Series H-34.
10. Simoni-Wastila L, Zuckerman IH, Singhal PK, et al. National estimates of exposure to prescription drugs with addiction potential in community-dwelling elders. Subst Abus. 2005;26:33-42.
11. Reducing tobacco use: a report of the Surgeon General. Office of the Surgeon General. Public Health Service. U.S. Department of Health and Human Services. August 9, 2000. Available at: http://www.surgeongeneral.gov/library/tobacco_use. Accessed November 19, 2009.
12. Blow FC. Substance abuse among older adults. Treatment improvement protocol (TIP) series 26. Substance Abuse and Mental Health Services Administration. Public Health Service. U.S. Department of Health and Human Services. Rockville, MD: Center for Substance Abuse Treatment; June 1998. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hssamhsatip&part=A48302. Accessed November 19, 2009.
13. Finfgeld-Connett DL. Treatment of substance misuse in older women: using a brief intervention model. J Gerontol Nurs. 2004;30(8):30-37.
14. Di Bari M, Silvestrini G, Chiarlone M, et al. Features of excessive alcohol drinking in older adults distinctively captured by behavioral and biological screening instruments. An epidemiological study. J Clin Epidemiol. 2002;55:41-47.
15. Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2007. MMWR. 2008;57(45):1221-1226.
16. Treating tobacco use and dependence: 2008 update. Office of the Surgeon General. Public Health Service. U.S. Department of Health and Human Services. Available at: http://www.surgeongeneral.gov/tobacco. Accessed November 19, 2009.
17. Starosta A, Leeman R, Volpicelli J. The BRENDA model: integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. J Psychiatr Pract. 2006;12(2):80-89.
18. Fleming M, Manuwell L, Barry K, et al. Brief physician advice for alcohol problems in older adults: a randomized community-based trial. J Fam Pract. 1999;48(5):378-384.
19. Barry KL, Blow FC, Cullinane P, et al. The effectiveness of implementing a brief alcohol intervention with older adults in community settings. Washington, DC: National Council on Aging; 2006.
20. Oslin D. Evidence-based treatment of geriatric substance abuse. Psychiatr Clin North Am. 2005;28:897-911.
21. Oslin D, Liberto JG, O’Brien J, et al. Naltrexone as an adjunctive treatment for older patients with alcohol dependence. Am J Geriatr Psychiatry. 1997;5:324-332.
22. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treatment of alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-1651.
23. Firoz S, Carlson G. Characteristics and treatment outcome of older methadone-maintenance patients. Am J Geriatr Psychiatry. 2004;12(5):539-541.
24. Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51-60.
25. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287-313.
1. Patterson TL, Jeste DV. The potential impact of the baby boom generation on substance abuse among elderly persons. Psychiatr Serv. 1999;50(9):1184-1188.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Oslin D, Pettinati H, Volpicelli J. Alcoholism treatment adherence. Older age predicts better adherence and drinking outcomes. Am J Geriatr Psychiatry. 2002;10(6):740-747.
4. Kashner TM, Rodell DI, Ogden SR, et al. Outcomes and costs of two VA inpatient treatment programs for older alcoholic patients. Hosp Community Psychiatry. 1992;43:985-989.
5. Alcohol and aging. Alcohol Alert, issue 40; April 1998. National Institute on Alcohol Abuse and Alcoholism. National Institutes of Health. Available at: http://pubs.niaaa.nih.gov/publications/aa40.htm. Accessed November 19, 2009.
6. Cyr MG, Wartman SA. The effectiveness of routine screening questions in the detection of alcoholism. JAMA. 1988;259(1):51-54.
7. O’Connell H, Chin AV, Hamilton F, et al. A systematic review of the utility of self-report alcohol screening instruments in the elderly. Int J Geriatr Psychiatry. 2004;19:1074-1086.
8. Simoni-Wastila L, Yang HK. Psychoactive drug abuse in older adults. Am J Geriatr Pharmacother. 2006;4:380-394.
9. Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2008. DHHS Publication SMA08-4343, NSDUH Series H-34.
10. Simoni-Wastila L, Zuckerman IH, Singhal PK, et al. National estimates of exposure to prescription drugs with addiction potential in community-dwelling elders. Subst Abus. 2005;26:33-42.
11. Reducing tobacco use: a report of the Surgeon General. Office of the Surgeon General. Public Health Service. U.S. Department of Health and Human Services. August 9, 2000. Available at: http://www.surgeongeneral.gov/library/tobacco_use. Accessed November 19, 2009.
12. Blow FC. Substance abuse among older adults. Treatment improvement protocol (TIP) series 26. Substance Abuse and Mental Health Services Administration. Public Health Service. U.S. Department of Health and Human Services. Rockville, MD: Center for Substance Abuse Treatment; June 1998. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hssamhsatip&part=A48302. Accessed November 19, 2009.
13. Finfgeld-Connett DL. Treatment of substance misuse in older women: using a brief intervention model. J Gerontol Nurs. 2004;30(8):30-37.
14. Di Bari M, Silvestrini G, Chiarlone M, et al. Features of excessive alcohol drinking in older adults distinctively captured by behavioral and biological screening instruments. An epidemiological study. J Clin Epidemiol. 2002;55:41-47.
15. Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2007. MMWR. 2008;57(45):1221-1226.
16. Treating tobacco use and dependence: 2008 update. Office of the Surgeon General. Public Health Service. U.S. Department of Health and Human Services. Available at: http://www.surgeongeneral.gov/tobacco. Accessed November 19, 2009.
17. Starosta A, Leeman R, Volpicelli J. The BRENDA model: integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. J Psychiatr Pract. 2006;12(2):80-89.
18. Fleming M, Manuwell L, Barry K, et al. Brief physician advice for alcohol problems in older adults: a randomized community-based trial. J Fam Pract. 1999;48(5):378-384.
19. Barry KL, Blow FC, Cullinane P, et al. The effectiveness of implementing a brief alcohol intervention with older adults in community settings. Washington, DC: National Council on Aging; 2006.
20. Oslin D. Evidence-based treatment of geriatric substance abuse. Psychiatr Clin North Am. 2005;28:897-911.
21. Oslin D, Liberto JG, O’Brien J, et al. Naltrexone as an adjunctive treatment for older patients with alcohol dependence. Am J Geriatr Psychiatry. 1997;5:324-332.
22. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treatment of alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-1651.
23. Firoz S, Carlson G. Characteristics and treatment outcome of older methadone-maintenance patients. Am J Geriatr Psychiatry. 2004;12(5):539-541.
24. Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51-60.
25. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287-313.
Adolescents in crisis: When to admit for self-harm or aggressive behavior
Ms. R, age 17, has a history of major depression, obsessive-compulsive disorder, and self-harm through superficial cutting of her arms and inguinal region. She reports that 10 days ago she ingested 7 times her prescribed fluoxetine dosage of 20 mg/d and aripiprazole dosage of 2 mg/d because she no longer wanted to feel emotional pain. She did not tell anyone she did this or seek medical attention.
Ms. R complains of chronic difficulties with her stepfather, who she describes as alcoholic. She feels her depression is worsening and support from her mother has deteriorated. Ms. R’s parents say they are trying to respond to their daughter, but she will not talk with them and some nights she does not return home. Ms. R admits to staying overnight in local mall parking lots to be alone. Her psychiatrist recommends acute inpatient care for Ms. R’s safety.
Admitting an adolescent such as Ms. R to a psychiatric inpatient facility may be necessary to address a crisis. Interdependent links among the patient, family, and support network complicate the determination of whether an adolescent requires inpatient care. To make the best decision, a psychiatrist needs to understand the youth’s difficulties within family, school, and community.
Who needs inpatient care?
Inpatient treatment remains an important part of the continuum of care for adolescent psychiatric treatment.1 Inpatient treatment typically is reserved for patients whose psychiatric disorder impairs multiple areas of functioning or poses a significant danger to self or others and for whom less-restrictive treatment resources are not appropriate or available.2 The number of psychiatric hospitalizations for adolescents is increasing, although lengths of stay are decreasing.3,4
Psychiatric inpatient care is appropriate for patients who require 24-hour nursing care and psychiatric monitoring to stabilize symptoms when they are in acute crisis and have a high risk of harm, and for initiation of treatments required for stabilization and integration into a less-restrictive setting.5 The decision to admit an adolescent rests on:
- the clinician’s ability to evaluate the risk of harm and functional status
- how much support the family and/or caregivers can provide
- the clinician’s knowledge of treatment resources available to the adolescent and family.6
Exploring suicide risk
Understanding potential lethality of suicidal thought and intent is complex and requires assessing suicidal behavior, the patient’s past and current intent, the risk of engaging in or repeating a suicide act, the underlying diagnosis, and protective factors. To quantify imminent suicide risk, directly address suicidality when interviewing an adolescent, progressing from past thoughts to current intent, plan, and ability to carry out such a plan (Table 1) .7
Planning and lethality. Also examine the patient’s degree of planning for a suicide attempt, efforts to avoid discovery and rescue, and his or her perceived lethality of a suicide attempt or plan. Patients who develop a coherent plan that would successfully avoid discovery clearly are at highest risk. Lethality of method is frequently misunderstood—especially among younger individuals—and thus their perception of the dangerousness of an attempt is more important than reality. Previous suicide attempts and chronic suicidality with recent escalation imply greater risk.
Motivation. Exploring the feelings that motivate a suicide attempt, intent, or ideation will help assess risk. Common motivations include:
- escaping from stress or hopelessness from perceived intolerable circumstances
- rejoining a dead loved one
- getting notice or attention from a parent, romantic interest, or other important individual
- injuring others around them.
Serious suicide risk may persist if the motivating feelings are not addressed satisfactorily.7
Unclear signals. An adolescent who expresses a clear intent to die, has a plausible plan, and is unable to work with or rejects caregivers’ attempts to help is at high risk and requires a secure setting, such as hospitalization. Typically, however, patients do not give such clear indicators; in these cases, consider other factors.
Unstable and unpredictable behavior implies serious short-term risk. Factors that indicate difficulties in a patient’s ability to maintain a safety plan include:
- a history of multiple suicide attempts or escalating seriousness of ideation
- inability to be truthful and form an alliance with the clinician
- difficulties in expressing and regulating emotions
- presence or likelihood of intoxication.
Psychosis, command hallucinations, high impulsivity, cycling associated with bipolar disorder, and substance abuse also are associated with high suicide risk.8
The clinician must determine whether an adolescent can form an alliance to report suicidal ideation, intent, or plan to a family member or other responsible adult, and if the family/caregivers are willing and capable of providing support, supervision, and compliance with future treatment recommendations that will ensure safety. If the answers are no, the patient requires hospitalization.
Table 1
Suggested questions for assessing adolescent suicidality
| Have you had thoughts of hurting yourself? |
| Have you ever tried to hurt yourself? |
| Have you ever wished you were not alive? |
| Have you had thoughts of taking your life? |
| Have you done things that are so dangerous that you knew you might get hurt or die? |
| Have you ever tried to kill yourself? |
| Have you had recent thoughts of killing yourself? |
| Do you have a plan to kill yourself? |
| Are the methods to kill yourself available to you? |
| Do you have access to guns? |
| Source: Adapted from reference 7 |
CASE CONTINUED: Unsafe at home
Ms. R feels she cannot be safe at home and cannot reliably form an alliance with her mother and stepfather to discuss whether her self-harm behaviors would escalate to serious injury or death. As a result, she is admitted to a psychiatric hospital. Inpatient care includes family intervention and a plan to intensify outpatient therapy. When Ms. R is discharged after 6 days, she reports improved mood and ability to contract with her family.
Aggressive behaviors
Besides suicidality, aggressive and combative behaviors in adolescents may lead to psychiatric referral.9–11 Overt homicidal ideation is not common; typically, patients exhibit escalating, disruptive, aggressive episodes in the home, school, or community that pose risk to themselves or others. Families seek clinical help because they feel unable to keep their child safe at home.
Aggressive behavior is linked to multiple patient factors, such as male gender, history of abuse and neglect, out-of-home placement in community systems, developmental disorders, mental retardation, disruptive behavior disorders, and learning disabilities. Aggressive behavior may include planned proactive situational-reactive or impulsive aggression, or it can stem from an altered mental status caused by illicit drug intoxication, medications, psychosis, or severe mood disorders.9-12
Psychiatric hospitalization of aggressive adolescents raises safety concerns, and some practitioners perceive that treatment is ineffective for these patients. However, high rates of psychiatric comorbidity and indications that positive outcomes are possible suggest that many aggressive youth can benefit from intervention.1,11
Because of the crisis nature of acute aggression and the often conflicted, hidden, and stressful situations these patients and families or caregivers are experiencing, hospitalization often is needed to stabilize the adolescent.
Assessment work with family/caregivers is vital because patients typically minimize the intensity of their aggressive behavior. Use a structured scale—such as the modified Overt Aggression Scale—to help quantify the severity of aggressive episodes, determine dangerousness, and establish a common language and measurement among caregivers, patients, and clinicians.13
The family/caregivers’ capacity and willingness to provide a safe environment, to avoid triggering events, and to provide support to de-escalate a potential crisis also determine if safety can be maintained in the home or if hospitalization is required. Hospitalization may be appropriate if the adolescent’s aggressive behavior substantially endangers the patient or others, is increasing in intensity, exceeds the ability to be managed in the home or living environment, and cannot be maintained in available less-restrictive settings.
In addition to the patient’s potential for suicidal or aggressive behavior, consider other aspects of potential harm, such as:
- unintentional harm associated with altered mental status from psychosis or intoxication
- the adolescent’s impulsivity or judgment in situations he or she is likely to encounter
- the patient’s ability to recognize potential threats and take appropriate action for safety
- severely impaired self-care.14
The Child and Adolescent Service Intensity Instrument can be used to help determine the level of care an adolescent patient requires ( Box ).14
CASII can help determine appropriate care for teens
The Child and Adolescent Service Intensity Instrument (CASII) can help you determine what level of care is most appropriate for your adolescent patient. This scale—developed by a work group of the American Academy of Child and Adolescent Psychiatry (AACAP)—links clinical assessment with standardized levels of care. It includes scoring in 6 dimensions:
- risk and harm
- functional status
- co-occurrence of conditions
- recovery environment
- resiliency and response to services
- primary caretaker involvement in services.
Scores are combined to generate a recommend level of service intensity from 0 (basic services) to 7 (24-hour psychiatric management—admission to a hospital or locked residential unit).
The AACAP strongly encourages clinicians to receive training to use the CASII and provides 1-and 2-day courses.
Source: Reference 14
Comorbid conditions
Comorbid medical illness, substance use disorders, and cognitive disability are common complications in determining the level of care for an adolescent in crisis. Active or passive noncompliance with treatment for medical conditions can pose an immediate or chronic threat to the individual and may represent a method of self-harm. Medical comorbidities and care requirements frequently preclude quick access to services such as group homes, therapeutic foster programs, and residential treatment. Hospitalization often is required to stabilize psychiatric conditions and medical illness.
CASE REPORT: Multiple comorbidities
Ms. P, age 16, has type 1 diabetes mellitus, posttraumatic stress disorder from early physical and sexual abuse, and an IQ of 49. She presents after repeated arguments and physical confrontations with her mother, with whom she lives. She has been caught hoarding high-sugar foods.
The most recent fight is over Ms. P wanting to consume large amounts of candy. She has been hospitalized twice for diabetic ketoacidosis in the last 6 months. Her most recent blood sugar levels ranged from 250 to 500 mg/dL. Ms. P states she is angry at her mother and will hit her if she tries to control her diet. She says she doesn’t care if she gets sick, but her recognition of medical complications is limited.
Developmental delays may complicate treatment for psychiatric illness or impair an adolescent’s ability to understand the dangerousness of his or her behaviors.15 Communication barriers make it challenging to assess risk or the patient’s ability to comply with a safety plan. In patients with developmental delay who live in the community, external structure, monitoring, and the ability to manage crises depends on the family/caregivers. Strongly consider hospitalization if an adolescent’s developmental delay has a serious adverse effect on managing the psychiatric condition, causing increased risk of harm to self or others.
Substance use frequently accompanies adolescent psychiatric illness and may pose severe risk by disinhibiting impulse control, exacerbating mood symptoms, altering mental status, or causing intoxication or withdrawal syndromes. Substance use also carries inherent risks, such as contracting human immunodeficiency virus or other blood-borne infections.
Substance use is well-documented as a severe risk factor for suicide and suicide attempts7,8 and frequently is associated with violence.16 Hospitalization may be the safest way to manage an adolescent who exhibits escalating substance use that complicates management of the psychiatric illness or indicates progressive endangering behavior.
Functional assessment
In addition to exploring risk of self-harm, aggressive behaviors, and medical comorbidities, evaluate the adolescent’s ability to function in interpersonal relationships, self-care, and school. A pattern of severe or worsening functional impairment often indicates illness progression or that management or supports are not meeting the patient’s needs.
Strongly consider hospitalizing patients who demonstrate serious deterioration in interpersonal relationships with peers, adults, or family, as evidenced by escalating threats, episodic violence, or disorganized communication. Additional concerns include severe social withdrawal, neglect of self-care appropriate to developmental level, and inability to perform academically despite appropriate accommodations.
Identify impaired physical functions. When severe medical complications accompany anorexia nervosa or other psychiatric illness, hospitalization is needed to ensure the patient’s safety and to begin appropriate assessment and treatment ( Table 2 ).17
Table 2
Adolescents with eating disorders: Admission criteria
| Heart rate near 40 bpm |
| Orthostasis (pulse change >20 bpm or blood pressure drop of >10 to 20 mm Hg from sitting to standing) |
| Hypotension (blood pressure <80/50 mm Hg) |
| Electrolyte imbalance (hypokalemia, hypophosphatemia, hypomagnesemia) |
| Weight <85% of ideal body weight |
| Acute weight decline with food refusal |
| Suicidal ideation |
| Needs supervision during and after all meals and in bathrooms because of disabling purging |
| Suitability of pediatric vs psychiatric unit depends on level of medical care required and respective units’ ability to manage eating disorders |
| Source: Adapted from reference 17 |
Family and environmental factors
The decision to admit an adolescent to a psychiatric hospital or provide a home treatment plan often hinges on the ability and willingness of the patient’s family/caregivers and support systems to meet the patient’s needs. Consider whether family functioning has been disrupted by a parent’s illness, death, divorce, medical problems, psychiatric illness, substance abuse, or financial stress. If you suspect abuse or violence in the home, observe reporting laws in your jurisdiction and intervene with the family to ensure the adolescent’s safety. Hospitalization may be the best means of providing safety during an investigation.
Determine if the family or primary caregivers are able to meet the adolescent’s developmental, material, and emotional requirements, and if help from treatment or support services or community resources could provide these needs. If not, hospitalization likely is required.
CASE CONTINUED: Risk of physical harm
Ms. P is admitted to the psychiatric hospital because her mother reports that in the past week she and her daughter have had 2 physical altercations—resulting from arguments about her daughter’s dietary intake—that caused injuries. She does not feel she can keep her daughter safe. Ms. P’s mother states she feels she is poorly trained in diabetic care and cannot provide the medical intervention her daughter needs.
Know your system
Child and adolescent psychiatric services are in great demand but often fall far short in meeting these needs across ethnic and socioeconomic groups. Availability of resources differs by geographic location and payer source.18,19 Community-funded mental health varies considerably. The best organizations offer a complete system of care, including outpatient therapy, medication management, case management, wraparound services, respite care, group homes, residential programs, and crisis programs.
Be familiar with local and regional programs and methods of accessing them. For patients who have access to a system of care, timely mobilization of appropriate resources often can avoid a hospital admission and place a patient in a less restrictive setting. Such options, however, frequently are not available to patients covered by commercial payers. For them, the decision typically is reduced to whether the family can manage the patient at home; if the family is unable to ensure safety, the adolescent is hospitalized.
Knowing the capability of available inpatient programs is essential to making an appropriate referral. Consider the level of medical care the psychiatric unit can provide and the accessibility of medical consultation services—both primary and medical subspecialty. Specialized programs for young people with comorbid severe cognitive delays, eating disorders, or forensic difficulties also assist with effective management. The inpatient unit’s collaboration and communication with outpatient providers frequently determines the success of the patient’s transition to less restrictive care.
- Child and Adolescent Service Intensity Instrument (CASII). www.aacap.org/cs/root/member_information/practice_information/casii.
Drug brand names
- Aripiprazole • Abilify
- Fluoxetine • Prozac
Disclosure
Dr. Sorter reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Green J, Jacobs B, Beecham J, et al. Inpatient treatment in child and adolescent psychiatry—a prospective study of health gain and costs. J Child Psychol Psychiatry. 2007;48(12):1259-1267.
2. American Academy of Child and Adolescent Psychiatry. Inpatient hospital treatment of children and adolescents. Policy statement. June 1989. Available at: http://www.aacap.org/cs/root/policy_statements/inpatient_hospital_treatment_of_children_
and_adolescents. Accessed November 19, 2009.
3. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in U.S. community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96.
4. Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62:107-114.
5. Sharfstein SS. Goals of inpatient treatment for psychiatric disorders. Annu Rev Med. 2009;60:393-403.
6. Thienhaus OJ. The decision to admit. In: Thienhaus OJ, ed. Manual of clinical hospital psychiatry. Washington, DC: American Psychiatric Press, Inc; 1995:3–16.
7. Shaffer D, Pfeffer CR. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7):24S-51S.
8. Spirito A, Espposito-Smythers C. Attempted and completed suicide in adolescence. Ann Rev Clin Psychol. 2005;2:237-266.
9. Jenson PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309-322.
10. Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808-820.
11. Dean AJ, Duke SG, Scott J, et al. Physical aggression during admission to a child and adolescent inpatient unit: predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42(6):536-543.
12. Masters KJ, Bellonci C. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2):4S-25S.
13. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. VI: scales assessing externalizing behaviors. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1143-1170.
14. Child and adolescent service intensity instrument user’s manual. Version 3.0. Washington, DC: American Academy of Child and Adolescent Psychiatry; 2007.
15. Lee P, Friedlander R. Attention-deficit and disruptive behavior disorders. In: Fletcher R, Loschen E, Stavrakaki C, et al, eds. Diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: National Association for the Dually Diagnosed; 2007:127–144.
16. Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623-632.
17. American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163 (7 suppl):4-54.
18. Sturm R, Ringel JS, Andreyeva T. Geographic disparities in children’s mental health care. Pediatrics. 2003;112(4):e308-e315.
19. Katoaka SM, Zhang L, Wells KB. Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. Am J Psychiatry. 2002;159:1548-1555.
Ms. R, age 17, has a history of major depression, obsessive-compulsive disorder, and self-harm through superficial cutting of her arms and inguinal region. She reports that 10 days ago she ingested 7 times her prescribed fluoxetine dosage of 20 mg/d and aripiprazole dosage of 2 mg/d because she no longer wanted to feel emotional pain. She did not tell anyone she did this or seek medical attention.
Ms. R complains of chronic difficulties with her stepfather, who she describes as alcoholic. She feels her depression is worsening and support from her mother has deteriorated. Ms. R’s parents say they are trying to respond to their daughter, but she will not talk with them and some nights she does not return home. Ms. R admits to staying overnight in local mall parking lots to be alone. Her psychiatrist recommends acute inpatient care for Ms. R’s safety.
Admitting an adolescent such as Ms. R to a psychiatric inpatient facility may be necessary to address a crisis. Interdependent links among the patient, family, and support network complicate the determination of whether an adolescent requires inpatient care. To make the best decision, a psychiatrist needs to understand the youth’s difficulties within family, school, and community.
Who needs inpatient care?
Inpatient treatment remains an important part of the continuum of care for adolescent psychiatric treatment.1 Inpatient treatment typically is reserved for patients whose psychiatric disorder impairs multiple areas of functioning or poses a significant danger to self or others and for whom less-restrictive treatment resources are not appropriate or available.2 The number of psychiatric hospitalizations for adolescents is increasing, although lengths of stay are decreasing.3,4
Psychiatric inpatient care is appropriate for patients who require 24-hour nursing care and psychiatric monitoring to stabilize symptoms when they are in acute crisis and have a high risk of harm, and for initiation of treatments required for stabilization and integration into a less-restrictive setting.5 The decision to admit an adolescent rests on:
- the clinician’s ability to evaluate the risk of harm and functional status
- how much support the family and/or caregivers can provide
- the clinician’s knowledge of treatment resources available to the adolescent and family.6
Exploring suicide risk
Understanding potential lethality of suicidal thought and intent is complex and requires assessing suicidal behavior, the patient’s past and current intent, the risk of engaging in or repeating a suicide act, the underlying diagnosis, and protective factors. To quantify imminent suicide risk, directly address suicidality when interviewing an adolescent, progressing from past thoughts to current intent, plan, and ability to carry out such a plan (Table 1) .7
Planning and lethality. Also examine the patient’s degree of planning for a suicide attempt, efforts to avoid discovery and rescue, and his or her perceived lethality of a suicide attempt or plan. Patients who develop a coherent plan that would successfully avoid discovery clearly are at highest risk. Lethality of method is frequently misunderstood—especially among younger individuals—and thus their perception of the dangerousness of an attempt is more important than reality. Previous suicide attempts and chronic suicidality with recent escalation imply greater risk.
Motivation. Exploring the feelings that motivate a suicide attempt, intent, or ideation will help assess risk. Common motivations include:
- escaping from stress or hopelessness from perceived intolerable circumstances
- rejoining a dead loved one
- getting notice or attention from a parent, romantic interest, or other important individual
- injuring others around them.
Serious suicide risk may persist if the motivating feelings are not addressed satisfactorily.7
Unclear signals. An adolescent who expresses a clear intent to die, has a plausible plan, and is unable to work with or rejects caregivers’ attempts to help is at high risk and requires a secure setting, such as hospitalization. Typically, however, patients do not give such clear indicators; in these cases, consider other factors.
Unstable and unpredictable behavior implies serious short-term risk. Factors that indicate difficulties in a patient’s ability to maintain a safety plan include:
- a history of multiple suicide attempts or escalating seriousness of ideation
- inability to be truthful and form an alliance with the clinician
- difficulties in expressing and regulating emotions
- presence or likelihood of intoxication.
Psychosis, command hallucinations, high impulsivity, cycling associated with bipolar disorder, and substance abuse also are associated with high suicide risk.8
The clinician must determine whether an adolescent can form an alliance to report suicidal ideation, intent, or plan to a family member or other responsible adult, and if the family/caregivers are willing and capable of providing support, supervision, and compliance with future treatment recommendations that will ensure safety. If the answers are no, the patient requires hospitalization.
Table 1
Suggested questions for assessing adolescent suicidality
| Have you had thoughts of hurting yourself? |
| Have you ever tried to hurt yourself? |
| Have you ever wished you were not alive? |
| Have you had thoughts of taking your life? |
| Have you done things that are so dangerous that you knew you might get hurt or die? |
| Have you ever tried to kill yourself? |
| Have you had recent thoughts of killing yourself? |
| Do you have a plan to kill yourself? |
| Are the methods to kill yourself available to you? |
| Do you have access to guns? |
| Source: Adapted from reference 7 |
CASE CONTINUED: Unsafe at home
Ms. R feels she cannot be safe at home and cannot reliably form an alliance with her mother and stepfather to discuss whether her self-harm behaviors would escalate to serious injury or death. As a result, she is admitted to a psychiatric hospital. Inpatient care includes family intervention and a plan to intensify outpatient therapy. When Ms. R is discharged after 6 days, she reports improved mood and ability to contract with her family.
Aggressive behaviors
Besides suicidality, aggressive and combative behaviors in adolescents may lead to psychiatric referral.9–11 Overt homicidal ideation is not common; typically, patients exhibit escalating, disruptive, aggressive episodes in the home, school, or community that pose risk to themselves or others. Families seek clinical help because they feel unable to keep their child safe at home.
Aggressive behavior is linked to multiple patient factors, such as male gender, history of abuse and neglect, out-of-home placement in community systems, developmental disorders, mental retardation, disruptive behavior disorders, and learning disabilities. Aggressive behavior may include planned proactive situational-reactive or impulsive aggression, or it can stem from an altered mental status caused by illicit drug intoxication, medications, psychosis, or severe mood disorders.9-12
Psychiatric hospitalization of aggressive adolescents raises safety concerns, and some practitioners perceive that treatment is ineffective for these patients. However, high rates of psychiatric comorbidity and indications that positive outcomes are possible suggest that many aggressive youth can benefit from intervention.1,11
Because of the crisis nature of acute aggression and the often conflicted, hidden, and stressful situations these patients and families or caregivers are experiencing, hospitalization often is needed to stabilize the adolescent.
Assessment work with family/caregivers is vital because patients typically minimize the intensity of their aggressive behavior. Use a structured scale—such as the modified Overt Aggression Scale—to help quantify the severity of aggressive episodes, determine dangerousness, and establish a common language and measurement among caregivers, patients, and clinicians.13
The family/caregivers’ capacity and willingness to provide a safe environment, to avoid triggering events, and to provide support to de-escalate a potential crisis also determine if safety can be maintained in the home or if hospitalization is required. Hospitalization may be appropriate if the adolescent’s aggressive behavior substantially endangers the patient or others, is increasing in intensity, exceeds the ability to be managed in the home or living environment, and cannot be maintained in available less-restrictive settings.
In addition to the patient’s potential for suicidal or aggressive behavior, consider other aspects of potential harm, such as:
- unintentional harm associated with altered mental status from psychosis or intoxication
- the adolescent’s impulsivity or judgment in situations he or she is likely to encounter
- the patient’s ability to recognize potential threats and take appropriate action for safety
- severely impaired self-care.14
The Child and Adolescent Service Intensity Instrument can be used to help determine the level of care an adolescent patient requires ( Box ).14
CASII can help determine appropriate care for teens
The Child and Adolescent Service Intensity Instrument (CASII) can help you determine what level of care is most appropriate for your adolescent patient. This scale—developed by a work group of the American Academy of Child and Adolescent Psychiatry (AACAP)—links clinical assessment with standardized levels of care. It includes scoring in 6 dimensions:
- risk and harm
- functional status
- co-occurrence of conditions
- recovery environment
- resiliency and response to services
- primary caretaker involvement in services.
Scores are combined to generate a recommend level of service intensity from 0 (basic services) to 7 (24-hour psychiatric management—admission to a hospital or locked residential unit).
The AACAP strongly encourages clinicians to receive training to use the CASII and provides 1-and 2-day courses.
Source: Reference 14
Comorbid conditions
Comorbid medical illness, substance use disorders, and cognitive disability are common complications in determining the level of care for an adolescent in crisis. Active or passive noncompliance with treatment for medical conditions can pose an immediate or chronic threat to the individual and may represent a method of self-harm. Medical comorbidities and care requirements frequently preclude quick access to services such as group homes, therapeutic foster programs, and residential treatment. Hospitalization often is required to stabilize psychiatric conditions and medical illness.
CASE REPORT: Multiple comorbidities
Ms. P, age 16, has type 1 diabetes mellitus, posttraumatic stress disorder from early physical and sexual abuse, and an IQ of 49. She presents after repeated arguments and physical confrontations with her mother, with whom she lives. She has been caught hoarding high-sugar foods.
The most recent fight is over Ms. P wanting to consume large amounts of candy. She has been hospitalized twice for diabetic ketoacidosis in the last 6 months. Her most recent blood sugar levels ranged from 250 to 500 mg/dL. Ms. P states she is angry at her mother and will hit her if she tries to control her diet. She says she doesn’t care if she gets sick, but her recognition of medical complications is limited.
Developmental delays may complicate treatment for psychiatric illness or impair an adolescent’s ability to understand the dangerousness of his or her behaviors.15 Communication barriers make it challenging to assess risk or the patient’s ability to comply with a safety plan. In patients with developmental delay who live in the community, external structure, monitoring, and the ability to manage crises depends on the family/caregivers. Strongly consider hospitalization if an adolescent’s developmental delay has a serious adverse effect on managing the psychiatric condition, causing increased risk of harm to self or others.
Substance use frequently accompanies adolescent psychiatric illness and may pose severe risk by disinhibiting impulse control, exacerbating mood symptoms, altering mental status, or causing intoxication or withdrawal syndromes. Substance use also carries inherent risks, such as contracting human immunodeficiency virus or other blood-borne infections.
Substance use is well-documented as a severe risk factor for suicide and suicide attempts7,8 and frequently is associated with violence.16 Hospitalization may be the safest way to manage an adolescent who exhibits escalating substance use that complicates management of the psychiatric illness or indicates progressive endangering behavior.
Functional assessment
In addition to exploring risk of self-harm, aggressive behaviors, and medical comorbidities, evaluate the adolescent’s ability to function in interpersonal relationships, self-care, and school. A pattern of severe or worsening functional impairment often indicates illness progression or that management or supports are not meeting the patient’s needs.
Strongly consider hospitalizing patients who demonstrate serious deterioration in interpersonal relationships with peers, adults, or family, as evidenced by escalating threats, episodic violence, or disorganized communication. Additional concerns include severe social withdrawal, neglect of self-care appropriate to developmental level, and inability to perform academically despite appropriate accommodations.
Identify impaired physical functions. When severe medical complications accompany anorexia nervosa or other psychiatric illness, hospitalization is needed to ensure the patient’s safety and to begin appropriate assessment and treatment ( Table 2 ).17
Table 2
Adolescents with eating disorders: Admission criteria
| Heart rate near 40 bpm |
| Orthostasis (pulse change >20 bpm or blood pressure drop of >10 to 20 mm Hg from sitting to standing) |
| Hypotension (blood pressure <80/50 mm Hg) |
| Electrolyte imbalance (hypokalemia, hypophosphatemia, hypomagnesemia) |
| Weight <85% of ideal body weight |
| Acute weight decline with food refusal |
| Suicidal ideation |
| Needs supervision during and after all meals and in bathrooms because of disabling purging |
| Suitability of pediatric vs psychiatric unit depends on level of medical care required and respective units’ ability to manage eating disorders |
| Source: Adapted from reference 17 |
Family and environmental factors
The decision to admit an adolescent to a psychiatric hospital or provide a home treatment plan often hinges on the ability and willingness of the patient’s family/caregivers and support systems to meet the patient’s needs. Consider whether family functioning has been disrupted by a parent’s illness, death, divorce, medical problems, psychiatric illness, substance abuse, or financial stress. If you suspect abuse or violence in the home, observe reporting laws in your jurisdiction and intervene with the family to ensure the adolescent’s safety. Hospitalization may be the best means of providing safety during an investigation.
Determine if the family or primary caregivers are able to meet the adolescent’s developmental, material, and emotional requirements, and if help from treatment or support services or community resources could provide these needs. If not, hospitalization likely is required.
CASE CONTINUED: Risk of physical harm
Ms. P is admitted to the psychiatric hospital because her mother reports that in the past week she and her daughter have had 2 physical altercations—resulting from arguments about her daughter’s dietary intake—that caused injuries. She does not feel she can keep her daughter safe. Ms. P’s mother states she feels she is poorly trained in diabetic care and cannot provide the medical intervention her daughter needs.
Know your system
Child and adolescent psychiatric services are in great demand but often fall far short in meeting these needs across ethnic and socioeconomic groups. Availability of resources differs by geographic location and payer source.18,19 Community-funded mental health varies considerably. The best organizations offer a complete system of care, including outpatient therapy, medication management, case management, wraparound services, respite care, group homes, residential programs, and crisis programs.
Be familiar with local and regional programs and methods of accessing them. For patients who have access to a system of care, timely mobilization of appropriate resources often can avoid a hospital admission and place a patient in a less restrictive setting. Such options, however, frequently are not available to patients covered by commercial payers. For them, the decision typically is reduced to whether the family can manage the patient at home; if the family is unable to ensure safety, the adolescent is hospitalized.
Knowing the capability of available inpatient programs is essential to making an appropriate referral. Consider the level of medical care the psychiatric unit can provide and the accessibility of medical consultation services—both primary and medical subspecialty. Specialized programs for young people with comorbid severe cognitive delays, eating disorders, or forensic difficulties also assist with effective management. The inpatient unit’s collaboration and communication with outpatient providers frequently determines the success of the patient’s transition to less restrictive care.
- Child and Adolescent Service Intensity Instrument (CASII). www.aacap.org/cs/root/member_information/practice_information/casii.
Drug brand names
- Aripiprazole • Abilify
- Fluoxetine • Prozac
Disclosure
Dr. Sorter reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. R, age 17, has a history of major depression, obsessive-compulsive disorder, and self-harm through superficial cutting of her arms and inguinal region. She reports that 10 days ago she ingested 7 times her prescribed fluoxetine dosage of 20 mg/d and aripiprazole dosage of 2 mg/d because she no longer wanted to feel emotional pain. She did not tell anyone she did this or seek medical attention.
Ms. R complains of chronic difficulties with her stepfather, who she describes as alcoholic. She feels her depression is worsening and support from her mother has deteriorated. Ms. R’s parents say they are trying to respond to their daughter, but she will not talk with them and some nights she does not return home. Ms. R admits to staying overnight in local mall parking lots to be alone. Her psychiatrist recommends acute inpatient care for Ms. R’s safety.
Admitting an adolescent such as Ms. R to a psychiatric inpatient facility may be necessary to address a crisis. Interdependent links among the patient, family, and support network complicate the determination of whether an adolescent requires inpatient care. To make the best decision, a psychiatrist needs to understand the youth’s difficulties within family, school, and community.
Who needs inpatient care?
Inpatient treatment remains an important part of the continuum of care for adolescent psychiatric treatment.1 Inpatient treatment typically is reserved for patients whose psychiatric disorder impairs multiple areas of functioning or poses a significant danger to self or others and for whom less-restrictive treatment resources are not appropriate or available.2 The number of psychiatric hospitalizations for adolescents is increasing, although lengths of stay are decreasing.3,4
Psychiatric inpatient care is appropriate for patients who require 24-hour nursing care and psychiatric monitoring to stabilize symptoms when they are in acute crisis and have a high risk of harm, and for initiation of treatments required for stabilization and integration into a less-restrictive setting.5 The decision to admit an adolescent rests on:
- the clinician’s ability to evaluate the risk of harm and functional status
- how much support the family and/or caregivers can provide
- the clinician’s knowledge of treatment resources available to the adolescent and family.6
Exploring suicide risk
Understanding potential lethality of suicidal thought and intent is complex and requires assessing suicidal behavior, the patient’s past and current intent, the risk of engaging in or repeating a suicide act, the underlying diagnosis, and protective factors. To quantify imminent suicide risk, directly address suicidality when interviewing an adolescent, progressing from past thoughts to current intent, plan, and ability to carry out such a plan (Table 1) .7
Planning and lethality. Also examine the patient’s degree of planning for a suicide attempt, efforts to avoid discovery and rescue, and his or her perceived lethality of a suicide attempt or plan. Patients who develop a coherent plan that would successfully avoid discovery clearly are at highest risk. Lethality of method is frequently misunderstood—especially among younger individuals—and thus their perception of the dangerousness of an attempt is more important than reality. Previous suicide attempts and chronic suicidality with recent escalation imply greater risk.
Motivation. Exploring the feelings that motivate a suicide attempt, intent, or ideation will help assess risk. Common motivations include:
- escaping from stress or hopelessness from perceived intolerable circumstances
- rejoining a dead loved one
- getting notice or attention from a parent, romantic interest, or other important individual
- injuring others around them.
Serious suicide risk may persist if the motivating feelings are not addressed satisfactorily.7
Unclear signals. An adolescent who expresses a clear intent to die, has a plausible plan, and is unable to work with or rejects caregivers’ attempts to help is at high risk and requires a secure setting, such as hospitalization. Typically, however, patients do not give such clear indicators; in these cases, consider other factors.
Unstable and unpredictable behavior implies serious short-term risk. Factors that indicate difficulties in a patient’s ability to maintain a safety plan include:
- a history of multiple suicide attempts or escalating seriousness of ideation
- inability to be truthful and form an alliance with the clinician
- difficulties in expressing and regulating emotions
- presence or likelihood of intoxication.
Psychosis, command hallucinations, high impulsivity, cycling associated with bipolar disorder, and substance abuse also are associated with high suicide risk.8
The clinician must determine whether an adolescent can form an alliance to report suicidal ideation, intent, or plan to a family member or other responsible adult, and if the family/caregivers are willing and capable of providing support, supervision, and compliance with future treatment recommendations that will ensure safety. If the answers are no, the patient requires hospitalization.
Table 1
Suggested questions for assessing adolescent suicidality
| Have you had thoughts of hurting yourself? |
| Have you ever tried to hurt yourself? |
| Have you ever wished you were not alive? |
| Have you had thoughts of taking your life? |
| Have you done things that are so dangerous that you knew you might get hurt or die? |
| Have you ever tried to kill yourself? |
| Have you had recent thoughts of killing yourself? |
| Do you have a plan to kill yourself? |
| Are the methods to kill yourself available to you? |
| Do you have access to guns? |
| Source: Adapted from reference 7 |
CASE CONTINUED: Unsafe at home
Ms. R feels she cannot be safe at home and cannot reliably form an alliance with her mother and stepfather to discuss whether her self-harm behaviors would escalate to serious injury or death. As a result, she is admitted to a psychiatric hospital. Inpatient care includes family intervention and a plan to intensify outpatient therapy. When Ms. R is discharged after 6 days, she reports improved mood and ability to contract with her family.
Aggressive behaviors
Besides suicidality, aggressive and combative behaviors in adolescents may lead to psychiatric referral.9–11 Overt homicidal ideation is not common; typically, patients exhibit escalating, disruptive, aggressive episodes in the home, school, or community that pose risk to themselves or others. Families seek clinical help because they feel unable to keep their child safe at home.
Aggressive behavior is linked to multiple patient factors, such as male gender, history of abuse and neglect, out-of-home placement in community systems, developmental disorders, mental retardation, disruptive behavior disorders, and learning disabilities. Aggressive behavior may include planned proactive situational-reactive or impulsive aggression, or it can stem from an altered mental status caused by illicit drug intoxication, medications, psychosis, or severe mood disorders.9-12
Psychiatric hospitalization of aggressive adolescents raises safety concerns, and some practitioners perceive that treatment is ineffective for these patients. However, high rates of psychiatric comorbidity and indications that positive outcomes are possible suggest that many aggressive youth can benefit from intervention.1,11
Because of the crisis nature of acute aggression and the often conflicted, hidden, and stressful situations these patients and families or caregivers are experiencing, hospitalization often is needed to stabilize the adolescent.
Assessment work with family/caregivers is vital because patients typically minimize the intensity of their aggressive behavior. Use a structured scale—such as the modified Overt Aggression Scale—to help quantify the severity of aggressive episodes, determine dangerousness, and establish a common language and measurement among caregivers, patients, and clinicians.13
The family/caregivers’ capacity and willingness to provide a safe environment, to avoid triggering events, and to provide support to de-escalate a potential crisis also determine if safety can be maintained in the home or if hospitalization is required. Hospitalization may be appropriate if the adolescent’s aggressive behavior substantially endangers the patient or others, is increasing in intensity, exceeds the ability to be managed in the home or living environment, and cannot be maintained in available less-restrictive settings.
In addition to the patient’s potential for suicidal or aggressive behavior, consider other aspects of potential harm, such as:
- unintentional harm associated with altered mental status from psychosis or intoxication
- the adolescent’s impulsivity or judgment in situations he or she is likely to encounter
- the patient’s ability to recognize potential threats and take appropriate action for safety
- severely impaired self-care.14
The Child and Adolescent Service Intensity Instrument can be used to help determine the level of care an adolescent patient requires ( Box ).14
CASII can help determine appropriate care for teens
The Child and Adolescent Service Intensity Instrument (CASII) can help you determine what level of care is most appropriate for your adolescent patient. This scale—developed by a work group of the American Academy of Child and Adolescent Psychiatry (AACAP)—links clinical assessment with standardized levels of care. It includes scoring in 6 dimensions:
- risk and harm
- functional status
- co-occurrence of conditions
- recovery environment
- resiliency and response to services
- primary caretaker involvement in services.
Scores are combined to generate a recommend level of service intensity from 0 (basic services) to 7 (24-hour psychiatric management—admission to a hospital or locked residential unit).
The AACAP strongly encourages clinicians to receive training to use the CASII and provides 1-and 2-day courses.
Source: Reference 14
Comorbid conditions
Comorbid medical illness, substance use disorders, and cognitive disability are common complications in determining the level of care for an adolescent in crisis. Active or passive noncompliance with treatment for medical conditions can pose an immediate or chronic threat to the individual and may represent a method of self-harm. Medical comorbidities and care requirements frequently preclude quick access to services such as group homes, therapeutic foster programs, and residential treatment. Hospitalization often is required to stabilize psychiatric conditions and medical illness.
CASE REPORT: Multiple comorbidities
Ms. P, age 16, has type 1 diabetes mellitus, posttraumatic stress disorder from early physical and sexual abuse, and an IQ of 49. She presents after repeated arguments and physical confrontations with her mother, with whom she lives. She has been caught hoarding high-sugar foods.
The most recent fight is over Ms. P wanting to consume large amounts of candy. She has been hospitalized twice for diabetic ketoacidosis in the last 6 months. Her most recent blood sugar levels ranged from 250 to 500 mg/dL. Ms. P states she is angry at her mother and will hit her if she tries to control her diet. She says she doesn’t care if she gets sick, but her recognition of medical complications is limited.
Developmental delays may complicate treatment for psychiatric illness or impair an adolescent’s ability to understand the dangerousness of his or her behaviors.15 Communication barriers make it challenging to assess risk or the patient’s ability to comply with a safety plan. In patients with developmental delay who live in the community, external structure, monitoring, and the ability to manage crises depends on the family/caregivers. Strongly consider hospitalization if an adolescent’s developmental delay has a serious adverse effect on managing the psychiatric condition, causing increased risk of harm to self or others.
Substance use frequently accompanies adolescent psychiatric illness and may pose severe risk by disinhibiting impulse control, exacerbating mood symptoms, altering mental status, or causing intoxication or withdrawal syndromes. Substance use also carries inherent risks, such as contracting human immunodeficiency virus or other blood-borne infections.
Substance use is well-documented as a severe risk factor for suicide and suicide attempts7,8 and frequently is associated with violence.16 Hospitalization may be the safest way to manage an adolescent who exhibits escalating substance use that complicates management of the psychiatric illness or indicates progressive endangering behavior.
Functional assessment
In addition to exploring risk of self-harm, aggressive behaviors, and medical comorbidities, evaluate the adolescent’s ability to function in interpersonal relationships, self-care, and school. A pattern of severe or worsening functional impairment often indicates illness progression or that management or supports are not meeting the patient’s needs.
Strongly consider hospitalizing patients who demonstrate serious deterioration in interpersonal relationships with peers, adults, or family, as evidenced by escalating threats, episodic violence, or disorganized communication. Additional concerns include severe social withdrawal, neglect of self-care appropriate to developmental level, and inability to perform academically despite appropriate accommodations.
Identify impaired physical functions. When severe medical complications accompany anorexia nervosa or other psychiatric illness, hospitalization is needed to ensure the patient’s safety and to begin appropriate assessment and treatment ( Table 2 ).17
Table 2
Adolescents with eating disorders: Admission criteria
| Heart rate near 40 bpm |
| Orthostasis (pulse change >20 bpm or blood pressure drop of >10 to 20 mm Hg from sitting to standing) |
| Hypotension (blood pressure <80/50 mm Hg) |
| Electrolyte imbalance (hypokalemia, hypophosphatemia, hypomagnesemia) |
| Weight <85% of ideal body weight |
| Acute weight decline with food refusal |
| Suicidal ideation |
| Needs supervision during and after all meals and in bathrooms because of disabling purging |
| Suitability of pediatric vs psychiatric unit depends on level of medical care required and respective units’ ability to manage eating disorders |
| Source: Adapted from reference 17 |
Family and environmental factors
The decision to admit an adolescent to a psychiatric hospital or provide a home treatment plan often hinges on the ability and willingness of the patient’s family/caregivers and support systems to meet the patient’s needs. Consider whether family functioning has been disrupted by a parent’s illness, death, divorce, medical problems, psychiatric illness, substance abuse, or financial stress. If you suspect abuse or violence in the home, observe reporting laws in your jurisdiction and intervene with the family to ensure the adolescent’s safety. Hospitalization may be the best means of providing safety during an investigation.
Determine if the family or primary caregivers are able to meet the adolescent’s developmental, material, and emotional requirements, and if help from treatment or support services or community resources could provide these needs. If not, hospitalization likely is required.
CASE CONTINUED: Risk of physical harm
Ms. P is admitted to the psychiatric hospital because her mother reports that in the past week she and her daughter have had 2 physical altercations—resulting from arguments about her daughter’s dietary intake—that caused injuries. She does not feel she can keep her daughter safe. Ms. P’s mother states she feels she is poorly trained in diabetic care and cannot provide the medical intervention her daughter needs.
Know your system
Child and adolescent psychiatric services are in great demand but often fall far short in meeting these needs across ethnic and socioeconomic groups. Availability of resources differs by geographic location and payer source.18,19 Community-funded mental health varies considerably. The best organizations offer a complete system of care, including outpatient therapy, medication management, case management, wraparound services, respite care, group homes, residential programs, and crisis programs.
Be familiar with local and regional programs and methods of accessing them. For patients who have access to a system of care, timely mobilization of appropriate resources often can avoid a hospital admission and place a patient in a less restrictive setting. Such options, however, frequently are not available to patients covered by commercial payers. For them, the decision typically is reduced to whether the family can manage the patient at home; if the family is unable to ensure safety, the adolescent is hospitalized.
Knowing the capability of available inpatient programs is essential to making an appropriate referral. Consider the level of medical care the psychiatric unit can provide and the accessibility of medical consultation services—both primary and medical subspecialty. Specialized programs for young people with comorbid severe cognitive delays, eating disorders, or forensic difficulties also assist with effective management. The inpatient unit’s collaboration and communication with outpatient providers frequently determines the success of the patient’s transition to less restrictive care.
- Child and Adolescent Service Intensity Instrument (CASII). www.aacap.org/cs/root/member_information/practice_information/casii.
Drug brand names
- Aripiprazole • Abilify
- Fluoxetine • Prozac
Disclosure
Dr. Sorter reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Green J, Jacobs B, Beecham J, et al. Inpatient treatment in child and adolescent psychiatry—a prospective study of health gain and costs. J Child Psychol Psychiatry. 2007;48(12):1259-1267.
2. American Academy of Child and Adolescent Psychiatry. Inpatient hospital treatment of children and adolescents. Policy statement. June 1989. Available at: http://www.aacap.org/cs/root/policy_statements/inpatient_hospital_treatment_of_children_
and_adolescents. Accessed November 19, 2009.
3. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in U.S. community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96.
4. Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62:107-114.
5. Sharfstein SS. Goals of inpatient treatment for psychiatric disorders. Annu Rev Med. 2009;60:393-403.
6. Thienhaus OJ. The decision to admit. In: Thienhaus OJ, ed. Manual of clinical hospital psychiatry. Washington, DC: American Psychiatric Press, Inc; 1995:3–16.
7. Shaffer D, Pfeffer CR. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7):24S-51S.
8. Spirito A, Espposito-Smythers C. Attempted and completed suicide in adolescence. Ann Rev Clin Psychol. 2005;2:237-266.
9. Jenson PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309-322.
10. Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808-820.
11. Dean AJ, Duke SG, Scott J, et al. Physical aggression during admission to a child and adolescent inpatient unit: predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42(6):536-543.
12. Masters KJ, Bellonci C. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2):4S-25S.
13. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. VI: scales assessing externalizing behaviors. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1143-1170.
14. Child and adolescent service intensity instrument user’s manual. Version 3.0. Washington, DC: American Academy of Child and Adolescent Psychiatry; 2007.
15. Lee P, Friedlander R. Attention-deficit and disruptive behavior disorders. In: Fletcher R, Loschen E, Stavrakaki C, et al, eds. Diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: National Association for the Dually Diagnosed; 2007:127–144.
16. Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623-632.
17. American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163 (7 suppl):4-54.
18. Sturm R, Ringel JS, Andreyeva T. Geographic disparities in children’s mental health care. Pediatrics. 2003;112(4):e308-e315.
19. Katoaka SM, Zhang L, Wells KB. Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. Am J Psychiatry. 2002;159:1548-1555.
1. Green J, Jacobs B, Beecham J, et al. Inpatient treatment in child and adolescent psychiatry—a prospective study of health gain and costs. J Child Psychol Psychiatry. 2007;48(12):1259-1267.
2. American Academy of Child and Adolescent Psychiatry. Inpatient hospital treatment of children and adolescents. Policy statement. June 1989. Available at: http://www.aacap.org/cs/root/policy_statements/inpatient_hospital_treatment_of_children_
and_adolescents. Accessed November 19, 2009.
3. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in U.S. community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96.
4. Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62:107-114.
5. Sharfstein SS. Goals of inpatient treatment for psychiatric disorders. Annu Rev Med. 2009;60:393-403.
6. Thienhaus OJ. The decision to admit. In: Thienhaus OJ, ed. Manual of clinical hospital psychiatry. Washington, DC: American Psychiatric Press, Inc; 1995:3–16.
7. Shaffer D, Pfeffer CR. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7):24S-51S.
8. Spirito A, Espposito-Smythers C. Attempted and completed suicide in adolescence. Ann Rev Clin Psychol. 2005;2:237-266.
9. Jenson PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309-322.
10. Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808-820.
11. Dean AJ, Duke SG, Scott J, et al. Physical aggression during admission to a child and adolescent inpatient unit: predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42(6):536-543.
12. Masters KJ, Bellonci C. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2):4S-25S.
13. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. VI: scales assessing externalizing behaviors. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1143-1170.
14. Child and adolescent service intensity instrument user’s manual. Version 3.0. Washington, DC: American Academy of Child and Adolescent Psychiatry; 2007.
15. Lee P, Friedlander R. Attention-deficit and disruptive behavior disorders. In: Fletcher R, Loschen E, Stavrakaki C, et al, eds. Diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: National Association for the Dually Diagnosed; 2007:127–144.
16. Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623-632.
17. American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163 (7 suppl):4-54.
18. Sturm R, Ringel JS, Andreyeva T. Geographic disparities in children’s mental health care. Pediatrics. 2003;112(4):e308-e315.
19. Katoaka SM, Zhang L, Wells KB. Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. Am J Psychiatry. 2002;159:1548-1555.
Borderline, bipolar, or both? Frame your diagnosis on the patient history
Borderline personality disorder (BPD) and bipolar disorder are frequently confused with each other, in part because of their considerable symptomatic overlap. This redundancy occurs despite the different ways these disorders are conceptualized: BPD as a personality disorder and bipolar disorder as a brain disease among Axis I clinical disorders.
BPD and bipolar disorder—especially bipolar II—often co-occur ( Box ) and are frequently misidentified, as shown by clinical and epidemiologic studies. Misdiagnosis creates problems for clinicians and patients. When diagnosed with BPD, patients with bipolar disorder may be deprived of potentially effective pharmacologic treatments.1 Conversely, the stigma that BPD carries—particularly in the mental health community—may lead clinicians to:
- not even disclose the BPD diagnosis to patients2
- lean in the direction of diagnosing BPD as bipolar disorder, potentially resulting in treatments that have little relevance or failure to refer for more appropriate psychosocial treatments.
To help you avoid confusion and the pitfalls of misdiagnosis, this article clarifies the distinctions between bipolar disorder and BPD. We discuss symptom overlap, highlight key differences between the constructs, outline diagnostic differences, and provide useful suggestions to discern the differential diagnosis.
between BPD and bipolar disorder*
1. Inability of current nosology to separate 2 distinct conditions
Relatively indistinct diagnostic boundaries confuse the differentiation of borderline personality disorder (BPD) and bipolar disorder (5 of 9 BPD criteria may occur with mania or hypomania). In this model, the person has 1 disorder but because of symptom overlap receives a diagnosis of both. Because structured interviews do not allow for subjective judgment or expert opinion, the result is the generation of 2 diagnoses when 1 may provide a more parsimonious and valid explanation.
2. BPD exists on a spectrum with bipolar disorder
The mood lability of BPD may be viewed as not unlike that seen with bipolar disorder.1 Behaviors displayed by patients with BPD are subsequently conceptualized as arising from their unstable mood. Supporting arguments cite family study data and evidence from pharmacotherapy trials of anticonvulsants, including divalproex, for rapid cycling bipolar disorder and BPD.2 Family studies have been notable for their failure to directly characterize family members, however, and clinical trials have been quite small. Further, treatment response may have very limited nosologic implications.
3. Bipolar disorder is a risk factor for BPD
4. BPD is a risk factor for bipolar disorder
Early emergence of a bipolar disorder (in preadolescent or adolescent patients) has been proposed to disrupt psychological development, leading to BPD. This adverse impact on personality development—the “scar hypothesis”3 —is supported by data showing greater risk of co-occurring BPD with earlier onset bipolar disorder.4 More important, prospective studies of patients with bipolar disorder show a greater risk for developing BPD.5
BPD also may be a risk factor for the development of bipolar disorder—the “vulnerability hypothesis.”3 Patients with BPD are more likely to develop bipolar disorder, even compared to patients with other personality disorders.5
5. Shared risk factors
BPD and bipolar disorder may be linked by shared risk factors, such as shared genes or trait neuroticism.3
*Some evidence supports each potential explanation, and they are not necessarily mutually exclusive
References
a. Akiskal HS. Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand. 2004;110(6):401-407.
b. Mackinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
c. Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60(2):79-88.
d. Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11(2):205-208.
e. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
Overlapping symptoms
Bipolar disorder is generally considered a clinical disorder or brain disease that can be understood as a broken mood “thermostat.” The lifetime prevalence of bipolar types I and II is approximately 2%.3 Approximately one-half of patients have a family history of illness, and multiple genes are believed to influence inheritance. Mania is the disorder’s hallmark,4 although overactivity has alternatively been proposed as a core feature.5 Most patients with mania ultimately experience depression6 ( Table 1 ).
No dimensional personality correlates have been consistently demonstrated in bipolar disorder, although co-occurring personality disorders—often the “dramatic” Cluster B type—are common4,7 and may adversely affect treatment response and suicide risk.8,9
Both bipolar disorder and BPD are associated with considerable risk of suicide or suicide attempts.10,11 Self-mutilation or self-injurious behavior without suicidal intent are particularly common in BPD.12 Threats of suicide—which may be manipulative or help-seeking—also are common in BPD and tend to be acute rather than chronic.13
Borderline personality disorder is characterized by an enduring and inflexible pattern of thoughts, feelings, and behaviors that impairs an individual’s psychosocial or vocational function. Its estimated prevalence is approximately 1%,14 although recent community estimates approach 6%.15 Genetic influences play a lesser etiologic role in BPD than in bipolar disorder.
Several of BPD’s common features ( Table 2 )—impulsivity, mood instability, inappropriate anger, suicidal behavior, and unstable relationships—are shared with bipolar disorder, but patients with BPD tend to show higher levels of impulsiveness and hostility than patients with bipolar disorder.16 Dimensional assessments of personality traits suggest that BPD is characterized by high neuroticism and low agreeableness.17 BPD also has been more strongly associated with a childhood history of abuse, even when compared with control groups having other personality disorders or major depression.18 The male-to-female ratio for bipolar disorder approximates 1:1;3 in BPD this ratio has been estimated at 1:4 in clinical samples19 and near 1:1 in community samples.15
BPD and bipolar disorder often co-occur. Evidence indicates ≤20% of patients with BPD have comorbid bipolar disorder20 and 15% of patients with bipolar disorder have comorbid BPD.21 Co-occurrence happens much more often than would be expected by chance. These similar bidirectional comorbidity estimates (15% to 20%) would not be expected for conditions of such differing prevalence (<1% vs 2% or more). This suggests:
- the estimated prevalence of bipolar disorder in BPD is too low
- the estimated prevalence of BPD in bipolar disorder samples is too high
- borderline personality disorder is present in >1% of the population
- bipolar disorder is less common
- some combination of the above.
Among these possibilities, the prevalence estimates of bipolar disorder are the most consistent. Several studies suggest that BPD may be much more common, with some estimates exceeding 5%.15
Table 1
Common signs and symptoms
associated with mania and depression in bipolar disorder
| (Hypo)mania | Depression |
|---|---|
| Elevated mood | Decreased mood |
| Irritability | Irritability |
| Decreased need for sleep | Anhedonia |
| Grandiosity | Decreased self-attitude |
| Talkativeness | Insomnia/hypersomnia |
| Racing thoughts | Change in appetite/weight |
| Increased motor activity | Fatigue |
| Increased sex drive | Hopelessness |
| Religiosity | Suicidal thoughts |
| Distractibility | Impaired concentration |
Table 2
Borderline personality disorder: Commonly reported features
| Impulsivity |
| Unstable relationships |
| Unstable self-image |
| Affective instability |
| Fear of abandonment |
| Recurrent self-injurious or suicidal behavior |
| Feelings of emptiness |
| Intense anger or hostility |
| Transient paranoia or dissociative symptoms |
Roots of misdiagnosis
The presence of bipolar disorder or BPD may increase the risk that the other will be misdiagnosed. When symptoms of both are present, those suggesting 1 diagnosis may reflect the consequences of the other. A diagnosis of BPD could represent a partially treated or treatment-resistant bipolar disorder, or a BPD diagnosis could be the result of several years of disruption by a mood disorder.
Characteristics of bipolar disorder have contributed to clinician bias in favor of that diagnosis rather than BPD ( Table 3 ).22,23 Bipolar disorder also may be misdiagnosed as BPD. This error may most likely occur when the history focuses excessively on cross-sectional symptoms, such as when a patient with bipolar disorder shows prominent mood lability or interpersonal sensitivity during a mood episode but not when euthymic.
Bipolar II disorder. The confusion between bipolar disorder and BPD may be particularly problematic for patients with bipolar II disorder or subthreshold bipolar disorders. The manias of bipolar I disorder are much more readily distinguishable from the mood instability or reactivity of BPD. The manic symptoms of bipolar I are more florid, more pronounced, and lead to more obvious impairment.
The milder highs of bipolar II may resemble the mood fluctuations seen in BPD. Further, bipolar II is characterized by a greater chronicity and affective morbidity than bipolar I, and episodes of illness may be characterized by irritability, anger, and racing thoughts.24 Whereas impulsivity or aggression are more characteristic of BPD, bipolar II is similar to BPD on dimensions of affective instability.24,25
When present in BPD, affective instability or lability is conceptualized as ultra-rapid or ultradian, with a frequency of hours to days. BPD is less likely than bipolar II to show affective lability between depression and euthymia or elation and more likely to show fluctuations into anger and anxiety.26
Nonetheless, because of the increased prominence of shared features and reduced distinguishing features, bipolar II and BPD are prone to misdiagnosis and commonly co-occur.
Table 3
Clinician biases that may favor a bipolar disorder diagnosis, rather than BPD
| Bipolar disorder is supported by decades of research |
| Patients with bipolar disorder are often considered more “likeable” than those with BPD |
| Bipolar disorder is more treatable and has a better long-term outcome than BPD (although BPD is generally characterized by clinical improvement, whereas bipolar disorder is more stable with perhaps some increase in depressive symptom burden) |
| Widely thought to have a biologic basis, the bipolar diagnosis conveys less stigma than BPD, which often is less empathically attributed to the patient’s own failings |
| A bipolar diagnosis is easier to explain to patients than BPD; many psychiatrists have difficulty explaining personality disorders in terms patients understand |
| BPD: borderline personality disorder |
| Source: References 22,23 |
History, the diagnostic key
A thorough and rigorous psychiatric history is essential to distinguish BPD from bipolar disorder. Supplementing the patient’s history with an informant interview is often helpful.
Because personality disorders are considered a chronic and enduring pattern of maladaptive behavior, focus the history on longitudinal course and not simply cross-sectional symptoms. Thus, symptoms suggestive of BPD that are confined only to clearly defined episodes of mood disturbance and are absent during euthymia would not warrant a BPD diagnosis.
Temporal relationship. A detailed chronologic history can help determine the temporal relationship between any borderline features and mood episodes. When the patient’s life story is used as a scaffold for the phenomenologic portions of the psychiatric history, one can determine whether any such functional impairment is confined to episodes of mood disorder or appears as an enduring pattern of thinking, acting, and relating. Exploring what happened at notable life transitions—leaving school, loss of job, divorce/separation—may be similarly helpful.
Family history of psychiatric illness may provide a clue to an individual’s genetic predisposition but, of course, does not determine diagnosis. A detailed family and social history that provides evidence of an individual’s function in school, work, and interpersonal relationships is more relevant.
Abandonment and identity issues. Essential to BPD is fear of abandonment, often an undue fear that those important to patients will leave them. Patients may go to extremes to avoid being “abandoned,” even when this threat is not genuine.27,28 Their insecure attachments often lead them to fear being alone. The patient with BPD may:
- make frantic phone calls or send text messages to a friend or lover seeking reassurance
- take extreme measures such as refusing to leave the person’s home or pleading with them not to leave.
Patients with BPD often struggle with identity disturbance, leading them to wonder who they are and what their beliefs and core values are.29 Although occasionally patients with bipolar disorder may have these symptoms, they are not characteristic of bipolar disorder.
Mood lability. The time course of changes in affect or mood swings also may help distinguish BPD from bipolar disorder.
- With bipolar disorder the shift typically is from depression to elation or the reverse, and moods are sustained. Manias or hypomanias are often immediately followed by a “crash” into depression.
- With BPD, “roller-coaster moods” are typical, mood shifts are nonsustained, and the poles often are anxiety, anger, or desperation.
Patients with BPD often report moods shifting rapidly over minutes or hours, but they rarely describe moods sustained for days or weeks on end—other than perhaps depression. Mood lability of BPD often is produced by interpersonal sensitivity, whereas mood lability in bipolar disorder tends to be autonomous and persistent.
Young patients. Assessment can be particularly challenging in young adults and adolescents because symptoms of an emerging bipolar disorder can be more difficult to distinguish from BPD.30 Patients this young also may have less longitudinal history to distinguish an enduring pattern of thinking and relating from a mood disorder. For these cases, it may be particularly important to classify the frequency and pattern of mood symptoms.
Affective dysregulation is a core feature of BPD and is variably defined as a mood reactivity, typically of short duration (often hours). Cycling in bipolar disorder classically involves a periodicity of weeks to months. Even the broadest definitions include a minimum duration of 2 days for hypomania.5
Mood reactivity can occur within episodes of bipolar disorder, although episodes may occur spontaneously and without an obvious precipitant or stressor. Impulsivity may represent more of an essential feature of BPD than affective instability or mood reactivity and may be of particular diagnostic relevance.
Treatment implications
When you are unable to make a clear diagnosis, describe your clinical reasoning and differential diagnosis in the assessment or formulation. With close follow-up, the longitudinal history and course of illness may eventually lead you to an accurate diagnosis.
There are good reasons to acknowledge both conditions when bipolar disorder and BPD are present. Proper recognition of bipolar disorder is a prerequisite to taking full advantage of proven pharmacologic treatments. The evidence base for pharmacologic management of BPD remains limited,31 but recognizing this disorder may help the patient understand his or her psychiatric history and encourage the use of effective psychosocial treatments.
Psychosocial treatments for bipolar disorder may target demoralization and circadian rhythms with sleep hygiene or social rhythms therapy. Acknowledging BPD:
- helps both clinician and patient to better understand the condition
- facilitates setting realistic treatment goals because BPD tends to respond to medication less robustly than bipolar disorder.
Recognizing BPD also allows for referral to targeted psychosocial treatments, including dialectical behavior therapy, mentalization-based treatment, or Systems Training for Emotional Predictability and Problem Solving (STEPPS).32-34
Related resources
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
- National Institute of Mental Health. Bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Systems Training for Emotional Predictability and Problem Solving (STEPPS). www.uihealthcare.com/topics/medicaldepartments/psychiatry/stepps/index.html.
Drug brand name
- Divalproex • Depakote, Depakene, others
Disclosures
Dr. Fiedorowicz reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Acknowledgment
The authors would like to thank Nancee Blum, MSW, and Nancy Hale, RN, for their assistance and expertise in the preparation of this article.
1. John H, Sharma V. Misdiagnosis of bipolar disorder as borderline personality disorder: clinical and economic consequences. World J Biol Psychiatry. 2007;1-4[epub ahead of print].
2. Lequesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004;10(3):170-176.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476-486.
5. Benazzi F. Testing new diagnostic criteria for hypomania. Ann Clin Psychiatry. 2007;19(2):99-104.
6. Solomon DA, Leon AC, Endicott J, et al. Unipolar mania over the course of a 20-year follow-up study. Am J Psychiatry. 2003;160(11):2049-2051.
7. Schiavone P, Dorz S, Conforti D, et al. Comorbidity of DSM-IV personality disorders in unipolar and bipolar affective disorders: a comparative study. Psychol Rep. 2004;95(1):121-128.
8. Fan AH, Hassell J. Bipolar disorder and comorbid personality psychopathology: a review of the literature. J Clin Psychiatry. 2008;69(11):1794-1803.
9. Garno JL, Goldberg JF, Ramirez PM, et al. Bipolar disorder with comorbid cluster B personality disorder features: impact on suicidality. J Clin Psychiatry. 2005;66(3):339-345.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Fiedorowicz JG, Leon AC, Keller MB, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763-771.
12. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13(3):179-185.
13. Zanarini MC, Frankenburg FR, Hennen J, et al. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. J Pers Disord. 2005;19(5):505-523.
14. Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590-596.
15. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
16. Wilson ST, Stanley B, Oquendo MA, et al. Comparing impulsiveness, hostility, and depression in borderline personality disorder and bipolar II disorder. J Clin Psychiatry. 2007;68(10):1533-1539.
17. Zweig-Frank H, Paris J. The five-factor model of personality in borderline and nonborderline personality disorders. Can J Psychiatry. 1995;40(9):523-526.
18. Zanarini MC, Frankenburg FR, Reich DB, et al. Adult experiences of abuse reported by borderline patients and Axis II comparison subjects over six years of prospective follow-up. J Nerv Ment Dis. 2005;193(6):412-416.
19. Zanarini MC, Frankenburg FR, Reich DB, et al. Violence in the lives of adult borderline patients. J Nerv Ment Dis. 1999;187(2):65-71.
20. McCormick B, Blum N, Hansel R, et al. Relationship of sex to symptom severity, psychiatric comorbidity, and health care utilization in 163 subjects with borderline personality disorder. Compr Psychiatry. 2007;48(5):406-412.
21. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
22. Paris J, Zweig-Frank H. A 27-year follow-up of patients with borderline personality disorder. Compr Psychiatry. 2001;42(6):482-487.
23. Coryell WH, Fiedorowicz JG, Solomon D, et al. Age transitions in the course of bipolar I disorder. Psychol Med. 2009;39(8):1247-1252.
24. Benazzi F. Borderline personality-bipolar spectrum relationship. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):68-74.
25. Critchfield KL, Levy KN, Clarkin JF. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. J Pers Disord. 2004;18(6):555-570.
26. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
27. Bray A. The extended mind and borderline personality disorder. Australas Psychiatry. 2008;16(1):8-12.
28. Gunderson JG. The borderline patient’s intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry. 1996;153(6):752-758.
29. Jorgensen CR. Disturbed sense of identity in borderline personality disorder. J Pers Disord. 2006;20(6):618-644.
30. Smith DJ, Muir WJ, Blackwood DH. Borderline personality disorder characteristics in young adults with recurrent mood disorders: a comparison of bipolar and unipolar depression. J Affect Disord. 2005;87(1):17-23.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006(1);CD005653.-
32. Lynch TR, Trost WT, Salsman N, et al. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181-205.
33. Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Pers Disord. 2004;18(1):36-51.
34. Blum N, St John D, Pfohl B, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165(4):468-478.
Borderline personality disorder (BPD) and bipolar disorder are frequently confused with each other, in part because of their considerable symptomatic overlap. This redundancy occurs despite the different ways these disorders are conceptualized: BPD as a personality disorder and bipolar disorder as a brain disease among Axis I clinical disorders.
BPD and bipolar disorder—especially bipolar II—often co-occur ( Box ) and are frequently misidentified, as shown by clinical and epidemiologic studies. Misdiagnosis creates problems for clinicians and patients. When diagnosed with BPD, patients with bipolar disorder may be deprived of potentially effective pharmacologic treatments.1 Conversely, the stigma that BPD carries—particularly in the mental health community—may lead clinicians to:
- not even disclose the BPD diagnosis to patients2
- lean in the direction of diagnosing BPD as bipolar disorder, potentially resulting in treatments that have little relevance or failure to refer for more appropriate psychosocial treatments.
To help you avoid confusion and the pitfalls of misdiagnosis, this article clarifies the distinctions between bipolar disorder and BPD. We discuss symptom overlap, highlight key differences between the constructs, outline diagnostic differences, and provide useful suggestions to discern the differential diagnosis.
between BPD and bipolar disorder*
1. Inability of current nosology to separate 2 distinct conditions
Relatively indistinct diagnostic boundaries confuse the differentiation of borderline personality disorder (BPD) and bipolar disorder (5 of 9 BPD criteria may occur with mania or hypomania). In this model, the person has 1 disorder but because of symptom overlap receives a diagnosis of both. Because structured interviews do not allow for subjective judgment or expert opinion, the result is the generation of 2 diagnoses when 1 may provide a more parsimonious and valid explanation.
2. BPD exists on a spectrum with bipolar disorder
The mood lability of BPD may be viewed as not unlike that seen with bipolar disorder.1 Behaviors displayed by patients with BPD are subsequently conceptualized as arising from their unstable mood. Supporting arguments cite family study data and evidence from pharmacotherapy trials of anticonvulsants, including divalproex, for rapid cycling bipolar disorder and BPD.2 Family studies have been notable for their failure to directly characterize family members, however, and clinical trials have been quite small. Further, treatment response may have very limited nosologic implications.
3. Bipolar disorder is a risk factor for BPD
4. BPD is a risk factor for bipolar disorder
Early emergence of a bipolar disorder (in preadolescent or adolescent patients) has been proposed to disrupt psychological development, leading to BPD. This adverse impact on personality development—the “scar hypothesis”3 —is supported by data showing greater risk of co-occurring BPD with earlier onset bipolar disorder.4 More important, prospective studies of patients with bipolar disorder show a greater risk for developing BPD.5
BPD also may be a risk factor for the development of bipolar disorder—the “vulnerability hypothesis.”3 Patients with BPD are more likely to develop bipolar disorder, even compared to patients with other personality disorders.5
5. Shared risk factors
BPD and bipolar disorder may be linked by shared risk factors, such as shared genes or trait neuroticism.3
*Some evidence supports each potential explanation, and they are not necessarily mutually exclusive
References
a. Akiskal HS. Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand. 2004;110(6):401-407.
b. Mackinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
c. Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60(2):79-88.
d. Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11(2):205-208.
e. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
Overlapping symptoms
Bipolar disorder is generally considered a clinical disorder or brain disease that can be understood as a broken mood “thermostat.” The lifetime prevalence of bipolar types I and II is approximately 2%.3 Approximately one-half of patients have a family history of illness, and multiple genes are believed to influence inheritance. Mania is the disorder’s hallmark,4 although overactivity has alternatively been proposed as a core feature.5 Most patients with mania ultimately experience depression6 ( Table 1 ).
No dimensional personality correlates have been consistently demonstrated in bipolar disorder, although co-occurring personality disorders—often the “dramatic” Cluster B type—are common4,7 and may adversely affect treatment response and suicide risk.8,9
Both bipolar disorder and BPD are associated with considerable risk of suicide or suicide attempts.10,11 Self-mutilation or self-injurious behavior without suicidal intent are particularly common in BPD.12 Threats of suicide—which may be manipulative or help-seeking—also are common in BPD and tend to be acute rather than chronic.13
Borderline personality disorder is characterized by an enduring and inflexible pattern of thoughts, feelings, and behaviors that impairs an individual’s psychosocial or vocational function. Its estimated prevalence is approximately 1%,14 although recent community estimates approach 6%.15 Genetic influences play a lesser etiologic role in BPD than in bipolar disorder.
Several of BPD’s common features ( Table 2 )—impulsivity, mood instability, inappropriate anger, suicidal behavior, and unstable relationships—are shared with bipolar disorder, but patients with BPD tend to show higher levels of impulsiveness and hostility than patients with bipolar disorder.16 Dimensional assessments of personality traits suggest that BPD is characterized by high neuroticism and low agreeableness.17 BPD also has been more strongly associated with a childhood history of abuse, even when compared with control groups having other personality disorders or major depression.18 The male-to-female ratio for bipolar disorder approximates 1:1;3 in BPD this ratio has been estimated at 1:4 in clinical samples19 and near 1:1 in community samples.15
BPD and bipolar disorder often co-occur. Evidence indicates ≤20% of patients with BPD have comorbid bipolar disorder20 and 15% of patients with bipolar disorder have comorbid BPD.21 Co-occurrence happens much more often than would be expected by chance. These similar bidirectional comorbidity estimates (15% to 20%) would not be expected for conditions of such differing prevalence (<1% vs 2% or more). This suggests:
- the estimated prevalence of bipolar disorder in BPD is too low
- the estimated prevalence of BPD in bipolar disorder samples is too high
- borderline personality disorder is present in >1% of the population
- bipolar disorder is less common
- some combination of the above.
Among these possibilities, the prevalence estimates of bipolar disorder are the most consistent. Several studies suggest that BPD may be much more common, with some estimates exceeding 5%.15
Table 1
Common signs and symptoms
associated with mania and depression in bipolar disorder
| (Hypo)mania | Depression |
|---|---|
| Elevated mood | Decreased mood |
| Irritability | Irritability |
| Decreased need for sleep | Anhedonia |
| Grandiosity | Decreased self-attitude |
| Talkativeness | Insomnia/hypersomnia |
| Racing thoughts | Change in appetite/weight |
| Increased motor activity | Fatigue |
| Increased sex drive | Hopelessness |
| Religiosity | Suicidal thoughts |
| Distractibility | Impaired concentration |
Table 2
Borderline personality disorder: Commonly reported features
| Impulsivity |
| Unstable relationships |
| Unstable self-image |
| Affective instability |
| Fear of abandonment |
| Recurrent self-injurious or suicidal behavior |
| Feelings of emptiness |
| Intense anger or hostility |
| Transient paranoia or dissociative symptoms |
Roots of misdiagnosis
The presence of bipolar disorder or BPD may increase the risk that the other will be misdiagnosed. When symptoms of both are present, those suggesting 1 diagnosis may reflect the consequences of the other. A diagnosis of BPD could represent a partially treated or treatment-resistant bipolar disorder, or a BPD diagnosis could be the result of several years of disruption by a mood disorder.
Characteristics of bipolar disorder have contributed to clinician bias in favor of that diagnosis rather than BPD ( Table 3 ).22,23 Bipolar disorder also may be misdiagnosed as BPD. This error may most likely occur when the history focuses excessively on cross-sectional symptoms, such as when a patient with bipolar disorder shows prominent mood lability or interpersonal sensitivity during a mood episode but not when euthymic.
Bipolar II disorder. The confusion between bipolar disorder and BPD may be particularly problematic for patients with bipolar II disorder or subthreshold bipolar disorders. The manias of bipolar I disorder are much more readily distinguishable from the mood instability or reactivity of BPD. The manic symptoms of bipolar I are more florid, more pronounced, and lead to more obvious impairment.
The milder highs of bipolar II may resemble the mood fluctuations seen in BPD. Further, bipolar II is characterized by a greater chronicity and affective morbidity than bipolar I, and episodes of illness may be characterized by irritability, anger, and racing thoughts.24 Whereas impulsivity or aggression are more characteristic of BPD, bipolar II is similar to BPD on dimensions of affective instability.24,25
When present in BPD, affective instability or lability is conceptualized as ultra-rapid or ultradian, with a frequency of hours to days. BPD is less likely than bipolar II to show affective lability between depression and euthymia or elation and more likely to show fluctuations into anger and anxiety.26
Nonetheless, because of the increased prominence of shared features and reduced distinguishing features, bipolar II and BPD are prone to misdiagnosis and commonly co-occur.
Table 3
Clinician biases that may favor a bipolar disorder diagnosis, rather than BPD
| Bipolar disorder is supported by decades of research |
| Patients with bipolar disorder are often considered more “likeable” than those with BPD |
| Bipolar disorder is more treatable and has a better long-term outcome than BPD (although BPD is generally characterized by clinical improvement, whereas bipolar disorder is more stable with perhaps some increase in depressive symptom burden) |
| Widely thought to have a biologic basis, the bipolar diagnosis conveys less stigma than BPD, which often is less empathically attributed to the patient’s own failings |
| A bipolar diagnosis is easier to explain to patients than BPD; many psychiatrists have difficulty explaining personality disorders in terms patients understand |
| BPD: borderline personality disorder |
| Source: References 22,23 |
History, the diagnostic key
A thorough and rigorous psychiatric history is essential to distinguish BPD from bipolar disorder. Supplementing the patient’s history with an informant interview is often helpful.
Because personality disorders are considered a chronic and enduring pattern of maladaptive behavior, focus the history on longitudinal course and not simply cross-sectional symptoms. Thus, symptoms suggestive of BPD that are confined only to clearly defined episodes of mood disturbance and are absent during euthymia would not warrant a BPD diagnosis.
Temporal relationship. A detailed chronologic history can help determine the temporal relationship between any borderline features and mood episodes. When the patient’s life story is used as a scaffold for the phenomenologic portions of the psychiatric history, one can determine whether any such functional impairment is confined to episodes of mood disorder or appears as an enduring pattern of thinking, acting, and relating. Exploring what happened at notable life transitions—leaving school, loss of job, divorce/separation—may be similarly helpful.
Family history of psychiatric illness may provide a clue to an individual’s genetic predisposition but, of course, does not determine diagnosis. A detailed family and social history that provides evidence of an individual’s function in school, work, and interpersonal relationships is more relevant.
Abandonment and identity issues. Essential to BPD is fear of abandonment, often an undue fear that those important to patients will leave them. Patients may go to extremes to avoid being “abandoned,” even when this threat is not genuine.27,28 Their insecure attachments often lead them to fear being alone. The patient with BPD may:
- make frantic phone calls or send text messages to a friend or lover seeking reassurance
- take extreme measures such as refusing to leave the person’s home or pleading with them not to leave.
Patients with BPD often struggle with identity disturbance, leading them to wonder who they are and what their beliefs and core values are.29 Although occasionally patients with bipolar disorder may have these symptoms, they are not characteristic of bipolar disorder.
Mood lability. The time course of changes in affect or mood swings also may help distinguish BPD from bipolar disorder.
- With bipolar disorder the shift typically is from depression to elation or the reverse, and moods are sustained. Manias or hypomanias are often immediately followed by a “crash” into depression.
- With BPD, “roller-coaster moods” are typical, mood shifts are nonsustained, and the poles often are anxiety, anger, or desperation.
Patients with BPD often report moods shifting rapidly over minutes or hours, but they rarely describe moods sustained for days or weeks on end—other than perhaps depression. Mood lability of BPD often is produced by interpersonal sensitivity, whereas mood lability in bipolar disorder tends to be autonomous and persistent.
Young patients. Assessment can be particularly challenging in young adults and adolescents because symptoms of an emerging bipolar disorder can be more difficult to distinguish from BPD.30 Patients this young also may have less longitudinal history to distinguish an enduring pattern of thinking and relating from a mood disorder. For these cases, it may be particularly important to classify the frequency and pattern of mood symptoms.
Affective dysregulation is a core feature of BPD and is variably defined as a mood reactivity, typically of short duration (often hours). Cycling in bipolar disorder classically involves a periodicity of weeks to months. Even the broadest definitions include a minimum duration of 2 days for hypomania.5
Mood reactivity can occur within episodes of bipolar disorder, although episodes may occur spontaneously and without an obvious precipitant or stressor. Impulsivity may represent more of an essential feature of BPD than affective instability or mood reactivity and may be of particular diagnostic relevance.
Treatment implications
When you are unable to make a clear diagnosis, describe your clinical reasoning and differential diagnosis in the assessment or formulation. With close follow-up, the longitudinal history and course of illness may eventually lead you to an accurate diagnosis.
There are good reasons to acknowledge both conditions when bipolar disorder and BPD are present. Proper recognition of bipolar disorder is a prerequisite to taking full advantage of proven pharmacologic treatments. The evidence base for pharmacologic management of BPD remains limited,31 but recognizing this disorder may help the patient understand his or her psychiatric history and encourage the use of effective psychosocial treatments.
Psychosocial treatments for bipolar disorder may target demoralization and circadian rhythms with sleep hygiene or social rhythms therapy. Acknowledging BPD:
- helps both clinician and patient to better understand the condition
- facilitates setting realistic treatment goals because BPD tends to respond to medication less robustly than bipolar disorder.
Recognizing BPD also allows for referral to targeted psychosocial treatments, including dialectical behavior therapy, mentalization-based treatment, or Systems Training for Emotional Predictability and Problem Solving (STEPPS).32-34
Related resources
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
- National Institute of Mental Health. Bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Systems Training for Emotional Predictability and Problem Solving (STEPPS). www.uihealthcare.com/topics/medicaldepartments/psychiatry/stepps/index.html.
Drug brand name
- Divalproex • Depakote, Depakene, others
Disclosures
Dr. Fiedorowicz reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Acknowledgment
The authors would like to thank Nancee Blum, MSW, and Nancy Hale, RN, for their assistance and expertise in the preparation of this article.
Borderline personality disorder (BPD) and bipolar disorder are frequently confused with each other, in part because of their considerable symptomatic overlap. This redundancy occurs despite the different ways these disorders are conceptualized: BPD as a personality disorder and bipolar disorder as a brain disease among Axis I clinical disorders.
BPD and bipolar disorder—especially bipolar II—often co-occur ( Box ) and are frequently misidentified, as shown by clinical and epidemiologic studies. Misdiagnosis creates problems for clinicians and patients. When diagnosed with BPD, patients with bipolar disorder may be deprived of potentially effective pharmacologic treatments.1 Conversely, the stigma that BPD carries—particularly in the mental health community—may lead clinicians to:
- not even disclose the BPD diagnosis to patients2
- lean in the direction of diagnosing BPD as bipolar disorder, potentially resulting in treatments that have little relevance or failure to refer for more appropriate psychosocial treatments.
To help you avoid confusion and the pitfalls of misdiagnosis, this article clarifies the distinctions between bipolar disorder and BPD. We discuss symptom overlap, highlight key differences between the constructs, outline diagnostic differences, and provide useful suggestions to discern the differential diagnosis.
between BPD and bipolar disorder*
1. Inability of current nosology to separate 2 distinct conditions
Relatively indistinct diagnostic boundaries confuse the differentiation of borderline personality disorder (BPD) and bipolar disorder (5 of 9 BPD criteria may occur with mania or hypomania). In this model, the person has 1 disorder but because of symptom overlap receives a diagnosis of both. Because structured interviews do not allow for subjective judgment or expert opinion, the result is the generation of 2 diagnoses when 1 may provide a more parsimonious and valid explanation.
2. BPD exists on a spectrum with bipolar disorder
The mood lability of BPD may be viewed as not unlike that seen with bipolar disorder.1 Behaviors displayed by patients with BPD are subsequently conceptualized as arising from their unstable mood. Supporting arguments cite family study data and evidence from pharmacotherapy trials of anticonvulsants, including divalproex, for rapid cycling bipolar disorder and BPD.2 Family studies have been notable for their failure to directly characterize family members, however, and clinical trials have been quite small. Further, treatment response may have very limited nosologic implications.
3. Bipolar disorder is a risk factor for BPD
4. BPD is a risk factor for bipolar disorder
Early emergence of a bipolar disorder (in preadolescent or adolescent patients) has been proposed to disrupt psychological development, leading to BPD. This adverse impact on personality development—the “scar hypothesis”3 —is supported by data showing greater risk of co-occurring BPD with earlier onset bipolar disorder.4 More important, prospective studies of patients with bipolar disorder show a greater risk for developing BPD.5
BPD also may be a risk factor for the development of bipolar disorder—the “vulnerability hypothesis.”3 Patients with BPD are more likely to develop bipolar disorder, even compared to patients with other personality disorders.5
5. Shared risk factors
BPD and bipolar disorder may be linked by shared risk factors, such as shared genes or trait neuroticism.3
*Some evidence supports each potential explanation, and they are not necessarily mutually exclusive
References
a. Akiskal HS. Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand. 2004;110(6):401-407.
b. Mackinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
c. Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60(2):79-88.
d. Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11(2):205-208.
e. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
Overlapping symptoms
Bipolar disorder is generally considered a clinical disorder or brain disease that can be understood as a broken mood “thermostat.” The lifetime prevalence of bipolar types I and II is approximately 2%.3 Approximately one-half of patients have a family history of illness, and multiple genes are believed to influence inheritance. Mania is the disorder’s hallmark,4 although overactivity has alternatively been proposed as a core feature.5 Most patients with mania ultimately experience depression6 ( Table 1 ).
No dimensional personality correlates have been consistently demonstrated in bipolar disorder, although co-occurring personality disorders—often the “dramatic” Cluster B type—are common4,7 and may adversely affect treatment response and suicide risk.8,9
Both bipolar disorder and BPD are associated with considerable risk of suicide or suicide attempts.10,11 Self-mutilation or self-injurious behavior without suicidal intent are particularly common in BPD.12 Threats of suicide—which may be manipulative or help-seeking—also are common in BPD and tend to be acute rather than chronic.13
Borderline personality disorder is characterized by an enduring and inflexible pattern of thoughts, feelings, and behaviors that impairs an individual’s psychosocial or vocational function. Its estimated prevalence is approximately 1%,14 although recent community estimates approach 6%.15 Genetic influences play a lesser etiologic role in BPD than in bipolar disorder.
Several of BPD’s common features ( Table 2 )—impulsivity, mood instability, inappropriate anger, suicidal behavior, and unstable relationships—are shared with bipolar disorder, but patients with BPD tend to show higher levels of impulsiveness and hostility than patients with bipolar disorder.16 Dimensional assessments of personality traits suggest that BPD is characterized by high neuroticism and low agreeableness.17 BPD also has been more strongly associated with a childhood history of abuse, even when compared with control groups having other personality disorders or major depression.18 The male-to-female ratio for bipolar disorder approximates 1:1;3 in BPD this ratio has been estimated at 1:4 in clinical samples19 and near 1:1 in community samples.15
BPD and bipolar disorder often co-occur. Evidence indicates ≤20% of patients with BPD have comorbid bipolar disorder20 and 15% of patients with bipolar disorder have comorbid BPD.21 Co-occurrence happens much more often than would be expected by chance. These similar bidirectional comorbidity estimates (15% to 20%) would not be expected for conditions of such differing prevalence (<1% vs 2% or more). This suggests:
- the estimated prevalence of bipolar disorder in BPD is too low
- the estimated prevalence of BPD in bipolar disorder samples is too high
- borderline personality disorder is present in >1% of the population
- bipolar disorder is less common
- some combination of the above.
Among these possibilities, the prevalence estimates of bipolar disorder are the most consistent. Several studies suggest that BPD may be much more common, with some estimates exceeding 5%.15
Table 1
Common signs and symptoms
associated with mania and depression in bipolar disorder
| (Hypo)mania | Depression |
|---|---|
| Elevated mood | Decreased mood |
| Irritability | Irritability |
| Decreased need for sleep | Anhedonia |
| Grandiosity | Decreased self-attitude |
| Talkativeness | Insomnia/hypersomnia |
| Racing thoughts | Change in appetite/weight |
| Increased motor activity | Fatigue |
| Increased sex drive | Hopelessness |
| Religiosity | Suicidal thoughts |
| Distractibility | Impaired concentration |
Table 2
Borderline personality disorder: Commonly reported features
| Impulsivity |
| Unstable relationships |
| Unstable self-image |
| Affective instability |
| Fear of abandonment |
| Recurrent self-injurious or suicidal behavior |
| Feelings of emptiness |
| Intense anger or hostility |
| Transient paranoia or dissociative symptoms |
Roots of misdiagnosis
The presence of bipolar disorder or BPD may increase the risk that the other will be misdiagnosed. When symptoms of both are present, those suggesting 1 diagnosis may reflect the consequences of the other. A diagnosis of BPD could represent a partially treated or treatment-resistant bipolar disorder, or a BPD diagnosis could be the result of several years of disruption by a mood disorder.
Characteristics of bipolar disorder have contributed to clinician bias in favor of that diagnosis rather than BPD ( Table 3 ).22,23 Bipolar disorder also may be misdiagnosed as BPD. This error may most likely occur when the history focuses excessively on cross-sectional symptoms, such as when a patient with bipolar disorder shows prominent mood lability or interpersonal sensitivity during a mood episode but not when euthymic.
Bipolar II disorder. The confusion between bipolar disorder and BPD may be particularly problematic for patients with bipolar II disorder or subthreshold bipolar disorders. The manias of bipolar I disorder are much more readily distinguishable from the mood instability or reactivity of BPD. The manic symptoms of bipolar I are more florid, more pronounced, and lead to more obvious impairment.
The milder highs of bipolar II may resemble the mood fluctuations seen in BPD. Further, bipolar II is characterized by a greater chronicity and affective morbidity than bipolar I, and episodes of illness may be characterized by irritability, anger, and racing thoughts.24 Whereas impulsivity or aggression are more characteristic of BPD, bipolar II is similar to BPD on dimensions of affective instability.24,25
When present in BPD, affective instability or lability is conceptualized as ultra-rapid or ultradian, with a frequency of hours to days. BPD is less likely than bipolar II to show affective lability between depression and euthymia or elation and more likely to show fluctuations into anger and anxiety.26
Nonetheless, because of the increased prominence of shared features and reduced distinguishing features, bipolar II and BPD are prone to misdiagnosis and commonly co-occur.
Table 3
Clinician biases that may favor a bipolar disorder diagnosis, rather than BPD
| Bipolar disorder is supported by decades of research |
| Patients with bipolar disorder are often considered more “likeable” than those with BPD |
| Bipolar disorder is more treatable and has a better long-term outcome than BPD (although BPD is generally characterized by clinical improvement, whereas bipolar disorder is more stable with perhaps some increase in depressive symptom burden) |
| Widely thought to have a biologic basis, the bipolar diagnosis conveys less stigma than BPD, which often is less empathically attributed to the patient’s own failings |
| A bipolar diagnosis is easier to explain to patients than BPD; many psychiatrists have difficulty explaining personality disorders in terms patients understand |
| BPD: borderline personality disorder |
| Source: References 22,23 |
History, the diagnostic key
A thorough and rigorous psychiatric history is essential to distinguish BPD from bipolar disorder. Supplementing the patient’s history with an informant interview is often helpful.
Because personality disorders are considered a chronic and enduring pattern of maladaptive behavior, focus the history on longitudinal course and not simply cross-sectional symptoms. Thus, symptoms suggestive of BPD that are confined only to clearly defined episodes of mood disturbance and are absent during euthymia would not warrant a BPD diagnosis.
Temporal relationship. A detailed chronologic history can help determine the temporal relationship between any borderline features and mood episodes. When the patient’s life story is used as a scaffold for the phenomenologic portions of the psychiatric history, one can determine whether any such functional impairment is confined to episodes of mood disorder or appears as an enduring pattern of thinking, acting, and relating. Exploring what happened at notable life transitions—leaving school, loss of job, divorce/separation—may be similarly helpful.
Family history of psychiatric illness may provide a clue to an individual’s genetic predisposition but, of course, does not determine diagnosis. A detailed family and social history that provides evidence of an individual’s function in school, work, and interpersonal relationships is more relevant.
Abandonment and identity issues. Essential to BPD is fear of abandonment, often an undue fear that those important to patients will leave them. Patients may go to extremes to avoid being “abandoned,” even when this threat is not genuine.27,28 Their insecure attachments often lead them to fear being alone. The patient with BPD may:
- make frantic phone calls or send text messages to a friend or lover seeking reassurance
- take extreme measures such as refusing to leave the person’s home or pleading with them not to leave.
Patients with BPD often struggle with identity disturbance, leading them to wonder who they are and what their beliefs and core values are.29 Although occasionally patients with bipolar disorder may have these symptoms, they are not characteristic of bipolar disorder.
Mood lability. The time course of changes in affect or mood swings also may help distinguish BPD from bipolar disorder.
- With bipolar disorder the shift typically is from depression to elation or the reverse, and moods are sustained. Manias or hypomanias are often immediately followed by a “crash” into depression.
- With BPD, “roller-coaster moods” are typical, mood shifts are nonsustained, and the poles often are anxiety, anger, or desperation.
Patients with BPD often report moods shifting rapidly over minutes or hours, but they rarely describe moods sustained for days or weeks on end—other than perhaps depression. Mood lability of BPD often is produced by interpersonal sensitivity, whereas mood lability in bipolar disorder tends to be autonomous and persistent.
Young patients. Assessment can be particularly challenging in young adults and adolescents because symptoms of an emerging bipolar disorder can be more difficult to distinguish from BPD.30 Patients this young also may have less longitudinal history to distinguish an enduring pattern of thinking and relating from a mood disorder. For these cases, it may be particularly important to classify the frequency and pattern of mood symptoms.
Affective dysregulation is a core feature of BPD and is variably defined as a mood reactivity, typically of short duration (often hours). Cycling in bipolar disorder classically involves a periodicity of weeks to months. Even the broadest definitions include a minimum duration of 2 days for hypomania.5
Mood reactivity can occur within episodes of bipolar disorder, although episodes may occur spontaneously and without an obvious precipitant or stressor. Impulsivity may represent more of an essential feature of BPD than affective instability or mood reactivity and may be of particular diagnostic relevance.
Treatment implications
When you are unable to make a clear diagnosis, describe your clinical reasoning and differential diagnosis in the assessment or formulation. With close follow-up, the longitudinal history and course of illness may eventually lead you to an accurate diagnosis.
There are good reasons to acknowledge both conditions when bipolar disorder and BPD are present. Proper recognition of bipolar disorder is a prerequisite to taking full advantage of proven pharmacologic treatments. The evidence base for pharmacologic management of BPD remains limited,31 but recognizing this disorder may help the patient understand his or her psychiatric history and encourage the use of effective psychosocial treatments.
Psychosocial treatments for bipolar disorder may target demoralization and circadian rhythms with sleep hygiene or social rhythms therapy. Acknowledging BPD:
- helps both clinician and patient to better understand the condition
- facilitates setting realistic treatment goals because BPD tends to respond to medication less robustly than bipolar disorder.
Recognizing BPD also allows for referral to targeted psychosocial treatments, including dialectical behavior therapy, mentalization-based treatment, or Systems Training for Emotional Predictability and Problem Solving (STEPPS).32-34
Related resources
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
- National Institute of Mental Health. Bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Systems Training for Emotional Predictability and Problem Solving (STEPPS). www.uihealthcare.com/topics/medicaldepartments/psychiatry/stepps/index.html.
Drug brand name
- Divalproex • Depakote, Depakene, others
Disclosures
Dr. Fiedorowicz reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Acknowledgment
The authors would like to thank Nancee Blum, MSW, and Nancy Hale, RN, for their assistance and expertise in the preparation of this article.
1. John H, Sharma V. Misdiagnosis of bipolar disorder as borderline personality disorder: clinical and economic consequences. World J Biol Psychiatry. 2007;1-4[epub ahead of print].
2. Lequesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004;10(3):170-176.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476-486.
5. Benazzi F. Testing new diagnostic criteria for hypomania. Ann Clin Psychiatry. 2007;19(2):99-104.
6. Solomon DA, Leon AC, Endicott J, et al. Unipolar mania over the course of a 20-year follow-up study. Am J Psychiatry. 2003;160(11):2049-2051.
7. Schiavone P, Dorz S, Conforti D, et al. Comorbidity of DSM-IV personality disorders in unipolar and bipolar affective disorders: a comparative study. Psychol Rep. 2004;95(1):121-128.
8. Fan AH, Hassell J. Bipolar disorder and comorbid personality psychopathology: a review of the literature. J Clin Psychiatry. 2008;69(11):1794-1803.
9. Garno JL, Goldberg JF, Ramirez PM, et al. Bipolar disorder with comorbid cluster B personality disorder features: impact on suicidality. J Clin Psychiatry. 2005;66(3):339-345.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Fiedorowicz JG, Leon AC, Keller MB, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763-771.
12. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13(3):179-185.
13. Zanarini MC, Frankenburg FR, Hennen J, et al. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. J Pers Disord. 2005;19(5):505-523.
14. Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590-596.
15. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
16. Wilson ST, Stanley B, Oquendo MA, et al. Comparing impulsiveness, hostility, and depression in borderline personality disorder and bipolar II disorder. J Clin Psychiatry. 2007;68(10):1533-1539.
17. Zweig-Frank H, Paris J. The five-factor model of personality in borderline and nonborderline personality disorders. Can J Psychiatry. 1995;40(9):523-526.
18. Zanarini MC, Frankenburg FR, Reich DB, et al. Adult experiences of abuse reported by borderline patients and Axis II comparison subjects over six years of prospective follow-up. J Nerv Ment Dis. 2005;193(6):412-416.
19. Zanarini MC, Frankenburg FR, Reich DB, et al. Violence in the lives of adult borderline patients. J Nerv Ment Dis. 1999;187(2):65-71.
20. McCormick B, Blum N, Hansel R, et al. Relationship of sex to symptom severity, psychiatric comorbidity, and health care utilization in 163 subjects with borderline personality disorder. Compr Psychiatry. 2007;48(5):406-412.
21. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
22. Paris J, Zweig-Frank H. A 27-year follow-up of patients with borderline personality disorder. Compr Psychiatry. 2001;42(6):482-487.
23. Coryell WH, Fiedorowicz JG, Solomon D, et al. Age transitions in the course of bipolar I disorder. Psychol Med. 2009;39(8):1247-1252.
24. Benazzi F. Borderline personality-bipolar spectrum relationship. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):68-74.
25. Critchfield KL, Levy KN, Clarkin JF. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. J Pers Disord. 2004;18(6):555-570.
26. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
27. Bray A. The extended mind and borderline personality disorder. Australas Psychiatry. 2008;16(1):8-12.
28. Gunderson JG. The borderline patient’s intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry. 1996;153(6):752-758.
29. Jorgensen CR. Disturbed sense of identity in borderline personality disorder. J Pers Disord. 2006;20(6):618-644.
30. Smith DJ, Muir WJ, Blackwood DH. Borderline personality disorder characteristics in young adults with recurrent mood disorders: a comparison of bipolar and unipolar depression. J Affect Disord. 2005;87(1):17-23.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006(1);CD005653.-
32. Lynch TR, Trost WT, Salsman N, et al. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181-205.
33. Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Pers Disord. 2004;18(1):36-51.
34. Blum N, St John D, Pfohl B, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165(4):468-478.
1. John H, Sharma V. Misdiagnosis of bipolar disorder as borderline personality disorder: clinical and economic consequences. World J Biol Psychiatry. 2007;1-4[epub ahead of print].
2. Lequesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004;10(3):170-176.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476-486.
5. Benazzi F. Testing new diagnostic criteria for hypomania. Ann Clin Psychiatry. 2007;19(2):99-104.
6. Solomon DA, Leon AC, Endicott J, et al. Unipolar mania over the course of a 20-year follow-up study. Am J Psychiatry. 2003;160(11):2049-2051.
7. Schiavone P, Dorz S, Conforti D, et al. Comorbidity of DSM-IV personality disorders in unipolar and bipolar affective disorders: a comparative study. Psychol Rep. 2004;95(1):121-128.
8. Fan AH, Hassell J. Bipolar disorder and comorbid personality psychopathology: a review of the literature. J Clin Psychiatry. 2008;69(11):1794-1803.
9. Garno JL, Goldberg JF, Ramirez PM, et al. Bipolar disorder with comorbid cluster B personality disorder features: impact on suicidality. J Clin Psychiatry. 2005;66(3):339-345.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Fiedorowicz JG, Leon AC, Keller MB, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763-771.
12. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13(3):179-185.
13. Zanarini MC, Frankenburg FR, Hennen J, et al. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. J Pers Disord. 2005;19(5):505-523.
14. Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590-596.
15. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
16. Wilson ST, Stanley B, Oquendo MA, et al. Comparing impulsiveness, hostility, and depression in borderline personality disorder and bipolar II disorder. J Clin Psychiatry. 2007;68(10):1533-1539.
17. Zweig-Frank H, Paris J. The five-factor model of personality in borderline and nonborderline personality disorders. Can J Psychiatry. 1995;40(9):523-526.
18. Zanarini MC, Frankenburg FR, Reich DB, et al. Adult experiences of abuse reported by borderline patients and Axis II comparison subjects over six years of prospective follow-up. J Nerv Ment Dis. 2005;193(6):412-416.
19. Zanarini MC, Frankenburg FR, Reich DB, et al. Violence in the lives of adult borderline patients. J Nerv Ment Dis. 1999;187(2):65-71.
20. McCormick B, Blum N, Hansel R, et al. Relationship of sex to symptom severity, psychiatric comorbidity, and health care utilization in 163 subjects with borderline personality disorder. Compr Psychiatry. 2007;48(5):406-412.
21. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
22. Paris J, Zweig-Frank H. A 27-year follow-up of patients with borderline personality disorder. Compr Psychiatry. 2001;42(6):482-487.
23. Coryell WH, Fiedorowicz JG, Solomon D, et al. Age transitions in the course of bipolar I disorder. Psychol Med. 2009;39(8):1247-1252.
24. Benazzi F. Borderline personality-bipolar spectrum relationship. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):68-74.
25. Critchfield KL, Levy KN, Clarkin JF. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. J Pers Disord. 2004;18(6):555-570.
26. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
27. Bray A. The extended mind and borderline personality disorder. Australas Psychiatry. 2008;16(1):8-12.
28. Gunderson JG. The borderline patient’s intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry. 1996;153(6):752-758.
29. Jorgensen CR. Disturbed sense of identity in borderline personality disorder. J Pers Disord. 2006;20(6):618-644.
30. Smith DJ, Muir WJ, Blackwood DH. Borderline personality disorder characteristics in young adults with recurrent mood disorders: a comparison of bipolar and unipolar depression. J Affect Disord. 2005;87(1):17-23.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006(1);CD005653.-
32. Lynch TR, Trost WT, Salsman N, et al. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181-205.
33. Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Pers Disord. 2004;18(1):36-51.
34. Blum N, St John D, Pfohl B, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165(4):468-478.
Clozapine for schizophrenia: Life-threatening or life-saving treatment?
Researchers in Finland surprised psychiatrists this year by announcing that clozapine “seems to be associated with a substantially lower mortality than any other antipsychotic.”1 This finding also surprised the researchers, who expected their 11-year study to link long-term use of second-generation (“atypical”) antipsychotics with increased mortality in patients with schizophrenia. Instead they found longer lives in patients who used antipsychotics (and particularly clozapine), compared with no antipsychotic use.
This study’s findings do not change clozapine’s association with potentially fatal agranulocytosis as well as weight gain, metabolic abnormalities, and other adverse effects. Clozapine also is difficult to administer ( Box 1 ),2 and patients must be enrolled in FDA-mandated registries (see Related Resources ). These obstacles might discourage you from offering clozapine to patients who could benefit from it ( Box 2 ).3-5
Why bother considering clozapine? Because recent data on decreased mortality, decreased suicidality, and control of aggressive behavior make clozapine a compelling choice for many patients. Careful attention to clozapine’s adverse effect profile is necessary, but you can manage these risks with appropriate monitoring.
Because of clozapine’s risk for leukopenia and agranulocytosis, frequent white blood cell count (WBC) monitoring is required. The risk of drug-induced blood dyscrasias has been shown to decrease over time, however, from 0.70/1,000 patient-years in the second 6 months of treatment to 0.39/1,000 patient-years after the first year.2
To start clozapine treatment, FDA guidelines require that the patient’s WBC must be at least 3,500 mm3, and the absolute neutrophil count (ANC) must be at least 2,000 mm3. For the first 6 months, patients receiving clozapine must have a weekly blood test for WBC and ANC.
The dispensing pharmacist must see the blood work result prior to releasing the drug to the patient. The blood draw date must be within the previous 7 days for the pharmacist to dispense a 1-week supply of clozapine.
Decreased monitoring over time. After 6 months of continuous therapy with clozapine without any interruptions because of a low WBC and/or ANC—defined as WBC 3 and/or ANC 3 or increased monitoring (when WBC 3 and/or ANC 3)—the patient’s blood monitoring may be done every 14 days and a 2-week supply of clozapine can be dispensed.
After 12 months of continuous clozapine therapy—6 months of continuous weekly monitoring, then 6 months of continuous biweekly monitoring—without any interruptions or increased monitoring, the patient may have blood monitoring done every 4 weeks and can receive a 4-week supply of clozapine.
One advantage of these monitoring requirements is that the increased frequency of visits can be used to foster greater patient engagement with treatment and promote a therapeutic alliance. Peer-led clozapine support groups, available in some communities, can facilitate adherence to monitoring requirements.
Clozapine was approved in the United States in 1989 for severely ill patients with schizophrenia who had not responded adequately to standard drug treatment. In 2002 it received an indication for patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state.
Off-label, clozapine has been commonly used for refractory bipolar disorder. Since 1998, it has been available in generic formulations and in a proprietary orally-disintegrating tablet formulation.
Dosing. The recommended target clozapine dosage is 300 to 450 mg/d. If an adequate response is not achieved, obtaining a plasma level might be helpful.3 Plasma levels ≥350 ng/mL constitute an optimal clozapine trial.
Not a ‘last resort.’ American Psychiatric Association treatment guidelines for schizophrenia state: “Because of clozapine’s superior efficacy, a trial of clozapine should be considered for a patient with a clinically inadequate response to antipsychotic treatment or for a patient with suicidal ideation or behavior. Besides clozapine, there are limited options for the many patients who have severe and significant residual symptoms even after antipsychotic monotherapy has been optimized, and none have proven benefits.”4
As additional evidence accumulates—including benefits regarding mortality and aggression—clozapine’s advantages support its clinical use earlier than as a “last resort.” In institutional settings, clozapine use has increased with the availability of additional data, such as from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).
In New York State Office of Mental Health hospitals, clozapine use increased from 20.6% of prescriptions in 2005 to 24.9% in 2007, compared with the other CATIE medications (olanzapine, quetiapine, risperidone, ziprasidone) and haloperidol.5 Whether clozapine use will increase in outpatient settings remains to be seen.
Potential for longer life?
The population-based, cohort study from Finland demonstrated that—contrary to popular belief—the introduction of atypical antipsychotics during the 1990s did not adversely affect mortality of patients with schizophrenia, at least in Finland.1
This study made specific drug comparisons and used perphenazine as the reference drug. The lowest risk for mortality was observed with clozapine, which showed a 26% relative advantage compared with perphenazine. Clozapine’s advantage was statistically significant when compared with all other antipsychotics tested.
The authors suggested provocatively that restrictions on clozapine use as a second- or third-line agent should be reassessed. A few caveats, however, might affect how one interprets this study or applies its findings to clinical practice:
- The main comparisons were for patients receiving outpatient antipsychotic monotherapy. No information was available about antipsychotics used during inhospital treatment.
- Only the most frequently used atypical antipsychotics (clozapine, olanzapine, oral risperidone, and quetiapine) or the most frequently prescribed first-generation antipsychotics (oral perphenazine, thioridazine, and oral haloperidol) were assessed individually.
- Data about patients’ marital status, diagnoses of substance abuse, socioeconomic status, and other social variables were not available.
- Not all antipsychotics were available throughout the study (quetiapine was the newest and available for the shortest time).
- The study population consisted of patients of all ages, including those under 20 and over 70 years of age. Although the number of deaths and mortality rates increased with age, causes of mortality may differ when younger and older persons are compared. A data supplement to the study—available at www.thelancet.com—contains information about odds ratios by age and other factors.
Recommendation. Consider clozapine earlier than as a “last resort” in the disease course of patients with schizophrenia. At the very least, routinely present clozapine to patients and their families as a possible treatment option.
Antiaggressive properties
Case series and retrospective studies have provided insights into clozapine’s antiaggressive properties, but the strongest evidence comes from a 12-week, double-blind, randomized trial that specifically enrolled patients with violent behavior.6 Clozapine, olanzapine, and haloperidol were directly compared in the treatment of assaults and other aggressive behaviors by physically assaultive in patients with schizophrenia and schizoaffective disorder:
- The Modified Overt Aggression Scale (MOAS) physical aggression score measured the number and severity of assaults.
- The Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms.
Recommendation. Offer clozapine as an option for patients with schizophrenia or schizoaffective disorder and persistent aggressive behavior. Another antipsychotic might not be “good enough.”
Reduced risk of suicidality
The International Suicide Prevention Trial (InterSePT) was a multicenter, randomized, 2-year clinical study that compared the risk for suicidal behavior in patients treated with clozapine vs olanzapine.7 Enrolled were 980 patients with schizophrenia or schizoaffective disorder who were considered at high risk for suicide because of past suicide attempts or current suicidal ideation. Approximately one-quarter had not responded adequately to previous treatment.
All patients were seen weekly for 6 months, then biweekly for 18 months. The weekly or biweekly contact required to monitor for clozapine-associated agranulocytosis was matched with a similar visit schedule for olanzapine-treated patients, during which clinicians obtained vital signs. Primary endpoints included suicide attempts (including death), hospitalization to prevent suicide, and a rating of “much worsening of suicidality” from baseline. Blinded raters, including an independent suicide monitoring board, determined when patients achieved endpoint criteria.
Patients receiving clozapine showed significantly less suicidal behavior than those treated with olanzapine (a 24% relative advantage in the hazard ratio for suicide attempts or hospitalizations to prevent suicide). Fewer patients in the clozapine group:
- attempted suicide (34 vs 55)
- required hospitalization (82 vs 107) or rescue interventions to prevent suicide (118 vs 155)
- required concomitant treatment with antidepressants (221 vs 258) or anxiolytics/soporifics (301 vs 331).
More deaths from suicide occurred in the clozapine group than the olanzapine group, but the numbers were small (5 vs 3) and the difference between clozapine and olanzapine on this outcome was not statistically significant (P=.73).
Recommendation. Clozapine is a first-line treatment for patients with schizophrenia or schizoaffective disorder who exhibit suicidal behavior. This is reflected in the drug’s product labeling.
Superior symptom management
CATIE findings. Phase 2 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) showed clozapine to be more effective than other atypical antipsychotics, as measured by time to all-cause discontinuation.8 Patients in this phase of CATIE had discontinued another atypical antipsychotic in phase 1, principally because of lack of adequate efficacy. In phase 2, they were re-randomized to receive open-label clozapine or double-blinded risperidone, olanzapine, or quetiapine.
Only 90 patients were included in the time-to-discontinuation analysis, yet the greater amount of time that patients remained on clozapine (median 10.5 months) compared with quetiapine (median 3.3 months) or risperidone (median 2.8 months) was statistically significant. Time to discontinuation because of inadequate therapeutic effect also was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone.
The NNT for the outcome of all-cause discontinuation for clozapine was 4 compared with risperidone and 3 compared with quetiapine. This means for every 4 or 3 patients randomly assigned to clozapine instead of risperidone or quetiapine, respectively, 1 additional patient successfully completed the CATIE trial on the original phase 2 medication.9 The NNT for clozapine vs olanzapine was 7, indicating a respectable effect size difference that might have been statistically significant if the sample size had been larger.
Meta-analyses support CATIE results. Clozapine’s greater efficacy (and effectiveness) compared with other antipsychotics as demonstrated in CATIE is supported by 2 meta-analyses:
- A systematic review of clinical trials between January 1953 and May 2002 found clozapine’s effect size in reducing symptoms for patients with schizophrenia was greater than that of any other antipsychotic.10
- In a similar but more recent meta-analysis of 150 double-blind, mostly short-term studies totaling 21,533 participants, clozapine showed the largest effect size when atypical antipsychotics were compared with first-generation antipsychotics.11
Caveats about clozapine
First-episode schizophrenia. Clozapine has been shown to be more effective than chlorpromazine in terms of time to remission and maintenance of remission for treatment-naïve patients with first-episode schizophrenia.13 Even so, most clinicians probably would not consider clozapine as a first-line treatment for an uncomplicated first-episode patient because of concerns about agranulocytosis. When genetic testing becomes available to determine individual risk for agranulocytosis, perhaps clozapine will be used earlier in the disease course.14
The patient’s ethnicity may influence the risk of adverse effects, as observed in the study examining clozapine’s antiaggressive effect;6 African-American patients receiving antipsychotics—and particularly clozapine—may be more likely to develop metabolic abnormalities than patients in other ethnic groups.15 Carefully monitor all patients receiving clozapine for metabolic adverse effects, and be prepared to institute remediative psychosocial, lifestyle, and adjunctive medication interventions, such as statins.
16
Table
Common adverse effects of clozapine
| Adverse effect | Frequency* |
|---|---|
| Hypersalivation | 31% to 48% |
| Drowsiness/sedation/somnolence | 39% to 46% |
| Weight increase | 31% |
| Tachycardia | 25% |
| Dizziness/vertigo | 19% to 27% |
| Constipation | 14% to 25% |
| Seizures | 5% (can be higher with doses approaching 900 mg/d); slow titration needed |
| *Pooled data from clinical trials reporting percentage of patients taking clozapine who experienced adverse effects | |
| Source: Prescribing information for Clozaril® brand clozapine tablets. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf. Accessed October 27, 2009 | |
Adjunctive treatments. Patients with a low baseline white blood cell count (WBC) and/or absolute neutrophil count (ANC) may benefit from adjunctive lithium treatment to increase their WBC, as demonstrated in case reports.18
When no other alternatives were clinically feasible, chronic treatment with granulocyte colony-stimulating factor (filgrastim) has been used successfully for some patients whose clozapine course was interrupted because of a low WBC and/or ANC.19
- Clozapine product information (as revised July 2009): www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf.
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
- Teva: www.clozapineregistry.com.
- Clozaril: www.clozarilcare.com.
- Caraco: www.caracoclozapine.com.
- FazaClo: www.fazacloregistry.com.
- Mylan: www.mylan-clozapine.com.
- Chlorpromazine • Thorazine
- Clozapine • Clozaril, FazaClo
- Filgrastim • Neupogen
- Haloperidol • Haldol
- Lithium • Lithobid, others
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Pfizer Inc., Schering-Plough Corporation, and Vanda Pharmaceuticals. No writing assistance or external financial support was utilized in the preparation of this review article.
1. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627 [online-only data supplement available with the article at ].
2. Schulte PFJ. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40:683-688.
3. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harvard Rev Psychiatry. 2002;10:280-291.
4. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
5. Citrome L, Jaffe A, Martello D, et al. Did CATIE influence antipsychotic use? Psychiatr Serv. 2008;59(5):476.-
6. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
7. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.Erratum in: Arch Gen Psychiatry. 2003;60(7):735.
8. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
9. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
10. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
14. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics. 2008;9(8):1101-1111.
15. Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res. 2009;110(1-3):95-102.
16. Citrome L, Vreeland B. Schizophrenia, obesity, and antipsychotic medications: what can we do? Postgrad Med. 2008;120(2):18-33.
17. Annamraju S, Sheitman B, Saik S, et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479-483.
18. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
19. Mathewson KA, Lindenmayer JP. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapine-induced neutropenia. J Clin Psycho pharmacol. 2007;27(6):714-715.
Researchers in Finland surprised psychiatrists this year by announcing that clozapine “seems to be associated with a substantially lower mortality than any other antipsychotic.”1 This finding also surprised the researchers, who expected their 11-year study to link long-term use of second-generation (“atypical”) antipsychotics with increased mortality in patients with schizophrenia. Instead they found longer lives in patients who used antipsychotics (and particularly clozapine), compared with no antipsychotic use.
This study’s findings do not change clozapine’s association with potentially fatal agranulocytosis as well as weight gain, metabolic abnormalities, and other adverse effects. Clozapine also is difficult to administer ( Box 1 ),2 and patients must be enrolled in FDA-mandated registries (see Related Resources ). These obstacles might discourage you from offering clozapine to patients who could benefit from it ( Box 2 ).3-5
Why bother considering clozapine? Because recent data on decreased mortality, decreased suicidality, and control of aggressive behavior make clozapine a compelling choice for many patients. Careful attention to clozapine’s adverse effect profile is necessary, but you can manage these risks with appropriate monitoring.
Because of clozapine’s risk for leukopenia and agranulocytosis, frequent white blood cell count (WBC) monitoring is required. The risk of drug-induced blood dyscrasias has been shown to decrease over time, however, from 0.70/1,000 patient-years in the second 6 months of treatment to 0.39/1,000 patient-years after the first year.2
To start clozapine treatment, FDA guidelines require that the patient’s WBC must be at least 3,500 mm3, and the absolute neutrophil count (ANC) must be at least 2,000 mm3. For the first 6 months, patients receiving clozapine must have a weekly blood test for WBC and ANC.
The dispensing pharmacist must see the blood work result prior to releasing the drug to the patient. The blood draw date must be within the previous 7 days for the pharmacist to dispense a 1-week supply of clozapine.
Decreased monitoring over time. After 6 months of continuous therapy with clozapine without any interruptions because of a low WBC and/or ANC—defined as WBC 3 and/or ANC 3 or increased monitoring (when WBC 3 and/or ANC 3)—the patient’s blood monitoring may be done every 14 days and a 2-week supply of clozapine can be dispensed.
After 12 months of continuous clozapine therapy—6 months of continuous weekly monitoring, then 6 months of continuous biweekly monitoring—without any interruptions or increased monitoring, the patient may have blood monitoring done every 4 weeks and can receive a 4-week supply of clozapine.
One advantage of these monitoring requirements is that the increased frequency of visits can be used to foster greater patient engagement with treatment and promote a therapeutic alliance. Peer-led clozapine support groups, available in some communities, can facilitate adherence to monitoring requirements.
Clozapine was approved in the United States in 1989 for severely ill patients with schizophrenia who had not responded adequately to standard drug treatment. In 2002 it received an indication for patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state.
Off-label, clozapine has been commonly used for refractory bipolar disorder. Since 1998, it has been available in generic formulations and in a proprietary orally-disintegrating tablet formulation.
Dosing. The recommended target clozapine dosage is 300 to 450 mg/d. If an adequate response is not achieved, obtaining a plasma level might be helpful.3 Plasma levels ≥350 ng/mL constitute an optimal clozapine trial.
Not a ‘last resort.’ American Psychiatric Association treatment guidelines for schizophrenia state: “Because of clozapine’s superior efficacy, a trial of clozapine should be considered for a patient with a clinically inadequate response to antipsychotic treatment or for a patient with suicidal ideation or behavior. Besides clozapine, there are limited options for the many patients who have severe and significant residual symptoms even after antipsychotic monotherapy has been optimized, and none have proven benefits.”4
As additional evidence accumulates—including benefits regarding mortality and aggression—clozapine’s advantages support its clinical use earlier than as a “last resort.” In institutional settings, clozapine use has increased with the availability of additional data, such as from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).
In New York State Office of Mental Health hospitals, clozapine use increased from 20.6% of prescriptions in 2005 to 24.9% in 2007, compared with the other CATIE medications (olanzapine, quetiapine, risperidone, ziprasidone) and haloperidol.5 Whether clozapine use will increase in outpatient settings remains to be seen.
Potential for longer life?
The population-based, cohort study from Finland demonstrated that—contrary to popular belief—the introduction of atypical antipsychotics during the 1990s did not adversely affect mortality of patients with schizophrenia, at least in Finland.1
This study made specific drug comparisons and used perphenazine as the reference drug. The lowest risk for mortality was observed with clozapine, which showed a 26% relative advantage compared with perphenazine. Clozapine’s advantage was statistically significant when compared with all other antipsychotics tested.
The authors suggested provocatively that restrictions on clozapine use as a second- or third-line agent should be reassessed. A few caveats, however, might affect how one interprets this study or applies its findings to clinical practice:
- The main comparisons were for patients receiving outpatient antipsychotic monotherapy. No information was available about antipsychotics used during inhospital treatment.
- Only the most frequently used atypical antipsychotics (clozapine, olanzapine, oral risperidone, and quetiapine) or the most frequently prescribed first-generation antipsychotics (oral perphenazine, thioridazine, and oral haloperidol) were assessed individually.
- Data about patients’ marital status, diagnoses of substance abuse, socioeconomic status, and other social variables were not available.
- Not all antipsychotics were available throughout the study (quetiapine was the newest and available for the shortest time).
- The study population consisted of patients of all ages, including those under 20 and over 70 years of age. Although the number of deaths and mortality rates increased with age, causes of mortality may differ when younger and older persons are compared. A data supplement to the study—available at www.thelancet.com—contains information about odds ratios by age and other factors.
Recommendation. Consider clozapine earlier than as a “last resort” in the disease course of patients with schizophrenia. At the very least, routinely present clozapine to patients and their families as a possible treatment option.
Antiaggressive properties
Case series and retrospective studies have provided insights into clozapine’s antiaggressive properties, but the strongest evidence comes from a 12-week, double-blind, randomized trial that specifically enrolled patients with violent behavior.6 Clozapine, olanzapine, and haloperidol were directly compared in the treatment of assaults and other aggressive behaviors by physically assaultive in patients with schizophrenia and schizoaffective disorder:
- The Modified Overt Aggression Scale (MOAS) physical aggression score measured the number and severity of assaults.
- The Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms.
Recommendation. Offer clozapine as an option for patients with schizophrenia or schizoaffective disorder and persistent aggressive behavior. Another antipsychotic might not be “good enough.”
Reduced risk of suicidality
The International Suicide Prevention Trial (InterSePT) was a multicenter, randomized, 2-year clinical study that compared the risk for suicidal behavior in patients treated with clozapine vs olanzapine.7 Enrolled were 980 patients with schizophrenia or schizoaffective disorder who were considered at high risk for suicide because of past suicide attempts or current suicidal ideation. Approximately one-quarter had not responded adequately to previous treatment.
All patients were seen weekly for 6 months, then biweekly for 18 months. The weekly or biweekly contact required to monitor for clozapine-associated agranulocytosis was matched with a similar visit schedule for olanzapine-treated patients, during which clinicians obtained vital signs. Primary endpoints included suicide attempts (including death), hospitalization to prevent suicide, and a rating of “much worsening of suicidality” from baseline. Blinded raters, including an independent suicide monitoring board, determined when patients achieved endpoint criteria.
Patients receiving clozapine showed significantly less suicidal behavior than those treated with olanzapine (a 24% relative advantage in the hazard ratio for suicide attempts or hospitalizations to prevent suicide). Fewer patients in the clozapine group:
- attempted suicide (34 vs 55)
- required hospitalization (82 vs 107) or rescue interventions to prevent suicide (118 vs 155)
- required concomitant treatment with antidepressants (221 vs 258) or anxiolytics/soporifics (301 vs 331).
More deaths from suicide occurred in the clozapine group than the olanzapine group, but the numbers were small (5 vs 3) and the difference between clozapine and olanzapine on this outcome was not statistically significant (P=.73).
Recommendation. Clozapine is a first-line treatment for patients with schizophrenia or schizoaffective disorder who exhibit suicidal behavior. This is reflected in the drug’s product labeling.
Superior symptom management
CATIE findings. Phase 2 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) showed clozapine to be more effective than other atypical antipsychotics, as measured by time to all-cause discontinuation.8 Patients in this phase of CATIE had discontinued another atypical antipsychotic in phase 1, principally because of lack of adequate efficacy. In phase 2, they were re-randomized to receive open-label clozapine or double-blinded risperidone, olanzapine, or quetiapine.
Only 90 patients were included in the time-to-discontinuation analysis, yet the greater amount of time that patients remained on clozapine (median 10.5 months) compared with quetiapine (median 3.3 months) or risperidone (median 2.8 months) was statistically significant. Time to discontinuation because of inadequate therapeutic effect also was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone.
The NNT for the outcome of all-cause discontinuation for clozapine was 4 compared with risperidone and 3 compared with quetiapine. This means for every 4 or 3 patients randomly assigned to clozapine instead of risperidone or quetiapine, respectively, 1 additional patient successfully completed the CATIE trial on the original phase 2 medication.9 The NNT for clozapine vs olanzapine was 7, indicating a respectable effect size difference that might have been statistically significant if the sample size had been larger.
Meta-analyses support CATIE results. Clozapine’s greater efficacy (and effectiveness) compared with other antipsychotics as demonstrated in CATIE is supported by 2 meta-analyses:
- A systematic review of clinical trials between January 1953 and May 2002 found clozapine’s effect size in reducing symptoms for patients with schizophrenia was greater than that of any other antipsychotic.10
- In a similar but more recent meta-analysis of 150 double-blind, mostly short-term studies totaling 21,533 participants, clozapine showed the largest effect size when atypical antipsychotics were compared with first-generation antipsychotics.11
Caveats about clozapine
First-episode schizophrenia. Clozapine has been shown to be more effective than chlorpromazine in terms of time to remission and maintenance of remission for treatment-naïve patients with first-episode schizophrenia.13 Even so, most clinicians probably would not consider clozapine as a first-line treatment for an uncomplicated first-episode patient because of concerns about agranulocytosis. When genetic testing becomes available to determine individual risk for agranulocytosis, perhaps clozapine will be used earlier in the disease course.14
The patient’s ethnicity may influence the risk of adverse effects, as observed in the study examining clozapine’s antiaggressive effect;6 African-American patients receiving antipsychotics—and particularly clozapine—may be more likely to develop metabolic abnormalities than patients in other ethnic groups.15 Carefully monitor all patients receiving clozapine for metabolic adverse effects, and be prepared to institute remediative psychosocial, lifestyle, and adjunctive medication interventions, such as statins.
16
Table
Common adverse effects of clozapine
| Adverse effect | Frequency* |
|---|---|
| Hypersalivation | 31% to 48% |
| Drowsiness/sedation/somnolence | 39% to 46% |
| Weight increase | 31% |
| Tachycardia | 25% |
| Dizziness/vertigo | 19% to 27% |
| Constipation | 14% to 25% |
| Seizures | 5% (can be higher with doses approaching 900 mg/d); slow titration needed |
| *Pooled data from clinical trials reporting percentage of patients taking clozapine who experienced adverse effects | |
| Source: Prescribing information for Clozaril® brand clozapine tablets. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf. Accessed October 27, 2009 | |
Adjunctive treatments. Patients with a low baseline white blood cell count (WBC) and/or absolute neutrophil count (ANC) may benefit from adjunctive lithium treatment to increase their WBC, as demonstrated in case reports.18
When no other alternatives were clinically feasible, chronic treatment with granulocyte colony-stimulating factor (filgrastim) has been used successfully for some patients whose clozapine course was interrupted because of a low WBC and/or ANC.19
- Clozapine product information (as revised July 2009): www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf.
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
- Teva: www.clozapineregistry.com.
- Clozaril: www.clozarilcare.com.
- Caraco: www.caracoclozapine.com.
- FazaClo: www.fazacloregistry.com.
- Mylan: www.mylan-clozapine.com.
- Chlorpromazine • Thorazine
- Clozapine • Clozaril, FazaClo
- Filgrastim • Neupogen
- Haloperidol • Haldol
- Lithium • Lithobid, others
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Pfizer Inc., Schering-Plough Corporation, and Vanda Pharmaceuticals. No writing assistance or external financial support was utilized in the preparation of this review article.
Researchers in Finland surprised psychiatrists this year by announcing that clozapine “seems to be associated with a substantially lower mortality than any other antipsychotic.”1 This finding also surprised the researchers, who expected their 11-year study to link long-term use of second-generation (“atypical”) antipsychotics with increased mortality in patients with schizophrenia. Instead they found longer lives in patients who used antipsychotics (and particularly clozapine), compared with no antipsychotic use.
This study’s findings do not change clozapine’s association with potentially fatal agranulocytosis as well as weight gain, metabolic abnormalities, and other adverse effects. Clozapine also is difficult to administer ( Box 1 ),2 and patients must be enrolled in FDA-mandated registries (see Related Resources ). These obstacles might discourage you from offering clozapine to patients who could benefit from it ( Box 2 ).3-5
Why bother considering clozapine? Because recent data on decreased mortality, decreased suicidality, and control of aggressive behavior make clozapine a compelling choice for many patients. Careful attention to clozapine’s adverse effect profile is necessary, but you can manage these risks with appropriate monitoring.
Because of clozapine’s risk for leukopenia and agranulocytosis, frequent white blood cell count (WBC) monitoring is required. The risk of drug-induced blood dyscrasias has been shown to decrease over time, however, from 0.70/1,000 patient-years in the second 6 months of treatment to 0.39/1,000 patient-years after the first year.2
To start clozapine treatment, FDA guidelines require that the patient’s WBC must be at least 3,500 mm3, and the absolute neutrophil count (ANC) must be at least 2,000 mm3. For the first 6 months, patients receiving clozapine must have a weekly blood test for WBC and ANC.
The dispensing pharmacist must see the blood work result prior to releasing the drug to the patient. The blood draw date must be within the previous 7 days for the pharmacist to dispense a 1-week supply of clozapine.
Decreased monitoring over time. After 6 months of continuous therapy with clozapine without any interruptions because of a low WBC and/or ANC—defined as WBC 3 and/or ANC 3 or increased monitoring (when WBC 3 and/or ANC 3)—the patient’s blood monitoring may be done every 14 days and a 2-week supply of clozapine can be dispensed.
After 12 months of continuous clozapine therapy—6 months of continuous weekly monitoring, then 6 months of continuous biweekly monitoring—without any interruptions or increased monitoring, the patient may have blood monitoring done every 4 weeks and can receive a 4-week supply of clozapine.
One advantage of these monitoring requirements is that the increased frequency of visits can be used to foster greater patient engagement with treatment and promote a therapeutic alliance. Peer-led clozapine support groups, available in some communities, can facilitate adherence to monitoring requirements.
Clozapine was approved in the United States in 1989 for severely ill patients with schizophrenia who had not responded adequately to standard drug treatment. In 2002 it received an indication for patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state.
Off-label, clozapine has been commonly used for refractory bipolar disorder. Since 1998, it has been available in generic formulations and in a proprietary orally-disintegrating tablet formulation.
Dosing. The recommended target clozapine dosage is 300 to 450 mg/d. If an adequate response is not achieved, obtaining a plasma level might be helpful.3 Plasma levels ≥350 ng/mL constitute an optimal clozapine trial.
Not a ‘last resort.’ American Psychiatric Association treatment guidelines for schizophrenia state: “Because of clozapine’s superior efficacy, a trial of clozapine should be considered for a patient with a clinically inadequate response to antipsychotic treatment or for a patient with suicidal ideation or behavior. Besides clozapine, there are limited options for the many patients who have severe and significant residual symptoms even after antipsychotic monotherapy has been optimized, and none have proven benefits.”4
As additional evidence accumulates—including benefits regarding mortality and aggression—clozapine’s advantages support its clinical use earlier than as a “last resort.” In institutional settings, clozapine use has increased with the availability of additional data, such as from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).
In New York State Office of Mental Health hospitals, clozapine use increased from 20.6% of prescriptions in 2005 to 24.9% in 2007, compared with the other CATIE medications (olanzapine, quetiapine, risperidone, ziprasidone) and haloperidol.5 Whether clozapine use will increase in outpatient settings remains to be seen.
Potential for longer life?
The population-based, cohort study from Finland demonstrated that—contrary to popular belief—the introduction of atypical antipsychotics during the 1990s did not adversely affect mortality of patients with schizophrenia, at least in Finland.1
This study made specific drug comparisons and used perphenazine as the reference drug. The lowest risk for mortality was observed with clozapine, which showed a 26% relative advantage compared with perphenazine. Clozapine’s advantage was statistically significant when compared with all other antipsychotics tested.
The authors suggested provocatively that restrictions on clozapine use as a second- or third-line agent should be reassessed. A few caveats, however, might affect how one interprets this study or applies its findings to clinical practice:
- The main comparisons were for patients receiving outpatient antipsychotic monotherapy. No information was available about antipsychotics used during inhospital treatment.
- Only the most frequently used atypical antipsychotics (clozapine, olanzapine, oral risperidone, and quetiapine) or the most frequently prescribed first-generation antipsychotics (oral perphenazine, thioridazine, and oral haloperidol) were assessed individually.
- Data about patients’ marital status, diagnoses of substance abuse, socioeconomic status, and other social variables were not available.
- Not all antipsychotics were available throughout the study (quetiapine was the newest and available for the shortest time).
- The study population consisted of patients of all ages, including those under 20 and over 70 years of age. Although the number of deaths and mortality rates increased with age, causes of mortality may differ when younger and older persons are compared. A data supplement to the study—available at www.thelancet.com—contains information about odds ratios by age and other factors.
Recommendation. Consider clozapine earlier than as a “last resort” in the disease course of patients with schizophrenia. At the very least, routinely present clozapine to patients and their families as a possible treatment option.
Antiaggressive properties
Case series and retrospective studies have provided insights into clozapine’s antiaggressive properties, but the strongest evidence comes from a 12-week, double-blind, randomized trial that specifically enrolled patients with violent behavior.6 Clozapine, olanzapine, and haloperidol were directly compared in the treatment of assaults and other aggressive behaviors by physically assaultive in patients with schizophrenia and schizoaffective disorder:
- The Modified Overt Aggression Scale (MOAS) physical aggression score measured the number and severity of assaults.
- The Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms.
Recommendation. Offer clozapine as an option for patients with schizophrenia or schizoaffective disorder and persistent aggressive behavior. Another antipsychotic might not be “good enough.”
Reduced risk of suicidality
The International Suicide Prevention Trial (InterSePT) was a multicenter, randomized, 2-year clinical study that compared the risk for suicidal behavior in patients treated with clozapine vs olanzapine.7 Enrolled were 980 patients with schizophrenia or schizoaffective disorder who were considered at high risk for suicide because of past suicide attempts or current suicidal ideation. Approximately one-quarter had not responded adequately to previous treatment.
All patients were seen weekly for 6 months, then biweekly for 18 months. The weekly or biweekly contact required to monitor for clozapine-associated agranulocytosis was matched with a similar visit schedule for olanzapine-treated patients, during which clinicians obtained vital signs. Primary endpoints included suicide attempts (including death), hospitalization to prevent suicide, and a rating of “much worsening of suicidality” from baseline. Blinded raters, including an independent suicide monitoring board, determined when patients achieved endpoint criteria.
Patients receiving clozapine showed significantly less suicidal behavior than those treated with olanzapine (a 24% relative advantage in the hazard ratio for suicide attempts or hospitalizations to prevent suicide). Fewer patients in the clozapine group:
- attempted suicide (34 vs 55)
- required hospitalization (82 vs 107) or rescue interventions to prevent suicide (118 vs 155)
- required concomitant treatment with antidepressants (221 vs 258) or anxiolytics/soporifics (301 vs 331).
More deaths from suicide occurred in the clozapine group than the olanzapine group, but the numbers were small (5 vs 3) and the difference between clozapine and olanzapine on this outcome was not statistically significant (P=.73).
Recommendation. Clozapine is a first-line treatment for patients with schizophrenia or schizoaffective disorder who exhibit suicidal behavior. This is reflected in the drug’s product labeling.
Superior symptom management
CATIE findings. Phase 2 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) showed clozapine to be more effective than other atypical antipsychotics, as measured by time to all-cause discontinuation.8 Patients in this phase of CATIE had discontinued another atypical antipsychotic in phase 1, principally because of lack of adequate efficacy. In phase 2, they were re-randomized to receive open-label clozapine or double-blinded risperidone, olanzapine, or quetiapine.
Only 90 patients were included in the time-to-discontinuation analysis, yet the greater amount of time that patients remained on clozapine (median 10.5 months) compared with quetiapine (median 3.3 months) or risperidone (median 2.8 months) was statistically significant. Time to discontinuation because of inadequate therapeutic effect also was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone.
The NNT for the outcome of all-cause discontinuation for clozapine was 4 compared with risperidone and 3 compared with quetiapine. This means for every 4 or 3 patients randomly assigned to clozapine instead of risperidone or quetiapine, respectively, 1 additional patient successfully completed the CATIE trial on the original phase 2 medication.9 The NNT for clozapine vs olanzapine was 7, indicating a respectable effect size difference that might have been statistically significant if the sample size had been larger.
Meta-analyses support CATIE results. Clozapine’s greater efficacy (and effectiveness) compared with other antipsychotics as demonstrated in CATIE is supported by 2 meta-analyses:
- A systematic review of clinical trials between January 1953 and May 2002 found clozapine’s effect size in reducing symptoms for patients with schizophrenia was greater than that of any other antipsychotic.10
- In a similar but more recent meta-analysis of 150 double-blind, mostly short-term studies totaling 21,533 participants, clozapine showed the largest effect size when atypical antipsychotics were compared with first-generation antipsychotics.11
Caveats about clozapine
First-episode schizophrenia. Clozapine has been shown to be more effective than chlorpromazine in terms of time to remission and maintenance of remission for treatment-naïve patients with first-episode schizophrenia.13 Even so, most clinicians probably would not consider clozapine as a first-line treatment for an uncomplicated first-episode patient because of concerns about agranulocytosis. When genetic testing becomes available to determine individual risk for agranulocytosis, perhaps clozapine will be used earlier in the disease course.14
The patient’s ethnicity may influence the risk of adverse effects, as observed in the study examining clozapine’s antiaggressive effect;6 African-American patients receiving antipsychotics—and particularly clozapine—may be more likely to develop metabolic abnormalities than patients in other ethnic groups.15 Carefully monitor all patients receiving clozapine for metabolic adverse effects, and be prepared to institute remediative psychosocial, lifestyle, and adjunctive medication interventions, such as statins.
16
Table
Common adverse effects of clozapine
| Adverse effect | Frequency* |
|---|---|
| Hypersalivation | 31% to 48% |
| Drowsiness/sedation/somnolence | 39% to 46% |
| Weight increase | 31% |
| Tachycardia | 25% |
| Dizziness/vertigo | 19% to 27% |
| Constipation | 14% to 25% |
| Seizures | 5% (can be higher with doses approaching 900 mg/d); slow titration needed |
| *Pooled data from clinical trials reporting percentage of patients taking clozapine who experienced adverse effects | |
| Source: Prescribing information for Clozaril® brand clozapine tablets. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf. Accessed October 27, 2009 | |
Adjunctive treatments. Patients with a low baseline white blood cell count (WBC) and/or absolute neutrophil count (ANC) may benefit from adjunctive lithium treatment to increase their WBC, as demonstrated in case reports.18
When no other alternatives were clinically feasible, chronic treatment with granulocyte colony-stimulating factor (filgrastim) has been used successfully for some patients whose clozapine course was interrupted because of a low WBC and/or ANC.19
- Clozapine product information (as revised July 2009): www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf.
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
- Teva: www.clozapineregistry.com.
- Clozaril: www.clozarilcare.com.
- Caraco: www.caracoclozapine.com.
- FazaClo: www.fazacloregistry.com.
- Mylan: www.mylan-clozapine.com.
- Chlorpromazine • Thorazine
- Clozapine • Clozaril, FazaClo
- Filgrastim • Neupogen
- Haloperidol • Haldol
- Lithium • Lithobid, others
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Pfizer Inc., Schering-Plough Corporation, and Vanda Pharmaceuticals. No writing assistance or external financial support was utilized in the preparation of this review article.
1. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627 [online-only data supplement available with the article at ].
2. Schulte PFJ. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40:683-688.
3. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harvard Rev Psychiatry. 2002;10:280-291.
4. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
5. Citrome L, Jaffe A, Martello D, et al. Did CATIE influence antipsychotic use? Psychiatr Serv. 2008;59(5):476.-
6. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
7. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.Erratum in: Arch Gen Psychiatry. 2003;60(7):735.
8. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
9. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
10. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
14. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics. 2008;9(8):1101-1111.
15. Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res. 2009;110(1-3):95-102.
16. Citrome L, Vreeland B. Schizophrenia, obesity, and antipsychotic medications: what can we do? Postgrad Med. 2008;120(2):18-33.
17. Annamraju S, Sheitman B, Saik S, et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479-483.
18. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
19. Mathewson KA, Lindenmayer JP. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapine-induced neutropenia. J Clin Psycho pharmacol. 2007;27(6):714-715.
1. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627 [online-only data supplement available with the article at ].
2. Schulte PFJ. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40:683-688.
3. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harvard Rev Psychiatry. 2002;10:280-291.
4. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
5. Citrome L, Jaffe A, Martello D, et al. Did CATIE influence antipsychotic use? Psychiatr Serv. 2008;59(5):476.-
6. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
7. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.Erratum in: Arch Gen Psychiatry. 2003;60(7):735.
8. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
9. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
10. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
14. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics. 2008;9(8):1101-1111.
15. Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res. 2009;110(1-3):95-102.
16. Citrome L, Vreeland B. Schizophrenia, obesity, and antipsychotic medications: what can we do? Postgrad Med. 2008;120(2):18-33.
17. Annamraju S, Sheitman B, Saik S, et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479-483.
18. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
19. Mathewson KA, Lindenmayer JP. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapine-induced neutropenia. J Clin Psycho pharmacol. 2007;27(6):714-715.
Mindfulness-based interventions: Effective for depression and anxiety
Mr. A, age 45, reports irritability, loss of interest, sleep disturbance, increased self-criticism, and decreased self care during the last month after a promotion at work. He has a history of 3 major depressive episodes, 1 of which required hospitalization. For the last 2 years his depressive symptoms had been successfully managed with escitalopram, 10 mg/d, plus bupropion, 150 mg/d. Mr. A wants to discontinue these medications because of sexual dysfunction. He asks if nonpharmacologic strategies might help.
One option to consider for Mr. A is mindfulness-based cognitive therapy (MBCT), which was originally developed to help prevent depressive relapse. MBCT also can reduce depression and anxiety symptoms. More recently, MBCT was shown to help individuals discontinue antidepressants after recovering from depression.
Regular mindfulness meditation has been shown to result in structural brain changes that may help explain how the practice effectively addresses psychiatric symptoms ( Box ). With appropriate training, psychiatrists can help patients reap the benefits of this cognitive treatment.
Regular mindfulness practice has been shown to increase cortical thickness in areas associated with attention, interoception, and sensory processing, such as the prefrontal cortex and right anterior insula.a This supports the hypothesis that mindfulness is a way of attuning the mind to one’s internal processes, and that this involves the same social neural circuits involved in interpersonal attunement—middle prefrontal regions, insula, superior temporal cortex, and the mirror neuron system.b
Amygdala responses. Mindfulness improves affect regulation by optimizing prefrontal cortex regulation of the amygdala. Recent developments in understanding the pathophysiology of depression have highlighted the lack of engagement of left lateral-ventromedial prefrontal circuitry important for the down-regulation of amygdala responses to negative stimuli.c Dispositional mindfulness is associated with greater prefrontal cortical activation and associated greater reduction in amygdala activity during affect labeling tasks, which results in enhanced affect regulation in individuals with higher levels of mindfulness.d
Left-sided anterior activation. Other researchers have examined mindfulness’ role in maintaining balanced prefrontal asymmetry. Relative left prefrontal activation is related to an affective style characterized by stronger tendencies toward positive emotional responses and approach/reward oriented behavior, whereas relative right-sided activation is associated with stronger tendencies toward negative emotional responses and avoidant/withdrawal oriented behavior.
One study found significant increases in left-sided anterior activation in mindfulness-based stress reduction participants compared with controls.e Similarly, in a study evaluating the effect of mindfulness-based cognitive therapy (MBCT) on frontal asymmetry in previously suicidal individuals, MBCT participants retained a balanced pattern of prefrontal activation, whereas the treatment-as-usual group showed significant deterioration toward decreased relative left frontal activation. These findings suggest a protective effect of the mindfulness intervention.f
Source: For references to studies described here see this article at CurrentPsychiatry.com
What is mindfulness meditation?
Meditation refers to a variety of practices that intentionally focus attention to help the practitioner disengage from unconscious absorption in thoughts and feelings. Unlike concentrative meditation—in which practitioners focus attention on a single object such as a word (mantra), body part, or external object—in mindfulness meditation participants bring their attention to a wide range of objects (such as breath, body, emotions, or thoughts) as they appear in moment-by-moment awareness.
Mindfulness is a nonjudgmental, present-centered awareness in which each thought, feeling, or sensation that arises in the attentional field is acknowledged and accepted as it is.1-3 Bishop et al4 defined a 2-component model of mindfulness:
- self-regulating attention of immediate experience, thereby allowing for increased recognition of mental events in the present moment
- adopting an orientation of curiosity, openness, and acceptance toward one’s experiences in each moment.
Mindfulness-based interventions
Buddhist and Western psychology inform the theoretical framework of most mindfulness-based clinical interventions, such as:
- acceptance and commitment therapy (ACT)
- dialectical behavioral therapy (DBT)
- mindfulness-based stress reduction (MBSR)
- MBCT.
Because mindfulness is only 1 of several components of ACT and DBT,5 this review focuses on MBCT and MBSR, in which teaching mindfulness skills is the central focus of treatment.
MBCT and MBSR. MBCT incorporates many aspects of the manualized MBSR treatment program developed for managing chronic pain.6,7 MBSR is devoted almost entirely to cultivating mindfulness through:
- formal mindfulness meditation practices such as body scan (intentionally bringing awareness to bodily sensations), mindful stretching, and mindfulness of breath/body/sounds/thoughts
- informal practices, including mindfulness of daily activities such as eating.1
MBSR typically involves 8 to 10 weekly group sessions of 2 to 2.5 hours with 10 to 40 participants with heterogeneous or homogenous clinical presentations. At each session, patients are taught mindfulness skills and practices. Typically, a full day of meditation practice on a weekend follows session 5 or 6. Participants also engage in a daily meditation practice and homework exercises directed at integrating awareness skills into daily life.
Meta-analytic and narrative reviews generally support MBSR’s efficacy for a wide range of clinical presentations, including improved quality of life for chronic pain and cancer patients.5,8-11 Variability in the methodologic rigor of clinical trials of mindfulness-based interventions—such as lack of active control groups and small sample sizes—limits the strength of these studies’ conclusions, however.8
MBCT integrates the mindfulness training of MBSR with cognitive therapy techniques ( Table 1 ) to prevent the consolidation of ruminative, negative thinking patterns that contribute to depressive relapse.2 These cognitive therapy techniques include:
- psychoeducation about depression symptoms and automatic thoughts
- exercises designed to demonstrate the cognitive model
- identifying activities that provide feelings of mastery and/or pleasure
- creating a specific relapse prevention plan.
In addition, MBCT introduces a new informal meditation—the 3-minute breathing space—to facilitate present-moment awareness in upsetting everyday situations.
Evidence supporting MBCT comes from randomized, controlled trials (RCTs) and uncontrolled trials ( Table 2 ).12-18 A systematic review of RCTs supported using MBCT in addition to usual care to prevent depressive relapse in individuals with a history of ≥3 depressive episodes.19 Since that review was published, a large RCT (123 patients) comparing antidepressant medication alone to antidepressants plus adjunctive MBCT with support to taper/discontinue antidepressant therapy found:
- MBCT comparable to maintenance antidepressant medication in preventing depressive relapse for individuals with ≥3 depressive episodes
- no difference in cost between these 2 treatments.12
In this study, MBCT was more effective than maintenance pharmacotherapy in reducing residual depressive symptoms and in improving quality of life; 75% in the MBCT group discontinued antidepressants. MBCT is included in the United Kingdom’s National Institute for Clinical Excellence Clinical Practice Guidelines for Depression20 for prevention of recurrent depression.
RCTs and uncontrolled studies have shown that MBCT reduces depressive and anxious symptoms in individuals suffering from mood disorders. In an open-label pilot study of MBCT’s efficacy in reducing depressive symptoms in patients with treatment-resistant depression and ≥3 depressive episodes, 61% of patients achieved a post-MBCT Beck Depression Inventory-II (BDI-II) score <14, which represents normal or near-normal mood (mean BDI-II scores decreased from 24.3 to 13.9; effect size 1.04).17
Mindfulness for other psychiatric conditions. A review by Toneatto and Nguyen21 of MBSR in the treatment of anxiety and depression symptoms in a range of clinical populations concluded that the evidence supporting a beneficial effect was equivocal. On the other hand, several uncontrolled studies and 1 RCT indicate that mindfulness-based treatments can reduce symptoms in other psychiatric conditions, including eating disorders,22 generalized anxiety disorder,23 bipolar disorder,24 and attention-deficit/hyperactivity disorder.25 Many of these studies were developed to target mood and anxiety symptoms by linking mindfulness and symptom management; this differs from MBSR, which focuses on stress reduction. Methodologically rigorous studies are necessary to evaluate mindfulness-based treatments in these and other psychiatric conditions.
Table 1
Skills and practices taught in mindfulness training
| MBCT session themes | Mindfulness skill | Associated practices |
|---|---|---|
| ‘Automatic pilot’ (acting without conscious awareness) | Awareness of automatic pilot Awareness of body | Mindful eating Body scan (intentionally bringing awareness to bodily sensations) |
| Dealing with barriers | Awareness of how the chatter of the mind influences feelings and behaviors | Body scan Short breathing meditation |
| Mindfulness of the breath | Awareness of breath and body | Breathing meditation 3-minute breathing space Mindful yoga |
| Staying present | Awareness of attachment and aversion | Breathing meditation Working with intense physical sensations |
| Acceptance | Acceptance of thoughts and emotions as fleeting events | Explicit instructions to practice acceptance are included in the breathing meditation and the 3-minute breathing space |
| Thoughts are not facts | Decentering or re-perceiving | Sitting meditation (awareness of thoughts) |
| How can I best take care of myself? | Awareness of signs of relapse; develop more flexible, deliberate responses at time of potential relapse | 3-minute coping breathing space |
| Dealing with future depression | Awareness of intention | Identifying coping strategies to address barriers to maintaining practice |
| MBCT: mindfulness-based cognitive therapy | ||
| Source: Reference 2 | ||
Table 2
Evidence of reduced depressive symptoms, anxiety with MBCT
| Study | Patients | Findings |
|---|---|---|
| Randomized controlled trials | ||
| Kuyken et al, 200812 | 123 patients with recurrent depression treated with antidepressants received maintenance antidepressants alone or adjunctive MBCT with support to taper/discontinue antidepressant therapy | Adjunctive MBCT was as effective as maintenance antidepressants in reducing relapse/recurrence rates but more effective in reducing residual depressive symptoms and improving quality of life; 75% in the MBCT group discontinued antidepressants |
| Kingston et al, 200713 | 19 outpatients with residual depressive symptoms following a depressive episode assigned to MBCT or treatment as usual | MBCT significantly reduced depressive symptoms, and these improvements were maintained over a 1-month follow-up period |
| Williams et al, 200814 | 14 patients with bipolar disorder who had no manic episodes in the last 6 months and ≤1 week of depressive symptoms in the last 8 weeks | MBCT resulted in a significant reduction in anxiety scores on the BAI compared with wait-list controls |
| Uncontrolled trials | ||
| Eisendrath et al, 200815 | 15 patients with treatment-resistant depression (failure to remit with ≥2 antidepressant trials) | MBCT significantly reduced anxiety and depression; increased mindfulness and decreased rumination and anxiety were associated with decreased depression |
| Finucane and Mercer, 200616 | 13 patients with recurrent depression or recurrent depression and anxiety | MBCT significantly reduced depression and anxiety scores on BDI-II and BAI |
| Kenny and Williams, 200717 | 46 depressed patients who had not fully responded to standard treatments | MBCT significantly reduced depression scores |
| Ree and Craigie, 200718 | 26 outpatients with mood and/or anxiety disorders | MBCT significantly improved symptoms of depression, anxiety, stress, and insomnia; improvements in insomnia were maintained at 3-month follow-up |
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory; MBCT: mindfulness-based cognitive therapy | ||
CASE CONTINUED: Explaining the potential benefits
You inform Mr. A that MBCT has been shown to improve acute mild-to-moderate depressive symptoms, may decrease his risk of depressive relapse by 50%26 and could help him discontinue his medications.12 He asks how mindfulness exercises will help his symptoms.
How mindfulness works
The assumption that increased mindfulness mediates treatment outcomes4 has been addressed systematically only recently, following the development of operational definitions of mindfulness and self-report mindfulness measures, including the:
- Mindful Attention Awareness Scale (MAAS)27
- Five Facet Mindfulness Questionnaire (FFMQ)12
- Toronto Mindfulness Scale (TMS).28
Uncontrolled studies using these measures demonstrated that self-reported mindfulness increased following MBSR28,29 and MBCT15,18 in individuals with general stress, anxiety disorder or primary depression, cancer, chronic pain disorder, diabetes, and multiple sclerosis. Accumulating evidence from 1 RCT30 and 2 other uncontrolled studies28,31 demonstrates that mindfulness is associated with symptom reduction following MBSR.
Researchers have begun to focus on how mindfulness skills reduce symptoms. Baer9 proposed several mechanisms, including:
- cognitive change
- improved self-management
- exposure to painful experiences leading to reduced emotional reactivity.
Cognitive change—also called meta-cognitive awareness—is the development of a “distanced “or “decentered” perspective in which patients experience their thoughts and feelings as “mental events” rather than as true, accurate versions of reality. This is thought to introduce a “space” between perception and response that enables patients to have a reflective—rather than a reflexive or reactive—response to situations, which in turn reduces vulnerability to psychological processes that contribute to emotional suffering. Some preliminary evidence suggests that MBCT-associated increases in metacognitive awareness reduce risk of depressive relapse.32
Teaching mindfulness
Guidelines for psychiatrists who wish to become MBCT instructors suggest undergoing formal teacher development training, attending a 7- to 10-day meditation retreat, and establishing your own daily mindfulness practice ( Table 3 ).33 Segal et al2 also recommend recognized training in counseling, psychotherapy, or as a mental health professional, as well as training in cognitive therapy and having experience leading psychotherapy groups.
The recommendation that a mindfulness teacher should practice meditation derives from the view that instructors teach from their own meditation experience and embody the attitudes they invite participants to practice. In an RCT, patients of psychotherapists in training (PiTs) who practiced meditation had greater symptom reductions than those of PiTs who did not engage in meditation.34
To cultivate your own mindfulness practice, consider enrolling in an MBSR group, participating in an MBCT training retreat (see Related Resources ), or attending a mindfulness meditation retreat.
Although patient access to MBCT and MBSR programs has been increasing, formal MBSR/MBCT group programs led by trained therapists are limited. Patients can go through an MBSR/MBCT book with a trained clinician or listen to audio recordings with guided meditation instructions. Alternately, they can join a meditation sitting group or an insight meditation correspondence course ( Table 4 ).
Table 3
Recommended process for becoming an MBCT instructor
| Complete a 5-day residential MBCT training program |
| Attend a 7- to 10-day residential mindfulness meditation retreat |
| Establish your own daily mindfulness meditation practice |
| Undergo professional training in cognitive therapy |
| Gain experience leading psychotherapy groups |
| MBCT: mindfulness-based cognitive therapy |
| Source: References 2,33 |
Table 4
Useful mindfulness resources for interested patients
| Insight Meditation Society: www.dharma.org |
| Kabat-Zinn J. MBSR meditation CDs/tapes: www.stressreductiontapes.com |
| Recordings of meditation (dharma) talks: www.dharmaseed.org |
| Salzberg S, Goldstein J. Insight meditation: an in-depth correspondence course. Louisville, CO: Sounds True, Inc; 2004 |
| Williams M, Teasdale J, Segal Z, et al. The mindful way through depression: freeing yourself from chronic unhappiness. New York, NY: Guilford Press; 2007 |
CASE CONTINUED: Daily mindfulness practice
Mr. A enrolls in and completes a group MBCT program. He rearranges his schedule to include 30 minutes of formal mindfulness practice daily. During an office visit after completing the MBCT course, he describes decreased irritability and self-criticism, newfound self-acceptance, an increased ability to tolerate previously distressing affect, and the ability to set realistic expectations of himself, particularly in light of increased responsibilities at work. He also reports an increased sense of engagement in and reward in his personal life.
Several months later he requests and successfully completes an antidepressant taper and has no recurrence of depressive episodes at 18-month follow-up. He participates in monthly meditation groups to support his home practice.
- Germer CK, Siegel R, Fulton PR, eds. Mindfulness and psychotherapy. New York, NY: Guilford Press; 2005.
- Mindfulness-based cognitive therapy. www.mbct.com; www.mbct.co.uk; www.bangor.ac.uk/mindfulness.
- Center for Mindfulness in Medicine, Health Care, and Society. www.umassmed.edu/cfm.
- Neurobiology of mindfulness. www.mindfulness-matters.org.
- Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: Norton; 2007.
- University of California, San Diego Center for Mindfulness. http://cme.ucsd.edu/mindfulness.
Drug brand names
- Bupropion • Wellbutrin
- Escitalopram • Lexapro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors would like to thank Amanda Yu for her assistance in preparing the manuscript.
1. Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Dell Publishing; 1990.
2. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
3. Shapiro SL, Schwartz GE. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;15:128-134.
4. Bishop SR, Lau MA, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pr. 2004;11:230-241.
5. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211-237.
6. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33-47.
7. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190.
8. Bishop SR. What do we really know about mindfulness-based stress reduction? Am Psychosom Soc. 2002;64:71-83.
9. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10(2):125-143.
10. Grossman P, Nieman L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
11. Salmon P, Sephton S, Weissbecker I, et al. Mindfulness meditation in clinical practice. Cog Behav Ther. 2004;11(4):434-446.
12. Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psych. 2008;76(6):966-978.
13. Kingston T, Dooley B, Bates A, et al. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007;80:193-203.
14. Williams J, Alatiq Y, Crance C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
15. Eisendrath SJ, Delucchi K, Bitner R, et al. Mindfulness-based cognitive therapy for treatment resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319-320.
16. Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14.-
17. Kenny MA, Williams JGM. Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45(3):617-625.
18. Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Cog Psychother. 2007;24(2):70-86.
19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psych. 2007;75(6):1000-1005.
20. National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical guideline 23. 2004. Available at: http://www.nice.org.uk/CG023NICEguideline. Accessed September 30, 2009.
21. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry. 2007;52(4):260-266.
22. Kristeller JL, Hallett B. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357-363.
23. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
24. Williams J, Alatiq Y, Crane C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
25. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
26. Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31-40.
27. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848.
28. Lau MA, Bishop SR, Segal ZV, et al. The Toronto Mindfulness Scale: development and validation. J Clin Psychol. 2006;62:1445-1467.
29. Carmody J, Reed G, Kristeller J, et al. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64(4):393-403.
30. Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol. 2008;64(7):840-862.
31. Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23-33.
32. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psych. 2002;70:275-287.
33. Lau MA, Segal ZV. Mindfulness based cognitive therapy as a relapse prevention approach to depression. In: Witkiewitz K, Marlatt A, eds. Evidence-based relapse prevention. Oxford, UK: Elsevier Press; 2007:73–90.
34. Grepmair L, Mitterlehner F, Loew T, et al. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double-blind, controlled study. Psychother Psychosom. 2007;76:332-338.
Mr. A, age 45, reports irritability, loss of interest, sleep disturbance, increased self-criticism, and decreased self care during the last month after a promotion at work. He has a history of 3 major depressive episodes, 1 of which required hospitalization. For the last 2 years his depressive symptoms had been successfully managed with escitalopram, 10 mg/d, plus bupropion, 150 mg/d. Mr. A wants to discontinue these medications because of sexual dysfunction. He asks if nonpharmacologic strategies might help.
One option to consider for Mr. A is mindfulness-based cognitive therapy (MBCT), which was originally developed to help prevent depressive relapse. MBCT also can reduce depression and anxiety symptoms. More recently, MBCT was shown to help individuals discontinue antidepressants after recovering from depression.
Regular mindfulness meditation has been shown to result in structural brain changes that may help explain how the practice effectively addresses psychiatric symptoms ( Box ). With appropriate training, psychiatrists can help patients reap the benefits of this cognitive treatment.
Regular mindfulness practice has been shown to increase cortical thickness in areas associated with attention, interoception, and sensory processing, such as the prefrontal cortex and right anterior insula.a This supports the hypothesis that mindfulness is a way of attuning the mind to one’s internal processes, and that this involves the same social neural circuits involved in interpersonal attunement—middle prefrontal regions, insula, superior temporal cortex, and the mirror neuron system.b
Amygdala responses. Mindfulness improves affect regulation by optimizing prefrontal cortex regulation of the amygdala. Recent developments in understanding the pathophysiology of depression have highlighted the lack of engagement of left lateral-ventromedial prefrontal circuitry important for the down-regulation of amygdala responses to negative stimuli.c Dispositional mindfulness is associated with greater prefrontal cortical activation and associated greater reduction in amygdala activity during affect labeling tasks, which results in enhanced affect regulation in individuals with higher levels of mindfulness.d
Left-sided anterior activation. Other researchers have examined mindfulness’ role in maintaining balanced prefrontal asymmetry. Relative left prefrontal activation is related to an affective style characterized by stronger tendencies toward positive emotional responses and approach/reward oriented behavior, whereas relative right-sided activation is associated with stronger tendencies toward negative emotional responses and avoidant/withdrawal oriented behavior.
One study found significant increases in left-sided anterior activation in mindfulness-based stress reduction participants compared with controls.e Similarly, in a study evaluating the effect of mindfulness-based cognitive therapy (MBCT) on frontal asymmetry in previously suicidal individuals, MBCT participants retained a balanced pattern of prefrontal activation, whereas the treatment-as-usual group showed significant deterioration toward decreased relative left frontal activation. These findings suggest a protective effect of the mindfulness intervention.f
Source: For references to studies described here see this article at CurrentPsychiatry.com
What is mindfulness meditation?
Meditation refers to a variety of practices that intentionally focus attention to help the practitioner disengage from unconscious absorption in thoughts and feelings. Unlike concentrative meditation—in which practitioners focus attention on a single object such as a word (mantra), body part, or external object—in mindfulness meditation participants bring their attention to a wide range of objects (such as breath, body, emotions, or thoughts) as they appear in moment-by-moment awareness.
Mindfulness is a nonjudgmental, present-centered awareness in which each thought, feeling, or sensation that arises in the attentional field is acknowledged and accepted as it is.1-3 Bishop et al4 defined a 2-component model of mindfulness:
- self-regulating attention of immediate experience, thereby allowing for increased recognition of mental events in the present moment
- adopting an orientation of curiosity, openness, and acceptance toward one’s experiences in each moment.
Mindfulness-based interventions
Buddhist and Western psychology inform the theoretical framework of most mindfulness-based clinical interventions, such as:
- acceptance and commitment therapy (ACT)
- dialectical behavioral therapy (DBT)
- mindfulness-based stress reduction (MBSR)
- MBCT.
Because mindfulness is only 1 of several components of ACT and DBT,5 this review focuses on MBCT and MBSR, in which teaching mindfulness skills is the central focus of treatment.
MBCT and MBSR. MBCT incorporates many aspects of the manualized MBSR treatment program developed for managing chronic pain.6,7 MBSR is devoted almost entirely to cultivating mindfulness through:
- formal mindfulness meditation practices such as body scan (intentionally bringing awareness to bodily sensations), mindful stretching, and mindfulness of breath/body/sounds/thoughts
- informal practices, including mindfulness of daily activities such as eating.1
MBSR typically involves 8 to 10 weekly group sessions of 2 to 2.5 hours with 10 to 40 participants with heterogeneous or homogenous clinical presentations. At each session, patients are taught mindfulness skills and practices. Typically, a full day of meditation practice on a weekend follows session 5 or 6. Participants also engage in a daily meditation practice and homework exercises directed at integrating awareness skills into daily life.
Meta-analytic and narrative reviews generally support MBSR’s efficacy for a wide range of clinical presentations, including improved quality of life for chronic pain and cancer patients.5,8-11 Variability in the methodologic rigor of clinical trials of mindfulness-based interventions—such as lack of active control groups and small sample sizes—limits the strength of these studies’ conclusions, however.8
MBCT integrates the mindfulness training of MBSR with cognitive therapy techniques ( Table 1 ) to prevent the consolidation of ruminative, negative thinking patterns that contribute to depressive relapse.2 These cognitive therapy techniques include:
- psychoeducation about depression symptoms and automatic thoughts
- exercises designed to demonstrate the cognitive model
- identifying activities that provide feelings of mastery and/or pleasure
- creating a specific relapse prevention plan.
In addition, MBCT introduces a new informal meditation—the 3-minute breathing space—to facilitate present-moment awareness in upsetting everyday situations.
Evidence supporting MBCT comes from randomized, controlled trials (RCTs) and uncontrolled trials ( Table 2 ).12-18 A systematic review of RCTs supported using MBCT in addition to usual care to prevent depressive relapse in individuals with a history of ≥3 depressive episodes.19 Since that review was published, a large RCT (123 patients) comparing antidepressant medication alone to antidepressants plus adjunctive MBCT with support to taper/discontinue antidepressant therapy found:
- MBCT comparable to maintenance antidepressant medication in preventing depressive relapse for individuals with ≥3 depressive episodes
- no difference in cost between these 2 treatments.12
In this study, MBCT was more effective than maintenance pharmacotherapy in reducing residual depressive symptoms and in improving quality of life; 75% in the MBCT group discontinued antidepressants. MBCT is included in the United Kingdom’s National Institute for Clinical Excellence Clinical Practice Guidelines for Depression20 for prevention of recurrent depression.
RCTs and uncontrolled studies have shown that MBCT reduces depressive and anxious symptoms in individuals suffering from mood disorders. In an open-label pilot study of MBCT’s efficacy in reducing depressive symptoms in patients with treatment-resistant depression and ≥3 depressive episodes, 61% of patients achieved a post-MBCT Beck Depression Inventory-II (BDI-II) score <14, which represents normal or near-normal mood (mean BDI-II scores decreased from 24.3 to 13.9; effect size 1.04).17
Mindfulness for other psychiatric conditions. A review by Toneatto and Nguyen21 of MBSR in the treatment of anxiety and depression symptoms in a range of clinical populations concluded that the evidence supporting a beneficial effect was equivocal. On the other hand, several uncontrolled studies and 1 RCT indicate that mindfulness-based treatments can reduce symptoms in other psychiatric conditions, including eating disorders,22 generalized anxiety disorder,23 bipolar disorder,24 and attention-deficit/hyperactivity disorder.25 Many of these studies were developed to target mood and anxiety symptoms by linking mindfulness and symptom management; this differs from MBSR, which focuses on stress reduction. Methodologically rigorous studies are necessary to evaluate mindfulness-based treatments in these and other psychiatric conditions.
Table 1
Skills and practices taught in mindfulness training
| MBCT session themes | Mindfulness skill | Associated practices |
|---|---|---|
| ‘Automatic pilot’ (acting without conscious awareness) | Awareness of automatic pilot Awareness of body | Mindful eating Body scan (intentionally bringing awareness to bodily sensations) |
| Dealing with barriers | Awareness of how the chatter of the mind influences feelings and behaviors | Body scan Short breathing meditation |
| Mindfulness of the breath | Awareness of breath and body | Breathing meditation 3-minute breathing space Mindful yoga |
| Staying present | Awareness of attachment and aversion | Breathing meditation Working with intense physical sensations |
| Acceptance | Acceptance of thoughts and emotions as fleeting events | Explicit instructions to practice acceptance are included in the breathing meditation and the 3-minute breathing space |
| Thoughts are not facts | Decentering or re-perceiving | Sitting meditation (awareness of thoughts) |
| How can I best take care of myself? | Awareness of signs of relapse; develop more flexible, deliberate responses at time of potential relapse | 3-minute coping breathing space |
| Dealing with future depression | Awareness of intention | Identifying coping strategies to address barriers to maintaining practice |
| MBCT: mindfulness-based cognitive therapy | ||
| Source: Reference 2 | ||
Table 2
Evidence of reduced depressive symptoms, anxiety with MBCT
| Study | Patients | Findings |
|---|---|---|
| Randomized controlled trials | ||
| Kuyken et al, 200812 | 123 patients with recurrent depression treated with antidepressants received maintenance antidepressants alone or adjunctive MBCT with support to taper/discontinue antidepressant therapy | Adjunctive MBCT was as effective as maintenance antidepressants in reducing relapse/recurrence rates but more effective in reducing residual depressive symptoms and improving quality of life; 75% in the MBCT group discontinued antidepressants |
| Kingston et al, 200713 | 19 outpatients with residual depressive symptoms following a depressive episode assigned to MBCT or treatment as usual | MBCT significantly reduced depressive symptoms, and these improvements were maintained over a 1-month follow-up period |
| Williams et al, 200814 | 14 patients with bipolar disorder who had no manic episodes in the last 6 months and ≤1 week of depressive symptoms in the last 8 weeks | MBCT resulted in a significant reduction in anxiety scores on the BAI compared with wait-list controls |
| Uncontrolled trials | ||
| Eisendrath et al, 200815 | 15 patients with treatment-resistant depression (failure to remit with ≥2 antidepressant trials) | MBCT significantly reduced anxiety and depression; increased mindfulness and decreased rumination and anxiety were associated with decreased depression |
| Finucane and Mercer, 200616 | 13 patients with recurrent depression or recurrent depression and anxiety | MBCT significantly reduced depression and anxiety scores on BDI-II and BAI |
| Kenny and Williams, 200717 | 46 depressed patients who had not fully responded to standard treatments | MBCT significantly reduced depression scores |
| Ree and Craigie, 200718 | 26 outpatients with mood and/or anxiety disorders | MBCT significantly improved symptoms of depression, anxiety, stress, and insomnia; improvements in insomnia were maintained at 3-month follow-up |
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory; MBCT: mindfulness-based cognitive therapy | ||
CASE CONTINUED: Explaining the potential benefits
You inform Mr. A that MBCT has been shown to improve acute mild-to-moderate depressive symptoms, may decrease his risk of depressive relapse by 50%26 and could help him discontinue his medications.12 He asks how mindfulness exercises will help his symptoms.
How mindfulness works
The assumption that increased mindfulness mediates treatment outcomes4 has been addressed systematically only recently, following the development of operational definitions of mindfulness and self-report mindfulness measures, including the:
- Mindful Attention Awareness Scale (MAAS)27
- Five Facet Mindfulness Questionnaire (FFMQ)12
- Toronto Mindfulness Scale (TMS).28
Uncontrolled studies using these measures demonstrated that self-reported mindfulness increased following MBSR28,29 and MBCT15,18 in individuals with general stress, anxiety disorder or primary depression, cancer, chronic pain disorder, diabetes, and multiple sclerosis. Accumulating evidence from 1 RCT30 and 2 other uncontrolled studies28,31 demonstrates that mindfulness is associated with symptom reduction following MBSR.
Researchers have begun to focus on how mindfulness skills reduce symptoms. Baer9 proposed several mechanisms, including:
- cognitive change
- improved self-management
- exposure to painful experiences leading to reduced emotional reactivity.
Cognitive change—also called meta-cognitive awareness—is the development of a “distanced “or “decentered” perspective in which patients experience their thoughts and feelings as “mental events” rather than as true, accurate versions of reality. This is thought to introduce a “space” between perception and response that enables patients to have a reflective—rather than a reflexive or reactive—response to situations, which in turn reduces vulnerability to psychological processes that contribute to emotional suffering. Some preliminary evidence suggests that MBCT-associated increases in metacognitive awareness reduce risk of depressive relapse.32
Teaching mindfulness
Guidelines for psychiatrists who wish to become MBCT instructors suggest undergoing formal teacher development training, attending a 7- to 10-day meditation retreat, and establishing your own daily mindfulness practice ( Table 3 ).33 Segal et al2 also recommend recognized training in counseling, psychotherapy, or as a mental health professional, as well as training in cognitive therapy and having experience leading psychotherapy groups.
The recommendation that a mindfulness teacher should practice meditation derives from the view that instructors teach from their own meditation experience and embody the attitudes they invite participants to practice. In an RCT, patients of psychotherapists in training (PiTs) who practiced meditation had greater symptom reductions than those of PiTs who did not engage in meditation.34
To cultivate your own mindfulness practice, consider enrolling in an MBSR group, participating in an MBCT training retreat (see Related Resources ), or attending a mindfulness meditation retreat.
Although patient access to MBCT and MBSR programs has been increasing, formal MBSR/MBCT group programs led by trained therapists are limited. Patients can go through an MBSR/MBCT book with a trained clinician or listen to audio recordings with guided meditation instructions. Alternately, they can join a meditation sitting group or an insight meditation correspondence course ( Table 4 ).
Table 3
Recommended process for becoming an MBCT instructor
| Complete a 5-day residential MBCT training program |
| Attend a 7- to 10-day residential mindfulness meditation retreat |
| Establish your own daily mindfulness meditation practice |
| Undergo professional training in cognitive therapy |
| Gain experience leading psychotherapy groups |
| MBCT: mindfulness-based cognitive therapy |
| Source: References 2,33 |
Table 4
Useful mindfulness resources for interested patients
| Insight Meditation Society: www.dharma.org |
| Kabat-Zinn J. MBSR meditation CDs/tapes: www.stressreductiontapes.com |
| Recordings of meditation (dharma) talks: www.dharmaseed.org |
| Salzberg S, Goldstein J. Insight meditation: an in-depth correspondence course. Louisville, CO: Sounds True, Inc; 2004 |
| Williams M, Teasdale J, Segal Z, et al. The mindful way through depression: freeing yourself from chronic unhappiness. New York, NY: Guilford Press; 2007 |
CASE CONTINUED: Daily mindfulness practice
Mr. A enrolls in and completes a group MBCT program. He rearranges his schedule to include 30 minutes of formal mindfulness practice daily. During an office visit after completing the MBCT course, he describes decreased irritability and self-criticism, newfound self-acceptance, an increased ability to tolerate previously distressing affect, and the ability to set realistic expectations of himself, particularly in light of increased responsibilities at work. He also reports an increased sense of engagement in and reward in his personal life.
Several months later he requests and successfully completes an antidepressant taper and has no recurrence of depressive episodes at 18-month follow-up. He participates in monthly meditation groups to support his home practice.
- Germer CK, Siegel R, Fulton PR, eds. Mindfulness and psychotherapy. New York, NY: Guilford Press; 2005.
- Mindfulness-based cognitive therapy. www.mbct.com; www.mbct.co.uk; www.bangor.ac.uk/mindfulness.
- Center for Mindfulness in Medicine, Health Care, and Society. www.umassmed.edu/cfm.
- Neurobiology of mindfulness. www.mindfulness-matters.org.
- Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: Norton; 2007.
- University of California, San Diego Center for Mindfulness. http://cme.ucsd.edu/mindfulness.
Drug brand names
- Bupropion • Wellbutrin
- Escitalopram • Lexapro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors would like to thank Amanda Yu for her assistance in preparing the manuscript.
Mr. A, age 45, reports irritability, loss of interest, sleep disturbance, increased self-criticism, and decreased self care during the last month after a promotion at work. He has a history of 3 major depressive episodes, 1 of which required hospitalization. For the last 2 years his depressive symptoms had been successfully managed with escitalopram, 10 mg/d, plus bupropion, 150 mg/d. Mr. A wants to discontinue these medications because of sexual dysfunction. He asks if nonpharmacologic strategies might help.
One option to consider for Mr. A is mindfulness-based cognitive therapy (MBCT), which was originally developed to help prevent depressive relapse. MBCT also can reduce depression and anxiety symptoms. More recently, MBCT was shown to help individuals discontinue antidepressants after recovering from depression.
Regular mindfulness meditation has been shown to result in structural brain changes that may help explain how the practice effectively addresses psychiatric symptoms ( Box ). With appropriate training, psychiatrists can help patients reap the benefits of this cognitive treatment.
Regular mindfulness practice has been shown to increase cortical thickness in areas associated with attention, interoception, and sensory processing, such as the prefrontal cortex and right anterior insula.a This supports the hypothesis that mindfulness is a way of attuning the mind to one’s internal processes, and that this involves the same social neural circuits involved in interpersonal attunement—middle prefrontal regions, insula, superior temporal cortex, and the mirror neuron system.b
Amygdala responses. Mindfulness improves affect regulation by optimizing prefrontal cortex regulation of the amygdala. Recent developments in understanding the pathophysiology of depression have highlighted the lack of engagement of left lateral-ventromedial prefrontal circuitry important for the down-regulation of amygdala responses to negative stimuli.c Dispositional mindfulness is associated with greater prefrontal cortical activation and associated greater reduction in amygdala activity during affect labeling tasks, which results in enhanced affect regulation in individuals with higher levels of mindfulness.d
Left-sided anterior activation. Other researchers have examined mindfulness’ role in maintaining balanced prefrontal asymmetry. Relative left prefrontal activation is related to an affective style characterized by stronger tendencies toward positive emotional responses and approach/reward oriented behavior, whereas relative right-sided activation is associated with stronger tendencies toward negative emotional responses and avoidant/withdrawal oriented behavior.
One study found significant increases in left-sided anterior activation in mindfulness-based stress reduction participants compared with controls.e Similarly, in a study evaluating the effect of mindfulness-based cognitive therapy (MBCT) on frontal asymmetry in previously suicidal individuals, MBCT participants retained a balanced pattern of prefrontal activation, whereas the treatment-as-usual group showed significant deterioration toward decreased relative left frontal activation. These findings suggest a protective effect of the mindfulness intervention.f
Source: For references to studies described here see this article at CurrentPsychiatry.com
What is mindfulness meditation?
Meditation refers to a variety of practices that intentionally focus attention to help the practitioner disengage from unconscious absorption in thoughts and feelings. Unlike concentrative meditation—in which practitioners focus attention on a single object such as a word (mantra), body part, or external object—in mindfulness meditation participants bring their attention to a wide range of objects (such as breath, body, emotions, or thoughts) as they appear in moment-by-moment awareness.
Mindfulness is a nonjudgmental, present-centered awareness in which each thought, feeling, or sensation that arises in the attentional field is acknowledged and accepted as it is.1-3 Bishop et al4 defined a 2-component model of mindfulness:
- self-regulating attention of immediate experience, thereby allowing for increased recognition of mental events in the present moment
- adopting an orientation of curiosity, openness, and acceptance toward one’s experiences in each moment.
Mindfulness-based interventions
Buddhist and Western psychology inform the theoretical framework of most mindfulness-based clinical interventions, such as:
- acceptance and commitment therapy (ACT)
- dialectical behavioral therapy (DBT)
- mindfulness-based stress reduction (MBSR)
- MBCT.
Because mindfulness is only 1 of several components of ACT and DBT,5 this review focuses on MBCT and MBSR, in which teaching mindfulness skills is the central focus of treatment.
MBCT and MBSR. MBCT incorporates many aspects of the manualized MBSR treatment program developed for managing chronic pain.6,7 MBSR is devoted almost entirely to cultivating mindfulness through:
- formal mindfulness meditation practices such as body scan (intentionally bringing awareness to bodily sensations), mindful stretching, and mindfulness of breath/body/sounds/thoughts
- informal practices, including mindfulness of daily activities such as eating.1
MBSR typically involves 8 to 10 weekly group sessions of 2 to 2.5 hours with 10 to 40 participants with heterogeneous or homogenous clinical presentations. At each session, patients are taught mindfulness skills and practices. Typically, a full day of meditation practice on a weekend follows session 5 or 6. Participants also engage in a daily meditation practice and homework exercises directed at integrating awareness skills into daily life.
Meta-analytic and narrative reviews generally support MBSR’s efficacy for a wide range of clinical presentations, including improved quality of life for chronic pain and cancer patients.5,8-11 Variability in the methodologic rigor of clinical trials of mindfulness-based interventions—such as lack of active control groups and small sample sizes—limits the strength of these studies’ conclusions, however.8
MBCT integrates the mindfulness training of MBSR with cognitive therapy techniques ( Table 1 ) to prevent the consolidation of ruminative, negative thinking patterns that contribute to depressive relapse.2 These cognitive therapy techniques include:
- psychoeducation about depression symptoms and automatic thoughts
- exercises designed to demonstrate the cognitive model
- identifying activities that provide feelings of mastery and/or pleasure
- creating a specific relapse prevention plan.
In addition, MBCT introduces a new informal meditation—the 3-minute breathing space—to facilitate present-moment awareness in upsetting everyday situations.
Evidence supporting MBCT comes from randomized, controlled trials (RCTs) and uncontrolled trials ( Table 2 ).12-18 A systematic review of RCTs supported using MBCT in addition to usual care to prevent depressive relapse in individuals with a history of ≥3 depressive episodes.19 Since that review was published, a large RCT (123 patients) comparing antidepressant medication alone to antidepressants plus adjunctive MBCT with support to taper/discontinue antidepressant therapy found:
- MBCT comparable to maintenance antidepressant medication in preventing depressive relapse for individuals with ≥3 depressive episodes
- no difference in cost between these 2 treatments.12
In this study, MBCT was more effective than maintenance pharmacotherapy in reducing residual depressive symptoms and in improving quality of life; 75% in the MBCT group discontinued antidepressants. MBCT is included in the United Kingdom’s National Institute for Clinical Excellence Clinical Practice Guidelines for Depression20 for prevention of recurrent depression.
RCTs and uncontrolled studies have shown that MBCT reduces depressive and anxious symptoms in individuals suffering from mood disorders. In an open-label pilot study of MBCT’s efficacy in reducing depressive symptoms in patients with treatment-resistant depression and ≥3 depressive episodes, 61% of patients achieved a post-MBCT Beck Depression Inventory-II (BDI-II) score <14, which represents normal or near-normal mood (mean BDI-II scores decreased from 24.3 to 13.9; effect size 1.04).17
Mindfulness for other psychiatric conditions. A review by Toneatto and Nguyen21 of MBSR in the treatment of anxiety and depression symptoms in a range of clinical populations concluded that the evidence supporting a beneficial effect was equivocal. On the other hand, several uncontrolled studies and 1 RCT indicate that mindfulness-based treatments can reduce symptoms in other psychiatric conditions, including eating disorders,22 generalized anxiety disorder,23 bipolar disorder,24 and attention-deficit/hyperactivity disorder.25 Many of these studies were developed to target mood and anxiety symptoms by linking mindfulness and symptom management; this differs from MBSR, which focuses on stress reduction. Methodologically rigorous studies are necessary to evaluate mindfulness-based treatments in these and other psychiatric conditions.
Table 1
Skills and practices taught in mindfulness training
| MBCT session themes | Mindfulness skill | Associated practices |
|---|---|---|
| ‘Automatic pilot’ (acting without conscious awareness) | Awareness of automatic pilot Awareness of body | Mindful eating Body scan (intentionally bringing awareness to bodily sensations) |
| Dealing with barriers | Awareness of how the chatter of the mind influences feelings and behaviors | Body scan Short breathing meditation |
| Mindfulness of the breath | Awareness of breath and body | Breathing meditation 3-minute breathing space Mindful yoga |
| Staying present | Awareness of attachment and aversion | Breathing meditation Working with intense physical sensations |
| Acceptance | Acceptance of thoughts and emotions as fleeting events | Explicit instructions to practice acceptance are included in the breathing meditation and the 3-minute breathing space |
| Thoughts are not facts | Decentering or re-perceiving | Sitting meditation (awareness of thoughts) |
| How can I best take care of myself? | Awareness of signs of relapse; develop more flexible, deliberate responses at time of potential relapse | 3-minute coping breathing space |
| Dealing with future depression | Awareness of intention | Identifying coping strategies to address barriers to maintaining practice |
| MBCT: mindfulness-based cognitive therapy | ||
| Source: Reference 2 | ||
Table 2
Evidence of reduced depressive symptoms, anxiety with MBCT
| Study | Patients | Findings |
|---|---|---|
| Randomized controlled trials | ||
| Kuyken et al, 200812 | 123 patients with recurrent depression treated with antidepressants received maintenance antidepressants alone or adjunctive MBCT with support to taper/discontinue antidepressant therapy | Adjunctive MBCT was as effective as maintenance antidepressants in reducing relapse/recurrence rates but more effective in reducing residual depressive symptoms and improving quality of life; 75% in the MBCT group discontinued antidepressants |
| Kingston et al, 200713 | 19 outpatients with residual depressive symptoms following a depressive episode assigned to MBCT or treatment as usual | MBCT significantly reduced depressive symptoms, and these improvements were maintained over a 1-month follow-up period |
| Williams et al, 200814 | 14 patients with bipolar disorder who had no manic episodes in the last 6 months and ≤1 week of depressive symptoms in the last 8 weeks | MBCT resulted in a significant reduction in anxiety scores on the BAI compared with wait-list controls |
| Uncontrolled trials | ||
| Eisendrath et al, 200815 | 15 patients with treatment-resistant depression (failure to remit with ≥2 antidepressant trials) | MBCT significantly reduced anxiety and depression; increased mindfulness and decreased rumination and anxiety were associated with decreased depression |
| Finucane and Mercer, 200616 | 13 patients with recurrent depression or recurrent depression and anxiety | MBCT significantly reduced depression and anxiety scores on BDI-II and BAI |
| Kenny and Williams, 200717 | 46 depressed patients who had not fully responded to standard treatments | MBCT significantly reduced depression scores |
| Ree and Craigie, 200718 | 26 outpatients with mood and/or anxiety disorders | MBCT significantly improved symptoms of depression, anxiety, stress, and insomnia; improvements in insomnia were maintained at 3-month follow-up |
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory; MBCT: mindfulness-based cognitive therapy | ||
CASE CONTINUED: Explaining the potential benefits
You inform Mr. A that MBCT has been shown to improve acute mild-to-moderate depressive symptoms, may decrease his risk of depressive relapse by 50%26 and could help him discontinue his medications.12 He asks how mindfulness exercises will help his symptoms.
How mindfulness works
The assumption that increased mindfulness mediates treatment outcomes4 has been addressed systematically only recently, following the development of operational definitions of mindfulness and self-report mindfulness measures, including the:
- Mindful Attention Awareness Scale (MAAS)27
- Five Facet Mindfulness Questionnaire (FFMQ)12
- Toronto Mindfulness Scale (TMS).28
Uncontrolled studies using these measures demonstrated that self-reported mindfulness increased following MBSR28,29 and MBCT15,18 in individuals with general stress, anxiety disorder or primary depression, cancer, chronic pain disorder, diabetes, and multiple sclerosis. Accumulating evidence from 1 RCT30 and 2 other uncontrolled studies28,31 demonstrates that mindfulness is associated with symptom reduction following MBSR.
Researchers have begun to focus on how mindfulness skills reduce symptoms. Baer9 proposed several mechanisms, including:
- cognitive change
- improved self-management
- exposure to painful experiences leading to reduced emotional reactivity.
Cognitive change—also called meta-cognitive awareness—is the development of a “distanced “or “decentered” perspective in which patients experience their thoughts and feelings as “mental events” rather than as true, accurate versions of reality. This is thought to introduce a “space” between perception and response that enables patients to have a reflective—rather than a reflexive or reactive—response to situations, which in turn reduces vulnerability to psychological processes that contribute to emotional suffering. Some preliminary evidence suggests that MBCT-associated increases in metacognitive awareness reduce risk of depressive relapse.32
Teaching mindfulness
Guidelines for psychiatrists who wish to become MBCT instructors suggest undergoing formal teacher development training, attending a 7- to 10-day meditation retreat, and establishing your own daily mindfulness practice ( Table 3 ).33 Segal et al2 also recommend recognized training in counseling, psychotherapy, or as a mental health professional, as well as training in cognitive therapy and having experience leading psychotherapy groups.
The recommendation that a mindfulness teacher should practice meditation derives from the view that instructors teach from their own meditation experience and embody the attitudes they invite participants to practice. In an RCT, patients of psychotherapists in training (PiTs) who practiced meditation had greater symptom reductions than those of PiTs who did not engage in meditation.34
To cultivate your own mindfulness practice, consider enrolling in an MBSR group, participating in an MBCT training retreat (see Related Resources ), or attending a mindfulness meditation retreat.
Although patient access to MBCT and MBSR programs has been increasing, formal MBSR/MBCT group programs led by trained therapists are limited. Patients can go through an MBSR/MBCT book with a trained clinician or listen to audio recordings with guided meditation instructions. Alternately, they can join a meditation sitting group or an insight meditation correspondence course ( Table 4 ).
Table 3
Recommended process for becoming an MBCT instructor
| Complete a 5-day residential MBCT training program |
| Attend a 7- to 10-day residential mindfulness meditation retreat |
| Establish your own daily mindfulness meditation practice |
| Undergo professional training in cognitive therapy |
| Gain experience leading psychotherapy groups |
| MBCT: mindfulness-based cognitive therapy |
| Source: References 2,33 |
Table 4
Useful mindfulness resources for interested patients
| Insight Meditation Society: www.dharma.org |
| Kabat-Zinn J. MBSR meditation CDs/tapes: www.stressreductiontapes.com |
| Recordings of meditation (dharma) talks: www.dharmaseed.org |
| Salzberg S, Goldstein J. Insight meditation: an in-depth correspondence course. Louisville, CO: Sounds True, Inc; 2004 |
| Williams M, Teasdale J, Segal Z, et al. The mindful way through depression: freeing yourself from chronic unhappiness. New York, NY: Guilford Press; 2007 |
CASE CONTINUED: Daily mindfulness practice
Mr. A enrolls in and completes a group MBCT program. He rearranges his schedule to include 30 minutes of formal mindfulness practice daily. During an office visit after completing the MBCT course, he describes decreased irritability and self-criticism, newfound self-acceptance, an increased ability to tolerate previously distressing affect, and the ability to set realistic expectations of himself, particularly in light of increased responsibilities at work. He also reports an increased sense of engagement in and reward in his personal life.
Several months later he requests and successfully completes an antidepressant taper and has no recurrence of depressive episodes at 18-month follow-up. He participates in monthly meditation groups to support his home practice.
- Germer CK, Siegel R, Fulton PR, eds. Mindfulness and psychotherapy. New York, NY: Guilford Press; 2005.
- Mindfulness-based cognitive therapy. www.mbct.com; www.mbct.co.uk; www.bangor.ac.uk/mindfulness.
- Center for Mindfulness in Medicine, Health Care, and Society. www.umassmed.edu/cfm.
- Neurobiology of mindfulness. www.mindfulness-matters.org.
- Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: Norton; 2007.
- University of California, San Diego Center for Mindfulness. http://cme.ucsd.edu/mindfulness.
Drug brand names
- Bupropion • Wellbutrin
- Escitalopram • Lexapro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors would like to thank Amanda Yu for her assistance in preparing the manuscript.
1. Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Dell Publishing; 1990.
2. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
3. Shapiro SL, Schwartz GE. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;15:128-134.
4. Bishop SR, Lau MA, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pr. 2004;11:230-241.
5. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211-237.
6. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33-47.
7. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190.
8. Bishop SR. What do we really know about mindfulness-based stress reduction? Am Psychosom Soc. 2002;64:71-83.
9. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10(2):125-143.
10. Grossman P, Nieman L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
11. Salmon P, Sephton S, Weissbecker I, et al. Mindfulness meditation in clinical practice. Cog Behav Ther. 2004;11(4):434-446.
12. Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psych. 2008;76(6):966-978.
13. Kingston T, Dooley B, Bates A, et al. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007;80:193-203.
14. Williams J, Alatiq Y, Crance C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
15. Eisendrath SJ, Delucchi K, Bitner R, et al. Mindfulness-based cognitive therapy for treatment resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319-320.
16. Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14.-
17. Kenny MA, Williams JGM. Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45(3):617-625.
18. Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Cog Psychother. 2007;24(2):70-86.
19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psych. 2007;75(6):1000-1005.
20. National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical guideline 23. 2004. Available at: http://www.nice.org.uk/CG023NICEguideline. Accessed September 30, 2009.
21. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry. 2007;52(4):260-266.
22. Kristeller JL, Hallett B. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357-363.
23. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
24. Williams J, Alatiq Y, Crane C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
25. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
26. Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31-40.
27. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848.
28. Lau MA, Bishop SR, Segal ZV, et al. The Toronto Mindfulness Scale: development and validation. J Clin Psychol. 2006;62:1445-1467.
29. Carmody J, Reed G, Kristeller J, et al. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64(4):393-403.
30. Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol. 2008;64(7):840-862.
31. Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23-33.
32. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psych. 2002;70:275-287.
33. Lau MA, Segal ZV. Mindfulness based cognitive therapy as a relapse prevention approach to depression. In: Witkiewitz K, Marlatt A, eds. Evidence-based relapse prevention. Oxford, UK: Elsevier Press; 2007:73–90.
34. Grepmair L, Mitterlehner F, Loew T, et al. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double-blind, controlled study. Psychother Psychosom. 2007;76:332-338.
1. Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Dell Publishing; 1990.
2. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
3. Shapiro SL, Schwartz GE. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;15:128-134.
4. Bishop SR, Lau MA, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pr. 2004;11:230-241.
5. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211-237.
6. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33-47.
7. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190.
8. Bishop SR. What do we really know about mindfulness-based stress reduction? Am Psychosom Soc. 2002;64:71-83.
9. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10(2):125-143.
10. Grossman P, Nieman L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
11. Salmon P, Sephton S, Weissbecker I, et al. Mindfulness meditation in clinical practice. Cog Behav Ther. 2004;11(4):434-446.
12. Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psych. 2008;76(6):966-978.
13. Kingston T, Dooley B, Bates A, et al. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007;80:193-203.
14. Williams J, Alatiq Y, Crance C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
15. Eisendrath SJ, Delucchi K, Bitner R, et al. Mindfulness-based cognitive therapy for treatment resistant depression: a pilot study. Psychother Psychosom. 2008;77(5):319-320.
16. Finucane A, Mercer SW. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006;6:14.-
17. Kenny MA, Williams JGM. Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45(3):617-625.
18. Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Cog Psychother. 2007;24(2):70-86.
19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psych. 2007;75(6):1000-1005.
20. National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical guideline 23. 2004. Available at: http://www.nice.org.uk/CG023NICEguideline. Accessed September 30, 2009.
21. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry. 2007;52(4):260-266.
22. Kristeller JL, Hallett B. An exploratory study of a meditation-based intervention for binge eating disorder. J Health Psychol. 1999;4(3):357-363.
23. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
24. Williams J, Alatiq Y, Crane C, et al. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107(2):275-279.
25. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746.
26. Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31-40.
27. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848.
28. Lau MA, Bishop SR, Segal ZV, et al. The Toronto Mindfulness Scale: development and validation. J Clin Psychol. 2006;62:1445-1467.
29. Carmody J, Reed G, Kristeller J, et al. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64(4):393-403.
30. Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol. 2008;64(7):840-862.
31. Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23-33.
32. Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psych. 2002;70:275-287.
33. Lau MA, Segal ZV. Mindfulness based cognitive therapy as a relapse prevention approach to depression. In: Witkiewitz K, Marlatt A, eds. Evidence-based relapse prevention. Oxford, UK: Elsevier Press; 2007:73–90.
34. Grepmair L, Mitterlehner F, Loew T, et al. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double-blind, controlled study. Psychother Psychosom. 2007;76:332-338.
Late-life depression: Managing mood in patients with vascular disease
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.